Uterine artery embolization for symptomatic uterine fibroids
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study: EMMY Trial. Design: randomized controlled trial. Attending gynaecologist contacted the trial bureau by telephone, where the participant was registered and randomly assigned (1:1) to UAE or hysterectomy, using a computer‐based minimization scheme (‘balancing procedure’), and stratified for study centre. The randomisation result was recorded electronically | |
| Participants | Participants: women were enrolled from five university hospitals and 29 general hospitals. Of 349 eligible participants, 177 were randomised: 88 were allocated UAE and 89 hysterectomy. The majority of participants refusing participation did so for a strong preference for hysterectomy (58%) or for UAE (21%). After randomisation, seven women in the UAE group and 14 women in the hysterectomy group refused the allocated treatment. The mean age was 44.6 years (UAE group) and 45.4 years (hysterectomy group). Participants suffered from menorrhagia for a median of 24 months. The majority of women had multiple fibroids. Fibroid volumes were higher in the hysterectomy group. Logistic regression analysis did not reveal baseline characteristics that could predict randomisation outcome, confirming successful randomisation | |
| Interventions | Study group: after randomisation, 7 (8%) women declined UAE. UAE was successfully performed in 72 of 81 women, 5 of whom had a unilateral procedure because of single‐sided arterial blood flow to the fibroid (procedural success rate: 88.9%). The remaining 11.1% consisted of 5 women (6.2%) with a unilateral procedure (caused by technical failure on the other side) and 4 women (4.9%) with bilateral UAE failure | |
| Outcomes | Primary endpoint: Evaluation of re‐intervention rates at 5 years: menstrual characteristics, menorrhagia, quality of life measures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (1:1) using a computer‐based minimization scheme |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, re‐intervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | High risk | After randomisation, 92% of randomised women were analysed in the UAE group (81/88) and 84,3% in the hysterectomy group (75/89). At 5 years, there were further dropouts: 85% in the UAE group (75/88) and 78.7% in the hysterectomy group (70/89) |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Low risk | No other potential source of bias identified |
| Methods | Study: FUME Trial. Design: randomized controlled trial. Sealed opaque envelopes, random numbers generated by computer. Blocks of 10 | |
| Participants | Participants: 163 women were randomised: 82 were allocated UAE and 81 myomectomy. After randomisation, eight women in the UAE group who withdrew ‐ six had myomectomy, 1 had hysterectomy and one was lost to follow‐up, and eight women in the myomectomy group withdrew ‐ four had hysterectomy, two were converted to hysterectomy at time of surgery and two others lost. During follow‐up there were a further 11 lost from the UAE group and 14 lost from the myomectomy group. The mean age was 44 years (UAE group) and 43 years (hysterectomy group). The UAE group had slightly larger fibroid volumes Exclusion criteria included fibroids attached to the uterus by a narrow pedicle, or the whole fibroid mass being so large that it extended beyond the level of the umbilicus, or documented allergy to radiographic contrast medium, or a history of recent or ongoing pelvic inflammatory disease. Women also were excluded if they were not prepared to accept surgery as a treatment option, if they were pregnant, or if they were actively planning or trying to conceive | |
| Interventions | Study group: UAE performed by or supervised by same experienced interventional radiologist | |
| Outcomes | Primary endpoint: quality of life measures at one year using the Uterine Fibroid Symptom and Quality of Life (UFS‐QOL) questionnaire. Other endpoints: evaluation of re‐intervention rates at 2 years, complications | |
| Notes | Fertility as an outcome was not collected as the ethics committee did not approve UAE for women who wished to conceive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomised using the sealed opaque envelope technique, using random numbers generated by computer" |
| Allocation concealment (selection bias) | Low risk | "Women were randomised using the sealed opaque envelope technique, using random numbers generated by computer" |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, reintervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | High risk | After randomisation, 23% of randomised women excluded from analysis in the UAE group (19/82) and 27% in the myomectomy group (22/81) |
| Selective reporting (reporting bias) | High risk | No suggestion of selective reporting. Fertility as an outcome was not collected as the ethics committee did not approve UAE for women who wished to conceive. Findings for QoL differed according to whether change scores or end scores were used, but both were reported in the review |
| Other bias | High risk | There were baseline differences between the groups in QoL and although these were reported as not statistically significant, these do represent high risk |
| Methods | Study design: randomised controlled trial. Randomisation was performed in a 1:1 ratio according to a computer‐generated schedule. The method of surgery was by open surgery in all cases | |
| Participants | Participants: women over the age of 18 were enrolled from 1 hospital. 127 women were randomised with 63 of them undergoing embolization and 64 undergoing surgery (10 hysterectomies and 54 myomectomies) | |
| Interventions | Study group: 63 women were allocated to UAE and all underwent the procedure | |
| Outcomes | Primary outcome measure: quality of life (36‐Item Short‐Form General Health Survey (SF‐36) and complications. The SF‐36 scores were presented at 6 month follow‐up while the complications were reported after a maximum follow‐up of 42 months. Secondary outcome measures: hospital stay, recovery time, satisfactory rate, recommending rate, pain at 24 hours and additional invasive procedures including hysterectomy or repeated embolization | |
