Número de embriones a transferir después de la fertilización in vitro o de la inyección intracitoplasmática de espermatozoides
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Randomised controlled trial Single centre USA | |
| Participants | Inclusion criteria: women undergoing fresh assisted reproductive technology (ART); age < 35 years, day 3 follicle stimulating hormone (FSH) < 10 miu/ml; no history of poor response, no more than 1 previous in vitro fertilisation (IVF) failure, no uterine cavity abnormalities and no contraindication to treatment medications or procedures. Exclusion criteria: patients with uterine abnormalities such as submucous fibroid, endometrial polyps, uterine septum or significant uterine arcuate anomaly were not excluded if they were corrected hysteroscopically and post‐procedure sono‐infusion‐hysterogram (SIH) was normal. | |
| Interventions | Intervention (n = 50): single fresh blastocyst transfer; if unsuccessful, a frozen double blastocyst transfer was done. Control (n = 50): fresh double blastocyst transfer was done. However, since both arms received double blastocyst transfer, we did not take the data from second cycle. | |
| Outcomes | Live birth rate, cumulative live birth rate, clinical pregnancy rate, multiple pregnancy rate, implantation rate, miscarriage rate, ectopic rate | |
| Notes | The study was first published as a conference abstract. Subsequently, the study was published in peer reviewed journal. The authors were contacted for clarification. The authors replied to all study related queries. The trial was registered (ISRCTN69937179). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized at the time of blastocyst transfer by computer generated table”. |
| Allocation concealment (selection bias) | Low risk | Envelopes were prepared by research assistant who was not involved in recruitment, consent, assignment or treatment. Group assignment was placed in sequentially numbered, identical sealed envelopes. The subject group assignment was blinded from the all study staff (nurse coordinator, nurses, embryologists, physicians) by placing the group assignment in sequentially numbered, sealed identical envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Author reply: no blinding for clinician or embryologist. We categorised the study to be at high risk for performance bias. |
| Blinding of outcome assessment (detection bias) | Low risk | The clinician or embryologist were not blinded. However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | Authors mention all the dropouts and exclusions. All the randomised women are accounted for in the flow chart. The attrition after randomisation was minimal (1 in intervention vs none in comparison). |
| Selective reporting (reporting bias) | Low risk | The trial was registered (ISRCTN69937179) and pre‐stated outcomes were reported in the manuscript |
| Other bias | High risk | Partially funded by Ferring Pharmaceuticals. The authors provided trial registry number, the trial was retrospectively registered. In the baseline characteristics, the proportion of excellent blastocysts transferred were significantly higher in SET group. This can potentially influence the outcomes. Duration of infertility was mentioned. The planned sample size was 200 (as per trial registry information), but just 100 women were randomised. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial | |
| Participants | Female age < 35 yrs if no previous ART pregnancy, < 40 if previous ART pregnancy. At least 4 good‐quality embryos or at least 3 if previous ART pregnancy successful 27 women randomised | |
| Interventions | Cleavage‐stage transfer: SET (n = 13) versus DET (n = 14) Eligibility into the trial was restricted to a single cycle of treatment. All subsequent cycles of treatment were performed under conditions of routine care. | |
| Outcomes | Cumulative live birth, twin live birth, clinical ongoing pregnancy (fetal heartbeat), complications during pregnancy, delivery and neonatal period, perinatal mortality and morbidity, use of neonatal intensive care | |
| Notes | Unpublished trial. This study was stopped because its implementation immediately and substantially altered patients’ decision making, which more than tripled the rates of elective single embryo transfer during the study period, and reduced participation rates (M Davies, University of Adelaide, personal communication). Funded by National Health and Medical Research Council Grant no: 158006) (M Davies, University of Adelaide, personal communication) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Pre‐randomised envelopes were used and stored in the laboratory, opened in numerical order |
| Blinding of participants and personnel (performance bias) | Low risk | Patients were not informed of the number of embryos transferred nor the number of embryos suitable for freezing until immediately after their embryo transfer, doctors were also not informed of the randomisation until after their patients' embryo transfer, database manager and data analyser were also blinded until completion of data analysis by using codes to represent the 2 treatment groups. The code was held by an independent third party We categorised the study at low risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Patients were not informed of the number of embryos transferred nor the number of embryos suitable for freezing until immediately after their embryo transfer, doctors were also not informed of the randomisation until after their patients' embryo transfer, database manager and data analyser were also blinded until completion of data analysis by using codes to represent the 2 treatment groups. The code was held by an independent third party We categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the protocol were reported |
| Other bias | Unclear risk | Day of randomisation on day of embryo transfer |
| Study characteristics | ||
| Methods | Randomised controlled trial Single centre Spain | |
| Participants | Included women were recipients between 18 and 50 years, undergoing first or second synchronised fresh oocyte donation cycle with minimum of 5 embryos with at least 2 good‐quality embryos on day 3 after oocyte retrieval Exclusion: Medical indication for single embryo transfer (Turner syndrome, uterine pathology/surgery, diabetes, hypertension, cardiovascular disease, serious general disease) and severe male factor | |
| Interventions | Intervention (SET, n = 34): Elective single embryo transfer (since at least 2 good quality embryos on day 3 was entry criteria), They were transferred at cleavage stage Control (DET, n = 31): Elective DET, Control had at least 2 good‐quality embryos with 5 available embryos on day 3 as entry eligibility. They were transferred at cleavage stage Subsequent frozen cycle did not follow randomised numbers. They were DET in frozen cycle as per unit policy. They were not included in the analysis | |
