Bifosfonatos y otros agentes óseos para el cáncer de mama
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Adjuvant zoledronic acid study. Open‐label, randomised, placebo‐controlled phase III trial | |
| Participants | N = 1803 women Baseline characteristics: similar between groups. Median age (45 years in both arms); > stage II (21.7% zoledronic acid, 21.2% no zoledronic acid), node‐positive (30.4% zoledronic acid, 30.4% no zoledronic acid); no women on adjuvant chemotherapy | |
| Interventions | 2 x 2 factorial design (randomised 1:1:1:1) Goserelin (3.6 mg monthly) plus either tamoxifen (20 mg daily) or anastrozole (1 mg daily) With or without zoledronic acid 4 mg every 6 months (protocol amended late 2000 from 8 mg to 4 mg every 6 months) | |
| Outcomes | Primary endpoint: DFS (local or regional recurrence, cancer in contralateral breast, distant metastasis, second primary carcinoma, or death from any cause) Secondary endpoints: RFS, OS, measures of BMD Exploratory endpoint: BMFS, safety | |
| Notes | Statistics: powered to detect a HR of 1.8 with 90% power and 95% confidence between tamoxifen and anastrozole. ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated adaptive randomisation method" |
| Allocation concealment (selection bias) | Low risk | "Assign treatment groups via an automated telephone service" |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Low risk | In this open‐label trial, no investigators, staff at participating centres, or participants were masked to treatment group; however, individuals analysing disease recurrence from laboratory results were masked to treatment group. All events underwent double central medical review with masked source data, and only histopathology reports or appropriate imaging were regarded as acceptable for confirmation of disease recurrence |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Multi‐centre, phase III, prospective, randomised, double‐blind, parallel assignment. Accrual from Dec 2006 to July 2013, 58 centres Austria/Sweden | |
| Participants | N = 3420 women Post‐menopausal women ≥ 45 years with EBC; ER and/or PgR positive; currently on or will commence non‐steroidal aromatase inhibitor (anastrozole, letrozole) Baseline characteristics: similar between groups. Mean tumour size: 3.81 cm zoledronic acid group; 3.56 cm control group | |
| Interventions | Denosumab 60 mg (n = 1711) or placebo (n = 1709) subcutaneously every 6 months. | |
| Outcomes | Primary endpoint: time to first clinical fracture Secondary endpoints: incidence of new fractures, BMD changes, DFS, BMFS, OS | |
| Notes | clinicaltrials.gov/ct2/show/NCT00556374 The primary endpoint was time from randomisation to first clinical fracture, analysed by ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly permuted block design with block sizes 2 and 4, stratified by type of hospital regarding Hologic device for DXA scans, previous aromatase inhibitor use, and baseline bone mineral density" |
| Allocation concealment (selection bias) | Low risk | "assigned by an interactive voice response system" |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients, treating physicians, investigators, data managers, and all study personnel were masked to treatment allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | "Clinical follow‐up, including fracture assessment and other diagnostic restaging procedures when indicated, was done at least every 6 months until the primary analysis data cutoff date on March 26, 2014, and annually thereafter. Patients remained on trial medication until up to 6 months after the primary analysis data cutoff date was reached. The assessments of the patients and the recording of adverse events followed the protocol‐defined regular schedule" |
| Incomplete outcome data (attrition bias) | Low risk | Good compliance, low numbers lost to follow‐up and ITT analysis Denosumab: 95% (per protocol N = 1636/ ITT N = 1711) Placebo: 96% (per protocol N = 1646 / ITT N = 1709) DFS and OS data immature, follow‐up ongoing |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were addressed |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | (Neo)adjuvant zoledronic acid study. Randomised, open‐label trial. Patients from Siteman Cancer Center, USA (2003‐2006) | |
| Participants | N = 120 | |
| Interventions | 4 mg zoledronate every 3 weeks for 1 year (commencing with first dose of chemotherapy) or open‐label control | |
| Outcomes | Primary endpoint: DTC in bone marrow at baseline and 3 months. DTCs were measured by bone marrow collection from each anterior iliac crest. It was defined as anti‐pan‐cytokeratin (CK) antibody‐positive, morphologically consistent cells as viewed by two independent pathologists Secondary endpoints: bone‐turnover markers, measured at baseline, 3 months and 12 months; BMD, measured at baseline and 12 months | |
| Notes | Statistics: the study was designed with > 80% power and 0.05 significance level to detect a 20% to 26% difference in DTCs at baseline compared to 3 months Both DFS and OS categorical event data not published and cannot be extracted from either 2‐year (Aft 2010) or 5‐year (Aft 2012) follow‐up publications. Study authors contacted to provide data. Trialists kindly provided unpublished trial data to the Cochrane Review team. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation by formal probability model and implemented with SAS process plan generated by statistician |
| Allocation concealment (selection bias) | Low risk | Allocation placed in sequentially numbered, opaque envelopes in locked cabinet, only accessible to study's patient co‐ordinator after enrolment |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label thus no blinding to participant |
| Blinding of outcome assessment (detection bias) | Low risk | "the interpreting pathologists were masked to study group". So, the primary endpoint was measured with blinding and minimised detection bias. No mention of blinding of radiology assessments, which were secondary outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | At 3 months, 109 of the 118 participants (92.3%) were assessable for DTCs, which is the primary endpoint. At 12 months, only 79 participants (67%) were assessable for DTCs. A negative outcome was assigned to participants with missing data points. For all other outcomes of interest in this review, there were no significant differences in attrition between the groups and reasons for any withdrawal were provided. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were addressed. RFS data were also included |
| Other bias | Unclear risk | DTCs is a difficult endpoint, which may or may not correlate directly with clinically evident bone metastases. It was therefore not included in the formal meta‐analysis |
| Methods | Pooled updated report (2000) from 2 prospective, multicentre, randomised, double‐blind, placebo‐controlled studies | |
| Participants | N = 751 Women with stage IV BC and osteolytic bone metastases | |
| Interventions | 2‐h infusion of iv pamidronate 90 mg or placebo every 3‐4 weeks for up to 24 cycles | |
| Outcomes | Skeletal morbidity rate (events/year), bone pain, analgesic use, QoL (Spitzer scale), ECOG performance status, bone biochemical markers, time to first skeletal complication and survival. Skeletal complications are defined as radiation to bone, pathological fractures, surgery to bone, spinal cord compression or hypercalcaemia | |
| Notes | AREDIA Protocol 18 (n = 372) published separately in Theriault 1999. Aredia Protocol 19 (n = 382), two‐year results, was published separately in June 1998 by Hortobagyi in Journal of Clinical Oncology. Pooled analysis performed after testing for heterogeneity between studies 18 and 19 (or for having a SRE) using Breslow Day Test indicated homogeneity (P = 0.19) Analysis by ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assigned randomly in equal numbers with computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (patients and study personnel): "infusions were prepared by the study pharmacist at each site according to a site specific, computer‐generated randomisation list" |
| Blinding of outcome assessment (detection bias) | Low risk | Other study personnel, as well as the patients and investigators, remained unaware of the treatment assigned. Double‐blind study drug administration was continued throughout the entire course of the study for each participant. The radiologic bone surveys were reviewed by a central radiologist who was unaware of the treatment assignment of individual participants. |
| Incomplete outcome data (attrition bias) | Low risk | Only 115 of 367 participants (31.1%) in the pamidronate group and 100 of 387 participants (25.8%) in the placebo group completed 24 months of study ITT analysis was performed for the entire randomised population. All participants were included in the survival and safety analyses. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were addressed |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | AZURE 2014 (BIG 01/04), adjuvant zoledronic acid study. Academic study run by the National Institute for Health Research National Cancer Research Network (NIHR NCRN) in the UK, involving 174 participating centres (UK, Ireland, Australia, Spain, Portugal, Thailand and Taiwan) | |
| Participants | N = 3360 Women with resected stage II/III BC. 205 women with T3/4 disease or N1 disease who were undergoing neoadjuvant chemotherapy were recruited to the neoadjuvant arm study. Baseline characteristics: similar between groups. T3/4 (17.1% zoledronic acid, 17% no zoledronic acid); N2/3 (36.2% zoledronic acid, 35.9% no zoledronic acid); endocrine therapy alone (4.5% both arms), chemotherapy alone (21.5% both arms). Endocrine plus chemotherapy (73.9% zoledronic acid, 74.1% no zoledronic acid) | |
| Interventions | Randomised to receive systemic adjuvant therapy +/‐ intervention: concurrent zoledronic acid iv over 15 min every 3‐4 weeks for 6 doses, every 3 months for 8 doses, then every 6 months for 5 doses or no zoledronic acid, for the duration of 5 years. Participants on the neoadjuvant arm sub‐study were randomised to standard neoadjuvant chemotherapy +/‐ zoledronic acid 4 mg every 3‐4 weeks for 6 doses. Postoperatively, participants randomised to active arm continued on zoledronic acid every 3 months for 8 doses then 6 months for 5 doses | |
| Outcomes | Primary endpoint: DFS (chest wall recurrence + regional recurrence + distant recurrence + death without recurrence) Secondary endpoints: invasive DFS, OS, BMFS, safety, translational endpoints | |
| Notes | Statistics: statistically powered (20% beta and 5% alpha) to detect a 17% reduction in DFS with a lower boundary of efficacy of 0.833 and upper boundary of lack of efficacy of 0.936. ITT analysis. Follow‐up: 59 months (Coleman 2010; see AZURE 2014). Trialists also kindly provided unpublished trial data to the Cochrane Review team. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation method |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | The primary end point of the study was DSF. The secondary end point was OS. Unlikely to be affected by bias. The follow‐up schedule for both the zoledronic acid group and the control group included clinical assessment, physical examination, monitoring for adverse events, and measurement of hematologic, renal, and hepatic function. Investigations for possible recurrence were clinically directed as deemed appropriate by the treating physician. Routine follow‐up imaging was not mandated. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Only 2/681 participants (0.1%) in the zoledronate group had missing data |
| Selective reporting (reporting bias) | Low risk | All major endpoints addressed. Translational endpoint not yet reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Double‐blind, placebo‐controlled study | |
| Participants | N = 466 | |
| Interventions | iv ibandronate: 2 mg injection or 6 mg by 1‐2 hr infusion or placebo injections or infusions monthly for up to 2 years | |
| Outcomes | Bone events: pathological fractures, hypercalcaemic episodes, bone complications requiring radiotherapy or surgery. Average SREs per person, time to first SRE, proportion of participants experiencing ≥ 1 SRE, time periods without SRE, QoL assessed using EORTC‐QLQ‐30 scale, bone pain assessed using a 5‐point scale, survival | |
| Notes | Event rate results expressed as events per patient year. Results are from abstract presentation (Body 1999). Updated complete study is in preparation for publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups so randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded to placebo and ibandronate but not between 2 mg and 6 mg |
| Blinding of outcome assessment (detection bias) | Unclear risk | The primary outcome was number of 12‐week periods with new bone complications and secondary outcomes were bone pain, analgesic use and safety. No mention of blinding of investigators when assessing vertebral fractures on radiographs |
| Incomplete outcome data (attrition bias) | Low risk | Primary and secondary efficacy analyses were conducted using ITT population. Adverse events, death and refusal of treatment were the main reasons for withdrawals but these were similar across groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Pooled results from 2 double‐blind, placebo‐controlled studies (MF4414 and MF4434) | |
| Participants | N = 564 Patients with BCBM | |
| Interventions | Oral ibandronate 50 mg or ibandronate 20 mg or placebo for up to 96 weeks. Only ibandronate 50 mg and placebo data were reported. The original design included 20 mg and 50 mg oral ibandronate arms. The pooled data on the 50 mg and placebo arms has been published in full. Earlier reports had indicated superiority in the 50 mg ibandronate arm, making it the recommended dose for clinical use | |
| Outcomes | Skeletal morbidity period rate (vertebral fractures, non vertebral fractures, irradiation to bone, surgery to bone) in aggregate and for each component evaluated by skeletal morbidity period rate, bone pain, QoL assessed using EORTC‐QLQ‐30 | |
| Notes | The primary study endpoint was skeletal morbidity period rate, which was the number of 12‐week periods with new skeletal complications, divided by the total observation time in periods. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Most frequent reasons for withdrawals were reported for both groups and the percentages of withdrawals were similar across both groups |
| Selective reporting (reporting bias) | High risk | Ibandronate 20 mg data were not reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, phase III study Baseline characteristics of the 2 groups were comparable | |
| Participants | N = 1822 participants (breast n = 833, prostate n = 674, myeloma n = 270 and other n = 45) | |
| Interventions | Zoledronic acid iv 4 mg every 4 weeks for up to 2 years or zoledronic acid iv 4 mg every 12 weeks for up to 2 years | |
| Outcomes | Primary: proportion of participants with ≥ 1 SRE within 2 years after randomisation Secondary: pain assessment (Brief Pain Inventory), ECOG status, ONJ, renal toxicity, skeletal morbidity rate, bone turnover assessed by serum N‐telopeptide (NTX), proportion of participants having ≥ one SRE within 24 months after randomisation for the subgroup of participants with BC, prostate cancer and multiple myeloma | |
| Notes | clinicaltrials.gov/ct2/show/NCT00869206 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation, parallel assignment". Baseline characteristics were comparable between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | The abstract states that 833 participants were included but reports data only on 820 participants. No further details provided |
| Selective reporting (reporting bias) | Unclear risk | The conference abstract reports most of the outcomes per the clinical trials registry record except for pain intensity score, ECOG performance status and skeletal morbidity rate. |
| Other bias | Unclear risk | No information, information only available in abstract form |
| Methods | Open‐label, randomised, controlled study | |
| Participants | N = 295 | |
| Interventions | Chemotherapy or chemotherapy and iv pamidronate 45 mg every 3 weeks until progressive disease in bone | |
| Outcomes | Time to progressive bone disease, bone pain, complications of bone metastases (hypercalcaemia, pathological fractures, episodes of radiotherapy or surgery), sclerotic response, analgesic use, response of extraskeletal metastases, WHO performance status | |
| Notes | A blinded, extra‐mural review was undertaken in each country. The results at extra mural review were referred to in this review. 83 participants included in efficacy analysis by ITT. 224 of these assessed at extra‐mural review. 268 participants had baseline pain scores. All 295 evaluated for survival | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | Participants remained in the active phase of the trial until they developed progressive disease in bone on radiograph and/or bone scan. Progressive disease in bone was diagnosed by a designated trial radiologist at each centre who was unaware of the participant's treatment. Participants were also discontinued if they developed a calcium level > 2.75 mmol/L that required specific therapy, or if they received corticosteroids for > 3 weeks. |
| Incomplete outcome data (attrition bias) | High risk | 12 participants excluded from the efficacy analysis (6 per arm), ITT analysis was "not feasible for these patients" as no imaging (6), no bone metastases at external review (2), baseline X‐rays performed 2 months prior to randomisation (2), no treatment (1) and pamidronate given for hypercalcaemia (1) |
| Selective reporting (reporting bias) | Low risk | All endpoints addressed |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Adjuvant study. Randomised, non‐placebo‐controlled study. Single‐institution study (University Hospital Heidelberg 1990‐1995) | |
| Participants | N = 302 T1‐T4, N0‐2 primary BC with positive immunocytochemical detection of tumour cells in bone marrow. Baseline characteristics: similar between groups. T3 and T4 (17% clodronate, 16% no clodronate), node‐positive disease (51% clodronate, 54% no clodronate), endocrine therapy alone (41% clodronate, 38% no clodronate), chemotherapy alone (25% clodronate, 28% no clodronate), combination therapy (16% clodronate, 15% no clodronate) | |
| Interventions | Oral clodronate 1600 mg orally/d for 2 years or no clodronate. Adjuvant systemic therapy based on German Adjuvant Breast Cancer Group/St Gallen Consensus Conference guidelines | |
| Outcomes | Primary endpoints: incidence of distant metastases: bone and visceral Secondary endpoints: length of time to bone and visceral metastases, OS | |
| Notes | Statistics: study was powered to detect 10% difference between study groups Follow‐up: examination every 3‐4 months during the 2‐year period. Chest radiographs, bone scans, liver ultrasound and mammography carried out annually. ITT analysis. Median follow‐up of 8.5 years (Diel 2008) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open label study |
| Blinding of outcome assessment (detection bias) | Low risk | The pattern of metastasis was analysed at the end of the study. Bone lesions seen on radiographs were assessed by 2 independent radiologists |
| Incomplete outcome data (attrition bias) | Low risk | 3/145 participants in the control group and 15/157 participants in the clodronate group were excluded with reasons provided. All participants were included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | All endpoints reported; 3rd analysis to date (103 months' follow‐up) which includes 290 of the original 302 participants |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, open‐label, multicenter comparison study | |
| Participants | N = 361 Women with BC with osteolytic bone metastases | |
| Interventions | Clodronate 2400 mg/d orally or 900 mg clodronate iv every 3 weeks or 60 mg pamidronate iv every 3 weeks, over 2 years | |
| Outcomes | Skeletal complications: vertebral fractures, pain; adverse events | |
| Notes | The intervention was in addition to the participant's usual cytotoxic regimen. Results presented in abstract form only (Diel 1999). 318 participants evaluated after a median follow‐up of 18 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". No baseline characteristics information given in the abstract |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given about outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information, information only available in abstract form |
| Methods | Phase III, randomised, 1:1 open‐label study | |
| Participants | N = 527 Postmenopausal women with early‐stage (surgically resectable stage I, II, or IIIa) ER and/or PgR receptor–positive BC as well as baseline LS and TH T scores of 2.0 or greater, who had been on adjuvant letrozole 2.5 mg daily for 5 years No baseline characteristics were reported, except for no adjuvant chemotherapy (47.5% upfront group, versus 47.0% delayed group) | |
| Interventions | Upfront: zoledronic acid 4 mg every 6 months for 5 years Delayed start: triggered by post‐baseline LS or TH T score decreased to < ‐2.0; any clinical, nontraumatic fracture; or asymptomatic vertebral fracture identified at 36 months), zoledronic acid 4 mg every 6 months for 5 years | |
| Outcomes | Primary endpoint: percentage change in LS BMD at 12 months | |
| Notes | Statistics: this was predominantly a BMD study with disease recurrence as one of its pre‐specified endpoints. 12‐month follow‐up reported. ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised, using an interactive voice‐response system, to either immediate zoledronate, which was initiated along with adjuvant letrozole, or to delayed zoledronate, to be initiated only after 1 of the following events was reported" |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system used |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given about outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis (Coleman 2009). Similar drop‐out rates across groups at 12 months with reasons provided. Immediate zoledronate acid: 13.5%; delayed zoledronate acid: 12.6% |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, placebo‐controlled study | |
| Participants | N = 34 Women with BC with osteolytic bone metastases | |
| Interventions | Oral clodronate (Cl2MDP) 1600 mg daily for 1 year or oral placebo | |
| Outcomes | Bone mineralisation, hypercalcaemia, incidence of new bone metastases, fractures | |
| Notes | Basic cancer therapy consisted of tamoxifen in all participants. Chemotherapy was added during the course of the trial in 16/17 participants per arm for progressive disease. Initial findings were reported in Elomaa 1983. Updated reports were in Elomma 1987 and Elomaa 1988 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated". No information was given about baseline characteristics |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Single‐blinded: "placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given about outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information. Endpoints were not specified |
| Other bias | Unclear risk | Unclear |
| Methods | Phase II trial of denosumab in people with bone metastases from BC, prostate cancer and multiple myeloma. Second‐line trial. Bone marker study. 26 centres in Europe and North America. Open‐label trial | |
| Participants | N = 111 (N = 46 for BC subgroup) Patients with BC, prostate cancer with bone metastases and multiple myeloma, who had high urinary N‐telopeptide (uNTx) (> 50 nM) despite iv bisphosphonate treatment > 8 weeks. Exclusion criteria included > 2 SRE, radiotherapy to bones within 2 weeks, radioisotopes to bones within 8 weeks, unresolved toxicities (> grade 2) | |
| Interventions | iv bisphosphonates every 4 weeks x 6 (clinician's choice: zoledronic acid or pamidronate) or sc injections of denosumab 180 mg every 4 weeks or denosumab 180 mg every 12 weeks for 25 weeks | |
| Outcomes | Primary endpoint: proportion of participants with uNTx < 50 nM at week 13 Secondary endpoints: proportion of participants with uNTx < 50 nM at week 25, time to reduction of uNTx to < 50; duration of uNTx < 50; percent change of serum C‐telopeptide (sCTx) from baseline to week 25, percent change of uNTx from baseline to week 25, incidence of hypercalcaemia; proportion of participants experiencing SREs, and the time to first on‐study SRE, exploratory biomarker measurement | |
| Notes | Unpublished data of SRE endpoint from BC subgroup only was supplied by Amgen Pharmaceuticals, which enabled this study to be included and analysed Follow‐up of 57 weeks (2 years' follow‐up for optional ongoing extension phase study) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | Primary outcome was biochemical analysis, unlikely to be affected by knowledge of treatment group |
| Incomplete outcome data (attrition bias) | High risk | 2 participants did not receive bisphosphonates, and 1 participant did not receive denosumab. 4 participants in denosumab group did not have uNTx measurement post‐baseline. These were not included in final efficacy analysis (non‐ITT analysis) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Phase III, open‐label, 2 x 2 factorial design | |
| Participants | N = 2994 BC, N1‐2, M0, post‐surgery | |
| Interventions | Randomisation A: (A1) sequential epirubicin‐taxol‐cyclophosphamide or (A2) epirubicin‐cyclophosphamide Taxol‐Xeloda Randomisation B: (B1) ibandronate 50 mg/d for 2 years or (B2) no ibandronate | |
| Outcomes | Primary endpoint: DFS (A1 versus A2, B1 versus B2) Secondary endpoint: OS, event‐free survival in hormone sensitive/insensitive subgroups and N0, compliance, safety (A1 versus A2, B1 versus B2), rate of responders, incidence of primary (A1 versus A2), prognostic markers | |
| Notes | Trialists kindly provided unpublished data on study outcomes by menopausal status. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated permutated block randomisation" p3535 2:1 randomisation ibandronate (n = 2015) to observation (n = 1008) |
| Allocation concealment (selection bias) | Low risk | "Eligibility was centrally confirmed ... computer‐generated permutated block randomization" p.3535 |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given about outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Modified ITT analysis. Analysis conducted on those commencing study treatment. Very small number of participants did not commence treatment & were excluded from ITT analysis (ibandronate 19/2015 = 0.9%; observation 10/1008 = 1%) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes of ibandronate analysis reported. Analysis relating to randomisation A to be reported in companion paper. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | N = 150 BCBM Baseline characteristics: only demographics described. No comparison of baseline characteristics between treatment and control arms | |
| Interventions | 6 mg iv ibandronate or placebo every 4 weeks for 24 months | |
| Outcomes | Primary endpoint: proportion of participants with SRE (defined as pathologic fracture, spinal cord compression, radiation therapy to bone, change in anti‐neoplastic therapy and surgery to bone) Secondary endpoints: time to first SRE, skeletal morbidity rate (events/year) and time to progression of bone lesions. | |
| Notes | Statistics: limited information about power and target HR. Alpha value of 5% was taken for consideration of statistical significance Other treatment and follow‐up: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Baseline characteristics restricted to description of demographics between treatment arms only |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were probably blinded. Primary efficacy end point was the proportion of participants with SREs, which were defined as pathologic fracture, spinal cord compression, radiation therapy to bone, change in anti‐neoplastic therapy and surgery to bone |
| Incomplete outcome data (attrition bias) | Unclear risk | No information about missing data or ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified endpoints were reported. However, safety was only briefly described without the complete list of AEs |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Phase III, randomised, double‐blind, multicentre trial | |
| Participants | N = 114 Pre‐menopausal early BC women on adjuvant chemotherapy Baseline characteristics: majority stage II patients (slightly more stage I in placebo group 38% versus 29%, more stage II in treatment group 67% versus 54%), 66% hormone receptor‐positive | |
