| 1 All‐cause mortality – main analysis Show forest plot | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.84, 4.77] |
|
| 2 All‐cause mortality – sensitivity analysis intention to treat (ITT) worse case: missing participants counted as events Show forest plot | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.96, 4.67] |
|
| 3 All‐cause mortality – subgroup analysis: older and recent studies Show forest plot | 6 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.84, 4.77] |
|
| 3.1 Older studies, higher dose | 4 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [0.85, 8.83] |
| 3.2 More recent studies, lower dose | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
| 4 All‐cause mortality – sensitivity analysis: persistent atrial fibrillation Show forest plot | 5 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.73, 4.53] |
|
| 5 All‐cause mortality – sensitivity analysis: low risk of bias studies Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
|
| 6 All‐cause mortality – sensitivity analysis: studies > 200 participants Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.34, 4.92] |
|
| 7 Withdrawals due to adverse effects – main analysis Show forest plot | 7 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.87, 2.78] |
|
| 8 Withdrawals due to adverse effects – subgroup analysis: older and recent studies Show forest plot | 7 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.87, 2.78] |
|
| 8.1 Older studies, higher dose | 5 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.29, 7.22] |
| 8.2 More recent studies, lower dose | 2 | 1234 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.27] |
| 9 Withdrawals due to adverse effects – sensitivity analysis: persistent atrial fibrillation Show forest plot | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.99, 4.87] |
|
| 10 Withdrawals due to adverse effects – sensitivity analysis: low risk of bias studies Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
|
| 11 Withdrawals due to adverse effects – sensitivity analysis: studies > 200 participants Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.09] |
|
| 12 Proarrhythmia – main analysis Show forest plot | 7 | 1676 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.95, 4.41] |
|
| 13 Proarrhythmia – subgroup analysis: older and recent studies Show forest plot | 7 | 1677 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.96, 4.42] |
|
| 13.1 Older studies, higher dose | 5 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.87, 11.32] |
| 13.2 More recent studies, lower dose | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
| 14 Proarrhythmia – sensitivity analysis: persistent atrial fibrillation Show forest plot | 5 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.93, 7.53] |
|
| 15 Proarrhythmia – sensitivity analysis: low risk of bias studies Show forest plot | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
|
| 16 Proarrhythmia – sensitivity analysis: studies > 200 participants Show forest plot | 2 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.24] |
|
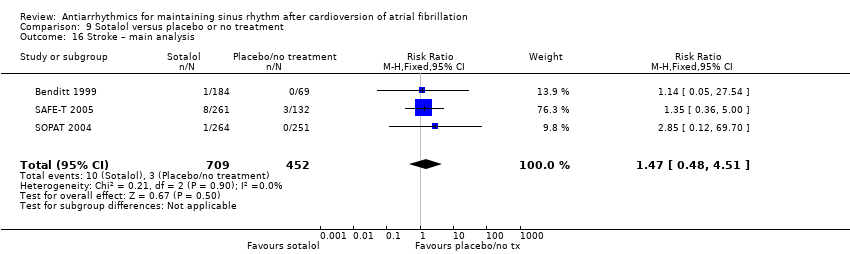
| 17 Stroke – main analysis Show forest plot | 4 | 1107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.25, 3.83] |
|
| 18 Stroke – sensitivity analysis: persistent atrial fibrillation Show forest plot | 3 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.19, 4.01] |
|
| 19 Stroke – sensitivity analysis: low risk of bias studies Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 20 Stroke – sensitivity analysis: studies > 200 participants Show forest plot | 1 | | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
| 21 Atrial fibrillation recurrence – main analysis Show forest plot | 7 | 1624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.78, 0.88] |
|
| 22 Atrial fibrillation recurrence – sensitivity analysis: persistent atrial fibrillation Show forest plot | 5 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.70, 0.85] |
|
| 23 Atrial fibrillation recurrence – sensitivity analysis: low risk of bias studies Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.80, 0.91] |
|
| 24 Atrial fibrillation recurrence – sensitivity analysis: studies > 200 participants Show forest plot | 2 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.92] |
|