Intervenciones para mejorar el cumplimiento de la higiene de manos en la atención al paciente
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: ITS Study period: 16‐week baseline period (June to Sept 2008) followed by a 16‐week post‐intervention period (Oct 6 2008 to Jan 24 2009) then 75‐week maintenance period (Jan 25 2009 to July 4 2010) | |
| Participants | All healthcare workers in a 17‐bed medical ICU | |
| Interventions | Video cameras recorded attempts at hand hygiene; feedback was given to staff in a variety of ways including continuous display of hand hygiene rates on electronic boards in hallways and detailed summaries sent to managers by email | |
| Outcomes | Hand hygiene compliance, defined as percentage of hand hygiene opportunities where hand hygiene was attempted within 10 seconds before or after access to a room | |
| Notes | Appropriate analysis for ITS Third‐party auditors remotely assessed video recordings Funding source: New York State Department of Health Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Presence of video cameras so staff aware of being monitored |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were not assessed blindly, although third‐party auditors were used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there were other campaigns, outbreaks, changes in staffing etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after intervention |
| Methods | Design: ITS Study period: May 2008‐April 2011 6 month baseline period, 7 month intervention period, 11 month follow up | |
| Participants | Europe. 13 ICUs | |
| Interventions | Multimodal campaign based on WHO 5 Moments | |
| Outcomes | Direct observation of hand hygiene; not clear for how long or how often | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: European Commission Declaration of interest: None They also conducted a cluster‐randomised trial related to screening and barrier use which did not have hand hygiene as an outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | High risk | Data collectors were nurses from the study units trained in data collection |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in the different study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Other changes occurred in phase 3 of the study re screening for MRSA and other pathogens, plus concurrent use of barrier and contact precautions |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
| Methods | Non‐randomised trial in 1 centre in the USA Study period: 3 observation days in a 3‐week period: day 1, day 14, day 21. Dates not stated. | |
| Participants | All healthcare workers | |
| Interventions | Visual light as reminder | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random allocation |
| Allocation concealment (selection bias) | Low risk | Room assigned to be intervention or control room prior to start of study |
| Blinding of participants and personnel (performance bias) | High risk | Participants were aware of observer and purpose of the light |
| Blinding of outcome assessment (detection bias) | High risk | Blinding was not possible |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | No baseline hand hygiene compliance assessed |
| Baseline characteristics | High risk | No report of characteristics of patients, staff or room set‐up |
| Protection from contamination | High risk | The same staff entered both rooms and were aware of the light cue in the intervention room |
| Methods | Design: cluster‐randomised trial Study period: Dates not stated Baseline period of 14 weeks, then phase 2 was 6 weeks (real‐time reminders) then phase 3 was 4 weeks (added individual feedback) | |
| Participants | Healthcare workers in cardiology ward and SICU | |
| Interventions | Wireless monitoring system of hand hygiene with real‐time reminders and individual feedback Control: no intervention | |
| Outcomes | Compliance with hand hygiene measured by system ABHR use (L per bed day) | |
| Notes | Inappropriate analysis: Unclear reporting of regression Electronic monitoring so observer effect not a concern Funding source: Centre for Integration of Medicine and Innovative Technology which is licensed to HandGenix and by the Agency for Science, Technology and Research (Singapore). The equipment and its installation was paid for by HandGenix Declaration of interest: One of the co‐authors, S.Schiefen, holds shares in HandGenix | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was by profession and performed at start of study |
| Blinding of participants and personnel (performance bias) | High risk | Visible and audible wireless technology so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Observers were members of the study team and not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Slightly more non‐participation in control group but this was unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Unclear risk | Those in control group could potentially hear reminder beep given to those in intervention group |
| Methods | Design: stepped‐wedge cluster‐randomised trial Study period: Campagin rolled out in all centres between December 2004‐June 2005; data were collected from October 1, 2006‐December 31, 2009. 36 month trial overall, with units added to intervention at different periods in time | |
| Participants | Healthcare workers in acute care and ICU: 60 wards in 16 hospitals | |
| Interventions | Feedback and personalised action planning plus National 'Clean Your Hands' campaign Control: 'Clean Your Hands' campaign only | |
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis for stepped wedge Funding source: Patient Safety Research Programme and Trustees of the Royal Free Hospital Declaration of interest: Cookson and Stone have received consultancy fees from GoJo industries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospitals were given a number, then the numbers were randomly allocated to arm using a research randomiser website |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the ward and was done at the start of the study |
| Blinding of participants and personnel (performance bias) | High risk | Included feedback and personalised action planning so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were assessed blindly |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing data (missed opportunities) unlikely to be very different in different arms Difficult to compare loss to follow‐up in both groups because of their different composition of types of units |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Only 12 wards participated in MRSA swabbing but all participated in hand hygiene assessment |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported; they reported relative changes from baseline with baseline as reference point |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Individualised unit‐based intervention so even if control units heard about it, they could not have the intervention |
| Methods | Design: pair‐matched cluster‐randomised trial Study period: Dates not stated Pre‐test: hand hygiene observations over a 2‐week period with no sign Post‐test: hand hygiene observations over a two 2‐week period with 1 of 2 signs displayed | |
| Participants | 3 categories of healthcare workers: MDs, nurses, and ancillary workers | |
| Interventions | 1 of 2 signs displayed. Signs had message related to personal consequences or to patient consequences | |
| Outcomes | Hand hygiene compliance | |
| Notes | Incorrect analysis: analysed by units rather than matched analysis Covert observation so observer effect unlikely to be a threat Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the ward and was done at the start of the study |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were aware of the signs but were not informed of the research underway |
| Blinding of outcome assessment (detection bias) | Low risk | Observers were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be different in each arm. All units remained in study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline hand hygiene rates for all 3 types of healthcare workers |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Participants were aware of the signs but were not informed of the research underway |
| Methods | Design: ITS Study period: Baseline for 2 months in November ‐ December 2009 and multimodal campaign to end of 2010. Then in autumn 2011 an e‐learning hand hygiene game was added; it was moved from ward to ward on a mobile station. Data collected until end of first quarter of 2012 | |
| Participants | Healthcare workers in 1 hospital | |
| Interventions | An e‐learning hand hygiene game: 1 week per unit, twice in1 year. Staff members had multiple opportunities to use it during that time on unit | |
