Delayed antibiotic prescriptions for respiratory infections
Abstract
Background
Concerns exist regarding antibiotic prescribing for respiratory tract infections (RTIs) owing to adverse reactions, cost, and antibacterial resistance. One strategy to reduce antibiotic prescribing is to provide prescriptions, but advise delay in antibiotic use with the expectation that symptoms will resolve first. This is an update of a Cochrane Review published in 2007, and updated in 2010 and 2013. This is was previously a living systematic review. Searches were run and screened monthly since May 2017.
Objectives
To evaluate the effects on clinical outcomes, antibiotic use, antibiotic resistance, and patient satisfaction of advising a delayed prescription of antibiotics in respiratory tract infections.
Search methods
For this 2017 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 4, 2017), which includes the Cochrane Acute Respiratory Infection Group's Specialised Register; Ovid MEDLINE (2013 to 25 May 2017); Ovid Embase (2013 to 2017 Week 21); EBSCO CINAHL Plus (1984 to 25 May 2017); Web of Science (2013 to 25 May 2017); WHO International Clinical Trials Registry Platform (1 September 2017); and ClinicalTrials.gov (1 September 2017).
Selection criteria
Randomised controlled trials involving participants of all ages defined as having an RTI, where delayed antibiotics were compared to immediate antibiotics or no antibiotics. We defined a delayed antibiotic as advice to delay the filling of an antibiotic prescription by at least 48 hours. We considered all RTIs regardless of whether antibiotics were recommended or not.
Data collection and analysis
We used standard Cochrane methodological procedures. Three review authors independently extracted and collated data. We assessed the risk of bias of all included trials. We contacted trial authors to obtain missing information.
Main results
For this 2017 update we added one new trial (405 participants) with uncomplicated acute respiratory infection. We included 11 studies (3555 participants). These 11 studies involved acute respiratory infections including acute otitis media (three studies), streptococcal pharyngitis (three studies), cough (two studies), sore throat (one study), common cold (one study), and a variety of RTIs (one study). Five studies involved only children, two only adults, and four included both adults and children. Six studies were conducted in a primary care setting, three in paediatric clinics, and two in emergency departments.
Studies were well reported, and appeared to be of moderate certainty evidence. Randomisation was not adequately described in two trials. Four trials blinded the outcomes assessor, and three included blinding of participants and doctors. We conducted meta‐analysis for antibiotic use and patient satisfaction.
We found no differences among delayed, immediate, and no prescribed antibiotics for clinical outcomes in the three studies that recruited participants with cough. For the outcome of fever with sore throat, three of the five studies favoured immediate antibiotics, and two found no difference. For the outcome of pain related to sore throat, two studies favoured immediate antibiotics, and three found no difference. One study compared delayed antibiotics with no antibiotic for sore throat, and found no difference in clinical outcomes.
Three studies included participants with acute otitis media. Of the two studies with an immediate antibiotic arm, one study found no difference for fever, and the other study favoured immediate antibiotics for pain and malaise severity on Day 3. One study including participants with acute otitis media compared delayed antibiotics with no antibiotics and found no difference for pain and fever on Day 3.
Two studies recruited participants with common cold. Neither study found differences for clinical outcomes between delayed and immediate antibiotic groups. One study favoured delayed antibiotics over no antibiotics for pain, fever, and cough duration (moderate certainty evidence for all clinical outcomes).
There were either no differences for adverse effects or results favoured delayed antibiotics over immediate antibiotics (low certainty evidence) with no significant differences in complication rates.
Delayed antibiotics resulted in a significant reduction in antibiotic use compared to immediate antibiotics prescription (odds ratio (OR) 0.04, 95% confidence interval (CI) 0.03 to 0.05). However, a delayed antibiotic was more likely to result in reported antibiotic use than no antibiotics (OR 2.55, 95% CI 1.59 to 4.08; moderate certainty evidence).
Patient satisfaction favoured delayed over no antibiotics (OR 1.49, 95% CI 1.08 to 2.06). There was no significant difference in patient satisfaction between delayed antibiotics and immediate antibiotics (OR 0.65, 95% CI 0.39 to 1.10; moderate certainty evidence).
None of the included studies evaluated antibiotic resistance.
Authors' conclusions
For many clinical outcomes, there were no differences between prescribing strategies. Symptoms for acute otitis media and sore throat were modestly improved by immediate antibiotics compared with delayed antibiotics. There were no differences in complication rates. Delaying prescribing did not result in significantly different levels of patient satisfaction compared with immediate provision of antibiotics (86% versus 91%; moderate certainty evidence). However, delay was favoured over no antibiotics (87% versus 82%). Delayed antibiotics achieved lower rates of antibiotic use compared to immediate antibiotics (31% versus 93%; moderate certainty evidence). The strategy of no antibiotics further reduced antibiotic use compared to delaying prescription for antibiotics (14% versus 28%).
Delayed antibiotics for people with acute respiratory infection reduced antibiotic use compared to immediate antibiotics, but was not shown to be different to no antibiotics in terms of symptom control and disease complications. Where clinicians feel it is safe not to prescribe antibiotics immediately for people with respiratory infections, no antibiotics with advice to return if symptoms do not resolve is likely to result in the least antibiotic use while maintaining similar patient satisfaction and clinical outcomes to delaying prescription of antibiotics. Where clinicians are not confident in using a no antibiotic strategy, a delayed antibiotics strategy may be an acceptable compromise in place of immediate prescribing to significantly reduce unnecessary antibiotic use for RTIs, and thereby reduce antibiotic resistance, while maintaining patient safety and satisfaction levels.
Editor note: This was previously a living systematic review. Searches were run and screened monthly since May 2017. The review authors have decided to cease maintaining this review in living systematic mode as a reasonable level of certainty has been reached in the existing evidence.
PICO
Plain language summary
Delayed antibiotic prescriptions for respiratory tract infections
Review question
Does delaying antibiotic prescription compared to immediate prescription or no antibiotics decrease the number of antibiotics taken for people with respiratory tract infections (RTIs) including sore throat, middle ear infection, cough (bronchitis), and the common cold?
Background
Prescribing too many antibiotics increases the risk of adverse reactions and results in higher healthcare costs and increased antibacterial resistance. One strategy to reduce unnecessary antibiotic prescribing is to provide an antibiotic prescription, but with advice to delay filling the prescription. The prescriber assesses that immediate antibiotics are not immediately required, expecting that symptoms will resolve without antibiotics.
We included all RTIs regardless of whether antibiotics were indicated or not. We also evaluated antibiotic use, patient satisfaction, antibiotic resistance, reconsultation rates, and use of supplemental therapies. This is an update of a review first published in 2007 and updated in 2010, 2013, and 2017.
Search date
Evidence is current to 25th May 2017.
Study characteristics
We included 12 trials with a total of 3555 participants evaluating prescribing strategies for people with respiratory tract infections. Ten of these studies compared strategies of delaying antibiotics with immediate antibiotics. Four studies compared delayed antibiotics with no antibiotics. Of the 11 studies, five included only children (1173 participants), two included only adults (594 participants), and four included children and adults (1761 participants). The studies investigated a variety of respiratory tract infections. One study involving 405 participants was new for this update.
Study funding sources
Two studies were funded by pharmaceutical companies, two studies did not describe the funding sources, and the remaining seven studies were funded by state institutions or specialist college.
Key results
There were no differences between immediate, delayed, and no antibiotics for many symptoms including fever, pain, feeling unwell, cough, and runny nose. The only differences were small and favoured immediate antibiotics for relieving pain, fever, and runny nose for sore throat; and pain and feeling unwell for middle ear infections. Compared to no antibiotics, delayed antibiotics led to a small reduction in how long pain, fever, and cough persisted in people with colds. There was little difference in antibiotic adverse effects, and no significant difference in complications.
Patient satisfaction was similar for people who trialled delayed antibiotics (86% satisfied) compared to immediate antibiotics (91% satisfied), but was greater than no antibiotics (87% versus 82% satisfied). Antibiotic use was greatest in the immediate antibiotic group (93%), followed by delayed antibiotics (31%), and no antibiotics (14%).
In the first month after the initial consultation, two studies indicated that participants were no more likely to come back and see the doctor for delayed or immediate prescribing groups. Excluding the first month, one study found that participants were no more likely to return to see the doctor in the 12 months after the delayed or immediate prescription for another respiratory infection, and another study found that participants were more likely to come back and see the doctor in the next 12 months if they had had an immediate prescription compared to a delayed prescription.
Two studies including children with acute otitis media reported on the use of other medicines in delayed and immediate antibiotic groups. There was no difference in the use of ibuprofen, paracetamol, and otic drops in one study. In the other study, fewer spoons of paracetamol were used in the immediate antibiotic group compared with the delayed antibiotic group on the second and third day after the child's initial presentation. No included studies evaluated herbal or other forms of complementary medicine.
No included studies evaluated antibiotic resistance.
Certainty of the evidence
Overall, the certainty of the evidence was moderate according to GRADE assessment.
When doctors feel it is safe not to immediately prescribe antibiotics, advising no antibiotics but to return if symptoms do not resolve, rather than delayed antibiotics, will result in lower antibiotic use. However, patient satisfaction may be greater when a delayed prescribing strategy is used. Using a delayed antibiotic strategy will still result in a significant reduction in antibiotic use compared to the use of immediate antibiotics.
Editorial note: This was previously a living systematic review. Searches were run and screened monthly since May 2017. The review authors have decided to cease maintaining this review in living systematic mode as a reasonable level of certainty has been reached in the existing evidence.
Authors' conclusions
Summary of findings
| Delayed antibiotics compared to immediate antibiotics for respiratory infections | ||||||
| Patient or population: respiratory infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with immediate antibiotics | Risk with delayed antibiotics | |||||
| Clinical outcomes | 10 included studies contributing data to this comparison measured clinical outcomes. For the 4 studies including participants with cough or common cold there was no evidence of difference for clinical outcomes. 5 studies included clinical outcome data for the presentation of sore throat, and for most clinical outcomes we found no evidence of difference. 2 studies measured clinical outcomes for participants with acute otitis media with 1 finding no evidence of difference in clinical outcomes, and the other favouring immediate antibiotics for malaise and pain severity on Day 3. There were sufficient outcome data to pool results for some clinical outcome measures. For participants with otitis media and sore throat, results favoured immediate antibiotics over delayed antibiotics for reducing pain and malaise severity on Day 3. For participants with common cold and otitis media, there was no evidence of differences in the number of participants with fever on Days 3 to 6 | ‐ | 2419 | ⊕⊕⊕⊝ |
| |
| Antibiotic use: delayed versus immediate antibiotics | 930 per 1000 | 348 per 1000 | OR 0.04 | 1963 | ⊕⊕⊕⊝ |
|
| Patient satisfaction: delayed versus immediate antibiotics | 909 per 1000 | 866 per 1000 | OR 0.65 | 1633 | ⊕⊕⊕⊝ |
|
| Reconsultation rate: delayed versus immediate antibiotics | 109 per 1000 | 113 per 1000 | OR 1.04 | 379 | ⊕⊕⊕⊝ |
|
| Adverse effects of antibiotics (Adverse effects) | The outcome of diarrhoea was measured by 4 studies and results favoured delayed antibiotics in 2 studies, and there was no evidence of difference the other 2. The outcome of vomiting was measured by 3 studies with no evidence of difference in 2, and results favouring immediate antibiotics in a third. The results for rash, measured by 2 studies, were sufficiently homogenous to conduct meta‐analysis, and results showed no evidence of difference | ‐ | 1303 | ⊕⊕⊝⊝ |
| |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded 1 level because more than half of studies were not adequately blinded and did not adequately report allocation concealment | ||||||
| Delayed antibiotics compared to no antibiotics for respiratory infections | ||||||
| Patient or population: respiratory infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no antibiotics | Risk with delayed antibiotics | |||||
| Clinical outcomes (clinical outcomes) | 4 studies measured clinical outcomes for this comparison. 2 studies recruited participants with sore throat, one study recruited participants with otitis media, and 1 study recruited participants with cough, and for these studies there was no evidence of differences found. 1 study recruited participants with the common cold, and found results favouring delayed antibiotics for pain, fever, and cough duration, but no evidence of difference for nasal mucosity | ‐ | 955 | ⊕⊕⊕⊝ |
| |
| Antibiotic use: delayed versus no antibiotics | 137 per 1000 | 287 per 1000 | OR 2.55 | 1241 | ⊕⊕⊕⊝ |
|
| Patient satisfaction: delayed versus no antibiotics | 824 per 1000 | 875 per 1000 | OR 1.49 | 1235 | ⊕⊕⊕⊝ |
|
| Adverse effects of antibiotics (adverse effects) | 2 studies measured adverse effects. 1 recruited participants with sore throat, and 1 with otitis media. Neither study found any difference in adverse effects | ‐ | 566 | ⊕⊕⊕⊝ |
| |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded 1 level for inadequate blinding for all studies, and allocation concealment not adequately reported for more than half of studies | ||||||
Background
Description of the condition
Over the past 70 years antimicrobials have transformed medicine, greatly reducing morbidity and mortality. However, the development of resistance to antimicrobials has increased substantially in recent decades. Each year in the USA, at least 2 million people acquire infections with antibiotic‐resistant bacteria, causing approximately 23,000 deaths (CDC 2017). The most significant cause for the development of resistance is considered to be excessive and inappropriate use of antibiotics for both humans, Goossens 2005; Sun 2012, and animals (Kempf 2016). A number of recent systematic reviews suggest that antibiotics only slightly modify the course of respiratory tract infections (RTIs) including acute otitis media (Venekamp 2015), sore throat (Spinks 2013), and acute bronchitis (Smith 2014), and have no effect on the common cold (Arroll 2013). Despite this, most antibiotics continue to be prescribed in primary care and mainly for people with RTIs (Goossens 2005; WHO 2014).
Description of the intervention
Strategies to reduce inappropriate antibiotic prescribing aim to reduce antibiotic resistance, adverse drug‐related events, and healthcare costs (AHRQ 2016).
One strategy is to advise patients to delay filling prescriptions, and to fill it only if symptoms persist or deteriorate. delayed antibiotics have been advocated as a means of demonstrating to patients that antibiotics are not always necessary, without making them feel under‐serviced (Arroll 2002b). Two ways of using this strategy have been deployed: giving the patient the antibiotic prescription (with instructions not to use unless there is deterioration), and making the prescription available at the clinic (to be picked up in the event of deterioration).
How the intervention might work
Delaying antibiotics may provide a feeling of safety for both patient and clinician should illness deteriorate. This intervention provides the safety of having a prescription of antibiotics available, yet an educational way of experiencing whether the illness resolves spontaneously without their use.
A systematic review showed that using delayed antibiotics for people with RTIs significantly reduced antibiotic prescribing (Arroll 2003a). The reduction ranged from a risk ratio (RR) of 0.77 (95% confidence interval (CI) 0.73 to 0.81) to RR 0.25 (95% CI 0.19 to 0.34) (Dowell 2001; Little 1997).
Why it is important to do this review
The delayed antibiotic strategy has been advocated as a safety net for avoiding rare but important complications of initially uncomplicated RTIs, and reducing antibiotic use, while enabling adequate control of symptoms and providing high levels of patient satisfaction (Little 2005b).
This review asked specifically what effect delayed antibiotics have on clinical outcomes for people with RTIs compared to immediate antibiotic provision and no antibiotics. It also evaluated the available data on antibiotic use, patient satisfaction, and antibiotic resistance for three prescribing strategies (delayed antibiotics, immediate antibiotics, and no antibiotics). This is a Cochrane Review update (Spurling 2007; Spurling 2010; Spurling 2013).
While previous versions of this systematic review have not supported the strategy of delayed antibiotic prescribing over no antibiotics, recommendations for delay persist in international guidelines, and continue to be discussed in the literature (De la Poza Abad 2016; NICE 2016).
A 2016 review that investigated strategies to improve antibiotic prescribing for people with uncomplicated RTIs prepared for the Agency for Healthcare Research and Quality in the USA highlighted the need for ongoing, systematic evaluation of these strategies, and the importance of ensuring that policy and practice is informed by a strong and up‐to‐date evidence base (AHRQ 2016). AHRQ 2016 also highlighted the need for further research reporting on resistance.
This was previously a living systematic review. Searches were run and screened monthly since May 2017. The review authors have decided to cease maintaining this review in living systematic mode as a reasonable level of certainty has been reached in the existing evidence.
Objectives
To evaluate the effects on clinical outcomes, antibiotic use, antibiotic resistance, and patient satisfaction of advising a delayed prescription of antibiotics in respiratory tract infections.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full text, those published as abstract only, and unpublished data. Open randomised trials that did not include blinding were accepted for inclusion.
Types of participants
We included adults and children diagnosed with RTIs.
Types of interventions
We included trials that investigated use of the following.
-
Delayed antibiotic use, defined as a strategy involving the use of or advice to use antibiotics more than 48 hours after the initial consultation.
-
Immediate antibiotic use, defined as the immediate use of a prescription of oral antibiotics given at the initial consultation.
-
No antibiotic use, defined as no prescription of antibiotics at the initial consultation.
Types of outcome measures
Primary outcomes
We aimed to compare delayed antibiotics with immediate antibiotics and delayed antibiotics with no antibiotics.
-
Clinical outcomes for sore throat, acute otitis media, bronchitis (cough), and common cold (we included duration and severity measures for the following symptoms: pain, malaise, fever, cough, and rhinorrhoea).
-
Antibiotic use.
-
Patient satisfaction (measured on a four‐ to six‐point Likert scale; we defined satisfaction as including moderately satisfied, very satisfied, and extremely satisfied).
-
Antibiotic resistance.
Secondary outcomes
-
Adverse effects of antibiotics.
-
Complications of disease.
-
Reconsultation.
-
Use of other therapies such as simple analgesia, e.g. paracetamol and ibuprofen.
Search methods for identification of studies
Electronic searches
For this 2017 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 4, to 25 May, 2017), which includes the Cochrane Acute Respiratory Infection Group's Specialised Register; Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily, and Ovid MEDLINE (2013 to 25 May 2017); Ovid Embase Classic+Embase (2013 to 2017 Week 21), EBSCO CINAHL Plus (1984 to 25 May 2017); Web of Science (2013 to 25 May 2017); WHO International Clinical Trials Registry Platform (1 September 2017); and ClinicalTrials.gov (1 September 2017).
In previous versions of this review, we searched MEDLINE using keywords and MeSH terms in conjunction with the highly sensitive search strategy designed by Cochrane for identifying RCTs (Dickersin 1994). We applied no trial filters for this update. Search strategies for all five databases can be found in Appendix 1.
We applied no language restrictions in any of the electronic database searches, but applied date restrictions to most of the databases, as this was an updated search.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We planned to contact experts in the field to identify additional unpublished materials. This was previously a living systematic review. Searches were run and screened monthly since May 2017. The review authors have decided to cease maintaining this review in living systematic mode as a reasonable level of certainty has been reached in the existing evidence.
Data collection and analysis
Selection of studies
Two review authors (RFo, GS) independently screened titles and abstracts of all potential studies identified by the search for inclusion in the review. We retrieved the full‐text study reports, and three review authors (CDM, LD, GS) independently screened the full texts and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or by consulting a third review author (RFo). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process and completed a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that was piloted on at least one study in the review. Two review authors (LD, CDM) extracted study characteristics from the included studies. We extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (LD, CDM) independently extracted outcome data from the included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author. One review author (RFo) transferred data into Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (GS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (LD, CDM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving third review author (GS). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided quotes from the study together with a justification for our judgement in 'Risk of bias' tables. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in 'Risk of bias' tables.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in Differences between protocol and review.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate the treatment effects (RevMan 2014). We used odds ratio for dichotomous outcomes and mean differences or standardised mean differences for continuous outcomes.
We undertook meta‐analyses only where this was meaningful, that is if treatments, participants, and the underlying clinical question were sufficiently similar for pooling to make sense.
Unit of analysis issues
The unit of analysis for each outcome was the individual study participant.
Dealing with missing data
We planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when we identified a study as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We also planned that if numerical outcome data were missing, such as standard deviations or correlation coefficients, and they were not obtainable from the study authors, we would calculate these from other available statistics, such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, we planned to report this and explore for possible causes in subgroup analysis.
Assessment of reporting biases
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We have reported much of the data in this review as a narrative synthesis describing outcome measures. As previously indicated, we pooled results where heterogeneity was satisfactorily low. We have conducted meta‐analysis where results were sufficiently homogenous.
Subgroup analysis and investigation of heterogeneity
We considered subgroup analyses for all outcomes and included year of publication, clinical presentation, setting, and differences in the intervention. We considered subgroup analyses for studies including only children versus those including only adults where data were available.
We described two subgroup analyses that showed differences in outcomes. We further explored heterogeneity of antibiotic use in delayed antibiotic arms in analyses of different delay strategy methods; we also investigated heterogeneity of patient satisfaction with respect to blinding of outcome assessors and participants.
Sensitivity analysis
We conducted sensitivity analysis according to risk of bias.
Summary of findings and assessment of the certainty of the evidence
We created two summary of findings tables. One table dealt with the comparison of delayed antibiotics versus immediate antibiotics and included clinical outcomes, antibiotics use, patient satisfaction, adverse effects of antibiotics, and reconsultation rates (summary of findings Table 1). The second table deals with the comparison of delayed antibiotics versus no antibiotics, and included clinical outcomes, antibiotics use, patient satisfaction, and adverse effects of antibiotics. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for these outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT ). We justified all decisions to down‐ or upgrade the certainty of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
When we identify new evidence (studies, data or information) that meets the review inclusion criteria, we will immediately assess risk of bias and extract the data and incorporate it in the synthesis, as appropriate.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We identified 432 records in database searching, and 155 records remained after duplicates were removed. We removed 134 records that were clearly not relevant based on title alone, leaving 21 records. We retrieved 21 full‐text reports, and of these 11 met our inclusion criteria (Figure 1).
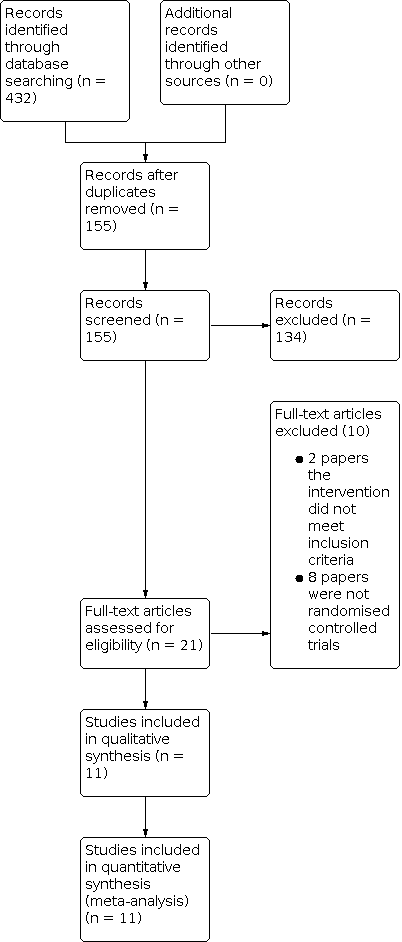
Study flow diagram.
Included studies
We included 11 trials involving a total of 3555 participants. Ten trials compared immediate provision of antibiotics with delayed antibiotics; four trials investigated sore throat (pharyngitis); two trials considered acute otitis media (AOM); two evaluated cough (bronchitis); one investigated common cold; and one included a number of acute upper RTIs.
Of the 11 included trials, 1357 participants were randomised to receive delayed antibiotics. In 10 of these trials, 1168 participants were allocated to receive immediate antibiotics, and in four trials 564 participants were allocated to receive no antibiotics. Four studies compared the prescribing strategy of no antibiotics with delayed antibiotics (Chao 2008; De la Poza Abad 2016; Little 1997; Little 2005a). These four trials investigated the presentations of pharyngitis/sore throat (De la Poza Abad 2016; Little 1997), bronchitis (cough) (De la Poza Abad 2016; Little 2005a), AOM (Chao 2008), and the common cold/rhinosinusitis (De la Poza Abad 2016). Please see the Characteristics of included studies table for details of the included trials.
Motives for studying delayed antibiotics
Early studies of sore throat were designed as efficacy trials to identify the rate of relapse of group A beta‐haemolytic streptococcus (GABHS) throat in immediate versus delayed antibiotic groups (El‐Daher 1991; Gerber 1990; Pichichero 1987). Subsequent trials comparing delayed antibiotics and immediate antibiotics were conducted with a view to evaluate the use of delayed antibiotics to reduce the use of antibiotics for upper respiratory tract infections (Arroll 2002a; De la Poza Abad 2016; Dowell 2001; Little 1997; Little 2001; Spiro 2006).
Population
Of the 11 included studies, five included only children (Chao 2008; El‐Daher 1991; Little 2001; Pichichero 1987; Spiro 2006), two included only adults (De la Poza Abad 2016; Dowell 2001), and four included both adults and children (Arroll 2002a; Gerber 1990; Little 1997; Little 2005a).
Setting
Of the 11 included studies, six were conducted in a primary care setting (Arroll 2002a; De la Poza Abad 2016; Dowell 2001; Little 1997; Little 2001; Little 2005a), three in paediatric clinics (El‐Daher 1991; Gerber 1990; Pichichero 1987), and two in emergency departments (Chao 2008; Spiro 2006).
Excluded studies
Two of the studies identified in searches were extensions of previously included studies (Little 2006; Moore 2009). We excluded one RCT because it compared usual delayed antibiotics with a post‐dated script for delayed antibiotics, and did not include either an immediate antibiotic or a no antibiotic arm (Worrall 2010). We excluded one study because it investigated information leaflets rather than prescribing strategies (Agnew 2013). We excluded a total of 10 studies; the other eight studies were not RCTs (Cates 1999; De la Poza Abad 2013; Fischer 2009; Ghebrehewet 2020; Little 2014; Newson 2009; Siegel 2003; Vouloumanou 2009).
Risk of bias in included studies
Overall, we assessed the included studies as at low risk of bias. Studies were most likely to be assessed as at unclear or moderate risk of bias for the domains of allocation concealment and blinding. Almost all studies showed a low risk of bias for all other domains. We assessed randomisation of studies as low risk for all of the included studies except for two, for which the randomisation was unclear. We assessed allocation concealment as low risk of bias for four studies, unclear for two studies, and high risk of bias for the five remaining studies. We assessed blinding as low risk of bias in three studies and high risk of bias for the remaining eight studies. For incomplete data, we assessed 10 studies as at low risk of bias and the remaining study as at high risk of bias. We assessed selective reporting as low risk of bias in 10 studies and unclear in one study. We detected no other biases apart from bias associated with funding source. Two studies were funded by pharmaceutical companies and were assessed as at high risk of bias. We assessed two studies for which the funding source was not described as at unclear risk of bias. The remaining seven studies were funded by state institutions or specialist college and were assessed as at low risk of bias. Summaries of the risk of bias in included studies are provided in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies reported using random number tables or computer‐generated randomisation and were assessed as at low risk of bias. Two studies did not describe randomisation methods and were assessed as at unclear risk of bias (El‐Daher 1991; Little 1997). Four trials described adequate allocation concealment using opaque envelopes and were assessed as at low risk of bias (Arroll 2002a; Little 2001; Little 2005a; Spiro 2006). We assessed the remaining studies as at unclear or high risk of bias.
Blinding
Seven studies attempted to blind some or all aspects of the study, that is participants, prescribing doctors, and outcome assessors were blinded. We assessed three studies as at low risk of bias because they attempted to blind participants and prescribing doctors without indicating if the outcome assessor was blinded (Arroll 2002a; El‐Daher 1991; Pichichero 1987). In one study, participants were informed only that they would be given one of two sets of instructions about taking antibiotics for their colds. Participants read an information sheet and completed a consent form. Participants were thus blinded to what the other group would take (Arroll 2002a). Two studies used placebo (tablets) to blind participants (El‐Daher 1991; Pichichero 1987). We assessed the remaining eight studies as at high risk of bias in this domain. Of these eight studies, the outcomes assessor, but not participants or prescribing doctors, were blinded in four studies (Chao 2008; Dowell 2001; Little 2005a; Spiro 2006). No blinding was reported in the other four studies (De la Poza Abad 2016; Gerber 1990; Little 1997; Little 2001).
Incomplete outcome data
We assessed one study as at high risk of bias for incomplete data reporting because the numbers of participants enrolled did not match the numbers of participants analysed, and this disparity was not explained (El‐Daher 1991). We assessed all other studies as at low risk of bias, with no or very small numbers of participant dropout.
Selective reporting
Gerber 1990 reported all clinical outcomes as one aggregated outcome and was assessed as at unclear risk of bias. We assessed all of the other studies as at low risk of bias because they reported on their predetermined outcome measures.
Other potential sources of bias
Six included studies received grants from research bodies funded by the national government where the trial was conducted (Arroll 2002a; De la Poza Abad 2016; Little 1997; Little 2001; Little 2005a; Spiro 2006). One study received funding from their relevant specialist college (Dowell 2001). We assessed these seven studies as at low risk of bias. We assessed two studies as at high risk of bias because they received funding from pharmaceutical companies. One study, El‐Daher 1991, was funded by Biochemie GmbH and the local university. Another study, Pichichero 1987, was funded by both a philanthropic organisation and a pharmaceutical company (Eli Lilly). Two studies did not describe the funding source (Chao 2008; Gerber 1990), and we have assessed them as at unclear risk of bias.
Effects of interventions
See: Summary of findings 1 Delayed antibiotics compared to immediate antibiotics for respiratory infections; Summary of findings 2 Delayed antibiotics compared to No antibiotics for respiratory infections
We assessed the effects of interventions using all 11 included studies. Details of the interventions are presented in Table 1 as per reporting recommendations published in 2017 (Hoffmann 2017). Assessing the effectiveness of antibiotic prescribing strategies was complicated by the heterogeneity of RTIs considered by the included studies. This heterogeneity is important because clinical outcomes are known to be influenced by antibiotics in different ways depending on the type of RTI. For example, antibiotics have been shown to reduce pain in otitis media (Venekamp 2015), but make no difference to the symptoms of the common cold (Kenealy 2013). Additionally, authors of studies measuring the same RTI reported clinical outcomes in a variety of ways which could not readily be compared even after we obtained raw study data. However, we did combine the outcomes of pain (Days 3 to 6; Analysis 1.1, Analysis 1.2), malaise (Days 3 to 6; Analysis 2.1, Analysis 2.2), and fever (Days 3 to 6; Analysis 3.1, Analysis 3.2), and conducted meta‐analysis where this was not precluded by heterogeneity. Other clinical outcomes are presented in Table 2 for the comparison of delayed antibiotics versus immediate antibiotics, and in Table 3 for the comparison of delayed antibiotics versus no antibiotics.
| Author Year | Disease | Participants | Trial outcomes | Materials and procedures for clinicians delivering intervention | Clinicians delivering intervention | How intervention was delivered to participants | Where intervention was delivered | When and how much | Tailoring | Modified during trial? | Checks of fidelity? | Fidelity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common cold | Any age | Antibiotic use, satisfaction, and symptoms of delayed prescribing | Antibiotic prescription (deemed appropriate by treating GP). Procedure not detailed | 15 GPs | Delayed: to fill prescription after 3 days if symptoms not improved Immediate: usual care | 1 general practice, New Zealand | Once, at index consultation; delayed group asked to wait 3 days | Participants advised to return to GP if symptoms worsened. | None reported | Not detailed | ‐ | |
| Acute otitis media | Children (2 to 12 years) | Antibiotic use | 2 forms of discharge instruction sheet provided by clinicians to patients: 1) completion of all: when to return for medical care (after 2 to 3 days); how to use complimentary symptom drugs 2) comparison: as above + prescription to fill if still unwell at 2 to 3 days | 14 emergency department physicians | Not detailed | Emergency department of an urban public hospital in the USA | Once, at index consultation | Provided with complimentary optional ibuprofen or paracetamol +/‐ benzocaine otic drops at index consultation | None reported | None | None | |
| Acute uncomplicated respiratory infection | Adults | Symptom duration and severity, antibiotic use, patient satisfaction, patients’ beliefs in antibiotic effectiveness | Physician structured script and patient information sheet about self limiting natural history of respiratory infection, pros and cons of antibiotics used with patients. Antibiotic prescription as indicated | GPs | 4 groups of antibiotic prescription use: 1) immediate; 2) delayed, patient‐led prescription; 3) delayed, prescription collection; 4) none. Delayed = 3 days | 23 primary care centres in 4 regions in Spain | Once, at index consultation; delayed prescription collection group could collect after 3 days if needed | All advised to return if no improvement or worsening after 5 days (pharyngitis) or 10 days (other infections). Central phone follow‐up if symptoms persisted | None reported | None | None | |
| Acute uncomplicated cough | Adults (> 16 years) | Symptom duration, prescription uptake, patient satisfaction, patient enablement subsequent consultation rates | Antibiotic prescription of GP's choice provided or lodged at reception. | 48 GPs | Immediate: usual care delayed: collect prescription after 1 week if required (within 2 weeks) | 22 general practices in Scotland, UK | Once, at index consultation; delayed prescription group asked to wait 1 week | Nil | None reported | Date scripts collected by delayed group | 35% (12/34) waited 7 days as asked; mean wait 6 days (range 1 to 10). | |
| GABHS | Children (4 to 14 years) | Signs and symptoms, antibody titre, subsequent episodes | Immediate group: supplied with 2 days of penicillin, then 8 days of penicillin on Day 3. delayed group: supplied with 2 days of placebo, then 10 days of penicillin on Day 3 | Physician | Immediate: 2 days penicillin, then 8 days penicillin delayed: 2 days placebo, then 10 days penicillin | Paediatric clinics at Jordan University of Science and Technology, Jordan | At index consultation, then re‐examined on Day 3 | Paracetamol as needed | None reported | None reported | None reported | |
| GABHS pharyngitis | Children / adolescents (2 to 22 years) | Positive follow‐up throat cultures, recurrences, symptomatic recurrences, or new acquisitions | Immediate group: supplied with 10‐day course of dose appropriate penicillin V. Delayed group: instructed to wait 48 hours before commencing 10‐day course of penicillin. Telephone follow‐up 24 hours later in both groups and next 24 hours for delayed group to advise commencement | Not reported (implied treating physicians) | Immediate: usual care delayed: wait 48 hours before commencing penicillin | 1 private paediatric practice in the USA | At index consultation and telephone follow‐up 24 and 48 hours afterwards | Further 10‐day courses of penicillin if further GABHS pharyngitis | None reported | Urine sample at Day 9, mailed after drying for analysis | No report of urine sample compliance results | |
| Sore throat | ≥ 4 years | Duration of symptoms, satisfaction and compliance with and perceived efficacy of antibiotics, time off school or work | Immediate group given 10‐day prescription of dose appropriate penicillin V. Delayed group offered antibiotics but could collect prescription if symptoms not settled within 3 days. GP standard advice sheets provided to participants | 25 GPs | 3 groups of antibiotic prescriptions: 1) immediate: usual care; 2) no antibiotics; 3) delayed: to collect within 3 days. | 11 general practices, England, UK | At index consultation; delayed prescription group within 3 days | Erythromycin if sensitive to penicillin. Analgesics or antipyretics allowed. | None reported | GP documented prescription on sheet. Patient daily diary until symptom‐free and medication finished | GPs’ compliance: immediate: 99%; no ABs: 2%; delayed: 5% left with script AB use: immediate: 99%; no: 13%; delayed: 31% | |
| Acute otitis media | Children (0.5 to 10 years) | Symptom resolution, absence from school or nursery, paracetamol consumption | Immediate group prescribed amoxicillin. Delayed group asked to delay 3 days before using prescription, and then only if necessary. GP used standardised advice sheets specific to each group | 42 GPs | Immediate: usual care delayed: wait 3 days to collect prescription | General practices in Scotland, UK | At index consultation; delayed prescription group asked to wait 3 days | Antipyretics were allowed. | None reported | Patient diary | No | |
| Acute uncomplicated lower respiratory tract infection | ≥ 3 years | Symptom duration and severity, antibiotic use, satisfaction, belief in antibiotics | Immediate group: prescription for 10 days amoxicillin. Delayed group: prescription written and left at reception for patient to retrieve if wanted (but advised to wait 14 days). Leaflet groups: 1‐page information leaflet covering natural history of illness, when to seek further help. All groups: statement about analgesics, natural history of illness, and prescribing strategy read out by physicians | 37 GPs | 6 groups (factorial): 1) no antibiotics, no leaflet; 2) delayed antibiotics, no leaflet; 3) immediate antibiotics, no leaflet; 4) no antibiotics, leaflet; 5) delayed antibiotics and leaflet; 6) immediate antibiotics and leaflet. Delay = 14 days | General practices, England, UK | At index consultation; 14 days for delayed prescription group | Erythromycin if allergic to penicillin. Antipyretics allowed. | None reported | Reported antibiotic use in diary | 96% immediate group; 20% delayed group; 16% no ABs group | |
| Sore throat (presumed GABHS) | Children (4 to 18 years) | Symptomatic response, recurrent infections | Drugs supplied directly to patients. Usual care 10‐day course penicillin V. Delayed group provided with placebo for first 3 days, then penicillin | Study nurse | Immediate: usual care delayed: placebo for 3 days then penicillin | Primary care paediatric practice in the USA | At index consultation | Antibiotic (tablet or suspension). Antipyretics were allowed | None reported | Check drug bottles at 3 days and 3 weeks. Test urine at 3 days for antibiotic | Confirmed in 98% cases (drug bottles); no ABs used in placebo group | |
| Acute otitis media | Children (0.5 to 12 years) | Antibiotic use, clinical symptoms, adverse outcomes, days off school or work, unscheduled medical visits, parents’ comfort with management | Provision of written prescription for antibiotics valid for 3 days. Wait‐and‐see prescription group given written and verbal instructions to only fill prescription if no improvement or worsening 2 days after emergency room visit | Emergency department clinicians | Immediate: usual care Wait‐and‐see prescription: wait 2 days | Paediatric emergency department in the USA | At index consultation and within 3 days if prescription filled | Ibuprofen and otic drops as needed. Primary care contact if worsening | None reported | Verification of filling of prescription by phone call to designated pharmacies for 28% of the sample | All instances of no filling of prescription confirmed by pharmacies, and 90% confirmation of parent report of prescription filled |
ABs: antibiotics
GABHS: group A beta‐haemolytic streptococcus
GP: general practitioner
| Study | Outcome | Delay | Immediate | Favours | Result (95% CI) |
|---|---|---|---|---|---|
| Sore throat | |||||
| Fever severity on Day 3 | 37.2 (SD 1.2, n = 55) | 36.8 (SD 0.6, n = 59) | Immediate antibiotics | MD 0.40 (95% CI 0.05 to 0.75) | |
| Malaise severity on Day 3 | 1.3 (SD 1.0, n = 55) | 1.1 (SD 0.7, n = 59) | No difference | MD 0.20 (95% CI ‐0.11 to 0.51) | |
| Pain severity on Day 3 | 1.6 (SD 1.4, n = 55) | 1.3 (SD 1.3, n = 59) | No difference | MD 0.30 (95% CI ‐0.15 to 0.75) | |
| Compliance | 55/55 | 59/59 | No difference | 100% in both groups | |
| Recurrence rate | ‐ | ‐ | No difference | Data not available | |
| Compliance | 44/50 | 59/63 | Delayed antibiotics | 88% in immediate group and 93% in delayed group | |
| Vomiting | 57/118 | 4/111 | Immediate antibiotics | OR 25.00 (95% CI 8.65 to 72.25) | |
| Pain on Day 3 | 106/118 | 42/111 | Immediate antibiotics | OR 14.51 (95% CI 7.14 to 29.50) | |
| Malaise on Day 3 | 45/118 | 4/111 | Immediate antibiotics | OR 16.49 (95% CI 5.68 to 47.83) | |
| Fever severity on Day 3 | 38.0 °C (SD 2.0, n = 118) | 37.1 °C (SD 1.0, n = 111) | Immediate antibiotics | SMD 0.58 (95% CI 0.31 to 0.84) | |
| Vomiting | 15/179 | 18/215 | No difference | OR 1.00 (95% CI 0.49 to 2.05) | |
| Diarrhoea | 23/179 | 23/215 | No difference | OR 1.23 (95% CI 0.67 to 2.28) | |
| Rash | 11/180 | 14/215 | No difference | OR 0.93 (95% CI 0.41 to 2.11) | |
| Stomachache | 48/180 | 66/215 | No difference | OR 0.82 (95% CI 0.53 to 1.27) | |
| Fever (> 37.0 °C) | Unavailable | Unavailable | Immediate antibiotics | Data not available | |
| Pain | Unavailable | Unavailable | No difference | Data not available | |
| Cough | Unavailable | Unavailable | No difference | Data not available | |
| Malaise | Unavailable | Unavailable | No difference | Data not available | |
| Analgesic use | Unavailable | Unavailable | No difference | Data not available | |
| Time off work | Unavailable | Unavailable | No difference | Data not available | |
| Pain duration (delayed prescription at time of visit) | 5.7 days (SD 5.1, n = 45) | 4.4 days (SD 2.4, n = 47) | No difference | MD 1.30 (95% CI ‐0.34 to 2.94) | |
| Pain duration (delayed prescription requiring collection) | 7.4 days (SD 6.3, n = 46) | 4.4 days (SD 2.4, n = 47) | Immediate antibiotics | MD 3.00 (95% CI ‐1.03 to 4.95) | |
| Fever duration (delayed prescription at time of visit) | 3.1 days (SD 1.8, n = 45) | 2.9 days (SD 1.7, n = 47) | No difference | MD ‐0.20 (95% CI ‐0.52 to 0.92) | |
| Fever duration (delayed prescription requiring collection) | 3.4 days (SD 2.4, n = 46) | 2.9 days (SD 1.7, n = 47) | No difference | MD 0.50 (95% CI ‐0.35 to 1.35) | |
| Cough duration (delayed prescription at time of visit) | 8.1 days (SD 5.9, n = 45) | 8.1 days (SD 5.7, n = 47) | No difference | MD ‐2.50 (95% CI ‐5.52 to 0.52) | |
| Cough duration (delayed prescription requiring collection) | 8.2 days (SD 6.9, n = 46) | 8.1 days (SD 5.7, n = 47) | No difference | MD ‐2.40 (95% CI ‐5.59 to 0.79) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 7.2 days (SD 4.3, n = 45) | 5.4 days (SD 3.9, n = 47) | Immediate antibiotics | MD ‐1.80 (95% CI 0.12 to 3.48) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 9.7 days (SD 8.3, n = 46) | 8.9 days (SD 6.5, n = 46) | Immediate antibiotics | MD 4.30 (95% CI 1.65 to 6.95) | |
| Acute otitis media | |||||
| Diarrhoea | 14/150 | 25/135 | Delayed antibiotics | OR 0.45 (95% CI 0.22 to 0.91) | |
| Rash | 8/150 | 6/135 | No difference | OR 1.21 (95% CI 0.41 to 2.58) | |
| Participants with pain on Day 3 | 28/111 | 15/101 | No difference | OR 1.93 (95% CI 0.96 to 3.88) | |
| Participants with pain on Day 7 | 3/111 | 0/101 | No difference | OR 6.55 (95% CI 0.33 to 128.35) | |
| Participants with malaise on Day 3 | 45/150 | 19/135 | Immediate antibiotics | OR 2.62 (95% CI 1.44 to 4.76) | |
| Malaise severity Day 3 | 0.8 (SD 1.7, n = 150) | 0.4 (SD 1.0, n = 134) | Immediate antibiotics | MD 0.43 (95% CI 0.11 to 0.75) | |
| Malaise severity on Day 7 | 2.2 (SD 2.0, n = 150) | 1.5 (SD 1.2, n = 135) | No difference | MD 0.01 (95% CI ‐0.11 to 0.13) | |
| Pain severity on Day 3 | 2.6 (SD 2.1, n = 111) | 1.8 (SD 1.4, n = 102) | Immediate antibiotics | MD 0.75 (95% CI 0.26 to 1.24) | |
| Pain severity on Day 7 | 1.17 (SD 0.75, n = 111) | 1.05 (SD 0.38, n = 101) | No difference | MD 0.12 (95% CI ‐0.04 to 0.28) | |
| Paracetamol consumption | 2.3 spoons | 1.7 spoons | Immediate antibiotics | MD 0.59 (95% CI 0.25 to 0.93) | |
| Last day of crying | 2.2 days | 1.5 days | Immediate antibiotics | MD 0.69 (95% CI 0.31 to 1.07) | |
| Episodes of earache in the 3 months since randomisation | Unavailable | Unavailable | No difference | OR 0.89 (95% CI 0.48 to 1.65) | |
| Episodes of earache over 1 year | Unavailable | Unavailable | No difference | OR 1.03 (95% CI 0.60 to 1.78) | |
| Pain day 4 to 6 | 85/132 | 89/133 | No difference | OR 0.89 (95% CI 0.54 to 1.48) | |
| Fever day 4 to 6 | 42/132 | 46/133 | No difference | OR 0.88 (95% CI 0.53 to 1.47) | |
| Vomiting | 15/132 | 15/133 | No difference | OR 1.01 (95% CI 0.47 to 2.16) | |
| Diarrhoea | 10/132 | 31/133 | Delayed antibiotics | OR 0.27 (95% CI 0.13 to 0.58) | |
| Cough | |||||
| Clinical outcomes | Unavailable | Unavailable | No difference | Data not available | |
| Clinical outcomes | Unavailable | Unavailable | No difference | Data not available | |
| Pain duration (delayed prescription at time of visit) | 11.0 days (SD 8.0, n = 32) | 10.5 days (SD 8.0, n = 32) | No difference | MD 0.50 (95% CI ‐0.34 to 4.42) | |
| Pain duration (delayed prescription requiring collection) | 8.9 days (SD 6.9, n = 32) | 10.5 days (SD 8.0, n = 32) | No difference | MD ‐1.60 (95% CI ‐5.26 to 2.06) | |
| Fever duration (delayed prescription at time of visit) | 5.6 days (SD 5.9, n = 32) | 4.1 days (SD 5.7, n = 32) | No difference | MD 1.50 (95% CI ‐1.34 to 4.34) | |
| Fever duration (delayed prescription requiring collection) | 4.7 days (SD 4.6, n = 32) | 4.1 days (SD 5.7, n = 32) | No difference | MD 0.60 (95% CI ‐1.94 to 3.14) | |
| Cough duration (delayed prescription at time of visit) | 15.6 days (SD 8.8, n = 32) | 13.0 days (SD 7.0, n = 32) | No difference | MD 2.60 (95% CI ‐1.30 to 6.50) | |
| Cough duration (delayed prescription requiring collection) | 12 days (SD 5.6, n = 32) | 13.0 days (SD 7.0, n = 32) | No difference | MD ‐1.00 (95% CI ‐4.11 to 2.11) | |
| Common cold | |||||
| Participants with fever on Day 3 | 5/67 | 6/62 | No difference | OR 0.75 (95% CI 0.22 to 2.6) | |
| Participants with fever on Day 7 | 3/67 | 4/62 | No difference | OR 0.68 (95% CI 0.15 to 3.17) | |
| Participants with diarrhoea | 11/67 | 12/62 | No difference | OR 0.79 (95% CI 0.53 to 1.19) | |
| Participants with pain on Day 3 | 13/61 | 9/58 | No difference | OR 1.47 (95% CI 0.58 to 3.77) | |
| Participants with pain on Day 7 | 1/61 | 3/58 | No difference | OR 0.31 (95% CI 0.03 to 3.03) | |
| Participants with cough on Day 3 | 54/67 | 51/62 | No difference | OR 0.90 (95% CI 0.37 to 2.18) | |
| Participants with cough on Day 7 | 41/61 | 43/58 | No difference | OR 0.72 (95% CI 0.32 to 1.58) | |
| Fever severity on Day 3 | 36.2 °C (SD 0.7, n = 61) | 36.4 °C (SD 0.6, n = 58) | No difference | MD ‐0.24 (95% CI ‐0.48 to 0.00) | |
| Fever severity on Day 7 | 36.0 °C (SD 0.8, n = 59) | 36.3 °C (SD 0.6, n = 60) | Delayed antibiotics | MD ‐0.32 (95% CI ‐0.57 to ‐0.07) | |
| Pain duration (delayed prescription at time of visit) | 8.4 days (SD 8.2, n = 29) | 6.7 days (SD 4.5, n = 20) | No difference | MD 1.70 (95% CI ‐1.88 to 5.28) | |
| Pain duration (delayed prescription requiring collection) | 10.1 days (SD 7.5, n = 20) | 6.7 days (SD 4.5, n = 20) | No difference | MD 3.40 (95% CI ‐0.43 to 7.23) | |
| Fever duration (delayed prescription at time of visit) | 3.0 days (SD 1.2, n = 29) | 5.3 days (SD 6.2, n = 20) | No difference | MD ‐2.30 (95% CI ‐5.05 to 0.45) | |
| Fever duration (delayed prescription requiring collection) | 4.2 days (SD 3.0, n = 20) | 5.3 days (SD 6.2, n = 20) | No difference | MD ‐1.10 (95% CI ‐4.12 to 1.92) | |
| Cough duration (delayed prescription at time of visit) | 8.3 days (SD 5.2, n = 29) | 7.6 days (SD 5.6, n = 20) | No difference | MD ‐0.70 (95% CI ‐2.40 to 3.80) | |
| Cough duration (delayed prescription requiring collection) | 6.4 days (SD 4.6, n = 20) | 7.6 days (SD 5.6, n = 20) | No difference | MD ‐1.20 (95% CI ‐4.38 to 1.98) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 15.2 days (SD 9.7, n = 29) | 13.0 days (SD 8.8, n = 20) | No difference | MD 2.20 (95% CI ‐3.03 to 7.43) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 10.7 days (SD 7.2, n = 20) | 13.0 days (SD 8.8, n = 20) | No difference | MD ‐2.30 (95% CI ‐7.28 to 2.68) | |
CI: confidence interval
MD: mean difference
OR: odds ratio
SD: standard deviation
SMD: standardised mean difference
| Study | Outcome | Delay | No antibiotics | Favours | Result (with 95% CI) |
|---|---|---|---|---|---|
| Sore throat | |||||
| Pain duration (delayed prescription at time of visit) | 5.7 days (SD 5.1, n = 45) | 7.8 days (SD 6.0, n = 46) | No difference | MD ‐2.10 (95% CI ‐4.39 to 0.19) | |
| Pain duration (delayed prescription requiring collection) | 7.4 days (SD 6.3, n = 46) | 7.8 days (SD 6.0, n = 46) | No difference | MD ‐0.40 (95% CI ‐2.91 to 2.11) | |
| Fever duration (delayed prescription at time of visit) | 3.1 days (SD 1.8, n = 45) | 3.2 days (SD 2.5, n = 46) | No difference | MD 0.10 (95% CI 0.99 to 0.79) | |
| Fever duration (delayed prescription requiring collection) | 3.4 days (SD 2.4, n = 46) | 3.2 days (SD 2.5, n = 46) | No difference | MD 0.20 (95% CI ‐0.80 to 1.20) | |
| Cough duration (delayed prescription at time of visit) | 8.1 days (SD 5.9, n = 45) | 10.6 days (SD 8.6, n = 46) | No difference | MD 0.0 (95% CI ‐2.37 to 2.37) | |
| Cough duration (delayed prescription requiring collection) | 8.2 days (SD 6.9, n = 46) | 10.6 days (SD 8.6, n = 46) | No difference | MD 0.10 (95% CI ‐2.48 to 2.68) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 7.2 days (SD 4.3, n = 45) | 8.9 days (SD 6.5, n = 45) | No difference | MD ‐1.70 (95% CI ‐3.96 to 0.56) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 9.7 days (SD 8.3, n = 46) | 8.9 days (SD 6.5, n = 46) | No difference | MD 0.80 (95% CI ‐2.25 to 3.85) | |
| Clinical outcomes | Unavailable | Unavailable | No difference | Unavailable | |
| Acute otitis media | |||||
| Fever day 3 | 18/106 | 8/100 | No difference | OR 1.45 (95% CI 0.50 to 4.24) | |
| Pain day 3 | 26/106 | 29/100 | No difference | OR 0.64 (95% CI 0.29 to 1.38) | |
| Cough | |||||
| Pain duration (delayed prescription at time of visit versus no antibiotics) | 11 days (SD 8.0, n = 32) | 12.2 days (SD 8.0, n = 32) | No difference | MD ‐1.20 (95% CI ‐5.07 to 2.67) | |
| Pain duration (delayed prescription requiring collection versus no antibiotics) | 8.9 days (SD 6.9, n = 32) | 12.2 days (SD 7.8, n = 32) | No difference | MD ‐3.30 (95% CI ‐6.91 to 0.31) | |
| Fever duration (delayed prescription at time of visit versus no antibiotics) | 5.6 days (SD 5.9, n = 32 | 7.2 days (SD 7.9, n = 32) | No difference | MD ‐1.60 (95% CI ‐8.82 to 5.62) | |
| Fever duration (delayed prescription requiring collection versus no antibiotics) | 4.7 days (SD 4.6, n = 32) | 7.2 days (SD 7.9, n = 32) | No difference | MD ‐2.50 (95% CI ‐5.67 to 0.67) | |
| Cough duration (delayed prescription at time of visit versus no antibiotics) | 15.6 days (SD 8.8, n = 32) | 15.1 days (SD 7.6, n = 32) | No difference | MD ‐0.50 (95% CI ‐3.53 to 4.53) | |
| Cough duration (delayed prescription requiring collection versus no antibiotics) | 12.0 days (SD 5.6, n = 32) | 15.1 days (SD 7.6, n = 32) | No difference | MD ‐3.10 (95% CI ‐6.37 to 0.17) | |
| Common cold | |||||
| Pain duration (delayed prescription at time of visit versus no antibiotics) | 8.4 days (SD 8.2, n = 29) | 13.7 days (SD 6.7, n = 19) | Delayed antibiotics | MD ‐5.30 (95% CI ‐9.54 to ‐1.06) | |
| Pain duration (delayed prescription requiring collection versus no antibiotics) | 10.1 days (SD 7.5, n = 20) | 13.7 days (SD 6.7, n = 19) | No difference | MD ‐3.60 (95% CI ‐8.06 to 0.86) | |
| Fever duration (delayed prescription at time of visit versus no antibiotics) | 3.0 days (SD 1.2, n = 29) | 9.0 days (SD 8.9, n = 19) | Delayed antibiotics | MD ‐6.00 (95% CI ‐10.03 to ‐1.97) | |
| Fever duration (delayed prescription requiring collection versus no antibiotics) | 4.2 days (SD 3, n = 20) | 9.0 days (SD 8.9, n = 19) | Delayed antibiotics | MD ‐4.80 (95% CI ‐9.01 to ‐0.59) | |
| Cough duration (delayed prescription at time of visit versus no antibiotics) | 8.3 days (SD 5.2, n = 29) | 11.7 days (SD 6.4, n = 19) | No difference | MD ‐3.40 (95% CI ‐6.84 to 0.04) | |
| Cough duration (delayed prescription requiring collection versus no antibiotics) | 6.4 days (SD 4.6, n = 20) | 11.7 days (SD 6.4, n = 19) | Delayed antibiotics | MD ‐5.30 (95% CI ‐8.81 to ‐1.79) | |
| Nasal mucosity duration (delayed prescription at time of visit versus no antibiotics) | 15.2 days (SD 9.7, n = 29) | 15.2 days (SD 7.5, n = 19) | No difference | MD ‐0.0 (95% CI ‐4.88 to 4.88) | |
| Nasal mucosity (delayed prescription requiring collection versus no antibiotics) | 10.7 days (SD 7.2, n = 20) | 15.2 days (SD 7.5, n = 19) | No difference | MD ‐4.50 (95% CI ‐9.12 to 0.12) | |
CI: confidence interval
MD: mean difference
OR: odds ratio
SD: standard deviation
De la Poza Abad 2016 divided its delayed antibiotic arm into two parts, that is a patient‐led prescription strategy and a prescription collection strategy. The patient‐led prescription strategy involved the doctor providing the patient with a prescription that they could fill at a pharmacy if they decided they needed to take antibiotics based on their assessment of their symptoms. The prescription collection strategy involved patients returning to the primary care health service to collect their prescription, and then filling it at a pharmacy if they decided they required antibiotics based on their assessment of their symptoms. The clinical outcomes of this study are presented in Table 2 and Table 3.
Regarding the other primary outcomes, we conducted meta‐analyses for antibiotic use (Analysis 4.1, Analysis 4.2) and patient satisfaction (Analysis 5.1, Analysis 5.2). No data were available for antibiotic resistance.
The secondary outcomes of adverse effects of antibiotics (Analysis 6.1, Analysis 6.2, Analysis 6.3) and reconsultation (Analysis 7.1) are presented with meta‐analysis where there was sufficient homogeneity of included study data.
Subgroup analysis
For most subgroups, there were insufficient data to justify subgroup analysis. However, we did analyse the two different strategies of delaying antibiotics (prescription at consult with advice to delay and return to collect prescription). Regarding study population, two studies included only adult participants (De la Poza Abad 2016; Dowell 2001), and neither study contributed data that could be compared with other studies. Five studies included only child participants (Chao 2008; El‐Daher 1991; Little 2001; Pichichero 1987; Spiro 2006); when these studies were analysed separately there were no changes to important outcome results except for the outcome of patient satisfaction. However, just one study involving only children measured patient satisfaction for delayed antibiotics versus immediate antibiotics (Little 2001). Additionally, just one study involving only children measured patient satisfaction for delayed antibiotics versus no antibiotics (Chao 2008). We have reported the results of the subgroup analysis for patient satisfaction below in the appropriate section.
Primary outcomes
1. Clinical outcomes for sore throat, acute otitis media, bronchitis, and common cold
The results for clinical outcomes were based on moderate certainty evidence according to GRADE assessment, and are summarised in summary of findings Table 1 for delayed versus immediate antibiotics, and summary of findings Table 2 for delayed versus no antibiotics.
Sore throat
Five included studies specifically examined sore throat (N = 1573) (De la Poza Abad 2016; El‐Daher 1991; Gerber 1990; Little 1997; Pichichero 1987).
Delayed antibiotics versus immediate antibiotics
Pain was not significantly different for delayed and immediate antibiotic groups in three studies (N = 939) (Gerber 1990; Little 1997; Pichichero 1987) (Table 2). In one study (El‐Daher 1991), pain was reported by a higher proportion of participants in the delayed antibiotic group (N = 118) on Day 3 compared to the immediate antibiotic group (N = 111) with an odds ratio (OR) of 14.51 (95% confidence interval (CI) 7.14 to 29.50) (Table 2). Participants in the delayed antibiotic arms (N = 91) of the study by De la Poza Abad 2016 reported longer pain duration than participants in the immediate antibiotic arm (N = 94) with a mean difference (MD) of 2.01 days (95% CI 0.75 to 3.26). For participants given a script at the time of consultation this difference was smaller with a MD of 1.30 days (95% CI ‐0.34 to 2.94) than for participants required to return to pick up the script where the MD was 3.00 days (95% CI ‐1.03 to 4.95) (Table 2).
Two studies measured malaise (Day 3) for delayed and immediate antibiotic groups, with one study finding no evidence of difference in malaise severity on Day 3 (N = 114) (Table 2) (Pichichero 1987). The other study detected a much higher proportion of participants with malaise on Day 3 in the delayed antibiotic group (N = 118) compared to the immediate antibiotic group (N = 111), with an OR of 16.49 (95% CI 5.68 to 47.83) (Table 2) (El‐Daher 1991).
Five studies measured fever for delayed and immediate antibiotics groups (N = 1573) (De la Poza Abad 2016; El‐Daher 1991; Gerber 1990; Little 1997; Pichichero 1987). Two studies did not report fever in a way that could be readily compared with other studies (Gerber 1990; Little 1997). Two studies found fever severity on Day 3 to be higher for participants in the delayed antibiotic group than in the immediate antibiotic group (N = 343) (El‐Daher 1991; Pichichero 1987), with a pooled MD of 0.53 °C (95% CI 0.31 to 0.74) (N = 343) (Analysis 1.1). One study found that the median number of days of fever experienced by participants in the delayed antibiotic group (N = 235) was one day longer than for the immediate antibiotic group (N = 247) (P = 0.04) (Little 1997). However, in one study (N = 405) (De la Poza Abad 2016), the number of days with fever was not significantly different for participants in the delayed antibiotic group compared to the immediate antibiotic group (Table 2).
Delayed antibiotics versus no antibiotics
Two studies that recruited participants with sore throat compared the prescribing strategy of delayed antibiotics with no antibiotics (N = 1117) (De la Poza Abad 2016; Little 1997). These studies found no evidence of difference in any clinical outcome between these two prescribing strategies (Table 3).
Complications
Data on complications of sore throat such as rheumatic fever, poststreptococcal glomerulonephritis, and peritonsillar abscess were not reported in any of the five studies evaluating sore throat for the three prescribing strategies of immediate, delayed, and no antibiotics.
Acute otitis media
Three included studies recruited participants with AOM (N = 830) (Chao 2008; Little 2001; Spiro 2006).
Delayed antibiotics versus immediate antibiotics
Two studies (N = 598) compared the prescribing strategies of delayed antibiotics versus immediate antibiotics for AOM (Little 2001; Spiro 2006). One of these studies (N = 283) measured pain and fever on Days 4 to 6 and found no evidence of difference (Table 2) (Spiro 2006). In the other study (N = 315) (Little 2001), pain and malaise on Day 3 were reported by a greater proportion of participants randomised to the delayed antibiotics group compared to the immediate antibiotics group (Table 2) (Little 2001). Further analysis of earache from one trial found that the delayed antibiotic prescribing strategy did not significantly increase risk of earache at three months (OR 0.89, 95% CI 0.48 to 1.65) or one year (OR 1.03, 95% CI 0.60 to 1.78) (Little 2006).
Delayed antibiotics versus no antibiotics
Only one study compared delayed antibiotics with no antibiotics (N = 232) (Chao 2008). In this study, no significant difference was detected for the outcomes of pain or fever for participants in delayed antibiotic and immediate antibiotic groups (Table 3). This trial also advised participants in the no antibiotic arm to return in two to three days if symptoms did not resolve (Chao 2008).
Complications
Data on complications of AOM such as mastoiditis, rheumatic fever, and poststreptococcal glomerulonephritis were not reported in any of the three studies evaluating AOM for the prescribing strategies of immediate and delayed antibiotics. However, Spiro 2006 and Chao 2008 reported that no serious adverse events had occurred in participants in their studies (N = 515).
Bronchitis (cough)
Delayed antibiotics versus immediate antibiotics
Three studies examined the prescribing strategies of immediate versus delayed antibiotics for the clinical presentation of cough (N = 1401) (De la Poza Abad 2016; Dowell 2001; Little 2005a). None of the studies found any difference in clinical outcomes including pain, fever, and cough (Table 2).
Delayed antibiotics versus no antibiotics
De la Poza Abad 2016 and Little 2005a (N = 1212) also evaluated delayed antibiotics versus no antibiotics, finding no evidence of difference in clinical outcomes (Table 3).
Complications
One participant in the no antibiotic group (N = 273) of one study developed pneumonia, and recovered with antibiotics in hospital (Little 2005a). Another study (N = 405) reported that there were no evidence of differences in complication rates between the delayed and immediate antibiotic groups (De la Poza Abad 2016). The third study (N = 189) did not report on complications in the immediate and delayed antibiotic groups (Dowell 2001).
Common cold
Delayed antibiotics versus immediate antibiotics
Two studies examined immediate antibiotics versus delayed antibiotics (N = 534) and found no evidence of difference between the two prescribing strategies for fever, cough, pain, malaise, and rhinorrhoea except for the outcome of fever severity on Day 7 which favoured delayed antibiotics (Table 2) (Arroll 2002a; De la Poza Abad 2016).
Delayed antibiotics versus no antibiotics
De la Poza Abad 2016 (N = 405) compared delayed antibiotics with no antibiotics and found a reduction in pain duration in the patient‐led prescription delayed antibiotic strategy and reductions in fever and cough duration for both delay strategies (patient‐led prescription and prescription collection) compared with no antibiotics (Table 3). There was no evidence of difference between delayed and no antibiotic prescribing groups for the outcome of nasal mucosity (Table 3).
Pooling of clinical outcomes (delayed versus immediate antibiotics)
Sufficient study data were available to allow the pooling of results for the outcomes of pain (Days 3 to 6), pain severity (Day 3), malaise (Day 3), malaise severity (Day 3), fever (Days 3 to 6), and fever severity (Day 3) for the comparison of delayed versus immediate antibiotics. We conducted meta‐analysis for study data where results were sufficiently homogenous. Data were insufficient to pool results for the comparison delayed versus no antibiotics.
Pain
There was significant heterogeneity of study data for the outcome of pain on Days 3 to 6 (Analysis 1.1). For three studies there was no evidence of difference examining the clinical conditions of common cold and otitis media (Arroll 2002a; Little 2001; Spiro 2006). One study that included participants with sore throat favoured immediate antibiotics (El‐Daher 1991). Meta‐analysis for the two studies that measured pain severity on Day 3 found in favour of immediate antibiotics with an MD of 0.35 (95% CI 0.13 to 0.57) (Analysis 1.2).
Malaise
There was significant heterogeneity of study data for the outcome of malaise on Day 3 (Analysis 2.1). However, both studies found in favour of immediate antibiotics. One study included participants with otitis media (Little 2001), the other participants with sore throat (El‐Daher 1991). Meta‐analysis of the two studies measuring malaise severity on Day 3 found in favour of immediate antibiotics with an MD of 0.29 (95% CI 0.09 to 0.48) (Analysis 2.2). One of these studies recruited participants with sore throat (Pichichero 1987), the other participants with AOM (Little 2001).
Fever
Two studies provided data that could be combined for the outcome of fever on Days 3 to 6 (Arroll 2002a; Spiro 2006). Meta‐analysis of these data found no evidence of difference with an OR of 0.86 (95% CI 0.54 to 1.38) (Analysis 3.1). The three studies providing data on fever severity on Day 3 provided heterogenous results. One study including participants with the common cold found no evidence of difference in fever severity on Day 3 with an MD of ‐0.24 (95% CI ‐0.48 to ‐0.00) (Arroll 2002a). Two studies found results favouring immediate antibiotics; both studies included participants with sore throat (Analysis 3.2). The first study was Pichichero 1987 (MD 0.40, 95% CI 0.05 to 0.75), and the second was El‐Daher 1991 (MD 0.90, 95% CI 0.50 to 1.30) (Analysis 3.2).
2. Antibiotic use
Delayed antibiotics versus immediate antibiotics
The three included studies published before 1992 investigated the concern that immediate antibiotics for streptococcal pharyngitis might impair the body's immune response and predispose the patient to a relapse of pharyngitis (El‐Daher 1991; Gerber 1990; Pichichero 1987). Antibiotic use in both immediate and delayed antibiotic groups was close to 100% as intended. Seven of the included studies published after 1992 (N = 2840) evaluated delayed antibiotics as a way to reduce antibiotic use for respiratory infections compared to immediate antibiotics (Arroll 2002a; De la Poza Abad 2016; Dowell 2001; Little 1997; Little 2001; Little 2005a; Spiro 2006). All seven studies found that antibiotic use was significantly reduced in the delayed antibiotic group compared to the immediate antibiotic group. There were significant differences in the way antibiotics were delayed, which may have resulted in the marked heterogeneity of this result. Of the eight studies published after 1991, four had the delayed script kept at reception to be picked up (N = 2023) (Dowell 2001; Little 1997; Little 2001; Little 2005a), while in three the script was issued to patients with instructions to delay (N = 644) (Arroll 2002a; Chao 2008; Spiro 2006). De la Poza Abad 2016 was specifically designed to determine the relative efficacy and safety of two delayed strategies: one where the delayed script was kept at the primary care centre to be picked up (prescription collection) and one where the script was issued to patients with instructions to delay (patient‐led prescription). For the delayed arms of the five studies where the script was left at reception, antibiotics were used in 27% of cases (196/718) compared with use of antibiotics in 38% of cases (154/403) where antibiotics were issued to patients with instructions to delay (Analysis 4.1). One included study compared delayed antibiotics with no antibiotics and did not include an immediate antibiotic prescribing arm (Chao 2008). Of the eight trials conducted after 1992 that included a delayed antibiotic arm, we found 350 prescriptions filled out for 1121 participants (31.2%) (Analysis 4.1). Pooled results of these studies showed that delayed antibiotics resulted in a significant reduction in antibiotic use compared to immediate antibiotics (OR 0.04, 95% CI 0.03 to 0.05) (Analysis 4.1). This evidence is moderate certainty according to GRADE assessment (summary of findings Table 1).
Seven trials published after 1992 provided immediate antibiotic arms measuring this outcome, resulting in 882 out of 948 participants (93.0%) filling prescriptions (Analysis 4.1).
Delayed antibiotics versus no antibiotics
Four studies compared delayed antibiotics with no antibiotics (N = 1241) (Chao 2008; De la Poza Abad 2016; Little 1997; Little 2005a). Pooled results of these studies showed that 77 out of 564 participants in the no antibiotic arms filled scripts (13.7%). More participants in the delayed antibiotic groups filled prescriptions compared with the no antibiotic groups (OR 2.55, 95% CI 1.59 to 4.08) (Analysis 4.2). This evidence is moderate certainty according to GRADE assessment (summary of findings Table 2).
3. Patient satisfaction
Delayed antibiotics versus immediate antibiotics
Patient satisfaction was measured in six (of eight) studies since 1992 (N = 1663) that evaluated delayed prescribing (Analysis 5.1) (Arroll 2002a; De la Poza Abad 2016; Dowell 2001; Little 1997; Little 2001; Little 2005a). The pooled result for all six studies showed no evidence of difference between the number of participants in the delayed antibiotic group who were satisfied or very satisfied compared to the immediate antibiotic group (OR 0.65, 95% CI 0.39 to 1.10) (Analysis 5.1). For the same outcome, we obtained a similar OR of 0.62 (95% CI 0.38 to 1.01) for the three studies that included elements of blinding (N = 1125) (Arroll 2002a; Dowell 2001; Little 2005a). Similarly, the three studies without any blinding (N = 1432) found an OR for this outcome of 0.64 (95% CI 0.27 to 1.55) (De la Poza Abad 2016; Little 1997; Little 2001). For the six studies addressing this outcome, 91% of participants in the immediate antibiotics arms were satisfied or very satisfied compared with 86% of participants in the delayed antibiotics arms. The one study that involved only child participants found in favour of immediate antibiotics, with an OR of 0.32 (95% CI 0.16 to 0.65) (Little 2001). These results are based on moderate certainty evidence according to GRADE assessment (summary of findings Table 1).
Delayed antibiotics versus no antibiotics
Four studies examined patient satisfaction for delayed antibiotics compared with no antibiotics (N = 1234) (Chao 2008; De la Poza Abad 2016; Little 1997; Little 2005a). The pooled result of all four studies showed that more participants were satisfied or very satisfied in the delayed antibiotic group compared with the no antibiotic group (OR 1.49, 95% CI 1.08 to 2.06) (Analysis 5.2). The number needed to treat with delayed antibiotics rather than no antibiotics to achieve a satisfied or very satisfied patient is 22.5. Fixed‐effect and random‐effects analyses gave similar results. The two trials that blinded the outcome assessor found a similar OR for this outcome (OR 1.42, 95% CI 0.92 to 2.19) (N = 1039) (Chao 2008; Little 2005a). Similarly, the two unblinded trials found an OR of 1.58 (95% CI 0.97 to 2.55) (N = 1117) (De la Poza Abad 2016; Little 1997). For the four studies addressing this outcome, 87% of participants in the delayed antibiotic group were satisfied or very satisfied compared with 82% in the no antibiotics group. The one study that involved only child participants found no evidence of difference, with an OR of 2.00 (95% CI 0.65 to 6.18) (Chao 2008). These results are based on moderate certainty evidence according to GRADE assessment (summary of findings Table 2).
4. Antibiotic resistance
None of the included studies evaluated antibiotic resistance.
Secondary outcomes
1. Adverse effects of antibiotics
Seven studies reported on the adverse effects of antibiotics (N = 2707) (Arroll 2002a; Chao 2008; El‐Daher 1991; Little 1997; Little 2001; Little 2005a; Spiro 2006).
Delayed antibiotics versus immediate antibiotics
Heterogeneity of outcomes for adverse events may be due to differences in antibiotic prescribing recommendations for different RTIs. This is likely to have contributed to the heterogeneity evident for these outcomes, preventing pooling of results except for the outcome of rash, for which there was no significant difference (OR 1.03, 95% CI 0.54 to 1.97). Overall results for adverse effects comparing delayed and immediate antibiotics are presented for the outcomes of vomiting (N = 888) (Analysis 6.1), diarrhoea (N = 1073) (Analysis 6.2), and rash (N = 1027) (Analysis 6.3). The evidence presented below is low certainty evidence according to GRADE assessment owing to concerns about bias from lack of blinding, concerns about allocation concealment, and heterogeneity of outcome data (summary of findings Table 1).
Sore throat
Little 1997 found no evidence of difference for diarrhoea, vomiting, rash, and stomachache for participants in delayed and immediate antibiotic groups. El‐Daher 1991 found more vomiting associated with delayed compared to immediate antibiotics.
Acute otitis media
Little 2001 and Spiro 2006 found reduced diarrhoea in the delayed antibiotic group. Spiro 2006 found no evidence of difference between delayed and immediate antibiotics for vomiting, and Little 2001 found no evidence of difference for rash.
Cough
Little 2005a found no evidence of difference for adverse effects.
Common cold
There was no significant difference between delayed and immediate antibiotic groups for diarrhoea, a potential adverse effect of antibiotics (Arroll 2002a).
Delayed antibiotics versus no antibiotics
There were too few studies measuring adverse effects of antibiotics for the comparison of delayed versus no antibiotics to justify pooling results. Little 1997 (N = 712) found no evidence of difference for the outcome of vomiting in participants with sore throat (OR 0.68, 95% CI 0.34 to 1.36). Little 1997 also found no evidence of difference for the outcome of diarrhoea (OR 1.57, 95% CI 0.80 to 3.07). In the study by Chao 2008 (N = 232) of children with AOM there were no reports of diarrhoea in either the delayed or no antibiotics group. Little 1997 found no evidence of difference for the outcome of rash between delayed antibiotics and no antibiotics (OR 0.51, 95% CI 0.24 to 1.10). These results were assessed as moderate certainty evidence according to GRADE assessment (summary of findings Table 2).
2. Complications of disease
There was no significant difference in complication rates between the three prescribing strategies. Five studies reported on complications or serious adverse effects (N = 1856) (Arroll 2002a; Chao 2008; De la Poza Abad 2016; Little 2005a; Spiro 2006). More details of disease complications are reported above under clinical outcomes for each disease category.
3. Reconsultation rates
Reconsultation rates were similar between delayed and immediate antibiotic groups in two studies. Pooling resulted in an OR of 1.04 (95% CI 0.55 to 1.98) (N = 379) (Analysis 7.1). Subsequent consultation rates in the 12 months (excluding the first month) were also similar between delayed and immediate antibiotic groups in one study (Little 2001). Participants with sore throat in one study were more likely to intend to consult again if they received immediate antibiotics compared to those who received delayed antibiotics (Little 1997). These results are based on moderate certainty evidence according to GRADE assessment (summary of findings Table 1).
4. Use of other therapies
Three studies reported on use of other medicines (N = 1802) (Little 1997; Little 2001; Spiro 2006). In one study (Little 1997), there was no evidence of difference in analgesic use for participants with sore throat presenting to primary care in immediate, delayed, and no antibiotic prescribing groups. Two studies looked at analgesic use in children with AOM. One study evaluating children presenting to primary care found less paracetamol was consumed in the immediate antibiotic group compared with the delayed antibiotic group (Little 2001). The other study, which evaluated children presenting to an emergency department, found no evidence of difference between groups in paracetamol and ibuprofen use (Spiro 2006).
Discussion
Summary of main results
Results for clinical outcomes were often heterogeneous. For most outcomes there was no evidence of difference between delayed antibiotics and both immediate and no antibiotic prescribing strategies. Insufficient data precluded pooling of study data for the comparison of delayed and no antibiotics. Where data could be pooled for the strategies of delayed and immediate antibiotics, results favoured immediate antibiotics for pain severity on Day 3 (participants presented with otitis media and sore throat) and malaise severity on Day 3 (participants presented with otitis media and sore throat). There was no evidence of differences in the number of participants with fever on Days 3 to 6 (participants presented with the common cold and otitis media). All strategies appear to have similar safety with no advantage for delayed antibiotics over either no antibiotics or immediate antibiotics for disease complications. delayed and no antibiotic strategies markedly reduced the use of antibiotics for RTIs compared to immediate antibiotics. The least antibiotic use was in the no antibiotic group, followed by delayed and then immediate antibiotic groups. The number needed to treat to prevent one antibiotic prescription using the delay strategy was 1.6 compared to immediate antibiotics. The number needed to treat to prevent one antibiotic prescription using a no antibiotic strategy compared to a delayed antibiotic strategy was 7.0. Patient satisfaction was highest in the immediate antibiotic group, with 91% being moderately satisfied, very satisfied, or extremely satisfied with the consultation. The delayed antibiotic group was more satisfied (87%) than the no antibiotic group (83%). These high satisfaction results may reflect patient involvement in studies, where treating physicians were more thorough in their explanations than usual (Hawthorne effect) (French 1950; Levitt 2011). No data were available regarding antibiotic resistance.
Overall completeness and applicability of evidence
Studies comparing delayed and immediate antibiotics have been performed with two different motives. The studies of Pichichero 1987, Gerber 1990, and El‐Daher 1991 were concerned that immediate antibiotics for streptococcal pharyngitis might impair the body's immune response and predispose the patient to a relapse of pharyngitis. These studies are useful for determining the effect of delayed versus immediate antibiotics on the clinical course of suspected streptococcal pharyngitis. Seven of the remaining studies were conducted to determine if the strategy of delayed antibiotics reduces the number of prescriptions filled for RTIs while maintaining patient safety and satisfaction (Arroll 2002a; De la Poza Abad 2016; Dowell 2001; Little 1997; Little 2001). The most recent study, De la Poza Abad 2016, further aimed to explore the relative efficacy and safety of two delayed prescribing strategies.
Useful data were collected for many symptom outcomes in all studies but were not always reported in a way that could be analysed or compared with other studies. This problem was partially overcome by obtaining raw data from some trial authors. The eight studies conducted after 1992 all reported useful data on antibiotic use, and seven reported useful data on patient satisfaction.
Four trials compared delayed antibiotics with no antibiotics.
There were no data on levels of antibiotic resistance.
Quality of the evidence
All but one trial, El‐Daher 1991, were adequately randomised and accounted for incomplete data. El‐Daher 1991 did find large differences for clinical outcomes for sore throat in favour of immediate antibiotics compared to delayed antibiotics.
The assessed interventions did not lend themselves to blinding. However, three trials attempted to blind participants and doctors (Arroll 2002a; El‐Daher 1991; Pichichero 1987). In four studies the outcomes assessor was blinded, but neither participants nor caregivers were blinded (Chao 2008; Dowell 2001; Little 2005a; Spiro 2006).
Otherwise, studies were well reported. The GRADE assessments of the meta‐analyses of outcomes for antibiotic use and patient satisfaction were moderate (summary of findings Table 1; summary of findings Table 2). GRADE assessments of clinical outcome data and reconsultation rates were moderate (summary of findings Table 1, summary of findings Table 2). GRADE assessments of adverse effects of antibiotics for the comparison of delayed antibiotics versus immediate antibiotics was low owing to concerns about lack of blinding, inadequate reporting of allocation concealment, and heterogeneity of results (summary of findings Table 1).
Potential biases in the review process
Heterogeneity of RCTs was one limitation of this review. Heterogeneity may have resulted from variable clinical presentations, differences in delay method, differences in antibiotic use, and certainty of included studies. Potential for type I error (falsely positive results) is another limitation of this review given the large number of reported clinical outcome results. For example, multiple outcome measures are reported for the clinical outcomes comparing delayed and immediate antibiotic groups.
Agreements and disagreements with other studies or reviews
Findings for certain clinical outcomes may have been anticipated. Systematic reviews on antibiotics for sore throat and AOM found that the time of greatest benefit for symptoms is apparent at Days 3 or 4 after treatment was started (Spinks 2013; Venekamp 2015). Delaying antibiotics by 48 hours or more would thus overshoot this zenith. Nor is it surprising that we found more adverse reactions to antibiotics from immediate antibiotics in line with known adverse events from comparison RCTs with no antibiotics.
We found the greatest difference in clinical outcomes in the only trial of delayed antibiotics conducted in a country not considered to be a high‐income economy according to the World Bank at the time of publication (World Bank 2017). El‐Daher 1991 favoured immediate antibiotics over delayed antibiotics. This trial was also the least methodologically sound, but it highlighted that concerns expressed about delayed antibiotics for children, the elderly, and those with language or cultural difficulties may also need to be extended to lower socioeconomic populations (Datta 2008; Johnson 2007).
A parallel RCT of people with acute infective conjunctivitis similarly reported shortest symptom duration with immediate antibiotics, followed by delayed and then no antibiotics (the last resulting in least antibiotic use). There was no evidence of difference between groups for patient satisfaction (Everitt 2006).
Worrall 2010 compared delayed prescriptions dated either the day of the office visit or two days later, but did not compare delayed with either immediate or no antibiotics. This study demonstrated no significant difference between groups in terms of antibiotic use.
Randomised controlled trials comparing delayed with no antibiotics and concluding that they were both acceptable alternatives to immediate antibiotics as a means of reducing antibiotic prescriptions led to a recommendation for delayed instead of no antibiotics to address concerns about risks of complications (Little 2001; Little 2005a; Little 2005b). Doctors worried about the risk of serious infective complications consequent to adopting a no antibiotic rather than delayed antibiotic strategy might take comfort from a UK observational study showing that reduced prescribing resulted in no increase in admissions to hospital for peritonsillar abscess or rheumatic fever (Sharland 2005), although mastoiditis might be a risk at the rate of 2500 children needing to be treated with antibiotics to prevent one case (Van Zuijlen 2001). Just over a third (35%) of parents in the AOM trials used their delayed script, suggesting that the number of delayed scripts required to prevent one case of mastoiditis would be significantly higher than 2500 (Chao 2008; Little 2001; Spiro 2006). A large cohort study (28,883 participants) recruiting people with symptoms and signs of lower RTI found no evidence of difference in hospitalisation or death regardless of antibiotic prescribing strategies, which included immediate, delayed, and no antibiotics (Little 2017). However, an even larger cohort study (1.82 million participants) recruited people with a diagnosis of upper respiratory tract infection, and compared hospitalisation (primary outcome) rates for both delayed and immediate antibiotics (van Staa 2020). Participants who had a delay in antibiotic prescription experienced a 52% increased risk of hospitalisation (adjusted hazard ratio 1.52, 95% confidence interval (CI) 1.43 to 1.62) which was equivalent to a number needed to harm of 1357 compared to immediate antibiotics. This non‐randomised cohort study is important owing to its large size and statistical power. However, the authors only collected data on actual delay of antibiotic prescription, so it is not known as to what extent the results reflect delayed antibiotics as a clinical prescribing strategy. Nevertheless, it does raise concerns about the small increased risks of hospitalisation associated with delayed antibiotics (van Staa 2020). Doctors often find it difficult to identify patients at risk of serious complications from respiratory infections (Kumar 2003). Patients probably perform even less well, despite their self confidence in making this decision if given a delayed antibiotic prescription. This concern is supported by empirical data: respiratory disease severity does not correlate with patients' immediate preference for an antibiotic prescription (Macfarlane 1997). We did not find any significant difference for complication rates between prescribing strategies.
There is little controversy within published guidelines that immediate antibiotics are recommended for patients who appear to be seriously unwell, fit multiple criteria indicating bacterial tonsillitis, are under six months of age with AOM, have bilateral AOM, or have AOM with otorrhoea (Tan 2008). American guidelines also recommend immediate antibiotics for children under the age of two with definite AOM (OMTG 2004). It seems then that for the majority of respiratory infections that do not meet these criteria, clinicians have the option of delayed or no antibiotics. Where doctors are confident in not prescribing antibiotics, it seems clear that no antibiotics will result in the least antibiotic use, and therefore less antibiotic resistance. Concerns about patient and doctor satisfaction with no antibiotics appear to be driving the use of a delayed strategy. Some doctors use the delay strategy to reduce antibiotic use, empower patients, and save the patient time and money without jeopardising the doctor‐patient relationship (Arroll 2002b). A qualitative study found that while some participants appreciated the option of controlling the decision as to whether and when to take antibiotics, others expected "the physician to decide" (Arroll 2002b). One physician expressed concern that patients might view delayed prescribing as physician incompetence, which was substantiated by comments from some patients. In this review, we found higher levels of patient satisfaction with a strategy of delayed antibiotics compared with no antibiotics (number needed to treat for an additional beneficial outcome: 22.5 patients). Shared decision‐making and education campaigns for doctors have been proposed as ways of helping doctors and patients avoid unnecessary antibiotic use (Butler 2001; Legare 2007; Sung 2006). One suggestion is that delayed antibiotics may in time become redundant as doctors and their patients become more reassured of the safety of not using antibiotics (Arroll 2003b). Meanwhile, a delayed antibiotics strategy may be an acceptable compromise to reduce antibiotics prescribing for RTIs and thereby reduce antibiotic resistance.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Pain: delayed versus immediate antibiotics, Outcome 1: Number of participants with pain on Days 3 to 6

Comparison 1: Pain: delayed versus immediate antibiotics, Outcome 2: Pain severity on Day 3

Comparison 1: Pain: delayed versus immediate antibiotics, Outcome 3: Duration of malaise

Comparison 1: Pain: delayed versus immediate antibiotics, Outcome 4: Duration of pain symptoms

Comparison 2: Malaise: delayed versus immediate antibiotics, Outcome 1: Number of people with malaise on Day 3

Comparison 2: Malaise: delayed versus immediate antibiotics, Outcome 2: Malaise severity on Day 3

Comparison 3: Fever: delayed versus immediate antibiotics, Outcome 1: Fever on Days 3 to 6

Comparison 3: Fever: delayed versus immediate antibiotics, Outcome 2: Fever severity on Day 3

Comparison 4: Antibiotic use, Outcome 1: Antibiotic use: delayed versus immediate antibiotics

Comparison 4: Antibiotic use, Outcome 2: Antibiotic use: delayed versus no antibiotics

Comparison 5: Patient satisfaction, Outcome 1: Patient satisfaction: delayed versus immediate antibiotics

Comparison 5: Patient satisfaction, Outcome 2: Patient satisfaction: delayed versus no antibiotics

Comparison 6: Adverse events, Outcome 1: Vomiting: delayed versus immediate antibiotics

Comparison 6: Adverse events, Outcome 2: Diarrhoea: delayed versus immediate antibiotics

Comparison 6: Adverse events, Outcome 3: Rash: delayed versus immediate antibiotics