| Interventions | iv zoledronic acid 4 mg every 3 months or placebo for 12 months Other treatment: 80% on chemotherapy, 60% on tamoxifen, 26% on aromatase inhibitors. All participants on calcium and vitamin D | |
| Outcomes | Primary endpoint: LS BMD at 24 and 52 weeks after initiation of chemotherapy Secondary endpoints: Percentage change in TH and femoral neck BMD, changes in CTX (serum C‐telopeptide of type I collagen, a marker of bone resorption) and BSAP (bone‐specific alkaline phosphatase, a marker of bone formation) at 24 and 52 weeks | |
| Notes | Statistics: study was 90% powered (5% alpha) to detect a difference of 3% change in LS BMD Per‐protocol analysis (114 randomised, 85 completed 12‐month evaluation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permutated block |
| Allocation concealment (selection bias) | Low risk | Central site enrolment |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Reasons were provided for participants who withdrew from the study and were generally similar across groups |
| Selective reporting (reporting bias) | High risk | All pre‐specified endpoints were addressed. However, recurrence was not actually an endpoint but was mentioned. Since recurrence was mentioned in the manuscript, we included this study. However, it was unlikely that the study was powered to detect difference in recurrence between arms. |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, placebo‐controlled multi‐centre study | |
| Participants | N = 404 Women with BC with skeletal metastases and expected survival > 3 months | |
| Interventions | iv pamidronate 60 mg every 3‐4 weeks up to 2 years or iv placebo | |
| Outcomes | SREs (symptoms e.g. pain, hypercalcaemia, fractures, radiotherapy, surgery, change in antitumoural therapy) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permutated blocks of 6" |
| Allocation concealment (selection bias) | Low risk | "Numbered packages" |
| Blinding of participants and personnel (performance bias) | Low risk | "The packages are delivered to hospital pharmacy with package number and patient identification to the study centre". All pharmacy staff, nurses, physicians and patients were blinded to treatment. Blinded treatment was not uncoded at treatment discontinuation unless the SAE was reported" |
| Blinding of outcome assessment (detection bias) | Low risk | The incidence of skeletal symptoms events (e.g. fractures, hypercalcaemia) was recorded every 3 months but the article did not describe by whom. The article also described "all medication was also recorded by a nurse" |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were analysed. No missing outcome data reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, double‐blind, placebo‐controlled study | |
| Participants | N = 133 Women with recurrent BC but no skeletal metastases | |
| Interventions | Oral clodronate 1600 mg daily for 3 years or identical oral placebo | |
| Outcomes | Incidence of skeletal metastases, complications of skeletal metastases e.g. hypercalcaemia, bone pain, fractures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated". Baseline characteristics were similar between groups so randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Low risk | Randomisation was controlled at an independent centre, pre‐randomisation numbering system |
| Blinding of participants and personnel (performance bias) | Low risk | Single‐blinded with an identical placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Bone scintigraphy and skeletal X‐rays (hands, pelvis, skull, lateral lumbar, and thoracic spine) were obtained at 6‐month intervals and read blindly |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were analysed. "No significant difference in the number of patients withdrawn from the study" between groups (p. 664) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study. | |
| Participants | N = 228 Japanese women with stage IV BC with ≥ 1 osteolytic bone metastasis | |
| Interventions | iv zoledronic acid (4 mg) or placebo every 4 weeks for 12 months | |
| Outcomes | SREs, incidence, rate, time‐to‐event; toxicity and pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised with dynamic balancing method" |
| Allocation concealment (selection bias) | Low risk | Registered by facsimile and verified by central office, which then contacted the individual centre |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | All radiologic assessments, including vertebral fractures, were conducted by a blinded radiographic assessment committee |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were analysed except for 1 participant in the placebo group (with reason provided) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Prospective, randomised, controlled, open‐label study | |
| Participants | N = 100 BCBM | |
| Interventions | Oral clodronate 800 mg twice/d for 2 years or open control The dose of clodronate was increased to 1600 mg twice/d at first progression in bone and therapy was stopped if subsequent bone progression occurred. The intervention was in addition to underlying systemic treatment for BC: chemotherapy, endocrine therapy or both | |
| Outcomes | SREs (hypercalcaemia, fractures, radiotherapy), pain, QoL. QoL was assessed using the EORTC‐QLQ‐C30 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (blocks of 10) by computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open control |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were analysed except for 1 participant who was excluded from the statistical analysis because the diagnosis of bone metastases remained unsettled |
| Selective reporting (reporting bias) | Low risk | All pre‐specified endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Open‐label, randomised, controlled study. Participants recruited from Denmark, Sweden and Iceland from 1990‐1996 | |
| Participants | N = 953 Women with resectable adenocarcinoma of the breast and without distant metastases, in 3 groups: pre‐menopausal women with grade II/III malignancy, without lymph node metastases and primary tumour ≤ 5 cm, independent of hormone receptor status pre‐menopausal women with axillary lymph node metastases or primary tumour > 5 cm, with negative hormone receptor status post‐menopausal women with axillary lymph node metastases or primary tumour > 5 cm, with negative hormone receptor status Baseline characteristics: similar between treatment arms. > 70% axillary lymph node with metastases; > 20 mm: 57% pamidronate, 57% control; Grade 3: 37% pamidronate, 39% control; ER‐positive: 13% pamidronate, 17% control Other treatment: adjuvant chemotherapy (cyclophosphamide, methotrexate and 5‐fluouracil (CMF), or cyclophosphamide, epirubicin and 5‐fluouracil (CEF)). Loco‐regional radiotherapy as per local guidelines. Endocrine therapy was to be avoided. | |
| Interventions | Oral pamidronate 150 mg twice/d for 4 years or no adjuvant therapy | |
| Outcomes | Endpoints: SREs, safety, BMD, survival | |
| Notes | Statistics: alpha, beta values and expected HRs were not specified. Multivariate analyses were performed between the 3 groups, tumour size, nodal status, type of surgery, histological type and grade, hormone receptor status, centre and treatment regimen Follow‐up: for the first year, every 12 weeks a clinical visit. For years 2‐5, every 6 months a clinical visit. For years 6‐10, an annual visit. Routine biochemistry was measured at each treatment, at 24 and 48 weeks, then twice/year for 3 years. Pelvic and spinal X‐rays were performed every 6 months and bone scans every year for 4 years. BMD was measured in a Swedish subgroup. 10 years of follow‐up. Categorical DFS and OS outcome data not published. Study authors contacted for data, including by menopausal status. Trialists kindly provided unpublished trial data to the Cochrane Review team. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open control |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | In the pamidronate arm, 460 allocated with pamidronate, 450 assessed for bone recurrence, 337 assessed for fractures, all participants assessed for OS (417 treated as per protocol). In the control arm, 493 allocated with pamidronate, 469 assessed for bone recurrence, 365 assessed for fractures, all participants assessed for OS (467 treated as per protocol). Ten participants from pamidronate arm and 14 from control arm were lost to follow‐up (˜3%). ITT analysis was performed and the results were similar to adjusted‐for‐protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary endpoints were not specified but both effects (recurrence, fracture, survival) and side‐effects were reported |
| Other bias | High risk | Participants were not allowed to be on endocrine therapy. However, 17% of participants in control arm versus 13% in pamidronate arm were ER‐positive. This may potentially bias results against the control arm since these participants were not treated optimally |
| Methods | Double‐blinded, active‐controlled, randomised phase II trial. International trial with 56 centres involved in Europe, North America and Australia | |
| Participants | N = 255 Women with BCBM, ECOG 0‐2 Baseline characteristics: overall balanced between the 6 arms. Higher rate of no SRE in the arm with 180 mg every 12 weeks denosumab (80%), although there was no difference in the rate of SRE between bisphosphonate and total denosumab (65% versus 66%) | |
| Interventions | Randomised 1:6 ratio to receive sc injection of denosumab (30 mg, 120 mg, 180 mg) or every 12 weeks (60 mg, 180 mg), or open‐label iv bisphosphonate (zoledronic acid, pamidronate or ibandronate) every 4 weeks During the 32‐week off‐treatment period, participants could choose to receive iv bisphosphonate, which was considered standard of care therapy | |
| Outcomes | Primary endpoint: percentage change of week 13 urinary NTx/Cr ratio from baseline Secondary endpoints: percentage change of week 26 urinary NTx/ Cr ratio from baseline, proportion of participants with > 65% reduction of NTx/Cr from baseline, median time to achieve this reduction, percentage of participants experiencing on‐study SRE (defined as fracture, surgery or radiation to bone, or spinal cord compression), safety | |
| Notes | Statistics: powered to detect a +/‐ 5.1% difference in primary endpoint with 95% CI Follow‐up: throughout the treatment period, serum chemistries and denosumab concentrations, urinary NTx/Cr levels were measured periodically. Off‐treatment, there were 4 visits for assessment of NTx/Cr level and safety. Total follow‐up period of 57 weeks (25 weeks of treatment and 32 weeks of follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "Partially double‐blind". Participants received either sc denosumab and placebo to maintain blinding to the dose, or iv infusion of bisphosphonate |
| Blinding of outcome assessment (detection bias) | Low risk | The primary end point of the study, reported previously, was the percentage change from baseline to week 13 in uNTx/Cr (16). Additional efficacy end points were the percentage change from baseline to week 25 in uNTx/Cr, the proportion of patients who achieved a >65% reduction in uNTx/Cr from baseline, and the median time to achieve this reduction. The percentage of patients experiencing an SRE (fracture, surgery or radiation to bone, or spinal cord compression) while on the study was also evaluated. No mention of blinding of outcome assessment, but unlikely to influence outcome assessment |
| Incomplete outcome data (attrition bias) | Unclear risk | 12% of iv bisphosphonate group and 6% of denosumab group discontinued their trial by week 13 analysis of primary endpoint; 30% of iv bisphosphonate group and 33% of denosumab group did not continue to week 57 final assessment. Neither CONSORT diagram nor explicit information about how missing data was addressed were available |
| Selective reporting (reporting bias) | Low risk | All the bone marker endpoints, SRE and safety parameters were reported |
| Other bias | Unclear risk | This was akin to a dose‐finding extended phase Ib/II trial. Whilst the primary endpoint urinary NTx/Cr at 13 weeks was reported separately for bisphosphonate and each of the 5 doses of denosumab, the secondary SRE endpoint was reported in aggregate (bisphosphonate vs all doses for denosumab). The standard dose of denosumab was now recognised at 120 mg monthly. It was difficult to know from this trial the true effect of standard‐dose denosumab against zoledronic acid |
| Methods | Randomised, placebo‐controlled trial | |
| Participants | N = 73 Women with BC with previously untreated locally advanced disease or metastases but no bone or central nervous system metastases 90% had stage III disease | |
| Interventions | Oral clodronate 800 mg twice/d or placebo for 2 years | |
| Outcomes | Incidence of bone and visceral metastases, time to progression, survival | |
| Notes | 10 participants not evaluable because of treatment < 2 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind with placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind. Bone scans were taken every 6 months or earlier if the participant was symptomatic. The outcomes were bone metastases, visceral metastases or death. |
| Incomplete outcome data (attrition bias) | High risk | 10/72 participants not evaluable because of "short duration of therapy (2 months)" |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, open‐label study, placebo‐controlled in the first week only during iv phase of treatment | |
| Participants | N = 38; N = 33 evaluated Normocalcaemic women with BCBM | |
| Interventions | Clodronate (Cl2MDP) 300 mg/d/iv or placebo for 7 d, then clodronate 100 mg/d/im for 3 weeks followed by 100 mg/im on alternate days for ≥ a further 2 months or no additional treatment Treatment was in addition to specific anti‐tumour therapy | |
| Outcomes | Laboratory tests of calcium metabolism, bone pain and radiological response (X‐rays and bone scan). The incidence of hypercalcaemia and fractures was recorded in evaluable participants. Pain was assessed during the first week using the Scott‐Huskisson visual‐analogue method | |
| Notes | Skeletal endpoints were described in 21/33 evaluable participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Placebo was administered, but at different regimen to treatment, so it was not effectively blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Biochemical tests were completed and unlikely to be influenced by the lack of blinding. However the other outcome measures were self‐reported pain intensity and number of bone lesions that may have been affected by no blinding |
| Incomplete outcome data (attrition bias) | Low risk | Two participants from arm A and 1 participant from arm B were not evaluated, but it was a negligible number. No ITT analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, controlled, phase III (open label). Germany and Austrian study | |
| Participants | N = 693 (enrolment) Participants with residual invasive tumour (ypT1‐4 and/or ypNþ) after ≥ 4 cycles of anthracycline‐taxane‐containing neoadjuvant chemotherapy | |
| Interventions | Zoledronate 4 mg iv for 5 years or observation. Zoledronate was given every 4 weeks for the first 6 months, every 3 months for the following 2 years, and every 6 months for the last 2.5 years | |
| Outcomes | Primary outcome: DFS Secondary outcomes: event‐free survival with respect to interval between surgery and randomisation, BMFS, OS, predictive value of primary breast tumour response to postoperative treatment, prognostic impact of chemotherapy induced amenorrhoea in premenopausal women, toxicity | |
| Notes | Trialists also kindly provided unpublished trial data to the Cochrane Review team. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients eligibility was centrally confirmed and block randomisation was used to randomise the patients after stratification for centre, time interval between surgery and entering the clinical trial (within 3 months, within 1 year, within 2 years, within 3 years), age at study entry (<50, or >50 years) and receptor content in diagnostic core or surgical biopsy" |
| Allocation concealment (selection bias) | Low risk | Block randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes were DFS, OS and toxicity. OS and DFS endpoints are less likely to be affected by unblinding |
| Incomplete outcome data (attrition bias) | Low risk | Low loss to follow‐up (1.5%); similar in both arms. Analyses were ITT |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in the prospectively registered trial (NCT00512993) were covered in the clinical trial report |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study. Local & systemic treatment at discretion of investigators | |
| Participants | N = 3323 Operable stage I‐III BC (T1‐3, N0‐2, M0). Age ≥ 50 years (65%); white (83%). T1 (67%), T2 (27%); N0 (75%), N1 (18%), N2 (6%) ER and/or PgR positive (78%), ER/PR negative (22%) Endocrine alone (31%), chemo alone (21%), both (44%) | |
| Interventions | Clodronate 1600 mg/d for 3 years (n = 1662) or placebo (n = 1661) | |
| Outcomes | Primary endpoint: DFS Secondary endpoints: skeletal metastases, OS, RFS, incidence of non‐skeletal metastases | |
| Notes | Poor adherence ‐ by the "end of the 3‐year therapeutic period, 60% (992/1647) of women assigned placebo and 56% (919/1640) of those allocated clodronate remained on study drugs " p737 Median follow up 90 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation with a biased‐coin minimisation approach to generate a treatment assignment on entry Stratified participants (within every centre) by age (< 50 and ≥ 50 years), number of positive axillary nodes (0, 1–3, and ≥ 4), and hormone receptor status (both ER and PgR negative, or one or both receptors positive) |
| Allocation concealment (selection bias) | Low risk | "Biased coin minimisation approach on study entry "p735 |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo controlled |
| Blinding of outcome assessment (detection bias) | Low risk | All participants, clinicians who treated and assessed protocol doctors were masked to treatment group assignment |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Very small number excluded as lost to follow‐up: clodronate: 7/1662; placebo: 5/1661 |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoints reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Prospective, randomised, double‐blind, multicenter non‐inferiority trial | |
| Participants | N = 433 Women with bone metastases from BC who previously received ≥ 9 doses of iv bisphosphonates (zoledronic acid or pamidronate) during the first 10‐15 months of therapy Baseline characteristics were comparable between arms | |
| Interventions | Randomised (1:1) to receive zoledronate iv 4 mg every 4 week or every 12 weeks (placebo between zoledronate doses to maintain blind) for 1 year | |
| Outcomes | Primary: proportion of participants who experienced ≥ 1 SRE. Primary analysis was non‐inferiority (pre‐defined margin of 10%) for the difference in SRE rates Secondary endpoints: time to first SRE, skeletal morbidity rate (SMR), bone pain score, change in bone turnover markers, and safety | |
| Notes | Conference abstract: Hortobagyi 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" with no further details provided. Baseline characteristics were comparable between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind (participant, investigator)" as per clinical trial registry record |
| Blinding of outcome assessment (detection bias) | Low risk | Double blind |
| Incomplete outcome data (attrition bias) | Unclear risk | The number of participants who completed the study did not match the denominators for certain outcomes (e.g. bone pain). Awaiting details from full trial publication |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the trial registry record; if not, reasons were provided (e.g. too few events to report median) |
| Other bias | Low risk | Appears to be free of other sources of bias |
| Methods | Double‐blind placebo‐controlled trial. | |
| Participants | N = 173 (updated data provided for N = 185) Patients with BCBM | |
| Interventions | Oral clodronate 800 mg twice/d or placebo for 3 years | |
| Outcomes | Hypercalcaemia, fractures and radiotherapy required for bone pain | |
| Notes | Analysis by ITT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Pre‐randomized numbering system" |
| Allocation concealment (selection bias) | Low risk | Controlled by independent centre: "pre‐randomized numbering system whereby patients, allocated a number in the order in which they presented, were prescribed the corresponding numbered medication package at each center at 3‐month intervals" |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled. The clinicians, nursing staff, and pharmacy staff at each participating hospital were unaware of the treatment allocation of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Biochemical measures were the primary outcome. Nonvertebral fractures were diagnosed and recorded by the trial radiologists at each centre. A research assistant based at the University of Sheffield travelled to each centre to perform vertebral and metacarpal morphometry. Unlikely to be aware of treatment groups |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants were analysed. The number of withdrawals were reported and similar across both groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Double‐blind, placebo‐controlled randomised study. Multi‐national, multi‐centred study | |
| Participants | N = 1069 Pre‐ and post‐menopausal women with primary operable BC Baseline characteristics: similar between groups. Median age (53 years for both groups), stage III (9% clodronate, 10% placebo), axillary lymph node involvement (37% clodronate, 38% placebo) | |
| Interventions | Clodronate 1600 mg/d orally or placebo for 2 years | |
| Outcomes | Primary endpoint: incidence of bone metastases over 5‐year study period Secondary endpoints: OS, non‐skeletal relapse | |
| Notes | Statistics: study was powered (5% beta, 5% alpha) to detect a 25% reduction in bone metastases over 5 years Analysis by ITT. Follow‐up of 5.6 years (final analysis, Powles 2006) Follow‐up: clinical laboratory tests every 3 months for the first year, every 6 months between 2‐5 years. All participants were assessed for bone metastases at 2 years and 5 years (bone scan, skeletal X‐ray, CT or MRI if indicated) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized by means of numerically ordered and coded packages..." |
| Allocation concealment (selection bias) | Low risk | "Centralised blinded code" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | The primary outcome was time to first bone metastases and secondary outcomes were OS and occurrence of skeletal relapses. "Bone metastases were diagnosed by isotopic bone scan, skeletal X‐rays and CT or MRI if required. The final diagnosis of bone metastases and subsequent audits of the data were always performed blinded to the patient's study medication" (pg. 3) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. All participants included in the analysis and no missing outcome data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Double‐blind phase III comparison study | |
| Participants | N = 1130 Women with ABC and ≥ 1 bone metastasis and patients with stage III multiple myeloma | |
| Interventions | iv zoledronic acid (4 mg or 8 mg) or pamidronate 90 mg iv every 3‐4 weeks for 12 months Participants in the 8 mg zoledronic acid arm, had zoledronic acid subsequently reduced to 4 mg because of concern over possible toxicity | |
| Outcomes | SREs: incidence at 13 months, morbidity, time‐to‐event, bone pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐dummy infusions; double‐blind, but pharmacists at each hospital were aware of the medications given |
| Blinding of outcome assessment (detection bias) | Low risk | The outcomes were proportion of participants who experienced ≥ 1 SRE, in addition to AEs, serious AEs and laboratory data. It is unlikely that any potential unblinding would affect the types of outcomes assessed |
| Incomplete outcome data (attrition bias) | Low risk | 99.7% of participants were analysed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported. Participants initially randomised to 8 mg zoledronic acid were given 4 mg after protocol amendment in 2000. The potential bias was mitigated by analysing the 4 mg zoledronic acid and 8 mg/4 mg zoledronic acid separately |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Adjuvant clodronate study. Randomised, open‐label, controlled trial. Single institution study (Helsinki University Hospital, Finland 1990‐1993) | |
| Participants | N = 299 (282 in analysis as 17 participants excluded from analysis due to major protocol violation) Women with primary operable node‐positive BC. T1‐3, N1‐2, M0 Baseline characteristics: similar between groups. Median age (52 years for both groups), T3 (6% of all participants), N2/3 (24% of all participants), adjuvant chemotherapy (54%), adjuvant endocrine therapy (46%) Other treatments: all participants received post‐operative radiotherapy (50 Gy/25 fractions) to breast and regional lymph nodes, and adjuvant systemic therapy: premenopausal 6 cycles CMF and postmenopausal anti‐oestrogens (randomised to tamoxifen or toremifene for 3 years) | |
| Interventions | Clodronate 1600 mg daily for 3 years or open control | |
| Outcomes | Primary endpoint: incidence of bone metastases (and visceral metastases) Secondary endpoints: survival, DFS Follow‐up: bone scan at 1, 2, 3, 5 and 10 years. Clinical investigation and laboratory tests every 4‐6 months for the first 5 years and at 10‐year visit | |
| Notes | Statistics: study was powered (beta 20%) to detect a 10% to 15% difference between arms Analysis by ITT. 10‐year follow‐up data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | "Clinical investigation and basic laboratory tests were repeated every 4 to 6 months with a radiologic examination if necessary. Investigators performing bone scans and radiologic examinations were blinded to treatment allocation" (pg.11) |
| Incomplete outcome data (attrition bias) | Low risk | ITT. No missing data for the final population of 282 |
| Selective reporting (reporting bias) | Low risk | Outcomes were not specified in methodology; however, all expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, phase III trial. International trial involving 322 centres in Europe, North America, America, South America, Japan, Australia, India and South Africa. Double‐blind, double‐dummy, active controlled trial | |
| Participants | N= 2049 Women with BC with prior or current radiological evidence of ≥ 1 bone metastasis, ECOG 0‐2 Baseline characteristics: similar between groups. 37% of participants in each group had prior SRE. Oestrogen receptor/progesterone receptor‐positive in 71% of participants on zoledronic acid, 72% of participants on denosumab, HER2 in 18% of participants in both groups. 21% of participants in each group had lung metastases, 18% (zoledronic acid) and 21% (denosumab) participants had liver metastases Other treatment: all chemotherapy and hormonal therapies were allowed | |
| Interventions | Randomised to sc denosumab 120 mg and iv placebo every 4 weeks, or iv zoledronic acid 4 mg and sc injection of placebo every 4 weeks | |
| Outcomes | Primary endpoint: first on‐study SRE (non‐inferiority test). SRE was defined as pathologic fracture, radiation therapy to bone, surgery to bone, or spinal cord compression) Secondary endpoints: first on‐study (superiority test), time to first and subsequent on‐study SREs, safety endpoint. Follow‐up: clinic visits every 4 weeks with skeletal surveys (X‐rays) every 12 weeks to assess fractures. Other radiological assessments (CT or MRI) are allowed as part of standard care. All radiological assessment were confirmed by 2 radiologists independently through blinded central radiology review | |
| Notes | Statistics: the study was 97% powered with 95% confidence (alpha 5%, beta 3%) to detect its non‐inferiority endpoint, set at HR of 0.9. The study was 90% powered with 95% confidence (alpha 5%, beta 10%) to detect its superiority endpoint, set at HR of 0.8 ITT analysis. Follow‐up of 34 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blinded, double‐dummy" |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes were SRE (defined as pathologic fracture, radiation therapy to bone, surgery to bone or spinal cord compression). "Fractures were assessed by skeletal surveys (x‐rays) every 12 weeks or by radiographic assessments (x‐ray, computed tomography, or magnetic resonance imaging) during the course of standard care and were identified or confirmed independently by ≥ two radiologists through blinded central radiology review". "Spinal cord compression events were also confirmed by blinded central radiology review" (pg. 5133) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. All participants included in efficacy analysis. Number of participants who discontinued were reported with no significant differences evidence between groups (as per CONSORT flowchart) |