| Outcomes | Hand hygiene compliance | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | E‐learning hand hygiene game stations used so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | E‐learning hand hygiene game stations used and visible so observers aware of intervention |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | None noted |
| Intervention independent | High risk | Extra ABHR stations added during the study period and there were 2 interventions occurring at the same time: 1) multimodal and 2) e‐learning hand hygiene game |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
| Methods | Design: cluster‐randomised trial with 2 intervention groups and 1 control group Study period: Hand hygiene observations occurred at baseline, intervention, 1 month post‐intervention and 4 months post‐intervention Duration of observation periods were not reported but totaled 333 hours between November 2009 and July 2010 | |
| Participants | Healthcare workers in 18 long‐term care facilities | |
| Interventions | WHO multimodal strategy including posters, reminders, education, pocket‐sized bottles of ABHR for personal use, and feedback. In addition, 1 test group received powdered disposable gloves and the other test group received powderless disposable gloves Control: 2‐hour health talk | |
| Outcomes | Hand hygiene compliance, defined as proportion of hand hygiene opportunities resulting in compliant action. Number of respiratory outbreaks and MRSA infections requiring hospital admission | |
| Notes | Logistic regression with GEE to account for clustering but did not compare changes between arms so inappropriate analysis Funding source: Centre for Health Protection, Hong Kong SAR, China Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 72 homes allocated to arm with a random‐number generator, then called in randomly selected order until 6 homes successfully recruited per group |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study |
| Blinding of participants and personnel (performance bias) | High risk | Included posters and feedback so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were not assessed blindly |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Possible selection bias as unclear who refused/did not have a chance to participate |
| Baseline outcomes | Unclear risk | Some difference in baseline hand hygiene compliance in the 3 groups (19.5, 27 and 22) |
| Baseline characteristics | Unclear risk | There were gender differences and a difference in the proportion of residents with dementia between arms |
| Protection from contamination | Low risk | Allocation was by institution |
| Methods | Design: RCT Questionnaires and observations done at baseline and at 4 months post‐intervention | |
| Participants | Nurses throughout a hospital | |
| Interventions | Education, mainly universal precautions | |
| Outcomes | % of nurses washing hands before and after patient contact Also evaluated knowledge scores, prevalence of Hepatitis B immunisation, self‐reported behaviours related to blood‐borne pathogens and universal precautions, self‐reported needlestick and sharps injury, and observed behaviours related to handling used needles. | |
| Notes | Intervention successful after 4 months Appropriate analysis Funding source: No information given Declaration of Interest: No information given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how were randomly selected to participate nor randomly allocated to group |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done at the start of the study but method was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Education and questionnaire were very specific so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Researchers did not specify if observers were blinded |
| Incomplete outcome data (attrition bias) | Low risk | 98% follow‐up achieved in both groups Missing data (missed opportunities) unlikely to be different in both arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar at baseline |
| Baseline characteristics | Low risk | Similar at baseline |
| Protection from contamination | Unclear risk | Participants worked in same institution so may have communicated with each other |
| Methods | Design: cluster‐randomised trial Study period:September 2008‐November 2009 Baseline (T1), then observations immediately after intervention (T2) then observations 6 months after end of intervention (T3) | |
| Participants | Nurses in patient wards | |
| Interventions | Multimodal: education, individual feedback, posters/signs, ABHR, admin support, staff involvement, adequate supplies Control: state of the art (no admin support or staff involvement) | |
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis Observer effect not a concern as participants did not know what was being observed Funding source:Netherlands Organisation for Health Research and Development Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was by unit at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) | High risk | Leaders directed strategy so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Analysts were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms 10 intervention wards did not complete intervention; they did an ITT analysis so the loss to follow‐up may have resulted in underestimation of effect but not bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | H1N1 influenza publicity may have influenced hand hygiene |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Individualised unit‐based intervention so even if control units heard about it, they could not have the intervention |
| Methods | RCT in an ICU Study period: November 2012‐January 2013 | |
| Participants | All healthcare workers | |
| Interventions | Olfactory cue and visual cues | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Not stated Declaration of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of observation periods (not individuals) were assigned to type of intervention using a random‐number generator |
| Allocation concealment (selection bias) | Low risk | Blocks assigned to intervention or control group prior to start of study |
| Blinding of participants and personnel (performance bias) | Unclear risk | They would have noticed signs and scent but authors did not specify whether they knew the purpose of the study |
| Blinding of outcome assessment (detection bias) | High risk | Not possible to blind observers |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | They did not collect data on number of visitors vs healthcare workers and unclear if their behaviour would be different |
| Baseline outcomes | Low risk | Single unit |
| Baseline characteristics | Low risk | Single unit |
| Protection from contamination | Unclear risk | Single unit, unclear if staff would have different behavior at end of 1 intervention period that could have affected performance when a different intervention occurred |
| Methods | Design: ITS Study period: March 2008‐July 2010 Baseline: 6 ‐ 7 months, Intervention 12 months, washout 6 months | |
| Participants | 33 wards, 10 hospitals, all healthcare workers | |
| Interventions | WHO multimodal | |
| Outcomes | Hand hygiene compliance, no feedback Also studied MRSA screening and decolonisation, with MRSA rates as outcome of primary interest | |
| Notes | Appropriate analysis for ITS ( segmented multilevel logistic regression) Funding source: European Commission 6th framework programme Declaration of interest: Harbarth is a member of the speakers' bureau for bioMerieux and Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Included posters and managerial support so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Observers were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Introduction of MRSA screening programme; 10 hospitals over 25 months with no report of whether there were other campaigns, outbreaks etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after |
| Methods | Design: Cluster‐randomised trial Study period: January 2009 to December 2009 3‐month baseline (first observation) then follow‐up (second observation) 6 months after intervention, although duration of data collection in the latter period was not specified | |
| Participants | Healthcare workers in 11 primary healthcare centres | |
| Interventions | Multimodal strategy based on WHO: posters, education sessions, and availability of ABHR Control: no intervention | |
| Outcomes | Hand hygiene compliance, defined as number of hand hygiene opportunities taken by number of opportunities observed | |
| Notes | Unit of analysis error: analysed by healthcare worker type, not cluster, and inappropriate correction for missing data 10 opportunities were observed for each healthcare worker at each observation period Unlikely observer effect as participants did not know what outcome was being measured Funding source: Istituto de Salud Carlos III, Ministry of Health of Spain Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | EPIDAT3 program used to randomly select centres for each arm (reported in previous article listed in references) |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the centre and performed at the start of study |
