Presión extratorácica negativa continua o presión positiva continua en las vías respiratorias en comparación con la asistencia respiratoria convencional para la insuficiencia respiratoria hipoxémica aguda en niños
Resumen
Antecedentes
La insuficiencia respiratoria hipoxémica aguda (IRHA) es una causa importante de mortalidad y morbilidad en los niños. Actualmente, la asistencia respiratoria con presión positiva es la atención estándar; sin embargo, se asocia con complicaciones. En estudios en animales y en estudios no controlados en humanos, se han demostrado ciertos efectos beneficiosos con la asistencia respiratoria con presión extratorácica negativa continua (PENC) o la asistencia respiratoria con presión positiva continua en las vías respiratorias con el uso de procedimientos no invasivos (CPAP‐Ni).
Objetivos
Evaluar la efectividad de la PENC o la CPAP‐Ni en comparación con la asistencia respiratoria convencional en niños (de al menos un mes de vida y menores de 18 años de edad) con IRHA debida a causas no cardiogénicas para mejorar la mortalidad o la morbilidad asociada con la IRHA.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL 2013, número 6, MEDLINE (enero de 1966 hasta junio, semana 3, 2013), EMBASE (1980 hasta julio de 2013) y en CINAHL (1982 hasta julio de 2013).
Criterios de selección
Se incluyeron ensayos clínicos aleatorios o cuasialeatorios sobre la PENC o la CPAP‐Ni versus el tratamiento estándar (incluida la asistencia respiratoria con presión positiva) que incluían niños (de al menos un mes de vida y menores de 18 años de edad en el momento de la asignación al azar) que cumplían con los criterios para el diagnóstico de IRHA, con al menos uno de los resultados informados.
Obtención y análisis de los datos
El riesgo de sesgo de los estudios incluidos se evaluó mediante la ocultación de la asignación, el cegamiento de la intervención, la completitud del seguimiento y el cegamiento de las mediciones de resultado. Se extrajeron los datos sobre las medidas de resultado relevantes y se calculó el tamaño del efecto mediante el cociente de riesgos (CR) y la diferencia de riesgos (DR) con los intervalos de confianza (IC) del 95%.
Resultados principales
Se identificaron dos estudios elegibles: uno sobre CPAP y uno sobre PENC (publicado como resumen). Ambos fueron estudios no cegados, con un riesgo de sesgo incierto debido principalmente a la falta de información adecuada para su evaluación. El estudio de CPAP incluyó a 37 niños que se asignaron a máscaras de oxígeno y CPAP e informó mejorías en la frecuencia respiratoria y en la saturación de oxígeno en ambos brazos después de 30 minutos de aplicación. El estudio de PENC se publicó como resumen e incluyó a 33 lactantes con bronquiolitis. En el estudio de PENC, hubo una reducción de la fracción de oxígeno inspirado (FiO2) (menor del 30% después de una hora de iniciado el tratamiento) en cuatro participantes del grupo de PENC en comparación con ninguno del grupo control (CR 10,7; IC del 95%: 0,6 a 183,9). En el grupo control, un lactante requirió CPAP y asistencia respiratoria mecánica, mientras que todos los lactantes del grupo de PENC fueron tratados sin intubación (CR para ambos resultados 0,40; IC del 95%: 0,02 a 9,06). Ninguno de los ensayos informó sobre la mortalidad. Ninguno de los ensayos incluidos informó eventos adversos.
Conclusiones de los autores
Faltan ensayos controlados bien diseñados sobre las formas no invasivas de asistencia respiratoria en los niños con IRHA. Se necesitan estudios que evalúen los resultados mortalidad, evitación de la intubación y sus complicaciones asociadas, estancia hospitalaria y comodidad de los pacientes.
PICOs
Resumen en términos sencillos
Presión extratorácica negativa continua o presión positiva continua de las vías respiratorias para niños con insuficiencia respiratoria aguda e insuficiencia de oxígeno
Los niños desarrollan insuficiencia respiratoria e insuficiencia de oxígeno cuando presentan enfermedades respiratorias infecciosas o no infecciosas. La presión extratorácica negativa continua (PENC) que mantiene los pulmones abiertos al crear una presión negativa sobre el tórax o la presión positiva continua de las vías respiratorias (del inglés CPAP) que mantiene los pulmones abiertos al proporcionar presión positiva en los pulmones durante todas las fases de la respiración, se utilizan para ayudar a aumentar los niveles sanguíneos de oxígeno y así reducir el daño orgánico y el riesgo de muerte. Sin embargo, la seguridad y la eficacia de estos métodos de asistencia respiratoria son inciertos. Las búsquedas para esta revisión se actualizaron en julio de 2013.
