Pneumococcal conjugate vaccines for preventing otitis media
Abstract
Background
Acute otitis media (AOM) is a very common respiratory infection in early infancy and childhood. The marginal benefits of antibiotics for AOM in low‐risk populations in general, the increasing problem of bacterial resistance to antibiotics and the huge estimated direct and indirect annual costs associated with otitis media (OM) have prompted a search for effective vaccines to prevent AOM.
Objectives
To assess the effect of pneumococcal conjugate vaccines (PCVs) in preventing AOM in children up to 12 years of age.
Search methods
We searched CENTRAL (2013, Issue 11), MEDLINE (1995 to November week 3, 2013), EMBASE (1995 to December 2013), CINAHL (2007 to December 2013), LILACS (2007 to December 2013) and Web of Science (2007 to December 2013).
Selection criteria
Randomised controlled trials (RCTs) of PCVs to prevent AOM in children aged 12 years or younger, with a follow‐up of at least six months after vaccination.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data.
Main results
We included 11 publications of nine RCTs (n = 48,426 children, range 74 to 37,868 per study) of 7‐ to 11‐valent PCV (with different carrier proteins). Five trials (n = 47,108) included infants, while four trials (n = 1318) included children aged one to seven years that were either healthy (one study, n = 264) or had a previous history of upper respiratory tract infection (URTI), including AOM. We judged the methodological quality of the included studies to be moderate to high. There was considerable clinical diversity between studies in terms of study population, type of conjugate vaccine and outcome measures. We therefore refrained from pooling the results.
In three studies, the 7‐valent PCV with CRM197 as carrier protein (CRM197‐PCV7) administered during early infancy was associated with a relative risk reduction (RRR) of all‐cause AOM ranging from ‐5% in high‐risk children (95% confidence interval (CI) ‐25% to 12%) to 7% in low‐risk children (95% CI 4% to 9%). Another 7‐valent PCV with the outer membrane protein complex of Neisseria meningitidis (N. meningitidis) serogroup B as carrier protein, administered in infancy, did not reduce overall AOM episodes, while a precursor 11‐valent PCV with Haemophilus influenzae (H. influenzae) protein D as carrier protein was associated with a RRR of all‐cause AOM episodes of 34% (95% CI 21% to 44%).
A 9‐valent PCV (with CRM197 carrier protein) administered in healthy toddlers was associated with a RRR of (parent‐reported) OM episodes of 17% (95% CI ‐2% to 33%). CRM197‐PCV7 followed by 23‐valent pneumococcal polysaccharide vaccination administered after infancy in older children with a history of AOM showed no beneficial effect on first occurrence and later AOM episodes. In a study in older children with a previously diagnosed respiratory tract infection, performed during the influenza season, a trivalent influenza vaccine combined with placebo (TIV/placebo) led to fewer all‐cause AOM episodes than vaccination with TIV and PCV7 (TIV/PCV7) when compared to hepatitis B vaccination and placebo (HBV/placebo) (RRR 71%, 95% CI 30% to 88% versus RRR 57%, 95% CI 6% to 80%, respectively) indicating that CRM197‐PCV7 after infancy may even have negative effects on AOM.
Authors' conclusions
Based on current evidence of the effects of PCVs for preventing AOM, the licensed 7‐valent CRM197‐PCV7 has modest beneficial effects in healthy infants with a low baseline risk of AOM. Administering PCV7 in high‐risk infants, after early infancy and in older children with a history of AOM, appears to have no benefit in preventing further episodes. Currently, several RCTs with different (newly licensed, multivalent) PCVs administered during early infancy are ongoing to establish their effects on AOM. Results of these studies may provide a better understanding of the role of the newly licensed, multivalent PCVs in preventing AOM. Also the impact on AOM of the carrier protein D, as used in certain pneumococcal vaccines, needs to be further established.
PICO
Plain language summary
Vaccination against a bacterium called pneumococcus for preventing middle ear infection
Review question
We reviewed the evidence about the effect of vaccination against pneumococcus (a type of bacterium) on preventing middle ear infections in children.
Background
Middle ear infection, or otitis media, is one of the most common respiratory infections in childhood. Infection with Streptococcus pneumoniae (pneumococcus) is a frequent cause of middle ear infection. Vaccination against pneumococcus with pneumococcal conjugate vaccines (PCVs) is primarily introduced to protect young children against severe pneumococcal infections, such as meningitis and pneumonia. We wanted to discover whether vaccination with PCV also leads to fewer middle ear infections in children.
Study characteristics
This review included evidence up to 3 December 2013. Nine trials with a total of 48,426 children were included; five trials included 47,108 infants, while four trials included 1318 children at a later age, i.e. aged one to seven years, who were either healthy (one trial, 264 children) or had previous upper respiratory tract infections, including middle ear infections. All trials had a long follow‐up, varying from 6 to 40 months.
Key outcomes
When vaccinating against seven different serotypes of pneumococcus (7‐valent PCV) during early infancy, the occurrence of middle ear infections either increased by 5% or decreased by 6% to 7%. One study in infants used 11 serotypes of pneumococcus together with a carrier protein from another bacterium (Haemophilus influenzae); this decreased the occurrence of middle ear infections by 34%.
Children with a history of middle ear infections do not seem to benefit from 7‐valent PCV when immunised at an older age (after infancy).
Quality of the evidence
We judged the quality of the evidence for 7‐valent PCV in early infancy to be high (further research is very unlikely to change our confidence in the estimate of effect), while we judged the quality of the evidence for multivalent (more than seven different serotypes) PCV to be moderate (further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate), as this evidence is derived from only one trial. We judged the quality of the evidence for 7‐valent PCV in older children with a history of middle ear infections to be high.
Future studies on the effects of PCV in infants, with broader serotype coverage (more than seven different serotypes), are likely to provide more understanding of the role of PCV in preventing middle ear infections.