| Notes | Power calculation was not carried out. In the surgical group, the majority of the procedures were myomectomies but impact on fertility was not one of the outcomes of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to study groups according to a computer‐generated schedule" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, re‐intervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | Low risk | After randomisation, 98.4% (62/63) were analysed in the UAE group and 96.9% (62/64) in the surgical group |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Unclear risk | Power calculation not carried out |
| Methods | Study design: prospective randomised controlled trial. Randomization was performed by means of a computer‐generated random numbers. Patients with odd integers were placed into the embolization group and those with even numbers into the myomectomy group | |
| Participants | Participants: 121 women with uterine fibroid or fibroids and unfinished reproductive plans were randomised: 58 were allocated embolization and 63 myomectomies; 120 women finished a six month follow‐up and 3 women dropped out of the trial. The mean age was 32.4 years (UAE group) and 32.0 years (myomectomy group) Of the 121 participants, 110 were symptomatic (90.9%). 66 were nulligravidae (54.5%), 35 were sterile (28.9%; 11 in embolization and 24 in myomectomy group; P < 0.05), 18 had miscarried in the past (14.9%) and 51 had another subfertility factor other than myoma (42.1%) Country: Czech Republic Inclusion criteria: 1) age up to 40 years; 2) planned pregnancy; 3) ultrasound verified intramural fibroids of at least 4 cm in greatest diameter (in the case of more fibroids, the largest being at least 4 cm); 4) serum concentration of FSH under 30 IU/L (on the third day of the menstrual cycle) Exclusion criteria: 1) type 0 and type 1 submucous myomas and subserous myomas; 2) size of largest fibroid greater than 12 cm in greatest diameter on ultrasound or a uterus greater than the 4th month of pregnancy on palpation; 3) previous surgical or medical treatment; 4) suspected uterine sarcoma; 5) significant illness that would contraindicate pregnancy; 6) lack of consent | |
| Interventions | Study group: Bilateral UAE Control group: After randomisation, 63 women underwent myomectomy, (42 laparoscopic, 21 open). The type and route of access were left at the discretion of the attending gynaecologist | |
| Outcomes |
| |
| Notes | 40 women after myomectomy and 26 women after UAE have tried to conceive | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients marked with odd integers were placed into the E group (embolization) and patients given even numbers by the computer were located into the Mgroup (myomectomy). In other words, a random number has been generated a new for every new patient; none of the researchers could therefore either know or predict the next number (there was no pre‐created list of numbers)." |
| Allocation concealment (selection bias) | Low risk | "Patients marked with odd integers were placed into the E group (embolization) and patients given even numbers by the computer were located into theMgroup (myomectomy). In other words, a random number has been generated anew for every new patient; none of the researchers could therefore either know or predict the next number (there was no pre‐created list of numbers)." |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, re‐intervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | Low risk | After randomisation, 100% (58/58) were analysed in the UAE group and 98.4% (6263) in the myomectomy group. At 12 months there were 2 further dropouts in the UAE group giving a follow‐up rate of 96.6% |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Low risk | No other potential source of bias identified |
| Methods | Study design: randomized, parallel, controlled clinical trial. Method of randomisation: Zelen design which is random allocation prior to seeking consent. The randomisation was stratified 2:1 in favour of UAE and generated by computer sealed number envelopes | |
| Participants | Participants: women aged 35 to 57 years. 57 women were randomised. 38 women to study group who were told of UAE and hysterectomy. 19 women to control group who were informed of hysterectomy only | |
| Interventions | Study group: 38 women informed of the study and offered the option of undergoing hysterectomy or UAE. 37 women elected to undergo UAE and one hysterectomy | |
| Outcomes | Evaluation of efficiency: total length of hospital stay after UAE and hysterectomy | |
| Notes | The analysis is different for different outcomes. The length of hospital stay in the two study arms were compared on an intent‐to‐treat basis. However, due to the participants electing different treatments to those they were allocated to, effectiveness and safety were evaluated on the basis of the actual treatment received (treatment received analysis) and hence, this was excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random patient assignments were generated by computer and kept in sealed, numbered envelopes" |
| Allocation concealment (selection bias) | Low risk | "The random patient assignments were generated by computer and kept in sealed, numbered envelopes" |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, re‐intervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | High risk | The analysis is different for different outcomes. Per protocol analysis used |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Low risk | No other potential source of bias identified |
| Methods | Study design: randomised controlled trial. Randomisation was performed by means of a computer‐generated schedule (permuted blocks). This was stratified by centre and women were randomly assigned (2:1) to UAE or surgery (hysterectomy or myomectomy). The method of surgery was not specified | |
| Participants | Participants: women over the age of 18 were enrolled from 27 hospitals. 157 women were randomised with 106 of them undergoing embolization and 51 undergoing surgery | |