Se incluyeron dos estudios en la revisión: un estudio de PENC incluyó a 33 pacientes menores de un año de edad que presentaban bronquiolitis y un estudio de CPAP incluyó a 37 pacientes que presentaban enfermedad relacionada con la fiebre del dengue. Ambos estudios informaron mejorías a corto plazo, pero no se encuentran disponibles informes sobre resultados clínicamente significativos. No fue posible evaluar la seguridad de los enfoques por el escaso número de pacientes en ambos estudios. Ambos estudios presentan problemas metodológicos y no tienen poder estadístico suficiente (incluyeron muy pocos pacientes para detectar una diferencia significativa). Ninguno de los ensayos incluidos informó eventos adversos. Se necesitan estudios controlados multicéntricos bien diseñados con números adecuados de lactantes que evalúen resultados clínicamente importantes, ya que no es posible hacer comentarios sobre la seguridad de la intervención debido a que no se evaluó en los estudios actuales. La limitación principal de esta revisión es que cuenta con un número muy limitado de estudios que incluyen una muestra muy pequeña de niños.
Authors' conclusions
Summary of findings
| Continuous negative extrathoracic pressure ventilation compared with control for acute hypoxaemic respiratory failure in children | |||
| Patient or population: children with acute hypoxaemic respiratory failure Settings: hospital Intervention: continuous negative extrathoracic pressure Comparison: control | |||
| Outcomes | Relative effect | No. of participants | Quality of the evidence |
| Less than 30% FiO2 after 1 hour of therapy | RR 10.69 (0.62 to 183.85) | 33 (1) | ⊕⊝⊝⊝ |
| Intermittent positive pressure ventilation during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ |
| CPAP during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| CPAP = continuous positive extrathoracic pressure 1 = Very wide confidence intervals 2= The outcome is only reported after one hour of treatment which is a very short period for any clinically meaningful assessment 3= Limitation in study design as the results are reported in an abstract and it was not possible to assess the study adequately (see assessment of risk of biases) | |||
Background
Description of the condition
Acute hypoxaemic respiratory failure (AHRF) is associated with high mortality and morbidity (Peters 1998). The underlying cause of AHRF is the major determinant of mortality rather than the severity of AHRF itself (Peters 1998). Major causes of AHRF in children include respiratory infection, non‐infectious respiratory diseases and interstitial pneumonitis and associated multi‐system failure. Vulnerable children may be immunodeficient, born prematurely or those with neuromuscular disorders.
Description of the intervention
Management of AHRF requires treatment of the primary cause. It also requires respiratory support, for which there are various methods including oxygen administration, continuous distending pressure (CDP) ventilation or both. CDP can be applied via positive or negative pressure ventilation. Positive pressure support can be achieved by non‐invasive or invasive continuous positive airway pressure (Ni‐CPAP or iCPAP) or positive pressure ventilation (PPV). CPAP is achieved with a face mask, nasal prongs, nasopharyngeal tube or endotracheal tube using a conventional ventilator or CPAP driver. PPV is achieved via an endotracheal tube attached to a conventional ventilator.
The purpose of CPAP is to avoid airway collapse even during the expiration phase in order to improve oxygenation (Alexander 1979). It reduces mortality and the rate of intubation in patients with cardiogenic pulmonary oedema (Pang 1998). Earlier use of CPAP was shown to be effective in increasing oxygenation in preterm infants with hyaline membrane disease (Allen 1977). Delivered via an endotracheal tube, CPAP became the most widely used form of respiratory support. More recently, with modern, often micro‐processor‐controlled, continuous negative pressure ventilators, CPAP has been delivered without endotracheal intubation.
How the intervention might work
Continuous negative extrathoracic pressure ventilation (CNEP) is an alternative, non‐invasive form of respiratory support. It is applied externally to the thorax using a negative pressure chamber with a seal around the neck to produce lung distension. CNEP was the only way of providing respiratory support prior to the advent of modern ventilators (Woollam 1976a; Woollam 1976b). In a piglet model of acute lung injury, both CNEP and positive end expiratory pressure (PEEP) were found to have similar effects on pulmonary function and the cardiovascular system (Easa 1994). Skaburskis 1987 demonstrated in a dog model of acute lung injury that CNEP provided similar improvements to positive end expiratory pressure ventilation in oxygenation without cardiac depression. Adams 1992 showed that CNEP improved oxygenation with maintenance of cardiac output in piglets with normal lungs. CNEP reduced the risks associated with intubation such as the introduction of pathogens into the lungs, impedance to venous return to heart and pulmonary circulation, and the subsequent increase in pulmonary vascular resistance (Raine 1993). Successful use of CNEP has been reported in patients with central hypoventilation (Hartmann 1994b), cystic fibrosis (Klonin 2000), postcardiac surgery (Penny 1991) and patients with phrenic nerve palsy (Raine 1992). The disadvantages of CNEP include technical difficulties, obstructed venous flow from the upper half of the body due to the neck seal and difficult access for care of the patient (McGettigan 1998).
