Infusión continua versus intermitente para la prevención de la pérdida de función de los catéteres intravenosos periféricos utilizados para la administración de fármacos en recién nacidos
Resumen
Antecedentes
El uso de cánulas intravenosas periféricas es frecuente en los recién nacidos. Muchos requieren un catéter intravenoso solamente para fármacos y no para líquidos. Actualmente hay poca uniformidad acerca de los métodos utilizados para mantener la permeabilidad de la cánula.
Objetivos
El objetivo de esta revisión fue determinar el mejor método para mantener los catéteres intravenosos utilizados en recién nacidos para medicaciones intravenosas solamente: infusión intermitente o continua
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, The Cochrane Library, número 2, 2004), CINAHL (desde 1982 hasta junio de 2004) y en MEDLINE (desde 1966 hasta junio de 2004) .
Criterios de selección
Ensayos controlados aleatorizados que compararon la infusión continua con la intermitente para mantener la permeabilidad de las cánulas intravenosas. Las unidades de asignación al azar podían incluir catéteres o lactantes individuales.
Obtención y análisis de los datos
Tres revisores evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos.
Resultados principales
Dos estudios fueron elegibles para inclusión. Solamente en un estudio estuvo disponible una de las medidas de resultado principales: la duración de la permeabilidad para la primera cánula utilizada en el recién nacido fue apenas mayor que la del grupo de infusión continua, pero no significativamente, con una diferencia de medias de ‐4,3 horas (IC del 95%: ‐18,2 a 9,7).
En el segundo estudio, solo estuvo disponible uno de los resultados principales: la media (DE) del número de cánulas utilizadas por recién nacido en las primeras 48 horas fue menor en el grupo de infusión intermitente, con una diferencia de medias de ‐0,76 cánulas (IC del 95%: ‐1,37 a ‐0,15). No hubo resultados disponibles para cualquiera de los otros resultados principales: en el artículo publicado, los resultados se informaron por catéter en lugar de por recién nacido, algunos de los lactantes recibieron más de un catéter intravenoso (39 recién nacidos recibieron un número desconocido de catéteres). La duración general de la permeabilidad de la cánula fue significativamente mayor en el grupo de infusión intermitente, con una duración media de la permeabilidad en el grupo de infusión intermitente de 2,1 días (DE: 1,0) en comparación con el grupo de infusión continua, donde la duración media de la permeabilidad fue 1,0 días (DE: 0,5) ‐ valor de p: 0,0003 de la prueba t de Student.
Conclusiones de los autores
Es difícil establecer conclusiones fiables debido a la manera en que se analizaron e informaron los datos en los dos estudios incluidos. La fiabilidad de los resultados es incierta. Sin embargo, además de la precaución al interpretar estos datos, también se debe señalar que el uso de infusiones intermitentes no se asoció en estos estudios a una menor vida útil de la cánula o a otras desventajas, lo que proporcionó cierto apoyo al uso de la infusión intermitente de las cánulas en una población seleccionada en las unidades neonatales.
PICOs
Resumen en términos sencillos
Infusión continua versus intermitente para la prevención de la pérdida de función de los catéteres intravenosos periféricos utilizados para la administración de fármacos en recién nacidos
Todavía se desconoce la mejor manera de mantener el funcionamiento del catéter intravenoso de un recién nacido.
Se intentó determinar cuál era la mejor manera para mantener el catéter intravenoso de un recién nacido abierto y en funcionamiento, si era al permitir el fluido de una cantidad continua de líquido intravenoso a través del mismo (infusión continua) o al introducir una cantidad pequeña de líquido a través del mismo cada pocas horas (infusión intermitente) solamente. Un estudio no mostró diferencias entre los dos enfoques para mantener abierto y en funcionamiento el catéter intravenoso de un recién nacido, y un estudio mostró una ventaja para la infusión intermitente. Sin embargo, los estudios presentaron algunos problemas en la forma en la que se analizaron y publicaron los datos. Por consiguiente, no se tiene completa seguridad sobre cuán fiables son los resultados y se deben realizar estudios de investigación adicionales.
