Antikoagulansi (produljenog djelovanja) za sprječavanje venske tromboembolije nastale uslijed totalne zamjene kuka, koljena, ali i oporavka nakon samog prijeloma kuka
Abstract
Background
The optimal duration of thromboprophylaxis after total hip or knee replacement, or hip fracture repair remains controversial. It is common practice to administer prophylaxis using low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH) until discharge from hospital, usually seven to 14 days after surgery. International guidelines recommend extending thromboprophylaxis for up to 35 days following major orthopaedic surgery but the recommendation is weak due to moderate quality evidence. In addition, recent oral anticoagulants that exert effect by direct inhibition of thrombin or activated factor X lack the need for monitoring and have few known drug interactions. Interest in this topic remains high.
Objectives
To assess the effects of extended‐duration anticoagulant thromboprophylaxis for the prevention of venous thromboembolism (VTE) in people undergoing elective hip or knee replacement surgery, or hip fracture repair.
Search methods
The Cochrane Vascular Information Specialist searched the Specialised Register (last searched May 2015) and CENTRAL (2015, Issue 4). Clinical trials databases were searched for ongoing or unpublished studies.
Selection criteria
Randomised controlled trials assessing extended‐duration thromboprophylaxis (five to seven weeks) using accepted prophylactic doses of LMWH, UFH, vitamin K antagonists (VKA) or direct oral anticoagulants (DOAC) compared with short‐duration thromboprophylaxis (seven to 14 days) followed by placebo, no treatment or similar extended‐duration thromboprophylaxis with LMWH, UFH, VKA or DOACs in participants undergoing hip or knee replacement or hip fracture repair.
Data collection and analysis
We independently selected trials and extracted data. Disagreements were resolved by discussion. We performed fixed‐effect model meta‐analyses with odds ratios (ORs) and 95% confidence intervals (CIs). We used a random‐effects model when there was heterogeneity.
Main results
We included 16 studies (24,930 participants); six compared heparin with placebo, one compared VKA with placebo, two compared DOAC with placebo, one compared VKA with heparin, five compared DOAC with heparin and one compared anticoagulants chosen at investigators' discretion with placebo. Three trials included participants undergoing knee replacement. No studies assessed hip fracture repair.
Trials were generally of good methodological quality. The main reason for unclear risk of bias was insufficient reporting. The quality of evidence according to GRADE was generally moderate, as some comparisons included a single study, low number of events or heterogeneity between studies leading to wide CIs.
We showed no difference between extended‐duration heparin and placebo in symptomatic VTE (OR 0.59, 95% CI 0.35 to 1.01; 2329 participants; 5 studies; high quality evidence), symptomatic deep vein thrombosis (DVT) (OR 0.73, 95% CI 0.39 to 1.38; 2019 participants; 4 studies; moderate quality evidence), symptomatic pulmonary embolism (PE) (OR 0.61, 95% CI 0.16 to 2.33; 1595 participants; 3 studies; low quality evidence) and major bleeding (OR 0.59, 95% CI 0.14 to 2.46; 2500 participants; 5 studies; moderate quality evidence). Minor bleeding was increased in the heparin group (OR 2.01, 95% CI 1.43 to 2.81; 2500 participants; 5 studies; high quality evidence). Clinically relevant non‐major bleeding was not reported.
We showed no difference between extended‐duration VKA and placebo (one study, 360 participants) for symptomatic VTE (OR 0.10, 95% CI 0.01 to 1.94; moderate quality evidence), symptomatic DVT (OR 0.13, 95% CI 0.01 to 2.62; moderate quality evidence), symptomatic PE (OR 0.32, 95% CI 0.01 to 7.84; moderate quality evidence) and major bleeding (OR 2.89, 95% CI 0.12 to 71.31; low quality evidence). Clinically relevant non‐major bleeding and minor bleeding were not reported.
Extended‐duration DOAC showed reduced symptomatic VTE (OR 0.20, 95% CI 0.06 to 0.68; 2419 participants; 1 study; moderate quality evidence) and symptomatic DVT (OR 0.18, 95% CI 0.04 to 0.81; 2459 participants; 2 studies; high quality evidence) compared to placebo. No differences were found for symptomatic PE (OR 0.25, 95% CI 0.03 to 2.25; 1733 participants; 1 study; low quality evidence), major bleeding (OR 1.00, 95% CI 0.06 to 16.02; 2457 participants; 1 study; low quality evidence), clinically relevant non‐major bleeding (OR 1.22, 95% CI 0.76 to 1.95; 2457 participants; 1 study; moderate quality evidence) and minor bleeding (OR 1.18, 95% CI 0.74 to 1.88; 2457 participants; 1 study; moderate quality evidence).
We showed no difference between extended‐duration anticoagulants chosen at investigators' discretion and placebo (one study, 557 participants, low quality evidence) for symptomatic VTE (OR 0.50, 95% CI 0.09 to 2.74), symptomatic DVT (OR 0.33, 95% CI 0.03 to 3.21), symptomatic PE (OR 1.00, 95% CI 0.06 to 16.13), and major bleeding (OR 5.05, 95% CI 0.24 to 105.76). Clinically relevant non‐major bleeding and minor bleeding were not reported.
We showed no difference between extended‐duration VKA and heparin (one study, low quality evidence) for symptomatic VTE (OR 1.64, 95% CI 0.85 to 3.16; 1279 participants), symptomatic DVT (OR 1.36, 95% CI 0.69 to 2.68; 1279 participants), symptomatic PE (OR 9.16, 95% CI 0.49 to 170.42; 1279 participants), major bleeding (OR 3.87, 95% CI 1.91 to 7.85; 1272 participants) and minor bleeding (OR 1.33, 95% CI 0.64 to 2.76; 1279 participants). Clinically relevant non‐major bleeding was not reported.
We showed no difference between extended‐duration DOAC and heparin for symptomatic VTE (OR 0.70, 95% CI 0.28 to 1.70; 15,977 participants; 5 studies; low quality evidence), symptomatic DVT (OR 0.60, 95% CI 0.11 to 3.27; 15,977 participants; 5 studies; low quality evidence), symptomatic PE (OR 0.91, 95% CI 0.43 to 1.94; 14,731 participants; 5 studies; moderate quality evidence), major bleeding (OR 1.11, 95% CI 0.79 to 1.54; 16,199 participants; 5 studies; high quality evidence), clinically relevant non‐major bleeding (OR 1.08, 95% CI 0.90 to 1.28; 15,241 participants; 4 studies; high quality evidence) and minor bleeding (OR 0.95, 95% CI 0.82 to 1.10; 11,766 participants; 4 studies; high quality evidence).
Authors' conclusions
Moderate quality evidence suggests extended‐duration anticoagulants to prevent VTE should be considered for people undergoing hip replacement surgery, although the benefit should be weighed against the increased risk of minor bleeding. Further studies are needed to better understand the association between VTE and extended‐duration oral anticoagulants in relation to knee replacement and hip fracture repair, as well as outcomes such as distal and proximal DVT, reoperation, wound infection and healing.
PICOs
Laički sažetak
Antikoagulansi tijekom duljeg vremenskog razdoblja za sprječavanje venske tromboze ili plućne embolije nakon zamjene kuka ili koljena
Dosadašnje spoznaje
Bolesnici koji se podvrgavaju kirurškom zahvatu imaju povećan rizik za razvoj krvnih ugrušaka u venama. Ti ugrušci mogu biti u dubokim venama noge (duboka venska tromboza, DVT) ili putovati do pluća (plućna embolija, PE). Venska tromboembolija (VTE) je skupni naziv za DVT i PE. Postupci za sprječavanje nastanka krvnih ugrušaka (profilaksa) nakon kirurškog zahvata mogu smanjiti rizik nastanka venskih ugrušaka nakon operacije. Te moguće prednosti, međutim, moraju se uzeti u obzir zajedno s rizikom od nastanka krvarenja. Optimalna duljina trajanja profilakse nakon totalne zamjene kuka ili koljena ili pak nakon prijeloma kuka ostaje kontroverzna. Uobičajen je praksa dati profilaksu koristeći lijekove kao što su niskomolekularni heparin i nefrakcionirani heparin (antikoagulansi) do otpuštanja iz bolnice i tijekom minimalno 14 dana nakon kirurškog zahvata. Trenutne međunarodne smjernice preporučuju produljenu profilaksu do 35 dana nakon velikog ortopedskog zahvata, ali priznaju da je preporuka slaba zbog umjerene kvalitete dokaza. Osim toga, novi oralni antikoagulansi (direktni oralni antikoagulansi, DOAC) pokazuju potencijalnu korist kao što je oralno uzimanje tableta umjesto injekcija, izostanak potrebe za praćenjem koncentracije lijeka u krvi i malo poznatih interakcija (međudjelovanja s drugim lijekovima). Interes je za ovu temu, dakle, i dalje vrlo visok.
Značajke istraživanja i ključni rezultati
U ovom Cochrane sustavnom pregledu uključeno je 16 studija s ukupno 24.930 randomiziranih (slučajno raspoređenih) sudionika (podatci iz studija objavljenih do svibnja 2015.god.). Glavni su rezultati od interesa bili simptomatski (stanja u kojima se u pacijenata bilježe simptomi) VTE, uključujući DVT i PE i krvarenje (veliko, ne veće klinički značajno i manje krvarenje). Šest je studija usporedilo heparin s placebom, jedna je usporedila vitamin K antagonist (VKA) varfarin s placebom, dvije DOAC s placebom, jedna VKA s heparinom, 5 ih je usporedilo DOAC s heparinom, a jedna je usporedila različite tretmane antikoagulansima s placebom. Samo su tri ispitivanja uključila sudionike koji su podvrgnuti zamjeni koljena, a nijedna studija nije uključila sudionike koji prolaze kroz oporavak uslijed prijeloma kuka.
Za usporedbu heparina naspram placeba (šest studija) nisu pronađene nikakve razlike između pojedinih skupina za simptomatski VTE, simptomatski DVT, simptomatski PE i veliko krvarenje. Manja su krvarenja češće zabilježena u heparinskoj skupini. Klinički značajno krvarenje nije zabilježeno.
Usporedba VKA s placebom (jedna studija) i usporedba placeba s antikoagulansima (jedna studija) nisu pokazale nikakve razlike između pojedinih skupina za simptomatski VTE, simptomatski DVT, simptomatski PE i veliko krvarenje. Klinički značajno krvarenje i manje krvarenje nisu opisani.
Za usporedbu DOAC naspram placeba (dvije studije), smanjeni simptomi VTE i simptomatski DVT pronađeni su u korist DOAC, ali nikakve razlike nisu pronađene za simptomatski PE, veće krvarenje, klinički značajno krvarenje i manje krvarenje.
Uspoređujući VKA produljenog djelovanja i LMWH produljenog djelovanja (jedna studija), nije bilo razlike između pojedinih skupina za simptomatski VTE, simptomatski DVT, simptomatski PE, veće krvarenje i manje krvarenje. Klinički značajno ne veće krvarenje nije opisano.
Uspoređujući DOAC produljenog trajanja s LMWH produljenog trajanja (5 studija) , nije bilo razlike između pojedinih skupina za simptomatski VTE, simptomatski DVT, simptomatski PE, veće krvarenje, klinički značajno krvarenje i manje krvarenje.
Kvaliteta dokaza
Sve u svemu, uključene su studije bile dobre metodološke kvalitete. Većina studija imala je malo rizika za pristranost zbog ustroja studije i načina opisivanja podataka. Problemi koji se tiču studija uglavnom su uočeni po pitanju nedostatnog opisivanja važnih detalja o istraživanju. Kvaliteta je dokaza uglavnom bila umjerena ili zato što je samo jedna studija bila uključena u usporedbi, zbog malog broja događaja ili zato što je bilo mnogo razlika između rezultata, što znači da je rezultate bilo teško protumačiti. Potrebna su daljnja istraživanja kako bismo bolje razumjeli vezu između VTE i oralnih antikoagulansa produljenog djelovanja za zamjenu koljena ili oporavak uslijed prijeloma kuka, kao i ishode kao što je DVT ispod i iznad koljena, ponovna operacija, infekcija rane i ozdravljenje.
Authors' conclusions
Summary of findings
| Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with heparin | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.59 | 2329 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 20 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.73 | 2019 | ⊕⊕⊕⊝ | — | |
| 24 per 1000 | 18 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.61 | 1595 | ⊕⊕⊝⊝ | — | |
| 6 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 0.59 | 2500 | ⊕⊕⊕⊝ | — | |
| 4 per 1000 | 2 per 1000 | |||||
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Bleeding ‐ minor | Study population | OR 2.01 | 2500 | ⊕⊕⊕⊕ | — | |
| 46 per 1000 | 88 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, low number of events leading to imprecision of results | ||||||
| Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.10 | 360 | ⊕⊕⊕⊝ | — | |
| 23 per 1000 | 2 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.13 | 360 | ⊕⊕⊕⊝ | — | |
| 17 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.32 | 360 | ⊕⊕⊕⊝ | — | |
| 6 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 2.89 | 360 | ⊕⊕⊝⊝ | no events recorded in placebo group | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study only so heterogeneity could not be assessed | ||||||
| DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.20 | 2419 | ⊕⊕⊕⊝ | — | |
| 12 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.18 | 2459 | ⊕⊕⊕⊕ | — | |
| 9 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.25 | 1733 | ⊕⊕⊝⊝ | — | |
| 5 per 1000 | 1 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 1.00 | 2457 | ⊕⊕⊝⊝ | — | |
| 1 per 1000 | 1 per 1000 | |||||
| Bleeding‐ clinically relevant non‐major | Study population | OR 1.22 | 2457 | ⊕⊕⊕⊝ | — | |
| 27 per 1000 | 33 per 1000 | |||||
| Bleeding ‐ minor | Study population | OR 1.18 | 2457 | ⊕⊕⊕⊝ | — | |
| 28 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity cannot be assessed | ||||||
| Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with anticoagulant (chosen at investigators' discretion) | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.50 | 557 | ⊕⊕⊝⊝ | — | |
| 14 per 1000 | 7 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.33 | 557 | ⊕⊕⊝⊝ | — | |
| 11 per 1000 | 4 per 1000 | |||||
| Symptomatic PE | Study population | OR 1.00 | 557 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 5.05 | 557 | ⊕⊕⊝⊝ | no major bleeding recorded in the placebo groups | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity could not be assessed | ||||||
| Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 1.64 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 38 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 1.36 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 31 per 1000 | |||||
| Symptomatic PE | see comment | OR 9.16 | 1279 | ⊕⊕⊝⊝ | no cases of symptomatic PE reported in the heparin study arm | |
| Bleeding ‐ major | Study population | OR 3.87 | 1272 | ⊕⊕⊝⊝ | — | |
| 16 per 1000 | 58 per 1000 | |||||
| Bleeding ‐ clinically indicated non‐major Treatment duration 28 ‐ 42 days | see comment | — | — | — | clinically indicated non‐major bleeding events not reported in single included study in this comparison | |
| Bleeding ‐ minor | Study population | OR 1.33 | 1279 | ⊕⊕⊝⊝ | — | |
| 20 per 1000 | 27 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, single study so heterogeneity could not be assessed | ||||||
| DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.70 | 15977 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.60 | 15977 | ⊕⊕⊝⊝ | — | |
| 3 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.91 | 14731 | ⊕⊕⊕⊝ | — | |
