Tratamiento para la necrosis ósea avascular en pacientes con enfermedad de células falciformes
Resumen
Antecedentes
La necrosis ósea avascular es una complicación frecuente y grave de la enfermedad de células falciformes y su tratamiento no está estandarizado. Ésta es una actualización de una revisión Cochrane publicada anteriormente.
Objetivos
Determinar la repercusión de cualquier procedimiento quirúrgico comparado con otras intervenciones quirúrgicas o procedimientos no quirúrgicos, sobre la necrosis ósea avascular en pacientes con enfermedad de células falciformes en cuanto a la eficacia y la seguridad.
Métodos de búsqueda
Se realizaron búsquedas en el Registro de Ensayos de Hemoglobinopatías perteneciente al Grupo Cochrane de Fibrosis Quística y Trastornos Genéticos (Cochrane Cystic Fibrosis and Genetic Disorders Group Haemoglobinopathies Trials Register) que incluyeron las referencias identificadas mediante búsquedas exhaustivas en bases de datos electrónicas y búsquedas manuales en revistas pertinentes y libros de resúmenes de actas de congresos. Se buscaron ensayos adicionales, tanto en los registros de ensayos en curso como en las listas de referencias de los documentos identificados por la estrategia de búsqueda.
Fecha de la búsqueda más reciente en el registro de ensayos de hemoglobinopatías del Grupo: 17 setiembre 2019.
Criterios de selección
Ensayos clínicos aleatorizados que compararon tratamientos específicos para la necrosis ósea avascular en pacientes con enfermedad de células falciformes.
Obtención y análisis de los datos
Cada autor extrajo los datos de forma independiente y evaluó la calidad de los ensayos. La calidad de la evidencia se evaluó mediante GRADE. Dado que sólo se identificó un ensayo, no fue posible realizar metanálisis.
Resultados principales
Un ensayo (46 participantes) fue eligible para la inclusión. Luego de la asignación al azar, se retiraron ocho participantes debido principalmente a que rehusaron participar en el ensayo. Al final del ensayo, se analizaron los datos de 38 participantes. Después de una media del seguimiento de tres años, la descompresión del núcleo de la cadera y la fisioterapia no mostraron mejoría clínica en comparación con la fisioterapia sola con la utilización de la puntuación del ensayo original (una mejoría de 18,1 puntos para los pacientes con tratamiento de intervención versus un mejoría de 15,7 puntos con tratamiento control). No se sabe con certeza si hay alguna diferencia entre los grupos con respecto a las complicaciones graves (dolor de cadera, cociente de riesgos 0,95 (intervalo de confianza del 95%: 0,56 a 1,60; crisis vaso‐oclusivas, riesgos relativos 1,14 (intervalo de confianza del 95%: 0,72 a 1,80; calidad muy baja de la evidencia); y síndrome torácico agudo, riesgos relativos 1,06 (intervalo de confianza del 95%: 0,44 a 2,56; calidad muy baja de la evidencia)). Este ensayo no informó resultados sobre la mortalidad o calidad de vida.
Conclusiones de los autores
No se encontró evidencia de que agregar la descompresión del núcleo de la cadera a la fisioterapia logre una mejoría clínica en los pacientes con enfermedad de células falciformes con necrosis ósea avascular en comparación con la fisioterapia sola. Sin embargo, se destaca que la conclusión se basa en un ensayo con altas tasas de desgaste. Se necesitan ensayos controlados aleatorizados para evaluar la función de la descompresión del núcleo de la cadera para esta enfermedad. Las variables de evaluación se deben centrar en la experiencia subjetiva de los participantes (p.ej., calidad de vida y dolor), así como en medidas de "tiempo hasta el evento" más objetivas (p.ej., mortalidad, supervivencia, longevidad de la cadera). La disponibilidad de participantes para lograr un poder estadístico adecuado para el ensayo será una consideración clave para la elección de las variables de evaluación.
PICO
Resumen en términos sencillos
Los tratamientos para los pacientes con enfermedad de células falciformes con irrigación sanguínea deficiente a un área ósea provocan la muerte ósea
Pregunta de la revisión
Se revisaron los efectos de los tratamientos para la necrosis ósea avascular en pacientes con enfermedad de células falciformes.
Antecedentes
Muchos pacientes con enfermedad de células falciformes presentan muerte ósea debido a la pérdida temporal o permanente de la irrigación sanguínea a partes de sus huesos. Lo anterior puede ser muy doloroso. Los huesos habitualmente afectados son los huesos del muslo en la articulación de la cadera y los huesos del brazo en la articulación del hombro. El objetivo del tratamiento es detener el dolor y mantener una articulación móvil. Los tratamientos incluyen mantener la articulación en reposo, fisioterapia, uso de alivio del dolor, reemplazos articulares e injertos óseos. Sin embargo, las complicaciones de la cirugía pueden ser más frecuentes en los pacientes con enfermedad de células falciformes. Ésta es una actualización de una revisión Cochrane publicada anteriormente.
