Anti‐inflammatory treatment for carditis in acute rheumatic fever
Abstract
Background
Rheumatic heart disease remains an important cause of acquired heart disease in developing countries. Although prevention of rheumatic fever and management of recurrences have been well established, optimal management of active rheumatic carditis remains unclear. This is an update of a review published in 2003, and previously updated in 2009 and 2012.
Objectives
To assess the effects, both harmful and beneficial, of anti‐inflammatory agents such as aspirin, corticosteroids and other drugs in preventing or reducing further valvular damage in patients with acute rheumatic fever.
Search methods
We searched the Cochrane Central Register of Controlled Trials (2013, Issue 9 of 12), MEDLINE (Ovid, 1948 to 2013 October Week 1), EMBASE (Ovid, 1980 to 2013 Week 41) and Latin American Caribbean Health Sciences Literature (LILACS) (1982 to 17 October 2013). We last searched Index Medicus (1950 to April 2001) in 2001. We checked reference lists of identified studies and applied no language restrictions.
Selection criteria
Randomised controlled trials comparing anti‐inflammatory agents (e.g. aspirin, steroids, immunoglobulins, pentoxifylline) versus placebo or controls, or comparing any of the anti‐inflammatory agents versus one another, in adults and children with acute rheumatic fever diagnosed according to Jones, or modified Jones, criteria. The presence of cardiac disease one year after treatment was the major outcome criterion selected.
Data collection and analysis
Two review authors extracted data and assessed risk of bias using the methodology outlined in the Cochrane Handbook of Systematic Reviews of Interventions. Standard methodological procedures as expected by The Cochrane Collaboration were used.
Main results
No new studies were included in this update. Eight randomised controlled trials involving 996 people were selected for inclusion in the review. Researchers compared several steroidal agents such as corticotrophin, cortisone, hydrocortisone, dexamethasone, prednisone and intravenous immunoglobulin versus aspirin, placebo or no treatment. Six trials were conducted between 1950 and 1965; one was done in 1990 and the final study was published in 2001. Overall there were no observed significant differences in risk of cardiac disease at one year between corticosteroid‐treated and aspirin‐treated groups (six studies, 907 participants, risk ratio 0.87, 95% confidence interval 0.66 to 1.15). Similarly, use of prednisone (two studies, 212 participants, risk ratio 1.13, 95% confidence interval 0.52 to 2.45) compared with aspirin did not reduce the risk of heart disease after one year. Investigators in five studies did not report adverse events. The three studies reporting on adverse events reported substantial adverse events. However, all results should be interpreted with caution because of the age of the studies and the substantial risk of bias.
Authors' conclusions
Little evidence of benefit was found when corticosteroids or intravenous immunoglobulins were used to reduce the risk of heart valve lesions in patients with acute rheumatic fever. The antiquity of most of the trials restricted adequate statistical analysis of the data and acceptable assessment of clinical outcomes by current standards. In addition, risk of bias was substantial, so results should be viewed with caution. New randomised controlled trials in patients with acute rheumatic fever are warranted to assess the effects of corticosteroids such as oral prednisone and intravenous methylprednisolone and the effects of other new anti‐inflammatory agents. Advances in echocardiography will allow more objective and precise assessments of cardiac outcomes.
PICO
Plain language summary
Anti‐inflammatory treatment in the form of corticosteroids and immunotherapy to prevent heart damage resulting from rheumatic fever
This is an update of a review published in 2003 and previously updated in 2009 and 2012. For this latest update, the search was rerun on 17 October 2013, and no new studies were found. Rheumatic fever is a late complication of a type of throat infection caused by streptococcal bacteria. It is an immune system disease that can lead to inflammatory disease of the heart (carditis), joints, brain and skin. Carditis can cause heart failure and death. Various anti‐inflammatory drugs have been used to treat carditis, including corticosteroids, aspirin and immunoglobulins (immune therapy using antibodies). No new trials were identified in this update. This review includes eight trials with 996 participants. Evidence shows little effect of corticosteroids over aspirin in preventing cardiac disease after one year (six studies, 907 participants, risk ratio 0.87, 95% confidence interval 0.66 to 1.15). Several steroidal agents such as corticotrophin, cortisone, hydrocortisone and dexamethasone were compared with aspirin before 1966, and prednisone and immunoglobulins were compared with placebo in studies from 1990 and 2001, respectively. Most studies did not report on adverse events, but those that did reported complications due to corticosteroids including weight gain, enlarged facial features and acne. Trials were generally old (six of the eight trials were conducted between 1955 and 1965), small and of poor quality and had high risk of bias. For this reason, results should be interpreted with caution.
Authors' conclusions
Background
Pathogenesis
Rheumatic fever is a delayed complication of pharyngeal infection with group A beta‐haemolytic streptococci. Infection with this organism results in a diffuse inflammatory disease of the heart, joints, brain, blood vessels and subcutaneous tissue. Carditis is not seen at the time of the streptococcal infection and appears only after a latent period of about three weeks. This serves as evidence against the direct role of streptococcal products in the pathogenesis of rheumatic fever. This latent period parallels the time required for development of an immune response (Abraham 1991). The molecular mimicry between certain parts of the streptococcus and tissues of the host may lead to a cross‐reactive immunity, in which the immune system produces an antibody response to various components of the streptococcal organism, as well as to certain tissues of the patient, particularly the heart. Immunological hyper‐responsiveness in some patients, together with disturbed helper and suppressor T‐cell function, leads to a complex immune system disturbance, which results in acute rheumatic fever and consequent cardiac damage (Williams 1982). Further evidence of an immunological mechanism for the pathogenesis of acute rheumatic fever is found in the presence of myocardial and endocardial deposits of immunoglobulin and complement in these patients (Kaplan 1964). In addition, rabbit antisera against certain group A streptococci have been shown to react with human heart preparations, indicating cross‐reaction between the streptococcus and the human heart (Kaplan 1963). More recently, the pathogenic role of inflammatory cytokines in rheumatic fever in the form of tumour necrosis factor (TNF)‐alpha, interleukin (IL)‐8 and IL‐6 has been described (Yegin 1997). Substantial evidence pointing to the inflammatory nature of the disease has resulted in use of anti‐inflammatory agents such as corticosteroids and aspirin to treat the disease. Newer therapies such as intravenous immunoglobulin (IVIG) and pentoxifylline have been used to modulate cytokine expression and suppression of activated cytotoxic T cells (Narin 2003; Voss 2001).