| Outcomes | Live birth rate, cumulative live birth, clinical pregnancy rate, implantation rates, multiple pregnancy rate, miscarriage rate | |
| Notes | The authors responded to all the study‐related queries The trial was terminated prematurely due to unacceptable high levels of multiple pregnancies in control arm The trial was registered with clinical trial registry (NCT01228474) Funding details were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “based on a computer generated simple randomization list”. |
| Allocation concealment (selection bias) | Low risk | “One or two embryos were transferred based on computer generated simple randomization list with concealed allocation created by statistical and epidemiology unit” Authors reply "We allocated the patients to a hidden random sequence through a computer‐generated simple randomization list" |
| Blinding of participants and personnel (performance bias) | High risk | Author reply: no blinding for clinician or embryologist We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Author reply: no blinding for clinician or embryologist However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No attrition reported after randomisation in either group |
| Selective reporting (reporting bias) | Low risk | The trial was registered (ClinicalTrials.gov Identifier: NCT01228474) and pre‐stated outcomes were reported in the manuscript |
| Other bias | Unclear risk | Baseline characteristics were comparable in both the groups The trial was prematurely terminated. The trial was terminated (at 65) before reaching the planned sample size (n = 160) due to high number of multiple births in the comparison |
| Study characteristics | ||
| Methods | Randomised controlled trial, computer‐generated random sequence, n = 23 women analysed | |
| Participants | Inclusion criteria: all women receiving IVF or intracytoplasmic sperm injection (ICSI) treatment with an optimal chance of achieving pregnancy, i.e. women aged less than 37 years, first or second cycle of treatment, 4 or more good quality embryos at the time of embryo transfer Exclusion criteria: women undergoing pre‐implantation genetic diagnosis, or assisted hatching, or a history of recurrent miscarriage | |
| Interventions | Cleavage‐stage transfer: SET fresh + multiple SET frozen (n = 11) versus DET fresh + multiple DET frozen (n = 12) Both groups: if a pregnancy does not result in the fresh cycle, women will be encouraged to return for replacement of frozen‐thawed embryos in subsequent cycles over the next 12 months | |
| Outcomes | Cumulative live birth, twin live birth, clinical pregnancy (at least 1 gestational sac with heartbeat), biochemical pregnancy (positive test), miscarriage, ectopic pregnancy preterm delivery, low birth weight, congenital abnormality | |
| Notes | Unpublished trial. This study was stopped because of poor recruitment (planned for 700 women, enrolled only 23) Funded by the Wellcome Trust (UK) (grant ref: 067469) and the Bertarelli Foundation (Switzerland) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation performed by the embryologist (call to the Aberdeen Fertility Centre) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded, couples and clinician or nurse who performed the embryo transfer were blinded to the number of embryos transferred We categorised the study at low risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded, couples and clinician or nurse who performed the embryo transfer were blinded to the number of embryos transferred However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the protocol were reported |
| Other bias | Unclear risk | Duration of infertility not reported. |
| Study characteristics | ||
| Methods | Randomised controlled trial. 48 women randomised | |
| Participants | Women aged up to 43 years, undergoing IVF and embryo transfer with their own oocytes. Day 3 FSH no more than 10 mIU/ml, E2 under 80 pg/ml, hysteroscopically normal endometrial cavity, at least 10 follicles over 12 mm in diameter on day of hCG administration | |
| Interventions | Blastocyst stage transfer: Single versus double blastocyst transfer | |
| Outcomes | Ongoing pregnancy (defined as gestational sac with cardiac activity noted on ultrasound exam at least 4.5 weeks after embryo transfer), multiple gestation | |
| Notes | Supported in part by grants from Organon International and Vitrolife AB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding of participants and personnel (performance bias) | High risk | Blinding status not stated We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding status not stated However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts mentioned, but results presented as percentages so it is unclear whether all women were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Live birth not reported |
| Other bias | Unclear risk | Baseline characteristics (indication for IVF, age, baseline ovarian reserve) similar. Duration of infertility not reported |
| Study characteristics | ||
| Methods | Randomised controlled trial. States external concealment for concealment of allocation. Good‐quality embryos transferred, morphology of good‐quality embryos defined. Protocols for ovarian stimulation, oocyte retrieval, insemination and embryo transfer clearly described. Natural progesterone used for luteal phase support. Semen was prepared using mini‐percoll gradient prior to insemination. Medi‐Cult medium used for embryo culture. Wallace embryo transfer catheter was used for transfer. Embryo transfer was performed on day 3, 64 to 67 hours after insemination, results expressed using 95% confidence intervals analysis 53 women randomised | |
| Participants | First IVF/ICSI cycle. Female age < 34 years. Average duration of infertility 3.5 years | |
| Interventions | 1 embryo transfer versus 2 embryo transfer | |
| Outcomes | Clinical pregnancy rate, live birth rate, multiple pregnancy rate per woman or couple and implantation rates | |
| Notes | Method of randomisation not mentioned. Blinding not stated. Power calculation not reported. Intention‐to‐treat analysis not performed. Withdrawals and dropouts not mentioned clearly. Indication for treatment not mentioned. Previous treatment not mentioned Sponsored by the Foundation Marguerite‐Marie Delacroix, dedicated to the prevention of cerebral palsy, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | States external concealment |
| Blinding of participants and personnel (performance bias) | High risk | Blinding status not stated We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding status not stated However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Reports live birth and multiple pregnancy rates |
