Evaluación del riesgo biomédico como ayuda para el abandono del hábito de fumar
Resumen
Antecedentes
Una posible estrategia para aumentar las tasas de abandono del hábito de fumar podría ser proporcionar a los fumadores información acerca de los efectos o los posibles efectos biomédicos de fumar, como la medición del monóxido de carbono exhalado (CO), la función pulmonar, o la predisposición genética al cáncer de pulmón u otras enfermedades.
Objetivos
El principal objetivo fue determinar la eficacia de proporcionar información a los fumadores sobre sus mediciones de monóxido de carbono (CO) exhalado, los resultados de la espirometría, la detección por la imagen de la placa aterosclerótica y la predisposición genética a las enfermedades relacionadas con el tabaco como una ayuda para abandonar el hábito de fumar.
Métodos de búsqueda
Para la actualización más reciente, se realizaron búsquedas en el registro especializado del Grupo Cochrane de Adicción al Tabaco (Cochrane Tobacco Addiction Group) en marzo de 2018 y clinicalTrials.gov y la ICTRP de la OMS en septiembre de 2018 para estudios añadidos desde la última actualización en 2012.
Criterios de selección
Los criterios de inclusión de la revisión fueron: un diseño de ensayo controlado aleatorio; participantes fumadores en ese momento; intervenciones basadas en pruebas biomédicas para aumentar tasas de abandono del hábito de fumar; grupos control que recibían los demás componentes de la intervención; y una medida de resultado de la tasa de abandono del hábito de fumar al menos seis meses después del comienzo de la intervención.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. Los resultados se expresaron como cociente de riesgos (CR) del abandono del hábito con intervalos de confianza (IC) del 95%. Cuando fue apropiado, los estudios se agruparon mediante un modelo de efectos aleatorios Mantel‐Haenszel.
Resultados principales
Se incluyeron 20 ensayos que utilizaron una variedad de intervenciones de pruebas biomédicas; un ensayo incluyó dos intervenciones, para un total de 21 intervenciones. Se incluyeron 9262 participantes, todos adultos fumadores. Todos los estudios incluyeron a fumadores adultos, tanto hombres como mujeres, en diferentes etapas de cambio y motivación para el abandono del hábito de fumar. Se consideró que todos los estudios excepto tres tuvieron riesgo alto o incierto de sesgo en al menos un dominio. Se agruparon los estudios en tres categorías de acuerdo con el tipo de biorretroalimentación proporcionada: retroalimentación sobre el riesgo de la exposición (cinco estudios); retroalimentación sobre el riesgo de enfermedades relacionadas con el tabaquismo (cinco estudios); y retroalimentación sobre los daños del tabaquismo (11 estudios). No hubo evidencia de aumento de las tasas de abandono a partir de la retroalimentación sobre la exposición al riesgo, que consistió principalmente en retroalimentación sobre la medición del CO, en cinco ensayos agrupados (CR 1,00; IC del 95%: 0,83 a 1,21; I2 = 0%; n = 2368). La retroalimentación sobre el riesgo de enfermedad relacionada con el tabaquismo, incluidos cuatro estudios que evaluaron la retroalimentación sobre los marcadores genéticos para el riesgo de cáncer y un estudio con retroalimentación sobre los marcadores genéticos para el riesgo de enfermedad de Crohn, no mostró un beneficio en el abandono del hábito de fumar (CR 0,80; IC del 95%: 0,63 a 1,01; I2 = 0%; n = 2064). La retroalimentación sobre el daño relacionado con el tabaquismo, incluidos nueve estudios que evaluaron la espirometría con o sin retroalimentación sobre la edad pulmonar y dos estudios sobre la retroalimentación sobre la ecografía carotídea, tampoco mostró un beneficio (CR 1,26; IC del 95%: 0,99 a 1,61; I2 = 34%; n = 3314). Sólo un estudio comparó directamente múltiples formas de medición con una sola forma de medición y no detectó una diferencia significativa en el efecto entre la medición del CO más la susceptibilidad genética al cáncer de pulmón y la medición del CO solamente (CR 0,82; IC del 95%: 0,43 a 1,56; n = 189).
Conclusiones de los autores
Existen pocas pruebas acerca de los efectos de la evaluación de los riesgos biomédicos como ayuda para dejar de fumar. Los resultados más prometedores se relacionan con la espirometría y el ultrasonido carotídeo, donde las pruebas de certeza moderada, limitadas por la imprecisión y el riesgo de sesgo, no detectaron un beneficio estadísticamente significativo, pero los intervalos de confianza no alcanzaron uno de forma muy estrecha, y la estimación puntual favoreció la intervención. Un análisis de sensibilidad con la exclusión de los estudios con alto riesgo de sesgo detectó un beneficio. Las pruebas de certeza moderada limitadas por el riesgo de sesgo no detectaron un efecto de la retroalimentación sobre la exposición al tabaquismo mediante el seguimiento del CO. Las pruebas de certeza baja limitadas por el riesgo de sesgo e imprecisión no detectaron un beneficio de la retroalimentación sobre el riesgo relacionado con fumar por pruebas de marcadores genéticos. No hay pruebas suficientes para evaluar la hipótesis de que los tipos múltiples de evaluación son más efectivos que las formas únicas de evaluación.
PICO
Resumen en términos sencillos
¿Dar a las personas retroalimentación acerca de los efectos del hábito de fumar sobre su cuerpo los ayuda a abandonar el hábito?
Antecedentes
La evaluación de los riesgos biomédicos es el proceso de proporcionar a los fumadores información acerca de los efectos del hábito de fumar sobre su cuerpo. Los efectos físicos del tabaquismo pueden ser evaluados usando varias medidas, y algunas personas piensan que esto podría ser usado como una herramienta para animar a las personas a dejar de fumar. Se revisaron la evidencia acerca de si dar a los adultos fumadores retroalimentación acerca de los efectos del hábito de fumar sobre su cuerpo los ayuda a abandonar el hábito.
