Topical treatments for chronic plaque psoriasis
Información
- DOI:
- https://doi.org/10.1002/14651858.CD005028.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 marzo 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Piel
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
The following contributions were made by the authors stated.
Link with editorial base and co‐ordinate contributions from co‐authors (AM).
Draft protocol (AM with contributions from MC, GD, GE, and JM).
Run searches (adherence) (AM).
Identify relevant titles and abstracts from searches, i.e. broad screen (AM and JM).
Obtain copies of trials (AM).
Select which trials to include (AM, JM, and MC as arbitrator when necessary).
Extract data from trials (AM, JM, and HH).
Enter data into RevMan (AM).
Carry out analysis (AM and JM)
Interpret analysis (AM, JM, and HH).
Draft final review (AM with contribution from MC, GD, HH, and JM).
Update review (AM, JM, HH, and MC).
Sources of support
Internal sources
-
Funding from Centre for Reviews and Dissemination to update review (2002) for UK products only, UK.
-
Award from University of York Fund for Staff on Fixed‐Term Contracts, UK.
External sources
-
Grant from Crookes Healthcare Ltd. to do original systematic review (1999), UK.
-
Grant from the Psoriasis Association to update review (2011), Other.
Declarations of interest
Mike Cork: "I gave a lecture for Leo Pharmaceuticals about psoriasis and atopic eczema in 2012."
Anne R Mason: none declared
Gordon Dooley: none declared
James Mason: none declared
Helen Hancock: none declared
The clinical referee for this review, Dr Phyllis Spuls, stated the following potential conflict of interest on her comments form: "I have participated in an advisory board of LEO Pharma to give guidance to general practitioners regarding what to do if patients used calcipotriol, which has been removed from the market. I am involved as a Principal Investigator in many clinical trials with systemic agents for psoriasis and now in one for the improvement of adherence to Dovobet."
Acknowledgements
The Cochrane Skin Group editorial base wishes to thank Luigi Naldi who was the Key Editor for this review; Jo Leonardi‐Bee and Philippa Middleton who were the Statistical and Methods Editors, respectively; the clinical referees, Phyllis Spuls and Steven Chow; and the consumer referee, Carolyn Hughes.
The review team are indebted to Jane Harrison (formerly of the Centre for Reviews and Dissemination, University of York) for devising the original search strategies in 1999, Julie Glanville (formerly of the Centre for Reviews and Dissemination, University of York) for running the effectiveness and adverse events search strategies in 2002 and 2005, Kate Light and Kath Wright (Centre for Reviews and Dissemination, University of York) for updating the searches in 2008 and 2011, and Finola Delamere and Liz Doney of the Cochrane Skin Group for searching the Cochrane Skin Group's Specialist Skin Trials Register. We also acknowledge the valuable contribution made by Gladys Edwards, who co‐authored a previous version of this review.
Last but not least, we would like to thank the authors and sponsors who provided unpublished data, which has greatly enriched this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Mar 28 | Topical treatments for chronic plaque psoriasis | Review | Anne R Mason, James Mason, Michael Cork, Gordon Dooley, Helen Hancock | |
| 2009 Apr 15 | Topical treatments for chronic plaque psoriasis | Review | Anne R Mason, James Mason, Michael Cork, Gordon Dooley, Gladys Edwards | |
| 2004 Jan 26 | Topical treatments for chronic plaque psoriasis | Protocol | Anne R Mason, James Mason, Michael Cork, Gordon Dooley, Gladys Edwards | |
Differences between protocol and review
There are some differences between the protocol and the review.
1. In our protocol, we stated our intention to adjust for the precision of findings from within‐patient studies for within‐patient correlation. However, we were unsuccessful in our attempts to identify estimates of this correlation from published or unpublished sources. We therefore undertook sensitivity analysis to investigate differences between within‐patient and between‐patient studies.
2. In our protocol, we listed three primary outcome measures for data extraction. In the review, we also included the Patient Assessment of Global Improvement.
3. In our protocol, we stated that there would be no language restrictions when searching for publications. However, the search for longer‐term studies of adverse events included a restriction to publications in English.
4. In our protocol, we stated our intention that studies meeting only some of the inclusion criteria stated above would be listed as excluded studies. However, as large numbers of studies would need to be listed, this was not feasible. Therefore, we listed as excluded studies only those studies that we deemed potentially eligible for inclusion and for which we retrieved full papers, but which we subsequently found to fail to meet the inclusion criteria.
5. Throughout the text, we replaced all references to vitamin D3 with 'vitamin D analogues'.
6. Under Types of studies, we relaxed the condition that studies were of at least two weeks duration. In the same section, we added the following sentence: "If no useful effectiveness, withdrawal or adverse events data were available, either from the published paper or from sponsors or trialists, we excluded the study."
7. Under Types of interventions, we added the sentence "The potency of topical corticosteroids was based on classifications from a previous review (Mason 2002b)".
8. Under Methods, Selection of studies, and our explanations of the studies excluded, we added the searches for studies exploring adverse events and compliance studies.
9. Under Methods/Data extraction and management, we added the phrase 'between‐patient design'.
10. Under Methods/Assessment of risk of bias in included studies, we removed the phrase 'in each arm'.
11. Under Methods/Unit of analysis issues/'Summarising primary outcomes with standardised mean differences', we revised the text in this section to include the PAGI outcome and to provide a fuller explanation of our analytic approach.
12. Under Methods/Unit of analysis issues/Secondary outcomes, we gave an explanation regarding the different method of analysis used.
13 Under Methods/Data collection and analysis/Sensitivity analysis, we added text to describe the different types of sensitivity analysis undertaken.
14 We replaced 'standardised weighted mean difference' with 'standardised mean difference' throughout the text to reflect Cochrane terminology.
15. Under Methods/Data and analyses/Subgroup analysis and investigation of heterogeneity, we inserted two paragraphs to explain our approach to statistical heterogeneity.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Topical;
- Adrenal Cortex Hormones [adverse effects, *therapeutic use];
- Bone Density Conservation Agents [adverse effects, *therapeutic use];
- Chronic Disease;
- Facial Dermatoses [drug therapy];
- Psoriasis [*drug therapy];
- Randomized Controlled Trials as Topic;
- Scalp Dermatoses [drug therapy];
- Vitamin D [adverse effects, analogs & derivatives, *therapeutic use];
Medical Subject Headings Check Words
Humans;
PICO
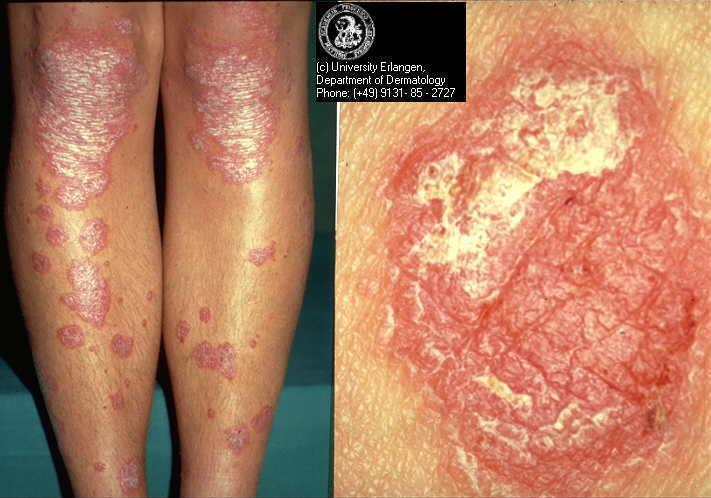
Chronic plaque psoriasis
Source: Dermis Dermatology Atlas Online (used with permission)
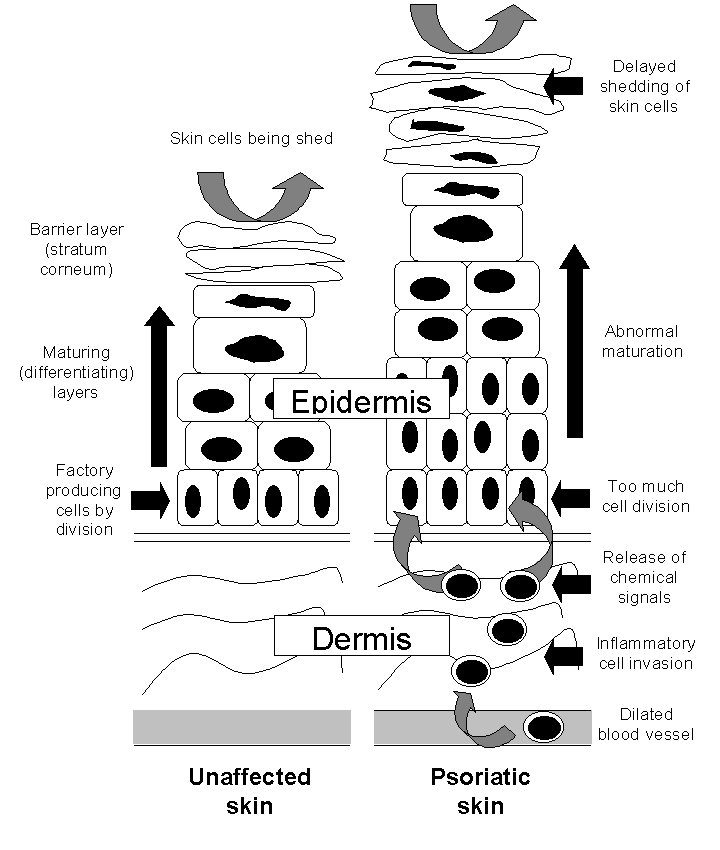
The epidermis in the skin of people with and without psoriasis

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
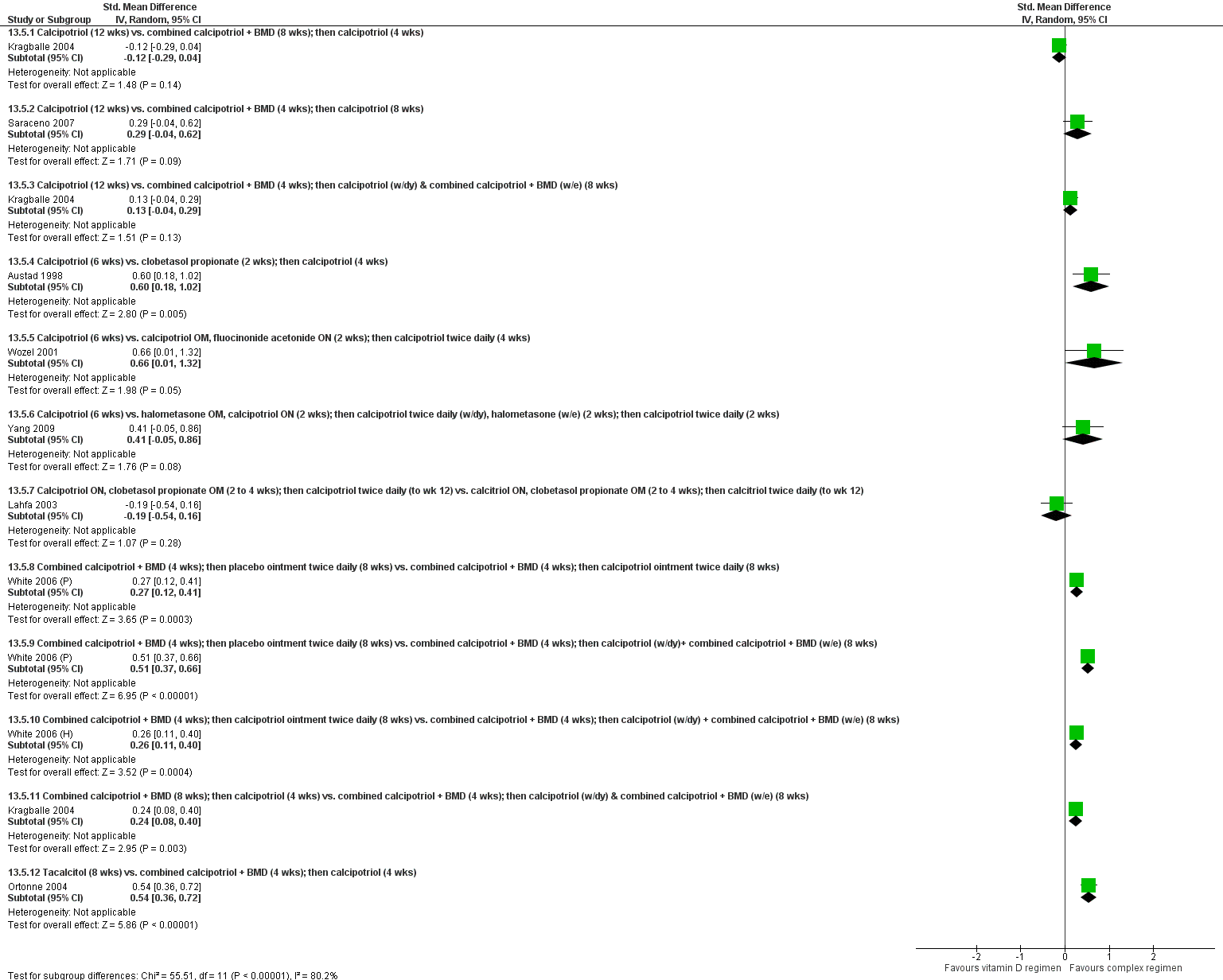
Forest plot of comparison: 13 Vitamin D alone or in combination vs. other treatments: complex regimens, outcome: 13.5 Combined end point (IAGI/TSS/PASI/PAGI).

Forest plot of comparison: 14 Vitamin D alone or in combination vs. other treatment: long term studies (>24wks), outcome: 14.5 Combined end point (IAGI/TSS/PASI/PAGI).

Forest plot of comparison: 18 Scalp psoriasis: placebo‐controlled trials, outcome: 18.5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 1 Vitamin D analogues versus placebo, Outcome 1 IAGI.

Comparison 1 Vitamin D analogues versus placebo, Outcome 2 TSS.
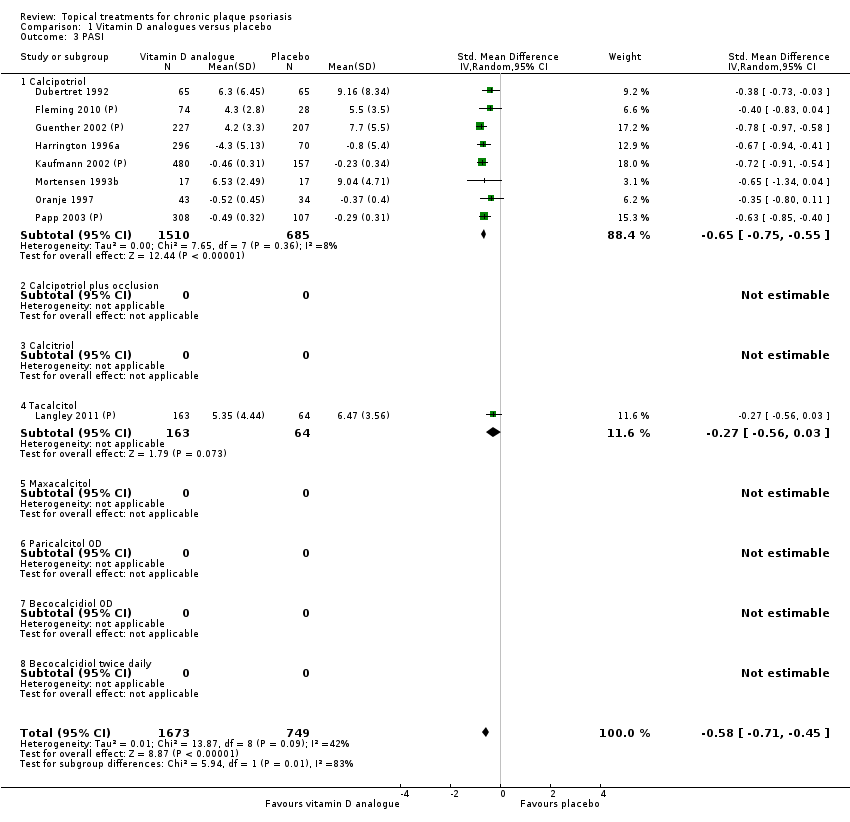
Comparison 1 Vitamin D analogues versus placebo, Outcome 3 PASI.

Comparison 1 Vitamin D analogues versus placebo, Outcome 4 PAGI.

Comparison 1 Vitamin D analogues versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
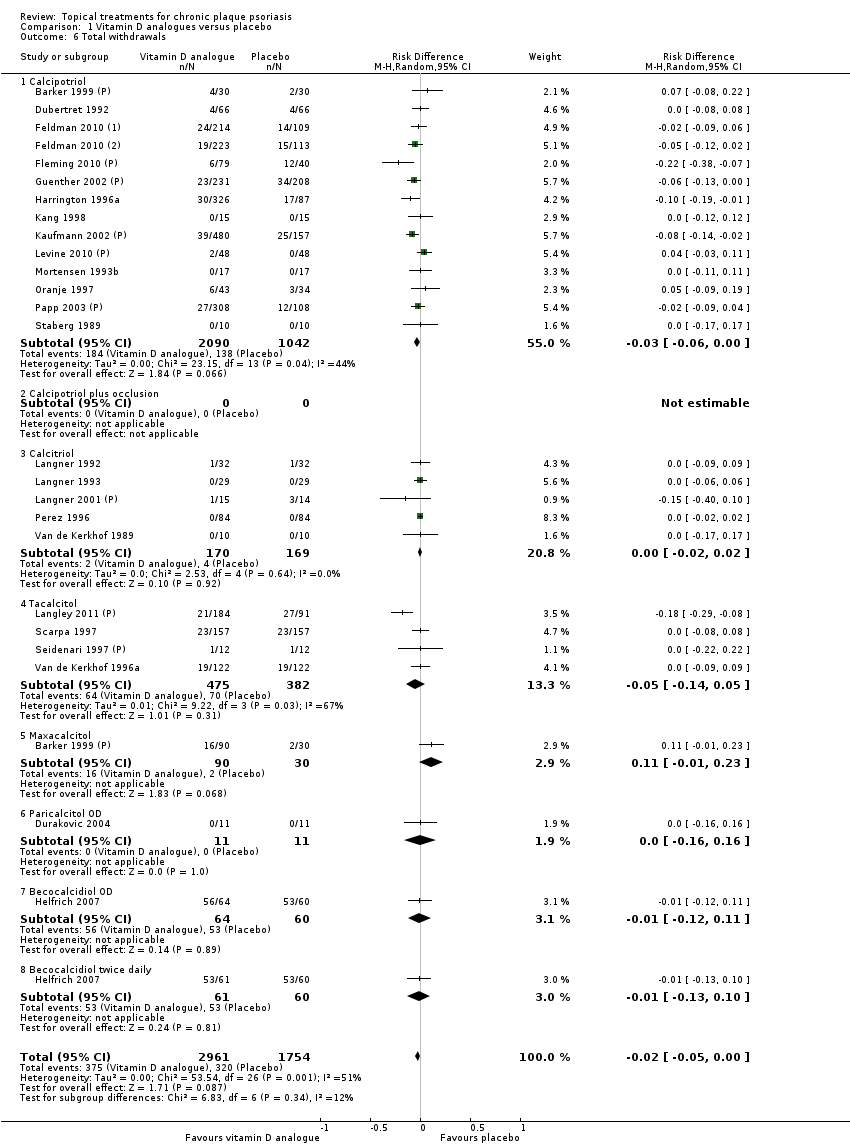
Comparison 1 Vitamin D analogues versus placebo, Outcome 6 Total withdrawals.
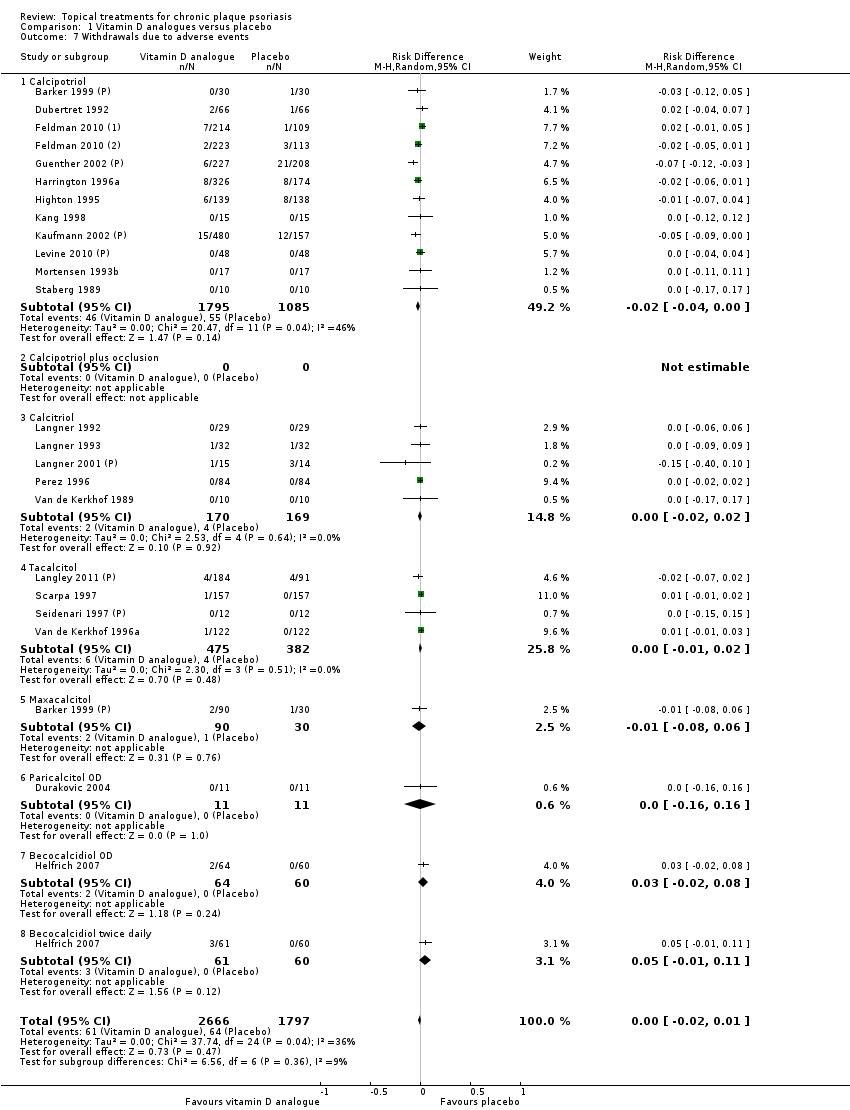
Comparison 1 Vitamin D analogues versus placebo, Outcome 7 Withdrawals due to adverse events.
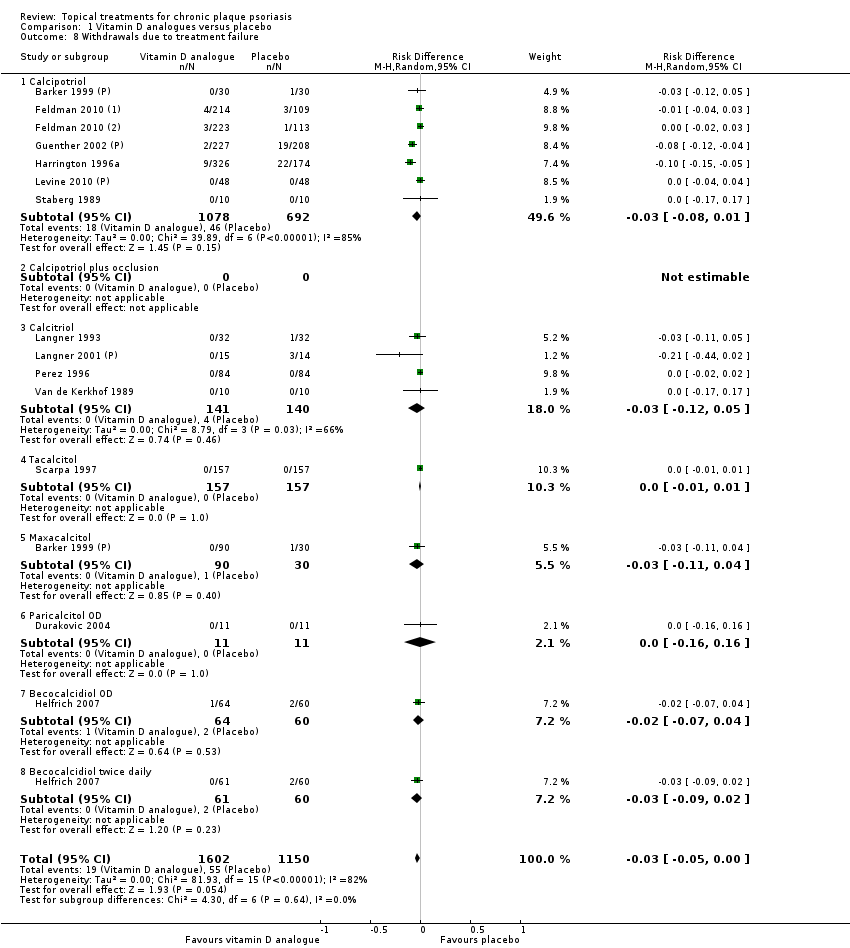
Comparison 1 Vitamin D analogues versus placebo, Outcome 8 Withdrawals due to treatment failure.

Comparison 1 Vitamin D analogues versus placebo, Outcome 9 Adverse events (local).
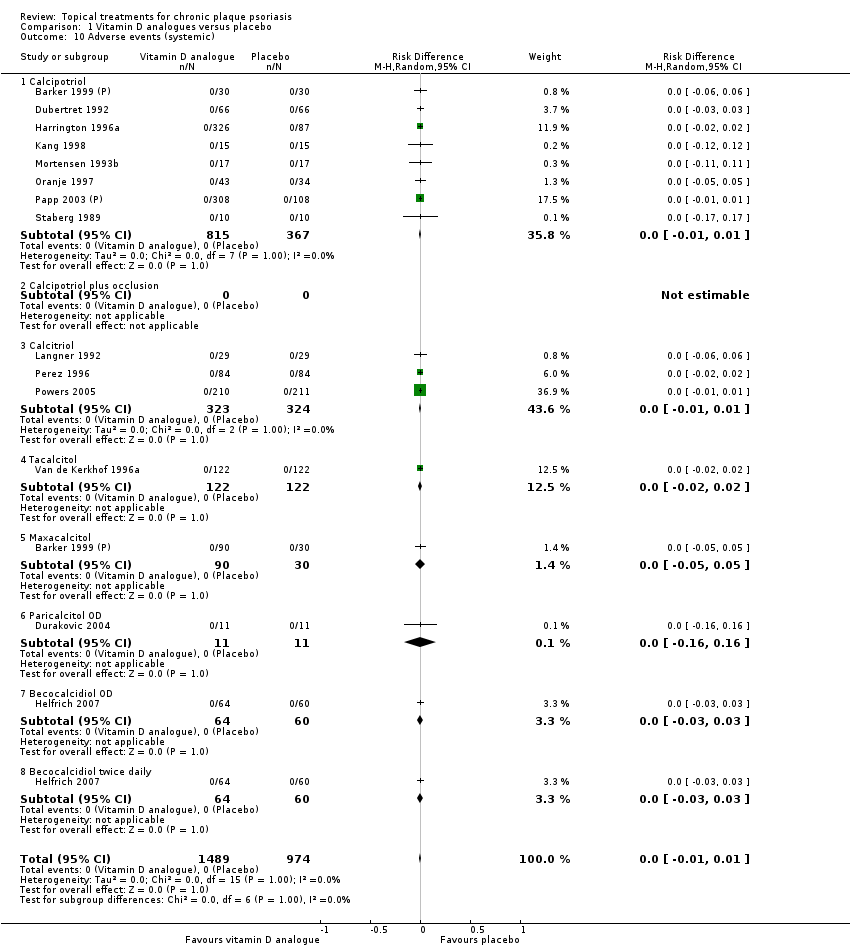
Comparison 1 Vitamin D analogues versus placebo, Outcome 10 Adverse events (systemic).

Comparison 2 Corticosteroid (potent) versus placebo, Outcome 1 IAGI.
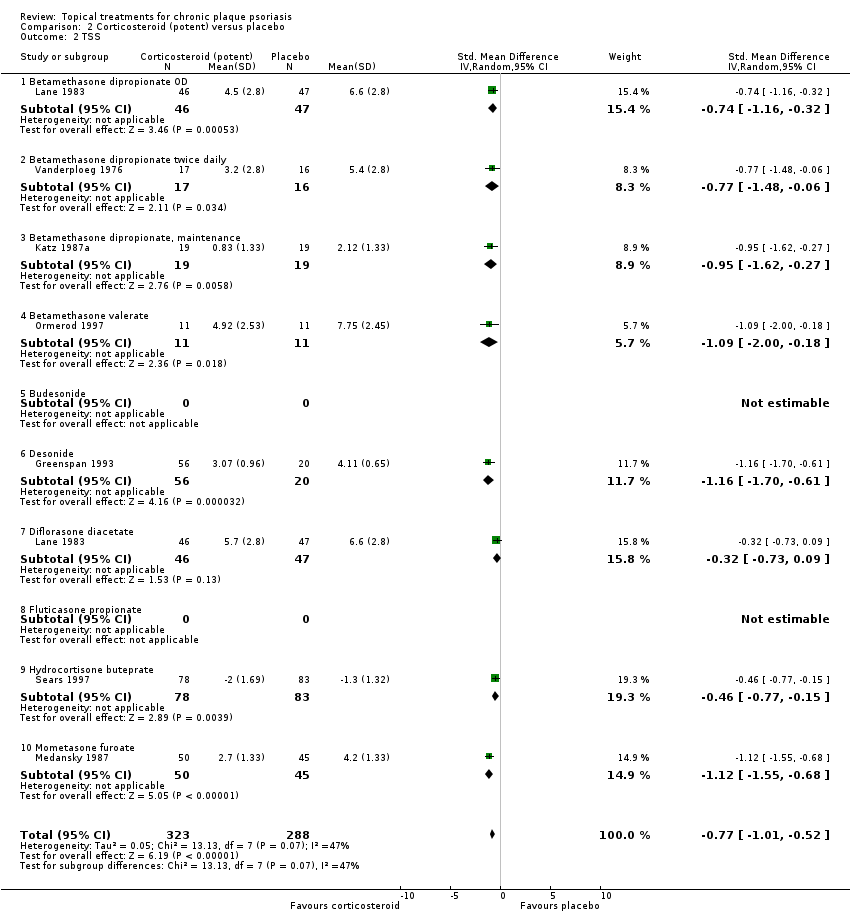
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 2 TSS.
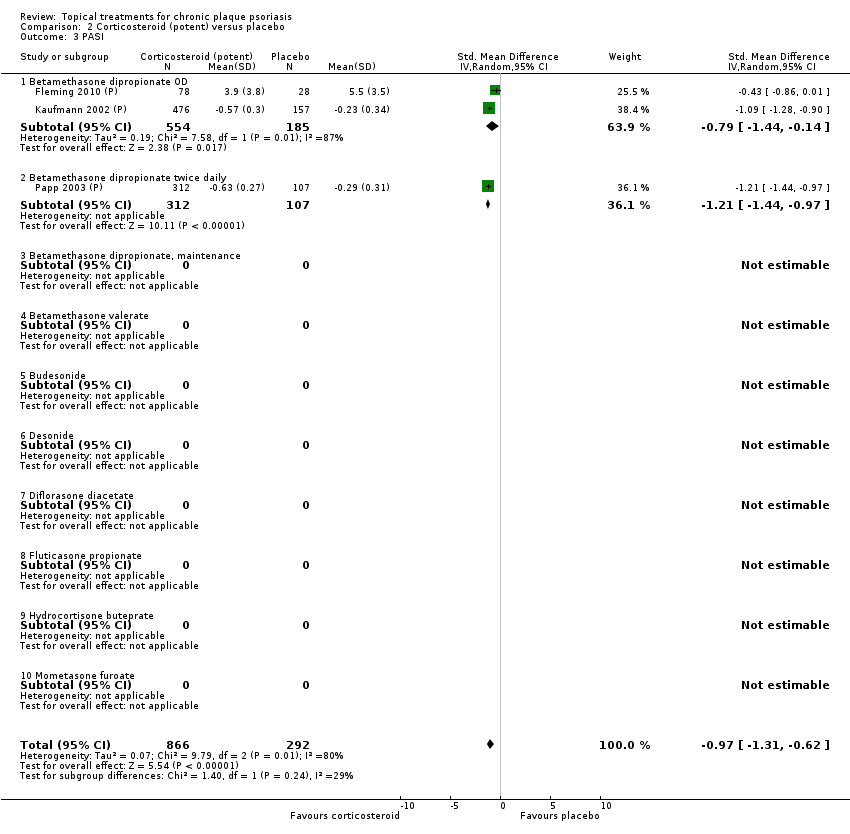
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 3 PASI.

