Antagonistas de la vitamina K versus heparina de bajo peso molecular para el tratamiento a largo plazo de la tromboembolia venosa sintomática
Resumen
Antecedentes
Las personas con tromboembolia venosa (TEV) generalmente son tratadas durante cinco días con heparina no fraccionada intravenosa o heparina subcutánea de bajo peso molecular (HBPM), seguido de tres meses de antagonistas de la vitamina K (AVK). El tratamiento con AVK requiere mediciones regulares en el laboratorio y conlleva el riesgo de hemorragias; algunos pacientes tienen contraindicaciones para dicho tratamiento. Se ha propuesto que el tratamiento con HBPM reduce el riesgo de complicaciones hemorrágicas. Ésta es una segunda actualización de una revisión publicada por primera vez en 2001.
Objetivos
El propósito de esta revisión fue evaluar la eficacia y la seguridad del tratamiento a largo plazo (tres meses) con HBPM versus el tratamiento a largo plazo (tres meses) con AVK para la TEV sintomática.
Métodos de búsqueda
Para esta actualización el especialista en información del Grupo Cochrane Vascular (Cochrane Vascular Information Specialist, CIS) buscó en el registro especializado (última búsqueda en noviembre de 2016) y en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials; CENTRAL; 2016, Número 10); el especialista en información de Cochrane Vascular también buscó en estudios en curso en registros de ensayos clínicos.
Criterios de selección
Ensayos controlados aleatorizados que compararon HBPM versus AVK para el tratamiento a largo plazo (tres meses) de la TEV sintomática. Dos autores de la revisión, de forma independiente, evaluaron los ensayos para inclusión y la calidad metodológica.
Obtención y análisis de los datos
Los autores de la revisión, de forma independiente, extrajeron los datos y evaluaron el riesgo de sesgo. Se resolvieron los desacuerdos mediante debate y se realizó el metanálisis mediante modelos de efectos fijos con odds ratios (OR) de Peto e intervalos de confianza (IC) del 95%. Los resultados de interés fueron la TEV recurrente, la hemorragia grave y la mortalidad. Se utilizó GRADE para evaluar la calidad general de la evidencia que apoyaba estos resultados.
Resultados principales
Dieciséis ensayos, con un total de 3299 participantes, cumplieron los criterios de inclusión. Según GRADE, la calidad de la evidencia fue moderada en el caso de la TEV recurrente, baja en el caso de la hemorragia grave y moderada en el caso de la mortalidad. La calidad de la evidencia se disminuyó por la imprecisión (TEV recurrente, mortalidad) y por el riesgo de sesgo y la inconsistencia (hemorragia grave).
No se encontraron diferencias claras en la TEV recurrente entre la HBPM y los AVK (OR de Peto 0,83; intervalo de confianza (IC) del 95%: 0,60 a 1,15; p = 0,27; 3299 participantes; 16 estudios; evidencia de calidad moderada). Se encontró menos hemorragia con la HBPM que con los AVK (OR de Peto 0,51; IC del 95%: 0,32 a 0,80; P = 0,004; 3299 participantes; 16 estudios; evidencia de baja calidad). Sin embargo, al comparar sólo los estudios de alta calidad para la hemorragia, no se observaron diferencias claras entre la HBPM y los AVK (OR de Peto 0,62; IC del 95%: 0,36 a 1,07; P = 0,08; 1872 participantes; siete estudios). No se encontraron diferencias claras entre la HBPM y la AVK en cuanto a la mortalidad (OR de Peto 1,08; IC del 95%: 0,75 a 1,56; p = 0,68; 3299 participantes; 16 estudios; evidencia de calidad moderada).
Conclusiones de los autores
La evidencia de calidad moderada no muestra diferencias claras entre la HBPM y los AVK en la prevención de la TEV sintomática y la muerte después de un episodio de TVP sintomática. La evidencia de baja calidad sugiere que hay menos casos de hemorragia grave con la HBPM que con los AVK. Sin embargo, la comparación de solo estudios de alta calidad para la hemorragia no muestra diferencias claras entre la HBPM y los AVK. La HBPM puede suponer una alternativa para algunos pacientes, por ejemplo, los que se residen en zonas geográficamente inaccesibles, los que no pueden o son reacios a visitar el servicio de trombosis con regularidad, y para quienes los AVK están contraindicados.
PICOs
Resumen en términos sencillos
Antagonistas de la vitamina K o heparina de bajo peso molecular para el tratamiento a largo plazo de los coágulos sanguíneos sintomáticos
Antecedentes
Los coágulos sanguíneos (tromboembolia venosa) a veces causan bloqueos en las venas después de la cirugía, durante el reposo en cama o de forma espontánea. Estos coágulos pueden ser mortales cuando se desplazan hasta los pulmones. Los antagonistas de la vitamina K (AVK), el 99% de los cuales consisten en warfarina, son eficaces para prevenir la formación de nuevos coágulos sanguíneos, porque diluyen la sangre. Las heparinas de bajo peso molecular (HBPM) son medicamentos que diluyen la sangre y se utilizan en personas con riesgo de hemorragia grave, personas que no pueden tomar antagonistas de la vitamina K y mujeres embarazadas.
Propósito de la revisión
Evaluar los efectos beneficiosos y perjudiciales del tratamiento a largo plazo (tres meses) de la tromboembolia venosa con HBPM en comparación con el tratamiento a largo plazo con AVK.
Resultados clave
Esta revisión sistemática de 16 ensayos con un total combinado de 3299 participantes (actual hasta noviembre de 2016) no encontró diferencias claras en los coágulos sanguíneos recurrentes y las muertes entre la HBPM y los AVK, y observó menos episodios hemorrágicos con la HBPM que con los AVK. Sin embargo, la comparación de solo estudios de alta calidad para la hemorragia no reveló diferencias claras entre la HBPM y los AVK.
Calidad de la evidencia
La calidad de la evidencia para los resultados de coágulos sanguíneos recurrentes y muerte fue moderada. La calidad de esta evidencia se redujo debido al bajo número de eventos informados, lo que llevó a la imprecisión. En cuanto al resultado de la hemorragia, la calidad de la evidencia fue baja debido a la incoherencia entre los estudios y al riesgo de sesgo. Es necesario continuar la investigación sobre el tratamiento a largo plazo de los coágulos de sangre en las venas con HBPM y AVK.
Conclusiones de los autores
Esta revisión no encontró diferencias claras en los coágulos sanguíneos recurrentes y las muertes entre la HBPM y los AVK, y observó menos episodios hemorrágicos con la HBPM que con los AVK. Sin embargo, cuando solo se compararon estudios de alta calidad para la hemorragia, no se observaron diferencias claras entre la HBPM y los AVK. La HBPM puede ofrecer una alternativa para algunos pacientes, por ejemplo, los que se encuentran en zonas geográficamente inaccesibles, los que no pueden o son reacios a visitar el servicio de trombosis con regularidad, y aquellos para los que tomar AVK puede ser perjudicial.
Authors' conclusions
Summary of findings
| LMWH compared with VKA for long term treatment of symptomatic VTE | ||||||
| Patient or population: patients with symptomatic VTE requiring long term treatment (3 months) for symptomatic VTE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with VKA | Risk with LMWH | |||||
| Incidence of recurrent VTE (treatment duration 3 months) | Study population | Peto OR 0.83 | 3299 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 42 per 1000 | |||||
| Incidence of major bleeding (treatment duration 3 months) | Study population | Peto OR 0.51 | 3299 | ⊕⊕⊝⊝ | ||
| 29 per 1000 | 15 per 1000 | |||||
| Mortality (treatment duration 3 months) | Study population | Peto OR 1.08 | 3299 | ⊕⊕⊕⊝ | ||
| 35 per 1000 | 37 per 1000 | |||||
| * The basis for the assumed risk with VKA for 'Study population' was the average risk in the VKA group (i.e. total number of participants with events divided by total number of participants in the VKA group included in the meta‐analysis). The risk in the LMWH group (and its 95% confidence interval) is based on assumed risk in the VKA group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of bias due to no blinding but not downgraded, as analysis excluding studies deemed of low methodological quality confirms no clear differences between LMWH and VKA | ||||||
Background
Description of the condition
Venous thromboembolism (VTE) is defined as formation of thrombus in the deep veins, most commonly in the legs (deep vein thrombosis, or DVT), and/or subsequent embolisation of all or part of the thrombus to the pulmonary circulation (pulmonary embolisation, or PE). DVT of the lower limbs may be associated with localised pain, swelling, and erythema, as well as with development of pulmonary emboli (PE) and later occurrence of post‐thrombotic syndrome (PTS; persistent swelling, erythema, and ulceration). PE presents acutely with shortness of breath, pain on inspiration, tachycardia, and right heart overload, and, if untreated, can lead to circulatory collapse and death; over the longer term, PE can cause chronic post‐thrombotic pulmonary hypertension. In this era of more liberal central venous catheterisation, DVT may more often involve the upper extremities. Rarely, other venous circulation (within cerebral veins, portal and mesenteric veins, etc.) can be affected.
In addition to DVT and PE, thrombus can form in the superficial veins, where it is associated with local pain and inflammation (superficial venous thrombosis). This event tends to be associated with lower rates of mortality and morbidity than are seen with DVT, although some patients may be at higher risk of DVT formation depending on the location of the clot (Chengelis 1996; Nasr 2015).
Venous thromboembolism (VTE) comprises DVT and PE and can occur spontaneously. However, risk factors for VTE are many and include periods of inactivity, dehydration, hospitalisation, trauma, clotting disorders and previous thrombosis, varicose veins with phlebitis, pregnancy, use of oral combined hormonal contraceptives, malignancy, obesity, smoking, and advanced age (Anderson 2003; NICE 2010).
The incidence of VTE in mostly Caucasian populations is between 100 and 200 per 100,000 person‐years (Heit 2015; White 2003). Of these, it is estimated that 45 to 117 per 100,000 person‐years are due to DVT (without PE) and 29 to 78 per 100,000 person‐years to PE (with or without DVT) (Heit 2015). Recurrent VTE occurs in approximately 7.4% of patients at one year and in 30.4% of patients by 10 years (Cushman 2007; Heit 2015; White 2003).
Description of the intervention
The primary aim of treatment of symptomatic VTE is prevention of its recurrence, including prevention of potentially fatal PE. Clinical guidelines provide recommendations for treatment of VTE in different settings (Kearon 2016; NICE 2012). In general, anticoagulation is the recommended treatment of choice. The recommended initial treatment consists of a direct oral anticoagulant (with or without initial parenteral anticoagulation as indicated) or a parenteral anticoagulant given in conjunction with a VKA. Long term therapy (usually for a minimum duration of three months of anticoagulation) is indicated for treatment of acute VTE.
Prolonged use of a VKA has proven efficacy in comparison with placebo and low‐dose heparin (unfractionated heparin) for treatment of VTE (Hull 1979; Lagerstedt 1985). Use of adjusted therapeutic doses of subcutaneous unfractionated heparin is as effective as use of a VKA for preventing recurrence of symptomatic VTE, but both require regular laboratory monitoring (Hull 1982b). Usual practice is to administer VKAs to achieve an international normalised ratio (INR) of 2.0 to 3.0 (Hull 1982a). However, use of VKAs continues to present considerable risk of major bleeding (approximately 3% to 4%) during the first three months of treatment (Hutten 1999). Moreover, for some patients, it is difficult to achieve a stable INR in the therapeutic range, and this leads to increased risk of bleeding complications.
