Taxanes for adjuvant treatment of early breast cancer
Abstract
Background
Adjuvant chemotherapy improves survival in premenopausal and postmenopausal women with early breast cancer. Taxanes are highly active chemotherapy agents used in metastatic breast cancer. Review authors examined their role in early breast cancer. This review is an update of a Cochrane Review first published in 2007.
Objectives
To assess the effects of taxane‐containing adjuvant chemotherapy regimens for treatment of women with operable early breast cancer.
Search methods
For this review update, we searched the Specialised Register of the Cochrane Breast Cancer Group, MEDLINE, Embase, CENTRAL (2018, Issue 6), the WHO International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov on 16 July 2018, using key words such as 'early breast cancer' and 'taxanes'. We screened reference lists of other related literature reviews and articles, contacted trial authors, and applied no language restrictions.
Selection criteria
Randomised trials comparing taxane‐containing regimens versus non‐taxane‐containing regimens in women with operable breast cancer were included. Studies of women receiving neoadjuvant chemotherapy were excluded.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias and quality of the evidence using the GRADE approach. Hazard ratios (HRs) were derived for time‐to‐event outcomes, and meta‐analysis was performed using a fixed‐effect model. The primary outcome measure was overall survival (OS); disease‐free survival (DFS) was a secondary outcome measure. Toxicity was represented as odds ratios (ORs), and quality of life (QoL) data were extracted when present.
Main results
This review included 29 studies (27 full‐text publications and 2 abstracts or online theses). The updated analysis included 41,911 randomised women; the original review included 21,191 women. Taxane‐containing regimens improved OS (HR 0.87, 95% confidence interval (CI) 0.83 to 0.92; high‐certainty evidence; 27 studies; 39,180 women; 6501 deaths) and DFS (HR, 0.88, 95% CI 0.85 to 0.92; high‐certainty evidence; 29 studies; 41,909 women; 10,271 reported events) compared to chemotherapy without a taxane. There was moderate to substantial heterogeneity across studies for OS and DFS (respectively).
When a taxane‐containing regimen was compared with the same regimen without a taxane, the beneficial effects of taxanes persisted for OS (HR 0.84, 95% CI 0.77 to 0.92; P < 0.001; 7 studies; 10,842 women) and for DFS (HR 0.84, 95% CI 0.78 to 0.90; P < 0.001; 7 studies; 10,842 women). When a taxane‐containing regimen was compared with the same regimen with another drug or drugs that were substituted for the taxane, a beneficial effect was observed for OS and DFS with the taxane‐containing regimen (OS: HR 0.80, 95% CI 0.74 to 0.86; P < 0.001; 13 studies; 16,196 women; DFS: HR 0.83, 95% CI 0.78 to 0.88; P < 0.001; 14 studies; 16,823 women). Preliminary subgroup analysis by lymph node status showed a survival benefit with taxane‐containing regimens in studies of women with lymph node‐positive disease only (HR 0.83, 95% CI 0.78 to 0.88; P < 0.001; 17 studies; 22,055 women) but less benefit in studies of women both with and without lymph node metastases or with no lymph node metastases. Taxane‐containing regimens also improved DFS in women with lymph node‐positive disease (HR 0.84, 95% CI 0.80 to 0.88; P < 0.001; 17 studies; 22,055 women), although the benefit was marginal in studies of women both with and without lymph node‐positive disease (HR 0.95, 95% CI 0.88 to 1.02; 9 studies; 12,998 women) and was not apparent in studies of women with lymph node‐negative disease (HR 0.99, 95% CI 0.86 to 1.14; 3 studies; 6856 women).
Taxanes probably result in a small increase in risk of febrile neutropenia (odds ratio (OR) 1.55, 95% CI 0.96 to 2.49; moderate‐certainty evidence; 24 studies; 33,763 women) and likely lead to a large increase in grade 3/4 neuropathy (OR 6.89, 95% CI 3.23 to 14.71; P < 0.001; moderate‐certainty evidence; 22 studies; 31,033 women). Taxanes probably cause little or no difference in cardiotoxicity compared to regimens without a taxane (OR 0.87, 95% CI 0.56 to 1.33; moderate‐certainty evidence; 23 studies; 32,894 women). Seven studies reported low‐quality evidence for QoL; overall, taxanes may make little or no difference in QoL compared to chemotherapy without a taxane during the follow‐up period; however, the duration of follow‐up differed across studies. Only one study, which was conducted in Europe, provided cost‐effectiveness data.
Authors' conclusions
This review of studies supports the use of taxane‐containing adjuvant chemotherapy regimens, with improvement in overall survival and disease‐free survival for women with operable early breast cancer. This benefit persisted when analyses strictly compared a taxane‐containing regimen versus the same regimen without a taxane or the same regimen with another drug that was substituted for the taxane. Preliminary evidence suggests that taxanes are more effective for women with lymph node‐positive disease than for those with lymph node‐negative disease. Considerable heterogeneity across studies probably reflects the varying efficacy of the chemotherapy backbones of the comparator regimens used in these studies. This review update reports results that are remarkably consistent with those of the original review, and it is highly unlikely that this review will be updated, as new trials are assessing treatments based on more detailed breast cancer biology.
PICO
Plain language summary
Taxane‐containing chemotherapy for women after surgery for early breast cancer
What is the aim of this review?
The aim of this Cochrane Review was to find out if adding taxane drugs to standard chemotherapy improves survival and is safe for women with early breast cancer. Cochrane Review authors collected and analysed all relevant studies to answer these questions and found 29 studies.
Key messages
Adding a taxane drug to standard chemotherapy improved survival (women lived longer) and reduced the chance of cancer returning in women with operable early breast cancer, but the use of taxanes probably led to increased risk of some side effects such as febrile neutropenia (low white cell count with fever) and neuropathy (damage to the nerves).
What was studied in this review?
Early breast cancer is cancer that has not spread beyond the breast or nearby lymph nodes. It may be curable with surgery alone, but there is a risk that after surgery the breast cancer may return. Chemotherapy and radiotherapy are needed after surgery to achieve a cure.
A combination of chemotherapy drugs, rather than one drug by itself, is usually used to treat early breast cancer.
One class of chemotherapy drugs commonly used is taxanes. Taxanes act by stalling the cellular processes that are needed for cells to divide. This action causes cancer cells to stop dividing and slows the growth of cancer or kills the cells. Two main taxane drugs are available ‐ paclitaxel and docetaxel.
The practice of adding taxanes to standard chemotherapy has increased over the last 10 years as data from clinical trials have become available. There is a need to review these data to find out the benefits of these drugs, any side effects of the drugs, and how treatment is affecting a woman's overall well‐being (quality of life).
What are the main results of this review?
Review authors found 29 relevant studies involving 41,911 women. These studies compared chemotherapy that contained a taxane against chemotherapy that did not contain a taxane. Around half of the studies used paclitaxel, and the other half used docetaxel. The decision whether to use paclitaxel or docetaxel generally was based on the availability of these drugs in the hospital. Researchers gave these drugs by injection into a vein.
The women's health was monitored for at least 12 months from the start of the study. Some studies monitored women for 10 years.
Review authors found that adding a taxane drug to chemotherapy:
• improves survival and reduces the risk of cancer coming back compared to chemotherapy with no taxane;
• probably leads to an increased chance of some side effects compared to chemotherapy with no taxane. Side effects that are more likely to occur due to taxanes are febrile neutropenia (low white cell count with fever) and neuropathy (damage to the nerves);
• probably makes little or no difference in heart function compared to chemotherapy with no taxane; and
• may make little or no difference in quality of life for women compared to chemotherapy with no taxane. Seven of 29 studies provided information on the quality of life of women.
Very little information is available on the costs of adding a taxane to chemotherapy; only one study, which was conducted in Europe, reported cost‐effectiveness data.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to July 2018.
Authors' conclusions
Summary of findings
| Taxane‐containing chemotherapy compared to any chemotherapy without taxane for early breast cancer | ||||||
| Patient or population: women with early breast cancer (operable, stages I to IIIA) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with any chemotherapy without taxanes | Risk with taxane‐containing chemotherapy | |||||
| Overall survival | Low risk of death | HR 0.87 | 39,180 | ⊕⊕⊕⊕ | Additional analyses (including specific taxane, scheduling, treatment duration, doses, node positive, and risk of bias) showed equivalent efficacy | |
| 80 per 1000* | 70 per 1000 | |||||
| High risk of death | ||||||
| 200 per 1000* | 176 per 1000 | |||||
| Disease‐free progression | Low risk of recurrence | HR 0.88 | 41,909 | ⊕⊕⊕⊕ | As above for overall survival | |
| 140 per 1000* | 124 per 1000 | |||||
| High risk of recurrence | ||||||
| 320 per 1000* | 288 per 1000 | |||||
| Quality of life | Not estimable. In general, there did not seem to be differences in quality of life scores between groups at long‐term follow‐up | ‐ | (7 studies) | ⊕⊕⊝⊝ | Studies used the validated EORTC‐C30 questionnaire and measures were patient‐reported | |
| Febrile neutropenia | Study population | OR 1.43 | 34,154 | ⊕⊕⊕⊝ | There was a higher incidence of febrile neutropenia with docetaxel‐containing regimens | |
| 56 per 1000 | 78 per 1000 | |||||
| Neuropathy (including grade 3/4 sensory or motor neuropathy, or both) | Study population | OR 6.89 (3.23 to 14.71) | 31,033 | ⊕⊕⊕⊝ | A test for subgroup differences by taxane type was not significant | |
| 6 per 1000 | 37 per 1,000 | |||||
| Cardiotoxicity (including grade 3/4 and congestive cardiac failure) | Study population | OR 0.87 | 32,894 | ⊕⊕⊕⊝ | Risk of cardiotoxicity was reduced with lower planned dose of anthracycline | |
| 9 per 1000 | 8 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHeterogeneity was detected (I² = 59%) mainly due to variations in chemotherapy backbones. The quality of evidence was not downgraded as variations in chemotherapy are likely to occur in clinical practice. | ||||||
Background
Description of the condition
Breast cancer is a major cause of morbidity and mortality among women worldwide. In 2012, an estimated 1.67 million new cases and over 522,000 deaths occurred (Ferlay 2015). Depending on the stage of early breast cancer, five‐year relative survival rates can range from 90% (stage II) to almost 100% (stage 0 or stage I) (AIHW 2012).
The primary treatment for early breast cancer is local, and surgery with or without radiotherapy is recommended for women with operable early breast cancer (NCCN 2007). Adjuvant polychemotherapy following surgery improves survival among premenopausal and postmenopausal women with early breast cancer (EBCTCG 2005).
Description of the intervention
Two taxanes are commercially available: paclitaxel (Taxol®, Bristol‐Myers Squibb) and docetaxel (Taxotere®, Sanofi‐Aventis). Extensive research has led to the creation of new second‐generation taxanes and additional non‐taxane microtubule‐targeting chemotherapies. Currently two new taxanes have been approved by the FDA: nab paclitaxel (Abraxane®, Celgene), which was approved in 2005 for treatment of refractory, relapsed, or metastatic breast cancer; and cabazitaxel (Jevtana®, Sanofi), which was approved in 2010 for use in hormone‐refractory metastatic prostate cancer. Additionally, two non‐taxane microtubule‐targeting agents have received FDA approval for use in breast cancer: ixabepilone (Ixempra®, Bristol‐Myers Squibb) in 2007, and eribulin (Halaven®, Eisai Co., Ltd.) in 2010.
The most common side effects differ slightly between the two available taxanes. Both agents cause neutropenia (low neutrophil count, a subset of white blood cells) and thrombocytopenia (low platelet count), as well as fatigue, nausea and vomiting, hair loss, diarrhoea, mouth ulcers, and joint and muscle pain. Paclitaxel also causes hypersensitivity reactions (skin rash and reactions to infusion of the agent) and peripheral neuropathy. Docetaxel also causes skin and nail changes and fluid accumulation (oedema). Both drugs can cause febrile neutropenia (serious infection due to low neutrophil count), which occasionally can be life‐threatening or fatal. Supportive therapies can modify many of these side effects.
How the intervention might work
Taxanes are cytotoxic chemotherapy agents that affect cellular structures needed for cancer cells to divide ‐ the microtubules. In a normal cell cycle, cells form microtubules at the beginning of cell division, and the microtubules are broken down when the cell stops dividing. Taxanes stabilise the microtubules, preventing them from breaking down normally. This causes the cancer cells to stop dividing, potentially slowing the growth of cancer or killing the cells.
Why it is important to do this review
Taxanes are among the most active agents in metastatic breast cancer (Bishop 1999; Chan 1999; Ghersi 2005); they are widely used (Crown 2002). Their incorporation into adjuvant regimens for early breast cancer has increased in recent years as mature data from clinical trials have become available. For this review update, data on an additional 19,416 participants were available, with time‐to‐event data provided for most of the randomised participants. Other systematic reviews have examined this topic: Bria 2006 and Qin 2011; however, these reviews did not include risk of bias assessments for the included studies as per Cochrane's risk of bias tool and did not grade the overall quality of evidence for each main outcome. An update of the efficacy and safety of taxanes in the form of an updated Cochrane Review seems warranted given the availability of mature follow‐up data and new trial data.
Objectives
To assess the effects of taxane‐containing adjuvant chemotherapy regimens for treatment of women with operable early breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials are included. Quasi‐randomised trials were excluded.
We included studies with full‐text publications and studies published in abstract form only. We excluded studies available only as protocols and those published without the outcome measures of interest in this review.
Types of participants
We included women of any age with histologically confirmed operable breast cancer (stages I to IIIA).
We excluded women who received neoadjuvant chemotherapy. We included studies that included both women who received adjuvant chemotherapy and women who received neoadjuvant chemotherapy if data for the two groups were reported separately.
Types of interventions
We defined an intervention as any chemotherapy regimen that contains a taxane.
We defined a comparator as any chemotherapy regimen that does not contain a taxane.
Comparisons included the following.
-
Question 1. Taxane‐containing regimen versus the same regimen without a taxane.
-
Question 2. Any taxane‐containing regimen versus any regimen without a taxane.
-
Question 3. Any taxane‐containing regimen versus the same regimen with another drug or drugs that were substituted for the taxane.
Endocrine treatment and targeted therapy were allowed if the same treatment was given to all groups.
The taxane drugs used were paclitaxel and docetaxel.
Types of outcome measures
Primary outcomes
-
Overall survival (OS), defined as time from randomisation/study entry until death from any cause
Secondary outcomes
-
Disease‐free survival (DFS), defined as time from date of randomisation to first date of a local, regional, or distant relapse, diagnosis of a second primary cancer, or death from any cause
-
Toxicity, defined by World Health Organization (WHO)/National Cancer Institute of Canada (NCIC) toxicity criteria
-
Quality of life (QoL), assessed by validated or trial‐specific instruments such as the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire
-
Cost‐effectiveness
Search methods for identification of studies
Electronic searches
We searched the following databases on 16 July 2018.
-
Specialised Register of the Cochrane Breast Cancer Group. Details of the search strategy used by the Group for identification of studies and the procedure used to code references are outlined in the Group's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Studies coded as 'early breast cancer' and 'chemotherapy' on the Specialised Register were extracted and combined with the keywords 'taxol', 'docetaxel', and 'paclitaxel'. A search was carried out for the following text words: 'taxane', 'taxol', 'taxotere', 'paclitaxel', 'paxene', 'nsc‐12973', 'docetaxel', 'anzatax', 'taxanes', 'taxoids', and 'taxoid'.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) (see Appendix 1).
-
MEDLINE (via OvidSP) (see Appendix 2).
-
Embase (via Embase.com) (see Appendix 3).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal for all prospectively registered and ongoing trials (see Appendix 4).
-
ClinicalTrials.gov register (clinicaltrials.gov) for additional unpublished and ongoing studies (see Appendix 5).
Searching other resources
We searched the reference lists of other related literature reviews and articles.
We performed handsearching for abstracts published from 1995 to 2006 for presentations at the American Society of Clinical Oncology Annual Scientific Meeting, and up until 2009 for the San Antonio Breast Cancer Symposium.
Data collection and analysis
Selection of studies
In the original review and review update, two review authors (original review: AN, TF; review update: MW, LB) applied the selection criteria to each trial publication (if full publication available) or abstract (full publication not available). A third review author was available to resolve any disagreements regarding eligibility (review update: NW).
We have recorded excluded studies in the Characteristics of excluded studies table.
We applied no language restrictions.
Data extraction and management
