Вмешательства при передозировке парацетамола (ацетаминофена)
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | "Parallel randomised clinical trial." | |
| Participants | Inclusion criteria: > 18 years, paracetamol ingestion > 7.5 g over the preceding 24 hr. Exclusion criteria: presentation later than 24 hr after ingestion, coingestion of cholinergic drugs, decreased level of consciousness at presentation, primary hepatic encephalopathy, status epilepticus after acetylcysteine administration, history of asthma and anaphylactoid reactions. Oral group vs IV group: Number of participants randomised: 33 vs 33. Age (mean (SD)) (years): 27.76 (9.52) vs 24.61 (5.95). Interval between ingestion and treatment (mean (SD) (hr): 11.88 (7.04) vs 12.21 (7.02). Paracetamol plasma level on admission (mean (SD)) (μg/mL): 78.09 (64.12) vs 72.06 (61.26). Amount of paracetamol ingested (mean (SD)) (mg/kg): 160.78 (28.61) vs 170.81 (17.73). Additional characteristics: no difference between serum AST, ALT, bilirubin or creatinine between the 2 groups. Not reported in either group: number of participants taking additional drugs or consuming additional alcohol, number of participants excluded before randomisation. | |
| Interventions | IV group: 20‐hr protocol: first dose 150 mg/kg over 15 min, second dose 50 mg/kg over 4 hr and third dose 100 mg/kg over 16 hr. Oral group: 72‐hr protocol: first dose 140 mg/kg followed by 17 maintenance doses of 70 mg/kg every 4 hr. | |
| Outcomes | Outcomes: liver enzymes (AST, ALT, bilirubin, PT; measured daily). Adverse effects: nausea, vomiting, flushing, rash, pruritus, dyspnoea, tachycardia, cough, wheeze, hypotension (systolic BP < 100 mmHg within 2 hr of administration), and bronchospasm. | |
| Notes | No statistically significant difference in AST, ALT, bilirubin, and PT in oral and IV group at 24, 48, and 72 hr. Nausea and hypotension were significantly more prevalent in oral compared to IV treatment group. Nausea: 19 (57.6%) in oral group vs 11 (33.3%) in IV group. Translated from Persian (Farsi). Author contacted but no reply to verify issues with translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: translation: "sampling was conducted using randomised blocks of four." Comment: no mention of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not recorded. Comment: no mention of whether there was knowledge of the fixed block randomisation, which might have revealed what the next allocation had to be for the last 1 or 2 people in each block. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not feasible given nature of intervention by 2 different routes. |
| Blinding of outcome assessment (detection bias) | High risk | High risk of bias for reporting adverse effects such as nausea or vomiting. Low risk of bias for reporting of LFTs. |
| Incomplete outcome data (attrition bias) | Unclear risk | Mean values shown for several tests for 72 hr, unclear if all participants were still in trial or if some had been discharged. |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered. Unclear what the primary and secondary outcomes of the study were, although there were no significant differences except in adverse effects which related to route of administration. |
| Other bias | Unclear risk | Power: translation quote: "using the sample size formula for comparing difference in means the sample size was set at 30 in each group and 10% added to each group." Unclear what test(s) and time point(s) this referred to and what data were used for determining variance and what difference was considered significant. Not recorded were number assessed for randomisation or number excluded. Participants were 'excluded' from IV arm if anaphylactoid reactions unresponsive to decreasing the administration rate and given oral acetylcysteine (unclear if these were still included based on intention to treat analysis principles when examining outcomes). |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | High risk | Judged as high risk. |
| Methods | Double‐blind randomised clinical trial. | |
| Participants | 3 hospitals: Royal Infirmary (Edinburgh), Royal Victoria Infirmary (Newcastle), Aberdeen Royal Infirmary. Inclusion criteria: acute paracetamol overdose and needed treatment with acetylcysteine on the basis of standard UK guidance for management. Exclusion criteria: people aged < 16 years; detained under Mental Health Act; known permanent cognitive impairment; life‐threatening illness; pregnant women; previous participation in study; considered to have unreliable history of paracetamol overdose; presenting > 36 hr after overdose (24 hr up to May 2011) of a single acute paracetamol overdose; presenting after taking staggered paracetamol overdose (defined as when overdose of paracetamol was taken over a period > 2 hr (1 hr up May 2011); anticoagulants (e.g. warfarin) in therapeutic doses or in overdose; people who, in the opinion of the responsible clinician/ nurse, were unlikely to complete the full course of acetylcysteine e.g. expressing wish to self‐discharge: people who, in the opinion of the responsible clinician/nurse, were unable to complete the initial questionnaire themselves or with nurse assistance; history of hypersensitivity to 5HT3 antagonists; non‐English speaking people. Number assessed for randomisation: 1539 suitable for acetylcysteine treatment. Number excluded before randomisation: 1170. Total number randomised: 369. Ondansetron‐modified: Number randomised: 55 (54 analysed). Age (median) (years): 29. Weight (median) (kg): 70. Number (%) of participants with interval between ingestion and treatment, < 8 hr: 32 (58%). Number of participants with paracetamol plasma level on admission (mean): not reported instead % of participants in a set range. Number (%) of participants with ingested paracetamol ≥ 16 g: 28 (51%). Number (%) of participants taking additional drugs: 25 (45%). Number (%) of participants consuming additional alcohol: 28 (51%). Number of participants excluded after randomisation: 1 withdrawn pretreatment. Ondansetron‐standard: Number randomised: 56 (55 analysed). Age (median) (years): 32. Weight (median) (kg): 68. Number (%) of participants with interval between ingestion and treatment, < 8 hr: 33 (59%). Number (%) of participants with ingested paracetamol ≥ 16 g: 29 (52%). Number (%) of participants taking additional drugs: 32 (57%). Number (%) of participants consuming additional alcohol: 30 (54%). Number of participants excluded after randomisation: 1. Placebo‐modified: Number of participants randomised: 55 (54 analysed). Age (median) (years): 36. Weight (median) (kg): 70. Number (%) of participants with interval between ingestion and treatment, < 8 hr: 32 (58%). Number (%) of participants with ingested paracetamol ≥ 16 g: 30 (55%). Number (%) of participants taking additional drugs: 31 (56%). Number (%) of participants consuming additional alcohol: 24 (44%). Number of participants excluded after randomisation: 1. Placebo‐standard: Number of participants randomised: 56 (54 analysed). Age (median) (years): 33. Weight (kg): 70. Number (%) of participants with interval between ingestion and treatment, < 8 hr: 31 (55%). Number (%) of participants with ingested paracetamol ≥ 16 g: 29 (52%). Number (%) of participants taking additional drugs: 39 (70%). Number (%) of participants consuming additional alcohol: 29 (52%). Number of participants excluded after randomisation: 2. | |
| Interventions | Ondansetron‐modified group: ondansetron 4 mg IV pretreatment and the modified (shorter) acetylcysteine regimen. Ondansetron‐standard group: ondansetron 4 mg IV pretreatment and the standard acetylcysteine regimen. Placebo‐modified group: placebo IV pretreatment and modified (shorter) acetylcysteine regimen. Placebo‐standard group: placebo IV pretreatment and standard acetylcysteine regimen. Acetylcysteine regimens used: UK standard schedule (20.25 hr): 150 mg/kg in 200 mL over 15 min, then 50 mg/kg in 500 mL over 4 hr, then 100 mg/kg in 1000 mL over 16 hr. Modified (shorter) protocol (12 hr): 100 mg/kg in 200 mL over 2 hr, then 200 mg/kg in 1 L over 10 hr, then 0.5 L of 5% dextrose to 20.25 hr. | |
| Outcomes | Primary outcome: absence of vomiting, retching, or need for rescue antiemetic at 2 hr. Secondary outcomes: up to 12 hr: proportion of participants without nausea (Likert scale), vomiting or retching up to 12 hr and anaphylactoid reactions > 50% increase in ALT over admission. | |
| Notes | Vomiting, or retching, or rescue antiemetics were significantly lower in participants receiving modified regimen compared to standard regimen and in participants treated with ondansetron versus placebo. Secondary outcome of nausea, vomiting, or retching up to 12 hr was less common in the shorter modified regimen and participants pretreated with ondansetron. Fewer people in the modified regimen had severe reactions requiring interruption to treatment. Participants pretreated with ondansetron had increased frequency of 50% increase in ALT. 2 protocol adjustments: extended time for paracetamol ingested from 1 hr to 2 hr to assist recruitment and second change in new UK guidance in September 2010 changed to 100 mg/L paracetamol nomogram line for recruitment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "2x2 factorial trial design." "Randomisation by minimisation to achieve balance (1:1:1:1 allocation), according to the following prognostic factors: reported paracetamol dose (<16g or ≥16g); risk factors for paracetamol – induced hepatic toxic effects, and time to presentation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Online program for randomisation." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Ondansetron and saline placebo ampoules identical in appearance." "Acetylcysteine not masked due to ethical and practical concerns." |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding of acetylcysteine regimens hence, high risk of bias for the standard vs modified regimens for detection of adverse reactions such as anaphylactoid reaction and nausea and vomiting. But low risk for mortality or liver injury. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "if any patient had missing data for an outcome variable, we removed them from formal statistical analysis at that time point." Only 5/222 participants unable to provide primary outcome data. |
| Selective reporting (reporting bias) | Low risk | Outcome measures published in trial protocol, subsequently reported in final paper. |
| Other bias | Low risk | Intention to treat: "analysis was done according to randomised treatment group, irrespective of adherence to treatment." Power: "To achieve at least 80% power to detect a relative risk of 0.6 for the proportion of patients with retching or vomiting within 2 hours (from 60% in the treated group to 36% in the placebo group), 91 patients needed to be enrolled in each group"… "to allow for a potential higher drop‐outs/noncompliance rate … planned to include 250 patients, 125 randomised to ondansetron and 125 to placebo. This was to ensure 50 patients in each of the four groups." Note: NOT powered for efficacy or non‐inferiority: modified vs conventional regimen IV acetylcysteine. Note: 2 protocol amendments: "Extended the time allowed for ingestion of paracetamol from 1h to 2h to assist recruitment…most patients found to ingest large single overdoses over a period of 2h." "Second, after new UK guidance was issued in September 2012, we used the 100mg/l paracetamol nomogram line for recruitment in all patients." |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Low risk | Judged as low risk. |
