Progestogen for preventing miscarriage
Información
- DOI:
- https://doi.org/10.1002/14651858.CD003511.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 octubre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
David Haas: is the guarantor of the review; prepared the 2013 update; performed independent data extraction and quality assessment of the included trials, and commented on all drafts of the review.
Patrick Ramsey: performed independent data extraction and quality assessment of included trials for the updates; commented on the drafts for this update.
Declarations of interest
None known related to the review topic. A portion of Dr Haas's time and effort are supported by a grant from the US National Institutes of Health Obstetric‐Fetal Pharmacology Units research network grant #5U10HD063094.
Acknowledgements
The review authors would like to thank Lynn Hampson for her search of the Cochrane Pregnancy and Childbirth Group's Trials Register.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Nov 20 | Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology | Review | David M Haas, Taylor J Hathaway, Patrick S Ramsey | |
| 2018 Oct 09 | Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology | Review | David M Haas, Taylor J Hathaway, Patrick S Ramsey | |
| 2013 Oct 31 | Progestogen for preventing miscarriage | Review | David M Haas, Patrick S Ramsey | |
| 2008 Apr 23 | Progestogen for preventing miscarriage | Review | David M Haas, Patrick S Ramsey | |
| 2003 Oct 20 | Progestogen for preventing miscarriage | Review | R M Oates‐Whitehead, David M Haas, J AK Carrier | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO

Funnel plot of comparison: 1 Progestogen versus placebo/no treatment, outcome: 1.1 Miscarriage (all trials).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
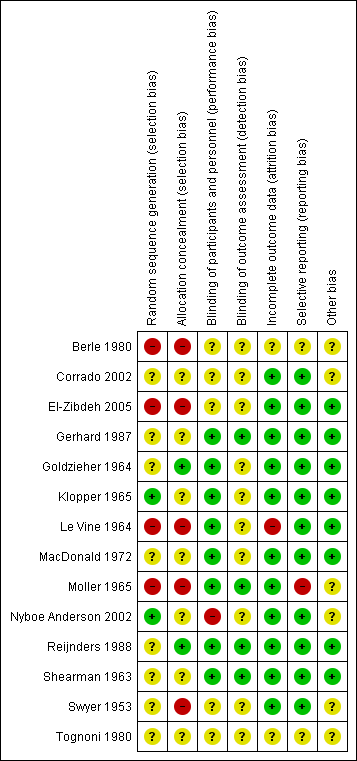
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 1 Miscarriage (all trials).
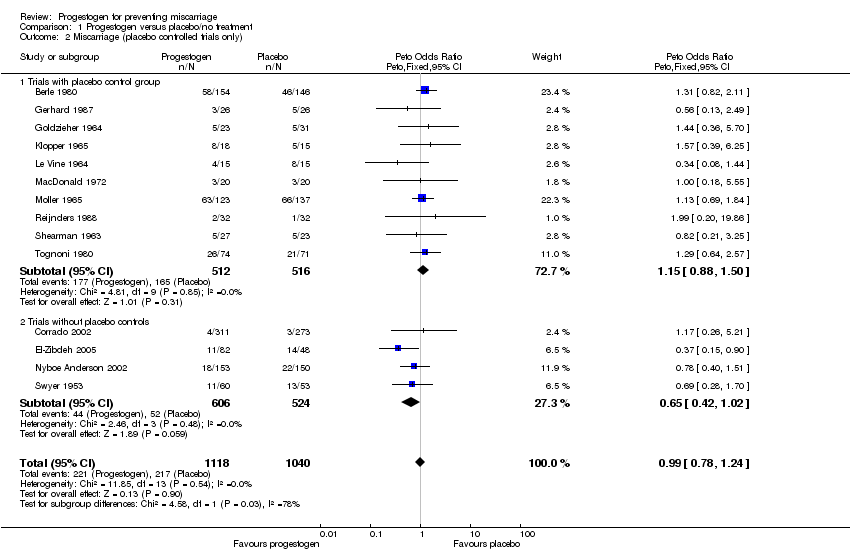
Comparison 1 Progestogen versus placebo/no treatment, Outcome 2 Miscarriage (placebo controlled trials only).

