Psihološke terapije za kronični posttraumatski stresni poremećaj (PTSP) u odraslih
Abstract
Background
Post‐traumatic stress disorder (PTSD) is a distressing condition, which is often treated with psychological therapies. Earlier versions of this review, and other meta‐analyses, have found these to be effective, with trauma‐focused treatments being more effective than non‐trauma‐focused treatments. This is an update of a Cochrane review first published in 2005 and updated in 2007.
Objectives
To assess the effects of psychological therapies for the treatment of adults with chronic post‐traumatic stress disorder (PTSD).
Search methods
For this update, we searched the Cochrane Depression, Anxiety and Neurosis Group's Specialised Register (CCDANCTR‐Studies and CCDANCTR‐References) all years to 12th April 2013. This register contains relevant randomised controlled trials from: The Cochrane Library (all years), MEDLINE (1950 to date), EMBASE (1974 to date), and PsycINFO (1967 to date). In addition, we handsearched the Journal of Traumatic Stress, contacted experts in the field, searched bibliographies of included studies, and performed citation searches of identified articles.
Selection criteria
Randomised controlled trials of individual trauma‐focused cognitive behavioural therapy (TFCBT), eye movement desensitisation and reprocessing (EMDR), non‐trauma‐focused CBT (non‐TFCBT), other therapies (supportive therapy, non‐directive counselling, psychodynamic therapy and present‐centred therapy), group TFCBT, or group non‐TFCBT, compared to one another or to a waitlist or usual care group for the treatment of chronic PTSD. The primary outcome measure was the severity of clinician‐rated traumatic‐stress symptoms.
Data collection and analysis
We extracted data and entered them into Review Manager 5 software. We contacted authors to obtain missing data. Two review authors independently performed 'Risk of bias' assessments. We pooled the data where appropriate, and analysed for summary effects.
Main results
We include 70 studies involving a total of 4761 participants in the review. The first primary outcome for this review was reduction in the severity of PTSD symptoms, using a standardised measure rated by a clinician. For this outcome, individual TFCBT and EMDR were more effective than waitlist/usual care (standardised mean difference (SMD) ‐1.62; 95% CI ‐2.03 to ‐1.21; 28 studies; n = 1256 and SMD ‐1.17; 95% CI ‐2.04 to ‐0.30; 6 studies; n = 183 respectively). There was no statistically significant difference between individual TFCBT, EMDR and Stress Management (SM) immediately post‐treatment although there was some evidence that individual TFCBT and EMDR were superior to non‐TFCBT at follow‐up, and that individual TFCBT, EMDR and non‐TFCBT were more effective than other therapies. Non‐TFCBT was more effective than waitlist/usual care and other therapies. Other therapies were superior to waitlist/usual care control as was group TFCBT. There was some evidence of greater drop‐out (the second primary outcome for this review) in active treatment groups. Many of the studies were rated as being at 'high' or 'unclear' risk of bias in multiple domains, and there was considerable unexplained heterogeneity; in addition, we assessed the quality of the evidence for each comparison as very low. As such, the findings of this review should be interpreted with caution.
Authors' conclusions
The evidence for each of the comparisons made in this review was assessed as very low quality. This evidence showed that individual TFCBT and EMDR did better than waitlist/usual care in reducing clinician‐assessed PTSD symptoms. There was evidence that individual TFCBT, EMDR and non‐TFCBT are equally effective immediately post‐treatment in the treatment of PTSD. There was some evidence that TFCBT and EMDR are superior to non‐TFCBT between one to four months following treatment, and also that individual TFCBT, EMDR and non‐TFCBT are more effective than other therapies. There was evidence of greater drop‐out in active treatment groups. Although a substantial number of studies were included in the review, the conclusions are compromised by methodological issues evident in some. Sample sizes were small, and it is apparent that many of the studies were underpowered. There were limited follow‐up data, which compromises conclusions regarding the long‐term effects of psychological treatment.
PICOs
Laički sažetak
Psihološke terapije za kronični posttraumatski stresni poremećaj (PTSP) u odraslih
Uvod: Posttraumatski stresni poremećaj (PTSP) može se pojaviti nakon traumatičnog događaja. Obilježavaju ga simptomi ponovnog proživljavanja traume (u obliku noćnih mora, prisjećanja na traumatični događaj i uznemirujućih misli), izbjegavanja svega što podsjeća na traumatični događaj, negativnih promjena misli i raspoloženja te simptomi pretjerane pobuđenosti (osjećaj kao da si na rubu, poteškoće sa spavanjem i koncentriranjem, razdražljivost i osjećaj ljutnje).
Prethodna istraživanja su podržala upotrebu individualnih kognitivno‐bihevioralnih terapija usmjerenih na traumu (TFCBT) te desenzibilizacije pomoću pokreta očiju i reprocesiranja (EDMR) u liječenju PTSP‐a. TFCBT je oblik kognitivno‐bihevioralne terapije (CBT) koji uključuje brojne tehnike za pomaganje osobi u savladavanju traumatičnog događaja. To je kombinacija kognitivne terapije usmjerene na mijenjanje načina na koji osoba razmišlja i bihevioralne terapije koja nastoji promijeniti način na koji osoba postupa. TFCBT pomaže osobi doći do uvjeta traume kroz izlaganje sjećanjima tog događaja. EDMR je psihološka terapija koja nastoji pomoći osobi u ponovnom obrađivanju (reprocesiranju) sjećanja na traumatični događaj. Terapija uključuje prizivanje uznemirujućih traumatičnih slika, vjerovanja i tjelesnih senzacija u misli, pri čemu terapeut usmjerava očne pokrete osobe s jedne strane na drugu. Identificiraju se pozitivniji pogledi na traumatična sjećanja s ciljem zamjenjivanja onih koja izazivaju probleme.
TFCBT i EDMR se trenutno preporučuju kao terapije izbora po smjernicama kao što su one koje objavljuje Nacionalni institut za zdravlje i kliničku izvrsnost Ujedinjenog Kraljevstva (NICE).
Značajke istraživanja: Ovaj Cochrane sustavni pregled predstavlja najnovije dokaze iz 70 studija koje uključuju ukupno 4761 osobu.
Zaključci: Dokazi iz studija kontinuirano potvrđuju djelotvornost individualne kognitivno‐bihevioralne terapije usmjerene na traumu (TFCBT), EDMR, non‐TFCBT i grupne TFCBT u liječenju kroničnog PTSP‐a kod odraslih. Ostale psihološke terapije neusmjerene na traumu nisu značajno smanjivale simptome PTSP‐a. Postoje dokazi da su individualna TFCBT, EDMR i non‐TFCBT podjednako učinkovite neposredno nakon tretmana u liječenju PTSP‐a. Neki dokazi pokazuju da su TFCBT i EDMR uspješnije od non‐TFCBT u razdoblju od jednog do četiri mjeseca nakon tretmana, ali da su individualna TFCBT, EDMR i non‐TFCBT učinkovitije od ostalih terapija. Nije pronađen neki poseban sukob interesa u uključenim studijama.
Kvaliteta dokaza: Iako je uključen pozamašan broj studija u ovom pregledu, svaka od njih uključila je mali broj ispitanika dok su neke od njih i loše osmišljene. Procjenjuje se da je ukupna kvaliteta navedenih studija vrlo niska te bi se i rezultati ovog sustavnog pregleda trebali protumačit is oprezom. Nedovoljno je dokaza koji pokazuju jesu li psihološke terapije štetne ili ne.
Authors' conclusions
Summary of findings
| Trauma‐focused CBT/Exposure therapy versus Waitlist/Usual Care | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Trauma Focused CBT/ Exposure Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician‐rated in the intervention groups was | 1.62 standard deviations lower | 1256 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.64 | 1756 | ⊕⊝⊝⊝ | ||
| 130 per 1000 | 189 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 124 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Trauma‐focused CBT/Exposure therapy compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | Trauma Focused CBT/ Exposure Therapy | |||||
| Severity of PTSD Symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician‐rated in the intervention groups was | 267 | ⊕⊝⊝⊝ | |||
| Leaving the study early for any reason | Study population | RR 1.19 | 312 | ⊕⊝⊝⊝ | ||
| 154 per 1000 | 183 per 1000 | |||||
| Moderate | ||||||
| 154 per 1000 | 183 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Trauma‐focused CBT/Exposure therapy compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Therapies | Trauma Focused CBT/Exposure Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | ‐0.48 standard deviations lower | 612 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.39 | 762 | ⊕⊝⊝⊝ | ||
| 142 per 1000 | 198 per 1000 | |||||
| Moderate | ||||||
| 138 per 1000 | 192 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| EMDR compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 1.17 standard deviations lower | 183 | ⊕⊝⊝⊝ | ||
| Leaving study early for any reason | Study population | RR 1.05 | 227 | ⊕⊝⊝⊝ | ||
| 178 per 1000 | 188 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 182 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| EMDR compared with Trauma‐focused CBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Trauma Focused CBT | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 0.03 standard deviations lower | 327 | ⊕⊝⊝⊝ | ||
| Leaving study early for any reason | Study population | RR 1.00 | 400 | ⊕⊝⊝⊝ | ||
| 279 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 257 per 1000 | 247 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged as posing a high risk of bias | ||||||
| EMDR compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 0.35 standard deviations lower | 53 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.03 | 84 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 144 per 1000 | |||||
| Moderate | ||||||
| 91 per 1000 | 94 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies reported insufficient information to judge risk of bias | ||||||
| EMDR compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Therapies | EMDR | |||||
| Leaving study early for any reason | Study population | RR 1.48 | 127 | ⊕⊝⊝⊝ | ||
| 32 per 1000 | 47 per 1000 | |||||
| Moderate | ||||||
| 32 per 1000 | 48 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study was judged to pose a high risk of bias. The other study reported insufficient information for a judgement to be made | ||||||
| Non‐TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Non‐TFCBT Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 106 | ⊕⊝⊝⊝ | |||
| Leaving the study early due to any reason | Study population | RR 1.96 | 141 | ⊕⊝⊝⊝ | ||
| 78 per 1000 | 153 per 1000 | |||||
| Moderate | ||||||
| 83 per 1000 | 163 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient data to judge risk of bias | ||||||
| Non‐TFCBT compared with other therapies for chronic post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | ||||||
| Severity of PTSD symptoms ‐ Clincian‐rated | See comment | See comment | Not estimable | 25 | ⊕⊝⊝⊝ | |
| Leaving the study early for any reason | See comment | See comment | Not estimable | 31 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Group TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Group TFCBT | ||||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 185 | ⊕⊝⊝⊝ | |||
| Leaving the study early for any reason | Study population | RR 1.21 | 573 | ⊕⊝⊝⊝ | ||
| 262 per 1000 | 317 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 242 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient information to judge risk of bias | ||||||
| Group CBT (trauma focused) compared with Group CBT (non‐trauma focused) for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Group CBT (non‐trauma focused) | Group CBT (trauma focused) | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | See comment | See comment | Not estimable | 325 | ⊕⊝⊝⊝ | |
| Leaving the study early for any reason | See comment | See comment | Not estimable | 360 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient information to judge risk of bias | ||||||
| Other therapies compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Other therapies | |||||
| Severity of PTSD symptoms ‐ Clinician | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 112 | ⊕⊝⊝⊝ | |||
| Leaving the study early due to any reason | Study population | RR 2.45 | 211 | ⊕⊝⊝⊝ | ||
| 74 per 1000 | 181 per 1000 | |||||
| Moderate | ||||||
| 72 per 1000 | 176 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small sample sizes | ||||||
| Group non‐TFCBT compared to Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Group nonTFCBT | |||||
| Leaving the study early for any reason | See comment | See comment | Not estimable | 47 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Only one study. Risk of bias high/unclear in several domains. | ||||||
Background
Description of the condition
Post‐traumatic stress disorder (PTSD) is a well recognised psychiatric disorder that occurs following a major traumatic event. Characteristic symptoms include re‐experiencing phenomena such as nightmares and recurrent distressing thoughts of the event, avoidance of talking or being reminded of the traumatic event, negative alterations in thoughts and mood, and hyperarousal symptoms including sleep disturbance, increased irritability and hypervigilance. PTSD can be diagnosed after a one‐month duration of symptoms. The condition is considered chronic once symptoms have been present for three months. PTSD is a relatively common condition. The National Co‐morbidity Survey (Kessler 1995) found that 7.8% of 5877 American adults had suffered from PTSD at some time in their lives. When data were examined from individuals who had been exposed to a traumatic event, rates of PTSD varied according to the type of stressor. For example, physical assaults amongst women led to a lifetime prevalence of 29% and combat experience amongst men to a lifetime prevalence of 39%.
Description of the intervention
Psychological therapies have been advocated as being effective in the treatment of PTSD since its conception. Various forms of psychological therapy have been used including exposure therapy (Creamer 2004), cognitive therapy (Ehlers 2005; Resick 1992), psychodynamic psychotherapy (Brom 1989) and eye movement desensitisation and reprocessing (EMDR) (Shapiro 1989b). Exposure therapy usually involves asking the participant to relive the trauma imaginally. This is often done by creating a detailed present tense account of exactly what happened, making an audio tape recording or transcript of it and asking the individual to listen to/read this over and over again. Another form of exposure therapy involves exposing participants to cues associated with the traumatic event (for example, graded re‐exposure to car travel following a road traffic accident). Trauma‐focused cognitive therapy involves helping the individual to identify distorted thinking patterns regarding themselves, the traumatic incident and the world. Individuals are encouraged to challenge their thoughts by weighing up available evidence and through the utilisation of various techniques by the therapist, including specific questioning that leads the individual to challenge distorted views. EMDR involves the PTSD sufferer focusing on a traumatic image, thought, emotion and a bodily sensation whilst receiving bilateral stimulation, most commonly in the form of eye movements. Non‐TFCBT usually focuses on techniques for the reduction of anxiety. The most widely used protocol for anxiety reduction in PTSD is stress inoculation training (SIT), which teaches skills for managing stress, such as relaxation, thought stopping and guided dialogue. It provides the opportunity to practise acquired skills gradually, across a variety of settings. Psychodynamic psychotherapy focuses on integrating the traumatic experience into the life experience of the individual as a whole; childhood issues are often felt to be important.
How the intervention might work
The psychological therapies considered by this review stem from various theoretical perspectives. Individual protocols describe how therapies might work in detail. TFCBT protocols draw on four core components emphasised in varying degrees: 1) psycho‐education; 2) anxiety management; 3) exposure; and 4) cognitive restructuring. Earlier therapies tended to be more behaviourally based, focusing heavily on exposure work. Cognitive components have become more prominent over time, in line with the popularity of information processing accounts of the disorder. Exposure plays an important role in many TFCBT protocols. It may be carried out in vivo (real life), or imaginally. It is common for both to be used in the treatment of PTSD, to target internally and externally feared stimuli. The rationale behind the use of imaginal exposure varies according to the specific TFCBT protocol. Imaginal exposure is based on principles of habituation (the reduction of anxiety after prolonged exposure) or information processing (allowing re‐evaluation of old information and incorporation of new information into the trauma memory), or a combination of both. In addition, cognitive restructuring has an important role, seeking to identify and modify dysfunctional thoughts by testing and challenging self‐held beliefs, based on the assumption that these usually unquestioned thoughts are distorted or unhelpful. EMDR is an integrative trauma‐focused therapy encompassing elements from various effective psychotherapies in a structured protocol drawn from an information processing model of PTSD. There is no agreed mechanism by which EMDR is thought to operate. Shapiro 1989b discovered EMDR accidentally. Her account implicates personal experience of rapid eye movements easing distress. On the basis of this experience, Shapiro elaborated EMDR for the treatment of Vietnam war veterans and abuse sufferers. It is suggested that bilateral stimulation aids the processing of traumatic memories. Non‐TFCBT interventions such as SIT operate by teaching the individuals techniques to minimise and control their anxiety. In terms of other psychological therapies considered by this review, psychodynamic psychotherapy places emphasis on the unconscious mind, aiming to resolve inner conflict arising from the traumatic event. Person‐centred therapy/supportive counselling allows the individual to talk through problems and resolve difficulties with minimal guidance and direction from the therapist. The therapist is accepting and non‐judgemental. This style encourages the individual to feel comfortable in the expression of feelings, facilitating positive change. Unlike trauma‐focused therapies, person‐centred/supportive counselling does not encourage exploration of the trauma memory.
For a summary of psychological models of PTSD providing the rationale for many of the treatment approaches considered here, see Brewin 2003.
Why it is important to do this review
It is apparent that PTSD causes clinically significant suffering and that developing effective interventions is important. Earlier versions of this review and other meta‐analyses have found psychological therapies to be effective (e.g. Bradley 2005), with trauma‐focused treatments being more effective than non‐trauma‐focused treatments (Bisson 2007a). This is an update of a Cochrane review first published in 2005 and updated in 2007, bringing together current evidence concerning the psychological treatment of chronic PTSD in adults. Other Cochrane Collaboration reviews have considered single‐session psychological 'debriefing' to prevent PTSD (Rose 2002), multiple‐session early psychological interventions for the prevention of PTSD (Roberts 2009), early psychological therapies to treat acute traumatic stress symptoms (Roberts 2010), pharmacological treatments (Stein 2006), combined pharmacotherapy and psychological therapies for PTSD (Hetrick 2010), and psychological therapies for the treatment of PTSD in children and adolescents (Gillies 2012).
Objectives
To assess the effects of psychological therapies for the treatment of adults with chronic post‐traumatic stress disorder (PTSD).
Methods
Criteria for considering studies for this review
Types of studies
We include randomised controlled trials (RCTs) of psychological therapies for chronic PTSD in adults. Cluster‐RCTs and cross‐over trials were considered eligible for inclusion. We did not use sample size, language or publication status to determine whether or not a study was included.
Types of participants
Age
This review considered studies of adults only (aged 18 or over).
Diagnosis
Any individual suffering from chronic traumatic stress symptoms, i.e. with a duration of three months or more. At least 70% of participants were required to be diagnosed as suffering from PTSD according to DSM‐III (APA 1980), DSM‐IIIR (APA 1987), DSM‐IV (APA 2000),ICD‐9 (WHO 1979) or ICD‐10 (WHO 1992) criteria, by means of a structured interview or diagnosis by a clinician. There was no restriction on the basis of severity of PTSD symptoms or the type of traumatic event.
Co‐morbidities
There was no restriction on the basis of comorbidity, although we required that PTSD be the primary diagnosis.
Setting
There was no restriction on the basis of study setting.
Types of interventions
This review considered any psychological therapy designed to reduce symptoms of chronic PTSD. Group interventions were considered separately from those delivered on an individual basis. Psychological therapy provided in a group format is clinically distinct from its individually‐delivered counterparts, not least due to the reduced therapist 'dose' associated with group therapy.
Experimental interventions
-
Individual trauma‐focused cognitive behavioural therapy (TFCBT): any psychological therapy that predominantly used trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques. This category included exposure therapy. Examples of therapies within this category are cognitive therapy (Ehlers 2005), cognitive processing therapy (Resick 1992) and prolonged exposure (Foa 2000).
-
Eye movement desensitisation and reprocessing (Shapiro 1989b).
-
Non‐trauma‐focused CBT: any psychological therapy that predominantly used non‐trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques, for example stress inoculation training (SIT) (Meichenbaum 1988).
-
Group TFCBT: any approach delivered in a group setting that predominantly used trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques.
-
Group non‐TFCBT: any approach delivered in a group that predominantly used non‐trauma‐focused cognitive, behavioural or cognitive‐behavioural techniques.
-
Other psychological therapy: any psychological therapy that predominantly used non‐trauma‐focused techniques that would not be considered cognitive, behavioural or cognitive‐behavioural techniques. This category comprised non‐directive/supportive/person‐centred counselling (Rogers 1961), hypnotherapy, psychodynamic therapy (Brom 1989) and present‐centred treatment.
Comparator interventions
-