Comparison 2 Corticosteroid (potent) versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
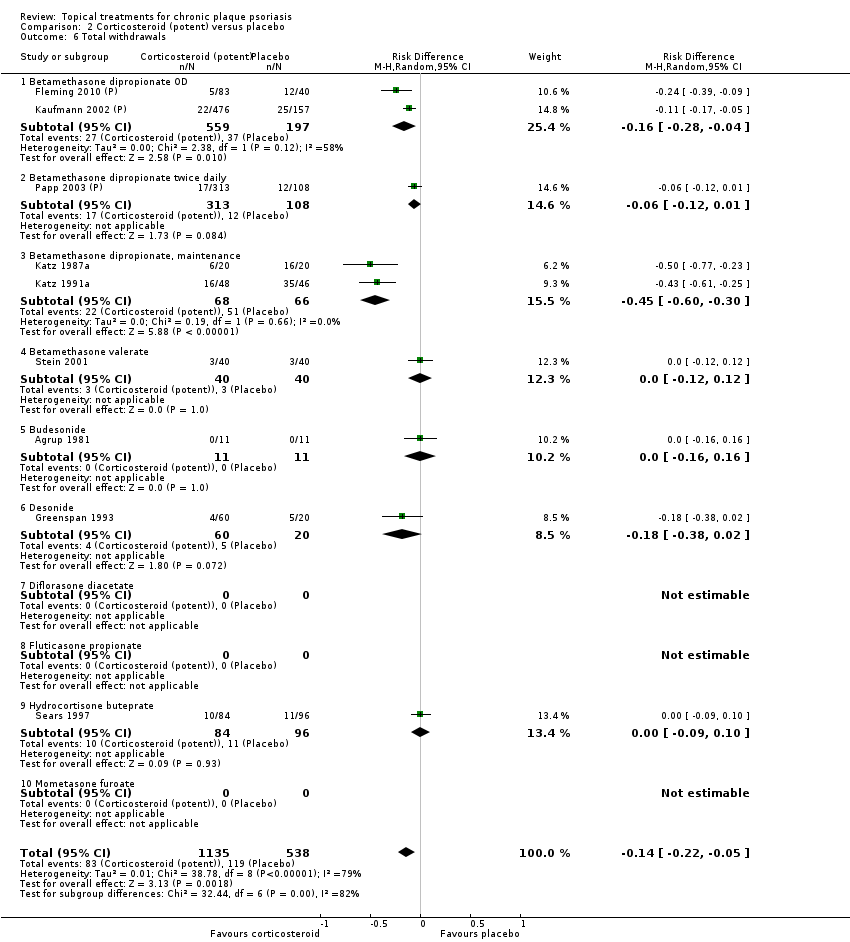
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 6 Total withdrawals.

Comparison 2 Corticosteroid (potent) versus placebo, Outcome 7 Withdrawals due to adverse events.

Comparison 2 Corticosteroid (potent) versus placebo, Outcome 8 Withdrawals due to treatment failure.

Comparison 2 Corticosteroid (potent) versus placebo, Outcome 9 Adverse events (local).
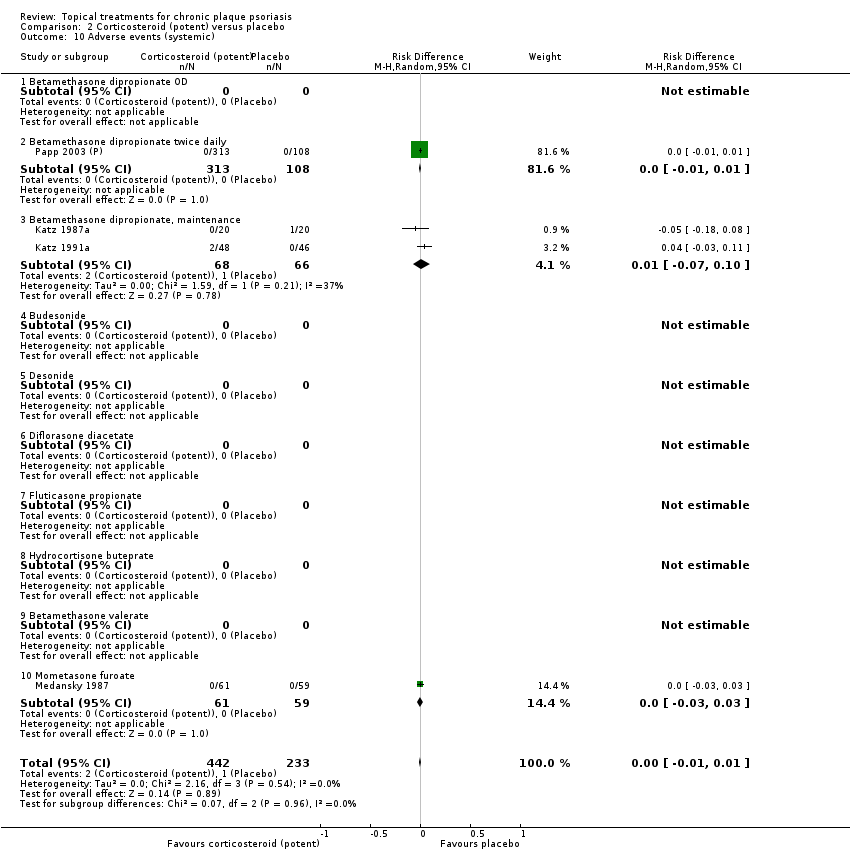
Comparison 2 Corticosteroid (potent) versus placebo, Outcome 10 Adverse events (systemic).
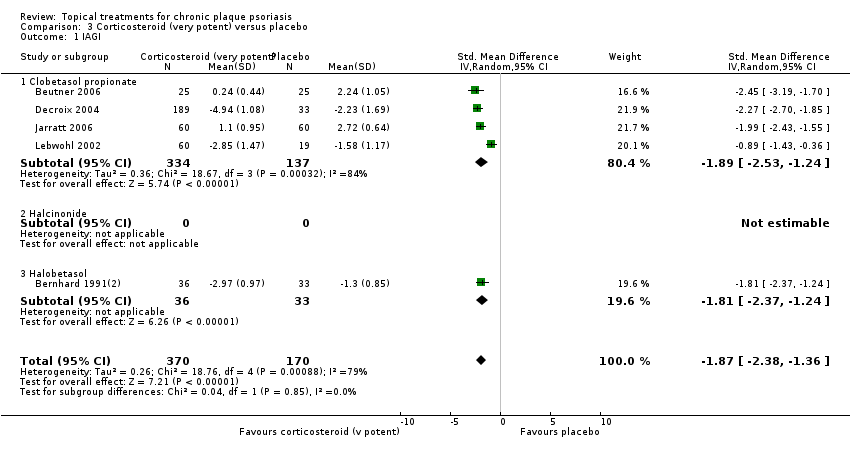
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 1 IAGI.

Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 2 TSS.

Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 4 PAGI.
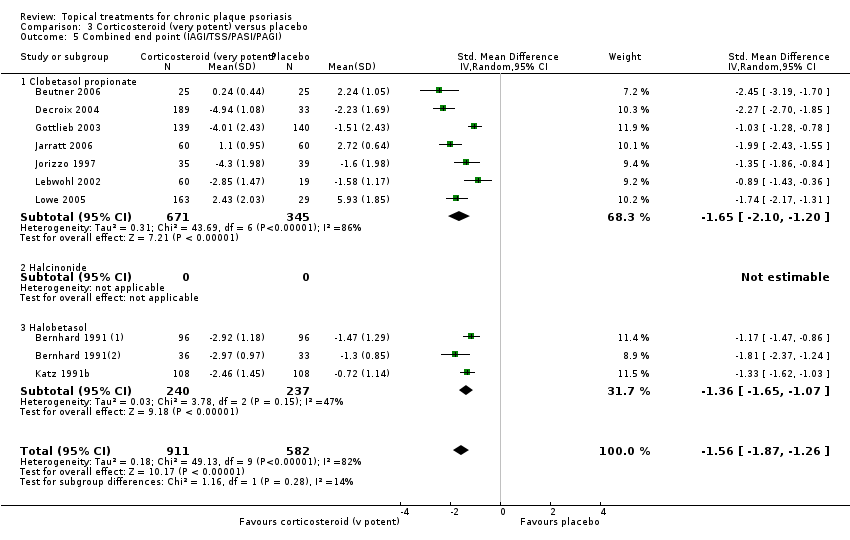
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 6 Total withdrawals.
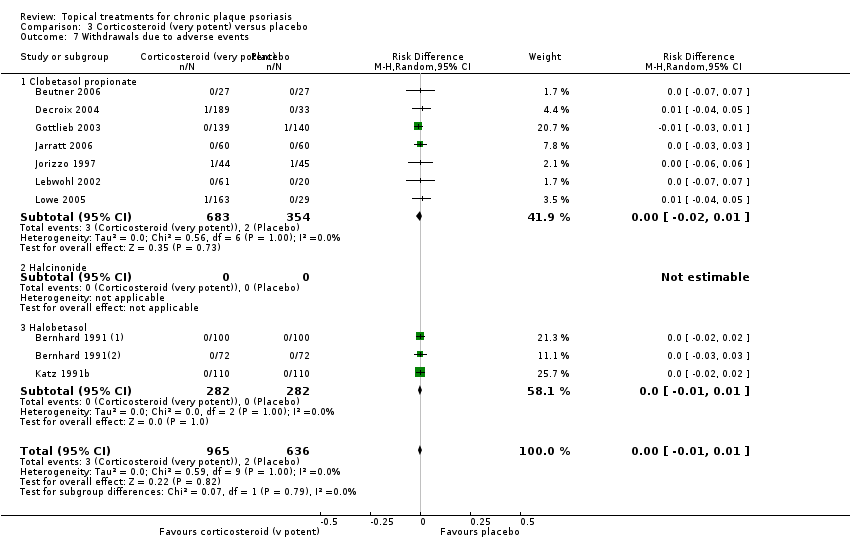
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 7 Withdrawals due to adverse events.
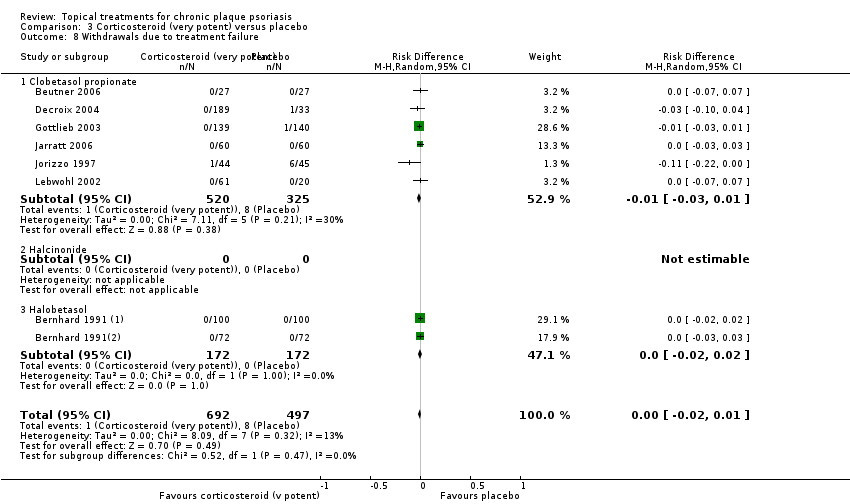
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 8 Withdrawals due to treatment failure.

Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 9 Adverse events (local).
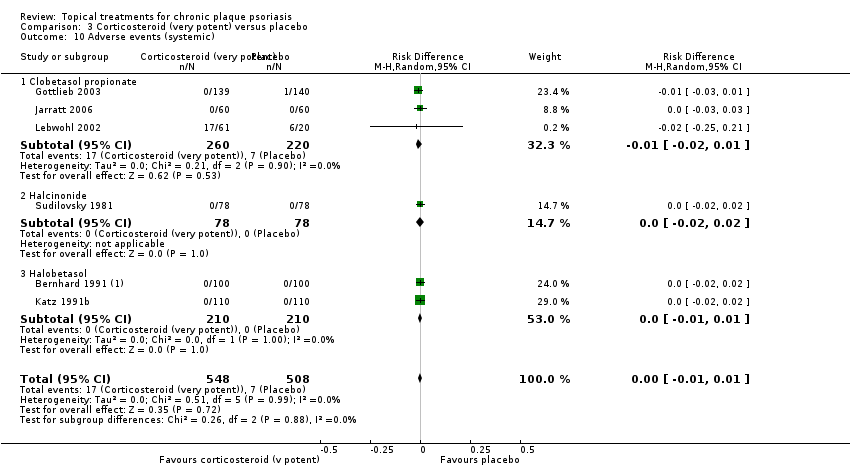
Comparison 3 Corticosteroid (very potent) versus placebo, Outcome 10 Adverse events (systemic).

Comparison 4 Dithranol versus placebo, Outcome 2 TSS.

Comparison 4 Dithranol versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 4 Dithranol versus placebo, Outcome 6 Total withdrawals.
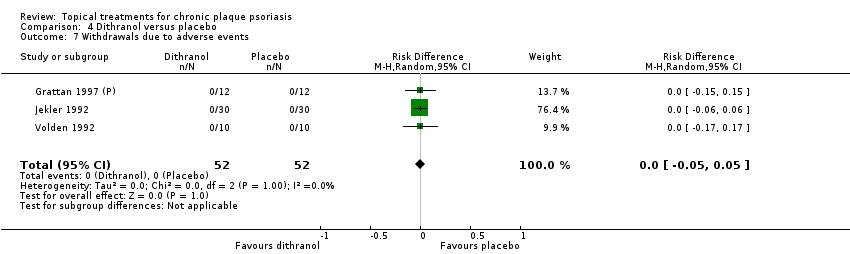
Comparison 4 Dithranol versus placebo, Outcome 7 Withdrawals due to adverse events.

Comparison 4 Dithranol versus placebo, Outcome 8 Withdrawals due to treatment failure.

Comparison 4 Dithranol versus placebo, Outcome 9 Adverse events (local).

Comparison 4 Dithranol versus placebo, Outcome 10 Adverse events (systemic).
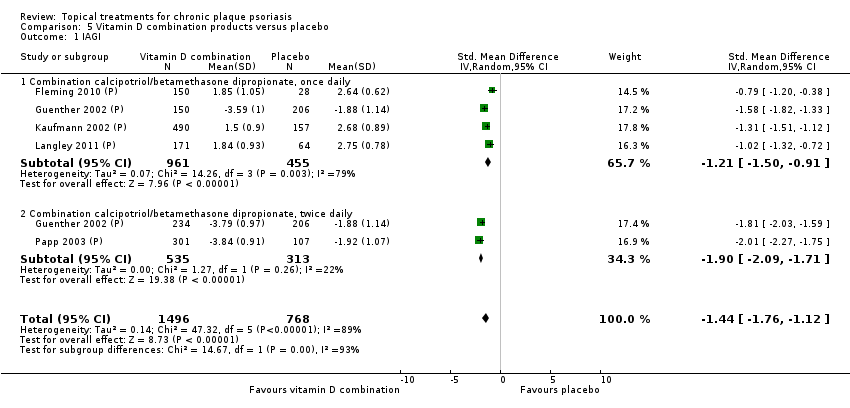
Comparison 5 Vitamin D combination products versus placebo, Outcome 1 IAGI.

Comparison 5 Vitamin D combination products versus placebo, Outcome 3 PASI.
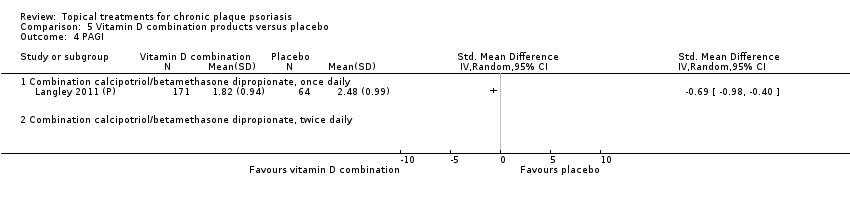
Comparison 5 Vitamin D combination products versus placebo, Outcome 4 PAGI.

Comparison 5 Vitamin D combination products versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 5 Vitamin D combination products versus placebo, Outcome 6 Total withdrawals.

Comparison 5 Vitamin D combination products versus placebo, Outcome 7 Withdrawals due to adverse events.

Comparison 5 Vitamin D combination products versus placebo, Outcome 8 Withdrawals due to treatment failure.
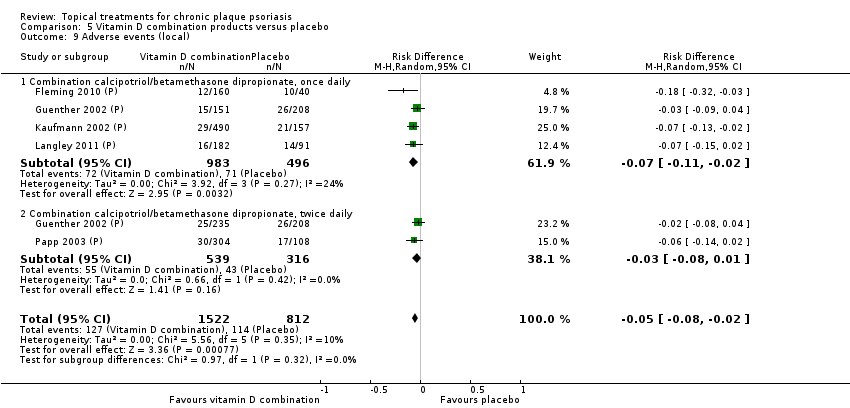
Comparison 5 Vitamin D combination products versus placebo, Outcome 9 Adverse events (local).

Comparison 5 Vitamin D combination products versus placebo, Outcome 10 Adverse events (systemic).

Comparison 6 Other treatment versus placebo, Outcome 1 IAGI.
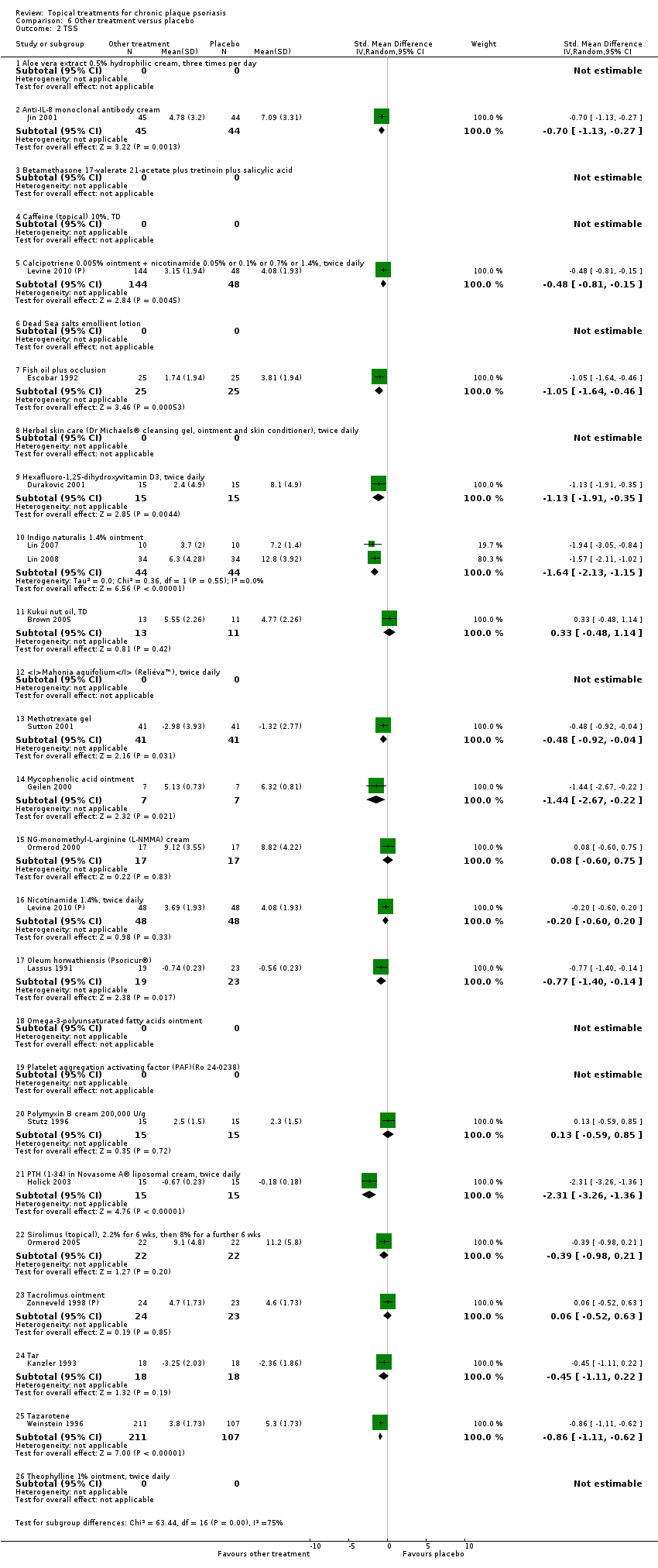
Comparison 6 Other treatment versus placebo, Outcome 2 TSS.

Comparison 6 Other treatment versus placebo, Outcome 3 PASI.

Comparison 6 Other treatment versus placebo, Outcome 4 PAGI.

Comparison 6 Other treatment versus placebo, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 6 Other treatment versus placebo, Outcome 6 Total withdrawals.
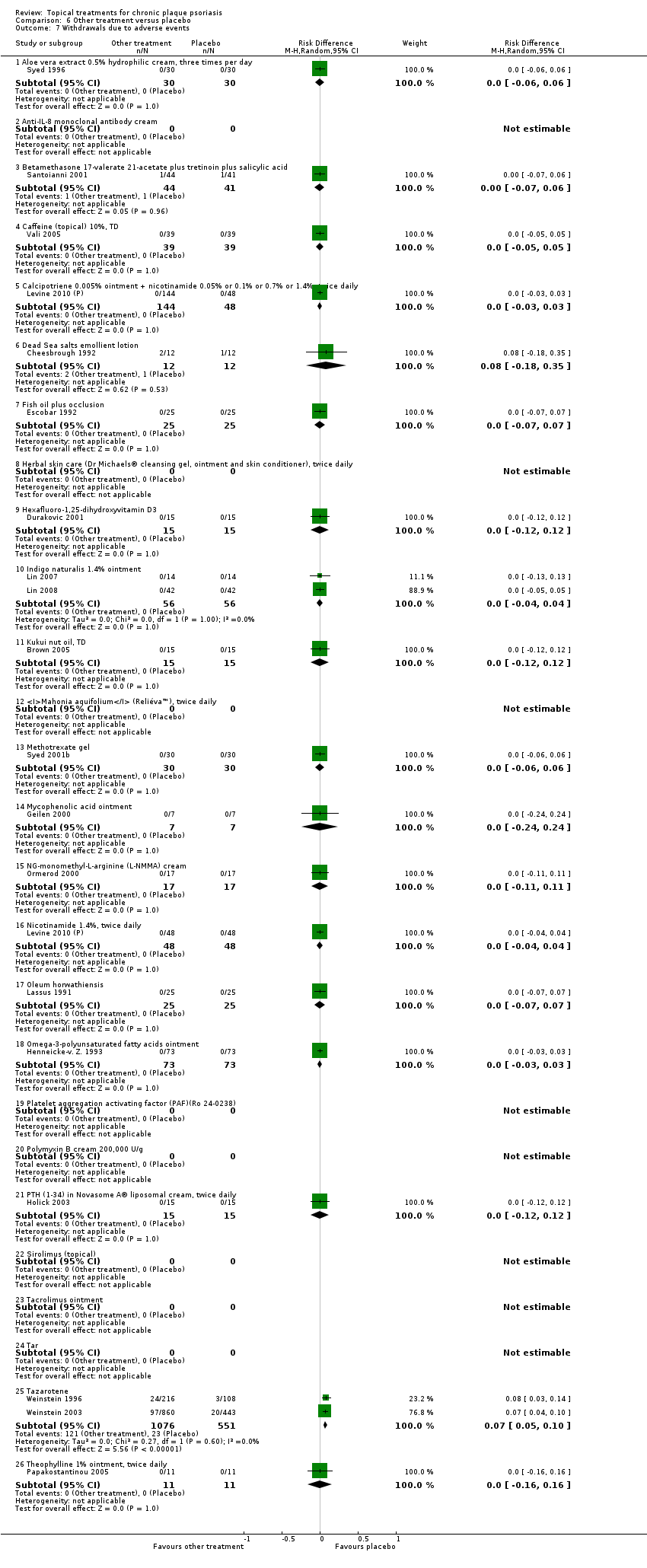
Comparison 6 Other treatment versus placebo, Outcome 7 Withdrawals due to adverse events.

Comparison 6 Other treatment versus placebo, Outcome 8 Withdrawals due to treatment failure.

Comparison 6 Other treatment versus placebo, Outcome 9 Adverse events (local).
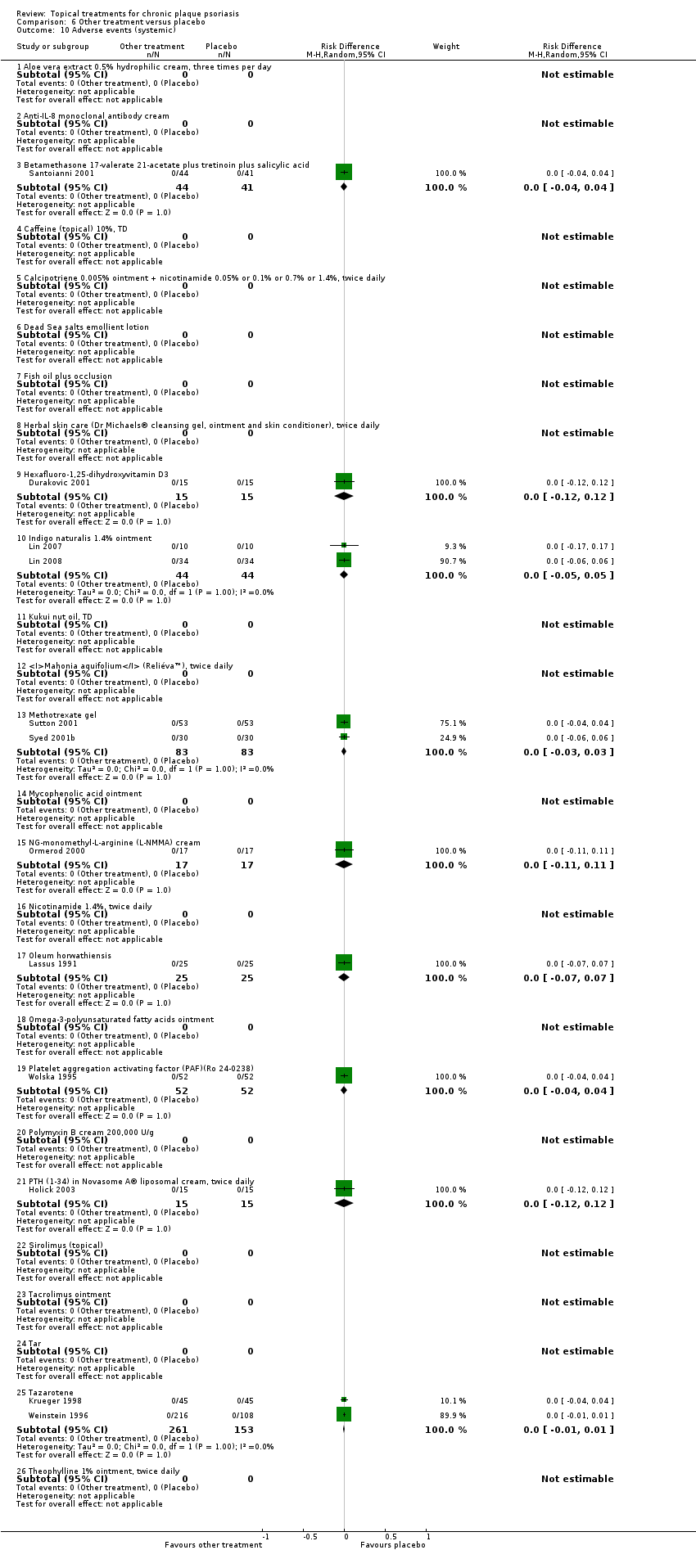
Comparison 6 Other treatment versus placebo, Outcome 10 Adverse events (systemic).

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 1 IAGI.
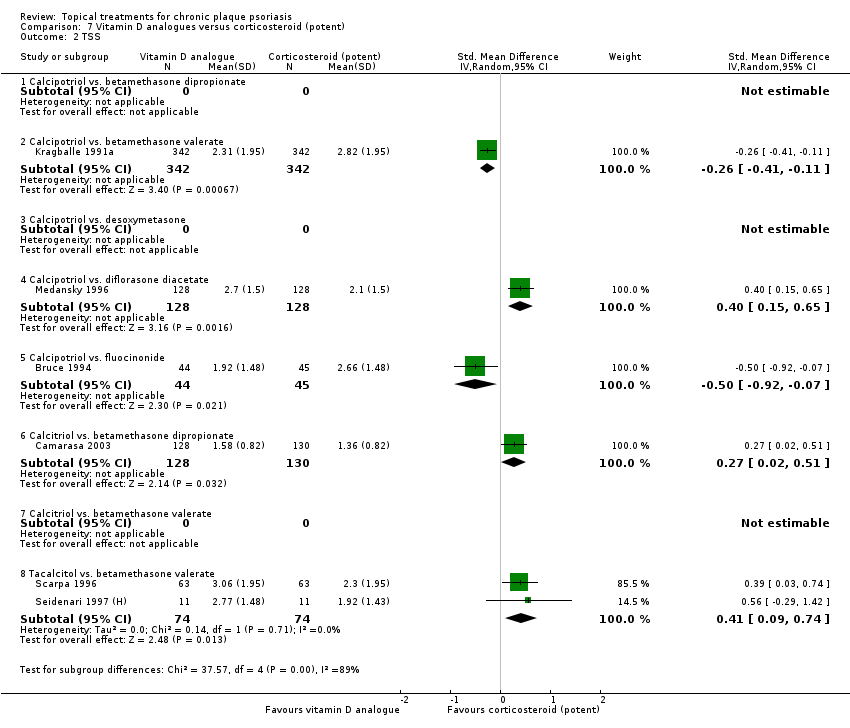
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 2 TSS.
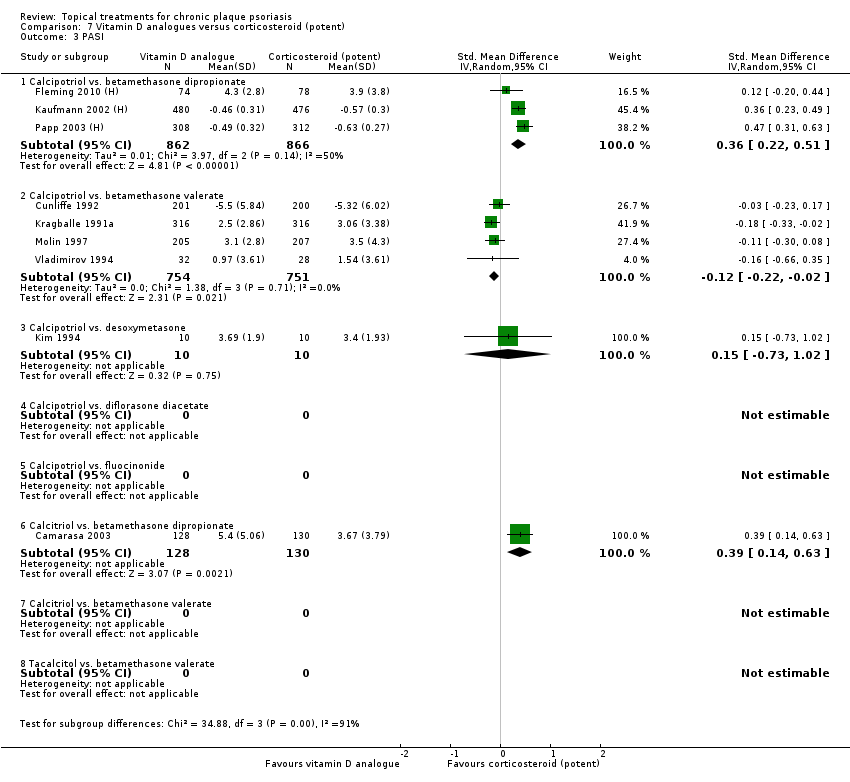
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 3 PASI.
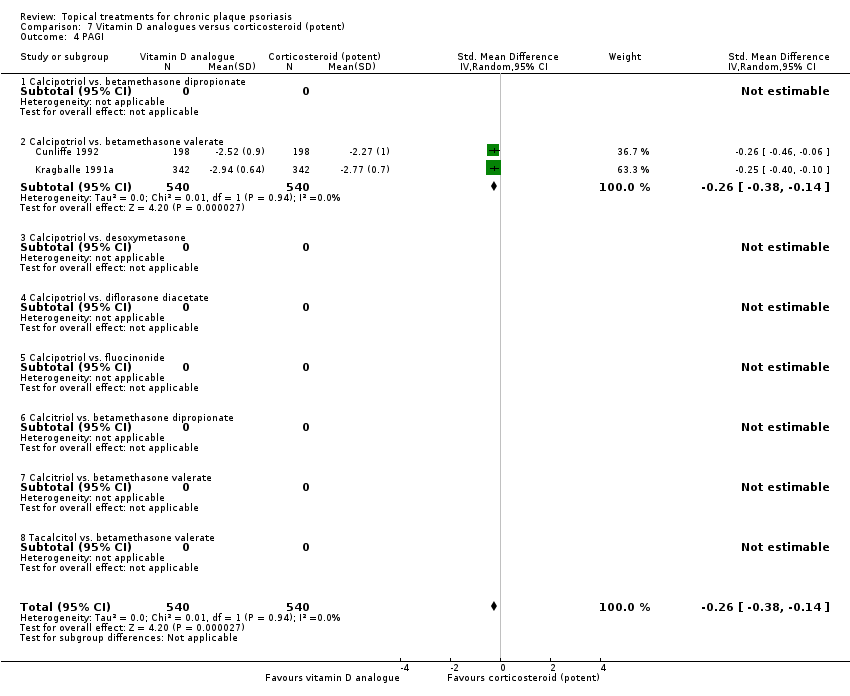
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 4 PAGI.

