Profilaxis antibiótica para el desgarro perineal de tercer y cuarto grado durante el parto vaginal
Resumen
Antecedentes
Del 1% al 8% de las mujeres sufre desgarros perineales de tercer grado (lesión del esfínter anal) y desgarros perineales de cuarto grado (lesión de la mucosa rectal) durante el parto vaginal, y estos desgarros son más frecuentes después del parto con fórceps (28%) y de las episiotomías de la línea media. Los desgarros de tercer y cuarto grado se pueden contaminar con bacterias del recto, lo que aumenta significativamente las probabilidades de infección de la herida perineal. Los antibióticos profilácticos pueden ser importantes para prevenir esta infección.
Objetivos
Evaluar la efectividad de la profilaxis antibiótica para reducir la morbilidad materna y los efectos secundarios en el desgarro perineal de tercer y cuarto grado durante el parto vaginal.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Especializado de Ensayos Controlados del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (31 agosto 2014) y en las listas de referencias de los artículos recuperados.
Criterios de selección
Ensayos controlados con asignación aleatoria que compararan los resultados de los antibióticos profilácticos versus placebo o ningún antibiótico en el desgarro perineal de tercer y cuarto grado durante el parto vaginal.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los informes de los ensayos para su inclusión y el riesgo de sesgo, extrajeron los datos y comprobaron su exactitud.
Resultados principales
Se identificó e incluyó un ensayo (147 mujeres de un tamaño de muestra preplanificado de 310 mujeres) que comparó el efecto del antibiótico profiláctico (una sola dosis de cefalosporina de segunda generación ‐ cefotetano o cefoxitina, 1 g por vía intravenosa) en las complicaciones de la herida perineal postparto en los desgarros perineales de tercer o cuarto grado en comparación con el placebo. Las complicaciones de las heridas perineales (apertura de la herida y secreción purulenta) hasta el chequeo postparto de dos semanas alcanzaron el 8,2% y el 24,1% en los grupos de tratamiento y control respectivamente (riesgo relativo (RR) 0,34; intervalo de confianza [IC] del 95%: 0,12 a 0,96). Sin embargo, la alta tasa de citas fallidas puede limitar la generalización de los resultados. El riesgo general de sesgo fue bajo, excepto por los datos de resultado incompletos. La calidad de la evidencia utilizando GRADE fue moderada para la tasa de infección a las dos semanas de postparto, y baja para la tasa de infección a las seis semanas de postparto.
Conclusiones de los autores
Aunque los datos indican que los antibióticos profilácticos ayudan a prevenir las complicaciones de la herida perineal después del desgarro perineal de tercer y cuarto grado, las pérdidas durante el seguimiento fueron muy altas. Los resultados se deben interpretar con precaución, ya que sólo se basaron en un pequeño ensayo.
PICOs
Resumen en términos sencillos
Profilaxis antibiótica para el desgarro perineal de tercer y cuarto grado durante el parto vaginal
Antecedentes
La mayoría de las mujeres son capaces de dar a luz sin daños graves en el perineo. Sin embargo, el traumatismo perineal grave, que afecta al músculo o al tejido en el canal posterior, se produce en el 1% al 8% de las mujeres que dan a luz y es frecuente cuando se usan fórceps. Hay una mayor probabilidad de infección cuando esto sucede. El dolor en esta área puede repercutir no sólo en la actividad diaria de una mujer, sino también en su relación con su bebé y su pareja.
Cuando una mujer presenta un desgarro perineal grave durante el parto vaginal, se cree que hay un mayor riesgo de infección. Los antibióticos a menudo se recetan para prevenir la infección.
Pregunta de la revisión Evaluar la efectividad de la profilaxis con antibióticos para prevenir la infección de la herida perineal en comparación con el placebo o ningún tratamiento para prevenir la infección de la herida perineal y comparar diferentes regímenes de antibióticos.
Principales hallazgos
La revisión identificó un ensayo con 147 mujeres. Este ensayo se realizó para explorar el beneficio de los antibióticos profilácticos habituales (grupo de intervención) versus placebo (grupo de control) para mujeres con desgarros perineales severos. El resultado mostró menos complicaciones de heridas perineales en el grupo de intervención a las dos semanas de postparto. No hubo diferencias estadísticamente significativas en las complicaciones de la herida perineal antes del alta y a las seis semanas después del parto.
El estudio incluido se terminó antes de alcanzar el tamaño de muestra previsto y tuvo una alta tasa de pérdida durante el seguimiento
Calidad de la evidencia
El estudio incluido era de alta calidad metodológica, excepto por el seguimiento incompleto. Se evaluó la reducción de la infección de la herida perineal en el desgarro de tercer o cuarto grado mediante la profilaxis con antibióticos como de baja a moderada calidad de la evidencia. Sin embargo, los resultados se basan en un ensayo pequeño y hubo muchas pérdidas en el seguimiento. Se necesitan más estudios de investigación.
