妊娠37週未満で正期産域前に破水した女性に対する妊娠アウトカム改善のための計画的早期分娩と待機的管理の比較
アブストラクト
背景
早産前期破水(PPROM)の管理は、PPROM後すぐに分娩を開始させる場合も、経過観察(待機的管理)する場合もある。どちらの方法が母児にとって最も有益であるのかは不明である。ここでは2010年に発表されたコクランレビュー(Buchanan 2010)をアップデートした。
目的
胎児、新生児、母体の健康を目的として、妊娠24週以降、37週未満に早期前期破水した女性に対する、計画的早期分娩と待機的管理を比較し、その効果を評価する。
検索戦略
Cochrane Pregnancy and Childbirth Group's Trials Register(2016年9月30日)および検索した研究の文献リストを調べた。
選択基準
妊娠37週未満におけるPPROMの女性を対象に、待機管理と早期分娩を比較しているランダム化比較試験。準ランダム化試験を除外した。
データ収集と分析
2名のレビューアが独立して本レビューへ組み込む試験の選択を行い、また方法論の質を評価した。2名のレビューアが独立してデータを抽出した。それぞれのデータの精度が確認された。GRADEアプローチを用いてエビデンスの質を評価した。
主な結果
本レビューでは12件の研究(女性3617人と児3628人)を対象とした。プライマリーアウトカムである、新生児敗血症(リスク比(RR)0.93、95%信頼区間(CI)0.66〜1.30、12件の試験、児3628人、中等度のエビデンス)、血液培養陽性の新生児感染(RR 1.24、95%CI 0.70~2.21、7件の試験、児2925人)について早期分娩と期待的管理に明確な差はなかった。しかし、早期分娩は呼吸窮迫症候群(RDS)の発生率が増加した(RR 1.26、95%CI 1.05~1.53、12件の試験、児3622人、高度のエビデンス)。早期分娩はまた、帝王切開率の増加と関連していた(RR 1.26、95%CI 1.11~1.44、12件の試験、児3620人、高度のエビデンス)。
早期分娩と待機的管理を比較した場合、セカンダリー周産期アウトカムである、総周産期死亡率(RR 1.76、95%CI 0.89~3.50、11件の試験、児3319人)、子宮内死亡(RR 0.45、95%CI 0.13~1.57)に差はなかった。しかし、早期分娩は新生児死亡率の上昇(RR 2.55、95%CI 1.17~5.56、11件の試験、児3316人)、換気の必要性(RR 1.27、95%CI 1.02~1.58、7件の試験、児2895人、高度のエビデンス)と関連していた。早期分娩に無作為に割り付けられた女性の児は、待機的管理に無作為に割り付けた女性の児よりも、早い在胎週数で出生していた(平均差(MD)‐0.48週、95%CI ‐0.57~‐0.39、8件の試験、児3139人)。新生児集中治療室への入室は、早期分娩の児に多くみられた(RR 1.16、95%CI 1.08~1.24、4件の試験、児2691人、中等度のエビデンス)。
母体に関するセカンダリーアウトカムの評価では、早期分娩は絨毛羊膜炎の減少(RR 0.50、95%CI 0.26~0.95、8件の試験、女性1358人、中等度のエビデンス)、子宮内膜炎の増加(RR 1.61 、95%CI 1.00~2.59、7件の試験、女性2980人)と関連していたことが分かった。介入の必要性に関して、早期分娩に無作為に割り付けられた女性は、分娩誘発をより多く実施されていた(RR 2.18、95%CI 2.01~2.36、4件の試験、女性2691人)。早期分娩に無作為に割り付けられた女性は、入院期間がより短かかった(MD ‐1.75日、95%CI ‐2.45~‐1.05、6件の試験、女性2848人、中等度のエビデンス)。
サブグループ解析では、妊娠34週以降の待機的管理により、特にRDSや母体感染に関しての母体と新生児のアウトカムの向上がみられた。予防的抗生剤の使用は、待機的管理に無作為に割り付けられた女性で母体感染の減少に有効であることが示された。
総合的に、12件の試験全てのバイアスのリスクは低いもしくは不明と評価された。方法論の十分な説明がなかったいくつかの研究は、バイアスのリスクは不明とした。5件の研究に、バイアスのリスクが高いと評価された1つもしくは2つの領域があった。GRADEプロファイルでは、すべての重要なアウトカムに渡って、エビデンスの質は、中程度〜高度と示された。
著者の結論
このアップデートレビューには、5件のランダム化比較試験(2927人)を追加したが、妊娠37週未満のPPROMの女性における早期分娩と待機的管理の比較において、新生児敗血症の発生に臨床的に重要な違いはなかった。早期計画分娩では、新生児RDSの発生、人工換気の必要性、新生児死亡、子宮内膜炎、新生児集中治療室への入室、帝王切開での分娩率の上昇がみられたが、絨毛膜羊膜炎の発生は減少していた。早期分娩群に無作為に割付けられた女性は、分娩誘発のリスクが上昇したが、入院期間は減少していた。早期分娩に無作為に割り付けた女性の児は、より短い在胎期間で出生しやすかった。
妊娠継続が禁忌でない妊娠37週未満のPPROMにおいて、注意深いモニタリングによる待機的管理の方針は、母児のよりよいアウトカムと関連していた。
今後の研究の方向性として、どちらのグループのPPROMの女性も、待機的管理による利益を得ないよう測定することを目的とすべきである。これは、入院時妊娠週数、コルチコステロイドの投与、および異常な腟微生物細菌叢に関するサブグループ解析によって測定できる。研究はまた、児の長期的な神経発達を評価する必要がある。
PICOs
一般語訳
妊娠37週より前に子宮収縮なく破水した場合、すぐに児を出産するか、陣痛が始まるのを待つか、どちらが児のためにより良いのか?
論点
妊娠37週未満で子宮収縮を伴わず破水した場合の管理方法には、すぐに児を分娩するか、自然な陣痛発来を待つかの2つの選択肢がある。両方の選択肢のリスクとベネフィットを慎重に考える必要がある。
重要である理由
正期産域前の出生では、呼吸障害や新生児集中治療室の長期滞在など未熟性に関する障害の機会が増加しうる。しかし、子宮内にとどまることは、深刻な健康問題や死につながりうる、母児の感染症を生じるかもしれない。このレビューの目的は、どちらが最良の選択肢かを調べることである。
どのようなエビデンスが得られたか?
早期前期破水の女性3617人を含む12試験を対象とした。女性は早期出産または、待機的管理(分娩を待つ)のいずれかにランダムに振り分けられた。女性は妊娠25以降、37週未満であった。研究は1977年~2013年に16カ国で行われた。全体として、12件の研究では、バイアスのリスクが低いか不明であると評価され、エビデンスの質は中程度から高度だった。
新生児の感染や出生前の児の死亡率に2群間に差はなかった。しかし、早期出生では、新生児が呼吸するために余分な助けを必要とし、呼吸障害だけでなく出産後の新生児死亡リスクが増加した。早期計画分娩群の児は待機的管理群の児に比べ、新生児集中治療に入院することが多く、より早期に出生した。早期分娩では、帝王切開率、分娩誘発率や子宮内膜の感染リスクは増加したが、羊膜の感染リスクは減少した。待機的管理群となった女性の入院期間はより長かった。
意味するもの
児がすぐに出生すべき理由がない場合に、妊娠37週未満で破水した女性が自然な陣痛発来を待機することは、より健康的なアウトカムを求めるために最良の選択肢である。
Authors' conclusions
Summary of findings
| Planned early birth compared to expectant management for preterm prelabour rupture of membranes prior to 37 weeks' gestation | ||||||
| Patient or population: women with preterm prelabour rupture of membranes prior to 37 weeks' gestation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Expectant management | Planned early birth | |||||
| Neonatal infection/sepsis | Study population | RR 0.93 | 3628 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 34 per 1000 | |||||
| Neonatal respiratory distress syndrome | Study population | RR 1.26 | 3622 | ⊕⊕⊕⊕ | ||
| 84 per 1000 | 109 per 1000 | |||||
| Need for ventilation | Study population | RR 1.27 | 2895 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 110 per 1000 | |||||
| Admission to neonatal intensive care | Study population | RR 1.16 | 2691 | ⊕⊕⊕⊝ | ||
| 428 per 1000 | 497 per 1000 | |||||
| Caesarean section | Study population | RR 1.26 | 3620 | ⊕⊕⊕⊕ | ||
| 172 per 1000 | 217 per 1000 | |||||
| Chorioamnionitis | Study population | RR 0.50 | 1358 | ⊕⊕⊕⊝ | ||
| 103 per 1000 | 51 per 1000 | |||||
| Length of hospital stay (maternal) | The mean length of hospital stay (maternal) in the expectant group was | The mean length of hospital stay (maternal) in the early birth group was 1.75 days lower | MD ‐1.75 (‐2.45 to ‐1.05) | 2848 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Wide confidence interval crossing the line of no effect, and the lines of appreciable benefit and harm. | ||||||
Background
Description of the condition
Preterm prelabour rupture of the membranes (PPROM) occurs when there is rupture of the membranes prior to term and prior to the onset of labour. PPROM complicates pregnancy for 1% to 2% of all women and is associated with 30% to 40% of preterm births (less than 37 weeks) (Arias 1982; Lee 2001; Mercer 2000; Mercer 2005).
Description of the intervention
The purpose of this review is to determine the optimal management for women and their babies with pregnancies complicated by PPROM. This could either be planned delivery soon after rupture of the membranes or expectant management. Planned early birth involves the birth of the baby near to the time of rupture of the membranes and may be by induction of labour or caesarean section. Expectant management involves observation of the mother and baby and awaiting the spontaneous onset of labour in the absence of any complications that may necessitate delivery. Women with PPROM who are managed expectantly may then have the delivery of their baby planned for term if labour has not ensued.
How the intervention might work
The management of PPROM is dependent upon the gestation at which rupture of the membranes occurs. The health benefits for the fetus in continuing a pregnancy after PPROM may be considerable, particularly in the late second and early third trimesters. However, there is currently no consensus as to the optimal management of PPROM in women in whom the fetus is relatively mature, at gestations near to term such as in the late second trimester and third trimester of pregnancy. The aim of care for women with PPROM is to maximise the benefits of further fetal maturity while avoiding the potential harms of remaining in utero.
There are treatments such as antibiotics and antenatal corticosteroids that can reduce associated complications for the mother and baby in pregnancies complicated by PPROM. The use of antibiotics in PPROM significantly improves neonatal and maternal morbidity including prolongation of pregnancy, reduction in neonatal infection, reduced need for oxygen therapy and less risk of abnormal cerebral ultrasound (Kenyon 2001; Kenyon 2003). In addition, antenatal corticosteroids have been shown to reduce the risk of neonatal respiratory distress, intraventricular haemorrhage (bleeding within the ventricles of the baby's brain) and neonatal death in the preterm neonate (Roberts 2006). These beneficial effects of corticosteroids also apply to women with PPROM (Harding 2001).
Why it is important to do this review
There are recognised maternal and fetal risks associated with PPROM. These complications decrease the nearer to term that PPROM occurs. The recognised complications include ascending infection, cord prolapse (prolapsing of the umbilical cord through the cervix), intrapartum fetal distress and abruption (premature detachment of the placenta from the uterine wall) (Gonen 1989; Major 1995; Mercer 2003). It may be that managing PPROM expectantly by awaiting the spontaneous onset of labour increases the risk to the fetus of these complications. In particular prolonged exposure to intrauterine infection is of major concern for the neonate. In fact it has been demonstrated that neonatal sepsis is twice as common in the setting of PPROM compared with preterm birth after preterm labour with intact membranes (Seo 1992).
There are potential risks associated with planned early delivery in pregnancies complicated by PPROM between 30 and 37 weeks' gestation. In particular the attendant risks of iatrogenic prematurity associated with birth before term but greater than 30 weeks' gestation are significant. These complications may include respiratory distress (Jones 2000; Lewis 1996), sepsis, necrotising enterocolitis (injury to the bowel of newborn babies), intraventricular haemorrhage, prolonged stays in the neonatal nursery, difficulty with thermoregulation and difficulty with breastfeeding (Engle 2008; Robertson 1992). These complications are less common when delivery occurs after 32 weeks' gestation (Mercer 2003). A number of retrospective studies have similarly found a decrease in neonatal morbidity associated with birth at 34 weeks' gestation (Lewis 1996; Neerhof 1999). The incidence of respiratory distress syndrome, hyperbilirubinaemia (high bilirubin within in the babies' blood which results in a yellow discolouration of the neonates' skin referred to as jaundice) and duration of stay in the neonatal nursery was significantly reduced in infants born after 34 weeks' gestation compared with those born before 34 weeks (Lewis 1996; Neerhof 1999). Infants born beyond 34 weeks' gestation do have better outcomes than those born prior to 34 weeks, however those babies born between 34 and 37 weeks' gestation are still physiologically immature and as such do have significantly increased morbidity and mortality as compared with those infants born at term (Engle 2007; Engle 2008).
The previous version of this review (Buchanan 2010), which included seven trials and 690 women, found there was insufficient evidence available at that time to guide clinical practice and that all included trials had methodological weaknesses.