| 2 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 1.11 | 16199 | ⊕⊕⊕⊕ | — | |
| 8 per 1000 | 9 per 1000 | |||||
| Bleeding ‐ clinically relevant, non‐major | Study population | OR 1.08 | 15241 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 36 per 1000 | |||||
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 0.95 | 11766 | ⊕⊕⊕⊕ | — | |
| 66 per 1000 | 63 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for inconsistency (heterogeneity, I2 = 55%) | ||||||
Background
Description of the condition
Venous thromboembolism describes the formation of a clot in the deep veins (deep vein thrombosis or DVT ‐ usually of the lower extremities) and the subsequent embolisation of the clot to the pulmonary circulation (pulmonary embolisation or PE) or both. DVT of the lower limbs is associated with localised pain, swelling and erythema as well as the development of pulmonary emboli (PE), and the more localised and chronic post thrombotic syndrome. PE presents with shortness of breath, pain on inspiration, tachycardia and right heart overload, and if untreated, can lead to circulatory collapse and death.
The incidence of VTE, in mostly white populations, is between 100 and 200 per 100,000 person years (Heit 2015; White 2003). Of these, it is estimated that 45 to 117 per 100,000 person years are due to DVT (without PE) and 29 to 78 per 100,000 person years are due to PE (with or without DVT) (Heit 2015). Recurrent VTE occurs in approximately 7.4% of patients by one year, rising to 30.4% of patients by 10 years (Cushman 2007; Heit 2015; White 2003).
Although DVT and PE can occur spontaneously, there are many risk factors for VTE, including periods of inactivity, dehydration, hospitalisation, trauma, clotting disorders and previous thrombosis, varicose veins with phlebitis, pregnancy, oral combined hormonal contraceptives, malignancy, obesity, smoking and age (NICE 2010).
Prophylactic strategies in those deemed to be at risk (for example those undergoing surgical procedures or prolonged hospital inpatient stays) are recommended by international guidelines published by the National Institute for Health and Care Excellence (NICE) (NICE 2010; NICE 2012), The American College of Chest Physicians (ACCP) (Guyatt 2012) and the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2010) and include the use of anticoagulation such as LMWH, UFH, oral direct factor Xa inhibitor (rivaroxaban), oral direct thrombin inhibitor (dabigatran), pentasaccharides (fondaparinux), and mechanical compression such as compression stockings and intermittent pneumatic compression devices.
Description of the intervention
It is common practice to administer prophylaxis until discharge from hospital, and for a minimum of seven to 14 days after surgery. However, in patients receiving in‐hospital prophylaxis, the prevalence of venographic DVT (a blood clot in the leg detected by venography) is still 15% to 30% at the time of hospital discharge (Eriksson 1997; Mohr 1993; Nurmohamed 1992) while an additional 10% to 25% of patients develop new asymptomatic DVT during the next three to four weeks (Dahl 1997; Fragmin Trial; French Study; Hirsh 1998). It is estimated, based on the available literature, that fewer than 10% of patients with venographically documented DVT will develop symptomatic VTE.
Extended‐duration prophylaxis is prophylaxis which starts at admission and continues well beyond discharge, typically for an additional 21 to 28 days after discharge, leading to a period of prophylaxis of approximately 35 days (NICE 2010). Randomised trials have demonstrated that extending prophylaxis beyond the time of hospital discharge substantially reduces the risk of developing new asymptomatic thrombi at 30 to 45 days (Dahl 1997; Fragmin Trial; French Study) and this has led these investigators to recommend that prophylaxis of longer duration should be used in all patients undergoing total hip replacement (THR).
However, two prospective studies, conducted in patients without known proximal DVT at the time of discharge from hospital, demonstrated that the incidence of new out‐of‐hospital symptomatic DVT or pulmonary embolism (PE) without extended‐duration prophylaxis was only approximately 2% after three months of follow‐up (Leclerc 1998; Robinson 1997). This data suggest that the majority of asymptomatic thrombi remain clinically silent irrespective of whether extended‐duration prophylaxis is given. Meanwhile, the impact of extended‐duration prophylaxis on symptomatic VTE remains to be clarified. Heit 2000 showed that a significant reduction in symptomatic VTE could not be demonstrated and therefore concluded that prophylaxis confined to the in‐hospital phase is adequate in most patients.
Why it is important to do this review
Current ACCP and NICE guidelines (Guyatt 2012; NICE 2010) for the prevention of VTE recommend extending thromboprophylaxis for up to 35 days following major orthopaedic surgery but recognise that the recommendation is weak due to moderate quality evidence (Guyatt 2012). In addition, recent oral anticoagulants that exert effect by direct inhibition of thrombin or activated factor X lack the need for monitoring and have few known drug interactions. Differences between anticoagulants for the extended duration also warrant further investigation. Interest in this topic therefore remains high.
Objectives
To assess the effects of extended‐duration anticoagulant thromboprophylaxis for the prevention of venous thromboembolism (VTE) in people undergoing elective hip or knee replacement surgery, or hip fracture repair.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies which assessed extended duration of anticoagulant thromboprophylaxis for the prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair.
We defined extended‐duration thromboprophylaxis as thromboprophylaxis of duration of five to seven weeks.
We included studies which used objective methods (e.g. ultrasound, venogram, V‐Q scan) to confirm the diagnosis of symptomatic and asymptomatic VTE (DVT or PE). We included data on asymptomatic DVT from these studies only if screening was performed using ascending lower‐limb contrast venography. We included open‐label, as well as double‐blind studies.
Types of participants
Patients undergoing elective total hip or knee replacement surgery or hip fracture repair. Patients undergoing revisions of a previous hip or knee replacement were included.
Types of interventions
We included studies which assessed extended‐duration thromboprophylaxis (five to seven weeks) using accepted prophylactic doses of anticoagulants LMWH, UFH, vitamin K antagonists or direct oral anticoagulants (DOACs) compared with short duration thromboprophylaxis therapy (seven to 14 days) followed by placebo or no treatment or similar extended duration of thromboprophylaxis therapy with anticoagulants LMWH, UFH, vitamin K antagonists or DOACs. Other antithrombotic therapy used in the prevention of VTE in major orthopaedic surgery was excluded.
Types of outcome measures
Primary outcomes
Symptomatic VTE (including DVT and PE)
Secondary outcomes
Total VTE (symptomatic or asymptomatic)
Asymptomatic DVT
Asymptomatic proximal and distal DVT
All‐cause mortality
Adverse events
Bleeding events (major, clinically relevant non‐major bleeding and minor bleeding)
Reoperation
Wound infection
Wound healing
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (last searched May 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 4, part of The Cochrane Library, www.cochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial databases for details of ongoing and unpublished studies;
World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/
ClinicalTrials.gov http://clinicaltrials.gov/
ISRCTN Register http://www.isrctn.com/
Searching other resources
We searched references sections of relevant reports for further studies.
Data collection and analysis
Selection of studies
Studies identified in the search were individually evaluated by two review authors (RF and MS) based on title, abstract or full report for possible inclusion and any disagreements resolved by discussion.
Data extraction and management
Two review authors (RF and MS) independently extracted data on study characteristics, efficacy and safety outcomes. Extracted information included study design, country, setting, intention‐to‐treat methods, number of participants randomised, number of participants excluded post‐randomisation, losses to follow up, age, sex, inclusion criteria, exclusion criteria, treatment and control details and duration of treatment, primary and secondary outcomes, funding, and how VTE outcomes were confirmed using objective methods. Data extracted for study outcomes included symptomatic VTE (DVT and PE), total VTE (symptomatic and asymptomatic), asymptomatic DVT documented by ascending lower limb contrast venography (stratified by proximal (popliteal vein and above) and distal (calf vein) DVT, where possible), all‐cause mortality, adverse events and bleeding events (major, clinically relevant non‐major and minor bleeding). We also extracted data for reoperation, wound infection and wound healing. We accepted the primary study authors' definitions for DVT, PE and bleeding events. Any disagreements were resolved through discussion.
Assessment of risk of bias in included studies
RF and MS independently assessed the methodological quality of included studies using the 'Risk of bias' tool according to Higgins 2011. The following domains were assessed: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias. We classified the domains as low risk, high risk, or unclear risk according to Higgins 2011. Disagreements were resolved by discussion.
Measures of treatment effect
We divided studies into six groups based on the treatment profile.
-
Heparin (LMWH or UFH) versus placebo
-
Vitamin K antagonists versus placebo
-
DOACs versus placebo
-
Anticoagulant chosen at investigators' discretion versus placebo
-
Vitamin K antagonists versus heparin (LMWH or UFH)
-
DOACs versus heparin (LMWH or UFH)
We pooled data for each of the comparisons for the outcomes of symptomatic VTE, total VTE, asymptomatic DVT documented by ascending lower limb contrast venography, all‐cause mortality, adverse events, bleeding events, reoperation, wound infection and wound healing. The pooled data for each outcome were used to create a meta‐analysis by calculating odds ratios (ORs) with 95% confidence intervals (CIs), as all outcomes were dichotomous. We used fixed‐effect models, unless there was evidence of a large amount of heterogeneity (see Assessment of heterogeneity), in which case a random‐effects model was implemented.
Unit of analysis issues
The unit of analysis was the individual patient.
Dealing with missing data
Where appropriate we used all randomised participants for the analysis. However, many of the included studies had many participants excluded after randomisation, creating a large disparity between the number of participants randomised and the number available for assessment of VTE at the end of the study. After discussion between the review authors, we decided it was generally inappropriate to include all randomised participants in our analysis, and would use the 'intention‐to‐treat' populations as reported by the studies. This population generally consisted of all participants that received treatment and had evaluable testing of VTE at the end of the study. If these values were not available, we utilised the reported per‐protocol data. Where necessary, we contacted study authors to provide missing data.
Assessment of heterogeneity
A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect. For each included meta‐analysis we obtained a value comparing the test statistic with a Chi2 distribution. To help readers assess the consistency of results of studies in a meta‐analysis, Review Manager software (RevMan) (RevMan 2014) includes a method (I2 statistic) that describes the percentage of total variation across studies due to heterogeneity rather than by chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (Higgins 2003). For the purposes of this review, if a meta‐analysis was found to have an I2 value of > 50%, we calculated the ORs using a random‐effects model instead of a fixed‐effect model.
Assessment of reporting biases
To detect reporting bias we planned to construct funnel plots for meta‐analyses that included at least 10 studies, as funnel plots with fewer than 10 studies lack the power to distinguish chance from real asymmetry (Egger 1997).
Data synthesis
We planned to use fixed‐effect models for each meta‐analysis to pool data, unless there was evidence of heterogeneity (see Assessment of heterogeneity), in which case we planned to use a random‐effects model to derive the ORs and 95% CIs.
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses for the primary outcome symptomatic VTE:
-
hip replacement versus knee replacement versus hip fracture repair;
-
according to duration of in‐hospital prophylaxis (up to 10 days, 10 to 14 days, 15 days or more) in trials comparing extended anticoagulant thromboprophylaxis versus placebo or no treatment;
-
performing mandatory discharge venography versus not performing mandatory discharge venography in trials comparing extended anticoagulant thromboprophylaxis versus placebo or no treatment;
-
patients undergoing revisions of a previous hip or knee replacement.
Sensitivity analysis
We conducted sensitivity analyses to further explore the robustness of our results. To identify any study that may have exerted a disproportionate influence on the summary treatment effect, we removed studies from the analysis that accounted for over 50% of the weighted summary statistic, where three or more studies were included, to see if these heavily‐weighted studies altered the findings. We also planned to examine the effect of excluding studies that were at high risk of bias from the analysis, based on the 'Risk of bias' tool. We planned to carry out sensitivity analysis only when two or more studies remained in the analysis after the removal of the studies in question.
Summary of findings
We constructed a 'Summary of findings' table for each comparison using the GRADEpro GDT software (GRADEpro GDT 2015) to present the main findings of the review. We included the outcomes symptomatic VTE, symptomatic DVT, symptomatic PE, major bleeding, clinically relevant non‐major bleeding and minor bleeding in the 'Summary of findings' table. We calculated assumed control intervention risks from the mean number of events in the control groups of the selected studies for each outcome. The system developed by the Grading of Recommendation, Assessment, Development and Evaluation Working Group (GRADE working group) was used for grading the quality of evidence as high, moderate, low and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of publication bias (Atkins 2004). For completeness, additional 'Summary of findings' tables were created for the remaining outcomes of this review and presented in the appendices.
Results
Description of studies
Results of the search
See Figure 1

