Progestogen for preventing miscarriage
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Unit of randomization: pregnancy. 300 women randomized, 0 exclusions, 300 women analyzed. Source of funding: not stated. | |
| Participants | Women up to 20 weeks' gestation presenting with bleeding. Age: 12% less than 21, 61% between 21 and 30, 17% between 31 and 35, and 9% older than 35. Location: Germany. | |
| Interventions | 90% of the treatment group received allylestrenol (15 mg‐20 mg orally per day). 10% received 250 mg of hydroxyprogesterone caproate intramuscularly every day or every 2 days. Placebo: yes. Duration: not stated. | |
| Outcomes | Miscarriage. | |
| Notes | Language of publication: German. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized. |
| Allocation concealment (selection bias) | High risk | Alternating treatments. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Unclear risk | Unknown. |
| Methods | Unit of randomization: pregnancy. 616 women randomized, 32 exclusions, 584 women analyzed. Source of funding: not stated. | |
| Participants | Women undergoing mid‐second trimester amniocentesis. Age: 36.4 +/‐ 3.6 in the progestogen group and 36.5 +/‐ 4.7 in the control group. Location: Italy. | |
| Interventions | 200 mg/day IM of natural progesterone for 3 days following amniocentesis, followed by 340 mg IM hydroxyprogesterone caproate twice a week until completion of the second week from the time of amniocentesis. Placebo: no. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Reported all outcomes. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes. |
| Other bias | Unclear risk | Unclear from methods. |
| Methods | Unit of randomization: pregnancy. 180 women randomized, 0 exclusions, 180 women analyzed. Source of funding: not stated. | |
| Participants | Women (< 35 years old) with at least 3 consecutive unexplained abortions with same husband with a new pregnancy. Age: treatment 22% age 20‐24 years, 36% age 25‐29 years, 41.5% age 30‐34 year, control: 21% age 20‐24 years, 48% age 25‐29 years, 31% age 30‐34 years. Location: Jordan. | |
| Interventions | 10mg BiD oral dydrogesterone, 5000 IU IM hCG every 4 days, or no treatment. Placebo: no, control group had no treatment. Duration: until miscarriage or 12th gestational week. | |
| Outcomes | Miscarriage, preterm delivery, fetal malformations, perinatal death (not analyzed in review: hospitalization for vaginal bleeding). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by day of the week. |
| Allocation concealment (selection bias) | High risk | Allocation by day of the week. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Does not appear to be incomplete data. |
| Selective reporting (reporting bias) | Low risk | None noted. |
| Other bias | Low risk | None noted. |
| Methods | Unit of randomization: pregnancy. 64 women randomized. | |
| Participants | Women with vaginal bleeding in pregnancy and a closed os. Age: treatment mean age ‐ 29.2, placebo mean age 28.7. Location: Germany. | |
| Interventions | 25 mg BiD progesterone vaginal suppositories. Placebo: yes. Duration: until miscarriage or 14 days from the cessation of symptoms. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | "code note broken until completion of the study, so that both staff and patients did not know which of the preparations contained the hormones." |
| Blinding of outcome assessment (detection bias) | Low risk | As above. |
| Incomplete outcome data (attrition bias) | Low risk | Reasonable. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. 54 women randomized, 0 women excluded, 54 women analyzed. Source of funding: Upjohn. | |
| Participants | Women who had either: All women had to have a urinary pregnanediol of less than 5 mg/day before 8 weeks' gestation and/or less than 7 mg/day by 14 weeks' gestation. Location: USA. | |
| Interventions | 10 mg/day of oral medroxyprogesterone. Placebo: yes. Duration: not stated. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered bottles. Code not broken until end. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Does not appear to be incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None apparent. |
| Methods | Unit of randomization: pregnancy. 33 women randomized, 33 women analyzed. Source of funding: not stated. | |
| Participants | Women who had 2 or more miscarriages, no pregnancy beyond 28 weeks' gestation, were less than 10 weeks into the current pregnancy and with no other obvious causes of miscarriage. | |