Authors' conclusions
Summary of findings
| Pneumococcal conjugate vaccine compared with control intervention for preventing acute otitis media | ||||
| Patient or population: children aged 12 years or younger and a follow‐up after vaccinations of at least 6 months Settings: open population Intervention: multivalent PCVs Comparison: control treatment | ||||
| Outcomes | VE ‐ relative effect (95% CI)* | No of participants | Quality of the evidence | Comments |
| Frequency of all‐cause AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR: ‐5% to 7% | 42,140 (4) | ⊕⊕⊕⊕ | Results are derived from 1 very large trial (Black 2000/Fireman 2003) and 3 trials of approximately equal size (944 to 1666 participants) (Eskola 2001; Kilpi 2003; O'Brien 2008) Lowest efficacy was found in high‐risk children (O'Brien 2008) |
| Frequency of all‐cause AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 34% (21 to 44) | 4968 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial (Prymula 2006) Part of the effect may be related to the protein D to which the polysaccharides are conjugated in the vaccine PD‐PCV11, demonstrated to reduce non‐typeable H. influenzae by 35% (95% CI 2 to 57) AOM incidence rate in control group was low compared to the other studies on the effect on PCV7 in infants and the absolute risk difference was small (Table 1) |
| Frequency of all‐cause AOM CRM197‐PCV9 administered in healthy toddlers Follow‐up 24 months | RRR 17% (‐2 to 33) | 264 (1) | ⊕⊕⊕⊝ | Results derived from 1 trial of moderate methodological quality (Dagan 2001). Uncertainty about the effect size (statistically non‐significant effect) and outcome measure (parent‐reported OM) |
| Frequency of all‐cause AOM PCV7 administered in older children with a known history of AOM Follow‐up 6 to 26 months | RRR ‐29% to 57% | 1054 (3) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Veenhoven 2003; Van Kempen 2006) and 1 trial of moderate methodological quality (Jansen 2008). The 2 high‐quality trials found no beneficial effect of PCV in preventing AOM recurrences, while the other trial found PCV7/TIV not to be superior to TIV/placebo in preventing AOM during the influenza season |
| Frequency of pneumococcal AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR 20% to 34% | 1233 (2) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Eskola 2001/Palmu 2009; Kilpi 2003) |
| Frequency of pneumococcal AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 52% (37 to 63) | 281 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial in which myringotomy was performed in all children (Prymula 2006) |
| GRADE Working Group grades of evidence *Results include both ITT and PP results; 95% CI lacking in case of multiple studies (range of effect estimates presented as we refrained from pooling). | ||||
| AOM: acute otitis media | ||||
Background
Description of the condition
Acute otitis media (AOM), defined as middle ear effusion accompanied by one or more signs of acute inflammation in the middle ear, such as otalgia, otorrhoea, fever or irritability, is one of the most common diseases in childhood, imposing a large burden on public health. It has a peak incidence in six to 11‐month‐old infants (Teele 1989). By the age of one year, approximately 60% of infants have experienced at least one AOM episode and by the age of two years up to 5% of all children have experienced recurrent episodes of AOM, defined as three or more AOM episodes in six months or four or more in one year (Kvaerner 1997; Teele 1989). The three main bacterial pathogens isolated from the middle ear fluid of children with AOM, collected before the widespread use of pneumococcal conjugate vaccines (PCVs), were Streptococcus pneumoniae (S. pneumoniae) (25% to 39%), (non‐typeable) Haemophilus influenzae (H. influenzae) (12% to 23%) and Moraxella catarrhalis (M. catarrhalis) (4% to 15%) (Bluestone 1992; Heikkinen 1999; Jacobs 1998; Luotonen 1981). Recent studies have shown that nationwide implementation of PCVs may have changed the frequency of the causative otopathogens involved in AOM towards pneumococcal serotypes not included in the vaccines and non‐typeable H. influenzae (ntHi) (Casey 2013; Coker 2010; Somech 2011; Wiertsema 2011).
Description of the intervention
The marginal benefits of antibiotics for AOM in low‐risk populations (Rovers 2006; Spiro 2008; Venekamp 2013), the increasing problem of bacterial resistance against antibiotics (Arason 1996; Del Castillo 1998; Dagan 2000; Goossens 2007) and the high estimated direct and indirect annual costs associated with otitis media (OM) (Boonacker 2011; Kaplan 1997; Niemela 1999) have prompted a search for effective vaccines to prevent AOM.
How the intervention might work
With S. pneumoniae (pneumococcus) being the prime bacterial cause of AOM and childhood pneumonia, and one of the most common causes of invasive bacterial disease such as meningitis, research has focused on the prevention of pneumococcal infections with pneumococcal vaccines. Pneumococcal polysaccharide vaccines (PPVs) have been available for decades but have been shown to be poorly immunogenic in children below two years of age, who are most prone to pneumococcal infections. The first pneumococcal conjugate vaccines (PCVs), in which the pneumococcal capsular serotypes are covalently conjugated to carrier proteins, were developed in the 1990s and proved to be adequately immunogenic in infants and toddlers (Dagan 1997; Eskola 1999; Shinefield 1999). No further attention will be paid to the effect of PPVs, which were described in a prior version of this review (Straetemans 2003).
Why it is important to do this review
With AOM being amongst the most common diseases in early childhood, the need for a vaccine to prevent effectively AOM is high. Over the past decades various randomised controlled trials (RCTs) have been performed to assess the effects of pneumococcal vaccination to prevent AOM. From 2009 onwards, two multivalent PCVs (10‐ and 13‐valent PCVs) have been licensed and are being implemented in nationwide immunisation programmes worldwide (WHO 2012). These new vaccines may have an increased benefit in preventing AOM (Marom 2014; O'Brien 2009). As such, it is important to provide an up‐to‐date systematic review on the effects of pneumococcal vaccination on preventing AOM. This review is an update of a Cochrane review first published in 2002 (Straetemans 2002), and updated in 2004 (Straetemans 2004) and 2009 (Jansen 2009).
Objectives
To assess the effect of pneumococcal conjugate vaccines (PCVs) in preventing AOM in children up to 12 years of age.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of PCVs with prevention of AOM as an outcome in children aged 12 years or younger and a follow‐up for at least six months.
Types of participants
Children up to 12 years of age.
Types of interventions
Multivalent PCVs.
Types of outcome measures
Primary outcomes
Frequency of all‐cause AOM episodes defined as AOM irrespective of causative pathogen, as we considered this to be most relevant for children, parents and physicians.
Secondary outcomes
-
Frequency of pneumococcal AOM.
-
Frequency of pneumococcal serotype‐specific AOM.
-
Frequency of recurrent AOM (defined as three or more episodes in the last six months or four or more in the last year).
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 11), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1995 to November week 3, 2013); EMBASE (1995 to December 2013); CINAHL (2007 to December 2013); LILACS (2007 to December 2013) and Web of Science (2007 to December 2013).
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4) and Web of Science (Appendix 5).
MEDLINE (Ovid)
1 exp Otitis Media/
2 otitis media.tw.
3 aom.tw.
4 or/1‐3
5 Pneumococcal Vaccines/
6 Vaccines, Conjugate/
7 Bacterial Vaccines/
8 (pneumococc* adj5 (vaccin* or conjugat* or immuni*)).tw,nm.
9 pcv*.tw,nm.
10 or/5‐9
11 4 and 10
Searching other resources
To increase the yield of relevant studies we reviewed the reference lists of all studies and review articles retrieved. We imposed no language restrictions on the searches. We checked ClinicalTrials.gov (http://clinicaltrials.gov/) and WHO ICTRP (http://www.who.int/trialsearch) for completed and ongoing trials (3 December 2013).
Data collection and analysis
Selection of studies
Three review authors (ACF, RPV, CWB) independently screened titles and abstracts obtained from the database searches and reviewed the full text of the potentially relevant titles and abstracts against the inclusion criteria. We resolved disagreements by discussion.
Data extraction and management
Two review authors (ACF, RPV) independently extracted data from the included studies. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (ACF, RPV) independently assessed the methodological quality of the included trials. We resolved any disagreements by discussion. We assessed the methodological quality of included studies using the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We judged the following domains as high, low or unclear risk of bias: random sequence generation (selection bias), concealment of allocation (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. We presented the results of the 'Risk of bias' assessment in a 'Risk of bias' graph (Figure 1) and a 'Risk of bias' summary (Figure 2).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For both our primary and secondary outcomes we extracted the relative risks and their accompanying 95% CIs. Vaccine efficacy was estimated as 1 minus the relative risk (relative risk reduction (RRR)).
Unit of analysis issues
We included all types of RCTs. In case of cluster‐randomised trials, we considered potential differences between the intervention effects being estimated.
Dealing with missing data
We primarily presented data based on the intention‐to‐treat (ITT) principle, i.e. all data were analysed in the group to which the participants were originally allocated. As a secondary analysis, we presented data based on the per‐protocol analysis. For each trial, we determined the number of missing data and whether the authors took duration of follow‐up (and censoring) of individual participants into account in their statistical analyses.
