Macrólidos versus placebo para el asma crónica
Resumen
Antecedentes
El asma es una enfermedad crónica en la que la inflamación de las vías respiratorias causa sibilancias sintomáticas, tos y dificultad respiratoria. Los macrólidos son antibióticos con actividades antimicrobianas y antiinflamatorias que se han administrado a largo plazo para controlar los síntomas del asma.
Objetivos
Evaluar los efectos de los macrólidos en comparación con placebo para el tratamiento del asma crónica.
Métodos de búsqueda
Se realizaron búsquedas en el registro especializado del Grupo Cochrane de Vías respiratorias (Cochrane Airways Group) hasta marzo de 2021. También se realizaron búsquedas manuales en las bibliografías de revisiones publicadas anteriormente y en resúmenes de congresos y se estableció contacto con los autores de los estudios. Se incluyeron en la búsqueda registros publicados en cualquier idioma.
Criterios de selección
Ensayos clínicos controlados aleatorizados (ECA) que incluyeron a niños y adultos con asma crónica tratados con macrólidos versus placebo durante cuatro o más semanas. Los desenlaces principales fueron las exacerbaciones que requirieron hospitalización, las exacerbaciones graves (exacerbaciones que requirieron visitas al servicio de urgencias [SU] o corticosteroides sistémicos, o ambos), las escalas de síntomas, el cuestionario de control del asma (CCA, con una puntuación de 0, totalmente controlado, a 6, gravemente descontrolado), el cuestionario de calidad de vida del asma (CCVA, con una puntuación de 1 a 7, en el que las puntuaciones más altas indican una mejor calidad de vida), las inhalaciones de medicación de rescate por día, el flujo espiratorio máximo matutino y vespertino (FEM; litros por minuto), el volumen espiratorio forzado en un segundo (VEF 1; litros), la hiperreactividad bronquial y la dosis de corticoides orales. Los desenlaces secundarios fueron los eventos adversos (incluida la mortalidad), los retiros, los eosinófilos en sangre, los eosinófilos en esputo, la proteína catiónica de eosinófilos (PCE) en suero y la PCE en esputo.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, examinaron todos los registros identificados en las búsquedas y luego revisaron el texto completo de todos los artículos potencialmente pertinentes antes de extraer los datos por duplicado de todos los estudios incluidos. Según el protocolo, se utilizó un modelo de efectos fijos. Se realizó un análisis de sensibilidad para los análisis con alta heterogeneidad (I2 mayor del 30%). Para evaluar la certeza del conjunto de evidencia se utilizó el método GRADE.
Resultados principales
Veinticinco estudios cumplieron los criterios de inclusión y asignaron al azar a 1973 participantes a recibir macrólidos o placebo durante al menos cuatro semanas. La mayoría de los estudios incluidos informaron datos de adultos (media de edad de 21 a 61 años) con asma persistente o grave, mientras que cuatro estudios incluyeron niños. Todos los participantes fueron reclutados en ámbitos ambulatorios. Los criterios de inclusión, las intervenciones y los desenlaces fueron muy variables.
La evidencia indica que los macrólidos probablemente proporcionan una reducción de tamaño moderado en las exacerbaciones que requieren hospitalizaciones en comparación con el placebo (odds ratio [OR] 0,47; intervalo de confianza [IC] del 95%: 0,20 a 1,12; estudios = 2, participantes = 529; evidencia de certeza moderada). Los macrólidos probablemente reducen las exacerbaciones que requieren visitas al SU o tratamiento con corticosteroides sistémicos (cociente de tasas [CT] 0,65; IC del 95%: 0,53 a 0,80; estudios = 4, participantes = 640; evidencia de certeza moderada). Los macrólidos podrían reducir los síntomas (medidos en escalas de síntomas) (diferencia de medias estandarizada [DME] ‐0,46; IC del 95%: ‐0,81 a ‐0,11; estudios = 4, participantes = 136; evidencia de certeza muy baja). Los macrólidos podrían dar lugar a una pequeña mejoría en el CCA (DME ‐0,17; IC del 95%: ‐0,31 a ‐0,03; estudios = 5, participantes = 773; evidencia de certeza baja). Los macrólidos podrían tener un efecto escaso o nulo sobre el CCVA (diferencia de medias [DM] 0,24; IC del 95%: 0,12 a 0,35; estudios = 6, participantes = 802; evidencia de certeza muy baja). En el caso del CCA y del CCVA, el efecto indicado de los macrólidos versus placebo no alcanzó una diferencia mínima clínicamente importante (DMCI, 0,5 para el CCA y el CCVA) (CCA: evidencia de certeza baja; CCVA: evidencia de certeza muy baja). Debido a la alta heterogeneidad (I2 > 30%), se realizaron análisis de sensibilidad sobre los resultados anteriores, que redujeron el tamaño de los efectos indicados al disminuir la ponderación de los estudios grandes y de calidad alta.
Los macrólidos podrían producir un efecto pequeño en comparación con placebo en la reducción de la necesidad de medicación de rescate (DM ‐0,43 inhalaciones/día; IC del 95%: ‐0,81 a ‐0,04; estudios = 4, participantes = 314; evidencia de certeza baja). Los macrólidos podrían aumentar el VEF1, pero el efecto está casi con seguridad por debajo de un nivel perceptible para los pacientes (DM 0,04 l; IC del 95%: 0 a 0,08; estudios = 10, participantes = 1046; evidencia de certeza baja). No fue posible agrupar los desenlaces para la hiperreactividad bronquial inespecífica o la dosis más baja tolerada de corticosteroides orales (en personas que requirieron corticosteroides orales al inicio). No hubo evidencia de una diferencia en los eventos adversos graves (incluida la mortalidad), aunque menos de la mitad de los estudios informaron el desenlace (OR 0,80; IC del 95%: 0,49 a 1,31; estudios = 8, participantes = 854; evidencia de certeza baja). El informe de los efectos adversos específicos fue demasiado inconsistente entre los estudios para un análisis significativo.
Conclusiones de los autores
La evidencia existente indica un efecto de los macrólidos en comparación con placebo sobre la tasa de exacerbaciones que requieren hospitalización. Los macrólidos probablemente reducen las exacerbaciones graves (que requieren una visita al SU o tratamiento con corticosteroides sistémicos) y podrían reducir los síntomas. Sin embargo, no es posible descartar la posibilidad de otros efectos beneficiosos o perjudiciales porque la calidad de la evidencia es muy baja debido a la heterogeneidad entre los pacientes y las intervenciones, la imprecisión y los sesgos de notificación. Los resultados se basaron principalmente en un ECA bien diseñado y con un poder estadístico suficiente, e indican que la azitromicina podría reducir la tasa de exacerbación y mejorar las puntuaciones de los síntomas en el asma grave.
La revisión destaca la necesidad de que los investigadores informen sobre los desenlaces con exactitud y de acuerdo con definiciones estándar. Los macrólidos pueden reducir la tasa de exacerbación en personas con asma grave. En los ensayos futuros se podría evaluar si este efecto se mantiene en todos los fenotipos de asma grave, la comparación con los nuevos fármacos biológicos, si los efectos persisten o disminuyen tras el cese del tratamiento y si los efectos se asocian con biomarcadores de infección.
PICO
Resumen en términos sencillos
¿Se deben administrar macrólidos para el asma crónica?
Punto principal: la evidencia existente indica un efecto beneficioso de los macrólidos en comparación con placebo para reducir las exacerbaciones que requieren hospitalización y las exacerbaciones graves (definidas como exacerbaciones que requieren visita al servicio de urgencias/tratamiento con corticosteroides sistémicos). El efecto de los macrólidos sobre otros desenlaces clínicos relevantes, como las escalas de síntomas y la función pulmonar, aún no está claro.
Antecedentes
El asma es una enfermedad crónica en la cual la inflamación de las vías respiratorias causa tos, sibilancias y problemas respiratorios. Probablemente hay diferentes razones para esta inflamación y motivos por los cuales persiste, y podrían ser necesarios diferentes tratamientos. La infección en los pulmones podría ser una causa, y los macrólidos son un tipo de antibiótico que se podrían utilizar a largo plazo como una forma de mejorar los síntomas en estas personas.
Cómo se respondió la pregunta
Se buscaron estudios sobre adultos o niños con asma a los que se les administró un macrólido o placebo (tratamiento simulado) durante al menos cuatro semanas para determinar si mejoraban los síntomas y disminuía la probabilidad de que tuvieran una crisis de asma, a menudo denominada "exacerbación". La búsqueda más reciente de estudios se realizó en marzo de 2021. Tras encontrar todos los estudios relevantes, se recogió información sobre las crisis de asma que requirieron ingreso hospitalario, las crisis de asma que necesitaron tratamiento con corticosteroides orales, las puntuaciones de los síntomas, el control del asma, la calidad de vida, varias medidas de la función pulmonar, la necesidad de inhaladores de rescate, los efectos secundarios graves y las medidas de la actividad del asma en sangre y esputo (moco).
Datos encontrados
Se encontraron 25 estudios, incluidos dos nuevos que se habían publicado desde la última búsqueda realizada en 2015. En general, poco más de 2000 pacientes recibieron un macrólido o placebo. Hubo muchos problemas en la forma en la que se describieron los estudios y en la forma en la que se informaron los datos, lo que hizo que se considerara que la evidencia en general fue de calidad baja, lo que disminuyó la confianza en la mayoría de los resultados. Los estudios fueron bastante diferentes entre sí, por ejemplo en cuanto a la gravedad del asma de las personas, el tipo de macrólido que se les administró y la duración del periodo de tratamiento.
Esta revisión mostró que los macrólidos fueron mejores que placebo en la reducción de las exacerbaciones y podrían ser beneficiosos en algunas personas para la mejoría de los síntomas del asma, el control del asma, la calidad de vida del asma y algunas medidas de la función pulmonar, pero no se sabe en qué medida ni para quiénes. Según un estudio bien realizado, el macrólido azitromicina podría tener algún beneficio en las personas con asma grave, pero en general los resultados de esta revisión no apoyan el uso de macrólidos para todo el asma de cualquier grado o gravedad. No hubo informes de efectos secundarios graves de los macrólidos, pero 16 estudios no informaron si se produjo alguno.
Authors' conclusions
Summary of findings
| Macrolide versus placebo for chronic asthma | ||||||
| Patient or population: adults and children with chronic asthma Drugs used were clarithromycin, azithromycin, roxithromycin and troleandomycin. Macrolide was given once or twice daily in most studies for 4–52 weeks (median 8 weeks). Treatment durations were calculated as weighted means of the studies included in each analysis. | ||||||
| Outcomesa | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed comparator risk** | Corresponding risk | |||||
| Placebo | Macrolide | |||||
| Exacerbation requiring hospitalisation Weighted mean study duration: 45 weeks | 61 per 1000 | 29 per 1000 (13 to 68) | OR 0.47 (0.20 to 1.12) | 529 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | — |
| Severe exacerbations (requiring ED visits or systemic steroids, or both) Number of people having ≥ 1 exacerbations requiring an ED visit or systemic steroids, or both. Classification varied across studies. Weighted mean study duration: 35 weeks | 410 per 1000 | 311 per 1000 | Rate ratio 0.65 | 640 | ⊕⊕⊕⊝ | — |
| Asthma symptoms – symptom scales (various scales; lower score = better) Weighted mean study duration: 10 weeks | The mean change in symptom scales in the intervention group compared to the control group was a reduction; 0.46 SD lower (0.81 SD lower to 0.11 SD lower) | — | 136 | ⊕⊝⊝⊝ | The SMD is a Cohen's effect size and can be interpreted as moderate (< 0.4 = small, 0.40–0.70 = moderate, > 0.70 = large). | |
| Asthma control (Asthma Control Questionnaire) Scored 0–6 (lower scores indicate improvement in asthma control) Weighted mean study duration: 34 weeks | The mean change in asthma control in the intervention group compared to the mean change in control group was a greater reduction;0.17 SD lower (0.31 SD lower to 0.03 SD lower) | — | 773 | ⊕⊕⊝⊝ | The SMD is a Cohen's effect size and can be interpreted as small. | |
| Asthma quality of life (AQLQ) Scored 1–7 (higher scores indicate improvement in quality of life) Weighted mean study duration: 35 weeks | The mean change on the AQLQ scale in the control group was an increase from baseline; 0.31 points higher** | The mean change in AQLQ in the intervention group compared to the mean change in the control group was an increase; 0.24 points higher (0.12 higher to 0.35 higher) | — | 802 | ⊕⊝⊝⊝ Very lowc,d,h | MCID: 0.5 points |