| Interventions | 50 mg BiD of oral cyclopentyl enol ether of progesterone. Placebo: yes. Duration: not stated. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random schedules generated by statistician. |
| Allocation concealment (selection bias) | Unclear risk | Given according to a random schedule. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded design ‐ neither patients nor physician knew which of the 2 they were getting. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated but likely blinded also. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. Source of funding not stated. | |
| Participants | Women who had had 3 consecutive miscarriages, were less than 16 weeks' gestation and with no signs of threatened miscarriage in the current pregnancy. Age: 20‐42. Location: USA. | |
| Interventions | 500 mg/week IM of hydroxyprogesterone caproate. Duration: until miscarriage or the 36th week of gestation. Placebo: yes. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating group A and B. |
| Allocation concealment (selection bias) | High risk | Alternating group A and B. |
| Blinding of participants and personnel (performance bias) | Low risk | Stated double blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | High risk | 56 women randomized, 26 women excluded, 30 women analyzed. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. 40 women randomized, 0 women excluded, 40 women analyzed. Source of funding: grant from N.V. Philips‐Duphar. | |
| Participants | Women with 2 or more consecutive proven abortions and then subsequent pregnancy with cervical mucus ferning present. | |
| Interventions | 2 x 5 mg tablets of dydrogesterone tablets 3 times daily, increased to 4 tablets 3 times daily if ferning persisted, no duration specified. Placebo: yes. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Stated double blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. 40 women randomized, 0 women excluded, 40 women analyzed. Source of funding: preparations were supplied by Leo Pharmaceuticals. | |
| Participants | Women with a positive pregnancy test. Age: not reported. Location: Denmark. | |
| Interventions | 20 mg/day of oral medroxyprogesterone for 3 days, followed by 10 mg/day for 11 days. Then from 1961‐1962, 40 mg/day of oral medroxyprogesterone for 3 days, followed by 20 mg/day for 11 days. Then from 1962‐1964, 80 mg/day of oral medroxyprogesterone for 3 days, followed by 40 mg/day for 7 days, followed by 20 mg for 7 days. Placebo: yes. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | According to admission numbers ‐ even and odd. |
| Allocation concealment (selection bias) | High risk | According to admission numbers ‐ even and odd. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded given as "A" and "B" preparations. Code not broken until completion of analysis. |
| Blinding of outcome assessment (detection bias) | Low risk | As above. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | High risk | Excluded women who aborted within 24 hours of admission. |
| Other bias | Unclear risk | Dose increased during the 4.5 years of the study. Analyzed in results ‐ likely not a risk of bias. |
| Methods | Unit of randomization: pregnancy. 303 women randomized, 0 excluded, 303 women analyzed. Source of funding: not stated. | |
| Participants | Women having undergone IVF or ICSI with a positive pregnancy test 14 days after transfer. Age: 32.1 +/‐ 4.1 in the progestogen group and 32.2 +/‐ 4.3 in the control group. Location: Denmark. | |
| Interventions | 200 mg TiDs vaginal suppositories. Placebo: no control group received no treatment. Duration: 3 weeks. | |
| Outcomes | Miscarriage. | |
| Notes | After IVF or ICSI. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | The participants knew whether they were stopping the medication or not and the providers likely knew as well. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Unclear risk | The patient's other physician may have given progesterone later. These data are not included. |
| Methods | Unit of randomization: pregnancy. 64 women randomized, 0 women excluded, 64 women analyzed. Source of funding: Schering. | |
| Participants | Women who fell into 1 or more of the following criteria: Evidence of a viable fetus at 6 weeks of pregnancy was required to be enrolled in the trial. Age: not stated. Location: Netherlands. | |
