Metadona para el dolor por cáncer
Resumen
Antecedentes
Esta es una actualización de una revisión publicada originalmente en 2004 y actualizada por primera vez en 2007. Esta versión incluye cambios significativos para adecuarla a los requisitos metodológicos actuales. La metadona es un opiáceo sintético que plantea algunos desafíos en el ajuste de la dosis y se reconoce que causa arritmias potencialmente mortales en algunos pacientes. Tiene un lugar en el tratamiento de los pacientes que no pueden tolerar otros opiáceos, pero solamente se debería iniciar por médicos experimentados. Esta revisión es una de una serie de revisiones sobre los opiáceos para el dolor por cáncer.
Objetivos
Evaluar la efectividad y la tolerabilidad de la metadona como un analgésico en pacientes adultos y niños con dolor por cáncer.
Métodos de búsqueda
Para esta actualización se hicieron búsquedas en CENTRAL, MEDLINE, Embase, CINAHL y clinicaltrials.gov, hasta mayo de 2016, sin restricciones de idioma. También se verificaron las listas de referencias de los artículos relevantes.
Criterios de selección
Se buscaron los ensayos controlados aleatorizados que compararan la metadona (cualquier formulación y por cualquier vía) con comparadores activos o placebo en pacientes con dolor por cáncer.
Obtención y análisis de los datos
Todos los autores estuvieron de acuerdo con respecto a los estudios seleccionados para inclusión. Cuando hubo dudas acerca de la elegibilidad se recuperó el texto completo. Un autor de la revisión extrajo los datos, que fueron verificados por un segundo autor de la revisión. No hubo suficientes datos comparables para el metanálisis. Se obtuvo información sobre el efecto de la metadona en la intensidad del dolor o el alivio del dolor, el número o la proporción de participantes con "dolor leve". Se extrajeron los datos sobre los retiros y otros eventos adversos. Se buscó información específicamente sobre los eventos adversos relacionados con el apetito, la sed y la somnolencia. La calidad de la evidencia se evaluó con los criterios GRADE y se creó una tabla de "Resumen de los hallazgos".
Resultados principales
Se revisaron las decisiones tomadas en la versión anterior de esta revisión y se excluyeron cinco estudios que se habían incluido previamente. La búsqueda actualizada identificó un nuevo estudio. Esta revisión incluye seis estudios con 388 participantes. No se identificaron estudios en niños.
Los estudios incluidos difirieron tanto en sus métodos y comparaciones que no fue posible realizar un resumen de los resultados. Sólo un estudio (103 participantes) informó específicamente el número de participantes con un nivel determinado de alivio del dolor, en este caso una reducción de al menos el 20%, similar en los grupos de metadona y morfina. Al utilizar un resultado de "dolor leve, como máximo", la metadona fue similar a la morfina en efectividad, y la mayoría de los participantes que pudieron tolerar la metadona lograron un "dolor leve, como máximo". Los retiros por eventos adversos con la metadona fueron poco frecuentes (12/202) y similares en otros grupos. Las muertes fueron poco frecuentes, excepto en un estudio en el que la mayoría de los participantes murieron, independientemente del grupo de tratamiento. En el caso de eventos adversos específicos, la somnolencia fue más frecuente con la metadona que con la morfina, mientras que la sequedad bucal fue más frecuente con la morfina que con la metadona. Ninguno de los estudios informó efectos adversos sobre el apetito.
Se consideró que la calidad de la evidencia fue baja a moderada debido al riesgo de sesgo y la escasez de datos. Para los eventos adversos específicos, se consideró que la calidad de la evidencia fue muy baja, disminuida debido al riesgo de sesgo, la escases de los datos y la poca precisión, ya que se utilizaron sustitutos para el apetito, la sed y la somnolencia.
No se dispone de datos sobre el uso de la metadona en niños.
Conclusiones de los autores
Sobre la base de evidencia de calidad baja, la metadona es un fármaco que tiene efectos analgésicos beneficiosos similares a los de la morfina y tiene una función en el tratamiento del dolor por cáncer en pacientes adultos. Otros opiáceos como la morfina y el fentanilo son más fáciles de administrar, pero pueden ser más caros que la metadona en muchas economías.
PICO
Resumen en términos sencillos
Metadona (un medicamento opiáceo) para tratar a los pacientes con dolor por cáncer
Conclusión
La metadona administrada por la boca produjo un buen alivio del dolor en la mayoría de los pacientes adultos con dolor por cáncer moderado o intenso.
Antecedentes
Un paciente de dos o tres con cáncer presentará un dolor de intensidad moderada o grave. El dolor tiende a empeorar a medida que evoluciona el cáncer. La metadona se ha utilizado durante muchos años como uno de los diferentes analgésicos para el dolor por cáncer.