| Interventions | Study group: 106 women were allocated to UAE. 101 of these underwent the procedure with technical failure encountered in three | |
| Outcomes | Primary outcome measure: quality of life (36‐Item Short‐Form General Health Survey (SF‐36). Secondary outcome measures: time until resumption of usual activities (we have used the data for when women started driving their car as a resumption to normal activities), satisfaction score, pain score at 24 hours, any complications and treatment failure. Ovarian failure has also been reported at 1 year. Pregnancy outcomes were reported at 5 year follow‐up. The study was not set up or powered to assess this outcome and there were only 8 myomectomies in the surgical group of 51 women | |
| Notes | The original target of 200 women was reduced to 150 because of difficulties in recruitment which reduced the power to 80%. The data were presented in median and inter‐quartile ranges as the milestone data were very skewed. After contacting the authors they released the mean and standard deviations of the data on the understanding that these data are included in this review with this caveat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (2:1) using a computer‐generated schedule |
| Allocation concealment (selection bias) | Low risk | Remote telephone randomisation |
| Blinding (performance bias and detection bias) | Unclear risk | No blinding, but unclear how much this would affect relatively objective outcomes (e.g. live birth, complications, re‐intervention) |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | Low risk | After randomisation, 89.6% (95/106) were analysed in the UAE group and 88.2% (45/51) in the surgical group |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Low risk | No other potential source of bias identified |
| Methods | Single‐centre, prospective, randomised trial. Enrolled and assigned eligible participants to UAE or hysterectomy using sealed envelopes (1:1 ratio). Recruitment and randomisation were performed at the same gynaecology outpatient clinic visit | |
| Participants | Of 137 eligible women, 57 (41.6%) were randomised (UAE, N = 27; hysterectomy, N = 30). All Caucasians, were recruited to the prospective follow‐up study between 2002 and 2007 Inclusion criteria were women's subjective symptoms, which had to be severe enough to warrant consideration of hysterectomy, and only women agreeing to hysterectomy, if necessary, were included in the study Exclusion criteria were suspected genital tract malignancy, adnexal pathological features (suspected tumour or sactosalpinx), acute pelvic inflammatory disease, fertility preservation, uterovaginal prolapse requiring treatment, previous reactions to contrast media, renal impairment, and leiomyomas suitable for hysteroscopic myomectomy (single leiomyoma over 50% in the cavum uteri and 5 cm or less in size) | |
| Interventions | Embolisation procedure: shortly after selective catheterization of both uterine arteries from right femoral artery access, embolization was performed with calibrated microsphere particles (550–700 μm; EmboSphere; BioSphere Medical, Louvres, France) until near‐stasis was observed in the ascending segment of the uterine artery. In tortuous, small or spastic uterine arteries, catheterization was performed with a 2.1‐ French microcatheter to ensure free‐flow embolization. An Angio‐Seal closure device was routinely used. The same interventional radiologist performed all interventions (HM, with 2 years’ experience in UAE at the beginning of the trial). After the intervention, women were observed in a recovery room for 4–6 h, after which they were transferred to the gynaecology ward for further care Hysterectomy: the type of hysterectomy and route of access were not standardised and left to the discretion of the attending gynaecologist, in order to maintain the protocol as close to that of daily practice as possible. Hysterectomy was performed as an abdominal hysterectomy, vaginal hysterectomy or laparoscopic‐assisted hysterectomy. General anaesthesia was used in all operations | |
| Outcomes | The primary endpoint was improvement of symptoms; secondary endpoints were procedural characteristics, major complications, time to discharge from hospital, length of sick leave, re‐interventions required, and satisfaction with treatment at 2 year follow‐up. The following symptoms were recorded: duration and severity of menstrual flow (no periods, mild, moderate, severe; with moderate or severe indicating menorrhagia), dysmenorrhoea, pressure symptoms of the bladder, bowel, or back, increased urinary frequency, urinary stress incontinence, and non‐menstrual related lower abdominal pain. Menstrual flow was recorded severe when it prevented everyday activities, caused anaemia, and extra large pads or tampons (change every 1 to 2 h) were needed. Complete blood count, ferritin, haematocrit, follicle‐stimulating hormone and estrogen levels were ordered | |
| Notes | Power calculation not carried out. Sample size is small. Attempts to randomise more women in a reasonable time failed because of difficulties in recruitment, and recruitment was stopped after 5 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details reported, states "The same gynaecologist discussed treatment options with the patient and enrolled and assigned eligible participants to UAE or hysterectomy using sealed envelopes (1:1 ratio)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details reported, states "The same gynaecologist discussed treatment options with the patient and enrolled and assigned eligible participants to UAE or hysterectomy using sealed envelopes (1:1 ratio)." |
| Blinding (performance bias and detection bias) | Unclear risk | Carried out in same gynaecology outpatient clinic |
| Blinding (performance bias and detection bias) | High risk | No blinding, which was likely to affect subjective outcomes (e.g. satisfaction rate, quality of life) |
| Incomplete outcome data (attrition bias) | Low risk | After randomisation, 96.35 (26/27) were analysed in the UAE group and 96.7% (29/30) in the hysterectomy group. One patient from the UAE group withdrew consent for follow‐up 1 day after UAE, and one patient from the hysterectomy group died from cerebral infarct 13 months after the hysterectomy |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all expected outcomes reported |