There is consensus on the management of term pregnancies with PROM (prelabour rupture of the membranes) (Middleton 2017). This Cochrane review found that fewer women in the planned compared with the expectant management groups had chorioamnionitis (inflammation of the fetal membranes) and/or endometritis (a postpartum infection of the lining of the womb) (average risk ratio (RR) 0.49, 95% confidence interval (CI) 0.33 to 0.72, eight trials, 6864 women). Additionally, early birth appeared to reduce the likelihood of definite or probable early‐onset neonatal sepsis (RR 0.73, 95% CI 0.58 to 0.92, sixteen trials, 7314 infants). There was no clear difference in the mode of birth between the groups.
It is evident that there is an increased incidence of chorioamnionitis associated with expectant management in women with PROM (Hannah 1996). Histological evidence of chorioamnionitis is present in up to 50% of women who deliver preterm and is often not associated with clinical symptoms or signs. Chorioamnionitis is a known significant risk factor for the development of both cystic periventricular leukomalacia (cystic changes around the ventricles of the babies brain) and cerebral palsy (Gaudet 2001; Wu 2000). Therefore, the question remains as to whether there is an increased chance of an adverse neurological outcome in those infants whose mothers are managed expectantly with PPROM by increasing their duration of exposure to often subclinical chorioamnionitis.
The objective of this review is to assess and further define the optimal management for women with PPROM prior to 37 weeks' gestation.
Objectives
To assess the effect of planned early birth versus expectant management for women with preterm prelabour rupture of the membranes between 24 and 37 weeks' gestation for fetal, infant and maternal well being.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all identified randomised controlled trials (RCTs) comparing planned early birth versus expectant management for women with preterm prelabour rupture of the membranes (PPROM) prior to 37 weeks' gestation. We did not consider quasi‐randomised studies for inclusion in the review. We also assessed for inclusion studies that were presented in abstract form only.
In studies in which gestational ages overlapped the less‐than‐37‐week gestation inclusion criteria, we attempted to extract gestational age‐specific data from the studies. We also attempted to contact researchers to provide further information.
Types of participants
Women with PPROM before 37 weeks' gestation with no specific maternal or fetal contraindications to expectant management.
Types of interventions
Planned early birth compared with expectant management.
Planned early birth is planned birth soon after PPROM. The mode of birth may either be via induction of labour by any means and a vaginal birth, or by caesarean section.
Expectant management involves planning to wait for birth until the baby is at term.
Types of outcome measures
Primary outcomes
Neonatal infection/sepsis:
-
proven neonatal infection with positive blood culture within 48 hours of birth;
-
proven neonatal infection with positive blood culture 48 hours or more after birth.
Respiratory distress syndrome
Caesarean section
Secondary outcomes
Fetal/perinatal outcomes
Perinatal death
Intrauterine death
Cord prolapse
Gestational age at birth
Neonatal outcomes
Neonatal death
Suspected neonatal infection
Treatment with antibiotics
Treatment with surfactant
Need for ventilation
Days of neonatal ventilation
Duration of oxygen therapy
Oxygen therapy at 36 weeks' postmenstrual age
Cord arterial pH
Birthweight
Apgar score less than 7 at five minutes
Abnormality on cerebral ultrasound:
-
cystic periventricular leukomalacia;
-
cerebroventricular haemorrhage (including grade of intraventricular haemorrhage).
Necrotising enterocolitis
Admission to neonatal intensive care unit
Admission to neonatal intensive care unit after 24 hours
Length of stay in neonatal intensive care unit
Days from birth to discharge home from hospital
Disability at time of childhood follow‐up
Maternal outcomes
Chorioamnionitis
Endometritis
Postpartum fever
Placental abruption
Induction of labour
Mode of induction of labour
Use of epidural anaesthesia
Vaginal birth
Operative vaginal birth
Caesarean section for fetal distress
Duration of hospitalisation:
-
days of antenatal hospitalisation;
-
days of postnatal hospitalisation.
Maternal satisfaction:
-
views of care;
-
preferences of care;
-
presence of postnatal depression.
Breastfeeding:
-
whether breastfeeding established;
-
time after birth breastfeeding established.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 September 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Two people screen search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
For the 2010 update (Buchanan 2010), we carried out additional author searching. See Appendix 1 for details.
Searching other resources
We searched reference lists of trials and other review articles. We contacted researchers to provide further information as required. We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Buchanan 2010.
For this update, we used the following methods for assessing the 11 additional reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third review author.
Data extraction and management
We designed a form to extract data based on Cochrane Pregnancy and Childbirth recommendations. For eligible studies, two review authors (DB and JM) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author (KL). One author (DB) entered data into Review Manager 5 (RevMan) software (RevMan 2014) and two others checked for accuracy (JM, KL).
David P van der Ham was Chief Investigator and first author of the PPROMEXIL trials and so JM and DB were responsible for data extraction and assessment of all trial reports relating to this study. Jonathan Morris was the Chief Investigator and first author for the NHMRC‐ (National Health and Medical Research Council) funded PPROMT trial and so data was assessed and extracted independently by PM.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (DB and KL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We have assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We have assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We have assessed the methods as:
-
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of evidence using the GRADE approach
For this update we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
-
Neonatal infection/sepsis
-
Neonatal respiratory distress syndrome
-
Need for ventilation
-
Admission to neonatal intensive care
-
Caesarean section
-
Chorioamnionitis
-
Length of hospital stay (maternal)
We used the GRADEproGDT (GRADEpro Guideline Development Tool) to import data from RevMan 5.3 (RevMan 2014) to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates if appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
We considered trials including twin pregnancies for inclusion in the review. For trials that included twin pregnancies, for fetal outcomes the denominator used for analysis was the number of pregnancies. However, for neonatal outcomes the denominator used for analysis was the number of individual babies randomised.
Cluster‐randomised trials
Cluster‐randomised trials were not eligible for inclusion in this review.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 50%), we planned to explore it by pre‐specified subgroup analysis (Deeks 2011).
Assessment of reporting biases
In this update, if there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform an exploratory analyses to investigate it (Sterne 2011).
Data synthesis
We carried out statistical analysis using the RevMan software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I² tests.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
-
Corticosteroid usage versus no corticosteroid usage
-
Gestational age at randomisation with stratification into:
-
less than 30 weeks' gestation
-
30 to 33 plus 6 weeks' gestation
-
34 to 37 weeks' gestation
-
-
Antibiotic usage versus no antibiotic usage
-
Time from randomisation to early birth: less than 24 hours versus greater than 24 hours
We used the following outcomes in subgroup analyses.
Fetal/neonatal outcomes
Neonatal infection
Neonatal infection confirmed with positive blood culture
Respiratory distress syndrome
Maternal outcomes
Caesarean section
Chorioamnionitis
Endometritis
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We included all eligible trials in the initial analysis and planned to carry out sensitivity analyses to evaluate the effect of trial quality. We also planned to perform a sensitivity analysis based on the randomisation process, assessing the presence of blinding of assessors to the primary outcome, assessing the quality of treatment allocation and assessing the presence of losses to follow‐up.
Results
Description of studies
Refer to the Characteristics of included studies and Characteristics of excluded studies tables for further detailed information on individual studies.
Results of the search
The search of the Cochrane Pregnancy and Childbirth's Trials Register retrieved 14 additional reports for consideration in this updated review, which contributed four additional studies to the review. Five of the reports were based on one study (Morris 2016), six on another (Van der Ham 2012a), four on the third (Van der Ham 2012b) and one on the fourth (Koroveshi 2013). We moved one study from 'excluded studies' in the first review to 'included studies' (Eroiz‐Hernandez 1997) in this update as the authors felt the study fitted the inclusion criteria after translation into English. We moved another study from 'ongoing' to 'excluded' as the trial was terminated prematurely due to poor recruitment (Lacaze 2006). One study remained ongoing as we were unable to ascertain the current status (Pasquier 2006). See Figure 1.