Why it is important to do this review
A previous systematic review showed that continuous distending pressure (CPAP or CNEP) for respiratory distress syndrome in neonates reduced mortality in preterm infants despite an increased rate of pneumothoraces (Ho 2010). They included three studies of CNEP (pressure varied between 4 and 14 cm) and three studies of CPAP (applied via face mask) in their review. Uncontrolled clinical trials have shown beneficial effects of CNEP with or without assisted ventilation (Chernick 1972; Cvetnic 1990; Outerbridge 1972). A cross‐over trial of intermittent mandatory ventilation with CNEP compared with PEEP for neonatal hypoxaemia showed that rescue therapy with CNEP was effective in infants with refractory hypoxaemia and may avoid extracorporeal life support therapy (Cvetnic 1992). Gappa 1994 observed improvements in pulmonary mechanics in infants recovering from neonatal respiratory distress syndrome. CNEP may avoid the need for extracorporeal life support in infants with severe lung disease and pulmonary hypertension (Sills 1989). In adults, CNEP was shown to be associated with side effects such as apnoea or hypopnoea (Levy 1992), lower oesophageal sphincter dysfunction (Marino 1992), predisposition to the risk of aspiration and increased venous return (Borelli 1998). These side effects can be harmful to children with certain disorders such as Duchenne muscular dystrophy.
We undertook this review because the use of CNEP or Ni‐CPAP compared to PPV for AHRF has not previously been systematically evaluated in terms of relative effectiveness in children.
Objectives
To assess the effectiveness of CNEP or Ni‐CPAP compared to conventional ventilation in children (at least one month old and less than 18 years of age) with AHRF due to non‐cardiogenic causes for improving the mortality or morbidity associated with AHRF.
AHRF was defined by any of the following definitions:
-
alveolar‐arterial oxygenation gradient more than 100; or
-
arterial oxygen tension (PaO2)/fraction of inspired oxygen (FiO2) ratio less than 200 in the presence of respiratory symptoms; or
-
PaO2 less than 10 kPa with an FiO2 more than 0.5 with bilateral diffuse infiltrates on chest X‐ray in the absence of cardiogenic causes.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomised controlled trials (RCTs) or quasi‐RCTs comparing CNEP (all forms of CNEP: constant pressure, variable pressure or either) or Ni‐CPAP with standard therapy (PPV with endotracheal intubation) in AHRF. We also intended to include studies with multiple cross‐over arms. We included results from published abstracts.
Types of participants
Children (at least one month old and less than 18 years) at the time of onset of therapy who had AHRF as outlined above.
Types of interventions
CNEP via a chamber enclosing the thorax and lower body with or without associated assisted PPV or Ni‐CPAP by mask, nasal prong or nasopharyngeal tube (without assisted PPV) compared with standard care (including PPV with endotracheal intubation). We included all studies with a comparison of CNEP and PPV irrespective of pressure applied.
Types of outcome measures
Primary outcomes
-
Mortality (both early and late, defined as less than and greater than 30 days after the diagnosis).
-
Improvement in oxygenation (at 24‐hourly intervals up to one week) as measured by oxygenation index (mean airway pressure x fractional concentration of oxygen x 100)/arterial oxygen tension (PaO2)) and hypoxia score (arterial oxygen tension/fractional inspired oxygen concentration ratio (PaO2/FiO2)) or improvement in PaO2 with reduction of FiO2 after starting the therapy.
-
Failure (use of any additional form of assisted ventilation).
Secondary outcomes
-
Improvement in partial pressure of carbon dioxide in arterial blood (PaCO2).
-
Pulmonary morbidity as judged by pulmonary air leak (any air leak, gross air leak including pneumothorax) and duration of oxygen therapy.
-
Number of apnoeic episodes.
-
Episodes of aspiration pneumonia.
-
Development of cardiovascular compromise leading to congestive heart failure.
-
Skin ulceration or destruction of nasal columella associated with CPAP with nasal prongs.
-
Duration of stay in the intensive care unit (ICU).
-
Duration of stay in the hospital.
-
Long‐term survival, neurodevelopmental outcomes and health‐related quality of life as defined by the primary authors.
These outcomes included all the possible and clinically relevant outcomes associated with AHRF in children. Oxygenation index and hypoxia score (PaO2/FiO2) were used as outcome measurements, as the former takes into account the extent of mechanical ventilation support needed, while the latter indicates the degree of intrapulmonary oxygen exchange.
Search methods for identification of studies
Electronic searches
For this 2013 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 6) which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2011 to June week 3, 2013), EMBASE (January 2011 to July 2013) and CINAHL (January 2011 to July 2013). The previous update was in January 2011.