| Other bias | Unclear risk | Power calculation not carried out |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Compared two similar methods of uterine artery occlusion i.e. laparoscopic bilateral occlusion of uterine arteries versus uterine artery embolization |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Treating Fibroids with either Embolisation or Myomectomy to Measure the Effect on quality of life among women wishing to avoid hysterectomy: The FEMME study |
| Methods | Multicentre randomised trial comparing myomectomy to UAE. Follow‐up at 6 weeks, 6 months, 12 months, 2 and 4 years |
| Participants | 216 women to be randomised Inclusion criteria: all criteria must be met. Women with symptomatic fibroids who do not wish to have a hysterectomy; Women with symptomatic fibroids who would ordinarily be offered a myomectomy; Women must be considered suitable for either treatment (myomectomy or embolisation); Clinical team uncertain as to which treatment is indicated; Written informed consent Exclusion criteria: refusal to accept hysterectomy, even as a result of an intra‐operative complication; recent or ongoing pelvic inflammatory disease; significant adenomyosis, as identified by TVUS or CEMRI. Concurrent adenomyosis where fibroids are believed to the predominant cause of symptoms will be eligible; Positive pregnancy test; Refusal to accept surgery or embolisation as treatment option; Postmenopausal, as defined as greater than one year since previous menstrual period; Suspected malignancy; Women aged under 18 years old; Unable to provide informed consent due to incapacity (as defined by Mental Capacity Act 2005 or Adults with Incapacity (Scotland) Act 2000); A non‐English speaker where translation or interpretation facilities are insufficient to guarantee informed consent; A previous myomectomy via a laparotomy or a previous embolization |
| Interventions | Myomectomy or UAE |
| Outcomes | Primary: quality of life (UFS‐QOL). Secondary: effect on menstrual bleeding, pregnancy outcomes, need for further treatment and adverse events, ovarian function and reserve |
| Starting date | March 2012 to October 2014 |
| Contact information | Dr William McKinnon Tel: +44 (0) 121 414 8335 |
| Notes | ISRCTN: 70772394. Registry accessed 26 November 2014. Funder is NIHR. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with treatment up to 24 months Show forest plot | 6 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.59, 1.48] |
| Analysis 1.1 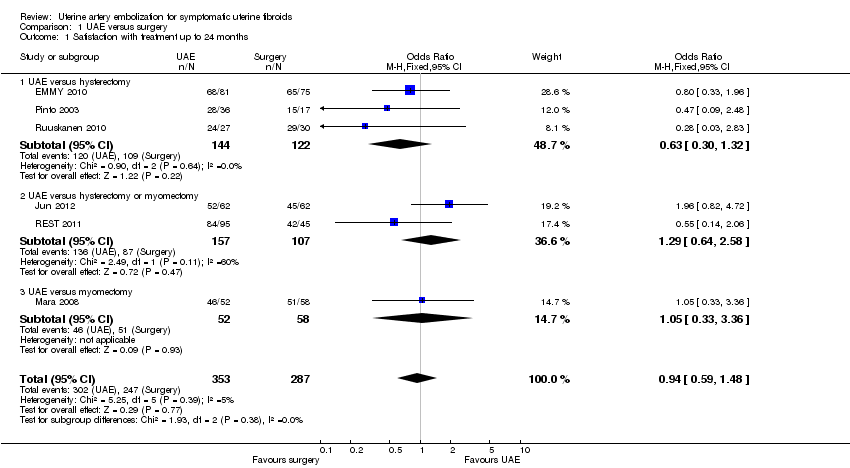 Comparison 1 UAE versus surgery, Outcome 1 Satisfaction with treatment up to 24 months. | ||||
| 1.1 UAE versus hysterectomy | 3 | 266 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.30, 1.32] |
| 1.2 UAE versus hysterectomy or myomectomy | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.64, 2.58] |
| 1.3 UAE versus myomectomy | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.33, 3.36] |
| 2 Satisfaction with treatment at 5 years Show forest plot | 2 | 295 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.45, 1.80] |
| Analysis 1.2 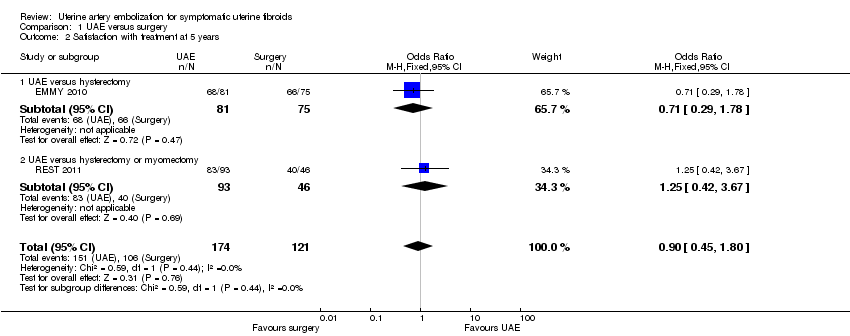 Comparison 1 UAE versus surgery, Outcome 2 Satisfaction with treatment at 5 years. | ||||
| 2.1 UAE versus hysterectomy | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.29, 1.78] |
| 2.2 UAE versus hysterectomy or myomectomy | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.42, 3.67] |
| 3 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 UAE versus surgery, Outcome 3 Live birth. | ||||
| 3.1 UAE versus myomectomy | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.84] |
| 4 Adverse events: intraprocedural complications Show forest plot | 4 | 452 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| Analysis 1.4 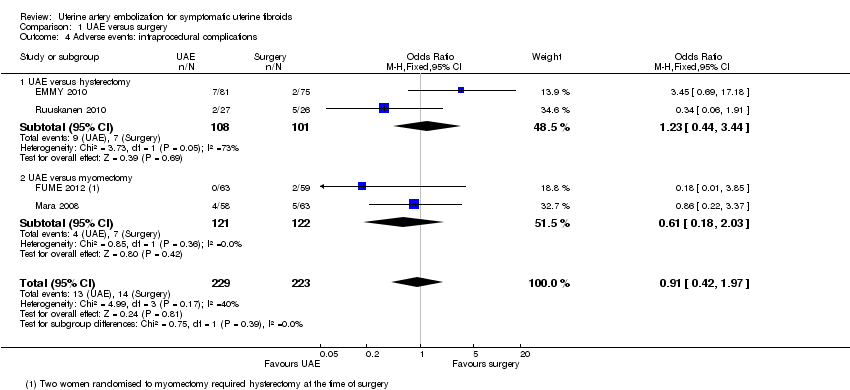 Comparison 1 UAE versus surgery, Outcome 4 Adverse events: intraprocedural complications. | ||||
| 4.1 UAE versus hysterectomy | 2 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.44, 3.44] |
| 4.2 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.03] |
| 5 Adverse events: Need for blood transfusion Show forest plot | 2 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| Analysis 1.5  Comparison 1 UAE versus surgery, Outcome 5 Adverse events: Need for blood transfusion. | ||||
| 5.1 UAE versus hysterectomy | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 5.2 UAE versus myomectomy | 1 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.47] |
| 6 Adverse events: minor postprocedural complications Show forest plot | 6 | 735 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.41, 2.81] |
| Analysis 1.6 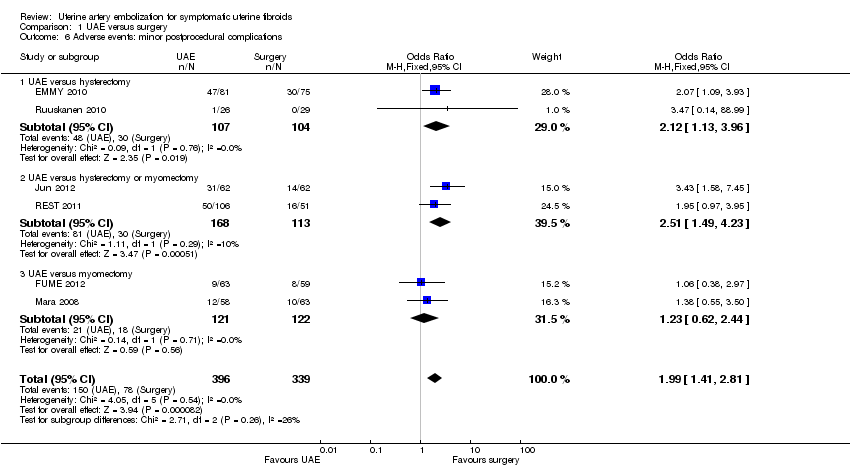 Comparison 1 UAE versus surgery, Outcome 6 Adverse events: minor postprocedural complications. | ||||
| 6.1 UAE versus hysterectomy | 2 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.13, 3.96] |
| 6.2 UAE versus hysterectomy or myomectomy | 2 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.49, 4.23] |
| 6.3 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.44] |
| 7 Adverse events: major postprocedural complications within one year Show forest plot | 5 | 611 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.26] |
| Analysis 1.7  Comparison 1 UAE versus surgery, Outcome 7 Adverse events: major postprocedural complications within one year. | ||||
| 7.1 UAE versus hysterectomy | 2 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.22, 4.58] |
| 7.2 UAE versus hysterectomy or myomectomy | 1 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.30, 1.74] |
| 7.3 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.50] |
| 8 Adverse events: later minor postprocedural complications Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 UAE versus surgery, Outcome 8 Adverse events: later minor postprocedural complications. | ||||
| 8.1 UAE versus hysterectomy or myomectomy (to 5 years) | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.73, 4.93] |