| Methods | Randomised clinical trial. | |
| Participants | Inclusion criteria: all participants admitted within 17 hr of paracetamol ingestion, who were hepatitis B surface antigen negative, had no history of pre‐existing liver disease and paracetamol level > 200 mg/L (4 hr) line. Cysteamine group vs non‐cysteamine (control) group: Number of participants randomised: 18 vs 20. Paracetamol "index": concentration by which the participant exceeded, the theoretical "safe" upper limit, indicated by the line at the time when plasma‐paracetamol was measured. Paracetamol "index" (mean) (mg/L): 72 vs 98 (difference between the 2 groups significant). Further analysis of participants under 30 years of age: Early cysteamine treatment (< 9 hr postingestion) (mean) (mg/L): 43 vs 138 (P < 0.01). Late cysteamine treatment (> 9 hr postingestion) (mean) (mg/L): 75 vs 67. Amount of ingested paracetamol (mean) (g): 28 vs 32. Following data not reported in either group: age, male:female ratio, number of participants taking additional drugs or consuming additional alcohol, number of participants excluded after randomisation. | |
| Interventions | Cysteamine group: cysteamine given as described by Prescott 1973 except that it was dissolved in 5% dextrose and injected, or added to 5% dextrose infusion, using a Millipore filter attached to a syringe. Control group: supportive treatment: 5% dextrose, 2 L to 3 L daily, with added vitamins and potassium if necessary. | |
| Outcomes | Mortality, maximum AST, maximum serum bilirubin, maximum PT, liver biopsy findings, maximum serum‐ferritin, renal function, serum amylase, and adverse events. | |
| Notes | 1 death in each treatment group. No difference between the 2 groups in maximum bilirubin and minimum PT. Difference for PT in subgroup of participants aged < 30 years, treated in < 9 hr, was statistically significance. Cysteamine group: late and early presenters had significantly lower serum AST compared to the control group. Liver biopsy: more grade III changes in the control group; however, participants with high plasma paracetamol concentrations regardless of treatment were more likely to have grade III changes. Douglas and colleagues did not provide the time interval between ingestion of paracetamol and treatment in either groups. 4 of the participants were also included in Hamlyn 1981 study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | First stratified into 1 of 4 groups and then "Patients were randomly allocated to receive cysteamine or not using a table of random numbers. Using a table of random numbers, it was possible that one or other group could become weighted with cases receiving only one of the treatment regimens. Adjustment was made therefore, so that every six patients in each group included three who had received cysteamine." Comment: process outlined suggested there may have been rejection of certain patterns of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | As above. Comment: unclear how the adjustment was done and whether this would have maintained allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding described. |
| Blinding of outcome assessment (detection bias) | Low risk | Low risk as outcomes measured mortality and LFT values. |
| Incomplete outcome data (attrition bias) | Low risk | Data seemed complete with individual data points presented for most participants. |
| Selective reporting (reporting bias) | Low risk | 2 groups different at baseline but noted by authors. Quote: "However, the difference for prothrombin in the young early group just reaches statistical significance (P=0.05)." Comment: there was some focus on subgroup analysis that did not seem justified given how small the study was and that it was examining effects not significant across all participants. |
| Other bias | Low risk | Intention to treat/power/premature stopping: Power: not recorded but authors noted. Quote: "our trial is on too small a scale to permit any conclusions about the effect of cysteamine on mortality rates." Comment: multiple statistical tests were done on various subgroups, without any statistical adjustment for multiple comparisons. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | High risk | Judged as high risk. |
| Methods | Randomised clinical trial. April 2009 to September 2010. | |
| Participants | Inclusion criteria: people with paracetamol poisoning aged ≥ 18 years, with time from ingestion to admission < 8 hr. Exclusion criteria: people who vomited twice after oral acetylcysteine was given (these people were excluded and were managed with IV acetylcysteine only), pregnant women, and risk factors for hepatic toxicity (e.g. hepatic cirrhosis, chronic ethanol ingestion, usage of substances that induce cytochrome P450). IV group vs oral + IV group: Number of participants randomised: 25 vs 25 (10 excluded presumably as vomited more than twice as per exclusion criteria). Age (mean) (years): 23.78 vs 24.46. Amount of ingested paracetamol (mean) (mg): 12,337.5 vs 11,290. Percentage of participants taking additional drugs: 42.3% vs 60%. Number of participants excluded after randomisation: 0 vs 10. Additional information: Percentage of participants vomited pre hospital: 60.7% vs 26.7%. Percentage of participants with no signs or symptoms before acetylcysteine: 10.7% vs 40%. Not recorded in either group: paracetamol plasma level on admission, interval between ingestion and treatment, number of participants consuming additional alcohol. | |
| Interventions | Ingestion < 4 hr received gastric evacuation and charcoal 1 g/kg in 200 mL water. IV group: IV acetylcysteine with 150 mg/kg infused in 200 mL of 5% dextrose over 30 min, followed by a 4‐hr infusion of 50 mg/kg of acetylcysteine in 500 mL of 5% dextrose and 16 hr of 100 mg/kg in 1 L of 5% dextrose. Oral + IV group: acetylcysteine 140 mg/kg in 200 mL of 5% dextrose orally then IV acetylcysteine 50 mg/kg in 500 mL of 5% dextrose every 4 hr then 100 mg/kg in 1 L of 5% dextrose in 16 hr. If vomiting occurred in any participant within 1 hr after the ingestion of the oral acetylcysteine, then metoclopramide 10 mg IM and oral acetylcysteine given at the same dose again. Oral acetylcysteine in the form of a 600 mg tablet. | |
| Outcomes | Anaphylactoid reaction defined as nausea and vomiting, dyspnoea, flushing, > 1 symptoms: | |
| Notes | IV group vs oral + IV group: Anaphylactoid reaction defined as nausea and vomiting, dyspnoea, flushing, > 1 symptoms: No signs or symptoms: 86.7% vs 39.3%. At least 1 sign of anaphylactoid reaction: 60.7% vs 13.3%. Most common symptom was nausea and vomiting: 28.5% vs 13.3%. Flushing or dyspnoea: 3.6% vs 0%. Nausea and vomiting was noted as a symptom of acetylcysteine administration but participants were excluded from oral + IV group if they vomited twice after oral acetylcysteine (10 participants excluded) and should have been analysed. Author contacted to get further details, particularly given the outcome data could not be extracted from the report. However, responses did not clarify any of the above issues including what the absolute numbers were with adverse events for randomised participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated in two groups randomly." Comment: randomisation sequence generation not recorded. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocated in two groups randomly." Comment: process of randomisation not recorded. |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded, primary outcome adverse reactions so high potential for bias. |
| Incomplete outcome data (attrition bias) | High risk | 40% of enrolled participants in one group excluded; unclear whether data from these participants were presented. Percentages of most outcomes reported (not numbers); however, these were not the multiples of 4 expected if data on all 25 participants randomised were included in the denominator. Therefore, there appears to be a missing data for most outcomes but it was not apparent how much were missing and how these were reported. |
| Selective reporting (reporting bias) | High risk | Quote: "patients who vomit two times after oral acetylcysteine was given (these patients were excluded and were managed with IV NAC only)." 10/25 participants oral + IV group postrandomisation. Outcomes for these participants not reported. |
| Other bias | High risk | Intention to treat: "group B [oral + IV group] (25 patients). 10 patients of group B were excluded from our study." Results given for 25 (IV group) and 15 (oral + IV group). Absolute numbers not given in results and unclear if percentages in oral + IV group results were out of 25 or 15. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | High risk | Judged as high risk. |
| Methods | Randomised clinical trial. | |
| Participants | Inclusion criteria: all participants seen in the Liver Unit with a plasma paracetamol level > 200 µg/L at any time in the first 12 hr after overdosage. Exclusion criteria: not reported. Haemoperfusion group vs supportive group: Number of participants: 8 vs 8. Age (mean) (years): 31 vs 35. Time elapsed between ingestion and presentation at the department (mean) (min): 300 vs 180. Paracetamol plasma level on admission (mean (SE)) (mg/L): 305 (46). Amount of ingested paracetamol (g): 56 vs 34. Additional characteristics: Initial plasma half‐life (hr): 7 vs 5. Initial AST level (U/L): 827 vs 142. Initial PT (seconds): 12 vs 2.2. Initial plasma bilirubin (mg/100 mL): 1.85 vs 1.27. Not reported in either group: male:female ratio, number of participants taking additional drugs or consuming additional alcohol. | |
| Interventions | All participants were treated by gastric lavage when first seen and fresh frozen plasma and fluid were administrated as clinically indicated. Haemoperfusion group: 2 catheters (14 French gauge 50 cm length) positioned in saphenous vein (under local anaesthesia and x‐ray guided) and attached to a perfusion column. Charcoal used was covered with a thin coating of polyhydroxyethyl‐methacrylate. Participants were heparinised before with an IV loading dose of 2000 units 10 min before the procedure, and thereafter a constant infusion pump delivered 1500 heparin units/ hr to 2000 heparin units/hr. Haemoperfusion was continued until participant's paracetamol level was < 30 µg/mL. Supportive group: gastric lavage when first seen, and fresh frozen plasma and fluid administrated as clinically indicated. | |
| Outcomes | Mortality. Fall in paracetamol level vs time after ingestion. Number of participants experiencing any adverse events. | |