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We also combined the search with the search strategy developed by Boluyt 2008 to identify child studies. See Appendix 1 for details of the CENTRAL and MEDLINE search strategy used in this update. We adapted the search strategy for EMBASE (Appendix 2) and CINAHL (Appendix 3). The MEDLINE search strategy used prior to the 2011 update is in Appendix 4.
Searching other resources
We searched ClinicalTrials.gov and WHO ICTRP for completed and ongoing trials (2 July 2013). We also searched published abstracts from the meetings of the American Thoracic Society and Pediatric Critical Care Meetings (1992 to 2010) and bibliographies of identified articles, and we contacted field experts. We imposed no language or publications restrictions. Two review authors (PS, AO) independently reviewed the searches, evaluated the titles and abstracts of all identified studies, and retrieved full texts for assessment. We reached agreement by consensus, or the third review author (JS) resolved the disagreement. All three review authors verified data entry into the Review Manager software (RevMan 2012).
Data collection and analysis
Selection of studies
We reached agreement about trial inclusion by consensus. One author (PS) selected articles from an initial list of articles for detailed evaluation. Two authors (PS, AO) performed a detailed evaluation of selected studies and resolved discrepancies by involving the third author (JS).
Data extraction and management
Two review authors (PS, JS) independently extracted data using custom‐designed data collection forms. A third review author (AO) resolved discrepancies.
Assessment of risk of bias in included studies
Each review author performed an independent assessment of trials for methodological quality according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We independently assessed each identified trial for methodological quality with respect to: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting and other bias. We included this information in the Characteristics of included studies table and completed the 'Risk of bias' tables, addressing the above methodological issues.
1. Random sequence generation. For each included study, we described the method used to generate the allocation sequence as:
-
low risk of bias (any truly random process, for example, random number table, computer random number generator);
-
high risk of bias (any non‐random process, for example, odd or even date of birth, hospital or clinic record number); or
-
unclear risk of bias (insufficient information about the sequence generation process to permit judgement of 'low risk' or 'high risk').
2. Allocation concealment. For each included study, we described the method used to conceal the allocation sequence as:
-
low risk of bias (for example, telephone or central randomisation, consecutively numbered, sealed, opaque envelopes);
-
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or
-
unclear risk of bias (insufficient information to permit judgement of 'low risk' or 'high risk').
3. Blinding of participants, personnel and outcome assessors. For each included study, we described the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the methods as:
-
low risk of bias, high risk of bias or unclear risk of bias for participants;
-
low risk of bias, high risk of bias or unclear risk of bias for study personnel; and
-
low risk of bias, high risk of bias or unclear risk of bias for outcome assessors and specific outcomes assessed.
4. Incomplete outcome data. For each included study and for each outcome we described the completeness of data including attrition and exclusions from the analysis. We addressed whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
-
low risk of bias (< 20% missing data);
-
high risk of bias (> 20% missing data); or
-
unclear risk of bias.
5. Selective outcome reporting. For each included study, we assessed the possibility of selective outcome reporting bias as:
-
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study's pre‐specified outcomes have been reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest are reported incompletely and so cannot be used, study fails to include results of a key outcome that would have been expected to have been reported); or
-
unclear risk of bias.
6. Other sources of bias. For each included study, we noted any important concerns regarding other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design). We assessed whether each study was free of other problems that could put it at risk of bias as follows:
-
yes (low risk of bias);
-
no (high risk of bias); or
-
unclear risk of bias.
Measures of treatment effect
We used RevMan 5.2 (RevMan 2012) for statistical analysis. We used the statistical parameters risk ratio (RR), risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB), number needed to treat for an additional harmful outcome (NNTH) and mean difference (MD) when appropriate. We used 95% confidence intervals (CIs) for estimates of treatment effects.
Unit of analysis issues
We used data from one patient only once, even if the patient received the intervention more than once, to avoid dependency of data.
Dealing with missing data
We contacted primary trial authors for missing information; however, we did not receive any response.
Assessment of heterogeneity
Clinical heterogeneity is described in the Characteristics of included studies table. We tested for study heterogeneity using the I2 statistic, to assess the appropriateness of combining studies. Because of significant heterogeneity between the two trials with respect to the intervention, we did not combine them in meta‐analyses.
Assessment of reporting biases
We planned to use funnel plots to assess for publication bias if appropriate.
