Terlipresina versus placebo o ninguna intervención para pacientes con cirrosis y síndrome hepatorrenal
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, multicentre RCT | |
| Participants | Participants had cirrhosis and type 1 hepatorenal syndrome based on the 2007 International Club of Ascites criteria. | |
| Interventions | Terlipressin/albumin versus placebo/albumin for a maximum of 14 days. The investigators discontinued treatment if serum creatinine was below 1.5 mg/dL at the initiation of renal replacement therapy or liver transplantation. Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: 90 days | |
| Country of origin | United States and Canada | |
| Inclusion period | October 2010 to February 2013 | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel using placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessors using placebo |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in the analyses based on the intention‐to‐treat principle. Information about clinical outcomes was available for all participants. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported as described in the protocol and online trial registration. |
| For‐profit funding | High risk | Ikaria Therapeutics LLC funded the trial. |
| Other bias | Low risk | No other biases identified. |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Double‐blind, single‐centre RCT. | |
| Participants |
| |
| Interventions | Terlipressin versus placebo Terlipressin
| |
| Outcomes | Duration of follow‐up: end of treatment | |
| Country of origin | France | |
| Inclusion period | Not described | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | We did not include the trial in our analyses of hepatorenal syndrome because we did not have information about the outcome measure from the first period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Identical coded drug containers |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel achieved through double‐blinding using terlipressin placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment achieved through double‐blinding using terlipressin placebo. |
| Incomplete outcome data (attrition bias) | High risk | The trial report states that the analyses excluded 3 participants. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. |
| For‐profit funding | High risk | The trial received funding from Ferring S.A., France. |
| Other bias | Low risk | No other biases identified. |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, multicentre RCT | |
| Participants |
| |
| Interventions | Terlipressin/albumin versus albumin for a maximum of 15 days Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: 3 months after treatment | |
| Country of origin | Spain | |
| Inclusion period | January 2002 to February 2006 | |
| Proportion with type 1 hepatorenal syndrome | 56% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel (open trial) |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment (open trial) |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up, and all participants included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. Primary outcome measures:
|
| For‐profit funding | Low risk | The trial did not receive funding from pharmaceutical companies (reported in the discussion). |
| Other bias | Low risk | No other biases identified. |
| Overall risk of bias (mortality) | Low risk | Low risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, multicentre RCT | |
| Participants |
| |
| Interventions | Terlipressin/albumin versus albumin for 19 days Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: 6 months after hospital discharge | |
| Country of origin | Italy | |
| Inclusion period | December 2002 to December 2005 | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | Participants with recurrence of hepatorenal syndrome after the initial treatment received terlipressin and albumin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel (open trial) |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment (open trial) |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. Primary outcome measure: resolution of hepatorenal syndrome defined as normalisation of creatinine. |
| For‐profit funding | Unclear risk | Funding not reported. Email requesting information about funding sent 17 February 2016. |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, single‐centre RCT. | |
| Participants |
| |
| Interventions | Terlipressin/albumin versus albumin for a maximum of 15 days Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: 180 days after treatment | |
| Country of origin | Italy | |
| Inclusion period | June 2004 to March 2006 | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel (open trial) |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment (open trial) |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. |
| For‐profit funding | Unclear risk | Funding not reported. Email requesting information about funding sent 17 February 2016. |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Double‐blind, multicentre RCT | |
| Participants |
| |
| Interventions | Terlipressin/albumin versus placebo/albumin for a maximum of 14 days Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: 6 months | |
| Country of origin | United States, Germany, and Russia | |
| Inclusion period | June 2004 to September 2006 | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel achieved through double‐blinding using terlipressin placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment achieved through double‐blinding using terlipressin placebo. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analyses including all participants randomised are reported. No outcomes reported after censoring. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. Primary outcome measure: treatment success at day 14 defined as normalisation of serum creatinine on 2 measurements with at least 48‐hour intervals and no dialysis, death, or recurrence of hepatorenal syndrome type 1 before day 15 |
| For‐profit funding | High risk | Industry funding (Orphan Therapeutics) |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, single‐centre RCT | |
| Participants |
| |
| Interventions | Terlipressin/albumin versus placebo/albumin for 15 days Terlipressin
Albumin
| |
| Outcomes | Duration of follow‐up: end of treatment | |
| Country of origin | India | |
| Inclusion period | Not described | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation through an independent statistician |
