Intervenciones para proteger la función renal en el período perioperatorio
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cardiac surgery patients, with pre‐existing chronic kidney disease were studied (GFR <60ml/min.1.73m2). Excluded patients in severe renal failure, emergency surgery and intravenous contrast within 4 days. | |
| Participants | Intervention group (N‐acetylcysteine group), n= 50, Age: mean=70, SD=9. Control group (matching placebo), n=52, Age: mean=72, SD=9. | |
| Interventions | Oral N‐acetyl cysteine. 14 doses, twice daily. Started 1 day before surgery, (3 doses before surgery and 11 doses after surgery). Matching placebo for same duration and time used. The 2 groups were comparable. | |
| Outcomes | 30 day mortality, acute kidney injury, acute renal injury needing haemodialysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by the investigational (?) pharmacist. Block randomization (blocks of 10). |
| Allocation concealment (selection bias) | Low risk | Participants, researchers and clinicians blinded to treatment assignment. |
| Blinding (performance bias and detection bias) | Low risk | Participants, researchers and clinicians (including data collecting nurse) were blinded. Drug packets matched in volume, colour, consistency and transparency and given mixed with fruit juice to mask taste. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Consecutive patients for coronary artery bypass graft (CABG); randomization done, but method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. | |
| Interventions | Glutathione 200 mg/kg IV before bypass and same dose repeated during 1st and 2nd postoperative days. | |
| Outcomes | Urine output, creatinine clearance, fractional excretion of sodium | |
| Notes | No response to letter for details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Randomly assigned’ into two groups. No details of randomization given |
| Allocation concealment (selection bias) | High risk | D ‐ Not used |
| Blinding (performance bias and detection bias) | High risk | None described; control group had no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Methods | Consecutive patients for CABG; randomization method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Diltiazem group n = 13, age, mean = 54.5, SD = 1.8; control group n = 10, age, mean = 54.2, SD = 1.6 | |
| Interventions | Diltiazem 0.1 mg/kg bolus, followed by infusion of 2 mcg/kg/min until end of aortic cross clamping, followed by nasogastric administration every 8 hours for 24 hrs; control group received 5% dextrose in the same manner | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | No response to letter for details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Patients were randomized into either diltiazem or no treatment groups'; no details of randomization method |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) | High risk | None described; control group had no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Methods | Patients having CABG; randomization by card allocation; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Off pump n = 25, age, mean = 59.4, SD = 10.5; control (on pump), n = 26, age, mean = 63.8, SD = 6.7 | |
| Interventions | On pump and off pump used for revascularization of coronary arteries | |
| Outcomes | Creatinine clearance | |
| Notes | Inadequate response to letter from the contact author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Prospectively randomized by card allocation’ |
| Allocation concealment (selection bias) | Unclear risk | ‘Prospectively randomized by card allocation’; no further details given on allocation |
| Blinding (performance bias and detection bias) | High risk | Not discussed in text |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed |
| Methods | Cardiac surgery patients, over 18 yrs, elective or urgent surgery in patients with chronic renal impairment (preop CCl of under 40ml/min). Excluded renally crippled patients (on dialysis), pregnant or sensitivity to drug used. | |
| Participants | 3 intervention groups: Fenoldopam group – 19, NAC group – 20, NAC+ Fenoldopam group – 21, Placebo group ‐ 19. Ages: mean, SD G1 – 77.2, 1.2; G2 – 73.8, 2.2; G3 – 73.5, 2.0; G4 ‐ 72.4, 2.0 Sex (Male/ Female): G1 – 12 / 7; G2 – 15 / 5; G3 ‐ 12 / 9; G4 ‐ 13 / 6 | |
| Interventions | Four groups of participants. Group 1: Fenoldopam 0.1 mcg/kg/min at induction and continued for 48 hrs Group 2: N‐acetyl cysteine orally 600mg per day 1 day preop and on the morning of surgery and the night of surgery. Group 3: Fenoldopam and N‐acetylcysteine together Group 4: Placebo: Normal saline instead of fenoldopam, for 48 hrs (‘double blinded’). Taste controlled placebo instead of NAC, same time | |
| Outcomes | Mortality, acute renal injury (renal replacement therapy), creatinine clearance (Cockroft‐Gault) | |
| Notes | results reported as mean and SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by pharmacy dept; method of randomization uncertain |
| Allocation concealment (selection bias) | Unclear risk | No specific mention of allocation concealment except to say ‘double‐blinded’. |
| Blinding (performance bias and detection bias) | Unclear risk | No specific mention of who all were blinded; reports as ‘Double‐blinded’, placebo controlled trial. Not sure if blinding was adequate |
| Incomplete outcome data (attrition bias) | Low risk | Reports one withdrawal |
| Methods | Patients having CABG; randomization done, method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine 0.5 mcg/kg/min, n = 10, age, mean = 60, SD = 7.1; dopamine 1 mcg/kg/min, n = 10, age, mean = 62, SD = 8.2; dopamine 2 mcg/kg/min, n = 10, age, mean = 62, SD = 10.2; placebo, n = 14, age, mean = 62, SD = 6.3 | |
| Interventions | Different doses of diltiazem, after induction of anaesthesia and for 24 hours post‐operation | |
| Outcomes | Creatinine clearance | |
| Notes | Did not contact authors; old study and no additional information required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Placebo controlled prospective study’; no description of randomization |
| Allocation concealment (selection bias) | High risk | None described |
| Blinding (performance bias and detection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Methods | Patients undergoing cardiac surgery with cardiopulmonary bypass; randomization done using list from hospital pharmacy; allocation concealment method unclear; blinding of patients, researchers and care givers is unknown, but strong possibility; study of moderate methodological quality | |
| Participants | Cardiac surgery. diltiazem group, n = 12, age, mean = 72, range, 69‐76; placebo group, n = 12; age, mean = 73, range, 69‐74. All participants had high serum creatinine | |
| Interventions | Diltiazem 0. 25 mg/kg infusion for 15 min, followed by infusion of 1.7 mcg/kg/min for 24 hours | |
| Outcomes | Glomerular filtration rate (GFR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were consented and randomized; method of randomization not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Not used |
| Incomplete outcome data (attrition bias) | Low risk | Accounted for drop outs |
| Methods | High risk patients undergoing cardiac surgery; randomization by pharmacy by permuted block strategy (alternate blocks of 4 or 6); Allocation concealed by central randomization and drug (or placebo) dispensed by pharmacy by colour and consistency matching; everyone blinded to the nature of drugs | |
| Participants | Cardiac surgery. N‐acetyl cysteine, 4 doses or 600mg or placebo 4 doses of 5% dextrose. Intervention group, n = 148, age, mean = 68.9, SD = 8.9; placebo group, n = 147; age = 69.2, SD = 9.7. Three patients from NAC group and 4 patients from placebo group withdrawn | |
| Interventions | N‐acetyl cysteine or placebo given. 1st dose after induction of anaesthesia, 2nd dose at end of bypass, 3rd dose at 12 hrs in ICU and 4th dose at 24hrs | |
| Outcomes | Data available only on mortality and acute renal injury | |
| Notes | Contacted the authors successfully for data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by pharmacy trial co‐ordinator using a permitted block strategy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was using central randomization with drugs prepared by pharmacy |
| Blinding (performance bias and detection bias) | Low risk | Quadruple blinded (patients, clinicians, data collectors and data analyst) |
| Incomplete outcome data (attrition bias) | Low risk | Account for drop outs in the trial |
| Methods | Patients for cardiac surgery; randomization by computer generated random number tables by Pharmacy; allocation concealment clearly stated. Patients, researchers and care givers were blinded; study with good methodological quality | |
| Participants | Cardiac surgery. Mannitol group, n = 26, age, mean = 64.3, SD = 8.9; Dopamine group, n = 25, age, mean = 63.8, SD = 9.8; mannitol + dopamine group, n = 25, age, mean = 63.4, SD = 7.8 (this group excluded in review); Placebo group, n = 24, age, mean = 63.3, SD = 8.8; | |
| Interventions | Mannitol 1 g/kg into pump, dopamine 2 mcg/kg/min during surgery or both together as treatment; saline as placebo | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No further information sought from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Prospective randomized double‐blinded and placebo controlled study’. Computer generated random number tables were used |
| Allocation concealment (selection bias) | Unclear risk | Does not specifically describe it, but quite likely it was concealed allocation |
| Blinding (performance bias and detection bias) | Low risk | Blinded manner; drug or saline supplied by the dept investigational pharmacy in a blinded manner; additive for the CPB circuit prime (mannitol or saline, supplied similarly) |
| Incomplete outcome data (attrition bias) | Low risk | All allocated patients completed the trial (withdrawals before allocation) |
| Methods | Adults over 18yrs having cardiac surgery under cardiopulmonary bypass and having pre‐existing renal insufficiency (CCl <60ml/min) were studied. Exclcuded shocked patients and patients with aortic dissection. | |
| Participants | Intervention group: n=17; Age: mean=77, SD=10; Sex: M=12, F=5. Control group: n=19; Age: mean=78, SD=7; Sex: M=10, F=9. | |
| Interventions | Nesiritide infusion 0.005 mcg/kg/min, from start of induction of anaesthesia for 24 hrs. Control group received 'placebo', but not sure what it was. The 2 groups were not comparable. | |
| Outcomes | Mortality, acute renal injury (needing dialysis), and creatinine clearance (Cochroft‐Gault formula) | |
| Notes | Data available on plasma cystatin. plasma aldosterone, plasma cGMP and plasma BNP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomized’; no details provided; described as ‘double‐blind, placebo‐controlled proof of concept trial’ |
| Allocation concealment (selection bias) | Unclear risk | No details provided apart from 'double‐blind, placebo‐controlled proof of concept trial’ |
| Blinding (performance bias and detection bias) | Unclear risk | No details provided apart from 'double‐blind, placebo‐controlled proof of concept trial’ |
| Incomplete outcome data (attrition bias) | Low risk | Reports 4 withdrawals from trial |
| Methods | Patients undergoing robot‐assisted laparoscopic radical prostatectomy (RALRP). Excluded patients with chronic renal insufficiency preop | |
| Participants | Intervention group: n=50; Age: mean=67, SD=6. Control group: n=50; Age: mean=68, SD=4 | |
| Interventions | Intervention group: Nicardipine infusion ‐ after induction of anaesthesia infusion 0.5mcg/kg/min until end of surgery. Control group: Normal saline infusion at same rate. Both groups were comparable. | |
| Outcomes | Mortality (mention only), calculated GFR, urine output | |
| Notes | Also reports 'renal insufficiency', based on eGFR values only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization method used |
| Allocation concealment (selection bias) | Unclear risk | Computer allocation, no further details given (likely to be adequate) |
| Blinding (performance bias and detection bias) | High risk | Not described except the statement ‘investigator blinded to the study group evaluated the postoperative data’; likely to be inadequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Elective cardiac surgery patients with high creatinine levels (> 1.5mg/dl) (patients with chronic renal failure), more than 70yrs, diabetes on insulin or previous cardiac surgery (at least one of these factors present = high risk pt). Excluded patients who were renal cripples (those on dialysis) and allergy to drug used | |
| Participants | Intervention group: n=95; Age: mean=70.3, SD=7.6; Sex: M=61; F=34 Control group: n=98; Age: mean=69.6, SD=10.4; Sex: M=63; F=35 | |
| Interventions | Fenoldopam 0.1mcg/kg/min infusion immediately before incision and continued for 24 hrs. Control group: normal saline at the same rate for 24 hrs. Groups were comparable. | |
| Outcomes | Creatinine clearance (Cochroft‐Gault formula), urine output | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization from a computer list, in an envelope |
| Allocation concealment (selection bias) | Low risk | Sealed envelope used; ‘All personnel and patients were blinded to the assignment’ |
| Blinding (performance bias and detection bias) | Low risk | Blinded nurse, not involved with study, prepared the drug/placebo in identical 50ml filled syringes, ‘All personnel and patients were blinded to the assignment’ |
| Incomplete outcome data (attrition bias) | Low risk | One participant lost to follow‐up |
| Methods | Patients having CABG; randomization & double blinding stated, but method not specified; method of allocation concealment is unclear; blinding of patients, researchers and care givers is unknown, but possible it was adequate; poor methodological study | |
| Participants | Coronary artery bypass surgery. Captopril group n = 8, age, mean = 56, SD = 3; control group n = 8, age, mean = 60, SD =2 | |
| Interventions | Captopril 100 mg orally tid for 2 days preoperatively, last dose being 2 hrs before surgery; placebo tablets for control group | |
| Outcomes | Renal plasma flow | |
| Notes | Old study; no attempt made to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to two groups'; No details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; no description of method of allocation |
| Blinding (performance bias and detection bias) | Unclear risk | No details on blinding except ‘double‐blind fashion’ |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given in text |
| Methods | Patients having abdominal aortic aneurysm (AAA) surgery; randomization and double blinding of treatments done, but method not specified; method of allocation concealment is unclear; blinding of patients, researchers and care givers is unknown, but possible it was adequate; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Enalapril group n = 8, age, mean = 58, SD = 4; nicardipine group n = 8; age, mean = 63, SD = 3; control group n = 8, age, mean = 63, SD = 1 | |
| Interventions | Enalapril 10 mg orally BD for 2 days preoperation; placebo capsules for control group | |
| Outcomes | GFR, renal plasma flow; fractional excretion of sodium | |
| Notes | Old study; no attempt made to contact authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; No details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; No description of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No details on blinding except ‘double blind fashion’ |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given in text |
| Methods | Patients with pre‐operative renal dysfunction (Creatinine clearance less than 50ml/min) having CABG; randomization method not specified; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine group n = 12, age, mean = 60.3, SD = 12.3; control group n = 12, age, mean = 61.3, SD = 8.9; dopamine and SNP group, n = 12, age, mean = 54.2, SD = 8.7 (this group excluded from review) | |
| Interventions | Dopamine infusion 2.5 mcg/kg/min during the operation (unsure for how long); control group had no treatment | |
| Outcomes | Creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | Old study; no attempt made to contact authors. Excluded parallel treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly divided into three groups’; no description of randomization |
| Allocation concealment (selection bias) | High risk | No description of allocation method |
| Blinding (performance bias and detection bias) | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No details in text |
| Methods | Idiopathic scoliosis surgery patients; randomization method not specified; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Corrective spinal surgery for scoliosis. Dopamine group, n = 15, age, mean = 14.6, SD = 3.6; control group, n = 15, age, mean = 12.1, SD = 2.8 | |
| Interventions | Dopamine infusion 3 mcg/kg/min after induction for 24 hrs; control group received 5% dextrose for 24 hrs | |
| Outcomes | Urine output, fractional excretion of sodium | |
| Notes | Unable to contact the author; | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated’ into three groups; no description of randomization method |
| Allocation concealment (selection bias) | High risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given |
| Methods | Consecutive patients for abdominal aortic surgery; randomization by random card method; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but possible; poor methodological quality study | |
| Participants | Corrective abdominal aortic surgery. Dextran 60 group, n = 10, age, mean = 62, SD = 10.4; Ringer's lactate group, n = 10, age, mean = 66.1, SD = 13.7 | |
| Interventions | Dextran 60 infusion during operation and Ringer's lactate solution during surgery, ratio being 1:3 for the solutions | |
| Outcomes | Urine output; mortality data | |
| Notes | Too old study to get any more information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Randomized to either treatment group’ by pulling a card from a previously prepared deck |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given in text |
| Methods | Consecutive patients for abdominal aortic surgery; randomization method unclear; allocation concealment not used; blinding of patients and care givers is unknown, researcher blinded; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Dopamine group n = 12, age, mean = 63; placebo n = 12, age, mean = 60 | |
| Interventions | Dopamine infusion 3 mcg/kg/min during operation and for 24 hrs | |
| Outcomes | Urine output, GFR, renal plasma flow, free water clearance | |
| Notes | No reply from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors who was unaware of the treatment allocation; method of randomization is not clear in text |
| Allocation concealment (selection bias) | Low risk | Not sure of any allocation concealment, but likely |