Fecha de la búsqueda
La evidencia está actualizada hasta: 17 setiembre 2019.
Características de los estudios
Se encontró un ensayo elegible, publicado en 2006, que analizó los datos de 38 pacientes de 32 centros de tratamiento diferentes de los EE.UU. El ensayo comparó un tratamiento de cirugía y fisioterapia con la fisioterapia por sí sola. Este ensayo no mostró que el agregado de la cirugía a un régimen de fisioterapia pudiera mejorar el resultado en los pacientes con enfermedad de células falciformes y necrosis avascular.
Resultados clave
Después de un seguimiento medio de tres años, la combinación de cirugía y terapias físicas no mostró una mejoría clínica en comparación con la terapia física sola. Dado que los resultados son imprecisos, no se sabe si la combinación de cirugía y fisioterapia tiene un efecto importante sobre el dolor de cadera, las crisis vaso‐oclusivas y el síndrome torácico agudo. Los autores de los ensayos no informaron sobre la mortalidad y la calidad de vida.
Calidad de la evidencia
El número limitado de participantes incluidos en el estudio condujo a resultados imprecisos, por lo tanto, la confianza en los resultados es muy baja.
Authors' conclusions
Summary of findings
| Treatment for avascular necrosis of bone in people with sickle cell disease | ||||||
| Patient or population: patients with avascular necrosis in people with sickle cell disease Settings: hospital Intervention: hip core decompression and physical therapy Comparison: physical therapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Physical therapy alone | Hip core decompression and physical therapy | |||||
| Pain | 619 per 1000 | 588 per 1000 | RR 0.95 | 38 | ⊕⊝⊝⊝ | |
| Vaso‐occlusive crisis | Population | RR 1.14 | 38 | ⊕⊝⊝⊝ | ||
| 619 per 1000 | 706 per 1000 | |||||
| Low risk population | ||||||
| Medium risk population | ||||||
| Acute chest syndrome | Population | RR 1.06 | 38 | ⊕⊝⊝⊝ | ||
| 333 per 1000 | 353 per 1000 | |||||
| Low risk population | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a46 (46/176; 26%) of the eligible participants were enrolled in the study. After randomization, 8 participants (6 in intervention group and 2 in control group) withdrew from the study; 6 of them because the parents or the individual themselves, declined to participate and two were withdrawn at the discretion of the main investigator. Therefore, 38 participants (38 hips; 17 in Intervention group and 21 in control group) completed the treatment protocol. bThere were few participants and few events. Therefore, the CIs were wide. | ||||||
Background
Description of the condition
Sickle cell disease (SCD) is a group of genetic hemoglobin disorders (Pauling 1949; Bunn 1997; Serjeant 2001) which have their origins in sub‐Saharan Africa and the Indian sub‐continent (Stuart 2004; Weatherall 2006). Population mobility has spread the disorders through Europe, Asia and the Americas.The term SCD includes sickle cell anaemia (Hb SS), hemoglobin S combined with hemoglobin C (Hb SC), hemoglobin S associated with β thalassemia (Sβ0 Thal and Sβ+ Thal) and other double heterozygous conditions which cause clinical disease (Saunthararajah 2004; Serjeant 2001; Weatherall 2006). Hemoglobin S combined with normal hemoglobin (A) is known as sickle trait (AS), is asymptomatic and therefore not part of this review. The pathophysiology of SCD has been reviewed extensively (Hebbel 2004; Steinberg 2006; Stuart 2004; Weatherall 2006). Although SCD is primarily a defect of red blood cells (a hematological defect), the changes in the red blood cells result in damage to blood vessels (vasculopathy) (Hebbel 2004) and severe osteo‐articular injuries are often observed in the evolution of the SCD (Kim 2002; Meddeb 2003; Aguilar 2005b). One of these injuries is avascular necrosis (AVN) or osteonecrosis; defined as massive necrosis of bone and bone marrow occurring as the only, or largely predominant, abnormality (Lafforgue 2006), and its pathophysiology, with or without SCD, has been reviewed by many researchers (Lafforgue 2006; Hernigou 2006).