| 8.2 UAE versus myomectomy (1 month‐2 years) | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.56, 5.94] |
| 9 Adverse events: major postprocedural complications Show forest plot | 3 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| Analysis 1.9  Comparison 1 UAE versus surgery, Outcome 9 Adverse events: major postprocedural complications. | ||||
| 9.1 UAE versus hysterectomy or myomectomy (to 5 years) | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 9.2 UAE versus myomectomy (1 month‐2 years) | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Further interventions within 2 years Show forest plot | 6 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.72 [2.28, 6.04] |
| Analysis 1.10 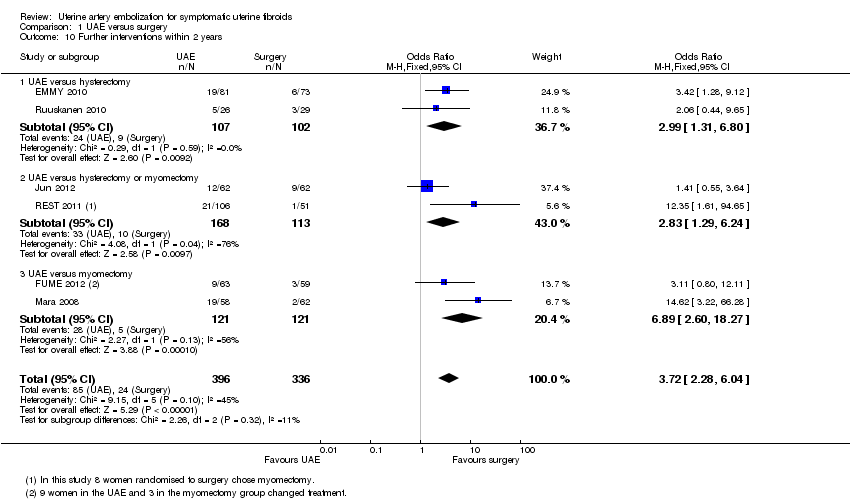 Comparison 1 UAE versus surgery, Outcome 10 Further interventions within 2 years. | ||||
| 10.1 UAE versus hysterectomy | 2 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.99 [1.31, 6.80] |
| 10.2 UAE versus hysterectomy or myomectomy | 2 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.29, 6.24] |
| 10.3 UAE versus myomectomy | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.89 [2.60, 18.27] |
| 11 Further interventions within 5 years Show forest plot | 2 | 289 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.79 [2.65, 12.65] |
| Analysis 1.11  Comparison 1 UAE versus surgery, Outcome 11 Further interventions within 5 years. | ||||
| 11.1 UAE versus hysterectomy | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.41, 8.30] |
| 11.2 UAE versus hysterectomy or myomectomy | 1 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 20.34 [2.68, 154.59] |
| 12 Unscheduled readmission rate within 4‐6 weeks Show forest plot | 2 | 278 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.22] |
| Analysis 1.12  Comparison 1 UAE versus surgery, Outcome 12 Unscheduled readmission rate within 4‐6 weeks. | ||||
| 12.1 UAE versus hysterectomy | 1 | 157 | Risk Difference (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.38] |
| 12.2 UAE versus myomectomy | 1 | 121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.07] |
| 13 Cost: duration of procedure (minutes) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 UAE versus surgery, Outcome 13 Cost: duration of procedure (minutes). | ||||
| 13.1 UAE versus hysterectomy | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐16.40 [‐26.04, ‐6.76] |
| 13.2 UAE versus myomectomy | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐49.7 [‐58.76, ‐40.64] |
| 14 Cost: length of hospital stay (days) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 UAE versus surgery, Outcome 14 Cost: length of hospital stay (days). | ||||
| 14.1 UAE versus hysterectomy | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 UAE versus hysterectomy or myomectomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 UAE versus myomectomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Cost: resumption of normal activities (days) Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 UAE versus surgery, Outcome 15 Cost: resumption of normal activities (days). | ||||
| 15.1 UAE versus hysterectomy | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐22.85 [‐27.30, ‐18.40] |
| 15.2 UAE versus hysterectomy or myomectomy | 2 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐13.68 [‐16.05, ‐11.30] |
| 15.3 UAE versus myomectomy | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐13.60, ‐6.80] |
| 16 FSH levels >40 IU/L (within 2 years) Show forest plot | 2 | 297 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.94] |
| Analysis 1.16  Comparison 1 UAE versus surgery, Outcome 16 FSH levels >40 IU/L (within 2 years). | ||||
| 16.1 UAE versus hysterectomy | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.32, 1.54] |
| 16.2 UAE versus hysterectomy or myomectomy | 1 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.64, 8.68] |
| 17 FSH levels >10 IU/L (within 6 months) Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.8 [0.97, 23.64] |
| Analysis 1.17 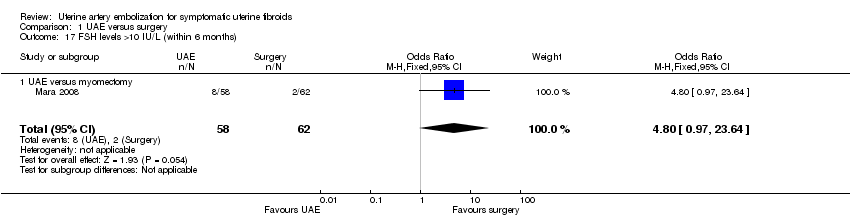 Comparison 1 UAE versus surgery, Outcome 17 FSH levels >10 IU/L (within 6 months). | ||||
| 17.1 UAE versus myomectomy | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.8 [0.97, 23.64] |
| 18 Fibroid recurrence within 2 years Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.38, 4.57] |
| Analysis 1.18  Comparison 1 UAE versus surgery, Outcome 18 Fibroid recurrence within 2 years. | ||||
| 18.1 UAE versus myomectomy | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.38, 4.57] |
| 19 Pregnancy Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.85] |
| Analysis 1.19  Comparison 1 UAE versus surgery, Outcome 19 Pregnancy. | ||||
| 19.1 UAE versus myomectomy | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.85] |
| 20 UAE versus myomectomy: Health related quality of life at one year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20 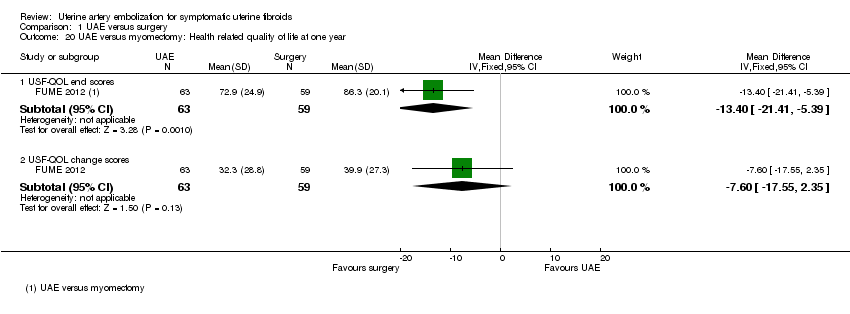 Comparison 1 UAE versus surgery, Outcome 20 UAE versus myomectomy: Health related quality of life at one year. | ||||
| 20.1 USF‐QOL end scores | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | ‐13.40 [‐21.41, ‐5.39] |
| 20.2 USF‐QOL change scores | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐17.55, 2.35] |
| 21 UAE versus hysterectomy or myomectomy: SF‐36 within 1 year Show forest plot | 2 | 1405 | Mean Difference (IV, Fixed, 95% CI) | 9.14 [8.04, 10.23] |
| Analysis 1.21  Comparison 1 UAE versus surgery, Outcome 21 UAE versus hysterectomy or myomectomy: SF‐36 within 1 year. | ||||
| 21.1 Physical function | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 7.77 [5.83, 9.70] |
| 21.2 Social function | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 6.14 [2.71, 9.58] |
| 21.3 Mental Health | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [9.50, 14.50] |
| 21.4 Emotional role | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 10.28 [7.96, 12.60] |
| 21.5 Vitality | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 8.75 [6.09, 11.41] |
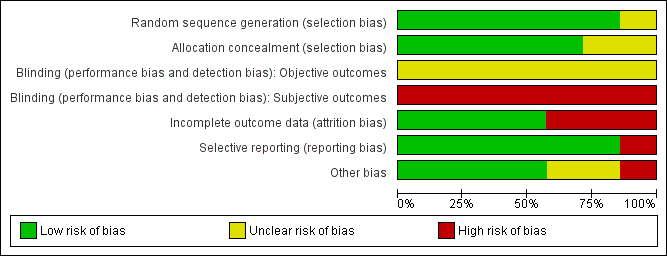
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 UAE versus surgery, outcome: 1.1 Satisfaction with treatment up to 24 months.