| Rescue medication (puffs/day) Weighted mean study duration: 17 weeks | The mean rescue medication use in the control group was 1.08 puffs/day** | The mean change in rescue medication puffs/day in the intervention group compared to the mean change in the control group was reduction; 0.43 fewer puffs (0.81 fewer to 0.04 fewer) | — | 314 | ⊕⊕⊝⊝ | — |
| FEV1 (L) Weighted mean study duration: 28 weeks | The mean FEV1 in the control group was 2.49 L** | The mean change in FEV1 (L) the intervention group compared to the control group was an increase; 0.04 L higher (0 L to 0.08 L higher) | — | 1046 | ⊕⊕⊝⊝ | Although there is no universally accepted MCID for FEV1 in asthma, variability within a single testing session can be up to 0.12 L (data from a mixed pool of respiratory patients; Enright 2004). |
| Serious adverse events (including mortality) Weighted mean study duration: 16 weeks | 93 per 1000 | 76 per 1000 | OR 0.80 | 854 | ⊕⊕⊝⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Assumed risk for continuous outcomes were calculated as weighted means of the scores in the control group. Note: Sutherland 2010 could not be included in the rescue medication or quality of life calculations because they reported only mean difference between groups; Brusselle 2013 was not included in the FEV1 calculation because it was the only change score; Cameron 2013 was not included in the asthma control or quality of life calculations because it was the only study reporting absolute endpoint scores rather than change from baseline. AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; ED: emergency department; FEV1: forced expiratory volume in one second; MCID: minimal clinically important difference; OR: odds ratio; RCT: randomised controlled trial; SD: standard deviation. | ||||||
| GRADE domains: study limitations, consistency of effect, imprecision, indirectness and publication bias. GRADE Working Group grades of evidence (Schünemann 2021). | ||||||
| aTwo primary outcomes (morning and evening peak expiratory flow) are not presented. Bronchial hyperresponsiveness could not be pooled in a meta‐analysis and is described narratively in the review. Studies did not report lowest tolerated oral corticosteroid dose well and there were no data to analyse. | ||||||
Background
Description of the condition
Asthma is an inflammatory disease of the airways characterised by chronic inflammation, bronchial hyperresponsiveness and paroxysmal attacks of wheezing. It affects people of every age, but frequently the disease occurs in childhood, especially in those who are atopic. It is estimated that asthma may affect between 1% and 18% of the general population, and it represents a significant cause of morbidity and costs for healthcare systems. Furthermore, the control of the disease is difficult to achieve in people with severe asthma, and even in people with milder asthma it may be hampered by poor adherence to treatments and lack of access to healthcare (GINA 2021).
Different phenotypes of the disease are recognised and under investigation. Current guidelines recommend tailoring asthma treatment according to a stepwise approach, considering severity of symptoms and response to treatment (GINA 2021).
Recently, the role of short‐acting bronchodilators in intermittent asthma was revised, and a combination of inhaled corticosteroids (ICS) and formoterol as needed is now recommended in people with mild asthma. Persistent asthma is treated with regular ICS, longer‐acting bronchodilators, or both (GINA 2021). More recent therapies include anti‐leukotrienes in mild‐to‐moderate asthma, humanised antibodies targeting immunoglobulin E (omalizumab), interleukin (IL)‐5 (mepolizumab, reslizumab, benralizumab), and IL‐4/‐13 (dupilumab), which are currently only recommended in severe asthma with markers of a phenotype likely to respond (e.g. raised blood eosinophils for the anti‐IL‐5 agents) (GINA 2021; Olin 2014).
Description of the intervention
Macrolides are a class of antibiotics that are widely used in the treatment of various infectious diseases, including respiratory tract infections (Alvarez‐Elcoro 1999). The first studies on macrolides in people with asthma suggested a steroid‐sparing effect (Nelson 1993), while other reports have demonstrated an anti‐inflammatory effect of this class of antibiotics, whereby macrolides seem to decrease bronchial hyperresponsiveness associated with eosinophilic inflammation (Amayasu 2000). A host‐directed anti‐inflammatory effects was also postulated (Spagnolo 2013). Macrolides are effective in the long‐term treatment of cystic fibrosis, diffuse panbronchiolitis and chronic obstructive pulmonary disease (COPD), and they are not associated with an increased risk of adverse events (Cai 2011; Spagnolo 2013).
However, a potential drawback of longer‐term antibiotic use for asthma is the development of bacterial resistance by strains that normally colonise the airways. Macrolide use in healthy volunteers led to pharyngeal carriage of macrolide‐resistant streptococci (Malhotra‐Kumar 2007), which is of particular concern for the wider community. Similar concerns were raised by studies in COPD (Brill 2015). Furthermore, the potential for arrhythmias due to QTc prolongation, potential ototoxicity and hepatotoxicity from macrolide long‐term use was highlighted in a British Thoracic Society document (BTS 2020).
How the intervention might work
Macrolides have anti‐inflammatory and antimicrobial properties that may improve asthma symptoms in two ways: by reducing airways inflammation directly and by controlling intracellular infection, which may trigger and maintain inflammation (Black 1997; Black 2000; Kawasaki 1998). Their anti‐inflammatory potential has been linked to their action on pro‐inflammatory cytokines and chemokines causing inflammation, which was highlighted by the results of the previous versions of this systematic review (Richeldi 2002; Richeldi 2005; Kew 2015). In vivo and in vitro studies of human and animal models have demonstrated that macrolides suppress the production of cytokines such as ILs and inhibit neutrophil adhesion to epithelial cells, the respiratory burst of neutrophils and the secretion of mucous from human airways (Adachi 1996; Hinks 2021; Konno 1994; Koyama 1998).
Older macrolides such as troleandomycin were investigated for a steroid‐sparing effect, related to reduced hepatic glucocorticoid metabolism (Nelson 1993).
The potential benefit of their antimicrobial action for people with asthma was suggested after observational studies identified intracellular bacterial infection (i.e. Chlamydophila pneumoniae or Mycoplasma pneumoniae) as a possible trigger of bronchial inflammation (Kraft 1998). Gencay 2001 subsequently demonstrated that people with asthma had a higher frequency of C pneumoniae antibodies than matched controls. Longitudinal studies showed no clear effect of infection with C pneumoniae on the incidence of asthma, but people who had an infection and developed asthma showed a faster decline in lung function (Pasternack 2005). Furthermore, in children with asthma, M pneumoniae detection in respiratory samples was associated with poorer asthma control (Wood 2013). Studies in animal models seem to point to an important role of the infection with C pneumoniae in the early phases of life in the pathogenesis of severe asthma (Essilfie 2015; Hansbro 2014).
Why it is important to do this review
Macrolides represent a relatively inexpensive intervention that may improve control of inflammation and clinical outcomes in people with chronic asthma.
Objectives
To assess the effects of macrolides compared with placebo for managing chronic asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel and cross‐over randomised controlled trials (RCTs).
Types of participants
Children and adults with chronic asthma.
Types of interventions
Macrolides, administered for four or more weeks versus placebo. We pooled data from studies comparing different macrolide therapies.
Types of outcome measures
Primary outcomes
-
Exacerbations requiring hospitalisation.
-
Severe exacerbations (defined as requiring an emergency department (ED) visits or short‐course of systemic steroids, or both).
-
Asthma symptoms, control and quality of life scores.
-
Asthma medication requirements (need for rescue medications).
-
Lung function, including morning and evening peak expiratory flow (PEF) and forced expiratory volume in one second (FEV1).
-
Non‐specific bronchial hyperresponsiveness (to histamine or methacholine).
-
Lowest tolerated oral corticosteroid dose (in people requiring oral corticosteroids at baseline).
Secondary outcomes
-
Number and type of serious adverse events (including mortality).
-
Number of study withdrawals.
-
Eosinophil count in peripheral blood samples, sputum samples or both.
-
Eosinophilic cationic protein (ECP) measurements in serum and sputum.
Search methods for identification of studies
Electronic searches
Search methods used in the previous version of this review are detailed in Appendix 1. The previously published version included searches up to April 2015. The search period for this update was April 2015 to 31 March 2021.
We searched the Cochrane Airways Trials Register on 31 March 2021. At that time the Register contained studies identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org), all years to Issue 3, 2021;
-
weekly searches of MEDLINE (OvidSP), 1946 to 26 March 2021;
-
weekly searches of Embase (OvidSP), 1974 to week 12 2021;
-
monthly searches of PsycINFO (OvidSP), 1967 to March week 4 2021;
-
monthly searches of CINAHL (EBSCO) (Cumulative Index to Nursing and Allied Health Literature), 1937 to 15 March 2021;
-
monthly searches of AMED (EBSCO) (Allied and Complementary Medicine), all years to 11 March 2021;
-
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 2. See Appendix 3 for search terms used to identify studies for this review. We did not restrict our search by language or type of publication.
We also searched the following trials registries on 31 March 2021:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Searching other resources
We surveyed review articles and bibliographies identified from the primary papers for additional references and RCTs.
Data collection and analysis
Selection of studies
Two review authors (KU and GF) independently screened the abstracts of articles identified using the search strategy above, retrieving the full text for articles that appeared to fulfil the inclusion criteria. Two review authors (KU and GF) independently reviewed and categorised each article identified as included or excluded. When there was disagreement or doubt, a third review author (KK) assessed the article and helped to reach a consensus. We presented a PRISMA diagram to illustrate the flow of studies through the selection process (Moher 2009).
Data extraction and management
We used a data collection form to collect study characteristics and outcome data. We piloted the form on at least one study in the review. Two review authors (KU and GF) extracted the following study characteristics from included studies, when available.
-
Methods: study design, total duration of study, details of any run‐in period, number of study centres and location, study setting, withdrawals and date of study.
-
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (KU and GF) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by involving a third review author (KK). Two review authors (KK and KU) transferred data into the Review Manager 5 (Review Manager 2014). Three review authors (KU, LG and GF) double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two review authors (KK and GF) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion and by involving another review author (KU). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
Each potential source of bias was graded as high, low or unclear and justified with a quote from the study report in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from for a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trial list, we noted this in the risk of bias table.
For treatment effects, we considered the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR), incidence rate data as rate ratio (RaR) and continuous data as mean difference (MD; where studies used the same scales) or standardised mean difference (SMD; where studies used different scales). We entered data presented as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges. We analysed data from cross‐over trials using generic inverse variance (GIV). We pooled results from cross‐over trials and parallel trials. Where studies presented raw data and adjusted analyses (e.g. accounting for baseline differences), we used the adjusted analyses.