Predisposition and susceptibility
Occurrence and spread of the streptococcal organism are influenced by the age of the patient, seasonality and socio‐economic conditions. A superimposed genetic predisposition also probably exists (Guilherme 1991; Olmez 1993). Supporting evidence for an underlying immunological susceptibility for developing acute rheumatic fever is found in the higher antibody response observed in rheumatic patients compared with non‐rheumatic individuals following administration of influenza vaccine, and the finding of isologous red blood cells in these patients (Barrett 1984). A non‐HLA B cell antigen, known as the D8/17 lymphocyte alloantigen, has also been identified in patients with rheumatic fever (Ganguly 1992; Khanna 1989). A test to detect this B cell antigen marker can aid in the diagnosis of rheumatic fever and can help detect siblings who are at risk of developing the disease (Herdy 1992).
Epidemiology
The epidemiology of acute rheumatic fever and of its complication rheumatic heart disease has changed since the first assessment of its impact, as recorded in this review for the first time in 2003. The literature at that time indicated that more than 12 million people were affected by rheumatic fever worldwide and approximately 400,000 deaths annually result from rheumatic heart disease (WHO/ISCF 1995). The incidence of rheumatic fever had declined in industrialised countries since the 1950s, with a recorded annual incidence of around 0.5 cases per 100,000 children of school age. A resurgence in the incidence of rheumatic fever has been reported in industrialised countries such as the intermountain area of the United States (Veasy 1987). In developing countries, rheumatic fever appeared to remain an endemic disease, with annual incidence ranging from 100 to 200 per 100,000 school‐aged children (Olivier 2000). In South Africa, a survey of 12,050 school children in 1972 revealed a prevalence rate of rheumatic heart disease of 6.9/1000, with a peak rate of 19.2/1000 in children aged 15 to 18 years (McLaren 1975). Rheumatic fever occurred at a much younger age in developing countries, and severe chronic valvular heart disease occurred earlier (Halim 1961; Padmavati 1978). The more recent estimate of the global burden of rheumatic heart disease publication in 2013 critically reviewed the previous burden of disease estimates, suggesting that very few populations worldwide have been sufficiently studied to allow for proper prevalence and incidence estimates. Nevertheless the number of definitive cases of rheumatic heart disease has been shown to remain relatively consistent in recent studies in multiple diverse populations, at a prevalence of three to eight per 1000 (Global Burden 2013).
Diagnosis of rheumatic fever
The most commonly used criteria for the diagnosis of rheumatic fever include the modified Jones criteria (AHA 1965; Dajani 1992). Diagnosis depends on the presence of events classified as major and minor manifestations of the disease. Concern about over‐diagnosis of acute rheumatic fever was the main motivation for the creation of the original Jones criteria almost 60 years ago (Jones 1944). To increase specificity, these criteria have been revised four times ‐ in 1955, 1965, 1984 and 1992 (Shiffman 1995). The latest revision of the Jones criteria (Dajani 1992) suggests that the probability of acute rheumatic fever is high when the following are found.
-
Evidence of a preceding streptococcal infection, usually measured by elevation of the antistreptolysin O titre together with:
-
two major manifestations (arthritis, carditis, chorea, erythema marginatum and subcutaneous nodules); or
-
one major and two minor manifestations (fever, arthralgia, high C‐reactive protein (CRP) or elevated erythrocyte sedimentation rate (ESR) and a prolonged PR interval on electrocardiogram).
-
CRP and ESR are also helpful for monitoring inflammatory activity in patients with acute rheumatic fever. However, the ESR does vary according to erythrocyte count and the presence of concomitant heart failure.
Carditis
Carditis, the most serious major manifestation of rheumatic fever, may culminate in chronic valvular disease and can lead to heart failure and, ultimately, death. Carditis usually has no associated symptoms and often is identified only during clinical examination of a patient who presents with fever, arthritis or chorea (involuntary, jerky movements of the limbs). Clinical features reflect involvement of various layers of the heart, viz, endocardium, including the valves, the myocardium or the pericardium, and frequently all three layers of the heart. The heart valves are the most commonly affected structures. The presence or absence of carditis is an important determinant of the course and prognosis of acute rheumatic fever. The prognosis worsens with increasing severity of the initial carditis and with recurrences (El‐Said 1998). Bed rest contributes to reduced rheumatic activity (Barlow 1990).
