Índice biespectral para mejorar la conciencia intraoperatoria y la recuperación posoperatoria temprana en pacientes adultos
Resumen
Antecedentes
El uso de signos clínicos, o gas anestésico espiratorio final (GAEF), puede no ser fiable para medir el componente hipnótico de la anestesia y puede dar lugar a una sobredosis o a una dosis insuficiente, lo que puede provocar efectos adversos debido a una anestesia demasiado profunda o demasiado ligera. La conciencia intraoperatoria, aunque no es común, puede conducir a alteraciones psicológicos graves, y los métodos alternativos para monitorizar la profundidad de la anestesia pueden reducir la incidencia de eventos graves. El índice biespectral (IBE) es una escala numérica basada en la actividad eléctrica del cerebro. El uso de un monitor IBE para guiar la dosis de anestésico puede tener ventajas sobre los signos clínicos o el GAEF. Esta es una actualización de una revisión publicada por última vez en 2014.
Objetivos
Evaluar la efectividad del IBE para reducir el riesgo de conciencia intraoperatoria y los tiempos de recuperación temprana de la anestesia general en pacientes adultos sometidos a cirugía.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE, Embase y Web of Science el 26 de marzo de 2019. Se buscó en los registros de ensayos clínicos y en la literatura gris, y se realizaron búsquedas manuales en las listas de referencias de los estudios incluidos y las revisiones relacionadas.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) y cuasialeatorizados en los que se utilizó el IBE para guiar la anestesia en comparación con la práctica estándar, que fueron los signos clínicos o el gas anestésico espiratorio final (GAEF) para guiar la dosis anestésica. Se incluyeron participantes adultos sometidos a cualquier tipo de cirugía con anestesia general, independientemente de si los participantes incluidos tenían un alto riesgo de conciencia intraoperatoria. Sólo se incluyeron los estudios en los que los investigadores intentaron evaluar la efectividad del IBE para su función en la monitorización de la profundidad intraoperatoria de la anestesia o las posibles mejorías en los tiempos de recuperación temprana de la anestesia.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron los estudios para inclusión, extrajeron los datos y evaluaron el riesgo de sesgo. La certeza de la evidencia se evaluó con GRADE.
Resultados principales
Se incluyeron 52 estudios con 41 331 participantes; dos estudios fueron cuasialeatorizados y los estudios restantes fueron ECA. Todos los estudios incluyeron participantes sometidos a cirugía con anestesia general. Tres estudios reclutaron sólo participantes con alto riesgo de conciencia intraoperatoria, mientras que dos estudios reclutaron específicamente un grupo de participantes no seleccionados. Los datos se analizaron según dos grupos de comparación: IBE versus signos clínicos e IBE versus GAEF. Cuarenta y ocho estudios utilizaron los signos clínicos como método de comparación, que incluyó la valoración de la anestesia según criterios como la presión arterial o la frecuencia cardíaca y seis estudios utilizaron el GAEF para guiar la anestesia. Aunque los valores objetivo del IBE difirieron entre los estudios, todos estaban dentro de un rango de valores entre 40 y 60.
IBE versus signos clínicos
Se encontró evidencia de certeza baja de que la anestesia guiada por el IBE puede reducir el riesgo de conciencia intraoperatoria en una población quirúrgica no seleccionada o con alto riesgo de conciencia (odds ratio [OR] de Peto 0,36; IC del 95%: 0,21 a 0,60; I2 = 61%; 27 estudios; 9765 participantes). Sin embargo, los eventos fueron poco frecuentes y sólo cinco de los 27 estudios informaron incidencias; se encontró que cuando se utilizó el IBE las incidencias de conciencia intraoperatoria fueron de tres por 1000 (IC del 95%: 2 a 6 por 1000) en comparación con nueve por 1000 cuando la anestesia se guió por los signos clínicos. De los cinco estudios con datos de eventos, uno incluyó participantes con alto riesgo de sensibilización y uno incluyó participantes no seleccionados, cuatro utilizaron un cuestionario estructurado para la evaluación y dos utilizaron un proceso de adjudicación para identificar el conocimiento confirmado o definitivo.
Los tiempos de recuperación temprana también mejoraron cuando se utilizó el IBE. Se encontró evidencia de certeza baja de que el IBE puede reducir el tiempo de apertura de los ojos según una diferencia de medias (DM) 1,78 minutos (IC del 95%: ‐2,53 a ‐1,03 minutos; 22 estudios; 1494 participantes), el tiempo hasta la orientación según una DM 3,18 minutos (IC del 95%: ‐4,03 a ‐2,33 minutos; seis estudios; 273 participantes) y el tiempo transcurrido hasta el alta de la unidad de cuidados posanestésicos (UCPA) según una DM 6,86 minutos (IC del 95%: ‐11,72 a ‐2 minutos; 13 estudios; 930 participantes).
IBE versus GAEF
Una vez más, los eventos de conciencia intraoperatoria fueron muy poco frecuentes y no se encontró evidencia de una diferencia en las incidencias de conciencia intraoperatoria en dependencia de si la anestesia fue guiada por IBE o por GAEF en una población quirúrgica no seleccionada o con alto riesgo de conciencia (OR de Peto 1,13; IC del 95%: 0,56 a 2,26; 2= 37%; cinco estudios; 26 572 participantes; evidencia de certeza baja). Las incidencias de conciencia intraoperatoria fueron de uno por 1000 en ambos grupos. Sólo tres de cinco estudios informaron eventos, dos incluyeron participantes con alto riesgo de sensibilización y uno incluyó participantes no seleccionados, todos utilizaron un cuestionario estructurado para la evaluación y un proceso de adjudicación para identificar el conocimiento confirmado o definitivo.
Un estudio grande (15 452 participantes) informó sobre una reducción del tiempo transcurrido hasta el alta de la UCPA en una mediana de tres minutos menos, y se consideró que la certeza de esta evidencia era baja. Ningún estudio midió o informó el tiempo transcurrido hasta la apertura de los ojos ni el tiempo hasta la orientación.
Certeza de la evidencia
Se utilizó GRADE para disminuir la calidad de la evidencia de todos los resultados a certeza baja. La incidencia de conciencia intraoperatoria es tan poco frecuente que, a pesar de la inclusión de algunos estudios multicéntricos grandes en los análisis, se consideró que las estimaciones del efecto eran imprecisas. Además, los análisis incluyeron estudios que se consideraron con limitaciones debido a algunas evaluaciones de sesgo alto o incierto y en todos los estudios no fue posible cegar a los anestesistas a los diferentes métodos de monitorización de la profundidad de la anestesia.
Los estudios a menudo no informaron una definición clara de la conciencia intraoperatoria. Los puntos temporales de medición difirieron y los métodos utilizados para identificar la conciencia intraoperatoria también difirieron y era de esperar que algunas herramientas de evaluación fueran más completas que otras.
Conclusiones de los autores
La conciencia intraoperatoria es poco frecuente y, a pesar de identificar un gran número de estudios elegibles, la evidencia de la efectividad del uso del IBE para guiar la profundidad anestésica no es precisa. Se encontró que la anestesia guiada por el IBE en comparación con los signos clínicos puede reducir el riesgo de conciencia intraoperatoria y mejorar los tiempos de recuperación temprana en los pacientes sometidos a cirugía con anestesia general, pero no se encontró evidencia de una diferencia entre la anestesia guiada por el IBE y la anestesia guiada por GAEF. Se encontraron seis estudios en espera de clasificación y dos estudios en curso; la inclusión de estos estudios en actualizaciones futuras puede aumentar la certeza de la evidencia.
PICO
Resumen en términos sencillos
Índice biespectral (IBE) para mejorar la conciencia intraoperatoria y la recuperación posoperatoria temprana en pacientes adultos
Antecedentes
Durante la cirugía con anestesia general, el anestesista ajustará la cantidad de anestésicos para asegurar que el paciente permanezca inconsciente. Este ajuste se realiza en función de signos clínicos, como la frecuencia cardíaca o la presión arterial del paciente, o el gas anestésico espiratorio final (GAEF) en el caso de la anestesia que se administra en forma de gas, que es una medida de la cantidad de gas restante después que el paciente espira. Sin embargo, el uso de estos métodos solos puede aumentar la probabilidad de que el paciente reciba muy poca o demasiada anestesia. La conciencia intraoperatoria, un evento angustioso en el que el paciente puede estar lo suficientemente consciente para recordar los eventos durante la cirugía, es muy poco frecuente y puede ser causada por muy poca anestesia. Demasiado anestésico puede hacer que se necesite más tiempo para lograr la recuperación completa. El índice biespectral (IBE) es una escala de medición basada en la actividad eléctrica en el cerebro, y mediante el uso de un monitor de actividad cerebral durante la anestesia, el anestesista puede utilizar esta escala para informar la cantidad de anestesia a administrar al paciente.
Esta es una actualización de una revisión que se publicó anteriormente en 2014.
Características de los estudios
La evidencia está actualizada hasta el 26 de marzo de 2019. Se encontraron 52 estudios con 41 331 participantes. Seis estudios están a la espera de clasificación (porque no se dispuso de información suficiente para evaluarlos) y dos están en curso. Todos los estudios incluyeron pacientes sometidos a cirugía con anestesia general. Tres estudios incluyeron sólo pacientes con alto riesgo de conciencia intraoperatoria y dos estudios incluyeron sólo pacientes que no se seleccionaron según el alto riesgo de conciencia intraoperatoria. Cuarenta y ocho estudios compararon la anestesia guiada por el IBE con la anestesia guiada por signos clínicos y seis estudios compararon la anestesia guiada por el IBE con la anestesia guiada por GAEF.