Características de los estudios
En esta revisión actualizada, se intentó determinar en qué medida funciona la metadona, cuántos pacientes presentaron efectos secundarios y cuán graves fueron dichos efectos (por ejemplo, si fueron tan graves que los participantes dejaron de tomar la metadona).
En mayo de 2016, se encontraron sólo seis estudios con 388 participantes adultos. A menudo los estudios fueron pequeños y compararon muchas preparaciones diferentes.
Hallazgos clave
Para el alivio del dolor no pareció haber mucha diferencia entre la metadona y la morfina. En la mayoría de los pacientes, el dolor se redujo de moderado o intenso a leve o nulo con la metadona. La metadona se asocia con algunos efectos no deseados, principalmente somnolencia, estreñimiento y sequedad bucal. Estos efectos pueden ser lo suficientemente graves como para impedir que los pacientes tomen metadona. No se dispone de datos sobre el uso de la metadona en los niños.
Sería bueno que hubiera más consistencia en el diseño de los estudios, y especialmente, en su informe, que debería incluir información sobre los efectos no deseados y el resultado del dolor reducido a niveles tolerables (dolor leve, como máximo) para que los pacientes con cáncer no presenten molestias relacionadas con el dolor.
Calidad de la evidencia
La calidad de la evidencia de los estudios se calificó con el uso de cuatro niveles: muy baja, baja, moderada o alta. La evidencia de calidad muy baja significa que existe una menor seguridad en los resultados. La evidencia de calidad alta significa que existe mucha seguridad en cuanto a los resultados. La calidad de la evidencia fue baja o muy baja.
Authors' conclusions
Summary of findings
| Methadone compared to morphine for cancer pain | ||||||
| Patient or population: people with cancer pain | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect | No of participants | Quality of the evidence | Comments |
| Morphine | Methadone | |||||
| Participant‐reported pain intensity | Pain intensity scores: One study (103 participants) reported > 20% improvement in pain scores for 76% of morphine and 75% methadone participants in those that completed No worse than mild pain (pain score of 3/10 or less after treatment): One study (54 participants) reported all achieved no worse than mild pain based on mean pain scores. Two studies (148 participants) reported mean pain scores very close to a score of 3 | ⊕⊕⊝⊝ | Downgraded two points for reasons stated | |||
| Adverse events: appetite, thirst, somnolence | Not estimable | Not estimable | Not estimable | 342 | ⊕⊝⊝⊝ | Downgraded three points for reasons stated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1 Risk of bias: random allocation and allocation concealment unclear, all had sample size of less than 200 per treatment arm. | ||||||
Background
This is the second update of the review entitled 'Methadone for Cancer Pain' first published in 2004, and updated in 2007 (Nicholson 2007). This version includes substantial changes to bring it in line with current methodological requirements for Cochrane reviews. This review is one of a suite of reviews on opioids for cancer pain.
Description of the condition
Cancer‐related pain is a common problem. Worldwide prevalence data almost two decades ago suggested that there were 17 million people living with cancer (Payne 1998). Assessments of the prevalence of pain from nationwide studies in different countries have revealed consistent results, indicating that 30% to 40% of patients in active therapy experience pain, with this rate increasing to 70% to 90% in patients with advanced and progressive disease. Van den Beuken‐van Everdingen estimated a prevalence of pain in excess of 50% for patients at all stages and with all types of cancer (van den Beuken‐van Everdingen 2007).
Cancer‐related pain may be caused by the tumour pressing on adjacent organs/tissues, or by the tumour invading the tissue and damaging it.
Pain has a significant impact on function. Uncontrolled pain is incompatible with satisfactory quality of existence and it is well recognised that persistent pain impairs daily life and social interaction. Studies highlight the increased risk of anxiety, depression and even suicidal ideation in patients with uncontrolled pain (Graner 2016).
Description of the intervention
Methadone is a synthetic opioid in the structural class of diphenylpropylamines, which was developed in the 1930s. It is a potent agonist at the mu‐opioid and delta‐opioid receptors. The high affinity of methadone for mu‐receptors (key mediators in supra‐spinal analgesia) and delta‐receptors (probably important in spinal analgesia) has resulted in it being recommended for use as an analgesic in cancer pain.
Most clinical practice and research in most countries uses a racaemic mixture of two isomers, levorotatory (L) methadone and dextrorotatory (D) methadone, although in Germany L‐methadone alone is used (Bruera 2002). L‐methadone is 8 to 50 times more potent than D‐methadone in humans and is believed to be almost entirely responsible for its analgesic properties (Fainsinger 1993).