Authors' conclusions
Background
Peripheral intravenous cannula use is common in newborn babies admitted to neonatal nurseries (Ward 1993). A significant number of these babies only require intermittent intravenous drug therapy and do not require supplemental fluids or other continuous drug infusions. Intravenous cannulation and therapy may require the baby to be separated from its mother, at least initially, and can delay the establishment of maternal infant bonding (Malcolm 2000; WHO 1998).
Insertion of peripheral intravenous cannulas in neonates can be a stressful experience for the baby, the new parent and the medical/nursing staff (Yeo 1998; Olds 2000; Cotton 1998). It is therefore desirable once the intravenous cannula is inserted that its patency be maintained for as long as possible. Each cannula should last as long as possible and for any given period of treatment a minimum number of cannulas should be used. To achieve this the cannula can be infused continuously with fluid at a low rate or flushed intermittently (usually every 4‐8 hours) (Cotton 1998).
Continuous infusions require more nursing time and equipment and impede access of the mother to the infant but the cannula might last longer and have fewer complications such as extravasation or dislodgment. Intermittent flushing would decrease nursing time and equipment and allow greater access of the mother to her infant, but may decrease cannula life by blockage due to clotting. Currently there is little uniformity between neonatal nurseries as to which method is used and evidence is required to decide which method, if any, best maintains intravenous access.
There is limited evidence that the following factors may increase the risk of intravenous cannula failure: certain drugs (such as gentamicin or aminophylline) (Moclair 1995); size of cannula (Danek 1992).
A different question as to whether the continuous infusion fluid or the intermittent flush solution should contain heparin has been considered in a separate Cochrane review (Shah 2004). Shah et al concluded that "no conclusive evidence is available...to evaluate the effectiveness of heparin to prolong PIV [peripheral intravenous] cannula life in the neonatal population". Only eight eligible studies were identified and only five commented on duration of catheter use. Due to significant clinical heterogeneity and heterogeneity in treatment effect no recommendation could be made as to whether use of heparin was advisable or not without further research.
As with all invasive procedures, there is a significant risk of introducing infection into the newborn when inserting a peripheral intravenous catheter. Septicaemia, although an infrequent complication could potentially have a lethal outcome. It is therefore desirable that catheter patency be maintained as long as possible thus reducing the number of skin breakages made in the baby and ultimately decreasing the risk of infection.
Objectives
Primary Objectives
-
To determine whether continuous infusion or intermittent flushing is better with regard to maintaining patency of intravenous cannulas.
Secondary Objectives
-
To determine which method resulted in fewer complications (infection, dislodgment, extravasation, phlebitis).
-
To determine which method has the greater cost.
-
To determine which method is better for reducing the time to initiate and attain full suck feeding.
We also aim to determine if the outcomes above were different by:
-
Which drug is being administered (antibiotics versus non antibiotics, aminoglycosides versus non aminoglycosides)
-
The size of the cannula used for intravenous therapy (24G or smaller versus 22G or bigger)
Methods
Criteria for considering studies for this review
Types of studies
Only randomised controlled trials of adequate quality in which continuous infusion is compared to intermittent flushing to maintain patency of intravenous cannulas in neonates will be included. Quasi randomised studies will not be used. Units of randomisation might include individual catheters or individual babies.
Types of participants
Newborn infants receiving intermittent intravenous drug treatment but not for continuous drug infusions or supplemental fluids.
Newborn infants are: term infants < 29 days old, preterm infants up to a corrected age of 44 weeks, or infants cared for in a neonatal unit.
Types of interventions
Continuous infusion of peripheral intravenous catheters with dextrose and/or saline solutions, with or without heparin.
Intermittent flushes of peripheral intravenous catheters with saline or heparinised saline given at intervals greater than every second hour.
Studies which allow the use of peripheral intravenous catheters for blood sampling will not be included.