| Notes | 1 death in the haemoperfusion group. Plasma clearance of paracetamol by charcoal column was variable and small. No clinical problems. Liver damage in most participants was mild but the haemoperfusion group had more evidence of hepatic dysfunction with a higher mean bilirubin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: method for generating sequence not detailed. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocated by a system of sealed envelopes." "Although the two groups were randomly allocated those receiving supportive therapy alone had ingested fewer tablets, were first seen earlier following the overdose, had a lower mean level of plasma paracetamol and a shorter initial drug half‐life." Comment: not specified if sequentially numbered, opaque or any other process used to prevent subversion. There was a marked imbalance in severity. |
| Blinding of participants and personnel (performance bias) | Low risk | Not feasible given the nature of the interventions. |
| Blinding of outcome assessment (detection bias) | Low risk | Not blinded, but low risk for primary outcomes of mortality and paracetamol concentration. |
| Incomplete outcome data (attrition bias) | Low risk | Does not appear to have any missing data (very small trial). |
| Selective reporting (reporting bias) | Low risk | No selective reporting apparent. |
| Other bias | Unclear risk | No power calculation provided. Very small trial which as written focused largely on kinetic outcomes. Intention to treat not detailed. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Judged as high risk. |
| Methods | Prospective, randomised, controlled trial. | |
| Participants | 2 hospitals: Newcastle (Royal Victoria Infirmary) and London (Guy's Hospital). Continuation of previously reported trial (Douglas 1976a). 40 participants: 9 (London), 31 (Newcastle) (4 from Newcastle in previous trial Douglas 1976a). Inclusion criteria: paracetamol level above semi‐logarithmic '200' line and treatment within 10 hr of ingestion. Exclusion criteria: children, alcohol dependency, known liver disease, and pregnant woman. No differences between the 3 treatment groups in terms of age, paracetamol ingested, delay to treatment, or paracetamol index (natural logarithm of the perpendicular distance from the blood paracetamol value to the 200‐line). Cysteamine and supportive group: 14 randomised. Age (mean (SD)) (years): 29.3 (14.9). Interval between ingestion and treatment (mean (SD)) (hr): 7.9 (1.9). Paracetamol index (mean (SD)): 0.444 (0.223). Amount of ingested paracetamol (mean (SD)) (g): 33.6 (16.0). Methionine and supportive group: 13 randomised. Age (mean (SD)) (years): 28.4 (14.2). Interval between ingestion and treatment (mean (SD)) (hr): 7.3 (1.6). Paracetamol index (mean (SD)): 0.525 (0.378). Amount of ingested paracetamol (mean (SD)) (g): 42.4 (25.2). Supportive treatment group: 13 participants. Age (mean (SD)) (years): 25.5 (10.8). Interval between ingestion and treatment (mean (SD)) (hr): 6.7 (2.2), this is the interval between ingestion and gastric lavage/treatment. Paracetamol index (mean (SD)): 0.671 (0.297). Amount of ingested paracetamol (mean (SD)) (g): 27.9 (13.0). Not reported in all groups: number of participants taking additional drugs, number of participants consuming additional alcohol, number of participants excluded after randomisation. | |
| Interventions | 3 treatment groups: all participants received gastric lavage and supportive treatment (10% dextrose, added vitamins with potassium). Cysteamine + supportive group (N): supportive therapy + cysteamine in Newcastle. Cysteamine + supportive group (L): supportive therapy + cysteamine in London. Methionine + supportive group (N): supportive therapy + methionine in Newcastle. Methionine + supportive group (L): supportive therapy + methionine in London. Supportive treatment group (N): supportive therapy only in Newcastle. They did not treat any participant with only supportive care in London. Cysteamine IV as an immediate loading dose through a Millipore filter, followed by slow IV infusion for 20 hr up to a total base‐equivalent dose of 3.6 g. Methionine orally, 2.5 g every 4 hr to a total dose of 10 g. Supportive treatment: IV 10% dextrose with vitamins. Metoclopramide 10 mg IM administrated for severe or persistent vomiting. | |
| Outcomes | Peak serum AST (LFTs for at least 4 days), maximum serum bilirubin, maximum PT. Renal function, amylase, electrocardiogram, lactate dehydrogenase and creatinine kinase measured daily. Liver biopsy in 20 participants. Number of participants experiencing any adverse events. | |
| Notes | 1 death in the supportive group. Significant difference in favour of active treatment for AST. Significantly lower numbers of participants with grade III (severe) necrosis with both cysteamine + supportive and methionine + supportive groups. Continuation of Douglas 1976a, now restricted to 10 hr postoverdose. Hamlyn and colleagues did not provide the SD of the mean in any of their results. 4 participants included were from the earlier trial reported by Douglas 1976a. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We employed, nevertheless, a balanced block randomisation to facilitate frequent trial monitoring. Patients were admitted to the trial by sealed envelope allocation, based upon random number tables, to one of three treatment groups in Newcastle, or one of two treatment groups in London. These comprised: supportive therapy only (S‐Newcastle only), supportive therapy + cysteamine (C) and supportive therapy + methionine (M). In order to avoid age bias, the randomisation also included adjustment for the numbers of under‐ and over‐30s in each group." Comment: 3 different methods for generating random allocation appear to be mentioned (simple randomisation, blocked randomisation, and minimisation). |
| Allocation concealment (selection bias) | High risk | Quote: "admitted to the trial by sealed envelope allocation." 1 centre randomised to only 2 groups of the study. Yet the numbers in each group were similar. Unclear how the method described above would be consistent with the randomisation process outlined above. Not mentioned if envelopes were sequentially numbered. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned and 2 different routes of administration (oral and IV) with a substance with a strong odour suggests this would be impossible. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded but outcomes measured included mortality and biochemical markers. Biochemical markers detecting difference may be biased by frequency of testing. Regarding outcome of liver biopsy unclear risk of bias, how it was determined which participants to biopsy. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned of participants had missing outcome data, but only 1 site performed liver biopsies. |
| Selective reporting (reporting bias) | Low risk | No selective reporting apparent. |
| Other bias | Unclear risk | Intention to treat/power/premature stopping: Power: quote: "in patients with severe hepatic necrosis the arithmetic mean peak AST is approximately 3000U/l +/‐ SD 1000U/l and it may be shown that, to show a reduction with treatment to the mild liver damage level of 35 U/l (Hamlyn et al 1978) significant at the two‐tailed 5% level and type II error 5%, 12 paired comparison are needed." "end point of this trial coincided with the commercial introduction of intravenous N‐acetylcysteine." Unclear if the trial terminated prematurely in response to the introduction of IV acetylcysteine. A sample size calculation based on paired comparisons seemed inappropriate for a parallel group study with 3 groups. Information on intention to treat not provided. Numbers excluded before randomisation not reported. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Judged as high risk. |
| Methods | Randomised clinical trial. | |
| Participants | Inclusion criteria: plasma paracetamol level that fell above a line on a semilog graph joining values of 1.3 mmol/L (200 mg/mL) 2 hr after ingestion and 0.5 mol/L (80 mg/mL) 12 hr after ingestion. Seen within 10 hr of paracetamol overdose. Exclusion criteria: not reported. Males: 18 (all participants). Females: 34 (all participants). Cysteamine group vs dimercaprol group: Number of participants randomised: 26 vs 26. Interval between ingestion and treatment (mean) (hr): 7.7 vs 7.9. Paracetamol plasma level on admission (mean) (mg/L): 295 vs 269. In both groups following data not reported: age, amount of ingested paracetamol, number of participants taking additional drugs, number of participants consuming additional alcohol, number of participants excluded after randomisation. Noted in earlier published data Gazzard 1975b, they noted no difference between the 2 groups in terms of initial paracetamol level. | |
| Interventions | Interventions: all received gastric lavage and supportive treatment. Cysteamine group: infusion of cysteamine hydrochloride freshly prepared for each participant, IV through a Millipore filter 0.22 mm in a dose of 2 g in 20 mL of water. A further 1.2 g dissolved in 1500 mL 5% dextrose was given over the next 20 hr. Dimercaprol group: deep IM injection in a dose of 4 mg/kg bodyweight every 4 hr for 24 hr, then 3 mg/kg every 4 hr for 24 hr. Liver biopsy was performed on 16 participants when PT had returned to normal. | |
| Outcomes | Mortality, maximum AST, maximum serum bilirubin, maximum PT, liver biopsy finding (16 participants) and adverse effects of treatment. | |
| Notes | Peak abnormalities in serum bilirubin and PT were greater in participants treated with dimercaprol as was the severity of hepatic necrosis found on liver biopsy. 1 participant died in the dimercaprol group. Noted in earlier published data (Gazzard 1975b), no difference between the 2 groups in term of initial paracetamol level. Note earlier published results: Gazzard 1975b and Hughes 1976. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were allocated at random to treatment with cysteamine or dimercaprol." Sequence generation not mentioned. |
| Allocation concealment (selection bias) | High risk | Author correspondence: randomisation by envelopes, not concealed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No mention of blinding and 2 different routes of administration (IM and IV). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not blinded but outcomes measured were mortality and biochemical markers. Potential for detection bias depending on frequency of measuring biochemical markers. |
| Incomplete outcome data (attrition bias) | Low risk | Author correspondence: outcomes were prospectively collected by the researchers. |
| Selective reporting (reporting bias) | Low risk | No selective reporting apparent. |
| Other bias | Unclear risk | Powering or intention to treat not specified. The first 2 reports of the study were interim analyses, the trial did not seem to have had any prespecified power analysis and stopped when the authors deemed other treatments were more promising. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Judged as high risk. |
| Methods | Randomised clinical trial. | |
