Reposo en cama en los embarazos con feto único para prevenir el parto prematuro
Appendices
Appendix 1. Search Terms
Authors wrote and ran the following:
CENTRAL
-
#1 PREGNANCY (MeSH)
-
#2 PREGNAN*
-
#3 PERINATOLOGY (MeSH)
-
#4 PERINATOLOGY
-
#5 LABOR‐PREMATURE (MeSH)
-
#6 PREMATURE
-
#7 PRETERM
-
#8 BED‐REST (MeSH)
-
#9 (BED next REST)
-
#10 REST*
-
#11 BED REST
-
#12 ((((((#1 or #2) or #3) or #4) or #5) or #6) or #7)
-
#13 (((#8 or #9) or #10) or #11)
-
#14 (#12 and #13)
MEDLINE SEARCH
-
#1 Premature Birth
-
#2 Obstetric Labor premature
-
#3 Perinatology
-
#4 Pregnancy
-
#5 "Premature Birth"[MeSH]
-
#6 "Obstetric Labor, Premature"[MeSH]
-
#7 "Perinatology"[MeSH])
-
#8 "Pregnancy"[MeSH]
-
#9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
-
#10 bed rest
-
#11 "Bed Rest"[MeSH]
-
#12 (#10 or #11)
-
#13 (#9 and #12)
EMBASE SEARCH
-
#1 'premature'/exp OR premature
-
#2 'preterm baby'
-
#3 preterm AND labor
-
#4 premature AND labor
-
#5 bed AND rest
-
#6 rest
-
#7 (((#1 or #2) or #3) or #4)
-
#8 (#5 or #6)
-
#9 (#7 and #8)
LILACS SEARCH
-
#1 "PREMATURE BIRTH"
-
#2 "PRETERM LABOR"
-
#3 "PRETERM"
-
#4 “REST”
-
#5 "REST‐ACTIVITY"
-
#6 "REST‐EXERCISE"
-
#7 “BED REST”
-
#8 ((#1 or #2) or #3)
-
#9 (((#4 or #5) or #6) or #7)
-
#10 (#8 and #9)
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Hobel 1994.
Two reviewers independently assessed the trials for inclusion and methodological quality. The two reviewers resolved any disagreement by consensus or, if necessary, by a third reviewer.
We assessed the methodological quality of included trials using the methods described in the Cochrane Reviewers' Handbook (Clarke 2000).
Allocation concealment was categorised as:
(a) adequate;
(b) uncertain; or
(c) inadequate.
Blinding and completeness of follow‐up were assessed for each outcome using the following criteria: for completeness of follow‐up: (a) less than 3% of participants excluded, (b) 3% to 9.9% of participants excluded, (c) 10% to 19.9% of participants excluded or (d) 20% or more of participants excluded. For blinding of outcome assessment: (a) single blinding, (b) no blinding or blinding not mentioned.
We extracted the data independently using a previously prepared data extraction form. The results were expressed as relative risks for dichotomous outcomes or weighted mean difference for continuous variables, and included 95% confidence intervals using the Cochrane Review Manager software (RevMan 2000).
We included studies irrespective of their methodological quality. In the case of significant heterogeneity among study outcomes, we performed a sensitivity analysis and based our conclusions on the results of studies with the best methodological quality.
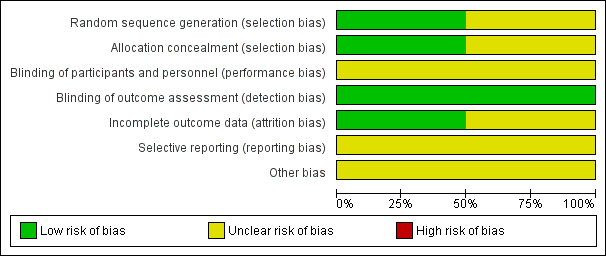
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Bed rest versus no intervention, Outcome 1 Preterm birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Preterm birth Show forest plot | 1 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.62, 1.37] |

