Avoidance of bottles during the establishment of breast feeds in preterm infants
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005252.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 18 octubre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
CT Collins wrote the protocol, searched for studies, extracted data, analysed data and wrote the review.
J Gillis contributed to the protocol, extracted data and commented on drafts of the review.
AJ McPhee contributed to the protocol and commented on drafts of the review.
H Suganuma extracted data and commented on drafts of the review.
M Makrides contributed to the protocol and commented on drafts of the review.
Sources of support
Internal sources
-
South Australian Health and Medical Research Institute, Adelaide, South Australia, Australia.
-
Neonatal Medicine and Special Care Baby Unit, Women's and Children's Hospital, North Adelaide, South Australia, Australia.
-
Salary for Carmel Collins was drawn from an MS McLeod Postdoctoral Research Fellowship (MS McLeod Research Fund, Women’s and Children’s Hospital Research Foundation), Australia.
-
Salary for Maria Makrides was drawn from a National Health and Medical Research Council Principal Research Fellowship (APP1061704), Australia.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
CT Collins and AJ McPhee were investigators responsible for one of the studies included in this review (Collins 2004). J Gillis is a clinical nurse in the Special Care Baby Unit, where one of the included studies was undertaken (Collins 2004).
Acknowledgements
We gratefully thank the authors of Kliethermes 1999 and Gilks 2004 for providing additional information about their studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Oct 21 | Avoidance of bottles during the establishment of breastfeeds in preterm infants | Review | Elizabeth Allen, Alice R Rumbold, Amy Keir, Carmel T Collins, Jennifer Gillis, Hiroki Suganuma | |
| 2016 Oct 18 | Avoidance of bottles during the establishment of breast feeds in preterm infants | Review | Carmel T Collins, Jennifer Gillis, Andrew J McPhee, Hiroki Suganuma, Maria Makrides | |
| 2016 Sep 30 | Avoidance of bottles during the establishment of breast feeds in preterm infants | Review | Carmel T Collins, Jennifer Gillis, Andrew J McPhee, Hiroki Suganuma, Maria Makrides | |
| 2008 Oct 08 | Avoidance of bottles during the establishment of breast feeds in preterm infants | Review | Carmel T Collins, Maria Makrides, Jennifer Gillis, Andrew J McPhee | |
| 2005 Apr 20 | Avoidance of bottles during the establishment of breast feeds in preterm infants | Protocol | Carmel T Collins, Maria Makrides, Jennifer Gillis, Andrew J McPhee | |
Differences between protocol and review
We added the methods and plan for Summary of findings tables and GRADE recommendations, which were not included in the original protocol.
For the 2016 review, we added infection events as an outcome.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Infant, Newborn;
PICO

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
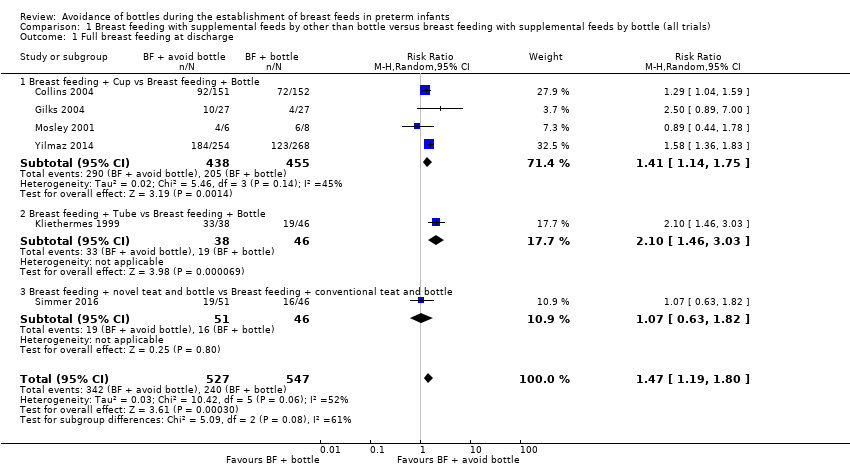
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 1 Full breast feeding at discharge.