| Selective reporting (reporting bias) | Low risk | All endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, controlled, phase III trial (open label). US study | |
| Participants | N = 6097 participants Women with stage I‐IIIa BC receiving adjuvant therapy. Median age: 53 years. 58% postmenopausal or aged ≥ 50 years | |
| Interventions | Zoledronate iv 4 mg monthly for 6 months then 3‐monthly for 30 months or oral clodronate 1600 mg/d 36 months or oral ibandronate 50 mg/d for 36 months | |
| Outcomes | Primary outcomes: histological confirmation of disease recurrence, site of first disease recurrence, DFS, OS, Zubrod performance status Secondary outcomes: time to progression, tolerability, participant's compliance, bone markers, dental substudy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomised" but no further details provided in the abstracts |
| Allocation concealment (selection bias) | Unclear risk | No information provided in abstract |
| Blinding of participants and personnel (performance bias) | High risk | No masking (as per clinical trials registry record) |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract states that analysis would be ITT but no further details provided |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes reported in abstract form |
| Other bias | Unclear risk | Insufficient information to judge |
| Methods | Randomised, open‐label study. Multicentre study in USA from 2000‐2007 | |
| Participants | N = 68 Post‐menopausal women with stage II/III adenocarcinoma of the breast | |
| Interventions | Randomised to zoledronic acid 4 mg iv every 12 weeks for 4 cycles or observation Baseline characteristics: imbalance in the rate of T1 and T2 disease (T1: 39% zoledronic acid, 2% control; T2: 30% zoledronic acid, 56% control). Imbalance in the rate of N1 and N2/3 disease (N1: 41% zoledronic acid, 15% control; N2‐3: 56% zoledronic acid, 78% control) Other treatment: adjuvant chemotherapy needed for 33/36 zoledronic acid and 31/32 control participants, and adjuvant radiation needed for 24/36 zoledronic acid and 26/32 control participants. Use of calcium and vitamin D were permitted but not mandated in the study | |
| Outcomes | Endpoints: BMD measurement, toxicities DFS and OS | |
| Notes | Statistics: the study was 80% powered with 0.05 alpha to detect a mean BMD change (lumbar spine) of ≥ 1.75% between zoledronic acid and observation ITT analysis. Follow‐up of 8 years. Follow‐up: BMD was measured at baseline, 6 and 12 months. Toxicity evaluated on day 1 in clinic and 1 week by telephone after treatment. Other ancillary tests as per clinician's discretion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomised", but baseline characteristics were very different between groups so randomisation was deemed to be not complete |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes were BMD (measured by DXA devices), death, disease recurrence and toxicity. "BMD results were reviewed by a single physician specializing in bone mass measurement" (p 3). The paper did not mention whether the physician was aware of treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. In both arms, 6 participants did not complete the study with reasons provided |
| Selective reporting (reporting bias) | Low risk | Endpoints were not pre‐specified, but all possible endpoints from a BMD trial were included. DFS and OS endpoints were provided by investigator from contacting first author |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised 1:1:1, parallel‐group, double‐blind, placebo‐controlled | |
| Participants | N = 435 (Study MF4434) Patients with histologically confirmed BC and radiographically confirmed bone metastases | |
| Interventions | 3 arms: oral ibandronate 50 mg/d for 96 weeks (n = 148) oral ibandronate 20 mg/d for 96 weeks (n = 144) placebo (n = 143) | |
| Outcomes | SREs reported, bone pain, analgesic use. SREs reported as Skeletal Morbidity Period Rate (SMPR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled, double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. The percentage of early withdrawals was similar across groups |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Double‐blind, randomised, controlled study | |
| Participants | N = 144 Patients with BC and osteolytic bone metastases | |
| Interventions | Oral clodronate 1600 mg/d or placebo for up to 12 months | |
| Outcomes | Time to bone event (hypercalcaemia, new bone pain, radiotherapy required to relieve bone pain, pathological fractures or death due to bone metastases), pain intensity. Pain intensity assessed using a visual pain scale | |
| Notes | Publication in French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised". Baseline characteristics were similar between groups so randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided about outcome assessors |
| Incomplete outcome data (attrition bias) | High risk | 7 participants not analysed (3 from placebo group, 4 from treatment group), including 1 in clodronate group that developed pulmonary lymphangitis 16 days after starting treatment, and 2 from placebo group who died from myocardial infarction and hypercalcaemia within 30 days of starting placebo. Not ITT analysis, and the missing participants' data described was clearly of importance to the analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, non‐placebo‐controlled study | |
| Participants | N = 161 Women with BCBM | |
| Interventions | Indefinite oral pamidronate 150 mg twice/d or open control Initial pamidronate dose was 300 mg twice/d from July 1983‐February 1985 (N = 48 on pamidronate) but because of gastrointestinal toxicity, was reduced to 150 mg twice/d for the remainder of study until March 1988 (final participant enrolled) | |
| Outcomes | Morbidity to bone: hypercalcaemia, severe bone pain needing radiotherapy or surgery, pathological or imminent fractures, event‐free survival, QoL | |
| Notes | Final analysis of data was first presented in Van Holten‐Verzantvoort 1987. QoL was reported separately in 144 participants (Van Holten‐Verzantvoort 1991). Analysis by ITT. Those receiving high‐dose pamidronate were not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised performed separately per participating centre" (14) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Bone scans and radiographs were reviewed by an independent experienced radiologist ...for skeletal disease progression, stabilisation or remission according to the World Health Organization (WHO) criteria. The reviewer was blinded for the supportive treatment given (pamidronate or control)."..."Two of the 14 participating centers could not make radiologic examinations available to central review" (pg.493) |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Only 2 participants in the pamidronate group were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Randomised, multi‐centre, open, controlled study | |
| Participants | N = 124 Women with BC with either established extra‐skeletal metastases or locally advanced disease but no bone metastases | |
| Interventions | Indefinite pamidronate 150 mg orally twice/d or open control. 6 participants received 300 mg twice/d and were included in the ITT analysis Anti‐tumour therapy was freely allowed | |
| Outcomes | Skeletal morbidity: hypercalcaemia, severe bone pain needing radiotherapy or surgery, pathological fracture, change in systemic therapy for bone metastases, QoL; event‐free period | |
| Notes | ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned per participation centre" (9) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Low risk | "Consecutive investigations were reviewed for the first development of bone metastases by two expert readers blinded for clinical data" (p. 451) |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis. Early withdrawal of participants in the pamidronate group (15/65) only due to gastro‐intestinal complaints; an additional 19/65 participants in the pamidronate group (29.2%) and 5/59 (18.5%) in the control group withdrew due to reported reasons |
| Selective reporting (reporting bias) | Low risk | All endpoints were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Phase III prospective, randomised, open‐label, non‐inferiority trial, 1995‐1999 | |
| Participants | N = 321 Women with confirmed bone metastases from BC > 18 years with ≥1 bone metastasis, histologically confirmed BC, ECOG performance status of 0–2, approximate life expectancy of > 6 months | |
| Interventions | 375 randomly assigned to 1/3 treatment groups: 60 mg pamidronate intravenously every 3 weeks (N = 129) 900 mg clodronate intravenously every 3 weeks (N = 120) 2400 mg oral clodronate daily (n = 126) | |
| Outcomes | Primary: "compare the side effects of oral versus intravenous BP treatment " Secondary: "assess their clinical effectiveness." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Assessments were performed at baseline and every 3 months thereafter. Outcomes assessed included adverse events, participant compliance, pain development and occurrence of pathologic fractures. Given the number of self‐reported outcomes and no information about assessment of pathologic fractures, we assessed this study to be potentially at high risk of bias |
| Incomplete outcome data (attrition bias) | High risk | High dropout rate, 14% (54/375 randomised); unclear if differential between arms; unclear flow diagram |
| Selective reporting (reporting bias) | Unclear risk | Median follow‐up 15 months; but recruitment completed in 1999, publication delayed 17 years to 2016 Trial not registered |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Z‐FAST 2012, one of the triplet adjuvant zoledronic acid studies. Participants were from 94 US and Canadian community‐based centres. Open‐label, randomised, placebo‐controlled study | |
| Participants | N = 602 Postmenopausal women with early‐stage (surgically resectable stage I, II, or IIIa) ER and/or PR–positive BC as well as baseline LS and TH T scores of ≥ 2.0, who were on adjuvant letrozole 2.5 mg orally every day for 5 years Baseline characteristics: similar between groups. Median age 60 years in both arms; all participants hormone receptor‐positive; no information on stages or characteristics of BC; no adjuvant chemotherapy (54.3% upfront group, 51.7% delayed group) (Coleman 2009) | |
| Interventions | Upfront zoledronic acid 4 mg every 6 months (after randomisation) or delayed start zoledronic acid (defined by post‐baseline LS or TH T score decreased to < –2.0; any clinical, non‐traumatic fracture occurred; or asymptomatic vertebral fracture identified at 36 months) for 5 years | |
| Outcomes | Primary endpoint: difference in percentage change in LS BMD from baseline to 12 months Secondary endpoints: percentage change difference in LS BMD from baseline to 24, 36, and 60 months; percentage change difference in TH BMD from baseline to 12, 24, 36 and 60 months; percentage change differences in serum N‐telopeptide and serum bone‐specific alkaline phosphatase concentrations from baseline to 12, 24, 36 and 60 months; fracture incidence at 36 months; time‐to‐disease recurrence, and rate of decrease in LS and TH BMD during the study. OS or death was not a pre‐specified endpoint | |
| Notes | Statistics: This was predominantly a BMD study with disease recurrence as one of its pre‐specified endpoints. However, the study authors reported that "the study was not powered to detect a difference in the incidence of clinical fractures or BC relapse" Z‐FAST 2012. Sites of recurrences reported ITT analysis. Follow‐up was 61 months (Coleman 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random assignment". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Central reader (BioImaging Technologies Inc, Newtown, PA) analysed all DEXA scans for the efficacy analysis |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. 301 participants in each group. 300 in ITT population: "1 patient erroneously randomised in each group" |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
| Methods | Phase III, open‐label, randomised, controlled non‐inferiority trial. UK trial, 99 centres | |
| Participants | N = 1404 Women ≥ 18 years with metastatic BC and ≥ 1 documented bone lesion, performance status ECOG 0‐2 | |
| Interventions | Oral ibandronate 50 mg/d continuous vs zoledronate 4 mg every 3‐4 weeks, for 96 weeks | |
| Outcomes | Primary outcome: SRE Secondary outcomes: time to first SRE, proportion of participants with SRE, OS, pain, QoL, toxicity, health resource usage | |
| Notes | Per‐protocol analysis included 654 participants in the ibandronic‐acid group and 672 in the zoledronic‐acid group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned (1:1 ratio) ... by use of a computer‐generated randomisation list at the Wales Cancer Trials Unit (WCTU). Randomisation was stratified, within blocks of size four, according to whether the patient was currently receiving chemotherapy, hormone therapy, or had had a previous skeletal‐related event within the last 3 months or had planned radiotherapy." |
| Allocation concealment (selection bias) | Low risk | "Research nurses (who recruited the patients) telephoning the WCTU, where randomisation and treatment allocation was done by a trial/data manager interacting with a computerised system. " |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding of outcome assessors mentioned. The primary endpoint for non‐inferiority was frequency and timing of SREs. A SRE was a composite event defined as one of: requirement for orthopaedic surgery, vertebroplasty, or radiotherapy to bone; symptomatic vertebral fracture; pathological non‐vertebral fracture; spinal‐cord compression; and hypercalcaemia of malignancy |
| Incomplete outcome data (attrition bias) | Low risk | High levels of attrition in both arms (withdrawal, treatment discontinuation, death), but comprehensively documented and balanced between arms. ITT analysis |
| Selective reporting (reporting bias) | Low risk | All primary and secondary endpoints presented |
| Other bias | Low risk | Study appears to be free of other sources of bias |
| Methods | Open‐label, multicentre, randomised 1:1, phase III study. 132 centres in 28 countries (Europe, Asia‐Pacific, Middle East, Latin America) | |
| Participants | N = 1065 Postmenopausal women with early‐stage (surgically resectable stage I‐IIIA) ER‐ and/or PR–positive BC, baseline LS and TH T scores ≥ 2.0, on adjuvant letrozole 2.5 mg daily for 5 years Baseline: median age 57; 78% white, performance status ECOG 0 (89%), 1 (10%); stage I (60%), II‐III (40%); primary tumour: < T2 (60%), ≥ T2 (40%); axillary nodal status: negative (43%), positive (57%); adjuvant chemotherapy: no (46%), yes (54%) | |
| Interventions | Immediate: zoledronic acid iv 4 mg every 6 months (< 4 weeks from randomisation) vs Delayed: zoledronic acid iv 4 mg every 6 months started at post‐baseline LS or TH T score decreased to < –2.0; any clinical, nontraumatic fracture occurred; or asymptomatic vertebral fracture | |
| Outcomes | Primary endpoint: percentage change in LS BMD at 12 months Secondary endpoints: "percentage change difference in TH BMD from baseline to each assessment, 3‐year fracture incidence, time to disease recurrence (local relapse or distant metastasis), OS, and safety" | |
| Notes | Predominantly a BMD study designed and powered to study "the effect of immediate and delayed treatment on change in BMD." Final efficacy analysis at 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". Baseline characteristics were similar between groups; randomisation appeared to be achieved |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information about blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis. Recurrence data complete. However BMD data incomplete at 36 months: only 314/434 participants on early‐treatment group and 319/434 on the delayed‐treatment group had both baseline and 36‐month BMD data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes reported |
| Other bias | Low risk | A sensitivity analysis censoring delayed‐treatment group at the first dose of zoledronic acid was also performed so to preclude the time difference of treatment as a confounding factor. The results before and after censoring were similar |
| Methods | Open‐label, non‐inferiority, phase 3 randomised 1:1 trial. Conducted in 62 Italian centres | |
| Participants | N = 425 Women > 18 years with metastatic BC and ≥ 1 radiologically documented bone metastasis, having completed 12‐15 months of iv zoledronic acid every 3‐4 weeks | |
| Interventions | 4 mg iv zoledronic acid every 4 weeks for 12 months (N = 205) or 4 mg iv zoledronic acid every 12 weeks for 12 months (N = 216) All participants received daily calcium (500 mg) and vitamin D (400‐500 IU) | |
| Outcomes | Primary endpoint: skeletal morbidity rate (SREs per participant per year) Secondary endpoints: incidence of each SRE per year, proportion of participants who had SREs, time to first SRE, bone pain, use of analgesics, N‐telopeptide of type I collagen concentration, and safety | |
| Notes | SRE rate for control group anticipated to be 0.91 events per participants per year. Pre‐defined non‐inferiority HR 0.67 (SRE rate 0.56), 420 participants needed to detect non‐inferiority with 80% power (one‐sided α = 0·025) Actual control rate was lower at 0.26 events per participant per year, but observed pooled standard deviation of study was 1/3 that estimated in power calculations. To maintain study power, "non‐inferiority margin was reduced, according to the ratio of the two estimated SDs, to 0·19." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random list generated by an independent statistician through a validated computer programme" |
| Allocation concealment (selection bias) | Low risk | "Allocated by the investigator to the smallest available random number of the list ... Sealed envelopes containing the randomisation code for each patient were produced and sent to centres: the investigators opened them sequentially when assigning a new patient" |
| Blinding of participants and personnel (performance bias) | High risk | Open label |
| Blinding of outcome assessment (detection bias) | High risk | Nobody involved in the study was masked to treatment allocation. |
| Incomplete outcome data (attrition bias) | Low risk | Only 149/209 (71.3%) in every‐12‐weeks intervention group and 142/216 (65.7%) in every‐4‐weeks control group completed the 12‐month study period. Analysed by ITT |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported |
| Other bias | Low risk | Study appeared to be free of other sources of bias |
ABC: advanced breast cancer
AE: adverse event
BC: breast cancer
BCBM: breast cancer with bone metastases
BMD: bone mineral density
BMFS: bone metastasis‐free survival
CK: anti‐pan‐cytokeratine (CK) antibody
CT: computed tomography
CTR: control
DFS: disease‐free survival
DMB: denosumab
DTC: detectable tumour cells
EBC: early breast cancer
ECOG: Eastern cooperative oncology group
ER: oestrogen receptor
G‐CSF: granulocyte colony‐stimulating factor
HR: hazard ratio
im: intramuscular
ITT: intention‐to‐treat
iv.: intravenous
LS: lumbar spine
MRI: magnetic resonance imaging
ONJ: osteonecrosis of the jaw
PgR: progesterone receptor
RFS: recurrence‐free survival
OS: overall survival
QoL: quality of life
sc: subcutaneous
SRE: skeletal‐related event
sCTx: serum C‐Telopeptide
TH: total hip
uNTx: urinary N‐telopeptide
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Evaluated short‐term anti‐tumour effects of neoadjuvant chemotherapy plus or minus zoledronate in women with invasive breast cancer, evaluating biological endpoints including apoptosis, proliferation and angiogenesis | |
| Study population was made up of patients with myeloma and breast cancer. Results were not stratified according to disease. Data for breast cancer were requested but not received | |
| The study was a BMD trial with no specific information about recurrence or death other than this: "There were two deaths due to recurrence of breast cancer, one in each group." It did not mention the types of recurrence for these patients, overall recurrence or overall survival. For the endpoint of meta‐analysis which specifically addressed locoregional/bone/visceral metastases and death, this information was not specific enough to be incorporated in the meta‐analysis | |
| Inadequate randomisation and allocation concealment in this adjuvant pamidronate trial (initial assignment made after coin toss by clinical nurse, alternating assignment of participants thereafter) | |
| BMD study for EBC (risedronate 35 mg orally, weekly or placebo). Recurrence mentioned briefly to be no different between treatment arms, but absolute numbers were not reported, nor did the manuscript expand on this in results or discussion section. Data for recurrence was requested but not received | |
| A BMD study randomising women to risedronate or placebo for 1 year but no SRE endpoints were discussed | |
| This was a small randomised study (N = 51) with a mixed study population, although breast cancer patients were included. However, outcomes were reported for the whole population only | |
| This Japanese adjuvant pamidronate study was a non‐randomised study with treatment assignment based on patient preference | |
| Study report from Saarto 2004 study examining the impact of adjuvant clodronate on survival outcomes, stratified by postoperative baseline matrix metalloproteinase ‐ 2 levels (low, high). Participants were stratified by MMP‐2 status and the effect of oral clodronate was compared on both groups. The mortality data from the combined cohort (Saarto 2004) was reported in Pavlakis 2005 update | |
| Randomised phase II trial of neoadjuvant trial of zoledronate vs placebo; it did not include DFS or OS endpoints | |
| A subset of patients with biomarker and BMD measured in Powles 2006 (851/1069) were reported in an analysis that correlated BMD, bone turnover markers and bone metastases. However, the bone metastases incidence of the ITT population was not reported | |
| Randomised phase II study, BMD endpoints only Methods: changes in BMD and trabecular bone score were assessed in 70 participants who were recruited in the double‐blind, placebo‐controlled ProBONE‐II trial and randomised to receive either zoledronate (N = 34) or placebo (N = 36) for 2 years. The changes were assessed at baseline and at 12 and 24 months after treatment initiation | |
| Histological study describing the effect of adjuvant clodronate on bone biopsies obtained from a small subset (N = 63) of consenting participants within included adjuvant study by Saarto 2004 (N = 299). No additional clinical outcomes were reported | |
| BMD, safety & tolerability endpoints only Single‐blind, randomised, placebo‐controlled phase II study designed to evaluate the impact of oral ibandronate (150 mg monthly) on BMD in osteopenic women on AIs in adjuvant setting | |
| Bone substudy of IBIS‐II primary prevention trial of anastrozole. Primary endpoint BMD. The double‐blind IBIS‐II trial recruited 3864 healthy, postmenopausal women at increased risk of breast cancer and randomly allocated them oral anastrozole or placebo. 1410 (36%) postmenopausal women were then enrolled in a bone substudy and stratified at baseline according to their lowest baseline T score at spine or femoral neck (stratum I: T score ≥ ‐1.0; stratum II: T score ≥ ‐2.5 but l< ‐1.0; stratum III: T score < ‐2.5 but > ‐4.0). Women in stratum I were monitored only; women in stratum III were all given risedronate (35 mg/week). Women in stratum II were randomly assigned (1:1) to risedronate (35 mg/week) or placebo | |
| Primary endpoints were biochemical: urinary calcium, hydroxyproline, serum calcium. Effect on bone pain was reported but only in a qualitative fashion | |
| No SRE outcomes were reported. Effect of clodronate on BMD only | |
| No SRE outcomes were reported. Effect of clodronate on BMD only | |
| Detailed QoL analysis in a whole breast cancer patient population within the including zoledronate versus pamidronate study by Rosen 2004. No additional comparative data were provided between the treatment arms |
BMD: bone mineral density
EBC: early breast cancer
ITT: intention‐to‐treat
QoL: quality of life
SRE: skeletal‐related event
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, open‐label study |
| Participants | Women with advanced breast cancer and radiographically confirmed bone metastases |
| Interventions | Standard schedule: zoledronic acid iv over 15 min once every 3‐4 weeks for 24 months |
| Outcomes | Primary outcomes: fractures, radiotherapy to bone, hypercalcaemia, orthopedic surgery and spinal cord compression Secondary outcomes: quality of life, clinical burden of skeletal complications, pain, performance status and analgesic use, incidence of new bone metastases, overall survival, bisphosphonate use and expenditure on administration, health care utilisation and clinical utility of the "point of care" test for N‐telopeptides (NTx) excretion |
| Notes | Study start date: March 2006. Estimated enrolment: 1500 |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized feasibility study of de‐escalated (every 12 weeks) versus standard (every 3 to 4 weeks) intravenous pamidronate in women with low‐risk bone metastases from breast cancer |
| Methods | Pilot, randomised phase II, non‐inferiority trial |
| Participants | Patients receiving intravenous bisphosphonates for ≥ 3 months and with low‐risk baseline serum C‐telopeptide (CTx) levels (< 600 ng/L) |
| Interventions | Control: 90 mg pamidronate iv every 3‐4 weeks Intervention (de‐escalated): 90 mg pamidronate iv every 12 weeks |
| Outcomes | CTx, bone alkaline phosphatase, and pain scores (Brief Pain Inventory and Functional Assessment of Cancer Therapy‐Bone Pain) were collected every 12 weeks for 48 weeks |
| Starting date | |
| Contact information | E. Amir, Division of Medical Oncology and Hematology, University of Toronto, Princess Margaret Hospital, Toronto ON, M5G 2M9, Canada |
| Notes |
| Trial name or title | Study of denosumab as adjuvant treatment for women with high risk early breast cancer receiving neoadjuvant or adjuvant therapy |
| Methods | Randomised phase III, double‐blind, placebo‐controlled trial |
| Participants | Patients with EBC |
| Interventions | Denosumab sc 120 mg 6‐monthly or placebo for 5 years. All participants take oral calcium and vitamin D for 5 years |
| Outcomes | Primary endpoint: bone metastases‐free survival Secondary endpoints: DFS, OS, distant RFS, safety |
| Starting date | 2010 |
| Contact information | ClinicalTrials.gov identifier: NCT01077154. Amgen Call Center: 866‐572‐6436 |
| Notes | International multi‐centre trial. Estimated enrolment to be completed by October 2016 with 4500 participants |
| Trial name or title | Zoledronic acid combined with adjuvant tamoxifen with or without ovarian function suppression in premenopausal early breast cancer patients |
| Methods | Premenopausal females who had undergone primary surgery for stage I, II ER‐positive and/or PR‐positive BC with < 10 positive lymph nodes. All participants were scheduled for standard tamoxifen 20 mg/d for five years plus goserelin 3.6 mg every 28 days |
| Participants | Premenopausal EBC patients (n = 300), median follow up 98.4 months (range 14‐120) |
| Interventions | Randomised to zoledronic acid 4 mg every 6 months for 3 years (group A) and without zoledronic acid (group B) |
| Outcomes | Primary: toxicity and DFS Secondary: OS |
| Starting date | April 2005‐March 2012 |
| Contact information | |
| Notes | SABCS Dec 2015: Abstract P5‐15‐04 "Adding ZOL [zoledronic acid] to endocrine therapy strongly suggests improved DFS versus endocrine therapy alone (90% versus 85% for an absolute increase of 5%). There were fewer disease recurrences in the ZOL group versus no ZOL group (12% vs. 16%) with the greatest reductions in the loco‐regional recurrence (3% vs. 5%), distant metastasis (6% vs. 7%) and bone metastasis (3% vs. 5%). Conclusion: ZOL with adjuvant endocrine therapy were generally well tolerated with no reports of renal failure or osteonecrosis of the jaw. So, a twice yearly ZOL enhanced the efficacy of adjuvant endocrine treatment, and this benefit is maintained for long time" We contacted study authors unpublished data |
| Trial name or title | The impact of skeletal‐related events on pain interference in patients with advanced breast cancer and bone metastases |
