Antibióticos para la pulpitis irreversible
Resumen
Antecedentes
La pulpitis irreversible, que se caracteriza por dolor agudo e intenso, es una de las razones más frecuentes por la que los pacientes asisten a consultorios odontológicos de urgencia. Además de la extracción del diente, la forma habitual de aliviar el dolor de la pulpitis irreversible es perforar el diente, extraer la pulpa (nervio) inflamada y limpiar el conducto radicular. Sin embargo, un número significativo de dentistas sigue prescribiendo antibióticos para aliviar el dolor de la pulpitis irreversible. Esta revisión actualiza la versión anterior publicada en 2016.
Objetivos
Evaluar los efectos de los antibióticos sistémicos para la pulpitis irreversible.
Métodos de búsqueda
Se buscaron estudios en el Registro de ensayos del Grupo Cochrane de Salud Oral (Cochrane Oral Health's Trials Register) (hasta el 18 febrero 2019), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials; CENTRAL, 2019, número 1) en la Cochrane Library (18 de febrero de 2019), MEDLINE Ovid (de 1946 hasta el 18 de febrero de 2019), Embase Ovid (de 1980 hasta el 18 de febrero de 2019), el US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (18 de febrero de 2019), y en la World Health Organization International Clinical Trials Registry Platform (el 18 de febrero de 2019). No hubo restricciones de idioma en las búsquedas de las bases de datos electrónicas.
Criterios de selección
Ensayos controlados aleatorizados que compararan el alivio del dolor con antibióticos y analgésicos sistémicos, versus placebo y analgésicos en la fase preoperatoria aguda de la pulpitis irreversible.
Obtención y análisis de los datos
Tres autores de la revisión seleccionaron los estudios y extrajeron los datos de forma independiente. La calidad de la evidencia de los estudios incluidos se evaluó mediante GRADE. No fue posible realizar el agrupamiento de los datos y se presenta un resumen descriptivo.
Resultados principales
En esta actualización, no se ha podido incluir ningún ensayo adicional. Un ensayo de bajo riesgo de sesgo, que evaluó la penicilina oral combinada con analgésicos versus placebo con analgésicos, con 40 participantes, se incluyó en una actualización anterior de la revisión. La certeza de la evidencia se consideró baja para los diferentes resultados. El resultado primario fue el dolor (intensidad/duración) y el alivio del dolor informados por el paciente. Hubo una distribución paralela cercana de las calificaciones del dolor tanto en la intervención (mediana 6,0; rango intercuartil 10,5) como en el placebo (mediana 6,0; rango intercuartil 9,5) durante el período de estudio de siete días. No hubo evidencia suficiente para afirmar o refutar un beneficio de la penicilina para la intensidad del dolor. No hubo diferencias significativas en el número total medio de comprimidos de ibuprofeno durante el período de estudio: 9,20 (desviación estándar [DE] 6,02) en el grupo de penicilina versus 9,60 (DE 6,34) en el grupo de placebo; diferencia de medias ‐0,40; intervalo de confianza (IC) del 95%: ‐4,23 a 3,43; P = 0,84). Lo anterior se dio igualmente para el número total medio de comprimidos de Tylenol: 6,90 (DE 6,87) en el grupo de penicilina versus 4,45 (DE 4,82) en el grupo de placebo; diferencia de medias 2,45 (IC del 95%: ‐1,23 a 6,13; P = 0,19). El resultado secundario del informe de los eventos adversos no se consideró en este estudio.
Conclusiones de los autores
Esta revisión Cochrane, basada en un ensayo con poco poder estadístico que incluyó una muestra pequeña y que se evaluó como en riesgo bajo de sesgo, refleja que no hay evidencia suficiente para determinar si los antibióticos alivian o no el dolor en comparación con la ausencia de administración de antibióticos. Los resultados de esta revisión confirman la necesidad de ensayos adicionales con una muestra más grande y metodológicamente sólidos que puedan aportar evidencia adicional sobre si los antibióticos, prescritos en la fase preoperatoria, pueden afectar los resultados del tratamiento para la pulpitis irreversible.
PICO
Resumen en términos sencillos
Administración de antibióticos para la odontalgia intensa (pulpitis irreversible)
Pregunta de la revisión
¿Los antibióticos orales son efectivos y seguros para tratar el dolor en la pulpitis irreversible (inflamación del nervio dentro del diente / nervio dañado)?
Antecedentes
La pulpitis irreversible ocurre cuando la pulpa dentaria (tejido dentro del diente que contiene el nervio) presenta un daño que no puede repararse. Se caracteriza por dolor intenso (odontalgia), suficiente para despertar a alguien por la noche, y se considera una de las razones más frecuentes por la cual los pacientes asisten a consultorios odontológicos de urgencia. Cualquier diente puede verse afectado, no está restringida a grupos etarios concretos, y generalmente ocurre como un resultado directo de la caries dental, rajaduras en el diente o un traumatismo.
El “estándar de atención” para la pulpitis irreversible ‐ extracción inmediata de la pulpa del diente afectado ‐ ahora es aceptado ampliamente y todavía en ciertas partes del mundo se siguen prescribiendo antibióticos.
Características de los estudios
La evidencia sobre la cual se basa esta revisión se actualizó el 18 de febrero de 2019. Se incluyó un estudio con 40 pacientes con pulpitis irreversible (daño en el nervio). Hubo dos grupos de 20 pacientes, un grupo fue tratado con penicilina de 500 mg, el otro con placebo (ningún principio activo) cada seis horas durante un período de siete días. Además, todos los participantes recibieron analgésicos (ibuprofeno y paracetamol [acetaminofeno] combinados con codeína).