Study flow diagram
Included studies
We have included 12 randomised controlled trials in this review, which comprise 3617 women and 3628 babies (Cox 1995; Eroiz‐Hernandez 1997; Garite 1981; Iams 1985; Koroveshi 2013; Mercer 1993; Morris 2016; Naef 1998; Nelson 1985; Spinnato 1987; Van der Ham 2012a; Van der Ham 2012b). One study was performed in Albania (Koroveshi 2013), one in Mexico (Eroiz‐Hernandez 1997), two in the Netherlands (Van der Ham 2012a; Van der Ham 2012b), and one, though based in Australia, included a total of 11 countries: Australia, Argentina, Brazil, Egypt, New Zealand, Norway, Poland, Romania, South Africa, UK and Uruguay (Morris 2016). The other seven studies were performed in the USA. All but one of the included studies were reported in English, but we were able to translate the Mexican study (Eroiz‐Hernandez 1997) from Spanish to English. Five studies recruited women from multiple sites (Garite 1981; Iams 1985; Morris 2016; Van der Ham 2012a; Van der Ham 2012b), while the remainder recruited from a single centre only (Cox 1995; Eroiz‐Hernandez 1997; Koroveshi 2013; Mercer 1993; Nelson 1985; Naef 1998; Spinnato 1987). The studies were performed between 1977 and 2016. As Koroveshi 2013 was a published abstract only, this study provided limited information for this review and as a result has been excluded from most of the following discussion about participants, interventions and outcomes. Outcomes were included in the analysis only.
A summary of the trials is provided (Table 1).
| Trial | Sample size | Gestational age for inclusion (weeks) | Co‐interventions | Fetal lung maturity tested |
| 129 (131 babies) 61 ED (62 babies) 68 EM (69 babies) | 30 to 34 |
| No | |
| 58 30 ED 28 EM | 28 to 34 |
| Yes, if positive excluded from randomisation | |
| 160 80 ED 80 EM | 28 to 34 |
| Yes: if L/S mature excluded from randomisation and delivered | |
| 73 38 ED 35 EM | 28 to 34 |
| Yes: if mature L/S excluded from randomisation and delivered | |
| 307 157 ED 150 EM | 34 to 37 |
| No | |
| 93 46 ED 47 EM | 32 to 36+ 6 |
| Yes: included if mature L/S | |
| 1835 923 ED 912 EM | 34 to 36+ 6 |
| No | |
| 120 57 ED 63 EM | 34 to 36+ 6 |
| No | |
| 68 22 ED and steroids 22 ED and no steroids 24 EM | 28 to 34 |
| No | |
| 47 26 to ED 21 to EM | 25 to 36 |
| Yes: included if mature L/S | |
| 532 (538 babies) 266 ED (268 babies) 266 EM (270 babies) | 34 to 36+6 |
| No | |
| 195 (198 babies) 100 ED (100 babies) 95 EM (98 babies) | 34 to 36+6 |
| No |
ED: early delivery
EM: expectant management
L/S: lecithin‐sphingomyelin
Participants
The studies included women with pregnancies complicated by preterm prelabour rupture of the membranes (PPROM) of differing gestational ages. Eroiz‐Hernandez 1997, Garite 1981, Iams 1985 and Nelson 1985 included women with PPROM between 28 and 34 weeks' gestation, while Spinnato 1987 included women from 25 to 36 weeks' gestation. Cox 1995 included women with PPROM at 30 to 34 weeks' gestation. Mercer 1993 included women from 32 to 36 weeks' gestation, while Koroveshi 2013, Morris 2016, Naef 1998; Van der Ham 2012a and Van der Ham 2012b included women from 34 weeks' gestation to 36 weeks' plus six days' gestation. Three trials (Cox 1995; Van der Ham 2012a; Van der Ham 2012b) included twin pregnancies for a total of three pairs of twins randomised to early birth and eight pairs randomised to expectant management. One study (Eroiz‐Hernandez 1997), although inclusion criteria specified singleton pregnancies only, reported outcomes for two sets of twins in each group in the analysis.
Importantly, the entry criteria also differed between the studies. Eroiz‐Hernandez 1997, Garite 1981, Iams 1985, Mercer 1993 and Spinnato 1987 required amniotic fluid sampling to assess fetal pulmonary maturity prior to study entry. Garite 1981 and Iams 1985 used a mature lecithin‐sphingomyelin (L/S) as an exclusion criteria and then treated the early birth group with corticosteroids, while in contrast Mercer 1993 and Spinnato 1987 used a mature L/S as an inclusion criteria and did not use antenatal corticosteroids. Eroiz‐Hernandez 1997 excluded women with 'positive' fetal lung maturity tests based on "tap*, clements* and 650 nm spectrophotometry" (*we were unsure of the definition of these tests, which may have been an error in translation) and used a different fetal lung maturity protocol of intravenous aminophylline for each arm of the trial. They also required an amniotic fluid index of greater than 5 cm for trial inclusion. Cox 1995, Morris 2016, Nelson 1985, Naef 1998, Van der Ham 2012a and Van der Ham 2012b did not require documented pulmonary maturity prior to study entry. Exclusion criteria included active labour, chorioamnionitis or non‐reassuring fetal status as assessed at the time of randomisation in all the studies. Morris 2016 included an additional broadly defined exclusion as being any other contraindications to continuing the pregnancy, while Van der Ham 2012a and Van der Ham 2012b more specifically included additional exclusion criteria as being: monochorionic multiple pregnancy; major fetal anomalies; haemolysis; elevated liver enzymes and low platelets (HELLP) syndrome; and severe pre‐eclampsia (see Table 1).
All of the studies defined determination of gestational age for inclusion by menstrual history or early ultrasound examination to give the most accurate clinical estimate. In the absence of an accurate clinical estimate, seven of the studies used ultrasound at the time of presentation to hospital to date the pregnancy (Garite 1981; Morris 2016; Naef 1998; Nelson 1985; Spinnato 1987; Van der Ham 2012a; Van der Ham 2012b).
All of the trials defined ruptured membranes by clinical assessment with a sterile speculum examination and visualising amniotic fluid passing through the cervical os and pooling in the posterior fornix of the vagina. Six of the trials (Garite 1981; Iams 1985; Mercer 1993; Naef 1998; Nelson 1985; Spinnato 1987) also confirmed the presence of PPROM with a Nitrazine test to demonstrate an alkaline pH and or ferning of a specimen of vaginal fluid on microscopy. Eroiz‐Hernandez 1997 used 'cristallography' or the 'flame test' as an additional diagnosis.
Six of the included trials (Cox 1995; Garite 1981; Iams 1985; Mercer 1993; Naef 1998; Spinnato 1987) did not allow digital cervical examinations to be performed in the absence of labour.
Intervention
The intervention assessed by these studies was the effect of early birth on maternal and fetal well being. The timing of intervention for early birth differed between the studies. The mean latency from PROM to birth in four studies (Cox 1995; Mercer 1993; Naef 1998; Spinnato 1987) indicated that birth was planned as soon as practicable from randomisation and less than 24 hours, although this was not explicitly stated in all the trials. One study (Eroiz‐Hernandez 1997) did not indicate when birth was intended but results indicated a median latency of three days. Nelson 1985 planned for early birth between 24 and 48 hours after initial rupture of membranes and 24 hours after initiation of steroid therapy, while Garite 1981 defined planned early birth as 48 hours after treatment with corticosteroids. Iams 1985 had an even longer delay in the early birth group and planned for early birth 48 to 72 hours after PPROM and initiation of steroid treatment. Morris 2016 defined timing of birth as birth scheduled as close to randomisation as possible and preferably within 24 hours. In both Van der Ham 2012a and Van der Ham 2012b women were randomised if not spontaneously delivered within 24 hours after initial rupture of membranes and women randomised to early birth were induced within 24 hours after randomisation.
The only study with a control arm of early birth for PPROM was Eroiz‐Hernandez 1997. As a result, for the discussion and analysis, we flipped the definition and results for the 'treatment' group and the 'control' group in this study to be consistent with the rest of the studies, which all defined the control arm as being expectant management. The intention of expectant management was defined in the majority of studies as waiting until spontaneous labour or until there was medical indication to facilitate birth, such as infection or fetal distress. The exceptions were Eroiz‐Hernandez 1997, Koroveshi 2013 and Nelson 1985, which did not specify the intent of expectant management, although Eroiz‐Hernandez 1997 did indicate in an outcomes table that the reasons for delivery in the expectant management group were onset of labour, infection, oligohydramnios and fetal distress. Iams 1985, Morris 2016, Spinnato 1987, Van der Ham 2012a and Van der Ham 2012b allowed women to be discharged home at the discretion of the attending physician or according to local protocol, while the remainder of the studies required the women in the trial to be hospitalised until birth.
The co‐interventions also differed between the included studies. Cox 1995, Mercer 1993, Naef 1998 and Spinnato 1987 did not treat the women with corticosteroids or tocolysis. Garite 1981 and Iams 1985 treated women in the early birth groups with corticosteroids and tocolysis as required. Nelson 1985 randomised women to steroids or no steroid therapy in the early birth group. These women in the early birth group were also treated with tocolytics. Morris 2016 used tocolytics, antibiotics and corticosteroids according to local protocol, whereas Van der Ham 2012a and Van der Ham 2012b only used tocolytics and antibiotics according to local protocol, but gave corticosteroids to women with PPROM less than 34 weeks' gestation. Eroiz‐Hernandez 1997 used tocolytics as well as a fetal lung maturity protocol of intravenous aminophylline in the early birth arm, which was repeated weekly in the women randomised to expectant management. Antibiotics were not used unless there were signs of chorioamnionitis, in which case birth was indicated. Naef 1998 was the only trial in which prophylactic antibiotics were used for all women. In this trial all women were treated with ampicillin.
Outcomes
Neonatal infection was variably defined in the studies. Cox 1995, Garite 1981 and Spinnato 1987 did not document their criteria required for documenting neonatal infection while Eroiz‐Hernandez 1997, Iams 1985, Mercer 1993, Morris 2016, Naef 1998, Nelson 1985, Van der Ham 2012a and Van der Ham 2012b required a positive culture of blood, cerebrospinal fluid or urine in addition to clinical features of sepsis for diagnosis.
Chorioamnionitis was defined in all of the studies as maternal temperature associated with uterine tenderness, maternal or fetal tachycardia, or both, and/or foul smelling amniotic fluid in the absence of any other cause of identifiable infection. None of the studies confirmed the presence of clinical chorioamnionitis pathologically with a histological examination of the placenta and fetal membranes. Eroiz‐Hernandez 1997 additionally included in their diagnosis a leucocyte count of 15,000 in maternal blood at the start of the study or a 50% increase from the baseline reading.
Excluded studies
We excluded 16 studies from the review (see Characteristics of excluded studies). Fayez 1978 used a quasi‐randomisation schema in which women were randomised to either early birth or expectant management based on odd or even hospital record numbers. We also excluded Parsons 1989 and Bergstrom 1991 as these were prospective but not randomised trials.
We excluded six trials (Cararach 1994; Gloeb 1989; Griffith‐Jones 1990; Ladfors 1996; Mateos 1998; Van Heerden 1996) because the gestational age criteria for trial entry included women both prior to term and at term. We attempted to obtain information of the subgroup of women with PPROM prior to term in these trials; however, this was not successful.
We excluded another five studies because they assessed interventions other than the effect of birth on maternal and fetal well‐being in women with PPROM (Decavalas 1995; El‐Qarmalawi 1990; Haghighi 2006; Miodovnik 1988; Perez 1992).
We excluded one study because it was available in abstract form only and did not quantify outcomes that we could include in a meta‐analysis (Makhlouf 1997).
Lacaze 2006 was moved from 'ongoing' in the previous review to 'excluded' in this review as the trial had been terminated due to poor recruitment and there were no outcome data available.
Risk of bias in included studies
Please see Figure 2 and Figure 3 for summary of risk of bias assessments.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
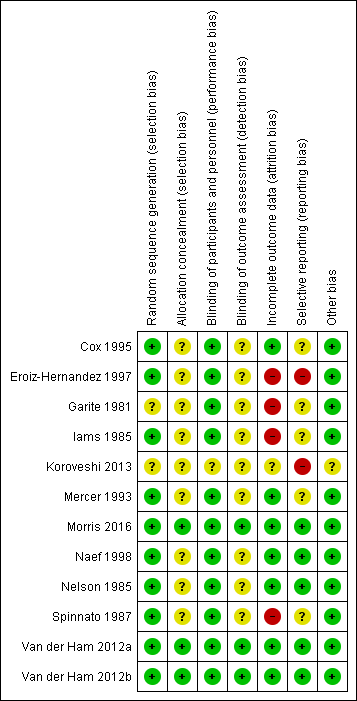
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
The trials were of variable methodological quality but overall of low to unclear risk of bias.
We assessed publication bias using funnel plots (Sterne 2011). We noted no visual asymmetry (Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11).
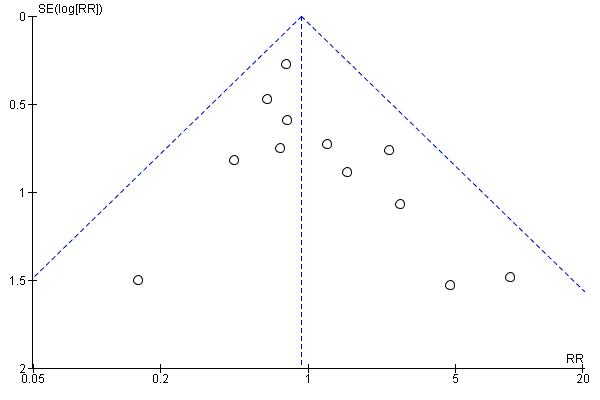
Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.1 Neonatal infection/sepsis

