Tratamiento anticonvulsivo para el estado epiléptico
Resumen
Antecedentes
El estado epiléptico es una urgencia médica asociada con una mortalidad y una morbilidad significativas, que requiere tratamiento inmediato y efectivo.
Objetivos
(1) Determinar si algún anticonvulsivo determinado es más efectivo o más seguro de utilizar en el estado epiléptico, en comparación con otro y con placebo.
(2) Delinear las razones de los desacuerdos en la literatura con respecto a los regímenes de tratamiento recomendados y destacar las áreas para los estudios de investigación futuros.
Métodos de búsqueda
Para la última actualización de esta revisión, se hicieron búsquedas en las siguientes bases de datos electrónicas el 15 de agosto de 2013: el Registro Especializado del Grupo Cochrane de Epilepsia (Cochrane Epilepsy Group's Specialized Register), CENTRAL The Cochrane Library julio de 2013, número 7, y MEDLINE (Ovid) 1946 hasta el 15 de agosto de 2013.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados con participantes con estado epiléptico premonitorio, temprano, establecido o refractario que utilizaran una asignación al tratamiento verdaderamente aleatoria o cuasialeatoria.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los ensayos para inclusión, evaluaron la calidad y extrajeron los datos.
Resultados principales
Se incluyeron 18 estudios con 2755 participantes. Pocos estudios utilizaron las mismas intervenciones. El diazepam fue mejor que placebo para reducir el riesgo de fracaso en el cese de las convulsiones (riesgo relativo [RR] 0,73; IC del 95%: 0,57 a 0,92), la necesidad de asistencia respiratoria (RR 0,39; IC del 95%: 0,16 a 0,94) o la continuación del estado epiléptico que requiriera el uso de un fármaco diferente o anestesia general (RR 0,73; IC del 95%: 0,57 a 0,92). El lorazepam fue mejor que placebo para disminuir el riesgo de fracaso en el cese de las convulsiones (RR 0,52; IC del 95%: 0,38 a 0,71) y el riesgo de continuación del estado epiléptico que requiriera el uso de un fármaco diferente o anestesia general (RR 0,52; IC del 95%: 0,38 a 0,71). El lorazepam fue mejor que el diazepam para reducir el riesgo de fracaso en el cese de las convulsiones (RR 0,64; IC del 95%: 0,45 a 0,90) y mostró un riesgo menor de continuación del estado de mal epiléptico que requiriera un fármaco diferente o anestesia general (RR 0,63; IC del 95%: 0,45 a 0,88). El lorazepam intravenoso fue mejor que la fenitoína intravenosa en cuanto al riesgo de fracaso en el cese de las convulsiones (RR 0,62; IC del 95%: 0,45 a 0,86). El gel de diazepam fue mejor que el gel placebo para reducir el riesgo de fracaso en el cese de las convulsiones (RR 0,43, IC del 95%: 0,30 a 0,62)
Para el tratamiento prehospitalario, el midazolam intramuscular es al menos tan efectivo como (probablemente más efectivo que) el lorazepam intravenoso en el control de las convulsiones (RR 1,16; IC del 95%: 1,06 a 1,27) y la frecuencia de la hospitalización (RR 0,88; IC del 95%: 0,79 a 0,97) o los ingresos en cuidados intensivos (RR 0,79; IC del 95%: 0,65 a 0,96). No está claro si el valproato intravenoso es mejor que la fenitoína intravenosa para reducir el riesgo de fracaso en el cese de las convulsiones (RR 0,75; IC del 95%: 0,28 a 2,00). El levetiracetam y el lorazepam fueron igualmente eficaces para cesar las convulsiones (RR 0,97; IC del 95%: 0,44 a 2,13). Los resultados de otras comparaciones de tratamientos anticonvulsivos no estuvieron claros debido a estudios únicos con pocos participantes.
El grupo de evidencia proveniente de ensayos aleatorizados para guiar las decisiones clínicas es pequeño. No se sabe con certeza si algún tratamiento anticonvulsivo es mejor que otro en cuanto a los efectos adversos, debido a los pocos estudios y participantes identificados. La calidad de la evidencia de los estudios incluidos no es sólida, pero parece aceptable. No fue posible evaluar el riesgo de sesgo de los dominios informe incompleto de los resultados (sesgo de desgaste) e informe selectivo de los resultados (sesgo de selección) debido a la falta de claridad de los informes de los autores de los estudios.
Conclusiones de los autores
El lorazepam intravenoso es mejor que el diazepam intravenoso o la fenitoína intravenosa sola para el cese de las convulsiones. El lorazepam intravenoso también conlleva un menor riesgo de continuación del estado epiléptico que requiera un fármaco diferente o anestesia general en comparación con el diazepam intravenoso. El lorazepam intravenoso y el diazepam son mejores que placebo para los mismos resultados. Para el tratamiento prehospitalario, el midazolam IM pareció más efectivo que el lorazepam IV para el cese de las convulsiones, la frecuencia de hospitalización y los ingresos en la UCI; sin embargo, no estuvo claro si el riesgo de recurrencia de las convulsiones difirió entre los tratamientos. Los resultados de otras comparaciones de tratamientos anticonvulsivos entre sí tampoco estuvieron claros. Se requieren definiciones universalmente aceptadas del estado epiléptico premonitorio, temprano, establecido y refractario. El gel de diazepam fue mejor que el gel placebo para reducir el riesgo de fracaso en el cese de las convulsiones. Los resultados de otras comparaciones de tratamientos anticonvulsivos no estuvieron claros debido a estudios únicos con pocos participantes.
PICO
Resumen en términos sencillos
Tratamiento anticonvulsivo para el estado epiléptico
Algunos pacientes desarrollan una actividad eléctrica anormal y excesiva de las células nerviosas del cerebro. Esto se denomina actividad convulsiva y puede afectar a una pequeña zona del cerebro o a todo el cerebro, provocando una disfunción repentina de las estructuras implicadas, como sacudidas de los miembros. La actividad convulsiva suele provocar movimientos espasmódicos (convulsiones) y suele durar unos minutos. Cuando hay más de 30 minutos de actividad convulsiva continua o se producen dos o más convulsiones seguidas sin recuperación de la plena conciencia entre dos convulsiones, la afección se denomina estado epiléptico, que es una urgencia médica. Se han estudiado muchos fármacos para el tratamiento de esta afección. Esta revisión encontró que el lorazepam intravenoso (inyectado en una vena) es mejor que el diazepam o la fenitoína para el control inmediato del estado epiléptico. En el tratamiento de las crisis epilépticas que se producen en forma serial, el diazepam en gel administrado por vía rectal es efectivo para controlar las crisis. El lorazepam intravenoso es mejor que el diazepam intravenoso o la fenitoína para el control inmediato del estado epiléptico. Para el tratamiento prehospitalario, el midazolam intramuscular es tan eficaz como (probablemente más eficaz que) el lorazepam intravenoso en el control de las convulsiones y la frecuencia de hospitalización o los ingresos en cuidados intensivos. Es necesario realizar más estudios sobre otros fármacos utilizados habitualmente para esta afección.
Authors' conclusions
Summary of findings
| Lorazepam IV versus diazepam IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam IV | |||||
| Non‐cessation of seizures | Study population | RR 0.64 | 264 | ⊕⊕⊕⊝ | ||
| 381 per 1000 | 244 per 1000 | |||||
| Moderate | ||||||
| 219 per 1000 | 140 per 1000 | |||||
| Requirement for ventilatory support | Study population | RR 0.73 | 264 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 91 per 1000 | |||||
| Adverse effects | Study population | See comment | 264 | ⊕⊝⊝⊝ | Risks were calculated from pooled risk differences | |
| 82 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 103 per 1000 | 64 per 1000 | |||||
| Continuation of status requiring a different drug or general anaesthesia | Study population | RR 0.63 | 264 | ⊕⊕⊕⊝ | ||
| 388 per 1000 | 244 per 1000 | |||||
| Moderate | ||||||
| 250 per 1000 | 158 per 1000 | |||||
| Death | Study population | See comment | 203 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences | |
| 30 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 22 per 1000 | 37 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Of the three studies, one (Appleton 1995) used odd/even dates for randomisation, clearly incurring high risk of bias. Two studies did not conceal allocation, incurring high risk of bias (Mehta 2007; Misra 2011) . . Furthermore an additional eight studies (Agarwal 2007; Chamberlain 1997; Chen 2011; McCormick 1999; Pellock 1998; Rossetti 2011; Shaner 1988; Sreenath 2010) did not provide clear information regarding generation of random sequence and/or allocation concealment.Taken together, there is enough risk of bias to downgrade the quality of evidence. | ||||||
| Lorazepam IV versus diazepam plus phenytoin IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam plus phenytoin IV | |||||
| Non‐cessation of seizures | Study population | RD ‐0.04 | 370 | ⊕⊕⊝⊝ | ||
| 227 per 1000 | 179 per 1000 | |||||
| Moderate | ||||||
| 221 per 1000 | 175 per 1000 | |||||
| Adverse effects | Study population | RR 0.85 | 370 | ⊕⊕⊕⊝ | ||
| 290 per 1000 | 246 per 1000 | |||||
| Moderate | ||||||
| 281 per 1000 | 239 per 1000 | |||||
| Deaths | See comment | See comment | Not estimable | 178 | See comment | Reported in a single study |
| Requirement of Ventilatory support | See comment | See comment | Not estimable | 178 | See comment | Reported in a single study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Even though one study did not have a clear concealment of allocation, this is possibly a lack of reporting. Lack of blinding in this study is unlikely to bias the outcome assessment as the outcomes are clearly detectable and objective. | ||||||
| Diazepam gel versus placebo gel for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Diazepam gel versus placebo gel | |||||
| Non‐cessation of seizures | Study population | RR 0.43 | 165 | ⊕⊕⊕⊝ | ||
| 716 per 1000 | 308 per 1000 | |||||
| Moderate | ||||||
| 716 per 1000 | 308 per 1000 | |||||
| Adverse effects | Study population | RR 1.5 | 165 | ⊕⊕⊕⊝ | ||
| 250 per 1000 | 375 per 1000 | |||||
| Moderate | ||||||
| 248 per 1000 | 372 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One placebo‐controlled study did not describe the method of sequence generation, but probably this is a limitation of writing style. Use of placebo will tend to limit any bias due to this. | ||||||
Background
Description of the condition