Study flow diagram.
Included studies
We included a total of 16 studies with 24,930 randomised participants (ADVANCE 3; Barrellier 2010; Dahl 1997; DaPP Study; EXTEND Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003; Prandoni 2002; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial; SACRE Study; Zhang 2014). Nearly all the included randomised studies were multicentre, with just three recruiting from only a single centre (French Study; Prandoni 2002; Zhang 2014). Three studies were conducted in France (Barrellier 2010; French Study; SACRE Study), one study in Norway (Dahl 1997), one in Denmark (DaPP Study), one in the US (Heit 2000) and another in the US and Canada (Fragmin Trial), one in Germany and Czech Republic (Kolb 2003), one in Italy (Prandoni 2002), one in China (Zhang 2014) and six that involved centres in multiple countries, ranging from 16 up to 27 countries (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The duration of treatment ranged between the studies, with the majority having treatment periods for an average of 35 days (ADVANCE 3; Barrellier 2010; Dahl 1997; DaPP Study; EXTEND Study; Fragmin Trial; French Study; RECORD 1 Trial; RECORD 2 Trial; Zhang 2014). Both RE‐NOVATE II Trial and RE‐NOVATE Trial had treatment periods of 28 to 35 days. One study had a four‐week post‐discharge period on top of a median nine‐day hospital phase (Prandoni 2002). Two studies had six‐week treatment periods (Heit 2000; SACRE Study), with the final study having a treatment period "up to 42 days" (Kolb 2003).
Heparin versus placebo
A total of six trials with 3221 participants compared heparin with placebo (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003). Four of the studies evaluated participants undergoing total hip replacement (Dahl 1997; DaPP Study; Fragmin Trial; French Study), two evaluated a combined group of hip and knee replacements (Heit 2000; Kolb 2003). Heit 2000 presented results for the hip and knee replacement groups separately, while Kolb 2003 presented results for the combined group only.
Five included studies evaluated LMWH versus placebo: three evaluated dalteparin (Dahl 1997; DaPP Study; Fragmin Trial), one evaluated enoxaparin (French Study) and one evaluated the certoparin (Kolb 2003). Heit 2000 compared the anticoagulant ardeparin sodium versus placebo. It should be noted that ardeparin has been removed from the market in the US since 2000, but not for reasons of efficacy or safety (Dotzel 2002).
Duration of the in‐hospital/initial phase ranged from four days to 14 days. Heit 2000 reported four to 10 days, while Dahl 1997 and DaPP Study reported seven days for the initial phase. The French Study and Kolb 2003 had 14 days for their initial phase. The Fragmin Trial did not report this timeframe. At the time of discharge from the hospital or at the end of the initial phase Dahl 1997, Fragmin Trial and the French Study had mandatory venography to test for DVT. The DaPP Study did state that they identified those with DVT at the end of the initial phase, but did not state their methods. While Heit 2000 and Kolb 2003 did not use objective methods to test for DVT at the end of the initial phase of the trial.
Vitamin K antagonists versus placebo
One trial with 360 participants compared the VKA antagonist warfarin with placebo (Prandoni 2002). Prandoni 2002 evaluated participants undergoing total hip replacement. Duration of the in‐hospital/initial phase had a median of nine days (Prandoni 2002). At the time of discharge from the hospital Prandoni 2002 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial.
DOACs versus placebo
Two trials with 2549 participants compared DOACs with placebo (RECORD 2 Trial; Zhang 2014). Both studies evaluated participants undergoing total hip replacement (RECORD 2 Trial; Zhang 2014).
The RECORD 2 Trial compared the oral anticoagulant rivaroxaban with enoxaparin in the initial phase, then the enoxaparin group began taking placebo. Zhang 2014 evaluated rivaroxaban compared with no treatment. Duration of the in‐hospital/initial phase was reported by Zhang 2014 as seven days for the initial phase while RECORD 2 Trial reported 10 to 14 days. At the end of the initial phase Zhang 2014 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial. The RECORD 2 Trial did not use objective methods to test for DVT at the end of the initial phase of the trial.
Anticoagulant treatments chosen at the investigators' discretion versus placebo
One trial with 857 participants compared thromboprophylaxis using anticoagulant treatments chosen at the investigators' discretion with placebo in participants undergoing total knee replacement (Barrellier 2010). The anticoagulation treatment was either heparin, enoxaparin, dalteparin, tinzaparin, nadroparin or fondaparinux (Barrellier 2010). Duration of the in‐hospital/initial phase was reported as 10 days. At the end of the initial phase Barrellier 2010 performed ultrasonography on all participants to determine incidence of DVT before the second phase of the trial.
Vitamin K antagonists versus heparin
One study with a total of 1289 randomised participants evaluated acenocoumarol versus reviparin sodium (SACRE Study). All participants began on the LMWH reviparin sodium until 3 ± 1 days after surgery when participants were randomised to either continue on reviparin sodium or cross over to acenocoumarol for six weeks. Participants showing clinical signs or symptoms of DVT, PE or major bleeding were not randomised. It is not clear whether those experiencing clinical signs or symptoms of DVT and PE at the randomisation stage underwent objective testing using venography or duplex scanning. The SACRE Study randomised participants undergoing a total hip replacement.
DOACs versus heparin
Five studies with a total of 16,654 randomised participants evaluated DOAC versus heparin therapy (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). All five studies evaluated participants undergoing a total hip replacement. One study compared apixaban versus enoxaparin (ADVANCE 3). One study evaluated ximelagatran versus enoxaparin (EXTEND Study), although this study was terminated early due to safety concerns with ximelagatran. Two studies compared dabigatran etexilate with enoxaparin, one with two doses of dabigatran of 220 mg and 150 mg (RE‐NOVATE Trial) and the other only evaluated the 220 mg dosage (RE‐NOVATE II Trial). Rivaroxaban was compared to enoxaparin in one study (RECORD 1 Trial). As these five studies compared two different anticoagulant treatments and randomised participants prior to surgery, there was no initial phase and objective assessment of VTE after initial phase as seen in the studies described above comparing anticoagulants versus placebo.
Excluded studies
A total of seven studies were assessed as excluded (Comp 2001; EPCAT II; Kristensen 1990; Manganelli 1998; NPHDO Study Group; PENTHIFRA PLUS Study; Swedish Study). Six studies had extended duration, but did not meet the five week minimum requirement (Comp 2001; EPCAT II; Manganelli 1998; NPHDO Study Group; PENTHIFRA PLUS Study; Swedish Study). Kristensen 1990 did not evaluate a treatment comparison within the scope of this review: heparin plus indomethacin versus heparin plus placebo.
A total of 175 records were deemed not relevant mainly due to the duration of the thromboprophylaxis being 10 to 14 days and not extended duration.
Risk of bias in included studies
See Figure 2 and Figure 3 for further information on risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall risk of bias was moderate to low with the largest concerns being some studies either not reporting on listed outcomes or reporting outcomes they did not pre‐define, leading to selective reporting bias, and other bias, mainly stemming from early termination or potential conflicts of interest due to the involvement of pharmaceutical companies.
Allocation
Adequate random sequence generation was described in the majority of studies, but two studies did not provide enough detail to make an assessment, and were rated as unclear (Dahl 1997; Kolb 2003).
Likewise, nearly all studies provided enough information to determine if allocation concealment was adequate, yet four studies did not give enough detail (Dahl 1997; DaPP Study; Kolb 2003; Zhang 2014).
Blinding
Performance bias was generally low as most studies were double‐blind and utilised a placebo. However, four studies were given an unclear rating as one study was open‐label (Barrellier 2010) and three studies did not state either way if they were open‐label or double‐blind, but as no placebo was acknowledged, they were most likely open‐label (Prandoni 2002; SACRE Study; Zhang 2014). It is unclear if knowing the treatment would have an effect on the outcomes.
All studies, except for two (Kolb 2003; Zhang 2014) used a blinded assessor or committee to evaluate at least some, if not all, outcomes. Kolb 2003 and Zhang 2014 may have used blinded assessors, but no details were given.
Incomplete outcome data
Twelve of the 16 included studies had low risk of attrition bias as all participants were accounted for in the analysis, and thorough explanations were given for exclusions. Four studies, while all participants were accounted for, did not give adequate explanations for why some participants stopped taking their medication or never started (EXTEND Study; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial).
Selective reporting
The majority of studies had no evidence of reporting bias, but two were rated as high risk of bias because one study had bleeding events listed as an outcome but did not fully report the number of events (Barrellier 2010) and the other reported on several outcomes that were not listed as a pre‐planned outcome (Dahl 1997). The EXTEND Study was given an unclear rating as defined outcomes were not reported on after early termination of the trial.
Other potential sources of bias
Two studies were given a high risk of other bias as one study was terminated early due to safety issues and also the funding pharmaceutical company was highly involved in the study design, data collection and analysis (EXTEND Study), and the other study was also terminated early because they had a very high statistical significance with the first 360 enrolled participants (Prandoni 2002). A further four studies also had pharmaceutical companies as funding bodies that were very involved in the design, data collection and analysis of the studies, which could be a conflict of interest (ADVANCE 3; RECORD 1 Trial; RECORD 2 Trial; RE‐NOVATE II Trial).
Effects of interventions
See: Summary of findings for the main comparison Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair; Summary of findings 2 Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair; Summary of findings 3 DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair; Summary of findings 4 Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair; Summary of findings 5 Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair; Summary of findings 6 DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair
Heparin versus placebo
See summary of findings Table for the main comparison and Appendix 2
Symptomatic VTE (DVT and PE)
Five studies assessed symptomatic VTE (Dahl 1997; Fragmin Trial; French Study; Heit 2000; Kolb 2003) and the fixed‐effect model found no difference between the study arms: OR 0.59, 95% CI 0.35 to 1.01; participants = 2329; studies = 5; I2 = 0%; high quality evidence; Analysis 1.1.