Resultados clave
Se encontró evidencia de certeza baja de que la anestesia guiada por el IBE puede reducir el riesgo de conciencia intraoperatoria. Sin embargo, los eventos fueron poco frecuentes y sólo cinco de 27 estudios informaron incidencias. Cuando se utilizó la anestesia guiada por el IBE, se encontraron tres por 1000 incidencias de conciencia intraoperatoria menos en comparación con nueve por 1000 incidencias cuando la anestesia estuvo guiada por signos clínicos. Además, se encontró evidencia de certeza baja de que el IBE puede mejorar la recuperación: el tiempo trascurrido hasta que los pacientes abrieran los ojos fue menor, al igual que el tiempo hasta la orientación y el tiempo para ser dados de alta de la unidad de cuidados posanestésicos.
No se encontró evidencia de una diferencia en las incidencias de conciencia intraoperatoria en dependencia de si la anestesia fue guiada por el IBE o por la GAEF, aunque, de nuevo, hubo pocas incidencias de conciencia (una por 1000 en cada grupo). Sólo un estudio que comparó el IBE con la anestesia guiada por GAEF midió los tiempos de recuperación; esta evidencia de certeza baja mostró que el alta de la unidad de cuidados posanestésicos fue más temprana si la anestesia fue guiada por el IBE. Ningún estudio que comparara el IBE con la anestesia guiada por GAEF midió el tiempo transcurrido hasta la apertura de los ojos o el tiempo hasta la orientación.
Certeza de la evidencia
Se utilizó GRADE para disminuir la calidad de la evidencia de todos los resultados a certeza baja. La incidencia de conciencia intraoperatoria es muy poco frecuente y, aunque se encontraron algunos estudios grandes, se concluye que aún la evidencia no es precisa. Además, se consideró que muchos estudios tuvieron limitaciones debido a los riesgos de sesgo altos o inciertos. Por ejemplo, todos los anestesistas sabían que utilizaban un monitor adicional del IBE y no se puede estar seguro de cómo este hecho afectaba a la práctica estándar de los anestesistas.
Además, se observó que algunos estudios no informaron una definición clara de la conciencia intraoperatoria. Los puntos temporales de medición difirieron y los métodos utilizados para identificar la conciencia intraoperatoria también difirieron y era de esperar que algunas herramientas de evaluación fueran más completas que otras.
Conclusión
La conciencia intraoperatoria es poco frecuente y, a pesar de encontrar un gran número de estudios elegibles, la evidencia de la efectividad del uso del IBE para guiar la profundidad anestésica es poco precisa. Se encontró evidencia de certeza baja de que la anestesia guiada por el IBE en comparación con la anestesia guiada por signos clínicos puede reducir el riesgo de conciencia intraoperatoria y mejorar los tiempos de recuperación temprana en los pacientes sometidos a cirugía con anestesia general. No se encontró evidencia de una diferencia entre la anestesia guiada por el IBE y la anestesia guiada por GAEF, y también se consideró que esta evidencia era de certeza baja.
Authors' conclusions
Summary of findings
| BIS compared to clinical signs for intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with clinical sides | Risk with BIS | |||||
| Occurrence of intraoperative awareness Time points of measure after surgery: 2 to 6 hours;12 hours; 1 day; 2 days; 3 days; 14 days; 30 days; or time point was not reported Measurement tools: simple questioning; interviews; or structured questionnaires | Study population | Peto OR 0.36 | 9765 | ⊕⊕⊝⊝ | Only 5 of 27 studies included incidences of awareness. Of these 5 studies: 4 used a structured questionnaire, and 1 used an interview method; 2 used an adjudication process to categorise incidences of awareness as 'confirmed' or 'definite'; participants in 1 study were at high risk of awareness, in 1 study were unselected, and in the remaining studies risk of awareness was not specified | |
| 9 per 1,000 | 3 per 1,000 | |||||
| Time to eye opening (in minutes) | ‐ | MD 1.78 minutes lower | ‐ | 1494 | ⊕⊕⊝⊝ | |
| Time to orientation (in minutes) | ‐ | MD 3.18 lower | ‐ | 273 | ⊕⊕⊝⊝ | |
| Time to discharge from the PACU (in minutes) | ‐ | MD 6.86 lower | ‐ | 930 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. We downgraded by one level for imprecision; whilst we noted a narrow CI, the effect was dominated by two large trials (with two different populations selected according to the likelihood of intraoperative awareness) and we found many studies with zero events in both arms. We conducted sensitivity analysis to explore alternative statistical models to account for zero events in both arms as well as rare events and found more conservative estimates when we used a random‐effects model, thus reducing our certainty in the estimate. | ||||||
| BIS compared to ETAG for improving intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ETAG | Risk with BIS | |||||
| Occurrence of intraoperative awareness Time points of measure after surgery: 24 hours; 24 to 72 hours; 72 hours; 30 days; 18 hours after extubation in the ICU Measurement tools: structured interviews; or structured questionnaire | Study population | Peto OR 1.13 | 26,572 | ⊕⊕⊝⊝ | Only 3 of these studies included incidences of awareness. Of these 3 studies: all used a structured questionnaire; all used an adjudication process to categorise incidences of awareness as 'definite'; 2 studies included only participants who were at high risk for intraoperative awareness, and 1 study included participants who were unselected | |
| 1 per 1,000 | 1 per 1,000 | |||||
| Time to eye opening | ‐ | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | |
| Time to orientation | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | ||
| Time to discharge from the PACU (in minutes) | Median (IQR): 98 minutes (66 to 140 minutes) | Median (IQR) 3 minutes lower (2 minutes lower to 2 minutes lower) | ‐ | 15,452 (1 study) | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for imprecision; despite a large number of participants, events were very rare (one per 1000 in the intervention and the comparison group) and the confidence interval for this effect was wide. In addition, we downgraded by one level for study limitations owing to the inclusion of some studies with unclear and high risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. | ||||||
Background
Description of the condition
The practice of anaesthesia is based on the concept of components of anaesthesia resulting from separate pharmacological actions of multiple agent administration (Kissin 1997). Many anaesthesiologists rely on somatic signs (motor responses, changes in respiratory pattern) and autonomic signs (tachycardia (abnormally rapid heart rate), hypertension (abnormally high blood pressure), lacrimation (flow of tears), sweating) to guide the dosages of anaesthetic agents in order to achieve the basic goals of anaesthetic management; that is unconsciousness (hypnotic effects), blockade of somatic motor responses, and suppression of autonomic responses to noxious stimulation. However, these clinical signs are not reliable measures of the conscious state of anaesthetized patients (Mahla 1997). The use of these clinical signs in judging the dosages of anaesthetic agents can lead to either overdosage or underdosage, which can result in adverse effects due to too deep or too light anaesthesia. Furthermore, there has been much concern regarding intraoperative awareness, which is an uncommon phenomenon occurring in about 0.1% to 0.2% of the general surgical population (Sebel 2004), but which can lead to a serious psychological disturbance called post‐traumatic stress disorder (PTSD), resulting in major depression and suicide. The incidence may approach 1% in surgical patients at high risk for intraoperative awareness such as patients with poor cardiac reserve, or undergoing cardiac surgery or caesarean section, where doses of anaesthetics have to be reduced to a light level of anaesthesia (Mashour 2012; Myles 2004). From a review of reported cases of intraoperative awareness, too light anaesthesia could account for 87% of the cases (Ghoneim 2009). Hence, strategies to provide optimal anaesthesia depth are required to avoid too light anaesthesia.
Description of the intervention
The bispectral index (BIS) is a dimensionless numerical scale for measuring brain electrical activity. It is derived from cerebral electrical activity (an electroencephalogram (EEG)) captured from the scalp surface at the forehead to reflect the sedative and hypnotic components of anaesthesia (Rampil 1998; Schneider 2010). Its value is a number within a range between 0 to 100, where 0 represents 'no detectable brain electrical activity' and 100 represents 'awake state'.
How the intervention might work
BIS has been recommended to guide doses of anaesthetics to achieve optimal depth of anaesthesia in individual patients. This is in order to avoid unnecessarily deep or too light anaesthesia due to overdosage or underdosage of the hypnotic medications during maintenance and recovery from anaesthesia (Schneider 2010; Sebel 2001). The recommended range of BIS is between 40 to 60 during maintenance of anaesthesia (Avidan 2011; Myles 2004) and 55 to 70 at 15 minutes prior to the end of surgery (Gan 1997).
Why it is important to do this review
Several studies have been conducted to assess the effect of BIS monitoring on the utilization of currently available anaesthetic agents, such as propofol, desflurane and sevoflurane (Gan 1997; Johansen 1998; Nelskyla 2001; Song 1997). A survey was conducted among anaesthesiologists regarding the routine use of BIS monitoring in anaesthesia (Johansen 1998). Although the majority of the respondents found that the monitor was easy to use, and it provided useful information, their comments revealed some ambivalence towards hypnotic titration using a BIS monitor. Most respondents felt that no changes occurred in their individual drug usage. Some respondents who reported a change in their practice felt that the hypnotic medication use might decrease, while analgesic and haemodynamic control agent use might increase. A previous study by Song and colleagues (Song 1997) reported increased use of mivacurium in the BIS‐targeted group. Badrinath 1999 reported an increase in the use of intraoperative opioids in the BIS‐guided group. The increased use of either a muscle relaxant or an opioid analgesic might relate to the ability to maintain 'lighter' planes of anaesthesia with BIS, to avoid movement and increased blood pressure or heart rate during the operation.