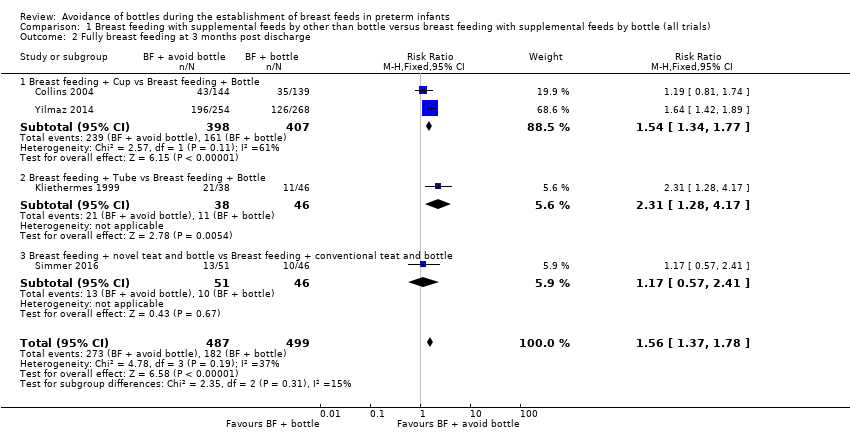
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 2 Fully breast feeding at 3 months post discharge.
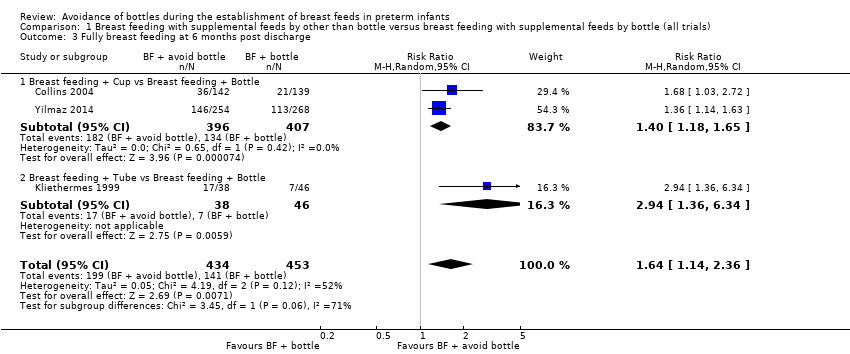
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 3 Fully breast feeding at 6 months post discharge.
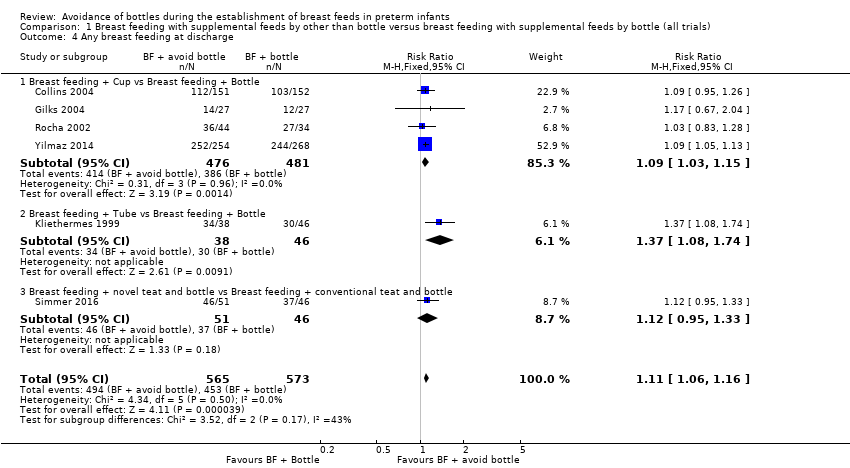
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 4 Any breast feeding at discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 5 Any breast feeding at 3 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 6 Any breast feeding at 6 months post discharge.

Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 7 Days to reach full sucking feeds.