Methadone is available as a lipophilic hydrochloride salt and is available as formulations for oral, rectal and parenteral administration (Fainsinger 1993; Ripamonti 1997). It is well absorbed by all routes. Oral administration is followed by rapid gastrointestinal absorption with measurable plasma levels at 30 minutes. The peak plasma levels after an oral dose occur at four hours and begin to decline 24 hours after dosing. Oral bioavailability is high, generally over 85% (79% ± 21 in studies). The recommended dose to be given parenterally is between 50% and 80% of the oral dose (Gannon 1997; Davis 2001). Although local toxicity associated with subcutaneous administration has been reported (Bruera 1991), in many cases this is manageable (Mathew 1999).
Elimination of methadone is mediated by hepatic oxidative biotransformation, renal N‐demethylation, and urinary and faecal clearance. Chronic administration results in increased metabolite to methadone ratios, suggesting that autoinduction of hepatic microsomal enzymes occurs. Renal impairment is not thought to impair clearance; methadone may be useful in the management of pain in patients with renal failure. Some drug interactions are significant (Davis 2001).
Methadone has also been demonstrated in animal studies to have antagonist activity at the N‐methyl‐D‐aspartate (NMDA) receptor, resulting in interest in the clinical application of the drug in neuropathic pain syndromes. A combination of NMDA receptor antagonism and opioid agonism might provide valuable analgesic effects with fewer side effects than other analgesics (Gorman 1997).
How the intervention might work
Single dose studies have shown potency marginally greater than morphine; repeated administration results in greater potency. Methadone's properties of high oral bioavailability, rapid onset of analgesic effect, long half‐life (resulting in infrequent dosing schedules), lack of active metabolites, low rate of induction of tolerance, and low cost are characteristics that result in its use in the management of pain in cancer patients (Fainsinger 1993; Hanks 1998; Twycross 1998).
The perceived drawbacks of methadone include high potential for accumulation leading to delayed toxicity, highly variable pharmacokinetics between individuals, relative ease of use of modified‐release morphine preparations, possible drug interactions, and concerns over dose titration and conversion from other opioids (Fainsinger 1993). Potential side effects of methadone include constipation, drowsiness, confusion, nausea, hypotension, miosis (constriction of pupils), antidiuresis, exacerbation of asthma, and respiratory depression. Clinical situations where particular caution is advised include:
-
use in the elderly;
-
pain only partially responsive to methadone, where there is a risk of rapid dose escalation;
-
pain thought to have a predominantly psychological component; and
-
people in whom sensitivity to low doses of opioids has previously been demonstrated.
Particular concern has grown in recent years about two aspects of methadone pharmacology: interactions with other drugs and also the potential for prolongation of the QT interval (a measure of cardiac function) resulting in the potentially fatal arrhythmia called torsade de pointes (van den Beuken‐van Everdingen 2013). Weschules, Bain and Richeimer (Weschules 2008) conducted a systematic review of the literature to quantify the available evidence relating to methadone‐related drug interactions. They concluded that the evidence base was not well developed and much of the available literature consisted of case reports and case series considering inpatients being managed on methadone maintenance treatment programmes for opioid substance misuse. These patients had a high rate of HIV infection (and associated prescribing) and many of them were tobacco smokers with consequent potential induction of the cytochrome P1A2 enzyme, which may confound conclusions. Whilst the authors urge caution in extrapolating the findings of their review to the chronic and cancer pain patient populations, they draw attention to possible interactions of interest to pain management clinicians in a table summarising those attributed to interactions with anticonvulsants (phenytoin, phenobarbital and carbamazepine), selective serotonin reuptake inhibitor (SSRI) antidepressants (fluvoxamine, fluoxetine, paroxetine, sertraline and citalopram), tricyclic antidepressants (desipramine, amitriptyline, imipramine) and benzodiazepine anxiolytics (diazepam and alprazolam) (Weschules 2008). They also highlight concern that genetic polymorphism associated with the cytochrome‐P enzymes may have a bearing on both methadone metabolism per se, and the occurrence ‐ and potential severity ‐ of methadone‐related drug interactions.
