Agonistas adrenérgicos alfa 2 para la prevención de complicaciones cardíacas en adultos sometidos a cirugía
Resumen
Antecedentes
La respuesta al estrés quirúrgico desempeña un rol importante en la patogenia de las complicaciones cardíacas perioperatorias. Los agonistas adrenérgicos alfa 2 atenúan esta respuesta y pueden prevenir las complicaciones cardíacas.
Objetivos
Determinar la eficacia y la seguridad de los agonistas adrenérgicos α2 para reducir la mortalidad y las complicaciones cardíacas en adultos sometidos a cirugía cardíaca y a cirugía no cardíaca.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL (2017, Número 4), MEDLINE (1950 a abril, semana 4, 2017), Embase (1980 a mayo de 2017), Science Citation Index, registros de ensayos clínicos y listas de referencias de los artículos incluidos.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios que compararon los agonistas adrenérgicos α2 (clonidina, dexmedetomidina o mivazerol) con placebo o con agonistas adrenérgicos no α2. Los ensayos incluidos tenían que evaluar la eficacia y la seguridad de los agonistas adrenérgicos α2 para la prevención de la mortalidad perioperatoria o las complicaciones cardíacas (o ambas), o medir uno o más resultados relevantes (es decir, muerte, infarto de miocardio, insuficiencia cardíaca, accidente cerebrovascular agudo, taquiarritmia supraventricular e isquemia miocárdica).
Obtención y análisis de los datos
Dos autores evaluaron de forma independiente la calidad de los ensayos, extrajeron los datos e introdujeron de forma independiente en la computadora los datos resumidos. Se estableció contacto con los autores de los estudios para obtener información adicional. En los ensayos se recogió información sobre los efectos adversos. Los estudios incluidos se evaluaron mediante la herramienta Cochrane "Riesgo de sesgo", y la calidad de la evidencia subyacente a los efectos agrupados del tratamiento se evaluó mediante la metodología GRADE. Debido a la heterogeneidad clínica entre la cirugía cardíaca y no cardíaca, estos subgrupos se analizaron por separado. Se expresaron los efectos del tratamiento como cocientes de riesgos (CR) agrupados con intervalos de confianza (IC) del 95%.
Resultados principales
Se incluyeron 47 ensayos con 17 039 participantes. De estos estudios, 24 ensayos solo incluyeron a participantes sometidos a cirugía cardíaca, 23 solo incluyeron a participantes sometidos a la cirugía no cardíaca y ocho solo incluyeron a participantes sometidos a cirugía vascular. El agonista adrenérgico α2 estudiado fue clonidina en 21 ensayos, dexmedetomidina en 24 ensayos y mivazerol en dos ensayos.
En la cirugía no cardíaca, hubo evidencia de alta calidad de que los agonistas adrenérgicos α2 dieron lugar a un riesgo similar de mortalidad por todas las causas en comparación con los grupos control (1,3% con agonistas adrenérgicos α2 versus 1,7% con control; CR 0,80; IC del 95%: 0,61 a 1,04; participantes = 14 081; estudios = 16). Además, el riesgo de mortalidad cardíaca fue similar entre los grupos de tratamiento (0,8% con agonistas adrenérgicos α2 versus 1,0% con control; CR 0,86; IC del 95%: 0,60 a 1,23; participantes = 12 525; estudios = 5, evidencia de alta calidad). El riesgo de infarto de miocardio probablemente fue similar entre los grupos de tratamiento (CR 0,94; IC del 95%: 0,69 a 1,27; participantes = 13 907; estudios = 12, evidencia de calidad moderada). No hubo efectos asociados sobre el riesgo de accidente cerebrovascular (CR 0,93; IC del 95%: 0,55 a 1,56; participantes = 11 542; estudios = 7; evidencia de alta calidad). Por el contrario, los agonistas adrenérgicos α2 quizás aumenten los riesgos de bradicardia clínicamente significativa (CR 1,59; IC del 95%: 1,18 a 2,13; participantes = 14 035; estudios = 16) e hipotensión (CR 1,24; IC del 95%: 1,03 a 1,48; participantes = 13 738; estudios = 15), sobre la base de evidencia de calidad moderada.
No hubo evidencia suficiente para determinar el efecto de los agonistas adrenérgicos α2 sobre la mortalidad por todas las causas en la cirugía cardíaca (CR 0,52; IC del 95%: 0,26 a 1,04; participantes = 1947; estudios = 16) ni el infarto de miocardio (CR 1,01; IC del 95%: 0,43 a 2,40; participantes = 782; estudios = 8), sobre la base de evidencia de calidad moderada. Hubo una muerte cardíaca en el brazo de clonidina en un estudio de 22 participantes. Según datos muy limitados, los agonistas adrenérgicos alfa 2 pueden haber reducido el riesgo de accidente cerebrovascular (CR 0,37; IC del 95%: 0,15 a 0,93; participantes = 1175; estudios = 7; eventos de resultado = 18; evidencia de baja calidad). Por el contrario, los agonistas adrenérgicos α2 aumentaron el riesgo de bradicardia del 6,4% al 12,0% (CR 1,88; IC del 95%: 1,35 a 2,62; participantes = 1477; estudios = 10; evidencia de calidad moderada), pero el efecto sobre la hipotensión no estuvo claro (CR 1,19; IC del 95%: 0,87 a 1,64; participantes = 1413; estudios = 9; evidencia de baja calidad).
Estos resultados no se modificaron de manera cualitativa en los análisis de subgrupos y los análisis de sensibilidad.
Conclusiones de los autores
Esta revisión concluye que los agonistas adrenérgicos α2 profilácticos en general no evitan la muerte perioperatoria ni las complicaciones cardíacas graves. Para la cirugía no cardíaca, hay evidencia de calidad moderada a alta de que estos agentes no evitan la muerte, el infarto de miocardio ni el accidente cerebrovascular. Por el contrario, hay evidencia de calidad moderada de que estos agentes tienen efectos adversos importantes, a saber, aumentan los riesgos de hipotensión y bradicardia. Para la cirugía cardíaca, hay evidencia de calidad moderada de que los agonistas adrenérgicos α2 no tienen un efecto sobre el riesgo de mortalidad o infarto de miocardio, y que aumentan el riesgo de bradicardia. La calidad de la evidencia no fue adecuada para establecer conclusiones con respecto a los efectos de los agonistas 2‐alfa sobre el accidente cerebrovascular o la hipotensión durante la cirugía cardíaca.
PICOs
Resumen en términos sencillos
Uso de agonistas adrenérgicos alfa 2 para prevenir las complicaciones cardíacas después de la cirugía mayor
Pregunta de la revisión
¿Los agonistas adrenérgicos alfa 2 (clonidina, dexmedetomidina y mivazerol) reducen el número de muertes y las complicaciones cardíacas cuando se administran alrededor del momento de la cirugía?
Antecedentes
Las complicaciones relacionadas con el corazón pueden ser mortales o prolongar la estancia hospitalaria después de la cirugía. Cada año, acerca de 300 000 000 de personas se someten a una cirugía mayor, de los que 9 000 000 presentan complicaciones cardíacas graves. Estas complicaciones pueden ocurrir en parte porque la cirugía provoca un estrés grande en el corazón. Este estrés puede causar hipertensión y frecuencias cardíacas elevadas durante la cirugía, y ninguna es buena para el corazón. Los agonistas adrenérgicos alfa 2 son un grupo de fármacos que pueden prevenir que la presión arterial y la frecuencia cardíaca aumenten durante la cirugía. Por lo tanto, estos fármacos también pueden proteger el corazón del estrés de la cirugía. Se deseaba determinar si administrar estos fármacos alrededor del momento de la cirugía puede proteger al corazón del estrés de la cirugía y, por lo tanto, prevenir las complicaciones cardíacas graves.
Características de los estudios
Se encontraron 47 estudios que se publicaron hasta mayo de 2017. En estos estudios, participaron 17 039 adultos sometidos a cirugía mayor. En 24 estudios participaron 2672 adultos sometidos a cirugía cardíaca. En 23 estudios participaron 14 367 adultos sometidos a una cirugía mayor diferente de la cirugía cardíaca. Cuarenta estudios compararon agonistas adrenérgicos alfa 2 con tratamiento simulado (placebo). Los otros siete estudios los compararon con otros fármacos. Veintiún estudios probaron un fármaco agonista adrenérgico alfa 2 llamado clonidina, 24 estudiaron otro fármaco llamado dexmedetomidina y dos estudiaron otro fármaco llamado mivazerol. La duración del fármaco agonista adrenérgico alfa 2 estudiado varió desde una dosis antes de la cirugía hasta tres días de tratamiento. La mayoría de los pacientes que participaron en estos estudios fueron hombres, y la edad promedio estuvo entre 60 a 70 años de edad. Catorce estudios informaron haber recibido dinero de la empresa que preparó el fármaco que se probó en el mismo estudio. Otros 15 estudios no informaron la procedencia del dinero necesario para financiar el estudio. El número de pacientes que participaron en cada estudio varió desde 20 hasta 10 000 participantes. Diecinueve estudios incluyeron más de 100 participantes.
Resultados clave
Se encontró que en general los agonistas adrenérgicos alfa 2 no proporcionaron efectos beneficiosos claros para evitar la muerte ni las complicaciones graves después de la cirugía. En los pacientes sometidos a cirugías mayores diferentes de la cirugía cardíaca, los agonistas adrenérgicos alfa 2 no disminuyeron las probabilidades de morir, presentar un ataque cardíaco o presentar un accidente cerebrovascular después de la cirugía. No se encontró evidencia suficiente de que, en los pacientes sometidos a cirugía cardíaca, los adrenérgicos alfa 2 disminuyeran el riesgo de muerte o presentar un ataque cardíaco después de la cirugía. Hubo alguna evidencia muy limitada de que estos fármacos podrían prevenir los accidentes cerebrovasculares después de la cirugía cardíaca. No obstante, se necesitan más estudios de investigación antes de poder tener la seguridad de que los agonistas adrenérgicos alfa 2 verdaderamente proporcionan este efecto beneficioso. Estos fármacos también tienen algunos efectos secundarios importantes. Los pacientes que recibieron agonistas adrenérgicos alfa 2 tuvieron muchas más probabilidades de tener presiones arteriales bajas o frecuencias cardíacas bajas durante o después de la cirugía.
Calidad de la evidencia
Se evaluó la calidad de todos los estudios identificados mediante una herramienta especializada denominada criterios GRADE. En general se encontró que la mayoría de la evidencia en estos estudios fue de calidad moderada o alta. Por lo tanto, según estos resultados es posible estar razonablemente seguros de que los agonistas adrenérgicos alfa 2 no son útiles para reducir el número de muertes o de complicaciones cardíacas graves que suceden después de la cirugía.
