Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants
Información
- DOI:
- https://doi.org/10.1002/14651858.CD002311.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Dr Onland and Dr van Kaam have full access to all of the data in the review and take responsibility for the integrity of the data and the accuracy of the data analysis.
-
Study concept and design: Onland, van Kaam
-
Acquisition of data: Onland, van Kaam
-
Analysis and interpretation of data: Onland, Offringa, van Kaam
-
Drafting of the manuscript: Onland, van Kaam
-
Critical revision of the manuscript for important intellectual content: Onland, Offringa, van Kaam
-
Stastical analysis: Onland
-
Study supervision: Offringa, van Kaam
Sources of support
Internal sources
-
Department of Neonatology, AMC, Amsterdam, Netherlands.
-
Department of Pediatric Clinical Epidemiology, AMC, Netherlands.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C.
Declarations of interest
Wes Onland: No financial disclosure to be declared. No potential conflicts of interest known.
Martin Offringa: No financial disclosure to be declared. No potential conflicts of interest known.
Anton van Kaam: No financial disclosure to be declared. No potential conflicts of interest known.
Acknowledgements
Dr P Lister, Dr R Iles, Dr B Shaw, Dr F Ducharme for writing the previous version of this review, 'Inhaled steroids for neonatal chronic lung disease'.
Dr DSchwartz, Dr T Giep, Prof M Silverman, and Dr S Brudno provided additional information for the previous version of this review.
Prof Silverman and Dr Jonsson provided precious additional data for this updated version of the review.
Ms D Haughton, Ms Y Montagne, Ms C Ovelman, Ms J Spano, and Prof R Soll of Cochrane Neonatal.
Version history
| Published | Title | Stage | Authors | Version |
| 2022 Dec 15 | Late (≥ 7 days) inhaled corticosteroids to reduce bronchopulmonary dysplasia in preterm infants | Review | Wes Onland, Martin Offringa, Anton Kaam | |
| 2017 Aug 24 | Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants | Review | Wes Onland, Martin Offringa, Anton van Kaam | |
| 2012 Apr 18 | Late (≥ 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants | Review | Wes Onland, Martin Offringa, Anton van Kaam | |
| 2010 Jan 20 | Inhaled steroids for neonatal chronic lung disease | Review | Paula Lister, Richard Iles, Ben NJ Shaw, Francine M Ducharme | |
| 1999 Oct 25 | Inhaled steroids for neonatal chronic lung disease | Review | Paula Lister, Richard Iles, Ben NJ Shaw, Francine Ducharme | |
Differences between protocol and review
Given the paucity of data, we failed to perform the sensitivity analyses examining the potential influence of treatment variation (type and dose of inhalation corticosteroid, duration of treatment, and delivery system).
Notes
Editorial responsibility for the review 'Inhaled steroids for neonatal chronic lung disease' has been transferred to the Cochrane Neonatal Review Group from the Cochrane Airways Group.
A new team of review authors has been assigned: Dr Wes Onland, Dr Martin Offringa, and Dr Anton Van Kaam.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Administration, Inhalation;
- Anti‐Inflammatory Agents [*administration & dosage];
- Beclomethasone [administration & dosage];
- Bronchopulmonary Dysplasia [etiology, *prevention & control];
- Budesonide [administration & dosage];
- Dexamethasone [administration & dosage];
- Fluocinolone Acetonide [administration & dosage, analogs & derivatives];
- Fluticasone [administration & dosage];
- Glucocorticoids [*administration & dosage];
- Infant, Premature;
- Pneumonia [complications, *drug therapy];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans; Infant, Newborn;
PICO

Flow of inclusion of randomised controlled trials in different phases of search.

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
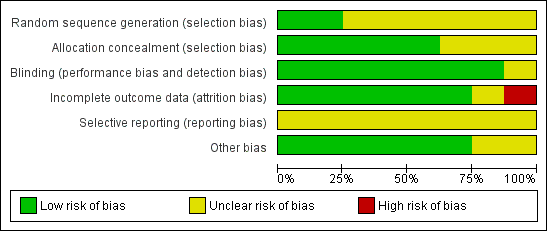
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
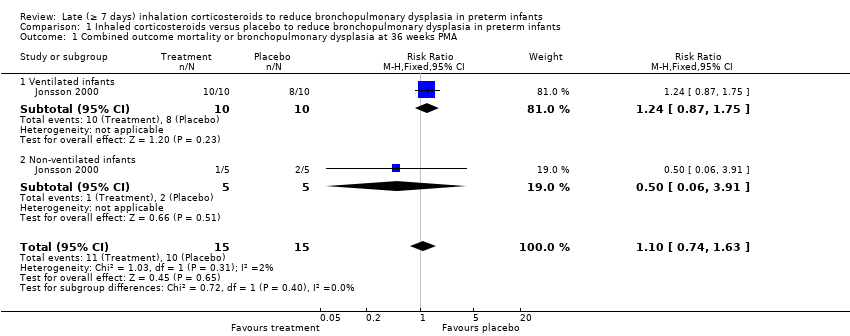
Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 1 Combined outcome mortality or bronchopulmonary dysplasia at 36 weeks PMA.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 2 Mortality at 28 days PNA.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 3 Mortality at 36 weeks PMA.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 4 Mortality at hospital discharge.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 5 Bronchopulmonary dysplasia at 28 days PNA.
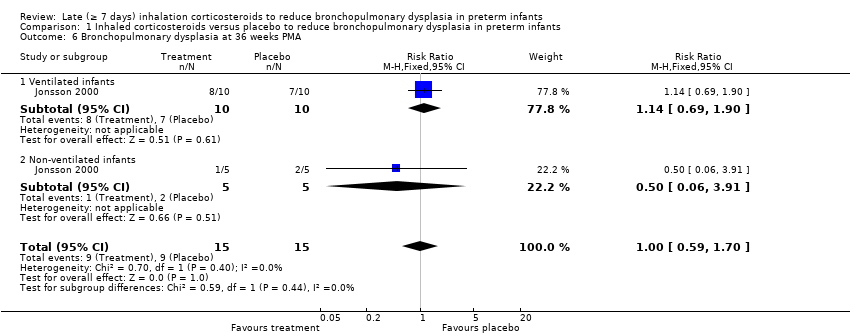
Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 6 Bronchopulmonary dysplasia at 36 weeks PMA.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 7 Combined outcome mortality and bronchopulmonary dysplasia at 28 days PNA.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 8 Failure to extubate day 7.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 9 Failure to extubate day 14.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 10 Failure to extubate at the latest reported moment.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 11 Days of mechanical ventilation.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 12 Days of supplemental oxygen.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 13 Open‐label intravenous corticosteroids.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 14 Sepsis (clinical suspected or culture proven).