Características de los estudios
Esta revisión incluye 20 estudios que utilizaron varias mediciones. Un estudio incluyó dos mediciones, para un total de 21 mediciones evaluadas. Las principales medidas de retroalimentación que evaluamos fueron el nivel de monóxido de carbono en la respiración de las personas (un signo de tabaquismo actual), medidas de la función pulmonar (un signo de daño pulmonar por fumar), pruebas genéticas para proporcionar riesgo individual de cáncer, y ultrasonido de las arterias principales del cuello para medir la cantidad de placa (un factor de riesgo de accidente cerebrovascular). Se agruparon los estudios en tres categorías de acuerdo con el tipo de retroalimentación que recibieron las personas: retroalimentación sobre la exposición al tabaquismo (cinco estudios); retroalimentación sobre el riesgo de una persona para las enfermedades relacionadas con el tabaquismo (cinco estudios); y retroalimentación sobre los daños del tabaquismo (11 estudios). Los estudios incluyeron un total de 9262 personas. Todos los participantes eran fumadores adultos, y se incluyeron tanto hombres como mujeres (aunque un estudio realizado en una clínica para veteranos del ejército incluyó sólo un 4% de mujeres). La mayoría de los estudios se realizaron en la atención primaria o ambulatoria. Casi todos los estudios duraron al menos seis meses. Las pruebas informadas están actualizadas hasta marzo de 2018.
Resultados clave
No se halló evidencia de que dar a los fumadores retroalimentación acerca de su exposición al tabaco, su riesgo genético de padecer enfermedades relacionadas con el tabaco o los efectos del hábito de fumar sobre su cuerpo los ayuda a abandonar el hábito. Los resultados más prometedores fueron de dar retroalimentación a las personas sobre el daño que el fumar hace a sus cuerpos. Los estudios no informaron sobre daños o efectos secundarios de proporcionar retroalimentación. Sin embargo, dada la naturaleza de las mediciones (análisis de pulmón o de sangre), es de esperar que el riesgo de daños sea bajo.
Certeza de la evidencia
Debido a los problemas con la forma en que se realizaron algunos de los estudios, es probable que la investigación adicional cambie estas conclusiones.
Authors' conclusions
Summary of findings
| Biomedical risk assessment compared with standard care or minimal intervention for smoking cessation | |||||
| Patient or population: people who smoke | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
| Risk with control | Risk with biomedical risk assessment | ||||
| Feedback on smoking exposure Smoking cessation at longest follow‐up over 6 months | Study population | RR 1.00 (0.83 to 1.21) | 2368 | ⊕⊕⊕⊝1 moderate | |
| 153 per 1000 | 153 per 1000 (127 to 185) | ||||
| Feedback on smoking‐related risk Smoking cessation at longest follow‐up over 6 months | Study population | RR 0.80 (0.63 to 1.01) | 2064 | ⊕⊕⊝⊝1,2 low | |
| 130 per 1000 | 104 per 1000 (82 to 131) | ||||
| Feedback on smoking‐related harm Smoking cessation at longest follow‐up over 6 months | Study population | RR 1.26 (0.99 to 1.61) | 3314 | ⊕⊕⊕⊝3 moderate | |
| 117 per 1000 | 147 per 1000 (116 to 188) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded one level for risk of bias: three out of five studies at high risk of bias, and remaining studies at unclear risk of bias. | |||||
Background
Description of the condition
Smoking is the leading cause of preventable mortality and morbidity in industrialised countries (CDC 2014; Phillips 1995; Sargeant 2001; Yach 2000), and increasingly the smoking epidemic is affecting low‐ and middle‐income countries. This is particularly true for vascular and respiratory diseases, as well as for cancer (DHHS 2004; Doll 2004; Thun 2013). Stopping smoking prolongs life and reduces morbidity (DHHS 1990; Jha 2013). Despite increasing scientific knowledge about health hazards due to cigarette consumption, there is still an increase in prevalence in many countries (Bilano 2015). The gap between knowledge and smoking cessation has been attributed, in part, to smokers' underestimation of their personal risks of smoking‐related illness (Krosnick 2017; Lerman 1993; Romer 2001). Although many smokers who are successful in quitting do so on their own (Livingstone‐Banks 2019; Schwartz 1987), an increasing proportion use specific aids to support a quit attempt.
Description of the intervention
Evidence is growing on how to help smokers to quit (Fiore 2008; Hartmann‐Boyce 2018; Kottke 1988; Lancaster 2017; Stead 2013; West 2000). Interventions that have been shown to help quitting smoking include individual or group counselling, pharmacological therapies, and possibly some forms of self‐help materials. Another possible strategy for increasing quit rates is to provide feedback on the physical effects of smoking by physiological measurements. We can conceptually distinguish, in this respect, three different types of feedback: the first one explores biomarkers of smoking exposure (cotinine, carbon monoxide); the second one gives information on smoking‐related disease risk (e.g. lung cancer susceptibility according to cytochrome P450 2D6 (CYP2D6) genotyping) (Audrain 1997); and the third one depicts smoking‐related harm (e.g. atherosclerotic plaque, impaired lung function) (Buist 2002).
How the intervention might work
The rationale for such interventions is to provide personalised motivational feedback to promote risk awareness and to accelerate smoking behaviour change (Curry 1993; Miller 1991). Recognition of personal susceptibility to the adverse effects of smoking may be an important step in the pathway to smoking cessation (McClure 2001; Weinberger 1981; Young 2010). Indeed, most theories related to health behaviour changes (e.g. self‐determination theory, theory of planned behaviour, health belief model, transtheoretical model, social cognitive theory, social ecological model, common‐sense model) have in common that they recognise an important role to the person's understanding of the potential negative consequences of a given behaviour (Hale 2007; Joseph 2016).
Why it is important to do this review
Individual studies have produced conflicting data on the effect of physiological feedback (Bovet 2002; Buffels 2006; Lerman 1993; McBride 2000; Parkes 2008; Rodondi 2012). This review systematically examined data on smoking cessation rates from randomised controlled trials using feedback on the physiological effect of smoking or on genetic susceptibility to smoking‐related diseases.
Objectives
The main objective was to determine the efficacy of providing smokers with feedback on their exhaled carbon monoxide (CO) measurement, spirometry results, atherosclerotic plaque imaging, and genetic susceptibility to smoking‐related diseases in helping them to quit smoking.
The hypotheses to be examined were as follows.
-
Feedback on personal characteristics indicating that the effects of smoking, or susceptibility to smoking‐related illness, increases rates of smoking cessation.
-
Multiple types of measurement (e.g. spirometry and exhaled CO measurement used together) are more effective for smoking cessation than a single form of measurement.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials.
Types of participants
We included studies of any individuals who smoked and who participated in smoking cessation programmes, or in screening for respiratory disease, or in health checkups. There were no restrictions on participant age, motivation to quit, or whether participants were pregnant, were hospitalised, or were suffering from coexistent illness.
