Fast‐track cardiac care for adult cardiac surgical patients
Abstract
Background
Fast‐track cardiac care is a complex intervention involving several components of care during cardiac anaesthesia and in the postoperative period, with the ultimate aim of early extubation after surgery, to reduce length of stay in the intensive care unit and in the hospital. Safe and effective fast‐track cardiac care may reduce hospital costs. This is an update of a Cochrane review first published in 2003, updated in 2012 and updated now in 2016.
Objectives
To determine the safety and effectiveness of fast‐track cardiac care compared with conventional (not fast‐track) care in adult patients undergoing cardiac surgery. Fast‐track cardiac care intervention includes administration of low‐dose opioid‐based general anaesthesia or use of a time‐directed extubation protocol, or both.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5), MEDLINE (January 2012 to May 2015), Embase (January 2012 to May 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; January 2012 to May 2015) and the Institute for Scientific Information (ISI) Web of Science (January 2012 to May 2015), along with reference lists of articles, to identify additional trials. We applied no language restrictions.
Selection criteria
We included all randomized controlled trials of adult cardiac surgical patients (coronary artery bypass grafts, aortic valve replacement, mitral valve replacement) that compared fast‐track cardiac care and conventional (not fast‐track) care groups. We focused on the following fast‐track interventions, which were designed for early extubation after surgery: administration of low‐dose opioid‐based general anaesthesia during cardiac surgery and use of a time‐directed extubation protocol after surgery. The primary outcome was risk of mortality. Secondary outcomes included postoperative complications, reintubation within 24 hours of surgery, time to extubation, length of stay in the intensive care unit and in the hospital, quality of life after surgery and hospital costs.
Data collection and analysis
Two review authors independently assessed trial quality and extracted study data. We contacted study authors for additional information. We calculated a Peto odds ratio (OR) for risk of mortality and used a random‐effects model to report risk ratio (RR), mean difference (MD) and 95% confidence intervals (95% CIs) for all secondary outcomes.
Main results
We included 28 trials (4438 participants) in the updated review. We considered most participants to be at low to moderate risk of death after surgery. We assessed two studies as having low risk of bias and 11 studies high risk of bias. Investigators reported no differences in risk of mortality within the first year after surgery between low‐dose versus high‐dose opioid‐based general anaesthesia groups (OR 0.53, 95% CI 0.25 to 1.12; eight trials, 1994 participants, low level of evidence) and between a time‐directed extubation protocol versus usual care (OR 0.80, 95% CI 0.45 to 1.45; 10 trials, 1802 participants, low level of evidence).
Researchers noted no significant differences between low‐dose and high‐dose opioid‐based anaesthesia groups in the following postoperative complications: myocardial infarction (RR 0.98, 95% CI 0.48 to 1.99; eight trials, 1683 participants, low level of evidence), stroke (RR 1.17, 95% CI 0.36 to 3.78; five trials, 562 participants, low level of evidence) and tracheal reintubation (RR 1.77, 95% CI 0.38 to 8.27; five trials, 594 participants, low level of evidence).
Comparisons with usual care revealed no significant differences in the risk of postoperative complications associated with a time‐directed extubation protocol: myocardial infarction (RR 0.59, 95% CI 0.27 to 1.31; eight trials, 1378 participants, low level of evidence), stroke (RR 0.85, 95% CI 0.33 to 2.16; 11 trials, 1646 participants, low level of evidence) and tracheal reintubation (RR 1.34, 95% CI 0.74 to 2.41; 12 trials, 1261 participants, low level of evidence).
Although levels of heterogeneity were high, low‐dose opioid anaesthesia was associated with reduced time to extubation (reduction of 4.3 to 10.5 hours, 14 trials, 2486 participants, low level of evidence) and length of stay in the intensive care unit (reduction of 0.4 to 7.0 hours, 12 trials, 1394 participants, low level of evidence). Use of a time‐directed extubation protocol was associated with reduced time to extubation (reduction of 3.7 to 8.8 hours, 16 trials, 2024 participants, low level of evidence) and length of stay in the intensive care unit (reduction of 3.9 to 10.5 hours, 13 trials, 1888 participants, low level of evidence). However, these two fast‐track care interventions were not associated with reduced total length of stay in the hospital (low level of evidence).
Authors' conclusions
Low‐dose opioid‐based general anaesthesia and time‐directed extubation protocols for fast‐track interventions have risks of mortality and major postoperative complications similar to those of conventional (not fast‐track) care, and therefore appear to be safe for use in patients considered to be at low to moderate risk. These fast‐track interventions reduced time to extubation and shortened length of stay in the intensive care unit but did not reduce length of stay in the hospital.
PICO
Plain language summary
Fast‐track interventions of low‐dose opioid‐based general anaesthesia and early tracheal extubation in adults undergoing cardiac surgery
Review question
Fast‐track cardiac care involves early removal, within eight hours of heart surgery, of the tube that provides mechanical breathing support (called early tracheal extubation) to enable cardiac surgery. This review examined evidence on the effectiveness and safety of fast‐track care compared with conventional (not fast‐track) care. We have updated the published evidence that we identified in 2012. It is now current to March 2016.
Background
In the past, adults were given high‐dose opioid‐based anaesthesia for cardiac surgery and were provided with mechanical breathing support overnight in an intensive care unit after surgery. Now, many surgical units remove the tube that provides mechanical breathing support when the patient is on the operating table or within hours after cardiac surgery. They use time‐directed protocols for removing breathing support. Some patients recover in an intensive care unit (ICU) or in a dedicated unit outside the ICU. It is important to improve hospital efficiency by using safe fast‐track interventions.
Study characteristics
We found 28 relevant randomized controlled studies, conducted between 1994 and 2015. Most of the 4438 adults who participated in these studies were undergoing first‐time elective coronary artery graft bypass or valve replacement surgery, or both. They were at low to moderate risk of death after surgery. Eighteen studies examined the use of low‐dose opioid‐based general anaesthesia. Sixteen studies assessed how effective the protocols were in guiding staff to remove the tube that provided breathing support within eight hours after surgery.
Key findings and quality of evidence
We found no differences in risk of death in the first year after surgery (18 trials, 3796 participants) nor in complications after surgery such as the need to replace the tracheal tube after surgery (17 trials, 1855 participants) and occurrence of myocardial infarction (16 trials, 3061 participants) or stroke (16 trials, 2208 participants), when we examined both types of interventions. Occurrences of acute renal failure, major bleeding, sepsis and wound infection also were not different. We rated the quality of evidence as low for both mortality and postoperative complications.
Tracheal tubes were removed from adults in the fast‐track care group up to a half day earlier than for those in the conventional care group. The fast‐track group spent less time in the intensive care unit, but length of time spent in the hospital was similar between groups. The quality of evidence was low because of study limitations and unexplained variation in study findings. Large trials were few, and only one trial was designed to study postoperative effects of myocardial infarction, stroke or death.
Our results did not apply to ‘high‐risk' patients who had multiple concurrent health problems or to settings in which a short‐acting opioid (remifentanil) was used for general anaesthesia.
Conclusion
Fast‐track cardiac care is safe in patients considered to be at low to moderate risk of death after surgery.
Authors' conclusions
Summary of findings
| Low‐dose opioid‐based general anaesthesia compared with high‐dose opioid‐based general anaesthesia for adults undergoing cardiac surgery | ||||||
| Patient or population: adult cardiac surgical patients Intervention: low‐dose opioid‐based general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with high‐dose opioid‐based general anaesthesia | Risk with low‐dose opioid‐based general anaesthesia | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.53 | 1994 | ⊕⊕⊝⊝ | No death recorded in 3 trials | |
| 10 per 1000 | 5 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 16 per 1000 | |||||
| High | ||||||
| 110 per 1000 | 61 per 1000 | |||||
| Postoperative myocardial infarction | Study population | RR 0.98 | 1683 | ⊕⊕⊝⊝ | No postoperative myocardial infarction recorded in 2 trials | |
| 30 per 1000 | 30 per 1000 | |||||
| Postoperative stroke | Study population | RR 1.17 | 562 | ⊕⊕⊝⊝ | No stroke recorded in 1 trial | |
| 18 per 1000 | 21 per 1000 | |||||
| Postoperative tracheal reintubation | Study population | RR 1.77 | 594 | ⊕⊕⊝⊝ | No reintubation recorded in 3 trials | |
| 7 per 1000 | 12 per 1000 | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 5.2 to 35.1 | Mean time to extubation (hours) in the intervention group was 7.4 lower (10.51 lower to 4.29 lower). | ‐ | 2486 | ⊕⊕⊝⊝ | |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 2.6 to 112.8. | Mean length of intensive care unit stay (hours) in the intervention group was 3.7 lower (6.98 lower to 0.41 lower). | ‐ | 1394 | ⊕⊕⊝⊝ | |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 27.0. | Mean length of hospital stay (days) in the intervention group was 0.3 lower (1.04 lower to 0.43 higher). | ‐ | 913 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOf the 8 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). bOptimal information size not met (downgrade 1 point owing to imprecision). cOf the 5 trials, 1 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 5 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). eOf the 14 trials, 4 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). fUnexplained reasons for high heterogeneity. gOf the 12 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). hOf the 8 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). | ||||||
| Time‐directed extubation protocol compared with usual care for adults undergoing cardiac surgery | ||||||
| Patient or population: adult cardiac surgical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with time‐directed extubation protocol | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.80 | 1802 | ⊕⊕⊝⊝ | No deaths recorded in 4 trials. Low to moderate heterogeneity (I2 = 37%) may be explained by the inclusion of a trial (Reyes 1997) that had the highest rate of mortality of all trials considered. When excluded, the OR changed to 0.31 (95% CI 0.11 to 0.90, P = 0.03, I2 = 0%). | |
| 10 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 24 per 1000 | |||||
| High | ||||||
| 110 per 1000 | 90 per 1000 | |||||
| Postoperative myocardial infarction | Study population | RR 0.59 | 1378 | ⊕⊕⊝⊝ | No postoperative myocardial infarction was recorded in 1 trial. | |
| 61 per 1000 | 36 per 1000 | |||||
| Postoperative stroke | Study population | RR 0.85 | 1646 | ⊕⊕⊝⊝ | No stroke was recorded in 2 trials. | |
| 12 per 1000 | 10 per 1000 | |||||
| Postoperative reintubation | Study population | RR 1.34 | 1261 | ⊕⊕⊝⊝ | No reintubation was recorded in 3 trials. | |
| 28 per 1000 | 38 per 1000 | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 3.4 to 18.9. | Mean time to extubation (hours) in the intervention group was 6.25 lower (8.84 lower to 3.67 lower). | ‐ | 2024 | ⊕⊕⊝⊝ | No variation in time to extubation in the early extubation group in 1 trial |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 17.9 to 95.0. | Mean length of intensive care unit stay (hours) in the intervention group was 7.16 lower (10.45 lower to 3.88 lower). | ‐ | 1888 | ⊕⊕⊝⊝ | No variation in length of ICU stay in early extubation group in 1 trial |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 13.0. | Mean length of hospital stay (days) in the intervention group was 0.44 lower (1.04 lower to 0.16 higher). | ‐ | 1334 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOf the 10 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). bOptimal information size not met (downgrade 1 point owing to imprecision). cOf the 8 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 11 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 12 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). fUnexplained reasons for high heterogeneity. gOf the 16 trials, 6 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). hOf the 13 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). iOf the 8 trials, 2 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). | ||||||
Background
Description of the condition
In the past, cardiac surgical patients were ventilated overnight following surgery and were given a regimen of high‐dose opioid‐based anaesthesia and postoperative analgesia (Hawkes 2003). However, in the early 1990s, fast‐track cardiac anaesthesia (FTCA) was introduced to address the increasing demand for cardiac surgery with limited medical facilities and available resources. Although the volume of coronary artery bypass grafting (CABG) surgery performed in the United States peaked in 1998, 219,000 patients underwent a total of 397,000 CABG procedures in 2010 (American Heart Association 2016). In many units, patients are now extubated (i.e. the tube that allows mechanical breathing support is removed) on the operating table or within hours after cardiac surgery via time‐directed formalized weaning protocols, and they recover in a dedicated unit outside the intensive care unit (ICU) setting (Ender 2008; Nougarede 2004; Probst 2014; Salah 2015) as part of a fast‐track programme.