Since 1977, several articles and abstracts regarding the utility of BIS have been published by numerous medical researchers and academic institutions. It has been suggested that close titration of anaesthetic effect with the BIS monitor may improve some measures of patient outcomes and operating suite efficiency. However, the results are still contradictory across studies. Many studies (Anez 2001; Boztuğ 2006; Gan 1997; Kreuer 2003; Muralidhar 2008; Tufano 2000) have reported a significant improvement in anaesthetics delivery in terms of reduced anaesthetic consumption or requirements and improved recovery profiles, but some studies (Bruhn 2005; Kreuer 2005; Luginbuhl 2003; Zohar 2006) have failed to demonstrate these effects.
Nowadays, the impact of BIS monitoring on the incidence of intraoperative awareness is a matter of interest in anaesthesia practice. The optimisation of the depth of anaesthesia may avoid too light anaesthesia which may result in intraoperative awareness. However, because of the low incidence of intraoperative awareness in an unselected surgical population undergoing surgeries with low risk of intraoperative awareness, an extremely large number of patients would be needed to determine the effects of BIS on awareness (Mashour 2012; O'Connor 2001). Questions regarding the utility of BIS, particularly to assess whether it is beneficial in reducing incidences of intraoperative awareness and improving early postoperative recovery are important in the clinical practice of anaesthesia. Additional studies have been published since the last update of this Cochrane Review (Punjasawadwong 2014), and therefore this review includes the most up‐to‐date evidence for the effectiveness of BIS.
Objectives
To assess the effectiveness of bispectral index (BIS) to reduce the risk of intraoperative awareness and early recovery times from general anaesthesia in adults undergoing surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) or quasi‐randomized trials comparing the use of the bispectral index (BIS) with either clinical signs or end‐tidal anaesthetic gas (ETAG) as the standard practice in the titration of anaesthetic agents, regardless of the language of publication of the articles.
We did not include studies with publications that were retracted from journals. And we excluded articles that were only available as abstracts if they were published early than 2005.
Types of participants
We included men and women over 18 years of age undergoing any type of surgery under general anaesthesia. We included participants who were selected because they were at high risk of intraoperative awareness (using any criteria specified by the study authors), and participants who were unselected, or for whom the study investigators did not specify risk of awareness.
Types of interventions
We included studies with at least two arms, which used BIS to guide the dose of an intravenous anaesthetic, a hypnotic, or a volatile anaesthetic and compared it with standard practice, which was either clinical signs or ETAG to guide the anaesthetic dose.
Therefore, the review included the following two comparison groups.
-
BIS‐guided anaesthesia versus clinical signs‐guided anaesthesia.
-
BIS‐guided anaesthesia versus ETAG‐guided anaesthesia
We included only studies in which investigators aimed to evaluate the effectiveness of BIS for its role in monitoring intraoperative depth of anaesthesia or potential improvements in early recovery times from anaesthesia.
Types of outcome measures
We included fewer outcomes in the updated review (see Differences between protocol and review). For the primary outcome, we included any events (or lack of events) which were described using the term 'intraoperative awareness', regardless of the time of measure, whether formal collection tools were used or whether reported events were subject to an adjudication process.
in the event that study authors differentiated data for intraoperative awareness as definite or confirmed awareness and possible awareness, we included only data in the analysis that were defined as confirmed or definite awareness.
Primary outcomes
-
Occurrence of intraoperative awareness
Secondary outcomes
-
Time to eye opening
-
Time to orientation
-
Time to discharge from the postanaesthesia care unit (PACU)
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). We applied no restrictions to language or publication status. We sourced the following databases for relevant trials:
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2019; Issue 3)
-
MEDLINE (Ovid SP: 1946 to 26 March 2019)
-
Embase (Ovid SP; 1974 to 26 March 2019)
-
Web of Science (SCI‐EXPANDED; 1900 to 26 March 2019)
We developed a subject‐specific search strategy in MEDLINE and other listed databases. The search strategy was developed in consultation with the Information Specialist for the Cochrane Anaesthesia Review Group. Search strategies can be found in: Appendix 1; Appendix 2; Appendix 3; Appendix 4.
We searched the following clinical trial registers for ongoing and unpublished trials.
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/; on 20 June 2019)
-
ClinicalTrials/gov (www.clinicaltrials.gov; on 7 June 2019)
Searching other resources
We carried out citation searching of identified included studies published since 2013 in Web of Science on 10 June 2019 (apps.webofknowledge.com). We conducted a search of grey literature using Opengrey on 20 June 2019 (www.opengrey.eu/). In addition, we scanned reference lists of relevant systematic reviews which were published since 2010.
Data collection and analysis
Two review authors (SL, and MP or LF) independently selected studies and extracted data from new included studies. We compared decisions at each stage. In cases of disagreement, we reassessed the respective studies to reach consensus.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence software to screen results of the search of titles and abstracts and identify potentially relevant studies (Covidence 2019). We sourced the full texts of all potentially relevant studies and considered whether they met the inclusion criteria (see Criteria for considering studies for this review). We reviewed abstracts at this stage and included these in the review only if they provided sufficient information and relevant results that included denominator figures for the intervention and control groups. Because of changes made to the review inclusion criteria (see Differences between protocol and review), we re‐evaluated all studies previously included in the review.
We recorded the number of papers retrieved at each stage and reported this information using a PRISMA flow chart (Figure 1). We did not report details of all studies excluded during the evaluation of full‐text articles; we reported in the review brief details of only closely related but excluded articles.
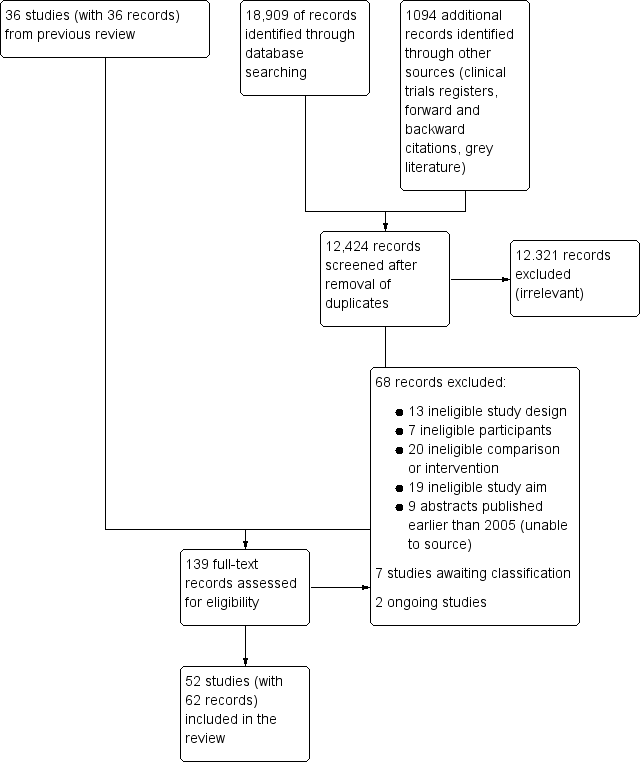
Study flow diagram. Search conducted in March 2019.
Data extraction and management
We used a data extraction form to collect information and outcome data from studies (Appendix 5). We collected the following information.
-
Methods: type of study design, setting; dates of study; funding sources and study author declarations of interest.
-
Participants: number randomized to each group; number of losses; number analysed in each group; baseline characteristics (age, gender, American Society of Anesthesiologists (ASA) physical status or other measure of health status; body mass index (BMI); weight; height; type of surgery; and duration of anaesthesia).
-
Intervention: details of BIS target values; details of control group; anaesthetic agents; experience of anaesthetist.
-
Outcomes: data for all reported review outcomes to include study author definitions, measurement tools and time points.
We considered the applicability of information from individual studies and generalizability of the data to our intended study population.
In multi‐arm studies, we did not collect data on any groups that were not eligible for inclusion in the review.
Assessment of risk of bias in included studies
We assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We considered the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants, personnel, and outcomes assessors (performance and detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective outcome reporting (reporting bias).
-
Other risks of bias.
For each domain, two review authors (SL, and MP or LF) judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using three measures ‐ high, low, or unclear risk of bias. We recorded this in 'Risk of bias' tables and presented summary 'Risk of bias' figures (Figure 2; Figure 3).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Blank spaces indicate that we did not conduct 'Risk of bias' assessments because studies did not report review outcomes.
Measures of treatment effect
We collected dichotomous data for intraoperative awareness. We collected continuous data for recovery outcomes, which were time to eye opening, time to orientation, and time to discharge from the postanaesthesia care unit (PACU).
We reported dichotomous data as Peto odds ratios (OR) to compare groups and continuous data as a mean difference (MD). We reported 95% confidence intervals (CIs).
Unit of analysis issues
The review included multi‐arm studies, in which more than one type of anaesthesia was included as separate study groups, or in which comparison groups included a clinical signs group and an ETAG‐guided group.
For multi‐arm studies that included study groups with different types of anaesthesia, we combined data for these groups for dichotomous data (occurrence of intraoperative awareness), and we selected the anaesthetic group which had the most conservative result for continuous data (recovery times). In subgroup analysis, we included each group separately according to type of anaesthetic agent.
We reported data separately for different comparison groups, and therefore there was no unit of analysis considerations for multi‐arm studies that included study groups with clinical signs and ETAG‐guided anaesthesia.