Conclusiones de los autores
Summary of findings
| Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery | ||||||
| Patient or population: adults undergoing non‐cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.80 | 14,081 | ⊕⊕⊕⊕ | ‐ | |
| 17 per 1000 | 13 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause.) | Study population | RR 0.86 | 12,525 | ⊕⊕⊕⊕ | ‐ | |
| 10 per 1000 | 8 per 1000 | |||||
| Myocardial infarction (within 30‐days after surgery: as detected on an electrocardiogram or trans‐oesophageal echocardiogram) | Study population | RR 0.94 | 13,907 | ⊕⊕⊕⊝ | ‐ | |
| 59 per 1000 | 55 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.93 | 11,542 | ⊕⊕⊕⊕ | ‐ | |
| 5 per 1000 | 4 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.59 | 14,035 | ⊕⊕⊕⊝ | ‐ | |
| 75 per 1000 | 119 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.24 | 13,738 | ⊕⊕⊕⊝ | ‐ | |
| 304 per 1000 | 377 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lacked proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Indirectness not serious. Intervention (mivazerol) used in one large study not available for clinical use. Not downgraded. 3Evidence of publication bias in funnel plot of analysis. Downgraded by one level. 4Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded by one level. | ||||||
| Alpha‐2 adrenergic agonists compared to control in cardiac surgery | ||||||
| Patient or population: adults undergoing cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.52 | 1947 | ⊕⊕⊕⊝ | ‐ | |
| 21 per 1000 | 11 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | 1 death from 12 participants in clonidine arm, and no deaths in 10 participants in control arm. | Not estimable | 22 (1 RCT) | Not estimable | We did not GRADE evidence for this outcome as accurate estimation of RRs is not possible for such low event rates. | |
| Myocardial infarction (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | Study population | RR 1.01 | 782 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 1000 | 21 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.37 | 1175 | ⊕⊕⊝⊝ | Total of 18 acute stokes reported, with 14 in control group and 4 in treatment group. | |
| 24 per 1000 | 9 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.88 | 1477 | ⊕⊕⊕⊝ | ‐ | |
| 64 per 1000 | 120 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.19 | 1413 | ⊕⊕⊝⊝ | ‐ | |
| 332 per 1000 | 395 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lack proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Serious imprecision, because analysis was below optimal information size and confidence interval includes significant benefit and harm. Downgraded by one level. 3Very serious imprecision, because analysis is below optimal information size and number of events was very small. Downgraded by two levels. 4Serious imprecision, because analysis was below optimal information size. Downgraded by one level 5Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded one level. | ||||||
Antecedentes
Descripción de la afección
Las complicaciones cardíacas perioperatorias son un importante problema de salud para los 312 000 000 de pacientes que se someten anualmente a una cirugía mayor en todo el mundo (Meara 2015). Por ejemplo, cerca del 3% de los pacientes que se someten a cirugía mayor no cardíaca presentan infarto de miocardio (IM) perioperatorio (VISION 2014). Las complicaciones cardíacas graves como el IM dan lugar a un aumento en la mortalidad, la estancia hospitalaria y los costos (Fleischmann 2003; Force 1990; VISION 2014). La respuesta al estrés quirúrgico puede desempeñar una función importante en la patogenia de estas complicaciones. Específicamente, el estrés quirúrgico estimula el sistema nervioso simpático, que a su vez da lugar a mayores niveles plasmáticos de norepinefrina y epinefrina (Halter 1997). Estos efectos producen una elevación de la presión arterial y de la frecuencia cardíaca, lo que puede predisponer al miocardio a la isquemia, especialmente en los pacientes con disminución de la reserva del flujo sanguíneo coronario.
Descripción de la intervención
Los agonistas adrenérgicos alfa 2 (α2) se unen selectivamente a los receptores adrenérgicos presinápticos α2 para activar un mecanismo de retroalimentación negativo que inhibe el flujo simpático central (Muzi 1992). Estos receptores están localizados sobre todo en el sistema nervioso central, específicamente en el tronco encefálico y el locus coeruleus. La activación de estos receptores da lugar a hipotensión, bradicardia, sedación central, ansiólisis y analgesia. Tres agonistas adrenérgicos α2 específicos se han evaluado en los pacientes sometidos a cirugía, a saber, clonidina, dexmedetomidina y mivazerol. La clonidina y la dexmedetomidina están disponibles para uso clínico, mientras que la administración del mivazerol se ha limitado a los ensayos clínicos. La clonidina tiene una vida media de 12 a 18 horas con biodisponibilidad excelente, lo que hace posible su administración una vez al día en forma de comprimido oral o parche transdérmico. También está disponible una preparación intravenosa (IV) de clonidina. La dexmedetomidina tiene una vida media más corta de solo dos horas y una biodisponibilidad variable, lo que hace que sea más conveniente para la administración como venoclisis continua (Flood 2015). De manera similar, el mivazerol también se administra como venoclisis continua (Oliver 1999).
De qué manera podría funcionar la intervención
Como se indicó anteriormente, los agonistas adrenérgicos α2 inhiben el flujo simpático central. En consecuencia, pueden atenuar las anomalías hemodinámicas perioperatorias (Ellis 1994; McSPI‐Europe 1997; Talke 1995), y, quizás, también prevenir las complicaciones cardíacas. Además, la clonidina tiene la capacidad única de reducir la actividad simpática sin inhibir el barorreflejo, que es fundamental para la respuesta a las fluctuaciones en el volumen de sangre circulante que a menudo se produce durante la cirugía (Muzi 1992). No obstante, los agonistas adrenérgicos α2 tienen efectos colaterales importantes, como hipotensión y bradicardia (Biccard 2008). Estos efectos hemodinámicos pueden tener consecuencias clínicamente importantes para los pacientes sometidos a cirugía. Por ejemplo, en el ensayo controlado aleatorio (ECA) Perioperative Ischemic Evaluation ‐ 1 (POISE‐1), el bloqueo β perioperatorio aumentó el riesgo de bradicardia, hipotensión, accidente cerebrovascular agudo y muerte (POISE 2008). Como los agonistas adrenérgicos α2 producen beneficios potenciales y efectos secundarios, una revisión sistemática cuantitativa puede ayudar a determinar su eficacia y seguridad general.
Por qué es importante realizar esta revisión
Anteriormente se han publicado revisiones sistemáticas de la administración perioperatoria de los agonistas adrenérgicos α2 (Biccard 2008; Nishina 2002; Stevens 2003). Sin embargo, dos de ellas se limitaron a agonistas adrenérgicos α2 individuales, a saber, clonidina (Nishina 2002) y dexmedetomidina (Biccard 2008). La otra revisión se limitó a estudios publicados antes de 2002 (Stevens 2003). Por lo tanto, se justifica una revisión sistemática según la metodología Cochrane. La revisión actual es una actualización de una revisión Cochrane anterior que incluyó estudios publicados antes de agosto de 2008. (Wijeysundera 2009). Esta actualización se consideró necesaria, en parte, debido a la publicación del ECA más grande hasta la fecha de agonistas adrenérgicos α2 perioperatorios, el ensayo Perioperative Ischemic Evaluation ‐ 2 (POISE‐2) (Devereaux 2014a).
Objetivos
Determinar la eficacia y la seguridad de los agonistas adrenérgicos α2 para reducir la mortalidad y las complicaciones cardíacas en adultos sometidos a cirugía cardíaca y a cirugía no cardíaca.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron ECA publicados.
Tipos de participantes
Se incluyeron pacientes adultos (con 18 años de edad o más) sometidos a una cirugía bajo anestesia general, anestesia neuraxial o ambas. Se excluyeron las intervenciones quirúrgicas realizadas bajo anestesia local o bloqueo nervioso periférico solo, porque tales procedimientos generalmente se asocian con un riesgo muy bajo de mortalidad y morbilidad. También se excluyó la cirugía realizada en embarazadas, receptores de trasplantes de órganos o pacientes con abstinencia del consumo de sustancias. Los procedimientos de trasplante de órganos pueden asociarse con un alto riesgo de mortalidad no relacionado con causas cardiovasculares, lo que enmascara los beneficios potenciales de los agonistas adrenérgicos α2.
Tipos de intervenciones
La intervención experimental debe haber incluido la administración de clonidina, mivazerol o dexmedetomidina antes de la cirugía (en el transcurso de 24 horas), durante la cirugía o después de la cirugía (en el transcurso de 48 horas). La medicación debe haber sido administrada por vía IV, intramuscular, oral o transdérmica. No hubo limitaciones en cuanto a la dosis, la duración o la frecuencia de la intervención.
Se permitieron intervenciones activas en el grupo de comparación solo si se consideró que el comparador tenía un efecto mínimo a ningún efecto sobre los resultados primarios o secundarios. Por ejemplo, en un ensayo en que la dexmedetomidina se evaluó principalmente para la función de proporcionar sedación posoperatoria después de una cirugía mayor, la comparación con propofol se consideró razonable.
Tipos de medida de resultado
Los ensayos incluidos tenían que evaluar la eficacia o la seguridad de los agonistas adrenérgicos α2 para la reducción de la mortalidad perioperatoria o las complicaciones cardíacas, o ambas. Los estudios se incluyeron si midieron uno o más resultados relevantes, incluidos la muerte, el IM, la insuficiencia cardíaca (IC), el accidente cerebrovascular agudo, la taquiarritmia supraventricular (TSV) o la isquemia miocárdica. Además, se incluyeron los estudios con objetivos similares a los de la presente revisión, incluso si estos estudios no informaron ningún evento de resultado relevante (es decir, muerte, IM, IC, accidente cerebrovascular agudo, TSV, isquemia miocárdica).
Resultados primarios
-
Mortalidad por todas las causas en el transcurso de los 30 días posteriores a la cirugía: cualquier muerte informada. También se documentó el período para la evaluación del resultado de cada ensayo.
Resultados secundarios
-
Mortalidad cardíaca en el transcurso de los 30 días posteriores a la cirugía: muerte súbita o muerte como resultado de una causa cardíaca identificable primariamente. También se documentó el período para la evaluación del resultado de cada ensayo.
-
IM en el transcurso de 30 días después de la cirugía: definición según el estudio individual (con los criterios específicos empleados documentados). También se documentó el período para la evaluación del resultado de cada ensayo.
-
Isquemia miocárdica en el transcurso de 30 días después de la cirugía: detectada en un electrocardiograma (ECG) o en un ecocardiograma transesofágico (con los criterios específicos empleados documentados). También se documentó el período para la evaluación del resultado de cada ensayo.
-
TSV en el transcurso de 30 días después de la cirugía: TSV, fibrilación auricular o flúter auricular. También se documentó el período para la evaluación del resultado de cada ensayo.
-
IC en el transcurso de 30 días después de la cirugía: diagnóstico clínico de IC o necesidad de apoyo posoperatorio con balón de contrapulsación intraaórtico (aplicable solo a la cirugía cardíaca). También se documentó el período para la evaluación del resultado de cada ensayo.
Efectos adversos del tratamiento
-
Accidente cerebrovascular agudo en el transcurso de 30 días después de la cirugía: nuevo déficit neurológico focal con signos y síntomas de más de 24 horas de duración. También se documentó el período para la evaluación del resultado de cada ensayo.
Efectos fisiológicos del tratamiento
-
Bradicardia que requirió tratamiento farmacológico o marcapaso durante el período de administración del fármaco de estudio.
-
Hipotensión que requirió tratamiento con fármacos inotrópicos o vasopresores durante el período de administración del fármaco de estudio.
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 4), MEDLINE (1950 to April week 4 2017), Embase (1980 to May 2017), the Science Citation Index and reference lists of articles. The Ovid platform was used for searching the electronic databases.
We searched MEDLINE using the search terms presented in Appendix 1. We then limited the studies to those identified simultaneously by a highly sensitive search strategy for identifying RCTs in MEDLINE (Dickersin 1994). Our search strategies for CENTRAL and Embase are presented in Appendix 1.
Búsqueda de otros recursos
We entered all trials selected for inclusion into the Science Citation Index to identify any additional relevant articles. The bibliographies of all included articles and published reviews were searched to identify any other potentially relevant studies for inclusion. Additionally, we searched clinical trial registries, namely ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), for published studies meeting our inclusion criteria. These additional searches were completed in May 2017.