| Blinding of participants and personnel (performance bias) | High risk | Included reminder posters so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Observer was blinded (reported in discussion) and participants were unaware hand hygiene was being observed |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Additional measures taken for H1N1 |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Similar types of healthcare workers but types of patients seen at the centres not reported and baseline characteristics of the units were not reported |
| Protection from contamination | Low risk | Intervention was by centre |
| Methods | Design: Cluster‐randomised trial Study period: 3 month baseline assessment (October ‐ December 2006) then trial was conducted for 1 year (June 2007 ‐ May 2008) with assessments conducted weekly (5 randomly‐selected 15‐minute periods per week per unit) | |
| Participants | All healthcare workers on 30 adult hospital wards in 3 acute care hospitals | |
| Interventions | Performance feedback (pooled not individual), small‐group teaching seminars, posters and pamphlets, unit‐generated target adherence level and approaches to increase awareness of hand hygiene Control: ABHR dispensers installed | |
| Outcomes | Adherence to hand hygiene: considered successful if hand hygiene occurred when it was deemed necessary (using WHO indications for hand hygiene) and if duration of hand hygiene met pre‐set criteria. Incidence of hospital‐acquired MRSA colonisation (cases per 1000 patient‐days) | |
| Notes | Appropriate analysis: unit of analysis for hand hygiene was at the level of the clusters Funding source: Physicians’ Services Incorporated Foundation of Ontario, Canada and Swiss National Science Foundation Grant Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using random numbers table; statistician was not part of study team |
| Allocation concealment (selection bias) | Low risk | Allocation was by unit and performed at start of study |
| Blinding of participants and personnel (performance bias) | High risk | Included posters and performance feedback so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcomes were assessed blindly |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | ABHR dispensers installed hospital wide during study; 1 MRSA outbreak |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Only reported that sinks and ABHR availability were similar; no comparison of patients, staffing, etc |
| Protection from contamination | High risk | Authors suggested contamination of control group likely; control units were in same hospitals as intervention groups |
| Methods | ITS in one hospital Study period: Pre‐intervention January‐September 2011; intervention October 2011‐July 2012; post‐intervention August 2012‐May 2014 | |
| Participants | All healthcare workers | |
| Interventions | Multimodal: education and training; promotion; use of visual cues, covert direct observation of hand hygiene by peers; rewards; alerts to the immediate supervisor; and regular reports to leadership | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Unclear if analysis was appropriate for ITS but reported only compliance per period Funding source: Not stated Declaration of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Observers not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there were other campaigns, outbreaks, changes in staffing etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
| Methods | Non‐randomised trial in 1 hospital Study period: November 2015‐March 2016 Lebanon | |
| Participants | Nurses | |
| Interventions | Incentives in 1 intervention arm, and audit feedback in separate intervention arm vs education in control group | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Not stated Declaration of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random allocation |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done at the start of the study but method was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) | Low risk | Auditors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline hand hygiene compliance |
| Baseline characteristics | Unclear risk | Reported as similar but no supporting data provided |
| Protection from contamination | Unclear risk | Unclear if staff moved from unit to unit and would have been aware of feedback |
| Methods | Design: RCT with cross‐over Study period: Dates not stated. Each participant was randomised to receive either the intervention or control first, was monitored for all activities with 1 patient (up to 120 minutes), then within a month was re‐monitored in the opposite arm | |
| Participants | Anaesthesiologists and CRNAs | |
| Interventions | Placement of ABHR dispenser on cart + wall vs wall only | |
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis Observer effect not a concern since participants did not know what outcome was being measured Funding source: GoJo provided the alcohol product and dispensers Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator used to select OR, then group allocation determined by electronic files based on previous block randomisation |
| Allocation concealment (selection bias) | Low risk | Participants assigned to start as intervention or control prior to start of study, then evaluated within 30 days in opposite allocation; did not know what outcome was being assessed |
| Blinding of participants and personnel (performance bias) | Unclear risk | ABHR dispenser was visible on cart but researchers said that participants were not aware of what was being assessed |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were not assessed blindly |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported |
| Baseline characteristics | Low risk | Similar baseline characteristics |
| Protection from contamination | High risk | Participants were assessed once with intervention and once with control conditions but were blinded to outcome being assessed. They may have learned to look for ABHR on the cart when in the intervention arm first, affecting behaviour when they crossed over to the control arm |
| Methods | Design: ITS Study period: Pre‐intervention: 3 quarters in 2006; intervention over 2 quarters in 2007; follow‐up over 10 quarters in 2007 ‐ 2009 | |
| Participants | 1 multi‐state healthcare system with 166 hospitals and 116 outpatient surgery and endoscopy centres | |
| Interventions | Available ABHR, ongoing education, letters for awareness | |
| Outcomes | ABHR use in ounces per adjusted patient‐day | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Bundle for MRSA reinforced hand hygiene so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Variable pre‐intervention ABHR use in different centres; introduction of MRSA screening, barrier precautions, cleaning and disinfection |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
| Methods | Stepped‐wedge cluster‐randomised trial in 11 ICUs in hospitals A new intervention unit was added each month, and a new intervention component was added each month in each intervention unit. Argentina | |
| Participants | All healthcare workers | |
| Interventions | Multimodal intervention with stepped introduction of leadership support, availability of ABHR, reminders, story boards, and unit feedback | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Patient Safety Small Grant Program, WHO, Switzerland Declaration of interest: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Participants assigned to intervention or control group once a month as next units added to intervention |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | High risk | Some differences in baseline; authors identified sites as heterogeneous |
| Baseline characteristics | Unclear risk | Variation reported but impact unclear |
| Protection from contamination | Unclear risk | Authors identified that contamination could not be ruled out |
| Methods | ITS in 1 centre Study period; July 2008‐May 2014, with interventions introduced or altered between July 2010 and July 2013 USA | |
| Participants | Physicians | |
| Interventions | Multimodal intervention with audit, role modelling, feedback, education, visual cues, direct physician engagement, incentives, and adequate resources | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Inappropriate analysis for an ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Participants aware of intervention |
| Blinding of outcome assessment (detection bias) | High risk | Observers not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in time period |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there other campaigns, outbreaks, changes in staffing etc; additional interventions added for physicians |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
