Patientenschulungen zur Verhütung von diabetischen Fußgeschwüren
Abstract
Background
Ulceration of the feet, which can result in loss of limbs and even death, is one of the major health problems for people with diabetes mellitus.
Objectives
To assess the effects of patient education on the prevention of foot ulcers in patients with diabetes mellitus.
Search methods
We searched The Cochrane Wounds Group Specialised Register (searched 03 September 2014); The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8).
Selection criteria
Prospective randomised controlled trials (RCTs) that evaluated educational programmes for preventing foot ulcers in people with diabetes mellitus.
Data collection and analysis
Two review authors independently undertook data extraction and assessment of risk of bias. Primary end points were foot ulceration or ulcer recurrence and amputation.
Main results
Of the 12 RCTs included, the effect of patient education on primary end points was reported in only five. Pooling of outcome data was precluded by marked, mainly clinical, heterogeneity. One of the RCTs showed reduced incidence of foot ulceration (risk ratio (RR) 0.31, 95% confidence interval (CI) 0.14 to 0.66) and amputation (RR 0.33, 95% CI 0.15 to 0.76) during one‐year follow‐up of diabetes patients at high risk of foot ulceration after a one‐hour group education session. However, one similar study, with lower risk of bias, did not confirm this finding (RR amputation 0.98, 95% CI 0.41 to 2.34; RR ulceration 1.00, 95% CI 0.70 to 1.44). Three other studies, also did not demonstrate any effect of education on the primary end points, but were most likely underpowered. Patients' foot care knowledge was improved in the short term in five of eight RCTs in which this outcome was assessed, as was patients' self‐reported self‐care behaviour in the short term in seven of nine RCTs. Callus, nail problems and fungal infections improved in only one of five RCTs. Only one of the included RCTs was at low risk of bias.
Authors' conclusions
In some trials, foot care knowledge and self reported patient behaviour seem to be positively influenced by education in the short term. Yet, based on the only two sufficiently powered studies reporting the effect of patient education on primary end points, we conclude that there is insufficient robust evidence that limited patient education alone is effective in achieving clinically relevant reductions in ulcer and amputation incidence.
PICOs
Laienverständliche Zusammenfassung
Schulung von Menschen mit Diabetes zur Fußpflege als Beitrag zur Verringerung von Fußgeschwüren und Amputationen.
Fußgeschwüre (offene Wunden) sind bei Menschen mit Diabetes weit verbreitet, vor allem bei denjenigen, die Probleme an den Nervenfasern (periphere Neuropathie), Probleme mit der Durchblutung der Beine (periphere arterielle Verschlusskrankheit (PaVK)) oder beides haben. Bei Menschen mit diabetischen Geschwüren kann es notwendig werden, einen Teil der Gliedmaße chrirurgisch zu entfernen (Amputation). Fußgeschwüre führen nicht nur bei den Betroffenen zu körperlicher Behinderung und Verlust von Lebensqualität, sie stellen auch eine volkswirtschaftliche Belastung dar (Gesundheitsausgaben, Arbeitsunfähigkeit). Aus verschiedenen Gründen gilt es daher, dem Auftreten von Geschwüren vorzubeugen. In diesem Review von Studien mit einem hochwertigen Studiendesign wurde festgestellt, dass sich die Fußpflegekenntnisse von Diabetikern und ihr Verhalten kurzfristig zu verbessern scheinen, wenn sie darüber aufgeklärt werden, dass sie sich um ihre Füße kümmern müssen. Für die Annahme, dass Schulungen allein, ohne zusätzliche präventive Maßnahmen, die Häufigkeit von Geschwüren und Amputationen effektiv vermindern, besteht keine ausreichende Evidenz.
Authors' conclusions
Background
Ulceration of the foot is one of the major health problems for people with diabetes mellitus. It is estimated to affect 15% to 25% of people with diabetes at some time in their lives (Singh 2005). Foot ulceration can result in marked physical disability and reduction of quality of life (Nabuurs‐Franssen 2005; Vileikyte 2001), not to mention limb loss and even death (Robbins 2008). Diabetic foot ulcers precede 25% to 90% of all amputations (Global Lower Extremity Amputation Study Group 2000; Pecoraro 1990). The risk of a lower extremity amputation in people with diabetes is therefore much higher than in people without diabetes (Canavan 2008; Icks 2009).
Several factors are involved in the development of foot ulcers, including peripheral neuropathy, PVD, limited joint mobility and repeated trauma from abnormal load distribution on the foot (Dinh 2005; Edmonds 2006). The underlying causes of foot ulcers are usually irreversible and chronically progressive. Therefore, 70% of healed foot ulcers recur within five years (Apelqvist 1993). Moreover, treatment itself is very challenging and often needs to be long lasting. It requires not only expert interference, orthopaedic appliances and antimicrobial drugs but also costly topical dressings and inpatient care (Boulton 2004; Cavanagh 2005; Edmonds 2006; Jeffcoate 2003; Singh 2005). Not surprisingly, this leads to substantial economic burden. Healing of a single ulcer costs approximately USD17,500 (1998 US dollars) (Ragnarson Tennvall 2004). In cases where lower extremity amputation is required, health care is even more expensive: USD30,000 to USD33,500 (Ragnarson Tennvall 2004). These costs do not even represent the total economic burden, since costs related to loss of productivity, preventive efforts, rehabilitation and home care should also be considered. When all this is taken into account, 7% to 20% of total expenditure on diabetes in North America and Europe might be attributable to diabetic foot ulceration (Boulton 2005).