| Other bias | Unclear risk | Duration of infertility reported. Indication for treatment not mentioned. Previous treatment not mentioned |
| Study characteristics | ||
| Methods | 2‐centre randomised controlled trial. Randomisation performed before embryo quality was known 45 women randomised | |
| Participants | Patients on the waiting list for IVF/ICSI. Women > 38 years and had an indication for IVF/ICSI either for the first time or after a previous IVF/ICSI childbirth | |
| Interventions | Cleavage stage transfer (day 3 or 4): 2 embryo transfer in the first 3 cycles versus 3 embryo transfer in the first 3 treatment cycles | |
| Outcomes | Cumulative live birth rate, live birth rate, multiple pregnancy rate | |
| Notes | Chi² test and Mann–Whitney U test used for analysis. Randomisation was performed before information on embryo quality was available. Power calculation not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Remote: "Randomization was carried out using sealed envelopes opened by the study coordinator on the phone" |
| Allocation concealment (selection bias) | Low risk | Remote: "Randomization was carried out using sealed envelopes opened by the study coordinator on the phone" |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not mentioned. We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding not mentioned However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All 45 women analysed by intention to treat |
| Selective reporting (reporting bias) | Low risk | Reports cumulative live birth rate, live birth rate, multiple pregnancy rate. |
| Other bias | Low risk | Duration of infertility reported |
| Study characteristics | ||
| Methods | Single‐centre RCT | |
| Participants | Women attending IVF clinic: 169 analysed (212 cycles) | |
| Interventions | Cleavage‐stage transfer (day 2): 2 versus 3 embryo transfer, number of cycles unclear | |
| Outcomes | Clinical pregnancy (gestational sac), ongoing pregnancy, live birth, multiple pregnancy | |
| Notes | 'Per cycle' data only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described; "patients were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not mentioned We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding not mentioned However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Dropouts and withdrawals not reported, 'per cycle' data only |
| Selective reporting (reporting bias) | Unclear risk | Reports expected outcomes, but only as 'per cycle' data |
| Other bias | Unclear risk | No information reported about baseline characteristics |
| Study characteristics | ||
| Methods | Randomised controlled trial 107 women randomised | |
| Participants | First IVF/ICSI cycle. Female age < 35 years, FSH < 10IU/L. At least 1 good‐quality embryo should be available | |
| Interventions | Cleavage‐stage transfer (day 3): SET (2 cycles) versus DET transfer | |
| Outcomes | Clinical pregnancy rate, live birth rate, multiple pregnancy rates and miscarriage rates per woman/couple. | |
| Notes | Good quality embryos transferred, but morphologic characteristics not defined clearly. Embryo transfer took place on day 3 after insemination. Patients and physicians not blinded to treatment. Power calculation reported. Details of those lost to follow‐up given. Duration of infertility and indication for treatment provided. Protocols for IVF/ICSI described. Methods of statistical analysis mentioned Chi² test and Student's t‐test were used for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | "Allocation to the randomized group by an opaque, sealed envelope took place just before embryo transfer by the laboratory personnel to maintain concealment to the last moment". Does not specify that envelopes were consecutively numbered. |
| Blinding of participants and personnel (performance bias) | High risk | Patients and physicians not blinded to treatment We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Patients and physicians not blinded to treatment However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women analysed |
| Selective reporting (reporting bias) | Low risk | Reports cumulative live birth rate, live birth rate, multiple pregnancy rate |
| Other bias | Low risk | Duration of infertility reported |
| Study characteristics | ||
| Methods | Randomised controlled trial Single centre Spain | |
| Participants | Inclusion criteria: women undergoing IVF, < 38 years, BMI 19 to 29 kg/m²; FSH < 15 mIU/ml on day 3; first or second cycle with previous attempt with positive pregnancy test. Exclusion criteria: patients were excluded if infertility > 5 years; had previous surgery (fibroid, endometriosis, hydrosalphinx); uterine malformations; repeated spontaneous abortions (2 or more). | |
| Interventions | Intervention (n = 84): 2 transfers; first fresh single embryo transfer followed by frozen SET if unsuccessful. In some cases of OHSS with freeze all, single embryo transfer done in frozen cycle. Control (n = 91): fresh double embryo transfer, on day 2 or 3 If freeze all for OHSS, then only 1 cycle of frozen transfer with 2 embryos. | |
| Outcomes | Live birth rate, cumulative live birth rate, clinical pregnancy rate, ongoing pregnancy rate, multiple pregnancy rate, miscarriage rate. | |
| Notes | Authors replied to all the data related queries satisfactorily. The trial was registered under clinical trial registry (NCT01909570). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated randomization numbers...” |
| Allocation concealment (selection bias) | High risk | The allocation concealment was not described in the manuscript. Author reply: randomisation was carried out through a list of random numbers, a single embryologist had access to this list of random numbers. |
| Blinding of participants and personnel (performance bias) | High risk | No clear mention of blinding in the manuscript. Author reply: Embryologist performed the interview for randomisation. Embryologist had the information about group allotment. The study was double blind until the day of transfer to clinicians, patients, nurses and embryologists. Due to lack of blinding of clinician and embryologist on the day of embryo transfer, we categorised the study at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | No clear mention of blinding in the manuscript. However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | The attrition was similar in both the arms. Intention‐to‐treat analysis was done. We categorised as low risk for attrition bias. |
| Selective reporting (reporting bias) | Low risk | The trial was registered (ClinicalTrials.gov Identifier: NCT01909570). All the prespecified outcomes were reported in the final manuscript. |