Types of interventions
We included studies of any intervention in which a physical measurement, such as exhaled carbon monoxide (CO), spirometry, atherosclerotic plaque imaging, or genetic testing, was used as a way to increase smoking cessation rate. We considered studies in which reporting of these measurements was the only component of an intervention, or was tested as an adjunct to another intervention such as counselling, where the control group received all other components except for the reporting of such measurements. We excluded trials in which the effect of biological measurements was confounded by the use of other components in the active intervention.
Types of outcome measures
The main outcome measure was abstinence from smoking, measured at least six months after the start of the intervention. We used the most conservative measure of quitting at the longest follow‐up, preferring biochemically validated results where available. We counted participants lost to follow‐up as continuing smokers. We excluded studies that did not provide data on cessation but instead measured intermediate outcomes such as withdrawal symptoms.
Search methods for identification of studies
For this update we searched the Cochrane Tobacco Addiction Group Specialized Register on 27 March 2018 and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) on 3 September 2018 for studies that involved any use of a biomedical test as part of an intervention. At the time of the search, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), Issue 1, 2018; MEDLINE (via Ovid) to update 20180209; Embase (via Ovid) to week 201807; and PsycINFO (via Ovid) to update 20180212. See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources.
We used the following topic‐related keywords and text terms to identify potentially relevant studies:
patient education, patient compliance, patient counselling, persuasive communication, spirometry, respiratory function, bronchospirometry, carbon monoxide, forced expiratory flow rates, obstructive lung diseases, genetic testing, genetic susceptibility, genetic predisposition, biomarker, feedback.
See Appendix 1 for the full strategy.
Data collection and analysis
Two review authors (out of CC, RB, YM, KS) independently prescreened all search results (abstracts) for possible inclusion or as useful background. Studies were selected for full‐text assessment if retained by at least one of the review authors. Two review authors (out of CC, RB, YM, KS) then independently assessed the selected articles for inclusion, resolving any discrepancies by consensus. We have recorded reasons for the non‐inclusion of studies in the Characteristics of excluded studies table.
Each review author then extracted and compared the data from the selected studies. This stage included an evaluation of risk of bias. Four review authors independently assessed each study according to the presence and quality of the randomisation process, whether or not trialists and assessors were 'blinded', whether the analysis was appropriate to the study design, and the description of withdrawals and dropouts. We used Covidence for the screening and data extraction (Covidence).
We extracted data, where available, on the following.
-
Country and setting (e.g. primary care, community, hospital outpatient/inpatient)
-
Recruitment
-
Method of selection of participants (e.g. willingness to make a quit attempt)
-
Definition of smoker used
-
Method of randomisation
-
Allocation concealment
-
Smoking and demographic characteristics of participants (e.g. average age, sex, average number of cigarettes smoked per day)
-
Description of the experimental and control interventions (provider, length, number of visits, etc.)
-
Outcomes, including definition of abstinence used, and biochemical validation of cessation
-
Proportion of participants with follow‐up data
-
Whether or not data were analysed on an intention‐to‐treat basis
-
Appropriateness of statistical approach
-
Declaration of interest and source of funding
Data synthesis
We expressed results as a risk ratio (RR) for smoking cessation with 95% confidence intervals (CI). Where appropriate, we pooled studies using a Mantel‐Haenszel random‐effects method. We assessed heterogeneity using the I2 statistic.
'Summary of findings' table
We created a 'Summary of findings' table for our primary outcome in accordance with standard Cochrane methodology. We assessed the certainty of evidence using the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias).
Results
Description of studies
Results of the search
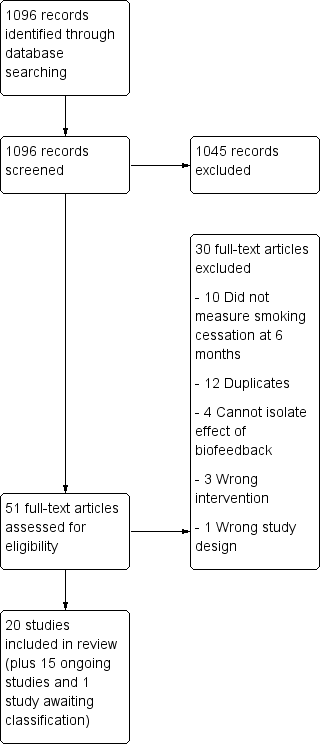
For this update we found 1096 records for screening, from which we identified five new trials, for a total of 20 trials of 9262 people that met our inclusion criteria. The flow of studies is reported in a PRISMA flow diagram (Figure 1). We found 15 studies that were potentially eligible and either ongoing (ACTRN12618000291280; IRCT2017080435257N1; NCT02658032; NCT02840513; NCT02991781; NCT03521141; NCT03583203), or finished but with no published data and no information retrievable through contacting study authors (Martin Lujan 2011; Martin Lujan 2016; NCT01186016; NCT02431611; NCT03377738; Pita Fernandez 2015; Ripoll 2012; Muhammad 2015). Details of these studies can be found in the Characteristics of ongoing studies table. One study was ongoing, but there was insufficient information to assess its eligibility, therefore we assessed it as awaiting classification (NCT02351167).

Study flow diagram for 2019 update.
Included studies
One included study tested two interventions (Audrain 1997). Out of the 21 interventions, five tested feedback on smoking exposure, each measuring the effect of exhaled CO measurements (Audrain 1997; Brunette 2013; Jamrozik 1984; Sanders 1989; Shahab 2011). Five studies tested feedback on smoking‐related disease risk; of these, four tested feedback about genetic susceptibility to cancer (Audrain 1997; Hishida 2010; Ito 2006; Nichols 2014), and one tested feedback about genetic susceptibility to Crohn's disease (Hollands 2012). In Audrain 1997, the intervention was feedback about genetic susceptibility combined with CO measurement, which could either be compared to a control group of CO measurement alone, or to a control group without biomarker feedback, thereby testing the combination of the two interventions. Eleven studies assessed feedback on smoking‐related harm: four tested the combination of exhaled CO measurement and spirometry (McClure 2009; Risser 1990; Sippel 1999; Walker 1985); five tested the effect of spirometry alone (Buffels 2006; Irizar Aramburu 2013; Segnan 1991), or with the addition of feedback on lung age (Drummond 2014; Parkes 2008); two tested the effect of undergoing an ultrasonography of carotid arteries (and femoral arteries for Bovet 2002) with photographic demonstration of atherosclerotic plaques when present (Bovet 2002; Rodondi 2012).