In 1989 one of the main five‐year targets of the European Declaration of St. Vincent was to reduce amputations caused by diabetes mellitus by 50% (St Vincent Declaration 1989). In order to reach these goals, international guidelines underline the need to reduce the incidence of foot ulceration by preventive efforts. This not only includes optimising metabolic control and identification and screening of people at high risk for diabetic foot ulceration, but also patient education in order to promote foot self‐examination and foot care knowledge (American Diabetes Association 2007; Frykberg 2006; IDF clinical guidelines task force 2005).
Population‐based research suggests that a meaningful reduction in the incidence of amputations caused by diabetes mellitus has already been achieved. Before the European Assembly in St. Vincent, the risk ratio (RR) of a lower extremity amputation was still 15 times higher in people with diabetes mellitus than in people without diabetes mellitus (Most 1983). This RR has since dropped to 8.8 (95% CI 7.3 to 10.7) in men and 5.7 (95% CI 4.3 to 7.6) in women in one study (Icks 2009) and to 7.7 (95% CI 5.0 to 12.9) in another (Canavan 2008).
However, it cannot be inferred from these figures that current preventive efforts are effective, since the reduction in amputation incidence may also have resulted from improvements in ulcer treatment. In this review of randomised controlled trials (RCTs) we, therefore, evaluate the effect of education of people with diabetes aiming to promote foot self‐care and to prevent the occurrence of foot lesions. Although this type of prevention is nowadays widely advocated and implemented in standard practice, the evidence for the effectiveness is still scarce. Several review articles on the diabetic foot, which include education among the prevention strategies discussed, have been published (Armstrong 1998; Assal 1985; Bild 1989; Boulton 1995; Bowering 2001; Edmonds 1996; Larsson 1995; Levin 1995; Majid 2000; Mason 1999; Mayfield 1998; Rith‐Najarian 2000; Singh 2005; Wu 2007). However, only three of these reviews were systematic (Majid 2000; Mason 1999; Singh 2005) and most of these reviews dealt primarily with uncontrolled studies. Furthermore, only two of these reviews assessed the methodological quality of the included studies (Majid 2000; Mason 1999). The overall conclusion of these review articles was that education is effective for the prevention of diabetic foot ulceration, but consequently this conclusion must be treated with care; especially since previous systematic reviews of patient education for adults with, for example, asthma and neck pain, have suggested that health outcomes were unlikely to be improved by limited patient education (Gibson 2002; Haines 2009).
Thus, after reviewing the available evidence, we decided to perform a systematic review of the effectiveness of (components of) education programmes targeted at people with diabetes with the aim of preventing foot ulceration.
Objectives
To assess the effects of patient education on the prevention of foot ulcers in patients with diabetes mellitus.
Methods
Criteria for considering studies for this review
Types of studies
Prospective RCTs evaluating educational programmes for the prevention of foot ulcers in people with diabetes mellitus. We excluded studies that were solely aimed at optimising blood glucose concentration. An explicit focus on foot care was required.
Types of participants
Studies involving people aged 18 years or older with type 1 or type 2 diabetes mellitus in any healthcare setting.
Types of interventions
Studies of educational programmes (or programmes that include education) aiming to reduce the incidence of foot ulceration in people with diabetes mellitus.
Studies where foot‐care education was part of a larger educational programme (e.g. on diabetes in general) were eligible, but education on foot care had to contrast with the control intervention. The foot care education could also be part of a more comprehensive diabetic foot programme, but in these cases patient education on foot care had to be the main contrast with the control intervention. All types of control intervention were considered for inclusion in the review.
Types of outcome measures
Primary outcomes
-
Foot ulceration or ulcer recurrence.
-
Amputation.
Secondary outcomes
Clinical outcomes:
-
callus development,
-
resolution of callus,
-
fungal infection,
-
number and duration of hospital admissions for diabetic foot problems.
Process outcomes:
-
foot care knowledge scores,
-
patients' behaviour assessment scores.
Trials were included if secondary outcomes only were reported.
Search methods for identification of studies
Electronic searches
The search methods section of the third update of this review can be found in Appendix 2
For this fourth update we searched the following electronic databases:
-
The Cochrane Wounds Group Specialised Register, comprising references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings (searched 03 September 2014);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 8).
The following search strategy was used in The Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor: [Education] explode all trees
#2 patient near/3 education*:ti,ab,kw
#3 diabetes near/3 education*:ti,ab,kw
#4 patient near/3 information:ti,ab,kw
#5 education* near/2 program*:ti,ab,kw
#6 (foot next care) or footcare:ti,ab,kw
#7 leaflet* or booklet* or pamphlet* or "poster" or "posters":ti,ab,kw
#8 (written or printed or oral) near/3 information:ti,ab,kw
#9 academic next detailing:ti,ab,kw
#10 training next program*:ti,ab,kw
#11 algorithm* or (decision next tree*):ti,ab,kw
#12 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11)
#13 MeSH descriptor: [Foot Ulcer] explode all trees
#14 MeSH descriptor: [Diabetic Foot] explode all trees
#15 diabet* near/3 ulcer*:ti,ab,kw
#16 diabet* near/3 (foot or feet):ti,ab,kw
#17 diabet* near/3 amputat*:ti,ab,kw
#18 diabet* near/3 wound*:ti,ab,kw
#19 diabet* near/3 defect*:ti,ab,kw
#20 (#13 or #14 or #15 or #16 or #17 or #18 or #19)
#21 (#12 and #20)
No date or language restrictions were applied.
Searching other resources
The bibliographies of all retrieved and relevant publications identified by these strategies and the list of articles that cited previous versions of this review were searched for further studies.