For the original review and review update, two review authors (original review: AN, TF; review update: MW, LB) independently extracted data from the included studies. If required, a third review author (NW) was available to resolve any discrepancies regarding extraction of quantitative data. We collected information on study design, participants (including hormone receptor status and nodal involvement), settings, interventions, primary and secondary outcomes, follow‐up, and sources of funding. For studies with more than one publication, we extracted data from these publications, and we considered the final or updated version of each study as the primary reference.
Assessment of risk of bias in included studies
For the review update, we used Cochrane's 'Risk of bias' assessment tool to assess potential sources of bias in the included studies (Higgins 2011). Two review authors (MW, LB) independently assessed the potential risk of bias for each study and resolved any differences in judgement through discussion. The domains assessed were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. We assigned ratings of 'high', 'low', or 'unclear' risk of bias to each domain for each included study in keeping with the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Among phase III oncology studies, open‐label studies are common due to the difficulty involved in concealing different chemotherapy schedules and toxicities. The blinding of outcome assessment domain was therefore grouped with outcome measures most unlikely or most likely to be influenced by lack of blinding. Outcomes were segregated into (1) overall survival, (2) disease‐free survival and toxicity, and (3) quality of life.
Measures of treatment effect
The primary outcome for this review was overall survival, and the secondary outcome was disease‐free survival, with both considered as time‐to‐event outcomes. Hazard ratios (HRs) and variances were extracted from trial publications, when available. If not reported, statistics were extracted from publications via the methods described by Parmar et al using other summary statistics, or from data from published Kaplan‐Meier curves in the original review (Parmar 1998). Numbers at risk were adjusted based on estimated minimum and maximum follow‐up times. When these were not reported, minimum follow‐up was estimated from parameters given, including date of final accrual, date of study closure, date of submission, and estimated time to complete treatment. Maximum follow‐up time was similarly estimated from date of first accrual, date of analysis, date of submission, and last event on the time‐to‐event curve. For the review update, if required, we calculated summary statistics indirectly using the methods outlined by Tierney (Tierney 2007; indirect methods were recorded in the Notes section in the Characteristics of included studies tables). All efficacy analyses used an intention‐to‐treat population when this was reported. A pooled HR was calculated using observed (O) minus expected (E) event numbers, and variance from each trial was derived as above in a fixed‐effect model (Yusuf 1991).
The treatment effect was also analysed by subgroups for hormone receptor status. In this case, HRs and confidence intervals (CIs) reported in hormone receptor‐positive women and hormone receptor‐negative women were analysed, when available.
For two studies (ADEBAR; Taxit 216), missing data were estimated using the formula HR = [(taxane events)/(taxane participants)]/[(control events)/(control participants)]. For ADEBAR, this formula was used to estimate the number of participants per treatment group, and for Taxit 216, the formula was used to estimate the number of events per treatment group for overall survival and for disease‐free survival.
Toxicity data were extracted from each trial by one or two review authors (original review: RV; review update: MW, LB); when possible, this was done for the treated population rather than the intention‐to‐treat population. As definitions of toxic events varied between trials, events were extracted and summarised to best reflect clinically important outcomes. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each toxicity via a random‐effects model, when that toxicity was reported in four or more trials. For this review, toxicity was abstracted only from the primary publication used to report efficacy or from a publication solely on toxicity, even when other published abstracts had reported separately on toxicity.
Quality of life (QoL) data were collected using the EORTC Common Toxicity Criteria (CTC) questionnaire, and four of the six trials reported data in a full publication. No attempt was made to statistically synthesise QoL data, which are summarised and reported qualitatively.
Pharmacoeconomic data were reported for only one trial, and a description was provided in the Results section.
Unit of analysis issues
Three trials were three‐arm studies (ECTO; NCIC‐CTG MA21a and NCIC‐CTG MA21b; UK TACT). For ECTO, data from two of the three arms were used for this review (the third being a neoadjuvant treatment arm). For NCIC CTG MA21, the control group was halved to allow a comparison with each of the two taxane arms (NCIC‐CTG MA21a; NCIC‐CTG MA21b). For UK TACT, the two control arms (epirubicin (e)‐cyclophosphamide, methotrexate, and fluorouracil (CMF) and fluorouracil/epirubicin/cyclophosphamide (FEC)) were combined.
Two trials were four‐arm studies (BIG 2‐98; CALGB 40101). For BIG 2‐98, the two control arms were combined, as were the two taxane arms. However, for the analysis related to sequential versus concurrent anthracycline/taxane, data comparing uncombined study arms were used (concurrent control vs concurrent taxane, and sequential control vs sequential taxane). For CALGB 40101, the two control arms (4‐ and 6‐cycle regimens) were combined, and the two taxane arms (four‐ and six‐cycle regimens) were combined.
Dealing with missing data
When data were missing, we contacted the original investigators (by written correspondence) to request missing data. For the review update, we contacted the following trialists for summary statistics, numbers of events for each treatment arm (for overall survival or disease‐free survival), and clarification on whether HRs were adjusted or unadjusted: ADEBAR; BIG 2‐98; CALGB 40101; E2197; FinHer; GEICAM 9906; GONO MIG‐5; HORG; Kader; NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01; Roy; Taxit 216; UK TACT. We received additional data from the trialists for seven studies: ADEBAR; CALGB 40101; E2197; FinHer; GEICAM 9906; GONO MIG‐5; HORG.
Assessment of heterogeneity
Heterogeneity was assessed by using the Chi² test and the I² statistic, as well as visual inspection of forest plots. The graphical representation of data was inspected; if confidence intervals for the results of individual studies had poor overlap, this generally indicated the presence of statistical heterogeneity.
We interpreted the I² statistic as per guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0% to 40% might not be important; 30% to 60% represented moderate heterogeneity; 50% to 90% represented substantial heterogeneity; and 75% to 100% represented considerable heterogeneity.
Assessment of reporting biases
We followed the recommendations for testing for funnel plot asymmetry as described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Funnel plot asymmetry may be due to reporting bias; we addressed this possibility in the Results and Discussion sections of the review.
Data synthesis
For dichotomous outcome data (i.e. toxicity), we used a random‐effects (Mantel‐Haenszel method) model.
For time‐to‐event outcome data (i.e. overall survival and disease‐free‐survival), we used a fixed‐effect (exp[(O‐E)/Var] method) analysis. In the case of hormone receptor status subgroup analysis, we analysed the pooled HR using fixed‐effect (generic inverse variance method) analysis.
We performed all analyses using Review Manager software (RevMan).
Summary of findings
We used the GRADE approach to assess the quality of evidence for the following six main outcomes: mortality (overall survival), risk of recurrence (disease‐free survival), quality of life, febrile neutropenia, neuropathy (grade 3/4), and cardiotoxicity. We used GRADEproGDT software to develop the 'Summary of findings' table and followed GRADE guidance (GRADEproGDT; Schünemann 2011). Two review authors (MW, LB) graded the quality of the evidence for this review update.
To calculate absolute risk of the control group for time‐to‐event outcomes, we estimated the event rate at a specific time point (five years for OS and DFS) from the Kaplan‐Meier curves or reported event rates. We used a range for baseline event rates (i.e. low‐risk and high‐risk participants). We entered these estimated values into GRADEproGDT, and the corresponding absolute risks for the intervention group with low‐ and high‐risk subgroups at five years were automatically populated by GRADEproGDT.
Subgroup analysis and investigation of heterogeneity
We performed the following post hoc subgroup analyses for overall survival and disease‐free‐survival.
-
Type of taxane (paclitaxel or docetaxel).
-
Sequential or concurrent anthracycline and taxane.
-
Addition or substitution of a taxane.
-
Node positive only, node positive and negative, or node negative only.
-
Longer or the same duration of chemotherapy.
-
Fewer than four or four or more cycles of taxane.
-
Hormone receptor status.
We also conducted post‐hoc subgroup analyses by type of taxane for febrile neutropenia and neuropathy, because neutropenia and neuropathy are toxicities commonly seen when taxanes are used.
Sensitivity analysis
We performed the following sensitivity analyses.
-
Publication status: fully published trials versus trials published in abstract form only.
-
Differences in the definition of DFS: DFS versus relapse‐free survival (RFS); DFS versus time to recurrence (TTR).
-
Risk of bias assessments: low versus high/unclear risk of bias. Studies with more than five of the nine domain judgements with unclear/high risk of bias were assigned an overall assessment of unclear/high risk.
Results
Description of studies
Results of the search
For this review update, searching yielded 5118 records from the Specialised Register of the Cochrane Breast Cancer Group, MEDLINE, Embase, and CENTRAL, on 16 July 2018. Searching relevant review papers revealed an additional 16 records, and searching WHO ICTRP and ClinicalTrials.gov revealed one potentially eligible ongoing study. After removing duplicates, we screened the titles and abstracts of the 2802 remaining records and excluded 2735 of them based on information found in the abstract alone. We further assessed the full‐text articles or ongoing trial records for 67 records. We excluded nine records after full‐text review and provided reasons in the Characteristics of excluded studies table.
Of the 58 remaining records, 33 records related to 17 new studies (ADEBAR; Boccardo; CALGB 40101; DEVA; ELDA; GEICAM 2003‐02; GEICAM 9805; GOIM 9902; GONO MIG‐5; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01; Roy; Sakr; TITAN; UK TACT), 14 related to updated data for nine previously included studies (BCIRG 001; BIG 2‐98; E2197; ECTO; FinHer; GEICAM 9906; PACS 01; Taxit 216; US Oncology 9735), eight related to four studies classified as studies 'awaiting classification' due to insufficient reporting of the number of events per treatment arm and relevant effect estimates (EC‐DOC; Kader; EORTC 10041/BIG 3‐04 MINDACT; PACS 04), and three were classified as 'ongoing' studies (NNBC3; NCT01966471; NCT02549677).
The original Cochrane Review identified 23 potentially eligible studies: 12 included studies, three studies 'awaiting classification', and eight ongoing studies (refer to Ferguson 2007). When studies from the original review and those from the review update were combined, review authors had 37 potentially eligible studies involving 29 included studies (referring to 30 treatment comparisons), four studies awaiting inclusion, and four ongoing studies (see PRISMA flowchart: Figure 1). The PRISMA flowchart for the original review can be found in the previously published version of this review (Ferguson 2007).
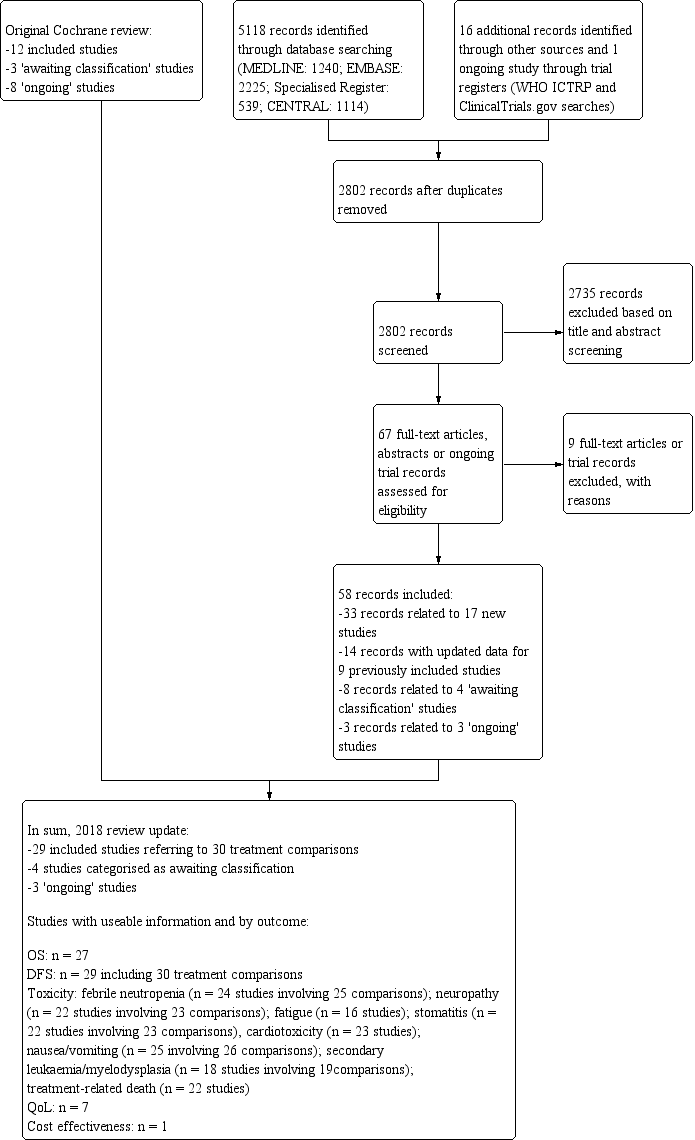
Review update: study flow diagram.
Of the 29 included studies, 27 studies published efficacy data in peer‐reviewed journals (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; DEVA; E2197; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; PACS 01; Roy; Sakr; TITAN; UK TACT; US Oncology 9735), one study had been reported only in abstract form (RAPP‐01), and for one study results were reported in an online thesis (Taxit 216). For seven studies, we received additional information or clarification of data from the trialists (ADEBAR; CALGB 40101; E2197; FinHer; GEICAM 9906; GONO MIG‐5; HORG).
Since publication of the original review, two studies formally categorised as 'awaiting assessment' ‐ ADEBAR and DEVA ‐ and six studies categorised as 'ongoing' studies ‐ GEICAM 9805; GOIM 9902; GONO MIG‐5; NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01; UK TACT ‐ have become included studies. We excluded one previously 'awaiting assessment' study (CALGB 9640: in the review update reclassified as SWOG S9623) due to confounders in the taxane (dose‐dense treatment) and non‐taxane (transplantation) treatment arms.
Included studies
See Characteristics of included studies.
For the updated review, we included 29 studies. Of these, seven studies addressed Question 1 (taxane‐containing regimen vs the same regimen without a taxane; BIG 2‐98; CALGB 9344; ECTO; GOIM 9902; HeCOG; NSABP B‐28; Taxit 216), nine addressed Question 2 (any taxane‐containing regimen vs any regimen without a taxane; ADEBAR; Boccardo; CALGB 40101; ELDA; GONO MIG‐5; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; UK TACT), and 15 addressed Question 3 (any taxane‐containing regimen vs the same regimen with another drug or drugs substituted for the taxane; BCIRG 001; BIG 2‐98; DEVA; E2197; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; PACS 01; RAPP‐01; Roy; Sakr; TITAN; UK TACT; US Oncology 9735). It is noted that BIG 2‐98 addressed Questions 1 and 3, and UK TACT addressed Questions 2 and 3.
The NCIC‐CTG MA21 study, which was a three‐arm study with two taxane‐containing arms, reported sufficient data that the study was split into NCIC‐CTG MA21a (comparing the taxane regimen of epirubicin/cyclophosphamide followed by paclitaxel (EC‐T) vs fluorouracil/epirubicin/cyclophosphamide (FEC)) and NCIC‐CTG MA21b (comparing the taxane regimen of doxorubicin/cyclophosphamide followed by paclitaxel (AC‐T) vs FEC).
Of the eight studies classified as 'awaiting classification' or 'ongoing', four addressed Question 2 (EC‐DOC; EORTC 10041/BIG 3‐04 MINDACT; NCI‐H99‐0038; NCT01966471) and four addressed Question 3 (Kader; NCT02549677; NNBC3; PACS 04).
Characteristics of patients
Axillary lymph node involvement
The included studies recruited patient populations with varying risk profiles. Seventeen trials entered participants who were positive for axillary node metastases (i.e. > 85% of included participants were lymph node positive; ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216). Nine studies included participants both with (node positive) and without (node negative) pathologically involved axillary lymph nodes (E2197; ECTO; ELDA; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01; TITAN; UK TACT; US Oncology 9735). Three studies entered participants who were node negative (100%: GEICAM 2003‐02; GEICAM 9805; 94%: CALGB 40101). In GEICAM 2003‐02 and GEICAM 9805, participants also had a high‐risk factor for recurrence according to 1998 St. Gallen criteria. Baseline characteristics of participants included in the eligible studies are presented in the Characteristics of included studies table.
Menopausal status
Both premenopausal and postmenopausal women were included in all studies except for three studies (DEVA; ELDA; ICE II‐GBG 52), which included only postmenopausal patients. HeCOG excluded postmenopausal women with hormone receptor‐positive tumours and fewer than four positive axillary nodes.
Hormone receptor status
In nearly all studies, more than 58% of participants had tumours testing positive for oestrogen and/or progesterone receptors, except for TITAN, which included participants with triple‐negative breast cancer.
Interventions used in the trials
Twenty‐nine studies involved 41,911 women who were randomised to treatment groups: 21,791 to a taxane‐containing arm, and 20,120 to a non‐taxane‐containing arm.
Chemotherapy
Thirteen of the 29 included studies used paclitaxel (Boccardo; CALGB 40101; CALGB 9344; ECTO; GEICAM 2003‐02; GEICAM 9906; GONO MIG‐5; HeCOG; ICE II‐GBG 52; NCIC‐CTG MA21; NSABP B‐28; Roy; TITAN). The remaining 16 included studies used docetaxel (ADEBAR; BCIRG 001; BIG 2‐98; DEVA; E2197; ELDA; FinHer; GEICAM 9805; GOIM 9902; HORG; PACS 01; RAPP‐01; Sakr; Taxit 216; US Oncology 9735; UK TACT).
All studies except four used an anthracycline in both taxane‐containing and non‐taxane‐containing arms (CALGB 40101; ELDA; ICE II‐GBG 52; US Oncology 9735). US Oncology 9735 compared docetaxel and cyclophosphamide to doxorubicin and cyclophosphamide, CALGB 40101 compared paclitaxel alone to the doxorubicin plus cyclophosphamide regimen; ELDA compared docetaxel alone to cyclophosphamide, methotrexate, and fluorouracil, and ICE II‐GBG 52 compared paclitaxel and capecitabine against epirubicin, cyclophosphamide or cyclophosphamide, methotrexate, and fluorouracil.
The taxane and anthracycline were administered either sequentially ‐ ADEBAR; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216; TITAN; UK TACT ‐ or concurrently ‐ BCIRG 001; BIG 2‐98; E2197; ECTO; GEICAM 9805; GONO MIG‐5; RAPP‐01. BIG 2‐98 randomised participants to four arms ‐ two control and two containing taxanes ‐ to simultaneously examine the effects of concurrent versus sequential administration of taxane and anthracycline. Updated published data from BIG 2‐98 provided the summary statistics for each group.
The total planned dose of anthracycline was the same in both arms in 10 studies (BCIRG 001; CALGB 9344; E2197; FinHer; GEICAM 9805; GOIM 9902; GONO MIG‐5; NCIC‐CTG MA21a; NSABP B‐28; Taxit 216); it was lower for the taxane‐containing arm in 14 studies (ADEBAR; BIG 2‐98; Boccardo; DEVA; ECTO; GEICAM 2003‐02; GEICAM 9906; HeCOG; HORG; PACS 01; RAPP‐01; Roy; Sakr; TITAN; UK TACT). UK TACT compared the taxane arm against two different control regimens of FEC or E‐CMF; however the total planned dose of anthracycline was lower in the taxane‐containing arm than in either of the control arms. NCIC‐CTG MA21b used doxorubicin in the taxane arm and epirubicin in the control arm, so doses of anthracycline used were not comparable. HeCOG administered dose‐dense chemotherapy in both taxane‐containing and non‐taxane‐containing arms. Dose density was unlikely to be a confounding factor, and the trial was included.