Waitlist, treatment as usual, symptom monitoring, repeated assessment or other minimal attention control group;
-
An alternative psychological treatment.
Types of outcome measures
Primary outcomes
-
Reduction in the severity of PTSD symptoms using a standardised measure (i.e. a test that is administered and scored in a consistent way) rated by a clinician (e.g. the Clinician Administered PTSD Symptom Scale (Blake 1995)).
-
Drop‐out rates.
Secondary outcomes
-
Severity of self‐reported traumatic stress symptoms using a standardised measure (e.g. the Impact of Event Scale (Horowitz 1979)).
-
Severity of depressive symptoms (e.g. the Beck Depression Inventory (Beck 1961)).
-
Severity of anxiety symptoms using scales (e.g. the Spielberger State Trait Anxiety Inventory (Spielberger 1973)).
-
PTSD diagnosis after treatment.
-
Any adverse effects, e.g. increased PTSD symptoms.
We produced hierarchies of the standardised measures, based on their frequency of use within the included studies. Where a trial reported data from two or more measures of the same outcome, we used only data from the measure ranked highest.
Timing of outcome assessment
We conducted separate pair‐wise meta‐analyses of follow‐up data at one to four months, five to eight months, nine months to one year, and over one year.
Search methods for identification of studies
Electronic searches
The Cochrane, Depression, Anxiety and Neurosis Group's Specialised Register (CCDANCTR)
The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintain two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References Register contains over 33,000 reports of randomised controlled trials in depression, anxiety and neurosis. Approximately 65% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies Register and records are linked between the two registers through the use of unique Study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Co‐ordinator for further details. Reports of trials for inclusion in the Group's registers are collated from routine (weekly), generic searches of OVID MEDLINE (1950‐), EMBASE (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review‐specific searches of additional databases. Reports of trials are also sourced from international trials registers c/o the World Health Organization’s trials portal (ICTRP), ClinicalTrials.gov, drug companies, the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCDAN's generic search strategies can be found on the Group's website.
We searched the CCDANCTR (Studies and References Registers, all years to 12th April 2013) on condition alone, using the following terms:
(PTSD or posttrauma* or post‐trauma* or “post trauma*” or (combat and disorder*))
Searching other resources
Grey literature
We searched abstracts from meetings of the European and International Societies of Traumatic Stress Studies. We also searched websites and discussion fora related to PTSD.
Handsearching
We handsearched the Journal of Traumatic Stress and the ISTSS Treatment Guidelines (Foa 2000; Foa 2009) for relevant articles.
Reference lists
We scrutinised the reference lists of included studies for additional studies meeting the inclusion criteria.
Correspondence
The main correspondence was with the UK NICE guidelines development group who kindly shared the results of their searches and communications (see Acknowledgements).
Data collection and analysis
Selection of studies
Two review authors independently read abstracts of all potential trials identified through the search strategy. If we felt an abstract represented a possible RCT, each review author independently read the full report to determine if the trial met the inclusion criteria. Any differences prompted re‐evaluation of the study, and we discussed disagreements with a third review author to reach a consensus.
Data extraction and management
We extracted data from published reports and entered them into Review Manager 5 Software (RevMan 5). We contacted authors to obtain missing information. Extracted data included demographic details of participants, details of the traumatic event, the randomisation process, the therapy used, and outcome data.
Main comparisons
-
Individual TFCBT versus waitlist/usual care
-
Individual TFCBT versus non‐TFCBT
-
Individual TFCBT versus other therapies
-
EMDR versus waitlist/usual care
-
EMDR versus individual TFCBT
-
EMDR versus non‐TFCBT
-
EMDR versus other therapies
-
Non‐TFCBT versus waitlist/usual care
-
Non‐TFCBT versus other therapy
-
Group TFCBT versus waitlist/usual care
-
Group TFCBT versus group non‐TFCBT
-
Other therapies versus waitlist/usual care
-
Group non‐TFCBT versus waitlist/usual care
-
Individual TFCBT versus group TFCBT
-
Individual TFCBT versus group non‐TFCBT
-
EMDR versus group TFCBT
-
EMDR versus group non‐TFCBT
-
Individual non‐TFCBT versus group TFCBT
-
Individual non‐TFCBT versus group non‐TFCBT
Assessment of risk of bias in included studies
We assessed all included studies for risk of bias, using the standard approach described in the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). This considered (1) sequence allocation for randomisation; (2) allocation concealment; (3) blinding of personnel and assessors; (4) incomplete outcome data; (5) selective reporting; and (6) any other notable risks of bias. Each was judged to pose a 'high', 'low' or 'unclear' risk of bias. Two review authors conducted the assessments, discussing any disagreements with a third review author to reach a consensus.
1. Sequence allocation randomisation
Randomisation was judged as posing a 'low' risk of bias if each participant had an equal chance of being randomised to a group. Low‐risk methods included referring to a random number table, use of a computerised random number generator, shuffling sealed envelopes or throwing a dice. Methods judged as posing a 'high' risk of bias included use of a sequence generated by e.g. date of birth, clinic number, date of admission to the study, or allocation by availability of the intervention. When insufficient information was given to permit judgement of high or low risk of bias, we describe the study as being at an 'unclear' risk of bias attributable to sequence allocation.
2. Allocation concealment
Concealment of intervention allocation was said to be at 'low' risk when there was no chance of the investigator foreseeing a participant's assignment. Methods judged as posing a low risk of bias included central allocation (e.g. by telephone or the internet) or sequentially numbered opaque sealed envelopes. High‐risk methods included alternation or rotation of assignment or using an open random allocation schedule (e.g. a list of random numbers). When insufficient information was reported to permit judgement of high or low risk, we describe the study as being at an 'unclear' risk of bias attributable to allocation concealment.
3. Blinding of personnel and assessors
It is not possible to blind either the participants or those administering psychological interventions. However, outcome assessors can be blinded. Those studies which blinded assessors were deemed to be at a low risk of bias, those which did not were judged as being at a high risk of bias. When insufficient information was reported to permit judgement of high or low risk, we describe the study as being at an 'unclear' risk of bias attributable to blinding.
4. Incomplete outcome data
We judged incomplete data to have been handled appropriately when reported completely, including attrition rates and exclusions, with consideration of the issue in terms of analysing the data. Low risk of bias was associated with no missing outcome data, or the use of data imputed using appropriate methods. Methods judged as posing a high risk of bias included analyses that considered only the data of treatment‐completers, or potentially inappropriate application of simple imputation. When insufficient information was reported to permit judgement of high or low risk, we describe the study as being at an 'unclear' risk of bias attributable to incomplete data.
5. Selective reporting
We judged a study to be at a low risk of bias if a protocol was available and all prespecified outcomes were reported. If there was no available protocol, the study was assigned a low risk in this domain if it was clear that the published reports included all expected outcomes, including those prespecified. When insufficient information was reported to permit judgement of high or low risk, we describe the study as being at an 'unclear' risk of bias attributable to selective reporting.
6. Other notable risks of bias
We noted any other potential threats to validity and judged them to be at a high or low risk of bias. We describe them as being at 'unclear' risk of bias when there was insufficient information to assess additional risk of bias.
Measures of treatment effect
Continuous data
We analysed continuous outcomes as standardised mean differences (SMDs) to allow ease of comparison across studies. All outcomes are presented using 95% confidence intervals (CIs).
Dichotomous data
We analysed categorical outcomes as risk ratios (RRs), these being more widely used in medical practice than odds ratios. All outcomes are presented using 95% confidence intervals.
Unit of analysis issues
Cross‐over trials
We included only outcome data from the first randomisation period when a study adopted a cross‐over design.
Studies with multiple treatment groups
If the trial had three (or more) arms, we undertook pair‐wise meta‐analysis with each arm, depending upon the nature of the intervention in each arm and the relevance to the review objectives. We avoided multiple comparisons as far as possible, to limit the risk of false positive results. When a study had three or more arms that were relevant to the review we considered the appropriateness of combining data from two arms if therapies were sufficiently similar or of using data from the arms of the trial which fit closest to the review objective. Decisions followed guidance provided by the Cochrane Handbook (Higgins 2011).
Cluster‐randomised trials
We had planned to include cluster‐RCTs, although none was identified by the searches to date. In future updates of the review, the methodology for dealing with these types of studies will follow that outlined by the Cochrane Handbook (Higgins 2011). We will adjust sample sizes using an estimate of the intracluster or intraclass correlation coefficient (ICC), which describes the 'similarity' of individuals within the same cluster. We will derive this from the trial if possible, or from another source, such as a similar study, or from a resource providing examples of ICCs.
Dealing with missing data
We contacted authors to obtain any data missing from the published report of included studies. We used intention‐to‐treat (ITT) data where possible, but used data from completers analyses when ITT data were not available.
Assessment of heterogeneity
We initially assessed studies included within each comparison for clinical heterogeneity in terms of variability in the experimental and control interventions, participants and settings, and outcomes. To further assess heterogeneity, we used both the I² statistic (Higgins 2003) and the Chi² test of heterogeneity, as well as visual inspection of the forest plots.
Assessment of reporting biases
When sufficient studies were available, we constructed funnel plots and scrutinised them for signs of asymmetry. Since reporting bias is just one possible reason for observed asymmetry, we also considered alternative explanations.
Data synthesis
We pooled data from more than one study using a fixed‐effect meta‐analysis, except where heterogeneity was present, in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We performed clinical heterogeneity subgroup analyses (when sufficient data were available) as follows:
-
Type of traumatic event (combat‐related trauma versus rape and sexual assault versus other civilian trauma)
-
Participant characteristics (men only versus women only)
Sensitivity analysis
We conducted sensitivity analyses to assess the effect of high or unclear risk of bias in any of the following domains:
-
Sequence generation
-
Allocation concealment
-
Blinding of outcome assessment
These analyses were performed for comparisons including 10 or more studies.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search yielded 1477 articles for consideration. We reviewed abstracts and obtained full‐text copies for 129 potentially relevant studies. Seventy RCTs (79 comparisons) met the inclusion criteria for the review. Figure 1 presents a flow diagram for study selection.
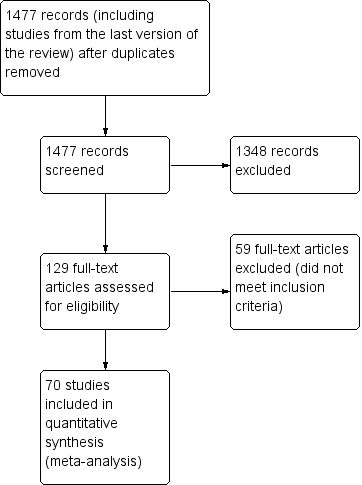
Study flow diagram.
Included studies
Design
All included studies were RCTs.
Sample sizes
The number of participants randomised in the trials ranged from 9 (Ready 2010) to 360 (Schnurr 2003). Eleven studies included sample sizes of over 100: Brom 1989 (n = 112); Cloitre 2010 (n = 104); Foa 2005 (n = 171); Krakow 2000 (n = 169); Krakow 2001 (n = 114); Mueser 2008 (n = 108); Nijdam 2012 (n = 140); Power 2002 (n = 105); Resick 2002 (n = 121); Schnurr 2003 (n = 360); Schnurr 2007 (n = 284).
Settings
USA (33 studies), UK (6 studies), Australia (7 studies), Netherlands (4 studies), Hawaii (3 studies), Germany (3 studies), Turkey (2 studies), Sweden (2 studies), Uganda (2 studies), Japan (1 study), Romania (1 study), Thailand (1 study), Canada (1 study), Portugal (1 study), Cambodia (1 study), China (1 study), Spain (1 study).
Participants
The study populations were varied and often not directly comparable (i.e. there was significant clinical heterogeneity). Studies included individuals traumatised by combat (13 studies), sexual assault (13 studies), war/persecution (6 studies), road traffic accidents (4 studies), earthquake (3 studies), childhood sexual abuse (3 studies), political detainment (1 study), terrorism (1 study), sexual or physical assault (1 study) and serving in the police force. The remainder of the studies included individuals traumatised by various traumatic events (24 studies).
Time post‐trauma
All studies included individuals at least three months after the trauma. The range was large, from three months to over 40 years (Bichescu 2007). There was often a wide range of times since trauma included in individual studies.
Interventions
In order to present the results in a meaningful way, we decided to pool data that used a similar theoretical methodology. This was conducted a priori, resulting in the establishment of six groups: individual TFCBT, non‐TFCBT, EMDR, group TFCBT, group non‐TFCBT and other therapies (psychodynamic therapy, hypnotherapy, supportive counselling and present‐centred counselling).
1. Trauma‐focused cognitive behavioural therapy: Forty‐nine studies considered individual TFCBT ‐ Adenauer 2011; Asukai 2010; Basoglu 2005; Basoglu 2007; Bichescu 2007; Blanchard 2003; Brom 1989; Bryant 2003; Bryant 2011; Cloitre 2002; Cloitre 2010; Cooper 1989; Devilly 1999; Duffy 2007; Dunne 2012; Echeburua 1997; Ehlers 2005; Fecteau 1999; Feske 2008; Foa 1991; Foa 1999; Foa 2005; Forbes 2012; Galovski 2012; Gamito 2010; Gersons 2000; Hensel‐Dittmann 2011; Hinton 2005; Ironson 2002; Keane 1989; Kubany 2003; Kubany 2004; Lindauer 2005; McDonagh 2005; Marks 1998; Monson 2006; Mueser 2008; Neuner 2004; Neuner 2008; Neuner 2010; Paunovic 2011; Peniston 1991; Power 2002; Ready 2010; Resick 2002; Schnurr 2007; Taylor 2003; Vaughan 1994; Nijdam 2012; Zang 2013.
2. Non‐TFCBT: Eight studies considered non‐TFCBT ‐ Carlson 1998; Echeburua 1997; Foa 1991; Foa 1999; Kearney 2013; Marks 1998; Taylor 2003; Vaughan 1994.
3. Eye Movement Desensitisation and Reprocessing: Sixteen studies considered eye movement desensitisation and reprocessing ‐ Carlson 1998; Devilly 1998; Devilly 1999; Hogberg 2007; Ironson 2002; Jensen 1994; Lee 2002; Marcus 1997; Power 2002; Rothbaum 1997; Rothbaum 2005; Scheck 1998; Taylor 2003; Vaughan 1994; Nijdam 2012.
4. Group TFCBT: Ten studies considered group TFCBT ‐ Beck 2009; Chard 2005; Classen 2001; Hinton 2011; Hollifield 2007; Kearney 2013; Krakow 2000; Krakow 2001; Schnurr 2003; Zlotnick 1997.
5. Group non‐TFCBT: One study considered group non‐TFCBT: Schnurr 2003
6. Other therapies: Nine studies considered other therapies (supportive counselling, present‐centred therapy, hypnotherapy and psychodynamic therapy) ‐ Blanchard 2003; Brom 1989; Bryant 2003; Cloitre 2010; Feske 2008; Foa 1991; McDonagh 2005; Ready 2010; Schnurr 2007.
Comparisons
The included trials compared
(i) Psychological therapy versus waitlist or usual care control (some studies allowed the control group to receive pharmacological treatments and/or psychological therapies that were not being considered specifically);
(ii) Psychological therapy versus other psychological therapy.
We made the following specific comparisons:
1. Individual TFCBT versus waitlist/usual care:Adenauer 2011; Asukai 2010; Basoglu 2005; Basoglu 2007; Bichescu 2007; Blanchard 2003; Brom 1989; Cloitre 2002; Cooper 1989; Duffy 2007; Dunne 2012; Ehlers 2003; Ehlers 2005; Fecteau 1999; Foa 1991; Foa 1999; Foa 2005; Forbes 2012; Galovski 2012; Gamito 2010; Gersons 2000; Hinton 2005; Keane 1989; Kubany 2003; Kubany 2004; Lindauer 2005; McDonagh 2005; Monson 2006; Mueser 2008; Neuner 2010; Paunovic 2011; Peniston 1991; Power 2002; Resick 2002; Vaughan 1994; Wells 2012; Zang 2013.
2. Individual TFCBT versus non‐TFCBT:Echeburua 1997; Foa 1991; Foa 1999; Hensel‐Dittmann 2011; Marks 1998; Taylor 2003; Vaughan 1994.
3.Individual TFCBT versus other therapies:Blanchard 2003; Brom 1989; Bryant 2003; Bryant 2011; Cloitre 2010; Feske 2008; Foa 1991; McDonagh 2005; Neuner 2004; Ready 2010; Schnurr 2007.
4. EMDR versus waitlist/usual care:Carlson 1998; Devilly 1998; Hogberg 2007; Jensen 1994; Power 2002; Rothbaum 1997; Rothbaum 2005; Vaughan 1994.
5. EMDR versus individual TFCBT:Devilly 1999: Ironson 2002: Lee 2002: Nijdam 2012; Power 2002: Rothbaum 2005; Taylor 2003; Vaughan 1994.
6. EMDR versus non‐TFCBT:Carlson 1998; Taylor 2003; Vaughan 1994.
7. EMDR versus other therapy:Marcus 1997; Scheck 1998.
8. Individual non‐TFCBT versus waitlist/usual care:Carlson 1998; Foa 1991; Foa 1999; Vaughan 1994; Wells 2012.
9. Non‐TFCBT versus other therapy:Foa 1991.
10. Group TFCBT versus waitlist/usual care:Beck 2009; Chard 2005; Hinton 2011; Hollifield 2007; Krakow 2001, Krakow 2000, Zlotnick 1997.
11. Group TFCBT versus group non‐TFCBT:Schnurr 2003.
12.Other therapies versus waitlist/usual care:Blanchard 2003; Brom 1989; Foa 1991; McDonagh 2005.
13. Group non‐TFCBT versus waitlist/usual care: no studies
14. Individual TFCBT versus group TFCBT: no studies
15. Individual TFCBT versus group non‐TFCBT: no studies
16. EMDR versus group TFCBT: no studies
17. EMDR versus group non‐TFCBT: no studies
18. Individual non‐TFCBT versus group TFCBT: no studies
19. Individual non‐TFCBT versus group non‐TFCBT: no studies
Outcomes
Primary outcomes were reduction in severity of clinician‐rated PTSD symptoms and drop‐out rate. Secondary outcome measures were severity of self‐reported PTSD symptom, severity of depressive symptoms, severity of anxiety symptoms, PTSD diagnosis after treatment and adverse side effects (such as increased PTSD symptoms).
Excluded studies
We excluded studies if they did not satisfy the inclusion criteria. Some studies made no formal diagnosis of PTSD (Abbasnejad 2007; Classen 2001; Classen 2010; Cole 2007; Edmond 1999; Edmond 2004; Falsetti 2001; Ginzberg 2009; Hiari 2005;, Knaevelsrud 2007; Lange 2001; Lange 2003; Litz 2007; Price 2007; Ryan 2005; Shapiro 1989a; Sloan 2004; Sloan 2005), and in other studies fewer than 70% of participants met full diagnostic criteria for the disorder (Davis 2007; Difede 2007a; DuHamel 2010; Maercker 2006; Rabe 2008; Van Emmerik 2008; Wilson 1995). Other reasons for excluding specific studies were a duration of less than three months following trauma (Echeburua 1996; some participants within, Foa 2006; Spence 2011), treatment did not target traumatic stress symptoms (Dunn 2007; Chemtob 1997), comparison of two psychological therapies in the same category (Arntz 2007; Mithoefer 2011; Paunovic 2001; Tarrier 1999; Watson 1997), the intervention was not a psychological therapy (Gidron 1996), including individuals under 18 years of age (Jaberghaderi 2004; Najavits 2006; Schaal 2009), comparison of two non‐TFCBT therapies, and comparison with pharmacotherapy (Frommberger 2004; Rothbaum 2006).
See Characteristics of excluded studies table for further details.
Studies awaiting classification
Krupnick 2008 is awaiting classification as we need to obtain further information.
Risk of bias in included studies
A graphical representation of the overall risk of bias for each domain and each study is available in Figure 2 and Figure 3 respectively.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
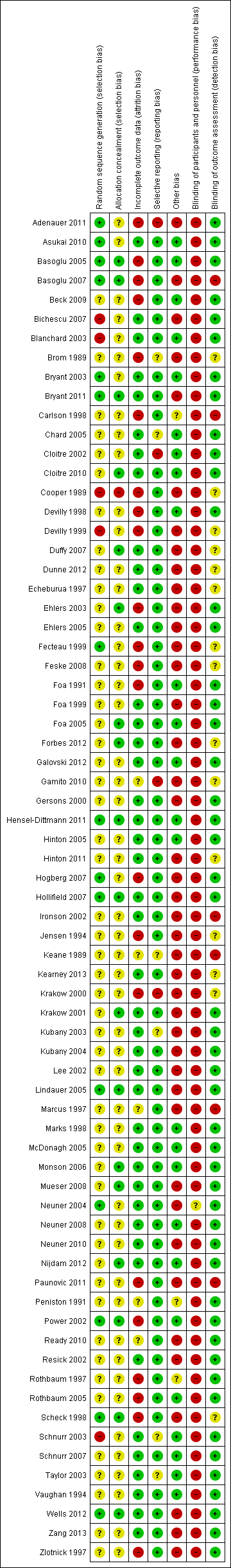
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence allocation randomisation
Fifteen studies reported a method of allocation that we felt to be appropriate, and which we judged to be at low risk of bias (Adenauer 2011; Asukai 2010; Basoglu 2005; Basoglu 2007; Bryant 2003; Bryant 2011; Fecteau 1999; Hensel‐Dittmann 2011; Hogberg 2007; Hollifield 2007; Lindauer 2005; Neuner 2004; Power 2002; Scheck 1998; Wells 2012). We judged the method of allocation to be at high risk of bias in six studies (Bichescu 2007; Blanchard 2003; Cooper 1989; Devilly 1999; Ehlers 2003; Schnurr 2003). The remainder of the studies reported insufficient information for us to make a judgement.
Allocation concealment
Many studies did not provide full details of the method of randomisation and we therefore judged concealment to be at unclear risk of bias in the majority. Fifteen studies reported adequate allocation concealment, representing a low risk of bias (Basoglu 2005; Basoglu 2007; Bryant 2011; Cloitre 2010; Duffy 2007; Ehlers 2003; Foa 2005; Forbes 2012; Krakow 2001; Lindauer 2005; Monson 2006; Mueser 2008; Nijdam 2012; Power 2002; Scheck 1998). We judged one study to be at high risk in terms of failure to conceal allocation (Cooper 1989). None of the other studies reported any methods of concealing allocation and we judged them to be at unclear risk of bias in this domain.
Blinding
In common with all studies of psychological therapies a double‐blind methodology is virtually impossible, as it is clear to the participant what treatment they are receiving. However, a well‐designed study should ensure blinding of the assessor of outcome measures. In six of the included studies, the assessor was aware of the participant's allocation (Basoglu 2005; Carlson 1998; Ironson 2002; Keane 1989; Marcus 1997; Paunovic 2011). It was unclear whether or not the assessor was blind in 14 studies (Brom 1989; Cooper 1989; Duffy 2007; Dunne 2012; Echeburua 1997; Fecteau 1999; Feske 2008; Forbes 2012; Gamito 2010; Hinton 2011; Jensen 1994; Kearney 2013; Krakow 2000; Scheck 1998).
Incomplete outcome data
Loss to follow‐up
