Intervenciones para proteger la función renal en el período perioperatorio
Appendices
Appendix 1. Search strategies employed
Search strategy for MEDLINE (Ovid SP)
1 exp Kidney Failure/ or exp Kidney Failure Acute/ or exp Kidney Failure Chronic/ or exp Kidney Function Tests/ or exp Glomerular Filtration Rate/ or exp Renal Circulation/ or exp Renal Plasma Flow/ or exp Renal Insufficiency/ or kidney.ti,ab. or (glomerul* adj3 filtration).mp. or (renal adj3 (failure or protect* or function*)).mp. or kidney function test*.mp. or renal function test*.mp. or free water clearance.mp. or fractional excretion of sodium.mp. or (urine adj3 (output or flow)).mp.
2 exp Angiotensin Converting Enzyme Inhibitors/ or exp Fluid Therapy/ or exp Infusions Intravenous/ or exp Angiotensin Converting Enzyme Inhibitors/ or exp diuretics/ or exp mannitol/ or exp Furosemide/ or exp Dopamine/ or exp Dopamine Agonists/ or (diuretic* or mannitol or frusemide or furosemide).mp. or (fluid* adj3 therap*).mp. or (intravenous adj3 fluid*).mp. or hydration.ti,ab. or angiotensin converting enzyme inhibitor*.mp. or ACE inhibitor*.mp. or dopamin*.ti,ab.
3 exp Perioperative Care/ or exp Intraoperative Period/ or exp Intraoperative Care/ or exp Intraoperative Complications/ or (peri?operativ* or intra?operativ*).ti,ab.
4 1 and 2 and 3
5 reno?protect*.af.
6 4 or 5 (2513)
7 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
8 6 and 7
Search strategy for EMBASE (Ovid SP)
1 exp kidney failure/ or exp kidney failure/ or exp kidney function test/ or exp glomerulus filtration rate/ or exp kidney circulation/ or exp kidney clearance/ or exp kidney plasma flow/ or exp urine flow rate/ or exp urine volume/ or kidney.ti,ab. or (glomerul* adj3 filtration).mp. or (renal adj3 (failure or protect* or function*)).mp. or kidney function test*.mp. or renal function test*.mp. or free water clearance.mp. or fractional excretion of sodium.mp. or (urine adj3 (output or flow)).mp.
2 exp dipeptidyl carboxypeptidase inhibitor/ or exp fluid therapy/ or intravenous drug administration/ or exp diuretic agent/ or exp diuretic agent/ or exp mannitol/ or exp furosemide/ or exp dopamine/ or exp dopamine receptor stimulating agent/ or (diuretic* or mannitol or frusemide or furosemide).mp. or (fluid* adj3 therap*).mp. or (intravenous adj3 fluid*).mp. or hydration.ti,ab. or angiotensin converting enzyme inhibitor*.mp. or ACE inhibitor*.mp. or dopamin*.ti,ab.
3 exp perioperative period/ or exp intraoperative period/ or exp peroperative care/ or (peri?operativ* or intra?operativ*).ti,ab.
4 1 and 2 and 3
5 reno?protect*.ti,ab.
6 4 or 5
7 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
8 6 and 7
Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Acute Kidney Injury explode all trees
#2 MeSH descriptor Kidney Failure, Chronic explode all trees
#3 MeSH descriptor Kidney Function Tests explode all trees
#4 MeSH descriptor Glomerular Filtration Rate explode all trees
#5 MeSH descriptor Renal Circulation explode all trees
#6 MeSH descriptor Renal Plasma Flow, Effective explode all trees
#7 MeSH descriptor Renal Insufficiency explode all trees
#8 kidney
#9 glomerul* near filtration
#10 renal near (failure or protect* or function*)
#11 kidney function test*
#12 renal function test*
#13 free water clearance
#14 (fractional excretion) of sodium
#15 urine near (output or flow)
#16 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15)
#17 MeSH descriptor Angiotensin‐Converting Enzyme Inhibitors explode all trees
#18 diuretic* or mannitol or frusemide or furosemide
#19 MeSH descriptor Fluid Therapy explode all trees
#20 fluid* near therap*
#21 MeSH descriptor Infusions, Intravenous explode all trees
#22 (intravenous near fluid*) or hydration