Following reports of sudden death in people taking methadone in the early to mid 2000s, interest in prolongation of the QT interval by methadone has arisen. This risk has received specific attention in published guidance about use of methadone for pain in cancer and palliative care patients. For example, the editors of the Palliative Care Formulary (Twycross 2014) included a specific reference to methadone risks in the chapter on QT prolongation in the Fourth Edition which was not present in the Third edition. Cruciani reviewed the available literature in an attempt to answer the questions of whether, and when, ECG examination should be undertaken in patients prescribed methadone (Cruciani 2008). From an examination of available studies published between 1973 and 2007, and considering the evidence in favour of performing ECG testing set against those that raise questions about the significance of cardiac toxicity related to methadone, it was concluded that there are reasons to be cautious and a low threshold for recommending ECG testing is advised. Pending further evidence, Cruciani's recommendations are that ECG examination should be conducted at baseline (methadone initiation), on dose escalation and on addition of relevant medication in the following situations:
-
patients co‐administered methadone with drugs that are substrates of the cytochrome‐P3A4 or cytochrome‐P2D26 enzymes;
-
patients treated with drugs which may block the HERG (human ether‐a‐go‐go related gene) protein in the potassium channel of cardiac tissue, including some macrolide antibiotics (erythromycin, clarithromycin), antipsychotics (chlorpromazine, haloperidol, olanzapine, risperidone) and antidepressants (tricyclics and sertraline and venlafaxine);
-
patients who are medically frail or who have other risk factors for prolonged QT interval including cardiac disease, hypokalaemia, hypomagnesaemia and family history of sudden death.
The Palliative Care Formulary seeks to put this into a clinical context (Twycross 2014), also recommending baseline ECG and repeated ECG on dose titration or other relevant circumstances, with methadone discontinuation in favour of an alternative opioid being advocated if the QT interval is prolonged to greater than 500 ms. The authors do emphasise the need for clinical judgment over the balance of burden versus benefit when weighing the risk, and the importance of communication of this with the patient and those close to them.
Why it is important to do this review
Methadone is considered to be a useful analgesic for the management of moderate to severe cancer pain. The earlier review in 2007 indicated that it had similar efficacy to morphine, but adverse events may be problematic with repeated dosing, based on limited evidence. Standards for systematic reviews have strengthened since then and it is important to identify any new studies and reassess the evidence for methadone's place in managing cancer pain.
This review is one of a suite of reviews on cancer pain and will be incorporated into an overview of opioids for cancer pain.
Objectives
To determine the effectiveness and tolerability of methadone as an analgesic in adults and children with cancer pain.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised controlled trials (RCTs). We required full journal publication, with the exception of online clinical trial results summaries of otherwise unpublished clinical trials and abstracts with sufficient data for analysis. We did not include short abstracts (usually meeting reports). In this update we excluded studies with treatment groups of fewer than 10 participants.
Types of participants
Inclusion criteria
-
Male and female adults and children with cancer pain
-
Any pain with a malignant etiology
-
Etiology may be primary or secondary malignancy, solid or haematological tumours
-
Pain of at least moderate intensity
-
Participants being treated in any setting
Exclusion criteria
-
People taking methadone for suppression of cough
-
People taking, or who have previously taken, methadone for rehabilitation from opioid dependence
Types of interventions
-
Methadone given specifically for relief of cancer‐related pain, at any dose and by any route
-
Methadone compared with placebo or any other active comparison
Types of outcome measures
We sought to asses the outcome of 'no worse than mild pain' and the impact of methadone on consciousness, appetite and thirst. These are issues of concern to patients and their relatives and care providers, highlighted in the UK but of international relevance. (DH 2013;Ma 2016; Wiffen 2014).
Primary outcomes
-
Participant‐reported pain intensity or pain relief, or both, measured using a visual analogue scale, verbal rating scale or numerical rating scale
-
Participants with 'no worse than mild pain' on treatment
We did not use physician or carer assessments of pain.
Secondary outcomes
-
Participants with adverse events. We collected information on all reported adverse events, but were particularly interested in consciousness; drowsiness and confusion; loss of appetite; thirst; constipation; nausea and vomiting; and respiratory effects.
-
Withdrawals for any reason including non‐compliance, adverse events, and death
Search methods for identification of studies
We searched electronic databases for published studies and clinicaltrails.gov for unpublished studies.
Electronic searches
For this update we searched the four databases listed below, without language restrictions.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Register of Studies Online) on 4 May 2016
-
MEDLINE (via Ovid) January 2006 to 4 May 2016
-
Embase (via Ovid) January 2006 to 4 May 2016
-
CINAHL (via EBSCO) to 4 May 2016
For the original review in 2004 and the first update in 2007, we also searched CancerLit, which has been retired and the Cochrane Pain, Palliative and Supportive Care Trials Register, which is no longer updated.
The search strategies for the four databases can be found in Appendix 1.
Searching other resources
For this update, we searched the clinical trials registry ClinicalTrials.gov up to May 2016.
Data collection and analysis
Selection of studies
PW and SD independently screened the title and abstract (where available) of all studies identified by the searches and selected studies that appeared to satisfy the inclusion criteria. Where there was any uncertainty, we requested the full text. We also reviewed all the included studies from the 2007 version of the review. All authors agreed the studies to be included. See Figure 1 for a flow chart of the screening process (Moher 2009).