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 6 Total withdrawals.

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 7 Withdrawals due to adverse events.

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 8 Withdrawals due to treatment failure.
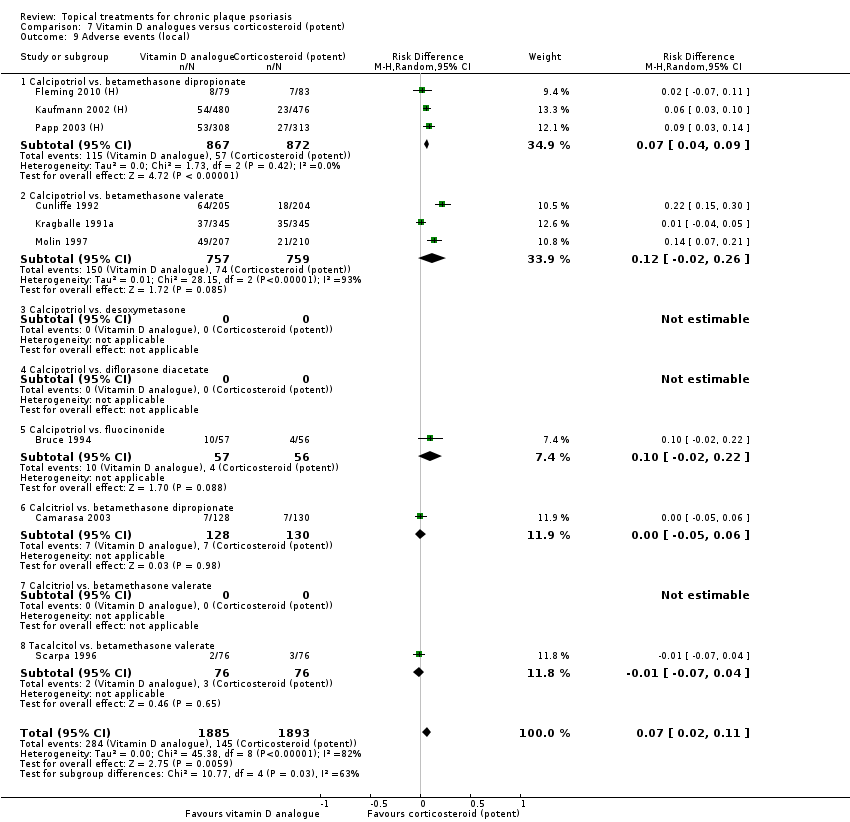
Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 9 Adverse events (local).

Comparison 7 Vitamin D analogues versus corticosteroid (potent), Outcome 10 Adverse events (systemic).

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 1 IAGI.
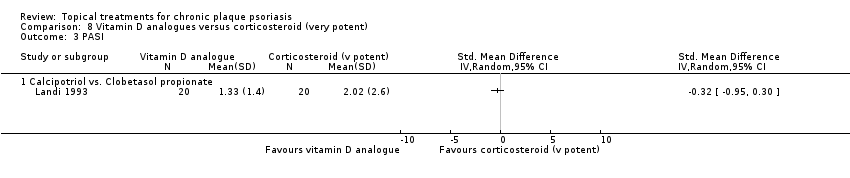
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 3 PASI.
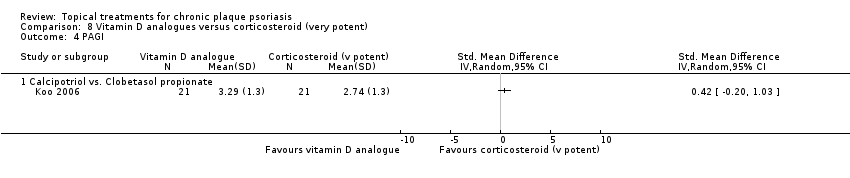
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 4 PAGI.
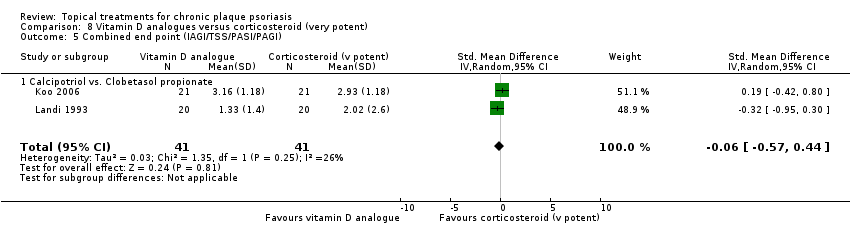
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 6 Total withdrawals.

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 7 Withdrawals due to adverse events.
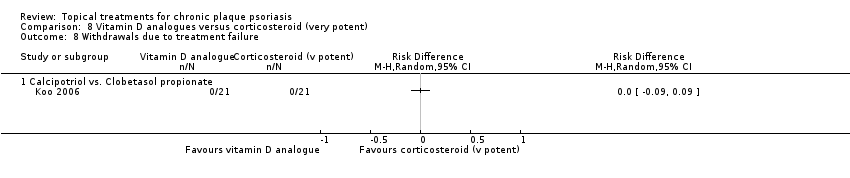
Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 8 Withdrawals due to treatment failure.

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 9 Adverse events (local).

Comparison 8 Vitamin D analogues versus corticosteroid (very potent), Outcome 10 Adverse events (systemic).
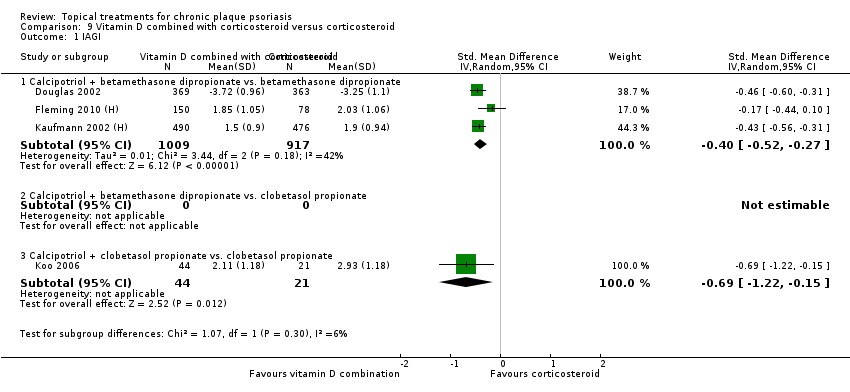
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 1 IAGI.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 2 TSS.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 3 PASI.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 4 PAGI.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
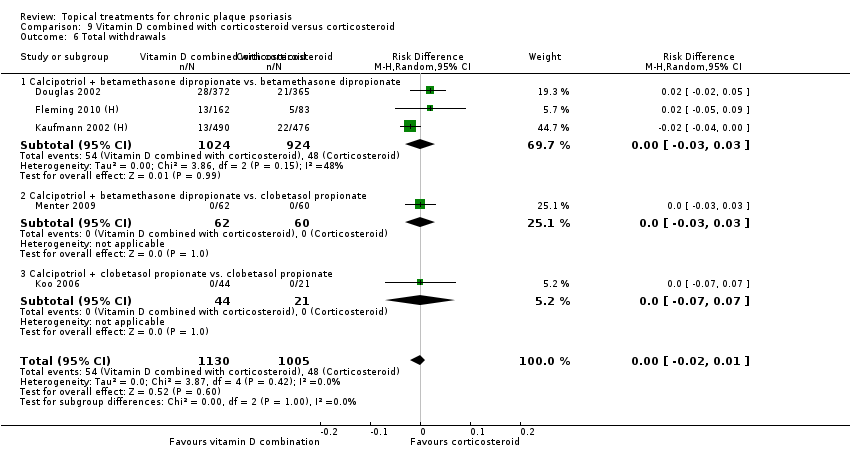
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 6 Total withdrawals.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 7 Withdrawals due to adverse events.

Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 8 Withdrawals due to treatment failure.
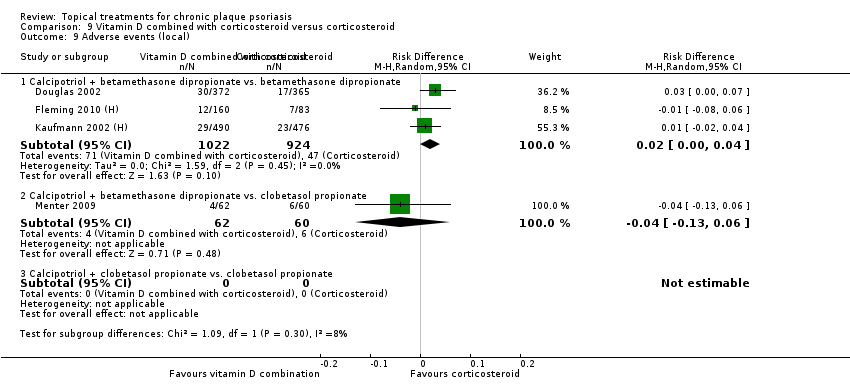
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 9 Adverse events (local).
Comparison 9 Vitamin D combined with corticosteroid versus corticosteroid, Outcome 10 Adverse events (systemic).

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 1 IAGI.
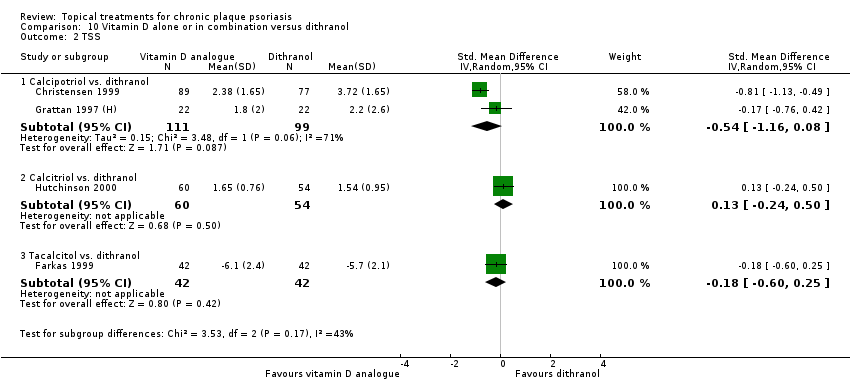
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 2 TSS.

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 3 PASI.

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 4 PAGI.

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 6 Total withdrawals.

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 7 Withdrawals due to adverse events.
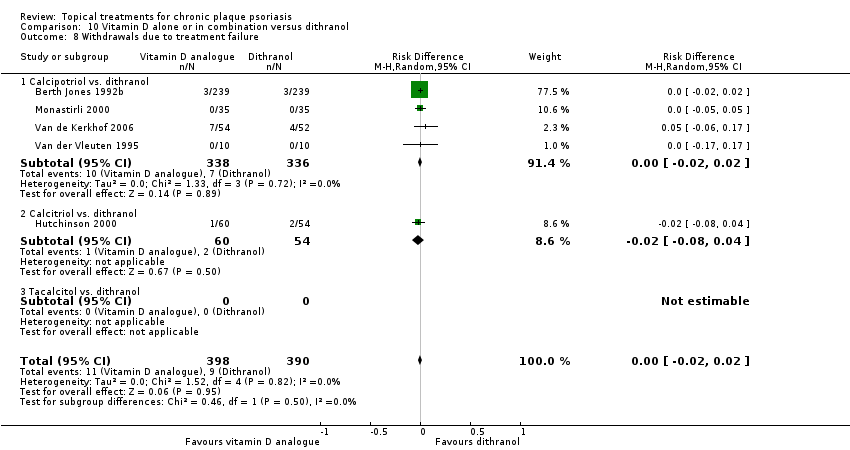
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 8 Withdrawals due to treatment failure.
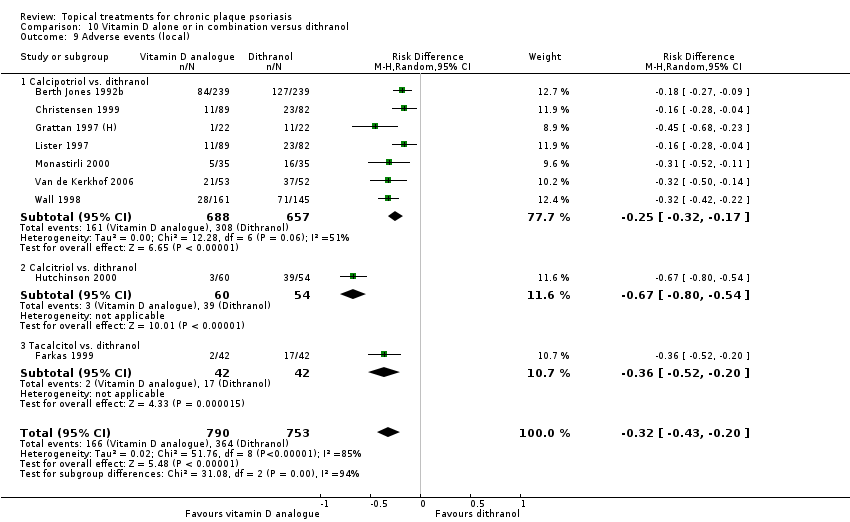
Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 9 Adverse events (local).

Comparison 10 Vitamin D alone or in combination versus dithranol, Outcome 10 Adverse events (systemic).

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 1 IAGI.

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 2 TSS.

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 3 PASI.
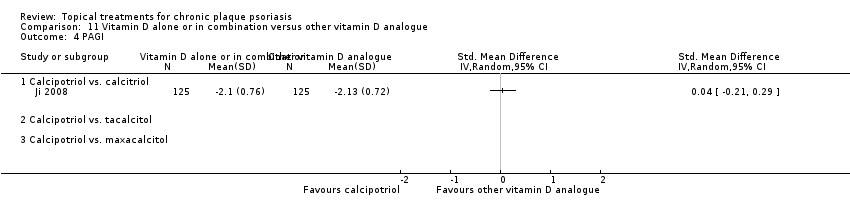
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 4 PAGI.

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 6 Total withdrawals.

Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 7 Withdrawals due to adverse events.
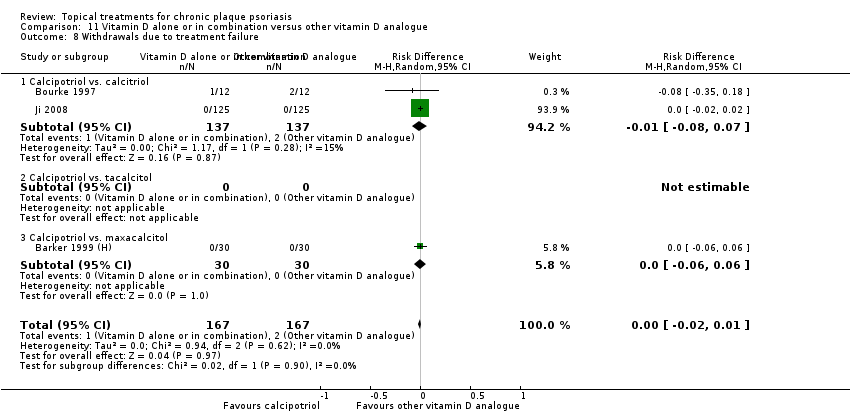
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 8 Withdrawals due to treatment failure.
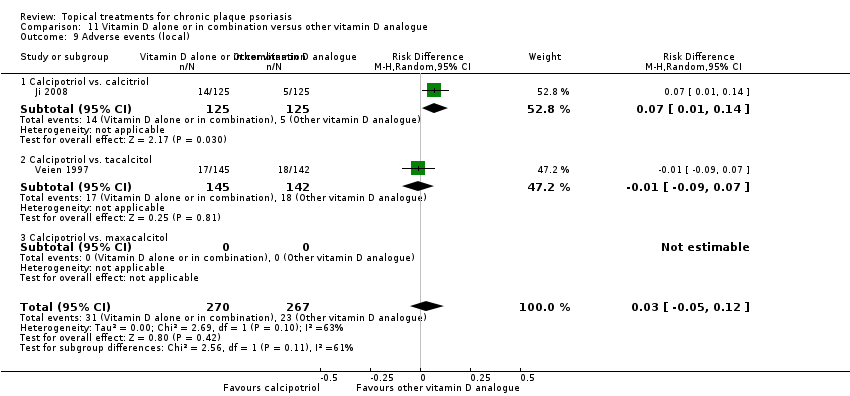
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 9 Adverse events (local).
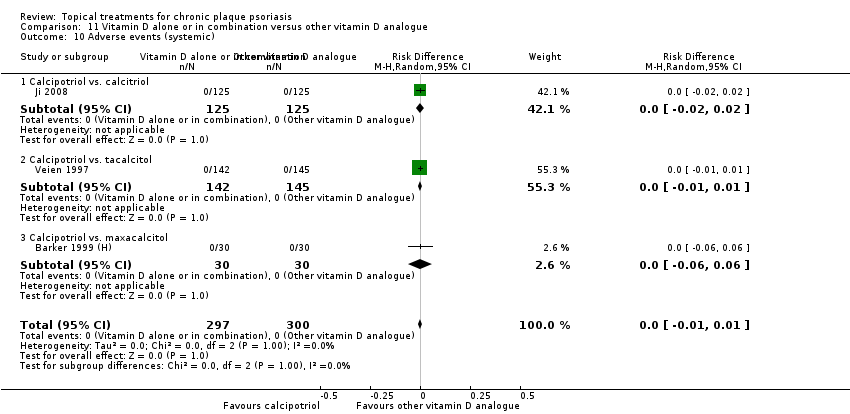
Comparison 11 Vitamin D alone or in combination versus other vitamin D analogue, Outcome 10 Adverse events (systemic).

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 1 IAGI.

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 2 TSS.
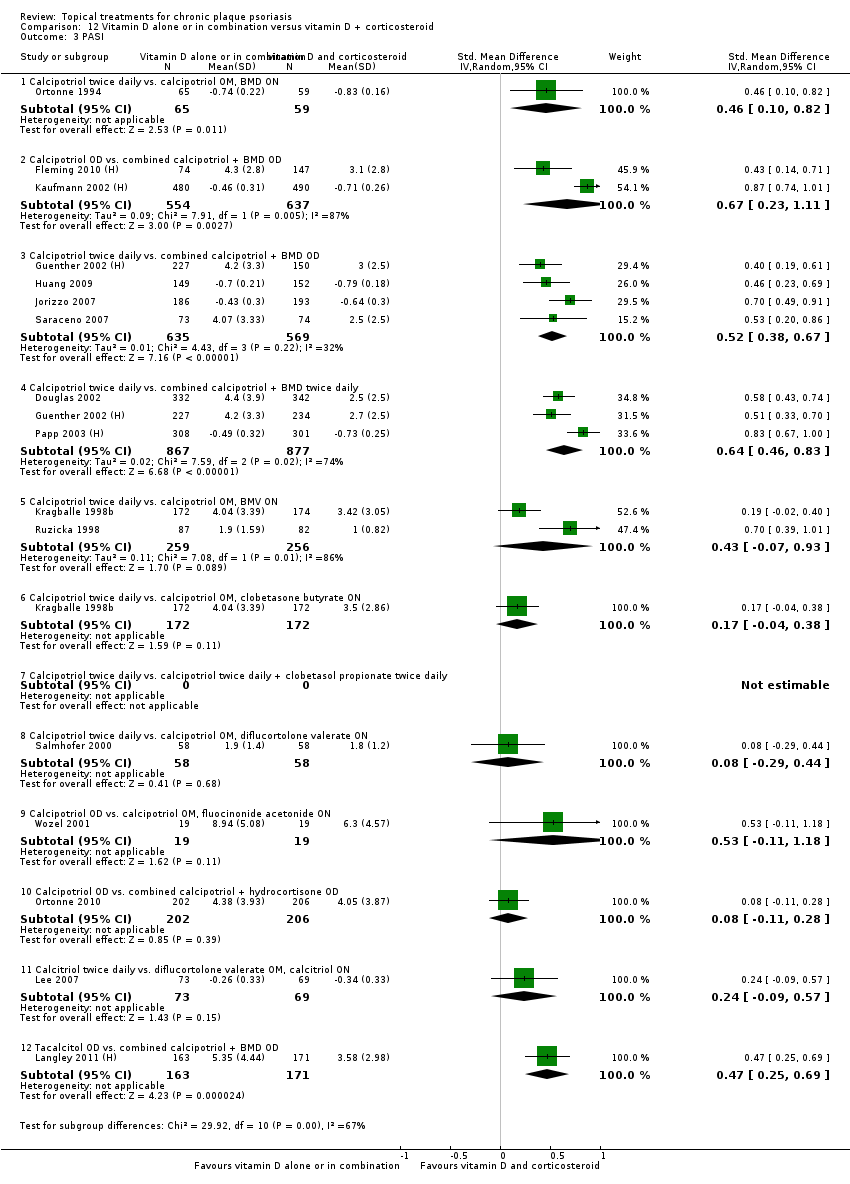
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 3 PASI.
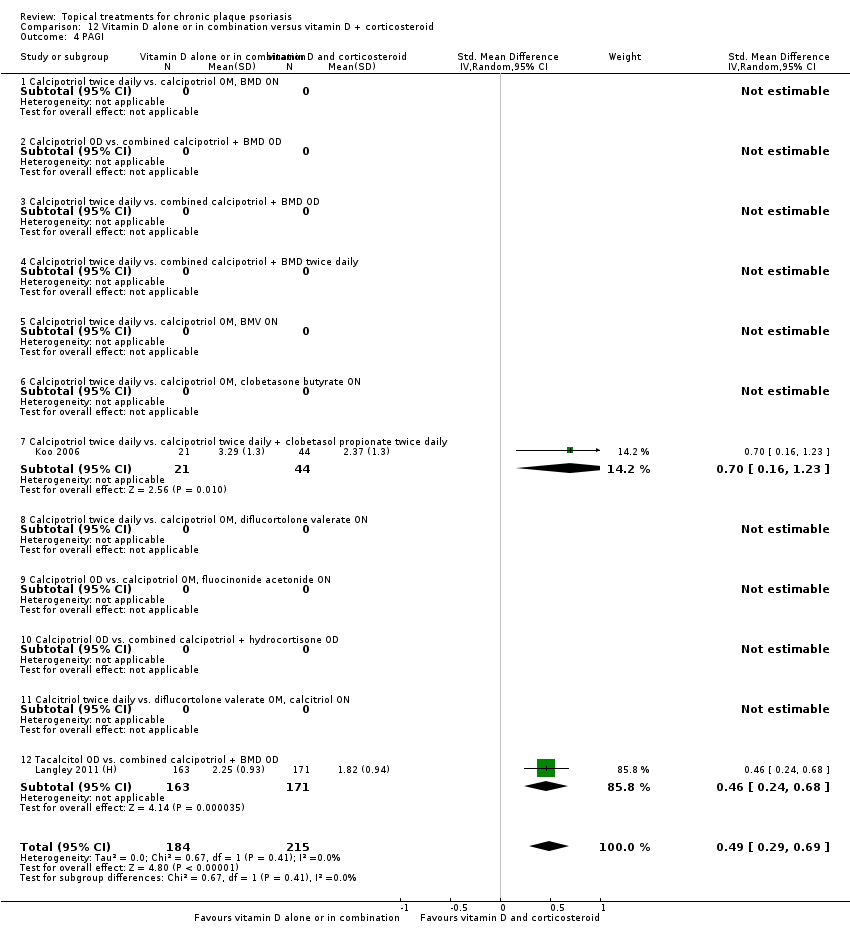
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 4 PAGI.

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 6 Total withdrawals.

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 7 Withdrawals due to adverse events.
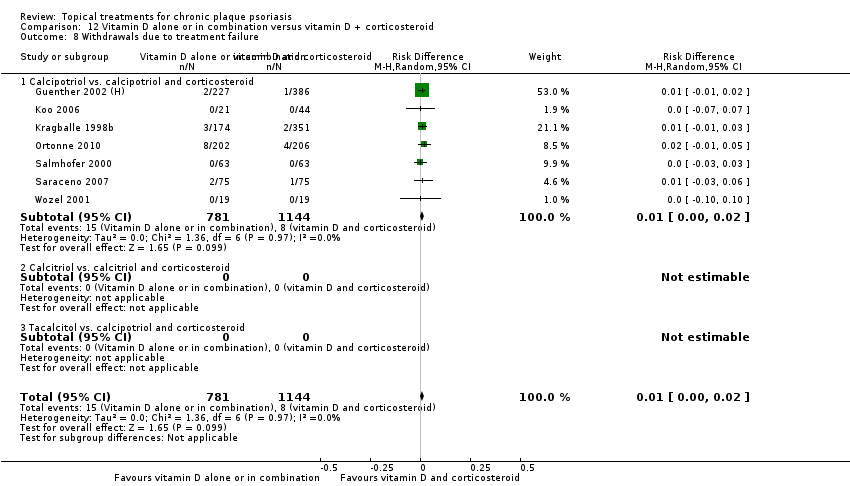
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 8 Withdrawals due to treatment failure.

Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 9 Adverse events (local).
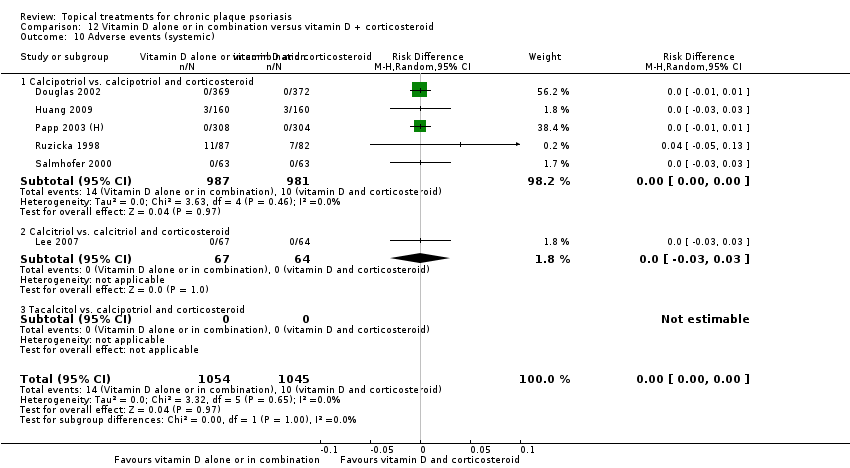
Comparison 12 Vitamin D alone or in combination versus vitamin D + corticosteroid, Outcome 10 Adverse events (systemic).
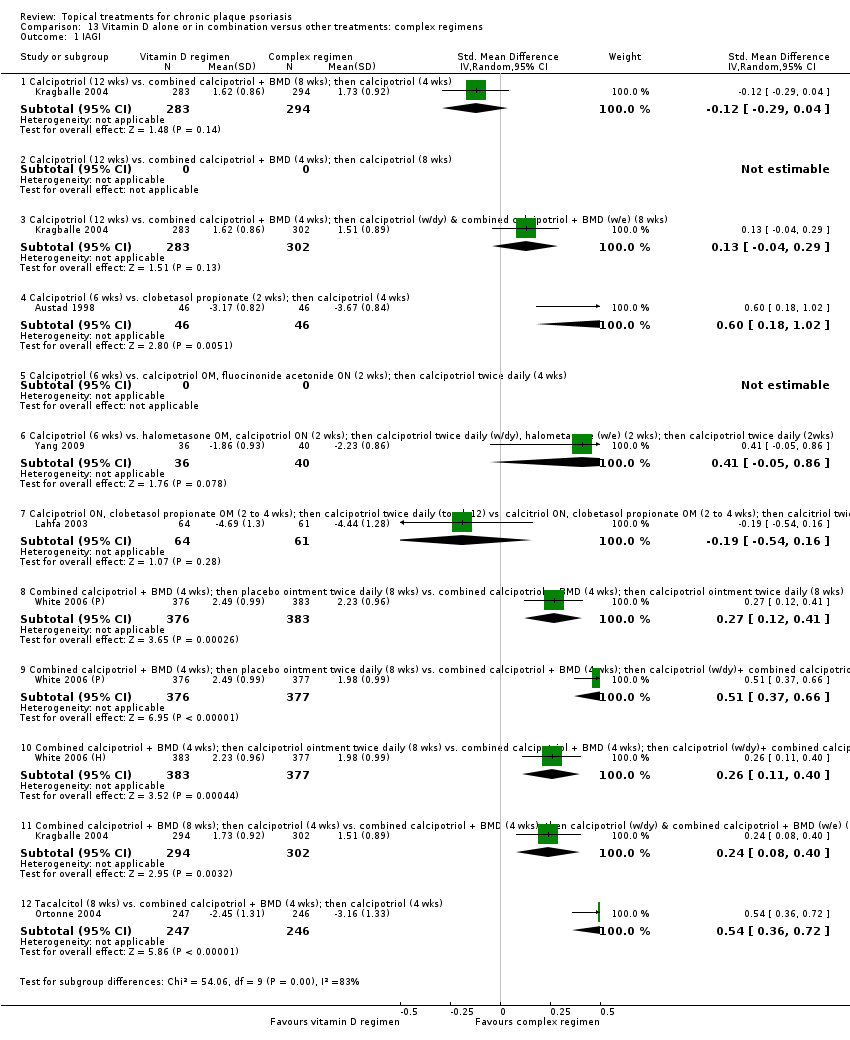
Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 1 IAGI.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 2 TSS.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 3 PASI.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 4 PAGI.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 6 Total withdrawals.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 7 Withdrawals due to adverse events.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 8 Withdrawals due to treatment failure.

Comparison 13 Vitamin D alone or in combination versus other treatments: complex regimens, Outcome 9 Adverse events (local).

Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 1 IAGI.

Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 6 Total withdrawals.

Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 7 Withdrawals due to adverse events.

Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 8 Withdrawals due to treatment failure.
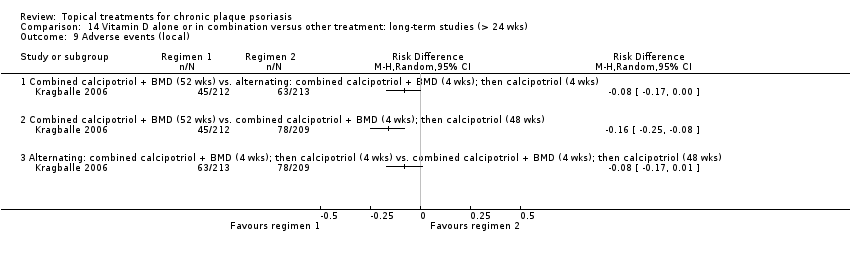
Comparison 14 Vitamin D alone or in combination versus other treatment: long‐term studies (> 24 wks), Outcome 9 Adverse events (local).
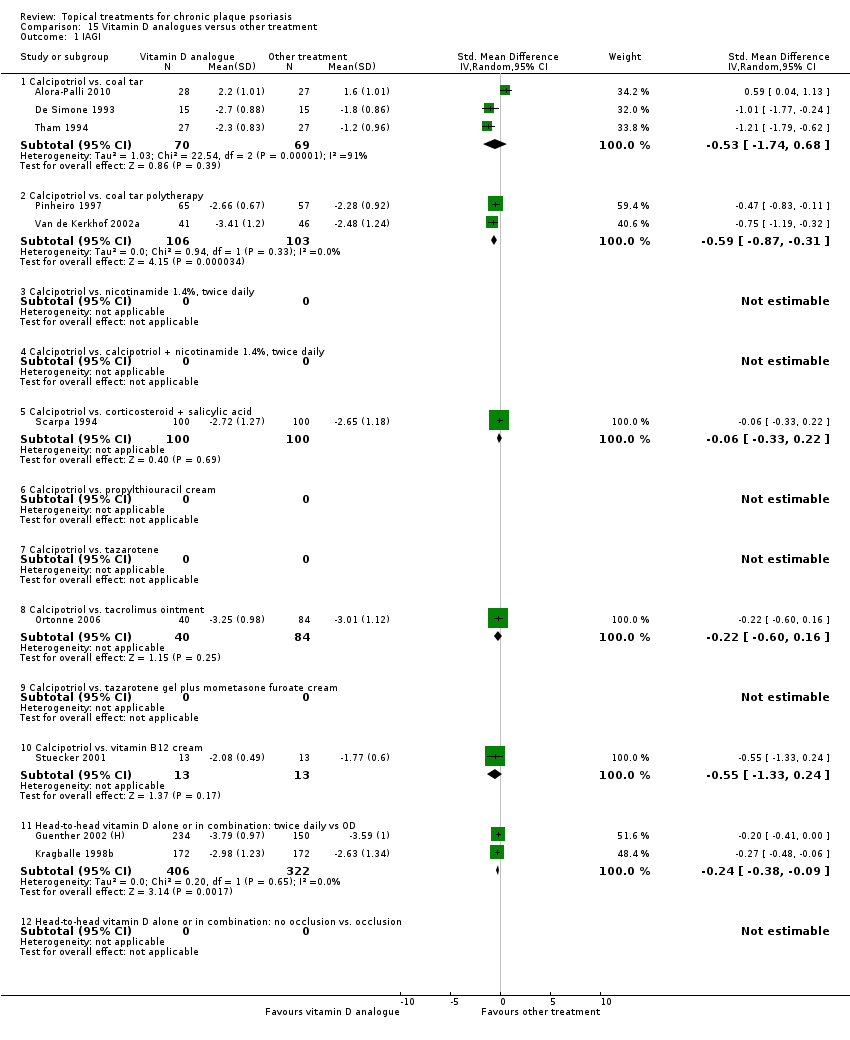
Comparison 15 Vitamin D analogues versus other treatment, Outcome 1 IAGI.
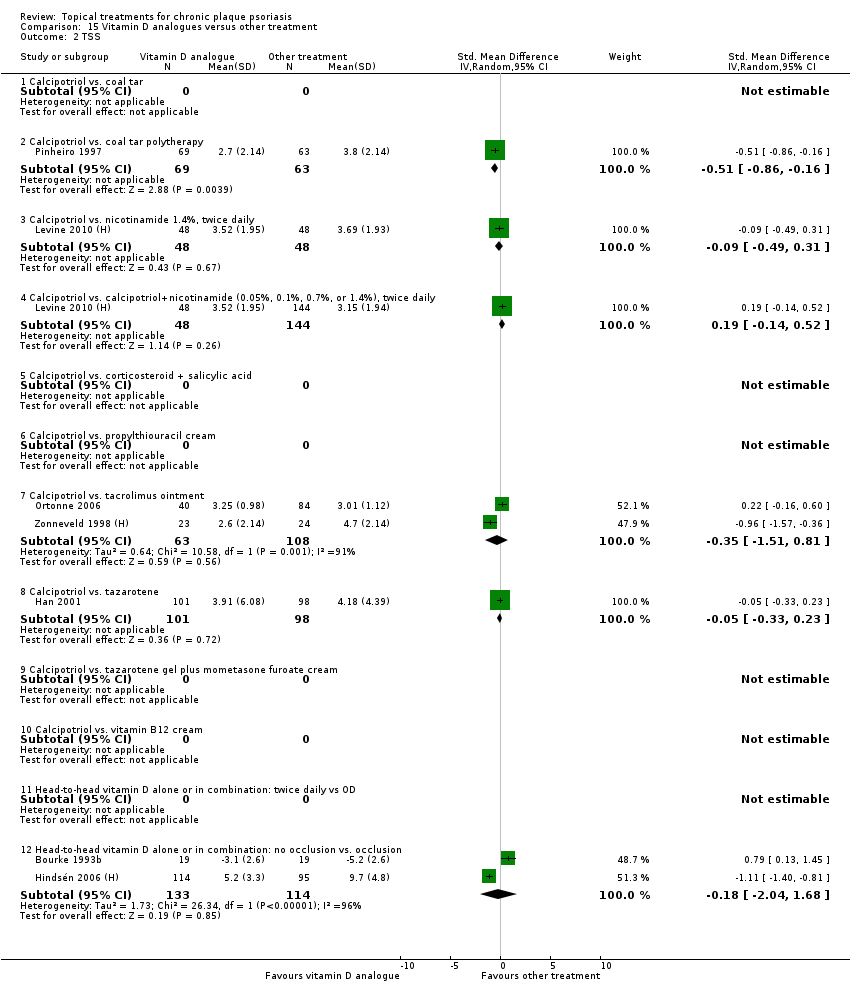
Comparison 15 Vitamin D analogues versus other treatment, Outcome 2 TSS.