Resultados clave
Los antibióticos no parecen reducir significativamente la odontalgia causada por la pulpitis irreversible. Además, no hubo diferencias en el número total de comprimidos de ibuprofeno o Tylenol utilizados durante el período de estudio entre ambos grupos. La administración de penicilina no reduce significativamente la percepción de dolor, la percepción de percusión (golpes suaves en el diente) o la cantidad de medicación para el dolor requerida por los pacientes con pulpitis irreversible. No hubo ningún informe sobre las reacciones o los eventos adversos.
Certeza de la evidencia
Este estudio incluyó un número pequeño de participantes y la certeza de la evidencia sobre los diferentes resultados se consideró baja. Actualmente no hay evidencia suficiente para poder decidir si los antibióticos ayudan en este trastorno. Esta revisión destaca la necesidad de más estudios de mejor de calidad sobre la administración de antibióticos para la pulpitis irreversible.
Authors' conclusions
Summary of findings
| Antibiotics for irreversible pulpitis | ||||||
| Patient or population: patients with irreversible pulpitis Control: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics | |||||
| Patient‐reported pain intensity Follow‐up: 7 days | The in between‐group differences (median ± IQR) in SPID for the penicillin group were 6.0 ± 10.5, and for placebo 6.0 ± 9.5 The SPPID for the penicillin group was 3.5 ± 7.5 and for placebo 2.0 ± 7.0 | 40 | ⊕⊕⊝⊝ | The in‐between group differences in SPID and SPPID were not statistically significant2 | ||
| Patient‐reported pain relief | Outcome not reported | |||||
| Total number of ibuprofen tablets Follow‐up: 7 days | The mean total number of ibuprofen tablets in the control groups was 9.60 tablets | The mean total number of ibuprofen tablets in the intervention groups was 0.40 lower | ‐ | 40 | ⊕⊕⊝⊝ | The administration of penicillin over placebo did not appear to significantly reduce the quantity of ibuprofen consumed for irreversible pulpitis |
| Total number of paracetamol (acetaminophen) + codeine tablets Follow‐up: 7 days | The mean total number of acetaminophen + codeine tablets in the control groups was 4.45 tablets | The mean total number of acetaminophen + codeine tablets in the intervention groups was 2.45 higher | ‐ | 40 | ⊕⊕⊝⊝ | The administration of penicillin over placebo did not appear to significantly reduce the quantity of Tylenol consumed for irreversible pulpitis |
| Number of adverse events | Outcome not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 2 levels for very serious imprecision (very small sample size). | ||||||
Background
Dental emergencies are extremely common. A survey conducted in the UK recorded that 16% of the population had experienced toothache in the preceding year (Steele 2011). Non‐traumatic dental condition visits account for 1.4% of all emergency department visits in the USA and have shown an annual rise of 4% (from 1% in 1997 to 1.7% in 2007) (Onkunseri 2012). Dental caries (tooth decay) is the result of bacterial attack on a tooth and is the precursor to irreversible pulpitis, which is considered to be an immune system mediated event affecting the dental pulp (nerve) rather than an infective condition of the pulp (Bergenholtz 1990). Acute and intense pain are the most typical presenting symptoms of irreversible pulpitis. It occurs more commonly in vital teeth beneath deep caries before the bacteria have even reached the pulp (Hahn 1991). Thus the involved tooth will usually have an extensive restoration (filling) or caries or both under which death of the pulp may occur quite quickly or which may take years to occur even if the dental caries is removed (Tronstad 1991).
Description of the condition
Irreversible pulpitis is caused by a vital inflamed pulp which is incapable of healing (Hargreaves 2015). The symptoms are a continuum and can vary but usually include a history of spontaneous pain which may also involve an exaggerated response to hot or cold that lingers after the stimulus is removed (Soames 1998). Any tooth may be affected by irreversible pulpitis, it is not restricted to particular age groups and occurs as a direct result of dental caries, a cracked tooth, or trauma. The involved tooth is usually not sensitive to percussion, and palpation tests do not produce an untoward reaction. The characteristics of irreversible pulpitis are a vital pulp which responds to cold and electric pulp testing. A number of variations of irreversible pulpitis have been recognised (Hargreaves 2015). These include acute, subacute, chronic, partial or total, infected or sterile, however it is not possible to clearly differentiate these except by histopathological methods.
Description of the intervention
Although not indicated by current guidance, it is recognised that a range of oral antibiotics with differing dosing regimens may in fact be prescribed (SDCEP 2016). Antibiotics commonly prescribed by dentists include: ß‐lactams (amoxicillin, penicillin V, co‐amoxiclav), macrolides, tetracyclines, clindamycin, and metronidazole (Dar‐Odeh 2010).
How the intervention might work
Pulpitis is an inflammatory reaction of the pulp and often occurs without any evidence of bacteria in the pulp chamber. Antibiotics have bactericidal or bacteriostatic properties or both, and are used widely to control or eliminate bacteria, but the mode of action and extent to which antibiotics have an anti‐inflammatory or analgesic effect in irreversible pulpitis remains less clear.