| Methods | Randomised, double‐blind, double‐dummy, placebo‐controlled trial |
| Participants | Advanced BC and bone metastases |
| Interventions | Randomised 1:1 to receive monthly denosumab 120 mg sc or zoledronic acid 4 mg iv, (adjusted for renal function) |
| Outcomes | Primary: the impact of SREs on pain interference in patients with BCBM |
| Starting date | |
| Contact information | Lesley Fallowfield: [email protected] |
| Notes |
| Trial name or title | FemZone trial: a randomized phase II trial comparing neoadjuvant letrozole and zoledronic acid with letrozole in primary breast cancer patients |
| Methods | Prospective randomised phase II trial |
| Participants | Randomly assigned to receive either LET 2.5 mg/d (N = 79) or the combination of LET 2.5 mg/d and a total of 7 infusions of zoledronic acid 4 mg every 4 weeks (N = 89) for 6 months. Primary endpoint was clinical response rate as assessed by mammogram readings. The study was terminated prematurely due to insufficient recruitment. |
| Interventions | Randomly assigned to receive either LET 2.5 mg/d (N = 79) or the combination of LET 2.5 mg/d and a total of 7 infusions of zoledronic acid 4 mg every 4 weeks (N = 89) for 6 months |
| Outcomes | Primary endpoint was clinical response rate as assessed by mammogram readings |
| Starting date | Terminated early because of poor recruitment. Exploratory analysis reported at this stage |
| Contact information | Peter A Fasching: ed.negnalre‐[email protected] |
| Notes | EUDRA CT: EUCTR2004‐004007‐37‐DE |
| Trial name or title | A study of hormonal adjuvant treatment effect on bone mineral density in early breast cancer patients |
| Methods | Randomised, controlled, phase III (open‐label), 3‐arm |
| Participants | Any BC with M0 disease, post‐surgery with indication of adjuvant hormone therapy |
| Interventions | Arm A: tamoxifen 20 mg/d and/or triptorelin 3.75 mg every month (for pre‐menopausal women) for 5 years, or arm B: letrozole 2.5 mg/d and/or triptorelin 3.75 mg every month (for pre‐menopausal women) for 5 years or arm C (experimental): letrozole 2.5 mg/d and/or triptorelin 3.75 mg every month (for pre‐menopausal women) and zoledronic acid every 6 months for 5 years |
| Outcomes | Primary outcomes: BMD (at 12 months), DSF in pre‐menopausal participants Secondary outcomes: BMD yearly, DSF in post‐menopausal participants, OS, toxicity, biomarker |
| Starting date | March 2004 |
| Contact information | ClinicalTrials.gov identifier: NCT00412022. Andrea De Matteis, Giuseppe D'Aiuto, Francesco Perrone, National Cancer Institute, Naples |
| Notes | Italian study. Enrolment (450/1271) expected completion March 2013 |
| Trial name or title | ODYSSEY |
| Methods | Randomised, double‐blind, phase IV study (post‐marketing) |
| Participants | BC patients with high‐risk bone metastases (prior SRE, bone progression, bone pain or levels of bone turnover marker serum C‐telopeptide (sCTX) > 400 ng/L) despite > 3 months of pamidronate (PAM) use |
| Interventions | Randomised in a double‐blind manner to either switch to zoledronate (ZA) or continue on PAM every 4 weeks for 12 weeks |
| Outcomes | Primary outcome: proportion of participants achieving a fall in sCTX at 12 weeks Secondary outcomes were pain control (Brief Pain Inventory and FACT‐BP) and toxicity |
| Starting date | Aug 2012 |
| Contact information | PI: Dr Mark Clemons, The Ottawa Hospital |
| Notes | Clinicaltrials.gov: NCT01907880 |
| Trial name or title | Efficacy and safety of denosumab from a phase III, randomized, active‐controlled study compared with zoledronic acid in patients of Asian ancestry with bone metastases from solid tumours |
| Methods | Phase III, double‐blind, denosumab (DmAb) vs zoledronic acid (ZA) |
| Participants | "Patients >18 years who had a confirmed solid tumor, evidence of 1 bone metastasis and ECOG score 0‐2 were enrolled." "485 (DmAb = 326, ZA = 159) patients were randomized; 90% of patients had either completed the study or withdrawn by planned data cut‐off (29 February 2016). Mean (SD) age of patients was 53.9 (11.38) years; 67% patients were women, 93% Chinese, 50% had BC and 27% had non‐small cell lung cancer." |
| Interventions | Methods: "Randomized (2:1) to receive either DmAb 120 mg subcutaneously every 4 weeks (Q4W) or ZA 4 mg intravenously Q4W for 49 weeks and are being followed up to Wk 73." |
| Outcomes | Primary: markers of bone turnover (% change in uNTx/uCr) from baseline to 13 weeks Secondary: changes in bone‐specific ALP; first on‐study SRE |
| Starting date | |
| Contact information | Not specified |
| Notes | Results: the mean change in uNTx/uCr from baseline to Wk 13 was ‐81.9% for DmAb and ‐75.2% for ZA (ANCOVA; P < 0.0001). The median change in S‐BALP from baseline to week 13 was ‐36.8% (DmAb) and ‐30.3% (ZA) (P = 0.027). Rate of developing any on‐study SRE within the first year after initialising treatment was lower in participants receiving DmAb vs ZA (4.9% vs 6.3%) without statistical significance. Incidence of AEs was similar in DmAb and ZA groups (89% vs 91%), with most common AEs being anaemia (25% vs 24%), white blood cell count decreased (21% vs 24%), and pyrexia (13% vs 21%); overall incidence of serious AEs: 14% vs 9%. One serious AE (muscular weakness) was reported as related to study treatment. Conclusions: DmAb was found to be superior than ZA in reducing uNTx/uCr overall and Chinese patients. No new safety concerns were identified with DmAb |
| Trial name or title | Disease‐free survival and Ki67 analysis of a randomized controlled trial comparing zoledronic acid plus chemotherapy with chemotherapy alone as a neoadjuvant treatment in patients with HER2‐negative primary breast cancer |
| Methods | Addition of zoledronate to neoadjuvant chemotherapy |
| Participants | Women with stage IIA‐IIIB HER‐2‐negative BC |
| Interventions | Experimental: CTZ group ‐ chemotherapy (3 x FEC, 12 x weekly paclitaxel) followed by zoledronic acid Control: CT group ‐ chemotherapy only (3 x FEC, 12 x weekly paclitaxel) |
| Outcomes | DFS Pathologic complete response (pCR) rates between baseline Ki67 high (20% and > 20%) with Ki67 low (< 20%) in ER‐positive cohort |
| Starting date | N = 188 participants accrued between March 2010‐April 2012 |
| Contact information | |
| Notes | Miura D et al. SABCS 2013. [PD3‐7] |
| Trial name or title | Investigating denosumab as add‐on neoadjuvant treatment for hormone receptor‐negative, RANK‐positive or RANK‐negative primary breast cancer and two different nab‐Paclitaxel schedules‐2x2 factorial design |
| Methods | "Denosumab will be tested in patients with HR‐ primary breast cancer in addition to neoadjuvant chemotherapy (NACT)" Methods: GeparX will randomise 778 patients to NACT +/‐ denosumab (120 mg sc every 4 weeks for 6 cycles), stratified by lymphocyte predominant BC (< 50% vs > 50% stromal tumor infiltrating lymphocytes [TILs]), HER2 status, and epirubicin/cyclophosphamide (EC, every 2 weeks vs every 3 weeks). Secondarily participants will be randomised to the backbone treatment of nab‐paclitaxel (nP) 125 mg/m2 weekly + EC or nP 125 mg/m2 day 1 and 8 every 22 days + EC, stratified by the first randomisation. Carboplatin will be given in triple negative (TNBC) and trastuzumab + pertuzumab in HER2+ BC |
| Participants | Patients with primary cT1c‐cT4a‐d BC, centrally confirmed HR‐ and centrally assessed HER2, Ki‐67, TIL and RANK status on core biopsy can be enrolled |
| Interventions | NACT +/‐ denosumab (120 mg sc every 4 weeks for 6 cycles) |
| Outcomes | Primary: pCR (ypT0 ypN0) rates of NACT +/‐ Dmab Secondary: interaction of denosumab treatment with RANK expression; pCR rates per arm for both randomisations in TNBC and HER2+ BC; pCR rates in RANK high vs low; other pCR definitions for both randomisations; response rates; breast conservation rates; toxicity and compliance; and survival |
| Starting date | April 2016 |
| Contact information | Contact: Sherko Kümmel, MD ++49 201 174 ext 33003 s.kuemmel@kliniken‐essen‐mitte.de |
| Notes | NCT02682693 |
| Trial name or title | Study in elderly patients with early breast cancer |
| Methods | Randomised, phase III, (open‐label) |
| Participants | Node‐positive BC after surgery, ≥ 65 years |
| Interventions | Oral ibandronate 50 mg daily/ iv ibandronate 6 mg every 4 weeks for 2 years; or Oral ibandronate 50 mg daily/ im ibandronate 6 mg every 4 weeks for 2 years and capecitabine 2000 mg/m2 day 1‐14 every 22 days x 6 cycles |
| Outcomes | Primary endpoint: any relapse Secondary endpoints: OS, premature discontinuation, completed months of ibandronate, change of preference of ibandronate application, osteoporosis, toxicity, QoL (EORTC Q30) |
| Starting date | June 2004 |
| Contact information | ClinicalTrials.gov identifier: NCT00196859. Horst Mochnatzki, Birgit Raasch |
| Notes | German study. Estimated enrolment completed by October 2010 with 1500 patients |
| Trial name or title | Zoledronate or ibandronate in preventing bone problems in women with stage IV breast cancer that has spread to the bone |
| Methods | Randomised, controlled, phase III trial (open‐label) |
| Participants | Stage IV BCBM |
| Interventions | Oral ibandronate day 1‐28 or zoledronate every 28 days for up to 18 courses |
| Outcomes | Primary endpoint: SRE Secondary endpoints: time to SRE, pain score, performance status, toxicity |
| Starting date | May 2006 |
| Contact information | ClinicalTrials.gov identifier: NCT00301886. Saul Rivkin, Swedish Cancer Institute at Swedish Medical Center ‐ First Hill Campus |
| Notes | Enrolment completed (N = 466), awaiting results |
| Trial name or title | Zometa and circulating vascular endothelial growth factor (VEGF) in breast cancer patients with bone metastasis |
| Methods | Randomised, controlled, phase III trial (open‐label) |
| Participants | BCBM |
| Interventions | Zoledronate 4 mg every 4 weeks or zoledronic acid 1 mg weekly |
| Outcomes | Primary endpoint: circulating vascular endothelial growth factor levels Secondary endpoints: time to first SRE, time to bone progression disease, progression‐free survival, OS |
| Starting date | November 2006 |
| Contact information | ClinicalTrials.gov identifier: NCT00524849. Xichun Hu, Fudan University Cancer Hospital |
| Notes | Chinese Trial. Enrolment completed January 2010 (N = 60), awaiting results |
| Trial name or title | Effect of zoledronic acid as anti‐cancer treatment in metastatic breast cancer patients |
| Methods | Randomised, controlled, phase IV (open‐label), 3 arms |
| Participants | BCBM |
| Interventions | Arm A: zoledronate (months 1‐6) for participants with no bone metastases; or Arm B: zoledronate (months 7‐12) for participants with no bone metastases; or Arm C: zoledronate (months 1 to 18) for participants with bone metastases |
| Outcomes | Primary endpoint: progression‐free survival Secondary endpoints: proportion of circulating tumour cells, time to disease progression, biomarker, functional assessment |
| Starting date | May 2010 |
| Contact information | ClinicalTrials.gov identifier: NCT01129336. Novartis pharmaceutical |
| Notes | US study. Estimated enrolment to be completed by November 2012 for 280 participants |
| Trial name or title | Phase III randomized trial with neoadjuvant chemotherapy (TAC) with or without zoledronic acid for patients with HER2‐negative large resectable or stage II or III breast cancer (BC)—A Dutch Breast Cancer Trialists’ Group (BOOG) study |
| Methods | National, multicenter, randomised study |
| Participants | Stage II/III, measurable, HER2‐negative BC and absence of prior bisphosphonate usage |
| Interventions | Comparing the efficacy of TAC (docetaxel, Adriamycin and cyclophosphamide iv) CT followed by G‐CSF on day 2 with or without zoledronic acid 4 mg im, every 3 weeks |
| Outcomes | Primary: pCR rate |
| Starting date | April 2010 |
| Contact information | Judith Kroep, MD and NCT01099436 |
| Notes |
| Trial name or title | Prevention of symptomatic skeletal events with denosumab administered every 4 weeks versus every 12 weeks: a non‐inferiority phase III trial |
| Methods | Open‐label, randomised, phase III non‐inferiority trial |
| Participants | Patients with breast or prostate cancer with bone metastases and adequate organ function are eligible. This trial is open for international collaboration |
| Interventions | Denosumab 12 0mg every 12 weeks versus 120 mg every 4 weeks |
| Outcomes | Primary endpoint: time to first on‐trial symptomatic skeletal events (SSE; clinically significant pathological fracture, radiation therapy to bone, surgery to bone or spinal cord compression) Secondary endpoints: safety, time to subsequent on‐trial SSE, QoL, health economic outcomes, and change in bone turnover markers |
| Starting date | July 2014 |
| Contact information | Andrea Fuhrer ‐ [email protected] |
| Notes |
| Trial name or title | Multi‐centre prospective randomised phase III study to the comparison of FEC docetaxel chemotherapy versus FEC docetaxel‐gemcitabine chemotherapy, as well as 2 versus 5 years of zoledronate therapy in the adjuvant therapy of patients with breast cancer |
| Methods | Randomised controlled trial (2 x 2 factorial design) |
| Participants | Stage I‐IIIa (pT1‐4, N1‐3, M0 or high risk pN0) |
| Interventions | Randomised to 3 cycles of epirubicin‐fluorouracil‐cyclophosphamide followed by 3 cycles docetaxel (FEC‐D), then endocrine therapy + zoledronic acid 2 years or 5 years; or randomised to 3 cycles of FEC followed by 3 cycles of gemcitabine‐docetaxel (DG), then endocrine therapy + zoledronic acid 2 years or 5 years |
| Outcomes | Primary endpoint: time to recurrence Secondary endpoints: distant DSF, OS, QoL, SREs, safety, prognostic and predictive value of minimal residual disease |
| Starting date | June 2005 |
| Contact information | Reference URL www.success‐studie.de/a/study.htm. Professor W. Janni, Medical Center of the Heine's University of Dusseldorf |
| Notes | German study. Enrolment completed March 2007 (N = 3754), awaiting results |
| Trial name or title | A phase II, multicenter trial evaluating the efficacy of de‐escalated bisphosphonate therapy in metastatic breast cancer patients at low‐risk of skeletal‐related events |
| Methods | |
| Participants | Women with BC and radiologic, scintigraphic‐ and/or biopsy‐confirmed bone metastases who had received ≥ 3 months of 3–4 weekly iv pamidronate |
| Interventions | All study participants were switched from 3–4‐weekly to 12‐weekly pamidronate |
| Outcomes | Exploratory biomarkers, pain, any SREs |
| Starting date | October 2010 |
| Contact information | Addison CL. Registered with Ontario Cancer Trials (October 2013) and www.canadiancancertrials.ca (10‐047) |
| Notes |
AE: adverse events
BC: breast cancer
BCBM: breast cancer with bone metastasis
BMD: bone mineral density
CT: chemotherapywww.success‐studie.de/a/study.htm
DFS: disease‐free survival
EBC: early breast cancer
ER: oestrogen receptor
FEC: fluorouracil, epirubicin, cyclophosphamide
iv: intravenous
M0: no clinical or radiographic evidence of distant metastases
OS: overall survival
pN0: no regional lymph node metastasis identified histologically
QoL: quality of life
RFS: recurrence‐free survival
SABCS: San Antonio Breast Cancer Symposium
SRE: skeletal‐related event
sc: subcutaneous
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bone metastases Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Early Breast Cancer (EBC), Outcome 1 Bone metastases. | ||||
| 1.1 Bisphosphonate vs control | 11 | 15005 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.99] |
| 1.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.19] |
| 2 Bone metastases by bisphosphonate Show forest plot | 14 | 17195 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.97] |
| Analysis 1.2  Comparison 1 Early Breast Cancer (EBC), Outcome 2 Bone metastases by bisphosphonate. | ||||
| 2.1 Zoledronate 4 mg iv every 4 weeks | 8 | 8267 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.60, 0.99] |
| 2.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 2.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.88, 1.50] |
| 2.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.13] |
| 3 Visceral recurrence Show forest plot | 13 | 17092 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.17] |
| Analysis 1.3 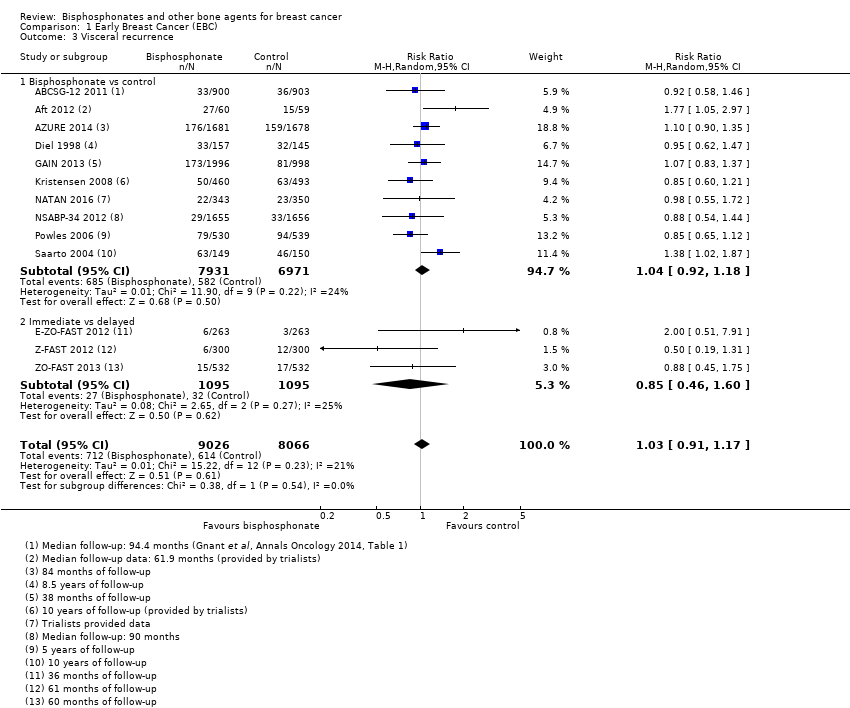 Comparison 1 Early Breast Cancer (EBC), Outcome 3 Visceral recurrence. | ||||
| 3.1 Bisphosphonate vs control | 10 | 14902 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 3.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.60] |
| 4 Locoregional recurrence Show forest plot | 11 | 15721 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| Analysis 1.4  Comparison 1 Early Breast Cancer (EBC), Outcome 4 Locoregional recurrence. | ||||
| 4.1 Bisphosphonate vs control | 8 | 13531 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 4.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.48] |
| 5 Overall recurrence Show forest plot | 14 | 17196 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| Analysis 1.5  Comparison 1 Early Breast Cancer (EBC), Outcome 5 Overall recurrence. | ||||
| 5.1 Bisphosphonate vs control | 11 | 15005 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.13] |
| 5.2 Immediate vs delayed | 3 | 2191 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.46] |
| 6 Overall recurrence by bisphosphonate Show forest plot | 14 | 17196 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| Analysis 1.6 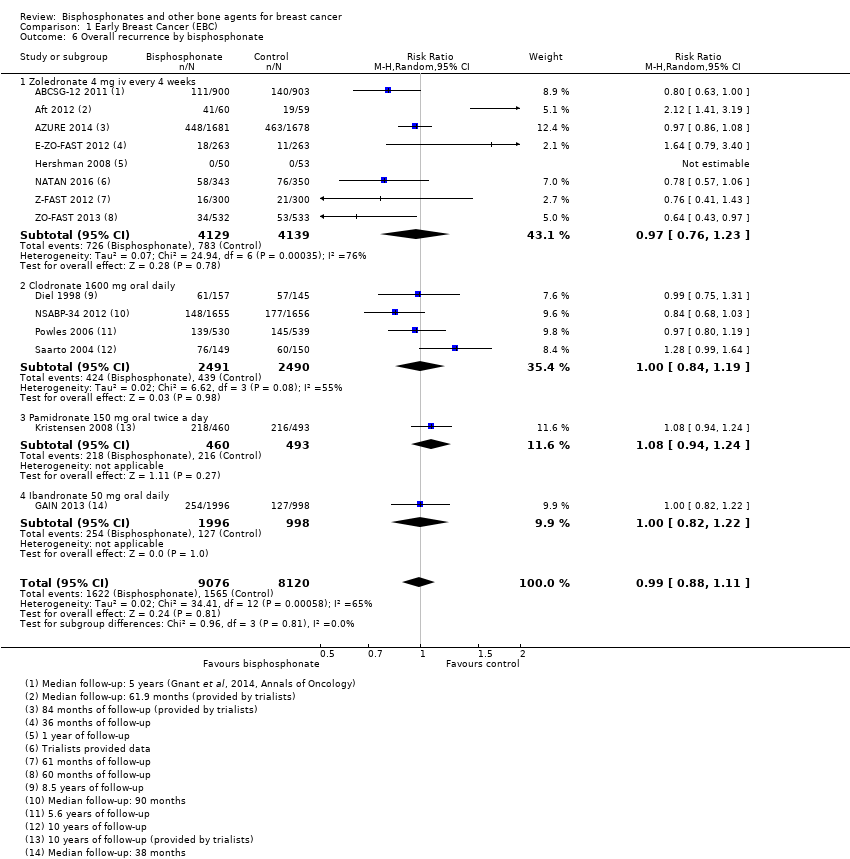 Comparison 1 Early Breast Cancer (EBC), Outcome 6 Overall recurrence by bisphosphonate. | ||||
| 6.1 Zoledronate 4 mg iv every 4 weeks | 8 | 8268 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.23] |
| 6.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 6.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 6.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.82, 1.22] |
| 7 Overall survival: time‐to‐event outcome Show forest plot | 10 | 15013 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| Analysis 1.7  Comparison 1 Early Breast Cancer (EBC), Outcome 7 Overall survival: time‐to‐event outcome. | ||||
| 7.1 Bisphosphonate vs control | 9 | 13949 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.83, 0.99] |
| 7.2 Immediate vs delayed bisphosphonate | 1 | 1064 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.42, 1.13] |
| 8 Overall survival: dichotomous outcome Show forest plot | 12 | 16028 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| Analysis 1.8 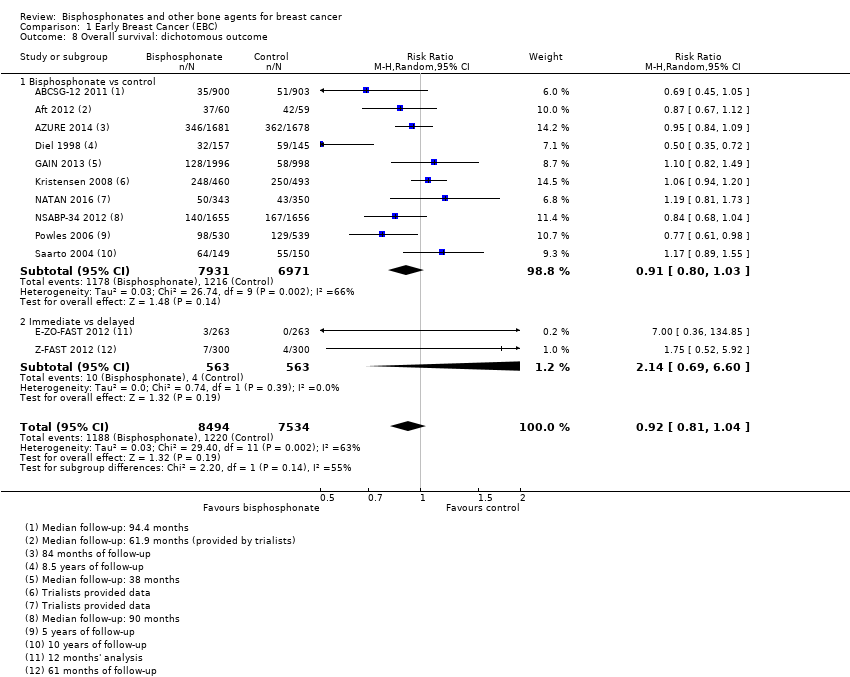 Comparison 1 Early Breast Cancer (EBC), Outcome 8 Overall survival: dichotomous outcome. | ||||
| 8.1 Bisphosphonate vs control | 10 | 14902 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
| 8.2 Immediate vs delayed | 2 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.69, 6.60] |
| 9 Overall survival by bisphosphonate: time‐to‐event outcome Show forest plot | 10 | 15013 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| Analysis 1.9 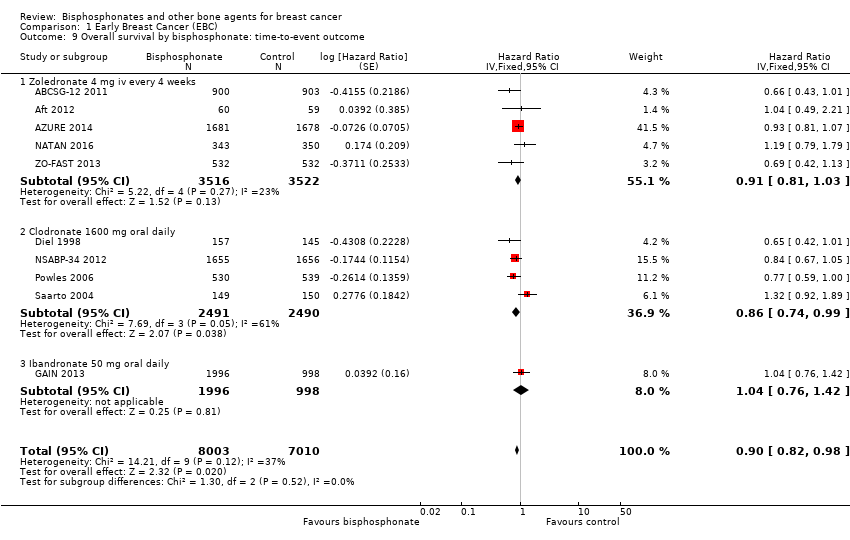 Comparison 1 Early Breast Cancer (EBC), Outcome 9 Overall survival by bisphosphonate: time‐to‐event outcome. | ||||
| 9.1 Zoledronate 4 mg iv every 4 weeks | 5 | 7038 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 9.2 Clodronate 1600 mg oral daily | 4 | 4981 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 9.3 Ibandronate 50 mg oral daily | 1 | 2994 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.76, 1.42] |
| 10 Overall survival by bisphosphonate: dichotomous outcome Show forest plot | 12 | 16028 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| Analysis 1.10  Comparison 1 Early Breast Cancer (EBC), Outcome 10 Overall survival by bisphosphonate: dichotomous outcome. | ||||
| 10.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7100 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.11] |
| 10.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 10.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.20] |
| 10.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.49] |
| 11 Overall survival by menopausal status: time‐to‐event outcome Show forest plot | 9 | 14906 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| Analysis 1.11 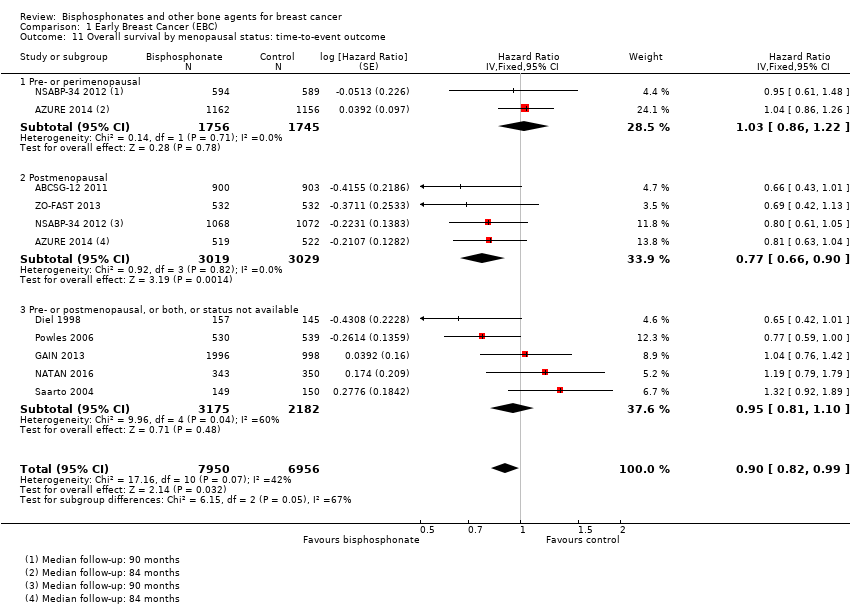 Comparison 1 Early Breast Cancer (EBC), Outcome 11 Overall survival by menopausal status: time‐to‐event outcome. | ||||
| 11.1 Pre‐ or perimenopausal | 2 | 3501 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| 11.2 Postmenopausal | 4 | 6048 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 11.3 Pre‐ or postmenopausal, or both, or status not available | 5 | 5357 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 12 Overall survival by menopausal status: dichotomous outcome Show forest plot | 12 | 16011 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| Analysis 1.12 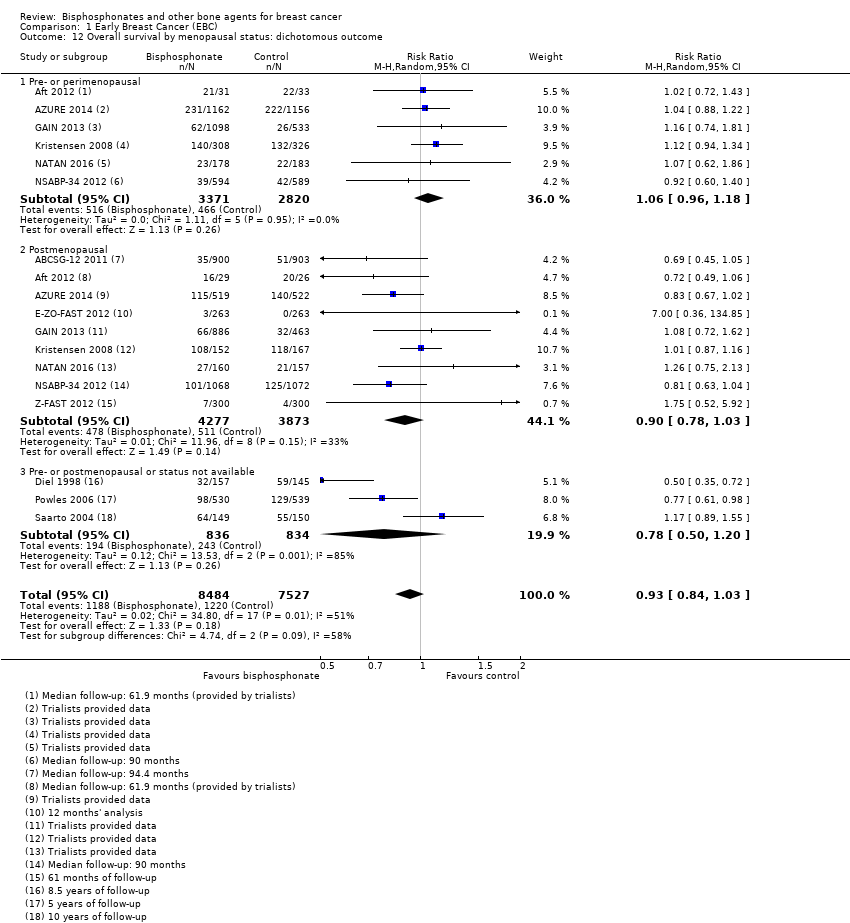 Comparison 1 Early Breast Cancer (EBC), Outcome 12 Overall survival by menopausal status: dichotomous outcome. | ||||
| 12.1 Pre‐ or perimenopausal | 6 | 6191 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.96, 1.18] |
| 12.2 Postmenopausal | 9 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 12.3 Pre‐ or postmenopausal or status not available | 3 | 1670 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.20] |
| 13 Disease‐free survival: time‐to‐event outcome Show forest plot | 9 | 14242 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| Analysis 1.13  Comparison 1 Early Breast Cancer (EBC), Outcome 13 Disease‐free survival: time‐to‐event outcome. | ||||
| 13.1 Bisphosphonate vs control | 7 | 12578 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 13.2 Immediate vs delayed bisphosphonate | 2 | 1664 | Hazard Ratio (Fixed, 95% CI) | 0.72 [0.52, 1.01] |
| 14 Disease‐free survival: dichotomous outcome Show forest plot | 10 | 15195 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| Analysis 1.14 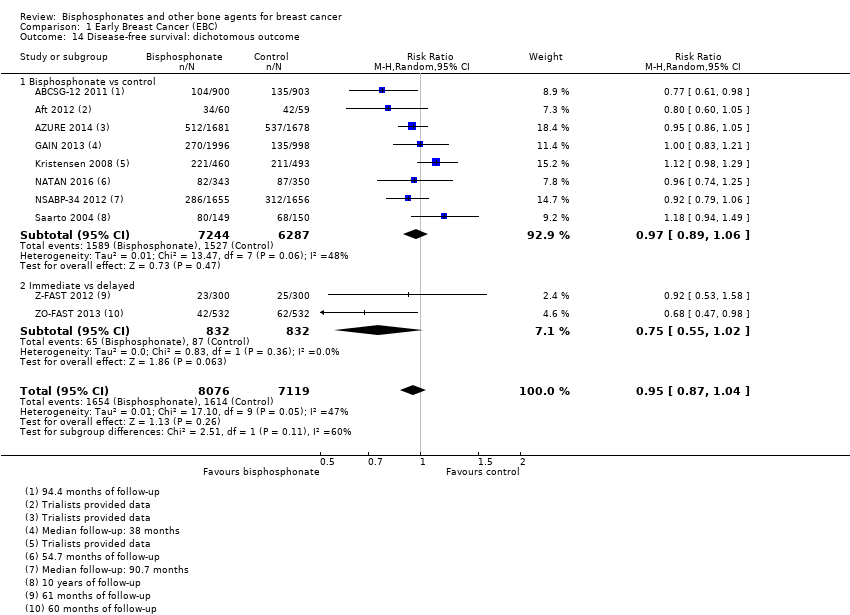 Comparison 1 Early Breast Cancer (EBC), Outcome 14 Disease‐free survival: dichotomous outcome. | ||||
| 14.1 Bisphosphonate vs control | 8 | 13531 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 14.2 Immediate vs delayed | 2 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 15 Disease‐free survival by bisphosphonate: time‐to‐event outcome Show forest plot | 9 | 14242 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| Analysis 1.15  Comparison 1 Early Breast Cancer (EBC), Outcome 15 Disease‐free survival by bisphosphonate: time‐to‐event outcome. | ||||
| 15.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7638 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.80, 0.98] |
| 15.2 Clodronate 1600 mg oral daily | 2 | 3610 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 15.3 Ibandronate 50 mg oral daily | 1 | 2994 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 16 Disease‐free survival by bisphosphonate: dichotomous outcome Show forest plot | 10 | 15202 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| Analysis 1.16 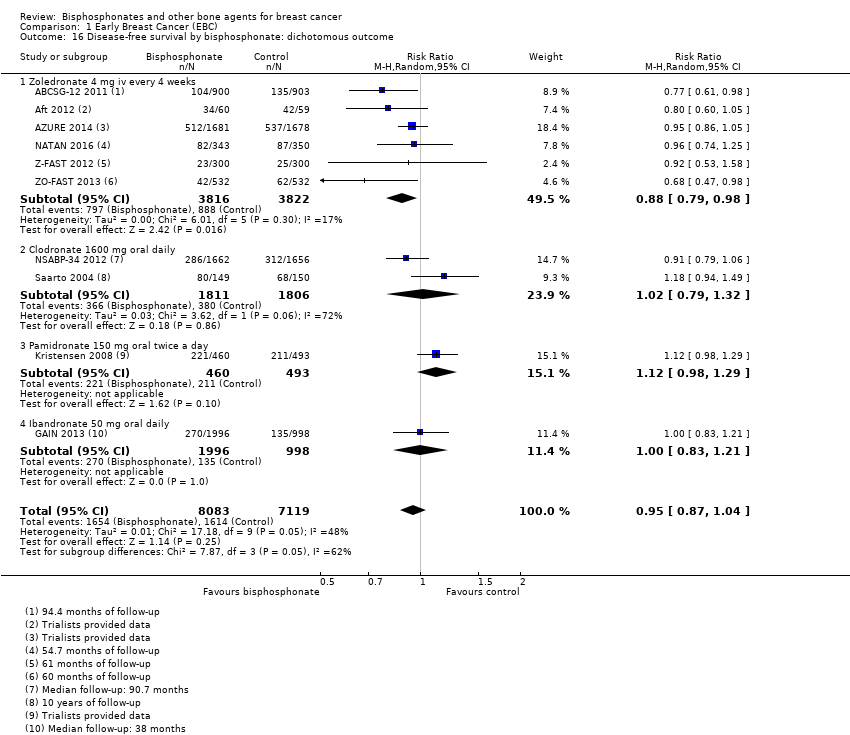 Comparison 1 Early Breast Cancer (EBC), Outcome 16 Disease‐free survival by bisphosphonate: dichotomous outcome. | ||||
| 16.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7638 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 16.2 Clodronate 1600 mg oral daily | 2 | 3617 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 16.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.29] |
| 16.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.83, 1.21] |
| 17 Disease‐free survival by menopausal status: time‐to‐event outcome Show forest plot | 8 | 14106 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| Analysis 1.17  Comparison 1 Early Breast Cancer (EBC), Outcome 17 Disease‐free survival by menopausal status: time‐to‐event outcome. | ||||
| 17.1 Pre‐ or perimenopausal | 4 | 5493 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 17.2 Postmenopausal | 7 | 8314 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.74, 0.91] |
| 17.3 Pre‐ or postmenopausal or status not available | 1 | 299 | Hazard Ratio (Fixed, 95% CI) | 1.53 [1.11, 2.11] |
| 18 Disease‐free survival by menopausal status: dichotomous outcome Show forest plot | 10 | 15150 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| Analysis 1.18 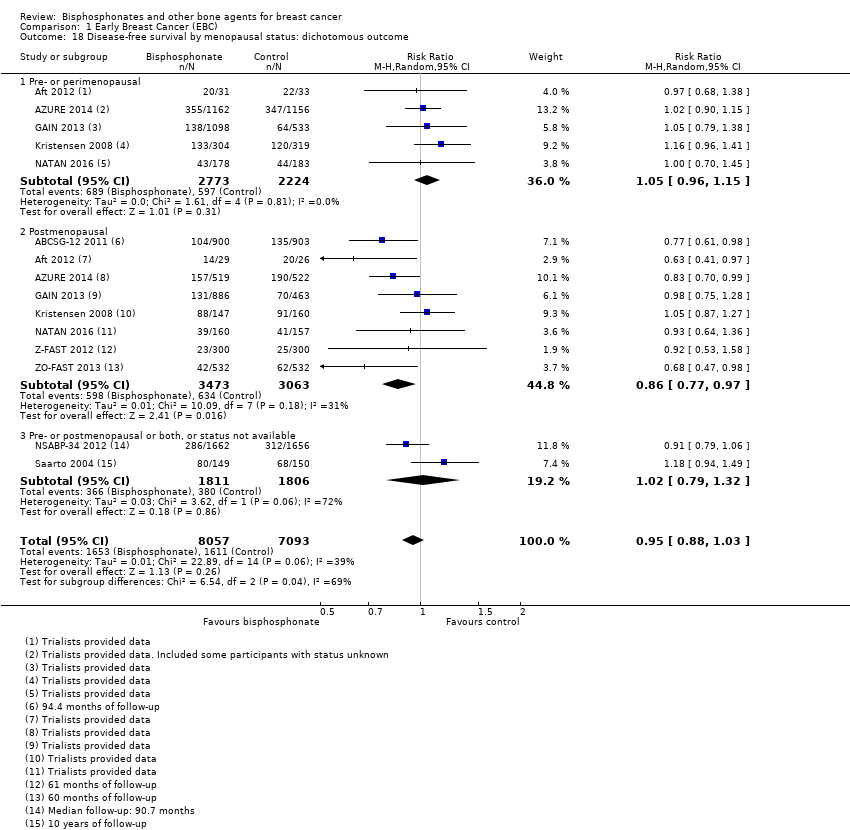 Comparison 1 Early Breast Cancer (EBC), Outcome 18 Disease‐free survival by menopausal status: dichotomous outcome. | ||||
| 18.1 Pre‐ or perimenopausal | 5 | 4997 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 18.2 Postmenopausal | 8 | 6536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] |
| 18.3 Pre‐ or postmenopausal or both, or status not available | 2 | 3617 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 19 Fracture incidence Show forest plot | 10 | 13212 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.90] |
| Analysis 1.19  Comparison 1 Early Breast Cancer (EBC), Outcome 19 Fracture incidence. | ||||
| 19.1 Bisphosphonate vs control | 6 | 7602 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.54, 1.08] |
| 19.2 Denosumab vs placebo | 1 | 3420 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.67] |
| 19.3 Immediate vs delayed bisphosphonate | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.57, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bone metastases Show forest plot | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.43] |
| Analysis 2.1  Comparison 2 Advanced Breast Cancer (ABC), Outcome 1 Bone metastases. | ||||
| 2 Overall survival Show forest plot | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.09] |
| Analysis 2.2 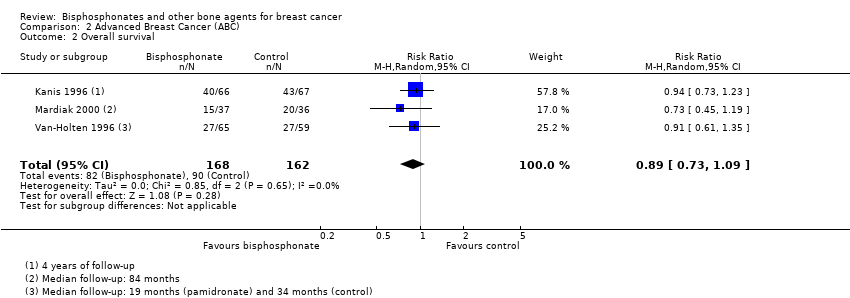 Comparison 2 Advanced Breast Cancer (ABC), Outcome 2 Overall survival. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SREs: bisphosphonate vs placebo/observation (including hypercalcaemia) Show forest plot | 8 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.95] |
| Analysis 3.1  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 1 SREs: bisphosphonate vs placebo/observation (including hypercalcaemia). | ||||
| 2 SREs: bisphosphonate vs placebo/observation (excluding hypercalcaemia) Show forest plot | 9 | 2810 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.95] |
| Analysis 3.2 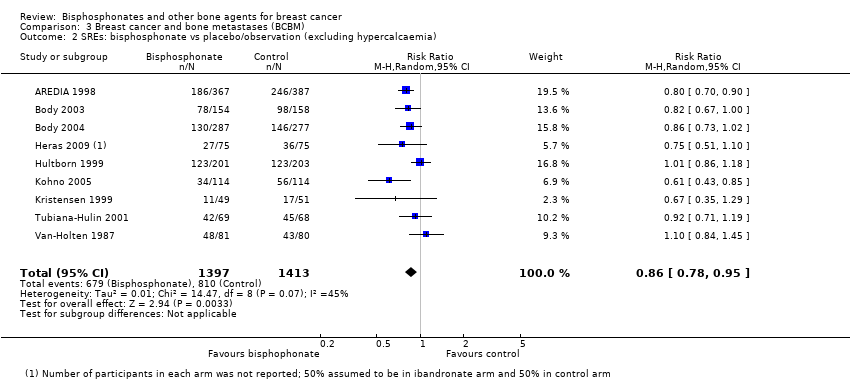 Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 2 SREs: bisphosphonate vs placebo/observation (excluding hypercalcaemia). | ||||
| 3 SREs: by route of administration Show forest plot | 11 | 3219 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.78, 0.91] |
| Analysis 3.3  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 3 SREs: by route of administration. | ||||
| 3.1 Intravenous bisphosphonates | 6 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.95] |
| 3.2 Oral bisphosphonates | 5 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.93] |
| 4 SREs: by bisphosphonate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 4 SREs: by bisphosphonate. | ||||
| 4.1 Zoledronate 4 mg iv | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.82] |
| 4.2 Pamidronate 90 mg iv | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.88] |
| 4.3 Ibandronate 6 mg iv | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.96] |
| 4.4 Clodronate 1600 mg oral | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.96] |
| 4.5 Ibandronate 50 mg oral | 1 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 4.6 Pamidronate 300 mg oral | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 5 SREs: denosumab vs bisphosphonate Show forest plot | 3 | 2345 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.72, 0.85] |
| Analysis 3.5  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 5 SREs: denosumab vs bisphosphonate. | ||||
| 6 SREs: standard vs reduced frequency bone‐targeted agent Show forest plot | 3 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| Analysis 3.6  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 6 SREs: standard vs reduced frequency bone‐targeted agent. | ||||
| 7 Median time to SRE Show forest plot | 9 | 2891 | Median Ratio (Fixed, 95% CI) | 1.43 [1.29, 1.58] |
| Analysis 3.7  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 7 Median time to SRE. | ||||
| 7.1 Bisphosphosphonate vs placebo/observation | 9 | 2891 | Median Ratio (Fixed, 95% CI) | 1.43 [1.29, 1.58] |
| 8 Overall survival Show forest plot | 7 | 1935 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| Analysis 3.8  Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 8 Overall survival. | ||||
| 8.1 Intravenous bisphosphonate vs placebo/observation | 3 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 8.2 Oral bisphosphonate vs placebo/observation | 4 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |

Study flow diagram
ABC: advanced breast cancer; ASCO: American Society of Clinical Oncology; BCBM: breast cancer with bone metastases; EBC: early breast cancer; SABCS: San Antonio Breast Cancer Symposium

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 3 Early breast cancer (EBC), outcome: 3.1 Incidence of bone metastases in EBC: bisphosphonate versus control
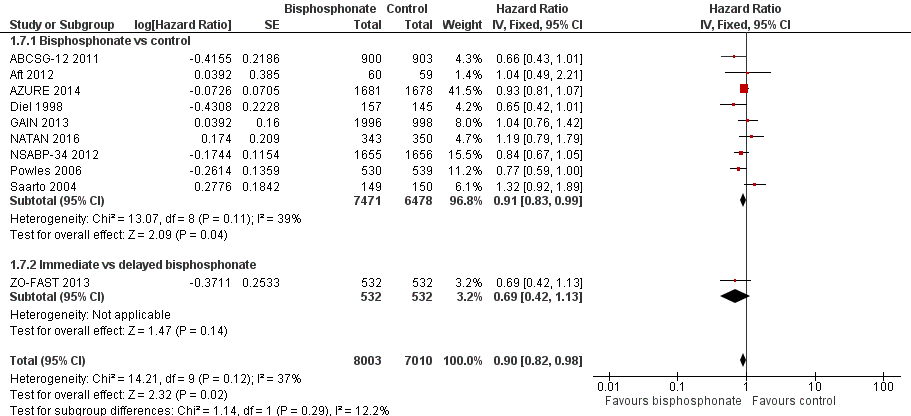
Forest plot of comparison: 1 Early Breast Cancer (EBC), outcome: 1.7 Overall survival: time‐to‐event outcome.