Data collection and analysis
Selection of studies
Full copies of potentially eligible studies were obtained and two review authors (GV and DK or GV and JD), acting independently, decided on inclusion or exclusion. In case of disagreement, consensus was reached by discussion between three review authors (GV, DK and JD).
Data extraction and management
We extracted details of eligible studies and summarised them using a data extraction sheet. We recorded the content of the educational package, plus the content of the total programme, if education was merely one component. Data from multiple reports of individual studies were extracted and the primary reference identified (Borges 2004; Rönnemaa 1997). We recorded all outcomes if different but relevant outcomes were available from different publications of the same RCT. Data regarding the interventions studied, type of outcome measures, duration of follow‐up, loss to follow‐up and outcomes were extracted by two review authors (GV and DK or GV and JD) independently. In case of disagreement, consensus was reached by discussion between three review authors (GV, DK and JD).
Assessment of risk of bias in included studies
We assigned two review authors (JD and DK or GV) to assess each study independently using The Cochrane Collaboration tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains, namely: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 1 for details of criteria on which the judgement was based). Because blinding of patients and healthcare providers does not appear to be feasible considering the nature of the interventions studied, judgement was solely based on the information provided about blinding of outcome assessors. Blinding and completeness of outcome data were assessed for each outcome separately. Any disagreements were discussed in a consensus meeting. We completed a 'Risk of bias' table for each eligible study (see Characteristics of included studies).
We assessed risk of bias using a 'Risk of bias summary' figure (see Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity may guide the weight the reader may give to the results of the particular studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
We reported separately for each study. Depending on the available data we aimed to present the results for binary outcomes (e.g. ulceration or amputation) as RR with corresponding 95% confidence intervals (CI) and the results for continuous data (e.g. callus diameter) as mean differences with corresponding 95% CIs.
Dealing with missing data
If data were missing from reports, we then attempted to contact the study authors. We were successful in contacting the authors of Corbett 2003; Rönnemaa 1997 and Mazzuca 1986, and additional data on effect sizes were obtained.
Data synthesis
Because substantial statistical and methodological heterogeneity between studies was observed, all results were presented in a qualitative summary (Reed 2005).
Subgroup analysis and investigation of heterogeneity
Possible sources of variation among studies that would require pre‐planned stratified analysis were:
-
character of patient groups (e.g. patients at high risk for foot ulceration compared with patients at low risk; patients with a history of foot ulceration compared with patients without, etc.),
-
health care setting,
-
risk of bias of studies,
-
outcome measures used,
-
type of intervention (e.g. brief compared with intensive programmes; education tailored to the individual needs compared with standardised education programmes),
-
nature of contrast (e.g. intervention compared with control intervention, patient education plus co‐intervention A compared with intervention A alone, intervention compared with no intervention).
Results
Description of studies
Results of the search
For this fourth update of the original review article (Other published versions of this review) no additional RCTs were identified. One study (Zhenghua 2011) previously placed in the Studies awaiting classification was moved to the Excluded studies, because no full text report was published. One other study (Gershater 2011) remained in the Studies awaiting classification, because the study report is still pending.
Included studies
Twelve RCTs are included in this review and are described in the Characteristics of included studies and summarised below.
Healthcare settings
Four RCTs were performed in a community‐based care setting (Cisneros 2010; Corbett 2003; Rettig 1986; Rönnemaa 1997): patients were recruited from a home care organisation register (Corbett 2003), hospital records (Rettig 1986), the national drug imbursement register (Rönnemaa 1997) or the National Health System database (Cisneros 2010). The care settings of three studies were classified as primary care (Bloomgarden 1987; Frank 2003; Mazzuca 1986): one was performed in a diabetes outpatient clinic in the USA (Bloomgarden 1987), one in a country hospital's outpatient clinic in the USA (Frank 2003) and one in an academic general medicine outpatient clinic in the USA. Four studies were performed in a secondary care setting: one in an outpatient clinic in Australia (Barth 1991), two in secondary outpatient care in the USA (Kruger 1992; Malone 1989) and one in secondary outpatient care in the UK (Lincoln 2008). Finally, one study setting could not be categorised with any of the above, because it was performed in the emergency departments of two community hospitals in the USA (Borges 2004).
Participants' risk of foot ulceration
In four of the 12 included RCTs, patients were at high risk of foot ulceration (Cisneros 2010; Corbett 2003; Lincoln 2008; Malone 1989). In Malone 1989 all patients were referred to podiatry or vascular surgery prior to study entry and had foot infection, ulceration or prior amputation. In Lincoln 2008 all participants had newly healed diabetic foot ulcers. In Corbett 2003 patients were excluded if they had a history of foot amputation, but it follows from the presence of important risk factors at baseline that the studied population was at high risk for foot ulceration: loss of protective sensation was present in 70%, impaired lower extremity circulation in 67% and foot deformity in 50%. Finally, in Cisneros 2010 all patients had diabetic neuropathy and most had additional risk factors such as plantar overpressure, deformity and previous foot ulceration. In four RCTs patients were at low or medium risk of foot ulceration (Barth 1991; Bloomgarden 1987; Frank 2003; Rönnemaa 1997). In Rönnemaa 1997 patients were even excluded if they had any need for podiatry. Finally in four RCTs, the level of risk for ulceration or amputation could not be determined (Borges 2004; Kruger 1992; Mazzuca 1986; Rettig 1986).