Authors' conclusions
Summary of findings
| Antibiotic prophylaxis versus no treatment for perineal tear for third‐ and fourth‐degree perineal tear during vaginal birth | ||||||
| Patient or population: Women with third‐ and fourth‐degree perineal tear during vaginal birth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic prophylaxis versus no treatment for perineal tear | |||||
| Infection rate at 2 weeks postpartum ‐ perineal wound infection in third‐ or fourth‐degree tear | Study population | RR 0.34 | 107 | ⊕⊕⊕⊝ | ||
| 241 per 1000 | 82 per 1000 | |||||
| Moderate | ||||||
| 241 per 1000 | 82 per 1000 | |||||
| Infection rate at 6 weeks postpartum ‐ perineal wound infection in third‐ or fourth‐degree tear | Study population | RR 0.38 | 128 | ⊕⊕⊝⊝ | ||
| 192 per 1000 | 73 per 1000 | |||||
| Moderate | ||||||
| 192 per 1000 | 73 per 1000 | |||||
| Fever or puerperal febrile morbidity | Not estimable | 0 study | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small sample size and not met optimal information size (OIS) criteria. | ||||||
Background
Description of the condition
Episiotomy is the incision in the perineal area to enlarge the vaginal orifice for easier vaginal birth. It consists of two types: midline or median and mediolateral style. Many studies have shown that routine episiotomy can cause such problems as persistent perineal pain, unsatisfied postpartum sexual function, and increasing anal sphincter injury leading to faecal or flatus incontinence (Christianson 2003; Jones 2000; Labrecque 1997; Nager 2001; Signorello 2001). Pain in this area can impact not only on a woman's daily activity but also on her relationship with her baby and her partner. A restrictive episiotomy policy (strict criteria to perform episiotomy only in proper cases such as short stature mother with short perineum, large size baby, or imminent severe laceration of perineum) has been in place for many years and is strongly supported by a Cochrane systematic review (Carroli 1999). In addition, prenatal perineal massage has also been introduced into practice to prevent perineal tears (Davidson 2000; Eason 2000; Johanson 2000; Labrecque 1999). One randomised controlled trial failed to show the effectiveness of perineal massage but concluded that it did no harm (Stamp 2001). A Cochrane review concluded that antenatal digital perineal massage reduces the incidence of perineal trauma (mainly episiotomies) (Beckmann 2013).
Most women are able to give birth without serious damage to the perineum, but 1% to 8% of women suffer severe perineal tears (anal sphincter injury, with or without rectal mucosa injury) during vaginal birth (De Leeuw 2001; Riskin‐Mashiah 2002; Samuelsson 2000; Samuelsson 2002; Sultan 1994). These tears are more common after operative vaginal birth, especially when forceps are used. The incidence of severe perineal lacerations after the use of forceps has been reported as 21% for third‐degree and 7% for fourth‐degree tears (Bofill 1996). Other risk factors include race (Asian women have the highest risk, perhaps due to small size mother and short perineum), midline episiotomy (short distance to anal sphincter), nulliparity (lesser elasticity than a multiparous mother), and high birthweight baby (De Leeuw 2001; Goldberg 2003; Homsi 1994; Jones 2000; Labrecque 1997; Nager 2001; Sultan 1994).
Description of the intervention
When a woman has a severe perineal tear during vaginal birth, there is thought to be an increased risk of infection. Laceration of the vagina and perineum during vaginal birth are classified as first‐, second‐, third‐ and fourth‐degree tears. First‐degree tears involve the vaginal mucosa and connective tissue. Second‐degree tears involve the vaginal mucosa, connective tissue and underlying muscles. Third‐degree tears involve a complete transection of anal sphincter and fourth‐degree tears involve the rectal mucosa (Cunningham 2001; WHO 2003). When the rectal mucosa is ruptured, the wound is classified as contaminated (Waddell 1994) or clean‐contaminated (Mangram 1999). Antibiotic prophylaxis is generally used where wounds have become, or are likely to become, contaminated, such as in colorectal surgery (Oates 1986; Song 1998). A Cochrane review has also shown antibiotic prophylaxis to be effective in reducing puerperal morbidity after cesarean section (Smaill 2002). On the other hand, a Cochrane review on antibiotic prophylaxis after operative vaginal birth could not conclude its effectiveness (Liabsuetrakul 2004).