Dealing with missing data
We did not re‐include missing data by using imputation methods; we used the number of analysed participants as reported by study authors. In the previous version of the review (Punjasawadwong 2014), we contacted study authors to obtain missing data; owing to time limitations in the preparation of this update, we did not contact study authors in the case of missing data.
We did not recalculate the standard deviations (SDs) for studies reporting continuous outcomes as medians with ranges or interquartile ranges (IQR). In this update, we reported data with median values in the notes section of the relevant table in Characteristics of included studies.
Assessment of heterogeneity
We assessed whether evidence of inconsistency was apparent in our results by considering heterogeneity. We assessed clinical and methodological heterogeneity by comparing similarities in our included studies between study designs, participants, interventions, and outcomes, and used the data collected from the full‐text reports (as stated in Data collection and analysis). We explored clinical and methodological heterogeneity through subgroup analysis. We assessed statistical heterogeneity by calculating the Chi² test or the I² statistic and judged any heterogeneity above an I² value of 50% and a Chi² P value less than or equal to 0.05 to indicate moderate to substantial statistical heterogeneity (Higgins 2011).
In addition to looking at statistical results, we considered point estimates and overlap of CIs. If CIs overlap, then results are more consistent. However, combined studies may show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source the published protocols for each of our included studies by using the results from our clinical trial register searches. We compared published protocols with published study results to assess the risk of selective reporting bias. In addition, we appraised reporting bias through visual assessment of funnel plots for outcomes for which we found more than 10 studies (Egger 1997). We only included figures of funnel plots in the review in which we identified possible reporting bias from visual inspection.
Data synthesis
We presented a statistical summary of treatment effects in the absence of significant clinical or methodological heterogeneity. We used the statistical calculator in ReMan 5 to perform meta‐analysis (Review Manager 14).
For the occurrence of intraoperative awareness, we used the Peto method to pool ORs across studies; this method accounted for the extremely rare events for this outcome. For continuous outcomes, we calculated the mean difference (MD); we used a random‐effects model to account for potential variability in types of surgeries between studies (Borenstein 2010).
We calculated CIs at 95% and used a P value less than or equal to 0.05 to judge whether a result was statistically significant. We considered imprecision in the results of analyses by assessing the CI around an effects measure; a wide CI would suggest a higher level of imprecision in our results. A small number of studies may also reduce precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
In subgroup analysis, we evaluated the type of anaesthetic agent which was used in the maintenance of anaesthesia (propofol, desflurane, isoflurane, sevoflurane). We conducted subgroup analysis for outcomes in which we found more than 10 studies (Higgins 2011).
Sensitivity analysis
We explored the potential effect of decisions made as part of the review process. In each sensitivity analysis, we compared the effect estimate with the main analysis. We reported these effect estimates only if they indicated a difference in interpretation of the effect. We performed the following sensitivity analyses.
-
We excluded studies that we judged to have a high or unclear risk of selection bias (for sequence generation).
-
We excluded studies that we judged to have a high risk of attrition bias because of a loss of more than 10% participants, or loss which was unbalanced between groups, or which was unexplained.
We found a large number of studies that reported intraoperative awareness with zero events in both arms. We used the Peto OR in the primary analysis of intraoperative awareness but made a post‐hoc decision to evaluate the effect of these zero‐event data by using alternative statistical methods. In sensitivity analysis, we used risk ratios (RR) with both a Mantel‐Haenszel and Inverse Variance method; we also evaluated these methods using a fixed‐effect and a random‐effects model. We did not use a risk difference method, because this method is unsuitable when events are rare (Bradburn 2007).
'Summary of findings' table and GRADE
One review author (SL) used the GRADE system to assess the certainty of the body of evidence associated with the following outcomes (Guyatt 2008).
-
Occurrence of intraoperative awareness
-
Time to eye opening
-
Time to orientation
-
Time to discharge from the PACU
The GRADE approach appraises the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and risk of publication bias.
We constructed 'Summary of findings' tables using GRADE profiler software for two comparisons (gradepro.org).
-
BIS‐guided anaesthesia versus clinical signs‐guided anaesthesia
-
BIS‐guided anaesthesia versus ETAG‐guided anaesthesia
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
Results of the search
After the removal of duplicates from the search results, we screened 12,424 titles and abstracts, which included forward and backward citation searches, clinical trials registers and grey literature. We re‐evaluated previously included studies alongside 103 articles sourced as full‐text reports and, therefore, assessed eligibility of 139 articles. See Figure 1.
Included studies
See Characteristics of included studies.
We included 52 studies with 41,331 participants (Ahmad 2003; Alimian 2016; Anez 2001; Arbabpour 2015; Assare 2002; Avidan 2008; Avidan 2011; Başar 2003; Boztuğ 2006; Bresil 2013; Bruhn 2005; Ellerkmann 2010; Fakhr 2014; Gan 1997; Georgakis 2000; Guo 2015; Ibraheim 2008; Jain 2016; Kabukcu 2012; Kamal 2009; Kamali 2017a; Karaca 2014; Khoshrang 2016; Kim 2003; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Masuda 2002; Morimoto 2002; Mozafari 2014; Muralidhar 2008; Myles 2004; Nelskyla 2001; Paventi 2001; Payas 2013; Persec 2012; Puri 2003; Rahul 2015; Raksakietisak 2016; Recart 2003; Savli 2005; Shafiq 2012; Siampalioti 2015; Song 1997; Sudhakaran 2018; Tufano 2000; White 2004; Wong 2002; Zhang 2011; Zhang 2016; Zohar 2006). Two studies were quasi‐randomized (Anez 2001; Shafiq 2012); the remaining studies were randomized controlled trials (RCTs). We included three studies for which we could only source the abstract and this limited the details of study characteristics that we were able to extract (Georgakis 2000; Kabukcu 2012; Raksakietisak 2016). We sourced the full text of all the remaining studies. We did not seek translation of five studies (Arbabpour 2015; Kim 2003; Masuda 2002; Morimoto 2002; Savli 2005), and details of study characteristics and outcome data in these studies were limited to data in the English abstract or tables within the main text.
This review includes 22 new studies (Alimian 2016; Arbabpour 2015; Bresil 2013; Fakhr 2014; Georgakis 2000; Guo 2015; Jain 2016; Kabukcu 2012; Kamali 2017a; Karaca 2014; Khoshrang 2016; Kim 2003; Mozafari 2014; Payas 2013; Persec 2012; Rahul 2015; Raksakietisak 2016; Savli 2005; Shafiq 2012; Siampalioti 2015; Sudhakaran 2018; Zhang 2016). The remaining studies were previously included in Punjasawadwong 2014.
Study population
All studies included participants undergoing surgery under general anaesthesia. In one study, participants were undergoing procedures using regional anaesthesia combined with general anaesthesia (Ellerkmann 2010).
General anaesthesia was maintained by propofol, or by sevoflurane, desflurane or isoflurane, and in one study, halothane was used (Jain 2016). Only three studies used laryngeal masks (LMA); these were during surgical procedures with a duration of less than one hour (Anez 2001; Assare 2002; Zohar 2006).
Five studies included more than one comparison group according to the type of anaesthetic agent (Luginbuhl 2003; Muralidhar 2008; Siampalioti 2015; Song 1997; Tufano 2000). The type of anaesthetic agents was at the discretion of the attending anaesthetists in four studies (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004); in Avidan 2008 and Avidan 2011 agents were only volatile. We were uncertain of the type of anaesthetic agent in Arbabpour 2015.
Three studies recruited only participants who were at high risk of intraoperative awareness (Avidan 2008; Avidan 2011; Myles 2004), whilst two studies specifically recruited an unselected participant group (Mashour 2012; Zhang 2011). In general, we found most studies did not report whether the population group was at risk. However, in the findings of the 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia (NAP5 2014), some factors may increase risk of intraoperative awareness: female gender; age (younger adults); obesity; seniority of anaesthetists (junior trainees); history of accidental awareness; out of hours operating; emergencies; type of surgery (obstetric, cardiac, thoracic, neurosurgery); and use of neuromuscular blockade. We collected information on studies that had at least one risk factor and reported this information in Appendix 6. However, we did not think this information alone was sufficient to categorise these studies as having participants at high risk of awareness.
Study setting
All studies were conducted in a hospital setting and seven were multi‐centre studies (Avidan 2008; Avidan 2011; Bruhn 2005; Mashour 2012; Myles 2004; Rahul 2015; Zhang 2011).
Interventions and comparisons
All studies included an intervention group in which a BIS monitor was used to guide anaesthesia. In six studies, this was compared with ETAG‐guided anaesthesia (Avidan 2008; Avidan 2011; Jain 2016; Mashour 2012; Muralidhar 2008; Sudhakaran 2018); one of these studies included comparisons with both ETAG‐guided anaesthesia, and anaesthesia guided by clinical signs (Sudhakaran 2018). One study described that the control group participants had anaesthesia guided by both clinical signs and end‐tidal concentration in the form of minimum alveolar concentration;(MAC) (Shafiq 2012); we categorised this study as belonging to our first comparison group (BIS versus clinical signs). The remaining studies included comparisons with only clinical signs. We could not be certain of other monitoring methods used in the included studies; our information was limited to the details reported in published reports. Therefore, it is possible that some studies in which anaesthesia was guided by clinical signs may also have been guided by ETAG. Similarly, because it was not always clearly reported, we could not be certain whether audible alarms were used for ETAG‐guided anaesthesia.
The large number of participants in this review was dominated by five large studies; two of these studies compared BIS with clinical signs and in total included 7772 participants (Myles 2004; Zhang 2011), and three studies compared BIS with ETAG and had 29,642 participants (Avidan 2008; Avidan 2011; Mashour 2012).