Valvular sequelae
Rheumatic fever complicates untreated group A beta‐haemolytic streptococcal infection in 0.1% to 0.3% of the general population and in 3% of epidemics in closed communities (Siegel 1961); second episode attack rates as high as 65% have been reported (Taranta 1964). Inflammation of components of the heart, such as the mitral and aortic valve leaflets and the chordae of the mitral valve, is the most common manifestation of rheumatic carditis (Ayoub 1995). Recurrent attacks of rheumatic fever result in increasing damage to the valves. Damaged valves may regurgitate (fail to close properly and leak) or may become stenotic (fail to open properly). Mitral regurgitation is the most common heart defect (85%) identified in children and adolescents; it results from fibrosis and contracture of the valve leaflets, which prevents proper apposition during systole. The aortic valve is affected in 54% of patients, and the tricuspid and pulmonary valves in less than 5% (Markowitz 1972).
Surgery
Open heart surgery is needed in patients with severely regurgitant or stenotic valve lesions. Heart valves can be repaired or replaced, if deemed to be non‐repairable. Replacement of the valve with a prosthetic heart valve would require the patient to take anticoagulant treatment, usually in the form of warfarin, for life. The dosage of warfarin is crucial, and close monitoring of blood levels is needed to avoid thrombus formation on the prosthetic valve. Control of warfarin at therapeutic levels is a problem in developing countries, where many patients come from poor urban or rural settings with limited access to adequate medical care. In addition, the cost of heart surgery in patients with severely damaged valves is exorbitant and is a drain on the meagre health resources of poor countries.
Treatment controversies
Recommended therapies for acute rheumatic fever include bed rest, penicillin and anti‐inflammatory agents.
Steroids
At present, no clear consensus has been reached about the place of steroids in preventing rheumatic heart disease. Some practitioners recommend their use for patients with moderate carditis and heart failure (Abraham 1991; Ayoub 1995; El‐Said 1998). Steroids have been shown to favourably affect clinical response while reducing the erythrocyte sedimentation rate. In addition, patients receiving steroids had a shorter hospital stay than those treated with aspirin (Human 1984). Other reports suggest that heart failure in patients with active rheumatic carditis occurs as a result of a haemodynamically severe valvular lesion that can be corrected only surgically ‐ not by giving steroids (Kingsley 1987). Steroids are never a "lifesaving measure" in patients with a severe valvular lesion. It is argued that steroids are likely to make the tissues more friable and the task of the surgeon more difficult. Although steroids are used frequently, their use has not been shown to induce improvement in patients with fulminating rheumatic carditis (Barlow 1990; Kingsley 1987).
Aspirin (acetylsalicylic acid)
Controversy surrounds the value of aspirin in patients with rheumatic fever. Data from several studies do not show an advantage of steroids over aspirin in the treatment of patients with mild or moderate carditis. It is recommended that aspirin be used in patients with mild or moderate carditis, and that steroids be reserved for patients with severe carditis, particularly those with pancarditis and cardiac failure. Use of steroids in these patients is preferred because steroids produce a more prompt anti‐inflammatory effect (Ayoub 1995).
Why it is important to do this review
Rheumatic heart disease remains a major cause of morbidity and mortality in low‐income communities (Olivier 2000). It is clear that antibiotic treatment for streptococcal pharyngitis prevents the development of rheumatic fever (DiSciascia 1980), and that antibiotic prophylaxis prevents recurrence (Massell 1988). However, no consensus has been reached on the optimal management of established active carditis to prevent complications, which may include valvular heart disease and death.
In view of the many controversies and uncertainties surrounding the use of anti‐inflammatory agents in patients with acute rheumatic fever, an up‐to‐date systematic review is indicated to clarify the most effective treatment strategy.
A glossary of terms used throughout this review is provided in Appendix 1.
Objectives
To assess the effects, both harmful and beneficial, of anti‐inflammatory agents such as aspirin, corticosteroids and other drugs in preventing or reducing further valvular damage in patients with acute rheumatic fever.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) in which participants were randomly assigned to receive anti‐inflammatory drugs for acute rheumatic carditis and were followed up for at least three months. We applied no language limitations.
Types of participants
Trials included children and adults with a clinical diagnosis of a first or new episode of rheumatic fever, based on Jones or modified Jones criteria (AHA 1965; Dajani 1992; Jones 1944), and associated cardiac disease at the time of study enrolment. The diagnosis of cardiac disease was based on the presence of mitral and/or aortic regurgitation and/or pericarditis and/or heart failure identified by clinical examination and auscultation, or echocardiography in later studies. Participants were in‐patients, and some were subsequently followed up as out‐patients following discharge.
Types of interventions
Selected studies were those in which the following interventions were compared.
-
Aspirin versus other anti‐inflammatory drugs or placebo.
-
Steroids versus other anti‐inflammatory drugs or placebo.
-
Other anti‐inflammatory drugs versus placebo.
-
Anti‐inflammatory drugs versus no treatment.
Therapy included anti‐inflammatory drugs of any type, administered by any route (i.e. intravenously, intramuscularly, orally) regardless of dosage or number of doses given. Controls included no treatment (Dorfman 1961), placebo infusion of dextrose and saline (Voss 2001) or placebo tablets (Haffejee 1990).
Types of outcome measures
Primary outcomes
-
Valvular lesions developing in participants with previously normal valves.
-
Worsening of valvular lesions in participants with valves damaged during previous attacks of rheumatic fever.
Secondary outcomes
-
Reduction in hospital stay.
-
Rate of decrease of erythrocyte sedimentation rate and/or C‐reactive protein.
Search methods for identification of studies
We searched for randomised and quasi‐randomised trials related to the topic of "Anti‐inflammatory treatment of acute rheumatic fever".
We searched the following databases for this update on 17 October 2013.
-
The Cochrane Central Register of Controlled Trials Register (CENTRAL) (2013, Issue 9 of 12).
-
MEDLINE (Ovid, 1948 to October Week 1 2013).
-
EMBASE (Ovid, 1980 to 2013 Week 41).