How the intervention might work
A woman contracting infection after a severe perineal tear may also be at risk of other morbidities as a result of the tear, such as haematoma, dyspareunia (painful sexual intercourse), incontinence and recto‐vaginal fistula (Crawford 1993; Homsi 1994; Labrecque 1997; Nager 2001; Signorello 2001; Sorensen 1988; Sultan 2002; WHO 2003). While some authorities recommend that prophylactic antibiotics be used for severe perineal tears (WHO 2003), others have recommended against this course of action (Whitfield 1995). As widespread use of antibiotics may contribute to antibiotic‐resistant bacteria (Towers 1998; Weinstein 1996), the over‐use of antibiotics is being discouraged by many groups.
Why it is important to do this review
Antibiotic prophylaxis is a low‐cost, accessible intervention that may prevent considerable maternal morbidity. It is therefore important to establish the benefits of prophylactic antibiotics for infection after severe perineal tears, and also to assess whether there are any adverse effects on mother or infant, by systematically reviewing the evidence.
Objectives
To assess the effectiveness of antibiotic prophylaxis for reducing maternal morbidity and side effects in third‐ and fourth‐degree perineal tears during vaginal birth.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and cluster‐randomised controlled trials. We did not plan to include quasi‐randomised controlled, cross‐over trials or include studies that were presented only as abstracts.
Types of participants
Mothers with third‐ and fourth‐degree perineal tears as a result of vaginal birth.
Types of interventions
Antibiotic regimens used to prevent perineal wound infection compared either with placebo, or no treatment; antibiotic treatment in women who get infection only; and comparisons between different antibiotic regimens.
Types of outcome measures
Primary outcomes
-
Fever or puerperal febrile morbidity (body temperature of 38ºC or higher occurring on any two occasions in the first 10 days postpartum, exclusion of the first 24 hours).
-
Perineal wound complications, including infection (oedematous, erythematous, wound edge with pain, serosanguinous or frankly purulent material), or wound dehiscence (wound separation).
Secondary outcomes
-
Serious infectious complications (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis, or death attributed to infection).
-
Pain (wound pain score or variously measured by authors).
-
Woman's comfort (unable to sit down or breast feed) while in hospital and six weeks postpartum.
-
Length of hospital stay for mother.
-
Adverse reaction (such as allergic reaction, anaphylaxis, diarrhoea).
-
Maternal‐infant interactions including breastfeeding.
-
Sexual function including dyspareunia (pain on sexual intercourse), sexual satisfaction, sexual sensation, time to resuming sexual intercourse.
-
Woman's satisfaction.
-
Recto‐vaginal fistula (hole between the vagina and rectum).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional searching carried out by authors for the initial version of the review (Buppasiri 2005), see:Appendix 1.]
Searching other resources
We checked the references of retrieved articles to locate other relevant trials.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous update, please see Buppasiri 2010. For this update no new studies were identified from the updated search. However, full risk of bias was performed for the included study and the quality of the evidence was assessed using the GRADE approach.
In future updates, the full methods outlined in Appendix 2 will be used.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for the study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). There were no disagreements. We would have resolved any disagreements through discussion or, if required, we would have involved a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for the one included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomisation participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the included study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update (2014) ,the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison.
-
Fever or puerperal febrile morbidity.
-
Perineal wound infection or perineal wound disruption.
The GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes were produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
The updated search in December 2009 identified two reports of one study (Duggal 2008) ‐ seeCharacteristics of included studies for details. An updated search in June 2014 did not identify any new trials.
Risk of bias in included studies
The one included study was terminated before it reached the pre‐planned sample size and had a high rate of loss to follow‐up (Figure 1).
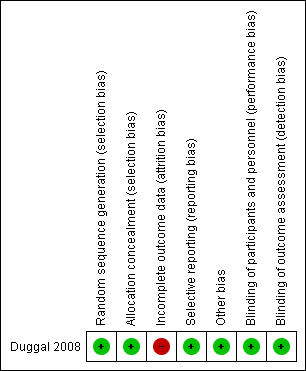
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Procedures for adequate allocation concealment were well described in the study. We judged this study to be at low risk of bias for selection bias.
Blinding
The process of blinding was described. The women, investigator and assessor were all blinded. We judged this study to be at low risk of bias for performance and detection bias.
Incomplete outcome data
The planned number of women to be recruited was 310 (155 per arm). The actual number of women recruited was 147 (44.5%, 64 in treatment arm and 83 in control group). The study was terminated before the planned number of women was achieved. The loss to follow‐up rate was high (27%, 40 in 147). The study was therefore considered to be at moderate risk of attrition bias for attrition bias.
Selective reporting
The study was considered to be at low risk of selective reporting because the planned outcome measurement was prospectively registered in clinical trial registration.
Other potential sources of bias
We considered that it was unlikely that there would be other sources of potential bias so this domain was judged to be at low risk of bias.