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.1).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Symptomatic DVT
Four studies assessed symptomatic DVT (distal or proximal) (Dahl 1997; Fragmin Trial; French Study; Heit 2000) and the fixed‐effect model found no difference between the study arms: OR 0.73, 95% CI 0.39 to 1.38; participants = 2019; studies = 4; I2 = 0%; moderate quality evidence; Analysis 1.2.
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.2).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Symptomatic PE
The fixed‐effect model evaluating three studies for symptomatic PE (Dahl 1997; French Study; Heit 2000), showed no difference between heparin or placebo (OR 0.61, 95% CI 0.16 to 2.33; participants = 1595; studies = 3; I2 = 49%; low quality evidence) Analysis 1.3.
Subgroup analysis by hip or knee replacement showed no differences between the subgroups and no differences between heparin and placebo were observed for the individual surgery groups (Analysis 1.3).
The findings did not change in studies with an in‐hospital or initial phase of 10 to 14 days, or with less than 10 days of in‐hospital/initial phase or when the studies that did not have mandatory, objective detection of DVT at hospital discharge were excluded (data analyses not shown).
Regarding sensitivity analysis, Dahl 1997 was found to account for 64.1% of the participants within this outcome, but after its removal no difference was seen in the findings.
Total VTE (symptomatic or asymptomatic)
For the six reporting studies (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003), the fixed‐effect model found decreased odds of any VTE event in favour of heparin (OR 0.39, 95% CI 0.28 to 0.56; participants = 2544; studies = 6; I2 = 0%; high quality evidence) Analysis 1.4.
For the five studies reporting on hip replacement, the fixed‐effect model found decreased odds in favour of heparin (OR 0.37, 95% CI 0.25 to 0.56; participants = 1511; studies = 5; I2 = 0%) Analysis 1.4. The single study reporting on knee replacement found no difference between the study arms (OR 0.78, 95% CI 0.21 to 2.92; participants = 723) Analysis 1.4. The single study reporting on a combined group of hip or knee replacement participants found decreased odds in favour of heparin (OR 0.38, 95% CI 0.16 to 0.90; participants = 310) Analysis 1.4.
When comparing the subgroup of studies evaluating 10 to 14 day in‐hospital/initial phase treatment duration and those that included objective DVT screening at discharge, the findings were still in favour of heparin treatment (data analyses not shown).
Asymptomatic DVT
In the five studies that reported on asymptomatic DVT (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Kolb 2003), the fixed‐effect model showed a decreased odds favouring the heparin treatment group (OR 0.38, 95% CI 0.24 to 0.60; participants = 1304; studies = 5; I2 = 0%; high quality evidence) Analysis 1.5.
When evaluating hip replacement only, the four included studies retained a decreased odds of asymptomatic DVT (OR 0.35, 95% CI 0.21 to 0.58; participants = 994; studies = 4; I2 = 0%) Analysis 1.5. The single study reporting on a combined group of hip or knee replacement participants found no difference between the study arms (OR 0.54, 95% CI 0.19 to 1.52; participants = 310) Analysis 1.5.
When comparing the subgroup of studies evaluating 10 to 14 day in‐hospital/initial phase treatment duration and those that included objective DVT screening at discharge, the findings were still in favour of heparin treatment (data analyses not shown).
Asymptomatic proximal DVT
None of the included studies reported on asymptomatic proximal DVT. Many did report asymptomatic or proximal DVT separately, but given the provided data, it was not possible to determine which events fell into both categories.
Asymptomatic distal DVT
No studies reported asymptomatic distal DVT. See above description. Many did report asymptomatic or distal DVT separately, but given the provided data, it was not possible to determine which events fell into both categories.
All‐cause mortality
For the five included studies that evaluated all‐cause mortality for heparin versus placebo (Dahl 1997; Fragmin Trial; French Study; Heit 2000; Kolb 2003), there was no difference between the study arms (OR 1.01, 95% CI 0.31 to 3.26; participants = 2518; studies = 5; I2 = 0%; moderate quality evidence) Analysis 1.6.
There was also no difference in the four studies evaluating participants undergoing hip replacement (OR 0.56, 95% CI 0.11 to 2.75; participants = 1485; studies = 4; I2 = 0%) Analysis 1.6, or in the one study undergoing knee replacement (OR 0.98, 95% CI 0.06 to 15.65; participants = 723) Analysis 1.6. The single study reporting on a combined group of hip or knee replacement participants also found no difference between the study arms (OR 4.69, 95% CI 0.22 to 98.42; participants = 310) Analysis 1.6.
When only evaluating studies that had an in‐hospital/initial phase of 10 to 14 days or studies that included objective DVT verification at discharge, there was still no difference between study arms (data analysis not shown).
Adverse events
Only two studies evaluating participants undergoing hip replacement reported on adverse events (DaPP Study; French Study) and found no difference between study arms (OR 1.06, 95% CI 0.68 to 1.64; participants = 460; I2 = 4%; moderate quality evidence Analysis 1.7), although it should be noted that there was minimal or no description of the criteria for adverse event reporting, and it varied greatly between the two reporting studies.
Too few studies remained for subgroup analysis by initial phase duration or objective DVT verification at discharge (data analyses not shown).
Bleeding events (major, clinically relevant non‐major, minor)
Major bleeding was reported in five studies (DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003) and minor bleeding was reported in five studies (DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003). Clinically relevant non‐major bleeding was not reported by the included studies comparing heparin and placebo.
There was no difference in major bleeding between the study arms (OR 0.59, 95% CI 0.14 to 2.46; participants = 2500; studies = 5; I2 = 0%; moderate quality evidence Analysis 1.8.
For participants undergoing hip replacement, there was no difference in major bleeding (OR 0.32, 95% CI 0.03 to 3.10; participants = 1494; studies = 4; I2 = 0% Analysis 1.8. There was no difference in major bleeding in participants undergoing knee replacement (OR 0.99, 95% CI 0.14 to 7.06; participants = 696; studies = 1; I2 = 0% Analysis 1.8). The single study reporting on a combined group of hip or knee replacement participants reported no major bleeding in either study arm (0/161 heparin versus 0/149 placebo).
There was an increased odds of minor bleeding in the heparin treatment group compared with placebo (OR 2.01, 95% CI 1.43 to 2.81; participants = 2500; studies = 5; I2 = 0%; high quality evidence) Analysis 1.9.
For participants undergoing hip replacement, there was an increased odds of minor bleeding in the heparin treatment group compared with placebo (OR 2.25, 95% CI 1.53 to 3.30; participants = 1494; studies = 4; I2 = 0%) Analysis 1.9. There was no difference in minor bleeding in participants undergoing knee replacement (OR 1.23, 95% CI 0.58 to 2.59; participants = 696; studies = 1 Analysis 1.9). The single study reporting on a combined group of hip and knee replacement participants also reported no difference in minor bleeding in both study arms (OR 2.79, 95% CI 0.11 to 69.13; participants = 310; studies = 1; I2 = 0%) Analysis 1.9.
There were no changes to the findings when only comparing studies with a in‐hospital/initial phase of 10 to 14 days or studies that had objective DVT testing at discharge (data analyses not shown).
Reoperation
The DaPP Study reported that two participants required reoperation but did not report from which study arm. Dahl 1997 reported reoperation due to bleeding to be a safety outcome but did not present data. The Fragmin Trial, Heit 2000 and Kolb 2003 did not report on reoperation. The French Study reported that no reoperations were required following bleeding Analysis 1.10.
Wound infection
DaPP Study, French Study, Heit 2000 and Kolb 2003 did not report on wound infection. Dahl 1997 reported that the majority of adverse events were luxation of prosthesis and infection but did not report specific numbers for the study arms. The Fragmin Trial reported wound infection as part of complications associated with wound haematoma but no specific details for infection were provided.
Wound healing
None of the six studies comparing heparin with placebo reported wound healing (Dahl 1997; DaPP Study; Fragmin Trial; French Study; Heit 2000; Kolb 2003).
Vitamin K antagonists versus placebo
One study evaluated vitamin K antagonist versus placebo (Prandoni 2002), in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. We found no difference between the treatment groups for any of the evaluated outcomes except for total VTE. See summary of findings Table 2 and Appendix 3.
Symptomatic VTE (DVT and PE)
No difference between VKA and placebo was observed for symptomatic VTE (OR 0.10, 95% CI 0.01 to 1.94; participants = 360; moderate quality evidence) Analysis 2.1.
Symptomatic DVT
No difference between VKA and placebo was observed for symptomatic DVT (OR 0.13, 95% CI 0.01 to 2.62; participants = 360; moderate quality evidence) Analysis 2.2.
Symptomatic PE
No difference between VKA and placebo was observed for symptomatic PE (OR 0.32, 95% CI 0.01 to 7.84; participants = 360; moderate quality evidence) Analysis 2.3.
Total VTE (symptomatic or asymptomatic)
Prandoni 2002 showed decreased odds of any VTE event in favour of VKA versus placebo (OR 0.10, 95% CI 0.01 to 0.81; participants = 360; moderate quality evidence) Analysis 2.4.
Asymptomatic DVT
Prandoni 2002 did not report on asymptomatic DVT.
Asymptomatic proximal DVT
Prandoni 2002 did not report on asymptomatic proximal DVT.
Asymptomatic distal DVT
Prandoni 2002 did not report on asymptomatic distal DVT.
All‐cause mortality
Prandoni 2002 reported no deaths in either study arm (0/184 VKA versus 0/176 placebo) Analysis 2.5.
Adverse events
Prandoni 2002 reported no adverse events in either study arm (0/184 VKA versus 0/176 placebo) Analysis 2.6.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the study arms in major bleeding (OR 2.89, 95% CI 0.12 to 71.31; participants = 360; low quality evidence) Analysis 2.7. Prandoni 2002 did not report clinically relevant non‐major bleeding or minor bleeding.
Reoperation
Prandoni 2002 did not report on reoperation.
Wound infection
Prandoni 2002 did not report on wound infection
Wound healing
Prandoni 2002 did not report on wound healing.
DOACs versus placebo
Two studies evaluated DOACs versus placebo (RECORD 2 Trial; Zhang 2014) in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. Zhang 2014 reported assessment of symptomatic DVT only and is therefore only included in the analysis for symptomatic DVT. See summary of findings Table 3 and Appendix 4.
Symptomatic VTE (DVT and PE)
The RECORD 2 Trial reported on symptomatic VTE showing a reduced odds of VTE in favour of the DOAC treatment (OR 0.20, 95% CI 0.06 to 0.68; participants = 2419; moderate quality evidence) Analysis 3.1.