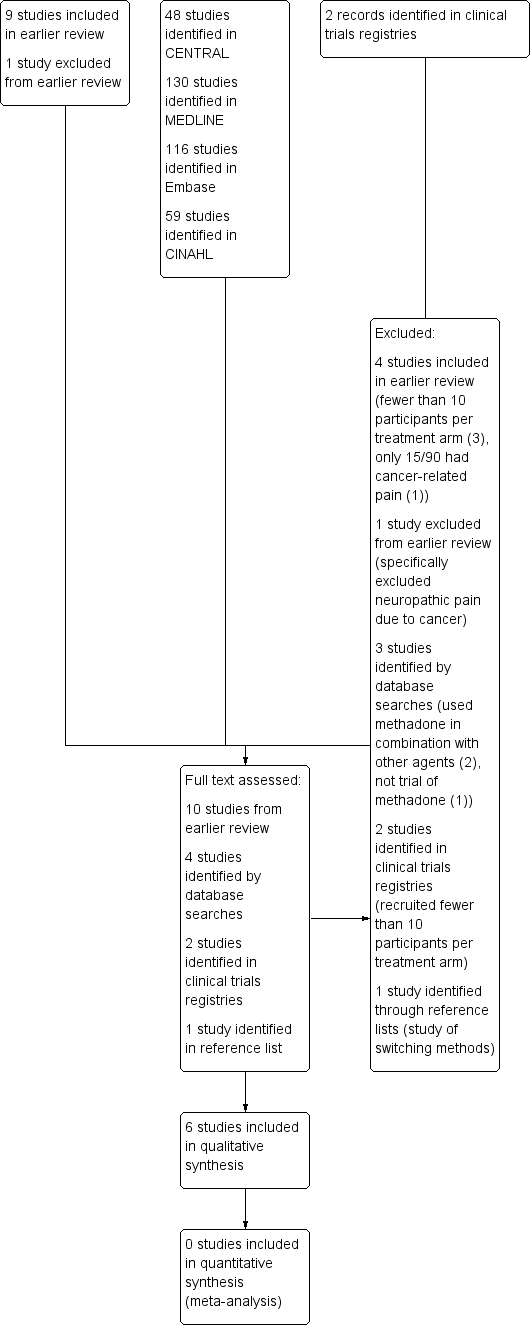
Study flow diagram
Data extraction and management
One author extracted data from the included studies into a standardised data collection sheet to include, where available:
-
publication details;
-
method, including trial design, allocation concealment, blinding and duration;
-
patient demographic details ‐ population, number of patients, age, sex, location of study (inpatient or outpatient);
-
details of malignant primary and secondary diagnoses, and previous and current drug history;
-
details of pain characteristics and types, range of intensity and how determined, assessed and documented;
-
description of the intervention (route, dose, etc) and control;
-
measures of effect (e.g. patient‐reported pain scores, patient satisfaction, quality‐of‐life scores);
-
adverse events, withdrawals, and dropouts.
A second author checked the data extraction before entry into Cochrane software Review Manager (RevMan 2014).
Assessment of risk of bias in included studies
All authors assessed all included studies for risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, and resolved any disagreements by discussion (Higgins 2011). The effect of risk of bias for included studies is shown in Figure 2 and Figure 3.
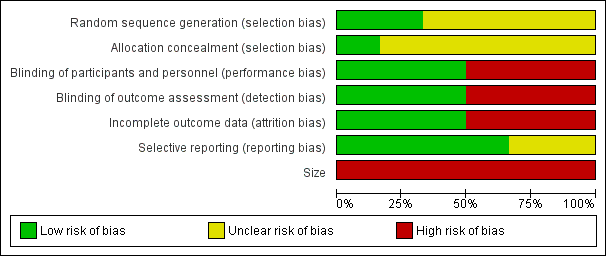
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
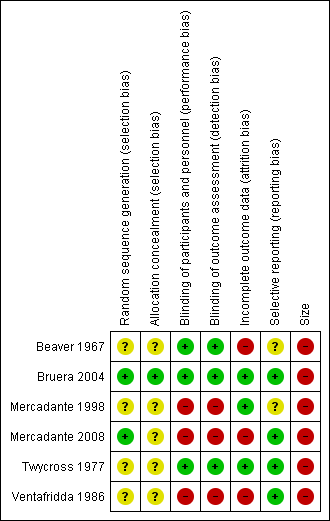
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process: random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a nonrandom process, which were therefore at high risk of bias (odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether the intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively‐numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation, which were therefore at high risk of bias (open list).