| Participants | Inclusion criteria: participants with paracetamol‐induced fulminant hepatic failure who had not already received acetylcysteine. Exclusion criteria: not reported. Acetylcysteine) group vs 'placebo' group: Number of participants randomised: 25 vs 25. Age (mean) (years): 33 vs 34. Male:female ratio: 12:13 vs 9:16. Interval between ingestion and admission to Liver Unit (mean) (hr): 53 vs 56. Additional characteristics: Serum creatinine (mean) (mmol/L): 246 vs 247. Arterial pH (mean): 7.39 vs 7.39. PT (mean) (seconds, control time 15 seconds):115 vs 140. Not reported in either group: paracetamol plasma level on admission, amount of ingested paracetamol, number of participants taking additional drugs, number of participants consuming additional alcohol, number of participants excluded after randomisation. | |
| Interventions | Acetylcysteine group: acetylcysteine IV infusion 150 mg/kg in 200 mL 5% dextrose over 15 min, followed by 50 mg/kg in 500 mL 5% dextrose over 4 hr, then 100 mg/L over 16 hr. Final infusion rate continued until recovery from encephalopathy or death. 'Placebo' group: equivalent amount of 5% dextrose without acetylcysteine. If needed, all participants received additional intensive liver care: maintenance of intravascular pressures, renal support (haemodialysis), treatment (mannitol, hyperventilation, and thiopentone) for raised intracranial pressure, elective ventilation, and muscle relaxant for grade 4 encephalopathy. | |
| Outcomes | Mortality, cerebral oedema, hypotension requiring inotropic support, and renal failure. Liver function as assessed by PT and degree of encephalopathy. | |
| Notes | Rate of survival significantly higher in acetylcysteine group (12/25 (48%)) vs placebo group (5/25 (20%)). Treatment group had a lower incidence of cerebral oedema, fewer participants developed hypotension needing inotropic support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised on admission to the liver failure unit by opening one of 50 identical sealed envelopes containing an allocation." No mention of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not specified if sealed envelopes were sequentially numbered, opaque, or any other process to prevent subversion. |
| Blinding of participants and personnel (performance bias) | High risk | Multiple other interventions, not specified. |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "treatment with acetylcysteine could not be blind because the solution has an easily pungent aroma." Outcomes measured were mortality, inotrope requirement and biochemical markers, so high risk of bias. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not detailed. |
| Selective reporting (reporting bias) | Low risk | Quote: "two woman who had been randomised to acetylcysteine group underwent orthotropic liver transplantation, for the statistical analysis we assumed that they would have died." |
| Other bias | Unclear risk | Quote: Power "in order to detect a 40% difference in survivals … we had calculated that we would need to recruit 25 patients in each group to give a 90% power of achieving 5% significance. The retrospective study of late treatment with acetylcysteine in patients after paracetamol overdose who subsequently developed fulminant hepatic failure had suggested that such a difference in survival might be achieved." (Harrison 1990). Harrison 1990: "Mortality was 37% in patients who received acetylcysteine 10‐36 h after the overdose, compared with 58% in patients not given the antidote." Comment: a power calculation actually based on the referenced study would thus have had approximately 4 times as many participants. This suggests the power calculation may be post hoc or the study was stopped early. |
| Overall bias assessment (mortality) | Unclear risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Judged as high risk. |
| Methods | Multicentre randomised clinical trial. | |
| Participants | Inclusion criteria: people with paracetamol poisoning who required the administration of acetylcysteine, as assessed by acceptable practice guidelines, and included criteria such as serum paracetamol level, amount ingested, tests of liver injury, or a combination of the 3. Exclusion criteria: hypersensitivity to acetylcysteine. Number assessed: Number randomised: 223. Number excluded after randomisation: 43 excluded; 42 as incomplete study data or notes, or both. 15‐min acetylcysteine infusion group vs 60‐min acetylcysteine infusion group: Number of participants randomised: 109 vs 71. Age (mean) (years): female: 30.2; male: 33.6 vs female: 26.4; male: 31.9. Weight (mean) (kg): female: 63.7; male: 80.0 vs female: 63.6; male: 80.7. Number of participants where acetylcysteine started < 8 hr: 33 vs 25. Number of participants where acetylcysteine started > 8 hr: 74 vs 38. Not reported in either group: interval between ingestion and treatment, paracetamol plasma level on admission, amount of ingested paracetamol, number of participants taking additional drugs or consuming additional alcohol. | |
| Interventions | 15‐min regimen: 150 mg/kg IV acetylcysteine in 200 mL of 5% dextrose over 15 min (loading). 60‐min regimen: 150 mg/kg IV acetylcysteine in 200mL of 5% dextrose over 60 min (loading). Both groups received the same 4 hr (50 mg/kg IV acetylcysteine in 500 mL of 5% dextrose) and 16 hr infusion (100 mg/kg IV acetylcysteine in 1000 mL of 5% dextrose). | |
| Outcomes | Adverse events during and after IV acetylcysteine, in particular anaphylactoid reaction, 30 min intervals for the first 4 hr and subsequently 2, 4, and 8 hr intervals, ceasing at 24 hr after acetylcysteine administration. Blood samples: LFT, paracetamol, and coagulation test baseline and 12 hr intervals until the participant was discharged. | |
| Notes | An adverse event occurred during or after acetylcysteine administration for 82 (75%) participants in the 15‐min group and 43 (61%) participants in the 60‐min treatment group. There were drug‐related adverse events in 49 (45%) participants in the 15‐min group and 27 (38%) participants in the 60‐min group; P = 0.36. Comparison of the 15‐min and 60‐min groups for ALT, AST, and INR revealed no statistically significant difference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The 500 randomization slips (250 with "15‐minute" and 250 with "60‐minute") were placed in a closed box. When an eligible patient was enrolled, the duty pharmacist allocated the listed treatment to that site and patient, which resulted in unblocked random allocation." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Of the 180 evaluable patients, 109 patients were randomised to the 15‐minute treatment arm, and 71 patients were randomised to the 60‐minute treatment arm." The third‐party randomising (the poisons centre duty pharmacist) were not adequately concealed; however, those collecting the treatment presumably were unable to determine what the allocation assigned to their participant would be. The study allocation was quite unbalanced raising concerns about bias in allocation. |
| Blinding of participants and personnel (performance bias) | Low risk | Not feasible given nature of intervention. |
| Blinding of outcome assessment (detection bias) | High risk | Study was unblinded, outcomes included adverse events and biochemical markers. Potential for bias as assessors not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | High risk | Quote: potential for bias as 42 participants excluded after randomisation. "223 patients were randomised; of these, 181 patients had evaluable hospital notes and study data.", "42 patients were excluded because of incomplete medical records" and "Further 3 excluded for efficacy as incomplete data." A large number excluded because of incomplete hospital notes. Comment: analysis not able to be done by intention to treat on all randomised participants and a large number of participants excluded for missing data. |
| Selective reporting (reporting bias) | Unclear risk | "Judgments of the attribution of an event to the study drug were made independently by 2 of the investigators, with consideration of the clinical events and assessments surrounding the event, whether medication was administered to treat the event, and whether any other action was implemented." Comment: unclear whether bias might have been introduced in this process of adjudicating on events. |
| Other bias | Unclear risk | Power: quote: "Initial sample size estimation indicated that 249 patients were required for each arm of the study to detect an approximate halving of the rate, with 80% power. An incidence of 9% was estimated using the literature evidence available at study design." Early cessation of study: quote: "The initial research plan included 500 patients. The study was terminated in 2003 with 180 evaluable patients because of the difficulty in obtaining data in a reasonable time frame." "At a formal consensus meeting, the investigators concluded that a reduction in the observed rate of anaphylactoid reaction (from 25% to 10%) was required to justify a change in the guidelines for the initial reaction in 180 patients was only 4.3% (standard error = 5.5%). A sample size of more than 1,000 patients in each arm would be required to show the observed difference to be significant at an equal to 0.05 with 80% power." Comment: early cessation for futility is not best practice but unlikely to have led to a biased estimate of treatment differences. Not recorded mean paracetamol level or mean paracetamol dose ingested, in each group. Known that increased adverse events from IV acetylcysteine at lower paracetamol concentrations, unknown if difference between the 2 groups. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Judged as high risk. |
| Methods | Multicentre, double‐blind, randomised clinical study. | |
| Participants | Inclusion: aged ≥ 12 years; any person requiring treatment with acetylcysteine for acute acetaminophen toxicity. Exclusion criteria: history of allergy or hypersensitivity to acetylcysteine or any component of Acetadote, exposed to investigational drugs within 30 days before Clinical Trial Material (CTM) administration, pregnant or nursing, baseline ALT or AST > 1000 U/L or INR > 2, on dialysis, had congestive heart failure or renal failure such that the volume of the study drug administration would render the participant unsuitable for the study. Acetylcysteine without ethylenediaminetetraacetic acid vs control: Number randomised: 7 vs 10. Male:female ratio: 5:2 vs 7:3. | |
| Interventions | Acetylcysteine without EDTA group: Acetadote EF (ethylenediaminetetraacetic acid ‐ free) (new formulation) 200 mg/kg in 1000 mL diluent over 4 hr; then 100 mg/kg in 1000 mL diluent over 16 hr. Control group: Acetadote (old formulation) 150 mg/kg in 200 mL diluent over 60 min; then Acetadote 50 mg/kg in 500 mL diluent over 4 hr; then Acetadote 100 mg/kg in 1000 mL diluent over 16 hr. | |
| Outcomes | Primary outcome: hepatotoxicity ALT or AST > 1000 U/L. Secondary outcomes: need for continued treatment beyond the standard 21‐hr dosing regimen, adverse events, anaphylactoid reaction in the first 1 hr. Acetylcysteine without EDTA vs control: 7 started 5 completed vs 10 started 8 completed. Adverse events: 2 vs 1. Number of participants withdrawn: 0 vs 1. | |