Status epilepticus is defined as a condition in which there is either more than 30 minutes of continuous seizure activity; or two or more sequential seizures without recovery of full consciousness between the seizures. Status epilepticus is a medical emergency. It is associated with an overall mortality of 8% in children and 30% in adults (Epilepsy Foundation of America 1993). An additional 5% to 10% of people experiencing this condition have permanent sequelae, such as a permanent vegetative state or cognitive difficulties. Approximately 12% to 30% of adults with a new diagnosis of epilepsy present in status epilepticus (Lowenstein 1998). Some authors, depending upon the duration of seizure activity, stipulate three stages of the condition: early, established and refractory. Early status epilepticus consists of the first 30 minutes of the epileptic state during which physiological mechanisms compensate for the greatly enhanced metabolic activity. Established status epilepticus is defined as the stage beyond 30 minutes, where the status continues despite early stage treatment. It is during this phase that physiological compensation mechanisms begin to fail. If seizures continue for 60 to 90 minutes after the initiation of therapy the stage of refractory status is said to have been reached. Status epilepticus may be convulsive (with limb stiffness and jerking) or non‐convulsive (without limb stiffness and jerking). Though convulsive status epilepticus is associated with a higher mortality and morbidity than non‐convulsive status epilepticus, both require prompt and effective treatment. However, the most effective treatment regimen is not clear from the literature. Different experts give different recommendations regarding the best treatment regimen for status epilepticus (Lowenstein 1998; Prasad 1995; Epilepsy Foundation of America 1993) and the evidence base of many of these recommendations is often unclear. We conducted a systematic review of all the randomised controlled trials that we could identify to summarise the existing evidence, to delineate the reasons for disagreements and to highlight areas requiring further research.
Description of the intervention
Several drugs are available as interventions to treat status epilepticus.
Interventions for the treatment of status epilepticus cover a range of drugs, some of which may be safe, while others may be associated with serious adverse events. As status epilepticus progresses sequentially through premonitory, early, established and refractory stages, accordingly, treatment starts with benzodiazepines but may, in appropriate situations, require anaesthetic agents. The need to terminate the status requires the use of such interventions that enter the brain rapidly, and have a long half‐life to prevent recurrence of seizures. Diazepam, lorazepam and midazolam, being lipid soluble, enter the brain rapidly, but diazepam is rapidly taken up by fatty tissues and hence has a shorter redistribution half‐life (one hour) compared to half‐lives of midazolam (two hours) and lorazepam (14 hours). As a result, duration of action of diazepam is only 0.25 to 0.5 hour, whereas for lorazepam it is 12 to 24 hours. For long‐term maintenance therapy, drugs that can be administered in both parenteral and oral routes are required, such as sodium valproate, phenytoin, phenobarbitone etc.
How the intervention might work
The mechanism of action varies from one drug to another. Benzodiazepines (diazepam, lorazepam, midazolam) mainly potentiate GABA induced chloride influx. The most important mechanism for phenobarbitone is GABA receptor mediated synaptic inhibition. Phenytoin has a stabiliSing influence on the neuronal membrane through prolongation of the inactivated state of voltage sensitive neuronal sodium channel. Sodium valproate acts through multiple mechanisms: frequency dependent prolongation of sodium channel inactivation, attenuation of calcium mediated transient currents, augmentation of GABA. Benzodiazepines have a relatively short duration of action, and hence for maintenance of action, phenytoin, sodium valproate, or phenobarbitone are used or added to benzodiazepines.
Why it is important to do this review
Status epilepticus is a medical emergency with a significant mortality, but like many acute medical emergencies it is a challenging topic for randomised controlled trials. A regularly updated systematic review is required to summarise current evidence in order to inform both treatment guidelines and the research agenda.
Objectives
The primary objective of the review was to synthesise the available evidence from randomised controlled trials (RCTs):
-
to determine whether a particular anticonvulsant is more effective or safer in controlling status epilepticus compared with another drug or placebo;
-
to delineate reasons for disagreements regarding recommended treatment and highlight areas for future research.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials using a truly random or quasi‐random allocation of treatment were included in this review if they included people with premonitory, early, established or refractory status epilepticus (see below for definitions).
Types of participants
For a study to be included in the review, study participants were required to have premonitory or early stage status epilepticus or established status epilepticus. The premonitory phase was referred to as the period during which seizures became increasingly frequent or severe but the condition did not meet the definition of status epilepticus. Early status was defined as the first 30 minutes of seizure activity. Established status epilepticus was defined as a condition with either more than 30 minutes of continuous seizure activity or two or more sequential seizures without recovery of full consciousness between the seizures. If seizure activity remains uncontrolled for one to two hours, in spite of first‐line treatment, then the participant is considered to be in refractory status epilepticus (Shorvon 2001).
Both convulsive as well as non‐convulsive status epilepticus were considered for this review.
Types of interventions
Studies comparing any anticonvulsant drug against placebo or another anticonvulsant drug were included in the review. For the premonitory stage of status epilepticus, interventions included diazepam (intravenous or intrarectal); lorazepam (intravenous); paraldehyde (intramuscular or intrarectal) or midazolam (intravenous, intramuscular or intrarectal). For early or established status epilepticus, drug interventions included lorazepam; diazepam; phenytoin; fosphenytoin; lignocaine; paraldehyde or clonazepam. For refractory status epilepticus, thiopentone; propofol; pentobarbitone; isoflurane or etomidate were included.
Types of outcome measures
The outcomes considered depended on the stage of status epilepticus at which the treatment was tested. For the purpose of this review, our intention was to analyse treatment failure as the primary outcome. We intended to define treatment failure for the various stages as any of the following.
For premonitory stage
-
Development of status epilepticus or death.
For early or established status epilepticus
-
Death.
-
Continuation of status epilepticus requiring use of a different drug or general anaesthesia for control.
-
Long‐term disabling sequelae, defined as persistent neurological deficits severe enough to require dependence on some other person for activities of daily living (walking, toileting, bathing, dressing and eating) at six months after randomisation.
-
Need for ventilatory support.
-
Incomplete recovery before discharge, defined as inability to attain pre‐status epilepticus state at the time of discharge. This outcome was included because most people attain their pre‐status epilepticus state before discharge and are not followed up subsequently. People who do not recover need to be followed up in order to judge recovery or persistent deficit.
We used the term 'non‐cessation of seizures' rather than cessation of seizures (used in the various studies) to maintain uniformity since all the other outcomes were unfavourable outcomes. Some of the studies described data on continuation of status epilepticus requiring a different drug or general anaesthesia separately, hence this has been taken as a separate outcome for analysis.
For refractory status epilepticus
-
Death.
-
Long‐term sequelae defined as dependence for activities of daily living (walking, toileting, bathing, dressing and eating) assessed at six months or beyond, but if studies reported only one or three months after onset, this was treated as a long‐term outcome.
Other outcomes which were considered for separate analyses in this review
-
Complications such as infections; renal failure; respiratory failure.
-
Adverse effects of drugs. The following were considered in the safety analysis:
-
hypotension, defined as a systolic blood pressure below 90 mm Hg recorded while the drug was being administered or within 24 hours of the last dose;
-
respiratory depression, defined as the occurrence of apnoea or need for intubation;
-
cardiac arrest (diagnosed clinically) or bradyarrhythmias including heart block, documented on an electrocardiogram.
-
We intended to analyse outcomes separately and as a composite outcome (treatment failure) because the latter would have allowed more power. This was not possible because many participants had more than one outcome measured during the course of treatment and the studies presented the number of outcomes observed but not the number of participants having one or more of these outcomes. We also considered an analysis of the number of outcomes as a continuous variable but the studies did not present standard deviations for these data and, therefore, did not permit meta‐analysis.
Search methods for identification of studies
This search was run for the original review in July 2005 and subsequent searches have been run in January 2010, January 2011, February 2012, and August 2013. For the initial review, the following databases were searched:
(1) Cochrane Epilepsy Group Specialized Register (December 2009);
(2) Cochrane Central Database of Controlled Trials (CENTRAL) (The Cochrane Library 2009, Issue 4) using the strategy outlined in Appendix 2;
(3) MEDLINE (1950 to December week 4, 2009) using the strategy outlined in Appendix 3;
In addition, we searched EMBASE (1966 ‐ January 2003) for the original version of this review, but we no longer have access to this database.