| Other bias | Low risk | Important baseline variables were similar in both the groups. Duration of infertility mentioned. Received institutional grant (Instituto Carlos III, code number: FIS09‐1968). No other source of bias detected. |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial 144 women randomised | |
| Participants | Fresh IVF/ICSI treatment who had/not had more than 1 previous failed treatment. Frozen embryo transfers were analysed separately. At least 4 good‐quality embryos should be available for inclusion in the trial. | |
| Interventions | Cleavage‐stage transfer: 1 embryo transfer (n = 74) versus 2 embryo transfer (n = 70). Good‐quality embryos transferred. Morphology of good‐quality embryos described clearly. Protocols for IVF/ICSI clearly defined. Effectiveness of 1 versus 2 embryo transfer in frozen replacement cycles analysed separately. All centres involved used various age limits for inclusion of women. Embryos cultured in Medi‐Cult medium. IVF‐500 medium or Sydney IVF medium (Cook IVF) catheters were used for embryo transfer. Embryo transfer performed 46 to 50 hours after oocyte recovery. Natural progesterone used for luteal phase support. Chi² test and 2‐tailed t‐tests used for statistical analysis | |
| Outcomes | Reports clinical pregnancy rate, live birth rate, multiple pregnancy rates per woman/couple. Implantation and miscarriage rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table, balanced in sets of 10 |
| Allocation concealment (selection bias) | Unclear risk | Not clear: allocation done by laboratory personnel |
| Blinding of participants and personnel (performance bias) | High risk | Not stated We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Not stated However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All women included in analysis |
| Selective reporting (reporting bias) | Low risk | Reports cumulative live birth rate, live birth rate, multiple pregnancy rate |
| Other bias | Unclear risk | Duration of infertility not mentioned |
| Study characteristics | ||
| Methods | Single‐centre RCT | |
| Participants | ART candidates referred to university clinic, 298 analysed | |
| Interventions | 1 cycle of double embryo transfer (155 analysed) versus triple embryo transfer (143 analysed). Day of transfer not reported | |
| Outcomes | Clinical pregnancy (fetal heart on ultrasound); multiple pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated: "the subjects were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not mentioned We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding not mentioned However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | High risk | Women who did not follow the prescribed drug regimen or who had OHSS were excluded (numbers not reported). 3 women with ectopic pregnancy also excluded ‐ not stated which group they were in |
| Selective reporting (reporting bias) | Unclear risk | Live birth not reported |
| Other bias | Unclear risk | Duration of infertility not mentioned |
| Study characteristics | ||
| Methods | Randomised open‐label controlled trial, designed to show equivalence Patients were informed on day 3 of embryo culture of the assigned group by their physician. Randomised women were allowed to change group if they did not feel confident and expressed a desire to modify the day or number of transferred embryos. Both ITT and per protocol analysis reported | |
| Participants | Inclusion criteria Women requesting fertility treatment, aged under 38 years, and first trial of in vitro fertilisation or intracytoplasmic sperm injection. At least 4 good‐quality embryos on day 3 of embryo development Exclusion criteria Patients who underwent pre‐implantation genetic diagnosis or oocyte donation treatments were excluded. Patients were also excluded if the sperm was not obtained from an ejaculate sample 199 women randomised | |
| Interventions | Day 3 of embryo culture: Cleavage stage SET (n = 50) Cleavage stage DET (n = 49) Day 5 of embryo culture: Blastocyst stage SET (n = 50) Blastocyst stage DET (n = 50) The number of embryos transferred on subsequent thawed embryo cycles was determined independently of the randomised group the patient belonged to. Protocols for IVF, embryo culture, transfer and freezing reported in detail in study publication | |
| Outcomes | Multiple birth, live birth, patient acceptance | |
| Notes | In press December 2012 Study enrolment ceased before planned sample size (n = 412) due to change in embryo cryopreservation programme at IVI Seville. Sponsored by the Instituto Valenciano de Infertilidad, Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of web site Randomization.com to generate randomly permuted blocks of 8 subjects per block |
| Allocation concealment (selection bias) | Low risk | The randomisation was kept in a locked drawer in the administration office where the clinical staff who enrolled participants had no access. The assigned group was requested by phone |
| Blinding of participants and personnel (performance bias) | High risk | Open label We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Open label However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Low risk | ITT outcomes reported for all women randomised |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Groups well‐balanced at baseline High proportion of participants changed groups (mostly from SET to DET): Cleavage‐stage SET = 30 (50 randomised) Cleavage‐stage DET = 71 (49 randomised) Blastocyst‐stage SET = 37 (50 randomised) Blastocyst‐stage DET = 57 (50 randomised) Study data were analysed by intention to treat (as reported in this review) and also per protocol |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial 661 women randomised | |
| Participants | First or second IVF cycle who had at least 2 embryos of good quality available for transfer or freezing. Female age < 36 years. Duration and cause for infertility mentioned | |
| Interventions | Transfer on day 2 (93%), day 3 (5%) (cleavage stage), or day 5 (2% to 3%) (blastocyst stage) a. 1 embryo transfer (n = 330) versus 2 embryo transfer (n = 331) | |
| Outcomes | Clinical pregnancy rate, live birth rate, multiple pregnancy rates and miscarriage rates per woman/couple. | |
| Notes | Power calculation performed. Good‐quality embryos transferred, morphologic characteristics defined clearly. Embryo transfer took place on day 2, 3 or 5 days after oocyte retrieval. Women lost to follow‐up mentioned. Fisher's non‐parametric permutation test and Fisher's exact test used for statistical analysis and 95% confidence intervals calculated. 8 women in each group (2.4%) had blastocyst transfer at day 5 Supported by a grant from Serono Nordic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation at a ratio of 1:1 |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study We categorised the study to be at low risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind study We categorised the study to be at low risk for detection bias |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women analysed |