Drop‐out rates were high in many of the studies and reasons for attrition were generally poorly reported. We judged 21 studies to be at high risk of bias for incomplete outcome data (Adenauer 2011; Basoglu 2005; Basoglu 2007; Beck 2009; Brom 1989; Carlson 1998; Cooper 1989; Devilly 1998; Devilly 1999; Ehlers 2003; Fecteau 1999; Feske 2008; Foa 1991; Hogberg 2007; Jensen 1994; Krakow 2000; Paunovic 2011; Power 2002; Rothbaum 1997; Rothbaum 2005; Scheck 1998; Zlotnick 1997). It was unclear how missing data were handled in six studies (Gamito 2010; Keane 1989; Marcus 1997; Peniston 1991; Ready 2010). We judged the remainder to have dealt with drop‐outs appropriately, i.e. at low risk of bias.
Selective reporting
It was difficult to draw any meaningful conclusions with regards to the issue of selective reporting. Very few of the included studies had published protocols available. It was therefore impossible to know whether prespecified outcome measures were adequately reported. The majority of studies adequately reported all outcomes outlined within the Methods section of the trial report.
Other potential sources of bias
A strength of the majority of the studies was having clear objectives, but sample sizes were often small and the follow‐up period was limited. The treatments delivered were reasonably well‐described and the majority of studies reported on the credentials and experience of therapists. A high proportion of the included studies made some assessment of treatment adherence. Most studies used well‐validated outcome measures although there was considerable variation in the actual measures used. For practical and ethical reasons there were rarely follow‐up data available from waitlist groups. We could not rule out potential researcher allegiance in many of the studies. A number of the included trials were of interventions that were evaluated by their originators.
Effects of interventions
See: Summary of findings for the main comparison Trauma‐focused CBT/Exposure therapy compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 2 Trauma‐focused CBT/Exposure therapy compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 3 Trauma‐focused CBT/Exposure therapy compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 4 EMDR compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 5 EMDR compared with Trauma‐focused CBT for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 6 EMDR compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 7 EMDR compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 8 Non‐TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 9 Non‐TFCBT compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 10 Group TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 11 Group TFCBT compared with Group non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 12 Other therapies compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults; Summary of findings 13 Group non‐TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults
The full results are contained in the Tables and are summarised below.
Comparison 1. Individual TFCBT/Exposure therapy versus waitlist/usual care
37 studies including 1830 participants contributed to this comparison.
Primary outcomes
1.1. Clinician‐rated PTSD symptoms
Twenty‐eight studies considered this outcome with a total of 1256 individuals (Analysis 1.1). There was heterogeneity between these trials (Chi² = 237.95, P = 0.00001; I² = 89%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (standardised mean difference (SMD) ‐1.62; 95% confidence interval (CI) ‐2.03 to ‐1.21).
At 1‐ to 4‐month follow‐up four studies compared this outcome with 336 individuals (Analysis 1.2). There was heterogeneity between these trials (Chi² = 52.30; P < 0.00001; I² = 94%). The individual TFCBT group did significantly better than the waitlist/usual care group (SMD ‐1.18; 95% CI ‐2.20 to ‐0.17).
At 5‐ to 8‐month follow‐up three studies compared this outcome with 192 individuals (Analysis 1.3). There was little heterogeneity between these trials (Chi² = 0.68; P = 0.71; I² = 0%). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐0.47; 95% CI ‐0.77 to ‐0.18).
At 9‐ to 12‐month follow‐up one study reported this outcome with 109 individuals (Analysis 1.4). The individual TFCBT group did significantly better than the waitlist/usual care group (SMD ‐0.78; 95% CI ‐1.18 to ‐0.39).
1.2. Drop‐outs
Thirty‐four studies with a total of 1776 individuals recorded whether individuals left the study early for any reason by group (Analysis 1.5). There was considerable clinical heterogeneity between these trials. The individual TFCBT group did significantly worse than the waitlist/usual care group (RR 1.64; 95% CI 1.30 to 2.06).
Secondary outcomes
1.3 Self‐reported PTSD symptoms
Seventeen studies considered this outcome with a total of 686 individuals (Analysis 1.1.4). There was heterogeneity between these trials (Chi² = 82.15; P < 0.00001; I² = 81%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.60; 95% CI ‐2.02 to ‐1.18).
At 1‐ to 4‐month follow‐up two studies compared this outcome with 181 individuals (Analysis 1.6). There was marked heterogeneity between these trials (Chi² = 40.04; P = 0.00001; I² = 98%). There were no significant differences between groups (SMD ‐3.03; 95% CI ‐6.51 to 0.45).
At 5‐ to 8‐month follow‐up two studies compared this outcome with 208 individuals (Analysis 1.7). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐0.61; 95% CI ‐0.90 to ‐0.32).
At 9‐ to 12‐month follow‐up one study compared this outcome with 121 individuals (Analysis 1.8). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐1.22; 95% CI ‐1.61 to ‐0.83).
1.4 Depression
Twenty‐nine studies considered this outcome with a total of 1233 individuals (Analysis 1.9). There was heterogeneity between these trials (Chi² = 174.84, P < 0.00001; I² = 84%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.31; 95% CI ‐1.65 to ‐0.98).
At 1‐ to 4‐month follow‐up seven studies compared this outcome with 413 individuals (Analysis 1.10). There was heterogeneity between these trials (Chi² = 41.24; P < 0.00001; I² = 85%). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐0.75; 95% CI ‐1.33 to ‐0.18).
At 5‐ to 8‐month follow‐up two studies compared this outcome with 150 individuals (Analysis 1.11). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐0.50; 95% CI ‐0.82 to ‐0.17).
At 9‐ to 12‐month follow‐up one study compared this outcome with 108 individuals (Analysis 1.12). The individual TFCBT group did better than the waitlist/usual care group (SMD ‐0.60; 95% CI ‐0.99 to ‐0.21).
1.5 Anxiety
Eighteen studies considered this outcome with a total of 664 individuals (Analysis 1.13). There was heterogeneity between these trials (Chi² = 27.43; P = 0.04; I² = 38%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐0.82; 95% CI ‐1.03 to ‐0.61).
At 1‐to 4‐month follow‐up three studies compared this outcome with 189 individuals (Analysis 1.14). There was little heterogeneity between trials (Chi² = 2.16; P = 0.34; I² = 7%). The TFCBT group did better than the waitlist/usual care group (SMD ‐0.32; 95% CI ‐0.60 to ‐0.03).
At 9‐ to 12‐month follow‐up one study compared this outcome with 108 individuals (Analysis 1.15). There was no difference between the two groups (SMD ‐0.33; 95% CI ‐0.71 to 0.05).
1.6 PTSD diagnosis after treatment
Nineteen studies with a total of 910 individuals reported this outcome (Analysis 1.18). There was significant heterogeneity between these trials (Chi² = 101.56; P < 0.00001; I² = 82%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group (RR 0.51; 95% CI 0.41 to 0.64).
1.7 Adverse effects
No studies formally considered adverse effects.
Comparison 2. TFCBT versus non‐TFCBT
Seven studies including 267 participants contributed to this comparison.
Primary outcomes
2.1 Clinician‐rated PTSD symptoms
Seven studies considered this outcome with a total of 267 individuals (Analysis 2.1). There was evidence of heterogeneity between these trials (Chi² = 11.25; P = 0.08; I² = 47%), and we used a random‐effects model to pool the data. There was no difference between TFCBT and non‐TFCBT in terms of clinician‐rated PTSD symptoms immediately after treatment (SMD ‐0.27; 95% CI ‐0.63 to 0.10).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 127 individuals (Analysis 2.2). There was evidence of heterogeneity between these trials (Chi² = 7.30; P = 0.12; I² = 45%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the non‐TFCBT group (SMD ‐0.51; 95% CI ‐1.00 to ‐0.01).
At 5‐ to 8‐month follow‐up two studies compared this outcome with 48 individuals (Analysis 2.3). There was evidence of heterogeneity between these trials (Chi² = 3.32; P = 0.07; I² = 70%) and we used a random‐effects model to pool the data. Based on these two trials, there was no difference between TFCBT and non‐TFCBT in terms of clinician‐rated PTSD symptoms at five to eight months (SMD ‐1.00; 95% CI ‐2.17 to 0.17).
At 9‐ to 12‐month follow‐up two studies compared this outcome with 48 individuals (Analysis 2.4). There was no evidence of heterogeneity between these trials (Chi² = 0.08; P = 0.78; I² = 0%) and we used a random‐effects model to pool the data. Based on these two trials, there was no difference between TFCBT and non‐TFCBT in terms of clinician‐rated PTSD symptoms at 9 to 12 months (SMD ‐12.93; 95% CI ‐18.72 to ‐7.14).
2.2 Drop‐outs
Seven studies with a total of 312 individuals recorded whether individuals left the study early for any reason by group (Analysis 2.5). There was no difference between the TFCBT group and the non‐TFCBT on this measure (RR 1.19; 95% CI 0.71 to 2.0).
Secondary outcomes
2.3 Self‐reported PTSD symptoms
Three studies considered this outcome with a total of 127 individuals (Analysis 2.6). There was no evidence of significant heterogeneity between these trials. The individual TFCBT group did better than the non‐TFCBT group (SMD ‐0.37; 95% CI ‐0.74 to 0.01).
At 1‐ to 4‐month follow‐up two studies considered this outcome with a total of 54 individuals (Analysis 2.7). There was no significant heterogeneity between these trials (Chi² = 0.78; P = 0.38; I² = 0%). There was no difference between the TFCBT group and the non‐TFCBT on this measure (SMD ‐0.44; 95% CI ‐0.99 to 0.10).
2.4 Depression
Six studies considered this outcome with a total of 189 individuals (Analysis 2.8). There was no evidence of heterogeneity between these trials (Chi² = 4.23; P = 0.52; I² = 0%). There was no difference between the individual TFCBT group and the non‐TFCBT group immediately after treatment (SMD ‐0.27; 95% CI ‐0.56 to 0.03).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 147 individuals (Analysis 2.9). There was no evidence of significant heterogeneity between these trials (Chi² = 4.47; P = 0.35; I² = 11%). There was no difference between the individual TFCBT group and the non‐TFCBT group (SMD ‐0.28; 95% CI ‐0.62 to 0.06).
At 5‐ to 8‐month follow‐up two studies compared this outcome with 48 individuals (Analysis 2.10). There was no evidence of significant heterogeneity between these trials (Chi² = 0.26; P = 0.61; I² = 0%). The individual TFCBT group did better than the non‐TFCBT group (SMD ‐0.71; 95% CI ‐1.30 to ‐0.12).
At 9‐ to 12‐month follow‐up one study compared this outcome with 20 individuals (Analysis 2.11). The individual TFCBT group did better than the non‐TFCBT group (SMD ‐8.00; 95% CI ‐14.44 to ‐1.86).
2.5 Anxiety
Four studies considered this outcome with a total of 127 individuals (Analysis 2.12). There was no evidence of significant heterogeneity between these trials (Chi² = 3.12; P = 0.37; I² = 4%). There was no difference between the individual TFCBT group and the non‐TFCBT group (SMD ‐0.12; 95% CI ‐0.49 to 0.26).
At 1‐ to 4‐month follow‐up four studies considered this outcome with a total of 117 individuals (Analysis 2.13). There was evidence of heterogeneity between these trials (Chi² = 5.56; P = 0.13; I² = 46%). There was no difference between the individual TFCBT group and the non‐TFCBT group (SMD ‐0.24; 95% CI ‐0.79 to 0.30).
At 5‐ to 8‐month follow‐up one study compared this outcome with 20 individuals (Analysis 2.14). There was no difference between the individual TFCBT group and the non‐TFCBT group (SMD ‐0.62; 95% CI ‐1.52 to 0.28).
At 9‐ to 12‐month follow‐up one study compared this outcome with 20 individuals (Analysis 2.15). There were no significant differences between groups (SMD ‐0.88; 95% CI ‐1.81 to 0.04).
2.6 PTSD diagnosis after treatment
Six studies with a total of 284 individuals reported this outcome (Analysis 2.16). There was no difference between the individual TFCBT group and the stress non‐TFCBT group (RR 0.83; 95% CI 0.60 to 1.17), using a random‐effects model due to moderate heterogeneity (Chi² = 8.59; P = 0.13; I² = 42%).
2.7 Adverse effects
No studies formally considered adverse effects.
Comparison 3. TFCBT versus other therapies
Eleven studies including 762 participants contributed to this comparison.
Primary outcomes
3.1 Clinician rated PTSD symptoms
Ten studies considered this outcome with a total of 625 individuals (Analysis 3.1). There was evidence of heterogeneity between these trials (Chi² = 29.33; P = 0.0006; I² = 69%) and we used a random‐effects model to pool the data The individual TFCBT group did better than the 'other therapies' group immediately after treatment (SMD ‐0.48; 95% CI ‐0.83 to ‐0.14).
At 1‐ to 4‐month follow‐up eight studies considered this outcome with a total of 548 individuals (Analysis 3.2). There was evidence of significant heterogeneity between these trials (Chi² = 15.13; P = 0.03; I² = 54%). The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.34; 95% CI ‐0.64 to ‐0.04).
At 5‐ to 8‐month follow‐up four studies compared this outcome with 434 individuals (Analysis 3.3). There was evidence of significant heterogeneity between these trials (Chi² = 19.43; P = 0.0002; I² = 85%), and we used a random‐effects model to pool the data. There were no differences between groups (SMD ‐0.58; 95% CI ‐1.20 to 0.04).
At 9‐ to 12‐month follow‐up two studies compared this outcome with 90 individuals (Analysis 3.4). There was significant heterogeneity between these trials (Chi² = 1.75; P = 0.19; I² = 43%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.76; 95% CI ‐1.35 to ‐0.17).
3.2 Drop‐outs
Eleven studies with a total of 762 individuals reported this outcome (Analysis 3.5). There was little heterogeneity between these trials. 'Other therapies' did better than TFCBT (RR 1.39; 95% CI 1.01 to 1.92).
Secondary outcomes
3.3 Self‐reported PTSD symptoms
Six studies considered this outcome with a total of 574 individuals (Analysis 3.6). There was evidence of heterogeneity between these trials (Chi² = 39.39; P < 0.00001; I² = 87%) and we used a random‐effects model to pool the data. The individual TFCBT group did better than the 'other therapies' group immediately after treatment (SMD ‐0.60; 95% CI ‐1.15 to ‐0.06).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 526 individuals (Analysis 3.7). There was no evidence of heterogeneity between these trials. The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.29; 95% CI ‐0.47 to ‐0.12).
At 5‐ to 8‐month follow‐up three studies compared this outcome with 338 individuals (Analysis 3.8). There was significant heterogeneity between these trials (Chi² = 16.14; P = 0.0003; I² = 88%) and we used a random‐effects model to pool the data. There were no differences between groups (SMD ‐0.90; 95% CI ‐2.05 to 0.25).
At 9‐ to 12‐month follow‐up two studies compared this outcome with 90 individuals (Analysis 3.9). There was no evidence of heterogeneity between these trials (Chi² = 0.26; P = 0.61; I² = 0%). Based on these two trials, there was no difference between TFCBT and 'other therapies' in terms of clinician‐rated PTSD symptoms (SMD ‐2.66; 95% CI ‐5.71 to 0.38).
3.4 Depression
Nine studies considered this outcome with a total of 570 individuals (Analysis 3.10). There was evidence of heterogeneity between these trials (Chi² = 13.34; P = 0.10; I² = 40%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.37; 95% CI ‐0.63 to ‐0.11).
At 1‐ to 4‐month follow‐up seven studies considered this outcome with a total of 510 individuals (Analysis 3.11). There was heterogeneity between these trials (Chi² = 13.28; P = 0.04; I² = 55%). There was no difference between the groups (SMD ‐0.29; 95% CI ‐0.62 to 0.03).
At 5‐ to 8‐month follow‐up five studies considered this outcome with a total of 443 individuals (Analysis 3.12). There was heterogeneity between these trials (Chi² = 12.31; P = 0.02; I² = 68%), and we used a random‐effects model to pool the data. There were no differences between groups (SMD ‐0.43; 95% CI ‐0.87 to 0.01).
At 9‐ to 12‐month follow‐up
3.5 Anxiety
Seven studies considered this outcome with a total of 539 individuals (Analysis 3.14). There was heterogeneity between these trials (Chi² = 14.75; P = 0.02; I² = 59%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.45; 95% CI ‐0.77 to ‐0.12).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 454 individuals (Analysis 3.15). There was heterogeneity between these trials (Chi² = 8.54; P = 0.07; I² = 53%), and we used a random‐effects model to pool the data. There was no difference between the individual TFCBT and the 'other therapies' groups (SMD ‐0.33; 95% CI ‐0.68 to 0.02).
Two trials reported this outcome at 5‐ to 8‐month follow‐up with a total of 329 individuals (Analysis 3.16). There was heterogeneity between these trials (Chi² = 10.28; P = 0.001; I² = 90%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.56; 95% CI ‐1.69 to 0.58).
3.6 PTSD diagnosis after treatment
Seven studies with a total of 358 individuals reported this outcome (Analysis 3.17). There was evidence of significant heterogeneity between these trials (Chi² = 9.54; P = 0.15; I² = 37%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the 'other therapies' group (RR 0.75; 95% CI 0.59 to 0.96).
3.7 Adverse effects
No studies formally considered adverse effects.
Comparison 4. EMDR versus waitlist/usual care
Eight studies including 301 participants contributed to this comparison.
Primary outcomes
4.1 Clinician‐rated PTSD symptoms
Six studies considered this outcome with a total of 183 individuals (Analysis 4.1). There was evidence of heterogeneity between these trials (Chi² = 31.52; P = 0.00001; I² = 84%) and we used a random‐effects model to pool the data. The EMDR group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.17; 95% CI ‐2.04 to ‐0.30).
4.2 Drop‐outs
Seven studies with a total of 227 individuals recorded whether individuals left the study early for any reason by group (Analysis 4.2). There was no evidence of heterogeneity between these trials. There was no difference between the EMDR and waitlist/usual care groups (RR 1.05; 95% CI 0.62 to 1.79).
Secondary outcomes
4.3 Self‐reported PTSD symptoms
Six studies considered this outcome with a total of 159 individuals (Analysis 4.3). There was heterogeneity between these trials (Chi² = 30.66; P < 0.00001; I² = 84%) and we used a random‐effects model to pool the data. There was no difference between studies (SMD ‐0.80; 95% CI ‐1.68 to 0.07).
4.4 Depression
Seven studies considered this outcome with a total of 226 individuals (Analysis 4.4). There was heterogeneity between these trials (Chi² = 9.61; P = 0.14; I² = 38%), and we used a random‐effects model to pool the data. The EMDR group did better than the control group (SMD ‐1.15; 95% CI ‐1.52 to ‐0.78).
4.5 Anxiety
Six studies considered this outcome with a total of 160 individuals (Analysis 4.5). There was no evidence of significant heterogeneity between these trials (Chi² = 3.20; P = 0.67; I² = 0%). The EMDR group did better than the control group (SMD ‐1.02; 95% CI ‐1.36 to ‐0.69).
4.7 PTSD diagnosis after treatment
Six studies with a total of 209 individuals reported this outcome (Analysis 4.6). There was no significant heterogeneity between these trials. The EMDR group did better than the controls (RR 0.51; 95% CI 0.42 to 0.62).
4.6 Adverse effects
No studies formally considered adverse effects.
Comparison 5. EMDR versus individual TFCBT/Exposure therapy
Eight studies including 400 participants contributed to this comparison.
Primary outcomes
5.1 Clinician‐rated PTSD symptoms
Seven studies considered this outcome with a total of 327 individuals (Analysis 5.1). There was evidence of heterogeneity between these trials (Chi² = 16.49; P = 0.01; I² = 64%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.03; 95% CI ‐0.43 to 0.38).
At 1‐ to 4‐month follow‐up three studies considered this outcome with a total of 76 individuals (Analysis 5.2). There was significant heterogeneity between these trials (Chi² = 5.56; P = 0.06; I² = 64%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.19; 95% CI ‐0.97 to 0.58).
5.2 Drop‐outs
Eight studies with a total of 400 individuals recorded whether individuals left the study early for any reason by group (Analysis 5.3). There was little heterogeneity (I² = 3%) between these trials. There was no difference between groups (RR 1.00; 95% CI 0.74 to 1.35).
Secondary outcomes
5.3 Self‐reported PTSD symptoms
Seven studies considered this outcome with a total of 306 individuals (Analysis 5.4). There was heterogeneity between these trials (Chi² = 8.82; P = 0.18; I² = 32%). There was no significant difference between the EMDR group and the individual TFCBT group immediately after treatment (SMD ‐0.30; 95% CI ‐0.60 to 0.01).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 111 individuals (Analysis 5.5). There was evidence of heterogeneity (Chi² = 8.46; P = 0.08; I² = 53%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.04; 95% CI ‐0.61 to 0.52).
At 9‐ to 12‐mont follow‐up
5.4 Depression
Eight studies considered this outcome with a total of 346 individuals (Analysis 5.6. There was heterogeneity between these trials (Chi² = 24.04; P = 0.001; I² = 71%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.31; 95% CI ‐0.75 to 0.13).
At 1‐ to 4‐month follow‐up five studies considered this outcome with a total of 111 individuals (Analysis 5.7). There was heterogeneity between these trials (Chi² = 6.93; P = 0.14; I² = 42%). There was no difference between groups (SMD ‐0.13; 95% CI ‐0.64 to 0.38).
5.5 Anxiety
Four studies considered this outcome with a total of 236 individuals (Analysis 5.8). There was little heterogeneity between these trials (Chi² = 1.94; P = 0.58; I² = 0%). The EMDR group did better than the TFCBT group (SMD ‐0.28; 95% CI ‐0.53 to ‐0.02).
At 1‐ to 4‐month follow‐up two studies considered this outcome with a total of 48 individuals (Analysis 5.9). There was little heterogeneity between these trials (Chi² = 0.70; P = 0.40; I² = 0%). There was no difference between groups (SMD 0.24; 95% CI ‐0.33 to 0.81).
5.6 PTSD diagnosis after treatment
Eight studies with a total of 400 individuals reported this outcome (Analysis 5.10). There was heterogeneity between these trials (Chi² = 15.56; P = 0.03; I² = 55%), and we used a random‐effects model to pool the data. There was no difference between the groups (RR 0.95; 95% CI 0.74 to 1.22).
5.7 Adverse effects
No studies formally considered adverse effects.
Comparison 6. EMDR versus non‐TFCBT
Three studies including 84 participants contributed to this comparison.
Primary outcomes
6.1 Clinician‐rated PTSD symptoms