Symptomatic DVT
A reduced odds of symptomatic DVT in favour of DOAC treatment was observed (OR 0.18, 95% CI 0.04 to 0.81; participants = 2459; studies = 2; I2 = not applicable; high quality evidence) Analysis 3.2
Symptomatic PE
No difference between DOACs and placebo was observed for symptomatic PE (OR 0.25, 95% CI 0.03 to 2.25; participants = 1733; studies = 1; low quality evidence) Analysis 3.3.
Total VTE (symptomatic or asymptomatic)
The RECORD 2 Trial showed decreased odds of any VTE event in favour of DOAC treatment versus placebo (OR 0.19, 95% CI 0.11 to 0.33; moderate quality evidence) Analysis 3.4.
Asymptomatic DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic DVT.
Asymptomatic proximal DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic proximal DVT.
Asymptomatic distal DVT
The RECORD 2 Trial and Zhang 2014 do not report on asymptomatic distal DVT.
All‐cause mortality
There were no differences between the study arms in all‐cause mortality (OR 0.33, 95% CI 0.07 to 1.66; participants = 1733; studies = 1; low quality evidence Analysis 3.5.
Adverse events
The RECORD 2 Trial reported no differences in adverse events (OR 0.87, 95% CI 0.74 to 1.03; participants = 2457; moderate quality evidence) Analysis 3.6.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences in any of the bleeding event categories reported by RECORD 2 Trial; major bleeding (OR 1.00, 95% CI 0.06 to 16.02; participants = 2457; low quality evidence Analysis 3.7); clinically relevant bleeding (OR 1.22, 95% CI 0.76 to 1.95; participants = 2457; moderate quality evidence Analysis 3.8); minor bleeding (OR 1.18, 95% CI 0.74 to 1.88; participants = 2457; moderate quality evidence Analysis 3.9.
Reoperation
The RECORD 2 Trial reported reoperation following bleeding; no cases of reoperation (0/1228 DOAC versus 0/1229 placebo) were reported Analysis 3.10.
Wound infection
The RECORD 2 Trial reported no differences in post‐operative wound infections (OR 1.34, 95% CI 0.46 to 3.86; participants = 2457; low quality evidence) Analysis 3.11.
Wound healing
The RECORD 2 Trial and Zhang 2014 did not report wound healing.
Anticoagulant treatments chosen at the investigators' discretion versus placebo
One study evaluated anticoagulant treatment chosen at the investigators' discretion versus placebo (Barrellier 2010) in participants undergoing knee replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. The anticoagulation treatment was either heparin, enoxaparin, dalteparin, tinzaparin, nadroparin or fondaparinux, at the discretion of the investigator at the study location. See summary of findings Table 4 and Appendix 5.
Symptomatic VTE (DVT and PE)
No difference between anticoagulant treatment and placebo was observed for symptomatic VTE (OR 0.50, 95% CI 0.09 to 2.74; participants = 557; low quality evidence) Analysis 4.1.
Symptomatic DVT
No difference between anticoagulant treatment and placebo was observed for symptomatic DVT (OR 0.33, 95% CI 0.03 to 3.21; participants = 557; low quality evidence) Analysis 4.2.
Symptomatic PE
No difference between anticoagulant treatment and placebo was observed for symptomatic PE (OR 1.00, 95% CI 0.06 to 16.13; participants = 557; low quality evidence) Analysis 4.3.
Total VTE (symptomatic or asymptomatic)
The Barrellier 2010 study showed decreased odds of any VTE event in favour of anticoagulant treatment versus placebo (OR 0.26, 95% CI 0.14 to 0.50; participants = 557; moderate quality evidence) Analysis 4.4.
Asymptomatic DVT
Barrellier 2010 showed a significant difference in new cases of asymptomatic DVT favouring anticoagulant treatment compared to placebo (OR 0.26, 95% CI 0.13 to 0.54; participants = 557; moderate quality evidence) Analysis 4.5. Barrellier 2010 reported on asymptomatic distal DVT cases only.
Asymptomatic proximal DVT
Barrellier 2010 did not report asymptomatic proximal DVT.
Asymptomatic distal DVT
Barrellier 2010 reported asymptomatic distal DVT as described above (Analysis 4.6).
All‐cause mortality
Barrellier 2010 reported no deaths in either study arm (0/422 anticoagulant treatment versus 0/420 placebo) Analysis 4.7.
Adverse events
Barrellier 2010 did not report adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the treatment groups in major bleeding (OR 5.05, 95% CI 0.24 to 105.76; participants = 557; low quality evidence). Analysis 4.8. Barrellier 2010 did not report clinically relevant non‐major bleeding or minor bleeding.
Reoperation
Barrellier 2010 reported on reoperation as part of their definition of major bleeding outcome (bleeding leading to reoperation) but did not report this data separately.
Wound infection
Barrellier 2010 did not report on wound infection.
Wound healing
Barrellier 2010 did not report on wound healing.
Vitamin K antagonists versus heparin
One study evaluated vitamin K antagonists versus heparin (SACRE Study) in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. We found no difference between the treatment groups for any of the evaluated outcomes. See summary of findings Table 5 and Appendix 6.
Symptomatic VTE (DVT and PE)
No difference was observed in symptomatic VTE between the treatment groups (OR 1.64, 95% CI 0.85 to 3.16; participants = 1279; low quality evidence) Analysis 5.1.
Symptomatic DVT
There was also no difference between the treatment groups for symptomatic DVT (OR 1.36, 95% CI 0.69 to 2.68; participants = 1279; low quality evidence) Analysis 5.2.
Symptomatic PE
No cases of PE were reported in the heparin group (0/643) compared with 4 cases in the VKA group (4/636) . No difference was found between the treatment groups for the outcome of symptomatic PE (OR 9.16, 95% CI 0.49 to 170.42; participants = 1279; low quality evidence) Analysis 5.3.
Total VTE (symptomatic or asymptomatic)
There was no difference in total VTE between the treatment groups (OR 1.64, 95% CI 0.85 to 3.16; participants = 1279; low quality evidence) Analysis 5.4 .
Asymptomatic DVT
The SACRE Study did not report asymptomatic DVT.
Asymptomatic proximal DVT
The SACRE Study did not report asymptomatic proximal DVT.
Asymptomatic distal DVT
The SACRE Study did not report asymptomatic distal DVT.
All‐cause mortality
No cases of death were reported in the heparin group (0/643) compared with 2 cases in the VKA group (2/636). There was no difference in all‐cause mortality between the treatment groups (OR 5.07, 95% CI 0.24 to 105.83; participants = 1279; low quality evidence) Analysis 5.5.
Adverse events
The SACRE Study did not report adverse events.
Bleeding events (major, clinically relevant non‐major, minor)
There were no differences between the treatment groups in major or minor bleeding (OR 3.87, 95% CI 1.91 to 7.85; participants = 1272; low quality evidence, Analysis 5.6, and OR 1.33, 95% CI 0.64 to 2.76; participants = 1279; low quality evidence Analysis 5.7, respectively). The SACRE Study did not report clinically relevant non‐major bleeding.
Reoperation
The SACRE Study reported on reoperation as part of their definition of major bleeding outcome (bleeding leading to reoperation; reoperation was required in 11 participants (two in the reviparin group and nine in the acecoumarol group (OR 4.60, 95% CI 0.99 to 21.38; participants = 1279; low quality evidence).
Wound infection
The SACRE Study did not report wound infection.
Wound healing
The SACRE Study did not report wound healing.
DOACs versus heparin
Five studies evaluated DOACs versus heparin therapy (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The studies evaluating DOACs versus heparin were only performed in participants undergoing hip replacement, and therefore no subgroup analysis between hip and knee replacement was undertaken. As these studies compared two different anticoagulant treatments and randomised participants prior to surgery, there was no initial phase and objective assessment of VTE after the initial phase. Therefore these subgroup analyses were not possible. We found no difference between the treatment groups for most of the evaluated outcomes. Due to heterogeneity in the data, some of the meta‐analyses in this treatment profile used random‐effects models. See summary of findings Table 6 and Appendix 7.
Symptomatic VTE (DVT and PE)
The random‐effects model evaluating five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) found no difference in symptomatic VTE between the treatment groups (OR 0.70, 95% CI 0.28 to 1.70; participants = 15,977; I2 = 55%; low quality evidence) Analysis 6.1.
Symptomatic DVT
There was no difference between the treatment groups for symptomatic DVT, as evaluated by the random‐effects model in five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.60, 95% CI 0.11 to 3.27; participants = 15,977; I2 = 65%; low quality evidence) Analysis 6.2.
Symptomatic PE
No difference was found between the DOAC and LMWH treatment groups for the outcome of symptomatic PE (OR 0.91, 95% CI 0.43 to 1.94; participants = 14,731; studies = 5; I2 = 0%; moderate quality evidence) Analysis 6.3.
Total VTE (symptomatic or asymptomatic)
There was significant difference in total VTE in the random‐effects model evaluating four studies favouring DOACs (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.53, 95% CI 0.29 to 0.97; participants = 12,447; I2 = 87%; moderate quality evidence) Analysis 6.4.
Asymptomatic DVT
In the random‐effects model, there was no difference in asymptomatic DVT between treatment groups in the two reporting studies (ADVANCE 3; RE‐NOVATE Trial) (OR 0.56, 95% CI 0.19 to 1.59; participants = 6559; I2 = 92%; low quality evidence) Analysis 6.5.
Asymptomatic proximal DVT
Only a single study (RE‐NOVATE Trial) reported on asymptomatic proximal DVT (OR 0.73, 95% CI 0.46 to 1.15; participants = 2704; moderate quality evidence) Analysis 6.6.
Asymptomatic distal DVT
As for asymptomatic proximal DVT, a single study (RE‐NOVATE Trial) reported on asymptomatic distal DVT (OR 1.22, 95% CI 0.75 to 1.99; participants = 2639; moderate quality evidence) Analysis 6.7.
All‐cause mortality
For the five reporting studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), there was no difference in all‐cause mortality between the treatment groups based on the fixed‐effect model ((OR 1.63, 95% CI 0.64 to 4.16; participants = 14,966; studies = 5; I2 = 0%; moderate quality evidence) Analysis 6.8. Regarding sensitivity analysis, the RECORD 1 Trial was found to account for 56% of the participants within this outcome, but after its removal no difference was seen in the findings.
Adverse events
There was also no difference in adverse events between treatment groups in the three studies reporting adverse events (RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) (OR 0.96, 95% CI 0.88 to 1.05; participants = 9908; studies = 3; I2 = 0%; high quality evidence) Analysis 6.9. Regarding sensitivity analysis, the RECORD 1 Trial was found to account for 50.8% of the participants within this outcome, but after its removal no difference was seen in the findings.
Bleeding events (major, clinically relevant non‐major, minor)
Major bleeding was reported in five studies (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), clinically relevant non‐major bleeding was reported in four studies (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial), and minor bleeding was reported in four studies (ADVANCE 3; EXTEND Study; RE‐NOVATE II Trial; RE‐NOVATE Trial).