Types of outcome measures
Primary Outcomes
-
Duration of cannula patency (hours) for the first cannula used per infant
-
Number of cannulas used during primary treatment course in individual infants
-
Number of cannulas used during first 48 hours in individual infants
Secondary Outcomes
-
Duration of cannula patency (hours) in individual infants (for all cannulas as averaged during the treatment course or during the first 24, 48, 72 hours of treatment )
-
Proportion of infants with loss of cannula function
‐ blockage
‐ extravasation
‐ phlebitis
‐ dislodgment
‐ infection
-
Cost (dollars)
-
Mortality (at discharge, 28 days or one year)
-
Proportion of infants with delayed suck feeding (defined as either i. has not initiated suck feeding within 12 hours of birth, ii. requires supplemental nasogastric tube feeding)
-
Proportion of infants not breast feeding (at discharge, 28 days, three months or six months)
-
Infections (positive blood cultures or as defined in individual studies)
-
Maternal‐infant bonding (however defined in individual studies)
Search methods for identification of studies
The standard search strategy for the Cochrane Neonatal Review Group was used. See: Neonatal Review Group search strategy. We searched the following electronic databases: The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2004), CINAHL (from 1982 ‐ June 2004 ) and MEDLINE (from 1966 ‐ June 2004).
Searches of the electronic databases were based on the following search terms:
MeSH terms: infusions, intravenous OR injections, intravenous;
OR text words: "IV treatment" OR "IV therapy" OR "IV drug" OR "IV medication" OR "intravenous treatment" OR "intravenous therapy" OR "intravenous drug" OR "intravenous medication" OR "intra‐venous treatment" OR "intra‐venous therapy" OR "intra‐venous drug" OR "intra‐venous medication";
NOT MeSH term: Substance Abuse, Intravenous.
AND
MeSH term: infant, newborn OR text word "neonate"
AND
The highly sensitive search strategy developed by Kay Dickersin to identify RCTs (Dickersin 1994)
We also searched previous reviews including cross references. We also used the results of searches of abstracts, conference and symposia proceedings as done by the Cochrane Neonatal Review Group. No language restrictions were applied. Published or un‐published data were considered.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials: standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. At least two of the reviewers worked independently to search for and assess trials for inclusion and methodological quality. Studies were assessed using the following key criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow up and blinding of outcome measurement. The reviewers extracted data independently. Differences were resolved by discussion. An attempt was made to contact study investigators for additional information or data.
Data analysis:
For individual trials: for continuous variables such as duration of cannula patency, mean differences, and 95% confidence intervals were to be reported. For categorical outcomes such as mortality, the relative risks (RR) and 95% confidence intervals will be reported.
For pooled results: for continuous variables, weighted mean differences (WMD) and 95% confidence intervals will be reported. For categorical outcomes, the relative risks (RR) and 95% confidence intervals will be reported. For significant findings, the risk difference (RD) and number needed to treat (NNT) will also to be reported. Each treatment effect will be tested for heterogeneity to help determine suitability for pooling of results in a meta‐analysis. The fixed effects model will be used for meta‐analysis.
Results
Description of studies
Two studies were identified for inclusion in this review (Kalyn 2000; Taylor 1989). Methods and clinical details including participants, interventions and outcomes are given in Table 1.
Included studies
Kalyn et al (Kalyn 2000) performed a multi‐centre randomised controlled trial. Infants were randomised either to a continuous infusion of 0.5‐1 mL/hr of 10% Dextrose or intermittent flushes with 0.5‐1 mL every 6 hrs of 0.9% non heparinised saline. The infants were randomly assigned to their groups using a computer generated random number. Blinding of the interventions was obviously not done. Infants receiving intermittent drug therapies via a peripheral intravenous catheter were eligible for inclusion in the study but infants receiving continuous drug infusions or supplemental fluids were not. A total of 95 neonates (42 to intermittent flush group and 53 to continuous infusion group) were cannulated with 238 catheters (84 to intermittent flush group and 154 to the continuous infusion group) during the study period. The primary diagnosis for all the infants was sepsis. Follow‐up continued until the intravenous catheter was either removed because intravenous medication was stopped or the catheter was no longer working; the infant was transferred elsewhere; or the infant required maintenance intravenous fluids. The two groups were all well matched for demographic and clinical variables, including birth weight, gestational age, the site and size of the catheters and types of drugs being administered. The duration of patency of each catheter was recorded as well as the reasons for loss of patency: phlebitis, occlusion, leaking and infiltration. The outcomes were reported per catheter used.