| Blinding (performance bias and detection bias) | High risk | Not described any blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given in text |
| Methods | Patients for abdominal aortic surgery | |
| Participants | Abdominal aortic surgery. Felodipine group n = 11, age, mean = 65; control group n = 12, age, mean = 60 | |
| Interventions | Felodipine 5 mg slow release tab for 5 days preoperatively, last dose 1‐2 hrs before surgery; placebo tablets as control | |
| Outcomes | Urine output, GFR, renal plasma flow, free water clearance, fractional excretion of sodium | |
| Notes | No reply from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomiztion and drug or placebo preparation done by drug company; method not described was central and likely to be good |
| Allocation concealment (selection bias) | Unclear risk | Not sure of any allocation concealment, but likely possibility |
| Blinding (performance bias and detection bias) | Unclear risk | Possible, but does not describe blinded tables |
| Incomplete outcome data (attrition bias) | Low risk | One patient had additional drugs, but not excluded |
| Methods | CABG patients, randomized into four groups. Method of randomization unclear; allocation concealment or blinding not mentioned. Methodological quality poor | |
| Participants | Aortocoronary bypass surgery. Patients into four groups; Group (1), controls with normal renal function: n = 12; age: mean = 62.6, SD = 8.0; group (2), dopexamine infusion in patients with normal renal function: n = 12; age: mean = 64.0, SD = 7.5; group (3), controls with abnormal renal function; n = 12; age, mean = 62.4, SD = 7.5; group (4), dopexamine infusion in patients with abnormal renal function: n = 12; age, mean = 65.4, SD = 8.1 | |
| Interventions | Dopexamine 1 mcg/kg/min after induction of anaesthesia, until the end of surgery in groups 2 and 4; not sure how the controls were treated | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated into two groups, but does not describe the randomization method |
| Allocation concealment (selection bias) | High risk | Does not describe the allocation concealment |
| Blinding (performance bias and detection bias) | High risk | Does not mention blinding |
| Incomplete outcome data (attrition bias) | Low risk | All patients accounted for in calculations |
| Methods | CABG patients. Patients were randomized, but method of randomization and allocation concealment not described | |
| Participants | CABG patients. All patients received dopamine 2mg/kg/min infusion. Group (1) verapamil 5mg added to prime solution: n = 25; age, mean = 58.3, SE = 1.9; group (2), nimodipine 1‐15mcg/kg/min during bypass; n = 25; age, mean = 56.1, SE = 2.6; group (3), control group; normal saline infusion only; n = 25; age, mean = 56.5, SE = 2.0 | |
| Interventions | Verapamil 5 mg in prime in group (1); group (2) received infusion of nimodipine 1‐15 mcg/kg/min during bypass; group (3), control group received normal saline only | |
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of allocation not described |
| Blinding (performance bias and detection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of blinding not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described in text |
| Methods | CABG patients; randomized by opaque sealed envelopes; allocation concealment unclear; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery surgery. Dopamine group n = 12, age, mean = 53.2, SD = 10.9; mannitol group, n = 12, age, mean = 55.4, SD = 8.4; control group, n = 12, age, mean = 53.7, SD = 8.3 | |
| Interventions | Dopamine 3 mcg/kg/min started after induction, until end of operation; mannitol 1 mg/kg/hr from induction unto end of operation; no treatment for control group | |
| Outcomes | Urine output | |
| Notes | No reply so far from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated into 3 groups’; method of allocation concealment not described |
| Blinding (performance bias and detection bias) | High risk | Randomly allocated into 3 groups’; method of blinding not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Does not describe any dropouts |
| Methods | Patients for CABG, with renal dysfunction (preoperative creatinine more than 2.5mg/dl) | |
| Participants | CABG in patients with renal dysfunction. Preoperative dialysis group, n = 21; age, mean = 58.1, SD = 11.8; control group (no preoperative dialysis), n = 23; age, mean = 54.3, SD = 11.1 | |
| Interventions | Preoperative haemodialysis in the intervention group; control group had no preoperative haemodialysis. | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization done by the last digit of the medical record number of patient (Quasi‐randomization) |
| Allocation concealment (selection bias) | High risk | ‘Patients were prospectively allocated into 2 groups’ |
| Blinding (performance bias and detection bias) | High risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not given in text |
| Methods | CABG. Used retrospective review of data from a randomized, double blinded, trial. No details are given about the method of randomization or allocation concealment, but confirms double‐blind status. Methodology poor | |
| Participants | CABG patients, age: mean = 66, SD = 9. Intervention group received N‐acetyl cysteine during bypass, n = 20; placebo group, n = 20, not sure what they received | |
| Interventions | N‐acetyl cysteine, 100 mg/kg in prime, followed by 20 mg/kg per hour until end of bypass. Unsure what placebo group received | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Retrospective chart review of a randomized trial in 2003, which used computer generated allocation list (randomly permuted blocks of random size) provided by dept of Medical Statistics |
| Allocation concealment (selection bias) | Unclear risk | Computer generated allocation list (randomly permuted blocks of random size) provided by dept of Medical Statistics |
| Blinding (performance bias and detection bias) | Low risk | Drugs were supplied in identical looking glass vials containing drug or placebo. |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions described in text |
| Methods | Patients for biliary surgery (on patients with some renal impairment); randomization method not detailed; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Biliary tract surgery. Mannitol group n = 17, age, mean = 65.9, SD = 12; control group n = 14, age, mean = 68.5, SD = 9.9 | |
| Interventions | Mannitol 50 g intravenously 1 hour preoperation and for 2 days; Control treatment had no treatment | |
| Outcomes | Urine output, GFR | |
| Notes | Study too old to try to obtain details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Prospectively randomized’; no details of method of randomization |
| Allocation concealment (selection bias) | Unclear risk | ’Prospectively randomized’; no details of method of allocation |
| Blinding (performance bias and detection bias) | Unclear risk | ’Prospectively randomized’; no details of method of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Fate of participants discussed |
| Methods | Elective cardiac surgery patients with high creatinine (>150 mcmol/l), older patients, diabetes on insulin or previous cardiac surgery (at least one of these factors present = high risk patient, but none on dialysis) | |
| Participants | Intervention group: n=30; Age: mean= 68.9, SD=9.7; Sex: M=23, F=7 Control group: n=30; Age: mean=68.3; SD=9.3; Sex: M=21, F=9 | |
| Interventions | Intervention group: N‐Acetyl cysteine infusion immediately after induction at dose of 150mg/kg over 15 min, followed by continuous infusion of 50mg/kg over 4 hours, then 100mg/kg over 20hrs. Control group: Normal saline at the same rate for 24 hrs | |
| Outcomes | Mortality, urine output, acute renal injury (needing postop renal replacement therapy) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using Microsoft Excel‐based random number generation to create a randomization list, in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by quadruple‐blinding (patients, clinicians, data collectors and data analysers) were unaware of groups or treatment |
| Blinding (performance bias and detection bias) | Low risk | Quadruple‐blinding (patients, clinicians, data collectors and data analysers were blinded) |
| Incomplete outcome data (attrition bias) | Low risk | None missed |
| Methods | Elective cardiac surgery patients (CPB), with pre‐existing renal dysfunction (creatinine >120mmol/L), high risk patients (no patients on dialysis) | |
| Participants | Intervention group: n=50; Age: mean=71.5, SD=9.2; Sex: M=30; F=20 Control group: n=50; Age: mean=70.6; SD=9.5; Sex: M=33, F=17 | |
| Interventions | Intervention group: Sodium bicarbonate 0.5mmol/kg in 250ml 5% dextrose bolus immediately after induction of anaesthesia, followed by continuous infusion of 0.15mmol/kg in 1000ml of 5% dextrose over 23 hrs: (Total of 4mmol/kg in 24 hrs) Control group: Sodium chloride infusion in a similar fashion for same period (same volume infused) | |
| Outcomes | Mortality (hospital) and requirement for renal replacement therapy | |
| Notes | No missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel based random number generation, with blocks of 10; central randomization by dept of Pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by central randomization, blinding to all researchers, patients and others. Allocation revealed only after data analysis. |
| Blinding (performance bias and detection bias) | Low risk | Both infusions were in separate shrink‐wrapped black plastic bags that were identical in appearance (blinded to patients, anaesthetists, surgeons ICU personnel and nurses and others |
| Incomplete outcome data (attrition bias) | Low risk | One in each group |
| Methods | Patients for AAA; randomization and blinding mentioned in text, but not detailed; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but possible; overall poor methodological quality study | |
| Participants | Abdominal aortic surgery. Fenoldopam group n = 14, age, mean = 70, SD = 5; control group n = 13, age, mean = 69, SD = 6 | |
| Interventions | Fenoldopam infusion 0.1 mcg/kg/min (only during aortic cross clamping); placebo for control group | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | No response from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Random allocation used’; method of randomization not given |
| Allocation concealment (selection bias) | Unclear risk | ‘Random allocation used’; method of allocation not given |
| Blinding (performance bias and detection bias) | High risk | Blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | Exclusion described |
| Methods | Emergency abdominal surgery, age >50yrs. Excluded night cases, patients on lithium and those having vascular surgery | |
| Participants | Intervention: n=14; Age: median=66, range=56‐75; Sex: M=11, F=3 Control: n=15; Age: median=64, range=51‐76; Sex: M=12, F=3 | |
| Interventions | Intervention: Optimization of intraoperative fluids using arterial line and Lidco cardiovascular monitoring and gave fluid boluses of 250ml 6% hydroxyethyl starch over 15 min done as necessary Control: Standard care, fluids decided by clinicians in Operating Theatre. Groups not comparable | |
| Outcomes | Mortality (30 days) and renal morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized’, but no details given |
| Allocation concealment (selection bias) | Unclear risk | Allocated to control and intervention group using opaque envelopes immediately before surgery; not sure if allocation concealment was adequate |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | 1 died before operation in the intervention group |
| Methods | Elective repair of AAA. Excluded patients with renal insufficiency (creatinine >130mmol/l) and those who had renal artery clamping done during surgery | |
| Participants | Intervention group: n=34; Age: mean=66, SD=10; Sex: M=27; F=7 Control group: n=35; Age: mean=67, SD=10; Sex: M=27, F=8 | |
| Interventions | Intervention: N‐Acetyl cysteine infusion, 150mg/kg NAC in 250ml 5% dextrose in 20min (bolus) after induction of anaesthesia, followed by 150mg/kg in 250ml 5% dextrose, infused over 24 hrs. Control: 250ml 5% dextrose in 20min, followed by 250ml 5% dextrose infusion for 24hrs | |
| Outcomes | Mortality, patients needing renal replacement therapy (dialysis), urine output, urinary NAG/creatinine ratio, urinary albumin/ creatinine ratio, plasma Cystatin C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized in blocks of 10, done by hospital pharmacy, no details given |
| Allocation concealment (selection bias) | Low risk | Allocation was done by hospital pharmacy. None of the clinical and study personnel was aware of study allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Likely that there was blinding, though not detailed in text |
| Incomplete outcome data (attrition bias) | Unclear risk | One patient withdrew from study intraoperatively, does not mention which group, though most likely the intervention group (as seen from the numbers in each group) |
| Methods | Cardiac surgery patients, with eGFR >30ml/min, LVEF <0.50 and a minimum of 2 lesions undergoing CABG. Excluded patients with CCF, cardiogenic shock, unstable angina, MI and renal cripples (on dialysis or creatinine >300mmol/l) Morbidly obese | |
| Participants | Intervention group: n=124; Age: mean=60.8, SD=10.8. Sex: M=81, F=43 Control group: n=116; Age: mean=61.3, SD=9.7; Sex: M=72, F=44 | |
| Interventions | Intervention: Sodium nitroprusside (SNP) infusion. SNP started with onset of rewarming 0.1mcg/kg/hr, until end of CPB. SNP in 50ml blinded syringe Control: Normal saline in 50ml blinded syringe | |
| Outcomes | Mortality, renal injury, eGFR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization done by statistician |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed, envelopes |
| Blinding (performance bias and detection bias) | Low risk | SNP and saline in uniformly appearing 50ml syringes, blinded to surgeons, perfusionists and nurses; the investigators did not know the details |
| Incomplete outcome data (attrition bias) | Low risk | Reported as none |
| Methods | CABG patients. Three groups, randomization not described, no indication of concealment allocation, but indicated double‐blind status. Methodological quality poor | |
| Participants | CABG. Pentoxyfylline group, n = 14, age, mean = 61.7, SD = 8.0; gamma‐hydroxybutyrate group, n = 13, age, mean = 62.3, SD = 3.9; control group, n = 13, age, mean = 62.9, SD = 6.2 | |
| Interventions | Pentoxyphylline 1 mg/kg bolus, followed by 1 mg/kg/hour during operation. Gamma hydroxybutyrate bolus of 25 mg/kg, followed by 25 mg/kg/hour during operation. Control group received normal saline infusion | |
| Outcomes | Creatinine clearance, fractional excretion of sodium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by computer |
| Allocation concealment (selection bias) | High risk | Not described in detail |
| Blinding (performance bias and detection bias) | High risk | Not described in detail |
| Incomplete outcome data (attrition bias) | High risk | Not described |
| Methods | Patients for CABG; randomized and double‐blinded study; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Theophylline group n = 28, age, mean = 60.4, SD = 10.1; control group n = 28, age, mean = 60.3, SD = 8.1 | |
| Interventions | Theophylline bolus of 4 mg/kg over 30 min, followed by infusion of 0.25 mg/kg/hr for 96 hrs; control group received saline | |
| Outcomes | GFR | |
| Notes | No response from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized to receive one of two treatments |
| Allocation concealment (selection bias) | High risk | No details given |
| Blinding (performance bias and detection bias) | High risk | No details given |
| Incomplete outcome data (attrition bias) | Unclear risk | Early termination of study in 33 of 56 patients; Intention to treat (ITT) analysis used |
| Methods | Patients for CABG; randomization method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Clonidine group n = 23, age, mean = 58, SD = 7; control n = 25, age, mean = 57, SD = 2 | |
| Interventions | Preoperative infusion of clonidine 4 mcg/kg over 15 min, 1 hr before surgery; placebo in control group | |
| Outcomes | Urine volume, creatinine clearance | |
| Notes | No response from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated into two groups in a double‐blinded randomized fashion'; no details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated into 2 groups in a double‐blinded randomized fashion'; no details of allocation given |
| Blinding (performance bias and detection bias) | Unclear risk | 'Allocated into 2 groups in a double‐blinded randomized fashion'; no details of blinding given |
| Incomplete outcome data (attrition bias) | Low risk | Two patients were excluded |
| Methods | Patients undergoing cardiac surgery; randomization by sealed envelopes; allocation concealment unclear, but strong possibility; blinding of patients, researchers and care givers done; good methodological quality study | |
| Participants | Cardiac surgery. Dopamine group n = 42, age, mean = 63, SD = 10; frusemide (furosemide) group n = 41, age, mean = 63, SD = 10; control group n = 40, age, mean = 65, SD = 10 | |
| Interventions | Dopamine infusion 2 mcg/kg/min for 48 hrs; 05 mcg/kg/min frusemide infusion for 48 hrs; saline in control group | |
| Outcomes | Urine volume, creatinine clearance, fractional excretion of sodium | |
| Notes | Same study (with alternative treatment) is quoted in "Lassnigg 2000A" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no further details of randomization |
| Allocation concealment (selection bias) | Low risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no further details of allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no details of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Three patients were excluded from analysis |
| Methods | Abdominal aortic repair patients. Randomization done, but method not specified. Allocation concealment unclear or not done. Poor methodological quality | |
| Participants | AAA repair. Intervention is extraperitoneal approach; n = 10; age, mean = 69.8, SEM=3.1. Control group is intraperitoneal approach; n=10; age, mean=74.3, SEM=2.5 | |
| Interventions | Extraperitoneal approach for AAA repair compared with transperitoneal approach | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Recruited patients were allocated to one of two groups’; no details on randomization |
| Allocation concealment (selection bias) | Unclear risk | ‘Recruited patients were allocated to one of 2 groups’; no details on allocation |
| Blinding (performance bias and detection bias) | High risk | ‘Recruited patients were allocated to one of 2 groups’; no details of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Two patients accounted for |