The trials were conducted in different settings: 11 trials took place in general practice (Buffels 2006; Irizar Aramburu 2013; Jamrozik 1984; Nichols 2014; Parkes 2008; Sanders 1989; Segnan 1991), or outpatient clinics (Bovet 2002; Ito 2006; Rodondi 2012; Sippel 1999); one study took place in the community, recruiting first‐degree relatives of probands with Crohn's disease (Hollands 2012); two took place in smoking cessation clinics (Audrain 1997; Walker 1985); one was conducted in a health promotion clinic for army veterans (Risser 1990); one was conducted in a mental health clinic (Brunette 2013); one was conducted in a company (Hishida 2010); and three took place in research institutions (Drummond 2014; McClure 2009; Shahab 2011). Seven trials took place in the USA (Audrain 1997; Brunette 2013; Drummond 2014; McClure 2009; Risser 1990; Sippel 1999; Walker 1985), six in the UK (Hollands 2012; Jamrozik 1984; Nichols 2014; Parkes 2008; Sanders 1989; Shahab 2011), two in Japan (Hishida 2010; Ito 2006), and one trial each took place in Italy (Segnan 1991), Belgium (Buffels 2006), the Seychelles Islands (Bovet 2002), Switzerland (Rodondi 2012), and Spain (Irizar Aramburu 2013).
Methods of recruitment were heterogeneous between studies. Among the six studies conducted in general practice, one recruited patients at their first visit (Jamrozik 1984); another screened outpatients on specific days (Segnan 1991); two screened patients during the recruitment period (Buffels 2006; Sanders 1989); and two invited known smokers by post (Parkes 2008; Nichols 2014). One study recruited smokers among outpatients in primary care clinics (Sippel 1999); one study recruited outpatients at a cancer centre hospital (Ito 2006); and one study recruited patients referred to a mental health treatment clinic (Brunette 2013). Four studies recruited smokers by media advertisement (Audrain 1997; Rodondi 2012; Shahab 2011; Walker 1985). The remaining studies recruited smokers participating in a health survey (Bovet 2002); employees who identified themselves as smokers in a questionnaire used for an annual workplace checkup (Hishida 2010); veterans that responded to a mailed invitation to attend a health promotion clinic (Risser 1990); and participants of a cohort study of people with a history of injecting drugs (Drummond 2014). Two studies combined diverse methods of recruitment. One study used media advertisement, outpatient recruitment, data from health plan records, a quitline register, and a purchased e‐mail list of smokers (McClure 2009), and another asked probands to identify first‐degree relatives through three routes, first approaching probands receiving care through hospital services, secondly contacting them by mail using Crohn's disease databases at 42 participating hospitals, and finally placing advertisements in the newsletters of associations (Hollands 2012). One study did not specify the method of recruitment (data were obtained after contacting the study author; the study is not yet published) (Irizar Aramburu 2013). Participation rates (i.e. the proportion of those approached who agreed to take part in the trial) were seldom recorded.
All studies included male and female adults who were smokers at the time of inclusion. Only 10 studies provided a definition of being a smoker at the time of inclusion (Audrain 1997; Bovet 2002; Drummond 2014; Hollands 2012; Irizar Aramburu 2013; Ito 2006; McClure 2009; Nichols 2014; Rodondi 2012; Shahab 2011). The mean age of the participants when given varied between 31.7 and 53.0 years. The proportion of women in the trials varied between 4% and 65%. The mean number of cigarettes smoked per day varied between 11.9 and 29.2. The mean number of cigarettes smoked per day tended to be highest in the trials set in a smoking cessation clinic (29.2 per day in Walker 1985 and 22.7 per day in Audrain 1997) or among veterans (23.5 per day in Risser 1990). Levels of nicotine addiction as assessed by the Fagerström score were provided in three studies (Heatherton 1991), and ranged from 3.5 to 5.4 (Drummond 2014; Nichols 2014; Rodondi 2012); proportions of participants in the various stages of change according to Prochaska and Di Clemente were only provided in five studies (Prochaska 1983), with the proportion of participants in the preparation stage ranging from 17% to 37.5% (Audrain 1997; Ito 2006; McClure 2009; Parkes 2008; Sippel 1999).
The therapist delivering the intervention was a physician in five trials (Bovet 2002; Buffels 2006; Irizar Aramburu 2013; Jamrozik 1984; Segnan 1991); a nurse in four trials (Hishida 2010; Risser 1990; Rodondi 2012; Sanders 1989); a specific study staff member in seven trials (Audrain 1997; Drummond 2014; Ito 2006; Parkes 2008; Shahab 2011; Sippel 1999; Walker 1985); a trained health educator or research counsellor in two trials (Hollands 2012; McClure 2009); the principal investigator with help from trained smoking cessation practitioners in one study (Nichols 2014); and the intervention and feedback was web based in one study (Brunette 2013). Further details on the included studies can be found in the Characteristics of included studies tables.
Excluded studies
For this update, we excluded 31 studies at full‐text screening, and listed a total of 61 excluded studies. The primary reasons for exclusion were because the effect of the biomedical assessment could not be evaluated separately from the rest of the cessation intervention; the study had less than six months follow‐up; or the study was not a true randomised trial. The excluded studies along with reasons for their exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We judged all but three included studies to be at high or unclear risk of bias in at least one domain (Parkes 2008; Rodondi 2012; Segnan 1991). 'Risk of bias' judgements for each study are displayed in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only six of the 20 included trials reported an adequate procedure for both randomisation and allocation concealment (Brunette 2013; Drummond 2014; Hollands 2012; Parkes 2008; Rodondi 2012; Segnan 1991). Eight studies did not report the method of randomisation, or provided insufficient information to assume that allocation was adequately concealed (Audrain 1997; Bovet 2002; Buffels 2006; McClure 2009; Nichols 2014; Risser 1990; Shahab 2011; Walker 1985). Five studies reported inadequate allocation sequences, with allocation by day, week, or month of attendance (Hishida 2010; Ito 2006; Jamrozik 1984; Sanders 1989), or by odd‐/even‐numbered questionnaire at the time of check‐in (Sippel 1999). In one of these studies, 120 participants were allocated to the wrong group and were excluded from further analysis (Sanders 1989).
