Nurse‐led versus doctor‐led care for bronchiectasis
Abstract
Background
Specialist nursing roles to manage stable disease populations are being used to meet the needs of both patients and health services. With increasing cost pressures on health departments, alternative models such as nurse‐led care are gaining momentum as a substitute for traditional doctor‐led care. This review evaluates the safety, effectiveness, and health outcomes of nurses practising in autonomous roles while using advanced practice skills, within the context of bronchiectasis management in subacute, ambulatory, and/or community care.
Objectives
To compare the effectiveness of nurse‐led care versus doctor‐led care in the management of stable bronchiectasis.
Search methods
We searched the Cochrane Airways Group Specialised Register and bibliographies of selected papers in addition to grey literature such as electronic clinical trials registries. Searches were current as of March 2018.
Selection criteria
Randomised controlled trials were eligible for inclusion in the review.
Data collection and analysis
Two reviewers extracted and entered data from included studies. Primary outcomes were numbers of exacerbations requiring treatment with antibiotics, hospital admissions, and emergency department attendances.
Main results
We included one United Kingdom (UK) study in the review. In this randomised controlled trial, a total of 80 participants, with a mean age of 58 years, were treated for 12 months by a specialist nurse or doctor, then were crossed over to the other clinician for the next 12 months. Two participants died during the study period. Six participants failed to cross over to nurse‐led care because of unstable bronchiectasis. Overall, the level of study completion was high.
Data show no difference in the numbers of exacerbations requiring treatment with antibiotics (rate ratio 1.09, 95% confidence interval (CI) 0.91 to 1.30, 80 participants, moderate‐certainty evidence). Investigators reported more hospital admissions in the nurse‐led care group (rate ratio 1.52, 95% CI 1.04 to 2.23, 80 participants, moderate‐certainty evidence) and did not report emergency department attendance.
For secondary outcomes, participants in the nurse‐led care group used more healthcare resources during the first year of the trial. Increased admissions and greater use of resources made treatment costs for nurse‐led groups' higher. Total costs for both years of the study were £8,464 and £5,228 for nurse‐led care compared with doctor‐led care. However, by the second year, treatment costs were almost equitable between the two groups, which may reflect the nurses' learning of how to better treat people with bronchiectasis. No statistically significant changes were observed in quality of life, exercise capacity, mortality, or lung function. Wide confidence intervals led to uncertainty regarding these results. Adverse events were not an outcome for this review.
Authors' conclusions
This update of the review shows that only one trial met review criteria. Review authors were unable to demonstrate effectiveness of nurse‐led care compared with doctor‐led care on the basis of findings of a single study. The included study reported no significant differences, but limited evidence means that differences in clinical outcomes between nurse‐led care and usual care within the setting of a specialist clinic remain unclear. Further research is required to determine whether nurse‐led care is cost‐effective, if guidelines and protocols for bronchiectasis management are followed does this increases costs and how effective nurse‐led management of bronchiectasis is in other clinical settings such as inpatient and outreach.
PICO
Plain language summary
Nurse specialist care for bronchiectasis
Background
Bronchiectasis is a long‐term lung disease. The main symptom is cough that produces phlegm and results in recurrent chest infections. As the disease gets worse, people have poor quality of life and eventually may develop respiratory failure ‐ a condition in which the body is not able to control oxygen and carbon dioxide levels properly.
Review question
We wanted to find out if nurses are able to manage the care of people with bronchiectasis as well as doctors. We looked for randomised controlled trials comparing nurse‐led care with doctor‐led care.
Study characteristics
We found one study from the United Kingdom involving 80 people with bronchiectasis. The study was completed in 2002, when management of bronchiectasis was different from today. Participants were divided into two groups: One group of outpatients was observed for a 12‐month period under the care of the specialist nurse, and the other under care of the doctor. After 12 months, these participants swapped groups.
Key results
We found no significant differences between nurse‐led and doctor‐led care in terms of lung function, infective flareups (exacerbations), or quality of life. In the first year of the study we noted increased costs for nurse‐led care with more hospital admissions and greater use of antibiotic injections.
Certainty of evidence
The certainty of evidence in the one included study was satisfactory, given that the study design meant participants knew which group they belonged to.
Bottom line
More research is required to determine how nurse specialists compare with doctors in providing safe and effective treatment for patients with stable bronchiectasis.
This Cochrane plain language summary was up‐to‐date as of March 2018.
Authors' conclusions
Summary of findings
| Nurse‐led care compared with doctor‐led care for management of bronchiectasis | ||||||
| Patient or population: management of bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with doctor‐led care | Risk with nurse‐led care | |||||
| Exacerbations Assessed by clinician identified or participant self‐reported Follow‐up: 12 months | Mean rate of infective exacerbations was 3.1 per patient per year. | 0.28 per patient per year higher | 1.09 (95% CI 0.91 to 1.30) | 80 | ⊕⊕⊝⊝ | |
| Hospital admissions (per patient per year). | Mean admission per patient per year was 1.02. | 1.55 per patient per year higher | 1.52 (95% CI 1.03 to 2.23) | 80 | ⊕⊕⊝⊝ | More admissions in nurse‐led care. All nurse‐led care admissions approved by consultant. Protocol followed by nurse regarding management |
| Emergency department attendance | See comment. | See comment. | See comment. | See comment. | See comment. | Not reported |
| Mortality | Two participants died ‐ 1 from each care group ‐ after 12‐month assessment. | See comment. | ⊕⊕⊝⊝ | |||
| Cost‐effectiveness Total cost for duration of study and difference in cost for first and second years Cost scale: £ per participant | Total costs £5428 Cost difference £274 higher in second year | Total costs £8464 Cost difference £1940 lower in second year | ⊕⊕⊝⊝ | Costs may be reduced over time through a learning effect. | ||
| Quality of life, measured with SGRQ ‐ total scores | Unreported | MD 1.7 higher | 79 | ⊕⊕⊝⊝ | Participants reported fewer symptoms and less impact on daily life with nurse‐led care, but data show no clinical or statistically significant differences between nurse‐led and doctor‐led care. | |
| Exercise capacity: 12MWT | Mean exercise capacity: 12MWT was 746 m. | MD 18 m greater | 80 | ⊕⊕⊝⊝ | No significant differences in distance walked between nurse‐led and doctor‐led care | |
| FEV1 | Mean FEV1 was 69.5% predicted. | MD 0.2% predicted higher | 80 | ⊕⊕⊝⊝ | Nil significant differences in percentage predicted FEV1 between nurse‐led and doctor‐led care | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aCannot rule out carryover effects from cross‐over trial. No reported information at first 12‐month time period before cross‐over. May have had a learned effect that resulted in fewer exacerbations and hospitalisations and better quality of life. This may have led to better lung function and exercise capacity. Marked down one point for risk of bias. bAge of study, small number of participants, and uncertainty, with some results based on wide confidence intervals. Marked down one point for imprecision. cCannot rule out selective reporting with the decision not to cross‐over 6 participants after first 12 months. No longer considered stable bronchiectasis. Already marked down for risk of bias previously, so not downgraded again based on this reason. | ||||||
Background
Description of the condition
Bronchiectasis is a lung condition characterised by the radiological finding of permanent abnormal dilatation of one or more bronchi (Boyton 2012; Kim 2012; King 2011). Patients with bronchiectasis have a persistent productive cough and recurrent infection and often develop airway colonisation with opportunistic micro‐organisms (Abo‐Leyah 2017; McShane 2013).
Diagnosis of bronchiectasis is confirmed by high‐resolution computed tomography (HRCT) (King 2010). Diagnosis is based on this radiological finding along with presenting clinical symptoms of cough and sputum production (Abo‐Leyah 2017; Feldman 2011).
Many causes of bronchiectasis are known. These include damage to the airway associated with foreign body, past significant respiratory infections, genetic disorders, abnormal host defences, and autoimmune disease (Katzenstein 1982; Kim 2012). The underlying cause may also be idiopathic (Boyton 2012).
Clinically, patients present with symptoms of increased sputum production and recurrent infections (Kim 2012; King 2010). Other symptoms may include shortness of breath, mild to moderate airflow limitation, and haemoptysis. As the condition progresses, patients may experience poorer health status and quality of life and increased functional disability (Boyton 2012; Wong 2012). This may result in weight loss and increased airflow obstruction and may lead to further complications of respiratory failure and right‐sided heart failure (Boyton 2012).
Prevalence
Previously referred to as an orphan disease and thought to be uncommon, bronchiectasis may be more common than was previously thought, but its true incidence remains unclear (Chalmers 2017; Chang 2008). In the past, global incidence data for bronchiectasis were derived from hospital admission coding (McShane 2013; Ringshausen 2013). Disease registries have been established globally over the past five years; these provide a more accurate picture of bronchiectasis populations around the world (Chalmers 2017). In the UK, the incidence of bronchiectasis in 2013 was 35.17/100,000 women and 26.92/100,000 men (Quint 2016). Point prevalence data for the same year show that cases of bronchiectasis in women (566.1/100,000) and men (485.5/100,000) younger than 40 remain uncommon and reach higher prevalence in older age (Quint 2016). In the United States bronchiectasis prevalence appears to have an 8.74% annual increase with the number of aging bronchiectasis patients reported to be contributing to this rise (Aksamit 2017). Sex and age may contribute to the pathogenesis of bronchiectasis, and more females and elderly individuals are given the diagnosis (King 2010).
Bronchiectasis occurs independently of other respiratory diseases but may coexist and may have similar features to other respiratory chronic diseases, leading to a possible delay in diagnosis (Chang 2015; King 2010). Researchers have noted overlap of chronic obstructive pulmonary disease (COPD) and bronchiectasis, and phenotyping in both diseases has become a topic of discussion. Additional investigations are required to determine the underlying relationships and interactions of coexisting respiratory diseases (Liu 2014: Martinez‐Garcia 2017).
Bronchiectasis prevalence is higher in indigenous population groups, and bronchiectasis has been identified as an important cause of childhood morbidity (Chang 2010). Indigenous examples of higher prevalence include Aboriginal Australians (1470 cases/100,000 population), Alaskan Natives (1400 cases/100,000 population), and Canadian Inuit in the Qikiqtani Region (202/100,000) (Boyton 2012; Chang 2010; Das 2015). Higher mortality rates have been reported among Pacific (17.8/100,000) and Maoris children (4.8/100,000) than among New Zealand children of European and other ethnicity; however significant mortality does not seem to begin until adulthood (Twiss 2005). Childhood lower respiratory tract infections and environmental factors of tobacco exposure and overcrowding in the home are thought to contribute to higher rates of bronchiectasis (Das 2015).
Management
There remains a lack of certain evidence from large clinical research trials to support bronchiectasis management (Chalmers 2017). Development and evolution of guidelines for the management of bronchiectasis have continued since the release of the Spanish guideline in 2008 (Chalmers 2017; Martinez‐Garcia 2018). Guidelines based on available evidence and expert opinion have been produced by the British Thoracic Society (BTS) and The Australian and New Zealand Thoracic Society (TSANZ) (Chang 2015; Pasteur 2010), and, more recently, the European Respiratory Society, the Spanish Society of Pulmonology, and the Saudi Thoracic Society (Al‐Jahdali 2017; Martinez‐Garcia 2018; Polverino 2017). Management includes symptom control, prevention of acute exacerbations/infections, and limitation of disease progression (Feldman 2011; King 2010; Lavery 2007), and aims to support a healthy lifestyle incorporating good nutrition, non‐smoking, and regular exercise including referral to available pulmonary rehabilitation programmes (Scullion 2013). Other preventative strategies such as immunisation and infection control practices can minimise future infection risk (King 2010).
Research
The development of new treatments and targeted therapies for bronchiectasis has been slow; research trials have struggled with recruitment numbers because of coexisting conditions in a rare disease, and effectiveness of treatment for individuals with coexisting conditions remains unknown (Chalmers 2016). Duration of antibiotic treatment has been the focus of research and guideline recommendations; difficulties surround consensus, but agreement has been reached regarding the value of sputum testing at the beginning of antibiotic use (Polverino 2017). Use of long‐term antibiotics, particularly macrolides with 'antibiotic' and 'anti‐inflammatory' properties, has shown promise for reducing the frequency of exacerbations; however care in screening for non‐tuberculous mycobacteria (NTM) is needed to avoid issues of macrolide resistance in NTM (Abo‐Leyah 2017). Specific tools have been developed to assess the severity of bronchiectasis these include the FACED score (forced expiratory volume in one second (FEV1) % predicted (F), age (A), chronic colonisation by Pseudomonas aeruginosa (C), extension of the disease by radiological assessment (E), and dyspnoea (D)), eFACED (FACED score including significant exacerbations(e)), and Bronchiectasis Severity Index (BSI) and a quality of life measure (Quality of Life ‐ Bronchiectasis questionnaire); additional trials are required to validate their wider use (Chalmers 2015; Minov 2015).
Description of the intervention
Traditionally, disease management has been a medically co‐ordinated activity encompassing diagnosis, clinical assessment, medication prescription, radiography, and pathology and other investigative testing, with goals of optimising treatment and monitoring disease (Nathan 2006).
It has been over 50 years since the introduction of specialist nursing roles in the United States; Canada and the United Kingdom were close behind in introducing similar models that have since been rolled out across the world (Donald 2014). Reference to these nurses encompasses different names across the world, including specialist nurse, nurse practitioner, clinical specialist nurse, and nursing consultant (Brodsky 2008). Different countries have required varying levels of skill and education to support these roles (Brodsky 2008; Niziol 2008). Nursing extended practice roles are predominantly complementary to, or are used to substitute for, the usual (medical) model of care (Donald 2014).
Within respiratory medicine, the respiratory nurse specialist role evolved in the early 1980s, initially to meet the needs of patients in terms of rehabilitation and medication support, and have targeted disease‐specific areas such as cystic fibrosis, asthma, COPD, and occupational lung disease (Fletcher 2007; Niziol 2008).
Specialist nurses have advanced through additional education and training to encompass roles previously within the domain of the physician, resulting in blurring of professional boundaries and provision of alternative models of care to the traditional medical model (Niziol 2008). These initiatives have been embraced by nurses, their medical colleagues, and health funders (Brodsky 2008).
How the intervention might work
Nurse‐led consultation involves the specialist nurse taking on the management role for stable disease as an alternative to the traditional doctor‐led care model. This is not a new concept within chronic disease, and disease specialties are reviewing cost‐effectiveness and equivalency of care with nurse‐led models (Kilpatrick 2014). Studies specific to nurse‐led care in bronchiectasis are limited; studies in respiratory medicine are presented below.
For asthma, a six‐month randomised controlled trial saw 154 participants randomised to doctor‐led or nurse‐led care (Nathan 2006). Outcomes studied included numbers of exacerbations, changes in peak flow, quality of life (Asthma 20) questionnaire scores, and clinic attendance (Nathan 2006). Follow‐up asthma care provided by the nurse specialist was as safe and effective as that provided by the physician when a suitably trained nurse used structured interventions including similar outpatient clinic timing and access to independent prescribing (Nathan 2006).
For COPD, a review of types of nurse‐led consultations showed nurses in advanced practice roles recommending both pharmacological and non‐pharmacological treatment and autonomously functioning in diagnostic and follow‐up roles (Fletcher 2013). A randomised controlled trial involving 187 participants looked at the effects on patient outcomes of transferring outpatient doctor care to a respiratory nurse for stable patients with COPD (Vrijhoef 2007). This study showed that nurses working under a protocol were effective in improving patients' subjective knowledge and satisfaction. Nurse‐led and doctor‐led care were comparable for FEV1, body mass index (BMI), smoking status, health status, objective knowledge, and compliance, but cost increases for additional consultations were noted (Vrijhoef 2007).
For moderate to severe obstructive sleep apnoea, a multi‐centre randomised controlled non‐inferiority trial compared health outcomes of nurse‐led care versus doctor‐led care. The nurse‐led approach used a simplified diagnostic and management model to initiate in‐home sleep study and treatment and to manage follow‐up. Data showed that nurse‐led care was not inferior to physician‐led care in continuous positive airway pressure (CPAP) adherence, quality of life, and patient satisfaction (Antic 2009), but offered an effective strategy to reduce wait times for sleep study and to free up physician clinics; also, costs were reduced and access to treatment and devices was improved for trial participants (Antic 2009).
Specialist nurses in the role of alternative providers of usual (medical) care have previously proved mostly equivalent for outcomes related to patients and health systems (Kilpatrick 2014). Evidence suggests that cost savings and resource use may be improved through the use of specialist nurses in an outpatient context (Kilpatrick 2014).
Why it is important to do this review
Specialist nursing roles are gaining traction within the current health service climate, but little is known about outcomes of nurse‐led care compared with outcomes of care delivered by doctors and the cost implications of using either model. Systematic reviews examining specialist nursing roles in a variety of healthcare settings and specialisations are amassing a growing body of knowledge (Donald 2014). To date, inconsistencies in reporting of study methods and differences in nursing education, roles, and experiences have made it difficult to discern any formal conclusions, other than that more rigorous research is required (Donald 2014). This systematic review seeks to evaluate currently available evidence from randomised controlled trials exploring management of bronchiectasis ‐ both chronic and acute episodes ‐ within the context of a nurse‐led model.
Objectives
To compare the effectiveness of nurse‐led care versus doctor‐led care in the management of stable bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of parallel and cross‐over design. We considered papers published in all languages.
Types of participants
We included studies in which adult or child participants had computed tomography‐defined bronchiectasis.
Types of interventions
Interventions include specialist care managed or delivered by a nurse who provides chronic disease management for bronchiectasis through a minimum of two contacts over separate days. Excluded were studies solely focused on inpatient or immediate postprocedural care. This systematic review compared nurse‐led care versus the usual care delivery model of doctor‐led care.
Types of outcome measures
Primary outcomes
-
Exacerbations requiring treatment with antibiotics* (self‐reported and physician/specialist nurse reported)
-
Hospital admissions
-
Emergency department attendance
Secondary outcomes
-
Cost‐effectiveness
-
Quality of life measures
-
Satisfaction (patient and general practitioner (GP))
-
Exercise capacity
-
Mortality
-
Lung function, such as FEV1 and forced vital capacity (FVC)
*Nurse was accredited to prescribe antibiotics; post‐clinic review nurse met with consultant regarding all clinical decisions.
Patients were taught signs and symptoms that indicate when antibiotics for exacerbation of bronchiectasis should be initiated. They self‐reported their use of antibiotics between clinic appointments.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
-
Weekly searches of MEDLINE Ovid SP 1946 to date.
-
Weekly searches of Embase Ovid SP 1974 to date.
-
Monthly searches of PsycINFO Ovid SP.
-
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO.
-
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
-
Handsearches of the proceedings of major respiratory conferences.
We identified studies contained in the Trials Register using search strategies based on the scope of Cochrane Airways. We have provided details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We conducted the latest search in March 2018.
Searching other resources
We searched the bibliography of the included study for relevant trials that were not identified by the search strategy. We searched online clinical trials registries, including the International Standard Registered Clinical/social sTudy Number (ISRCTN) Registry from Controlled Clinical Trials (www.controlled‐trials.com), government registries (clinical trials.gov), and the International Clinical Trials Registry Platform (ICTRP) search portal of World Health Organization (WHO) registries (www.who.int/trialsearch), for completed and ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (KL and KR) independently scanned the titles, abstracts, and keywords of papers identified from the searches. We retrieved articles of potential relevance and reviewed the full text for consideration of inclusion.
We reached consensus (after discussion) on whether all independently classified citations and full‐text studies obtained should be included or excluded.
Both review authors (KL and KR) then applied inclusion criteria to determine which papers should be included in the review and should undergo data extraction. Inclusion criteria were developed on the basis of types of studies, participants, interventions, and outcomes identified.
Data extraction and management
One of the two review authors extracted all study data, the second review author verified the data. KL and KVC independently performed the risk of bias assessment.
Assessment of risk of bias in included studies
Two review authors (KL and KVC) independently assessed the included study for risk of bias related to:
-
sequence generation;
-
allocation sequence concealment;
-
blinding of participants and personnel;
-
blinding at outcome measurement;
-
incomplete outcome data;
-
selective outcome reporting; and
-
other reporting biases.
Measures of treatment effect
We extracted continuous and dichotomous outcome data and would have analysed them using standard statistical techniques with a fixed‐effect model had we identified a sufficient number of included studies for pooling in the meta‐analysis. If significant heterogeneity was found, we would have used a random‐effects model. For continuous outcomes, we would have calculated mean differences (MDs) with 95% confidence intervals (CIs) and would have pooled values as MDs or standardised mean differences (SMDs) for rates presented as rate ratios. For dichotomous outcomes, we would have calculated risk ratios (RRs) with 95% CIs.
We would have performed a narrative synthesis for each study had we included more than one. We would have combined all trials using Review Manager software.
Unit of analysis issues
We considered a mixture of cross‐over and parallel studies for inclusion in the review, with the potential for unit of analysis issues to occur had we found sufficient studies for pooling of results. We planned to use the generic inverse variance (GIV) method (by entering effect estimates and their standard errors) to adjust for unit of analysis errors when meta‐analysing the data, as per Section 7.7.7 of the Cochrane Handbook for Systematic Reviews and Interventions, had we found more than one study to allow for meta‐analysis (Higgins 2011).