| Blinding of participants and personnel (performance bias) | Unclear risk | The authors state that the trial is single‐blind, but do not describe the method of blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | The authors state that the trial is single‐blind, but do not describe the method of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | There were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. |
| For‐profit funding | Unclear risk | Funding not reported. |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, single‐centre RCT | |
| Participants | Participant characteristics (n = 50) not reported. | |
| Interventions | Terlipressin versus no intervention for 15 days Terlipressin
| |
| Outcomes | Duration of follow‐up: 15 days | |
| Country of origin | China | |
| Inclusion period | Not reported | |
| Proportion with type 1 hepatorenal syndrome | 100% | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel (open trial) |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment (open trial) |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear reporting of losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. Primary outcome measure: reversal of hepatorenal syndrome |
| For‐profit funding | Unclear risk | Funding not reported. |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
| Methods | Open, multicentred RCT. | |
| Participants | Participant characteristics not reported. | |
| Interventions | Terlipressin/albumin versus albumin for 10 days (range 8 to 12 days)
| |
| Outcomes | Not reported | |
| Country of origin | Pakistan | |
| Inclusion period | Not reported | |
| Proportion with type 1 hepatorenal syndrome | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | No blinding of participants or personnel (open trial) |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment (open trial) |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome measures reported. |
| For‐profit funding | Unclear risk | Not described |
| Other bias | Low risk | No other biases |
| Overall risk of bias (mortality) | High risk | High risk of bias |
| Overall risk of bias (non‐mortality outcomes) | High risk | High risk of bias |
RCTs: randomised clinical trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT on noradrenaline/albumin versus terlipressin/albumin for hepatorenal syndrome. | |
| RCT comparing different modes of administering terlipressin/albumin for hepatorenal syndrome. | |
| RCT on terlipressin/albumin versus midodrine/octreotide/albumin for hepatorenal syndrome. | |
| RCT comparing terlipressin versus midodrine/octreotide for people with hepatorenal syndrome. | |
| RCT comparing captopril/octreotide versus octreotide published in abstract form. | |
| RCT on noradrenalin/albumin versus terlipressin/albumin for hepatorenal syndrome. | |
| Observational study on terlipressin for hepatorenal syndrome. | |
| Cross‐over trial on octreotide for hepatorenal syndrome. | |
| RCT on noradrenalin/albumin versus terlipressin/albumin for hepatorenal syndrome. | |
| RCT on dopamine versus terlipressin/albumin for hepatorenal syndrome. | |
| RCT comparing low and high‐dose of terlipressin for hepatorenal syndrome type 1. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A placebo‐controlled, double‐blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin |
| Methods | Study type: interventional |
| Participants | Cirrhosis, ascites, and hepatorenal syndrome type 1 |
| Interventions | Drug: terlipressin Drug: placebo |
| Outcomes | Confirmed hepatorenal syndrome reversal: the percentage of participants with 2 serum creatinine values of ≤ 133 µmol/L (1.5 mg/dL) at least 48 hours apart, on treatment, and without intervening renal replacement therapy or liver transplant. |
| Starting date | September 2010 |
| Contact information | Diane Stebbins [email protected] |
| Notes | Estimated enrolment: 180 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 9 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| Analysis 1.1  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 1 Mortality. | ||||
| 1.1 Type 1 hepatorenal syndrome | 7 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.98] |
| 1.2 Type 1 or 2 hepatorenal syndrome | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2 Mortality in randomised clinical trials evaluating terlipressin and albumin Show forest plot | 7 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| Analysis 1.2  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 2 Mortality in randomised clinical trials evaluating terlipressin and albumin. | ||||
| 3 Hepatorenal syndrome Show forest plot | 7 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.82] |
| Analysis 1.3  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 3 Hepatorenal syndrome. | ||||
| 3.1 Type 1 hepatorenal syndrome | 6 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.87] |
| 3.2 Type 2 hepatorenal syndrome | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.08] |
| 3.3 Type 1 or 2 hepatorenal syndrome | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 4 Serious adverse events, total number Show forest plot | 9 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.21] |
| Analysis 1.4  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 4 Serious adverse events, total number. | ||||
| 5 Serious adverse events, types Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 5 Serious adverse events, types. | ||||
| 5.1 Cardovascular adverse events | 4 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 7.26 [1.70, 31.05] |
| 5.2 Circulatory overload | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.59, 5.17] |
| 5.3 Gastrointestinal bleeding | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.05] |
| 5.4 Hepatic encephalopathy | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.68, 1.47] |
| 5.5 Respiratory distress/acidosis | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.84, 4.60] |
| 5.6 Bacterial inflections | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.43] |
| 6 Non‐serious adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 6 Non‐serious adverse events. | ||||
| 6.1 Total number | 5 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.58, 2.68] |
| 6.2 Abdominal pain | 4 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.97, 2.43] |
| 6.3 Chest pain | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 99.34] |
| 6.4 Livedo reticularis | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.10] |
| 6.5 Diarrhoea | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 5.76 [2.19, 15.15] |