Due to the demonstrative nature of the intervention, participants and those delivering the intervention could not be blinded to allocation, therefore we did not assess performance bias, and assessed detection bias independent of blinding. We judged the risk of detection bias to be low if abstinence was biochemically verified, or if the intervention and control groups received similar amounts of face‐to‐face contact. If abstinence was not biochemically verified and the intervention group received more face‐to‐face contact than the control group, then we judged the risk of detection bias to be high because the results may be prone to differential misreport. We judged only one study to be at high risk of detection bias based on these criteria (Hishida 2010); we assessed the remaining studies as at low risk of bias.
Three trials used urinary cotinine level to validate smoking cessation at follow‐up (Parkes 2008; Sanders 1989; Segnan 1991). One study used the same validation procedure but only on a subsample of self‐reported ex‐smokers (Jamrozik 1984). Two studies used expired air carbon monoxide (Risser 1990; Walker 1985). One study used serum cotinine level (Rodondi 2012); one study used saliva cotinine (Hollands 2012); and two studies used both exhaled CO and salivary cotinine (Drummond 2014; Nichols 2014). Nine studies did not use any biochemical validation (Audrain 1997; Bovet 2002; Brunette 2013; Buffels 2006; Hishida 2010; Ito 2006; McClure 2009; Shahab 2011; Sippel 1999). Only seven studies explicitly mentioned that assessors were blinded to allocation at the time of outcome determination (Bovet 2002; Brunette 2013; Parkes 2008; Risser 1990; Rodondi 2012; Shahab 2011; Sippel 1999).
In three studies (Audrain 1997; Nichols 2014; Walker 1985), it was not possible to determine the initial allocation of the participants who were subsequently lost to follow‐up, and the analysis had to be performed per protocol. This may have overestimated cessation rates for the individual studies, but impact on meta‐analysis results were probably limited given the limited number of participants in those three studies. In one study (Hishida 2010), those who were allocated to the intervention group but who subsequently declined to participate in biomarker testing were included in the baseline characteristics table, but were excluded from further analyses.
Effects of interventions
Results are given as risk ratios (RRs) for smoking cessation at the latest recorded follow‐up time (six to 12 months) between intervention and control groups, with 95% confidence intervals (CI). An RR greater than one favours the intervention group. We classified trials into three analyses by type of intervention: those that provided feedback on smoking exposure, those that provided feedback on smoking‐related disease risk, and those that provided feedback on smoking‐related harms. Results of the analyses are reproduced in Figure 3, Figure 4, and Figure 5.

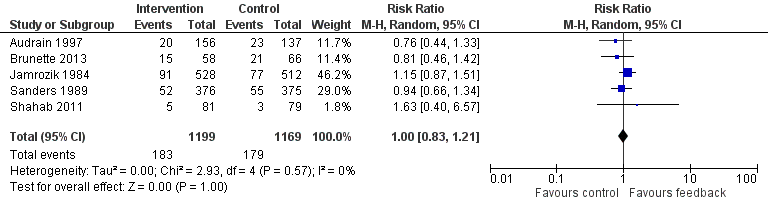
Forest plot of comparison: 1 All interventions, outcome: 1.1 Smoking cessation ‐ feedback on smoking exposure.
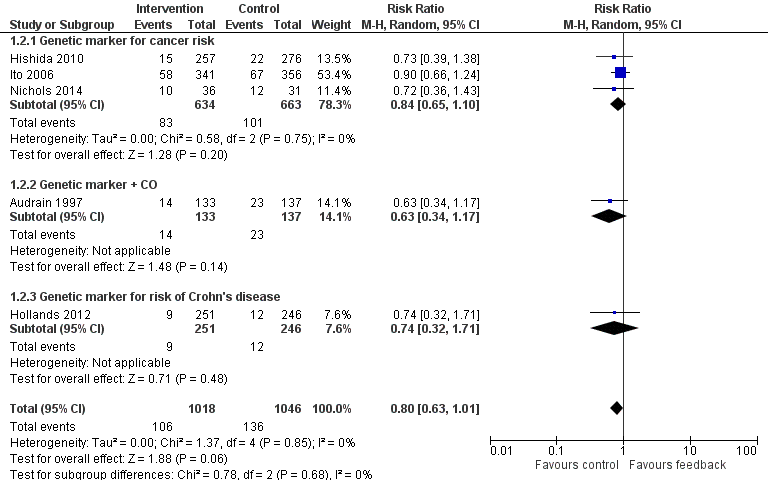
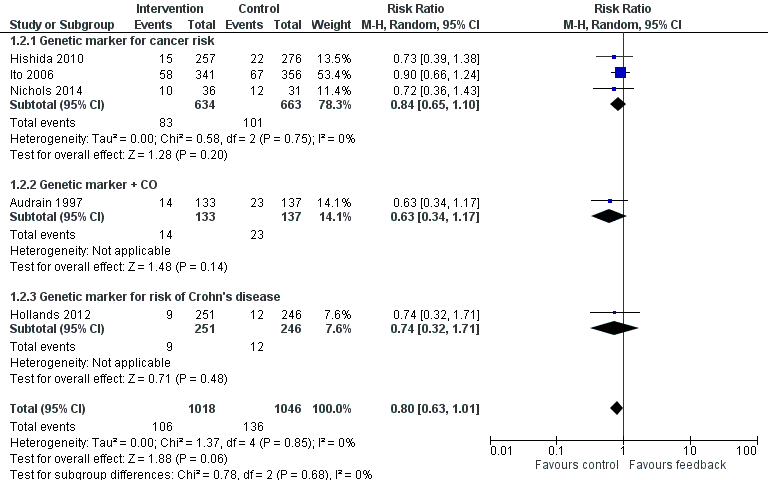
Forest plot of comparison: 1 All interventions, outcome: 1.2 Smoking cessation ‐ feedback on smoking‐related disease risk.

Forest plot of comparison: 1 All interventions, outcome: 1.3 Smoking cessation ‐ feedback on smoking‐related harm.
Feedback on smoking exposure
Five studies isolated the effect of demonstrating levels of exhaled CO on smoking cessation rate (Audrain 1997; Brunette 2013; Jamrozik 1984; Sanders 1989; Shahab 2011). There was no evidence of a significant benefit from these pooled studies (RR 1.00, 95% CI 0.83 to 1.21; I2 = 0%; n = 2368; Analysis 1.1; Figure 3).
Feedback on smoking‐related disease risk
Three trials studied measurements of genetic susceptibility to smoking‐related cancer (Hishida 2010; Ito 2006; Nichols 2014), and one measured the genetic susceptibility to Crohn's disease (Hollands 2012). We pooled all five trials and found no evidence of a significant benefit from these pooled studies (RR 0.80, 95% CI 0.63 to 1.01; I2 = 0%; n = 2064; Analysis 1.2; Figure 4).
