Glucocorticoides para la laringotraqueobronquitis (o crup) en niños
Resumen
Antecedentes
Los glucocorticoides son la base del tratamiento de la laringotraqueobronquitis. La evidencia existente muestra que los glucocorticoides son eficaces en el tratamiento de la laringotraqueobronquitis en niños. Sin embargo, es imperativo actualizar la evidencia sobre su relevancia clínica en esta enfermedad. Esta es una actualización de una revisión publicada por primera vez en 1999 y actualizada en 2004, 2011 y 2018.
Objetivos
Investigar los efectos y la seguridad de los glucocorticoides en el tratamiento de la laringotraqueobronquitis en niños de hasta 18 años de edad.
Métodos de búsqueda
Se hicieron búsquedas en La Biblioteca Cochrane, que incluye el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2022 número 9), Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations y Ovid MEDLINE (1946 hasta el 4 de marzo de 2022), Embase (Ovid) (1974 hasta el 4 de marzo de 2022). También se realizaron búsquedas en la ICTRP de la OMS y en ClinicalTrials.gov el 4 de marzo de 2022.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) en niños (de 18 años o menos) con laringotraqueobronquitis. El efecto de los glucocorticoides se evaluó en comparación con lo siguiente: placebo, cualquier otro agente farmacológico, cualquier otro glucocorticoide, cualquier combinación de otros glucocorticoides, administrados por diferentes formas de administración o administrados en diferentes dosis. Los estudios incluidos debían haber evaluado al menos uno de los desenlaces principales de esta revisión (definidos como el cambio en la puntuación del crup o las nuevas visitas a consulta, los [re]ingresos hospitalarios o ambos) o los desenlaces secundarios (definidos como la duración de la estancia hospitalaria o en los servicios de urgencias, la mejoría del paciente, el uso de tratamientos adicionales o los eventos adversos).
Obtención y análisis de los datos
Los autores de la revisión extrajeron los datos de forma independiente, con la comprobación de otro autor de la revisión. Los datos se introdujeron en Review Manager 5 para el metanálisis. Dos autores de la revisión evaluaron de forma independiente el riesgo de sesgo de los estudios mediante la herramienta Cochrane de riesgo de sesgo. Dos autores de la revisión evaluaron la certeza de la evidencia para los desenlaces principales mediante el método GRADE.
Resultados principales
Esta revisión actualizada incluye 45 ECA con un total de 5888 niños, un aumento de dos ECA con 1323 niños desde la última actualización. También se identificó un estudio en curso y otro pendiente de clasificación. La mayoría de los estudios (98%) se consideraron con riesgo de sesgo alto o incierto.
Cualquier glucocorticoide comparado con placebo
En comparación con placebo, los glucocorticoides podrían dar lugar a mayores reducciones de la puntuación del crup después de dos horas (diferencia de medias estandarizada [DME] ‐0,65; intervalo de confianza [IC] del 95%: ‐1,13 a ‐0.18; siete ECA, 426 niños; evidencia de certeza baja); de seis horas (DME ‐0,76; IC del 95%: ‐1,12 a ‐0,40; 11 ECA, 959 niños; evidencia de certeza baja); y de 12 horas (DME ‐1,03; IC del 95%: ‐1,53 a ‐0,53; ocho ECA, 571 niños; evidencia de certeza baja). La evidencia del cambio en la puntuación del crup después de 24 horas es muy incierta (DME ‐0,86; IC del 95%: ‐1,40 a ‐0,31; ocho ECA, 351 niños; evidencia de certeza muy baja).
Un glucocorticoide comparado con otro glucocorticoide
Hubo poca o ninguna diferencia entre la prednisolona y la dexametasona en la reducción de la puntuación del crup a las dos horas a partir de la puntuación inicial (DME 0,06; IC del 95%: ‐0,06 a 0,18; un ECA, 1231 niños; evidencia de certeza alta). Probablemente hubo poca o ninguna diferencia entre la prednisolona y la dexametasona en la reducción de la puntuación del crup a las seis horas a partir de la puntuación inicial (DME 0,21; IC del 95%: ‐0,21 a 0,62; un ECA, 99 niños; evidencia de certeza moderada). Sin embargo, es posible que la dexametasona reduzca las nuevas visitas a consulta o los (re)ingresos por laringotraqueobronquitis a casi la mitad (razón de riesgos [RR] 0,55; IC del 95%: 0,28 a 1,11; cuatro ECA, 1537 niños; evidencia de certeza moderada), y mostró una reducción del 28% en el uso de glucocorticoides suplementarios como tratamiento adicional (RR 0,72; IC del 95%: 0,53 a 0,97; dos ECA, 926 niños).
Dexametasona administrada a diferentes dosis
En comparación con 0,15 mg/kg, 0,60 mg/kg de dexametasona probablemente redujo la gravedad del laringotraqueobronquitis evaluada por la escala de puntuación del crup a las 24 horas a partir de la puntuación inicial (DME 0,63; IC del 95%: 0,16 a 1,10; un ECA, 72 niños; evidencia de certeza moderada); sin embargo, este no fue el caso a las dos horas (DME ‐0,27; IC del 95%: ‐0,76 a 0,22; dos ECA, 861 niños; evidencia de certeza alta). Probablemente no hubo una reducción a las seis horas (DME ‐0,45; IC del 95%: ‐1,26 a 0,35; tres ECA, 178 niños; evidencia de certeza moderada) y la evidencia a las 12 horas es muy incierta (DME ‐0,60; IC del 95%: ‐4,39 a 3,19; dos ECA, 113 niños; evidencia de certeza muy baja). Hubo poca o ninguna diferencia entre las dosis de dexametasona en las nuevas visitas o (re)ingresos de los niños o ambos (RR 0,91; IC del 95%: 0,71 a 1,17; tres ECA, 949 niños; evidencia de certeza alta) o en la duración de la estancia hospitalaria o en el servicio de urgencias (diferencia de medias 0,12; IC del 95%: ‐0,32 a 0,56; dos ECA, 892 niños). La necesidad de tratamientos adicionales, como epinefrina (RR 0,78; IC del 95%: 0,34 a 1,75; dos ECA, 885 niños); intubación (diferencia de riesgos 0,00; IC del 95%: ‐0,00 a 0,00; dos ECA, 861 niños); o la administración de glucocorticoides suplementarios (RR 0,77; IC del 95%: 0,51 a 1,15; dos ECA, 617 niños), tampoco difirió entre las dosis de dexametasona.
Hubo niveles de heterogeneidad de moderados a altos en los análisis de la mayoría de las comparaciones. Se observaron eventos adversos en algunas de las comparaciones notificadas en la revisión.
Conclusiones de los autores
La evidencia de que los glucocorticoides reducen los síntomas de la laringotraqueobronquitis a las dos horas, acortan la estancia hospitalaria y reducen la tasa de nuevas visitas o (re)ingresos no ha cambiado en esta actualización. Una dosis menor de 0,15 mg/kg de dexametasona podría ser tan eficaz como la dosis estándar de 0,60 mg/kg. Se necesitan más ECA para reforzar la evidencia de la efectividad de la dexametasona a dosis bajas de 0,15 mg/kg para tratar la laringotraqueobronquitis.
PICO
Resumen en términos sencillos
Glucocorticoides para la laringotraqueobronquitis (o crup) en niños
Pregunta de la revisión
¿Cuál es la eficacia y la seguridad de los glucocorticoides en el tratamiento de los niños con laringotraqueobronquitis?
Antecedentes
Los virus respiratorios son la principal causa de la laringotraqueobronquitis en niños. Provoca una inflamación de la garganta y las vías respiratorias, lo que puede dificultar la respiración. Los niños también presentan un tipo especial de tos denominada tos perruna. Los glucocorticoides son tipos de corticoides que ayudan a reducir la inflamación, facilitando así la respiración de los niños con esta enfermedad.
Esta es una actualización de una revisión publicada por primera vez en 1999 y actualizada en 2004, 2011 y 2018.
Fecha de la búsqueda
La evidencia está actualizada hasta el 4 de marzo de 2022.
Características de los estudios
Se incluyeron dos estudios nuevos con 1323 niños, para un total de 45 estudios con 5888 niños de cero a 18 años publicados entre 1964 y 2021. Los tres tipos de glucocorticoides utilizados en los nuevos estudios fueron la budesonida, la dexametasona y la prednisolona. El estudio más reciente comparó la eficacia de la budesonida y la dexametasona. El otro estudio nuevo comparó la eficacia de la dexametasona y la prednisolona, así como una dosis pequeña de dexametasona (0,15 mg/kg) frente a 0,60 mg/kg de dexametasona. Se añadieron los datos del nuevo estudio que comparó las dosis de dexametasona con los estudios incluidos anteriormente que analizaron la misma comparación.
Fuentes de financiación de los estudios
Entre las fuentes de financiación se incluyeron el gobierno (11%), entidades académicas o de investigación (7%), la industria (18%) o fundaciones (9%). Más de la mitad de los estudios (55%) no informaron acerca de las fuentes de financiación.
Resultados clave
En comparación con la prednisolona, la dexametasona no mostró mejoría en la puntuación de la laringotraqueobronquitis a las dos y seis horas de acudir al hospital o al servicio de urgencias, y probablemente redujo casi a la mitad las nuevas visitas o los (re)ingresos por la enfermedad. Añadir de glucocorticoides suplementarios favoreció a la dexametasona frente a la prednisolona. En comparación con 0,15 mg/kg de dexametasona, la dosis estándar de 0,60 mg/kg probablemente redujo la gravedad de la laringotraqueobronquitis evaluada con la escala de puntuación de la laringotraqueobronquitis a las 24 horas de acudir al hospital o al servicio de urgencias. Sin embargo, no se encontraron diferencias importantes entre los grupos en la escala de puntuación de laringotraqueobronquitis a las 2, 6 o 12 horas, en las nuevas visitas o (re)ingresos de los niños, ni en la duración de la estancia en el hospital o en el servicio de urgencias. La necesidad de tratamientos adicionales como el uso de otros medicamentos como adrenalina, glucocorticoides suplementarios o el uso de un tubo para ayudar a respirar no difirió entre 0,15 mg/kg y 0,60 mg/kg de dexametasona. No se notificaron episodios adversos graves derivados del uso de glucocorticoides en los nuevos estudios incluidos.
Conclusiones
No ha cambiado la evidencia de que los glucocorticoides reducen los síntomas de la laringotraqueobronquitis a las dos horas, acortan la estancia hospitalaria y reducen la tasa de nuevas visitas a consulta o (re)ingresos en comparación con el placebo (tratamiento falso). Una pequeña dosis de dexametasona de 0,15 mg/kg podría ser tan eficaz como la dosis estándar de 0,60 mg/kg. Se necesitan más estudios para reforzar la evidencia de la efectividad de la dexametasona a dosis bajas de 0,15 mg/kg para tratar la laringotraqueobronquitis. Se concluye que los glucocorticoides son eficaces en el tratamiento de la laringotraqueobronquitis en niños.
Certeza de la evidencia
La mayoría de los estudios (98%) tenían problemas relacionados con los métodos, con la manera de presentar la información o ambos. En el caso de cualquier glucocorticoide comparado con placebo, se redujo la certeza de la evidencia para el cambio en la puntuación de la laringotraqueobronquitis después de 2, 6, 12 y 24 horas y las nuevas visitas o (re)ingresos debido a la variabilidad de los estudios, la imprecisión y la inconsistencia de los resultados de los estudios, así como el riesgo de sesgo. Hay escasa evidencia de que el sesgo de notificación influyera en los resultados de esta revisión con respecto a las nuevas visitas o los (re)ingresos, o ambos. Se encontraron amenazas similares a la certeza de la evidencia en las otras comparaciones de esta revisión, que incluyen preocupaciones relacionadas con el riesgo de sesgo y la inconsistencia e imprecisión de los resultados de los estudios.
Authors' conclusions
Summary of findings
| Any glucocorticoid compared to placebo for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Placebo | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −1.50 to −0.81. | The mean change in croup score was 0.65 standard deviations in favour | ‐ | 426 | ⊕⊕⊝⊝ | A standard deviation of 0.65 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.23 to −0.65. | The mean change in croup score was 0.76 standard deviations in favour | ‐ | 959 | ⊕⊕⊝⊝ | A standard deviation of 0.76 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −7.62 to −1.00. | The mean change in croup score was 1.03 standard deviations in favour | ‐ | 571 | ⊕⊕⊝⊝ | A standard deviation of 1.03 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score was −2.56 to −1.05. | The mean change in croup score was 0.86 standard deviations in favour | ‐ | 351 | ⊕⊝⊝⊝ | A standard deviation of 0.86 represents a large difference between groups. |
| Return visits or (re)admissions or both | 204 per 1000 | 106 per 1000 | RR 0.52 | 1679 | ⊕⊕⊝⊝ |
|
| Adverse events | 13/26 (50%) studies reported collecting adverse events data, and 8/13 (62%) reported no serious adverse events. Bjornson 2004 reported 7 instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported 1 child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported 7 secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported 1 child with pneumonitis in the placebo group (1/13, 7.7%) and 2 children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported 1 instance of exacerbated symptoms, 5 children with emotional distress, 2 with vomiting, and 1 instance of eye irritation in the budesonide group (9/42, 21.4%), and 3 instances of exacerbated symptoms, 6 children with emotional distress, 3 with vomiting, 2 rashes, and 1 instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%). | 1399 (13 RCTs) | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. | ||||||
| Any glucocorticoid compared to epinephrine for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Epinephrine | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −4.24 to −3.74. | The mean change in croup score was 0.77 standard deviations not in favour | ‐ | 130 | ⊕⊝⊝⊝ | A standard deviation of 0.77 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −1.25 to −1.10. | The mean change in croup score was 0.10 standard deviations in favour | ‐ | 63 | ⊕⊝⊝⊝ | A standard deviation of 0.10 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.86 to −1.45. | The mean change in croup score was 0.07 standard deviations in favour | ‐ | 129 | ⊕⊕⊝⊝ | A standard deviation of 0.07 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score was −4.40 to −2.01. | The mean change in croup score was 0.17 standard deviations not in favour | ‐ | 129 | ⊕⊕⊝⊝ | A standard deviation of 0.17 represents a small difference between groups. |
| Return visits or (re)admissions or both | 0 per 1000 | 0 per 1000 | RD 0.00 | 130 | ⊕⊕⊝⊝ |
|
| Adverse events | 3/4 (75%) studies reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported 5 cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported 4 cases of tremor and tachycardia (4/25, 16%) in the epinephrine group. | 162 | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 87%), and variation in point estimates. There was minimal overlap of the confidence intervals. | ||||||
Background
Description of the condition
Croup is a common childhood respiratory disease that often leads to frequent emergency department (ED) visits (Bjornson 2008). It is a spectrum of diseases including laryngotracheitis, laryngotracheobronchitis, and laryngotracheobronchopneumonitis (Sizar 2021). Patients may present with sudden onset of a seal‐like barking cough, often accompanied by stridor, voice hoarseness, and respiratory distress (Bjornson 2008). As with many other acute respiratory diseases, croup can be mild, moderate, or severe in presentation. In brief, the pathophysiology of croup involves upper‐airway obstruction due to generalised inflammation of the airways, triggered by viral infection (especially the parainfluenza virus, which accounts for over 75% of infections) (Bjornson 2013). Whilst croup is a self‐limiting viral infection, the burden of frequent hospitalisation contributes significantly to healthcare utilisation (Bjornson 2013; Denny 1983). Croup accounts for 7% and 3% of hospitalisation in children under five and children between six months and three years in North America (Johnson 2014; Weinberg 2009). Likewise, one European study found that 16% of children aged five to eight years old had suffered from croup at least once, and 5% had experienced recurrent croup (Van Bever 1999).
Description of the intervention
The clinical benefits of glucocorticoids in the management of croup are well documented in the literature (Griffin 2000; Kairys 1989). Unlike the controversies that existed in the 1970s concerning the treatment of croup (Cherry 1979), many clinical guidelines now support the use of glucocorticoids (Alberta Medical Association 2008). Glucocorticoids have also been shown to decrease the rate and length of hospitalisation, return visits, and admission to intensive care unit in children with croup (Brown 2002; Geelhoed 1996b; Kairys 1989). Studies have also continued to highlight the effectiveness of glucocorticoids in reducing the severity of croup (Brown 2002).
How the intervention might work
One of the cardinal features of inflammation is oedema or swelling. Whilst there are associated generalised swellings of the airway in croup, inflammation and oedema of the subglottic larynx (the narrowest part of the paediatric airway) and trachea, especially near the cricoid cartilage, are most clinically significant (Cherry 2008). Glucocorticoids have anti‐inflammatory properties through which they reduce croup‐related mucosal oedema and inflammation and as such reduce the associated difficulty in breathing (Cherry 2008).
Why it is important to do this review
Systematic reviews of randomised controlled trials (RCTs) on the use of glucocorticoid for the treatment of croup have contributed significantly to the evidence around the management of croup to date. The first Cochrane Review on this study question included 24 RCTs that examined the effectiveness of treating croup with glucocorticoids (Ausejo 2000). A few other reviews have been conducted since to update the existing evidence (Gates 2018; Russell 2004; Russell 2011). The current review is necessary to incorporate new evidence to help strengthen or refute the findings of previous reviews on this study question. As there is a growing debate about the lowest effective dose of glucocorticoid in the management of croup (Alshehr 2005; Chub‐Uppakarn 2007; Dobrovoljac 2009), this review aimed to address this, and to update the existing evidence on the effect of glucocorticoids on croup.
Objectives
To investigate the effects and safety of glucocorticoids in the treatment of croup in children aged 18 years and below.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs in child health research that met our inclusion criteria irrespective of language, publication status, trial conduct and reporting quality, or risk of bias. We excluded all other study designs.
Types of participants
We included RCTs on children aged 18 years and below diagnosed with croup, pseudo croup, or laryngotracheitis. We defined croup as a syndrome consisting of hoarseness, barking cough, and stridor, where an alternative diagnosis of acute stridor had been excluded. We included both inpatients and outpatients, and defined children admitted to the emergency department as outpatients.
Types of interventions
We included studies where the intervention was the use of one or more glucocorticoids via any route of drug administration. There were no restrictions on the type or dose of glucocorticoid administered. We defined the control as the use of a placebo or any other active pharmacologic agent. We considered the following scenarios: the use of any glucocorticoid compared to placebo, glucocorticoid compared to epinephrine, or one glucocorticoid compared to one or a combination of other glucocorticoids, or glucocorticoids given by different modes of administration, or glucocorticoids given in different doses. We excluded studies if none of the treatment groups received one or more glucocorticoids.
Types of outcome measures
We included RCTs that measured on one or more of our primary or secondary outcomes. We excluded studies that failed to meet all of our inclusion criteria.
Primary outcomes
-
Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours.
-
Return visits or (re)admissions to the hospital, or both.
Secondary outcomes
-
Length of stay in the hospital or emergency department.
-
Patient improvement at 2, 6, 12, and/or 24 hours (yes or no, as reported in the individual studies).
-
The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids.
-
Any adverse events.
Search methods for identification of studies
Electronic searches
We adopted the search strategy developed by a research librarian in the previous review (Gates 2018) on 4 March 2022 (Appendix 1). The update searches were conducted by the librarian Mê‐Linh Lê. We included subject headings and keywords for croup and glucocorticoids and restricted the search to RCTs. We searched the Cochrane Library, which includes the Cochrane Central Register of Controlled Trials (CENTRAL; 2022, Issue 9), Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 4 March 2022), and Embase (Ovid) (1974 to 4 March 2022).
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int/) and ClinicalTrials.gov (clinicaltrials.gov) on 4 March 2022 (Appendix 1). We scanned the reference lists of relevant systematic reviews identified during screening and the included studies to identify additional relevant primary studies.
Data collection and analysis
Selection of studies
We transferred the citations identified via the search to Rayyan software after de‐duplication (Ouzzani 2016). Three review authors (CT, AK, MR) independently screened the identified citations for eligibility using a two‐stage sifting approach to review the title, abstract, and full‐text article. Any disagreements were resolved by discussion or by involving another review author (AA) when necessary.
Data extraction and management
Three review authors (CT, AK, MR) independently extracted the data, which were all in the English language. We used Microsoft Excel to manage data extraction (Microsoft Excel). We leveraged the data extraction form used in our previous review (Gates 2018). The details of the data extracted based on participant characteristics, experimental and control interventions, and primary and secondary outcomes have all been previously published (Gates 2018). Any disagreements during data extraction were resolved by discussion or by involving another review author (AA) when necessary.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool to assess risk of bias of the included studies (Higgins 2011b). We judged the risk of bias for each study as low, high, or unclear for seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. We determined the overall risk of bias as follows: low when all domains were judged as low risk; unclear when one or more domains were judged as unclear risk; and high when one or more domains were judged as high risk. Two review authors (CT, MR) independently assessed risk of bias, resolving any disagreements by discussion or by involving another review author (AA) when necessary.
Measures of treatment effect
We added relevant data from the included studies into Review Manager 5 for analysis (Review Manager 2020). We computed the effect of treatment using the random‐effects model.
Croup scores were reported as the Westley score (Westley 1978), the telephone outpatient (TOP) score (Bjornson 2016), the Downes and Raphaelly score (Downes 1975), or various author‐created scales. We therefore used standardised mean differences (SMDs) to combine the outcome for any croup score. A treatment effect (difference between treatment means) divided by its measurement variation (e.g. a pooled standard deviation) gives the SMD. We did not find effect estimates to be significantly different between Westley and other croup scores, so we included studies that reported any croup score in the subgroup analyses. Of note, a decrease in Westley score of one point from baseline is thought to be a clinically important change.
We expressed length of stay as mean differences (MDs) and calculated an overall MD. We calculated risk ratios (RRs) for binary data (i.e. return visits or (re)admissions (or both), patient improvement, use of additional treatments). We calculated risk differences (RDs) where outcomes had zero events in both groups. For return visits or (re)admissions (or both), we calculated the number needed to treat for an additional beneficial outcome (NNTB) for significant results. Because there was substantial variation in control group event rates between studies, we reported the NNTB for the mean control group rate, as well as for the smallest and largest control group rate observed.
We reported data on adverse events narratively.
Unit of analysis issues
As reported in Gates 2018, we calculated the change from baseline croup score in 28 (62%) studies where the change from baseline measures was not reported directly (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
We pooled counts, means, and variances using standard formulae for seven (15%) studies that contained more than one experimental treatment group (Cetinkaya 2004; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Luria 2001; Parker 2019). One study by Geelhoed (Geelhoed 1995a; Geelhoed 1995b), and another by Skowron (Skowron 1966a; Skowron 1966a and b; Skowron 1966b), presented the results of two individual trials in one publication. We treated these as separate comparisons in the analyses and used pooled counts only when they were reported as such in the publications.
Dealing with missing data
When they were not directly reported, we estimated the variances for continuous data in accordance with the work of Abrams 2005 and Follmann 1992. Using standard formulae, we imputed standard deviations from standard errors in three (7%) studies (Alshehr 2005; Johnson 1998; Von Mühlendahl 1982), ranges in three (7%) studies (Alshehr 2005; Roorda 1998; Super 1989), 95% confidence intervals (CIs) in two (4%) studies (Fitzgerald 1996; Klassen 1998), and interquartile ranges (IQRs) in three (7%) studies (Johnson 1996; Klassen 1994; Klassen 1998). When the change in croup score from baseline was not directly reported (n = 14, 31%), we derived the variance of the change assuming a correlation of 0.5 between pre‐ and post‐treatment scores (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fitzgerald 1996; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
In 11 (26%) studies, data from which to impute variances for change in croup score or length of stay were inadequate; for these studies we substituted average variances from other studies in the main analysis (Cetinkaya 2004; Dobrovoljac 2012; Eboriadou 2010; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Kuusela 1988; Massicotte 1973; Roberts 1999; Skowron 1966a; Skowron 1966b). Furukawa and colleagues assert that when the number of studies with imputed data within a meta‐analysis is relatively small, variance data can be safely borrowed from other studies and still provide accurate results (Furukawa 2006). For certain outcomes only one study was included in the comparison, and that study did not report a variance estimate; in such a case we did not calculate a point estimate of effect (Cetinkaya 2004; Duman 2005; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Rittichier 2000).
We substituted medians for means in nine (20%) studies (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1998; Parker 2019; Super 1989; Von Mühlendahl 1982). When data for our prespecified time points (2, 6, 12, and 24 hours from baseline) were not reported, we used time points close to these if available. We substituted one hour for two hours in one study (Dobrovoljac 2012); four hours for six hours in 12 (28%) studies (Alshehr 2005; Amir 2006; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973); five hours or discharge for six hours in one study (Johnson 1998); and 14 hours for 12 hours in one study (Massicotte 1973).
Assessment of heterogeneity
In keeping with Gates 2018, we assessed heterogeneity quantitatively with the Chi² test for heterogeneity and the I² statistic (Higgins 2002). The I² statistic indicates the per cent variability due to between‐study (or interstudy) variability as opposed to within‐study (or intrastudy) variability. We considered an I² of less than 40% to be low (potentially unimportant), 30% to 60% to be moderate, 50% to 90% to be substantial, and 75% to 100% to be considerable (Higgins 2011a, Section 9.5.2).
Assessment of reporting biases
In addition to visually inspecting the funnel plots, we used the rank correlation test and weighted regression for the detection of publication bias (Begg 1994; Egger 1997; Light 1984). We used more than one method because the relative merits of the methods are not well established.
Data synthesis
We used random‐effects models to combine treatment effects regardless of quantified heterogeneity for the analyses of all outcomes.
Subgroup analysis and investigation of heterogeneity
We explored heterogeneity between studies using subgroup analyses for the primary outcomes of change in croup score from baseline to 2, 6, 12, and 24 hours, and return visits or (re)admissions or both, using the Chi² test for subgroup differences in meta‐analysis. We explored heterogeneity by croup score, by inpatient or outpatient status, and by glucocorticoid.
Sensitivity analysis
In some analyses, we imputed variance data for most of the included RCTs (e.g. any glucocorticoid compared to placebo, change in croup score after two hours). We undertook sensitivity analyses for these and all other analyses containing imputed variance data using the largest, smallest, and average variances from the other included RCTs. As per protocol, we did not undertake any additional sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables for our two main comparisons (any glucocorticoid compared to placebo and any glucocorticoid compared to epinephrine) for the primary outcomes: change in croup score at 2, 6, 12, and 24 hours from baseline, and return visits or (re)admissions or both. The findings for the two main comparisons have not changed since the previous version of the review, as no new data were identified in the current update (Gates 2018). As per protocol, we created summary of findings tables for the remaining comparisons; however, in order not to detract from the two main comparisons, these are included in the Additional tables section. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it relates to the studies that contributed data to the meta‐analyses (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the reader's understanding where necessary.
Results
Description of studies
Results of the search
We identified 100 records in the 2022 update search (Figure 1). We retrieved 83 citations from the database searches and 17 records from trial registers, from which we identified and removed 41 duplicates. We screened 59 records by title and abstract and excluded 49 citations. We screened 10 full‐text articles of which six were excluded, with reasons for their exclusion provided. A flow diagram illustrating the 2022 update selection process is shown in Figure 1. We added two new RCTs with 1323 children (Huang 2021; Parker 2019), one ongoing study (IRCT20190914044765N1), and one study awaiting classification (Chen 2018). This updated review includes 45 RCTs with a total of 5888 children.

Flow diagram of study selection for this review.
Included studies
Participant and trial characteristics
We identified 42 studies (93%) published in English, and one each in French (Massicotte 1973), Spanish (Martinez Fernandez 1993), and Danish (Vad Pedersen 1998). Four studies (9%) included children with mild croup (Bjornson 2004; Geelhoed 1996a; Luria 2001, Parker 2019). Twenty‐three studies (51%) assessed outpatient children (n = 22 emergency department visits, n = 1 physician office visits) (Alshehr 2005; Amir 2006; Bjornson 2004; Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996a; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Parker 2019; Rittichier 2000; Soleimani 2013; Sparrow 2006). Twenty‐three studies (51%) assessed hospitalised children (Chub‐Uppakarn 2007; Eden 1964; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Parker 2019; Roberts 1999; Roorda 1998; Skowron 1966a; Skowron 1966b; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982).
Thirty‐two studies (71%) were two‐armed trials (Alshehr 2005; Amir 2006; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Eden 1964; Eden 1967; Fitzgerald 1996; Garbutt 2013; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Johnson 1996; Klassen 1994; Klassen 1996; Koren 1983; Leipzig 1979; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Soleimani 2013; Sparrow 2006; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982); eight studies (18%) were three‐armed trials (Duman 2005; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Klassen 1998; Luria 2001; Parker 2019); and three studies (7%) were four‐armed trials (Cetinkaya 2004; Kuusela 1988; Martinez Fernandez 1993). Two studies (4%) included two individual two‐armed trials each (Geelhoed 1995a; Geelhoed 1995b; Skowron 1966a; Skowron 1966b).
Characteristics of the comparisons
Twenty‐six studies (58%) investigated any glucocorticoid compared to placebo. Of these, 15 (58%) investigated dexamethasone (Bjornson 2004; Cruz 1995; Dobrovoljac 2012; Eden 1967; Geelhoed 1996a; James 1969; Johnson 1996; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Skowron 1966a and b; Super 1989; Von Mühlendahl 1982); four (15%) investigated budesonide (Godden 1997; Husby 1993; Klassen 1994; Roberts 1999); three (12%) investigated prednisolone (Eden 1964; Massicotte 1973; Tibballs 1992); one (4%) investigated fluticasone (Roorda 1998); and three (12%) investigated both dexamethasone and budesonide (Cetinkaya 2004; Geelhoed 1995c; Johnson 1998). Four studies (10%) investigated any glucocorticoid compared to epinephrine. Of these, one investigated budesonide (Fitzgerald 1996); two investigated dexamethasone (Kuusela 1988; Martinez Fernandez 1993); and one investigated both dexamethasone and beclomethasone (Eboriadou 2010).
Thirteen studies (29%) investigated one glucocorticoid compared to another glucocorticoid. Of these, one investigated budesonide compared to dexamethasone (Huang 2021); six investigated dexamethasone compared to budesonide (Cetinkaya 2004; Duman 2005; Geelhoed 1995c; Johnson 1998; Klassen 1998; Vad Pedersen 1998); one investigated dexamethasone compared to betamethasone (Amir 2006); one investigated dexamethasone compared to beclomethasone (Eboriadou 2010); and four investigated dexamethasone compared to prednisolone (Fifoot 2007; Garbutt 2013; Parker 2019; Sparrow 2006). Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. Of these, one investigated dexamethasone and budesonide compared to a combination of dexamethasone and budesonide (Klassen 1998), and two investigated dexamethasone compared to a combination of dexamethasone and budesonide (Geelhoed 2005; Klassen 1996).
Five studies (11%) investigated dexamethasone using different modes of administration. Of these, four investigated oral compared to intramuscular dexamethasone (Cetinkaya 2004; Donaldson 2003; Rittichier 2000; Soleimani 2013), and one investigated oral compared to nebulised dexamethasone (Luria 2001). Four studies investigated dexamethasone given in different doses. Of these, three investigated 0.60 mg/kg compared to 0.15 mg/kg dexamethasone (Alshehr 2005; Chub‐Uppakarn 2007; Fifoot 2007), and one investigated both 0.60 mg/kg compared to 0.30 mg/kg and 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Geelhoed 1995a; Geelhoed 1995b).
Reported outcomes: primary outcomes
Sixteen studies (35%) reported a two‐hour change in croup score (Amir 2006; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Parker 2019; Roberts 1999; Roorda 1998); 20 studies (44%) reported a six‐hour change in croup score (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Roorda 1998; Vad Pedersen 1998; Von Mühlendahl 1982); 12 studies (27%) reported a 12‐hour change in croup score (Alshehr 2005; Chub‐Uppakarn 2007; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982); and 11 studies (24%) reported a 24‐hour change in croup score (Alshehr 2005; Cetinkaya 2004; Fitzgerald 1996; Godden 1997; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989). Of the 30 studies (67%) that reported a change in croup score, 18 (60%) used a validated score (the Westley score or a modified Westley score) (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Fifoot 2007; Godden 1997; Husby 1993; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Parker 2019; Rittichier 2000; Roorda 1998; Super 1989); 11 (37%) used author‐created scales (Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 2005; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Vad Pedersen 1998; Von Mühlendahl 1982); and one used the score by Downes 1975 (Eboriadou 2010). The studies by Bjornson 2004 and Garbutt 2013 used another validated score, the telephone outpatient (TOP) score, to measure clinical improvement. The TOP score is a two‐item, three‐point score used to assess the presence of stridor and barky cough by asking parents about their child's symptoms in the previous 24 hours (Bjornson 2016). Twenty‐seven studies (60%) reported return visits or (re)admissions to the hospital or both (Alshehr 2005; Amir 2006; Bjornson 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Parker 2019; Rittichier 2000; Roberts 1999; Skowron 1966a; Skowron 1966a and b; Skowron 1966b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998).
Reported outcomes: secondary outcomes
A total of 13 studies (29%) reported length of stay in the hospital or emergency department (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Klassen 1998; Kuusela 1988; Leipzig 1979; Parker 2019; Roorda 1998; Skowron 1966a; Skowron 1966a and b; Skowron 1966b; Sparrow 2006; Super 1989). Twelve studies (27%) reported patient improvement; of these, one reported improvement after two hours (Roberts 1999); eight reported improvement after six hours (Eden 1964; Eden 1967; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973; Roberts 1999); six reported improvement after 12 hours (Eden 1964; Eden 1967; James 1969; Massicotte 1973; Roberts 1999; Super 1989); and seven reported improvement after 24 hours (Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; James 1969; Roberts 1999; Super 1989). About two‐thirds of the included studies (n = 30) reported the use of additional treatments; of these, 12 reported intubation/tracheotomies (Chub‐Uppakarn 2007; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; James 1969; Johnson 1996; Johnson 1998; Leipzig 1979; Parker 2019; Roorda 1998; Skowron 1966a; Skowron 1966a and b; Skowron 1966b); four reported the use of antibiotics (Husby 1993; James 1969; Koren 1983; Rittichier 2000); 14 reported the use of supplemental glucocorticoids (Dobrovoljac 2012; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Parker 2019; Rittichier 2000; Roorda 1998; Super 1989; Vad Pedersen 1998); 22 reported the use of epinephrine (Amir 2006; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Koren 1983; Parker 2019; Rittichier 2000; Roberts 1999; Sparrow 2006; Super 1989; Tibballs 1992); and five reported the use of a mist tent (Alshehr 2005; Johnson 1996; Klassen 1996; Rittichier 2000; Super 1989). Twenty‐four studies reported collecting adverse events data, of which eight reported serious adverse events following the administration of glucocorticoids (namely secondary bacterial infections, e.g. pneumonia, otitis media) (Alshehr 2005; Bjornson 2004; Johnson 1996; Klassen 1998; Kuusela 1988; Parker 2019; Roberts 1999; Super 1989), and 16 reported no serious adverse events (Chub‐Uppakarn 2007; Duman 2005; Eden 1967; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Huang 2021; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Sparrow 2006; Tibballs 1992; Vad Pedersen 1998).
Funding
The included studies received funding from government (11%), academic (7%), industry (18%), and foundations (9%) sources. However, more than half (55%) of the included studies did not report any funding sources.
Excluded studies
We excluded six studies following the searches in 2022 (Figure 1). Gursanscky 2019 and Tyler 2022 were not randomised trials; Lee 2019 and Meskina 2019 were randomised trials that did not investigate glucocorticoids; and Faraji‐Goodarzi 2018 was a randomised trial that did not report any relevant outcomes. See Characteristics of excluded studies table.
We edited the excluded studies list to remove legacy excluded studies that evidently did not meet the inclusion criteria (e.g. letters, commentaries, summaries, case studies). We made this change to comply with current Cochrane standards for methods and reporting. We excluded 38 studies in this 2022 updated review.
Ongoing studies
We identified one ongoing study, IRCT20190914044765N1, and one study awaiting classification, Chen 2018 (Figure 1). We will assess these studies for inclusion in a future update.
Risk of bias in included studies
We presented the risk of bias of all included studies as assessed using the Cochrane risk of bias tool in Figure 2 and Figure 3. We judged the overall risk of bias to be low in one study (Garbutt 2013), unclear in 32 studies (Alshehr 2005; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; James 1969; Johnson 1996; Johnson 1998; Klassen 1996; Klassen 1998; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Parker 2019; Roorda 1998; Skowron 1966a and b; Sparrow 2006; Super 1989; Tibballs 1992; Von Mühlendahl 1982), and high in 12 studies (Amir 2006; Cetinkaya 2004; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fitzgerald 1996; Geelhoed 1995c; Klassen 1994; Rittichier 2000; Roberts 1999; Soleimani 2013; Vad Pedersen 1998). Rationales for our risk of bias judgements are provided in the risk of bias tables in the Characteristics of included studies table.

Risk of bias graph for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged risk of bias for random sequence generation to be low in 27 studies (60%) and unclear in 18 studies (40%). The 17 studies at unclear risk of bias were described as randomised; however, the method for generating the randomisation sequence was unclear or not reported (Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Godden 1997; Huang 2021; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Martinez Fernandez 1993; Roorda 1998; Skowron 1966a and b; Soleimani 2013; Von Mühlendahl 1982). Randomisation was adequately described in the remaining 27 studies. We judged risk of bias for allocation concealment to be low in 19 studies (42%) and unclear in 26 studies (58%); in the latter studies, there was insufficient information reported in the publication to determine whether or not the groups to which the children were allocated could have been foreseen (Amir 2006; Cetinkaya 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; Koren 1983; Leipzig 1979; Rittichier 2000; Roorda 1998; Skowron 1966a and b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998; Von Mühlendahl 1982). Allocation concealment was adequate in the remaining 19 studies.
Blinding
We judged risk of bias for blinding of participants and personnel to be low in 26 studies (58%), unclear in 11 studies (24%), and high in eight studies (18%). Of the eight studies at high risk of bias, four appeared to be open‐label (Amir 2006; Duman 2005; Rittichier 2000; Vad Pedersen 1998). Cetinkaya 2004 did not explicitly describe any measures taken to blind participants and personnel from treatment assignment, and any blinding could have been broken. Personnel were not blinded in Fitzgerald 1996. In Eboriadou 2010, the treatments were clearly distinguishable, and the method for blinding was not described even though the study was termed "double‐blind". In Soleimani 2013, only the outcome assessor was blinded. Of the 11 studies assessed as at unclear risk of bias, seven were described as double‐blind without any further details regarding who was blinded or how blinding was achieved (Eden 1964; Geelhoed 1996a; Huang 2021; Husby 1993; Leipzig 1979; Roorda 1998; Von Mühlendahl 1982). In Donaldson 2003, Geelhoed 1995a, Geelhoed 1995c, and Johnson 1998, blinding was attempted, but we judged that the blinding could have been broken; however, it was unclear how often this could have occurred. The remaining studies included satisfactory descriptions of how participants and personnel were blinded.
We judged risk of bias for blinding of outcome assessment to be low in 27 studies (60%), unclear in 13 studies (29%), and high in five studies (11%). For 22 studies (49%), there was no mention of a third‐party outcome assessor, so the judgement for outcome assessment was carried over from blinding of participants and personnel (Cetinkaya 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996a; Geelhoed 2005; Godden 1997; Huang 2021; Husby 1993; Koren 1983; Kuusela 1988; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Sparrow 2006; Tibballs 1992). Of the remaining studies, we judged two as at high risk of bias because outcome assessors were not blinded (Amir 2006; Vad Pedersen 1998). We judged seven studies as at unclear risk of bias: in Donaldson 2003, Johnson 1998, and Rittichier 2000, blinding of the outcome assessors was attempted, but we judged that the blinding could have been broken, although it was unclear how often this could have occurred; the studies by Leipzig 1979, Roorda 1998, and Von Mühlendahl 1982 were described as double‐blind, but it was unclear if the outcome assessors were blinded; and in Soleimani 2013, the outcome assessor was described as blinded, but it was unclear how or if the blinding could have been broken. The remaining studies provided satisfactory descriptions of how outcome assessors were blinded.
Incomplete outcome data
We judged risk of bias for incomplete outcome data to be low in 27 studies (60%), unclear in 14 studies (31%), and high in four studies (9%). The four studies at high risk of bias reported large losses to follow‐up that were imbalanced between groups (Dobrovoljac 2012; Geelhoed 1995c; Klassen 1994; Roberts 1999). Dobrovoljac 2012 and Roberts 1999 used the last observation carried forward (LOCF) method to estimate endpoint outcome values. Regarding the studies at unclear risk of bias, in one study the number of children analysed was not reported (Amir 2006), and in seven studies it was either unclear to which group the children who were lost to follow‐up had been allocated, or whether or not the losses to follow‐up were balanced between groups (Cruz 1995; Eden 1964; Johnson 1996; Huang 2021; Kuusela 1988; Rittichier 2000; Soleimani 2013; Von Mühlendahl 1982). In four studies, losses to follow‐up ranged from 13% to 17% (Fifoot 2007; Luria 2001; Soleimani 2013; Super 1989). In Fitzgerald 1996, loss to follow‐up was 5%, and the LOCF method was used to estimate endpoint outcome values. In Parker 2019, 11% of participants were missing at one‐hour croup assessment with unexplained exclusion reasons. We judged risk of bias due to incomplete outcome data not a concern for the remaining studies.
Selective reporting
We judged risk of bias for selective reporting to be low in three studies (7%) and unclear in 42 studies (93%). In the three studies at low risk of bias, the outcomes in the trial registers matched those reported in the publications (Fifoot 2007; Garbutt 2013; Parker 2019). For the remaining 42 studies, no protocol or trial registry was cited in the publication or located via online searches. In all cases, the outcomes reported in the methods matched those reported in the results section of the publications.
Other potential sources of bias
We judged risk of bias from other sources to be low in 35 studies (78%), unclear in eight studies (18%), and high in two studies (4%). In the two studies at high risk of bias, there was a baseline imbalance in croup score (Amir 2006; Vad Pedersen 1998). For six studies at unclear risk of bias, there was the potential for bias in participant selection because some children were not enrolled due to manpower constraints, failure of the emergency department to contact the research team, or because the emergency department was busy (Geelhoed 1995a; Geelhoed 1995b; Godden 1997; Klassen 1994; Klassen 1996; Klassen 1998; Sparrow 2006). In one study at unclear risk of bias, baseline data were not presented, therefore it was not possible to estimate whether or not baseline imbalances existed between groups (Skowron 1966a and b). For the remaining study at unclear risk of bias, participants were enrolled more than once (Parker 2019).
Effects of interventions
See: Summary of findings 1 Any glucocorticoid compared to placebo for croup; Summary of findings 2 Any glucocorticoid compared to epinephrine for croup
See summary of findings Table 1 and summary of findings Table 2.
Comparison 1: Any glucocorticoid compared to placebo
See summary of findings Table 1.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to placebo, glucocorticoids may have resulted in greater reductions in croup score after two hours (standardised mean difference (SMD) −0.65, 95% confidence interval (CI) −1.13 to −0.18; P = 0.007, I² = 81%; 7 RCTs, 426 children; low‐certainty evidence; Analysis 1.1); six hours (SMD −0.76, 95% CI −1.12 to −0.40; P < 0.001, I² = 83%; 11 RCTs, 959 children; low‐certainty evidence; Analysis 1.2); and 12 hours (SMD −1.03, 95% CI −1.53 to −0.53; P < 0.001, I² = 86%; 8 RCTs, 571 children; low‐certainty evidence; Analysis 1.3). The evidence for change in croup score after 24 hours is very uncertain (SMD −0.86, 95% CI −1.40 to −0.31; P = 0.002, I² = 81%; 8 RCTs, 351 children; very low‐certainty evidence; Analysis 1.4).
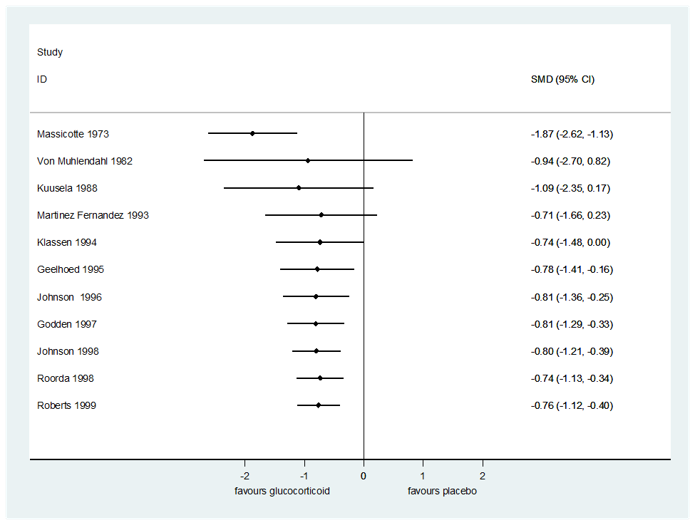
There were no subgroup differences in reductions in croup score by score (Westley 1978 or otherwise) (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4) or by inpatient or outpatient status (Analysis 1.5; Analysis 1.6; Analysis 1.7) at any time point. At two hours, there was no subgroup difference in effect by glucocorticoid (Chi² = 5.65, P = 0.06, I² = 64.6%; Analysis 1.8). At six hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 11.46, P = 0.009, I² = 73.8%; Analysis 1.9), accounted for by the larger reduction in croup score for prednisolone (SMD −1.87, 95% CI −2.62 to −1.13; P < 0.001; 1 RCT, 42 children) compared to budesonide (SMD −0.81, 95% CI −1.04 to −0.58; P < 0.001, I² = 0%; 5 RCTs, 333 children) and dexamethasone (SMD −0.62, 95% CI −1.17 to −0.08; P = 0.03, I² = 85%; 6 RCTs, 567 children). Fluticasone did not show an effect (SMD 0.06, 95% CI −0.89 to 1.02; P = 0.90; 1 RCT, 17 children). At 12 hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 10.08, P = 0.006, I² = 80.2%; Analysis 1.10), accounted for by the larger reduction in croup score for prednisolone (SMD −2.40, 95% CI −3.26 to −1.55; P < 0.001; 1 RCT, 39 children) compared to budesonide (SMD −0.97, 95% CI −1.26 to −0.68; P < 0.001, I² = 0%; 3 RCTs, 209 children) and dexamethasone (SMD −0.85, 95% CI −1.55 to −0.15; P = 0.02, I² = 84%; 5 RCTs, 323 children). At 24 hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 9.02, P = 0.01, I² = 77.8%; Analysis 1.11). Although larger reductions in croup score were observed with budesonide (SMD −1.40, 95% CI −1.88 to −0.93; P < 0.001, I² = 0%; 2 RCTs, 89 children) and dexamethasone (SMD −0.89, 95% CI −1.55 to −0.22; P = 0.009, I² = 81%; 6 RCTs, 245 children) compared to placebo, fluticasone did not show an effect (SMD 0.21, 95% CI −0.75 to 1.17; P = 0.67; 1 RCT, 17 children).
2. Return visits or (re)admissions to the hospital or both
Compared to placebo, glucocorticoids may have reduced the rate of return visits or (re)admissions to the hospital or both by almost half (risk ratio (RR) 0.52, 95% CI 0.36 to 0.75; P < 0.001, I² = 52%; 10 RCTs, 1679 children; low‐certainty evidence; Analysis 1.12). There were no subgroup differences in effect by glucocorticoid (budesonide or dexamethasone, Analysis 1.13); by inpatient or outpatient status (Analysis 1.12); or by croup severity (mild or moderate croup, Analysis 1.14).
The number needed to treat for an additional beneficial outcome (NNTB) is presented in Table 1. The NNTB was 7 children (95% CI 5 to 12) for the mean placebo group rate (30.62%). The NNTB was 102 children (95% CI 78 to 179) for the smallest placebo group rate (2.06%). Lastly, the NNTB was 3 children (95% CI 2 to 5) for the largest placebo group rate (72.00%).
| Baseline rate (%) | NNTB (95% CI) |
|---|---|
| Mean baseline rate | |
| 30.62 | 7 (5 to 12) |
| Smallest baseline rate | |
| 2.06 | 102 (78 to 179) |
| Largest baseline rate | |
| 72.00 | 3 (2 to 5) |
NNTB: number needed to treat for an additional beneficial outcome
Secondary outcomes
1. Length of stay in the hospital or emergency department
Compared to those given a placebo, children treated with glucocorticoids spent fewer hours in the hospital (mean difference (MD) −14.90, 95% CI −23.58 to −6.22; P < 0.001, I² = 54%; 8 RCTs, 476 children; Analysis 1.15). All of the included studies investigated inpatients. There was no subgroup difference in effect by glucocorticoid (budesonide, dexamethasone, or fluticasone; Analysis 1.16).
2. Patient improvement at 2, 6, 12, and/or 24 hours
Only one study investigated patient improvement two hours after the administration of glucocorticoids compared to placebo. Roberts 1999 studied 82 hospitalised children aged six months to eight years with moderate to severe croup who were given budesonide or placebo, and observed no difference in improvement after two hours (RR 1.81, 95% CI 0.96 to 3.40; P = 0.07; 1 RCT, 82 children; Analysis 1.17). Compared to placebo, glucocorticoids were associated with improvement in a greater proportion of children after six hours (RR 1.45, 95% CI 1.12 to 1.88; P = 0.005, I² = 34%; 6 RCTs, 332 children; Analysis 1.18); 12 hours (RR 1.33, 95% CI 1.09 to 1.62; P = 0.005, I² = 53%; 6 RCTs, 340 children; Analysis 1.19); and 24 hours (RR 1.28, 95% CI 1.01 to 1.61; P = 0.04, I² = 75%; 5 RCTs, 251 children; Analysis 1.20).
Only inpatients were included in the 12‐hour analysis (Analysis 1.19). There were no subgroup differences in estimates of effect by inpatient or outpatient status at six or 24 hours (Analysis 1.18; Analysis 1.20). There were no subgroup differences in effect by glucocorticoid at six hours (budesonide, dexamethasone, or prednisolone; Analysis 1.21), 12 hours (budesonide, dexamethasone, or prednisolone; Analysis 1.22), or 24 hours (dexamethasone or prednisolone; Analysis 1.23).
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference between children treated with glucocorticoids and those given placebo in the use of antibiotics (risk difference (RD) 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 3 RCTs, 202 children; Analysis 1.24); the use of epinephrine (RD −0.03, 95% CI −0.08 to 0.01; P = 0.16, I² = 45%; 9 RCTs, 709 children; Analysis 1.25); the rate of intubation/tracheostomy (RD 0.00, 95% CI −0.01 to 0.01; P = 0.79, I² = 0%; 11 RCTs, 1090 children; Analysis 1.26); the use of a mist tent (RD −0.20, 95% CI −0.87 to 0.47; P = 0.55, I² = 95%; 2 RCTs, 84 children; Analysis 1.27); or the use of supplemental glucocorticoids (RR 0.61, 95% CI 0.36 to 1.03; P = 0.07, I² = 10%; 6 RCTs, 305 children; Analysis 1.28).
4. Any adverse events
Of the 26 studies that investigated any glucocorticoid compared to placebo, 13 reported collecting adverse events data. Of these, eight reported no serious adverse events (Eden 1967; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Tibballs 1992). Bjornson 2004 reported seven instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported one child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported seven secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported one child with pneumonitis in the placebo group (1/13, 7.7%) and two children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported one instance of exacerbated symptoms, five children with emotional distress, two with vomiting, and one instance of eye irritation in the budesonide group (9/42, 21.4%), and three instances of exacerbated symptoms, six children with emotional distress, three with vomiting, two rashes, and one instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%).
Comparison 2: Any glucocorticoid compared to epinephrine
See summary of findings Table 2.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to epinephrine, we do not know if there was no difference in the change in croup score following treatment with glucocorticoids after two hours (SMD 0.77, 95% CI −0.24 to 1.77; P = 0.13, I² = 87%; 2 RCTs, 130 children; very low‐certainty evidence; Analysis 2.1) and six hours (SMD −0.10, 95% CI −1.18 to 0.97; P = 0.85, I² = 78%; 2 RCTs, 63 children; very low‐certainty evidence; Analysis 2.2). There may be no difference between groups at 12 hours (SMD −0.07, 95% CI −0.57 to 0.43; P = 0.78, I² = 47%; 3 RCTs, 129 children; low‐certainty evidence; Analysis 2.3) or 24 hours (SMD 0.17, 95% CI −0.18 to 0.51; P = 0.35, I² = 0%; 3 RCTs, 129 children; low‐certainty evidence; Analysis 2.4).
The analyses at six hours (Analysis 2.2), 12 hours (Analysis 2.3), and 24 hours (Analysis 2.4) included only inpatients. At two hours, there was a subgroup difference in effect by inpatient or outpatient status (Chi² = 7.44, P = 0.006, I² = 86.6%; Analysis 2.1). For outpatients, glucocorticoids were less effective at reducing croup score compared to epinephrine after two hours (SMD 1.29, 95% CI 0.73 to 1.84; P < 0.001; 1 RCT, 64 children). No difference was detected between the two treatments for inpatients (SMD 0.26, 95% CI −0.22 to 0.75; P = 0.29; 1 RCT, 66 children).
At two hours, there was a subgroup difference in effect by glucocorticoid (Chi² = 7.37, P = 0.03, I² = 72.9%; Analysis 2.5). Epinephrine was more effective at reducing croup score compared to beclomethasone (SMD 1.41, 95% CI 0.62 to 2.19; P < 0.001; 1 RCT, 33 children) and dexamethasone (SMD 1.13, 95% CI 0.35 to 1.91; P = 0.005; 1 RCT, 31 children). At this time point, there was no difference in reduction in croup score between budesonide and epinephrine (SMD 0.26, 95% CI −0.22 to 0.75; P = 0.29; 1 RCT, 66 children). The 12‐ and 24‐hour analyses investigated budesonide and dexamethasone, and there were no subgroup differences in effect (Analysis 2.6; Analysis 2.7).
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 and Fitzgerald 1996 investigated return visits and (re)admissions, respectively, following the administration of glucocorticoids (dexamethasone and beclomethasone, and budesonide, respectively) compared to epinephrine. Both studies may not have reported any events (RD 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 2 RCTs, 130 children; low‐certainty evidence; Analysis 2.8).
Secondary outcomes
1. Length of stay in the hospital or emergency department
Kuusela 1988 investigated length of stay for 32 children hospitalised with croup who were treated with dexamethasone, epinephrine, a combination of dexamethasone and epinephrine, or placebo. There was no difference in hours spent in the hospital between children treated with dexamethasone and those treated with epinephrine (MD −10.00, 95% CI −33.89 to 13.89; P = 0.41; 1 RCT, 32 children; Analysis 2.9).
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Fitzgerald 1996 investigated the use of additional treatments for children aged six months to six years admitted to the hospital with croup who were treated with budesonide or epinephrine. There was no difference in the proportion of children who required additional epinephrine between groups (RR 0.30, 95% CI 0.03 to 2.69; P = 0.28; 1 RCT, 66 children; Analysis 2.10). No child was intubated (RD 0.00, 95% CI −0.06 to 0.06; P = 1.00; 1 RCT, 66 children; Analysis 2.11). There was no difference between groups in the proportion of children who required supplemental glucocorticoids (RR 0.83, 95% CI 0.48 to 1.43; P = 0.49; 1 RCT, 66 children; Analysis 2.12).
4. Any adverse events
Of the four studies that investigated glucocorticoids compared to epinephrine, three reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported five cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported four cases of tremor and tachycardia (4/25, 16%) in the epinephrine group.
Comparison 3: Dexamethasone compared to budesonide
See Table 2.
| Dexamethasone compared to budesonide for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Budesonide | Dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −2.93 to −2.00. | The mean change in croup score was 0.46 standard deviations in favour (0.79 more to 0.13 more). | ‐ | 326 | ⊕⊕⊝⊝ | A standard deviation of 0.46 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.07 to −2.33. | The mean change in croup score was 0.75 standard deviations in favour (1.19 more to 0.30 more). | ‐ | 84 | ⊕⊕⊝⊝ | A standard deviation of 0.75 represents a large difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.69 | 374 | ⊕⊕⊕⊝ |
| |
| 122 per 1000 | 84 per 1000 (49 to 149) | |||||
| Adverse events | 4/6 (67%) studies reported collecting adverse events data, and 3/4 (75%) studies reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and 1 case each of hives and violent behaviour in the dexamethasone group (2/69, 2.9%). | 335 (4 RCTs) | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
aWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias. Allocation concealment was unclear in two studies; blinding was unclear in two studies; and one study was unblinded. There was a baseline imbalance in croup score in one study.
bWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 51%), and variation in point estimates.
cWe downgraded by one level for risk of bias. The contributing studies were at high risk of bias. Allocation concealment was unclear in both studies; blinding was unclear in one study, and the other study was unblinded. There was a baseline imbalance in croup score in one study.
dWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
eWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included a null effect as well as considerable benefit for dexamethasone compared to budesonide.
fWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
gWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Compared to budesonide, dexamethasone may have resulted in a greater reduction in croup score after six hours (SMD −0.46, 95% CI −0.79 to −0.13; P = 0.006, I² = 51%; 4 RCTs, 326 children; low‐certainty evidence; Analysis 3.1) and 12 hours (SMD −0.75, 95% CI −1.19 to −0.30; P = 0.001, I² = 0%; 2 RCTs, 84 children; low‐certainty evidence; Analysis 3.2). The analysis at 12 hours included only inpatients (Analysis 3.2). At six hours, there was no subgroup difference in effect by inpatient or outpatient status (Analysis 3.1).
2. Return visits or (re)admissions to the hospital or both
There was probably no difference in the rate of return visits or (re)admissions to the hospital (or both) between dexamethasone and budesonide groups (RR 0.69, 95% CI 0.40 to 1.22; P = 0.20, I² = 0%; 5 RCTs, 374 children; moderate‐certainty evidence; Analysis 3.3). There were no subgroup differences in effect by inpatient or outpatient status (Analysis 3.3).
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no difference in hours spent in the hospital or emergency department between children treated with dexamethasone and those treated with budesonide (MD −0.51, 95% CI −1.28 to 0.25; P = 0.15, I² = 51%; 2 RCTs, 184 children; Analysis 3.4). There was no subgroup difference in effect by inpatient or outpatient status (Analysis 3.4).
2. Patient improvement at 2, 6, 12, and/or 24 hours
Klassen 1998 investigated response to treatment, defined as a two‐point improvement in croup score, amongst 198 children aged three months to five years who were treated with budesonide, dexamethasone, or a combination of budesonide and dexamethasone in the emergency department for croup. There was no difference in response to treatment between those treated with dexamethasone and those treated with budesonide (RR 1.12, 95% CI 0.93 to 1.34; P = 0.22; 1 RCT, 134 children; Analysis 3.5).
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Compared to those treated with budesonide, children treated with dexamethasone were at a reduced risk of needing treatment with epinephrine (RR 0.45, 95% CI 0.21 to 0.96; P = 0.04, I² = 0%; 4 RCTs, 321 children; Analysis 3.6). Geelhoed 1995c and Johnson 1998 investigated the need for intubation/tracheostomy amongst children treated with dexamethasone or budesonide for croup. There were no events in either study (RD 0.00, 95% CI −0.04 to 0.04; P = 1.00, I² = 0%; 2 RCTs, 145 children; Analysis 3.7). There was no difference in the need for additional glucocorticoids between children treated with dexamethasone and those treated with budesonide (RR 0.48, 95% CI 0.18 to 1.32; P = 0.15, I² = 0%; 3 RCTs, 240 children; Analysis 3.8).
4. Any adverse events
Of the six studies investigating dexamethasone compared to budesonide, three (50%) reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%) and one case each of hives and violent behaviour in the dexamethasone group (2/69, 2.9%).
Comparison 4: Dexamethasone compared to beclomethasone
See Table 3.
| Dexamethasone compared to beclomethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Beclomethasone | Dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RD 0.00 | 39 | ⊕⊕⊕⊝ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Adverse events (no events) | Eboriadou 2010 reported no adverse events related to the glucocorticoids. | 39 (1 RCT) | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
cWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 investigated return visits to the emergency department amongst 39 children aged six months to five years treated with dexamethasone or beclomethasone for croup. There were probably no children that returned for additional care (RD 0.00, 95% CI −0.09 to 0.09; P = 1.00; 1 RCT, 39 children; moderate‐certainty evidence; Analysis 4.1).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies investigated the use of additional treatments for this comparison.
4. Any adverse events
Eboriadou 2010 investigated this comparison and reported no adverse events related to the glucocorticoids.
Comparison 5: Dexamethasone compared to betamethasone
See Table 4.
| Dexamethasone compared to betamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Betamethasone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score from 1 study was −1.68. | The mean change in croup score was 0.62 units in favour (1.17 more to 0.06 more). | ‐ | 52 | ⊕⊕⊝⊝ |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −1.89. | The mean change in croup score was 0.67 units in favour (1.23 more to 0.11 more). | ‐ | 52 | ⊕⊕⊝⊝ |
| Return visits or (re)admissions or both | Study population | RR 0.95 | 52 | ⊕⊕⊝⊝ | |
| 731 per 1000 | 694 per 1000 (490 to 979) | ||||
| Adverse events | Amir 2006 did not report collecting adverse events data. | 52 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score.
bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
cWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score.
dWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit or harm for dexamethasone compared to betamethasone.
eWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Amir 2006 investigated reduction in croup score for 52 children aged six months to six years were treated with dexamethasone or betamethasone in the emergency department for croup. Compared to betamethasone, dexamethasone may have resulted in a greater reduction in croup score after two hours (SMD −0.62, 95% CI −1.17 to −0.06; P = 0.03; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.1) and six hours (SMD −0.67, 95% CI −1.23 to −0.11; P = 0.02; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.2).
2. Return visits or (re)admissions to the hospital or both
Amir 2006 investigated re‐examinations by a primary care physician amongst 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. There may have been no difference in the rate of re‐examinations between dexamethasone and betamethasone groups (RR 0.95, 95% CI 0.67 to 1.34; P = 0.76; 1 RCT, 52 children; low‐certainty evidence; Analysis 5.3).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Amir 2006 investigated the need for epinephrine amongst 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. The risk for needing epinephrine was higher for those treated with dexamethasone compared to those treated with betamethasone (RR 2.11, 95% CI 1.18 to 3.76; P = 0.01; 1 RCT, 52 children; Analysis 5.4).
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 6: Dexamethasone compared to prednisolone
See Table 5.
| Dexamethasone compared to prednisolone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Prednisolone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score from 1 study was −0.89. | The mean change in croup score was 0.06 units not in favour (0.06 more to 0.18 less). | ‐ | 1231 | ⊕⊕⊕⊕ |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −2.35. | The mean change in croup score was 0.21 units not in favour (0.21 more to 0.62 less). | ‐ | 99 | ⊕⊕⊕⊝ |
| Return visits or (re)admissions or both | Study population | RR 0.55 | 1537 | ⊕⊕⊕⊝ | |
| 212 per 1000 | 117 per 1000 | ||||
| Adverse events | Fifoot 2007, Garbutt 2013, and Sparrow 2006 reported no serious adverse events related to the glucocorticoids. Parker 2019 reported 1 case of insomnia (1/411, 0.24%) and 13 cases of vomiting (13/411, 3.3%) in the prednisolone group, and 29 cases of vomiting (29/820, 3.5%), 1 case of 30‐second febrile convulsion (1/820, 0.1%), and 1 case of hyperactivity (1/820, 0.1%) in the dexamethasone group. | 1550 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
bWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 59%), and variation in point estimates.
cWe downgraded by one level for imprecision. Narrative synthesis conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Two trials compared the effect of dexamethasone versus prednisolone. Parker 2019 found little to no difference at two‐hour postbaseline croup score assessment (SMD 0.06, 95% CI −0.06 to 0.18; P = 0.32; 1 RCT, 1231 children; high‐certainty evidence; Analysis 6.1). Fifoot 2007 found likely little to no difference between groups at six‐hour postbaseline croup score assessment (SMD 0.21, 95% CI −0.21 to 0.62; P = 0.33; 1 RCT, 99 children; moderate‐certainty evidence; Analysis 6.2).
2. Return visits or (re)admissions to the hospital or both
Dexamethasone probably reduced the rate of return visits or readmissions for croup by about half when compared to prednisolone (RR 0.55, 95% CI 0.28 to 1.11; P = 0.09, I² = 59%; 4 RCTs, 1537 children; moderate‐certainty evidence; Analysis 6.3).
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was little to no difference between groups in length of stay in the hospital or emergency department (MD −0.02, 95% CI −0.42 to 0.39; P = 0.94, I² = 12%; 2 RCTs, 1363 children; Analysis 6.4).
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
We found no difference in the addition of epinephrine to the treatment received by children treated with dexamethasone versus those treated with prednisolone (RR 0.90, 95% CI 0.50 to 1.64; P = 0.74, I² = 0%; 3 RCTs, 1463 children; high‐certainty evidence; Analysis 6.5). No child required intubation (RD 0.00, 95% CI −0.00 to 0.00; P = 1.00; 1 RCT, 1231 children; Analysis 6.6). However, there was a 28% reduction in the use of supplemental glucocorticoids as an additional treatment between children who received dexamethasone and those who received prednisolone (RR 0.72, 95% CI 0.53 to 0.97; P = 0.03, I² = 0%; 2 RCTs, 926 children; Analysis 6.7).
4. Any adverse events
Although not specific to this comparison, Parker 2019 reported a few adverse events in four participants: one participant in the dexamethasone group had a febrile convulsion, and one participant in the prednisolone group had insomnia. Unlike Parker 2019, three trials did not report serious adverse events (Fifoot 2007; Garbutt 2013; Sparrow 2006).
Comparison 7: Budesonide compared to dexamethasone
See Table 6.
| Budesonide compared to dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone | Budesonide | ||||
| Adverse events | Huang 2021 reported no adverse events.
| 92 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies reported on change in clinical croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
No studies reported on return visits or (re)admission to the hospital (or both) for this comparison.
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies reported on length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies reported on the use of additional treatments for this comparison.
4. Any adverse events
Huang 2021 investigated the effect of inhaled budesonide versus dexamethasone in children with acute infectious laryngitis and found no adverse condition following treatment. The study authors did not report on any other outcomes relevant to this review.
Comparison 8: Budesonide and dexamethasone compared to dexamethasone
See Table 7.
| Budesonide and dexamethasone compared to dexamethasone for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Dexamethasone | Budesonide and dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.24 to −1.80. | The mean change in croup score was 0.05 standard deviations not in favour (0.19 more to 0.30 less). | ‐ | 255 | ⊕⊕⊕⊝ | A standard deviation of 0.05 represents a minimal difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.91 | 254 | ⊕⊕⊝⊝ |
| |
| 100 per 1000 | 91 per 1000 | |||||
| Adverse events | 1/3 (33%) studies reported collecting adverse events data. Klassen 1998 reported no adverse events in either the dexamethasone group or the dexamethasone and budesonide group. | 133 (1 RCTs) | ⊕⊕⊕⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
bWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and a significant benefit or harm for dexamethasone and budesonide compared to dexamethasone alone.
cWe downgraded by one level for imprecision. Narrative sythesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
There was probably no difference in reduction in croup score after six hours between children treated with combined dexamethasone and budesonide versus those treated with dexamethasone alone (SMD 0.05, 95% CI −0.19 to 0.30; P = 0.67, I² = 0%; 3 RCTs, 255 children; moderate‐certainty evidence; Analysis 7.1). There was no difference in effect by inpatient or outpatient status (Analysis 7.1).
2. Return visits or (re)admissions to the hospital or both
There may have been no difference in the rate of admissions or return visits between children treated with combined dexamethasone and budesonide versus those treated with dexamethasone alone (RR 0.91, 95% CI 0.45 to 1.83; P = 0.79, I² = 0%; 3 RCTs, 254 children; low‐certainty evidence; Analysis 7.2). There was no subgroup difference in effect by inpatient or outpatient status (Analysis 7.2).
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no difference in hours spent in the hospital or emergency department amongst children treated with dexamethasone and budesonide versus those treated with dexamethasone alone (MD 0.44, 95% CI −0.05 to 0.92; P = 0.08, I² = 0%; 2 RCTs, 204 children; Analysis 7.3). There were no subgroup differences in effect by inpatient or outpatient status (Analysis 7.3).
2. Patient improvement at 2, 6, 12, and/or 24 hours
After six hours, there was no difference in the clinical improvement of children treated with dexamethasone and budesonide versus those treated with dexamethasone alone (RR 1.11, 95% CI 0.65 to 1.90; P = 0.70; 2 RCTs, 183 children; Analysis 7.4). This analysis only included outpatients (Analysis 7.4).
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference in the need for epinephrine (RR 1.42, 95% CI 0.27 to 7.39; P = 0.67, I² = 0%; 2 RCTs, 183 children; Analysis 7.5); a mist tent (RR 1.07, 95% CI 0.69 to 1.65; P = 0.77; 1 RCT, 50 children; Analysis 7.6); or supplemental glucocorticoids (RR 1.10, 95% CI 0.07 to 16.66; P = 0.95, I² = 66%; 2 RCTs, 182 children; Analysis 7.7) amongst children treated with dexamethasone and budesonide versus those treated with dexamethasone alone.
4. Any adverse events
Klassen 1998 reported no adverse events in either the dexamethasone group or the dexamethasone and budesonide group.
Comparison 9: Budesonide and dexamethasone compared to budesonide
See Table 8.
| Budesonide and dexamethasone compared to budesonide for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Budesonide | Budesonide and dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −2.30. | The mean change in croup score was 0.18 units in favour (0.52 more to 0.17 less). | ‐ | 129 | ⊕⊕⊕⊝ |
| Return visits or (re)admissions or both | Study population | RD 0.00 | 129 | ⊕⊕⊕⊝ | |
| 0 per 1000 | 0 per 1000 | ||||
| Adverse events | Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group. | 129 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
Klassen 1998 investigated children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was probably no difference in reduction in croup score after six hours amongst children treated with combined dexamethasone and budesonide versus those treated with budesonide alone (SMD −0.18, 95% CI −0.52 to 0.17; P = 0.32; 1 RCT, 129 children; moderate‐certainty evidence; Analysis 8.1).
2. Return visits or (re)admissions to the hospital or both
Klassen 1998 investigated return visits to the emergency department amongst children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There were probably no events in either treatment group (RD 0.00, 95% CI −0.03 to 0.03; P = 1.00; 1 RCT, 129 children; moderate‐certainty evidence; Analysis 8.2).
Secondary outcomes
1. Length of stay in the hospital or emergency department
Klassen 1998 investigated hours spent in the emergency department amongst children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There was no difference in length of stay amongst children treated with dexamethasone and budesonide versus those treated with budesonide alone (MD 0.25, 95% CI −0.36 to 0.86; P = 0.42; 1 RCT, 129 children; Analysis 8.3).
2. Patient improvement at 2, 6, 12, and/or 24 hours
Klassen 1998 investigated response to treatment, defined as a two‐point reduction in croup score, amongst children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no difference in response to treatment amongst children treated with dexamethasone and budesonide versus those treated with budesonide alone (RR 0.97, 95% CI 0.79 to 1.20; P = 0.80; 1 RCT, 129 children; Analysis 8.4).
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Klassen 1998 investigated the need for additional treatments amongst children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no difference between groups in the need for epinephrine (RR 1.02, 95% CI 0.15 to 6.99; P = 0.99; 1 RCT, 129 children; Analysis 8.5) or supplemental glucocorticoids (RR 1.31, 95% CI 0.52 to 3.29; P = 0.57; 1 RCT, 129 children; Analysis 8.6).
4. Any adverse events
Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group.
Comparison 10: Oral compared to intramuscular dexamethasone
See Table 9.
| Oral dexamethasone compared to intramuscular dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Intramuscular dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.81 | 440 | ⊕⊕⊕⊝ | |
| 259 per 1000 | 210 per 1000 | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for imprecision. The effect estimate included both a null effect and substantial benefit for oral compared to intramuscular dexamethasone.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
There was probably no difference in the rate of return visits or admissions following treatment with oral dexamethasone compared to intramuscular dexamethasone (RR 0.81, 95% CI 0.58 to 1.12; P = 0.21, I² = 0%; 3 RCTs, 440 children; moderate‐certainty evidence; Analysis 9.1). The analysis only included outpatients (Analysis 9.1).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
Donaldson 2003 investigated clinical improvement, defined as parents' assessment that their child's condition had improved at least somewhat after 24 hours, amongst children aged three to 84 months treated in the emergency department with oral or intramuscular dexamethasone for croup. There was no difference between groups in rate of clinical improvement (RR 1.07, 95% CI 0.95 to 1.19; P = 0.27; 1 RCT, 95 children; Analysis 9.2).
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
There was no difference in the need for antibiotics (RR 0.14, 95% CI 0.02 to 1.15; P = 0.07; 1 RCT, 277 children; Analysis 9.3); epinephrine (RR 0.94, 95% CI 0.71 to 1.24; P = 0.64, I² = 0%; 2 RCTs, 372 children; Analysis 9.4); a mist tent (RR 1.34, 95% CI 0.31 to 5.89; P = 0.70; 1 RCT, 277 children; Analysis 9.5); or supplemental glucocorticoids (RR 1.10, 95% CI 0.50 to 2.41; P = 0.81; 1 RCT, 277 children; Analysis 9.6) amongst children treated with oral dexamethasone versus those treated with intramuscular dexamethasone.
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 11: Oral compared to nebulised dexamethasone
See Table 10.
| Oral dexamethasone compared to nebulised dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Nebulised dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.39 | 176 | ⊕⊕⊕⊝ | |
| 209 per 1000 | 81 per 1000 | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Luria 2001 investigated returns to medical care of children aged six months to six years following treatment with oral or nebulised dexamethasone in the emergency department for croup. There were probably fewer return visits to medical care amongst those treated with oral dexamethasone versus those treated with nebulised dexamethasone (RR 0.39, 95% CI 0.17 to 0.89; P = 0.03; 1 RCT, 176 children; moderate‐certainty evidence; Analysis 10.1).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
No studies investigated the use of additional treatments for this comparison.
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 12: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg
See Table 11.
| Dexamethasone 0.30 mg/kg compared to dexamethasone 0.15 mg/kg for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.30 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 0.94 | 60 | ⊕⊕⊝⊝ | |
| 34 per 1000 | 32 per 1000 | ||||
| Adverse events | Geelhoed 1995b did not report collecting adverse events data. | 60 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential harm for 0.30 mg/kg compared to 0.15 mg/kg dexamethasone.
bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995b investigated re‐presentations to medical care for croup amongst children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone. There may have been no difference in the rate of re‐presentations to medical care between groups (RR 0.94, 95% CI 0.06 to 14.27; P = 0.96; 1 RCT, 60 children; low‐certainty evidence; Analysis 11.1).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Geelhoed 1995b investigated the need for additional treatments amongst children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.43, 95% CI 0.19 to 0.98; P = 0.05; 1 RCT, 60 children; Analysis 11.2). No child required supplemental glucocorticoids (RD 0.00, 95% CI −0.06 to 0.06; P = 1.00; 1 RCT, 60 children; Analysis 11.3).
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg
See Table 12.
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.30 mg/kg for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone 0.30 mg/kg | Dexamethasone 0.60 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 1.40 | 60 | ⊕⊕⊝⊝ | |
| 69 per 1000 | 97 per 1000 | ||||
| Adverse events | Geelhoed 1995a did not report collecting adverse events data. | 60 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential for harm for 0.60 mg/kg compared to 0.30 mg/kg dexamethasone.
bWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
No studies investigated change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995a investigated re‐presentations to medical care for croup amongst children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone. There may have been no difference in the rate of re‐presentations to medical care amongst children treated with 0.60 mg/kg versus 0.30 mg/kg dexamethasone (RR 1.40, 95% CI 0.25 to 7.81; P = 0.70; 1 RCT, 60 children; low‐certainty evidence; Analysis 12.1).
Secondary outcomes
1. Length of stay in the hospital or emergency department
No studies investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies investigated clinical improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
Geelhoed 1995a investigated the need for additional treatments amongst children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.78, 95% CI 0.27 to 2.28; P = 0.65; 1 RCT, 60 children; Analysis 12.2) or supplemental glucocorticoids (RR 2.81, 95% CI 0.12 to 66.40; P = 0.52; 1 RCT, 60 children; Analysis 12.3).
4. Any adverse events
No studies investigated adverse events for this comparison.
Comparison 14: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg
See Table 13.
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.15 mg/kg for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.60 mg/kg | |||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −1.05 to −0.75. | The mean change in croup score was 0.27 standard deviations in favour | ‐ | 861 | ⊕⊕⊕⊕ | A standard deviation of 0.14 represents a small difference between groups. |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.10 to −2.09. | The mean change in croup score was 0.45 units in favour (1.26 more to 0.35 less). | ‐ | 178 | ⊕⊕⊕⊝ |
|
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.50 to −2.95. | The mean change in croup score was 0.60 units in favour (4.39 more to 3.19 less). | ‐ | 113 | ⊕⊝⊝⊝ |
|
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score from 1 study was −4.00. | The mean change in croup score was 0.63 units not in favour (0.16 less to 1.10 less). | ‐ | 72 | ⊕⊕⊕⊝ |
|
| Return visits or (re)admissions or both | Study population | RR 0.91 | 949 | ⊕⊕⊕⊕ |
| |
| 208 per 1000 | 189 per 1000 | |||||
| Adverse events | Parker 2019 reported 16 cases of vomiting (16/410, 4.0%) and 1 case of 30 seconds of febrile convulsion (1/410, 0.2%) in the 0.60 mg/kg dexamethasone group, and 13 cases of vomiting (13/410 (3.3%), 1 case of stridor (1/410, 0.2%), and 1 case of hyperactivity (1/410, 0.2%) in the 0.15 mg/kg dexamethasone group. Alshehr 2005 reported 1 case of bacterial tracheitis and 2 cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%) and no adverse events in the 0.15 mg/kg dexamethasone group. Chub‐Uppakarn 2007 and Fifoot 2007 reported no adverse events in either treatment group. | 170 | ⊕⊕⊕⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
bWe downgraded by two levels level for inconsistency. There was considerable heterogeneity (I² = 99%), and variation in point estimates. The 95% confidence intervals did not overlap.
cWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit and harm for 0.60 mg/kg compared to 0.15 mg/kg dexamethasone.
dWe downgraded by one level for imprecision. Narrative synthesis was conducted, estimates are not precise.
Primary outcomes
1. Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours
There was no reduction at two hours (SMD −0.27, 95% CI −0.76 to 0.22; P = 0.28, I² = 62%; 2 RCTs, 861 children; high‐certainty evidence; Analysis 13.1). There was probably no difference at six hours (SMD −0.45, 95% CI −1.26 to 0.35; P = 0.27, I² = 85%; 3 RCTs, 178 children; moderate‐certainty evidence; Analysis 13.2). We do not know if there was no difference at 12 hours (SMD −0.60, 95% CI −4.39 to 3.19; P = 0.76, I² = 98%; 2 RCTs, 113 children; very low‐certainty evidence; Analysis 13.3). We found that treating children with 0.60 mg/kg versus 0.15 mg/kg dose of dexamethasone probably reduced the severity of croup scores at 24‐hour postbaseline score (SMD 0.63, 95% CI 0.16 to 1.10; P = 0.009; 1 RCT, 72 children; moderate‐certainty evidence; Analysis 13.4).
2. Return visits or (re)admissions to the hospital or both
There was little to no difference in return visits or (re)admissions or both between children treated with dexamethasone 0.60 mg/kg versus 0.15 mg/kg (RR 0.91, 95% CI 0.71 to 1.17; P = 0.48, I² = 0%; 3 RCTs, 949 children; high‐certainty evidence; Analysis 13.5).
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was little to no difference in hours spent at the outpatient clinic between children treated with dexamethasone 0.60 mg/kg versus 0.15 mg/kg (MD 0.12, 95% CI −0.32 to 0.56; P = 0.59, I² = 0%; 2 RCTs, 892 children; Analysis 13.6).
2. Patient improvement at 2, 6, 12, and/or 24 hours
No studies reported on patient improvement for this comparison.
3. The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids
We found no difference in the need of additional treatments between treating children with croup with dexamethasone at 0.60 mg/kg versus 0.15 mg/kg in cases of epinephrine use (RR 0.78, 95% CI 0.34 to 1.75; P = 0.54, I² = 0%; 2 RCTs, 885 children; Analysis 13.7); intubation (RD 0.00, 95% CI −0.00 to 0.00; P = 1.00, I² = 0%; 2 RCTs, 861 children; Analysis 13.8); or use of supplemental glucocorticoids (RR 0.77, 95% CI 0.51 to 1.15; P = 0.19, I² = 0%; 2 RCTs, 617 children; Analysis 13.9).
4. Any adverse events
Of the four studies investigating dexamethasone at 0.60 mg/kg versus dexamethasone at 0.15 mg/kg, two (50%) reported no adverse events in either treatment group (Chub‐Uppakarn 2007; Fifoot 2007). Parker 2019 reported 16 cases of vomiting (16/410, 4.0%) and one case of 30 seconds of febrile convulsion (1/410, 0.2%) in the 0.60 mg/kg dexamethasone group, and 13 cases of vomiting (13/410, 3.3%), one case of stridor (1/410, 0.2%), and one case of hyperactivity 30 minutes after the dose (1/410, 0.2%) in the 0.15 mg/kg dexamethasone group. Alshehr 2005 reported one case of bacterial tracheitis and two cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%), and no adverse events in the 0.15 mg/kg dexamethasone group.
Publication bias
The publication bias for change in croup score (at six hours), and return visits or (re)admissions to the hospital (or both), for glucocorticoids compared to placebo remains the same as in the previous version of this review (Gates 2018). Insufficient numbers of included studies precluded testing for publication bias for any of the other comparisons or outcomes.
Discussion
This updated review includes 45 RCTs with a total of 5888 children. This is an increase of two RCTs with 1323 children from the last update (Huang 2021; Parker 2019). Parker 2019 reported relevant data to update the existing evidence on the effects of glucocorticoid compared to prednisolone, and the optimal dosage of glucocorticoid for the treatment of croup. Huang 2021 reported only on adverse events related to the use of budesonide compared to dexamethasone for croup.
Summary of main results
Any glucocorticoid compared to placebo
Twenty‐six studies investigated glucocorticoids compared to placebo. Glucocorticoids may have reduced the symptoms of croup within two hours of treatment, with the effect lasting at least 24 hours. The effect was dependent on the glucocorticoid administered. Budesonide and dexamethasone reduced the symptoms of croup within two hours of treatment, with the effect lasting at least 24 hours. One trial showed that prednisolone reduced the symptoms of croup within six hours, with the effect lasting at least 12 hours. One trial showed that fluticasone did not reduce the symptoms of croup after two, six, or 24 hours compared to placebo. The certainty of the evidence for the effect of glucocorticoids compared to placebo for reducing the symptoms of croup from two to 12 hours was low, as there was considerable between‐study heterogeneity in effect estimates (summary of findings Table 1). The certainty of the evidence for the effect of glucocorticoids compared to placebo for reducing the symptoms of croup after 24 hours was very low, as there was considerable between‐study heterogeneity in the magnitude and direction of the effect (summary of findings Table 1).
Compared to placebo, both budesonide and dexamethasone may have reduced the rate of return visits and/or (re)admissions to the hospital or emergency department. The certainty of the evidence for the effect of glucocorticoids in reducing the rate of return visits or (re)admissions or both was low, as there was considerable between‐study heterogeneity in the effect estimates (summary of findings Table 1).
Compared to placebo, glucocorticoids reduced length of stay in the hospital by approximately 15 hours and resulted in clinical improvement in a greater proportion of children after six hours. The effect lasted at least 24 hours. There was little to no difference in the need for additional treatments between children treated with glucocorticoids and those treated with placebo. Treatment with glucocorticoids was infrequently associated with serious adverse events.
Any glucocorticoid compared to epinephrine
Four studies investigated glucocorticoids compared to epinephrine. We do not know if there was no difference in the reduction in symptoms of croup for children treated with epinephrine compared to those treated with glucocorticoids at two and six hours. There may not have been a reduction in croup symptoms at 12 or 24 hours following administration of glucocorticoids or epinephrine. After two hours, the effect was dependent on the glucocorticoid administered. Epinephrine resulted in greater reductions in symptoms of croup compared to beclomethasone and dexamethasone. There was little no difference in reduction in croup symptoms between epinephrine and budesonide two hours after treatment. The certainty of the evidence for the effect of glucocorticoids compared to epinephrine for reducing the symptoms of croup was very low to low. The sample sizes for the six‐, 12‐, and 24‐hour analyses were small, and there was considerable between‐study heterogeneity in effect estimates for the six‐hour analysis. There was considerable between‐study heterogeneity in the magnitude and direction of the effect estimates for the two‐hour analysis; the sample size for the comparison was small; and the pooled effect estimate was imprecise (summary of findings Table 2).
There may have been no difference in the rate of return visits or (re)admissions or both following treatment with glucocorticoids compared with epinephrine. The certainty of the evidence for the effect of glucocorticoids compared to epinephrine for reducing the rate of return visits or (re)admissions or both was low, as the sample size did not meet the optimal information size, and the contributing studies reported no events (summary of findings Table 2).
There was little to no difference in length of stay in the hospital for children treated with glucocorticoids compared to those treated with epinephrine, nor were there any differences between groups in the need for additional treatments. One study reported a 31.3% rate of secondary bacterial infections amongst children treated with dexamethasone. Another study reported a 16% rate of tremor and tachycardia amongst children treated with epinephrine.
One glucocorticoid compared to another glucocorticoid
Thirteen studies investigated one glucocorticoid compared to another glucocorticoid. Compared to budesonide, dexamethasone may have resulted in greater reductions in symptoms of croup after six and 12 hours. The certainty of the evidence for the effect of dexamethasone compared to budesonide for reducing the symptoms of croup was low, as the contributing studies were all at high or unclear risk of bias; there was substantial between‐study heterogeneity in effect estimates for the six‐hour analysis; and the sample size did not meet the optimal information size for the 12‐hour analysis (Table 2). Compared to betamethasone, dexamethasone may have resulted in greater reductions in symptoms of croup after two and six hours. The certainty of the evidence for the effect of dexamethasone compared to betamethasone for reducing the symptoms of croup was low, as the only study contributing to the analysis was at high risk of bias and had a small sample size (Table 4). There was no difference in the reduction in symptoms of croup two hours following treatment with dexamethasone or prednisolone, and likely no difference at six hours. The certainty of the evidence for the effect of dexamethasone compared to prednisolone for reducing the symptoms of croup was moderate, as the only study that contributed to the analysis had a small sample size (Table 5).
There was probably no difference between dexamethasone and budesonide in the rate of return visits or (re)admissions or both. The certainty of the evidence for the effect of dexamethasone compared to budesonide for reducing the rate of return visits or (re)admissions or both was moderate, as few events were reported, and the effect estimate included the null effect as well as considerable benefit for dexamethasone compared to budesonide (Table 2). There was probably no difference between dexamethasone and beclomethasone, and there may have been no difference between dexamethasone and betamethasone in the rate of return visits or (re)admissions or both. The certainty of the evidence for the effect of dexamethasone compared to beclomethasone for reducing the rate of return visits or (re)admissions or both was moderate, as the only study that contributed to the analysis had a small sample size and reported no events (Table 3). The certainty of the evidence for the effect of dexamethasone compared to betamethasone for reducing the rate of return visits or (re)admissions or both was low, as only one small study contributed to the analysis, and the effect estimate included the null effect as well as appreciable benefit and harm (Table 4). Compared to prednisolone, dexamethasone probably reduced the rate of return visits or (re)admissions or both by about 50%. The certainty of the evidence for the effect of dexamethasone compared to prednisolone for reducing the rate of return visits or (re)admissions or both was moderate, as the sample size did not reach the optimal information size (Table 5). The addition of data from Parker 2019 attenuated the magnitude of the difference (61%) previously reported in this comparison category.
There was no difference in length of stay in the hospital or emergency department between children treated with dexamethasone compared to budesonide, or dexamethasone compared to prednisolone. One study showed no difference in clinical improvement between children treated with dexamethasone and those treated with budesonide. Compared to those treated with budesonide, children treated with dexamethasone were at a reduced risk for needing epinephrine. There was no difference between children treated with dexamethasone and those treated with budesonide in need for intubation or supplemental glucocorticoids. Compared to those treated with betamethasone, children treated with dexamethasone were at a increased risk for needing epinephrine. There was no difference between children treated with dexamethasone and those treated with prednisolone in the need for epinephrine. However, there was a 28% reduction in the use of supplemental glucocorticoids as an additional treatment. Adverse events were infrequently reported.
Huang 2021 was the only study that investigated the effect of inhaled budesonide versus dexamethasone. The study authors found no adverse condition following the treatment of infectious laryngitis with inhaled budesonide or dexamethasone. They did not report on change in clinical croup scores between baseline and 2, 6, 12, and/or 24 hours, or any other outcomes relevant to this review.
One glucocorticoid compared to a combination of glucocorticoids
Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. There was probably no difference in reduction in symptoms of croup for children treated with dexamethasone compared to combined dexamethasone and budesonide, and probably no difference for children treated with budesonide compared to combined budesonide and dexamethasone. The certainty of the evidence for the effect of dexamethasone compared to dexamethasone and budesonide for reducing the symptoms of croup was moderate, as the sample size for the analysis did not meet the optimal information size (Table 8). The certainty of the evidence for the effect of budesonide compared to budesonide and dexamethasone for reducing the symptoms of croup was moderate (Table 8), as only one small study contributed to the analysis.
There may have been no difference in rate of return visits or (re)admissions to the hospital or both following treatment with dexamethasone compared to combined dexamethasone and budesonide, and there was probably no difference for this outcome following treatment with budesonide compared to combined budesonide and dexamethasone. The certainty of the evidence for the effect of dexamethasone compared to dexamethasone and budesonide for reducing the rate of return visits or (re)admissions or both was low (Table 7), as the sample size for the analysis did not meet the optimal information size; there were few events; and the estimate was imprecise. The certainty of the evidence for the effect of budesonide compared to budesonide and dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate (Table 8), as only one small study contributed to the analysis.
There was no difference in hours spent in the hospital or emergency department, clinical improvement, or the need for additional treatments for children treated with dexamethasone compared to those treated with combined dexamethasone and budesonide, nor for children treated with budesonide compared to combined budesonide and dexamethasone. Only one study collected adverse events data, which included one case (1.5%) of oral thrush in the budesonide group and no events in the budesonide and dexamethasone group (Klassen 1998).
Glucocorticoids given by different modes of administration
Five studies investigated dexamethasone given by different modes of administration. There was probably no difference in the rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to those treated with intramuscular dexamethasone. There was probably a reduced rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to those treated with nebulised dexamethasone. The certainty of the evidence for the effect of oral compared to intramuscular dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate, as the contributing studies reported few events, and the estimate was imprecise (Table 9). The certainty of the evidence for the effect of oral compared to nebulised dexamethasone for reducing the rate of return visits or (re)admissions or both was moderate, as only one study contributed to the analysis, and the sample size did not meet the optimal information size (Table 10).
There was no difference in clinical improvement or in the need for additional treatments between children treated with oral dexamethasone and those treated with intramuscular dexamethasone. None of the studies comparing dexamethasone given by different modes of administration reported collecting adverse events data.
Dexamethasone given in different doses
Five studies investigated dexamethasone given in different doses. There was no reduction in croup score after two hours for inpatients treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone. There was probably no reduction in croup score after six hours for children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone. After 12 hours, we do not know if there was no difference in the change in croup score amongst children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. The effect differed by inpatient and outpatient status. One study showed that there was probably a reduction in croup score with 0.60 mg/kg after 24 hours (Alshehr 2005).
In inpatients, the 0.60 mg/kg dose resulted in a greater reduction in croup score after 12 hours, whereas in outpatients, the 0.15 mg/kg dose was more effective. One study investigated change in croup score after 24 hours for inpatients treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone (Alshehr 2005). Children treated with 0.15 mg/kg probably experienced greater reductions in croup score after 24 hours compared to those treated with 0.60 mg/kg dexamethasone. The certainty of the evidence for the effect of 0.60 mg/kg dexamethasone compared to 0.15 mg/kg dexamethasone for reducing croup score was very low to high (Table 13). The six‐hour analysis included three studies, but the sample size did not meet the optimal information size. In the 12‐hour analysis, there was considerable between‐study heterogeneity in effect estimates, and the sample sizes did not meet the optimal information size. In the 12‐hour analysis, the pooled effect estimate included the null effect as well as appreciable benefit and harm. The 24‐hour analysis included only one study with a small sample size.
There may have been no difference in the rate of return visits or (re)admissions or both for children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone and 0.60 mg/kg compared to 0.30 mg/kg dexamethasone. There was no difference in the rate of return visits or (re)admissions for children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. The certainty of the evidence was low for the effect of 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Table 11), and 0.60 mg/kg compared to 0.30 mg/kg dexamethasone (Table 12), for reducing the rate of return visits or (re)admissions or both for croup, as the analysis included only one small study that reported few events, and the effect estimate included benefit, the null effect, and potential for harm. The certainty of the evidence for the effect of 0.60 mg/kg compared to 0.15 mg/kg dexamethasone on return visits or (re)admissions or both was high (Table 13).
Likewise, we found no difference in length of stay in the hospital or emergency department for children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. There was no difference in the need for additional treatments between children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone; 0.60 mg/kg compared to 0.30 mg/kg dexamethasone; or 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. Adverse events were infrequently reported for the 0.15 mg/kg and 0.60 mg/kg doses of dexamethasone.
Overall completeness and applicability of evidence
We searched for RCTs that compared glucocorticoids to placebo, or any other active pharmacologic treatment for croup. However, in this update we found only two new studies that investigated one glucocorticoid compared to another glucocorticoid (Huang 2021; Parker 2019), and one study that investigated glucocorticoids given in different doses (Parker 2019). Overall, the number of included studies was large (n = 45), of which 26 (58%) investigated glucocorticoids compared to placebo. Only four studies investigated glucocorticoids compared to epinephrine; 13 investigated one glucocorticoid compared to another glucocorticoid; three investigated one glucocorticoid compared to a combination of glucocorticoids; five investigated glucocorticoids given by different modes of administration; and five investigated glucocorticoids given in different doses. Most studies (67%) reported a change in croup score for at least one time point, and 58% used the Westley croup score (Westley 1978), which has been shown to be a valid and reliable measure of croup severity. Most studies (51%) investigated outpatients presenting to emergency departments or outpatient clinics, generally with mild to moderate croup. In a study conducted by Rosychuk 2010 that described the epidemiology of croup presentation to emergency departments within the Alberta, Canada emergency databases, less than 6% of children presenting to the emergency department with croup symptoms required hospitalisation. We have therefore presented subgroup analyses by inpatient or outpatient setting as a form of sensitivity analysis because of the possible overrepresentation of studies with inpatient cases of croup. However, the findings from these subgroup analyses should be interpreted with caution.
We found no evidence of publication bias for our two primary outcomes: change in croup score (at six hours), and return visits or (re)admissions to the hospital or both for glucocorticoids compared to placebo.
Certainty of the evidence
This systematic review included 45 RCTs of 5888 children. Most studies were at unclear or high overall risk of bias (98%). We assessed risk of bias for random sequence generation as low in 60% of studies. The allocation sequence was adequately concealed between randomisation and assignment to treatment groups in 42% of studies. We were unable to ascertain whether the conduct of these studies was methodologically flawed. However, based on the information provided in the publications, we cannot exclude the possibility of selection bias. Empirically, selection bias has been associated with exaggerated estimates of treatment effects (Jüni 2001; Wood 2008). Inadequate allocation concealment is more likely to result in biased estimates of treatment effects when the outcomes of a study are subjective (Wood 2008). Croup score, one of our primary outcomes, is typically assessed by the healthcare provider, and interobserver variability has been reported to be fair to moderate (Chan 2001). Hartling 2014 demonstrated that the association between selection bias and the estimate of treatment effects may not hold true for RCTs in child health. We are therefore uncertain as to how selection bias may have impacted our results.
More than half (58%) of the included studies were at low risk of bias for blinding of participants and personnel, and 60% were at low risk for blinding of outcome assessors. Many of the studies judged as at unclear risk of bias for the blinding domains were described as "blind" or "double‐blind". However, details about who was blinded or how (or both) were omitted from the publications. Whilst it is possible that these studies were well conducted but inadequately reported, we cannot confidently exclude the potential for performance and detection bias. In eight (18%) studies, participants and personnel were not blinded. All of these studies but one investigated glucocorticoids given via different modes of administration (e.g. orally, intramuscularly, nebulised), therefore blinding participants and personnel to the treatment assignment would not have been feasible. Studies that are not blinded or that are inadequately blinded can result in exaggerated estimates of treatment effects (Wood 2008). This association may not be true for RCTs in child health (Hartling 2014), therefore we are uncertain as to how the inclusion of unblinded or inadequately blinded trials may have impacted our results.
Most studies (60%) were at low risk of bias for incomplete outcome data. Although most studies (93%) were at unclear risk of bias for selective reporting, the outcomes reported in the results matched those reported in the methods sections of the publications in most cases.
For the comparison any glucocorticoid versus placebo, we detected between‐study heterogeneity in point estimates of effect as well as heterogeneity in the pooled estimates of effect by glucocorticoid for change in croup score. For this reason, we downgraded the certainty of the evidence for change in croup score after 2, 6, 12, and 24 hours. With respect to the estimates for individual glucocorticoids, after two hours the between‐study estimates for budesonide were heterogeneous. Two studies showed a clear benefit for dexamethasone, whilst Johnson 1996 showed the potential for no difference in effect between dexamethasone and placebo. Between‐study estimates for the effectiveness of budesonide compared to placebo after 6, 12, and 24 hours showed a consistent beneficial effect. For dexamethasone, between‐study estimates were highly heterogeneous at all time points and included the potential for benefit, no effect, or harm compared to placebo. In future updates of this review, we may use meta‐regression analyses to explore factors that could explain at least some of the observed heterogeneity (e.g. the 'effective' dosage of the active comparator). If such an analysis is deemed important to clinicians and researchers, it should be planned and documented a priori before future updates of this review. Only one very small study (N = 17) investigated croup score for fluticasone compared to placebo 2, 6, and 24 hours after treatment (Roorda 1998). Another single study (N = 42) investigated croup score for prednisolone compared to placebo 6 and 12 hours after treatment (Massicotte 1973). We caution against drawing any conclusions based on the evidence from these small, single studies.
Accounting for the pooled estimates of effect by glucocorticoid, the test for subgroup differences between the effects of budesonide, dexamethasone, and fluticasone two hours following their administration indicated marginal differences in croup scores (P = 0.06). Whilst fluticasone (based on one study) compared to placebo showed no reduction in croup scores (P = 0.36), the pooled effect estimate for budesonide indicated a reduction in croup scores (P = 0.005), and a marginal reduction for dexamethasone (P = 0.06). There was a subgroup difference in effect between budesonide, dexamethasone, fluticasone, and prednisolone six hours following their administration (P = 0.009). This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone, and fluticasone (based on one study) had no effect (P = 0.90). There was a subgroup difference in effect between budesonide, dexamethasone, and prednisolone 12 hours following their administration (P = 0.006). This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone. There was a subgroup difference in effect between budesonide, dexamethasone, and fluticasone 24 hours following their administration (P = 0.01). This was accounted for by the fact that the effect estimate for fluticasone (based on one study) indicated no effect (P = 0.36), whilst the pooled estimates for budesonide and dexamethasone both showed beneficial effects.
For the comparison any glucocorticoid versus placebo, we also downgraded the certainty of the evidence for return visits or (re)admissions or both due to inconsistency. There was little evidence that publication bias influenced our results for return visits or (re)admissions or both.
Similar threats to the certainty of the evidence were present in the other 12 comparisons in this review, including concerns regarding risk of bias, inconsistency, and imprecision. Aside from the comparison of glucocorticoids versus placebo, for which seven to 11 RCTs made up the analyses for the primary outcomes, all of the other comparisons included between one and five studies. Combined with the fact that the studies mostly included small samples of children (median n = 72, interquartile range 54 to 99), many analyses had to be downgraded due to imprecision, as the optimal information size criteria were not met. Since many of the analyses contained only one or two small RCTs, we caution against drawing any firm conclusions from the results of these few small studies. There exist very few within‐study comparisons of one glucocorticoid compared to another, of glucocorticoids given by different modes of administration, or of different doses of the same glucocorticoid.
Potential biases in the review process
We know that the risk of bias of studies used in a meta‐analysis is crucial to the certainty of evidence produced. This may affect the translation and uptake of research evidence to practice. We used the Cochrane risk of bias tool to assess the risk of bias of all included studies, and judged most of the studies as at unclear or high risk of bias. We were unable to update some of the other comparisons due to a lack of new data. These comparison categories are highlighted in the Results section. The lack of new data in these areas may signal areas where more RCTs on glucocorticoids and croup are needed. We also know that some comparisons may not warrant new RCTs at this time because of the considerable high‐certainty evidence that is available. To the best of our knowledge, 26 RCTs have compared any glucocorticoids versus placebo, and the findings have been consistently in support of the use of glucocorticoids in the treatment of croup (Gates 2018). That said, other areas that may benefit from more RCTs are comparisons around the lowest optimal glucocorticoids dose, and the most effective mode of administration.
Agreements and disagreements with other studies or reviews
This update strengthens evidence from previous review publications that supported the use of a lower dose of glucocorticoids (Chub‐Uppakarn 2007; Geelhoed 1995). It would be preferable to treat croup with the lowest effective dose of glucocorticoids to limit steroid exposure in these children. Data from Parker 2019 and Chub‐Uppakarn 2007 suggest that 0.15 mg/kg is as effective in the treatment of croup as 0.60 mg/kg. Their findings are also supported by Geelhoed 1995, which found no difference in return visits or (re)admissions amongst children treated for croup using 0.30 mg/kg compared to 0.15 mg/kg. Likewise, there was no difference between the two treatment groups in the need for additional treatment with epinephrine or supplemental glucocorticoid.

Cumulative meta‐graph by year for change in croup score six hours after treatment for any glucocorticoid compared to placebo.

Cumulative meta‐graph by year for return visits or (re)admissions or both for any glucocorticoid compared to placebo.

Flow diagram of study selection for this review.

Risk of bias graph for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary for studies included in the 2022 update synthesis: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Any glucocorticoid compared to placebo, Outcome 1: Croup score (change baseline ‐ 2 hours) by score

Comparison 1: Any glucocorticoid compared to placebo, Outcome 2: Croup score (change baseline ‐ 6 hours) by score

Comparison 1: Any glucocorticoid compared to placebo, Outcome 3: Croup score (change baseline ‐ 12 hours) by score

Comparison 1: Any glucocorticoid compared to placebo, Outcome 4: Croup score (change baseline ‐ 24 hours) by score

Comparison 1: Any glucocorticoid compared to placebo, Outcome 5: Croup score (change baseline ‐ 2 hours) by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 6: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 7: Croup score (change baseline ‐ 24 hours) by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 8: Croup score (change baseline ‐ 2 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 9: Croup score (change baseline ‐ 6 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 10: Croup score (change baseline ‐ 12 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 11: Croup score (change baseline ‐ 24 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 12: Return visits or (re)admissions or both by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 13: Return visits or (re)admissions or both by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 14: Return visits or (re)admissions or both by croup severity

Comparison 1: Any glucocorticoid compared to placebo, Outcome 15: Length of stay by inpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 16: Length of stay by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 17: Improvement (at 2 hours) by inpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 18: Improvement (at 6 hours) by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 19: Improvement (at 12 hours) by inpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 20: Improvement (at 24 hours) by inpatient/outpatient

Comparison 1: Any glucocorticoid compared to placebo, Outcome 21: Improvement (at 6 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 22: Improvement (at 12 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 23: Improvement (at 24 hours) by glucocorticoid

Comparison 1: Any glucocorticoid compared to placebo, Outcome 24: Additional treatments: antibiotics

Comparison 1: Any glucocorticoid compared to placebo, Outcome 25: Additional treatments: epinephrine

Comparison 1: Any glucocorticoid compared to placebo, Outcome 26: Additional treatments: intubation/tracheostomy

Comparison 1: Any glucocorticoid compared to placebo, Outcome 27: Additional treatments: mist tent

Comparison 1: Any glucocorticoid compared to placebo, Outcome 28: Additional treatments: supplemental glucocorticoids

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 1: Croup score (change baseline ‐ 2 hours) by inpatient/outpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 2: Croup score (change baseline ‐ 6 hours) by inpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 3: Croup score (change baseline ‐ 12 hours) by inpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 4: Croup score (change baseline ‐ 24 hours) by inpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 5: Croup score (change baseline ‐ 2 hours) by glucocorticoid

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 6: Croup score (change baseline ‐ 12 hours) by glucocorticoid

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 7: Croup score (change baseline ‐ 24 hours) by glucocorticoid

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 8: Return visits or (re)admissions or both by inpatient/outpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 9: Length of stay by inpatient

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 10: Additional treatments: epinephrine

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 11: Additional treatments: intubation/tracheostomy

Comparison 2: Any glucocorticoid compared to epinephrine, Outcome 12: Additional treatments: supplemental glucocorticoids

Comparison 3: Dexamethasone compared to budesonide, Outcome 1: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient

Comparison 3: Dexamethasone compared to budesonide, Outcome 2: Croup score (change baseline ‐ 12 hours) by inpatient

Comparison 3: Dexamethasone compared to budesonide, Outcome 3: Return visits or (re)admissions or both by inpatient/outpatient

Comparison 3: Dexamethasone compared to budesonide, Outcome 4: Length of stay by inpatient/outpatient

Comparison 3: Dexamethasone compared to budesonide, Outcome 5: Improvement (at 6 hours) by outpatient

Comparison 3: Dexamethasone compared to budesonide, Outcome 6: Additional treatments: epinephrine

Comparison 3: Dexamethasone compared to budesonide, Outcome 7: Additional treatments: intubation/tracheostomy

Comparison 3: Dexamethasone compared to budesonide, Outcome 8: Additional treatments: supplemental glucocorticoids

Comparison 4: Dexamethasone compared to beclomethasone, Outcome 1: Return visits or (re)admissions or both by outpatient

Comparison 5: Dexamethasone compared to betamethasone, Outcome 1: Croup score (change baseline ‐ 2 hours) by outpatient

Comparison 5: Dexamethasone compared to betamethasone, Outcome 2: Croup score (change baseline ‐ 6 hours) by outpatient

Comparison 5: Dexamethasone compared to betamethasone, Outcome 3: Return visits or (re)admissions or both by outpatient

Comparison 5: Dexamethasone compared to betamethasone, Outcome 4: Additional treatments: epinephrine

Comparison 6: Dexamethasone compared to prednisolone, Outcome 1: Croup score (change baseline ‐ 2 hours) by outpatient

Comparison 6: Dexamethasone compared to prednisolone, Outcome 2: Croup score (change baseline ‐ 6 hours) by outpatient

Comparison 6: Dexamethasone compared to prednisolone, Outcome 3: Return visits or (re)admissions or both by outpatient

Comparison 6: Dexamethasone compared to prednisolone, Outcome 4: Length of stay by outpatient

Comparison 6: Dexamethasone compared to prednisolone, Outcome 5: Additional treatments: epinephrine

Comparison 6: Dexamethasone compared to prednisolone, Outcome 6: Additional treatments: intubation/tracheotomy

Comparison 6: Dexamethasone compared to prednisolone, Outcome 7: Additional treatments: supplemental glucocorticoids

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 1: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 2: Return visits or (re)admissions or both by inpatient/outpatient

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 3: Length of stay by inpatient/outpatient

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 4: Improvement (at 6 hours) by outpatient

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 5: Additional treatments: epinephrine

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 6: Additional treatments: mist tent

Comparison 7: Budesonide and dexamethasone compared to dexamethasone, Outcome 7: Additional treatments: supplemental glucocorticoids

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 1: Croup score (change baseline ‐ 6 hours) by outpatient

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 2: Return visits or (re)admissions or both by outpatient

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 3: Length of stay by outpatient

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 4: Improvement (at 6 hours) by outpatient

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 5: Additional treatments: epinephrine

Comparison 8: Budesonide and dexamethasone compared to budesonide, Outcome 6: Additional treatments: supplemental glucocorticoids

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 1: Return visits or (re)admissions or both by outpatient

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 2: Improvement (at 24 hours) by outpatient

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 3: Additional treatments: antibiotics

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 4: Additional treatments: epinephrine

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 5: Additional treatments: mist tent

Comparison 9: Oral compared to intramuscular dexamethasone, Outcome 6: Additional treatments: supplemental glucocorticoids

Comparison 10: Oral compared to nebulised dexamethasone, Outcome 1: Return visits or (re)admissions or both by outpatient

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 1: Return visits or (re)admissions or both by outpatient

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 2: Additional treatments: epinephrine

Comparison 11: Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 3: Additional treatments: supplemental glucocorticoids

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 1: Return visits or (re)admissions or both by outpatient

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 2: Additional treatments: epinephrine

Comparison 12: Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 3: Additional treatments: supplemental glucocorticoids

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 1: Croup score (Westley) (change baseline ‐ 2 hours) by inpatient/outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 2: Croup score (change baseline ‐ 6 hours) by inpatient/outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 3: Croup score (change baseline ‐ 12 hours) by inpatient/outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 4: Croup score (change baseline ‐ 24 hours) by outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 5: Return visits or (re)admissions or both by outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 6: Length of stay by outpatient

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 7: Additional treatments: epinephrine

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 8: Additional treatments: intubation/tracheotomy

Comparison 13: Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 9: Additional treatments: supplemental glucocorticoids
| Any glucocorticoid compared to placebo for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Placebo | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −1.50 to −0.81. | The mean change in croup score was 0.65 standard deviations in favour | ‐ | 426 | ⊕⊕⊝⊝ | A standard deviation of 0.65 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.23 to −0.65. | The mean change in croup score was 0.76 standard deviations in favour | ‐ | 959 | ⊕⊕⊝⊝ | A standard deviation of 0.76 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −7.62 to −1.00. | The mean change in croup score was 1.03 standard deviations in favour | ‐ | 571 | ⊕⊕⊝⊝ | A standard deviation of 1.03 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score was −2.56 to −1.05. | The mean change in croup score was 0.86 standard deviations in favour | ‐ | 351 | ⊕⊝⊝⊝ | A standard deviation of 0.86 represents a large difference between groups. |
| Return visits or (re)admissions or both | 204 per 1000 | 106 per 1000 | RR 0.52 | 1679 | ⊕⊕⊝⊝ |
|
| Adverse events | 13/26 (50%) studies reported collecting adverse events data, and 8/13 (62%) reported no serious adverse events. Bjornson 2004 reported 7 instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported 1 child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported 7 secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported 1 child with pneumonitis in the placebo group (1/13, 7.7%) and 2 children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported 1 instance of exacerbated symptoms, 5 children with emotional distress, 2 with vomiting, and 1 instance of eye irritation in the budesonide group (9/42, 21.4%), and 3 instances of exacerbated symptoms, 6 children with emotional distress, 3 with vomiting, 2 rashes, and 1 instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%). | 1399 (13 RCTs) | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. | ||||||
| Any glucocorticoid compared to epinephrine for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Epinephrine | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −4.24 to −3.74. | The mean change in croup score was 0.77 standard deviations not in favour | ‐ | 130 | ⊕⊝⊝⊝ | A standard deviation of 0.77 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −1.25 to −1.10. | The mean change in croup score was 0.10 standard deviations in favour | ‐ | 63 | ⊕⊝⊝⊝ | A standard deviation of 0.10 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.86 to −1.45. | The mean change in croup score was 0.07 standard deviations in favour | ‐ | 129 | ⊕⊕⊝⊝ | A standard deviation of 0.07 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score was −4.40 to −2.01. | The mean change in croup score was 0.17 standard deviations not in favour | ‐ | 129 | ⊕⊕⊝⊝ | A standard deviation of 0.17 represents a small difference between groups. |
| Return visits or (re)admissions or both | 0 per 1000 | 0 per 1000 | RD 0.00 | 130 | ⊕⊕⊝⊝ |
|
| Adverse events | 3/4 (75%) studies reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported 5 cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported 4 cases of tremor and tachycardia (4/25, 16%) in the epinephrine group. | 162 | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 87%), and variation in point estimates. There was minimal overlap of the confidence intervals. | ||||||
| Baseline rate (%) | NNTB (95% CI) |
|---|---|
| Mean baseline rate | |
| 30.62 | 7 (5 to 12) |
| Smallest baseline rate | |
| 2.06 | 102 (78 to 179) |
| Largest baseline rate | |
| 72.00 | 3 (2 to 5) |
| NNTB: number needed to treat for an additional beneficial outcome | |
| Dexamethasone compared to budesonide for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Budesonide | Dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −2.93 to −2.00. | The mean change in croup score was 0.46 standard deviations in favour (0.79 more to 0.13 more). | ‐ | 326 | ⊕⊕⊝⊝ | A standard deviation of 0.46 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.07 to −2.33. | The mean change in croup score was 0.75 standard deviations in favour (1.19 more to 0.30 more). | ‐ | 84 | ⊕⊕⊝⊝ | A standard deviation of 0.75 represents a large difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.69 | 374 | ⊕⊕⊕⊝ |
| |
| 122 per 1000 | 84 per 1000 (49 to 149) | |||||
| Adverse events | 4/6 (67%) studies reported collecting adverse events data, and 3/4 (75%) studies reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and 1 case each of hives and violent behaviour in the dexamethasone group (2/69, 2.9%). | 335 (4 RCTs) | ⊕⊕⊝⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias. Allocation concealment was unclear in two studies; blinding was unclear in two studies; and one study was unblinded. There was a baseline imbalance in croup score in one study. | ||||||
| Dexamethasone compared to beclomethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Beclomethasone | Dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RD 0.00 | 39 | ⊕⊕⊕⊝ | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Adverse events (no events) | Eboriadou 2010 reported no adverse events related to the glucocorticoids. | 39 (1 RCT) | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | |||||
| Dexamethasone compared to betamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Betamethasone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score from 1 study was −1.68. | The mean change in croup score was 0.62 units in favour (1.17 more to 0.06 more). | ‐ | 52 | ⊕⊕⊝⊝ |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −1.89. | The mean change in croup score was 0.67 units in favour (1.23 more to 0.11 more). | ‐ | 52 | ⊕⊕⊝⊝ |
| Return visits or (re)admissions or both | Study population | RR 0.95 | 52 | ⊕⊕⊝⊝ | |
| 731 per 1000 | 694 per 1000 (490 to 979) | ||||
| Adverse events | Amir 2006 did not report collecting adverse events data. | 52 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score. | |||||
| Dexamethasone compared to prednisolone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Prednisolone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score from 1 study was −0.89. | The mean change in croup score was 0.06 units not in favour (0.06 more to 0.18 less). | ‐ | 1231 | ⊕⊕⊕⊕ |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −2.35. | The mean change in croup score was 0.21 units not in favour (0.21 more to 0.62 less). | ‐ | 99 | ⊕⊕⊕⊝ |
| Return visits or (re)admissions or both | Study population | RR 0.55 | 1537 | ⊕⊕⊕⊝ | |
| 212 per 1000 | 117 per 1000 | ||||
| Adverse events | Fifoot 2007, Garbutt 2013, and Sparrow 2006 reported no serious adverse events related to the glucocorticoids. Parker 2019 reported 1 case of insomnia (1/411, 0.24%) and 13 cases of vomiting (13/411, 3.3%) in the prednisolone group, and 29 cases of vomiting (29/820, 3.5%), 1 case of 30‐second febrile convulsion (1/820, 0.1%), and 1 case of hyperactivity (1/820, 0.1%) in the dexamethasone group. | 1550 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | |||||
| Budesonide compared to dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone | Budesonide | ||||
| Adverse events | Huang 2021 reported no adverse events.
| 92 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded one level for imprecision. Narrative synthesis was conducted, estimates are not precise. | |||||
| Budesonide and dexamethasone compared to dexamethasone for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Dexamethasone | Budesonide and dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.24 to −1.80. | The mean change in croup score was 0.05 standard deviations not in favour (0.19 more to 0.30 less). | ‐ | 255 | ⊕⊕⊕⊝ | A standard deviation of 0.05 represents a minimal difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.91 | 254 | ⊕⊕⊝⊝ |
| |
| 100 per 1000 | 91 per 1000 | |||||
| Adverse events | 1/3 (33%) studies reported collecting adverse events data. Klassen 1998 reported no adverse events in either the dexamethasone group or the dexamethasone and budesonide group. | 133 (1 RCTs) | ⊕⊕⊕⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | ||||||
| Budesonide and dexamethasone compared to budesonide for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Budesonide | Budesonide and dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score from 1 study was −2.30. | The mean change in croup score was 0.18 units in favour (0.52 more to 0.17 less). | ‐ | 129 | ⊕⊕⊕⊝ |
| Return visits or (re)admissions or both | Study population | RD 0.00 | 129 | ⊕⊕⊕⊝ | |
| 0 per 1000 | 0 per 1000 | ||||
| Adverse events | Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group. | 129 | ⊕⊕⊕⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | |||||
| Oral dexamethasone compared to intramuscular dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Intramuscular dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.81 | 440 | ⊕⊕⊕⊝ | |
| 259 per 1000 | 210 per 1000 | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for imprecision. The effect estimate included both a null effect and substantial benefit for oral compared to intramuscular dexamethasone. | |||||
| Oral dexamethasone compared to nebulised dexamethasone for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Nebulised dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.39 | 176 | ⊕⊕⊕⊝ | |
| 209 per 1000 | 81 per 1000 | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | |||||
| Dexamethasone 0.30 mg/kg compared to dexamethasone 0.15 mg/kg for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.30 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 0.94 | 60 | ⊕⊕⊝⊝ | |
| 34 per 1000 | 32 per 1000 | ||||
| Adverse events | Geelhoed 1995b did not report collecting adverse events data. | 60 | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential harm for 0.30 mg/kg compared to 0.15 mg/kg dexamethasone. | |||||
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.30 mg/kg for croup | |||||
| Patient or population: children with croup | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Dexamethasone 0.30 mg/kg | Dexamethasone 0.60 mg/kg | ||||
| Return visits or (re)admissions or both | Study population | RR 1.40 | 60 | ⊕⊕⊝⊝ | |
| 69 per 1000 | 97 per 1000 | ||||
| Adverse events | Geelhoed 1995a did not report collecting adverse events data. | 60 | ⊕⊝⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential for harm for 0.60 mg/kg compared to 0.30 mg/kg dexamethasone. | |||||
| Dexamethasone 0.60 mg/kg compared to dexamethasone 0.15 mg/kg for croup | ||||||
| Patient or population: children with croup | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments** | |
|---|---|---|---|---|---|---|
| Dexamethasone 0.15 mg/kg | Dexamethasone 0.60 mg/kg | |||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 2 hours) | The mean change in croup score was −1.05 to −0.75. | The mean change in croup score was 0.27 standard deviations in favour | ‐ | 861 | ⊕⊕⊕⊕ | A standard deviation of 0.14 represents a small difference between groups. |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 6 hours) | The mean change in croup score was −3.10 to −2.09. | The mean change in croup score was 0.45 units in favour (1.26 more to 0.35 less). | ‐ | 178 | ⊕⊕⊕⊝ |
|
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 12 hours) | The mean change in croup score was −3.50 to −2.95. | The mean change in croup score was 0.60 units in favour (4.39 more to 3.19 less). | ‐ | 113 | ⊕⊝⊝⊝ |
|
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (Follow‐up: 24 hours) | The mean change in croup score from 1 study was −4.00. | The mean change in croup score was 0.63 units not in favour (0.16 less to 1.10 less). | ‐ | 72 | ⊕⊕⊕⊝ |
|
| Return visits or (re)admissions or both | Study population | RR 0.91 | 949 | ⊕⊕⊕⊕ |
| |
| 208 per 1000 | 189 per 1000 | |||||
| Adverse events | Parker 2019 reported 16 cases of vomiting (16/410, 4.0%) and 1 case of 30 seconds of febrile convulsion (1/410, 0.2%) in the 0.60 mg/kg dexamethasone group, and 13 cases of vomiting (13/410 (3.3%), 1 case of stridor (1/410, 0.2%), and 1 case of hyperactivity (1/410, 0.2%) in the 0.15 mg/kg dexamethasone group. Alshehr 2005 reported 1 case of bacterial tracheitis and 2 cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%) and no adverse events in the 0.15 mg/kg dexamethasone group. Chub‐Uppakarn 2007 and Fifoot 2007 reported no adverse events in either treatment group. | 170 | ⊕⊕⊕⊝ |
| ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Croup score (change baseline ‐ 2 hours) by score Show forest plot | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 1.1.1 Westley score | 5 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.44, 0.01] |
| 1.1.2 Non‐Westley score | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.93, ‐0.10] |
| 1.2 Croup score (change baseline ‐ 6 hours) by score Show forest plot | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 1.2.1 Westley score | 5 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.02, ‐0.56] |
| 1.2.2 Non‐Westley score | 6 | 623 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.43, ‐0.18] |
| 1.3 Croup score (change baseline ‐ 12 hours) by score Show forest plot | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.53, ‐0.53] |
| 1.3.1 Westley score | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.56, ‐0.53] |
| 1.3.2 Non‐Westley score | 6 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.45, ‐0.30] |
| 1.4 Croup score (change baseline ‐ 24 hours) by score Show forest plot | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 1.4.1 Westley score | 4 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.72, ‐0.37] |
| 1.4.2 Non‐Westley score | 4 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.56, 0.16] |
| 1.5 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient Show forest plot | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 1.5.1 Inpatient | 5 | 301 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.44, ‐0.16] |
| 1.5.2 Outpatient | 2 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.93, 0.29] |
| 1.6 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient Show forest plot | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 1.6.1 Inpatient | 8 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.22, ‐0.23] |
| 1.6.2 Outpatient | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.11, ‐0.56] |
| 1.7 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient Show forest plot | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 1.7.1 Inpatient | 7 | 291 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.46, ‐0.19] |
| 1.7.2 Outpatient | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.71, ‐0.48] |
| 1.8 Croup score (change baseline ‐ 2 hours) by glucocorticoid Show forest plot | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.10, ‐0.22] |
| 1.8.1 Budesonide | 4 | 246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.71, ‐0.30] |
| 1.8.2 Dexamethasone | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.00, 0.03] |
| 1.8.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.52, 1.42] |
| 1.9 Croup score (change baseline ‐ 6 hours) by glucocorticoid Show forest plot | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.07, ‐0.41] |
| 1.9.1 Budesonide | 5 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.04, ‐0.58] |
| 1.9.2 Dexamethasone | 6 | 567 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.08] |
| 1.9.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.89, 1.02] |
| 1.9.4 Prednisolone | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.62, ‐1.13] |
| 1.10 Croup score (change baseline ‐ 12 hours) by glucocorticoid Show forest plot | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.51, ‐0.56] |
| 1.10.1 Budesonide | 3 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.26, ‐0.68] |
| 1.10.2 Dexamethasone | 5 | 323 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.55, ‐0.15] |
| 1.10.3 Prednisolone | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐3.26, ‐1.55] |
| 1.11 Croup score (change baseline ‐ 24 hours) by glucocorticoid Show forest plot | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.41, ‐0.37] |
| 1.11.1 Budesonide | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.88, ‐0.93] |
| 1.11.2 Dexamethasone | 6 | 245 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.55, ‐0.22] |
| 1.11.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.75, 1.17] |
| 1.12 Return visits or (re)admissions or both by inpatient/outpatient Show forest plot | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.75] |
| 1.12.1 Inpatient | 3 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.30] |
| 1.12.2 Outpatient | 7 | 1356 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 1.13 Return visits or (re)admissions or both by glucocorticoid Show forest plot | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| 1.13.1 Budesonide | 4 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.90] |
| 1.13.2 Dexamethasone | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.81] |
| 1.14 Return visits or (re)admissions or both by croup severity Show forest plot | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.76] |
| 1.14.1 Mild croup | 3 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.95] |
| 1.14.2 Moderate croup | 7 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.86] |
| 1.15 Length of stay by inpatient Show forest plot | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 1.15.1 Inpatient | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 1.16 Length of stay by glucocorticoid Show forest plot | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.55 [‐22.70, ‐6.41] |
| 1.16.1 Budesonide | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐15.29 [‐26.89, ‐3.69] |
| 1.16.2 Dexamethasone | 6 | 328 | Mean Difference (IV, Random, 95% CI) | ‐18.25 [‐27.87, ‐8.62] |
| 1.16.3 Fluticasone | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐12.34, 21.94] |
| 1.17 Improvement (at 2 hours) by inpatient Show forest plot | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 1.17.1 Inpatient | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 1.18 Improvement (at 6 hours) by inpatient/outpatient Show forest plot | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 1.18.1 Inpatient | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.96, 1.90] |
| 1.18.2 Outpatient | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.16, 2.74] |
| 1.19 Improvement (at 12 hours) by inpatient Show forest plot | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.19.1 Inpatient | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.20 Improvement (at 24 hours) by inpatient/outpatient Show forest plot | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 1.20.1 Inpatient | 4 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.43] |
| 1.20.2 Outpatient | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.14, 3.51] |
| 1.21 Improvement (at 6 hours) by glucocorticoid Show forest plot | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 1.21.1 Budesonide | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.19, 2.32] |
| 1.21.2 Dexamethasone | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.76, 2.72] |
| 1.21.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.69, 2.62] |
| 1.22 Improvement (at 12 hours) by glucocorticoid Show forest plot | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 1.22.1 Budesonide | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.08, 1.84] |
| 1.22.2 Dexamethasone | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.06, 2.18] |
| 1.22.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.55] |
| 1.23 Improvement (at 24 hours) by glucocorticoid Show forest plot | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 1.23.1 Dexamethasone | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.05, 1.84] |
| 1.23.2 Prednisolone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| 1.24 Additional treatments: antibiotics Show forest plot | 3 | 202 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 1.25 Additional treatments: epinephrine Show forest plot | 9 | 709 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 1.26 Additional treatments: intubation/tracheostomy Show forest plot | 11 | 1090 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 1.27 Additional treatments: mist tent Show forest plot | 2 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.87, 0.47] |
| 1.28 Additional treatments: supplemental glucocorticoids Show forest plot | 6 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient Show forest plot | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.24, 1.77] |
| 2.1.1 Inpatient | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 2.1.2 Outpatient | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.73, 1.84] |
| 2.2 Croup score (change baseline ‐ 6 hours) by inpatient Show forest plot | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 2.2.1 Inpatient | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 2.3 Croup score (change baseline ‐ 12 hours) by inpatient Show forest plot | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.3.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.4 Croup score (change baseline ‐ 24 hours) by inpatient Show forest plot | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.4.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.5 Croup score (change baseline ‐ 2 hours) by glucocorticoid Show forest plot | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.13, 1.63] |
| 2.5.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 2.5.2 Dexamethasone | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.35, 1.91] |
| 2.5.3 Beclomethasone | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 1.41 [0.62, 2.19] |
| 2.6 Croup score (change baseline ‐ 12 hours) by glucocorticoid Show forest plot | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 2.6.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.47, 0.50] |
| 2.6.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.09, 0.82] |
| 2.7 Croup score (change baseline ‐ 24 hours) by glucocorticoid Show forest plot | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 2.7.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.27, 0.70] |
| 2.7.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.38, 0.61] |
| 2.8 Return visits or (re)admissions or both by inpatient/outpatient Show forest plot | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 2.8.1 Inpatient | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.8.2 Outpatient | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.9 Length of stay by inpatient Show forest plot | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐33.89, 13.89] |
| 2.9.1 Inpatient | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.00 [‐33.89, 13.89] |
| 2.10 Additional treatments: epinephrine Show forest plot | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 2.69] |
| 2.11 Additional treatments: intubation/tracheostomy Show forest plot | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 2.12 Additional treatments: supplemental glucocorticoids Show forest plot | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient Show forest plot | 4 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.13] |
| 3.1.1 Inpatient | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.04, ‐0.22] |
| 3.1.2 Outpatient | 2 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.90, 0.18] |
| 3.2 Croup score (change baseline ‐ 12 hours) by inpatient Show forest plot | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 3.2.1 Inpatient | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 3.3 Return visits or (re)admissions or both by inpatient/outpatient Show forest plot | 5 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.22] |
| 3.3.1 Inpatient | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.14, 2.79] |
| 3.3.2 Outpatient | 3 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.30] |
| 3.4 Length of stay by inpatient/outpatient Show forest plot | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.28, 0.25] |
| 3.4.1 Inpatient | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.93, ‐0.07] |
| 3.4.2 Outpatient | 1 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 3.5 Improvement (at 6 hours) by outpatient Show forest plot | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 3.5.1 Outpatient | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 3.6 Additional treatments: epinephrine Show forest plot | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.96] |
| 3.7 Additional treatments: intubation/tracheostomy Show forest plot | 2 | 145 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 3.8 Additional treatments: supplemental glucocorticoids Show forest plot | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| 4.1.1 Outpatient | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.09, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Croup score (change baseline ‐ 2 hours) by outpatient Show forest plot | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.06] |
| 5.1.1 Outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.06] |
| 5.2 Croup score (change baseline ‐ 6 hours) by outpatient Show forest plot | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.23, ‐0.11] |
| 5.2.1 Outpatient | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.23, ‐0.11] |
| 5.3 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 5.3.1 Outpatient | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 5.4 Additional treatments: epinephrine Show forest plot | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.18, 3.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Croup score (change baseline ‐ 2 hours) by outpatient Show forest plot | 1 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.1.1 Outpatient | 1 | 1231 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.06, 0.18] |
| 6.2 Croup score (change baseline ‐ 6 hours) by outpatient Show forest plot | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.21, 0.62] |
| 6.2.1 Outpatient | 1 | 99 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.21, 0.62] |
| 6.3 Return visits or (re)admissions or both by outpatient Show forest plot | 4 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.11] |
| 6.3.1 Outpatient | 4 | 1537 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.11] |
| 6.4 Length of stay by outpatient Show forest plot | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.39] |
| 6.4.1 Outpatients | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.42, 0.39] |
| 6.5 Additional treatments: epinephrine Show forest plot | 3 | 1463 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.50, 1.64] |
| 6.6 Additional treatments: intubation/tracheotomy Show forest plot | 1 | 1231 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 6.7 Additional treatments: supplemental glucocorticoids Show forest plot | 2 | 926 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient Show forest plot | 3 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.19, 0.30] |
| 7.1.1 Inpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.30, 0.63] |
| 7.1.2 Outpatient | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.39] |
| 7.2 Return visits or (re)admissions or both by inpatient/outpatient Show forest plot | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.83] |
| 7.2.1 Inpatient | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.46, 2.29] |
| 7.2.2 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.13, 2.60] |
| 7.3 Length of stay by inpatient/outpatient Show forest plot | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.05, 0.92] |
| 7.3.1 Inpatient | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.75, 4.15] |
| 7.3.2 Outpatient | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.94] |
| 7.4 Improvement (at 6 hours) by outpatient Show forest plot | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 7.4.1 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 7.5 Additional treatments: epinephrine Show forest plot | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.39] |
| 7.6 Additional treatments: mist tent Show forest plot | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.65] |
| 7.7 Additional treatments: supplemental glucocorticoids Show forest plot | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 16.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Croup score (change baseline ‐ 6 hours) by outpatient Show forest plot | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.52, 0.17] |
| 8.1.1 Outpatient | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.52, 0.17] |
| 8.2 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 8.2.1 Outpatient | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.03] |
| 8.3 Length of stay by outpatient Show forest plot | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 8.3.1 Outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 8.4 Improvement (at 6 hours) by outpatient Show forest plot | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 8.4.1 Outpatient | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 8.5 Additional treatments: epinephrine Show forest plot | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.15, 6.99] |
| 8.6 Additional treatments: supplemental glucocorticoids Show forest plot | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.52, 3.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 9.1 Return visits or (re)admissions or both by outpatient Show forest plot | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 9.1.1 Outpatient | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 9.2 Improvement (at 24 hours) by outpatient Show forest plot | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 9.2.1 Outpatient | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 9.3 Additional treatments: antibiotics Show forest plot | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 9.4 Additional treatments: epinephrine Show forest plot | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 9.5 Additional treatments: mist tent Show forest plot | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.31, 5.89] |
| 9.6 Additional treatments: supplemental glucocorticoids Show forest plot | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 10.1 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
| 10.1.1 Outpatient | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 11.1 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 11.1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 11.2 Additional treatments: epinephrine Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.98] |
| 11.3 Additional treatments: supplemental glucocorticoids Show forest plot | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 12.1 Return visits or (re)admissions or both by outpatient Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 12.1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 12.2 Additional treatments: epinephrine Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.28] |
| 12.3 Additional treatments: supplemental glucocorticoids Show forest plot | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 66.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 13.1 Croup score (Westley) (change baseline ‐ 2 hours) by inpatient/outpatient Show forest plot | 2 | 861 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.76, 0.22] |
| 13.1.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.25, 0.00] |
| 13.1.2 Outpatient | 1 | 820 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.23, 0.04] |
| 13.2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient Show forest plot | 3 | 178 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.26, 0.35] |
| 13.2.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.13, ‐0.74] |
| 13.2.2 Outpatient | 2 | 137 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.35, 0.32] |
| 13.3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient Show forest plot | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐4.39, 3.19] |
| 13.3.1 Inpatient | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.55 [‐3.39, ‐1.71] |
| 13.3.2 Outpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 1.32 [0.81, 1.83] |
| 13.4 Croup score (change baseline ‐ 24 hours) by outpatient Show forest plot | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.16, 1.10] |
| 13.4.1 Outpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [0.16, 1.10] |
| 13.5 Return visits or (re)admissions or both by outpatient Show forest plot | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 13.5.1 Outpatient | 3 | 949 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.71, 1.17] |
| 13.6 Length of stay by outpatient Show forest plot | 2 | 892 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.32, 0.56] |
| 13.6.1 Outpatient | 2 | 892 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.32, 0.56] |
| 13.7 Additional treatments: epinephrine Show forest plot | 2 | 885 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.34, 1.75] |
| 13.8 Additional treatments: intubation/tracheotomy Show forest plot | 2 | 861 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.00, 0.00] |
| 13.9 Additional treatments: supplemental glucocorticoids Show forest plot | 2 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.15] |