Data synthesis
We synthesised data using the standard methods of the Cochrane Acute Respiratory Infections Group (ARI Group). We included the RR, RD, NNTB and NNTH, derived from 1/RD. We calculated the MD for continuous outcomes. We analyzed studies using the fixed‐effect model with the Cochrane RevMan 5.2 software (RevMan 2012). As studies were heterogeneous, we did not undertake meta‐analyses but descriptively summarised the results.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses on the basis of aetiology of AHRF and according to types of therapy, i.e. CNEP and Ni‐CPAP. We planned to undertake a sensitivity analysis by trial quality (randomised or quasi‐randomised). We planned a priori subgroup analyses as follows:
-
children with acute respiratory failure with no underlying illness;
-
children with respiratory failure with underlying conditions (we planned to divide this group into further groups if sufficient numbers of children were found in any group, such as children with neuromuscular diseases, restrictive or obstructive lung diseases, and children with immunodeficiency);
-
if a sufficient number of studies using a similar pressure of CNEP or PPV were available, we planned a subgroup analysis with an arbitrary cut‐off level of studies comparing CPAP greater than 5 and CPAP less than or equal to 5, and CNEP greater than 15 cm of water and less than or equal to 15 cm of water; and
-
Ni‐CPAP given via various methods.
Sensitivity analysis
If required, we planned a sensitivity analysis based on indication and method of non‐invasive support.
Results
Description of studies
Results of the search
From the initial search we screened 197 titles and for further determination of eligibility, we read their abstracts. We retrieved 18 studies for detailed evaluation and of these two studies met the eligibility criteria (Cam 2002; Hartmann 1994a). In this review update we retrieved 182 records from the searches of the electronic literature. However, no trials were eligible for inclusion.
Included studies
The Cam 2002 study included 37 children (18 received CPAP and 18 received oxygen via mask) who had dengue shock syndrome. The inclusion criteria were infants with a clinical diagnosis of dengue shock syndrome who had acute respiratory failure, defined as failure to respond to 40% oxygen given via nasal cannula, evidenced by (a) cyanosis, oxygen saturation < 93 or PaO2 < 70 mm Hg; (b) respiratory rate > 50 breaths/minute; or (c) severe chest retraction and nasal flaring. CPAP was started at 6 cm water pressure delivered through a Beneviste valve connected to binasal prongs at a FiO2 of 60%. The oxygen mask group received oxygen via a face mask with reservoir bag at a flow rate of 6 to 8 l/min resulting in a FiO2 of 60% to 80%. The management of infants in both groups remained similar except for the intervention. The outcome reported was stabilisation of the participant with a PaO2 > 80 mm Hg after 30 minutes of treatment.
The Hartmann 1994a study was published in abstract form and included 33 infants (15 received CNEP and 18 were controls) under one year of age. The inclusion criteria were a clinical diagnosis of bronchiolitis and oxygen requirement of greater than or equal to 40% to maintain oxygen saturation between 96% and 99%. CNEP was started at ‐6 cm water pressure. Weaning from CNEP was left at the discretion of the clinicians. The management of infants in the control group was unclear from the abstract. The outcomes reported were reduction in oxygen requirement within one hour of initiating the intervention, need for intubation or CPAP, and duration of CNEP.
Excluded studies
We identified two additional relevant reports (Linney 1997; Samuels 1989); these were reports from a single institution. The Linney 1997 study was published in abstract format. This was a report of the use of CNEP ventilation in 27 infants with bronchiolitis. The study was performed in an uncontrolled manner without randomisation. The Samuels 1989 study reported on 88 infants and young children with respiratory failure due to various causes. These patients received negative pressure ventilation via a purpose‐built respirator in a non‐randomised fashion. Neither study met the entry criteria specified a priori for this review. Neither of these reports were of randomised or non‐randomised studies.
In an updated search we identified one RCT of non‐invasive positive pressure ventilation in children with lower airway obstruction (Thill 2004). The children in this study did not meet the criteria for acute hypoxaemic respiratory failure. In the subsequent review update (Shah 2008) we identified a further eight studies relevant to the objectives of the review (Codazzi 2006; Katz 2004; Padman 2004; Palombini 2004; Prado 2005; Rodriguez 2002; Shime 2006; Thia 2007). Of these eight, five studies were single‐centre and single‐arm studies (Codazzi 2006; Padman 2004; Palombini 2004; Prado 2005; Shime 2006) and one (Katz 2004) was a retrospective case‐control study. Rodriguez 2002 performed a RCT of CPAP in patients with post‐extubation laryngitis and Thia 2007 performed a RCT of children with bronchiolitis and hypercapnia. Neither of these two studies met the criteria for hypoxaemic respiratory failure. In this current review update we identified another four single‐arm studies (Carretero 2008; Chidini 2010; Essouri 2008; Stucki 2009) and one double‐arm study (Sanabria 2008) comparing CPAP delivery by two methods (helmet versus mask). We have provided the reasons for exclusion of these studies in the Characteristics of excluded studies table.
Risk of bias in included studies
Both included studies in this review were open‐label studies. The risk of bias is summarised in Figure 1 and Figure 2. One was a study of CPAP (Cam 2002) whereas the other was a study of CNEP (Hartmann 1994a).