For the most recent update of this review, we searched the following electronic databases for published trials:
(1) Cochrane Epilepsy Group Specialized Register (15 August 2013) using the strategy outlined in Appendix 1;
(2) Cochrane Central Database of Controlled Trials (CENTRAL) ,The Cochrane Library July 2013, Issue 7) using the strategy outlined in Appendix 2;
(3) MEDLINE (Ovid, 1946 to 15/08/2013) using the strategy outlined in Appendix 3.
We did not apply any language restrictions to our search.
All resulting titles and abstracts were scanned and any relevant articles were followed up.
Data collection and analysis
Two review authors (MP, PRK) independently selected the trials to be included in the review. Any disagreements were resolved by seeking an independent opinion of the third review author (KA‐R).
The methodological quality of each trial was assessed by two review authors (MP, PRK). The following criteria were included: randomisation method; baseline comparability of the trial arms; blinding; and whether the published data permitted an intention‐to‐treat analysis. Data were independently extracted by two review authors and cross‐checked. Data on the number of participants with each outcome event, by allocated treatment group, were sought to allow an intention‐to‐treat analysis.
We assessed the risk of bias as low, high or unclear risk of bias. We evaluated the following characteristics using the Cochrane 'Risk of bias' tool.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
The trials comparing the same drugs were combined, whereas those comparing different drugs were analysed separately.
Our intention was to carry out our primary analysis on the risk of treatment failure. However, it was not possible to extract data on this outcome because the same participants were probably counted more than once in the various outcomes presented in the studies. Clearly some individuals had more than one of the above outcomes. For example, a person might require anaesthesia and ventilation then recover incompletely and may have long‐term disabling sequelae. Our intention was to carry out separate analyses for premonitory stage, early status epilepticus, established status epilepticus and refractory status epilepticus. However, the definitions used in the different studies were both variable (seeTable 1) and often unclear.
| Study | Definition |
| Continuous or repeated seizure activity > 5 minutes without recovery of consciousness. | |
| Leppik 1983 (mixed) | (a) Generalised tonic‐clonic status: three or more generalised tonic‐clonic seizures in one hour; two or more generalised seizures in rapid succession without recovery of consciousness; (b) absence status: confusional state with generalised 3 Hz spike wave pattern on EEG; (c) complex partial status: confusional state, clinical seizure or both with focal EEG abnormality; (d) elementary status: partial seizures without loss of consciousness. |
| Pellock 1998 (premonitory) | Acute repetitive seizures (no definition). |
| Remy 1991 (mixed) | Successive partial seizures for at least 20 minutes or two generalised tonic‐clonic seizures within 20 minutes. |
| Shaner 1988 (established/mixed) | Generalised convulsive status epilepticus: (a) a history of 30 minutes of continuous generalised convulsive seizures and witnessed generalised seizures in the emergency room; (b) a history of 30 minutes of recurrent generalised convulsive seizures but failure to attain baseline mental status between seizures and witnessed generalised seizures in the emergency room; (c) a history of 3 or more generalised convulsive seizures in one hour in patients with obtundation prior to the onset of status epilepticus and witnessed generalised convulsive seizures in the emergency room; (d) uncertain history of seizures but generalised convulsive seizures continuously for more than 5 minutes as witnessed in the emergency room. |
| Singhi 2002 (refractory) | Motor seizures uncontrolled after 2 doses of diazepam and phenytoin infusion. |
| McCormick 1999 (unclear) | No definition. |
| Chamberlain 1997 (premonitory) | Motor seizures of at least 10 minutes duration. |
| Appleton 1995 (unclear) | No definition. |
| Treiman 1998 (premonitory) | Overt generalised convulsive status: two or more generalised convulsions or continuous convulsive activity > 10 minutes. |
| Cereghino 2002 (premonitory) | Multiple seizures of complex partial or generalised type within an observation period between 12 ‐ 24 hours. |
| Two or more convulsive seizures without full recovery of consciousness between the seizures or continuous convulsions lasting for more than five minutes. | |
| 30 minutes of continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures. | |
| More than five minutes of continuous seizures or two or more discrete seizures between which there is incomplete recovery of consciousness. | |
| Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. | |
| Generalized convulsions continuing for minimum of five minutes. | |
| Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. | |
| Continuous convulsions for longer than five minutes or having convulsions at the time of treatment after having intermittent seizures without regaining consciousness for longer than five minutes. | |
| Continuous convulsive activity lasting for 5 minutes or more. |
Potential causes of heterogeneity were assessed by examining differences between trials in respect to trial design; participant population; intervention and outcome. We tested for heterogeneity between trial results for each outcome using a chi squared (Chi2) test. If the test for heterogeneity was statistically non‐significant, then the results from the different trials were combined to obtain a summary estimate of effect (and the corresponding confidence interval (CI)) using a fixed‐effect model. We used risk ratio (RR) as the measure of choice for our analyses, but for some outcomes there were zero events in all the arms of some studies. In such situations we used risk difference (RD) to ensure inclusion of the data in our meta‐analysis. Using RR would exclude such studies from analysis because confidence intervals cannot be calculated around RR when there is a zero event in both the arms of the study.
Assessment of statistical heterogeneity was supplemented using the I2 statistic, which provides an estimate of the percentage of variability due to heterogeneity rather than a sampling error.
Interpretation of I2 for heterogeneity is as follows.
-
0% to 40%: may not be important.
-
30% to 60%: represents moderate heterogeneity.
-
50% to 90%: represents substantial heterogeneity.
-
75% to 100%: represents considerable heterogeneity.
Results
Description of studies
Results of the search
In the original review, the search yielded 28 studies that could potentially be included. After close scrutiny, 10 studies met the inclusion criteria, and the remaining 18 were excluded. For the current update, the search yielded 27 studies that could potentially be included. 17 out of these 27 studies were excluded after screening the abstracts. The full texts of the remaining 10 studies were screened and out of those, eight met the inclusion criteria.The current update includes eight new studies (Agarwal 2007; Ahmad 2006; Chen 2011; Mehta 2007; Misra 2011; Rossetti 2011; Silbergleit 2012; Sreenath 2010).
Included studies
Eighteen studies are included with a total of 2755 participants. Of the 18 studies included in this review, five studied participants with premonitory status (Alldredge 2001; Cereghino 2002; Chamberlain 1997; Pellock 1998; Treiman 1998), one established (Shaner 1988), three refractory (Agarwal 2007; Mehta 2007; Singhi 2002), and one mixed status epilepticus (Leppik 1983). Two studies did not clearly define the status (Appleton 1995; McCormick 1999). This made it difficult to analyse studies according to type of status epilepticus. Ten studies included only adults (Alldredge 2001; Cereghino 2002; Leppik 1983; Pellock 1998; Shaner 1988; Treiman 1998; Chen 2011;Misra 2011; Rossetti 2011), and six only children (Appleton 1995; Chamberlain 1997; McCormick 1999; Singhi 2002;Ahmad 2006;Sreenath 2010). Two studies included both adults and children (Agarwal 2007; Silbergleit 2012) The type of status epilepticus included varied from study to study: twelve generalised tonic‐clonic (Alldredge 2001; Appleton 1995; Shaner 1988; Treiman 1998;Agarwal 2007; Ahmad 2006; Chen 2011;Mehta 2007; Misra 2011; Rossetti 2011; Silbergleit 2012; Sreenath 2010); and three mixed (Cereghino 2002; Leppik 1983; Singhi 2002). Three studies (Chamberlain 1997; McCormick 1999; Pellock 1998) did not describe the type of status epilepticus. Time‐since‐onset of status epilepticus also was variable, from minutes to hours in the different studies.
Three studies had more than two arms: (four arms in two studies (Appleton 1995; Treiman 1998) and three in one (Alldredge 2001)). There was difficulty in data extraction in one of these (Appleton 1995), so that only two arms were included in our analysis. Three studies had placebo arms (Alldredge 2001; Cereghino 2002; Pellock 1998) all in premonitory status epilepticus, described by two authors as acute repetitive seizures (Cereghino 2002; Pellock 1998). Two studies compared intrarectal diazepam gel with placebo (Cereghino 2002; Pellock 1998); and one study had lorazepam, diazepam, and placebo arms (Alldredge 2001), with the interventions all administered intravenously. Eight studies compared two or more active drugs. All studies except three (two intrarectal and one intramuscular (IM) midazolam in one arm) used intravenous (IV) administration of drugs. Twenty different comparisons were available but only five (lorazepam versus diazepam, both administered intravenously; lorazepam versus diazepam plus phenytoin; diazepam plus phenytoin versus phenobarbitone, administered intravenously; diazepam intrarectal gel versus placebo gel, valproate versus diazepam intravenously) included more than one study to permit a meta‐analysis. In three studies (Alldredge 2001; Cereghino 2002; Pellock 1998) drugs were administered in the prehospital phase.
All participants were followed up only during their hospital stay. No study had post‐discharge follow‐up. All studies had cessation of status epilepticus and adverse effects as outcomes. Death was an outcome in five comparisons. Other outcomes studied were requirement for ventilatory support (seven comparisons) and continuation of status epilepticus requiring another drug or general anaesthesia (five comparisons).