Description of the intervention
The label 'fast‐track' originally referred to the use of low‐dose opioid‐based general anaesthesia in cardiac surgical patients to carry connotations of excitement and rapid advancement (Silbert 2009). Although no standard definition of FTCA is known, it is generally accepted that it involves the use of a combination of short‐acting hypnotic drugs with reduced doses of opioids, or the use of short‐acting opioids such as remifentanil (Myles 2003; van Mastrigt 2006b), with the ultimate aim of extubation within eight hours after cardiac surgery. Several authors arbitrarily defined the criteria for early extubation within eight hours (Berry 1998; Michalopoulos 1998), but no physiological or pathological reasons have been proposed to explain why this time point was adopted.
Normothermic temperature management and use of an extubation protocol with the intention to extubate a patient within a specified time period are considered fast‐track strategies (van Mastrigt 2006b).
In this systematic review, fast‐track cardiac care is defined as a complex intervention involving several components of care during cardiac anaesthesia and in the postoperative period, with the ultimate aim of early extubation after surgery to reduce length of stay in the ICU and in the hospital. These components of fast‐track cardiac care include administration of low‐dose opioid‐based general anaesthesia, use of a time‐directed extubation protocol, or both. Although safe and effective fast‐track cardiac care may reduce hospital costs, the incidence of fast‐track failure after cardiac surgery ranges from 11% (Lee 2013) to 16% (Constantinides 2006).
How the intervention might work
Early tracheal extubation after surgery is a key component of fast‐track cardiac management. Early extubation reduces the patient's length of stay in the ICU and in the hospital, resulting in reduced hospital costs and improved hospital efficiency (Hawkes 2003). Although high intraoperative opioid doses are used to suppress hormonal and metabolic stress responses to surgery, the opioids may have cumulative effects and can depress respiration and prolong ventilation times (Maddali 2006), whereas combining a short‐acting hypnotic with a low‐dose opioid‐based anaesthesia can avoid these problems without compromising patient recovery. A time‐directed extubation protocol improves efficiency of practice by following an expert consensus guideline to reduce variations in (1) decisions about when the patient is ready for weaning (the process leading to discontinuation of mechanical ventilation support), (2) the process of reducing ventilatory support and (3) criteria for deciding whether patients are ready to be extubated (Blackwood 2014).
Why it is important to do this review
In an earlier version of this Cochrane review (Hawkes 2003), which included six trials, review authors found no evidence of a difference between early and conventionally late extubated patients for the following outcomes: risk of mortality in ICU (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.42 to 1.52); risk of mortality at 30 days after surgery (RR 1.20, 95% CI 0.63 to 2.27); risk of myocardial ischaemia (RR 0.96, 95% CI 0.71 to 1.30); and risk of reintubation within 24 hours of surgery (RR 5.93, 95% CI 0.72 to 49.14). Times spent in ICU and in hospital were significantly shorter for patients who were extubated early (‐7.02 hours, 95% CI ‐7.42 to ‐6.61; ‐1.08 days, 95% CI ‐1.35 to ‐0.82, respectively) (Hawkes 2003).
In another systematic review of the safety and effectiveness of FTCA in 10 trials (Myles 2003), the FTCA group spent less time in ICU (‐5.4 hours, 95% CI ‐10.5 to ‐0.3) than the conventional group given opioid‐based anaesthesia. However, investigators reported no significant reduction in hospital stay (‐0.61 days, 95% CI ‐1.51 to 0.28). Risk of mortality was similar between FTCA (1.2%) and conventional care (2.7%) groups (RR 0.51, 95% CI 0.23 to 1.13) (Myles 2003). A meta‐regression of randomized clinical trials of fast‐track treatment in cardiac patients showed that the introduction of an early extubation protocol was an independent predictor of decreased ICU stay and hospital stay (van Mastrigt 2006b).
In the last Cochrane review update of 25 trials (n = 4118) (Zhu 2012), we found no differences in risk of mortality within the first year after surgery between low‐dose and high‐dose opioid‐based general anaesthesia groups (RR 0.58, 95% CI 0.28 to 1.18), and between early extubation protocol versus usual care groups (RR 0.84, 95% CI 0.40 to 1.75). We noted no significant differences between low‐dose and high‐dose opioid‐based anaesthesia groups for the following postoperative complications: myocardial infarction (RR 0.98, 95% CI 0.48 to 1.99), reintubation (RR 1.77, 95% CI 0.38 to 8.27), acute renal failure (RR 1.19, 95% CI 0.33 to 4.33), major bleeding (RR 0.48, 95% CI 0.16 to 1.44) and stroke (RR 1.17, 95% CI 0.36 to 3.78). Comparison with usual care revealed no significant differences in risk of the following postoperative complications associated with a time‐directed extubation protocol: myocardial infarction (RR 0.94, 95% CI 0.55 to 1.60), reintubation (RR 1.91, 95% CI 0.90 to 4.07), acute renal failure (RR 0.77, 95% CI 0.19 to 3.10), major bleeding (RR 0.80, 95% CI 0.45 to 1.44), stroke (RR 0.87, 95% CI 0.31 to 2.46), major sepsis (RR 1.25, 95% CI 0.08 to 19.75) and wound infection (RR 0.67, 95% CI 0.25 to 1.83). Although levels of heterogeneity were high, both low‐dose opioid anaesthesia and use of time‐directed extubation protocols were associated with reductions in time to extubation (3.0 to 10.5 hours) and in length of stay in the intensive care unit (0.4 to 8.7 hours). However, these fast‐track care interventions were not associated with reductions in total length of stay in the hospital. One high‐quality cost‐effectiveness analysis conducted in a randomized controlled trial showed that early extubation was likely to be cost‐effective.
The rationale for conducting this Cochrane review update was to include findings from the most recent trials on risks and benefits of interventions commonly used as part of a fast‐track cardiac programme.
Objectives
To determine the safety and effectiveness of fast‐track cardiac care compared with conventional (not fast‐track) care in adult patients undergoing cardiac surgery. Fast‐track cardiac care intervention includes administration of low‐dose opioid‐based general anaesthesia or use of a time‐directed extubation protocol, or both.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing fast‐track care (interventions with the aim of early extubation within eight hours after surgery) with conventional (not fast‐track) care when extubation occurred after eight hours following cardiac surgery. We focused on RCTs that compared the use of low‐dose opioid‐based general anaesthesia versus high‐dose opioid‐based general anaesthesia, and early extubation based on time‐directed protocols versus usual care for extubation.
Types of participants
We included adults undergoing cardiac surgery (CABG, aortic valve procedures, mitral valve procedures or a combination of these) with or without cardiopulmonary bypass. We excluded studies that involved children (age limits defined by each study) or participants undergoing surgery for aortic aneurysm repair.
Types of interventions
As in the previous reviews (Hawkes 2003; Zhu 2012), we chose eight hours as the defined time limit for early tracheal extubation because this definition was frequently presented in the literature published in the 1990s. As rapid advances and changes in cardiac anaesthesia and surgical techniques have occurred since the 1990s, we did not exclude studies that compared interventions designed for early extubation (within four hours after surgery).
For the purpose of this review, FTCA involves the use of low‐dose opiate (fentanyl ≤ 20 μg/kg or equivalent) (Myles 2003) or short‐acting opioid supplemented with propofol or etomidate, or volatile anaesthesia with or without a protocol for early extubation within eight hours. Conventional cardiac anaesthesia was defined by the use of high‐dose opioids (fentanyl ≥ 20 μg/kg or equivalent) with propofol or etomidate, or volatile anaesthesia with or without a protocol for extubation within a specified time after surgery. We excluded trials with remifentanil as, unlike other opioids, it has a short half‐life and does not accumulate after prolonged administration (Howie 2003); its use in cardiac surgery has been reviewed elsewhere (Greco 2012). We excluded studies that examined major regional blockade (epidural or intrathecal), as the effectiveness of thoracic epidural in cardiac surgery has been reviewed in another Cochrane systematic review (Svircevic 2013). We also excluded studies that compared normothermia and hypothermia during cardiopulmonary bypass in adult cardiac surgery, as the risks and benefits have been reviewed elsewhere (Ho 2011).
Types of outcome measures
If a study did not report any of the following prespecified outcome data, we excluded that study from the systematic review.
Primary outcomes
Mortality
1. Risk of mortality in the ICU.
2. Risk of hospital mortality.
3. Risk of mortality at 30 days.
4. Risk of mortality at one year.
5. Risk of mortality at any time point.
Secondary outcomes
Postoperative complications
For the following postoperative complications, we used individual study definitions, which may vary between trials.