#23 angiotensin converting enzyme inhibitor*
#24 ACE inhibitor*
#25 MeSH descriptor Diuretics explode all trees
#26 MeSH descriptor Mannitol explode all trees
#27 MeSH descriptor Furosemide explode all trees
#28 MeSH descriptor Dopamine explode all trees
#29 MeSH descriptor Dopamine Agonists explode all trees
#30 dopamin*
#31 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30)
#32 MeSH descriptor Perioperative Care explode all trees
#33 MeSH descriptor Intraoperative Care explode all trees
#34 MeSH descriptor Intraoperative Complications explode all trees
#35 MeSH descriptor Intraoperative Period explode all trees
#36 perioperativ* or intraoperativ*
#37 (#32 OR #33 OR #34 OR #35 OR #36)
#38 (#16 AND #31 AND #37)
Appendix 2. data extraction form
Data extraction form
Study ID:
Language: English/
What was the surgical procedure?
What was the study intervention?
How many participants were studied?
How many in the intervention group?
How many in the control group?
Were the inclusion criteria clearly defined?
Were the exclusion criteria clearly defined?
Age group Intervention Control
Male:Female numbers:
Bias:
Was there randomization of allocation in the groups?
Was there adequate information about randomization?
Was there allocation concealment?
Was the allocation concealment adequate?
Were there any withdrawals from the study?
How many people withdrew from each group?
Was there blinding in the study?
Study details:
What was the actual nature of the intervention?
When did the intervention start and finish?
What was the actual nature of the control group?
When did the control group start and finish?
Were the two groups treated equally?
What were the outcomes studied?
Mortality
Acute renal failure
Urine output
Creatinine clearance
Free water clearance
Fractional excretion of sodium
Renal blood flow
Urinary microalbumin:creatinine ratio
Urinary NAG:creatinine ratio
Urinary RBOP:creatinine ratio
Urinary NGAL:creatinine ratio
Plasma cystatin C
When were the outcomes measured?
Preoperative Postoperative: 24 hours Postoperative: 48 hours Postoperative: 72 hours Postoperative: day (note)
Was the outcome assessment blinded?
Was there intention‐to‐treat analysis?
Are mean and standard deviation given?
Other measures of presentation of data
Graphic data?
Results:
Mean and SD Other measures: (please specify clearly)
Remarks:
Drug company sponsorship?
Other comments:
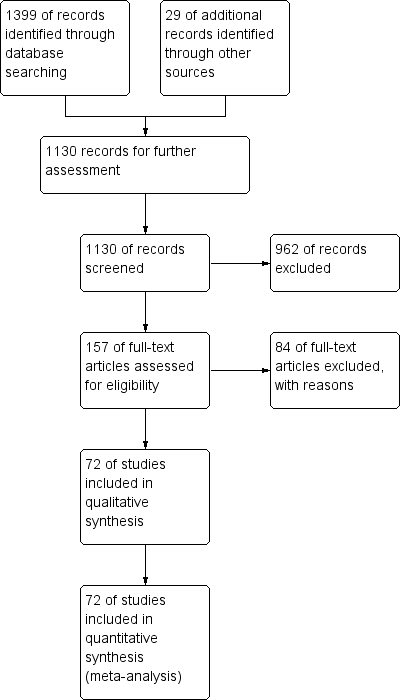
Study flow diagram, as of December 2012.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across 78 included studies.
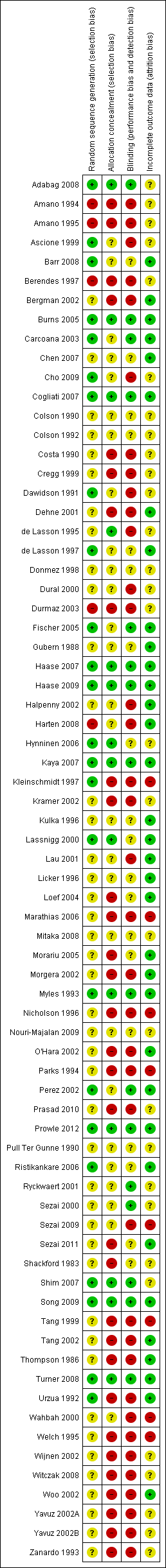
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.1 Mortality.
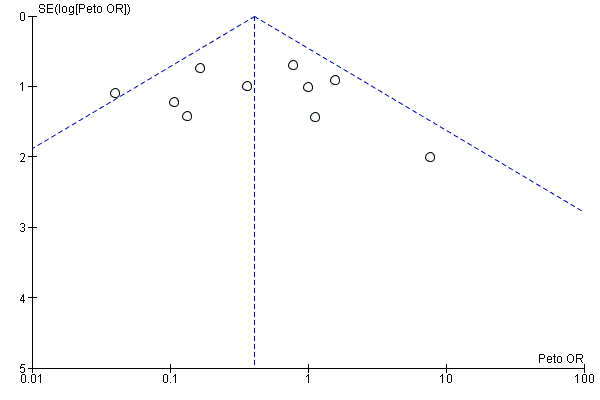
Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.2 Acute renal injury.
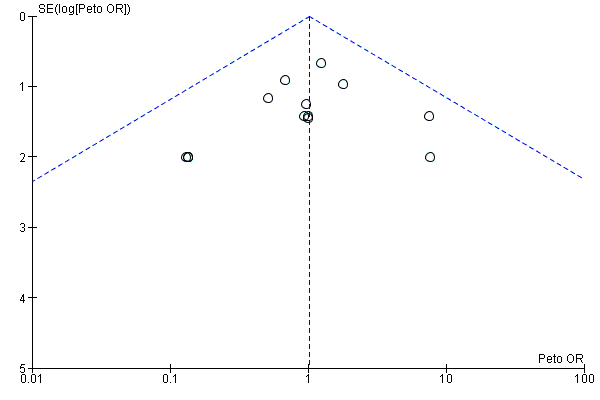
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.1 Reported mortality, low risk of bias studies only.
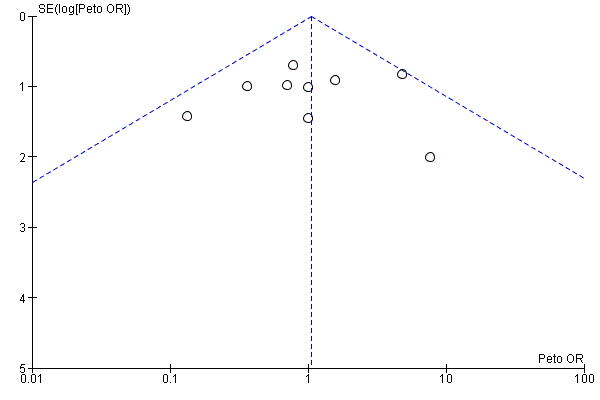
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.2 Acute renal injury, requiring dialysis, low risk of bias studies only.
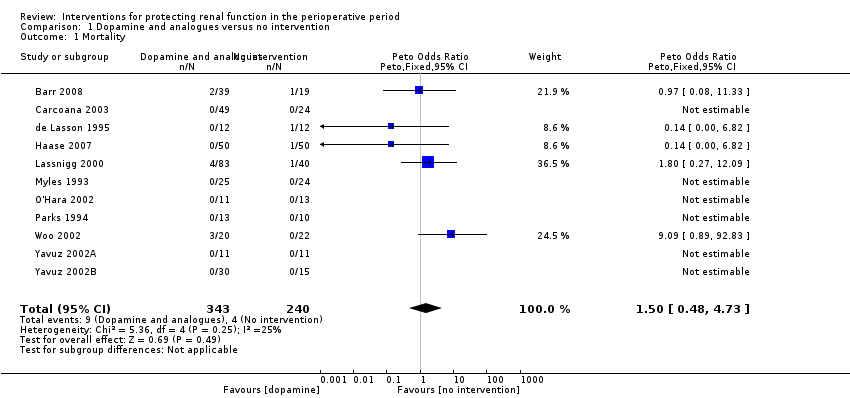
Comparison 1 Dopamine and analogues versus no intervention, Outcome 1 Mortality.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 2 Acute renal injury.
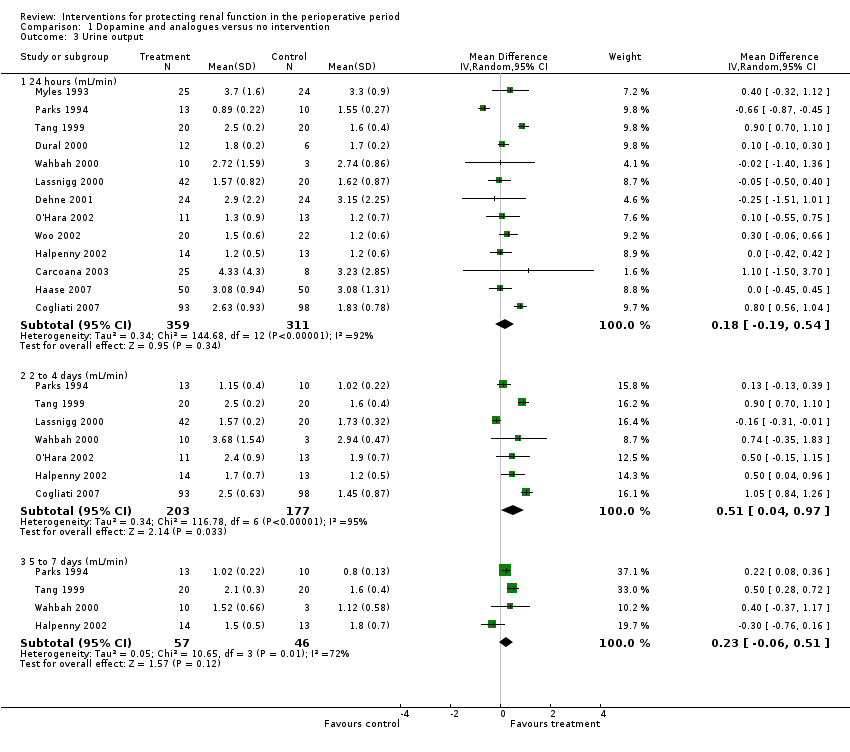
Comparison 1 Dopamine and analogues versus no intervention, Outcome 3 Urine output.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 4 Creatinine clearance.
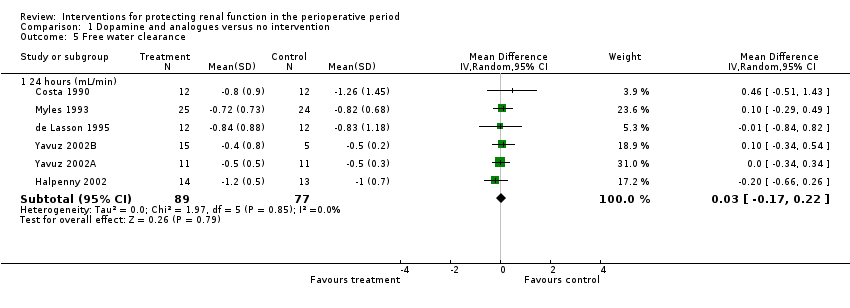
Comparison 1 Dopamine and analogues versus no intervention, Outcome 5 Free water clearance.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 6 Fractional excretion of sodium.

