Escisión mesorrectal total abierta versus laparoscópica para el cáncer rectal
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT Sao Paulo, Brazil Inclusion period: September 1997 to September 2000 | |
| Participants | n = 28 (LTME n = 13; OTME n= 15) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | No primary outcome stated | |
| Notes | Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomised to undergo treatment" |
| Allocation concealment (selection bias) | Unclear risk | Unknown, moment of randomisation unknown |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not described, intention‐to‐treat not described |
| Selective reporting (reporting bias) | High risk | No primary outcome stated, not all data given as described in Methods section No sample size calculation |
| Other bias | High risk | Published in a non‐peer‐reviewed journal, Low diversity with distal rectal cancer only Surgeon's experience unknown |
| Blinding of participants and personnel (performance bias) | Unclear risk | Surgical procedure described according to TME Postoperative protocol unknown |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Single‐centre RCT Milan, Italy Inclusion period: unknown | |
| Participants | n = 168 (LTME n = 83; OTME n = 85) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Short‐term postoperative morbidity | |
| Notes | Enlarged subgroup from Braga 2002 Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list, sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Opened before induction of anaesthesia |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Survival given per stage, not combined and only in Kaplan‐Meier curve Sample size calculation performed |
| Other bias | Low risk | Same surgical team, "well experienced" |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure according to TME |
| Blinding of outcome assessment (detection bias) | Low risk | Complications registered by independent team, |
| Methods | Multicentre RCT (30 academic centres) Belgium, Canada, Denmark, Germany, The Netherlands, Spain, South Korea, Sweden Inclusion period: January 2004 to May 2010 | |
| Participants | n = 1044 (LTME n = 699; OTME n = 345) | |
| Interventions | Laparoscopic vs open TME LAR (%): 70 vs 77 Colon (%): 0 Neoadjuvant therapy: radiotherapy 59% vs 58%, chemotherapy 32% vs 34% | |
| Outcomes | Primary outcome: local recurrences at 3 years (will be published later) Secondary outcomes: Operating time, conversion rate, blood loss, postoperative recovery of gastrointestinal function, postoperative pain medication, length of hospital stay, morbidity and mortality within 28 days after surgery, histopathological outcomes and anastomotic leakage. | |
| Notes | COLOR 2 b 2011 presents a local subgroup of 40 participants focusing on inflammatory response markers Funding or conflicts of interest: Funding by Ethicon Endo‐Surgery Europe, Swedish Cancer Foundation, West Gothia Region and Sahlgrenska University Hospital. The authors declare to have no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised via online register with randomisation list stratified for centre, tumour location and preoperative radiotherapy |
| Allocation concealment (selection bias) | Low risk | Central randomisation after registration in database |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Sample size calculation performed |
| Other bias | Low risk | Surgeon's technique and resection quality assessed prior to enrolment |
| Blinding of participants and personnel (performance bias) | Low risk | Local standardised postoperative protocols |
| Blinding of outcome assessment (detection bias) | Unclear risk | Objective measurements |
| Methods | Single‐centre RCT (University Hospital) Amsterdam, The Netherlands Inclusion period: June 2006 to December 2008 | |
| Participants | n = 40 (LTME n = 22; OTME n = 18) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcomes :Postoperative inflammatory response (IL‐6, IL‐8, CRP), immune status (WBC, HLA‐DR), stress response (cortisol, prolactin, growth hormone) | |
| Notes | Local subgroup of COLOR 2 a 2013 Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised within COLOR II trial, computer‐generated list, stratified for preoperative radiotherapy and location of tumour |
| Allocation concealment (selection bias) | Low risk | Randomisation after entry of participant details in database |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No sample size calculation for this sub study |
| Other bias | High risk | Surgeon's experience: unknown |
| Blinding of participants and personnel (performance bias) | Low risk | Standardised postoperative protocol per hospital |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measurements |
| Methods | Two‐centre RCT Hong Kong, China Inclusion period: September 1993 to October 2002 | |
| Participants | n = 403 (LTME n = 203; OTME n = 200) | |
| Interventions | Laparoscopic vs open | |
| Outcomes | Primary outcome: 5‐year disease‐free survival, | |
| Notes | Short‐term data rectosigmoid subgroup Funding or conflicts of interest: The authors declare no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Kept concealed by an independent operating theatre co‐ordinator |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Sample size calculation performed Ratio between sigmoid and rectal cancer not given |
| Other bias | High risk | High diversity rectosigmoid carcinoma, ratio not given Surgeon's experience: "Skilled in both laparoscopic and open colorectal surgery" |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure described Standardised postoperative protocol (no enhanced recovery programme) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Two‐centre RCT Hong Kong, China Number of patients assessed for eligibility but not randomised unknown Inclusion period: September 1993 to October 2002 | |
| Participants | n = 153 (LTME n = 76; OTME n = 77) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Long‐term morbidity (adhesion‐related obstruction, incisional hernia) | |
| Notes | Long‐term data upper rectal cancer subgroup of Hong Kong a 2004 Short‐term mortality not included in long‐term morbidity and mortality analysis Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Low diversity with upper rectal subgroup |
| Other bias | High risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Single‐centre RCT Hong Kong, China Inclusion period: September 1996 to April 1998 | |
| Participants | n = 34 (LTME n = 17 ; OTME n = 17 ) | |
| Interventions | Laparoscopic vs open | |
| Outcomes | Primary outcome: cytokine and CRP response | |
| Notes | Smaller subgroup rectosigmoid Hong Kong a 2004 Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | No missing data reported, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Sample size calculated |