Comparison 7: Reconsultation rate, Outcome 1: Reconsultation rate: delayed versus immediate antibiotics
| Delayed antibiotics compared to immediate antibiotics for respiratory infections | ||||||
| Patient or population: respiratory infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with immediate antibiotics | Risk with delayed antibiotics | |||||
| Clinical outcomes | 10 included studies contributing data to this comparison measured clinical outcomes. For the 4 studies including participants with cough or common cold there was no evidence of difference for clinical outcomes. 5 studies included clinical outcome data for the presentation of sore throat, and for most clinical outcomes we found no evidence of difference. 2 studies measured clinical outcomes for participants with acute otitis media with 1 finding no evidence of difference in clinical outcomes, and the other favouring immediate antibiotics for malaise and pain severity on Day 3. There were sufficient outcome data to pool results for some clinical outcome measures. For participants with otitis media and sore throat, results favoured immediate antibiotics over delayed antibiotics for reducing pain and malaise severity on Day 3. For participants with common cold and otitis media, there was no evidence of differences in the number of participants with fever on Days 3 to 6 | ‐ | 2419 | ⊕⊕⊕⊝ |
| |
| Antibiotic use: delayed versus immediate antibiotics | 930 per 1000 | 348 per 1000 | OR 0.04 | 1963 | ⊕⊕⊕⊝ |
|
| Patient satisfaction: delayed versus immediate antibiotics | 909 per 1000 | 866 per 1000 | OR 0.65 | 1633 | ⊕⊕⊕⊝ |
|
| Reconsultation rate: delayed versus immediate antibiotics | 109 per 1000 | 113 per 1000 | OR 1.04 | 379 | ⊕⊕⊕⊝ |
|
| Adverse effects of antibiotics (Adverse effects) | The outcome of diarrhoea was measured by 4 studies and results favoured delayed antibiotics in 2 studies, and there was no evidence of difference the other 2. The outcome of vomiting was measured by 3 studies with no evidence of difference in 2, and results favouring immediate antibiotics in a third. The results for rash, measured by 2 studies, were sufficiently homogenous to conduct meta‐analysis, and results showed no evidence of difference | ‐ | 1303 | ⊕⊕⊝⊝ |
| |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded 1 level because more than half of studies were not adequately blinded and did not adequately report allocation concealment | ||||||
| Delayed antibiotics compared to no antibiotics for respiratory infections | ||||||
| Patient or population: respiratory infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with no antibiotics | Risk with delayed antibiotics | |||||
| Clinical outcomes (clinical outcomes) | 4 studies measured clinical outcomes for this comparison. 2 studies recruited participants with sore throat, one study recruited participants with otitis media, and 1 study recruited participants with cough, and for these studies there was no evidence of differences found. 1 study recruited participants with the common cold, and found results favouring delayed antibiotics for pain, fever, and cough duration, but no evidence of difference for nasal mucosity | ‐ | 955 | ⊕⊕⊕⊝ |
| |
| Antibiotic use: delayed versus no antibiotics | 137 per 1000 | 287 per 1000 | OR 2.55 | 1241 | ⊕⊕⊕⊝ |
|
| Patient satisfaction: delayed versus no antibiotics | 824 per 1000 | 875 per 1000 | OR 1.49 | 1235 | ⊕⊕⊕⊝ |
|
| Adverse effects of antibiotics (adverse effects) | 2 studies measured adverse effects. 1 recruited participants with sore throat, and 1 with otitis media. Neither study found any difference in adverse effects | ‐ | 566 | ⊕⊕⊕⊝ |
| |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
| aDowngraded 1 level for inadequate blinding for all studies, and allocation concealment not adequately reported for more than half of studies | ||||||
| Author Year | Disease | Participants | Trial outcomes | Materials and procedures for clinicians delivering intervention | Clinicians delivering intervention | How intervention was delivered to participants | Where intervention was delivered | When and how much | Tailoring | Modified during trial? | Checks of fidelity? | Fidelity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Common cold | Any age | Antibiotic use, satisfaction, and symptoms of delayed prescribing | Antibiotic prescription (deemed appropriate by treating GP). Procedure not detailed | 15 GPs | Delayed: to fill prescription after 3 days if symptoms not improved Immediate: usual care | 1 general practice, New Zealand | Once, at index consultation; delayed group asked to wait 3 days | Participants advised to return to GP if symptoms worsened. | None reported | Not detailed | ‐ | |
| Acute otitis media | Children (2 to 12 years) | Antibiotic use | 2 forms of discharge instruction sheet provided by clinicians to patients: 1) completion of all: when to return for medical care (after 2 to 3 days); how to use complimentary symptom drugs 2) comparison: as above + prescription to fill if still unwell at 2 to 3 days | 14 emergency department physicians | Not detailed | Emergency department of an urban public hospital in the USA | Once, at index consultation | Provided with complimentary optional ibuprofen or paracetamol +/‐ benzocaine otic drops at index consultation | None reported | None | None | |
| Acute uncomplicated respiratory infection | Adults | Symptom duration and severity, antibiotic use, patient satisfaction, patients’ beliefs in antibiotic effectiveness | Physician structured script and patient information sheet about self limiting natural history of respiratory infection, pros and cons of antibiotics used with patients. Antibiotic prescription as indicated | GPs | 4 groups of antibiotic prescription use: 1) immediate; 2) delayed, patient‐led prescription; 3) delayed, prescription collection; 4) none. Delayed = 3 days | 23 primary care centres in 4 regions in Spain | Once, at index consultation; delayed prescription collection group could collect after 3 days if needed | All advised to return if no improvement or worsening after 5 days (pharyngitis) or 10 days (other infections). Central phone follow‐up if symptoms persisted | None reported | None | None | |
| Acute uncomplicated cough | Adults (> 16 years) | Symptom duration, prescription uptake, patient satisfaction, patient enablement subsequent consultation rates | Antibiotic prescription of GP's choice provided or lodged at reception. | 48 GPs | Immediate: usual care delayed: collect prescription after 1 week if required (within 2 weeks) | 22 general practices in Scotland, UK | Once, at index consultation; delayed prescription group asked to wait 1 week | Nil | None reported | Date scripts collected by delayed group | 35% (12/34) waited 7 days as asked; mean wait 6 days (range 1 to 10). | |
| GABHS | Children (4 to 14 years) | Signs and symptoms, antibody titre, subsequent episodes | Immediate group: supplied with 2 days of penicillin, then 8 days of penicillin on Day 3. delayed group: supplied with 2 days of placebo, then 10 days of penicillin on Day 3 | Physician | Immediate: 2 days penicillin, then 8 days penicillin delayed: 2 days placebo, then 10 days penicillin | Paediatric clinics at Jordan University of Science and Technology, Jordan | At index consultation, then re‐examined on Day 3 | Paracetamol as needed | None reported | None reported | None reported | |
| GABHS pharyngitis | Children / adolescents (2 to 22 years) | Positive follow‐up throat cultures, recurrences, symptomatic recurrences, or new acquisitions | Immediate group: supplied with 10‐day course of dose appropriate penicillin V. Delayed group: instructed to wait 48 hours before commencing 10‐day course of penicillin. Telephone follow‐up 24 hours later in both groups and next 24 hours for delayed group to advise commencement | Not reported (implied treating physicians) | Immediate: usual care delayed: wait 48 hours before commencing penicillin | 1 private paediatric practice in the USA | At index consultation and telephone follow‐up 24 and 48 hours afterwards | Further 10‐day courses of penicillin if further GABHS pharyngitis | None reported | Urine sample at Day 9, mailed after drying for analysis | No report of urine sample compliance results | |
| Sore throat | ≥ 4 years | Duration of symptoms, satisfaction and compliance with and perceived efficacy of antibiotics, time off school or work | Immediate group given 10‐day prescription of dose appropriate penicillin V. Delayed group offered antibiotics but could collect prescription if symptoms not settled within 3 days. GP standard advice sheets provided to participants | 25 GPs | 3 groups of antibiotic prescriptions: 1) immediate: usual care; 2) no antibiotics; 3) delayed: to collect within 3 days. | 11 general practices, England, UK | At index consultation; delayed prescription group within 3 days | Erythromycin if sensitive to penicillin. Analgesics or antipyretics allowed. | None reported | GP documented prescription on sheet. Patient daily diary until symptom‐free and medication finished | GPs’ compliance: immediate: 99%; no ABs: 2%; delayed: 5% left with script AB use: immediate: 99%; no: 13%; delayed: 31% | |
| Acute otitis media | Children (0.5 to 10 years) | Symptom resolution, absence from school or nursery, paracetamol consumption | Immediate group prescribed amoxicillin. Delayed group asked to delay 3 days before using prescription, and then only if necessary. GP used standardised advice sheets specific to each group | 42 GPs | Immediate: usual care delayed: wait 3 days to collect prescription | General practices in Scotland, UK | At index consultation; delayed prescription group asked to wait 3 days | Antipyretics were allowed. | None reported | Patient diary | No | |
| Acute uncomplicated lower respiratory tract infection | ≥ 3 years | Symptom duration and severity, antibiotic use, satisfaction, belief in antibiotics | Immediate group: prescription for 10 days amoxicillin. Delayed group: prescription written and left at reception for patient to retrieve if wanted (but advised to wait 14 days). Leaflet groups: 1‐page information leaflet covering natural history of illness, when to seek further help. All groups: statement about analgesics, natural history of illness, and prescribing strategy read out by physicians | 37 GPs | 6 groups (factorial): 1) no antibiotics, no leaflet; 2) delayed antibiotics, no leaflet; 3) immediate antibiotics, no leaflet; 4) no antibiotics, leaflet; 5) delayed antibiotics and leaflet; 6) immediate antibiotics and leaflet. Delay = 14 days | General practices, England, UK | At index consultation; 14 days for delayed prescription group | Erythromycin if allergic to penicillin. Antipyretics allowed. | None reported | Reported antibiotic use in diary | 96% immediate group; 20% delayed group; 16% no ABs group | |
| Sore throat (presumed GABHS) | Children (4 to 18 years) | Symptomatic response, recurrent infections | Drugs supplied directly to patients. Usual care 10‐day course penicillin V. Delayed group provided with placebo for first 3 days, then penicillin | Study nurse | Immediate: usual care delayed: placebo for 3 days then penicillin | Primary care paediatric practice in the USA | At index consultation | Antibiotic (tablet or suspension). Antipyretics were allowed | None reported | Check drug bottles at 3 days and 3 weeks. Test urine at 3 days for antibiotic | Confirmed in 98% cases (drug bottles); no ABs used in placebo group | |
| Acute otitis media | Children (0.5 to 12 years) | Antibiotic use, clinical symptoms, adverse outcomes, days off school or work, unscheduled medical visits, parents’ comfort with management | Provision of written prescription for antibiotics valid for 3 days. Wait‐and‐see prescription group given written and verbal instructions to only fill prescription if no improvement or worsening 2 days after emergency room visit | Emergency department clinicians | Immediate: usual care Wait‐and‐see prescription: wait 2 days | Paediatric emergency department in the USA | At index consultation and within 3 days if prescription filled | Ibuprofen and otic drops as needed. Primary care contact if worsening | None reported | Verification of filling of prescription by phone call to designated pharmacies for 28% of the sample | All instances of no filling of prescription confirmed by pharmacies, and 90% confirmation of parent report of prescription filled | |
| ABs: antibiotics | ||||||||||||
| Study | Outcome | Delay | Immediate | Favours | Result (95% CI) |
|---|---|---|---|---|---|
| Sore throat | |||||
| Fever severity on Day 3 | 37.2 (SD 1.2, n = 55) | 36.8 (SD 0.6, n = 59) | Immediate antibiotics | MD 0.40 (95% CI 0.05 to 0.75) | |
| Malaise severity on Day 3 | 1.3 (SD 1.0, n = 55) | 1.1 (SD 0.7, n = 59) | No difference | MD 0.20 (95% CI ‐0.11 to 0.51) | |
| Pain severity on Day 3 | 1.6 (SD 1.4, n = 55) | 1.3 (SD 1.3, n = 59) | No difference | MD 0.30 (95% CI ‐0.15 to 0.75) | |
| Compliance | 55/55 | 59/59 | No difference | 100% in both groups | |
| Recurrence rate | ‐ | ‐ | No difference | Data not available | |
| Compliance | 44/50 | 59/63 | Delayed antibiotics | 88% in immediate group and 93% in delayed group | |
| Vomiting | 57/118 | 4/111 | Immediate antibiotics | OR 25.00 (95% CI 8.65 to 72.25) | |
| Pain on Day 3 | 106/118 | 42/111 | Immediate antibiotics | OR 14.51 (95% CI 7.14 to 29.50) | |
| Malaise on Day 3 | 45/118 | 4/111 | Immediate antibiotics | OR 16.49 (95% CI 5.68 to 47.83) | |
| Fever severity on Day 3 | 38.0 °C (SD 2.0, n = 118) | 37.1 °C (SD 1.0, n = 111) | Immediate antibiotics | SMD 0.58 (95% CI 0.31 to 0.84) | |
| Vomiting | 15/179 | 18/215 | No difference | OR 1.00 (95% CI 0.49 to 2.05) | |
| Diarrhoea | 23/179 | 23/215 | No difference | OR 1.23 (95% CI 0.67 to 2.28) | |
| Rash | 11/180 | 14/215 | No difference | OR 0.93 (95% CI 0.41 to 2.11) | |
| Stomachache | 48/180 | 66/215 | No difference | OR 0.82 (95% CI 0.53 to 1.27) | |
| Fever (> 37.0 °C) | Unavailable | Unavailable | Immediate antibiotics | Data not available | |
| Pain | Unavailable | Unavailable | No difference | Data not available | |
| Cough | Unavailable | Unavailable | No difference | Data not available | |
| Malaise | Unavailable | Unavailable | No difference | Data not available | |
| Analgesic use | Unavailable | Unavailable | No difference | Data not available | |
| Time off work | Unavailable | Unavailable | No difference | Data not available | |
| Pain duration (delayed prescription at time of visit) | 5.7 days (SD 5.1, n = 45) | 4.4 days (SD 2.4, n = 47) | No difference | MD 1.30 (95% CI ‐0.34 to 2.94) | |
| Pain duration (delayed prescription requiring collection) | 7.4 days (SD 6.3, n = 46) | 4.4 days (SD 2.4, n = 47) | Immediate antibiotics | MD 3.00 (95% CI ‐1.03 to 4.95) | |
| Fever duration (delayed prescription at time of visit) | 3.1 days (SD 1.8, n = 45) | 2.9 days (SD 1.7, n = 47) | No difference | MD ‐0.20 (95% CI ‐0.52 to 0.92) | |
| Fever duration (delayed prescription requiring collection) | 3.4 days (SD 2.4, n = 46) | 2.9 days (SD 1.7, n = 47) | No difference | MD 0.50 (95% CI ‐0.35 to 1.35) | |
| Cough duration (delayed prescription at time of visit) | 8.1 days (SD 5.9, n = 45) | 8.1 days (SD 5.7, n = 47) | No difference | MD ‐2.50 (95% CI ‐5.52 to 0.52) | |
| Cough duration (delayed prescription requiring collection) | 8.2 days (SD 6.9, n = 46) | 8.1 days (SD 5.7, n = 47) | No difference | MD ‐2.40 (95% CI ‐5.59 to 0.79) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 7.2 days (SD 4.3, n = 45) | 5.4 days (SD 3.9, n = 47) | Immediate antibiotics | MD ‐1.80 (95% CI 0.12 to 3.48) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 9.7 days (SD 8.3, n = 46) | 8.9 days (SD 6.5, n = 46) | Immediate antibiotics | MD 4.30 (95% CI 1.65 to 6.95) | |
| Acute otitis media | |||||
| Diarrhoea | 14/150 | 25/135 | Delayed antibiotics | OR 0.45 (95% CI 0.22 to 0.91) | |
| Rash | 8/150 | 6/135 | No difference | OR 1.21 (95% CI 0.41 to 2.58) | |
| Participants with pain on Day 3 | 28/111 | 15/101 | No difference | OR 1.93 (95% CI 0.96 to 3.88) | |
| Participants with pain on Day 7 | 3/111 | 0/101 | No difference | OR 6.55 (95% CI 0.33 to 128.35) | |
| Participants with malaise on Day 3 | 45/150 | 19/135 | Immediate antibiotics | OR 2.62 (95% CI 1.44 to 4.76) | |
| Malaise severity Day 3 | 0.8 (SD 1.7, n = 150) | 0.4 (SD 1.0, n = 134) | Immediate antibiotics | MD 0.43 (95% CI 0.11 to 0.75) | |
| Malaise severity on Day 7 | 2.2 (SD 2.0, n = 150) | 1.5 (SD 1.2, n = 135) | No difference | MD 0.01 (95% CI ‐0.11 to 0.13) | |
| Pain severity on Day 3 | 2.6 (SD 2.1, n = 111) | 1.8 (SD 1.4, n = 102) | Immediate antibiotics | MD 0.75 (95% CI 0.26 to 1.24) | |
| Pain severity on Day 7 | 1.17 (SD 0.75, n = 111) | 1.05 (SD 0.38, n = 101) | No difference | MD 0.12 (95% CI ‐0.04 to 0.28) | |
| Paracetamol consumption | 2.3 spoons | 1.7 spoons | Immediate antibiotics | MD 0.59 (95% CI 0.25 to 0.93) | |
| Last day of crying | 2.2 days | 1.5 days | Immediate antibiotics | MD 0.69 (95% CI 0.31 to 1.07) | |
| Episodes of earache in the 3 months since randomisation | Unavailable | Unavailable | No difference | OR 0.89 (95% CI 0.48 to 1.65) | |
| Episodes of earache over 1 year | Unavailable | Unavailable | No difference | OR 1.03 (95% CI 0.60 to 1.78) | |
| Pain day 4 to 6 | 85/132 | 89/133 | No difference | OR 0.89 (95% CI 0.54 to 1.48) | |
| Fever day 4 to 6 | 42/132 | 46/133 | No difference | OR 0.88 (95% CI 0.53 to 1.47) | |
| Vomiting | 15/132 | 15/133 | No difference | OR 1.01 (95% CI 0.47 to 2.16) | |
| Diarrhoea | 10/132 | 31/133 | Delayed antibiotics | OR 0.27 (95% CI 0.13 to 0.58) | |
| Cough | |||||
| Clinical outcomes | Unavailable | Unavailable | No difference | Data not available | |
| Clinical outcomes | Unavailable | Unavailable | No difference | Data not available | |
| Pain duration (delayed prescription at time of visit) | 11.0 days (SD 8.0, n = 32) | 10.5 days (SD 8.0, n = 32) | No difference | MD 0.50 (95% CI ‐0.34 to 4.42) | |
| Pain duration (delayed prescription requiring collection) | 8.9 days (SD 6.9, n = 32) | 10.5 days (SD 8.0, n = 32) | No difference | MD ‐1.60 (95% CI ‐5.26 to 2.06) | |
| Fever duration (delayed prescription at time of visit) | 5.6 days (SD 5.9, n = 32) | 4.1 days (SD 5.7, n = 32) | No difference | MD 1.50 (95% CI ‐1.34 to 4.34) | |
| Fever duration (delayed prescription requiring collection) | 4.7 days (SD 4.6, n = 32) | 4.1 days (SD 5.7, n = 32) | No difference | MD 0.60 (95% CI ‐1.94 to 3.14) | |
| Cough duration (delayed prescription at time of visit) | 15.6 days (SD 8.8, n = 32) | 13.0 days (SD 7.0, n = 32) | No difference | MD 2.60 (95% CI ‐1.30 to 6.50) | |
| Cough duration (delayed prescription requiring collection) | 12 days (SD 5.6, n = 32) | 13.0 days (SD 7.0, n = 32) | No difference | MD ‐1.00 (95% CI ‐4.11 to 2.11) | |
| Common cold | |||||
| Participants with fever on Day 3 | 5/67 | 6/62 | No difference | OR 0.75 (95% CI 0.22 to 2.6) | |
| Participants with fever on Day 7 | 3/67 | 4/62 | No difference | OR 0.68 (95% CI 0.15 to 3.17) | |
| Participants with diarrhoea | 11/67 | 12/62 | No difference | OR 0.79 (95% CI 0.53 to 1.19) | |
| Participants with pain on Day 3 | 13/61 | 9/58 | No difference | OR 1.47 (95% CI 0.58 to 3.77) | |
| Participants with pain on Day 7 | 1/61 | 3/58 | No difference | OR 0.31 (95% CI 0.03 to 3.03) | |
| Participants with cough on Day 3 | 54/67 | 51/62 | No difference | OR 0.90 (95% CI 0.37 to 2.18) | |
| Participants with cough on Day 7 | 41/61 | 43/58 | No difference | OR 0.72 (95% CI 0.32 to 1.58) | |
| Fever severity on Day 3 | 36.2 °C (SD 0.7, n = 61) | 36.4 °C (SD 0.6, n = 58) | No difference | MD ‐0.24 (95% CI ‐0.48 to 0.00) | |
| Fever severity on Day 7 | 36.0 °C (SD 0.8, n = 59) | 36.3 °C (SD 0.6, n = 60) | Delayed antibiotics | MD ‐0.32 (95% CI ‐0.57 to ‐0.07) | |
| Pain duration (delayed prescription at time of visit) | 8.4 days (SD 8.2, n = 29) | 6.7 days (SD 4.5, n = 20) | No difference | MD 1.70 (95% CI ‐1.88 to 5.28) | |
| Pain duration (delayed prescription requiring collection) | 10.1 days (SD 7.5, n = 20) | 6.7 days (SD 4.5, n = 20) | No difference | MD 3.40 (95% CI ‐0.43 to 7.23) | |
| Fever duration (delayed prescription at time of visit) | 3.0 days (SD 1.2, n = 29) | 5.3 days (SD 6.2, n = 20) | No difference | MD ‐2.30 (95% CI ‐5.05 to 0.45) | |
| Fever duration (delayed prescription requiring collection) | 4.2 days (SD 3.0, n = 20) | 5.3 days (SD 6.2, n = 20) | No difference | MD ‐1.10 (95% CI ‐4.12 to 1.92) | |
| Cough duration (delayed prescription at time of visit) | 8.3 days (SD 5.2, n = 29) | 7.6 days (SD 5.6, n = 20) | No difference | MD ‐0.70 (95% CI ‐2.40 to 3.80) | |
| Cough duration (delayed prescription requiring collection) | 6.4 days (SD 4.6, n = 20) | 7.6 days (SD 5.6, n = 20) | No difference | MD ‐1.20 (95% CI ‐4.38 to 1.98) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 15.2 days (SD 9.7, n = 29) | 13.0 days (SD 8.8, n = 20) | No difference | MD 2.20 (95% CI ‐3.03 to 7.43) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 10.7 days (SD 7.2, n = 20) | 13.0 days (SD 8.8, n = 20) | No difference | MD ‐2.30 (95% CI ‐7.28 to 2.68) | |
| CI: confidence interval | |||||
| Study | Outcome | Delay | No antibiotics | Favours | Result (with 95% CI) |
|---|---|---|---|---|---|
| Sore throat | |||||
| Pain duration (delayed prescription at time of visit) | 5.7 days (SD 5.1, n = 45) | 7.8 days (SD 6.0, n = 46) | No difference | MD ‐2.10 (95% CI ‐4.39 to 0.19) | |
| Pain duration (delayed prescription requiring collection) | 7.4 days (SD 6.3, n = 46) | 7.8 days (SD 6.0, n = 46) | No difference | MD ‐0.40 (95% CI ‐2.91 to 2.11) | |
| Fever duration (delayed prescription at time of visit) | 3.1 days (SD 1.8, n = 45) | 3.2 days (SD 2.5, n = 46) | No difference | MD 0.10 (95% CI 0.99 to 0.79) | |
| Fever duration (delayed prescription requiring collection) | 3.4 days (SD 2.4, n = 46) | 3.2 days (SD 2.5, n = 46) | No difference | MD 0.20 (95% CI ‐0.80 to 1.20) | |
| Cough duration (delayed prescription at time of visit) | 8.1 days (SD 5.9, n = 45) | 10.6 days (SD 8.6, n = 46) | No difference | MD 0.0 (95% CI ‐2.37 to 2.37) | |
| Cough duration (delayed prescription requiring collection) | 8.2 days (SD 6.9, n = 46) | 10.6 days (SD 8.6, n = 46) | No difference | MD 0.10 (95% CI ‐2.48 to 2.68) | |
| Nasal mucosity duration (delayed prescription at time of visit) | 7.2 days (SD 4.3, n = 45) | 8.9 days (SD 6.5, n = 45) | No difference | MD ‐1.70 (95% CI ‐3.96 to 0.56) | |
| Nasal mucosity duration (delayed prescription requiring collection) | 9.7 days (SD 8.3, n = 46) | 8.9 days (SD 6.5, n = 46) | No difference | MD 0.80 (95% CI ‐2.25 to 3.85) | |
| Clinical outcomes | Unavailable | Unavailable | No difference | Unavailable | |
| Acute otitis media | |||||
| Fever day 3 | 18/106 | 8/100 | No difference | OR 1.45 (95% CI 0.50 to 4.24) | |
| Pain day 3 | 26/106 | 29/100 | No difference | OR 0.64 (95% CI 0.29 to 1.38) | |
| Cough | |||||
| Pain duration (delayed prescription at time of visit versus no antibiotics) | 11 days (SD 8.0, n = 32) | 12.2 days (SD 8.0, n = 32) | No difference | MD ‐1.20 (95% CI ‐5.07 to 2.67) | |
| Pain duration (delayed prescription requiring collection versus no antibiotics) | 8.9 days (SD 6.9, n = 32) | 12.2 days (SD 7.8, n = 32) | No difference | MD ‐3.30 (95% CI ‐6.91 to 0.31) | |
| Fever duration (delayed prescription at time of visit versus no antibiotics) | 5.6 days (SD 5.9, n = 32 | 7.2 days (SD 7.9, n = 32) | No difference | MD ‐1.60 (95% CI ‐8.82 to 5.62) | |
| Fever duration (delayed prescription requiring collection versus no antibiotics) | 4.7 days (SD 4.6, n = 32) | 7.2 days (SD 7.9, n = 32) | No difference | MD ‐2.50 (95% CI ‐5.67 to 0.67) | |
| Cough duration (delayed prescription at time of visit versus no antibiotics) | 15.6 days (SD 8.8, n = 32) | 15.1 days (SD 7.6, n = 32) | No difference | MD ‐0.50 (95% CI ‐3.53 to 4.53) | |
| Cough duration (delayed prescription requiring collection versus no antibiotics) | 12.0 days (SD 5.6, n = 32) | 15.1 days (SD 7.6, n = 32) | No difference | MD ‐3.10 (95% CI ‐6.37 to 0.17) | |
| Common cold | |||||
| Pain duration (delayed prescription at time of visit versus no antibiotics) | 8.4 days (SD 8.2, n = 29) | 13.7 days (SD 6.7, n = 19) | Delayed antibiotics | MD ‐5.30 (95% CI ‐9.54 to ‐1.06) | |
| Pain duration (delayed prescription requiring collection versus no antibiotics) | 10.1 days (SD 7.5, n = 20) | 13.7 days (SD 6.7, n = 19) | No difference | MD ‐3.60 (95% CI ‐8.06 to 0.86) | |
| Fever duration (delayed prescription at time of visit versus no antibiotics) | 3.0 days (SD 1.2, n = 29) | 9.0 days (SD 8.9, n = 19) | Delayed antibiotics | MD ‐6.00 (95% CI ‐10.03 to ‐1.97) | |
| Fever duration (delayed prescription requiring collection versus no antibiotics) | 4.2 days (SD 3, n = 20) | 9.0 days (SD 8.9, n = 19) | Delayed antibiotics | MD ‐4.80 (95% CI ‐9.01 to ‐0.59) | |
| Cough duration (delayed prescription at time of visit versus no antibiotics) | 8.3 days (SD 5.2, n = 29) | 11.7 days (SD 6.4, n = 19) | No difference | MD ‐3.40 (95% CI ‐6.84 to 0.04) | |
| Cough duration (delayed prescription requiring collection versus no antibiotics) | 6.4 days (SD 4.6, n = 20) | 11.7 days (SD 6.4, n = 19) | Delayed antibiotics | MD ‐5.30 (95% CI ‐8.81 to ‐1.79) | |
| Nasal mucosity duration (delayed prescription at time of visit versus no antibiotics) | 15.2 days (SD 9.7, n = 29) | 15.2 days (SD 7.5, n = 19) | No difference | MD ‐0.0 (95% CI ‐4.88 to 4.88) | |
| Nasal mucosity (delayed prescription requiring collection versus no antibiotics) | 10.7 days (SD 7.2, n = 20) | 15.2 days (SD 7.5, n = 19) | No difference | MD ‐4.50 (95% CI ‐9.12 to 0.12) | |
| CI: confidence interval | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Number of participants with pain on Days 3 to 6 Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Pain severity on Day 3 Show forest plot | 2 | 327 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.13, 0.57] |
| 1.3 Duration of malaise Show forest plot | 1 | 294 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.86, 1.06] |
| 1.4 Duration of pain symptoms Show forest plot | 1 | 588 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.17, 0.29] |
| 1.4.1 Pharyngitis | 1 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.21, 0.81] |
| 1.4.2 Acute otitis media | 1 | 294 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.76, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Number of people with malaise on Day 3 Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Malaise severity on Day 3 Show forest plot | 2 | 398 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.09, 0.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Fever on Days 3 to 6 Show forest plot | 2 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.38] |
| 3.2 Fever severity on Day 3 Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Antibiotic use: delayed versus immediate antibiotics Show forest plot | 8 | 2257 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.02, 0.04] |
| 4.1.1 Antibiotic use: delayed (prescription at time of visit) versus immediate antibiotics | 4 | 841 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.04, 0.09] |
| 4.1.2 Antibiotic use: delayed (prescription collection) versus immediate antibiotics | 5 | 1416 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.01, 0.03] |
| 4.2 Antibiotic use: delayed versus no antibiotics Show forest plot | 4 | 1241 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [1.59, 4.08] |
| 4.2.1 Antibiotic use: delayed (prescription at time of visit) versus no antibiotics | 2 | 353 | Odds Ratio (M‐H, Random, 95% CI) | 3.84 [2.18, 6.76] |
| 4.2.2 Antibiotic use: delayed (prescription collection) versus no antibiotics | 3 | 888 | Odds Ratio (M‐H, Random, 95% CI) | 2.05 [1.11, 3.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Patient satisfaction: delayed versus immediate antibiotics Show forest plot | 7 | 1927 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.29] |
| 5.2 Patient satisfaction: delayed versus no antibiotics Show forest plot | 4 | 1235 | Odds Ratio (M‐H, Random, 95% CI) | 1.49 [1.08, 2.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Vomiting: delayed versus immediate antibiotics Show forest plot | 3 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Diarrhoea: delayed versus immediate antibiotics Show forest plot | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.3 Rash: delayed versus immediate antibiotics Show forest plot | 2 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Reconsultation rate: delayed versus immediate antibiotics Show forest plot | 2 | 379 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.55, 1.98] |

