Intervencije za smanjivanje koncentracije homocisteina u krvnoj plazmi pacijenata na dijalizi
Abstract
Background
People with end‐stage kidney disease (ESKD) have high rates of cardiovascular events. Randomised controlled trials (RCTs) of homocysteine‐lowering therapies have not shown reductions in cardiovascular event rates in the general population. However, people with kidney disease have higher levels of homocysteine and may have different mechanisms of cardiovascular disease. We performed a systematic review of the effect of homocysteine‐lowering therapies in people with ESKD.
Objectives
To evaluate the benefits and harms of established homocysteine lowering therapy (folic acid, vitamin B6, vitamin B12) on all‐cause mortality and cardiovascular event rates in patients with ESKD.
Search methods
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review.
Selection criteria
Studies conducted in people with ESKD that reported at least 100 patient‐years of follow‐up and assessed the effect of therapies that are known to have homocysteine‐lowering properties were included.
Data collection and analysis
Two authors independently extracted data using a standardised form. The primary outcome was cardiovascular mortality. Secondary outcomes included all‐cause mortality, incident cardiovascular disease (fatal and nonfatal myocardial infarction and coronary revascularisation), cerebrovascular disease (stroke and cerebrovascular revascularisation), peripheral vascular disease (lower limb amputation), venous thromboembolic disease (deep vein thrombosis and pulmonary embolism), thrombosis of dialysis access, and adverse events. The effects of homocysteine‐lowering therapies on outcomes were assessed with meta‐analyses using random‐effects models. Prespecified subgroup and sensitivity analyses were conducted.
Main results
We included six studies that reported data on 2452 participants with ESKD. Interventions investigated were folic acid with or without other vitamins (vitamin B6, vitamin B12). Participants' mean age was 48 to 65 years, and proportions of male participants ranged from 50% to 98%.
Homocysteine‐lowering therapy probably leads to little or no effect on cardiovascular mortality (4 studies, 1186 participants: RR 0.93, 95% CI 0.70 to 1.22). There was no evidence of heterogeneity among the included studies (I² = 0%). Homocysteine‐lowering therapy had little or no effect on all‐cause mortality or any other of this review's secondary outcomes. All prespecified subgroup and sensitivity analyses demonstrated little or no difference. Reported adverse events were mild and there was no increase in the incidence of adverse events from homocysteine‐lowering therapies (3 studies, 1248 participants: RR 1.12, 95% CI 0.51 to 2.47; I2 = 0%). Overall, studies were assessed as being at low risk of bias and there was no evidence of publication bias.
Authors' conclusions
Homocysteine‐lowering therapies were not found to reduce mortality (cardiovascular and all‐cause) or cardiovascular events among people with ESKD.
PICOs
Laički sažetak
Intervencije za smanjivanje koncentracije homocisteina u krvnoj plazmi pacijenata na dijalizi
Dosadašnje spoznaje
Ljudi s uznapredovalom bubrežnom bolešću često razviju srčanu bolest, koja je ujedno i najčešći razlog smrti tih ljudi. Visoka koncentracija aminokiseline homocisteina u krvi je rizični čimbenik razvoja srčane bolesti u tih bolesnika. Često se koriste terapije koje smanjuju razinu homocisteina u krvi (npr. folna kiselina, vitamin B6 i vitamin B12), ali prednosti i nedostatci takve terapije još nisu potpuno jasni. Cilj ovog Cochrane sustavnog pregleda bio je procijeniti nedostatke i prednosti liječenja čiji je cilj smanjivanje plazmatske koncentracije homocisteina u ljudi s uznapredovalom bubrežnom bolešću koji su na dijalizi.
Obilježja studija
Temeljem pretraživanja literature objavljene do siječnja 2016. godine, nađeno je 6 randomiziranih kontroliranih pokusa, s ukupnim brojem od 2452 sudionika u dobi između 48 i 65 godina.
Ključni rezultati
Pronađeno je da terapije za smanjivanje razine homocisteina nisu imale koristan učinak u liječenju srčanih bolesti u bolesnika s uznapredovalom bubrežnom bolešću koji su na dijalizi Te terapjije nisu pokazale koristan učinak na smanjenje stope smrtnih slučajeva povezanih sa srčanom bolešću. Međutim, terapiju smanjivanja razine homocisteina pacijenti su općenito dobro podnosili jer ima blage nuspojave.
Kvaliteta dokaza
Sveukupno, studije su ocijenjene visoko kvalitetnima.