Two studies considered this outcome with a total of 53 individuals (Analysis 6.1). There was little heterogeneity between these trials (Chi² = 0.69; P = 0.41; I² = 0%). There was no difference between groups (SMD ‐0.35; 95% CI ‐0.90 to 0.19).
At 1‐ to 4‐month follow‐up three studies considered this outcome with a total of 71 individuals (Analysis 6.2). There was heterogeneity between these trials (Chi² = 6.16; P = 0.05; I² = 68%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.74; 95% CI ‐1.64 to 0.15).
6.2 Drop‐outs
Three studies with a total of 84 individuals recorded whether individuals left the study early for any reason by group (Analysis 6.3). There was little heterogeneity between these trials. There was no difference between groups (RR 1.03; 95% CI 0.37 to 2.88).
Secondary outcomes
6.3 Self‐reported PTSD symptoms
Three studies considered this outcome with a total of 75 individuals (Analysis 6.4). There was little heterogeneity between these trials (Chi² = 0.64; P = 0.73; I² = 0%). There was no difference between groups (SMD ‐0.40; 95% CI ‐0.86 to 0.06).
At 1‐ to 4‐month follow‐up three studies considered this outcome with a total of 75 individuals (Analysis 6.5). There was little heterogeneity between these trials (Chi² = 0.89; P = 0.64; I² = 0%).The EMDR group did significantly better than the non‐TFCBT group immediately after treatment (SMD ‐0.52; 95% CI ‐0.98 to ‐0.05).
6.4 Depression
Three studies considered this outcome with a total of 75 individuals (Analysis 6.6). There was little heterogeneity between these trials. (Chi² = 1.02; P = 0.60; I² = 0%). The EMDR group did better than the non‐TFCBT group (SMD ‐0.67; 95% CI ‐1.14 to ‐0.20).
At 1‐ to 4‐month follow‐up three studies considered this outcome with a total of 75 individuals (Analysis 6.7). There was heterogeneity between these trials. (Chi² = 3.45; P = 0.18; I² = 42%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.25; 95% CI ‐0.86 to 0.36).
6.5 Anxiety
Two studies considered this outcome with a total of 45 individuals (Analysis 6.8). There was little heterogeneity between these trials (Chi² = 0.37; P = 0.54; I² = 0%). The EMDR group did better than the non‐TFCBT group (SMD ‐0.75; 95% CI ‐1.36 to ‐0.13).
At 1‐ to 4‐month follow‐up two studies considered this outcome with a total of 45 individuals (Analysis 6.9). There was heterogeneity between these trials (Chi² = 8.09; P = 0.004; I² = 88%), and we used a random‐effects model to pool the data. There was no difference between groups (SMD ‐0.42; 95% CI ‐2.21 to 1.37).
6.6 PTSD diagnosis after treatment
Three studies with a total of 84 individuals reported this outcome (Analysis 6.10). There was significant heterogeneity between these trials. There was no difference between groups ((RR 0.71; 95% CI 0.41 to 1.22).
6.7 Adverse effects
No studies formally considered adverse effects.
Comparison 7. EMDR versus 'other therapies'
Two studies including 127 participants contributed to this comparison.
Primary outcomes
7.1 Clinician rated PTSD symptoms
No studies formally considered this outcome.
7.2 Drop‐outs
Two studies with a total of 127 individuals recorded whether individuals left the study early for any reason by group (Analysis 7.1). There was little heterogeneity between these trials. There was no difference between groups (RR 1.48; 95% CI 0.26 to 8.54).
Secondary outcomes
7.3 Self‐reported PTSD symptoms
Two studies considered this outcome with a total of 124 individuals (Analysis 7.2). There was little heterogeneity between these trials (Chi² = 0.19; P = 0.66; I² = 0%). The EMDR group did better than the 'other therapies' group immediately after treatment (SMD ‐0.84; 95% CI ‐1.21 to ‐0.47).
7.4 Depression
Two studies considered this outcome with a total of 127 individuals (Analysis 7.3). There was little heterogeneity between these trials (Chi² = 0.05; P = 0.82; I² = 0%). The EMDR group did better than the 'other therapies' group (SMD ‐0.67; 95% CI ‐1.03 to ‐0.32).
7.5 Anxiety
Two studies considered this outcome with a total of 126 individuals (Analysis 7.4). There was little heterogeneity between these trials (Chi² = 0.10; P = 0.75; I² = 0%). The EMDR group did better than the 'other therapies' group (SMD ‐0.72; 95% CI ‐1.08 to ‐0.36).
7.6 PTSD diagnosis after treatment (Analysis 7.5)
7.7 Adverse effects
No studies formally considered adverse effects.
Comparison 8. Non‐TFCBT versus waitlist/usual care
Five studies including 141 participants contributed to this comparison.
Primary outcomes
8.1 Clinician‐rated PTSD symptoms
Four studies considered this outcome with a total of 106 individuals (Analysis 8.1). There was heterogeneity between these trials (Chi² = 4.62; P = 0.2; I² = 35%), and we used a random‐effects model to pool the data. The non‐TFCBT group did better than the controls (SMD ‐1.22; 95% CI ‐1.76 to ‐0.69).
8.2 Drop‐outs
Four studies with a total of 121 individuals recorded whether individuals left the study early for any reason by group (Analysis 8.2). There was no significant heterogeneity between these trials (Chi² = 1.10; P = 0.89; I² = 0%). There was no difference between the groups (RR 1.96; 95% CI 0.70 to 5.48).
Secondary outcomes
8.3 Self‐reported PTSD symptoms
Two studies considered this outcome with a total of 44 individuals (Analysis 8.3). There was considerable heterogeneity between the groups (Chi² = 11.83; P = 0.0006; I² = 92%), and we used a random‐effects model to pool the data. There was no difference between the non‐TFCBT group and the waitlist/usual care group immediately after treatment (SMD ‐0.86; 95% CI ‐3.27 to 1.55).
8.4 Depression
Five studies considered this outcome with a total of 129 individuals (Analysis 8.4). There was heterogeneity between these trials (Chi² = 7.04; P = 0.13; I² = 43%), and we used a random‐effects model to pool the data. The non‐TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐0.93; 95% CI ‐1.43 to ‐0.42).
8.5 Anxiety
Four studies considered this outcome with a total of 102 individuals (Analysis 8.5). There was no heterogeneity between these trials (Chi² = 2.38; P = 0.50; I² = 0%). Non‐TFCBT did better than the waitlist/usual care group immediately after treatment (SMD ‐0.83; 95% CI ‐1.24 to ‐0.42).
8.6 PTSD diagnosis after treatment
Four studies with a total of 121 individuals reported this outcome (Analysis 8.6). There was heterogeneity between these trials (Chi² = 6.15; P = 0.10; I² = 51%), and we used a random‐effects model to pool the data. The non‐TFCBT group did better than the waitlist/usual care group (RR 0.65; 95% CI 0.50 to 0.86).
8.7 Adverse effects
No studies formally considered adverse effects.
Comparison 9. Non‐TFCBT versus other therapies
One study including 31 participants contributed to this comparison.
Primary outcomes
9.1 Clinician‐rated PTSD symptoms
One study considered this outcome with a total of 25 individuals (Analysis 9.1). The stress non‐TFCBT group did better than the other therapies group immediately after treatment (SMD ‐1.22; 95% CI ‐2.09 to ‐0.35).
At 1‐ to 4‐month follow‐up one study considered this outcome with a total of 18 individuals (Analysis 9.2). The stress non‐TFCBT group did no better than the 'other therapies' group (SMD ‐0.38; 95% CI ‐1.31 to 0.55).
9.2 Drop‐outs
One study with a total of 31 individuals recorded whether individuals left the study early for any reason by group (Analysis 9.3). There was no difference between groups (RR 0.82; 95% CI 0.20 to 3.46).
Secondary outcomes
9.3 Self‐reported PTSD symptoms
No studies considered this outcome.
9.4 Depression
One study considered this outcome with a total of 25 individuals (Analysis 9.4). There was no difference between groups (SMD ‐0.51; 95% CI ‐1.31 to 0.30).
At 1‐ to 4‐month follow‐up one study considered this outcome with a total of 18 individuals (Analysis 9.5). There was no difference between groups (SMD ‐0.48; 95% CI ‐1.42 to 0.46).
9.5 Anxiety
One study considered this outcome with a total of 25 individuals (Analysis 9.6). There was no difference between groups (SMD ‐0.51; 95% CI ‐1.32 to 0.29).
At 1‐ to 4‐month follow‐up one study considered this outcome with a total of 18 individuals (Analysis 9.7). There was no difference between groups (SMD ‐0.68; 95% CI ‐1.64 to 0.28).
9.6 PTSD diagnosis after treatment
One study with a total of 31 individuals reported this outcome (Analysis 9.8). There was no difference between groups (RR 0.58; 95% CI 0.30 to 1.11).
9.7 Adverse effects
No studies formally considered adverse effects.
Comparison 10. Group TFCBT versus waitlist/usual care
Seven studies including 573 participants contributed to this comparison.
Primary outcomes
10.1 Clinician‐rated PTSD symptoms
Three studies considered this outcome with a total of 185 individuals (Analysis 10.1). There was heterogeneity between these trials (Chi² = 15.60; P = 0.0004; I² = 87%), and we used a random‐effects model to pool the data. The Group TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.28; 95% CI ‐2.25 to ‐0.31).
At 5‐ to 8‐month follow‐up one study compared this outcome with 97 individuals (Analysis 10.2). The Group TFCBT group did better than the waitlist/usual care group (SMD ‐0.72; 95% CI ‐1.14 to ‐0.31).
10.2 Drop‐outs
Seven studies with a total of 573 individuals recorded whether individuals left the study early for any reason by group (Analysis 10.3). There was no significant heterogeneity between these trials (Chi² = 2.28; P = 0.81; I² = 0%). There was no difference between the Group TFCBT group and the waitlist/usual care group (RR 1.21; 95% CI 0.94 to 1.55).
Secondary outcomes
10.3 Self‐reported PTSD symptoms
Six studies considered this outcome with a total of 274 individuals (Analysis 10.4). There was heterogeneity between these trials (Chi² = 17.53; P = 0.004; I² = 71%), and we used a random‐effects model to pool the data. The Group TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.20; 95% CI ‐1.70 to ‐0.69).
At 1‐ to 4‐month follow‐up two studies compared this outcome with 73 individuals (Analysis 10.5). There was some heterogeneity between these trials (Chi² = 1.45; P = 0.23; I² = 31%). The Group TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.14; 95% CI ‐1.78 to ‐ 0.50).
10.4 Depression
Three studies with a total of 137 individuals reported this outcome (Analysis 10.6). There was heterogeneity between these trials (Chi² = 9.91; P = 0.007; I² = 80%), and we used a random‐effects model to pool the data. The Group TFCBT group did significantly better than the waitlist/usual care group (SMD ‐1.15; 95% CI ‐1.98 to ‐0.32).
At 1‐ to 4‐month follow‐up one study compared this outcome with 49 individuals (Analysis 10.7). The Group TFCBT group did better than the waitlist/usual care group (SMD ‐0.62; 95% CI ‐1.00 to ‐0.24).
10.5 Anxiety
Three studies with a total of 106 individuals reported this outcome (Analysis 10.8). There was little heterogeneity between these trials. The Group TFCBT group did significantly better than the waitlist/usual care group immediately after treatment (SMD ‐0.66; 95% CI ‐1.06 to ‐0.27).
At 1‐ to 4‐month follow‐up two studies compared this outcome with 61 individuals (Analysis 10.9). There was little heterogeneity between these trials (Chi² = 1.20; P = 0.27; I² = 17%). The Group TFCBT group did significantly better than the waitlist/usual care group (SMD ‐0.43; 95% CI ‐0.72 to ‐0.14).
10.6 PTSD diagnosis after treatment
One study with a total of 48 individuals reported this outcome (Analysis 10.10).There was no significant difference between the Group TFCBT group and the waitlist/usual care group (RR 0.56; 95% CI 0.31 to 1.01).
10.7 Adverse effects
No studies formally considered adverse effects.
Comparison 11. Group TFCBT versus group non‐TF CBT
One study including 360 participants contributed to this comparison.
Primary outcomes
11.1 Clinician‐rated PTSD symptoms
One study considered this outcome with a total of 325 individuals (Analysis 11.1). There was no difference between the Group trauma‐focused CBT and Group non‐trauma‐focused CBT (SMD ‐0.12; 95% CI ‐0.34 to 0.10).
11.2 Drop‐outs
One study with a total of 360 individuals recorded whether individuals left the study early for any reason by group (Analysis 11.2). There was no significant difference between the Group trauma‐focused CBT and Group non‐trauma‐focused CBT groups (RR 1.38; 95% CI 1.00 to 1.90).
Secondary outcomes
11.3 Self‐reported PTSD symptoms
No studies considered this outcome.
11.4 Depression
No studies considered this outcome.
11.5 Anxiety
No studies considered this outcome.
11.6 PTSD diagnosis after treatment
One study with a total of 360 individuals reported this outcome (Analysis 11.3). There was no difference between the Group trauma‐focused CBT and Group non‐trauma‐focused CBT groups (RR 0.98; 95% CI 0.83 to 1.16).
11.7 Adverse effects
No studies formally considered adverse effects.
Comparison 12. Other therapies versus waitlist/usual care
Four studies including 211 participants contributed to this comparison.
Primary outcomes
12.1 Clinician‐rated PTSD symptoms
Three studies considered this outcome with a total of 112 individuals (Analysis 12.1). There was no heterogeneity between these trials (Chi² = 1.61; P = 0.45; I² = 0%). The 'other therapies' group did better than the waitlist/usual care group immediately after treatment (SMD ‐0.58; 95% CI ‐0.96 to ‐0.20).
12.2 Drop‐outs
Four studies with a total of 211 individuals recorded whether individuals left the study early for any reason by group (Analysis 12.2). There was no heterogeneity between these trials. The waitlist group did better than the 'other therapies' group (RR 2.45; 95% CI 0.99 to 6.10).
Secondary outcomes
12.3 Self‐reported PTSD symptoms
Two studies considered this outcome with a total of 132 individuals (Analysis 12.3). There was no heterogeneity between these trials (Chi² = 0.29; P = 0.59; I² = 0%). The 'other therapies' group did better than the waitlist/usual care group immediately after treatment (SMD ‐0.61; 95% CI ‐0.98 to ‐0.24).
12.4 Depression
Three studies considered this outcome with a total of 112 individuals (Analysis 12.4). There was little heterogeneity between these trials (Chi² = 2.62; P = 0.27; I² = 24%). The 'other therapies' group did better than the waitlist/usual care group immediately after treatment (SMD ‐0.45; 95% CI ‐0.83 to ‐0.07).
12.5 Anxiety
Four studies considered this outcome with a total of 193 individuals (Analysis 12.5). There was no heterogeneity between these trials (Chi² = 0.70; P = 0.87; I² = 0%). The 'other therapies' group did significantly better than the waitlist/usual care group immediately after treatment (SMD ‐0.52; 95% CI ‐0.82 to ‐0.22).
12.6 PTSD diagnosis after treatment
Four studies with a total of 210 individuals reported this outcome (Analysis 12.6). There was heterogeneity between these trials (Chi² = 8.34; P = 0.04; I² = 64%), and we used a random‐effects model to pool the data. There was no difference between the 'other therapies' and the waitlist/usual care group (RR 0.80; 95% CI 0.61 to 1.05).
12.7 Adverse effects
No studies formally considered adverse effects.
Comparison 13. Group non‐TFCBT versus waitlist/usual care
One study including 47 participants contributed to this comparison.
Primary outcomes
13.1 Clinician‐rated PTSD symptoms
No studies considered this outcome.
13.2 Drop‐outs
One study with a total of 47 individuals recorded whether individuals left the study early for any reason by group (Analysis 13.1). There was no difference between groups (RR 1.76; 95% CI 0.17 to 18.11).
Secondary outcomes
13.3 Self‐reported PTSD symptoms
One study considered this outcome with a total of 47 individuals (Analysis 13.2). There was no difference between groups (SMD ‐0.49; 95% CI ‐1.07 to 0.09).
At 1‐ to 4‐month follow‐up one study considered this outcome with a total of 47 individuals (Analysis 13.3). There was no difference between groups (SMD ‐0.40; 95% CI ‐0.98 to 0.18).
13.4 Depression
One study considered this outcome with a total of 47 individuals (Analysis 13.4). There was no difference between groups (SMD ‐0.06; 95% CI ‐0.64 to 0.51).
At 1‐ to 4‐month follow‐up one study considered this outcome with a total of 47 individuals (Analysis 13.5). There was no difference between groups (SMD ‐0.03; 95% CI ‐0.60 to 0.54).
13.5 Anxiety
No studies considered this outcome.
13.6 PTSD diagnosis after treatment
No studies considered this outcome.
13.7 Adverse effects
No studies formally considered adverse effects.
Comparison 14. Individual TFCBT versus group TFCBT
No studies made this comparison.
Comparison 15. Individual TFCBT versus group non‐TFCBT
No studies made this comparison.
Comparison 16. EMDR versus group TFCBT
No studies made this comparison.
Comparison 17. EMDR versus group non‐TFCBT
No studies made this comparison.
Comparison 18. Individual non‐TFCBT versus group TFCBT
No studies made this comparison.
Comparison 19. Individual non‐TFCBT versus group non‐TFCBT
No studies made this comparison.
19. Risk of bias sensitivity analysis
19.1 Comparison 1. Individual TFCBT/Exposure therapy versus waitlist/usual care
Clinician‐rated PTSD symptoms
Only one trial ( Basoglu 2005) including 59 participants had a low risk of bias for sequence generation, allocation concealment and blinding of the outcome assessor. Analysis restricted only to this trial (Analysis 1.17) showed no difference between TFCBT and waitlist/usual care (SMD ‐0.44; 95% CI ‐0.95 to 0.08), which differs from the overall meta‐analysis, which showed a statistically significant difference in favour of TFCBT (SMD ‐1.62; 95% CI ‐2.03 to ‐1.21). However, the intervention was a single session of TFCBT.
Drop‐outs
The intervention was a single session of TFCBT. On this basis, drop‐out was not a relevant comparison.
19.2 Comparison 2. TFCBT versus non‐TFCBT
Fewer than 10 studies made this comparison.
19.3 Comparison 3. TFCBT versus other therapies
Clinician‐rated PTSD symptoms
Only one trial (Bryant 2003) including 58 participants had a low risk of bias in terms of sequence generation, allocation concealment and blinding of the outcome assessor. Analysis restricted only to this trial (Analysis 3.19) favoured TFCBT (SMD ‐1.52; 95% CI ‐2.14 to ‐0.89).
Drop‐outs
One study with a total of 58 participants had a low risk of bias in terms of sequence generation, allocation concealment and blinding of the outcome assessor. There was no difference between TFCBT and other therapy when the analysis was restricted to this study (Analysis 3.20) (RR 1.50; 95% CI 0.47 to 4.8).
19.4 Comparison 4. EMDR versus waitlist/usual care
Fewer than 10 studies made this comparison.
19.5 Comparison 5. EMDR versus individual TFCBT/Exposure therapy
Fewer than 10 studies made this comparison.
19.6 Comparison 6. EMDR versus non‐TFCBT
Fewer than 10 studies made this comparison.
19.7 Comparison 7. EMDR versus other therapies
Fewer than 10 studies made this comparison.
19.8 Comparison 8. Non‐TFCBT versus waitlist/usual care
Fewer than 10 studies made this comparison.
19.9 Comparison 9. Non‐TFCBT versus other therapies
Fewer than 10 studies made this comparison.
19.10 Comparison 10. Group TFCBT versus waitlist/usual care
Fewer than 10 studies made this comparison.
19.11 Comparison 11. Group TFCBT versus group non‐TFCBT
Fewer than 10 studies made this comparison.
19.12 Comparison 12. Other therapies versus waitlist/usual care
Fewer than 10 studies made this comparison.
19.13 Comparison 13. Group non‐TFCBT versus waitlist/usual care
Fewer than 10 studies made this comparison.
20. Clinical heterogeneity sensitivity analyses
In order to explore clinical heterogeneity, we conducted two subgroup analyses for the two comparisons including more than 10 studies.
20.1 Comparison 1. Individual TFCBT/Exposure therapy versus waitlist/usual care
Twenty‐eight studies considered this outcome with a total of 1256 individuals (Analysis 1.1.1). There was heterogeneity between these trials (Chi² = 237.95; P = 0.00001; I² = 89%), and we used a random‐effects model to pool the data. The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.62; 95% CI ‐2.03 to ‐1.21).
Women‐only studies
Nine studies met this criterion, with a total of 562 women (Analysis 1.1.2). There was heterogeneity between these trials (Chi² = 37.39; P = 0.00001; I² = 79%), and we used a random‐effects model to pool the data.The individual TFCBT group did better than the waitlist/usual care group immediately after treatment (SMD ‐1.83; 95% CI ‐2.31 to ‐1.36), demonstrating a larger difference in favour of individual TFCBT than in the overall analyses.
Studies excluding Vietnam war veterans
Twenty‐seven studies met this criterion, with a total of 1232 individuals (Analysis 1.1.3). There was heterogeneity between these trials (Chi² = 230.39; P = 0.00001; I² = 89%), and we used a random‐effects model to pool the data.The individual TFCBT group did significantly better than the waitlist/usual care group immediately after treatment (SMD ‐1.67; 95% CI ‐2.09 to ‐1.25), demonstrating little difference from the overall analyses. Excluding these two trials made little difference to the observed statistically significant heterogeneity or to the effect estimate.
20.2 Comparison 3. TFCBT versus other therapies
Ten studies considered this outcome with a total of 608 individuals (Analysis 3.1). There was evidence of heterogeneity between these trials (Chi² = 25.08; P = 0.003; I² = 64%), and we used a random‐effects model to pool the data The individual TFCBT group did better than the 'other therapies' group immediately after treatment (SMD ‐0.46; 95% CI ‐0.79 to ‐0.13).
Women‐only studies (sexual assault/abuse)
Three studies met this criterion, with a total of 129 women (Analysis 3.21). There was no difference between groups (SMD 0.03; 95% CI ‐0.42 to 0.47).
Studies excluding Vietnam war veterans
Nine studies met this criterion, with a total of 599 individuals (Analysis 3.22).The individual TFCBT group did better than the 'other therapies' group (SMD ‐0.46; 95% CI ‐0.80 to ‐0.11), demonstrating minimal difference from the overall analyses. Excluding this one trial made little difference to the observed statistically significant heterogeneity or to the effect estimate.
20.3 Publication bias
All the studies identified for this review were published, and many of the trials were undertaken relatively recently. We explored the potential effects of publication bias using funnel plots. We constructed two funnel plots using data from the individual TFCBT versus waitlist/usual care comparison (comparison 1), one involving continuous data in the primary outcome, and the second involving dichotomous data in a secondary outcome. We also looked at the TFCBT versus 'other therapies' comparison (3.1).
The first funnel plot examined the measure of clinician‐rated PTSD symptoms (see Figure 4) and was roughly symmetrical with the exception of one outlier and an absence of smaller studies demonstrating no difference or a difference in favour of waitlist/usual care. This suggests the possibility of publication bias. As the studies become less precise the results of the studies tended be more variable and scattered to either side of the more precise larger studies. The same was found for the comparison of TFCBT with other therapies (see Figure 5).