-
Latin American and Caribbean Health Sciences Literature (LILACS) (1982 to 17 October 2013).
The following searches were not updated and were last searched in 2001 and 2011, respectively.
-
Index Medicus (1950 to April 2001).
-
PubMed using the terms "Rheumatic fever and treatment" (2007 to September 2011).
We applied no language restrictions. We used the Cochrane sensitivity‐maximising RCT filter for MEDLINE (updated search in 2013) (Lefebvre 2011) and an adaptation of this filer for EMBASE. See Appendix 2 for details of the search strategies used in 2013, Appendix 3 for strategies used for the 2011 update and Appendix 4 for the original searches conducted in 2002.
We checked references in the identified studies.
Data collection and analysis
Selection of studies
One review author (AMC) initially selected 33 papers according to relevance by reviewing titles and abstracts. Two review authors then independently analysed selected studies for predetermined inclusion criteria. We resolved disagreements regarding selection through discussion. We have undertaken three updates since initial publication of the review, and we found none of the 507 references in 2009, none of the 78 references in 2011 and none of the 816 references in 2013 to be eligible for inclusion.
Data extraction and management
We extracted various study characteristics and outcome measures, which we entered onto a predesigned data extraction form as follows.
-
Methods: study design, method of randomisation, allocation concealment, blinding of investigators, inclusion and exclusion criteria.
-
Population: recruitment, sample size, age, gender.
-
Intervention: agent, dose, timing and duration of therapy, co‐interventions.
-
Control: participants, agent and dose.
-
Outcome: timing of outcome, reported outcomes.
One review author (AMC) performed data entry and analysis and checked with a second review author.
Assessment of risk of bias in included studies
We assessed risk of bias in the following areas using the methodology of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Incomplete outcome data.
-
Selective reporting.
Measures of treatment effect
We used risk ratios (RRs) and 95% confidence intervals (CIs) as calculated for dichotomous data.
Assessment of heterogeneity
-
We assessed homogeneity between studies using Chi2 and I2 tests. We pooled and analysed results using random‐effects models.
-
We explored reasons for heterogeneity and the most appropriate conclusions drawn from the observations.
Data synthesis
We pooled the data using a random‐effects model.
Results
Description of studies
Results of the search
One review author (AMC) initially selected 33 papers, eight of which were included for analysis. We checked a further 507 references in 2009, 78 references in 2011 and 816 references in 2013, and found that none described studies that were eligible for inclusion in the review.
Included studies
We identified eight randomised controlled trials for this review. We present study details in the Characteristics of included studies table.
Length of trials
Studies provided long‐term follow‐up from one to six years.
Participants
The eight included studies reported on 966 participants. All studies, except RFWP 1955, included participants with a first episode of acute rheumatic fever. Acute rheumatic fever was diagnosed according to Jones criteria 1944 (RFWP 1955), Jones criteria 1965 (Haffejee 1990; Voss 2001) or Jones criteria 1992 (Voss 2001). The other five studies (CRFSG 1960; CRFSG 1965; Dorfman 1961; Massell 1961; Stolzer 1955) did not state the criteria used but were not excluded from analysis, because, despite this deficiency, it is unlikely that they used criteria other than Jones or modified Jones to make a diagnosis of acute rheumatic fever. Six studies enrolled children as participants, with mean age younger than 12 years in each case (range eight to 11 years) (CRFSG 1960; CRFSG 1965; Dorfman 1961; Haffejee 1990; RFWP 1955; Voss 2001). Massell 1961 did not indicate the age of study participants, and Stolzer (Stolzer 1955) reported on a study population that included adult male airmen only. Five of the studies were conducted in the United States (CRFSG 1960; CRFSG 1965; Dorfman 1961; Massell 1961; Stolzer 1955), one was a joint study between the United States and the United Kingdom (RFWP 1955), one study was reported from New Zealand (Voss 2001) and one from South Africa (Haffejee 1990).
Inclusion criteria
CRFSG 1960 required participants to have moderate to severe carditis and time to treatment within 28 days of onset of the illness; CRFSG 1965 and Voss 2001 included patients with carditis and polyarthritis. Duration of aspirin treatment varied in the CRFSG 1965 study, depending on whether participants presented with carditis or polyarthritis; Dorfman 1961 randomly assigned participants following a diagnosis of acute rheumatic fever and required that therapy be initiated within 18 days of onset of the illness; Haffejee 1990 and Massell 1961 required participants to have a diagnosis of carditis only; RFWP 1955 included three groups, which consisted of participants with a normal heart, participants with carditis and those with preexisting heart disease; Stolzer 1955 required a diagnosis of acute rheumatic fever only.
Study size
RFWP 1955 was by far the largest study (497 participants), and Haffejee 1990 randomly assigned only 35 participants. Three studies included between 50 and 100 participants (CRFSG 1960; Massell 1961; Voss 2001), and the remaining three studies included between 101 and 160 participants (CRFSG 1965; Dorfman 1961; Stolzer 1955).
Interventions
Trialists provided a wide range of corticosteroid treatments, including ACTH (corticotrophin), cortisone, prednisone, dexamethasone and hydrocortisone. Dosage, route of administration, interval between symptoms and treatment and duration of treatment varied greatly. Aspirin (acetylsalicylic acid) and placebo were used as comparisons. Two studies (RFWP 1955; Stolzer 1955) examined outcomes of using ACTH and cortisone versus aspirin. Massell 1961 combined four drugs (prednisone, dexamethasone, ACTH and cortisone) into a steroid category and compared this versus aspirin. Separate data were not provided for each drug. The study included five allocation groups. Only participants in groups 4 and 5 who were randomly assigned were included in the analysis. Non‐randomly assigned participants in groups 1, 2 and 3 who were given "no therapy", "small‐dose hormone" and "large‐dose hormone" were not included in the analysis. Prednisone was compared with aspirin in the CRFSG 1960 and CRFSG 1965 RCTs, whereas Dorfman 1961 chose four treatment groups, which included hydrocortisone alone, aspirin alone, hydrocortisone and aspirin together and "no specific treatment". Haffejee 1990 examined the outcomes of participants given prednisone versus placebo, and Voss 2001 studied the effects of intravenous immunoglobulin versus placebo.