Assessment of heterogeneity
Meta‐analysis by pooling the results of the different trials is only useful and justified when trials show satisfactory clinical homogeneity in terms of study population, setting, intervention and outcome measures. We assessed clinical diversity between trials by reviewing the differences across trials. There was considerable clinical diversity between trials. First, there were differences in the timing of PCV administration, i.e. trials administering PCV during infancy and trials administering PCV later in life. As such, study populations varied from healthy infants to those at high risk of AOM. Second, the number of pneumococcal serotypes present in the vaccines, the type of conjugate method used and co‐administration of non‐bacterial vaccines differed across trials. For example, the protein D carrier, as used in one trial assessing the effect of PD‐PCV11 (Prymula 2006), has been shown to be beneficial in reducing AOM caused by non‐typeableH. influenzae (ntHi) (Prymula 2006), and as such may have an impact on all‐cause AOM beyond pneumococcal AOM. Furthermore, co‐administration of a trivalent flu vaccine with CRM197‐PCV7, as used in another trial (Jansen 2008), may have substantial impact on all‐cause AOM. Finally, large differences in outcome assessments and AOM definitions were observed, varying from 'passive' (chart review at end of the trial) versus 'active' (parents were instructed to visit a physician in case of AOM symptoms) outcome assessments. As a consequence, AOM incidence in the control groups varied widely across the studies administering PCV during infancy, i.e. from 0.13 to 1.3 episodes per person‐year. Therefore, we decided that a meta‐analysis was not appropriate.
Assessment of reporting biases
For each study, we searched the Internet and ClinicalTrials.gov (http://clinicaltrials.gov/) for available study protocols to determine whether all a priori defined outcomes have been reported in the publications.
Data synthesis
As we refrained from pooling (see Assessment of heterogeneity section), we reported the effect estimates as presented by the individual trials. Where possible we reported the incidences of the various outcomes in the study arms together with the vaccine efficacy estimates, with 95% confidence intervals (CIs). However, due to limitations of the data, we reported alternative statistical measures in some instances.
We will briefly describe the methods we would have used if we had pooled the results. The generalised Cox proportional hazard method proposed by Andersen 1982 is regarded as the most appropriate to assess the effect of PCVs on AOM (Jahn‐Eimermacher 2007). Under the assumption that the hazard rate is proportional between both groups over time and that the risk of AOM is not affected by previous episodes (although this assumption is not true), this model takes all available information into account; that is, all episodes (also the recurrent ones), differences in individual patient follow‐up time and time until a case of AOM (Jahn‐Eimermacher 2007). However, information on individual follow‐up time until the first, second, third, etc. case of AOM is hard to obtain for each study to be included in the meta‐analysis. Poisson regression is based on the assumption of a constant risk of AOM over time and that this risk is not affected by previous episodes of AOM. This method only requires the total follow‐up time and total number of episodes and appears therefore a more feasible method for meta‐analysis. Furthermore, Poisson regression seems not to be affected by the deviation from a constant risk over time, having very similar results for the effect of PCVs on AOM to the Anderson‐Gill approach (Jahn‐Eimermacher 2007). For Poisson regression, the treatment effect is measured as a rate ratio defined as follows: (total AOM episodes in pneumococcal vaccination group divided by the number of children in the pneumococcal vaccination group multiplied by the follow‐up time in months) divided by (total AOM episodes in control group divided by the number of children in the control group multiplied by the follow‐up time in months) (McCullagh 1989).
Subgroup analysis and investigation of heterogeneity
Since the effect of PCVs on AOM may be influenced by the age at which the PCV was administered and the occurrence of previous AOM episodes, we described the studies accordingly, that is, those with vaccination in early infancy versus those with vaccination later in childhood.
Sensitivity analysis
As mentioned, the clinical diversity between the studies was large and therefore we decided not to meta‐analyse the trials nor conduct a sensitivity analysis on, for example: risk status (age, number of previous episodes), outcome measurement or adjustments for clustering in the case of a cluster‐randomised trial.
Results
Description of studies
See the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
This is an update of a Cochrane review first published in 2002 (Straetemans 2002) and updated in 2004 (Straetemans 2004) and 2009 (Jansen 2009). In the 2009 review, which included studies up to November 2007, eight publications were found eligible, concerning a total of seven RCTs (Black 2000/Fireman 2003; Dagan 2001; Eskola 2001; Kilpi 2003; Prymula 2006; Van Kempen 2006; Veenhoven 2003). The studies of Eskola 2001 and Kilpi 2003 are part of the FinOM Vaccine Trial (three parallel‐group trials using the same control group (hepatitis B vaccine) but two different treatment groups, each with a different type of 7‐valent pneumococcal conjugate vaccine) and we therefore regarded them as two 'separate' trials in this review.
With the updated search (November 2007 to December 2013) we retrieved 171 records. Removing duplicates left 165 records. After screening titles and abstracts, we identified seven potentially eligible studies. After obtaining the full text of these papers, we excluded two studies as they studied the effect of pneumococcal vaccination on either otitis media with effusion (OME) (Le 2007) or suppurative otitis media (Roy 2011). Furthermore, one RCT studying the effect of PCV on (recurrent) AOM was excluded as the children in the control group did not receive any type of vaccination (PCV versus no vaccination), while for outcome assessment non‐blinded parents were instructed to visit the ear, nose and throat (ENT) department whenever they suspected an episode of AOM; the parental threshold to consult ENT departments may be lower in children allocated to the control treatment (no vaccination) than in those allocated to PCV, which may have introduced (detection) bias (Gisselsson Solen 2011). Finally, one trial was excluded (Jokinen 2012), as this study was a re‐analysis of the Eskola 2001 study and did not include new outcome data useful to this review. This left three new publications eligible for inclusion (Jansen 2008; O'Brien 2008; Palmu 2009). One study (Palmu 2009) was an additional analysis of the previously included Eskola 2001 study. We did not identify any additional eligible trials after scanning the reference lists of the full‐text papers and relevant systematic reviews. In searching ClinicalTrials.gov, we identified five ongoing RCTs (NCT00466947; NCT00861380; NCT01545375; NCT01735084; NCT01174849) (see also the Characteristics of ongoing studies table).
Included studies
The 11 included trials in this review originate from nine RCTs in total: (1) Black 2000/Fireman 2003; (2) Dagan 2001; (3) Eskola 2001/Palmu 2009; (4) Jansen 2008; (5) Kilpi 2003; (6) O'Brien 2008; (7) Prymula 2006; (8) Van Kempen 2006; (9) Veenhoven 2003.
Study designs
Of these trials, eight were standard, individually randomised trials, while one trial was a cluster‐randomised trial (O'Brien 2008).
Study populations (early infancy versus later in life)
Five trials (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006) included healthy infants and studied the effect of PCV administered in early infancy (first dose before the age of 12 months) on otitis media (OM), while the other four trials (Dagan 2001; Jansen 2008; Van Kempen 2006; Veenhoven 2003) assessed the effects of PCV administered at a later age on OM in either healthy infants (Dagan 2001), or in children with a known history of respiratory disease, including OM (Jansen 2008; Van Kempen 2006; Veenhoven 2003).
Interventions (type of PCV used)
In all nine RCTs the control group received a control vaccine. In six trials the 7‐valent PCV containing the polysaccharides of seven serotypes (4, 6B, 9V, 14, 18C, 19F and 23F) coupled to the carrier protein CRM197 (a non‐toxic mutant of diphtheria toxin) (CRM197‐PCV7) was used as the intervention (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Jansen 2008; O'Brien 2008; Van Kempen 2006; Veenhoven 2003). In two of these studies a booster dose with 23‐valent PPV (containing capsular polysaccharides of the serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F and 33F) was given to all children (Van Kempen 2006; Veenhoven 2003), while in one trial CRM197‐PCV7 was administered with a trivalent inactivated influenza vaccine (TIV) (Jansen 2008). The other three studies (Dagan 2001; Kilpi 2003; Prymula 2006) used different interventions, i.e. a 9‐valent PCV containing the capsular polysaccharides of serotypes 1 and 5 besides those included in CRM197‐PCV7, conjugated to CRM197 (CRM197‐PCV9) (Dagan 2001), a 7‐valent PCV with the outer membrane complex of N. meningitidis serogroup B as protein carrier (OMPC‐PCV7) (Kilpi 2003) and a subgroup of these children received a PPV23 as a booster dose and an 11‐valent PCV containing the capsular polysaccharides of serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F, conjugated to protein D, which is a surface lipoprotein of H. influenzae (PD‐PCV11) (Prymula 2006).