| Methods | Infrarenal aortic surgery; randomization method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but likely; overall poor methodological quality study | |
| Participants | Abdominal aortic surgery. Enalapril group n = 11, age, mean = 69; control group n = 9, age, mean = 68 | |
| Interventions | Enalapril bolus 50 mcg/kg injection 25 min before anaesthesia; saline injection in control group | |
| Outcomes | Urine output, creatinine clearance, renal plasma flow, free water clearance, fractional excretion of sodium | |
| Notes | Contacted authors to confirm or exclude duplicate reporting; no response; used only full publication in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of allocation given |
| Blinding (performance bias and detection bias) | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of blinding given |
| Incomplete outcome data (attrition bias) | Low risk | Two patients were excluded from trial |
| Methods | CABG surgery. Method of randomization unclear, allocation concealment not stated, double‐blind status stated. Methodological quality moderately poor | |
| Participants | CABG surgery. Dexamethasone group, n = 10, age, mean = 67.7, range = 58‐76; control group, n = 10, age, mean = 59.6, range = 47‐76 | |
| Interventions | Dexamethasone 1 mg/kg before induction of anaesthesia, followed by 0.5 mg/kg 8 hours later. Control group received a placebo, nature of which is unsure | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized in a double blind fashion’; no details of randomization given |
| Allocation concealment (selection bias) | High risk | 'Randomized in a double blind fashion’; no details of allocation given |
| Blinding (performance bias and detection bias) | Unclear risk | 'Randomized in a double blind fashion’; no details of blinding given |
| Incomplete outcome data (attrition bias) | Low risk | 'All patients completed the trial' |
| Methods | Cardiac surgery (open heart) in patients with high creatinine. Randomization done, but method unclear. Allocation concealment not stated. Poor methodological quality | |
| Participants | Open heart surgery in patients with renal impairment (high creatinine). Intervention was fluid hydration preoperatively for 12 hours using half normal saline; n = 30; age, mean = 64, SEM = 1.7. Control group had fluid restriction for 12 hours preoperatively; n = 15; age, mean = 64.2, SEM = 2.8 | |
| Interventions | Preoperative fluid hydration using half isotonic saline, 1 ml/kg/hour for 12 hours; control group had no fluid hydration | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used a 2:1 ratio of randomization process, patients were randomized into groups; no other details of randomization given |
| Allocation concealment (selection bias) | High risk | Patients were randomized into groups; no other details of allocation given |
| Blinding (performance bias and detection bias) | High risk | Used a 2:1 ratio of randomization process, patients were randomized into groups; no details of blinding |
| Incomplete outcome data (attrition bias) | High risk | Not described |
| Methods | Repair of AAA (elective) in patients over 20yrs. Excluded patients on chronic dialysis and those with a preop creatinine >3.0mg/dl | |
| Participants | Intervention group: n=20; Age: mean=69.4, SD=7.7; Sex: M=18, F=2 Control group: n=20; Age: mean=73.3, SD=8.6; Sex: M=17, F=3 | |
| Interventions | Intervention: hANP (Atrial natriuretic peptide) infusion, 0.01mcg/kg/min starting dose (to prevent hypotension), increasing by 0.01mcg/kg/min every 10min until dose of 0.05mcg/kg/min is reached. Start infusion of hANP just before cross clamping and continued for 48hrs Control group: Placebo infusion started at 2ml/hr, increased by 2ml every 10min, until 10ml/hr | |
| Outcomes | Acute renal injury (needing dialysis), creatinine clearance, urinary NAG, urine volume | |
| Notes | No data in paper for creatinine clearance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized into two groups’; not sure what method of randomization was used |
| Allocation concealment (selection bias) | Unclear risk | Not sure how the allocation was done |
| Blinding (performance bias and detection bias) | Unclear risk | ‘Blind infusion was performed’; not sure about blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described in paper |
| Methods | CABG patients. Intervention dexamethasone IV; randomized, but method of randomization unclear. Allocation concealment not stated, but patients were double‐blinded. Methodological quality poor | |
| Participants | CABG patients. Intervention was dexamethasone and control group received a placebo, the nature of which is unclear. Dexamethasone group; n = 10; age, mean = 67.8, 95% CI = 63.4‐72.1. Control group, n = 10; age, mean = 59.5; 95% CI = 53.4‐65.5 | |
| Interventions | Dexamethasone 1 mg/kg at induction, followed by 0.5 mg/kg 8 hours later; placebo in the control group at the same time | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; no other details of randomization provided |
| Allocation concealment (selection bias) | High risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; no description of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; but no other details of blinding provided |
| Incomplete outcome data (attrition bias) | Low risk | 'All patients completed the trial' |
| Methods | High risk patients for CABG; randomization not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Prostaglandin group n = 17, age, mean = 62, SD = 5.5; control group n = 17, age, mean = 61, SD = 7 | |
| Interventions | Prostaglandin infusion 2 ng/kg/min at start of anaesthesia and for 48 hrs; control group treatment not described | |
| Outcomes | Urine volume, creatinine clearance | |
| Notes | No response to letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no other details given |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no mention of allocation |
| Blinding (performance bias and detection bias) | High risk | ‘Patients were randomized’; no mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Two people were excluded from analysis |
| Methods | Patients for CABG; randomization by random number generated by pharmacy; allocation concealment adequate; blinding of patients, researchers and care givers are adequate; good methodological quality study | |
| Participants | Coronary artery bypass surgery. dopamine group n = 25, age, mean = 62.2, SD = 8; control group n = 24, age, mean = 61, SD = 10 | |
| Interventions | Dopamine infusion 3 mcg/kg/min for 24 hrs; 5% dextrose infusion for control group | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No need for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by use of a table of random numbers; ‘prospective double‐blind randomized trial’ |
| Allocation concealment (selection bias) | Low risk | Coded 50ml syringes from the pharmacy, with contents remaining unknown to investigators until the end of trial; allocation concealed |
| Blinding (performance bias and detection bias) | Low risk | Coded 50ml syringes from the pharmacy, with contents remaining unknown to investigators until the end of trial; blinded |
| Incomplete outcome data (attrition bias) | Low risk | 3 withdrawals before start of trial |
| Methods | Consecutive patients for AAA surgery; randomization by sealed envelope (random number); allocation concealment adequate; blinding of patients done, but that of researchers and care givers unknown, but is possible; moderate methodological quality study | |
| Participants | Abdominal aortic surgery. Mannitol group n = 15, age, mean = 68; control group n = 13, age, mean = 71 | |
| Interventions | Mannitol 0.3 g/kg before cross‐clamp; normal saline for control group | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Need for further information uncertain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Prospective randomized trial’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Prospective randomized trial’; no details on allocation |
| Blinding (performance bias and detection bias) | High risk | ‘Prospective randomized trial’; no details on blinding |
| Incomplete outcome data (attrition bias) | High risk | None reported |
| Methods | Elective CABG surgery patients with renal impairment (GFR < 60ml/min), age >18yrs. Excluded emergency CABG surgery patients | |
| Participants | Intervention group: n=30; Age: mean=65, SD=9.5; Sex: M=17, F=13 Control group: n=30; Age: mean=61, SD=7.9; Sex: M=14, F=16 | |
| Interventions | Intervention: Vitamin E 100 units QID and allopurinol 100mg BD, for 3‐5 days preop Control group: Had no treatment | |
| Outcomes | Mortality, ARF (needing dialysis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomized’; no further details |
| Allocation concealment (selection bias) | Unclear risk | No indication of allocation concealment, but for statement ‘to prevent bias surgeons, nurses, and lab technicians were blinded to patient assignment’ |
| Blinding (performance bias and detection bias) | Unclear risk | Possible: states, ‘to prevent bias surgeons, nurses, and lab technicians were blinded to patient assignment’ |
| Incomplete outcome data (attrition bias) | Unclear risk | None indicated in text |
| Methods | Partial nephrectomy in patients with single kidney; Randomization mentioned in text, no details given; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Partial nephrectomy. Dopamine group n = 13, age, mean = 64.6, SD = 8; Control group n = 11, age, mean = 62.4, SD = 8.8 | |
| Interventions | Dopamine infusion 3 mcg/kg/min during surgery and for 1 hr afterwards; no intervention in control group | |
| Outcomes | Urine output, GFR, renal blood flow | |
| Notes | No response to letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Prospective randomized study’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | 'Prospective randomized study’; no details on allocation |
| Blinding (performance bias and detection bias) | High risk | 'Prospective randomized study’; no details on blinding |
| Incomplete outcome data (attrition bias) | Low risk | 11 out of 35 excluded |
| Methods | Surgery for obstructive jaundice; randomized, but no details given. allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective surgery for obstructive jaundice. Control group, n = 10, age not given; dopamine group, n = 13, age not given | |
| Interventions | Control group had pre‐op IV fluids and frusemide on induction; dopamine group had the above + infusion of dopamine 3 mcg/kg/min for 48 hours | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Old study, no real need for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly allocated into two groups’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomly allocated into 2 groups’; no details on allocation |
| Blinding (performance bias and detection bias) | High risk | ‘Patients were randomly allocated into 2 groups’; no details on blinding |
| Incomplete outcome data (attrition bias) | High risk | Not disclosed |
| Methods | Laparoscopic colorectal surgery; randomization done by use of sealed envelopes; allocation concealment not used; blinding of patients, researchers done, but that of care givers is unknown; overall good methodological quality study (unfortunately no relevant observations useful for this review) | |
| Participants | Elective laparoscopic colorectal surgery patients. Dopamine group, n = 19, age, mean = 64.3, SD = 9.4; control group, n = 18, age, mean = 61.3, SD = 16.7 | |
| Interventions | Dopamine infusion, 2 mcg/kg/min during the operation; control group received saline in the same manner | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Mailed authors for data because only the difference is given in the text. the data from authors contains details only during and for 2 hours after the operations. Continuous data not suitable for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization performed by aleatorized numbers prepared in closed envelopes'. |
| Allocation concealment (selection bias) | Unclear risk | No details on concealment of allocation except ‘Randomization performed by aleatorized numbers prepared in closed envelopes’; possible to have concealment of allocation |
| Blinding (performance bias and detection bias) | Low risk | Drug or placebo given with an identical container in a double blind manner and the volume of drug or saline were same. |
| Incomplete outcome data (attrition bias) | Low risk | 4 patients were excluded |
| Methods | Elective OP‐CABG surgery patients, with baseline creatinine >133mcmol/l. Inclusion and exclusion criteria detailed in text. | |
| Participants | Intervention group: n=35; Age: mean=55.6, SD=10.2. Sex: M=25; F=10 Control group: n=35; Age: mean=57.8, SD=9.4; Sex: M=28, F=7 | |
| Interventions | Intervention: N‐actylcysteine (NAC). Oral NAC 600mg BD on preop day, followed by IV NAC 600mg prior to induction of anaesthesia and IV NAC 600mg BD for 2 postop days (total dose of NAC = 4.8g) Control: No treatment | |
| Outcomes | Postop renal dysfunction (judged by rise of more than 44 mmol/l or 25% in creatinine from preop values), GFR (Cockroft‐Gault formula) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, prospective, open label study; random number generated from a random number table |
| Allocation concealment (selection bias) | High risk | No concealment of assignment discussed |
| Blinding (performance bias and detection bias) | High risk | No blinding discussed |
| Incomplete outcome data (attrition bias) | Unclear risk | Four people excluded after randomization |
| Methods | Elective high risk cardiac surgery patients (Patients with high creatinine >1.2mg/dl, older than 70yrs,lCHF,lLV EF<35%, diabetes on insulin or previous cardiac surgery; at least one of these factors present) | |
| Participants | Intervention group: n=50; mean age= 69.0; SD=11.1; Sex: M = 33; F=17 Control group: n=50; mean age= 67.3; SD=10.8; Sex: M = 37; F=13 | |
| Interventions | Intervention group: Atorvastatin 40 mg orally a day before surgery and 3 further doses orally in the post op days 1,2 and 3 Control group: Matching placebo for same duration and time | |
| Outcomes | Mortality; Acute kidney injury needing dialysis; Rise in Serum creatinine levels; Urinary NGAL, and urinary NGAL/urinary creatinine ratio; Rise in serum transminases and serum creatine kinase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by the hospital pharmacy clinical trials coordinator. Microsoft excel –based random number generator‐permuted block strategy with blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Allocation stratified into two groups based on preop use of statins. Allocation concealed to patients, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators |
| Blinding (performance bias and detection bias) | Low risk | "Double blind”. Atorvastatin or placebo medication was prepared in capsules of identical appearance and blinded to patients, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators |
| Incomplete outcome data (attrition bias) | Low risk | Eight in intervention group and seven in control |
| Methods | Patients undergoing AAA surgery; randomized, method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Aortic surgery for aneurysm. Preoperative hydration group, n = 11, age, mean = 65, SD, 9. Control group, n = 8, mean = 71, SD = 10 | |
| Interventions | Treatment group optimally hydrated preoperatively (guided by PCWP); control treatment no special treatment | |
| Outcomes | Creatinine clearance | |
| Notes | Unlikely to get authors to respond after this long after the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random assignment into two groups'; no further details of randomization |
| Allocation concealment (selection bias) | Unclear risk | 'Random assignment into 2 groups'; the anaesthesiologist was aware of the allocation and treatment received; no further details on allocation |
| Blinding (performance bias and detection bias) | Unclear risk | 'Random assignment into 2 groups'; the anaesthesiologist was aware of the allocation and treatment received; no further details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No details provided |
| Methods | Cardiac surgery patients with high creatinine levels (abnormal renal function). Randomization and allocation concealment methods not stated, but done by hospital pharmacy. Double‐blind status stated. Methodological quality moderately good | |
| Participants | Cardiac surgery (bypass) patients. N‐acetyl cysteine group, n = 38, age, mean = 72, range = 44‐87; control group, n = 42, age, mean = 69, range = 51‐81 | |
| Interventions | N‐acetyl cysteine group received loading dose of the drug 150 mg/kg in 15 min, followed by 50 mg/kg for next 4 hours, thereafter 100 mg/kg for next 16 hours. Placebo group received similar amount of saline (0.9%) over the same time | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’ |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; but no further details of allocation concealment provided in text |
| Blinding (performance bias and detection bias) | Unclear risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; no details of blinding provided |
| Incomplete outcome data (attrition bias) | Low risk | Three patients were excluded |
| Methods | Cardiac surgery patients; randomized, but method not specified; allocation concealment unclear; blinding of patients, researchers and care givers is unknown, but is likely; overall poor methodological quality study | |
| Participants | Cardiac surgery (CABG). Enalapril group n = 7, mean = 60.1, SD = 3.6; control group n = 7, age, mean = 66.3, SD = 4.2 | |
| Interventions | Enalapril, 1 mg, 6 hourly for 2 days | |
| Outcomes | Urine output, GFR, renal plasma flow | |
| Notes | Unable to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were allocated in a randomized double‐blind fashion to two groups’; no further details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on allocation method |
| Blinding (performance bias and detection bias) | Low risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No drop outs detailed in text |
| Methods | CABG patients. Randomization done, but details unclear. Allocation concealment not detailed. Blinding is done. Methodological quality moderately good | |
| Participants | CABG patients. Atrial natriuretic peptide group, n=20, age, mean = 62.1, SD = 7.9; control group, n = 20, age, mean = 64.8, SD = 5.2 | |
| Interventions | Intervention group received atrial natriuretic peptide infusion, 0.03‐0.05 mcg/kg/min for 20 hours, starting during operation, then reduced to 0.02 mcg/kg/min for another 4 hours. Control group received placebo (nature not described). All patients received dopamine and dobutamine at the end of bypass | |
| Outcomes | Urine output, GFR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on allocation method |
| Blinding (performance bias and detection bias) | Low risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on blinding, but likely to be adequate |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described, but probably there were no dropouts |