Forest plot of comparison: 1 UAE versus surgery, outcome: 1.2 Satisfaction with treatment at 5 years.

Forest plot of comparison: 1 UAE versus surgery, outcome: 1.3 Live birth.

Forest plot of comparison: 1 UAE versus surgery, outcome: 1.10 Further interventions within 2 years.

Comparison 1 UAE versus surgery, Outcome 1 Satisfaction with treatment up to 24 months.

Comparison 1 UAE versus surgery, Outcome 2 Satisfaction with treatment at 5 years.

Comparison 1 UAE versus surgery, Outcome 3 Live birth.

Comparison 1 UAE versus surgery, Outcome 4 Adverse events: intraprocedural complications.

Comparison 1 UAE versus surgery, Outcome 5 Adverse events: Need for blood transfusion.

Comparison 1 UAE versus surgery, Outcome 6 Adverse events: minor postprocedural complications.

Comparison 1 UAE versus surgery, Outcome 7 Adverse events: major postprocedural complications within one year.
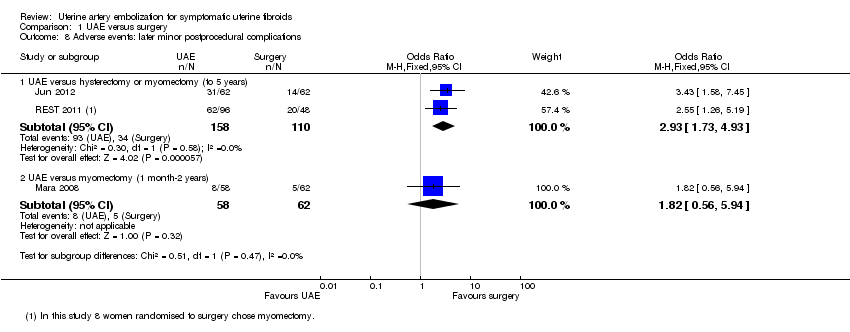
Comparison 1 UAE versus surgery, Outcome 8 Adverse events: later minor postprocedural complications.

Comparison 1 UAE versus surgery, Outcome 9 Adverse events: major postprocedural complications.
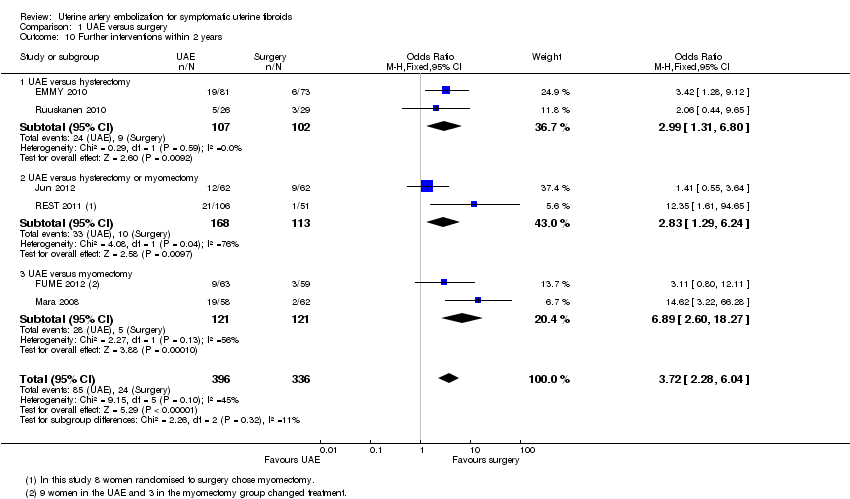
Comparison 1 UAE versus surgery, Outcome 10 Further interventions within 2 years.

Comparison 1 UAE versus surgery, Outcome 11 Further interventions within 5 years.