| Selective reporting (reporting bias) | Low risk | Cumulative live birth rate, live birth rate, multiple pregnancy rate |
| Other bias | Unclear risk | No mean duration of infertility given. 8 women in each group (2.4%) had blastocyst transfer at day 5 |
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial. Computer‐generated randomisation at a ratio of 1:1 27 women randomised | |
| Participants | Female age ≥ 36 years. First or second IVF/ICSI cycle. At least 2 good‐quality embryos available | |
| Interventions | Transfer at cleavage stage (23/27; 85%) or blastocyst stage (4/27; 15%) DET fresh versus SET fresh + SET frozen | |
| Outcomes | Reports live birth rate per woman, multiple live birth per woman | |
| Notes | Unpublished trial, pilot study, part of a thesis Supported by a grant from Serono Nordic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation at a ratio of 1:1 |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind study We categorised the study to be at low risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind study We categorised the study to be at low risk for detection bias |
| Incomplete outcome data (attrition bias) | Low risk | Women lost to follow‐up mentioned ITT performed |
| Selective reporting (reporting bias) | Low risk | Reports cumulative live birth rate, live birth rate, multiple pregnancy rate |
| Other bias | Unclear risk | No mean duration of infertility given |
| Study characteristics | ||
| Methods | Randomised controlled trial 308 women randomised | |
| Participants | First IVF cycle. Participants had to have at least 2 oocytes (2PN embryos) | |
| Interventions | Cleavage‐stage transfer (day 2 or 3): 1 embryo versus 2 embryo transfer | |
| Outcomes | Reports clinical pregnancy rate, multiple pregnancy rate per woman/couple | |
| Notes | Randomisation performed immediately prior to embryo transfer, but method of randomisation not stated. Patient population was stratified with respect to female age (< 38 and > 38 years), fertilisation technique (IVF/ICSI). Power calculation performed. Number lost to follow‐up mentioned. Duration and cause for infertility mentioned. Analysis of variance (ANOVA) with Tukey's multiple test procedure and Chi² test were used for statistical analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | "by using a nontransparent box containing the sealed opaque envelopes, the randomization procedure was blinded". Does not state that envelopes were consecutively numbered |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded study We categorised the study to be at low risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded study We categorised the study to be at low risk for detection bias |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women included in analysis |
| Selective reporting (reporting bias) | Low risk | Reports pregnancy rate, multiple pregnancy rate, miscarriage rate |
| Other bias | Unclear risk | Duration of infertility not provided |
| Study characteristics | ||
| Methods | Randomised controlled trial 56 women included in analysis | |
| Participants | Fresh IVF/ICSI cycle. Frozen embryo transfers analysed separately. Age ≤ 35 years. Cleavage rate ≥ 70% for IVF. Good‐quality embryos transferred. Morphological characteristics of good‐quality embryos defined. Study and control groups were comparable in terms of age, number of hMG ampoules required for ovarian stimulation, mean number of oocytes obtained and the number of embryos obtained. Indications for IVF was also comparable in both groups. Protocols for IVF/ICSI defined. HCG and natural progesterone used for luteal phase support. IVF using donor sperm was also included and the number of patients who used donor sperm for IVF was also comparable in the 2 groups. Patients who had a single, successful previous IVF attempt were also included | |
| Interventions | Cleavage stage transfer: 2 (n = 28) versus 4 (n = 28) embryo transfer | |
| Outcomes | Clinical pregnancy rate, live birth rate and multiple pregnancy rate per woman/couple | |
| Notes | Method of randomisation not mentioned. Blinding not stated. Allocation concealment not clear. Power calculation not reported. Intention‐to‐treat analysis not performed. Details of withdrawals, dropouts not given. Duration of infertility and indication for treatment not provided. Methods of statistical analysis not clearly mentioned. Embryo culture medium and catheter used for embryo transfer not described. Day of embryo transfer also unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Not stated We categorised the study to be at high risk for performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | Not stated However, due to objective nature of outcomes, we categorised the study at low risk for detection bias for all outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Details of withdrawals, dropouts not given. |
| Selective reporting (reporting bias) | Low risk | Reports live birth rate and multiple pregnancy rate per woman/couple |
| Other bias | Unclear risk | Day of embryo transfer also unclear |
ART ‐ assisted reproductive technology; SET ‐ single embryo transfer; DET ‐ double embryo transfer; ICSI ‐ intracytoplasmic sperm injection; IVF ‐ in vitro fertilisation; mIU/ml ‐ milli international unit/ millilitre; RCT ‐ randomised controlled trial; ITT ‐ intention to treat; BMI ‐ body mass index; 2 PN ‐ 2 pronuclei; OHSS ‐ ovarian hyperstimulation syndrome; E2 ‐ estradiol; kg/m2 ‐ kilograms/ metre 2; pg/ml ‐ picograms/ millilitre
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| RCT comparing convention IVF and SET vs modified natural cycle IVF vs 3 cycles of IUI | |
| Non‐randomised study of double blastocyst transfer versus single blastocyst plus frozen transfers. NB: same publication also includes Livingstone 2001. | |
| Secondary analysis looking at decision‐making process. | |
| Review article on methodological considerations in decision making | |
| Compares cleavage versus blastocyst transfer. On average more embryos were transferred in the cleavage‐stage group but this was not prespecified policy. | |
| Compares quantitative chromosome‐screened SET (pre implantation genetic screening) versus morphology‐based DET. | |
| Compares preimplantation genetic screening SET vs morphology based DET along with cost analysis | |
| Compares obstetric outcomes of pregnancies following preimplantation genetic screening SET vs morphology‐based DET | |