| Methods | CABG surgery. No patients with renal impairment (determined by Cr <1.3 mg/dl and CCr <80ml/min) were included | |
| Participants | Intervention group: n=251; Age: mean=65.6, SD=0.6; Sex: M=193, F=58 Control group: n=253; Age: mean=66.3, SD=0.6; Sex: M=205, F=48 | |
| Interventions | Intervention: hANP (Human atrial natriuretic peptide) infusion of hANP 0.02mcg/kg/min from start of CPB, reduced to 0.01mcg/kg/min after start of oral medications and then stopped after 12hrs Control: Normal saline infusion in the same fashion | |
| Outcomes | ARF needing dialysis, mortality, creatinine clearance, fractional excretion of sodium, free water clearance, urine output | |
| Notes | Too many confounders in the intervention and control groups such as use of dopamine infusion in some patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated into 2 groups by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | 'Randomly allocated by drawing lots’; no other details |
| Blinding (performance bias and detection bias) | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) | High risk | No mention of dropouts in the text |
| Methods | Randomly allocated into 2 groups by lottery method | |
| Participants | Intervention group: n=141; age: 68.8, SD: 6.7; Males: 123/141 Control group: n=144; age: 68.8, SD: 7.8; Males 128/144 | |
| Interventions | Intervention: Carperitide (hANP) infusion, 0.01mcg/kg/min for over 2 days Control: Saline infusion for similar period | |
| Outcomes | Mortality, Acute renal injury needing dialysis, calculated GFR | |
| Notes | Poor quality study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Lottery method' |
| Allocation concealment (selection bias) | High risk | No evidence of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | None used |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts discussed |
| Methods | Patients for aortic reconstruction; randomized by random number method; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective aortic reconstruction surgery. Hypertonic saline group, n = 30, age, mean = 60.5, SD = 8.2; Ringer's lactate group, n = 28, age, mean= 61.7, SD = 8.5 | |
| Interventions | Hypertonic saline intraoperatively and Ringer's lactate solution intraoperatively | |
| Outcomes | Urine output, creatinine clearance, fractional excretion of sodium | |
| Notes | Very old study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned by random number to one of 2 groups; no details on randomization |
| Allocation concealment (selection bias) | High risk | Patients were assigned by random number to one of 2 groups; no details on concealment of allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Patients were assigned by random number to one of 2 groups; no details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described |
| Methods | Cardiac surgery (off‐pump coronary artery surgery). Randomization using computer generated randomization table. Allocation concealment not stated, but blinding seems adequate. Methodological quality moderately good | |
| Participants | Off‐pump coronary artery surgery. Mannitol group, n = 25, age, mean = 63, SD = 8; control group, n = 25, age, mean = 63, SD = 8 | |
| Interventions | Mannitol 0.5 g/kg in 10 min during grafting; control group 2.5 ml/kg normal saline during the same time period | |
| Outcomes | Acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomly allocated to one of 2 groups using a computer generated randomization table' |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to one of 2 groups using a computer generated randomization table; no further details on allocation concealment; likely to be adequate |
| Blinding (performance bias and detection bias) | Low risk | All medical personnel involved in the study were blinded to the contents of the infusion bottle. |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts recorded |
| Methods | Cardiac surgery patients, mostly off pump CABG. | |
| Participants | Adults undergoing CABG | |
| Interventions | EPO 300u/kg given immediately following induction of anaesthesia. Same volume of normal saline given as placebo. | |
| Outcomes | Acute kidney injury was primary outcome (serum creatinine rise of more than 50%); urine output and creatinine clearance (calculated) | |
| Notes | EPO study. All enrolled patients completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by research unit of hospital. Randomization was stratified by creatinine levels |
| Allocation concealment (selection bias) | Low risk | Allocation was via Internet |
| Blinding (performance bias and detection bias) | Low risk | None of the clinicians, patients or researchers were aware of the nature of the drugs; matching syrings of EPO and normal saline used |
| Incomplete outcome data (attrition bias) | Low risk | All patients completed the trial |
| Methods | Consecutive patients for CABG; randomization method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine group n = 20, age, mean = 61, SD = 10.3; control group n = 20, age, mean = 56.3, SD = 8.7 | |
| Interventions | Dopamine infusion, 2.5‐4 mcg/kg/min for 48 hrs; control group without any intervention | |
| Outcomes | Urine output | |
| Notes | No need to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospectively randomized; no details given |
| Allocation concealment (selection bias) | High risk | No details of allocation provided in text |
| Blinding (performance bias and detection bias) | High risk | No details of blinding provided in the text |
| Incomplete outcome data (attrition bias) | High risk | No dropouts recorded |
| Methods | Coronary artery surgery. Randomization done, but method not clear. No evidence of allocation concealment or blinding. Methodological quality poor | |
| Participants | Coronary artery surgery. Beating heart surgery group, n = 20, age, mean = 64.8, SD = 6.9; conventional bypass group (control), n = 20, age, mean = 62.1, SD = 9.3 | |
| Interventions | Intervention is off‐pump coronary artery surgery and control group is on pump coronary artery surgery | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomized'; no further details on randomization |
| Allocation concealment (selection bias) | High risk | 'Patients were randomized'; no further details on allocation |
| Blinding (performance bias and detection bias) | High risk | 2 different types of procedures; no blinding possible |
| Incomplete outcome data (attrition bias) | Low risk | 5 people were subsequently excluded from trial |
| Methods | Surgery for obstructive jaundice. Randomized, but method of randomization, allocation concealment and blinding not stated. Methodological quality poor | |
| Participants | Surgery for obstructive jaundice. Oral ursodeoxycholic acid group, n = 20, age, mean = 57.0, range = 18‐72. Control group, n = 20, age, mean = 56.5, range = 45‐78 | |
| Interventions | Intervention oral ursodeoxycholic acid 900 mg, 8 hourly for 48 hours in the immediate preoperative period. Control group had no additional treatment | |
| Outcomes | Mortality, acute renal injury, creatinine clearance | |
| Notes | Too old study to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no more details |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no account of allocation method |
| Blinding (performance bias and detection bias) | High risk | ‘Patients were randomized’; no details on blinding |
| Incomplete outcome data (attrition bias) | Low risk | ‘There were no withdrawals’ |
| Methods | Patients for elective AAA (infrarenal) repair. Excluded patients on steroids, diabetic patients and those with CRF (Creatinine >150mcmol/l) | |
| Participants | Intervention group: n=10; Age: mean=69.1, SD=5.4; Sex: not described Control group: n=10; Age: mean=71.9, SD=6.0; Sex: not described | |
| Interventions | Intervention: Methyl prednisolone 10mg/kg in 500ml 5% dextrose, infusion over 30min, but mentions only that infusion was given ‘during the surgery’; not sure when Control group: Received 5% dextrose solution | |
| Outcomes | Mortality, NAG/creatinine ratio | |
| Notes | Also did serum creatinine, cytokines, alpha‐1 microglobulin/ creatinine ratio and albumin/ creatinine ratio | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done using computer generated randomization list |
| Allocation concealment (selection bias) | Low risk | Computer generated randomization list placed in sealed envelopes and opened in numerical order by a third party preparing the study infusion |
| Blinding (performance bias and detection bias) | Low risk | Third party prepared the infusion. The infusions were such that the volumes were equal in the bag and identical colour and the contents of bag were indistinguishable; the infusion was done over 30min to avoid any haemodynamic effects of treatment |
| Incomplete outcome data (attrition bias) | Low risk | Yes, none lost to follow‐up |
| Methods | CABG surgery. Quasi randomization by using last digits of their notes, no allocation concealment or blinding | |
| Participants | CABG. Phenylephrine group, n = 7, age, mean = 55, SD = 7. Control group, n = 14, age, mean = 54, SD = 7 | |
| Interventions | Intervention is to maintain mean perfusion pressure above 70 mmHg during surgery. Control group had no intervention | |
| Outcomes | Acute renal injury, urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | To old study to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned into one of 2 groups, according to the last digit of their clinical history number (Quasi‐randomization) |
| Allocation concealment (selection bias) | High risk | Allocation by last digit of clinical history number is impossible to conceal |
| Blinding (performance bias and detection bias) | High risk | No report of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 'All patients completed the trial' |
| Methods | Patients for biliary surgery; randomization method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Biliary tract surgery. Dopamine group n = 10, age, median = 50, range = 37‐60; control group n = 10, age, median = 44.5, range = 36‐60; dopamine + mannitol group n = 10, age, median = 51, range = 44‐58; dopamine + frusemide (furosemide) group n = 10, age, median = 61, range = 55‐71 (excluded the last 2 groups from review) | |
| Interventions | Dopamine infusion 2.5 mcg/kg/min during surgery and for 2 days; control group had no treatment | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No need to contact authors. Excluded 2 parallel treatment groups in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly allocated into 4 equal groups’; no further details on randomization |
| Allocation concealment (selection bias) | Unclear risk | 'Patients were randomly allocated into 4 equal groups’; no further details on allocation |
| Blinding (performance bias and detection bias) | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) | High risk | None described |
| Methods | Infrarenal aortic surgery; Randomization method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Dopexamine group n = 15, age, mean = 63.5; control group n = 17, age, mean = 62.1 | |
| Interventions | Dopexamine 2 mcg/kg/min infusion during surgery; saline in control group | |
| Outcomes | Creatinine clearance | |
| Notes | Need to contact authors uncertain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly assigned’; no further details on randomization method used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomly assigned’; no further details on allocation method used |
| Blinding (performance bias and detection bias) | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) | High risk | None described |
| Methods | AAA surgery (infrarenal). Randomized, but method of randomization, allocation concealment and blinding not stated in the text. Methodological quality poor | |
| Participants | Elective AAA repair. Antioxidant group, n = 20, age, mean = 67, range = 51‐75; control group, n = 22, age, mean = 70, range = 59‐82 | |
| Interventions | Intervention was multiple antioxidant therapy as follows: vitamin E 200 mg orally for 5 days before surgery + vitamin C 200 mg orally on morning of surgery + allopurinol 300 mg orally 1 day before surgery and 300 mg at induction + N‐acetyl cysteine 150 mg/kg bolus, followed by infusion of 200 mg/kg over 12 hours preoperatively + mannitol 10%, 500 ml over 12 hours from the time of surgery. Control group had no intervention | |
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no further details on method of randomization used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no details on method of allocation used |
| Blinding (performance bias and detection bias) | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | One death described, but not of any dropouts |
| Methods | Elective CPB surgery patients studied. Males with creatinine >150mcmol/L and females >130mcmol/l CABG or valve surgery were included. Excluded patients with unstable angina, EF <35%, renal cripple (on dialysis) or renal transplant patients. | |
| Participants | Intervention: n=10; Age: mean=67.7, SD=9.0; Sex: M=8, F=2 Control: n=10; Age: mean=65.8, SD=10.6; Sex: M=8, F=2 | |
| Interventions | Intervention: Infusion of nifedipine, from start of surgery and for 24hrs; Nifedipine infusion rate was 0.25 – 0.60 mcg/kg/min (adjusted according to BP) Control: No treatment | |
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were ‘randomized’. It appears that the anaesthesiologist ‘randomly drew an envelope with the assigned treatment’ |
| Allocation concealment (selection bias) | High risk | Allocation concealment was possible only for patients and statistician |
| Blinding (performance bias and detection bias) | High risk | No; control had no treatment; only the patients and statistician were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | None described |
| Methods | Consecutive patients undergoing cardiac surgery; block randomized with sealed envelopes; allocation concealment described in follow‐up correspondence; blinding of patients done, but researchers and care givers not blinded; moderate methodological quality of study | |
| Participants | Elective cardiac surgery patients (with high risk of postoperative renal dysfunction). Dopamine group, n = 20, age, mean = 64.5, range 58‐82; control group, n = 22, age, mean = 66.5, range 48‐84) | |
| Interventions | Dopamine infusion 3 mcg/kg/min during operation and for 48 hrs post‐operation; saline infusion for the control group | |
| Outcomes | Data on urine output obtained following correspondence | |
| Notes | Contacted the author and obtained excellent response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no further details on method of randomization used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no details on method of allocation used |
| Blinding (performance bias and detection bias) | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) | Low risk | Eight patients were excluded due to death or major complications |
| Methods | Elective CABG patients; randomized, but method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective CABG patients. Dopamine group, n = 11, age, mean = 55.7, SD = 5.2; control group, n = 11, age, mean = 56.4, SD = 9.5 | |
| Interventions | Dopamine 2 mcg/kg/min infusion, started 24 hrs before surgery and continued for 48 hrs after surgery; control group had no treatment | |
| Outcomes | Creatinine clearance, free water clearance | |
| Notes | Contacted the author (e‐mail), but no response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were prospectively randomized’; no details on method of randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients were prospectively randomized’; no details on method of allocation |
| Blinding (performance bias and detection bias) | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | States no deaths; no description of any dropouts |
| Methods | CABG patients; randomized, but method of randomization unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective CABG patients. Control group, n = 15, age, mean = 61.3, SD = 8.3; dopamine group, n = 15, age, mean = 58.3, SD = 5.3; diltiazem group, n = 15, age, mean = 60.3, SD = 7.1; dopamine + diltiazem group, n = 15, age, mean = 58.7, SD = 7.1 (excluded this group from review) | |
| Interventions | Dopamine and diltiazem 2 mcg/kg/min, started 24 hrs pre‐operation and continued for 48 hrs post‐operation; no mention of how control group was treated | |
| Outcomes | Creatinine clearance, free water clearance | |
| Notes | Contacted (e‐mail) to author, no response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients randomized into four groups’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients randomized into four groups’; no description of allocation |
| Blinding (performance bias and detection bias) | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | 'No mortality' is described, but not any suggestion of dropouts |
| Methods | CABG surgery. Randomization done, but method of randomization, allocation concealment and blinding not described. Methodological quality poor | |
| Participants | Diltiazem infusion (2 doses): Group 1, diltiazem 1 mcg/kg/min, n = 11, age, mean = 58.1, SD = 10.7; Group 2, diltiazem 2 mcg/kg/min, n = 12, age, mean = 58.3, SD = 5.8; control group, n = 12, age, mean = 57.8, SD = 10.1 | |
| Interventions | Intervention, group 1, diltiazem 1 mcg/kg/min and group 2, diltiazem 2 mcg/kg/min, both started after chest opening, until 24 hours in ICU. Control group had no additional treatment | |
| Outcomes | Acute renal injury, urine output, creatinine clearance | |
| Notes | Old study, unlikely to get more data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly assigned’; no further details of randomization |
| Allocation concealment (selection bias) | High risk | ‘Randomly assigned’; no further details of allocation |
| Blinding (performance bias and detection bias) | High risk | No blinding described |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts described |
CABG = coronary artery bypass graft; AAA = abdominal aortic aneurysm; GFR = glomerular filtration rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Treatment only for very short duration during surgery | |
| Not a randomized controlled trial | |
| Treatment started only after operation | |
| There is no control group | |
| Treatment started only in the postoperative period | |
| A phase 2 trial, looking for side effects; no controls | |
| No control group without treatment in the trial | |
| Not relevant to the review | |
| This is not a randomized controlled trial | |
| No control group without treatment in the trial | |
| Not a randomized controlled trial | |
| Not very relevant to the review; no randomization | |
| The control group received active treatment with dopamine, dobutamine or frusemide; some controls started in postoperative period only | |