Obtención y análisis de los datos
Selección de los estudios
Two authors (DD, AS) independently performed literature searches for potentially relevant RCTs. All identified published full papers and abstracts were assessed independently for inclusion by the same two authors. We applied no language restrictions. We documented the reasons for exclusion for all excluded studies. We resolved all disagreements by consensus or involvement of a third author (DNW).
Extracción y manejo de los datos
Two authors (DD, AS) independently extracted data from the included studies on a predesigned data abstraction form (Appendix 2). These same two authors independently entered all data into Review Manager 5 (RevMan 2014). We were not blinded to study authors, institution or journal when performing data abstraction. Where necessary, we contacted authors of published trials to provide any additional information required for the analyses (see Methods of the review).
Evaluación del riesgo de sesgo de los estudios incluidos
Two authors (DD, AS) independently evaluated the quality of all included trials using the criteria recommended by the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group. These criteria emphasize the adequacy of allocation concealment, randomization, blinding and intention‐to‐treat (ITT) analysis. Each included study was evaluated using the Cochrane 'Risk of bias' tool (Higgins 2011a). We were not blinded to study authors, institution or journal when performing quality assessment.
Medidas del efecto del tratamiento
We performed all statistical analyses using Review Manager 5 (RevMan 2014). Given that all outcomes and adverse effects were dichotomous, all treatment effects were expressed as pooled risk ratios (RR) with 95% confidence intervals (CI).
Cuestiones relativas a la unidad de análisis
We excluded cross‐over trials and cluster randomized trials in this review. If a study had multiple treatment arms, comparisons were made between α‐2 adrenergic agonist and placebo, or between α‐2 adrenergic agonist and inactive control.
Manejo de los datos faltantes
If a study had missing relevant data in the published report, we attempted to contact the study authors up to three times to obtain these data. If data were missing due to participant attrition, and imputation methods were not used in the published report, we employed complete case analysis when importing the data.
Evaluación de la heterogeneidad
We measured heterogeneity using the I2 statistic: the proportion of total variation explained by between‐study variation as opposed to chance (Higgins 2002; Higgins 2003). Higher I2 statistics imply more heterogeneity between studies than would be expected by chance alone.
Evaluación de los sesgos de notificación
We carried out funnel plot analyses to assess for publication bias (Egger 1997), with formal tests for asymmetry being performed only if meta‐analyses pooled data from 10 or more studies (Higgins 2011b).
Síntesis de los datos
Given the clinical heterogeneity between cardiac and non‐cardiac surgery, we conducted analyses for these two subgroups separately. If an individual study included both cardiac and non‐cardiac surgery procedures, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent cardiac surgical procedures, the specific study was allocated to the cardiac surgery subgroup. Conversely, the study was allocated to the non‐cardiac surgery subgroup if greater than 75% of participants underwent non‐cardiac surgical procedures. In all other cases, the specific study was excluded from the review. In the presence of low heterogeneity (I2 statistic 25% or less) (Higgins 2003), pooled RRs were calculated using the fixed‐effect model. In the presence of moderate‐to‐significant heterogeneity (I2 statistic greater than 25%) (Higgins 2003), we used the random‐effects model and carried out post‐hoc analyses to attempt to explain the heterogeneity.
Análisis de subgrupos e investigación de la heterogeneidad
A priori, we planned several subgroup analyses to determine the potential influence of the surgical procedure, the specific α‐2 adrenergic agonist employed and coexistent therapies on the overall results. Subgroup‐specific results were only calculated if there were two or more studies within the subgroup. These subgroup analyses were as follows.
-
Treatment effects of α‐2 adrenergic agonists on mortality (all‐cause and cardiac‐cause), MI and ischaemia based on the type of non‐cardiac surgical procedure, namely vascular versus non‐vascular non‐cardiac surgery. If a variety of surgical procedures were included in a study, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent the same class of surgery, the specific study was allocated to that specific subgroup. Failing that, the specific study was excluded from the subgroup analysis based on procedure type. We used statistical tests of interaction to assess for the presence of any subgroup effects.
-
We calculated treatment effects for each of clonidine, mivazerol and dexmedetomidine on mortality (all‐cause and cardiac‐cause), and MI in non‐cardiac surgery. Statistical tests of interaction were used to assess for the presence of any subgroup effects.
Análisis de sensibilidad
We planned several sensitivity analyses a priori to characterize the influence of study quality and outcome definitions on the overall results.
-
We restricted the meta‐analyses to the subset of studies that clearly reported methods for blinding and allocation concealment.
-
We determined the effect of α‐2 adrenergic agonists on MI in the subset of RCTs that strictly defined MI a priori as either significant new Q waves on an ECG or significant elevations in enzymatic markers of cardiac injury (MB isoenzyme of creatinine kinase, troponin‐I, troponin‐T).
-
We determined the effect of α‐2 adrenergic agonists on myocardial ischaemia in the subset of RCTs that strictly defined ischaemia a priori as ST segment depression or elevation of 0.1 mV or greater for one minute or longer.
In addition, we performed four additional post‐hoc analyses.
-
Significant statistical heterogeneity was identified when calculating the pooled effect of α‐2 adrenergic agonists on hypotension during non‐cardiac surgery. To explore potential explanations for this heterogeneity, we conducted subgroup analyses based on the specific agent (i.e. clonidine, mivazerol or dexmedetomidine) in the included trials. A statistical test of interaction was used to assess for the presence of a subgroup effect.
-
During the course of the review, we identified several very large included RCTs that might have highly influenced the overall pooled estimates. Therefore, we conducted a sensitivity analysis that excluded these very large RCTs.
-
Mivazerol is an experimental α‐2 adrenergic agonist that was studied in several relatively large trials, but never proceeded through the approval process for clinical use. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials that evaluated mivazerol.
-
Several relevant studies were conducted prior to 1997, during a period when perioperative practice might not necessarily be generalizable to contemporary practice. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials where data were collected prior to 1997.
'Summary of findings' tables and GRADE
To characterize the confidence in the pooled estimated treatment effects better, we used GRADE methodology to assess the quality of evidence (Guyatt 2008). We generated 'Summary of findings' tables that separately presented pooled treatment effect estimates for the subgroups of participants who underwent non‐cardiac surgery and cardiac surgery. To facilitate this process, data from the meta‐analyses in Review Manager 5 (RevMan 2014), were initially exported into GRADEpro. The GRADE approach rates quality of evidence as high, moderate, low or very low (GRADE Handbook 2013). Since all data included in this review were from RCTs, the quality of evidence for each outcome of interest was initially rated as high level, and then potentially downgraded up to three levels based on any deficiencies in the quality of the underlying evidence. The quality of evidence underlying each pooled treatment effect estimate was assessed with respect to the risk of bias, inconsistency, indirectness, imprecision and publication bias (Balshem 2011). The anticipated risk for comparison of each outcome was determined based on the event rate in the control group. Only outcomes judged as critically important, based on their impact on patient health or clinical decision‐making, were chosen for presentation in the 'Summary of findings' tables. In this present review, we included the following outcomes in the 'Summary of findings' tables, provided that relevant estimated pooled treatment effects were present: all‐cause mortality, cardiac mortality, MI, acute stroke, bradycardia and hypotension.
Results
Description of studies
Results of the search
Our search results are presented in Figure 1. The authors identified 3099 separate papers in the literature search and three additional papers from other sources and in total read 389 papers in full.
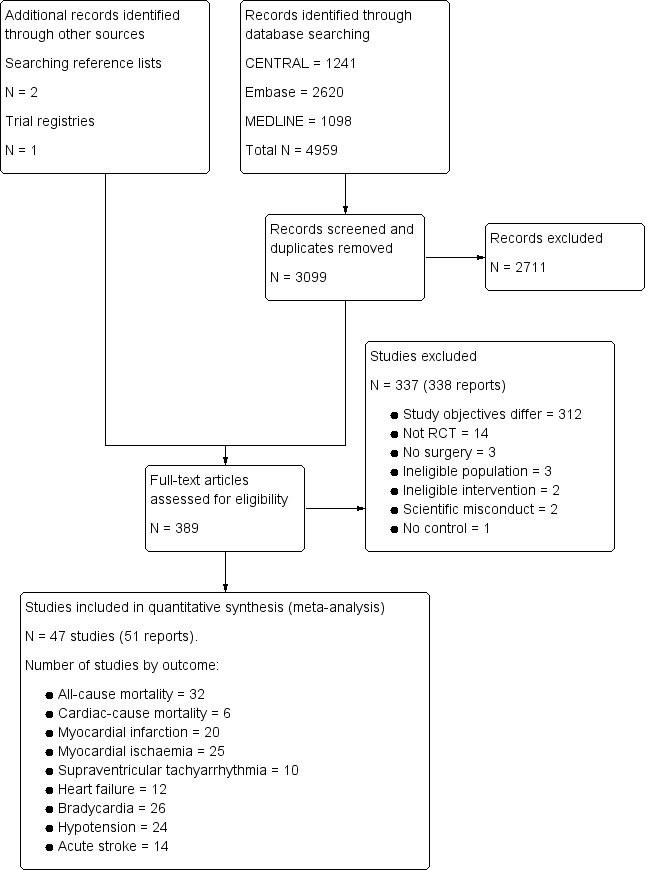
Study flow diagram.
Included studies
We included 47 trials, which encompassed 17,039 participants (Abi‐Jaoude 1993; Ammar 2016; Bergese 2010; Chi 2016; Cho 2016; Corbett 2005; Devereaux 2014a; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ellis 1994; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Lee 2013a; Li 2017; Lipszyc 1991; Liu 2016; Loick 1999; Matot 2000; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Patel 2016; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). These studies are described in detail in the Characteristics of included studies tables.
Twenty four studies with 2672 participants involved cardiac surgery alone (Abi‐Jaoude 1993; Ammar 2016; Chi 2016; Cho 2016; Corbett 2005; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ghignone 1986; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Li 2017; Liu 2016; Loick 1999; Myles 1999; Park 2014; Patel 2016; Quintin 1993; Ren 2013; Shehabi 2009; Venn 1999). In all cases, the procedure involved was coronary artery bypass graft surgery or valve replacement surgery.
Of the 23 studies with 14,367 participants that involved non‐cardiac surgery (Bergese 2010; Devereaux 2014a; Ellis 1994; Ghignone 1987; Lee 2013a; Lipszyc 1991; Matot 2000; McSPI‐Europe 1997; Oliver 1999; Pawlik 2005; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002), eight involved vascular procedures exclusively (Lipszyc 1991; McSPI‐Europe 1997; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Talke 1995; Talke 2000), and seven involved non‐vascular procedures exclusively (Ghignone 1987; Lee 2013a; Matot 2000; Pawlik 2005; Venn 2001; Viviano 2012; Xu 2014). One non‐cardiac surgery study presented subgroup‐specific results for both vascular and non‐vascular procedures (Oliver 1999).
Sample size
The sample sizes of the included trials ranged from 20 participants to 10,010 participants. Fourteen studies had fewer than 50 participants (Abi‐Jaoude 1993; Dorman 1993; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Lipszyc 1991; Matot 2000; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Talke 1995; Talke 2000; Venn 2001), 14 studies had 50 to 100 participants (Ammar 2016; Chi 2016; Corbett 2005; El‐Kerdawy 2004; Ellis 1994; Jalonen 1997; Khalil 2013; Lee 2013a; Liu 2016; Loick 1999; Patel 2016; Viviano 2012; Xu 2014; Yin 2002), and 19 studies had greater than 100 participants (Bergese 2010; Cho 2016; Devereaux 2014a; Djaiani 2016; Herr 2003; Kim 2014a; Li 2017; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Venn 1999; Wallace 2004; Wijeysundera 2014a).