In as many as 50% of the people with SCD, avascular necrosis (AVN) or osteonecrosis (disease resulting from the temporary or permanent loss of the blood supply to the bones) can cause chronic pain, particularly in the head of the femur (Vichinsky 1999; Mukisi‐Mukaza 2011; Mahadeo 2011; Mouba 2011). The head of the humerus can also be affected by AVN (Pande 1998; Poignard 2012). It has been reported that the prevalence of humeral head necrosis occurs with equal frequency to hip necrosis and this reported frequency has varied from 2% to 17% in several studies of people with SS disease (Serjeant 1992). Humeral and femoral head avascular necroses tend to be associated with each other, the combination occurring in 8 out of 10 people with SCD (Serjeant 1992). Occasionally, the temporomandibular joint can also be involved (Baykul 2004; el‐Sabbagh 1989). Vertebral bone compression and collapse induced by AVN in people with SCD has also been reported (Babhulkar 1998; Emodi 2001; Sadat‐Ali 1994).
The diagnosis of AVN must be precise, as the condition is complex requiring specific treatment according to the grade of joint involvement (Hattrup 1998; Plancher 1997). There are a number of classifications or staging systems used for grading joint involvement. Some authors suggest that the ideal outcome is a pain‐free preserved joint with good mobility (Plancher 1997).
Description of the intervention
The non‐surgical approach includes observation, analgesics and limiting weight bearing over the joint. The surgical options include joint reconstruction (femoral head replacement, cup arthroplasty, replacement of the articular surface, and total hip replacement) as well as nucleus decompression, bone graft, vascularised bone grafts, electrical stimulation and osteotomy. There is more experience in treating AVN in the femoral head than in the humerus. In the latter, Hattrup reported the indications for replacement to be similar to those for any arthroplasty, i.e. pain and dysfunction that do not respond to non‐surgical measures (Hattrup 1998).
Why it is important to do this review
Joint replacement is not risk‐free and complications (intra‐operative bleeding, infection, loosening of the prosthesis and early loss of the joint replacement (Hernigou 1993)) may be more frequent in people with SCD. The treatment of AVN for people with SCD is not standardised. People with SCD suffering orthopedic complications require a multidisciplinary management (Sathappan 2006). This review, therefore, aims to address the question: what is the efficacy and safety of the surgical approaches compared to the available non‐surgical treatments for people with SCD suffering from AVN?
This is an update of a previously published Cochrane Review (Martí‐Carvajal 2004; Martí‐Carvajal 2009; Martí‐Carvajal 2014; Martí‐Carvajal 2016).
Objectives
To determine if any of the following treatments have any impact on avascular necrosis of bone in people with SCD in both the short‐ and the long‐term (efficacy, safety, and adverse events): to compare any surgical with any non‐surgical intervention, including combinations of surgical and non‐surgical treatment. For this review, when available from the study reports, we accepted the definitions of short‐ and long‐terms provided by the studies' authors.
Also, we sought to address the following with regard to the affected joint or bone and the stage of AVN:
-
the role of decompression;
-
the relative effectiveness of surgical approaches (only applicable for studies comparing two surgical approaches);and
-
types of prosthesis (glued or not, with or without bone grafts, etc.).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐randomized controlled trials (published or unpublished). Trials using quasi‐randomization methods such as alternation were included if there was sufficient evidence that the treatment and comparison groups were comparable in terms of clinical status.
Types of participants
People of all ages and both sexes, in any setting, with SCD‐related AVN. Diagnoses of SCD should be proven by electrophoresis and sickle solubility test, with family studies or deoxyribonucleic acid (DNA) tests as appropriate.
Types of interventions
Trials in which one surgical approach has been compared to another, or to a non‐surgical approach, or where two non‐surgical approaches have been compared.
Surgical treatment currently included: joint reconstruction (femoral head replacement, cup arthroplasty, articular surface replacement and total hip replacement); and its alternatives (nucleus decompression, bone graft, vascularized bone grafts and osteotomy).
Non‐surgical treatment currently included: observation; analgesic drugs; electrical stimulation; physiotherapy; resting of the joint; red blood cell transfusion and stem cell transplant; treatment to prevent sickling; and bisphosphonates such as pamidronate.
Types of outcome measures
Primary outcomes
-
Pain
-
presence and level of pain
-
use of analgesia during the first two weeks (doses of opioids will be converted into morphine equivalents to facilitate comparisons)
-
weak opioids, e.g. codeine and tramadol
-
strong opioids, e.g. morphine and pethidine
-
acetaminophen and non‐steroidal anti‐inflammatory drugs (NSAIDs) (open or selective Cox 2‐inhibitors)
-
other analgesics
-
-
-
Death and cause of death
-
Adverse events, the events will be classified as: immediate (less than 24 hours post‐intervention); early (one to eight days after intervention); and long‐term (more than eight days after intervention). Our definition of immediate, early, and long term is arbitrary.