| Methods | Design: cluster‐randomised trial Study period: March 2003‐February 2004 4‐month baseline, intervention period of 5 months | |
| Participants | Healthcare workers in 10 community hospitals | |
| Interventions | Multimodal, customised to the unit: education, feedback at the unit level, posters/signs, ABHR, admin support, staff involvement, recognition and rewards programme (candy, buttons) Control: usual infection control practices | |
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Mixed effects logistic regression: appropriate analysis Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) | High risk | Individualised campaigns so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes were assessed blindly but local observers were used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms 1 withdrew early from the control group but this was unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported; they compared absolute changes from baseline |
| Baseline characteristics | High risk | No baseline characteristics reported |
| Protection from contamination | Low risk | Allocation was by institution |
| Methods | Cluster‐randomised trial in 1 centre Study period: Baseline period April 1, 2009‐June 30, 2010; intervention period July 1, 2010 ‐June 30, 2012. Switzerland | |
| Participants | All healthcare workers | |
| Interventions | Enhanced feedback or enhanced feedback with patient participation vs standard WHO‐based multimodal intervention | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Swiss National Science Foundation Declaration of interest: None declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence with block randomisation of wards to groups |
| Allocation concealment (selection bias) | Low risk | Participants assigned to intervention or control group prior to start of study |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) | High risk | Not possible to blind as posters used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline outcomes |
| Baseline characteristics | Unclear risk | Allocated by strata so patient characteristics likely similar but no data provided on healthcare workers or physical layout |
| Protection from contamination | Unclear risk | Unclear if staff moved from control to intervention wards; identified by authors in discussion as a possibility |
| Methods | Design: ITS Study period: Baseline: 2004 ‐ 2009; Programme launch over 12‐month period (late 2009 ‐ late 2010); active accountability phase from late 2010 to fall 2012 USA | |
| Participants | Healthcare workers in 1 centre | |
| Interventions | Leadership goal‐setting, financial incentives for centre, expanded hand hygiene observation programme including feedback to individuals, system‐wide marketing campaign | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Appropriate analysis for ITS Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Leaders were involved so participants were aware of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Observers were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | They did not report whether or not there were other campaigns, outbreaks etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
| Methods | Design: ITS Study period: February 2000 ‐ September 2006; VigiGerme® campaign occurred in spring 2003 and the Clean Care is Safer Care occurred in autumn 2005 | |
| Participants | Healthcare workers throughout hospital | |
| Interventions | Social marketing campaign (VigiGerme®) aimed at Standard Precautions in 2003 and Clean Care is Safer Care campaign in 2005. The campaigns were not described but were based on the Geneva campaign model which included the five components recommended in the WHO Guidelines 2009 | |
| Outcomes | Volume of hand hygiene products (litres per 100 patient‐days) Also measured new MRSA isolates per 100 patient‐days, newC. difficile isolates per 100 patient‐days, defined daily dose of antibiotics per 100 patient‐days | |
| Notes | Analysis appropriate for ITS Funding source: None Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Pocket‐sized ABHR given so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Multiple interventions occurred over the 7‐year period including 2 infection control programmes, so very likely there were confounding factors |
| Shape of effect pre‐specified | Low risk | Point of analysis same as point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after the intervention |
| Methods | Design: ITS Duration: 2004‐2006, with 24 months of data collection following start of each campaign Geneva: pre‐intervention July‐October 2004; intervention October 2004‐May 2005 Washington: pre‐intervention July‐November 2004; intervention November 2004‐May 2005 Australia | |
| Participants | All healthcare workers in multiple units | |
| Interventions | 3 separate interventions: 1) Simple substitutions: ABHR for soap, and 1 type of ABHR for another 2) Geneva campaign: based on the Geneva campaign (Pittet 2000) that existed at the time which consisted of all of the elements later included in the WHO Guidelines 2009 3) Washington campaign: based on a campaign that had taken place in Washington (Larson 2000) and consisted of the elements later included in the WHO Guidelines 2009 with informal feedback during the staff involvement in all aspects of design and implementation | |
| Outcomes | Product use (electronic count of soap/AHBR dispensers) | |
| Notes | Appropriate analysis for ITS Funding source: None Declaration of interest: No information given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) | High risk | Staff involved in developing campaign so participants aware of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | They did not comment on whether there were other changes, outbreaks etc. |
| Shape of effect pre‐specified | Low risk | Point of analysis same as point of intervention |
| Intervention had no effect on data collection | Low risk | Data collection method same before and after |
| Methods | Design: cluster‐randomised trial Study period: intervention period April 1‐15, 2007; baseline assessment over 3 months; post intervention assessments over 36‐37 days starting April 16, 2007. Monthly monitoring for 3 months, then gave feedback to both intervention and control groups, then monitored for another 4 months | |
| Participants | Hong Kong Healthcare workers in 6 long term care facilities | |
| Interventions | Multimodal: education, feedback to group in one session, posters, individual ABHR, pens as reminder Control: basic life support workshop | |
| Outcomes | Observed compliance to hand hygiene | |
| Notes | Unit of analysis error: analysed at level of individual not cluster Funding source: Grant to Support Academic Activities for Public Health and Social Medicine from the Chinese University of Hong Kong and by Vickmans Laboratories which supplied the pocket‐sized alcohol containers of hand rub Declaration of interest: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) | High risk | Included reminders so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes were not assessed blindly (reported in discussion) |
| Incomplete outcome data (attrition bias) | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | A feedback session took place in both intervention and control units 3 months after intervention |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Higher proportion with severe disabilities in treatment group and they had fewer handwashing sinks |
| Protection from contamination | Unclear risk | 43% of intervention group staff left by end of study and new staff may not have received education |
ABHR: alcohol‐based hand rub
C.difficile: Clostridium difficile
CRNA: certified registered nurse anaesthetist
GEE: generalised estimating equation
ICU: intensive care unit
ITS: interrupted time series
MRSA: methicillin‐resistant Staphylococcus aureus
OR: operating room
RCT: randomised (controlled) trial
SICU: surgical intensive care unit
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| ITS design with insufficient data collection points | |
| ITS design with unclear intervention period | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with insufficient data collection points | |
| ITS design with inadequate data collection points | |
| CBA study design with 1 nonequivalent control group. | |
| ITS design with inadeqate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with inadeqate data collection points | |
| CBA study design with 1 nonequivalent control group. | |
| IITS design with inadeqate data collection points | |
| ITS design with unclear intervention period | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points and unclear intervention period | |