There were no differences in any of the bleeding event categories within the studies reporting on bleeding events; major bleeding, OR 1.11, 95% CI 0.79 to 1.54; participants = 16,199; studies = 5; I2 = 21%, high quality evidence Analysis 6.10; clinically relevant bleeding OR 1.08, 95% CI 0.90 to 1.28; participants = 15,241; studies = 4; I2 = 7%, high quality evidence) Analysis 6.11; minor bleeding OR 0.95, 95% CI 0.82 to 1.10; participants = 11,766; studies = 4; I2 = 0%, high quality evidence Analysis 6.12.
For minor bleeding, ADVANCE 3 accounted for 53.3% of the participants, and when removed in a sensitivity analysis there were no differences in the association.
Reoperation
Four studies reported on reoperation relating to bleeding (ADVANCE 3; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial) showing no differences between the study arms (OR 1.06, 95% CI 0.34 to 3.24; participants = 15241; studies = 4; I2 = 0%; moderate quality evidence) Analysis 6.13.
The RE‐NOVATE Trial accounted for > 50 % of the weight and when removed in a sensitivity analysis there were no differences in the association.
Wound infection
Three studies reported on wound infection (RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial). The RE‐NOVATE Trial reported one participant who died of septicaemia and GI bleeding following ischaemic bowel resection but reported no other cases of wound infection. Pooling RECORD 1 Trial and RE‐NOVATE II Trial showed no differences in wound infections between the treatment arms (OR 0.89, 95% CI 0.46 to 1.72; participants = 6446; studies = 2; I2 = 0%; moderate quality evidence) Analysis 6.14.
Wound healing
None of the five studies included in this comparison reported on wound healing (ADVANCE 3; EXTEND Study; RECORD 1 Trial; RE‐NOVATE II Trial; RE‐NOVATE Trial).
Reporting bias, subgroup and sensitivity analysis
We did not construct funnel plots for meta analyses because the meta analyses did not include at least 10 studies.
We planned to apply subgroup analysis to examine participants undergoing revisions of a previous hip or knee replacement or hip fracture repair, but we unfortunately found this to be impossible because these data were not reported separately. As the duration of the in‐hospital/initial phase ranged from four days to 14 days, subgroup analysis for 15 days or more was not required. Results of the other planned subgroup analyses are reported above. We did not consider any studies to be of low quality based on the 'Risk of bias' tool and therefore did not perform the planned sensitivity analysis for this reason.
Discussion
Summary of main results
We have shown that extended‐duration prophylaxis with DOACs in patients undergoing major hip or knee replacement surgery significantly reduces the risk of symptomatic VTE. This benefit is achieved with no excess adverse events or major bleeding but with increased minor bleeding. A significant reduction in PE or mortality could not be shown but the direction of the treatment effect for these outcomes was consistent with that seen for the primary outcome, suggesting that a similar reduction for these outcomes might be expected.
Our findings of a significant treatment benefit of extended‐duration prophylaxis for prevention of symptomatic VTE are based on a pooled analysis of both hip and knee replacement trials. However, our meta‐analysis cannot draw a fully informed conclusion for participants undergoing knee replacement as far fewer studies evaluated these types of participants separately, as compared with hip replacement.
When comparing extended‐duration anticoagulants (DOACs and VKA) versus extended‐duration LMWHs there were no differences between the treatments in symptomatic and asymptomatic VTE and showed decreased odds in total VTE for DOACs versus LMWH. There was also no difference in mortality, adverse events or bleeding events. These findings support the use of less invasive oral anticoagulants versus the more invasive LMWHs which often have to be administered by injection into subcutaneous tissue. However, it needs to be kept in mind that several of the meta‐analyses were underpowered and showed heterogeneity. We hope with the inclusion of new studies in future updates of this review we will be better able to draw stronger conclusions regarding these treatment comparisons.
One widely recognised impediment to the more widespread use of effective prophylaxis in patients undergoing hip or knee replacement is concern about the risk of bleeding. In this regard, the data from our review are reassuring in that, even with sustained use for up to six weeks, prophylactic‐dose anticoagulants is not associated with an excess in major bleeding in all comparisons except for the comparison of VKA versus heparin.
Overall completeness and applicability of evidence
This review included a total of 16 studies with nearly 25,000 randomised participants. Despite this large number of included studies some comparisons included a single study only, or few events were recorded, which led to wide confidence intervals, and difficulties interpreting the data.
There are other antithrombotic therapies besides the ones evaluated in this review that might be used for prevention of VTE in major orthopaedic surgery or out‐of‐hospital prophylaxis, for example aspirin, which were not addressed in this review. We plan to include these in future updates of this review.
Although compliance with extended‐duration prophylaxis seemed to be 90% or higher in most trials included in our meta‐analyses, the definition of compliance varied among the studies and not all studies reported the level of compliance with randomised treatment allocation that was achieved. However, the probable impact of lack of compliance is to reduce the power of a randomised trial to detect a significant treatment benefit. Therefore an even greater benefit of extended‐duration prophylaxis might be realised in populations in which higher levels of compliance are achieved.
Wang 2014 reported that surgical site infections and reoperations in the three months following joint replacements may be associated with anticoagulant use and this may be a barrier to extended thromboprophylaxis but our meta‐analyses are underpowered to draw conclusions on this issue due to a lack of reporting of wound infections, wound healing and reoperations in the studies included in this review.
Quality of the evidence
Despite examining the totality of the evidence by pooling results from all the available properly randomised trials, the total number of participants randomly assigned and the number of outcome events was modest. Therefore, our meta‐analyses potentially lacked statistical power to provide precise estimates of frequency and treatment effect for clinically important outcomes such as PE. However, DVT and PE represent clinical manifestations of the same underlying disease process. Therefore, strategies that are effective for the prevention of DVT, especially those proximally located, are likely also to be effective for the prevention of non‐fatal and fatal PE.
There was a lot of variation in the design of studies included in our meta‐analyses. However, differences among trials are inevitable since individual trials look at different populations with different treatment protocols, and there is always some heterogeneity, even within individual trials (Lau 1998; Thompson 1994). Differences in trial design do not necessarily preclude pooling of their results since, in a meta‐analysis, individual participants are directly compared only with other participants within the same trial, and not across the trials. The validity of our approach is further supported by the external consistency of our findings with the results of individual trials.
The quality of the evidence varied by comparison and was on average moderate. Heterogeneity was found between studies comparing DOAC and heparin, and for some outcomes, such as PE and mortality, few events were recorded during the study periods leading to imprecision. For three comparisons, only one study was identified. See summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6 for further details.
Potential biases in the review process
Every effort was made to limit potential biases in the review through duplication of study selection, data extraction and assessment of risk of bias by two review authors. Also, we utilised pre‐designed data extraction forms and discussed any disagreements and undertook a meticulous and exhaustive search for both published and unpublished studies.
Despite these techniques to reduce bias, there are still areas of concern. One issue was with the total numbers of participants used in the meta‐analyses. Most of the included studies had large numbers of participants that were excluded after randomisation. This was due mainly to detecting DVT at discharge from the hospital, non‐compliance, adverse events and non‐evaluable objective testing of DVT. Due to these large numbers of exclusions it was inappropriate to use all randomised participants, and we therefore attempted to use the most reliable intention‐to‐treat population as reported by the study authors. This method required us to adapt the numbers used by the study authors, which did vary between studies.
We did not stratify according to the use of mechanical prophylaxis since, in most studies, the use of mechanical prophylaxis was left to the discretion of the local investigator and is usually not reported separately in the final study report. However, this should not lead to any bias because the use of mechanical methods is likely to be balanced between the two groups as a result of proper use of randomisation.
Agreements and disagreements with other studies or reviews
A recently published meta‐analysis evaluated extended duration using new oral anticoagulants versus extended duration using LMWHs (Liew 2014). The four included studies are also included in our review. For symptomatic VTE they found a risk ratio in favour of oral anticoagulants of 0.42 (95% CI 0.21 to 0.86). Although Liew 2014 included RE‐NOVATE Trial in their review, they did not include this study in the evaluation of symptomatic VTE, which led to the major differences in our findings, as the two dabigatran treatment groups had a large number of VTEs, compared with the LMWH group. Liew 2014 also did not include the SACRE Study. Liew 2014 found no difference in mortality or bleeding events, which is similar to our own review.
A meta‐analysis published in 2001 compared extended‐duration prophylaxis to short duration followed by placebo or no treatment (Eikelboom 2001). Eikelboom 2001 included nine studies, five of which were also included in our review and the other four we excluded because we deemed they did not meet the five‐week treatment‐duration requirement for this review. Eikelboom 2001's findings were similar to ours, with a decreased odds of symptomatic DVT in the extended duration treatment group (OR 0.38, 95% CI 0.24 to 0.61), no difference in major bleeding but an increase in minor bleeding.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Heparin versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).
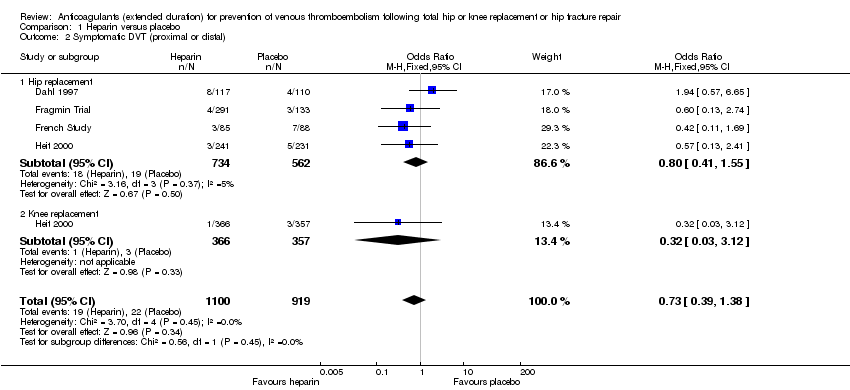
Comparison 1 Heparin versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 1 Heparin versus placebo, Outcome 3 Symptomatic PE.