Comparison 15 Vitamin D analogues versus other treatment, Outcome 3 PASI.

Comparison 15 Vitamin D analogues versus other treatment, Outcome 4 PAGI.
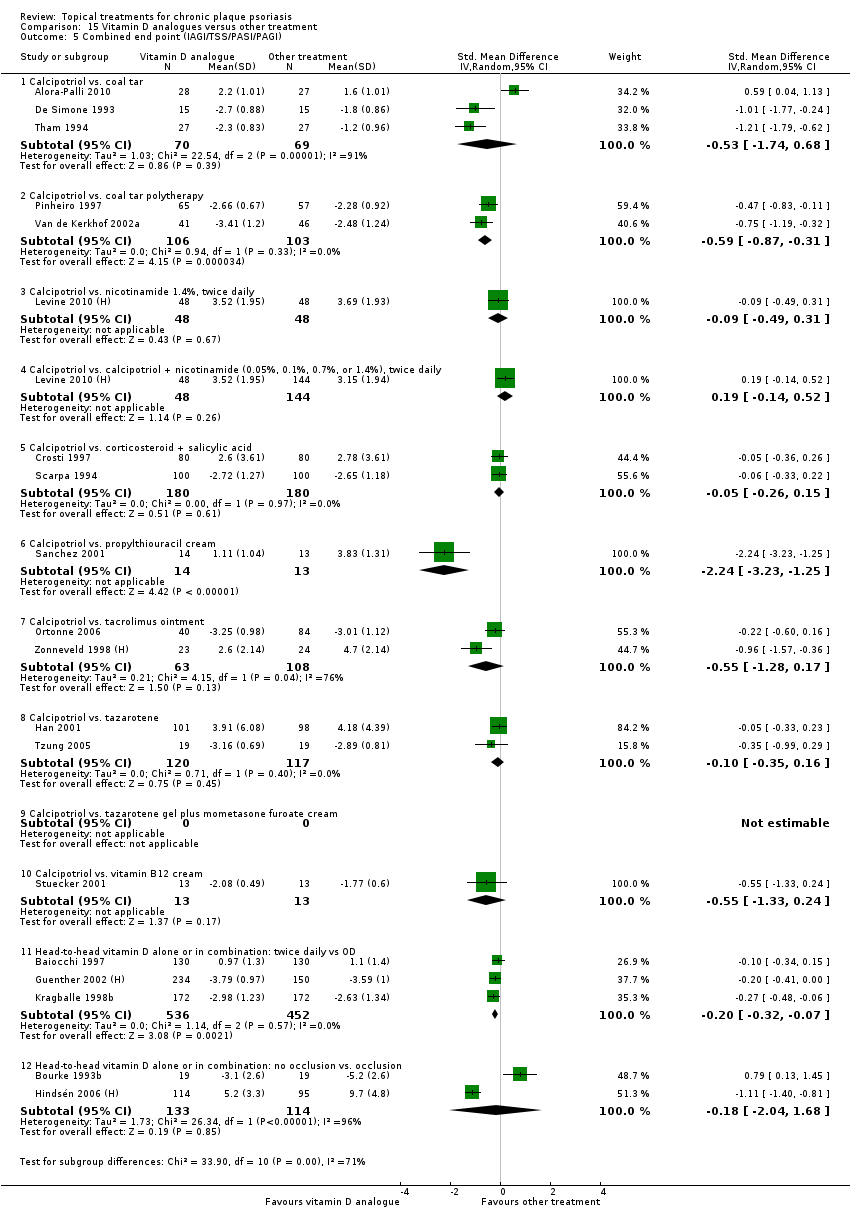
Comparison 15 Vitamin D analogues versus other treatment, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 15 Vitamin D analogues versus other treatment, Outcome 6 Total withdrawals.
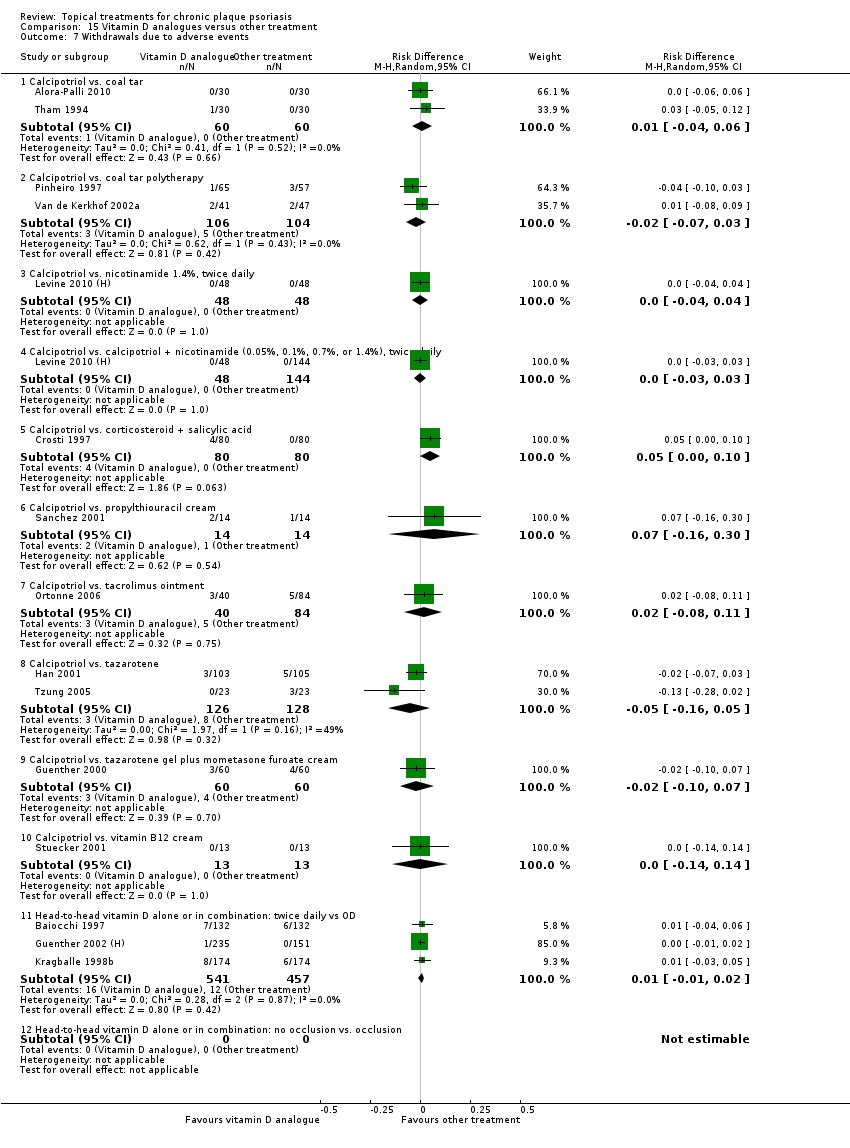
Comparison 15 Vitamin D analogues versus other treatment, Outcome 7 Withdrawals due to adverse events.

Comparison 15 Vitamin D analogues versus other treatment, Outcome 8 Withdrawals due to treatment failure.

Comparison 15 Vitamin D analogues versus other treatment, Outcome 9 Adverse events (local).
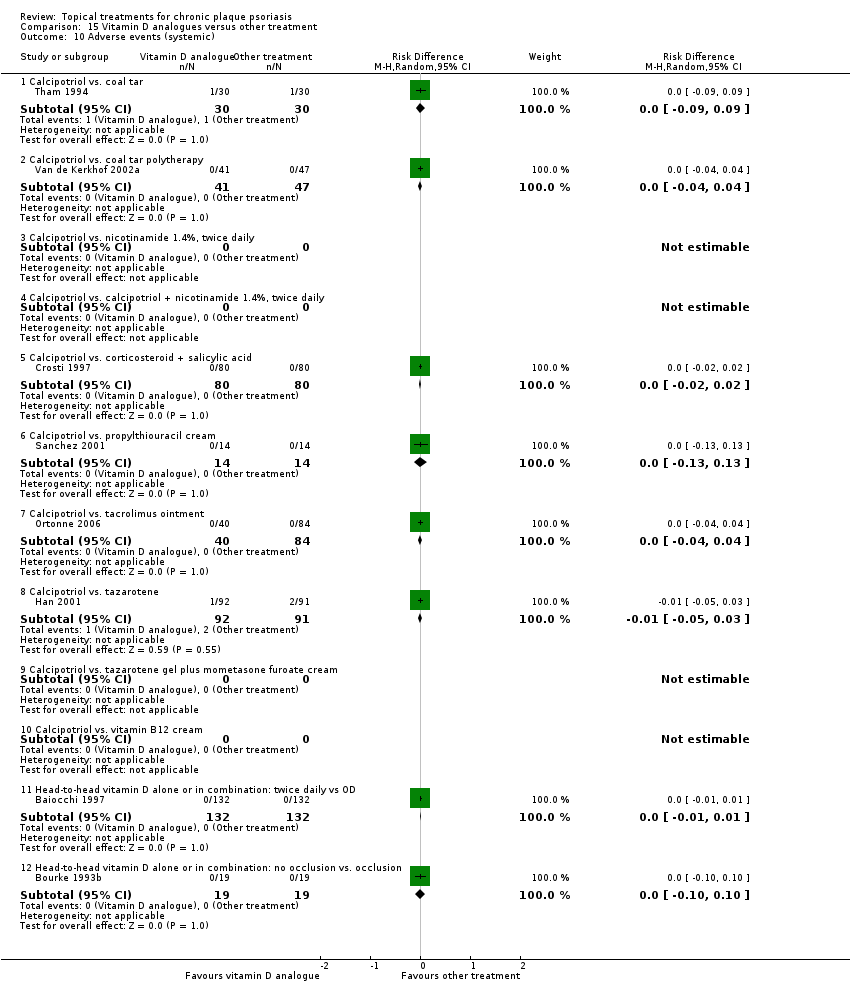
Comparison 15 Vitamin D analogues versus other treatment, Outcome 10 Adverse events (systemic).

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 1 IAGI.

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 2 TSS.

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 3 PASI.

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 4 PAGI.

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 6 Total withdrawals.
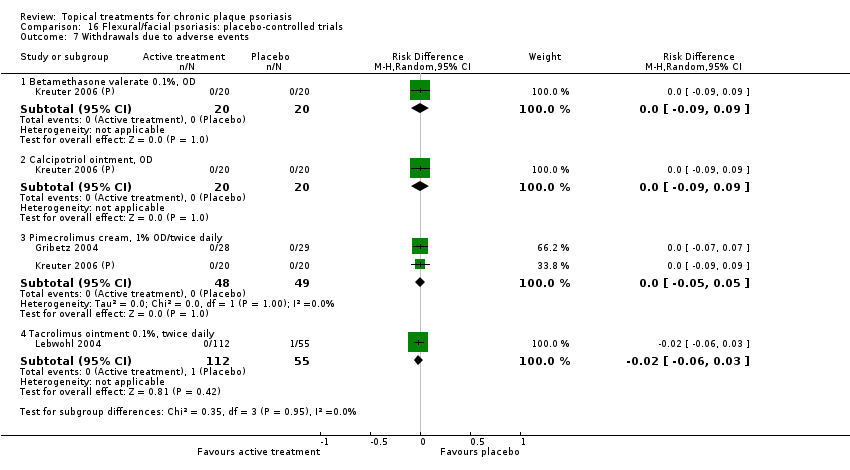
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 7 Withdrawals due to adverse events.

Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 8 Withdrawals due to treatment failure.
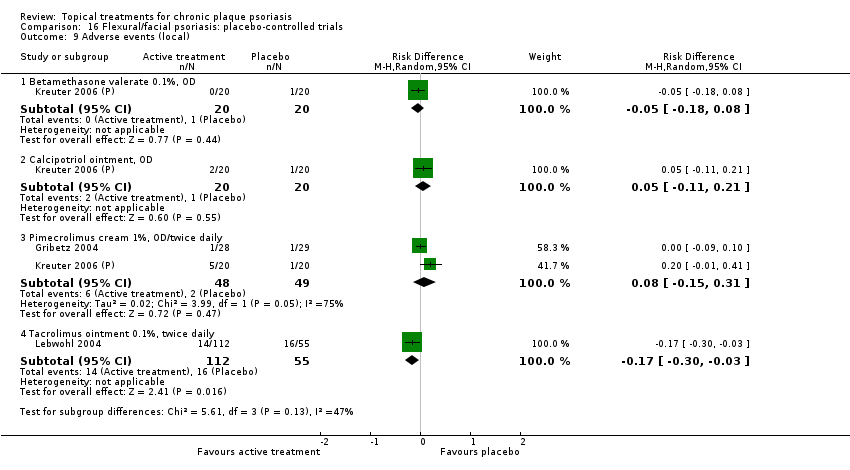
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 9 Adverse events (local).
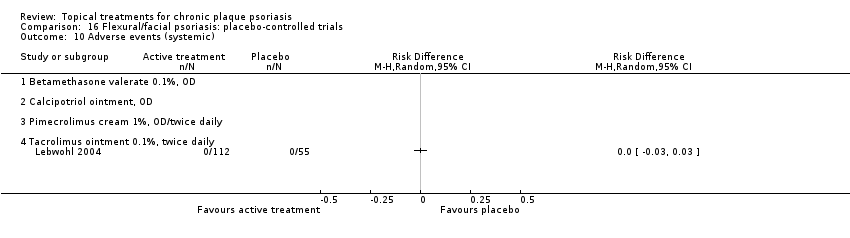
Comparison 16 Flexural/facial psoriasis: placebo‐controlled trials, Outcome 10 Adverse events (systemic).

Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 1 IAGI.

Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 2 TSS.

Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 3 PASI.

Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
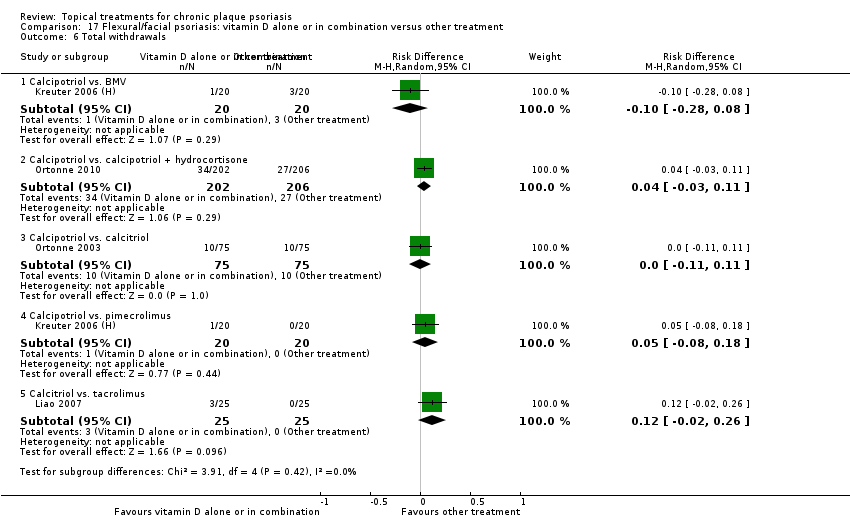
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 6 Total withdrawals.
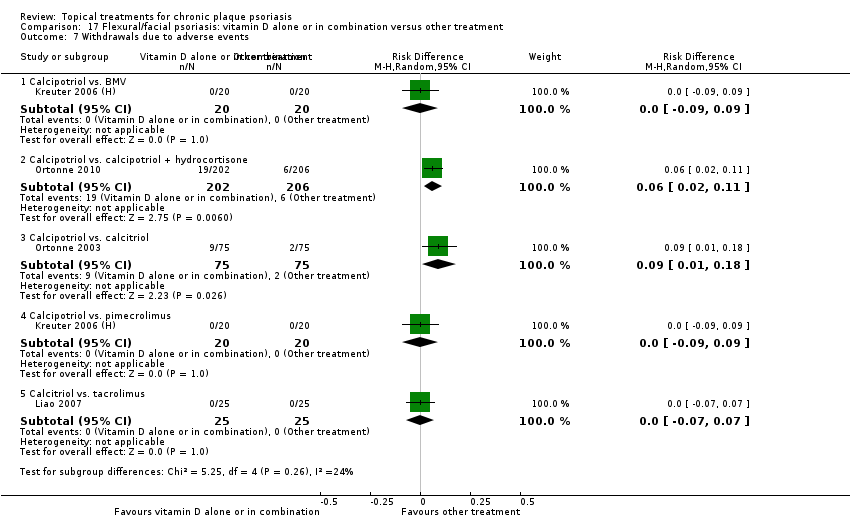
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 7 Withdrawals due to adverse events.

Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 8 Withdrawals due to treatment failure.
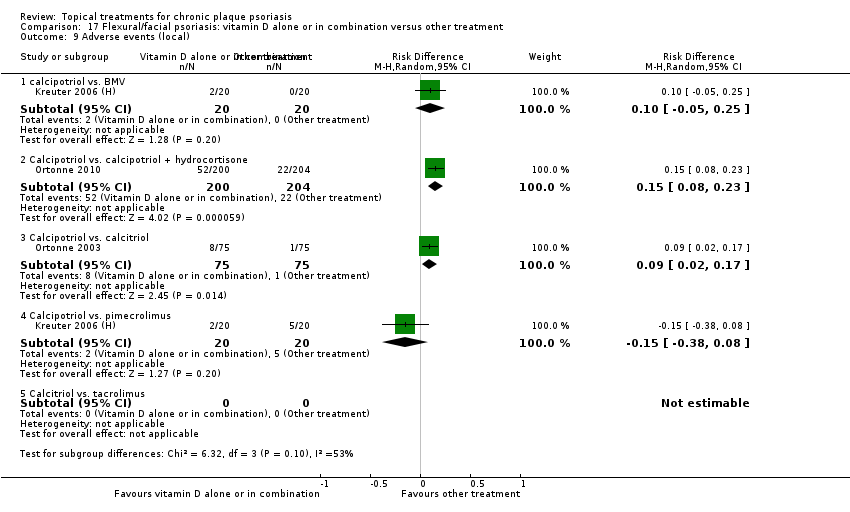
Comparison 17 Flexural/facial psoriasis: vitamin D alone or in combination versus other treatment, Outcome 9 Adverse events (local).

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 1 IAGI.
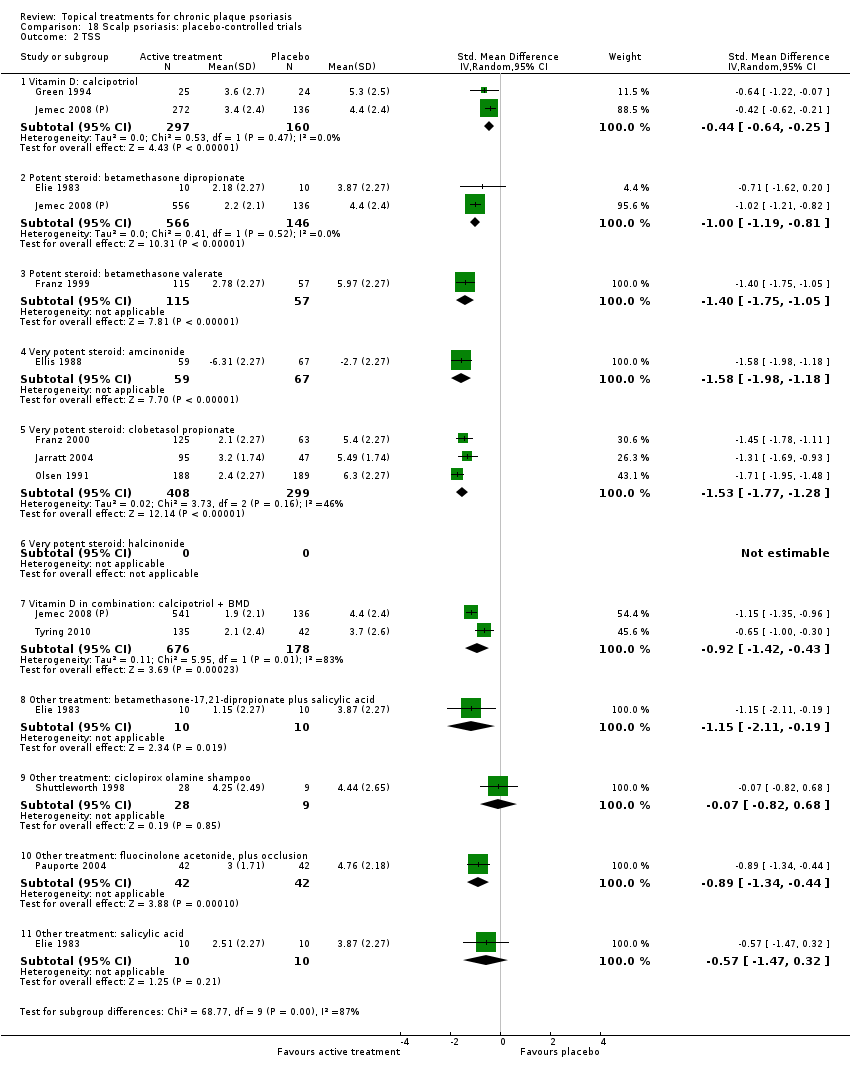
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 2 TSS.

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 4 PAGI.

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 6 Total withdrawals.
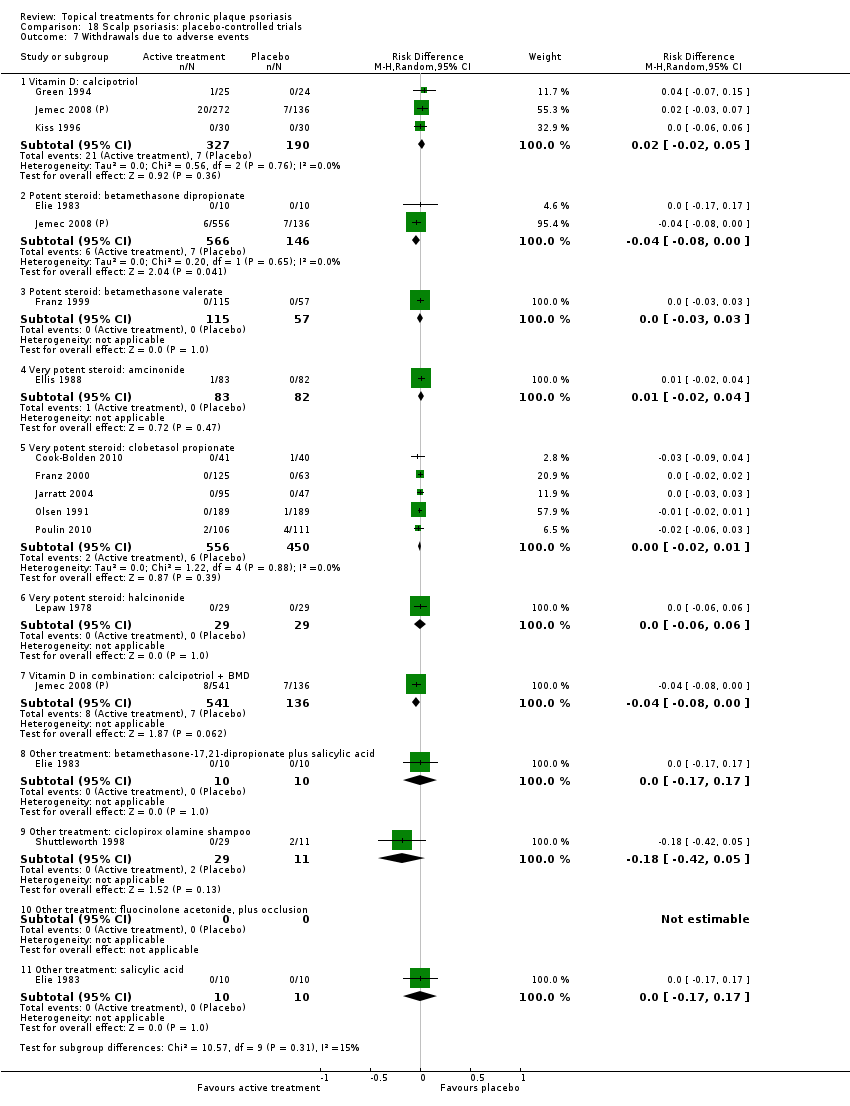
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 7 Withdrawals due to adverse events.
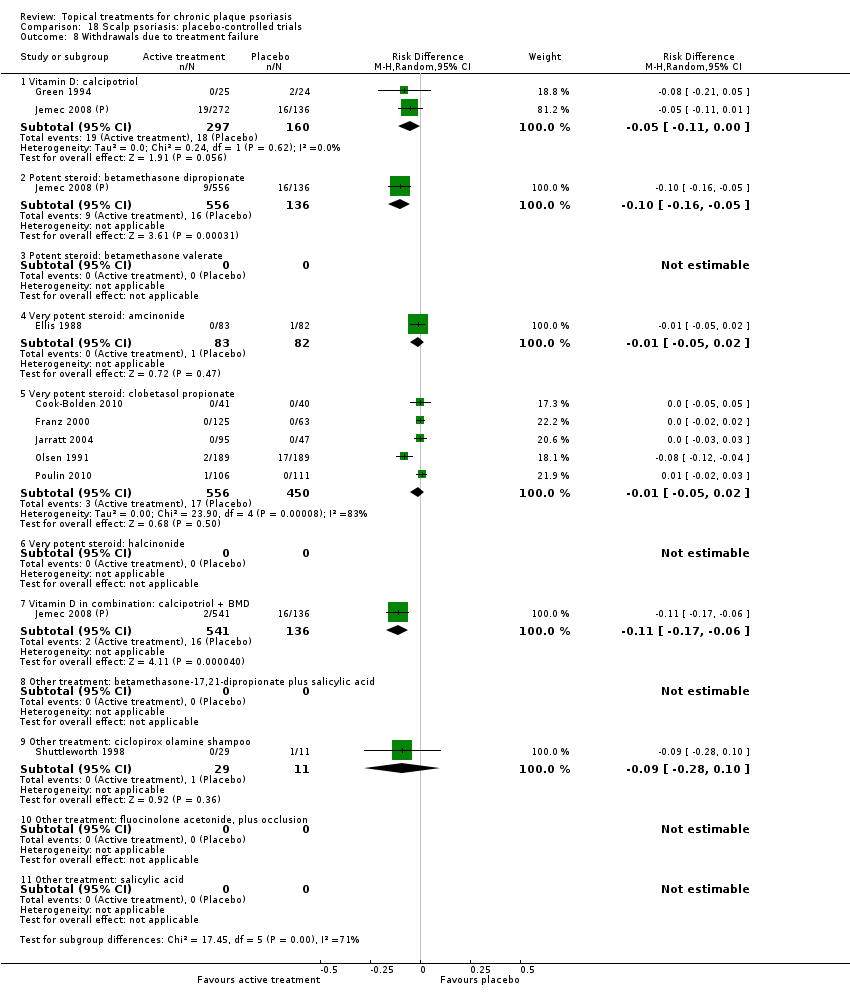
Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 8 Withdrawals due to treatment failure.

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 9 Adverse events (local).

Comparison 18 Scalp psoriasis: placebo‐controlled trials, Outcome 10 Adverse events (systemic).

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 1 IAGI.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 2 TSS.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 4 PAGI.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 5 Combined end point (IAGI/TSS/PASI/PAGI).
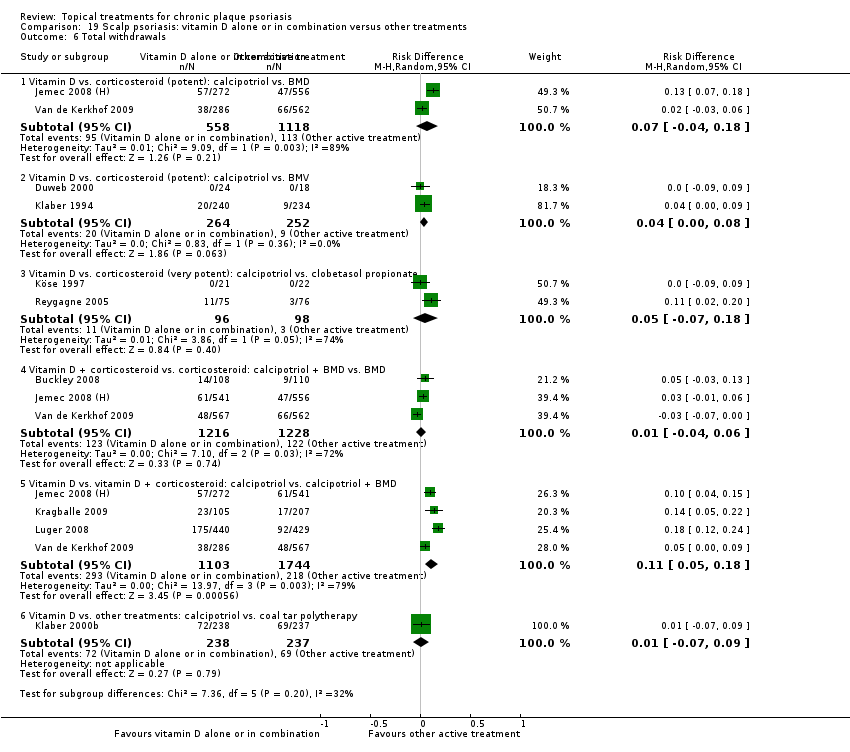
Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 6 Total withdrawals.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 7 Withdrawals due to adverse events.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 8 Withdrawals due to treatment failure.

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 9 Adverse events (local).