Effects of interventions
Single‐dose, second‐generation cephalosporin intravenously (cefotetan or cefoxitin, 1 g, intravenously, or clindamycin, 900 mg intravenously if allergic to penicillin, in 100 mL of saline) was used as the intervention to prevent perineal wound infection or disruption in the third‐ or fourth‐degree perineal tear compared with placebo (100 mL of normal saline intravenously) .
The primary outcome of the included study was gross disruption or purulent discharge at the site of perineal repair by two weeks postpartum.
Perineal wound complications at two weeks postpartum in the treatment and control groups were four of 49 (8.2%) and 14 of 58 (24.1%) respectively (P = 0.037, risk ratio (RR) 0.34, 95% confidence interval (CI) 0.12 to 0.96) (Analysis 1.1).
Perineal wound infection before discharge and at six weeks postpartum was also reported. There were no perineal wound complications before hospital discharge in both groups. One hundred and twenty‐eight women were checked at six weeks postpartum (19 of 147 (12.9%) did not come for follow‐up at six weeks). There were perineal wound complications in four out of 55 (7.3%) and 14 out of 73 (19.2%) women in the treatment and control groups respectively, (P = 0.07, RR 0.38, 95% CI 0.13 to 1.09) (Analysis 1.2).
Discussion
Summary of main results
There were marginally significantly fewer perineal wound complications at two weeks' postpartum in the intervention group. There was no statistically significant difference in perineal wound complications before discharge and at six weeks' postpartum. However, loss to follow‐up was 27.2% and 12.9 % at two and six weeks postpartum respectively.
Overall completeness and applicability of evidence
The one included study, conducted in a developed country, with a relatively high loss of follow‐up, provides insufficient data to address the objectives of this review.
Quality of the evidence
The included study was of high methodological quality; most of the domains were judged to be at low risk of bias except for incomplete outcome data, which we assessed as at high risk of bias. We assessed perineal wound infection in third‐ or fourth‐degree tear reduced significantly by antibiotic prophylaxis at two weeks' postpartum as moderate quality of the evidence and six weeks' postpartum as low quality of the evidence due to the small sample size and because the confidence interval overlaps 'no effect' (summary of findings Table for the main comparison). We could not assess "1. Fever or puerperal febrile morbidity" because the outcome was not reported in the one included study. The inadequate sample size and high loss to follow‐up rate do not allow us to draw a firm conclusion about the benefit of antibiotic prophylaxis in third‐ or fourth‐degree perineal tear after vaginal birth.
Potential biases in the review process
We adhered to the Cochrane Pregnancy and Childbirth Group search strategies and review process. We are not aware of any potential bias in the review process.
Agreements and disagreements with other studies or reviews
There are no other studies or reviews addressing this clinical question.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Antibiotic prophylaxis versus no treatment for perineal tear, Outcome 1 Infection rate at 2 weeks postpartum.

Comparison 1 Antibiotic prophylaxis versus no treatment for perineal tear, Outcome 2 Infection rate at 6 weeks postpartum.
| Antibiotic prophylaxis versus no treatment for perineal tear for third‐ and fourth‐degree perineal tear during vaginal birth | ||||||
| Patient or population: Women with third‐ and fourth‐degree perineal tear during vaginal birth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic prophylaxis versus no treatment for perineal tear | |||||
| Infection rate at 2 weeks postpartum ‐ perineal wound infection in third‐ or fourth‐degree tear | Study population | RR 0.34 | 107 | ⊕⊕⊕⊝ | ||
| 241 per 1000 | 82 per 1000 | |||||
| Moderate | ||||||
| 241 per 1000 | 82 per 1000 | |||||
| Infection rate at 6 weeks postpartum ‐ perineal wound infection in third‐ or fourth‐degree tear | Study population | RR 0.38 | 128 | ⊕⊕⊝⊝ | ||
| 192 per 1000 | 73 per 1000 | |||||
| Moderate | ||||||
| 192 per 1000 | 73 per 1000 | |||||
| Fever or puerperal febrile morbidity | Not estimable | 0 study | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small sample size and not met optimal information size (OIS) criteria. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infection rate at 2 weeks postpartum Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.96] |
| 1.1 Perineal wound infection in third‐ or fourth‐degree tear | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.96] |
| 1.2 Perineal wound infection in third‐degree tear | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Perineal wound infection in fourth‐degree tear | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Infection rate at 6 weeks postpartum Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.09] |
| 2.1 Perineal wound infection in third‐ or fourth‐degree tear | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.13, 1.09] |
| 2.2 Perineal wound infection in third‐degree tear | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Perineal wound infection in fourth‐degree tear | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