Interventions
Three RCTs compared the effectiveness of a patient education programme on diabetes in general (which included a component of foot care education) compared with usual care (Bloomgarden 1987; Mazzuca 1986; Rettig 1986). In one of these studies, the content of the educational programme was preset and consisted of nine group patient education sessions, one of which was about foot care and skin hygiene (Bloomgarden 1987). In the other two studies, the content of the educational programme was tailored to patients' individual needs (Mazzuca 1986; Rettig 1986). In Mazzuca 1986 those educational needs were identified using a set of safety‐level objectives selected by a multidisciplinary group of healthcare professionals. These objectives covered seven areas of patient education, of which foot care was one. In the other study areas of diabetes self‐management, patients most in need of improvement were determined using an assessment instrument (100 short answer and yes‐no questions, brief patient demonstrations of urine testing and insulin injection techniques) (Rettig 1986). In both studies, participants received a variable number of educational sessions.
In addition to Mazzuca 1986 and Rettig 1986, there were two more RCTs that adopted the concept of education tailored to patients' individual needs, but in these studies the educational interventions were only directed at improving foot care (Borges 2004; Corbett 2003) and they were less intensive than in Mazzuca 1986 and Rettig 1986. In Corbett 2003 a single 10‐ to 20‐minute educational session, combined with written instructions, was compared with no intervention, and in Borges 2004 a 15‐minute educational session after a risk assessment for foot ulceration was compared with risk assessment alone and with no intervention at all.
In the remaining six RCTs, an intensive foot care education programme was compared with a less proactive intervention (Barth 1991; Cisneros 2010; Frank 2003; Kruger 1992; Lincoln 2008; Malone 1989; Rönnemaa 1997). This, however, does not mean that the interventions in these studies were all similar. In two studies the intervention was only one hour of patient education, reinforced by hand‐outs (Lincoln 2008; Malone 1989). In Malone 1989, this was compared with routine patient education, and in Lincoln 2008 with written instructions only. In Rönnemaa 1997, patients in the intervention group also received 45 minutes of foot care education, but this was combined with a variable number of follow‐up visits at a podiatry clinic. The control intervention consisted of written instructions on foot care only. In the other studies, the educational interventions were more comprehensive, more intensive or both. In Frank 2003, for example, the intervention comprised viewing a videotape about proper foot care, a 30‐ to 40‐minute individualised educational session, a foot ulceration risk assessment, hand‐outs, a foot care checklist, a bag of foot care supplies and weekly reminder telephone calls. Control group patients received a foot ulceration risk assessment only. In Cisneros 2010 people in the intervention group received four 90‐minute therapeutic education sessions on the complications of diabetes, disease treatment, inspection and foot hygiene and choice and use of proper footwear. In addition, participants received footwear after completion of the educational programme. This was compared with routine care. In two other studies a basic educational programme on diabetes in general was provided to all study participants and supplemented by specific foot care education in the intervention group (Barth 1991; Kruger 1992). In Barth 1991, the general diabetes education consisted of 14 hours of group education and included a one‐hour lecture on foot care. In addition to that, the intervention group followed nine hours of group education about the diabetic foot (Barth 1991). In Kruger 1992, the general diabetes education consisted of a one‐week course, which included viewing an instructional videotape on foot care. In the intervention group this was supplemented with a 'hands‐on' foot care approach, a patient education kit and daily foot care sheets (Kruger 1992).
Duration of follow‐up
The median time to follow‐up was six months (Barth 1991; Kruger 1992; Rettig 1986), ranging from only four weeks (Borges 2004; Frank 2003) to seven years (Rönnemaa 1997).
Excluded studies
Eighteen studies are excluded from the review. Six studies are not randomised controlled studies (Dargis 1999; Davidson 2000; Litzelman 1997; Pieber 1995; Ward 1999; Wooldridge 1996). Four studies did not report an educational programme that includes patient education aimed at reducing diabetic foot ulcers (De Weerdt 1991; Donohoe 2000; Reichard 1993; Vinicor 1985). Foot care education was not a unique intervention in five studies (Litzelman 1993; McCabe 1998; McMurray 2002; Nesari 2010; Plank 2003), one study investigated education initially directed at wound healing (Fresenius 2009) and one study (Glasgow 1992) did not report any outcomes relevant to this review.
Risk of bias in included studies
The risk of bias of most included studies was high, except for one RCT (Lincoln 2008). Details are presented in a 'Risk of bias' table for each eligible study (see Characteristics of included studies) and in a 'Risk of bias summary' figure (see Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. Judgements on the six items were made by two review authors independently for each of the 12 studies. There was initial disagreement on six items (percentage of agreement 92%). All disagreements were resolved by discussion without needing to consult the third review author.
Allocation
True randomisation with allocation concealment was evident in only two of the included RCTs. In Corbett 2003 the sequence was determined by drawing labelled consent forms that were covered with opaque stickers and shuffled. In Lincoln 2008, the sequence was based on a computer‐generated list held by an independent randomisation centre that was contacted by telephone each time a person was randomised. Randomisation was inadequate in three studies. In Frank 2003 group allocation was determined by drawing papers from an envelope, but this envelope was not sealed. Moreover, in Kruger 1992 and Malone 1989, people were allocated to the experimental and control group on alternating weeks and the last digit of their Social Security number respectively, which are both considered non‐random. Insufficient information on randomisation and allocation concealment was provided in the remaining studies.
Blinding
Outcome assessment was blinded in four RCTs (Barth 1991; Borges 2004; Lincoln 2008; Rettig 1986), unblinded in three (Cisneros 2010; Frank 2003; Mazzuca 1986) and not described in the remaining studies. Inadequate blinding may affect subjectively measured secondary outcomes more than foot ulcer and amputation incidence.
Incomplete outcome data
The withdrawal/dropout rate was unacceptably high in six RCTs (Borges 2004; Cisneros 2010; Corbett 2003; Kruger 1992; Mazzuca 1986; Rettig 1986). In only one of the RCTs an intention‐to‐treat (ITT) analysis was performed for primary outcomes (Lincoln 2008).