Five trials permitted granulocyte colony‐stimulating factor (G‐CSF) as primary prophylaxis. CALGB 9344 used primary prophylaxis with G‐CSF and ciprofloxacin with doxorubicin dosed at 90 mg/m², and this was not different between taxane and non‐taxane arms. HeCOG used G‐CSF during each cycle (days 3 to 10) with the same dose of G‐CSF given in the two treatment arms. TITAN stated that CSF could be used as per the American Society of Clinical Oncology (ASCO) guidelines, and its use was similar in the two treatment arms. GEICAM 9805 used primary prophylactic antibiotics for all participants receiving the taxane‐containing regimen and gave primary prophylactic G‐CSF after a protocol amendment. In NCIC‐CTG MA21 (i.e. NCIC‐CTG MA21a), G‐CSF was used as primary prophylaxis in the EC‐T taxane arm but not in the control arm. Use of G‐CSF as primary prophylaxis was identified as a potential confounder when it was used in only one treatment arm. In two studies (DEVA; FinHer), G‐CSF was recommended in the case of febrile neutropenia.
This review did not include neoadjuvant chemotherapy studies. ECTO enrolled patients before surgery and included a neoadjuvant treatment arm. The review included only the two adjuvant chemotherapy arms of this study, one if which contained a taxane drug in the treatment regimen and one that did not.
When the taxane‐containing chemotherapy regimens of the included studies was considered, the duration of chemotherapy varied across trials. The addition of extra cycles of chemotherapy to the taxane‐containing regimen has been considered as a potential confounding factor in favour of the taxane‐containing arms of these studies. Fifteen studies, involving 16 treatment comparisons, included chemotherapy treatment arms of equal duration (ADEBAR; BCIRG 001; CALGB 40101; E2197; ECTO; ELDA; FinHer; GEICAM 9805; NCIC‐CTG MA21a and NCIC‐CTG MA21b; PACS 01; RAPP‐01; Roy; Sakr; TITAN; US Oncology 9735), and eight studies used a taxane‐containing arm that delivered a longer duration or more cycles of chemotherapy than were used in the control arm (CALGB 9344; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NSABP B‐28; Taxit 216). Three studies involved a taxane‐containing arm of shorter chemotherapy duration than the non‐taxane‐containing arm (DEVA; Boccardo; GONO MIG‐5). In BIG 2‐98, a four‐arm study, the two taxane‐containing arms were of different duration: the sequential taxane arm was longer than the sequential control arm, and the concurrent taxane arm was the same duration as the concurrent control arm. UK TACT, a three‐arm study, provided two different control regimens whereby one control arm delivered a longer duration of chemotherapy than the second control arm. The control arms were not analysed separately and therefore were not included in analyses related to duration of chemotherapy.
Endocrine therapy
Tamoxifen 20 mg daily for five years for women with oestrogen receptor (ER)‐ and/or progesterone receptor (PR)‐positive tumours was used in most studies, with the following exceptions. HeCOG treated all hormone receptor‐positive premenopausal women with a gonadotropin‐releasing hormone (GnRH) analogue for one year in addition to tamoxifen; ECTO used tamoxifen for all participants before June 2000, but a subsequent protocol amendment mandated tamoxifen only for hormone receptor‐positive women; PACS 01 initially required tamoxifen treatment post chemotherapy only for postmenopausal women with hormone receptor‐positive or ‐negative tumours and amended the protocol in 1998 to require tamoxifen for premenopausal women with hormone receptor‐positive tumours; TITAN included women with triple‐negative breast cancer; in NSABP B‐28, all women older than 50 years of age at the time of surgery received tamoxifen regardless of hormone receptor status, and only those with positive hormone receptor status received tamoxifen if they were younger than 50 years of age. NSABP B‐28 also commenced tamoxifen concurrently with chemotherapy. It was not clear from published information whether any other included studies also commenced tamoxifen concurrently with chemotherapy. In eight studies, tamoxifen was initially given to women with ER‐ and/or PR‐positive tumours, but from 2003 to 2007, several protocol amendments allowed postmenopausal women to switch from tamoxifen to aromatase inhibitors (DEVA; E2197; FinHer; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; NCIC‐CTG MA21a and NCIC‐CTG MA21b; UK TACT). GEICAM 2003‐02 allowed postmenopausal women with hormone receptor‐positive tumours to receive aromatase inhibitors as initial adjuvant therapy or after tamoxifen. All studies used uniform policies for hormonal treatment in both control and experimental arms.
Radiotherapy
Radiotherapy to the breast, when reported, was required when breast‐conserving surgery was performed. This information was not specified in the DEVA and GONO MIG‐5 study reports. Additional radiotherapy was 'as per institution' in BCIRG 001, BIG 2‐98, Boccardo, CALGB 40101, E2197, FinHer, GEICAM 9805, HORG, ICE II‐GBG 52, NCIC‐CTG MA21a and NCIC‐CTG MA21b, Sakr, TITAN, UK TACT, and US Oncology 9735. In the TITAN study, in some cases MammoSite brachytherapy radiation was permitted if immediately after surgery and before treatment. In ADEBAR, all participants received adjuvant radiotherapy given following completion of chemotherapy or intermittently after completion of 50% of chemotherapy. In Roy, participants received locoregional external beam radiotherapy following their modified radical mastectomy. Axillary or chest wall radiotherapy was given to participants with four or more positive axillary nodes or a primary tumour (T) > 5 cm in GOIM 9902, HeCOG, and GEICAM 9906. ECTO also gave chest wall radiotherapy to women with a primary T4 tumour. Radiation to the chest wall, supraclavicular area, and internal mammary chain was recommended following mastectomy in PACS 01. In CALGB 9344, axillary or chest wall radiotherapy was not permitted.
Targeted therapy
One study used secondary randomisation to examine the additional question of the addition of trastuzumab to adjuvant chemotherapy for women with human epidermal growth factor receptor 2 (HER2)‐positive tumours following adjuvant chemotherapy (FinHer). UK TACT allowed women with HER2‐positive tumours to enter clinical trials for trastuzumab, and CALGB 40101 and NCIC‐CTG MA21 (NCIC‐CTG MA21a and NCIC‐CTG MA21b) recommended trastuzumab for HER2‐positive tumours after 2005. ELDA included women with HER2‐positive tumours (19% of the population) who received adjuvant trastuzumab for one year after chemotherapy based on results from the HERA trial.
Outcomes assessed in trials
The median follow‐up for participants ranged from 24 months in Roy to 163 months (estimated follow‐up) in GONO MIG‐5.
Four studies reported overall survival as a primary outcome (Boccardo; GONO MIG‐5; NSABP B‐28; US Oncology 9735), and 26 studies reported DFS (or RFS, distant disease‐free survival (DDFS) if reported) as the primary outcome. Both NSABP B‐28 and US Oncology 9735 had two primary outcomes (overall survival and DFS).
Not all trials reported data on all outcomes for this review. Two studies did not report complete data on overall survival and were not included for this part of the meta‐analysis (NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01). For NCIC‐CTG Ma21, the number of events had not been reached to conduct a formal statistical comparison for survival analysis, and for RAPP‐01, overall survival data were not reported in abstract form nor in the full trial publication. All trials reported on DFS; however the terminology and definitions used differed slightly between trials (Table 1). Definitions were the same for both experimental and control arms of each trial, and, for the purposes of this review, all breast cancer recurrence events under the different definitions were combined.
| Study | Description | Definition |
| DFS | Revised to iDFS (in 2016) in line with Standardized Definitions for Efficacy End Points (STEEP), where DFS referred to all invasive ipsilateral, regional, contralateral, and distant disease recurrences, second primary tumours, and death from any cause as events, and all non‐invasive in situ cancer events were excluded | |
| DFS | Time from randomisation to date of clinical relapse (histological or radiological evidence), a second cancer (except skin cancer other than melanoma or carcinoma in situ of breast or cervix), or death | |
| DFS | Time from randomisation until first date of local, regional, or distant relapse; diagnosis of a second primary cancer, including contralateral invasive breast cancer; or death from any cause | |
| RFS | Time from date of random assignment to date of locoregional and/or distant release | |
| RFS | Time from study entry until local recurrence, distant relapse, or death without relapse, whichever occurred first | |
| DFS | Time from randomisation to date of first locoregional recurrence, first distant metastasis, or death from any cause | |
| DFS | Time from date of randomisation to locoregional recurrence, distant recurrence, new primary breast cancer, or death | |
| Freedom from progression | Not reported in trial publication | |
| DFS | Interval between randomisation and locoregional or distant relapse or contralateral invasive breast cancer or second primary invasive non‐breast cancer or ipsilateral or contralateral in situ ductal carcinoma or death without cancer, whichever occurred first | |
| DFS | Time from date of random assignment to date of invasive breast cancer recurrence, invasive contralateral breast cancer, or death from any cause | |
| Recurrence‐free survival | Time from randomisation to date of detection of local or distant relapse (histological or radiological evidence) or contralateral invasive breast cancer or death without recurrence | |
| DFS | Time from random assignment to date of local, regional, or metastatic relapse; date of a second primary malignancy; or death form any cause, whichever occurred first | |
| DFS | Time from date of randomisation to date of local, regional, or metastatic relapse; diagnosis of second primary cancer or death from any cause | |
| DFS | According to the definition of invasive disease‐free survival (IDFS) in the STEEP system | |
| DFS | Time from randomisation to first relapse (local, regional, distant), contralateral breast cancer, or death from any cause | |
| EFS | Time from date of randomisation to date of local recurrence, distant metastases, contralateral breast cancer, second primary malignancy, or death from any cause, whichever occurred first | |
| DFS | Time from randomisation until local recurrence, distant relapse, or death (without relapse) | |
| DFS | Time from randomisation to date of breast cancer recurrence (local, regional, or distant), invasive contralateral breast cancer, non‐breast second primary cancer, or death from any cause | |
| iDFS and DDFS | Any local invasive or distant recurrence of breast cancer, any contralateral breast cancer, any second malignancy, and any death irrespective of its cause for iDFS | |
| RFS | Time from random assignment to time of recurrence of the primary breast cancer (local, nodal, metastatic). Patients with contralateral breast cancer, second malignancy, non‐disease‐related death were censored | |
| RFS | Time from random assignment to time of recurrence of the primary breast cancer (local, nodal, metastatic). Patients with contralateral breast cancer, second malignancy, non‐disease‐related death were censored | |
| DFS | Time from randomisation until local, regional, or distant treatment failure, contralateral breast cancer, non‐breast second primary cancer, or death | |
| DFS | Time from randomisation until first relapse (local, regional, or distant), contralateral breast cancer, or death from any cause | |
| TTR | Time to locoregional relapse, contralateral breast cancer, or distant metastasis, whichever occurs first | |
| DFS | Time from study entry to first local recurrence or distant metastasis, or death as a result of any cause | |
| DFS | Time from randomisation until first relapse (locoregional or distant), contralateral breast cancer, or death from any cause | |
| DFS | Time from randomisation to first invasive relapse, new primary breast cancer (ipsilateral or contralateral), or death form any cause | |
| DFS | Time from date of randomisation to local or distant recurrence or contralateral breast cancer or second primary malignancy or death from any case, whichever occurred first | |
| DFS | Time between randomisation and date of first documented disease recurrence or death from any cause | |
| DFS | Time from first dose of chemotherapy until date of any recurrence of breast cancer, a new second breast cancer or any other type of cancer, death due to any cause without relapse or recurrence of breast cancer, or date of last patient contact |
DFS: disease‐free survival
EFS: event‐free survival
RFS: relapse‐free survival
TTR: time to recurrence
All 29 studies reported some toxicity data; however data from one study could not be extracted and included in this review (Roy). This study presented toxicity data per week of treatment.
Seven studies presented quality of life measures (ADEBAR; BCIRG 001; DEVA; ELDA; GEICAM 9805; HeCOG; UK TACT). GONO MIG‐5, CALGB 40101, and NCIC‐CTG MA21 also listed quality of life as a secondary outcome; however these data have yet to be reported.
Excluded studies
Nine studies were excluded from this review update. One record reported results for GEICAM 9906 but was found to be a trial commentary (Dang), and another record reported on the prognostic and predictive significance of subtyping in the BCIRG 001 study (Hugh). Two studies involved neoadjuvant chemotherapy: in Albert, adjuvant data were unable to be separated from neoadjuvant data, and in NSABP B‐27, all participants received neoadjuvant chemotherapy before receiving adjuvant taxane. In four studies it was observed that all treatment arms received taxane therapy (NCT02838225; Sparano 2015; SWOG S0221; Wildiers). In SWOG S9623, dose‐dense chemotherapy in the taxane‐containing arm and high‐dose escalated chemotherapy with autologous haematopoietic progenitor cell transplantation in the non‐taxane arm were viewed as confounders, and the study was excluded. See Characteristics of excluded studies.
Risk of bias in included studies
Refer to Figure 2 for a summary of the risk of bias judgements for the included studies for each risk of bias domain.
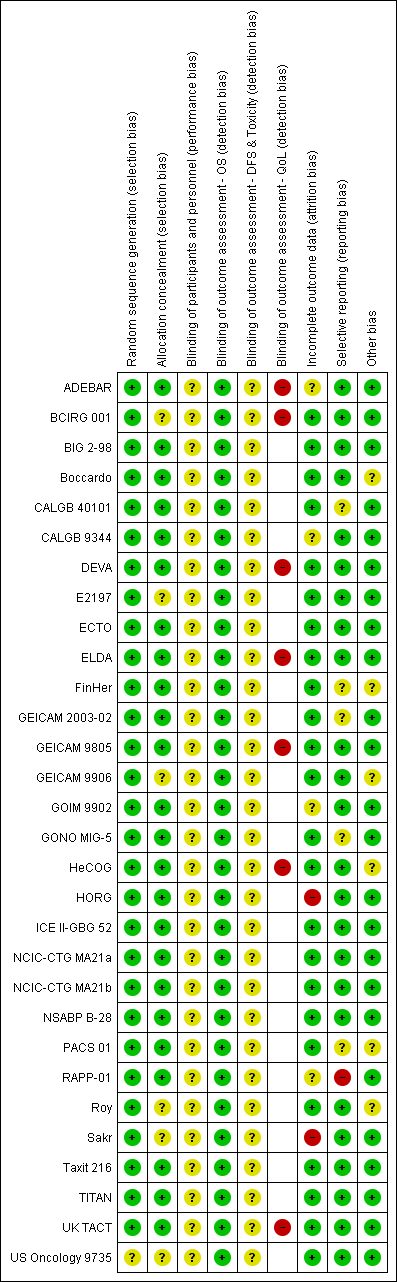
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The 29 studies, which related to 30 treatment comparisons, were described as randomised. The method of random sequence generation was described adequately (i.e. with low risk of bias) in 28 studies referring to 29 treatment comparisons (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; DEVA; E2197; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; GOIM 9902; HeCOG; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; PACS 01; RAPP‐01; Roy; Sakr; Taxit 216; TITAN; UK TACT). These studies reportedly used stratified randomisation, permuted block design, or minimisation. It was not possible to accurately assess the method of random sequence generation in one study owing to lack of information presented in the published trial report (US Oncology 9735). This study was classified as having unclear risk of bias.
Twenty‐three of the 29 studies were at low risk of bias for allocation concealment. These studies described central randomisation systems (computer or telephone/fax) (BIG 2‐98; Boccardo; CALGB 9344; DEVA; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GOIM 9902; HeCOG; HORG; ICE II‐GBG 52; NCIC‐CTG MA21 (NCIC‐CTG MA21a and NCIC‐CTG MA21b); NSABP B‐28; PACS 01; RAPP‐01; Taxit 216;TITAN; UK TACT). Six studies did not describe methods of allocation concealment or did not provide sufficient detail in the trial publication or abstract and were judged as having unclear risk of bias (BCIRG 001; E2197; GEICAM 9906; Roy; Sakr; US Oncology 9735).
Blinding
Twenty‐one studies were described as 'open label' or 'non‐blinded' (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; DEVA; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; GONO MIG‐5; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; PACS 01; Roy; Taxit 216; TITAN; UK TACT), and eight studies provided no information in the abstract or trial publication to allow a firm conclusion on whether they were 'open‐label' studies (E2197; GOIM 9902; HeCOG; HORG; NSABP B‐28; RAPP‐01; Sakr; US Oncology 9735). Performance bias due to lack of blinding of participants and personnel could not be ruled out, and these 29 studies were judged as having unclear risk of bias for this domain.
Detection bias was assessed by grouping outcomes with similar risks of bias: (1) overall survival, (2) disease‐free survival and toxicity, and (3) quality of life. For overall survival, lack of blinding was perceived as unlikely to have an impact on this outcome assessment. Therefore all studies were perceived to be at low risk of bias. For outcome measures that were more likely to be influenced by lack of blinding, that is, disease‐free survival and toxicity, we assessed whether outcome assessments were confirmed through imaging and biochemical tests and reviewed by independent panels/adjudication committees in each study. All 29 included studies were at unclear risk of bias because these outcomes were measured through scans and blood tests with no independent clinical review group. Quality of life measures were likely to be affected by lack of blinding to treatment. Seven of the ten studies that had planned to collect QoL data actually reported these data (data provided: ADEBAR; BCIRG 001; DEVA; ELDA; GEICAM 9805; HeCOG; UK TACT; data not reported: CALGB 40101; GONO MIG‐5; NCIC‐CTG MA21a and NCIC‐CTG MA21b). Quality of life questionnaires were completed by participants; the seven studies that reported these data were therefore considered to be at high risk.
Incomplete outcome data
Twenty‐three studies described intention‐to‐treat analysis and minimal patient loss to follow‐up that was accounted for; therefore we judged them to be at low risk of bias: BCIRG 001, BIG 2‐98, Boccardo, CALGB 40101, DEVA, E2197, ECTO, ELDA, FinHer, GEICAM 2003‐02, GEICAM 9805, GEICAM 9906, GONO MIG‐5, HeCOG, ICE II‐GBG 52, NCIC‐CTG MA21a and NCIC‐CTG MA21b, NSABP B‐28, PACS 01, Roy, Taxit 216, TITAN, UK TACT, and US Oncology 9735. Four studies were judged as having unclear risk of bias due to insufficient or no information provided for their analysis plan (ADEBAR; CALGB 9344; GOIM 9902; RAPP‐01), and two studies were considered at high risk of bias for this domain due to no intention‐to‐treat analyses and missing data with no reasons provided (HORG; Sakr).