Outcomes
All studies provided data on long‐term follow‐up of at least one year. Chosen outcomes varied between studies. CRFSG 1960 included the presence of both mitral and aortic incompetence on auscultation at one year as the outcome following treatment. CRFSG 1965 specified cardiac outcomes as "heart disease"; Dorfman 1961 assessed participants for the presence of apical systolic murmurs, apical diastolic murmurs and basal diastolic murmurs on auscultation; Haffejee 1990 determined the presence of murmurs, the need for surgery and death at follow‐up of 15 months to six years (mean three years and 11 months); Massell 1961 assessed the presence of "signs of rheumatic heart disease at one year"; RFWP 1955 analysed all murmurs, including functional murmurs, present at one year. Only significant apical systolic and basal diastolic murmurs diagnosed on auscultation and detected in this study were included in the review meta‐analysis; Stolzer 1955 made a distinction between the presence of apical systolic, apical diastolic and aortic diastolic murmurs at 14 months, and Voss 2001 assessed the presence of "carditis", defined by the presence of an aortic or mitral murmur diagnosed clinically, and aortic or mitral regurgitation diagnosed using echocardiography, one year after treatment.
Multiple outcomes such as a systolic murmur at the apex and an apical diastolic murmur were documented in several participants. All cardiac outcomes, including murmurs, documented one year after treatment were grouped together as "cardiac disease" in the review meta‐analysis. Other outcomes such as ESR, CRP values and duration of hospital stay in response to anti‐inflammatory therapy were too varied and inconsistent between studies for performance of a meta‐analysis.
Drug duration and dosage
Studies used not only different drugs but also various dosages and durations of therapy, for example, prednisone and aspirin were given for 12 weeks by CRFSG 1960; prednisone for one to two weeks and aspirin for eight weeks by CRFSG 1965; hydrocortisone and aspirin for 12 weeks by Dorfman 1961; prednisone and placebo for two to 42 days depending on clinical response by Haffejee 1990; ACTH, cortisone, prednisone, dexamethasone and aspirin for 12 weeks by Massell 1961; ACTH, cortisone and aspirin for five weeks by RFWP 1955; cortisone, corticotrophin and aspirin for six weeks by Stolzer 1955; and IVIG and placebo on four occasions over a 28‐day period by Voss 2001.
Oral hydrocortisone (Dorfman 1961) was given at a dose of 250 mg/d initially (for 96 hours) depending on whether the participant weighed more or less than 80 lb, and then at 100 mg/d × eight weeks, with subsequent tapering over three weeks. Intramuscular cortisone (Stolzer 1955) was prescribed at a dose of 300 mg/d initially (for one day) and then was tapered over six weeks. RFWP 1955 treated participants with ACTH 120 USP units (for four days) and intramuscular cortisone 300 mg initially (for one day) with doses tapered over six weeks. Prednisone was given at a dose of 60 mg/d initially (for three weeks) and was tapered over nine weeks and given at 3 mg/lb for seven days by CRFSG 1960 and CRFSG 1965, respectively.
Excluded studies
We initially excluded 25 studies because they were not randomised trials or did not meet the outcome analysis criteria (see Characteristics of excluded studies). We excluded two RCTs because follow‐up was provided for less than three months (Camara 2002; Rowe 1953). Three were review studies (Bywaters 1956; Bywaters 1961; Naik 2002), one was a letter (Marshall 1989), another a retrospective analysis (Uziel 2000), one was a study on people who did not have carditis (Paz 2006) and the remaining 17 studies examined the use of steroidal agents for treatment of acute rheumatic fever in a non‐randomised fashion. One study (Herdy 2012) met some of the search criteria regarding use of steroids in patients with rheumatic carditis, but this study was a follow‐up retrospective analysis and therefore did not meet inclusion criteria.
Risk of bias in included studies
Random sequence generation
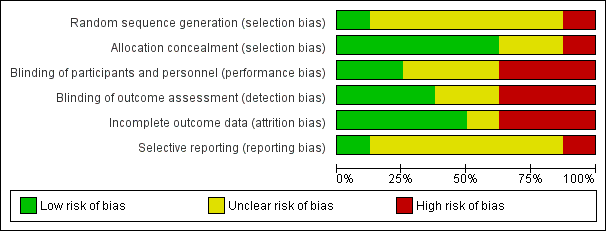
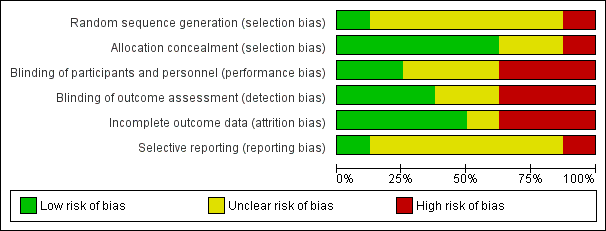
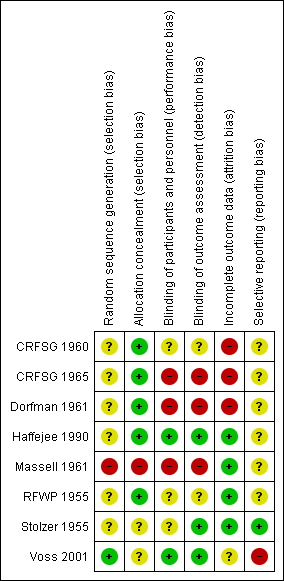
All studies claimed to be randomised, but only one was judged to have low risk of bias (Voss 2001). Six did not state how the study was randomised and were at unknown risk of bias (CRFSG 1960; CRFSG 1965; Dorfman 1961; Haffejee 1990; RFWP 1955; Stolzer 1955). One study included participants who were not randomly assigned and was at high risk of bias (Massell 1961) (Figure 1; Figure 2).