| Other bias | High risk | High diversity with sigmoid and rectum carcinoma, ratio not given Surgeon's experience: "Skilled in both laparoscopic and open colorectal surgery" |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measurements |
| Methods | Single‐centre RCT Hong Kong, China Inclusion period: June 1998 to August 1999 | |
| Participants | n = 40 (LTME n = 20; OTME n = 20) | |
| Interventions | Laparoscopic vs open | |
| Outcomes | Primary outcome: lymphocyte subpopulation and natural killer cell cytotoxicity | |
| Notes | Smaller subgroup rectosigmoid Hong Kong a 2004 Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | Low risk | no missing data reported, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Sample size calculated |
| Other bias | High risk | High diversity rectosigmoid carcinoma, ratio not given Surgeon's experience: "Skilled in both laparoscopic and open colorectal surgery" |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measurements |
| Methods | Multicenter RCT (3 centres) Seoul, South Corea Inclusion period: April 2006 to August 2009 | |
| Participants | n = 340 (LTME n = 170; OTME n =170) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: 3‐year disease‐free survival | |
| Notes | Long‐term data expected in 2013 Funding or conflicts of interest: National cancer centre, South Corea. The authors declared no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone trial co‐ordinator, block permutation approach |
| Allocation concealment (selection bias) | Unclear risk | Telephone trial co‐ordinator, moment of randomisation unknown |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | No missing data Sample size calculation performed |
| Other bias | Low risk | Low diversity with mid/low rectal cancer cT3N0‐2 |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure according to TME Standardised postoperative protocol, no enhanced recovery |
| Blinding of outcome assessment (detection bias) | Low risk | Pathologists blinded |
| Methods | Single‐centre RCT Yeovil, United Kingdom January 2002 to March 2004 | |
| Participants | n = 62 (LTME n = 41; OTME n = 19) (rectal n = 19) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Hospital stay | |
| Notes | Funding or conflicts of interest: National Health Service Developments in the Organization of Care Projects Grant. Yeovil District Hospital has received funds from Ethicon Endosurgery to support postgraduate training in | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Telephone trial co‐ordinator, moment of randomisation unclear |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Data reported according to methods described No sample size for these outcomes calculated |
| Other bias | Unclear risk | High diversity, all colorectal patients Single surgeon, experience unknown |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure described according to TME Postoperative protocol according to enhanced recovery programme |
| Blinding of outcome assessment (detection bias) | Low risk | Data collection team |
| Methods | Single‐centre RCT Taiyuan, China Number of patients assessed for eligibility but not randomised: 3 Inclusion period: May 2004 and April 2008 | |
| Participants | n = 343 (LTME n = 169; OTME n = 174) Inclusion criteria: rectal cancer confirmed by pathological examination, written informed consent. Suitable for LAR or APR. Exclusion criteria: metastatic disease, BMI > 30, acute intestinal obstruction, previous abdominal surgery, neoadjuvant chemotherapy Age (y): 57.3 vs 57.4 (mean) Dukes stage (%): A 5.3 vs 4.0; B 42.6 vs 48.3; C 52.1 vs 47.7; D 0 vs 0 Tumour location: rectum Follow‐up: 44 months (median) | |
| Interventions | Laparoscopic versus open TME APR (%): 49.1 vs 40.2 LAR (%): 50.9 vs 59.8 Colon(%): 0 (neo)adjuvant therapy: neoadjuvant excluded, adjuvant unknown | |
| Outcomes | Primary outcome: 3‐year survival Secondary outcomes: Number of lymph nodes removed, length of specimen, distance between inferior border of tumour and incised margin in LAR, time to first discharge, bowel movement and fluid intake, infectious complications, anastomotic leakage, anastomotic stenosis, deep vein thrombosis, 1‐year survival | |
| Notes | Funding or conflicts of interest: No competing financial interests declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not mentioned |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes, day before surgery |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Sample size calculation not performed |
| Other bias | Unclear risk | Distance for anal verge unknown Single surgical team |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure not described, TME principles followed Standardised postoperative protocol (no enhanced recovery programme) |
| Blinding of outcome assessment (detection bias) | Low risk | Complications assessed by reviewer unaware of treatment group |
| Methods | Single‐centre RCT Hangzhou, China Inclusion period: February 2005 and October 2008 | |
| Participants | n = 186 (LTME n = 98; OMTE n = 88) | |
| Interventions | Laparoscopic vs open TME, hand‐assisted | |
| Outcomes | Primary outcome: "safety and efficacy" | |
| Notes | Hand‐assisted laparoscopy Funding or conflicts of interest: The authors declared no conflicts of interest in relation to this article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | High risk | Unknown |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not described, intention‐to‐treat irrelevant with no conversions |
| Selective reporting (reporting bias) | High risk | Sample size calculation not performed |
| Other bias | Unclear risk | Distance from anal verge unknown Single surgical team, experience unknown |
| Blinding of participants and personnel (performance bias) | High risk | Surgical procedure described, TME unknown No standardised postoperative protocol |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Single‐centre RCT Murcia, Spain Inclusion period: January 2002 and February 2007 | |
| Participants | n = 204 (LTME n = 103; OTME n = 101) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: number of lymph nodes harvested Secondary outcomes: 2‐ and 5‐year local recurrence, survival, circumferential margin involvement, complication rate, hospital stay | |
| Notes | Funding or conflicts of interest: The authors declare no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelope until day of operation |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up described, intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Non‐radical resections excluded from analysis Sample size calculation performed |
| Other bias | Unclear risk | Single surgical team, experience unknown |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure described according to TME |
| Blinding of outcome assessment (detection bias) | Low risk | Single experienced pathologist |
| Methods | Multicenter RCT (27 centres) Inclusion period: July 1996 to July 2002 | |
| Participants | n = 794 (381 rectal LTME n = 253; OTME n = 128 ) | |
| Interventions | Laparoscopic vs open colorectal surgery | |
| Outcomes | Primary outcomes: resection margins, Dukes C2 tumours, in‐hospital mortality, 3 and 5 year OS/DFS and local recurrence | |
| Notes | Short term results, short‐term costs, 3‐year, 5‐year and 10‐year data of the CLASICC Trial across 5 different publications. Funding or conflicts of interest: The authors declare to have no conflict of interest. The trial was funded by the UK Medical Research Council. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone trial co‐ordinator, stratified by surgeon, site of surgery, presence of metastases and preoperative radiotherapy |
| Allocation concealment (selection bias) | Low risk | Telephone trial co‐ordinator |
| Incomplete outcome data (attrition bias) | Low risk | Loss to follow‐up and missing data described, intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | High rate of missing participant and pathological data, up to 13% Sample size calculation performed, but not reached |
| Other bias | Low risk | High diversity with colorectal cancer patients, specific rectal cancer data published separately Surgeons' experience: a minimum of 20 laparoscopic resections |
| Blinding of participants and personnel (performance bias) | High risk | Surgical procedure according to surgeons current practice |
| Blinding of outcome assessment (detection bias) | Low risk | Data monitoring committee |
| Methods | Multicenter RCT (27 centres) Inclusion period: July 1996 to July 2002 | |
| Participants | n = 148 (LTME n = 98; OTME n =50), n = 347 including laparoscopic colon group | |
| Interventions | Laparoscopic colon versus laparoscopic rectal versus open rectal | |
| Outcomes | Primary outcome: Overall function score for sexual and bladder function | |
| Notes | Subgroup of MRC CLASICC a 2005 Converted patients analysed as open surgery Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Most data only addressed in text, numbers not given Sample size calculated for questionnaire outcome |
| Other bias | Unclear risk | No other bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unknown |
| Blinding of outcome assessment (detection bias) | Low risk | Validated questionnaires |
| Methods | Single‐centre RCT Singapore Inclusion period: March 1997 to August 1999 | |
| Participants | n = 236 (LTME n = 118; OTME n = 118) | |
| Interventions | Laparoscopic vs open colorectal surgery | |
| Outcomes | Primary outcome: T‐cell number | |
| Notes | Singapore subgroup MRC CLASICC a 2005 Funding or conflicts of interest: Funding by the National Reseach Council Singapore, no statement on conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation |
| Allocation concealment (selection bias) | Low risk | Blocks of 6 and 4 |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis, missing data addressed Sample size calculation performed |
| Selective reporting (reporting bias) | High risk | High rate of missing data (12 participants preoperative, 44 postoperative) |
| Other bias | Unclear risk | 1:1 randomisation, in contrast to 2:1 randomisation in CLASICC Trial Surgeons' experience as MRC CLASICC a 2005 > 20 procedures |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure described according to TME |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measurements |
| Methods | Single‐centre RCT Hong Kong, China Inclusion period: September 1994 to February 2005 | |
| Participants | n = 99 (LTME n = 51; OTME n = 48) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Analgesic requirement and postoperative recovery | |
| Notes | Low and mid rectal cancer subgroup Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Kept concealed by an independent operating theatre co‐ordinator |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis, loss to follow‐up described |
| Selective reporting (reporting bias) | Low risk | Follow‐up for participants alive Sample size calculation performed |
| Other bias | Low risk | Surgeons' experience: "surgeons experienced in both laparoscopic and colorectal surgery" |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure, TME unknown Standardised postoperative protocol, no enhanced recovery |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Multicenter RCT (3 centres) Crete and Athens, Greece Inclusion period: unknown | |
| Participants | n = 73 (LTME n = 34; OTME n = 39) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Oncological clearance (number of lymph nodes) | |
| Notes | Funding or conflicts of interest: No statement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | High risk | Unknown |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up and intention‐to‐treat not described Only one outcome Limited details on inclusion and exclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No sample size calculation |
| Other bias | High risk | Significantly less anastomoses and more ileostomies in the laparoscopic group Surgeon's experience: "most experienced surgeon" |
| Blinding of participants and personnel (performance bias) | Low risk | Surgical procedure described according to TME Postoperative protocol irrelevant for outcome |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding described |
| Methods | Single‐centre RCT Sichuan, China Inclusion period: June 2001 to September 2002 | |
| Participants | n = 171 (LTME n = 82; OMTE n = 89) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome: Feasibility and efficacy and short‐term outcomes | |
| Notes | Funding or conflicts of interest: Funded by a National Outstanding Youth Foundation of China grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The grouping was randomised |
| Allocation concealment (selection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | High risk | Loss to follow‐up and intention‐to‐treat analysis unknown, conversion rate unknown |
| Selective reporting (reporting bias) | High risk | No sample size calculation |
| Other bias | Unclear risk | Surgeons' experience: 4 colorectal surgeons, experience unknown |
| Blinding of participants and personnel (performance bias) | High risk | No standardised postoperative protocol described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Single‐centre RCT Shijiazhuang, China Inclusion period: December 2004 to April 2007 | |
| Participants | n = 71 (LTME n = 36; OTME n = 35) | |
| Interventions | Laparoscopic vs open TME | |
| Outcomes | Primary outcome not stated Outcomes: Body temperature, WBC count, CRP level, Cortisol level, IL‐6 level, VAS score at ‐1, 1, 3 and 5 days | |
| Notes | Article translated from Chinese Funding or conflicts of interest: Science and Research Fund of The Second Hospital of Hebei Medical University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) | High risk | Conversion and intention‐to‐treat unknown |
| Selective reporting (reporting bias) | Unclear risk | No sample size calculation |
| Other bias | High risk | Surgeon's experience: unknown |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unknown |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measurements |
APR: abdominoperineal resection
AR: anterior resection
ASA: American Society of Anaesthesiologists
CI: Confidence interval
CRP: C‐reative protein
CT: computed tomography
EORTC: European Organization for the Research and Treatment of Cancer
FAP: familial adenomatous polyposis
FSFI: Female sexual function index
HLA‐DR: Human Leukocyte Antigen D related
HNPCC: hereditary non‐polyposis colorectal cancer
HRQL: health‐related quality of life
IIEF: Internation index of erectile function
I‐PSS: International prostate symptom score
LAR: lower anterior resection
MRI: magnetic resonance imaging
QLQ‐CR38: Quality of life questionnaire ‐ colorectal cancer‐specific
TME: total mesorectal excision
VAS: visual analogue scale
WBC: white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Colorectal benign disease included, extended subgroup of rectal cancer participants described in Braga 2007 | |
| Data on colorectal participants, extended subgroup of rectal cancer participants described in Braga 2007 | |
| Colon cancer including rectosigmoid, rectal cancer excluded. | |
| Mid and low rectum excluded, number of upper rectum within proctosigmoid group unclear. | |
| Unknown number of rectal cancer participants included (anterior resection, left and right colectomy) | |
| Colorectal participants, unknown number of rectal carcinoma > 12 cm included. | |
| Only partially randomised and no intention‐to‐treat analysis | |
| No TME performed (D3 lymphadenectomy) | |
| Benign disease included, no separate analysis on rectal cancer. | |
| Almost all participants were randomised within 2 other trials (MRC CLASICC a 2005; King 2006), not fully randomised | |
| Comparison between CLASICC Trial data and national registry | |
| Surgeon in steep learning curve during study. Significant differences in outcome between early and late inclusion groups. No numerical outcomes provided in abstract, no full‐text available. | |
| Benign disease and familial polyposis coli participants included | |
| Sphincter‐preserving resection with TME is exclusion criterion | |
| Economic comparison between UK and USA trials | |
| Non‐randomised, single arm phase II trial |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A La CaRT: Australasian Laparoscopic Cancer of the Rectum Trial A phase III prospective randomised trial comparing laparoscopic‐assisted resection versus open resection for rectal cancer |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: Histological diagnosis of adenocarcinoma of the rectum (<15cm from the anal verge as measured by rigid sigmoidoscopy), T 1‐3 N0 M0, T1‐3 N1 M0 or T1‐3 N0‐1 M1 disease as determined by pre‐treatment CT scans and pelvic MRI or EUS. For patients with T3 or N1 disease, completion of pre‐operative 5FU‐based chemotherapy and/or radiation therapy. Capecitabine may be substituted for 5FU, Age >18 years, ECOG Performance Status: 0, 1 or 2, Written informed consent, Life expectancy of at least 12 weeks. Exclusion criteria: Medical or psychiatric conditions that compromise the patient's ability to give informed consent or comply with the study protocol. Pregnancy or breast feeding. Any uncontrolled concurrent medical condition. Any co‐morbid disease that would increase risk of morbidity. Participation in any investigational drug study within the previous 4 weeks. Evidence of T4 disease extending to circumferential margin of rectum or invading adjacent organs. Evidence of systemic disease (cardiovascular, renal, hepatic, etc.) that would preclude surgery, or other severe incapacitating disease, ASA IV or ASA V. History of conditions that would preclude use of a laparoscopic approach (e.g. multiple previous major laparotomies, severe adhesions). Concurrent or previous invasive pelvic malignancy (cervical, uterine and rectal) within five years prior to registration. |
| Interventions | Laparoscopic‐assisted resection versus open resection |
| Outcomes | To determine whether laparoscopic‐assisted resection is not inferior to open rectal resection as a safe, effective oncologic approach to rectal cancer and secondary from a patient related benefit perspective, based on morbidity, mortality associated with surgery, disease‐free survival and disease recurrence and quality of life |
| Starting date | March 2010 |
| Contact information | Dr. Andrew Stevenson, c/o A La CaRT Trial Coordinator NHMRC Clinical Trials Centre Locked Bag 77, Camperdown, 1450, Australia. [email protected] |
| Notes | Patient recruitment ongoing |
| Trial name or title | A phase III prospective randomized trial comparing laparoscopic‐assisted resection versus open resection for rectal cancer ‐ ACOSOG Z6051 |
| Methods | Randomised controlled trial |
| Participants | Inclusion: Histologically confirmed adenocarcinoma of the rectum (<12 cm from the anal verge), T3, N0, M0 or T1‐3, N1‐2, M0 disease by pre‐neoadjuvant therapy CT scans and pelvic MRI or transrectal ultrasound. Completed neoadjuvant fluorouracil‐based chemotherapy and/or radiotherapy within the past 4 weeks (Capecitabine may have been substituted for fluorouracil), ECOG performance status 0 ‐ 2, |
| Interventions | Laparoscopic versus open rectal surgery |
| Outcomes | Primary outcomes: Circumferential margin > 1 mm, Distal resected margin > 2 cm (or > 1 cm with clear frozen section in the low rectum), Completeness of total mesorectal excision |
| Starting date | August 2008 |
| Contact information | James Fleshman, MD. American College of Surgeons Oncology Group. [email protected] |
| Notes | Patient recruitment ongoing until Dec 2013 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐free survival Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Survival and recurrences, Outcome 1 Disease‐free survival. | ||||
| 1.1 10‐year | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.06] |
| 1.2 5‐year | 4 | 943 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.38] |
| 1.3 3‐year | 1 | 326 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.74] |
| 2 Overall survival Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Survival and recurrences, Outcome 2 Overall survival. | ||||
| 2.1 10‐year | 2 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 2.2 5‐year | 4 | 987 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.87, 1.52] |
| 2.3 3‐year | 2 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.42] |
| 3 Local recurrences Show forest plot | 8 | 1538 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.39] |
| Analysis 1.3  Comparison 1 Survival and recurrences, Outcome 3 Local recurrences. | ||||
| 3.1 5‐year | 5 | 963 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.81] |
| 3.2 3‐year | 3 | 575 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.56] |
| 4 Distant recurrences Show forest plot | 6 | 1341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.32] |
| Analysis 1.4  Comparison 1 Survival and recurrences, Outcome 4 Distant recurrences. | ||||
| 5 Wound/port site metastases Show forest plot | 7 | 2130 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.75, 10.20] |
| Analysis 1.5  Comparison 1 Survival and recurrences, Outcome 5 Wound/port site metastases. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||
| 1 Lymph nodes retrieved Show forest plot | 11 | 3682 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.13, 0.26] | ||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 Surgical data, Outcome 1 Lymph nodes retrieved. | ||||||||||||||||||||||||||||||||||||
| 2 CRM positivity Show forest plot | 8 | 2313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.40] | ||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 Surgical data, Outcome 2 CRM positivity. | ||||||||||||||||||||||||||||||||||||
| 3 Duration of surgery Show forest plot | 12 | 3840 | Mean Difference (IV, Random, 95% CI) | 37.48 [27.80, 47.15] | ||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 Surgical data, Outcome 3 Duration of surgery. | ||||||||||||||||||||||||||||||||||||
| 4 Incision length Show forest plot | 4 | 1488 | Mean Difference (IV, Random, 95% CI) | ‐12.83 [‐14.87, ‐10.80] | ||||||||||||||||||||||||||||||||
| Analysis 2.4  Comparison 2 Surgical data, Outcome 4 Incision length. | ||||||||||||||||||||||||||||||||||||
| 5 Conversion rate Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| Analysis 2.5
Comparison 2 Surgical data, Outcome 5 Conversion rate. | ||||||||||||||||||||||||||||||||||||
| 6 Blood loss Show forest plot | 8 | 2615 | Mean Difference (IV, Random, 95% CI) | ‐101.78 [‐147.57, ‐55.98] | ||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 Surgical data, Outcome 6 Blood loss. | ||||||||||||||||||||||||||||||||||||
| 7 Transfusion requirement Show forest plot | 5 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.62] | ||||||||||||||||||||||||||||||||
| Analysis 2.7  Comparison 2 Surgical data, Outcome 7 Transfusion requirement. | ||||||||||||||||||||||||||||||||||||
| 8 Intraoperative morbidity Show forest plot | 4 | 1618 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.18] | ||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 Surgical data, Outcome 8 Intraoperative morbidity. | ||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30‐day morbidity (total) Show forest plot | 11 | 3397 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| Analysis 3.1  Comparison 3 Short‐term morbidity and mortality, Outcome 1 30‐day morbidity (total). | ||||
| 2 Wound infection Show forest plot | 10 | 3337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.93] |
| Analysis 3.2  Comparison 3 Short‐term morbidity and mortality, Outcome 2 Wound infection. | ||||
| 3 Bleeding Show forest plot | 5 | 1181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.93] |
| Analysis 3.3  Comparison 3 Short‐term morbidity and mortality, Outcome 3 Bleeding. | ||||
| 4 Urinary complications Show forest plot | 8 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.83, 1.81] |
| Analysis 3.4  Comparison 3 Short‐term morbidity and mortality, Outcome 4 Urinary complications. | ||||
| 5 Pneumonia Show forest plot | 8 | 2668 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.83, 2.09] |
| Analysis 3.5  Comparison 3 Short‐term morbidity and mortality, Outcome 5 Pneumonia. | ||||
| 6 Anastomotic leakage Show forest plot | 10 | 2505 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| Analysis 3.6  Comparison 3 Short‐term morbidity and mortality, Outcome 6 Anastomotic leakage. | ||||
| 7 Need for reoperation Show forest plot | 7 | 2316 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.20] |
| Analysis 3.7  Comparison 3 Short‐term morbidity and mortality, Outcome 7 Need for reoperation. | ||||
| 8 30‐day mortality Show forest plot | 11 | 3812 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.32] |
| Analysis 3.8  Comparison 3 Short‐term morbidity and mortality, Outcome 8 30‐day mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Analgesia use (number of doses) Show forest plot | 5 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.93, ‐0.27] |
| Analysis 4.1  Comparison 4 Postoperative recovery, Outcome 1 Analgesia use (number of doses). | ||||
| 2 Day 1 pain score (VAS) Show forest plot | 3 | 776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.04, ‐0.44] |
| Analysis 4.2  Comparison 4 Postoperative recovery, Outcome 2 Day 1 pain score (VAS). | ||||
| 3 Hospital stay (days) Show forest plot | 11 | 3084 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐3.22, ‐1.10] |
| Analysis 4.3  Comparison 4 Postoperative recovery, Outcome 3 Hospital stay (days). | ||||
| 4 Time to normal diet (days) Show forest plot | 8 | 2109 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.80, ‐0.23] |
| Analysis 4.4  Comparison 4 Postoperative recovery, Outcome 4 Time to normal diet (days). | ||||
| 5 Time to first defecation (days) Show forest plot | 8 | 2893 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.17, ‐0.54] |
| Analysis 4.5  Comparison 4 Postoperative recovery, Outcome 5 Time to first defecation (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incisional hernia Show forest plot | 3 | 508 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.32, 2.21] |
| Analysis 5.1  Comparison 5 Long term morbidity, Outcome 1 Incisional hernia. | ||||
| 2 Intestinal obstruction Show forest plot | 3 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.75] |
| Analysis 5.2  Comparison 5 Long term morbidity, Outcome 2 Intestinal obstruction. | ||||
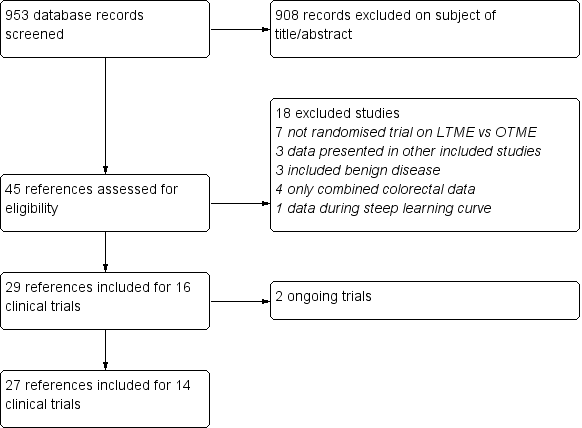
Study selection flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 2 Survival and recurrences, outcome: 2.1 Disease free survival.
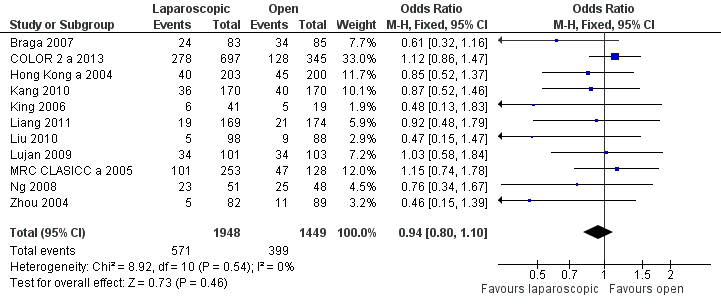
Forest plot of comparison: 4 Short term morbidity and mortality, outcome: 4.1 30d morbidity (total).

Forest plot of comparison: 5 Post op recovery, outcome: 5.3 Hospital stay.

Comparison 1 Survival and recurrences, Outcome 1 Disease‐free survival.

Comparison 1 Survival and recurrences, Outcome 2 Overall survival.
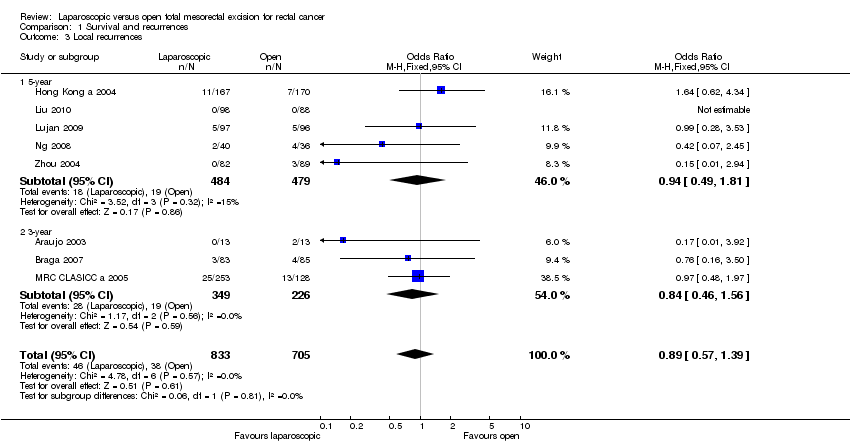
Comparison 1 Survival and recurrences, Outcome 3 Local recurrences.

Comparison 1 Survival and recurrences, Outcome 4 Distant recurrences.

Comparison 1 Survival and recurrences, Outcome 5 Wound/port site metastases.
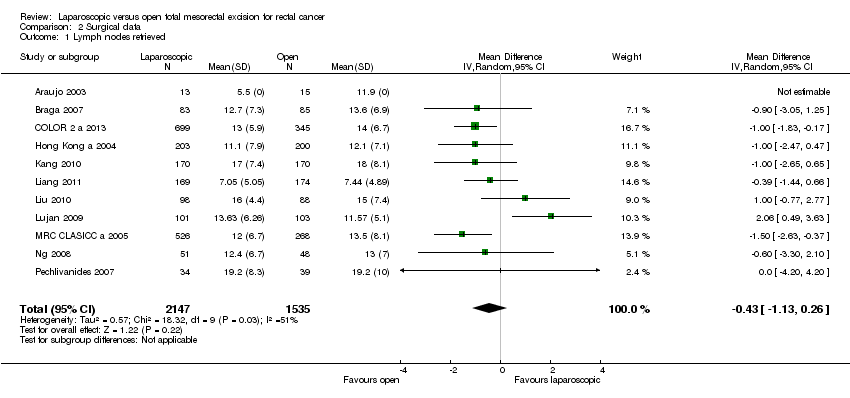
Comparison 2 Surgical data, Outcome 1 Lymph nodes retrieved.

Comparison 2 Surgical data, Outcome 2 CRM positivity.

Comparison 2 Surgical data, Outcome 3 Duration of surgery.

Comparison 2 Surgical data, Outcome 4 Incision length.
| Study | |
| Araujo 2003 | 0 (0/13) |
| Braga 2007 | 7.2 (6/83) |
| COLOR 2 a 2013 | 17 (121/695) |
| Hong Kong a 2004 | 23.2 (47/203) |
| Kang 2010 | 1.2 (2/170) |
| King 2006 | 7.3 (3/41) |
| Liang 2011 | 0.5 (1/169) |
| Liu 2010 | 0 (0/98) |
| Lujan 2009 | 7.9 (8/101) |
| MRC CLASICC a 2005 | 33.9 (82/242) |
| Ng 2008 | 9.8 (5/51) |
| Pechlivanides 2007 | 2.9 (1/34) |
| Zhou 2004 | Unknown |
| Zhou 2007 | Unknown |
Comparison 2 Surgical data, Outcome 5 Conversion rate.
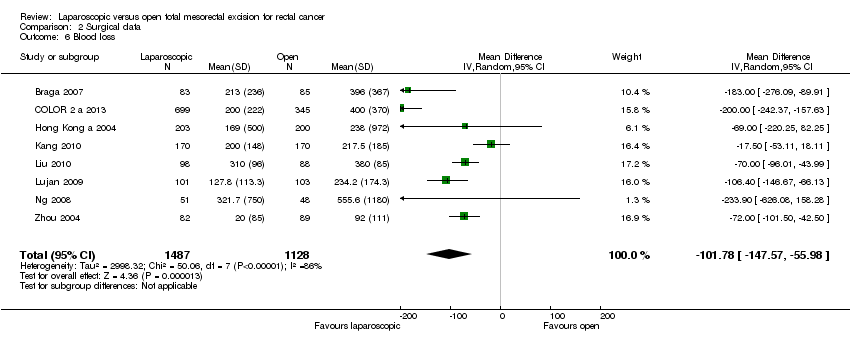
Comparison 2 Surgical data, Outcome 6 Blood loss.
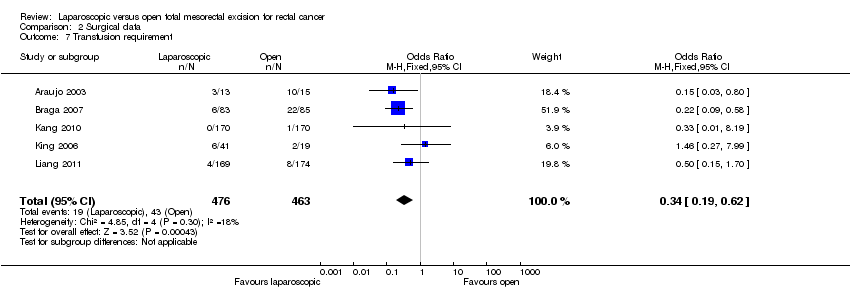
Comparison 2 Surgical data, Outcome 7 Transfusion requirement.

Comparison 2 Surgical data, Outcome 8 Intraoperative morbidity.
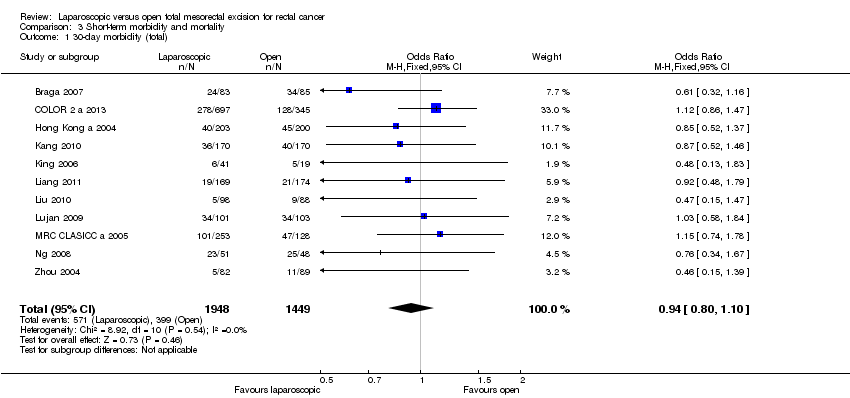
Comparison 3 Short‐term morbidity and mortality, Outcome 1 30‐day morbidity (total).
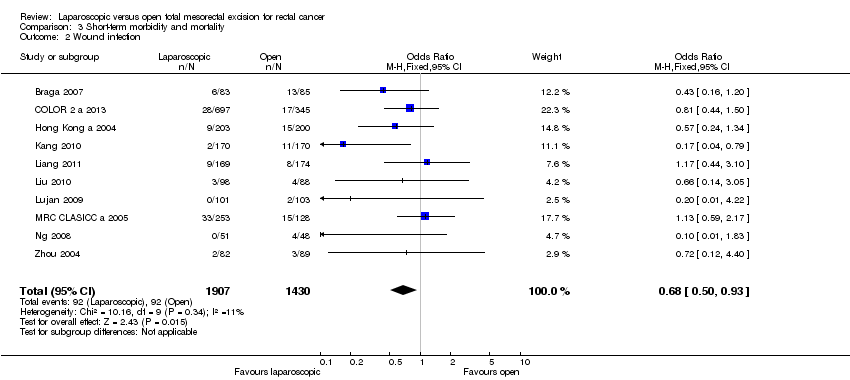
Comparison 3 Short‐term morbidity and mortality, Outcome 2 Wound infection.

Comparison 3 Short‐term morbidity and mortality, Outcome 3 Bleeding.
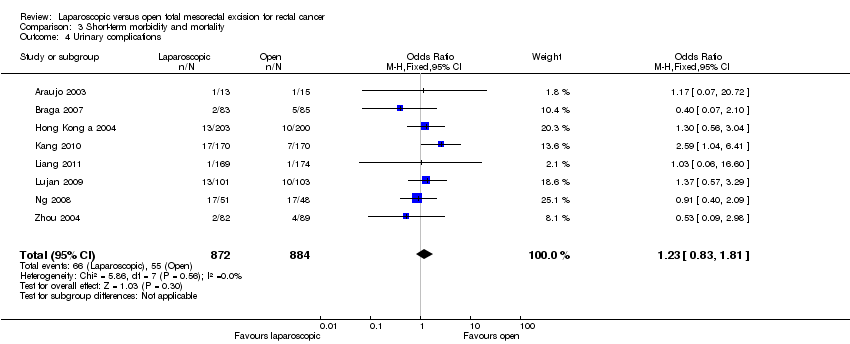
Comparison 3 Short‐term morbidity and mortality, Outcome 4 Urinary complications.