| Interventions | 500 mg/week IM of hydroxyprogesterone caproate given IM. Placebo: yes. Duration: from 7 weeks' gestation to 12 weeks' gestation. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Sequentially coded ampules supplied by central location. |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Not specifically stated but likely blinded. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. 50 women randomized. Source of funding: preparations supplied by Schering. | |
| Participants | Women having had 2 or more consecutive abortions and who had low or falling pregnanediol levels. Exclusions: women with uterine malformations. Age: not stated. Location: London. | |
| Interventions | Up to 8 weeks' gestation ‐ 250 ml/week IM hydroxyprogesterone; Placebo: yes. | |
| Outcomes | Miscarriage. | |
| Notes | Source of funding: preparations supplied by Schering. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Low risk | Solution A and B ‐ the "correct identity of these substances is not known to any person taking part in this study." |
| Blinding of outcome assessment (detection bias) | Low risk | As above. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Low risk | None. |
| Methods | Unit of randomization: pregnancy. 113 women were enrolled, 0 women were excluded, 113 women were analyzed. Source of funding: not stated. | |
| Participants | Women having had 2 or more consecutive miscarriages before 12 weeks' gestation. Exclusions: women with any other known complicating factor (positive Wassermann reaction, extensive cervical tear, uterine malformation, associated medical disease etc). Age: not stated. Location: London. | |
| Interventions | 6 x 25 mg progesterone pellets inserted within the gluteal muscle either: Placebo: no but had a no‐treatment control group. Duration: unclear. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | High risk | There were 2 centers in this study. 1 allocated by alternation. It was stated in the paper that the other center used "randomisation". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | None. |
| Other bias | Unclear risk | Treatment duration not clearly stated. |
| Methods | Unit of randomization: pregnancy. Number of women randomized: 145. Source of funding: not stated. | |
| Participants | Women with threatened miscarriage up until 14 weeks' gestation. Age: not stated. Location: Italy. | |
| Interventions | Oral allylestrenol 10 mg/day Placebo: yes. Duration: 8 weeks. | |
| Outcomes | Miscarriage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Only states allocated "at random". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Not stated. |
| Other bias | Unclear risk | Not stated. Brief report in a letter. |
BiD: twice daily
CI: confidence interval
hCG: human chorionic gonadotropin
ICSI: intracytoplasmic sperm injection
IM: intramuscular
IU: international unit
IVF: in vitro fertilisation
TiDs: 3 times daily
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Outcomes of this study were not applicable to this review. Treatment was not given to women until 38 weeks' gestation. The outcome measured was time from onset of labour to delivery. | |
| No method of randomization was used for this study. | |
| No method of randomization was used for this study. | |
| It is unclear as to whether there was any randomization. The authors of this review attempted, but failed, to contact the trial authors. | |
| The intervention considered in this study is progesterone in association with immunotherapy rather than progesterone alone. | |
| In this study, progesterone was not taken during pregnancy. | |
| This study is not an RCT. | |
| This study was terminated before the results were of sufficient size to statistically analyze. Therefore, data are incomplete. | |
| The outcome measure of this study was preterm delivery rather than miscarriage. | |
| Comparison of stopping progestin at 12 weeks vs continuing. Wrong comparison. | |
| This study was excluded because progesterone was given after 20 weeks' gestation to prevent preterm labour. | |
| The active intervention given in this study is a combination of progesterone and oestrogen, rather than progesterone alone. | |
| This study is not an RCT. | |
| Wrong population and intervention. | |
| This study is not an RCT. | |
| This study compares intramuscular progesterone versus intravaginal progesterone in the luteal phase followed by all participants receiving progesterone and oestrogen on the day prior to ovocyte puncture. There is no placebo or 'no treatment' control and the treatment given after commencement of pregnancy is a combination of progesterone and oestrogen rather than progesterone alone. | |
| This trial was conducted to ascertain the efficacy of progesterone to prevent preterm birth rather than miscarriage. | |
| The outcome of this study is not relevant to the current systematic review. Progesterone was not given to prevent miscarriage and was not given until the 30/40. |
RCT: randomized controlled trial
VS: versus
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | PROMISE. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Habitual abortion study. |
| Methods | |
| Participants | Pregnant women with history of 3 idiopathic miscarriages. |
| Interventions | Oral dydrogesterone versus placebo. |
| Outcomes | Change in IFN/IL‐10 ratio. |
| Starting date | 2003 |
| Contact information | Gereon Raddatz, [email protected] |
| Notes |
| Trial name or title | The prevention of miscarriage study (PROMISE). |
| Methods | |
| Participants | Pregnant women with history of at least 3 spontaneous miscarriages with same partner and negative standard evaluation. |
| Interventions | 20 mg daily oral dydrogesterone versus placebo tablet. |
| Outcomes | Change in Interferon‐gamma/Interleukin‐10 ratio from baseline and 'pregnancy outcomes'. |
| Starting date | |
| Contact information | Dr. Katharina Walch, Vienna, Austria, [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Miscarriage (all trials) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| Analysis 1.1  Comparison 1 Progestogen versus placebo/no treatment, Outcome 1 Miscarriage (all trials). | ||||
| 2 Miscarriage (placebo controlled trials only) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| Analysis 1.2  Comparison 1 Progestogen versus placebo/no treatment, Outcome 2 Miscarriage (placebo controlled trials only). | ||||
| 2.1 Trials with placebo control group | 10 | 1028 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 2.2 Trials without placebo controls | 4 | 1130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.42, 1.02] |
| 3 Miscarriage (women with previous recurrent miscarriage only) Show forest plot | 10 | 1287 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| Analysis 1.3  Comparison 1 Progestogen versus placebo/no treatment, Outcome 3 Miscarriage (women with previous recurrent miscarriage only). | ||||
| 3.1 Women with a history of 3 or more prior miscarriages | 4 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 3.2 Women with a history of 2 or more prior miscarriage | 7 | 450 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 3.3 Women with unspecified prior miscarriages presenting with threatened miscarriage | 3 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.84, 1.63] |
| 4 Miscarriage (by route of administration versus placebo) Show forest plot | 11 | 1600 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.65, 1.17] |
| Analysis 1.4  Comparison 1 Progestogen versus placebo/no treatment, Outcome 4 Miscarriage (by route of administration versus placebo). | ||||
| 4.1 Oral versus placebo/control | 5 | 517 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.65, 1.40] |
| 4.2 Intramuscular versus placebo/control | 4 | 728 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.36, 1.68] |
| 4.3 Vaginal versus placebo/control | 2 | 355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.40, 1.35] |
| 5 Preterm birth Show forest plot | 7 | 946 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.67, 1.81] |
| Analysis 1.5  Comparison 1 Progestogen versus placebo/no treatment, Outcome 5 Preterm birth. | ||||
| 6 Neonatal death Show forest plot | 5 | 445 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.72, 2.64] |
| Analysis 1.6  Comparison 1 Progestogen versus placebo/no treatment, Outcome 6 Neonatal death. | ||||
| 7 Fetal genital abnormalities/virilisation Show forest plot | 4 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.64 [0.15, 385.21] |
| Analysis 1.7  Comparison 1 Progestogen versus placebo/no treatment, Outcome 7 Fetal genital abnormalities/virilisation. | ||||

Funnel plot of comparison: 1 Progestogen versus placebo/no treatment, outcome: 1.1 Miscarriage (all trials).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
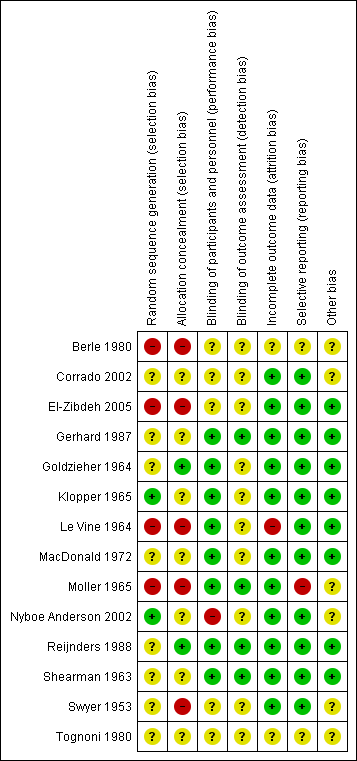
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 1 Miscarriage (all trials).
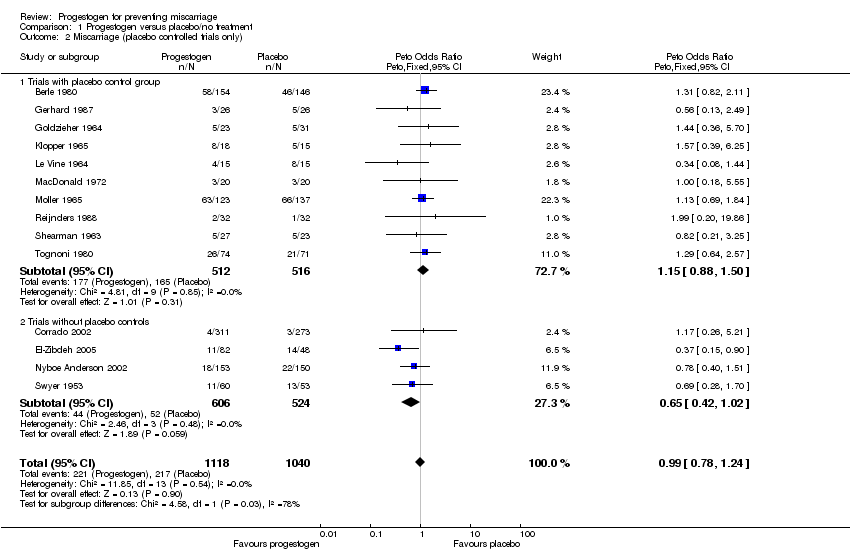
Comparison 1 Progestogen versus placebo/no treatment, Outcome 2 Miscarriage (placebo controlled trials only).

Comparison 1 Progestogen versus placebo/no treatment, Outcome 3 Miscarriage (women with previous recurrent miscarriage only).
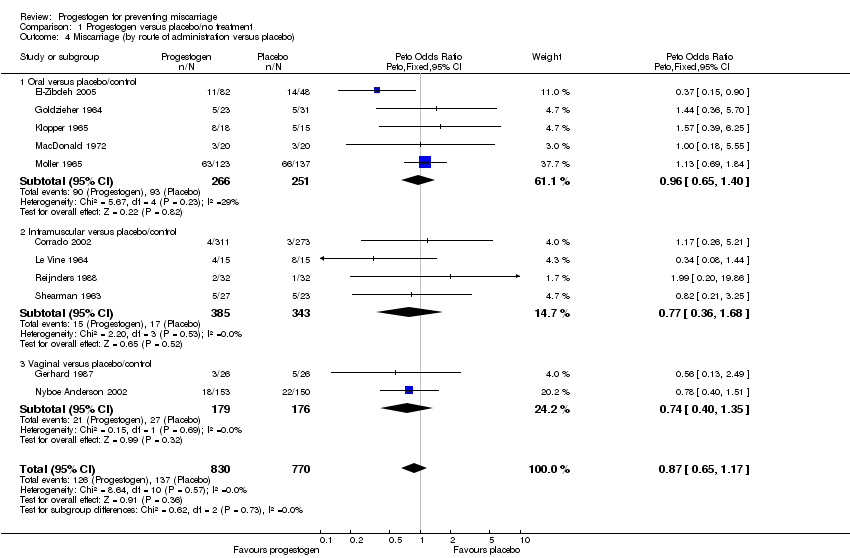
Comparison 1 Progestogen versus placebo/no treatment, Outcome 4 Miscarriage (by route of administration versus placebo).
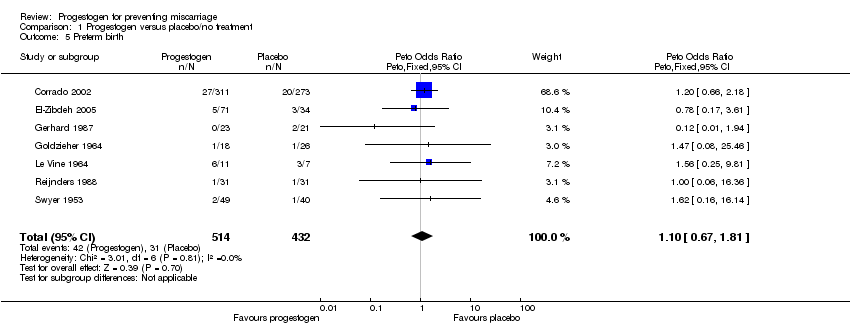
Comparison 1 Progestogen versus placebo/no treatment, Outcome 5 Preterm birth.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 6 Neonatal death.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 7 Fetal genital abnormalities/virilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Miscarriage (all trials) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| 2 Miscarriage (placebo controlled trials only) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| 2.1 Trials with placebo control group | 10 | 1028 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 2.2 Trials without placebo controls | 4 | 1130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.42, 1.02] |
| 3 Miscarriage (women with previous recurrent miscarriage only) Show forest plot | 10 | 1287 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 3.1 Women with a history of 3 or more prior miscarriages | 4 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 3.2 Women with a history of 2 or more prior miscarriage | 7 | 450 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 3.3 Women with unspecified prior miscarriages presenting with threatened miscarriage | 3 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.84, 1.63] |
| 4 Miscarriage (by route of administration versus placebo) Show forest plot | 11 | 1600 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.65, 1.17] |
| 4.1 Oral versus placebo/control | 5 | 517 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.65, 1.40] |
| 4.2 Intramuscular versus placebo/control | 4 | 728 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.36, 1.68] |
| 4.3 Vaginal versus placebo/control | 2 | 355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.40, 1.35] |
| 5 Preterm birth Show forest plot | 7 | 946 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.67, 1.81] |
| 6 Neonatal death Show forest plot | 5 | 445 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.72, 2.64] |
| 7 Fetal genital abnormalities/virilisation Show forest plot | 4 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.64 [0.15, 385.21] |