Funnel plot of comparison: 1 Individual trauma‐focused CBT/Exposure therapy vs waitlist/usual care, outcome: 3.1 Severity of PTSD symptoms ‐ clinician‐rated.

Funnel plot of comparison: 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, outcome: 3.1 Severity of PTSD symptoms ‐ clinician‐rated.
The second funnel plot using data from the individual TFCBT versus waitlist/usual care comparison examined the dichotomous measure of PTSD diagnosis after treatment (see Figure 6). This funnel plot shows that larger studies demonstrated smaller differences between individual TFCBT and waitlist/usual care, and also suggests an absence of smaller studies demonstrating no difference or a difference in favour of waitlist/usual care. This funnel plot therefore shows some evidence of publication bias. It is possible that, due to the greater likelihood of publication of positive studies, the true difference between groups is smaller than is suggested by this review.

Funnel plot of comparison: 1 Individual trauma‐focused CBT/Exposure therapy vs waitlist/usual care, outcome: 1.7 PTSD diagnosis after treatment.
Discussion
Summary of main results
We include 70 studies of 4761 participants in this review. We have created a 'Summary of findings' table for each of the 13 comparisons we were able to conduct. We assessed the quality of the evidence for each comparison as very low. Statistically, individual trauma‐focused cognitive behavioural therapy (TFCBT) and eye movement desensitisation and reprocessing (EMDR) did better than waitlist/usual care in reducing clinician‐assessed post‐traumatic stress disorder (PTSD) symptoms. Non‐TFCBT did significantly better than waitlist/usual care and other therapies. Other therapies did significantly better than waitlist/usual care control, as did group‐TFCBT. There was no difference between group non‐TFCBT and waitlist/usual care. There was no statistically significant difference between individual TFCBT, EMDR and non‐TFCBT immediately post‐treatment although there was some evidence that individual TFCBT and EMDR are superior to non‐TFCBT at between one and four months following treatment, and that individual TFCBT, EMDR and non‐TFCBT were more effective than other therapies. There was some evidence of greater drop‐out in active treatment groups. The considerable unexplained heterogeneity observed in these comparisons, and the potential impact of publication bias on these data, suggest the need for caution in interpreting the results of this review.
Trauma‐focused cognitive behavioural therapy
There was very low quality evidence that individual TFCBT was better than waitlist/usual care in reducing traumatic stress symptoms and associated symptoms of depression and anxiety. The overall standardised mean difference for traumatic stress symptoms post‐treatment represents an effect size generally accepted as indicating a strong positive effect. After exploration of heterogeneity this finding remains robust although there is statistically significant heterogeneity present in all analyses. There is not enough evidence to confirm whether this advantage is maintained over time. There is evidence that individual TFCBT is a more effective treatment than non‐trauma‐focused therapies classed as 'other therapies'. There is also evidence that individual TFCBT is superior to non‐TFCBT, although this is not apparent immediately after treatment.
Eye movement desensitisation and reprocessing
There was very low quality evidence that EMDR was better than waitlist/usual care in reducing traumatic stress symptoms and additionally associated symptoms of depression and anxiety. The overall standardised mean differences for clinician‐rated and self‐rated traumatic stress symptoms post‐treatment represents a strong positive effect size. There is not enough evidence to determine whether these improvements are maintained in the long‐term as there were no follow‐up comparisons of EMDR and waitlist groups. There were limited data to suggest a better outcome than non‐TFCBT at between one‐ and four‐month follow‐up. EMDR appeared to have similar effectiveness to individual TFCBT in the studies that compared them directly. There was some evidence that EMDR was a more effective treatment than other therapies.
Non‐trauma‐focused cognitive behavioural therapy
There was very low quality evidence that non‐TFCBT was better than waitlist/usual care in reducing traumatic stress symptoms and additionally associated symptoms of depression and anxiety. It is worth noting that the meta‐analyses of non‐TFCBT included fewer participants than TFCBT and EMDR, and demonstrated high heterogeneity, indicating that these results should be interpreted with caution. There was some evidence that non‐TFCBT is a more effective treatment than other non‐trauma‐focused therapies, but this was from the results of one study only. Non‐TFCBT was less effective than TFCBT at follow‐up. Whilst this might indicate insufficient statistical power, it may also indicate that non‐TFCBT provides only temporary relief of symptoms, or that TFCBT provides a basis for continued improvement.
Other therapies
There was very low quality evidence that other therapies were better than waitlist/usual care in reducing traumatic stress symptoms and associated symptoms of depression and anxiety. As stated above, other therapies were statistically significantly worse in terms of the primary outcome measure of reducing traumatic stress symptoms when directly compared with individual TFCBT and non‐TFCBT. There was a statistically significant difference in favour of other therapies in comparison with individual TFCBT in terms of drop‐out. This finding suggests the possibility of non‐trauma‐focused therapies being easier to tolerate than individual TFCBT, although this finding is based on studies of variable quality.
Group TFCBT
There was very low quality evidence that group TFCBT was better than waitlist/usual care in reducing traumatic stress symptoms. There was no difference between group TFCBT and non‐trauma‐focused group CBT.
Group non‐TFCBT
There was no evidence that group non‐TFCBT was any better than waitlist/usual care in reducing traumatic stress symptoms or depression immediately after treatment or at four‐month follow‐up in the one study making this comparison.
Drop‐outs
Individual TFCBT and other therapies both did worse than waitlist/usual care on this outcome measure. There was also some evidence that individual TFCBT incurred a greater drop‐out rate than other therapies.
Adverse effects
Unfortunately no studies reported adverse effects. It is well recognised that these may occur, such as increased re‐experiencing following exposure treatment (e.g. Pitman 1991), and the absence of any reporting of them is of major concern.
Anxiety and depression
Symptoms of anxiety and depression generally improved in line with improvements in traumatic stress symptoms. This is no surprise for treatments such as cognitive restructuring where many of the approaches used for PTSD would also be used for anxiety and depression. However other treatments such as exposure therapy do not address depressive symptoms per se yet still appeared to reduce depressive and anxiety symptoms. This suggests that the anxiety and depressive symptoms found in many PTSD sufferers in these studies were secondary to the PTSD rather than being discrete conditions requiring specific treatment.
Heterogeneity
The forest plots of the pooled results demonstrated statistically significant heterogeneity between the studies. For example, heterogeneity levels of P < 0.00001 were observed in several analyses of the primary outcome measure. There are likely to be several factors that contribute to this heterogeneity. There is clearly considerable clinical diversity within the studies considered. We attempted to explore this by performing subgroup analyses on the primary outcome measure of individual TFCBT versus waitlist/usual care. Those studies including only women, all of whom had been sexually or non‐sexually assaulted, produced more positive results than the overall results. Possible explanations include the treatments having been superior, women being more responsive to individual TFCBT than men, traumatisation by assault being more responsive to individual TFCBT, a combination of these and/or other factors. Those studies that did not include only Vietnam war veterans produced a slightly more positive result than all studies. However there was only one study excluded from this subgroup analysis, which may therefore lack power to show a real difference; great caution must be exercised in interpreting this finding.
Overall completeness and applicability of evidence
This review considers randomised controlled trials (RCTs) of a wide range of psychological therapies for chronic PTSD, including individual TFCBT, EMDR, non‐TFCBT, group TFCBT, group non‐TFCBT, and other psychological therapies (see Types of interventions). Studies included participants from different countries and backgrounds, who had been exposed to a variety of different traumatic events (see Types of participants). The majority of studies reported on the use of qualified and experienced therapists, and a high proportion included assessment of adherence to a treatment protocol (see Characteristics of included studies). Many factors, however, limit the generalisability of conclusions reached by this review. Most studies to date have been conducted in the USA, Canada, Australia and Europe, limiting generalisability of results to the rest of the world. Although we have included the full range of psychological therapies for PTSD considered by RCTs to date, insufficient data preclude meta‐analysis of a number of outcomes in some treatment groups. There are many more studies of individual TFCBT than other therapies, for example, those taking a person‐centred only or psychodynamic approach, which were considered within a single category. The majority of studies did not include participants with comorbid psychiatric diagnoses and substance dependence, excluding individuals who are arguably more difficult to treat. This may have resulted in the exclusion of individuals with complicated histories and the experience of multiple traumatic events. Generalising the results to more complex presentations of the disorder is therefore problematic. There were very limited long‐term follow‐up data, especially for waitlist groups. This prevents determination of the long‐term efficacy of therapies, but the incremental improvement of the active treatment groups in the trials with longer follow‐ups suggests that benefits are maintained. Comparison on the basis of trauma type, study setting and participant variables would be an interesting addition to future versions of this review.
Most studies reported on drop‐outs by group; drop‐out rates are likely to be influenced by adverse effects along with other factors. The major distinction between the treatments classed as TFCBT and those classed as 'other therapies' was the inclusion of exposure‐based components. Since the treatments that included exposure had a significantly greater drop‐out rate, this raises questions surrounding the tolerability of exposure work. Although it is well documented that exposure‐based treatments can cause short‐term exacerbation of symptoms (e.g. Tarrier 1999), we have little understanding of how this impacts on drop‐out rates. Given that the included studies provide little disclosure of adverse events and few explanations for drop‐outs, it is difficult to ascertain why participants were less able to tolerate exposure‐based treatments. This is an important finding and one that should stimulate the development of interventions that are more acceptable to those who receive them.
Quality of the evidence
Methodological quality varied considerably (see Characteristics of included studies). Risk of bias was high or unclear for multiple domains in a large proportion of older studies, especially those published before 2000. Most studies rated as having a low risk of bias were of individual TFCBT. In a large proportion of studies, the information provided by the published report was insufficient to determine the risk of bias associated with key methodological indicators. There was considerable unexplained heterogeneity for some analyses and the quality of evidence for each of the comparisons was rated as very low.
As with all psychological therapy trials, there are issues with the control groups. The development of a 'psychological therapy placebo' is very difficult, if not impossible, as is blinding of participants and therapists. It is possible that the efficacy of psychological therapies is stronger than suggested by the data, as in several studies the waitlist/usual care group received some contact and the expectation that they would be treated, which may have been therapeutic. It is, however, also possible that waitlist groups do worse than usual care groups because they do not expect to improve until they receive the active therapy.
Potential biases in the review process
The review followed guidelines set out by The Cochrane Collaboration (Higgins 2011). Two authors independently read all the candidate studies, assessed them for inclusion, and rated them for risk of bias. We discussed any disagreements with a third review author with the aim of reaching a consensus. This will have minimised potential bias, but several unavoidable issues remain. For example, all studies included in the review were published, resulting in the possibility of publication bias. We explored and confirmed this using funnel plots (Figures 4, 5 and 6). Several studies reported incomplete data, although we contacted authors to obtain missing results where possible. Although we searched numerous online databases systematically, scrutinised reference lists, contacted experts in the field, and handsearched relevant additional sources, we cannot rule out the possibility of missed RCTs. There was a great deal of heterogeneity between trials of the psychological therapies included within specific categories. This was especially notable within the 'other therapies' group. Although all were examples of non‐trauma‐focused psychological therapies that were not based on the principles of CBT, interventions included psychodynamic therapy, hypnotherapy, supportive counselling and present‐centred counselling, which differ in terms of proposed active ingredients and the way in which they are delivered. There was also considerable statistical heterogeneity evident in many of the comparisons. In circumstances where heterogeneity was thought to be an issue, we used a random‐effects model, and reported this. In addition, therapies within the 'other therapies' category were not always delivered with the intention of being as effective as the other treatment under study, but to act as a control (e.g.Bryant 2003; Bryant 2011; Feske 2008). It is clear that TFCBT performed very well versus waitlist/usual care comparison, but it must also be considered that this comparison included by far the most studies. It is fair to say that other categories (e.g. other therapies) may have performed better had they been represented by a larger number of studies. Finally, it is worth noting that there were differences in terms of acceptance into treatment protocols (for example, the requirement of diagnosis through structured interview versus self‐reported measures), which is likely to have resulted in some studies that were overly inclusive and others that exercised more conservative guidelines.
Agreements and disagreements with other studies or reviews
This review supports the efficacy of psychological therapy for chronic PTSD, which is consistent with the results of many other reviews (e.g. Bisson 2007b; Bradley 2005; Van Etten 1988). It is also consistent with current practice guidelines which recommend individual TFCBT and EMDR as frontline treatments for the disorder (ACPMH 2007; NICE 2005).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Individual trauma‐focused CBT/Exposure therapy vs waitlist/usual care, outcome: 3.1 Severity of PTSD symptoms ‐ clinician‐rated.