Demographics of sample
The mean age of participants in most studies was 60 to 70 years. In addition, the ratio of men to women in the included studies was skewed, with trials generally recruiting disproportionally more men (Characteristics of included studies table).
Intervention and comparators
The number of studies that assessed dexmedetomidine was 24, clonidine was 21 and mivazerol was two. Treatment duration ranged from a single preoperative dose to a 72‐hour course of treatment. With the exception of seven studies (Corbett 2005; Djaiani 2016; Herr 2003; Liu 2016; Park 2014; Shehabi 2009; Venn 2001), all trials compared α‐2 adrenergic agonists against inactive control. Of the four studies with active controls, one compared dexmedetomidine to morphine (Shehabi 2009), whereas the remainder were comparisons of dexmedetomidine versus propofol.
All studies that evaluated dexmedetomidine employed the IV route of administration. Dexmedetomidine was administered intraoperatively in 15 studies, with administration being continued postoperatively in nine of them. The duration of postoperative administration varied across these nine studies, ranging from continuation until arrival to the critical care unit, to continuation for 48 hours. Nine additional studies investigated dexmedetomidine that was administered entirely after surgery in the critical care unit. Both studies of mivazerol administered the drug IV starting from the intraoperative period, with continuation until 72 hours after surgery.
There was considerable variation in the administration regimens used in trials that assessed clonidine. It was administered intraoperatively by the IV route in three studies, with one of these studies also administering an oral loading dose before surgery. A single study used IV clonidine that was administered only preoperatively (i.e. 30 minutes prior to surgery). Four studies employed clonidine administered using the combination of an oral preoperative loading dose, and subsequent maintenance via the transdermal route for 72 hours. Finally, 12 studies administered clonidine orally before surgery, with three of them administering an additional intraoperative dose via the nasogastric route.
Funding
Thirty‐two studies reported their funding sources, whereas 15 did not (Abi‐Jaoude 1993; Chi 2016; Cho 2016; El‐Kerdawy 2004; Ghignone 1986; Ghignone 1987; Lipszyc 1991; Loick 1999; Myles 1999; Park 2014; Pluskwa 1991; Quintin 1993; Ren 2013; Stuhmeier 1996; Viviano 2012). Fourteen studies reported operational funding from pharmaceutical companies (Bergese 2010; Djaiani 2016; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Li 2017; McSPI‐Europe 1997; Oliver 1999; Quintin 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001), and the remaining 18 studies reported that no pharmaceutical funds were used to complete the research (Ammar 2016; Corbett 2005; Devereaux 2014a; Dorman 1993; Ellis 1994; Khalil 2013; Kim 2014a; Lee 2013a; Liu 2016; Matot 2000; Patel 2016; Pawlik 2005; Shehabi 2009; Soliman 2016; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). Several studies in the latter group reported that a pharmaceutical company supplied the study drug as in‐kind support, and explicitly stated no further funds were received from the company.
Excluded studies
After the full‐text articles were reviewed, we excluded 337 studies. The reasons for these exclusions are presented in the Characteristics of excluded studies table, as well as the study flow diagram (Figure 1). The most common reason for exclusion was study objectives that differed from this present review (312 excluded studies). In these cases, the focus of these studies was to answer a question unrelated to the efficacy or safety of α‐2 adrenergic agonists for reducing mortality or cardiac complications (e.g. assessing the efficacy of these drugs for providing analgesia). Of the remaining articles, 14 were excluded because the experimental design was not an RCT, three were excluded since participants did not undergoing surgery and three were excluded due to an ineligible population. Two studies could not be classified into either the cardiac or non‐cardiac surgery subgroups, and were therefore excluded (Martin 2003; Triltsch 2002). A further two studies were excluded because the intervention was administered via an ineligible route (Nader 2009; Tzortzopoulou 2009), while one study was excluded due to lack of a control arm (Moghadam 2012). Three reports of two individual studies were excluded due to concerns about scientific misconduct (Boldt 1996; Wahlander 2005). In each of these cases, a lead author was found to have conducted scientific misconduct (Anon 2013; Rasmussen 2011; Wise 2013). Notably, both studies had been included in the previous 2009 version of this review (Wijeysundera 2009), at which point these issues with scientific misconduct had not yet been identified (Boldt 1996; Wahlander 2005).
Studies awaiting classification
No studies are currently awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
The methodological quality of included studies is shown in the 'Risk of bias' figures (Figure 2; Figure 3). A visual summary of judgements about the quality and risk of bias for each trial is presented in Figure 3. Details explaining the judgements for each domain are presented in the 'Risk of bias' tables (Characteristics of included studies).
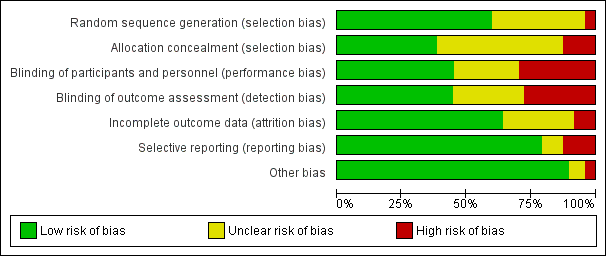
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
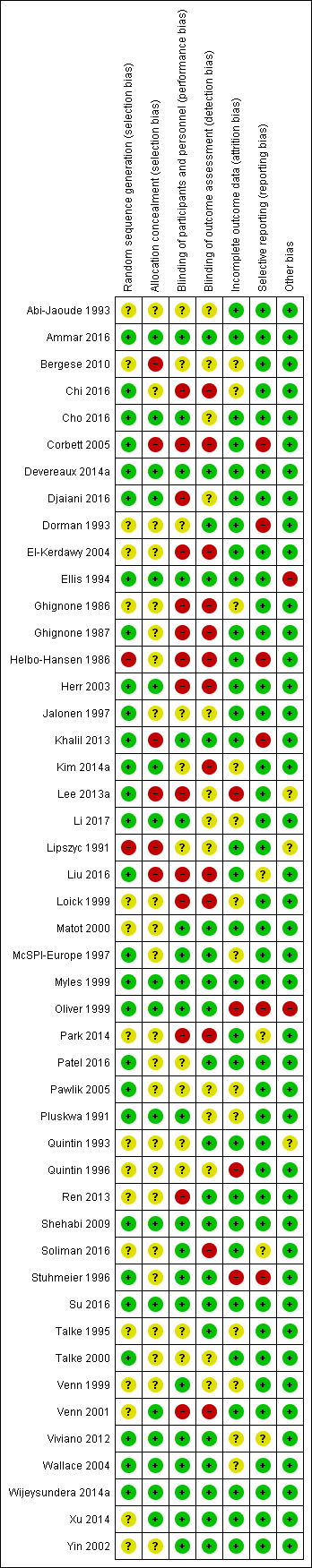
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 47 included trials, only 28 were judged to have adequate methods of generating allocation sequences. Of the remaining 19 studies, two trials used methods likely to produces bias (Helbo‐Hansen 1986; Lipszyc 1991), while the remaining trials were classified as having unclear risk of bias because the methods were not described in adequate detail. Concealment of allocation sequence was generally poor with only 18 studies reporting methods associated with low risk of bias, while six studies described methods associated with a high risk of bias (Bergese 2010; Corbett 2005; Khalil 2013; Lee 2013a; Lipszyc 1991; Liu 2016). Only 16 studies reported adequate allocation sequence generation and allocation concealment.
Blinding
Although 31 studies described themselves as double‐blind, only 21 clearly reported adequate methods for how blinding was achieved. Of the remaining 26 studies, 14 were open‐label and therefore assessed to be high risk of bias, while the others were judged to have an unclear risk of bias. Outcome assessment was blinded in 21 trials, and therefore judged to be at low risk of bias. Only 16 trials demonstrated blinding of participants, personnel and outcome assessors.
Incomplete outcome data
Thirty trials reported no exclusions, exclusions deemed to be appropriate and ITT analysis. For four trials, exclusions (Lee 2013a; Oliver 1999; Quintin 1996; Stuhmeier 1996), were either not reported or judged as being excessive enough to likely cause bias. The remainder either failed to use ITT analysis or adequately account for exclusions. Only 11 studies reported a flow diagram of participants in the trial (Bergese 2010; Chi 2016; Devereaux 2014a; Kim 2014a; Lee 2013a; Li 2017; Liu 2016; Shehabi 2009; Su 2016; Viviano 2012; Wijeysundera 2014a), as is recommended in the CONSORT statement (Schulz 2010).
Selective reporting
Of the 47 trials, 37 demonstrated concordance between outcomes discussed in the methods or protocol and the outcomes reported. Four studies were judged to be of unclear risk of bias because they reported adverse events without discussing any surveillance methods (Liu 2016; Park 2014; Soliman 2016; Viviano 2012). The remaining six studies either failed to report major outcomes, or reported major outcomes not discussed in the relevant methods sections (Corbett 2005; Dorman 1993; Helbo‐Hansen 1986; Khalil 2013; Oliver 1999; Stuhmeier 1996).
Other potential sources of bias
Five trials had other sources of bias classified as unclear risk or high risk. Two of the trials had high risk of bias due to significant changes in their methods during the trial recruitment phase. One trial terminated early (Ellis 1994), while the other changed its selection criteria (Oliver 1999). Three trials were classified as having unclear risk of bias, because two trials (Lipszyc 1991; Quintin 1993), were being published only in abstract form (therefore lacking complete peer‐review), and another lacked reproducible selection criteria (Lee 2013a).
Effects of interventions
See: Summary of findings for the main comparison Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery; Summary of findings 2 Alpha‐2 adrenergic agonists compared to control in cardiac surgery
Non‐cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 210 events (1.5%) among 14,081 participants. Alpha‐2 adrenergic agonists had no statistically significant reduction in all‐cause mortality (RR 0.80, 95% CI 0.61 to 1.04, P = 0.10), without any measurable heterogeneity (I2 = 0%) (Analysis 1.1). The quality of this evidence was high (summary of findings Table for the main comparison).
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Five studies reported cardiac‐related deaths, with 114 events (0.9%) among 12,525 participants. Alpha‐2 adrenergic agonists did not cause a statistically significant reduction in cardiac‐related mortality (RR 0.86, 95% CI 0.60 to 1.23, P = 0.41) with low measurable heterogeneity (I2 = 16%) (Analysis 1.2). The quality of evidence was high (summary of findings Table for the main comparison).
2. Myocardial infarction within 30 days after surgery (definition as per individual study)
Twelve studies reported MIs, with 835 events (6.0%) among 13,907 participants. Alpha‐2 adrenergic agonists were not associated with any statistically significant difference in the risk of MI (RR 0.94, 95% CI 0.69 to 1.27, P = 0.67) with moderate heterogeneity (I2 = 37%) (Analysis 1.3). The quality of evidence was moderate (summary of findings Table for the main comparison).
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Twelve studies reported myocardial ischaemia, with 291 events (21.1%) among 1379 participants. Alpha‐2 adrenergic agonists did not significantly reduced the risk of ischaemia (RR 0.73, 95% CI 0.53 to 1.02, P = 0.06; I2 = 45%) (Analysis 1.4).