We undertook meta‐analyses only where meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense and made decisions about this by consensus among review authors.
Where a trial reported multiple arms, we included only the relevant arms but reported all arms in the Characteristics of included studies table. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
If change from baseline and endpoint scores were available for continuous data, we used change from baseline unless most studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end‐of‐study treatment measurement.
When both an analysis using only participants who completed the trial and an analysis that imputed data for participants who were randomised but did not provide endpoint data (e.g. last observation carried forward) were available, we used the latter.
Unit of analysis issues
We combined events data using ORs or RaRs (number of participants or number of events) according to which measure would allow us to include the most studies. We used ORs for exacerbations requiring hospitalisation, serious adverse events (including mortality) (Peto OR) and withdrawal. We used RaRs for severe exacerbations (defined as exacerbations requiring ED visits or systemic steroids, or both). For continuous data in cross‐over trials, we entered data using GIV from suitable adjusted analyses to account for the trial's design.
Dealing with missing data
We assessed the potential for bias in each trial as a result of participants dropping out of the intervention prematurely. Where this was thought to introduce serious bias, we removed the studies in a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified high heterogeneity (e.g. I2 greater than 30%), we reported it and performed a sensitivity analysis with a random‐effects model.
Assessment of reporting biases
We were unable to pool more than 10 trials for any of the primary outcomes, so were unable to examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a fixed‐effect model for all analyses, as we expected limited variation in effects due to differences in study populations and methods.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses based on serological response or positivity to polymerase chain reaction (PCR) for C pneumoniae.
Sensitivity analysis
We performed a sensitivity analysis with a random‐effects model in case of high heterogeneity (I2 greater than 30%).
Summary of findings and assessment of the certainty of the evidence
The summary of findings table included the following outcomes: number of exacerbations requiring hospitalisation; severe exacerbations (requiring ED visits or short‐course systemic steroids, or both); asthma symptoms (including symptom scores, asthma control and Asthma Quality of Life Questionnaire (AQLQ)); asthma medication requirements (as reliever); lung function (including morning and evening PEF and FEV1); non‐specific bronchial hyperresponsiveness; serious adverse events; withdrawal; blood and sputum eosinophils; and ECP in serum and sputum.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. Except for serious adverse events, we did not perform GRADE ratings on the secondary outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the certainty of the evidence using footnotes and made comments to aid reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The literature search of previous versions of this review up to April 2015 identified 137 citations. Out of the 38 references identified in the search between 2007 and 2015, duplicate sifting of the titles and abstracts alone identified 33 potentially eligible studies for inclusion in the systematic review. Among them, review authors were concordant in identifying 16 RCTs to be included in the previous update of the systematic review (Kew 2015). Six of the 16 RCTs were identified in a previous meta‐analysis (Tong 2015); these Chinese trials were only listed in China's biomedical databases. The authors of this review were able to confirm key study characteristics in order to include them, although risk of bias could not be properly assessed.
For this updated review, the search extended to March 2021. We identified 55 new references. Seven titles referring to abstracts presented at congresses were duplicates of other studies already included in the review, while one title (Gibson 2019) was a subgroup analysis of another RCT (Gibson 2017) included in this update, leaving 20 references for screening. We excluded four based on the abstract alone and 13 after screening the full‐text of the original manuscripts, leaving two new studies eligible for inclusion in the systematic review and meta‐analyses (Wan 2016; Gibson 2017). One study is ongoing, no results available. See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables. The study flow of the new included studies is presented in Figure 1.
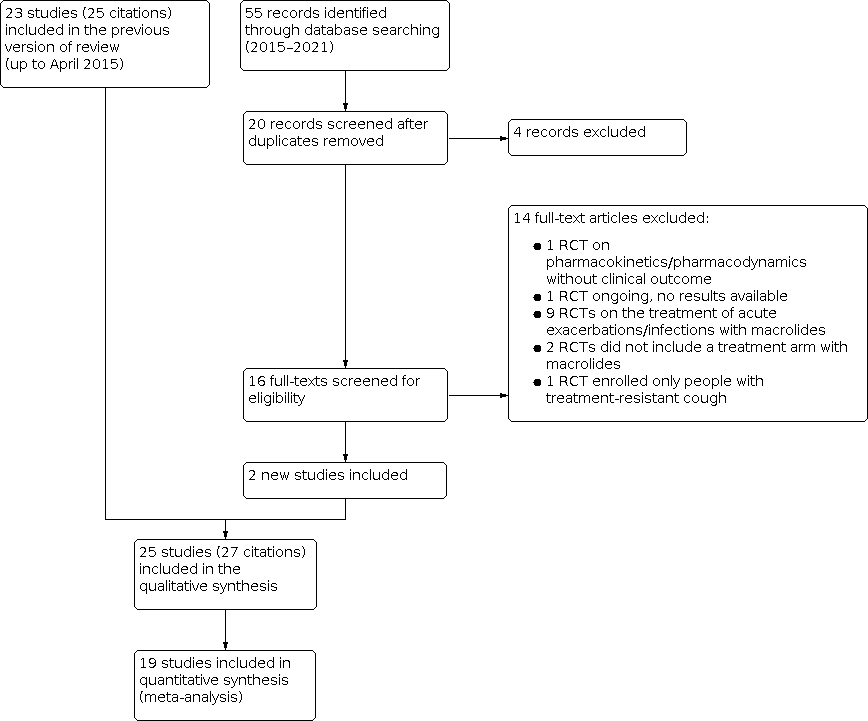
Study flow diagram.
Included studies
We included 25 RCTs (Amayasu 2000; Belotserkovskaya 2007; Black 2001; Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; He 2009; Kamada 1993; Kapoor 2010; Kostadima 2004; Kraft 2002; Nelson 1993; Piacentini 2007; Shoji 1999; Simpson 2008; Strunk 2008; Sutherland 2010; Wan 2016; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013). For brief descriptions of the included studies, refer to the Characteristics of included studies table. For a summary of study characteristics and a narrative on the main results of each study, see Table 1 and Appendix 4.
| Study ID | Country | Number of participants | Design | Duration (weeks) | Macrolide dose and schedule | Mean age (years) | % Male | % on ICS | % Predicted FEV1 |
|---|---|---|---|---|---|---|---|---|---|
| Japan and USA | 17 | C, R, DB, PC | 8 | Clarithromycin 200 mg twice daily | 38.5 | 52.9 | 0.0 | 76.2 | |
| Russia | 51 | P, R | 8 | Azithromycin (unknown dose) | NR | NR | NR | NR | |
| Multinational | 219 | P, R, DB, PC | 6 | Roxithromycin 150 mg twice daily | 41.0 | 47.5 | 80.8 | 77.1 | |
| Belgium | 109 | P, R, DB, PC | 26 | Azithromycin 250 mg once daily for 5 days then 3 times per week | 53.0 (median) | 38.5 | 100a | 82.5 | |
| UK | 77 | P, R, DB, PC | 12 | Azithromycin 250 mg once daily | 44.6 | 48.1 | 85.7 | 78.3 | |
| Australia | 420 | P, R, DB, PC | 48 | Azithromycin 500 mg 3 times per week | 60.5 | 39.3 | 99.8 | 72.9 | |
| USA, Canada | 45 | P, R, DB, PC | 6 | Azithromycin 600 mg once daily for 3 days then weekly | 47.7 | 48.9 | 80.0 | NR | |
| USA | 75 | P, R, DB, PC | 12 | Azithromycin 600 mg once daily for 3 days then weekly | 46.6 | 32.0 | 72.0 | NR | |
| China | 40 | P, R, PC | 12 | Azithromycin 250 mg twice weekly | 34.5 | NR | NR | NR | |
| USA | 19 | P, R, DB, PC | 12 | Troleandomycin 250 µg once daily + OCS | 12.5 | 63.2 | 100 | NR | |
| India | 40 | C, R, DB, PC | 6 | Roxithromycin 150 mg once daily | NR | NR | NR | NR | |
| Greece | 75 | P, R, DB, PC | 8 | Clarithromycin 250 mg twice daily or 3 times daily | 43.7 | 47.1 | 100 | 85.3 | |
| USA | 55 | P, R, DB, PC | 6 | Clarithromycin 500 mg twice daily | 33.4 | 49.1 | 32.7 | 69.3 | |
| USA | 75 | P, R, DB, PC | 52 | Troleandomycin 250 mg once daily + OCS | NR | 33.3 | 0 | NR | |
| Italy | 16 | P, R, DB, PC | 8 | Azithromycin 10 mg/kg once daily 3 days per week | 13.4 | 75 | 100 | 78.9 | |
| Japan | 14 | C, R, DB, PC | 8 | Roxithromycin 150 mg twice daily | 39.6 | 42.9 | 0.0 | 75.0 | |
| Australia | 45 | P, R, DB, PC | 8 | Clarithromycin 500 mg twice daily | 57.6 | 48.9 | NRb | 70.7 | |
| USA | 55c | P, R, DB, PC | 30 | Azithromycin 250 mg or 500 mg once daily | 11.2 | 58.2 | 100a | 101.9 | |
| USA | 92 | P, R, DB, PC | 16 | Clarithromycin 500 mg twice daily | 39.4 | 43.5 | NR | 76.0 | |
| Taiwan | 58 | P, R, PC | 4 | Clarithromycin 5 mg/kg daily | 10.1 | 60.3 | 100 | 79.7 | |
| China | 45 | P, R, PC | 8 | Clarithromycin 500 mg twice daily | NR | NR | NR | NR | |
| China | 58 | P, R, PC | 52 | Azithromycin 250 mg twice weekly | 29.0 | NR | NR | NR | |
| China | 210 | P, R, PC | 12 | Roxithromycin 150 mg twice daily | 34.1 | NR | NR | NR | |
| China | 40 | P, R, PC | 4 | Roxithromycin 150 mg twice daily | 38.5 | NR | NR | NR | |
| China | 60 | P, R, PC | 9 | Azithromycin 100 mg once daily | NR | NR | NR | NR |
C: cross‐over; DB: double‐blind; FEV1 : forced expiratory volume in one second; ICS: inhaled corticosteroid; NR: not reported; OCS: oral corticosteroids; P: parallel; PC: placebo‐controlled; R: randomised.
aAll participants were taking long‐acting beta2‐agonist + ICS combination.
b82.2% were taking long‐acting beta2‐agonist + ICS combination.
c19 participants were not included in the review because they were randomised to a third group who received montelukast.
The 25 included studies reported a great variability in type of participants (ranging from intermittent aspirin‐induced asthma to severe asthma), interventions (different type of macrolides, administration scheme and doses in most of the studies) and outcomes recorded.
Design
All studies were RCTs using placebo controls, and most were described as double‐blind. There were 22 parallel group studies and three cross‐over studies (Amayasu 2000; Kapoor 2010; Shoji 1999). Median study duration was 15.2 weeks (range four to 52 weeks). Two studies were reported in the form of abstracts from congresses, with a very limited amount of data available (Belotserkovskaya 2007; Kapoor 2010); the results of one new included study could not be used due to very poor reporting (Wan 2016), and only basic information was available from six Chinese studies (He 2009; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013) included in the previous version of this review (Kew 2015).
Participants
The studies included 1991 participants of whom 1973 were relevant to this review (Strunk 2008 included a third group of 18 people receiving a treatment that was outside the protocol of this review). All participants had an asthma diagnosis, which was generally established according to the guidelines in use at the time of the studies (ATS 1987; GINA 1995; GINA 2002; GINA 2007; GINA 2010; GINA 2014); these are similar to the current international guidelines for what concerns main diagnosis and grading of severity of asthma (GINA 2021).
Four studies assessed the effects of macrolide treatment in children with asthma (Kamada 1993; Piacentini 2007; Strunk 2008; Wan 2016), and the rest recruited adults with asthma (aged over 18 years). The studies varied according to GINA 2014/GINA 2020/GINA 2021 criteria for asthma severity, and often there was very little information about baseline severity.
Most studies included participants with persistent mild‐to‐severe asthma, while one included participants with mild asthma (step 1 according to GINA 2021) (Amayasu 2000), and one used people with aspirin‐induced intermittent asthma (Shoji 1999).
Five studies investigated the role of macrolides in people with evidence of C pneumoniae or M pneumoniae infection, based on serological (Black 2001; Hahn 2006; Wan 2016) or molecular (Kraft 2002; Sutherland 2010) methods. The remaining studies did not investigate the presence of these co‐infections or of other concomitant co‐infections, although we could not confirm this in the non‐English language papers. Cameron 2013 investigated the effect of macrolides in adult smokers with persistent asthma, while Brusselle 2013, Simpson 2008, and Gibson 2017 considered the effect of macrolides in people with eosinophilic and non‐eosinophilic asthma.
Interventions
Five studies compared roxithromycin with placebo (Black 2001; Kapoor 2010; Shoji 1999; Xiao 2013; Yan 2008); seven studies compared clarithromycin with placebo (Amayasu 2000; Kostadima 2004; Kraft 2002; Simpson 2008; Sutherland 2010; Wang 2014; Wan 2016); 11 studies investigated the effect of azithromycin (Belotserkovskaya 2007; Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; He 2009; Piacentini 2007; Strunk 2008; Wang 2012; Zhang 2013), and two studies assessed the effects of troleandomycin in addition to oral steroid therapy as part of a steroid‐tapering protocol (Kamada 1993; Nelson 1993).
Outcomes
Six studies did not appear in any of the quantitative syntheses (Belotserkovskaya 2007; Black 2001; Kapoor 2010; Wan 2016; Wang 2012; Zhang 2013), and two more only contributed to the bronchial hyperresponsiveness summary of results and withdrawal (Piacentini 2007; Simpson 2008).