Why it is important to do this review
There is limited and what appears to be largely anecdotal evidence to support the routine prescribing of antibiotics for irreversible pulpitis. It is likely that the practice of prescribing of antibiotics may have arisen due to a misconception of the natural pathological process of pulpitis (pain resolves when the pulp devitalises irrespective of antibiotic use), or the perception that antibiotics should be prescribed prophylactically in anticipation of pain arising prior to endodontic treatment. Either of these approaches may have promoted the inappropriate prescribing of antibiotics for endodontic emergencies. A study conducted in the USA of members of the American Association of Endodontists (AAE) surveyed their prescribing practices and reported that 16.7% of the specialist endodontists prescribed antibiotics for cases of irreversible pulpitis (Yingling 2002). A similar study in Spain with members of the Spanish Endodontic Society found a figure of 40% (Rodriguez‐Núñez 2009). General dental practitioners are often the first point of contact for patients with irreversible pulpitis and although one study conducted in Belgium reported that a smaller proportion (4.3%) of general dentists continue to prescribe antibiotics for irreversible pulpitis (Mainjot 2009), another study conducted in Spain indicated that a substantial number (86%) of respondents continue to do so (Segura‐Egea 2010). A more recent study in UK general dental practices found 19.4% of dental antibiotics were prescribed for irreversible pulpitis (Cope 2016), whilst a UK dental hospital's acute dental care department audit found 70% of antibiotics prescribed for pulpitis in the first cycle of a clinical audit and 20% in the second cycle (Chopra 2014). To further investigate the prescribing behaviours of the general dentists and endodontists, we conducted a survey with the same question as of this review and found that one in every four dentists would prescribe antibiotics, when not needed (Agnihotry 2014a). In a more recent study (Cope 2016), it was found out that more than half of the sample size of general dentists (65.6%) prescribed antibiotics when there was no evidence of spreading infection.
Unnecessary use of antibiotics is driving the global increase in drug‐resistant infections and action is required across government and society including health care and agriculture (O'Neill 2016). Antibiotics are the corner stone of modern medicine; without effective antibiotics the success of major surgery and cancer chemotherapy will be compromised (WHO 2018). Life‐threatening infections caused by Klebsiella pneumoniae which are now resistant to the last resort treatment (carbapenems) have spread worldwide; treatment failure to the last resort of medicine for gonorrhoea (third generation cephalosporin antibiotics) has been confirmed in at least 10 countries (Australia, Austria, Canada, France, Japan, Norway, Slovenia, South Africa, Sweden, and the UK (WHO 2018). By 2050, it is expected that deaths from antimicrobial resistant infections will be higher than from cancer (O'Neill 2016). Although the inappropriate prescribing of antibiotics for endodontic emergencies has received much attention (Agnihotry 2014; Fouad 1996; Palmer 2003; Segura‐Egea 2018), it remains unclear to what extent this may have contributed to the development of resistant strains of bacteria and the growing problem of antibiotic resistance (CDC 2008; SMAC 1997).
Other adverse events associated with dental antibiotic use have been reported, including its contribution to the incidence of Clostridium difficile in the community (Bye 2017). Antibiotic‐related colitis caused by C diff is associated with significant morbidity and can be life threatening, especially for elderly and medically compromised patients (Beacher 2015). Increasing rates of anaphylaxis to antibiotics have also been reported (Turner 2015).
Irreversible pulpitis, at least in the early phase, is not normally accompanied by the clinical signs of bacterial infection, i.e. swelling and tenderness of adjacent mucosa, which more generally manifests itself after the pulp has become necrotic and the infected pulpal tissues pass into the periapical region (Cope 2018). Although some dentists continue to prescribe antibiotics, there appears to be very limited evidence that penicillin reduces pain, percussion sensitivity, or the amount of analgesics required in untreated teeth diagnosed with irreversible pulpitis (Nagle 2000).
Immediate pulpectomy is now widely accepted as the 'standard of care' for irreversible pulpitis (Segura‐Egea 2018; Walton 2009) and yet in certain parts of the world antibiotics continue to be prescribed. We consider that a systematic review is still necessary to provide further evidence of the effects of antibiotics and ultimately more clarity and guidance in the management of this clinical condition.This review updates the previous version published in 2016 (Agnihotry 2016).
Objectives
To assess the effects of systemic antibiotics for irreversible pulpitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were considered in this review.
Types of participants
We included adult patients who were over the age of 18 and presented with a single tooth with a clinical diagnosis of irreversible pulpitis.
Types of interventions
Active interventions
Administration of any systemic antibiotic at any dosage and any analgesic at any dosage prescribed in the acute preoperative phase of irreversible pulpitis.
Control
Administration of placebo and any analgesic, at any dosage, prescribed in the acute preoperative phase of irreversible pulpitis.
Types of outcome measures
Primary outcomes
-
Patient‐reported pain (intensity/duration) and pain relief measured on a categorical scale in the preoperative phase of irreversible pulpitis.
Secondary outcomes
-
Type, dose, and frequency of medication required for pain relief.
-
Any adverse effects related to any clinically diagnosed hypersensitivity or other reactions to either the antibiotics or analgesics.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions.
For this update we searched the following databases:
-
Cochrane Oral Health's Trials Register (searched 18 February 2019) (Appendix 1);
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1) in the Cochrane Library (searched 18 February 2019) (Appendix 2);
-
MEDLINE Ovid (1946 to 18 February 2019) (Appendix 3);
-
Embase Ovid (1980 to 18 February 2019) (Appendix 4).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid.
Searching other resources
The following trial registries were searched for ongoing studies (see Appendix 5 for the search strategy):
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (searched 18 February 2019);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 18 February 2019).