We pooled the three trials testing feedback on the genetic susceptibility to smoking‐related cancers and found no evidence of a significant benefit from these pooled studies (RR 0.84, 95% CI 0.65 to 1.10; I2 = 0%; n = 1297; Analysis 1.2.1). Hollands 2012 did not detect a benefit from feedback on genetic susceptibility to Crohn's disease (in relatives of individuals suffering from Crohn's disease) (RR 0.74, 95% CI 0.32 to 1.71; n = 497).
Feedback on smoking‐related harms
Eleven studies provided feedback on smoking‐related harm. Pooling all 11 studies narrowly missed a significant effect on smoking cessation (RR 1.26, 95% CI 0.99 to 1.61; I2 = 34%; n = 3314; Analysis 1.3; Figure 5). However, a sensitivity analysis removing the three studies at high risk of bias did detect an effect (RR 1.36, 95% CI 1.07 to 1.74; I2 = 24%; n = 2710; analysis not shown).
Five studies provided feedback based on spirometry results (Buffels 2006; Drummond 2014; Irizar Aramburu 2013; Parkes 2008; Segnan 1991). There was no evidence of a significant benefit from spirometry with or without feedback on lung age when the five studies were pooled (RR 1.24, 95% CI 0.83 to 1.84; I2 = 38%; n = 1728; Analysis 1.3.1). Four trials used exhaled CO measurement and spirometry together (McClure 2009; Risser 1990; Sippel 1999; Walker 1985). None of these trials detected a significant effect independently, and there was no evidence of a significant benefit when these studies were pooled (RR 1.27, 95% CI 0.74 to 2.18; I2 = 47%; n = 895; Analysis 1.3.2). Two trials tested ultrasonography of carotid (and femoral) arteries (Bovet 2002; Rodondi 2012). There was no evidence of a significant benefit from these pooled studies (RR 1.56, 95% CI 0.67 to 3.66; I2 = 66%; n = 691; Analysis 1.3.3).
Multiple versus single forms of measurement
Only one study directly compared multiple forms of measurement with a single form of measurement (Audrain 1997). The study did not detect a significant difference in effect between measurement of CO plus genetic susceptibility to lung cancer and measurement of CO only (RR 0.82, 95% CI 0.43 to 1.56; n = 189; Analysis 2.1). The other included studies were too clinically heterogenous to allow indirect comparisons of multiple versus single forms of measurement.
Discussion
Summary of main results
Due to the scarcity of evidence of sufficient quality, we cannot make definitive statements about the effectiveness of biomedical risk assessment as an aid for smoking cessation. Most studies were small and did not detect significant effects. We pooled studies based on the type of feedback they provided. The most promising results relate to spirometry and carotid ultrasound, where moderate‐certainty evidence, limited by imprecision and risk of bias, did not detect a statistically significant benefit, but confidence intervals very narrowly missed one, and the point estimate favoured the intervention. A sensitivity analysis removing those studies at high risk of bias did detect a benefit. Moderate‐certainty evidence limited by risk of bias did not detect an effect of feedback on smoking exposure by CO monitoring. Low‐certainty evidence, limited by risk of bias and imprecision, did not detect a benefit from feedback on smoking‐related risk by genetic marker testing. There is insufficient evidence with which to evaluate the hypothesis that multiple types of assessment are more effective than single forms of assessment. Results are summarised in summary of findings Table for the main comparison.
Only two studies detected statistically significant intervention effects. One of these was a trial in primary care that used spirometry and immediate feedback of the results using a graphical display (Parkes 2008). Intervention participants were told their "lung age" if this was older than their chronological age. The control group also had spirometry and feedback, but the results were given by letter in terms of standard measures of lung function, with no mention of lung age. All participants were advised to quit and offered a referral to intensive support. Contact time was longer in the intervention group because of the verbal feedback. Giving the spirometry results in terms of lung age resulted in an increase in the cessation rate at 12 months from 6.4% to 13.6%. In considering the strength of evidence and generalisability of this trial, it should also be noted that the outcome was limited to point‐prevalence abstinence, and information was lacking about the comparability of the study sample with the entire recruitment population. Another study, retrieved in the current update, also assessed spirometry with lung age feedback (compared with spirometry results in a standardised written format) but in a research cohort of intravenous drug users (Drummond 2014). The sample size was small (50 participants), and the study did not show a significant benefit of this approach.
The other study with a statistically significant positive effect used pictures of ultrasound photographs of atherosclerotic plaques (Bovet 2002). Participants were randomised to undergo ultrasonography (n = 74) or not (n = 79). Those with ultrasonographic demonstration of atherosclerotic plaques (n = 20) were given photographs of their plaques with a relevant explanation, whereas the others did not benefit from any further procedure. This study's external validity can be questioned, as the sample was made up predominantly of male light smokers (average 10 to 12 cigarettes a day). Another study using a similar feedback method had a larger sample (536 smokers), a larger proportion of women (45%), and heavier smokers as participants (an average of 20 cigarettes smoked per day) (Rodondi 2012). This study did not show a statistically significant effect of the intervention. The authors noted in their discussion that they included only smokers with a motivation to quit and that they used a very intensive smoking cessation programme in both the intervention and control groups.
While the evidence does not directly support the use of biomedical risk assessment for smoking cessation, more research is needed before we can be confident in this result.
Overall completeness and applicability of evidence, and limitations of the review
Cochrane methods are designed to minimise bias in the review process. However, due to the nature of the intervention, there were some difficulties in finding suitable evidence. In most of the included studies, the biomedical testing component was added to intensive quit‐smoking sessions, with counselling lasting up to 60 minutes and completed by written material and reinforcement sessions or follow‐up phone calls. The incremental effect of biomedical risk assessment might have been diluted by the high intensity of the 'standard' care used. It is also possible that the changes in motivational stages induced by biomedical risk assessment are too subtle to be characterised as directly leading to a successful quit attempt. Data from those studies do not permit verification of this hypothesis. However, given the increasing evidence that the transtheoretical model of behaviour change is not a mandatory prerequisite for a smoking cessation intervention to be effective (Cahill 2010; Riemsma 2003), we do not consider this lack of intermediate data to be a major concern.