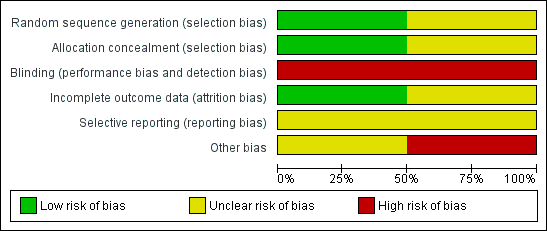
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
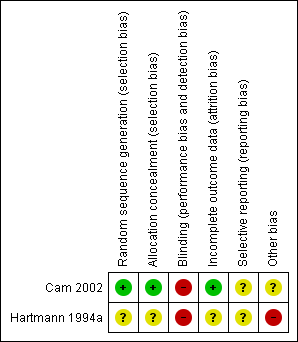
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In Cam 2002 randomisation was performed using sealed envelopes which were randomly numbered. This was an open‐label trial. Forty‐eight participants were enrolled but 11 were later excluded (two with pneumonia, three were comatose and six had negative serodiagnosis of dengue fever). We reported outcomes for the remaining 37 infants.
In Hartmann 1994a the randomisation procedure was not reported. Masking of the intervention was practically not possible, and information regarding masking of assessment of outcome was not provided in the abstract. Outcomes were reported for all infants enrolled in the study but only some of the outcomes specified in this review were reported.
Allocation
Cam 2002 stated that envelopes were randomly numbered, whereas Hartmann 1994a did not provide information regarding allocation generation.
Blinding
Neither studies masked the intervention.
Incomplete outcome data
Both studies reported data on all eligible infants.
Selective reporting
Cam 2002 reported data in the first 30 minutes after the intervention for some of the outcomes. Hartmann 1994a reported data on completion of the trial for some of the outcomes.
Other potential sources of bias
Data from Hartmann 1994a have not been published in full.
Effects of interventions
Primary outcomes
1. Mortality
This outcome was not reported in either included trial.
2. Improvement in oxygenation
In the Cam 2002 study 37 children were enrolled (19 in the oxygen mask group and 18 in the CPAP group). After 30 minutes of starting the intervention, the respiratory rate decreased significantly in the CPAP group (a mean of 55 breaths/minute at the start decreasing to a mean of 48 breaths/minute at the end of 30 minutes, compared to a mean of 60 breaths/minute at the start decreasing to a mean of 58 breaths/minute at the end of 30 minutes in the oxygen mask group; P < 0.05). Oxygen saturation increased significantly in both groups (mean of 93% at the start improving to a mean of 96% at the end of 30 minutes in the CPAP group compared to a mean of 94% at the start in the oxygen mask group improving to a mean of 98% at the end of 30 minutes in the oxygen mask group; P < 0.05). PaO2 improved significantly in both groups (a mean of 65 mm Hg at the start increasing to a mean of 120 mm Hg at the end of 30 minutes in the CPAP group, compared to a mean of 91 mm Hg at the start increasing to a mean of 146 mm Hg at the end of 30 minutes in the oxygen mask group; P < 0.01).
In the Hartmann 1994a study 15 infants were enrolled in the CNEP group and 18 infants were enrolled in the control group. After one hour of intervention the oxygen requirement was reduced to less than or equal to 30% in four infants in the CNEP group, while none of the infants in the control group had such an improvement (typical RR 10.7, 95% CI 0.6 to 183.9 and RD 0.27, 95% CI 0.02 to 0.51) (Analysis 1.1). No further respiratory parameters were reported in the study.
3. Failure (use of any additional form of assisted ventilation)
In the Cam 2002 study all participants in the CPAP group met the success criteria, whereas one participant in the oxygen mask group failed to respond and subsequently responded when given CPAP. After the initial 30 minutes, 12 out of 18 participants in the oxygen mask group worsened and then improved after CPAP institution. Four participants in the CPAP group worsened in the later phase and required mechanical ventilation.
In the Hartmann 1994a study none of the infants in the CNEP group required assisted ventilation or nasal CPAP, while one infant each required assisted ventilation and nasal CPAP in the control group (typical RR for both outcomes 0.40, 95% CI 0.02 to 9.06 and RD ‐0.06, 95% CI ‐0.21 to 0.10) (Analysis 2.1; Analysis 2.2). The median duration of CNEP was five (range one to seven) days.
Secondary outcomes
1. Improvement in partial pressure of carbon dioxide in arterial blood
This outcome was not reported in either included trial.
2. Pulmonary morbidity as judged by pulmonary air leak (any air leak, gross air leak including pneumothorax) and duration of oxygen therapy
This outcome was not reported in either included trial.
3. Number of apnoeic episodes
This outcome was not reported in either included trial.
4. Episodes of aspiration pneumonia
This outcome was not reported in either included trial.