Only handsearching done as part of the Cochrane worldwide handsearching programme and uploaded to CENTRAL was included.
We searched reference lists of relevant articles and clinical trials in an attempt to identify any potential or additional studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Three review authors independently assessed the titles and abstracts of studies resulting from the searches. All irrelevant records were excluded and only details of potential studies were noted. Full copies were obtained of all relevant and potentially relevant studies which appeared to meet the inclusion criteria, or when there were insufficient data in the title and abstract to make a clear decision. Studies not matching our inclusion criteria were excluded and their details and reasons for their exclusion were noted in the Characteristics of excluded studies table in Review Manager (RevMan) (Review Manager 2014).
Data extraction and management
Study details were entered into the Characteristics of included studies table. We collected outcome data using a predetermined form and entered them into RevMan. The review authors only included data if there was an independently reached consensus. All disagreements were resolved by discussion.
We extracted the following details.
-
Study methods: method of allocation, masking of participants and outcomes.
-
Participants: country of origin, sample size, age, sex, inclusion and exclusion criteria.
-
Intervention: type of antibiotic.
-
Control: analgesic, placebo or nil.
-
Outcomes: primary and secondary outcomes as described in the Types of outcome measures section of this review.
Assessment of risk of bias in included studies
Each of two review authors then graded the selected studies separately according to the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011) (Higgins 2011). The gradings were compared and any inconsistencies between the review authors were discussed and resolved.
The following domains were assessed as at 'low risk' of bias (i.e. plausible bias unlikely to seriously alter the results), 'unclear' (i.e. uncertain risk of bias, plausible risk of bias that raises some doubts about the results), or 'high risk' of bias (plausible bias that seriously weakens confidence in the results):
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, personnel and outcomes assessors);
-
incomplete outcome data;
-
selective outcome reporting; and
-
other sources of bias.
We categorised and reported the overall risk of bias in the included study according to the following:
-
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
-
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
-
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
These assessments are reported for the included study in the Characteristics of included studies table.
Measures of treatment effect
The trialists in Nagle 2000 used sum of pain intensity difference (SPID) and sum of pain percussion intensity difference (SPPID) to assess between‐group differences. Values were expressed as medians with interquartile ranges and were analysed using the Mann‐Whitney‐Wilcoxon test. Each patient was asked to rate their pain on a scale from 0 to 3 (0 = no pain; 1 = mild pain, pain that was recognizable but not discomforting; 2 = moderate pain, pain that was discomforting but bearable; 3 = severe pain, pain that caused considerable discomfort and was difficult to bear). Patients were asked to rate the pain to percussion using the same scale. SPID is defined as the sum of pain intensity differences weighted by the length of the interval since the previous observation. These assessments were made at wake‐up time over the seven‐day study period. We were unsuccessful in our attempts to contact the investigators to provide us with means or ranges of the minimum and maximum scores for SPID and SPPID, and therefore we were unable to calculate and present means, standard deviations and confidence intervals for these outcomes. These have been discussed narratively based on the data as reported in the study (see Effects of interventions).
We have presented the continuous outcomes on the original scale as reported in the study for our secondary outcome 'number of painkillers' together with their associated 95% confidence intervals (CIs). These data were analysed in RevMan (Review Manager 2014) using a random‐effects model.
For future studies we will present continuous outcomes on the original scale as reported in each individual study. If similar outcomes were reported using different scales, we would convert these to standardized mean differences (SMD).
We will present dichotomous outcomes as risk ratios (RR), and if found significant, we would convert them to the number needed to treat (NNT) to find one success. We will report all outcomes' data with their associated 95% CIs and analyse the data using a random‐effects model in RevMan, with a general inverse variance (DerSimonian and Laird method), unless stated otherwise. In cases where only medians are presented with ranges, the mean is estimated by the median, and the variance using the range and the number of observations (Hozo 2005).
Unit of analysis issues
It is possible that studies included in future updates may present data from repeated observations on participants which may lead to unit of analysis errors, if so we will follow the advice provided in section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
There were no missing data in the single included study. For future updates, if data are missing attempts will be made to contact the trial investigators.
Assessment of heterogeneity
There was only one single trial and therefore no assessments were made.
If further studies are included in future updates, we will assess clinical heterogeneity by examining the characteristics of the studies, the similarity between the types of participants, the interventions and outcomes as specified in the criteria for included studies. Statistical heterogeneity will be assessed using a Chi2 test and the I2 statistic where I2 values over 60% indicate moderate to substantial heterogeneity (Higgins 2011). If this could be explained by clinical reasoning and a coherent argument can be made for combining the studies, we will enter these into a meta‐analysis. In cases where the heterogeneity could not be adequately explained, the data will not be pooled. A cut off P value of > 0.10 would be used to determine statistical significance.
Assessment of reporting biases
If a sufficient number (> 10) of trials investigating similar interventions are identified for inclusion in future updates of this review, publication bias will be assessed according to the recommendations on testing for funnel plot asymmetry as described in section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry is identified, we will try to assess other possible causes and these will be explored in the discussion if appropriate.
Data synthesis
If further studies are included the following methods of data synthesis will apply. Data will be analysed using RevMan and reported according to Cochrane criteria. Pooling of data will only occur if the included studies have similar interventions involving similar participants. We will present risk ratios for outcomes and odds ratios for adverse effect outcomes. The risk ratio is the ratio of the risk of an event in the two groups whereas the odds ratio is the ratio of the odds of an adverse event in the intervention group to the odds of an event in the control group. Additionally, any data obtained from visual analogue scales and any categorical outcomes will be transformed into dichotomous data prior to analysis if appropriate. Risk ratios, the number needed to treat and their 95% confidence intervals will be calculated for all dichotomous data.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of studies with moderate to substantial heterogeneity (as defined above) are identified we will carry out subgroup analyses based on different antibiotics and dosing regimens.