Two studies reported data on exacerbations requiring hospitalisation (Brusselle 2013; Gibson 2017), and four on severe exacerbations (defined as exacerbations requiring ED visits or systemic steroids, or both) as an outcome, but the definition of 'severe exacerbation' used in the different studies was variable and sometimes unclear (Brusselle 2013; Gibson 2017; Kostadima 2004; Strunk 2008). The meta‐analysis of Tong 2015 did not include exacerbations as an outcome but explicitly confirmed that the Chinese studies did not report this outcome either. Data from these studies only contributed to one meta‐analysis (FEV1). We narratively summarised data that could not be meta‐analysed for the relevant outcomes.
Most studies reported measures of symptoms, asthma control or quality of life, but the analyses were limited by the way data were reported and by the use of different scales. We did not consider a meta‐analysis of all these measures to be valid or the subsequent results to be interpretable in any meaningful way, so we chose only to meta‐analyse those that we knew would be similar. We used SMDs for the 'symptom scale' meta‐analysis, which still made the effect and its precision difficult to interpret.
Four studies reported data about change in rescue medication as puffs per day in a way that could be included in meta‐analysis (Brusselle 2013; Cameron 2013; Hahn 2006; Sutherland 2010).
Most of the studies reported measures of lung function such as FEV1 or PEF, but only 10 reported data for FEV1 (Amayasu 2000; Cameron 2013; Gibson 2017; He 2009; Kraft 2002; Shoji 1999; Sutherland 2010; Wang 2014; Xiao 2013; Yan 2008), four reported morning PEF (Brusselle 2013; Cameron 2013; Kamada 1993; Sutherland 2010), and three reported for evening PEF (Brusselle 2013; Kamada 1993; Sutherland 2010) that could be pooled. There were some issues with selective reporting that prevented studies from being included in the analyses, such as data only being presented graphically or without a measure of variance (e.g. Black 2001; Wan 2016). It was often unclear when the measures were taken (i.e. pre‐ or post‐bronchodilator), but when the information was available, we recorded it in the analysis footnotes. Brusselle 2013 reported percentage FEV1, but their data could not be combined with the other studies, which reported the outcome in litres. We combined the data made available to us from Tong 2015 for He 2009, Wang 2014, Xiao 2013, and Yan 2008. We were also provided with data for peak flow for Wang 2014, Xiao 2013, and Yan 2008, but the data were a different order of magnitude to the other studies, and it did not make sense to pool them.
Nine studies considered bronchial hyperresponsiveness, but there was variation in the measures used and the way the data were reported, which meant it was not possible to meta‐analyse the data (Amayasu 2000; Cameron 2013; Kamada 1993; Kostadima 2004; Nelson 1993; Piacentini 2007; Shoji 1999; Simpson 2008; Sutherland 2010).
Most studies considered adverse events, but only eight explicitly reported serious adverse events (Amayasu 2000; Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; Kamada 1993; Sutherland 2010). While it was not ideal to include the dichotomous cross‐over data without adjusting them to account for matched pairs, there were no events in Amayasu 2000, so it did not contribute to the pooled effect.
Ten studies reported study withdrawal (Brusselle 2013; Gibson 2017; Hahn 2006; Hahn 2012; Kamada 1993; Kostadima 2004; Nelson 1993; Simpson 2008; Strunk 2008; Sutherland 2010).
Eight studies reported the effect of macrolides on markers of inflammation related to asthma activity, but they used different measures, which could not be pooled in one analysis (Amayasu 2000; Cameron 2013; Kraft 2002; Nelson 1993; Piacentini 2007; Shoji 1999; Simpson 2008; Yan 2008). There were also some issues with data accuracy or incomplete reporting that reduced our confidence in the reliability of the data. The separate analyses include very small participant numbers, mostly from the two cross‐over studies.
Two studies considered the steroid‐sparing effect of macrolides (Kamada 1993; Nelson 1993).
Excluded studies
We excluded 14 studies from the review after viewing the full papers. Reasons for exclusion are reported in the Characteristics of excluded studies table and Figure 1.
Previous versions of this systematic review excluded 17 studies after reading the full papers (Kew 2015; Richeldi 2005), so a total of 31 studies are excluded.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We found one ongoing study (NCT02517099).
Risk of bias in included studies
There was considerable uncertainty relating to study methodology due to insufficient reporting in the published reports. This was particularly true for the selection bias domains, but also for blinding of outcome assessment and attrition bias. We had concerns about incomplete and selective reporting of the results for most of the studies and generally considered there to be a high risk for bias because only a few studies reported data in a way that could be pooled in meta‐analysis. Summaries of the risk of bias judgements for each study are presented in Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Pharmaceutical industries financed at least five included studies (Amayasu 2000; Cameron 2013; Hahn 2006; Hahn 2012; Kamada 1993); which could raise the risk of publication bias; this could not be ascertained in the non‐English language papers. The authors of Tong 2015 provided us with information about study quality for the studies that were not available in English.
Allocation
We deemed 13 studies at low risk of bias for random sequence generation, including seven of the English language studies (Brusselle 2013; Gibson 2017; Hahn 2006; Hahn 2012; Piacentini 2007; Simpson 2008; Wan 2016) and six in Tong 2015 (He 2009; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013). For the rest, the methods for random sequence generation was unclear.
Only five studies were at low risk for adequate allocation concealment (Brusselle 2013; Gibson 2017; Hahn 2006; Hahn 2012; Wan 2016); the studies from the Tong 2015 review were not assessed for this criterion because it is not considered in the Jadad 1996 system, so we had to rate those studies as unclear, and the 14 other studies did not adequately describe the methods used.
Blinding
Most studies described as double‐blind and placebo‐controlled contained adequate descriptions of the blinding of participants and personnel, but methods were unclear in nine studies (He 2009; Wan 2016; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013, including two in an abstract form: Belotserkovskaya 2007; Kapoor 2010). Blinding of outcome assessment was adequate in seven studies (Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; Kraft 2002; Piacentini 2007; Sutherland 2010).
The same study that we rated high for performance bias also carried a high risk for detection bias (Strunk 2008).
Incomplete outcome data
Six studies had a high risk of attrition bias (Gibson 2017; Hahn 2006; Hahn 2012; Kostadima 2004; Nelson 1993; Sutherland 2010), five carried a low risk (Black 2001; Brusselle 2013; Kraft 2002; Simpson 2008; Strunk 2008), and the risk was unclear for the other 14 studies.
Selective reporting
We considered three studies at low risk of bias (Brusselle 2013; Hahn 2006; Hahn 2012). We judged eight studies at high risk of selective reporting (Belotserkovskaya 2007; Black 2001; Cameron 2013; Kamada 1993; Kapoor 2010; Simpson 2008; Strunk 2008; Sutherland 2010). This was mostly due to insufficient reporting of numerical data, which meant they could not be pooled in meta‐analysis. We rated 14 studies as unclear for this domain, including the six non‐English language studies, which we could not assess fully.
Overall, it is likely that reporting biases had a significant effect on the completeness of the meta‐analyses in this systematic review.
Other potential sources of bias
We judged Kamada 1993 to be at high risk of bias because the report showed significant baseline imbalances between groups, and this may have been an issue in some of the other trials that included very small number of participants. We judged eight studies to be at unclear risk of bias (Hahn 2012; He 2009; Kapoor 2010; Kostadima 2004; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013).
Effects of interventions
See: Summary of findings 1 Macrolides compared to placebo for chronic asthma
We present the data with the order of outcomes listed in the methods. Evidence certainty was varied. We downgraded most for indirectness due to differences in the study populations and the way outcomes were defined, and for risk of bias due to uncertainty of randomisation procedures and high risk of attrition bias. Appendix 4 presents a narrative on each study, except for the six that we could not assess fully (He 2009; Wang 2012; Wang 2014; Xiao 2013; Yan 2008; Zhang 2013).
Primary outcomes
Exacerbations requiring hospitalisation
Two studies reported exacerbations requiring hospitalisation, with 24 events (four recorded in Brusselle 2013; 20 recorded in Gibson 2017). The meta‐analysis suggests macrolides make a moderately sized but non‐significant reduction in exacerbation requiring hospitalisation compared to placebo (OR 0.47, 95% CI 0.20 to 1.12; I2 = 0%; participants = 529; Analysis 1.1; Figure 4) (95% CI cross the line of zero effect). One study is equivocal and this effect is driven by the larger study. The evidence was of moderate certainty.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.1 Exacerbation requiring hospitalisation.
Severe exacerbations
Four studies reported data on severe exacerbations (defined as exacerbations requiring ED visits or short‐course of systemic steroids, or both) (Brusselle 2013; Gibson 2017; Kostadima 2004; Strunk 2008). The pooled effect of these studies showed a benefit of macrolides over placebo (RaR 0.65, 95% CI 0.53 to 0.80; I2 = 37%; participants = 640; Analysis 1.2; Figure 5). The results were mostly driven by the data from Gibson 2017 (weight 81.6%). Apart from this study, the evidence from the other studies was very low quality, being downgraded for indirectness and imprecision. Overall the evidence was of moderate certainty.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.2 Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids.
Asthma symptoms, control and quality of life scores
Symptom scales used across studies varied and were mostly not psychometrically validated. Data from four studies could be combined in meta‐analysis (Amayasu 2000; Hahn 2006; Hahn 2012; Kamada 1993). There was suggestion of a benefit of macrolides compared with placebo (SMD −0.46, 95% CI −0.81 to −0.11; I2 = 70%; participants = 156; Analysis 1.3; Figure 6). We downgraded the evidence for high heterogeneity (I2 = 70%), use of scales that were not psychometrically validated and small numbers in the analysis, meaning the evidence was of very low certainty.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.3 Symptom scales.
Five studies reported measures of asthma control, mostly the Asthma Control Questionnaire (ACQ) (Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2012; Sutherland 2010). There was a small benefit of macrolide over placebo in reducing symptoms (SMD −0.17, 95% CI −0.31 to −0.03; I2 = 35%; participants = 773; Analysis 1.4; Figure 7), although the decrease in the symptom score did not reach the minimal clinically important difference (MCID) (0.5 for the ACQ, Juniper 2005). We considered the evidence to be low after downgrading for uncertainties with randomisation procedures and different populations with regards to severity of asthma.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.4 Asthma Control.
Six studies reported quality of life measured with the AQLQ (Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; Sutherland 2010). There was an improvement of quality of life with macrolides over placebo, but it was under the MCID (MD 0.24, 95% CI 0.12 to 0.35; I2 = 55%; participants = 802; Analysis 1.5; Figure 8) (MCID: 0.5; Juniper 1994). We considered the evidence to be very low certainty after downgrading it for imprecision, uncertainties with randomisation procedures and different populations with regards to severity of asthma.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.5 Asthma Quality of Life Questionnaire (AQLQ).
Need for rescue medications
Four studies reported need for rescue medication (Brusselle 2013; Cameron 2013; Hahn 2006; Sutherland 2010). Analysis indicated macrolides reduced in the need for rescue medications compared to placebo (MD −0.43 puffs per day, 95% CI −0.81 to −0.04; I2 = 0%; participants = 314; Analysis 1.6; Figure 9). The evidence was low certainty, being downgraded for uncertainties in the randomisation procedure and indirectness.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.6 Rescue medication puffs/day.
Lung function (morning and evening peak expiratory flow and forced expiratory volume in one second)