Funnel plot of comparison: 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, outcome: 3.1 Severity of PTSD symptoms ‐ clinician‐rated.

Funnel plot of comparison: 1 Individual trauma‐focused CBT/Exposure therapy vs waitlist/usual care, outcome: 1.7 PTSD diagnosis after treatment.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 1 Severity of PTSD symptoms ‐ clinician.
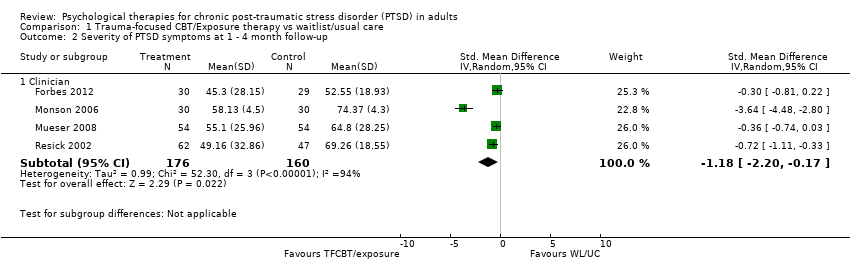
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 2 Severity of PTSD symptoms at 1 ‐ 4 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 3 Severity of PTSD symptoms at 5 ‐ 8 month follow‐up..

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 4 Severity of PTSD symptoms 9 ‐ 12 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 5 Leaving the study early for any reason.
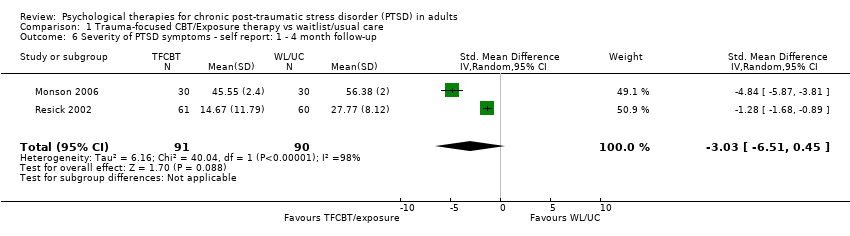
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 6 Severity of PTSD symptoms ‐ self report: 1 ‐ 4 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 7 Severity of PTSD symptoms ‐ self report: 5 ‐ 8 months).
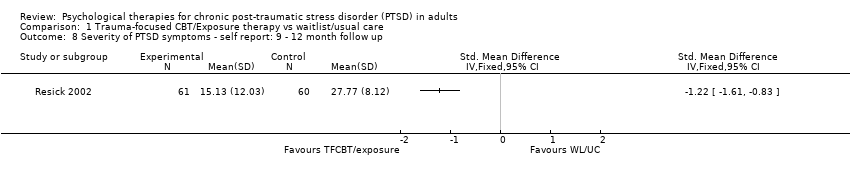
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 8 Severity of PTSD symptoms ‐ self report: 9 ‐ 12 month follow up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 9 Depression.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 10 Depression 1 ‐ 4 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 11 Depression 5 ‐ 8 month follow‐up.
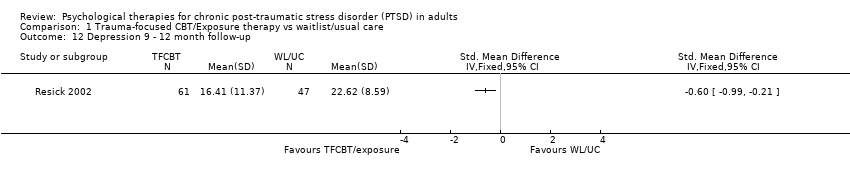
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 12 Depression 9 ‐ 12 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 13 Anxiety.
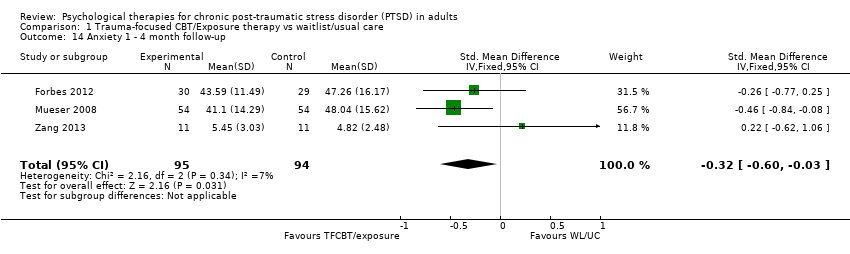
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 14 Anxiety 1 ‐ 4 month follow‐up.
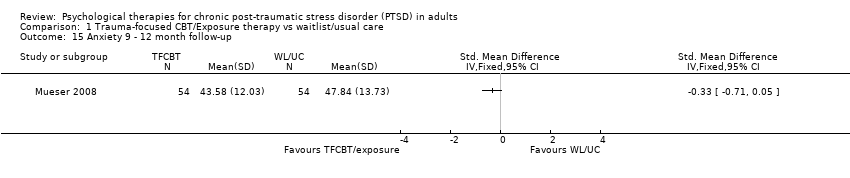
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 15 Anxiety 9 ‐ 12 month follow‐up.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 16 Exploration of publication bias.
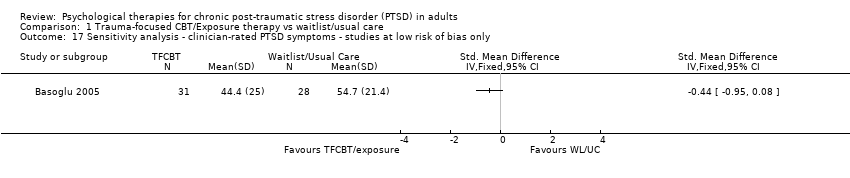
Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 17 Sensitivity analysis ‐ clinician‐rated PTSD symptoms ‐ studies at low risk of bias only.

Comparison 1 Trauma‐focused CBT/Exposure therapy vs waitlist/usual care, Outcome 18 PTSD diagnosis after treatment.

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 1 Severity of PTSD Symptoms ‐ clinician.

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1 ‐ 4 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 3 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (5 ‐ 8 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 4 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (9 ‐ 12 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 5 Leaving the study early for any reason.

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 6 Severity of PTSD symptoms ‐ self report.

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 7 Severity of PTSD symptoms ‐ self report ‐ follow‐up (1‐4 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 8 Depression.
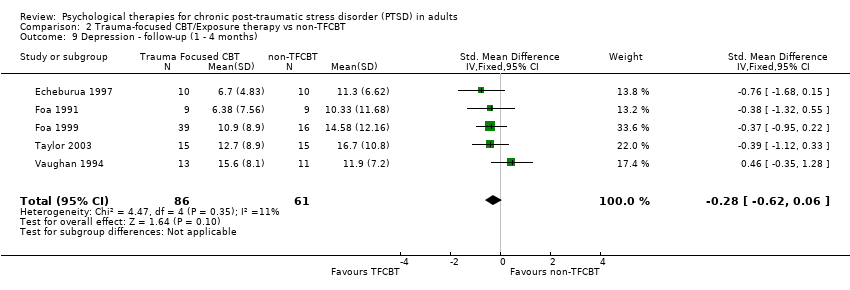
Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 9 Depression ‐ follow‐up (1 ‐ 4 months).
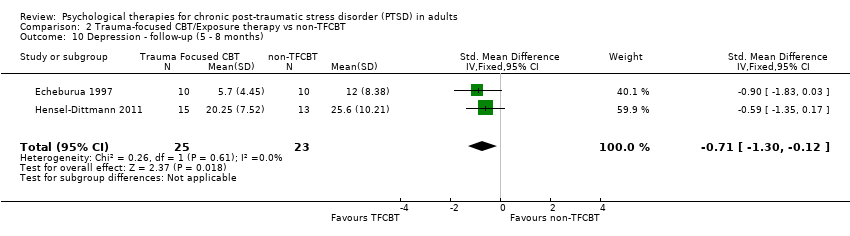
Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 10 Depression ‐ follow‐up (5 ‐ 8 months).
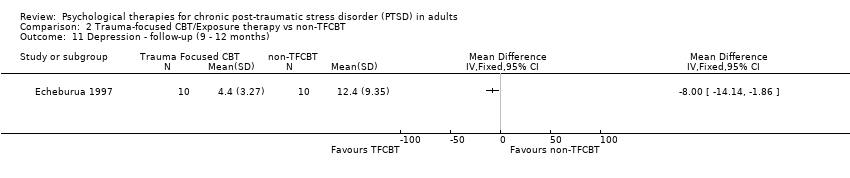
Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 11 Depression ‐ follow‐up (9 ‐ 12 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 12 Anxiety.

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 13 Anxiety ‐ follow‐up (1 ‐ 4 months).
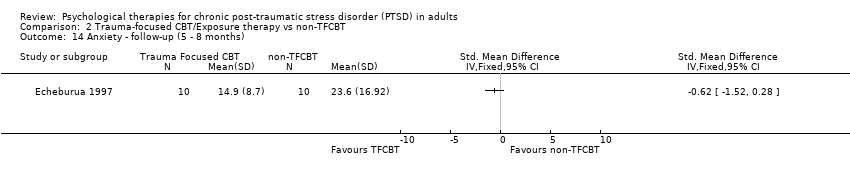
Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 14 Anxiety ‐ follow‐up (5 ‐ 8 months).
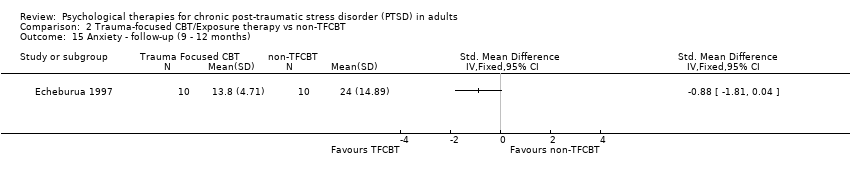
Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 15 Anxiety ‐ follow‐up (9 ‐ 12 months).

Comparison 2 Trauma‐focused CBT/Exposure therapy vs non‐TFCBT, Outcome 16 PTSD diagnosis after treatment.
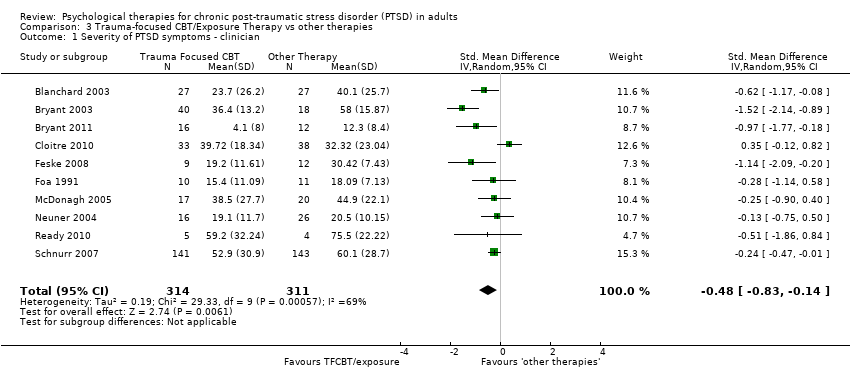
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 1 Severity of PTSD symptoms ‐ clinician.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 2 Severity of PTSD symptoms ‐ clinician ‐ 1 ‐ 4 month follow‐up.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 3 Severity of PTSD symptoms ‐ clinician ‐ 5 ‐ 8 month follow‐up.
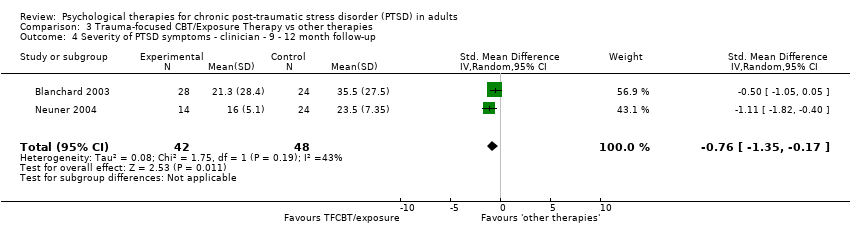
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 4 Severity of PTSD symptoms ‐ clinician ‐ 9 ‐ 12 month follow‐up.
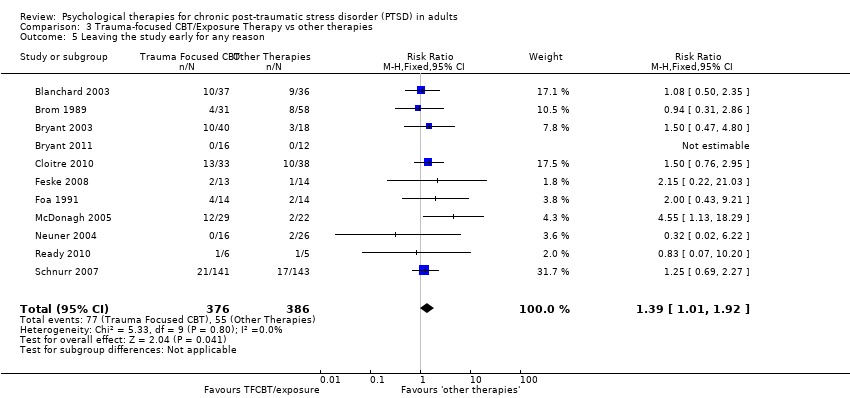
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 5 Leaving the study early for any reason.
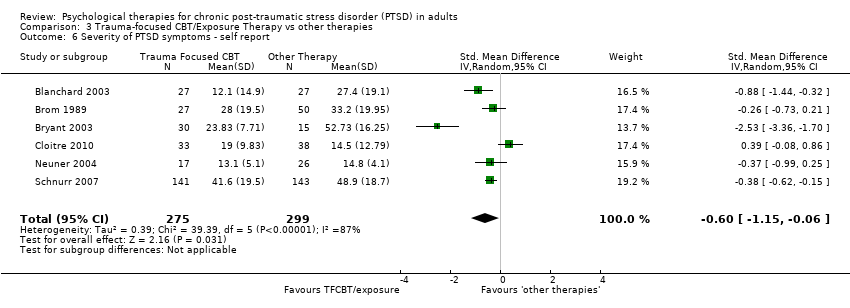
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 6 Severity of PTSD symptoms ‐ self report.
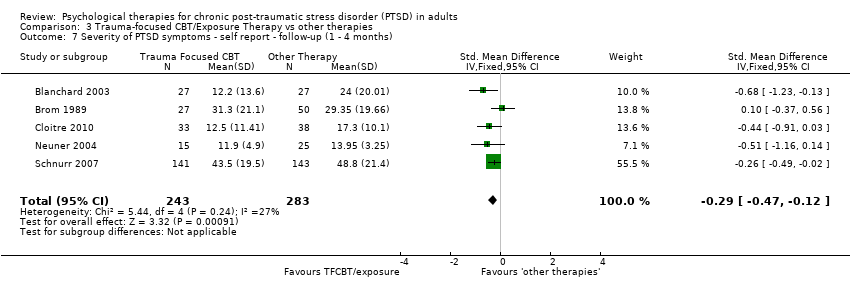
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 7 Severity of PTSD symptoms ‐ self report ‐ follow‐up (1 ‐ 4 months).

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 8 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (5 ‐ 8 months).

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 9 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (9 ‐ 12 months).

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 10 Depression ‐ self report.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 11 Depression ‐ self‐report ‐ follow‐up (1‐4 months).
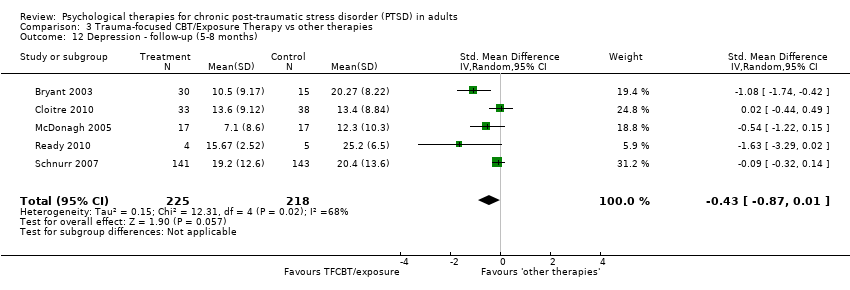
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 12 Depression ‐ follow‐up (5‐8 months).
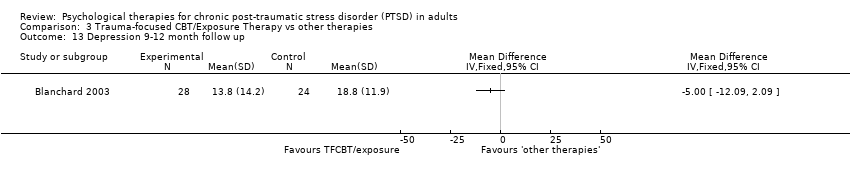
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 13 Depression 9‐12 month follow up.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 14 Anxiety ‐ self report.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 15 Anxiety ‐ self‐report ‐ follow‐up (1 ‐ 4 months).

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 16 Anxiety ‐ follow‐up (5 ‐ 8 months).
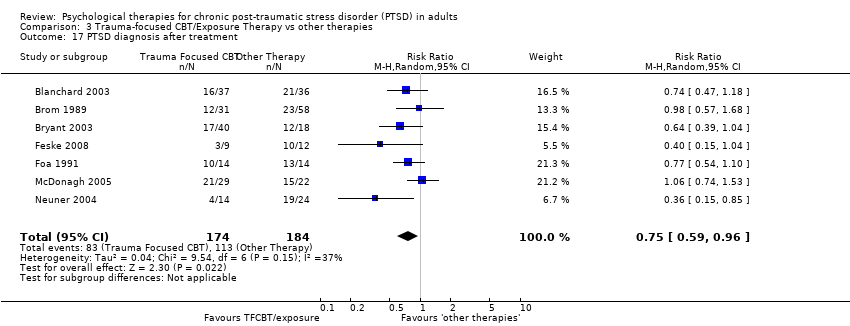
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 17 PTSD diagnosis after treatment.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 18 Severity of PTSD symptoms ‐ clinician ‐ 13‐24 month follow‐up.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 19 Sensitivity analysis ‐ PTSD symptoms ‐ studies at low risk of bias only.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 20 Sensitivity analysis ‐ drop‐out ‐ studies at low risk of bias only.
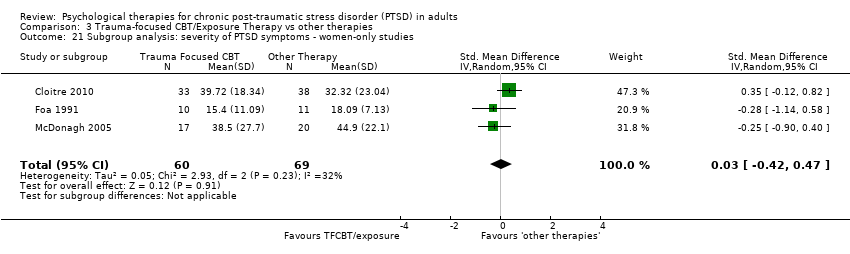
Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 21 Subgroup analysis: severity of PTSD symptoms ‐ women‐only studies.