Outcomes
Five studies applied a standardised diagnosis of AOM (Eskola 2001/Palmu 2009; Kilpi 2003; Prymula 2006; Van Kempen 2006; Veenhoven 2003), one study used standardised AOM registration forms to be completed by GPs (Jansen 2008), whereas in two studies AOM episodes were extracted from a computerised data source containing all visits registered by physicians (Black 2000/Fireman 2003; O'Brien 2008). Another study assessed parent‐reported AOM episodes (Dagan 2001). Six studies additionally assessed the effect of PCVs on (serotype‐specific) pneumococcal AOM (Black 2000/Fireman 2003; Eskola 2001; Kilpi 2003; O'Brien 2008; Prymula 2006; Veenhoven 2003). Three studies cultured middle ear fluid from all AOM episodes (Eskola 2001; Kilpi 2003; Prymula 2006), whereas one study only cultured it from the first AOM episode by myringotomy or from spontaneously draining ears (Veenhoven 2003). Two other studies assessed the effect on reported cultures that were taken in cases of spontaneously draining ears (Black 2000/Fireman 2003; O'Brien 2008). Three studies reported on the effects of PCVs on recurrent AOM (Black 2000/Fireman 2003; Eskola 2001; Prymula 2006). Three of the included studies had all types of OM (including but not exclusively AOM) as an outcome (Black 2000/Fireman 2003; Dagan 2001; O'Brien 2008). Since the effect of PCVs on AOM may be influenced by the age at which the PCV was administered and the occurrence of previous episodes of AOM, we will further describe the studies accordingly; that is, those with vaccination in infancy versus those vaccinated later in childhood.
PCV administered in early infancy (first dose before the age of 12 months)
In the Northern California Kaiser Permanente (NCKP) trial (Black 2000/Fireman 2003), the Finnish Otitis Media (FinOM) trial (Eskola 2001/Palmu 2009) and the trial among Navajo and White Mountain Apache children (O'Brien 2008), the treatment group was administered CRM197‐PCV7, at the age of two, four and six months and 12 to 15 months. In the NCKP trial (Black 2000/Fireman 2003), infants were enrolled over a period of almost three years and had a follow‐up time varying from about eight months to 30 months (Black 2000) and 42 months (Fireman 2003), respectively. The trial was originally designed to investigate the effect of CRM197‐PCV7 on invasive pneumococcal disease, with OM as a secondary outcome. Clinical diagnoses of OM were obtained from a computerised database collecting department‐specific diagnosis checklists routinely marked by emergency physicians and paediatricians in the NCKP population. All clinical diagnoses of 'otitis media', 'otitis media, acute', 'middle ear effusion', 'otitis media, serous' or 'otitis media with effusion' were included.
The FinOM trial primarily aimed to assess the effect of CRM197‐PCV7 on AOM (Eskola 2001/Palmu 2009). Infants were followed until the age of 24 months and parents were encouraged to bring their child to the study clinic (established specifically for the purpose) for evaluation of symptoms suggesting respiratory infection or AOM. The diagnosis of AOM was standardised. In recent years, an additional analysis was performed as part of the Eskola 2001 trial, including pneumolysin‐PCR positive AOM (Palmu 2009).
The trial among Navajo and White Mountain Apache children was a cluster‐randomised trial primarily aiming to assess the safety and efficacy of CRM197‐PCV7 on invasive pneumococcal disease (O'Brien 2003). These children have some of the highest rates of invasive pneumococcal disease and otitis media in the world. Clinical and culture‐proven OM were secondary outcomes measured in this trial and were assessed, at trial completion, by retrospective chart review. OM visits, as in every visit made by the study children through to two years of age and documented by their treating physician, were evaluated. An OM visit was defined as a visit for 'otitis media', 'acute otitis media', 'bilateral OM', 'chronic OM', 'OM with perforation', 'otorrhoea', 'pressure equalising tube placement', 'perforation tympanic membrane', 'serous OM' and 'bullous myringitis'. Further sub‐categorisation was performed on AOM (either 'acute otitis media' or 'bilateral otitis media'), severe episodes, number of medical visits and pressure equalising tube placement.
Kilpi 2003 describes another part of the FinOM trial in which the index group was administered another 7‐valent PCV, containing capsular polysaccharides of the same seven serotypes as used in the Eskola 2001 study, i.e. OMPC‐PCV7. Additionally, 22% of the children assigned to OMPC‐PCV7 received PPV23 at the age of 12 months instead of a fourth OMPC‐PCV7 dose. The follow‐up and outcome measure was similar to Eskola 2001.
Finally, in Prymula 2006 (POET trial), an 11‐valent PCV was administered at the ages of three, four, five months and 12 to 15 months, conjugated to protein D (PD‐PCV11). Follow‐up continued until the age of 24 to 27 months. The primary aim of the trial was to assess the effect on AOM and parents were advised to consult their paediatrician if their child was sick, had ear pain or had spontaneous ear discharge. The diagnosis of AOM was standardised.
PCV administered at a later age (first dose administered after 12 months of age)
Dagan 2001 assessed the effect of CRM197‐PCV9 on AOM in healthy day‐care attendees aged 12 to 35 months. The vaccine was administered twice in 12‐ to 17‐month‐olds and once in 18‐ to 35‐month‐olds. The study was undertaken to examine the effect on respiratory infections. In 18 encounters during the two‐year follow‐up period that started one month after complete immunisation, parents were questioned about illness episodes, including OM episodes. The OM diagnosis was not physician‐confirmed and not standardised.
Van Kempen 2006 and Veenhoven 2003 assessed the effect of pneumococcal vaccination on AOM in children aged one to seven years with a history of at least two AOM episodes in the year prior to study entry. CRM197‐PCV7 was administered twice in one to two year‐olds and once in two to seven year‐olds followed by PPV23 six to seven months later. Children with underlying illnesses, including immuno‐compromising conditions, were excluded. Both studies had a similar design and were conducted in parallel, but were analysed separately due to differences in study population (children included in Van Kempen 2006 had a more severe history of AOM and more frequent tympanostomy tube placement prior to study entry). Follow‐up lasted about 24 months. Parents were instructed to visit the study clinics or their family physician, otolaryngologists or paediatrician for assessment in case of symptoms suggesting AOM. Physicians registered signs and symptoms of every AOM episode on standard registration forms. The diagnosis of AOM was standardised.
In Jansen 2008, children aged between 18 and 72 months were randomly assigned in blocks of three in a 1:1:1 ratio to either (1) two doses of CRM197‐PCV7 (six months apart) administered together with a trivalent inactivated influenza vaccine (TIV), (2) TIV plus placebo or (3) hepatitis B virus vaccine plus placebo. The main outcome measure was febrile respiratory tract infections including AOM during the influenza season. All children were eligible if they had a previous history of physician‐diagnosed respiratory tract infections (RTI). This history included 'acute otitis media', 'cough', acute upper RTI', 'sinusitis', 'acute tonsillitis', 'acute laryngitis/tracheitis', 'acute bronchitis/bronchiolitis', 'influenza', 'pneumonia', 'pleurisy/pleural effusion' and 'other respiratory infections', all recorded according to the International Classification of Primary Care (ICPC). Each parent was instructed to keep a daily diary, recording signs or symptoms associated with RTI that began 14 days after the second dose and continued for six to 18 months (depending on year of inclusion). The parent was also instructed to measure the child's body temperature with a validated tympanic thermometer provided by the study centre. All GP visits were recorded as well and the GP was instructed to complete a form including diagnosis and treatment.