Comparison 1 Dopamine and analogues versus no intervention, Outcome 7 Renal plasma flow (24 hours).

Comparison 2 Diuretics versus no intervention, Outcome 1 Mortality.
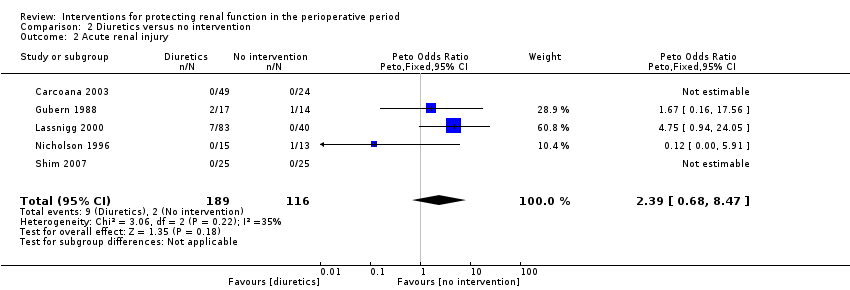
Comparison 2 Diuretics versus no intervention, Outcome 2 Acute renal injury.
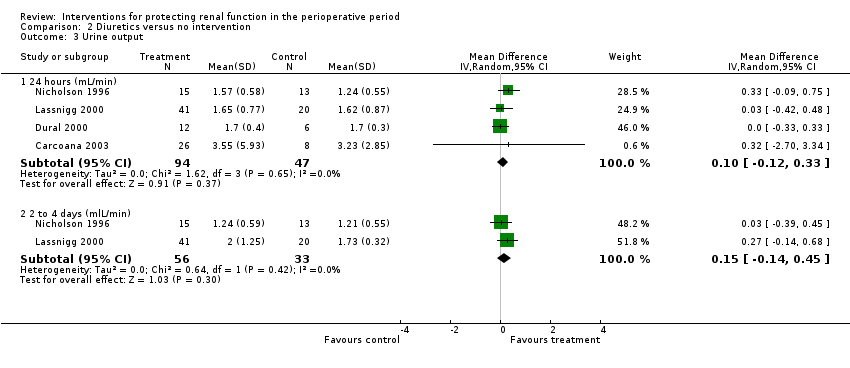
Comparison 2 Diuretics versus no intervention, Outcome 3 Urine output.

Comparison 2 Diuretics versus no intervention, Outcome 4 Creatinine clearance.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 1 Mortality.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 2 Acute renal injury.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 3 Urine output.
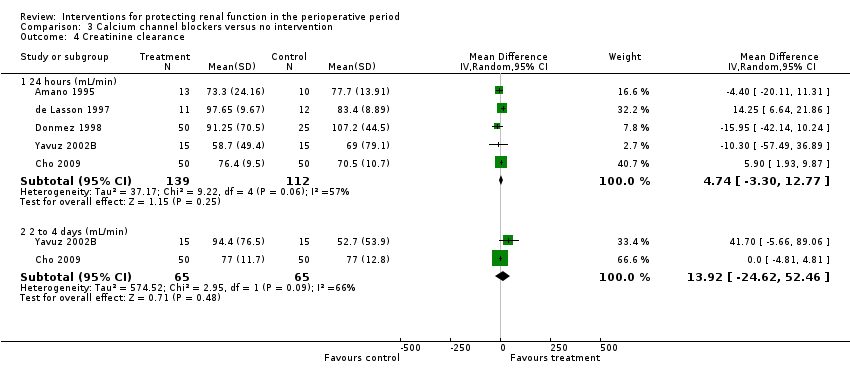
Comparison 3 Calcium channel blockers versus no intervention, Outcome 4 Creatinine clearance.

Comparison 3 Calcium channel blockers versus no intervention, Outcome 5 Free water clearance.
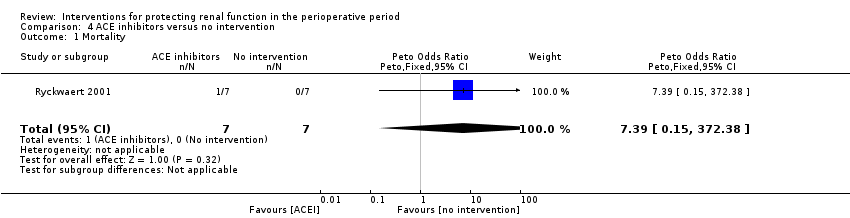
Comparison 4 ACE inhibitors versus no intervention, Outcome 1 Mortality.

Comparison 4 ACE inhibitors versus no intervention, Outcome 2 Acute renal injury.

Comparison 4 ACE inhibitors versus no intervention, Outcome 3 Renal plasma flow.
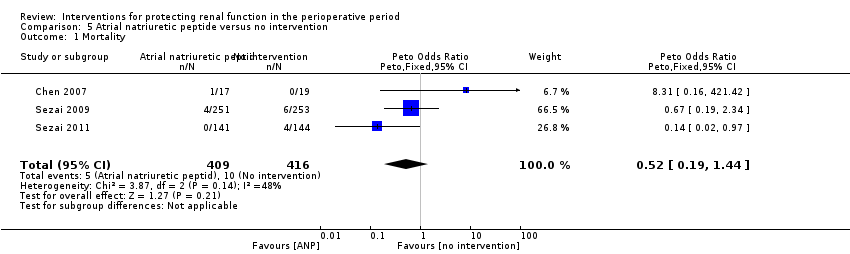
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 1 Mortality.
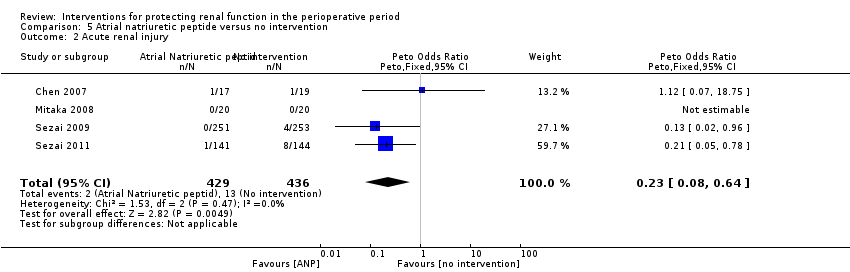
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 2 Acute renal injury.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 3 Urine output at 24 hours.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 4 Creatinine clearance, 24 hours.

Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 5 Creatinine clearance, 2 to 3 days.
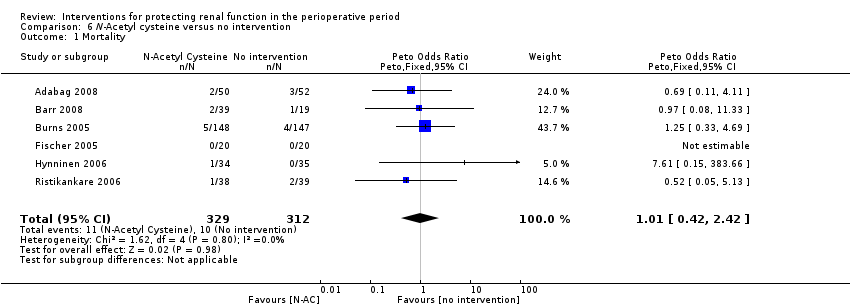
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 1 Mortality.
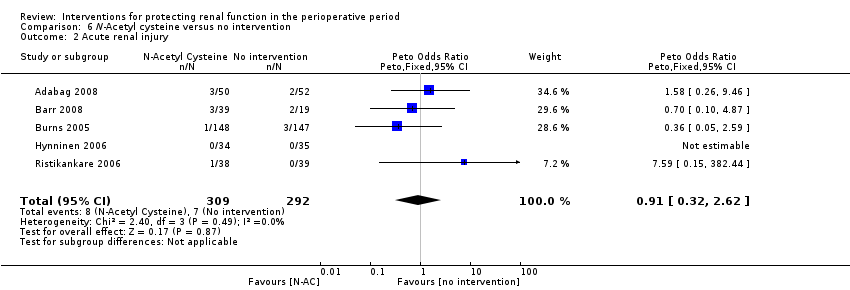
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 2 Acute renal injury.

Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 3 Urine output, 24 hours.
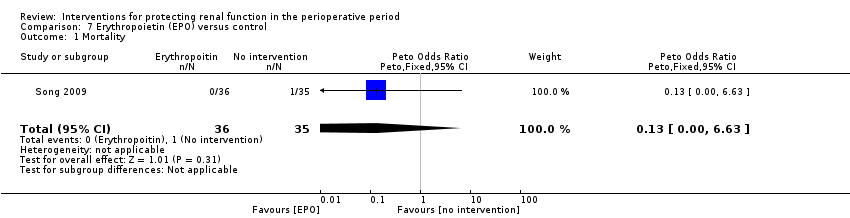
Comparison 7 Erythropoietin (EPO) versus control, Outcome 1 Mortality.

Comparison 7 Erythropoietin (EPO) versus control, Outcome 2 Acute renal injury.
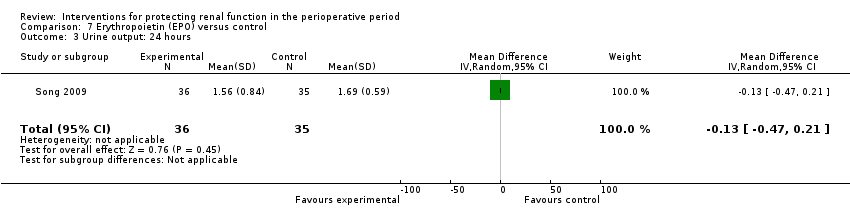
Comparison 7 Erythropoietin (EPO) versus control, Outcome 3 Urine output: 24 hours.
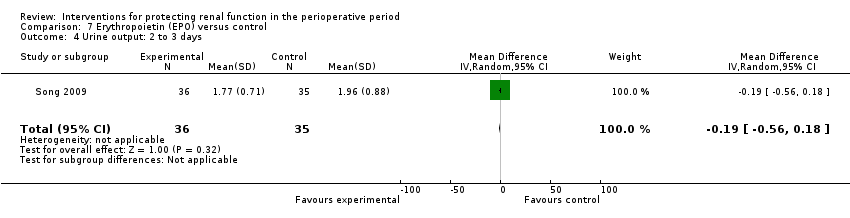
Comparison 7 Erythropoietin (EPO) versus control, Outcome 4 Urine output: 2 to 3 days.

Comparison 7 Erythropoietin (EPO) versus control, Outcome 5 Urine output: 5 to 7 days.

Comparison 8 Intravenous fluid versus control, Outcome 1 Mortality.
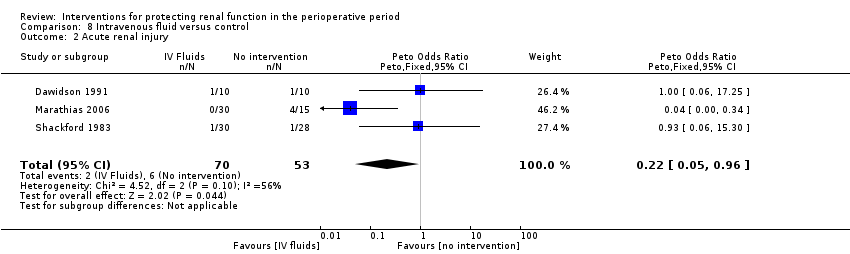
Comparison 8 Intravenous fluid versus control, Outcome 2 Acute renal injury.
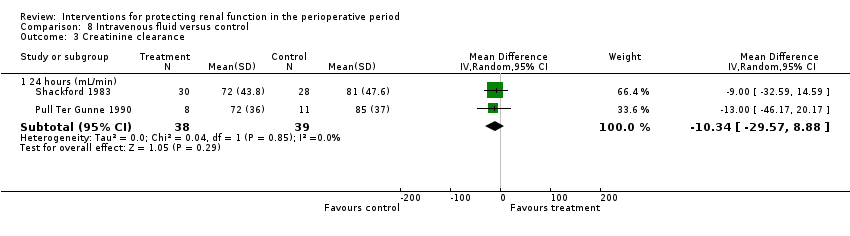
Comparison 8 Intravenous fluid versus control, Outcome 3 Creatinine clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 1 Mortality.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 2 Acute renal injury.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 3 Urine output.
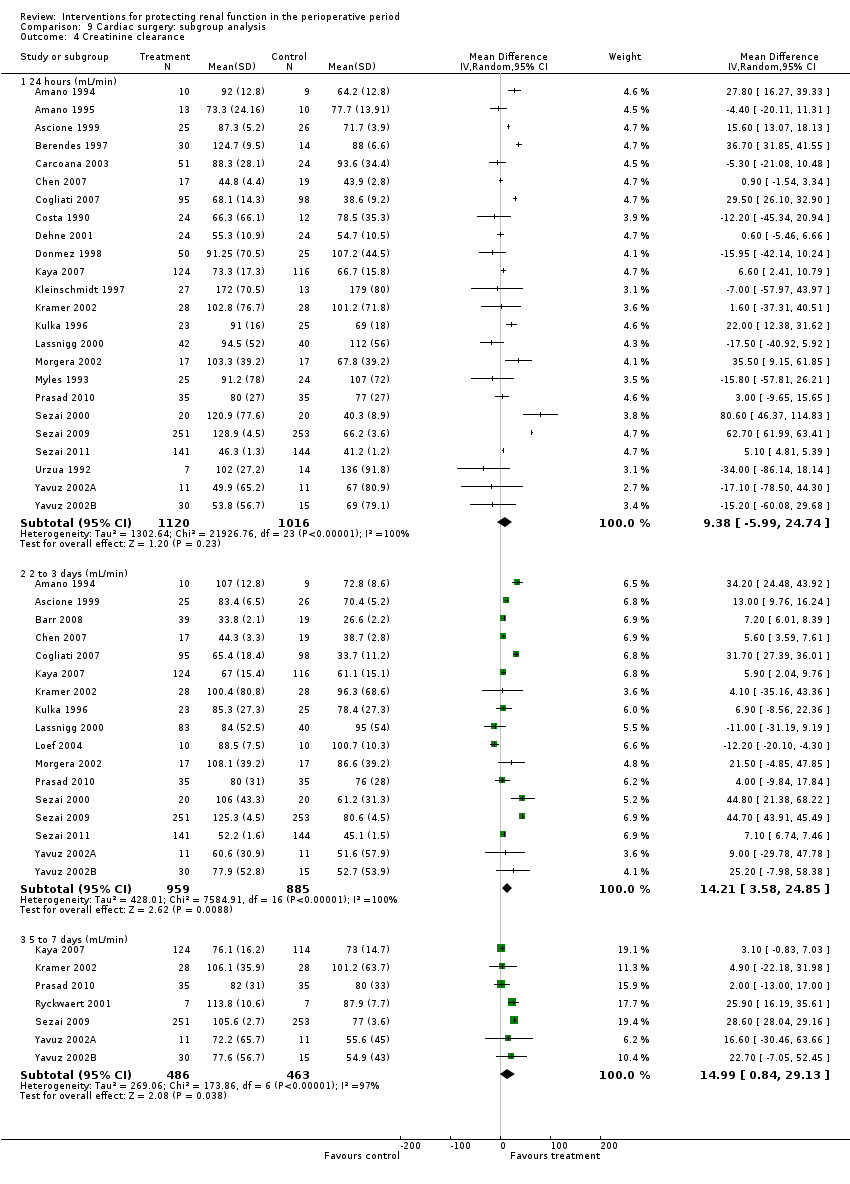
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 4 Creatinine clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 5 Free water clearance.