Excluded studies
Eleven studies are now excluded from the review. The reasons for exclusion were: poor data presentation (three studies), publication of only the study protocol (one study), a non‐randomised comparative clinical trial (one study), a case study with only one participant (one study), and participants not having status epilepticus (one study), poor randomisation method (one study), comparing two doses of the same drug (one study) (for details seeExcluded studies).
Risk of bias in included studies
Six studies (Alldredge 2001; Cereghino 2002; Leppik 1983; Pellock 1998; Silbergleit 2012; Treiman 1998) used a similar‐looking placebo or comparison drug. With similar‐looking interventions and blinding the recruiting person and the person administering the intervention should not know the group to which the next participant is going to be assigned. Thus, the similarity in appearance concealed the allocation in these studies. In addition, three studies (Rossetti 2011; Shaner 1988; Sreenath 2010) used sealed envelopes to conceal allocation in the randomisation process but whether the envelopes were opaque and serially numbered is unclear from the study reports. The rest of the studies (Agarwal 2007; Appleton 1995; Chamberlain 1997; Chen 2011; McCormick 1999; Mehta 2007; Misra 2011) did not mention any attempt to conceal randomisation. Studies with a similar‐looking placebo or comparison drug were assumed to be blinded but nine studies (Ahmad 2006; Appleton 1995; Chen 2011; Mehta 2007; Misra 2011; Rossetti 2011; Shaner 1988; Singhi 2002; Sreenath 2010) did not have blinding of carers or outcome assessors. The follow‐up was restricted to the period of the hospital admissions in all the studies.
Three studies (Appleton 1995; Leppik 1983; Shaner 1988) did not report the number of participants having the events; rather they counted the number of events in the arms. This made it difficult to extract data from these studies for meta‐analysis.
Please refer to Figure 1 and Figure 2.
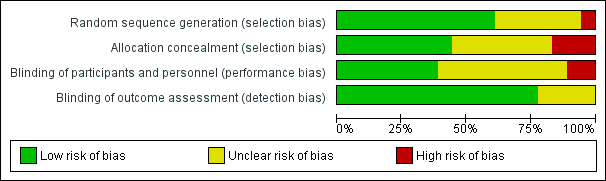
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
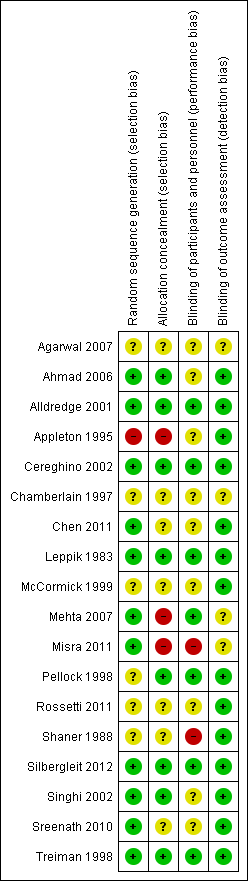
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Summary of findings for the main comparison Lorazepam IV versus diazepam IV for status epilepticus; Summary of findings 2 Lorazepam IV versus diazepam plus phenytoin IV for status epilepticus; Summary of findings 3 Diazepam gel versus placebo gel for status epilepticus
All disagreements were resolved through discussion. Poor data presentation encountered included studies not mentioning the total number of participants studied, or presenting only the number of outcomes observed rather than the number of participants with the outcomes.
The data extraction was difficult because of heterogeneity in the definition of status epilepticus (seeTable 1) and the type of data presented. Very few studies used the same interventions. We could combine data from eleven studies over five different outcomes. Even here, the definitions used by different authors varied and we assumed that the type of participants were similar. We presented the rest of the studies separately.
The results are presented according to the comparisons used.
Comparison 1: Lorazepam IV versus diazepam IV
There were three studies with 289 participants (Alldredge 2001; Appleton 1995; Leppik 1983). The outcome of death was available in two studies (203 participants). There was no statistically significant difference in deaths between the two groups (5/103 versus 3/100 participants; risk difference (RD) 0.02, 95% confidence interval (CI) ‐0.04 to 0.08). Compared with diazepam, lorazepam had statistically significant lower risk of non‐cessation of seizures (32/130 versus 51/134 participants; risk ratio (RR) 0.64, 95% CI 0.45 to 0.90) and of continuation of status epilepticus requiring a different drug or general anaesthesia (32/130 participants versus 52/134; RR 0.63, 95% CI 0.45 to 0.88).
There was a statistically non‐significant trend favouring lorazepam for reducing requirement for ventilatory support (12/130 versus 17/134 participants; RR 0.73, 95% CI 0.36 to 1.49) and adverse effects (7/130 versus 11/134 participants; RD ‐0.03, 95% CI ‐0.10 to 0.03).
Comparison 2: Lorazepam IV versus placebo IV
There was one study (Alldredge 2001) with 137 participants. Compared with placebo, lorazepam had a statistically significant lower risk of non‐cessation of seizures (27/66 versus 56/71 participants; RR 0.52, 95% CI 0.38 to 0.71) and of continuation of status epilepticus requiring a different drug or general anaesthesia (27/66 versus 56/71 participants; RR 0.52, 95% CI 0.38 to 0.71). There was a statistically non‐significant but strong trend favouring lorazepam for the following outcomes: death (5/66 versus 11/71 participants; RR 0.49, 95% CI 0.18 to 1.33); requirement for ventilatory support (7/66 versus 16/71 participants; RR 0.47, 95% CI 0.21 to 1.07) and adverse effects (7/66 versus 16/71 participants; RR 0.47, 95% CI 0.21 to 1.07).
Comparison 3: Lorazepam IV versus diazepam plus phenytoin IV
There were two studies (Sreenath 2010,Treiman 1998) with 370 participants. The outcome of non‐cessation of seizures and adverse effects were available in both studies. There was a statistically non‐significant trend favouring lorazepam for both non‐cessation of seizures (34/187 versus 42/183 participants; RD ‐0.04, CI ‐0.35 to 0.26) and adverse effects (46/187 versus 53/183 participants; RR 0.85, 95% CI 0.63 to 1.15). The outcome of death and requirement of ventilatory support were available in one study. There was no statistically significant difference for the outcome of death (0/88 versus 0/90 participants and requirement of ventilatory support (0/88 versus 0/90).
Comparison 4: Lorazepam IV versus phenobarbitone IV
A single study (Treiman 1998) with 188 participants showed no statistically significant difference in the risk of non‐cessation of seizures (34/97 versus 38/91 participants; RR 0.84, 95% CI 0.58 to 1.21) or adverse effects (42/97 versus 46/91 participants; RR 0.86, 95% CI 0.63 to 1.16) between the two drugs.
Comparison 5: Lorazepam IV versus phenytoin IV
There was only one study (Treiman 1998) with 198 participants. Risk of non‐cessation of seizures was less with lorazepam compared with phenytoin (34/97 versus 57/101, RR 0.62, 95% CI 0.45 to 0.86). There was no statistically significant difference between the two groups regarding adverse effects (42/97 versus 44/101, RR 0.99, 95% CI 0.72 to 1.37).
Comparison 6: Midazolam IV versus lorazepam IV
There was a single small study (McCormick 1999) with 27 participants. This study reported a statistically non‐significant trend favouring midazolam regarding the following outcomes: non‐cessation of seizures (1/15 versus 4/12 participants; RR 0.20, 95% CI 0.03 to 1.56); requirement for ventilatory support (1/15 versus 2/12 participants; RR 0.40, 95% CI 0.04 to 3.90) and adverse effects (1/15 versus 2/12 participants; RR 0.40, 95% CI 0.04 to 3.90) and continuation of status epilepticus requiring a different drug or general anaesthesia (1/15 versus 4/12 participants; RR 0.20, 95% CI 0.03 to 1.56).
Comparison 7: Midazolam IV versus diazepam IV
There was a single study (Singhi 2002) with 40 participants. There was no statistically significant difference between the two groups regarding the following outcomes: non‐cessation of seizures (3/21 versus 2/19 participants; RR 1.36, 95% CI 0.25 to 7.27); requirement for ventilatory support (11/21 versus 9/19 participants; RR 1.11, 95% CI 0.59 to 2.07) and adverse effects (8/21 versus 9/19 participants; RR 0.80, 95% CI 0.39 to 1.66). There was a statistically non‐significant trend favouring diazepam for the outcome of death (8/21 versus 2/19 participants; RR 3.62, 95% CI 0.87 to 14.97).
Comparison 8: Midazolam IM versus diazepam IV
A small single study (Chamberlain 1997) of 24 participants showed no statistically significant difference between the two groups for the following outcomes: non‐cessation of seizures (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01); requirement for ventilatory support (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01); adverse effects (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01) and continuation of status epilepticus requiring a different drug or general anaesthesia (1/13 versus 1/11 participants; RR 0.85, 95% CI 0.06 to 12.01).
Comparison 9: Diazepam IV versus placebo IV
One hundred and thirty nine participants in a single study (Alldredge 2001) were analysed. Most of the outcomes significantly favoured diazepam: non‐cessation of seizures (39/68 versus 56/71 participants; RR 0.73, 95% CI 0.57 to 0.92); death (3/68 versus 11/71 participants; RR 0.28, 95% CI 0.08 to 0.98); requirement for ventilatory support (6/68 versus 16/71 participants; RR 0.39, 95% CI 0.16 to 0.94) and continuation of status requiring a different drug or general anaesthesia (39/68 versus 56/71 participants; RR 0.73, 95% CI 0.57 to 0.92). There was a non‐significant trend favouring diazepam for adverse effects (7/68 versus 16/71 participants; RR 0.46, 95% CI 0.20 to 1.04).
Comparison 10: Diazepam gel versus placebo gel
There were two studies (Cereghino 2002; Pellock 1998) with a total of 165 participants. The risk of non‐cessation of seizures was significantly less with diazepam gel compared with placebo gel (24/77 versus 63/88 participants; RR 0.43, 95% CI 0.30 to 0.62). For adverse effects there was a strong but statistically non‐significant trend towards the placebo gel (29/77 versus 22/88 participants; RR 1.50, 95% RR 0.94 to 2.37).
Comparison 11: Leviteracetam IV versus Lorazepam IV
There was one study (Misra 2011) with a total of 79 participants. Both levetiracetam and lorazepam were equally effective in aborting seizures (9/38 versus 10/41, RR 0.97, 95% CI 0.44 to 2.13).
Comparison 12: Diazepam plus phenytoin IV versus phenobarbitone IV