Excluded studies
Four studies were excluded for various reasons (Gisselsson Solen 2011; Jokinen 2012; Le 2007; Roy 2011) (see also Characteristics of excluded studies table).
Risk of bias in included studies
We judged the methodological quality of the included studies to be moderate to high. For further details on the risk of bias in included studies see the 'Risk of bias' graph (Figure 1) and 'Risk of bias' summary (Figure 2).
Allocation
Concealment of allocation was adequately described in six of the nine included trials, while in the other three trials it was unclear due to insufficient information (Black 2000/Fireman 2003; Jansen 2008; Veenhoven 2003). We judged random sequence generation to be adequate in five of the nine trials, while in four trials insufficient information was provided on the method of random sequence generation used (Black 2000/Fireman 2003; Dagan 2001; Jansen 2008; Van Kempen 2006).
Blinding
Authors of all studies indicated that the studies were double‐blinded, but for two of the nine trials insufficient information was provided on how blinding was performed (Black 2000/Fireman 2003; Prymula 2006).
Incomplete outcome data
Overall, we judged the risk of bias due to incomplete outcome data to be low. In the Fireman 2003 study (extension of Black 2000), we judged the risk of attrition bias to be high, as 27% in the PCV group and 26% in the control group did not stay in the Kaiser Permanente healthcare database to the end of follow‐up (April 1999), while missing data were either substantial or unclear in two trials (Jansen 2008; Palmu 2009), and in one trial the distribution across treatment groups of those who were not included in the primary efficacy analysis was unclear (O'Brien 2008).
Almost all trials did take duration of follow‐up of individuals into account by using either the generalised Cox proportional hazard method (Black 2000/Fireman 2003; Eskola 2001; Kilpi 2003; Prymula 2006; Van Kempen 2006; Veenhoven 2003), or Poisson regression analyses (Jansen 2008; O'Brien 2008). One trial used a Chi2 test to compare rates of AOM, which is considered suboptimal (Dagan 2001).
Selective reporting
In three trials, we judged the risk of reporting bias to be low as prespecified (primary and secondary) outcomes were listed in ClinicalTrials.gov (Jansen 2008; O'Brien 2008; Prymula 2006). O'Brien 2008 used a subset of the 4476 children that were included in the cluster‐randomised trial to estimate the efficacy of PCV on the main outcome of the trial, i.e. invasive pneumococcal disease for a retrospective chart review on OM. Of the 4476 children that were included in the main study (O'Brien 2003), 944 were randomly selected and 856 met the chart review criteria (O'Brien 2008). In four trials no study protocol was identified (Black 2000/Fireman 2003; Dagan 2001; Van Kempen 2006; Veenhoven 2003), while in two trials the study protocol was uploaded at ClinicalTrials.gov but after the completion of the study (Eskola 2001/Palmu 2009; Kilpi 2003).
Other potential sources of bias
In four trials we detected no other potential sources of bias (Dagan 2001; Eskola 2001/Palmu 2009; Van Kempen 2006; Veenhoven 2003). In three trials study enrolment was stopped as a result of interim analyses (Black 2000/Fireman 2003; O'Brien 2008; Prymula 2006). These interim analyses were prespecified and performed by independent data safety monitoring boards; we therefore judged the risk of bias as low. In one trial a sample of the children (22%) assigned to OMPC‐PCV7 received PPV23 at the age of 12 months instead of a fourth OMPC‐PCV7 dose. However, it was unclear how it was known that only those particular children should receive that intervention. Moreover, in one trial PCV was administered together with a influenza vaccine (CRM197‐PCV7/TIV) (Jansen 2008). As such, it is not possible to determine the effect of PCV only in this study.
Effects of interventions
See: Summary of findings for the main comparison
The main results are described in summary of findings Table for the main comparison.
In total, we included nine trials with 48,426 children, ranging from 74 to 37,868 participants per study. Five trials included infants who received primary vaccinations before six months of age (47,108 participants in total) (Black 2000/Fireman 2003; Eskola 2001/Palmu 2009; Kilpi 2003; O'Brien 2008; Prymula 2006); one study included day‐care attendees aged 12 to 35 months (264 participants) (Dagan 2001), two trials included one to seven year‐olds with a history of acute otitis media (AOM) (457 participants) (Van Kempen 2006; Veenhoven 2003), and one trial included children aged 18 to 72 months with a previously diagnosed respiratory tract infection (RTI) (597 participants) (Jansen 2008).
We present the results of the individual trials as reported in the published papers since meta‐analysis was inappropriate due to substantial differences between studies. Therefore, the statistical methods by which the data were analysed in each study are briefly assessed. Black 2000/Fireman 2003, Eskola 2001, Kilpi 2003, Prymula 2006, Van Kempen 2006 and Veenhoven 2003 all used the generalised Cox proportional hazard method proposed by Andersen 1982, currently regarded as the most optimal for analysing this kind of data (see Data synthesis). Dagan 2001 compared rates of AOM, but rather than comparing them by Poisson regression (which would presumably yield results similar to those obtained with the Anderson approach), the Chi2 test was used, which is suboptimal for comparing rates. Jansen 2008 used Poisson regression to compare rates of AOM, accounting for the potential dependency of observations between individuals. O'Brien 2008 was a cluster‐randomised trial. The incidence rate ratio was calculated with a Poisson regression with sandwich variance estimation to account for within‐community correlation.
Effect estimates of pneumococcal conjugate vaccines (PCVs) on all AOM episodes, pneumococcal AOM and recurrent AOM are summarised in Table 1, Table 2 and Table 3, respectively.
| Intention‐to‐treat | Per‐protocol | ||||||
| Episodes/person year | VE expressed as relative reduction in risk (95% CI) | Episodes/person year | Incidence rate difference ‐ episodes per person year (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||
| Treatment | Control | Treatment | Control | ||||
| PCV administered in early infancy | |||||||
| ‐ ‐ | ‐ ‐ | 6% (4 to 9) 6% (4 to 8) | ‐ ‐ | ‐ ‐ | ‐ ‐ | 7% (4 to 10) 7% (4 to 9) | |
| ‐ | ‐ | ‐ | 1.16 | 1.24 | ‐0.08 ˜ | 6% (‐4 to 16) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐1% (‐12 to 10) | |
| ‐ | ‐ | ‐ | 0.08 | 0.13 | ‐0.04 ˜ | 34% (21 to 44) | |
| 1.4 | 1.4 | ‐5% (‐25 to 12)# | 1.3 | 1.3 | 0.0 (‐0.13 to 0.14) | 0% (‐21 to 17) | |
| PCV administered at a later age | |||||||
| ‐ | ‐ | ‐ | 0.66 | 0.79 | ‐0.14 (‐0.29 to 0.02) | 17% (‐2 to 33) | |
| ‐ | ‐ | ‐25% (‐57 to 1) | 1.1 | 0.83 | 0.27 ˜ | ‐29% (‐62 to ‐2) | |
| ‐ | ‐ | ‐ | 0.78 | 0.67 | 0.11 ˜ | ‐16% (‐96 to 31) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 57% (6 to 80)^ | |
CI: confidence interval; HBV: hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; TIV: trivalent influenza vaccine; VE: vaccine efficacy.