| Notes | Risk of bias table could not be completed as "study was terminated prematurely due to lack of enrolment." Limited study results published on the ClinicalTrials.gov database. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unable to assess as "study was terminated prematurely due to lack of enrolment", limited protocol published on ClinicalTrials.gov database. |
| Allocation concealment (selection bias) | Unclear risk | Unable to assess as study was terminated prematurely. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unable to assess as study was terminated prematurely. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unable to assess as study was terminated prematurely. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to assess as "study was terminated prematurely due to lack of enrolment," limited results published on ClinicalTrials.gov database. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess as "study was terminated prematurely due to lack of enrolment," limited results published on ClinicalTrials.gov database. |
| Other bias | Unclear risk | Unable to assess as study was terminated prematurely. |
| Overall bias assessment (mortality) | Unclear risk | Risk of bias table could not be completed as "study was terminated prematurely due to lack of enrolment." Limited study results published on the ClinicalTrials.gov database. Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | Unclear risk | Risk of bias table could not be completed as "study was terminated prematurely due to lack of enrolment." Limited study results published on the ClinicalTrials.gov database. Judged as high risk. |
| Methods | Randomised clinical trial. | |
| Participants | Inclusion criteria: people aged ≥ 16 years who had ingested paracetamol ≥ 5 g within 4 hr of admission. Exclusion criteria: depressed conscious level, or with a condition such as previous gastric surgery that might preclude the use of any 1 of the treatment methods. Number of participants randomised: 60. Age (mean) (years): 25.7 (range 16‐62). Male:female ratio: 16:44 (numbers not given for each group but said to be similar age and sex). Time elapsed between ingestion and presentation at the department (mean) (min): 123 (range 30‐240). Paracetamol plasma level on admission (mean) (mg/L): supportive group: 90 vs activated charcoal group: 135 vs gastric lavage group: 160 vs ipecacuanha group: 110. Amount of ingested paracetamol (g): not reported. Number of participants taking additional drugs: 12. Number of participants consuming additional alcohol: 21. Number of participant excluded that were assessed: not reported. | |
| Interventions | Gastric lavage group: gastric lavage using a 36 FG tube. Activated charcoal group: activated charcoal carried out with Carbomix to drug ratio of 10:1. Ipecacuanha group: ipecacuanha syrup 30 mL, was repeated after 30 min if there was no response. No intervention group: no intervention to limit absorption (this group was treated in a different hospital in Derby, UK). Group was stopped for ethical reasons after only 5 participants were treated. | |
| Outcomes | Plasma paracetamol levels measured on samples taken from an indwelling cannula prior to any treatment, and following treatment at 60, 90, 150 min after the first sample. Percentage change between first and last plasma level used as a measure of effectiveness. Fall in plasma paracetamol concentration vs time. | |
| Notes | Activated charcoal more effective in lowering plasma paracetamol levels than either gastric lavage or ipecacuanha. In the intervention group, the plasma paracetamol concentration increased during treatment in 4/5 participants and led to cessation of the supportive treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated into one of four treatment groups." Not documented how randomisation sequence was generated. |
| Allocation concealment (selection bias) | High risk | "Ethical committee approval was obtained at both hospitals and the inclusion of a group who did not receive absorption limiting treatment was also approved at Derby." The above makes it clear that for allocation to the no intervention group there was no concealment that this was only possible at 1 hospital. Otherwise randomisation process not detailed enough to determine if there was allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned. Unclear whether there were missing data on randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Further, multiple methods could be used for the outcome of change in paracetamol level over time. They did not report if this resulted in any clinically relevant difference; for example, in the number requiring treatment with IV acetylcysteine or clinical outcome. |
| Other bias | Unclear risk | Power: not mentioned. Early cessation: quote: "Group 4 (no treatment group, Derby) was stopped for ethical reasons when the serum paracetamol levels increased between the first and last samples in four out of five patients." Intention‐to‐treat analysis: not mentioned. |
| Overall bias assessment (mortality) | High risk | Judged as high risk. |
| Overall bias assessment (non‐mortality outcomes) | High risk | Judged as high risk. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BP: blood pressure;
hr: hour; IM: intramuscular; INR: international normalised ratio; IU: international units; IV: intravenous; min: minute; LFT: liver function test; NAC: N‐acetylcysteine; PT: prothrombin time; SD: standard deviation; SE: standard error; vs: versus.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Human volunteer study. | |
| Review article. | |
| Observational study and meta‐analysis. | |
| Quasi‐randomised study, randomised according to month of the year. Rates of adverse effects used. | |
| Single armed study, all participants given ondansetron. | |
| Trial in people taking overdose of any medications, no subgroup analysis of paracetamol. | |
| Human volunteer study. | |
| Not randomised. Study including human volunteers. | |
| Commentary. | |
| Case report and meta‐analysis. | |
| Human volunteer study. | |
| Editorial. | |
| Letter regarding Kerr 2005. | |
| Randomised clinical trial of heparin in people with raised INR secondary to paracetamol‐induced liver necrosis. Excluded as per method of search, not to examine interventions that were treating secondary complication of liver failure, such as coagulopathy. | |
| Abstract from meeting confirmed with author same data as Hughes 1977. | |
| Randomised clinical trial of fresh frozen plasma in people with a raised INR . Excluded as per method of search, not to examine interventions that are treating secondary complication of liver failure, such as coagulopathy. | |
| Update. | |
| Abstract for the Hamlyn 1981 trial that was included. | |
| Retrospective chart review. | |
| Case report. | |
| Same data as Hughes 1977, correspondence with author confirms this. | |
| Review article. | |
| Abstract, early data from Keays 1991. | |
| Participants are liver failure from a non‐paracetamol cause. | |
| A study of gastric emptying in people with overdose, no subgroup analysis of individual drugs, so unable to obtain paracetamol data. | |
| Commentary. | |
| Review. | |
| Animal rat study and human volunteer study. | |
| Observational case series. | |
| Randomised clinical trial of charcoal haemoperfusion in people with acute liver failure. Excluded as per method of search, not to examine interventions that are treating secondary complication of liver failure. | |
| Human volunteer study. | |
| Randomised clinical trial of an albumin dialysis system, Molecular Adsorbent Recirculating System (MARS). Excluded as per method not to investigate liver support devices. | |
| Observational prospective case series. |
INR: international normalised ratio.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Methionine versus no intervention, Outcome 1 Mortality. | ||||
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.2 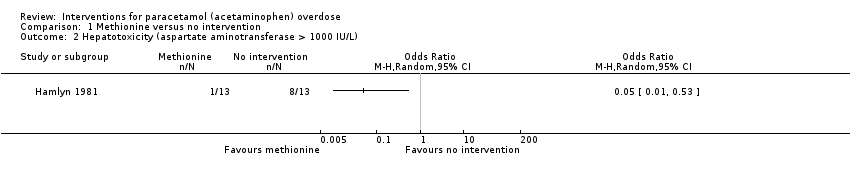 Comparison 1 Methionine versus no intervention, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.22] |
| Analysis 2.1  Comparison 2 Cysteamine versus no intervention, Outcome 1 Mortality. | ||||
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 2 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.35] |
| Analysis 2.2  Comparison 2 Cysteamine versus no intervention, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 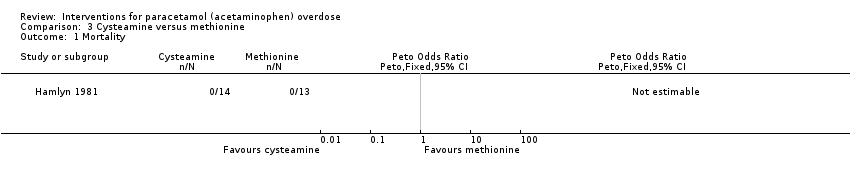 Comparison 3 Cysteamine versus methionine, Outcome 1 Mortality. | ||||
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 1 | 27 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.05, 16.46] |
| Analysis 3.2  Comparison 3 Cysteamine versus methionine, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Cysteamine versus dimercaprol, Outcome 1 Mortality. | ||||
| 2 Maximum alanine aminotransferase (IU/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Cysteamine versus dimercaprol, Outcome 2 Maximum alanine aminotransferase (IU/L). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1 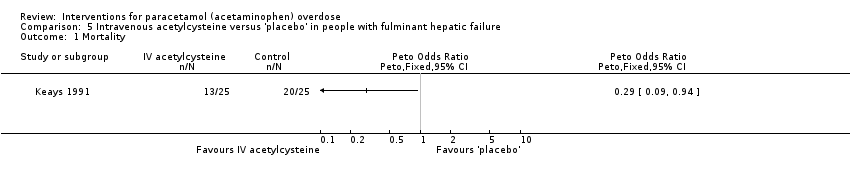 Comparison 5 Intravenous acetylcysteine versus 'placebo' in people with fulminant hepatic failure, Outcome 1 Mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1 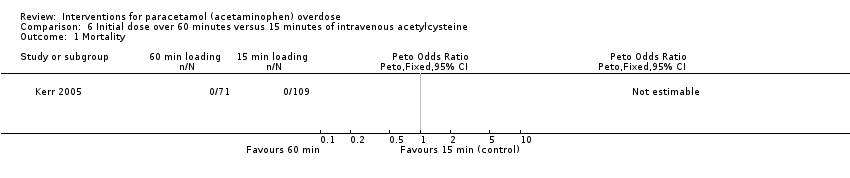 Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 1 Mortality. | ||||
| 2 Hepatotoxicity Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 2 Hepatotoxicity. | ||||
| 3 Any adverse event Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 3 Any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 1 Mortality. | ||||
| 2 Hepatotoxicity Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 2 Hepatotoxicity. | ||||
| 3 Vomiting, retching, or antiemetics from 0 to 2 hour Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 3 Vomiting, retching, or antiemetics from 0 to 2 hour. | ||||
| 4 Vomiting, retching, or antiemetics 0 to 12 hour Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.4  Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 4 Vomiting, retching, or antiemetics 0 to 12 hour. | ||||
| 5 Anaphylactoid symptoms (all) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 5 Anaphylactoid symptoms (all). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.1 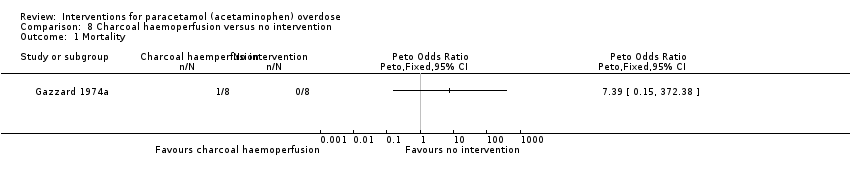 Comparison 8 Charcoal haemoperfusion versus no intervention, Outcome 1 Mortality. | ||||
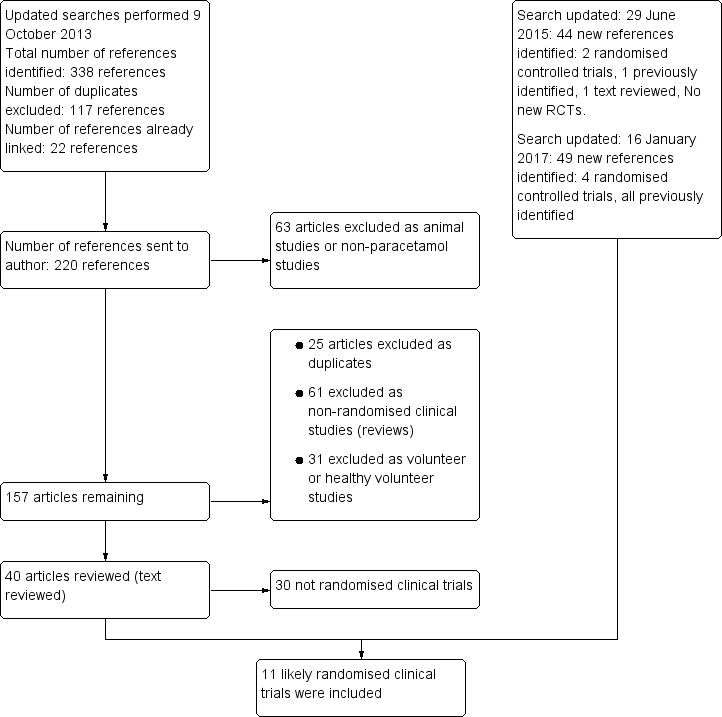
Flow chart: search strategy and results.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis of cysteamine versus control on hepatotoxicity defined as aspartate aminotransferase (AST) above 1000 IU/L. The diversity‐adjusted required information size (DARIS) was 982 participants based on a proportion of 53% with the outcome in the control group (Pc); a risk reduction of 20%; an alpha (a) of 2.5%; a beta (b) of 20% (equivalent to a power of 80%); and an assumed diversity of 20%. As demonstrated the trial sequential monitoring boundaries for benefit, harm, or futility were crossed by the cumulative Z value.