Comparison 1 UAE versus surgery, Outcome 12 Unscheduled readmission rate within 4‐6 weeks.
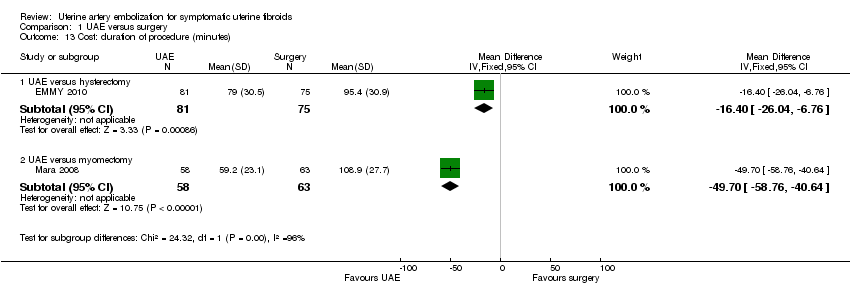
Comparison 1 UAE versus surgery, Outcome 13 Cost: duration of procedure (minutes).

Comparison 1 UAE versus surgery, Outcome 14 Cost: length of hospital stay (days).

Comparison 1 UAE versus surgery, Outcome 15 Cost: resumption of normal activities (days).
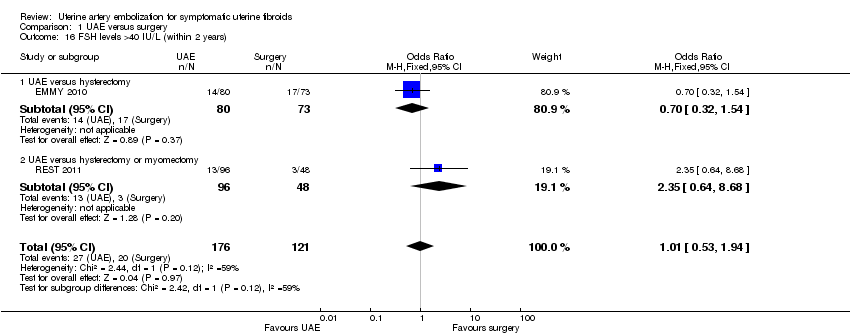
Comparison 1 UAE versus surgery, Outcome 16 FSH levels >40 IU/L (within 2 years).
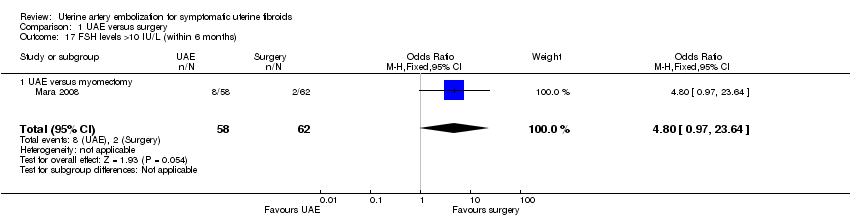
Comparison 1 UAE versus surgery, Outcome 17 FSH levels >10 IU/L (within 6 months).

Comparison 1 UAE versus surgery, Outcome 18 Fibroid recurrence within 2 years.

Comparison 1 UAE versus surgery, Outcome 19 Pregnancy.