How the intervention might work
Long term treatment of symptomatic VTE with low‐molecular‐weight heparin (LMWH) has been proposed to minimise risk of bleeding complications. Comparison of LMWH versus unfractionated heparin for initial treatment of symptomatic VTE reveals that LMWH is associated with a reduction in major bleeding (Hettiarachchi 1998), and that treatment with LMWH is less frequently complicated by thrombocytopaenia (Warkentin 1995) and osteoporosis (Kelton 1995; Monreal 1994); also, these compounds do not require laboratory monitoring.
Why it is important to do this review
If the efficacy and safety of LMWH are found to be comparable with those of VKAs, LMWH could be used for long term treatment of symptomatic VTE. This would be especially important for patients in whom VKAs are contraindicated or impractical, for example, pregnant women and those living in geographically inaccessible places.
Objectives
The purpose of this review was to evaluate the efficacy and safety of long term treatment (three months) with LMWH versus long term treatment (three months) with VKAs for symptomatic VTE.
Methods
Criteria for considering studies for this review
Types of studies
We included trials that randomly allocated participants to long term (three months) treatment with VKAs or LMWH.
Types of participants
We included trials involving participants with symptomatic venous thromboembolism (VTE). We excluded trials that exclusively included participants with active malignancy and symptomatic VTE because this is the topic of another Cochrane review (Akl 2014). In addition, we excluded trials when investigators did not use objective tests to confirm the diagnosis of deep venous thrombosis (DVT) (such as venography, ultrasound, or any sequence of tests that results in a high positive predictive value for the diagnosis of symptomatic DVT) or the diagnosis of pulmonary embolism (PE) (such as high‐probability ventilation‐perfusion lung scan or pulmonary angiography).
Types of interventions
We included trials comparing VKAs versus LMWH for long term (three months) treatment of symptomatic VTE. Trials were included if the initial treatment for symptomatic VTE consisted of LMWH or unfractionated heparin for 5 to 10 days.
Types of outcome measures
Primary outcomes
-
Incidence of recurrent symptomatic VTE during three months of allocated treatment
-
Occurrence of major bleeding complications during three months of allocated treatment
-
Mortality during three months of allocated treatment
To confirm an episode of suspected recurrent VTE, we considered the following criteria as constituting a positive diagnosis of recurrent symptomatic DVT.
-
Extension of an intraluminal filling defect on a venogram.
-
New intraluminal filling defect.
-
Extension of non‐visualisation of proximal veins in the presence of a sudden cut‐off defect on a venogram seen on at least two projections.
When no previous venogram was available for comparison, we considered an intraluminal filling defect as sufficient. When no venogram was available, we accepted abnormal results of compression ultrasonography in an area where compression had previously been normal, or a substantial increase in the diameter of the thrombus during full compression at the popliteal or femoral vein (Koopman 1996; Levine 1996). When neither a venogram nor an ultrasonographic trial was available, a change in the results of impedance plethysmography from normal to abnormal, accompanied by a change from a negative to a positive result on a D‐dimer test, was acceptable.
To confirm an episode of suspected recurrent PE, we accepted the following criteria.
-
New intraluminal filling defect.
-
Extension of an existing defect.
-
Sudden cut‐off of vessels > 2.5 mm in diameter on a pulmonary angiogram.
When no prior pulmonary angiogram was available, an intraluminal filling defect or a sudden cut‐off of vessels > 2.5 mm in diameter on a pulmonary angiogram was sufficient. When no pulmonary angiogram was available, we accepted a defect of ≥ 75% of a segment on the perfusion scan with normal ventilation. When the ventilation‐perfusion scan was non‐diagnostic (and no pulmonary angiogram was available), satisfaction of the above criteria for DVT was acceptable. Pulmonary embolism demonstrated at autopsy was also acceptable.
We classified haemorrhages as major if they were clinically overt and associated with a fall in haemoglobin level ≥ 2 g/dL (1.6 mM); clinically overt and leading to transfusion of ≥ 2 units of packed cells; intracranial; retroperitoneal; leading directly to death; or leading to interruption of antithrombotic treatment or (re)operation.
We excluded studies that evaluated bleeding if definitions of major and minor bleeding were unclear.
Secondary outcomes
-
Incidence of recurrent symptomatic VTE during additional six to nine months after cessation of allocated three months of treatment for symptomatic VTE
-
Occurrence of major bleeding complications during additional six to nine months after cessation of allocated three months of treatment for symptomatic VTE
-
Mortality during additional six to nine months after cessation of allocated three months of treatment for symptomatic VTE
We considered additional long term outcomes for inclusion in the review when these were available.
Search methods for identification of studies
We applied no language restrictions on publications and no restrictions regarding status of publications.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
-
Cochrane Vascular Specialised Register (11 November 2016).
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 10) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Allied and Complementary Medicine Database (AMED), and through handsearching of relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies (11 November 2016).
-
ClinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2.
Searching other resources
We searched the reference lists of articles retrieved by electronic searches for additional citations. We contacted trialists for further information when data were missing, or when we had doubts about whether we should include specific trials in the review.
Data collection and analysis
Selection of studies
At least two members of the current review author team (AA, AST, MS) independently scrutinised trials for eligibility and resolved disagreements by discussion. We obtained full versions of articles that potentially met our inclusion criteria upon review of titles or abstracts and assessed these trials independently against the inclusion criteria. We have presented the reason for exclusion of each study in the Characteristics of excluded studies table.
Data extraction and management
We reviewed eligible articles and extracted and recorded summary information on forms developed by Cochrane Vascular. We sought the following information: participant characteristics (age, gender, comorbidities); number of participants in each treatment arm; duration of therapy; type of anticoagulant (vitamin K antagonist/LMWH); and incidence and timing of recurrent VTE, major bleeding complications, and mortality. When important information was not reported, we contacted trial authors.
Assessment of risk of bias in included studies
Two review authors working independently (AA, MS) used the 'Risk of bias tool' as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias, judging each item to be at low, unclear, or high risk of bias according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions.
We then classified trials into two categories. Category I trials were those with high methodological quality, that is, clearly concealed randomisation and double‐blind treatment or blinded assessment of outcome measures. Category II trials were those with lower methodological quality, that is, unclear or clearly not concealed randomisation or blind outcome assessment. We sought all information regarding adequacy of the randomisation process, allocation concealment, blinding, intention‐to‐treat analysis, and completeness of follow‐up.
Measures of treatment effect
We used Review Manager 5.3 as provided by Cochrane to analyse data. For dichotomous outcomes, we have presented results of statistical analysis as Peto odds ratios (Peto ORs) with 95% confidence intervals (CIs).
Unit of analysis issues
Participating individuals were the unit of analysis.
Dealing with missing data
When necessary, we contacted the authors of included trials to clarify data and provide missing values.
Assessment of heterogeneity
We conducted all analyses on an intention‐to‐treat basis. When individual trials did not use intention‐to‐treat analysis, we analysed data (absolute numbers) as provided in the included trial report.
We assessed trial heterogeneity using the I2 statistic, which describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). When we identified heterogeneity (I2 > 50%), we investigated reasons for this.
Assessment of reporting biases
We used asymmetry in funnel plots to assess reporting bias when we included more than 10 studies in the meta‐analysis (Higgins 2011).
Data synthesis
We combined calculated Peto ORs from individual trials across trials, giving weight to the number of events reported in each of the two treatment groups for each separate trial. This approach assumes the use of a fixed‐effect analysis model (Collins 1987; Mantel 1959).
We performed separate analyses for all trials combined and for trials of high methodological quality (Category I) (see Assessment of risk of bias in included studies).
Subgroup analysis and investigation of heterogeneity
We performed separate analyses for trials that used similar initial treatment in both trial arms and for those that used different treatment regimens during initial treatment for PE or DVT (i.e. LMWH vs unfractionated heparin for initial treatment of symptomatic VTE ‐ a potential source of confounding). In addition, we performed analyses for symptomatic PE and symptomatic DVT to explore the effects of vitamin K antagonists on these two different disease components of symptomatic VTE.
Sensitivity analysis
The primary analysis included data on all trial participants during the period of randomly allocated treatment. We performed sensitivity analyses to explore the effect that risk of bias had on estimates of treatment effects by excluding studies classified as category II trials (trials with low methodological quality, i.e. unclear or clearly not concealed randomisation or no blind outcome assessment; see Assessment of risk of bias in included studies).
'Summary of findings'
We presented the main findings of this review regarding quality of evidence, magnitude of effects of interventions examined, and sum of available data on primary outcomes (Types of outcome measures) in a 'Summary of findings' table, according to GRADE principles as described by Higgins 2011 and Atkins 2004. We developed a 'Summary of findings' table for the comparison 'LMWH versus VKA during allocated treatment (category I and II studies)' and used GRADEpro (GRADEproGDT) software (http://www.guidelinedevelopment.org/) to facilitate preparation of the 'Summary of findings' table.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
See Figure 1.