Comparison 1 Heparin versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 1 Heparin versus placebo, Outcome 5 Asymptomatic DVT.

Comparison 1 Heparin versus placebo, Outcome 6 All‐cause mortality.

Comparison 1 Heparin versus placebo, Outcome 7 Adverse events.

Comparison 1 Heparin versus placebo, Outcome 8 Bleeding ‐ major.

Comparison 1 Heparin versus placebo, Outcome 9 Bleeding ‐ minor.
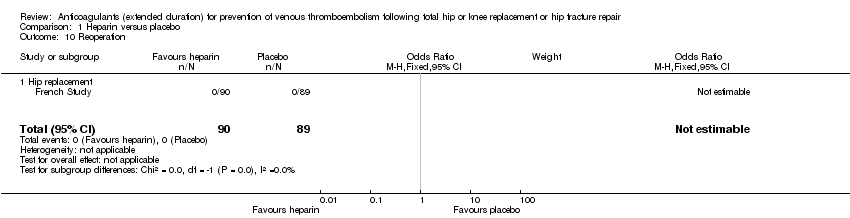
Comparison 1 Heparin versus placebo, Outcome 10 Reoperation.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 2 Vitamin K antagonists versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 2 Vitamin K antagonists versus placebo, Outcome 3 Symptomatic PE.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
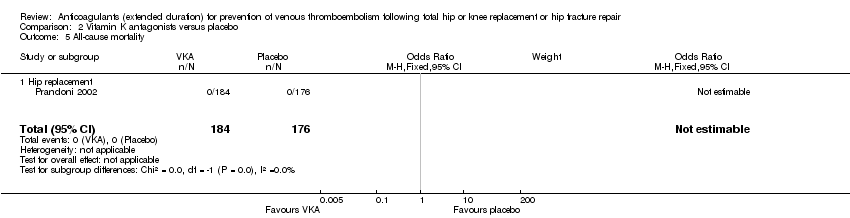
Comparison 2 Vitamin K antagonists versus placebo, Outcome 5 All‐cause mortality.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 6 Adverse events.

Comparison 2 Vitamin K antagonists versus placebo, Outcome 7 Bleeding ‐ major.

Comparison 3 DOAC versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 3 DOAC versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 3 DOAC versus placebo, Outcome 3 Symptomatic PE.

Comparison 3 DOAC versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
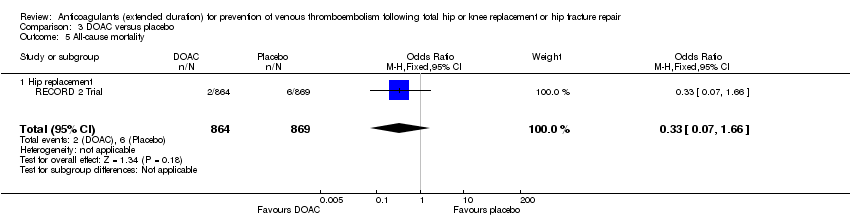
Comparison 3 DOAC versus placebo, Outcome 5 All‐cause mortality.

Comparison 3 DOAC versus placebo, Outcome 6 Adverse events.

Comparison 3 DOAC versus placebo, Outcome 7 Bleeding ‐ major.

Comparison 3 DOAC versus placebo, Outcome 8 Bleeding‐ clinically relevant non‐major.

Comparison 3 DOAC versus placebo, Outcome 9 Bleeding ‐ minor.

Comparison 3 DOAC versus placebo, Outcome 10 Reoperation.

Comparison 3 DOAC versus placebo, Outcome 11 Wound infection.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 3 Symptomatic PE.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 4 Total VTE (symptomatic and asymptomatic).
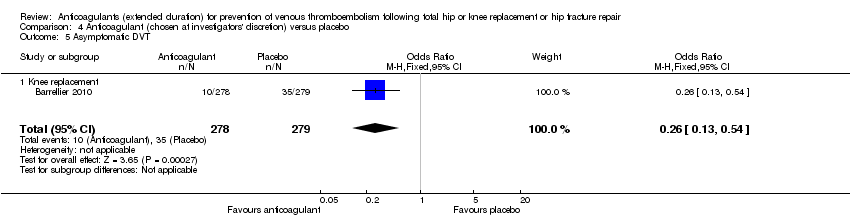
Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 5 Asymptomatic DVT.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 6 Asymptomatic distal DVT.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 7 All‐cause mortality.

Comparison 4 Anticoagulant (chosen at investigators' discretion) versus placebo, Outcome 8 Bleeding ‐ major.
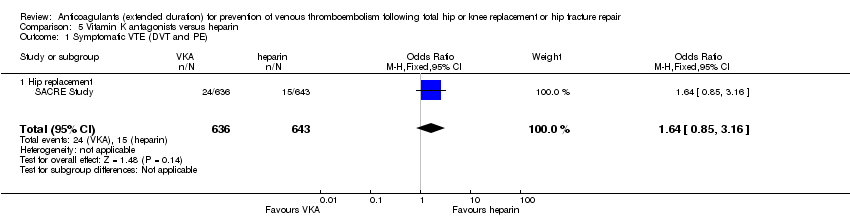
Comparison 5 Vitamin K antagonists versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 5 Vitamin K antagonists versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).
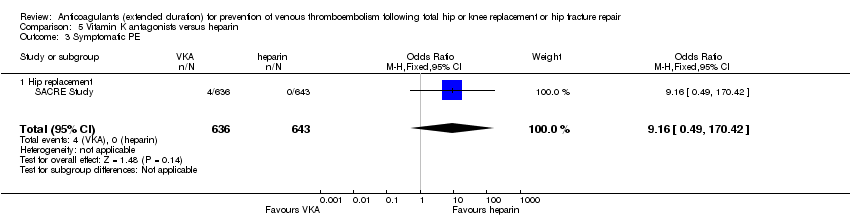
Comparison 5 Vitamin K antagonists versus heparin, Outcome 3 Symptomatic PE.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).

Comparison 5 Vitamin K antagonists versus heparin, Outcome 5 All‐cause mortality.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 6 Bleeding ‐ major.

Comparison 5 Vitamin K antagonists versus heparin, Outcome 7 Bleeding ‐ minor.
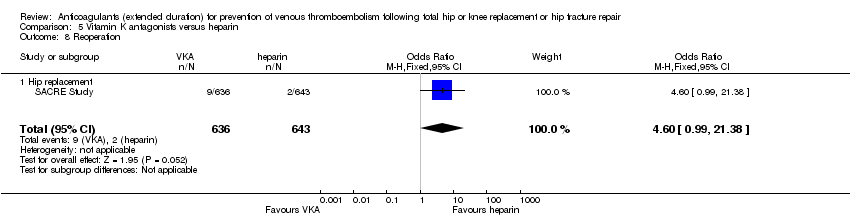
Comparison 5 Vitamin K antagonists versus heparin, Outcome 8 Reoperation.

Comparison 6 DOAC versus heparin, Outcome 1 Symptomatic VTE (DVT and PE).

Comparison 6 DOAC versus heparin, Outcome 2 Symptomatic DVT (proximal or distal).

Comparison 6 DOAC versus heparin, Outcome 3 Symptomatic PE.

Comparison 6 DOAC versus heparin, Outcome 4 Total VTE (symptomatic and asymptomatic).
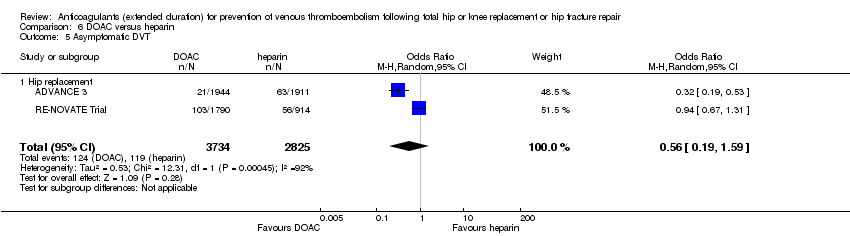
Comparison 6 DOAC versus heparin, Outcome 5 Asymptomatic DVT.

Comparison 6 DOAC versus heparin, Outcome 6 Asymptomatic proximal DVT.
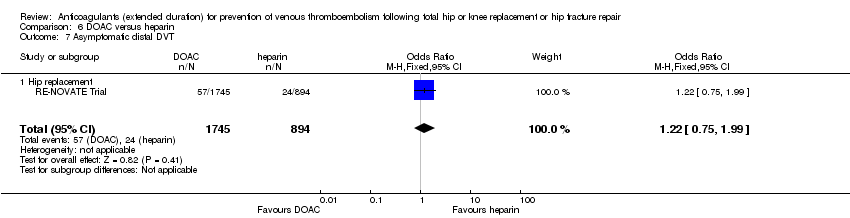
Comparison 6 DOAC versus heparin, Outcome 7 Asymptomatic distal DVT.

Comparison 6 DOAC versus heparin, Outcome 8 All‐cause mortality.

Comparison 6 DOAC versus heparin, Outcome 9 Adverse events.

Comparison 6 DOAC versus heparin, Outcome 10 Bleeding ‐ major.

Comparison 6 DOAC versus heparin, Outcome 11 Bleeding ‐ clinically relevant, non‐major.