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Two studies reported SVTs, with one event (2.3%) among 44 participants. Both studies evaluated dexmedetomidine. Since there was no events reported in one of the studies (Venn 2001), pooled estimates were not calculated. The remaining trial showed no effect of α‐2 adrenergic agonists on SVT (RR 1.11, 95% CI 0.05 to 24.07) (Analysis 1.5) (Talke 1995).
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure
Eight studies reported episodes of HF, with 107 events (1.0%) among 10,802 participants. There was no significant reduction in congestive heart failure (CHF) with perioperative α‐2 adrenergic agonist use (RR 1.21, 95% CI 0.83 to 1.75, P = 0.32), with negligible heterogeneity (I2 = 3%) (Analysis 1.6).
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute strokes, with 56 strokes (0.5%) among 11,542 participants. Alpha‐2 adrenergic agonists had no significant effect on acute stroke (RR 0.93, 95% CI 0.55 to 1.56 P = 0.79) with no measurable heterogeneity (I2 = 0%) (Analysis 1.7). The quality of evidence for effects on acute stroke was high (summary of findings Table for the main comparison).
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Sixteen studies reported bradycardia, with 1349 events (9.6%) in 14,035 participants. Within these 16 studies, α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.59, 95% CI 1.18 to 2.13, P = 0.002), albeit with substantial heterogeneity (I2 = 53%) (Analysis 1.8). The quality of evidence for treatment effects on bradycardia was moderate (summary of findings Table for the main comparison).
2. Hypotension requiring treatment with inotropes or vasopressors
Fifteen studies reported hypotension, with 4766 events (34.7%) in 13,738 participants. Alpha‐2 adrenergic agonists caused a significant increase in the risk of perioperative hypotension (RR 1.24, 95% CI 1.03 to 1.48, P = 0.02), albeit with substantial heterogeneity (I2 = 54%) (Analysis 1.9). Based on a post‐hoc subgroup analysis, the choice of drug may explain this heterogeneity (Analysis 4.4). Specifically, there was statistically significant evidence of subgroup effects based on whether the studies evaluated clonidine, dexmedetomidine or mivazerol (test of interaction P < 0.001). Clonidine significantly increased the risk of hypotension (RR 1.29, 95% CI 1.23 to 1.35, P < 0.001). Dexmedetomidine was also associated with an increased risk (RR 1.81, 95% CI 1.07 to 3.06, P = 0.03). Conversely, mivazerol did not increase the risk of hypotension (RR 0.95, 95% CI 0.82 to 1.10, P = 0.48). Clonidine and mivazerol subgroup analyses had no measurable heterogeneity (I2 = 0%), whereas the dexmedetomidine subgroup analysis demonstrated significant heterogeneity (I2 = 50%). The quality of evidence for treatment effects on hypotension was moderate (summary of findings Table for the main comparison).
Cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 29 events (1.5%) among 1949 participants. Alpha‐2 adrenergic agonists did not result in a statistically significant reduction in all‐cause mortality (RR 0.52, 95% CI 0.26 to 1.04, P = 0.06), without any measurable heterogeneity (I2 = 0%) (Analysis 2.1). The quality of this evidence was moderate (summary of findings Table 2).
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Only one study reported cardiac mortality, with 1 event among the 12 participants in the clonidine arm and no events among the 10 participants in the control arm (Loick 1999). Thus, no pooled analysis was performed.
2. Myocardial infarction within 30 days after surgery: definition as per individual study
Eight studies reported MIs, with 16 events (2.0%) among 782 participants. Alpha‐2 adrenergic agonists were not associated with reduced risk of MI (RR 1.01, 95% CI 0.43 to 2.40, P = 0.98) in an analysis with no heterogeneity (I2 = 0%) (Analysis 2.2). The quality of evidence was moderate (summary of findings Table 2).
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Thirteen studies reported myocardial ischaemia, with 243 events (21.4%) among 1134 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of ischaemia (RR 0.69, 95% CI 0.56 to 0.86, P < 0.001) with no heterogeneity (I2 = 0%) (Analysis 2.3).
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Six studies reported SVTs, with 79 events (7.7%) among 1044 participants. Alpha‐2 adrenergic agonists had no significant effect on the risk of SVT (RR 0.77, 95% CI 0.50 to 1.16, P = 0.21) with low measurable heterogeneity (I2 = 24%) (Analysis 2.4).
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure or need for postoperative intra‐aortic balloon pump support
Four studies reported 38 HF events (6.9%) among 549 participants. Alpha‐2 adrenergic agonists had no statistically significant effect on the risk of HF (RR 0.90, 95% CI 0.49 to 1.63, P = 0.72) with no measurable heterogeneity (I2 = 0%) (Analysis 2.5).
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute stroke, with 18 events (1.5%) among 1175 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of acute stroke (RR 0.37, 95% CI 0.15 to 0.93, P = 0.03; I2 = 0%) (Analysis 2.6). The quality of evidence was low (summary of findings Table 2).
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Ten studies reported episodes of bradycardia, with 136 events (9.2%) among 1477 participants. Pooled analysis demonstrated that α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.88, 95% CI 1.35 to 2.62, P = 0.0002) with no heterogeneity (I2 = 0%) (Analysis 2.7). The quality of evidence was moderate (summary of findings Table 2).
2. Hypotension requiring treatment with inotropes or vasopressors
Nine studies reported 494 episodes of hypotension (35%) among 1413 participants. Alpha‐2 adrenergic agonists did not significantly increase the risk of hypotension (RR 1.19, 95% CI 0.87 to 1.64, P = 0.28) in an analysis with substantial heterogeneity (I2 = 72%) (Analysis 2.8). The quality of evidence was low (summary of findings Table 2).
Subgroup analyses
Vascular versus non‐vascular non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on procedure type (i.e. vascular versus non‐vascular procedures) with respect to the outcomes of all‐cause mortality (test of interaction P = 0.17; Analysis 3.1), cardiac mortality (test of interaction P = 0.13; Analysis 3.2), MI (test of interaction P = 0.13; Analysis 3.3), and myocardial ischaemia (test of interaction P = 0.17; Analysis 3.4).
Drug (i.e. clonidine, mivazerol or dexmedetomidine) evaluated in non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on the specific α‐2 adrenergic agonist evaluated with respect to the outcomes of all‐cause mortality (test of interaction P = 0.50) (Analysis 4.1), and MI (test of interaction P = 0.48) (Analysis 4.3). Conversely, there was a statistically significant subgroup effect with respect to cardiac mortality (test of interaction P = 0.05) (Analysis 4.2). In these subgroup analyses, mivazerol significantly reduced cardiac mortality (RR 0.51, 95% CI 0.27 to 0.98, P = 0.04), whereas clonidine did not (RR 1.12, 95% CI 0.71 to 1.75, P = 0.63). There were insufficient studies that reported myocardial ischaemia as an outcome for dexmedetomidine or mivazerol to facilitate drug‐specific subgroup analysis for the outcome.
Sensitivity analyses
Studies that clearly reported blinding and concealed allocation
The pooled effects of α‐2 adrenergic agonists on all‐cause mortality (RR 0.68, 95% CI 0.41 to 1.11, P = 0.12; participants = 13,066; studies = 7; Analysis 5.1), MI (RR 1.08, 95% CI 0.95 to 1.23, P = 0.26; participants = 13,026; studies = 6; Analysis 5.2), and myocardial ischaemia (RR 0.77, 95% CI 0.40 to 1.48, P = 0.43; participants = 412; studies = 3; Analysis 5.3) were qualitatively similar when analyses were restricted to trials that clearly reported methods for blinding and allocation concealment.
Strict definitions of myocardial infarction and ischaemia
When analyses were restricted to trials that strictly defined MI on ECG or enzymatic criteria, pooled treatment effects in non‐cardiac surgery (RR 0.98, 95% CI 0.70 to 1.36, P = 0.90; participants = 13,003; studies = 8) and cardiac surgery (RR 0.76, 95% CI 0.19 to 2.98, P = 0.69; participants = 275; studies = 3) were qualitatively unchanged (Analysis 6.1). When analyses were restricted to studies that strictly defined events of myocardial ischaemia, the effects in non‐cardiac surgery remained non‐significant (RR 0.76, 95% CI 0.54 to 1.07, P = 0.12; participants = 1175; studies = 9). In cardiac surgery, the sensitivity analysis continued to demonstrate a reduction in the risk of ischaemia (RR 0.71, 95% CI 0.55 to 0.91, P = 0.007; participants = 820; studies = 8) (Analysis 6.2).
Influence of two large trials
The overall results of this review are likely highly influenced by two large RCTs in non‐cardiac surgery, one of which assessed mivazerol (Oliver 1999), while the other assessed clonidine (Devereaux 2014a). Therefore, we performed a post‐hoc sensitivity analysis that excluded these studies. After excluding these two trials, treatment effect on all‐cause mortality became statistically significant (RR 0.45, 95% CI 0.22 to 0.93, P = 0.03; participants = 2174; studies = 14; Analysis 7.1). Conversely, the effect on cardiac mortality (RR 0.47, 95% CI 0.10 to 2.25, P = 0.35; participants = 618; studies = 3; Analysis 7.2), and MI (RR 0.56, 95% CI 0.25 to 1.25, P = 0.16; participants = 2000; studies = 10; Analysis 7.3) were statistically non‐significant, albeit with more optimistic individual point estimates (i.e. pooled treatment effects shifted towards larger risk reductions).
Excluding drugs not introduced into clinical practice (i.e. mivazerol)
In post‐hoc sensitivity analyses excluding the two trials that evaluated mivazerol (McSPI‐Europe 1997; Oliver 1999), there was no change in pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, SVT, HF, stroke, bradycardia, or hypotension (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 8.9). Conversely, the pooled treatment effect on ischaemia became statistically significant (RR 0.68, 95% CI 0.48 to 0.97, P = 0.03; participants = 1079; studies = 11; I2 = 40%) (Analysis 8.4), albeit in an analysis with moderate heterogeneity and relatively few participants.
Restricting studies more representative of contemporary perioperative practice
When analyses pertaining to non‐cardiac surgery were restricted to studies that collected data within the previous 20 years, there was no change in the pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, HF or stroke (Analysis 9.1; Analysis 9.2; Analysis 9.3; Analysis 9.5; Analysis 9.6). Nonetheless, exclusion of older studies resulted in a significant reduction in the risk of myocardial ischaemia (RR 0.51, 95% CI 0.28 to 0.93, P = 0.03; participants = 634; studies = 6; I2 = 48%) in an analysis with moderate heterogeneity (Analysis 9.4). In cardiac surgery, exclusion of older studies resulted in no substantive effect on the pooled treatment effects for MI, myocardial ischaemia, SVT, HF or stroke (Analysis 10.2; Analysis 10.3; Analysis 10.4; Analysis 10.5; Analysis 10.6). Conversely, the pooled treatment effect on all‐cause mortality became statistically significant (RR 0.47, 95% CI 0.23 to 0.97; participants = 1782; studies = 13; I2 = 0%) (Analysis 10.1).
Funnel plots
Funnel plots of included studies revealed no obvious publication bias with regard to the outcome of mortality (Figure 4), but some possible bias with regard to MI (Figure 5). Since this analysis pooled results from only nine studies, formal statistical testing for asymmetry was not conducted.