Authors' conclusions
Background
Description of the condition
Dialysis‐dependent end‐stage kidney disease (ESKD) patients have reduced life expectancy, with annual mortality of 14.8% in Australia (McDonald 2007), and 16.7% in the USA (USRDS 2007). Chronic kidney disease (CKD) is an independent and powerful risk factor for cardiovascular disease (Go 2004; Weiner 2004). Cardiovascular mortality is significant in CKD (de Jager 2009). For dialysis‐dependent ESKD patients, death from cardiovascular disease is 10 to 100 times higher than in age and sex‐matched controls (Foley 1998).
The increased prevalence of cardiovascular disease among ESKD patients is not completely accounted for by the presence of traditional risk factors such as hypertension, dyslipidaemia, diabetes mellitus, smoking, and left ventricular hypertrophy (Baigent 2000). Thus, there has been increasing investigation of nontraditional risk factors such as anaemia, hyperparathyroidism and hyperhomocysteinaemia.
Description of the intervention
Homocysteine is an amino acid produced from the metabolism of methionine. It is believed to play a role in the pathogenesis of atherosclerosis by damaging the endothelium and promoting clotting (Eikelboom 1999). Epidemiological studies have shown homocysteine to be an independent risk factor for cardiovascular disease (HSC 2002). Elevated homocysteine level is a predictor of vascular disease including stroke, myocardial infarction (MI, heart attack), atherosclerosis (thickening of artery walls), arterial and venous thrombosis (clotting) and cardiovascular death in the general population (HSC 2002; Wald 2002) and in people with ESKD (Chauveau 1993; Moustapha 1998; Robinson 1996).
In contrast to the well‐documented association between homocysteine levels and vascular events in the general population, the association between homocysteine levels and risk for atherothrombotic disease is not consistent among the ESKD population. Some investigators have described an inverse association between homocysteine levels and clinical outcomes in the ESKD patients with low levels of homocysteine being associated with worse outcomes (Kalantar‐Zadeh 2004; Wrone 2001). However, even a mild increase in the homocysteine level appears to be a vascular risk factor in the general population and there is high prevalence of hyperhomocysteinaemia in ESKD. Thus, the paradoxical reverse association between homocysteine and clinical outcome in ESKD patients does not as such refute a possible role for homocysteine in the vascular pathogenesis. Furthermore, it has also been shown that association of hyperhomocysteinaemia and worse clinical outcomes persists in ESKD patients without chronic inflammation‐malnutrition state (Ducloux 2006). These authors postulate that the burden of chronic inflammation‐malnutrition might mask the true relationship between hyperhomocysteinaemia and cardiovascular risk among dialysis‐dependent ESKD patients.
How the intervention might work
Because kidney metabolism is the primary means by which homocysteine is cleared, plasma homocysteine has a strong inverse correlation with estimated glomerular filtration rate (Freidman 2001). Among patients with ESKD, the prevalence of hyperhomocysteinaemia is 85% to 100% (Bostom 1999).
In classical homocystinuria, plasma total homocysteine concentrations are very high (100 to 400 μmol/L), and untreated patients die prematurely from venous thromboembolism and malignant arterial disease. Long‐term treatment aimed at lowering homocysteine levels has been extremely effective in reducing the potentially life‐threatening vascular risk in these patients modified by agents that lower homocysteine such as folic acid and vitamins B6 and B12. Of the homocysteine‐lowering therapies, folic acid is the most effective and consistent in reducing homocysteine levels in ESKD (van Guldener 2006).
Why it is important to do this review
A limited number of studies of homocysteine‐lowering therapy in ESKD patients have been conducted which individually have been unable to find a statistically significant effect on surrogate markers of cardiovascular disease, cardiovascular events or all‐cause mortality (ASFAST 2004; HOST Study 2004; Vianna 2007; Wrone 2004). The picture is confused by the inability of studies to normalise homocysteine levels in most patients, despite the administration of larger doses of folic acid than in studies of other population groups (ASFAST 2004; Gonin 2005).
Although there is no RCT evidence to support folic acid or vitamin B interventions, multivitamin supplementation for patients with ESKD is commonly practiced. The K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients promote the administration of folate, vitamins B6 and B12 to compensate for dialysate losses and prevent elevation of homocysteine on the grounds of cardiovascular risk (K/DOQI 2005). The group also acknowledged the lack of evidence in the area and state that further data are required regarding the effect of vitamin therapy on clinical outcomes. Furthermore potential harms associated with folic acid supplements have not been excluded (Zoccali 2010).