Taylor et al (Taylor 1989) did a randomised controlled trial in infants who were admitted to the 'intermediate care' nursery who either:
1. required intravenous medications but no additional intravenous fluids, or
2. had an umbilical arterial catheter in situ and required an intravenous cannula for medications.
The infants were randomised either to a continuous infusion group where the intravenous line was kept patent by a continuous infusion of 10% dextrose (without heparin) at a rate of 1.5 to 3.0 ml/hr; or intermittent flushes where the intravenous cannula was kept patent with a heparin lock (0.5 ml of heparinised saline) given every six hours or after injection of medications. The method of randomisation was not stated. Blinding of the interventions was obviously not done. One infant in the intermittent flush group was excluded post randomisation because the "... catheter was needed only briefly, not allowing for time for data collection". Data were collected on a total of 39 neonates (22 to intermittent flush group and 17 to continuous infusion group) who were cannulated with an unknown number of catheters. The infants were enrolled in the study until they no longer required intravenous access. The two groups were all well matched for demographic and clinical variables, including birth weight and gestational age. Outcome measures included the number of days the infant was enrolled in the study; quantitation of parental medications, blood products and intravenous fluids; number of line infiltrations; duration of cannula patency (this seemed to have been measured for each cannula regardless of the number used in each infant); and the number of times the infant was removed from the incubator to be held by a nurse or parent.
Excluded Studies
A number of studies were found which examined the duration of peripheral intravenous catheter patency with or without heparin intermittent flushes (Danek 1992; Hanrahan 2000; LeDuc 1997; McMullen 1993; Nelson 1998). However, none compared intermittent flushes with continuous infusions and were therefore not included in the review. This is the subject of another review by Shah et al (Shah 2004).
Risk of bias in included studies
Kalyn's study (Kalyn 2000): the randomisation method was by a computer generated random number to assign infants by alternate sequential series; allocation was performed in a blind manner by using sealed opaque envelopes to assign each infant to either group; blinding of intervention was not done; follow‐up was complete and outcome assessments were not blinded.
Taylor's study (Taylor 1989): the randomisation method was not stated; allocation was performed in a blind manner by using sealed opaque envelopes to assign each infant to either group; blinding of intervention was not done; follow‐up was complete and outcome assessments were not blinded.
Effects of interventions
Two studies (Kalyn 2000; Taylor 1989) were eligible for inclusion.
Kalyn 2000
The lead author of Kalyn et al (Kalyn 2000) was contacted for further information regarding this study: additional data were available on the duration of patency for the first catheter used per infant.
Primary outcomes
-
Duration of cannula patency for the first cannula used per infant was slightly longer in the continuous infusion group, but not significantly so, with a mean difference of ‐4.3 hours (95% CI ‐18.2 to 9.7). These unpublished data were provided by the lead author of the study (Kalyn 2000).
-
Number of cannulas used during primary treatment course in individual infants ‐ individual outcome not available
-
Number of cannulas used during the first 48 hrs in individual infants ‐ individual outcome not available
Secondary outcomes
-
Duration of cannula patency in individual infants for all catheters as averaged during the treatment course:
In the published report (Kalyn 2000) results were reported per catheter rather than per infant, a number of infants received more than one intravenous catheter (95 infants received 238 catheters in total). No statistically significant difference was found between the two groups for duration of cannula patency. The mean duration of patency in the intermittent flush group was 45.2 hours (SD 29.3) and in the continuous infusion group 48.7 hours (SD 41.1) ‐ Mann‐Whitney U test P value 0.13. However, whilst the mean duration of cannula patency in each group was similar, there were 84 cannulas used in the intermittent flush group and 154 cannulas used in the continuous infusion group. It is difficult to reconcile that mean duration of cannula use was not different; yet almost twice as many cannulas were used in the continuous infusion group. The average total duration of cannula use for infants in the continuous infusion group must have been longer (although data for this are not available). And therefore any differences between the two groups is prone to significant bias.