There were two studies (Shaner 1988; Treiman 1998) with a total of 222 participants. For the outcomes of death and requirement for ventilatory support, data were available in only one study (36 participants). There was no statistically significant difference between the two groups for the following outcomes: requirement for ventilatory support (6/18 versus 6/18 participants; RR 1.00, 95% CI 0.40 to 2.52); adverse effects (57/113 versus 55/109 participants, RR 1.00, 95% CI 0.77 to 1.30) and death (0/18 versus 0/18 participants; RD 0.00, 95% CI ‐0.10 to 0.10). For non‐cessation of seizures, the test for heterogeneity was significant and the type of status epilepticus studied was different, hence the two studies were analysed separately for this outcome. There was a weak statistically non‐significant trend favouring phenobarbitone in one of the studies (Shaner 1988) (8/18 versus 2/18 participants; RR 4.00, 95% CI 0.98 to 16.30). In the other larger study (Treiman 1998), there was no statistically significant difference between the two groups for non‐cessation of seizures (42/95 versus 38/91 participants; RR 1.06, 95% CI 0.76 to 1.47).
Comparison 13: Diazepam plus phenytoin IV versus phenobarbitone IV (premonitory status)
The was a single study (Treiman 1998) with 196 participants. There was no statistically significant difference between the two groups for cessation of seizures ( 42/95 versus 38/91 RR 1.06, 95% CI 0.76 to 1.47).
Comparison 14: Diazepam plus phenytoin IV versus phenytoin IV
There was a single study (Treiman 1998) with 196 participants. The study reported a statistically non‐significant trend favouring diazepam plus phenytoin for non‐cessation of seizures (42/95 versus 57/101 participants; RR 0.78, 95% CI 0.59 to 1.04). There was no statistically significant difference between the two groups for adverse effects (48/95 versus 44/101 participants; RR 1.16, 95% CI 0.86 to 1.56).
Comparison 15: Phenobarbitone IV versus phenytoin IV
There was a single study ( Treiman 1998) with 186 participants. There was a statistically non‐significant trend favouring phenobarbitone for non‐cessation of seizures (38/91 versus 51/95 participants; RR 0.78, 95% CI 0.57 to 1.06). There was no statistically significant difference between the two groups for adverse effects (46/91 versus 44/95 participants; RR 1.09, 95% CI 0.81 to 1.47).
Comparison 16: Lorazepam intranasal versus paraldehyde IM
There was a single study (Ahmad 2006) with 160 participants. There was no statistically significant difference between two groups for cessation of seizures (60/80 versus 49/80 participants; RR 1.22, 95% CI 0.99 to 1.52). There was no statistically significant difference between two groups for deaths (15/80 versus 13/80 participants; RR 1.15, 95% CI 0.59 to 2.27).
Comparison 17:Valproate IV versus Phenytoin IV
There was one study (Agarwal 2007) with a total of 100 participants. The study reported a statistically non‐significant trend favouring Intravenous valproate for reducing risk of non‐cessation of seizures (6/50 versus 8/50, RR 0.75, 95% CI 0.28 to 2.00).
Comparison 18: Midazolam IM versus lorazepam IV
There was a single study (Silbergleit 2012) with 893 participants. The study reported a statistically significant difference favouring midazolam for cessation of seizures (329/448 versus 282/445; RR 1.16, 95% CI 1.06 to 1.27), for requirement for intensive care unit (ICU) admission (128/448 versus 161/445 participants; RR 0.79, 95% CI 0.65 to 0.96), and for requirement for hospitalisation (258/448 versus 292/445 participants; RR 0.88, 95% CI 0.79 to 0.97).There was no statistically significant difference between the two groups for following outcomes: endotracheal Intubation (63/448 versus 64/445 participants; RR 0.98, 95% CI 0.71 to 1.35); recurrence of seizures (51/448 versus 47/445 participants; RR 1.08, 95% CI 0.74 to 1.57); adverse effects (16/448 versus 18/445 participants; RR 0.88, 95% CI 0.46 to 1.71): for ICU stay (mean difference (MD) 1.60, 95% CI ‐0.23 to 3.43, P =0.09); and length of hospital stay (MD 1.20, 95% CI ‐0.24 to 2.64, P =0.10).
Comparison 19: Valproate IV versus diazepam IV infusion
There were two studies (Chen 2011; Mehta 2007) with 106 participants. There was a statistically non‐significant trend favouring diazepam for non‐cessation of seizures (19/50 versus 19/56 participants; RR 1.16, 95% CI 0.71 to 1.89). There was a statistically significant difference between the two groups for the adverse effect of hypotension (0/50 versus 12/56, RR 0.08, 95% CI 0.01 to 0.58)
Comparison 20: Propofol IVversus barbiturates IV
There was a single study (Rossetti 2011) with 23 participants. There was no statistically significant difference between the two groups in any of the following outcomes: refractory status epilepticus (RSE) controlled with first course of drug (6/14 versus 2/9 participants; RR 1.93, 95% CI 0.49 to 7.54); RSE treated subsequently (4/8 versus 5/7 participants; RR 0.70, 95% CI 0.30 to 1.62); thrombotic/embolic complication (0/14 versus 0/9 participants); mortality (6/14 versus 3/9 participants; RR 1.29, 95% CI 0.43 to 3.88); functional outcome at three weeks (5/14 versus 3/9 participants; RR 1.07, 95% CI 0.34 to 3.42); infection requiring antibiotics (7/14 versus 6/9 participants; RR 0.75, 95% CI 0.37 to 1.51).
Discussion
Our review demonstrated that there are few reported randomised studies on drug interventions used in status epilepticus. Considering that the condition is relatively frequent in neurological practice, it is surprising that we found only 18 studies with analysable data. For status epilepticus, carrying out randomised controlled trials (RCTs) in the emergency situation may be difficult; particularly when the patient is unconscious, which makes getting rapid consent to join a trial difficult. This review highlights the need to conduct more randomised studies in status epilepticus.
The review has demonstrated several areas requiring attention in future research in status epilepticus. A universally acceptable definition of premonitory, early, established and refractory status needs to be agreed upon and used consistently by investigators. Agreement on the definition of outcomes and method of data presentation is also desirable to facilitate meta‐analysis. In particular, reports should provide the number of participants having each outcome and the denominator in analyses should be the number of participants rather than the number of episodes of status epilepticus.
The time frame required of the administration of interventions would be an important element for study/comparison in the future. A table has been added depicting the time since onset of status to administration of drug and since administration of drug to cessation of seizures as reported for drugs in respective studies (Table 2).
| Lorazepam lV | Diazepam‐phenytoin IV | Diazepam IV | Paraldehyde IM | Valproate IV | Levetiracetam IV | Midazolam IV infusion | Midazolam IM | |
| Time since onset of status to administration of drug (min) | 30 (10‐441) (Mehta 2007) | 120 (35.5‐252) (Ahmad 2006) | 30 (5.5‐147) (Mehta 2007) | |||||
| 31.3 (16.8‐45.8) (Alldredge 2001) | ||||||||
| Time since administration of drug to seizure cessation (min) | 0.3 (0.25‐0.38) (Sreenath 2010) | 0.3 (0.255‐0.4) (Sreenath 2010) | 15.8 (2.8‐28.8) (Singhi 2002) (for RSE) | 8 (5‐21) (Ahmad 2006) | ‐ | ‐ | 15.9 (6.3‐25.5) (Singhi 2002) (for RSE) | 3.3 (Silbergleit 2012) |
| 7.5 (4.5‐11.5) (Ahmad 2006) | ||||||||
| 1.6 (Silbergleit 2012) |
IM: intramuscular injection
IV: administered intravenously
RSE: refractory status epilepticus
Summary of main results
Even with limited data, the results may be summarised as follows.
-
Diazepam was better than placebo for cessation of seizures: there was a lower risk of requirement for ventilatory support and continuation of status epilepticus requiring a different drug or general anaesthesia with diazepam.
-
Lorazepam was better than placebo for cessation of seizures and carried a lower risk for continuation of status epilepticus requiring a different drug or general anaesthesia.
-
Lorazepam was better than diazepam for cessation of seizures and had a lower risk for continuation of status epilepticus requiring a different drug or general anaesthesia.
-
Lorazepam was better than phenytoin for cessation of seizures.
5. For pre hospital treatment, midazolam IM was as effective and probably better than lorazepam IV for cessation of seizures, frequency of hospitalisation and ICU admissions but not for risk of recurrence of seizures.
No definitive conclusions were possible with other comparisons. However, statistically non‐significant trends were found for the following.
-
Lorazepam IV versus diazepam IV ‐ trend favouring lorazepam for ventilatory support and adverse effects.
-
Lorazepam IV versus placebo ‐ trend favouring lorazepam for death, requirement for ventilatory support and adverse effects.
-
Lorazepam IV versus diazepam plus phenytoin IV ‐ trend favouring lorazepam for cessation of seizures and adverse effects.
-
Midazolam versus lorazepam IV ‐ trend favouring midazolam for cessation of seizures, need for ventilatory support, adverse effects and continuation of status epilepticus requiring a different drug or general anaesthesia.
-
Midazolam versus diazepam IV ‐ trend favouring diazepam for reduced number of deaths.
-
Diazepam versus placebo gel ‐ trend towards fewer adverse effects with placebo gel.
-
Diazepam plus phenytoin versus phenytoin IV ‐ trend favouring diazepam plus phenytoin for cessation of seizures.
-
Phenobarbitone versus phenytoin IV ‐ trend favouring phenobarbitone for cessation of seizures.
Overall completeness and applicability of evidence
Considering the frequency and seriousness of the condition, the body of randomised evidence available to guide clinical decisions is small. The only clinically applicable conclusions appear to favour lorazepam or midazolam as initial treatment of status epilepticus. Of these, adequate evidence is available only in favour of midazolam IM. However, this result is applicable only for pre hospital treatment. Once a patient has reached hospital, the intravenous (IV) route is the preferred route, for which evidence seems to favour lorazepam.
Quality of the evidence
The quality of evidence is not strong, but appears acceptable.
Potential biases in the review process
Several criteria to assess risk of bias could not be assessed because of unclear reporting by the authors. Attempts to contact the authors for clarification did not succeed. This may have underestimated/overestimated the risk of bias in the studies.
Agreements and disagreements with other studies or reviews
Seperate systematic reviews for drugs like levetiracetam have appeared in literature but their results lack precision because of the small number of studies and events.
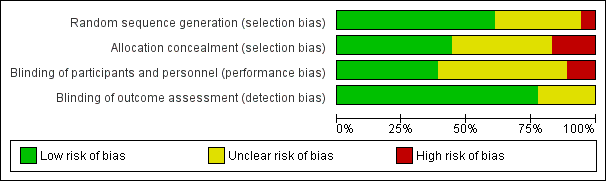
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Lorazepam IV versus diazepam IV, Outcome 1 Non‐cessation of seizures.