-
Blinding of participants, personnel, and outcome assessments (checking for possible performance and detection bias). We assessed the methods used to blind study participants, personnel, and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding: identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how blinding was achieved); high risk of bias (study not blinded).
-
Incomplete outcome data (attrition bias). We assessed the risk of bias from missing data as: low risk of bias (ITT analysis, or all participants accounted for with adequate reasons for loss and equally distributed between groups); low risk of bias (minimal loss of data); unclear risk of bias (other issues, such as cross‐over studies); high risk of bias (completer analysis, attrition 10% or more).
-
Selective reporting (reporting bias). We assessed the risk of reporting bias as: low risk of bias (all intended outcomes reported); unclear risk of bias (any anomaly in reporting, such as participants contributing more than one set of data, or some outcomes not participant‐reported); high risk of bias (prespecified outcome of interest not reported).
-
Size (checking for possible biases confounded by small size). Small studies may overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised. We considered studies to be at low risk of bias if they had 200 participants or more per treatment arm, at unclear risk if they had 50 to 200 participants per treatment arm, and at high risk if they had fewer than 50 participants per treatment arm.
Measures of treatment effect
It was not possible to combine studies for meta‐analysis to measure any treatment effect.
Unit of analysis issues
It was not possible to combine studies for meta‐analysis and therefore no unit of analysis issues were encountered.
Dealing with missing data
We took a decision not to impute missing data.
Assessment of heterogeneity
No statistical assessment of heterogeneity was made as no meta‐analysis was possible.
Assessment of reporting biases
We carried out extensive searches to identify relevant studies, but were unable to carry out any formal test for publication or other reporting biases.
Data synthesis
We did not pool data.
Grading of evidence
This section is taken from Cochrane Drugs and Alcohol recommended text. The overall quality of the evidence for each outcome was assessed using the GRADE system, and presented in summary of findings Table for the main comparison, to present the main findings of a review in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grade of evidence:
-
High: we are very confident that the true effect lies close to that of the estimate of the effect;
-
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different;
-
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect;
-
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We downgraded the evidence for:
-
serious (‐1) or very serious (‐2) limitation to study quality;
-
important inconsistency (‐1);
-
some (‐1) or major (‐2) uncertainty about directness;
-
imprecise or sparse data (‐1);
-
high probability of reporting bias (‐1).
In addition, where there were circumstances where the overall rating for a particular outcome needed to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used last observation carried forward (LOCF) imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by 3 levels, to very low quality. In circumstances where there were no data reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings in a transparent and simple tabular format. We have included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of participant‐reported pain intensity and adverse events ‐ appetite, thirst and somnolence.
Subgroup analysis and investigation of heterogeneity
We expected from previous versions of this review that the included studies would be heterogeneous and planned to undertake subgroup analysis based upon variations in methods such as titration schedule, route of administration, different comparators, and duration of studies. However no data were available for pooled analysis.
Sensitivity analysis
No sensitivity analysis was planned, or possible.
Results
Description of studies
Results of the search
We reviewed all the included and excluded studies in the earlier review, and carried out updated searches to May 2016. After deduplication and screening we assessed the full texts of 10 studies from the earlier review and four new studies identified by database searching. We subsequently identified one further potential paper from reference lists (Moksnes 2011) but this was excluded. Searches of ClinicalTrials.gov identified two studies, both of which had been terminated due to slow accrual, and were excluded from this review.
A flow chart of the process is shown in Figure 1 (Moher 2009).
Included studies
In this updated review, we included six studies, with 388 participants. Five of the nine included studies from the 2006 update are in this latest version (Beaver 1967; Bruera 2004; Mercadante 1998; Twycross 1977; Ventafridda 1986). One new study has been added (Mercadante 2008).
All studies were of adult participants with various types of cancer who required strong opioids to control their pain. Where reported the mean (or median) age was 48 to 65 years (range 18 to 87), and there were slightly more women than men in the studies (1.3:1). Most participants had previously been treated with World Health Organization (WHO) step 1 and 2 opioids (Ventafridda 1987), with inadequate response, but some had previously received step 3 opioids. Studies generally allowed titration to achieve the optimum balance of pain relief and adverse events. One study examined single doses of both intramuscular (IM) and oral morphine with some participants undergoing more than one round of treatments (Beaver 1967). The remaining studies used oral morphine.
Details of the individual studies are in the Characteristics of included studies table.