*Cluster‐randomised trial.
#Defined as primary efficacy analysis. Analysis not entirely according to intention‐to‐treat principle as 88/944 children were not included in analysis because of not meeting strict chart review criteria.
^Index group: TIV/PCV7, control: HBV/placebo; VE TIV/placebo versus HBV/placebo: 71% (95% 30% to 88%), i.e. larger VE TIV/placebo versus HBV/placebo then TIV/PCV7 versus HBV/placebo.
˜ 95% CI could not be calculated as person‐time across treatment groups was not reported.
Note: negative values for VE expressed as relative reduction in risk represent an increase in the risk for AOM.
| Intention‐to‐treat | Per‐protocol | |||||||
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||||||
| Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | |
| PCV administered in infancy | ||||||||
| ‐ ‐ | 65% P = 0.04 ‐ | ‐ ‐ | ‐ ‐ | ‐ ‐ | 67% P = 0.08 ‐ | ‐ ‐ | ‐ ‐ | |
| ‐ ‐ | 54% (41 to 64) ‐ | ‐ ‐ | ‐ ‐ | 34% (21 to 45) 20% (7 to 31) | 57% (44 to 67) ‐ | 51% (27 to 67) ‐ | ‐33% (‐80 to 1) ‐ | |
| ‐ | ‐ | ‐ | ‐ | 25% (11 to 37) | 56% (44 to 66) | ‐5% (‐47 to 25) | ‐27% (‐70 to 6) | |
| ‐ | ‐ | ‐ | ‐ | 52% (37 to 63) | 58% (41 to 69) | 66% (22 to 85) | 9% (‐64 to 49) | |
| O'Brien 2008* # | ‐ | 64% (‐34 to 90) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| PCV administered at a later age | ||||||||
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | 34% P = 0.22 | 52% P = 0.21 | ‐ | 21% P = 0.44 | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
VE: vaccine efficacy; PCV: pneumococcal conjugate vaccine; MEF: middle ear fluid.
*Cluster‐randomised trial.
#MEF collected from spontaneous draining ears; in the other studies MEF was routinely collected during AOM episodes through paracentesis.
^Additional analysis of Eskola 2001 including pneumococcal AOM by a positive culture or PCR.
Note: negative values represent an increase in the risk of AOM.
| Intention‐to‐treat | Per‐protocol | |
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |
| PCV administered in infancy | ||
| 9% (4 to 14) 10% (7 to 13) | 9% (3 to 15) ‐ | |
| 9% (‐12 to 27) | 16% (‐6 to 35) | |
| ‐ | ‐ | |
| ‐ | 56% (‐2 to 81) | |
| ‐ | ‐ | |
| PCV administered at a later age | ||
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
PCV: pneumococcal conjugate vaccine; VE: vaccine efficacy.
*Cluster‐randomised trial.
Note: negative values represent an increase in the risk of recurrent AOM.
Primary outcome
1. Frequency of all‐cause AOM episodes (defined as AOM irrespective of causative pathogen)
Effect of PCV administered in early infancy
CRM197‐PCV7 reduced overall AOM episodes by ‐5% in high‐risk children to 6% in low‐risk children in intention‐to‐treat (ITT) analyses and by 0% in high‐risk children to 7% in low‐risk children in per‐protocol analyses (Black 2000/Fireman 2003; Eskola 2001; O'Brien 2008), whereas OMPC‐PCV7 appeared to have no effect on overall AOM episodes (Kilpi 2003). In a per‐protocol analysis, PD‐PCV11 led to a 34% (95% confidence interval (CI) 21% to 44%) relative reduction in AOM episodes (Prymula 2006). However, the AOM incidence rate in the control group was low compared to the other studies and the absolute risk difference was small (Table 1).
Effect of PCV administered at a later age
In per‐protocol analyses, CRM197‐PCV9 administered in healthy 12‐ to 35‐month‐olds reduced overall otitis media (OM) episodes by 17% (Dagan 2001), while CRM197‐PCV7 followed by PPV23 in one to seven‐year‐olds with a history of AOM did not reduce the occurrence of further AOM episodes (Van Kempen 2006; Veenhoven 2003). CRM197‐PCV7 administered together with a trivalent influenza vaccine (CRM197‐PCV7/TIV) reduced overall AOM episodes by 57% (95% CI 6% to 80%) in per‐protocol analysis (Jansen 2008), as compared to hepatitis B (HBV)/placebo vaccination. However, the vaccine efficacy of trivalent influenza vaccine (TIV)/placebo, as compared to HBV/placebo, on overall AOM, appeared to be even larger, i.e. 71% (95% CI 30% to 88%).
Secondary outcomes
1. Frequency of pneumococcal AOM
Effect of PCV administered in early infancy
The efficacy of PCVs for pneumococcal AOM varied from 25% for OMPC‐PCV7 (Kilpi 2003), 20% to 34% for CRM197‐PCV7 (Eskola 2001/Palmu 2009), to 52% for PD‐PCV11 (Prymula 2006). CRM197‐PCV7 and PD‐PCV11 also seemed to reduce AOM caused by the so‐called cross‐reactive serotypes which are non‐vaccine serotypes with a serogroup that is included in the vaccine (Eskola 2001; Prymula 2006), while OMPC‐PCV7 failed to show cross‐protection (Kilpi 2003). Although not statistically significant, CRM197‐PCV7 and OMPC‐PCV7 were associated with an increase in non‐vaccine‐type AOM (replacement) (Eskola 2001; Kilpi 2003) and H. influenzae AOM, while PD‐PCV11 did not show pneumococcal replacement and showed a vaccine efficacy of 35% against AOM caused by H. influenzae (Prymula 2006).
Effect of PCV administered at a later age
Only one study reported the effect of CRM197‐PCV7 followed by PPV23 on pneumococcal AOM (Veenhoven 2003). In per‐protocol analysis, pneumococcal AOM was reduced by 34%, while non‐vaccine‐type AOM was reduced by 21%, although none of the estimates was statistically significant (because of small numbers).
2. Frequency of pneumococcal serotype‐specific AOM
Effect of PCV administered in early infancy
The effect of CRM197‐PCV7 on vaccine‐type pneumococcal AOM varied from 54% to 65% in ITT analyses (Black 2000/Fireman 2003; Eskola 2001; O'Brien 2008), and from 57% to 67% in per‐protocol analyses (Black 2000/Fireman 2003; Eskola 2001). In per‐protocol analyses, OMPC‐PCV7 appeared to reduce vaccine‐type AOM by 56% (Kilpi 2003), while PD‐PCV11 led to a 58% relative reduction in vaccine‐type AOM (Prymula 2006).
Effect of PCV administered at a later age
In per‐protocol analysis, CRM197‐PCV7 followed by PPV23 reduced pneumococcal serotype‐specific AOM by a statistically non‐significant 52% (Veenhoven 2003). None of the other trials reported on pneumococcal (serotype‐specific) AOM.
3. Frequency of recurrent AOM (defined as three or more episodes in the last six months or four or more in the last year)
Effect of PCV administered in early infancy
CRM197‐PCV7 seemed to reduce recurrent AOM by 9% to 10% in ITT analyses (Black 2000/Fireman 2003; Eskola 2001), whereas the administration of PD‐PCV11 was associated with a statistically non‐significant decrease of 56% in recurrent AOM in per‐protocol analysis (Prymula 2006).
Effect of PCV administered at a later age
None of the four trials in older children reported the effect of PCV on recurrent AOM.
Discussion
Summary of main results
Clinical diversity between the nine included randomised controlled trials (RCTs), in terms of study population, number of pneumococcal serotypes present in the vaccines, type of conjugate method used, co‐administration of non‐bacterial vaccines and outcome measures, was considerable and we therefore did not pool results. The main findings are summarised in the summary of findings Table for the main comparison.