Comparison 1 Lorazepam IV versus diazepam IV, Outcome 2 Requirement for ventilatory support.

Comparison 1 Lorazepam IV versus diazepam IV, Outcome 3 Adverse effects.
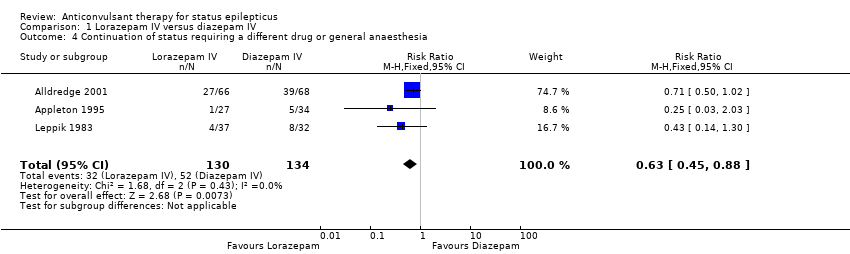
Comparison 1 Lorazepam IV versus diazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.

Comparison 1 Lorazepam IV versus diazepam IV, Outcome 5 Death.

Comparison 2 Lorazepam IV versus placebo IV, Outcome 1 Non‐cessation of seizures.

Comparison 2 Lorazepam IV versus placebo IV, Outcome 2 Requirement for ventilatory support.

Comparison 2 Lorazepam IV versus placebo IV, Outcome 3 Adverse effects.

Comparison 2 Lorazepam IV versus placebo IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.

Comparison 2 Lorazepam IV versus placebo IV, Outcome 5 Death.

Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 1 Non‐cessation of seizures.

Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 2 Adverse effects.

Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 3 Deaths.

Comparison 3 Lorazepam IV versus diazepam plus phenytoin IV, Outcome 4 Requirement of Ventilatory support.

Comparison 4 Lorazepam IV versus phenobarbitone IV, Outcome 1 Non‐cessation of seizures.

Comparison 4 Lorazepam IV versus phenobarbitone IV, Outcome 2 Adverse effects.

Comparison 5 Lorazepam IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.

Comparison 5 Lorazepam IV versus phenytoin IV, Outcome 2 Adverse effects.

Comparison 6 Midazolam IV versus lorazepam IV, Outcome 1 Non‐cessation of seizures.

Comparison 6 Midazolam IV versus lorazepam IV, Outcome 2 Requirement for ventilatory support.

Comparison 6 Midazolam IV versus lorazepam IV, Outcome 3 Adverse effects.

Comparison 6 Midazolam IV versus lorazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.

Comparison 7 Midazolam IV versus diazepam IV, Outcome 1 Non‐cessation of seizures.

Comparison 7 Midazolam IV versus diazepam IV, Outcome 2 Requirement for ventilatory support.

Comparison 7 Midazolam IV versus diazepam IV, Outcome 3 Adverse effects.

Comparison 7 Midazolam IV versus diazepam IV, Outcome 4 Death.

Comparison 8 Midazolam IM versus diazepam IV, Outcome 1 Non‐cessation of seizures.

Comparison 8 Midazolam IM versus diazepam IV, Outcome 2 Requirement for ventilatory support.

Comparison 8 Midazolam IM versus diazepam IV, Outcome 3 Adverse effects.

Comparison 8 Midazolam IM versus diazepam IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.

Comparison 9 Diazepam IV versus placebo IV, Outcome 1 Non‐cessation of seizures.

Comparison 9 Diazepam IV versus placebo IV, Outcome 2 Requirement for ventilatory support.

Comparison 9 Diazepam IV versus placebo IV, Outcome 3 Adverse effects.

Comparison 9 Diazepam IV versus placebo IV, Outcome 4 Continuation of status requiring a different drug or general anaesthesia.

Comparison 9 Diazepam IV versus placebo IV, Outcome 5 Death.

Comparison 10 Diazepam gel versus placebo gel, Outcome 1 Non‐cessation of seizures.

Comparison 10 Diazepam gel versus placebo gel, Outcome 2 Adverse effects.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 1 Non‐cessation of seizure.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 2 Siezure recurrence at 1‐24 h.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 3 Hypotension.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 4 Respiratory failure.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 5 Death.

Comparison 11 Levetiracetam versus lorazepam IV, Outcome 6 Ventilatory need.

Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 1 Non‐cessation of seizures.

Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 2 Adverse effects.

Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 3 Requirement for ventilatory support.

Comparison 12 Diazepam plus phenytoin IV versus phenobarbitone IV, Outcome 4 Death.

Comparison 13 Diazepam plus phenytoin IV versus phenobarbitone IV (premonitory status), Outcome 1 Non‐cessation of seizures.

Comparison 14 Diazepam plus phenytoin IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.
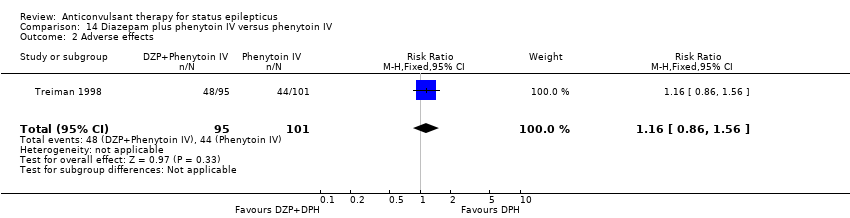
Comparison 14 Diazepam plus phenytoin IV versus phenytoin IV, Outcome 2 Adverse effects.

Comparison 15 Phenobarbitone IV versus phenytoin IV, Outcome 1 Non‐cessation of seizures.
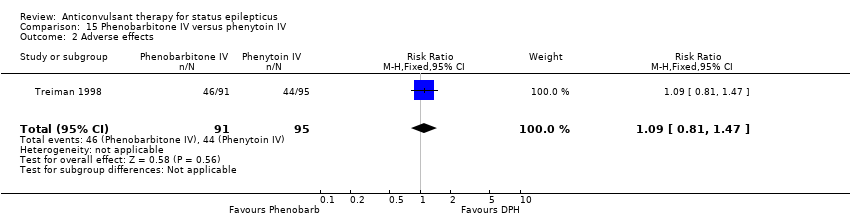
Comparison 15 Phenobarbitone IV versus phenytoin IV, Outcome 2 Adverse effects.

Comparison 16 Lorazepam intranasal versus paraldehyde IM, Outcome 1 Cessation of seizures.

Comparison 16 Lorazepam intranasal versus paraldehyde IM, Outcome 2 Deaths.

Comparison 17 Valproate versus phenytoin IV, Outcome 1 Non‐cessation of seizure.

Comparison 17 Valproate versus phenytoin IV, Outcome 2 Adverse effects.

Comparison 17 Valproate versus phenytoin IV, Outcome 3 Deaths.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 1 Cessation of seizures.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 2 Endotracheal Intubation.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 3 Requirement of Hospitalisation.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 4 ICU Admissions.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 5 Recurrence of seizure.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 6 Adverse effects.
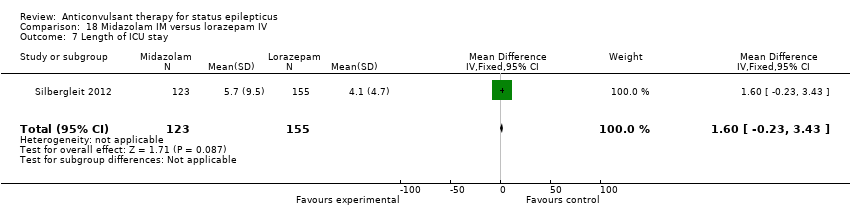
Comparison 18 Midazolam IM versus lorazepam IV, Outcome 7 Length of ICU stay.

Comparison 18 Midazolam IM versus lorazepam IV, Outcome 8 Length of Hospital stay.

Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 1 Non‐cessation of seizure.

Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 2 Hypotension.

Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 3 Relapse of seizure.

Comparison 19 Valproate IV versus diazepam IV infusion, Outcome 4 Respiratory depression.
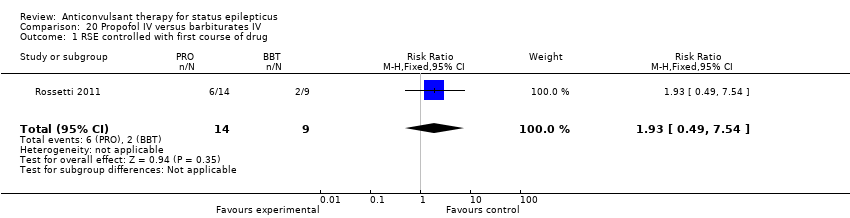
Comparison 20 Propofol IV versus barbiturates IV, Outcome 1 RSE controlled with first course of drug.

Comparison 20 Propofol IV versus barbiturates IV, Outcome 2 RSE treated subsequently.

Comparison 20 Propofol IV versus barbiturates IV, Outcome 3 Thrombotic/embolic complications.

Comparison 20 Propofol IV versus barbiturates IV, Outcome 4 Mortality.

Comparison 20 Propofol IV versus barbiturates IV, Outcome 5 Functional outcome at 3 weeks.