1. Risk of postoperative myocardial infarction.
2. Risk of stroke.
3. Risk of acute renal failure.
4. Risk of major bleeding.
5. Risk of major sepsis.
6. Risk of wound infection.
7. Risk of reintubation.
Patient‐centred outcomes
1. Quality of life at one month.
2. Quality of life at one year.
Service outcomes
1. Time to extubation.
2. ICU length of stay.
3. Hospital length of stay.
4. Inpatient costs (USD).
Costs were estimated at 2015 USD values. The reported currency was converted to 2015 USD with the ‘CCEMG – EPPI‐Centre Cost Converter’ (v.1.5) (a free web‐based tool available at http://eppi.ioe.ac.uk/costconversion/default.aspx).
We performed a separate meta‐analysis for each of the primary and secondary outcomes listed above for the following interventions used in fast‐track cardiac care.
1. Low‐dose opioid‐based general anaesthesia versus high‐dose opioid‐based general anaesthesia.
2. Early extubation via a time‐directed protocol versus usual extubation care.
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant trials.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5) (The Cochrane Library); see Appendix 1.
-
MEDLINE (Ovid SP) (January 2012 to 28 May 2015); see Appendix 2.
-
Embase (Ovid SP) (January 2012 to 28 May 2015); see Appendix 3.
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost) (January 2012 to 28 May 2015); see Appendix 4.
-
Institute for Scientific Information (ISI) Web of Science (January 2012 to 28 May 2015); see Appendix 5.
We used medical subject heading (MeSH) terms for MEDLINE and other headings appropriate to other databases, such as ‘cardiac surgery', ‘extubation' and ‘fast‐track'. We combined our subject search filter with the Cochrane highly sensitive search strategy for identifying RCTs, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), when searching in MEDLINE. We adopted this RCT filter to searches in other databases and applied no language restrictions.
Searching other resources
We searched the reference lists of retrieved articles, trials and reviews (Blackwood 2014; Meades 2001; Myles 2003; van Mastrigt 2006b). We also searched the World Health Organization Clinical Trials Registry and ClinicalTrial.gov on 29 December 2015.
Data collection and analysis
Selection of studies
We selected trials included in the systematic review on the basis of search strategy. Two review authors (WTW and AL) independently scanned the titles and abstracts of reports identified by electronic searches to produce a list of possibly relevant studies. We used the Rayyan application to manage the screening process (Elmagarmid 2014). We obtained full‐text versions, and two review authors (WTW and AL) used a standardized data collection form to independently assess them for inclusion. We resolved disagreements between review authors by meetings for discussion.
Data extraction and management
Two review authors (VKWL and AL) independently extracted data using the Cochrane Anaesthesia, Critical and Emergency Care Review Group data extraction form adapted for this review. We collected data on the types and doses of drugs used in anaesthesia, as well as on patient population and type of cardiac surgery.
Assessment of risk of bias in included studies
Two review authors (VKWL and AL) independently assessed the quality of studies by applying the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We graded risk of bias for each study in the domains of sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and comparison of baseline characteristics entered into a ‘Risk of bias' table (Higgins 2011). We graded each domain as ‘yes' (low risk of bias), ‘no' (high risk of bias) or ‘unclear' (uncertain risk of bias) according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We defined a high‐quality trial as one in which all domains were considered to be at low risk of bias, and a low‐quality trial as a trial in which one or more of these domains were rated as having high risk of bias.
We used the GRADE approach to rate the overall quality of evidence for seven outcomes (death at any time after surgery, postoperative myocardial infarction, postoperative stroke, postoperative tracheal reintubation, time to extubation, length of intensive care unit stay, length of hospital stay) (Summary of findings table 1; Summary of findings table 2) as high, moderate, low or very low (Guyatt 2011). We downgraded the quality of evidence from high if we noted study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates and potential publication bias (Guyatt 2011). We upgraded the quality of evidence when we observed a large effect (RR < 0.5 or RR > 2) in the absence of plausible confounders (Higgins 2011).
Measures of treatment effect
Summary estimates reported included risk ratio (RR), mean difference (MD) and associated 95% confidence intervals (95% CIs). For rare outcomes, such as death, we estimated Peto's odd ratio (OR) to combine data as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If more than one group met intervention or control group criteria, we combined the data to create a single pair‐wise comparison. For dichotomous outcomes, we summed both sample sizes and numbers of participants with events across groups (Higgins 2011). For continuous outcomes, we combined means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In Slogoff and Keat's study (Slogoff 1989), we combined enflurane, halothane and isoflurane groups into a single early extubation group.
Unit of analysis issues
None.
Dealing with missing data
We contacted the first authors of included trials to obtain missing data that were necessary for meta‐analysis. We calculated missing standard deviations from standard errors, confidence intervals and interquartile ranges, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We calculated standard deviations for service outcomes in studies by van Mastrigt and colleagues using published confidence intervals (van Mastrigt 2006a, 2010).
Assessment of heterogeneity
As only randomized controlled trials were included in this systematic review, we would not expect methodological heterogeneity to be problematic. We observed clinical heterogeneity in the interventions compared, but if we found a comparable body of trials amenable to meta‐analysis, we calculated a summary estimate and displayed pooled results graphically. We assessed statistical heterogeneity between trials by using the I2 statistic. We defined low, moderate and high levels of heterogeneity as I2 values of 25%, 50% and 75%, respectively (Higgins 2003). When we found evidence of large heterogeneity, we attempted to explain the reason for it and rechecked the data for possible data entry errors.
Assessment of reporting biases
Using STATA statistical software (Stata Corporation, College Station, Texas, USA, version 14), we constructed a contour‐enhanced funnel plot to correctly identify publication bias separate from other causes of funnel plot asymmetry when we included more than 10 trials (Peters 2008). This was performed for mortality at any time with opioid‐based cardiac anaesthesia and with time‐directed extubation protocols. We used the Egger's test to test for funnel plot asymmetry with time to extubation outcomes (Egger 1997).
Data synthesis
We used a DerSimonian and Laird random‐effects model and Review Manager 5.3 software to combine data for continuous and dichotomous outcomes. We reported risk ratio (RR), mean difference (MD), 95% confidence interval (CI) and P value. However, we calculated Peto odds ratio (OR) and 95% CI were calculated in pooling mortality data, as many studies reported no deaths in either or both trial arms, and we expected the event rate to be low.
Subgroup analysis and investigation of heterogeneity
We undertook exploratory a priori subgroup analyses for trials examining the use of time‐directed protocols for early extubation. In an attempt to assess the safety associated with fast‐track recovery units, we compared subgroups for early extubation in settings inside and outside ICU and risk of reintubation. To test whether subgroups were different from one another, we tested the interaction using Review Manager 5.3 software (Deeks 2010).
Sensitivity analysis
We conducted a sensitivity analysis for trials with low risk of bias to estimate the robustness of results for mortality at any time within one year in studies examining FTCA and time‐directed extubation protocols.
Summary of findings
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2011). The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the outcome being assessed (Guyatt 2011). Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of the effect estimates and risk of publication bias (Guyatt 2011). Using the GRADE software, we constructed 'Summary of findings' tables for comparison of the following specific outcomes: mortality, postoperative myocardial infarction, stroke, tracheal reintubation, time to extubation, length of ICU stay and length of hospital stay.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search identified six studies for full‐text review. For this systematic review, we brought forward 62 trials (25 included and 37 excluded) from our previous Cochrane review (Zhu 2012). The process is shown in Figure 1. We found no ongoing studies.
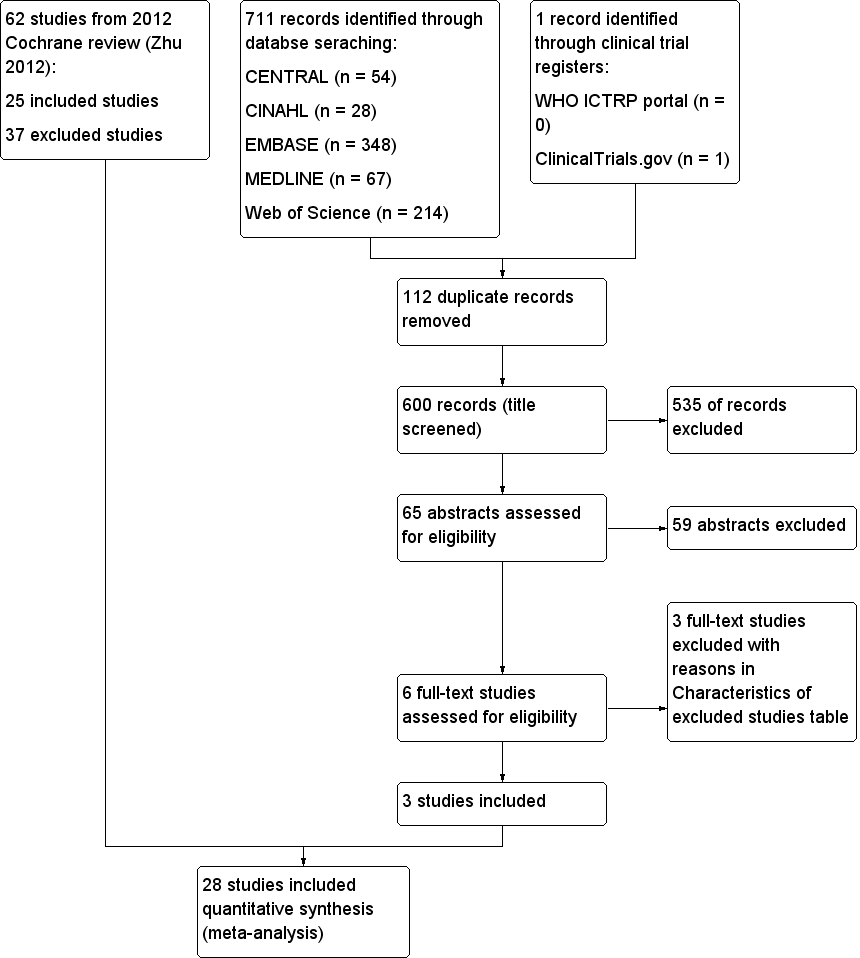
Study flow diagram.
Included studies
We included in the updated review a total of 28 RCTs involving 4438 participants. One trial was multi‐centred (Silbert 2006). The sample size of these studies ranged from 35 (Nicholson 2002) to 1012 (Slogoff 1989). The trials were conducted in the United States (Engoren 1998; Slogoff 1989), Canada (Cheng 1996a,1996b, 2003; Dumas 1999; Nicholson 2002; Quasha 1980), Australia (Myles 1997; Myles 2002; Silbert 1998; Silbert 2006), France (Nougarede 2004), Italy (Simeone 2002), United Kingdom (Bell 1994; Berry 1998; Sherry 1996), Netherlands (van Mastrigt 2006a, 2010), Spain (Reyes 1997), Sweden (Pettersson 2004), Greece (Michalopoulos 1998), Oman (Maddali 2006), Japan (Kadoi 2003; Sakaida 1998), Hong Kong (Gruber 2008; Zhu 2015), Germany (Probst 2014), Egypt (Salah 2015) and Taiwan (Chang 2007; Lu 2003). Most participants were undergoing first‐time elective coronary artery graft bypass or valvular replacement surgical procedures, or both. In two studies (Nicholson 2002; Reyes 1997), participants were undergoing emergency, semi urgent or urgent cardiac surgery. Two studies recruited participants with low cardiac output (Bell 1994; Sherry 1996).