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 24 hours of treatment ‐ the study did not report on this outcome
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 48 hours of treatment ‐ the study did not report on this outcome
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 72 hours of treatment ‐ the study did not report on this outcome
-
Proportion of infants with loss of cannula function due to:
‐blockage ‐ individual outcome not available
‐extravasation ‐ individual outcome not available
‐phlebitis ‐ individual outcome not available
‐dislodgment ‐ individual outcome not available
‐infection ‐ individual outcome not available
A significant difference (Chi squared test P < 0.001) was found when the two catheter groups were compared with respect to the removal or loss of patency of catheters. The results were presented by catheter rather than by infant although it was stated that results were also analysed by infant and similar results were obtained. The catheters in the intermittent flush group were less likely to infiltrate, leak or cause phlebitis (35.7%) than in the continuous infusion group. The continuous infusion group, however, were less likely to occlude (9.1%) than the intermittent flush group (25%). In the intermittent flush group 39% of catheters (compared with 24% in the continuous infusion group) did not have infiltration/phlebitis/leaking/occlusion because the catheter was either removed because intravenous medication was stopped, the infant was transferred elsewhere or the infant required maintenance intravenous fluids.
-
Cost (dollars) ‐ individual outcome not available
-
Mortality ‐ individual outcome not available
-
Proportion of infants with delayed suck feeding ‐ individual outcome not available
-
Infections ‐ individual outcome not available
-
Maternal ‐ infant bonding‐individual outcome not available
Taylor 1989
The lead author of Taylor et al (Taylor 1989) was contacted for further information regarding this study: additional data were available on post‐randomisation exclusions, the number of cannulas used during the first 48 hours in individual infants, and loss of cannula function.
Primary outcomes
-
Duration of cannula patency for the first cannula ‐ individual outcome not available
-
Number of cannulas used during primary treatment course in individual infants ‐ individual outcome not available
-
Number of cannulas used during the first 48 hrs in individual infants:
The mean (SD) number cannulas used per infant in the first 48 hours was 1.59 (0.59) in the intermittent flush group and 2.35 (1.17) in the continuous infusion group. The mean difference was ‐0.76 cannulas (95% CI ‐1.37 to ‐0.15).
Secondary outcomes
-
Duration of cannula patency in individual infants for all catheters as averaged during the treatment course:
In the published report (Taylor 1989) results were reported per catheter rather than per infant, a number of infants received more than one intravenous catheter (39 infants received an unknown number of catheters). The duration of cannula patency was significantly longer in the intermittent flush group. The mean duration of patency in the intermittent flush group was 2.1 days (SD 1.0) and in the continuous infusion group 1.0 days (SD 0.5) ‐ Student's t test P value 0.0003.
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 24 hours of treatment ‐ the study did not report on this outcome
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 48 hours of treatment ‐ the study did not report on this outcome
-
Duration of cannula patency (hours) in individual infants for all cannulas as averaged during the first 72 hours of treatment ‐ the study did not report on this outcome
-
Proportion of infants with loss of cannula function due to:
‐blockage ‐ outcome not available
‐extravasation ‐ outcome not available except for first 48 hours (see below)
‐phlebitis ‐ nil noted in either group
‐dislodgment ‐ outcome not available except for first 48 hours (see below)
‐infection ‐ nil noted in either group
The authors reported extravasation (i.e., subcutaneous infiltration) as the number of infiltrations per day. There was a mean (SD) of 0.7 (0.6) infiltrations per day in the continuous infusion group and 0.3 (0.2) in the intermittent flush group (Student's t test P value 0.0015).
-
Cost (dollars) ‐ individual outcome not available
-
Mortality ‐ individual outcome not available
-
Proportion of infants with delayed suck feeding ‐ individual outcome not available
-
Infections ‐ individual outcome not available
-
Maternal ‐ infant bonding‐individual outcome not available
The study also reported the following outcomes:
-
volume of fluid infused through the cannula ‐ mean (SD) of 28.7 (11) ml/kg/day in the continuous infusion group and 7.7 (6.8) ml/kg/day in the intermittent flush group (Student's t test P <0.0001);
-
number of times the infant was removed from the incubator by parent or nurse ‐ mean (SD) of 1.9 (1.3) times per day in the continuous infusion group and 1.2 (1.5) times per day in the intermittent flush group (Student's t test P value 0.2);
-
a score consisting of a "subjective evaluation regarding ease of handling and maintaining intravenous patency" (a scale of 1 to 5 with 1 having maximum ease of handling and maintaining intravenous patency and 5 having maximum problems with ease of handling and maintaining intravenous patency) ‐ mean (SD) score of 3.8 (1.1) in the continuous infusion group and 1.7 (0.7) in the intermittent flush group (Student's t test P value 0.0001).