Sensitivity analysis
We had expected to be able to conduct sensitivity analyses to assess the robustness of our review results by repeating the analysis with the following adjustments: exclusion of studies at high risk of bias and unpublished studies. However, as there was only a single trial that matched our inclusion criteria no sensitivity analyses were carried out.
Summary of findings
We developed a 'Summary of findings' table following GRADE methods (GRADE 2004) and using GRADEproGDT software (GRADEpro GDT 2015) for the following outcomes listed according to priority.
-
Patient‐reported pain intensity (sum pain intensity differences and sum pain percussion intensity differences).
-
Patient‐reported pain relief.
-
Total number of ibuprofen tablets.
-
Total number of paracetamol (acetaminophen) + codeine tablets.
-
Number of adverse events.
The certainty of the body of evidence was assessed with reference to the overall risk of bias of the included study, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, and the magnitude of the effect. We categorised the certainty of the body of evidence for each outcome as high, moderate, low, or very low.
Results
Description of studies
Results of the search
The search strategy used in the first version of this review in 2005 identified 39 references of which all but four were excluded from further analysis. Full‐text copies of these four papers were obtained for further assessment. Only one study (Nagle 2000) met the inclusion criteria and is included in the review. No additional studies were identified for inclusion based on subsequent updated searches in February 2009, September 2013, January 2016, or February 2019 (Figure 1).
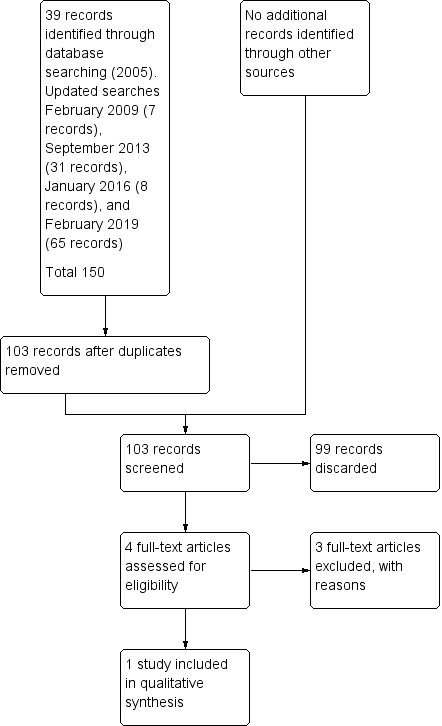
Study flow diagram.
Included studies
Methods
Nagle 2000 is a randomised double‐blind placebo‐controlled clinical trial conducted in the emergency department of a university dental college in the USA.
Participants and setting
Forty adult patients, 17 male, 23 female, with an age range of 30 to 34 years who had presented as an emergency with spontaneous moderate to severe pain associated with a tooth, participated in this study. All of the teeth were vital and responsive to an electric pulp tester (EPT) and to Endo Ice and displayed percussion sensitivity. The diagnosis of irreversible pulpitis was confirmed by a radiographically widened periodontal ligament space (see Additional Table 1).
| Penicillin | Placebo | |
| Initial pain (median & interquartile range) | 2.00 ± 0.00 | 2.00 ± 1.00 |
| Initial percussion pain (median & interquartile range) | 2.00 ± 0.50 | 2.00 ± 1.00 |
| Pain ratings: moderate | 65% | 80% |
| Pain ratings: severe | 35% | 20% |
| Percussion pain ratings: mild | 20% | 25% |
| Percussion pain ratings: moderate | 50% | 65% |
| Percussion pain ratings: severe | 30% | 10% |
Intervention
Twenty participants were allocated to antibiotic and analgesic and 20 to placebo and analgesic. The participants received a seven‐day oral dose (28 capsules each to be taken every six hours) of either penicillin (500 mg) or a placebo control in which the participants and trialists were double‐blinded. They also received a supply of pain medication consisting of ibuprofen 600 mg; paracetamol (acetaminophen) with codeine 30 mg (Tylenol). No operative endodontic treatment was performed during the course of the study.
Outcomes
The primary outcome for this review was pain relief in the preoperative phase of irreversible pulpitis. Participants in this study were requested to complete a seven‐day diary in which they recorded pain, percussion pain, and the quantity and type of pain medication taken. Pain was assessed using a short ordinal numerical scale graded from 0 to 3 (see Measures of treatment effect). Additionally, the patients were asked to use the same scale to rate pain on percussion which was achieved by tapping the affected tooth with a finger. The pain scale used in this trial had been used in previous pain studies which were referenced by the trialists of the included study.
The secondary outcome was the type and dose of pain medication required to achieve pain relief. The participants in this study were instructed to initially take one tablet of the ibuprofen every four to six hours as needed for pain and to take the Tylenol (two tablets every four to six hours) only if the ibuprofen did not relieve their pain. Each participant received a seven‐day diary to record their symptoms and the number and type of pain medication taken. No assessments of adverse effects to either the antibiotics or analgesics were considered or reported by the investigators.