| CBA study inadequate control, no baseline | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| Non‐randomised trial with inadequate control group | |
| Cross‐over CBA design with only 1 intervention group and 1 control group | |
| CBA design with only 1 intervention group and 1 control group | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| CBA design with 2 intervention groups but only 1 control group | |
| ITS design with inadeqate data collection points | |
| IITS design with inadeqate data collection points | |
| Non‐randomised clinical trial with inadequate control group | |
| CBA study design with 1 nonequivalent control group | |
| CBA study design with 1 nonequivalent control group | |
| CBA study design with 1 nonequivalent control group | |
| CBA design with only 1 intervention group and 1 control group | |
| ITS design with inadeqate data collection points | |
| ITS design with inadequate data collection points | |
| Non‐randomised trial, no baseline data, inadequate control group | |
| CBA design with only 1 intervention group and 1 control group | |
| CBA design with only 1 intervention group and 1 control group | |
| CBA design with only 1 intervention group and 1 control group | |
| ITS design with inadeqate data collection points | |
| CBA design with only 1 intervention group and 1 control group | |
| CBA study design with 1 nonequivalent control group | |
| ITS design with inadequate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with inadequate data collection points | |
| ITS design with unclear intervention period | |
| ITS design with inadequate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points and unclear intervention period | |
| Cross‐over CBA design with only 1 intervention group and 1 control group | |
| ITS design with no clear intervention period | |
| ITS design with inadequate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| ITS design with inadeqate data collection points | |
| CBA study with only 1 control group | |
| ITS design with inadequate data collection points | |
| ITS design with inadequate data collection points | |
| CBA design with only 1 intervention group and 1 control group | |
| CBA design with only 1 intervention group and 1 control group |
CBA: controlled before‐after
ITS: interrupted time series
RCT: randomised (controlled) trial

Study flow diagram.

Risk of bias graph for non‐ITS studies (RCTs, NRCTs, and CBAs)

Risk of bias summary for non‐ITS studies (RCTs, NRCTs, and CBAs)
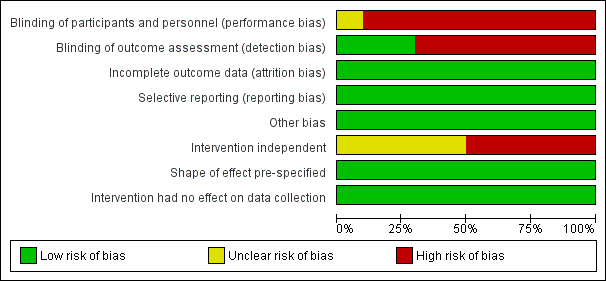
Risk of bias graph for ITS studies

Risk of bias summary for ITS studies
| Overview: interventions compared with different or no interventions for improving hand hygiene compliance in healthcare workers or reducing infection or colonisation rates | ||||
| Patient or population: Healthcare workers Settings: Hospitals, nursing homes and long‐term care facilities Intervention: Strategies varied by study Comparison: Varied by study | ||||
| Types of Interventions1 | Impact | Outcomes and Certainty of the evidence (GRADE) 2 | ||
| Hand Hygiene Compliance3 | Change in infection rates4 | Change in colonisation rates4 | ||
| Multimodal, not WHO‐based5: contains some strategies recommended by WHO | Multimodal interventions that include some but not all strategies recommended in the WHO guidelines may slightly improve hand hygiene compliance and may slightly reduce infection rates (low certainty of evidence). | ⊕⊕⊝⊝ (5 studies) | ⊕⊕⊝⊝ (3 studies) | ‐‐‐ |
| Multimodal, WHO‐based: contains all strategies recommended by WHO | It is uncertain whether multimodal interventions that include all strategies recommended in the WHO guidelines improve hand hygiene compliance or reduces infection because the certainty of this evidence is very low. Such multimodal interventions may slightly reduce colonization rates (low certainty of evidence) | ⊕⊝⊝⊝ (5 studies) | ⊕⊝⊝⊝ (2 studies) | ⊕⊕⊝⊝ (2 studies) |
| Multimodal, WHO‐enhanced: contains all strategies recommended by WHO and additional ones | Multimodal interventions that contain all strategies recommended in the WHO guidelines plus additional strategies may slightly improve hand hygiene compliance (low certainty of evidence). It is uncertain whether such multimodal interventions reduce infection rates because the certainty of this evidence is very low | ⊕⊕⊝⊝ (6 studies) | ⊕⊝⊝⊝ (1 study) | ‐‐‐ |
| Performance feedback | Performance feedback may improve hand hygiene compliance (low certainty of evidence) and probably slightly reduces infection and colonisation rates | ⊕⊕⊝⊝ (6 studies) | ⊕⊕⊕⊝ (1 study) | ⊕⊕⊕⊝ (1 study) |
| Education | Education may improve hand hygiene compliance (low certainty of evidence) | ⊕⊕⊝⊝ (2 studies) | ‐‐‐ | ‐‐‐ |
| Cues | Cues such as signs or scent may slightly improve hand hygiene compliance (low certainty of evidence) | ⊕⊕⊝⊝ (3 studies) | ‐‐‐ | ‐‐‐ |
| Placement of ABHR | Placement of ABHR close to point of use probably slightly improves hand hygiene compliance (moderate certainty of evidence). | ⊕⊕⊕⊝ (1 study) | ‐‐‐ | ‐‐‐ |
| GRADE Working Group grades of evidence | ||||
| 1Studies evaluated different strategies or combinations of strategies. | ||||
| Multimodal interventions (not WHO‐based) compared with no intervention for promotion of hand hygiene or reduction of infection or colonisation rates | |||
| Patient or population: Healthcare workers Settings: Long‐term care, primary care, hospital Intervention: Multimodal with some but not all of the strategies recommended by WHO; strategies varied by study Comparison: No hand hygiene promotion | |||
| Outcomes | Impact | Studies | Certainty of the evidence |
| Hand hygiene compliance | In the RCTs, the absolute differences in hand hygiene compliance compared to baseline ranged from 1.9 to 37.7 percentage points in intervention groups and from 0.3 to 11.9 in control groups. The ITS reported an adjusted OR of 1.19, 95% CI 1.01 to 1.42 favouring the intervention | 4 RCTs, 1 ITS 24 long‐term care facilities, 10 hospitals, 11 ICUs and 11 primary healthcare units | ⊕⊕⊝⊝ |
| Infection rates | 1 RCT reported reduced respiratory outbreaks and MRSA infections requiring hospitalisation (IRR 0.12 to 0.61) favouring the intervention, while 1 ITS study reported no reduction in MRSA clinical isolates or infection. 1 RCT reported reductions of 0.27 to 0.77 cases per 1000 resident‐days in serious infections, pneumonia and death in the intervention group compared to no change or an increase of 0.57 cases per 1000 resident‐days in the control group | 2 RCT, 1 ITS 24 long‐term care facilities, 10 hospitals, | ⊕⊕⊝⊝ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence | |||
| 1Evidence downgraded from high to low due to non‐randomised evidence (one of five studies); high risk of bias (all studies have two or more sources of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. | |||
| WHO‐based multimodal interventions compared with some or no interventions for promotion of hand hygiene or reduction of infection or colonisation rates | |||
| Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Multimodal with all five strategies recommended by WHO: ABHR at point of care, education, performance feedback, reminders, and administrative support. Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence |
| Hand hygiene compliance | The absolute difference in hand hygiene compliance between intervention and control group was 6.3 percentage points in the RCT. One ITS reported a difference of 17 percentage points in hand hygiene compliance compared to baseline, while another ITS reported no change on medicine units and a RR of 1.56, 95% CI 1.29 to 1.89 in IDUs favouring intervention. One ITS in a multistate system reported an increase of 27.45 ounces of ABHR per adjusted bed‐day. One ITS did not report estimates of change | 1 RCT, 4 ITS 1 multistate system with 166 hospitals, 5 hospitals and 13 ICUs | ⊕⊝⊝⊝ |
| Infection rates | 1 ITS reported a decrease in blood stream infections of 0.191 cases per 1000 line‐days and a decrease in ventilator‐associated pneumonia of 0.538 cases per 1000 ventilator days. 1 ITS reported that MRSA decreased by 0.03 clinical isolates for each litre of ABHR per 100 patient‐days but there was no change in C. difficile | 2 ITS 3 hospitals and 13 ICUs | ⊕⊝⊝⊝ |
| Colonisation rates | 1 RCT reported no difference in MRSA colonisation. 1 ITS reported a slight decrease in MRSA acquisition (IRR 0.976 favouring intervention) but no change in VRE or HRE acquisition. | 1 RCT, 1 ITS 1 multistate system with 166 hospitals, 1 hospital | ⊕⊕⊝⊝ |
| GRADE Working Group grades of evidence | |||
| 1Evidence downgraded from high to very low due to non‐randomised evidence (four of five studies); high risk of bias (four of five studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. | |||
| WHO‐enhanced multimodal interventions compared with some or no interventions for promoting hand hygiene | |||
| Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Multimodal with all of the strategies recommended by WHO, plus additional interventions. Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence |
| Hand hygiene compliance | 1 RCT and one ITS reported an increase in hand hygiene compliance with RR of 1.48 to 1.64 favouring intervention. 1 RCT reported increases in hand hygiene compliance of 20.1 to 28.4 percentage points in the intervention group compared to a decrease of 0.7 to 3.1 in the control. 1 ITS reported an increase in hand hygiene compliance of 2% per month during the intervention compared to < 1% a month before and after the intervention, while another ITS reported hand hygiene compliance of 83% ‐ 95% post‐intervention compared to 38% ‐ 100% at baseline, with variation by unit. 1 ITS did not report estimates of change | 2 RCTs, 4 ITS 15 hospitals | ⊕⊕⊝⊝ |
| Infection rates | 1 ITS reported no change in MRSA clinical isolates or in C. difficile | 1 ITS 1 hospital | ⊕⊝⊝⊝ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence Abbreviations:C. difficile: Clostridium difficile; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial; RR: risk ratio; WHO: World Health Organization | |||
| 1Evidence downgraded from high to low due to non‐randomised evidence (four of six studies; high risk of bias (five of six studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. 2Evidence downgraded from high to very low due to non‐randomised evidence and high risk of bias (two sources of high risk of bias). | |||
| Performance feedback compared with some or no interventions for promoting hand hygiene | |||
| Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Feedback with additional strategies such as focus on leadership; varied by study Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence |
| Observed hand hygiene compliance | 1 RCT and 1 NRCT reported increases in hand hygiene compliance of 0 ‐ 61 percentage points in intervention groups compared to no changes or a slight decrease of 4 percentage points in control groups. 2 RCTs reported ORs of 1.61 to 2.09 favouring intervention. 1 ITS reported a weekly increase in hand hygiene compliance of 4% after an initial increase of 17.5%, while 1 ITS reported an increase of 37 percentage points during the active accountability phase of the study | 3 RCTs, 1 NRCT, 2 ITS 21 hospitals | ⊕⊕⊝⊝ |
| Infection rates | 1 RCT reported reduced primary bloodstream infection in the enhanced feedback group (0.71, 95% CI 0.54 to 0.95) and control group (0.57, 95% CI 0.40 to 0.80) with little change in the enhanced feedback + patient participation group (1.02, 95% CI 0.78 to 1.34). Period prevalence of HCAIs was also reduced in the enhanced feedback group (0.91, 95% CI 0.68 to 1.23), with little change in the enhanced feedback + patient participation group (1.05, 95% CI 0.78 to 1.40) and an increase in the control group (1.33, 95% CI 0.94 to 1.88) | 1 RCT 1 hospital | ⊕⊕⊕⊝ |
| Colonisation rates | 1 RCT reported reduced colonisation with MRSA in the enhanced feedback group (0.79, 95% CI 0.66 to 0.95) and the enhanced feedback + patient participation group (0.82, 95% CI 0.67 to 0.99), as well as in the control group (0.92, 95% CI 0.77 to 1.13) | 1 RCT 1 hospital | ⊕⊕⊕⊝ |
| GRADE Working Group grades of evidence Abbreviations: CI: confidence interval; HCAIs: healthcare‐associated infections; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; NRCT: non‐randomised (controlled) trial; OR: odds ratio; RCT: randomised (controlled) trial | |||
| 1Evidence downgraded from high to low due to non‐randomised evidence (three of six studies); high risk of bias (two or more sources in all studies), and inconsistency in effect sizes between studies and within multi‐unit studies. | |||
| Education compared with no education for promotion of hand hygiene | |||
| Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Education; content and delivery methods varied by study Comparison: No education | |||
| Outcomes | Impact | Studies | Certainty of the evidence |
| Observed hand hygiene compliance | 1 RCT reported increases of 16.3 to 24.5 percentage points in the proportion of nurses in the intervention group who complied with recommendations for hand hygiene, depending on moment of hand hygiene evaluated, compared to no changes or a decrease of 4.1 percentage points in the control group. 1 ITS reported an increase in hand hygiene compliance as a proportion of opportunities of 42 percentage points | 1RCT and 1 ITS 2 hospitals | ⊕⊕⊝⊝ |
| Infection rates | Not reported. | ‐ | ‐ |
| Colonisation rates | Not reported. | ‐ | ‐ |
| GRADE Working Group grades of evidence Abbreviations: ITS: interrupted time series; RCT: randomised (controlled) trial | |||
| 1Evidence downgraded from high to low due to non‐randomised evidence (one of two studies); and risk of bias (high and unclear). | |||
| Cues compared with no cue or different cue for promotion of hand hygiene | |||
| Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Signs or scent as cue Comparison: No cue or different signs | |||
| Outcomes | Impact | No of Participants | Certainty of the evidence |
| Observed hand hygiene compliance | 1 RCT reported an increase in hand hygiene of 8.51 percentage points for the patient consequences sign compared to a slight decrease of 0.29 percentage points for the personal consequences sign. 1 RCT reported increases in hand hygiene compliance of 31.9 and 6.7 percentage points for the scent cue and sign of stern male eyes respectively, and a decrease of 5 percentage points for the sign with female eyes. One NRCT reported an increase of 7 percentage points in hand hygiene compliance with the light cue on day 2 compared to 9 percentage points with no light cue, whereas on day 3 compared to day 1 there was no difference with the light cue and an increase of 16 percentage points with no light cue | 2 RCTs, 1 NRCT 3 hospitals | ⊕⊕⊝⊝ |
| Infection rates | Not reported | ‐ | ‐ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence Abbreviations: NRCT: non‐randomised (controlled) trial; RCT: randomised (controlled) trial | |||
| 1Evidence downgraded from high to low due to non‐randomised evidence (one of three studies); risk of bias (all studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies. | |||
| Placement of ABHR on cart compared with placement of ABHR on wall for promotion of hand hygiene | |||
| Patient or population: Anaesthesiologists and CRNAs Settings: Acute care surgical Intervention: Placement of ABHR on anaesthesia cart Comparison: Placement of ABHR on wall of anaesthesia room | |||
| Outcomes | Impact | No of Participants | Certainty of the evidence |
| Observed hand hygiene compliance | 1 RCT reported an increase of 0.3 hand hygiene events an hour in the intervention group compared to the control group | 1 RCT 1 hospital | ⊕⊕⊕⊝ |
| Infection rates | Not reported | ‐ | ‐ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence Abbreviations: ABHR: alcohol‐based hand rub; CRNA: certified registered nurse anaesthetist; RCT: randomised (controlled) trial | |||
| 1Evidence downgraded from high to moderate due to high risk for bias (two sources of high risk and two sources of unclear risk ). | |||
| Study | Comparison | Estimate of compliance | Measure of difference or change |
| Intervention: Multimodal, not WHO | |||
| Cluster‐randomised trial Intervention: Multimodal not WHO · Also had study arms with powdered or powderless gloves Control: 2‐hour health talk | Outcome: Hand hygiene compliance Inappropriate analysis: GEE but did not compare changes between arms Observed mean hand hygiene compliance: Intervention with powdered gloves: · Baseline: 27.0% · 1 month post: 59.2% · 4 months post: 60.6% Intervention with powderless gloves: · Baseline: 22.2% · 1 month post: 59.9% · 4 months post: 48.6% Control: · Baseline: 19.5% · 1 month post: 19.8% · 4 months post: 21.6% | Not reported by researchers Calculated differences1 in percentage points between baseline and 1 month: · intervention with powdered gloves: 32.2 · intervention with powderless gloves: 37.7 · control: 0.3 Calculated differences1 in percentage points between baseline and 4 months: · intervention with powdered gloves: 33.6 · intervention with powderless gloves: 26.4 · control: 2.1 | |
| ITS · 6 ‐ 7 month baseline · Intervention: Multimodal not WHO · 12 month intervention phase · 6‐month washout period · Control wards: no hand hygiene promotion | Outcome: Hand hygiene compliance Intervention wards · Baseline: 49.3% (95% CI 47.2% to 51.4%) · Intervention phase: 63.8% (95% CI 62.3% to 64.4%) Control wards: · Baseline: 30.5% (95% CI 28.7% to 32.4%) · Washout period: 23.9% (95% CI 22.0% to 25.9%) | Segmented regression analysis: · Increase after start of hand hygiene promotion: adjusted OR 1.19, 95% CI 1.01 to 1.42 · Decrease of 9% per month in washout period after campaign ended: adjusted OR 0.91, 95% CI 0.85 to 0.97 | |