Selective reporting
Twenty‐three studies, relating to 24 treatment comparisons, reported results for outcomes listed in the methods section of the trial publication (Boccardo; DEVA; GOIM 9902; HORG; Roy; Sakr; Taxit 216) or provided a trial registration record with listed outcomes found in the methods and results sections of the trial publication (ADEBAR; BCIRG 001; BIG 2‐98; CALGB 40101; CALGB 9344; E2197; ECTO; ELDA; GEICAM 9805; GEICAM 9906; HeCOG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; TITAN; UK TACT; US Oncology 9735). In the remaining six studies, there was partial reporting of results (i.e. for QoL) or some changes were noted in the primary or secondary outcomes (CALGB 40101; FinHer; GEICAM 2003‐02; GONO MIG‐5; PACS 01); these studies were ranked at unclear risk of bias for this domain. RAPP‐01 was judged as having high risk of bias for this domain, as data related to overall survival were not reported, although OS was listed as a secondary outcome in the trial publication.
Other potential sources of bias
Treatment groups were well balanced in most studies (low risk of bias: ADEBAR; BCIRG 001; BIG 2‐98; CALGB 40101; CALGB 9344; DEVA; E2197; ECTO; ELDA; GEICAM 2003‐02; GEICAM 9805; GOIM 9902; GONO MIG‐5; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; RAPP‐01; Sakr; Taxit 216; TITAN; UK TACT; US Oncology 9735). When minor baseline imbalances (such as differences in tumour size and in hormone receptor status across treatment groups) were reported, these were considered by the review authors to be unlikely to bias trial outcomes; these trials therefore were ranked as having unclear risk (Boccardo; FinHer; GEICAM 9906; HeCOG; PACS 01; Roy).
Effects of interventions
Refer to summary of findings Table for the main comparison.
Overall survival
High‐quality evidence was obtained from 27 studies for analysis of overall survival (OS). The NCIC‐CTG MA21 ‐ NCIC‐CTG MA21a; NCIC‐CTG MA21b ‐ and RAPP‐01 studies did not report sufficient OS data to allow indirect calculations of the hazard ratio (HR) and confidence interval (CI). A total of 39,180 women were included in the overall survival analysis, with 6501 deaths reported. Taxane‐containing regimens improved survival when compared to non‐taxane‐containing controls (HR 0.87, 95% CI 0.83 to 0.92; P < 0.001; high‐certainty evidence; Analysis 1.1; Figure 3). Heterogeneity across trials was moderate (heterogeneity I² = 35%; P = 0.04).
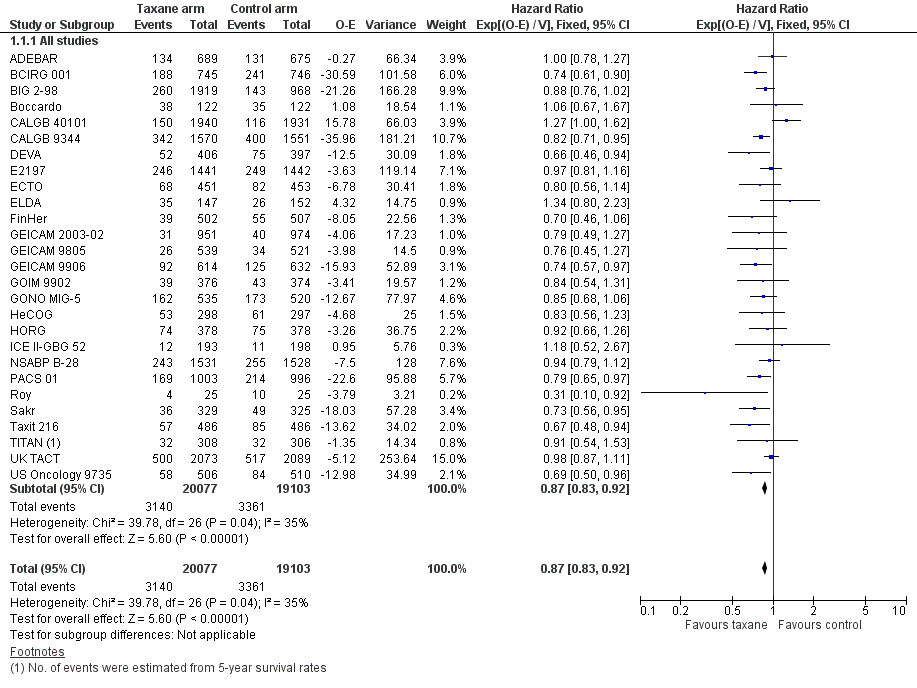
Forest plot of comparison: 1 Overall effect of taxanes, outcome: 1.1 Overall survival ‐ all studies.
Disease‐free survival
High‐certainty evidence was obtained from all 29 studies, involving 30 comparisons, for analysis of disease‐free survival (DFS). In this review, DFS analysis included freedom from progression (as measured in ECTO), time to recurrence (as measured in RAPP‐01), event‐free survival (as per GONO MIG‐5), and recurrence‐free survival (as measured in Boccardo, CALGB 40101, FinHer, and NCIC‐CTG MA21a and NCIC‐CTG MA21b). Although FinHer reported relapse‐free survival (RFS) and GONO MIG‐5 reported event‐free survival (EFS), these definitions were considered similar to those reported as DFS in other studies. Two studies ‐ ADEBAR; BCIRG 001 ‐ specifically excluded ductal carcinoma in situ (DCIS) and one study included DCIS in its DFS definition (ELDA). See Table 1 for the definition of DFS used in each study.
A total of 41,909 women from 29 studies were included in the DFS analysis, with 10,271 reported events. Taxane‐containing treatment improved disease‐free survival compared to control (HR 0.88, 95% CI 0.85 to 0.92; P < 0.001; high‐certainty evidence; Analysis 1.2; Figure 4). Heterogeneity across studies was substantial (heterogeneity I² = 59%; P < 0.001).
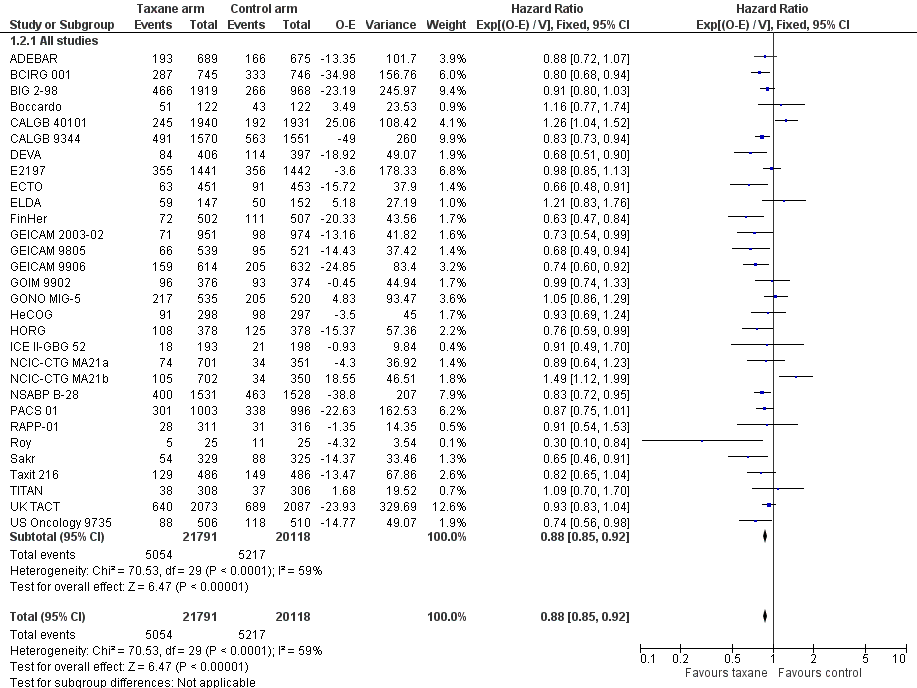
Forest plot of comparison: 1 Overall effect of taxanes, outcome: 1.2 Disease‐free survival: all studies.
We noted differences among the definitions used by each study, with death and contralateral breast cancer at times not counted among DFS events; in the first instance, we judged that this would have a minor impact on DFS analysis. To test this, we conducted a post‐hoc sensitivity analysis while excluding Boccardo, CALGB 40101, NCIC‐CTG MA21a and NCIC‐CTG MA21b and RAPP‐01. The result did not change significantly (HR 0.86, 95% CI 0.82 to 0.89; P < 0.001; Analysis 1.3), and heterogeneity was moderate (I² = 40%; P = 0.02). The definition of freedom from progression in ECTO would typically be consistent with DFS as defined in the other included studies; therefore we did not conduct a sensitivity analysis.
Subgroup analysis
Several post‐hoc subgroup analyses were performed in a side‐by‐side observational manner and are reported below. This exercise has limitations, although it was undertaken to address clinically relevant questions. Tests for interaction have not been performed as no differences between subgroups have been postulated.
Type of taxane
The two taxane drugs were analysed in separate groups in this post‐hoc analysis to assess whether there was a difference in efficacy between them.
Sixteen studies used docetaxel: 15 studies provided sufficient data for OS analyses (ADEBAR; BCIRG 001; BIG 2‐98; DEVA; E2197; ELDA; FinHer; GEICAM 9805; GOIM 9902; HORG; PACS 01; Sakr; Taxit 216; UK TACT; US Oncology 9735), and all 16 studies provided DFS data for analyses (ADEBAR; BCIRG 001; BIG 2‐98; DEVA; E2197; ELDA; FinHer; GEICAM 9805; GOIM 9902; HORG; PACS 01; RAPP‐01; Sakr; Taxit 216; UK TACT; US Oncology 9735). The remaining 13 studies used paclitaxel; OS data were available for 12 studies (Boccardo; CALGB 40101; CALGB 9344; ECTO; GEICAM 2003‐02; GEICAM 9906; GONO MIG‐5; HeCOG; ICE II‐GBG 52; NSABP B‐28; Roy; TITAN), and DFS data were available for 13 studies with 14 treatment comparisons (Boccardo; CALGB 40101; CALGB 9344; ECTO; GEICAM 2003‐02; GEICAM 9906; GONO MIG‐5; HeCOG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; Roy; TITAN). Both types of taxane showed a significant HR favouring taxane treatment over controls for both OS and DFS.
Overall survival
The docetaxel group included 22,105 women, and the paclitaxel group included 17,075 women. The HR for the docetaxel group was 0.86 (95% CI 0.81 to 0.92; P < 0.001; Analysis 2.1) with moderate heterogeneity (I² = 37%; P = 0.07), which was comparable to the paclitaxel group at HR 0.89 (95% CI 0.82 to 0.96; P = 0.003; Analysis 2.1) with moderate heterogeneity (I² = 37%; P = 0.10).
Disease‐free survival
The docetaxel group included 22,730 women, and the paclitaxel group had 19,179 women. The HR for the docetaxel group was 0.87 (95% CI 0.82 to 0.91; P < 0.001; Analysis 2.2) with moderate heterogeneity (I² = 39%; P = 0.06), and the HR for the paclitaxel group was 0.91 (95% CI 0.85 to 0.96; P = 0.002; Analysis 2.2) with substantial heterogeneity (I² = 71%; P < 0.001).
Weekly versus three‐weekly taxane
A post‐hoc analysis was conducted to examine the difference in efficacy between weekly versus three‐weekly administered taxanes. All studies that administered docetaxel did so using a three‐weekly regimen, except ELDA, which administered weekly docetaxel, so efficacy data for docetaxel were not further examined. Two studies that administered paclitaxel were not included in this post‐hoc analysis as they could not be classified as weekly or three‐weekly regimens: CALGB 40101 administered paclitaxel either fortnightly or in three‐weekly cycles, and HeCOG used fortnightly paclitaxel for all women in the taxane group.
Overall survival
Four studies (4176 women) provided a regimen of weekly paclitaxel in the taxane arm (GEICAM 2003‐02; GEICAM 9906; ICE II‐GBG 52; TITAN). Analysis of these studies yielded an HR of 0.80, favouring the taxane arm (95% CI 0.65 to 0.98; P = 0.7; Analysis 3.1), with no heterogeneity (P = 0.70). Six studies (8433 women) administered paclitaxel every three weeks (Boccardo; CALGB 9344; ECTO; GONO MIG‐5; NSABP B‐28; Roy), with HR of 0.86 favouring taxane (95% CI 0.78 to 0.95; P = 0.002; Analysis 3.1), and with no significant heterogeneity (I² = 15%; P = 0.32).
Disease‐free survival
Four studies (4176 women) that administered weekly paclitaxel ‐ GEICAM 2003‐02; GEICAM 9906; ICE II‐GBG 52; TITAN ‐ gave a combined HR of 0.79 favouring the taxane (95% CI 0.67 to 0.92; P = 0.003; Analysis 3.2), with no heterogeneity (I² = 0%; P = 0.42). Six studies (8433) provided a regimen of paclitaxel administered three‐weekly (Boccardo; CALGB 9344; ECTO; GONO MIG‐5; NSABP B‐28; Roy). Analysis of these studies revealed an HR of 0.85 (95% CI 0.79 to 0.92; P < 0.001; Analysis 3.2), with significant heterogeneity (I² = 62%; P < 0.02).
Sequential or concurrent taxane and anthracycline
Taxane treatment was given sequentially or concurrently with anthracycline, and it is unclear whether this scheduling impacts efficacy. Analysis was performed to examine this question. Toxicity differences between the two schedules are discussed separately.
Overall survival
Eighteen studies (24,764 women) administered the taxane and anthracycline sequentially in the experimental arm (ADEBAR; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216; TITAN; UK TACT). Analysis of these studies demonstrated an HR of 0.86 favouring the taxane‐containing group (95% CI 0.81 to 0.91; P < 0.001; Analysis 4.1), with no significant heterogeneity (I² = 17%; P = 0.25). Six studies (8839 women) administered the taxane and anthracycline concurrently in the experimental arm (BCIRG 001; BIG 2‐98; E2197; ECTO; GEICAM 9805; GONO MIG‐5). Analysis of these studies revealed an HR of 0.86, favouring the taxane‐containing group (95% CI 0.78 to 0.94; P = 0.002; Analysis 4.1), with no heterogeneity (P = 0.37).
Disease‐free survival
Nineteen studies with 20 treatment comparisons (26,866 women) administered the taxane and anthracycline sequentially in the experimental arm (ADEBAR; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216; TITAN; UK TACT). The estimated HR from analysis of these studies was 0.86, favouring the taxane‐containing group (95% CI 0.82 to 0.90; P < 0.001; Analysis 4.2), with moderate heterogeneity (I² = 51%; P = 0.005). Seven studies (9466 women) administered the taxane and anthracycline concurrently in the experimental arm (BCIRG 001; BIG 2‐98; E2197; ECTO; GEICAM 9805; GONO MIG‐5; RAPP‐01). The estimated HR was 0.89, favouring the taxane‐containing group (95% CI 0.83 to 0.97; P < 0.001; Analysis 4.2), with moderate heterogeneity (I² = 51%; P = 0.05). BIG 2‐98 reported both sequential and concurrent administration arms; thus separately reported arms were included in this analysis.
Addition of taxane or substitution of taxane
This analysis separated groups into those where a taxane was added to control chemotherapy studies (Question 1) and those where a taxane was substituted for part of the control chemotherapy (Question 3). This analysis was performed, as it has been postulated that benefit from taxane treatment could be due in part to the addition of an extra non‐cross‐resistant drug rather than to superior efficacy of the taxane itself.
Overall survival
Data were available for seven studies (10,842 women) designed such that the experimental arm received a taxane administered in addition to control chemotherapy (BIG 2‐98; CALGB 9344; ECTO; GOIM 9902; HeCOG; NSABP B‐28; Taxit 216). Analysis of these studies yielded an HR of 0.84, favouring the taxane‐containing group (95% CI 0.77 to 0.92; P < 0.001; Analysis 5.1), with no heterogeneity (P = 0.60). Data were available for 13 (16,196 women) of 15 eligible studies, and these studies were designed with the experimental arm given a taxane substituted for one or more of the drugs from the control (BCIRG 001; BIG 2‐98; DEVA; E2197; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; PACS 01; Roy; Sakr; TITAN; US Oncology 9735). This group also had an HR of 0.80 in favour of the taxane‐containing treatments (95% CI 0.74 to 0.86; P < 0.001; Analysis 5.1), with no significant heterogeneity (I² = 6%; P = 0.38).
Disease‐free survival
Seven studies (10,842 women) were designed such that the experimental arm received a taxane administered in addition to control chemotherapy (BIG 2‐98; CALGB 9344; ECTO; GOIM 9902; HeCOG; NSABP B‐28; Taxit 216). For these studies, an HR of 0.84 favoured the taxane‐containing group (95% CI 0.78 to 0.90; P < 0.001), with no heterogeneity (P = 0.66). Fourteen studies included 16,823 women and were designed with the experimental arm receiving a taxane substituted for one or more of the drugs from the control (BCIRG 001; BIG 2‐98; DEVA; E2197; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; PACS 01; RAPP‐01; Roy; Sakr; TITAN; US Oncology 9735). This group also had an HR of 0.83, in favour of taxane‐containing treatments (95% CI 0.78 to 0.88; P < 0.001), with moderate heterogeneity (I² = 47%; P = 0.03).
Duration of chemotherapy
Studies have been examined post‐hoc to examine whether a longer duration of chemotherapy in the taxane arm may explain the observed improvement in outcomes. Groups were divided according to duration of total planned treatment rather than the total number of planned cycles to account for variation in cycle length between studies. BIG 2‐98 included two taxane‐containing arms ‐ one with longer duration and the other with the same duration as the control arm. As these arms were reported separately for OS and DFS, they are included in the analyses below.
Overall survival
Nine studies (13,865 women) included a taxane‐containing experimental arm that was of longer duration than the control arm (BIG 2‐98; CALGB 9344; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NSABP B‐28; Taxit 216). Analysis of these studies revealed an HR of 0.84, favouring the taxane‐containing group (95% CI 0.77 to 0.91; P < 0.001; Analysis 6.1), with no heterogeneity (P = 0.69). Fourteen studies (18,660 women) were designed with a taxane‐containing experimental arm of the same duration as the control arm (ADEBAR; BCIRG 001; BIG 2‐98; CALGB 40101; E2197; ECTO; ELDA; FinHer; GEICAM 9805; PACS 01; Roy; Sakr; TITAN; US Oncology 9735). This group also had an HR of 0.87 in favour of taxane‐containing treatments (95% CI 0.81 to 0.94; P < 0.001; Analysis 6.1), with moderate heterogeneity (I² = 52%; P = 0.01).
Disease‐free survival
Nine studies (13,865 women) were designed to include a taxane‐containing experimental arm of longer duration than the control arm (BIG 2‐98; CALGB 9344; GEICAM 2003‐02; GEICAM 9906; GOIM 9902; HeCOG; HORG; NSABP B‐28; Taxit 216). The HR for DFS was 0.83, favouring the taxane‐containing group (95% CI 0.77 to 0.88; P < 0.001; Analysis 6.2), with no heterogeneity (P = 0.85). Sixteen studies (17 treatment comparisons) involving 21,391 women were designed with the taxane‐containing arm having the same duration as the control arm (ADEBAR; BCIRG 001; BIG 2‐98; CALGB 40101; E2197; ECTO; ELDA; FinHer; GEICAM 9805; NCIC‐CTG MA21a and NCIC‐CTG MA21b; PACS 01; RAPP‐01; Roy; Sakr; TITAN; US Oncology 9735). An HR of 0.90 in favour of the taxane‐containing arms was found for this group of studies (95% CI 0.85 to 0.96; P < 0.001; Analysis 6.2), with substantial heterogeneity (I² = 70%; P < 0.001).
Number of cycles of taxane‐containing chemotherapy
This post‐hoc analysis examined studies that administered three cycles of the taxane drug in comparison with studies that used four or more cycles of taxane in the experimental arm. This was done to determine if the number of cycles of taxane impacted efficacy. This analysis had limitations due to heterogeneity between studies with the use of different control regimens and varying doses and scheduling of the taxane drug. BIG 2‐98 included two taxane arms ‐ one administered three cycles of taxane, and the other four. These arms were separately reported for DFS and therefore were included separately in this analysis. Although TITAN administered paclitaxel weekly for 12 weeks, this is often considered as three weeks of paclitaxel (weekly) multiplied by four, and thus is classified as four cycles of taxane. GEICAM 2003‐02 and GEICAM 9906 used eight weekly doses of paclitaxel and could not be classified in either group. These results were not included in the analysis.
Overall survival
Seven studies (6551 women) used three cycles of taxane treatment in the experimental arm (BIG 2‐98; DEVA; FinHer; HeCOG; PACS 01; Roy; Sakr), reporting an HR of 0.77 favouring the taxane‐containing group (95% CI 0.69 to 0.86; P < 0.001; Analysis 7.1), with no heterogeneity (P = 0.60). Nineteen studies (29,458 women) ‐ ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; E2197; ECTO; ELDA; GEICAM 9805; GOIM 9902; GONO MIG‐5; HORG; ICE II‐GBG 52; NSABP B‐28; Taxit 216; TITAN; UK TACT; US Oncology 9735 ‐ used four or more cycles of taxane and found an HR of 0.91 in favour of taxane‐containing treatments (95% CI 0.86 to 0.96; P < 0.001; Analysis 7.1), with moderate heterogeneity (I² = 32%; P = 0.09).
Disease‐free survival
Seven studies (6551 women) used three cycles of taxane treatment in the taxane arm (BIG 2‐98; DEVA; FinHer; HeCOG; PACS 01; Roy; Sakr). Analysis of these studies revealed an HR of 0.80, favouring the taxane‐containing group (95% CI 0.73 to 0.88; P < 0.001; Analysis 7.2), with moderate heterogeneity (I² = 48%; P = 0.07). Twenty‐one studies (32,187 women) with 22 treatment comparisons ‐ ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; E2197; ECTO; ELDA; GEICAM 9805; GOIM 9902; GONO MIG‐5; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; RAPP‐01; Taxit 216; TITAN; UK TACT; US Oncology 9735 ‐ used four or more cycles of taxane and also found an HR for DFS of 0.91 in favour of taxane‐containing treatments (95% CI 0.87 to 0.95; P < 0.001; Analysis 7.2), with moderate heterogeneity (I² = 57%; P < 0.001).
Lymph node status
Variations in inclusion criteria between studies may have had an impact on the risk of recurrence. Analysis was performed to examine whether there was any indication that the benefit of taxane‐containing treatment was greater in studies that included only lymph node‐positive women as compared to studies that included both lymph node‐negative and lymph node‐positive women. It was identified that studies allowing participation of women without lymph node involvement generally required other high‐risk features for inclusion.
Overall survival