Excluded studies
We revised the excluded studies list for this update and this version of the review now excludes 11 studies. Four studies were included in the earlier review (Ferrer‐Brechner 1984; Gourlay 1986; Grochow 1989; Matts 1964), and one was excluded (Morley 2003). The new excluded studies were two studies identified in ClinicalTrials.gov (both excluded because they recruited only a single participant, NCT00573937; NCT00726830); Cubero 2010 and Lauretti 2013 (both used methadone in combination with other agents); Raptis 2013 (not a trial of methadone); and Moksnes 2011 (a study of switching methods).
See Characteristics of excluded studies for further details.
Risk of bias in included studies
Two authors independently assessed risk of bias for each study using the criteria outlined in Assessment of risk of bias in included studies. Summaries of the risk of bias assessment are provided in Figure 2 and Figure 3. The risks of bias for the included studies are described here.
Allocation
All studies were randomised, but only two adequately described the method used to generate the random sequence (Bruera 2004; Mercadante 2008). One study described the method used to conceal allocation of the random sequence and we judged this to be at low risk of bias (Bruera 2004). The remaining studies were judged at unclear risk of bias.
Blinding
Three studies adequately described the method used to maintain blinding (Beaver 1967; Bruera 2004; Twycross 1977). Two of the studies were open‐label and used commercial products so we judged them to be at high risk of bias (Mercadante 1998; Mercadante 2008). One study did not describe blinding and used different dosing regimens, and we judged it to be at high risk of bias (Ventafridda 1986).
Incomplete outcome data
We judged three studies to be at low risk of bias for this domain (Bruera 2004; Mercadante 1998; Twycross 1977), and two studies to be at high risk because they analysed only those who completed the study (Mercadante 2008; Ventafridda 1986). Beaver 1967 reported on participants who completed both cross‐over phases, and we judged this study to be at high risk of bias.
Selective reporting
We judged two studies to be at an unclear risk of bias (Beaver 1967, Mercadante 1998); all others were judged to be at low risk for this domain.
Other potential sources of bias
We judged all the studies to be at high risk due to small numbers of participants per treatment arm.
Effects of interventions
See: Summary of findings for the main comparison Methadone compared to Morphine for cancer pain
A summary of results is presented in Appendix 2.
Efficacy
1. Methadone compared with oral morphine
a) Intramuscular (IM) methadone compared with IM morphine
A study by Beaver 1967 looked at 37 participants in a double‐blind double‐dummy cross‐over comparisons of IM methadone and IM morphine. Pain relief was good in both groups at six hours, with higher doses producing 'no worse than mild pain'. Methadone was found to be slightly more potent than morphine.
b) Oral methadone compared with immediate‐release oral morphine
Beaver 1967 also examined the efficacy of oral methadone and oral morphine in the IM study discussed above. However the study report does not provide separate data for the oral drugs. In a study of 66 participants by Ventafridda 1986, all who completed 14 days of treatment (n = 54) achieved 'no worse than mild pain'. We judged this to be very low‐quality evidence because of the small size of the study, the lack of blinding, and use of completer analysis.
c) Oral methadone compared with modified‐release oral morphine
Three studies (215 participants) compared oral methadone with oral morphine, modified‐release (MR) (Bruera 2004; Mercadante 1998; Mercadante 2008).
Bruera 2004 found that methadone at a daily dose of 15 mg was not superior to 60 mg/day of morphine MR. Pain reduction of at least 20% from baseline was the main pain outcome (methadone 24/49, morphine 30/54 at day 29). We judged this to be low‐quality evidence because of the small size of the treatment groups. We could not derive data for 'no worse than mild pain'.
Mercadante 1998 reported mean pain scores that were just above 'no worse than mild pain' (See Appendix 2). In the second study by Mercadante (Mercadante 2008) similar mean pain scores were reported for participants who did not drop out (completer analysis). We judged this to be low‐quality evidence because of the small size of the treatment groups, lack of blinding, and use of completer analysis in one study.
2. Oral methadone compared with transdermal (TD) fentanyl
In a three‐arm study by Mercadante 2008, there were no reported differences in pain intensity between the methadone and TD fentanyl groups for participants who did not drop out.
3. Oral methadone compared with diamorphine and cocaine combination
Twycross 1977 compared methadone with a diamorphine‐cocaine combination in 46 participants. The study examined the relative efficacy of the two interventions but no evaluable results were reported. Diamorphine‐cocaine combinations are no longer used so the study is of historical interest only.
Subgroup analysis
There were insufficient data to carry out any subgroup analysis.
Other efficacy measures
None of the studies reported quality‐of‐life or treatment‐satisfaction outcomes.