Funnel plot of comparison: 1 Early Breast Cancer (EBC), outcome: 1.7 Overall survival: time‐to‐event outcome.

Forest plot of comparison: 1 Early Breast Cancer (EBC), outcome: 1.11 Overall survival by menopausal status: time‐to‐event outcome.

Funnel plot of comparison: 1 Early Breast Cancer (EBC), outcome: 1.13 Disease‐free survival: time‐to‐event outcome.

Forest plot of comparison: 2 Advanced breast cancer (ABC), outcome: 2.1 Incidence of bone metastases in ABC (Stage III/IV)

Forest plot of comparison: 1 Breast cancer and Bone Metastases (BCBM), outcome: 1.2 Overall risk of SREs in BCBM: bisphosphonate versus control (excluding hypercalcaemia).

Forest plot of comparison: 1 Breast cancer with bone metastases (BCBM), outcome: 1.3 Overall risk of skeletal events in BCBM: denosumab versus bisphosphonate

Comparison 1 Early Breast Cancer (EBC), Outcome 1 Bone metastases.

Comparison 1 Early Breast Cancer (EBC), Outcome 2 Bone metastases by bisphosphonate.
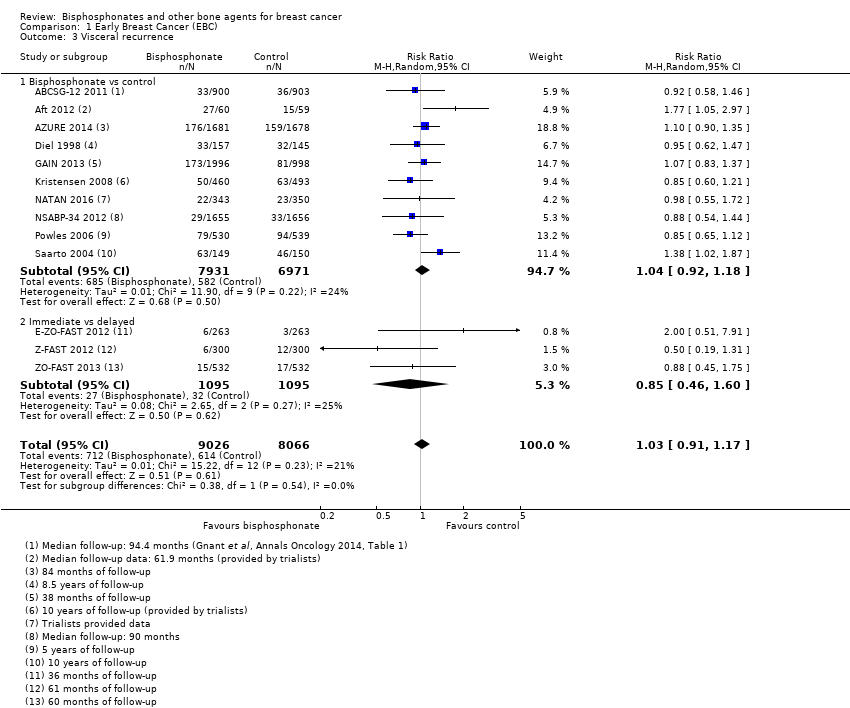
Comparison 1 Early Breast Cancer (EBC), Outcome 3 Visceral recurrence.