In light of the burden of cardiovascular disease for patients with ESKD, the effectiveness or otherwise of any potentially effective interventions needs to be clearly established. This review aimed to assess the benefits and harms of homocysteine lowering therapy among people with ESKD to guide decision making and improve outcomes for patients.
Objectives
To evaluate the benefits and harms of established homocysteine lowering therapy (folic acid, vitamin B6, vitamin B12) on all‐cause mortality and cardiovascular event rates in patients with ESKD.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alteration, use of alternate medical records, date of birth or other predictable methods) looking at the use of established homocysteine lowering therapy, with a minimum of 100 patient years were included to reduce the risk of reporting or publication bias. Sequential and cross‐over studies were excluded.
Types of participants
We included adults (aged over 18 years) with ESKD defined as those requiring maintenance dialysis. Patients with functioning kidney transplants were excluded.
Types of interventions
Studies randomising patients to therapies which have proven efficacy in lowering homocysteine levels were included. Studies of regimens in which the main mechanism of action is thought not to be homocysteine lowering were excluded (e.g. simvastatin plus folic acid, N‐acetyl cysteine). We investigated the following comparisons.
-
Homocysteine‐lowering therapy versus placebo or usual care
-
Higher dose homocysteine‐lowering therapy versus lower dose homocysteine‐lowering therapy
-
Any schedule of treatment
-
Any route of treatment.
Types of outcome measures
Primary outcomes
-
Cardiovascular mortality
Secondary outcomes
-
All‐cause mortality
-
Cardiovascular disease
-
Fatal and nonfatal MI
-
Coronary revascularisation
-
-
Cerebrovascular disease
-
Stroke
-
Cerebrovascular revascularisation
-
-
Peripheral vascular disease and venous thromboembolic disease
-
Lower limb amputation
-
Deep vein thrombosis (DVT) and pulmonary embolism (PE)
-
-
Kidney‐specific outcomes
-
Thrombosis of dialysis access
-
-
Adverse events from folic‐based therapy
-
Gastrointestinal events
-
Dermatological events
-
Neurological events
-
Malignancy incidence and mortality
-
Any self‐reported adverse events.
-
Search methods for identification of studies
Electronic searches
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
-
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of review articles, relevant studies and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Steps in data collection and analyses are outlined below.
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, and studies that were not applicable were discarded. However, studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and the full text as necessary of these studies to determine which studies satisfy the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one RCT existed, only the publication with the most complete data were to be included; however, we did not find any duplicate publications. Disagreements were resolved by consultation.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all‐cause mortality, MI, coronary revascularisation, cardiovascular death, stroke, cerebrovascular revascularisation, lower limb amputation, thrombosis of arteriovenous (AV) access, DVT, PE), results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Dealing with missing data
Any further information required from the original author was to be requested by written correspondence and any relevant information obtained in this manner was included in the review. Attrition rates, such as drop‐outs, losses to follow‐up and withdrawals were to be investigated. Issues of missing data and imputation methods (e.g. last‐observation‐carried‐forward) were to be critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Publication bias was assessed using a funnel plot (Egger 1997). Attrition bias was assessed using the loss/event ratio.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted to explore possible sources of heterogeneity using inverse variance according to the following characteristics.
-
Study intervention
-
Exposure to folic acid among control group participants
-
Proportion of participants with diabetes mellitus
-
Proportion of participants with cardiac disease
-
Study duration follow‐up
-
Study event number.
We aimed to analyse the ability of interventions to normalise homocysteine levels in a subgroup of studies that enrolled patients with elevated homocysteine levels. Plausible explanations for variations in treatment effect were explored using subgroup analyses based on study quality and length of follow‐up.
Sensitivity analysis
Sensitivity analyses were conducted to ensure conclusions are robust to decisions made during the review process such as inclusion criteria and imputing of missing data. Where outcomes sought were reported in insufficient detail to allow meta‐analysis and further information was not forthcoming from triallists, we planned to summarise these outcomes and assess with descriptive techniques.
We planned to calculate the number of persons needed to treat to avoid one cardiovascular death if adequate data were available; however, our non‐significant results did not permit making this calculation.
Results
Description of studies
Detailed description of study search results and description of studies is outlined below.