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.3 Respiratory distress syndrome

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.4 Caesarean section

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.5 Perinatal mortality
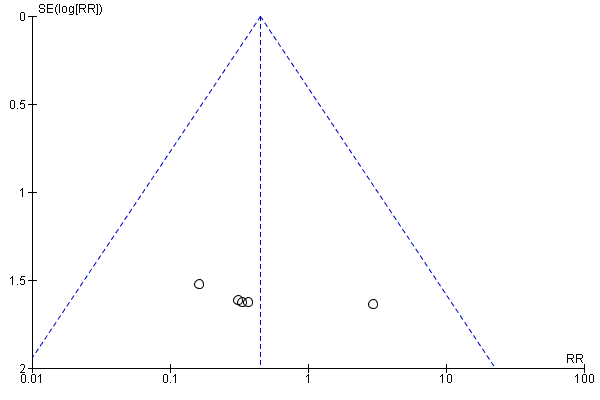
Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.6 Intrauterine death

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.9 Neonatal death
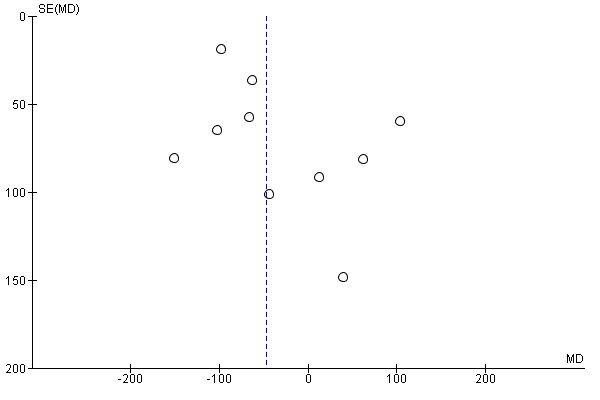
Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.15 Birthweight (g)
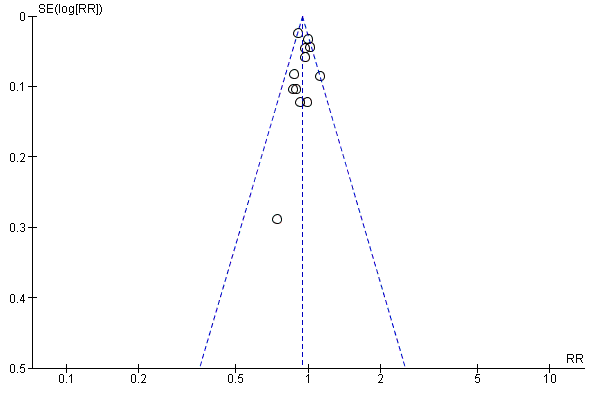
Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.31 Vaginal birth
Sensitivity analysis
We did not perform sensitivity analyses for this version of the review, due to very small differences in the potential risk of bias between the included studies. We assessed heterogeneity with subgroup analyses of outcomes.
Allocation
Random sequence generation
Ten of the 12 included trials discussed the method of randomisation (Cox 1995; Eroiz‐Hernandez 1997; Iams 1985; Mercer 1993; Morris 2016; Naef 1998; Nelson 1985; Spinnato 1987; Van der Ham 2012a; Van der Ham 2012b). This involved computer‐generated randomisation sequences, randomisation cards and random number tables. Garite 1981 and Koroveshi 2013 did not report on the method of randomisation.
Allocation concealment
Allocation concealment was unclear in all of the studies except Morris 2016, Van der Ham 2012a and Van der Ham 2012b each of which were considered low risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
Blinding was not possible due to the intervention, however this is likely low risk of bias due to objective and specific assessment criteria for outcomes, where lack of blinding would not affect treatment decisions or other aspects of care. However, for one trial (Koroveshi 2013), the risk was unclear as the assessment criteria for outcomes was not mentioned.
Blinding of outcome assessment (detection bias)
Blinding of outcome assessors was only performed in three of the included trials (Morris 2016; Van der Ham 2012a; Van der Ham 2012b). In one trial (Garite 1981), only radiologists for reviewing X‐rays prior to diagnosis of hyaline membrane disease were blinded as to treatment allocation. It is not clear in the other trials whether outcome assessors were blinded to treatment allocation.
Incomplete outcome data
All of the trials reported on short‐term outcomes. The only trials that assessed any maternal or neonatal outcomes after discharge from hospital were Van der Ham 2012a, which reported on neurodevelopmental outcomes at two years of age, and Morris 2016, which reported on maternal satisfaction and breastfeeding duration greater than 12 weeks. There was incomplete outcome data in four of the studies (Eroiz‐Hernandez 1997; Garite 1981; Iams 1985; Spinnato 1987). Eroiz‐Hernandez 1997 showed results inconsistent with the number randomised. There was no explanation to account for the inconsistencies and incomplete data. Garite 1981 removed a fetal death that occurred in the expectant management group from the denominator of neonatal outcomes. Iams 1985 excluded five women from analysis after randomisation. Three of these women were randomised to the expectant management group and were discharged home and they subsequently delivered their babies in another hospital and were excluded from analysis. Another mother and baby were excluded (also in this trial) due to failure to complete steroid therapy, and an additional neonate was excluded post‐randomisation due to the presence of congential abnormalities. Spinnato 1987 excluded 15 women on case review after randomisation. Seven of these women were excluded for preterm labour, four for protocol violation and four others for unspecified reasons. In addition, two perinatal deaths resulting from lethal congenital anomalies were excluded in this trial after randomisation.
Selective reporting
Selective reporting bias was considered high in two of the trials. Although Koroveshi 2013 was a published abstract only, in which three outcomes as well as secondary unspecified outcomes were reported, the full paper has not yet been published despite completion of the trial in 2011. In Eroiz‐Hernandez 1997, outcomes were only reported in results, and not pre‐specified. Although the inclusion criteria was singleton pregnancy only, results for caesarean section indicate there were two sets of twins in each group, which was not reported.
Other potential sources of bias
As Koroveshi 2013 was a published abstract only, it was unclear as to whether or not there were other sources of bias. We did not identify other potential sources of bias in any of the other included studies.
Effects of interventions
We included 12 randomised controlled trials in this review. This included 3617 women and 3628 babies with preterm premature rupture of the membranes randomised to either planned early birth or expectant management.
Primary outcomes
Fetal/neonatal outcomes
Infection
We identified no clear differences in the primary outcomes of probable or definite neonatal sepsis, or both (risk ratio (RR) 0.93, 95% confidence interval (CI) 0.66 to 1.30, 12 trials, 3628 babies, evidence graded moderate, Analysis 1.1), or proven neonatal infection with positive blood culture (RR 1.24, 95% CI 0.70 to 2.21, seven trials, 2925 babies, Analysis 1.2). No separate data were available for proven neonatal infection with a positive blood culture specifically within 48 hours of birth or after 48 hours of birth. Therefore, we further categorised neonatal sepsis into subgroups of: neonatal sepsis proven with positive blood culture (and included those trials that specified their sepsis outcome was defined by a positive culture); presumed sepsis; and neonatal treatment with antibiotics.
Three trials (Mercer 1993; Van der Ham 2012a; Van der Ham 2012b) reported on suspected neonatal sepsis and found a reduction associated with early birth (RR 0.56, 95% CI 0.36 to 0.88, 829 babies, Analysis 1.10).
Respiratory distress syndrome
Early birth increased the incidence of respiratory distress syndrome (RDS) (RR 1.26, 95% CI 1.05 to 1.53, 12 trials, 3622 babies, evidence graded high, Analysis 1.3). Included in this outcome is Spinnato 1987 who included RDS in the definition of "transient pulmonary insufficiency" along with respiratory insufficiency of prematurity and transient tachypnoea of the newborn. Three trials included a sub‐category of severe respiratory distress, which showed no difference between the two groups (RR 1.54, 95% CI 0.80 to 2.97, three trials, 321 babies, Analysis 1.21). However, the definition of severe respiratory distress differed between the trials, and the numbers were too small to adequately assess this outcome.
Maternal outcomes
Caesarean section
Early birth was associated with an increased rate of caesarean section (RR 1.26, 95% CI 1.11 to 1.44, 12 trials, 3620 women, evidence graded high, Analysis 1.4).
Secondary outcomes
Fetal outcomes
There was no clear difference in the overall perinatal mortality (RR 1.76, 95% CI 0.89 to 3.50, 11 trials, 3319 babies, Analysis 1.5), or intrauterine deaths (RR 0.45, 95% CI 0.13 to 1.55, 11 trials, 3321 babies, Analysis 1.6) when comparing early birth with expectant management. There was no difference in incidence of cord prolapse (RR 1.24, 95% CI 0.33 to 4.61, four trials, 2722 babies, Analysis 1.7). Babies of women randomised to early birth tended to be born at a gestational age lower than those randomised to expectant management (mean difference (MD) ‐0.48 weeks, 95% CI ‐0.57 to ‐0.39, eight trials, 3139 babies, Analysis 1.8).
Neonatal outcomes
Neonatal mortality
Early birth was associated with a higher rate of neonatal death (RR 2.55, 95% CI 1.17 to 5.56, 11 trials, 3316 babies, Analysis 1.9). There was a disproportionate increased number of deaths in the planned early delivery arm of one trial (Eroiz‐Hernandez 1997). This was the only trial comparing a fetal lung maturity protocol using intravenous aminophylline every eight hours in the early‐birth arm and weekly in the expectant‐management arm.
Neonatal morbidity
Early birth was associated with an increased need for neonatal ventilation (RR 1.27, 95% CI 1.02 to 1.58, seven trials, 2895 babies, evidence graded high, Analysis 1.12) and increased the arterial pH in the umbilical cord to above the normal range (MD 0.09, 95% CI 0.07 to 0.11, one trial, 120 babies, Analysis 1.14).
There were no clear differences between the groups in other measures of neonatal morbidities, including neonatal treatment with antibiotics (average RR 0.86, 95% CI 0.63 to 1.19, four trials, 2638 babies, Tau² = 0.08, I²= 80%,Analysis 1.11), days of oxygen therapy (RR ‐3.05, 95% CI ‐6.92 to 0.82, one trial, 73 babies, Analysis 1.13), birthweight (MD ‐47.10 g, 95% CI ‐96.00 to 1.80), 10 trials, 3263 babies, Tau² = 2260.76, I² = 44%, Analysis 1.15), Apgar score less than seven at five minutes (RR 0.96, 95% CI 0.54 to 1.69, five trials, 2700 babies, Analysis 1.16), abnormality on cerebral ultrasound (RR 1.90, 95% CI 0.52 to 6.92, three trials, 271 babies, Analysis 1.17), periventricular leukomalacia (RR 1.00, 95% CI 0.14 to 6.99, two trials, 707 babies, Analysis 1.18), cerebroventricular haemorrhage (RR 1.19, 95% CI 0.40 to 3.52, six trials, 1095 babies, Analysis 1.19), and necrotising enterocolitis (RR 0.81, 95% CI 0.25 to 2.62, six trials, 2842 babies, Analysis 1.20).
There was substantial heterogeneity between the trials in assessing neonatal treatment with antibiotics as demonstrated by an I² of 80%. This was likely due to the differences in clinical practice guidelines between sites regarding antibiotic use. There was also high heterogeneity for birthweight which was likely due to the differences in gestational age at randomisation between trials.
Neonatal hospitalisation
Admission to neonatal intensive care (RR 1.16, 95% CI 1.08 to 1.24, four trials, 2691 babies, evidence graded moderate, Analysis 1.22) was higher for those babies randomised to early birth. However, the length of stay in neonatal intensive care for those babies who were admitted to the neonatal intensive care unit (NICU) was no different between the two groups (MD ‐0.17 days, 95% CI ‐1.62 to 1.27, four trials, 2121 babies, Tau² = 1.65, I² = 85%, Analysis 1.23). This may suggest that although fewer babies in the expectant management arm were admitted to NICU, their length of stay in NICU was longer. Furthermore, the duration of days in NICU for one trial (Iams 1985) was considerably longer than the other three trials that assessed this outcome. This may be attributed to the earlier gestation at randomisation (28 to 34 weeks as compared to 34 to 36 + 6 weeks) as well as being the oldest of the four trials (1985). Changes in clinical practice over time may have influenced time spent in NICU. Two trials (Van der Ham 2012a; Van der Ham 2012b) were excluded from this outcome on the advice of the study author as there was uncertainty regarding the denominator used for the analysis. Overall duration of neonatal hospitalisation (MD 0.67 days, 95% CI ‐0.28 to 1.61, six trials, 2832 babies, Tau² = 0.80, I² = 71%, Analysis 1.24) was also no different between those babies in the early birth group compared to the expectant management group.
Heterogeneity for all these outcomes was high. This was likely due to the differences in clinical practice as evidenced by the large number of countries and sites represented in this review. Many hospitals routinely admit babies where the pregnancy is complicated by PPROM to NICU for observation for a specified period of time, whereas others do not.
Long‐term disability
There were no clear differences in neurodevelopmental outcomes at two years of age between children in the early birth group compared to children in the expectant when assessed by the Child Behaviour Checklist (CBCL) (RR 0.64, 95% CI 0.26 to 1.59, one trial, 199 babies, Analysis 1.38) or the Ages and Stages Questionnaire (ASQ) (RR 0.61, 95% CI 0.35 to 1.05, one trial, 228 babies, Analysis 1.39). However, the trial was not powered for this outcome and with a 45% follow‐up rate, the numbers may be insufficient for an overall effect.
Maternal outcomes
Infection
In assessing maternal outcomes, we found that early birth was associated with a decreased rate of chorioamnionitis (average RR 0.50, 95% CI 0.26 to 0.95, eight trials, 1358 women, evidence graded moderate, Tau² = 0.36, I² = 48%, Analysis 1.25). However, early birth was associated with an increased rate of endometritis (RR 1.61, 95% CI 1.00 to 2.59, seven trials, 2980 women, Analysis 1.26). There was no difference between early planned birth and expectant management in the risk of postpartum fever (RR 0.52, 95% CI 0.26 to 1.03, one trial, 1835 women, Analysis 1.27).
There was substantial heterogeneity between the trials in assessing chorioamnionitis as demonstrated by an I² of 48%. To analyse the source of heterogeneity we performed a number of subgroup analyses. The subgroup analysis comparing those trials in which early birth occurred less than 24 hours after randomisation reduced chorioamnionitis (RR 0.22, 95% CI 0.17 to 0.55, three trials, 342 women), while no difference was seen when planned birth was 24 hours or more from randomisation (RR 1.10, 95% CI 0.62 to 1.95, five trials, 1016 women). An interaction test for this difference between subgroups was significant in assessing the subgroup of early birth on chorioamnionitis: Chi² = 6.02, df = 1 (P = 0.01), I² = 83.4%, Analysis 5.5). Likewise, the subgroup analysis for gestational age at randomisation showed a reduction in chorioamnionitis in women greater than 34 weeks' gestation (RR 0.26, 95% CI 0.12 to 0.57, three trials, 847 women) compared to women less than 34 weeks' gestation (RR 0.77, 95% CI 0.45 to 1.30, four trials, 418 women). However, the interaction test for subgroup differences was not significant, Analysis 3.5.
Other complications
There were no clear differences in rates of placental abruption between the two groups (RR 1.19, 95% CI 0.36 to 3.87, one trial, 1835 women, Analysis 1.28).
Mode of birth
As expected due to the intervention, women randomised to early birth had a higher chance of having an induction of labour as compared to women randomised to expectant management (RR 2.18, 95% CI 2.01 to 2.36, four trials, 2691 women, Analysis 1.29). In Analysis 1.30, the results were probably in favour of the expectant management group, with more women in the planned early birth group using spinal/epidural anaesthesia (average RR 1.28, 95% CI 0.99 to 1.65, three trials, 2562 women, Tau² = 0.03, I² = 57%,Analysis 1.30). The high heterogeneity for this outcome was likely due to the differences in clinical practice between sites regarding the availability and use of epidural/spinal anaesthesia. The chances of a vaginal birth were lower in women randomised to early birth (RR 0.94, 95% CI 0.91 to 0.97, 12 trials, 3618 women, Analysis 1.31), although the rate of operative vaginal birth showed no difference (RR 0.85, 95% CI 0.67 to 1.10, four trials, 2685 women, Analysis 1.32). Although caesarean section rates were higher in the early birth group, if the reason for the caesarean section was fetal distress, the difference between the early birth group and the expectant management group was not apparent (RR 0.89, 95% CI 0.66 to 1.20, seven trials, 2918 women, Analysis 1.33).
Duration of maternal hospital stay