| Cluster‐randomised trial Intervention: Multimodal not WHO Control: No intervention | Outcome: Hand hygiene compliance Inappropriate analysis: Analysed at level of individual not cluster; inappropriate correction for missing data Mean observed hand hygiene compliance: Intervention group: Baseline: 7.98%, 95% CI 4.5 to 10.2 6 months post: 32.74 (no CI reported) Control group: Baseline: 8.26% (95% CI: 6.2‐11.6) 6 months post: 11.86 (no CI reported) | Not reported by researchers Calculated differences1 in percentage points between baseline and 6 months post‐intervention: · intervention group: 24.76 · control group: 3.6 | |
| Stepped wedge RCT Intervention: Multimodal Not WHO Control: No intervention | Outcome: Hand hygiene compliance Variation by site: · Pre: 47.2% to 79.8% · Post: 57.0% to 93.9% | Absolute difference range: 1.9 to 26.7 Intervention effect: OR 1.17, 95% CI 1.13 to 1.22 Intervention effect adjusted by time: OR 1.08, 95% CI 1.03 to 1.14 | |
| Cluster‐randomised trial Intervention: Multimodal not WHO Control: Basic life support workshop | Outcome: Hand hygiene compliance Inappropriate analysis: Analysed at level of individual not cluster Mean observed hand hygiene compliance (handwashing or ABHR use): Intervention group: Baseline: 25.8% Post‐intervention: 33.3% 7 months post: 36.7% Control group: Baseline: 25.8% Post‐intervention: 30.0% 7 months post: 37.7% | Not reported by researchers Calculated differences1 in percentage points between baseline and post intervention: · intervention group: 7.5 · control group: 4.2 Calculated differences1 in percentage points between baseline and 7 months post‐intervention: · intervention group: 10.9 · control group: 11.9 | |
| Intervention: Multimodal, WHO based | |||
| ITS Intervention: WHO based multimodal | Outcome: Observed mean hand hygiene compliance: · Baseline: 52% · Optimised hand hygiene plus CHG bathing: 69% · Addition of MRSA screening and contact precautions: 77% | Inappropriate analysis: No statistical analysis done Calculated difference1 in percentage points: · between baseline and optimised hand hygiene plus CHG bathing: 17 · between baseline and addition of MRSA screening and contact precautions: 25 | |
| Cluster‐randomised trial Intervention: WHO based multimodal Control: addition of ABHR | Outcome: Hand hygiene compliance Intervention: · Pre: 15.8% · Post: 48.2% Control: · Pre: 15.9% · Post 42.6% | Mean difference between groups at post‐test: · 6.3%, 95% CI 4.3% to 8.4% | |
| ITS Intervention: WHO‐based multimodal | Outcome: Mean ounces of ABHR per adjusted pt‐day · Pre intervention: 21.3 · Post intervention: 48.75 | Inappropriate analysis: No statistical analysis done Calculated difference1 between pre and post intervention: 27.45 ounces of ABHR per adjusted patient‐day | |
| Interventions: Multimodal, WHO‐enhanced and WHO based | |||
| ITS VigiGerme campaign:WHO‐enhanced multimodal Clean Care is Safer Care campaign: WHO‐based multimodal | Outcome: ABHR in litres per 100 patient‐days Did not report actual volume | Increases in both VigiGerme and Clean Care campaigns via ARIMA modelling; no estimates of effect reported Overall increase in ABHR from 1.303 L/100 patient days to 2.016 L/patient days, but did not report by programme | |
| ITS Washington programme: WHO‐enhanced multimodal Geneva programme: WHO based multimodal | Outcome: Electronic count of hand hygiene measured number of times ABHR dispensed from count Actual counts were not reported Noted that initial compliance was high in IDU | GEE analysis: Washington program: increase in hand hygiene relative to baseline: RR 1.48 (95% CI: 1.2‐1.81) Geneva on medicine units: no increase in hand hygiene Geneva in IDU: increase in hand hygiene relative to baseline: RR 1.56, 95% CI 1.29 to 1.89 | |
| Intervention: Multimodal, WHO‐enhanced | |||
| Cluster‐randomised trial Intervention: WHO‐enhanced multimodal Control: State of the art multimodal | Outcome: Observed mean hygiene compliance Intervention: · Pre: 20% · Post: 53% · 6 months: 53% Control: · Pre: 23% · Post: 42% · 6 months: 46% | OR of 1.64, 95% CI 1.33 to 2.02 in favour of team leader support | |
| ITS · 9‐month baseline Intervention: Multimodal WHO‐enhanced · 10‐month intervention period · 22‐month post‐intervention | Outcome: Hand hygiene compliance · Baseline: 72.7% (range: 62.5% to 86.2%) · Intervention period: 79.7% (range not reported) · Post: 93.2% (range 7.9% to 97.7%) | Inappropriate reporting of analysis for ITS · During intervention, average increase was 2% per month · Before‐after intervention, average increase was < 1% a month | |
| ITS · 2‐year baseline Intervention: Multimodal WHO‐enhanced · 3‐year intervention period · 10‐month post‐intervention | Outcome: Hand hygiene compliance Inappropriate analysis for ITS All healthcare workers: · During intervention: 85% to 92% · Pre‐intervention: variation (38% ‐ 100% but < 80% most months) · Post‐intervention: 83% ‐ 95% but most > 85% MDs: · During intervention: 75% ‐ 83% · Not reported for other time periods | Not reported by researchers Because of the considerable variation by unit, it was not possible for the review authors to calculate a difference1 in percentage points between pre‐ and post‐intervention | |
| Cluster‐randomised trial Intervention: WHO‐enhanced multimodal Control: Usual activities | Outcome: Observed mean hand hygiene compliance Actual compliance rates were not reported | Hand hygiene before and after patient contact, mean difference per group: Intervention: · 20.1% (range: 7.8% ‐ 35.5%) Control: · ‐3.1% (range: ‐6.3% ‐ +5.9%) Hand hygiene before or after patient contact, mean difference per group: Intervention: · 28.4% (range: 17.8% ‐ 38.2%) Control: · ‐0.7% (range: ‐16.7% ‐ +20.7%) | |
| 1 Where researchers did not report differences, the review authors calculated the differences based on the data reported by the researchers and summarised in the column "estimate of compliance". | |||
| Study | Comparison | Estimate of compliance | Measure of difference or change |
| Intervention: Performance feedback | |||
| ITS · 16‐week baseline · Intervention: video recording and feedback of hand hygiene rates · 16‐week post · 75‐week maintenance | Outcome: Observed mean hand hygiene compliance: Baseline: 6.5% (weekly range: 3.5% to 9.8%) Post‐feedback period: 81.6% (weekly range: 30.8% to 91.2%) Maintenance phase: 87.9% (weekly range: 83.5% to 91.6%) | Segmented regression analysis: · In week after start of intervention, estimated increase in compliance of 17.5% with additional 4% increase in each following week · In maintenance period, small weekly decrease of ‐0.04% | |
| Cluster‐randomised trial Intervention: wireless monitoring and feedback Control: No intervention | Outcome: Mean hand hygiene compliance on entry as recorded by electronic monitor: Intervention group: · Baseline: 28% (21% ‐ 37%) · Phase 2: real time reminders: 33% (25% ‐ 41%) · Phase 3: feedback: 28% (16% ‐ 40%) Control group: · Baseline: 28% (21% ‐ 37%) · Phase 2: real time reminders: 26% (22% ‐ 32%) · Phase 3: feedback: 24% (19% ‐ 33%) Similar increases in compliance on exit Variation by study ward, professional category and opportunity load | Unclear reporting of regression Not reported by researchers Calculated differences1 in percentage points between baseline and phase 2 real time reminders: · intervention group: 5 · control group: ‐2 Calculated differences1 in percentage points between baseline and phase 3 feedback: · intervention group: 0 · control group: ‐4 | |
| Stepped‐wedge RCT Intervention: feedback and personalised action planning Control: Clean Your Hands campaign | Outcomes reported: · Estimated relative change in liquid soap procurement · Hand hygiene compliance Estimates of volume of soap use or observed hand hygiene compliance were not reported | Estimated relative change in liquid soap: ACE: 1.133, 95% CI 0.987 to 1.3) ITU: 1.314, 95% CI 1.114 to 1.548 Absolute increase in compliance: ACE wards: · 13% if pre‐hand hygiene compliance was 50% · 10% if pre‐hand hygiene compliance was 70% ITU wards · 18% if pre‐hand hygiene compliance was 50% · 13% if pre‐hand hygiene compliance was 70% OR (compared to baseline) ACE wards: · 1.67, 95% CI 1.26 to 2.22 ITU wards: · 2.09, 95% CI 1.55 to 2.81 | |
| NRCT Intervention 1: Incentive Intervention 2: Audit and feedback Control: Usual hand hygiene campaign | Outcome: Hand hygiene compliance Variation by week: · Baseline all groups: 16% ‐ 20% · During intervention 1: 60% at week 8 and 77% at week 14 · During intervention 2: 43% at week 8 and 51% at week 14 · Control group: unchanged from baseline Decreased post‐intervention at week 21: · Intervention 1: 34% · Intervention 2: 48% · Control: unchanged | Not reported by researchers Calculated differences1 in percentage points between baseline and week 8: · intervention 1: 40 ‐ 44 · intervention 2: 23 ‐ 27 · control group: unchanged Calculated differences1 in percentage points between baseline and week 14: · intervention 1: 57 ‐ 61 · intervention 2: 31 ‐ 35 · control group: unchanged | |