Trial Sequential Analysis of acetylcysteine versus placebo on mortality. The diversity‐adjusted required information size (DARIS) is 375 participants based on a proportion of 80% with the outcome in the control group (Pc); a risk reduction of 20% (Peto OR: POR); an alpha (a) of 2.5%; a beta (b) of 20% (equivalent to a power of 80%); and an assumed diversity of 20%. As demonstrated the trial sequential monitoring boundaries for benefit, harm, or futility were crossed by the cumulative Z value.

Trial Sequential Analysis of 15‐min infusion of acetylcysteine versus 60‐min infusion of acetylcysteine on any adverse event. The diversity‐adjusted required information size (DARIS) is 820 participants based on a proportion of 60% with the outcome in the control group (Pc); a risk reduction of 20%; an alpha (a) of 2.5%; a beta (b) of 20% (equivalent to a power of 80%); and an assumed diversity of 20%. As demonstrated the trial sequential monitoring boundaries for harm, benefit, or futility were crossed by the cumulative Z value.

Comparison 1 Methionine versus no intervention, Outcome 1 Mortality.

Comparison 1 Methionine versus no intervention, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L).

Comparison 2 Cysteamine versus no intervention, Outcome 1 Mortality.
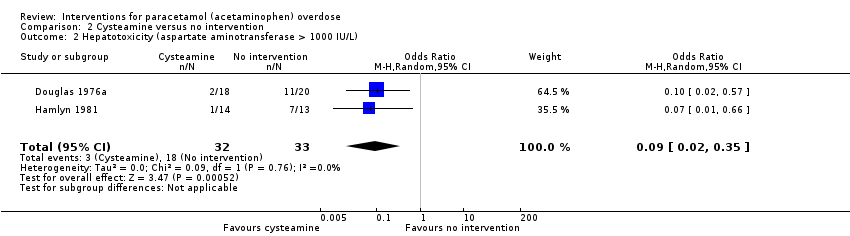
Comparison 2 Cysteamine versus no intervention, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L).