Women randomised to early birth showed a decrease in the total length of hospitalisation (MD ‐1.75 days, 95% CI ‐2.45 to ‐1.05, six trials, 2848 women, Tau² = 0.41, I² = 63%, Analysis 1.34), including the length of antenatal hospitalisation (MD ‐6.30 days, 95% CI ‐9.67 to ‐2.93, one trial, 73 women, Analysis 1.33). This result remained consistent in trials which allowed for antenatal discharge between randomisation and birth (MD ‐1.64 days, 95% CI ‐3.06 to ‐0.23, two trials, 213 women, Tau² = 0.65, I² = 58%, Analysis 1.36). The differences in patient admission management between different sites and countries likely accounted for the high heterogeneity regarding duration of maternal hospital stay.
Time from randomisation to birth
As expected, the time from randomisation to birth was shorter for those randomised to early birth (MD ‐79.48 hours, 95% CI ‐88.27 to ‐70.69, three trials, 2571 women, Analysis 1.37).
Satisfaction and breastfeeding
There was no difference in overall maternal satisfaction relating to their birth experience when comparing early birth with expectant management (RR 0.99, 95% CI 0.86 to 1.13, one trial, 493 women, Analysis 1.40). Likewise there was no difference between the two groups in the number of women who continued to breast feed for longer than 12 weeks following birth (RR 0.95, 95% CI 0.80 to 1.12, one trial, 415 women, Analysis 1.41).
Subgroup analyses
Antenatal corticosteroids
The trials differed in their use of antenatal corticosteroids in randomised women. Five trials (Cox 1995; Eroiz‐Hernandez 1997; Mercer 1993; Naef 1998; Spinnato 1987) did not give antenatal corticosteroids to any of their randomised participants. Two trials (Garite 1981; Iams 1985) gave corticosteroids to the women randomised to early birth and not to women randomised to expectant management. One trial (Nelson 1985) gave corticosteroids only to one group of women randomised to early birth and not to the second group or to the expectant management group. One trial (Morris 2016) used corticosteroids according to local protocol, and two trials (Van der Ham 2012a; Van der Ham 2012b) gave corticosteroids to women who ruptured their membranes prior to 34 weeks' gestation. No trials gave antenatal corticosteroids to all their randomised participants. Koroveshi 2013 did not stipulate corticosteroid usage.
There was no evidence of a difference between subgroups for corticosteroid use for the following outcomes: neonatal infection, Analysis 2.1, neonatal infection confirmed with positive culture, Analysis 2.2, RDS, Analysis 2.3, caesarean section, Analysis 2.4, chorioamnionitis, Analysis 2.5, and endometritis, Analysis 2.6, between trials in which no antenatal corticosteroids were used and trials where some antenatal corticosteroids were used.
Gestational age
Five trials (Koroveshi 2013; Morris 2016; Naef 1998; Van der Ham 2012a; Van der Ham 2012b) randomised women greater than 34 weeks' gestation, and five trials (Cox 1995; Eroiz‐Hernandez 1997; Garite 1981; Iams 1985; Nelson 1985) randomised women less than 34 weeks' gestation. Two trials (Mercer 1993; Spinnato 1987) included women before and after 34 weeks' gestation.
The test for subgroup differences were not significant for neonatal infection (Analysis 3.1), neonatal infection confirmed with positive culture (Analysis 3.2), RDS (Analysis 3.3), caesarean section, (Analysis 3.4), and chorioamnionitis (Analysis 3.5) between trials that randomised women after 34 weeks' gestation compared to before 34 weeks' gestation. There was a decrease in endometritis in women randomised to early delivery in trials greater than 34 weeks' gestation (RR 0.37, 95% CI 0.10 to 1.40, three trials, 2562 women) compared to women randomised in trials less than 34 weeks' gestation (RR 2.23, 95% CI 1.29 to 3.84, four trials, 418 women). The test for subgroup differences showed: Chi² = 5.99, df = 1 (P = 0.01), I² = 83.3%, Analysis 3.6. There were overall effect differences in RDS and chorioamnionitis showing an increase in RDS in early delivery in trials that recruited women greater than 34 weeks' gestation (RR 1.45, 95% CI 1.10 to 1.90, five trials, 2992 babies), and a decrease in chorioamnionitis in expectant management in trials that recruited women greater than 34 weeks' gestation (RR 0.26, 95% CI 0.12 to 0.57, three trials, 847 women). This may suggest better infant and maternal outcomes related to expectant management after 34 weeks' gestation. However, these results must be interpreted with caution as there were considerably fewer women in the subgroup less than 34 weeks' gestation compared to more than 34 weeks' gestation.
Prophylactic antibiotics
One trial (Naef 1998) gave prophylactic antibiotics to all women randomised to the trial. Seven trials (Cox 1995; Eroiz‐Hernandez 1997; Garite 1981; Iams 1985; Mercer 1993; Nelson 1985; Spinnato 1987) did not use prophylactic antibiotics. Three trials (Morris 2016; Van der Ham 2012a; Van der Ham 2012b) used prophylactic antibiotics according to local protocol which differed between sites. Morris 2016 provided individual participant data regarding the use of antibiotics for the outcome of neonatal sepsis. Koroveshi 2013 did not mention whether they used prophylactic antibiotics.
The test for subgroup differences were not significant for neonatal infection (Analysis 4.1), neonatal infection confirmed with positive blood culture (Analysis 4.2), RDS (Analysis 4.3), caesarean section (Analysis 4.4), or chorioamnionitis (Analysis 4.5), between trials whether all, none or some women received prophylactic antibiotics. However, there was an increase in endometritis in women randomised to early birth in trials that did not use prophylactic antibiotics (RR 2.23, 95% CI 1.29 to 3.84, four trials, 418 women) as compared to trials where some women received prophylactic antibiotics (RR 0.37, 95% CI 0.10 to 1.40, three trials, 2562 women). The test for subgroup differences showed: Chi² = 5.99, df = 1 (P = 0.01), I² = 83.3%, Analysis 4.6. Overall effect differences showed a decrease in chorioamnionitis in expectant management when all or some prophylactic antibiotics were used, suggesting that antibiotics were effective in reducing maternal infections. Again, the results for this subgroup analysis must be interpreted with caution due to the considerably increased number of women in the 'some antibiotics' category compared to those who did not receive antibiotics. Independent participant data analysis would be useful to assess this more comprehensively.
Timing of birth
The timing of intervention for early birth differed between the studies. The mean latency from PROM to birth in four studies (Cox 1995; Mercer 1993; Naef 1998;Spinnato 1987) indicated that early birth was planned for less than 24 hours, even though this was not explicitly stated. One study (Eroiz‐Hernandez 1997) did not indicate when birth was intended but results indicated a median latency of three days. Three studies (Garite 1981; Iams 1985; Nelson 1985) planned for women in the early birth arm to give birth more than 24 hours after randomisation. Three studies (Morris 2016; Van der Ham 2012aVan der Ham 2012b) intended for women randomised to early birth to have labour initiated within 24 hours. However, the mean latency from randomisation to birth in these trials ranged from 33.9 to 39 hours. Koroveshi 2013 did not indicate or provide data to support timing of early birth. Because of the lack of information related to 'intention' of timing of delivery in four of the trials, we based subgroup analysis on mean latency results rather than intended timing of early birth.
Subgroup analysis by timing of birth showed the tests for subgroup differences were not significant for neonatal sepsis (Analysis 5.1), neonatal infection confirmed with positive blood culture (Analysis 5.2), RDS (Analysis 5.3), caesarean section (Analysis 5.4), and endometritis (Analysis 5.6) between trials where early birth occurred less than 24 hours after randomisation as compared to trials where early birth occurred more than 24 hours after randomisation. However, chorioamnionitis was reduced in women randomised to early birth when planned birth occurred within 24 hours (RR 0.25, 95% CI 0.10 to 0.61, three trials, 342 women) as compared to when planned birth occurred more than 24 hours from randomisation (RR 0.76, 95% CI 0.41 to 1.42, five trials, 1016 women). The test for subgroup differences showed: Chi² = 4.06, df = 1 (P = 0.04), I² = 75.4%, Analysis 5.5.
Outcomes not able to be assessed
There were no data related to secondary neonatal outcomes of treatment with surfactant, days of neonatal ventilation, oxygen therapy at 36 weeks' postmenstrual age, or admission to NICU after 24 hours.
There were no data related to secondary maternal outcomes of mode of induction of labour and days of postnatal hospitalisation.
Discussion
Summary of main results
The existing evidence arose from clinical trials in which the protocols differed in their management of women with preterm prelabour rupture of the membranes (PPROM), particularly the use of prophylactic antibiotics, use of corticosteroids, timing of early birth and gestational age at trial entry. However, subgroup analyses of these four factors did not alter the result of the primary outcome of sepsis, which indicated that there was no difference in the incidence of neonatal sepsis between women who gave birth immediately or were managed expectantly in PPROM prior to 37 weeks' gestation.
Planned early birth was associated with an increase in the incidence of neonatal RDS, neonatal mortality and the likelihood of birth by caesarean section. Babies in the early birth arm were more likely to be admitted to neonatal intensive care unit, and receive ventilatory support.
Maternal outcomes indicated early birth was associated with an increased likelihood of induction of labour and endometritis, which was reduced with the use of antenatal antibiotics, and a decreased incidence of chorioamnionitis. The clinical significance of the decreased likelihood of chorioamnionitis following early planned birth but an increased risk of endometritis is difficult to interpret. Chorioamnionitis was defined clinically and by those not blinded to treatment allocation so there is a possibility of bias in reporting this outcome. Long term follow‐up studies are necessary to ascertain whether there are any sequelae beyond the neonatal period that may result from these exposures. Chorioamnionitis was decreased when randomisation occurred greater than 34 weeks of gestation and if birth occurred within 24 hours of randomisation. However, not all trials assessed this outcome on women randomised to early birth as the presence of chorioamnionitis was an exclusion criteria. The length of time between randomisation and birth as well as overall length of maternal hospitalisation was longer in women randomised to expectant management. The three trials which reported on suspected neonatal infection showed an increase in babies who were managed expectantly, however the numbers were too small to be interpreted with confidence.
Overall completeness and applicability of evidence
The applicability of findings from the meta‐analysis to other populations and settings is limited by several factors. Firstly, the gestational age for inclusion into the studies was often wide. As the perinatal complications associated with PPROM change with gestational age, the management of women with a pregnancy complicated by PPROM requires a stratified approach based largely on gestational age. However, the addition of four trials with inclusion criteria between 34 and 37 weeks' gestation provided some meaningful gestational age‐based management for women at 34 or more weeks' gestation.
Secondly, a number of trials included co‐interventions in addition to the timing of birth such as corticosteroids, tocolysis and antibiotics which may also limit the applicability of these findings. Since the publication of these earlier trials it has been clearly demonstrated that a number of these co‐interventions are of benefit for the mother and baby in the setting of PPROM. There is a beneficial effect for both the mother and the baby in the use of prophylactic erythromycin in the setting of PPROM (Kenyon 2001; Kenyon 2003) similar to the demonstrated beneficial effects of antenatal corticosteroids for women with PPROM, particularly for the neonate at gestations less than 34 weeks (Harding 2001). Only one of these trials (Naef 1998) used prophylactic antibiotics for all women. However, Morris 2016 provided data for those women who did receive prophylactic antibiotics that contributed a substantial number towards the analysis. None of the trials used corticosteroids for both the early birth and expectant management groups. Using corticosteroids for both the early birth and expectant management groups in women at gestations less than 34 weeks would have provided clinically meaningful information and would have removed the confounder of steroids in assessing the effect of early birth on maternal and neonatal outcomes.
Quality of the evidence
Overall, all 12 studies were assessed as being at low or unclear risk of bias. Some of the studies lacked an adequate description of methods and the risk of bias could only be assessed as unclear. In five of the studies there were one or two domains where the we judged the risk of bias as high. However, this was unlikely to change the magnitude of effect. We used GRADE profiling to assess neonatal outcomes of infection/sepsis, RDS, need for ventilation, and admission to NICU: and maternal outcomes of caesarean section, chorioamnionitis, and length of hospital stay. The results show the quality of evidence across all critical outcomes to be moderate to high. Downgrading was mainly due to imprecision where few events resulted in wide confidence intervals, and inconsistency as evidenced by heterogeneity.
Potential biases in the review process
The inclusion criteria for this review were intentionally broad, with the aim of being able to better examine all of the possible evidence available. These trials differed with respect to inclusion criteria, co‐interventions and the timing of the early birth intervention. The results provided by the subgroup analysis should be interpreted with caution due to the large differences in numbers of women between the subgroups.
We acknowledge that there was the potential for bias at all stages in the reviewing process. We attempted to minimise bias in a number of ways; for example, two review authors independently carried out data extraction and assessed risk of bias. David P van der Ham was Chief Investigator and first author of the PPROMEXIL trials and so JM and DB were responsible for data extraction and assessment of all trial reports relating to this study. Jonathan Morris was the Chief Investigator and first author for the NHMRC‐ (National Health and Medical Research Council) funded PPROMT trial and so data was assessed and extracted independently by PM. However, we acknowledge that such assessments involve subjective judgments, and another review team may not have agreed with all of our decisions.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews addressing this question. Most of the studies included in this review concur with expectant management as not having an increased risk to neonatal outcomes. The exceptions are Mercer 1993 and Naef 1998 which concluded that early birth resulted in decreased infection for both mothers and infants. However, these trials were underpowered to adequately assess this outcome.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.1 Neonatal infection/sepsis