Most studies defined clinical signs as using parameters such as heart rate or systolic blood pressure. One study used a standardised scoring system (PRST: systolic blood pressure, heart rate, sweating and tears) to evaluate depth of anaesthesia (Rahul 2015).
BIS target values varied in each study but all were within a range of 40 to 60.
Only four studies reported the experience of the attending anaesthetist, which was described as a quote: "experienced anaesthesiologist" (Ellerkmann 2010), "supervised by a faculty anaesthetist" (Gan 1997), greater than one year (Başar 2003), and greater than five years (Wong 2002). The remaining studies did not describe the experience of the attending anaesthetist.
Outcomes
Five studies did not report outcomes relevant to the review (Ahmad 2003; Bresil 2013; Jain 2016; Payas 2013; Raksakietisak 2016). The remaining studies reported measured at least one of the review outcomes.
For the measurement of intraoperative awareness, we noted that studies did not report a clear definition of intraoperative awareness. In addition, the time point of measurement varied between studies and included one or more measurement taken from as early as the time in the PACU to several days, and up to 30 days, after anaesthesia. In addition, the methods or tools used to collect the information were not always reported, and were described in limited terms such as 'an interview', or in more comprehensive terms (for example, by including a specific standardised questionnaire such as structured modified Brice questionnaire (Brice 1970).
Funding
Five studies reported financial support or included study authors who had received fees (for example for consultancy work), from companies involved in the manufacture of BIS monitors (Ahmad 2003; Bruhn 2005; Gan 1997; Myles 2004; Wong 2002). Twenty‐one studies were either not funded or funded from independent sources (Alimian 2016; Avidan 2008; Avidan 2011; Bresil 2013; Fakhr 2014; Kamali 2017a; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Mozafari 2014; Nelskyla 2001; Payas 2013; Persec 2012; Rahul 2015; Raksakietisak 2016; Recart 2003; Sudhakaran 2018; White 2004; Zohar 2006). We could not ascertain funding sources from the remaining studies.
Excluded studies
We excluded 68 studies during full‐text review and the reasons for these exclusions are described in Figure 1.
In order to improve usability of the review, we have only reported details of 19 of these 68 excluded studies in the review (Aceto 2015; Aimé 2006; Chan 2013; Chiu 2007; Hachero 2001; Kamali 2017b; Karwacki 2014; Kaval 2015; Kerssens 2009; Nitzschke 2014; Panagopoulou 2000; Quesada 2016; Radtke 2013; Rüsch 2018; Samarkandi 2004; Shahrbazi 2008; Struys 2001; Vretzakis 2005; Zhou 2018). We excluded these 19 studies because their study aims did not match the review aim. See Characteristics of excluded studies.
Of these 19 studies, five studies were included in the previous version of the review (Aimé 2006; Chiu 2007; Hachero 2001; Samarkandi 2004; Struys 2001); the decision to exclude these five studies was due to a change in the review criteria (see Differences between protocol and review).
We did not include in the review studies that were excluded during previous versions of the review (Punjasawadwong 2007; Punjasawadwong 2014).
Studies awaiting classification
Six studies are awaiting classification (Aksun 2007; Croci 2014; CTRI/2018/03/012457; Golmohammadi 2014; Jeong 2002; Qu 2011). We were unable to source the full text of two studies from current library sources and the abstracts contained insufficient information to assess eligibility (Aksun 2007; Qu 2011). Croci 2014 was published only as an abstract and, similarly this contained insufficient information to assess eligibility. We found one completed study in a clinical trial register but the clinical trial register did not include study results and therefore, we await publication of the full report (CTRI/2018/03/012457). Two studies require translation to assess eligibility (Golmohammadi 2014; Jeong 2002).
Ongoing studies
We found two ongoing studies (Martins 2013; NCT03571945). One study is a protocol, previously included in the review as 'awaiting classification' (Martins 2013); because the status is recorded in the clinical trial register as unknown we have assumed that it is still ongoing. This study compares BIS with clinical signs in people undergoing coronary artery bypass graft. The other ongoing and multi‐centre study aims to recruit 2000 participants and will compare BIS‐guided anaesthesia with anaesthesia guided by ETAG in participants undergoing elective surgery lasting more than 30 minutes (NCT03571945).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3.
We only conducted 'Risk of bias' assessments on studies that measured or reported review outcomes. Blank spaces in Figure 2 and Figure 3 indicate that these studies did not measure or report review outcomes.
Allocation
Two quasi‐randomized studies were at high risk of selection bias because of methods used for sequence generation and allocation concealment (Anez 2001; Shafiq 2012). In addition, we noted differences between the number of participants allocated to each group and differences between groups in one study which we also judged to have a high risk of bias for sequence generation (Zhang 2011).
Twenty‐two studies reported insufficient detail of methods to randomize participants and we judged these studies to have an unclear risk of bias for sequence generation (Alimian 2016; Arbabpour 2015; Assare 2002; Başar 2003; Georgakis 2000; Ibraheim 2008; Kamal 2009; Kamali 2017a; Karaca 2014; Kim 2003; Masuda 2002; Morimoto 2002; Muralidhar 2008; Nelskyla 2001; Paventi 2001; Rahul 2015; Recart 2003; Savli 2005; Tufano 2000; White 2004; Zhang 2016; Zohar 2006). The remaining studies reported adequate methods to randomize participants and we judged these to have a low risk of bias for sequence generation.
Four studies reported adequate methods to conceal allocation and we judged these to have a low risk of bias (Avidan 2011; Boztuğ 2006; Gan 1997; Myles 2004). The remaining studies did not report sufficient methods to conceal allocation to enable us to judge the risk of bias for allocation concealment.
Blinding
Whilst some studies reported that participants were blinded to group allocation, we based our judgement for risk of performance bias according to whether relevant personnel were blinded; this was because we expected that knowledge of group assignment was unlikely to influence participants. In all studies, it was not feasible to blind anaesthetists to the two methods used to guide anaesthesia in this review. Therefore we judged all studies to have a high risk of performance bias. As well as the risk that anaesthetists may alter their methods of providing anaesthesia depending on the group to which they are allocated, this type of study has a performance bias risk related to 'learning contamination' bias. Learning contamination bias is the risk of changing clinical practice in the parallel control or unmonitored group by using the information from the BIS group (Roizen 1994).
Intraoperative awareness is a self‐reported outcome; however, we did not consider the blinding of participants to influence the measurement of intraoperative awareness. We expected that most studies used an anaesthetist who interviewed the participants postoperatively (either informally or using a standardised questionnaire) and, in this review, we classed the interviewer as the outcome assessor as the terms used to ask questionnaires may be subject to bias. Nineteen studies reported that outcome assessors were blinded (Avidan 2008; Avidan 2011; Bruhn 2005; Fakhr 2014; Gan 1997; Kamali 2017a; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mashour 2012; Myles 2004; Paventi 2001; Recart 2003; Siampalioti 2015; White 2004; Wong 2002; Zhang 2011; Zohar 2006). The remaining studies did not report whether outcome assessors were blinded.
Incomplete outcome data
In four studies, we noted a loss of more than 10% participants or that the loss of participants was imbalanced between groups (Gan 1997; Mashour 2012; Morimoto 2002; Zhang 2011); we judged these studies to have a high risk of attrition bias. We could not be certain whether all participants were accounted for in two studies; therefore, we judged these studies to have an unclear risk of attrition bias (Arbabpour 2015; Fakhr 2014). The remaining studies had no apparent participant loss, or the loss of participants was fewer than 10%, and we judged these studies to have a low risk of attrition bias.
Selective reporting
Two studies were prospectively registered with a clinical trial register (Avidan 2011; Mashour 2012). We judged Mashour 2012 to have a low risk of reporting bias because outcomes in the published report were the same as those in the clinical trial register documents. We could not be certain whether a risk of reporting bias was evident in Avidan 2011 because several outcomes listed in the clinical trial register documents were not included in the published report.
Four studies were retrospectively registered with a clinical trial register (Alimian 2016; Avidan 2008; Khoshrang 2016; Persec 2012), and we could not be certain whether registration was prospective or retrospective in one study (Siampalioti 2015); it was not feasible to use these documents to effectively assess risk of reporting bias. The remaining studies did not report clinical trial registration or study protocol publication, and therefore, it was similarly not feasible to effectively assess risk of reporting bias.
Other potential sources of bias
We were unable to assess risks of other sources of bias in those studies for which we did not seek translation (Arbabpour 2015; Kim 2003; Masuda 2002; Morimoto 2002; Savli 2005), or in studies that were reported only as abstracts (Georgakis 2000; Kabukcu 2012); therefore, we used an unclear judgement for other risks of bias. Similarly, we used an unclear judgement in two studies in which study characteristics were poorly reported (for example, with no baseline characteristics table) (Kamali 2017a; Tufano 2000).
We noted some important baseline imbalances between groups in three studies and we could not be certain of the influence of these imbalances on the outcome data (Avidan 2008; Persec 2012; Rahul 2015).
Effects of interventions
See: Summary of findings 1 Bispectral index compared to clinical signs for improving intraoperative awareness and early postoperative recovery; Summary of findings 2 BIS compared to ETAG for improving intraoperative awareness and early postoperative recovery
1. BIS versus clinical signs
Occurrence of intraoperative awareness
Twenty‐nine studies measured intraoperative awareness (Anez 2001; Assare 2002; Bruhn 2005; Ellerkmann 2010; Fakhr 2014; Guo 2015; Ibraheim 2008; Kabukcu 2012; Kamal 2009; Kamali 2017a; Karaca 2014; Kim 2003; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mozafari 2014; Myles 2004; Paventi 2001; Persec 2012; Puri 2003; Rahul 2015; Recart 2003; Song 1997; Sudhakaran 2018; White 2004; Wong 2002; Zhang 2011; Zhang 2016; Zohar 2006). In two studies, data were measured but were unclearly reported or were not reported (Fakhr 2014; Paventi 2001).
Events were rare, and only five of these studies included incidences of awareness (Kamali 2017a; Mozafari 2014; Myles 2004; Puri 2003; Zhang 2011). Two of these studies were large, multi‐centre trials ‐ one included only participants at high risk of intraoperative awareness (Myles 2004), and one included an unselected population (Zhang 2011). Four of these five studies used a structured questionnaire to collect data on awareness, and one study reported that participants were interviewed (with no additional details). Two of these five studies used an adjudication process to judge whether descriptions of awareness were possible or confirmed, and we included data only for confirmed reports of awareness.
We found that BIS‐guided anaesthesia may reduce the risk of intraoperative awareness in a surgical population that were at unselected or at high risk of awareness (Peto odds ratio (OR) 0.36, 95% confidence interval (CI) 0.21 to 0.60; I2 = 61%; 27 studies; 9765 participants; low‐certainty evidence; Analysis 1.1). We used GRADE to downgrade the certainty of the evidence by two levels. We downgraded by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies, it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. We downgraded by one level for imprecision; whilst we noted a narrow CI, the effect was dominated by two large trials (with two different populations selected according to the likelihood of intraoperative awareness) and we found many studies with zero events in both arms. We conducted sensitivity analysis to explore alternative statistical models to account for zero events in both arms as well as rare events and found more conservative estimates when we used a random‐effects model, thus reducing our certainty in the estimate. See summary of findings Table 1.
Recovery time to eye opening
Twenty‐seven studies measured time to eye opening (Anez 2001; Başar 2003; Boztuğ 2006; Bruhn 2005; Ellerkmann 2010; Gan 1997; Georgakis 2000; Ibraheim 2008; Kamal 2009; Karaca 2014; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Masuda 2002; Morimoto 2002; Myles 2004; Nelskyla 2001; Paventi 2001; Puri 2003; Recart 2003; Savli 2005; Shafiq 2012; Siampalioti 2015; Tufano 2000; White 2004; Wong 2002; Zohar 2006). In two studies, time was measured but not reported (Georgakis 2000; Zohar 2006). We did not combine data in analysis from studies that reported time to eye opening as median values (Myles 2004; Paventi 2001; Tufano 2000).
For Siampalioti 2015, a multi‐arm study, we included data only for participants in which sevoflurane was used for anaesthesia; the effect for these participants showed a more conservative estimate.
We found that BIS‐guided anaesthesia may reduce time to eye opening by mean difference (MD)1.78 minutes (95% CI ‐2.53 to ‐1.03 minutes; I2 = 83%; 22 studies; 1494 participants; low‐certainty evidence; Analysis 1.2). We used GRADE to downgrade the certainty of the evidence by one level for inconsistency owing to the substantial statistical heterogeneity in this effect, and by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies, it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See summary of findings Table 1.
Recovery time to orientation
Eight studies measured time to orientation (Fakhr 2014; Kamal 2009; Nelskyla 2001; Paventi 2001; Savli 2005; Song 1997; White 2004; Wong 2002). In Fakhr 2014, data were measured but not reported. We did not combine data in analysis from studies that reported time to orientation as median values (Paventi 2001). In Song 1997, data were included separately according to the type of volatile anaesthetic (sevoflurane and desflurane); we included data for participants who were given sevoflurane as this presented a more conservative finding.
We found that BIS‐guided anaesthesia may reduce time to orientation by MD 3.18 minutes (95% CI ‐4.03 to ‐2.33 minutes; I2 = 41%; 6 studies; 273 participants; low‐certainty evidence; Analysis 1.3). We used GRADE to downgrade the certainty of the evidence by one level for imprecision as the evidence was from few studies with few participants, and by one level for study limitation owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See summary of findings Table 1.
Time to discharge from the postanaesthesia care unit (PACU)
Seventeen studies measured time to discharge from the PACU (Alimian 2016; Anez 2001; Arbabpour 2015; Boztuğ 2006; Bruhn 2005; Fakhr 2014; Gan 1997; Kamal 2009; Khoshrang 2016; Masuda 2002; Morimoto 2002; Myles 2004; Recart 2003; Song 1997; White 2004; Wong 2002; Zohar 2006). We did not include data for one study in which time was reported as median values (Myles 2004), nor for three studies in which data were not reported or were reported unclearly (Arbabpour 2015; Fakhr 2014; Khoshrang 2016). In Song 1997, data were included separately according to the type of volatile anaesthetic (sevoflurane and desflurane); we included data for participants who were given sevoflurane as this presented a more conservative finding.
We found that BIS‐guided anaesthesia may reduce time to discharge from the PACU by MD 6.86 minutes (95% CI ‐11.72 to ‐2.00; I2 = 79%; 13 studies; 930 participants; low‐certainty evidence; Analysis 1.4). We used GRADE to downgrade the certainty of the evidence by one level for inconsistency owing to the substantial statistical heterogeneity in this effect, and by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See summary of findings Table 1.
From visual inspection of a funnel plot for these outcome data, we noted the possibility of publication bias (Figure 4).

Funnel plot of comparison: 1 BIS versus clinical sides, outcome: 1.4 Time to discharge from the PACU (minutes).
Subgroup analysis
Type of agent used to guide anaesthesia
We did not include Myles 2004 in this subgroup analysis because the type of anaesthetic was at the discretion of the attending anaesthetists, and therefore may include all types.
Occurrence of intraoperative awareness: the effect for propofol was consistent with our primary analysis, showing a reduction in intraoperative awareness when propofol was guided by BIS (Peto OR 0.24, 95% CI 0.10 to 0.60; I2 = 0%; 10 studies; 5784 participants). The evidence for isoflurane was inconsistent and showed no evidence of a reduction in intraoperative awareness when isoflurane was guided by BIS (Peto OR 0.58, 95% CI 0.26 to 1.28; I2 = 74%; 4 studies; 637 participants). None of the studies in which desflurane or sevoflurane was given reported events. Analysis 2.1.
Recovery time to eye opening: this subgroup analysis included both propofol and sevoflurane groups in Siampalioti 2015. The effect for time to eye opening was consistent with our primary analysis, showing a reduction in time to eye opening for BIS‐guided anaesthesia with: propofol (MD ‐2.13 minutes, 95% CI ‐3.82 to ‐0.43 minutes; I2 = 89%; 8 studies; 680 participants); isoflurane (MD ‐2.45 minutes, 95% CI ‐4.80 to ‐0.09 minutes; I2 = 73%; 3 studies; 150 participants); and sevoflurane (MD ‐1.52 minutes, 95% CI ‐2.60 to ‐0.44 minutes; I2 = 83%; 8 studies; 392 participants). We found no evidence of a difference in time to eye opening when desflurane was used (MD ‐0.51 minutes, 95% CI ‐1.44 to 0.42 minutes; I2 = 38%; 4 studies; 322 participants). Analysis 2.2.
Recovery time to orientation: we did not conduct subgroup analysis for this outcome because the primary analysis included too few studies.
Recovery time to discharge from the PACU: this subgroup analysis included both sevoflurane and desflurane anaesthetic groups in Song 1997. We noted differences between subgroups in this analysis (Chi² = 57.54, df = 13, P < 0.00001). Whilst studies in which propofol was used showed a reduction in time to discharge from the PACU which was consistent with the primary analysis (MD ‐5.42 minutes, 95% CI ‐9.36 to ‐1.48 minutes; I2 = 0%; 4 studies; 398 participants), we found that the evidence when volatile agents were used was inconsistent: desflurane (MD ‐14.76 minutes, 95% CI ‐29.61 to 0.09 minutes; I2 = 88%; 4 studies; 272 participants); isoflurane (MD ‐14.00 minutes, 95% CI ‐34.12 to 6.12 minutes; 1 study; 60 participants); sevoflurane (MD ‐5.99 minutes, 95% CI ‐13.34 to 1.36 minutes; I2 = 83%; 5 studies; 230 participants). Analysis 2.4.
Sensitivity analysis
Unclear or high risk of selection bias for sequence generation
-
Occurrence of intraoperative awareness: only 14 studies included in the primary analysis had a low risk of selection bias (Bruhn 2005; Ellerkmann 2010; Guo 2015; Kabukcu 2012; Kreuer 2003; Kreuer 2005; Luginbuhl 2003; Mozafari 2014; Myles 2004; Persec 2012; Puri 2003; Song 1997; Sudhakaran 2018; Wong 2002). When we excluded the remaining studies, analysis demonstrated no evidence of an effect (Peto OR 0.60 (95% CI 0.29 to 1.23; 14 studies; 3654 participants); however, we noted that this sensitivity analysis included only three studies with event data, of which one small study had findings which were inconsistent with other studies and which we could not explain (Mozafari 2014).
-
Time to eye opening: only 10 studies included in the primary analysis had a low risk of selection bias (Boztuğ 2006; Bruhn 2005; Ellerkmann 2010; Gan 1997; Khoshrang 2016; Kreuer 2003; Kreuer 2005; Puri 2003; Siampalioti 2015; Wong 2002). When the remaining studies were excluded, we found no difference in the interpretation of the effect.
-
Time to orientation: only two studies included in the primary analysis had a low risk of selection bias (Song 1997; Wong 2002). When we excluded the remaining studies, we found no difference in the interpretation of the effect.
-
Time to discharge from the PACU: only five studies included in the primary analysis had a low risk of selection bias (Boztuğ 2006; Bruhn 2005; Gan 1997; Song 1997; Wong 2002). When we excluded the remaining studies, we found no evidence of an effect (MD ‐1.62 minutes (95% CI ‐5.96 to 2.72); 519 participants).
Unclear or high risk of attrition bias
-
Occurrence of intraoperative awareness: we excluded one study with a high risk of attrition bias (Zhang 2011). This did not alter the interpretation of the effect.
-
Time to eye opening: we excluded two studies with a high risk of attrition bias (Gan 1997; Morimoto 2002). This did not alter the interpretation of the effect.
-
Time to orientation: no studies included in the primary analysis had an unclear or high risk of attrition bias.
-
Time to discharge from the PACU: we excluded two studies with a high risk of attrition bias (Gan 1997; Morimoto 2002). This did not alter the interpretation of the effect.
Zero event data
Analysis of the occurrence of intraoperative awareness included 22 studies with zero events in both arms. We evaluated alternative statistical tools and methods using the calculator in Review Manager 14. We report the effect of these sensitivity analyses in Appendix 7. Based on a fixed‐effect model, using a RR with either Mantel‐Haenszel or Inverse Variance did not alter the interpretation of the effect. Although we evaluated the effect using a random‐effects model, this model is less appropriate for evidence of rare events (Higgins 2011). Based on a random‐effects model, we found a more conservative estimate which indicated no evidence of a difference in intraoperative awareness when the Mantel‐Haenszel method was used (RR 0.32, 95% CI 0.10 to 1.01; I2 = 62%), and when the Inverse Variance method was used (RR 0.32, 95% CI 0.10 to 1.00; I2 = 60%).
2. BIS versus ETAG
Occurrence of intraoperative awareness
Five studies measured and reported intraoperative awareness (Avidan 2008; Avidan 2011; Mashour 2012; Muralidhar 2008; Sudhakaran 2018). This surgical population included participants who were at high risk of intraoperative awareness (Avidan 2008; Avidan 2011), who were unselected (Mashour 2012), or for whom risk of awareness was not specified (Muralidhar 2008; Sudhakaran 2018).
Events were rare and only three of these studies included incidences of awareness. Of the three studies with event data, all used a structured Brice questionnaire to collect data on awareness, and all used an adjudication process to categorise incidences of awareness as possible or definite; we included in analysis data for definite awareness.
We found no evidence of a difference in incidences of intraoperative awareness according to whether anaesthesia was guided by BIS or by ETAG (Peto OR 1.13, 95% CI 0.56 to 2.26; I2 = 37%; 26,572 participants; low‐certainty evidence; Analysis 3.1). We used GRADE to downgrade the certainty of the evidence by one level for imprecision; despite a large number of participants, events were very rare (one per 1000 in the intervention and the comparison group) and the confidence interval for this effect was wide. In addition, we downgraded by one level for study limitations owing to the inclusion of some studies with unclear and high risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. See summary of findings Table 2.
Recovery time to eye opening
No studies reported time to eye opening.
Recovery time to orientation
No studies reported time to orientation.
Time to discharge from the PACU
One study measured and reported time to discharge from the PACU (Mashour 2012). Study authors reported a reduction in readiness for discharge from the PACU when BIS‐guided anaesthesia was used, with a median interquartile range (IQR)) duration of 95 minutes (64 to 138 minutes) for participants having BIS‐guided anaesthesia (6076 participants) compared to a median (IQR) duration of 98 minutes (66 to 140 minutes) for participants having ETAG‐guided anaesthesia (9376 participants). We used GRADE to downgrade this evidence to low certainty. We downgraded by two levels for imprecision because the evidence was from one study in which the IQR of time spent in the PACU was wide in both groups.
Subgroup analysis
We did not conduct subgroup analysis for the comparison BIS versus ETAG because we found too few studies in the primary analysis.
Sensitivity analysis
Unclear or high risk of selection bias for sequence generation
Occurrence of intraoperative awareness: all studies in the primary had a low risk of selection bias (Mashour 2012).
Time to discharge from the PACU: data for this outcome were from a single study which we judged to have a low risk of selection bias (Mashour 2012).
Unclear or high risk of attrition bias
Occurrence of intraoperative awareness: we excluded one study with a high risk of attrition bias (Mashour 2012). This did not alter the interpretation of the effect.
Time to discharge from the PACU: data for this outcome was from a single study which we judged to have a high risk of attrition bias (Mashour 2012).
Zero event data
Analysis of the occurrence of intraoperative awareness included two studies with zero events in both arms. We evaluated alternative statistical tools and methods using the calculator in Review Manager 14. We report the effect of these sensitivity analyses in Appendix 7. We found that alternative statistical tools did not alter the interpretation of the effect for this outcome.
Discussion
Summary of main results
We found 52 studies that compared bispectral index (BIS)‐guided anaesthesia with either clinical signs or end‐tidal anaesthetic gas (ETAG). Studies included participants undergoing any type of surgery under general anaesthesia. Three studies included only participants who were at high risk of intraoperative awareness, and two studies included only unselected participants. Whilst some studies included participants who had one or more factor that may increase the risk of intraoperative awareness (according to NAP5 2014), we did not categorise these studies as at high risk of intraoperative awareness.
We included two comparison groups in the review: BIS‐guided anaesthesia compared with anaesthesia guided by clinical signs, and BIS‐guided anaesthesia compared with anaesthesia guided by ETAG.
We found low‐certainty evidence that BIS‐guided anaesthesia compared to clinical signs may reduce the incidence of intraoperative awareness. However, incidences of awareness were rare. Incidences, when BIS was used, were only six per 1000 fewer than in the clinical signs group. We found low‐certainty evidence that early recovery times may be reduced when BIS was used; the time to eye opening, to orientation, and to discharge from the PACU was shorter for all studies in which BIS was used.
For BIS‐guided anaesthesia compared to ETAG‐guided anaesthesia, we found no evidence of a difference in the incidence of intraoperative awareness. Again, we found few incidences of intraoperative awareness (1 per 1000 in both groups) and we judged this evidence to be low certainty. Only one study in which BIS was compared with ETAG‐guided anaesthesia measured the time to discharge from the PACU, and this study reported median values which showed a reduction in time to discharge from the PACU for BIS‐guided anaesthesia (low‐certainty evidence). No studies comparing BIS‐ to ETAG‐guided anaesthesia measured or reported the time to eye opening and the time to orientation.
Overall completeness and applicability of evidence
We identified 52 studies with 41,331 participants. All participants were undergoing surgery under general anaesthesia. Most studies did not specify whether participants were selected according to their risk of intraoperative awareness, and we noted that some characteristics of included participants and the methods used in their anaesthesia indicated at least one risk factor for awareness. These factors, identified in the most recent NAP5 audit (NAP5 2014), included female gender, obesity, type of surgery (obstetric, cardiac, thoracic, neurosurgery), and the use of neuromuscular blockade. Whilst experience of the anaesthetist may be relevant to the risk of intraoperative awareness, we found that this was poorly reported in studies, and no studies reported that attending anaesthetists in the trials had a junior level of experience.
Most studies compared BIS with clinical signs to monitor the depth of anaesthesia with only six studies comparing BIS with ETAG.
We attempted to account for differences between studies in terms of the type surgery by using a random‐effects model in the analysis of the recovery time points. However, we noted a moderate to substantial statistical heterogeneity in most of our primary analyses. We expected that this was inevitable because of the broad inclusion criteria regarding type of surgery. These differences in surgery type may increase the duration of general anaesthesia and the subsequent recovery times, and we believed that this statistical heterogeneity was unavoidable in the review.
Quality of the evidence
We used GRADE to downgrade the certainty of the evidence for all outcomes in this review to low. Whilst we found a large number of studies that compared BIS with anaesthesia guided by clinical signs, incidences of intraoperative awareness were so infrequent with most studies reporting no events in either group. We reported the effect estimate as Peto odds ratio (OR) which accounted for rare events, but when we used alternative statistical methods using random‐effects models, we found more conservative estimates, thus we believed the evidence was imprecise. Similarly, although evidence comparing BIS to ETAG, included a larger number of participants, events were from only three studies and were infrequent.
We also used GRADE to downgrade the certainty of the evidence owing to study limitations. We used the 'Risk of bias' tool to assess some studies as having an unclear risk of bias, often because these studies reported no information on which to base a confident judgement. This study design prohibits blinding of the attending anaesthetists and therefore, we judged all studies to have a high risk of performance bias. We expected that some anaesthetists who were assigned a BIS monitor may continue to base their judgements of a patient's depth of anaesthesia on standard clinical practices rather than BIS, or that they may alter their standard anaesthetic practice in other ways when using the BIS monitor.
We also noted that intraoperative awareness was often not clearly defined in studies and without a precise definition we could not be certain that the incidences (or lack of incidences) were comparable in the analyses. Similarly, the time points at which intraoperative awareness was measured (for example in the postanaesthesia care unit (PACU), or on the first, second, or third postoperative day, or up to 30 days postoperatively) were not comparable, nor the methods of data collection (for example, using a simple question or using a recognised data collection tool such as the Brice questionnaire, and whether reports of awareness were evaluated for being possible or definite).
Potential biases in the review process
We conducted a thorough search in the update and used two review authors to assess study eligibility, extract data, and assess risk of bias in included studies; therefore, we reduced potential bias in the review process.
In updating this review, we made minor changes to the review inclusion criteria. We decided to exclude studies that did not aim to address our review question. As a result of this decision, we excluded five previously included studies. As these five studies, and other similar studies identified during the search, did not address our review aims, it was not expected that this decision affected data for the relevant outcomes in the review. In addition, we re‐evaluated the previous review outcomes. In order to improve the focus of the review, we reduced the number of outcomes and included only those that measured the success of anaesthesia in terms of a reduction in the risk of intraoperative awareness and an optimum early recovery; for usability, we selected only three measures of recovery (time to eye opening, time to orientation, and time to discharge from the PACU). The decision to reduce the number of outcomes may introduce bias into the review process, however, we believed that this decision improved the usability of the review.
In addition, we were unable to source all texts from current British Library sources, and we did not seek translation of some studies, and therefore the review includes six studies awaiting classification. We could not be certain whether these studies included relevant data for the review.
For the primary outcome (the occurrence of intraoperative awareness), we found many studies with zero events in both arms. We limited the choice of appropriate meta‐analytic tools to those available in Review Manager 14, and used Peto OR in the primary analysis. We conducted sensitivity analysis using alternative statistical methods in Review Manager 14. Alternative methods, such as Bayesian meta‐analysis, may offer a more robust method to account for rare events as well as studies with zero events in both arms (Cheng 2016).
Agreements and disagreements with other studies or reviews
A recent meta‐analysis conducted by Gao and colleagues (Gao 2018), combined data from the five largest studies in this review (Avidan 2008; Avidan 2011; Mashour 2012; Myles 2004; Zhang 2011). Whilst their findings demonstrated that intraoperative awareness did not appear to be associated with BIS monitoring, this result combined studies of both ETAG‐ and clinical signs‐guided comparison groups. Similarly, the Cochrane Review by Messina and colleagues also combined both comparison groups (Messina 2016). We noted that Messina 2016 gave greater emphasis to the definition of different types of awareness, and the methods by which data were collected. As we had not specified this criteria, and the primary outcome in our review was for a broader criteria for intraoperative awareness, we subsequently included more small studies in the analysis. We do not think that the result in Messina 2016 is comparable with our own findings.
In relation to recovery measures, our findings are consistent with other systematic reviews which indicate that anaesthesia guided by BIS monitoring improves early postoperative recovery with a shorter time to eye opening and to orientation demonstrated in Chiang 2018 and Oliveira 2017, and a reduced time in the PACU for participants undergoing ambulatory surgery in Liu 2004.

Study flow diagram. Search conducted in March 2019.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Blank spaces indicate that we did not conduct 'Risk of bias' assessments because studies did not report review outcomes.

Funnel plot of comparison: 1 BIS versus clinical sides, outcome: 1.4 Time to discharge from the PACU (minutes).

Comparison 1: BIS versus clinical sides, Outcome 1: Occurrence of intraoperative awareness

Comparison 1: BIS versus clinical sides, Outcome 2: Time to eye opening (minutes)

Comparison 1: BIS versus clinical sides, Outcome 3: Time to orientation (minutes)

Comparison 1: BIS versus clinical sides, Outcome 4: Time to discharge from the PACU (minutes)

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 1: Occurrence of intraoperative awareness

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 2: Time to eye opening (minutes)

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 3: Time to orientation (minutes)

Comparison 2: BIS versus clinical signs: subgroup by type of anaesthetic, Outcome 4: Time to discharge from the PACU stay (minutes)

Comparison 3: BIS versus ETAG, Outcome 1: Occurrence of intraoperative awareness
| BIS compared to clinical signs for intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with clinical sides | Risk with BIS | |||||
| Occurrence of intraoperative awareness Time points of measure after surgery: 2 to 6 hours;12 hours; 1 day; 2 days; 3 days; 14 days; 30 days; or time point was not reported Measurement tools: simple questioning; interviews; or structured questionnaires | Study population | Peto OR 0.36 | 9765 | ⊕⊕⊝⊝ | Only 5 of 27 studies included incidences of awareness. Of these 5 studies: 4 used a structured questionnaire, and 1 used an interview method; 2 used an adjudication process to categorise incidences of awareness as 'confirmed' or 'definite'; participants in 1 study were at high risk of awareness, in 1 study were unselected, and in the remaining studies risk of awareness was not specified | |
| 9 per 1,000 | 3 per 1,000 | |||||
| Time to eye opening (in minutes) | ‐ | MD 1.78 minutes lower | ‐ | 1494 | ⊕⊕⊝⊝ | |
| Time to orientation (in minutes) | ‐ | MD 3.18 lower | ‐ | 273 | ⊕⊕⊝⊝ | |
| Time to discharge from the PACU (in minutes) | ‐ | MD 6.86 lower | ‐ | 930 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for study limitations owing to the inclusion of some studies with unclear risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. We downgraded by one level for imprecision; whilst we noted a narrow CI, the effect was dominated by two large trials (with two different populations selected according to the likelihood of intraoperative awareness) and we found many studies with zero events in both arms. We conducted sensitivity analysis to explore alternative statistical models to account for zero events in both arms as well as rare events and found more conservative estimates when we used a random‐effects model, thus reducing our certainty in the estimate. | ||||||
| BIS compared to ETAG for improving intraoperative awareness and early postoperative recovery | ||||||
| Population: adults undergoing any type of surgery under general anaesthesia; types of anaesthesia included propofol, desflurane, isoflurane, and sevoflurane; people were either selected for being at high risk of intraoperative awareness, were unselected, or study authors did not report risk of awareness in the included participants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with ETAG | Risk with BIS | |||||
| Occurrence of intraoperative awareness Time points of measure after surgery: 24 hours; 24 to 72 hours; 72 hours; 30 days; 18 hours after extubation in the ICU Measurement tools: structured interviews; or structured questionnaire | Study population | Peto OR 1.13 | 26,572 | ⊕⊕⊝⊝ | Only 3 of these studies included incidences of awareness. Of these 3 studies: all used a structured questionnaire; all used an adjudication process to categorise incidences of awareness as 'definite'; 2 studies included only participants who were at high risk for intraoperative awareness, and 1 study included participants who were unselected | |
| 1 per 1,000 | 1 per 1,000 | |||||
| Time to eye opening | ‐ | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | |
| Time to orientation | ‐ | ‐ | ‐ | We found no studies that measured or reported this outcome | ||
| Time to discharge from the PACU (in minutes) | Median (IQR): 98 minutes (66 to 140 minutes) | Median (IQR) 3 minutes lower (2 minutes lower to 2 minutes lower) | ‐ | 15,452 (1 study) | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for imprecision; despite a large number of participants, events were very rare (one per 1000 in the intervention and the comparison group) and the confidence interval for this effect was wide. In addition, we downgraded by one level for study limitations owing to the inclusion of some studies with unclear and high risks of bias, and in all studies it was not possible to blind anaesthetists to the different methods of depth of anaesthesia monitoring leading to a high risk of performance bias throughout. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Occurrence of intraoperative awareness Show forest plot | 27 | 9765 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.21, 0.60] |
| 1.2 Time to eye opening (minutes) Show forest plot | 22 | 1494 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐2.53, ‐1.03] |
| 1.3 Time to orientation (minutes) Show forest plot | 6 | 273 | Mean Difference (IV, Random, 95% CI) | ‐3.18 [‐4.03, ‐2.33] |
| 1.4 Time to discharge from the PACU (minutes) Show forest plot | 13 | 930 | Mean Difference (IV, Random, 95% CI) | ‐6.86 [‐11.72, ‐2.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Occurrence of intraoperative awareness Show forest plot | 26 | 7302 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.1.1 Propofol | 10 | 5784 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.24 [0.10, 0.60] |
| 2.1.2 Desflurane | 7 | 474 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.1.3 Isoflurane | 4 | 637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.26, 1.28] |
| 2.1.4 Sevoflurane | 7 | 407 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Not estimable |
| 2.2 Time to eye opening (minutes) Show forest plot | 22 | 1544 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.40, ‐0.95] |
| 2.2.1 propofol | 8 | 680 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.82, ‐0.43] |
| 2.2.2 desflurane | 4 | 322 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.44, 0.42] |
| 2.2.3 isoflurane | 3 | 150 | Mean Difference (IV, Random, 95% CI) | ‐2.45 [‐4.80, ‐0.09] |
| 2.2.4 sevoflurane | 8 | 392 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐2.60, ‐0.44] |
| 2.3 Time to orientation (minutes) Show forest plot | 7 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐3.15 [‐3.70, ‐2.61] |
| 2.3.1 propofol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2.3.2 desflurane | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐4.23, ‐0.97] |
| 2.3.3 isoflurane | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐5.92, ‐1.28] |
| 2.3.4 sevoflurane | 5 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐3.20 [‐3.80, ‐2.60] |
| 2.4 Time to discharge from the PACU stay (minutes) Show forest plot | 13 | 960 | Mean Difference (IV, Random, 95% CI) | ‐6.26 [‐10.68, ‐1.84] |
| 2.4.1 propofol | 4 | 398 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐9.36, ‐1.48] |
| 2.4.2 desflurane | 4 | 272 | Mean Difference (IV, Random, 95% CI) | ‐14.76 [‐29.61, 0.09] |
| 2.4.3 isoflurane | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐14.00 [‐34.12, 6.12] |
| 2.4.4 sevoflurane | 5 | 230 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐13.34, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Occurrence of intraoperative awareness Show forest plot | 5 | 26572 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.56, 2.26] |