Comparison 9 Cardiac surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
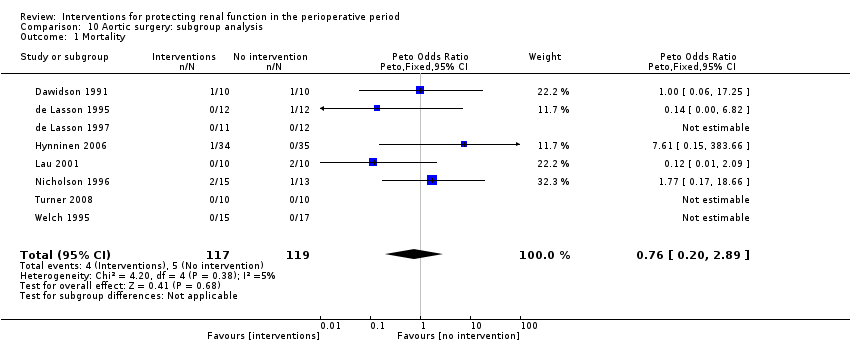
Comparison 10 Aortic surgery: subgroup analysis, Outcome 1 Mortality.
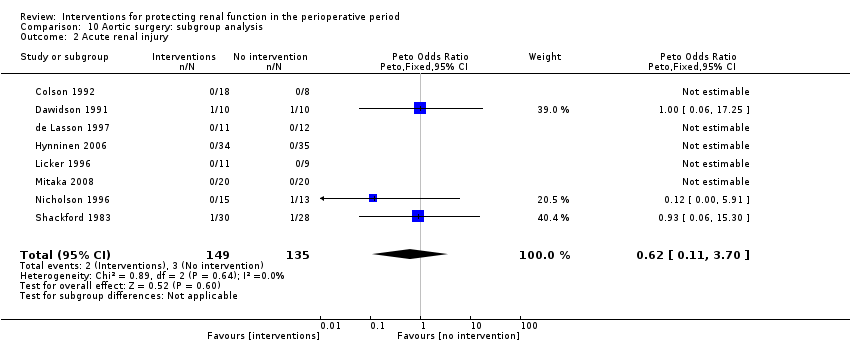
Comparison 10 Aortic surgery: subgroup analysis, Outcome 2 Acute renal injury.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 3 Urine output.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 4 Creatinine clearance.
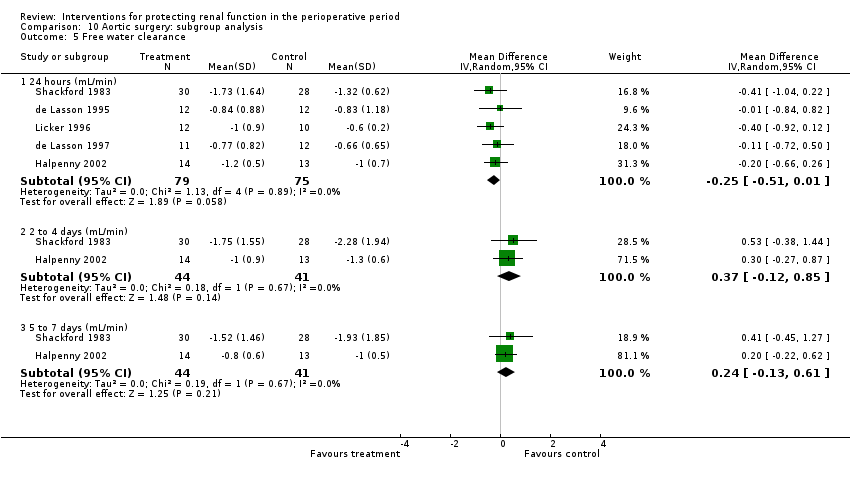
Comparison 10 Aortic surgery: subgroup analysis, Outcome 5 Free water clearance.

Comparison 10 Aortic surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
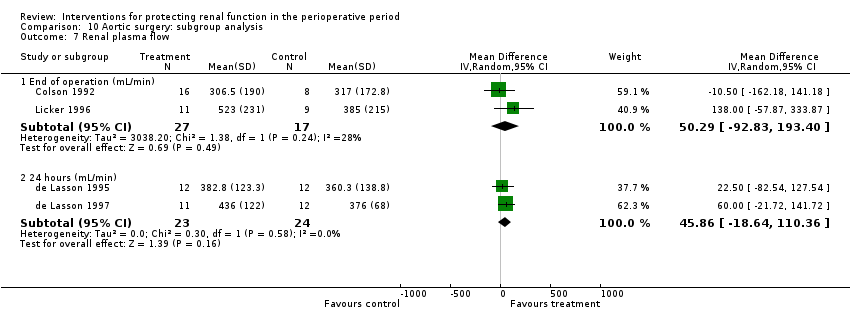
Comparison 10 Aortic surgery: subgroup analysis, Outcome 7 Renal plasma flow.
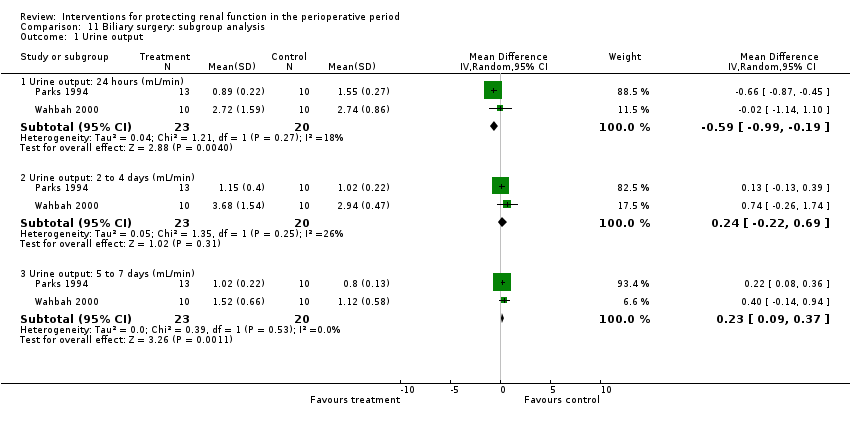
Comparison 11 Biliary surgery: subgroup analysis, Outcome 1 Urine output.
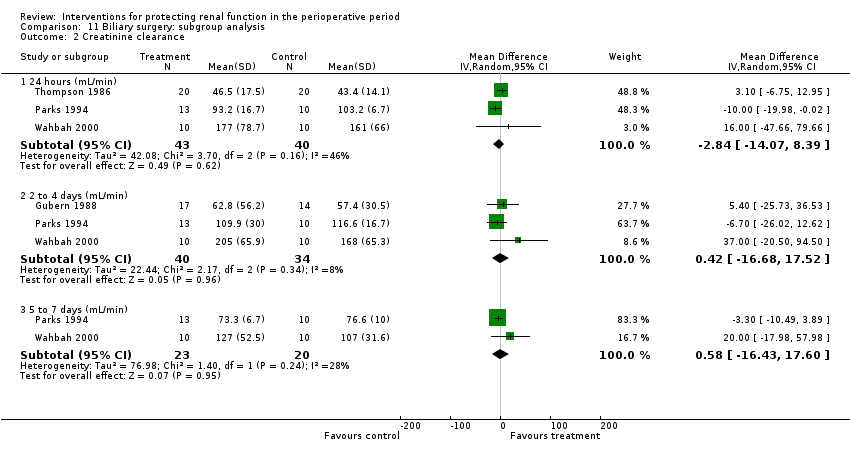
Comparison 11 Biliary surgery: subgroup analysis, Outcome 2 Creatinine clearance.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 1 Mortality.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 2 Acute renal injury.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 3 Urine output.

Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 4 Creatinine clearance.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 1 Reported mortality, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 2 Acute renal injury, requiring dialysis, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 3 Urine output at 24 hours, low risk of bias studies only.

Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 4 Creatinine clearance at 24 hours, low risk of bias studies only.
| Interventions for protecting renal function in patients with pre‐existing renal impairment who are undergoing surgery | ||||||
| Patient or population: patients with pre‐existing renal impairment Settings: perioperative period (7 days) Intervention: interventions to protect the kidneys during the perioperative period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Various interventions | |||||
| Mortality in patients with pre‐existing renal impairment As reported in the included trials Folliow‐up: 7 days | Study population | OR 0.74 (0.36 to 1.52) | 959 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality | |
| 38 per 1000 | 29 per 1000 | |||||
| Moderate | ||||||
| 20 per 1000 | 15 per 1000 (8 to 30) | |||||
| Acute renal injury in patients with pre‐existing renal impairment As reported in the included trials Follow‐up: 1 to 7 days | Study population | OR 0.40 (0.22 to 0.76) | 979 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 62 per 1000 | 28 per 1000 | |||||
| Moderate | ||||||
| 40 per 1000 | 18 per 1000 (9 to 32) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence: | ||||||
| aOnly six of the 10 studies showed low risk of bias. | ||||||
| Interventions to protect the kidneys during the perioperative period in patients undergoing surgery: low ROB studies only | ||||||
| Patient or population: patients undergoing surgery Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Interventions to protect the kidneys: low ROB cases only | |||||
| Reported mortality, low risk of bias studies only | Study population | OR 1.01 | 1604 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality | |
| 23 per 1000 | 23 per 1000 | |||||
| Moderate | ||||||
| 20 per 1000 | 20 per 1000 | |||||
| Acute renal injury, low‐risk studies only | Study population | OR 1.05 | 1550 | ⊕⊝⊝⊝ | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 23 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence: | ||||||
| aOnly six of the 10 studies showed low risk of bias. | ||||||
| Study ID | Randomization | Allocation concealment | Blinding | Withdrawals recorded | Overall quality |
| Randomly assigned by the investigational pharmacist Block randomization (blocks of 10) | Participants, researchers and clinicians blinded to treatment assignment
| Participants, researchers and clinicians (including data collecting nurse) blinded Drug packets matched in volume, colour, consistency and transparency and given mixed with fruit juice to mask taste | Not reported | Good | |
| ‘Randomly assigned’ into two groups | None described | None described; control group had no treatment | Not described | Poor | |
| ‘Patients were randomized into either diltiazem or no treatment' | Not described | None described; control group had no treatment | Not described | Poor | |
| ‘Prospectively randomized by card allocation’ | ‘Prospectively randomized by card allocation’ | Not used | Not discussed | Moderate | |
| Randomization done by pharmacy department; method of randomization uncertain Not sure about adequacy of randomization | No specific mention of allocation concealment except to say ‘double‐blinded’ Allocation concealment inadequate | No specific mention of who all were blinded; ‘double‐blinded’, placebo‐controlled trial Not sure whether blinding was adequate | One withdrawal from study reported | Moderate | |
| ‘Placebo controlled prospective study’; no randomization | None described | None described | Not described | Poor | |
| ‘Consented and were randomized’ | Not described | Not used | 3 participants (2, 1) not operated upon; 2 participants excluded from final analysis because of clinical management changes | Poor | |
| Randomization done by pharmacy trial co‐ordinator using a permitted block strategy | Allocation concealment using central randomization with drugs prepared by pharmacy | Quadruple‐blinded (participants, clinicians, data collectors and data analyst) placebo‐controlled study | Clearly accounted for (5 in intervention group and 2 in control group) | Good | |
| ‘Prospective randomized double‐blinded and placebo‐controlled study’ Computer‐generated random number tables | Not specifically described, but quite likely it was concealed allocation | Blinded manner; drug or saline supplied by the department investigational pharmacy in a blinded manner + additive for the CPB circuit prime (mannitol or saline, supplied similarly) | All allocated participants completed the trial (withdrawals before allocation) | Good | |
| ‘Randomized’; no details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ | Four withdrawals from trial reported
| Poor | |
| Computer‐generated randomization method used | Computer allocation, no further details given | Not described except by the statement, ‘investigator blinded to the study group evaluated the postoperative data’ | Not reported | Moderate | |
| Randomization from a computer list, in an envelope | Sealed envelope used ‘All personnel and patients were blinded to the assignment’ | Blinded nurse, not involved with study, prepared the drug/ placebo in identical 50 mL filled syringes ‘All personnel and patients were blinded to the assignment’ | 1 participant | Good | |
| Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method | Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment | No details on blinding except ‘double‐blind fashion’ | Not given | Poor | |
| Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method | Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment | No details on blinding except ‘double‐blind fashion’ | Not given | Poor | |
|
| Participants with renal dysfunction (CCl < 50 mL/min) ‘Randomly divided into 3 groups’; no description of randomization | No description of allocation | No description of blinding | Not given | Poor |
| ‘Randomly allocated’ into 3 groups; no description of randomization | No description of allocation | No description of blinding | Not given | Poor | |
| ‘Randomized to either treatment group’ by pulling a card from a previously prepared deck | No description of allocation concealment | No details on blinding | Not given | Poor | |
| ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation
| ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation; no description of allocation concealment
| No blinding described | Not given | Poor | |
| Randomization and drug or placebo preparation provided by drug company; method not described, but likely to be good | Not sure of any allocation concealment, but likely possible | Possible, but blinded tables not described | 1 participant had additional drugs but was not excluded | Moderate | |
| Randomly allocated into 2 groups, randomization method not described | Allocation concealment not described | Blinding not mentioned | All participants accounted for in calculations | Poor | |
| ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor | |
| ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor | |
| Randomization done by the last digit of the medical record number of participant (quasi‐randomization) | ‘Patients were prospectively allocated into 2 groups’ No details given | Not given | Not given | Poor | |
| Retrospective chart review of a randomized trial in 2003, which used computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Drugs supplied in identical looking glass vials containing drug or placebo | Exclusions described in text | Good | |
|
| ’Prospectively randomized’; no details of method of randomization | ’Prospectively randomized’; no details of method of allocation | ’Prospectively randomized’; no details of method of blinding | Fate of participants discussed | Poor |
| Random assignment of participants using Microsoft Excel‐based random number generation to create a randomization list, in blocks of 10 | Allocation concealment ensured by quadruple‐blinding (participants, clinicians, data collectors and data analysers were unaware of groups or treatment) | Quadruple‐blinding (participants, clinicians, data collectors and data analysers were blinded) | 0 participants | Good | |
| Microsoft Excel‐based random number generation, with blocks of 10; central randomization by department of pharmacy | Allocation concealment achieved by central randomization, blinding to all researchers, participants and others. Allocation revealed only after data analysis | Both fluids in separate shrink‐wrapped black plastic bags that were identical in appearance (blinded to participants, anaesthetists, surgeons, ICU personnel, nurses and others) | 1 in each group | Good | |
| ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | 1 participant excluded from the trial | Poor | |
| ‘Randomized’, but no details given | Allocated to control and intervention groups using opaque envelopes immediately before surgery; not sure whether allocation was maintained | No blinding | 1 died before operation (intervention group) | Poor | |
| Random assignment in blocks of 10 done by hospital pharmacy, no details given | Allocation done by hospital pharmacy Clinical and study personnel not aware of study allocation | Blinding quite likely, although not detailed in text | 1 participant withdrew from study intraoperatively (does not mention which group, although most likely the intervention group-1 less in that group) | Moderate | |
| Computer‐generated randomization done by statistician | Sequentially numbered, sealed envelopes
| SNP and saline in uniformly appearing 50 mL syringes, blinded to surgeons, perfusionists and nurses; investigators did not know the details | None | Good | |
| Randomization by computer | Not described in detail | Not described in detail | No detailed description | Poor | |
| Participants randomly assigned to receive 1 of 2 treatments | No details given | No details given | Early termination of study in 33 of 56 participants; ITT used | Poor | |
| Allocated into 2 groups in a double‐blinded random fashion; no details of randomization given | Allocated into 2 groups in a double‐blinded random fashion; no details of allocation given | Allocated into 2 groups in a double‐blinded random fashion; no details of blinding given | 2 participants excluded | Poor | |
| Placebo‐controlled randomized double‐blind trial; block randomization done and sealed envelopes used | Placebo‐controlled randomized double‐blind trial; block randomizations done with the use of sealed envelopes; no further details on allocation concealment provided | Placebo‐controlled randomized double‐blind trial; no other details of blinding provided | 3 participants excluded from analysis | Moderate | |
| ‘Recruited patients were allocated to one of 2 groups’; no details on randomization | ‘Recruited patients were allocated to one of 2 groups’; no details on allocation concealment | ‘Recruited patients were allocated to one of 2 groups’; no details on blinding provided | 2 participants accounted for | Poor | |
| 'Patients were allocated in a randomized double‐blind manner’; no details of randomization given | 'Patients were allocated in a randomized double‐blind manner’; no details of allocation concealment given | 'Patients were allocated in a randomized double‐blind manner’; no details of blinding given | 2 participants excluded from the trial | Poor | |
| ‘Randomized in a double‐blind fashion’; no details of randomization given | ‘Randomized in a double‐blind fashion’; no details of allocation given | ‘Randomized in a double‐blind fashion’; no details of blinding given | All participants completed the trial | Poor | |
| Used a 2:1 ratio in randomization process, participants randomly assigned into groups; no other details of randomization given | Participants randomly assigned into groups; no other details of allocation given | Participants randomly assigned into groups; no other details of blinding given | Not given | Poor | |
| ‘Patients were randomized into 2 groups’; not sure what method of randomization was used | Not sure how allocation was performed | ‘Blind infusion was performed’; not sure about blinding | None indicated | Poor | |
| Designed as a prospective double‐blind placebo‐controlled randomized trial; no other details of randomization provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of allocation concealment provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of blinding provided | All participants competed the trial | Poor | |
| ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | 2 participants excluded from analysis | Poor | |
| Randomly assigned with the use of a table of random numbers; ‘prospective double‐blind randomized trial’ | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; allocation concealed | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; blinded | 3 withdrawals before start of trial | Good | |
| ‘Prospective randomized trial’; no further details on randomization | ‘Prospective randomized trial’; no further details on allocation | ‘Prospective randomized trial’; no details on blinding | None reported | Poor | |
| ‘Patients were randomized’; no further details | No indication of allocation concealment, but for statement, ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ | Possible: ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ | None indicated in text | Poor | |
| ‘Prospective randomized study’; no further details on randomization given | ‘Prospective randomized study’; no further details on allocation | ‘Prospective randomized study’; no further details on blinding | 11 of 35 excluded | Poor | |
| ‘Patients were randomly allocated into 2 groups’; no further details on randomization | ‘Patients were randomly allocated into 2 groups’; no further details on allocation | ‘Patients were randomly allocated into 2 groups’; no further details on blinding | Not disclosed | Poor | |
| Randomization performed by aleatorized numbers prepared in closed envelopes | No details on concealment of allocation except ‘Randomization performed by aleatorized numbers prepared in closed envelopes’ | Drug or placebo given with an identical container in a double‐blind manner with the same volume of drug or saline | 4 participants excluded | Moderate | |
| Randomized, prospective, open‐label study Random number generated from a random number table | No concealment of assignment | No blinding | 4 excluded after randomization? | Poor | |
| Random assignment by the hospital pharmacy clinical trials co‐ordinator Microsoft Excel–based random number generator permuted block strategy with blocks of 10 | Allocation stratified into 2 groups based on pre‐op use of statins Allocation concealed to participants, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators | 'Double‐blind'. Atorvastatin or placebo medication prepared in capsules of identical appearance
| 8 in intervention group and 7 in control group | Good | |
| Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details; the anaesthesiologist was aware of the allocation and treatment received | No details provided | Poor | |
| ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’ | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’, but no details of allocation concealment provided | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; no details of blinding provided | 3 participants excluded | Moderate | |
| ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of randomization given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of allocation given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of blinding given | No dropouts detailed in text | Poor | |
| ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on randomization method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on allocation method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on blinding | Not described, but study probably had no dropouts | Poor | |
| Randomly allocated into 2 groups by drawing lots | ‘Randomly allocated by drawing lots’ No other details | No evidence of blinding | No mention in the text | Poor | |
| Randomly allocated into 2 groups by lottery method | 'Randomly allocated into 2 groups'; no evidence of concealment of allocation | No blinding discussed | Dropouts discussed | Poor | |
| Participants were assigned by random number to 1 of 2 groups; no details on randomization given | Participants were assigned by random number to 1 of 2 groups; no details on concealment of allocation given | Participants assigned by random number to 1 of 2 groups; no details on blinding | No dropouts | Poor | |
| Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table | Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table; no further details on allocation concealment given | All medical personnel involved in the study blinded to the contents of the infusion bottle | No dropouts recorded | Moderate | |
| Block randomization developed by research centre Randomization stratified by serum creatinine levels | Allocation via Internet using predetermined randomization | Participants, healthcare clinicians and researchers blinded | None | Good | |
| Prospectively randomly assigned | No details on allocation provided in text | No details of blinding provided in text | No dropouts recorded | Poor | |
| Participants randomly assigned; no further details on randomization given | Participants randomly assigned; no further details on allocation given | 2 different types of procedures; no blinding possible | 5 participants subsequently excluded from trial | Poor | |
| ‘Patients were randomized’; no more details | ‘Patients were randomized’; no more details | No details provided | ‘There were no withdrawals’ | Poor | |
| Random assignment done with use of computer‐generated randomization list | Computer‐generated randomization list placed in sealed envelopes and opened in numerical order by a third party, who prepared the study infusion | Third party prepared the infusion. Infusions were such that volumes were equal in the bag and of identical colour, and contents of the bag were indistinguishable; the infusion was done over 30 minutes to avoid haemodynamic effects of treatment | Yes, none lost | Good | |
| Participants randomly assigned into 1 of 2 groups, according to the last digit of their clinical history number (quasi‐randomization) | No description of concealment of allocation | No report of blinding | All participants completed | Poor | |
| ‘Patients were randomly allocated into 4 equal groups’; no further details on randomization | Patients were randomly allocated into 4 equal groups’; no further details on allocation | No description of blinding | None described | Poor | |
| ‘Patients were randomly assigned’; no further details on randomization method used | ‘Patients were randomly assigned’; no further details on allocation method used | No description of blinding | None described | Poor | |
| ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | One death described | Poor | |
| Participants were ‘randomized’ It appears that the anaesthesiologist ‘randomly drew an envelope with the assigned treatment’ | Allocation concealment was possible only for participants and the statistician | No; control received no treatment Participants and the statistician were blinded | Not described | Poor | |
| ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | 8 participants excluded because of death or major complications | Poor | |