| No data relevant to the review | |
| Not a randomized controlled trial | |
| Data not relevant to the review | |
| Data not relevant to the review | |
| A retrospective study only | |
| Follow up only for 12 hours | |
| The intervention started in the postoperative period only | |
| Postoperative study in the intensive care unit | |
| This is an observational study, not a randomized controlled trial or controlled trial | |
| Started in the postoperative period in the intensive care unit | |
| Data not relevant to the review | |
| Not a randomized controlled study; there is only treatment and no controls | |
| No control group | |
| No control group | |
| This is a postoperative study | |
| Started the intervention only towards the end of operation and continued only for 16 hours | |
| Not a randomized controlled trial | |
| Study lasted only 6‐14 hours | |
| Open study, with inadequate randomization; used multiple interventions (frusamide) in both groups | |
| Retrospective study | |
| Mostpatients received other interventions such as dopamine and frusamide | |
| Not a randomized controlled trial | |
| This is a conference abstract; data published as Kulka 1996 (included) | |
| No control groups | |
| There were no control group patients | |
| Not a randomized controlled trial | |
| This is not a randomized controlled trial or controlled trial | |
| Control group had received active treatment | |
| No relevant data for the review | |
| The intervention is not relevant for the review | |
| We could not exclude if this study contained the same data as in Licker 1996; hence excluded this publication from analysis | |
| Postoperative study | |
| Not a randomized controlled trial | |
| No control group; intervention mostly in the intensive care unit | |
| The follow up was only for 12 hrs | |
| No control group patients in the trial | |
| Only two treatment groups, no control group | |
| The data not relevant to the review | |
| Not a randomized controlled trial | |
| Not a randomized controlled trial | |
| Study started in the postoperative period only | |
| No randomization or controls | |
| Conference abstract only; data published as O'Hara 2002 (included) | |
| No control group | |
| Not a randomized controlled trial | |
| The treatment using intravenous hydration is only during the preoperative period; no randomization | |
| Randomization method inadequate; two treatments were given to the treatment groups (both dopamine and mannitol) | |
| Postoperative study | |
| No control group | |
| Intervention started only in intensive care unit | |
| No control group; only 2 treatments | |
| No relevant data for the review | |
| Study did not look at renal function | |
| No clear intervention in the study | |
| Not a randomized controlled trial | |
| Abstract publication only; data published as Ryckwaert 2001 (included) | |
| Study of intravenous fluid administration in the preoperative period only; no relevant data reported | |
| Not a randomized controlled trial | |
| Study started in intensive care unit | |
| There is no relevant data for the review | |
| Multiple measures used for the purpose of renal protection in the intervention group (mannitol + dopamine or frusemide) | |
| There is no renal data in the study | |
| Not a randomized controlled trial | |
| Used only retrospective controls | |
| No control group | |
| Not a randomized controlled trial | |
| No control group | |
| No control group | |
| It is a group registry only and not a RCT | |
| The renal function tests were done only for very short periods of time | |
| Not relevant to the review |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | Patients undergoing partial nephrectomy of solitary kidney |
| Interventions | Fenoldopam and placebo |
| Outcomes | |
| Notes | Awaiting full publication; unable to contact authors |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 11 | 583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.48, 4.73] |
| Analysis 1.1  Comparison 1 Dopamine and analogues versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 10 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.44, 4.23] |
| Analysis 1.2  Comparison 1 Dopamine and analogues versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Dopamine and analogues versus no intervention, Outcome 3 Urine output. | ||||
| 3.1 24 hours (mL/min) | 13 | 670 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.19, 0.54] |
| 3.2 2 to 4 days (mL/min) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.04, 0.97] |
| 3.3 5 to 7 days (mL/min) | 4 | 103 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.51] |
| 4 Creatinine clearance Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Dopamine and analogues versus no intervention, Outcome 4 Creatinine clearance. | ||||
| 4.1 24 hours (mL/min) | 14 | 616 | Mean Difference (IV, Random, 95% CI) | 7.17 [‐5.53, 19.86] |
| 4.2 2 to 4 days (mL/min) | 9 | 459 | Mean Difference (IV, Random, 95% CI) | 7.31 [‐6.19, 20.82] |
| 4.3 5 to 7 days (mL/min) | 5 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐13.63, 6.98] |
| 5 Free water clearance Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Dopamine and analogues versus no intervention, Outcome 5 Free water clearance. | ||||
| 5.1 24 hours (mL/min) | 6 | 166 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Dopamine and analogues versus no intervention, Outcome 6 Fractional excretion of sodium. | ||||
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow (24 hours) Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 75.36 [‐63.27, 213.98] |
| Analysis 1.7  Comparison 1 Dopamine and analogues versus no intervention, Outcome 7 Renal plasma flow (24 hours). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [0.80, 7.74] |
| Analysis 2.1  Comparison 2 Diuretics versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 5 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.39 [0.68, 8.47] |
| Analysis 2.2  Comparison 2 Diuretics versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Diuretics versus no intervention, Outcome 3 Urine output. | ||||
| 3.1 24 hours (mL/min) | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.33] |
| 3.2 2 to 4 days (mlL/min) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.14, 0.45] |
| 4 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Diuretics versus no intervention, Outcome 4 Creatinine clearance. | ||||
| 4.1 24 hours (mL/min) | 3 | 123 | Mean Difference (IV, Random, 95% CI) | ‐18.02 [‐41.78, 5.75] |
| 4.2 2 to 4 days (mL/min) | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐14.76, 19.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.1  Comparison 3 Calcium channel blockers versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 6 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.01, 1.17] |
| Analysis 3.2  Comparison 3 Calcium channel blockers versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Calcium channel blockers versus no intervention, Outcome 3 Urine output. | ||||
| 3.1 Urine output: 24 hours (mL/min) | 4 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.02, 0.45] |
| 4 Creatinine clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Calcium channel blockers versus no intervention, Outcome 4 Creatinine clearance. | ||||
| 4.1 24 hours (mL/min) | 5 | 251 | Mean Difference (IV, Random, 95% CI) | 4.74 [‐3.30, 12.77] |
| 4.2 2 to 4 days (mL/min) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 13.92 [‐24.62, 52.46] |
| 5 Free water clearance Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Calcium channel blockers versus no intervention, Outcome 5 Free water clearance. | ||||
| 5.1 24 hours (mL/min) | 3 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| Analysis 4.1  Comparison 4 ACE inhibitors versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 3 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.2  Comparison 4 ACE inhibitors versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Renal plasma flow Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 ACE inhibitors versus no intervention, Outcome 3 Renal plasma flow. | ||||
| 3.1 RPF: end of operation (mL/min) | 3 | 62 | Mean Difference (IV, Random, 95% CI) | 46.37 [‐68.61, 161.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| Analysis 5.1  Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 4 | 865 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.08, 0.64] |
| Analysis 5.2  Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Urine output at 24 hours Show forest plot | 3 | 584 | Mean Difference (IV, Random, 95% CI) | 0.42 [0.18, 0.67] |
| Analysis 5.3  Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 3 Urine output at 24 hours. | ||||
| 4 Creatinine clearance, 24 hours Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 35.23 [‐0.48, 70.94] |
| Analysis 5.4  Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 4 Creatinine clearance, 24 hours. | ||||
| 5 Creatinine clearance, 2 to 3 days Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 27.30 [4.36, 50.23] |
| Analysis 5.5  Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 5 Creatinine clearance, 2 to 3 days. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 641 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.42, 2.42] |
| Analysis 6.1  Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 5 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.32, 2.62] |
| Analysis 6.2  Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 2 Acute renal injury. | ||||
| 3 Urine output, 24 hours Show forest plot | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.24, 0.60] |
| Analysis 6.3  Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 3 Urine output, 24 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.63] |
| Analysis 7.1  Comparison 7 Erythropoietin (EPO) versus control, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 7.2  Comparison 7 Erythropoietin (EPO) versus control, Outcome 2 Acute renal injury. | ||||
| 3 Urine output: 24 hours Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.47, 0.21] |
| Analysis 7.3  Comparison 7 Erythropoietin (EPO) versus control, Outcome 3 Urine output: 24 hours. | ||||
| 4 Urine output: 2 to 3 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.18] |
| Analysis 7.4  Comparison 7 Erythropoietin (EPO) versus control, Outcome 4 Urine output: 2 to 3 days. | ||||
| 5 Urine output: 5 to 7 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.22] |
| Analysis 7.5  Comparison 7 Erythropoietin (EPO) versus control, Outcome 5 Urine output: 5 to 7 days. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.16, 3.42] |
| Analysis 8.1  Comparison 8 Intravenous fluid versus control, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 3 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.96] |
| Analysis 8.2  Comparison 8 Intravenous fluid versus control, Outcome 2 Acute renal injury. | ||||
| 3 Creatinine clearance Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.3  Comparison 8 Intravenous fluid versus control, Outcome 3 Creatinine clearance. | ||||
| 3.1 24 hours (mL/min) | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐29.57, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 26 | 2390 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.56, 1.64] |
| Analysis 9.1  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 31 | 2504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| Analysis 9.2  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.3  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 3 Urine output. | ||||
| 3.1 24 hours (mL/min) | 17 | 1475 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.17, 0.36] |
| 3.2 2 to 3 days (mL/min) | 9 | 1058 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.13, 0.54] |
| 4 Creatinine clearance Show forest plot | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.4  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 4 Creatinine clearance. | ||||
| 4.1 24 hours (mL/min) | 24 | 2136 | Mean Difference (IV, Random, 95% CI) | 9.38 [‐5.99, 24.74] |
| 4.2 2 to 3 days (mL/min) | 17 | 1844 | Mean Difference (IV, Random, 95% CI) | 14.21 [3.58, 24.85] |
| 4.3 5 to 7 days (mL/min) | 7 | 949 | Mean Difference (IV, Random, 95% CI) | 14.99 [0.84, 29.13] |
| 5 Free water clearance Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.5  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 5 Free water clearance. | ||||
| 5.1 24 hours (mL/min) | 7 | 700 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.19] |
| 5.2 2 to 3 days (mL/min) | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.30, ‐0.28] |
| 6 Fractional excretion of sodium Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 9.6  Comparison 9 Cardiac surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium. | ||||
| 6.1 24 hours (%) | 8 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 8 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.20, 2.89] |
| Analysis 10.1  Comparison 10 Aortic surgery: subgroup analysis, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 8 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.11, 3.70] |
| Analysis 10.2  Comparison 10 Aortic surgery: subgroup analysis, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 10.3  Comparison 10 Aortic surgery: subgroup analysis, Outcome 3 Urine output. | ||||
| 3.1 24 hours (mL/min) | 7 | 227 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 3.2 2 to 3 days (mL/min) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.58] |
| 3.3 5 to 7 days (mL/min) | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 4 Creatinine clearance Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 10.4  Comparison 10 Aortic surgery: subgroup analysis, Outcome 4 Creatinine clearance. | ||||
| 4.1 24 hours (mL/min) | 9 | 323 | Mean Difference (IV, Random, 95% CI) | 7.99 [‐0.77, 16.74] |
| 4.2 2 to 3 days (mL/min) | 5 | 195 | Mean Difference (IV, Random, 95% CI) | 11.62 [‐6.13, 29.37] |
| 4.3 5 to 7 days (mL/min) | 4 | 116 | Mean Difference (IV, Random, 95% CI) | ‐12.85 [‐26.41, 0.72] |
| 5 Free water clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 10.5  Comparison 10 Aortic surgery: subgroup analysis, Outcome 5 Free water clearance. | ||||
| 5.1 24 hours (mL/min) | 5 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.51, 0.01] |
| 5.2 2 to 4 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.12, 0.85] |
| 5.3 5 to 7 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.13, 0.61] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.6  Comparison 10 Aortic surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium. | ||||
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 10.7  Comparison 10 Aortic surgery: subgroup analysis, Outcome 7 Renal plasma flow. | ||||
| 7.1 End of operation (mL/min) | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 50.29 [‐92.83, 193.40] |
| 7.2 24 hours (mL/min) | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 45.86 [‐18.64, 110.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urine output Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 11.1  Comparison 11 Biliary surgery: subgroup analysis, Outcome 1 Urine output. | ||||
| 1.1 Urine output: 24 hours (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.19] |
| 1.2 Urine output: 2 to 4 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.22, 0.69] |
| 1.3 Urine output: 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.09, 0.37] |
| 2 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 11.2  Comparison 11 Biliary surgery: subgroup analysis, Outcome 2 Creatinine clearance. | ||||
| 2.1 24 hours (mL/min) | 3 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐14.07, 8.39] |
| 2.2 2 to 4 days (mL/min) | 3 | 74 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐16.68, 17.52] |
| 2.3 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐16.43, 17.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 959 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.36, 1.52] |
| Analysis 12.1  Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 1 Mortality. | ||||
| 2 Acute renal injury Show forest plot | 11 | 979 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.76] |
| Analysis 12.2  Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 2 Acute renal injury. | ||||
| 3 Urine output Show forest plot | 4 | 707 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.16, 0.85] |
| Analysis 12.3  Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 3 Urine output. | ||||
| 3.1 Urine output, 24 hours | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.12, 0.81] |
| 3.2 Urine output, 2 to 3 days | 2 | 291 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.78, 1.65] |
| 4 Creatinine clearance Show forest plot | 4 | 646 | Mean Difference (IV, Random, 95% CI) | 10.65 [0.04, 21.27] |
| Analysis 12.4  Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 4 Creatinine clearance. | ||||
| 4.1 Creatinine clearance, 24 hours | 4 | 347 | Mean Difference (IV, Random, 95% CI) | 7.78 [‐10.39, 25.94] |
| 4.2 Creatinine clearance, 2 to 3 days | 3 | 299 | Mean Difference (IV, Random, 95% CI) | 14.16 [‐6.20, 34.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reported mortality, low risk of bias studies only Show forest plot | 19 | 1604 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| Analysis 13.1  Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 1 Reported mortality, low risk of bias studies only. | ||||
| 2 Acute renal injury, requiring dialysis, low risk of bias studies only Show forest plot | 16 | 1550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.55, 2.03] |
| Analysis 13.2  Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 2 Acute renal injury, requiring dialysis, low risk of bias studies only. | ||||
| 3 Urine output at 24 hours, low risk of bias studies only Show forest plot | 11 | 798 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
| Analysis 13.3  Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 3 Urine output at 24 hours, low risk of bias studies only. | ||||
| 4 Creatinine clearance at 24 hours, low risk of bias studies only Show forest plot | 9 | 817 | Mean Difference (IV, Random, 95% CI) | 6.59 [‐3.53, 16.72] |
| Analysis 13.4  Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 4 Creatinine clearance at 24 hours, low risk of bias studies only. | ||||
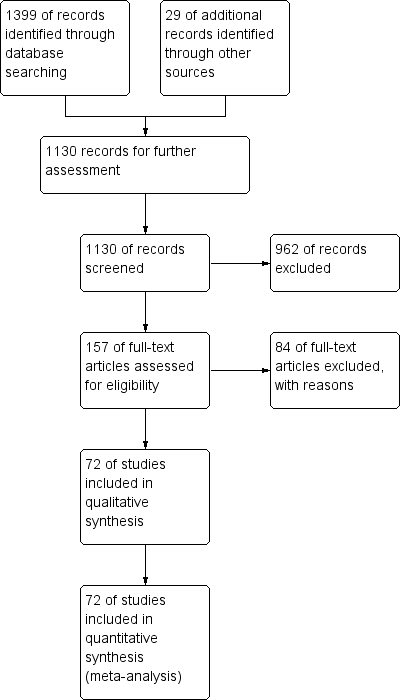
Study flow diagram, as of December 2012.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across 78 included studies.
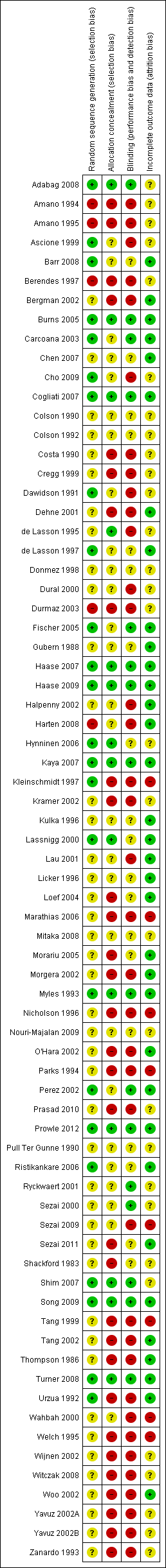
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.1 Mortality.
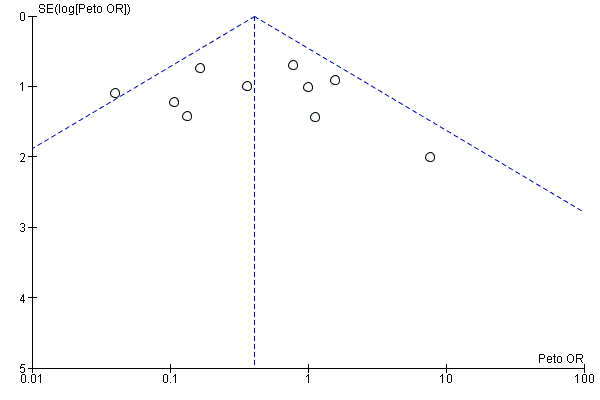
Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.2 Acute renal injury.
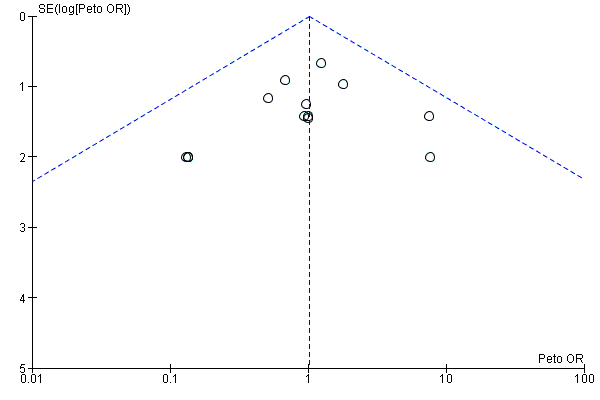
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.1 Reported mortality, low risk of bias studies only.
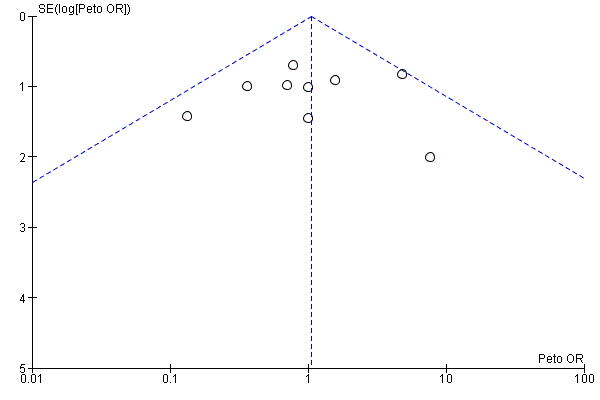
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.2 Acute renal injury, requiring dialysis, low risk of bias studies only.