Eighteen studies involved the use of low‐dose opioid‐based anaesthesia (Bell 1994; Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Probst 2014; Sakaida 1998; Sherry 1996; Silbert 1998; Silbert 2006; Slogoff 1989; Zhu 2015). Sixteen studies involved the use of time‐directed extubation protocols (Bell 1994; Berry 1998; Cheng 1996a,1996b, 2003; Dumas 1999; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Pettersson 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015).
Participants were extubated in the ICU, except in three studies in which early extubation occurred on the operating table (Nougarede 2004; Salah 2015), or in the postanaesthesia care unit (Nicholson 2002). Three studies provided follow‐up for participants for one year (Cheng 1996a,1996b, 2003; Silbert 2006; van Mastrigt 2006a, 2010); the remaining trials followed participants during their ICU stay or until the end of their hospital stay.
Unpublished data for four studies (Myles 2002; Reyes 1997; Sakaida 1998; Zhu 2015), were included in this updated review. We received no unpublished data after writing to the authors of new included trials.
Excluded studies
A total of 40 studies did not meet the inclusion criteria for the reasons shown in the Characteristics of excluded studies table.
Ongoing studies
We found no ongoing studies.
Studies awaiting classification
We found no studies awaiting classification.
Risk of bias in included studies
See Characteristics of included studies. Two studies had low risk of bias (Cheng 1996a,1996b, 2003; van Mastrigt 2006a, 2010), as all key domains were rated 'yes'. Eleven studies had high risk of bias (Bell 1994; Dumas 1999; Engoren 1998; Gruber 2008; Maddali 2006; Quasha 1980; Salah 2015; Sakaida 1998; Sherry 1996; Silbert 1998; Zhu 2015), as one or more domains were rated 'no'. A 'Risk of bias' graph and summary are provided in Figure 2 and Figure 3, respectively.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten studies used computer‐generated random numbers to determine allocation sequences (Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Maddali 2006; Reyes 1997; Salah 2015; Silbert 1998; Slogoff 1989; van Mastrigt 2006a, 2010; Zhu 2015), and three studies used random number tables (Myles 1997; Myles 2002; Silbert 2006). One study reported inadequate allocation concealment (Engoren 1998).
Blinding
Four studies did not use blinding (Gruber 2008; Maddali 2006; Silbert 1998; Zhu 2015).
Incomplete outcome data
One study omitted two participants from the outcome analysis (Quasha 1980).
Selective reporting
Three studies did not report mortality and complications outcomes (Bell 1994; Sakaida 1998; Sherry 1996), and one study showed inconsistency in outcome reporting (Silbert 1998).
Other potential sources of bias
Demographic and intraoperative characteristics were comparable in most studies.
Effects of interventions
See: Summary of findings for the main comparison Low‐dose opioid‐based GA vs high‐dose opioid‐based GA; Summary of findings 2 Time‐directed extubation protocol vs usual care
Dose of opioid‐based cardiac anaesthesia
Primary outcomes
Mortality (Analysis 1.1)
Investigators reported short‐term (ICU and hospital stay) and long‐term (one‐year) mortality. Three studies recorded no deaths (Engoren 1998; Michalopoulos 1998; Myles 1997). Berry 1998 showed no significant differences between low‐dose and high‐dose opioid groups for risk of death in the ICU (OR 0.13, 95% CI 0.01 to 2.06). The overall risk of death at hospital discharge was less than 2%, and researchers found no significant differences between low‐dose and high‐dose opioid groups (OR 0.58, 95% CI 0.24 to 1.39; seven trials, 1896 participants) (Analysis 1.1). At one‐year follow‐up, risk of death was similar between groups (OR 0.55, 95% CI 0.17 to 1.82; two trials, 446 participants) in two studies (Cheng 1996a,1996b, 2003; Silbert 2006) (Analysis 1.1).
When death was reported at several time periods within a study, we used the data from the longest follow‐up. Data from 1994 participants show that eight trials examined the risk of mortality after surgery at any time within a year (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Myles 1997; Silbert 1998; Silbert 2006; Slogoff 1989). We found no heterogeneity between the eight studies (I2 = 0%) (Analysis 1.1) and similar risk of death between groups (OR 0.53, 95% CI 0.25 to 1.12, P = 0.10). We downgraded the evidence from high to low because two trials had one or more high risk of bias domains along with imprecision.
As the meta‐analysis included fewer than 10 studies, we did not construct a contour‐enhanced funnel plot graph to examine the presence of publication bias. Sensitivity analysis showed that the risk of mortality at any time within a year in a study with low risk of bias was not different between groups (OR 0.36, 95% CI 0.05 to 2.61) (Cheng 1996a,1996b, 2003).
Secondary outcomes
Postoperative complications (Analysis 1.2)
Myocardial infarction (Analysis 1.2.1)
The overall incidence of postoperative myocardial infarction was 3.3% (95% CI 2.5% to 4.2%). Two studies stated that no participants had postoperative myocardial infarction (Michalopoulos 1998; Silbert 1998). Eight studies involving 1683 participants reported no significant difference in the risk of postoperative myocardial infarction between low‐dose and high‐dose opioid groups (RR 0.98, 95% CI 0.48 to 1.99; P = 0.96) (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Myles 1997; Myles 2002; Silbert 1998; Slogoff 1989). Heterogeneity between studies was low (I2 = 6%). We downgraded the evidence from high to low because of imprecision and because two trials had one or more domains at high risk of bias.
Stroke (Analysis 1.2.2)
The overall incidence of postoperative stroke was 2.0% (95% CI 1.0% to 3.4%). One study reported no participants with stroke after surgery (Michalopoulos 1998). The risk of stroke after surgery was similar between low‐dose and high‐dose opioid groups (RR 1.17, 95% CI 0.36 to 3.78; P = 0.80), and we found no heterogeneity (I2 = 0%) between the five studies involving 562 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Michalopoulos 1998; Myles 2002) (Analysis 1.2). We downgraded the evidence from high to low because of imprecision, and because one trial had one or more high risk of bias domains.
Acute renal failure (Analysis 1.2.3)
The overall incidence of acute renal failure after surgery was 1.8% (95% CI 0.9% to 3.3%). Michalopoulos 1998 reported no participants with acute renal failure after surgery. We found no significant difference in the risk of postoperative acute renal failure between low‐dose and high‐dose opioid groups (RR 1.19, 95% CI 0.33 to 4.33; P = 0.79) in four studies involving 492 participants (Cheng 1996a,1996b, 2003; Kadoi 2003; Michalopoulos 1998; Myles 2002) (Analysis 1.2). We downgraded the quality of evidence to moderate owing to imprecision.
Major bleeding (Analysis 1.2.4)
The overall incidence of major bleeding after cardiac surgery was 5.5% (95% CI 3.7% to 7.9%). Researchers reported no significant difference in the risk of major bleeding after surgery between low‐dose and high‐dose opioid groups (RR 0.48, 95% CI 0.16 to 1.44; P = 0.19), and heterogeneity between studies was low (I2 = 27%) in a random‐effects model of four studies involving 469 participants (Berry 1998; Cheng 1996a,1996b, 2003; Lu 2003; Michalopoulos 1998) (Analysis 1.2). We downgraded the quality of evidence to moderate owing to imprecision.
Major sepsis
Michalopoulos 1998 reported no participants with major sepsis.
Wound infection
Michalopoulos 1998 reported no wound infection. Myles 2002 observed no significant difference in the risk of postoperative wound infection between low‐dose and high‐dose opioid groups (RR 2.00, 95% CI 0.19 to 20.61).
Reintubation within 24 hours of surgery (Analysis 1.2.5)
No participants required reintubation in three studies (Engoren 1998; Michalopoulos 1998; Silbert 1998). The overall risk of reintubation in the low‐dose opioid group was 1.4% (95% CI 0.4% to 3.2%). This risk of reintubation in the low‐dose opioid group was not significantly higher than risk in the high‐dose opioid group (RR 1.77, 95% CI 0.38 to 8.27; P = 0.47) in five studies involving 594 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Michalopoulos 1998; Silbert 1998) (Analysis 1.2). These studies were homogenous (I2 = 0%). We downgraded the evidence from high to low because of imprecision, and because two trials had one or more high risk of bias domains.
Service outcomes (Analysis 1.3)
Time to extubation (Analysis 1.3.1)
Two studies did not report interquartile range around the median time to extubation (Bell 1994; Sherry 1996). In Bell 1994, investigators extubated the low‐dose opioid group 8.54 hours before the high‐dose opioid group (P < 0.0005). Researchers in Sherry 1996 extubated the low‐dose opioid group 4.84 hours before the high‐dose opioid group, but whether this difference was significant was not clear. We did not include these two studies in the time to extubation meta‐analysis.
Comparison with the high‐dose opioid group revealed that the low‐dose opioid group was associated with a reduction in time to extubation (MD ‐7.40 hours, 95% CI ‐10.51 to ‐4.29; P < 0.0001) in 14 studies involving 2486 participants (Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Sakaida 1998; Silbert 1998; Silbert 2006; Slogoff 1989). The mean difference in time to extubation ranged from ‐27.20 hours (95% CI ‐33.21 to ‐21.19) (Lu 2003), favouring the low‐dose opioid group, to 1.02 hours (95% CI ‐0.76 to 2.80) (Engoren 1998), favouring the high‐dose opioid group. The Egger's test showed no evidence of funnel plot asymmetry (P = 0.40). Heterogeneity among the 14 studies was high (total n = 2486) when results were pooled (I2 = 99%) (Analysis 1.3). We downgraded the evidence from high to low because four trials had one or more high risk of bias domains and high heterogeneity among the studies was not explained.