Selective reporting
Study protocols were not sought and therefore not available for review, but most trial reports listed all expected outcomes in both the methods and the results section. Only Bloomgarden 1987 reported additional outcomes in the results section.
Other potential sources of bias
Co‐interventions were avoided or similar in two studies (Borges 2004; Lincoln 2008). The adherence to the interventions reached an acceptable level in five RCTs (Borges 2004; Cisneros 2010; Corbett 2003; Frank 2003; Lincoln 2008). The most important baseline prognostic indicators were clearly incomparable in two RCTs (Barth 1991; Bloomgarden 1987), sufficiently similar in two others (Frank 2003; Lincoln 2008) and inadequately described in the remaining RCTs.
The eligibility criteria with regard to risk for foot ulceration were sufficiently described in only five RCTs (Borges 2004; Corbett 2003; Frank 2003; Lincoln 2008; Malone 1989).
Effects of interventions
Additional data are presented in Table 1. Results of studies are summarised below in a study‐by‐study qualitative synthesis.
| Study ID | Primary outcomes | Secondary outcomes |
| No primary outcomes reported | Foot problems requiring treatment: Foot care knowledge: Foot care routine compliance: | |
| Ulcer or amputation: people with callus, nail dystrophy or fungal infection at baseline: intervention 2/37 vs control 3/63 people with an ulcer or amputation at baseline: intervention 6/7 vs control 11/13 | Callus, nail dystrophy and fungal infection: Behaviour assessment scores: | |
| No primary outcomes reported | Patients' self‐reported behaviour assessment scores: Observed self‐care behaviour: Foot care knowledge scores: | |
| Ulcer incidence: people without a history of foot ulceration: intervention 8/21 vs control 8/14 (P = 0.317) Patient with a history of foot ulceration: intervention 1/8 vs control 5/8 (P = 0.119) All people: difference between the survival curves of intervention and control (P = 0.362) (HR not reported) | No secondary outcomes reported | |
| No primary outcomes reported | Foot care knowledge scores: Foot care practice scores: | |
| No primary outcomes reported | Foot care knowledge scores: Patients' behaviour assessment: | |
| No primary outcomes reported | Foot status: Foot care knowledge scores: Behaviour assessment: | |
| Ulcer incidence: Amputation rate: | Behaviour assessment scores: | |
| Ulcer incidence: Amputation rate: | No secondary outcomes reported | |
| No primary outcomes reported | Foot care knowledge scores: | |
| No primary outcomes reported | Foot appearance scores (mean ±standard error): Foot care knowledge scores: Foot care skills scores: | |
| Amputation: 7‐year follow‐up: intervention 1 vs control 0 Foot ulceration: 7‐year follow‐up: intervention 1 vs control 1 | Callus development: Calcaneal region:
Other regions:
7‐year follow‐up: Calcaneal region:
Other regions:
Foot care knowledge scores:
7‐year follow‐up:
Patients' behaviour assessment scores:
7‐year follow‐up:
| |
| Abbreviations: CI = confidence interval, ns = no statistical significance, RA = group that received risk assessment only, RR = risk ratio, SD = standard deviation. | ||
We attempted to estimate pooled effect sizes of the primary outcomes of two seemingly similar studies (Lincoln 2008; Malone 1989), but this was precluded by inconsistencies in the unit of analysis (one analysed the number of limbs and the other the number of people), unequal methodological quality and considerable statistical heterogeneity. Pooling of the remaining studies was not attempted because of considerable clinical heterogeneity.
1. Foot care education as part of general diabetes education compared with usual care (3 RCTs)
Primary outcomes
The incidence of foot ulceration or amputation was only reported in Bloomgarden 1987: 146 patients had no foot lesion at the initial evaluation and since only two severe foot lesions (ulceration or amputation) were observed in both the intervention and the control group during follow‐up of approximately 1.5 years, the effect was not significant. Also in the subgroup of patients with callus, nail dystrophy or fungal infection at baseline (n = 100) and in the subgroup of patients with a previous ulcer or amputation at baseline (n = 20), no significant effects of the intervention were observed.
Secondary outcomes
After six months' follow‐up, Rettig 1986 reported that foot care knowledge scores were significantly higher in the intervention group (mean score: 62 ± 1.7 SE) compared with the control group (mean score: 53 ± 1.8 SE) (P = 0.001), but this had not resulted in positive effects on foot appearance and foot care skills score. Mazzuca 1986 reported no significant improvements in foot care knowledge, and Bloomgarden 1987 found no significant improvements in behaviour assessment scores, neither in the occurrence of callus, fungal infection and nail dystrophy.
It should be noted that in both Mazzuca 1986 and Bloomgarden 1987, adherence to the intervention and follow‐up were poor. In Mazzuca 1986, only 52% of patients were followed up, and only 67% of patients requiring foot care completed treatment. Bloomgarden 1987 reported that 77% of patients who gave consent completed follow‐up, but only 50% of patients in the intervention group adhered to the intervention. Rettig 1986 did not report adherence to the intervention.
2. Foot care education tailored to educational needs compared with no intervention (2 RCTs)
Primary outcomes
Not reported in any of the included RCTs.
Secondary outcomes
After a 10‐ to 20‐minute individualised foot care education session at home, Corbett 2003 found that participants in the intervention group (n = 19) had significantly greater foot care knowledge (P = 0.03) and improved self‐care practices (P = 0.007) compared with participants in the control group (n = 16). This study, however, was limited by a small sample size and short duration of follow‐up (six weeks). In Borges 2004, self‐reported behaviour assessment scores were significantly improved after one month of follow‐up in both the intervention group, who received a 15‐minute education session (P < 0.01), and in the control group, who received no intervention at all (P < 0.05). Four of 16 foot self‐care behaviours were significantly more frequently observed in the intervention group compared with the control group. Paradoxically, foot care knowledge increased only in the control group but not in the intervention group.