Comparison 1 UAE versus surgery, Outcome 20 UAE versus myomectomy: Health related quality of life at one year.
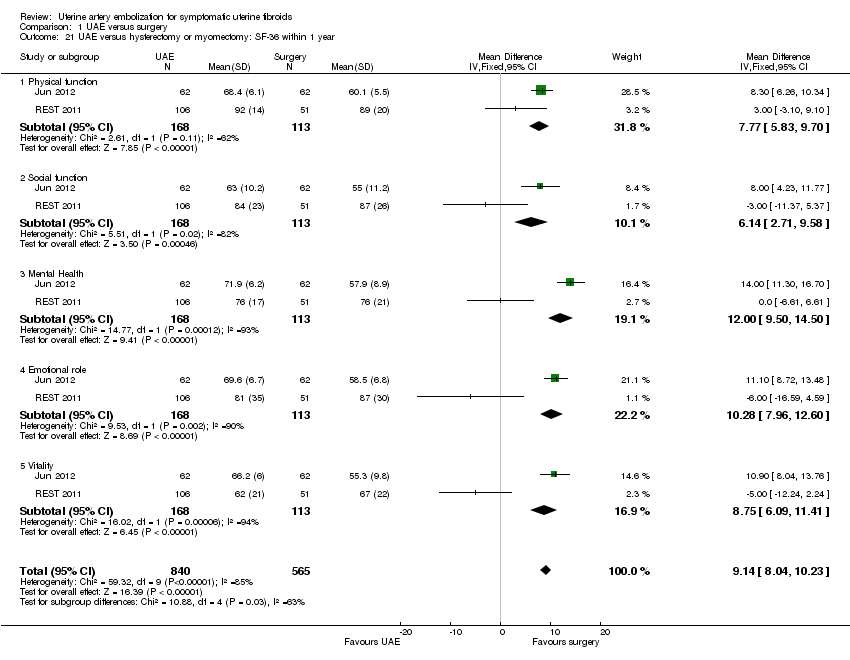
Comparison 1 UAE versus surgery, Outcome 21 UAE versus hysterectomy or myomectomy: SF‐36 within 1 year.
| UAE compared to surgery for symptomatic uterine fibroids | ||||||
| Population: women with symptomatic uterine fibroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgery | UAE | |||||
| Satisfaction with treatment up to 24 months | 861 per 1000 | 853 per 1000 | OR 0.94 | 640 | ⊕⊕⊕⊝ | |
| Satisfaction with treatment at 5 years | 876 per 1000 | 864 per 1000 | OR 0.90 | 295 | ⊕⊕⊕⊝ | |
| Live birth ‐ UAE versus myomectomy | 475 per 1000 | 190 per 1000 | OR 0.26 | 66 | ⊕⊝⊝⊝ | |
| Adverse events: intra‐procedural complications | 63 per 1000 | 57 per 1000 | OR 0.91 | 452 | ⊕⊕⊝⊝ | |
| Adverse events: minor post‐procedural complications within one year | 230 per 1000 | 373 per 1000 | OR 1.99 | 735 | ⊕⊕⊕⊝ | |
| Adverse events: major post‐procedural complications within one year | 69 per 1000 | 46 per 1000 | OR 0.65 | 611 | ⊕⊕⊕⊝ | |
| Further interventions within 2 years | 71 per 1000 | 222 per 1000 | OR 3.72 | 732 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies unblinded 2Two studies did not fully explain randomisation and allocation concealment, but quality not downgraded for this as their omission from analysis did not substantially change the findings 3 Wide confidence intervals compatible with substantial harm from UAE or with no effect 4Includes only those trial participants who wished to conceive (66/121) 5One study did not fully explain methods of randomisation and allocation concealment, but omission of this study did not substantially affect the findings 6Low event rate 7Wide confidence intervals compatible with substantial harm or benefit from either intervention, or with no effect 8Some statistical heterogeneity (I2 = 45%); quality not downgraded for this as direction of effect is consistent | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Satisfaction with treatment up to 24 months Show forest plot | 6 | 640 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.59, 1.48] |
| 1.1 UAE versus hysterectomy | 3 | 266 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.30, 1.32] |
| 1.2 UAE versus hysterectomy or myomectomy | 2 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.64, 2.58] |
| 1.3 UAE versus myomectomy | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.33, 3.36] |
| 2 Satisfaction with treatment at 5 years Show forest plot | 2 | 295 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.45, 1.80] |
| 2.1 UAE versus hysterectomy | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.29, 1.78] |
| 2.2 UAE versus hysterectomy or myomectomy | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.42, 3.67] |
| 3 Live birth Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 UAE versus myomectomy | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.84] |
| 4 Adverse events: intraprocedural complications Show forest plot | 4 | 452 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.42, 1.97] |
| 4.1 UAE versus hysterectomy | 2 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.44, 3.44] |
| 4.2 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.03] |
| 5 Adverse events: Need for blood transfusion Show forest plot | 2 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.52] |
| 5.1 UAE versus hysterectomy | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 5.2 UAE versus myomectomy | 1 | 121 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.47] |
| 6 Adverse events: minor postprocedural complications Show forest plot | 6 | 735 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.41, 2.81] |
| 6.1 UAE versus hysterectomy | 2 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.13, 3.96] |
| 6.2 UAE versus hysterectomy or myomectomy | 2 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.49, 4.23] |
| 6.3 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.62, 2.44] |
| 7 Adverse events: major postprocedural complications within one year Show forest plot | 5 | 611 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.26] |
| 7.1 UAE versus hysterectomy | 2 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.22, 4.58] |
| 7.2 UAE versus hysterectomy or myomectomy | 1 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.30, 1.74] |
| 7.3 UAE versus myomectomy | 2 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.50] |
| 8 Adverse events: later minor postprocedural complications Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 UAE versus hysterectomy or myomectomy (to 5 years) | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.73, 4.93] |
| 8.2 UAE versus myomectomy (1 month‐2 years) | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.56, 5.94] |
| 9 Adverse events: major postprocedural complications Show forest plot | 3 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 9.1 UAE versus hysterectomy or myomectomy (to 5 years) | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.18] |
| 9.2 UAE versus myomectomy (1 month‐2 years) | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Further interventions within 2 years Show forest plot | 6 | 732 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.72 [2.28, 6.04] |
| 10.1 UAE versus hysterectomy | 2 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.99 [1.31, 6.80] |
| 10.2 UAE versus hysterectomy or myomectomy | 2 | 281 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.83 [1.29, 6.24] |
| 10.3 UAE versus myomectomy | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.89 [2.60, 18.27] |
| 11 Further interventions within 5 years Show forest plot | 2 | 289 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.79 [2.65, 12.65] |
| 11.1 UAE versus hysterectomy | 1 | 145 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.41, 8.30] |
| 11.2 UAE versus hysterectomy or myomectomy | 1 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 20.34 [2.68, 154.59] |
| 12 Unscheduled readmission rate within 4‐6 weeks Show forest plot | 2 | 278 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.22] |
| 12.1 UAE versus hysterectomy | 1 | 157 | Risk Difference (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.38] |
| 12.2 UAE versus myomectomy | 1 | 121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.07] |
| 13 Cost: duration of procedure (minutes) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 UAE versus hysterectomy | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐16.40 [‐26.04, ‐6.76] |
| 13.2 UAE versus myomectomy | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐49.7 [‐58.76, ‐40.64] |
| 14 Cost: length of hospital stay (days) Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 UAE versus hysterectomy | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 UAE versus hysterectomy or myomectomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 UAE versus myomectomy | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Cost: resumption of normal activities (days) Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 UAE versus hysterectomy | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐22.85 [‐27.30, ‐18.40] |
| 15.2 UAE versus hysterectomy or myomectomy | 2 | 220 | Mean Difference (IV, Fixed, 95% CI) | ‐13.68 [‐16.05, ‐11.30] |
| 15.3 UAE versus myomectomy | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐10.20 [‐13.60, ‐6.80] |
| 16 FSH levels >40 IU/L (within 2 years) Show forest plot | 2 | 297 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.53, 1.94] |
| 16.1 UAE versus hysterectomy | 1 | 153 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.32, 1.54] |
| 16.2 UAE versus hysterectomy or myomectomy | 1 | 144 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.64, 8.68] |
| 17 FSH levels >10 IU/L (within 6 months) Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.8 [0.97, 23.64] |
| 17.1 UAE versus myomectomy | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.8 [0.97, 23.64] |
| 18 Fibroid recurrence within 2 years Show forest plot | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.38, 4.57] |
| 18.1 UAE versus myomectomy | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.38, 4.57] |
| 19 Pregnancy Show forest plot | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.85] |
| 19.1 UAE versus myomectomy | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.85] |
| 20 UAE versus myomectomy: Health related quality of life at one year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 USF‐QOL end scores | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | ‐13.40 [‐21.41, ‐5.39] |
| 20.2 USF‐QOL change scores | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | ‐7.60 [‐17.55, 2.35] |
| 21 UAE versus hysterectomy or myomectomy: SF‐36 within 1 year Show forest plot | 2 | 1405 | Mean Difference (IV, Fixed, 95% CI) | 9.14 [8.04, 10.23] |
| 21.1 Physical function | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 7.77 [5.83, 9.70] |
| 21.2 Social function | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 6.14 [2.71, 9.58] |
| 21.3 Mental Health | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 12.00 [9.50, 14.50] |
| 21.4 Emotional role | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 10.28 [7.96, 12.60] |
| 21.5 Vitality | 2 | 281 | Mean Difference (IV, Fixed, 95% CI) | 8.75 [6.09, 11.41] |