| Compares cleavage versus blastocyst transfer. On average more embryos were transferred in the cleavage‐stage group but this was not prespecified policy | |
| Compares cleavage versus blastocyst transfer. On average more embryos were transferred in the cleavage‐stage group but this was not prespecified policy | |
| Not randomised controlled trial | |
| Individualised participant data (IPD) meta analysis | |
| In vitro maturation (IVM) cycles; retrospective study | |
| The ovarian stimulation regimes used for the 2 randomised groups (SET versus DET) were significantly different | |
| Compared sequential day 3 and day 5 transfer with single‐day 5 embryo transfer | |
| Non randomised design | |
| Compares cleavage versus blastocyst transfer. On average more embryos were transferred in the cleavage‐stage group but this was not prespecified policy | |
| No comparison of interest ‐ compares double cleavage‐stage embryo versus single blastocyst‐stage embryo. Mentioned in same paper as Bowman 2004 | |
| Retrospective cohort study | |
| RCT comparing 3 to 5 cleavage‐stage versus 1 to 3 blastocyst‐stage embryos | |
| Quasi‐randomised trial ‐ days of week used | |
| Comparing morphology based screening (SET) vs pre implantation genetic screening SET | |
| Compares cleavage versus blastocyst transfer. On average more embryos were transferred in the cleavage‐stage group but this was not prespecified policy | |
| Pre‐implantation genetic screening cost analysis study | |
| Review article | |
| Not randomised controlled trial | |
| Orginal trial was included which included randomised group evaluating SET vs DET. Current trial looked at cumulative live birth following subsequent frozen transfer of 1 or 2 embryos (not randomised in 1 or 2 embryos) on both arms | |
| Cost effectiveness study | |
| Not randomised controlled trial | |
| Compared time lapse and preimplantation genetic screening versus only time lapse screening | |
| Compared fresh conventional IVF DET vs minimal stimulation frozen SET cycles |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Cumulative live birth Show forest plot | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| Analysis 2.1  Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 1: Cumulative live birth | ||||
| 2.1.1 SET + 1 FET versus DET (x1) (cleavage stage) | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 2.1.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.84] |
| 2.2 Multiple pregnancy Show forest plot | 4 | 985 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.08, 0.21] |
| Analysis 2.2  Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 2: Multiple pregnancy | ||||
| 2.2.1 SET + 1 FET versus DET (x1) (cleavage stage) | 3 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.08, 0.22] |
| 2.2.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.54] |
| 2.3 Clinical pregnancy rate Show forest plot | 3 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| Analysis 2.3  Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 3: Clinical pregnancy rate | ||||
| 2.3.1 SET + 1 FET versus DET (x1) (cleavage stage) | 2 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 2.3.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 2.4 Miscarriage Show forest plot | 2 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [0.93, 4.95] |
| Analysis 2.4  Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 4: Miscarriage | ||||
| 2.4.1 SET + 1 FET versus DET (x1) (cleavage stage) | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [0.85, 9.67] |
| 2.4.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.52, 5.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Live birth Show forest plot | 12 | 1904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.75] |
| Analysis 3.1  Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 1: Live birth | ||||
| 3.1.1 SET (x1) versus DET (x1) (cleavage stage) | 11 | 1704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.76] |
| 3.1.2 SET (x1) versus DET (x1) (blastocyst stage) | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 3.2 Multiple pregnancy Show forest plot | 13 | 1952 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.12, 0.22] |
| Analysis 3.2  Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 2: Multiple pregnancy | ||||
| 3.2.1 SET (x1) versus DET (x1) (cleavage stage) | 11 | 1704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.11, 0.22] |
| 3.2.2 SET (x1) versus DET (x1) (blastocyst stage) | 3 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.09, 0.36] |
| 3.3 Clinical pregnancy rate Show forest plot | 10 | 1860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.77] |
| Analysis 3.3  Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 3: Clinical pregnancy rate | ||||
| 3.3.1 SET (x1) versus DET (x1) (cleavage stage) | 8 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.78] |
| 3.3.2 SET (x1) versus DET (x1) (blastocyst stage) | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.62, 0.88] |
| 3.4 Miscarriage Show forest plot | 7 | 1560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.66, 1.42] |
| Analysis 3.4  Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 4: Miscarriage | ||||
| 3.4.1 SET (x1) versus DET (x1) (cleavage stage) | 6 | 1460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.65, 1.43] |
| 3.4.2 SET (x1) versus DET (x1) (blastocyst stage) | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.24, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Live or cumulative live birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 1: Live or cumulative live birth | ||||
| 4.1.1 DET (x1) versus TET (x1) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.68] |
| 4.1.2 DET (x1) versus four embryo transfer (x1) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.05] |
| 4.1.3 DET (x2) versus TET (x2) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.92] |
| 4.1.4 DET (x3) versus TET (x3) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.71] |
| 4.2 Multiple pregnancy Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 2: Multiple pregnancy | ||||
| 4.2.1 DET versus TET | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.14, 0.93] |
| 4.2.2 DET versus four embryo transfer | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.11, 1.88] |
| 4.3 Clinical pregnancy Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 3: Clinical pregnancy | ||||
| 4.3.1 DET (x1) versus TET (x1) | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.06] |
| 4.3.2 DET (x1) versus four embryo transfer (x1) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.26] |
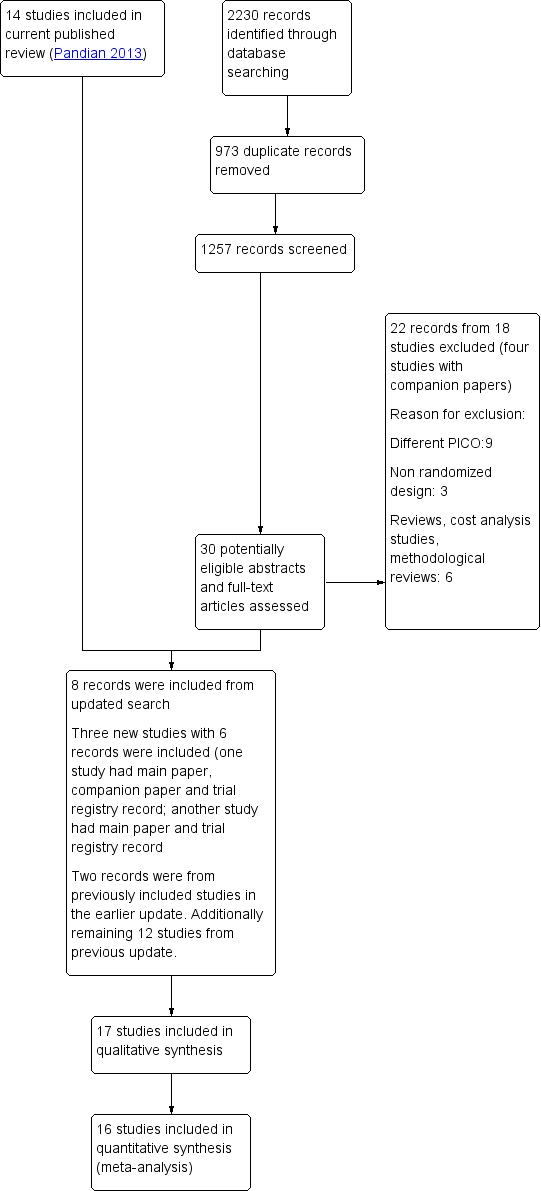
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 2 Repeated SET (mixed policies) versus multiple ET in a single cycle, outcome: 2.1 Cumulative live birth.