-
prevalence and timing of joint loosening
-
bone fracture
-
infections
-
intra‐operative bleeding
-
complications of SCD during hospital admission (e.g. painful crises, acute chest syndrome and stroke)
-
related to analgesia
-
Secondary outcomes
-
Quality of life
-
general function (such as ability to continue in employment)
-
mobility
-
psychological measures
-
-
Time in hospital
-
total time spent in hospital
-
number of visits to hospital during the first eight weeks post‐op
-
-
Joint function: range of movement
It was planned that outcome data would be grouped into those measured at up to one month, then at one, three, six, twelve months and annually thereafter. If outcome data were recorded at other time periods, then consideration would have been given to examining these as well.
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
Relevant trials were identified from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND avascular necrosis.
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (Clinical Trials) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 17 September 2019.
We also searched the following trial registries for ongoing trials (17 Septemebr 2019) (Appendix 1):
-
ClinicalTrials.gov (clinicaltrials.gov/);
-
WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
Searching other resources
The bibliographic references of all retrieved literature were reviewed for additional reports of trials.
Data collection and analysis
Selection of studies
The authors screened the search results for potentially relevant trials and independently assessed them for inclusion or exclusion, using a pre‐designed eligibility form based on the inclusion criteria. We resolved disagreements through discussion to reach a consensus.
Data extraction and management
Arturo Martí‐Carvajal (AMC) and Ivan Solà (IS) designed a standard data extraction form and Luis Agreda Pérez (LAP) validated it. Each author used this form to extract data from each relevant study. We independently extracted information from the papers and AMC contacted the lead author of the included trial for further information (NOTSCA 2006). We extracted information on study design and participant characteristics (age, sex, type of SCD (SS, SC, Sbβ+ and Sbβ0), the joint involved, and AVN score).
AMC entered the data into the Review Manager software, which was checked by IS and LAP (RevMan 2011). The Characteristics of included studies table was completed using the 'Sheet to enter data for performing a Cochrane Review' software (Zavala 2006).
Assessment of risk of bias in included studies
Two review authors (AMC, IS) assessed every study using a simple form and followed the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following domains as having either a low, unclear or high risk of bias:
-
randomization;
-
concealment of allocation;
-
blinding (of participants, assessors and providers of care);
-
incomplete outcome data.
1. Randomization
'Low risk' (if a person who was not otherwise involved in the recruitment of participants performed one of the following procedures): if the allocation sequence was, for example, generated by a computer or random number table, by drawing of lots, by tossing of a coin, by the shuffling of cards, or the throwing of a dice.
'Unclear risk': if the trial was described as randomized, but the method used for the allocation sequence generation was not described.
'High risk': if a system involving dates, names, or admittance numbers were used for the allocation of participants.
2. Concealment of allocation
'Low risk': participants and researchers were unaware of participants' future allocation to condition until after decisions about eligibility were made and informed consent was obtained.
'Unclear risk': allocation concealment measures were not described in detail.
'High risk': allocation was not concealed from either participants before informed consent or from researchers before decisions about inclusion were made.
3. Blinding (of participants, assessors and providers of care)
'Low risk': assessor blind to condition.
'Unclear risk': blinding of assessor not reported and information not available from researchers.
'High risk': assessor not blind to condition.
4. Incomplete outcome data
'Low risk': losses to follow‐up were equally distributed between treatment and comparison groups;
'Unclear risk': information about losses to follow‐up unavailable; and
'High risk': significant or unevenly distributed losses to follow‐up.
Measures of treatment effect
We used Review Manager software to enter and analyse the included data (RevMan 2011). For binary outcomes, we estimated the treatment effects using risk ratios (RR). For continuous outcomes, we estimated the treatment effects by calculating the mean differences. We could not examine data for skewness using the means and standard deviations because it is not appropriate for change from baseline measures (Higgins 2011).
As only one trial was identified, it was not possible to perform any pooled analysis. Below, we describe our methodological plan for when more trials are included in the review in the future.
Dealing with missing data
One author (AMC) contacted the lead author of the included trial for further information (Neumayr 2009 [pers comm]). If in future we require further data on any of the included trials we will contact the relevant authors. We will seek data on the number of participants by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up. If we are not able to do so, we will record for each trial, whether the results pertain to an intention‐to‐treat analysis or to a available‐cases analysis.
Assessment of heterogeneity
If in future, sufficient trials are included in the review, we will quantify the impact of statistical heterogeneity using the I² statistic, which describes the percentage of total variation across studies that are due to heterogeneity rather than sampling error (Higgins 2003).
Assessment of reporting biases
When sufficient trials are included in the review, we plan to use a funnel plot to explore the possibly of publication bias.
Data synthesis
If the identified studies are sufficiently comparable, we will summarize their findings using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
In case of significant heterogeneity, we will devote further research to identify possible causes of heterogeneity such as the impact of participants' characteristics. We will perform subgroup analyses by type of SCD (SS, SC, Sbβ+ and Sbβ0), age, setting, the joint involved, AVN score and type of surgery.
Sensitivity analysis
We also plan to perform a sensitivity analysis based on the methodological quality of the trials, including and excluding quasi‐randomized trials. We will perform further sensitivity analysis according to whether the trial results were calculated using intention‐to‐treat analysis (ITT) or not.
Results
Description of studies
Results of the search
We identified seven potentially eligible studies in the bibliographical searches, of which one (National Osteonecrosis Trial in Sickle Cell Anemia Study Group) was eligible for inclusion in the review (NOTSCA 2006).
Included studies
One trial (National Osteonecrosis Trial in Sickle Cell Anemia Study Group), including 46 participants was eligible for inclusion in the review (NOTSCA 2006). The included trial was first incorporated into the review in the 2008 update (NOTSCA 2006).
Trial design
The trial was conducted in 32 SCD centers across the USA, from June 1998 to June 2002 in people with SCD (hemoglobin‐SS, hemoglobin‐SC, or hemoglobin‐S/β0‐thalassemia). The authors of the included trial indicated that it was underpowered by stating "It is possible that our inability to demonstrate a difference in outcome between the two groups was due to a type‐2 statistical error. The sample size required to detect this 2.4‐point difference in mean change scores with a power of 80% and a type‐1 error of 0.05 would be more than 1000 in each group" (NOTSCA 2006)
Participants
Four hundred and twenty individuals were screened for osteonecrosis of the femoral head of the hip; 176 met the inclusion criteria; 46 (26%) of the eligible participants were enrolled in the study. The mean age of participant was 26 years. If participants had bilateral disease, the more symptomatic hip was included in the trial.
Interventions
Participants in the intervention group received hip core decompression and physical therapy. They were also admitted to hospital for one day preoperatively for evaluation, hydration and transfusion. Participants with hemoglobin‐SS or hemoglobin‐S/b0‐thalassemia underwent a single preoperative transfusion to bring the hemoglobin level to approximately 100 g/L. For participants with hemoglobin‐SC or hemoglobin‐S/b+‐thalassemia whose baseline hemoglobin concentration was higher than 100 g/L, partial exchange transfusion was performed to avoid raising the hemoglobin concentration to an unsafe level. The control group received physical therapy alone with matching based on the Steinberg stage, assigned at the local center. However, because there is some variation in the interpretation of the Steinberg staging system (especially in distinguishing Stage II from III), trial investigators included an expert panel who examined these grades and in some cases re‐graded the stages. We contacted the investigators for more information and were told that the panel were blind to the randomization and read the baseline films in a few batches at the end of the study. The investigators felt that consistency and reliability on the staging was important for analysis, especially since Stage IV was an exclusion criteria and below Stage IV was eligible for inclusion; the investigators confirmed that none of the study hips were Stage IV.
Outcomes
The main outcome measure was clinical improvement measured by the Children's Hospital Oakland Hip Evaluation Scale (CHOHES) score (Aguilar 2005a). This is a 100‐point scale for assessing pain, function, strength, and range of motion (Table 1). The secondary outcome was hip survival.
| CHOHES website |
For further details see the Characteristics of included studies table.
Excluded studies
Six studies have been excluded from the review. For further details see the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias of the included trial (NOTSCA 2006) is summarised in Figure 1 and Figure 2. This trial had some methodological inadequacy and following the risk of bias methodology listed above, we classified it as trial with high risk of bias.