Comparison 1 Early Breast Cancer (EBC), Outcome 4 Locoregional recurrence.
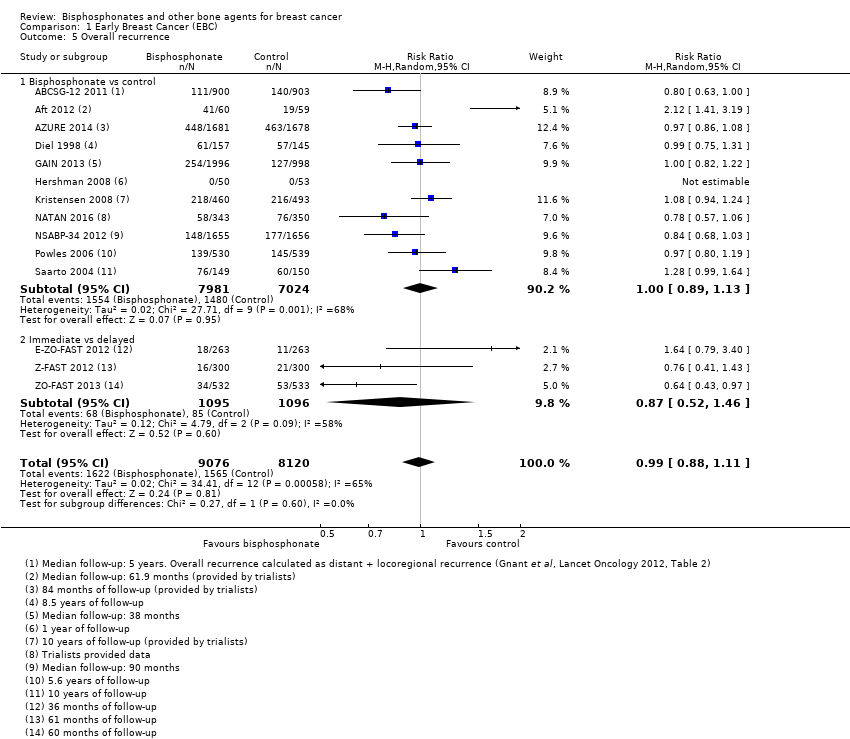
Comparison 1 Early Breast Cancer (EBC), Outcome 5 Overall recurrence.
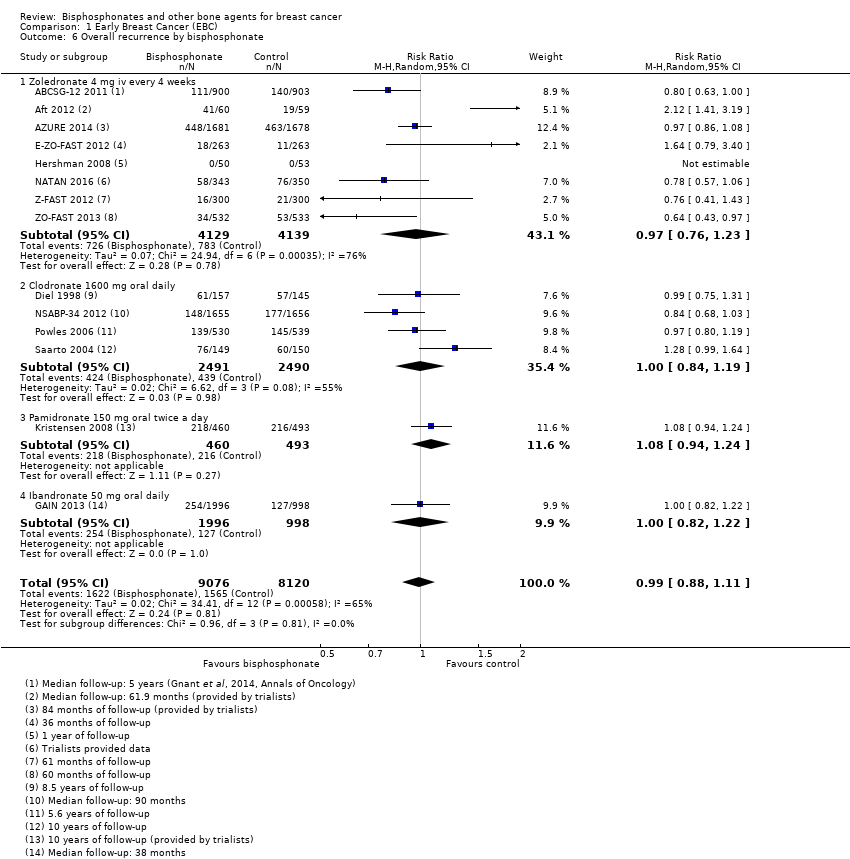
Comparison 1 Early Breast Cancer (EBC), Outcome 6 Overall recurrence by bisphosphonate.

Comparison 1 Early Breast Cancer (EBC), Outcome 7 Overall survival: time‐to‐event outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 8 Overall survival: dichotomous outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 9 Overall survival by bisphosphonate: time‐to‐event outcome.
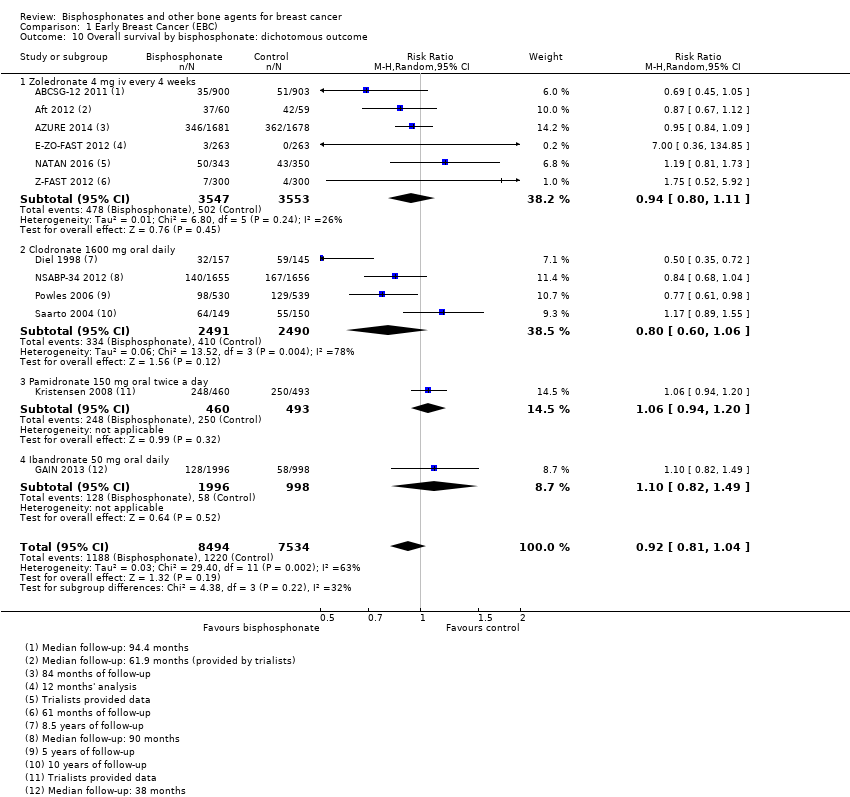
Comparison 1 Early Breast Cancer (EBC), Outcome 10 Overall survival by bisphosphonate: dichotomous outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 11 Overall survival by menopausal status: time‐to‐event outcome.
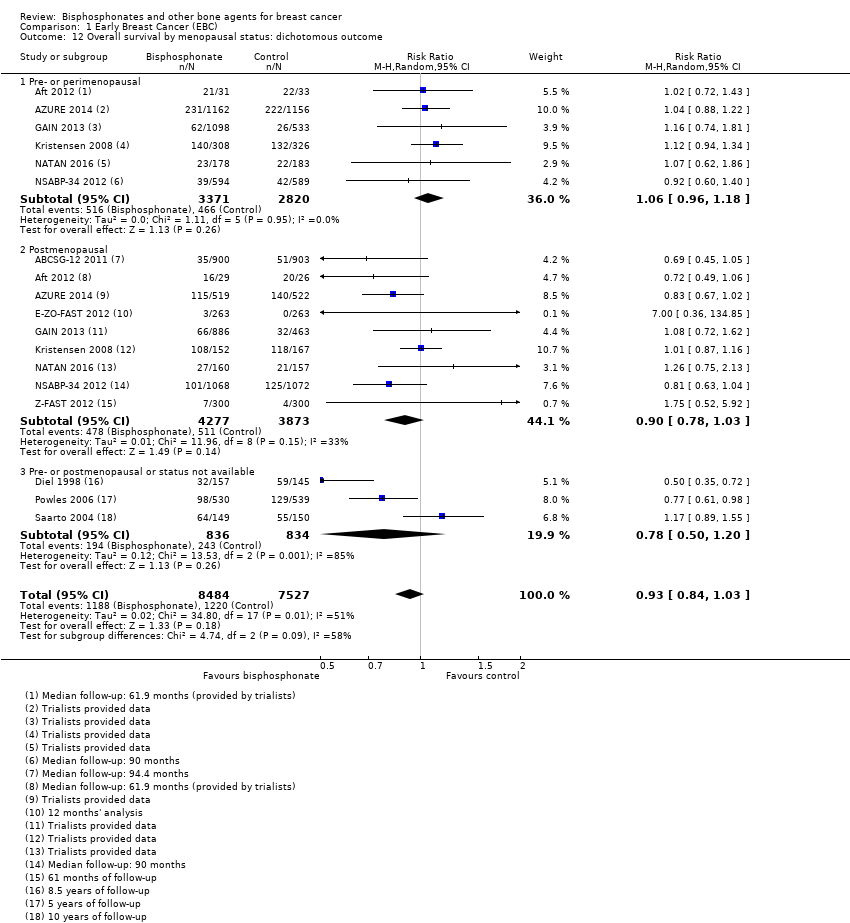
Comparison 1 Early Breast Cancer (EBC), Outcome 12 Overall survival by menopausal status: dichotomous outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 13 Disease‐free survival: time‐to‐event outcome.
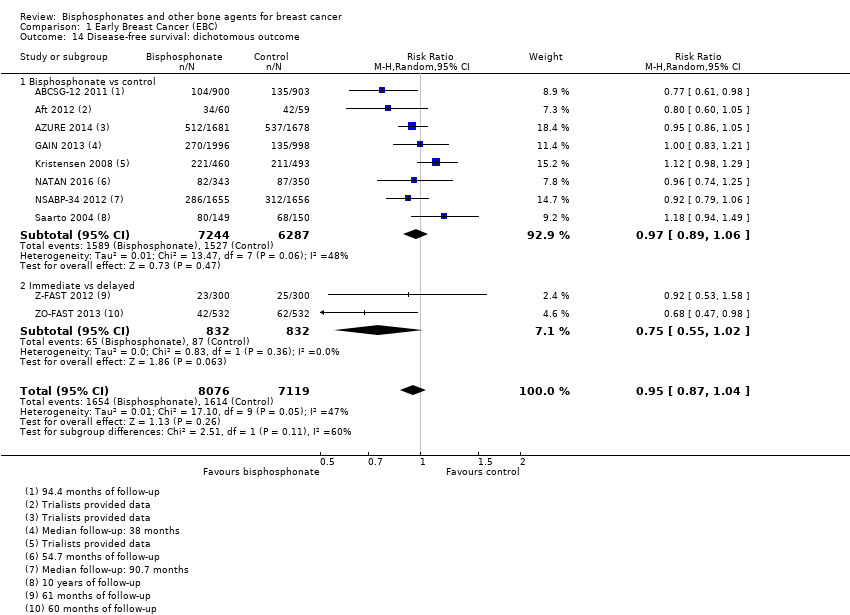
Comparison 1 Early Breast Cancer (EBC), Outcome 14 Disease‐free survival: dichotomous outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 15 Disease‐free survival by bisphosphonate: time‐to‐event outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 16 Disease‐free survival by bisphosphonate: dichotomous outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 17 Disease‐free survival by menopausal status: time‐to‐event outcome.

Comparison 1 Early Breast Cancer (EBC), Outcome 18 Disease‐free survival by menopausal status: dichotomous outcome.
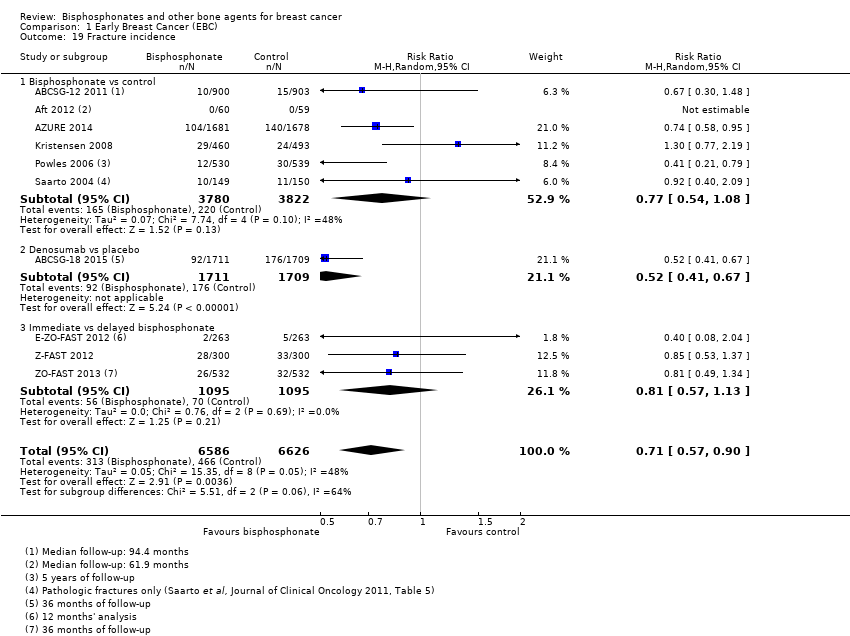
Comparison 1 Early Breast Cancer (EBC), Outcome 19 Fracture incidence.

Comparison 2 Advanced Breast Cancer (ABC), Outcome 1 Bone metastases.
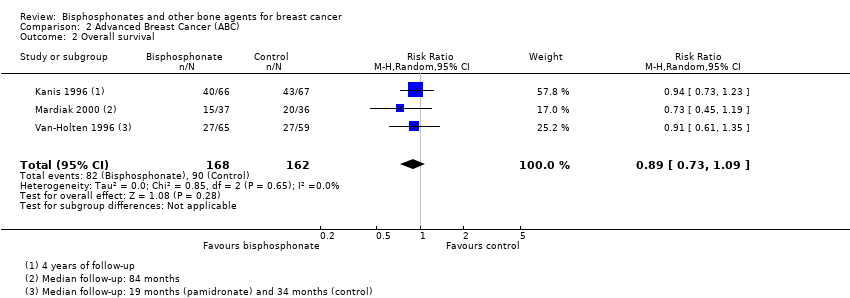
Comparison 2 Advanced Breast Cancer (ABC), Outcome 2 Overall survival.
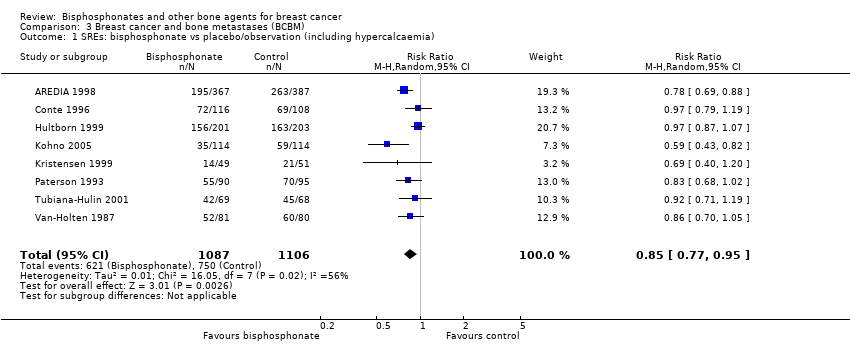
Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 1 SREs: bisphosphonate vs placebo/observation (including hypercalcaemia).
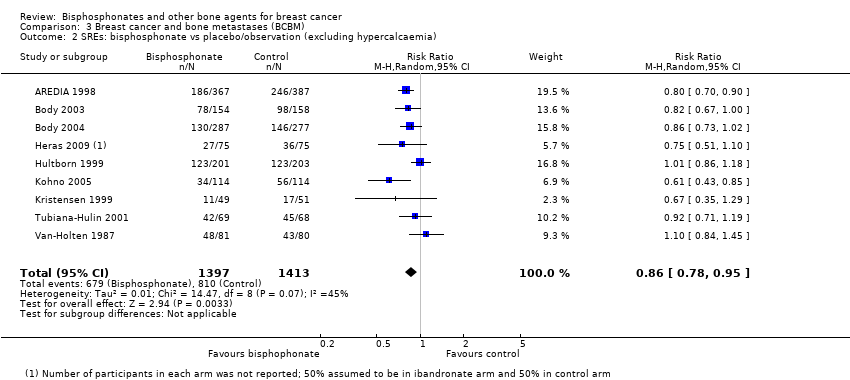
Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 2 SREs: bisphosphonate vs placebo/observation (excluding hypercalcaemia).
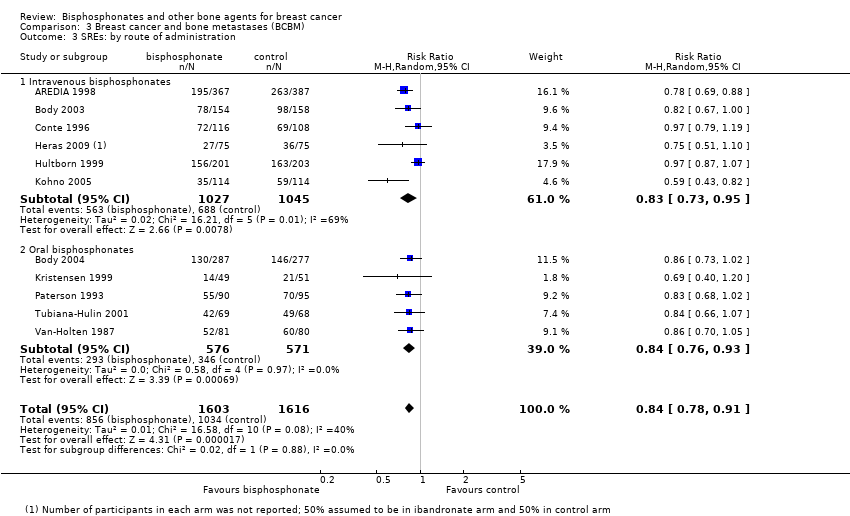
Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 3 SREs: by route of administration.

Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 4 SREs: by bisphosphonate.
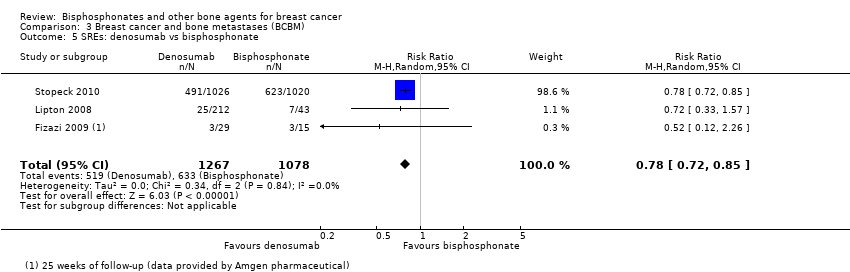
Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 5 SREs: denosumab vs bisphosphonate.

Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 6 SREs: standard vs reduced frequency bone‐targeted agent.
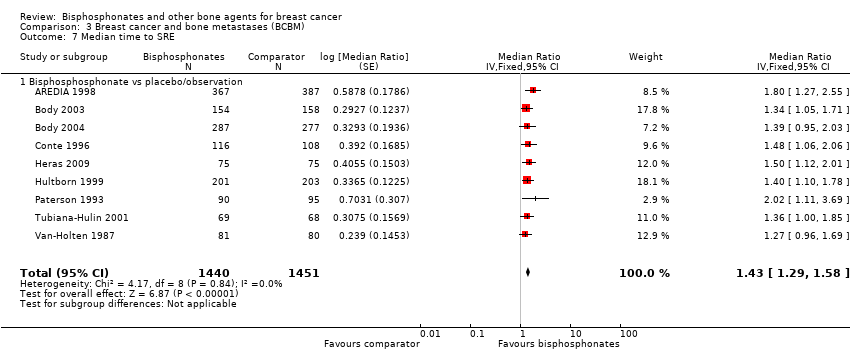
Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 7 Median time to SRE.

