Vacunas para la prevención de la gripe en adultos sanos
Resumen
Antecedentes
Las consecuencias de la gripe en los adultos incluyen principalmente el tiempo de ausentismo del trabajo. La vacunación de las embarazadas se recomienda a nivel internacional. Esta es una actualización de una revisión publicada en 2014. Esta revisión se actualizará en el futuro solo cuando se disponga de nuevos ensayos o vacunas. Los datos observacionales incluidos en las versiones anteriores de la revisión se han mantenido por razones históricas, pero no se han actualizado debido a que no han influido en las conclusiones de la revisión.
Objetivos
Evaluar los efectos (eficacia, efectividad y efectos perjudiciales) de las vacunas contra la gripe en adultos sanos, incluidas las embarazadas.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (CENTRAL; 2016, número 12), MEDLINE (enero 1966 hasta el 31 de diciembre 2016), Embase (1990 hasta el 31 de diciembre 2016), la International Clinical Trials Registry Platform de la OMS (ICTRP; 1 de julio 2017) y ClinicalTrials.gov (1 de julio 2017), y también se examinaron las bibliografías de los artículos recuperados.
Criterios de selección
Ensayos controlados aleatorizados (ECA) o ensayos controlados cuasialeatorizados que compararan las vacunas contra la gripe con placebo o ninguna intervención en individuos sanos de entre 16 a 65 años de edad con gripe adquirida de forma natural. Las versiones anteriores de esta revisión incluyeron estudios comparativos observacionales que evaluaron los efectos perjudiciales graves y poco frecuentes de los estudios de cohortes y de casos y controles. Debido a la calidad incierta de los estudios observacionales (es decir, no aleatorizados) y la falta de influencia en las conclusiones de la revisión, se decidió actualizar solo la evidencia aleatoria. Ya no se actualizan las búsquedas de estudios comparativos de observación.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos. Se calificó la certeza de la evidencia para los resultados clave (gripe, enfermedad similar a la gripe [ESG], hospitalización y efectos adversos) mediante los criterios GRADE.
Resultados principales
Se incluyeron 52 ensayos clínicos, con más de 80 000 personas, que evaluaron la seguridad y la efectividad de las vacunas contra la gripe. Se presentaron los resultados de 25 estudios que compararon la vacuna inactivada parenteral contra la gripe frente a grupos de control con placebo o de ningún tratamiento como los más relevantes para la toma de decisiones. Los estudios se realizaron durante temporadas de gripe únicas en América del Norte, América del Sur y Europa entre 1969 y 2009. No se considera que los estudios en riesgo alto de sesgo influyan en los hallazgos de los resultados, excepto en el caso de la hospitalización.
Las vacunas inactivadas contra la gripe probablemente reducen la gripe en los adultos sanos del 2,3% sin vacunación al 0,9% (riesgo relativo [RR] 0,41; intervalo de confianza [IC] del 95%: 0,36 a 0,47; 71 221 participantes; evidencia de certeza moderada), y probablemente reducen las ESG del 21,5% al 18,1% (RR 0,84; IC del 95%: 0,75 a 0,95; 25 795 participantes; evidencia de certeza moderada; 71 adultos sanos deben ser vacunados para evitar que uno de ellos contraiga gripe, y 29 adultos sanos deben ser vacunados para evitar que uno de ellos contraiga una ESG). La diferencia entre los valores de los dos números necesarios para vacunar (NNV) depende de la diferente incidencia de ESG y la gripe confirmada entre las poblaciones de estudio. La vacunación puede dar lugar a una pequeña reducción del riesgo de hospitalización en adultos sanos, del 14,7% al 14,1%, pero el IC es amplio y no descarta un gran beneficio (RR 0,96; IC del 95%: 0,85 a 1,08; 11 924 participantes; evidencia de certeza baja). Las vacunas pueden dar lugar a una reducción pequeña o a ninguna reducción de los días de ausentismo laboral (‐0,04 días, IC del 95%: ‐0,14 días a 0,06; evidencia de certeza baja). Las vacunas inactivadas causan un aumento de la fiebre del 1,5% al 2,3%.
Se identificó un ECA y un ensayo clínico controlado que evaluó los efectos de la vacunación en las embarazadas. La eficacia de la vacuna inactivada que contiene el pH1N1 contra la gripe fue del 50% (IC del 95%: 14% a 71%) en las madres (NNV 55), y del 49% (IC del 95%: 12% a 70%) en los niños de hasta 24 semanas (NNV 56). No se dispuso de datos sobre la eficacia contra la gripe estacional durante el embarazo. La evidencia de los estudios observacionales mostró que la efectividad de las vacunas de la gripe contra las ESG en las embarazadas era del 24% (IC del 95%: 11% a 36%, NNV 94) y contra la gripe en los recién nacidos de las mujeres vacunadas era del 41% (IC del 95%: 6% a 63%, NNV 27).
Las vacunas con virus vivos administradas en forma de aerosol tienen una efectividad general correspondiente a un NNV de 46. El rendimiento de las vacunas antipandémicas de una o dos dosis con virus enteros de 1968 a 1969 fue mayor (NNV 16) contra las ESG y (NNV 35) contra la gripe. El impacto de las hospitalizaciones en la pandemia de 1968 a 1969 fue limitado (NNV 94). La administración de las vacunas contra la pandemia, tanto estacionales como de 2009 durante el embarazo no tuvo un efecto significativo sobre el aborto o la muerte neonatal, aunque esta información se basó en conjuntos de datos de observación.
Conclusiones de los autores
Los adultos sanos que reciben la vacuna inactivada parenteral contra la gripe en lugar de ninguna vacuna probablemente experimentan menos gripe, de un poco más del 2% a un poco menos del 1% (evidencia de certeza moderada). También es probable que experimenten menos ESG después de la vacunación, pero el grado de beneficio cuando se expresa en términos absolutos varió en los distintos entornos. La variación en la protección contra la ESG puede deberse en parte a la clasificación inconsistente de los síntomas. La certeza de la evidencia de la pequeña reducción en las hospitalizaciones y en el tiempo de ausentismo laboral es baja. La protección contra la gripe y las ESG en las madres y los recién nacidos fue menor que los efectos observados en otras poblaciones consideradas en esta revisión.
Las vacunas aumentan el riesgo de varios eventos adversos, incluido un pequeño aumento de la fiebre, pero las tasas de náuseas y vómitos son inciertas. El efecto protector de la vacunación en las embarazadas y los recién nacidos también es muy moderado. En los estudios comparativos considerados en esta revisión no se encontró evidencia de una asociación entre la vacunación contra la gripe y los eventos adversos graves. Quince de los ECA incluidos fueron financiados por la industria (29%).
PICOs
Resumen en términos sencillos
Vacunas para prevenir la gripe en adultos sanos
Objetivo de la revisión
El objetivo de esta revisión Cochrane publicada por primera vez en 1999 fue resumir la investigación que analiza los efectos de la inmunización de adultos sanos con vacunas contra la gripe durante las temporadas de gripe. Se utilizó información de ensayos aleatorizados que compararan las vacunas con vacunas ficticias o nada. El interés se centró en los resultados de los estudios sobre las vacunas basadas en virus de la gripe inactivados, que se desarrollan eliminando el virus de la gripe con una sustancia química y se administran mediante una inyección a través de la piel. Se evaluaron los efectos de las vacunas en la reducción del número de adultos con gripe confirmada y el número de adultos que presentaban síntomas similares a los de la gripe, como dolor de cabeza, fiebre alta, tos y dolor muscular (enfermedad similar a la gripe o ESG). También se evaluó el ingreso al hospital y los efectos perjudiciales de las vacunas. Los datos observacionales incluidos en las versiones anteriores de la revisión se han mantenido por razones históricas, pero no se han actualizado debido a que no han influido en las conclusiones de la revisión.
¿Qué se estudió en esta revisión?
Más de 200 virus causan ESG, que produce los mismos síntomas (fiebre, dolor de cabeza, dolores, tos y secreción nasal) que la gripe. Sin pruebas de laboratorio, los médicos no pueden distinguir entre la ESG y la gripe debido a que ambas duran días y rara vez causan una enfermedad grave o la muerte. Los tipos de virus que contienen las vacunas contra la gripe suelen ser los que se espera que circulen en las temporadas de gripe siguientes, según las recomendaciones de la Organización Mundial de la Salud (vacuna estacional). La vacuna pandémica solo contiene la cepa del virus que es responsable de la pandemia (es decir, el Tipo A H1N1 para la pandemia de 2009 a 2010).
Resultados principales
Se encontraron 52 ensayos clínicos con más de 80 000 adultos. No fue posible determinar el impacto del sesgo en alrededor del 70% de los estudios incluidos debido a la información insuficiente de los detalles. Alrededor del 15% de los estudios incluidos estaban bien diseñados y realizados. El interés se centró en el informe de los resultados de 25 estudios que examinaron las vacunas inactivadas. Las vacunas contra la gripe inyectadas probablemente tienen un pequeño efecto protector contra la gripe y las ESG (evidencia de certeza moderada), ya que sería necesario vacunar a 71 personas para evitar un caso de gripe y sería necesario vacunar a 29 para evitar un caso de ESG. La vacunación puede tener un efecto poco o nada apreciable en las hospitalizaciones (evidencia de certeza baja) o en el número de días de ausentismo laboral.
No existe seguridad en cuanto a la protección que la vacuna inactivada contra la gripe ofreció a las embarazadas contra las ESG y la gripe, o por lo menos fue muy limitada.
La administración de vacunas estacionales durante el embarazo no mostró ningún efecto significativo sobre el aborto o la muerte neonatal, pero el conjunto de evidencia fue observacional.