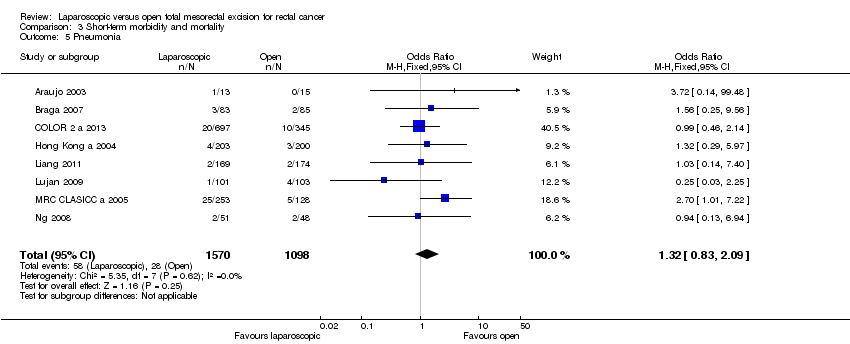
Comparison 3 Short‐term morbidity and mortality, Outcome 5 Pneumonia.
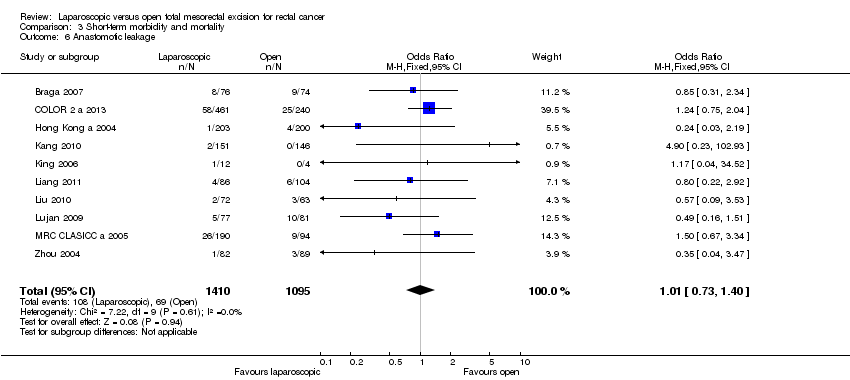
Comparison 3 Short‐term morbidity and mortality, Outcome 6 Anastomotic leakage.

Comparison 3 Short‐term morbidity and mortality, Outcome 7 Need for reoperation.

Comparison 3 Short‐term morbidity and mortality, Outcome 8 30‐day mortality.
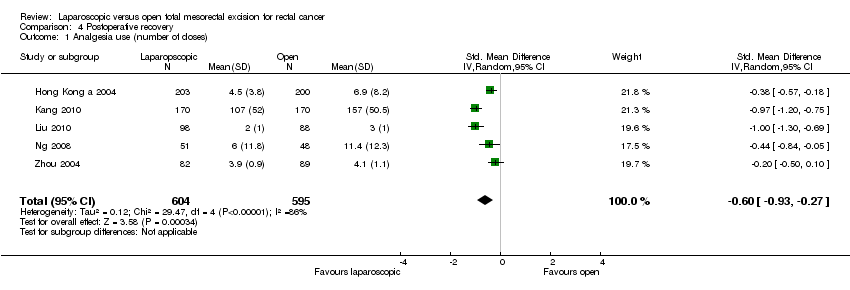
Comparison 4 Postoperative recovery, Outcome 1 Analgesia use (number of doses).
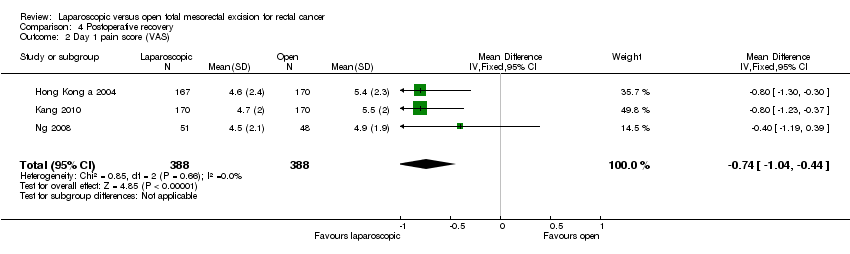
Comparison 4 Postoperative recovery, Outcome 2 Day 1 pain score (VAS).
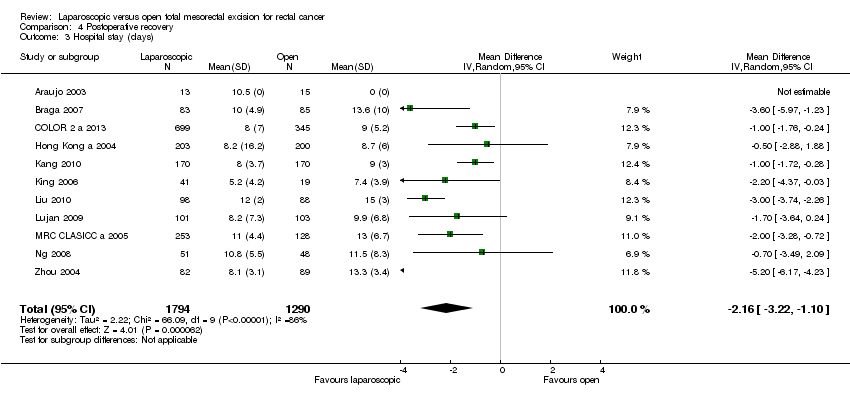
Comparison 4 Postoperative recovery, Outcome 3 Hospital stay (days).
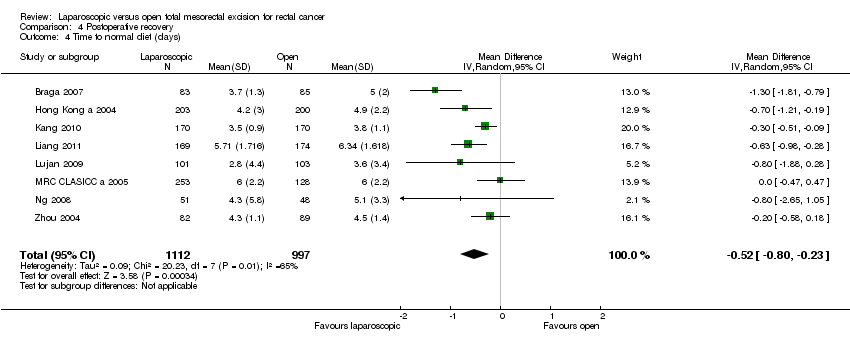
Comparison 4 Postoperative recovery, Outcome 4 Time to normal diet (days).

Comparison 4 Postoperative recovery, Outcome 5 Time to first defecation (days).

Comparison 5 Long term morbidity, Outcome 1 Incisional hernia.