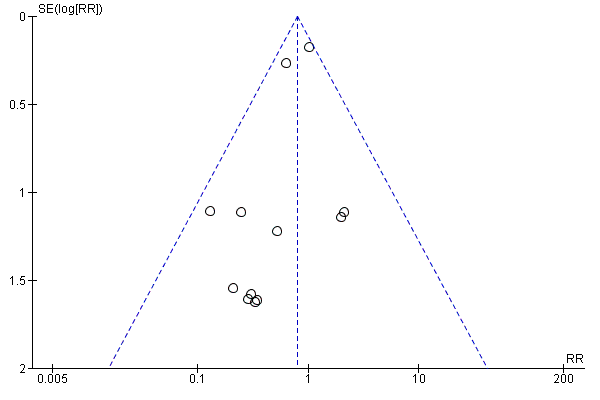
Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.1 All‐cause mortality.
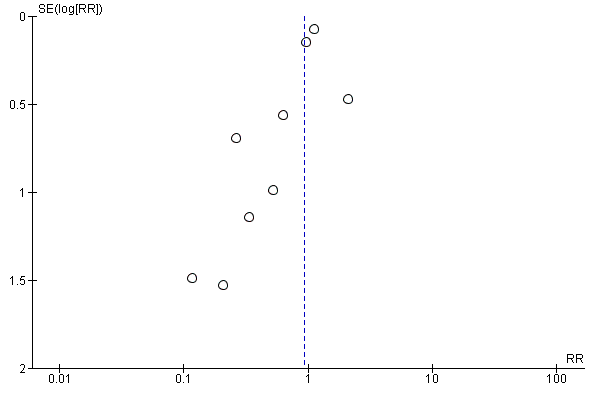
Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.3 Myocardial infarction.
Discusión
Resumen de los resultados principales
La presente revisión encontró evidencia de alta calidad de que los agonistas adrenérgicos α2 perioperatorios no redujeron el riesgo de mortalidad por todas las causas, mortalidad cardíaca o IM en los pacientes sometidos a cirugía cardíaca o no cardíaca (Tabla 1 Resumen de los hallazgos; Tabla 2 Resumen de los hallazgos). Estos resultados permanecieron estables en los análisis de sensibilidad limitados a los estudios que demostraron bajo riesgo de sesgo o utilizaron definiciones estrictas de IM. Además de la falta de cualquier efecto beneficioso sobre estos resultados clínicos, los agonistas adrenérgicos α2 también se asociaron con riesgos importantes, específicamente mayores tasas de hipotensión y bradicardia. Aunque estos efectos hemodinámicos no se asociaron con un aumento en el riesgo de accidente cerebrovascular agudo, los IC del 95% para este efecto agrupado fueron amplios (CR 0,93; IC del 95%: 0,55 a 1,56), por lo que no se excluye la posibilidad de un aumento moderado del riesgo de accidente cerebrovascular con los agonistas adrenérgicos α2 perioperatorios.
Compleción y aplicabilidad general de las pruebas
Los 47 ECA incluidos en esta revisión sistemática incorporaron 17 039 participantes, una amplia variedad de procedimientos quirúrgicos relevantes realizados en varios países diferentes y regímenes de dosis clínicamente relevantes de agonistas adrenérgicos α2 disponibles en la actualidad (es decir, clonidina, dexmedetomidina). Además, un número significativo de participantes en los estudios incluidos se sometió a cirugía en la última década. Por lo tanto, existe seguridad con respecto a que los resultados generales de la presente revisión sistemática, a saber, que los agonistas adrenérgicos α2 no reducen de manera significativa los riesgos de complicaciones cardiovasculares o mortalidad cuando se administran como profilaxis antes de una cirugía mayor no cardíaca o cardíaca, se pueden extrapolar razonablemente a la práctica perioperatoria contemporánea.
No obstante, el número de participantes dentro de subgrupos específicos no fue suficiente para evaluar de manera concluyente varios posibles efectos beneficiosos de los agonistas adrenérgicos α2, a saber, la prevención del accidente cerebrovascular, la isquemia miocárdica y la mortalidad por todas las causas después de la cirugía cardíaca. El análisis de subgrupos que evaluó los efectos sobre el accidente cerebrovascular durante la cirugía cardíaca fue pequeño; solo se incluyeron siete estudios que incorporaron 1175 participantes (Análisis 2.6). La estimación agrupada se basó en datos de baja calidad calculados en base a escasos eventos de resultado (es decir, 18 accidentes cerebrovasculares). Estudios de investigación anteriores han encontrado que en general los efectos del tratamiento se sobrestiman en los metanálisis que incluyen relativamente pocos eventos de resultado (Thorlund 2011). Consistente con esta posibilidad, la magnitud de la estimación agrupada fue de cierta manera poco verosímil (CR 0,37; IC del 95%: 0,15 a 0,93), pues indicó una reducción relativa del 63% en el riesgo de accidente cerebrovascular a partir de una única intervención perioperatoria. Por lo tanto, se necesitan estudios de investigación adicionales para determinar si los agonistas adrenérgicos α2 pueden reducir verdaderamente el riesgo de accidente cerebrovascular agudo después de la cirugía cardíaca.
De manera similar, un efecto agrupado estadísticamente significativo del tratamiento sobre la mortalidad por todas las causas solo se observó en un análisis de subgrupos post hoc limitado a los ensayos de cirugía cardíaca realizados después de 1997 (Análisis 10.1). Este subgrupo fue relativamente pequeño (13 estudios que incluyeron 1782 participantes), y la estimación agrupada se calculó en base a escasos eventos de resultado (es decir, 28 muertes) y la magnitud de la estimación agrupada fue de cierta manera poco verosímil (CR 0,47; IC del 95%: 0,23 a 0,97) para una intervención única. Se necesitan más estudios para evaluar el efecto de los agonistas adrenérgicos α2 sobre la mortalidad por todas las causas después de la cirugía cardíaca.
En otro análisis de subgrupos en la cirugía cardíaca, los agonistas adrenérgicos α2 también causaron una reducción significativa de la isquemia miocárdica perioperatoria (Análisis 2.3). No obstante, la isquemia miocárdica es un resultado indirecto, con limitaciones asociadas importantes (Svensson 2013). En especial, a falta de reducciones asociadas en resultados clínicamente importantes y relevantes para los pacientes, como la mortalidad o el IM, las reducciones aisladas de la isquemia miocárdica perioperatoria no son una justificación suficiente para utilizar los agonistas adrenérgicos α2 en la práctica clínica.
Calidad de la evidencia
Esta revisión sistemática fue respaldada por 47 ECA que incirporaron a 17 039 participantes. El tamaño de la muestra de los ECA incluidos varió ampliamente, de 20 a más de 10 000 participantes. Diecinueve estudios incluyeron más de 100 participantes, y solamente dos estudios incluyeron más de 1000 participantes (Devereaux 2014a; Oliver 1999). La gran mayoría de estos participantes (14 367) fueron incorporados a los 23 ensayos incluidos de cirugía no cardíaca. En comparación, en los 24 ECA restantes de cirugía cardíaca participaron 2672 participantes.
Sólo 16 estudios informaron métodos adecuados de generación de la secuencia aleatoria y la ocultación de la asignación. Además, aunque 31 estudios se describieron como "doble ciego", solo 21 estudios informaron métodos apropiados para lograr el cegamiento. No obstante, la mayoría de los participantes se incluyeron en estudios bien diseñados con métodos adecuados para la asignación y el cegamiento, por lo que tuvieron bajo riesgo de estar afectados por el sesgo de selección, el sesgo de realización y el sesgo de detección.
Los análisis relevantes para los resultados primarios y secundarios en los pacientes sometidos a cirugía no cardíaca en general fueron consistentes. Estos resultados se consideraron de calidad moderada a alta según la metodología GRADE (Tabla 1 Resumen de los hallazgos). Aunque muchos estudios no lograron informar métodos adecuados para evitar el riesgo de sesgo, fue poco probable que los resultados específicos se hayan afectado, por lo que no fue necesario disminuir la calidad. Además, hubo una posible amenaza de falta de direccionalidad porque el segundo ECA más grande evaluó el mivazerol (Oliver 1999), que no está disponible para uso clínico. Sin embargo, debido a la semejanza del mivazerol con la dexmedetomidina (que está disponible para uso clínico), se consideró que era probable que este riesgo no fuera grave. Por otra parte, los gráficos en embudo indicaron que los efectos agrupados del tratamiento sobre el IM fueron afectados por el sesgo de publicación (Figura 5). La asimetría en los gráficos en embudo se produjo por dos estudios pequeños con tamaños del efecto al parecer poco realistas (Ellis 1994; Stuhmeier 1996). Aunque la ponderación combinada de estos estudios fue menor del 3% en el análisis agrupado, la calidad de la evidencia se disminuyó en un nivel debido a la sospecha de sesgo de publicación para un resultado que se sabe que está afectado por el sesgo de realización (Análisis 1.3). Finalmente, la calidad de la evidencia para los efectos fisiológicos bradicardia e hipotensión se disminuyó debido a la heterogeneidad significativa (I2 mayor del 50%) en los análisis (Análisis 1.8; Análisis 1.9).
La calidad de la evidencia para los efectos de los agonistas adrenérgicos α2 en la cirugía cardíaca fue en general menor, debido en gran parte a la imprecisión, que se debió a que hubo significativamente menos participantes en los análisis agrupados (Tabla 2 Resumen de los hallazgos). La calidad de la evidencia para todos los resultados se disminuyó porque no se logró el tamaño de información óptimo de 2000 participantes, y los IC del 95% de las estimaciones agrupadas no descartaron efectos clínicamente significativos (GRADE Handbook 2013). Por lo tanto, la calidad de la evidencia de los análisis relevantes para la mortalidad por todas las causas y el IM fue moderada. Debido a que hubo muy pocos eventos de resultado en el análisis del accidente cerebrovascular agudo (es decir, 18 accidentes cerebrovasculares), la calidad se disminuyó otro nivel y se consideró baja. Finalmente, la presencia de imprecisión significativa en el análisis agrupado relevante para la hipotensión dio lugar a que la calidad de la evidencia se disminuyera a baja (I2 = 72%; Análisis 2.8). No obstante, la magnitud de la asociación entre los agonistas adrenérgicos α2 y la hipotensión en la cirugía cardíaca (CR 1,19; IC del 95%: 0,87 a 1,64) fue cualitativamente muy similar a la observada en la cirugía no cardíaca (CR 1,24; IC del 95%: 1,03 a 1,48), en la que la calidad de la evidencia fue moderada.
Sesgos potenciales en el proceso de revisión
Hubo varias limitaciones en el proceso de revisión. Primero, aunque la búsqueda fue exhaustiva e incluyó todos los índices médicos y los registros de ensayos clínicos principales, es probable que se hayan pasado por alto algunos ensayos publicados enumerados solamente en otros índices utilizados con menos frecuencia. No obstante, se considera que es poco probable que la estrategia de búsqueda haya pasado por alto algún estudio relevante de tamaño y calidad al menos moderados. Segundo, solamente se incluyeron los estudios que informaron datos de resultado específicos de subgrupos según el tipo de procedimiento quirúrgico (es decir, cirugía cardíaca versus no cardíaca). Por lo tanto, se excluyó cualquier estudio que no incluyera predominantemente procedimientos (más del 75%) de cualquiera de estos subgrupos de procedimientos quirúrgicos, a menos que fuera posible obtener de los autores los datos de los resultados específicos de subgrupos. En consecuencia, dos estudios por otra parte elegibles no se pudieron incluir en esta revisión sistemática (Martin 2003; Triltsch 2002). Con la exclusión de estos estudios, se equilibró el riesgo de sesgar los análisis con la pérdida de datos adicionales, y se eligió esto último para mantener la integridad de los análisis.