Mensajes clave
Las vacunas inactivadas pueden reducir la proporción de adultos sanos (incluidas las embarazadas) que padecen gripe y ESG, pero su impacto es moderado. No existe seguridad en cuanto a los efectos de las vacunas inactivadas en los días de ausentismo laboral ni en cuanto a las complicaciones graves de la gripe durante la temporada de gripe.
¿Cómo de actualizada está esta revisión?
La evidencia está actualizada hasta el 31 de diciembre 2016.
Conclusiones de los autores
Summary of findings
| Inactivated parenteral influenza vaccine compared to placebo or 'do nothing' for preventing influenza in healthy adults | ||||||
| Patient or population: healthy adults | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or 'do nothing' | Risk with inactivated parenteral influenza vaccine | |||||
| Influenza Timing of assessment: most studies tested vaccines over a single influenza season | Study population1 | RR 0.41 | 71,221 | ⊕⊕⊕⊝ | ||
| 23 per 1000 | 9 per 1000 | |||||
| Influenza‐like illness Timing of assessment: most studies tested vaccines over a single influenza season | Low1 | RR 0.84 | 25,795 | ⊕⊕⊕⊝ | ||
| 40 per 1000 | 34 per 1000 | |||||
| Moderate | ||||||
| 215 per 1000 | 181 per 1000 | |||||
| High | ||||||
| 910 per 1000 | 764 per 1000 | |||||
| Hospitalisations Timing of assessment: single influenza season | Study population1 | RR 0.96 | 11,924 | ⊕⊕⊝⊝ | ||
| 147 per 1000 | 141 per 1000 | |||||
| Time off work Timing of assessment: single influenza season | Study population1 | NA | 3726 (4 RCTs) | ⊕⊕⊝⊝ | ||
| Average number of days lost per person ranged from 0.2 to 2 days over the season. | Average reduction in working days lost following vaccination was 0.04 days fewer (0.14 fewer to 0.06 days more) | |||||
| Fever assessed by subjective report Timing of assessment: single influenza season | Study population1 | RR 1.55 | 23,850 | ⊕⊕⊕⊕ | ||
| 15 per 1000 | 23 per 1000 | |||||
| Nausea or vomiting Timing of assessment: single influenza season | Study population1 | RR 1.80 | 6315 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 66 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk calculated as the sum of events over total sample size from the control groups. For the outcome of influenza‐like illness, control group risk was stratified as low, moderate (or median), and high due to variation in risk groups across the studies. For the remaining outcomes, the control group risk was taken as aggregate. | ||||||
Antecedentes
Descripción de la afección
Las enfermedades respiratorias virales imponen una gran carga a la sociedad. La mayor parte de las enfermedades respiratorias virales (enfermedades tipo gripe) son causadas por numerosos agentes diferentes, clínicamente indistinguibles unos de otros. Una proporción variable de enfermedades tipo gripe (7% al 15% como promedio) es causada por los virus de la gripe y se conocen como gripe (Jefferson 2009a).
La gripe es una infección respiratoria aguda causada por un virus de la familia Orthomyxoviridae. Se conocen tres serotipos (A, B y C). La gripe causa un cuadro febril agudo con mialgias, cefalea y tos. A pesar de que la duración promedio del cuadro agudo es de tres días, la tos y el malestar pueden persistir durante semanas. Las complicaciones de la gripe incluyen otitis media, neumonía, neumonía bacteriana secundaria, exacerbaciones de la enfermedad respiratoria crónica y bronquiolitis en los niños. Además, la gripe puede provocar diversas complicaciones no respiratorias, como convulsiones febriles, síndrome de Reye y miocarditis (Treanor 2016; Wiselka 1994). Los esfuerzos para prevenir o minimizar la repercusión de la gripe estacional en la segunda parte del siglo XX se han concentrado en el uso de las vacunas. Debido a los cambios anuales en la configuración antigénica viral y la falta de protección que se prolongue de año en año, es necesario organizar anualmente una nueva campaña de vacunación, con un esfuerzo científico y logístico enorme para asegurar la producción y la entrega de las vacunas.
Descripción de la intervención
En la actualidad existen tres tipos de vacunas contra la gripe:
-
vacunas inactivas de virus enteros, que consisten en virus completos a los que se les ha "matado" o inactivado, de modo que no son infecciosos, pero conservan las propiedades antigénicas específicas de la cepa;
-
vacunas de subunidades de virus que se elaboran solo con antígenos superficiales (H y N); y
-
vacunas de virus fraccionados, en las que se fracciona la estructura viral mediante un agente separador.
Estas vacunas contienen antígenos de superficie e internos. Además, varios fabricantes no europeos producen vacunas de virus vivos atenuados. Tradicionalmente se cree que las vacunas de virus enteros no son tan bien toleradas debido a la presencia de un estrato lipídico en la superficie de las partículas virales (un residuo de la membrana celular huésped que recubre el virus, cuando se reproducen de la célula huésped).
La Food and Drug Administration (FDA) de los Estados Unidos aprobó recientemente una nueva vacuna recombinante (Flublok) que consiste en proteínas de hemaglutinina purificadas producidas en células insertables para su uso en adultos de entre 18 y 49 años de edad con antecedentes conocidos de alergia al huevo (ACIP 2015).
Las vacunas contra la gripe se fabrican en todo el mundo. Las variaciones antigénicas menores y los cambios antigénicos periódicos plantean problemas para la producción y la adquisición de vacunas, ya que se debe producir y adquirir una vacuna nueva que sea estrechamente compatible con la configuración antigénica circulante para el comienzo de cada nueva "temporada" de gripe. Para lograr estos requerimientos, la Organización Mundial de la Salud (OMS) ha establecido un sistema de vigilancia mundial que permite identificar y aislar las cepas virales que circulan en las diferentes regiones del mundo. Las prácticas centinelas recuperan las partículas virales de la nasofaringe de los pacientes con síntomas similares a los de la gripe y las muestras se envían rápidamente a los laboratorios de los centros nacionales de gripe (110 laboratorios de 79 países). Cuando se detectan cepas nuevas, se envían las muestras a uno de los cuatro centros de referencia de la OMS (Londres, Atlanta, Tokio y Melbourne) para realizarles un análisis antigénico. Posteriormente, la información sobre la cepa circulante se envía a la OMS, que en febrero de cada año recomienda, a través de un comité, las cepas que se deben incluir en la vacuna para la próxima «temporada». Los gobiernos individuales pueden o no seguir las recomendaciones de la OMS. Australia, Nueva Zelanda y más recientemente Sudáfrica, han seguido sus propias recomendaciones para el contenido de la vacuna. Por lo tanto, la vigilancia y la identificación temprana desempeñan un papel central en la composición de la vacuna.
De qué manera podría funcionar la intervención
Las vacunas funcionan al simular una infección y estimular al cuerpo a producir anticuerpos contra la amenaza, además de activar otros mecanismos de defensa. Cada campaña de vacunación ha establecido objetivos con los que se deben medir los efectos de la campaña. Quizás el documento más detallado que presenta la justificación de un programa preventivo integral es el del Advisory Committee on Immunization Practices (ACIP) de los EE.UU., publicado en 2006 (ACIP 2006). El documento identifica 11 categorías de personas con riesgo alto de complicaciones de la gripe, entre las que se encuentran los adultos sanos de 50 a 65 años de edad y los trabajadores de los servicios de salud. La justificación para la selección de políticas se basa en la gran carga que la gripe impone sobre las poblaciones y los efectos beneficiosos derivados de la vacunación. La disminución de los casos y las complicaciones (como el número excesivo de hospitalizaciones, el ausentismo laboral, la mortalidad y las visitas a los servicios sanitarios) y la interrupción de la transmisión son los principales argumentos para extender la vacunación a los adultos sanos de 50 a 65 años de edad (ACIP 2006).
La actualización del documento ACIP 2015 recomienda la vacunación sistemática para todas las personas a partir de los seis meses de edad sin contraindicaciones. Subraya la importancia de centrar los esfuerzos de vacunación, cuando los suministros para la vacunación son limitados, en los adultos sanos que tienen mayor riesgo de desarrollar complicaciones graves de la gripe, como:
-
personas de 50 años o más;
-
mujeres que están o estarán embarazadas durante la temporada de gripe;
-
personal sanitario;
-
contactos familiares y cuidadores de niños menores de cinco años y adultos de 50 años o más, con particular énfasis en vacunar a los contactos de los niños menores de seis meses; y
-
contactos familiares y cuidadores de pacientes con enfermedades que los exponen a un riesgo mayor de complicaciones graves a causa de la gripe (ACIP 2010; ACIP 2015; Grohskopf 2016).
Las embarazadas están incluidas entre los receptores prioritarios de la inmunización contra la gripe estacional en muchos países debido al riesgo de morbilidad asociada a la gripe durante el embarazo y a los posibles resultados neonatales adversos asociados a las infecciones por gripe materna (AIH 2013; DoH 2015; NACI 2014; STIKO 2010), y sobre la base de la evidencia de que la vacunación de las embarazadas protege a los recién nacidos de la gripe y de las hospitalizaciones relacionadas con la gripe (NACI 2014).
La vacuna inactivada contra la gripe podría ser administrada en cualquier estadio del embarazo, mientras que la vacuna de virus vivos no se autoriza durante el embarazo, ya que los datos disponibles acerca de la seguridad y la eficacia en las madres y los recién nacidos son muy limitados (ACIP 2010; DoH 2015).