Study flow diagram.
We identified one new study that was eligible for inclusion in this update (Perez‐de‐Llano 2010).
Included studies
In total, 16 trials that examined long term treatment of symptomatic VTE fulfilled our inclusion criteria (Beckman 2003; Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Kakkar 2003; Kucher 2005; Lopaciuk 1999; Lopez 2001; Massicotte 2003; Perez‐de‐Llano 2010; Pini 1994; Romera 2009; Veiga 2000). Three trials (Beckman 2003; Kucher 2005; Perez‐de‐Llano 2010) included only participants with symptomatic PE. One trial included participants with both symptomatic DVT and symptomatic PE (Massicotte 2003). The 12 remaining trials included participants with symptomatic DVT. See the Characteristics of included studies table for a detailed description of these trials.
The trials were performed in the following countries.
-
Canada (Hull 2007; Hull 2009; Massicotte 2003).
-
Germany (Hamann 1998).
-
Greece (Daskalopoulos 2005).
-
Italy (Pini 1994).
-
Poland (Lopaciuk 1999).
-
Spain (Gonzalez 1999; Lopez 2001; Perez‐de‐Llano 2010; Romera 2009; Veiga 2000).
-
USA (Beckman 2003; Kakkar 2003; Kucher 2005).
-
UK (Das 1996).
The 16 included trials recruited a total of 3299 participants. The number of participants in each trial ranged from 40 (Kucher 2005) to 737 (Hull 2007). Seven trials provided similar treatments in both arms (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Perez‐de‐Llano 2010; Pini 1994); the remaining nine trials allocated participants to different treatments provided in different trial arms (Beckman 2003; Hamann 1998; Kakkar 2003; Kucher 2005; Lopaciuk 1999; Lopez 2001; Massicotte 2003; Romera 2009; Veiga 2000). The 16 included trials were published between 1994 and 2010 (Beckman 2003; Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Kakkar 2003; Kucher 2005; Lopaciuk 1999; Lopez 2001; Massicotte 2003; Perez‐de‐Llano 2010; Pini 1994; Romera 2009; Veiga 2000).
Category I trials were those with high methodological quality, that is, clearly concealed randomisation and double‐blind treatment or blinded assessment of outcome measures. Category II trials were those with lower methodological quality, that is unclear or clearly not concealed randomisation or blind outcome assessment. We deemed that seven trials were category I trials (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003; Pini 1994) and the remaining nine trials were category II trials (Beckman 2003; Hamann 1998; Kakkar 2003; Kucher 2005; Lopaciuk 1999; Lopez 2001; Perez‐de‐Llano 2010; Romera 2009; Veiga 2000). For additional details on methodological quality, see the Risk of bias in included studies section.
Seven of the 16 trials included only participants with symptomatic DVT and used similar initial treatment in both treatment arms. These included two category I trials (Das 1996; Pini 1994) and five category II trials (Hamann 1998; Lopaciuk 1999; Lopez 2001; Romera 2009; Veiga 2000). The category II trial Kakkar 2003 randomised participants between three treatment arms; in one arm, initial treatment was intravenous unfractionated heparin followed by three months of VKA. The other two treatment arms initially treated participants with LMWH followed by a VKA or LMWH for 12 weeks. In the category I trials Gonzalez 1999, Daskalopoulos 2005, and Hull 2007, initial treatment in the LMWH arm consisted of subcutaneous LMWH, and initial treatment in the VKA arm consisted of a course of intravenous unfractionated heparin. Category I trial Hull 2009 included only participants with acute proximal DVT; initial treatment consisted of subcutaneous LMWH or subcutaneous LMWH plus warfarin. Among trials including only participants with symptomatic PE (Beckman 2003; Kucher 2005; Perez‐de‐Llano 2010), Beckman 2003 compared different initial treatments, but Kucher 2005 and Perez‐de‐Llano 2010 provided the same initial treatment, that is, subcutaneous LMWH. One trial included participants with both symptomatic DVT and symptomatic PE (Massicotte 2003) and provided different initial treatment for the two treatment groups.
Pini 1994 followed all participants for the entire follow‐up period and performed intention‐to‐treat analysis. Das 1996 reported that a total of 19 participants (18%) did not complete the trial according to the protocol; six participants in the LMWH group did not complete the three months of follow‐up (one death, one severe illness, two PE, one loss to follow‐up, one inadequate venogram); 13 participants in the VKA group did not complete the three months of follow‐up (three deaths, three severe illness, one PE, three losses to follow‐up, three inadequate venograms); and analyses of participant data were based on an intention‐to‐treat analysis. Gonzalez 1999 excluded 20 (11%) participants from the analysis: eight participants in the LMWH arm and 12 in the vitamin K antagonist arm. Investigators did not provide results of intention‐to‐treat analysis nor outcome data (in total, 12 participants had no second venogram, five participants received treatment that was not conducted properly, and three participants were lost to follow‐up). Lopaciuk 1999 excluded a total of nine participants after randomisation and performed no intention‐to‐treat analyses. Three participants in the LMWH group (one sudden death during initial treatment, one PE (day three) and vena caval filter insertion (day 14), and one initial treatment changed to unfractionated heparin) and six in the VKA group (two with an exclusion criterion overlooked (vein compression by arterial aneurysm), three consent withdrawals, and one initial treatment changed to thrombectomy) did not complete the trial according to the protocol. Kakkar 2003 reported that 54 participants were not included in the intention‐to‐treat analysis (evenly divided over the three treatment arms), six participants did not have a baseline venography, and in 48 participants symptomatic DVT was not confirmed independently by the baseline venogram. Daskalopoulos 2005 reported that a total of six participants were excluded before commencement of treatment (five in the LMWH arm and one in the VKA arm). Hull 2007 reported that a total of six participants did not complete the trial according to the protocol and provided intention‐to‐treat analysis of data for these participants. In the VKA arm, four participants did not complete the trial (one was lost to follow‐up and three withdrew consent); and in the LMWH arm, two participants did not complete the trial (one was lost to follow‐up and one withdrew consent). Hull 2009 reported that 3 of 480 participants were lost to follow‐up at 12 months (1 in the heparin group and 2 in the usual care group). Perez‐de‐Llano 2010 reported that eight participants did not complete the study protocol successfully; five participants (9.7%) randomised to heparin (metastatic cancer, allergy to heparin, vein thrombosis, and two unknown reasons) and three (6%) to VKA (metastatic cancer, inability to reach therapeutic INR, and one unknown reason). Hamann 1998, Veiga 2000, Lopez 2001, Beckman 2003, Kucher 2005, and Romera 2009 reported that all trial participants were followed‐up. Massicotte 2003 reported use of intention‐to‐treat analyses but excluded two participants ‐ one from each group, who did not receive study medications ‐ from these analyses. Two participants failed to meet inclusion criteria and two failed to meet exclusion criteria, but these participants did receive study medications and were left in the intention‐to‐treat analyses. Massicotte 2003 reported that eight participants (including one death) in the LMWH group and 14 (including 4 deaths) in the unfractionated heparin group withdrew from the study.
All included studies provided a minimum of three months of treatment. Three studies reported a treatment period of six months (Daskalopoulos 2005; Perez‐de‐Llano 2010; Romera 2009), and in three other studies some participants received three months of treatment and others six months of treatment (Hamann 1998; Lopez 2001; Veiga 2000). A total of 12 studies reported additional follow‐up after cessation of treatment ranging from 28 days to 9 months (Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Kakkar 2003; Lopaciuk 1999; Lopez 2001; Massicotte 2003; Pini 1994; Romera 2009; Veiga 2000).
Seven trials described quality of treatment with VKAs, defined as INR between 2.0 and 3.0 (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Lopez 2001; Perez‐de‐Llano 2010; Pini 1994; Veiga 2000); percentages are given in the Characteristics of included studies table. Beckman 2003 and Hull 2007 provided INRs for participants who had a major bleeding complication. Romera 2009 provided INRs for some of the participants with bleeding complications.
Excluded studies
In total, we excluded four studies. Reasons for exclusion included the following.
-
Non‐randomised trial (Vorobyeva 2009).
-
Composite endpoint trial (Ghirarduzzi 2009).
-
Subjective reporting (Hull 2001; Hull 2001a).
Risk of bias in included studies
See Figure 2 and Figure 3 for graphical presentations of risk of bias. Lack of detail was the main reason for the 'unclear' rating for most trials.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Nine trials described the method used to generate the random allocation sequence in sufficient detail, indicating low risk of bias (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Kucher 2005; Massicotte 2003; Perez‐de‐Llano 2010; Pini 1994). The randomisation method was unclear in seven trials (Beckman 2003; Hamann 1998; Kakkar 2003; Lopaciuk 1999; Lopez 2001; Romera 2009; Veiga 2000).
Only three trials adequately concealed allocation (Das 1996; Lopaciuk 1999; Veiga 2000). We determined that the remaining 13 trials had unclear risk of bias (Beckman 2003; Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Kakkar 2003; Kucher 2005; Lopez 2001; Massicotte 2003; Perez‐de‐Llano 2010; Pini 1994; Romera 2009).
Blinding
All included trials were at high risk of performance bias because they were open‐label trials.
Eleven trials were at low risk of detection bias because they reported adequate blinding of outcome assessments (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Kakkar 2003; Lopez 2001; Massicotte 2003; Pini 1994; Romera 2009; Veiga 2000). Two trials were at high risk of detection bias because they did not report blinded outcome assessment (Kucher 2005; Lopaciuk 1999); and three trials were at unclear risk of bias because it was unclear whether those collecting outcomes data were aware of the allocation (Beckman 2003; Hamann 1998; Perez‐de‐Llano 2010).
Incomplete outcome data
Risk of bias was low for 15 trials, as investigators followed‐up and reported on all participants (Beckman 2003; Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Kucher 2005; Lopaciuk 1999; Lopez 2001; Massicotte 2003; Perez‐de‐Llano 2010; Pini 1994; Romera 2009; Veiga 2000). We classified only Kakkar 2003 as having high risk of bias, as investigators did not follow up on 33% of randomised participants, as was required by the trial design.
Selective reporting
Fourteen trials were at low risk of bias, and two trials (Das 1996; Hamann 1998) had unclear risk of bias owing to insufficient information provided in trial reports.
Other potential sources of bias
Nine trials were free of other sources of bias (Beckman 2003; Das 1996; Kakkar 2003; Kucher 2005; Lopez 2001; Perez‐de‐Llano 2010; Pini 1994; Romera 2009; Veiga 2000). However, one trial was at unclear risk, as investigators provided insufficient information (Hamann 1998). We deemed Lopaciuk 1999 to be at unclear risk of other bias because study authors did not discuss three fatal peripheral or cardiovascular events in the acenocoumarol group, and because follow‐up treatments after planned three‐month outcomes differed between groups. Five (category I) trials had unclear risk, as results may have been confounded by differences in the initial treatment (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003).
Effects of interventions
Incidence of recurrent venous thromboembolism during active treatment
All 16 trials reported the occurrence of recurrent symptomatic VTE during the first three months after randomisation.
A total of 86 of 1702 participants (5.1%) in the VKA group had recurrent symptomatic VTE versus 70 of 1597 participants (4.4%) in the LMWH group. Pooled analysis showed no clear differences between treatment modalities for recurrent symptomatic VTE (Peto OR 0.83, 95% CI 0.60 to 1.15; P = 0.27; 3299 participants; 16 studies; moderate‐quality evidence) among participants with symptomatic VTE. Heterogeneity was assessed as I2 = 9% (Analysis 1.1; Figure 4).