Acuerdos y desacuerdos con otros estudios o revisiones
Hay varios posibles motivos por los que los efectos fisiológicos teóricamente beneficiosos de los agonistas adrenérgicos α2 no se tradujeron en una reducción de las tasas de complicaciones cardíacas posoperatorias graves. Primero, los agonistas adrenérgicos α2 quizás no hayan reducido de manera suficiente la frecuencia cardíaca para mitigar los riesgos de IM perioperatorio, como se ha propuesto previamente (Devereaux 2014a). Esta hipótesis está apoyada por la observación de que, aunque los bloqueadores β perioperatorios causaron bradicardia más significativa que los agonistas adrenérgicos α2, las tasas de IM perioperatorio se redujeron con los bloqueadores β pero no con los agonistas adrenérgicos α2 (Wijeysundera 2014b). Segundo, el mecanismo predominante subyacente del IM perioperatorio en muchos pacientes afectados podría no ser el aumento de la presión arterial y la frecuencia cardíaca inducida por la respuesta al estrés quirúrgico. Por ejemplo, alrededor del 30% de los pacientes con IM posoperatorio no presentan coronariopatía obstructiva significativa (Sheth 2015). Es poco probable que la limitación del aumento perioperatorio de la frecuencia cardíaca ayude a prevenir el IM en estos pacientes. Tercero, a un nivel poblacional, los efectos beneficiosos de la reducción de la frecuencia cardíaca en algunos pacientes sometidos a cirugía se podrían haber compensado con el número igual de pacientes que presentaron efectos nocivos de la hipotensión perioperatoria significativa.
En particular, los resultados generales de esta revisión en cuanto a la falta de grandes reducciones en el riesgo cardiovascular perioperatorio con los agonistas adrenérgicos α2 fue de cierta manera consistente con los resultados observados con el tratamiento profiláctico con otros agentes simpaticolíticos. Específicamente, se ha mostrado que los bloqueadores adrenérgicos β causan un efecto perjudicial neto en la cirugía no cardíaca (Wijeysundera 2014b). Aún está por verse si cualquier estrategia de disminuir de manera profiláctica las anomalías hemodinámicas con los agentes simpaticolíticos puede reducir con seguridad el riesgo cardíaco perioperatorio. De hecho, si los ECA futuros con regímenes alternativos de agentes simpaticolíticos evaluados previamente (es decir, agonistas adrenérgicos α2, bloqueadores adrenérgicos β) o agentes alternativos cronotrópicos negativos (p.ej. ivabradina) no logran mostrar un efecto beneficioso general neto, es posible que la estrategia general para la reducción del riesgo cardíaco perioperatorio deba desplazarse del tratamiento profiláctico al tratamiento temprano. Específicamente, en contraposición con la administración de estos fármacos a un grupo amplio de pacientes antes de la cirugía, los médicos podrían considerar la posibilidad de dirigir el tratamiento a los pacientes con alto riesgo identificados según los cambios isquémicos en el ECG o las concentraciones de troponina cardíaca elevadas poco después de la cirugía.
La comparación de la presente revisión sistemática sobre los agonistas adrenérgicos α2 perioperatorios con una revisión sistemática previa de los bloqueadores adrenérgicos β perioperatorios en la cirugía no cardíaca también arroja alguna luz sobre los mecanismos subyacentes del accidente cerebrovascular perioperatorio (Wijeysundera 2014b). Específicamente, los agonistas adrenérgicos α2 (CR 1,24; IC del 95%: 1,03 a 1,48) y los bloqueadores adrenérgicos β (CR 1,47; IC del 95%: 1,34 a 1,60) se asociaron con riesgos aproximadamente similares de hipotensión perioperatoria. A pesar de este semejanza en los efectos hemodinámicos, los bloqueadores β (CR 1,86; IC del 95%: 1,09 a 3,16) se asociaron con un aumento significativo en el riesgo de accidente cerebrovascular agudo perioperatorio, lo que no ocurrió con los agonistas adrenérgicos α2 (CR 0,93; IC del 95%: 0,55 a 1,56). Estos efectos contradictorios también son evidentes al comparar dos ECA perioperatorios individuales grandes de estas dos clases diferentes de fármacos, a saber, el ensayo POISE‐1 de metoprolol (POISE 2008) y el ensayo POISE‐2 de clonidina (Devereaux 2014a). Específicamente, mientras el metoprolol (cociente de riesgos instantáneos [CRI] 1,55; IC del 95%: 1,38 a 1,74) y la clonidina (CRI 1,32; IC del 95%: 1,24 a 1,40) causaron aumentos cualitativamente similares en las tasas de hipotensión, el metoprolol aumentó de manera significativa el riesgo de accidente cerebrovascular (CRI 2,17; IC del 95%: 1,26 a 3,74), lo que no ocurrió con la clonidina (CRI 1,06; IC del 95%: 0,54 a 2,05). Estos resultados indican que, a pesar de la asociación previamente observada entre la hipotensión perioperatoria y el accidente cerebrovascular (POISE 2008), es probable que los mecanismos subyacentes del accidente cerebrovascular agudo perioperatorio sean más complejos que sólo una disminución en la presión de perfusión. Se necesitan estudios de investigación adicionales para delinear mejor estos mecanismos y de ese modo informar el desarrollo de estrategias a para ayudar a prevenir esta complicación perioperatoria, a menudo devastadora (POISE 2008).
Es importante señalar que los agonistas adrenérgicos α2 perioperatorios tienen otros posibles efectos beneficiosos que pueden justificar su uso selectivo en algunos pacientes sometidos a cirugía. Específicamente, en una revisión sistemática anterior de 30 ECA pequeños que incluyeron 1792 participantes, estos agentes redujeron la intensidad del dolor posoperatorio y el consumo de morfina (Blaudszun 2012). El presente estudio proporcionó datos adicionales que apoyan la seguridad de la administración de los agonistas adrenérgicos α2 como tratamiento complementario para el control del dolor agudo posoperatorio, siempre que exista una atención adecuada a los riesgos asociados de hipotensión y bradicardia.
Fortalezas
La revisión actual tuvo varias fortalezas. La búsqueda bibliográfica fue extensa y abarcó todos los idiomas. Además, este estudio utilizó un grupo de datos significativamente mayor que las revisiones anteriores (Biccard 2008; Nishina 2002; Stevens 2003; Wijeysundera 2003; Wijeysundera 2009). Finalmente, se realizaron múltiples análisis de sensibilidad para evaluar la influencia de la calidad de los estudios, la definición de los resultados y el sesgo de publicación, en las conclusiones generales.
Limitaciones
La presente revisión tuvo varias limitaciones que se deben considerar. Primero, los resultados estuvieron influenciados en gran medida por dos ensayos grandes de mivazerol (Oliver 1999) y clonidina (Devereaux 2014a) en la cirugía no cardíaca. Aunque la exclusión de estos ensayos grandes del metanálisis dio lugar a estimaciones puntuales algo más optimistas de los efectos individuales agrupados del tratamiento, estas estimaciones agrupadas permanecieron no significativas desde el punto de vista estadístico. En segundo lugar, igual que en cualquier otra revisión sistemática, estos resultados pueden haber estado afectados por el sesgo de publicación. Aunque los gráficos en embudo no indicaron un sesgo obvio en el informe de la muerte (por todas las causas y de causa cardíaca), sí indicaron que podía estar presente el sesgo de informe para el IM. Tercero, el presente análisis agrupó ensayos de agonistas adrenérgicos α2 con diferente selectividad para sus receptores objetivo, a saber, los receptores adrenérgicos α2 y los receptores no adrenérgicos imidazolina (Khan 1999). Específicamente, el mivazerol y la dexmedetomidina tienen una selectividad considerablemente mayor para estos receptores objetivo que la clonidina. Además, al comparar los dos ensayos individuales grandes que dominan este metanálisis (Devereaux 2014a; Oliver 1999), la clonidina no tuvo efecto sobre la mortalidad y aumentó las tasas de hipotensión perioperatoria significativa, mientras que el mivazerol, un agonista adrenérgico α2 más selectivo que actualmente no está disponible para uso clínico, se asoció a tendencias hacia una reducción de la mortalidad y no tuvo efecto sobre las tasas de hipotensión. Es importante señalar que estos resultados contradictorios quizás se hayan debido también a los diferentes períodos en los que se realizaron los estudios, las diferencias en el diseño de los estudios, las diferencias en las características de los participantes o al azar. No obstante, los hallazgos contradictorios todavía indican que cualquier ECA futuro de agonistas adrenérgicos α2 para la reducción del riesgo cardíaco de los pacientes sometidos a cirugía se debe centrar en los agentes con selectividad mayor para los receptores adrenérgicos α2 como la dexmedetomidina o el mivazerol.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.1 All‐cause mortality.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.3 Myocardial infarction.
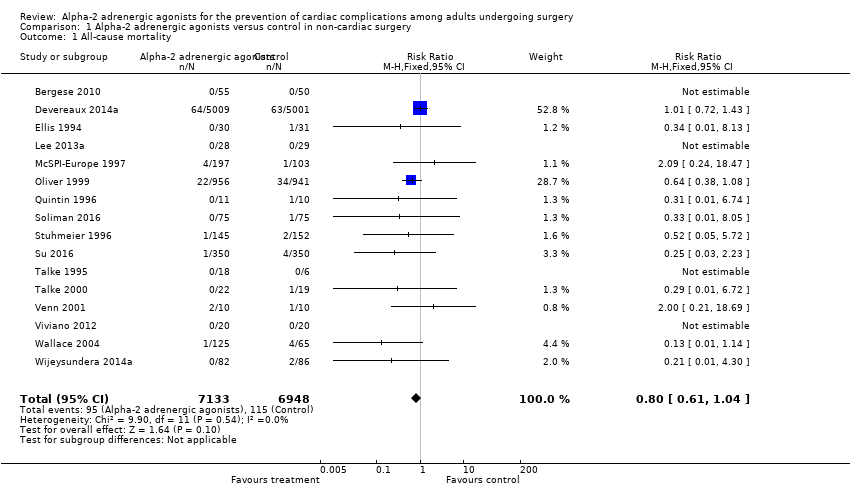
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.
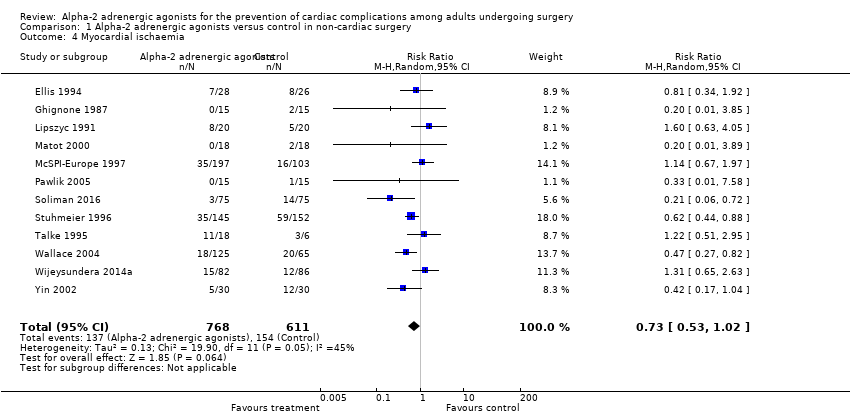
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 6 Heart failure.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 7 Acute stroke.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 8 Bradycardia.

Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 9 Hypotension.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 2 Myocardial infarction.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 3 Myocardial ischaemia.