Extra information available from the study investigators also include the following outcomes:
-
need for the cannula to be replaced during the entire study period ‐ 2/17 (12%) infants did not need their cannula to be replaced in the continuous infusion group, and 8/22 (36%) infants did not need their cannula replaced in the intermittent flush group;
-
loss of cannula function in the first 48 hours ‐ in the continuous infusion group 10 cannulas extravasated, two dislodged, five remained intact; in the intermittent flush group 11 cannulas extravasated, one dislodged, 10 remained intact.
Discussion
Even though one study showed no difference between the approaches for maintaining cannula patency and one showed an advantage for intermittent flushes, it is unfortunate that the way the data were analysed and reported in the two included studies makes the reliability of the results uncertain.
The reports (for both included studies) only included as their main outcome the average duration of cannula patency for all cannulas. That is, they reported duration of cannula patency for all cannulas in all infants in each treatment group averaged over the treatment course. Some infants would have had multiple cannulas and, therefore, the mean duration of cannula patency would include multiple measures of the duration of cannula patency in some infants with non‐independence of those multiple measures. This would invalidate the assumptions necessary to ensure the reliability of the statistical tests used.
In the study by Kalyn et al (Kalyn 2000) the number of catheters per number of neonates was difficult to interpret and additional unpublished data are not available to clarify this. The reasons for re‐cannulation of individual infants were not recorded in the study. However, the additional information that was provided by the study investigators for duration of patency for the first catheter used per infant did not show any significant difference between groups for this outcome.
Taylor et al found a longer duration of cannula patency (averaged over all catheters for that infant) with intermittent flushing and greater ease of handling of those infants. They also found that the mean number of cannulas used per infant in the first 48 hours was less for intermittent flushing. We had speculated that a baby requiring intermittent flushing of their intravenous line is far more portable than one with a continuous infusion line. We believed that this would make handling, feeding and caring for baby easier for mothers in neonatal nurseries and enable staff the opportunity to enhance maternal‐infant bonding opportunities. These outcomes were assessed by Taylor et al (Taylor 1989) using a subjective nursing score (unblinded assessment) and they did find that the infants having intermittent flushes were easier to care for.
It should be noted that Taylor et al's study compared intermittent flushing of the cannula with heparinised saline with a continuous infusion that did not contain heparin. The use of heparinised saline for the intermittent flush used to maintain cannula patency has been reviewed by Shah et al (Shah 2004). Their systematic review found five studies that compared intermittent flush with heparin and intermittent flush without heparin and reported the outcome of duration of cannula patency for the first cannula used per infant. There was no consistency of results for this outcome with two studies showing longer duration with heparin, one study showing longer duration without heparin and two studies showing no difference.
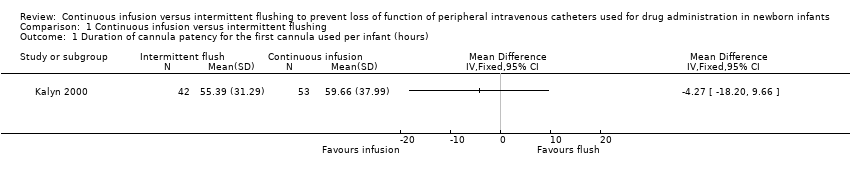
Comparison 1 Continuous infusion versus intermittent flushing, Outcome 1 Duration of cannula patency for the first cannula used per infant (hours).

Comparison 1 Continuous infusion versus intermittent flushing, Outcome 2 Number of cannulas used per infant in the first 48 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of cannula patency for the first cannula used per infant (hours) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Number of cannulas used per infant in the first 48 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