Comparison 19 Scalp psoriasis: vitamin D alone or in combination versus other treatments, Outcome 10 Adverse events (systemic).
| Acronym | Full name |
| BC | baseline comparability demonstrated (clinical/demographic) |
| BD | twice daily |
| BMD | betamethasone dipropionate |
| BMV | betamethasone valerate |
| BSA | Body Surface Area |
| Btw‐patient | Between‐patient |
| CI | confidence interval |
| dys | days |
| EQ‐5D | EuroQOL |
| FU | follow up (includes treatment period) |
| I² | heterogeneity statistic |
| IAGI | Investigator Assessment of Global Improvement (change score) |
| IGA | Investigator Global Assessment (static score) |
| IQR | interquartile range |
| ISGA | Investigator's Static Global Assessment Score |
| LAE | local adverse effects |
| LCD | liquor carbonis distillate |
| LF | loss to follow up (per cent of participants randomised, not contributing to primary outcome measure) |
| MEMS | Medication Event Monitoring System |
| mPASI | modified Psoriasis Area Severity Index |
| NA | not available/not applicable |
| NR | not reported |
| OD | once daily |
| OM | once in the morning |
| ON | once at night |
| ODS | overall disease severity |
| PAGI | Patient Assessment of Global Improvement (change score) |
| PASI | Psoriasis Area Severity Index |
| PDI | Psoriasis Disability Index |
| PGA | Patient Global Assessment (static score) |
| PMAQ‐3w | Medication Adherence Questionnaire, version 3W |
| pt | point |
| QOL | quality of life |
| RD | risk difference |
| SD | standard deviation |
| SMD | standardised mean difference |
| TCP | two‐compound product |
| TD | three times daily |
| TLPSS | Total Local Psoriasis Severity Score |
| TSS | Total Severity Score/total sum score |
| UV | ultra violet |
| VDRE | Vitamin D‐Responsive Element |
| wks | weeks |
| yrs | years |
| Outcome | Acronym | Construct | Scale, minimum | Scale, maximum | Notes |
| * Investigator's Assessment of Overall Global Improvement | IAGI | Improvement from baseline variably defined. Common taxonomy ranges from worse to cleared | 4‐pt | 7‐pt | Calculated means and standard deviations by assigning zero to 'worse' (or equivalent). Higher scores indicate greater improvement |
| Investigator's Global Assessment of Disease Severity | IGA | Static equivalent of the IAGI | 4‐pt | 7‐pt | Calculated means and standard deviations by assigning zero to 'clear' (or equivalent). Higher scores indicate more severe disease |
| Total Severity Score | TSS | Redness (erythema), thickness (infiltration) and scaling (sometimes also itching (pruritis)) of target plaque(s). Scored separately then summed | 0 to 3 | 0 to 24 | Also known as the Local Psoriasis Severity Index or the Total Sum Score. Higher scores indicate more severe disease |
| Psoriasis Area and Severity Index | PASI | Redness, thickness, and scaliness of the lesions (each graded on a 0 to 4 scale), weighted by the area of involvement (0 to 6) and summed | 0 to 68 (without head) | 0 to 72 (including head) | Higher scores indicate more severe disease |
| * Patient's Assessment of Overall Global Improvement | PAGI | Assessed as IAGI | 4‐pt | 7‐pt | Less often reported than IAGI. Majority of included trials use 5‐pt scale |
| Patient's Global Assessment of Disease Severity | PGA | Assessed as IGA | 4‐pt | 5‐pt | Rarely reported (5/177 studies) |
| * IAGI/PAGI data are entered as a negative values; thus, a reduction denotes a positive improvement for the active treatment consistent with TSS and PASI measures. | |||||
| Type of study/score | Placebo IAGI (change)/IGA (end point) | Placebo TSS | Placebo PASI | Placebo PAGI (change)/PGA (end point) | H2H IAGI (change)/IGA (end point) | H2H TSS | H2H PASI | H2H PAGI (change)/PGA (end point) |
| Between‐patient (end point) | 0.93 | 1.33 | 3.76 | 1.13 | 1.01 | 1.65 | 3.61 | 1.12 |
| Within‐patient (end point) | 1.08 | 1.49 | 7.17 | NA | NA | 1.50 | 2.58 | NA |
| Between‐patient (change) | 1.17 | 1.52 | 5.75 | 1.31 | 1.10 | 1.73 | 7.85 | 1.20 |
| Within‐patient (change) | 1.02 | 1.58 | NA | 1.53 | 0.96 | 1.94 | NA | 0.83 |
| Within‐patient (% change) | NA | 0.18 | NA | NA | NA | NA | NA | NA |
| Between‐patient (% change) | NA | NA | 0.37 | NA | NA | 0.13 | 0.33 | NA |
| Scalp between‐patient (end point) | 1.08 | 1.74 | NA | 1.06 | 1.06 | 1.94 | NA | 1.18 |
| Scalp within‐patient (end point) | 1.33 | NA | NA | NA | NA | NA | NA | NA |
| Scalp between‐patient (change) | 1.20 | NA | NA | 1.28 | 1.30 | 1.75 | NA | 1.20 |
| Scalp between‐patient (% change) | NA | NA | NA | NA | NA | 0.25 | NA | NA |
| NA: not available; H2H: head‐to‐head; IGA [PGA]: Investigator [Patient] Global Assessment of Disease Severity; IAGI [PAGI]: Investigator (patient) Assessment of Global Improvement; TSS: Total Severity Score; PASI: Psoriasis Area and Severity Index | ||||||||
| Comparison No. | Comparison Label | No. studies | Per cent studies with | No. |
| 01 | Vitamin D analogues vs. placebo | 30 | 60% | 4986 |
| 02 | Corticosteroid (potent) vs. placebo | 13 | 85% | 2216 |
| 03 | Corticosteroid (very potent) vs. placebo | 10 | 70% | 1264 |
| 04 | Dithranol vs. placebo | 3 | 0% | 47 |
| 05 | Vitamin D combination products vs. placebo | 5 | 100% | 2058 |
| 06 | Other treatment vs. placebo | 26 | 46% | 1450 |
| 07 | Vitamin D analogues vs. corticosteroid (potent) | 14 | 64% | 3542 |
| 08 | Vitamin D analogues vs. corticosteroid (very potent) | 2 | 100% | 82 |
| 09 | Vitamin D combined with corticosteroid vs. corticosteroid | 5 | 100% | 2113 |
| 10 | Vitamin D alone or in combination vs. dithranol | 8 | 88% | 1284 |
| 11 | Vitamin D alone or in combination vs. other vitamin D analogue | 4 | 75% | 513 |
| 12 | Vitamin D alone or in combination vs. vitamin D + corticosteroid | 17 | 94% | 5856 |
| 13 | Vitamin D alone or in combination vs. other treatments: complex regimens | 9 | 89% | 2936 |
| 14 | Vitamin D alone or in combination vs. other treatment: long‐term studies (> 24 wks) | 1 | 100% | 297 |
| 15 | Vitamin D analogues vs. other treatment | 19 | 68% | 2364 |
| 16 | Flexural/facial psoriasis: placebo‐controlled trials | 2 | 100% | 122 |
| 17 | Flexural/facial psoriasis: vitamin D alone or in combination vs. other treatment | 4 | 75% | 588 |
| 18 | Scalp psoriasis: placebo‐controlled trials | 14 | 93% | 3011 |
| 19 | Scalp psoriasis: vitamin D alone or in combination vs. other treatments | 12 | 100% | 5413 |
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol OD/BD | Effect size [CI] | (SMD ‐0.93; 95% CI ‐1.17 to ‐0.68) | (SMD ‐1.15; 95% CI ‐1.41 to ‐0.89) | (SMD ‐0.65; 95% CI ‐0.75 to ‐0.55) | (SMD ‐0.64; 95% CI ‐0.97 to ‐0.30) | (SMD ‐0.96; 95% CI ‐1.15 to ‐0.77) |
| 02 Calcipotriol plus occlusion | Effect size [CI] | NA | (SMD ‐0.15; 95% CI ‐0.44 to 0.14) | ‐ | ‐ | (SMD ‐0.15; 95% CI ‐0.44 to 0.14) |
| 03 Calcitriol OD/BD | Effect size [CI] | (SMD ‐1.03; 95% CI ‐1.71 to ‐0.36) | (SMD ‐1.22; 95% CI ‐2.38 to ‐0.07) | ‐ | (SMD ‐0.59; 95% CI ‐0.76 to ‐0.41) | (SMD ‐0.92; 95% CI ‐1.54 to ‐0.29) |
| 04 Tacalcitol OD | Effect size [CI] | (SMD ‐0.84; 95% CI ‐1.41 to ‐0.26) | (SMD ‐0.66; 95% CI ‐0.95 to ‐0.36) | (SMD ‐0.27; 95% CI ‐0.56 to 0.03) | (SMD ‐0.24; 95% CI ‐0.53 to 0.05) | (SMD ‐0.73; 95% CI ‐1.09 to ‐0.37) |
| 05 Maxacalcitol OD | Effect size [CI] | (SMD ‐1.43; 95% CI ‐1.91 to ‐0.96) | (SMD ‐1.61; 95% CI ‐2.10 to ‐1.12) | ‐ | ‐ | (SMD ‐1.43; 95% CI ‐1.91 to ‐0.96) |
| 06 Paricalcitol OD | Effect size [CI] | (SMD ‐1.66; 95% CI ‐2.66 to ‐0.67) | (SMD ‐2.15; 95% CI ‐3.24 to ‐1.06) | ‐ | ‐ | (SMD ‐1.66; 95% CI ‐2.66 to ‐0.67) |
| 07 Becocalcidiol OD | Effect size [CI] | (SMD ‐0.22; 95% CI ‐0.58 to 0.14) | (SMD ‐0.02; 95% CI ‐0.37 to 0.34) | ‐ | ‐ | (SMD ‐0.22; 95% CI ‐0.58 to 0.14) |
| 08 Becocalcidiol BD | Effect size [CI] | (SMD ‐0.67; 95% CI ‐1.04 to ‐0.30) | (SMD ‐0.46; 95% CI ‐0.83 to ‐0.10) | ‐ | ‐ | (SMD ‐0.67; 95% CI ‐1.04 to ‐0.30) |
| All treatments | Effect size [CI]; I² statistic | (SMD ‐0.95; 95% CI ‐1.17 to ‐0.74): I² statistic: 89.0% | (SMD ‐1.04; 95% CI ‐1.33 to ‐0.74) I² statistic: 93.0% | (SMD ‐0.58; 95% CI ‐0.71 to ‐0.45): I² statistic: 42.3% | (SMD ‐0.54; 95% CI ‐0.72 to ‐0.36): I² statistic: 55.5% | (SMD ‐0.90; 95% CI ‐1.07 to ‐0.72); I² statistic: 87.5% |
| ‐ | No. participants | 3771 | 2647 | 2357 | 1467 | 4986 |
| ‐ | Between‐patient design | 13 | 9 | 8 | 5 | 18 |
| ‐ | Within‐patient design | 7 | 10 | 1 | 0 | 12 |
| ‐ | Treatment duration | 4 wks to 12 wks | 4 wks to 12 wks | 3 wks to 8 wks | 8 wks to 8 wks | 3 wks to 12 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.11; 95% CI ‐1.58 to ‐0.64) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.80; 95% CI ‐0.96 to ‐0.63) |
| ‐ | Calcitriol, Perez 1996 removed | ‐ | ‐ | ‐ | (SMD ‐0.60; 95% CI ‐0.78 to ‐0.41) | |
| ‐ | Calcipotriol BD | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.02; 95% CI ‐1.23 to ‐0.82) |
| ‐ | Calcipotriol OD | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.76; 95% CI ‐1.13 to ‐0.40) |
| ‐ | correlation coefficient (rho) = 0 All trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.85; 95% CI ‐1.00 to ‐0.71); |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.87; 95% CI ‐1.01 to ‐0.72); |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.88; 95% CI ‐1.03 to ‐0.73); |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.91; 95% CI ‐1.07 to ‐0.75); |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Betamethasone dipropionate OD | Effect size [CI] | (SMD ‐0.81; 95% CI ‐0.98 to ‐0.64) | (SMD ‐0.74; 95% CI ‐1.16 to ‐0.32) | (SMD ‐0.79; 95% CI ‐1.44 to ‐0.14) | ‐ | (SMD ‐0.80; 95% CI ‐0.96 to ‐0.64) |
| 02 Betamethasone dipropionate BD | Effect size [CI] | (SMD ‐1.35; 95% CI ‐1.56 to ‐1.15) | (SMD ‐0.77; 95% CI ‐1.48 to ‐0.06) | (SMD ‐1.21; 95% CI ‐1.44 to ‐0.97) | ‐ | (SMD ‐1.35; 95% CI ‐1.56 to ‐1.15) |
| 03 Betamethasone dipropionate, maintenance | Effect size [CI] | (SMD ‐0.95; 95% CI ‐1.62 to ‐0.27) | ‐ | ‐ | (SMD ‐0.95; 95% CI ‐1.62 to ‐0.27) | |
| 04 Betamethasone valerate | Effect size [CI] | (SMD ‐1.41; 95% CI ‐1.92 to ‐0.90) | (SMD ‐1.09; 95% CI ‐2.00 to ‐0.18) | ‐ | ‐ | (SMD ‐1.33; 95% CI ‐1.78 to ‐0.89) |
| 05 Budesonide | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 06 Desonide | Effect size [CI] | (SMD ‐0.81; 95% CI ‐1.34 to ‐0.28) | (SMD ‐1.16; 95% CI ‐1.70 to ‐0.61) | ‐ | ‐ | (SMD ‐0.81; 95% CI ‐1.34 to ‐0.28) |
| 07 Diflorasone diacetate | Effect size [CI] | ‐ | (SMD ‐0.32; 95% CI ‐0.73 to 0.09) | ‐ | ‐ | (SMD ‐0.32; 95% CI ‐0.73 to 0.09) |
| 08 Fluticasone propionate | Effect size [CI] | (SMD ‐0.93; 95% CI ‐1.14 to ‐0.72) | ‐ | ‐ | ‐ | (SMD ‐0.93; 95% CI ‐1.14 to ‐0.72) |
| 09 Hydrocortisone buteprate | Effect size [CI] | ‐ | (SMD ‐0.46; 95% CI ‐0.77 to ‐0.15) | ‐ | ‐ | (SMD ‐0.46; 95% CI ‐0.77 to ‐0.15) |
| 10 Mometasone furoate | Effect size [CI] | (SMD ‐0.75; 95% CI ‐1.17 to ‐0.34) | (SMD ‐1.12; 95% CI ‐1.55 to ‐0.68) | ‐ | ‐ | (SMD ‐0.75; 95% CI ‐1.17 to ‐0.34) |
| All treatments | Effect size [CI]; I² statistic | (SMD ‐1.00; 95% CI ‐1.18 to ‐0.82); I² statistic: 57.6% | (SMD ‐0.77; 95% CI ‐1.01 to ‐0.52); I² statistic: 46.7% | (SMD ‐0.97; 95% CI ‐1.31 to ‐0.62); I² statistic: 79.6% | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72); I² statistic: 65.1% |
| ‐ | No. participants | 1867 | 553 | 1158 | 0 | 2216 |
| ‐ | Between‐patient design | 8 | 6 | 3 | 0 | 11 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 2 |
| ‐ | Treatment duration | 3 wks to 12 wks | 2 wks to 12 wks | 4 wks to 8 wks | ‐ | 2 wks to 12 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.33; 95% CI ‐1.78 to ‐0.89) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.85; 95% CI ‐1.03 to ‐0.67) |
| ‐ | correlation coefficient (rho) = 0 All trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72) I² statistic: 77.7% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.89; 95% CI ‐1.06 to ‐0.72) I² statistic: 78.0% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.90; 95% CI ‐1.07 to ‐0.73) I² statistic: 78.6% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.91; 95% CI ‐1.08 to ‐0.74) I² statistic: 80.2% |
| For acronyms, see Table 1. Both within‐patient trials compared betamethasone valerate with placebo. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Clobetasol propionate | Effect size [CI] | (SMD ‐1.89; 95% CI ‐2.53 to ‐1.24) | (SMD ‐1.35; 95% CI ‐1.80 to ‐0.89) | ‐ | (SMD ‐1.01; 95% CI ‐1.55 to ‐0.47) | (SMD ‐1.65; 95% CI ‐2.10 to ‐1.20) |
| 02 Halcinonide | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 03 Halobetasol | Effect size [CI] | (SMD ‐1.81; 95% CI ‐2.37 to ‐1.24) | ‐ | ‐ | (SMD ‐1.25; 95% CI ‐1.46 to ‐1.04) | (SMD ‐1.36; 95% CI ‐1.65 to ‐1.07) |
| All treatments | Effect size [CI], N, I² | (SMD ‐1.87; 95% CI ‐2.38 to ‐1.36); I² statistic: 78.7% | (SMD ‐1.35; 95% CI ‐1.80 to ‐0.89); I² statistic: 75.3% | ‐ | (SMD ‐1.22; 95% CI ‐1.42 to ‐1.02); I² statistic: 0% | (SMD ‐1.56; 95% CI ‐1.87 to ‐1.26); I² statistic: 81.7% |
| ‐ | No. participants | 515 | 545 | 0 | 283 | 1264 |
| ‐ | Between‐patient design | 4 | 3 | 0 | 1 | 7 |
| ‐ | Within‐patient design | 1 | 0 | 0 | 2 | 3 |
| ‐ | Treatment duration | 2 wks to 4 wks | 2 wks to 4 wks | 2 wks to 2 wks | 2 wks to 4 wks | |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐2.02 to ‐1.02) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.58; 95% CI ‐1.99 to ‐1.17) |
| ‐ | correlation coefficient (rho) = 0 All trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐1.80 to ‐1.24) I² statistic: 81.6% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.52; 95% CI ‐1.80 to ‐1.25) I² statistic: 82.2% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.53; 95% CI ‐1.80 to ‐1.26) I² statistic: 83.3% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.55; 95% CI ‐1.80 to ‐1.29) I² statistic: 85.9% |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Combination calcipotriol/betamethasone dipropionate, OD | Effect size [CI] | (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91) | ‐ | (SMD ‐1.14; 95% CI ‐1.57 to ‐0.70) | (SMD ‐0.69; 95% CI ‐0.98 to ‐0.40) | (SMD ‐1.21; 95% CI ‐1.50 to ‐0.91) |
| 02 Combination calcipotriol/betamethasone dipropionate, BD | Effect size [CI] | (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71) | ‐ | (SMD ‐1.41; 95% CI ‐1.86 to ‐0.97) | ‐ | (SMD ‐1.90; 95% CI ‐2.09 to ‐1.71) |
| All treatments | Effect size [CI], N, I² statistic | (SMD ‐1.44; 95% CI ‐1.76 to ‐1.12); I² statistic: 89.4% | ‐ | (SMD ‐1.24; 95% CI ‐1.53 to ‐0.95); I² statistic: 87.6% | (SMD ‐0.69; 95% CI ‐0.98 to ‐0.40); I² statistic: NA | (SMD ‐1.44; 95% CI ‐1.76 to ‐1.12); I² statistic: 89.4% |
| ‐ | No. participants | 2058 | 0 | 2056 | 235 | 2058 |
| ‐ | Between‐patient design | 5 | 0 | 5 | 1 | 5 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 4 wks to 8 wks | ‐ | 4 wks to 8 wks | 8 wks | 4 wks to 8 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Aloe vera extract | Effect size [CI] | ‐ | ‐ | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) | ‐ | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) |
| 02 Anti‐IL‐8 monoclonal antibody cream | Effect size [CI] | (SMD ‐0.59; 95% CI ‐1.01 to ‐0.16) | (SMD ‐0.70; 95% CI ‐1.13 to ‐0.27) | ‐ | ‐ | (SMD ‐0.59; 95% CI ‐1.01 to ‐0.16) |
| 03 Betamethasone 17‐valerate 21‐acetate plus tretinoine plus salicylic acid | Effect size [CI] | (SMD ‐0.76; 95% CI ‐1.21 to ‐0.31) | ‐ | (SMD ‐0.54; 95% CI ‐0.99 to ‐0.10) | (SMD ‐0.80; 95% CI ‐1.26 to ‐0.35) | (SMD ‐0.76; 95% CI ‐1.21 to ‐0.31) |
| 04 Caffeine (topical) 10%, TD | Effect size [CI] | ‐ | ‐ | (SMD ‐0.39; 95% CI ‐0.84 to 0.06) | ‐ | (SMD ‐0.39; 95% CI ‐0.84 to 0.06) |
| 05 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.48; 95% CI ‐0.81 to ‐0.15) | ‐ | ‐ | (SMD ‐0.48; 95% CI ‐0.81 to ‐0.15) |
| 06 Dead sea salts emollient lotion | Effect size [CI] | ‐ | ‐ | (SMD 0.57; 95% CI ‐0.36 to 1.51) | ‐ | (SMD 0.57; 95% CI ‐0.36 to 1.51) |
| 07 Fish oil plus occlusion | Effect size [CI] | ‐ | (SMD ‐1.05; 95% CI ‐1.64 to ‐0.46) | ‐ | ‐ | (SMD ‐1.05; 95% CI ‐1.64 to ‐0.46) |
| 08 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), BD | Effect size [CI] | (SMD ‐2.96; 95% CI ‐4.19 to ‐1.74) | ‐ | (SMD ‐2.96; 95% CI ‐4.19 to ‐1.74) | ||
| 09 Hexafluoro‐1,25‐dihydroxyvitamin D3 | Effect size [CI] | (SMD ‐0.62; 95% CI ‐1.35 to 0.12) | (SMD ‐1.13; 95% CI ‐1.91 to ‐0.35) | ‐ | ‐ | (SMD ‐0.62; 95% CI ‐1.35 to 0.12) |
| 10 Indigo naturalis 1.4% ointment | Effect size [CI] | (SMD ‐2.14; 95% CI ‐2.74 to ‐1.53) | (SMD ‐1.64; 95% CI ‐2.13 to ‐1.15) | ‐ | ‐ | (SMD ‐2.09; 95% CI ‐2.62 to ‐1.56) |
| 11 Kukui nut oil, TD | Effect size [CI] | (SMD 0.00; 95% CI ‐0.80 to 0.80) | (SMD 0.33; 95% CI ‐0.48 to 1.14) | (SMD ‐0.03; 95% CI ‐0.84 to 0.77) | (SMD 0.00; 95% CI ‐0.80 to 0.80) | (SMD 0.00; 95% CI ‐0.80 to 0.80) |
| 12 Mahonia aquifolium (Reliéva™), BD | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | (SMD ‐0.77; 95% CI ‐1.06 to ‐0.48) |
| 13 Methotrexate gel | Effect size [CI] | (SMD ‐0.56; 95% CI ‐1.01 to ‐0.12) | (SMD ‐0.48; 95% CI ‐0.92 to ‐0.04) | (SMD ‐1.58; 95% CI ‐2.16 to ‐0.99) | ‐ | (SMD ‐1.05; 95% CI ‐2.04 to ‐0.06) |
| 14 Mycophenolic acid ointment | Effect size [CI] | ‐ | (SMD ‐1.44; 95% CI ‐2.67 to ‐0.22) | ‐ | ‐ | (SMD ‐1.44; 95% CI ‐2.67 to ‐0.22) |
| 15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | Effect size [CI] | ‐ | (SMD 0.08; 95% CI ‐0.60 to 0.75) | ‐ | ‐ | (SMD 0.08; 95% CI ‐0.60 to 0.75) |
| 16 Nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.20; 95% CI ‐0.60 to 0.20) | ‐ | ‐ | (SMD ‐0.20; 95% CI ‐0.60 to 0.20) |
| 17 Oleum horwathiensis | Effect size [CI] | (SMD ‐0.02; 95% CI ‐0.63 to 0.58) | (SMD ‐0.77; 95% CI ‐1.40 to ‐0.14) | ‐ | ‐ | (SMD ‐0.02; 95% CI ‐0.63 to 0.58) |
| 18 Omega‐3‐polyunsaturated fatty acids ointment | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 19 Platelet aggregation activating factor (PAF) (Ro 24‐0238) | Effect size [CI] | (SMD ‐0.07; 95% CI ‐0.50 to 0.37) | ‐ | ‐ | (SMD ‐0.07; 95% CI ‐0.50 to 0.37) | |
| 20 Polymyxin B cream, 200,000 U/g | Effect size [CI] | ‐ | (SMD 0.13; 95% CI ‐0.59 to 0.85) | ‐ | ‐ | (SMD 0.13; 95% CI ‐0.59 to 0.85) |
| 21 PTH (1‐34) in Novasome A® liposomal cream, BD | Effect size [CI] | ‐ | (SMD ‐2.31; 95% CI ‐3.26 to ‐1.36) | ‐ | ‐ | (SMD ‐2.31; 95% CI ‐3.26 to ‐1.36) |
| 22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | Effect size [CI] | ‐ | (SMD ‐0.39; 95% CI ‐0.98 to 0.21) | ‐ | ‐ | (SMD ‐0.39; 95% CI ‐0.98 to 0.21) |
| 23 Tacrolimus ointment | Effect size [CI] | ‐ | (SMD 0.06; 95% CI ‐0.52 to 0.63) | ‐ | ‐ | (SMD 0.06; 95% CI ‐0.52 to 0.63) |
| 24 Tar | Effect size [CI] | ‐ | (SMD ‐0.45; 95% CI ‐1.11 to 0.22) | ‐ | ‐ | (SMD ‐0.45; 95% CI ‐1.11 to 0.22) |
| 25 Tazarotene | Effect size [CI] | ‐ | (SMD ‐0.86; 95% CI ‐1.11 to ‐0.62) | ‐ | ‐ | (SMD ‐0.86; 95% CI ‐1.11 to ‐0.62) |
| 26 Theophylline 1% ointment, BD | Effect size [CI] | ‐ | ‐ | (SMD ‐2.87; 95% CI ‐4.13 to ‐1.62) | ‐ | (SMD ‐2.87; 95% CI ‐4.13 to ‐1.62) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 364 | 907 | 529 | 105 | 1450 |
| ‐ | Between‐patient design | 4 | 5 | 8 | 2 | 12 |
| ‐ | Within‐patient design | 4 | 12 | 1 | 0 | 14 |
| ‐ | Treatment duration | 3 wks to 12 wks | 3 wks to 12 wks | 2 wks to 12 wks | 3 wks to 12 wks | 2 wks to 12 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. betamethasone dipropionate | Effect size [CI] | (SMD 0.43; 95% CI 0.28 to 0.58) | ‐ | (SMD 0.36; 95% CI 0.22 to 0.51) | ‐ | (SMD 0.43; 95% CI 0.28 to 0.58) |
| 02 Calcipotriol vs. betamethasone valerate | Effect size [CI] | (SMD ‐0.02; 95% CI ‐0.21 to 0.17) | (SMD ‐0.26; 95% CI ‐0.41 to ‐0.11) | (SMD ‐0.12; 95% CI ‐0.22 to ‐0.02) | (SMD ‐0.26; 95% CI ‐0.38 to ‐0.14) | (SMD ‐0.12; 95% CI ‐0.26 to 0.02) |
| 03 Calcipotriol vs. desoxymetasone | Effect size [CI] | ‐ | ‐ | (SMD 0.15; 95% CI ‐0.73 to 1.02) | ‐ | (SMD 0.15; 95% CI ‐0.73 to 1.02) |
| 04 Calcipotriol vs. diflorasone diacetate | Effect size [CI] | (SMD 0.27; 95% CI 0.02 to 0.52) | (SMD 0.40; 95% CI 0.15 to 0.65) | ‐ | ‐ | (SMD 0.27; 95% CI 0.02 to 0.52) |
| 05 Calcipotriol vs. fluocinonide | Effect size [CI] | (SMD ‐0.58; 95% CI ‐0.99 to ‐0.18) | (SMD ‐0.50; 95% CI ‐0.92 to ‐0.07) | ‐ | ‐ | (SMD ‐0.58; 95% CI ‐0.99 to ‐0.18) |
| 06 Calcitriol vs. betamethasone dipropionate | Effect size [CI] | (SMD 0.21; 95% CI ‐0.04 to 0.45) | (SMD 0.27; 95% CI 0.02 to 0.51) | (SMD 0.39; 95% CI 0.14 to 0.63) | ‐ | (SMD 0.21; 95% CI ‐0.04 to 0.45) |
| 07 Calcitriol vs. betamethasone valerate | Effect size [CI] | (SMD ‐0.19; 95% CI ‐0.91 to 0.53) | ‐ | ‐ | ‐ | (SMD ‐0.19; 95% CI ‐0.91 to 0.53) |
| 08 Tacalcitol vs. betamethasone valerate | Effect size [CI] | ‐ | (SMD 0.41; 95% CI 0.09 to 0.74) | ‐ | ‐ | (SMD 0.41; 95% CI 0.09 to 0.74) |
| All treatments | Effect size [CI]; I² statistic | (SMD 0.17; 95% CI ‐0.04 to 0.37); I² statistic: 83.4% | (SMD 0.11; 95% CI ‐0.22 to 0.44); I² statistic: 86.7% | (SMD 0.12; 95% CI ‐0.07 to 0.32); I² statistic: 86.2% | (SMD ‐0.26; 95% CI ‐0.38 to ‐0.14); I² statistic: 0% | (SMD 0.11; 95% CI ‐0.07 to 0.30); I² statistic: 85.6% |
| ‐ | No. participants | 2655 | 891 | 3185 | 738 | 3542 |
| ‐ | Between‐patient design | 7 | 2 | 7 | 1 | 9 |
| ‐ | Within‐patient design | 1 | 4 | 2 | 1 | 5 |
| ‐ | Treatment duration | 3 wks to 8 wks | 3 wks to 6 wks | 4 wks to 8 wks | 6 wks | 3 wks to 8 wks |
| Sensitivity analyses | Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.17; 95% CI ‐0.20 to 0.54) |
| ‐ | Between‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.11 to 0.31) |
| ‐ | correlation coefficient (rho) = 0 All trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.08 to 0.28); |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.10; 95% CI ‐0.07 to 0.28) |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.11; 95% CI ‐0.07 to 0.29) |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD 0.12; 95% CI ‐0.06 to 0.30) |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. clobetasol propionate | Effect size [CI] | (SMD 0.19; 95% CI ‐0.42 to 0.80) | ‐ | (SMD ‐0.32; 95% CI ‐0.95 to 0.30) | (SMD 0.42; 95% CI ‐0.20 to 1.03) | (SMD ‐0.06; 95% CI ‐0.57 to 0.44); I² statistic: 25.7% |
| All treatments | No. participants | 42 | 0 | 40 | 42 | 82 |
| ‐ | Between‐patient design | 1 | 0 | 1 | 1 | 2 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 2 wks | ‐ | 6 wks | 2 wks | 2 wks to 6 wks |
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | Effect size [CI] | (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27) | ‐ | (SMD ‐0.44; 95% CI ‐0.55 to ‐0.33) | ‐ | (SMD ‐0.40; 95% CI ‐0.52 to ‐0.27) |
| 02 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | Effect size [CI] | ‐ | (SMD 0.45; 95% CI 0.09 to 0.81) | ‐ | ‐ | (SMD 0.45; 95% CI 0.09 to 0.81) |
| 03 Calcipotriol + clobetasol propionate vs. clobetasol propionate | Effect size [CI] | (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15) | ‐ | ‐ | (SMD ‐0.28; 95% CI ‐0.80 to 0.24) | (SMD ‐0.69; 95% CI ‐1.22 to ‐0.15) |
| ‐ | Effect size [CI], I² | (SMD ‐0.41; 95% CI ‐0.53 to ‐0.29); I² statistic: 32.0% | (SMD 0.45; 95% CI 0.09 to 0.81): I² statistic: NA | (SMD ‐0.44; 95% CI ‐0.55 to ‐0.33) I² statistic: 22.4% | (SMD ‐0.28; 95% CI ‐0.80 to 0.24) I² statistic: NA | (SMD ‐0.26; 95% CI ‐0.52 to ‐0.00); I² statistic: 84.4% |
| ‐ | No. participants | 1991 | 122 | 1876 | 65 | 2113 |
| ‐ | Between‐patient design | 4 | 1 | 3 | 1 | 5 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 2 wks to 8 wks | 4 wks | 4 wks to 8 wks | 2 wks | 2 wks to 8 wks |
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. dithranol | Effect size [CI] | (SMD ‐0.43; 95% CI ‐0.85 to ‐0.01) | (SMD ‐0.54; 95% CI ‐1.16 to 0.08) | (SMD 0.73; 95% CI ‐0.55 to 2.00) | (SMD ‐0.05; 95% CI ‐0.90 to 0.80) | (SMD 0.07; 95% CI ‐0.57 to 0.71) |
| 02 Calcitriol vs. dithranol | Effect size [CI] | (SMD 0.51; 95% CI 0.13 to 0.88) | (SMD 0.13; 95% CI ‐0.24 to 0.50) | (SMD ‐0.21; 95% CI ‐0.58 to 0.16) | ‐ | (SMD 0.51; 95% CI 0.13 to 0.88) |
| 03 Tacalcitol vs. dithranol | Effect size [CI] | ‐ | (SMD ‐0.18; 95% CI ‐0.60 to 0.25) | (SMD ‐0.07; 95% CI ‐0.50 to 0.36) | ‐ | (SMD ‐0.18; 95% CI ‐0.60 to 0.25) |
| All treatments | Effect size [CI], I² statistic | (SMD ‐0.24; 95% CI ‐0.72 to 0.25); I² statistic:93.0% | (SMD ‐0.27; 95% CI ‐0.73 to 0.20); I² statistic: 80.6% | (SMD 0.36; 95% CI ‐0.33 to 1.04); I² statistic: 94.5% | (SMD ‐0.05; 95% CI ‐0.90 to 0.80); I² statistic: 92.5% | (SMD 0.09; 95% CI ‐0.44 to 0.63); I² statistic: 94.9% |
| ‐ | No. participants | 1108 | 386 | 796 | 544 | 1284 |
| ‐ | Between‐patient design | 5 | 3 | 5 | 2 | 7 |
| ‐ | Within‐patient design | 0 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 8 wks to 12 wks | 4 wks to 8 wks | 8 wks to 12 wks | 8 wks to 12 wks | 4 wks to 12 wks |
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. calcitriol | Effect size [CI] | (SMD 0.00; 95% CI ‐0.25 to 0.25) | (SMD ‐0.32; 95% CI ‐0.57 to ‐0.07) | (SMD ‐1.11; 95% CI ‐2.22 to 0.01) | (SMD 0.04; 95% CI ‐0.21 to 0.29) | (SMD ‐0.41; 95% CI ‐1.46 to 0.64) |
| 02 Calcipotriol vs. tacalcitol | Effect size [CI] | (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21) | (SMD ‐0.45; 95% CI ‐0.68 to ‐0.22) | ‐ | ‐ | (SMD ‐0.47; 95% CI ‐0.73 to ‐0.21) |
| 03 Calcipotriol vs. maxacalcitol | Effect size [CI] | (SMD 0.43; 95% CI ‐0.12 to 0.98) | (SMD 0.13; 95% CI ‐0.41 to 0.68) | ‐ | ‐ | (SMD 0.43; 95% CI ‐0.12 to 0.98) |
| All treatments | Effect size [CI], I² | (SMD ‐0.06; 95% CI ‐0.51 to 0.38); I² statistic: 82.2% | (SMD ‐0.31; 95% CI ‐0.55 to ‐0.06); I² statistic: 46.9% | (SMD ‐1.11; 95% CI ‐2.22 to 0.01); I² statistic: NA | (SMD 0.04; 95% CI ‐0.21 to 0.29); I² statistic: NA | (SMD ‐0.17; 95% CI ‐0.62 to 0.27); I² statistic: 78.5% |
| ‐ | No. participants | 498 | 563 | 15 | 250 | 513 |
| ‐ | Between‐patient design | 2 | 2 | 1 | 1 | 3 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 8 wks to 12 wks | 8 wks to 12 wks | 8 wks | 12 wks | 8 wks to 12 wks |
| Sensitivity analyses | TSS data from Ji 2008 used in combined end point: 01 Calcipotriol vs. calcitriol | ‐ | ‐ | ‐ | (SMD ‐0.52; 95% CI ‐1.19 to 0.15; I² statistic: 44.9%) | |
| ‐ | TSS data from Ji 2008 used in combined end point: all treatments | ‐ | ‐ | ‐ | (SMD ‐0.28; 95% CI ‐0.66 to 0.10; I² statistic: 70.6%) |
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol BD vs. calcipotriol OM, BMD ON | Effect size [CI] | (SMD 0.56; 95% CI 0.23 to 0.88) | ‐ | (SMD 0.46; 95% CI 0.10 to 0.82) | ‐ | (SMD 0.56; 95% CI 0.23 to 0.88) |
| 02 Calcipotriol OD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.66; 95% CI 0.31 to 1.02) | ‐ | (SMD 0.67; 95% CI 0.23 to 1.11) | ‐ | (SMD 0.66; 95% CI 0.31 to 1.02) |
| 03 Calcipotriol BD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.27; 95% CI 0.06 to 0.48) | (SMD 0.25; 95% CI 0.03 to 0.48) | (SMD 0.52; 95% CI 0.38 to 0.67) | ‐ | (SMD 0.43; 95% CI 0.20 to 0.66) |
| 04 Calcipotriol BD vs. combined calcipotriol + BMD BD | Effect size [CI] | (SMD 0.66; 95% CI 0.40 to 0.93) | ‐ | (SMD 0.64; 95% CI 0.46 to 0.83) | ‐ | (SMD 0.66; 95% CI 0.40 to 0.93) |
| 05 Calcipotriol BD vs. calcipotriol OM, BMV ON | Effect size [CI] | (SMD 0.27; 95% CI ‐0.19 to 0.74) | ‐ | (SMD 0.43; 95% CI ‐0.07 to 0.93) | ‐ | (SMD 0.27; 95% CI ‐0.19 to 0.74) |
| 06 Calcipotriol BD vs. calcipotriol OM, clobetasone butyrate ON | Effect size [CI] | (SMD 0.27; 95% CI 0.05 to 0.48) | ‐ | (SMD 0.17; 95% CI ‐0.04 to 0.38) | ‐ | (SMD 0.27; 95% CI 0.05 to 0.48) |
| 07 Calcipotriol BD vs. calcipotriol BD + clobetasol propionate BD | Effect size [CI] | (SMD 0.88; 95% CI 0.34 to 1.42) | ‐ | ‐ | (SMD 0.70; 95% CI 0.16 to 1.23) | (SMD 0.88; 95% CI 0.34 to 1.42) |
| 08 Calcipotriol BD vs. calcipotriol OM, diflucortolone valerate ON | Effect size [CI] | ‐ | ‐ | (SMD 0.08; 95% CI ‐0.29 to 0.44) | ‐ | (SMD 0.08; 95% CI ‐0.29 to 0.44) |
| 09 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | Effect size [CI] | ‐ | ‐ | (SMD 0.53; 95% CI ‐0.11 to 1.18) | ‐ | (SMD 0.53; 95% CI ‐0.11 to 1.18) |
| 10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | Effect size [CI] | (SMD 0.14; 95% CI ‐0.06 to 0.33) | ‐ | (SMD 0.08; 95% CI ‐0.11 to 0.28) | ‐ | (SMD 0.14; 95% CI ‐0.06 to 0.33) |
| 11 calcitriol BD vs. diflucortolone valerate OM, calcitriol ON | Effect size [CI] | ‐ | ‐ | (SMD 0.24; 95% CI ‐0.09 to 0.57) | ‐ | (SMD 0.24; 95% CI ‐0.09 to 0.57) |
| 12 Tacalcitol OD vs. combined calcipotriol + BMD OD | Effect size [CI] | (SMD 0.48; 95% CI 0.26 to 0.70) | ‐ | (SMD 0.47; 95% CI 0.25 to 0.69) | (SMD 0.46; 95% CI 0.24 to 0.68) | (SMD 0.48; 95% CI 0.26 to 0.70) |
| All treatments | Effect size [CI], I² statistic | (SMD 0.48; 95% CI 0.32 to 0.65), I² statistic: 86.9% | (SMD 0.25; 95% CI 0.03 to 0.48) | (SMD 0.47; 95% CI 0.34 to 0.59), I² statistic: 82.3% | (SMD 0.49; 95% CI 0.29 to 0.69), I² statistic: 0% | (SMD 0.46; 95% CI 0.33 to 0.59), I² statistic: 83.3% |
| ‐ | No. participants | 4791 | 301 | 5703 | 399 | 5856 |
| ‐ | Between‐patient design | 11 | 1 | 15 | 2 | 16 |
| ‐ | Within‐patient design | 0 | 0 | 1 | 0 | 1 |
| ‐ | Treatment duration | 2 wks to 8 wks | 4 wks | 2 wks to 12 wks | 2 wks to 8 wks | 2 wks to 12 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD ‐0.12; 95% CI ‐0.29 to 0.04) | ‐ | (SMD ‐0.04; 95% CI ‐0.19 to 0.11) | (SMD ‐0.14; 95% CI ‐0.30 to 0.02) | (SMD ‐0.12; 95% CI ‐0.29 to 0.04) |
| 02 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | Effect size [CI] | ‐ | ‐ | (SMD 0.29; 95% CI ‐0.04 to 0.62) | ‐ | (SMD 0.29; 95% CI ‐0.04 to 0.62) |
| 03 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.13; 95% CI ‐0.04 to 0.29) | ‐ | (SMD 0.10; 95% CI ‐0.05 to 0.25) | (SMD 0.10; 95% CI ‐0.06 to 0.26) | (SMD 0.13; 95% CI ‐0.04 to 0.29) |
| 04 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD 0.60; 95% CI 0.18 to 1.02) | (SMD 0.63; 95% CI 0.21 to 1.05) | ‐ | ‐ | (SMD 0.60; 95% CI 0.18 to 1.02) |
| 05 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol BD (4 wks) | Effect size [CI] | ‐ | ‐ | (SMD 0.66; 95% CI 0.01 to 1.32) | ‐ | (SMD 0.66; 95% CI 0.01 to 1.32) |
| 06 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol BD (w/dy), halometasone (w/e) (2 wks); then calcipotriol BD (2wks) | Effect size [CI] | (SMD 0.41; 95% CI ‐0.05 to 0.86) | ‐ | (SMD 1.13; 95% CI 0.64 to 1.62) | ‐ | (SMD 0.41; 95% CI ‐0.05 to 0.86) |
| 07 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol BD (to wk12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol BD (to wk12) | Effect size [CI] | (SMD ‐0.19; 95% CI ‐0.54 to 0.16) | ‐ | (SMD ‐0.27; 95% CI ‐0.62 to 0.09) | ‐ | (SMD ‐0.19; 95% CI ‐0.54 to 0.16) |
| 08 Combined calcipotriol + BMD (4 wks); then placebo ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment BD (8 wks) | Effect size [CI] | (SMD 0.27; 95% CI 0.12 to 0.41) | ‐ | (SMD 0.25; 95% CI 0.10 to 0.39) | (SMD 0.28; 95% CI 0.13 to 0.42) | (SMD 0.27; 95% CI 0.12 to 0.41) |
| 09 Combined calcipotriol + BMD (4 wks); then placebo ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.51; 95% CI 0.37 to 0.66) | ‐ | (SMD 0.59; 95% CI 0.45 to 0.74) | (SMD 0.71; 95% CI 0.56 to 0.85) | (SMD 0.51; 95% CI 0.37 to 0.66) |
| 10 combined calcipotriol + BMD (4 wks); then calcipotriol ointment BD (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.26; 95% CI 0.11 to 0.40) | ‐ | (SMD 0.30; 95% CI 0.16 to 0.45) | (SMD 0.44; 95% CI 0.29 to 0.58) | (SMD 0.26; 95% CI 0.11 to 0.40) |
| 11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | Effect size [CI] | (SMD 0.24; 95% CI 0.08 to 0.40) | ‐ | (SMD 0.15; 95% CI ‐0.01 to 0.30) | (SMD 0.23; 95% CI 0.07 to 0.39) | (SMD 0.24; 95% CI 0.08 to 0.40) |
| 12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD 0.54; 95% CI 0.36 to 0.72) | ‐ | (SMD 0.49; 95% CI 0.31 to 0.67) | (SMD 0.54; 95% CI 0.36 to 0.72) | (SMD 0.54; 95% CI 0.36 to 0.72) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 2755 | 46 | 2991 | 2508 | 2936 |
| ‐ | Between‐patient design | 6 | 0 | 8 | 4 | 8 |
| ‐ | Within‐patient design | 1 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 6 wks to 12 wks | 6 wks | 2 wks to 12 wks | 8 wks to 12 wks | 2 wks to 12 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | Effect size [CI] | (SMD ‐0.09; 95% CI ‐0.36 to 0.18) | ‐ | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.36 to 0.18) |
| 02 Combined calcipotriol+BMD (52 wks) vs. combined calcipotriol+ BMD (4 wks); then calcipotriol (48 wks) | Effect size [CI] | (SMD ‐0.18; 95% CI ‐0.47 to 0.10) | ‐ | ‐ | ‐ | (SMD ‐0.18; 95% CI ‐0.47 to 0.10) |
| 03 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | Effect size [CI] | (SMD ‐0.09; 95% CI ‐0.37 to 0.19) | ‐ | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.37 to 0.19) |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 297 | 0 | 0 | 0 | 297 |
| ‐ | Between‐patient design | 1 | 0 | 0 | 0 | 1 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 52 wks | ‐ | ‐ | ‐ | 52 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. coal tar | Effect size [CI] | (SMD ‐0.53; 95% CI ‐1.74 to 0.68) | ‐ | (SMD ‐0.10; 95% CI ‐1.54 to 1.35) | (SMD ‐0.10; 95% CI ‐1.54 to 1.35) | (SMD ‐0.53; 95% CI ‐1.74 to 0.68) |
| 02 Calcipotriol vs. coal tar polytherapy | Effect size [CI] | (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31) | (SMD ‐0.51; 95% CI ‐0.86 to ‐0.16) | (SMD ‐0.63; 95% CI ‐1.06 to ‐0.20) | (SMD ‐0.63; 95% CI ‐1.06 to ‐0.20) | (SMD ‐0.59; 95% CI ‐0.87 to ‐0.31) |
| 03 Calcipotriol vs. nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD ‐0.09; 95% CI ‐0.49 to 0.31) | ‐ | ‐ | (SMD ‐0.09; 95% CI ‐0.49 to 0.31) |
| 04 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, BD | Effect size [CI] | ‐ | (SMD 0.19; 95% CI ‐0.14 to 0.52) | ‐ | ‐ | (SMD 0.19; 95% CI ‐0.14 to 0.52) |
| 05 Calcipotriol vs. corticosteroid + salicylic acid | Effect size [CI] | (SMD ‐0.06; 95% CI ‐0.33 to 0.22) | ‐ | (SMD ‐0.05; 95% CI ‐0.36 to 0.26) | (SMD ‐0.49; 95% CI ‐0.79 to ‐0.20) | (SMD ‐0.05; 95% CI ‐0.26 to 0.15) |
| 06 Calcipotriol vs. propylthiouracil cream | Effect size [CI] | ‐ | ‐ | (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25) | ‐ | (SMD ‐2.24; 95% CI ‐3.23 to ‐1.25) |
| 07 Calcipotriol vs. tacrolimus ointment | Effect size [CI] | ‐ | (SMD ‐0.35; 95% CI ‐1.51 to 0.81) | (SMD ‐0.13; 95% CI ‐0.51 to 0.24) | (SMD ‐0.55; 95% CI ‐1.28 to 0.17) | |
| 08 Calcipotriol vs. tazarotene | Effect size [CI] | (SMD ‐0.22; 95% CI ‐0.60 to 0.16) | (SMD ‐0.05; 95% CI ‐0.33 to 0.23) | ‐ | (SMD ‐0.35; 95% CI ‐0.99 to 0.29) | (SMD ‐0.10; 95% CI ‐0.35 to 0.16) |
| 09 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| 10 Calcipotriol vs. vitamin B12 cream | Effect size [CI] | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) | ‐ | (SMD ‐0.01; 95% CI ‐0.78 to 0.75) | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) | (SMD ‐0.55; 95% CI ‐1.33 to 0.24) |
| 11 Head‐to‐head vitamin D alone or in combination: dosing | Effect size [CI] | (SMD ‐0.24; 95% CI ‐0.38 to ‐0.09) | ‐ | (SMD ‐0.12; 95% CI ‐0.25 to 0.00) | ‐ | (SMD ‐0.20; 95% CI ‐0.32 to ‐0.07) |
| 12 Head‐to‐head vitamin D alone or in combination: occlusion | Effect size [CI] | ‐ | (SMD ‐0.18; 95% CI ‐2.04 to 1.68) | ‐ | ‐ | (SMD ‐0.18; 95% CI ‐2.04 to 1.68) |
| All treatments | (not pooled) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 1386 | 898 | 1228 | 456 | 2364 |
| ‐ | Between‐patient design | 8 | 5 | 6 | 3 | 13 |
| ‐ | Within‐patient design | 2 | 2 | 3 | 3 | 6 |
| ‐ | Treatment duration | 4 wks to 12 wks | 6 wks to 12 wks | 4 wks to 12 wks | 4 wks to 12 wks | 4 wks to 12 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Betamethasone valerate 0.1%, OD | Effect size [CI] | ‐ | ‐ | (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) | ‐ | (SMD ‐2.83; 95% CI ‐3.79 to ‐1.88) |
| 02 Calcipotriol ointment, OD | Effect size [CI] | ‐ | ‐ | (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40) | ‐ | (SMD ‐1.08; 95% CI ‐1.77 to ‐0.40) |
| 03 Pimecrolimus cream, 1% OD/BD | Effect size [CI] | (SMD ‐1.07; 95% CI ‐1.69 to ‐0.45) | (SMD ‐1.37; 95% CI ‐1.95 to ‐0.79) | (SMD ‐0.62; 95% CI ‐1.27 to 0.02) | (SMD ‐0.65; 95% CI ‐1.24 to ‐0.06) | (SMD ‐0.86; 95% CI ‐1.30 to ‐0.41) |
| 04 Tacrolimus ointment 0.1%, BD | Effect size [CI] | ‐ | ‐ | ‐ | ‐ | ‐ |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 47 | 57 | 75 | 47 | 122 |
| ‐ | Between‐patient design | 1 | 1 | 1 | 1 | 2 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 8 wks | 8 wks | 4 wks | 8 wks | 4 wks to 8 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Calcipotriol vs. BMV | Effect size [CI] | ‐ | ‐ | (SMD 2.02; 95% CI 1.20 to 2.84) | ‐ | (SMD 2.02; 95% CI 1.20 to 2.84) |
| 02 Calcipotriol vs. calcipotriol + hydrocortisone | Effect size [CI] | (SMD 0.30; 95% CI 0.11 to 0.50) | ‐ | (SMD 0.32; 95% CI 0.12 to 0.51) | ‐ | (SMD 0.30; 95% CI 0.11 to 0.50) |
| 03 Calcipotriol vs. calcitriol | Effect size [CI] | ‐ | (SMD 0.61; 95% CI 0.28 to 0.94) | ‐ | ‐ | (SMD 0.61; 95% CI 0.28 to 0.94) |
| 04 Calcipotriol vs. pimecrolimus | Effect size [CI] | ‐ | ‐ | (SMD ‐0.53; 95% CI ‐1.17 to 0.11) | (SMD ‐0.53; 95% CI ‐1.17 to 0.11) | |
| 05 Calcitriol vs. tacrolimus | Effect size [CI] | (SMD 0.42; 95% CI ‐0.15 to 0.98) | (SMD 0.29; 95% CI ‐0.27 to 0.85) | ‐ | ‐ | (SMD 0.42; 95% CI ‐0.15 to 0.98) |
| All treatments | (no pooling) | ‐ | ‐ | ‐ | ‐ | ‐ |
| ‐ | No. participants | 457 | 124 | 464 | 0 | 588 |
| ‐ | Between‐patient design | 2 | 1 | 2 | 0 | 3 |
| ‐ | Within‐patient design | 0 | 1 | 0 | 0 | 1 |
| ‐ | Treatment duration | 6 wks to 8 wks | 6 wks | 4 wks to 8 wks | 0 | 4 wks to 8 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Vitamin D: calcipotriol | Effect size [CI] | (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16) | (SMD ‐0.44; 95% CI ‐0.64 to ‐0.25) | ‐ | (SMD ‐0.66; 95% CI ‐1.28 to ‐0.05) | (SMD ‐0.72; 95% CI ‐1.28 to ‐0.16) |
| 02 Potent steroid: betamethasone dipropionate | Effect size [CI] | (SMD ‐1.09; 95% CI ‐1.29 to ‐0.90) | (SMD ‐1.00; 95% CI ‐1.19 to ‐0.81) | ‐ | (SMD ‐1.23; 95% CI ‐1.43 to ‐1.03) | (SMD ‐1.09; 95% CI ‐1.29 to ‐0.90) |
| 03 Potent steroid: betamethasone valerate | Effect size [CI] | ‐ | (SMD ‐1.40; 95% CI ‐1.75 to ‐1.05) | ‐ | ‐ | (SMD ‐1.40; 95% CI ‐1.75 to ‐1.05) |
| 04 Very potent steroid: amcinonide | Effect size [CI] | (SMD ‐1.42; 95% CI ‐1.80 to ‐1.04) | (SMD ‐1.58; 95% CI ‐1.98 to ‐1.18) | ‐ | (SMD ‐0.97; 95% CI ‐1.33 to ‐0.61) | (SMD ‐1.42; 95% CI ‐1.80 to ‐1.04) |
| 05 Very potent steroid: clobetasol propionate | Effect size [CI] | (SMD ‐1.73; 95% CI ‐1.99 to ‐1.48) | (SMD ‐1.53; 95% CI ‐1.77 to ‐1.28) | ‐ | ‐ | (SMD ‐1.57; 95% CI ‐1.81 to ‐1.34) |
| 06 Very potent steroid: halcinonide | Effect size [CI] | (SMD ‐1.11; 95% CI ‐1.69 to ‐0.53) | ‐ | ‐ | ‐ | (SMD ‐1.11; 95% CI ‐1.69 to ‐0.53) |
| 07 Vitamin D in combination: calcipotriol + BMD | Effect size [CI] | (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32) | (SMD ‐0.92; 95% CI ‐1.42 to ‐0.43) | ‐ | (SMD ‐1.00; 95% CI ‐1.79 to ‐0.22) | (SMD ‐0.97; 95% CI ‐1.61 to ‐0.32) |
| 08 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | Effect size [CI] | (SMD ‐1.48; 95% CI ‐2.50 to ‐0.47) | (SMD ‐1.15; 95% CI ‐2.11 to ‐0.19) | ‐ | ‐ | (SMD ‐1.48; 95% CI ‐2.50 to ‐0.47) |
| 09 Other treatment: ciclopirox olamine shampoo | Effect size [CI] | ‐ | (SMD ‐0.07; 95% CI ‐0.82 to 0.68) | ‐ | (SMD ‐0.11; 95% CI ‐0.86 to 0.64) | (SMD ‐0.07; 95% CI ‐0.82 to 0.68) |
| 10 Other treatment: fluocinolone acetonide, plus occlusion | Effect size [CI] | (SMD ‐1.22; 95% CI ‐1.69 to ‐0.76) | (SMD ‐0.89; 95% CI ‐1.34 to ‐0.44) | ‐ | ‐ | (SMD ‐1.22; 95% CI ‐1.69 to ‐0.76) |
| 11 Other treatment: salicylic acid | Effect size [CI] | (SMD ‐0.86; 95% CI ‐1.79 to 0.06) | (SMD ‐0.57; 95% CI ‐1.47 to 0.32) | ‐ | ‐ | (SMD ‐0.86; 95% CI ‐1.79 to 0.06) |
| All treatments | No. participants | 2472 | 2897 | 0 | 1875 | 3011 |
| ‐ | Between‐patient design | 9 | 12 | 0 | 5 | 13 |
| ‐ | Within‐patient design | 1 | 0 | 0 | 0 | 1 |
| ‐ | Treatment duration | 2 wks to 8 wks | 2 wks to 8 wks | ‐ | 3 wks to 8 wks | 2 wks to 8 wks |
| Sensitivity analysis: potent corticosteroids | Effect size [CI]; I² statistic | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.18; 95% CI ‐1.40 to ‐0.96); I² statistic: 19.9% |
| Sensitivity analysis: very potent corticosteroids | Effect size [CI]; I² statistic | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.51; 95% CI ‐1.70 to ‐1.31); I² statistic: 37.5% |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | Effect size [CI] | (SMD 0.48; 95% CI 0.32 to 0.64) | (SMD 0.45; 95% CI 0.28 to 0.63) | ‐ | (SMD 0.56; 95% CI 0.31 to 0.81) | (SMD 0.48; 95% CI 0.32 to 0.64) |
| 02 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | Effect size [CI] | (SMD 0.37; 95% CI 0.20 to 0.55) | (SMD 0.09; 95% CI ‐0.09 to 0.27) | ‐ | (SMD 0.41; 95% CI 0.22 to 0.59) | (SMD 0.37; 95% CI 0.20 to 0.55) |
| 03 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | Effect size [CI] | ‐ | (SMD 0.37; 95% CI 0.05 to 0.69) | ‐ | ‐ | (SMD 0.37; 95% CI 0.05 to 0.69) |
| 04 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | Effect size [CI] | (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10) | (SMD ‐0.19; 95% CI ‐0.27 to ‐0.11) | ‐ | (SMD ‐0.17; 95% CI ‐0.25 to ‐0.09) | (SMD ‐0.18; 95% CI ‐0.26 to ‐0.10) |
| 05 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | Effect size [CI] | (SMD 0.64; 95% CI 0.44 to 0.84) | (SMD 0.70; 95% CI 0.56 to 0.84) | ‐ | (SMD 0.84; 95% CI 0.61 to 1.08) | (SMD 0.64; 95% CI 0.44 to 0.84) |
| 06 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | Effect size [CI] | (SMD ‐0.24; 95% CI ‐0.73 to 0.25) | (SMD ‐0.30; 95% CI ‐0.84 to 0.24) | ‐ | ‐ | (SMD ‐0.45; 95% CI ‐0.92 to 0.02) |
| All treatments | No. participants | 5175 | 4877 | 0 | 3742 | 5413 |
| ‐ | Between‐patient design | 10 | 11 | 0 | 6 | 12 |
| ‐ | Within‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Treatment duration | 4 wks to 52 wks | 4 wks to 8 wks | 0 | 4 wks to 8 wks | 4 wks to 52 wks |
| For acronyms, see Table 1. | ||||||
| Subcategory | Measure | 01 IAGI/IGA | 02 TSS | 03 PASI | 04 PAGI/PGA | 05 Combined end point |
| 01 Dithranol | Effect size [CI], N, I² statistic | ‐ | (SMD ‐1.06; 95% CI ‐1.66 to ‐0.46); I² statistic: 37.4% | ‐ | ‐ | (SMD ‐1.06; 95% CI ‐1.66 to ‐0.46); I² statistic: 37.4% |
| ‐ | No. participants | 0 | 47 | 0 | 0 | 47 |
| ‐ | Between‐patient design | 0 | 0 | 0 | 0 | 0 |
| ‐ | Within‐patient design | 0 | 3 | 0 | 0 | 3 |
| ‐ | Treatment duration | 3 wks to 8 wks | ‐ | ‐ | 3 wks to 8 wks | |
| ‐ | correlation coefficient (rho) = 0 All trials | ‐ | ‐ | ‐ | (SMD ‐0.98; 95% CI ‐1.56 to ‐0.41) I² statistic: 13.9% | |
| ‐ | rho = 0 Btw‐patient trials rho = 0.25 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.05; 95% CI ‐1.67 to ‐0.44) I² statistic: 35.4% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.50 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.12; 95% CI ‐1.75 to ‐0.48) I² statistic: 56.9% |
| ‐ | rho = 0 Btw‐patient trials rho = 0.75 Within‐patient trials | ‐ | ‐ | ‐ | ‐ | (SMD ‐1.17; 95% CI ‐1.81 to ‐0.52) I² statistic: 78.5% |
| For acronyms, see Table 1. | ||||||
| Study | Methods | Participants | Intervention(s) | Outcomes (AEs) | Summary findings | Notes | Allocation concealment |
| DESIGN: between‐patient patient delivery Concealment: unclear | N: 26 | Clobetasol propionate 0.05% shampoo, OD. Applied to dry scalp, rinsed off after 15 minutes Clobetasol propionate 0.05% gel, OD. Applied to dry scalp and left in. | Serum cortisol atrophogenicity (ultrasound measurement of skin thickness (epidermis + dermis) (mm), averaged over 3 sites of the scalp) ocular safety (intraocular pressure) DSS (10‐pt; sum of erythema, adherent desquamation, and plaque thickening; 0 (none) to 3 (severe) with half‐point ratings permitted) Patient‐reported ocular stinging (0 to 3) Compliance | Neither formulation had an impact on ocular safety, no report of ocular stinging. LAE: HPA suppression: Atrophy: Decrease in skin thickness from baseline: mean difference: Efficacy results of the 2 formulations were similar. Compliance with protocol was good in both groups. | Exploratory safety study Sponsored by Galderma Laboratories | Unclear | |
| DESIGN: within‐patient ALLOCATION: non‐random | N: 202 | Calcipotriol scalp solution 50 mcg/ml BD | Local AEs: serum calcium | Local AEs: | Sponsored by Leo Pharmaceuticals | Not applicable | |
| DESIGN: uncontrolled study Concealment: NA | STUDY A: | Study A: calcipotriol ointment 50 mcg/g BD up to 100 g/wk | Local AEs: | Study A: no significant trend in urine calcium excretion | Sponsorship not reported | Not applicable | |
| DESIGN: uncontrolled study ALLOCATION: non‐random Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: not described | N: 28 | STUDY A: 200 g calcipotriol ointment 50 mcg/g (wk 1) plus 300 g 50 mcg/g calcipotriol (wk 2) | Local AEs: | 5 participants developed hypercalcaemia during treatment, all had received a dose > 5 g/kg | Sponsorship not reported | Not applicable | |
| DESIGN: uncontrolled study | N: 305 % white: 91.8% | Clobetasol propionate 0.05% spray BD (2 to 4 wks); treatment responders (ODS < = 3) then treated with calcitriol 3 mg/g ointment (8 wks) | Pruritis, telangiectasias, burning/stinging (0 to 3), skin atrophy, folliculitis Overall disease severity (ODS) (5‐pt: 0 = clear to 4 = severe/very severe) based on erythema, scaling, and plaque elevation. Treatment success (change from baseline ODS > = 1 at wk 12)
| At 4 wks: skin atrophy 7/285 telangiectasias 2/285 stinging/Burning 39/285 folliculitis 11/285
At 12 wks: skin atrophy 2/235 telangiectasias 5/235 stinging/Burning 35/235 folliculitis 3/235
Any adverse event: 100/305
| Sponsored by Galderma laboratories | Not applicable | |
| DESIGN: within‐patient | N: 14 EXCLUSION CRITERIA: NR | Clobetasol propionate 0.05% ointment, BD Betamethasone valerate 0.1% ointment, BD | Local AEs: NR | Quantities used by study participants were small (mean: 7 g/wk) | Sponsorship not reported | Unclear | |
| DESIGN: uncontrolled study patient delivery ALLOCATION: NA Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described | N: 257 | Calcitriol 3 mcg/g BD | Local AEs: | Local AEs: | Sponsored by Solvay‐Duphar BV | Not applicable | |
| DESIGN: between‐patient ALLOCATION: random | N: 78 EXCLUSION CRITERIA: hypercalcaemia, bone, thyroid or parathyroid disease; topical therapy within previous 2 wks; systemic/phototherapy within previous 8 wks | Calcipotriol 50 mcg/g ointment BD, up to 120 g/wk | Local AEs: not assessed | No adverse effects on bone metabolism or calcium | Sponsored by Bristol‐Myers Squibb | Unclear | |
| DESIGN: between‐patient (retrospective study) ALLOCATION: non‐random | N: 28 EXCLUSION CRITERIA: NR | Previous prolonged treatment with topical fluorinated steroids | Local AEs: light/electron microscopy for examination of basal keratinocyte herniation (BKH); layers of basement membrane | Local AEs: light microscopy revealed no between‐group differences. Electron microscopy revealed multi‐layered, fragmented and disorganised basal laminae in the steroid group, which appeared to be correlated with duration of treatment. Fragmentation was not observed in the control group | Sponsorship not reported | Not applicable | |
| DESIGN: between‐patient ALLOCATION: random Method of randomisation: NS | N: 40 | Betamethasone dipropionate (0.05%) in optimised vehicle BD; Clobetasol 17 propionate (0.05%) ointment BD | Local AEs: not assessed Systemic AEs: morning plasma cortisol levels; 24‐hr urine steroid levels; FBC, blood chemistry, urinalysis | Temporary reversible suppression of the hypothalamic‐pituitary‐adrenal axis in 8/40 participants | Sponsorship not reported; 1 author from Schering Corporation | Unclear | |
| DESIGN: within‐patient ALLOCATION: random BLINDING: double‐blind (participant/investigator) | N: 30 | Betamethasone dipropionate (0.05%) in optimised vehicle BD (BMD) | Local AEs: skin surface microscopic examination with photographic documentation; oil and magnifying (8 x) lens to detect 'preatrophy' (visibility of subpapillary vascular plexus caused by thinning of epidermis and papillary dermis) | Local AEs: no serious adverse events observed with either treatment. Preatrophy identified in 20% of involved plaques (BMD: 11/59; CP: 12/59) and was more likely in females. In the test area (non‐psoriatic skin), 5% of plaques showed preatrophy (BMD: 2/30; CP: 1/30). Preatrophy appeared to be usually reversible following treatment cessation. | Sponsored by Schering Corporation | Unclear | |
| Phase II | DESIGN: uncontrolled BLINDING: open WITHDRAWAL/DROPOUT: | N: 32 | Clobetasol 0.05% foam BD Clobetasol 0.05% ointment BD (= 7 g/day, up to 50 g/wk)
| Maximal plasma concentration of clobetasol propionate
| Higher but non‐significant levels of clobetasol in ointment group | Supported by Stiefel Laboratories, Inc. Review of phase II studies on AD and psoriasis (clobetasol foam) | Not applicable |
| Phase III | DESIGN: between‐patient | N: 497 | Clobetasol 0.05% foam BD Clobetasol 0.05% ointment BD Placebo foam BD Face, scalp, and intertriginous areas excluded from treatment
| Treatment‐related adverse events:
ISGA (investigator's static global assessment score)(scale NR) | Atrophy: C foam: 2% (N = 253) Burning: C foam: 2% (N = 253) All treatment‐related adverse events: C foam: 8% (N = 253)
| Supported by Stiefel Laboratories Inc. Review of phase III studies on AD and psoriasis (clobetasol foam). | Unclear |
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random BLINDING: open WITHDRAWAL/DROPOUT: described | N: 15 EXCLUSION CRITERIA: hypercalcaemia, impaired renal/hepatic function, daily receiving > 400 i.u. vitamin D | Calcipotriol ointment 50 mcg/g BD (max: 100 g/wk) | Local AEs: patient report of adverse events | Local AEs: | Sponsorship not reported. Leo Pharmaceuticals supplied study medication | Not applicable | |
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random | N: 157 INCLUSION CRITERIA: chronic plaque psoriasis; aged 18 to 70; BSA 7% to 20%; laboratory parameters normal at baseline EXCLUSION CRITERIA: pregnancy or risk thereof; topical antipsoriatic therapy within previous 2 wks; systemic antipsoriatic therapy within previous 6 wks; retinoids within previous 52 wks.; history of hyperparathyroidism; concomitant use of drugs affecting calcium metabolism | Tacalcitol ointment, 4 mcg/g OD. No control | Local AEs: participants asked about adverse events. Tolerability assessed by investigator (4‐pt: 1 = excellent to 4 = poor). | Local AEs: | Sponsored by Hermal/BHI, Germany | Not applicable | |
| DESIGN: between‐patient ALLOCATION: random. | N: 40 EXCLUSION CRITERIA: history of sensitivity to study ingredients; topical antipsoriatics within previous 2 wks; UVB/PUVA within previous 8 wks; history of hypercalcaemia, recurrent illness | Initial regimen: all participants received 2 wks of calcipotriol (OM), halobetasol ointment (ON) | Local AEs: treatment‐related adverse events | Local AEs: | Sponsored by Westwood Squibb Pharmaceuticals | Unclear | |
| DESIGN: between‐patient patient delivery ALLOCATION: random | N: 50 | Open‐label phase: tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment for 6 wks. Once daily initially, then 'tapered'. | Local AEs: no. steroid‐specific side‐effects | Local AEs: no steroid‐specific side‐effects | Sponsorship: not reported | Unclear | |
| DESIGN: uncontrolled BLINDING: open | N: 1423 | Clobetasol 0.05% foam BD, up to 50 g/wk either as monotherapy or adjunctive to existing therapy
| Erythema, peeling/scaling, dryness, stinging/burning (0 none to 3 severe). Telangiectasia, skin atrophy, pruritus, folliculitis (absent/present)
Also assessed efficacy and QoL | Erythema: 23.7% Telangiectasia, skin atrophy, folliculitis: present in less than 1% of participants (findings not reported separately) Pruritus: 5.7% | Clobex Spray Community‐Based Research Assessment (COBRA) Sponsorship not reported | Not applicable | |
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random | N: 160 | Tacalcitol ointment 20 mcg/g OD (max: 10 g/day) | Local AEs: treatment‐related adverse events | Local AEs: | Sponsorship: not reported | Not applicable | |
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random BLINDING: open | N: 203 | Calcipotriol 50 mcg/g ointment | Loca AEs: self report of adverse events. | Local AEs: | Sponsored by Leo Pharmaceuticals | Not applicable | |
| DESIGN: uncontrolled open study ALLOCATION: non‐random | N: 167 TD: 52 wks; FU: 52 wks LF: 39 (23.4%) BC: NA Age: 49 (range: 20 to 85) Gender (per cent men): 60% Severity: PASI (modified): 8.1 (6.7SD) INCLUSION CRITERIA: chronic plaque psoriasis; previous response to calcipotriol; managed by specialists EXCLUSION CRITERIA: pregnancy or risk thereof; abnormal serum calcium or phosphate; impaired hepatic/renal function; concomitant oral calcium/vitamin D; systemic therapy within previous 8 wks; topical therapy within previous 4 wks | Calcipotriol 50 mcg/g ointment. Max dose: 100 g/wk; 2500 g/pa Face/scalp/neck excluded | Local AEs: self report of adverse events: mild, moderate, severe; unlikely, possibly, or probably treatment‐related | AE(L): 52/161 60 (46 considered to be treatment‐related) events reported by 52 of 161 participants. 1 participant developed a significant rise in serum calcium. No other abnormalities in haematology or biochemistry tests. 118/161 participants reported continuous medication use and 80% to 90% used it twice daily. Mean use: 35.1 g/wk ('initially') to 23.4 g/wk during last 6 mths | Sponsored by Leo Pharmaceuticals | Not applicable | |
| DESIGN: observational study using retrospective data (medical records, cancer registry) and prospective (survey) data (treatments/lifestyle). Multivariate proportional hazards regression. | N: 4315 | All topical, phototherapy, and systemic therapies. Focus of study is on coal tar:
| Any cancer Skin cancer Internal malignancies Specific tumour groups:
No statistically significant increased risk of any cancer. Hazard ratios: Any cancer: LCD: 0.85 (95% CI 0.60 to 1.19) Skin cancer: LCD: 1.35 (95% CI 0.53 to 3.44) | Analysis adjusted for smoking status, other treatments, skin type, alcohol consumption. However, data on duration of tar therapy, smoking status and alcohol consumption were missing for most participants | Not applicable‐ | ||
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random BLINDING: open | N: 58 | Part 1: double‐blind study (8 wks): tacalcitol 4 mcg/g ointment OD Placebo | Local AEs: occurrence of adverse events (duration, severity, and whether treatment‐related) | Local AEs: No case of hypercalcaemia | Sponsorship not reported | Not applicable | |
| van de Kerkhof 2002c (see also Lambert 2002) | DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random Method of randomisation: NA Concealment: NA BLINDING: open WITHDRAWAL/DROPOUT: described | Part 1: | Tacalcitol 4 mcg/g OD. Treatment discontinued during remission and restarted if relapse | Local AEs: number treatment‐related adverse events; withdrawals due to adverse events (WA); investigator assessment of tolerability; participant assessment of tolerability | Local AEs: | Sponsored by Hermal/BHI, Germany | Not applicable |
| DESIGN: uncontrolled study patient delivery ALLOCATION: non‐random BLINDING: open WITHDRAWAL/DROPOUT: described | N: 20 INCLUSION CRITERIA: EXCLUSION CRITERIA: use of topical steroids in previous 2 mths | Clobetasol propionate 0.05% cream, OD (weekends) plus calcipotriol 50 mcg/g ointment BD (weekdays) | Local AEs: clinical (naked eye) examination of psoriatic plaque and surrounding area | Overuse of topical steroids resulted in appearance of clinically unapparent but dermoscopically apparent linear telangiectasias. 7/20 participants failed to adhere to recommended steroid dosing schedules. Dermoscopic red lines not apparent in 15/20 participants. Dermoscopic red lines apparent in 5/20 participants, of whom 4 had overused topical steroid cream. Steroid discontinued in participants with red lines and there was complete resolution within 2 mths | Links compliance with adverse events | Not applicable | |
| DESIGN: uncontrolled study | N: 48 | 0.1% tazarotene gel, short contact therapy (applied OD for 20 minutes then rinsed off with water) | Pruritis (4‐pt: 0 = absent to 3 = severe) Burning (4‐pt: 0 = absent to 3 = severe) | At day 45: pruritis (0 to 3): 0.17 (0.38SD) 14/43 had mild pruritis
Burning (0 to 3) 0.17 (0.38SD) 14/43 14/43 had mild burning No participant withdrew because of irritation on treated lesion | Sponsorship not reported | Not applicable | |
| DESIGN: uncontrolled study patient delivery | N: 30 | Calcitriol 15 mcg/g ointment OD Quantity of study drug varied by group: Group 1 (N =12): 4% to 8% BSA treated (300 to 600 cm^2) Group 2 (N = 10): 8% to 15% BSA treated (600 to 1200 cm^2) Group 3 (N = 8): 15% to 30% BSA treated (1200 to 2400 cm^2) | IAGI (6‐pt) ECG Haematology, biochemistry, urine protein and glucose. Serum calcium, phosphorus, plasma PTH, serum 25‐hydroxyvitaim D, 1‐alpha,25dihydroxy‐vitaim D, 24 hr urine tests for calcium, creatinine and phosphorus. Compliance also assessed (medication weight) | Mean daily usage: 74.0 to 306.1 mcg. No systemic adverse events, no skin irritation. No clinically relevant changes in vital signs, haematology, biochemistry, urine or ECGs. IAGI (0 to 5): ‐3.57 (1.01SD, N = 30) | Usual dose is 3 mcg/g BD, max. 30 g daily Sponsored by Solvay Duphar | Not applicable | |
| per cent men: per cent male; AE(L): number local adverse events/number participants; AE(S): number systemic adverse events/number participants; AE: adverse events; BC: baseline comparability; BD: twice daily; BSA: body surface area; FU: follow up (includes TD); N: number enrolled; NA: not applicable; NR: not reported; OD: once daily; PASI: Psoriasis Area and Severity Index; PRN: as required; TD: treatment duration; TSS: Total Severity Score; WA: withdrawal due to adverse events | |||||||
| Study | Reason for exclusion |
| Follow‐up under 12 wks and not focused on adverse events | |
| Not psoriasis, short review (letter) | |
| Not a product included in our review (bexarotene gel 1%) | |
| Not focused on adverse events | |
| Evaluated add‐on clobetasol for participants treated concurrently with topical or systemic therapy | |
| Inadequate reporting of adverse events | |
| Small (N = 54) retrospective study using participant questionnaires ‐ aimed to identify teratogenetic effects of tar, but many women unable to recall whether tar used in pregnancy | |
| Not chronic plaque psoriasis: paediatric dermatology participants had eczema or "eczema–psoriasis overlap (atopic dermatitis with associated features of psoriasis)" | |
| Small uncontrolled short‐term and already reflected in results from main review | |
| Short‐term and already reflected in results from main review | |
| Follow‐up under 12 wks and not focused on adverse events | |
| Case study | |
| Adverse events not reported | |
| Short‐term (4 weeks) and brief mention of adverse events | |
| Short‐term, unclear if psoriasis, small numbers (N = 6) | |
| Participants were healthy volunteers | |
| Not about adverse events | |
| Not about adverse events |
| Study | Methods | Participants | Interventions | Outcomes (compliance | Summary findings | Notes | Allocation concealment |
| DESIGN: uncontrolled study | N: 10 | Topical salicylic acid 6% | Medication adherence: | Medication adherence measured by method 1 (electronic) much lower than by method 2 (patient log) | Sponsorship not reported | D | |
| DESIGN: within‐patient | N: 30 | Topical salicylic acid 6% plus 0.1% tacrolimus ointment BD | Medication adherence: | Adherence decreased over time. On the intervention side, a decrease in adherence rate of 10% was associated with a 1‐point increase in severity (P < 0.05). For the placebo‐treated side, adherence was not significantly correlated with changes in severity. | Sponsored by Fujisawa Healthcare, Inc. and by Wake Forest University School of Medicine. | B | |
| DESIGN: uncontrolled study | N: 29 | 6% salicylic acid gel BD | Impact of office visits on participants' adherence to topical treatment. | Adherence statistically significantly higher at time of office visit. | Sponsored in part by Astellas Pharma US, Inc. | D | |
| DESIGN: between‐patient | N: 881 | Calcipotriol plus reinforcement programme | Reinforcement therapeutic programme to enhance adherence: dermatologist provided participant education with explanation of disease characteristics and treatment efficacy and application, plus written information card | The reinforcement programme had no effect on treatment efficacy | Sponsorship not reported | B | |
| DESIGN: questionnaire survey (observational cross‐sectional study) | N: 1281 | Any antipsoriatic therapy | Compliance measured against PMAQ‐3w scale (patient medication adherence questionnaire): strict adherence to prescribed regimen over previous 3 days and last weekend | 73% reported non‐compliance with current treatment | Sponsorship not reported. | D | |
| DESIGN: open uncontrolled study | N: 109 | Any prescribed antipsoriatic therapy | Medication adherence: number prescribed doses taken/number prescribed doses prescribed (see Zaghloul 2004). | Mean adherence for topical therapy: 72% (31%SD) | Findings relate to any treatment for psoriasis (not just topical therapy) | D | |
| DESIGN: questionnaire survey (cross‐sectional uncontrolled study) | N: 120 | Any antipsoriatic therapy | Per cent complying with treatment (self report): scale not reported | 39 per cent reported non‐compliance (sometimes/never complying) with prescribed treatment. The non‐compliant group had a higher self‐rated disease severity, were younger, and had a younger age at onset. | Sponsorship not reported | D | |
| DESIGN: questionnaire survey (uncontrolled study) BLINDING: NA | N: 972 | Any topical antipsoriatic therapy | Per cent complying with frequency of application of prescribed topical therapies | 29% of responders reported that the prescriber did not specify dosage frequency. | Sponsorship not reported. | D | |
| DESIGN: questionnaire survey (uncontrolled study) | N: 839 | Any antipsoriatic therapy including topical treatments, photo(chemo)therapy and systemic therapy | Per cent complying with duration of prescribed treatment (topical therapies) | Per cent complying with duration of prescribed treatment (topical therapies): 71% | Sponsorship not reported | D | |
| DESIGN: within‐patient (see Notes) | N: 976 | Calcipotriol cream OM plus calcipotriol ointment ON | Compliance: | At wk 3, 72% of participants applied the regimen on most days. By wk 8, this statistic had fallen to 61% | Sponsorship not reported | D | |
| DESIGN: uncontrolled study | N: 294 | Topical, oral, or combined antipsoriatic medication | Medication adherence: | Medication adherence measured by method 1 (objective) much lower than by method 2 (patient self report). Mean rate: 60.6% (33.0%SD); (range: 0% to169%) | Authors report no relevant financial interests | D | |
| per cent men: per cent male; AE(L): number local adverse events/number participants; AE(S): number systemic adverse events/number participants; AE: adverse events; BC: baseline comparability; BD: twice daily; BSA: body surface area; FU: follow up (includes TD); N: number enrolled; NA: not applicable; NR: not reported; OD: once daily; PASI: Psoriasis Area and Severity Index; PRN: as required; TD: treatment duration; TSS: Total Severity Score; WA: withdrawal due to adverse events | |||||||
| Study | Reason for exclusion |
| Adherence not assessed | |
| Treatment guideline (not primary study) | |
| Review/think piece | |
| Review | |
| Study focused on non‐responsive participants rather than those that are specifically non‐compliant | |
| Review | |
| Think piece (not primary study) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 20 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol | 10 | 2287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.17, ‐0.68] |
| 1.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcitriol | 6 | 1120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.71, ‐0.36] |
| 1.4 Tacalcitol | 2 | 433 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.41, ‐0.26] |
| 1.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐1.91, ‐0.96] |
| 1.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.66, ‐0.67] |
| 1.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.14] |
| 1.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.04, ‐0.30] |
| 2 TSS Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol | 10 | 1208 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.41, ‐0.89] |
| 2.2 Calcipotriol plus occlusion | 1 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 2.3 Calcitriol | 4 | 1027 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐2.38, ‐0.07] |
| 2.4 Tacalcitol | 3 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.95, ‐0.36] |
| 2.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐2.10, ‐1.12] |
| 2.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐3.24, ‐1.06] |
| 2.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.37, 0.34] |
| 2.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.83, ‐0.10] |
| 3 PASI Show forest plot | 9 | 2422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.71, ‐0.45] |
| 3.1 Calcipotriol | 8 | 2195 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.75, ‐0.55] |
| 3.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Tacalcitol | 1 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.56, 0.03] |
| 3.5 Maxacalcitol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Paricalcitol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Becocalcidiol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Becocalcidiol twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 5 | 1467 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.72, ‐0.36] |
| 4.1 Calcipotriol | 2 | 439 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 4.2 Calcipotriol plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcitriol | 2 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.76, ‐0.41] |
| 4.4 Tacalcitol | 1 | 227 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.53, 0.05] |
| 4.5 Maxacalcitol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Paricalcitol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Becocalcidiol OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Becocalcidiol twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 30 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol | 17 | 3269 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.15, ‐0.77] |
| 5.2 Calcipotriol plus occlusion | 1 | 187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 5.3 Calcitriol | 7 | 1140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.54, ‐0.29] |
| 5.4 Tacalcitol | 4 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.09, ‐0.37] |
| 5.5 Maxacalcitol | 1 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐1.91, ‐0.96] |
| 5.6 Paricalcitol OD | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.66 [‐2.66, ‐0.67] |
| 5.7 Becocalcidiol OD | 1 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.58, 0.14] |
| 5.8 Becocalcidiol twice daily | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.04, ‐0.30] |
| 6 Total withdrawals Show forest plot | 25 | 4715 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.00] |
| 6.1 Calcipotriol | 14 | 3132 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 6.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Calcitriol | 5 | 339 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 6.4 Tacalcitol | 4 | 857 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.14, 0.05] |
| 6.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [‐0.01, 0.23] |
| 6.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 6.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.13, 0.10] |
| 7 Withdrawals due to adverse events Show forest plot | 23 | 4463 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.1 Calcipotriol | 12 | 2880 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.04, 0.00] |
| 7.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcitriol | 5 | 339 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 7.4 Tacalcitol | 4 | 857 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 7.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 7.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 8 Withdrawals due to treatment failure Show forest plot | 14 | 2752 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, 0.00] |
| 8.1 Calcipotriol | 7 | 1770 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 8.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Calcitriol | 4 | 281 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.12, 0.05] |
| 8.4 Tacalcitol | 1 | 314 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.04] |
| 8.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.04] |
| 8.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.02] |
| 9 Adverse events (local) Show forest plot | 19 | 4402 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 9.1 Calcipotriol | 11 | 2652 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 9.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcitriol | 4 | 917 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 9.4 Tacalcitol | 2 | 566 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 9.5 Maxacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 9.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.06, 0.08] |
| 9.8 Becocalcidiol twice daily | 1 | 121 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [0.00, 0.19] |
| 10 Adverse events (systemic) Show forest plot | 14 | 2463 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Calcipotriol | 8 | 1182 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Calcipotriol plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcitriol | 3 | 647 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.4 Tacalcitol | 1 | 244 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.5 Maxacalcitol | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 10.6 Paricalcitol OD | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 10.7 Becocalcidiol OD | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.8 Becocalcidiol twice daily | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 11 | 1904 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.18, ‐0.82] |
| 1.1 Betamethasone dipropionate OD | 2 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.98, ‐0.64] |
| 1.2 Betamethasone dipropionate twice daily | 4 | 537 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.56, ‐1.15] |
| 1.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Betamethasone valerate | 1 | 74 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐1.92, ‐0.90] |
| 1.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.34, ‐0.28] |
| 1.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Fluticasone propionate | 2 | 383 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.14, ‐0.72] |
| 1.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.17, ‐0.34] |
| 2 TSS Show forest plot | 7 | 611 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.01, ‐0.52] |
| 2.1 Betamethasone dipropionate OD | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.16, ‐0.32] |
| 2.2 Betamethasone dipropionate twice daily | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.48, ‐0.06] |
| 2.3 Betamethasone dipropionate, maintenance | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.62, ‐0.27] |
| 2.4 Betamethasone valerate | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐2.00, ‐0.18] |
| 2.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.70, ‐0.61] |
| 2.7 Diflorasone diacetate | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.73, 0.09] |
| 2.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Hydrocortisone buteprate | 1 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
| 2.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.55, ‐0.68] |
| 3 PASI Show forest plot | 3 | 1158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.31, ‐0.62] |
| 3.1 Betamethasone dipropionate OD | 2 | 739 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.44, ‐0.14] |
| 3.2 Betamethasone dipropionate twice daily | 1 | 419 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.44, ‐0.97] |
| 3.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Desonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Mometasone furoate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Betamethasone dipropionate OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Betamethasone dipropionate twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Betamethasone dipropionate, maintenance | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Desonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Fluticasone propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Hydrocortisone buteprate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Mometasone furoate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 15 | 2311 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.06, ‐0.72] |
| 5.1 Betamethasone dipropionate OD | 3 | 832 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.96, ‐0.64] |
| 5.2 Betamethasone dipropionate twice daily | 4 | 537 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.56, ‐1.15] |
| 5.3 Betamethasone dipropionate, maintenance | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.62, ‐0.27] |
| 5.4 Betamethasone valerate | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐1.78, ‐0.89] |
| 5.5 Budesonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Desonide | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.34, ‐0.28] |
| 5.7 Diflorasone diacetate | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.73, 0.09] |
| 5.8 Fluticasone propionate | 2 | 383 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.14, ‐0.72] |
| 5.9 Hydrocortisone buteprate | 1 | 161 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
| 5.10 Mometasone furoate | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.17, ‐0.34] |
| 6 Total withdrawals Show forest plot | 9 | 1673 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.22, ‐0.05] |
| 6.1 Betamethasone dipropionate OD | 2 | 756 | Risk Difference (M‐H, Random, 95% CI) | ‐0.16 [‐0.28, ‐0.04] |
| 6.2 Betamethasone dipropionate twice daily | 1 | 421 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.12, 0.01] |
| 6.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.38, 0.02] |
| 6.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Hydrocortisone buteprate | 1 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.10] |
| 6.10 Mometasone furoate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events Show forest plot | 9 | 1292 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 7.1 Betamethasone dipropionate OD | 1 | 633 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.02] |
| 7.2 Betamethasone dipropionate twice daily | 1 | 33 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.02, 0.17] |
| 7.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.1 [‐0.24, 0.04] |
| 7.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.11, 0.01] |
| 8 Withdrawals due to treatment failure Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Betamethasone dipropionate OD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Betamethasone dipropionate twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Betamethasone dipropionate, maintenance | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | ‐0.46 [‐0.61, ‐0.31] |
| 8.4 Betamethasone valerate | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.5 Budesonide | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.8 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8.10 Mometasone furoate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 10 | 2117 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, ‐0.00] |
| 9.1 Betamethasone dipropionate OD | 2 | 756 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.15, ‐0.04] |
| 9.2 Betamethasone dipropionate twice daily | 2 | 454 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.12, 0.03] |
| 9.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9.4 Betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Budesonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Desonide | 1 | 80 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9.7 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Fluticasone propionate | 1 | 383 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.05, 0.05] |
| 9.9 Hydrocortisone buteprate | 1 | 190 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.18, 0.07] |
| 9.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.23, 0.02] |
| 10 Adverse events (systemic) Show forest plot | 4 | 675 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 10.1 Betamethasone dipropionate OD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Betamethasone dipropionate twice daily | 1 | 421 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Betamethasone dipropionate, maintenance | 2 | 134 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.07, 0.10] |
| 10.4 Budesonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Desonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Fluticasone propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Hydrocortisone buteprate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Mometasone furoate | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 5 | 540 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.38, ‐1.36] |
| 1.1 Clobetasol propionate | 4 | 471 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.53, ‐1.24] |
| 1.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Halobetasol | 1 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.81 [‐2.37, ‐1.24] |
| 2 TSS Show forest plot | 3 | 545 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.80, ‐0.89] |
| 2.1 Clobetasol propionate | 3 | 545 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐1.80, ‐0.89] |
| 2.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Halobetasol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Halobetasol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 3 | 487 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.42, ‐1.02] |
| 4.1 Clobetasol propionate | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.55, ‐0.47] |
| 4.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Halobetasol | 2 | 408 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.46, ‐1.04] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 10 | 1493 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐1.87, ‐1.26] |
| 5.1 Clobetasol propionate | 7 | 1016 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.10, ‐1.20] |
| 5.2 Halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Halobetasol | 3 | 477 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐1.65, ‐1.07] |
| 6 Total withdrawals Show forest plot | 8 | 1181 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.10, 0.01] |
| 6.1 Clobetasol propionate | 7 | 1037 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.01] |
| 6.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Halobetasol | 1 | 144 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7 Withdrawals due to adverse events Show forest plot | 10 | 1601 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 7.1 Clobetasol propionate | 7 | 1037 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Halobetasol | 3 | 564 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Withdrawals due to treatment failure Show forest plot | 8 | 1189 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8.1 Clobetasol propionate | 6 | 845 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 8.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Halobetasol | 2 | 344 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 9 Adverse events (local) Show forest plot | 8 | 1265 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 9.1 Clobetasol propionate | 6 | 845 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 9.2 Halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Halobetasol | 2 | 420 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10 Adverse events (systemic) Show forest plot | 6 | 1056 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 10.1 Clobetasol propionate | 3 | 480 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 10.2 Halcinonide | 1 | 156 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.3 Halobetasol | 2 | 420 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS Show forest plot | 3 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.66, ‐0.46] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 3 | 94 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.66, ‐0.46] |
| 6 Total withdrawals Show forest plot | 4 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7 Withdrawals due to adverse events Show forest plot | 3 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8 Withdrawals due to treatment failure Show forest plot | 2 | 44 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9 Adverse events (local) Show forest plot | 3 | 94 | Risk Difference (M‐H, Random, 95% CI) | 0.26 [‐0.30, 0.82] |
| 10 Adverse events (systemic) Show forest plot | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.35, 0.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 5 | 2264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.76, ‐1.12] |
| 1.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1416 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.50, ‐0.91] |
| 1.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 848 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.09, ‐1.71] |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Combination calcipotriol/betamethasone dipropionate, once daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI Show forest plot | 5 | 2263 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐1.53, ‐0.95] |
| 3.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1414 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.57, ‐0.70] |
| 3.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 849 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐1.86, ‐0.97] |
| 4 PAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Combination calcipotriol/betamethasone dipropionate, once daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 5 | 2264 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐1.76, ‐1.12] |
| 5.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1416 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐1.50, ‐0.91] |
| 5.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 848 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.09, ‐1.71] |
| 6 Total withdrawals Show forest plot | 5 | 2340 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.17, ‐0.07] |
| 6.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1483 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.22, ‐0.09] |
| 6.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 857 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 7 Withdrawals due to adverse events Show forest plot | 3 | 1723 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.04] |
| 7.1 Combination calcipotriol/betamethasone dipropionate, once daily | 3 | 1280 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.03] |
| 7.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 443 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.14, ‐0.05] |
| 8 Withdrawals due to treatment failure Show forest plot | 1 | 802 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.12, ‐0.06] |
| 8.1 Combination calcipotriol/betamethasone dipropionate, once daily | 1 | 359 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] |
| 8.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 443 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] |
| 9 Adverse events (local) Show forest plot | 5 | 2334 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| 9.1 Combination calcipotriol/betamethasone dipropionate, once daily | 4 | 1479 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.11, ‐0.02] |
| 9.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 2 | 855 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 10 Adverse events (systemic) Show forest plot | 1 | 412 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Combination calcipotriol/betamethasone dipropionate, once daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Combination calcipotriol/betamethasone dipropionate, twice daily | 1 | 412 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Anti‐IL‐8 monoclonal antibody cream | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Caffeine (topical) 10%, TD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Dead Sea salts emollient lotion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Indigo naturalis 1.4% ointment | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Kukui nut oil, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.13 Methotrexate gel | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.17 Oleum horwathiensis (Psoricur®) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.26 Theophylline 1% ointment, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS Show forest plot | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.13, ‐0.27] |
| 2.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Caffeine (topical) 10%, TD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.81, ‐0.15] |
| 2.6 Dead Sea salts emollient lotion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Fish oil plus occlusion | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.64, ‐0.46] |
| 2.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.91, ‐0.35] |
| 2.10 Indigo naturalis 1.4% ointment | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐2.13, ‐1.15] |
| 2.11 Kukui nut oil, TD | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.48, 1.14] |
| 2.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Methotrexate gel | 1 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.92, ‐0.04] |
| 2.14 Mycophenolic acid ointment | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.67, ‐0.22] |
| 2.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.60, 0.75] |
| 2.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2.17 Oleum horwathiensis (Psoricur®) | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.40, ‐0.14] |
| 2.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.59, 0.85] |
| 2.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐3.26, ‐1.36] |
| 2.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.98, 0.21] |
| 2.23 Tacrolimus ointment | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.52, 0.63] |
| 2.24 Tar | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.11, 0.22] |
| 2.25 Tazarotene | 1 | 318 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.11, ‐0.62] |
| 2.26 Theophylline 1% ointment, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Anti‐IL‐8 monoclonal antibody cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Caffeine (topical) 10%, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Dead Sea salts emollient lotion, 30% | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Indigo naturalis 1.4% ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Kukui nut oil, twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Methotrexate gel | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Oleum horwathiensis (Psoricur®) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.26 Theophylline 1% ointment, twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Anti‐IL‐8 monoclonal antibody cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Caffeine (topical) 10%, TD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Dead Sea salts emollient lotion, 30% | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Fish oil plus occlusion | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Hexafluoro‐1,25‐dihydroxyvitamin D3, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Indigo naturalis 1.4% ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Kukui nut oil, TD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Methotrexate gel | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 Mycophenolic acid ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Nicotinamide 1.4%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Oleum horwathiensis (Psoricur®) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Polymyxin B cream 200,000 U/g | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.23 Tacrolimus ointment | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.24 Tar | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.25 Tazarotene | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.26 Theophylline 1% ointment, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 26 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.16, ‐0.99] |
| 5.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.01, ‐0.16] |
| 5.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 81 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.21, ‐0.31] |
| 5.4 Caffeine (topical) 10%, TD | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.84, 0.06] |
| 5.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.81, ‐0.15] |
| 5.6 Dead Sea salts emollient lotion, 30% | 1 | 19 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.36, 1.51] |
| 5.7 Fish oil plus occlusion | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.64, ‐0.46] |
| 5.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.96 [‐4.19, ‐1.74] |
| 5.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.35, 0.12] |
| 5.10 Indigo naturalis 1.4% ointment | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.09 [‐2.62, ‐1.56] |
| 5.11 Kukui nut oil, TD | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.80, 0.80] |
| 5.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.06, ‐0.48] |
| 5.13 Methotrexate gel | 2 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐2.04, ‐0.06] |
| 5.14 Mycophenolic acid ointment | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.67, ‐0.22] |
| 5.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.60, 0.75] |
| 5.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 5.17 Oleum horwathiensis (Psoricur®) | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.63, 0.58] |
| 5.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.50, 0.37] |
| 5.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.59, 0.85] |
| 5.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐3.26, ‐1.36] |
| 5.22 Sirolimus (topical), 2.2% for 6 wks, then 8% for a further 6 wks | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.98, 0.21] |
| 5.23 Tacrolimus ointment | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.52, 0.63] |
| 5.24 Tar | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.11, 0.22] |
| 5.25 Tazarotene | 1 | 318 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.11, ‐0.62] |
| 5.26 Theophylline 1% ointment, twice daily | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐4.13, ‐1.62] |
| 6 Total withdrawals Show forest plot | 23 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 6.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.09, 0.09] |
| 6.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.08] |
| 6.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.25 [‐0.06, 0.56] |
| 6.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.10 Indigo naturalis 1.4% ointment | 2 | 112 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 6.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.42, 0.15] |
| 6.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.32, ‐0.14] |
| 6.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 6.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 6.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 6.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.15, 0.15] |
| 6.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 6.20 Polymyxin B cream 200,000 U/g | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 6.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 6.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 6.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 7 Withdrawals due to adverse events Show forest plot | 19 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
| 7.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.18, 0.35] |
| 7.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.10 Indigo naturalis 1.4% ointment | 2 | 112 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 7.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 7.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.05, 0.10] |
| 7.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 8 Withdrawals due to treatment failure Show forest plot | 18 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 8.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.12, 0.29] |
| 8.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.10 Indigo naturalis 1.4% ointment | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 8.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 8.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 8.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.25 Tazarotene | 2 | 1627 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.04, 0.01] |
| 8.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 9 Adverse events (local) Show forest plot | 21 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9.2 Anti‐IL‐8 monoclonal antibody cream | 1 | 92 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.10, 0.14] |
| 9.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.07, 0.06] |
| 9.4 Caffeine (topical) 10%, TD | 1 | 78 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.03, 0.13] |
| 9.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [‐0.02, 0.27] |
| 9.6 Dead Sea salts emollient lotion | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.18, 0.35] |
| 9.7 Fish oil plus occlusion | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 9.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 1 | 24 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.44, 0.27] |
| 9.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [‐0.06, 0.33] |
| 9.10 Indigo naturalis 1.4% ointment | 2 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 9.11 Kukui nut oil, TD | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 9.12 Mahonia aquifolium (Reliéva™), twice daily | 1 | 200 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 9.13 Methotrexate gel | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9.14 Mycophenolic acid ointment | 1 | 14 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 9.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 9.16 Nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.07, 0.28] |
| 9.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 9.18 Omega‐3‐polyunsaturated fatty acids ointment | 1 | 146 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.05] |
| 9.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.19, 0.19] |
| 9.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 9.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.25 Tazarotene | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.26 Theophylline 1% ointment, twice daily | 1 | 22 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 10 Adverse events (systemic) Show forest plot | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Aloe vera extract 0.5% hydrophilic cream, three times per day | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Anti‐IL‐8 monoclonal antibody cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Betamethasone 17‐valerate 21‐acetate plus tretinoin plus salicylic acid | 1 | 85 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.4 Caffeine (topical) 10%, TD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriene 0.005% ointment + nicotinamide 0.05% or 0.1% or 0.7% or 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Dead Sea salts emollient lotion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Fish oil plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Herbal skin care (Dr Michaels® cleansing gel, ointment and skin conditioner), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Hexafluoro‐1,25‐dihydroxyvitamin D3 | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 10.10 Indigo naturalis 1.4% ointment | 2 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 10.11 Kukui nut oil, TD | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.12 Mahonia aquifolium (Reliéva™), twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.13 Methotrexate gel | 2 | 166 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.14 Mycophenolic acid ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.15 NG‐monomethyl‐L‐arginine (L‐NMMA) cream | 1 | 34 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 10.16 Nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.17 Oleum horwathiensis | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 10.18 Omega‐3‐polyunsaturated fatty acids ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.19 Platelet aggregation activating factor (PAF)(Ro 24‐0238) | 1 | 104 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.20 Polymyxin B cream 200,000 U/g | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.21 PTH (1‐34) in Novasome A® liposomal cream, twice daily | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 10.22 Sirolimus (topical) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.23 Tacrolimus ointment | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.24 Tar | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.25 Tazarotene | 2 | 414 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.26 Theophylline 1% ointment, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.28, 0.58] |
| 1.2 Calcipotriol vs. betamethasone valerate | 1 | 412 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.21, 0.17] |
| 1.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.52] |
| 1.5 Calcipotriol vs. fluocinonide | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.99, ‐0.18] |
| 1.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.45] |
| 1.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.91, 0.53] |
| 1.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. betamethasone valerate | 1 | 684 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.41, ‐0.11] |
| 2.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.15, 0.65] |
| 2.5 Calcipotriol vs. fluocinonide | 1 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.92, ‐0.07] |
| 2.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.51] |
| 2.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Tacalcitol vs. betamethasone valerate | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.09, 0.74] |
| 3 PASI Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.22, 0.51] |
| 3.2 Calcipotriol vs. betamethasone valerate | 4 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.22, ‐0.02] |
| 3.3 Calcipotriol vs. desoxymetasone | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.73, 1.02] |
| 3.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol vs. fluocinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.14, 0.63] |
| 3.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 2 | 1080 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.38, ‐0.14] |
| 4.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol vs. betamethasone valerate | 2 | 1080 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.38, ‐0.14] |
| 4.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol vs. fluocinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcitriol vs. betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 14 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1728 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.28, 0.58] |
| 5.2 Calcipotriol vs. betamethasone valerate | 4 | 1557 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.26, 0.02] |
| 5.3 Calcipotriol vs. desoxymetasone | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.73, 1.02] |
| 5.4 Calcipotriol vs. diflorasone diacetate | 1 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.02, 0.52] |
| 5.5 Calcipotriol vs. fluocinonide | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.99, ‐0.18] |
| 5.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.45] |
| 5.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.91, 0.53] |
| 5.8 Tacalcitol vs. betamethasone valerate | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.09, 0.74] |
| 6 Total withdrawals Show forest plot | 11 | 3995 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.03] |
| 6.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1739 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 6.2 Calcipotriol vs. betamethasone valerate | 3 | 1520 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 6.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Calcipotriol vs. diflorasone diacetate | 1 | 268 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.5 Calcipotriol vs. fluocinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 6.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.10, 0.23] |
| 6.8 Tacalcitol vs. betamethasone valerate | 2 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 7 Withdrawals due to adverse events Show forest plot | 9 | 3058 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.01] |
| 7.1 Calcipotriol vs. betamethasone dipropionate | 1 | 956 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 7.2 Calcipotriol vs. betamethasone valerate | 3 | 1520 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.01] |
| 7.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 Calcipotriol vs. fluocinonide | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 7.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.10, 0.23] |
| 7.8 Tacalcitol vs. betamethasone valerate | 2 | 180 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8 Withdrawals due to treatment failure Show forest plot | 5 | 1500 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8.1 Calcipotriol vs. betamethasone dipropionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Calcipotriol vs. betamethasone valerate | 2 | 1099 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Calcipotriol vs. fluocinonide | 1 | 113 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 8.7 Calcitriol vs. betamethasone valerate | 1 | 30 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 8.8 Tacalcitol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 9 | 3778 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.02, 0.11] |
| 9.1 Calcipotriol vs. betamethasone dipropionate | 3 | 1739 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.09] |
| 9.2 Calcipotriol vs. betamethasone valerate | 3 | 1516 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [‐0.02, 0.26] |
| 9.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Calcipotriol vs. fluocinonide | 1 | 113 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.02, 0.22] |
| 9.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.05, 0.06] |
| 9.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.8 Tacalcitol vs. betamethasone valerate | 1 | 152 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 10 Adverse events (systemic) Show forest plot | 6 | 2547 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.00, 0.00] |
| 10.1 Calcipotriol vs. betamethasone dipropionate | 1 | 621 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Calcipotriol vs. betamethasone valerate | 3 | 1516 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.00, 0.00] |
| 10.3 Calcipotriol vs. desoxymetasone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. diflorasone diacetate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol vs. fluocinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Calcitriol vs. betamethasone dipropionate | 1 | 258 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 10.7 Calcitriol vs. betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Tacalcitol vs. betamethasone valerate | 1 | 152 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Calcipotriol vs. Clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol vs. Clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.57, 0.44] |
| 5.1 Calcipotriol vs. Clobetasol propionate | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.57, 0.44] |
| 6 Total withdrawals Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals due to adverse events Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events (systemic) Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Calcipotriol vs. Clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1926 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.52, ‐0.27] |
| 1.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.22, ‐0.15] |
| 2 TSS Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI Show forest plot | 3 | 1876 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.55, ‐0.33] |
| 3.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1876 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.55, ‐0.33] |
| 3.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1926 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.52, ‐0.27] |
| 5.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.09, 0.81] |
| 5.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.22, ‐0.15] |
| 6 Total withdrawals Show forest plot | 5 | 2135 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 6.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1948 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 6.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | 65 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 7 Withdrawals due to adverse events Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure Show forest plot | 2 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 3 | 1946 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 9.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.06] |
| 9.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Calcipotriol + betamethasone dipropionate vs. betamethasone dipropionate | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Calcipotriol + betamethasone dipropionate vs. clobetasol propionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Calcipotriol + clobetasol propionate vs. clobetasol propionate | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. dithranol | 4 | 994 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.85, ‐0.01] |
| 1.2 Calcitriol vs. dithranol | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.13, 0.88] |
| 1.3 Tacalcitol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. dithranol | 2 | 210 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.16, 0.08] |
| 2.2 Calcitriol vs. dithranol | 1 | 114 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.50] |
| 2.3 Tacalcitol vs. dithranol | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.60, 0.25] |
| 3 PASI Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. dithranol | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcitriol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Tacalcitol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. dithranol | 2 | 544 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.90, 0.80] |
| 4.2 Calcitriol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Tacalcitol vs. dithranol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Calcipotriol vs. dithranol | 6 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Calcitriol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Tacalcitol vs. dithranol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total withdrawals Show forest plot | 7 | 615 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.01] |
| 6.1 Calcipotriol vs. dithranol | 5 | 417 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.04] |
| 6.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.25, 0.06] |
| 6.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.16, 0.11] |
| 7 Withdrawals due to adverse events Show forest plot | 7 | 1265 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.00] |
| 7.1 Calcipotriol vs. dithranol | 6 | 1151 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, 0.00] |
| 7.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 7.3 Tacalcitol vs. dithranol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Withdrawals due to treatment failure Show forest plot | 5 | 788 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 8.1 Calcipotriol vs. dithranol | 4 | 674 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
| 8.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 8.3 Tacalcitol vs. dithranol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 9 | 1543 | Risk Difference (M‐H, Random, 95% CI) | ‐0.32 [‐0.43, ‐0.20] |
| 9.1 Calcipotriol vs. dithranol | 7 | 1345 | Risk Difference (M‐H, Random, 95% CI) | ‐0.25 [‐0.32, ‐0.17] |
| 9.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | ‐0.67 [‐0.80, ‐0.54] |
| 9.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.36 [‐0.52, ‐0.20] |
| 10 Adverse events (systemic) Show forest plot | 4 | 746 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 10.1 Calcipotriol vs. dithranol | 2 | 548 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 10.2 Calcitriol vs. dithranol | 1 | 114 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.3 Tacalcitol vs. dithranol | 1 | 84 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. calcitriol | 1 | 246 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.25, 0.25] |
| 1.2 Calcipotriol vs. tacalcitol | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 1.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.12, 0.98] |
| 2 TSS Show forest plot | 3 | 589 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.55, ‐0.06] |
| 2.1 Calcipotriol vs. calcitriol | 1 | 250 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.57, ‐0.07] |
| 2.2 Calcipotriol vs. tacalcitol | 1 | 287 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 2.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.41, 0.68] |
| 3 PASI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Calcipotriol vs. calcitriol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcipotriol vs. tacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Calcipotriol vs. maxacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol vs. calcitriol | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Calcipotriol vs. tacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Calcipotriol vs. maxacalcitol | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 4 | 539 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.62, 0.27] |
| 5.1 Calcipotriol vs. calcitriol | 2 | 261 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.46, 0.64] |
| 5.2 Calcipotriol vs. tacalcitol | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.73, ‐0.21] |
| 5.3 Calcipotriol vs. maxacalcitol | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.12, 0.98] |
| 6 Total withdrawals Show forest plot | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 6.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 6.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 7 Withdrawals due to adverse events Show forest plot | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.06] |
| 7.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 7.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8 Withdrawals due to treatment failure Show forest plot | 3 | 334 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8.1 Calcipotriol vs. calcitriol | 2 | 274 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.08, 0.07] |
| 8.2 Calcipotriol vs. tacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9 Adverse events (local) Show forest plot | 2 | 537 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.05, 0.12] |
| 9.1 Calcipotriol vs. calcitriol | 1 | 250 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.01, 0.14] |
| 9.2 Calcipotriol vs. tacalcitol | 1 | 287 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.09, 0.07] |
| 9.3 Calcipotriol vs. maxacalcitol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) Show forest plot | 3 | 597 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.1 Calcipotriol vs. calcitriol | 1 | 250 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.2 Calcipotriol vs. tacalcitol | 1 | 287 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Calcipotriol vs. maxacalcitol | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.23, 0.88] |
| 1.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1194 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.31, 1.02] |
| 1.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 1 | 377 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.06, 0.48] |
| 1.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1804 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.40, 0.93] |
| 1.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.19, 0.74] |
| 1.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.48] |
| 1.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.34, 1.42] |
| 1.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.06, 0.33] |
| 1.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.26, 0.70] |
| 2 TSS Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI Show forest plot | 16 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.10, 0.82] |
| 3.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1191 | Std. Mean Difference (IV, Random, 95% CI) | 0.67 [0.23, 1.11] |
| 3.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 4 | 1204 | Std. Mean Difference (IV, Random, 95% CI) | 0.52 [0.38, 0.67] |
| 3.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1744 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.46, 0.83] |
| 3.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 515 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.07, 0.93] |
| 3.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.04, 0.38] |
| 3.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.44] |
| 3.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.11, 1.18] |
| 3.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.11, 0.28] |
| 3.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.57] |
| 3.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.25, 0.69] |
| 4 PAGI Show forest plot | 2 | 399 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.29, 0.69] |
| 4.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.16, 1.23] |
| 4.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.46 [0.24, 0.68] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 17 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol twice daily vs. calcipotriol OM, BMD ON | 1 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.23, 0.88] |
| 5.2 Calcipotriol OD vs. combined calcipotriol + BMD OD | 2 | 1194 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.31, 1.02] |
| 5.3 Calcipotriol twice daily vs. combined calcipotriol + BMD OD | 4 | 1204 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [0.20, 0.66] |
| 5.4 Calcipotriol twice daily vs. combined calcipotriol + BMD twice daily | 3 | 1804 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.40, 0.93] |
| 5.5 Calcipotriol twice daily vs. calcipotriol OM, BMV ON | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.19, 0.74] |
| 5.6 Calcipotriol twice daily vs. calcipotriol OM, clobetasone butyrate ON | 1 | 344 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.48] |
| 5.7 Calcipotriol twice daily vs. calcipotriol twice daily + clobetasol propionate twice daily | 1 | 65 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.34, 1.42] |
| 5.8 Calcipotriol twice daily vs. calcipotriol OM, diflucortolone valerate ON | 1 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.29, 0.44] |
| 5.9 Calcipotriol OD vs. calcipotriol OM, fluocinonide acetonide ON | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.11, 1.18] |
| 5.10 Calcipotriol OD vs. combined calcipotriol + hydrocortisone OD | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.06, 0.33] |
| 5.11 Calcitriol twice daily vs. diflucortolone valerate OM, calcitriol ON | 1 | 142 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.57] |
| 5.12 Tacalcitol OD vs. combined calcipotriol + BMD OD | 1 | 334 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.26, 0.70] |
| 6 Total withdrawals Show forest plot | 15 | 5494 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.02, 0.05] |
| 6.1 Talcipotriol vs. calcipotriol and corticosteroid | 13 | 4985 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.05] |
| 6.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 142 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.08, 0.10] |
| 6.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 367 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.11] |
| 7 Withdrawals due to adverse events Show forest plot | 13 | 4081 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.01, 0.03] |
| 7.1 Calcipotriol vs. calcipotriol and corticosteroid | 11 | 3572 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.01, 0.03] |
| 7.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 142 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 7.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 367 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 8 Withdrawals due to treatment failure Show forest plot | 7 | 1925 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.02] |
| 8.1 Calcipotriol vs. calcipotriol and corticosteroid | 7 | 1925 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.02] |
| 8.2 Calcitriol vs. calcitriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Tacalcitol vs. calcipotriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 15 | 5581 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.05, 0.08] |
| 9.1 Calcipotriol vs. calcipotriol and corticosteroid | 13 | 5084 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.04, 0.08] |
| 9.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 131 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [0.02, 0.19] |
| 9.3 Tacalcitol vs. calcipotriol and corticosteroid | 1 | 366 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.15] |
| 10 Adverse events (systemic) Show forest plot | 6 | 2099 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 10.1 Calcipotriol vs. calcipotriol and corticosteroid | 5 | 1968 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 10.2 Calcitriol vs. calcitriol and corticosteroid | 1 | 131 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.3 Tacalcitol vs. calcipotriol and corticosteroid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 1.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.29] |
| 1.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.18, 1.02] |
| 1.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.05, 0.86] |
| 1.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 1.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.12, 0.41] |
| 1.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.37, 0.66] |
| 1.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.40] |
| 1.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 1.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 2 TSS Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Calcipotriol (6 wks) vs. clobetasol propionate (2wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.21, 1.05] |
| 2.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 3.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 3.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 650 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 3.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.01, 1.32] |
| 3.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.64, 1.62] |
| 3.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.62, 0.09] |
| 3.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.10, 0.39] |
| 3.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.45, 0.74] |
| 3.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.16, 0.45] |
| 3.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 645 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.01, 0.30] |
| 3.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Std. Mean Difference (IV, Random, 95% CI) | 0.49 [0.31, 0.67] |
| 4 PAGI Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 4.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.26] |
| 4.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.13, 0.42] |
| 4.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.56, 0.85] |
| 4.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [0.29, 0.58] |
| 4.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.07, 0.39] |
| 4.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 577 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 5.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 143 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.04, 0.62] |
| 5.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 585 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.04, 0.29] |
| 5.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.18, 1.02] |
| 5.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.01, 1.32] |
| 5.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.05, 0.86] |
| 5.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 5.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.12, 0.41] |
| 5.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.37, 0.66] |
| 5.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.40] |
| 5.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 596 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.08, 0.40] |
| 5.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 493 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.36, 0.72] |
| 6 Total withdrawals Show forest plot | 9 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.00, 0.10] |
| 6.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.09, 0.17] |
| 6.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.03, 0.13] |
| 6.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 6.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 6.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 6.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.12, 0.11] |
| 6.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.03, 0.14] |
| 6.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.06, 0.17] |
| 6.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 6.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 644 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 6.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.01, 0.12] |
| 7 Withdrawals due to adverse events Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 7.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 7.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 7.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 759 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 753 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.02] |
| 7.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 760 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 8 Withdrawals due to treatment failure Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.10, 0.33] |
| 8.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| 8.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 8.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy)+ combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 9 Adverse events (local) Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.06, 0.17] |
| 9.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 649 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.05, 0.17] |
| 9.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 1 | 98 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.12] |
| 9.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 9.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 1 | 76 | Risk Difference (M‐H, Random, 95% CI) | 0.26 [0.07, 0.45] |
| 9.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 1 | 125 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 9.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 1 | 752 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 9.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 743 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 9.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 1 | 749 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 9.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 1 | 644 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 9.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | 501 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.01, 0.11] |
| 10 Adverse events (systemic) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcipotriol (12 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol (6 wks) vs. clobetasol propionate (2 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol (6 wks) vs. calcipotriol OM, fluocinonide acetonide ON (2 wks); then calcipotriol twice daily (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 Calcipotriol (6 wks) vs. halometasone OM, calcipotriol ON (2 wks); then calcipotriol twice daily (w/dy), halometasone (w/e) (2 wks); then calcipotriol twice daily (2 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Calcipotriol ON, clobetasol propionate OM (2 to 4 wks); then calcipotriol twice daily (to wk 12) vs. calcitriol ON, clobetasol propionate OM (2 to 4 wks); then calcitriol twice daily (to wk 12) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.8 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Combined calcipotriol + BMD (4 wks); then placebo ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Combined calcipotriol + BMD (4 wks); then calcipotriol ointment twice daily (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) + combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Combined calcipotriol + BMD (8 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (w/dy) & combined calcipotriol + BMD (w/e) (8 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.12 Tacalcitol (8 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 PASI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Total withdrawals Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals due to adverse events Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Withdrawals due to treatment failure Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events (local) Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse events (systemic) | 0 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Combined calcipotriol + BMD (52 wks) vs. alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Combined calcipotriol + BMD (52 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Alternating: combined calcipotriol + BMD (4 wks); then calcipotriol (4 wks) vs. combined calcipotriol + BMD (4 wks); then calcipotriol (48 wks) | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. coal tar | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.74, 0.68] |
| 1.2 Calcipotriol vs. coal tar polytherapy | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.87, ‐0.31] |
| 1.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 200 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.33, 0.22] |
| 1.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Calcipotriol vs. tazarotene | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.60, 0.16] |
| 1.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 1.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 2 | 728 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 1.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 TSS Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. coal tar | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. coal tar polytherapy | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.86, ‐0.16] |
| 2.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.49, 0.31] |
| 2.4 Calcipotriol vs. calcipotriol+nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.14, 0.52] |
| 2.5 Calcipotriol vs. corticosteroid + salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Calcipotriol vs. tacrolimus ointment | 2 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.51, 0.81] |
| 2.8 Calcipotriol vs. tazarotene | 1 | 199 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.23] |
| 2.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Calcipotriol vs. vitamin B12 cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐2.04, 1.68] |
| 3 PASI Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. coal tar | 2 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.54, 1.35] |
| 3.2 Calcipotriol vs. coal tar polytherapy | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.06, ‐0.20] |
| 3.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.36, 0.26] |
| 3.6 Calcipotriol vs. propylthiouracil cream | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.23, ‐1.25] |
| 3.7 Calcipotriol vs. tacrolimus ointment | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Calcipotriol vs. tazarotene | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.78, 0.75] |
| 3.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 989 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.00] |
| 3.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. coal tar | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.51 [‐2.12, ‐0.90] |
| 4.2 Calcipotriol vs. coal tar polytherapy | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.99, ‐0.13] |
| 4.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 186 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.79, ‐0.20] |
| 4.6 Calcipotriol vs. propylthiouracil cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.51, 0.24] |
| 4.8 Calcipotriol vs. tazarotene | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.99, 0.29] |
| 4.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 4.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. coal tar | 3 | 139 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.74, 0.68] |
| 5.2 Calcipotriol vs. coal tar polytherapy | 2 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.87, ‐0.31] |
| 5.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.49, 0.31] |
| 5.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.14, 0.52] |
| 5.5 Calcipotriol vs. corticosteroid + salicylic acid | 2 | 360 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.15] |
| 5.6 Calcipotriol vs. propylthiouracil cream | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.23, ‐1.25] |
| 5.7 Calcipotriol vs. tacrolimus ointment | 2 | 171 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.28, 0.17] |
| 5.8 Calcipotriol vs. tazarotene | 2 | 237 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.35, 0.16] |
| 5.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.33, 0.24] |
| 5.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 988 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.32, ‐0.07] |
| 5.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 2 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐2.04, 1.68] |
| 6 Total withdrawals Show forest plot | 15 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 6.2 Calcipotriol vs. coal tar polytherapy | 2 | 220 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 6.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.05, 0.09] |
| 6.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.06, 0.07] |
| 6.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.09, 0.17] |
| 6.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.16, 0.30] |
| 6.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.13 [‐0.25, ‐0.01] |
| 6.8 Calcipotriol vs. tazarotene | 2 | 254 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.10, 0.01] |
| 6.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.15, 0.08] |
| 6.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 6.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 1001 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events Show forest plot | 15 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 7.2 Calcipotriol vs. coal tar polytherapy | 2 | 210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 7.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 7.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.00, 0.10] |
| 7.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.16, 0.30] |
| 7.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.08, 0.11] |
| 7.8 Calcipotriol vs. tazarotene | 2 | 254 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.16, 0.05] |
| 7.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.10, 0.07] |
| 7.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 7.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 998 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Withdrawals due to treatment failure Show forest plot | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol vs. coal tar | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.2 Calcipotriol vs. coal tar polytherapy | 1 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.07] |
| 8.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.02] |
| 8.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 8.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 8.8 Calcipotriol vs. tazarotene | 1 | 208 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 8.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 1 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 8.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 3 | 998 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 8.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 11 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Calcipotriol vs. coal tar | 2 | 120 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.06, 0.10] |
| 9.2 Calcipotriol vs. coal tar polytherapy | 1 | 122 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.09, 0.20] |
| 9.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 1 | 96 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.32, 0.03] |
| 9.4 Calcipotriol vs. calcipotriol + nicotinamide (0.05%, 0.1%, 0.7%, or 1.4%), twice daily | 1 | 192 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 9.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.15] |
| 9.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.25, 0.11] |
| 9.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | ‐0.19 [‐0.37, ‐0.01] |
| 9.8 Calcipotriol vs. tazarotene | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 9.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.10 Calcipotriol vs. vitamin B12 cream | 1 | 26 | Risk Difference (M‐H, Random, 95% CI) | 0.23 [‐0.06, 0.52] |
| 9.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 2 | 731 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.05] |
| 9.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Calcipotriol vs. coal tar | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 10.2 Calcipotriol vs. coal tar polytherapy | 1 | 88 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.3 Calcipotriol vs. nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. calcipotriol + nicotinamide 1.4%, twice daily | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcipotriol vs. corticosteroid + salicylic acid | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 10.6 Calcipotriol vs. propylthiouracil cream | 1 | 28 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 10.7 Calcipotriol vs. tacrolimus ointment | 1 | 124 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 10.8 Calcipotriol vs. tazarotene | 1 | 183 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 10.9 Calcipotriol vs. tazarotene gel plus mometasone furoate cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Calcipotriol vs. vitamin B12 cream | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Head‐to‐head vitamin D alone or in combination: twice daily vs OD | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.12 Head‐to‐head vitamin D alone or in combination: no occlusion vs. occlusion | 1 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.10, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Betamethasone valerate 0.1%, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Calcipotriol ointment, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 TSS Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone valerate 0.1%, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Calcipotriol ointment, OD | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PASI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Betamethasone valerate 0.1%, OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Calcipotriol ointment, OD | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Pimecrolimus cream, 1% OD/twice daily | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Tacrolimus ointment 0.1%, twice daily | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 PAGI Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Betamethasone valerate 0.1%, OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol ointment, OD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Pimecrolimus cream, 1% OD/twice daily | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.24, ‐0.06] |
| 4.4 Tacrolimus ointment 0.1%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Betamethasone valerate 0.1%, OD | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.83 [‐3.79, ‐1.88] |
| 5.2 Calcipotriol ointment, OD | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.77, ‐0.40] |
| 5.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.30, ‐0.41] |
| 5.4 Tacrolimus ointment 0.1%, twice daily | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Total withdrawals Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.08, 0.28] |
| 6.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.14, 0.14] |
| 6.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.16, 0.04] |
| 6.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 7 Withdrawals due to adverse events Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.05, 0.05] |
| 7.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
| 8 Withdrawals due to treatment failure Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 8.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 8.3 Pimecrolimus cream, 1% OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 8.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.02] |
| 9 Adverse events (local) Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Betamethasone valerate 0.1%, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 9.2 Calcipotriol ointment, OD | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.11, 0.21] |
| 9.3 Pimecrolimus cream 1%, OD/twice daily | 2 | 97 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.15, 0.31] |
| 9.4 Tacrolimus ointment 0.1%, twice daily | 1 | 167 | Risk Difference (M‐H, Random, 95% CI) | ‐0.17 [‐0.30, ‐0.03] |
| 10 Adverse events (systemic) Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Betamethasone valerate 0.1%, OD | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Calcipotriol ointment, OD | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Pimecrolimus cream 1%, OD/twice daily | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Tacrolimus ointment 0.1%, twice daily | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.11, 0.50] |
| 1.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.15, 0.98] |
| 2 TSS Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Calcipotriol vs. calcitriol | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.28, 0.94] |
| 2.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.27, 0.85] |
| 3 PASI Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Calcipotriol vs. BMV | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 2.02 [1.20, 2.84] |
| 3.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.51] |
| 3.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Calcipotriol vs. pimecrolimus | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.17, 0.11] |
| 3.5 Calcitriol vs. tacrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Calcipotriol vs. calcitriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Calcitriol vs. tacrolimus | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Calcipotriol vs. BMV | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 2.02 [1.20, 2.84] |
| 5.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.11, 0.50] |
| 5.3 Calcipotriol vs. calcitriol | 1 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.28, 0.94] |
| 5.4 Calcipotriol vs. pimecrolimus | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.17, 0.11] |
| 5.5 Calcitriol vs. tacrolimus | 1 | 49 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.15, 0.98] |
| 6 Total withdrawals Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.28, 0.08] |
| 6.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 6.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.11, 0.11] |
| 6.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.08, 0.18] |
| 6.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [‐0.02, 0.26] |
| 7 Withdrawals due to adverse events Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.11] |
| 7.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.01, 0.18] |
| 7.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 7.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 8 Withdrawals due to treatment failure Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 8.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 8.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 8.5 Calcitriol vs. tacrolimus | 1 | 50 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 9 Adverse events (local) Show forest plot | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 calcipotriol vs. BMV | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 9.2 Calcipotriol vs. calcipotriol + hydrocortisone | 1 | 404 | Risk Difference (M‐H, Random, 95% CI) | 0.15 [0.08, 0.23] |
| 9.3 Calcipotriol vs. calcitriol | 1 | 150 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.02, 0.17] |
| 9.4 Calcipotriol vs. pimecrolimus | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.38, 0.08] |
| 9.5 Calcitriol vs. tacrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Adverse events (systemic) | 0 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Calcipotriol vs. BMV | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Calcipotriol vs. calcipotriol + hydrocortisone | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Calcipotriol vs. calcitriol | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Calcipotriol vs. pimecrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Calcitriol vs. tacrolimus | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.28, ‐0.16] |
| 1.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.29, ‐0.90] |
| 1.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 1.5 Very potent steroid: clobetasol propionate | 2 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐1.99, ‐1.48] |
| 1.6 Very potent steroid: halcinonide | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.69, ‐0.53] |
| 1.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.61, ‐0.32] |
| 1.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.50, ‐0.47] |
| 1.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.69, ‐0.76] |
| 1.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.79, 0.06] |
| 2 TSS Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.64, ‐0.25] |
| 2.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.19, ‐0.81] |
| 2.3 Potent steroid: betamethasone valerate | 1 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.75, ‐1.05] |
| 2.4 Very potent steroid: amcinonide | 1 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐1.98, ‐1.18] |
| 2.5 Very potent steroid: clobetasol propionate | 3 | 707 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐1.77, ‐1.28] |
| 2.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.42, ‐0.43] |
| 2.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.11, ‐0.19] |
| 2.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.82, 0.68] |
| 2.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.34, ‐0.44] |
| 2.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.47, 0.32] |
| 3 PASI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Vitamin D: calcipotriol | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Potent steroid: betamethasone dipropionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Very potent steroid: amcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Very potent steroid: clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Vitamin D in combination: calcipotriol + BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 Other treatment: salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Vitamin D: calcipotriol | 2 | 450 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.28, ‐0.05] |
| 4.2 Potent steroid: betamethasone dipropionate | 1 | 685 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐1.43, ‐1.03] |
| 4.3 Potent steroid: betamethasone valerate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.33, ‐0.61] |
| 4.5 Very potent steroid: clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Very potent steroid: halcinonide | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Vitamin D in combination: calcipotriol + BMD | 2 | 841 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.79, ‐0.22] |
| 4.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.86, 0.64] |
| 4.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.11 Other treatment: salicylic acid | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 13 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Vitamin D: calcipotriol | 2 | 457 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.28, ‐0.16] |
| 5.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.29, ‐0.90] |
| 5.3 Potent steroid: betamethasone valerate | 1 | 172 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.75, ‐1.05] |
| 5.4 Very potent steroid: amcinonide | 1 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 5.5 Very potent steroid: clobetasol propionate | 4 | 788 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐1.81, ‐1.34] |
| 5.6 Very potent steroid: halcinonide | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.69, ‐0.53] |
| 5.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.61, ‐0.32] |
| 5.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐2.50, ‐0.47] |
| 5.9 Other treatment: ciclopirox olamine shampoo | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.82, 0.68] |
| 5.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.69, ‐0.76] |
| 5.11 Other treatment: salicylic acid | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.79, 0.06] |
| 6 Total withdrawals Show forest plot | 13 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Vitamin D: calcipotriol | 3 | 517 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.08, 0.05] |
| 6.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.06] |
| 6.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.11, 0.11] |
| 6.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.10, 0.04] |
| 6.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.13, 0.13] |
| 6.7 Vitamin D in combination: calcipotriol + BMD | 2 | 854 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.16, ‐0.03] |
| 6.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.15 [‐0.38, 0.09] |
| 6.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 89 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 6.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Withdrawals due to adverse events Show forest plot | 13 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vitamin D: calcipotriol | 3 | 517 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.05] |
| 7.2 Potent steroid: betamethasone dipropionate | 2 | 712 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, ‐0.00] |
| 7.3 Potent steroid: betamethasone valerate | 1 | 172 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 7.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 7.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 7.7 Vitamin D in combination: calcipotriol + BMD | 1 | 677 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 7.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 7.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.42, 0.05] |
| 7.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.11 Other treatment: salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 8 Withdrawals due to treatment failure Show forest plot | 9 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Vitamin D: calcipotriol | 2 | 457 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.11, 0.00] |
| 8.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.05] |
| 8.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.4 Very potent steroid: amcinonide | 1 | 165 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 8.5 Very potent steroid: clobetasol propionate | 5 | 1006 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 8.6 Very potent steroid: halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.7 Vitamin D in combination: calcipotriol + BMD | 1 | 677 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.06] |
| 8.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 8.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 12 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Vitamin D: calcipotriol | 3 | 510 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 9.2 Potent steroid: betamethasone dipropionate | 2 | 703 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.01] |
| 9.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Very potent steroid: amcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 Very potent steroid: clobetasol propionate | 4 | 817 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 9.6 Very potent steroid: halcinonide | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| 9.7 Vitamin D in combination: calcipotriol + BMD | 2 | 831 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.13, 0.02] |
| 9.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 9.9 Other treatment: ciclopirox olamine shampoo | 1 | 40 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.24, 0.13] |
| 9.10 Other treatment: fluocinolone acetonide, plus occlusion | 1 | 89 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 9.11 Other treatment: salicylic acid | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.17, 0.17] |
| 10 Adverse events (systemic) Show forest plot | 4 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vitamin D: calcipotriol | 1 | 408 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.2 Potent steroid: betamethasone dipropionate | 1 | 692 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Potent steroid: betamethasone valerate | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Very potent steroid: amcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 Very potent steroid: clobetasol propionate | 2 | 385 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 10.6 Very potent steroid: halcinonide | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.7 Vitamin D in combination: calcipotriol + BMD | 2 | 843 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.8 Other treatment: betamethasone‐17,21‐dipropionate plus salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 Other treatment: ciclopirox olamine shampoo | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 Other treatment: fluocinolone acetonide, plus occlusion | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 Other treatment: salicylic acid | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 IAGI Show forest plot | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.32, 0.64] |
| 1.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.20, 0.55] |
| 1.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.10] |
| 1.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2581 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.44, 0.84] |
| 1.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 2 | 748 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.73, 0.25] |
| 2 TSS Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.28, 0.63] |
| 2.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 487 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.09, 0.27] |
| 2.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.05, 0.69] |
| 2.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.27, ‐0.11] |
| 2.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1978 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [0.56, 0.84] |
| 2.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 3 | 925 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 3 PASI | 0 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 PAGI Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1654 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.31, 0.81] |
| 4.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 1 | 468 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.22, 0.59] |
| 4.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2414 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.25, ‐0.09] |
| 4.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1952 | Std. Mean Difference (IV, Random, 95% CI) | 0.84 [0.61, 1.08] |
| 4.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Combined end point (IAGI/TSS/PASI/PAGI) Show forest plot | 11 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.32, 0.64] |
| 5.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 510 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.20, 0.55] |
| 5.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.05, 0.69] |
| 5.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.10] |
| 5.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2581 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.44, 0.84] |
| 5.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 3 | 835 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.92, 0.02] |
| 6 Total withdrawals Show forest plot | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.04, 0.18] |
| 6.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.00, 0.08] |
| 6.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.07, 0.18] |
| 6.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 6.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2847 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.05, 0.18] |
| 6.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 475 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.07, 0.09] |
| 7 Withdrawals due to adverse events Show forest plot | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 7.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 7.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [‐0.05, 0.15] |
| 7.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 7.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2847 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.09] |
| 7.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.02, 0.14] |
| 8 Withdrawals due to treatment failure Show forest plot | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1676 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 8.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 8.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 8.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2444 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 8.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 2535 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.01, 0.10] |
| 8.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Adverse events (local) Show forest plot | 10 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1652 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.04, 0.11] |
| 9.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 2 | 516 | Risk Difference (M‐H, Random, 95% CI) | 0.17 [0.01, 0.33] |
| 9.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | 0.19 [0.10, 0.28] |
| 9.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 3 | 2415 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 9.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 4 | 2801 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 9.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.24 [0.15, 0.33] |
| 10 Adverse events (systemic) Show forest plot | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMD | 2 | 1666 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.2 Vitamin D vs. corticosteroid (potent): calcipotriol vs. BMV | 1 | 474 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10.3 Vitamin D vs. corticosteroid (very potent): calcipotriol vs. clobetasol propionate | 1 | 151 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 10.4 Vitamin D + corticosteroid vs. corticosteroid: calcipotriol + BMD vs. BMD | 2 | 2216 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.5 Vitamin D vs. vitamin D + corticosteroid: calcipotriol vs. calcipotriol + BMD | 3 | 1970 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 10.6 Vitamin D vs. other treatments: calcipotriol vs. coal tar polytherapy | 1 | 445 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |