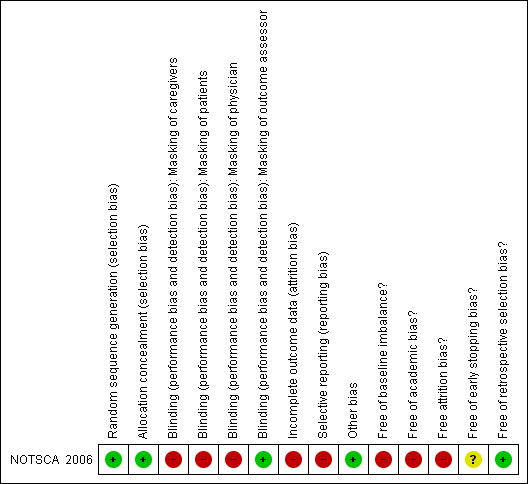
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
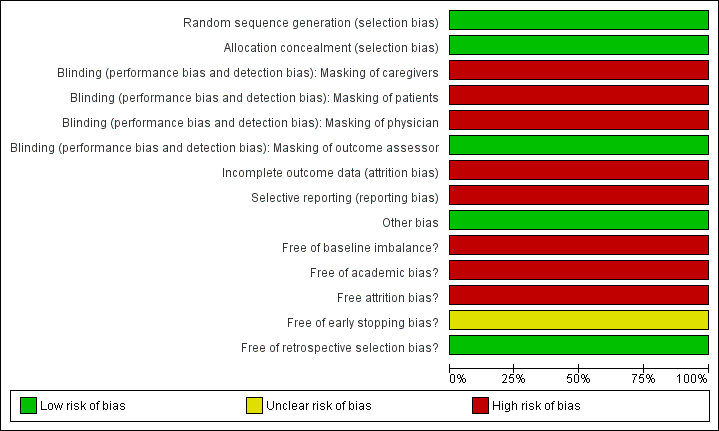
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Trial investigators confirmed that participants were randomized by stage of AVN (I, II, III; those with stage IV were excluded) to either the surgical or conservative‐therapy arm. A list of random numbers was generated for each stage of AVN (confirmed by contact with authors). This stratified assignment aimed to ensure equal numbers of participants with Stage I, Stage II and Stage III in each of the treatment arms (Table 2). Participating centers called the Co‐ordinating Center for arm assignment. If the initial clinical examination revealed AVN of the contralateral hip, the most symptomatic hip was randomized. The participant was randomized to surgery or conservative therapy based on the stage of the symptomatic hip.
| Stage | Definition |
| 0 | No radiographic evidence of osteonecrosis osteonecrosis of the femoral head. |
| I | Osteonecrosis of the femoral head on magnetic resonance imaging but normal findings on plain radiographs. |
| II | Sclerosis and lucencies of the femoral head seen on plain radiographs. |
| III | Evidence of flattening of the femoral head on plain radiographs. |
| IV | Collapse of the femoral head with degenerative changes in the joint space. |
After radiologic staging and informed consent was obtained, participating centers called the Co‐ordinating Center for arm assignment.
Therefore, randomization and allocation concealment are assessed as having a low risk of bias.
Blinding
The coordinating center’s radiology committee (three radiologists) were blinded to the reports from the participating centers. Blinding of other trial personnel and participants was not possible given treatments.
Incomplete outcome data
The study enrolled 46 participants. After randomization, eight participants (26% in experimental group (6/23) and 9% in control group (2/23)) withdrew from the trial, mainly because they declined to participate in the trial. Thirty‐eight participants (38 hips), 17 in experimental group (17/23) and 21(21/23) in control group, completed the treatment protocol. This trial used per protocol analysis (Figure 3). Given this imbalance between comparison groups regarding trial completion, the trial had a high risk of bias in this domain.
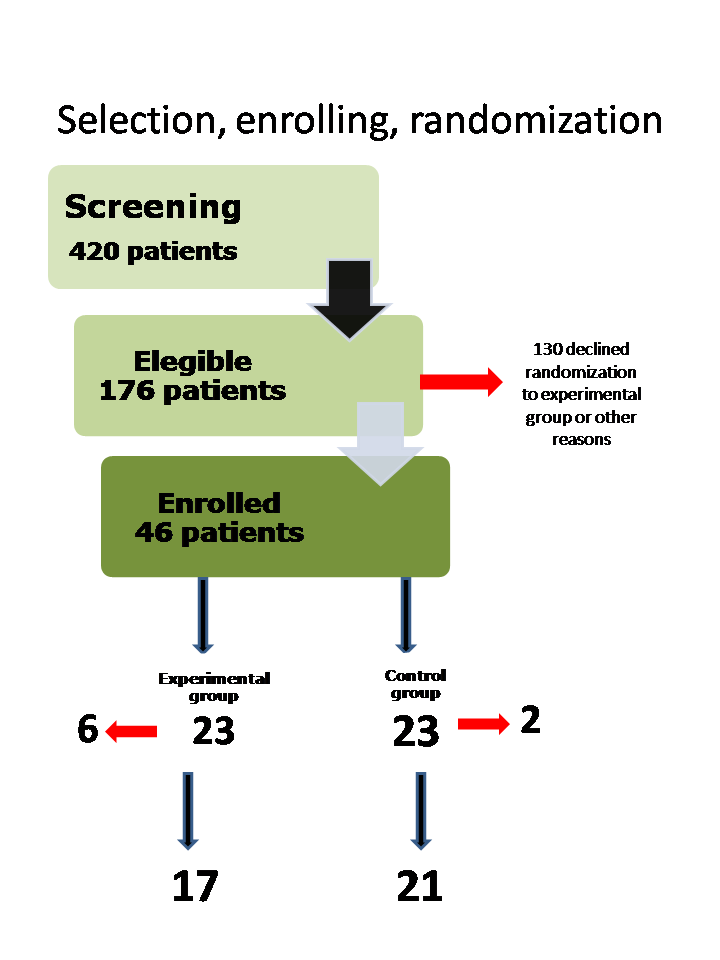
Selection, enrolment and randomization
Other potential sources of bias
Participants were graded at the local center according to Steinberg stage. However, because there is some variation in the interpretation of the Steinberg staging system (especially in distinguishing Stage II from III), trial investigators included an expert panel who examined these grades and in some cases re‐graded the stages. We contacted the investigators for more information and were told that the panel were blind to the randomization and read the baseline films in a few batches at the end of the study. The investigators felt that consistency and reliability on the staging was important for analysis, especially since Stage IV was an exclusion criteria and below Stage IV was eligible for inclusion; the investigators confirmed that none of the study hips were Stage IV (Neumayr 2009 [pers comm]). We are therefore happy that there is no potential risk of bias from the retrospective re‐grading of any participants.
Effects of interventions
See: Summary of findings for the main comparison
Results are based on one trial (38 participants) (NOTSCA 2006). The quality of the evidence has been graded for those outcomes included in the summary of findings table. For the definitions of these gradings, please refer to the summary of findings tables (summary of findings Table for the main comparison).
Primary outcomes
1. Pain
The included trial did not show differences in the pain measures between the groups compared.
The main outcome in the NOTSCA study was the clinical improvement in general function measured by means of the CHOHES score, a multi‐outcome clinical score that measured pain, but also joint motion, function and strength (NOTSCA 2006). The authors defined a clinical success with a improvement of 15 points at follow‐up. After an average of 27 months of follow‐up, participants in the intervention group showed a mean improvement of 18 points (mean (SD) 18.1 (19.8)) compared with an improvement of 15 points (mean (SD) 15.7 (19.6)) showed by those in the control group. The observed difference between groups was not significant. It is worthy to comment that it is likely that these results are skewed, as they only reported data for the participants who received one surgical intervention (the analyses excluded the participants who required total hip replacement).
However, authors reported pain as a complication related to the treatment received, and did not observe a significant difference between groups, RR 0.95 (95% CI 0.56 to 1.60) (very low‐quality evidence) (Analysis 1.1)
2. Death and cause of death
The included trial did not assess this outcome (NOTSCA 2006). There were three deaths (one in the intervention group and two in the control group); two from acute chest syndrome, and one from an unknown cause. However, these events occurred more than three years after study entry.
3. Adverse events
a. prevalence and timing of joint loosening
The trial investigators did not report any intra‐operative or immediate post‐operative complications in those participants receiving the hip core decompression (NOTSCA 2006). During the post‐operative period (30 days), two participants were re‐admitted to the hospital due to bilateral hip pain, and a further post‐operative infection was documented. Six of the randomized participants (15.8%) required an additional surgical intervention. Three participants assigned to the control group received hip core decompression (the assessed intervention) during the study. The surgical intervention failed in three participants of the intervention group and they underwent a total hip replacement due to the recurrent pain produced by an osteonecrosis of the femoral head.
b. bone fracture
This outcome was not reported in the included trial (NOTSCA 2006).
c. infections
Post‐operative infection was documented in one participant in intervention group. Drainage from of the hip core incision was performed on the 42nd post‐operative day.
d. intra‐operative bleeding
The average blood loss was reported to be 48 mL in the hip core decompression group.
e. complications of SCD during hospital admission, e.g. painful crises, acute chest syndrome and stroke
There was no significant difference between groups regarding vaso‐occlusive crisis, RR 1.14 (95% CI 0.72 to 1.80) (very low‐quality evidence) (Analysis 1.2), or acute chest syndrome, RR 1.06 (95% CI 0.44 to 2.56) (very low‐quality evidence) (Analysis 1.3). Stroke was not reported (NOTSCA 2006).
f. related to analgesia
This outcome was not reported by the trial authors (NOTSCA 2006).
Secondary outcomes
1. Quality of life
This outcome was not reported by the included trial (NOTSCA 2006).
2. Time in hospital
The length of stay for the participants who received hip core decompression was 4.6 days (range: 2 to 10 days) (NOTSCA 2006).
3. Joint function: range of movement
This outcome was measured by means of the CHOHES score in the NOTSCA study (NOTSCA 2006), as a measure of clinical improvement. The observed differences at the end of the study were not significant (see above in 'Primary outcome 1. Pain' for further details).
Discussion
This review has identified only one RCT which met the pre‐defined inclusion criteria (NOTSCA 2006). From this trial, we are very uncertain whether there is any difference between the comparison groups (hip core decompression and physical therapy versus physical therapy alone). Two reasons could explain these results. Firstly, this RCT was underpowered to detect a difference in treatments. Therefore, this RCT cannot be considered a 'negative trial' given that it was not sufficiently large enough to detect clinically relevant differences (Alderson 2004). Secondly, the trial had some methodological limitation regarding a significant imbalance in participant withdrawals.
The number of people worldwide who suffer from this severe complication of sickle cell disease, which results in chronic pain and deterioration of quality of life varies in different reports from 2.9% to 41%; indeed, AVN of the femoral head is quite common among Kuwaiti people with SCD (Marouf 2003), and people from Africa, the Mediterranean and the USA (Athanassiou 2002; Knox‐Macaulay 1983; Koduri 2001; Powars 2002). The natural history of AVN in people with SCD shows that symptomatic osteonecrosis of the hip has a high likelihood of leading to femoral head collapse, necessitating surgical intervention (Hernigou 2006; Ilyas 2002), but this point could be not confirmed in the light of the evidence identified in this review. There is an argument for early prevention and effective treatment for people with SCD with suspected AVN, but as yet no evidence base to support operative intervention. Surgical approaches have previously been reported to have many types of complications and therefore require proper evaluation (Al‐Mousawi 2002; Vichinsky 1999).
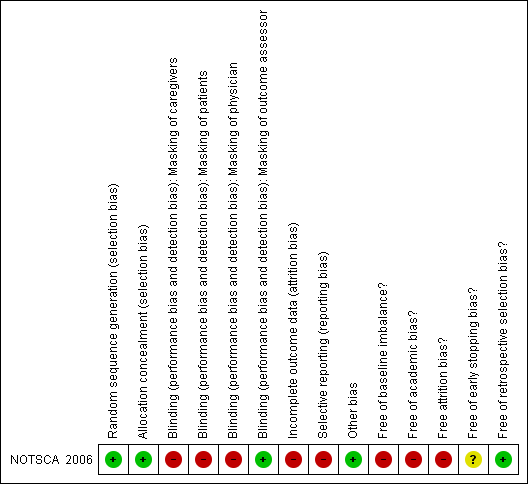
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Selection, enrolment and randomization

Comparison 1 Hip core decompression and physical therapy versus physical therapy alone, Outcome 1 Pain.

Comparison 1 Hip core decompression and physical therapy versus physical therapy alone, Outcome 2 Vaso‐occlusive crisis.

Comparison 1 Hip core decompression and physical therapy versus physical therapy alone, Outcome 3 Acute chest syndrome.
| Treatment for avascular necrosis of bone in people with sickle cell disease | ||||||
| Patient or population: patients with avascular necrosis in people with sickle cell disease Settings: hospital Intervention: hip core decompression and physical therapy Comparison: physical therapy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Physical therapy alone | Hip core decompression and physical therapy | |||||
| Pain | 619 per 1000 | 588 per 1000 | RR 0.95 | 38 | ⊕⊝⊝⊝ | |
| Vaso‐occlusive crisis | Population | RR 1.14 | 38 | ⊕⊝⊝⊝ | ||
| 619 per 1000 | 706 per 1000 | |||||
| Low risk population | ||||||
| Medium risk population | ||||||
| Acute chest syndrome | Population | RR 1.06 | 38 | ⊕⊝⊝⊝ | ||
| 333 per 1000 | 353 per 1000 | |||||
| Low risk population | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a46 (46/176; 26%) of the eligible participants were enrolled in the study. After randomization, 8 participants (6 in intervention group and 2 in control group) withdrew from the study; 6 of them because the parents or the individual themselves, declined to participate and two were withdrawn at the discretion of the main investigator. Therefore, 38 participants (38 hips; 17 in Intervention group and 21 in control group) completed the treatment protocol. bThere were few participants and few events. Therefore, the CIs were wide. | ||||||
| CHOHES website |
| Stage | Definition |
| 0 | No radiographic evidence of osteonecrosis osteonecrosis of the femoral head. |
| I | Osteonecrosis of the femoral head on magnetic resonance imaging but normal findings on plain radiographs. |
| II | Sclerosis and lucencies of the femoral head seen on plain radiographs. |
| III | Evidence of flattening of the femoral head on plain radiographs. |
| IV | Collapse of the femoral head with degenerative changes in the joint space. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Vaso‐occlusive crisis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Acute chest syndrome Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