Excluded studies
Three studies were excluded: a systematic review (Matthews 2003) which included a potential trial (Henry 2001) which was subsequently excluded as it investigated the effect of antibiotics on postoperative endodontic pain. One trial (Fouad 1996) was excluded as it combined the interventions with immediate operative endodontic treatment. We excluded Nusstein 2003 because it was a retrospective non‐experimental study, see Characteristics of excluded studies for further details.
Risk of bias in included studies
The single included study (Nagle 2000) met all of the criteria across all of the domains in Cochrane's tool for assessing the risk of bias, and therefore this study was considered to be at low risk of bias (plausible bias unlikely to seriously alter the results) (Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
In this study the intervention (penicillin) and control (placebo) groups were assigned before the experiment by using four‐digit numbers from a random number table. The method used to generate the allocation sequence was described in sufficient detail; therefore, this domain was judged as at low risk of bias.
Allocation concealment
To ensure adequate concealment only the random numbers were recorded on the data collection and postoperative diary sheets and it was unlikely that allocation could be foreseen and therefore this domain was judged as at low risk of bias.
Blinding
The measures used to blind study participants and personnel from knowledge of which intervention a participant received as well as blinding of outcomes assessors were described in sufficient detail. The medications were blinded, randomised, and packaged by a pharmacy. Each 500 mg gelatin capsule of either penicillin or placebo was identical in form. The 500 mg tablets of penicillin VK were ground into a powder and placed into the clear, unlabelled gelatin capsules. The white powder of the lactose placebo was indistinguishable from the white powder of the penicillin tablets when viewed through the capsule.
Incomplete outcome data
The report was complete and there were no missing data and this domain was judged as at low risk of bias.
Selective reporting
There was no evidence of selective outcome reporting and the outcomes listed in the methods section were comparable to the reported results. This was judged as at low risk of bias.
Other potential sources of bias
There was no evidence of other potential sources of bias in the report of the included trial.
Effects of interventions
See: Summary of findings for the main comparison Antibiotics for irreversible pulpitis
The single included study (Nagle 2000) did not provide sufficient data to perform a statistical analysis on the primary outcome and the only data presented are those which were published in the study. Unsuccessful attempts to obtain additional and individual level data from the trialists made it difficult to confirm the results presented in their study (see Measures of treatment effect).
Oral penicillin in combination with analgesics versus placebo with analgesics
Primary outcome: patient‐reported pain (intensity/duration) and pain relief
Baseline data indicated that all of the participants that entered the study had moderate to severe pain (Additional Table 1). After the first day of the study the average pain rating decreased and remained quite stable over the following six days. This initial decrease in pain may be considered to be due to the effect of the analgesics which was sustained by the gradual and progressive necrosis of the pulp. However, at the end of the study period and at the commencement of operative endodontic treatment it was found that 75% of the teeth in the penicillin group and 80% in the placebo were still vital.
There was a close parallel distribution of the pain ratings in both the intervention and placebo groups over the seven days. The following data were presented as medians with their interquartile range. The in between‐group differences in sum of pain intensity difference (SPID) for the penicillin group were 6.0 ± 10.5, and for placebo 6.0 ± 9.5, P = 0.776. The sum of pain percussion intensity difference (SPPID) for the penicillin group was 3.5 ±7.5 and placebo 2.0 ± 7.0, P = 0.290, with differences as assessed by the Mann‐Whitney‐Wilcoxon test considered by the investigators of the study to be statistically significant at P < 0.05 (Additional Table 2). (See Measures of treatment effect for additional information on these data.)
| Penicillin | Placebo | P value | |
| Sum of pain intensity difference (median and interquartile range) | 6.0 ± 10.5 | 6.0 ± 9.5 | 0.776 |
| Sum of percussion pain intensity difference (median and interquartile range) | 3.5 ± 7.5 | 2.0 ± 7.0 | 0.290 |
Secondary outcome: type, dose and frequency of medication required for pain relief
The number, percentage and average use and non‐use of ibuprofen and Tylenol are summarised in Additional Table 3.
On both day one and day two only one participant did not take either one or other of the analgesic medications. The number not taking any medication increased to three to four (15% to 20%) on day three, and two to six (10% to 30%) on day four. On the fifth to seventh days only four to seven (20% to 35%) did not take any additional pain medication. At day seven, 20% of the penicillin group and 35% of the placebo group took no additional analgesics.
There was no significant difference in the mean total number of ibuprofen tablets over the study period: 9.20 (standard deviation (SD) 6.02) in the penicillin group versus 9.60 (SD 6.34) in the placebo group; mean difference ‐0.40 (95% confidence interval (CI) ‐4.23 to 3.43; P = 0.84). The same was true for the mean total number of Tylenol tablets: 6.90 (SD 6.87) in the penicillin group versus 4.45 (SD 4.82) in the placebo group; mean difference 2.45 (95% CI ‐1.23 to 6.13; P = 0.19). There was insufficient evidence to determine whether penicillin reduced the quantity of analgesic medication or not.
| Day | Number ibuprofen | Number Tylenol | Nil pain medication |
| Day 1 | |||
| Penicillin | 17 (85%) | 10 (50%) | 1 (5%) |
| Number of tablets | 33 | 21 | 0 |
| Placebo | 16 (80%) | 8 (40%) | 0 |
| Number of tablets | 28 | 11 | 0 |
| Day 2 | |||
| Penicillin | 17 (85%) | 10 (50%) | 0 |
| Number of tablets | 30 | 28 | 0 |
| Placebo | 16 (80%) | 9 (45%) | 1 (5%) |
| Number of tablets | 31 | 18 | 0 |
| Day 3 | |||
| Penicillin | 13 (65%) | 9 (45%) | 4 (20%) |
| Number of tablets | 27 | 20 | 0 |
| Placebo | 15 (75%) | 8 (40%) | 3 (15%) |
| Number of tablets | 28 | 14 | 0 |
| Day 4 | |||
| Penicillin | 12 (60%) | 9 (45%) | 6 (30%) |
| Number of tablets | 24 | 23 | 0 |
| Placebo | 17 (85%) | 5 (25%) | 2 (10%) |
| Number of tablets | 28 | 8 | 0 |
| Day 5 | |||
| Penicillin | 12 (60%) | 8 (40%) | 7 (35%) |
| Number of tablets | 21 | 15 | 0 |
| Placebo | 16 (80%) | 7 (35%) | 3 (15%) |
| Number of tablets | 32 | 11 | 0 |
| Day 6 | |||
| Penicillin | 13 (65%) | 8 (40%) | 5 (25%) |
| Number of tablets | 24 | 15 | 0 |
| Placebo | 13 (65%) | 6 (30%) | 6 (30%) |
| Number of tablets | 24 | 13 | 0 |
| Day 7 | |||
| Penicillin | 14 (70%) | 10 (50%) | 4 (20%) |
| Number of tablets | 25 | 16 | 0 |
| Placebo | 11 (55%) | 7 (35%) | 7 (35%) |
| Number of tablets | 20 | 14 | 0 |
Secondary outcome: adverse events
Not assessed.
Discussion
Summary of main results
The results of this well constructed but underpowered trial of 20 participants in each study arm indicate that the administration of penicillin did not appear to significantly (P > 0.05) reduce either the pain perception, the percussion perception, or the quantity of analgesic medication required by patients with irreversible pulpitis. Our secondary outcome regarding adverse events or reactions was not addressed. The certainty of the evidence was rated low for the different outcomes. For further details see summary of findings Table for the main comparison.
The significance of the relatively common occurrence of toothache, the prevalence of inappropriate prescribing of antibiotics with the potential for producing antibiotic resistance and patient sensitisation cannot be underestimated. It was somewhat disappointing to see that only one single trial matched our inclusion criteria.
Overall completeness and applicability of evidence
The single included study (Nagle 2000) provides insufficient evidence that the administration of antibiotics is effective in relieving the pain from irreversible pulpitis. However, although we consider that the population, intervention, comparator to the intervention, and outcome of interest satisfy the clinical question of our review, the lack of further research since this study was conducted, which is still highly desirable, would appear to indicate that there is a wider acceptance that the 'standard of care' and appropriate management strategy for irreversible pulpitis is immediate extirpation of the pulp.
Quality of the evidence
The certainty of the evidence as summarised in summary of findings Table for the main comparison was rated as low. The most important reasons for downgrading for each outcome were: imprecision, mainly due to low sample size and the 95% confidence interval included no effect and the upper or lower confidence limit crossed the minimal important difference.
Limitations in study design and implementation
We did not identify any limitations in either the design or implementation of the study (Figure 2). However, adverse events were not addressed in this study.
Indirectness of the evidence
Although limited to a single study, the evidence can be directly generalized to the clinical scenario of the presentation of irreversible pulpitis.
Inconsistency of the results
The single included study did not allow any assessment of inconsistency of results.
Imprecision of the results
The single study with a low sample size included in this review provided limited amounts of data. Our primary outcome was downgraded due to small sample size, and due to the sparse data we were unable to further evaluate the imprecision of the results. However, for our secondary outcome we downgraded twice as the confidence intervals included no effect and both the upper and lower confidence limit crossed the minimal important difference.
Publication bias
Although it would be reasonable to assume that the comprehensive searches will have identified all existing randomised controlled trials, and thereby helped to limit bias in the conduct of this review, the absence of any published trials over the last 10 years creates a measure of uncertainty that there may be further and as yet unpublished studies which might add to the overall evidence.
Potential biases in the review process
We made every attempt to limit bias in the review process by ensuring a comprehensive search for potentially eligible studies. The authors' independent assessments of eligibility of studies for inclusion in this review minimised the potential for selection bias. The effects of language bias on the identification and selection of studies for inclusion in a systematic review is widely recognised; therefore, we ensured that language of publication was not used as an exclusion criterion.
Agreements and disagreements with other studies or reviews
Our electronic searches did identify two systematic reviews (Aminoshariae 2016; Matthews 2003) which offered strong confirmatory evidence that in the absence of systemic complications e.g. fever, lymphadenopathy, cellulitis or in immunocompromised patients, antibiotics alone have no place in the management of localised acute apical abscess. Furthermore, they stated that although the pain from acute apical abscess is as a result of an infective process, the infection is localised and that even in this terminal stage of irreversible pulpitis the use of antibiotics as a sole or concomitant therapy remains questionable.
In our search for additional studies and reviews, we also examined several clinical references and sources for guidelines and systematic reviews: Agency for Healthcare Research and Quality (www.ahrq.gov), National Guidelines Clearinghouse (www.guideline.gov), National Institute for Health and Care Excellence (www.nice.org.uk), Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/index.html), UK Database of Uncertainties about the Effects of Treatments (www.library.nhs.uk/duets), and UpToDate (www.uptodate.com/home). It was surprising to find that the majority did not address this clinical topic or provided very limited useful or current information that could aid clinical decision‐making.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Core elements | Issues to consider | Status of research for this review |
| Evidence (E) | What is the current state of the evidence? | This systematic review identified 1 randomised controlled trial |
| Population (P) | Diagnosis, disease stage, comorbidity, risk factors, gender, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Inclusion criteria
Exclusion criteria
|
| Intervention (I) | Type, frequency, dose, duration, prognostic factor | Any systemic antibiotic at any dosage and any analgesic at any dosage prescribed in the acute preoperative phase of irreversible pulpitis |
| Comparison (C) | Type, frequency, dose, duration, prognostic factor | Placebo and any analgesic, at any dosage, prescribed in the acute preoperative phase of irreversible pulpitis |
| Outcome (O) | Which clinical or patient‐related outcomes will the researcher need to measure, improve, influence, or accomplish? Which methods of measurement should be used? |
|
| Time stamp (T) | Date of literature search or recommendation | 18 February 2019 |
| Study type | What is the most appropriate study design to address the proposed question? |
|
| Antibiotics for irreversible pulpitis | ||||||
| Patient or population: patients with irreversible pulpitis Control: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics | |||||
| Patient‐reported pain intensity Follow‐up: 7 days | The in between‐group differences (median ± IQR) in SPID for the penicillin group were 6.0 ± 10.5, and for placebo 6.0 ± 9.5 The SPPID for the penicillin group was 3.5 ± 7.5 and for placebo 2.0 ± 7.0 | 40 | ⊕⊕⊝⊝ | The in‐between group differences in SPID and SPPID were not statistically significant2 | ||
| Patient‐reported pain relief | Outcome not reported | |||||
| Total number of ibuprofen tablets Follow‐up: 7 days | The mean total number of ibuprofen tablets in the control groups was 9.60 tablets | The mean total number of ibuprofen tablets in the intervention groups was 0.40 lower | ‐ | 40 | ⊕⊕⊝⊝ | The administration of penicillin over placebo did not appear to significantly reduce the quantity of ibuprofen consumed for irreversible pulpitis |
| Total number of paracetamol (acetaminophen) + codeine tablets Follow‐up: 7 days | The mean total number of acetaminophen + codeine tablets in the control groups was 4.45 tablets | The mean total number of acetaminophen + codeine tablets in the intervention groups was 2.45 higher | ‐ | 40 | ⊕⊕⊝⊝ | The administration of penicillin over placebo did not appear to significantly reduce the quantity of Tylenol consumed for irreversible pulpitis |
| Number of adverse events | Outcome not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded 2 levels for very serious imprecision (very small sample size). | ||||||
| Penicillin | Placebo | |
| Initial pain (median & interquartile range) | 2.00 ± 0.00 | 2.00 ± 1.00 |
| Initial percussion pain (median & interquartile range) | 2.00 ± 0.50 | 2.00 ± 1.00 |
| Pain ratings: moderate | 65% | 80% |
| Pain ratings: severe | 35% | 20% |
| Percussion pain ratings: mild | 20% | 25% |
| Percussion pain ratings: moderate | 50% | 65% |
| Percussion pain ratings: severe | 30% | 10% |
| Penicillin | Placebo | P value | |
| Sum of pain intensity difference (median and interquartile range) | 6.0 ± 10.5 | 6.0 ± 9.5 | 0.776 |
| Sum of percussion pain intensity difference (median and interquartile range) | 3.5 ± 7.5 | 2.0 ± 7.0 | 0.290 |
| Day | Number ibuprofen | Number Tylenol | Nil pain medication |
| Day 1 | |||
| Penicillin | 17 (85%) | 10 (50%) | 1 (5%) |
| Number of tablets | 33 | 21 | 0 |
| Placebo | 16 (80%) | 8 (40%) | 0 |
| Number of tablets | 28 | 11 | 0 |
| Day 2 | |||
| Penicillin | 17 (85%) | 10 (50%) | 0 |
| Number of tablets | 30 | 28 | 0 |
| Placebo | 16 (80%) | 9 (45%) | 1 (5%) |
| Number of tablets | 31 | 18 | 0 |
| Day 3 | |||
| Penicillin | 13 (65%) | 9 (45%) | 4 (20%) |
| Number of tablets | 27 | 20 | 0 |
| Placebo | 15 (75%) | 8 (40%) | 3 (15%) |
| Number of tablets | 28 | 14 | 0 |
| Day 4 | |||
| Penicillin | 12 (60%) | 9 (45%) | 6 (30%) |
| Number of tablets | 24 | 23 | 0 |
| Placebo | 17 (85%) | 5 (25%) | 2 (10%) |
| Number of tablets | 28 | 8 | 0 |
| Day 5 | |||
| Penicillin | 12 (60%) | 8 (40%) | 7 (35%) |
| Number of tablets | 21 | 15 | 0 |
| Placebo | 16 (80%) | 7 (35%) | 3 (15%) |
| Number of tablets | 32 | 11 | 0 |
| Day 6 | |||
| Penicillin | 13 (65%) | 8 (40%) | 5 (25%) |
| Number of tablets | 24 | 15 | 0 |
| Placebo | 13 (65%) | 6 (30%) | 6 (30%) |
| Number of tablets | 24 | 13 | 0 |
| Day 7 | |||
| Penicillin | 14 (70%) | 10 (50%) | 4 (20%) |
| Number of tablets | 25 | 16 | 0 |
| Placebo | 11 (55%) | 7 (35%) | 7 (35%) |
| Number of tablets | 20 | 14 | 0 |