Seventeen studies included women with positive axillary lymph node metastasis (22,055 women; 4152 deaths) (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216). Analysis of these studies revealed an HR of 0.83, favouring the taxane‐containing group (95% CI 0.78 to 0.88; P < 0.001; Analysis 8.1), with nominal heterogeneity (I² = 3%; P = 0.41). Seven studies included participants both with and without lymph node metastases (10,269 women; 1952 deaths) (E2197; ECTO; ELDA; ICE II‐GBG 52; TITAN; UK TACT; US Oncology 9735). Analysis of these studies yielded an HR of 0.95, with taxane‐containing groups resulting reporting little to no difference in survival compared to non‐taxane‐containing groups (95% CI 0.87 to 1.04; P = 0.26; Analysis 8.1), with no significant heterogeneity (I² = 12%, P = 0.34). Three studies included women with no lymph node metastases (6856 women; 397 deaths) (CALGB 40101; GEICAM 2003‐02; GEICAM 9805); researchers found an HR of 1.08, indicating that the taxane‐containing treatment group showed little to no difference in survival compared to the control group (95% CI 0.89 to 1.32; P = 0.43; Analysis 8.1), with substantial heterogeneity (I² = 62%; P = 0.07).
Disease‐free survival
Seventeen studies included participants with positive axillary lymph node metastasis (22,055 women; 6575 events) (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 9344; DEVA; FinHer; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG; NSABP B‐28; PACS 01; Roy; Sakr; Taxit 216). The estimated HR was 0.84, favouring the taxane‐containing group (95% CI 0.80 to 0.88; P < 0.001; Analysis 8.2), with moderate heterogeneity (I² = 32%; P = 0.10). Nine studies with 10 treatment comparisons included women both with and without lymph node involvement (12,998 women; 2929 events) (E2197; ECTO; ELDA; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; RAPP‐01; TITAN; UK TACT; US Oncology 9735). The estimated HR was 0.95 and did not demonstrate a difference in risk of disease progression between taxane and non‐taxane groups (95% CI 0.88 to 1.02; P = 0.15; Analysis 8.2), with moderate heterogeneity (I² = 55%; P = 0.02). Three studies ‐ CALGB 40101; GEICAM 2003‐02; GEICAM 9805 ‐ included only women with negative lymph nodes (6856 women; 767 events); the pooled analysis did not demonstrate a difference in risk of disease progression between taxane‐containing and control chemotherapy regimens, with an estimated HR of 0.99 (95% CI 0.86 to 1.14; P = 0.85; Analysis 8.2), with substantial heterogeneity (I² = 87%; P < 0.001).
Hormone receptor status
This post‐hoc analysis examined studies that reported treatment effects by subgroups for hormone receptor status. This analysis was performed to see whether there was any indication of benefit for taxane‐containing regimens in hormone receptor‐positive women as compared to women with hormone receptor‐negative tumours. Fifteen studies, 16 treatment comparisons, did not test or adequately report the effects of taxanes by hormone receptor subgroup for time‐to‐event analysis (ADEBAR; CALGB 40101; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9906; HeCOG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; RAPP‐01; Roy; Sakr; Taxit 216).
Overall survival
Five studies reported sufficient data for the subgroups of hormone receptor‐positive and ‐negative status (BCIRG 001; BIG 2‐98; GONO MIG‐5; PACS 01; TITAN). The subgroup of participants with hormone receptor‐positive tumours showed an HR of 0.79, in favour of taxane‐containing treatments (95% CI 0.70 to 0.89; P < 0.001; Analysis 9.1), with no heterogeneity (P = 0.61). The subgroup of participants with hormone receptor‐negative tumours showed that taxane‐containing regimens resulted in little to no difference in survival compared to non‐taxane‐containing regimens (HR 0.88, 95% CI 0.73 to 1.05; P = 0.15; Analysis 9.1), with no heterogeneity (P = 0.56).
Disease‐free survival
Eleven studies published sufficient data on hormone receptor subgroups (BCIRG 001; BIG 2‐98; Boccardo; CALGB 9344; DEVA; E2197; GEICAM 9805; GOIM 9902; HORG; UK TACT; US Oncology 9735). For the subgroup of participants with hormone receptor‐positive (or oestrogen receptor‐positive only in some studies: DEVA; E2197; GOIM 9902; HORG; UK TACT) tumours, taxane‐containing regimens appeared to reduce the risk of disease recurrence compared to non‐taxane‐containing regimens, with an HR of 0.91 (95% CI 0.85 to 0.97; P = 0.005; 11 studies; 3367 participants; Analysis 9.2), although with substantial heterogeneity (I² = 56%; P = 0.01). The subgroup of participants with hormone receptor‐negative tumours was found to have a similar result in favour of the taxane‐containing regimen, with an HR of 0.80 (95% CI 0.73 to 0.88; P < 0.001; 12 studies; 1581 participants; Analysis 9.2), with no heterogeneity (P = 0.87).
Publication status
For a sensitivity analysis conducted to assess publication bias, studies with fully published efficacy papers were examined as a separate group from those published in non‐peer‐reviewed format only (i.e. abstracts or online theses).
Overall survival
Twenty‐five studies (38,208 women) had a published efficacy paper (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; DEVA; E2197; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG: NSABP B‐28; PACS 01; Roy; Sakr; TITAN; UK TACT; US Oncology 9735), and the HR of 0.88 favoured taxane‐containing treatment (95% CI 0.84 to 0.92; P < 0.001; Analysis 10.1), with moderate heterogeneity (I² = 33%; P = 0.05). One study (972 women) ‐ Taxit 216 ‐ had data available in an online thesis and indicated that the treatment effect persisted with an HR of 0.67, in favour of taxane‐containing treatment (95% CI 0.48 to 0.94; P = 0.05) (Analysis 10.1).
Disease‐free survival
Twenty‐seven studies with 28 treatment comparisons (40,310 women) included in this analysis had a published efficacy paper (ADEBAR; BCIRG 001; BIG 2‐98; Boccardo; CALGB 40101; CALGB 9344; DEVA; E2197; ECTO; ELDA; FinHer; GEICAM 2003‐02; GEICAM 9805; GEICAM 9906; GOIM 9902; GONO MIG‐5; HeCOG; HORG; ICE II‐GBG 52; NCIC‐CTG MA21a and NCIC‐CTG MA21b; NSABP B‐28; PACS 01; Roy; Sakr; TITAN; UK TACT; US Oncology 9735), estimating the HR as 0.88, favouring the taxane‐containing group (95% CI 0.85 to 0.92; P < 0.001; Analysis 10.2), with substantial heterogeneity (I² = 62%; P < 0.001). Two studies (1599 women) had data available in abstracts or online theses (RAPP‐01; Taxit 216), reporting an HR for DFS of 0.84 (95% CI 0.67 to 1.04; P = 0.10; Analysis 10.2), with no heterogeneity (P = 0.42).
A funnel plot did not support any publication bias for the studies reviewed (Figure 5).
Funnel plot of comparison: 1 Overall effect of taxanes, outcome: 1.1 Overall survival ‐ all studies.
Toxicity
Toxic effects of taxane therapy have been well characterised and were expected to be observed in the taxane‐containing arms. We noted heterogeneity between studies with the use of different control chemotherapies and varying doses and scheduling of the taxane drug. This heterogeneity needs to be considered on a trial‐by‐trial basis to interpret the tolerability of each taxane‐containing regimen. Toxicity data were extracted and combined for analysis.
Of the 29 included studies, 28 provided extractable data on toxicity. No toxicity data could be extracted from Roy studies. Data were extracted and analysed for febrile neutropenia, grade 3 or 4 neuropathy (sensory and motor), grade 3 or 4 fatigue, grade 3 or 4 stomatitis, cardiotoxicity, grade 3 or 4 nausea and/or vomiting, and secondary leukaemia or myelodysplasia. These outcomes are illustrated in Analysis 11 (Analysis 11.1 to Analysis 11.10). Definitions of toxicity varied between studies, and the specific definitions from each study are reported in Table 2. When toxicity was reported as less than 1%, it was treated as 0% for the purposes of statistical comparison. It was not possible to determine the treated population (i.e. only those women receiving chemotherapy) in CALGB 9344. In this case, the randomised population was used for the denominator when the odds ratio was calculated.
| Trial | Cardiotoxicity | Febrile neutropenia | Neuropathy | Nausea/vomiting | Fatigue | Stomatitis |
| Grade 3/4 cardiac symptoms: NCI CTC (version 2) | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 neurological symptoms: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Congestive heart failure (cardiac function grade 3 or 4) | Reported as "febrile neutropenia": Protocol definition ‐ fever of grade 2 or more concomitant with grade 4 neutropenia requiring IV antibiotics, hospitalisation, or both | Grade 3/4 neurosensory effects: NCI CTC (version 1) | Grade 3/4 or severe vomiting: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiac function toxicity | Reported as "febrile neutropenia": protocol defined grade 4 neutropenia, fever with > 38 degrees Celsius, NCI CTC (version 1) | Grade 3/4 neurosensory effects: NCI CTC (version 1) | Grade 3/4 or severe vomiting: NCI CTC (version 1) | Grade 3 or higher asthenia: NCI CTC (version 1) | Grade 3 or higher stomatitis: NCI CTC (version 1) | |
| Grade 3/4 cardiotoxicity: WHO toxicity criteria | NR | Grade 3/4 peripheral neuropathy: WHO toxicity criteria | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| Grade 3 or higher cardiotoxicity (left ventricular systolic dysfunction, restrictive cardiomyopathy, general cardiac, cardiac deaths attributed to protocol treatment ‐ myocardial infarction and left ventricular dysfunction) | Reported as grade 3/4 febrile neutropenia: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | NR | |
| Congestive heart failure during treatment or post treatment follow‐up | NR | NR | NR | NR | NR | |
| Diagnosed congestive cardiac failure | Grade 3/4 febrile neutropenia: NCI CTC (version 2) | Grade 3/4 peripheral neuropathy: NCI CTC (version 2) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 congestive heart failure | Reported as "febrile neutropenia": toxicity criteria not specified | Grade 3/4 neuropathy (reported both sensory and motor) | Grade 3 or higher vomiting: toxicity criteria not specified | Grade 3 or higher fatigue: toxicity criteria not specified | Grade 3 or higher stomatitis: toxicity criteria not specified | |
| Symptomatic cardiac failure | NR | Grade 3 neuropathy reported only: NCI CTC (no version provided) | NR | NR | NR | |
| Grade 3/4 heart rhythm toxicity | Reported as "febrile neutropenia": NCT CTC (version 2) | Grade 3/4 neuropathy: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 mucositis: NCI CTC (version 2) | |
| NR | Reported as "neutropenic fever": NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiac dysfunction, Grade 4 cardiac ischaemia, Grade 3 arrhythmia | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 sensory neuropathy: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Grade 3/4 arrhythmia, Grade 3/4 congestive heart failure | Reported as "febrile neutropenia": protocol defined fever Grade 2 or higher with Grade 4 neutropenia requiring intravenous antibiotics, hospitalisation, or both | Grade 3/4 peripheral neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| NR | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 1) | Grade 3 peripheral sensory neuropathy: NCI CTC (version 1) | Grade 3/4 nausea and vomiting: NCI CTC (version 1) | Grade 3/4 fatigue: NCI CTC (version 1) | Grade 3/4 mucositis: NCI CTC (version 1) | |
| Reversible cardiotoxicity | Reported as "neutropenic fever" grade 3/4: NCI CTC (version 3) | Grade 3/4 neurotoxicity: NCI CTC (version 3) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 3) | NR | Grade 3/4 mucositis: NCI CTC (version 3) | |
| Grade 3/4 cardiotoxicity: WHO criteria | Reported as "febrile neutropenia" grade 3/4: WHO criteria | Grade 3/4 neurological toxicity: WHO criteria | Grade 3/4 nausea and vomiting: WHO criteria | NR | Grade 3/4 stomatitis: WHO criteria | |
| NR | Reported as "febrile neutropenia": WHO toxicity criteria | Grade 3/4 peripheral neuropathy: WHO toxicity criteria | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | Grade 3/4 fatigue: WHO toxicity criteria | NR | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neurotoxicity: NCI CTC (version 2) | Grade 3/4 nausea: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3 to 5 congestive heart failure and cardiac ischaemia | Reported as "febrile neutropenia" grade 1 to 5: NCI CTC (version 3) | Grade 3 to 5 sensory neuropathy: NCI CTC version 3 | Grade 3 to 5 vomiting: NCI CTC (version 3) | Grade 3 to 5 fatigue: NCI CTC (version 3) | Grade 3 to 5 mucositis, stomatitis, oesophagitis: NCI CTC (version 3) | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3 or higher cardiac dysfunction | NR | NR | NR | NR | NR | |
| Any serious adverse cardiac event | Reported as "febrile neutropenia": WHO toxicity criteria | NR | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| NR | Reported as "febrile neutropenia" defined as any Grade 3/4 neutropenia plus fever (> 38 degrees) requiring antibiotics: NCI CTC (version 2) | NR | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | NR | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Grade 3/4 cardiac event | Reported as "febrile neutropenia" Grade 3/4: WHO toxicity criteria | NR | Grade 3/4 nausea‐vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| NR | Reported as "febrile neutropenia" Grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy: NCI CTC (version 2) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | Grade 3/4 lethargy: NCI CTC (version 2) | Grade 3/4 stomatitis (mucositis): NCI CTC (version 2) | |
| Cardiac function toxicity | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Cardiac events | Reported as "febrile neutropenia": NCI CTC (version 3) | Grade 3/4 peripheral neuropathy: NCI CTC (version 3) | Grade 3/4 nausea (vomiting not reported): NCI CTC (version 3) | Grade 3/4 fatigue: NCI CTC (version 3) | NR | |
| Death due to cardiac event | Reported as "febrile neutropenia" Grade 3/4 : NCI CTC (version 1) | NR | Grade 3/4 vomiting: NCI CTC (version 1) | Grade 3/4 asthenia: NCI CTC (version 1) | Grade 3/4 stomatitis: NCI CTC (version 1) |
NCI CTC: National Cancer Institute Common Toxicity Criteria
NR: not reported
WHO: World Health Organziation
Febrile neutropenia
Twenty‐four studies with 25 treatment comparisons provided data on febrile neutropenia. Other myelosuppression toxicity data were reported in multiple studies (grade 3 to 4 neutropenia, grade 3 to 4 infection, and infection requiring antibiotics); however, febrile neutropenia was the most consistently reported outcome. Pooled analysis of these studies found that taxane‐containing regimens probably resulted in a small increase in risk of febrile neutropenia compared to non‐taxane‐containing regimens (OR 1.55, 95% CI 0.96 to 2.49; P = 0.07; 33,763 participants; 24 studies (25 treatment comparisons); moderate‐certainty evidence; Analysis 11.1; Figure 6). The risk was highest for studies that administered the taxane concurrently with an anthracycline (OR 5.76, 95% CI 3.36 to 9.87; P < 0.001; 8552 women; 6 studies; Analysis 11.1), with substantial heterogeneity (I² = 86%; P < 0.001) rather than sequential taxane and anthracycline treatment (OR 1.31, 95% CI 0.82 to 2.10; P = 0.26; 20,148 women; 15 studies (16 treatment comparisons); Analysis 11.1), also with substantial heterogeneity (I² = 93%; P < 0.001). There was little to no difference in the risk of febrile neutropenia among studies that compared a taxane replacement for an anthracycline versus control (OR 0.22, 95% CI 0.02 to 3.04; P = 0.26; 5063 women; 3 studies; Analysis 11.1), and heterogeneity was substantial (I² = 96%; P < 0.001). The test for differences between subgroups was significant (P < 0.001).
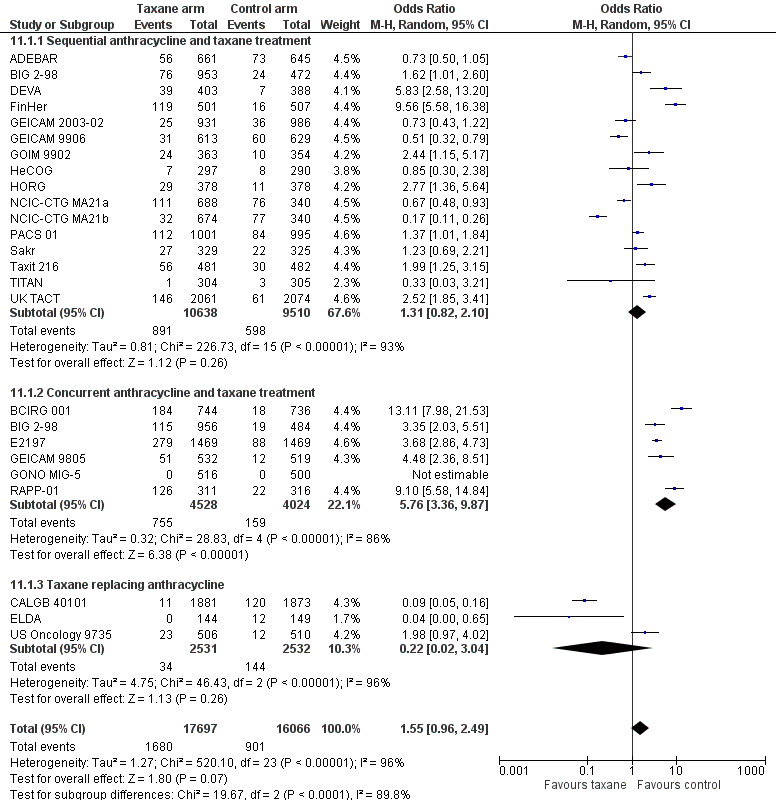
Forest plot of comparison: 11 Toxicities, outcome: 11.1 Febrile neutropenia by sequential or concurrent anthracycline/taxane.
A subgroup analysis indicated that increased risk of febrile neutropenia for taxane‐containing regimens (compared to non‐taxane regimens) appeared to be driven by docetaxel rather than paclitaxel (Analysis 11.2). Docetaxel‐containing regimens were likely to increase the risk of febrile neutropenia compared to non‐taxane regimens (OR 2.83, 95% CI 1.88 to 4.27; P < 0.001; 22,596 women; 16 studies; significant heterogeneity: I² = 92%; P < 0.001; Analysis 11.2.1), and paclitaxel‐containing regimens were likely to reduce the risk of febrile neutropenia compared to non‐taxane regimens (OR 0.36, 95% CI 0.19 to 0.67; P < 0.001; 11,558 women; 8 studies (9 treatment comparisons); substantial heterogeneity (I² = 88%; P < 0.001). The test for differences between subgroups was significant (P < 0.001).
Grade 3/4 neuropathy
Twenty‐two studies with 23 treatment comparisons reported grade 3 or 4 neuropathy. Of these, four studies provided data on neurosensory neuropathy only, six studies reported peripheral neuropathy only, seven studies reported sensory and motor neuropathy separately, and six reported neurotoxicity (no further specifics provided). Taxane‐containing regimens likely resulted in a large increase in neuropathy compared to controls (OR 6.89, 95% CI 3.23 to 14.71; P < 0.001; 31,033 women; moderate‐certainty evidence; Analysis 11.3), with substantial heterogeneity (I² = 82%; P < 0.001).
A subgroup analysis suggested that the increase in risk of grade 3 or 4 neuropathy for taxane‐containing regimens compared to non‐taxane regimens tended to be worse for women receiving paclitaxel than docetaxel (Analysis 11.4); however the confidence intervals were very wide. For women receiving paclitaxel, the OR was 11.93 (95% CI 3.59 to 39.70; 10 studies (11 treatment comparisons); 12,678 women; substantial heterogeneity; I² = 85%; P < 0.001; Analysis 11.4.2), and for docetaxel, the OR was 3.74 (95% CI 1.33 to 10.53; 11 studies, 18,355 women; substantial heterogeneity; I² = 79%; P < 0.001; Analysis 11.4.1). The test for difference between subgroups was not statistically significant (P = 0.15).
Grade 3/4 fatigue
Sixteen studies were pooled for analysis of grade 3 or 4 fatigue. Taxane‐containing regimens likely increased the risk of fatigue compared to non‐taxane‐containing regimens (OR 1.81, 95% CI 1.31 to 2.49; P < 0.001; 25,003 women; Analysis 11.5), with substantial heterogeneity (I2 = 83%; P < 0.001).
Grade 3/4 stomatitis
Twenty‐two studies with 23 treatment comparisons assessed grade 3 or 4 stomatitis. Pooled analysis of these studies revealed that taxane‐containing regimens likely resulted in little to no difference in stomatitis compared to controls (OR 1.29, 95% CI 0.93 to 1.78; P = 0.12; 22,648 women; Analysis 11.6), with substantial heterogeneity (I² = 81%; P < 0.001).
A subgroup analysis indicated that the docetaxel‐containing regimens were likely to increase the risk of stomatitis compared to control regimens (OR 1.73, 95% 1.28 to 2.35; 16 studies; 22648 participants), with substantial heterogeneity (I² = 73%; P < 0.001), and paclitaxel‐containing regimens were likely to result in little to no difference in grade 3/4 stomatitis compared to control regimens (OR 0.64, 95% CI 0.31 to 1.32; 7 studies; 6852 participants), with substantial heterogeneity (I² = 81%; P < 0.001). The test for difference between subgroups was significant (P = 0.01).
Cardiotoxicity
Twenty‐three studies provided extractable data on cardiotoxicity. Pooled analysis of these studies indicated that administering taxane‐containing regimens probably resulted in little to no difference in cardiotoxicity compared to non‐taxane‐containing regimens (OR 0.87, 95% CI 0.56 to 1.33; 32,894 participants; 23 studies; moderate‐quality evidence; Analysis 11.7). When the same planned dose of anthracycline was used in the taxane‐containing arm and in the control arm, there was no difference in the risk of cardiotoxicity between groups (HR 1.27, 95% CI 0.88 to 1.84; 14,967 women; 9 studies; Analysis 11.7), with little heterogeneity (I² = 24%; P = 0.24). Risk of cardiotoxicity was reduced in studies that provided a lower planned dose of anthracycline in the taxane arm than in the control arm (OR 0.39, 95% CI 0.18 to 0.86; 12,473 women; 10 studies; Analysis 11.7), with little heterogeneity (I² = 24%; P = 0.22). Four studies were designed with the taxane replacing the anthracycline and showed little to no difference in cardiotoxicity between treatment groups (OR 1.01, 95% CI 0.26 to 3.91; 5454 women; 4 studies; Analysis 11.7), with moderate heterogeneity (I² = 58%; P = 0.07). The test for differences between subgroups was significant (P = 0.03).
Grade 3/4 nausea and/or vomiting
Twenty‐five studies with 26 treatment comparisons were pooled for analysis of grade 3 or 4 nausea and/or vomiting. If studies did not report nausea/vomiting, we included data related to vomiting only if reported (refer to Table 2). Administration of taxane‐containing regimens likely resulted in little to no difference in nausea/vomiting compared to non‐taxane‐containing regimens (OR 0.83, 95% CI 0.67 to 1.04; 34,450 women; moderate‐certainty evidence; Analysis 11.8), with substantial heterogeneity (I² = 77%; P < 0.001).
Secondary leukaemia or myelodysplasia
A total of 86 cases of secondary leukaemia or myelodysplasia were reported from 18 studies, with 19 treatment comparisons: 39 cases from taxane‐containing regimens and 47 from control regimens. There was little to no difference in the risk of developing secondary leukaemia or myelodysplasia between groups (OR 0.85, 95% CI 0.54 to 1.33; 33,225 women; Analysis 11.9), with no heterogeneity (P = 0.62).
Two studies reported on the development of other second malignancies. In PACS 01, 14 women in the taxane‐containing arm and 20 women in the control arm developed a second cancer, and in HeCOG, this occurred in five women from the taxane‐containing arm and in four women from the control arm.
Treatment‐related death
Treatment‐related death was uncommon for both taxane‐containing and non‐taxane‐containing groups. Treatment‐related deaths were defined in the trials as "toxic death", "treatment‐related death", and "death occurring during treatment or within 30 days post‐treatment". In all, 68 treatment‐related deaths were recorded from 22 studies during chemotherapy: 35 from taxane‐containing regimens and 33 from control regimens. Administering taxane‐containing regimens resulted in little to no difference in treatment‐related deaths compared to non‐taxane‐containing regimens (OR 1.24, 95% CI 0.63 to 2.47; 34,882 women; 22 studies; Analysis 11.10), with little heterogeneity (I² = 26%, P = 0.17). One trial reported deaths without describing the causes (three women; CALGB 9344). Several studies reported additional deaths but excluded these as they were not considered related to treatment.
For the purposes of this review, we have focused on the aforementioned toxicities commonly reported across most trials. Other reported toxicities ranged from grade 3/4 myalgia or arthralgia, anaemia, allergy, or oedema, to neurotoxicity, but reporting on the frequency of all of these toxicities was beyond the remit of this review.
Quality of life
Seven studies reported low‐certainty evidence for quality of life (QoL) (ADEBAR; BCIRG 001; DEVA; ELDA; GEICAM 9805; HeCOG; UK TACT); details of these findings are summarised in Table 3. NCIC‐CTG MA21 ‐ NCIC‐CTG MA21a and NCIC‐CTG MA21b ‐ stated that QoL data would be reported in a separate article. BCIRG 001 demonstrated a transient reduction in both treatment arms; the reduction in QoL score was greater in the taxane‐containing regimen, but by first follow‐up, both treatment arms had returned to baseline. Similarly, in ADEBAR, both groups had decreased scores on the EORTC questionnaire used to assess quality of life in cancer patients (EORTC‐C30), and changes in scores on the breast cancer‐specific EORTC QoL questionnaire (EORTC BR23) over time were similar in the two groups. DEVA and HeCOG did not report any differences in QoL scores between treatment arms either at the beginning or at the end of chemotherapy. ELDA reported no differences in global QoL scores, functioning scales, and other items; however, there was worsening of systemic therapy side effects in the docetaxel group compared to the CMF group at the end of one or more cycles (ELDA). GEICAM 9805 reported decreased QoL in the taxane‐containing group compared to the control group, but this was resolved by week 44, and there were no significant differences between groups during the follow‐up period. UK TACT noted a reduction in QoL global, physical, emotional functioning, social functioning, and fatigue scores compared to the control group; more nausea and vomiting was reported in the control group than in the taxane‐containing group.
| Trial ID | Instruments used | Summary of findings |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 at baseline, before each course of chemotherapy, and at 4 weeks, 6 weeks, and 6 months after completion of chemotherapy | Both treatment groups had decreased EORTC‐C30 scores for physical, role, emotional, social, and cognitive functioning from baseline to 28 days after chemotherapy. Global health scores also decreased in this time frame for both groups. Changes in symptom scores were generally similar between treatment groups with the exception of nausea/vomiting and pain scores. Nausea/vomiting was worse for the FEC120 group than for the EC‐Doc group (+9.42 above baseline vs +1.88), and pain scores were worse in the EC‐Doc group (+9.18 for EC‐Doc vs ‐1.61 for FEC120). Changes in items on the EORTC BR23 over time were quantitatively and qualitatively similar in the 2 treatment groups | |
| Patients completed EORTC QLQ‐C30 (version 2.0) and the breast cancer‐specific QLQ‐BR23 (version 1.0) questionnaires at baseline, before cycles 3 and 5, at 3 to 4 weeks after completion, and at 6, 12, and 24 months | Both FAC and TAC arms led to a transient and statistically significant reduction in QoL scores, which returned to baseline levels at first follow‐up visit. QoL measures were similar between groups and were similar to baseline measures at 6 months and at the end of 2 years | |
| QoL results designed to study short‐ and long‐term toxicities on quality of life by treatment agent and duration. No further details provided | QoL to be reported in a companion study | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 in the clinic at baseline and 9 months, 2 years, and 5 years after random assignment | No significant difference in overall QoL or across any of the scales between treatments at 9 months, 2 years, or 5 years | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ BR23 at baseline, and at the end of the first, second, and third cycles of chemotherapy | No difference reported for QoL global functioning scales and other items. However there was worsening of systemic therapy side effects, future perspective, nausea and vomiting, diarrhoea, appetite loss, and upset by hair loss and body experience with docetaxel compared to CMF at the end of 1 or more cycles | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 at baseline after each chemotherapy cycle and at 6, 12, and 24 months after completion of chemotherapy | During chemotherapy, QoL decreased and was worse with TAC than with FAC, but this effect resolved by week 44. There were no statistically significant differences in QoL between TAC+G‐CSF and FAC after cycle 6 or during further follow‐up | |
| Trial registry record indicates that QoL will be compared across treatment arms. No further details provided | QoL information not yet reported | |
| Patients completed EORTC QLQ‐C30 questionnaire at baseline and on completion of chemotherapy | Only 23% of participants (72 participants in E‐T‐CMF and 67 participants in E‐CMF) completed baseline and end of chemotherapy questionnaires. There was no difference between the 2 treatment arms at the beginning or at the end of chemotherapy. Significant increase in nausea and vomiting for both groups. Social functioning significantly decreased in the taxane‐containing arm, and emotional functioning and pain significantly improved in the control arm | |
| NCIC‐CTGMA21 (NCIC‐CTG MA21a; NCIC‐CTG MA21b) | Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR within 7 days before random assignment and afterwards | QoL results will be reported in a separate article |
| Selected centres invited participants to quality of life substudy. These patients completed EORTC QLQ‐C30, QLQ‐BR23, and Hospital Anxiety and Depression Scale (HADS) before randomisation, before fifth and after eighth cycles, and at 9, 12, 18, and 24 months post chemotherapy | FEC‐D was associated with worse global QoL (P = 0.001), physical (P < 0.0001), role (P = 0.002), emotional functioning (P = 0.008), social functioning (P = 0.003), pain (P = 0.001), and fatigue (P = 0.006) compared to control. In contrast, there was more nausea and vomiting in the control group (P = 0.01) than in the FEC‐D group |
CMF: cyclophosphamide, methotrexate and fluorouracil
E‐CMF: epirubicin‐ cyclophosphamide, methotrexate and fluorouracil
EC‐Doc: epirubicin, cyclophosphamide‐docetaxel
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer (EORTC) core Quality of Life Questionnaire C30 (QLQ‐C30)
QLQ‐BR23: breast cancer‐specific Quality of Life Questionnaire BR23
E‐T‐CMF: epirubicin‐paclitaxel‐cyclophosphamide, methotrexate, fluorouracil
FAC: fluorouracil, doxorubicin, cyclophosphamide
FEC‐D: fluorouracil, epirubicin, cyclophosphamide ‐ docetaxel
TAC: docetaxel, doxorubicin, cyclophosphamide
Cost‐effectiveness
One study presented data on cost‐effectiveness, finding that the extra cost of anthracycline plus docetaxel treatment compared to anthracycline alone was offset by the lower rate of recurrence in the anthracycline plus docetaxel group (PACS 01). The cost per quality‐adjusted life‐year (QALY) gained by adding the taxane was reported to be €2372 (upper CI €55,515). This value was reported to be a cost‐effective alternative.
Sensitivity analysis
Low versus high or unclear risk of bias
Post‐hoc subgroup analyses were conducted to investigate treatment effects in studies with low risk of bias compared to studies with unclear/high risk of bias. Of the 29 studies, 23 studies (24 treatment comparisons) were considered to be at low risk overall. Six studies were grouped as having unclear or high risk of bias overall (FinHer; GEICAM 9906; PACS 01; Roy; Sakr; US Oncology 9735).
Overall survival
Analysis of low risk of bias studies demonstrated an HR of 0.90, favouring the taxane‐containing group (95% CI 0.86 to 0.95; P < 0.001; Analysis 12.1), with moderate heterogeneity (I² = 28%; P = 0.12). For the six studies categorised as having unclear or high risk of bias, the HR favoured taxane‐containing regimens (HR 0.74, 95 CI 0.65 to 0.83; P < 0.001; Analysis 12.1), with no heterogeneity (P = 0.68).
Disease‐free survival
Twenty‐three low risk of bias studies (24 treatment comparisons) had data available for this outcome and showed an HR of 0.90 in favour of taxane‐containing regimens (95% CI 0.87 to 0.94; P < 0.001; 35,935 women; Analysis 12.2). Heterogeneity was moderate (I² = 57%; P < 0.001). For the six studies judged as having unclear or high risk of bias, an HR of 0.76 favoured taxane‐containing regimens (95% CI 0.69 to 0.84; P < 0.001; 5974 women), with moderate heterogeneity (I² = 42%; P = 0.12).
Discussion
Summary of main results
This review update provides high‐quality evidence supporting the conclusion that use of a taxane drug as part of the adjuvant chemotherapy regimen following surgery for early‐stage breast cancer in women with moderate to high risk of recurrence leads to improvement in overall survival (OS) and disease‐free survival (DFS) (see summary of findings Table for the main comparison). Among these women, the hazard ratio for OS was 0.87, and for DFS 0.88, favouring use of a taxane as part of the adjuvant chemotherapy regimen when compared to adjuvant regimens that did not contain a taxane.
This review included 29 studies (totaling 41,911 randomised women, 6501 deaths, and 10,271 DFS events), which is an adequate number to establish conclusive results. With the addition of 17 new studies and updated follow‐up data from previously included studies, little evidence suggests that the hazard ratio will change over time. We identified three ongoing studies, and there may be further unpublished studies that have not been identified for this review update. It is possible that publication bias in favour of significant findings may have led to an overestimation of the overall treatment effect; however, the funnel plot does not support this.
Overall, taxane‐containing regimens were well tolerated but increased the risk of some adverse events. Moderate‐quality evidence shows that there was a difference in toxicity between taxane‐containing regimens and non‐taxane‐containing regimens. This is not unexpected based on established toxicity profiles for the taxane drugs. A small increase in rates of febrile neutropenia, neuropathy, and fatigue was evident for the taxane‐containing regimens. Researchers have noted no significant differences in the risk of developing cardiotoxicity, stomatitis, nausea and/or vomiting, secondary haematological or other second cancers, nor treatment‐related death. Less cardiotoxicity was seen with taxane‐containing regimens that employed less anthracycline than was seen with the control intervention. Seven studies reported quality of life (QoL) results. Overall, low‐quality evidence suggests no differences in QoL between groups during follow‐up.
The subgroup analysis looked at the relative efficacy for each type of taxane. For docetaxel and paclitaxel, the hazard ratio for OS was 0.86 (range 0.81 to 0.92) and 0.89 (range 0.82 to 0.96), respectively; and for DFS the hazard ratio was 0.87 (range 0.82 to 0.91) and 0.91 (range 0.85 to 0.96), respectively. No conclusions can be drawn about the relative efficacy of the two agents without a direct comparison. Such direct comparisons are underway, and some results have been published.
Overall completeness and applicability of evidence
This review provides preliminary evidence for restricting adjuvant taxane chemotherapy to lymph node‐positive women and shows that efficacy appeared equivalent in trials that included both node‐positive and node‐negative groups and only node‐negative groups. The absolute recurrence risk for an individual patient must be considered when one is making clinical decisions on the use of a taxane‐containing adjuvant chemotherapy regimen. Limited evidence suggests that taxane chemotherapy restricted to women with hormone receptor‐positive breast cancer could provide additional benefit in terms of survival, but for disease‐free survival, efficacy in trials of hormone receptor‐positive and hormone receptor‐negative groups is unclear. Further questions that are beyond the scope of this review include efficacy in women with more than four involved axillary lymph nodes and the role of human epidermal growth factor receptor 2 (HER2) in breast cancer treatment.
Quality of the evidence
This updated review contains 29 included studies involving over 41,000 women. High‐quality evidence supports the use of taxane‐containing regimens in the adjuvant setting with an increase in survival time and in time free of disease recurrence. Some clinical heterogeneity between studies was evident, with variation in choice of control chemotherapy, doses, and treatment scheduling. Post‐hoc analyses in a side‐by‐side comparison of pooled data were performed for several subgroups. Benefit appears to be no less when the taxane is substituted for part of the control regimen as opposed to adding it to the control regimen. This is also the case when taxane regimens of the same duration are compared with regimens of longer duration than the control regimen, when three cycles of taxane treatment rather than four or more are compared, and when sequential anthracycline and taxane combination is given rather than concurrent administration.
Potential biases in the review process
For the review update, trialists were contacted if data were not fully reported in the full‐text article or if no information aside from a conference proceeding abstract was available. We tested whether publication status (i.e. full text vs non‐peer‐reviewed information) had an impact on the effect estimate. It was reassuring that benefit from taxanes in the adjuvant setting persisted when data for overall survival were analysed; however, this benefit was not sustained for disease‐free survival, with data from abstracts, unpublished manuscripts, or online theses (related to four studies) showing no added benefit from taxanes compared to control interventions.
Agreements and disagreements with other studies or reviews
Results of this meta‐analysis are in accordance with the findings of previous meta‐analyses (Bria 2006; Qin 2011). The Bria 2006 meta‐analysis included nine studies with 15,598 and 15,074 women analysed for DFS and OS, respectively. Bria 2006 reported the risk ratios for DFS and OS as 0.86 (95% CI 0.81 to 0.90) and 0.87 (95% CI 0.81 to 0.93), in favour of the taxane‐containing treatment group (Bria 2006). The meta‐analysis by Bria et al included the MD Anderson CC trial, which was excluded from this Cochrane Review as published efficacy data did not distinguish results for adjuvant patients from those for neoadjuvant patients. Nineteen additional trials were included in the Cochrane Review, which provides even stronger supporting evidence for the efficacy of taxane‐containing regimens. Similarly, Qin 2011 reported a reduction in the number of deaths and in risk of disease recurrence for women receiving adjuvant taxane‐containing chemotherapy compared to those given chemotherapy without taxanes, and reported similar toxicity profiles as we have provided in our updated Cochrane Review.

Review update: study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Overall effect of taxanes, outcome: 1.1 Overall survival ‐ all studies.

Forest plot of comparison: 1 Overall effect of taxanes, outcome: 1.2 Disease‐free survival: all studies.

Funnel plot of comparison: 1 Overall effect of taxanes, outcome: 1.1 Overall survival ‐ all studies.

Forest plot of comparison: 11 Toxicities, outcome: 11.1 Febrile neutropenia by sequential or concurrent anthracycline/taxane.

Comparison 1 Overall effect of taxanes, Outcome 1 Overall survival ‐ all studies.

Comparison 1 Overall effect of taxanes, Outcome 2 Disease‐free survival: all studies.

Comparison 1 Overall effect of taxanes, Outcome 3 Disease‐free survival: as defined in Cochrane protocol.

Comparison 2 Type of taxane, Outcome 1 Overall survival.

Comparison 2 Type of taxane, Outcome 2 Disease‐free survival.

Comparison 3 Weekly or three‐weekly paclitaxel, Outcome 1 Overall survival.

Comparison 3 Weekly or three‐weekly paclitaxel, Outcome 2 Disease‐free survival.

Comparison 4 Sequential or concurrent anthracycline/taxane, Outcome 1 Overall survival.

Comparison 4 Sequential or concurrent anthracycline/taxane, Outcome 2 Disease‐free survival.

Comparison 5 Addition or substitution of taxane, Outcome 1 Overall survival.

Comparison 5 Addition or substitution of taxane, Outcome 2 Disease‐free survival.

Comparison 6 Duration of chemotherapy, Outcome 1 Overall survival.

Comparison 6 Duration of chemotherapy, Outcome 2 Disease‐free survival.

Comparison 7 Number of cycles of taxane‐containing chemotherapy, Outcome 1 Overall survival.

Comparison 7 Number of cycles of taxane‐containing chemotherapy, Outcome 2 Disease‐free survival.

Comparison 8 Lymph node status, Outcome 1 Overall survival.

Comparison 8 Lymph node status, Outcome 2 Disease‐free survival.

Comparison 9 Hormone receptor status, Outcome 1 Overall survival.

Comparison 9 Hormone receptor status, Outcome 2 Disease‐free survival.

Comparison 10 Publication status, Outcome 1 Overall survival.

Comparison 10 Publication status, Outcome 2 Disease‐free survival.

Comparison 11 Toxicities, Outcome 1 Febrile neutropenia by sequential or concurrent anthracycline/taxane.

Comparison 11 Toxicities, Outcome 2 Febrile neutropenia by type of taxane.

Comparison 11 Toxicities, Outcome 3 Neuropathy (grade 3/4).

Comparison 11 Toxicities, Outcome 4 Neuropathy (grade 3/4) by type of taxane.

Comparison 11 Toxicities, Outcome 5 Fatigue (grade 3/4).

Comparison 11 Toxicities, Outcome 6 Stomatitis (grade 3/4).

Comparison 11 Toxicities, Outcome 7 Cardiotoxicity (includes grade 3/4 and symptomatic CCF).

Comparison 11 Toxicities, Outcome 8 Nausea and/or vomiting (grade 3/4).

Comparison 11 Toxicities, Outcome 9 Secondary leukaemia/myelodysplasia.

Comparison 11 Toxicities, Outcome 10 Treatment‐related deaths.

Comparison 12 Risk of Bias, Outcome 1 Overall survival.

Comparison 12 Risk of Bias, Outcome 2 Disease‐free survival.
| Taxane‐containing chemotherapy compared to any chemotherapy without taxane for early breast cancer | ||||||
| Patient or population: women with early breast cancer (operable, stages I to IIIA) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with any chemotherapy without taxanes | Risk with taxane‐containing chemotherapy | |||||
| Overall survival | Low risk of death | HR 0.87 | 39,180 | ⊕⊕⊕⊕ | Additional analyses (including specific taxane, scheduling, treatment duration, doses, node positive, and risk of bias) showed equivalent efficacy | |
| 80 per 1000* | 70 per 1000 | |||||
| High risk of death | ||||||
| 200 per 1000* | 176 per 1000 | |||||
| Disease‐free progression | Low risk of recurrence | HR 0.88 | 41,909 | ⊕⊕⊕⊕ | As above for overall survival | |
| 140 per 1000* | 124 per 1000 | |||||
| High risk of recurrence | ||||||
| 320 per 1000* | 288 per 1000 | |||||
| Quality of life | Not estimable. In general, there did not seem to be differences in quality of life scores between groups at long‐term follow‐up | ‐ | (7 studies) | ⊕⊕⊝⊝ | Studies used the validated EORTC‐C30 questionnaire and measures were patient‐reported | |
| Febrile neutropenia | Study population | OR 1.43 | 34,154 | ⊕⊕⊕⊝ | There was a higher incidence of febrile neutropenia with docetaxel‐containing regimens | |
| 56 per 1000 | 78 per 1000 | |||||
| Neuropathy (including grade 3/4 sensory or motor neuropathy, or both) | Study population | OR 6.89 (3.23 to 14.71) | 31,033 | ⊕⊕⊕⊝ | A test for subgroup differences by taxane type was not significant | |
| 6 per 1000 | 37 per 1,000 | |||||
| Cardiotoxicity (including grade 3/4 and congestive cardiac failure) | Study population | OR 0.87 | 32,894 | ⊕⊕⊕⊝ | Risk of cardiotoxicity was reduced with lower planned dose of anthracycline | |
| 9 per 1000 | 8 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aHeterogeneity was detected (I² = 59%) mainly due to variations in chemotherapy backbones. The quality of evidence was not downgraded as variations in chemotherapy are likely to occur in clinical practice. | ||||||
| Study | Description | Definition |
| DFS | Revised to iDFS (in 2016) in line with Standardized Definitions for Efficacy End Points (STEEP), where DFS referred to all invasive ipsilateral, regional, contralateral, and distant disease recurrences, second primary tumours, and death from any cause as events, and all non‐invasive in situ cancer events were excluded | |
| DFS | Time from randomisation to date of clinical relapse (histological or radiological evidence), a second cancer (except skin cancer other than melanoma or carcinoma in situ of breast or cervix), or death | |
| DFS | Time from randomisation until first date of local, regional, or distant relapse; diagnosis of a second primary cancer, including contralateral invasive breast cancer; or death from any cause | |
| RFS | Time from date of random assignment to date of locoregional and/or distant release | |
| RFS | Time from study entry until local recurrence, distant relapse, or death without relapse, whichever occurred first | |
| DFS | Time from randomisation to date of first locoregional recurrence, first distant metastasis, or death from any cause | |
| DFS | Time from date of randomisation to locoregional recurrence, distant recurrence, new primary breast cancer, or death | |
| Freedom from progression | Not reported in trial publication | |
| DFS | Interval between randomisation and locoregional or distant relapse or contralateral invasive breast cancer or second primary invasive non‐breast cancer or ipsilateral or contralateral in situ ductal carcinoma or death without cancer, whichever occurred first | |
| DFS | Time from date of random assignment to date of invasive breast cancer recurrence, invasive contralateral breast cancer, or death from any cause | |
| Recurrence‐free survival | Time from randomisation to date of detection of local or distant relapse (histological or radiological evidence) or contralateral invasive breast cancer or death without recurrence | |
| DFS | Time from random assignment to date of local, regional, or metastatic relapse; date of a second primary malignancy; or death form any cause, whichever occurred first | |
| DFS | Time from date of randomisation to date of local, regional, or metastatic relapse; diagnosis of second primary cancer or death from any cause | |
| DFS | According to the definition of invasive disease‐free survival (IDFS) in the STEEP system | |
| DFS | Time from randomisation to first relapse (local, regional, distant), contralateral breast cancer, or death from any cause | |
| EFS | Time from date of randomisation to date of local recurrence, distant metastases, contralateral breast cancer, second primary malignancy, or death from any cause, whichever occurred first | |
| DFS | Time from randomisation until local recurrence, distant relapse, or death (without relapse) | |
| DFS | Time from randomisation to date of breast cancer recurrence (local, regional, or distant), invasive contralateral breast cancer, non‐breast second primary cancer, or death from any cause | |
| iDFS and DDFS | Any local invasive or distant recurrence of breast cancer, any contralateral breast cancer, any second malignancy, and any death irrespective of its cause for iDFS | |
| RFS | Time from random assignment to time of recurrence of the primary breast cancer (local, nodal, metastatic). Patients with contralateral breast cancer, second malignancy, non‐disease‐related death were censored | |
| RFS | Time from random assignment to time of recurrence of the primary breast cancer (local, nodal, metastatic). Patients with contralateral breast cancer, second malignancy, non‐disease‐related death were censored | |
| DFS | Time from randomisation until local, regional, or distant treatment failure, contralateral breast cancer, non‐breast second primary cancer, or death | |
| DFS | Time from randomisation until first relapse (local, regional, or distant), contralateral breast cancer, or death from any cause | |
| TTR | Time to locoregional relapse, contralateral breast cancer, or distant metastasis, whichever occurs first | |
| DFS | Time from study entry to first local recurrence or distant metastasis, or death as a result of any cause | |
| DFS | Time from randomisation until first relapse (locoregional or distant), contralateral breast cancer, or death from any cause | |
| DFS | Time from randomisation to first invasive relapse, new primary breast cancer (ipsilateral or contralateral), or death form any cause | |
| DFS | Time from date of randomisation to local or distant recurrence or contralateral breast cancer or second primary malignancy or death from any case, whichever occurred first | |
| DFS | Time between randomisation and date of first documented disease recurrence or death from any cause | |
| DFS | Time from first dose of chemotherapy until date of any recurrence of breast cancer, a new second breast cancer or any other type of cancer, death due to any cause without relapse or recurrence of breast cancer, or date of last patient contact | |
| DFS: disease‐free survival | ||
| Trial | Cardiotoxicity | Febrile neutropenia | Neuropathy | Nausea/vomiting | Fatigue | Stomatitis |
| Grade 3/4 cardiac symptoms: NCI CTC (version 2) | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 neurological symptoms: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Congestive heart failure (cardiac function grade 3 or 4) | Reported as "febrile neutropenia": Protocol definition ‐ fever of grade 2 or more concomitant with grade 4 neutropenia requiring IV antibiotics, hospitalisation, or both | Grade 3/4 neurosensory effects: NCI CTC (version 1) | Grade 3/4 or severe vomiting: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiac function toxicity | Reported as "febrile neutropenia": protocol defined grade 4 neutropenia, fever with > 38 degrees Celsius, NCI CTC (version 1) | Grade 3/4 neurosensory effects: NCI CTC (version 1) | Grade 3/4 or severe vomiting: NCI CTC (version 1) | Grade 3 or higher asthenia: NCI CTC (version 1) | Grade 3 or higher stomatitis: NCI CTC (version 1) | |
| Grade 3/4 cardiotoxicity: WHO toxicity criteria | NR | Grade 3/4 peripheral neuropathy: WHO toxicity criteria | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| Grade 3 or higher cardiotoxicity (left ventricular systolic dysfunction, restrictive cardiomyopathy, general cardiac, cardiac deaths attributed to protocol treatment ‐ myocardial infarction and left ventricular dysfunction) | Reported as grade 3/4 febrile neutropenia: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | NR | |
| Congestive heart failure during treatment or post treatment follow‐up | NR | NR | NR | NR | NR | |
| Diagnosed congestive cardiac failure | Grade 3/4 febrile neutropenia: NCI CTC (version 2) | Grade 3/4 peripheral neuropathy: NCI CTC (version 2) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 congestive heart failure | Reported as "febrile neutropenia": toxicity criteria not specified | Grade 3/4 neuropathy (reported both sensory and motor) | Grade 3 or higher vomiting: toxicity criteria not specified | Grade 3 or higher fatigue: toxicity criteria not specified | Grade 3 or higher stomatitis: toxicity criteria not specified | |
| Symptomatic cardiac failure | NR | Grade 3 neuropathy reported only: NCI CTC (no version provided) | NR | NR | NR | |
| Grade 3/4 heart rhythm toxicity | Reported as "febrile neutropenia": NCT CTC (version 2) | Grade 3/4 neuropathy: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 mucositis: NCI CTC (version 2) | |
| NR | Reported as "neutropenic fever": NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiac dysfunction, Grade 4 cardiac ischaemia, Grade 3 arrhythmia | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 sensory neuropathy: NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Grade 3/4 arrhythmia, Grade 3/4 congestive heart failure | Reported as "febrile neutropenia": protocol defined fever Grade 2 or higher with Grade 4 neutropenia requiring intravenous antibiotics, hospitalisation, or both | Grade 3/4 peripheral neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 fatigue: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| NR | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 1) | Grade 3 peripheral sensory neuropathy: NCI CTC (version 1) | Grade 3/4 nausea and vomiting: NCI CTC (version 1) | Grade 3/4 fatigue: NCI CTC (version 1) | Grade 3/4 mucositis: NCI CTC (version 1) | |
| Reversible cardiotoxicity | Reported as "neutropenic fever" grade 3/4: NCI CTC (version 3) | Grade 3/4 neurotoxicity: NCI CTC (version 3) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 3) | NR | Grade 3/4 mucositis: NCI CTC (version 3) | |
| Grade 3/4 cardiotoxicity: WHO criteria | Reported as "febrile neutropenia" grade 3/4: WHO criteria | Grade 3/4 neurological toxicity: WHO criteria | Grade 3/4 nausea and vomiting: WHO criteria | NR | Grade 3/4 stomatitis: WHO criteria | |
| NR | Reported as "febrile neutropenia": WHO toxicity criteria | Grade 3/4 peripheral neuropathy: WHO toxicity criteria | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | Grade 3/4 fatigue: WHO toxicity criteria | NR | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neurotoxicity: NCI CTC (version 2) | Grade 3/4 nausea: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3 to 5 congestive heart failure and cardiac ischaemia | Reported as "febrile neutropenia" grade 1 to 5: NCI CTC (version 3) | Grade 3 to 5 sensory neuropathy: NCI CTC version 3 | Grade 3 to 5 vomiting: NCI CTC (version 3) | Grade 3 to 5 fatigue: NCI CTC (version 3) | Grade 3 to 5 mucositis, stomatitis, oesophagitis: NCI CTC (version 3) | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3/4 cardiotoxicity | Reported as "febrile neutropenia" grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | NR | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Grade 3 or higher cardiac dysfunction | NR | NR | NR | NR | NR | |
| Any serious adverse cardiac event | Reported as "febrile neutropenia": WHO toxicity criteria | NR | Grade 3/4 nausea and/or vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| NR | Reported as "febrile neutropenia" defined as any Grade 3/4 neutropenia plus fever (> 38 degrees) requiring antibiotics: NCI CTC (version 2) | NR | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | NR | Grade 3/4 mucositis: NCI CTC (version 2) | |
| Grade 3/4 cardiac event | Reported as "febrile neutropenia" Grade 3/4: WHO toxicity criteria | NR | Grade 3/4 nausea‐vomiting: WHO toxicity criteria | NR | Grade 3/4 stomatitis: WHO toxicity criteria | |
| NR | Reported as "febrile neutropenia" Grade 3/4: NCI CTC (version 2) | Grade 3/4 neuropathy: NCI CTC (version 2) | Grade 3/4 nausea and/or vomiting: NCI CTC (version 2) | Grade 3/4 lethargy: NCI CTC (version 2) | Grade 3/4 stomatitis (mucositis): NCI CTC (version 2) | |
| Cardiac function toxicity | Reported as "febrile neutropenia": NCI CTC (version 2) | Grade 3/4 neuropathy (reported both sensory and motor): NCI CTC (version 2) | Grade 3/4 vomiting: NCI CTC (version 2) | Grade 3/4 asthenia: NCI CTC (version 2) | Grade 3/4 stomatitis: NCI CTC (version 2) | |
| Cardiac events | Reported as "febrile neutropenia": NCI CTC (version 3) | Grade 3/4 peripheral neuropathy: NCI CTC (version 3) | Grade 3/4 nausea (vomiting not reported): NCI CTC (version 3) | Grade 3/4 fatigue: NCI CTC (version 3) | NR | |
| Death due to cardiac event | Reported as "febrile neutropenia" Grade 3/4 : NCI CTC (version 1) | NR | Grade 3/4 vomiting: NCI CTC (version 1) | Grade 3/4 asthenia: NCI CTC (version 1) | Grade 3/4 stomatitis: NCI CTC (version 1) | |
| NCI CTC: National Cancer Institute Common Toxicity Criteria | ||||||
| Trial ID | Instruments used | Summary of findings |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 at baseline, before each course of chemotherapy, and at 4 weeks, 6 weeks, and 6 months after completion of chemotherapy | Both treatment groups had decreased EORTC‐C30 scores for physical, role, emotional, social, and cognitive functioning from baseline to 28 days after chemotherapy. Global health scores also decreased in this time frame for both groups. Changes in symptom scores were generally similar between treatment groups with the exception of nausea/vomiting and pain scores. Nausea/vomiting was worse for the FEC120 group than for the EC‐Doc group (+9.42 above baseline vs +1.88), and pain scores were worse in the EC‐Doc group (+9.18 for EC‐Doc vs ‐1.61 for FEC120). Changes in items on the EORTC BR23 over time were quantitatively and qualitatively similar in the 2 treatment groups | |
| Patients completed EORTC QLQ‐C30 (version 2.0) and the breast cancer‐specific QLQ‐BR23 (version 1.0) questionnaires at baseline, before cycles 3 and 5, at 3 to 4 weeks after completion, and at 6, 12, and 24 months | Both FAC and TAC arms led to a transient and statistically significant reduction in QoL scores, which returned to baseline levels at first follow‐up visit. QoL measures were similar between groups and were similar to baseline measures at 6 months and at the end of 2 years | |
| QoL results designed to study short‐ and long‐term toxicities on quality of life by treatment agent and duration. No further details provided | QoL to be reported in a companion study | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 in the clinic at baseline and 9 months, 2 years, and 5 years after random assignment | No significant difference in overall QoL or across any of the scales between treatments at 9 months, 2 years, or 5 years | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ BR23 at baseline, and at the end of the first, second, and third cycles of chemotherapy | No difference reported for QoL global functioning scales and other items. However there was worsening of systemic therapy side effects, future perspective, nausea and vomiting, diarrhoea, appetite loss, and upset by hair loss and body experience with docetaxel compared to CMF at the end of 1 or more cycles | |
| Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR23 at baseline after each chemotherapy cycle and at 6, 12, and 24 months after completion of chemotherapy | During chemotherapy, QoL decreased and was worse with TAC than with FAC, but this effect resolved by week 44. There were no statistically significant differences in QoL between TAC+G‐CSF and FAC after cycle 6 or during further follow‐up | |
| Trial registry record indicates that QoL will be compared across treatment arms. No further details provided | QoL information not yet reported | |
| Patients completed EORTC QLQ‐C30 questionnaire at baseline and on completion of chemotherapy | Only 23% of participants (72 participants in E‐T‐CMF and 67 participants in E‐CMF) completed baseline and end of chemotherapy questionnaires. There was no difference between the 2 treatment arms at the beginning or at the end of chemotherapy. Significant increase in nausea and vomiting for both groups. Social functioning significantly decreased in the taxane‐containing arm, and emotional functioning and pain significantly improved in the control arm | |
| NCIC‐CTGMA21 (NCIC‐CTG MA21a; NCIC‐CTG MA21b) | Patients completed EORTC QLQ‐C30 and the breast cancer‐specific QLQ‐BR within 7 days before random assignment and afterwards | QoL results will be reported in a separate article |
| Selected centres invited participants to quality of life substudy. These patients completed EORTC QLQ‐C30, QLQ‐BR23, and Hospital Anxiety and Depression Scale (HADS) before randomisation, before fifth and after eighth cycles, and at 9, 12, 18, and 24 months post chemotherapy | FEC‐D was associated with worse global QoL (P = 0.001), physical (P < 0.0001), role (P = 0.002), emotional functioning (P = 0.008), social functioning (P = 0.003), pain (P = 0.001), and fatigue (P = 0.006) compared to control. In contrast, there was more nausea and vomiting in the control group (P = 0.01) than in the FEC‐D group | |
| CMF: cyclophosphamide, methotrexate and fluorouracil | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival ‐ all studies Show forest plot | 27 | 39180 | Hazard Ratio (95% CI) | 0.87 [0.83, 0.92] |
| 1.1 All studies | 27 | 39180 | Hazard Ratio (95% CI) | 0.87 [0.83, 0.92] |
| 2 Disease‐free survival: all studies Show forest plot | 30 | 41909 | Hazard Ratio (95% CI) | 0.88 [0.85, 0.92] |
| 2.1 All studies | 30 | 41909 | Hazard Ratio (95% CI) | 0.88 [0.85, 0.92] |
| 3 Disease‐free survival: as defined in Cochrane protocol Show forest plot | 25 | 35063 | Hazard Ratio (95% CI) | 0.86 [0.82, 0.89] |
| 3.1 DFS ‐ excluding Boccardo, CALGB40101, NCIC‐CTG MA21 & RAPP‐01 | 25 | 35063 | Hazard Ratio (95% CI) | 0.86 [0.82, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 27 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Docetaxel | 15 | 22105 | Hazard Ratio (95% CI) | 0.86 [0.81, 0.92] |
| 1.2 Paclitaxel | 12 | 17075 | Hazard Ratio (95% CI) | 0.89 [0.82, 0.96] |
| 2 Disease‐free survival Show forest plot | 30 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Docetaxel | 16 | 22730 | Hazard Ratio (95% CI) | 0.87 [0.82, 0.91] |
| 2.2 Paclitaxel | 14 | 19179 | Hazard Ratio (95% CI) | 0.91 [0.85, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 10 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Weekly paclitaxel | 4 | 4176 | Hazard Ratio (95% CI) | 0.80 [0.65, 0.98] |
| 1.2 Three‐weekly paclitaxel | 6 | 8433 | Hazard Ratio (95% CI) | 0.86 [0.78, 0.95] |
| 2 Disease‐free survival Show forest plot | 10 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Weekly Paclitaxel | 4 | 4176 | Hazard Ratio (95% CI) | 0.79 [0.67, 0.92] |
| 2.2 Three‐weekly paclitaxel | 6 | 8433 | Hazard Ratio (95% CI) | 0.85 [0.79, 0.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 23 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Sequential anthracycline/taxane | 18 | 24764 | Hazard Ratio (95% CI) | 0.86 [0.81, 0.91] |
| 1.2 Concurrent anthracycline/taxane | 6 | 8839 | Hazard Ratio (95% CI) | 0.86 [0.78, 0.94] |
| 2 Disease‐free survival Show forest plot | 26 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Sequential anthracycline/taxane | 20 | 26866 | Hazard Ratio (95% CI) | 0.86 [0.82, 0.90] |
| 2.2 Concurrent anthracycline/taxane | 7 | 9466 | Hazard Ratio (95% CI) | 0.90 [0.83, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 19 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Addition of taxane ‐ question 1 | 7 | 10842 | Hazard Ratio (95% CI) | 0.84 [0.77, 0.92] |
| 1.2 Substitution of taxane ‐ question 3 | 13 | 16196 | Hazard Ratio (95% CI) | 0.80 [0.74, 0.86] |
| 2 Disease‐free survival Show forest plot | 20 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Addition of taxane ‐ question 1 | 7 | 10842 | Hazard Ratio (95% CI) | 0.84 [0.78, 0.90] |
| 2.2 Substitution of taxane ‐ question 3 | 14 | 16823 | Hazard Ratio (95% CI) | 0.83 [0.78, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 22 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Taxane‐containing arm longer duration than control arm | 9 | 13865 | Hazard Ratio (95% CI) | 0.84 [0.77, 0.91] |
| 1.2 Taxane‐containing arm same duration as control arm | 14 | 18660 | Hazard Ratio (95% CI) | 0.87 [0.81, 0.94] |
| 2 Disease‐free survival Show forest plot | 25 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Taxane‐containing arm longer duration than control arm | 9 | 13865 | Hazard Ratio (95% CI) | 0.83 [0.77, 0.88] |
| 2.2 Taxane‐containing arm same duration as control arm | 17 | 21391 | Hazard Ratio (95% CI) | 0.90 [0.85, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 25 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 3 cycles of taxane | 7 | 6551 | Hazard Ratio (95% CI) | 0.77 [0.69, 0.86] |
| 1.2 4 or more cycles of taxane | 19 | 29458 | Hazard Ratio (95% CI) | 0.91 [0.86, 0.96] |
| 2 Disease‐free survival Show forest plot | 28 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 3 cycles of taxane | 7 | 6551 | Hazard Ratio (95% CI) | 0.80 [0.73, 0.88] |
| 2.2 4 or more cycles of taxane | 22 | 32187 | Hazard Ratio (95% CI) | 0.91 [0.87, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 27 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Node‐positive‐only studies | 17 | 22055 | Hazard Ratio (95% CI) | 0.83 [0.78, 0.88] |
| 1.2 Node‐positive or ‐negative studies | 7 | 10269 | Hazard Ratio (95% CI) | 0.95 [0.87, 1.04] |
| 1.3 Node‐negative‐only studies | 3 | 6856 | Hazard Ratio (95% CI) | 1.08 [0.89, 1.32] |
| 2 Disease‐free survival Show forest plot | 30 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Node‐positive‐only studies | 17 | 22055 | Hazard Ratio (95% CI) | 0.84 [0.80, 0.88] |
| 2.2 Node‐positive or ‐negative studies | 10 | 12998 | Hazard Ratio (95% CI) | 0.95 [0.88, 1.02] |
| 2.3 Node‐negative‐only studies | 3 | 6856 | Hazard Ratio (95% CI) | 0.99 [0.86, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 5 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 1.1 Hormone receptor‐positive | 4 | Hazard Ratio (Fixed, 95% CI) | 0.79 [0.70, 0.89] | |
| 1.2 Hormone receptor‐negative | 5 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.73, 1.05] | |
| 2 Disease‐free survival Show forest plot | 12 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 Hormone receptor‐positive | 11 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.85, 0.97] | |
| 2.2 Hormone receptor‐negative | 12 | Hazard Ratio (Fixed, 95% CI) | 0.80 [0.73, 0.88] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 27 | Hazard Ratio (95% CI) | Subtotals only | |
| 1.1 Studies with published efficacy paper | 26 | 38208 | Hazard Ratio (95% CI) | 0.88 [0.84, 0.92] |
| 1.2 Studies with results from online thesis | 1 | 972 | Hazard Ratio (95% CI) | 0.67 [0.48, 0.94] |
| 2 Disease‐free survival Show forest plot | 30 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 Studies with published efficacy paper | 28 | 40310 | Hazard Ratio (95% CI) | 0.88 [0.85, 0.92] |
| 2.2 Studies with results in abstract format or in online thesis | 2 | 1599 | Hazard Ratio (95% CI) | 0.84 [0.67, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Febrile neutropenia by sequential or concurrent anthracycline/taxane Show forest plot | 24 | 33763 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.96, 2.49] |
| 1.1 Sequential anthracycline and taxane treatment | 16 | 20148 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.10] |
| 1.2 Concurrent anthracycline and taxane treatment | 6 | 8552 | Odds Ratio (M‐H, Random, 95% CI) | 5.76 [3.36, 9.87] |
| 1.3 Taxane replacing anthracycline | 3 | 5063 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 3.04] |
| 2 Febrile neutropenia by type of taxane Show forest plot | 25 | 34154 | Odds Ratio (M‐H, Random, 95% CI) | 1.43 [0.89, 2.31] |
| 2.1 Docetaxel | 16 | 22596 | Odds Ratio (M‐H, Random, 95% CI) | 2.83 [1.88, 4.27] |
| 2.2 Paclitaxel | 9 | 11558 | Odds Ratio (M‐H, Random, 95% CI) | 0.36 [0.19, 0.67] |
| 3 Neuropathy (grade 3/4) Show forest plot | 23 | 31033 | Odds Ratio (M‐H, Random, 95% CI) | 6.89 [3.23, 14.71] |
| 4 Neuropathy (grade 3/4) by type of taxane Show forest plot | 23 | 31033 | Odds Ratio (M‐H, Random, 95% CI) | 6.89 [3.23, 14.71] |
| 4.1 Docetaxel | 12 | 18355 | Odds Ratio (M‐H, Random, 95% CI) | 3.74 [1.33, 10.53] |
| 4.2 Paclitaxel | 11 | 12678 | Odds Ratio (M‐H, Random, 95% CI) | 11.93 [3.59, 39.70] |
| 5 Fatigue (grade 3/4) Show forest plot | 16 | 25003 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.31, 2.49] |
| 5.1 Docetaxel | 10 | 16503 | Odds Ratio (M‐H, Random, 95% CI) | 2.14 [1.65, 2.78] |
| 5.2 Paclitaxel | 6 | 8500 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.58, 2.84] |
| 6 Stomatitis (grade 3/4) Show forest plot | 23 | 29500 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.93, 1.78] |
| 6.1 Docetaxel | 16 | 22648 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [1.28, 2.35] |
| 6.2 Paclitaxel | 7 | 6852 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.32] |
| 7 Cardiotoxicity (includes grade 3/4 and symptomatic CCF) Show forest plot | 23 | 32894 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.33] |
| 7.1 Total planned dose of anthracycline the same in both taxane‐ and non‐taxane‐containing arms | 9 | 14967 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.88, 1.84] |
| 7.2 Total planned dose of anthracycline reduced in the taxane‐containing arm | 10 | 12473 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.86] |
| 7.3 Taxane replacing anthracycline | 4 | 5454 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.26, 3.91] |
| 8 Nausea and/or vomiting (grade 3/4) Show forest plot | 26 | 34450 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.67, 1.04] |
| 8.1 Docetaxel | 16 | 22648 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 8.2 Paclitaxel | 10 | 11802 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.57, 1.39] |
| 9 Secondary leukaemia/myelodysplasia Show forest plot | 19 | 33225 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 9.1 Docetaxel | 13 | 24911 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.60, 1.59] |
| 9.2 Paclitaxel | 6 | 8314 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.15, 1.32] |
| 10 Treatment‐related deaths Show forest plot | 22 | 34882 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.63, 2.47] |
| 10.1 Docetaxel | 15 | 26021 | Odds Ratio (M‐H, Random, 95% CI) | 1.54 [0.76, 3.15] |
| 10.2 Paclitaxel | 7 | 8861 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.14, 3.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall survival Show forest plot | 27 | 39180 | Hazard Ratio (95% CI) | 0.87 [0.83, 0.92] |
| 1.1 Low risk of bias | 21 | 33206 | Hazard Ratio (95% CI) | 0.90 [0.86, 0.95] |
| 1.2 Unclear or high risk of bias | 6 | 5974 | Hazard Ratio (95% CI) | 0.74 [0.65, 0.83] |
| 2 Disease‐free survival Show forest plot | 30 | 41909 | Hazard Ratio (95% CI) | 0.88 [0.85, 0.92] |
| 2.1 Low risk of bias | 24 | 35935 | Hazard Ratio (95% CI) | 0.90 [0.87, 0.94] |
| 2.2 Unclear or high risk of bias | 6 | 5974 | Hazard Ratio (95% CI) | 0.76 [0.69, 0.84] |