3. Intensive compared with brief educational interventions (6 RCTs)
Primary outcomes
In Malone 1989, 34 foot ulcers, 28 lower‐extremity amputations and 4 foot infections were observed during one year of follow‐up of 182 patients. A marked reduction in ulcer incidence (intervention 8, control 26) and amputation rate (intervention 7, control 21) was observed in the intervention group. It should be noted, however, that outcomes were reported per limb (n = 354) instead of per person (n = 182). Therefore, a single person could have had two events, although multiple events on the same limb were reported as one. This may have resulted in overestimation of the effect sizes (RR foot ulceration 0.31, 95% CI 0.14 to 0.66; 354 limbs, one trial, Analysis 1.1, and RR amputation 0.33, 95% CI 0.15 to 0.76, 354 limbs, one trial, Analysis 1.2).
These findings were not reproduced in the study by Lincoln 2008, in which 71 foot ulcers and 18 lower‐extremity amputations were observed during one‐year follow‐up of 172 participants. No effects of the intervention on primary outcomes were observed. The RR for foot ulceration was 1.00 (172 participants, one trial, Analysis 1.3) and the RR for lower extremity amputation was 0.98 (172 participants, one trial, Analysis 1.4).
In Cisneros 2010, 22 foot ulcers were observed in 51 people. The accompanying survival curve in the trial report showed a trend towards longer event‐free survival in intervention‐group participants, but this was not statistically significant (P = 0.362; hazard ratio (HR) not reported). In Rönnemaa 1997 ulcerations and amputations were reported, but these occurred too infrequently to be evaluated conclusively.
Secondary outcomes
In Lincoln 2008 a statistically significant increase in the persons behaviour assessment scores was reported in the intervention group (P = 0.03), but for this outcome only 72% of people were followed up. Moreover, the authors questioned the reliability of their assessment tool.
Rönnemaa 1997 found a significant increase in foot care knowledge in the intervention group after one year of follow‐up (P = 0.004). Also the persons behaviour assessment scores (5.4 increased to 7.0), the mean diameter of callosities (calcaneal region 40.5 mm decreased to 25.5 mm, P = 0.065; other regions 16.6 mm decreased to 11.4 mm, P < 0.001) and the presence of callus in other regions than the calcaneal region (54.4% decreased to 39.5%, P < 0.009) initially improved after the intervention. However, after seven years of follow‐up, the control group had made up all these arrears.
In Frank 2003, foot care knowledge was marginally but significantly better in the intervention than in the control group four weeks after the educational intervention. Adherence to one of the four daily foot care behaviours studied (wearing shoes and socks) was significantly more improved in the intervention group than in the control group, but this did not account for adherence to the other three foot care behaviours studied (checking feet, washing and drying feet, applying lotion).
In Barth 1991, already after one month of follow‐up a significant reduction of foot problems requiring treatment (P < 0.001) and an increase in foot care routine compliance (P < 0.001) was seen in the intervention group. Also foot care knowledge improved in both groups, but most in the intervention group (P < 0.001). Improvements were maintained until after six months of follow‐up, but the differences between intervention and control group diminished. Kruger 1992 also found almost similar improvements of foot care knowledge scores in both the intervention and control group (intervention 9.1 increased to 10.0, control 8.7 increased to 9.9) and no significant differences in the status of the participants' feet after six months of follow‐up. Some (daily foot washing, trimming of toenails), but not all foot care behaviours (daily foot inspection, use of pumice stones, keeping toenails shorter) improved in the intervention group. However, this study dealt with small groups (23 people in the intervention group and 27 in the control group), and also had a relatively high dropout rate (40%).
Discussion
Summary of main results
A wide range and combinations of patient educational interventions have been evaluated for the prevention of diabetic foot ulceration. These interventions varied from brief patient education to intensive patient education including demonstration and 'hands‐on' teaching.
The ultimate aim of foot care education for people with diabetes is to prevent foot ulceration and amputations. However, these end points were assessed in only five of the 12 RCTs (Table 1). The results of this review are presented in a study‐by‐study qualitative synthesis. Pooling of the results was precluded by marked heterogeneity (mainly clinical), because participants, types of interventions, types of control interventions, outcome measures, outcome assessment tools, duration of follow‐up and risk of bias varied widely between studies.
Only one RCT showed that, after one‐year follow‐up, the incidence of foot ulcers and amputations was lower in the group who received one hour of group education on the diabetic foot by a podiatrist compared with the group who received routine foot care education (Malone 1989). In this RCT, the number of legs instead of the number of people was taken as the unit of analysis (so‐called 'unit of analysis error') leading to an overestimation of the precision of the study and thus the ability to reach statistical significance. Moreover, an inadequate quasi‐randomised method was used for group allocation and it was not clear if baseline variables were comparable. Also, blinding of the outcome assessor and co‐interventions were not reported and ITT analysis was not attempted (Malone 1989). More importantly, the positive findings of this study are contradicted by the results of a more recent study that was included in this review (Lincoln 2008), which also studied a population at high risk for foot ulceration. Most other characteristics of this study were also similar to Malone 1989, although regular care, which was available to both the intervention and the control groups of both studies might have improved between 1989 and 2008 when the latter study was published. It can be argued that in Lincoln 2008, the educational intervention and the control intervention did not contrast enough to result in significantly different outcomes. Nevertheless, the risk of bias in Lincoln 2008 was very low. This study concluded that one hour of patient education compared with written instructions only had no beneficial effects on the incidence of foot ulceration and amputation rate after 12 months of follow‐up. The other three RCTs that reported the effect of patient education on foot ulceration and amputation demonstrated no effect either (Bloomgarden 1987; Cisneros 2010; Rönnemaa 1997). However, the overall event rate in the populations studied showed that these two studies were underpowered to show any effect on these primary outcome measures.
The present review demonstrates a positive short‐term effect of education on patients' foot care knowledge, which improved in five of the eight RCTs in which this outcome was assessed (Barth 1991; Corbett 2003; Frank 2003; Rettig 1986; Rönnemaa 1997). However, in the one RCT with longer follow‐up, the difference in foot care knowledge between intervention and control group had disappeared at seven years (Rönnemaa 1997). Similarly patients' behaviour at 6 to 18 months improved in seven of the nine RCTs in which this outcome was assessed (Barth 1991; Borges 2004; Corbett 2003; Frank 2003; Kruger 1992; Lincoln 2008; Rönnemaa 1997). Although behaviour assessment scores were still improved after seven years of follow‐up compared with the baseline measurements, the control group had made up arrears (Rönnemaa 1997). The assessment tools for measuring foot care knowledge and self‐care behaviour varied between studies, because there is currently no single standardised validated tool widely used for these purposes. We were therefore unable to evaluate the importance (clinical relevance) of the reported statistically significant improvements in foot care knowledge scores and self‐care behaviour assessment scores.
The effects on callus, nail problems and/or fungal infections were described in five studies. In only two of them were some positive effects at short‐term follow‐up reported (Barth 1991; Rönnemaa 1997), but the differences between intervention and control group were not maintained until final follow‐up. In three additional studies no benefit on these outcomes was achieved (Bloomgarden 1987; Kruger 1992; Rettig 1986). It may be possible that co‐interventions, such as podiatry care, influenced these outcomes. In Rönnemaa 1997 podiatry care was part of the experimental intervention. Therefore, people in the intervention group were reported to have seen a podiatrist more often (mean number of visits 4.7) than people in the control group (mean number of visits 0.4) after one year of follow‐up, and consequently an improvement on callus, nail problems and fungal infections was reported in the intervention group. During the last year of follow‐up, only 30.8% of the people in the intervention group still visited a podiatrist compared with 25.2% of the control group. The initial advantage of the intervention group had disappeared. A similar explanation accounts for the initial benefit of the intervention group in Barth 1991: after one month of follow‐up, 17 people in the intervention group compared with seven in the control group had consulted a podiatrist. Between the third and sixth month of follow‐up, these figures levelled to seven and eight people in each group, respectively. These examples show the importance of adequate reporting of co‐interventions, which was one of the shortcomings of the other three studies that reported these outcomes.
Overall completeness and applicability of evidence
The studies in this review that included people with diabetes at low or medium risk for foot ulceration recruited too few participants and followed them up for too short a period of time to be able to detect clinically important differences in primary outcomes. For example, in order to detect a 50% reduction in the incidence of diabetic foot ulceration, 430 to 870 participants would be required per treatment group (based on an annual incidence of foot ulceration in the general diabetes population of 2% to 4% per year or 4% to 8% over two years) (De Sonnaville 1997; Reenders 1993). The mean size of studies in this review that included people at low or medium risk for foot ulceration was 138 people per treatment group, ranging from 25 (Kruger 1992) to 266 (Mazzuca 1986) and with six months median time to follow‐up, indicating that none of these studies were actually sufficiently powered to detect differences in the long term on one of the primary outcomes. Unfortunately, the trials included in this review do not share a common set of characteristics (participants, educational methods, intensity of education to intervention and the control group, outcome measures, duration of follow‐up), thereby hindering present and future pooling.
Of the studies that reported the effect of foot care education on primary outcomes in a population at high risk, two were well‐powered (Lincoln 2008; Malone 1989). However, in these studies the educational programme under study was very limited, comprising only a single one‐hour educational session, reinforced by hand‐outs, compared with either routine patient education (Malone 1989) or written instructions only (Lincoln 2008). The conclusions of these studies were contradictory, but because the risk of bias was lower in Lincoln 2008, more weight should be given to the outcomes of this study. It shows that a limited educational intervention is not likely to result in improvement of ulcer incidence and amputation incidence. This, however, does not rule out effectiveness of more comprehensive and/or more intensive educational strategies, but these strategies were not studied.
In summary, evidence on the effectiveness of comprehensive and intensive patient education programmes to prevent diabetic foot ulceration is still needed. Below we make suggestions for future research (Implications for research).
Quality of the evidence
One of the most important findings of the present review is the high or unclear risk of bias in all but one of the included RCTs. This was mainly caused by insufficient reporting. Usually methodological flaws lead to an overestimation of the effect size. Therefore, the few positive effects that were found should be interpreted with caution. In addition, unknown and unregistered co‐interventions in the control groups of the included trials (e.g. podiatry care, unstructured patient education by the care provider) could have led to reductions in the effects of the experimental educational interventions. Finally, it must be stressed that foot care knowledge and patient behaviour were measured using subjective outcome measures and are therefore also prone to bias.
Potential biases in the review process
The clinical heterogeneity of the RCTs meant it was not possible to produce a funnel plot to assess the presence of publication bias. However, in general, publication bias would be likely to lead to an overestimation of the effects. In this review, most of the RCTs identified reported non‐significant findings and it is therefore unlikely that we overestimated any effect.
The availability of co‐interventions to those participating in the studies may have influenced the outcomes of the trials in this review. For example, in Rönnemaa 1997 and Barth 1991 the incidence of callus, nail problems and fungal infection were probably influenced by the availability of podiatry care (see Summary of main results). In most other studies, it is not reported which co‐interventions were available and whether they have influenced the outcomes. Furthermore, 'care as usual' has greatly improved and must no longer be mistaken for 'doing nothing'. Therefore, a limited educational intervention may add little to the existing knowledge of patients to result in any beneficial effects. This especially accounts for more recent studies like Lincoln 2008.
The studies included in this review used different tools to assess care knowledge and self‐care behaviour. Therefore, it was not possible to evaluate the importance (clinical relevance) of the reported statistically significant improvements in foot care knowledge scores and self‐care behaviour assessment scores. Foot care knowledge improved to some extent in five of eight studies, but consequently, this does not provide proof of effectiveness.
Agreements and disagreements with other studies or reviews
The conclusions of this systematic review contrast with those in earlier reviews on this topic (Armstrong 1998; Assal 1985; Bild 1989; Boulton 1995; Bowering 2001; Edmonds 1996; Larsson 1995; Levin 1995; Majid 2000; Mason 1999; Mayfield 1998; Rith‐Najarian 2000; Singh 2005; Wu 2007). According to these review articles, there was enough evidence to support the effectiveness of patient education for the prevention of diabetic foot ulceration. This systematic review of RCTs, however, provides a more complete and well‐considered overview of the available evidence, incorporating an evaluation of the risk of bias of the included studies.
This review was written in close conjunction with another review on the effectiveness of complex interventions for preventing diabetic foot ulceration (New Reference). That review included only five RCTs that were at high or unclear risk of bias. In agreement with this review, it was concluded that there is also no evidence to support the effectiveness of a multilevel integrated care approach for the prevention of diabetic foot ulceration (complex intervention, some of which included patient education), although this should be interpreted as a lack of evidence rather than evidence of no effect.
The RCTs included in this review show the same shortcomings as the RCTs included in a systematic review about the effectiveness of individual patient education for improvement of general metabolic control of people with diabetes mellitus (Duke 2009). The RCTs in that review were also small and had too many methodological flaws from which to draw firm conclusions.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 1 Foot ulcer incidence (1‐year follow‐up).
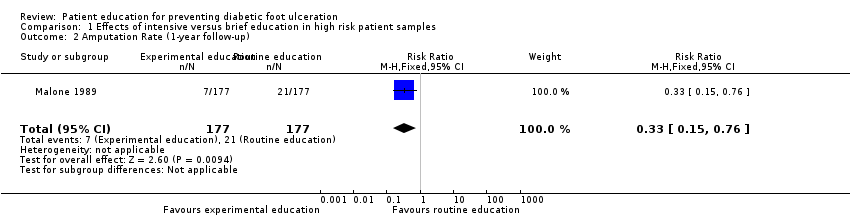
Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 2 Amputation Rate (1‐year follow‐up).

Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 3 Foot ulcer incidence (1‐year follow‐up).

Comparison 1 Effects of intensive versus brief education in high risk patient samples, Outcome 4 Amputation rate (1‐year follow‐up).
| Study ID | Primary outcomes | Secondary outcomes |
| No primary outcomes reported | Foot problems requiring treatment: Foot care knowledge: Foot care routine compliance: | |
| Ulcer or amputation: people with callus, nail dystrophy or fungal infection at baseline: intervention 2/37 vs control 3/63 people with an ulcer or amputation at baseline: intervention 6/7 vs control 11/13 | Callus, nail dystrophy and fungal infection: Behaviour assessment scores: | |
| No primary outcomes reported | Patients' self‐reported behaviour assessment scores: Observed self‐care behaviour: Foot care knowledge scores: | |
| Ulcer incidence: people without a history of foot ulceration: intervention 8/21 vs control 8/14 (P = 0.317) Patient with a history of foot ulceration: intervention 1/8 vs control 5/8 (P = 0.119) All people: difference between the survival curves of intervention and control (P = 0.362) (HR not reported) | No secondary outcomes reported | |
| No primary outcomes reported | Foot care knowledge scores: Foot care practice scores: | |
| No primary outcomes reported | Foot care knowledge scores: Patients' behaviour assessment: | |
| No primary outcomes reported | Foot status: Foot care knowledge scores: Behaviour assessment: | |
| Ulcer incidence: Amputation rate: | Behaviour assessment scores: | |
| Ulcer incidence: Amputation rate: | No secondary outcomes reported | |
| No primary outcomes reported | Foot care knowledge scores: | |
| No primary outcomes reported | Foot appearance scores (mean ±standard error): Foot care knowledge scores: Foot care skills scores: | |
| Amputation: 7‐year follow‐up: intervention 1 vs control 0 Foot ulceration: 7‐year follow‐up: intervention 1 vs control 1 | Callus development: Calcaneal region:
Other regions:
7‐year follow‐up: Calcaneal region:
Other regions:
Foot care knowledge scores:
7‐year follow‐up:
Patients' behaviour assessment scores:
7‐year follow‐up:
| |
| Abbreviations: CI = confidence interval, ns = no statistical significance, RA = group that received risk assessment only, RR = risk ratio, SD = standard deviation. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Foot ulcer incidence (1‐year follow‐up) Show forest plot | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.66] |
| 2 Amputation Rate (1‐year follow‐up) Show forest plot | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.76] |
| 3 Foot ulcer incidence (1‐year follow‐up) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.70, 1.44] |
| 4 Amputation rate (1‐year follow‐up) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.41, 2.34] |