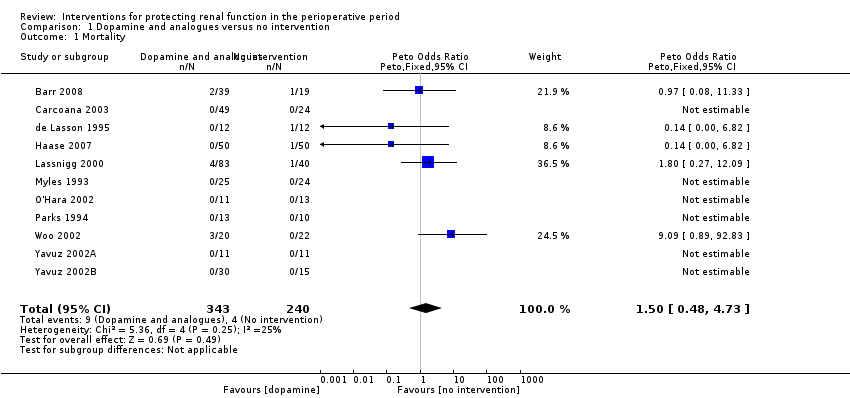
Comparison 1 Dopamine and analogues versus no intervention, Outcome 1 Mortality.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 2 Acute renal injury.
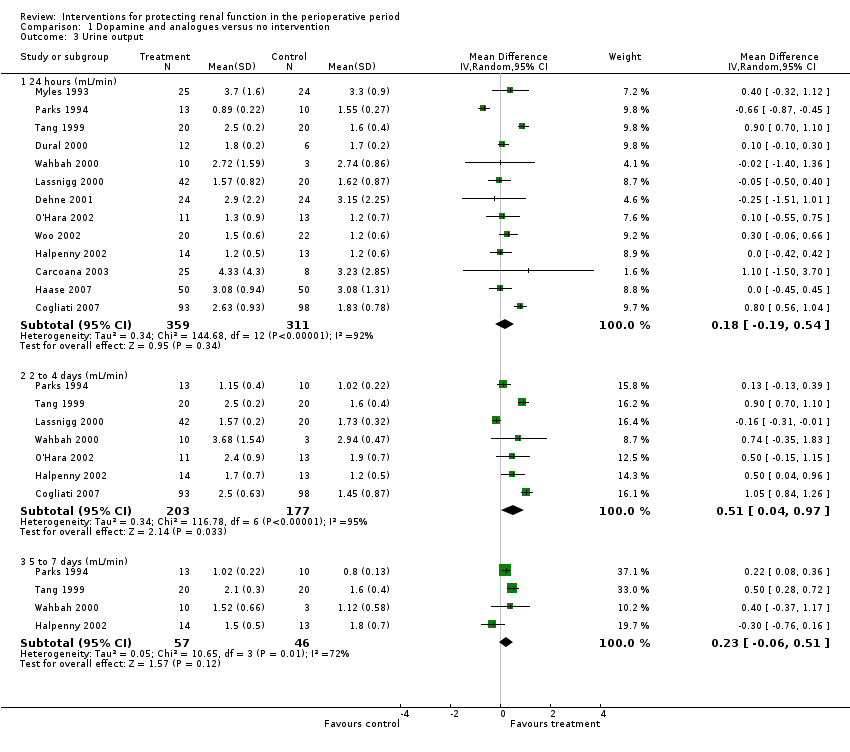
Comparison 1 Dopamine and analogues versus no intervention, Outcome 3 Urine output.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 4 Creatinine clearance.
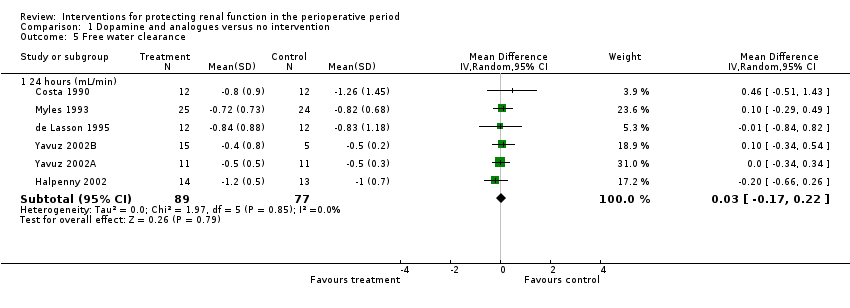
Comparison 1 Dopamine and analogues versus no intervention, Outcome 5 Free water clearance.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 6 Fractional excretion of sodium.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 7 Renal plasma flow (24 hours).

Comparison 2 Diuretics versus no intervention, Outcome 1 Mortality.
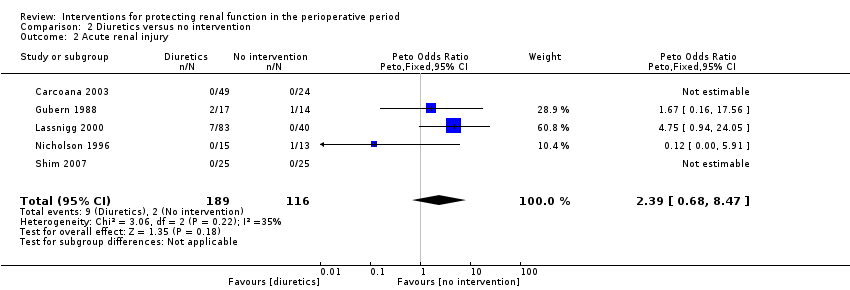
Comparison 2 Diuretics versus no intervention, Outcome 2 Acute renal injury.
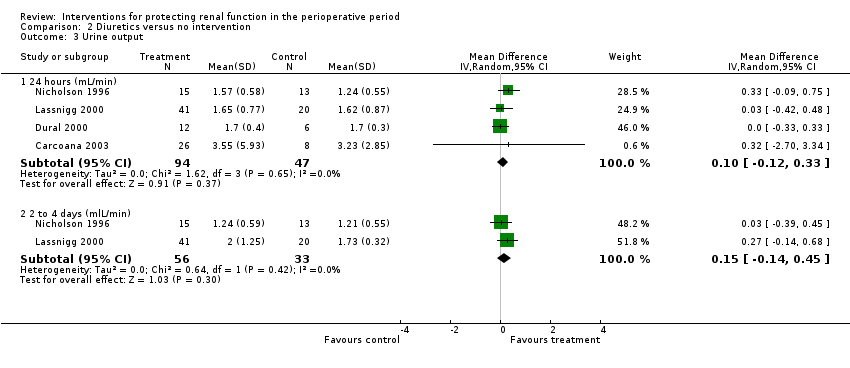
Comparison 2 Diuretics versus no intervention, Outcome 3 Urine output.

Comparison 2 Diuretics versus no intervention, Outcome 4 Creatinine clearance.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 1 Mortality.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 2 Acute renal injury.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 3 Urine output.
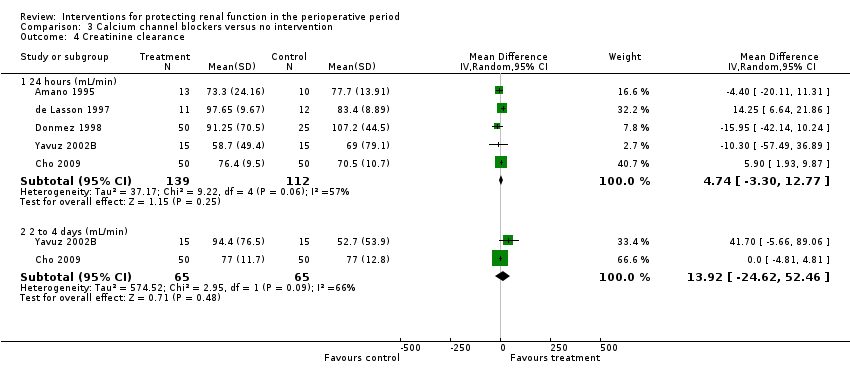
Comparison 3 Calcium channel blockers versus no intervention, Outcome 4 Creatinine clearance.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 5 Free water clearance.
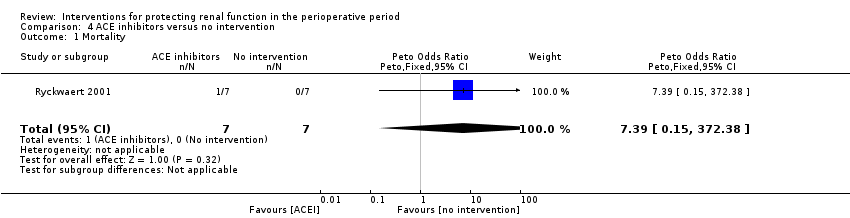
Comparison 4 ACE inhibitors versus no intervention, Outcome 1 Mortality.

Comparison 4 ACE inhibitors versus no intervention, Outcome 2 Acute renal injury.

Comparison 4 ACE inhibitors versus no intervention, Outcome 3 Renal plasma flow.
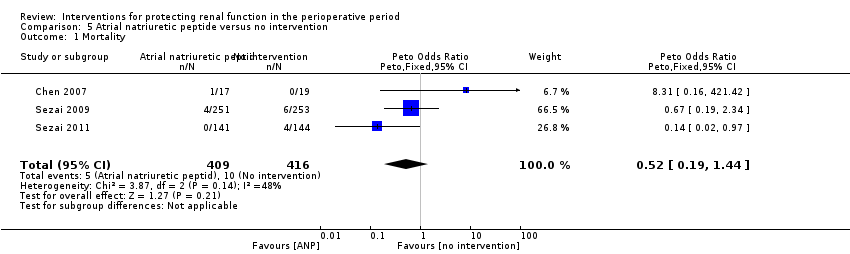
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 1 Mortality.
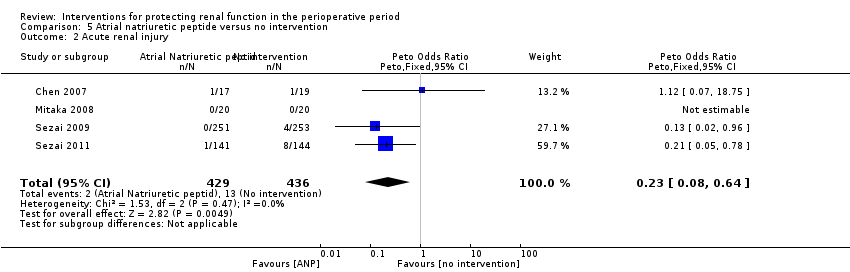
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 2 Acute renal injury.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 3 Urine output at 24 hours.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 4 Creatinine clearance, 24 hours.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 5 Creatinine clearance, 2 to 3 days.
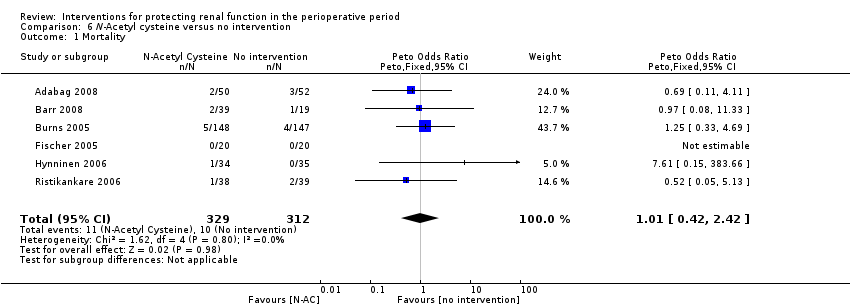
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 1 Mortality.
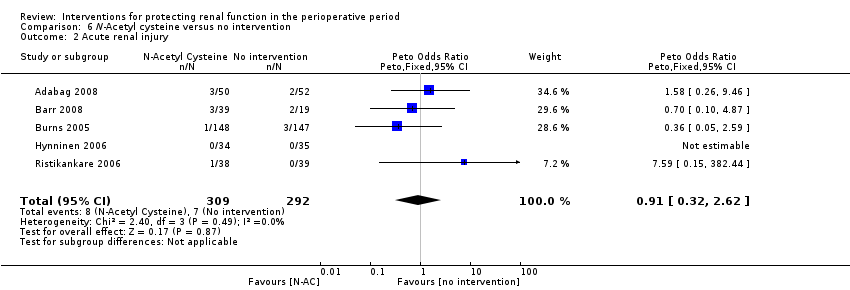
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 2 Acute renal injury.

Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 3 Urine output, 24 hours.
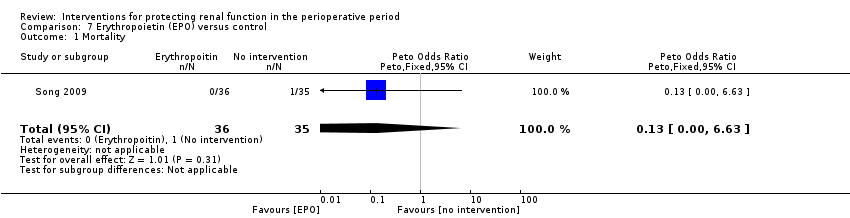
Comparison 7 Erythropoietin (EPO) versus control, Outcome 1 Mortality.

Comparison 7 Erythropoietin (EPO) versus control, Outcome 2 Acute renal injury.
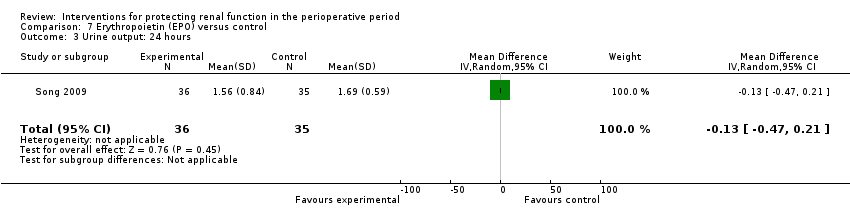
Comparison 7 Erythropoietin (EPO) versus control, Outcome 3 Urine output: 24 hours.
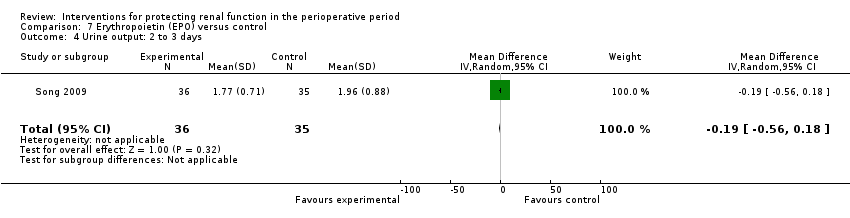
Comparison 7 Erythropoietin (EPO) versus control, Outcome 4 Urine output: 2 to 3 days.

Comparison 7 Erythropoietin (EPO) versus control, Outcome 5 Urine output: 5 to 7 days.

Comparison 8 Intravenous fluid versus control, Outcome 1 Mortality.
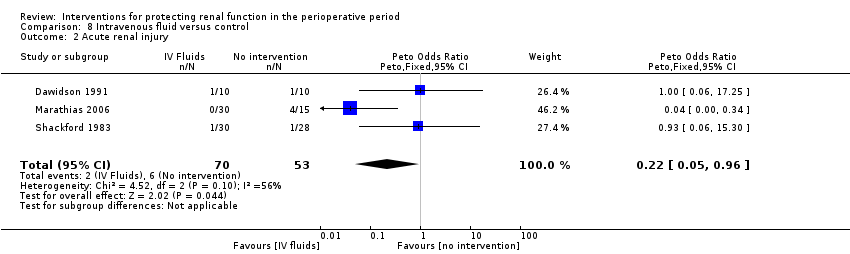
Comparison 8 Intravenous fluid versus control, Outcome 2 Acute renal injury.
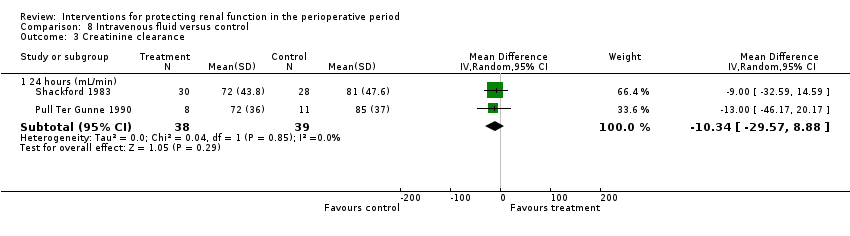
Comparison 8 Intravenous fluid versus control, Outcome 3 Creatinine clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 1 Mortality.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 2 Acute renal injury.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 3 Urine output.