Based on current evidence of the effects of pneumococcal conjugate vaccines (PCVs) for preventing acute otitis media (AOM), the licensed 7‐valent PCV has modest beneficial effects in healthy infants; in the studies in healthy infants, the licensed CRM197‐PCV7 was associated with a relative risk reduction (RRR) of overall AOM of ‐5% (in high‐risk children) to 7% (in low‐risk children) and OMPC‐PCV7 did not reduce overall AOM episodes. PD‐PCV11 showed a large reduction in all‐cause AOM episodes, i.e. 34%, compared to the PCV7 studies. In healthy toddlers, CRM197‐PCV9 was associated with a non‐significant RRR of 17% of parent‐reported OM episodes. Administering PCV7 in older children with a history of AOM appears to have no beneficial effect on preventing further AOM episodes.
In infants, PCV led to a substantial reduction in AOM caused by S. pneumoniae (RRR ranging from 20% to 52%). This beneficial effect seems to be mainly driven by the large effect of PCV on vaccine‐type pneumococcal AOM (RRR ranging from 54% to 67%). In contrast, no or even a negative contribution of PCV was observed for non‐vaccine‐type pneumococcal AOM (RRR ranging from ‐33% to 9%). For PCV7, there was a tendency toward replacement disease by non‐vaccine pneumococci as well as by other otopathogens, such as H. influenzae. OMPC‐PCV7 appeared to increase the proportion of AOM caused by M. catarrhalis (Kilpi 2003). This means that while PCVs are effective against vaccine‐serotype pneumococcal AOM, there is high potential for replacement by other pathogens. Although we are confident in the effect estimate for PCV7 administered in infancy on all‐cause AOM and pneumococcal AOM, uncertainty exists about the effect of conjugate vaccines that include more than seven pneumococcal serotypes and the impact of replacement by non‐vaccine‐type pneumococci or other otopathogens (summary of findings Table for the main comparison).
The large effect of PD‐PCV11 on all‐cause AOM found in the trial of Prymula 2006 may not be solely explained by the four additional serotypes covered by PD‐PCV11 compared to PCV7. Part of the effect might be related to the protein D, which has the potential to provide protection against H. influenzae strains causing AOM (Forsgren 2008), or may be secondary to the prevention of pneumococcal AOM in the first place with ntHi as a pathogen more frequently observed in previously inflamed middle ears (Dagan 2013; Kaur 2013). Furthermore, Prymula 2006 used a stringent outcome definition for AOM (children diagnosed with AOM by the paediatrician were subsequently referred to the otorhinolaryngologist for confirmation of diagnosis), which may have contributed to the low AOM incidence rate observed in Prymula 2006; this is about 10 times lower than the incidence reported by other studies in infancy. It might be that in Prymula 2006 more severe episodes of AOM were identified and consequently the effect may only apply to these more severe episodes. The case definition potentially also introduced a different selection of pathogens as early findings suggest that S. pneumoniae was associated with more severe episodes (Howie 1970). However, a subsequent post hoc analysis of Eskola 2001, using a case definition of AOM very close to the Prymula 2006 definition, showed only a slight impact on the vaccine efficacy estimates compared to the original case definitions (Palmu 2008).
The effect of a multivalent PCV, i.e. CRM197‐PCV9, on AOM was evaluated in healthy toddlers attending day‐care (Dagan 2001). As such, the effect of PCV9 in early life remains uncertain. In toddlers, PCV9 led to a non‐statistically significant RRR of all‐cause AOM of 17%. However, this result should be interpreted with caution as the outcome measure (parent‐reported AOM) and the statistics were suboptimal.
In AOM there is a high potential for replacement by other pathogens that are common colonisers of the nasopharynx. CRM197‐PCV7 is known to affect nasopharyngeal carriage of pneumococci, with a shift from vaccine‐type pneumococci to non‐vaccine‐type pneumococci and other bacteria that may have pathogenic potential (Block 2006; Casey 2013; Coker 2010; Eskola 2001; Obaro 1996; Somech 2011; Veenhoven 2003; Wiertsema 2011). Nasopharyngeal carriage results from a recent RCT with a commercially available 10‐valent protein D pneumococcal conjugate vaccine (PD‐PCV10) show similar bacterial colonisation patterns of vaccine‐type and non‐vaccine‐type pneumococci (H. influenzae,M. catarrhalis and Staphylococcus aureus (S. aureus)) to CRM197‐PCV7 in healthy Dutch children up to two years of age (Van den Bergh 2013). The middle ear is directly connected to the nasopharynx and by lowering the carriage of vaccine‐type pneumococci, a niche is created for other bacteria with a pathogenic potential (Block 2006; Veenhoven 2003; Veenhoven 2004). Future studies may provide more precise information about the extent and impact of such replacement in AOM when administering conjugate vaccines that include more than seven pneumococcal serotypes.
Overall completeness and applicability of evidence
The nine RCTs included in this review on the effect of PCV on AOM in children show large heterogeneity regarding study design (standard, individually randomised trials versus group randomised trial), population (age of administration of PCV and baseline risk of AOM), intervention (vaccine valency (7‐/9‐/11‐valent vaccines), carrier protein (CRM197, OMPC or PD), presence or absence of additional booster immunisation with PCV or PPV23, co‐administration of non‐bacterial vaccines and outcome assessment and definition (active surveillance for standardised physician‐diagnosed AOM, passive collection of diagnoses of AOM or parent‐reported AOM). Furthermore, in the infant studies on AOM focusing on bacteriology (Eskola 2001; Kilpi 2003; Prymula 2006), the control groups varied markedly in the proportions of S. pneumoniae, H. influenzae and M. catarrhalis in middle ear fluid, possibly related to time and geographic region as well as case definition, which will affect the result of all‐cause AOM episodes prevented by pneumococcal vaccines. Additionally, three studies included older otitis‐prone children, so the intervention was aimed at secondary or even tertiary prevention and not primary prevention (Jansen 2008; Van Kempen 2006; Veenhoven 2003). The reduced efficacy of CRM197‐PCV7 in children already with a history of AOM may be explained by an increased susceptibility to subsequent infections, not only with non‐vaccine‐type pneumococci but also other nasopharyngeal colonisers, due to 'damage' already suffered by the middle ear mucosa caused by prior AOM (Veenhoven 2003). Another explanation, although debated, could be the non‐protective, impaired antibody responses of children who are otitis‐prone (Pichichero 2013; Wiertsema 2012). Thus, it appears that the age at which PCV is administered, a history of AOM episodes, or both, modifies the effect of PCV on AOM, despite the fact that age alone could not be identified as a statistically significant effect modifier (Fireman 2003; Veenhoven 2003).
Quality of the evidence
We judged the methodological quality of the included studies to be moderate to high. Although we were not able to pool the results of the separate trials due to substantial clinical diversity, the overall results were consistent.
Potential biases in the review process
In this review, we strictly adhered to the prespecified review protocol. Three review authors independently searched all relevant electronic databases by using a search syntax comprising all relevant synonyms for PCV and AOM. Additionally, we performed a broad Internet search to identify potentially relevant articles. To increase the yield of relevant studies, we reviewed the reference lists of all identified studies and systematic reviews or meta‐analyses.