Study flow diagram.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Trial Sequential Analysis of eight randomised clinical trials (525 participants) evaluating terlipressin versus placebo or no intervention for people with hepatorenal syndrome on mortality. Data from Hadengue 1998 is not included due to lack of events. The analysis is made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of mortality of 61.5%, and a model variance ‐based heterogeneity correction of 30%. The risk ratio is 0.85 (95% confidence interval 0.70 to 1.02). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.

Trial Sequential Analysis of seven randomised clinical trials (510 participants) evaluating terlipressin versus placebo/no intervention for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis is made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 88%, and a heterogeneity correction of 70%. The risk ratio is 0.64 (95% confidence interval 0.46 to 0.89). The cumulative Z‐curve (blue line) crosses the diversity‐adjusted trial monitoring boundary for benefit during the fourth trial.

Trial Sequential Analysis of four randomised clinical trials (234 participants) evaluating terlipressin versus placebo or no intervention for people with hepatorenal syndrome on cardiovascular adverse events. The analysis is made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The risk ratio is 7.26 (95% confidence interval 1.70 to 31.05). The diversity‐adjusted trial monitoring boundary for harm is not included in the figure due to insufficient information. The estimated required information size is 4831 participants. Accordingly, with an accrued number of participants of 234, only 4.8% of the required number of participants has been achieved.
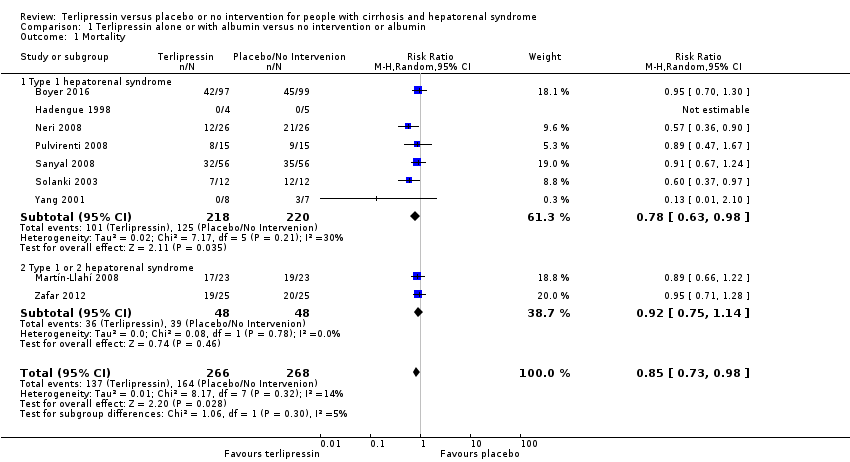
Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 1 Mortality.

Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 2 Mortality in randomised clinical trials evaluating terlipressin and albumin.

Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 3 Hepatorenal syndrome.

Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 4 Serious adverse events, total number.

Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 5 Serious adverse events, types.

Comparison 1 Terlipressin alone or with albumin versus no intervention or albumin, Outcome 6 Non‐serious adverse events.
| Terlipressin versus placebo or no intervention for hepatorenal syndrome. Administration of albumin allowed if administered to both the intervention and comparison group | ||||||
| Patient or population: people with hepatorenal syndrome Comparison: placebo or no intervention or albumin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or no intervention | Risk with terlipressin | |||||
| Mortality | Study population | RR 0.85 (0.73 to 0.98) | 534 | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) the results of the Trial Sequential Analysis | |
| 688 per 1000 | 536 per 1000 | |||||
| Moderate | ||||||
| 625 per 1000 | 488 per 1000 | |||||
| Hepatorenal syndrome | Study population | RR 0.63 (0.48 to 0.82) | 510 | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) all trials are judged as 'high risk of bias'. | |
| 879 per 1000 | 510 per 1000 | |||||
| Moderate | ||||||
| 875 per 1000 | 507 per 1000 | |||||
| Serious adverse events | Study population | RR 0.91 (0.68 to 1.21) | 534 (9 RCTs) | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) all RCTs are judged as high risk of bias. | |
| 85 per 1000 | 212 per 1000 | |||||
| Serious cardiovascular adverse events | Study population | RR 7.26 (1.70 to 31.05) | 234 | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) all RCTs are judged as high risk of bias | |
| 16 per 1000 | 111 per 1000 | |||||
| Abdominal pain | Study population | RR 1.54 (0.97 to 2.43) | 294 (4 RCTs) | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) all RCTs are judged as high risk of bias | |
| 149 per 1000 | 229 per 1000 | |||||
| Diarrhoea | Study population | RR 5.76 (2.19 to 15.15) | 240 (2 RCTs) | ⊕⊕⊝⊝ | Downgraded because of i) clinical heterogeneity and ii) all RCTs are judged as high risk of bias | |
| 33 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Not confirmed in analyses of randomised clinical trials (RCTs) with a low risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 9 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.98] |
| 1.1 Type 1 hepatorenal syndrome | 7 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.98] |
| 1.2 Type 1 or 2 hepatorenal syndrome | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2 Mortality in randomised clinical trials evaluating terlipressin and albumin Show forest plot | 7 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| 3 Hepatorenal syndrome Show forest plot | 7 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.48, 0.82] |
| 3.1 Type 1 hepatorenal syndrome | 6 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.47, 0.87] |
| 3.2 Type 2 hepatorenal syndrome | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.08] |
| 3.3 Type 1 or 2 hepatorenal syndrome | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.92] |
| 4 Serious adverse events, total number Show forest plot | 9 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.21] |
| 5 Serious adverse events, types Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Cardovascular adverse events | 4 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 7.26 [1.70, 31.05] |
| 5.2 Circulatory overload | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.59, 5.17] |
| 5.3 Gastrointestinal bleeding | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.05] |
| 5.4 Hepatic encephalopathy | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.68, 1.47] |
| 5.5 Respiratory distress/acidosis | 2 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [0.84, 4.60] |
| 5.6 Bacterial inflections | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.43] |
| 6 Non‐serious adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Total number | 5 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.58, 2.68] |
| 6.2 Abdominal pain | 4 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.97, 2.43] |
| 6.3 Chest pain | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 5.00 [0.25, 99.34] |
| 6.4 Livedo reticularis | 1 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.12, 72.10] |
| 6.5 Diarrhoea | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 5.76 [2.19, 15.15] |