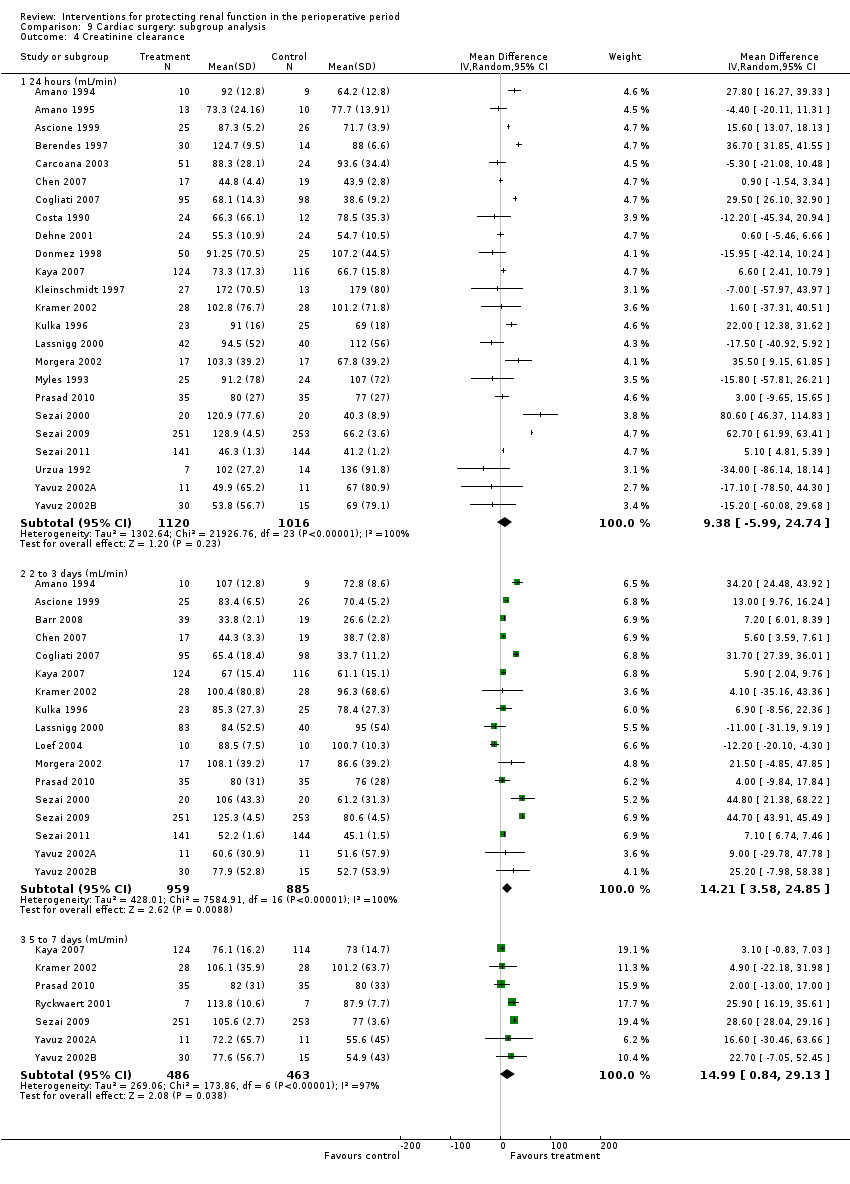
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 4 Creatinine clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 5 Free water clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
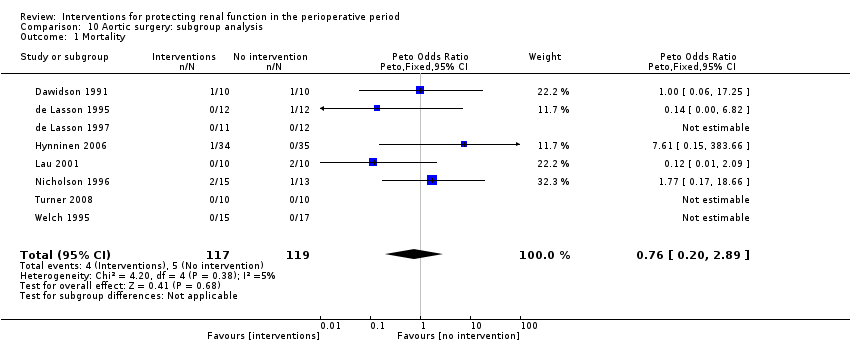
Comparison 10 Aortic surgery: subgroup analysis, Outcome 1 Mortality.
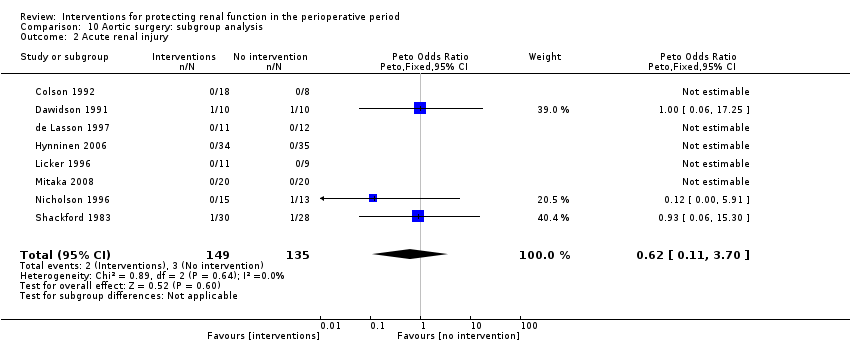
Comparison 10 Aortic surgery: subgroup analysis, Outcome 2 Acute renal injury.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 3 Urine output.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 4 Creatinine clearance.
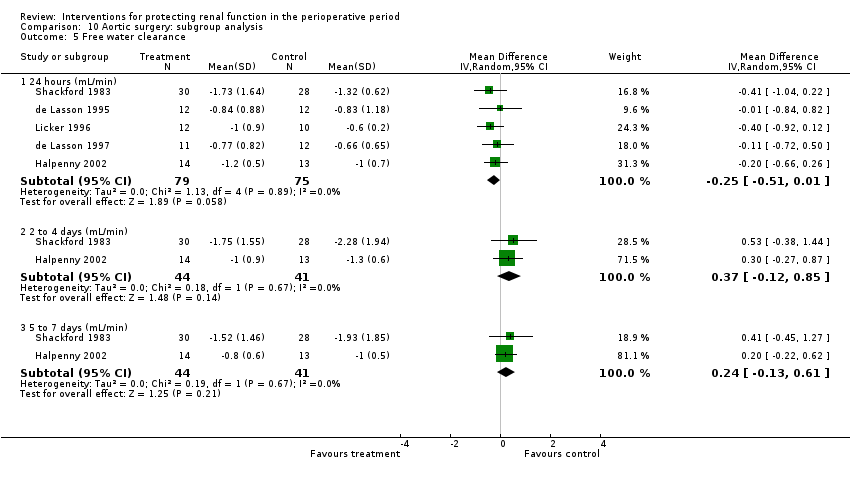
Comparison 10 Aortic surgery: subgroup analysis, Outcome 5 Free water clearance.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
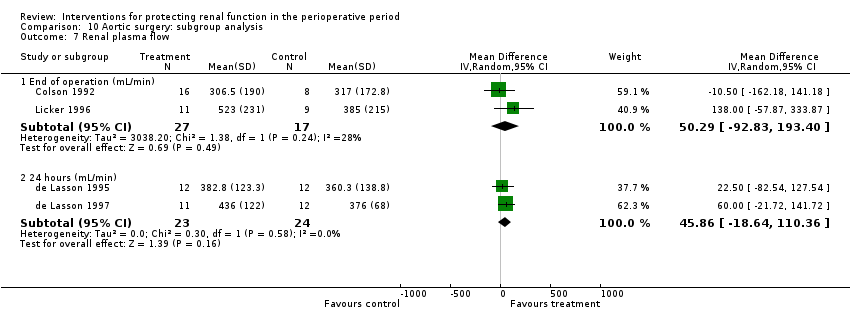
Comparison 10 Aortic surgery: subgroup analysis, Outcome 7 Renal plasma flow.
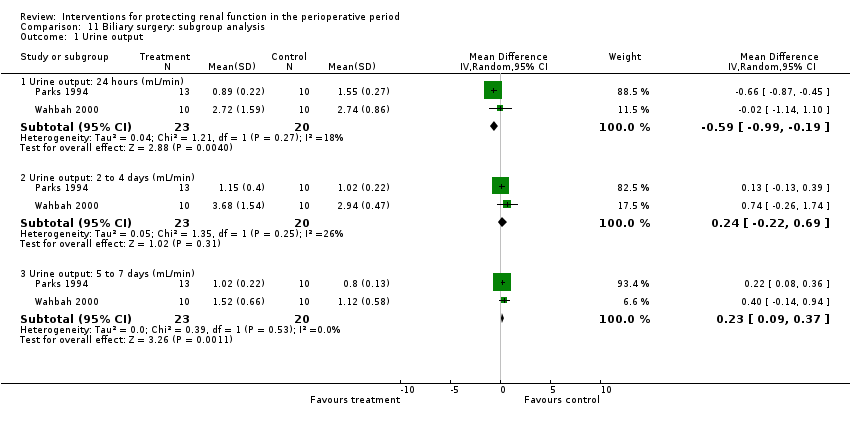
Comparison 11 Biliary surgery: subgroup analysis, Outcome 1 Urine output.
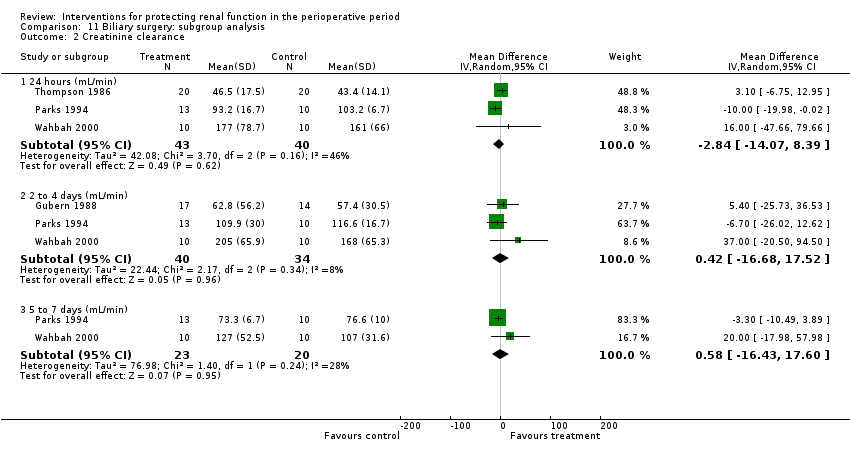
Comparison 11 Biliary surgery: subgroup analysis, Outcome 2 Creatinine clearance.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 1 Mortality.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 2 Acute renal injury.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 3 Urine output.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 4 Creatinine clearance.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 1 Reported mortality, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 2 Acute renal injury, requiring dialysis, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 3 Urine output at 24 hours, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 4 Creatinine clearance at 24 hours, low risk of bias studies only.
| Interventions for protecting renal function in patients with pre‐existing renal impairment who are undergoing surgery | ||||||
| Patient or population: patients with pre‐existing renal impairment Settings: perioperative period (7 days) Intervention: interventions to protect the kidneys during the perioperative period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Various interventions | |||||
| Mortality in patients with pre‐existing renal impairment As reported in the included trials Folliow‐up: 7 days | Study population | OR 0.74 (0.36 to 1.52) | 959 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality | |
| 38 per 1000 | 29 per 1000 | |||||
| Moderate | ||||||
| 20 per 1000 | 15 per 1000 (8 to 30) | |||||
| Acute renal injury in patients with pre‐existing renal impairment As reported in the included trials Follow‐up: 1 to 7 days | Study population | OR 0.40 (0.22 to 0.76) | 979 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 62 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 18 per 1000 (9 to 32) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence: | ||||||
| aOnly six of the 10 studies showed low risk of bias. | ||||||
| Interventions to protect the kidneys during the perioperative period in patients undergoing surgery: low ROB studies only | ||||||
| Patient or population: patients undergoing surgery Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Interventions to protect the kidneys: low ROB cases only | |||||
| Reported mortality, low risk of bias studies only | Study population | OR 1.01 | 1604 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality | |
| 23 per 1000 | 23 per 1000 | |||||
| Moderate | ||||||
| 20 per 1000 | 20 per 1000 | |||||
| Acute renal injury, low‐risk studies only | Study population | OR 1.05 | 1550 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 23 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| aOnly six of the 10 studies showed low risk of bias. | ||||||
| Study ID | Randomization | Allocation concealment | Blinding | Withdrawals recorded | Overall quality |
| Randomly assigned by the investigational pharmacist Block randomization (blocks of 10) | Participants, researchers and clinicians blinded to treatment assignment
| Participants, researchers and clinicians (including data collecting nurse) blinded Drug packets matched in volume, colour, consistency and transparency and given mixed with fruit juice to mask taste | Not reported | Good | |
| ‘Randomly assigned’ into two groups | None described | None described; control group had no treatment | Not described | Poor | |
| ‘Patients were randomized into either diltiazem or no treatment' | Not described | None described; control group had no treatment | Not described | Poor | |
| ‘Prospectively randomized by card allocation’ | ‘Prospectively randomized by card allocation’ | Not used | Not discussed | Moderate | |
| Randomization done by pharmacy department; method of randomization uncertain Not sure about adequacy of randomization | No specific mention of allocation concealment except to say ‘double‐blinded’ Allocation concealment inadequate | No specific mention of who all were blinded; ‘double‐blinded’, placebo‐controlled trial Not sure whether blinding was adequate | One withdrawal from study reported | Moderate | |
| ‘Placebo controlled prospective study’; no randomization | None described | None described | Not described | Poor | |
| ‘Consented and were randomized’ | Not described | Not used | 3 participants (2, 1) not operated upon; 2 participants excluded from final analysis because of clinical management changes | Poor | |
| Randomization done by pharmacy trial co‐ordinator using a permitted block strategy | Allocation concealment using central randomization with drugs prepared by pharmacy | Quadruple‐blinded (participants, clinicians, data collectors and data analyst) placebo‐controlled study | Clearly accounted for (5 in intervention group and 2 in control group) | Good | |
| ‘Prospective randomized double‐blinded and placebo‐controlled study’ Computer‐generated random number tables | Not specifically described, but quite likely it was concealed allocation | Blinded manner; drug or saline supplied by the department investigational pharmacy in a blinded manner + additive for the CPB circuit prime (mannitol or saline, supplied similarly) | All allocated participants completed the trial (withdrawals before allocation) | Good | |
| ‘Randomized’; no details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | Four withdrawals from trial reported
| Poor | |
| Computer‐generated randomization method used | Computer allocation, no further details given | Not described except by the statement, ‘investigator blinded to the study group evaluated the postoperative data’ | Not reported | Moderate | |
| Randomization from a computer list, in an envelope | Sealed envelope used ‘All personnel and patients were blinded to the assignment’ | Blinded nurse, not involved with study, prepared the drug/ placebo in identical 50 mL filled syringes ‘All personnel and patients were blinded to the assignment’ | 1 participant | Good | |
| Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method | Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment | No details on blinding except ‘double‐blind fashion’ | Not given | Poor | |
| Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method | Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment | No details on blinding except ‘double‐blind fashion’ | Not given | Poor | |
|
| Participants with renal dysfunction (CCl < 50 mL/min) ‘Randomly divided into 3 groups’; no description of randomization | No description of allocation | No description of blinding | Not given | Poor |
| ‘Randomly allocated’ into 3 groups; no description of randomization | No description of allocation | No description of blinding | Not given | Poor | |
| ‘Randomized to either treatment group’ by pulling a card from a previously prepared deck | No description of allocation concealment | No details on blinding | Not given | Poor | |
| ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation
| ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation; no description of allocation concealment
| No blinding described | Not given | Poor | |
| Randomization and drug or placebo preparation provided by drug company; method not described, but likely to be good | Not sure of any allocation concealment, but likely possible | Possible, but blinded tables not described | 1 participant had additional drugs but was not excluded | Moderate | |
| Randomly allocated into 2 groups, randomization method not described | Allocation concealment not described | Blinding not mentioned | All participants accounted for in calculations | Poor | |
| ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor | |
| ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor | |
| Randomization done by the last digit of the medical record number of participant (quasi‐randomization) | ‘Patients were prospectively allocated into 2 groups’ No details given | Not given | Not given | Poor | |
| Retrospective chart review of a randomized trial in 2003, which used computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Drugs supplied in identical looking glass vials containing drug or placebo | Exclusions described in text | Good | |
|
| ’Prospectively randomized’; no details of method of randomization | ’Prospectively randomized’; no details of method of allocation | ’Prospectively randomized’; no details of method of blinding | Fate of participants discussed | Poor |
| Random assignment of participants using Microsoft Excel‐based random number generation to create a randomization list, in blocks of 10 | Allocation concealment ensured by quadruple‐blinding (participants, clinicians, data collectors and data analysers were unaware of groups or treatment) | Quadruple‐blinding (participants, clinicians, data collectors and data analysers were blinded) | 0 participants | Good | |
| Microsoft Excel‐based random number generation, with blocks of 10; central randomization by department of pharmacy | Allocation concealment achieved by central randomization, blinding to all researchers, participants and others. Allocation revealed only after data analysis | Both fluids in separate shrink‐wrapped black plastic bags that were identical in appearance (blinded to participants, anaesthetists, surgeons, ICU personnel, nurses and others) | 1 in each group | Good | |
| ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | 1 participant excluded from the trial | Poor | |
| ‘Randomized’, but no details given | Allocated to control and intervention groups using opaque envelopes immediately before surgery; not sure whether allocation was maintained | No blinding | 1 died before operation (intervention group) | Poor | |
| Random assignment in blocks of 10 done by hospital pharmacy, no details given | Allocation done by hospital pharmacy Clinical and study personnel not aware of study allocation | Blinding quite likely, although not detailed in text | 1 participant withdrew from study intraoperatively (does not mention which group, although most likely the intervention group-1 less in that group) | Moderate | |
| Computer‐generated randomization done by statistician | Sequentially numbered, sealed envelopes
| SNP and saline in uniformly appearing 50 mL syringes, blinded to surgeons, perfusionists and nurses; investigators did not know the details | None | Good | |
| Randomization by computer | Not described in detail | Not described in detail | No detailed description | Poor | |
| Participants randomly assigned to receive 1 of 2 treatments | No details given | No details given | Early termination of study in 33 of 56 participants; ITT used | Poor | |
| Allocated into 2 groups in a double‐blinded random fashion; no details of randomization given | Allocated into 2 groups in a double‐blinded random fashion; no details of allocation given | Allocated into 2 groups in a double‐blinded random fashion; no details of blinding given | 2 participants excluded | Poor | |
| Placebo‐controlled randomized double‐blind trial; block randomization done and sealed envelopes used | Placebo‐controlled randomized double‐blind trial; block randomizations done with the use of sealed envelopes; no further details on allocation concealment provided | Placebo‐controlled randomized double‐blind trial; no other details of blinding provided | 3 participants excluded from analysis | Moderate | |
| ‘Recruited patients were allocated to one of 2 groups’; no details on randomization | ‘Recruited patients were allocated to one of 2 groups’; no details on allocation concealment | ‘Recruited patients were allocated to one of 2 groups’; no details on blinding provided | 2 participants accounted for | Poor | |
| 'Patients were allocated in a randomized double‐blind manner’; no details of randomization given | 'Patients were allocated in a randomized double‐blind manner’; no details of allocation concealment given | 'Patients were allocated in a randomized double‐blind manner’; no details of blinding given | 2 participants excluded from the trial | Poor | |
| ‘Randomized in a double‐blind fashion’; no details of randomization given | ‘Randomized in a double‐blind fashion’; no details of allocation given | ‘Randomized in a double‐blind fashion’; no details of blinding given | All participants completed the trial | Poor | |
| Used a 2:1 ratio in randomization process, participants randomly assigned into groups; no other details of randomization given | Participants randomly assigned into groups; no other details of allocation given | Participants randomly assigned into groups; no other details of blinding given | Not given | Poor | |
| ‘Patients were randomized into 2 groups’; not sure what method of randomization was used | Not sure how allocation was performed | ‘Blind infusion was performed’; not sure about blinding | None indicated | Poor | |
| Designed as a prospective double‐blind placebo‐controlled randomized trial; no other details of randomization provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of allocation concealment provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of blinding provided | All participants competed the trial | Poor | |
| ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | 2 participants excluded from analysis | Poor | |
| Randomly assigned with the use of a table of random numbers; ‘prospective double‐blind randomized trial’ | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; allocation concealed | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; blinded | 3 withdrawals before start of trial | Good | |
| ‘Prospective randomized trial’; no further details on randomization | ‘Prospective randomized trial’; no further details on allocation | ‘Prospective randomized trial’; no details on blinding | None reported | Poor | |
| ‘Patients were randomized’; no further details | No indication of allocation concealment, but for statement, ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ | Possible: ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ | None indicated in text | Poor | |
| ‘Prospective randomized study’; no further details on randomization given | ‘Prospective randomized study’; no further details on allocation | ‘Prospective randomized study’; no further details on blinding | 11 of 35 excluded | Poor | |