Biomedical risk assessment was also conceptualised as a part of multicomponent interventions (with components other than biomedical risk assessment) by some authors of studies identified by our search strategy (Borrelli 2002; Borrelli 2005; Ferketich 2012; Hajek 2002; Humerfelt 1998; Kotz 2009; Martucci 2010; McBride 2002; McClure 2013; Prokhorov 2008; Richmond 1986). Even though four of these studies demonstrated effects significantly favouring intervention versus control groups, they did not isolate the specific effect of biomedical feedback. The retrieved studies also did not provide us with sufficient data to adequately examine our second research hypothesis, that feedback with different types of measurements is more effective for smoking cessation than feedback of a single measurement.
Another possible explanation for the absence of effectiveness of biomedical risk assessment provided in addition to counselling could be the potentially counterproductive effect of communicating normal results to smokers. Six included studies provided some insight about smoking cessation rates in subgroups according to test results. Sippel 1999, Buffels 2006, and Parkes 2008 did not find any correlation between smoking cessation and abnormal spirometry results. Bovet 2002, which overall detected significantly higher cessation rates in participants offered ultrasonography, found a statistically non‐significant lower smoking cessation rate among the subgroup of participants without plaques at ultrasonography than among participants in the control group. Rodondi 2012 found that, in the ultrasonography group, cessation rates did not differ according to the presence or absence of atherosclerotic plaques. Parkes 2008 noted that anecdotally some smokers were motivated by having normal results because it helped them feel it was "not too late" to quit. Ito 2006 found some evidence that among participants without cancer, those told they had increased susceptibility to cancer were more likely to quit. This particular question, and the way to handle and communicate normal results, has yet to be answered.
Trials demonstrating smoking‐related harms using spirometry (especially when combined with lung age) or carotid ultrasound tended to show a stronger effect on smoking cessation, though this effect was not significant. This could be explained by the fact that direct and concrete demonstration of manifest harm might be more likely to motivate smokers to quit smoking, according to the Leventhal Common Sense Model (Hale 2007). It might be more straightforward for a smoker to establish a causal link between smoking and a direct and "noticeable" harm such as lung damage (increased lung age) or carotid plaques. Inversely, feedback on smoking exposure, using indirect and more obtuse measures such as CO, might not be sufficient to motivate a change in behaviour. Genetic feedback might be even more difficult to understand and counterproductive in the sense that people might believe that risks are not modifiable due to the deterministic aspect of genetics.
Two other trials used demonstration of smokers' children's exposure to environmental tobacco smoke by measuring the child's urinary cotinine level (abstinence was a secondary outcome) and demonstration of Latino caregivers' asthmatic children's exposure to secondhand CO (Borrelli 2010; Wakefield 2002), with RRs of 0.26 (95% CI 0.02 to 2.33) and 0.90 (95% CI 0.72 to 1.12), respectively. A third study evaluated the efficacy of personalised feedback during foetus ultrasound on pregnant smokers (Stotts 2009), and again did not detect a benefit (RR 1.31, 95% CI 0.66 to 2.58). We excluded these studies from our analysis because our research hypothesis was that smokers, although adept at estimating the risks of smoking in terms of morbidity and mortality, are prone to underestimate their own risk with regard to smoking (Lerman 1993; Romer 2001). It therefore seemed to us that providing biomarker feedback about someone else's health (even one's own children or foetus) would act differently and may not contribute to counteracting this personal optimistic bias. In any event, these trials did not detect benefits for the interventions tested.
A promising new approach could be for dental practitioners to use a point‐of‐care test for salivary nicotine metabolites. A short‐term randomised controlled trial compared smoking cessation after eight weeks among participants who received either counselling with immediate or delayed (at the end of the study) feedback on salivary nicotine level (Barnfather 2005). The risk ratio for smoking cessation is close to being statistically significantly in favour of those who received immediate feedback: RR of 3.40 (95% CI 1.00 to 11.61). Trials with longer‐term follow‐up are needed to confirm this effect (Coleman 2005).
Despite broad inclusion criteria regarding the type of participants, we found only scarce data exploring the effect of biomedical risk assessment on hospitalised patients or acutely ill individuals. It is possible that such a specific context and the presence of coexistent illnesses could facilitate a modification of risk perception. One study included participants both with and without cancer (Ito 2006). Reported subgroup analyses did not detect benefits either for participants with cancer (RR 0.65, 95% CI 0.43 to 1.02) or for participants without cancer (RR 1.22, 95% CI 0.63 to 2.36).
Some recent studies used connected devices such as mobile‐phone applications (ACTRN12618000291280; NCT02840513; NCT03583203), or provided web‐based feedback (Brunette 2013). Biofeedback on lung damage or risk of lung cancer, or both, is a well‐represented intervention in ongoing studies (NCT02658032; NCT03521141; NCT03583203).
Certainty of the evidence
The certainty of the evidence using GRADE criteria was low to moderate, depending on feedback category. The evidence on interventions providing feedback on risk exposure was of moderate certainty, limited by risk of bias because three of the five studies were at high risk of bias, and the remaining studies were at unclear risk of bias. The evidence on interventions providing feedback on smoking‐related disease risk was of low certainty, limited by risk of bias and imprecision because fewer than 300 events were recorded. The evidence on interventions providing feedback on smoking‐related harm through spirometry or carotid ultrasound was of moderate certainty, limited by imprecision and risk of bias because the confidence intervals only narrowly missed a statistically significant benefit, and a sensitivity analysis excluding studies at high risk of bias did detect one.
Agreements and disagreements with other studies or reviews
An earlier non‐systematic review was conducted on the use of biomarkers in smoking cessation (McClure 2001). This work aimed to review the theoretical rationale and empirical evidence regarding this practice, and therefore did not specifically focus on the assessment of the efficacy of biomarker feedback as a way to increase rates of long‐term smoking cessation. The review therefore included non‐randomised trials (Haddow 1991; Kilburn 1990; Loss 1979; Scott 1990); trials providing multicomponent interventions that precluded the isolation of the specific effect of biomarker feedback (Bauman 1983; Lerman 1997; Richmond 1986); trials comparing the effect of abnormal test results versus normal test results rather than test versus no test (Li 1984); and trials reporting outcomes other than smoking cessation. One study identified by McClure as "in press" appears never to have been published (Hoffman 1998), and we were unable to obtain further information despite several attempts to contact the authors. Four studies mentioned by McClure were also included in our review (Audrain 1997; Jamrozik 1984; Risser 1990; Walker 1985). Our review includes 16 trials not in the McClure review (Bovet 2002; Brunette 2013; Buffels 2006; Drummond 2014; Hishida 2010; Hollands 2012; Irizar Aramburu 2013; Ito 2006; McClure 2009; Nichols 2014; Parkes 2008; Rodondi 2012; Sanders 1989; Segnan 1991; Shahab 2011; Sippel 1999). When focusing on efficacy data, McClure 2001 concluded that biomarker feedback may enhance the likelihood of cessation because a trend for increased abstinence was found in three randomised trials (Hoffman 1998; Risser 1990; Walker 1985). The fact that two of these trials, Walker 1985; Risser 1990, are subject to major methodological limitations (small samples, inadequate randomisation procedures), and that the report of Hoffman 1998 remains unpublished, calls for great caution in drawing such conclusions.