5. Development of cardiovascular compromise leading to congestive heart failure
This outcome was not reported in either included trial.
6. Skin ulceration or destruction of nasal columella associated with CPAP with nasal prongs
This outcome was not reported in either included trial.
7. Duration of stay in the intensive care unit (ICU)
This outcome was not reported in either included trial.
8. Duration of stay in the hospital
This outcome was not reported in either included trial.
9. Long‐term survival, neurodevelopmental outcomes and health‐related quality of life as defined by primary authors
This outcome was not reported in either included trial.
Discussion
Summary of main results
The effectiveness of either continuous negative extrathoracic pressure (CNEP) or non‐invasive continuous positive airway pressure (Ni‐CPAP) in acute hypoxaemic respiratory failure (AHRF) in children has not been evaluated in detail. We included two studies in this review, one of which is published in an abstract form only (Hartmann 1994a). Cam 2002 studied the effectiveness of CPAP over a 30‐minute period in children with dengue fever and Hartmann 1994a studied the effectiveness of CNEP in children with AHRF over one hour. Short‐term improvements in outcomes were reported in both studies with improvement in haemodynamic parameters. The severity of the underlying illness in both studies is difficult to assess from the data provided because in one study (Cam 2002) only four participants required mechanical ventilation whereas in the second study (Hartmann 1994a) only one of the control group infants required assisted ventilation.
Overall completeness and applicability of evidence
Samuels 1989 was the first to report on the use of CNEP in this population. The participants were not randomised. Reduction in oxygen requirements was evident in 75 out of 88 participants after two hours and in 74 out of 88 participants after 48 hours. There was no comparison group so it is difficult to assess the effectiveness of CNEP on mortality or other significant outcomes. Linney 1997 published a report from the same institution on a subgroup of patients with bronchiolitis. The authors showed that CNEP helped in avoiding intubation in the majority of participants (26 out of 27). This was a non‐randomised study. Samuels 1996 reported on the use of CNEP in neonatal respiratory distress syndrome in a randomised controlled trial (RCT). Infants were randomised to standard therapy or standard therapy and CNEP. CNEP resulted in a reduction in the number of infants needing intubation (5%), reduced duration of oxygen therapy and subsequent reduction in the incidence of chronic lung disease of preterm infants. However, there was a statistically insignificant increase in mortality, cranial ultrasound abnormalities and pneumothoraces in the CNEP group.
Corrado 1998, in a retrospective case‐control study, compared CNEP and conventional ventilation in the treatment of acute respiratory failure in chronic obstructive pulmonary disease (COPD) patients and found negative pressure ventilation to be equally efficacious in reducing in‐hospital mortality. Corrado 2002, in a retrospective case‐control study, compared negative pressure ventilation and non‐invasive positive pressure ventilation in the treatment of acute chronic respiratory failure in adult patients with COPD in four intermediate respiratory intensive care units (ICUs) in Italy. Both ventilatory techniques were found to be equally effective in avoiding endotracheal intubation and death.
Gorini 2001 studied seven adult patients who had acute exacerbation of COPD using a microprocessor‐based negative pressure ventilator and found that CNEP in association with negative pressure ventilation improved ventilatory patterns, arterial blood gases and reduced the work of inspiratory muscles. Negative pressure ventilation has a limitation of triggering capability. Gorini 2002 studied a microprocessor‐based negative pressure ventilator capable of thermistor triggering in four normal participants and six patients with COPD. The authors found that this technique improved the synchrony between the patient and the negative pressure ventilator. Thus, in these uncontrolled studies negative pressure ventilation has been found to be equally as efficacious as conventional mechanical ventilation in adult patients with respiratory failure, however prospective studies comparing these interventions are lacking.
Soong 1993 studied 10 infants with bronchiolitis and impending respiratory failure in a non‐randomised study. They reported improvement in symptoms, signs and physiologic parameters (heart rate, respiratory rate, PaCO2 and oxygenation index) after two hours of application of CPAP.
Hilbert 2000 evaluated adult patients who were neutropenic and had AHRF treated by CPAP. They reported that CPAP was successful in avoiding intubation in 25% of the patients.
Use of CPAP in neonates has shown some benefits but the trials were conducted in the pre‐surfactant era (Ho 2010). Recently, Declaux 2000 in a RCT of CPAP in AHRF adults showed that after one hour of therapy the subjective response to treatment and PaO2/FiO2 ratio were better in the CPAP group compared to the standard therapy group, although this was not associated with a reduced rate of intubation or in‐hospital mortality.
The use of CPAP in paediatric AHRF is increasing. Several pilot studies (Essouri 2008; Sanabria 2008; Stucki 2009) were identified recently which have used different forms of CPAP devices such as helmet, nasal prongs or high‐flow nasal cannula to generate CPAP and effectively help patients to breath easily and support respiration during acute phases. The relative ease of application prior to invasive forms of ventilation has led clinicians to use CPAP without sufficient evidence. RCTs should be conducted in this population.