| ‘Patients were prospectively randomized’; no details on method of randomization used | ‘Patients were prospectively randomized’; no details on method of allocation used | No description of blinding | States no deaths; no description of dropouts | Poor | |
| ‘Patients randomized into 4 groups’; no further details on randomization given | ‘Patients randomized into 4 groups’; no description of allocation used | No description of blinding | No mortality described, but no suggestion of dropouts | Poor | |
| ‘Randomly assigned’; no further details of randomization given | ‘Randomly assigned’; no further details of allocation given | No blinding described | No dropouts described | Poor |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 11 | 583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.48, 4.73] |
| 2 Acute renal injury Show forest plot | 10 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.44, 4.23] |
| 3 Urine output Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 13 | 670 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.19, 0.54] |
| 3.2 2 to 4 days (mL/min) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.04, 0.97] |
| 3.3 5 to 7 days (mL/min) | 4 | 103 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.51] |
| 4 Creatinine clearance Show forest plot | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 14 | 616 | Mean Difference (IV, Random, 95% CI) | 7.17 [‐5.53, 19.86] |
| 4.2 2 to 4 days (mL/min) | 9 | 459 | Mean Difference (IV, Random, 95% CI) | 7.31 [‐6.19, 20.82] |
| 4.3 5 to 7 days (mL/min) | 5 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐13.63, 6.98] |
| 5 Free water clearance Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 6 | 166 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow (24 hours) Show forest plot | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 75.36 [‐63.27, 213.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [0.80, 7.74] |
| 2 Acute renal injury Show forest plot | 5 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.39 [0.68, 8.47] |
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.33] |
| 3.2 2 to 4 days (mlL/min) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.14, 0.45] |
| 4 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 3 | 123 | Mean Difference (IV, Random, 95% CI) | ‐18.02 [‐41.78, 5.75] |
| 4.2 2 to 4 days (mL/min) | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐14.76, 19.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute renal injury Show forest plot | 6 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.01, 1.17] |
| 3 Urine output Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Urine output: 24 hours (mL/min) | 4 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.02, 0.45] |
| 4 Creatinine clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 5 | 251 | Mean Difference (IV, Random, 95% CI) | 4.74 [‐3.30, 12.77] |
| 4.2 2 to 4 days (mL/min) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 13.92 [‐24.62, 52.46] |
| 5 Free water clearance Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 3 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 2 Acute renal injury Show forest plot | 3 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Renal plasma flow Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 RPF: end of operation (mL/min) | 3 | 62 | Mean Difference (IV, Random, 95% CI) | 46.37 [‐68.61, 161.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 2 Acute renal injury Show forest plot | 4 | 865 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.08, 0.64] |
| 3 Urine output at 24 hours Show forest plot | 3 | 584 | Mean Difference (IV, Random, 95% CI) | 0.42 [0.18, 0.67] |
| 4 Creatinine clearance, 24 hours Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 35.23 [‐0.48, 70.94] |
| 5 Creatinine clearance, 2 to 3 days Show forest plot | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 27.30 [4.36, 50.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 6 | 641 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.42, 2.42] |
| 2 Acute renal injury Show forest plot | 5 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.32, 2.62] |
| 3 Urine output, 24 hours Show forest plot | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.24, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.63] |
| 2 Acute renal injury Show forest plot | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Urine output: 24 hours Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.47, 0.21] |
| 4 Urine output: 2 to 3 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.18] |
| 5 Urine output: 5 to 7 days Show forest plot | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 4 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.16, 3.42] |
| 2 Acute renal injury Show forest plot | 3 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.96] |
| 3 Creatinine clearance Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐29.57, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 26 | 2390 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.56, 1.64] |
| 2 Acute renal injury Show forest plot | 31 | 2504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 3 Urine output Show forest plot | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 17 | 1475 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.17, 0.36] |
| 3.2 2 to 3 days (mL/min) | 9 | 1058 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.13, 0.54] |
| 4 Creatinine clearance Show forest plot | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 24 | 2136 | Mean Difference (IV, Random, 95% CI) | 9.38 [‐5.99, 24.74] |
| 4.2 2 to 3 days (mL/min) | 17 | 1844 | Mean Difference (IV, Random, 95% CI) | 14.21 [3.58, 24.85] |
| 4.3 5 to 7 days (mL/min) | 7 | 949 | Mean Difference (IV, Random, 95% CI) | 14.99 [0.84, 29.13] |
| 5 Free water clearance Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 7 | 700 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.19] |
| 5.2 2 to 3 days (mL/min) | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.30, ‐0.28] |
| 6 Fractional excretion of sodium Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 8 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 8 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.20, 2.89] |
| 2 Acute renal injury Show forest plot | 8 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.11, 3.70] |
| 3 Urine output Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 7 | 227 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 3.2 2 to 3 days (mL/min) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.58] |
| 3.3 5 to 7 days (mL/min) | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 4 Creatinine clearance Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 9 | 323 | Mean Difference (IV, Random, 95% CI) | 7.99 [‐0.77, 16.74] |
| 4.2 2 to 3 days (mL/min) | 5 | 195 | Mean Difference (IV, Random, 95% CI) | 11.62 [‐6.13, 29.37] |
| 4.3 5 to 7 days (mL/min) | 4 | 116 | Mean Difference (IV, Random, 95% CI) | ‐12.85 [‐26.41, 0.72] |
| 5 Free water clearance Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 5 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.51, 0.01] |
| 5.2 2 to 4 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.12, 0.85] |
| 5.3 5 to 7 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.13, 0.61] |
| 6 Fractional excretion of sodium Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 End of operation (mL/min) | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 50.29 [‐92.83, 193.40] |
| 7.2 24 hours (mL/min) | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 45.86 [‐18.64, 110.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Urine output Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Urine output: 24 hours (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.19] |
| 1.2 Urine output: 2 to 4 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.22, 0.69] |
| 1.3 Urine output: 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.09, 0.37] |
| 2 Creatinine clearance Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 24 hours (mL/min) | 3 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐14.07, 8.39] |
| 2.2 2 to 4 days (mL/min) | 3 | 74 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐16.68, 17.52] |
| 2.3 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐16.43, 17.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | 959 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.36, 1.52] |
| 2 Acute renal injury Show forest plot | 11 | 979 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.76] |
| 3 Urine output Show forest plot | 4 | 707 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.16, 0.85] |
| 3.1 Urine output, 24 hours | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.12, 0.81] |
| 3.2 Urine output, 2 to 3 days | 2 | 291 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.78, 1.65] |
| 4 Creatinine clearance Show forest plot | 4 | 646 | Mean Difference (IV, Random, 95% CI) | 10.65 [0.04, 21.27] |
| 4.1 Creatinine clearance, 24 hours | 4 | 347 | Mean Difference (IV, Random, 95% CI) | 7.78 [‐10.39, 25.94] |
| 4.2 Creatinine clearance, 2 to 3 days | 3 | 299 | Mean Difference (IV, Random, 95% CI) | 14.16 [‐6.20, 34.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reported mortality, low risk of bias studies only Show forest plot | 19 | 1604 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| 2 Acute renal injury, requiring dialysis, low risk of bias studies only Show forest plot | 16 | 1550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.55, 2.03] |
| 3 Urine output at 24 hours, low risk of bias studies only Show forest plot | 11 | 798 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
| 4 Creatinine clearance at 24 hours, low risk of bias studies only Show forest plot | 9 | 817 | Mean Difference (IV, Random, 95% CI) | 6.59 [‐3.53, 16.72] |