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation concealment
We assessed five studies as having low risk of allocation concealment bias (CRFSG 1960; CRFSG 1965; Dorfman 1961; Haffejee 1990; RFWP 1955), two as having unclear risk (Stolzer 1955; Voss 2001) and one as having high risk (Massell 1961) (Figure 1; Figure 2).
Blinding of participants and personnel
Blinding of study participants was clearly described in only three studies (Haffejee 1990; Voss 2001; CRFSG 1965); two studies used a double‐blind design (Haffejee 1990; Voss 2001) and were considered to be at low risk of bias. The third study, CRFSG 1965, used both a single‐blind and and a double‐blind design and was labelled as having a high risk of bias. Three studies had unclear risk of bias (CRFSG 1960; RFWP 1955; Stolzer 1955). Three studies were at high risk of bias: One used a consecutive single‐ and double‐blind study group (CRFSG 1965). One study (Dorfman 1961) specified that it was difficult for observers in this study to be unaware of group assignments because of the striking effects of corticosteroid therapy, and one included participants for which no blinding was applied (Massell 1961) (Figure 1; Figure 2).
Three studies had low risk of detection bias (Haffejee 1990; Stolzer 1955; Voss 2001), two had unclear risk of detection bias (CRFSG 1960; RFWP 1955) and three had high risk of detection bias (CRFSG 1965; Dorfman 1961; Massell 1961) (Figure 1; Figure 2).
Incomplete outcome data
No intention‐to‐treat analysis was undertaken in any of the trials, despite small numbers of participants 'crossing over' to other treatment groups within trials (CRFSG 1960; CRFSG 1965). Three participants in the carditis group in the CRFSG 1965 trial were changed from the acetylsalicylic acid (aspirin) to prednisone treatment. Four participants were moved from the aspirin group to the prednisone group in CRFSG 1960. One participant was initially randomly assigned to the aspirin group and then was changed to the prednisone group because he was critically ill. Three others were changed to prednisone because they had a poor response to aspirin. One of these participants subsequently died. In RFWP 1955, five participants were moved from the aspirin group to other treatment groups (three to ACTH, two to cortisone) because of severe illness. Of these, four participants were included for analysis under their original randomisation group (aspirin), and the other was excluded from the final analysis.
Withdrawal of participants or loss to follow‐up was well described in all studies. Between 3.2% (Voss 2001) and 16% (Dorfman 1961) of participants withdrew from the studies, died or were lost to follow‐up. We determined that four studies were at low risk of incomplete outcome bias (RFWP 1955; Haffejee 1990; Massell 1961; Stolzer 1955). One study had unclear risk of bias (Voss 2001), two were at high risk because participants were not analysed in the groups to which they were randomly assigned (CRFSG 1960; CRFSG 1965) and one study was at high risk because 16% of participants were lost (Dorfman 1961).
Selective reporting
Most studies did not report outcomes in the Methods and therefore were at unclear risk of reporting bias (CRFSG 1960; CRFSG 1965; Dorfman 1961; Haffejee 1990; Massell 1961; RFWP 1955). One study was at low risk (Stolzer 1955), and one was at high risk (Voss 2001) (Figure 1; Figure 2).
Overall risk of bias in most studies was high or unclear for all biases except allocation concealment and incomplete outcome bias, and most studies had unclear or high risk of bias for most types of bias.
Effects of interventions
We identified eight randomised controlled trials with dichotomous data describing cardiac outcomes in participants given anti‐inflammatory agents. All studies examined cardiac outcomes after at least one year. None of the studies undertook an intention‐to‐treat analysis or compared aspirin versus placebo.
Primary outcome ‐ cardiac disease after one year
Corticosteroids versus aspirin
Significant statistical heterogeneity was observed between trials (I2 = 69%, χ2 = 16.04, df = 5, P value = 0.0062; Analysis 1.1). Anti‐inflammatory agents studied included ACTH (corticotrophin), cortisone, dexamethasone, prednisone and aspirin. Pooled analysis showed no evidence of differences between steroidal agents and aspirin in preventing cardiac outcomes (RR 0.87, 95% CI 0.66 to 1.15; Analysis 1.1). Three studies showed some benefit of steroids over aspirin (Dorfman 1961; Massell 1961; Stolzer 1955), with only one (Dorfman 1961) showing statistical significance (RR 0.43, 95% CI 0.19 to 0.97). Three studies showed higher risk of cardiac disease at one year after treatment with corticosteroids compared with aspirin (CRFSG 1960; CRFSG 1965; RFWP 1955). One study stood out as the only study displaying some advantage for aspirin, although statistically insignificant, in reducing risk of cardiac disease compared with prednisone (RR 1.71, 95% CI 0.92 to 3.19) (CRFSG 1960).