Comparison 20 Propofol IV versus barbiturates IV, Outcome 6 Infections requiring antibiotics.
| Lorazepam IV versus diazepam IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam IV | |||||
| Non‐cessation of seizures | Study population | RR 0.64 | 264 | ⊕⊕⊕⊝ | ||
| 381 per 1000 | 244 per 1000 | |||||
| Moderate | ||||||
| 219 per 1000 | 140 per 1000 | |||||
| Requirement for ventilatory support | Study population | RR 0.73 | 264 | ⊕⊕⊝⊝ | ||
| 127 per 1000 | 93 per 1000 | |||||
| Moderate | ||||||
| 125 per 1000 | 91 per 1000 | |||||
| Adverse effects | Study population | See comment | 264 | ⊕⊝⊝⊝ | Risks were calculated from pooled risk differences | |
| 82 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 103 per 1000 | 64 per 1000 | |||||
| Continuation of status requiring a different drug or general anaesthesia | Study population | RR 0.63 | 264 | ⊕⊕⊕⊝ | ||
| 388 per 1000 | 244 per 1000 | |||||
| Moderate | ||||||
| 250 per 1000 | 158 per 1000 | |||||
| Death | Study population | See comment | 203 | ⊕⊕⊕⊝ | Risks were calculated from pooled risk differences | |
| 30 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 22 per 1000 | 37 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Of the three studies, one (Appleton 1995) used odd/even dates for randomisation, clearly incurring high risk of bias. Two studies did not conceal allocation, incurring high risk of bias (Mehta 2007; Misra 2011) . . Furthermore an additional eight studies (Agarwal 2007; Chamberlain 1997; Chen 2011; McCormick 1999; Pellock 1998; Rossetti 2011; Shaner 1988; Sreenath 2010) did not provide clear information regarding generation of random sequence and/or allocation concealment.Taken together, there is enough risk of bias to downgrade the quality of evidence. | ||||||
| Lorazepam IV versus diazepam plus phenytoin IV for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lorazepam IV versus diazepam plus phenytoin IV | |||||
| Non‐cessation of seizures | Study population | RD ‐0.04 | 370 | ⊕⊕⊝⊝ | ||
| 227 per 1000 | 179 per 1000 | |||||
| Moderate | ||||||
| 221 per 1000 | 175 per 1000 | |||||
| Adverse effects | Study population | RR 0.85 | 370 | ⊕⊕⊕⊝ | ||
| 290 per 1000 | 246 per 1000 | |||||
| Moderate | ||||||
| 281 per 1000 | 239 per 1000 | |||||
| Deaths | See comment | See comment | Not estimable | 178 | See comment | Reported in a single study |
| Requirement of Ventilatory support | See comment | See comment | Not estimable | 178 | See comment | Reported in a single study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Even though one study did not have a clear concealment of allocation, this is possibly a lack of reporting. Lack of blinding in this study is unlikely to bias the outcome assessment as the outcomes are clearly detectable and objective. | ||||||
| Diazepam gel versus placebo gel for status epilepticus | ||||||
| Patient or population: patients with status epilepticus | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Diazepam gel versus placebo gel | |||||
| Non‐cessation of seizures | Study population | RR 0.43 | 165 | ⊕⊕⊕⊝ | ||
| 716 per 1000 | 308 per 1000 | |||||
| Moderate | ||||||
| 716 per 1000 | 308 per 1000 | |||||
| Adverse effects | Study population | RR 1.5 | 165 | ⊕⊕⊕⊝ | ||
| 250 per 1000 | 375 per 1000 | |||||
| Moderate | ||||||
| 248 per 1000 | 372 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 One placebo‐controlled study did not describe the method of sequence generation, but probably this is a limitation of writing style. Use of placebo will tend to limit any bias due to this. | ||||||
| Study | Definition |
| Continuous or repeated seizure activity > 5 minutes without recovery of consciousness. | |
| Leppik 1983 (mixed) | (a) Generalised tonic‐clonic status: three or more generalised tonic‐clonic seizures in one hour; two or more generalised seizures in rapid succession without recovery of consciousness; (b) absence status: confusional state with generalised 3 Hz spike wave pattern on EEG; (c) complex partial status: confusional state, clinical seizure or both with focal EEG abnormality; (d) elementary status: partial seizures without loss of consciousness. |
| Pellock 1998 (premonitory) | Acute repetitive seizures (no definition). |
| Remy 1991 (mixed) | Successive partial seizures for at least 20 minutes or two generalised tonic‐clonic seizures within 20 minutes. |
| Shaner 1988 (established/mixed) | Generalised convulsive status epilepticus: (a) a history of 30 minutes of continuous generalised convulsive seizures and witnessed generalised seizures in the emergency room; (b) a history of 30 minutes of recurrent generalised convulsive seizures but failure to attain baseline mental status between seizures and witnessed generalised seizures in the emergency room; (c) a history of 3 or more generalised convulsive seizures in one hour in patients with obtundation prior to the onset of status epilepticus and witnessed generalised convulsive seizures in the emergency room; (d) uncertain history of seizures but generalised convulsive seizures continuously for more than 5 minutes as witnessed in the emergency room. |
| Singhi 2002 (refractory) | Motor seizures uncontrolled after 2 doses of diazepam and phenytoin infusion. |
| McCormick 1999 (unclear) | No definition. |
| Chamberlain 1997 (premonitory) | Motor seizures of at least 10 minutes duration. |
| Appleton 1995 (unclear) | No definition. |
| Treiman 1998 (premonitory) | Overt generalised convulsive status: two or more generalised convulsions or continuous convulsive activity > 10 minutes. |
| Cereghino 2002 (premonitory) | Multiple seizures of complex partial or generalised type within an observation period between 12 ‐ 24 hours. |
| Two or more convulsive seizures without full recovery of consciousness between the seizures or continuous convulsions lasting for more than five minutes. | |
| 30 minutes of continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures. | |
| More than five minutes of continuous seizures or two or more discrete seizures between which there is incomplete recovery of consciousness. | |
| Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. | |
| Generalized convulsions continuing for minimum of five minutes. | |
| Continuous, generalized, convulsive seizure lasting greater than 5 minutes, or two or more seizures during which patient does not return to baseline consciousness. | |
| Continuous convulsions for longer than five minutes or having convulsions at the time of treatment after having intermittent seizures without regaining consciousness for longer than five minutes. | |
| Continuous convulsive activity lasting for 5 minutes or more. |
| Lorazepam lV | Diazepam‐phenytoin IV | Diazepam IV | Paraldehyde IM | Valproate IV | Levetiracetam IV | Midazolam IV infusion | Midazolam IM | |
| Time since onset of status to administration of drug (min) | 30 (10‐441) (Mehta 2007) | 120 (35.5‐252) (Ahmad 2006) | 30 (5.5‐147) (Mehta 2007) | |||||
| 31.3 (16.8‐45.8) (Alldredge 2001) | ||||||||
| Time since administration of drug to seizure cessation (min) | 0.3 (0.25‐0.38) (Sreenath 2010) | 0.3 (0.255‐0.4) (Sreenath 2010) | 15.8 (2.8‐28.8) (Singhi 2002) (for RSE) | 8 (5‐21) (Ahmad 2006) | ‐ | ‐ | 15.9 (6.3‐25.5) (Singhi 2002) (for RSE) | 3.3 (Silbergleit 2012) |
| 7.5 (4.5‐11.5) (Ahmad 2006) | ||||||||
| 1.6 (Silbergleit 2012) | ||||||||
| IM: intramuscular injection | ||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.45, 0.90] |
| 2 Requirement for ventilatory support Show forest plot | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.36, 1.49] |
| 3 Adverse effects Show forest plot | 3 | 264 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.10, 0.03] |
| 4 Continuation of status requiring a different drug or general anaesthesia Show forest plot | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.88] |
| 5 Death Show forest plot | 2 | 203 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.04, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 2 Requirement for ventilatory support Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| 3 Adverse effects Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |
| 4 Continuation of status requiring a different drug or general anaesthesia Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.71] |
| 5 Death Show forest plot | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.18, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 2 | 370 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.35, 0.26] |
| 2 Adverse effects Show forest plot | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3 Deaths Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Requirement of Ventilatory support Show forest plot | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.21] |
| 2 Adverse effects Show forest plot | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.63, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.86] |
| 2 Adverse effects Show forest plot | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| 2 Requirement for ventilatory support Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 3.90] |
| 3 Adverse effects Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 3.90] |
| 4 Continuation of status requiring a different drug or general anaesthesia Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.25, 7.27] |
| 2 Requirement for ventilatory support Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.59, 2.07] |
| 3 Adverse effects Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.39, 1.66] |
| 4 Death Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [0.87, 14.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 2 Requirement for ventilatory support Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 3 Adverse effects Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 4 Continuation of status requiring a different drug or general anaesthesia Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.92] |
| 2 Requirement for ventilatory support Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.16, 0.94] |
| 3 Adverse effects Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.20, 1.04] |
| 4 Continuation of status requiring a different drug or general anaesthesia Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.92] |
| 5 Death Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.30, 0.62] |
| 2 Adverse effects Show forest plot | 2 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.94, 2.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizure Show forest plot | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.44, 2.13] |
| 2 Siezure recurrence at 1‐24 h Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.54] |
| 3 Hypotension Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 0.96] |
| 4 Respiratory failure Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 5 Death Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.51, 2.00] |
| 6 Ventilatory need Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.13, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.98, 16.30] |
| 2 Adverse effects Show forest plot | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 3 Requirement for ventilatory support Show forest plot | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.40, 2.52] |
| 4 Death Show forest plot | 1 | 36 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 2 Adverse effects Show forest plot | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.86, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizures Show forest plot | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.06] |
| 2 Adverse effects Show forest plot | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation of seizures Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.52] |
| 2 Deaths Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizure Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.28, 2.00] |
| 2 Adverse effects Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.16, 1.55] |
| 3 Deaths Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation of seizures Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.06, 1.27] |
| 2 Endotracheal Intubation Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.71, 1.35] |
| 3 Requirement of Hospitalisation Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| 4 ICU Admissions Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.96] |
| 5 Recurrence of seizure Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.57] |
| 6 Adverse effects Show forest plot | 1 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.71] |
| 7 Length of ICU stay Show forest plot | 1 | 278 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐0.23, 3.43] |
| 8 Length of Hospital stay Show forest plot | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.24, 2.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐cessation of seizure Show forest plot | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.71, 1.89] |
| 2 Hypotension Show forest plot | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.58] |
| 3 Relapse of seizure Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.83] |
| 4 Respiratory depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 RSE controlled with first course of drug Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.49, 7.54] |
| 2 RSE treated subsequently Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.30, 1.62] |
| 3 Thrombotic/embolic complications Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.43, 3.88] |
| 5 Functional outcome at 3 weeks Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.34, 3.42] |
| 6 Infections requiring antibiotics Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.37, 1.51] |