Adverse events
Specific adverse events
The most frequently observed adverse events were dry mouth, somnolence, and constipation. We have separately presented adverse events relating to appetite, consciousness and thirst, as well as more general adverse events in Appendix 3. No study reported the effects of the intervention on appetite. Sedation or somnolence was reported with both morphine and methadone; the results were inconsistent across studies, but the incidence was generally higher with methadone than with morphine. Dry mouth was reported in three studies (Beaver 1967; Mercadante 1998; Ventafridda 1986), but again results were not consistent. Both morphine and methadone can produce a dry mouth and, by association, thirst. The majority of studies reported nausea, vomiting, and constipation.
We judged the quality of the evidence relating to adverse events as very low because of the small size of the studies, lack of blinding and use of completer analysis in some studies, the small number of events, and indirectness, since surrogates for appetite, thirst and somnolence (such as sedation, drowsiness and dry mouth) were used.
Withdrawals due to adverse events, non compliance and death
Adverse event withdrawals were uncommon (12/202) and similar between groups.
There were no recorded withdrawals for noncompliance.
Deaths were uncommon except in one study where the majority of participants died irrespective of the treatment group (Twycross 1977).
Discussion
This is the second update of a review first published in 2004 and last updated in 2007. In this update, earlier decisions were revisited and current standards applied to the review.
Summary of main results
For studies in cancer pain, it is useful to know what proportion of people starting treatment are likely to be able to tolerate it, and what proportion of those who tolerate it are likely to obtain adequate pain relief. The studies did not report results in a way that unequivocally answered these questions.
Based on very limited amounts of data, there were no clear differences in participant‐reported pain intensity or pain relief between methadone and morphine or transdermal fentanyl: similar proportions of participants were able to tolerate each drug and achieve a level of pain that was probably similar to mild pain. All six included studies were small, and in addition, three used methods that put them at risk of bias. The results must therefore be interpreted with caution.
We were not able to obtain reliable data on the numbers of participants who achieved no worse than mild pain.
Adverse events were typical for opioids (e.g. sedation, somnolence, dry mouth, constipation) but inconsistently reported.
Overall completeness and applicability of evidence
Given the use of methadone in palliative care the amount of underpinning data is small. The most recent study is now eight years old and most studies were conducted before 2000. The more recent studies (251 participants randomised) compared methadone with either modified‐release morphine or transdermal fentanyl, which are commonly used in practice (Bruera 2004; Mercadante 1998; Mercadante 2008).
There were insufficient data to carry out any subgroup analysis. In particular, we were unable to investigate the influence of dose and titration regimen on either efficacy or tolerability. Included studies were underpowered to investigate serious adverse events, including arrhythmias.
We did not identify any studies in children.
Quality of the evidence
The evidence base identified by this review was small and limited in scope due to the small number of participants included and the diversity of the study methodologies and outcome reporting. Two of the more recent studies were at high risk of bias due to a lack of blinding (Mercadante 1998; Mercadante 2008).
Potential biases in the review process
We are unaware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
The search strategy for this updated review identified a systematic review conducted using Cochrane methods to examine the literature on methadone for cancer pain since the 2007 publication of this review (Good 2014). The review authors identified four randomised controlled trials. One is included in this update (Mercadante 2008). The other three were identified in our search strategy but have been allocated to the 'excluded studies' because they used methadone in combination with other agents (Lauretti 2013), or did not compare methadone with a different comparator. One examined outcomes for participants on a stable dose of morphine, who were switched to methadone with or without acetaminophen (paracetamol) (Cubero 2010). Good pain control and improved side‐effect burden and quality of life were reported, with a majority of participants expressing a preference for methadone treatment. However this was not a trial that examined methadone use in the way we defined for our review, which explains our decision to exclude it. The other study identified by Good 2014 compared two methods of switching to methadone (Moksnes 2011). Whilst interesting, the study addressed a different question from that considered by this review.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
| Methadone compared to morphine for cancer pain | ||||||
| Patient or population: people with cancer pain | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect | No of participants | Quality of the evidence | Comments |
| Morphine | Methadone | |||||
| Participant‐reported pain intensity | Pain intensity scores: One study (103 participants) reported > 20% improvement in pain scores for 76% of morphine and 75% methadone participants in those that completed No worse than mild pain (pain score of 3/10 or less after treatment): One study (54 participants) reported all achieved no worse than mild pain based on mean pain scores. Two studies (148 participants) reported mean pain scores very close to a score of 3 | ⊕⊕⊝⊝ | Downgraded two points for reasons stated | |||
| Adverse events: appetite, thirst, somnolence | Not estimable | Not estimable | Not estimable | 342 | ⊕⊝⊝⊝ | Downgraded three points for reasons stated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
| 1 Risk of bias: random allocation and allocation concealment unclear, all had sample size of less than 200 per treatment arm. | ||||||