Four studies reported morning PEF (Brusselle 2013; Cameron 2013; Kamada 1993; Sutherland 2010), and three studies reported evening PEF (Brusselle 2013; Cameron 2013; Kamada 1993; Sutherland 2010). The data for morning and evening PEF did not suggest a benefit of macrolide over placebo, and the evidence was very low certainty (morning PEF: MD 1.60 L/minute, 95% CI −10.35 to 13.56; I2 = 0%; participants = 289; Analysis 1.7; Figure 10; evening PEF: MD 1.00, 95% CI −13.65 to 15.65; I2 = 0%; participants = 212; Analysis 1.8; Figure 11). The evidence for both measures was downgraded due to issues with risk of bias, indirectness and imprecision.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.7 Morning PEF (L/minute).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.8 Evening PEF (L/minute).
Tong 2015 provided data for three additional studies, but it was not clear if they were morning or evening measurements (Wang 2014; Xiao 2013; Yan 2008). Moreover, the data were in a different order of magnitude to the other studies and had been combined using SMD, so we could not combine them. These three studies showed a benefit of macrolide over placebo, but the method of analysis meant it was difficult to contextualise.
Ten studies reported data on FEV1, that could be aggregated in a meta‐analysis (Amayasu 2000; Cameron 2013; Gibson 2017; He 2009; Kraft 2002; Shoji 1999; Sutherland 2010; Wang 2014; Xiao 2013; Yan 2008). There was a benefit of macrolide over placebo on FEV1 (MD 0.04 L, 95% CI 0 to 0.008; I2 = 66%; participants = 1046; Analysis 1.9; Figure 12). The evidence was of low certainty due to indirectness and four studies for which we were unable to properly assess risk of bias. It was not always clear whether the measurement was taken before or after a bronchodilator.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.9 FEV1 (L).
Non‐specific bronchial hyperresponsiveness
We could not compare the results reported for bronchial hyperresponsiveness in nine studies due to differences in the challenge agent (e.g. methacholine, hypertonic solution) and measurement (histamine provocative concentration causing a 20% (PC20) or 15% (PC15) drop in FEV1, results expressed as log) in the different studies. We present the unpooled data in Analysis 1.10. Three studies reported an effect of macrolides in reducing bronchial hyperresponsiveness compared to placebo (Amayasu 2000; Kostadima 2004; Sutherland 2010), while six studies reported no effect compared with placebo (Cameron 2013; Kamada 1993; Nelson 1993; Piacentini 2007; Shoji 1999; Simpson 2008).
Lowest tolerated oral corticosteroid dose (in people requiring oral corticosteroids at baseline)
Most studies either excluded people taking oral corticosteroids or recruited people who did not take them regularly. Two studies that recruited people taking regular oral corticosteroids reported that macrolides had a steroid‐sparing benefit (Analysis 1.11; Figure 13; Kamada 1993; Nelson 1993). However, there was a baseline imbalance in corticosteroid dose in Nelson 1993, which overstated the difference at endpoint. We chose not to combine the study results because it was unclear if the ways the doses were calculated were sufficiently similar for pooling to make sense.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.11 Oral corticosteroid dose.
Secondary outcomes
Serious adverse events
In general, macrolides were well tolerated, and there were no recorded deaths due to treatment with macrolides. Eight studies reported SAEs (Amayasu 2000; Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2006; Hahn 2012; Kamada 1993; Sutherland 2010). Meta‐analysis found no clear difference in the likelihood of SAEs in the treatment and placebo groups, but the effect was imprecise due to the rarity of events (Peto OR 0.80, 95% CI 0.49 to 1.31; I2 = 41%; participants = 854; Analysis 1.12; Figure 14).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.12 Serious adverse events (including mortality).
We rated the evidence to be of low certainty due to very serious imprecision in the estimate, risk of bias issues and possible indirectness.
Study withdrawals/dropouts
Ten studies reported withdrawals/dropouts (Brusselle 2013; Gibson 2017; Hahn 2006; Hahn 2012; Kamada 1993; Kostadima 2004; Nelson 1993; Simpson 2008; Strunk 2008; Sutherland 2010). Analysis suggested the likelihood of withdrawal from the studies was similar between participants taking macrolide and placebo (OR 1.06, 95% CI 0.76 to 1.48; I2 = 0%; participants = 984; Analysis 1.13).
Eosinophil counts in blood and sputum
One study reported blood eosinophils (Yan 2008). There was no difference between macrolide and placebo in blood eosinophils, but analysed data were only available as SMD, so we did not enter it with the two existing studies (Amayasu 2000; Shoji 1999). A meta‐analysis of these two small cross‐over studies showed a reduction of eosinophils in the blood of people with asthma treated with macrolides (MD −32.16, 95% CI −34.77 to −29.56; I2 = 12%; participants = 62; Analysis 1.14). Cameron 2013 investigated the effect of macrolides in sputum eosinophils in current smokers with asthma, and found a vastly different result from the two trials previously included in the analysis. The highly significant heterogeneity suggested that there was a data error (I2 = 97%). For this reason, data for the three studies have been displayed but not pooled (Analysis 1.15).
Eosinophil cationic protein in serum and sputum
Two studies reported serum and sputum ECP (Amayasu 2000; Shoji 1999). Macrolides appear to reduce the concentration of ECP both in serum and sputum (serum: MD −12.07, 95% CI −14.90 to −9.24; I2 = 0%; participants = 62; Analysis 1.16; sputum: MD −1.35, 95% CI −1.69 to −1.01; I2 = 0%; participants = 62; Analysis 1.17).
Subgroup analysis
Subgroup analysis based on serological response or positivity to PCR for C pneumoniae was not possible due to the scarcity and heterogeneity of data and methods.
Sensitivity analysis
Primary or secondary outcomes with high heterogeneity (I2 greater than 30%) were taken into the consideration for conducting the sensitivity analysis with a random‐effects model. The outcomes with high heterogeneity that suggested a beneficial effect of macrolide in primary analyses such as severe exacerbations: exacerbations requiring ED visits/systemic steroids (Analysis 1.2 versus Analysis 2.1), symptom scales (Analysis 1.3 versus Analysis 2.2), asthma control (Analysis 1.4 versus Analysis 2.3), AQLQ (Analysis 1.5 versus Analysis 2.4), and FEV1 (Analysis 1.9 versus Analysis 2.5) became analyses in which the 95% CIs crossed the line of no effect by changing the model to random‐effects. The reason for the change in effect estimate is decreasing the weighting of large studies such as Gibson 2017 (Analysis 2.1; Analysis 2.3; Analysis 2.4) and Hahn 2012 (Analysis 2.2) by changing the model to random effects.
Discussion
Summary of main results
Twenty‐five studies, involving 1973 participants given macrolide or placebo, met the inclusion criteria. The certainty of the evidence was generally low due to incomplete reporting of study methodology and clinical data, indirectness of study populations, risk of bias, and imprecision caused by small numbers of participants and events. Most studies reported data from people with persistent or severe asthma, but inclusion criteria, interventions and outcomes were highly variable, and there may have been selective or incomplete reporting.
Macrolides led to a moderately sized but non‐significant improvement compared to placebo when this was defined as exacerbations requiring hospital admission (OR 0.47, 95% CI 0.20 to 1.12; I2 = 0%; studies = 2, participants = 529), but this was driven by one study and the 95% CIs did not exclude the possibility of no effect. Not considering the heterogeneity among the studies, macrolides appeared beneficial compared to placebo for severe exacerbations (defined as requiring ED visits or systemic steroids, or both) (RaR 0.65, 95% CI 0.53 to 0.80; I2 = 37%; studies = 4, participants = 640), improvement on symptom scales (SMD −0.46, 95% CI −0.81 to −0.11; I2 = 70%, studies = 4, participants = 136), rescue medication use (MD −0.43 puffs per day, 95% CI −0.81 to −0.04), ACQ (SMD −0.17, 95% CI −0.31 to −0.03) and AQLQ (MD 0.24, 95% CI 0.12 to 0.35), although the variation in ACQ and AQLQ did not reach the MCID (for both scores, MCID = 0.50) (Juniper 2005; Juniper 1994). The improvement in FEV1 (MD 0.04 L, 95% CI 0.03 to 0.08) was very small and of doubtful clinical relevance. There is no agreed MCID for FEV1 but this is below any likely MCID. The other outcomes such as lung function were of very low quality and did not show a benefit of macrolide treatment (morning PEF: MD 1.60 L/minute, 95% CI −10.35 to 13.56; evening PEF: MD 1.00 L/minute, 95% CI −13.65 to 15.65). Measures of bronchial hyperresponsiveness were too varied to pool, but most studies showed no clear benefit of macrolide over placebo. Two studies with people taking regular oral corticosteroids suggested macrolides may have a steroid‐sparing effect in this population. Macrolides were well tolerated with respect to severe adverse events (Peto OR 0.80, 95% CI 0.49 to 1.31; I2 = 41%; studies = 8, participants = 854), although less than half of the studies reported the outcome which was approaching 10% in each group. Reporting of specific adverse effects was too patchy across studies to be analysed meaningfully. Number of withdrawals from the study was near to equal in both macrolide and placebo groups (OR 1.06, 95% CI 0.76 to 1.48; I2 = 0%; studies = 10, participants = 984), and quite high in both group (almost 20%). Biomarkers of asthma activity such as sputum and serum ECP, sputum and serum eosinophils were lower in people treated with macrolides, but this was not associated with clinical benefits.
Overall completeness and applicability of evidence
The available data do not support any generalised use of macrolides in clinical practice to improve clinical outcomes in people with persistent asthma, but we cannot rule out the possibility of benefit due to several shortcomings in the available studies. The potential benefit of macrolides for the wide range of phenotypes and for clinically relevant groups (e.g. smokers) remains to be confirmed.
The available data from the 25 RCTs included in the present review are difficult to interpret for several reasons. First, four different types of macrolides were used across the studies (roxithromycin, clarithromycin, azithromycin and troleandomycin), often with differences in dosage and frequency of administration. Second, participants with different severities of asthma were included: the oldest studies included participants who were taking long‐term oral steroids (Kamada 1993; Nelson 1993), which could reflect a severe population or outdated prescribing practice. One study included people with aspirin‐intolerant asthma (Shoji 1999), one included people with intermittent allergic asthma (Amayasu 2000), and another exclusively recruited smokers with asthma (Cameron 2013); all the other studies enrolled people with mild‐to‐severe persistent asthma, and we could not properly assess the populations of six Chinese studies. Seven studies tested participants for C pneumoniae or M pneumoniae infection, but all with different techniques and very different results (Black 2001; Hahn 2006; Kraft 2002; Simpson 2008; Strunk 2008; Sutherland 2010; Wan 2016). The scarcity of data in the primary analyses precluded any meaningful subgroup analyses to assess the possible effect of these factors. Third and perhaps most importantly, the outcomes measured were heterogeneous; reporting of exacerbations and definitions for exacerbations and their severity varied across the studies; asthma symptoms were recorded using a variety of non‐validated scales as well as the ACQ and AQLQ, with a great variability across the studies. Lung function and bronchial hyperresponsiveness were often assessed and reported using different methodologies or parameters.
Two studies showing some effect on symptoms and markers of eosinophil inflammation were unusual both in the participants they recruited and in their design. Both were cross‐over studies, one recruited people with allergic intermittent asthma (Amayasu 2000), and the other enrolled people whose asthma was aspirin‐induced (Shoji 1999).
Only four studies investigated the role of macrolides in children with asthma (Kamada 1993; Piacentini 2007; Strunk 2008; Wan 2016); unfortunately the great variability in the interventions, measurements and outcomes makes any firm conclusion on the role of macrolides in children impossible. Kamada 1993 suggested a potential role for troleandomycin as steroid‐sparing agent, while Strunk 2008 seemed instead to exclude any role of macrolides used in this way.
Since the last version of this review, two RCTs were published (Gibson 2017; Wan 2016). Wan 2016 explored the effect of macrolides versus placebo on lung function and eosinophil inflammation (measured as exhaled nitric oxide, peripheral blood eosinophil count and ECP). Unfortunately, the quality of reporting of this study did not allow us to include its results in any meta‐analysis. Nevertheless, the fact that Wan 2016 included only 58 participants mitigates the impact of the exclusion of its results on the overall conclusions of this review. In contrast, Gibson 2017 was a very well‐designed and conducted study, with a large sample‐size of well‐selected participants, most of them on high‐dose ICS and long‐acting beta‐agonist (LABA)/long‐acting muscarinic antagonists (LAMA), falling in the category severe asthma of the current GINA 2021 guidelines. A number of biological drugs such as mepolizumab, reslizumab, benralizumab (Farne 2017) and dupilumab (Castro 2018; Rabe 2018) have entered the market since 2015 and, together with omalizumab (Normansell 2014), are routinely used in clinical practice; their use is now recommended in severe eosinophilic asthma (GINA 2021).
The results of Gibson 2017 and of a subgroup analysis from the same study (Gibson 2019) may indicate that macrolides could be an alternative to biological drugs for severe forms of asthma: that would be valuable especially in low‐income countries, although the potential risk of resistance development would remain a serious caveat to their wide use.
Despite all the limitations and considering the heterogeneity, our systematic review and meta‐analysis found no benefit of macrolides over placebo on lung function. As discussed, this does not rule out the possibility for significant benefit or harm of macrolides given the shortcomings of the evidence described above. The results of this review might change if further well‐designed and appropriately powered RCTs are conducted, but at present, the evidence is not promising enough to support further research for a general use of macrolides, while there is a suggestion that research targeted at specific phenotypes (i.e. severe or non‐eosinophilic (or both) asthma) may be warranted.
Overall, the use of macrolides for at least four continuous weeks of treatment proved safe, with similar rates of severe adverse events when macrolides were compared with placebo. There were no reported deaths. Unfortunately, this outcome was not consistently reported across the studies, and internationally approved scales such as the Common Terminology Criteria for Adverse Events were not used (CTCAE 2020). Therefore, the evidence for this outcome was low.
Antibiotic resistance is of increasing concern and only two included studies investigated this (Brusselle 2013; Gibson 2017). Brusselle 2013 reported that 87% of azithromycin‐treated participants were colonised with erythromycin‐resistant streptococci, a statistically significant increase from baseline and in comparison with the placebo group, while Gibson 2017 reported no significant change on the occurrence of resistant strains.
These results suggest that spread of resistant strain is a real concern, and any further research should clearly measure and report resistance as an outcome.
Alongside this, the case for macrolide therapy contributing to better outcomes in those testing positive for C pneumoniae or M pneumoniae infection was mostly unconvincing (Belotserkovskaya 2007; Black 2001; Kraft 2002; Strunk 2008; Sutherland 2010; Wan 2016). The number of participants testing positive was much lower than expected in several studies, and subgroup analyses were often underpowered or post hoc.
Quality of the evidence
The overall certainty of the evidence is low. We had serious concerns about selective or incomplete reporting, under‐reporting or variation of study results; there was uncertainty regarding allocation procedures and blinding of outcome assessment, and all but four trials recruited fewer than 100 people.
Few studies reported clinical data well enough to be included in a meta‐analysis. Some studies reported outcomes of interest but not in a format that allowed the data to be combined with other studies, and other studies focused on non‐clinical outcomes when the use of macrolides was being tested to assess their mechanism of action and effect on biomarkers. Most outcomes were also downgraded for indirectness because some studies focused on specific populations, such as smokers or people with asthma of a particular severity, which varied across studies. Inconsistencies in the scales used or description of outcomes also made it difficult to meta‐analyse the data and reduced our confidence in the conclusions that could be drawn. Risk of bias was also an issue across most of the analyses, largely due to uncertainty as a result of insufficient reporting of methodology, but also as a result of failure to prevent or account for high or unbalanced dropout.
Evidence for outcomes such as exacerbations requiring hospitalisation and serious adverse events was very imprecise due to the length of the studies and the rarity of this type of events, so it was difficult to reach meaningful conclusions for these outcomes. Several studies excluded people at higher risk of adverse events including prolonged corrected QT interval on electrocardiogram (Brusselle 2013; Cameron 2013; Gibson 2017; Hahn 2012; Sutherland 2010; Wan 2016), abnormal liver function tests (Black 2001; Cameron 2013; Hahn 2012; Sutherland 2010), and hearing impairment (Gibson 2017); the effect of excluding these potential participants is uncertain. For other outcomes such as symptoms, quality of life and FEV1, the reporting made it difficult for us to assess the amount of variation in scales, properties, time of measurement, etc., and this uncertainty made the data difficult to interpret.
Potential biases in the review process
We did not contact most trial authors to obtain unpublished data or to clarify methodology. Only two of the studies were conducted in the last five years, and 14 were conducted over 10 years ago. We judged that the time taken to contact all authors and the anticipated low response rate due to study age would delay the publication of this update.
We found six studies listed in an existing systematic review conducted in China that did not appear in our searches (Tong 2015). While we did not limit our searches by language, they did not cover studies that are indexed in non‐English language databases. Since the Tong 2015 systematic review was published in English, we were able to contact the authors and extract sufficient information to confirm the eligibility of these trials. However, we did not have the resources to personally extract data or assess for risk of bias in these studies, and the information we were able to include were kindly provided by the authors of that systematic review and not directly from the studies themselves. The review authors were able to answer the questions we had about the studies and their outcomes, but these studies could not be assessed as rigorously as the other 19 included studies and we could not be certain that all data relevant to this review were included.
The definitions of severe exacerbation, symptom scales and quality of life were not always consistent across studies. This required a significant post‐hoc assessment of which outcomes could be pooled.
For peak flow and blood eosinophils, some of these studies could not be pooled with the others because they used a different unit of analysis. In these cases, we reported the results alongside the meta‐analysis results narratively. The main benefit of including these studies is the completeness of the evidence base, and checking study lists of existing meta‐analyses is part of the standard search procedures for Cochrane Reviews. Subtle differences in the methods between our own review and that of Tong 2015 (e.g. the way data were extracted, application of trial eligibility criteria) may also have introduced a potential bias, meaning we cannot be sure that all studies relevant to our review were identified and analysed in the same way. We considered the overall benefit of inclusion to outweigh the potential biases in light of the help provided to us by Hon Fang and the other authors of that review (Fan 2015).
Agreements and disagreements with other studies or reviews
Four other meta‐analyses have evaluated the treatment of asthma with long‐term macrolides (Hiles 2019; Reiter 2013; Tong 2015; Wang 2019). There are noticeable differences across the systematic reviews and meta‐analyses in the conclusions drawn, and this is likely a reflection of the choice of outcomes and methods of analysis. In particular, we chose not to pool results where we were uncertain of scale or measurement similarity in order to make the results as clinically meaningful as possible. Furthermore, more subtle differences in the eligibility criteria and the way scores were aggregated are likely to have contributed to differences in the results and conclusions, such as using SMD or MD, fixed or random effects, change from baseline or endpoint scores, merging multiple relevant study arms, etc. These differences entail difficulties with meta‐analysing and interpreting the body of evidence, which is quite heterogeneous.
The analysis by Reiter 2013 included 12 RCTs with a minimum treatment duration of three weeks, and reported a positive effect of macrolides on symptoms scores, quality of life, peak flow and bronchial hyperresponsiveness. Tong 2015 included 18 studies, including the six Chinese studies that were not identified by our search, and it reported a positive effect on several measures of lung function (FEV1, PEF and forced vital capacity (FVC)) and airways hyperresponsiveness, but not on other measures of lung function (percentage predicted FEV1 and percentage predicted FVC), symptoms, or quality of life. Wang 2019 explored only studies conducted with azithromycin and for at least three weeks of duration. This systematic review and meta‐analysis included eight RCTs, reporting a small but statistically significant increase in FEV1, but no change in exacerbation rate, quality of life measures, PEF or fractional exhaled nitric oxide (FeNO) in the treatment group compared to placebo.
Hiles 2019 performed an individual participant data meta‐analysis of three studies comparing azithromycin (treatment duration of at least eight weeks) versus placebo. The studies had to include data on exacerbations for a follow‐up of at least six months. Exacerbations were defined as need for at least three days of oral corticosteroids or antibiotic course, or visit to the ED, or admission to hospital. Most of the participants included in this meta‐analysis had severe asthma (320/529 participants, 60.5%). The meta‐analysis showed a reduction of exacerbations in this population, and in people an eosinophilic or non‐eosinophilic phenotype. Azithromycin did not have beneficial effects for secondary outcomes such as quality of life, lung function or biomarkers.
Reiter 2013 and Tong 2015 did not formally assess exacerbations, either because they were not included as an outcome (Tong 2015) or because the data were considered insufficient to do so (Reiter 2013). Wang 2019 explored the exacerbation frequency among the studies they included, but they did not report which definition they used for their analysis. Therefore, the results were difficult to contextualise. The results of Hiles 2019 were limited to people with severe asthma, and seem to confirm the findings of our systematic review and meta‐analysis for this specific group of patients.
The lack of hospitalisation and exacerbation data is a major shortcoming of the evidence base, considering that reducing the frequency of these events is the main premise of long‐term macrolides treatment.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.1 Exacerbation requiring hospitalisation.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.2 Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.3 Symptom scales.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.4 Asthma Control.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.5 Asthma Quality of Life Questionnaire (AQLQ).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.6 Rescue medication puffs/day.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.7 Morning PEF (L/minute).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.8 Evening PEF (L/minute).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.9 FEV1 (L).