Hydrocortisone versus aspirin
Dorfman 1961 was the sole randomised trial comparing oral hydrocortisone versus aspirin. A significant reduction in cardiac disease was reported at one year when hydrocortisone was compared with aspirin (RR 0.43, 95% CI 0.19 to 0.97). Although cardiac disease was significantly reduced with hydrocortisone, no significant difference between various treatment groups (hydrocortisone, aspirin, hydrocortisone and aspirin and no treatment) was observed in the development of new murmurs among participants with murmurs absent at the start of the study.
Prednisone versus aspirin
We used in this analysis data from two studies (CRFSG 1960; CRFSG 1965) with 212 participants. The pooled estimate showed no reduction in heart disease after one year when prednisone was used over aspirin (RR 1.13, 95% CI 0.52 to 2.45; Analysis 2.1).
Outcomes of participants with severe disease
Only five studies reported outcomes in participants with severe disease characterised by a grade 3 apical systolic murmur assessed clinically (CRFSG 1960; Dorfman 1961; Haffejee 1990; RFWP 1955; Voss 2001) or by use of echocardiographic criteria (Voss 2001) at the start of treatment. The number of participants with severe heart disease was small compared with the total number of participants. No evidence suggested a difference in reduction in heart disease after one year between corticosteroids and aspirin (three studies, 119 participants, RR 0.94, 95% CI 0.32 to 2.70; Analysis 1.2). Participants with severe heart disease in the CRFSG 1960 study failed to improve with prednisone or aspirin (risk ratio therefore was not estimable). One study in which only nine participants had severe disease showed hydrocortisone to be more beneficial in reducing the severity of cardiac disease compared with aspirin after one year (RR 0.17, 95% CI 0.03 to 1.00) (Dorfman 1961). The study with the greatest number of participants with severe disease (n = 89) found no statistically significant benefit for corticosteroid therapy compared with aspirin (RR 2.16, 95% Cl 0.97 to 4.81) (RFWP 1955).
Prednisone and intravenous immunoglobulin (IVIG) versus placebo
Effects of the two study medications were assessed separately. The benefit of using IVIG (Voss 2001) (RR 0.87, 95% CI 0.55 to 1.39) or prednisone (Haffejee 1990) (RR 1.78, 95% CI 0.95 to 3.34) over placebo to prevent cardiac disease in patients with acute rheumatic fever was not statistically significant.
Outcome of severe disease
Two studies confirmed that the effects of prednisone (RR 2.5, 95% Cl 0.42 to 14.83) or IVIG (RR 0.79, 95% Cl 0.31 to 1.96) were similar to those of placebo for participants with severe valvular disease (Haffejee 1990; Voss 2001).
Secondary outcomes
Outcomes such as reduction in hospital stay, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) were not reported by CRFSG 1960 and Massell 1961. CRFSG 1965 reported similar ESR levels in the prednisone and salicylate groups. Dorfman 1961 graphically depicted a prompt and striking drop in ESR with the use of hydrocortisone, which increased upon withdrawal of therapy. Aspirin also had a striking effect on ESR, but not as marked as that of hydrocortisone. Haffejee 1990 found ESR, CRP and sleeping pulse responses to be similar in prednisone and placebo groups. RFWP 1955 showed a more dramatic drop in ESR in the corticosteroid groups compared with the aspirin groups, but this increased sharply, although temporarily, in both corticosteroid groups after treatment was stopped, in contrast to a negligible change in the aspirin group. ESR eventually reached a similarly low level in all three groups at 13 weeks, and this level was maintained at one year. Corticotrophin had the most marked effect on reducing ESR compared with cortisone and aspirin in the Stolzer 1955 trial. Voss 2001 demonstrated that ESR measurements in the IVIG and placebo groups were similar at six weeks following the intervention. However, a significant difference was noted at one week and two weeks, with ESR significantly higher in the IVIG group.
Adverse effects of study drugs
Complications related to trial medication were not disclosed in five studies (CRFSG 1960; CRFSG 1965; Haffejee 1990; Stolzer 1955; Voss 2001). Untoward reactions were reported in the remaining three trials. Massell 1961 noted steroidal effects such as weight gain, moon face, buffalo hump, striae of the skin and acne. RFWP 1955 found that ACTH and cortisone groups had similar steroidal effects. Participants in the aspirin group experienced tinnitus, deafness, nausea and hyperventilation. Dorfman 1961 reported symptoms of nausea, emesis and tinnitus in some participants receiving aspirin. Two participants receiving hydrocortisone and aspirin developed gastric ulceration.
Concomitant therapy
Concomitant treatments were very similar in these trials. Bed rest, penicillin for 10 days, prophylactic antibiotics after 10 days, diuretics and digitalis (for heart failure) constituted standard auxiliary therapy in five studies (Dorfman 1961; Haffejee 1990; RFWP 1955; Stolzer 1955; Voss 2001). Bed rest may be an important co‐factor in the success of corticosteroid therapy. Although penicillin prophylaxis was provided in the CRFSG 1960 study, bed rest was not reported as part of the treatment regimen. Supplementary treatment was not indicated by CRFSG 1965 and Massell 1961.
Discussion
Summary of main results
None of the included trials showed consistent benefit with the use of corticosteroids, aspirin or IVIG in ameliorating or avoiding cardiac disease one year after treatment among participants with acute rheumatic fever. The combined analysis of corticosteroid agents ACTH, cortisone, prednisone, dexamethasone and hydrocortisone indicated a weak overall favourable outcome in preventing cardiac disease compared with aspirin (random risk ratio 0.87, 95% confidence interval 0.66 to 1.15). Thus, the data do not provide sufficient quantitative evidence to support the use of corticosteroid therapy, IVIG or aspirin to prevent cardiac disease in patients with acute rheumatic fever, if they offer no compelling reason for selection of corticosteroids over aspirin or vice versa.