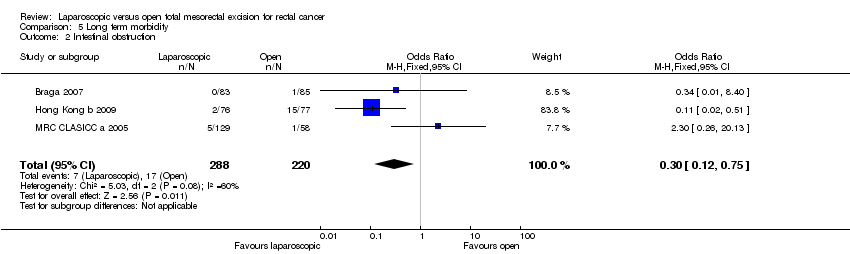
Comparison 5 Long term morbidity, Outcome 2 Intestinal obstruction.
| Laparoscopic versus open total mesorectal excision (TME) for rectal cancer | |||||
| Patient or population: people with Rectal Cancer | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Open TME | Laparoscopic TME | ||||
| Disease‐free survival at 5 years | 718 per 1000 | 722 per 1000 | OR 1.02 | 943 | ⊕⊕⊕⊝ |
| Overall survival at 5 years | 679 per 1000 | 709 per 1000 | OR 1.15 | 987 | ⊕⊕⊕⊝ |
| Local recurrences | 54 per 1000 | 48 per 1000 | OR 0.89 | 1538 | ⊕⊕⊕⊝ |
| Lymph nodes retrieved | The mean number of lymph nodes retrieved in the intervention groups was | 3682 | ⊕⊕⊕⊕ | ||
| CRM positivity | 61 per 1000 | 60 per 1000 | OR 0.99 | 2313 | ⊕⊕⊕⊝ |
| 30‐day morbidity (total) | 275 per 1000 | 263 per 1000 | OR 0.94 | 3397 | ⊕⊕⊕⊝ |
| Hospital stay (days) | The mean length of hospital stay in the intervention groups was | 3084 | ⊕⊕⊕⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Statistical inaccuracy with wide confidence interval at both sides 2Statistical inaccuracy with wide confidence interval at both sides, but a tendency for a higher overall survival for LTME 3Statistical inaccuracy with wide confidence interval at both sides, but a tendency for a lower recurrence rate for LTME 4Only 8 studies provided data on CRM positivity | |||||
| Study ID | n | Long‐term survival | 30‐day mortality | 30‐day morbidity | Long‐term morbidity | Lymphnodes | Gastrointestinal recovery | Pain | Bleeding | Length of hospital stay | Immune response | Quality of life | Cost |
| 28 | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | + | + | ‐ | ‐ | ‐ | |
| 168 | 5y/3y | + | + | + | + | + | ‐ | + | + | ‐ | + | + | |
| 1044 | ‐ | + | + | ‐ | + | + | + | + | + | ‐ | ‐ | ‐ | |
| 40 | ‐ | + | + | ‐ | + | ‐ | ‐ | + | + | + | ‐ | ‐ | |
| 403 | 5y | + | + | ‐ | + | + | + | + | + | ‐ | ‐ | + | |
| 153 | 10y | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 34 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ | |
| 40 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ | |
| 340 | ‐ | + | + | ‐ | + | + | + | + | + | ‐ | + | ‐ | |
| 19 | ‐ | + | + | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | + | + | |
| 343 | 3y | + | + | ‐ | + | + | ‐ | + | ‐ | ‐ | ‐ | ‐ | |
| 186 | ‐ | + | + | ‐ | + | ‐ | ‐ | + | + | ‐ | ‐ | ‐ | |
| 204 | 5y | + | + | ‐ | + | + | ‐ | + | + | ‐ | ‐ | ‐ | |
| 381 | 10y/5y/3y | + | + | ‐ | + | + | ‐ | ‐ | + | ‐ | + | ‐ | |
| 148 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | |
| 236 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ | |
| 99 | 5y | + | + | ‐ | + | + | + | + | + | ‐ | ‐ | + | |
| 73 | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 171 | ‐ | + | + | ‐ | ‐ | + | ‐ | + | + | ‐ | ‐ | ‐ | |
| 71 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | + | ‐ | ‐ |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Disease‐free survival Show forest plot | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 10‐year | 1 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.06] |
| 1.2 5‐year | 4 | 943 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.38] |
| 1.3 3‐year | 1 | 326 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.74] |
| 2 Overall survival Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 10‐year | 2 | 534 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 2.2 5‐year | 4 | 987 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.87, 1.52] |
| 2.3 3‐year | 2 | 682 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.70, 1.42] |
| 3 Local recurrences Show forest plot | 8 | 1538 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.39] |
| 3.1 5‐year | 5 | 963 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.49, 1.81] |
| 3.2 3‐year | 3 | 575 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.56] |
| 4 Distant recurrences Show forest plot | 6 | 1341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.32] |
| 5 Wound/port site metastases Show forest plot | 7 | 2130 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.75, 10.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lymph nodes retrieved Show forest plot | 11 | 3682 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.13, 0.26] |
| 2 CRM positivity Show forest plot | 8 | 2313 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.40] |
| 3 Duration of surgery Show forest plot | 12 | 3840 | Mean Difference (IV, Random, 95% CI) | 37.48 [27.80, 47.15] |
| 4 Incision length Show forest plot | 4 | 1488 | Mean Difference (IV, Random, 95% CI) | ‐12.83 [‐14.87, ‐10.80] |
| 5 Conversion rate Show forest plot | Other data | No numeric data | ||
| 6 Blood loss Show forest plot | 8 | 2615 | Mean Difference (IV, Random, 95% CI) | ‐101.78 [‐147.57, ‐55.98] |
| 7 Transfusion requirement Show forest plot | 5 | 939 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.19, 0.62] |
| 8 Intraoperative morbidity Show forest plot | 4 | 1618 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 30‐day morbidity (total) Show forest plot | 11 | 3397 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 2 Wound infection Show forest plot | 10 | 3337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.50, 0.93] |
| 3 Bleeding Show forest plot | 5 | 1181 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.93] |
| 4 Urinary complications Show forest plot | 8 | 1756 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.83, 1.81] |
| 5 Pneumonia Show forest plot | 8 | 2668 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.83, 2.09] |
| 6 Anastomotic leakage Show forest plot | 10 | 2505 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 7 Need for reoperation Show forest plot | 7 | 2316 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.20] |
| 8 30‐day mortality Show forest plot | 11 | 3812 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Analgesia use (number of doses) Show forest plot | 5 | 1199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.93, ‐0.27] |
| 2 Day 1 pain score (VAS) Show forest plot | 3 | 776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.04, ‐0.44] |
| 3 Hospital stay (days) Show forest plot | 11 | 3084 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐3.22, ‐1.10] |
| 4 Time to normal diet (days) Show forest plot | 8 | 2109 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.80, ‐0.23] |
| 5 Time to first defecation (days) Show forest plot | 8 | 2893 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.17, ‐0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incisional hernia Show forest plot | 3 | 508 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.32, 2.21] |
| 2 Intestinal obstruction Show forest plot | 3 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.75] |