Dealing with missing data
We evaluated missing information regarding participants on an available case analysis basis, as described in Chapter 16.2.2 of the Cochrane Handbook for Systematic Reviews and Interventions (Higgins 2011). If statistics essential for analysis were missing (e.g. group means and standard deviations for both groups were not reported) and could not be calculated from other data, we planned to contact the study authors to request missing data. We considered loss of participants that occurred before baseline to have no effect on eventual outcome data provided by the study. We assessed and discussed on an intention‐to‐treat basis any losses that occurred after baseline measurements had been taken.
Assessment of heterogeneity
Had we identified sufficient studies, we would have assessed statistical heterogeneity using a combination of tests, including an I² statistic and visual inspection of the data. If we had included 10 or more studies, we would have also used funnel plots. We would have considered the Der‐Simonian and Laird method of analysis presented with a P value less than 0.05 as statistically significant. In the presence of significant heterogeneity, we would have re‐analysed data using both fixed‐effect and random‐effects models.
Assessment of reporting biases
We planned to examined reporting biases by using a funnel plot, if we were able to meta‐analyse 10 or more studies.
Data synthesis
We analysed trial data using RevMan 5.1.
Subgroup analysis and investigation of heterogeneity
If we had included a sufficient number of studies, we would have performed the following subgroup analyses.
-
Hospital versus community‐based nursing care.
-
Adults versus children.
Sensitivity analysis
We planned to perform a sensitivity analysis to explore effects of bias derived from study methods on review findings. However, we did not conduct a sensitivity analysis because we included only one study, which we did not judge to be at high risk of bias for sequence generation and allocation concealment.
Results
Description of studies
Characteristics of included studies and Characteristics of excluded studies are reported in the respective tables.
Results of the search
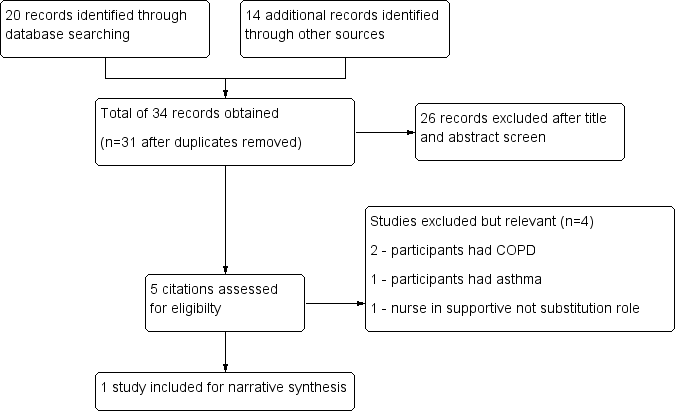
We identified 34 studies using search methods ‐ 20 citations from the literature search, and 14 through other source searches. A total of 31 studies remained when we had removed duplicates. We excluded 26 records after title and abstract screening. We considered five studies relevant and screened them for eligibility, then excluded four studies. Only one study met review inclusion criteria (Sharples 2002; see Figure 1).
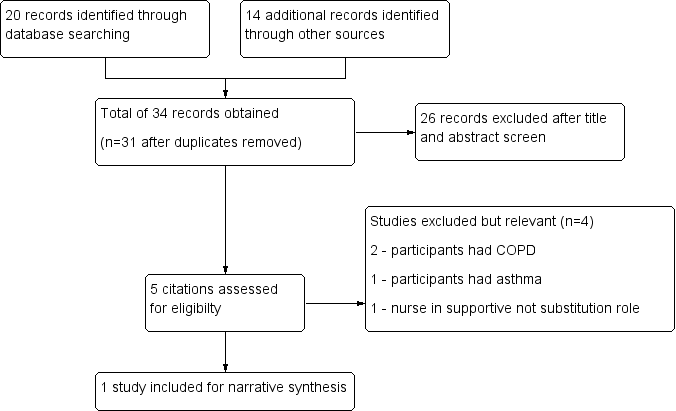
Study flow diagram.
Included studies
We have reported details of the included study in the Characteristics of included studies table and reasons for exclusion of four studies in the Characteristics of excluded studies table.
Study design
The included study ‐ Sharples 2002 ‐ investigated the efficacy of nurse specialist care in bronchiectasis using a randomised, single‐centre, cross‐over design. This study was conducted in the United Kingdom and was published in 2002.
Participant characteristics
A total of 149 patients with bronchiectasis from the Lung Defence Clinic were identified, 40 of whom were unsuitable for inclusion; seven additional participants declined or did not participate in the recruitment process. From the remaining 102 eligible participants, investigators randomised the first 80 participants to attend the clinic. All participants were ≥ 18 years of age, and mean participant age was 58.3 ± 13.3 years. The study included 55 female participants, and the diagnosis of bronchiectasis was confirmed for all participants by high‐resolution computed tomography (HRCT). Participants were recruited from a bronchiectasis outpatient clinic. Trials included only stable patients with an established management plan*. Exclusion criteria comprised a life expectancy of less than two years, expected transplant listing within the two‐year study period, FEV1 < 30% predicted, and other significant comorbidities that would modify the management of bronchiectasis. Data were obtained for 77 participants following two deaths and inability of one participant to complete tests.
Investigators allocated 39 participants to nurse‐led care in the intervention arm and the remaining 41 participants to doctor‐led care during the year‐long treatment period.
*The management plan was not specifically defined but was inclusive of best practice recommendations including physiotherapy, medication compliance, and use of antibiotics (Sharples 2002).
Intervention characteristics
Patients, on arrival to the outpatient clinic, received routine testing followed by consultation with a nurse practitioner or a doctor. Consultation involved clinical assessment, review of history, physical examination, and discussion of a treatment management plan for bronchiectasis. Appropriate changes to the management plan were made, and additional tests such as X‐ray and blood testing performed. The nurse practitioner or the doctor had the discretion to determine frequency of follow‐up appointments on the basis of a protocol that included weekly appointments for those given intravenous antibiotics at home, fortnightly appointments to assess results of antibiotic courses, and appointments every three to six months for routine monitoring of the patient's disease. When participants were randomised, they were assigned their appropriate contact person (nurse practitioner or doctor) and were encouraged to telephone that contact person with disease or management queries. The nurse practitioner had the same autonomy as the doctor to bring patient appointments forward and to recommend general practitioner review or emergency medication commencement. The nurse practitioner did not have authority to manage other systemic problems outside management of bronchiectasis, and admission to hospital for these issues was referred to the consultant. Additional education, referrals, and use of specific sputum clearance techniques were not reported.
To ensure patient safety, a supervision mechanism was included as part of the study design whereby the nurse practitioner had a detailed discussion with a consultant within 24 hours of the clinic to detail management decisions. If the consultant would have made a different management decision, the patient was contacted regarding a change in treatment.
Excluded studies
Four studies appeared relevant from the initial screening process; however on further investigation, they did not meet all criteria for inclusion. We excluded these studies from analysis for the following reasons. Two studies were randomised controlled trials involving patients with COPD (Bergner 1988; Cockcroft 1987). Both studies looked at home care nursing as the intervention, with nurses in a supportive role. Levy 2000 was a randomised controlled trial in which participants had asthma. The intervention patient education provided by a respiratory nurse was compared with no education and standard follow‐up with the general practitioner. Maa 2007 met criteria for inclusion in that this was a randomised controlled trial with participants who had bronchiectasis; however this was not a nurse‐led care comparison study. The nurse played a complementary role in supervising acupressure treatment.
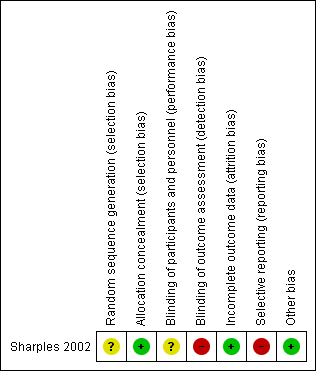
Risk of bias in included studies
Full details of our risk of bias judgements can be found in the "Risk of bias" section at the end of each Characteristics of included studies table and in Figure 2. Overall, the methodological certainty of the study was satisfactory. Two independent review authors (KL and KC) reached agreement regarding assessment of study certainty.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Review authors considered generation of randomisation sequence as having unclear risk in Sharples 2002. Investigators mentioned but did not describe randomisation. Review authors considered allocation concealment to be adequately reported in Sharples 2002. This presented low risk of bias with the use of numbered opaque envelopes.
Blinding
Blinding of participants and personnel did not occur; this was considered to confer unclear risk of bias because the design of this intervention made blinding impossible. The effect of knowing which groups the participant was assigned to and crossed over to is unknown.
Lack of blinding of assessment outcomes led to the conclusion of high risk of detection bias.
Incomplete outcome data
Overall the level of study completion was high. Two patient deaths had occurred after the first 12 months of the study; a patient given nurse‐led care died from perforated bowel, and a patient receiving doctor‐led care died from respiratory failure. Most participants completed clinical assessment. One patient was unable to complete lung function and exercise testing owing to a rib fracture not related to his bronchiectasis. Two other patients did not complete the exercise test; one at 12 months had a fractured toe, and one at 24 months was too sick to complete testing. Both of these participants had received doctor‐led care before the time of their assessment. Quality of life was not assessed in two different participants, one at 12 months and one at 24 months; both had received nurse‐led care during the assessment period. Six participants did not cross over to nurse‐led care in the second year, as they were no longer considered to have stable bronchiectasis. These participants were still included in the trial on an intention‐to‐treat basis. Trialists reported that a secondary analysis excluding participants produced almost identical results. These results were not published in the original article, and trial authors did not respond to attempts to contact them. They reported attrition with reasons and a high completion rate; therefore we judged this domain as having low risk of bias.
Selective reporting
Investigators tested changes between time periods; however, they observed effects with changes in the economic analysis in the second year of the trial and mentioned these results in the discussion but did not report them in the results. Owing to the cross‐over design with no washout period, we could not exclude the presence of a carryover effect. This may have an impact on data pertaining to exacerbations and hospital admissions in particular, but could also effect FEV1 and exercise capacity. Trialists performed post hoc analysis for carryover of clinical outcomes but stated that these results were non‐significant and did not report further on them. Attempts to seek clarification from study authors of post hoc analysis and first year data were unsuccessful. Cost changes between the first and second years of the study could be related to learned effect or selection effect, given that six participants did not cross over to nurse‐led care (Sharples 2002). For all of these reasons, we judged this domain as having high risk of bias.
Other potential sources of bias
We did not identify other potential sources of bias in this study.
Effects of interventions
See summary of findings Table for the main comparison for the main comparison of nurse‐led care versus doctor‐led care for management of stable bronchiectasis.
No meta‐analysis or sensitivity analysis was possible because only a single study was identified (Sharples 2002). To eliminate questions regarding carryover effects of doctor‐led/nurse‐led care, we attempted to contact the authors of this study to request data from the first 12 months of the study. We received no response, so we reviewed outcomes over the 24‐month study period including the 12‐month crossover time periods. We have presented narrative data from the published paper.
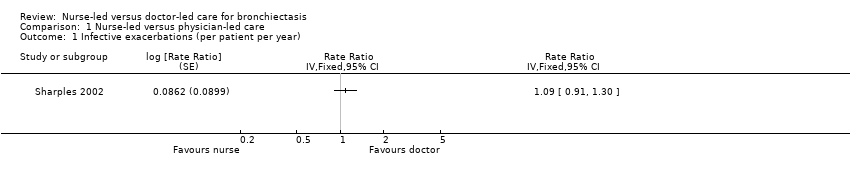
Exacerbations requiring treatment with antibiotics
Infective exacerbations were reported by patients and were not verified by the physician or nurse. The number of infective exacerbations experienced by participants during nurse practitioner‐led care was 262 in 79.4 patient‐years of follow‐up, compared with 238 in 77.8 years during doctor‐led care (rate ratio 1.09, 95% CI 0.91 to 1.30; low‐certainty evidence; Analysis 1.1).
Hospital admissions
During doctor‐led care, investigators reported 42 admissions to hospital compared with 66 during nurse‐led care (rate ratio 1.52, 95% CI 1.04 to 2.23; low‐certainty evidence; Analysis 1.2). Of these, 23 and 43 re‐admissions, respectively, were attributable to bronchiectasis (rate ratio 1.59, 95% CI 0.75 to 3.39; P = 0.22; Sharples 2002). Data show a statistically higher proportion of hospital admissions in nurse‐led care over the trial period.
Emergency department attendance
This was not reported.
Cost‐effectiveness
Researchers assessed hospital admission, use of medication, and clinic visits to determine cost‐effectiveness of nurse‐led versus doctor‐led care. Three drugs accounted for more than 80% of the difference in antibiotic utilisation, namely, intravenous meropenem and ceftazidime and nebulised colistin. These drugs are infrequently used but are costly; this meant that the slight increase in use among participants being cared for by the nurse was economically important. Most intravenous antibiotics were prescribed for treatment of patients with Pseudomonas infection, as per a well‐defined protocol. Medical staff pre‐authorised all hospital admissions and determined length of stay of these participants (Table 1). Nurse‐led care resulted in significantly higher costs per patient compared with doctor‐led care, largely owing to differences in the number of hospital admissions and increased use of intravenous and nebulised antibiotics. Total cost of nurse‐led care per patient in the first year was £5202, and total cost was £3262 in the second year. Costs of doctor‐led care per patient in the first year were £2577 and in the second year £2851 (Sharples 2002).
| Resource | Nurse‐led care (mean visits per participant) | Nurse‐led care (mean cost per participant, £) | Doctor‐led care (mean visits per participant) | Doctor‐led care (mean cost per participant, £) | Difference (SD, £) |
| Nurse‐led clinics | 4.61 | 180 | 0 | 0 | 180 (158) |
| Doctor‐led clinics | 0.45 | 25 | 4.48 | 217 | ‐192 (199) |
| Procedures | 0.13 | 61 | 0.11 | 54 | 7 (376) |
| Imaging | 1.14 | 47 | 0.76 | 45 | 1 (112) |
| Other tests | 24.58 | 260 | 18.94 | 222 | 37 (257) |
| Antibiotics (intravenous) | 23 (days) | 879 | 16 (days) | 523 | 356 (1452) |
| Antibiotics (oral) | 222 (days) | 684 | 201 (days) | 524 | 161 (695) |
| Bronchodilators | 461 (days) | 213 | 435 (days) | 193 | 20 (179) |
| Corticosteroids | 238 (days) | 278 | 219 (days) | 258 | 20 (181) |
| Other drugs | 212 (days) | 180 | 190 (days) | 155 | 25 (194) |
| Inpatient | 6.46 (days) | 1338 | 2.36 (days) | 477 | 861 (2755) |
| Day case | 0.11 | 43 | 0.05 | 16 | 27 (170) |
| GP visits | 1.11 | 20 | 1.40 | 26 | ‐6 (33) |
| Total | 4208 | 2711 | 1498 (688 to 2674) |
SD: standard deviation.
Nurse‐led clinic appointments lasted on average 26 minutes compared with 20 minutes for doctor‐led care (Sharples 2002). Nurses averaged 5.06 clinic visits per patient compared with 4.48 for doctors (Table 1).
Quality of life measures
Triaists administered St. George's Respiratory Questionnaire (SGRQ). Results show no significant differences but significant uncertainty because of wide confidence intervals for each of the scores for symptoms, control, or impact, or for total score (Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6). One patient in a nurse‐led care group refused to complete quality of life interviews at 12 months.
Satisfaction (patient and GP)
Study authors asked participants to rate their satisfaction with nurse‐led and doctor‐led care and analysed 12 individual statements related to the consultation. Statistically significant differences favoured the nurse practitioner in terms of communication and time spent with patients (Table 2).
| Comments | Nurse practitioner better, number, (%) | Doctor better, number (%) | P value |
| It was sometimes difficult to discuss your problems with the doctor/nurse practitioner. | 11/76 (14.5) | 1/76 (1.3) | 0.006 |
| The doctor/nurse practitioner explained clearly what is wrong. | 7/74 (9.5) | 0/74 (0) | 0.016 |
| The doctor/nurse practitioner examined you thoroughly when necessary. | 6/70 (8.6) | 0/70 (0) | 0.031 |
| The doctor/nurse practitioner should tell you more about your illness/condition and treatment. | 7/59 (11.9) | 3/59 (5.1) | 0.344 |
| The doctor/nurse practitioner made you feel at ease. | 2/75 (2.7) | 1/75 (1.3) | 1.000 |
| There was not enough time to discuss your problems with the doctor/nurse. | 10/74 (13.5) | 1/74 (1.4) | 0.012 |
| You felt confident the doctor/nurse practitioner knew about your medical history and your care. | 7/74 (9.5) | 1/74 (1.4) | 0.070 |
| Sometimes you felt that the doctor/nurse practitioner should listen more to what you said. | 5/69 (7.2) | 2/69 (2.9) | 0.453 |
| The doctor/nurse practitioner gave clear explanation about any tests that you needed. | 4/75 (5.3) | 1/75 (1.3) | 0.375 |
| You often came away from your appointment wishing you'd asked more questions. | 13/72 (18.1) | 9/72 (12.5) | 0.523 |
| You felt you were given a chance to have an active part when discussing your illness/condition. | 4/73 (5.5) | 0/73 (0.0) | 0.125 |
| There were frequent interruptions during your consultation. | 6/73 (8.2) | 3/73 (4.1) | 0.508 |
Exercise capacity
Results on exercise capacity were unclear owing to wide confidence intervals in distance walked during a 12‐minute walking test (12MWT) between people receiving nurse‐led care and those given doctor‐led care (low‐certainty evidence; Analysis 1.7).
Mortality
Two patients died after the 12‐month follow‐up. The patient given nurse‐led care died from a perforated bowel, and the patient receiving doctor‐led care died from end‐stage respiratory failure (low‐certainty evidence; Sharples 2002).
Lung function
Results show no statistically significant differences in FEV1/FVC percent predicted or distance walked between nurse‐led and doctor‐led care in the two treatment periods (low‐certainty evidence; Analysis 1.8; Analysis 1.9).
Discussion
Summary of main results
The primary aim of this review was to assess the effectiveness of nurse specialist care compared with traditional doctor‐led care for people with bronchiectasis. The evidence presented in this review is insufficient to show if nurse‐led or doctor‐led care is better, worse, or the same.
Sharples 2002 randomised 80 people with stable bronchiectasis to receive care from either the nurse practitioner or a physician for one year. After one year, the group crossed over to the other practitioner. This trial is now old, and since it was published, bronchiectasis guidelines have been produced for the first time. Outcome data related to infective exacerbation rate, quality of life, exercise capacity, and lung function show little between‐group difference but do not demonstrate equivalence. Patient satisfaction showed significant differences in favour of the nurse practitioner ‐ which trial authors postulated may be due to improved communication and increased time spent with patient (Sharples 2002). An increase in hospital admissions for nurse‐led care was evident when paired data from both arms of the trial were considered. Nurse‐led care in the first year of the study incurred increased costs from hospital admissions and use of antibiotics (Sharples 2002). A paucity of data contributed to wide confidence intervals for all outcomes, reflecting uncertainty in the results and low certainty of evidence.
The cross‐over design as applied to temporary effects such as the patient review is a suitable evaluation when directed to a stable chronic condition such as bronchiectasis. Advantages of this design include that each patient acts as his or her own control (eliminating participant variation amongst participants), fewer participants are required for assessment, and all participants receive the intervention (Higgins 2011). The potential for a carryover effect without a washout period between treatments cannot be excluded as a potential bias (Higgins 2011).
Overall completeness and applicability of evidence
Despite an extensive literature search and increasing utilisation of nursing specialist roles in health care, only one study conducted within this 15‐year time frame met the inclusion criteria for this review. Since 2002, bronchiectasis guidelines have been introduced and practices have changed. Additional research into classification of bronchiectasis through use of severity scores, as well as new inhaled antibiotic agents and successful use of macrolides as treatment options, have added to management strategies. Future development of targeted therapies in line with underlying causes of bronchiectasis are under investigation (Chalmers 2015).
Additional costs
Sharples 2002 reports significant additional resource use in nurse‐led care related to increased hospital admissions during the first year of the trial. However, the difference between nurse‐led care and doctor‐led care was substantially less in the second year of nurse specialist care. Study authors suggest this may be a learned effect, as the nurse became more familiar with the outpatient clinic management role. After receiving additional training, it was the nurses' first year of managing people in a clinic, but the doctors leading care groups had at least three years' experience. Trial authors postulated that further modification to the protocol may reduce the cost difference further (Sharples 2002). Few studies of good certainty have effectively shown that disease management can affect healthcare utilisation and costs (Ofman 2004). A broad systematic review of nurse‐led clinics did show some favourable results in terms of nurse‐led care cost‐effectiveness; however limited studies in this area have reduced the generalisability of these results (Randall 2017). Case management in nurse‐led clinics was also shown to increase cost‐effectiveness and to reduce hospital admission (Randall 2017).
Antibiotic stewardship
The role of stewardship in the use of antibiotics is to maximise clinical success and reduce unplanned consequences such as antibiotic resistance (Garau 2014). People with bronchiectasis who experience frequent exacerbations should have antibiotic choice guided by sputum testing for microscopy, culture, and sensitivity (Pasteur 2010; Polverino 2017). Colonisation with opportunistic organisms such as Pseudomonas can occur in bronchiectasis, and the decision to treat an individual with infection should be made using clinically objective measures (i.e. presence of temperature, general malaise) and, when possible, blood results indicating raised C‐reactive protein (CRP) or white cell count (WCC) (Brink 2016; Garau 2014).
The role of non‐pharmacological interventions such as self‐management/chronic disease support and review of sputum clearance techniques should be considered to optimise care (Garau 2014; Pasteur 2010).
Protocol use
Use of a protocol by the nurse may have led to increased costs through compliance with the recommended management pathway. No evidence suggests that doctor‐led care followed the same protocol. Variance in decision making between the three doctors involved in the study cannot be excluded (Sharples 2002). A management protocol would have been used in Sharples 2002 in the absence of bronchiectasis guidelines, which were not released until 2008 (Martinez‐Garcia 2018). Auditing of British Thoracic Society (BTS) guidelines for bronchiectasis management has revealed lack of adherence to the guideline (Hill 2012; Hill 2014). Few economic evaluations were reported before guideline release (Garrison 2016); however it is expected that better management through adherence to guidelines should lead to better health outcomes (Polverino 2017). Further evaluation of protocol use/guideline adherence by clinicians is recommended to determine whether costs reduce overtime and health outcomes are improved.
Hospital admissions
Hospital admissions were higher for the group receiving nurse‐led care. All admissions in nurse specialist care had to be authorised by a consultant, and all admissions were considered appropriate (Sharples 2002). This increase in cost may be attributed to the experience of the nurse compared with that of the doctor. The nurse practitioner had been trained to practice at an advanced level but required additional training in the specialist aspect of this disease before participating in the study. The doctor‐led care group included physicians with a minimum of two to three years of consultative experience in caring for patients with respiratory disorders.
Nurse training
The nursing role or its level of specialisation is not defined within the BTS guideline for bronchiectasis nor in the European Respiratory Society (ERS) monograph, but the BTS guideline suggests that nurses should be suitably trained to fulfil their role in the management of bronchiectasis (Floto 2011;Pasteur 2010). The advanced practice role of a specialist nurse in a tertiary setting in Sharples 2002 was acknowledged in the guideline (Pasteur 2010). Little has been written about nurses and their training in recent guideline releases. The Spanish guideline has identified some of the key nursing responsibilities, which include control of treatment adherence, assessment of medication tolerance, inhaled medication education, maintenance of equipment, intravenous antibiotic administration, disease monitoring with spirometry, and sputum clearance (Martinez‐Garcia 2018). Introduction of guidelines for management assists nurses in advanced or extended practice roles to provide translational health care through integration of guidelines into current practice when clinically judged appropriate (Branham 2014). The costs of nurse training were also included among study expenses.
Patient satisfaction
Patient satisfaction was greater for communication and time spent with patient when nurse‐led care was provided (Sharples 2002). Nurse interventions in complementary roles to doctor‐led care often include review of patient needs, education, self‐management, and additional referral to healthcare professionals (Kilpatrick 2014). This approach is likely to be incorporated into nurse‐led care models, accounting for some of the extra time that nurses spent with their patients (Vrijhoef 2007).
The generalisability of results presented in Sharples 2002 is limited to the Lung Defence Clinic because no similar studies have been conducted.
Quality of the evidence
The overall quality of the included study was low, but because review authors identified only one study during the literature, we have limited ability to conclude, beyond this study, that nurse‐led care in the management of stable bronchiectasis is more effective than doctor‐led care.
Potential biases in the review process
No significant biases were expected, or and none were found to occur during the review process. Criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions were strictly followed to limit potential biases during screening, data extraction, and data analysis (Higgins 2011). Two review authors independently assessed risk of bias and resolved conflicts by discussion with a third review author.
We attempted to correspond with study authors but received no reply. None of the authors of this review reported conflicts of interests, financial or otherwise.
Agreements and disagreements with other studies or reviews
We did not identify any new studies via updated searches; therefore our conclusions have not substantively changed in this update. We have updated the background section to present a contemporary picture of bronchiectasis and to provide examples of respiratory nurse‐led care versus doctor‐led care for a variety of respiratory diseases. Cochrane methods have changed since the review was last completed, and we have updated this review to bring it in line with current recommendations.
Respiratory specialist nurses may focus on a specific single disease or may have a broader chronic disease scope of practice (Fletcher 2013). For other respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and sleep disorders, specialist nurses have been shown to be comparable to doctors caring for stable patient groups (Antic 2009; Fletcher 2013; Nathan 2006). Expanding the scope of this review to make it inclusive of respiratory specialist nurses functioning in an alternative role to doctors in providing care may improve the yield of suitable studies and improve the body of evidence to permit a determination about specialist nurse care.
Study flow diagram.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Nurse‐led versus physician‐led care, Outcome 1 Infective exacerbations (per patient per year).

Comparison 1 Nurse‐led versus physician‐led care, Outcome 2 Admissions per patient per year.

Comparison 1 Nurse‐led versus physician‐led care, Outcome 3 SGRQ ‐ symptoms.
Comparison 1 Nurse‐led versus physician‐led care, Outcome 4 SGRQ ‐ control.

Comparison 1 Nurse‐led versus physician‐led care, Outcome 5 SGRQ ‐ impact.
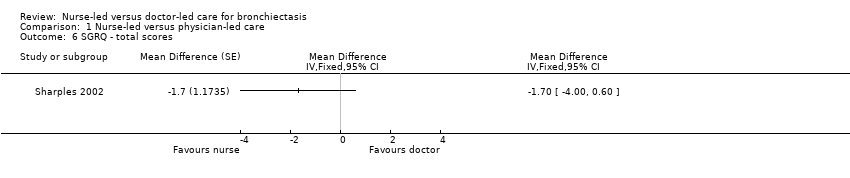
Comparison 1 Nurse‐led versus physician‐led care, Outcome 6 SGRQ ‐ total scores.
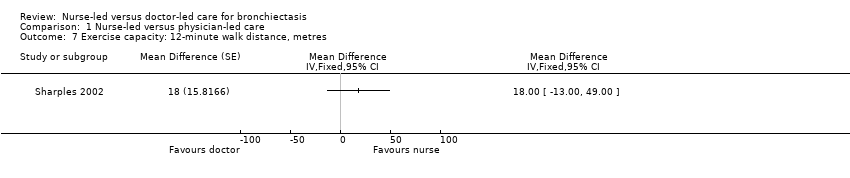
Comparison 1 Nurse‐led versus physician‐led care, Outcome 7 Exercise capacity: 12‐minute walk distance, metres.
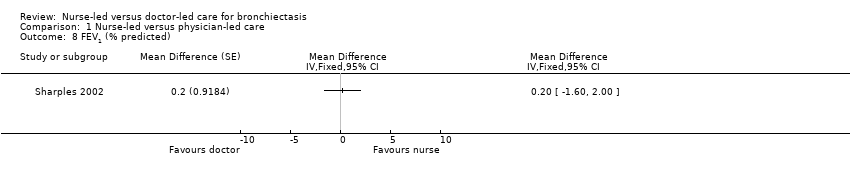
Comparison 1 Nurse‐led versus physician‐led care, Outcome 8 FEV1 (% predicted).