Results of the search
We searched Cochrane Kidney and Transplant's Specialised Register to 25 January 2016 through contact with the Information Specialist using search terms relevant to this review. Additionally, reference lists of review articles, relevant studies and clinical practice guidelines were also searched. This comprehensive search yielded 336 records. The titles and abstracts of these 336 records were assessed and 110 articles were excluded since they did not meet the prespecified review criteria. The full texts of the remaining 220 records were reviewed; six studies (34 records) met our review criteria (Figure 1). Prior to publication three additional studies were identified and these will be assessed in a future update of this review (NCT00004495; Soleimani 2011; Tayebi‐Khosroshahi 2013).

Flow diagram showing study selection
Included studies
We included six studies that involved 2452 participants with ESKD requiring dialysis (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). All studies were reported between 2003 and 2009.
Design
All included studies were parallel RCTs (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). Five studies were double‐blinded (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and one study followed an open‐label design (Righetti 2003).
Sample sizes
Sample sizes of included studies ranged from 88 participants (Righetti 2003) to 761 participants (HOST Study 2004).
Setting
The included studies were conducted in outpatient dialysis units (ASFAST 2004; Heinz 2009; HOST Study 2004; Righetti 2003; Vianna 2007; Wrone 2004). Two were single centre studies (Righetti 2003; Vianna 2007) and four were multicentre studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Wrone 2004). Studies were conducted in the USA (HOST Study 2004; Wrone 2004), Australia and New Zealand (ASFAST 2004), Germany (Heinz 2009), Brazil (Vianna 2007) and Italy (Righetti 2003). Mandatory folate fortification was present only in the USA at the time the studies were conducted. Three studies included ESKD patients on maintenance haemodialysis (Heinz 2009; Righetti 2003; Vianna 2007) only. Three studies included ESKD patients on both maintenance haemodialysis and peritoneal dialysis (ASFAST 2004; HOST Study 2004; Wrone 2004). Proportions of peritoneal dialysis patients were 28% (ASFAST 2004), 8.2% (Wrone 2004) or not reported (HOST Study 2004).
Participants
The mean age of study participants was between 48 and 65 years (Characteristics of included studies). The proportion of study participants who were male ranged from 50% to 98%, the proportion with a diagnosis of diabetes mellitus ranged from 19% to 55%, and the proportion with a history of cardiac disease ranged from 11% to 58%. Follow‐up ranged from 2.0 to 3.6 years. Participants consisted entirely of people with ESKD in four studies (Heinz 2009; Righetti 2003; Vianna 2007; Wrone 2004) and both ESKD and CKD in two studies (ASFAST 2004; HOST Study 2004). Specific outcome data on ESKD patients were obtained from the study authors of ASFAST 2004.
Interventions
Interventions investigated were folic acid with or without other vitamins (vitamin B6, vitamin B12). The dose of folic acid was variable from the equivalent daily dose of 5 mg to 40 mg. Wrone 2004 compared placebo versus low dose (5 mg) versus high dose (15 mg) folate therapy with the latter two arms combined for the purposes of this review. Comparator treatment was placebo (ASFAST 2004; HOST Study 2004; Vianna 2007), low dose folic acid (Heinz 2009; Wrone 2004) or usual care (Righetti 2003).
Outcomes
For ASFAST 2004, the primary outcomes were change in rate of progression of mean maximum carotid artery intimal media thickness, composite of MI, stroke, and death from cardiovascular cause. Secondary outcomes were all fatal and nonfatal cardiovascular events including MI, stroke, unstable angina, revascularisation, and peripheral vascular disease.
The primary outcome in Heinz 2009 was overall mortality. Secondary outcomes were occurrence of first fatal or nonfatal cardiovascular event (MI, unstable angina, coronary vascularisation procedure, sudden cardiac death, stroke, peripheral artery disease, PE, and thromboses). Shunt thromboses were not regarded as an end point.
The primary outcome in HOST Study 2004 was time to death from any cause. Secondary outcomes were time to MI, stroke, amputation of all or part of a lower extremity, and a composite of these three plus all‐cause mortality.
For Righetti 2003, the primary outcome was a composite cardiovascular end point (typical history of angina with abnormal myocardial scintigraphy or coronarography, fatal and nonfatal MI, symptomatic extracranial carotid stenosis resulting in carotid endarterectomy, fatal and nonfatal stroke and sudden cardiac arrest). There were no secondary outcomes.
The primary outcome in Vianna 2007 was a composite of new major cardiovascular events, including death from cardiovascular causes, nonfatal MI, cardiac arrhythmias, angina, heart failure, and cerebral vascular accident. Secondary outcome was the evaluation of the intima‐media thickness of the common carotid arteries.
For Wrone 2004, the primary outcomes were cardiovascular events (coronary artery intervention, MI, stroke, transient ischaemic attack, carotid endarterectomy, limb amputation) and mortality. The secondary outcome was vascular access thrombosis (among those with AV fistulae).
Excluded studies
Excluded studies are described (Characteristics of excluded studies). Reasons for exclusion were: wrong study design (4 studies); less than 100 patient‐years follow‐up (70 studies); wrong study population (3 studies); not homocysteine‐lowering interventions (19 studies); outcome data were not extractable (7 studies).
Risk of bias in included studies
A risk of bias graph and summary are presented in Figure 2 and Figure 3. Risk of selection bias related to random sequence generation and allocation concealment was low in 50% of the included studies. Risks attributed to performance, attrition, detection, and reporting biases were low in more than 65% of included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Method of sequence generation was assessed as being at low risk of bias in three studies (ASFAST 2004; HOST Study 2004; Righetti 2003) and unclear in three studies (Heinz 2009; Vianna 2007; Wrone 2004).
Allocation concealment was assessed as being at low risk of bias in three studies (ASFAST 2004; Righetti 2003; Wrone 2004) and unclear in three studies (Heinz 2009; HOST Study 2004; Vianna 2007).
Blinding
Performance bias (participants and study staff) was assessed as low in five studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and high in one study (Righetti 2003).
Detection bias (outcome assessors) was assessed as low in four studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007) and unclear in two studies (Righetti 2003; Wrone 2004).
Incomplete outcome data
Attrition bias was assessed as low in all six studies.
Selective reporting
Reporting bias was assessed as low in five studies (ASFAST 2004; Heinz 2009; HOST Study 2004; Vianna 2007; Wrone 2004) and unclear in one study (Righetti 2003).
Other potential sources of bias
Righetti 2003 was assessed as being at high risk of other bias due to the imbalance between the groups in the baseline data; all other studies were assessed as being at low risk of other potential biases.
Funnel plot analysis did not show publication bias (Figure 4).

Funnel plot of comparison: 2 Secondary outcomes, outcome: 2.1 all‐cause mortality
Effects of interventions
Primary outcomes
Cardiovascular mortality
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Vianna 2007) reported 173 cardiovascular mortality events among 1186 participants. Homocysteine‐lowering therapy had no overall effect on cardiovascular mortality (Analysis 1.1 (4 studies, 1186 participants): RR 0.93, 95% CI 0.70 to 1.22; I2 = 0%). There was no evidence of heterogeneity.
We planned to calculate the number of persons needed to treat to avoid one cardiovascular death if adequate data were available; however, our non‐significant results did not enable this calculation to be made.
Secondary outcomes
All‐cause mortality
The six included studies reported 719 all‐cause mortality events among 2447 participants. Homocysteine‐lowering therapy had no overall effect on all‐cause mortality (Analysis 2.1 (6 studies, 247 participants): RR 1.00, 95% CI 0.89 to 1.11; I2 = 0%). There was no evidence of heterogeneity.
Fatal and nonfatal myocardial infarction
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Wrone 2004) reported 75 fatal and nonfatal MI events among 1510 participants. Homocysteine‐lowering therapy had no overall effect on MI (Analysis 2.2 (4 studies; 1510 participants): RR 1.04, 95% CI 0.66 to 1.62; I2 = 0%). There was no evidence of heterogeneity.
Coronary revascularisation
Heinz 2009 and Wrone 2004 reported 44 coronary revascularisation events among 1160 participants. Homocysteine‐lowering therapy had no overall effect on coronary revascularisation (Analysis 2.3 (2 studies, 1160 participants): RR 0.83, 95% CI 0.22 to 3.14; I2 = 74%). Significant heterogeneity was observed.
Stroke
Four studies (ASFAST 2004; Heinz 2009; Righetti 2003; Wrone 2004) reported 77 stroke events among 1510 participants. Homocysteine‐lowering therapy had no overall effect on stroke (Analysis 2.4 (4 studies, 1510 participants): RR 0.89, 95% CI 0.57 to 1.40; I2 = 0%)). There was no evidence of heterogeneity.
Cerebrovascular revascularisation
Adequate data on this outcome were not available in published reports and further information was not forthcoming from the triallists.
Lower limb amputation
Adequate data on this outcome were not available in published reports and further information was not forthcoming from the triallists.
Deep vein thrombosis and pulmonary embolism
Heinz 2009 reported 13 DVT and PE events among 650 participants. Homocysteine‐lowering therapy had no overall effect on DVT and PE (Analysis 2.5: RR 1.15, 95% CI 0.39 to 3.39).
Thrombosis of dialysis access
HOST Study 2004 and Wrone 2004 reported 533 dialysis access thrombosis events among 1261 participants. Homocysteine‐lowering therapy had no overall effect dialysis access thrombosis (Analysis 2.6 (2 studies, 1261 participants): RR 1.00, 95% CI 0.88 to 1.14; I2 = 0%). There was no evidence of heterogeneity.
Adverse events from folic acid‐based therapy
Adverse event rates were reported in three studies (Heinz 2009; Righetti 2003; Wrone 2004, 1248 participants) where homocysteine‐lowering therapy had no effect on adverse event rates (Analysis 2.7 (3 studies, 1248 participants): (RR 1.12, 95% CI 0.51 to 2.47; I2 = 0%). Reported adverse events included nausea, abdominal discomfort, hunger and weight gain, skin rashes, headaches, fatigue, and paraesthesia. We were unable to complete planned separate analyses of gastrointestinal, dermatological, neurological and malignant events due to the small number of studies reporting these outcomes. Rate of withdrawal from study treatment due to adverse events was less than 1% among the included studies.
Subgroup analyses
Subgroup analyses were conducted to explore possible sources of heterogeneity according to the following characteristics.
Study intervention
The impact of intervention on cardiovascular events did not differ significantly (P = 0.649) when studies of folic acid alone (ASFAST 2004; Vianna 2007) were compared with studies of folic acid plus B group vitamins (Heinz 2009; Righetti 2003).
Exposure to folic acid among control group participants
The impact of intervention on cardiovascular events did not differ significantly (P = 0.557) when studies of low dose exposure in control group participants (Heinz 2009) were compared with studies of no low dose exposure in control group participants (ASFAST 2004; Righetti 2003; Vianna 2007).
Proportion of participants with diabetes mellitus
The impact of intervention on cardiovascular events did not differ significantly (P = 0.557) when studies that included more than a third of participants with diabetes mellitus (Heinz 2009) were compared with studies that included fewer than a third of participants with diabetes mellitus (ASFAST 2004; Righetti 2003; Vianna 2007).
Proportion of participants with cardiac disease
The impact of intervention on cardiovascular events did not differ significantly (P = 1.000) when studies that included more than a third of participants with cardiac disease (Heinz 2009; Righetti 2003) were compared with studies that included fewer than a third of participants with cardiac disease (ASFAST 2004; Vianna 2007).
Study follow‐up duration
The impact of intervention on cardiovascular events did not differ significantly (P = 0.649) when studies with more than 30 month follow‐up (ASFAST 2004) were compared with those that had with less than 30 months follow‐up (Heinz 2009; Vianna 2007; Righetti 2003).
Study event number
The impact of interventions on cardiovascular events did not differ significantly (P = 0.557) when studies with more than 150 events (Heinz 2009) were compared with those that had fewer than 150 events (ASFAST 2004; Vianna 2007; Righetti 2003).
We aimed to analyse the ability of interventions to normalise homocysteine level in a subgroup of studies that enrolled patients with elevated homocysteine levels. However, there was only one study (Vianna 2007) in which a significant proportion of patients in the active arm achieved normalisation of homocysteine levels. In this study, there was no difference in cardiovascular mortality between active and control arm participants.
Sensitivity analyses
The same results were observed with fixed‐effects and random‐effects models.
Plausible explanations for variations in treatment effect for primary outcome were explored using subgroup analyses based on study quality and length of follow‐up.
The impact of intervention on cardiovascular mortality did not differ significantly (P = 0.649 for heterogeneity) when studies with and without blinding of participants and study staff (ASFAST 2004; Heinz 2009; Vianna 2007) were compared.
The impact of interventions on cardiovascular mortality did not differ significantly (P = 0.657) when studies with median follow‐up over three years (ASFAST 2004) were compared with median follow‐up of fewer than three years.
Discussion
Summary of main results
In this systematic review including 2452 participants with ESKD, randomisation to folic acid‐based homocysteine‐lowering therapy did not affect cardiovascular mortality, all‐cause mortality, and incidence of cardiovascular events including MI, stroke, venous or access thrombosis. Null results were consistently observed across all prespecified subgroups and sensitivity analyses. Reported adverse events from these therapies were mild and rates of adverse events were variable across the studies. Overall, there was no evidence of harm defined by rates of adverse events in this population.
Overall completeness and applicability of evidence
Guidelines for ESKD patients routinely suggest supplementation with folic acid and B group vitamins (homocysteine‐lowering therapies) without lack of clear evidence (K/DOQI 2005), and many people with ESKD appear to be receiving folic acid supplementation (Andreucci 2004). Our findings suggest that folic acid‐based homocysteine‐lowering should not be used for cardiovascular prevention in people with kidney disease, a population in whom medication burden is often high.
Quality of the evidence
The strengths of our systematic review we that an important clinical question has been investigated focusing on a study population with ESKD, using rigorous methodology and study search strategy techniques. We found consistent null results in primary, secondary, subgroup and sensitivity analyses.
These findings have direct implications for millions of people with ESKD globally who are currently taking homocysteine‐lowering medications.
Risk of selection bias related to random sequence generation and allocation concealment was low in 50% of the included studies. Risks attributed to performance, attrition, detection, and reporting biases were low in more than 65% of included studies.
Potential biases in the review process
Limitations of our systematic review include reliance on tabular rather than individual patient level data. We focused on cardiovascular mortality as our primary outcome; however, this outcome was reported in only four of the six included studies. Competing risks for cardiovascular mortality were not analysed. Although funnel plot analysis suggested a low possibility of publication bias, this cannot be excluded based on the small number of included studies. We could not conduct separate analyses for peritoneal and haemodialysis patients since adequate data were not available. Overall, participants from the included studies were younger than the average ESKD population and this may limit generalisability.
Data on genetic polymorphisms that determine homocysteine‐lowering effects of therapies (Klerk 2002) were not available; however, these polymorphisms have been shown to have no effects among dialysis patients (Aucella 2005). Nevertheless, although lowering of homocysteine levels were noted in all studies, normalisation of homocysteine levels was rare, pointing to the possibility of folic acid resistance in ESKD patients (Robinson 1996) and possible need for higher doses. However, in studies where high dose (> 5 mg daily) folic acid was administered, similar null effects on outcomes were noted (ASFAST 2004; HOST Study 2004; Wrone 2004).
Recent data indicate that there may be non‐cardiovascular benefits of homocysteine lowering in the general population (such as reduced fracture risk) (Yang 2012) and our review did not evaluate effects on such outcomes.
Agreements and disagreements with other studies or reviews
Our findings were consistent with a recent meta‐analysis conducted in the general population for cardiovascular mortality, all‐cause mortality and MI (Huang 2012); however, our findings were discordant in terms of effects on stroke risk reduction.
Huang 2012 pooled data from 19 studies that included 47,921 participants and observed approximately 12% reduction in stroke risk in the general population. Although the analyses by Huang 2012 suffer from heterogeneity of patient populations in the pooled studies, other possible reasons for differences in stroke risk reduction between general and ESKD populations may relate to differences in biological risk factors for cerebrovascular disease in dialysis versus general populations (Kanbay 2010).
VITATOPS 2010 was conducted in patients who had experienced a recent stroke or transient ischaemic attack and found no significant difference for cardiovascular events (RR 0.91, 95% CI 0.82 to 1.00, P = 0.05) or stroke (RR 0.92, 95% CI 0.81 to 1.06).
There have been two recent meta‐analyses that evaluated the effects of homocysteine lowering therapies on cardiovascular events in patients with kidney disease. Jardine 2012 included 11 studies comprising 4389 patients with CKD, 2452 with ESKD, and 4110 with functioning kidney transplants. In Jardine 2012, folic acid‐based therapy did not reduce cardiovascular event incidence (RR 0.97, 95% CI 0.92 to 1.03, P = 0.326). Pan 2012 pooled 10 studies involving 4836 participants with CKD or ESKD. The investigators noted that the estimated relative risks were not significantly different for any cardiovascular outcomes and all‐cause mortality.
Our findings were consistent with these findings. In addition, we specifically derived outcome data on ESKD patients from studies that involved both CKD and ESKD patients (ASFAST 2004; HOST Study 2004). Considering these differences, we believe that the findings of our review are likely to be a better estimate of the true effect of folic acid‐based homocysteine‐lowering on cardiovascular outcomes in ESKD patients. In combination with results from Jardine 2012 and Pan 2012 we feel confident regarding our conclusions on null effect from homocysteine‐lowering therapies in ESKD patients and further solidify futility of such treatments as discussed in a recent editorial (Haynes 2012).