Comparison 1 Progestogen versus placebo/no treatment, Outcome 3 Miscarriage (women with previous recurrent miscarriage only).
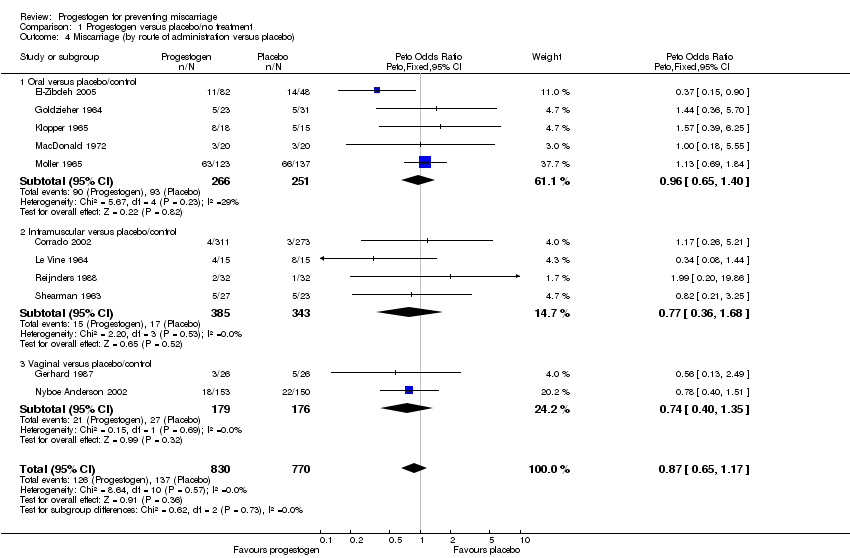
Comparison 1 Progestogen versus placebo/no treatment, Outcome 4 Miscarriage (by route of administration versus placebo).
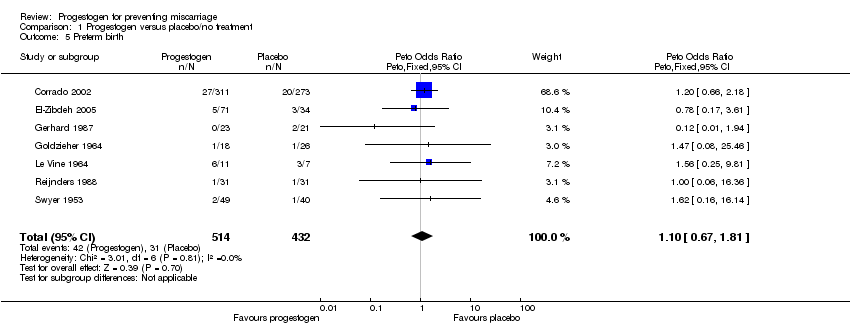
Comparison 1 Progestogen versus placebo/no treatment, Outcome 5 Preterm birth.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 6 Neonatal death.

Comparison 1 Progestogen versus placebo/no treatment, Outcome 7 Fetal genital abnormalities/virilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Miscarriage (all trials) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| 2 Miscarriage (placebo controlled trials only) Show forest plot | 14 | 2158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| 2.1 Trials with placebo control group | 10 | 1028 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 2.2 Trials without placebo controls | 4 | 1130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.42, 1.02] |
| 3 Miscarriage (women with previous recurrent miscarriage only) Show forest plot | 10 | 1287 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 3.1 Women with a history of 3 or more prior miscarriages | 4 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.21, 0.72] |
| 3.2 Women with a history of 2 or more prior miscarriage | 7 | 450 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 3.3 Women with unspecified prior miscarriages presenting with threatened miscarriage | 3 | 612 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.84, 1.63] |
| 4 Miscarriage (by route of administration versus placebo) Show forest plot | 11 | 1600 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.65, 1.17] |
| 4.1 Oral versus placebo/control | 5 | 517 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.65, 1.40] |
| 4.2 Intramuscular versus placebo/control | 4 | 728 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.36, 1.68] |
| 4.3 Vaginal versus placebo/control | 2 | 355 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.40, 1.35] |
| 5 Preterm birth Show forest plot | 7 | 946 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.67, 1.81] |
| 6 Neonatal death Show forest plot | 5 | 445 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.38 [0.72, 2.64] |
| 7 Fetal genital abnormalities/virilisation Show forest plot | 4 | 228 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.64 [0.15, 385.21] |