| Cluster‐randomised trial Intervention 1: Enhanced performance feedback Intervention 2: Enhanced performance feedback plus patient participation Control: Usual WHO‐based hand hygiene campaign | Outcome: Hand hygiene compliance Performance feedback: · Baseline: 65% · Intervention period:75% · Follow‐up:72% Feedback plus patient participation: · Baseline: 66% · Intervention period: 77% · Follow‐up: 72% Control: · Baseline: 66% · Intervention period: 73% · Follow‐up: 70% | Absolute change for performance feedback: · Intervention period: 10% with OR 1.61, 95% CI 1.41 to 1.84 · Follow‐up:7% with OR 1.38, 95% CI 1.19 to 1.60 Absolute change for feedback plus patient participation: · Intervention period: 11% with OR 1.73, 95% CI 1.51 to 1.98 · Follow‐up: 6% with OR 1.36, 95% CI 1.18 to 1.57 Absolute change for Control: · Intervention period: 7% with OR 1.41, 95% CI 1.21 to 1.63 · Follow‐up: 4% with OR 1.21, 95% CI 1.00 to 1.47 | |
| ITS · Baseline: 2004 ‐ 2009 · Intervention 2009 ‐ 10: feedback, leadership and incentives · Active accountability: 2010 ‐ 2012 | Outcome: observed hand hygiene compliance Baseline: 52% Intervention: 75% Active accountability phase: 89% | Segmented regression analysis done but no estimates of effect reported: · Increase in adherence in each phase · Changes in slope associated with each time period Calculated differences1 in percentage points between baseline and · intervention phase: 23 · active accountability phase 37 | |
| Intervention: Education | |||
| ITS Intervention: Education: E‐learning hand hygiene game | Outcome: Observed mean hand hygiene compliance: · in 12 months pre‐e‐learning game: 42% · in 12 months post‐e‐learning game: 84% | Appropriateness of analysis unclear: Did not specify statistical analysis done but only reported mean hand hygiene compliance Calculated differences1 in percentage points between pre and post: 42 | |
| RCT Intervention: Education sessions on hand hygiene and UP Control: No intervention | Outcome: % of nurses who performed hand hygiene Before patient contact: Intervention · Pre: 51.0% · Post: 85.7% Control · Pre: 53.1% · Post:53.1% After patient contact: Intervention · Pre: 75.5% · Post: 91.8% Control · Pre: 75.5% · Post: 71.4% | Not reported by researchers Calculated differences1 in percentage points for before pt contact: · intervention: 24.5 · control group: no change Calculated differences1 in percentage points for after patient contact: · intervention: 16.3 · control group: 4.1 | |
| Intervention: Cues | |||
| NRCT Intervention: Light cue over sink Comparison: no light cue | Outcome: Hand hygiene compliance Light cue: · Day 1: 23% · Day 2: 30% · Day 3: 23% No light cue: · Day 1: 7% · Day 2: 16% · Day 3: 23% | Not reported by researchers Calculated differences1 in percentage points between day 1 and day 2: · light cue: 7 · no light cue: 9 Calculated differences1 in percentage points between day 1 and day 3: · light cue: 0 · no light cue: 16 | |
| Pair‐matched cluster‐randomised trial Compared 2 signs: personal vs patient consequences as message | Outcome: Observed mean hand hygiene compliance: Personal consequences sign: Pre‐test: 80.0% Post‐test: 79.71% Patient consequences sign: Pre‐test: 80.69% Post‐test: 89.2% Variation by type of practitioner but all had greater increase in hand hygiene in response to patient consequences sign | Inappropriate analysis : Did not do a matched analysis Not reported by researchers Calculated differences1 in percentage points between pre and post test: · Personal consequences sign: ‐0.29 · Patient consequences sign: +8.51 | |
| RCT Intervention: Olfactory cue (scent) or signs with male or female eyes Comparison: baseline without cues | Outcome: Hand hygiene compliance · Baseline: 15.0% · Scent cue: 46.9% · Male eyes cue: 21.7% · Female eyes cue: 10.0% Some differences women vs men | Not reported by researcher Calculated differences1 in percentage points between pre‐ and post‐test: · Scent cue: +31.9 · Stern male eyes: +6.7 · Female eyes: ‐5 | |
| Intervention: Placement of ABHR | |||
| RCT with cross‐over Intervention: placement of ABHR on cart Control: ABHR on wall | Outcome: hand hygiene events per hour: Intervention: 0.84 Control: 0.54 | Difference was an increase of 0.3 events per hour | |
| 1 Where researchers did not report differences, the review authors calculated the differences based on the data reported by the researchers and summarized in the column "estimate of compliance". | |||
| Study/ Category* | Education | Feedback | Posters/ signs | ABHR | Admin | Staff | Other |
| Intervention: Multimodal, not WHO | |||||||
| Yes (detailed) | Individual and unit | Yes | Individual and point of care | No | No | Gloves with and without powder | |
| Yes | No | Yes | Yes | Yes | No | ‐‐‐ | |
| Yes (details) | No | Yes | Yes | ‐‐‐ | No | ‐‐‐ | |
| Yes | Unit level | Yes | Yes | Yes | No | Role modelling Direct MD encouragement Incentives for MDs | |
| Yes (details) | 1 session to both groups at 3 months | Yes | Individual | No | No | Pens as reminder | |
| Intervention: Multimodal, WHO based | |||||||
| WHO‐based | Yes | Unit level | Yes | Yes | Yes | Yes | ‐‐‐ |
| WHO‐based | Yes | Yes (at discretion) | Yes | Yes | Yes | No | ‐‐‐ |
| Geneva Intervention WHO‐based | Yes | Yes | Yes | Yes | Yes | No | ‐‐‐ |
| Intervention: Multimodal, WHO‐enhanced | |||||||
| Yes | Individual | Yes | Yes | Yes | Yes | Adequate supplies | |
| Yes | Individual and unit level | Yes | Yes | Yes | No | Rewards, alerts to immediate supervisor | |
| No | Unit level | Yes | Yes | Yes | No | ‐‐‐ | |
| Yes | Yes at unit level (variable) | Yes | Yes | Yes | Yes | Recognition and rewards programme (e.g. candy, buttons) | |
| Whitby 2008 : Washington Intervention | Yes | Informal | Yes | Yes | Yes (walk around by exec) | Yes | ‐‐‐ |
| Note: Vernaz 2008 and Derde 2014 did not describe their multimodal campaigns and are not included in this table. | |||||||
| Study | Design/ Intervention | Results |
| Intervention: Multimodal, not WHO | ||
| RCT | · Reduced respiratory outbreaks: IRR 0.12, 95% CI 0.01 to 0.93 · Reduced MRSA infections requiring hospitalisation: IRR 0.61, 95% CI 0.38 to 0.97 | |
| ITS | No reduction related to the hand hygiene promotion campaign alone in: · MRSA in clinical isolates: IRR 1.44, 95% CI 0.96 to 2.15 · MRSA infections: IRR 1.28, 95% CI 0.79 to 2.06 | |
| RCT | Reduced serious infections (cases per 1000 resident‐days): · Intervention group: pre: 1.42; post: 0.65 (difference: ‐0.77) · Control groups: pre: 0.49; post: 1.05 (difference: 0.56) Reduced pneumonia (cases per 1000 resident‐days) · Intervention group: pre: 0.91; post: 0.28 (difference: ‐0.63) · Control group: no change Reduced deaths per 1000 resident‐days: · Intervention group: pre: 0.37; post: 0.10 (difference: ‐0.27) · Control group: no change | |
| Intervention: Multimodal, WHO based | ||
| ITS | · Trend in MRSA acquisition following hand hygiene campaign: IRR 0.976, 95% CI 0.954 to 0.999; · No changes in acquisition of VRE or HRE | |
| RCT | No difference in MRSA colonisation (cases per 1000 patient‐days): · Intervention group: 0.30 · Control group: 0.31 | |
| ITS | MRSA CLABSI per 1000 line days: · Pre: .497 (difference: ‐0.191) · Post: .306 MRSA VAP per 1000 ventilator days: · Pre: 1.088 (difference: ‐0.538) · Post: 0.550 | |
| ITS | · MRSA decreased by 0.03 clinical isolates per 100 patient‐days for each litre of ABHR per 100 patient‐days · No change in C. difficile | |
| Intervention: Multimodal, WHO‐enhanced | ||
| ITS | · No change in MRSA clinical isolates · No change in C. difficile | |
| Intervention: Performance feedback | ||
| RCT | Primary bloodstream infection · Enhanced feedback: IRR 0.71, 95% CI 0.54 to 0.95 · Enhanced feedback + patient participation: IRR 1.02, 95% CI 0.78 to 1.34 · Control: IRR 0.57, 95% CI 0.40 to 0.80 Period prevalence of HCAIs · Enhanced feedback: IRR 0.91, 95% CI 0.68 to 1.23 · Enhanced feedback + patient participation: IRR 1.05, 95% CI 0.78 to 1.40 · Control: IRR 1.33, 95% CI 0.94 to 1.88 Colonisation with MRSA · Enhanced feedback: IRR 0.79, 95% CI 0.66 to 0.95 · Enhanced feedback + patient participation: IRR 0.82, 95% CI 0.67 to 0.99 · Control: IRR 0.92, 95% CI 0.77 to 1.13 | |
| AHBR: alcohol‐based hand rub; C. difficile: Clostridium difficile; CLABSI: central line‐associated blood stream infections; CI: confidence interval; HCAI: healthcare‐associated infection; HRE: highly‐resistant Enterobacteriaceae; IRR: incidence rate ratio; ITS: interrupted time series; MSRA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial; VAP: ventilator‐associated pneumonia; VRE: vancomycin‐resistant enterococci; WHO: World Health Organization | ||