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.11 Oral corticosteroid dose.

Forest plot of comparison: 1 Macrolide versus placebo, outcome: 1.12 Serious adverse events (including mortality).

Comparison 1: Macrolide versus placebo, Outcome 1: Exacerbation requiring hospitalisation

Comparison 1: Macrolide versus placebo, Outcome 2: Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids

Comparison 1: Macrolide versus placebo, Outcome 3: Asthma symptom scales

Comparison 1: Macrolide versus placebo, Outcome 4: Asthma control

Comparison 1: Macrolide versus placebo, Outcome 5: Asthma Quality of Life Questionnaire (AQLQ)

Comparison 1: Macrolide versus placebo, Outcome 6: Need for rescue medication puffs/day

Comparison 1: Macrolide versus placebo, Outcome 7: Morning PEF (L/minute)

Comparison 1: Macrolide versus placebo, Outcome 8: Evening PEF (L/minute)

Comparison 1: Macrolide versus placebo, Outcome 9: Forced expiratory volume in 1 second (FEV 1; L)
| Bronchial hyperresponsiveness (BHR) | |||
| Study | Measure of BHR (units) | Results | Conclusions |
|---|---|---|---|
| Amayasu 2000 | Methacholine challenge test (log PC20) | Clarithromycin: 2.96, standard deviation (SD) 0.57 Placebo: 2.60, SD 0.51 (P < 0.01) | Clarithromycin significantly reduced BHR in people with allergic intermittent asthma. |
| Cameron 2013 | Methacholine challenge test (log PC20) | Azithromycin: 0.20, SD 1.52 Placebo: 0.19, SD 1.29 (P < 0.93) | No effect of azithromycin in smokers with persistent asthma. |
| Kamada 1993 | Methacholine challenge test (PC20) | 16/19 participants completed the test at the beginning and end of the analysis. Data were reported graphically and not included in the main analysis | No significant difference at the end of the treatment was recorded among the 3 arms of the study. |
| Kostadima 2004 | Methacholine challenge test (PC20) | Median before and after the treatment: Clarithromycin 250 mg twice daily: 0.3 mg (interquartile range (IQR) 0.1 to 1) and 1.3 mg (IQR 0.6 to 2) mg (P < 0.001) Clarithromycin 250 mg 3 times daily: 0.4 mg (IQR 0.1 to 0.9) and 2.0 mg (IQR 2.0 to 2.0) (P < 0.001) Placebo: 0.4 mg (IQR 0.1 to 0.9) and 0.3 mg (IQR 0.1 to 0.6) mg (P not significant) | Compared to the baseline, there was a significant increase in the median PC20 in the 2 macrolide groups but not in the placebo group. |
| Nelson 1993 | Methacholine challenge test (PC20) | 11/27 participants in placebo group and 13/30 participants in troleandomycin group completed the test at the start and end of the study. Troleandomycin: +1.89 mg/mL Placebo: +0.55 mg/mL | No significant effect of troleandomycin was recorded in comparisons within and between the study groups. |
| Piacentini 2007 | Hypertonic saline challenge (dose–response slope) | Azithromycin: 2.75, SD 2.12 to 1.42, SD 1.54 (P < 0.02) Placebo: 1.48, SD 1.75 to 1.01, SD 1.38 (P = 0.21) | The reduction of BHR in the treatment group was driven by the change from the baseline in 3/9 participants. No significant difference observed in a comparison between the groups. Study in children. |
| Shoji 1999 | Sulpyrine inhalation testing (log PC20‐sulpyrine) | Roxithromycin: 1.18, SD 0.40 Placebo: 1.15, SD 0.43 | No significant improvement of BHR recorded within and between group comparisons. |
| Simpson 2008 | Hypertonic saline challenge (dose–response slope; DSR) | DSR before: 1.8 (IQR 0.6 to 6.4) and after clarithromycin: 1 (IQR 0.5 to 4.2) DSR before: 1 (IQR 0.6 to 3.2) and after placebo: 1 (IQR 0.5 to 3.3) | No significant improvement of BHR within and between group comparisons. |
| Sutherland 2010 | Methacholine challenge test (PC20 doubling dose) Analysis stratified for polymerase chain reaction (PCR) positivity for M pneumoniae or C pneumonia | Difference between clarithromycin and placebo groups: Irrespective of PCR status: +1.2, SD 0.5 (P = 0.01) In participants with positive PCR status: +0.9, SD 1.8 (P = 0.6) In participants with negative PCR status: +1.2, SD 0.5 (P = 0.02) | BHR was significantly improved by clarithromycin compared to placebo in the whole population and in the PCR‐negative groups, but not among the PCR‐positive participants. |
Comparison 1: Macrolide versus placebo, Outcome 10: Bronchial hyperresponsiveness (BHR)

Comparison 1: Macrolide versus placebo, Outcome 11: Oral corticosteroid dose

Comparison 1: Macrolide versus placebo, Outcome 12: Serious adverse events (including mortality)

Comparison 1: Macrolide versus placebo, Outcome 13: Withdrawal

Comparison 1: Macrolide versus placebo, Outcome 14: Blood eosinophils

Comparison 1: Macrolide versus placebo, Outcome 15: Sputum eosinophils

Comparison 1: Macrolide versus placebo, Outcome 16: Eosinophil cationic protein (ECP) in serum

Comparison 1: Macrolide versus placebo, Outcome 17: ECP in sputum

Comparison 2: Sensitivity analysis, Outcome 1: Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids

Comparison 2: Sensitivity analysis, Outcome 2: Symptom scales

Comparison 2: Sensitivity analysis, Outcome 3: Asthma control

Comparison 2: Sensitivity analysis, Outcome 4: Asthma Quality of Life Questionnaire (AQLQ)