Study flow diagram for 2019 update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Forest plot of comparison: 1 All interventions, outcome: 1.1 Smoking cessation ‐ feedback on smoking exposure.
Forest plot of comparison: 1 All interventions, outcome: 1.2 Smoking cessation ‐ feedback on smoking‐related disease risk.

Forest plot of comparison: 1 All interventions, outcome: 1.3 Smoking cessation ‐ feedback on smoking‐related harm.
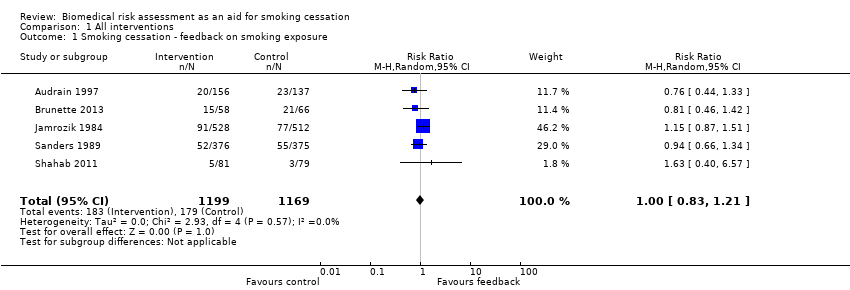
Comparison 1 All interventions, Outcome 1 Smoking cessation ‐ feedback on smoking exposure.
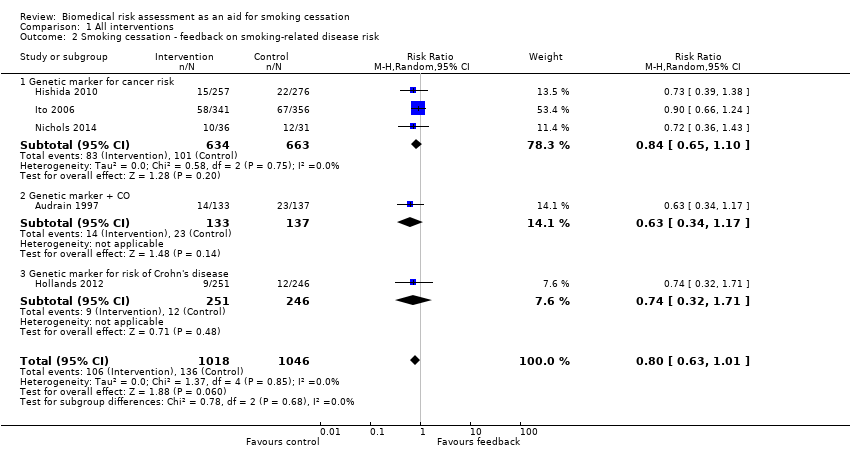
Comparison 1 All interventions, Outcome 2 Smoking cessation ‐ feedback on smoking‐related disease risk.

Comparison 1 All interventions, Outcome 3 Smoking cessation ‐ feedback on smoking‐related harm.
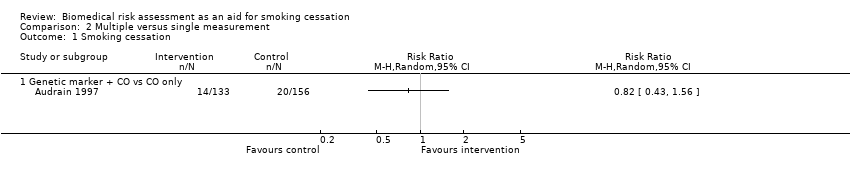
Comparison 2 Multiple versus single measurement, Outcome 1 Smoking cessation.

Comparison 3 All interventions, Outcome 1 Spirometry and/or lung age.
| Biomedical risk assessment compared with standard care or minimal intervention for smoking cessation | |||||
| Patient or population: people who smoke | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
| Risk with control | Risk with biomedical risk assessment | ||||
| Feedback on smoking exposure Smoking cessation at longest follow‐up over 6 months | Study population | RR 1.00 (0.83 to 1.21) | 2368 | ⊕⊕⊕⊝1 moderate | |
| 153 per 1000 | 153 per 1000 (127 to 185) | ||||
| Feedback on smoking‐related risk Smoking cessation at longest follow‐up over 6 months | Study population | RR 0.80 (0.63 to 1.01) | 2064 | ⊕⊕⊝⊝1,2 low | |
| 130 per 1000 | 104 per 1000 (82 to 131) | ||||
| Feedback on smoking‐related harm Smoking cessation at longest follow‐up over 6 months | Study population | RR 1.26 (0.99 to 1.61) | 3314 | ⊕⊕⊕⊝3 moderate | |
| 117 per 1000 | 147 per 1000 (116 to 188) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Downgraded one level for risk of bias: three out of five studies at high risk of bias, and remaining studies at unclear risk of bias. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking cessation ‐ feedback on smoking exposure Show forest plot | 5 | 2368 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2 Smoking cessation ‐ feedback on smoking‐related disease risk Show forest plot | 5 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.01] |
| 2.1 Genetic marker for cancer risk | 3 | 1297 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.10] |
| 2.2 Genetic marker + CO | 1 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.17] |
| 2.3 Genetic marker for risk of Crohn's disease | 1 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.32, 1.71] |
| 3 Smoking cessation ‐ feedback on smoking‐related harm Show forest plot | 11 | 3314 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.99, 1.61] |
| 3.1 Spirometry with or without lung age | 5 | 1728 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.84] |
| 3.2 CO and spirometry feedback | 4 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.74, 2.18] |
| 3.3 Carotid ultrasound | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.67, 3.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking cessation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Genetic marker + CO vs CO only | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Spirometry and/or lung age Show forest plot | 5 | 1728 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.83, 1.84] |
| 1.1 Spirometry with lung age | 2 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.62, 5.05] |
| 1.2 Spirometry without lung age | 3 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.48] |