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.1 incidence of recurrent VTE.
Although 15 trials showed no clear differences in recurrent VTE between LMWH and VKA treatment, one trial (Gonzalez 1999) found a difference in favour of LMWH treatment (Peto OR 0.38, 95% CI 0.17 to 0.86; 185 participants).
Twelve trials included only participants with symptomatic DVT. In these trials, a total of 82 of 1572 participants (5.2%) in the VKA group had recurrent symptomatic VTE versus 63 of 1449 participants (4.3%) in the LMWH group, showing no clear differences between treatment modalities for recurrent symptomatic VTE (Peto OR 0.79, 95% CI 0.57 to 1.11; P = 0.18; 3021 participants; 12 studies) among participants with symptomatic DVT. Heterogeneity was assessed as I2 = 8% (Analysis 2.1).
In contrast, among the three trials including only participants with symptomatic PE, none of 90 participants (0%) in the VKA group had recurrent symptomatic VTE versus 5 of 112 participants (4.5%) in the LMWH group, resulting in no clear differences between treatments for episodes of recurrent symptomatic VTE (Peto OR 5.70, 95% CI 0.91 to 35.60; P = 0.06; 202 participants; three studies) among participants with symptomatic PE. Heterogeneity was assessed as I2 = 0% (Analysis 3.1).
Consideration of category I trials (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003; Pini 1994) revealed that six trials included only participants with symptomatic DVT and the remaining trial (Massicotte 2003) included participants with both symptomatic DVT and PE. A total of 61 of 941 participants (6.5%) in the VKA arm had recurrent symptomatic VTE versus 49 of 931 participants (5.3%) allocated to LMWH treatment during three months of treatment. Analysis of pooled data showed no clear differences between treatment modalities for recurrent symptomatic VTE (Peto OR 0.80, 95% CI 0.54 to 1.18; P = 0.26; 1872 participants; seven studies). Heterogeneity was assessed as I2 = 16% (Analysis 4.1).
Five category I trials may have been confounded by differences in initial treatment (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003). Analysing these trials separately revealed no clear differences between treatment groups (Peto OR 0.68, 95% CI 0.44 to 1.03; P = 0.07; 1580 participants; five studies). Heterogeneity was assessed as I2 = 0% (Analysis 6.1). We considered in a separate analysis the two category I trials that compared a VKA versus LMWH for long term treatment of symptomatic VTE, using the same initial treatment in both arms (Das 1996; Pini 1994). Analysis of pooled data showed no clear differences in recurrent symptomatic VTE between treatments (Peto OR 1.95, 95% CI 0.74 to 5.19; P = 0.18; 292 participants; two studies). Heterogeneity was assessed as I2 = 0% (Analysis 5.1).
Incidence of recurrent symptomatic venous thromboembolism during the additional period of follow‐up after cessation of active treatment
Five category I trials (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Pini 1994) and five category II trials (Hamann 1998; Lopaciuk 1999; Lopez 2001; Romera 2009; Veiga 2000) evaluated a period of six to nine months after cessation of the allocated treatment. A total of 53 of 1296 participants (4.1%) in the VKA group versus 59 of 1296 participants (4.6%) in the arm allocated to LMWH treatment experienced an episode of recurrent symptomatic VTE. Combined analysis showed no clear differences in recurrent symptomatic VTE between treatment arms (Peto OR 1.12, 95% CI 0.77 to 1.64; P = 0.56; 2592 participants; 10 studies). Heterogeneity was assessed as I2 = 28% (Analysis 7.1).
A separate analysis for the category I trials (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Pini 1994) evaluating an additional period of nine months after cessation of the allocated treatment resulted in a total of 36 of 846 participants (4.3%) in the VKA arm versus 45 of 845 participants (5.3%) in the LMWH arm experiencing an episode of recurrent symptomatic VTE. Combined analysis showed no clear differences in thromboembolic complications between treatment modalities (Peto OR 1.26, 95% CI 0.81 to 1.98; P = 0.30; 1691 participants; five studies). Heterogeneity was assessed as I2 = 14% (Analysis 8.1). It should be noted that in Pini 1994, 34 of 94 participants used the VKA during an additional three months and 14 of 94 participants used the VKA for an additional nine months, whereas in the LMWH group all 93 participants stopped assigned treatment after three months. Furthermore, in Hull 2007, 250 of 368 participants in the VKA allocated treatment arm were treated with LMWH beyond the three months of allocated treatment and in the LMWH group 146 of 369 participants continued allocated treatment beyond the three months of allocated treatment. Hull 2009 reported that some participants in both treatment groups received ongoing warfarin after the first three months of allocated treatment.
The total 12‐month period of follow‐up (combining three months of active treatment and nine months of follow‐up) among five category I trials (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Pini 1994) included a total of 91 of 846 participants (10.8%) in the VKA group versus 87 of 845 participants (10.3%) in the LMWH group who had recurrent symptomatic VTE and showed no clear differences between treatment modalities for risk of recurrent symptomatic VTE (Peto OR 0.95, 95% CI 0.70 to 1.30; P = 0.75; 1691 participants; five studies) among participants with symptomatic PE. Heterogeneity was assessed as I2 = 58% (Analysis 10.1).
Analysis of pooled data from 10 category I and category II trials (Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Lopaciuk 1999; Lopez 2001; Pini 1994; Romera 2009; Veiga 2000) showed no clear differences between LMWH and VKA treatment for the total 12‐month period of follow‐up (Peto OR 0.88, 95% CI 0.67 to 1.15; P = 0.34; 2592 participants; 10 studies). Heterogeneity was assessed as I2 = 41% (Analysis 9.1).
Incidence of major bleeding during active treatment
All 16 category I and II trials reported the incidence of major bleeding during allocated treatment. Thirteen trials found no clear differences and only two trials found differences between groups (Beckman 2003; Lopez 2001). Lopez 2001 found a difference that favoured the LMWH group (Peto OR 0.12, 95% CI 0.02 to 0.89). This trial included only participants with DVT. Beckman 2003 found a difference that favoured the LMWH group (Peto OR 0.05, 95% CI 0.00 to 0.92). This trial included only participants with symptomatic PE.
Analysis of pooled trials showed major bleeding complications among 50 of 1702 participants (2.9%) in the VKA arm versus 25 of 1597 participants (1.6%) in the LMWH group. This difference favoured LMWH therapy for the outcome of major bleeding (Peto OR 0.51, 95% CI 0.32 to 0.80; P = 0.004; 3299 participants; 16 studies; low‐quality evidence). Heterogeneity was assessed as I2 = 0% (Analysis 1.2; Figure 5).
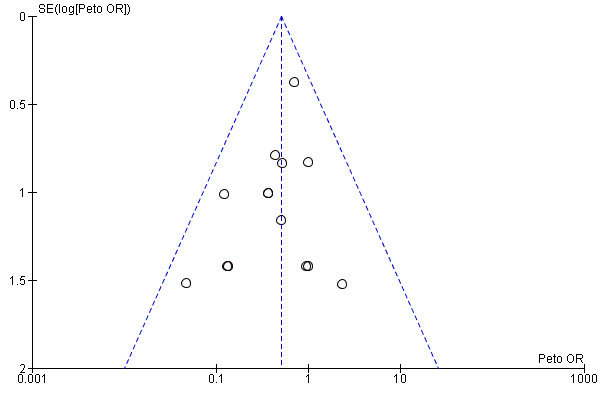
Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.2 incidence of major bleeding.
Analysing the 12 trials that included only participants with symptomatic DVT revealed that a total of 42 of 1572 participants (2.7%) in the VKA group had major bleeding versus 22 of 1449 participants (1.5%) in the LMWH group, showing a difference between treatment modalities that favoured LMWH for the outcome of major bleeding (Peto OR 0.54, 95% CI 0.33 to 0.88; P = 0.01; 3021 participants; 12 studies) among participants treated with symptomatic DVT. Heterogeneity was assessed as I2 = 0% (Analysis 2.2).
The three trials (Beckman 2003; Kucher 2005; Perez‐de‐Llano 2010) that included only participants with symptomatic PE observed major bleeding in a total of 3 of 90 participants (3.3%) in the VKA group versus 1 of 112 participants (0.9%) in the LMWH group, revealing no clear differences between treatments for the outcome of major bleeding (Peto OR 0.23, 95% CI 0.03 to 1.78; P = 0.16; 202 participants; three studies) among participants treated for symptomatic PE. Heterogeneity was assessed as I2 = 52% (Analysis 3.2).
Consideration of only category I trials (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003; Pini 1994) revealed that a total of 34 of 941 participants (3.6%) in the VKA arm versus 21 of 931 participants (2.3%) allocated to LMWH treatment experienced major bleeding during three months of treatment. Analysis of pooled data showed no clear differences between treatment modalities for the outcome of major bleeding (Peto OR 0.62, 95% CI 0.36 to 1.07; P = 0.08; 1872 participants; seven studies). Heterogeneity was assessed as I2 = 0% (Analysis 4.2).
We performed post hoc analyses to assess the subsets of participants with fatal haemorrhage and intracranial haemorrhage to determine how LMWH and VKA differ with respect to severe bleeds. We found no clear differences between LMWH and VKA among combined category I and category II studies and in category I studies only (results not shown).
For two category I trials providing the same initial treatment in both groups (Das 1996; Pini 1994) analysis of data showed no evidence of a difference in major bleeding incidence between treatment modalities (Peto OR 1.01, 95% CI 0.20 to 5.12; P = 0.99; 292 participants; two studies; I2 = not applicable) (Analysis 5.2). For five category I trials providing different initial treatments in the two groups (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003) analysis of data showed no clear differences between treatment modalities (Peto OR 0.59, 95% CI 0.33 to 1.04; P = 0.07; 1580 participants; five studies; I2 = 0%) (Analysis 6.2).
Incidence of major bleeding during the additional period of follow‐up after cessation of active treatment
No major bleeding occurred during the additional nine months of follow‐up (Analysis 7.2; Analysis 8.2).
Analysis of pooled data in nine category I and category II trials (Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Lopaciuk 1999; Lopez 2001; Pini 1994; Romera 2009; Veiga 2000) for the total 12 months of follow‐up showed major bleeding complications in 36 of 1056 participants (3.4%) in the VKA arm versus 20 of 1056 participants (1.9%) in the LMWH group. This difference was in favour of LMWH therapy (Peto OR 0.56, 95% CI 0.33 to 0.95; P = 0.03; 2112 participants; nine studies). Heterogeneity was assessed as I2 = 0% (Analysis 9.2).
The 12‐month period of follow‐up (combining three months active treatment and nine months follow‐up) in four category I trials (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Pini 1994) included a total of 25 of 606 participants (4.1%) in the VKA group versus 18 of 605 participants (3.0%) in the LMWH group who had a major bleeding incident, and showed no clear differences between treatment modalities (Peto OR 0.72, 95% CI 0.39 to 1.32; P = 0.28; 1211 participants; four studies). Heterogeneity was assessed as I2 = 0% (Analysis 10.2).
Mortality during active treatment
All 16 trials reported mortality during allocated treatment with no clear differences between treatment groups. Fifty‐nine of 1702 participants (3.5%) in the VKA treatment group died versus 62 of 1597 participants (3.9%) in the LMWH group, which produced a pooled Peto OR of 1.08 (95% CI 0.75 to 1.56; P = 0.68; 3299 participants; 16 studies; moderate‐quality evidence). Heterogeneity was assessed as I2 = 0% (Analysis 1.3; Figure 6). We obtained similar results when pooling only category I trial data (Peto OR 0.92, 95% CI 0.61 to 1.41; P = 0.71; 1872 participants; seven studies). Heterogeneity was assessed as I2 = 0% (Analysis 4.3).