Agreements and disagreements with other studies or reviews
Our main findings are in agreement with two other systematic reviews on the effect of PCV in children, indicating that PCV may provide some protection against otitis media, but that other factors could also have contributed to the observed effect estimates (Pavia 2009; Taylor 2012).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
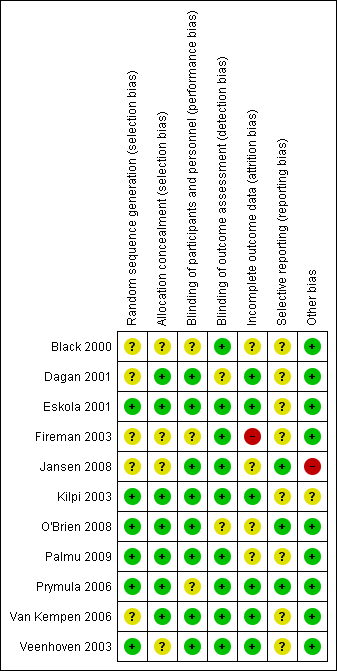
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Pneumococcal conjugate vaccine compared with control intervention for preventing acute otitis media | ||||
| Patient or population: children aged 12 years or younger and a follow‐up after vaccinations of at least 6 months Settings: open population Intervention: multivalent PCVs Comparison: control treatment | ||||
| Outcomes | VE ‐ relative effect (95% CI)* | No of participants | Quality of the evidence | Comments |
| Frequency of all‐cause AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR: ‐5% to 7% | 42,140 (4) | ⊕⊕⊕⊕ | Results are derived from 1 very large trial (Black 2000/Fireman 2003) and 3 trials of approximately equal size (944 to 1666 participants) (Eskola 2001; Kilpi 2003; O'Brien 2008) Lowest efficacy was found in high‐risk children (O'Brien 2008) |
| Frequency of all‐cause AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 34% (21 to 44) | 4968 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial (Prymula 2006) Part of the effect may be related to the protein D to which the polysaccharides are conjugated in the vaccine PD‐PCV11, demonstrated to reduce non‐typeable H. influenzae by 35% (95% CI 2 to 57) AOM incidence rate in control group was low compared to the other studies on the effect on PCV7 in infants and the absolute risk difference was small (Table 1) |
| Frequency of all‐cause AOM CRM197‐PCV9 administered in healthy toddlers Follow‐up 24 months | RRR 17% (‐2 to 33) | 264 (1) | ⊕⊕⊕⊝ | Results derived from 1 trial of moderate methodological quality (Dagan 2001). Uncertainty about the effect size (statistically non‐significant effect) and outcome measure (parent‐reported OM) |
| Frequency of all‐cause AOM PCV7 administered in older children with a known history of AOM Follow‐up 6 to 26 months | RRR ‐29% to 57% | 1054 (3) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Veenhoven 2003; Van Kempen 2006) and 1 trial of moderate methodological quality (Jansen 2008). The 2 high‐quality trials found no beneficial effect of PCV in preventing AOM recurrences, while the other trial found PCV7/TIV not to be superior to TIV/placebo in preventing AOM during the influenza season |
| Frequency of pneumococcal AOM PCV7 administered in early infancy Follow‐up 6 to 42 months | RRR 20% to 34% | 1233 (2) | ⊕⊕⊕⊕ | Results are derived from 2 high‐quality trials (Eskola 2001/Palmu 2009; Kilpi 2003) |
| Frequency of pneumococcal AOM PD‐PCV11 administered in early infancy Follow‐up 27 months | RRR 52% (37 to 63) | 281 (1) | ⊕⊕⊕⊝ | Results derived from 1 high‐quality trial in which myringotomy was performed in all children (Prymula 2006) |
| GRADE Working Group grades of evidence *Results include both ITT and PP results; 95% CI lacking in case of multiple studies (range of effect estimates presented as we refrained from pooling). | ||||
| AOM: acute otitis media | ||||
| Intention‐to‐treat | Per‐protocol | ||||||
| Episodes/person year | VE expressed as relative reduction in risk (95% CI) | Episodes/person year | Incidence rate difference ‐ episodes per person year (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||
| Treatment | Control | Treatment | Control | ||||
| PCV administered in early infancy | |||||||
| ‐ ‐ | ‐ ‐ | 6% (4 to 9) 6% (4 to 8) | ‐ ‐ | ‐ ‐ | ‐ ‐ | 7% (4 to 10) 7% (4 to 9) | |
| ‐ | ‐ | ‐ | 1.16 | 1.24 | ‐0.08 ˜ | 6% (‐4 to 16) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐1% (‐12 to 10) | |
| ‐ | ‐ | ‐ | 0.08 | 0.13 | ‐0.04 ˜ | 34% (21 to 44) | |
| 1.4 | 1.4 | ‐5% (‐25 to 12)# | 1.3 | 1.3 | 0.0 (‐0.13 to 0.14) | 0% (‐21 to 17) | |
| PCV administered at a later age | |||||||
| ‐ | ‐ | ‐ | 0.66 | 0.79 | ‐0.14 (‐0.29 to 0.02) | 17% (‐2 to 33) | |
| ‐ | ‐ | ‐25% (‐57 to 1) | 1.1 | 0.83 | 0.27 ˜ | ‐29% (‐62 to ‐2) | |
| ‐ | ‐ | ‐ | 0.78 | 0.67 | 0.11 ˜ | ‐16% (‐96 to 31) | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 57% (6 to 80)^ | |
| CI: confidence interval; HBV: hepatitis B vaccine; PCV: pneumococcal conjugate vaccine; TIV: trivalent influenza vaccine; VE: vaccine efficacy. *Cluster‐randomised trial. ˜ 95% CI could not be calculated as person‐time across treatment groups was not reported. Note: negative values for VE expressed as relative reduction in risk represent an increase in the risk for AOM. | |||||||
| Intention‐to‐treat | Per‐protocol | |||||||
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |||||||
| Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | Pneumococcal AOM | Vaccine‐type AOM | Cross‐reactive type AOM | Non‐vaccine‐type AOM | |
| PCV administered in infancy | ||||||||
| ‐ ‐ | 65% P = 0.04 ‐ | ‐ ‐ | ‐ ‐ | ‐ ‐ | 67% P = 0.08 ‐ | ‐ ‐ | ‐ ‐ | |
| ‐ ‐ | 54% (41 to 64) ‐ | ‐ ‐ | ‐ ‐ | 34% (21 to 45) 20% (7 to 31) | 57% (44 to 67) ‐ | 51% (27 to 67) ‐ | ‐33% (‐80 to 1) ‐ | |
| ‐ | ‐ | ‐ | ‐ | 25% (11 to 37) | 56% (44 to 66) | ‐5% (‐47 to 25) | ‐27% (‐70 to 6) | |
| ‐ | ‐ | ‐ | ‐ | 52% (37 to 63) | 58% (41 to 69) | 66% (22 to 85) | 9% (‐64 to 49) | |
| O'Brien 2008* # | ‐ | 64% (‐34 to 90) | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| PCV administered at a later age | ||||||||
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | 34% P = 0.22 | 52% P = 0.21 | ‐ | 21% P = 0.44 | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| VE: vaccine efficacy; PCV: pneumococcal conjugate vaccine; MEF: middle ear fluid. | ||||||||
| Intention‐to‐treat | Per‐protocol | |
| VE expressed as relative reduction in risk (95% CI) | VE expressed as relative reduction in risk (95% CI) | |
| PCV administered in infancy | ||
| 9% (4 to 14) 10% (7 to 13) | 9% (3 to 15) ‐ | |
| 9% (‐12 to 27) | 16% (‐6 to 35) | |
| ‐ | ‐ | |
| ‐ | 56% (‐2 to 81) | |
| ‐ | ‐ | |
| PCV administered at a later age | ||
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| ‐ | ‐ | |
| PCV: pneumococcal conjugate vaccine; VE: vaccine efficacy. | ||