Comparison 3 Cysteamine versus methionine, Outcome 1 Mortality.
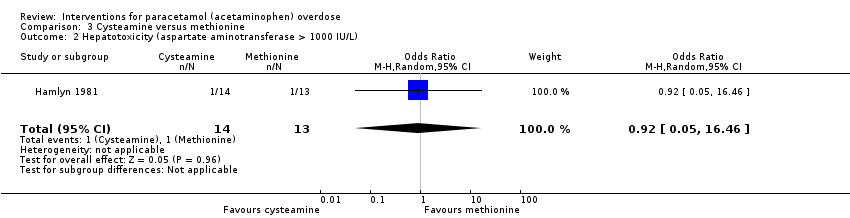
Comparison 3 Cysteamine versus methionine, Outcome 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L).

Comparison 4 Cysteamine versus dimercaprol, Outcome 1 Mortality.

Comparison 4 Cysteamine versus dimercaprol, Outcome 2 Maximum alanine aminotransferase (IU/L).

Comparison 5 Intravenous acetylcysteine versus 'placebo' in people with fulminant hepatic failure, Outcome 1 Mortality.

Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 1 Mortality.
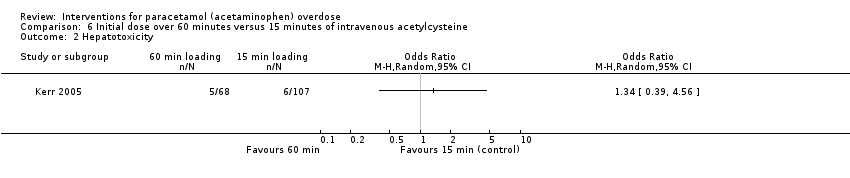
Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 2 Hepatotoxicity.

Comparison 6 Initial dose over 60 minutes versus 15 minutes of intravenous acetylcysteine, Outcome 3 Any adverse event.

Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 1 Mortality.

Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 2 Hepatotoxicity.
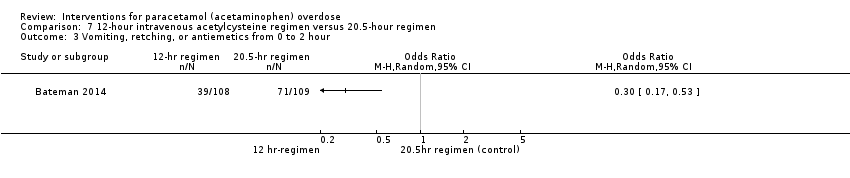
Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 3 Vomiting, retching, or antiemetics from 0 to 2 hour.

Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 4 Vomiting, retching, or antiemetics 0 to 12 hour.

Comparison 7 12‐hour intravenous acetylcysteine regimen versus 20.5‐hour regimen, Outcome 5 Anaphylactoid symptoms (all).

Comparison 8 Charcoal haemoperfusion versus no intervention, Outcome 1 Mortality.
| Methionine and supportive treatment compared with supportive treatment (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Supportive treatment | Methionine and supportive treatment | |||||
| Mortality | Study population | Peto OR 0.14 | 26 | ⊕⊝⊝⊝ | The Trial Sequential Analysis‐adjusted CI could not be estimated due to the paucity of data. | |
| 77 per 1000 | 12 per 1000 | |||||
| Hepatotoxicity | Study population | OR 0.05 | 26 | ⊕⊕⊝⊝ | ‐ | |
| 615 per 1000 | 74 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for risk of bias (concerns regarding randomisation sequence generation and allocation concealment probably compromised). | ||||||
| Cysteamine compared with no intervention (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Cysteamine | |||||
| Mortality | Study population | Peto OR 0.53 | 65 | ⊕⊝⊝⊝ | ‐ | |
| 61 per 1000 | 33 per 1000 | |||||
| Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) | Study population | OR 0.09 | 65 | ⊕⊕⊝⊝ | Trial Sequential Analysis‐adjusted CI ranged from 0.00 to 24.0. | |
| 545 per 1000 | 97 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (method of randomisation had potential for bias and allocation concealment not specified). | ||||||
| Cysteamine compared with dimercaprol (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dimercaprol | Cysteamine | |||||
| Mortality | Study population | Peto OR 0.14 | 52 | ⊕⊝⊝⊝ | ‐ | |
| 38 per 1000 | 6 per 1000 | |||||
| Mean maximum alanine aminotransferase (IU/L) | The mean maximum alanine aminotransferase (IU/L) in the dimercaprol was 754 | The mean maximum alanine aminotransferase (IU/L) in the cysteamine group was 722 (IU/L) | ‐ | 52 | ⊕⊕⊝⊝ | Difference ‐32.00 (95% CI ‐512.9 to 448.9). The difference between the 2 groups was not significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels because of serious imprecision (due to small sample studied, low number of deaths, and confidence intervals wide). | ||||||
| Cysteamine compared with methionine (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Methionine | Cysteamine | |||||
| Mortality | Study population | Not estimable | 27 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Hepatotoxicity (aspartate aminotransferase > 1000 U/L) | Study population | OR 0.92 | 27 | ⊕⊝⊝⊝ | ‐ | |
| 77 per 1000 | 71 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (concerns regarding randomisation and allocation concealment not specified). | ||||||
| Standard intravenous acetylcysteine regimen (20.5 hours) compared with shorter (12 hours) protocol for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard intravenous acetylcysteine regimen (20.25 hours) | Shorter (12‐hour protocol) | |||||
| Mortality | Study population | Not estimable | 222 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Hepatotoxicity | Study population | OR 0.67 (0.11 to 4.08) | 202 (1 RCT) | ⊕⊝⊝⊝ | ‐ | |
| 30 per 1000 | 20 per 1000 (3 to 111) | |||||
| Vomiting, retching, or antiemetics from 0‐2 hours | Study population | OR 0.30 (0.17 to 0.53) | 217 | ⊕⊕⊝⊝ | ‐ | |
| 651 per 1000 | 359 per 1000 | |||||
| Vomiting, retching, or antiemetics 0‐12 hours | Study population | OR 0.40 (0.22 to 0.75) | 203 | ⊕⊕⊝⊝ | ‐ | |
| 784 per 1000 | 593 per 1000 | |||||
| Anaphylactoid symptoms | Study population | OR 0.39 (0.21 to 0.70) | 208 | ⊕⊕⊝⊝ | ‐ | |
| 750 per 1000 | 539 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of indirectness (a large number of prospective participants excluded prior to randomisation: 1539 judged suitable for treatment, only 222 randomised). | ||||||
| Oral compared with intravenous acetylcysteine for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous acetylcysteine | Oral acetylcysteine | |||||
| Mortality | Study population | Not estimable | 66 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Hepatotoxicity | ‐ | ‐ | ‐ | ‐ | ‐ | Rates of hepatotoxicity not reported, only mean alanine aminotransferase between the 2 study groups. |
| Nausea | Study population | OR 2.71 | 66 | ⊕⊝⊝⊝ | ‐ | |
| 333 per 1000 | 575 per 1000 | |||||
| Vomiting | Study population | OR 2.10 | 66 | ⊕⊝⊝⊝ | ‐ | |
| 152 per 1000 | 273 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (due to randomisation details or concealment allocation were not specified, participants were excluded from IV group if they developed an anaphylactoid reaction unresponsive to decreasing the administration rate. Unclear whether these participants were analysed and should have been included as intention‐to‐treat). | ||||||
| Intravenous acetylcysteine compared with placebo in people with fulminant hepatic failure (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with fulminant hepatic failure secondary to paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Intravenous acetylcysteine | |||||
| Mortality | Study population | Peto OR 0.29 | 50 | ⊕⊕⊝⊝ | Trial Sequential Analysis‐adjusted CI ranged from 0.01 to 15.8. | |
| 800 per 1000 | 537 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (randomisation and allocation concealment unclear). | ||||||
| Initial infusion rate of intravenous acetylcysteine over 15 minutes compared with 60 minutes (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Initial infusion over 15 minutes | Initial infusion over 60 minutes | |||||
| Mortality | Study population | Not estimable | 180 | ⊕⊝⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Hepatotoxicity | Study population | OR 1.34 (0.39 to 4.56) | 175 | ⊕⊝⊝⊝ | ‐ | |
| 56 per 1000 | 74 per 1000 | |||||
| Any adverse event | Study population | OR 0.51 (0.27 to 0.96) | 180 | ⊕⊕⊝⊝ | Trial Sequential Analysis‐adjusted CI ranged from 0.36 to 11.0. | |
| 752 per 1000 | 608 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (possible bias due to method of randomisation via "randomisation slips" in a "closed box," many participants lost to follow‐up, and uneven numbers between the 2 treatment groups with many more participants in the 15‐minute infusion group). | ||||||
| Oral and intravenous acetylcysteine compared with intravenous acetylcysteine for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous acetylcysteine | Oral and intravenous acetylcysteine | |||||
| Mortality | Study population | Not estimable | 40 | ⊕⊝⊝⊝ | Primary outcome for this study was anaphylactoid reaction. Unable to analyse these results due to large number excluded from one arm.1 | |
| 0 per 1000 | 0 per 1000 | |||||
| Hepatotoxicity | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels because of serious risk of bias (randomisation sequence generation and allocation concealment not recorded, and a large number of participants excluded (10 excluded from the 25 randomised)). | ||||||
| Charcoal haemoperfusion compared with no intervention (randomised trials) for paracetamol (acetaminophen) overdose | ||||||
| Patient or population: people with paracetamol (acetaminophen) overdose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Charcoal haemoperfusion | |||||
| Mortality | Study population | Peto OR 7.39 | 16 | ⊕⊝⊝⊝ | Note very small numbers in this trial; only 8 in each group. With only 1 death in the charcoal haemoperfusion arm. The Trial Sequential Analysis‐adjusted CI could not be calculated. | |
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level because of risk of bias (randomisation sequence generation and allocation concealment not detailed). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.05, 5.22] |
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 2 | 65 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.02, 0.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Hepatotoxicity (aspartate aminotransferase > 1000 IU/L) Show forest plot | 1 | 27 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.05, 16.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Maximum alanine aminotransferase (IU/L) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Hepatotoxicity Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Any adverse event Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Hepatotoxicity Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Vomiting, retching, or antiemetics from 0 to 2 hour Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Vomiting, retching, or antiemetics 0 to 12 hour Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Anaphylactoid symptoms (all) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |

