Conservative management for postprostatectomy urinary incontinence
Abstract
Background
Urinary incontinence is common after radical prostatectomy and can also occur in some circumstances after transurethral resection of the prostate (TURP). Conservative management includes pelvic floor muscle training with or without biofeedback, electrical stimulation, extra‐corporeal magnetic innervation (ExMI), compression devices (penile clamps), lifestyle changes, or a combination of methods.
Objectives
To determine the effectiveness of conservative management for urinary incontinence up to 12 months after transurethral, suprapubic, laparoscopic, radical retropubic or perineal prostatectomy, including any single conservative therapy or any combination of conservative therapies.
Search methods
We searched the Cochrane Incontinence Group Specialised Register (5 February 2014), CENTRAL (2014, Issue 1), EMBASE (January 2010 to Week 3 2014), CINAHL (January 1982 to 18 January 2014), ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (both searched 29 January 2014), and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised controlled trials evaluating conservative interventions for urinary continence in men after prostatectomy.
Data collection and analysis
Two or more review authors assessed the methodological quality of the trials and abstracted data. We tried to contact several authors of included studies to obtain extra information.
Main results
Fifty trials met the inclusion criteria, 45 in men after radical prostatectomy, four trials after TURP and one trial after either operation. The trials included 4717 men of whom 2736 had an active conservative intervention. There was considerable variation in the interventions, populations and outcome measures. Data were not available for many of the pre‐stated outcomes. Men's symptoms improved over time irrespective of management.
There was no evidence from eight trials that pelvic floor muscle training with or without biofeedback was better than control for men who had urinary incontinence up to 12 months after radical prostatectomy; the quality of the evidence was judged to be moderate (for example 57% with urinary incontinence in the intervention group versus 62% in the control group, risk ratio (RR) for incontinence after 12 months 0.85, 95% confidence interval (CI) 0.60 to 1.22). One large multi‐centre trial of one‐to‐one therapy showed no difference in any urinary or quality of life outcome measures and had narrow CIs. It seems unlikely that men benefit from one‐to‐one PFMT therapy after TURP. Individual small trials provided data to suggest that electrical stimulation, external magnetic innervation, or combinations of treatments might be beneficial but the evidence was limited.
Amongst trials of conservative treatment for all men after radical prostatectomy, aimed at both treatment and prevention, there was moderate evidence of an overall benefit from pelvic floor muscle training versus control management in terms of reduction of urinary incontinence (for example 10% with urinary incontinence after one year in the intervention groups versus 32% in the control groups, RR for urinary incontinence 0.32, 95% CI 0.20 to 0.51). However, this finding was not supported by other data from pad tests. The findings should be treated with caution because the risk of bias assessment showed methodological limitations.
Men in one trial were more satisfied with one type of external compression device, which had the lowest urine loss, compared to two others or no treatment. The effect of other conservative interventions such as lifestyle changes remained undetermined as no trials involving these interventions were identified.
Authors' conclusions
The value of the various approaches to conservative management of postprostatectomy incontinence after radical prostatectomy remains uncertain. The evidence is conflicting and therefore rigorous, adequately powered randomised controlled trials (RCTs) which abide by the principles and recommendations of the CONSORT statement are still needed to obtain a definitive answer. The trials should be robustly designed to answer specific well constructed research questions and include outcomes which are important from the patient's perspective in decision making and are also relevant to the healthcare professionals. Long‐term incontinence may be managed by an external penile clamp, but there are safety problems.
PICO
Plain language summary
Conservative management for men with urinary incontinence after prostate surgery
Background information
The prostate is a male sex gland that surrounds the outlet of the bladder. Two main diseases of the prostate (cancer of the prostate, and benign (non‐cancerous) prostatic enlargement) can be treated by surgery but some men suffer leakage of urine (urinary incontinence) afterwards. Conservative treatments of the leakage such as pelvic floor muscle training with or without biofeedback or anal electrical stimulation are thought to help men control this leakage.
The main findings of the review
The review of trials found that there was conflicting evidence about the benefit of therapists teaching men to contract their pelvic floor muscles for either prevention or treatment of urine leakage after radical prostate surgery for cancer. However, information from one large trial suggested that men do not benefit from seeing a therapist to receive pelvic floor muscle training after transurethral resection (TURP) for benign prostatic enlargement. Overall, there was insufficient evidence to demonstrate a beneficial effect from pelvic floor muscle training.
Of three external compression devices tested, one penile clamp seemed to be better than the others.
Adverse effects
This one penile clamp needed to be used cautiously because of safety risks.
Any limitations of the review
In future updates it may be worth considering two separate reviews, looking separately at 'treatment' and 'prevention' trials. More research that is of better quality is also needed to assess conservative management.
Authors' conclusions
Summary of findings
| Treatment of UI after radical: PFMT ±biofeedback versus no treatment; for postprostatectomy urinary incontinence | ||||||
| Patient or population: patients with postprostatectomy urinary incontinence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 623 per 1000 | 529 per 1000 | RR 0.85 | 665 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICI‐SF) ‐ after first year | The mean urinary incontinence score (ici‐short form) ‐ after first year in the intervention groups was | 391 | ⊕⊕⊝⊝ | |||
| Adverse events | See comment | See comment | Not estimable | 138 | ⊕⊕⊕⊕ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide CI (0.60 to 1.22) | ||||||
| Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 63 per 1000 | 16 per 1000 | RR 0.26 | 413 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICIQ‐SF UI score) ‐ after 12 months | The mean urinary incontinence score (iciq‐short form ui score) ‐ after 12 months in the intervention groups was | 47 | ⊕⊕⊝⊝ | |||
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) ‐ after 12 months | See comment | See comment | Not estimable | 47 | ⊕⊕⊝⊝ | |
| Adverse events | 133 per 1000 | 77 per 1000 | RR 0.58 | 56 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear is 1/2 trials taking part in the meta‐analysis | ||||||
| Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy UI | ||||||
| Patient or population: patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: combinations of treatments versus no treatment | |||||
| Number of incontinent men with 3 to 6 months | 53 per 1000 | 150 per 1000 | RR 2.85 | 39 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) after 12 months | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events ‐ PFMT + anal EStim + BFB | 0 per 1000 | 0 per 1000 | RR 4.86 | 138 | ⊕⊕⊝⊝ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear | ||||||
| Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men within 6 to 12 months ‐ FES versus ExMI | 83 per 1000 | 167 per 1000 | RR 2 | 24 | ⊕⊝⊝⊝ | |
| Quality of Life Score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT | The mean quality of life score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT in the intervention groups was | 24 | ⊕⊕⊝⊝ | |||
| Adverse events PFMT + Anal EStim versus PFMT alone | 0 per 1000 | 0 per 1000 | RR 5 | 140 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment is unclear | ||||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men ‐ after 12 months | 321 per 1000 | 103 per 1000 | RR 0.32 | 373 | ⊕⊕⊕⊝ | |
| Quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months | The mean quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months in the intervention groups was | 105 | ⊕⊝⊝⊝ | |||
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear for Filocamo 2005 which contributes 84.2% weightage | ||||||
| Prevention of UI after radical: electric or magnetic energy versus no treatment for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of life score assessed using (ICIQ‐SF score) ‐ within 6 to 12 months | See comment | See comment | Not estimable | 32 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Prevention of UI after radical: combinations of treatments versus no treatment compared to for postprostatectomy UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: combinations of treatments versus no treatment | ||||||
| Number of incontinent men within 6 to 12 months ‐ PFMT + anal EStim + biofeedback versus no treatment | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐ SF UI score) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment are both unclear | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT pre and post‐operation versus PFMT post‐operation) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT pre and post‐operation versus PFMT post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | See comment | See comment | Not estimable | 367 | ⊕⊕⊕⊝ | |
| Quality of Life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events | See comment | See comment | Not estimable | 102 | ⊕⊕⊕⊕ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation is unclear 2/3 trials and allocation concealment is unclear in 1/3 trials | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | 71 per 1000 | 100 per 1000 | RR 1.4 | 58 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events | See comment | See comment | Not estimable | 68 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of Life Score assessed using (ICIQ‐SF) within 6 to 12 months | See comment | See comment | Not estimable | 34 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: Men with UI after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men‐ after 12 months | See comment | See comment | Not estimable | 1609 | ⊕⊕⊕⊝ | |
| Quality of life Score assessed using Score (ICIQ‐SF UI score) ‐ after 12 months | See comment | See comment | Not estimable | 397 | ⊕⊕⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment for UI | ||||||
| Patient or population: All men after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ within 3 to 6 months | 227 per 1000 | 116 per 1000 | RR 0.51 | 48 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) at 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
Background
Description of the condition
It is not uncommon for men to have urinary incontinence (UI) after prostatectomy. UI can be divided into three groups of urgency urinary incontinence (UUI), stress urinary incontinence (SUI) and mixed urinary incontinence (MUI). UUI is described by the International Continence Society (ICS) as the complaint of involuntary leakage of urine associated with a sudden desire to void urine (Altman 2013). SUI is defined as the involuntary leakage of urine with concurrent coughing, sneezing or physical exertion, whilst MUI, as the name suggests, is a mixture of the symptoms found in both of these types (Altman 2013). The reported frequency varies depending on the type of surgery and surgical technique (Grise 2001; Peyromaure 2002), the definition and quantification of incontinence (Grise 2001; Peyromaure 2002), the timing of the evaluation relative to the surgery, and who evaluates the presence or absence of incontinence (physician or patient) (Donnellan 1997; McCammon 1999). Furthermore, the costs associated with UI can be substantial. The annual cost to the National Health Service (NHS) in the UK for treating clinically significant storage symptoms in men was estimated to be GBP 303 million (Turner 2004) and the annual direct cost of UI in the US was estimated to be USD 3.8 billion (Wilson 2001).
The prevalence of UI after radical prostatectomy is widely reported, ranging from 2% to 60%, albeit at varying times after operation (Milsom 2009). For example, in one study at three months after radical prostatectomy (Donnellan 1997) 51% were subjectively wet (self‐report) but 36% were wet on pad testing (objective reporting). By 12 months, 20% were subjectively still wet but only 16% were classed as wet using objective criteria.
UI is less common after transurethral resection of the prostate (TURP) for benign prostate disease (Omar 2014) and most cases are due to persistent incontinence pre‐dating the surgery. Early UUI affects up to 30% to 40% of men but late SUI is rare affecting less than 0.5% of men (Rassweiler 2006). This is a less invasive operation than a radical prostatectomy and usually does not involve damage to pelvic nerves. Due to these clinical differences, we have analysed data relating to TURP separately.
After both types of operation the problem tends to improve with time, so that it declines and plateaus within one to two years postoperatively (Hunskaar 2002). However, some men are left with incontinence that persists for years afterwards.
Continence mechanisms
Urinary continence depends on a complex interaction of smooth and striated muscle fibres blended together to form the continence mechanism. Considerable debate has existed in the literature as to whether incontinence after prostatectomy is due to an effect on the detrusor (bladder) muscle or on the sphincter, as commonly these abnormalities coexist (Peyromaure 2002). New detrusor overactivity and intrinsic sphincter deficiency due to sphincteric injury (Ficazzola 1998; Groutz 2000; McGuire 1990) or weakness (Majoros 2006) are cited as the most important causes of persistent incontinence after radical prostatectomy. Debate continues on whether detrusor overactivity is a primary or secondary factor. Whereas some report overactivity as the primary cause of postprostatectomy incontinence (Golubuff 1995; Leach 1995) others argue strongly that even if other factors play a role, intrinsic sphincter deficiency is the primary cause of UI after radical prostatectomy (Aboseif 1996; Chao 1995; Groutz 2000; Gudziak 1996; Kondo 2002; Majoros 2006; Winters 1997).
Risk factors for postprostatectomy UI after radical prostatectomy include pre‐existing abnormalities of detrusor contractility (Leach 1995) and older age (Kondo 2002). This is possibly because in older men there is evidence of rhabdosphincter atrophy and neural degeneration (Burnett 1998; Chao 1995). Other risk factors include previous TURP (Jacobsen 2007); pre‐operative radiotherapy (Kondo 2002; Rainwater 1988); trauma; a spinal cord lesion; new obstruction due to recurrence, bladder neck contracture, or urethral stricture (Litwiller 1997); Parkinson's disease (Kondo 2002); dementia; and medications (Khan 1991). A surgeon's inadequate skill and expertise can determine post‐operative incontinence rates (Eastham 1996). In addition, having surgery in a hospital which performs fewer than 20 radical prostatectomies a year may be a factor (Albertsen 1997).
After TURP, UI is thought most likely to be due to pre‐existing abnormalities of bladder function, such as poor compliance or detrusor overactivity, rather than direct sphincter injury (Abrams 1991), possibly because removal of the prostatic tissue removed some of the protective mechanism for continence.
Description of the intervention
Many of the treatments used in current practice for postprostatectomy UI are 'conservative', which is usually considered as not involving drugs or surgery. Treatments such as biofeedback with surface intra‐anal probes are defined as non‐invasive in this context, as opposed to surgical interventions. Five categories of conservative management are considered in this review, both singly and in combination when appropriate.
1. Pelvic floor muscle training (PFMT)
This involves any method of training the pelvic floor muscles to contract. It includes teaching performance of an accurate voluntary pelvic floor muscle contraction using biofeedback and co‐ordinating and timing the contraction against increases in intra‐abdominal pressure, often called functional PFMT.
Traditionally, biofeedback involves the use of equipment to provide visual or auditory feedback about the pelvic floor muscle function to enable one to train, strengthen and increase endurance and co‐ordination of the pelvic floor muscle contractions. Simple auditory biofeedback can also be provided by the therapist informing the patient when a contraction is felt through digital anal examination during the pelvic floor muscle contraction. Additionally, pelvic floor muscle contraction electromyography (EMG) can be used as a surrogate for biofeedback, as well as for measuring the intra‐rectal pressure.
The theoretical basis of PFMT is that repeated, volitional contractions of selected pelvic floor muscles may improve their strength and efficiency during periods of increased intra‐abdominal pressure and can inhibit detrusor activity. In a systematic review of the literature on female UI, Berghmans and colleagues noted that a pelvic floor muscle contraction may raise the urethra and press it towards the symphysis pubis, prevent urethral descent, and improve structural support of the pelvic organs (Berghmans 1998). They further pointed out that PFMT may result in hypertrophy of the peri‐urethral striated muscles thereby increasing the 'external mechanical pressure' on the urethra.
2. Electrical stimulation (non‐invasive) delivered via surface electrodes
Electrical stimulation (ES) works by activating the motor fibres of the pudendal nerve, which can result in contraction of the pelvic floor muscles or the striated peri‐urethral musculature, supporting the intrinsic part of the urethral sphincter closing mechanism (Berghmans 2013). This may be important in the management of men with SUI by stimulating the intrinsic sphincter, strengthening the pelvic muscles and raising the patient's awareness of these muscles in a similar way to biofeedback. ES can also be helpful in men with detrusor overactivity or UUI because it can stimulate afferent fibres of the pudendal nerve, decreasing the sensation of urgency and inhibiting parasympathetic activity which results in a decrease in involuntary detrusor contractions (Berghmans 2013). Two types of non‐invasive ES are detailed below. The parameters of the ES used in studies vary depending on the type of UI and ES. Parameters include pulse width and duration, current intensity, stimulus frequency, current source, pulse shape, duration of treatment and total number of sessions, and rest to work ratio.
Anal electrical stimulation (ES)
Any type of ES using a non‐invasive surface anal probe designed for the therapy. The intention of ES is to facilitate contraction of the peri‐urethral striated muscle by inserting the probe into the anal canal (Jabs 2001).
Sticky patch electrodes, also called transcutaneous electrical nerve stimulation (TENS)
TENS is a low intensity, sensory nerve stimulation used for detrusor overactivity. It is delivered at various sites using patch electrodes. Sites include the sacral dermatomes, dorsal penile nerve, hamstring and quadriceps muscle, and the posterior tibial or perineal nerves (Berghmans 2013).
3. Lifestyle adjustment
This includes fluid adjustment, healthy diet, avoiding excessive caffeine, physical exercise, weight loss and cessation of smoking.
4. Extra‐corporeal magnetic innervation
This involves the use of a magnetic chair to stimulate contraction of the pelvic floor muscles and sacral nerve roots, without the discomfort of inserting an anal probe (Galloway 2000).
5. External penile compression devices (penile clamps)
These devices use an external clamp to achieve non‐surgical compression of the urethra.
Timing of the intervention
Conservative treatment can be started before or after surgery. In general, when it is delivered to all men (whether before or after) the aim is to prevent the development or persistence of UI. We have therefore distinguished between treatment of all men who do have UI (treatment) as opposed to a mixed population of men some of whom do not have UI (prevention).
How the intervention might work
All of these interventions, apart from lifestyle adjustment and a penile clamp, work by inducing contraction of pelvic muscles to increase their strength and efficiency, whilst improving co‐ordination and bladder control by inhibiting overactive detrusor activity. Repetitive contractions can raise urethral closure pressure at rest and during an increase in intra‐abdominal pressure.
Why it is important to do this review
The uncertainty about the benefit of conservative treatment for men with UI after prostate surgery was confirmed in the initial Cochrane review, first published in 1999 (Moore 1999b) and updated in 2001 (Moore 2001). The review originally only considered post‐operative PFMT, biofeedback and electrical stimulation. In a subsequent update (Hunter 2004) the review was broadened to include trials evaluating lifestyle adjustment, external penile compression devices and extracorporeal magnetic innervation. The most recent update also included trials on men after TURP (Hunter 2007) but still did not provide reliable evidence on the effects of conservative treatment. The current update includes 13 new trials.
Objectives
To determine the effectiveness of conservative management for urinary incontinence (UI) up to 12 months after transurethral or radical retropubic prostatectomy, including any single conservative therapy or any combination of conservative therapies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials and quasi‐randomised trials of conservative management to prevent or treat UI after TURP or radical prostatectomy were included. Trials were included if they used any single conservative therapy or any combination of conservative therapies. Other forms of clinical trials were excluded. Analysis of trials in men having radical prostatectomy was done separately from those in men having a TURP.
Types of participants
Adult men with UI following prostatectomy.
Types of interventions
PFMT; biofeedback (verbal or machine‐mediated); electrical stimulation (ES) via a surface electrode (e.g. anal probe ES, sticky patch electrode, transcutaneous electrical nerve stimulation (TENS)); extra‐corporeal magnetic innervation (ExMI); lifestyle adjustment; and external penile compression devices. These interventions could be compared with no treatment or with each other, alone or in combination.
The following comparisons were made for treatment or prevention of UI after prostatectomy.
Radical prostatectomy
Treatment (of men with UI after radical prostatectomy)
(1) Treatment of UI after radical prostatectomy: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(2) Treatment of UI after radical prostatectomy: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, transcutaneous electrical nerve stimulation (TENS), extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(3) Treatment of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment
(4) Treatment of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment
(5) Treatment of UI after radical prostatectomy: one treatment versus another active treatment
Prevention (of UI in men after radical prostatectomy)
(6) Prevention of UI after radical prostatectomy: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(7) Prevention of UI after radical prostatectomy: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(8) Prevention of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment
(9) Prevention of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment
(10) Prevention of UI after radical prostatectomy: one treatment versus another active treatment
TURP
Treatment (of men with UI after TURP)
(11) Treatment of UI after TURP: PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(12) Treatment of UI after TURP: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(13) Treatment of UI after TURP: lifestyle interventions versus no treatment or sham treatment
(14) Treatment of UI after TURP: combinations of treatments versus no treatment or sham treatment
(15) Treatment of UI after TURP: one treatment versus another active treatment
Prevention (of UI in men after TURP)
(16) Prevention of UI after TURP: pre or post‐operative PFMT plus or minus biofeedback versus no treatment or sham therapy or verbal instruction
(17) Prevention of UI after TURP: electric or magnetic energy (e.g. anal ES (EStim), perineal ES, TENS,extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment
(18) Prevention of UI after TURP: lifestyle interventions versus no treatment or sham treatment
(19) Prevention of UI after TURP: combinations of treatments versus no treatment or sham treatment
(20) Prevention of UI after TURP: one treatment versus another active treatment
Containment of urinary incontinence (UI) from any cause
(21) External penile compression devices (penile clamps) versus no treatment or sham treatment
We have not listed all possible comparisons here. As and when new trials address new comparisons these will be added to the review.
Pharmacological agents will be considered in separate reviews. Verbal or written instructions, as well as sham therapy, were considered as 'no treatment'. The use of the term 'sham therapy' in this review meant any therapy that could not influence the pelvic floor muscles such as placing an ES probe in the anus but not turning it on.
Types of outcome measures
Primary outcomes
-
Number of men reporting urinary incontinence (UI) after 12 months
-
Quality of life assessed using the International Consultation on Incontinence Questionnaire Urinary Incontinence Short Form (ICIQ‐UI‐SF) or (ICIQ‐SF)
-
Number of men reporting adverse effects
Secondary outcomes
1. Participant reported observations
-
Number of men reporting UI (number not cured, in the short, medium or long term)
-
Number of men with no improvement in UI (number not cured or improved)
-
Self‐report of satisfaction with method
-
Compliance
2. Quantification of symptoms
-
Standardised pad test (24 hour or 1 hour) measuring grams of urine lost
-
Frequency of micturitions per 24 hours
-
Number of pad or clothing changes (pad changes per 24 hours)
-
Frequency of UI from self‐report or diary (incontinent episodes per 24 hours)
3. Clinician reported urinary outcome measures
-
Objective or observed leakage
-
Urodynamic outcome measures
4. Quality of life
-
Impact of UI e.g. Incontinence Impact Questionnaire (Uebersax 1995)
-
General health status e.g. Short Form 36 (Ware 1993)
5. Adverse effects
-
Pain or discomfort
-
Other adverse outcomes as reported by individual trials and judged to be important
6. Health economics outcomes
-
Cost of intervention
-
Resource implications of differences in outcome
-
Cost effective analysis
7. Other outcomes
-
Non‐prespecified outcomes judged important when performing the review
The quality of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011a; Guyatt 2011b; Guyatt 2013; Guyatt 2013a). This approach divides the quality of evidence into four categories: high, moderate, low and very low. Randomised controlled trials (RCTs) start as high quality evidence and non‐randomised trials begin as low quality evidence. The quality of evidence can be rated down for RCTs and up or down for non‐RCTs depending on predefined characteristics. The factors considered when assessing the quality of evidence included:
-
limitations in study design and implementation;
-
indirectness of evidence;
-
unexplained heterogeneity or inconsistency of results;
-
imprecision of results;
-
high probability of publication bias.
Primary and secondary outcomes were classified as critical, important or not important for decision making from the man’s perspective. The GRADE working group strongly advises a maximum of seven outcomes in a systematic review (Guyatt 2011a). The critical outcomes for assessing quality of evidence included in this review were:
-
number of men reporting UI after 12 months;
-
quality of life assessed using the ICIQ‐UI‐SF;
-
number of men reporting adverse effects;
-
cost effective analysis.
Search methods for identification of studies
We did not impose any language or other limits on the searches. Details of the search methods used for the previous versions of this review can be found in Appendix 1 and Appendix 2.
Electronic searches
This review has drawn on the search strategy developed for the Incontinence Review Group. Relevant trials were identified from the Incontinence Review Group Specialised Register of controlled trials which is described, along with the Group's search strategy, in the Incontinence Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, and handsearching of journals and conference proceedings. The Incontinence Group Specialised Register was searched using the Group's own keyword system; the search terms used were:
({design.cct*} OR {design.rct*})
AND
({topic.urine.incon.postprost*})
(All searches were of the keyword field of Reference Manager 2012).
The date of the most recent search of the Specialised Register for this review was 5 February 2014. Most of the trials in the Incontinence Group Specialised Register are also contained in CENTRAL.
Specific searches were also performed for this update of the review.
-
CENTRAL (OvidSP) (2014, Issue 1) was searched on 26 February 2014.
-
EMBASE (OvidSP) (January 2010 to Week 3 2014) was searched on 20 January 2014.
-
CINAHL (EBSCOhost) (January 1982 to 18 January 2014) was searched on 22 January 2014.
-
ClinicalTrials.gov (via the Cochrane Register of Studies (CRS) interface) and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (both searched on 29 January 2014).
The strategies used to search these databases can be found in Appendix 3.
Searching other resources
Reference lists of relevant articles
The reference lists of relevant articles were searched for other possibly relevant trials.
Contact with investigators in the field
We contacted investigators to ask for other possibly relevant trials, published or unpublished.
Data collection and analysis
Comparisons of the outcomes of the chosen interventions with no treatment, with each other, and in combination were planned a priori for the review update. Data were not available for all planned comparisons. There was considerable diversity in the length of time interventions were carried out for and in the timing of outcome measurements relative to randomisation. The data were therefore reported at three monthly time points.
Selection of studies
The list of abstracts for each update was reviewed independently by two review authors and results compared. The full text articles of references or abstracts identified as potentially relevant by either review author were retrieved and reviewed by both. Reference lists of relevant review articles were reviewed to identify any further trials. References were assessed based on the population, interventions, control management, outcomes and overall study design. Using the full texts of the potentially relevant published studies and abstracts, the same two review authors independently reviewed the studies for relevance and inclusion. Authors were contacted for further data or clarification of methods. Disagreements were resolved through discussion; third party arbitration was not required.
Attempts were made to contact authors of trial reports if clarification was necessary. Studies were excluded from the review if they made comparisons other than those pre‐specified or if data were unavailable. Excluded studies were listed with reasons for their exclusion.
Data extraction and management
Data for the trials were extracted independently by two review authors using a standard form developed for this purpose. The following information was included:
-
study method and characteristics (design, method of randomisation, inclusion and exclusion criteria, withdrawals and dropouts);
-
participants (type of surgery, age, timing of randomisation, baseline incontinence or not);
-
type of intervention, timing (before or after surgery, or both) and duration of therapy, co‐interventions;
-
control (no treatment or sham therapy or other active treatment);
-
outcomes (types of outcome measures, reported outcomes, adverse events).
Extracted data were compared by two review authors for completeness and accuracy, and cross‐checked by another review author if necessary. Disagreements were resolved through discussion and review of the trial report. New data were entered using RevMan5 software.
Assessment of risk of bias in included studies
The risk of bias of the trials was assessed using the Cochrane 'risk of bias' tool.
The following methodological parameters were recorded:
1) identification of study as randomised or quasi‐randomised;
2) description of inclusion and exclusion criteria;
3) potential for selection bias (method of sequence generation, adequacy of random allocation concealment) rating;
4) potential for bias around the time of treatment or during outcome assessment (blinding of participants, personnel, outcome assessors);
5) potential for selection bias in the analysis (description of withdrawals, dropouts, participants lost to follow up, analysis based on intention to treat).
Measures of treatment effect
Analyses were based on available data from all included trials that were relevant to the comparisons and outcomes of interest. Meta‐analysis was undertaken where data were available from more than one study assessing the same outcome. A fixed‐effect model was used for calculations of pooled estimates and their 95% confidence intervals (CIs), or a random‐effects model if there was heterogeneity. For categorical outcomes we related the numbers reporting an outcome to the numbers at risk in each group to calculate a risk ratio (RR) with 95% CI. For continuous variables we used means and standard deviations to calculate a mean difference (MD) with 95% CI. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We reversed the direction of effect if needed to ensure consistency across trials. If data to calculate RRs or MDs were not given, we utilised the most detailed numerical data available to calculate the actual numbers or means and standard deviations (for example test statistics, P values).
Unit of analysis issues
The primary analysis was per man randomised.
Dealing with missing data
Analysis of the data was on an intention‐to‐treat basis to the furthest possible extent. This meant all participants were analysed in the groups to which they were randomised. If this was not the case, we considered whether to exclude the trial. Attempts were made to obtain missing data from the original trialists. However, if this was not possible data were reported as given in the studies, except if there was evidence of differential loss to follow up from the randomised groups. In that case, the use of imputation of missing data was considered. If trials reported sufficient detail to calculate MDs but gave no information on the associated standard deviation (SD), the outcome was assumed to have a SD equal to the highest SD from other trials within the same analysis.
Assessment of heterogeneity
Trials were only combined if they were thought to be clinically similar. We assessed heterogeneity between studies by visual inspection of plots of the data, the Chi2 test for heterogeneity and I2statistic (Higgins 2011). We used the thresholds for interpretation of the I2 statistic in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2003).
Assessment of reporting biases
Due to the difficulty of detecting and correcting for publication bias and other reporting biases, the authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being careful to watch for duplication of data. Funnel plots could not be utilised because there were fewer than 10 trials in each meta‐analysis.
Data synthesis
Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For dichotomous outcomes, data were summarised (for example number of people for whom an outcome is present or not) and risk ratios (RR) calculated with their 95% CIs. For continuous outcomes, each trial was summarised using the mean value for each group and SD, and combined as mean difference (MD) if the same scale (for example pad test in grams of urine) was used for the outcome measurement in more than one trial. A fixed‐effect model was used to calculate the summary statistic and the 95% CI. Heterogeneity was assessed visually and using the Chi2 test for heterogeneity and the I2 statistic (Higgins 2003). Forest plots were examined and potential sources influencing heterogeneity identified. Possible sources of heterogeneity were explored statistically through subgroup analysis. Where synthesis was deemed not appropriate, a narrative overview was planned.
Trials were combined if interventions were based on similar clinical criteria. To combine trial data, a meta‐analysis was conducted and a fixed‐effect model approach to the analysis was utilised unless there was evidence of heterogeneity across studies, in which case a random‐effects model was used.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on cancer stage but there were not enough data.
Sensitivity analysis
We planned to perform sensitivity analysis to investigate the effect of including or excluding trials at high risk of bias, however not enough trials were in the meta‐analysis.
Results
Description of studies
Results of the search
For the current update (2014) of the review 764 possibly relevant articles and abstracts were identified. Sources and numbers of potentially eligible titles were:
-
Incontinence Review Group Specialised Register (193);
-
CENTRAL (37);
-
updated search of EMBASE (354);
-
CINAHL (23);
-
ClinicalTrials.gov (125);
-
WHO ICTRP (32).
Overall 96 reports of 50 studies were included in the qualitative synthesis. Fifty‐nine reports of 27 studies were included in the quantitative synthesis. Four trials are awaiting further information from the authors (Crivellaro 2011;Delmastro 2010; Lilli 2006 NEW; Zhang 2007) and eight trials are ongoing (Burnett 2012; Burnett 2013;Fode 2012 NEW;Goode 2014;Mina 2013; Ng 2011;Terrone 2007; Zopf 2012).
Forty‐one reports of 36 studies were excluded and reasons are given in the 'Characteristics of excluded studies' table. The flow of the literature through the assessment process is shown in the PRISMA study flow chart (Figure 1).

PRISMA study flow diagram.
New included trials
After abstract and full text screening 13 relevant new trials (Ahmed 2012;Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Geraerts 2013;Ghanem 2013; Hou 2013; Laurienzo 2013; Marchiori 2010; Martini 2011; Morihiro 2011; Park 2012; Tienforti 2012) were identified. We also identified 12 new reports of the trials which were already identified in the previous update (Campbell 2012). The trialists were contacted for additional information and data.
One previously included trial published as an abstract was updated with data from a full publication (Centemero 2009).
Included studies
Types of populations
The trials included 4717 men, of whom 2736 had an active conservative intervention.
Surgery
Forty‐five trials involved patients undergoing radical prostatectomy (Ahmed 2012;Bales 2000;Burgio 2006;Centemero 2009;Dijkstra‐Eshuis 2013;Dubbelman 2004;Fader 2013;Filocamo 2005;Floratos 2002;Fode 2014;Franke 1998;Geraerts 2013;Ghanem 2013;Glazener RP 2011;Goode 2009;Hoffman 2005;Koo 2009;Laurienzo 2013;Liu 2008;Manassero 2007;Marchiori 2010;Mariotti 2009;Martini 2011;Mathewson‐Chapman 97;Moore 1999;Moore 2004;Moore 2008;Morihiro 2011;Nowak 2007;Opsomer 1994;Overgard 2008;Park 2012;Parekh 2003;Perissinotto 2008;Ribeiro 2008;Robinson 2008;Robinson 2009;Seleme 2008;Tienforti 2012;Tobia 2008;van Kampen 1998;Wille 2003;Yamanishi 2006;Yokoyama 2004;Zhang 2007).
One very small trial included one patient having a TURP while the rest were radical prostatectomy patients (Joseph 2000) but this was included in the radical prostatectomy group for analysis. Also, as all the men in this trial were incontinent for some time after surgery, they may have represented a group with persistent (longer than one to two years) UI. There were many potentially confounding variables in this trial, acknowledged by the author.
Four trials involved patients after TURP (Glazener TURP 2011;Hou 2013;Porru 2001;Tibaek 2007).
The trials involving post‐TURP patients only (Glazener TURP 2011; Hou 2013; Porru 2001; Tibaek 2007) were analysed separately from the trials amongst men having radical prostatectomy.
Continence status of populations
There was variation in continence status, which led to different populations being studied separately: those with persistent UI and those with all men undergoing surgery (many of whom were likely to recover continence spontaneously). The comparisons were therefore structured to reflect this: trials which included only men with post‐operative incontinence were deemed to be trials of treatment, while trials in which all men were treated (irrespective of continence status) were deemed to be trials of prevention.
-
Twenty‐three treatment trials enrolled only men with post‐operative UI (diagnosis of UI varied with recruitment time) (Dubbelman 2004; Fader 2013; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Moore 1999; Moore 2004; Moore 2008; Opsomer 1994; Robinson 2009; Seleme 2008; van Kampen 1998; Yamanishi 2006; Yokoyama 2004; Zhang 2007).
-
Twenty‐seven prevention trials included all men who underwent surgery, some of whom m.ay have been dry or become dry spontaneously (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Morihiro 2011; Nowak 2007; Overgard 2008; Park 2012; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003).
Timing of recruitment
As the populations and the type and timing of interventions varied so greatly among the trials, the decision was made by the authors to also identify the timing of the recruitment to the trials and the timing of the intervention (before or after surgery):
-
only post‐operative treatment for UI (Ahmed 2012; Dubbelman 2004; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Moore 1999; Moore 2008; Morihiro 2011; Nowak 2007; Overgard 2008; Park 2012; Ribeiro 2008; Robinson 2009; Seleme 2008; van Kampen 1998; Yokoyama 2004; Zhang 2007) or containment (Fader 2013; Moore 2004); and
-
pre‐operative recruitment of all men undergoing surgery, which included a pre‐operative intervention with or without a post‐operative intervention (Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Laurienzo 2013; Martini 2011; Mathewson‐Chapman 97; Parekh 2003; Perissinotto 2008; Porru 2001; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003; Yamanishi 2006).
Time of recruitment of participants to the trial relative to the time of their surgery also varied:
-
pre‐operatively (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013; Ghanem 2013; Hou 2013; Laurienzo 2013; Martini 2011; Mathewson‐Chapman 97; Moore 2008; Nowak 2007; Overgard 2008; Parekh 2003; Perissinotto 2008; Robinson 2008; Tibaek 2007; Tienforti 2012; Tobia 2008; Wille 2003);
-
within days or up to two weeks post‐operatively or after catheter removal (Dubbelman 2004; Filocamo 2005; Floratos 2002; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Hoffman 2005; Koo 2009; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Park 2012; Porru 2001; Ribeiro 2008; Robinson 2009; van Kampen 1998; Yamanishi 2006);
-
weeks to months after surgery (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007).
Types of interventions
In the included trials, there was considerable variation in the type and intensity of interventions. Table 1 gives the exact details of the interventions used in each trial. The duration of the treatment varied from four weeks up to one year. The interventions included:
| Study ID | Intervention | Control |
| A: At catheter removal received standard care of verbal and written instructions, instructed by physiotherapist to perform 3 sets of 15‐20 contractions daily, for a duration of 3‐5 seconds with a 6‐10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs
B: ES, treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks
C: PFMT + BFB + ES: Treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position
All patients were given a logbook to complete daily regarding self‐report of exercises | ||
| PFMT + biofeedback 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5‐10 seconds, 10‐15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery | No biofeedback training Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10‐15 repetitions). | |
| PFMT + biofeedback Single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2‐10 seconds periods separated by 2‐10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively | Usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician. | |
| Intervention A: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC teste (endurance and contraction quality), breathing coordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Intervention B: PFMT post‐operatively only | ||
| 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7‐10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe, All patients received PFMT + biofeedback or electrical stimulation, or both, if still incontinent after 6 weeks | Received written instructions on PFMT after catheter removal (7‐10 days after surgery) | |
| Nine or less sessions of physiotherapy guided pelvic floor exercises after surgery | Exercise instruction through information folder | |
| Formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting | No formal instruction | |
| Initiated after catheter removal, 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic | Instruction with verbal feedback and an information pamphlet with instructions to perform PFMT 50‐100 times daily at home | |
| Pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device. Instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, instructed to restart stimulation after catheter removal for 6 weeks All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment | Preoperative session guided PFMT | |
| Biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively | No treatment. | |
| Intervention A: PFMT + biofeedback 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery. Patients were instructed to carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair. Patients were also instructed to restart PFMT on day 4 after surgery while catheter was in situ Intervention B: Instructed to start PFMT on the day after catheter removal (e.g. 2‐3 weeks after surgery) All men: Received weekly individual guided exercise programme with digital or EMG biofeedback after surgery. Advice was given on how to contract pelvic floor muscles to prevent leakage during functional activities. When patients carried out the instructed 60 contractions, they were asked to colour in three squares in their diary to assess compliance | ||
| Pre‐operative PFMT for 2 weeks + postoperative PFMT programme | Postoperative PFMT programme only | |
| Intervention A: Behavioural therapy with PFMT for 8 weeks Intervention B: Behavioural therapy with biofeedback and electrical stimulation for 8 weeks Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups | No treatment | |
| Intervention A: perineal EStim plus physiotherapy (PFMT) Intervention B: anal EStim plus physiotherapy (PFMT) | PFMT alone | |
| Guided PFMT + biofeedback after catheter removal (2 days post‐operatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly | No description | |
| Intervention A: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Intervention B: Instruction in PFMT, squeezing of finger during digital rectal examination | ||
| ExMI, treatment sessions were for 20 minutes twice weekly for 8 weeks | PFMT alone | |
| A (15): Standard treatment with verbal instructions for PFMT B (17): Pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): Pre‐operative PFMT + ES during 10 physiotherapy sessions, ES was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive treatment post‐operatively | Instructed to start PFMT at home 15 weeks before surgery. | |
| Extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week | PFMT alone, instructions given to carry out 20mins x 3 a day. | |
| PFMT re‐education program, verbal feedback The training program involved active PFE. verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. the strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists | No treatment. | |
| Guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control | Received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform 30 contractions a day at home for the first month after catheter removal (16 days after surgery) | |
| PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrodes was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally | Instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow up visits. | |
| PFMT: 5 sessions of guided PFMT for 2‐3 weeks pre‐operatively and continued post‐operatively All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms | Postoperative standard care, written instructions for PFMT | |
| Pre‐operatively received further instruction and practice with PME protocol Home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT. | Post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed. | |
| Intervention A: PFMT alone Intervention B: PFMT plus rectal ES treated by one physiotherapist 30 minutes twice a week for 12 weeks Both included home exercises 3x/day gradually working up to 30 minutes per session lying, standing, sitting; strength, endurance, speed and control with maximum contractions of 5‐10 seconds, 10‐20 second relaxation and 12‐20 repetitions; submaximum contractions at 65‐75% of maximum strength with hold 20‐30 seconds and equal rest time, 8‐10 repetitions; speed was sets of quick repetitive contractions in a 10 second time span; control involved gradual recruitment to maximum contraction in 3 stages with 5 second hold at each stage and a slow release with rest 15‐30 seconds | oral and written information about PFMT pre and post‐ operatively (standard treatment) | |
| Each participant had 4 periods (each lasted 1 day) | ||
| Maximum 24 weekly, 30‐minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2‐3 times a day | Verbal and written information on PFME and weekly telephone contact by a urology nurse | |
| PFMT + sacral surface therapeutic electrical stimulation (ssTES), ssTES 2x a day for 15 minutes each, lasting 1 month after catheter removal (day 5) | PFME only, carried out alone | |
| Extra‐corporeal magnetic innervation (EXMI) based pelvic floor device | PFMT alone | |
| PFMT plus biofeedback plus electrical stimulation directed by physiotherapist | PFMT on their own without medical supervision. | |
| Instructions on PFMT and physiotherapy, 45 minutes weekly Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital | Instructions on PFMT alone. | |
| Two treatment sessions preoperatively. Session 1 consisted of PFMT in a hook lying position | No formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly. | |
| Patients performed Kegel exercises together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks Details of the combined exercise regime: Post‐operative weeks 1‐4 1) Education about postoperative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5‐8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot | In the control group, only Kegel exercises were performed | |
| Early pelvic floor rehabilitation program at home twice dally, Kegel exercises | No formal PFMT | |
| Initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) | Not specified | |
| PFMT plus BF weekly for 3 months | PFMT oral instructions only | |
| Intervention A: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Intervention B: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) | ||
| Intervention A: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Intervention B: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls | Routine brief verbal and written PFMT. | |
| Verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES | Verbal instruction and information on PFMT plus information on life style changes. | |
| One hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme | No pre operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward. | |
| PFMT + biofeedback Patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles the day before surgery and after catheter removal. They were also given oral and written instructions on Kegel exercises to be performed at home which involved three sets of contractions daily for 10 mins, contracting their pelvic floor while lying, sitting and standing. The frequency of contractions was recorded in a training diary and visits at monthly intervals after catheter removal involved assisted biofeedback and motivation for 20 min | No biofeedback training Received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT after catheter removal (e.g. 2‐3 weeks after surgery) | |
| PFMT | No PFMT | |
| 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1‐2 weeks for as long as UI persisted. 90 daily home exercises sitting, standing and lying. 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe | No formal PFMT instruction but saw the therapist at 1‐2 weeks and received placebo stimulation and information about aetiology of UI. | |
| Intervention A: PFMT alone Intervention B: PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Intervention C: PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after dischargeRegular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery | ||
| Oral PFMT plus ES for 15 minutes twice daily Instructed pre‐operatively PFMT by nurses and continued after catheter removal | Oral PFMT plus sham device. Instructed pre‐operatively PFMT by nurses and continued after catheter removal. | |
| Intervention A: anal electrode for 15 minutes twice a day for 1 month Intervention B: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks | PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice. | |
| PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist | PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes |
-
PFMT alone (Centemero 2009; Dubbelman 2004; Filocamo 2005; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Martini 2011; Park 2012; Perissinotto 2008; Porru 2001; Tobia 2008);
-
PFMT plus biofeedback (Bales 2000; Burgio 2006; Dijkstra‐Eshuis 2013; Floratos 2002; Franke 1998; Geraerts 2013; Hou 2013; Joseph 2000; Manassero 2007; Marchiori 2010; Mathewson‐Chapman 97; Moore 1999; Moore 2008; Overgard 2008; Parekh 2003; Ribeiro 2008; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012);
-
ESl with PFMT (Ahmed 2012; Hoffman 2005; Laurienzo 2013; Morihiro 2011; Wille 2003; Yamanishi 2006);
-
ES with PFMT and biofeedback (Ahmed 2012; Goode 2009; Mariotti 2009; Opsomer 1994; Seleme 2008; Wille 2003; Zhang 2007);
-
extra‐corporeal magnetic innervation (ExMI) with PFMT (Koo 2009; Liu 2008; Nowak 2007);
-
extra‐corporeal magnetic innervation (ExMI) with PFMT or ES with PFMT (Yokoyama 2004);
-
penile compression (Fader 2013; Moore 2004);
-
transcutaneous mechanical nerve stimulation by vibration with PFMT (Fode 2014).
No trials testing lifestyle changes alone were identified.
Types of comparators
There was considerable variation in the types of comparators. Table 1 provides the details of the comparators used in each trial. The comparators included:
-
no treatment, verbal or written instructions or sham therapy (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dubbelman 2004; Filocamo 2005; Franke 1998; Glazener RP 2011; Glazener TURP 2011; Hou 2013; Laurienzo 2013; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Moore 2004; Moore 2008; Morihiro 2011; Nowak 2007; Opsomer 1994; Overgard 2008; Park 2012; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; Tobia 2008; van Kampen 1998; Wille 2003; Yamanishi 2006; Yokoyama 2004);
-
active treatment (Ahmed 2012; Dijkstra‐Eshuis 2013; Floratos 2002; Fode 2014; Geraerts 2013; Ghanem 2013; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Laurienzo 2013; Moore 1999; Seleme 2008; Zhang 2007).
Types of outcome measures
There was a lack of consistency in the reporting of outcome measures. In terms of the primary outcomes of interest in this review these included:
-
number of men with incontinence, for radical surgery (Ahmed 2012; Bales 2000; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Floratos 2002; Fode 2014; Franke 1998; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Goode 2009; Manassero 2007; Marchiori 2010; Mariotti 2009; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Morihiro 2011; Opsomer 1994; Overgard 2008; Parekh 2003; Park 2012; Tobia 2008; van Kampen 1998; Yamanishi 2006) and for TURP (Glazener TURP 2011; Porru 2001; Tibaek 2007; Tienforti 2012);
-
number not cured (Zhang 2007) (assumed to indicate number of incontinent men);
-
time until continent (Fode 2014; Marchiori 2010; Mariotti 2009);
-
number of pad changes over 24 hours (Floratos 2002; Koo 2009; Mathewson‐Chapman 97; Ribeiro 2008; Tienforti 2012);
-
number of men using pads (Glazener RP 2011; Glazener TURP 2011);
-
number of incontinence episodes per day (Glazener RP 2011; Glazener TURP 2011; Goode 2009; Tienforti 2012);
-
pad test weights, grams of urine lost in 24 hours (Ahmed 2012; Geraerts 2013; Joseph 2000; Koo 2009; Mariotti 2009; Mathewson‐Chapman 97; Moore 1999; Moore 2008; Overgard 2008; Park 2012; Ribeiro 2008; Yamanishi 2006), 1 hour (Floratos 2002; Geraerts 2013; Hoffman 2005), 20 minutes (Wille 2003);
-
number with severe incontinence (pad test weight > 150 g) (Centemero 2009);
-
quality of life (condition‐specific such as incontinence scores): ICIQ‐SF score (Centemero 2009; Glazener RP 2011; Glazener TURP 2011; Park 2012; Ribeiro 2008; Yamanishi 2006), severity of UI (Zhang 2007), I‐QoL (Seleme 2008), ICI‐Q‐SF (Liu 2008), IIQ (Ahmed 2012; Ribeiro 2008), ICIQ‐SF QoL score (Glazener RP 2011; Glazener TURP 2011; Yamanishi 2006), EPIC‐UI (Goode 2009), KHQ (Dijkstra‐Eshuis 2013);
-
pelvic floor muscle strength (Overgard 2008);
-
carrying out PFMT or compliance (Glazener RP 2011; Glazener TURP 2011; Goode 2009; Overgard 2008; Zhang 2007).
Excluded studies
In total 36 studies were excluded. The majority of the studies that did not meet the inclusion criteria were excluded because the study design was not appropriate or the intervention was not relevant for the population of interest. See the Excluded studies table for a more detailed description.
Risk of bias in included studies
The assessment criteria of The Cochrane Collaboration assume that the avoidance of bias is best achieved by: a randomised trial with an adequate method of random sequence generation; secure concealment of allocation prior to formal entry; adequate blinding of patients, healthcare providers and outcome assessors; description of reasons and numbers of withdrawals and dropouts; and analysis on an intention‐to‐treat basis. None of the early trials fulfilled all these criteria. However recent trials have fared much better in terms of secure concealment of allocation and blinding but overall this continues to be problematic in many trials (Figure 2; Figure 3).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
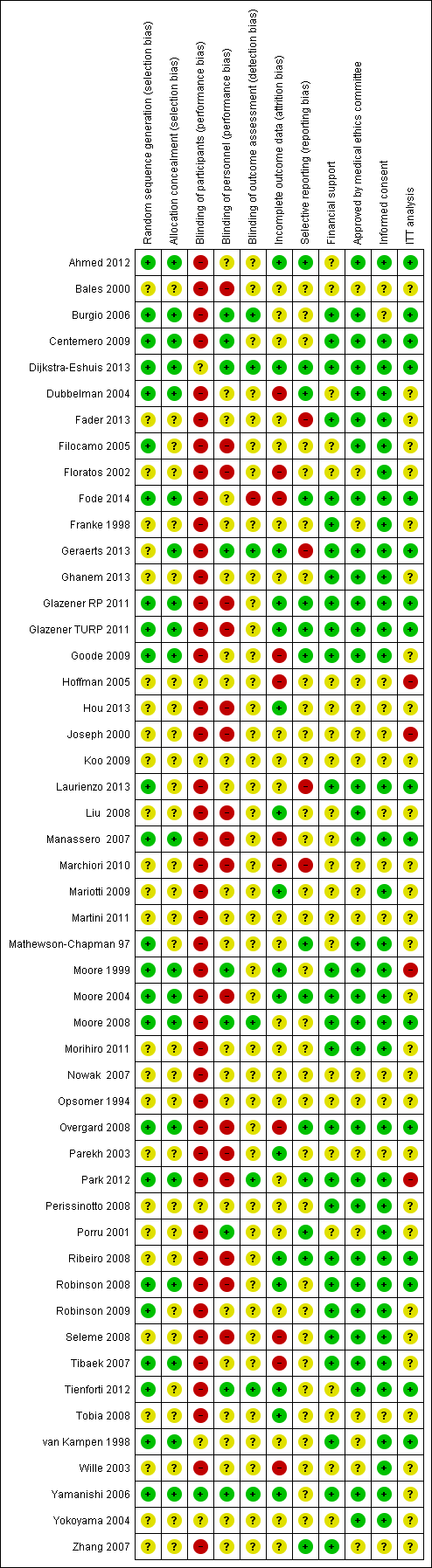
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation
Although all trials were identified as RCTs only 24 trials (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Fode 2014; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; van Kampen 1998; Yamanishi 2006) described a method of adequate sequence generation (for example computer generated random numbers) and were assessed as low risk of bias. The remainder did not provide enough information to make a judgement and were assessed as unclear.
Allocation concealment
Only 20 trials (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fode 2014; Geraerts 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Manassero 2007; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006) adequately described a technique of allocation concealment (for example sealed envelopes or computerised randomisation) and were assessed as low risk of bias. The remainder did not provide enough information to make a judgement and were assessed as unclear.
Blinding
Blinding was not described in most trials. In complex interventions such as physical therapy it is not possible to blind either the clinicians or the participants from the intervention, however, if blinding did not take place in trials they were judged to be at high risk of bias. This may have an impact on the outcome of interest and was considered while assessing the quality of evidence. Yamanishi 2006 used a sham device for the control group and this was the only trial that was deemed to be at low risk of bias in terms of blinding of participants.
In terms of blinding of personnel:
-
9 trials (Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Geraerts 2013; Moore 1999; Moore 2008; Porru 2001; Tienforti 2012; Yamanishi 2006) were deemed to be at low risk of bias;
-
17 trials (Bales 2000; Filocamo 2005; Floratos 2002; Glazener RP 2011; Glazener TURP 2011; Hou 2013; Joseph 2000; Liu 2008; Manassero 2007; Marchiori 2010; Moore 2004; Overgard 2008; Parekh 2003; Park 2012; Ribeiro 2008; Robinson 2008; Seleme 2008) were deemed to be at high risk of bias; and
-
23 trials (Ahmed 2012; Dubbelman 2004; Fader 2013; Fode 2014; Franke 1998; Ghanem 2013; Goode 2009; Hoffman 2005; Koo 2009; Laurienzo 2013; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Morihiro 2011; Nowak 2007; Opsomer 1994; Perissinotto 2008; Robinson 2009; Tobia 2008; van Kampen 1998; Wille 2003; Yokoyama 2004; Zhang 2007) were at unclear risk.
Burgio 2006; Moore 1999 and Moore 2008 indicated that a single therapist, blinded to control group outcomes, provided all treatment. Dijkstra‐Eshuis 2013 and Geraerts 2013 reported that the post‐operative physiotherapist was blinded to allocation and physical therapy provided by the pre‐operative therapist.
In terms of blinding of outcome assessment:
-
7 trials (Burgio 2006; Dijkstra‐Eshuis 2013; Geraerts 2013; Moore 2008; Park 2012; Tienforti 2012; Yamanishi 2006) were deemed to be at low risk of bias because they had outcome assessors who were not involved in the provision of the intervention or were not aware of allocation when entering data;
-
1 trial (Fode 2014) was found to be at high risk of bias; and
-
42 trials (Ahmed 2012; Bales 2000; Centemero 2009; Dubbelman 2004; Fader 2013; Filocamo 2005; Floratos 2002; Franke 1998; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Laurienzo 2013; Liu 2008; Manassero 2007; Marchiori 2010; Mariotti 2009; Martini 2011; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Morihiro 2011; Nowak 2007; Opsomer 1994; Overgard 2008; Parekh 2003; Perissinotto 2008; Porru 2001; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; Tobia 2008; van Kampen 1998; Wille 2003; Yokoyama 2004; Zhang 2007) were at unclear risk because this information was not provided.
Yamanishi 2006 used a sham device for the control group but there was no statement of whether assessors were aware of this or not.
Incomplete outcome data
Several trials gave no description or did not report dropouts (Centemero 2009; Ghanem 2013; Koo 2009; Marchiori 2010; Morihiro 2011; Perissinotto 2008; Ribeiro 2008; Robinson 2009; Seleme 2008; Yamanishi 2006; Yokoyama 2004), or did not have withdrawals or dropouts (Bales 2000; Liu 2008; Moore 2004; Tobia 2008).
All others reported the number of withdrawals or dropouts, although the reasons were not consistently reported and few, except Moore 2008 and Robinson 2008, discussed how this was dealt with in the analysis. In one trial, outcomes beyond eight weeks were not available for the control group because all the men were treated, and data were not available for over a third of the men in the other two intervention groups (Goode 2009). Two trials were thought to be at risk of bias because of differential dropout from the randomised groups (Dubbelman 2004; Manassero 2007). One trial (Marchiori 2010) that was judged to be at high risk of bias reported that the survey questionnaire used for one of their outcomes was completed correctly but returned by fewer than 10% of the men.
Six trials (Fader 2013; Martini 2011; Nowak 2007; Perissinotto 2008; Robinson 2008; Robinson 2009) did not provide any usable data. Three of these trials (Nowak 2007; Perissinotto 2008; Robinson 2009) did not report how many men were randomised to each group.
Selective reporting
There was significant difficulty in assessing selective outcome reporting because the protocols for most of the included trials were not available for assessment or could not be found. For a few of the trials, data were not available for some of the outcomes stated in the methods section.
Other potential sources of bias
Information about funding was available for 27 of the included studies (Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Fader 2013; Fode 2014; Franke 1998; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Moore 1999; Moore 2004; Moore 2008; Morihiro 2011; Overgard 2008; Park 2012; Perissinotto 2008; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006; Zhang 2007) and the studies were judged to be at low risk of bias. The rest of the trials were judged to be at unclear risk of bias because there was a lack of information even after contacting the authors.
Thirty‐two trials reported obtaining approval from a medical ethics committee (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fader 2013; Filocamo 2005; Fode 2014; Geraerts 2013; Ghanem 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Liu 2008; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Morihiro 2011; Overgard 2008; Park 2012; Perissinotto 2008; Ribeiro 2008; Robinson 2008; Robinson 2009; Seleme 2008; Tibaek 2007; Tienforti 2012; Yamanishi 2006; Yokoyama 2004) and were judged to be at low risk of bias. The remaining 18 trials did not report their source of medical ethical approval and were judged to be at unclear risk of bias after no further information was provided by the authors.
Fourteen trials were deemed to be at unclear risk of bias in terms of obtaining informed consent (Bales 2000; Burgio 2006; Hoffman 2005; Hou 2013; Joseph 2000; Koo 2009; Liu 2008; Marchiori 2010; Martini 2011; Nowak 2007; Opsomer 1994; Parekh 2003; Tobia 2008; Zhang 2007). These authors were contacted but no further information on this matter was provided. The other trials did report obtaining informed consent from patients and therefore were deemed to be at low risk of bias for this domain.
Effects of interventions
See: Summary of findings for the main comparison Treatment of UI after radical: PFMT ± biofeedback versus no treatment; for postprostatectomy urinary incontinence; Summary of findings 2 Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy urinary incontinence; Summary of findings 3 Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy urinary incontinence; Summary of findings 4 Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy urinary incontinence; Summary of findings 5 Prevention of UI after radical: PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence; Summary of findings 6 Prevention of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy urinary incontinence; Summary of findings 7 Prevention of UI after radical: combinations of treatments versus no treatment for postprostatectomy urinary incontinence; Summary of findings 8 Prevention of UI after radical: one active treatment versus another active treatment (PFMT pre and post‐operation versus PFMT post‐operation) for postprostatectomy urinary incontinence; Summary of findings 9 Prevention of UI after radical: one active treatment versus another active treatment (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for postprostatectomy urinary incontinence; Summary of findings 10 Prevention of UI after radical: one active treatment versus another active treatment (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for postprostatectomy urinary incontinence; Summary of findings 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence; Summary of findings 12 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment for postprostatectomy urinary incontinence
Radical prostatectomy: treatment of incontinent men after surgery
1. Treatment of UI after radical prostatectomy: post‐operative PFMT with or without biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 1)
Nine trials (Dubbelman 2004; Floratos 2002; Franke 1998; Manassero 2007; Glazener RP 2011; Goode 2009; Moore 1999; Moore 2008; van Kampen 1998) compared PFMT with or without biofeedback to no treatment (sham or verbal instruction) amongst men who had UI after radical prostatectomy. The quality of the evidence is given in summary of findings Table for the main comparison.
Differences between trials
All the men were incontinent at baseline.
In one trial (Manassero 2007) there was evidence of unexplained differential dropout from the control group (13 of 53 men, while there were no dropouts from the 54 in the intervention group). The missing men have therefore been assumed to be dry for the purpose of an intention‐to‐treat analysis. The other trials have been analysed as reported since dropouts (if any) were balanced between the groups.
Sources of heterogeneity
(1) Definition of incontinence varied with each trial:
-
more than 1 g urine on one hour pad test (Dubbelman 2004);
-
more than 8 g urine loss on 24 hour pad test (Moore 2008);
-
more than 2 g urine loss on one hour (van Kampen 1998) or 24 hour pad test (Moore 1999);
-
men who were not pad free (Franke 1998);
-
a visual analogue score of 10 = completely incontinent and 0 = completely continent (Manassero 2007); or
-
no leakage based on bladder diaries (Goode 2009).
(2) The type of PFMT regimens differed between the trials:
-
four trials (Dubbelman 2004; Goode 2009; Manassero 2007; Moore 1999) evaluated PFMT alone (without biofeedback);
-
three trials evaluated PFMT with biofeedback, verbal instruction (Manassero 2007; Moore 2008) or ES (van Kampen 1998);
-
two trials (Floratos 2002; Franke 1998) used PFMT with biofeedback via a perineal patch (surface) EMG.
Formal PFMT post‐operative sessions directed by a therapist ranged from: twice a week for 12 weeks (Moore 1999); three times a week for three weeks (Floratos 2002); in up to nine sessions (Dubbelman 2004); weekly for 24 weeks (Moore 2008); four sessions over eight weeks (Goode 2009); five sessions over 16 weeks (Franke 1998); to as long as the UI persisted (van Kampen 1998). Men received only four therapy sessions in three months in one of these trials (Glazener RP 2011) and men in another trial were seen weekly for up to six months (Moore 2008).
(3) Control interventions differed between the trials and included:
-
information (verbal or written) about PFMT only (Dubbelman 2004; Floratos 2002; Moore 1999; Moore 2008);
-
no treatment (Manassero 2007);
-
sham placebo PFMT and contact with therapist (van Kampen 1998);
-
monitoring of UI only (e.g. by bladder diary or phone calls) (Franke 1998; Goode 2009).
(4) The participants differed between the trials.
Two trials (Goode 2009; Moore 1999) recruited participants with persistent incontinence (some longer than one year) post‐operatively, and these participants may have differed from those enrolled pre‐operatively (Moore 2008) but still incontinent at four weeks after surgery) or from those recruited within a week or two of catheter removal (Dubbelman 2004;Floratos 2002;Glazener RP 2011; Manassero 2007; van Kampen 1998) or up to six weeks after radical prostatectomy (Franke 1998).
Incontinence in men and incontinence episodes
Because there was evidence of significant statistical heterogeneity between the trials included in this comparison (see below), meta‐analysis was carried out using a random‐effects model, therefore widening the CI. There were no significant differences at any time period in the UI rates, and the CIs were wide (for example RR for UI up to 12 months 0.91, 95% CI 0.73 to 1.14, Analysis 1.1.3; and after 12 months 57% with UI versus 62% in the control group, RR 0.85, 95% CI 0.60 to 1.22, Analysis 1.1.4). Only two trials (Manassero 2007; van Kampen 1998) favoured the treatment and of these, only one (van Kampen 1998) used biofeedback. The estimates from the other trials had CIs that did not rule out clinically important effects. Overall, as one of the pre‐defined GRADE‐specific outcomes, the quality of evidence for the outcome 'number of incontinent men after 12 months' was found to be moderate.
The meta‐analysis was dominated by the Glazener RP 2011 trial, which was a large pragmatic multi‐centre trial conducted in a context where information on PFMT was widely available. This showed no good evidence to support one‐to‐one training by a therapist (for example RR for UI after 12 months 0.98, 95% CI 0.87 to 1.09, Analysis 1.1.4) (Glazener RP 2011). This one large trial had narrow CIs which did not include a clinically significant difference, pre‐specified to be 15%. One other trial (Moore 2008) was in line with the Glazener RP 2011 findings but had wider CIs (RR 1.02, 95% CI 0.70 to 1.48, Analysis 1.1.4) (Moore 2008).
In one large trial (Glazener RP 2011), men did not report differences in UI episodes at any time period, based on urinary diary data (for example after 12 months MD 0.1, 95% CI ‐0.82 to 1.02, Analysis 1.2.4). Alternatively, one trial (Goode 2009) did report a significant difference, however this measurement was obtained at less than 3 months (MD ‐1.14, 95% CI ‐1.46 to ‐0.82, Analysis 1.2.1).
Use of pads
Use of pads could be considered to be a measure of more severe incontinence. There was no statistically significant difference in the number of men using pads in one large trial (40% in intervention group versus 42% in control group after 12 months, RR 0.94, 95% CI 0.72 to 1.22, Analysis 1.3) (Glazener RP 2011). Floratos 2002 used number of pad changes over 24 hours as the outcome measure, with no statistically significant difference in the MD between treatment and control groups at any time period (Analysis 1.4).
Urinary incontinence score and effect on quality of life
In one large trial (Glazener RP 2011), there was no evidence of a difference in the ICIQ‐SF (a composite score of frequency, amount and effect of UI on quality of life) at any time period after the intervention up to or beyond one year (MD after 12 months ‐0.5, 95% CI ‐1.35 to 0.35, Analysis 1.5) or quality of life as a single score from 0 to 10 (MD ‐0.30, 95% CI ‐0.73 to 0.13, Analysis 1.6), however the quality of evidence for this outcome was found to be low.
Pad tests
Two trials (Moore 1999; Moore 2008) reported 24 hour pad test results and one (Floratos 2002) reported a one hour pad test. Dubbelman 2004 and van Kampen 1998 also measured urine loss on a 24 hour pad test, but did not report SDs and therefore these data could not be included in the meta‐analysis. Amongst the two trials which gave 24 hour pad test data, there were no statistically significant differences between the groups at 3, 6 or 12 months, or after 12 months (Analysis 1.8). Similarly, using a one hour pad test (Floratos 2002), there were no statistically significant differences between the groups up to six months (Analysis 1.9). In the smaller trials (Floratos 2002; Moore 1999; Moore 2008) the SDs were often larger than the means, suggesting highly skewed data.
2. Treatment of UI after radical prostatectomy: post‐operative interventions using electric or magnetic energy (for example post‐operative anal ES, perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI) versus no treatment or sham treatment (Comparison 2)
Four trials were identified which addressed this comparison (Marchiori 2010; Moore 1999; Morihiro 2011; Yamanishi 2006). These trials compared anal ES with oral (verbal) PFMT. The control group in Moore's trial received oral information about PFMT only, whereas in Yamanishi's trial the control group also received sham ES. The quality of the evidence is given in summary of findings Table 2.
Number of incontinent men
In the short term (less than three months), there were fewer incontinent men in the intervention groups in two trials (64% versus 84% in the control groups, RR 0.77, 95% CI 0.60 to 0.98, Analysis 2.1.1) (Moore 1999; Yamanishi 2006) and the quality of the evidence for this outcome was deemed to be moderate. This remained the same at 6 to 12 months (19% versus 53% in the control groups, RR 0.37, 95% CI 0.18 to 0.73, Analysis 2.1.3) and after 12 months (7% versus 33% in the control groups of three trials, RR 0.26, 95% CI 0.09 to 0.74). However, the data were too few to be reliable in the longer term.
Adverse effects
One small trial (Yamanishi 2006) reported adverse effects, with two men in the active ES group and four men in the group receiving sham treatment reporting anal pain or discomfort. No statistically significant differences were found between the groups (RR 0.58, 95% CI 0.11 to 2.90, Analysis 2.2).
Pad test
There were no statistically significant differences between the groups on grams of urine lost (24 hour pad test) at any of the time points (Analysis 2.3). SSs were large, indicating skewed distribution of data, and the CIs were wide with evidence of significant statistical heterogeneity.
UI score
Men in the intervention group in one trial (Yamanishi 2006) had lower (better) UI scores using a quality of life outcome combined with amount and frequency of urine lost (for example MD ‐3.9, 95% CI ‐7.15 to ‐0.65, Analysis 2.4.3, at one year) though this did not quite reach statistical significance when quality of life was analysed on its own (MD ‐0.40, 95% CI ‐2.02 to 1.22, Analysis 2.5).
Time until continence achieved
Men achieved continence on average about 5 months sooner in the intervention group of one trial (MD ‐4.11 months, 95% CI ‐6 to ‐2.23, Analysis 2.6) (Yamanishi 2006).
3. Treatment of UI after radical prostatectomy: post‐operative lifestyle adjustment versus no treatment or sham treatment (Comparison 3)
No trials were identified.
4. Treatment of UI after radical prostatectomy: post‐operative combinations of treatments versus no treatment or sham treatment (Comparison 4)
Two trials reported using PFMT with anal ES as well as biofeedback (Goode 2009; Opsomer 1994) versus control management. Goode 2009 compared behavioural therapy comprising biofeedback and ES for eight weeks with a control group. Opsomer 1994 treated incontinent men in the intervention group with two sessions of ES with biofeedback as well as continuing the PFMT taught to both groups at six weeks after radical prostatectomy. The quality of the evidence is given in summary of findings Table 3.
Number of incontinent men
Goode 2009 reported fewer incontinent men in the intervention group compared with the control group (83% versus 94% in the control group at less than 3 months, RR 0.88, 95% CI 0.78 to 0.99, Analysis 4.1.1). In the other trial (Opsomer 1994), four men in total had incontinence at 3 to 6 months, with 3/20 in the intervention group and 1/19 in the control group, but this was not statistically significant (RR 2.85, 95% CI 0.32 to 25.07, Analysis 4.2). Overall, the quality of evidence for this outcome was very low.
Adverse events
There were two adverse events (haemorrhoidal irritation) reported by men receiving ES in one trial (Goode 2009), and the quality of evidence for this outcome was deemed to be of low quality with wide CIs indicating uncertainty (RR 4.86, 95% CI 0.24 to 99.39, Analysis 4.4.1).
5. Treatment of UI after radical prostatectomy: post‐operative use of one treatment versus another active treatment (Comparison 5)
Nine trials comparing one active treatment to another were identified (Floratos 2002; Goode 2009; Hoffman 2005; Joseph 2000; Koo 2009; Moore 1999; Seleme 2008; Yokoyama 2004; Zhang 2007).
-
PFMT plus anal ES (EStim) (Hoffman 2005; Moore 1999).
-
PFMT plus perineal ES (EStim) (Hoffman 2005).
-
PFMT plus visual biofeedback (Joseph 2000; Zhang 2007).
-
PFMT plus visual biofeedback plus support group (Zhang 2007).
-
PFMT plus oral (verbal) biofeedback (Joseph 2000).
-
PFMT plus biofeedback plus ES(Estim) (Goode 2009; Seleme 2008).
-
PFMT alone (Goode 2009; Hoffman 2005; Koo 2009; Moore 1999; Seleme 2008).
-
Extra‐corporeal Magnetic Innervation (ExMI) (Koo 2009).
The quality of the evidence is given in summary of findings Table 4.
Number of incontinent men
Four small trials provided data for this outcome (Goode 2009; Moore 1999; Yokoyama 2004; Zhang 2007). The definition of incontinence varied with each trial:
-
no urine loss recorded in bladder diaries (Goode 2009);
-
less than 8 g urine loss on 24 hour pad test (Moore 1999);
-
'urine loss' (Yokoyama 2004); and
-
use of pad or brief (Zhang 2007).
There was no difference in the incontinence rates in the trials at any time period, but CIs were wide, up to 3 months (RR 0.96, 95% CI 0.83 to 1.12, Analysis 5.1); 3 to 6 months (RR 0.59, 95% CI 0.33 to 1.05, Analysis 5.2); 6 to 12 months (RR 2, 95% CI 0.21 to 18.23, Analysis 5.3) and the quality of evidence was deemed to be of very low quality.
Pad tests
For the majority of the comparisons there were no statistically significant differences between the groups, SDs were large, indicating skewed distribution of data, and the CIs were wide.
However, men having extra‐corporeal magnetic innervation (ExMI) compared to PFMT alone had less urine loss on the 24 hour pad test at 3 to 6 months in one small trial (Koo 2009) (compared to PFMT alone, MD ‐36 g, 95% CI ‐55 to ‐17, Analysis 5.12.3) and used fewer pads per day (MD ‐0.5, 95% CI ‐0.79 to ‐0.21, Analysis 5.13.1) (Koo 2009).
Quality of life
In another small trial (Seleme 2008) men receiving PFMT plus biofeedback plus ES reported better quality of life using the Incontinence Quality of life score than those receiving PFMT alone (MD ‐28.63, 95% CI ‐34.60 to ‐22.66, Analysis 5.6.1).
In a third trial (Liu 2008), PFMT supplemented by extra‐corporeal magnetic innervation (ExMI) seemed to be better than PFMT alone in terms of quality of life assessed using the ICIQ‐SF score (MD ‐1.60, 95% CI ‐2.73 to ‐0.47, Analysis 5.7.1) but the quality of the evidence for this outcome was judged to be of low quality.
Adverse events
Two men in one trial (Goode 2009) had an adverse event with ES (haemorrhoidal irritation, RR 5, 95% CI 0.24 to 102.30, Analysis 5.8.1) but the evidence for this outcome was judged to be of low quality.
Radical prostatectomy: prevention of UI in all men having surgery, intervention before or after prostatectomy or both
6. Prevention of UI after radical prostatectomy: PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 6)
Ten trials addressed this comparison (Bales 2000; Burgio 2006; Filocamo 2005; Laurienzo 2013; Mathewson‐Chapman 97; Overgard 2008; Parekh 2003; Ribeiro 2008; Tienforti 2012; Tobia 2008). The quality of the evidence is given in summary of findings Table 5.
Differences between trials
The participants were not selected because they were incontinent so included a mixed population of men with and without incontinence after surgery.
Sources of heterogeneity
(1) The type of PFMT regimens differed between the trials:
-
PFMT plus biofeedback (Bales 2000; Burgio 2006; Laurienzo 2013; Mathewson‐Chapman 97; Parekh 2003; Ribeiro 2008; Tienforti 2012);
-
PFMT alone (Filocamo 2005; Overgard 2008; Tobia 2008).
Biofeedback was delivered via surface electrodes (Bales 2000) or via a digital or anal probe (Burgio 2006; Mathewson‐Chapman 97; Parekh 2003; Tienforti 2012). In one trial (Ribeiro 2008) the type of biofeedback was not described.
(2) Control interventions differed between the trials and included:
-
no treatment (Filocamo 2005; Mathewson‐Chapman 97; Parekh 2003; Tobia 2008);
-
post‐operative verbal or written instruction on PFMT only (Bales 2000; Laurienzo 2013; Overgard 2008; Ribeiro 2008; Tienforti 2012);
-
usual care with simple instructions to interrupt the stream when voiding (Burgio 2006).
(3) The timing of the interventions relative to surgery also varied:
-
two trials delivered an intervention before surgery only (Laurienzo 2013; Tobia 2008);
-
five trials delivered their intervention before and after surgery (Bales 2000; Burgio 2006; Parekh 2003; Mathewson‐Chapman 97; Tienforti 2012);
-
three trials delivered their intervention after surgery only (Filocamo 2005; Overgard 2008; Ribeiro 2008).
Number of men with UI
Data describing UI were reported by 8 of the 10 trials. While there was no statistically significant difference at 3 months (Analysis 6.1.1), there was evidence from the findings of this systematic review of an overall benefit from PFMT in the number of men with UI within 6 to 12 months (24% versus 52%, RR 0.51, 95% CI 0.35 to 0.75, Analysis 6.1.3) and after 1 year (10% versus 32%, RR 0.32, 95% CI 0.20 to 0.51, Analysis 6.1.4), but the quality of evidence was judged to be moderate. The data were driven mainly by two trials (Filocamo 2005; Overgard 2008), neither of which included biofeedback. One of these trials did not disclose details of allocation concealment (Filocamo 2005) and the other was small (Overgard 2008). The remaining trials showed conflicting results and there was statistically significant heterogeneity, hence the use of a random‐effects model.
Pad changes and pad tests
In the four trials which reported these outcomes (Filocamo 2005; Mathewson‐Chapman 97; Overgard 2008; Ribeiro 2008) there was statistical heterogeneity. One small trial favoured PFMT (Ribeiro 2008) but using a random‐effects model there was only a significant difference at 6 to 12 months (MD ‐15 g less urine loss on 24 hour pad test with treatment, 95% CI ‐18 to ‐11, Analysis 6.4.3). Men in the intervention group in this trial received PFMT plus biofeedback weekly for three months until they were continent or until three months. The findings from the Filocamo 2005 and Overgard 2008 trials (no significant difference in pad weights) was in contrast to their report of fewer incontinent men with active treatment (RR 0.32, 95% CI0.20 to 0.51, Analysis 6.1.4). However, the SDs were large and the CIs were wide. Laurienzo 2013 did not find a significant difference up to 12 months when using a 1 hour pad test (MD 19.80, 95% CI ‐9.15 to 48.75, Analysis 6.3) and comparing PFMT with no standard treatment.
Mean number of incontinence episodes per day
Tienforti 2012 favoured PFMT at all time points (MD ‐1.43, 95% CI ‐2.35 to ‐0.51, Analysis 6.5) when quantifying episodes of UI in men each day, with men in the intervention group suffering fewer mean numbers of episodes.
Quality of life
Quality of life was assessed using the ICIQ‐SF by two trials (Laurienzo 2013; Ribeiro 2008) but the quality of the evidence was found to be very low. No significant difference was found within 6 to 12 months (MD ‐0.69, 95% CI ‐3.19 to 1.81, Analysis 6.6).
Ribeiro 2008 also assessed quality of life using the IIQ, favouring the intervention at 3 to 6 months (MD ‐2.70, 95% CI ‐4.88 to ‐0.52, Analysis 6.7).
7. Prevention of UI after radical prostatectomy: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 7)
One small trial that delivered the intervention pre‐operatively only was identified (Laurienzo 2013). There was no significant difference using a 1 hour pad test at 6 to 12 months (MD ‐1.15, 95% CI ‐9.11 to 6.81, Analysis 7.1) or when assessing quality of life using the ICIQ‐SF (MD 1.60, 95% CI ‐2.15 to 5.35, Analysis 7.2), but the quality of evidence for this outcome was judged to be very low (summary of findings Table 6).
8. Prevention of UI after radical prostatectomy: lifestyle interventions versus no treatment or sham treatment (Comparison 8)
No trials were identified.
9. Prevention of UI after radical prostatectomy: combinations of treatments versus no treatment or sham treatment (Comparison 9)
Only one small trial (Mariotti 2009) looked at this comparison. Men in the intervention group received PFMT plus ES with biofeedback post‐operatively and men in the control group received verbal and written instructions on PFMT. There was a statistical difference with regards to:
-
number of incontinent men within 6 to 12 months (RR 0.10, 95% CI 0.01 to 0.73, Analysis 9.2);
-
using a 24 hour pad test (MD ‐24.30, 95% CI ‐45.02 to ‐3.58, Analysis 9.4); and
-
time until UI was regained (MD ‐1.50, 95% CI ‐2.44 to ‐0.56, Analysis 9.5).
However, the quality of evidence for the primary outcome (number of incontinent men) was found to be low (summary of findings Table 7).
Adverse events
Adverse events were not reported.
10. Prevention of UI after radical prostatectomy: one treatment versus another active treatment (Comparison 10)
Eight trials were identified (Ahmed 2012; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013Nowak 2007; Park 2012; Wille 2003). Five of these were new in this update (Ahmed 2012; Dijkstra‐Eshuis 2013; Fode 2014; Geraerts 2013; Park 2012) and one was updated with new information (Centemero 2009).
-
Ahmed 2012 was a three‐armed trial, with patients receiving PFMT plus TENS with biofeedback or TENS only or guided PFMT only.
-
Centemero 2009 compared PFMT before and after surgery with PFMT delivered after surgery only.
-
Dijkstra‐Eshuis 2013 compared pre‐operative guided PFMT with biofeedback versus post‐operative written instructions on PFMT; however all men received PFMT plus biofeedback plus ES if they were still incontinent after six weeks.
-
Fode 2014 compared PFMT + penile vibration before and after surgery with PFMT alone before and after surgery: all men received a phosphodiesterase type 5 (PDE5) inhibitor after the first month.
-
Geraerts 2013 compared PFMT plus biofeedback versus active PFMT.
-
Nowak 2007 compared extra‐corporeal magnetic innervation (ExMI) versus PFMT alone but did not provide any useable data.
-
Park 2012 compared post‐operative PFMT plus general exercise versus post‐operative PFMT alone.
-
Wille 2003, a three‐arm trial, compared PFMT plus ES versus PFMT plus ES plus anal probe biofeedback versus PFMT alone.
The trials were generally small and few were similar enough to combine in a meta‐analysis. The quality of the evidence is illustrated in summary of findings Table 8.
Number of incontinent men
This outcome was reported by six trials (Ahmed 2012; Centemero 2009; Dijkstra‐Eshuis 2013; Fode 2014; Ghanem 2013; Park 2012).
In one trial, Centemero 2009 reported fewer incontinent men at less than 3 months and within 3 to 6 months when PFMT was delivered pre and post‐operatively, compared with post‐operatively only, and this correlated with a statistically significant better quality of life score (MD ‐3.70, 95% CI ‐6.00 to ‐1.40, Analysis 10.15.1; MD ‐4.10, 95% CI ‐6.64 to ‐1.56, Analysis 10.16.1). However, when combined with the data from Geraerts 2013, who used the same interventions, there was no statistically significant difference between the interventions at 3 months (RR 0.86, 0.69 to 1.06, Analysis 10.1.1) or 3 to 6 months (RR 0.75, 95% CI 0.54 to 1.04, Analysis 10.2.1). It should be noted that the CIs were very wide.
Ahmed 2012 compared three different treatments (PFMT plus transcutaneous electrical stimulation with biofeedback; TENS only; and guided PFMT only) and found no statistically significant differences between the interventions in terms of number of men with UI (Analysis 10.1, Analysis 10.2; Analysis 10.3) except that at 6 to 12 months PFMT plus ES plus biofeedback proved to be significantly better than PFMT only (RR 0.10, 95% CI 0.01 to 0.76, Analysis 10.3.3).
One small trial (Park 2012) found that general exercise added to PFMT was statistically significantly better than PFMT alone within 3 to 6 months (RR 0.48, 95% CI 0.23 to 0.99, Analysis 10.2.3).
Four trials reported the number of incontinent men after 12 months (Dijkstra‐Eshuis 2013;Fode 2014;Geraerts 2013;Ghanem 2013). The quality of the evidence was moderate (summary of findings Table 8). Three of these trials, comparing pre and post‐operative PFMT to post‐operative PFMT only, found that more men were incontinent after 12 months when PFMT began before surgery (15.3% versus 10.7% with post‐operative training alone) but this did not reach statistical significance (RR 1.32, 95% CI 0.78 to 2.25, Analysis 10.4.1). The Fode 2014 study was too small to identify a difference between PFMT plus penile vibratory stimulation pre and post‐operatively compared with pre and post‐operative PFMT (Analysis 10.4.2).
Pad tests
In general, the short‐duration pad tests did not distinguish between the various interventions being compared, apart from in one trial. At 6 months (but not at 3 months), Wille 2003 found that PFMT plus anal ES both with and without extra biofeedback were both better than PFMT alone using a 20 minute pad test (MD urine lost ‐3 g, 95% CI ‐6 to ‐0.5 in both comparisons, Analysis 10.8.1 and Analysis 10.8.2), while there was little to choose between the two more intensive interventions (Analysis 10.8.3). However, the trial was small, the SDs large and the CIs wide.
Using a longer‐duration 24 hour pad test, the groups receiving ES were generally better than those only having PFMT or only having ES (Analysis 10.12; Analysis 10.13; Analysis 10.14) but the interventions were to dissimilar to combine. At three to six months, one small trial (Park 2012) did not find significant benefit when comparing PFMT plus general exercise with PFMT alone (Analysis 10.13.4).
Quality of life
ICIQ‐SF
The ICIQ‐SF score is a mixed measure of both incontinence severity and effect on quality of life. One small trial (Park 2012) found that there was a significant benefit in terms of the ICIQ‐SF and the intervention PFMT plus general exercise versus PFMT alone, but the evidence for this outcome was found to be very low (MD in scores ‐4.00, 95% CI ‐5.41 to ‐2.59, Analysis 10.16.2).
King’s Health Questionnaire
For all domains of the King’s Health Questionnaire, Dijkstra‐Eshuis 2013 did not find a statistically significant difference between PFMT given pre and post‐operatively and PFMT given post‐operatively only (Analysis 10.18).
SF‐36
In contrast, one trial (Park 2012) found that the intervention PFMT with general exercise was favoured at 3 to 6 months when using the health status measure SF‐36 (MD ‐9.00, 95% CI ‐11.17 to ‐6.83, Analysis 10.19.1) compared with PFMT alone. This may have been more of a measure of an effect of exercise on general health than on incontinence itself.
Adverse events
One trial (Fode 2014) was in the meta‐analysis and the authors stated that 5/30 men reported adverse effects in the intervention group using the group with a penile vibratory stimulation device. The quality of evidence for this outcome was deemed to be low. Adverse effects included:
-
red spots on the glans penis;
-
small laceration with some bleeding;
-
soreness;
-
frank pain.
TURP: treatment of incontinent men, after surgery
11. Treatment of UI after TURP: PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 11)
One large trial compared PFMT with or without biofeedback to no treatment (sham or verbal instruction) amongst men who had UI after TURP (Glazener TURP 2011). All the men were incontinent at randomisation, six weeks after surgery, and received four one‐to‐one sessions with a trained therapist over a three month period. The quality of the evidence is illustrated in summary of findings Table 11.
Incontinence in men and incontinence episodes
There were no significant differences at any time period in the incontinence rates (for example RR for incontinence up to 12 months 1.04, 95% CI 0.90 to 1.20, Analysis 11.1.3; and after 12 months, 65% with UI versus 62% in the control group, RR 1.05, 95% CI 0.91 to 1.23, Analysis 11.1.4). The evidence was judged to be moderate.
In one large trial (Glazener TURP 2011) men did not report differences in incontinence episodes at any time period, based on urinary diary data (for example after 12 months MD 0.2, 95% CI ‐0.27 to 0.67, Analysis 11.2).
Use of pads
Use of pads could be considered to be a measure of more severe incontinence. There was no statistically significant difference in the number of men using pads in one large trial (16% in intervention group versus 18% in control group after 12 months, RR 0.93, 95% CI 0.56 to 1.56, Analysis 11.3) (Glazener TURP 2011).
Urinary incontinence score and effect on quality of life
In one large trial (Glazener TURP 2011), there was no evidence of a difference in the ICIQ‐SF (a composite score of frequency, amount and effect of UI on quality of life) at any time period after the intervention up to or beyond one year (for example MD after 12 months ‐0.1, 95% CI ‐0.89 to 0.69, Analysis 11.4) or quality of life as a single score from 0 to 10 (MD ‐0.1, 95% CI ‐0.51 to 0.31, Analysis 11.5). The quality of evidence for this outcome was deemed to be low.
Adverse events
No adverse events were reported.
12. Treatment of UI after TURP: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 12)
No trials were identified.
13. Treatment of UI after TURP: lifestyle interventions versus no treatment or sham treatment (Comparison 13)
No trials were identified.
14. Treatment of UI after TURP: combinations of treatments versus no treatment or sham treatment (Comparison 14)
No trials were identified.
15. Treatment of UI after TURP: one treatment versus another active treatment (Comparison 15)
No trials were identified.
TURP: prevention of UI in all men having surgery, intervention before or after prostatectomy, or both
16. Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment or sham therapy or verbal instruction (Comparison 16)
Three small trials enrolled men before TURP for benign prostatic hyperplasia (Hou 2013; Porru 2001; Tibaek 2007). Men in the intervention groups in both trials received one session with a therapist before surgery to teach them the correct contractions (using verbal biofeedback) and they were expected to practice PFMT afterwards. In the second trial (Tibaek 2007), men also attended three group teaching sessions. The control groups received information only. The quality of the evidence is illustrated in summary of findings Table 12.
There were no statistically significant differences between the groups in the number of men with incontinence at less than 3 months or 3 to 6 months, but the CIs were wide and the quality of evidence was very low (< 3 months RR 0.60, 95% CI 0.21 to 1.77, Analysis 16.1.1; 3 to 6 months RR 0.51, 95% CI 0.14 to 1.89, Analysis 16.1.2).
Quality of life
One trial (Hou 2013) measured quality of life using a health status measure Short‐Form 36 (SF‐36) questionnaire at three to six months. No statistically significant differences were found on any of the domains apart from those associated with mental health (Analysis 16.2).
17. Prevention of UI after TURP: electric or magnetic energy (for example anal ES (EStim), perineal ES, TENS, extra‐corporeal magnetic innervation (ExMI)) versus no treatment or sham treatment (Comparison 17)
No trials were identified.
18. Prevention of UI after TURP: lifestyle interventions versus no treatment or sham treatment (Comparison 18)
No trials were identified.
19. Prevention of UI after TURP: combinations of treatments versus no treatment or sham treatment (Comparison 19)
No trials were identified.
20. Prevention of UI after TURP: one treatment versus another active treatment (Comparison 20)
No trials were identified.
Containment of UI (all men with residual UI)
21. External penile compression devices (penile clamps) versus no treatment or sham treatment (Comparison 21)
One trial compared three different penile compression devices (Cunningham clamp, U‐Tex Male Adjustable Tension Band, and C3 penile compression device) with a control period of no device (Moore 2004). A randomised block assignment was used with a multiple period cross‐over design, so that each of the 12 participants had a control period of no device and three periods in which the different devices were used.
All external compression devices reduced the weight of urine lost on a four hour pad test compared to the control period (P < 0.05, Analysis 21.2), but none completely eliminated urine loss. Satisfaction was based on ease of application, comfort and efficacy. The device preferred by the largest number of men (Analysis 21.1) was also that with the lowest urine loss (the Cunningham clamp) (Analysis 21.2).
Adverse events
The Cunningham clamp was also the device with the greatest reduction in systolic blood flow velocity (P < 0.05 versus control period, Analysis 21.3; Analysis 21.4), raising the possibility of safety issues if applied too tightly. In the trial, men were able to judge when to release the device. The authors recommended that its use should therefore be limited to men who were cognitively intact, aware of bladder filling, had normal genital sensation and intact penile skin, and had sufficient manual dexterity to open and close the device (Moore 2004).
In another trial with no useable data (Fader 2013), men provided qualitative information which suggested that pads were most highly rated compared with sheath catheters (P = 0.31), clamps (P < 0.01) and the body‐worn urinal (P < 0.001). The clamp was rated as more secure, less leaky and less restrictive on clothing choice than the others (P < 0.05) but was more painful than the rest (P < 0.002).
Discussion
This review incorporates a broad array of possible interventions under the umbrella term of conservative management of postprostatectomy UI. The populations studied included men undergoing prostatectomy for both benign (TURP) and malignant (radical prostatectomy) disease. The interventions were delivered pre‐operatively, post‐operatively or both. In some trials all the men were incontinent at baseline, while at least some were dry in other trials which recruited all men having surgery (these were classed as prevention of incontinence trials). Seven trials (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007) included men who had been incontinent for a considerable time after surgery while the rest recruited men around the time of surgery. More recent trials have focused on the pre‐operative or post‐operative period immediately after catheter removal. It is acknowledged that UI after prostatectomy will resolve over time in many men.
Conservative interventions tend to be resource‐intensive strategies that require people, equipment and clinic space, so administrators will look for evidence of efficacy. Funding has been an issue given the inconclusive nature of the evidence to date. For example, in the United States, the centres for both Medicare and Medicaid services have considered whether to withdraw funding for biofeedback and pelvic floor electrical stimulation (ES) in the treatment of UI of any etiology based on a lack of evidence regarding effectiveness. Through a lobbying effort from service providers and manufacturers, these modalities continued to be covered in the United States (Thompson 2002). However, as controversy about funding is likely to continue, there is a need for continued research in the area to determine which groups of patients are most likely to benefit from conservative interventions.
The findings of this review should continue to be treated with caution. The effectiveness of conservative measures in the longer term or in men with persistent UI remain inconclusive.
Summary of main results
Fifty trials met the inclusion criteria, 45 trials amongst men after radical prostatectomy, four trials after TURP, and one small trial which included one man with benign disease but was classed as a radical prostatectomy trial. There was considerable variation in the interventions, populations and outcome measures. Given this clinical heterogeneity it was decided to differentiate the trials and the comparisons, by type of surgery (TURP or radical prostatectomy) and by whether the intervention was partly preventative (in that not all men were incontinent, for example if all men before or after surgery were recruited, N = 27 trials), for treatment only (when all included men were incontinent at baseline, N = 23 trials) or for containment (external penile compression devices, N = 2 trials). Although the International Prostate Score (IPSS) was used in many of the trials, the authors felt that this questionnaire did not assess UI and therefore was not included in the outcome of quality of life.
Treatment trials for urinary incontinence after radical prostatectomy
Twenty‐one trials investigated the effects of PFMT versus no treatment or a variety of other means of stimulating the pelvic floor muscles. There was considerable clinical and statistical heterogeneity in the populations and the timing and frequency of the interventions, hence a random‐effects model was chosen for most of the comparisons where meta‐analysis was possible. Only two trials (Manassero 2007; van Kampen 1998) showed a statistically significant benefit from active treatment versus no treatment control groups (at 12 months and within 6 to 12 months respectively), and the other trials showed conflicting results. There was differential dropout from the control group in the Manassero 2007 trial (these men were assumed to be dry for analysis purposes). Additionally, men in the experimental group in the van Kampen 1998 trial received one session of PFMT in hospital before discharge and were then seen by a physiotherapist for one to two weeks, whereas those in the Manassero 2007 trial were taught PFMT by two urologists using verbal feedback and instructed to perform contractions at home. Because of the heterogeneity a random‐effects model was used, which led to wider confidence intervals (CIs).
Overall, there was not enough evidence to say whether or not PFMT with or without biofeedback was effective as the CIs were wide (for example number of men with incontinence in the intervention groups 193/339 (57%) versus 203/326 (62%) in the control groups, RR for incontinence after 12 months 0.85, 95% CI 0.60 to 1.22, Analysis 1.1.4).
The meta‐analysis was dominated by the Glazener RP 2011 trial, which was a large pragmatic multi‐centre trial conducted in a context where information on PFMT was widely available. This showed no good evidence to support one‐to‐one training by a therapist (for example RR for UI after 12 months 0.98, 95% CI 0.87 to 1.09, Analysis 1.1.4) (Glazener RP 2011). This one large trial had narrow CIswhich did not include a clinically significant difference, pre‐specified to be 15%. The only other large trial (Moore 2008) was in line with the Glazener RP 2011 findings but with wider CIs (RR 1.02, 95% CI 0.70 to 1.48, Analysis 1.1.4) (Moore 2008) despite a more intensive intervention. While men in the Glazener RP 2011 trial had four therapy sessions over three months, in the Moore 2008 trial men were seen weekly for up to six months. The findings in these two trials concurred despite different intensities of intervention, and the quality of evidence for this GRADE‐specific outcome was moderate. Data from quality of life measures and use of pads and pad tests supported the finding of no differences between intervention and control groups.
Three small trials provided data and the meta‐analysis suggested that ES was better than control interventions in terms of less incontinence, regaining continence more quickly and better quality of life, at least in the short term up to six months. The quality of evidence was deemed to be moderate, however less information was available for the longer term.
Individual small trials provided data to suggest that extra‐corporeal magnetic innervation (ExMI) or combinations of treatments might be beneficial but the evidence was limited.
Prevention trials for urinary incontinence after radical prostatectomy
Nineteen trials, some of which enrolled men before surgery and others all men as soon as the catheter was removed, included a mixed population of men with and without incontinence after surgery. Again a random‐effects model was chosen to compensate for the considerable clinical and statistical heterogeneity between the trials. Including the information from the quasi‐randomised trial (Filocamo 2005), the chance of incontinence appeared to be lower in the intervention groups in two trials with data after 12 months. The quality of evidence was judged to be moderate (number of men with UI after one year 10.2% versus 32.1% in the control groups, RR 0.32, 95% 0.20 to 0.51, Analysis 6.1.4).
The meta‐analysis of prevention trials included a number of small trials with wide CIs apart from Filocamo 2005, which was out of line with the others. This was the only large trial to favour the intervention group. The worry is that this trial may have been biased due to a lack of reporting on concealment of allocation.
One small trial (Ribeiro 2008) suggested that men were more likely to be carrying out PFMT, at least soon after the intervention (Analysis 6.9), though this was not reflected in significant differences in higher anal squeeze pressures (Analysis 6.8). Another trial of anal ES was too small to be conclusive (Laurienzo 2013). One small trial (Mariotti 2009) reported that adding anal ES and biofeedback to PFMT was beneficial. One further small trial (Wille 2003) found that PFMT plus anal ES with and without extra biofeedback were both better than PFMT alone at six months, but there was little to choose between the two more intensive interventions (Analysis 10.8). Tienforti 2012 found that pre‐operative PFMT was associated with a reduction in number of incontinence episodes per day, but this was a small trial and larger sample sizes would be needed to draw reliable conclusions.
Nine trials compared one active treatment with another active treatment. Overall there did not seem to be one intervention that proved to be statistically significantly better than another.
Treatment trials for urinary incontinence after TURP
One large trial addressed this comparison (Glazener TURP 2011), comparing four sessions of one‐to‐one therapy with standard management in a context where information about PFMT was widely available. The quality of evidence for the number of incontinent men was moderate but there were no differences between the groups in any of the outcome measures except for performance of PFMT, suggesting that the intervention had changed behaviour but not incontinence or other clinical outcomes.
Prevention trials for urinary incontinence after TURP
Three small trials enrolled men before TURP to receive a minimal PFMT intervention before and after surgery. There were no statistically significant differences in terms of number of incontinent men between the groups but the quality of evidence was deemed to be very low (Analysis 16.1).
Containment of urinary incontinence
One alternative intervention, a clamp fitted to the shaft of the penis, can be used to control unwanted leakage. Men in one trial reported a preference for one type of external compression device compared to two others or no treatment; a Cunningham clamp proved satisfactory to 10 of 12 men with intractable UI (Moore 2004). This may be a viable alternative for some cognitively capable men providing they take into account safety issues such as adequate sensation and the ability to remove the device when it feels too tight or the bladder is full. Another trial which compared pads, sheath catheters, body‐worn urinals and clamps also reported that men found the clamps most effective but painful (Fader 2013).
Men whose incontinence cannot be otherwise controlled can use absorbent pads (Fader 2007; Fader 2008) or a variety of external sheath devices with leg bags. An alternative is an indwelling urinary catheter (Jahn 2007; Moore 2007; Niël‐Weise 2005).
Lifestyle changes
The effect of other conservative interventions such as lifestyle changes remains undetermined as no trials involving these interventions were identified.
Overall completeness and applicability of evidence
Few trials used the primary outcomes of interest, patient reported symptoms and the standardised pad test. Most used a variety of subjective outcomes derived from patient reported symptoms to define continence. There were no trials which examined lifestyle adjustments in alleviating UI after prostatectomy.
Attrition bias may have played a role in the results of some of the included trials and therefore affected the outcome of this review. One of the smaller trials (Franke 1998) lost half of the randomised participants by the end of the data collection period. Although most of those trials that lost participants provided an explanation of these losses, none accounted for the missing data in their primary analyses. The intention‐to‐treat principle mandates, at minimum, that patients stay in the group to which they are randomised (Juni 2001), which the included trials appeared to do. It is also suggested that primary outcomes for all patients randomised to groups should be recorded or estimated if not available. Three of the included trials (Filocamo 2005; Moore 2008; Parekh 2003) reported an analysis using the intention‐to‐treat principle, and one trial (Burgio 2006) used survivor analysis in the original trial analysis. In one trial where there was clear evidence of differential dropout (Manassero 2007), the review authors elected to assume that the men whose data were missing were continent. However, attrition bias may have affected a number of the other trials which did not present relevant data or discuss the issue.
In 21 trials in this review, men who were all incontinent were analysed together. However, in seven of these trials (Goode 2009; Joseph 2000; Moore 1999; Moore 2004; Opsomer 1994; Seleme 2008; Zhang 2007) men had longstanding or persistent incontinence. It is possible that they might respond differently to the interventions compared to men recruited around the time of prostate surgery.
Quality of the evidence
Trial quality and methodological assessment
The quality of the estimated treatment effect of any intervention is determined partly by methodological assessment. Methodological flaws within the included trials of this review were assessed using the reports of the trials and therefore were reliant on the quality of reporting. Data were not available in all the trials for many of the pre‐stated outcomes. CIs tended to be wide except for the more recent large trials, and it was difficult to reliably identify or rule out a useful effect.
All trials claimed to be randomised, but only 24 out of 50 trials provided details of adequate sequence generation (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Filocamo 2005; Fode 2014; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Laurienzo 2013; Manassero 2007; Mathewson‐Chapman 97; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Robinson 2009; Tibaek 2007; Tienforti 2012; van Kampen 1998; Yamanishi 2006). Only 20 of the 50 trials provided details of adequate concealment of randomisation (Ahmed 2012; Burgio 2006; Centemero 2009; Dijkstra‐Eshuis 2013; Dubbelman 2004; Fode 2014; Geraerts 2013; Glazener RP 2011; Glazener TURP 2011; Goode 2009; Manassero 2007; Moore 1999; Moore 2004; Moore 2008; Overgard 2008; Park 2012; Robinson 2008; Tibaek 2007; van Kampen 1998; Yamanishi 2006) and were subsequently judged to be at low risk of selection bias. Additionally, blinding to PFMT was not possible, and blinding of outcome assessment appeared to be absent in many trials as it was not discussed. Therefore, many trials were judged to be at high risk of performance and detection bias.
The quality of the evidence was downgraded for the following.
-
Study design i.e. there was evidence of methodological flaws in the study design.
-
Indirectness i.e. a surrogate outcome was selected when a GRADE‐specific outcome was not reported.
-
Inconsistency, when there was evidence of statistical (either clinical or methodological) heterogeneity.
-
Imprecision, when the CIwas wide and crossed the line of no effect.
-
Publication bias. We planned to use funnel plot for publication bias, however, there were fewer than 10 trials in the meta‐analysis and the funnel plot could not be used.
The quality of the evidence for the critical outcomes ranged from moderate to very low, as evident in the summary of findings tables.
Potential biases in the review process
All relevant databases were searched and no language restriction was imposed during the search process, which enabled as many potentially eligible trials as possible to be included. Some reports of trials may not be published and therefore the full extent of the data may not have been obtained. One of the review authors was involved in four of the included trials and another review author was involved in two of the included trials. In order to account for this potential bias in the review process, data extraction and risk of bias assessment were performed by two independent review authors, one of whom was not involved in any of the included trials.
Agreements and disagreements with other studies or reviews
A systematic review conducted by Macdonald et al (MacDonald 2007) was identified which addressed conservative management of post‐prostatectomy urinary incontinence. Macdonald and colleagues included 11 trials (Bales 2000; Burgio 2006; Filocamo 2005; Floratos 2002; Franke 1998; Mathewson‐Chapman 97; Moore 1999; Parekh 2003; van Kampen 1998; Wille 2003; Yokoyama 2004), all of which were included in this review. They did not distinguish between treatment and prevention trials. Macdonald and colleagues' review analysed PFMT and PFMT with biofeedback, focusing on any additional benefit from biofeedback. The authors concluded that the use of guided PFMT was associated with superior patient outcomes compared with no treatment, which differs from the findings of this review. The Macdonald and colleagues review did not include more recent trials because the MEDLINE search only included trials up to 2006. Additionally, the conclusions made in Macdonald's review may have differed because the authors did not utilise the GRADE approach, suggesting the quality of the evidence was not assessed.
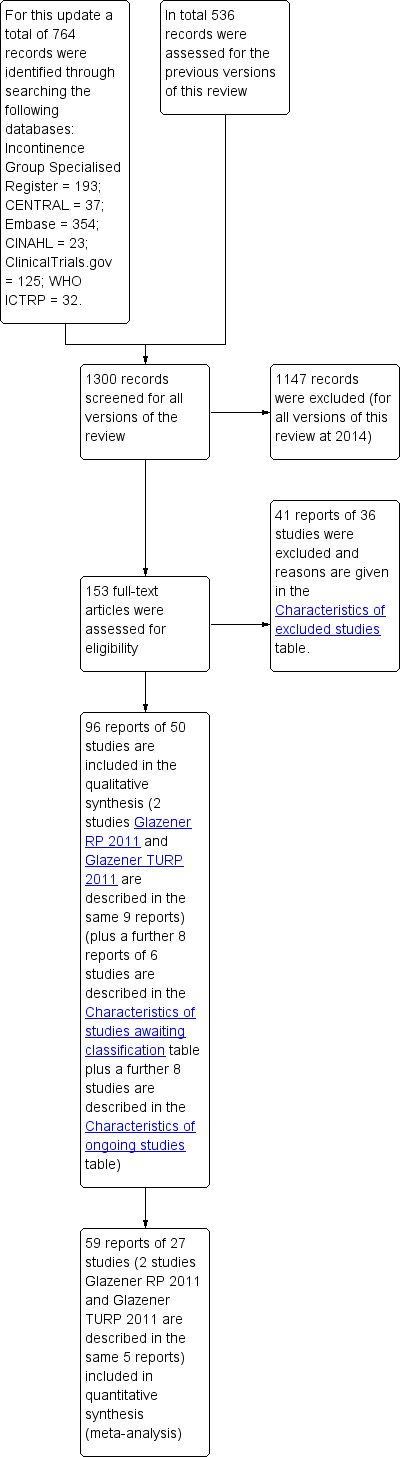
PRISMA study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
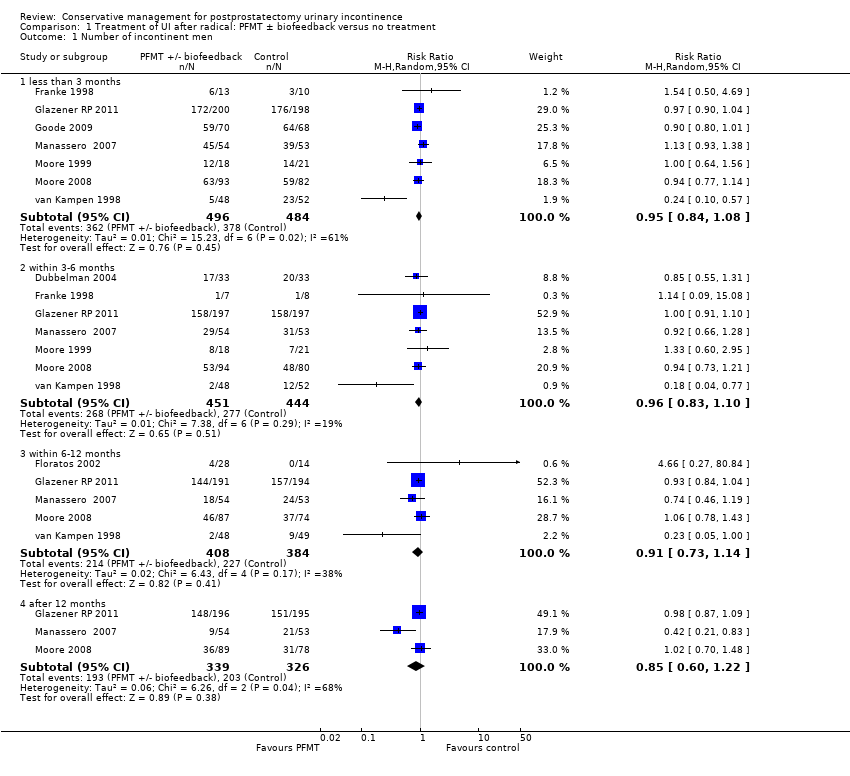
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.
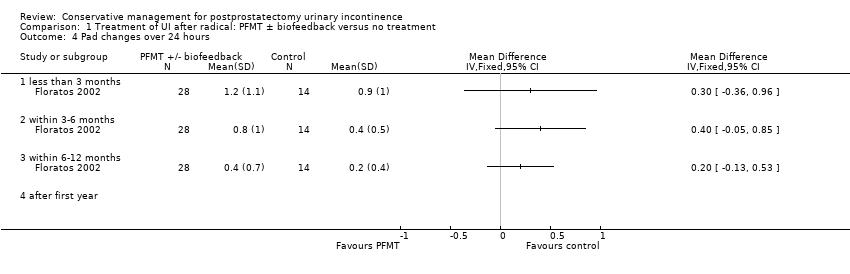
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 Pad changes over 24 hours.
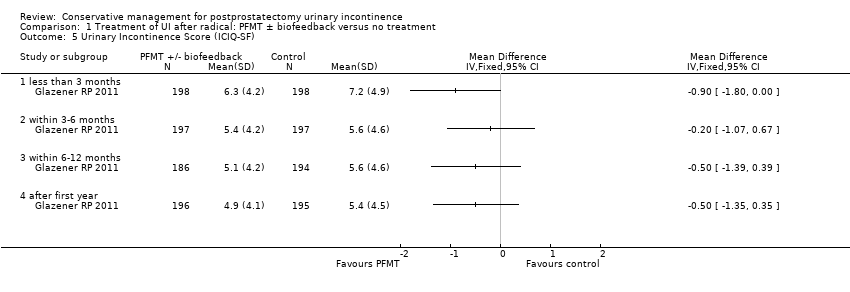
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Urinary Incontinence Score (ICIQ‐SF).
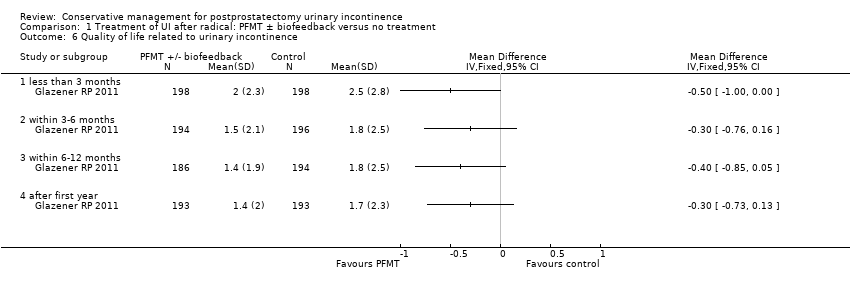
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Quality of life related to urinary incontinence.
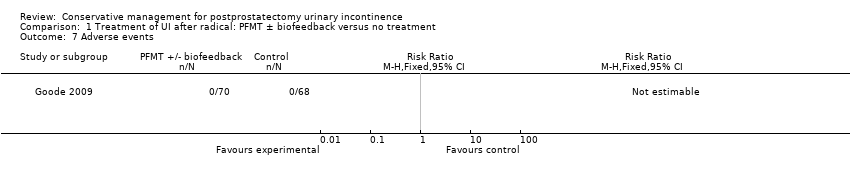
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Adverse events.
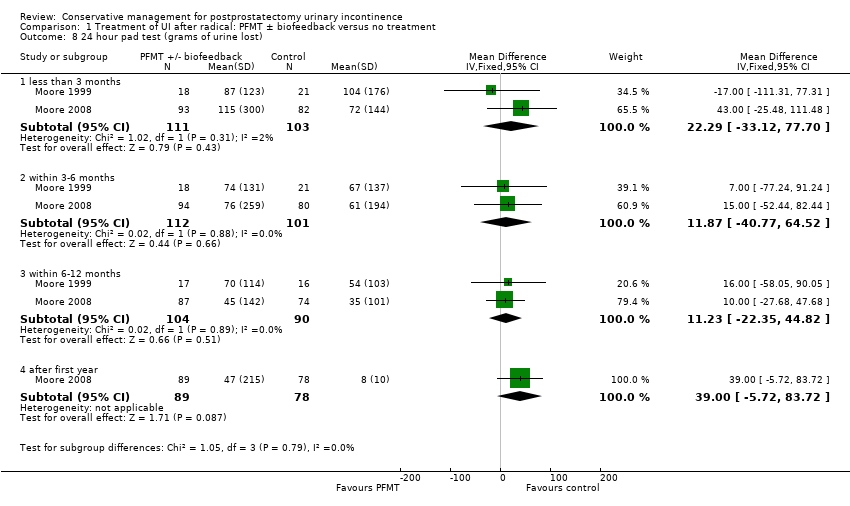
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 24 hour pad test (grams of urine lost).

Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 1 hour pad test (grams of urine lost).
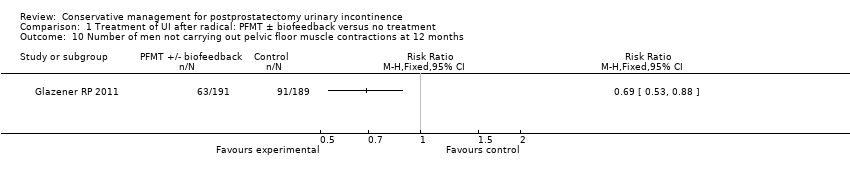
Comparison 1 Treatment of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men not carrying out pelvic floor muscle contractions at 12 months.

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 Number of incontinent men.

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 Adverse effects.
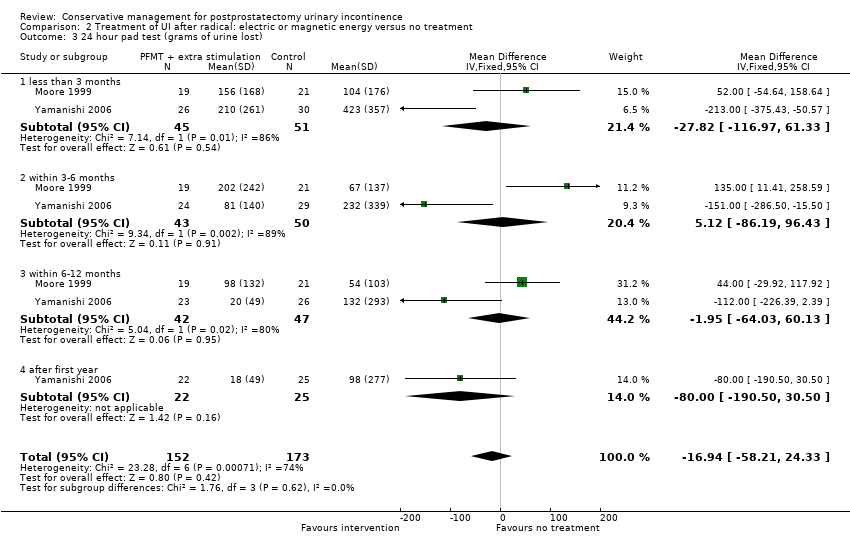
Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 3 24 hour pad test (grams of urine lost).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 4 Urinary Incontinence Score (ICIQ‐short form UI score).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form).

Comparison 2 Treatment of UI after radical: electric or magnetic energy versus no treatment, Outcome 6 Time until continent (months).

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men at < 3 months.

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 3‐6 months.
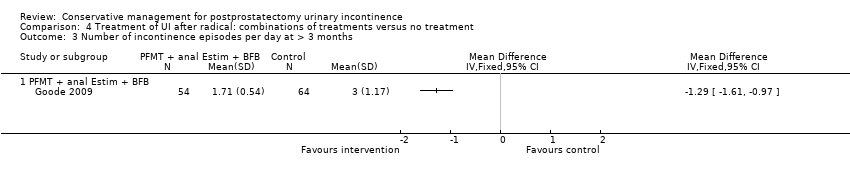
Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 3 Number of incontinence episodes per day at > 3 months.

Comparison 4 Treatment of UI after radical: combinations of treatments versus no treatment, Outcome 4 Adverse effects.
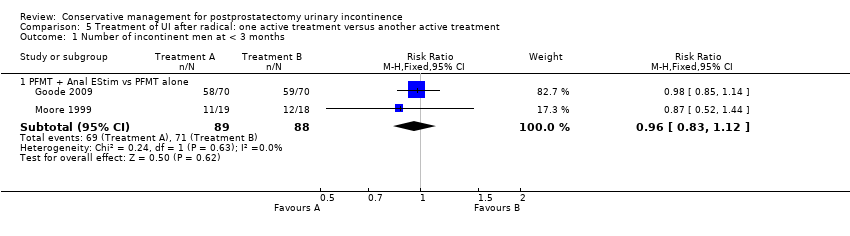
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
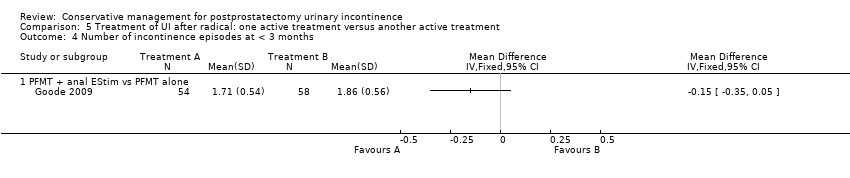
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinence episodes at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 5 Quality of Life Score (severity of UI) within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 6 Quality of Life Score (I‐QoL) within 6‐12 months.
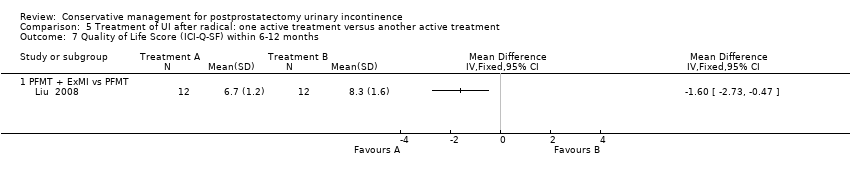
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months.
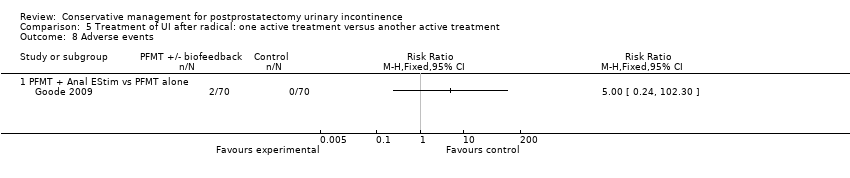
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 8 Adverse events.
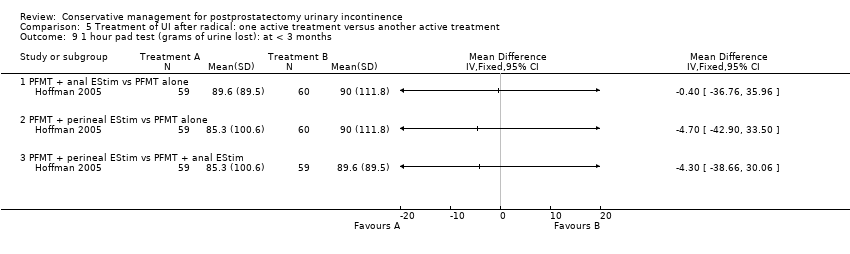
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost): at < 3 months.
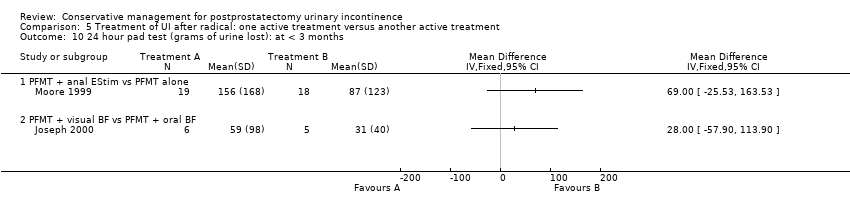
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 10 24 hour pad test (grams of urine lost): at < 3 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 11 24 hour pad test (grams of urine lost): within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost): within 3 to 6 months.
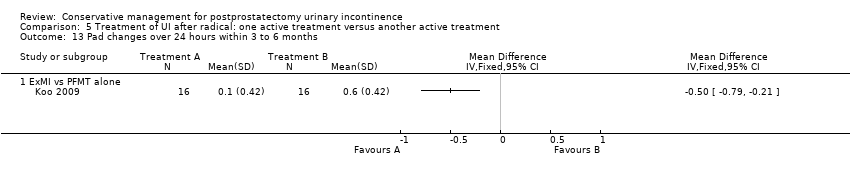
Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 13 Pad changes over 24 hours within 3 to 6 months.

Comparison 5 Treatment of UI after radical: one active treatment versus another active treatment, Outcome 14 Number of men not carrying out sufficient PFMT within 3 to 6 months.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 2 Pad changes over 24 hours.
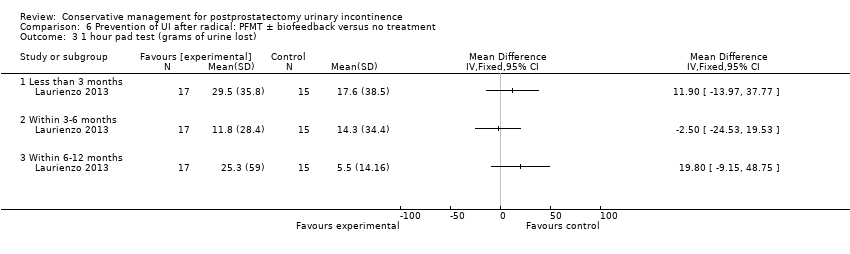
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 3 1 hour pad test (grams of urine lost).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 4 24 hour pad test (gm/24hrs).
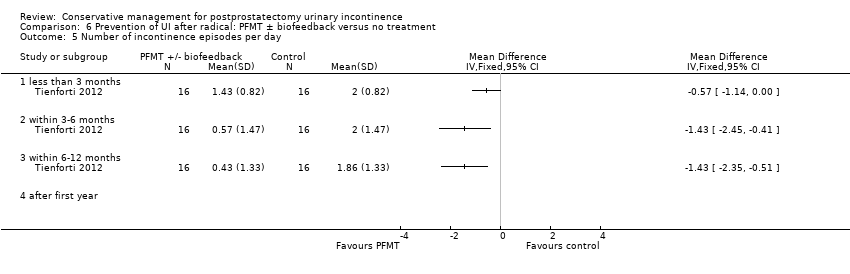
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 5 Number of incontinence episodes per day.
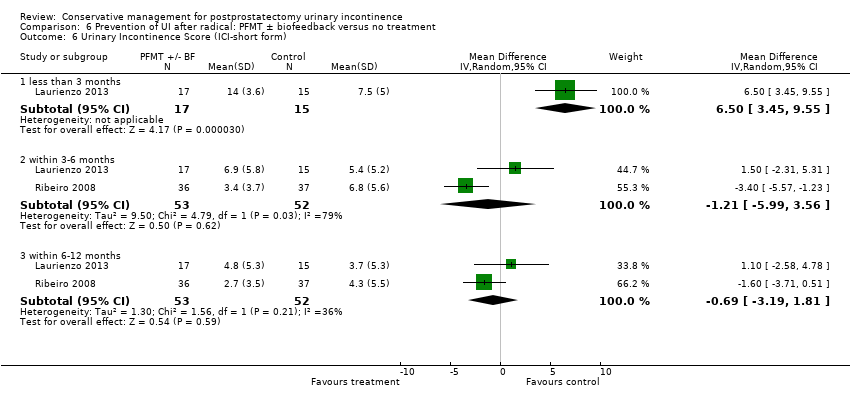
Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 6 Urinary Incontinence Score (ICI‐short form).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 7 Quality of Life Score (IIQ).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O).

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 9 Number of men not carrying out sufficient PFMT.

Comparison 6 Prevention of UI after radical: PFMT ± biofeedback versus no treatment, Outcome 10 Number of men having surgery for incontinence.
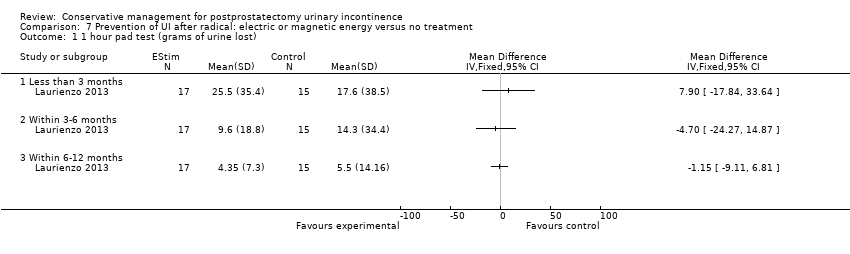
Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 1 1 hour pad test (grams of urine lost).

Comparison 7 Prevention of UI after radical: electric or magnetic energy versus no treatment, Outcome 2 ICIQ‐SF score.
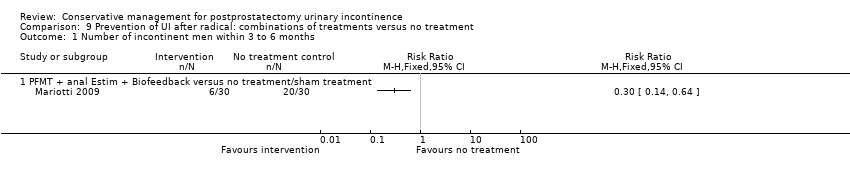
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 1 Number of incontinent men within 3 to 6 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 2 Number of incontinent men within 6 to 12 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 3 24 hour pad test (grams of urine lost) within 3 to 6 months.
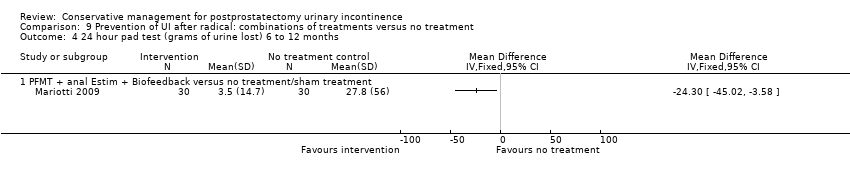
Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 4 24 hour pad test (grams of urine lost) 6 to 12 months.

Comparison 9 Prevention of UI after radical: combinations of treatments versus no treatment, Outcome 5 Time until continent (months).