Intensive care unit length of stay (Analysis 1.3.2)
Berry 1998 described little variability in length of stay in the ICU in the early extubation group. Although Bell 1994 reported no variability, participants in the low‐dose opioid group were discharged from the ICU sooner than participants in the high‐dose opioid group (median 4.5 hours; P = 0.005). Analysis of 12 studies involving 1394 participants revealed that the low‐dose opioid group was associated with shorter ICU length of stay (MD ‐3.70, 95% CI ‐6.98 to ‐0.41 hours; P = 0.03) (Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Sakaida 1998; Silbert 2006). The mean difference in ICU length of stay ranged from ‐60.00 hours (95% CI ‐81.04 to ‐38.96), favouring the low‐dose opioid group (Lu 2003), to 2.00 hours (95% CI ‐1.58 to 5.58), favouring the conventional extubation group (Engoren 1998). Heterogeneity between the 12 studies (total n = 1394) was high when pooled (I2 = 98%). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
Hospital length of stay (Analysis 1.3.3)
All studies except two (Cheng 1996a,1996b, 2003; Michalopoulos 1998) reported no significant differences in hospital length of stay between groups. When we pooled the eight studies involving 913 participants, we found that the length of stay in the hospital was not significantly different between low‐dose and high‐dose opioid groups (MD ‐0.30 days, 95% CI ‐1.04 to 0.43; P = 0.42). However, heterogeneity between the studies was large (I2 = 85%). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and high heterogeneity among studies could not be explained.
Cost
We did not pool studies, as each study measured costs on different aspects of cardiac anaesthesia care. The low‐dose opioid‐based general anaesthesia intervention was associated with a reduction in departmental cost savings for uncomplicated CABG surgery (MD 2015 USD ‐2016, 95% CI ‐3247 to ‐785) (Cheng 1996a,1996b, 2003). The total hospital cost in Myles 2002 was similar between the low‐dose opioid group (mean 2015 USD 15,744 ± 4234) and the high‐dose opioid group (mean 2015 USD 14,641 ± 3376), with MD of 2015 USD 1103 (95% CI ‐1142 to 3348). The total cost of drugs was similar between the low‐dose opioid group (mean 2015 USD 117 ± 32) and the high‐dose opioid group (mean 2015 USD 128 ± 47), with MD of 2015 USD 11 (95% CI ‐35 to 13). The cost of ICU nursingand of drugs used in the operating theatre and in the ICU in the low‐dose opioid group was not significantly different from the cose in the high‐dose opioid group (MD 2015 USD ‐93, 95% CI ‐7 to 192) (Sherry 1996).
Time‐directed extubation protocol
Primary outcomes
Mortality (Analysis 2.1)
Investigators reported no significant difference between early and usual care (late extubation) groups for risk of death in the ICU in two studies involving 370 participants (OR 0.87, 95% CI 0.19 to 3.88) (Analysis 2.1). Three studies recorded no deaths in hospital after surgery (Engoren 1998; Gruber 2008; Michalopoulos 1998). Pooling of data from showed that risk of death in the hospital after surgery was similar between groups (OR 0.23, 95% CI 0.05 to 1.04; five trials, 582 participants) (Analysis 2.1). At one month after surgery, risk of death was similar (< 4%) in early extubation and usual care (late extubation) groups (OR 1.13, 95% CI 0.59 to 2.19; four trials, 1122 participants) (Analysis 2.1).
When death was reported at several time periods within a study, we used the data from longest follow‐up. We pooled 10 studies involving 1802 participants for outcome analysis (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Pettersson 2004; Probst 2014; Reyes 1997; van Mastrigt 2006a, 2010; Zhu 2015). Heterogeneity between the studies was moderate (I2 = 37%) (Analysis 2.1). Researchers showed no difference in risk of mortality after surgery at any time within a year between early and late extubation groups (OR 0.80, 95% CI 0.45 to 1.45; P = 0.47) (Analysis 2.1). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and showed imprecision.
Sensitivity analysis showed that risk of mortality at any time within a year in a study with low risk of bias was not significantly different between early and late extubation groups (RR 0.37, 95% CI 0.05 to 2.62) (van Mastrigt 2006a, 2010). Heterogeneity in mortality at any time after surgery may be due in part to inclusion of the Reyes 1997 trial, which found exceptionally high mortality compared with other studies; when we excluded this study, the OR estimate favoured the time‐directed protocol group with much lower heterogeneity (OR 0.31, 95% CI 0.11 to 0.90; P = 0.03, I2 = 0%) and moderate quality of evidence (downgraded owing to study limitations). As fewer than 10 studies with estimates were included in the meta‐analysis, we did not construct a contour‐enhanced funnel plot graph to examine the presence of publication bias.
Secondary outcomes
Postoperative complications (Analysis 2.2)
Myocardial infarction (Analysis 2.2.1)
The overall incidence of postoperative myocardial infarction was 5.0% (95% CI 3.9% to 6.3%). One study stated that no participants had postoperative myocardial infarction (Michalopoulos 1998). We pooled eight studies involving 1378 participants for analysis and found no difference in risk of postoperative myocardial infarction between early extubation and usual care (late extubation) groups (RR 0.59, 95% CI 0.27 to 1.31; P = 0.20) (Analysis 2.2). Heterogeneity between studies was moderate (I2 = 39%). We downgraded the evidence from high to low because of imprecision, and because three trials had one or more high risk of bias domains.
Stroke (Analysis 2.2.2)
The overall incidence of postoperative stroke was 1.0% (95% CI 0.6% to 1.6%). Two studies reported that no participants had stroke after surgery (Dumas 1999; Michalopoulos 1998). Pooling of 11 studies involving 1646 participants for analysis revealed that risk of stroke after surgery was similar between time‐directed protocol and usual care groups (RR 0.85, 95% CI 0.33 to 2.16; P = 0.73) (Cheng 1996a,1996b, 2003; Dumas 1999; Engoren 1998; Gruber 2008; Michalopoulos 1998; Probst 2014; Quasha 1980; Reyes 1997; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015). We found no heterogeneity (I2 = 0%) between the 11 studies involving 1646 participants (Analysis 2.2), and we downgraded the evidence from high to low because of imprecision, and because five trials had one or more high risk of bias domains.
Acute renal failure (Analysis 2.2.3)
The overall incidence of postoperative acute renal failure was 1.0% (95% CI 0.6% to 1.6%). Two studies reported that no participants had acute renal failure after surgery (Gruber 2008; Michalopoulos 1998). Pooling of nine studies involving 1541 participants for analysis showed no difference in the risk of postoperative acute renal failure between early extubation and late extubation groups (RR 1.11, 95% CI 0.42 to 2.91; P = 0.84) and these trials were homogeneous (I2 = 0%) (Analysis 2.2). We downgraded the evidence from high to low because of imprecision and because three trials had one or more high risk of bias domains.
Major bleeding (Analysis 2.2.4)
The overall incidence of major bleeding after cardiac surgery was 4.4% (95% CI 3.4% to 5.7%). Investigators showed no difference in the risk of major bleeding after surgery between early extubation and usual care (late extubation) groups (RR 0.92, 95% CI 0.53 to 1.61; P = 0.77). We noted no heterogeneity (I2 = 0%) between 10 studies involving 1244 participants (Berry 1998; Cheng 1996a,1996b, 2003; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Quasha 1980; Salah 2015; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.2). We downgraded the evidence from high to low because of imprecision, and because four trials had one or more high risk of bias domains.
Major sepsis (Analysis 2.2.5)
The overall incidence of sepsis was 1.8% (95% CI 0.3% to 2.0%). Michalopoulos 1998 reported no participants with major sepsis. We found no difference in the risk of postoperative major sepsis between early extubation and usual care (late extubation) groups (RR 2.40, 95% CI 0.31 to 18.25; P = 0.40) in a random‐effects model of three studies involving 477 participants (Michalopoulos 1998; Reyes 1997; Zhu 2015) (Analysis 2.2). We found no heterogeneity between studies (I2 = 0%), and we downgraded the evidence from high to low because of imprecision, and because one trial had one or more high risk of bias domains.
Wound infection (Analysis 2.2.6)
The overall incidence of postoperative wound infection was 1.8% (95% CI 1.1% to 2.9%). We noted no difference in the risk of postoperative wound infection between early extubation and usual care (late extubation) groups (RR 0.67, 95% CI 0.25 to 1.83; P = 0.43) and no heterogeneity between the two trials (I2 = 0%) involving 868 participants (Reyes 1997; van Mastrigt 2006a, 2010) (Analysis 2.2). The level of evidence was moderate owing to imprecision.
Reintubation within 24 hours of surgery (Analysis 2.2.7)
No participants required reintubation in three studies (Engoren 1998; Michalopoulos 1998; Nougarede 2004). Reyes 1997 and Probst 2014 contributed to 42.8% and 32.1% of the total data pooled for this outcome, respectively. Risk of reintubation in the time‐directed extubation protocol group was 4.1% (95% CI 2.8% to 5.9%). This risk of reintubation in the time‐directed extubation protocol group was not significantly higher than risk in the usual care (late extubation) group (RR 1.34, 95% CI 0.74 to 2.41; P = 0.33) in 12 studies involving 1261 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015; Simeone 2002; Zhu 2015) (Analysis 2.2). We found no heterogeneity between studies (I2 = 0%) and no subgroup differences (P = 0.58) in risk of reintubation according to where extubation occurred (Figure 4). We downgraded the evidence from high to low because of imprecision, and because five trials had one or more high risk of bias domains.

Forest plot of comparison: 2 Time‐directed extubation protocols, outcome: 2.4 Subgroup analysis.
Patient‐centred outcome
Quality of life within one year
Only one study reported quality of life within one year using the visual analogue scale (0 to 100) in the EuroQoL Group Quality of Life Questionnaire (EQ5D) instrument (van Mastrigt 2006a, 2010). The change in quality of life from baseline (one day before surgery) to one month after surgery between early extubation (9.99 ± 21.76) and usual care (late extubation) groups (7.00 ± 18.99) was similar (MD 2.99, 95% CI ‐0.98 to 6.96; P = 0.14). The change in quality of life from baseline to one year after surgery between early extubation (5.20 ± 17.23) and usual care (late extubation) groups (6.45 ± 16.19) was also similar (MD ‐1.25, 95% CI ‐4.50 to 2.00; P = 0.45).
Service outcomes (Analysis 2.3)
Time to extubation (Analysis 2.3.1)
In the early extubation group in two studies (Nougarede 2004; Salah 2015), investigators extubated participants immediately after surgery. Participants were extubated in the ICU in all but two trials (Nicholson 2002; Probst 2014), in which participants were extubated in the postanaesthesia care unit. The MD in time to extubation was ‐2.60 hours (95% CI ‐2.88 to ‐2.32) in Nicholson 2002 to ‐6.47 hours (95%CI ‐7.32 to ‐5.62) in Probst 2014.
We pooled 16 studies involving 2024 participants for analysis (Berry 1998; Cheng 1996a,1996b, 2003; Dumas 1999; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Pettersson 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015, Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.3). Comparison with usual care revealed that use of a time‐directed extubation protocol was associated with a reduction in intubation time of ‐6.25 hours (95% CI ‐8.84 to ‐3.67; P < 0.001). The MD in the time to extubation group ranged from ‐16.00 hours (95% CI ‐17.64 to ‐14.36) (Quasha 1980), favouring the time‐directed extubation protocol group, to 1.02 hours (95% CI ‐0.76 to 2.80) (Engoren 1998), favouring the usual care group. The Egger's test showed no evidence of funnel plot asymmetry (P = 0.47). When pooled, heterogeneity between the 16 studies (total n = 2024) was high (I2 = 99%). We downgraded the evidence from high to low because six trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
Intensive care unit length of stay (Analysis 2.3.2)
We pooled 13 studies involving 1888 participants for analysis (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nougarede 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015, Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015). The time‐directed extubation protocol intervention group was associated with a shorter ICU length of stay (MD ‐7.16, 95% CI ‐10.45 to ‐3.88 hours; P = 0.000019) (Analysis 2.3). The MD in the ICU length of stay ranged from ‐37.62 hours (95% CI ‐52.38 to ‐22.86), favouring the time‐directed extubation protocol group (Salah 2015) to 2.00 hours (95% CI ‐1.68 to 5.58), favouring the usual care group (Engoren 1998). Heterogeneity between the 13 studies involving 1888 participants was high when pooled (I2 = 94%). We downgraded the evidence from high to low because five trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
Hospital length of stay (Analysis 2.3.3)
Two studies found a significant difference in hospital length of stay between groups (Cheng 1996a,1996b, 2003; Michalopoulos 1998). Pooling of the eight studies involving 1334 participants showed that length of stay in the hospital was not different between time‐directed extubation protocol and usual care groups (MD ‐0.44, 95% CI ‐1.04 to 0.16; P = 0.15) (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Pettersson 2004; Probst 2014; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.3). However, heterogeneity between studies was large (I2 = 77%). We downgraded the evidence from high to low because two trials had one or more high risk of bias domains, and because reasons for high heterogeneity among studies were not explained.
Cost
We did not pool studies, as each study measured costs on different aspects of fast‐track cardiac anaesthesia care. The early extubation intervention was associated with a reduction in departmental cost savings for uncomplicated CABG surgery (MD 2015 USD ‐2016, 95% CI ‐3247 to ‐785) (Cheng 1996a,1996b, 2003).
Van Mastright and colleagues (van Mastrigt 2006a, 2010) reported that early extubation was associated with a reduction in total hospital cost (nutrition, laundry, accommodation, cleaning, overheads, equipment, staff, material and medication) (MD 2015 USD ‐1102, 95% CI ‐2117 to ‐233). Furthermore, the cost‐effectiveness (cost/change in quality‐adjusted life months) analysis found that 98% of bootstrapped incremental cost‐effectiveness ratios showed greater improvement in quality of life and lower costs for participants with early extubationcompared with participants with late extubation (van Mastrigt 2006a, 2010).
Discussion
Summary of main results
In this update, we added to the body of evidence three trials (Probst 2014; Salah 2015; Zhu 2015) ‐ all on the effects of a time‐directed extubation protocol. In this systematic review of 28 randomized controlled trials involving 4438 particpants, we found that fast‐track cardiac care was associated with a similar risk of mortality after surgery when compared with conventional (not fast‐track) care (summary of findings Table for the main comparison; summary of findings Table 2). The incidence of mortality at any time after surgery was generally low (< 5%). We found moderate‐quality evidence of reduced risk of death associated with a time‐directed protocol intervention when a trial (Reyes 1997) with a high mortality rate of 8% was excluded from the main meta‐analysis. This post hoc sensitivity analysis is a new finding of this review.
We noted no increase in risk of myocardial infarction, acute renal failure, stroke, major bleeding, sepsis or wound infection (summary of findings Table for the main comparison; summary of findings Table 2), and found that the overall quality of evidence for postoperative complications was low. We also found evidence of low quality for the reduction in time to extubation (up to 11 hours earlier) and length of stay in the intensive care unit (ICU) (up to 10 hours earlier). It should be noted that patient transfer out of ICU to the ward is dependent on the availability of beds on the ward in some settings, which may explain the high heterogeneity observed. Clinically important reductions in length of stay in the hospital were not associated with fast‐track cardiac care, and we rated this evidence as low quality.
We found limited evidence on the effects of early extubation following a time‐directed protocol on hospital cost and on quality of life within one year after cardiac surgery. Nevertheless, a formal cost‐effectiveness analysis in one trial showed that early tracheal extubation was cost‐effective (van Mastrigt 2006a, 2010).
Overall completeness and applicability of evidence
We considered most of the study participants to be at low to moderate risk of operative mortality (based on the European system for cardiac operative risk evaluation criteria), and most were undergoing elective cardiac surgery. This may explain why low rates of mortality and postoperative complications were found in most trials included in this systematic review. We found few large trials, and all, except one (Slogoff 1989), were not designed to detect an effect on myocardial infarction, stroke or death. Thus, it is likely that this systematic review was underpowered to detect significant risk reductions. For example, a post hoc power analysis using G*Power software (Faul 2014) showed that the power to detect a difference in the risk of mortality at any time after surgery with the use of time‐directed extubation protocols was 11%.
Results of this review are unlikely to be applicable to high‐risk patients, who required significantly longer time to extubation (about one hour) than low‐risk patients when the same fast‐track cardiac anaesthesia technique was used in a cohort study of 1162 participants (Alhan 2003). Of note, length of stay in the ICU and in hospital was similar for high‐risk patients and low‐risk patients (Alhan 2003). Risk factors associated with fast‐track cardiac surgery protocol failure (ICU readmission or failure to directly transfer patients to an intermediate care unit after surgery) include preoperative American Society of Anesthesiologists' Physical Status > 3, New York Heart Association class > III and operative time > 267 minutes (Kiessling 2013).
Our systematic review highlights the paucity of high‐quality cost‐analysis studies on fast‐track cardiac care. A propensity‐matched cohort study of 652 participants showed that extubation in the operating room was associated with a 23% decrease in overall postoperative costs (mean difference 2014 USD ‐1013, 95% CI ‐1597 to ‐429) compared with extubation in the ICU within 12 hours after surgery (Badhwar 2014). Reductions in cost were due to shorter length of stay in the ICU and hospital with similar risks of postoperative complications among patients extubated in the operating room (Badhwar 2014).
Remifentanil, an opioid with rapid onset and offset of action, is used frequently in fast‐track cardiac anaesthesia (Greco 2012). The trials in this systematic review did not include the use of remifentanil; this limits the applicability of evidence to settings in which remifentanil is used as part of the fast‐track cardiac anaesthesia technique. A meta‐analysis of remifentanil trials in cardiac surgery showed that remifentanil was associated with reduced time to extubation and length of hospital stay with no increase in risk of mortality when compared with use of fentanyl or sufentanil during general anaesthesia (Greco 2012).
Quality of the evidence
The quality of evidence as rated by the GRADE approach is shown in summary of findings Table for the main comparison and summary of findings Table 2 (Guyatt 2011). A limitation of this systematic review is that 11 of the 28 trials had one or more high risk of bias domains. Blinding and selective reporting introduced the most common risks of bias. Overall, evidence on the association between fast‐track cardiac care and mortality was of low quality.
Evidence on the association between fast‐track cardiac care and major postoperative complications was of low quality, as review authors included high risk of bias trials in the various meta‐analyses and noted imprecision. Although we observed a clinically important difference between groups in time to extubation, the evidence was of low quality owing to inclusion of high risk of bias trials and large inconsistencies of effect between trials. Only one high‐quality trial examined changes in quality of life after cardiac surgery associated with a time‐directed extubation protocol (van Mastrigt 2006a, 2010).
Potential biases in the review process
We cannot rule out the presence of publication bias in this systematic review. We did not attempt to handsearch conference proceedings. As we identified fewer than 10 trials with risk estimates of mortality after surgery, it was impossible for review authors to assess publication bias using a contour‐enhanced funnel plot. Nevertheless, we found no funnel asymmetry for time to extubation outcomes for the comparison of opioid‐based general anaesthesia and time‐directed protocol interventions.
Caution is required in interpreting results of the post hoc sensitivity analysis on exclusion of a trial (Reyes 1997) from the meta‐analysis on risk of death at any time after surgery, and on use of time‐directed protocols (odds ratio (OR) 0.31, 95% confidence interval (CI) 0.11 to 0.90). We are unsure why the excluded trial (Reyes 1997) had a higher mortality rate than the other trials considered, irrespective of trial arm.
Agreements and disagreements with other studies or reviews
Although investigators measured mortality at different time points, the risk of mortality associated with fast‐track cardiac care in this systematic review did not change the conclusions reported in previous systematic reviews (Hawkes 2003; Meades 2001; Myles 2003; Zhu 2012). In a retrospective cohort of 7989 participants comparing low‐dose and high‐dose opioid‐based general anaesthesia (Svircevic 2009), the adjusted odds ratio for mortality was 0.92 (95% confidence interval (CI) 0.65 to 1.32). The significant post hoc sensitivity analysis result contrasts with findings of a consensus conference on fast‐track cardiac anaesthesia (FTCA) that found no significant reduction in risk of mortality in any of the trials screened (Landoni 2011).
Previous systematic reviews found no evidence of increased risk of major complications after surgery associated with FTCA (Myles 2003; Zhu 2012). Our relative risks of myocardial infarction, major sepsis, acute renal failure, stroke and major bleeding were similar, but showed greater precision. Therefore, we conclude that FTCA is safe and comparable with conventional (not fast‐track) care.
The time to extubation result in this systematic review was comparable with that in previous systematic reviews (Meades 2001; Myles 2003; Zhu 2012) and in a large retrospective cohort study of 7989 participants in which the fast‐track cardiac anaesthesia group was extubated six hours earlier than the high‐dose opioid group (Svircevic 2009). A previous review suggested that the introduction of an early extubation protocol, in conjunction with low‐dose opioid anaesthetic techniques and normothermic temperature management, would be essential for decreasing intensive care unit (ICU) and hospital stays (van Mastrigt 2006b).
This systematic review found four studies examining early extubation outside the ICU setting (Nicholson 2002; Nougarede 2004; Probst 2014; Salah 2015). Careful selection of patients for immediate extubation in the operating room rarely resulted in tracheal reintubation and was associated with shorter ICU and hospital length of stay in two propensity‐matched cohort studies (Chamchad 2010; Badhwar 2014).
As health‐related quality of life instruments and timing of these assessments varied in 10 studies, the authors of a previous review (van Mastrigt 2006b) did not pool the results. The main limitation of the previous review was that pain and cognitive function were the only main dimensions of quality of life considered in the postoperative period to six months after surgery (van Mastrigt 2006b). In contrast, we included a trial that used a valid and reliable generic health‐related quality of life tool (van Mastrigt 2006a, 2010) and found high quality of evidence for a small but non‐significant improvement in quality of life at one year after surgery associated with early extubation.
We did not pool our cost analyses but found that early extubation was likely to be cost‐effective in one high‐quality study (van Mastrigt 2006a, 2010). A propensity‐matched cohort study of 652 participants showed that extubation in the operating room was associated with a 23% decrease in overall postoperative costs (mean difference 2014 USD ‐1013, 95% CI ‐1597 to ‐429) compared with extubation in the ICU within 12 hours after surgery (Badhwar 2014).
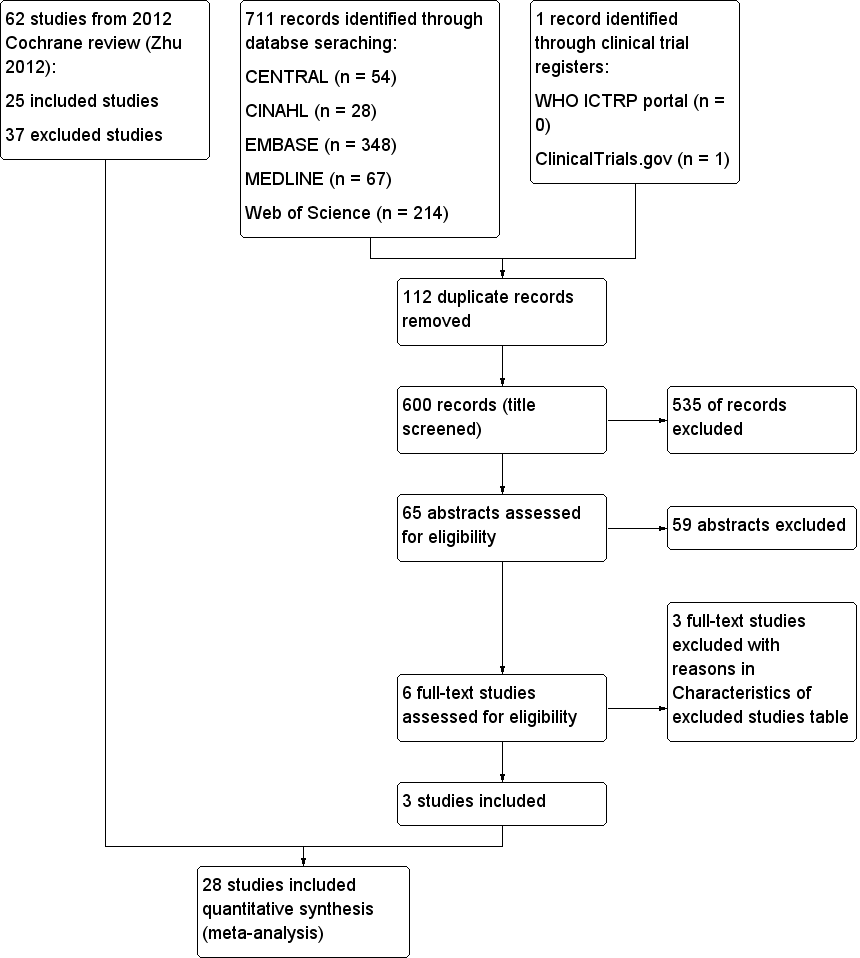
Study flow diagram.
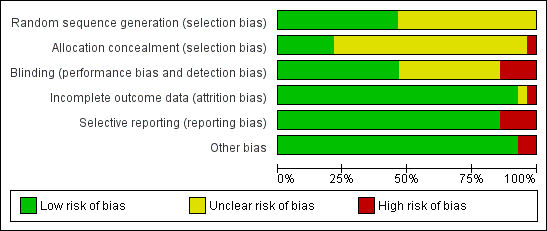
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
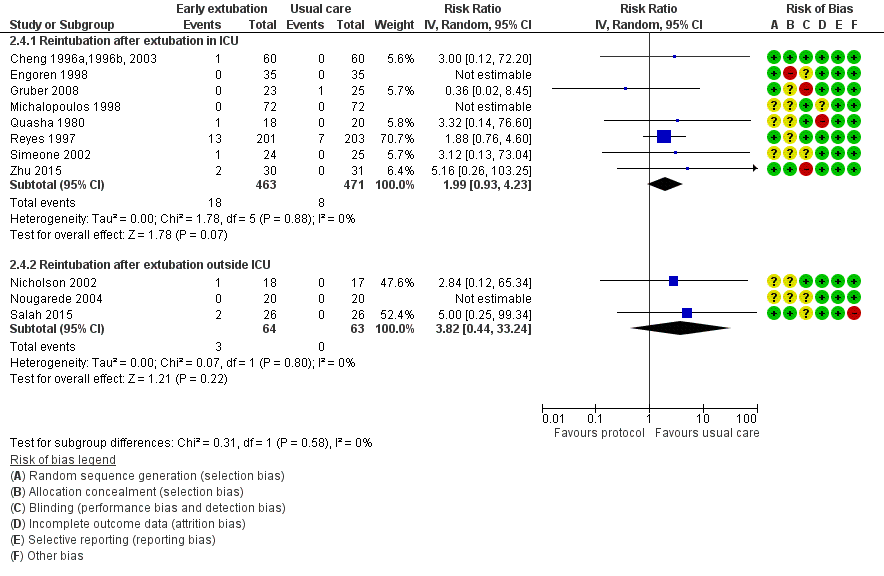
Forest plot of comparison: 2 Time‐directed extubation protocols, outcome: 2.4 Subgroup analysis.

Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 1 Mortality.

Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 2 Postoperative complications.
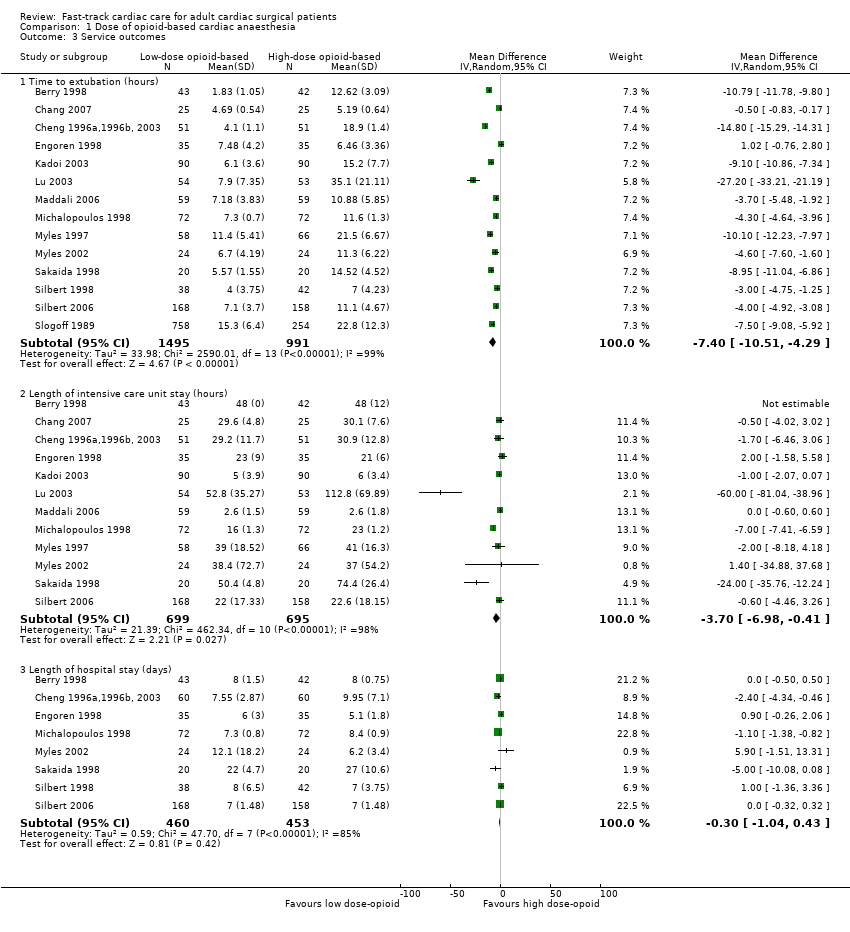
Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 3 Service outcomes.

Comparison 2 Time‐directed extubation protocol, Outcome 1 Mortality.
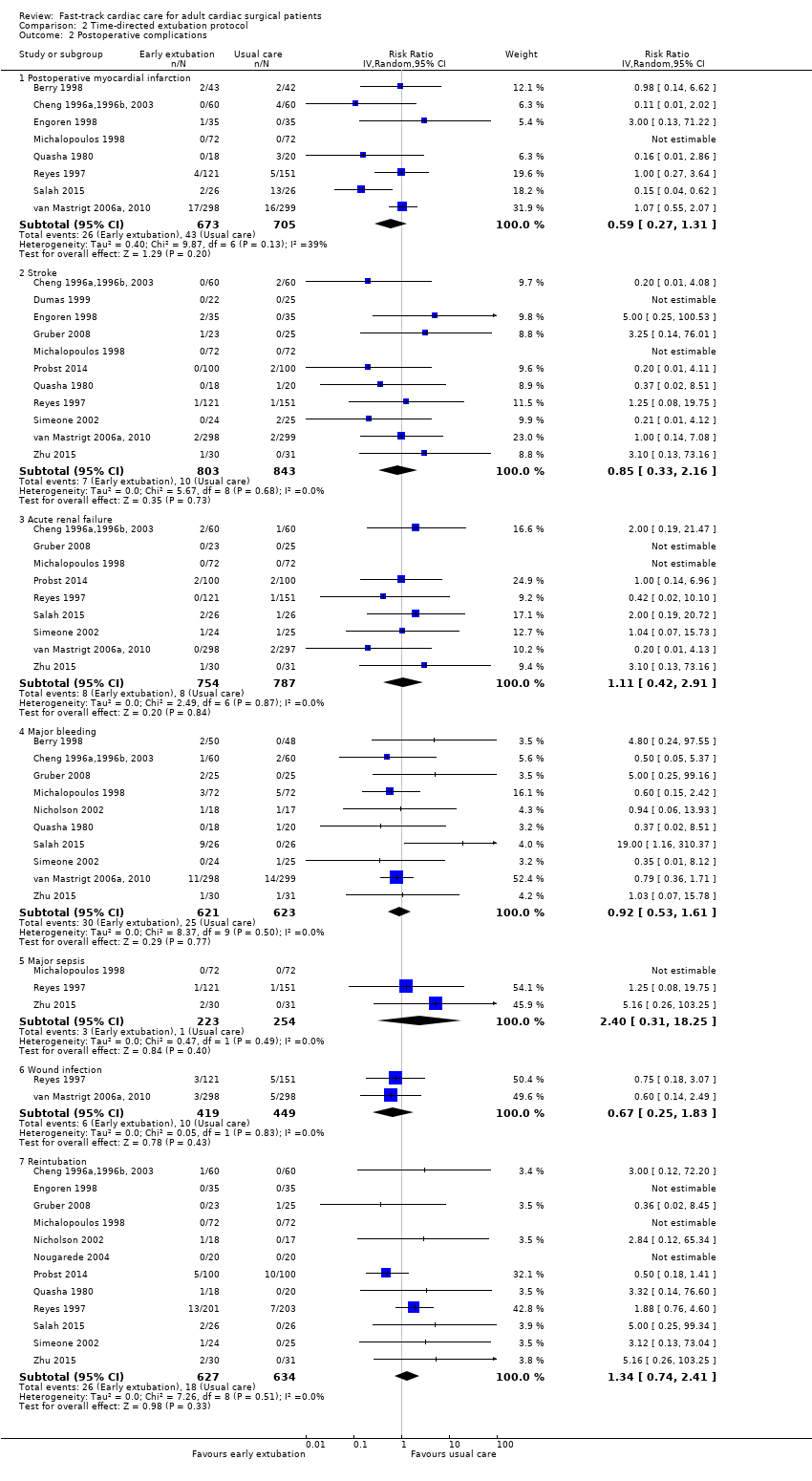
Comparison 2 Time‐directed extubation protocol, Outcome 2 Postoperative complications.

Comparison 2 Time‐directed extubation protocol, Outcome 3 Service outcomes.
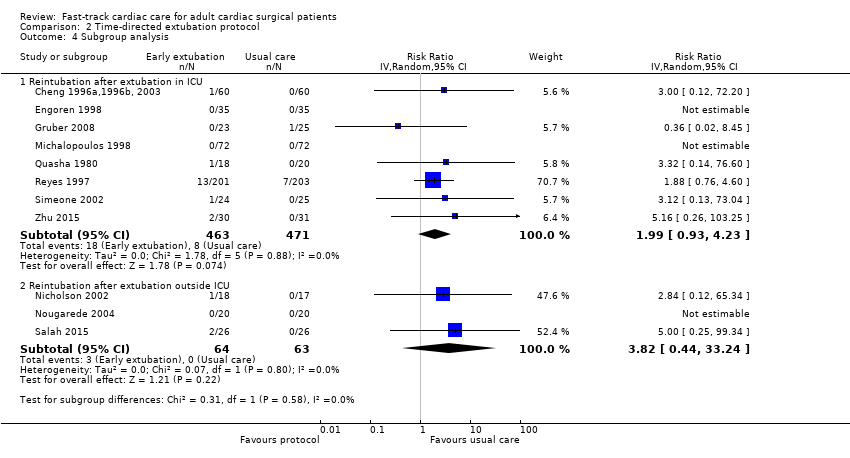
Comparison 2 Time‐directed extubation protocol, Outcome 4 Subgroup analysis.
| Low‐dose opioid‐based general anaesthesia compared with high‐dose opioid‐based general anaesthesia for adults undergoing cardiac surgery | ||||||
| Patient or population: adult cardiac surgical patients Intervention: low‐dose opioid‐based general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with high‐dose opioid‐based general anaesthesia | Risk with low‐dose opioid‐based general anaesthesia | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.53 | 1994 | ⊕⊕⊝⊝ | No death recorded in 3 trials | |
| 10 per 1000 | 5 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 16 per 1000 | |||||
| High | ||||||
| 110 per 1000 | 61 per 1000 | |||||
| Postoperative myocardial infarction | Study population | RR 0.98 | 1683 | ⊕⊕⊝⊝ | No postoperative myocardial infarction recorded in 2 trials | |
| 30 per 1000 | 30 per 1000 | |||||
| Postoperative stroke | Study population | RR 1.17 | 562 | ⊕⊕⊝⊝ | No stroke recorded in 1 trial | |
| 18 per 1000 | 21 per 1000 | |||||
| Postoperative tracheal reintubation | Study population | RR 1.77 | 594 | ⊕⊕⊝⊝ | No reintubation recorded in 3 trials | |
| 7 per 1000 | 12 per 1000 | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 5.2 to 35.1 | Mean time to extubation (hours) in the intervention group was 7.4 lower (10.51 lower to 4.29 lower). | ‐ | 2486 | ⊕⊕⊝⊝ | |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 2.6 to 112.8. | Mean length of intensive care unit stay (hours) in the intervention group was 3.7 lower (6.98 lower to 0.41 lower). | ‐ | 1394 | ⊕⊕⊝⊝ | |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 27.0. | Mean length of hospital stay (days) in the intervention group was 0.3 lower (1.04 lower to 0.43 higher). | ‐ | 913 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOf the 8 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). bOptimal information size not met (downgrade 1 point owing to imprecision). cOf the 5 trials, 1 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 5 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). eOf the 14 trials, 4 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). fUnexplained reasons for high heterogeneity. gOf the 12 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). hOf the 8 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations). | ||||||
| Time‐directed extubation protocol compared with usual care for adults undergoing cardiac surgery | ||||||
| Patient or population: adult cardiac surgical patients | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with time‐directed extubation protocol | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.80 | 1802 | ⊕⊕⊝⊝ | No deaths recorded in 4 trials. Low to moderate heterogeneity (I2 = 37%) may be explained by the inclusion of a trial (Reyes 1997) that had the highest rate of mortality of all trials considered. When excluded, the OR changed to 0.31 (95% CI 0.11 to 0.90, P = 0.03, I2 = 0%). | |
| 10 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 30 per 1000 | 24 per 1000 | |||||
| High | ||||||
| 110 per 1000 | 90 per 1000 | |||||
| Postoperative myocardial infarction | Study population | RR 0.59 | 1378 | ⊕⊕⊝⊝ | No postoperative myocardial infarction was recorded in 1 trial. | |
| 61 per 1000 | 36 per 1000 | |||||
| Postoperative stroke | Study population | RR 0.85 | 1646 | ⊕⊕⊝⊝ | No stroke was recorded in 2 trials. | |
| 12 per 1000 | 10 per 1000 | |||||
| Postoperative reintubation | Study population | RR 1.34 | 1261 | ⊕⊕⊝⊝ | No reintubation was recorded in 3 trials. | |
| 28 per 1000 | 38 per 1000 | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 3.4 to 18.9. | Mean time to extubation (hours) in the intervention group was 6.25 lower (8.84 lower to 3.67 lower). | ‐ | 2024 | ⊕⊕⊝⊝ | No variation in time to extubation in the early extubation group in 1 trial |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 17.9 to 95.0. | Mean length of intensive care unit stay (hours) in the intervention group was 7.16 lower (10.45 lower to 3.88 lower). | ‐ | 1888 | ⊕⊕⊝⊝ | No variation in length of ICU stay in early extubation group in 1 trial |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 13.0. | Mean length of hospital stay (days) in the intervention group was 0.44 lower (1.04 lower to 0.16 higher). | ‐ | 1334 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOf the 10 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). bOptimal information size not met (downgrade 1 point owing to imprecision). cOf the 8 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 11 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). dOf the 12 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). fUnexplained reasons for high heterogeneity. gOf the 16 trials, 6 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). hOf the 13 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). iOf the 8 trials, 2 had 1 high risk of bias domain (downgrade 1 point owing to study limitations). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in hospital after surgery | 7 | 1896 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.24, 1.39] |
| 1.2 Death at 1 year after surgery | 2 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.17, 1.82] |
| 1.3 Death at any time after surgery | 8 | 1994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.25, 1.12] |
| 2 Postoperative complications Show forest plot | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Postoperative myocardial infarction | 8 | 1683 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.48, 1.99] |
| 2.2 Stroke | 5 | 562 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.36, 3.78] |
| 2.3 Acute renal failure | 4 | 492 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.33, 4.33] |
| 2.4 Major bleeding | 4 | 469 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.16, 1.44] |
| 2.5 Reintubation | 5 | 594 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.38, 8.27] |
| 3 Service outcomes Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Time to extubation (hours) | 14 | 2486 | Mean Difference (IV, Random, 95% CI) | ‐7.40 [‐10.51, ‐4.29] |
| 3.2 Length of intensive care unit stay (hours) | 12 | 1394 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐6.98, ‐0.41] |
| 3.3 Length of hospital stay (days) | 8 | 913 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.04, 0.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in the intensive care unit | 2 | 370 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.19, 3.88] |
| 1.2 Death in hospital after surgery | 5 | 582 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.04] |
| 1.3 Death at 1 month after surgery | 4 | 1122 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.59, 2.19] |
| 1.4 Death at any time after surgery | 10 | 1802 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.45, 1.45] |
| 2 Postoperative complications Show forest plot | 15 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Postoperative myocardial infarction | 8 | 1378 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.27, 1.31] |
| 2.2 Stroke | 11 | 1646 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.33, 2.16] |
| 2.3 Acute renal failure | 9 | 1541 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.42, 2.91] |
| 2.4 Major bleeding | 10 | 1244 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.53, 1.61] |
| 2.5 Major sepsis | 3 | 477 | Risk Ratio (IV, Random, 95% CI) | 2.40 [0.31, 18.25] |
| 2.6 Wound infection | 2 | 868 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.25, 1.83] |
| 2.7 Reintubation | 12 | 1261 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.74, 2.41] |
| 3 Service outcomes Show forest plot | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Time to extubation (hours) | 16 | 2024 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐8.84, ‐3.67] |
| 3.2 Length of intensive care unit stay (hours) | 13 | 1888 | Mean Difference (IV, Random, 95% CI) | ‐7.16 [‐10.45, ‐3.88] |
| 3.3 Length of hospital stay (days) | 8 | 1334 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.04, 0.16] |
| 4 Subgroup analysis Show forest plot | 11 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Reintubation after extubation in ICU | 8 | 934 | Risk Ratio (IV, Random, 95% CI) | 1.99 [0.93, 4.23] |
| 4.2 Reintubation after extubation outside ICU | 3 | 127 | Risk Ratio (IV, Random, 95% CI) | 3.82 [0.44, 33.24] |