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials), outcome; 2.3 mortality.
For two category I trials providing the same initial treatment for both groups (Das 1996; Pini 1994) and five category I trials providing different initial treatments for the two groups (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003), pooled analyses did not show a clear difference in mortality between treatment modalities (Peto OR 0.89, 95% CI 0.29 to 2.68; P = 0.83; 292 participants; two studies; I2 = 0% (Analysis 5.3); and Peto OR 0.93, 95% CI 0.59 to 1.46; P = 0.76; 1580 participants; five studies; I2 = 0% (Analysis 6.3)).
For the 12 trials that considered participants with DVT and for the three trials that considered participants with PE, analysis revealed no clear differences in mortality between treatment modalities (Peto OR 1.10, 95% CI 0.75 to 1.60; P = 0.64; 3021 participants; 12 studies; I2 = 0% (Analysis 2.3); and Peto OR 5.39, 95% CI 0.51 to 57.36; P = 0.16; 202 participants; three studies; I2 = 0% (Analysis 3.3)).
Mortality during the additional period of follow‐up after cessation of active treatment
Five category I and five category II trials (Daskalopoulos 2005; Gonzalez 1999; Hamann 1998; Hull 2007; Hull 2009; Lopaciuk 1999; Lopez 2001; Pini 1994; Romera 2009; Veiga 2000) reported an extended follow‐up period for an additional six to nine months and found that 72 of 1296 participants (5.6%) in the VKA arm versus 72 of 1296 participants (5.6%) in the LMWH group (Peto OR 1.00, 95% CI 0.71 to 1.40; P = 1.00; 2592 participants; 10 studies) died. Heterogeneity was assessed as I2 = 0% (Analysis 7.3). We obtained similar results when considering only category I trials (Peto OR 1.06, 95% CI 0.72 to 1.55; P = 0.77; 1691 participants; five studies; I2 = 0%) (Analysis 8.3).
Analysis of mortality for the total 12‐month follow‐up period did not detect a clear difference between treatment modalities for the 10 category I and category II trials (Peto OR 1.09, 95% CI 0.84 to 1.43; P = 0.51; 2592 participants; 10 studies; I2 = 0% (Analysis 9.3)) nor for the five category I trials (Peto OR 1.05, 95% CI 0.78 to 1.42; P = 0.76; 1691 participants; five studies; I2 = 0% (Analysis 10.3)).
Discussion
Summary of main results
This review detected no clear differences between low‐molecular‐weight heparin (LMWH) and vitamin K antagonists (VKAs) for two of the three primary outcomes (recurrent symptomatic venous thromboembolism (VTE) and overall mortality). For the third outcome, major bleeding, data show a reduction in favour of LMWH (Peto odds ratio (OR) 0.51, 95% confidence interval (CI) 0.32 to 0.80; P = 0.004). However, pooling of data from category I trials alone (clearly concealed randomisation, double‐blind or blinded outcome assessment) revealed no clear differences in major bleeding between treatment groups (Peto OR 0.62, 95% CI 0.36 to 1.07; P = 0.08). Although this review found no evidence that LMWH has greater efficacy than VKAs for long term treatment of VTE, evidence shows that long term treatment of symptomatic VTE with LMWH versus long term treatment with VKAs may be safer with respect to major bleeding. The largest trial (Hull 2007) reported no clear differences for any of the three outcomes. Results were similar for recurrent symptomatic VTE, major bleeding, and mortality during an additional six to nine months after cessation of the allocated three months of treatment for symptomatic VTE.
In interpreting the findings of this review, one must consider several points. Five category I trials did not use the same initial treatment in both treatment arms, and these differences may threaten the validity of data derived from these trials (Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Hull 2009; Massicotte 2003). A previous report suggests that the inferior quality of initial unfractionated heparin treatment may be associated with higher recurrence of VTE during follow‐up (Hull 1997), and two trials included in the review did not report the quality of unfractionated heparin treatment (Daskalopoulos 2005; Gonzalez 1999). In the largest trial (Hull 2007), initial treatment with unfractionated heparin was adequate but produced no differences in effect between treatment modalities. Doses of the various LMWH compounds used in individual trials ranged from 100 IU/kg reviparin sodium to 175 anti‐Xa IU/kg tinzaparin and 4000 anti‐Xa IU enoxaparin. Daskalopoulos 2005 and Hull (Hull 2007; Hull 2009) used the same dose of LMWH for both initial and long term treatment of symptomatic VTE. Massicotte 2003 recruited only children and adjusted the dose accordingly. These five category I trials found no clear difference in the incidence of recurrent VTE between LMWH and VKAs (Peto OR 0.68, 95% CI 0.44 to 1.03; P = 0.07).
In contrast, the two category I trials that provided the same initial treatment for both treatment arms observed a trend in favour of VKAs for prevention of recurrent symptomatic VTE when using relatively low doses of LMWH during long term treatment of deep vein thrombosis (DVT) (Das 1996; Pini 1994); dosages were approximately twice those normally used for prophylaxis of symptomatic VTE and were not adjusted for weight. The relatively low dose used is reflected in the very low levels of anti‐Xa activity found after 22 hours (0.04 U/mL after injection of 4000 anti‐Xa IU enoxaparin) (Pini 1994).
Lopez 2001 included 25 participants (14 participants in the LMWH treatment group and 11 in the VKA treatment group; n = 158) with infrapopliteal DVT (calf vein thrombosis) diagnosed by duplex ultrasonography. Excluding these data from the sensitivity analysis did not affect findings. Two considerations are of interest here. First, duplex ultrasonography is not as sensitive and specific for distal thrombosis as it is for proximal DVT (Mitchell 1991). Second, the natural history of distal DVT is unclear. It is estimated, from trials of diagnosis, that approximately only 20% of calf vein thrombi develop into a proximal DVT within two weeks of presentation, whereas the remainder, which probably are small and self‐limiting, do not (Heijboer 1993; Huisman 1986; Huisman 1989; Hull 1985).
The difference in major bleeding complications favouring LMWH during allocated treatment of three months has to be considered with caution. Different LMWH compounds and relatively low doses of medication were used, as has been mentioned. Although a difference in bleeding incidence favouring LMWH was found when all trials were combined (Peto OR 0.51, 95% CI 0.32 to 0.80; P = 0.004), no clear difference between LMWH and VKA was observed (Peto OR 0.62, 95% CI 0.36 to 1.07; P = 0.08) when only category I trials were considered. These trials used higher dosages of LMWH. On the other hand, the only trial that found a difference in favour of LMWH treatment used the same dose of LMWH for initial treatment as for long term treatment of symptomatic DVT (Peto OR 0.12, 95% CI 0.02 to 0.89) (Lopez 2001). Post hoc analyses showed no clear differences between LMWH and VKA when subsets of fatal and intracranial haemorrhage were assessed.
Overall, the data show no substantial differences in efficacy for long term treatment of patients with DVT with LMWH or VKAs, but long term treatment with LMWH may cause fewer episodes of major bleeding than occur with VKA therapy.
Currently, many patients are treated at home with a course of subcutaneous LMWH administered by the patients themselves. After this initial treatment, patients continue with a three‐month course of VKA with the dose adjusted to achieve an international normalised ratio (INR) between 2.0 and 3.0. Treatments used in Das 1996 and Daskalopoulos 2005 do not represent current practice.
Important practical considerations involving mainly patient and local preferences also influence the choice between LMWH and VKAs. The major disadvantage of VKA treatment compared with LMWH treatment is the need for regular laboratory monitoring. Furthermore, VKA compounds have some major drug interactions, but drug interactions of LMWH are uncommon. In addition, treatment with LMWH is relatively safe during pregnancy (Sanson 1999). A major disadvantage of treatment with LMWH is that the patient has to self‐administer subcutaneous injections on a daily basis. Among the included trials, only a few participants stopped treatment with LMWH, mainly as the result of problems other than administration of subcutaneous injections. Das 1996 reported that 8% of patients refused to participate in the trial because of reluctance to administer subcutaneous injections by themselves.
Overall completeness and applicability of evidence
Participants with symptomatic VTE included in these trials constituted a representative mix of people with this disease. All trials included approximately similar participants; therefore, these data can be generalised to the wider population. Several published trials included only participants with a diagnosis of cancer who had symptomatic VTE. We did not include these trials in our review because participants do not represent the normal cohort of patients with a diagnosis of symptomatic VTE, and because this is the topic of another Cochrane review (Akl 2014).
Only scant data have been gathered on patients with symptomatic pulmonary embolisation (PE). This review includes data from only 202 people with PE, and review findings should be interpreted with caution.
Direct oral anticoagulants (DOACs) are changing the ways that patients are treated (Robertson 2015; Robertson 2015b). Therefore, in the future, as more and more patients are prescribed DOACs instead of VKA and LMWH, the analysis performed for this review may become outdated. We will reconsider the focus and future of this review in future updates.
Quality of the evidence
Fifty‐nine per cent of included patients (1872/3299) participated in trials with category I classification yielding evidence of highest quality on direct treatment comparisons.
All randomised controlled trials included in this systematic review were conducted in an unblinded manner because the two different interventions were delivered in different settings (hospital and home), making participant blinding impossible. When participant outcomes were assessed by individuals who were blind to treatment allocation, we considered threats to the validity of trial conclusions to be reduced. When it was not reported that those collecting outcome measures data were unaware of treatment allocation, trial findings may have been compromised. Trials in which allocation is not concealed and outcomes are not collected in a blind manner are essentially observational rather than experimental in nature. Analysis for only category I trials (clearly concealed randomisation, double‐blind or blinded outcome assessment) shows similar results to those of analyses for all studies combined, except for bleeding, which showed no clear differences between treatment groups.
We downgraded the quality of evidence for recurrent VTE and mortality to moderate owing to imprecision resulting from the small number of events and the relatively large confidence interval. We decided not to downgrade for risk of bias due to blinding because a sensitivity analysis excluding studies deemed of low methodological quality confirmed no clear differences between LMWH and VKA.
We downgraded the quality of evidence for major bleeding to low owing to risk of bias and inconsistency: a sensitivity analysis including only category I trials (clearly concealed randomisation, double‐blind or blinded outcome assessment) showed no clear differences between VKA and LMWH. Bleeding outcomes are more susceptible to biased outcome reporting than outcomes such as VTE and mortality, and only two studies (studies of low methodological quality) have reported less bleeding with LMWH; the remainder showed no clear differences because confidence intervals crossed the line of no effect. See summary of findings Table for the main comparison.
Potential biases in the review process
Methods used to conduct this review are described in detail in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); particular strengths include independent application of review eligibility criteria, independent data extraction and assessment of risk of bias, and assessment of quality of evidence according to GRADE. The Cochrane Vascular Information Specialist, who is highly skilled in identification of randomised controlled trials, devised and conducted the search strategies. We are confident that we have included all relevant studies. However, the possibility remains that some relevant trials, particularly in the 'grey' literature (e.g. conference proceedings), have been missed. Other potential biases in the review process are the missing information from three trials regarding aspects of their conduct (Massicotte 2003; Pini 1994; Romera 2009).
Agreements and disagreements with other studies or reviews
Three published systematic reviews have previously evaluated VKAs versus LMWH (Bochenek 2012; Ferretti 2006; Iorio 2003). Two reviews did not find a clear difference between LMWH and oral anticoagulants (Ferretti 2006; Iorio 2003).
Iorio 2003 reviewed the efficacy and safety of long term treatment of VTE with LMWH compared with oral anticoagulants and did not find a clear difference between treatment types in terms of assessment of recurrent VTE (odds ratio (OR) 0.66, 95% CI 0.41 to 1.07), major bleeding (OR 0.45, 95% CI 0.18 to 1.11), or total mortality (OR 1.19, 95% CI 0.78 to 1.83). This meta‐analysis included six trials (Das 1996; Gonzalez 1999; Lopaciuk 1999; Lopez 2001; Pini 1994; Veiga 2000) and one abstract (Hull 2000). We included all trials in our review except Hull 2000, which is a duplicate report (conference abstract) of the included trial Hull 2007.
Ferretti 2006 reviewed only recurrent VTE after treatment with LMWH compared with oral anticoagulants for people with VTE and found no clear differences between treatments when assessing recurrent symptomatic VTE (OR 1.29, 95% CI 0.82 to 2.02). This meta‐analysis included three trials of patients with cancer, which we excluded for the purpose of this Cochrane review, three other trials that we judged to be of high methodological quality (Das 1996; Gonzalez 1999; Pini 1994), four that we considered of lower methodological quality (Kakkar 2003; Lopaciuk 1999; Lopez 2001; Veiga 2000), and an abstract (Hull 2002) of the included trial Hull 2007.
The third systematic review did detect a difference between treatments. Bochenek 2012 reviewed the efficacy and safety of long term treatment of VTE with LMWH compared with oral anticoagulants and found a reduction in episodes of VTE (OR 0.75, 95% CI 0.59 to 0.97) and in the outcome major bleeding (OR 0.59, 95% CI 0.47 to 0.74). This review did not evaluate mortality as an outcome. The Bochenek 2012 review included six trials that we judged to be of high methodological quality (Das 1996; Daskalopoulos 2005; Gonzalez 1999; Hull 2007; Massicotte 2003; Pini 1994) and six trials that we judged to be of lower methodological quality (Beckman 2003; Kakkar 2003; Kucher 2005; Lopaciuk 1999; Lopez 2001; Veiga 2000). The meta‐analysis by Bochenek 2012 included two trials that considered only patients with cancer, who were excluded for the purpose of this Cochrane review. One of these studies showed a significant effect of LMWH heavily influencing the overall outcome and contributing approximately one‐third of the overall weight; this was likely the reason for the difference between Bochenek 2012 and the current Cochrane review.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.1 incidence of recurrent VTE.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials); outcome: 2.2 incidence of major bleeding.

Funnel plot of comparison: 2 LMWH versus VKA during three months of allocated treatment (category I and II trials), outcome; 2.3 mortality.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 2 Incidence of major bleeding.

Comparison 1 LMWH versus VKA during allocated treatment (category I and II trials) in participants with VTE, Outcome 3 Mortality.
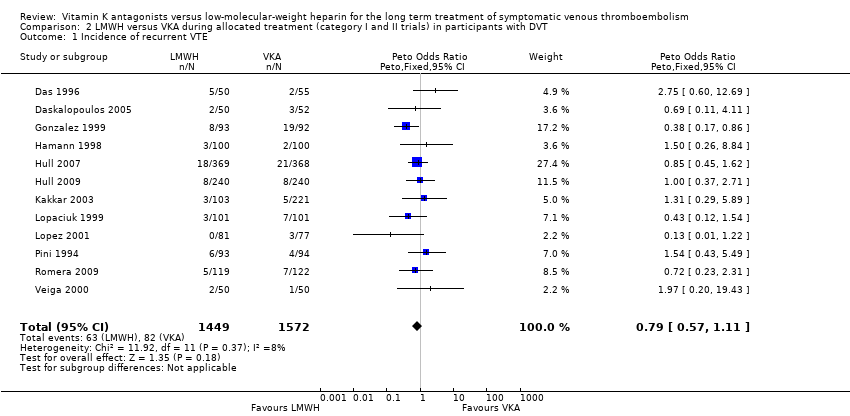
Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 1 Incidence of recurrent VTE.
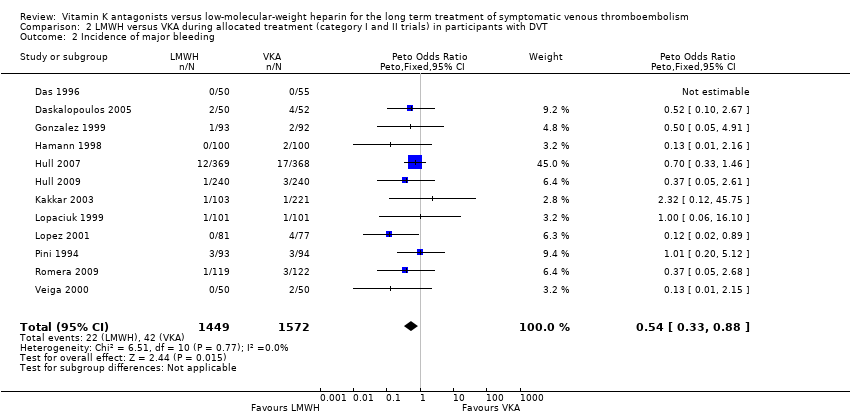
Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 2 Incidence of major bleeding.

Comparison 2 LMWH versus VKA during allocated treatment (category I and II trials) in participants with DVT, Outcome 3 Mortality.

Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 1 Incidence of recurrent VTE.

Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 2 Incidence of major bleeding.

Comparison 3 LMWH versus VKA during allocated treatment (category I and II trials) in participants with PE, Outcome 3 Mortality.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 1 Incidence of recurrent VTE.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 2 Incidence of major bleeding.

Comparison 4 LMWH versus VKA during allocated treatment (category I trials) in participants with VTE, Outcome 3 Mortality.

Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 1 Incidence of recurrent VTE.

Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 2 Incidence of major bleeding.

Comparison 5 Category I trials and the same initial treatment in both groups (unfractionated heparin or LMWH), Outcome 3 Mortality.

Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 1 Incidence of recurrent VTE.

Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 2 Incidence of major bleeding.
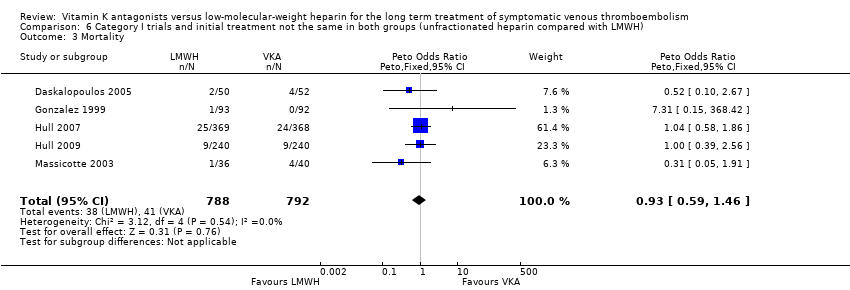
Comparison 6 Category I trials and initial treatment not the same in both groups (unfractionated heparin compared with LMWH), Outcome 3 Mortality.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding.

Comparison 7 LMWH versus VKA during additional follow‐up (category I and II trials), Outcome 3 Mortality.

Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE.

Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding.

Comparison 8 LMWH versus VKA during additional nine months of follow‐up (category I trials), Outcome 3 Mortality.

Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 1 Incidence of recurrent VTE.

Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 2 Incidence of major bleeding.

Comparison 9 LMWH versus VKA for total period of 12 months of follow‐up (category I and II trials), Outcome 3 Mortality.

Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 1 Incidence of recurrent VTE.

Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 2 Incidence of major bleeding.

Comparison 10 LMWH versus VKA for total period of 12 months of follow‐up (category I trials), Outcome 3 Mortality.
| LMWH compared with VKA for long term treatment of symptomatic VTE | ||||||
| Patient or population: patients with symptomatic VTE requiring long term treatment (3 months) for symptomatic VTE | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with VKA | Risk with LMWH | |||||
| Incidence of recurrent VTE (treatment duration 3 months) | Study population | Peto OR 0.83 | 3299 | ⊕⊕⊕⊝ | ||
| 51 per 1000 | 42 per 1000 | |||||
| Incidence of major bleeding (treatment duration 3 months) | Study population | Peto OR 0.51 | 3299 | ⊕⊕⊝⊝ | ||
| 29 per 1000 | 15 per 1000 | |||||
| Mortality (treatment duration 3 months) | Study population | Peto OR 1.08 | 3299 | ⊕⊕⊕⊝ | ||
| 35 per 1000 | 37 per 1000 | |||||
| * The basis for the assumed risk with VKA for 'Study population' was the average risk in the VKA group (i.e. total number of participants with events divided by total number of participants in the VKA group included in the meta‐analysis). The risk in the LMWH group (and its 95% confidence interval) is based on assumed risk in the VKA group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aHigh risk of bias due to no blinding but not downgraded, as analysis excluding studies deemed of low methodological quality confirms no clear differences between LMWH and VKA | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.60, 1.15] |
| 2 Incidence of major bleeding Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.32, 0.80] |
| 3 Mortality Show forest plot | 16 | 3299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.75, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.57, 1.11] |
| 2 Incidence of major bleeding Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.33, 0.88] |
| 3 Mortality Show forest plot | 12 | 3021 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.10 [0.75, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.70 [0.91, 35.60] |
| 2 Incidence of major bleeding Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.03, 1.78] |
| 3 Mortality Show forest plot | 3 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.39 [0.51, 57.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.54, 1.18] |
| 2 Incidence of major bleeding Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.36, 1.07] |
| 3 Mortality Show forest plot | 7 | 1872 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.61, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.74, 5.19] |
| 2 Incidence of major bleeding Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.20, 5.12] |
| 3 Mortality Show forest plot | 2 | 292 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.29, 2.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.44, 1.03] |
| 2 Incidence of major bleeding Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.33, 1.04] |
| 3 Mortality Show forest plot | 5 | 1580 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.59, 1.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.77, 1.64] |
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.71, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.26 [0.81, 1.98] |
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.72, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| 2 Incidence of major bleeding Show forest plot | 9 | 2112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.33, 0.95] |
| 3 Mortality Show forest plot | 10 | 2592 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.84, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of recurrent VTE Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.70, 1.30] |
| 2 Incidence of major bleeding Show forest plot | 4 | 1211 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.39, 1.32] |
| 3 Mortality Show forest plot | 5 | 1691 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.78, 1.42] |