Overall completeness and applicability of evidence
Both studies comparing anti‐inflammatory agents, oral prednisone and IVIG versus placebo identified no benefit from the use of active agents (Haffejee 1990; Voss 2001).
Two smaller studies (Dorfman 1961; Stolzer 1955) stood out because they confirmed benefit derived from the use of oral hydrocortisone and intramuscular cortisone, respectively, in preventing cardiac disease compared with aspirin. However, in the light of the absence of evidence of efficacy of aspirin (no trial comparing aspirin with placebo), the significance of these results is unknown.
Quality of the evidence
Type, dose and duration of corticosteroids and other medications varied between studies. Differences in outcomes between prednisone and hydrocortisone may be explained by the fact that prednisone is a less effective agent than hydrocortisone, or that corticosteroids need to be given for a longer period (12 weeks) to be effective, as was the case in the Dorfman 1961 study.
In at least three of the trials (CRFSG 1960; CRFSG 1965; RFWP 1955), participants 'crossed over' from one intervention to another. However, the meta‐analysis was performed by using the original randomisation groups in an attempt to adhere to 'intention‐to‐treat' principles.
Only five studies included participants with severe cardiac disease and documented outcomes following treatment with steroidal agents, IVIG or aspirin. Overall 152 participants were included in this subanalysis of only data from Dorfman 1961, and the fewest participants (n = 9) showed a statistical beneficial outcome following administration of oral hydrocortisone. The inclusion criteria of Dorfman 1961 did not include carditis, but methods of selection and assignment of cases appear to have resulted in satisfactory random assignment of participants with carditis to each group. Other severe outcomes such as death and the requirement for heart surgery were reported in only three studies (CRFSG 1960; Haffejee 1990; RFWP 1955). Investigators reported a total of eight deaths and five participants requiring surgery, with five deaths and four surgical interventions occurring in the corticosteroid group. The data refute a life‐saving role for corticosteroid therapy in patients with rheumatic carditis.
Secondary objectives of this review were to evaluate the effects of anti‐inflammatory treatment on hospital stay and on erythrocyte sedimentation rate and C‐reactive protein values. Reporting of these outcomes was too varied and inconsistent for adequate analysis. Corticosteroids appeared superior to aspirin in the rate at which ESR drops. However, this is not a compelling reason to choose one drug over another, as the significance of this rapid fall is not defined.
Five studies did not report on adverse events. The three studies that did so noted steroidal effects such as weight gain, moon face, buffalo hump, striae of the skin and acne and gastric ulceration. Participants in the aspirin group experienced tinnitus, deafness, nausea and hyperventilation.
Potential biases in the review process
Limitations of the review process and potential sources of bias in this review include lack of intention‐to‐treat analyses in the original studies, along with antiquated studies and the small number of trials available for analysis. Statistical heterogeneity between studies was significant. Major differences between studies were noted regarding patient diagnosis, inclusion criteria, interventions (various types, doses used, duration), outcomes (mainly type of outcome, such as systolic murmurs, diastolic murmurs and "carditis") and methodology, such as lack of blinding in some studies. For all these reasons, results should be interpreted with caution.
Agreements and disagreements with other studies or reviews
Prednisone continues to be recommended in contemporary reference textbooks for patients with carditis and acute rheumatic fever (Ayoub 1995; El‐Said 1998). The recommended duration of steroid treatment differs from that used in the studies mentioned above, for example, Ayoub 1995 suggests 2 mg/kg/d of prednisone for two weeks followed by gradual withdrawal over the following two weeks, and El‐Said 1998 recommends 2 mg/kg/d of prednisone or prednisolone for three to four weeks. In addition, Ayoub 1995 suggests that aspirin should be used in patients with carditis for four to eight weeks at a dose of 90 to 100 mg/kg/d, depending on clinical response. A more recent review (Kumar 2013) indicates that no significant change has been reported in the management of acute rheumatic fever over the past 50 years, and suggests that anti‐inflammatory agents such as aspirin and steroids, although they continue to be used to control rheumatic activity, do not cure rheumatic fever. The evidence base for these recommendations is clearly lacking.
Rheumatic fever and its valvular heart disease sequelae continue to be major causes of acquired heart disease in developing countries. Despite this, only one study was carried out in a developing country (Haffejee 1990), and the remaining seven studies took place in the USA, the United Kingdom and New Zealand. The number of randomised controlled trials examining treatment of acute rheumatic fever has diminished since the 1950s and 1960s in tandem with the decrease in prevalence of rheumatic heart disease in developed countries. The relevance and applicability to the current situation of data derived from studies performed 35 to 50 years ago in poorer countries remain pertinent because of the high incidence of rheumatic fever reported in industrialised countries during that period (Olivier 2000).
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Corticosteroids versus aspirin, Outcome 1 Cardiac disease after 1 year.

Comparison 1 Corticosteroids versus aspirin, Outcome 2 Outcome of severe cardiac disease after 1 year.

Comparison 2 Individual corticosteroids versus aspirin, Outcome 1 Cardiac disease after 1 year.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiac disease after 1 year Show forest plot | 6 | 907 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.15] |
| 2 Outcome of severe cardiac disease after 1 year Show forest plot | 3 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.32, 2.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiac disease after 1 year Show forest plot | 3 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.42, 1.76] |
| 1.1 Hydrocortisone versus aspirin | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.97] |
| 1.2 Prednisone versus aspirin | 2 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.52, 2.45] |