Comparison 3 Trauma‐focused CBT/Exposure Therapy vs other therapies, Outcome 22 Subgroup analysis: severity of PTSD symptoms ‐ clinician ‐ excluding Vietnam veterans.

Comparison 4 EMDR vs waitlist/usual care, Outcome 1 Severity of PTSD symptoms ‐ Clinician.
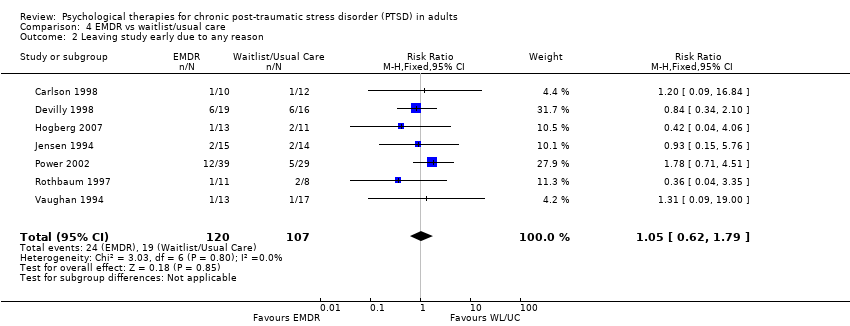
Comparison 4 EMDR vs waitlist/usual care, Outcome 2 Leaving study early due to any reason.

Comparison 4 EMDR vs waitlist/usual care, Outcome 3 Severity of PTSD symptoms ‐ self report.

Comparison 4 EMDR vs waitlist/usual care, Outcome 4 Depression.

Comparison 4 EMDR vs waitlist/usual care, Outcome 5 Anxiety.

Comparison 4 EMDR vs waitlist/usual care, Outcome 6 PTSD diagnosis after treatment.

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 1 Severity of PTSD symptoms ‐ clinician.

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1 ‐ 4 months).

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 3 Leaving study early for any reason.

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 4 Severity of PTSD symptoms ‐ self report.
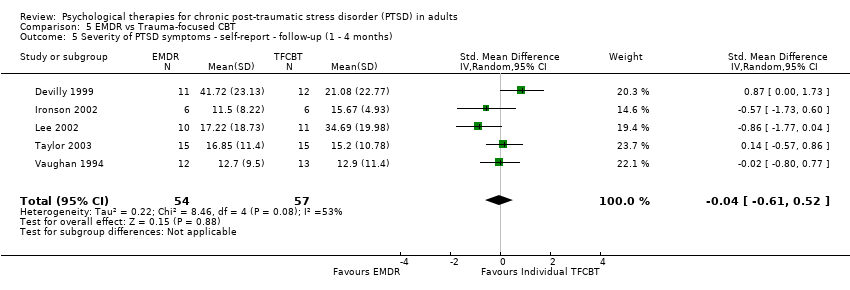
Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 5 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (1 ‐ 4 months).
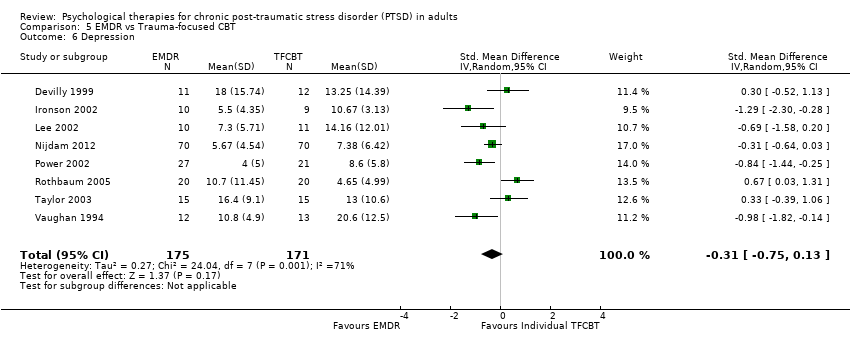
Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 6 Depression.
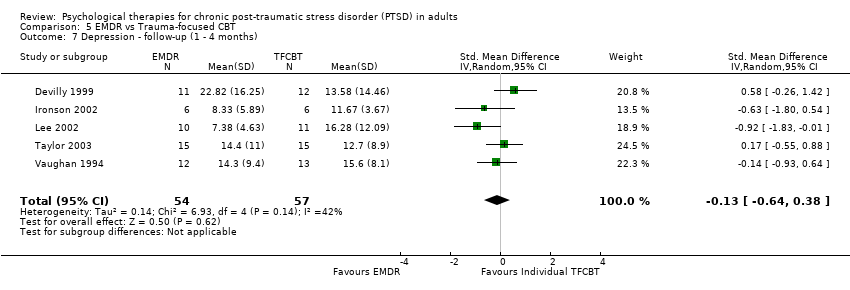
Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 7 Depression ‐ follow‐up (1 ‐ 4 months).
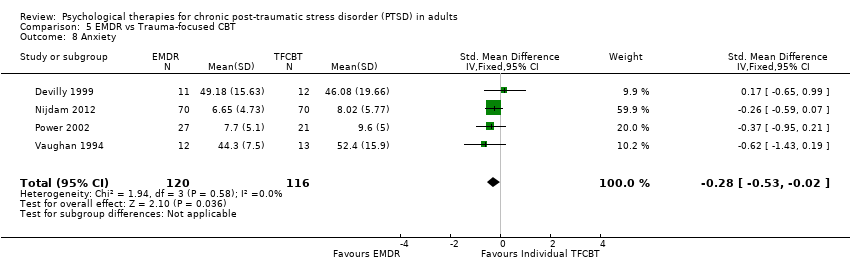
Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 8 Anxiety.

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 9 Anxiety ‐ follow‐up (1 ‐ 4 months).

Comparison 5 EMDR vs Trauma‐focused CBT, Outcome 10 PTSD diagnosis after treatment.

Comparison 6 EMDR vs non‐TFCBT, Outcome 1 Severity of PTSD symptoms ‐ clinician.

Comparison 6 EMDR vs non‐TFCBT, Outcome 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1‐4 months).

Comparison 6 EMDR vs non‐TFCBT, Outcome 3 Leaving the study early for any reason.
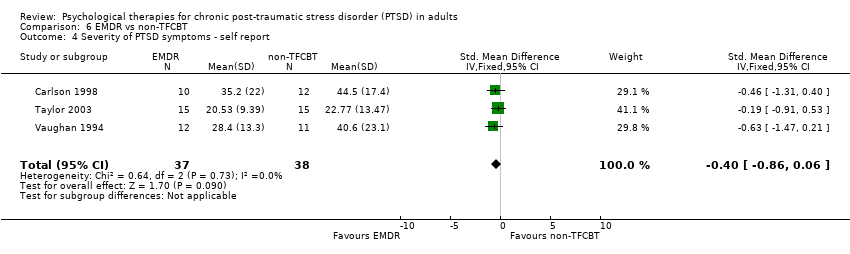
Comparison 6 EMDR vs non‐TFCBT, Outcome 4 Severity of PTSD symptoms ‐ self report.

Comparison 6 EMDR vs non‐TFCBT, Outcome 5 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (1 ‐ 4 months).
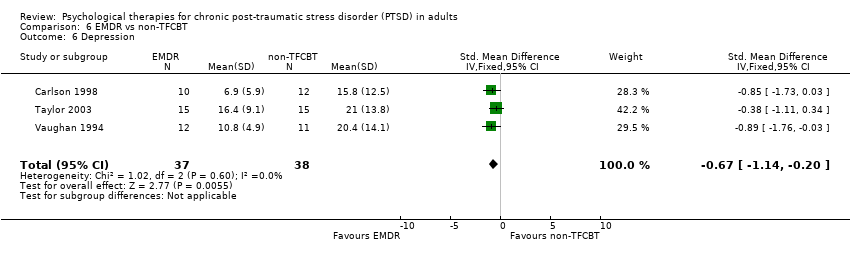
Comparison 6 EMDR vs non‐TFCBT, Outcome 6 Depression.

Comparison 6 EMDR vs non‐TFCBT, Outcome 7 Depression ‐ follow‐up (1 ‐ 4 months).

Comparison 6 EMDR vs non‐TFCBT, Outcome 8 Anxiety.
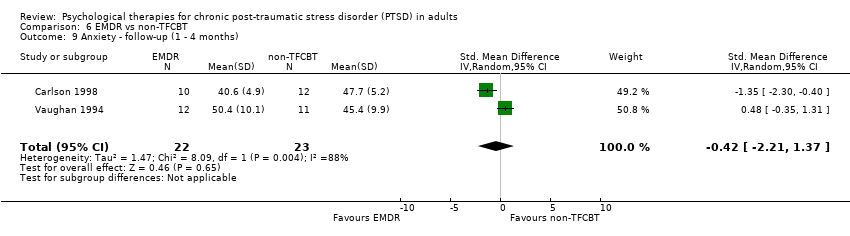
Comparison 6 EMDR vs non‐TFCBT, Outcome 9 Anxiety ‐ follow‐up (1 ‐ 4 months).
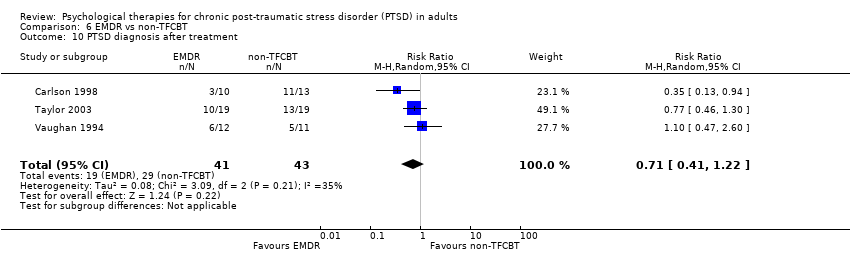
Comparison 6 EMDR vs non‐TFCBT, Outcome 10 PTSD diagnosis after treatment.
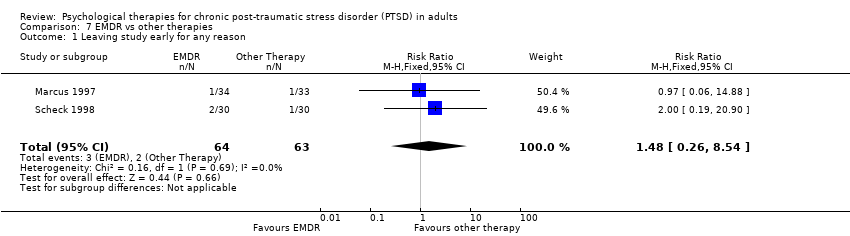
Comparison 7 EMDR vs other therapies, Outcome 1 Leaving study early for any reason.

Comparison 7 EMDR vs other therapies, Outcome 2 Severity of PTSD symptoms ‐ self report.
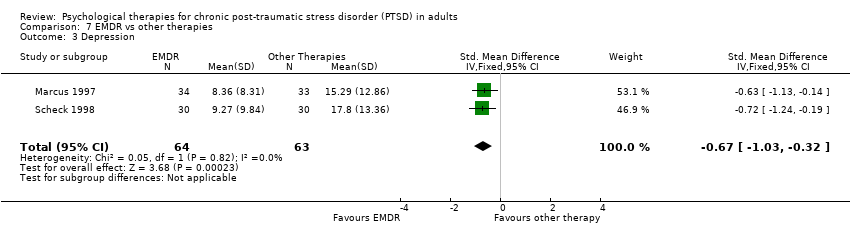
Comparison 7 EMDR vs other therapies, Outcome 3 Depression.

Comparison 7 EMDR vs other therapies, Outcome 4 Anxiety.
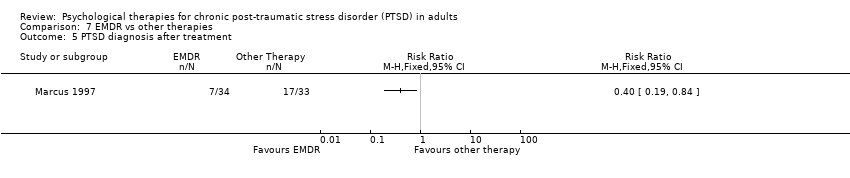
Comparison 7 EMDR vs other therapies, Outcome 5 PTSD diagnosis after treatment.
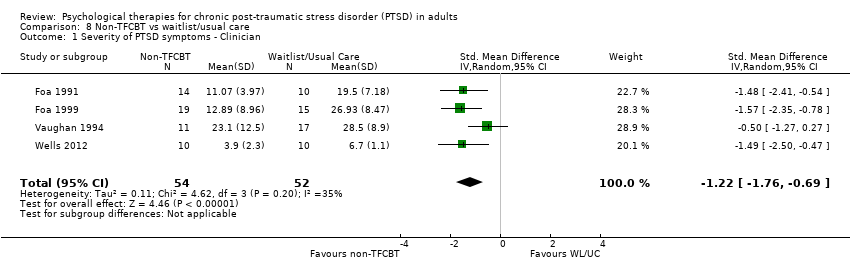
Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 1 Severity of PTSD symptoms ‐ Clinician.
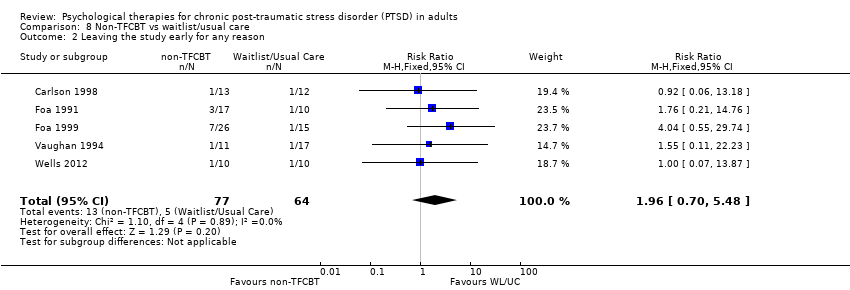
Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 2 Leaving the study early for any reason.

Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 3 Severity of PTSD symptoms ‐ Self‐report.

Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 4 Depression.

Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 5 Anxiety.

Comparison 8 Non‐TFCBT vs waitlist/usual care, Outcome 6 PTSD diagnosis after treatment.
Comparison 9 Non‐TFCBT vs other therapies, Outcome 1 Severity of PTSD symptoms ‐ Clincian.
Comparison 9 Non‐TFCBT vs other therapies, Outcome 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (3 months).
Comparison 9 Non‐TFCBT vs other therapies, Outcome 3 Leaving the study early for any reason.
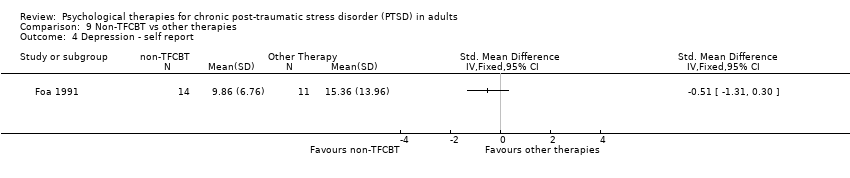
Comparison 9 Non‐TFCBT vs other therapies, Outcome 4 Depression ‐ self report.

Comparison 9 Non‐TFCBT vs other therapies, Outcome 5 Depression ‐ self report ‐ follow‐up (3 months).

Comparison 9 Non‐TFCBT vs other therapies, Outcome 6 Anxiety ‐ self report.

Comparison 9 Non‐TFCBT vs other therapies, Outcome 7 Anxiety ‐ self report ‐ follow‐up (3 months).

Comparison 9 Non‐TFCBT vs other therapies, Outcome 8 PTSD diagnosis after treatment.

Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 1 Clinician‐rated.
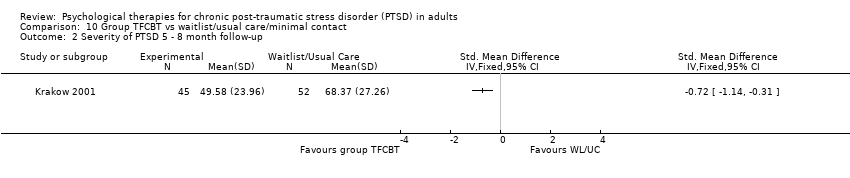
Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 2 Severity of PTSD 5 ‐ 8 month follow‐up.

Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 3 Leaving the study early for any reason.
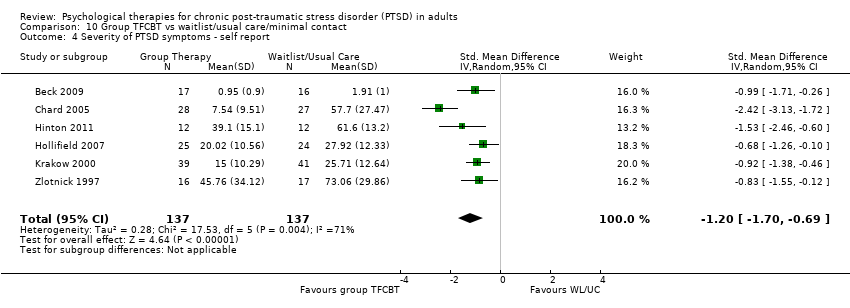
Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 4 Severity of PTSD symptoms ‐ self report.

Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 5 Severity of PTSD ‐ self report ‐ 1 ‐ 4 months.
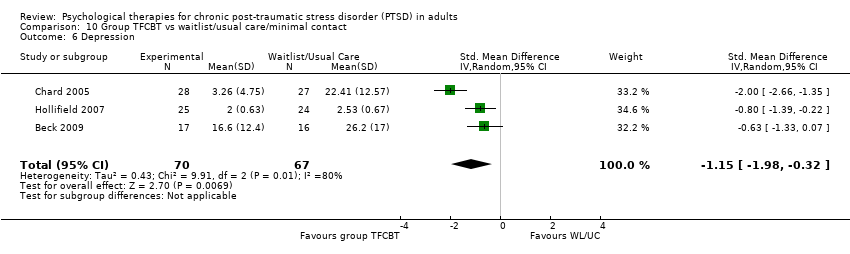
Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 6 Depression.
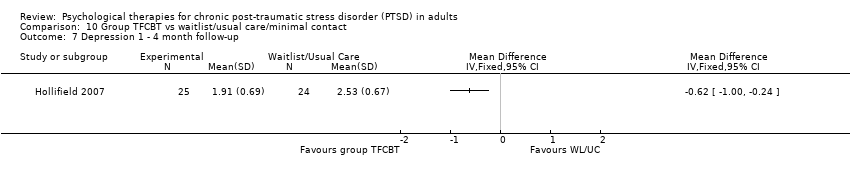
Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 7 Depression 1 ‐ 4 month follow‐up.
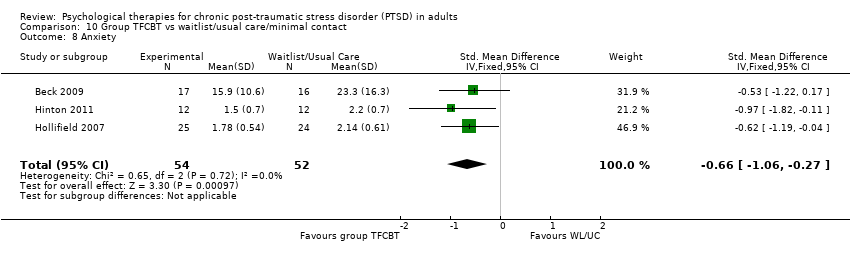
Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 8 Anxiety.

Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 9 Anxiety 1 ‐ 4 month follow‐up.

Comparison 10 Group TFCBT vs waitlist/usual care/minimal contact, Outcome 10 PTSD diagnosis after treatment.

Comparison 11 Group CBT (trauma‐focused) vs Group CBT (non‐trauma‐focused), Outcome 1 Severity of PTSD symptoms.

Comparison 11 Group CBT (trauma‐focused) vs Group CBT (non‐trauma‐focused), Outcome 2 Leaving the study early for any reason.
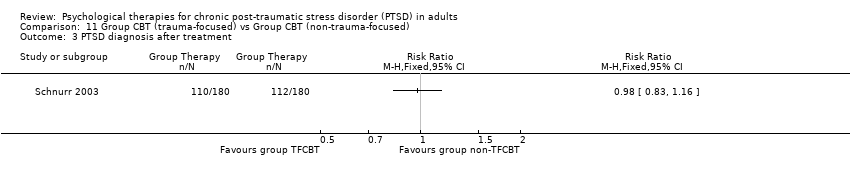
Comparison 11 Group CBT (trauma‐focused) vs Group CBT (non‐trauma‐focused), Outcome 3 PTSD diagnosis after treatment.
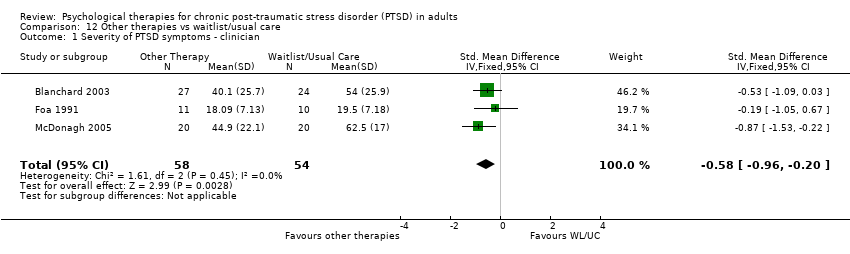
Comparison 12 Other therapies vs waitlist/usual care, Outcome 1 Severity of PTSD symptoms ‐ clinician.

Comparison 12 Other therapies vs waitlist/usual care, Outcome 2 Leaving the study early due to any reason.

Comparison 12 Other therapies vs waitlist/usual care, Outcome 3 Severity of PTSD symptoms ‐ self report.

Comparison 12 Other therapies vs waitlist/usual care, Outcome 4 Depression.

Comparison 12 Other therapies vs waitlist/usual care, Outcome 5 Anxiety ‐ Self report.
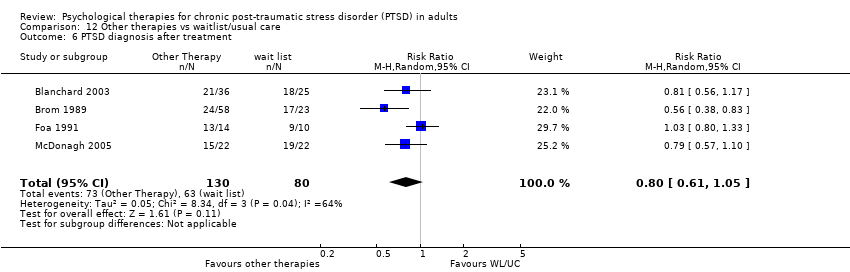
Comparison 12 Other therapies vs waitlist/usual care, Outcome 6 PTSD diagnosis after treatment.

Comparison 13 Group non‐TFCBT vs waitlist/usual care, Outcome 1 Leaving the study early for any reason.
Comparison 13 Group non‐TFCBT vs waitlist/usual care, Outcome 2 Severity of PTSD symptoms ‐ self report.
Comparison 13 Group non‐TFCBT vs waitlist/usual care, Outcome 3 Severity of PTSD symptoms ‐ self report 1 ‐ 4 months.
Comparison 13 Group non‐TFCBT vs waitlist/usual care, Outcome 4 Depression.
Comparison 13 Group non‐TFCBT vs waitlist/usual care, Outcome 5 Depression ‐ 1 ‐ 4 months.
| Trauma‐focused CBT/Exposure therapy versus Waitlist/Usual Care | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Trauma Focused CBT/ Exposure Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician‐rated in the intervention groups was | 1.62 standard deviations lower | 1256 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.64 | 1756 | ⊕⊝⊝⊝ | ||
| 130 per 1000 | 189 per 1000 | |||||
| Moderate | ||||||
| 85 per 1000 | 124 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Trauma‐focused CBT/Exposure therapy compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | Trauma Focused CBT/ Exposure Therapy | |||||
| Severity of PTSD Symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician‐rated in the intervention groups was | 267 | ⊕⊝⊝⊝ | |||
| Leaving the study early for any reason | Study population | RR 1.19 | 312 | ⊕⊝⊝⊝ | ||
| 154 per 1000 | 183 per 1000 | |||||
| Moderate | ||||||
| 154 per 1000 | 183 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Trauma‐focused CBT/Exposure therapy compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Therapies | Trauma Focused CBT/Exposure Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | ‐0.48 standard deviations lower | 612 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.39 | 762 | ⊕⊝⊝⊝ | ||
| 142 per 1000 | 198 per 1000 | |||||
| Moderate | ||||||
| 138 per 1000 | 192 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| EMDR compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 1.17 standard deviations lower | 183 | ⊕⊝⊝⊝ | ||
| Leaving study early for any reason | Study population | RR 1.05 | 227 | ⊕⊝⊝⊝ | ||
| 178 per 1000 | 188 per 1000 | |||||
| Moderate | ||||||
| 172 per 1000 | 182 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| EMDR compared with Trauma‐focused CBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Trauma Focused CBT | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 0.03 standard deviations lower | 327 | ⊕⊝⊝⊝ | ||
| Leaving study early for any reason | Study population | RR 1.00 | 400 | ⊕⊝⊝⊝ | ||
| 279 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 257 per 1000 | 247 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged as posing a high risk of bias | ||||||
| EMDR compared with non‐TFCBT for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | EMDR | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 0.35 standard deviations lower | 53 | ⊕⊝⊝⊝ | ||
| Leaving the study early for any reason | Study population | RR 1.03 | 84 | ⊕⊝⊝⊝ | ||
| 140 per 1000 | 144 per 1000 | |||||
| Moderate | ||||||
| 91 per 1000 | 94 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Studies reported insufficient information to judge risk of bias | ||||||
| EMDR compared with other therapies for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other Therapies | EMDR | |||||
| Leaving study early for any reason | Study population | RR 1.48 | 127 | ⊕⊝⊝⊝ | ||
| 32 per 1000 | 47 per 1000 | |||||
| Moderate | ||||||
| 32 per 1000 | 48 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One study was judged to pose a high risk of bias. The other study reported insufficient information for a judgement to be made | ||||||
| Non‐TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Non‐TFCBT Therapy | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 106 | ⊕⊝⊝⊝ | |||
| Leaving the study early due to any reason | Study population | RR 1.96 | 141 | ⊕⊝⊝⊝ | ||
| 78 per 1000 | 153 per 1000 | |||||
| Moderate | ||||||
| 83 per 1000 | 163 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient data to judge risk of bias | ||||||
| Non‐TFCBT compared with other therapies for chronic post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐TFCBT | ||||||
| Severity of PTSD symptoms ‐ Clincian‐rated | See comment | See comment | Not estimable | 25 | ⊕⊝⊝⊝ | |
| Leaving the study early for any reason | See comment | See comment | Not estimable | 31 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Some studies were judged to pose a high risk of bias | ||||||
| Group TFCBT compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Group TFCBT | ||||||
| Severity of PTSD symptoms ‐ Clinician‐rated | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 185 | ⊕⊝⊝⊝ | |||
| Leaving the study early for any reason | Study population | RR 1.21 | 573 | ⊕⊝⊝⊝ | ||
| 262 per 1000 | 317 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 242 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient information to judge risk of bias | ||||||
| Group CBT (trauma focused) compared with Group CBT (non‐trauma focused) for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Group CBT (non‐trauma focused) | Group CBT (trauma focused) | |||||
| Severity of PTSD symptoms ‐ Clinician‐rated | See comment | See comment | Not estimable | 325 | ⊕⊝⊝⊝ | |
| Leaving the study early for any reason | See comment | See comment | Not estimable | 360 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Insufficient information to judge risk of bias | ||||||
| Other therapies compared with Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Other therapies | |||||
| Severity of PTSD symptoms ‐ Clinician | The mean severity of PTSD symptoms ‐ clinician in the intervention groups was | 112 | ⊕⊝⊝⊝ | |||
| Leaving the study early due to any reason | Study population | RR 2.45 | 211 | ⊕⊝⊝⊝ | ||
| 74 per 1000 | 181 per 1000 | |||||
| Moderate | ||||||
| 72 per 1000 | 176 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Small sample sizes | ||||||
| Group non‐TFCBT compared to Waitlist/Usual Care for chronic post‐traumatic stress disorder (PTSD) in adults | ||||||
| Patient or population: Adults with PTSD for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Waitlist/Usual Care | Group nonTFCBT | |||||
| Leaving the study early for any reason | See comment | See comment | Not estimable | 47 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Only one study. Risk of bias high/unclear in several domains. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ clinician Show forest plot | 32 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Clinician PTSD | 28 | 1256 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.03, ‐1.21] |
| 1.2 Clinician PTSD severity women‐only subgroup analysis) | 9 | 562 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.31, ‐1.36] |
| 1.3 Clinician PTSD excluding studies of Vietnam War veterans | 27 | 1232 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐2.09, ‐1.25] |
| 1.4 Self report | 16 | 666 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐2.01, ‐1.14] |
| 2 Severity of PTSD symptoms at 1 ‐ 4 month follow‐up Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Clinician | 4 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐2.20, ‐0.17] |
| 3 Severity of PTSD symptoms at 5 ‐ 8 month follow‐up. Show forest plot | 3 | 192 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.77, ‐0.18] |
| 4 Severity of PTSD symptoms 9 ‐ 12 month follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Leaving the study early for any reason Show forest plot | 33 | 1756 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.30, 2.06] |
| 6 Severity of PTSD symptoms ‐ self report: 1 ‐ 4 month follow‐up Show forest plot | 2 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.03 [‐6.51, 0.45] |
| 7 Severity of PTSD symptoms ‐ self report: 5 ‐ 8 months) Show forest plot | 2 | 208 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐0.90, ‐0.32] |
| 8 Severity of PTSD symptoms ‐ self report: 9 ‐ 12 month follow up Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Depression Show forest plot | 28 | 1213 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.62, ‐0.94] |
| 10 Depression 1 ‐ 4 month follow‐up Show forest plot | 7 | 413 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.33, ‐0.18] |
| 11 Depression 5 ‐ 8 month follow‐up Show forest plot | 2 | 150 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.82, ‐0.17] |
| 12 Depression 9 ‐ 12 month follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Anxiety Show forest plot | 17 | 644 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.03, ‐0.59] |
| 14 Anxiety 1 ‐ 4 month follow‐up Show forest plot | 3 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.60, ‐0.03] |
| 15 Anxiety 9 ‐ 12 month follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16 Exploration of publication bias Show forest plot | 28 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Clinician | 28 | 1256 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.03, ‐1.21] |
| 17 Sensitivity analysis ‐ clinician‐rated PTSD symptoms ‐ studies at low risk of bias only Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18 PTSD diagnosis after treatment Show forest plot | 19 | 910 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.41, 0.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD Symptoms ‐ clinician Show forest plot | 7 | 267 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.63, 0.10] |
| 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 127 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.00, ‐0.01] |
| 3 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (5 ‐ 8 months) Show forest plot | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.17, 0.17] |
| 4 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (9 ‐ 12 months) Show forest plot | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐12.93 [‐18.72, ‐7.14] |
| 5 Leaving the study early for any reason Show forest plot | 7 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.71, 2.00] |
| 6 Severity of PTSD symptoms ‐ self report Show forest plot | 3 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.74, 0.01] |
| 7 Severity of PTSD symptoms ‐ self report ‐ follow‐up (1‐4 months) Show forest plot | 2 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.99, 0.10] |
| 8 Depression Show forest plot | 6 | 189 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.56, 0.03] |
| 9 Depression ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.62, 0.06] |
| 10 Depression ‐ follow‐up (5 ‐ 8 months) Show forest plot | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.30, ‐0.12] |
| 11 Depression ‐ follow‐up (9 ‐ 12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Anxiety Show forest plot | 4 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.49, 0.26] |
| 13 Anxiety ‐ follow‐up (1 ‐ 4 months) Show forest plot | 4 | 117 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.79, 0.30] |
| 14 Anxiety ‐ follow‐up (5 ‐ 8 months) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15 Anxiety ‐ follow‐up (9 ‐ 12 months) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16 PTSD diagnosis after treatment Show forest plot | 6 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ clinician Show forest plot | 10 | 625 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.83, ‐0.14] |
| 2 Severity of PTSD symptoms ‐ clinician ‐ 1 ‐ 4 month follow‐up Show forest plot | 8 | 548 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.64, ‐0.04] |
| 3 Severity of PTSD symptoms ‐ clinician ‐ 5 ‐ 8 month follow‐up Show forest plot | 4 | 434 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.20, 0.04] |
| 4 Severity of PTSD symptoms ‐ clinician ‐ 9 ‐ 12 month follow‐up Show forest plot | 2 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.35, ‐0.17] |
| 5 Leaving the study early for any reason Show forest plot | 11 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.01, 1.92] |
| 6 Severity of PTSD symptoms ‐ self report Show forest plot | 6 | 574 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.15, ‐0.06] |
| 7 Severity of PTSD symptoms ‐ self report ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 526 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.47, ‐0.12] |
| 8 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (5 ‐ 8 months) Show forest plot | 3 | 338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.05, 0.25] |
| 9 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (9 ‐ 12 months) Show forest plot | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐2.66 [‐5.71, 0.38] |
| 10 Depression ‐ self report Show forest plot | 9 | 570 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.63, ‐0.11] |
| 11 Depression ‐ self‐report ‐ follow‐up (1‐4 months) Show forest plot | 7 | 510 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.62, 0.03] |
| 12 Depression ‐ follow‐up (5‐8 months) Show forest plot | 5 | 443 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.87, 0.01] |
| 13 Depression 9‐12 month follow up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Anxiety ‐ self report Show forest plot | 7 | 539 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.77, ‐0.12] |
| 15 Anxiety ‐ self‐report ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 454 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.68, 0.02] |
| 16 Anxiety ‐ follow‐up (5 ‐ 8 months) Show forest plot | 2 | 329 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.69, 0.58] |
| 17 PTSD diagnosis after treatment Show forest plot | 7 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.96] |
| 18 Severity of PTSD symptoms ‐ clinician ‐ 13‐24 month follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19 Sensitivity analysis ‐ PTSD symptoms ‐ studies at low risk of bias only Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20 Sensitivity analysis ‐ drop‐out ‐ studies at low risk of bias only Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21 Subgroup analysis: severity of PTSD symptoms ‐ women‐only studies Show forest plot | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.42, 0.47] |
| 22 Subgroup analysis: severity of PTSD symptoms ‐ clinician ‐ excluding Vietnam veterans Show forest plot | 9 | 599 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.80, ‐0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ Clinician Show forest plot | 6 | 183 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.04, ‐0.30] |
| 2 Leaving study early due to any reason Show forest plot | 7 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.62, 1.79] |
| 3 Severity of PTSD symptoms ‐ self report Show forest plot | 6 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.68, 0.07] |
| 4 Depression Show forest plot | 7 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.52, ‐0.78] |
| 5 Anxiety Show forest plot | 6 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.36, ‐0.69] |
| 6 PTSD diagnosis after treatment Show forest plot | 6 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.42, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ clinician Show forest plot | 7 | 327 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.43, 0.38] |
| 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1 ‐ 4 months) Show forest plot | 3 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.97, 0.58] |
| 3 Leaving study early for any reason Show forest plot | 8 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.74, 1.35] |
| 4 Severity of PTSD symptoms ‐ self report Show forest plot | 7 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.60, 0.01] |
| 5 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.61, 0.52] |
| 6 Depression Show forest plot | 8 | 346 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.75, 0.13] |
| 7 Depression ‐ follow‐up (1 ‐ 4 months) Show forest plot | 5 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.64, 0.38] |
| 8 Anxiety Show forest plot | 4 | 236 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.53, ‐0.02] |
| 9 Anxiety ‐ follow‐up (1 ‐ 4 months) Show forest plot | 2 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.33, 0.81] |
| 10 PTSD diagnosis after treatment Show forest plot | 8 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.74, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ clinician Show forest plot | 2 | 53 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.90, 0.19] |
| 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (1‐4 months) Show forest plot | 3 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.64, 0.15] |
| 3 Leaving the study early for any reason Show forest plot | 3 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.37, 2.88] |
| 4 Severity of PTSD symptoms ‐ self report Show forest plot | 3 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.86, 0.06] |
| 5 Severity of PTSD symptoms ‐ self‐report ‐ follow‐up (1 ‐ 4 months) Show forest plot | 3 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.98, ‐0.05] |
| 6 Depression Show forest plot | 3 | 75 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.14, ‐0.20] |
| 7 Depression ‐ follow‐up (1 ‐ 4 months) Show forest plot | 3 | 75 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.86, 0.36] |
| 8 Anxiety Show forest plot | 2 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.36, ‐0.13] |
| 9 Anxiety ‐ follow‐up (1 ‐ 4 months) Show forest plot | 2 | 45 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐2.21, 1.37] |
| 10 PTSD diagnosis after treatment Show forest plot | 3 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.41, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving study early for any reason Show forest plot | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.26, 8.54] |
| 2 Severity of PTSD symptoms ‐ self report Show forest plot | 2 | 124 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.21, ‐0.47] |
| 3 Depression Show forest plot | 2 | 127 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.03, ‐0.32] |
| 4 Anxiety Show forest plot | 2 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.08, ‐0.36] |
| 5 PTSD diagnosis after treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ Clinician Show forest plot | 4 | 106 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐1.76, ‐0.69] |
| 2 Leaving the study early for any reason Show forest plot | 5 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.70, 5.48] |
| 3 Severity of PTSD symptoms ‐ Self‐report Show forest plot | 2 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐3.27, 1.55] |
| 4 Depression Show forest plot | 5 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.43, ‐0.42] |
| 5 Anxiety Show forest plot | 4 | 102 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐1.24, ‐0.42] |
| 6 PTSD diagnosis after treatment Show forest plot | 4 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.50, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ Clincian Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Severity of PTSD symptoms ‐ clinician ‐ follow‐up (3 months) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Leaving the study early for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Depression ‐ self report Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Depression ‐ self report ‐ follow‐up (3 months) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Anxiety ‐ self report Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Anxiety ‐ self report ‐ follow‐up (3 months) Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 PTSD diagnosis after treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinician‐rated Show forest plot | 3 | 185 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐2.25, ‐0.31] |
| 2 Severity of PTSD 5 ‐ 8 month follow‐up Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Leaving the study early for any reason Show forest plot | 7 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.55] |
| 4 Severity of PTSD symptoms ‐ self report Show forest plot | 6 | 274 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.70, ‐0.69] |
| 5 Severity of PTSD ‐ self report ‐ 1 ‐ 4 months Show forest plot | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.78, ‐0.50] |
| 6 Depression Show forest plot | 3 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.98, ‐0.32] |
| 7 Depression 1 ‐ 4 month follow‐up Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Anxiety Show forest plot | 3 | 106 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.06, ‐0.27] |
| 9 Anxiety 1 ‐ 4 month follow‐up Show forest plot | 2 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.72, ‐0.14] |
| 10 PTSD diagnosis after treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Leaving the study early for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 PTSD diagnosis after treatment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Severity of PTSD symptoms ‐ clinician Show forest plot | 3 | 112 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.96, ‐0.20] |
| 2 Leaving the study early due to any reason Show forest plot | 4 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.99, 6.10] |
| 3 Severity of PTSD symptoms ‐ self report Show forest plot | 2 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐0.98, ‐0.24] |
| 4 Depression Show forest plot | 3 | 112 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.83, ‐0.07] |
| 5 Anxiety ‐ Self report Show forest plot | 4 | 193 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.82, ‐0.22] |
| 6 PTSD diagnosis after treatment Show forest plot | 4 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.61, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Severity of PTSD symptoms ‐ self report Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Severity of PTSD symptoms ‐ self report 1 ‐ 4 months Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Depression Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Depression ‐ 1 ‐ 4 months Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