Comparison 3 Breast cancer and bone metastases (BCBM), Outcome 8 Overall survival.
| Bisphosphonates compared to placebo/observation for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo/observation | Risk with bisphosphonates | |||||
| Bone metastases | Study population | RR 0.86 | 15,005 | ⊕⊕⊕⊝ | Additional analysis of iv zoledronate or oral clodronate showed a treatment benefit when compared to placebo/control | |
| 90 per 1000 | 77 per 1000 | |||||
| Overall survival | 3‐year risk of deathb | HR 0.91 | 13,949 | ⊕⊕⊕⊕ | ||
| 80 per 1000 | 73 per 1000 | |||||
| Overall survival postmenopausal women | 50 per 1000c | 39 per 1000 | HR 0.77 | 6048 | ⊕⊕⊕⊕ | A sensitivity analysis removing ZO‐FAST 2013 (due to the control arm being delayed bisphosphonate) showed equivalent efficacy (HR 0.78, 95% CI 0.66 to 0.92, 3 studies, 4984 women) |
| Overall survival: pre‐ or perimenopausal women | 50 per 1000c | 51 per 1000 | HR 1.03 | 3501 | ⊕⊕⊕⊕ | |
| Disease‐free progression | 3‐year risk of recurrenced | HR 0.94 | 12578 | ⊕⊕⊕⊕ | ||
| 120 per 1000 | 113 per 1000 | |||||
| Disease‐free progression: postmenopausal women | 110 per 1000e | 91 per 1000 | HR 0.82 | 8314 | ⊕⊕⊕⊕ | A sensitivity analysis removing Z‐FAST 2012 and ZO‐FAST 2013 (due to the control arm being delayed bisphosphonate), showed equivalent efficacy (HR 0.83, 95% CI 0.74 to 0.93; 5 studies; 6650 women) |
| Disease‐free progression: pre‐ or perimenopausal women | 110 per 1000e | 111 per 1000 | HR 1.01 | 5493 | ⊕⊕⊕⊕ | |
| Fracture incidence | Study population | RR 0.77 | 7602 | ⊕⊕⊕⊝ | Three studies used iv bisphosphonate (zoledronate) and three studies used oral bisphosphonate (clodronate or pamidronate) compared to placebo | |
| 58 per 1000 | 44 per 1000 | |||||
| Osteonecrosis of the jaw (ONJ) | Bisphosphonates: approximately 35 events of ONJ were recorded in 7047 women Placebo/open: no events of ONJ were recorded in 6195 women | ‐ | 13,242 | ⊕⊕⊕⊕ | Six studies used iv bisphosphonates (zoledronate) and three studies used oral bisphosphonates (ibandronate or clodronate). Most ONJ events came from 2 studies using iv zoledronate (AZURE 2014 & NATAN 2016) | |
| Infusion‐related side effects | Seven studies reported 1 or 2 infusion‐related side‐effects (e.g. fever, fatigue, nausea or influenza‐type symptoms). Intravenous bisphosphonate (zoledronate) appeared to slightly increase the incidence of fever (in 3 out of 5 studies), fatigue (in 2 out of 3 studies) and nausea (in 2 out of 3 studies) compared to placebo. However the reporting of the grade toxicity was sometimes unspecified or on different scales. | ‐ | (7 RCTs) | ⊕⊕⊕⊝ | Fever: 6070 women (5 studies), fatigue: 2599 women (3 studies), nausea: 3825 women (3 studies), influenza‐type symptoms: 103 women (1 study) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOutcome assessors were either part of an independent adjudication committee or blinded to the treatment allocation in 5 out of 11 studies. We downgraded for risk of bias by 1 point because this outcome measure may be influenced by a lack of blinding in the other 6 studies. | ||||||
| Bisphosphonates compared to placebo/observation for women with advanced breast cancer without bone metastases | ||||||
| Patient or population: women with advanced breast cancer without bone metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo/observation | Risk with bisphosphonates | |||||
| Bone metastases | Study population | RR 0.96 | 330 | ⊕⊕⊕⊝ | ||
| 235 per 1000 | 225 per 1000 | |||||
| Median time to a skeletal‐related event (SRE) | We did not observe any statistically significant benefit using the bisphosphonate, oral clodronate. The median time to an SRE with clodronate was 28.4 months compared to 13.4 months with placebo (P = 0.42) | ‐ | 73 | ⊕⊕⊝⊝ | ||
| Overall survival | Risk of death | RR 0.89 | 330 | ⊕⊕⊕⊕ | ||
| 556 per 1000 | 494 per 1000 | |||||
| Quality of life | Similar quality‐of‐life scores with bisphosphonates (pamidronate) or no bisphosphonates | ‐ | 124 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded for imprecision because the confidence intervals included no effect and appreciable benefit and harm. | ||||||
| Bisphosphonates compared to placebo/observation for women with metastatic breast cancer with bone metastases | ||||||
| Patient or population: women with metastatic breast cancer with bone metastases | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo/observation | Risk with bisphosphonates | |||||
| Skeletal‐related event (SRE) | Study population | RR 0.86 | 2810 | ⊕⊕⊕⊕ | Additional analyses of iv or oral bisphosphonates vs placebo showed equivalent efficacy | |
| 640 per 1000 | 550 per 1000 | |||||
| Median time to a skeletal‐related event | Bisphosphonates significantly delayed the median time to an SRE compared to placebo/observation (in 11 out of 12 studies that reported results but not sufficiently to be included in a meta‐analysis). The median time to an SRE in the bisphosphonates group ranged from 8.7 to 20.8 months while the placebo group ranged from 4.9 to 14.9 months | Median ratio 1.43 (1.29 to 1.58) | 2891 | ⊕⊕⊕⊕ | Significant benefits were observed using iv bisphosphonates (7 studies) and oral bisphosphonates (4 studies) vs placebo | |
| Overall survival | Risk of death | RR 1.01 | 1935 | ⊕⊕⊕⊝ | Analyses of iv or oral bisphosphonates vs placebo showed similar results | |
| 575 per 1000 | 581 per 1000 | |||||
| Bone pain | Bisphosphonates significantly reduced bone pain compared to placebo (in 6 out of 11 studies). Bone pain was reduced with bisphosphonates in another 3 studies but the effect was not statistically significant or P value not reported | ‐ | 3297 | ⊕⊕⊕⊝ | Bone pain was assessed using a wide range of scales across studies and only 6 studies used a validated scale (e.g. Brief Pain Inventory). Significant benefits observed using iv bisphosphonates (3 studies) and oral bisphosphonates (3 studies) when compared to placebo | |
| Quality of life | Quality‐of‐life scores were better with bisphosphonates than placebo at comparable time‐points (in 3 out of 5 studies). Quality‐of‐life scores decreased during the studies as disease progressed | ‐ | 1888 | ⊕⊕⊕⊝ | The studies used validated questionnaires (in one study a trial‐specific but validated one) and unvalidated scales | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe did not downgrade for heterogeneity. This is because when two studies that used relatively low doses of pamidronate (45 mg or 60 mg) and contributed largely to the heterogeneity were removed from the meta‐analysis, the beneficial effect of bisphosphonates compared to placebo persisted. | ||||||
| Study | Treatment vs comparator | Age: mean & SDa | Menopausal status | ER status | Chemotherapy | Endocrine therapy |
| Zoledronate vs observation | Bisphosphonate: < 40 years: 19% | Premenopausal when recruited | ER‐positive: Bisphosphonate: 93% | Preoperative chemotherapy Bisphosphonate: 6% | NR | |
| Denosumab vs placebo | Denosumab: < 60 years: 30% < 60 years: 28% | Postmenopausal only | ER‐positive: Bisphosphonate: 99% | Neo/adjuvant therapy: Control: 25% | Endocrine therapy before randomisation: Bisphosphonate: 84% Control: 85% | |
| Zoledronate vs observation | Bisphosphonate: mean 50 (range 30‐68) years Observation: mean 49.1 (range 32‐69) years | Premenopausal Bisphosphonate: 52% Control: 56% Postmenopausal: Bisphosphonate: 48% Control: 44% | ER‐positive: Bisphosphonate: 53% Control: 58% | NR | NR | |
| Zoledronate vs observation | Bisphosphonate: 51.6 ± 9.9 years | Premenopausal: Control: 45% Postmenopausal: Bisphosphonate: 45% Control: 46% | ER‐positive: Bisphosphonate: 78% | Intended treatment chemotherapy plan: Bisphosphonate: 22% Control: 21% | Intended treatment endocrine therapy plan: Bisphosphonate: 5% | |
| Clodronate vs observation | Across both groups: Median 51 (range: 24‐78) years | Postmenopausal: Control: 61% | ER‐positive: Bisphosphonate: 66% Control: 58% | Adjuvant chemotherapy: | Adjuvant endocrine therapy: Control: 30% | |
| Ibandronate vs observation | Bisphosphonate: < 60 years: 81% | Premenopausal: Bisphosphonate: 48% Postmenopausal: Bisphosphonate: 51% | Hormone receptor‐positive: Bisphosphonate: 77% Control: 78% | NR | Adjuvant therapy: | |
| Zoledronate vs placebo | Bisphosphonate: 43 ± 6 years | Premenopausal only | Hormone receptor‐positive: Bisphosphonate: 74% | Bisphosphonates: Control: | Endocrine therapy after treatment: | |
| Pamidronate vs observation | Bisphosphonate: < 39 years: 16% 50‐59 years: 23% 60‐69 years: 15% Observation: < 39 years: 15% 50‐59 years: 23% 60‐69 years: 14% | Premenopausal: Bisphosphonate: 67% Control: 66% Postmenopausal: Bisphosphonate: 33% Control: 34% | ER‐positive: Bisphosphonate: 14% Control: 17% | NR | NR | |
| Zoledronate vs observation | Bisphosphonate: < 55 years: 67% > 55 years: 33% Observation: < 55 years: 66% | Premenopausal: Bisphosphonate: 22% | ER‐positive and/or PR‐positive: Bisphosphonate: 78% | NR | NR | |
| Clodronate vs placebo | Bisphosphonate: > 50 years: 64% Placebo: < 49 years: 36% > 50 years: 65% | NR | ER‐positive and/or PR‐positive: Bisphosphonate: 78% | Bisphosphonate: 21% Control: 21% | Bisphosphonate: 31% | |
| Clodronate vs placebo | Bisphosphonate: 52.8 ± 6 years 52.7 ± 10.5 years | Premenopausal: Bisphosphonate: 50% Control: 49% Postmenopausal: Bisphosphonate: 50% Control: 51% | ER‐positive: Bisphosphonate: 46% Control: 45% | Bisphosphonate: 16% | Tamoxifen: Bisphosphonate: 32% | |
| Clodronate vs observation | Bisphosphonate: 52 years (no SD provided) Observation: | Premenopausal: Bisphosphonate: 48% Postmenopausal: Bisphosphonate: 52% | ER‐positive: Bisphosphonate: 61% | Bisphosphonate: 50% Control: 58% | Pretreatment antioestrogen: Bisphosphonate: 50% Control: 58% | |
| Zoledronate vs clodronate vs ibandronate | Median 53 years (range not provided) | Postmenopausal or aged 50 plus: 58% | ER‐positive: | Planned adjuvant chemotherapy: 80% (not reported by group) | Planned endocrine therapy: | |
| Zoledronate vs observation | Across both groups: | Postmenopausal only | ER‐positive: Bisphosphonate: 81% | Any adjuvant chemotherapy: Bisphosphonate: 92% | Tamoxifen, other SERM or AI: Bisphosphonate: 75% Control: 72% | |
| Immediate vs delayed zoledronate | Immediate: median 58 (range 40‐81) years | Postmenopausal only | NR | Prior chemotherapy Immediate: 52% | NR | |
| Immediate vs delayed zoledronate | Immediate: 61.4 ± 9.28 years | Postmenopausal only | NR | NR | NR | |
| Immediate vs delayed zoledronate | Immediate: median 57 (range 36‐87) years | Postmenopausal only | NR | Prior adjuvant therapy | NR | |
| AI: aromatase inhibitor; ER: oestrogen receptor; NR: not reported; PR: progesterone receptor; SD: standard deviation; SERM: selective estrogen receptor modulator | ||||||
| Study | Treatment vs comparator | ONJ | Hypocalcaemia | Renal dysfunction | Drug‐related death | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | ||
| Zoledronate vs observation | 0/899 | 0/904 | NR | NR | 0/899 | 0/904 | 0/899 | 0/904 | |
| Denosumab vs placebo | 0/1709 | 0/1690 | 1/1709 | 3/1690 | 2a/1709 | 3a/1690 | 1/1709 | 0/1690 | |
| Zoledronate vs observation | 1/60 | 0/59 | NR | NR | 0/60 | 0/59 | NR | NR | |
| Zoledronate vs observation | 26/1685 | 0/1667 | NR | NR | 188/1685 | 158/1667 | 0/1685 | 0/1667 | |
| Clodronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate vs observation | 2/1832 | 0/968 | NR | NR | NR | NR | 0/1832 | 0/968 | |
| Zoledronate vs placebo | 0/50 | 0/53 | NR | NR | 0/50 | 0/53 | NR | NR | |
| Pamidronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs observation | 5/343 | 0/350 | NR | NR | 7b/343 | 4b/350 | 0/343 | 0/350 | |
| Clodronate vs placebo | 1/1612 | 0/1623 | 1/1612 (G3) | 2/1523 (G3/4) | NR | NR | 4/1612 | 7/1623 | |
| Clodronate vs placebo | 0/530 | 0/539 | NR | NR | 28/530 | 31/539 | 0/530 | 0/539 | |
| Clodronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs clodronate | Zoledronate: 24/2094 | Clodronate: 6/2151 | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs observation | 0/36 | 0/32 | 0c/36 | 0c/32 | NR | NR | NR | NR | |
| Immediate vs delayed zoledronate | 2/252 | 0/270 | NR | NR | 1/252 | 0/270 | 0/252 | 0/270 | |
| Immediate vs delayed zoledronate | 0/300 | 0/300 | NR | NR | 5/300 (G1 to 4) | 4/300 (G1 to 4) | 0/300 | 0/300 | |
| Immediate vs delayed zoledronate | 2/524 | 0/536 | NR | NR | 3/524 (G1/2) | 2/536 (G1/2) | 0/524 | 0/536 | |
| G: grade; n: number of events; N: number of women studied in each group; NR: not reported; ONJ: osteonecrosis of the jaw | |||||||||
| Study | Treatment vs comparator | Nausea | Fatigue | Fever | Influenza‐type symptoms | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | ||
| Zoledronate vs observation | 79/899 | 55/904 | 192/899 | 169/904 | 85/899 | 21/904 | NR | NR | |
| Denosumab vs placebo | 49/1709 | 42/1690 | 108/1709 | 98/1690 | 13/1709 | 8/1690 | 25/1709 | 20/1690 | |
| Zoledronate vs observation | NR | NR | NR | NR | 3/60 | 2/59 | NR | NR | |
| Zoledronate vs observation | NR | NR | NR | NR | 37/1685 | 24/1667 | NR | NR | |
| Clodronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs placebo | NR | NR | 24/50 | 29/53 | 11/50 | 10/53 | 21/50 | 21/53 | |
| Pamidronate vs observation | 324a/460 | 337a/493 | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs observation | NR | NR | 65b/343 | 36b/350 | 28b/343 | 1b/350 | NR | NR | |
| Clodronate vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Clodronate vs placebo | 143/530 | 161/539 | NR | NR | NR | NR | NR | NR | |
| Clodronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs clodronate | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate vs observation | NR | NR | NR | NR | NR | NR | NR | NR | |
| Immediate vs delated zoledronate | 17/252 | 14/270 | 38/252 | 50/270 | 17/252 | 0/270 | 15/252 | 3/270 | |
| Immediate vs delated zoledronate | 41/300 | 40/300 | 101/300 | 88/300 | 27/300 | 6/300 | NR | NR | |
| Immediate vs delated zoledronate | 46/524 | 42/536 | 84/524 | 81/536 | 78/524 | 15/536 | 45/524 | 8/536 | |
| n: number of events; N: number of women studied in each group; NR: not reported | |||||||||
| Study | Treatment | Comparator | Number of skeletal‐related events | Ratio: Bisphosphonate/comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Kanis 1996 | Clodronate 1600 mg oral | Placebo | 71 Event rate = event/100 patient years | 96.5 | 0.74 | P < 0.01 |
| Mardiak 2000 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| (N = 124) | Pamidronate 300 mg oral | Control | NR | NR | NR | NR |
| N: total number of women in the study; NR: not reported | ||||||
| Study | Treatment | Comparator | Median time to event (months) | Ratio ‐ Bisphosphonate/Comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Kanis 1996 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | No significant difference |
| Mardiak 2000 | Clodronate 1600 mg oral | Placebo | 28.4 | 13.4 | 2.1 | P = 0.42 |
| (N = 124) | Pamidronate 300 mg oral | Control | Not reached. First bone event was not within the first 36 months of the analysis | Not reached | ‐ | ‐ |
| N: total number of women in the study; NR: not reported | ||||||
| Study | Treatment | Comparator | Median survival time (months) | Ratio ‐ Bisphosphonate/comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Kanis 1996 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | Not significantly different |
| Mardiak 2000 | Clodronate 1600 mg oral | Placebo | 59.4 | 54.7 | 1.09 | P = 0.35 |
| (N = 124) | Pamidronate 300 mg oral | Control | NR | NR | NR | P = 0.30 |
| N: total number of women in the study; NR: not reported | ||||||
| Study | Questionnaires used | Summary of findings |
| NR | NR | |
| NR | NR | |
| Participants scored questionnaire items on a 4‐point scale (0 = none, 3 = very severe) | At baseline, mean scores were similar across the 2 groups however pamidronate had a worse score for fatigue compared to control. At follow‐up, the mean scores were similar in the 2 groups with similar worsening over time in mobility and gastrointestinal toxicity. There was no change in bone pain and fatigue over time or between the 2 groups | |
| NR: not reported | ||
| Study | Treatment vs comparator | Osteonecrosis of the jaw | Renal dysfunction | Bone pain | Drug‐related death | Additional comment | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | |||
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | 13a/66 | 17a/67 | NR | NR | No hypocalcaemia observed in either group | |
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | 1 participant with rash (clodronate); 2 participants with gastrointestinal toxicity (1 clodronate, 1 placebo); 1 participant with abdominal pain (placebo) | |
| Pamidronate 300 mg oral vs control | NR | NR | NR | NR | "...did not change over time and there was no effect of pamidronate treatment" | NR | NR | 4 participants with gastrointestinal intolerance in pamidronate group | ||
| n: number of events; N: number of women studied in each group; NR: not reported | ||||||||||
| Study | Treatment vs comparator | Nausea | Fatigue | Fever | Influenza‐type symptoms | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | ||
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Pamidronate 300 mg oral vs control | NR | NR | Scored 0.6 (worse than control arm) | Scored 0.3 | NR | NR | NR | NR | |
| n: number of events; N: number of women studied in each group; NR: not reported | |||||||||
| Study | Bisphosphonate | Comparator | No. of skeletal‐related events | Ratio: Bisphosphonate/comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Bisphosphonate vs placebo/open | ||||||
| AREDIA 1998 | Pamidronate 90 mg iv | Placebo | 2.4 | 3.7 | 0.65 | < 0.001 |
| (N = 312) | Ibandronate 6 mg iv | Placebo | 0.56 | 1.08 | 0.52 | 0.03 |
| Body 2004 | Ibandronate 50 mg oral | Placebo | 0.99 | 1.15 | 0.86 | 0.041 |
| Conte 1996 | Pamidronate 45 mg iv | Open | 135 | 169 | 0.80 | ‐ |
| Elomaa 1983 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| Heras 2009 | Ibandronate 6 mg iv | Placebo | NR | NR | NR | NR |
| Hultborn 1999 | Pamidronate 60 mg iv | Placebo | 0.98 Event rate = cumulative events/follow‐up | 1.41 | 0.70 | < 0.01 |
| Kohno 2005 | Zoledronate 4 mg iv | Placebo | 0.63 | 1.10 | 0.57 | 0.016 |
| Kristensen 1999 | Clodronate 800 mg oral, 2/d for 2 years | Open | 0.4 | 0.5 | 0.8 | ‐ |
| Martoni 1991 | Clodronate 300 mg oral | Placebo | NR | NR | NR | NR |
| Paterson 1993 | Clodronate 800 mg oral, 2/d for up to 3 years | Placebo | 218.6 | 304.8 | 0.72 | P < 0.001 |
| Tripathy 2004 | Ibandronate 50 mg oral | Placebo | 0.98 | 1.2 | 0.81 | 0.037 |
| Tubiana‐Hulin 2001 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| Van‐Holten 1987 | Pamidronate 150 mg oral, 2/d indefinitely | Open | 90 | 144 | 0.63 | 0.003 |
| Direct comparisons of different bisphosphonate regimens | ||||||
| (N = 318) | Pamidronate 60 mg iv | Clodronate 2400 mg oral or 900 mg iv | 16 Event = number of people with fractures | Clodronate oral = 11 Clodronate iv = 19 Event = number of people with fractures | NR | NR |
| Rosen 2004 | Zoledronate 4 mg iv | Pamidronate 90 mg iv | NR | NR | 0.81 | 0.037 |
| von Au 2016 | Pamidronate 60 mg iv | Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | 7.3% | 14.3% or 17.3% | NR | 0.07 (pamidronate versus clodronate oral) |
| ZICE 2014 | Ibandronate 50 mg oral | Zoledronate 4 mg iv | 0.507 | 0.425 | 1.19 | 0.035 |
| Bone‐targeted agents vs bisphosphonate | ||||||
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Bisphosphonate iv (clinician choice) | NR | NR | NR | NR |
| Lipton 2008 | Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) | Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | NR | NR | NR | NR |
| (N = 2046) | Denosumab 120 mg sc (iv placebo) | Zoledronate 4 mg iv (sc placebo) | 0.58 | 0.45 | 0.78 | 0.004 |
| Standard vs reduced bisphosphonate/bone agent | ||||||
| CALGB‐70604 2015 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR |
| (N = 403) | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | 0.46 | 0.50 | NR | NR |
| ZOOM 2013 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | 0.22 Event rate = skeletal morbidity rate (SRE/patient/year) Non‐inferiority not demonstrated | 0.26 | NR | NR |
| iv: intravenous; N: total number of women in each study; NR: not reported; sc: subcutaneous; SMPR: skeletal morbidity period rate; SRE: skeletal related event | ||||||
| Study | Bisphosphonate | Comparator | Median time to event (months) | Ratio: bisphosphonate/comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Bisphosphonate vs placebo/open | ||||||
| AREDIA 1998 | Pamidronate 90 mg iv | Placebo | 12.7 | 7 | 1.81 | < 0.001 |
| (N = 312) | Ibandronate 6 mg iv | Placebo | 12.65 | 8.28 | 1.34 | 0.018 |
| Body 2004 | Ibandronate 50 mg oral | Placebo | 20.8a | 14.9a | 1.39 | 0.089 |
| Conte 1996 | Pamidronate 45 mg iv | Open | 8.9 | 6 | 1.48 | 0.02 |
| Elomaa 1983 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| Heras 2009 | Ibandronate 6 mg iv | Placebo | 15.2a | 10.1a | 1.50 | 0.007 |
| Hultborn 1999 | Pamidronate 60 mg iv | Placebo | 11.8 | 8.4 | 1.4 | 0.006 |
| Kohno 2005 | Zoledronate 4 mg iv | Placebo | NR | 12^ | NR | 0.007 |
| Kristensen 1999 | Clodronate 800 mg oral, 2/d for 2 years | Open | NRb | NRb | NR | 0.015 |
| Martoni 1991 | Clodronate 300 mg oral | Placebo | NR | NR | NR | NR |
| Paterson 1993 | Clodronate 800 mg oral, 2/d for up to 3 years | Placebo | 9.9 | 4.9 | 2.02 | 0.022 |
| Tripathy 2004 | Ibandronate 50 mg oral | Placebo | 17.5a | 11.1a | 1.58 | NS |
| Tubiana‐Hulin 2001 | Clodronate 1600 mg oral | Placebo | 8.7 | 6.4 | 1.36 | 0.05 |
| Van‐Holten 1987 | Pamidronate 150 mg oral, 2/d indefinitely | Open | 14 | 11 | 1.27 | 0.10 |
| Direct comparisons of different bisphosphonate regimens | ||||||
| (N = 318) | Pamidronate 60 mg iv | Clodronate 2400 mg oral or 900 mg iv | NR | NR | NR | NR |
| Rosen 2004 | Zoledronate 4 mg iv | Pamidronate 90 mg iv | 10.3 | 5.8 | 0.56 | 0.013 |
| von Au 2016 | Pamidronate 60 mg iv | Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | NR | NR | NR | NR |
| ZICE 2014 | Ibandronate 50 mg oral | Zoledronate 4 mg iv | 22.4a | 22.9a | 1.034 | 0.7 |
| Bone‐targeted agents vs bisphosphonate | ||||||
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Bisphosphonate iv (clinician choice) | NR | NR | NR | NR |
| Lipton 2008 | Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) | Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | NR | NR | NR | NR |
| (N = 2046) | Denosumab 120 mg sc (iv placebo) | Zoledronate 4 mg iv (sc placebo) | Not yet reached | 26.4 | 0.82 | 0.01 |
| Standard vs reduced bisphosphonate/bone agent | ||||||
| CALGB‐70604 2015 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR |
| (N = 403) | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| ZOOM 2013 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| iv: intravenous; N: total number of women in each study; NR: not reported; NS: not significant; sc: subcutaneous; SMPR: skeletal morbidity period rate; SRE: skeletal‐related event. | ||||||
| Study | Bisphosphonate | Comparator | Median survival (months) | Ratio: bisphosphonate/comparator | P value reported | |
| Bisphosphonate | Comparator | |||||
| Bisphosphonate vs placebo/open | ||||||
| AREDIA 1998 | Pamidronate 90 mg iv | Placebo | 19.8 | 17.8 | 1.11 | 0.98 |
| (N = 312) | Ibandronate 6 mg iv | Placebo | 28.3a | 26.7a | 1.06 | NS |
| Body 2004 | Ibandronate 50 mg oral | Placebo | NR | NR | NR | NS |
| Conte 1996 | Pamidronate 45 mg iv | Open | 19.4 | 21 | 0.92 | NS |
| Elomaa 1983 | Clodronate 1600 mg oral | Placebo | 25 | 14 | 1.78 | 0.004 |
| Heras 2009 | Ibandronate 6 mg iv | Placebo | NR | NR | NR | NR |
| Hultborn 1999 | Pamidronate 60 mg iv | Placebo | 18.3 | 18.3 | 1.00 | NS |
| Kohno 2005 | Zoledronate 4 mg iv | Placebo | NR | NR | NR | NR |
| Kristensen 1999 | Clodronate 800 mg oral, 2/d for 2 years | Open | 18.3 | 18 | 1.02 | 0.97 |
| Martoni 1991 | Clodronate 300 mg oral | Placebo | NR | NR | NR | NR |
| Paterson 1993 | Clodronate 800 mg oral, 2/d for up to 3 years | Placebo | NR | NR | NR | 0.198 |
| Tripathy 2004 | Ibandronate 50 mg oral | Placebo | NR | NR | NR | NR |
| Tubiana‐Hulin 2001 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| Van‐Holten 1987 | Pamidronate 150 mg oral, 2/d indefinitely | Open | 25 | 24 | 1.04 | NS |
| Direct comparisons of different bisphosphonate regimens | ||||||
| (N = 318) | Pamidronate 60 mg iv | Clodronate 2400 mg oral or 900 mg iv | NR | NR | NR | NR |
| Rosen 2004 | Zoledronate 4 mg iv | Pamidronate 90 mg iv | NR | NR | NR | NR |
| von Au 2016 | Pamidronate 60 mg iv | Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | NR | NR | NR | NR |
| ZICE 2014 | Ibandronate 50 mg oral | Zoledronate 4 mg iv | 26.1 | 25.6 | 1.02 | 0.24 |
| Bone‐targeted agents vs bisphosphonate | ||||||
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Bisphosphonate iv (clinician choice) | NR | NR | NR | NR |
| Lipton 2008 | Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) | Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | NR | NR | NR | NR |
| (N = 2046) | Denosumab 120 mg sc (iv placebo) | Zoledronate 4 mg iv (sc placebo) | NR | NR | 0.95 | 0.49 |
| Standard vs reduced bisphosphonate/bone agent | ||||||
| CALGB‐70604 2015 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR |
| (N = 403) | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| ZOOM 2013 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| iv: intravenous; N: total number of women in each study; NR: not reported; NS: not significant; sc: subcutaneous | ||||||
| Study | Bisphosphonate | Comparator | Bone pain | Pain tool used | P value reported | |
| Bisphosphonate | Comparator | |||||
| Bisphosphonate vs placebo/open | ||||||
| AREDIA 1998 | Pamidronate 90 mg iv | Placebo | A significant difference in mean change from baseline pain score favouring pamidronate was first noted at 24 months | Reference to validation | P = 0.015 | |
| (N = 312) | Ibandronate 6 mg iv | Placebo | Significantly improved bone pain score over time favouring the ibandronate 6 mg group compared to placebo | 5‐point scale. No reference to validation | P < 0.001 | |
| Body 2004 | Ibandronate 50 mg oral | Placebo | At week 96, mean bone pain scores were significantly reduced from baseline with ibandronate compared to placebo (‐0.10, 95% CI ‐0.32 to 0.02 vs 0.20, 95% CI 0.07 to 0.34) | Participant‐rated scale | P = 0.001 | |
| Conte 1996 | Pamidronate 45 mg iv | Open | No significant difference between the groups at the predefined time points; most symptomatic variables showed a greater degree of improvement in the pamidronate group | 6‐point self‐assessment scale | NS | |
| Elomaa 1983 | Clodronate 1600 mg oral | Placebo | NR | NR | NR | NR |
| Heras 2009 | Ibandronate 6 mg iv | Placebo | NR | NR | NR | NR |
| Hultborn 1999 | Pamidronate 60 mg iv | Placebo | "...results favoured pamidronate however insignificant when corrected for the prestudy values" (page 3387) | Questionnaire & VAS | NS | |
| Kohno 2005 | Zoledronate 4 mg iv | Placebo | From weeks 4‐52, a chart of mean change in the BPI was statistically significant in favour of a reduction by zoledronic acid | BPI | NR | |
| Kristensen 1999 | Clodronate 800 mg oral, 2/d for 2 years | Open | No difference between groups using a physician‐rated scale (no reference to validation) | Physician‐rated scale. No reference to validation | NS | |
| Martoni 1991 | Clodronate 300 mg oral | Placebo | No significant difference | Scott‐Huskinsson Visual Analog method | NS | |
| Paterson 1993 | Clodronate 800 mg oral, 2/d for up to 3 years | Placebo | NR | NR | NR | NR |
| Tripathy 2004 | Ibandronate 50 mg oral | Placebo | From baseline to study end point, bone pain scores increased by +0.21 in the placebo group and a slight increase of +0.03 in the ibandronate 50 mg group | 4‐point scale | P = 0.201 | |
| Tubiana‐Hulin 2001 | Clodronate 1600 mg oral | Placebo | Significant reduction in pain in clodronate group compared to control group | Visual pain scale. No reference to validation | P = 0.01 | |
| Van‐Holten 1987 | Pamidronate 150 mg oral, 2/d indefinitely | Open | Bone scores were significantly higher in the control group with an early reduction in bone pain within the first 3 months of pamidronate. However, bone pain then increased significantly over time (P = 0.005) in both groups although more rapidly in the control than pamidronate group (P = 0.02) | 3 items on bone pain within a quality‐of‐life questionnaire designed specifically for this trial. Reliability of the questionnaire tested at first observation point of participants | P = 0.007 (pamidronate vs control at 3 months) | |
| Direct comparisons of different bisphosphonate regimens | ||||||
| (N = 318) | Pamidronate 60 mg iv | Clodronate 2400 mg oral or 900 mg iv | Trend to improvement with iv bisphosphonates (30% reduction with pamidronate iv, 25% reduction with clodronate iv) compared with oral clodronate (15%) | Pain tool not reported in abstract | NR | |
| Rosen 2004 | Zoledronate 4 mg iv | Pamidronate 90 mg iv | No difference | BPI | NS | |
| von Au 2016 | Pamidronate 60 mg iv | Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | Pain scores at baseline and final examinations were not significantly different among the groups. Overall, a slight increase in pain scores over time with no significant differences among the groups (P = 0.36) | VAS | NS | |
| ZICE 2014 | Ibandronate 50 mg oral | Zoledronate 4 mg iv | Pain scores reduced from baseline at 12 weeks and were maintained over 96 weeks. There was no difference between the groups | BPI | NS | |
| Bone‐targeted agents vs bisphosphonate | ||||||
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Bisphosphonate iv (clinician choice) | NR | NR | NR | NR |
| Lipton 2008 | Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) | Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | NR | NR | NR | NR |
| (N = 2046) | Denosumab 120 mg sc (iv placebo) | Zoledronate 4 mg iv (sc placebo) | Prolonged median time to develop moderate/severe pain from no pain on baseline (denosumab: zoledronate hazard ratio 0.78). Lower proportion of participants with moderate/severe pain from no pain on baseline (denosumab 14.8% vs zoledronate 26.7% at week 73) | BPI | P < 0.05 | |
| Standard vs reduced bisphosphonate/bone agent | ||||||
| CALGB‐70604 2015 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR |
| Fizazi 2009 | Denosumab 180 mg sc every 4 weeks | Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR |
| (N = 189) | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | Change from baseline in mean BPI score was 0.24 (standard deviation 1.976) in zoledronate every 4 weeks while the change from baseline score was 0.31 (standard deviation 2.099) in zoledronate every 12 weeks | BPI | NR | |
| ZOOM 2013 | Zoledronate 4 mg iv every 4 weeks | Zoledronate 4 mg iv every 12 weeks | Most people had a score < 4; median pain at rest and pain on movement scores were < 4 at all points in both groups | Validated 6‐point Verbal Rating Scale | NS | |
| BPI: Brief Pain Inventory; CI: confidence interval; iv: intravenous; N: total number of women in each study; NR: not reported; NS: not significantly different; sc: subcutaneous; VAS: visual analogue scale | ||||||
| Study | Questionnaires used | Summary of findings |
| Bisphosphonate vs placebo/open | ||
| Spitzer Quality‐of‐Life Index scores | "...quality of life scores worsened from baseline to the last visit in both groups, although less so in the pamidronate group (P = 0.057 and 0.088, respectively)" (page 1087) | |
| EORTC Quality of Life Scale ‐ Core 30 questionnaire (QLQ‐C30) | "...overall quality of life scores decreased to a lesser extent between baseline and last assessment for patients receiving 2 mg ibandronate (‐18.1) and 6 mg ibandronate (‐10.3) compared with patients receiving placebo (‐45.4)" (page 1709) | |
| EORTC QLQ‐C30 | Global quality of life scores decreased significantly during the study, though significantly less with ibandronate than with placebo (‐8.3, 95% CI ‐20.6 to 4.1 vs ‐26.8, 95% CI ‐39.4 to 14.3, P = 0.03) | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| EORTC QLQ‐C30 | "There was no significant difference between patients receiving clodronate and controls in the change from baseline to 3 or 6 months in any of the 17 quality‐of‐life variables" (page 71) | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| A questionnaire was developed specifically for the trial (validated 4‐point ordinal scale). The 4 items were related to mobility impairment, gastrointestinal toxicity, bone pain and fatigue | The mean mobility impairment score was higher in the control group than the pamidronate group (P = 0.03). Similarly, bone pain scores were higher in the control group compared to pamidronate (P = 0.007). No differences were noted in fatigue or gastrointestinal toxicity between the two groups | |
| Direct comparisons of different bisphosphonate regimens | ||
| NR | NR | |
| FACT‐G | No significant difference between groups. Quality‐of‐life data reported in conference presentation only | |
| NR | NR | |
| EORTC QLQ‐C30 | No difference | |
| Bone‐targeted agents vs bisphosphonate | ||
| NR | NR | |
| NR | NR | |
| FACT‐G | "...over monthly time points during an 18‐month period, an average of 10% more patients in the denosumab group compared with the zoledronic acid group had a clinically meaningful improvement in HRQoL ( > 5‐point increase in FACT‐G total score) over the course of the study. An average of 7% fewer patients in the denosumab group than in the zoledronic acid group had worsening of HRQoL on study" (page 7, Clinical Cancer Research) | |
| Standard vs reduced bisphosphonate/bone agent | ||
| NR | NR | |
| NR | NR | |
| NR | NR | |
| NR | NR | |
| CI: confidence interval; EORTC: European Organisation for the Research and Treatment of Cancer; NR: not reported | ||
| Study | Treatment vs comparator | Osteonecrosis of the jaw | Hypocalcaemia | Renal dysfunction | Drug‐related death | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | ||
| Bisphosphonate vs placebo/open | |||||||||
| Pamidronate 90 mg iv vs placebo | NR | NR | One participant had a "symptomatic hypocalcemia episode" (page 1088): 1/367 | 0/384 | NR | NR | 0/182 | 0/189 | |
| Ibandronate 6 mg iv vs placebo | NR | NR | NR | NR | No difference between ibandronate and control | 0/154 | 0/158 | ||
| Ibandronate 50 mg oral vs placebo | NR | NR | 27/286 | 14/277 | 15/286 "renal AEs". "No reports of serious AEs (renal failure) in the active treatment group" (page 1136) | 13/277 "renal AEs" | 0/286 | 0/277 | |
| Pamidronate 45 mg iv vs open | NR | NR | Transient asymptomatic hypocalcemia: 24/143 | Transient asymptomatic hypocalcaemia: 9/152 | NR | NR | NR | NR | |
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate 6 mg iv vs placebo | 0/75 | 0/75 | NR | NR | No comparable differences between ibandronate and control | NR | NR | ||
| Pamidronate 60 mg iv vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate 4 mg iv vs placebo | NR | NR | G1: 44/114, G2&3: 0/114; G4: 1/114 | G1: 8/113; G2&3: 0/113; G4: 1/113 | 0/114 | 0/113 | NR | NR | |
| Clodronate 800 mg oral, 2/d for 2 years vs open | NR | NR | 13/49 | 2/51 | NR | NR | NR | NR | |
| Clodronate 300 mg oral vs placebo | NR | NR | 0/19 | 0/19 | 0/19 | 0/19 | NR | NR | |
| Clodronate 800 mg oral bid for up to 3 years vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate 50 mg oral vs placebo | NR | NR | 10/148 | 6/143 | 10/148 | 6/143 | 0/148 | 0/143 | |
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Pamidronate 150 mg oral, 2/d indefinitely vs open | NR | NR | NR | NR | 1/81 "gradual deterioration in kidney function during 40 months of study" | 0/80 | NR | NR | |
| Direct comparisons of different bisphosphonate regimens | |||||||||
| Pamidronate 60 mg iv vs Clodronate 2400 mg oral or 900 mg iv | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate 4 mg iv vs Pamidronate 90 mg iv | NR | NR | NR | NR | Renal toxicity was greater in the zoledronate arm and was dependent on the dose and infusion time, compared to the pamidronate arm | NR | NR | ||
| Pamidronate 60 mg iv vs Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate 50 mg oral vs Zoledronate 4 mg iv | 5/704 | 9/697 | G3/4: 4/704 | G3/4: 4/697 | 172/704 "renal toxic effects" | 226/697 "renal toxic effects" | 3/704 | 4/697 | |
| Bone‐targeted agents vs bisphosphonate | |||||||||
| Denosumab 180 mg sc every 4 weeks or every 12 weeks vs Bisphosphonate iv (clinician choice) | NR | NR | G3/4: 7/73 | G/3/4: 1/35 | NR | NR | 0/73 | 0/35 | |
| Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) vs Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | 0/211 | 0/43 | NR | NR | No significant renal impairment in either arm | 0/211 | 0/43 | ||
| Denosumab 120 mg sc (iv placebo) vs Zoledronate 4 mg iv (sc placebo) | 26/1020 | 18/1013 | 62/1020 | 37/1013 | 50/1020. Renal failure: 2/1020 | 86/1013. Renal failure: 25/1013 | NR | NR | |
| Standard vs reduced bisphosphonate/bone agent | |||||||||
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | NR (reported for breast, prostate and multiple myeloma patients) | NR | NR | NR | NR | NR | NR | NR | |
| Denosumab 180 mg sc every 4 weeks vs Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR | "denosumab did not affect renal function" (page 1569). Data were not reported separately for denosumab every 4 weeks and every 12 weeks | 0/38 | 0/35 | ||
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | 2a/198 | 2a/202 | 1a/198 | 2a/202 | Renal failure: 0a/198 | Renal failure: 2a/202 | NR | NR | |
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | 4/209 | 3/216 | NR | NR | 1/209 "renal adverse event" | 2/216 "renal adverse event" | 0/209 | 0/216 | |
| AE: adverse event; G: grade; iv: intravenous; n: number of events; N: number of women studies in each group; NR: not reported; sc: subcutaneous | |||||||||
| Study | Treatment vs comparator | Nausea | GI events | Fatigue | Fever | ||||
| Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | Bone agent (n/N) | Comparator (n/N) | ||
| Bisphosphonate vs placebo/open | |||||||||
| Pamidronate 90 mg iv vs placebo | NR | NR | NR | NR | 147/367 | 112/386 | 51/367 | 19/386 | |
| Ibandronate 6 mg iv vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate 50 mg oral vs placebo | 10/286 | 4/277 | 6/286 | 2/277 | NR | NR | NR | NR | |
| Pamidronate 45 mg iv vs open | NR | NR | NR | NR | NR | NR | 7/143 | 5/152 | |
| Clodronate 1600 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Ibandronate 6 mg iv vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Pamidronate 60 mg iv vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate 4 mg iv vs placebo | 57/114 | 60/113 | 19/114 | 8/113 | 51/114 | 36/113 | 63/114 | 37/113 | |
| Clodronate 800 mg oral, 2/d for 2 years vs open | NR | NR | NR | NR | NR | NR | NR | NR | |
| Clodronate 300 mg oral vs placebo | NR | NR | NR | NR | NR | NR | NR | NR | |
| Clodronate 800 mg oral, 2/d for up to 3 years | NR | NR | Non‐specific GI symptoms: 2/85 | Non‐specific GI symptoms: 1/88 | NR | NR | NR | NR | |
| Ibandronate 50 mg oral vs placebo | 7/148 | 3/143 | Upper GIT events: 10% and similar to placebo | NR | NR | NR | NR | ||
| Clodronate 1600 mg oral vs placebo | 7/69 | 9/68 | 4/49 | 4/68 | 1/69 | 0/68 | NR | NR | |
| Pamidronate 150 mg oral, 2/d indefinitely vs open | NR | NR | 18/81 | 0/80 | NR | NR | NR | NR | |
| Direct comparisons of different bisphosphonate regimens | |||||||||
| Pamidronate 60 mg iv vs Clodronate 2400 mg oral or 900 mg iv | NR | NR | 14/112 | NR | NR | NR | NR | NR | |
| Zoledronate 4 mg iv vs Pamidronate 90 mg iv | 355/742 | 179/388 | NR | NR | 294/742 | 159/388 | 231/742 | 103/388 | |
| Pamidronate 60 mg iv vs Clodronate 900 mg iv every 3 weeks or 2400 mg/d oral | NR | NR | 14/109 | 900 mg iv every 3 weeks: 11/105; 2400 mg oral daily: 24/107 | NR | NR | NR | NR | |
| Ibandronate 50 mg oral vs Zoledronate 4 mg iv | G3/4: 41/704 | G3/4:38/697 | G3/4: 8/704 "dyspepsia" | G3/4: 2/697 "dyspepsia" | G3/4: 98/704 | G3/4: 97/697 | G3/4:12/704 | G3/4: 18/697 | |
| Bone‐targeted agents vs bisphosphonate | |||||||||
| Denosumab 180 mg sc every 4 weeks or every 12 weeks vs Bisphosphonate iv (clinician choice) | 17/73 | 7/35 | NR | NR | 8/73 | 4/35 | NR | NR | |
| Denosumab sc every 4 weeks (30 mg, 120 mg or 180 mg) or every 12 weeks (60 mg or 180 mg) vs Bisphosphonate iv (either zoledronate, pamidronate or ibandronate) | 36/211 | 8/43 | NR | NR | 28/211 | 5/43 | 13/211 | 10/43 | |
| Denosumab 120 mg sc (iv placebo) vs Zoledronate 4 mg iv (sc placebo) | 356/1020 | 384/1013 | NR | NR | 301/1020 | 324/1013 | 170/1020 | 247/1013 | |
| Standard vs reduced bisphosphonate/bone agent | |||||||||
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | NR | NR | NR | NR | NR | NR | NR | NR | |
| Denosumab 180 mg sc every 4 weeks vs Denosumab 180 mg sc every 12 weeks | NR | NR | NR | NR | NR | NR | NR | NR | |
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | 2/198 | 2/202 | 2/198 | 5/202 | 1/198 | 2/202 | 1/198 | 0/202 | |
| Zoledronate 4 mg iv every 4 weeks vs Zoledronate 4 mg iv every 12 weeks | G3/4: 24/209 | G3/4: 33/216 | 65/209 | 91/216 | G3/4:18/209 | G3/4: 19/216 | G3/4: 22/209 | G3/4: 28/216 | |
| G: grade; iv: intravenous; n: number of events; N: number of women studies in each group; NR: not reported; sc: subcutaneous | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bone metastases Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Bisphosphonate vs control | 11 | 15005 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 0.99] |
| 1.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.19] |
| 2 Bone metastases by bisphosphonate Show forest plot | 14 | 17195 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.97] |
| 2.1 Zoledronate 4 mg iv every 4 weeks | 8 | 8267 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.60, 0.99] |
| 2.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.70, 1.00] |
| 2.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.88, 1.50] |
| 2.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.13] |
| 3 Visceral recurrence Show forest plot | 13 | 17092 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.91, 1.17] |
| 3.1 Bisphosphonate vs control | 10 | 14902 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 3.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.60] |
| 4 Locoregional recurrence Show forest plot | 11 | 15721 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 4.1 Bisphosphonate vs control | 8 | 13531 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.85, 1.20] |
| 4.2 Immediate vs delayed | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.48] |
| 5 Overall recurrence Show forest plot | 14 | 17196 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 5.1 Bisphosphonate vs control | 11 | 15005 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.13] |
| 5.2 Immediate vs delayed | 3 | 2191 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.46] |
| 6 Overall recurrence by bisphosphonate Show forest plot | 14 | 17196 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.11] |
| 6.1 Zoledronate 4 mg iv every 4 weeks | 8 | 8268 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.23] |
| 6.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 6.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 6.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.82, 1.22] |
| 7 Overall survival: time‐to‐event outcome Show forest plot | 10 | 15013 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| 7.1 Bisphosphonate vs control | 9 | 13949 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.83, 0.99] |
| 7.2 Immediate vs delayed bisphosphonate | 1 | 1064 | Hazard Ratio (Fixed, 95% CI) | 0.69 [0.42, 1.13] |
| 8 Overall survival: dichotomous outcome Show forest plot | 12 | 16028 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 8.1 Bisphosphonate vs control | 10 | 14902 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.03] |
| 8.2 Immediate vs delayed | 2 | 1126 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.69, 6.60] |
| 9 Overall survival by bisphosphonate: time‐to‐event outcome Show forest plot | 10 | 15013 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.98] |
| 9.1 Zoledronate 4 mg iv every 4 weeks | 5 | 7038 | Hazard Ratio (Fixed, 95% CI) | 0.91 [0.81, 1.03] |
| 9.2 Clodronate 1600 mg oral daily | 4 | 4981 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 9.3 Ibandronate 50 mg oral daily | 1 | 2994 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.76, 1.42] |
| 10 Overall survival by bisphosphonate: dichotomous outcome Show forest plot | 12 | 16028 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 10.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7100 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.11] |
| 10.2 Clodronate 1600 mg oral daily | 4 | 4981 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.06] |
| 10.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.20] |
| 10.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.82, 1.49] |
| 11 Overall survival by menopausal status: time‐to‐event outcome Show forest plot | 9 | 14906 | Hazard Ratio (Fixed, 95% CI) | 0.90 [0.82, 0.99] |
| 11.1 Pre‐ or perimenopausal | 2 | 3501 | Hazard Ratio (Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| 11.2 Postmenopausal | 4 | 6048 | Hazard Ratio (Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 11.3 Pre‐ or postmenopausal, or both, or status not available | 5 | 5357 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 12 Overall survival by menopausal status: dichotomous outcome Show forest plot | 12 | 16011 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 12.1 Pre‐ or perimenopausal | 6 | 6191 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.96, 1.18] |
| 12.2 Postmenopausal | 9 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.03] |
| 12.3 Pre‐ or postmenopausal or status not available | 3 | 1670 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.20] |
| 13 Disease‐free survival: time‐to‐event outcome Show forest plot | 9 | 14242 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 13.1 Bisphosphonate vs control | 7 | 12578 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.87, 1.02] |
| 13.2 Immediate vs delayed bisphosphonate | 2 | 1664 | Hazard Ratio (Fixed, 95% CI) | 0.72 [0.52, 1.01] |
| 14 Disease‐free survival: dichotomous outcome Show forest plot | 10 | 15195 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 14.1 Bisphosphonate vs control | 8 | 13531 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.06] |
| 14.2 Immediate vs delayed | 2 | 1664 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.02] |
| 15 Disease‐free survival by bisphosphonate: time‐to‐event outcome Show forest plot | 9 | 14242 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 15.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7638 | Hazard Ratio (Fixed, 95% CI) | 0.89 [0.80, 0.98] |
| 15.2 Clodronate 1600 mg oral daily | 2 | 3610 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 15.3 Ibandronate 50 mg oral daily | 1 | 2994 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 16 Disease‐free survival by bisphosphonate: dichotomous outcome Show forest plot | 10 | 15202 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.04] |
| 16.1 Zoledronate 4 mg iv every 4 weeks | 6 | 7638 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.79, 0.98] |
| 16.2 Clodronate 1600 mg oral daily | 2 | 3617 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 16.3 Pamidronate 150 mg oral twice a day | 1 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.29] |
| 16.4 Ibandronate 50 mg oral daily | 1 | 2994 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.83, 1.21] |
| 17 Disease‐free survival by menopausal status: time‐to‐event outcome Show forest plot | 8 | 14106 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 17.1 Pre‐ or perimenopausal | 4 | 5493 | Hazard Ratio (Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 17.2 Postmenopausal | 7 | 8314 | Hazard Ratio (Fixed, 95% CI) | 0.82 [0.74, 0.91] |
| 17.3 Pre‐ or postmenopausal or status not available | 1 | 299 | Hazard Ratio (Fixed, 95% CI) | 1.53 [1.11, 2.11] |
| 18 Disease‐free survival by menopausal status: dichotomous outcome Show forest plot | 10 | 15150 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.03] |
| 18.1 Pre‐ or perimenopausal | 5 | 4997 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
| 18.2 Postmenopausal | 8 | 6536 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.77, 0.97] |
| 18.3 Pre‐ or postmenopausal or both, or status not available | 2 | 3617 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.32] |
| 19 Fracture incidence Show forest plot | 10 | 13212 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.90] |
| 19.1 Bisphosphonate vs control | 6 | 7602 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.54, 1.08] |
| 19.2 Denosumab vs placebo | 1 | 3420 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.41, 0.67] |
| 19.3 Immediate vs delayed bisphosphonate | 3 | 2190 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.57, 1.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Bone metastases Show forest plot | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.65, 1.43] |
| 2 Overall survival Show forest plot | 3 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SREs: bisphosphonate vs placebo/observation (including hypercalcaemia) Show forest plot | 8 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.95] |
| 2 SREs: bisphosphonate vs placebo/observation (excluding hypercalcaemia) Show forest plot | 9 | 2810 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.78, 0.95] |
| 3 SREs: by route of administration Show forest plot | 11 | 3219 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.78, 0.91] |
| 3.1 Intravenous bisphosphonates | 6 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.95] |
| 3.2 Oral bisphosphonates | 5 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.76, 0.93] |
| 4 SREs: by bisphosphonate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Zoledronate 4 mg iv | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.82] |
| 4.2 Pamidronate 90 mg iv | 1 | 754 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.69, 0.88] |
| 4.3 Ibandronate 6 mg iv | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.67, 0.96] |
| 4.4 Clodronate 1600 mg oral | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.71, 0.96] |
| 4.5 Ibandronate 50 mg oral | 1 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.02] |
| 4.6 Pamidronate 300 mg oral | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 5 SREs: denosumab vs bisphosphonate Show forest plot | 3 | 2345 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.72, 0.85] |
| 6 SREs: standard vs reduced frequency bone‐targeted agent Show forest plot | 3 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.26] |
| 7 Median time to SRE Show forest plot | 9 | 2891 | Median Ratio (Fixed, 95% CI) | 1.43 [1.29, 1.58] |
| 7.1 Bisphosphosphonate vs placebo/observation | 9 | 2891 | Median Ratio (Fixed, 95% CI) | 1.43 [1.29, 1.58] |
| 8 Overall survival Show forest plot | 7 | 1935 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 8.1 Intravenous bisphosphonate vs placebo/observation | 3 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.90, 1.16] |
| 8.2 Oral bisphosphonate vs placebo/observation | 4 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |