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 1 Number of incontinent men at < 3months.
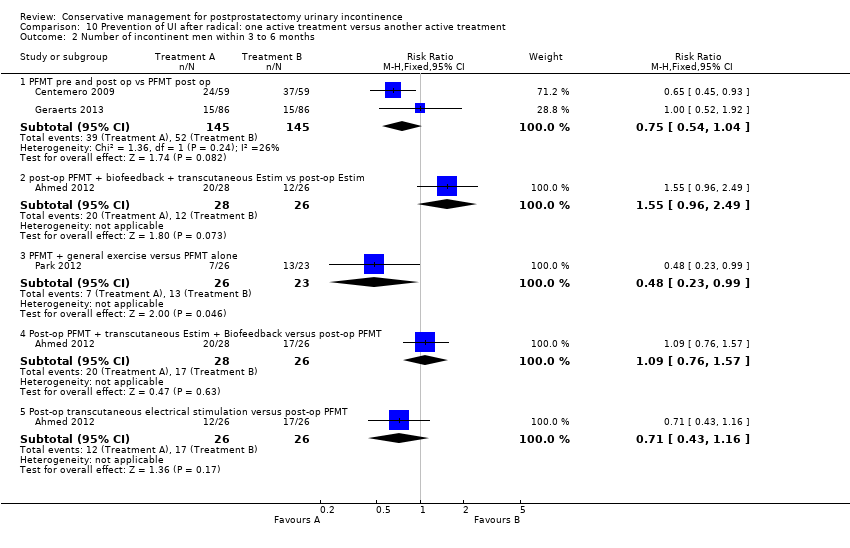
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 2 Number of incontinent men within 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 3 Number of incontinent men within 6 to 12 months.
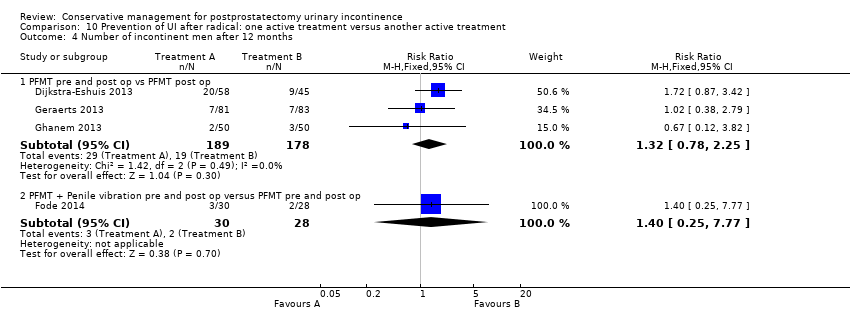
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 4 Number of incontinent men after 12 months.
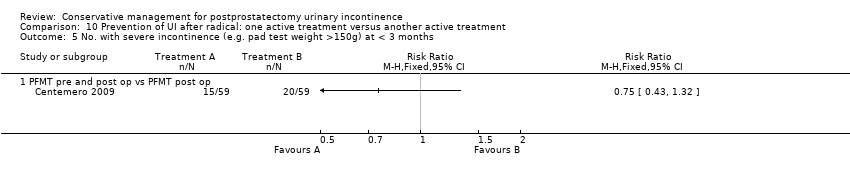
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months.
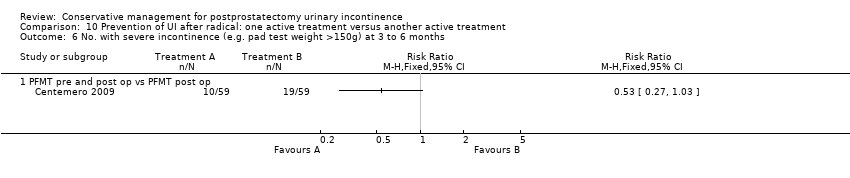
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 7 20 minute pad test (grams of urine lost): within 3 to 6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 8 20 minute pad test (grams of urine lost): within 6 to 12 months.
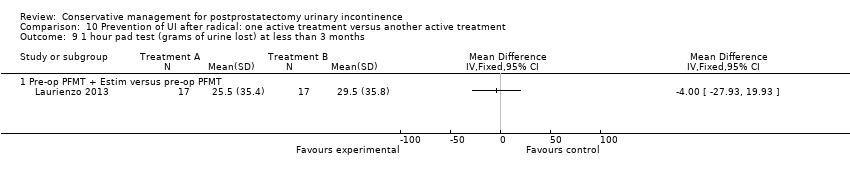
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 9 1 hour pad test (grams of urine lost) at less than 3 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 10 1 hour pad test (grams of urine lost) within 3‐6 months.
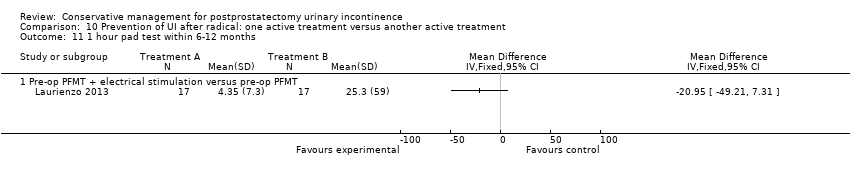
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 11 1 hour pad test within 6‐12 months.
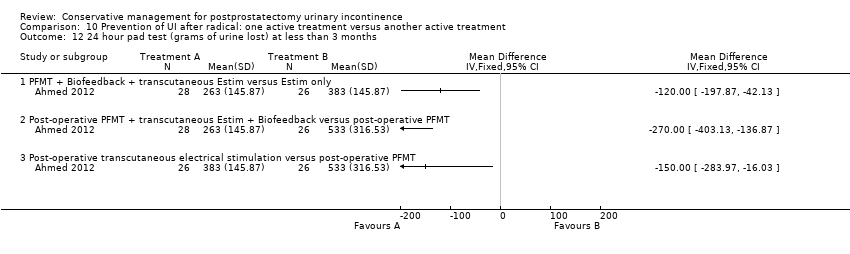
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 12 24 hour pad test (grams of urine lost) at less than 3 months.
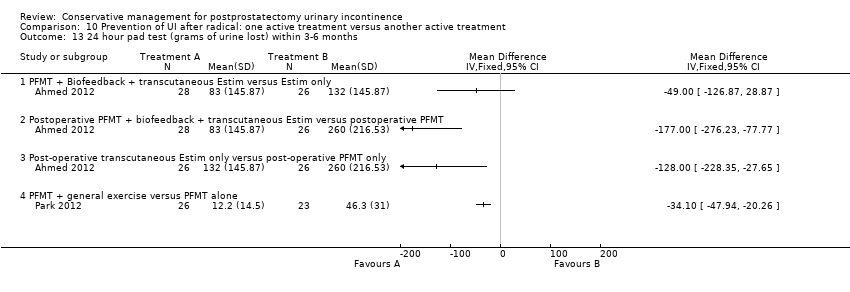
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 13 24 hour pad test (grams of urine lost) within 3‐6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 14 24 hour pad test (grams of urine lost) within 6‐12 months.
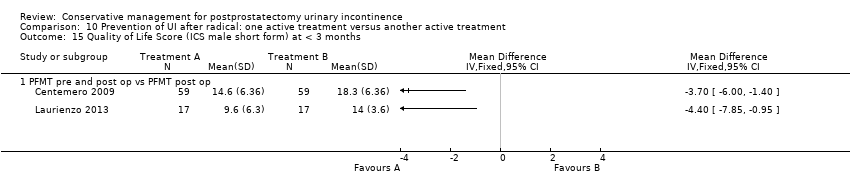
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 15 Quality of Life Score (ICS male short form) at < 3 months.
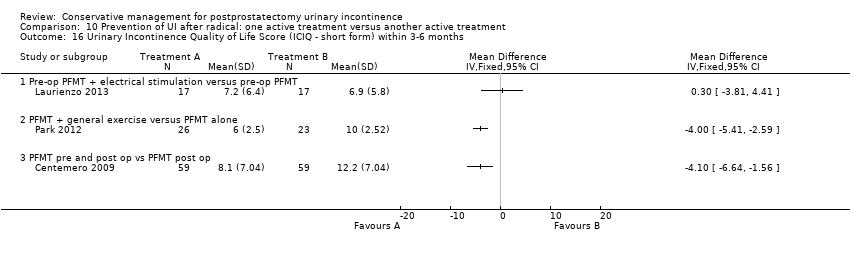
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months.

Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months.
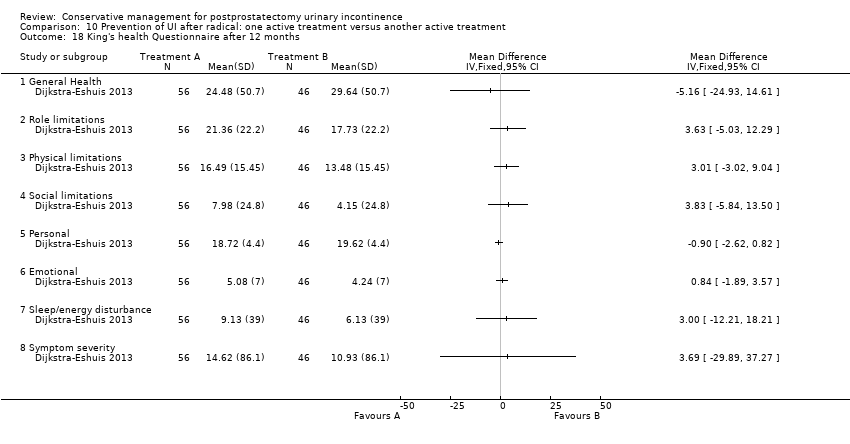
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 18 King's health Questionnaire after 12 months.
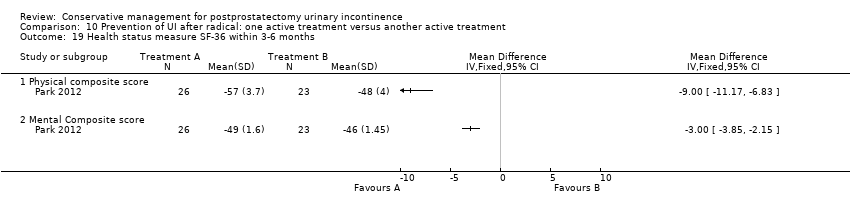
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 19 Health status measure SF‐36 within 3‐6 months.
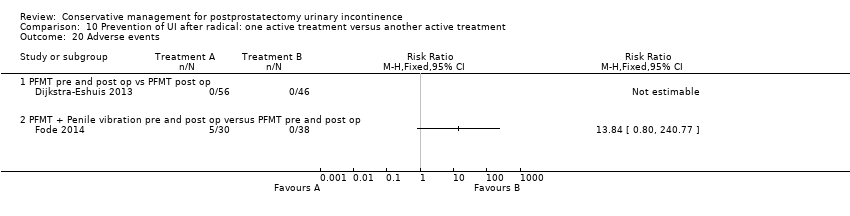
Comparison 10 Prevention of UI after radical: one active treatment versus another active treatment, Outcome 20 Adverse events.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 2 Number of incontinence episodes per day.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 3 Number of men using pads.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 4 Urinary Incontinence Score (ICI‐short form).

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 5 Quality of life related to urinary incontinence.

Comparison 11 Treatment of UI after TURP: PFMT ± biofeedback versus no treatment, Outcome 6 Number of men not carrying out pelvic floor muscle contractions at 12 months.
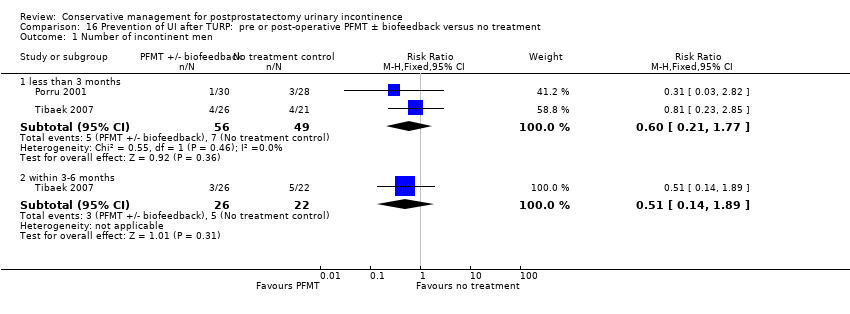
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 1 Number of incontinent men.
Comparison 16 Prevention of UI after TURP: pre or post‐operative PFMT ± biofeedback versus no treatment, Outcome 2 Health status measure SF‐36 within 3‐6 months.
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 0/12 | 0/12 | 2/12 | 10/12 |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 1 Number of men satisfied with device.
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | 122.8 gm (SD 130.8) | 53.3 gm (SD 65.7) | 32.3 gm (SD 24.3) | 17.1 gm (SD 21.3) |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 2 Mean urine loss (grams of urine on pad test).
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men | N=12 men | N=12 men | N=12 men |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 3 Penile Doppler blood flow (mean systolic velocity).
| Study | Control (no device) | U‐Tex | C3 | Cunningham |
| Moore 2004 | N=12 men | N=12 men | N=12 men | N=12 men |
Comparison 21 Containment of urinary incontinence from any cause: external penile compression devices (penile clamps) versus no treatment, Outcome 4 Penile Doppler blood flow (mean resistence to flow index).
| Treatment of UI after radical: PFMT ±biofeedback versus no treatment; for postprostatectomy urinary incontinence | ||||||
| Patient or population: patients with postprostatectomy urinary incontinence | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 623 per 1000 | 529 per 1000 | RR 0.85 | 665 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICI‐SF) ‐ after first year | The mean urinary incontinence score (ici‐short form) ‐ after first year in the intervention groups was | 391 | ⊕⊕⊝⊝ | |||
| Adverse events | See comment | See comment | Not estimable | 138 | ⊕⊕⊕⊕ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide CI (0.60 to 1.22) | ||||||
| Treatment of UI after radical: electric or magnetic energy versus no treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men ‐ after 12 months | 63 per 1000 | 16 per 1000 | RR 0.26 | 413 | ⊕⊕⊕⊝ | |
| Urinary Incontinence Score (ICIQ‐SF UI score) ‐ after 12 months | The mean urinary incontinence score (iciq‐short form ui score) ‐ after 12 months in the intervention groups was | 47 | ⊕⊕⊝⊝ | |||
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) ‐ after 12 months | See comment | See comment | Not estimable | 47 | ⊕⊕⊝⊝ | |
| Adverse events | 133 per 1000 | 77 per 1000 | RR 0.58 | 56 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear is 1/2 trials taking part in the meta‐analysis | ||||||
| Treatment of UI after radical: combinations of treatments versus no treatment for postprostatectomy UI | ||||||
| Patient or population: patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: combinations of treatments versus no treatment | |||||
| Number of incontinent men with 3 to 6 months | 53 per 1000 | 150 per 1000 | RR 2.85 | 39 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Quality of Life Score (ICIQ‐SF) after 12 months | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events ‐ PFMT + anal EStim + BFB | 0 per 1000 | 0 per 1000 | RR 4.86 | 138 | ⊕⊕⊝⊝ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment unclear | ||||||
| Treatment of UI after radical: one active treatment versus another active treatment for postprostatectomy UI | ||||||
| Patient or population: Patients with postprostatectomy UI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Treatment of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men within 6 to 12 months ‐ FES versus ExMI | 83 per 1000 | 167 per 1000 | RR 2 | 24 | ⊕⊝⊝⊝ | |
| Quality of Life Score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT | The mean quality of life score (ICI‐Q‐SF) within 6 to 12 months ‐ PFMT + ExMI versus PFMT in the intervention groups was | 24 | ⊕⊕⊝⊝ | |||
| Adverse events PFMT + Anal EStim versus PFMT alone | 0 per 1000 | 0 per 1000 | RR 5 | 140 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Random sequence generation and allocation concealment is unclear | ||||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men ‐ after 12 months | 321 per 1000 | 103 per 1000 | RR 0.32 | 373 | ⊕⊕⊕⊝ | |
| Quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months | The mean quality of life score assessed using (ICI‐SF UI score) ‐ within 6 to 12 months in the intervention groups was | 105 | ⊕⊝⊝⊝ | |||
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear for Filocamo 2005 which contributes 84.2% weightage | ||||||
| Prevention of UI after radical: electric or magnetic energy versus no treatment for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after radical: electric or magnetic energy versus no treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of life score assessed using (ICIQ‐SF score) ‐ within 6 to 12 months | See comment | See comment | Not estimable | 32 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Prevention of UI after radical: combinations of treatments versus no treatment compared to for postprostatectomy UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Prevention of UI after radical: combinations of treatments versus no treatment | ||||||
| Number of incontinent men within 6 to 12 months ‐ PFMT + anal EStim + biofeedback versus no treatment | See comment | See comment | Not estimable | 60 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐ SF UI score) ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation and allocation concealment are both unclear | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT pre and post‐operation versus PFMT post‐operation) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT pre and post‐operation versus PFMT post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | See comment | See comment | Not estimable | 367 | ⊕⊕⊕⊝ | |
| Quality of Life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events | See comment | See comment | Not estimable | 102 | ⊕⊕⊕⊕ | |
| Economic Analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Sequence generation is unclear 2/3 trials and allocation concealment is unclear in 1/3 trials | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) for | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (PFMT + penile vibration pre and post‐operation versus PFMT pre and post‐operation) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months | 71 per 1000 | 100 per 1000 | RR 1.4 | 58 | ⊕⊕⊝⊝ | |
| Quality of life Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) | Study population | Not estimable | 0 | See comment | ||
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events | See comment | See comment | Not estimable | 68 | ⊕⊕⊝⊝ | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after radical: one active treatment versus another active treatment compared to (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) for UI | ||||||
| Patient or population: All men after radical prostatectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| (pre‐operative PFMT + electrical stimulation versus pre‐operative PFMT) | Prevention of UI after radical: one active treatment versus another active treatment | |||||
| Number of incontinent men after 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Quality of Life Score assessed using (ICIQ‐SF) within 6 to 12 months | See comment | See comment | Not estimable | 34 | ⊕⊝⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Allocation concealment is unclear | ||||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment compared to for UI | ||||||
| Patient or population: Men with UI after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Treatment of UI after TURP: PFMT ±biofeedback versus no treatment | ||||||
| Number of incontinent men‐ after 12 months | See comment | See comment | Not estimable | 1609 | ⊕⊕⊕⊝ | |
| Quality of life Score assessed using Score (ICIQ‐SF UI score) ‐ after 12 months | See comment | See comment | Not estimable | 397 | ⊕⊕⊝⊝ | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment for UI | ||||||
| Patient or population: All men after TURP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prevention of UI after TURP: pre or post‐operative PFMT ±biofeedback versus no treatment | |||||
| Number of incontinent men ‐ within 3 to 6 months | 227 per 1000 | 116 per 1000 | RR 0.51 | 48 | ⊕⊝⊝⊝ | |
| Urinary Incontinence Score assessed using (ICIQ‐SF) or (ICIQ‐SF UI score) at 12 months ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic analysis using QALY ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Not applicable | ||||||
| Study ID | Intervention | Control |
| A: At catheter removal received standard care of verbal and written instructions, instructed by physiotherapist to perform 3 sets of 15‐20 contractions daily, for a duration of 3‐5 seconds with a 6‐10 second rest period, encouraged to perform exercises before functional activities such as sneezing, coughing, or lifting weight, also in the supine position, sitting, squatting and going up and down stairs
B: ES, treatment started one week after catheter removal, patients received 15 minutes of twice weekly electrical stimulation for 12 weeks
C: PFMT + BFB + ES: Treatment started one week after catheter removal, patients received twice weekly treatment with 15 minutes of electrical stimulation and 15 minutes of biofeedback for 12 weeks, instructed to perform 3 series of 10 rapid contractions, 3 sustained contractions of 5, 7 or 10 seconds and then 10 contractions during prolonged expiration in the supine position
All patients were given a logbook to complete daily regarding self‐report of exercises | ||
| PFMT + biofeedback 45 minute session with nurse trained in biofeedback. Patients were instructed to perform graded PFMT. Contractions of 5‐10 seconds, 10‐15 repetitions were performed with biofeedback (surface electrodes used to measure muscle strength). Advised to practice the exercises 4 times per day until surgery | No biofeedback training Written and brief verbal instructions from a nurse on how to perform PFMT (isolate muscle that stops urine flow, practice 4 times per day, 10‐15 repetitions). | |
| PFMT + biofeedback Single session of biofeedback (rectal probe to measure intra‐abdominal rectal pressure and external anal sphincter contraction) assisted behavioural training. Feedback and verbal instruction used to teach control of pelvic muscles. Taught to contract sphincter during 2‐10 seconds periods separated by 2‐10 seconds of relaxation, dependent on ability. Written instructions for daily at home practice of 45 PFM exercises daily (3 sessions of 15 exercises each time). Additionally instructed to slow or interrupt voiding once daily. Encouraged to exercise daily preoperatively, then resume when catheter removed post‐operatively | Usual care of brief verbal instructions post operatively to interrupt the voiding stream plus any instruction from physician. | |
| Intervention A: PFMT both pre and post‐operatively. A structured PFMT program 30 and 15 days before surgery, previous physiotherapist evaluation to provide the patients with feedback about the quality of pelvic floor muscle function, PC teste (endurance and contraction quality), breathing coordination, typify muscle contraction as tonic and modify incorrect physical attitudes. This was also repeated after the procedure Intervention B: PFMT post‐operatively only | ||
| 30 mins of guided PFMT + biofeedback weekly for 4 weeks before surgery, received written instructions to: carry out two sets of 30 contractions during abdominal breathing, one breath between each contraction; restart PFMT after catheter removal (7‐10 days after surgery) All men were seen before surgery by a physiotherapist, who explained relevant anatomy, anal visual inspection and digital palpation, biofeedback registration with rectal probe, All patients received PFMT + biofeedback or electrical stimulation, or both, if still incontinent after 6 weeks | Received written instructions on PFMT after catheter removal (7‐10 days after surgery) | |
| Nine or less sessions of physiotherapy guided pelvic floor exercises after surgery | Exercise instruction through information folder | |
| Formal instruction (3 treatment sessions plus at home exercises) in PFMT using verbal explanation, palpation and visualization of the base of the penis with a mirror, in different positions and prior to sneezing, coughing or lifting | No formal instruction | |
| Initiated after catheter removal, 15 treatment sessions (3 times per week for 30 minutes) of PFMT with EMG (surface) biofeedback in clinic | Instruction with verbal feedback and an information pamphlet with instructions to perform PFMT 50‐100 times daily at home | |
| Pre‐operative session guided PFMT + instruction on how to use penile vibratory stimulation device. Instructed to stimulate frenulum once daily, 10 seconds of stimulation then 10 second pause, repeated 10 times for 1 week pre‐operatively, instructed to restart stimulation after catheter removal for 6 weeks All men were offered a PDE5 inhibitor after 1 month post‐operatively and also received telephone contact to ensure compliance with treatment | Preoperative session guided PFMT | |
| Biofeedback (perineal patch EMG) enhanced PFMT; exercise treatment sessions at 6, 7, 9, 11, and 16 weeks post‐operatively | No treatment. | |
| Intervention A: PFMT + biofeedback 30 mins of guided PFMT + biofeedback weekly for 3 weeks before surgery. Patients were instructed to carry out 60 contractions a day at home; contract their pelvic floor while coughing, and sitting down or getting up from a chair. Patients were also instructed to restart PFMT on day 4 after surgery while catheter was in situ Intervention B: Instructed to start PFMT on the day after catheter removal (e.g. 2‐3 weeks after surgery) All men: Received weekly individual guided exercise programme with digital or EMG biofeedback after surgery. Advice was given on how to contract pelvic floor muscles to prevent leakage during functional activities. When patients carried out the instructed 60 contractions, they were asked to colour in three squares in their diary to assess compliance | ||
| Pre‐operative PFMT for 2 weeks + postoperative PFMT programme | Postoperative PFMT programme only | |
| Intervention A: Behavioural therapy with PFMT for 8 weeks Intervention B: Behavioural therapy with biofeedback and electrical stimulation for 8 weeks Behavioural therapy consisted of pelvic floor muscle exercises and bladder control strategies in both groups | No treatment | |
| Intervention A: perineal EStim plus physiotherapy (PFMT) Intervention B: anal EStim plus physiotherapy (PFMT) | PFMT alone | |
| Guided PFMT + biofeedback after catheter removal (2 days post‐operatively), instructed to: contract pelvic muscles for 5 seconds and relax for 10 seconds. After discharge, patients were instructed to carry out 5 mins of each PFE three times daily. Patients also received motivational telephone interviews once weekly | No description | |
| Intervention A: Instruction in PFMT including biofeedback with visual feedback as well as verbal to assist in identifying and discriminating muscles Intervention B: Instruction in PFMT, squeezing of finger during digital rectal examination | ||
| ExMI, treatment sessions were for 20 minutes twice weekly for 8 weeks | PFMT alone | |
| A (15): Standard treatment with verbal instructions for PFMT B (17): Pre‐operative guided PFMT, with 10 physiotherapy sessions: contractions of the pelvic floor muscles for 5 seconds in “dorsal decubitus” position for 10 times, in the same position with the waist elevated (10 times), lying down with legs adducted against a plastic ball performed 10 times and standing and flexing the hips to 60̊ (10 times) C (17): Pre‐operative PFMT + ES during 10 physiotherapy sessions, ES was with an anal probe lasting 15 minutes in total, and men also received guided PFMT and followed the same training regime as above Men did not receive treatment post‐operatively | Instructed to start PFMT at home 15 weeks before surgery. | |
| Extra‐corporeal magnetic innervation (ExMI), the frequency of the pulse field was 10Hz for 10 minutes, followed by a 3 minute rest and a second treatment of 50 Hz for 20 minutes. This was done twice a week | PFMT alone, instructions given to carry out 20mins x 3 a day. | |
| PFMT re‐education program, verbal feedback The training program involved active PFE. verbal feedback of the contraction was used to instruct the patients to correctly and selectively contract their pelvic muscles while relaxing the abdominal muscles. the strength of the pelvic floor muscles was measured by digital anal control using a score of 0 to 5 ( 0 = no contraction, 5 = good contraction against strong resistance) Initially home practice comprised 45 contractions (3 sessions of 15) per day at home, progressively increasing the number until 90 per day. This was taught by two experienced urologists | No treatment. | |
| Guided PFMT + biofeedback during first session, second session involved 10 sets of pelvic floor electrical stimulation lasting 15 mins each, instructed to: carry out three sets of 30 contractions a day at home for the first month after catheter removal (16 days after surgery) All men received oral and written information on pelvic floor anatomy and on PFME, pelvic floor muscle endurance assessed by digital anal control | Received oral and written information on pelvic floor anatomy and on PFME, instructed to: perform 30 contractions a day at home for the first month after catheter removal (16 days after surgery) | |
| PFMT plus ES and biofeedback twice a week for 6 weeks ES ‐ a surface electrodes was inserted into the anus and pulsed, the intensity was adequate to induce visual lifting of the levator ani and pubococcygeus muscle, considering the level of comfort to the patient Biofeedback ‐ via surface electrodes both perineal and abdominally | Instructions to conduct PFMT ‐ verbal and written instructions at catheter removal and follow up visits. | |
| PFMT: 5 sessions of guided PFMT for 2‐3 weeks pre‐operatively and continued post‐operatively All men underwent clinical examination of pelvic muscles function using digital perineal testing according to “AIPDA score” and evaluation of voiding symptoms | Postoperative standard care, written instructions for PFMT | |
| Pre‐operatively received further instruction and practice with PME protocol Home exercises and biofeedback (anal probe) (Incare 8900); practiced at home 3 times a week, starting with daily 15 PFMT and increasing by 10 every 4 weeks to a maximum of 35 PFMT. | Post‐operatively no further interventions until week 5 when pelvic muscle strength was assessed. | |
| Intervention A: PFMT alone Intervention B: PFMT plus rectal ES treated by one physiotherapist 30 minutes twice a week for 12 weeks Both included home exercises 3x/day gradually working up to 30 minutes per session lying, standing, sitting; strength, endurance, speed and control with maximum contractions of 5‐10 seconds, 10‐20 second relaxation and 12‐20 repetitions; submaximum contractions at 65‐75% of maximum strength with hold 20‐30 seconds and equal rest time, 8‐10 repetitions; speed was sets of quick repetitive contractions in a 10 second time span; control involved gradual recruitment to maximum contraction in 3 stages with 5 second hold at each stage and a slow release with rest 15‐30 seconds | oral and written information about PFMT pre and post‐ operatively (standard treatment) | |
| Each participant had 4 periods (each lasted 1 day) | ||
| Maximum 24 weekly, 30‐minute treatment protocol (30 min biofeedback‐assisted PFMT) and home exercise protocol of 2‐3 times a day | Verbal and written information on PFME and weekly telephone contact by a urology nurse | |
| PFMT + sacral surface therapeutic electrical stimulation (ssTES), ssTES 2x a day for 15 minutes each, lasting 1 month after catheter removal (day 5) | PFME only, carried out alone | |
| Extra‐corporeal magnetic innervation (EXMI) based pelvic floor device | PFMT alone | |
| PFMT plus biofeedback plus electrical stimulation directed by physiotherapist | PFMT on their own without medical supervision. | |
| Instructions on PFMT and physiotherapy, 45 minutes weekly Patients were instructed to perform 3 sets of contractions daily at home, in either a supine, sitting or standing position. Digital anal palpation to teach correct contractions, as well as oral and written instructions DVD of instructions given to those living too far from hospital | Instructions on PFMT alone. | |
| Two treatment sessions preoperatively. Session 1 consisted of PFMT in a hook lying position | No formal education on PFMT pre‐operatively, telephone or face to face follow‐up at least monthly. | |
| Patients performed Kegel exercises together with other types of exercises which included resistance training and pelvic flexibility. The intervention started 3 weeks after surgery and lasted 12 weeks Details of the combined exercise regime: Post‐operative weeks 1‐4 1) Education about postoperative symptoms 2) Performing Kegel exercises, recognizing the parapelvic muscles 3) Pelvic floor flexibility fitness: performing pelvic exercises while sitting on a ball Post‐operative weeks 5‐8 (ball exercises) 1) Performing pelvic exercises while sitting on a ball 2) Performing lower extremity exercises while placing a ball on the wall 3) Lifting a heel on the ball while standing face‐to‐face with the wall 4) Lifting up and down on the ball while spreading and bending legs 5) Performing flank exercises while having a ball in the hand 6) Squeezing the ball with the adductor muscles while lying on a table Post‐operative weeks 9‐12 (elastic band exercises) 1) Lifting the object with an elastic band lateral, anterior, and posterior to the patient’s arms 2) Lifting the legs and then spreading them while attaching an elastic band to the foot | In the control group, only Kegel exercises were performed | |
| Early pelvic floor rehabilitation program at home twice dally, Kegel exercises | No formal PFMT | |
| Initial visit before surgery, digital evaluation of pelvic muscle contraction strength. Verbal instruction, feedback and reinforcement on contraction was given to teach selective contraction of anal sphincter and relaxation of abdominal muscles. Verbal and written instruction given for home PFMT. Weekly digital anal reassessment and grading of pelvic muscle contraction by the therapist. Instructed to practice contractions 45 times per day (3 groups of 15 contractions) | Not specified | |
| PFMT plus BF weekly for 3 months | PFMT oral instructions only | |
| Intervention A: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 33%) plus PFMT for four weeks with biofeedback Intervention B: Brief verbal instruction in PFMT before operation and offer of one biofeedback session at 2 months after surgery (uptake 46%) | ||
| Intervention A: routine brief verbal and written PFMT plus one PFMT session and 3 weekly nurse phone calls Intervention B: routine brief verbal and written PFMT plus four BF enhanced PFMT sessions and 4 weekly nurse phone calls | Routine brief verbal and written PFMT. | |
| Verbal instruction and information on PFMT plus information on life style changes. Additional 15 physiotherapy sessions consisting of intensive PFMT with BF and ES | Verbal instruction and information on PFMT plus information on life style changes. | |
| One hour individual session with physiotherapist to teach correct contraction for PFMT, three 1 hour group lessons and home training programme | No pre operative physiotherapy. Information about anatomy and physiology and verbal instructions for 2 to 3 days after TURP in the ward. | |
| PFMT + biofeedback Patients received guided PFMT + biofeedback + information about the anatomy of pelvic floor muscles the day before surgery and after catheter removal. They were also given oral and written instructions on Kegel exercises to be performed at home which involved three sets of contractions daily for 10 mins, contracting their pelvic floor while lying, sitting and standing. The frequency of contractions was recorded in a training diary and visits at monthly intervals after catheter removal involved assisted biofeedback and motivation for 20 min | No biofeedback training Received standard care, oral and written instructions from urologist on PFMT, Instructed to: start PFMT after catheter removal (e.g. 2‐3 weeks after surgery) | |
| PFMT | No PFMT | |
| 1 session of PFMT in hospital before discharge and then saw the physiotherapist for 1‐2 weeks for as long as UI persisted. 90 daily home exercises sitting, standing and lying. 7 men unable to contract PFM or with weak contraction received electrical stimulation by anal probe | No formal PFMT instruction but saw the therapist at 1‐2 weeks and received placebo stimulation and information about aetiology of UI. | |
| Intervention A: PFMT alone Intervention B: PFMT + ES; PFMT as above plus instructed by dedicated in ES via surface anal electrode and bio‐impulser (biphasic pulse with 1 second bursts, 5 second pulse width, 2 second pulse trains Intervention C: PFMT + ES + biofeedback. As above plus biofeedback (anal probe) 15 minutes twice daily for 3 months All groups: PFMT by physiotherapist, 20‐30 minute sessions for 3 days, instructed to perform exercises twice daily for 3 months plus 3 week rehabilitation program after dischargeRegular interaction with health professional for 6 weeks after surgery, encouraged to performed treatment for 3 months post‐surgery | ||
| Oral PFMT plus ES for 15 minutes twice daily Instructed pre‐operatively PFMT by nurses and continued after catheter removal | Oral PFMT plus sham device. Instructed pre‐operatively PFMT by nurses and continued after catheter removal. | |
| Intervention A: anal electrode for 15 minutes twice a day for 1 month Intervention B: extra‐corporeal magnetic innervation, neocontrol system, treatment sessions 20 minutes, twice a week for 2 weeks | PFMT, digital anal teaching of correct contractions, then verbal and written instructions for home practice. | |
| PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes. Plus support group, 6 meetings in 3 months with a health psychologist | PFMT plus BF using rectal electrical sensor, initial 45 minute session with physical therapist then written instructions to carry out at home three times a day for 10 minutes |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 980 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 1.2 within 3‐6 months | 7 | 895 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.10] |
| 1.3 within 6‐12 months | 5 | 792 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.14] |
| 1.4 after 12 months | 3 | 665 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.22] |
| 2 Number of incontinence episodes per day Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 less than 3 months | 2 | 400 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.39, ‐0.78] |
| 2.2 within 3‐6 months | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.40, 1.00] |
| 2.3 within 6‐12 months | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.33, 0.93] |
| 2.4 after first year | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.82, 1.02] |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Score (ICIQ‐SF) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 24 hour pad test (grams of urine lost) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 less than 3 months | 2 | 214 | Mean Difference (IV, Fixed, 95% CI) | 22.29 [‐33.12, 77.70] |
| 8.2 within 3‐6 months | 2 | 213 | Mean Difference (IV, Fixed, 95% CI) | 11.87 [‐40.77, 64.52] |
| 8.3 within 6‐12 months | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | 11.23 [‐22.35, 44.82] |
| 8.4 after first year | 1 | 167 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [‐5.72, 83.72] |
| 9 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 1.2 within 3‐6 months | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.79] |
| 1.3 within 6‐12 months | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.73] |
| 1.4 after 12 months | 3 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.09, 0.74] |
| 2 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 24 hour pad test (grams of urine lost) Show forest plot | 2 | 325 | Mean Difference (IV, Fixed, 95% CI) | ‐16.94 [‐58.21, 24.33] |
| 3.1 less than 3 months | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐27.82 [‐116.97, 61.33] |
| 3.2 within 3‐6 months | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.12 [‐86.19, 96.43] |
| 3.3 within 6‐12 months | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐64.03, 60.13] |
| 3.4 after first year | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐190.50, 30.50] |
| 4 Urinary Incontinence Score (ICIQ‐short form UI score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Urinary Incontinence Quality of Life Score (ICIQ‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 3‐6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + Biofeedback vs no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinence episodes per day at > 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + BFB | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + BFB | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT + Anal EStim vs PFMT alone | 2 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.12] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 FES vs ExMI | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinence episodes at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of Life Score (severity of UI) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + BF + support group vs PFMT + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Quality of Life Score (I‐QoL) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT + BF + EStim vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Quality of Life Score (ICI‐Q‐SF) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + ExMI vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + Anal EStim vs PFMT alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost): at < 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 PFMT + perineal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 PFMT + perineal EStim vs PFMT + anal EStim | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 24 hour pad test (grams of urine lost): at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost): within 3 to 6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 PFMT + visual BF vs PFMT + oral BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Pad changes over 24 hours within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 ExMI vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Number of men not carrying out sufficient PFMT within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + BF + support group vs PFMT + BF | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 7 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.83, 1.06] |
| 1.2 within 3‐6 months | 7 | 697 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.75, 0.97] |
| 1.3 within 6‐12 months | 6 | 640 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.35, 0.75] |
| 1.4 after 12 months | 2 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.51] |
| 2 Pad changes over 24 hours Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6 ‐ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (gm/24hrs) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 less than 3 months | 3 | 424 | Mean Difference (IV, Random, 95% CI) | ‐78.19 [‐211.46, 55.07] |
| 4.2 within 3‐6 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐73.28 [‐196.42, 49.86] |
| 4.3 within 6‐12 months | 2 | 373 | Mean Difference (IV, Random, 95% CI) | ‐14.50 [‐18.36, ‐10.64] |
| 4.4 after first year | 2 | 378 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.81, ‐0.19] |
| 5 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Urinary Incontinence Score (ICI‐short form) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 less than 3 months | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 6.5 [3.45, 9.55] |
| 6.2 within 3‐6 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐5.99, 3.56] |
| 6.3 within 6‐12 months | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐3.19, 1.81] |
| 7 Quality of Life Score (IIQ) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 within 6‐12 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 after first year | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pelvic floor muscle strength (anal squeeze pressure, cm H2O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 less than 3 months | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of men not carrying out sufficient PFMT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 less than 3 months | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of men having surgery for incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 1 hour pad test (grams of urine lost) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 ICIQ‐SF score Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men within 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinent men within 6 to 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 PFMT + anal Estim + biofeedback versus no treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 24 hour pad test (grams of urine lost) within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 24 hour pad test (grams of urine lost) 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time until continent (months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT + anal Estim + Biofeedback versus no treatment/sham treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men at < 3months Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 PFMT pre and post op vs PFMT post op | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.06] |
| 1.2 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.26] |
| 1.3 PFMT + Biofeedback + transcutaneous Estim versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.11] |
| 1.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.22] |
| 2 Number of incontinent men within 3 to 6 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 PFMT pre and post op vs PFMT post op | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.54, 1.04] |
| 2.2 post‐op PFMT + biofeedback + transcutaneous Estim vs post‐op Estim | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.96, 2.49] |
| 2.3 PFMT + general exercise versus PFMT alone | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 2.4 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.76, 1.57] |
| 2.5 Post‐op transcutaneous electrical stimulation versus post‐op PFMT | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.16] |
| 3 Number of incontinent men within 6 to 12 months Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 post‐op PFMT + Biofeedback + transcutaneous Estim vs post‐op Estim | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of incontinent men after 12 months Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 PFMT pre and post op vs PFMT post op | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.78, 2.25] |
| 4.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.25, 7.77] |
| 5 No. with severe incontinence (e.g. pad test weight >150g) at < 3 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 No. with severe incontinence (e.g. pad test weight >150g) at 3 to 6 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 20 minute pad test (grams of urine lost): within 3 to 6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 20 minute pad test (grams of urine lost): within 6 to 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 PFMT + anal EStim vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 PFMT + anal EStim + BF vs PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 PFMT + anal EStim vs PFMT + anal EStim + BF | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 1 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Pre‐op PFMT + Estim versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 1 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 1 hour pad test within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 24 hour pad test (grams of urine lost) at less than 3 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Post‐operative PFMT + transcutaneous Estim + Biofeedback versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Post‐operative transcutaneous electrical stimulation versus post‐operative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 24 hour pad test (grams of urine lost) within 3‐6 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 PFMT + Biofeedback + transcutaneous Estim versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Postoperative PFMT + biofeedback + transcutaneous Estim versus postoperative PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Post‐operative transcutaneous Estim only versus post‐operative PFMT only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 24 hour pad test (grams of urine lost) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 PFMT + transcutaneous Estim + biofeedback versus Estim only | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Post‐op PFMT + transcutaneous Estim + Biofeedback versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Post‐op transcutaneous Estim versus post‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Quality of Life Score (ICS male short form) at < 3 months Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 PFMT pre and post op vs PFMT post op | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Urinary Incontinence Quality of Life Score (ICIQ ‐ short form) within 3‐6 months Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 PFMT + general exercise versus PFMT alone | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 PFMT pre and post op vs PFMT post op | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Urinary Incontinence Quality of Life Score (ICIQ‐short form) within 6‐12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Pre‐op PFMT + electrical stimulation versus pre‐op PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 King's health Questionnaire after 12 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Role limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.3 Physical limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.4 Social limitations | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.5 Personal | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.6 Emotional | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.7 Sleep/energy disturbance | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.8 Symptom severity | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Physical composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Mental Composite score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 PFMT pre and post op vs PFMT post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 PFMT + Penile vibration pre and post op versus PFMT pre and post op | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of incontinence episodes per day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of men using pads Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 less than 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 within 3‐6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 within 6‐12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 after 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Urinary Incontinence Score (ICI‐short form) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Quality of life related to urinary incontinence Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 less than 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 within 3‐6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 within 6‐12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 after first year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of men not carrying out pelvic floor muscle contractions at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinent men Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 less than 3 months | 2 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.21, 1.77] |
| 1.2 within 3‐6 months | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.14, 1.89] |
| 2 Health status measure SF‐36 within 3‐6 months Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Physical component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Physical functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Body pain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 General Health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Physical role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Mental health component | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mental role limitation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Vitality | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mental health | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Social functioning | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of men satisfied with device Show forest plot | Other data | No numeric data | ||
| 2 Mean urine loss (grams of urine on pad test) Show forest plot | Other data | No numeric data | ||
| 3 Penile Doppler blood flow (mean systolic velocity) Show forest plot | Other data | No numeric data | ||
| 4 Penile Doppler blood flow (mean resistence to flow index) Show forest plot | Other data | No numeric data | ||