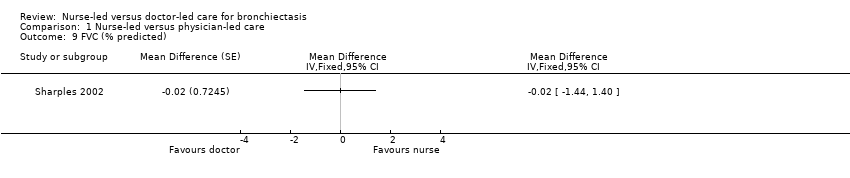
Comparison 1 Nurse‐led versus physician‐led care, Outcome 9 FVC (% predicted).
| Nurse‐led care compared with doctor‐led care for management of bronchiectasis | ||||||
| Patient or population: management of bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with doctor‐led care | Risk with nurse‐led care | |||||
| Exacerbations Assessed by clinician identified or participant self‐reported Follow‐up: 12 months | Mean rate of infective exacerbations was 3.1 per patient per year. | 0.28 per patient per year higher | 1.09 (95% CI 0.91 to 1.30) | 80 | ⊕⊕⊝⊝ | |
| Hospital admissions (per patient per year). | Mean admission per patient per year was 1.02. | 1.55 per patient per year higher | 1.52 (95% CI 1.03 to 2.23) | 80 | ⊕⊕⊝⊝ | More admissions in nurse‐led care. All nurse‐led care admissions approved by consultant. Protocol followed by nurse regarding management |
| Emergency department attendance | See comment. | See comment. | See comment. | See comment. | See comment. | Not reported |
| Mortality | Two participants died ‐ 1 from each care group ‐ after 12‐month assessment. | See comment. | ⊕⊕⊝⊝ | |||
| Cost‐effectiveness Total cost for duration of study and difference in cost for first and second years Cost scale: £ per participant | Total costs £5428 Cost difference £274 higher in second year | Total costs £8464 Cost difference £1940 lower in second year | ⊕⊕⊝⊝ | Costs may be reduced over time through a learning effect. | ||
| Quality of life, measured with SGRQ ‐ total scores | Unreported | MD 1.7 higher | 79 | ⊕⊕⊝⊝ | Participants reported fewer symptoms and less impact on daily life with nurse‐led care, but data show no clinical or statistically significant differences between nurse‐led and doctor‐led care. | |
| Exercise capacity: 12MWT | Mean exercise capacity: 12MWT was 746 m. | MD 18 m greater | 80 | ⊕⊕⊝⊝ | No significant differences in distance walked between nurse‐led and doctor‐led care | |
| FEV1 | Mean FEV1 was 69.5% predicted. | MD 0.2% predicted higher | 80 | ⊕⊕⊝⊝ | Nil significant differences in percentage predicted FEV1 between nurse‐led and doctor‐led care | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aCannot rule out carryover effects from cross‐over trial. No reported information at first 12‐month time period before cross‐over. May have had a learned effect that resulted in fewer exacerbations and hospitalisations and better quality of life. This may have led to better lung function and exercise capacity. Marked down one point for risk of bias. bAge of study, small number of participants, and uncertainty, with some results based on wide confidence intervals. Marked down one point for imprecision. cCannot rule out selective reporting with the decision not to cross‐over 6 participants after first 12 months. No longer considered stable bronchiectasis. Already marked down for risk of bias previously, so not downgraded again based on this reason. | ||||||
| Resource | Nurse‐led care (mean visits per participant) | Nurse‐led care (mean cost per participant, £) | Doctor‐led care (mean visits per participant) | Doctor‐led care (mean cost per participant, £) | Difference (SD, £) |
| Nurse‐led clinics | 4.61 | 180 | 0 | 0 | 180 (158) |
| Doctor‐led clinics | 0.45 | 25 | 4.48 | 217 | ‐192 (199) |
| Procedures | 0.13 | 61 | 0.11 | 54 | 7 (376) |
| Imaging | 1.14 | 47 | 0.76 | 45 | 1 (112) |
| Other tests | 24.58 | 260 | 18.94 | 222 | 37 (257) |
| Antibiotics (intravenous) | 23 (days) | 879 | 16 (days) | 523 | 356 (1452) |
| Antibiotics (oral) | 222 (days) | 684 | 201 (days) | 524 | 161 (695) |
| Bronchodilators | 461 (days) | 213 | 435 (days) | 193 | 20 (179) |
| Corticosteroids | 238 (days) | 278 | 219 (days) | 258 | 20 (181) |
| Other drugs | 212 (days) | 180 | 190 (days) | 155 | 25 (194) |
| Inpatient | 6.46 (days) | 1338 | 2.36 (days) | 477 | 861 (2755) |
| Day case | 0.11 | 43 | 0.05 | 16 | 27 (170) |
| GP visits | 1.11 | 20 | 1.40 | 26 | ‐6 (33) |
| Total | 4208 | 2711 | 1498 (688 to 2674) | ||
| SD: standard deviation. | |||||
| Comments | Nurse practitioner better, number, (%) | Doctor better, number (%) | P value |
| It was sometimes difficult to discuss your problems with the doctor/nurse practitioner. | 11/76 (14.5) | 1/76 (1.3) | 0.006 |
| The doctor/nurse practitioner explained clearly what is wrong. | 7/74 (9.5) | 0/74 (0) | 0.016 |
| The doctor/nurse practitioner examined you thoroughly when necessary. | 6/70 (8.6) | 0/70 (0) | 0.031 |
| The doctor/nurse practitioner should tell you more about your illness/condition and treatment. | 7/59 (11.9) | 3/59 (5.1) | 0.344 |
| The doctor/nurse practitioner made you feel at ease. | 2/75 (2.7) | 1/75 (1.3) | 1.000 |
| There was not enough time to discuss your problems with the doctor/nurse. | 10/74 (13.5) | 1/74 (1.4) | 0.012 |
| You felt confident the doctor/nurse practitioner knew about your medical history and your care. | 7/74 (9.5) | 1/74 (1.4) | 0.070 |
| Sometimes you felt that the doctor/nurse practitioner should listen more to what you said. | 5/69 (7.2) | 2/69 (2.9) | 0.453 |
| The doctor/nurse practitioner gave clear explanation about any tests that you needed. | 4/75 (5.3) | 1/75 (1.3) | 0.375 |
| You often came away from your appointment wishing you'd asked more questions. | 13/72 (18.1) | 9/72 (12.5) | 0.523 |
| You felt you were given a chance to have an active part when discussing your illness/condition. | 4/73 (5.5) | 0/73 (0.0) | 0.125 |
| There were frequent interruptions during your consultation. | 6/73 (8.2) | 3/73 (4.1) | 0.508 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infective exacerbations (per patient per year) Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Admissions per patient per year Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 3 SGRQ ‐ symptoms Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 SGRQ ‐ control Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 SGRQ ‐ impact Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 SGRQ ‐ total scores Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Exercise capacity: 12‐minute walk distance, metres Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 FEV1 (% predicted) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 FVC (% predicted) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |

