Opioid antagonists with minimal sedation for opioid withdrawal
Abstract
Background
Managed withdrawal is a necessary step prior to drug‐free treatment or as the endpoint of long‐term substitution treatment.
Objectives
To assess the effects of opioid antagonists plus minimal sedation for opioid withdrawal. Comparators were placebo as well as more established approaches to detoxification, such as tapered doses of methadone, adrenergic agonists, buprenorphine and symptomatic medications.
Search methods
We updated our searches of the following databases to December 2016: CENTRAL, MEDLINE, Embase, PsycINFO and Web of Science. We also searched two trials registers and checked the reference lists of included studies for further references to relevant studies.
Selection criteria
We included randomised and quasi‐randomised controlled clinical trials along with prospective controlled cohort studies comparing opioid antagonists plus minimal sedation versus other approaches or different opioid antagonist regimens for withdrawal in opioid‐dependent participants.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
Ten studies (6 randomised controlled trials and 4 prospective cohort studies, involving 955 participants) met the inclusion criteria for the review. We considered 7 of the 10 studies to be at high risk of bias in at least one of the domains we assessed.
Nine studies compared an opioid antagonist‐adrenergic agonist combination versus a treatment regimen based primarily on an alpha2‐adrenergic agonist (clonidine or lofexidine). Other comparisons (placebo, tapered doses of methadone, buprenorphine) made by included studies were too diverse for any meaningful analysis. This review therefore focuses on the nine studies comparing an opioid antagonist (naltrexone or naloxone) plus clonidine or lofexidine versus treatment primarily based on clonidine or lofexidine.
Five studies took place in an inpatient setting, two studies were in outpatients with day care, two used day care only for the first day of opioid antagonist administration, and one study described the setting as outpatient without indicating the level of care provided.
The included studies were heterogeneous in terms of the type of opioid antagonist treatment regimen, the comparator, the outcome measures assessed, and the means of assessing outcomes. As a result, the validity of any estimates of overall effect is doubtful, therefore we did not calculate pooled results for any of the analyses.
The quality of the evidence for treatment with an opioid antagonist‐adrenergic agonist combination versus an alpha2‐adrenergic agonist is very low. Two studies reported data on peak withdrawal severity, and four studies reported data on the average severity over the period of withdrawal. Peak withdrawal induced by opioid antagonists in combination with an adrenergic agonist appears to be more severe than withdrawal managed with clonidine or lofexidine alone, but the average severity over the withdrawal period is less. In some situations antagonist‐induced withdrawal may be associated with significantly higher rates of treatment completion compared to withdrawal managed with adrenergic agonists. However, this result was not consistent across studies, and the extent of any benefit is highly uncertain.
We could not extract any data on the occurrence of adverse events, but two studies reported delirium or confusion following the first dose of naltrexone. Delirium may be more likely with higher initial doses and with naltrexone rather than naloxone (which has a shorter half‐life), but we could not confirm this from the available evidence.
Insufficient data were available to make any conclusions on the best duration of treatment.
Authors' conclusions
Using opioid antagonists plus alpha2‐adrenergic agonists is a feasible approach for managing opioid withdrawal. However, it is unclear whether this approach reduces the duration of withdrawal or facilitates transfer to naltrexone treatment to a greater extent than withdrawal managed primarily with an adrenergic agonist.
A high level of monitoring and support is desirable for several hours following administration of opioid antagonists because of the possibility of vomiting, diarrhoea and delirium.
Using opioid antagonists to induce and accelerate opioid withdrawal is not currently an active area of research or clinical practice, and the research community should give greater priority to investigating approaches, such as those based on buprenorphine, that facilitate the transition to sustained‐release preparations of naltrexone.
PICO
Plain language summary
Use of opioid antagonists with minimal sedation to manage opioid withdrawal
Review question
We reviewed the evidence on the effects of opioid antagonists (naltrexone, naloxone) plus minimal sedation for managing withdrawal in people who are dependent on opioid drugs (for example, heroin or pharmaceutical opiates).
Background
Managed withdrawal, or detoxification, is a required first step for longer‐term treatments of opioid dependence. The combination of uncomfortable symptoms and intense craving makes completing withdrawal difficult for most people. The rationale underlying the use of opioid antagonists to induce withdrawal is that a faster transition from dependence to abstinence might make completing withdrawal easier. This review considered the effects of treatment with opioid antagonists versus other approaches for withdrawal.
Search date
The evidence is current to December 2016.
Study characteristics
We identified 10 studies, including six randomised controlled trials (where people are randomly put into one of two or more treatment groups) and four prospective cohort studies (where participants could choose which treatment they received) involving 955 opioid‐dependent participants. Four of the studies took place in the UK, three in the USA, two in Italy and one in Australia. Nine of the 10 studies compared treatment with an opioid antagonist (naltrexone or naloxone) plus an adrenergic agonist (clonidine or lofexidine) versus a regimen based on clonidine or lofexidine alone. Other comparisons (placebo, reducing doses of methadone, buprenorphine) made by included studies were too diverse for any meaningful analysis.
Four studies received some financial support from a pharmaceutical company.
Key results
We are uncertain whether peak withdrawal induced by opioid antagonists plus clonidine or lofexidine is more severe than withdrawal managed with clonidine or lofexidine alone, or whether the average severity over the withdrawal period is less, as the certainty of the evidence is very low.
Clinicians should warn people of the possibility of delirium in the first day of administration of naltrexone, particularly with higher doses (> 25 mg). People should also know that withdrawal will be moderately severe and that symptoms such as muscle aches, vomiting and diarrhoea, and insomnia are likely to persist despite medication.
Quality of the evidence
The studies included in this review were diverse and generally of very low quality. As a result there is considerable uncertainty about the value of approaches using opioid antagonists to induce opioid withdrawal as a means of managing withdrawal from opioid dependence.
Authors' conclusions
Summary of findings
| Antagonist‐adrenergic combination compared to adrenergic agonist for opioid withdrawal | |||
| Patient or population: opioid dependent adults | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Peak withdrawal severity ‐ Naltrexone | In 1 study peak withdrawal severity was similar for the 2 types of intervention. In the other study peak withdrawal was more severe in the group receiving antagonist‐adrenergic combination. | 184 | ⊕⊝⊝⊝ |
| Peak withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found more severe withdrawal with naloxone‐clonidine combination. | 91 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naltrexone | No difference in overall withdrawal severity for 2 studies; in 1 study overall severity significantly less for antagonist‐adrenergic combination. | 256 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found less severe overall withdrawal with naloxone‐lofexidine combination. | 49 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naltrexone | Completion rate with adrenergic agonist only ranged from 42% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 95% | 330 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naloxone | Completion rate with adrenergic agonist only ranged from 28% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 98% | 463 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| aOne study at high risk of bias. | |||
Background
Description of the condition
Dependence on opioid drugs is a major health and social issue in most societies. While only around 0.2% of adults report unsanctioned use of opioid drugs globally (Gowing 2015), these substances contribute more to the burden of disease than other illicit psychoactive drugs. The burden to the individual user and the community of opioid dependence arises from premature mortality and disability associated with dependent use (with the greatest impact in younger drug users; Gowing 2015), transmission of human immunodeficiency virus (HIV) and hepatitis C, healthcare costs, crime and law enforcement costs,and the less tangible costs of family disruption and lost productivity (Mark 2001).
Treatment is central to reducing the harms incurred by individuals and the community from opioid dependence. Managed withdrawal, or detoxification, by itself is not an effective treatment for dependence (Lipton 1983; Mattick 1996). Rates of withdrawal completion tend to be low, whereas rates of relapse to opioid use following detoxification are high (Broers 2000; Gossop 1989; Vaillant 1988). However, withdrawal remains a required first step for many forms of longer‐term treatment such as residential rehabilitation and naltrexone maintenance (Kleber 1982). It may also represent the endpoint of an extensive period of substitution treatment such as methadone maintenance. As such, managed withdrawal is an essential part of an effective and comprehensive treatment system.
The signs and symptoms of the opioid withdrawal syndrome include irritability, anxiety, apprehension, muscular and abdominal pains, chills, nausea, diarrhoea, yawning, lacrimation, sweating, sneezing, rhinorrhoea, general weakness, and insomnia. Symptoms of opioid withdrawal usually begin two to three half‐lives after the last opioid use, that is, 6 to 12 hours after taking short half‐life opioids such as heroin and morphine, and 36 to 48 hours after taking long half‐life opioids such as methadone. Following cessation of a short half‐life opioid, symptoms reach peak intensity within two to four days, with most of the obvious physical withdrawal signs no longer observable after 7 to 14 days. As with onset of withdrawal, its duration also varies with the half‐life of the opioid used and the history of regular use (Tetrault 2009). Opioid withdrawal is rarely life‐threatening or associated with significant aberrations of mental state (Farrell 1994), but the combination of uncomfortable symptoms and intense craving makes completing withdrawal difficult for most people (Mattick 1996; Tetrault 2009).
Description of the intervention
For many years routine procedures involved suppression of withdrawal with methadone and gradual reduction of the methadone dose (Kleber 1982). This approach derived from observations that the withdrawal syndrome from methadone was milder, though longer, than from morphine. Methadone's high oral bioavailability, efficacy and long duration of withdrawal relief (24 to 36 hours) were additional factors that contributed to the widespread use of methadone in specialist withdrawal programmes.
Ambivalence to using a drug of dependence to treat opioid dependence, government restrictions on prescriptions, and consumer dislike of the protracted nature of methadone withdrawal have all limited, to some extent, the use of methadone for managing opioid withdrawal (Farrell 1994). The alpha2‐adrenergic agonist clonidine has been widely used as a non‐opioid alternative for managing opioid withdrawal because of its capacity to ameliorate some signs and symptoms of opioid withdrawal (Gossop 1988), but it is associated with adverse effects of sedation and hypotension. Disatisfaction with methadone and clonidine have driven research into a variety of alternative approaches. This review focuses on approaches that involve administering opioid antagonists to induce withdrawal, in combination with medication to ameliorate withdrawal symptoms with minimal sedation.
How the intervention might work
The rationale underlying the use of opioid antagonists to induce withdrawal is that a faster transition from dependence to abstinence might increase rates of withdrawal completion. Initial experiments with opioid antagonists to induce withdrawal date back to the 1970s (Bearn 1999). While initial studies showed that the objective signs of withdrawal reduce rapidly with successive injections of naloxone (the only opioid antagonist available at that time), there was little further interest in the approach until clonidine became available. It seems likely that the use of antagonists alone was limited by poor acceptability to opioid users. Experience with the capacity of clonidine to ameliorate the signs and symptoms of opioid withdrawal led to studies investigating clonidine (and subsequently other alpha2‐adrenergic agonists as well as other medications) plus opioid antagonists for managing opioid withdrawal.
Rapid detoxification techniques have seen further development with the administration of opioid antagonists in conjunction with sedation or anaesthesia to suppress people's awareness of withdrawal (Bearn 1999; Simon 1997). This approach of anaesthetic‐assisted accelerated withdrawal is the subject of a separate Cochrane Review (Gowing 2010).
There is a complex range of variables that can potentially influence the course and subjective severity of withdrawal, including the type of opioid used, dose taken, concomitant use of other drugs including alcohol, duration of use, general physical health, and psychological factors, such as the reasons for undertaking withdrawal and fear of withdrawal (Farrell 1994; Phillips 1986; Preston 1985). Outcomes of a withdrawal episode may also be influenced by a prior period of substitution treatment, since such treatment is likely to result in a degree of stabilisation in health and social functioning that may facilitate successful withdrawal. Where information is available, we consider the influence of these variables.
The first, or acute, phase of withdrawal is followed by a period of six months or so of a secondary or protracted withdrawal syndrome. This protracted syndrome is characterised by a general feeling of reduced well‐being that is reflected in measurable abnormal physiological functioning. During this phase, people may experience strong periodic cravings for opioids. The malaise associated with protracted abstinence is thought to be a major factor in relapse (Satel 1993). The protracted nature of withdrawal typically lengthens the recovery period, in which a range of factors – both social‐ and treatment‐related – are at play. The types of intervention offered in the acute phase of withdrawal differ substantially from those offered afterwards to promote recovery and prevent relapse. These may include psychological and lifestyle counselling, support groups, and pharmacological and medical treatment. We thus excluded this long‐term aspect of treatment of opioid dependence from this review.
Why it is important to do this review
This review is one of a series relating to managing opioid withdrawal. Other reviews consider the use of alpha2‐adrenergic agonists (Gowing 2016), opioid antagonists under heavy sedation or anaesthesia (Gowing 2010), buprenorphine to manage opioid withdrawal (Gowing 2017), tapered doses of methadone (Amato 2013), treatments in inpatient versus other settings (Day 2005), detoxification treatments for adolescents (Minozzi 2014), and psychosocial and pharmacological treatments for opioid detoxification (Amato 2011).
Objectives
To assess the effects of opioid antagonists plus minimal sedation for opioid withdrawal. Comparators were placebo as well as more established approaches to detoxification, such as tapered doses of methadone, adrenergic agonists, buprenorphine and symptomatic medications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐randomised controlled clinical trials (CCTs) and prospective controlled cohort studies. To better control the risk of bias, it is desirable to limit systematic reviews to randomised and quasi‐randomised controlled trials. However, because there are relatively few RCTs of antagonist‐induced withdrawal, this review also includes prospective cohort studies.
Types of participants
Participants who were primarily opioid dependent, as defined by diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD) or by clinical assessment, who underwent managed withdrawal.
Types of interventions
Experimental interventions involved administering an opioid antagonist (naloxone, naltrexone or nalmefene) in the first three days of treatment or within three days of last opioid use to induce opioid withdrawal, in combination with medication to ameliorate the symptoms but with no or minimal sedation.
We excluded studies of interventions involving opioid antagonists under heavy sedation or anaesthesia. A separate Cochrane Review covers this approach (Gowing 2010).
Sedation differs from anaesthesia in that it is still possible to rouse the patient. In addition, the pharmacological agent used to induce sedation is usually different from anaesthetising agents. The studies included in this review involved either no sedation or light sedation as an adjunct to treatment to aid sleep or reduce anxiety.
Comparators involved tapered doses of methadone, an alpha2‐adrenergic agonist, buprenorphine, symptomatic medications or placebo to manage withdrawal, or antagonist‐based regimens differing in the type or dose regimen of opioid antagonist. For the purpose of this review, we define symptomatic medications as benzodiazepines, anti‐emetics, anti‐diarrhoeals, anti‐psychotics, anti‐spasmodics, muscle relaxants or non‐opioid analgesics, administered in combination as needed, or according to a defined regimen.
Types of outcome measures
Primary outcomes
We assessed the included studies on the basis of a number of measures.
-
Severity of the withdrawal syndrome experienced (peak withdrawal scores and average withdrawal score over the duration of withdrawal treatment).
-
Duration of treatment.
-
Nature and incidence of adverse effects.
-
Completion of treatment.
The rationale for antagonist‐induced withdrawal is that a faster transition from dependence to abstinence might increase rates of withdrawal completion. Hence we considered duration of treatment, in addition to rates of treatment completion to assess the extent to which the withdrawal process was shortened by the use of opioid antagonists.
It is difficult to differentiate treatment side effects from the signs and symptoms of opioid withdrawal. We have defined adverse effects as clinically significant signs and symptoms of opioid withdrawal (such as vomiting and diarrhoea) plus any incidents that are not typical components of the opioid withdrawal syndrome (delirium, hypotension, dry mouth).
Secondary outcomes
We also sought to assess data on the number of participants engaged in further treatment following completion of the withdrawal intervention. Managed withdrawal by itself is not an effective treatment for dependence. Hence we consider engagement in further treatment to be an outcome of interest. Engagement in naltrexone maintenance treatment is of particular interest as transition to naltrexone is the main reason for selecting antagonist‐induced withdrawal.
Search methods for identification of studies
All searches included non‐English language literature. We assessed studies with English abstracts on the basis of the abstract. If we thought the study was likely to meet inclusion criteria we translated it sufficiently to extract study methods and results.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11), MEDLINE (1946 to December week 1, 2016), Embase (1974 to 22 December 2016), PsycINFO (1806 to December week 3, 2016) and the Web of Science (1945 to 22 December 2016).
We developed a search strategy to retrieve references for all the Cochrane Reviews relating to the management of opioid withdrawal in one operation. We adapted this strategy to each of the major databases and the supporting platform. See Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5.
Searching other resources
We also searched:
-
the reference lists of all relevant papers to identify further studies;
-
some of the main electronic sources of ongoing trials: ISRCTN registry (www.isrctn.com ), ClinicalTrials.gov (clinicaltrials.gov/) and Trialsjournal.com (www.trialsjournal.com/);
-
conference proceedings likely to contain trials relevant to the review.
We contacted investigators to seek information about unpublished or incomplete trials.
Data collection and analysis
Selection of studies
One author (LG) assessed each potentially relevant study for inclusion according to the identified inclusion and exclusion criteria. All authors confirmed the inclusion and exclusion decisions.
Data extraction and management
We developed a form for recording data on the outcomes of interest, taking into account the different ways that studies might report such data. The outcomes specified in the form were:
-
intensity of withdrawal (peak withdrawal score, overall withdrawal score, number with severe withdrawal, other data such as amount of adjunct medication);
-
duration of withdrawal treatment (days in treatment, other data such as time of dropout);
-
adverse effects (number experiencing any adverse effects, number with hypotensive effects, number with delirium, number withdrawn from treatment due to adverse effects, other);
-
completion of treatment (number completing the scheduled period of treatment, number abstinent at completion of treatment);
-
postdetoxification (number engaged in further treatment, other such as number abstinent at follow‐up).
One author (LG) extracted key information, in consultation with other authors where there was any uncertainty. We contacted study authors if we required additional information to include studies in meta‐analyses.
Assessment of risk of bias in included studies
We assessed the risk of bias of included studies according to the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions and criteria developed by the Cochrane Drugs and Alcohol Group for assessing prospective observational studies (Higgins 2011; Appendix 6).
We evaluated seven specific methodological domains (namely, sequence generation, allocation concealment, blinding of participants and providers, blinding of outcome assessor, incomplete outcome data, selective outcome reporting, and other issues). For each study we analysed the seven domains as reported in the study and provided a final judgement on the likelihood of bias in terms of low, high or unclear risk. We based these judgements on the criteria indicated in Higgins 2011 and their applicability to the addiction field. We also incorporated criteria drawn from the Newcastle‐Ottawa Scale for observational studies (Wells 2010). We operationalised the 'Risk of bias' tables for assessing RCTs, CCTs and prospective observational studies using this approach.
We considered blinding for subjective and objective outcomes separately. Lack of blinding is a source of serious risk of bias for subjective outcomes but is less significant with objective outcomes such as treatment completion and duration. We only considered incomplete outcome data for intensity of withdrawal and the nature and incidence of adverse effects. Retention in treatment (duration of treatment) and completion of treatment are frequently primary outcome measures in addiction research. See Appendix 6 for a detailed description.
Details of the assessments of risk of bias are in the Characteristics of included studies.
Measures of treatment effect
For dichotomous data (e.g. number completing treatment), we calculated risk ratios (RR), and for continuous data with consistent methods of measurement (e.g. time in treatment), we calculated mean differences (MD). For continuous data with differences between studies in the method of measurement (e.g. withdrawal scores), we calculated standardised mean differences (SMD). We used 95% confidence intervals (CI) to express the uncertainty in each result.
Unit of analysis issues
Where there were trials with multiple arms, we excluded any arms that involved an intervention not defined by the inclusion criteria for this review. Where there were trials with multiple arms relevant to meta‐analyses, we considered whether it was appropriate to combine or separate the arms in analyses.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Chi2 test and its P value, by visual inspection of the forest plots and the I2 statistic. We regarded heterogeneity as substantial if the I2 was greater than 50% or the P value lower than 0.10 for the Chi2 test for heterogeneity. Following guidance in Higgins 2011, we distinguished the values to denote heterogeneity as unimportant (0% to 40%), moderate (30% to 60%), substantial (50% to 90%) and considerable (75% to 100%).
Data synthesis
We used Review Manager 5 for statistical analyses (RevMan 2014). In all analyses we used a random‐effects model.
We suppressed the calculation of overall effects in all meta‐analyses. Studies included in this review were heterogeneous in design (six randomised controlled trials and three prospective cohort studies) as well as in methods for assessing outcomes and study findings. This heterogeneity made the validity of calculating overall effects doubtful.
Subgroup analysis and investigation of heterogeneity
This review also aimed to consider the following potential sources of heterogeneity through subgroup analyses, as this approach is considered to be associated with less risk of bias: drug of dependence and severity of dependence (as indicated by duration and level of use); poly‐drug use; concurrent physical and psychiatric illness; precipitants to the withdrawal episode; the nature of the treatment setting; and the nature of adjunct treatment, including other medications to manage symptoms. Insufficient studies met the inclusion criteria to support such analyses.
Sensitivity analysis
We did not use risk of bias as a criterion for inclusion in the review. However, we intended to assess the impact of risk of bias through sensitivity analysis. This would have involved considering the overall estimate of effect when including or excluding studies at high risk of bias, but there were insufficient studies with data for such analyses to be undertaken.
Summary of findings table
We assessed the overall quality of the evidence for the primary outcome using the GRADE system. The Grading of Recommendation, Assessment, Development and Evaluation Working Group (GRADE) developed a system for grading the quality of evidence that takes into account issues related to both internal and external validity, such as directness, consistency, imprecision of results and publication bias (Guyatt 2008; Guyatt 2011; Oxman 2004).
The 'Summary of findings' table presents the main findings in a transparent and simple tabular format. In particular, they provide key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grades of evidence.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
We downgraded the certainty of evidence for the following reasons.
-
Serious (−1) or very serious (−2) study limitation for risk of bias.
-
Serious (−1) or very serious (−2) inconsistency between study results.
-
Some (−1) or major (−2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review).
-
Serious (−1) or very serious (−2) imprecision of the pooled estimate (−1).
-
Publication bias strongly suspected (−1).
We would have upgraded the certainty of evidence in observational studies for the following reasons.
-
Strong evidence of association – significant relative risk of more than 2.0 (< 0.5) based on consistent evidence from two or more observational studies, with no plausible confounders (+ 1).
-
Very strong evidence of association – significant relative risk of more than 5.0 (< 0.2) based on direct evidence with no major threats to validity (+ 2).
-
Evidence of a dose response gradient (+ 1).
-
All plausible confounders would have reduced the effect (+ 1).
Results
Description of studies
Results of the search
This is an update of a Cochrane Review that was first published in 2000 under the title 'Opioid antagonists and adrenergic agonists for the management of opioid withdrawal'. We substantially updated the review in 2009 to incorporate a modified search strategy and changed the title to the current version.
In the present update, we again modified the search strategy, adding the Web of Science to the databases searched. We also removed the term 'Metabolic Detoxication, Drug' from the MEDLINE and CENTRAL searches as this term now maps to 'metabolic inactivation', which is not relevant to this review. We have also amended the review to meet current Cochrane requirements and included a flow diagram of the search process (Figure 1).

Flow diagram of literature search
As indicated in the flow diagram (Figure 1), we identified 3573 records relevant to the management of opioid withdrawal in general. Of these, we excluded 3269 on the basis of title and abstract. We assessed 304 full‐text articles, excluding 244 without listing reasons and 47 records (37 studies) with reasons. Thirteen articles (10 studies) satisfied all the criteria to be included in the review. We had previously considered one of the included studies to be a secondary report of one of the other studies, but this update treats it as a separate, primary study. As a result, this update has 10 included studies, compared to 9 in the 2009 version. We did not identify any new studies relevant to the review published since 2009.
Included studies
Ten studies (13 reports) involving 955 participants met the inclusion criteria for this review (see Characteristics of included studies). In total, 458 participants received treatment with an opioid antagonist to induce withdrawal.
Six of the studies were randomised controlled trials (Arnold‐Reed 2005; Beswick 2003; Gerra 1995; Gerra 2000; O'Connor 1997; Umbricht 1999), and three were prospective cohort studies with participants able to choose which treatment modality they received (Bearn 2001; Buntwal 2000; O'Connor 1995). In McCambridge 2007, participants who did not consent to or were excluded from randomisation received methadone. We excluded this group from the review, so this study is effectively a randomised controlled trial.
Four studies received some financial support from a pharmaceutical company, four studies reported funding from government research grants, and two studies did not report funding arrangements.
Comparisons
Nine of the 10 studies that met the inclusion criteria compared an opioid antagonist‐adrenergic agonist combination treatment with a regimen based primarily on either an alpha2‐adrenergic agonist, clonidine (Arnold‐Reed 2005; Gerra 1995; Gerra 2000; O'Connor 1995; O'Connor 1997), or on an analogue of clonidine: lofexidine (Bearn 2001; Beswick 2003; Buntwal 2000; McCambridge 2007).
Studies comparing clonidine and lofexidine as the primary medication have reported similar effectiveness in terms of ameliorating signs and symptoms of opioid withdrawal. However, lofexidine is associated with fewer hypotensive side effects than clonidine (Gowing 2016). Gerra 2001 directly compared clonidine and lofexidine as adjunct medications in withdrawal induced by opioid antagonists. Outcomes were similar for the clonidine‐ and lofexidine‐based regimens in terms of craving levels, morphine metabolites in urine and dropout rate. However, participants receiving lofexidine showed lower levels of withdrawal symptoms, fewer mood problems, less sedation and less hypotensive effects. Overall we consider it reasonable to combine clonidine and lofexidine‐based regimens in analyses but note the potential for heterogeneity in analyses of withdrawal severity and adverse effects.
Like most included studies, this review focuses primarily on an opioid antagonist‐adrenergic agonist combination versus treatment based on an alpha2‐adrenergic agonist for withdrawal.
Four studies compared antagonist‐induced withdrawal with treatment regimens other than alpha2‐adrenergic agonists: in Gerra 1995 the comparator was placebo; in Gerra 2000, tapered doses of methadone; and in O'Connor 1997 and Umbricht 1999, a regimen based primarily on buprenorphine, although in O'Connor 1997 participants in the buprenorphine group were transferred to 25 mg naltrexone plus clonidine on day 4 of treatment.
Because of the small number of studies investigating each of these comparisons, we could draw few conclusions. We did not include these comparisons in analyses, therefore, but we do discuss them where relevant to the review.
Treatment setting
Of the 10 studies that met the inclusion criteria, 5 carried out detoxification on a full inpatient basis (Bearn 2001; Beswick 2003; Buntwal 2000; McCambridge 2007; Umbricht 1999), and two used an outpatient setting with day care (Gerra 1995; Gerra 2000). Arnold‐Reed 2005 and O'Connor 1995 used an outpatient setting with full day care for the first day of administration of naltrexone. O'Connor 1997 described the setting as outpatient without indicating whether it also involved an extended period of care on the first day of naltrexone treatment.
Four of the studies took place in the UK, three in the USA, two in Italy and one in Australia.
Participant characteristics
In 6 of the 10 studies that met the inclusion criteria, all participants were withdrawing from heroin. Participants in Umbricht 1999 were atypical for heroin users in the USA in that only 30% were injectors. Participants in four studies were using heroin, methadone or both (Bearn 2001; Beswick 2003; Buntwal 2000; McCambridge 2007). All participants in these studies were stabilised on methadone for three days prior to detoxification. The mean stabilisation doses were 32.0, 34.3 and 40.0 mg/day, respectively, for Bearn 2001, Beswick 2003 and Buntwal 2000, whereas McCambridge 2007 did not report them.
Opioid use is more common amongst men than women. Consistent with this, between 55% and 82% of participants in the included studies were men. (Beswick 2003 did not report details of sex.)
Treatment regimens
Six of the 10 included studies used a treatment regimen based on naltrexone. Four of these studies administered an initial dose of 12.5 mg, given orally as a single dose on day 1 of treatment in O'Connor 1995 and O'Connor 1997 or day 2 in Gerra 1995 and Umbricht 1999. Buntwal 2000 used a similar starting dose (14 mg) but administered it orally in five divided doses over the first day of treatment. Participants also received 0.8 mg naloxone intramuscularly (IM) just prior to the first oral dose of naltrexone (1 mg). Authors reported using this approach in case of a severe withdrawal reaction.
The treatment protocols of three studies called for a maintenance dose of 50 mg/day naltrexone to be reached on day 3 (Gerra 1995; O'Connor 1995; O'Connor 1997). In Buntwal 2000 participants were scheduled to receive 50 mg naltrexone in two divided doses on day 4, with a single daily dose of 50 mg from day 5. Umbricht 1999 discontinued use of buprenorphine on day 4, with a 50 mg dose of naltrexone scheduled for day 5.
Six studies used naloxone to induce withdrawal. In Bearn 2001, Beswick 2003 and McCambridge 2007, participants received a single injection of 0.8 mg naloxone in the morning of days 3 to 6. Bearn 2001 offered participants 25 mg of oral naltrexone on day 7, the day after completion of withdrawal treatment. the following day. Beswick 2003 and McCambridge 2007 did not report the use of naltrexone.
Participants in Gerra 1995 received 0.2 mg naloxone intravenously (IV) on day 2, then 0.4 mg twice a day on days 3 and 4, before commencing naltrexone 50 mg/day on day 5. Participants in Gerra 2000 received repeated IV injections of 0.04 mg naloxone to a total of 0.4 mg/day on days 1 and 2 of treatment. This regimen of repeated injections was followed by 5 mg oral naltrexone at 6:00 pm on day 1 and 50 mg on day 2.
Arnold‐Reed 2005 administered 0.8 mg naloxone in repeated doses until no significant withdrawal was apparent. After a rest of 20 to 30 minutes, participants received oral naltrexone in doses of 4 mg, 8 mg, 15 mg and 23 mg at 30‐minute intervals.
Because the naltrexone and naloxone regimens differ substantially, we performed separate analyses for each of these opioid antagonists. We included Arnold‐Reed 2005 in the naloxone group as this was the antagonist used to induce withdrawal.
Clonidine and lofexidine are usually administered orally. However, Gerra and colleagues used intravenous infusion in their two studies for administering clonidine as an adjunct to antagonist‐induced withdrawal. In Gerra 1995 they infused 0.15 mg clonidine three times a day, giving a total dose of 0.45 mg/day as an adjunct to either naloxone‐ or naltrexone‐based withdrawal. Gerra 2000 administered 0.15 mg clonidine six times a day in 100 mL saline for two days, with an additional oral dose of 0.15 mg in the evening. This equates to 1.05 mg/day for two days. The dose was reduced to 0.15 mg orally three times on day 3 (0.45 mg/day).
Other than Gerra 1995, all studies reported the use of a range of additional adjunct medications, including benzodiazepines, non‐steroidal anti‐inflammatory drugs (analgesics), anti‐emetics and muscle relaxants.
Excluded studies
After a detailed assessment, we excluded 37 studies (47 reports) that we considered potentially relevant to the review (see Figure 1 and Characteristics of excluded studies). The reasons for exclusion were: no treatment comparison (20 studies), retrospective data collection (2 studies), case reports other than controlled study design (4 studies), insufficient data on outcomes defined for this review (9 studies), comparison of interventions other than those defined by the inclusion criteria (6 studies); and a primary focus on aspects other than the management of withdrawal (4 studies). We excluded some studies for more than one reason.
Risk of bias in included studies
For summary results of the 'Risk of bias' assessment across the included studies for each domain, see Figure 2 and Figure 3. The following sections summarise our judgements of the risk of bias for the included studies.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
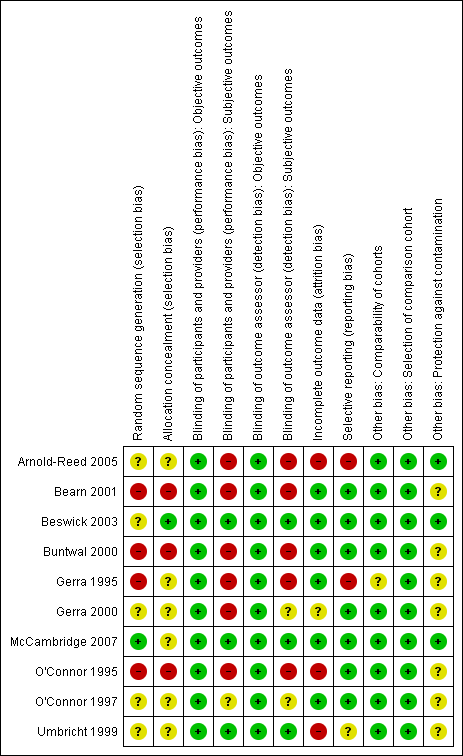
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
We judged 4 of the 10 studies to be at high risk of selection bias due to random sequence generation (Bearn 2001; Buntwal 2000; Gerra 1995; O'Connor 1995). We judged three studies to be at high risk of selection bias due to allocation concealment (Bearn 2001; Buntwal 2000; O'Connor 1995). We considered that only McCambridge 2007 was at low risk of selection bias due to sequence generation, and only Beswick 2003 was at low risk for allocation concealment. We considered the risk of bias to be unclear in the remaining studies.
Blinding
We deemed the risk of performance and detection bias for objective outcomes (duration and completion of treatment) to be low for all studies, as these outcomes are unlikely to be affected by an awareness of group allocation. This section therefore focuses on the risk of performance and detection bias in relation to subjective outcomes (intensity of withdrawal, occurrence and severity of adverse effects).
We judged six studies to be at high risk of performance bias for subjective outcomes (Arnold‐Reed 2005; Bearn 2001; Buntwal 2000; Gerra 1995; Gerra 2000; O'Connor 1995). We considered the risk of performance bias to be low in three studies (Beswick 2003; McCambridge 2007; Umbricht 1999), while it was unclear in O'Connor 1997.
We judged five studies to be at high risk of detection bias for subjective outcomes (Arnold‐Reed 2005; Bearn 2001; Buntwal 2000; Gerra 1995; O'Connor 1995). In three studies, we considered the risk of detection bias to be low (Beswick 2003; McCambridge 2007;Umbricht 1999), while in two studies the risk was unclear (Gerra 2000;O'Connor 1997).
Incomplete outcome data
Retention (duration of treatment) and completion of treatment are primary outcome measures for opioid withdrawal interventions. Hence we only considered the risk of bias due to incomplete data for the outcomes of intensity of withdrawal and adverse effects.
We deemed three studies to be at high risk of bias due to incomplete outcome data (Arnold‐Reed 2005; O'Connor 1995;Umbricht 1999). In Gerra 2000, the risk of bias due to incomplete outcome data was unclear, while it was low in the remaining studies.
Selective reporting
We judged two studies to have a high risk of bias due to selective reporting (Arnold‐Reed 2005; Gerra 1995), while the risk was unclear in Umbricht 1999. We judged the risk to be low for the other studies.
Other potential sources of bias
We considered that seven studies were at unclear risk of other bias due to insufficient information on the comparability of the groups in Gerra 1995 and unclear protection against contamination of the groups in Bearn 2001, Buntwal 2000, Gerra 1995, Gerra 2000, O'Connor 1995, O'Connor 1997 and Umbricht 1999.
Effects of interventions
We present results in two sections. The first considers the major comparison between withdrawal induced by an opioid antagonist‐adrenergic agonist combination and withdrawal managed primarily with an adrenergic agonist. The second section considers other comparisons. Within each section, we address the four types of outcome measures of interest: intensity of withdrawal syndrome, duration of withdrawal treatment, nature and incidence of adverse effects and completion of treatment and postdetoxification outcomes.
Antagonist‐adrenergic agonist combination versus adrenergic agonist
Severity of withdrawal
The capacity for and strength of meta‐analysis for this outcome was limited by:
-
the small number of studies reporting data on the severity of withdrawal;
-
different approaches to the assessment of the severity of withdrawal; and
-
different ways of presenting the data.
The two studies by O'Connor and colleagues used the same rating instrument comprising 24 items, each graded for severity on a four‐point scale (O'Connor 1995; O'Connor 1997). Gerra 1995 and Gerra 2000 used a scale that comprised nine items, primarily objective signs assessed by an observer and graded zero to five for severity. Bearn 2001, Beswick 2003 and Buntwal 2000 all used the Short Opiate Withdrawal Scale, which has 10 patient‐reported items, each graded zero to three for severity (Gossop 1990). Arnold‐Reed 2005 reported using part 1 of the Severity of Dependence Questionnaire to assess physical withdrawal. This scale usually asks respondents to consider a recent month and to assess the frequency with which they experienced 10 symptoms on waking, before the first dose of opiates. Presumably Arnold‐Reed 2005 adjusted this scale to record symptoms at the time of assessment.
Studies also varied in the way they reported assessments of withdrawal severity. Gerra 1995 reported total mean (standard deviation (SD)) withdrawal scores at time points of 48 hours and 72 hours after commencement of detoxification. O'Connor 1995 reported the withdrawal score at admission only. However, they reported the amount of oxazepam used by participants in each group during detoxification as an alternative indicator of severity. O'Connor 1997 reported the mean (SD) overall withdrawal score and the mean (SD) peak withdrawal score. Buntwal 2000 and Bearn 2001 both undertook an area‐under‐the‐curve analysis of daily withdrawal scores. Beswick 2003 and Gerra 2000 reported withdrawal scores only as graphs of mean daily scores and statistical analyses that were not suitable for inclusion in analyses. Arnold‐Reed 2005 assessed physical withdrawal immediately prior to and postdetoxification.
Gerra 1995 presented graphs of withdrawal scores at 24 hours, 48 hours and 72 hours after commencement of treatment, and a table of mean withdrawal score at 48 hours and 72 hours. The 24‐hour assessment point was prior to the administration of opioid antagonist. The score at 48 hours was higher than that at 72 hours for all except the placebo group. On this basis we have taken the 48‐hour score to represent the peak mean score, making it equivalent to the mean peak withdrawal scores reported in O'Connor 1997. We present data from these studies in Analysis 1.1. (Note that we have entered peak withdrawal data from Gerra 1995 for the group treated with clonidine twice: once for comparison with the naltrexone‐clonidine combination, and once for comparison with the naloxone‐clonidine combination. As we did not calculate an overall result, there is no double‐counting of these participants.)
Similarly, we have taken the data reported in O'Connor 1995 on oxazepam use during detoxification to be an indicator of overall withdrawal severity. While substantially different in nature, the mean overall withdrawal scores from O'Connor 1997 and the area‐under‐the‐curve data from Bearn 2001 and Buntwal 2000 similarly indicate the overall severity experienced. These data are therefore presented together in Analysis 1.2.
The studies that provide data on peak withdrawal severity provide quite different results (Gerra 1995; O'Connor 1997; Analysis 1.1). In O'Connor 1997 peak withdrawal severity was similar for the two groups [28 (SD 13.1) versus 29.9 (SD 14.9), using a scale with a maximum possible score of 72]. However, in Gerra 1995 peak withdrawal severity is significantly higher in the groups treated with an antagonist [22.88 (SD 11.06) for clonidine‐naltrexone and 15.22 (SD 9.49) for clonidine‐naloxone compared to 5.44 (SD 1.87) for the group treated with clonidine only, using a scale with a maximum possible score of 45].
Gerra 1995 and O'Connor 1997 differ in two aspects: the use of adjunct medications and the method for assessing withdrawal severity. These differences could explain the different results. Participants in O'Connor 1997 rated withdrawal using a 24‐item scale. This scale comprised a mix of signs and symptoms but was predominantly subjective. In Gerra 1995 observers rated each of nine items (pulse rate, tremor, rhinorrhoea, mydriasis, aching muscles, shiver, vomiting, anxiety and insomnia) from zero to five for severity. Graphical presentation of withdrawal at 48 hours (the first day of administration of the opioid antagonist) indicated a significant contribution to the score by pulse rate, an item that was not rated in O'Connor 1997.
Gerra 2000 assessed withdrawal severity with the same rating system used in Gerra 1995. Authors reported mean daily withdrawal scores graphically and in terms of statistical tests, not in a form that enabled data extraction. However, the graphs and statistical tests show no difference in peak withdrawal severity in the group treated with naloxone, naltrexone and clonidine compared to the group treated with clonidine alone. This finding suggests that the rating scale is not sufficient to explain the different results. The treatment regimen used in Gerra 2000 differed from those used in Gerra 1995 in the pattern of administration of naloxone and naltrexone, in the doses of clonidine, and in the use of adjunct medication. Gerra 1995 did not report use of adjunct medications, while in Gerra 2000, the group treated with naloxone and naltrexone received oxazepam, baclofen and ketoprofen over the two days of initial antagonist treatment. The authors note that this group, as a result, experienced more sedation and suggested that this countered the affective distress and dysphoria of withdrawal. O'Connor 1995 also used adjunct medications on the first day of antagonist administration, in this case oxazepam, ibuprofen, ketorolac and prochlorperazine. Hence it would seem likely that the differing result reported in Gerra 1995 compared to O'Connor 1997 and Gerra 2000 reflects the use of adjunct medications.
Beswick 2003 report mean daily withdrawal scores to be significantly lower for the group receiving naloxone and lofexidine compared to lofexidine alone, from day 5 (with the first injection of naloxone or placebo occurring on day 3). However, withdrawal was more pronounced in the naloxone‐lofexidine group one hour after injection of naloxone on day 3.
The four studies that provide data indicating overall withdrawal severity also produced differing results (Analysis 1.2). Two studies found overall withdrawal severity to be significantly less in the groups treated with an opioid antagonist combined with an adrenergic agonist, compared to those treated primarily with the adrenergic agonist alone (Bearn 2001; Buntwal 2000). However, data from O'Connor 1995 and O'Connor 1997 indicate no significant difference in overall withdrawal severity. These studies differed in:
-
the opioid antagonist used (Bearn 2001 used naloxone whereas the other three used naltrexone);
-
the adrenergic agonist used (Bearn 2001 and Buntwal 2000 used lofexidine, while O'Connor 1995 and O'Connor 1997 used clonidine);
-
the drug of dependence (in Bearn 2001 and Buntwal 2000 participants were stabilised on methadone for three days prior to withdrawal, while all participants in O'Connor 1995 and O'Connor 1997 were withdrawing from heroin);
-
the means of assessing withdrawal severity (Bearn 2001 and Buntwal 2000 used the 10‐item Short Opiate Withdrawal Scale with multiple assessments each day while O'Connor 1997 used a 24‐item scale completed once a day, and O'Connor 1995 only provided data on the use of oxazepam);
-
the means of calculating an overall statistic (Buntwal 2000 and Bearn 2001 undertook an area‐under‐the curve analysis while O'Connor 1997 calculated the mean overall score, and O'Connor 1995 provided mean overall doses of oxazepam); and
-
study design (O'Connor 1997 was a randomised controlled trial while the other studies were prospective cohort studies with participants able to choose the treatment received).
With only four studies, it was not possible to determine the extent to which these differences may be contributing to the variability in findings.
Duration of treatment
Bearn 2001 provided detoxification as part of a structured programme of inpatient care. While not specifically stated, this is likely to also be the case in Beswick 2003 and Buntwal 2000, which involved the same inpatient facility and research team. As a consequence of this approach, the duration of treatment reflects both detoxification and after‐care. There was no significant difference in any of these studies between the mean length of stay for the group receiving an antagonist‐based regimen and the group receiving an adrenergic agonist only, with the mean length of stay ranging from 15 days for the lofexidine‐naltrexone group in Buntwal 2000 to 17.5 days for the lofexidine‐naloxone group in Beswick 2003. McCambridge 2007 again involved the same inpatient facility and research team. In this study, participants in the lofexidine‐naloxone group remained in treatment for an average 10.2 days after detoxification, while those in the lofexidine‐placebo group remained in treatment for an average 6.7 days.
Arnold‐Reed 2005, O'Connor 1995, Gerra 1995 and Gerra 2000 did not report any data on the duration of treatment.
Nature and incidence of adverse effects
O'Connor 1995 reported that 4 of 68 (6%) participants treated with clonidine‐naltrexone experienced mild to moderate delirium on the first day of treatment, compared to none of the 57 treated with clonidine only. One of the clonidine‐only group experienced symptoms of hypotension, but this may have been due to an unrelated medical condition.
Bearn 2001 reported the development of a "transient, self‐limiting confusional state" in 2 of 26 (8%) of those treated with naltrexone and lofexidine. Buntwal 2000 reported that one participant in the lofexidine group experienced dizziness and was given a lower dose. Authors reported no other adverse effects. In both these studies prochlorperazine was used as an adjunct medication. Although used as an anti‐emetic, prochlorperazine has some anti‐psychotic activity which may have helped reduce delirium in these studies.
Arnold‐Reed 2005, Gerra 1995, Gerra 2000, McCambridge 2007 and O'Connor 1997 did not discuss adverse effects.
Completion of treatment and postdetoxification outcomes
Four studies reported data on treatment completion with naltrexone regimens versus an adrenergic agonist, and six studies with naloxone regimens, with data from Gerra 1995 included in both subcategories (Analysis 1.3). There is significant heterogeneity in these data. Two studies reported significantly higher rates of treatment completion for antagonist‐induced withdrawal compared to regimens based on alpha2‐adrenergic agonists alone (Arnold‐Reed 2005; O'Connor 1995), but in the remaining studies the difference was not significant.
Three of the nine studies providing data on treatment completion were prospective cohort studies and hence are at high risk of allocation bias (Bearn 2001; Buntwal 2000; O'Connor 1995). We consider Gerra 1995 to be at high risk of allocation bias, while the risk was unclear in O'Connor 1997 and Beswick 2003.
A further point of variability in the studies reporting data on completion of withdrawal was the definition of 'completion'. O'Connor 1995 and O'Connor 1997 defined completion by the administration of a 50 mg dose of naltrexone. Gerra 1995 did not define the endpoint, but they reported the number of participants who completed one week of treatment, at which time participants in all treatment groups were scheduled to have achieved a 50 mg dose of naltrexone. Gerra 2000 defined the endpoint as commencement of extended naltrexone treatment, which would also equate to achievement of a 50 mg dose of naltrexone.
The nature of the endpoint used in Gerra 2000 introduces some risk of bias favouring the naloxone‐clonidine group. Participants in this group were scheduled to receive 50 mg naltrexone at the end of the second day of treatment, whereas participants in the clonidine group did not commence naltrexone until day 6. However, this schedule reflects the way in which these treatment regimens would be used in a context of routine service delivery, so the comparison is realistic.
Bearn 2001, Beswick 2003, Buntwal 2000 and McCambridge 2007 stated the number of participants who completed treatment without defining how this was determined. However, these studies took place in an inpatient setting with regular urine screening throughout treatment. Hence, it is likely that completion of withdrawal was determined on the basis of abatement ofsigns and symptoms of withdrawal along with opioid‐negative urine tests.
Arnold‐Reed 2005 did not define treatment completion.
The lack of studies at low risk of bias along with the heterogeneity in study design and findings makes any conclusions on relative rates of completion of detoxification treatment highly uncertain.
Bearn 2001 reported that 17 of 26 (65%) participants treated with the naloxone‐lofexidine combination elected to take naltrexone on day 7. Naltrexone was continued for a mean of 4.5 days (range 1 to17). Participants in the comparison lofexidine group were not routinely offered naltrexone.
Buntwal 2000 reported that participants in the group treated with the naltrexone‐lofexidine combination continued to take naltrexone for a mean of 9.8 days (range 1 to 29). Three participants refused to continue naltrexone treatment while still receiving inpatient treatment.
Gerra 2000 reported that 15 of 32 participants (47%) treated with the clonidine‐naloxone combination had relapsed to opioid use at six months after detoxification, compared to 18 of 32 (56%) from the clonidine group. However, they reported no difference in opioid‐positive urine samples between the two groups at the six‐month follow‐up.
McCambridge 2007 reported that 9 of 45 (20%) in the lofexidine‐naloxone group were abstinent at follow‐up, compared to 4 of 46 (9%) in the lofexidine‐placebo group. Follow‐up occurred on average 8.7 months following discharge from treatment. These data are at some risk of attrition bias, with only 75% of study participants contacted at follow‐up.
Gerra 1995, O'Connor 1995 and O'Connor 1997 did not report any information on postdetoxification outcomes.
Other comparisons
Four studies compared antagonist‐induced withdrawal with other interventions.
-
Placebo (Gerra 1995).
-
Tapered doses of methadone (Gerra 2000).
-
Buprenorphine (Umbricht 1999).
-
Buprenorphine for three days followed by naltrexone plus clonidine (O'Connor 1997).
Severity of withdrawal
In Gerra 1995 withdrawal scores were significantly higher for the group receiving placebo than either of the groups treated with an opioid antagonist.
In Gerra 2000, peak withdrawal scores were higher and occurred later (day 12 compared to day 3) for the group treated with reducing doses of methadone compared to the group treated with the clonidine‐naloxone combination.
Participants in Umbricht 1999 received tapering doses of buprenorphine on days 1 to 4 of treatment (a total of 26 mg over the four days). In addition, one group received naltrexone 12.5 mg on day 2, increasing to 50 mg on day 5, while the comparison group commenced naltrexone 50 mg on day 8. Withdrawal severity was assessed primarily by observers (11 items, possible range of scores 0 to 30). These withdrawal ratings indicated that mean peak withdrawal severity was similar in the two groups but occurred at different times: 5.2 (SD 3.3) points on day 2 for the group commencing naltrexone on day 2, and 4.0 (SD 3.9) on day 8 for the group commencing naltrexone on day 8. Authors reported that withdrawal symptoms were of moderate intensity.
In O'Connor 1997, overall and peak withdrawal scores were significantly lower in the group receiving buprenorphine prior to naltrexone on day 4 compared to the group commencing naltrexone on day 1.
Duration of treatment
The only study to report data on the duration of treatment was Umbricht 1999. In this study the average length of stay was significantly shorter in the group commencing naltrexone on day 2: 5.9 (SD 0.5) days compared to 7.4 (SD 0.3) days for the comparison group that commenced naltrexone on day 8.
Nature and incidence of adverse effects
No studies reported data on adverse effects.
Completion of treatment and postdetoxification outcomes
In Gerra 2000, 24 of 32 participants (75%) treated with the clonidine‐naloxone combination accepted naltrexone treatment, compared with 9 of 24 (38%) of those treated with tapering doses of methadone. Six months after detoxification, 15 of 32 (47%) in the clonidine‐naloxone group and 29 of 42 (69%) in the methadone group had relapsed to opioid use.
In Umbricht 1999 there were more dropouts from the group administered naltrexone from day 2; 18 of 32 participants (56%) from this group completed the study, compared to 21 of 28 participants (75%) from the group administered naltrexone on day 8. However, in the absence of studies investigating similar treatment approaches, it is not possible to draw any conclusions as to the basis of this result. Umbricht 1999 did not report any postdetoxification outcomes.
In O'Connor 1997, the proportion of participants successfully completing detoxification was the same (81%) in the buprenorphine and antagonist‐induced withdrawal groups.
Discussion
Summary of main results
Severity of withdrawal
The small number of studies, the low quality of the evidence, variability in treatment regimen, and variability in the means of assessing and reporting withdrawal severity make it impossible to draw firm conclusions as to the nature and severity of withdrawal induced by an opioid antagonist‐adrenergic agonist combination compared to withdrawal managed primarily with an alpha2‐adrenergic agonist. However, the findings of the studies that met the inclusion criteria for this review suggest that, when combined with an alpha2‐adrenergic agonist, the severity of antagonist‐induced withdrawal is, at worst, similar to withdrawal managed primarily with an adrenergic agonist, and at best it is less severe. This is probably because of earlier resolution of the signs and symptoms of withdrawal.
Initial administration of an opioid antagonist, particularly naltrexone, may be associated with a peak in withdrawal severity that is greater than the peak that occurs with adrenergic agonist‐based regimens. This difference is most marked with the two antagonist‐based regimens used in Gerra 1995. This was the only study not to report the use of other adjunct medication in addition to the adrenergic agonist. The research team subsequently adopted the use of adjunct medication in Gerra 2000 and considered the higher degree of sedation that resulted to be a factor in countering the "affective distress and dysphoria of withdrawal".
The type of opioid used prior to detoxification could potentially influence the nature and severity of withdrawal. The small number of studies involving participants withdrawing from methadone and the variability in treatment regimens and means of assessing withdrawal, prevented any exploration of the effect of the drug of dependence on withdrawal severity based on studies included in this review. However, Bell 1999 reports the use of naltrexone with adjunct medication (flunitrazepam, clonidine, octreotide, buscopan, quinine) to induce withdrawal in 15 participants withdrawing from heroin and 15 withdrawing from methadone. On day 7, participants rated the acceptability of the treatment on a 10‐point Likert scale where zero was completely acceptable. Of those withdrawing from heroin, 82% (compared to 60% of those withdrawing from methadone) rated acceptability as less than five, indicating the procedure was more acceptable to those withdrawing from heroin compared to those withdrawing from methadone. On the same day, investigators asked participants to rate severity on a 10‐point Likert scale where zero is minimally severe. Of those withdrawing from heroin, 27% (compared to 7% of those withdrawing from methadone) rated severity as less than five. Participants withdrawing from methadone experienced more gastrointestinal symptoms than those withdrawing from heroin. These data suggest less severe symptoms in the cohort withdrawing from heroin, compared to those withdrawing from methadone.
Duration of treatment
Data reported on this aspect were minimal. Consequently it is currently not possible to determine whether antagonist‐induced withdrawal reduces the duration of withdrawal treatment.
Nature and incidence of adverse effects
In addition to vomiting and diarrhoea, some opioid‐dependent people may experience a degree of delirium or confusion following administration of an opioid antagonist. Only two of the studies included in this review reported delirium. However, six studies that did not meet the criteria for inclusion in this review reported delirium as an adverse effect of antagonist‐induced withdrawal (Bell 1999; Charney 1986; Golden 2004; London 1999; Senft 1991; Silverstone 1992). Other studies have reported delirium or confusion after accidental ingestion of naltrexone by people who are opioid dependent (Boyce 2003; Galloway 1993; Mannelli 1999; Tornabene 1974). In all cases the opioid antagonist administered was naltrexone, and in most cases the initial dose of naltrexone was 25 mg or greater. There are no reports of delirium as an adverse effect of treatment regimens involving low doses of naloxone, suggesting that the risk of delirium may be related to the dose and duration of action of the opioid antagonist. While the risk of delirium is probably reduced by the use of lower doses of naltrexone, it can still occur (Charney 1986; Silverstone 1992). The use of other drugs in addition to opioids, particularly benzodiazepines, is one possible risk factor (Bell 1999; Golden 2004).
Adverse effects of alpha2‐adrenergic agonists (dizziness, sedation, dry mouth) are likely to be experienced in the first few days of treatment when high doses of drug are required to ameliorate the withdrawal symptoms induced by the opioid antagonist. However, it appears that hypotension necessitating cessation of adrenergic agonist may be rare when the drug is administered in combination with an opioid antagonist, and even less likely with lofexidine than with clonidine, although London 1999 reported that 1 of 20 participants had a late hypotensive reaction to withdrawal induced by naltrexone administered in conjunction with lofexidine and a range of other adjunct medications.
Bell 1999 reported that vomiting was common with antagonist‐induced withdrawal, but participants treated with the drug octreotide did not require fluid replacement. London 1999, in their review of 20 cases of antagonist‐induced withdrawal, reported that two participants experienced severe vomiting, and five had severe diarrhoea. (The opioid drug, or drugs, from which participants were withdrawing was not specified.)
The studies included in this review did not report any other serious adverse effects.
While a number of studies considered for this review provided antagonist‐based treatment in an outpatient setting, all provided extended care at least for the first day on which the opioid antagonist was administered. This reflects the desirability of providing a high level of monitoring and support until patients are comfortable and development of delirium is considered unlikely (at least several hours after administration of the antagonist).
Completion of treatment and postdetoxification outcomes
The use of naltrexone maintenance in relapse prevention treatment for opioid‐dependent people has been limited by two factors: low rates of commencement on naltrexone, and low rates of retention in treatment (Minozzi 2006; Tucker 2000). Antagonist‐induced withdrawal with minimal sedation is seen as one way in which rates of commencement on naltrexone might be increased.
The data reported in this review suggest that in some situations, antagonist‐induced withdrawal may be associated with significantly higher rates of treatment completion compared to regimens based primarily on an adrenergic agonist, but results are inconsistent, and the extent of any benefit is highly uncertain.
As indicated in the Results, the studies varied in their definitions of treatment endpoint, with only some studies clearly reporting the number of participants achieving maintenance doses of naltrexone, and hence initiating naltrexone maintenance treatment. Hence it is not possible, on the basis of the studies included in this review, to determine whether antagonist‐induced withdrawal helps to increase rates of commencement in naltrexone maintenance treatment. However, any such benefit appears to be relatively short‐lived, with Buntwal 2000 and Bearn 2001 reporting the mean duration of naltrexone treatment to be less than 10 days, and Gerra 2000 reporting no differences in opioid‐positive samples at a six‐month follow‐up for the group detoxified by an antagonist‐based regimen compared to one based on an adrenergic agonist. McCambridge 2007 did not report data on engagement in naltrexone maintenance treatment but did report a higher rate of abstinence at follow‐up for the group receiving antagonist‐based treatment. The authors considered the difference in rates of abstinence at follow‐up to be generally associated with the length of stay postdetoxification rather than the detoxification procedure.
Treatment regimen
A notable feature of the studies included in the review is the variability in the treatment regimens based on opioid antagonists to induce withdrawal. Points of variability include:
-
the use of naloxone or naltrexone, or a combination of both, to induce withdrawal;
-
initial dose, frequency of administration, and rate of increase of the dose of opioid antagonist;
-
the medication used to ameliorate the signs and symptoms of withdrawal (most commonly clonidine or lofexidine but also buprenorphine); and
-
the nature and extent of use of additional adjunct medications.
Due to this degree of variability, it is not possible to describe a standard regimen for this form of antagonist‐induced withdrawal. The small number of studies also prevented sub‐comparisons to determine the effect of the above variations in treatment protocol.
It is of interest that two of the most recent studies adopted an approach of repeated small doses of opioid antagonist with a 50 mg dose of naltrexone achieved on day 4 and the end of day 2 (in Buntwal 2000 and Gerra 2000, respectively). The authors of these studies do not report the reasoning behind their choice of dose regimen; presumably clinical experience with opioid antagonists was a factor. This approach is also reminiscent of some of the earliest studies of antagonist‐induced withdrawal (e.g. Charney 1986; Kleber 1987).
Arnold‐Reed 2005, Bearn 2001, Beswick 2003 and Gerra 2000 used repeated doses of naloxone to induce withdrawal. Bearn 2001 and McCambridge 2007 administered a single dose of 0.8 mg IM in the mornings of the first three days after cessation of methadone. This antagonist regimen reduced the overall withdrawal severity compared to treatment with an adrenergic agonist alone, but Bearn and colleagues note that the response to naloxone was variable. Based on the graph of mean withdrawal score from Bearn 2001, the difference in overall withdrawal severity is unlikely to be clinically significant. The authors themselves note that the "addition of naloxone did not confer substantial benefit over lofexidine monotherapy".
In Gerra 2000, multiple doses of naloxone 0.04 mg IV were administered to a total of 0.4 mg/day for two days. Naltrexone was commenced with a single 5 mg dose on day 1, after the doses of naloxone were completed, and it increased to 50 mg by day 3. Graphs of mean daily withdrawal scores in Gerra 2000 show peak withdrawal severity on day 2. Gerra and colleagues note that mean withdrawal scores were higher for the group treated with opioid antagonist compared to those receiving a clonidine‐based regimen, but the difference was not statistically significant. This suggests that low doses of naloxone, while apparently having little effect on the course of withdrawal, may help to prepare opioid‐dependent people to tolerate subsequent doses of naltrexone.
Overall completeness and applicability of evidence
The studies included in this review provided some information on the severity of withdrawal induced by opioid antagonists with minimal sedation, but this information remains scarce. There is little information on the nature of withdrawal signs and symptoms experienced by patients, the duration of significant symptoms, the overall severity, or the acceptability to patients of this approach to the management of opioid withdrawal. The studies included in this review were too small to provide accurate information on the risks of adverse effects associated with antagonist‐induced withdrawal. It remains uncertain to what extent the type and dose of opioid antagonist, the use of adjunct medications, the person's drug use history (including use of heroin or methadone) may contribute to both withdrawal severity and adverse effects.
Buprenorphine has been shown to be more effective than clonidine or lofexidine for managing opioid withdrawal (Gowing 2017). Eight of the studies included in this review compared antagonist‐induced withdrawal with a regimen based primarily on an alpha2‐adrenergic agonist, and only two studies used buprenorphine as a comparison. Some authors have suggested that due to its pharmacological properties, buprenorphine can facilitate the transition from dependent opioid use to naltrexone maintenance treatment (O'Connor 1997). However, evidence of the effectiveness of antagonist‐induced withdrawal relative to withdrawal managed with buprenorphine is scarce and inconclusive.
Quality of the evidence
The available studies of antagonist‐induced withdrawal with minimal sedation are relatively small and have design limitations with consequent risk of bias. These limitations, together with diversity in the nature of outcomes and findings reported, make it impossible to draw firm conclusions. Both the extent of potential benefits and the risk of harms associated with antagonist‐induced withdrawal remain uncertain.
Potential biases in the review process
The review draws together studies of diverse design and some risk of internal bias. The review itself is consequently exposed to some risk of bias.

Flow diagram of literature search

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
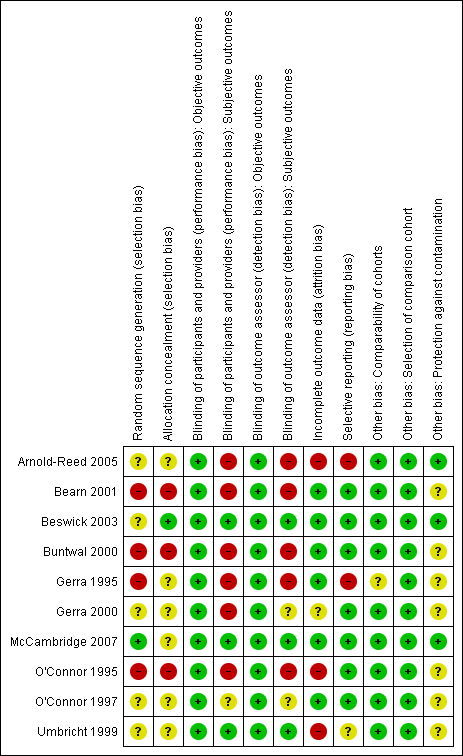
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 1 Peak withdrawal severity.
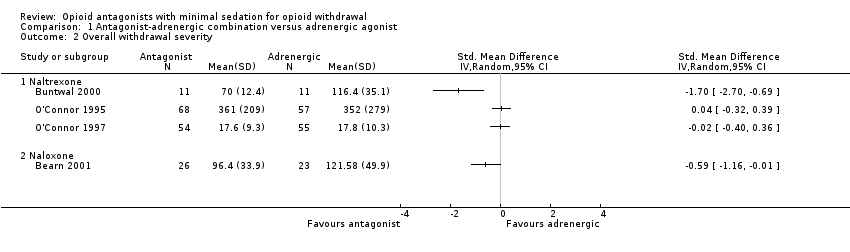
Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 2 Overall withdrawal severity.

Comparison 1 Antagonist‐adrenergic combination versus adrenergic agonist, Outcome 3 Completion rate.
| Antagonist‐adrenergic combination compared to adrenergic agonist for opioid withdrawal | |||
| Patient or population: opioid dependent adults | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Peak withdrawal severity ‐ Naltrexone | In 1 study peak withdrawal severity was similar for the 2 types of intervention. In the other study peak withdrawal was more severe in the group receiving antagonist‐adrenergic combination. | 184 | ⊕⊝⊝⊝ |
| Peak withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found more severe withdrawal with naloxone‐clonidine combination. | 91 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naltrexone | No difference in overall withdrawal severity for 2 studies; in 1 study overall severity significantly less for antagonist‐adrenergic combination. | 256 | ⊕⊝⊝⊝ |
| Overall withdrawal severity ‐ Naloxone | This comparison reported by only 1 study, which found less severe overall withdrawal with naloxone‐lofexidine combination. | 49 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naltrexone | Completion rate with adrenergic agonist only ranged from 42% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 95% | 330 | ⊕⊝⊝⊝ |
| Completion rate ‐ Naloxone | Completion rate with adrenergic agonist only ranged from 28% to 94%. Completion rate with antagonist‐adrenergic agonist combination ranged from 73% to 98% | 463 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| aOne study at high risk of bias. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peak withdrawal severity Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Naltrexone | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Overall withdrawal severity Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Naltrexone | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Naloxone | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Completion rate Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Naltrexone | 4 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Naloxone | 6 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