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.3 Respiratory distress syndrome

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.4 Caesarean section

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.5 Perinatal mortality

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.6 Intrauterine death

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.9 Neonatal death

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.15 Birthweight (g)

Funnel plot of comparison: 1 Any planned birth versus expectant management: by type, outcome: 1.31 Vaginal birth

Comparison 1 Any planned birth versus expectant management: by type, Outcome 1 Neonatal infection/sepsis.
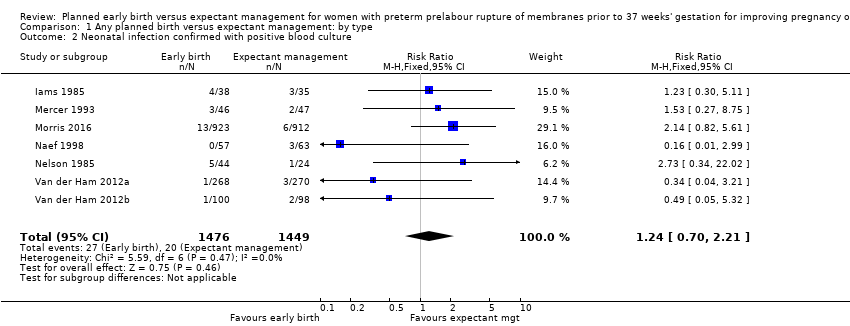
Comparison 1 Any planned birth versus expectant management: by type, Outcome 2 Neonatal infection confirmed with positive blood culture.
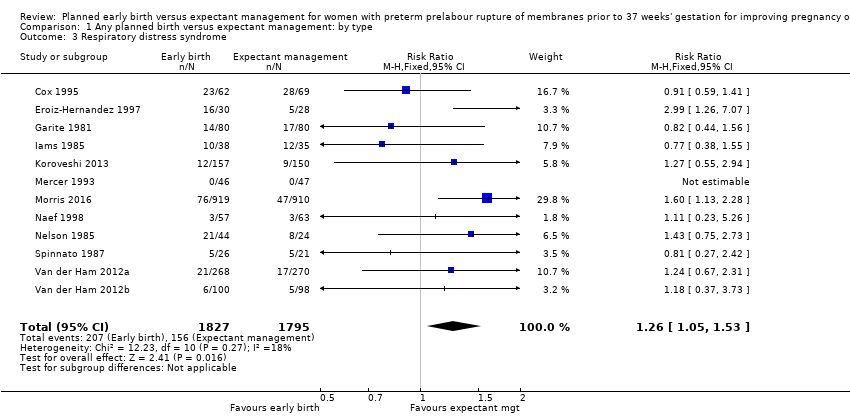
Comparison 1 Any planned birth versus expectant management: by type, Outcome 3 Respiratory distress syndrome.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 4 Caesarean section.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 5 Perinatal mortality.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 6 Intrauterine death.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 7 Cord prolapse.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 8 Gestational age at birth (weeks).
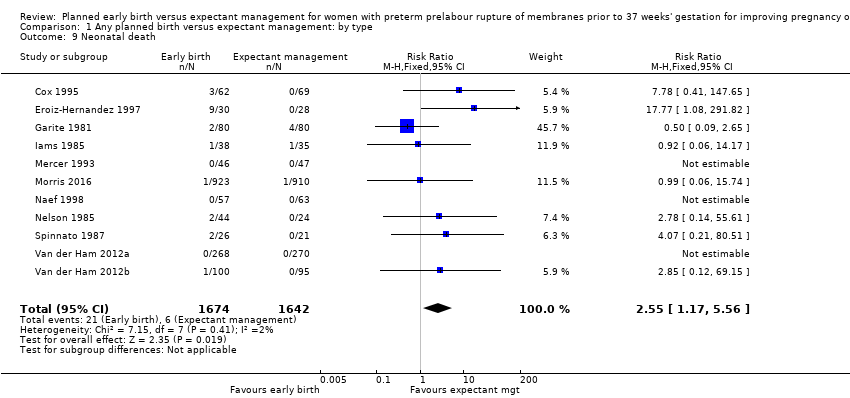
Comparison 1 Any planned birth versus expectant management: by type, Outcome 9 Neonatal death.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 10 Suspected neonatal infection.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 11 Neonatal treatment with antibiotics.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 12 Need for ventilation.
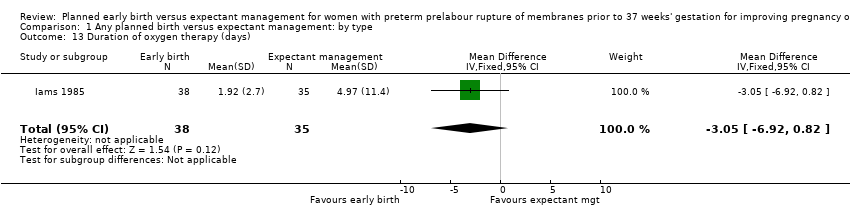
Comparison 1 Any planned birth versus expectant management: by type, Outcome 13 Duration of oxygen therapy (days).

Comparison 1 Any planned birth versus expectant management: by type, Outcome 14 Umbilical cord arterial pH.
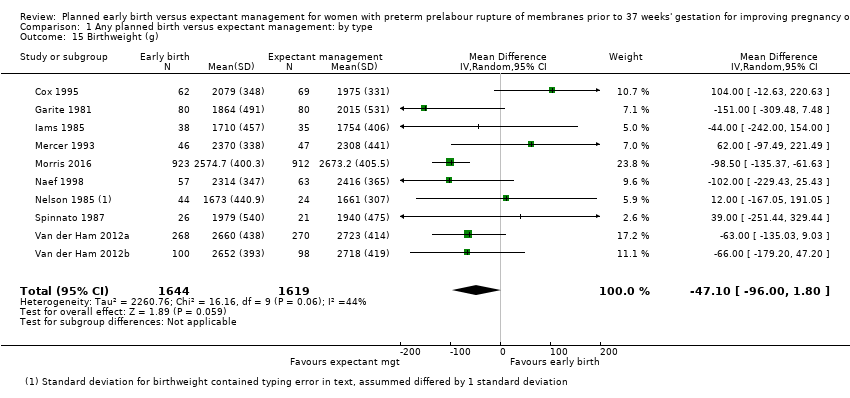
Comparison 1 Any planned birth versus expectant management: by type, Outcome 15 Birthweight (g).
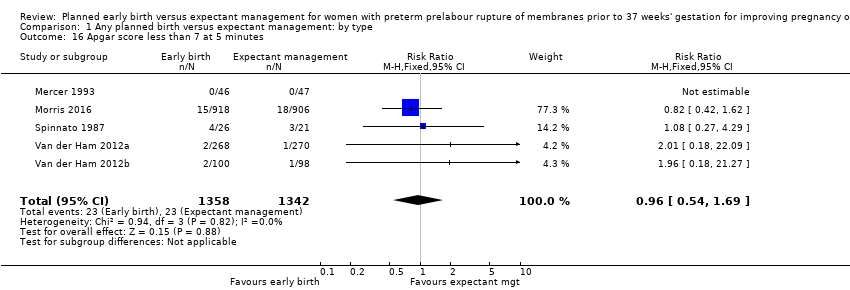
Comparison 1 Any planned birth versus expectant management: by type, Outcome 16 Apgar score less than 7 at 5 minutes.
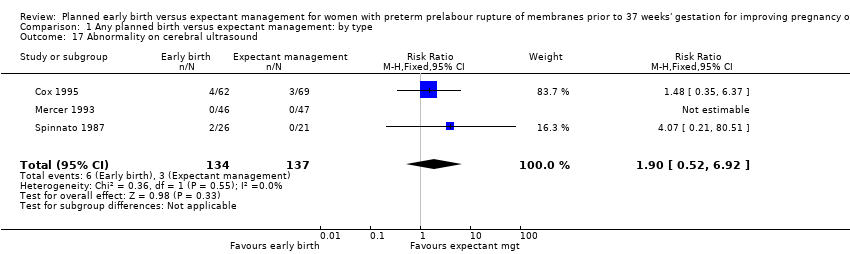
Comparison 1 Any planned birth versus expectant management: by type, Outcome 17 Abnormality on cerebral ultrasound.
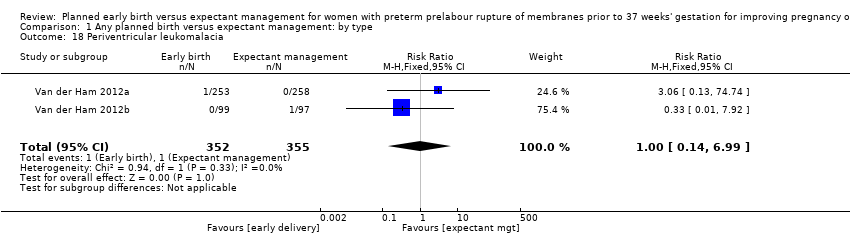
Comparison 1 Any planned birth versus expectant management: by type, Outcome 18 Periventricular leukomalacia.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 19 Cerebroventricular haemorrhage.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 20 Necrotising enterocolitis.
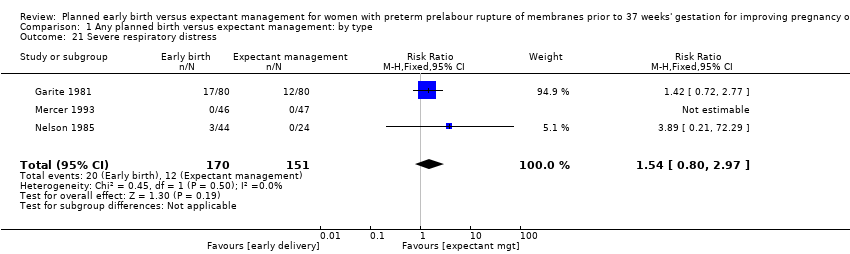
Comparison 1 Any planned birth versus expectant management: by type, Outcome 21 Severe respiratory distress.
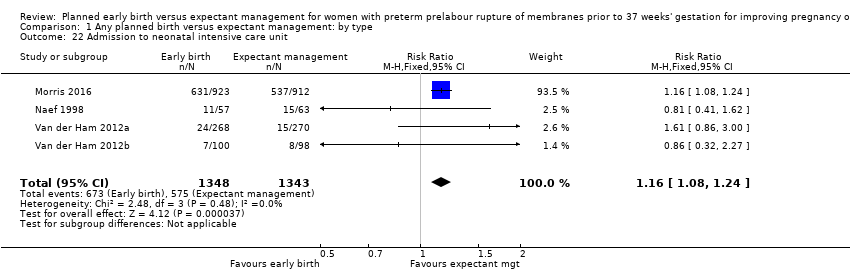
Comparison 1 Any planned birth versus expectant management: by type, Outcome 22 Admission to neonatal intensive care unit.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 23 Length of stay in neonatal intensive care unit (days).
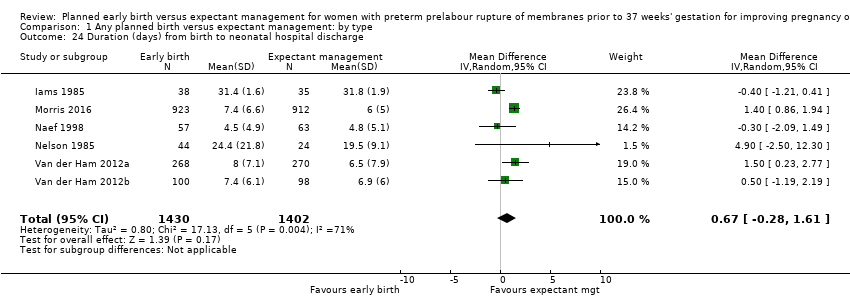
Comparison 1 Any planned birth versus expectant management: by type, Outcome 24 Duration (days) from birth to neonatal hospital discharge.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 25 Chorioamnionitis.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 26 Endometritis.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 27 Postpartum fever.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 28 Placental abruption.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 29 Induction of labour.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 30 Use of epidural/spinal anaesthesia.
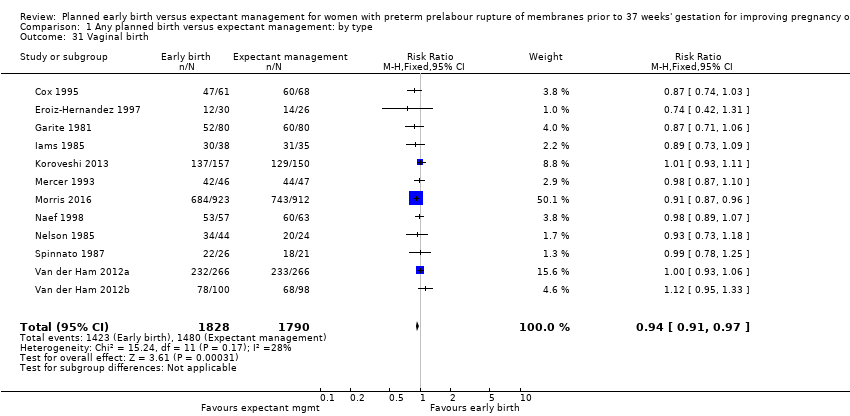
Comparison 1 Any planned birth versus expectant management: by type, Outcome 31 Vaginal birth.
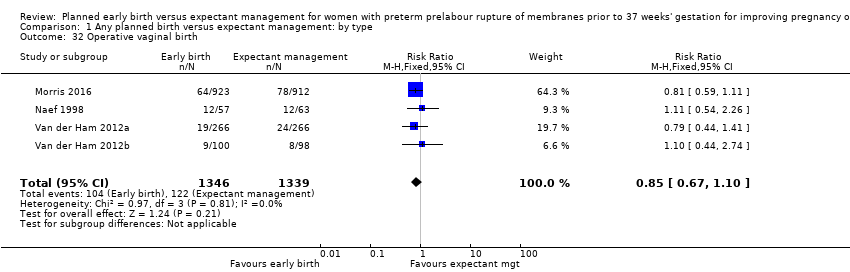
Comparison 1 Any planned birth versus expectant management: by type, Outcome 32 Operative vaginal birth.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 33 Caesarean section for fetal distress.
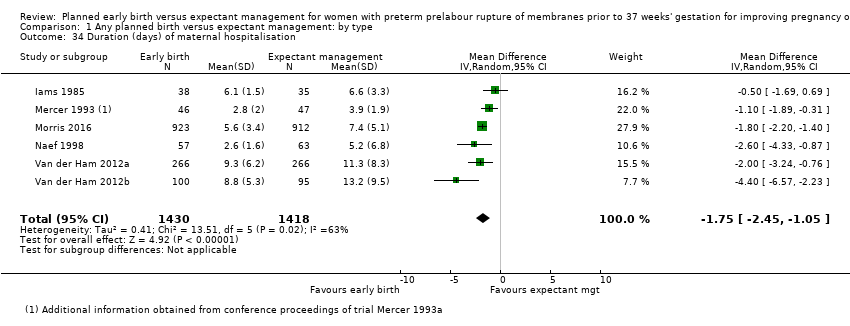
Comparison 1 Any planned birth versus expectant management: by type, Outcome 34 Duration (days) of maternal hospitalisation.
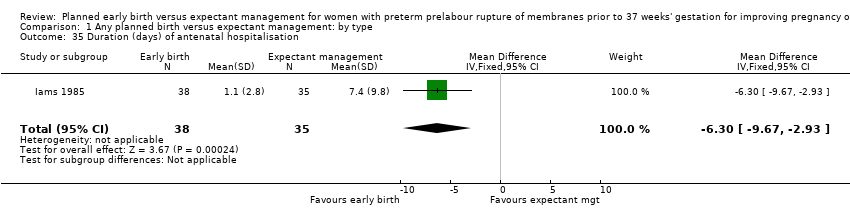
Comparison 1 Any planned birth versus expectant management: by type, Outcome 35 Duration (days) of antenatal hospitalisation.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 36 Duration (days) of maternal hospitalisation (excluding trials with antenatal discharge).

Comparison 1 Any planned birth versus expectant management: by type, Outcome 37 Time (hours) from randomisation to birth.
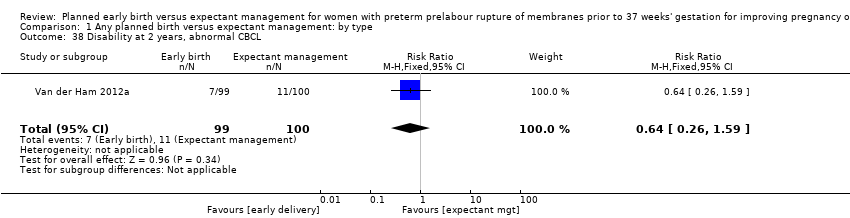
Comparison 1 Any planned birth versus expectant management: by type, Outcome 38 Disability at 2 years, abnormal CBCL.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 39 Disability at 2 years, abnormal ASQ.

Comparison 1 Any planned birth versus expectant management: by type, Outcome 40 Maternal satisfaction.
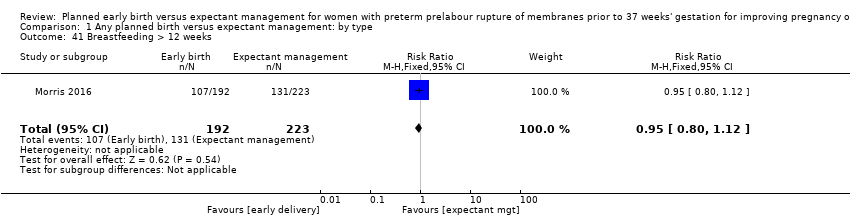
Comparison 1 Any planned birth versus expectant management: by type, Outcome 41 Breastfeeding > 12 weeks.

Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 1 Neonatal infection.

Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 2 Neonatal infection confirmed with positive culture.

Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 3 Respiratory distress syndrome.
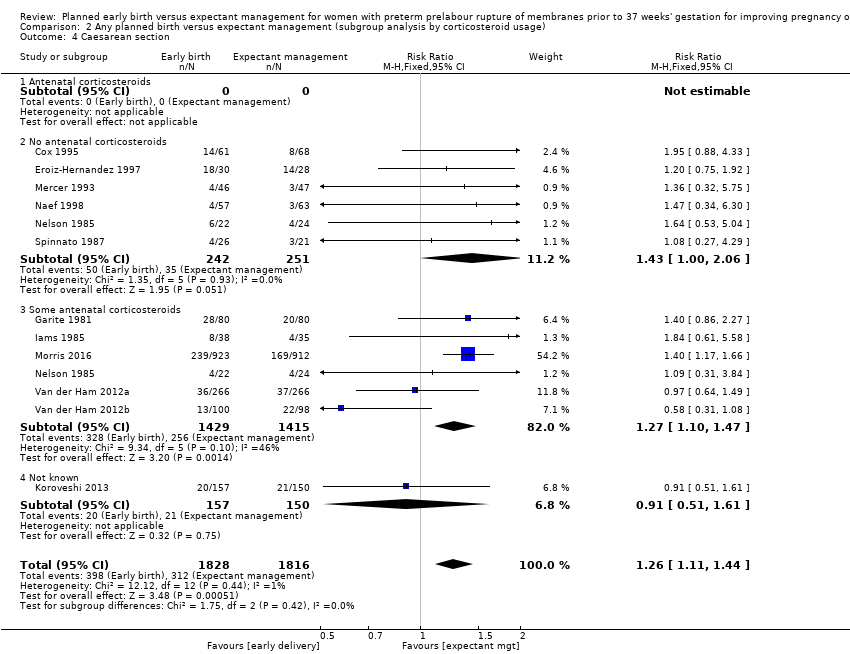
Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 4 Caesarean section.

Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 5 Chorioamnionitis.

Comparison 2 Any planned birth versus expectant management (subgroup analysis by corticosteroid usage), Outcome 6 Endometritis.

Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 1 Neonatal infection.

Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 2 Neonatal infection confirmed with positive culture.

Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 3 Respiratory distress syndrome.
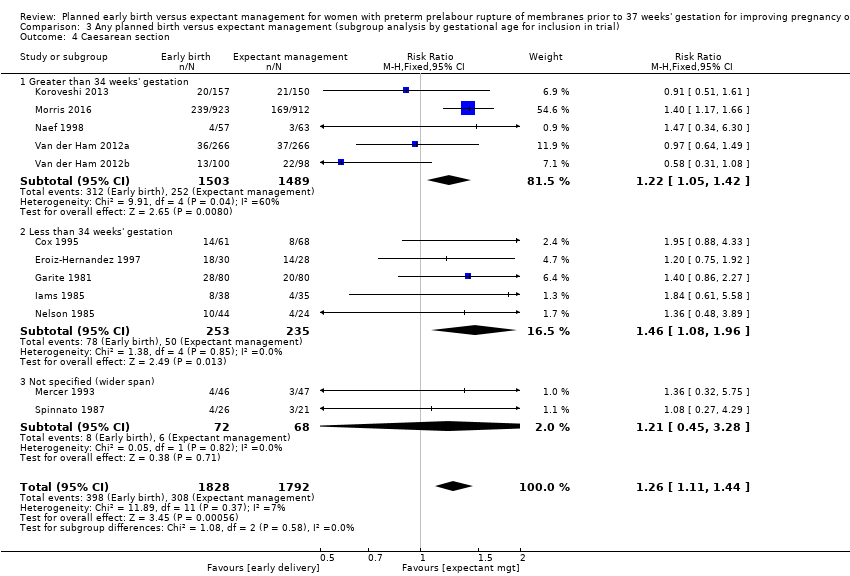
Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 4 Caesarean section.

Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 5 Chorioamnionitis.

Comparison 3 Any planned birth versus expectant management (subgroup analysis by gestational age for inclusion in trial), Outcome 6 Endometritis.

Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 1 Neonatal infection.

Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 2 Neonatal infection confirmed with positive culture.

Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 3 Respiratory distress syndrome.

Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 4 Caesarean section.

Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 5 Chorioamnionitis.
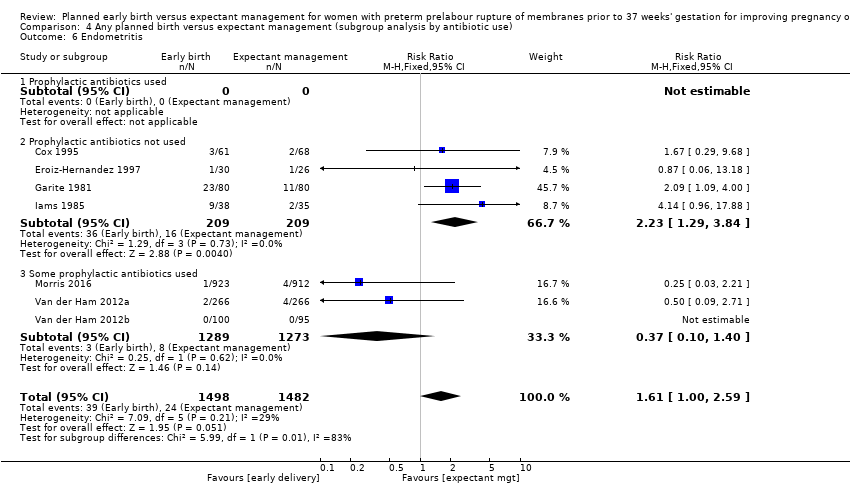
Comparison 4 Any planned birth versus expectant management (subgroup analysis by antibiotic use), Outcome 6 Endometritis.

Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 1 Neonatal infection.
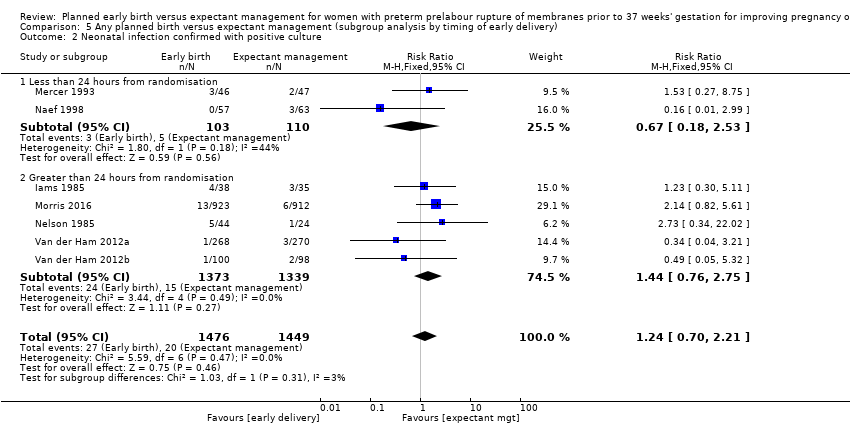
Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 2 Neonatal infection confirmed with positive culture.

Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 3 Respiratory distress syndrome.

Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 4 Caesarean section.
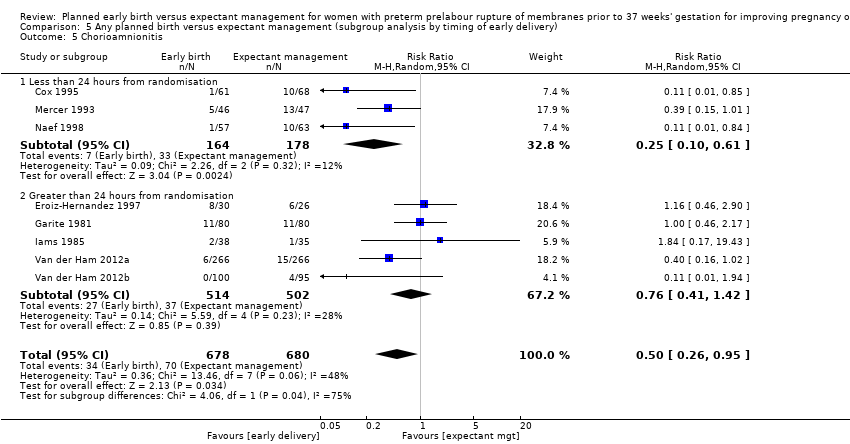
Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 5 Chorioamnionitis.

Comparison 5 Any planned birth versus expectant management (subgroup analysis by timing of early delivery), Outcome 6 Endometritis.
| Planned early birth compared to expectant management for preterm prelabour rupture of membranes prior to 37 weeks' gestation | ||||||
| Patient or population: women with preterm prelabour rupture of membranes prior to 37 weeks' gestation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Expectant management | Planned early birth | |||||
| Neonatal infection/sepsis | Study population | RR 0.93 | 3628 | ⊕⊕⊕⊝ | ||
| 37 per 1000 | 34 per 1000 | |||||
| Neonatal respiratory distress syndrome | Study population | RR 1.26 | 3622 | ⊕⊕⊕⊕ | ||
| 84 per 1000 | 109 per 1000 | |||||
| Need for ventilation | Study population | RR 1.27 | 2895 | ⊕⊕⊕⊕ | ||
| 86 per 1000 | 110 per 1000 | |||||
| Admission to neonatal intensive care | Study population | RR 1.16 | 2691 | ⊕⊕⊕⊝ | ||
| 428 per 1000 | 497 per 1000 | |||||
| Caesarean section | Study population | RR 1.26 | 3620 | ⊕⊕⊕⊕ | ||
| 172 per 1000 | 217 per 1000 | |||||
| Chorioamnionitis | Study population | RR 0.50 | 1358 | ⊕⊕⊕⊝ | ||
| 103 per 1000 | 51 per 1000 | |||||
| Length of hospital stay (maternal) | The mean length of hospital stay (maternal) in the expectant group was | The mean length of hospital stay (maternal) in the early birth group was 1.75 days lower | MD ‐1.75 (‐2.45 to ‐1.05) | 2848 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Wide confidence interval crossing the line of no effect, and the lines of appreciable benefit and harm. | ||||||
| Trial | Sample size | Gestational age for inclusion (weeks) | Co‐interventions | Fetal lung maturity tested |
| 129 (131 babies) 61 ED (62 babies) 68 EM (69 babies) | 30 to 34 |
| No | |
| 58 30 ED 28 EM | 28 to 34 |
| Yes, if positive excluded from randomisation | |
| 160 80 ED 80 EM | 28 to 34 |
| Yes: if L/S mature excluded from randomisation and delivered | |
| 73 38 ED 35 EM | 28 to 34 |
| Yes: if mature L/S excluded from randomisation and delivered | |
| 307 157 ED 150 EM | 34 to 37 |
| No | |
| 93 46 ED 47 EM | 32 to 36+ 6 |
| Yes: included if mature L/S | |
| 1835 923 ED 912 EM | 34 to 36+ 6 |
| No | |
| 120 57 ED 63 EM | 34 to 36+ 6 |
| No | |
| 68 22 ED and steroids 22 ED and no steroids 24 EM | 28 to 34 |
| No | |
| 47 26 to ED 21 to EM | 25 to 36 |
| Yes: included if mature L/S | |
| 532 (538 babies) 266 ED (268 babies) 266 EM (270 babies) | 34 to 36+6 |
| No | |
| 195 (198 babies) 100 ED (100 babies) 95 EM (98 babies) | 34 to 36+6 |
| No | |
| ED: early delivery | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal infection/sepsis Show forest plot | 12 | 3628 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 2 Neonatal infection confirmed with positive blood culture Show forest plot | 7 | 2925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.21] |
| 3 Respiratory distress syndrome Show forest plot | 12 | 3622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.05, 1.53] |
| 4 Caesarean section Show forest plot | 12 | 3620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.11, 1.44] |
| 5 Perinatal mortality Show forest plot | 11 | 3319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.89, 3.50] |
| 6 Intrauterine death Show forest plot | 11 | 3321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.13, 1.55] |
| 7 Cord prolapse Show forest plot | 4 | 2722 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.33, 4.61] |
| 8 Gestational age at birth (weeks) Show forest plot | 8 | 3139 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.57, ‐0.39] |
| 9 Neonatal death Show forest plot | 11 | 3316 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [1.17, 5.56] |
| 10 Suspected neonatal infection Show forest plot | 3 | 829 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.36, 0.88] |
| 11 Neonatal treatment with antibiotics Show forest plot | 4 | 2638 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.19] |
| 12 Need for ventilation Show forest plot | 7 | 2895 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.58] |
| 13 Duration of oxygen therapy (days) Show forest plot | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐3.05 [‐6.92, 0.82] |
| 14 Umbilical cord arterial pH Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.07, 0.11] |
| 15 Birthweight (g) Show forest plot | 10 | 3263 | Mean Difference (IV, Random, 95% CI) | ‐47.10 [‐96.00, 1.80] |
| 16 Apgar score less than 7 at 5 minutes Show forest plot | 5 | 2700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.54, 1.69] |
| 17 Abnormality on cerebral ultrasound Show forest plot | 3 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.52, 6.92] |
| 18 Periventricular leukomalacia Show forest plot | 2 | 707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 6.99] |
| 19 Cerebroventricular haemorrhage Show forest plot | 6 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.40, 3.52] |
| 20 Necrotising enterocolitis Show forest plot | 6 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.25, 2.62] |
| 21 Severe respiratory distress Show forest plot | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.80, 2.97] |
| 22 Admission to neonatal intensive care unit Show forest plot | 4 | 2691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.08, 1.24] |
| 23 Length of stay in neonatal intensive care unit (days) Show forest plot | 4 | 2121 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐1.62, 1.27] |
| 24 Duration (days) from birth to neonatal hospital discharge Show forest plot | 6 | 2832 | Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.28, 1.61] |
| 25 Chorioamnionitis Show forest plot | 8 | 1358 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.95] |
| 26 Endometritis Show forest plot | 7 | 2980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.00, 2.59] |
| 27 Postpartum fever Show forest plot | 1 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.03] |
| 28 Placental abruption Show forest plot | 1 | 1835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.36, 3.87] |
| 29 Induction of labour Show forest plot | 4 | 2691 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [2.01, 2.36] |
| 30 Use of epidural/spinal anaesthesia Show forest plot | 3 | 2562 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.99, 1.65] |
| 31 Vaginal birth Show forest plot | 12 | 3618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.91, 0.97] |
| 32 Operative vaginal birth Show forest plot | 4 | 2685 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.67, 1.10] |
| 33 Caesarean section for fetal distress Show forest plot | 7 | 2918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.20] |
| 34 Duration (days) of maternal hospitalisation Show forest plot | 6 | 2848 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐2.45, ‐1.05] |
| 35 Duration (days) of antenatal hospitalisation Show forest plot | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐6.30 [‐9.67, ‐2.93] |
| 36 Duration (days) of maternal hospitalisation (excluding trials with antenatal discharge) Show forest plot | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.06, ‐0.23] |
| 37 Time (hours) from randomisation to birth Show forest plot | 3 | 2571 | Mean Difference (IV, Fixed, 95% CI) | ‐79.48 [‐88.27, ‐70.69] |
| 38 Disability at 2 years, abnormal CBCL Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.26, 1.59] |
| 39 Disability at 2 years, abnormal ASQ Show forest plot | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.05] |
| 40 Maternal satisfaction Show forest plot | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.13] |
| 41 Breastfeeding > 12 weeks Show forest plot | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal infection Show forest plot | 12 | 3652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.68, 1.32] |
| 1.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 No antenatal corticosteroids | 6 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.48, 2.03] |
| 1.3 Some antenatal corticosteroids | 6 | 2850 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.41] |
| 1.4 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.55] |
| 2 Neonatal infection confirmed with positive culture Show forest plot | 7 | 2939 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.74, 2.23] |
| 2.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 No antenatal corticosteroids | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.18, 2.04] |
| 2.3 Some antenatal corticosteroids | 5 | 2680 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.85, 3.00] |
| 3 Respiratory distress syndrome Show forest plot | 12 | 3646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.07, 1.56] |
| 3.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 No antenatal corticosteroids | 6 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.83] |
| 3.3 Some antenatal corticosteroids | 6 | 2844 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.01, 1.63] |
| 3.4 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.55, 2.94] |
| 4 Caesarean section Show forest plot | 12 | 3644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.11, 1.44] |
| 4.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 No antenatal corticosteroids | 6 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.00, 2.06] |
| 4.3 Some antenatal corticosteroids | 6 | 2844 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.10, 1.47] |
| 4.4 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.61] |
| 5 Chorioamnionitis Show forest plot | 8 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.72] |
| 5.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 No antenatal corticosteroids | 4 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.67] |
| 5.3 Some antenatal corticosteroids | 4 | 960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.36, 1.06] |
| 6 Endometritis Show forest plot | 7 | 2980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.00, 2.59] |
| 6.1 Antenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 No antenatal corticosteroids | 2 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.32, 5.94] |
| 6.3 Some antenatal corticosteroids | 5 | 2795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.99, 2.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal infection Show forest plot | 12 | 3628 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 1.1 Greater than 34 weeks' gestation | 5 | 2998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.47, 1.07] |
| 1.2 Less than 34 weeks' gestation | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.74, 3.50] |
| 1.3 Not specified (wider span) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.65, 6.18] |
| 2 Neonatal infection confirmed with positive culture Show forest plot | 7 | 2925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.21] |
| 2.1 Greater than 34 weeks' gestation | 4 | 2691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.52, 2.20] |
| 2.2 Less than 34 weeks' gestation | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.52, 5.35] |
| 2.3 Not specified (wider span) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.27, 8.75] |
| 3 Respiratory distress syndrome Show forest plot | 12 | 3622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.05, 1.53] |
| 3.1 Greater than 34 weeks' gestation | 5 | 2992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.10, 1.90] |
| 3.2 Less than 34 weeks' gestation | 5 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.43] |
| 3.3 Not specified (wider span) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.27, 2.42] |
| 4 Caesarean section Show forest plot | 12 | 3620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.11, 1.44] |
| 4.1 Greater than 34 weeks' gestation | 5 | 2992 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.05, 1.42] |
| 4.2 Less than 34 weeks' gestation | 5 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.08, 1.96] |
| 4.3 Not specified (wider span) | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.45, 3.28] |
| 5 Chorioamnionitis Show forest plot | 8 | 1358 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.72] |
| 5.1 Greater than 34 weeks' gestation | 3 | 847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.57] |
| 5.2 Less than 34 weeks' gestation | 4 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.30] |
| 5.3 Not specified (wider span) | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.15, 1.01] |
| 6 Endometritis Show forest plot | 7 | 2980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.00, 2.59] |
| 6.1 Greater than 34 weeks' gestation | 3 | 2562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.40] |
| 6.2 Less than 34 weeks' gestation | 4 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.29, 3.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal infection Show forest plot | 12 | 3625 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 1.1 Prophylactic antibiotics used | 2 | 1702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.31] |
| 1.2 Prophylactic antibiotics not used | 8 | 880 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.81, 2.51] |
| 1.3 Some prophylactic antibiotics used | 2 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.30, 1.46] |
| 1.4 Not specified | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.55] |
| 2 Neonatal infection confirmed with positive culture Show forest plot | 7 | 2925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.21] |
| 2.1 Prophylactic antibiotics used | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.99] |
| 2.2 Prophylactic antibiotics not used | 3 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.62, 4.28] |
| 2.3 Some prophylactic antibiotics used | 3 | 2571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.62, 2.93] |
| 3 Respiratory distress syndrome Show forest plot | 12 | 3622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.56] |
| 3.1 Prophylactic antibiotics used | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.23, 5.26] |
| 3.2 Prophylactic antibiotics not used | 7 | 630 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.85, 1.45] |
| 3.3 Some prophylactic antibiotics used | 3 | 2565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.10, 1.99] |
| 3.4 Not specified | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.55, 2.94] |
| 4 Caesarean section Show forest plot | 12 | 3620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.14, 1.49] |
| 4.1 Prophylactic antibiotics used | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.34, 6.30] |
| 4.2 Prophylactic antibiotics not used | 7 | 628 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.08, 1.90] |
| 4.3 Some prophylactic antibiotics used | 3 | 2565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.12, 1.53] |
| 4.4 Not specified | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.61] |
| 5 Chorioamnionitis Show forest plot | 8 | 1358 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.95] |
| 5.1 Prophylactic antibiotics used | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 0.84] |
| 5.2 Prophylactic antibiotics not used | 5 | 511 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.34, 1.41] |
| 5.3 Some prophylactic antibiotics used | 2 | 727 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.86] |
| 6 Endometritis Show forest plot | 7 | 2980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.00, 2.59] |
| 6.1 Prophylactic antibiotics used | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Prophylactic antibiotics not used | 4 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.29, 3.84] |
| 6.3 Some prophylactic antibiotics used | 3 | 2562 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal infection Show forest plot | 12 | 3628 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 1.1 Less than 24 hours from randomisation | 4 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.41, 1.99] |
| 1.2 Greater than 24 hours from randomisation | 7 | 2930 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.41] |
| 1.3 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.25, 2.55] |
| 2 Neonatal infection confirmed with positive culture Show forest plot | 7 | 2925 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.70, 2.21] |
| 2.1 Less than 24 hours from randomisation | 2 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.53] |
| 2.2 Greater than 24 hours from randomisation | 5 | 2712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.76, 2.75] |
| 3 Respiratory distress syndrome Show forest plot | 12 | 3622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.56] |
| 3.1 Less than 24 hours from randomisation | 4 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.65, 1.50] |
| 3.2 Greater than 24 hours from randomisation | 7 | 2924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.10, 1.71] |
| 3.3 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.55, 2.94] |
| 4 Caesarean section Show forest plot | 12 | 3620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.11, 1.44] |
| 4.1 Less than 24 hours from randomisation | 4 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.90, 2.81] |
| 4.2 Greater than 24 hours from randomisation | 7 | 2924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.10, 1.46] |
| 4.3 Not known | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.61] |
| 5 Chorioamnionitis Show forest plot | 8 | 1358 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.95] |
| 5.1 Less than 24 hours from randomisation | 3 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.10, 0.61] |
| 5.2 Greater than 24 hours from randomisation | 5 | 1016 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.41, 1.42] |
| 6 Endometritis Show forest plot | 7 | 2980 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.00, 2.59] |
| 6.1 Less than 24 hours from randomisation | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.29, 9.68] |
| 6.2 Greater than 24 hours from randomisation | 6 | 2851 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.98, 2.63] |