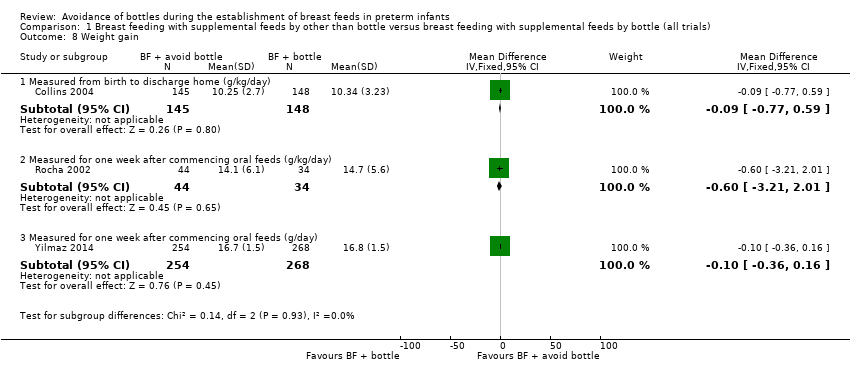
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 8 Weight gain.
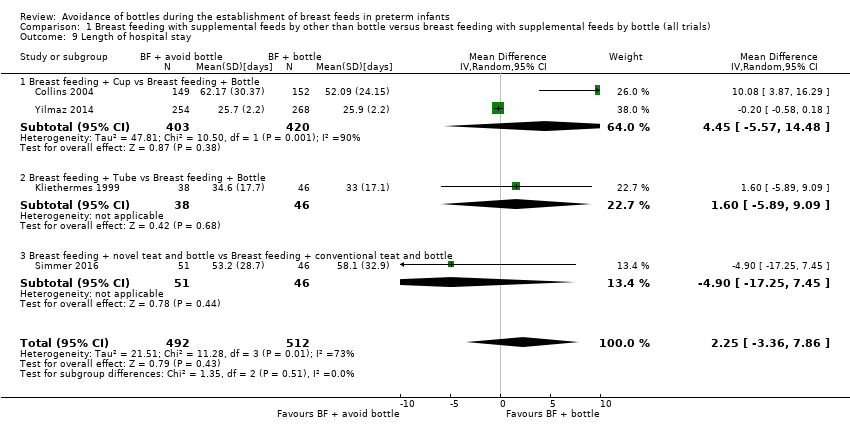
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 9 Length of hospital stay.
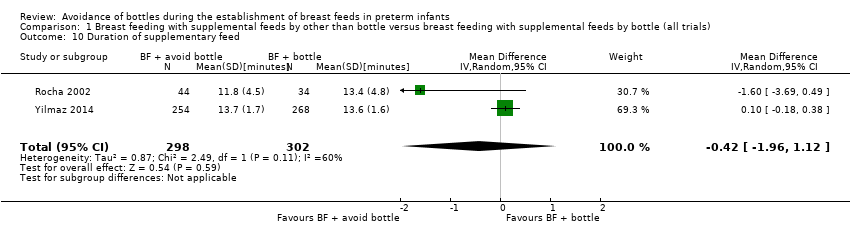
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 10 Duration of supplementary feed.
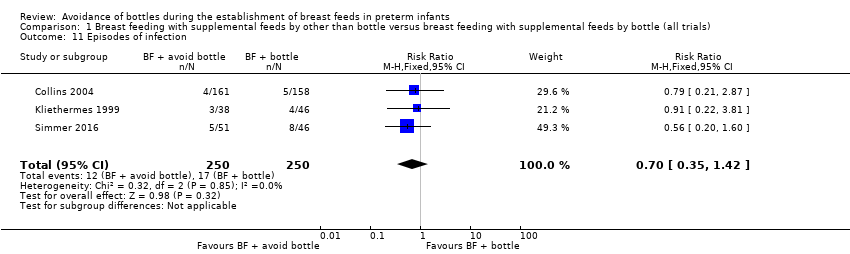
Comparison 1 Breast feeding with supplemental feeds by other than bottle versus breast feeding with supplemental feeds by bottle (all trials), Outcome 11 Episodes of infection.
| Breast feeding with supplemental feeds by other than bottle compared with breast feeding with supplemental feeds by bottle (all trials) in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with breast feeding with supplemental feeds by bottle (all trials) | Risk with breast feeding with supplemental feeds by other than bottle | |||||
| Full breast feeding at discharge | Study population | RR 1.47 | 1074 | ⊕⊕⊝⊝ | ||
| 44 per 100 | 64 per 100 | |||||
| Full breast feeding at 3 months post discharge | Study population | RR 1.56 | 986 | ⊕⊕⊕⊝ | ||
| 36 per 100 | 57 per 100 | |||||
| Full breast feeding at 6 months post discharge | Study population | RR 1.64 | 887 | ⊕⊕⊝⊝ | ||
| 31 per 100 | 51 per 100 | |||||
| Any breast feeding at discharge | Study population | RR 1.11 | 1138 | ⊕⊕⊕⊝ | ||
| 79 per 100 | 88 per 100 | |||||
| Any breast feeding at 3 months post discharge | Study population | RR 1.31 | 1063 | ⊕⊝⊝⊝ | ||
| 60 per 100 | 78 per 100 | |||||
| Any breast feeding at 6 months post discharge | Study population | RR 1.25 | 886 | ⊕⊝⊝⊝ | ||
| 45 per 100 | 56 per 100 | |||||
| Length of hospital stay | MD 2.25 higher | ‐ | 1004 | ⊕⊝⊝⊝ | ||
| Episodes of infection | Study population | RR 0.70 | 500 | ⊕⊕⊕⊝ | ||
| 7 per 100 | 5 per 100 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAttrition bias (14% and 15% attrition in two included studies). bModerate heterogeneity (I2 = 52%). cModerate heterogeneity (I2 = 73%). dModerate heterogeneity (I2 = 71%). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Full breast feeding at discharge Show forest plot | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.19, 1.80] |
| 1.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 893 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.14, 1.75] |
| 1.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.46, 3.03] |
| 1.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.63, 1.82] |
| 2 Fully breast feeding at 3 months post discharge Show forest plot | 4 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.78] |
| 2.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.34, 1.77] |
| 2.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.28, 4.17] |
| 2.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.57, 2.41] |
| 3 Fully breast feeding at 6 months post discharge Show forest plot | 3 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.14, 2.36] |
| 3.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.18, 1.65] |
| 3.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.36, 6.34] |
| 4 Any breast feeding at discharge Show forest plot | 6 | 1138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.16] |
| 4.1 Breast feeding + Cup vs Breast feeding + Bottle | 4 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [1.03, 1.15] |
| 4.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.08, 1.74] |
| 4.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.95, 1.33] |
| 5 Any breast feeding at 3 months post discharge Show forest plot | 5 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.01, 1.71] |
| 5.1 Breast feeding + Cup vs Breast feeding + Bottle | 3 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.89, 1.71] |
| 5.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.19, 2.41] |
| 5.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.80] |
| 6 Any breast feeding at 6 months post discharge Show forest plot | 3 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.10, 1.41] |
| 6.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
| 6.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.18, 3.64] |
| 7 Days to reach full sucking feeds Show forest plot | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 2.56 [‐7.17, 12.28] |
| 7.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 332 | Mean Difference (IV, Random, 95% CI) | 5.08 [‐6.43, 16.59] |
| 7.2 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐15.63, 7.63] |
| 8 Weight gain Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Measured from birth to discharge home (g/kg/day) | 1 | 293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.77, 0.59] |
| 8.2 Measured for one week after commencing oral feeds (g/kg/day) | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.21, 2.01] |
| 8.3 Measured for one week after commencing oral feeds (g/day) | 1 | 522 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 9 Length of hospital stay Show forest plot | 4 | 1004 | Mean Difference (IV, Random, 95% CI) | 2.25 [‐3.36, 7.86] |
| 9.1 Breast feeding + Cup vs Breast feeding + Bottle | 2 | 823 | Mean Difference (IV, Random, 95% CI) | 4.45 [‐5.57, 14.48] |
| 9.2 Breast feeding + Tube vs Breast feeding + Bottle | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.89, 9.09] |
| 9.3 Breast feeding + novel teat and bottle vs Breast feeding + conventional teat and bottle | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐17.25, 7.45] |
| 10 Duration of supplementary feed Show forest plot | 2 | 600 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐1.96, 1.12] |
| 11 Episodes of infection Show forest plot | 3 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.42] |