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 15 Patent ductus arteriosus.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 16 Hypertension (> 2 SD).

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 17 Necrotising enterocolitis.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 18 Intraventricular haemorrhage (any grade).

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 19 Days of hospitalisation.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 20 Airway resistance.

Comparison 1 Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants, Outcome 21 Dynamic lung compliance.
| Inhaled corticosteroids versus placebo to reduce bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with inhaled corticosteroids | |||||
| Combined outcome mortality or bronchopulmonary dysplasia at 36 weeks postmenstrual age | Study population | RR 1.10 | 30 | ⊕⊝⊝⊝ | ||
| 533 per 1000 | 587 per 1000 | |||||
| Mortality at 36 weeks postmenstrual age | Study population | RR 3.00 | 61 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Bronchopulmonary dysplasia at 36 weeks postmenstrual age | Study population | RR 1.00 | 30 | ⊕⊝⊝⊝ | ||
| 600 per 1000 | 600 per 1000 | |||||
| Open‐label intravenous corticosteroids | Study population | RR 0.51 | 74 | ⊕⊝⊝⊝ | ||
| 432 per 1000 | 320 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: No serious limitations. No downgrade. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Combined outcome mortality or bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.74, 1.63] |
| 1.1 Ventilated infants | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.87, 1.75] |
| 1.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 3.91] |
| 2 Mortality at 28 days PNA Show forest plot | 2 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 2.1 Ventilated infants | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 2.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality at 36 weeks PMA Show forest plot | 3 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.78] |
| 3.1 Ventilated infants | 3 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.78] |
| 3.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mortality at hospital discharge Show forest plot | 3 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.78] |
| 4.1 Ventilated infants | 3 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.78] |
| 4.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Bronchopulmonary dysplasia at 28 days PNA Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 5.1 Ventilated infants | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 5.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 6 Bronchopulmonary dysplasia at 36 weeks PMA Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.59, 1.70] |
| 6.1 Ventilated infants | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.69, 1.90] |
| 6.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 3.91] |
| 7 Combined outcome mortality and bronchopulmonary dysplasia at 28 days PNA Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.85, 1.18] |
| 7.1 Ventilated infants | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.83, 1.20] |
| 7.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 8 Failure to extubate day 7 Show forest plot | 5 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.98] |
| 9 Failure to extubate day 14 Show forest plot | 2 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.10, 1.33] |
| 10 Failure to extubate at the latest reported moment Show forest plot | 6 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.80] |
| 11 Days of mechanical ventilation Show forest plot | 3 | 45 | Mean Difference (IV, Fixed, 95% CI) | 3.42 [‐1.30, 8.13] |
| 12 Days of supplemental oxygen Show forest plot | 4 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐5.92, 7.07] |
| 12.1 Ventilated infants | 4 | 100 | Mean Difference (IV, Fixed, 95% CI) | 5.53 [‐3.99, 15.05] |
| 12.2 Non‐ventilated infants | 2 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐3.74 [‐12.63, 5.14] |
| 13 Open‐label intravenous corticosteroids Show forest plot | 4 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.00] |
| 13.1 Ventilated infants | 4 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.00] |
| 13.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Sepsis (clinical suspected or culture proven) Show forest plot | 5 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.50, 1.64] |
| 14.1 Ventilated infants | 5 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.77] |
| 14.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.75] |
| 15 Patent ductus arteriosus Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.20] |
| 15.1 Ventilated infants | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.20] |
| 16 Hypertension (> 2 SD) Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Ventilated infants | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Necrotising enterocolitis Show forest plot | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 Ventilated infants | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Non‐ventilated infants | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Intraventricular haemorrhage (any grade) Show forest plot | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.13, 2.82] |
| 18.1 Ventilated infants | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.13, 2.82] |
| 19 Days of hospitalisation Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐24.70 [‐41.75, ‐7.65] |
| 19.1 Ventilated infants | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐24.70 [‐41.75, ‐7.65] |
| 20 Airway resistance Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 21.40 [‐71.11, 113.91] |
| 21 Dynamic lung compliance Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.33, ‐0.11] |