Forest plot of comparison: 2 Repeated SET (mixed policies) versus multiple ET in a single cycle, outcome: 2.2 Multiple pregnancy.

Forest plot of comparison: 2 Single versus multiple (in a single cycle), outcome: 2.1 Live birth.

Funnel plot of comparison: 3 Single versus multiple (in a single cycle), outcome: 3.1 Live birth.

Forest plot of comparison: 2 Single versus multiple (in a single cycle), outcome: 2.2 Multiple pregnancy.

Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 1: Cumulative live birth

Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 2: Multiple pregnancy

Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 3: Clinical pregnancy rate

Comparison 2: Repeated SET (mixed policies) versus multiple ET in a single cycle, Outcome 4: Miscarriage

Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 1: Live birth

Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 2: Multiple pregnancy

Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 3: Clinical pregnancy rate

Comparison 3: Single versus multiple embryo transfer (in a single cycle), Outcome 4: Miscarriage

Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 1: Live or cumulative live birth

Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 2: Multiple pregnancy

Comparison 4: Double embryo transfer versus more than two embryos transferred, Outcome 3: Clinical pregnancy
| Repeated single embryo transfer (mixed policies) versus multiple embryo transfer in a single cycle of IVF or ICSI | |||||||
| Patient or population: transfer following in vitro fertilisation or intracytoplasmic sperm injection | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | ||
|---|---|---|---|---|---|---|---|
| Risk with multiple embryo transfer | Risk with repeated single (mixed policies) | ||||||
| Cumulative live birth | pooled | 420 per 1000 | 399 per 1000 | RR 0.95 | 985 | ⊕⊕⊝⊝ | |
| Cumulative live birth | SET + 1 FET versus DET (×1) (cleavage stage) | 421 per 1000 | 392 per 1000 | RR 0.93 | 878 | ⊕⊕⊝⊝ | |
| SET (×2) versus DET (×1) (cleavage stage) | 407 per 1000 | 464 per 1000 | RR 1.14 | 107 | ⊕⊝⊝⊝ | ||
| Multiple pregnancy | pooled | 127 per 1000 | 18 per 1000 | Peto odds ratio 0.13 | 985 | ⊕⊕⊕⊝ | |
| Multiple pregnancy | SET + 1 FET versus DET (×1) (cleavage stage) | 128 per 1000 | 19 per 1000 | Peto odds ratio 0.13 | 878 | ⊕⊕⊕⊝ | |
| SET (×2) versus DET (×1) (cleavage stage) | 111 per 1000 | 15 per 1000 | Peto odds ratio 0.12 | 107 | ⊕⊝⊝⊝ | ||
| Clinical pregnancy rate | pooled | 515 per 1000 | 489 per 1000 (432 to 556) | RR 0.95 (0.84 to 1.08) | 943 (3 RCTs) | ⊕⊕⊝⊝ | |
| Miscarriage rate | pooled | 76 per 1000 | 149 per 1000 (71 to 289) | Peto odds ratio 2.14 (0.93 to 4.95) | 282 (2 RCTs) | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 Very serious risk of bias, downgraded by 2 levels: high risk or unclear risk of bias for allocation concealment, high risk of bias for performance bias due to lack of blinding. | |||||||
| Single compared to multiple embryo transfer in a single cycle following in vitro fertilisation or intracytoplasmic sperm injection | ||||||
| Patient or population: transfer following in vitro fertilisation or intracytoplasmic sperm injection | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with multiple (in a single cycle) | Risk with Single | |||||
| Live birth | 463 per 1000 | 310 per 1000 | RR 0.67 | 1904 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 151 per 1000 | 28 per 1000 | Peto odds ratio 0.16 | 1952 | ⊕⊕⊕⊝ | |
| Clinical pregnancy | 547 per 1000 | 383 per 1000 (350 to 421) | RR 0.70 (0.64 to 0.77) | 1860 (10 RCTs) | ⊕⊕⊝⊝ | |
| Miscarriage rate | 72 per 1000 | 69 per 1000 (46 to 99) | Peto odds ratio 0.96 (0.66 to 1.42) | 1560 (7 RCTs) | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Very serious risk of bias, downgraded by 2 levels: unclear or high risk for allocation concealment, high risk for performance bias due to lack of blinding in majority of the included studies. | ||||||
| Study author and year | Age Eligibility criteria (mean participant age, where stated) | Duration of infertility | Previous failed cycle | Frozen cycles | Prim/Sec infertility | FSH | Quality of embryo |
|---|---|---|---|---|---|---|---|
| Under 38 years (mean age 33) | Mean 2.6 to 3.2 years | First IVF/ICSI cycle. | Frozen cycles included | Not stated | Not stated | Good | |
| less than 34 years | Average duration of infertility 3.5 years. | First IVF/ICSI cycle. | Not included | Unclear | Not mentioned | Good | |
| 38 to 45 years (mean age 41) | Average duration of infertility in DET group was 3.7(± 2.5) and in TET group was 3.2(± 2.4) years | First cycle and previous successful cycle | Not included | Yes | Not mentioned | Good | |
| Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Good | |
| < 35 years (mean age 30 to 31) | Not stated | First IVF/ICSI cycle or after previous successful cycle . | Not included | Yes | FSH < 10IU/L. | Good | |
| various, no age criteria, ranged between 22 to 40 years (mean age 31) | Not stated | women who had / not had more than 1 previous failed treatment. | Frozen cycles included | Yes, but not mentioned | Not mentioned | good | |
| Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Good | |
| < 36 years (mean age 31) | 0 to 12 years | First or second IVF cycle | Frozen cycles included | Yes | Not mentioned | Good, blastocysts included | |
| Unpublished trial, pilot study, part of a thesis | ≥ 36 years | 0 to 12 years | First or second IVF/ICSI cycle | Frozen cycles included | Yes | Not mentioned | At |
| Various ages, no criteria (mean age 33) | SET‐ 3.3 ± 1.8, DET‐ 3.3 ± 2.1 years | First IVF cycle | Not included | Yes | Not mentioned | Good | |
| ≤ 35 years | Not mentioned | First or previous successful cycle | Frozen cycles included | Yes | Not mentioned | good | |
| unpublished trial | Female age < 35 if no previous ART pregnancy, < 40 if | Not mentioned | First or previous successful cycle | Frozen cycles included | Yes | Not mentioned | At least 4 good‐quality |
| unpublished trial | ≤ 37 years | Not mentioned | First or second cycle of treatment | Frozen cycles included | Yes | Not mentioned | 4 or more good quality embryos available at the time of embryo transfer |
| < 35 years | SET ‐ 2.6 ± 1.6 years DET ‐ 3.2 ± 2.4 years | No more than 1 previous ART failure | Frozen cycles performed but not included in the analysis | Yes | Mentioned | At least 2 good‐quality blastocysts were available, | |
| oocyte donor recipients aged 18‐50 years | not mentioned | Undergoing first or second synchronised oocyte donation cycle | Frozen cycles performed but not included in the analysis | Not mentioned | Not mentioned | Minimum of 5 embryos with at least 2 good‐quality embryos on day 3 after oocyte retrieval | |
| < 38 years | SET ‐ 3.1 ± 1.1 DET ‐ 3.1 ± 1.0 | First or second cycle with previous attempt with positive pregnancy test | Frozen cycles included | Not mentioned | Not mentioned | good |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Cumulative live birth Show forest plot | 4 | 985 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 2.1.1 SET + 1 FET versus DET (x1) (cleavage stage) | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 2.1.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.70, 1.84] |
| 2.2 Multiple pregnancy Show forest plot | 4 | 985 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.08, 0.21] |
| 2.2.1 SET + 1 FET versus DET (x1) (cleavage stage) | 3 | 878 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.08, 0.22] |
| 2.2.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.03, 0.54] |
| 2.3 Clinical pregnancy rate Show forest plot | 3 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 2.3.1 SET + 1 FET versus DET (x1) (cleavage stage) | 2 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.11] |
| 2.3.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.81, 1.71] |
| 2.4 Miscarriage Show forest plot | 2 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.14 [0.93, 4.95] |
| 2.4.1 SET + 1 FET versus DET (x1) (cleavage stage) | 1 | 175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [0.85, 9.67] |
| 2.4.2 SET (x2) versus DET (x1) (cleavage stage) | 1 | 107 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [0.52, 5.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Live birth Show forest plot | 12 | 1904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.75] |
| 3.1.1 SET (x1) versus DET (x1) (cleavage stage) | 11 | 1704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.76] |
| 3.1.2 SET (x1) versus DET (x1) (blastocyst stage) | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.84] |
| 3.2 Multiple pregnancy Show forest plot | 13 | 1952 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.12, 0.22] |
| 3.2.1 SET (x1) versus DET (x1) (cleavage stage) | 11 | 1704 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.11, 0.22] |
| 3.2.2 SET (x1) versus DET (x1) (blastocyst stage) | 3 | 248 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.09, 0.36] |
| 3.3 Clinical pregnancy rate Show forest plot | 10 | 1860 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.77] |
| 3.3.1 SET (x1) versus DET (x1) (cleavage stage) | 8 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.78] |
| 3.3.2 SET (x1) versus DET (x1) (blastocyst stage) | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.62, 0.88] |
| 3.4 Miscarriage Show forest plot | 7 | 1560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.66, 1.42] |
| 3.4.1 SET (x1) versus DET (x1) (cleavage stage) | 6 | 1460 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.65, 1.43] |
| 3.4.2 SET (x1) versus DET (x1) (blastocyst stage) | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.24, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Live or cumulative live birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 DET (x1) versus TET (x1) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.68] |
| 4.1.2 DET (x1) versus four embryo transfer (x1) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.05] |
| 4.1.3 DET (x2) versus TET (x2) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.37, 1.92] |
| 4.1.4 DET (x3) versus TET (x3) | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.43, 1.71] |
| 4.2 Multiple pregnancy Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 DET versus TET | 2 | 343 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.14, 0.93] |
| 4.2.2 DET versus four embryo transfer | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.11, 1.88] |
| 4.3 Clinical pregnancy Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 DET (x1) versus TET (x1) | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.53, 1.06] |
| 4.3.2 DET (x1) versus four embryo transfer (x1) | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.47, 1.26] |