Comparison 6 DOAC versus heparin, Outcome 12 Bleeding ‐ minor.
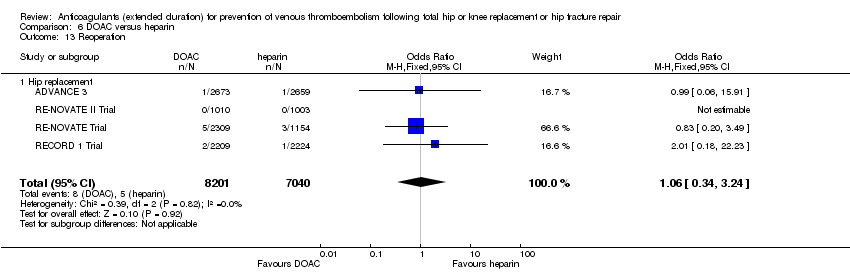
Comparison 6 DOAC versus heparin, Outcome 13 Reoperation.

Comparison 6 DOAC versus heparin, Outcome 14 Wound infection.
| Heparin compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with heparin | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.59 | 2329 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 20 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.73 | 2019 | ⊕⊕⊕⊝ | — | |
| 24 per 1000 | 18 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.61 | 1595 | ⊕⊕⊝⊝ | — | |
| 6 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 0.59 | 2500 | ⊕⊕⊕⊝ | — | |
| 4 per 1000 | 2 per 1000 | |||||
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Bleeding ‐ minor | Study population | OR 2.01 | 2500 | ⊕⊕⊕⊕ | — | |
| 46 per 1000 | 88 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, low number of events leading to imprecision of results | ||||||
| Vitamin K antagonists compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.10 | 360 | ⊕⊕⊕⊝ | — | |
| 23 per 1000 | 2 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.13 | 360 | ⊕⊕⊕⊝ | — | |
| 17 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.32 | 360 | ⊕⊕⊕⊝ | — | |
| 6 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 2.89 | 360 | ⊕⊕⊝⊝ | no events recorded in placebo group | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study only so heterogeneity could not be assessed | ||||||
| DOAC compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.20 | 2419 | ⊕⊕⊕⊝ | — | |
| 12 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.18 | 2459 | ⊕⊕⊕⊕ | — | |
| 9 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.25 | 1733 | ⊕⊕⊝⊝ | — | |
| 5 per 1000 | 1 per 1000 | |||||
| Bleeding ‐ major | Study population | OR 1.00 | 2457 | ⊕⊕⊝⊝ | — | |
| 1 per 1000 | 1 per 1000 | |||||
| Bleeding‐ clinically relevant non‐major | Study population | OR 1.22 | 2457 | ⊕⊕⊕⊝ | — | |
| 27 per 1000 | 33 per 1000 | |||||
| Bleeding ‐ minor | Study population | OR 1.18 | 2457 | ⊕⊕⊕⊝ | — | |
| 28 per 1000 | 32 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity cannot be assessed | ||||||
| Anticoagulants (chosen at investigators' discretion) compared to placebo for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with anticoagulant (chosen at investigators' discretion) | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.50 | 557 | ⊕⊕⊝⊝ | — | |
| 14 per 1000 | 7 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 0.33 | 557 | ⊕⊕⊝⊝ | — | |
| 11 per 1000 | 4 per 1000 | |||||
| Symptomatic PE | Study population | OR 1.00 | 557 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 4 per 1000 | |||||
| Bleeding ‐ major | see comment | OR 5.05 | 557 | ⊕⊕⊝⊝ | no major bleeding recorded in the placebo groups | |
| Clinically relevant non‐major bleeding | see comment | — | — | — | not reported | |
| Minor bleeding | see comment | — | — | — | not reported | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, results from a single study so heterogeneity could not be assessed | ||||||
| Vitamin K antagonists compared to heparin for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with vitamin K antagonists | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 1.64 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 38 per 1000 | |||||
| Symptomatic DVT (proximal or distal) | Study population | OR 1.36 | 1279 | ⊕⊕⊝⊝ | — | |
| 23 per 1000 | 31 per 1000 | |||||
| Symptomatic PE | see comment | OR 9.16 | 1279 | ⊕⊕⊝⊝ | no cases of symptomatic PE reported in the heparin study arm | |
| Bleeding ‐ major | Study population | OR 3.87 | 1272 | ⊕⊕⊝⊝ | — | |
| 16 per 1000 | 58 per 1000 | |||||
| Bleeding ‐ clinically indicated non‐major Treatment duration 28 ‐ 42 days | see comment | — | — | — | clinically indicated non‐major bleeding events not reported in single included study in this comparison | |
| Bleeding ‐ minor | Study population | OR 1.33 | 1279 | ⊕⊕⊝⊝ | — | |
| 20 per 1000 | 27 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level, single study so heterogeneity could not be assessed | ||||||
| DOAC compared to heparin for people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Patient or population: people requiring prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with heparin | Risk with DOAC | |||||
| Symptomatic VTE (DVT and PE) | Study population | OR 0.70 | 15977 | ⊕⊕⊝⊝ | — | |
| 4 per 1000 | 3 per 1000 | |||||
| Symptomatic DVT (proximal or distal) Treatment duration 28 ‐ 42 days | Study population | OR 0.60 | 15977 | ⊕⊕⊝⊝ | — | |
| 3 per 1000 | 2 per 1000 | |||||
| Symptomatic PE | Study population | OR 0.91 | 14731 | ⊕⊕⊕⊝ | — | |
| 2 per 1000 | 2 per 1000 | |||||
| Bleeding ‐ major Treatment duration 28 ‐ 42 days | Study population | OR 1.11 | 16199 | ⊕⊕⊕⊕ | — | |
| 8 per 1000 | 9 per 1000 | |||||
| Bleeding ‐ clinically relevant, non‐major | Study population | OR 1.08 | 15241 | ⊕⊕⊕⊕ | — | |
| 33 per 1000 | 36 per 1000 | |||||
| Bleeding ‐ minor Treatment duration 28 ‐ 42 days | Study population | OR 0.95 | 11766 | ⊕⊕⊕⊕ | — | |
| 66 per 1000 | 63 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for inconsistency (heterogeneity, I2 = 55%) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 2329 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 1.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.30] |
| 1.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 1.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.06] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 4 | 2019 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.39, 1.38] |
| 2.1 Hip replacement | 4 | 1296 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 2.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.12] |
| 3 Symptomatic PE Show forest plot | 3 | 1595 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.16, 2.33] |
| 3.1 Hip replacement | 3 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.56] |
| 3.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.24, 8.83] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 6 | 2544 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.28, 0.56] |
| 4.1 Hip replacement | 5 | 1511 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.56] |
| 4.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.21, 2.92] |
| 4.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.90] |
| 5 Asymptomatic DVT Show forest plot | 5 | 1304 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.60] |
| 5.1 Hip replacement | 4 | 994 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.21, 0.58] |
| 5.2 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.52] |
| 6 All‐cause mortality Show forest plot | 5 | 2518 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.26] |
| 6.1 Hip replacement | 4 | 1485 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.75] |
| 6.2 Knee replacement | 1 | 723 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.65] |
| 6.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.22, 98.42] |
| 7 Adverse events Show forest plot | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 7.1 Hip replacement | 2 | 460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.64] |
| 8 Bleeding ‐ major Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.46] |
| 8.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.10] |
| 8.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.06] |
| 8.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Bleeding ‐ minor Show forest plot | 5 | 2500 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.43, 2.81] |
| 9.1 Hip replacement | 4 | 1494 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.53, 3.30] |
| 9.2 Knee replacement | 1 | 696 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.58, 2.59] |
| 9.3 Hip or knee replacement | 1 | 310 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.79 [0.11, 69.13] |
| 10 Reoperation Show forest plot | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 179 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 1.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.94] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 2.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.62] |
| 3 Symptomatic PE Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 3.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.84] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 4.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 5 All‐cause mortality Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse events Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Bleeding ‐ major Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| 7.1 Hip replacement | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 71.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 1.1 Hip replacement | 1 | 2419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.68] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 2.1 Hip replacement | 2 | 2459 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.81] |
| 3 Symptomatic PE Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 3.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.25] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 4.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.11, 0.33] |
| 5 All‐cause mortality Show forest plot | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 5.1 Hip replacement | 1 | 1733 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.66] |
| 6 Adverse events Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 6.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 7 Bleeding ‐ major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 7.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.02] |
| 8 Bleeding‐ clinically relevant non‐major Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 8.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.76, 1.95] |
| 9 Bleeding ‐ minor Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 9.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 10 Reoperation Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Wound infection Show forest plot | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| 11.1 Hip replacement | 1 | 2457 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 1.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.74] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 2.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.21] |
| 3 Symptomatic PE Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 3.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.13] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 4.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 5 Asymptomatic DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 5.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6 Asymptomatic distal DVT Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 6.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.13, 0.54] |
| 7 All‐cause mortality Show forest plot | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Knee replacement | 1 | 842 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Bleeding ‐ major Show forest plot | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| 8.1 Knee replacement | 1 | 557 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.24, 105.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 1.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 2.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.69, 2.68] |
| 3 Symptomatic PE Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 3.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.16 [0.49, 170.42] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 4.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.85, 3.16] |
| 5 All‐cause mortality Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 5.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [0.24, 105.83] |
| 6 Bleeding ‐ major Show forest plot | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 6.1 Hip replacement | 1 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.87 [1.91, 7.85] |
| 7 Bleeding ‐ minor Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 7.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.64, 2.76] |
| 8 Reoperation Show forest plot | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| 8.1 Hip replacement | 1 | 1279 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.60 [0.99, 21.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptomatic VTE (DVT and PE) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 1.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.28, 1.70] |
| 2 Symptomatic DVT (proximal or distal) Show forest plot | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 2.1 Hip replacement | 5 | 15977 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.27] |
| 3 Symptomatic PE Show forest plot | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 3.1 Hip replacement | 5 | 14731 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.43, 1.94] |
| 4 Total VTE (symptomatic and asymptomatic) Show forest plot | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 4.1 Hip replacement | 4 | 12447 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.97] |
| 5 Asymptomatic DVT Show forest plot | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 5.1 Hip replacement | 2 | 6559 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.19, 1.59] |
| 6 Asymptomatic proximal DVT Show forest plot | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 6.1 Hip replacement | 1 | 2704 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.46, 1.15] |
| 7 Asymptomatic distal DVT Show forest plot | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 7.1 Hip replacement | 1 | 2639 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 1.99] |
| 8 All‐cause mortality Show forest plot | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 8.1 Hip replacement | 5 | 14966 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.64, 4.16] |
| 9 Adverse events Show forest plot | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 9.1 Hip replacement | 3 | 9908 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 10 Bleeding ‐ major Show forest plot | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 10.1 Hip replacement | 5 | 16199 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 11 Bleeding ‐ clinically relevant, non‐major Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 11.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.28] |
| 12 Bleeding ‐ minor Show forest plot | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 12.1 Hip replacement | 4 | 11766 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13 Reoperation Show forest plot | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 13.1 Hip replacement | 4 | 15241 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.34, 3.24] |
| 14 Wound infection Show forest plot | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |
| 14.1 Hip replacement | 2 | 6446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.72] |