The role of non‐invasive ventilation in modern clinical practice was evaluated at an international consensus conference (Evans 2001). Important issues were raised in terms of study designs. These included matching of patients in a non‐randomised fashion, the small sample size of available studies and the influence of this on confounding variables, the potential for missing undetected adverse effects in a subgroup population, practical problems associated with unblinded studies of increased care and surveillance of study participants and non‐standardised assessment of qualitative endpoints. Recommendations were made for identifying means of rapidly assessing patients who will benefit from non‐invasive ventilatory modes. In adult patients with AHRF, evidence was pointing towards the effectiveness of non‐invasive PPV in avoiding intubation, complications and mortality. However, the need for larger controlled trials was identified (Evans 2001). Recently there have been reports on the use of CNEP and CPAP. However, these were single‐centre studies with no comparative cohort or, if a comparative cohort was included, the participants did not meet the criteria for hypoxaemic respiratory failure. In light of these reports, proper studies are warranted as this practice continues to be implemented in a random fashion.
For future studies it is important to decide the importance of various outcomes. Reduction of in‐hospital mortality is a very important outcome and even a small reduction would be beneficial. However, as patients with the most severe disease are not going to be candidates for these experimental interventions, such trials would require very large sample sizes to achieve any significant reduction. Other outcomes of interest may include reduction in intubation rates, associated complications, duration of hospital stay and increased patient comfort. Standardised interventions in the control group and assessments are warranted to assess these outcomes. CNEP and Ni‐CPAP are perceived to be potentially useful in certain clinical situations where avoidance of intubation or early extubation and management by CNEP or Ni‐CPAP may be useful, but further studies are warranted.
Thus, there is insufficient evidence from randomised studies to conclude that CNEP is beneficial in AHRF in paediatric patients. Potential advantages, such as reduction in the intubation rate, intubation‐associated complications and patient comfort (less restlessness, discomfort or dyspnoea), make this modality appealing. However, properly conducted RCTs are needed to assess the benefits and risks.
Quality of the evidence
The quality of the CPAP study (Cam 2002) was relatively good, whereas the quality of the CNEP study (Hartmann 1994a) was difficult to assess as it was only published in abstract form. Both studies reported on short‐term outcomes only and had small sample sizes.
Potential biases in the review process
None.
Agreements and disagreements with other studies or reviews
Guidelines for non‐invasive management of respiratory failure have been published. However, most of the evidence stems from non‐randomised studies.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
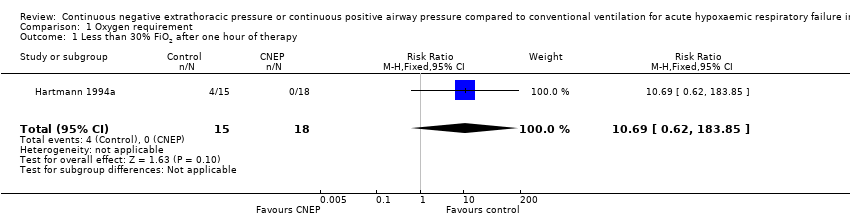
Comparison 1 Oxygen requirement, Outcome 1 Less than 30% FiO2 after one hour of therapy.

Comparison 2 Need for assisted ventilation (intermittent positive pressure ventilation or CPAP), Outcome 1 Intermittent positive pressure ventilation during hospital stay.

Comparison 2 Need for assisted ventilation (intermittent positive pressure ventilation or CPAP), Outcome 2 CPAP during hospital stay.
| Continuous negative extrathoracic pressure ventilation compared with control for acute hypoxaemic respiratory failure in children | |||
| Patient or population: children with acute hypoxaemic respiratory failure Settings: hospital Intervention: continuous negative extrathoracic pressure Comparison: control | |||
| Outcomes | Relative effect | No. of participants | Quality of the evidence |
| Less than 30% FiO2 after 1 hour of therapy | RR 10.69 (0.62 to 183.85) | 33 (1) | ⊕⊝⊝⊝ |
| Intermittent positive pressure ventilation during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ |
| CPAP during hospital stay | RR 0.40 (0.02 to 9.06) | 33 (1) | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| CPAP = continuous positive extrathoracic pressure 1 = Very wide confidence intervals 2= The outcome is only reported after one hour of treatment which is a very short period for any clinically meaningful assessment 3= Limitation in study design as the results are reported in an abstract and it was not possible to assess the study adequately (see assessment of risk of biases) | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Less than 30% FiO2 after one hour of therapy Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.69 [0.62, 183.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intermittent positive pressure ventilation during hospital stay Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.06] |
| 2 CPAP during hospital stay Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.06] |