Recent data regarding associations between hyperhomocysteinaemia and cardiovascular risk (Menon 2006; Suliman 2007) have questioned the earlier findings that linked hyperhomocysteinaemia with increased cardiovascular risk (Robinson 1996) and thus further strengthen our conclusions. In fact, hyperhomocysteinaemia in ESKD may be an illustration of reverse epidemiology with some authors finding elevated homocysteine levels associated with reduced cardiovascular risk (Kalantar‐Zadeh 2004) as has been described for factors such as obesity (Kalantar‐Zadeh 2005) in the population with ESKD. Regardless of the nature of the association reported by observational studies, our results clearly indicate that interventions to lower homocysteine levels in people with ESKD do not offer cardiovascular risk reduction and are likely to be futile. We found one study listed at clinicaltrials.gov with unknown recruitment status on this topic (NCT00004495); however, the enrolment goal for this study is listed as 84 and it is unlikely to significantly affect results of our analyses.

Flow diagram showing study selection

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 2 Secondary outcomes, outcome: 2.1 all‐cause mortality

Comparison 1 Primary outcome, Outcome 1 Cardiovascular mortality.

Comparison 2 Secondary outcomes, Outcome 1 All‐cause mortality.
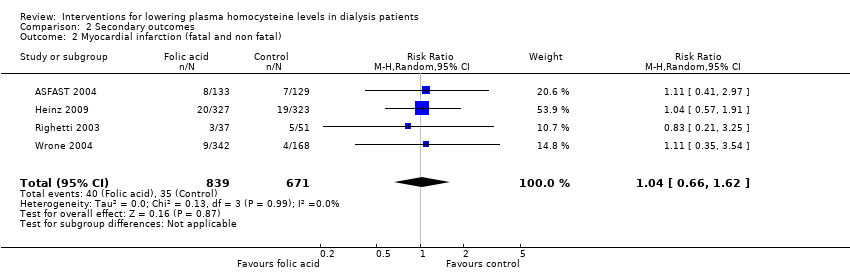
Comparison 2 Secondary outcomes, Outcome 2 Myocardial infarction (fatal and non fatal).
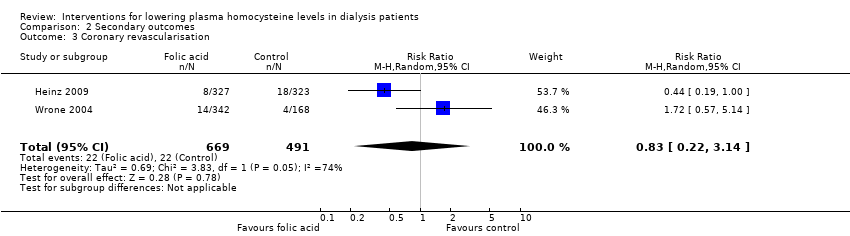
Comparison 2 Secondary outcomes, Outcome 3 Coronary revascularisation.

Comparison 2 Secondary outcomes, Outcome 4 Stroke.

Comparison 2 Secondary outcomes, Outcome 5 Deep venous thrombosis and pulmonary embolism.

Comparison 2 Secondary outcomes, Outcome 6 Thrombosis of dialysis access.
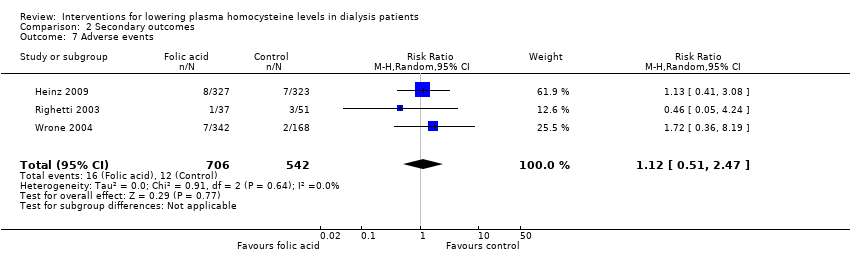
Comparison 2 Secondary outcomes, Outcome 7 Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiovascular mortality Show forest plot | 4 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 6 | 2447 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.11] |
| 2 Myocardial infarction (fatal and non fatal) Show forest plot | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.66, 1.62] |
| 3 Coronary revascularisation Show forest plot | 2 | 1160 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.22, 3.14] |
| 4 Stroke Show forest plot | 4 | 1510 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.40] |
| 5 Deep venous thrombosis and pulmonary embolism Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Thrombosis of dialysis access Show forest plot | 2 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.14] |
| 7 Adverse events Show forest plot | 3 | 1248 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.51, 2.47] |