| ‘Patients were randomly allocated into 2 groups’; no further details on randomization | ‘Patients were randomly allocated into 2 groups’; no further details on allocation | ‘Patients were randomly allocated into 2 groups’; no further details on blinding | Not disclosed | Poor | |
| Randomization performed by aleatorized numbers prepared in closed envelopes | No details on concealment of allocation except ‘Randomization performed by aleatorized numbers prepared in closed envelopes’ | Drug or placebo given with an identical container in a double‐blind manner with the same volume of drug or saline | 4 participants excluded | Moderate | |
| Randomized, prospective, open‐label study Random number generated from a random number table | No concealment of assignment | No blinding | 4 excluded after randomization? | Poor | |
| Random assignment by the hospital pharmacy clinical trials co‐ordinator Microsoft Excel–based random number generator permuted block strategy with blocks of 10 | Allocation stratified into 2 groups based on pre‐op use of statins Allocation concealed to participants, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators | 'Double‐blind'. Atorvastatin or placebo medication prepared in capsules of identical appearance
| 8 in intervention group and 7 in control group | Good | |
| Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details; the anaesthesiologist was aware of the allocation and treatment received | No details provided | Poor | |
| ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’ | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’, but no details of allocation concealment provided | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; no details of blinding provided | 3 participants excluded | Moderate | |
| ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of randomization given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of allocation given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of blinding given | No dropouts detailed in text | Poor | |
| ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on randomization method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on allocation method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on blinding | Not described, but study probably had no dropouts | Poor | |
| Randomly allocated into 2 groups by drawing lots | ‘Randomly allocated by drawing lots’ No other details | No evidence of blinding | No mention in the text | Poor | |
| Randomly allocated into 2 groups by lottery method | 'Randomly allocated into 2 groups'; no evidence of concealment of allocation | No blinding discussed | Dropouts discussed | Poor | |
| Participants were assigned by random number to 1 of 2 groups; no details on randomization given | Participants were assigned by random number to 1 of 2 groups; no details on concealment of allocation given | Participants assigned by random number to 1 of 2 groups; no details on blinding | No dropouts | Poor | |
| Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table | Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table; no further details on allocation concealment given | All medical personnel involved in the study blinded to the contents of the infusion bottle | No dropouts recorded | Moderate | |
| Block randomization developed by research centre Randomization stratified by serum creatinine levels | Allocation via Internet using predetermined randomization | Participants, healthcare clinicians and researchers blinded | None | Good | |
| Prospectively randomly assigned | No details on allocation provided in text | No details of blinding provided in text | No dropouts recorded | Poor | |
| Participants randomly assigned; no further details on randomization given | Participants randomly assigned; no further details on allocation given | 2 different types of procedures; no blinding possible | 5 participants subsequently excluded from trial | Poor | |
| ‘Patients were randomized’; no more details | ‘Patients were randomized’; no more details | No details provided | ‘There were no withdrawals’ | Poor | |
| Random assignment done with use of computer‐generated randomization list | Computer‐generated randomization list placed in sealed envelopes and opened in numerical order by a third party, who prepared the study infusion | Third party prepared the infusion. Infusions were such that volumes were equal in the bag and of identical colour, and contents of the bag were indistinguishable; the infusion was done over 30 minutes to avoid haemodynamic effects of treatment | Yes, none lost | Good | |
| Participants randomly assigned into 1 of 2 groups, according to the last digit of their clinical history number (quasi‐randomization) | No description of concealment of allocation | No report of blinding | All participants completed | Poor | |
| ‘Patients were randomly allocated into 4 equal groups’; no further details on randomization | Patients were randomly allocated into 4 equal groups’; no further details on allocation | No description of blinding | None described | Poor | |
| ‘Patients were randomly assigned’; no further details on randomization method used | ‘Patients were randomly assigned’; no further details on allocation method used | No description of blinding | None described | Poor | |
| ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | One death described | Poor | |
| Participants were ‘randomized’ It appears that the anaesthesiologist ‘randomly drew an envelope with the assigned treatment’ | Allocation concealment was possible only for participants and the statistician | No; control received no treatment Participants and the statistician were blinded | Not described | Poor | |
| ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | 8 participants excluded because of death or major complications | Poor | |
| ‘Patients were prospectively randomized’; no details on method of randomization used | ‘Patients were prospectively randomized’; no details on method of allocation used | No description of blinding | States no deaths; no description of dropouts | Poor | |
| ‘Patients randomized into 4 groups’; no further details on randomization given | ‘Patients randomized into 4 groups’; no description of allocation used | No description of blinding | No mortality described, but no suggestion of dropouts | Poor | |
| ‘Randomly assigned’; no further details of randomization given | ‘Randomly assigned’; no further details of allocation given | No blinding described | No dropouts described | Poor |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 11 | 583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.48, 4.73] |
| 2 Acute renal injury Show forest plot | 10 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.44, 4.23] |
| 3 Urine output Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 13 | 670 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.19, 0.54] |
| 3.2 2 to 4 days (mL/min) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.04, 0.97] |
| 3.3 5 to 7 days (mL/min) | 4 | 103 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.51] |
| 4 Creatinine clearance Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 14 | 616 | Mean Difference (IV, Random, 95% CI) | 7.17 [‐5.53, 19.86] |
| 4.2 2 to 4 days (mL/min) | 9 | 459 | Mean Difference (IV, Random, 95% CI) | 7.31 [‐6.19, 20.82] |
| 4.3 5 to 7 days (mL/min) | 5 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐13.63, 6.98] |
| 5 Free water clearance Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 6 | 166 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow (24 hours) Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 75.36 [‐63.27, 213.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [0.80, 7.74] |
| 2 Acute renal injury Show forest plot | 5 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.39 [0.68, 8.47] |
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.33] |
| 3.2 2 to 4 days (mlL/min) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.14, 0.45] |
| 4 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 3 | 123 | Mean Difference (IV, Random, 95% CI) | ‐18.02 [‐41.78, 5.75] |
| 4.2 2 to 4 days (mL/min) | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐14.76, 19.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute renal injury Show forest plot | 6 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.01, 1.17] |
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Urine output: 24 hours (mL/min) | 4 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.02, 0.45] |
| 4 Creatinine clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 5 | 251 | Mean Difference (IV, Random, 95% CI) | 4.74 [‐3.30, 12.77] |
| 4.2 2 to 4 days (mL/min) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 13.92 [‐24.62, 52.46] |
| 5 Free water clearance Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 3 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 2 Acute renal injury Show forest plot | 3 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Renal plasma flow Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 RPF: end of operation (mL/min) | 3 | 62 | Mean Difference (IV, Random, 95% CI) | 46.37 [‐68.61, 161.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 2 Acute renal injury Show forest plot | 4 | 865 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.08, 0.64] |
| 3 Urine output at 24 hours Show forest plot | 3 | 584 | Mean Difference (IV, Random, 95% CI) | 0.42 [0.18, 0.67] |
| 4 Creatinine clearance, 24 hours Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 35.23 [‐0.48, 70.94] |
| 5 Creatinine clearance, 2 to 3 days Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 27.30 [4.36, 50.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 641 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.42, 2.42] |
| 2 Acute renal injury Show forest plot | 5 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.32, 2.62] |
| 3 Urine output, 24 hours Show forest plot | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.24, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.63] |
| 2 Acute renal injury Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Urine output: 24 hours Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.47, 0.21] |
| 4 Urine output: 2 to 3 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.18] |
| 5 Urine output: 5 to 7 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.16, 3.42] |
| 2 Acute renal injury Show forest plot | 3 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.96] |
| 3 Creatinine clearance Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐29.57, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 26 | 2390 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.56, 1.64] |
| 2 Acute renal injury Show forest plot | 31 | 2504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 3 Urine output Show forest plot | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 17 | 1475 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.17, 0.36] |
| 3.2 2 to 3 days (mL/min) | 9 | 1058 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.13, 0.54] |
| 4 Creatinine clearance Show forest plot | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 24 | 2136 | Mean Difference (IV, Random, 95% CI) | 9.38 [‐5.99, 24.74] |
| 4.2 2 to 3 days (mL/min) | 17 | 1844 | Mean Difference (IV, Random, 95% CI) | 14.21 [3.58, 24.85] |
| 4.3 5 to 7 days (mL/min) | 7 | 949 | Mean Difference (IV, Random, 95% CI) | 14.99 [0.84, 29.13] |
| 5 Free water clearance Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 7 | 700 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.19] |
| 5.2 2 to 3 days (mL/min) | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.30, ‐0.28] |
| 6 Fractional excretion of sodium Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 8 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 8 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.20, 2.89] |
| 2 Acute renal injury Show forest plot | 8 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.11, 3.70] |
| 3 Urine output Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 7 | 227 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 3.2 2 to 3 days (mL/min) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.58] |
| 3.3 5 to 7 days (mL/min) | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 4 Creatinine clearance Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 9 | 323 | Mean Difference (IV, Random, 95% CI) | 7.99 [‐0.77, 16.74] |
| 4.2 2 to 3 days (mL/min) | 5 | 195 | Mean Difference (IV, Random, 95% CI) | 11.62 [‐6.13, 29.37] |
| 4.3 5 to 7 days (mL/min) | 4 | 116 | Mean Difference (IV, Random, 95% CI) | ‐12.85 [‐26.41, 0.72] |
| 5 Free water clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 5 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.51, 0.01] |
| 5.2 2 to 4 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.12, 0.85] |
| 5.3 5 to 7 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.13, 0.61] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 End of operation (mL/min) | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 50.29 [‐92.83, 193.40] |
| 7.2 24 hours (mL/min) | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 45.86 [‐18.64, 110.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urine output Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Urine output: 24 hours (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.19] |
| 1.2 Urine output: 2 to 4 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.22, 0.69] |
| 1.3 Urine output: 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.09, 0.37] |
| 2 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 24 hours (mL/min) | 3 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐14.07, 8.39] |
| 2.2 2 to 4 days (mL/min) | 3 | 74 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐16.68, 17.52] |
| 2.3 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐16.43, 17.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 959 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.36, 1.52] |
| 2 Acute renal injury Show forest plot | 11 | 979 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.76] |
| 3 Urine output Show forest plot | 4 | 707 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.16, 0.85] |
| 3.1 Urine output, 24 hours | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.12, 0.81] |
| 3.2 Urine output, 2 to 3 days | 2 | 291 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.78, 1.65] |
| 4 Creatinine clearance Show forest plot | 4 | 646 | Mean Difference (IV, Random, 95% CI) | 10.65 [0.04, 21.27] |
| 4.1 Creatinine clearance, 24 hours | 4 | 347 | Mean Difference (IV, Random, 95% CI) | 7.78 [‐10.39, 25.94] |
| 4.2 Creatinine clearance, 2 to 3 days | 3 | 299 | Mean Difference (IV, Random, 95% CI) | 14.16 [‐6.20, 34.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reported mortality, low risk of bias studies only Show forest plot | 19 | 1604 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| 2 Acute renal injury, requiring dialysis, low risk of bias studies only Show forest plot | 16 | 1550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.55, 2.03] |
| 3 Urine output at 24 hours, low risk of bias studies only Show forest plot | 11 | 798 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
| 4 Creatinine clearance at 24 hours, low risk of bias studies only Show forest plot | 9 | 817 | Mean Difference (IV, Random, 95% CI) | 6.59 [‐3.53, 16.72] |