Comparison 2: Sensitivity analysis, Outcome 5: Forced expiratory volume in 1 second (FEV 1; L)
| Macrolide versus placebo for chronic asthma | ||||||
| Patient or population: adults and children with chronic asthma Drugs used were clarithromycin, azithromycin, roxithromycin and troleandomycin. Macrolide was given once or twice daily in most studies for 4–52 weeks (median 8 weeks). Treatment durations were calculated as weighted means of the studies included in each analysis. | ||||||
| Outcomesa | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed comparator risk** | Corresponding risk | |||||
| Placebo | Macrolide | |||||
| Exacerbation requiring hospitalisation Weighted mean study duration: 45 weeks | 61 per 1000 | 29 per 1000 (13 to 68) | OR 0.47 (0.20 to 1.12) | 529 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | — |
| Severe exacerbations (requiring ED visits or systemic steroids, or both) Number of people having ≥ 1 exacerbations requiring an ED visit or systemic steroids, or both. Classification varied across studies. Weighted mean study duration: 35 weeks | 410 per 1000 | 311 per 1000 | Rate ratio 0.65 | 640 | ⊕⊕⊕⊝ | — |
| Asthma symptoms – symptom scales (various scales; lower score = better) Weighted mean study duration: 10 weeks | The mean change in symptom scales in the intervention group compared to the control group was a reduction; 0.46 SD lower (0.81 SD lower to 0.11 SD lower) | — | 136 | ⊕⊝⊝⊝ | The SMD is a Cohen's effect size and can be interpreted as moderate (< 0.4 = small, 0.40–0.70 = moderate, > 0.70 = large). | |
| Asthma control (Asthma Control Questionnaire) Scored 0–6 (lower scores indicate improvement in asthma control) Weighted mean study duration: 34 weeks | The mean change in asthma control in the intervention group compared to the mean change in control group was a greater reduction;0.17 SD lower (0.31 SD lower to 0.03 SD lower) | — | 773 | ⊕⊕⊝⊝ | The SMD is a Cohen's effect size and can be interpreted as small. | |
| Asthma quality of life (AQLQ) Scored 1–7 (higher scores indicate improvement in quality of life) Weighted mean study duration: 35 weeks | The mean change on the AQLQ scale in the control group was an increase from baseline; 0.31 points higher** | The mean change in AQLQ in the intervention group compared to the mean change in the control group was an increase; 0.24 points higher (0.12 higher to 0.35 higher) | — | 802 | ⊕⊝⊝⊝ Very lowc,d,h | MCID: 0.5 points |
| Rescue medication (puffs/day) Weighted mean study duration: 17 weeks | The mean rescue medication use in the control group was 1.08 puffs/day** | The mean change in rescue medication puffs/day in the intervention group compared to the mean change in the control group was reduction; 0.43 fewer puffs (0.81 fewer to 0.04 fewer) | — | 314 | ⊕⊕⊝⊝ | — |
| FEV1 (L) Weighted mean study duration: 28 weeks | The mean FEV1 in the control group was 2.49 L** | The mean change in FEV1 (L) the intervention group compared to the control group was an increase; 0.04 L higher (0 L to 0.08 L higher) | — | 1046 | ⊕⊕⊝⊝ | Although there is no universally accepted MCID for FEV1 in asthma, variability within a single testing session can be up to 0.12 L (data from a mixed pool of respiratory patients; Enright 2004). |
| Serious adverse events (including mortality) Weighted mean study duration: 16 weeks | 93 per 1000 | 76 per 1000 | OR 0.80 | 854 | ⊕⊕⊝⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Assumed risk for continuous outcomes were calculated as weighted means of the scores in the control group. Note: Sutherland 2010 could not be included in the rescue medication or quality of life calculations because they reported only mean difference between groups; Brusselle 2013 was not included in the FEV1 calculation because it was the only change score; Cameron 2013 was not included in the asthma control or quality of life calculations because it was the only study reporting absolute endpoint scores rather than change from baseline. AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; ED: emergency department; FEV1: forced expiratory volume in one second; MCID: minimal clinically important difference; OR: odds ratio; RCT: randomised controlled trial; SD: standard deviation. | ||||||
| GRADE domains: study limitations, consistency of effect, imprecision, indirectness and publication bias. GRADE Working Group grades of evidence (Schünemann 2021). | ||||||
| aTwo primary outcomes (morning and evening peak expiratory flow) are not presented. Bronchial hyperresponsiveness could not be pooled in a meta‐analysis and is described narratively in the review. Studies did not report lowest tolerated oral corticosteroid dose well and there were no data to analyse. | ||||||
| Study ID | Country | Number of participants | Design | Duration (weeks) | Macrolide dose and schedule | Mean age (years) | % Male | % on ICS | % Predicted FEV1 |
|---|---|---|---|---|---|---|---|---|---|
| Japan and USA | 17 | C, R, DB, PC | 8 | Clarithromycin 200 mg twice daily | 38.5 | 52.9 | 0.0 | 76.2 | |
| Russia | 51 | P, R | 8 | Azithromycin (unknown dose) | NR | NR | NR | NR | |
| Multinational | 219 | P, R, DB, PC | 6 | Roxithromycin 150 mg twice daily | 41.0 | 47.5 | 80.8 | 77.1 | |
| Belgium | 109 | P, R, DB, PC | 26 | Azithromycin 250 mg once daily for 5 days then 3 times per week | 53.0 (median) | 38.5 | 100a | 82.5 | |
| UK | 77 | P, R, DB, PC | 12 | Azithromycin 250 mg once daily | 44.6 | 48.1 | 85.7 | 78.3 | |
| Australia | 420 | P, R, DB, PC | 48 | Azithromycin 500 mg 3 times per week | 60.5 | 39.3 | 99.8 | 72.9 | |
| USA, Canada | 45 | P, R, DB, PC | 6 | Azithromycin 600 mg once daily for 3 days then weekly | 47.7 | 48.9 | 80.0 | NR | |
| USA | 75 | P, R, DB, PC | 12 | Azithromycin 600 mg once daily for 3 days then weekly | 46.6 | 32.0 | 72.0 | NR | |
| China | 40 | P, R, PC | 12 | Azithromycin 250 mg twice weekly | 34.5 | NR | NR | NR | |
| USA | 19 | P, R, DB, PC | 12 | Troleandomycin 250 µg once daily + OCS | 12.5 | 63.2 | 100 | NR | |
| India | 40 | C, R, DB, PC | 6 | Roxithromycin 150 mg once daily | NR | NR | NR | NR | |
| Greece | 75 | P, R, DB, PC | 8 | Clarithromycin 250 mg twice daily or 3 times daily | 43.7 | 47.1 | 100 | 85.3 | |
| USA | 55 | P, R, DB, PC | 6 | Clarithromycin 500 mg twice daily | 33.4 | 49.1 | 32.7 | 69.3 | |
| USA | 75 | P, R, DB, PC | 52 | Troleandomycin 250 mg once daily + OCS | NR | 33.3 | 0 | NR | |
| Italy | 16 | P, R, DB, PC | 8 | Azithromycin 10 mg/kg once daily 3 days per week | 13.4 | 75 | 100 | 78.9 | |
| Japan | 14 | C, R, DB, PC | 8 | Roxithromycin 150 mg twice daily | 39.6 | 42.9 | 0.0 | 75.0 | |
| Australia | 45 | P, R, DB, PC | 8 | Clarithromycin 500 mg twice daily | 57.6 | 48.9 | NRb | 70.7 | |
| USA | 55c | P, R, DB, PC | 30 | Azithromycin 250 mg or 500 mg once daily | 11.2 | 58.2 | 100a | 101.9 | |
| USA | 92 | P, R, DB, PC | 16 | Clarithromycin 500 mg twice daily | 39.4 | 43.5 | NR | 76.0 | |
| Taiwan | 58 | P, R, PC | 4 | Clarithromycin 5 mg/kg daily | 10.1 | 60.3 | 100 | 79.7 | |
| China | 45 | P, R, PC | 8 | Clarithromycin 500 mg twice daily | NR | NR | NR | NR | |
| China | 58 | P, R, PC | 52 | Azithromycin 250 mg twice weekly | 29.0 | NR | NR | NR | |
| China | 210 | P, R, PC | 12 | Roxithromycin 150 mg twice daily | 34.1 | NR | NR | NR | |
| China | 40 | P, R, PC | 4 | Roxithromycin 150 mg twice daily | 38.5 | NR | NR | NR | |
| China | 60 | P, R, PC | 9 | Azithromycin 100 mg once daily | NR | NR | NR | NR | |
| C: cross‐over; DB: double‐blind; FEV1 : forced expiratory volume in one second; ICS: inhaled corticosteroid; NR: not reported; OCS: oral corticosteroids; P: parallel; PC: placebo‐controlled; R: randomised. aAll participants were taking long‐acting beta2‐agonist + ICS combination. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Exacerbation requiring hospitalisation Show forest plot | 2 | 529 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.20, 1.12] |
| 1.2 Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids Show forest plot | 4 | 640 | Rate Ratio (IV, Fixed, 95% CI) | 0.65 [0.53, 0.80] |
| 1.3 Asthma symptom scales Show forest plot | 4 | 136 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.81, ‐0.11] |
| 1.4 Asthma control Show forest plot | 5 | 773 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.31, ‐0.03] |
| 1.5 Asthma Quality of Life Questionnaire (AQLQ) Show forest plot | 6 | 802 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [0.12, 0.35] |
| 1.6 Need for rescue medication puffs/day Show forest plot | 4 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.81, ‐0.04] |
| 1.7 Morning PEF (L/minute) Show forest plot | 4 | 289 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [‐10.35, 13.56] |
| 1.8 Evening PEF (L/minute) Show forest plot | 3 | 212 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐13.65, 15.65] |
| 1.9 Forced expiratory volume in 1 second (FEV 1; L) Show forest plot | 10 | 1046 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [0.00, 0.08] |
| 1.10 Bronchial hyperresponsiveness (BHR) Show forest plot | 9 | Other data | No numeric data | |
| 1.11 Oral corticosteroid dose Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Serious adverse events (including mortality) Show forest plot | 8 | 854 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.49, 1.31] |
| 1.13 Withdrawal Show forest plot | 10 | 984 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.48] |
| 1.14 Blood eosinophils Show forest plot | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐32.16 [‐34.77, ‐29.56] |
| 1.15 Sputum eosinophils Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16 Eosinophil cationic protein (ECP) in serum Show forest plot | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐12.07 [‐14.90, ‐9.24] |
| 1.17 ECP in sputum Show forest plot | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐1.69, ‐1.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Severe exacerbations: exacerbations requiring emergency department visits/systemic steroids Show forest plot | 4 | 640 | Rate Ratio (IV, Random, 95% CI) | 0.72 [0.48, 1.08] |
| 2.2 Symptom scales Show forest plot | 4 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.27, 0.11] |
| 2.3 Asthma control Show forest plot | 5 | 773 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.33, 0.06] |
| 2.4 Asthma Quality of Life Questionnaire (AQLQ) Show forest plot | 6 | 802 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.06, 0.36] |
| 2.5 Forced expiratory volume in 1 second (FEV 1; L) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.01, 0.15] | |