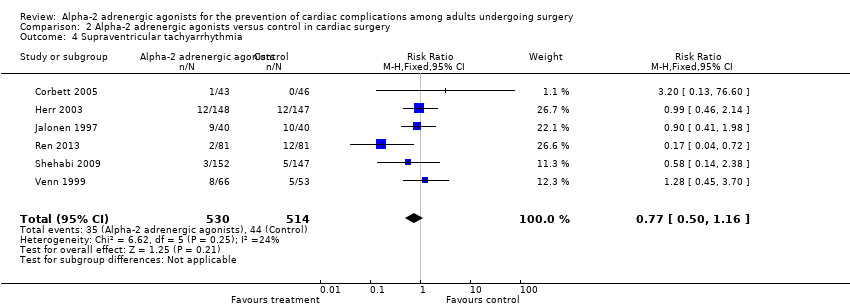
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 4 Supraventricular tachyarrhythmia.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 5 Heart failure.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 6 Acute stroke.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 7 Bradycardia.

Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 8 Hypotension.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 1 All‐cause mortality.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 2 Cardiac mortality.
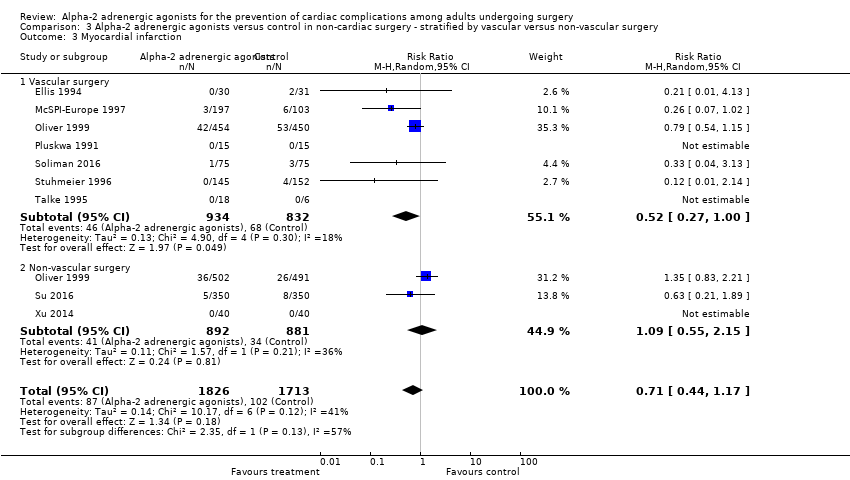
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 3 Myocardial infarction.

Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 4 Myocardial ischaemia.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.

Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 4 Hypotension.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 1 All‐cause mortality.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 2 Myocardial infarction.

Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 3 Myocardial ischaemia.

Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 1 Myocardial infarction.

Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 2 Myocardial ischaemia.

Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 1 All‐cause mortality.

Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 2 Cardiac mortality.

Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 3 Myocardial infarction.
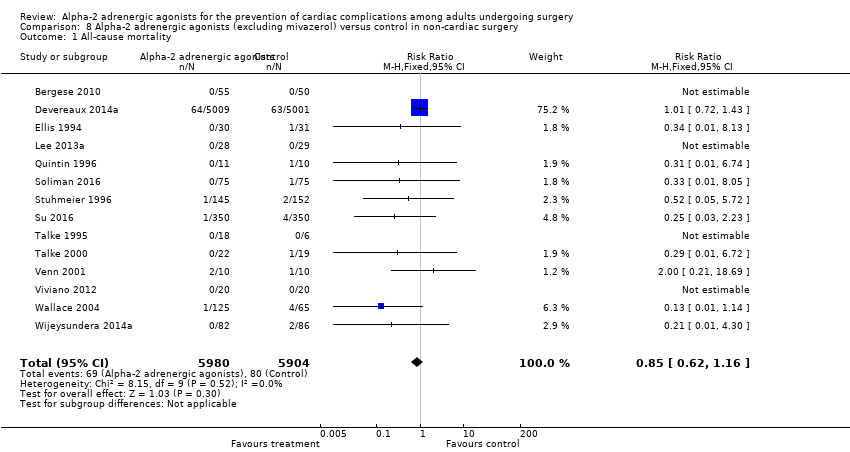
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 6 Heart failure.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 7 Acute stroke.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 8 Bradycardia.

Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 9 Hypotension.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 2 Cardiac mortality.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 3 Myocardial infarction.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 4 Myocardial ischaemia.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 5 Heart failure.

Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 6 Acute stroke.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 2 Myocardial infarction.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 3 Myocardial ischaemia.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 4 Supraventricular tachyarrhythmia.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 5 Heart failure.

Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 6 Acute stroke.
| Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery | ||||||
| Patient or population: adults undergoing non‐cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.80 | 14,081 | ⊕⊕⊕⊕ | ‐ | |
| 17 per 1000 | 13 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause.) | Study population | RR 0.86 | 12,525 | ⊕⊕⊕⊕ | ‐ | |
| 10 per 1000 | 8 per 1000 | |||||
| Myocardial infarction (within 30‐days after surgery: as detected on an electrocardiogram or trans‐oesophageal echocardiogram) | Study population | RR 0.94 | 13,907 | ⊕⊕⊕⊝ | ‐ | |
| 59 per 1000 | 55 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.93 | 11,542 | ⊕⊕⊕⊕ | ‐ | |
| 5 per 1000 | 4 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.59 | 14,035 | ⊕⊕⊕⊝ | ‐ | |
| 75 per 1000 | 119 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.24 | 13,738 | ⊕⊕⊕⊝ | ‐ | |
| 304 per 1000 | 377 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lacked proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Indirectness not serious. Intervention (mivazerol) used in one large study not available for clinical use. Not downgraded. 3Evidence of publication bias in funnel plot of analysis. Downgraded by one level. 4Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded by one level. | ||||||
| Alpha‐2 adrenergic agonists compared to control in cardiac surgery | ||||||
| Patient or population: adults undergoing cardiac surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
| All‐cause mortality (within 30‐days after surgery: any reported death) | Study population | RR 0.52 | 1947 | ⊕⊕⊕⊝ | ‐ | |
| 21 per 1000 | 11 per 1000 | |||||
| Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | 1 death from 12 participants in clonidine arm, and no deaths in 10 participants in control arm. | Not estimable | 22 (1 RCT) | Not estimable | We did not GRADE evidence for this outcome as accurate estimation of RRs is not possible for such low event rates. | |
| Myocardial infarction (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) | Study population | RR 1.01 | 782 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 1000 | 21 per 1000 | |||||
| Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) | Study population | RR 0.37 | 1175 | ⊕⊕⊝⊝ | Total of 18 acute stokes reported, with 14 in control group and 4 in treatment group. | |
| 24 per 1000 | 9 per 1000 | |||||
| Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) | Study population | RR 1.88 | 1477 | ⊕⊕⊕⊝ | ‐ | |
| 64 per 1000 | 120 per 1000 | |||||
| Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) | Study population | RR 1.19 | 1413 | ⊕⊕⊝⊝ | ‐ | |
| 332 per 1000 | 395 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias was not serious. Although multiple studies lack proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded. 2Serious imprecision, because analysis was below optimal information size and confidence interval includes significant benefit and harm. Downgraded by one level. 3Very serious imprecision, because analysis is below optimal information size and number of events was very small. Downgraded by two levels. 4Serious imprecision, because analysis was below optimal information size. Downgraded by one level 5Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 2 Cardiac mortality Show forest plot | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 3 Myocardial infarction Show forest plot | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Myocardial ischaemia Show forest plot | 12 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.53, 1.02] |
| 5 Supraventricular tachyarrhythmia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure Show forest plot | 8 | 10802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.75] |
| 7 Acute stroke Show forest plot | 7 | 11542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.56] |
| 8 Bradycardia Show forest plot | 16 | 14035 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.18, 2.13] |
| 9 Hypotension Show forest plot | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 2 Myocardial infarction Show forest plot | 8 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.43, 2.40] |
| 3 Myocardial ischaemia Show forest plot | 13 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.86] |
| 4 Supraventricular tachyarrhythmia Show forest plot | 6 | 1044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.16] |
| 5 Heart failure Show forest plot | 4 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 6 Acute stroke Show forest plot | 7 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.93] |
| 7 Bradycardia Show forest plot | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.35, 2.62] |
| 8 Hypotension Show forest plot | 9 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 13 | 3713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 1.1 Vascular surgery | 8 | 1798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.88] |
| 1.2 Non‐vascular surgery | 6 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.67] |
| 2 Cardiac mortality Show forest plot | 4 | 2515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.93] |
| 2.1 Vascular surgery | 4 | 1522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.79] |
| 2.2 Non‐vascular surgery | 1 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.35, 2.77] |
| 3 Myocardial infarction Show forest plot | 9 | 3539 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.17] |
| 3.1 Vascular surgery | 7 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 1.00] |
| 3.2 Non‐vascular surgery | 3 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.55, 2.15] |
| 4 Myocardial ischaemia Show forest plot | 9 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.17] |
| 4.1 Vascular surgery | 6 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.54, 1.29] |
| 4.2 Non‐vascular surgery | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 1.1 Clonidine | 7 | 10787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 1.2 Dexmedetomidine | 7 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.58] |
| 1.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.15] |
| 2 Cardiac mortality Show forest plot | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 2.1 Clonidine | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 2.2 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.98] |
| 3 Myocardial infarction Show forest plot | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.1 Clonidine | 6 | 10756 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.57, 1.92] |
| 3.2 Dexmedetomidine | 4 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.49] |
| 3.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.08] |
| 4 Hypotension Show forest plot | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
| 4.1 Clonidine | 7 | 10485 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.23, 1.35] |
| 4.2 Dexmedetomidine | 6 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.07, 3.06] |
| 4.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 7 | 13066 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.11] |
| 2 Myocardial infarction Show forest plot | 6 | 13026 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 3 Myocardial ischaemia Show forest plot | 3 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.40, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Myocardial infarction Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐cardiac surgery | 8 | 13003 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 1.2 Cardiac surgery | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.19, 2.98] |
| 2 Myocardial ischaemia Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐cardiac surgery | 9 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Cardiac surgery | 8 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 14 | 2174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 2 Cardiac mortality Show forest plot | 3 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.25] |
| 3 Myocardial infarction Show forest plot | 10 | 2000 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 14 | 11884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 2 Cardiac mortality Show forest plot | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 3 Myocardial infarction Show forest plot | 10 | 11710 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.59, 1.53] |
| 4 Myocardial ischaemia Show forest plot | 11 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.97] |
| 5 Supraventricular tachyarrhythmia Show forest plot | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure Show forest plot | 7 | 10502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 7 Acute stroke Show forest plot | 6 | 11242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 8 Bradycardia Show forest plot | 14 | 11838 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.17, 2.36] |
| 9 Hypotension Show forest plot | 13 | 11541 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 13378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.06] |
| 2 Cardiac mortality Show forest plot | 2 | 11907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 3 Myocardial infarction Show forest plot | 7 | 13195 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.24] |
| 4 Myocardial ischaemia Show forest plot | 6 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.93] |
| 5 Heart failure Show forest plot | 5 | 10424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.89, 2.03] |
| 6 Acute stroke Show forest plot | 5 | 11218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 13 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.97] |
| 2 Myocardial infarction Show forest plot | 4 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.03] |
| 3 Myocardial ischaemia Show forest plot | 7 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 4 Supraventricular tachyarrhythmia Show forest plot | 5 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.19] |
| 5 Heart failure Show forest plot | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.77] |
| 6 Acute stroke Show forest plot | 6 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.98] |