La European Medicines Agency (EMA) recientemente hizo cambios en el registro de las vacunas contra la gripe estacionales, prepandémicas y pandémicas (EMA 2014; Wijnans 2016). Los cambios se introdujeron en 2014; desencadenados por el reconocimiento de que las respuestas de los anticuerpos no son variables predictivas suficientes de la protección de campo, como han mostrado de manera sistemática las revisiones con el transcurso de los años. La mayoría de los datos de las vacunas contra la gripe incluidas en las revisiones son de vacunas registradas, y todavía la protección de campo que se ha logrado es moderada o insignificante. Además, había una falta de métodos de estandarización de los niveles de anticuerpos. Las nuevas reglas para los adultos y las personas de edad avanzada requieren la demostración de la no inferioridad de la respuesta de los anticuerpos (inmunogenicidad) por parte de una vacuna contra la gripe estacional candidata, en comparación con una establecida. Además, cuando se necesita una demostración de la eficacia clínica (ver Apéndice 1), la EMA promueve el uso mínimo de un placebo y promueve el uso de controles activos (como vacunas diferentes a las de la gripe), con la ESG (y los resultados relevantes de la reacción en cadena de la polimerasa [RCP]) como la variable de evaluación primaria. La efectividad clínica se debe evaluar mediante la realización (preferentemente prospectiva) de estudios de cohortes o estudios de casos y controles de pruebas negativas denominados secundarios («nested»), según el protocolo del European Centre for Disease Prevention and Control (ECDC) (Kissling 2009a; Kissling 2009b).
En la actualidad se requiere la vigilancia de los efectos perjudiciales, con un seguimiento de al menos seis meses de duración, y en la población general de personas de edad muy avanzada una base de datos de al menos 3000 personas expuestas a la vacuna. Deben recopilarse cuanto antes los datos mejorados de vigilancia de la vacuna, al comienzo de la campaña de vacunación cada año.
Por qué es importante realizar esta revisión
Debido a que el ciclo de producción de las vacunas contra la gripe es único (se analizan con resultados alternativos [estimulación de anticuerpos] antes de cada "temporada" de gripe), el rendimiento anterior es probablemente la única manera fiable de predecir el rendimiento futuro.
Es fundamental una evaluación precisa de los efectos (eficacia, efectividad y perfil de seguridad) de las vacunas contra la gripe que permita la elección racional entre estrategias alternativas. Esta revisión junto con las dos que la acompañan, Demicheli 2014 y Jefferson 2012, son revisiones a largo plazo. Se encuentran entre las de acceso más consistente en toda la Base de Datos Cochrane de Revisiones Sistemáticas, lo que confirma la importancia del tema y el interés que existe. Las actualizaciones periódicas, algunas de hace casi dos décadas, han permitido incluir un número cada vez mayor de estudios sobre los efectos de las vacunas contra la gripe y monitorizar su repercusión en las revisiones (Tabla 1).
Las revisiones no son metodológicamente homogéneas debido a que los métodos reflejan la historia y el desarrollo de las revisiones Cochrane. En particular, la inclusión de los estudios observacionales, que inicialmente estuvo favorecida para la evaluación de los efectos perjudiciales, ha sido fuente de discusión. En esta revisión, la evidencia aleatoria representa el 44% de los estudios considerados. Con objeto de aumentar la pertinencia de la revisión para los encargados de la toma de decisiones, en la actualización de 2007 de Jefferson se incluyeron estudios comparativos no aleatorizados que informaron sobre la evidencia de efectos perjudiciales graves o poco frecuentes (o ambos).
Históricamente, los estudios observacionales han sido de calidad metodológica deficiente y a menudo informan resultados conflictivos o paradójicos, lo que impide establecer conclusiones sólidas. Sin embargo, la inclusión de tipos de estudios particulares y el tamaño cada vez mayor de los grupos de datos no ha dado lugar a un cambio en la conclusión de las revisiones, y a su vez dan lugar a un volumen mucho mayor de trabajo. La anterior es la razón principal por la cual los autores, el grupo de revisión y los redactores Cochrane han decidido estabilizar las tres revisiones, o sea, no realizar actualizaciones habituales del grupo de datos observacionales y actualizar el grupo de datos aleatorizados si se cumplen determinadas condiciones en el futuro.
Por la misma razón, el contenido observacional de esta revisión y similares se ha mantenido como evidencia histórica del ciclo de vida de las revisiones.
Desde la actualización de esta revisión en 2014 (Jefferson 2014), se incluyó evidencia sobre la vacunación contra la gripe en embarazadas y recién nacidos.
Se planificó actualizar la evidencia aleatoria en esta revisión si en el futuro se cumple alguna o todas las condiciones a continuación:
-
existe disponibilidad de un ensayo que evalúe los efectos clínicos de la evolución de las tecnologías actuales;
-
se desarrolla un tipo nuevo de vacuna; o
-
se propone un paradigma causal nuevo creíble para la gripe.
Para una visión general de las tres revisiones, ver el editorial de portada en https://community.cochrane.org/news/why‐have‐three‐long‐running‐cochrane‐reviews‐influenza‐vaccines‐been‐stabilised.
Objetivos
Evaluar los efectos (eficacia, efectividad y efectos perjudiciales) de las vacunas contra la gripe en adultos sanos, incluidas las embarazadas.
Los «efectos» se definieron como sigue:
-
la eficacia se definió como la capacidad de las vacunas para prevenir la gripe A o B y sus complicaciones;
-
la efectividad, como la capacidad de las vacunas para prevenir las enfermedades similares a la gripe y sus consecuencias; y
-
los efectos perjudiciales como cualquier evento perjudicial potencialmente asociado con la exposición a las vacunas contra la gripe.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Cualquier ensayo controlado aleatorizado (ECA) o cuasialeatorizado que comparara las vacunas contra la gripe en humanos versus placebo o ninguna intervención, o que comparara tipos, dosis o esquemas de administración de la vacuna contra la gripe. Solamente se consideraron los estudios que evaluaron la protección a la gripe adquirida por exposición natural.
Con objeto de aumentar la pertinencia de la revisión para los encargados de la adopción de decisiones, en la actualización de 2007 de Jefferson se incluyeron estudios comparativos no aleatorizados si informaron de evidencia de la asociación entre las vacunas contra la gripe y efectos adversos graves, como el síndrome de Guillain‐Barré o síndromes oculorrespiratorios, o si informaron de datos sobre la eficacia o la efectividad de la administración de la vacuna durante el embarazo.
Se definieron como ECA los estudios en que parecía que los individuos (u otras unidades experimentales) incluidos en el estudio habían sido asignados definitiva o posiblemente de forma prospectiva a una de dos (o más) formas alternativas de atención sanitaria mediante asignación al azar. Un estudio se considera cuasialeatorizado cuando al parecer los individuos (u otras unidades experimentales), seguidos durante el mismo, han sido definitiva o posiblemente asignados al azar de forma prospectiva a una de dos (o más) formas alternativas de atención sanitaria, con el uso de un método cuasialeatorio de asignación (como la alternancia, la fecha de nacimiento o el número de historia clínica).
Tipos de participantes
Individuos sanos de 16 a 65 años de edad, independientemente de su estado de inmunidad contra la gripe. Se excluyeron los estudios que consideraron a más del 25% de los individuos fuera de este rango de edad. También se incluyeron embarazadas junto con los recién nacidos.
Tipos de intervenciones
Vacunas con virus vivos, atenuados o inactivados o fracciones de los mismos administradas por cualquier vía, independientemente de la configuración antigénica.
Tipos de medida de resultado
Resultados primarios
Clínica
-
Número y gravedad (complicaciones y días del trabajo) de los casos de gripe sintomática y de enfermedades similares a la gripe que ocurrieron en los grupos de vacuna y placebo.
Efectos perjudiciales
-
Número y gravedad de los efectos adversos (sistémicos y graves). Los efectos adversos sistémicos incluyen casos de malestar, náuseas, fiebre, artralgias, erupción cutánea, cefalea y signos más generalizados y graves, como efectos perjudiciales neurológicos.
-
Resultados maternos y resultados relacionados con el curso del embarazo. Los mismos incluyeron el aborto (espontáneo, interno, muerte fetal y mortinato), el parto prematuro (menos de 37 semanas) y la muerte materna.
-
Resultados neonatales: malformaciones congénitas (menores y mayores), muerte neonatal.
Resultados secundarios
-
Los efectos secundarios locales incluyeron induración, dolor y enrojecimiento en el sitio de inoculación.
Métodos de búsqueda para la identificación de los estudios
Búsquedas electrónicas
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) searched 31 December 2016 via the Cochrane Library), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (PubMed) (January 1966 to 31 December 2016); Embase (Elsevier) (1990 to 31 December 2016); WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en, 1 July 2017); and ClinicalTrials.gov (www.clinicaltrials.gov, 1 July 2017). See Appendix 2 for the search strategies used to identify trials.
See Appendix 3 for search strategies used prior to this 2017 update to identify observational studies. See Appendix 4 for strategies used in the 2010 update, and Appendix 5 for the MEDLINE search strategy used in 2004.
Búsqueda de otros recursos
In order to identify further trials, we read the bibliographies of retrieved articles and handsearched the journal Vaccine from its first issue to the end of 2009. The results of the handsearches are included in CENTRAL. In order to locate unpublished trials for the first edition of this review, we wrote to manufacturers and first or corresponding trial authors of studies in the review.
Obtención y análisis de los datos
Selección de los estudios
Two review authors (AR, CDP) independently excluded all initially identified and retrieved articles not fulfilling the inclusion criteria. In the case of disagreement, one review author (VD) acted as arbitrator. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Extracción y manejo de los datos
Two review authors (AR, CDP) performed data extraction using a data extraction form (Appendix 6). We checked and entered the data into Review Manager 5 software (RevMan 2014). We extracted data on the following:
-
methodological quality of studies;
-
study design (Appendix 7);
-
description of setting;
-
characteristics of participants;
-
description of vaccines (content and antigenic match);
-
description of outcomes;
-
publication status;
-
date of study;
-
location of study.
One review author (CDP) carried out statistical analyses.
We assumed an ILI case (specific definition) to be the same as a 'flu‐like illness' according to a predefined list of symptoms (such as the Centers for Disease Control and Prevention (CDC) case definition for surveillance) or 'upper respiratory illness' according to a predefined list of symptoms.
The laboratory confirmations of influenza cases we found were:
-
virus isolation from culture;
-
four‐fold antibody increase (haemagglutinin) in acute‐ or convalescent‐phase sera;
-
four‐fold antibody increase (haemagglutinin) in postvaccination‐ or postepidemic‐phase sera.
Evaluación del riesgo de sesgo de los estudios incluidos
Experimental studies (trials)
Two review authors (CDP, AR) independently assessed the methodological quality of the included studies using criteria from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In case of disagreement, one review author (VD) acted as arbitrator in assigning quality judgements.
We classified studies according to the following key domains for assessing risk of bias (Higgins 2011).
Random sequence generation
-
Low risk of bias: e.g. a table of random numbers or computer‐generated random numbers.
-
High risk of bias: e.g. alternation, date of birth, day of the week, or case record number.
-
Unclear risk of bias: if insufficient information was provided.
Allocation concealment
-
Low risk of bias: e.g. numbered or coded identical containers were administered sequentially; an onsite computer system that could only be accessed after entering the characteristics of an enrolled participant; or serially numbered, opaque, sealed envelopes, or sealed envelopes that were not sequentially numbered.
-
High risk of bias: e.g. an open table of random numbers.
-
Unclear risk of bias: if insufficient information was provided.
Blinding
-
Low risk of bias: if adequate double‐blinding (e.g. placebo vaccine) or single‐blinding (i.e. blinded outcome assessment) was used.
-
High risk of bias: if there was no blinding.
-
Unclear risk of bias: if insufficient information was provided.
Incomplete outcome data
Number of losses to follow‐up:
-
Low risk of bias: no missing data or the proportion of missing data compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate.
-
High risk of bias: when the proportion of missing data compared with observed event risk was large enough to induce clinically relevant bias in the intervention effect estimate.
-
Unclear risk of bias: if insufficient information was provided.
Non‐experimental studies
We carried out quality assessment of non‐randomised studies in relation to the presence of potential confounders, which could make interpretation of the results difficult. We evaluated the quality of case‐control (prospective and retrospective) and cohort studies using the appropriate Newcastle‐Ottawa Scales (NOS) (Appendix 8).
Using quality at the analysis stage as a means of interpreting the results, we assigned 'Risk of bias' categories (Higgins 2011):
-
Low risk of bias: plausible bias unlikely to seriously alter the results.
-
Unclear risk of bias: plausible bias that raises some doubt about the results.
-
High risk of bias: plausible bias that seriously weakens confidence in the results.
Medidas del efecto del tratamiento
We used the risk ratio (RR) and its 95% confidence interval (CI) as the summary measure. We calculated vaccine efficacy (or effectiveness) as VE = 1 ‐ RR, expressed as a percentage, for cohort and RCT/controlled clinical trial (CCT) studies. For case‐control studies we adopted an odds ratio (OR) with 95% CIs.
To enhance relevance to everyday practice, we also expressed the summary measure of the most reliable and significant comparisons (those from RCTs with influenza cases as an outcome by age group) as a risk difference (RD). This is a measure of absolute efficacy of the vaccines, which incorporates significant information such as the incidence in the control arm and allows the calculation of its reciprocal, the number needed to treat for an additional beneficial outcome (NNTB), or in this case, the number needed to vaccinate (NNV).
The NNV expresses the number of children needed to be vaccinated to prevent one case of influenza. The NNV can be computed as 1/RD. Since meta‐analysis estimates from RD are affected by statistical heterogeneity, we preferred to compute the NNV from the RD between assumed and corresponding risks. We used aggregate or median of the control group risks, giving a formula of: 1/(control event rate (CER) ‐ CER*RR).
We conducted quantitative synthesis of the evidence from observational studies using adjusted estimates, when these were available; in some cases we also used original data (unadjusted data) in order to compare meta‐analysis results from adjusted and unadjusted estimates.
We calculated hospital admission rates as the proportion of cases hospitalised for respiratory causes. We considered complications as the proportion of cases complicated by bronchitis, pneumonia, or otitis. We also considered working days lost due to episodes of sickness absence regardless of cause. Only five trials used working days lost as an outcome measure, of which four trials measured the work absence in terms of the difference in the average number of days lost in two arms of the trial (Analysis 1.7). These studies presented a standard error value measured accordingly. The remainder expressed work absence in terms of rate ratio, which does not allow the recalculation of the correct estimate of the standard error (aa Nichol 1999a). We therefore excluded this study from the pooled analysis.
We presented local symptoms separately from systemic symptoms. We have considered individual harms in the analysis, as well as a combined endpoint (any or highest symptom). We used all data included in the analysis as presented by the authors in the primary study, regardless of the number of dropouts. We decided on this approach (complete‐case scenario) because the majority of the included studies did not attempt to use an intention‐to‐treat analysis or mention the reasons for the loss to follow‐up, and they did not contain detailed information to allow estimations of the real number of participants.
Cuestiones relativas a la unidad de análisis
Several trials included more than one active vaccine arm. Where several active arms from the same trial were included in the same analysis, we split the placebo group equally between the different arms, so that the total number of participants in a single analysis did not exceed the actual number in the trials.
We found four different definitions of the 'epidemic period'.
-
Interval between the first and the last virus isolation in the community.
-
Interval during which the influenza virus was recovered from more than a stated percentage of ill participants.
-
Period during which an increase of respiratory illness of more than a stated percentage was recorded.
-
Winter period, taken as a proxy for the epidemic period.
We included data regardless of the definition of epidemic period used in the primary study. When data were presented for the epidemic period and the entire follow‐up period, we considered those that occurred during the former.
Manejo de los datos faltantes
For the first publication of this review (Demicheli 1999), we wrote to the trial authors and manufacturers to identify possible unpublished studies and missing data. The response was disappointing, and we desisted from any further attempts. Our analysis relies on existing data. Whenever possible we used the intention‐to‐treat population.
Evaluación de la heterogeneidad
We calculated the I2 statistic for each pooled estimate to assess the impact on statistical heterogeneity. The I2 statistic can be interpreted as the proportion of total variation among effect estimates that is due to heterogeneity rather than sampling error, and it is intrinsically independent from the number of studies. When the I2 statistic is less than 30%, there is little concern about statistical heterogeneity (Higgins 2011). We used random‐effects models throughout to take into account the between‐study variance in our findings (Higgins 2011). Variance is to be expected in influenza vaccine trials, as there are unpredictable systematic differences between trials regarding the circulating strains, degree of antigenic matching of the vaccine, type of vaccine, and the levels of immunity presented by different populations in different settings. Not all studies reported sufficient details to enable a full analysis of the sources of heterogeneity, but we were able to take into account vaccine matching and circulating strain.
Evaluación de los sesgos de notificación
Due to the limited number of studies in each comparison or subgroup, assessment of publication bias was not applicable, since the evidence presented in this review originated mainly from published data. For this reason, our results could be affected by publication bias.
The overall quality of the retrieved studies was poor and was affected by poor reporting or limited descriptions of the studies' designs. A detailed description is provided in the Risk of bias in included studies section of the review.
The main problems with influenza vaccine studies are their poor quality and discrepancies between the data presented, their conclusions, and the authors' recommendations.
Síntesis de los datos
We calculated all meta‐analyses using a random‐effects model due to expected variation in the efficacy and effectiveness of viral strain matching, and seasonal variation in virulence of the circulating influenza virus. We summarised evidence from non‐randomised studies (cohort and case‐control) according to Higgins 2011.
Análisis de subgrupos e investigación de la heterogeneidad
We carried out subgroup analyses according to the degree of matching with that year's World Health Organization (WHO) recommended content and with circulating viruses ("WHO recommended and matching" when known). WHO recommendations on the content of vaccines have been published since 1973. Different dosages and schedules of the vaccine and the presence of different adjuvants were not compared. We pooled data from the arms of trials comparing only vaccine composition or dosage in the analysis. We checked compliance of the study vaccine with the official antigenic content and potency recommendations by reviewing the WHO records whenever possible. In case of uncertainty due to ambiguity in the wording used (in the oldest trials), we took into account the opinion given by the trial authors. We classified the compliance of a live attenuated vaccine with the recommendations according to the antigenic comparability of the wild strains.
Since the degree of matching between vaccine and circulating strains could affect the effectiveness/efficacy of the vaccine, we analysed the data in separate subgroups according to this parameter. For serious adverse events, whenever possible we analysed data from pregnant women and the general population in separate subgroups. When case‐control studies reported safety outcomes, whenever possible we performed analyses in separate subgroups according to time since exposure. Finally, we carried out a separate analysis of trials carried out during the 1968 to 1969 (H3N2) pandemic and the 2009 to 2010 (H1N1) pandemic.
Análisis de sensibilidad
As it was not possible to identify all sources of heterogeneity, we decided to carry out a sensitivity analysis by applying fixed‐effect and random‐effects models to assess the impact of heterogeneity on our results. In order to assess the robustness of our conclusions, we performed a sensitivity analysis by excluding studies judged to be at high risk of bias for one domain or unclear risk of bias for two or more domains. We restricted sensitivity analyses to Summary of findings table 1 outcomes (see below). Historical versions of this review compared the results from the crude data with those from the adjusted data from observational studies (historical versions of this review only).
GRADE and 'Summary of findings' table
We restricted our focus in the 'Summary of findings' tables to the comparison of inactivated parenteral influenza vaccine with placebo or do nothing, which we regarded as the most commonly adopted strategy. We created a Summary of findings table 1 using the following outcomes: ILI, influenza, hospitalisations, time off work, fever, and nausea/vomiting. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014). We used the results from randomised studies and justified all decisions to down‐ or upgrade the quality of studies using footnotes, making comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The first publication of this review contained 20 trials (Demicheli 1999). In the second publication we included five additional trials (Demicheli 2004), and the third publication included 48 trials in total (Jefferson 2007). The fourth published update, Jefferson 2010, included two new trials (aa Beran 2009a; aa Beran 2009b), and excluded three new trials (Belongia 2009; Chou 2007; Khazeni 2009). The fourth update included 41 new study reports and excluded 63 new trials (Jefferson 2014). In this 2016 update we have included 20 new studies, excluded 21 new trials, and added two further trials (three data sets). One was newly identified (aa Mc Bride 2016a; aa Mc Bride 2016b), and one was included from the 'awaiting assessment' category (aa Treanor 2011).
Some of the included studies had more than two arms, comparing different vaccines, routes of administration, schedules, or dosages, or reported data from different settings and epidemic seasons. We split these studies into substudies (data sets). For the remainder of this review, the term 'study report' refers to the original study report, while the word 'data set' refers to the substudy; these substudies could refer either to different study arms, different influenza seasons, or different study designs. Risk of bias may be independently assessed for each substudy (or data set) study design.
More information about the division of study reports into data sets is given in the Characteristics of included studies table. In this 2016 updated review, we included a total of 160 studies (137 data sets), while we no longer updated searches for observational comparative studies (Figure 1). Trial register searches identified 18 completed trials with one or more corresponding publications, reporting methods and study design. All 18 trials had been identified and dealt with appropriately in our searches of journal publication databases.

Study flow diagram.
Included studies
We coded each trial on the basis of study design and the type of data contributed to the review as follows. The letter preceding the study represents the study design: (a) denotes RCTs, (b) denotes case‐control studies, and (c) denotes cohort studies. The second letter indicates the contribution to the evidence in the data set: (a) efficacy/effectiveness or (b) harms. So, for example, a case‐control study contributing safety or harms data is coded as (bb), and a trial contributing efficacy/effectiveness data is coded as (aa). A (p) code has been added to refer to the studies on vaccination during pregnancy.
Seasonal vaccines: efficacy or effectiveness
-
RCTs on inactivated parenteral vaccine: (22 studies/32 data sets) (aa Barrett 2011; aa Beran 2009a; aa Beran 2009b; aa Bridges 2000a; aa Bridges 2000b; aa Eddy 1970; aa Frey 2010; aa Hammond 1978; aa Jackson 2010a; aa Jackson 2010b; aa Keitel 1988a; aa Keitel 1988b; aa Keitel 1997a; aa Keitel 1997b; aa Keitel 1997c; aa Leibovitz 1971; aa Mcbride 2016a; aa Mcbride 2016b; aa Mesa Duque 2001; aa Mixéu 2002; aa Monto 2009; aa Nichol 1995; aa Ohmit 2006; aa Ohmit 2008; aa Powers 1995a; aa Powers 1995b; aa Powers 1995c; aa Tannock 1984; aa Treanor 2011; aa Weingarten 1988; aa Zhilova 1986a; aa Zhilova 1986b).
-
RCTs on live aerosol vaccine: (8 studies/12 data sets) (aa Edwards 1994a; aa Edwards 1994b; aa Edwards 1994c; aa Edwards 1994d; aa Monto 1982; aa Monto 2009; aa Nichol 1999a; aa Ohmit 2006; aa Ohmit 2008; aa Rytel 1977; aa Zhilova 1986a; aa Zhilova 1986b).
-
RCTs on inactivated aerosol vaccine: (one study/one data set) (aa Langley 2011).
Seasonal vaccines: safety (local and systemic harms)
-
RCTs on inactivated parenteral vaccine: (21 studies/22 data sets) (aa Barrett 2011; aa Bridges 2000a; aa Bridges 2000b; aa Frey 2010; aa Jackson 2010a; aa Mesa Duque 2001; aa Monto 2009; aa Nichol 1995; aa Ohmit 2006; aa Ohmit 2008; aa Powers 1995a; aa Tannock 1984; aa Treanor 2011; aa Weingarten 1988; ab Caplan 1977; ab El'shina 1996; ab Forsyth 1967; ab Goodeve 1983; ab Pyrhönen 1981; ab Rocchi 1979a; ab Saxen 1999; ab Scheifele 2003).
-
RCTs on live aerosol vaccine: (13 studies/14 data sets) (aa Monto 1982; aa Nichol 1999a; aa Ohmit 2006; aa Ohmit 2008; aa Rytel 1977; ab Atmar 1990; ab Betts 1977a; ab Evans 1976; ab Hrabar 1977; ab Keitel 1993a; ab Keitel 1993b; ab Lauteria 1974; ab Miller 1977; ab Rocchi 1979b).
-
RCTs on inactivated aerosol vaccine: (three studies/three data sets) (aa Langley 2011; ab Boyce 2000; ab Langley 2005).
We could not introduce two studies with live aerosol vaccine, ab Reeve 1982 and ab Spencer 1977, (each one a data set) into the harms analysis (secondary effects) because the data did not allow for quantitative analysis (systemic and local harms were reported given as cumulative in ab Spencer 1977 and data were not clearly reported in ab Reeve 1982).
Administration during pregnancy ‐ efficacy/effectiveness in mothers
-
Seasonal trivalent inactivated vaccine containing pH1N1 ‐ RCTs: (one study/one data set) (paa Madhi 2014).
-
2009 to 2010 pandemic: inactivated vaccine ‐ CCTs: (one study/one data set) (paa Ma 2014).
-
Seasonal inactivated vaccine ‐ cohort studies: (three studies/three data sets) (pca Ahrens 2014; pca Black 2004; pca Hulka 1964).
-
2009 to 2010 pandemic: inactivated vaccines ‐ cohort studies: (one study/one data set) (pca Yamada 2012).
Administration during pregnancy ‐ efficacy/effectiveness in newborns
-
Seasonal trivalent inactivated vaccine containing pH1N1 ‐ RCTs: (one study/one data set) (paa Madhi 2014).
-
Seasonal inactivated vaccine ‐ cohort studies on effectiveness (ILI): (three studies/three data sets) (pca Black 2004; pca Eick 2011; pca France 2006).
-
Seasonal inactivated vaccine ‐ cohort studies on efficacy (laboratory‐confirmed): (one study/one data set) (pca Eick 2011).
-
Seasonal inactivated vaccine ‐ case‐control on effectiveness (ILI): (two studies/two data sets) (pba Benowitz 2010; pba Poehling 2011).
Administration during pregnancy ‐ pregnancy‐related outcomes (abortion, congenital malformation, prematurity, neonatal death)
-
Seasonal inactivated vaccine ‐ cohort studies: (seven studies/seven data sets) (pca Ahrens 2014; pca Black 2004; pca Munoz 2005; pcb Dodds 2012; pcb Nordin 2014; pcb Omer 2011; pcb Sheffield 2012).
-
2009 to 2010 pandemic: inactivated vaccine ‐ cohort studies: (14 studies/14 data sets) (pcb Beau 2014; pcb Cleary 2014; pcb Fell 2012; pcb Håberg 2013; pcb Heikkinen 2012; pcb Källén 2012; pcb Launay 2012; pcb Lin 2012; pcb Ludvigsson 2013; pcb Oppermann 2012; pcb Pasternak 2012; pcb Richards 2013; pcb Rubinstein 2013; pcb Trotta 2014).
-
Seasonal trivalent inactivated vaccine containing pH1N1 ‐ cohort studies: (two studies/two data sets) (pcb Chambers 2013; pcb Louik 2013).
-
Seasonal inactivated vaccine ‐ case‐control: (one study/one data set) (pbb Irving 2013).
We did not introduce one study in the quantitative synthesis because it is the only study on the A/NJ/8/76 vaccine (pcb Deinard 1981). We also did not include the retrospective cohort study of pcb Toback 2012 in the analysis because it did not contain useful outcomes. Results of one cohort study was not included in the analysis as it was only commented on (pcb Cantu 2013).
Administration during pregnancy ‐ severe harms
One included cohort study assessed the association between seasonal vaccine exposure during pregnancy and the following harms within 42 days from administration: Guillain‐Barré syndrome, demyelinating diseases, and immune thrombocytopenic purpura (pcb Nordin 2013).
Severe harms ‐ general population
Guillain‐Barré syndrome
-
2009 to 2010 pandemic ‐ case‐control: (two studies/six data sets) (bb Dieleman 2011a; bb Dieleman 2011b; bb Dieleman 2011c; bb Dieleman 2011d; bb Dieleman 2011e; bb Grimaldi‐Bensouda 2011).
-
Seasonal inactivated vaccine ‐ case‐control: (one study/one data set) (bb Galeotti 2013).
-
Seasonal inactivated vaccine ‐ cohort studies: (two studies/four data sets) (cb Kaplan 1982; cb Lasky 1998).
We did not introduce one cohort study assessing the association between the A/NJ/8/76 vaccine and Guillain‐Barré syndrome into the analysis (cb Shonberger 1979).
Demyelinating diseases (optic neuritis or multiple sclerosis)
-
Seasonal inactivated vaccine ‐ case‐control: (four studies/four data sets) (bb DeStefano 2003; bb Hernan 2004; bb Payne 2006; bb Zorzon 2003).
-
2009 to 2010 pandemic ‐ cohort study: (one study/one data set) (cb Moro 2013).
Immune thrombocytopenic purpura
-
Seasonal inactivated vaccine ‐ case‐control: (two studies/two data sets) (bb Garbe 2012; bb Grimaldi‐Bensouda 2012).
Other serious adverse events
-
Oculo‐respiratory syndrome: randomised cross‐over trial (one study) (ab Scheifele 2003) and one case‐control study (bb Rouleau 2014).
-
Respiratory function: RCT (ab Atmar 1990).
-
Cutaneous melanoma: case‐control (bb Mastrangelo 2000).
-
Bell's palsy: case‐control (bb Mutsch 2004).
-
Cardiac arrest: case‐control (bb Siscovick 2000).
-
Acute myocardial infarction: case‐control (bb MacIntyre 2013)
-
Rheumatoid arthritis: case‐control (bb Ray 2011).
-
Neurological and autoimmune disorders: three cohort studies (cb Bardage 2011; cb O'Flanagan 2014; cb Persson 2014) and one case‐control (bb Dauvilliers 2013).
-
Other serious adverse events: cohort study (cb Baxter 2012).
Pandemic vaccine: efficacy or effectiveness
-
RCT on inactivated parenteral vaccine: (four studies/seven data sets) (aa Eddy 1970; aa Mogabgab 1970a; aa Mogabgab 1970b; aa Waldman 1969a; aa Waldman 1969b; aa Waldman 1972b; aa Waldman 1972d).
-
RCT on inactivated aerosol vaccine: (two studies/four data sets) (aa Waldman 1969c; aa Waldman 1969d; aa Waldman 1972a; aa Waldman 1972c).
-
RCT on live aerosol vaccine (one study/one data set) (aa Sumarokow 1971).
Excluded studies
We excluded 183 studies (see Characteristics of excluded studies table).
Risk of bias in included studies
Out of the 137 included studies (substudy or data set), we classified 16.1% (22/137) as at low risk of bias (12 RCTs, two case‐control, eight cohort studies); 17.5% (24/137) as at high risk of bias (seven RCTs, three case‐control, 14 cohorts); and 66.4% (91/137) either did not present sufficient information in one or more key domains or, although presenting a low risk of bias in a specific domain, scored at high risk of bias in one or more items used in the quality evaluation. Table 2 shows the summary quality assessment of all included studies, and graphical displays of the quality assessment are presented in Figure 2 and Figure 3. We have highlighted that each 'paper' could include more than one study (data set), and these different studies required separate quality assessment. The funding source can be referred only to a single paper.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Study design | High risk | Low risk | Unclear risk | Total |
| Case‐control | 3 | 2 | 18 | 23 |
| Cohort | 14 | 8 | 18 | 40 |
| RCT/CCT | 7 | 12 | 55 | 74 |
| Total | 24 | 22 | 91 | 137 |
CCT: controlled clinical trial
RCT: randomised controlled trial
This table displays the overall methodological quality assessment of the included studies described in the text and represented in extended form (with all items of the tools) in Figure 1.
Allocation
In the included trials allocation concealment was adequate (low risk of bias) in 21 studies (28.4%), inadequate (high risk of bias) in seven studies (9.5%), and unclear (unclear risk of bias) in 46 studies (62.2%).
Blinding
We judged blinding as at low risk of bias in 17 RCTs/CCTs (23%), high risk of bias in three RCTs/CCTs (4.1%), and unclear in 54 RCTs/CCTs (73%).
Incomplete outcome data
The majority of the included RCTs/CCTs reported insufficient information about loss to follow‐up (64 studies; 86.5%).
Selective reporting
The assessment of selective reporting bias presents several difficulties and would require review of the original study protocols for the included studies, which are mainly unavailable.
Other potential sources of bias
Few studies reported information on influenza circulation in the surrounding community, making interpretation of the results and assessment of their generalisability difficult.
It is now known that industry funding of influenza vaccine studies determines publication in high‐prestige journals and higher citation rates than other types of funding. In addition, industry funding is associated with optimistic conclusions, but the quality of the majority of influenza vaccine studies is low, irrespective of funding (Table 3). A previously cited review showed a complex web of interrelationships between these variables (Jefferson 2009b), but the impact of this on policymaking is unknown.
| Study design | Government, institutional, or public | Industry | Mixed | Total |
| Case‐control | 14 | 2 | 2 | 18 |
| Cohort | 33 | 5 | 2 | 40 |
| RCT/CCT | 32 | 15 | 5 | 52 |
| Total | 79 | 22 | 9 | 110 |
CCT: controlled clinical trial
RCT: randomised controlled trial
Case‐control studies ‐ quality assessment
-
Case selection (definition/representativeness): case identification is mainly performed by means of registers maintained at several healthcare organisations (HMO, Kaiser Permanente) or by hospital or GP (general practice) registers. A further case ascertainment is conducted by specialists in order to verify the agreement with the chosen case definition. In studies assessing vaccine efficacy, cases were identified using a laboratory test performed on all participants having symptoms. For 21 out of 23 (91%), we classified case selection and definition as at low risk of bias.
-
Control selection (definition): controls were selected from within the same registers used for case identification or from among participants living in the same catchment area of the hospitals in which the cases were identified. We classified control selection and definition as at low risk of bias for 10 out of 23 studies (43.4%), and unclear risk of bias for 11 out of 23 (47.8%).
-
Comparability: the most frequent method used to ensure comparability between cases and controls consisted of matching for age, gender, and index date (onset of symptoms for cases and GP visit for controls). Less frequently matching was also done for other possible parameters, such as the number of GP visits within a certain time interval, or by resorting to the use of a propensity score or multivariate models in order to reduce the impact of other possible confounders. Nevertheless, many studies (18 out of 23 (78.3%)) provided insufficient information to judge how comparable cases and controls effectively are.
-
Exposure ascertainment (same method of ascertainment for cases and controls/non‐response rate): for studies based on healthcare organisations or insurance registers, assessment of vaccine exposure was certified in the same registers. In other studies vaccine exposure was ascertained with a structured interview, and less frequently also with the recovering of the vaccination records. In many studies (15 out of 23 (65.2%)), ascertainment of the vaccine exposure was not fully reliable. For 7 out of 23 studies (30.4%), we judged exposure ascertainment as at low risk of bias.
Cohort studies ‐ quality assessment
-
Selection exposed cohort (definition/representativeness): the majority of the studies were retrospective and used a data linkage method to select the exposed cohort. In 20 out of 40 studies (50%), this procedure was insufficiently described.
-
Selection non‐exposed cohort (definition/ascertainment): most of the studies were based on record linkage and the identification of the non‐exposed cohort was done by considering the absence of vaccination records. However, insufficient detail was provided, therefore we classified such studies as at unclear risk of bias (18 out of 40 (45%)).
-
Comparability: in most of the included cohort studies matching procedures for the most probable confounders were applied using a multivariate model to ensure comparability between exposed and unexposed cohorts. A propensity score procedure was also sometimes used. Therefore in some studies only a few confounders were used to ensure comparability between exposed and non‐exposed cohorts. We classified seven studies as at low risk of bias (17.5%).
-
Assessment of outcome (demonstration that outcome of interest was not present at the start of the study/whether follow‐up was long enough for outcomes to occur/adequacy of follow‐up of cohorts): outcomes of interest were generally documented in the registries used to identify the study population, and consequently were almost always retrospectively assessed, thus we classified 11 out of 40 (27.5%) as at low risk of bias.
Effects of interventions
We constructed the Data and analyses tables according to the following criteria.
-
Inactivated parenteral influenza vaccine versus placebo or 'do nothing' (Comparison 01).
-
Live aerosol influenza vaccine versus placebo or 'do nothing' (Comparison 02).
-
Inactivated aerosol influenza vaccine versus placebo or 'do nothing' (Comparison 03).
-
Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy (Comparison 04).
-
Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies (Comparison 05).
-
Inactivated parenteral influenza vaccine versus placebo ‐ case‐control studies (Comparison 06).
-
Serious adverse events: Guillain‐Barré syndrome ‐ cohort studies (Comparison 07).
-
Serious adverse events: Guillain‐Barré syndrome ‐ case‐control studies (Comparison 08).
-
Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies (Comparison 09).
-
Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies (Comparison 10).
-
Serious adverse events: immune thrombocytopenic purpura ‐ cohort studies (Comparison 11).
-
Serious adverse events: immune thrombocytopenic purpura ‐ case‐control studies (Comparison 12).
-
1968 to 1969 pandemic: inactivated polyvalent parenteral influenza vaccine versus placebo (Comparison 13).
-
1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo (Comparison 14).
-
1968 to 1969 pandemic: inactivated polyvalent aerosol influenza vaccine versus placebo (Comparison 15).
-
1968 to 1969 pandemic: inactivated monovalent aerosol influenza vaccine versus placebo (Comparison 16).
-
1968 to 1969 pandemic: live aerosol influenza vaccine versus placebo (Comparison 17).
Evidence from RCTs/CCTs on vaccine efficacy/effectiveness in the general population is reported in Analyses 1 to 3. Evidence from RCTs/CCTs on vaccine efficacy/effectiveness in pregnancy is reported in Comparison 4. Evidence from observational studies in pregnancy is reported in Analyses 5 and 6.
Studies investigating the association between influenza vaccination and Guillain‐Barré syndrome were included in Comparison 7 (cohort on seasonal vaccine) and Comparison 8 (case‐control on H1N1 vaccine). In Comparison 8, we stratified studies according to three different exposure definitions according to the time between vaccination to onset of symptoms (any time, within seven weeks, over seven weeks). We have presented evidence for the association between seasonal vaccine and Guillain‐Barré syndrome from cohort studies in Comparison 7.
Studies investigating the association between influenza vaccination and multiple sclerosis and optic neuritis are included in Analyses 9 and 10 (cohort and case‐control studies ‐ demyelinating diseases).
Studies investigating the association between influenza vaccination and immune thrombocytopenic purpura are included in Analyses 11 and 12 (cohort and case‐control studies ‐ immune thrombocytopenic purpura).
We have constructed a 'Summary of findings' table for key outcomes (see summary of findings Table for the main comparison).
Inactivated parenteral influenza vaccine versus placebo or 'do nothing' (Comparison 01)
Inactivated parenteral vaccines probably have 59% efficacy in preventing confirmed influenza (risk ratio (RR) 0.41, 95% confidence interval (CI) 0.36 to 0.47; 71,221 participants; 25 studies, moderate‐certainty evidence) (Analysis 1.1). Based on the control group risk of 2.3%, 71 healthy adults need to be vaccinated in order to prevent one of them experiencing influenza. The effects were very similar when matching was absent or unknown. Since heterogeneity was very low (I2 = 17% for Analysis 1.2.1; I2 = 14% for Analysis 1.1.2), there were no differences when comparing the estimates obtained by using a fixed‐effect model with those from a random‐effects model. Restricting the analysis to studies at low risk of bias did not affect the direction or size of effect (see Table 4).
| Outcome (analysis) | All studies (primary analysis) | Studies at low risk of bias (sensitivity analysis) |
| Influenza (Analysis 1.1) | RR 0.41 (0.36 to 0.47) | RR 0.34 (0.25 to 0.45) |
| Influenza‐like illness (Analysis 1.2) | RR 0.84 (0.75 to 0.95) | RR 0.82 (0.69 to 0.98) |
| Hospitalisations (Analysis 1.8) | RR 0.96 (0.85 to 1.08) | RR 2.89 (0.12 to 70.68) |
| Fever (Analysis 1.11.2) | RR 1.55 (1.26 to 1.91) | RR 1.59 (1 to 2.53) |
| Nausea/vomiting (Analysis 1.11.5) | RR 1.80 (0.65 to 5.04) | RR 7.05 (1.61 to 30.87) |
RR: risk ratio
Inactivated parenteral vaccines probably have 16% effectiveness in preventing ILI (RR 0.84, 95% CI 0.75 to 0.95; 25,795 participants; 16 studies; moderate‐certainty evidence) (Analysis 1.2). There was wide variation in the control group risks, with risk differences in low‐, moderate‐, and high‐risk groups of 0.6%, 3.4%, and 14.6%. Based on the median (i.e. moderate risk) control group risk of 21.5%, 29 healthy adults need to be vaccinated to prevent one adult experiencing an ILI. For low‐ and high‐risk control group the corresponding NNVs were 167 and 7, respectively. Sensitivity analysis by risk of bias did not change the size or direction of effect (Table 4).
Results across the subgroups by matching criteria were very similar (I2 = 0%).
Based on the results from a single study (aa Bridges 2000b), physician visits appear 42% less frequent (95% CI 9% to 63%) in participants immunised with vaccines prepared with strains matching circulating viruses (Analysis 1.3.1), whereas there were no significant results when the degree of matching was unknown or absent (RR 1.28, 95% CI 0.90 to 1.83; Analysis 1.3.2). The overall effect was also not significant (RR 0.87, 95% CI 0.40 to 1.89) (Analysis 1.3). Even though the two data sets of aa Bridges 2000b showed very high heterogeneity (I2 = 87%), no difference arose when comparing the results from the fixed‐effect with the random‐effects model.
We observed a similar conflicting result when analysing the effect of inactivated vaccine administration on days of illness (Analysis 1.4), when the estimate (mean difference (MD)) obtained in good‐match conditions was compared with unknown or absent degree of matching. As a consequence of the high overall heterogeneity (I2 = 87%), the result obtained from the fixed‐effect model analysis (MD ‐0.31, 95% CI ‐0.54 to ‐0.07) differed from the result of the application of a random‐effects model (MD ‐0.21, 95% CI ‐0.98 to 0.56).
There seemed to be no effect on the time an antibiotic or drug was prescribed (Analysis 1.5; Analysis 1.6).
Four trials evaluated time off work, estimating that vaccination may save around 0.04 working days per person over a single influenza season. This result was affected by high levels of heterogeneity (I2 = 82%) but did not change depending on whether a fixed‐effect (MD ‐0.04, 95% CI ‐0.06 to ‐0.01) or random‐effects model (MD ‐0.04, 95% CI ‐0.14 to 0.06) (Analysis 1.7) was used. We rated the evidence as of low certainty.
Vaccination may have a small effect on hospitalisation (Analysis 1.8), but the CI was wide and does not rule out a large reduction in hospitalisation (RR 0.96, 95% CI 0.85 to 1.08; low‐certainty evidence). We found no evidence for cases of pneumonia.
Harms
Live parenteral influenza vaccines increase fever from 1.5% to 2.33% (RR 1.55, 95% CI 1.26 to 1.91; 23,850 participants; 13 studies; high‐certainty evidence) (Analysis 1.11.2). The rate of nausea or vomiting was low in the trials (4% in unvaccinated population versus 7% with vaccines), although we rated this evidence as low certainty due to wide CIs and possible impact of bias (see Table 4) (RR 1.80, 95% CI 0.65 to 5.04; 6315 participants; 4 trials) (Analysis 1.11.5).
Local tenderness and soreness were more than three times as common among parenteral vaccine recipients than among those in the placebo group (RR 3.13, 95% CI 2.44 to 4.02) (Analysis 1.10.1). There were also increases in erythema (RR 2.59, 95% CI 1.77 to 3.78; Analysis 1.10.2) and induration (RR 4.28, 95% CI 1.25 to 14.67) but not in arm stiffness. The combined local effects endpoint was significantly higher for those receiving the vaccine (RR 2.44, 95% CI 1.82 to 3.28; Analysis 1.10.5).
Myalgia was significantly associated with vaccination (RR 1.74, 95% CI 1.41 to 2.14) (Analysis 1.11.1), fatigue or indisposition (RR 1.19, 95% CI 1.05 to 1.36) (Analysis 1.11.4), and malaise (RR 1.51, 95% CI 1.18 to 1.92) (Analysis 1.11.6). The combined endpoint was not increased (RR 1.16, 95% CI 0.87 to 1.53; Analysis 1.11.7).
Live aerosol influenza vaccine versus placebo or 'do nothing' (Comparison 02)
Live aerosol vaccines have an overall efficacy of 53% (95% CI 38% to 65%), and the NNV is 39 (95% CI 32 to 54). Neither content nor matching appeared to affect their performance significantly. The vaccines have an effectiveness against ILI of 10% (95% CI 4% to 16%; NNV 46, 95% CI 29 to 115), and content and matching appeared not to affect their performance significantly (Analysis 2.2).
No evidence was available on complications (e.g. bronchitis, otitis media, pneumonia).
The effectiveness of the aerosol vaccines against ILI (with no clear definition) was significant only for vaccines with absent or unknown matching (37%, 95% CI 20% to 51%), and the NNV was 69 (95% CI 23 to 46) (Analysis 2.3).
The conclusions of this comparison were unaffected by analysis using either the fixed‐effect or random‐effects models.
Harms
Significantly more recipients experienced local symptoms after vaccine administration than after placebo administration (Analysis 2.4).
-
Upper respiratory infection (RR 1.66, 95% CI 1.22 to 2.27).
-
Cough (RR 1.51, 95% CI 1.08 to 2.10).
-
Coryza (RR 1.56, 95% CI 1.26 to 1.94).
-
Sore throat (RR 1.66, 95% CI 1.49 to 1.86).
-
Combined endpoint (any or highest symptom) (RR 1.56, 95% CI 1.31 to 1.87).
There was no significant increase in systemic harms (combined endpoint: any or highest symptom RR 1.40, 95% CI 0.82 to 2.38), although rates of myalgia (RR 2.47, 95% CI 1.26 to 4.85) and headache (RR 1.54, 95% CI 1.09 to 2.18) were higher in the vaccine group than in the placebo group (Analysis 2.5).
Inactivated aerosol influenza vaccine versus placebo or 'do nothing' (Comparison 03)
We could include no RCTs assessing the effectiveness of inactivated aerosol vaccines in preventing ILI; the only available evidence comes from studies carried out during the 1968 to 1969 pandemic (Analyses 12 to 16).
The efficacy of inactivated aerosol vaccine in preventing laboratory‐confirmed influenza (Analysis 3.1.1) was assessed in one RCT (aa Langley 2011), whose results do not show a statistically significant protective effect (RR 0.38, 95% CI 0.14 to 1.02).
Harms
None of the trials on inactivated aerosol vaccines reported significant harms.
Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy (Comparison 04)
In this analysis, we considered the results of one RCT (at low risk of bias) and one CCT (at high risk of bias) assessing the effect of vaccination during pregnancy on the prevention of influenza and ILI in both mother and newborns.
Vaccination with trivalent inactivated vaccine containing pH1N1 was weakly protective against influenza (RCT data only) in mothers within 24 weeks after delivery (RR 0.50, 95% CI 0.29 to 0.86; vaccine efficacy (or effectiveness) (VE) 50%, 95% CI 14% to 71%; NNV 55, 95% CI 39 to 198; Analysis 4.1), as well as among children born from a vaccinated mother until their first 24 weeks of life (VE 49%, 95% CI 12% to 70%; NNV 56, 95% CI 39 to 230; Analysis 4.3). Vaccination with monovalent pandemic or trivalent inactivated vaccine containing pH1N1 did not confer significant protection against ILI, either in mothers (RR 0.96, 95% CI 0.79 to 1.16; Analysis 4.2) or in newborns (RR 1.02, 95% CI 0.94 to 1.09; Analysis 4.4).
Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies (Comparison 05)
Based on unadjusted data from a cohort study (high risk of bias), 2009/2010 H1N1 monovalent pandemic vaccines (Analysis 5.1.1) provide a significant protective effect against ILI in pregnant women (RR 0.11, 95% CI 0.06 to 0.21; VE 89%, 95% CI 79% to 94%; NNV 54, 95% CI 51 to 61). Seasonal inactivated vaccine is not effective against ILI (RR 0.54, 95% CI 0.24 to 1.18; Analysis 5.1.2). Sensitivity analysis performed using the fixed‐effect model showed statistical significance, even for a modest protective effect (RR 0.76, 95% CI 0.64 to 0.89; NNV 94, 95% CI 63 to 205; VE 24%, 95% CI 11% to 36%).
The effectiveness of vaccination with seasonal inactivated vaccine during pregnancy for preventing ILI in newborns was not statistically significant, as the results are based on two cohort studies using either hazard ratio (HR) or RR adjusted estimates (Analysis 5.2.1 and Analysis 5.3.1, respectively). Efficacy against confirmed influenza (Analysis 5.3.2) is modest but has statistical significance (adjusted RR 0.59, 95% CI 0.37 to 0.94; NNV 27, 95% CI 18 to 185; VE 41%, 95% CI 6% to 63%).
Vaccination with the 2009/2010 H1N1 monovalent pandemic vaccine during pregnancy may not be associated with a higher risk of abortion (Analysis 5.4.1 and Analysis 5.4.2), congenital malformation (Analysis 5.4.3), or neonatal death (Analysis 5.4.9). From a meta‐analysis of seven cohort studies, preterm deliveries (before 37 weeks of gestation) occurred with slightly less frequency among women who were immunised with monovalent pandemic H1N1 vaccine during pregnancy compared to unvaccinated women (Analysis 5.4.5, adjusted OR 0.84, 95% CI 0.76 to 0.93). This result was not confirmed by two other cohort studies, which found no significant association (Analysis 5.4.6, adjusted HR 1.11, 95% CI 0.46 to 2.68) or by two other cohort studies that separately analysed vaccine administration during the first trimester of gestation with that during the second or third trimester (Analysis 5.4.6; Analysis 5.4.7; Analysis 5.4.8).
Cases of neonatal death and abortion were observed less frequently among women immunised with seasonal influenza vaccine (Analysis 5.5.1 and Analysis 5.5.4, both unadjusted estimates). We found no statistically relevant association between seasonal influenza vaccine exposure during pregnancy and prematurity or congenital malformations (Analysis 5.5.2; Analysis 5.5.3; Analysis 5.5.4). Two other cohort studies did not find any statistically significant association between exposure to seasonal trivalent inactivated vaccine containing pH1N1 and prematurity, whatever the trimester of gestation (Analysis 5.6). This finding was confirmed by one other retrospective cohort study, which was not included in the analysis (pcb Cantu 2013, adjusted RR 1.2, 95% CI 0.9 to 1.6).
The results of pcb Deinard 1981 are based on the follow‐up results of 189 pregnant women immunised with monovalent pandemic A/New Jersey/8/76 (either in split‐ or whole‐virus formulation) and 517 pregnant women who did not receive vaccination. The time of observation was extended up to the first eight weeks of life of the newborns. No statistically different incidence of maternal pregnancy outcomes or infant deaths was observed between vaccinated and unvaccinated groups. Statistical analysis (Chi2 test) showed no relation between immunisation history and presence of anomalities at the eighth week of life. We did not include this cohort study in the analysis, as the vaccine studied is no longer in use.
Inactivated parenteral influenza vaccine versus placebo ‐ case‐control studies (Comparison 06)
This analysis only included studies assessing the effect of vaccination against influenza during pregnancy. The incidence of ILI in pregnant women who were immunised with inactivated seasonal vaccine during pregnancy was not statistically different when compared with that observed among unvaccinated pregnant women (Analysis 6.1.1). However, the results of the analysis became statistically significant in sensitivity analysis using the fixed‐effect model, leading us to conclude that the results of this comparison were affected by the model used to perform the analysis.
One further case‐control study did not find a statistically significant association between exposure to seasonal inactivated vaccine in pregnancy and abortion cases (Analysis 6.2.1).
One retrospective cohort study attempted to assess the effect of live attenuated vaccine during pregnancy based on data from a health insurance database during six subsequent influenza seasons (pcb Toback 2012). A total of 834,999 pregnant women were identified, of whom 138 received live attenuated vaccine at any time during pregnancy. Claims for hospitalisation or visits to the emergency department within 42 days after immunisation were searched for, but all observed events were considered to be related to a normal physiological pregnancy and not to immunisation. The system used (claim data) would be unable to detect birth outcomes.
Serious adverse events: Guillain‐Barré syndrome ‐ cohort studies (Comparison 07)
Two cohort studies performed during two subsequent epidemic seasons investigated the possible association between exposure to seasonal inactivated vaccine in healthy adults and Guillain‐Barré syndrome onset within six weeks following immunisation. No significant association was found (Analysis 7.1.1). Administration of seasonal inactivated vaccine during pregnancy was not associated with Guillain‐Barré syndrome onset within six weeks from immunisation (Analysis 7.1.2).
The cohort of cb Shonberger 1979 was the first study that compared Guillain‐Barré syndrome cases by vaccination status and the national incidence in vaccinated and unvaccinated national cohorts after the suspension of the National Influenza Immunization Program in the winter of 1976 to 1977. At that time the monovalent inactivated swine vaccine A/New Jersey/8/76 had been administered. The attributable risk from vaccination was just below one case of Guillain‐Barré syndrome in every 100,000 vaccinations. We did not include this cohort study in the analysis as the vaccine studied is no longer in use.
Serious adverse events: Guillain‐Barré syndrome ‐ case‐control studies (Comparison 08)
In an analysis performed using the mean of unadjusted data relative to six data sets, exposure to monovalent H1N1 pandemic inactivated vaccine resulted in an apparent statistically significant association with Guillain‐Barré syndrome onset when administration took place within six weeks before symptoms occurred (odds ratio (OR) 2.22, 95% CI 1.14 to 4.31; Analysis 8.1.1). It should thus be taken into account that only one out of the six data sets showed a statistically significant association between vaccine exposure and Guillain‐Barré syndrome onset (bb Dieleman 2011e). When we performed a sensitivity analysis excluding this data set from the pooled estimate, the result was no longer significant. When the analysis was performed for vaccine exposure that occurred at any time before disease onset, there was no significant association (Analysis 8.1.2).
The analyses performed by pooling authors' estimates adjusted for several confounders (i.e. receipt of other vaccines, family history of autoimmune diseases, physician consultation during the previous year, and use of antibiotic, antiviral, or antipyretic agents) did not show a statistical association for exposure within six weeks (Analysis 8.2.1) before disease onset or for exposure at any time (Analysis 8.2.2).
Data from one other case‐control study confirmed that immunisation with seasonal inactivated vaccine is not significantly associated with the onset of Guillain‐Barré syndrome within six weeks after inoculation (Analysis 8.3) (bb Galeotti 2013).
Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies (Comparison 09)
In one cohort study the authors attempted to assess whether there was an association between exposure to inactivated trivalent seasonal influenza vaccine during pregnancy and several pathologies (e.g. Guillain‐Barré syndrome, demyelinating diseases, immune thrombocytopenic purpura) within six weeks after immunisation. Unadjusted estimates were calculated for an association with demyelinating diseases by using the number of cases observed among exposed and unexposed hemi‐cohorts, and indicated that there was no association (Analysis 9.1.2).
One cohort study assessed the safety of the H1N1 vaccine. No statistical association was found between vaccination with H1N1 monovalent pandemic vaccine and demyelinating diseases.
Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies (Comparison 10)
An association between exposure to seasonal inactivated vaccine and demyelinating diseases (including both multiple sclerosis and optic neuritis case definitions) in a healthy adult population was not statistically significant when we pooled unadjusted data from four case‐control studies (OR 0.96, 95% CI 0.79 to 1.17) (Analysis 10.1). Also, when we analysed adjusted data for each of the case definitions separately, the estimates remained non‐statistically significant for multiple sclerosis (Analysis 10.2) and for optic neuritis (Analysis 10.3).
Serious adverse events: immune thrombocytopenic purpura ‐ cohort studies (Comparison 11)
One cohort study aimed to assess whether there was an association between exposure to inactivated trivalent seasonal influenza vaccine during pregnancy and several pathologies (e.g. Guillain‐Barré syndrome, demyelinating diseases, immune thrombocytopenic purpura) within six weeks after immunisation. Neither the unadjusted (Analysis 11.2.2) nor adjusted estimates (Analysis 11.1.2) for an association with immune thrombocytopenic purpura were statistically significant.
Serious adverse events: immune thrombocytopenic purpura ‐ case‐control studies (Comparison 12)
Data analysis of two case‐control studies did not show a statistically significant association between immune thrombocytopenic purpura and seasonal influenza vaccine in any of the time frames considered (i.e. less than two months, six or 12 months between immunisation and disease onset), or when the data were pooled together (Analysis 12.2) (bb Garbe 2012; bb Grimaldi‐Bensouda 2012). We drew the same conclusions when analysis was performed using estimates adjusted for confounders (Analysis 12.1), and a sensitivity analysis carried out using either a random‐effects or fixed‐effect model did not change our conclusions, providing further confirmation of them. It should be observed that no data sets included in this comparison, with the exception of bb Garbe 2012, showed a statistical association between disease and influenza vaccination. It is possible that the ages of the participants (cases and controls) were different in these two studies, and that some elderly participants may have been included. Unlike bb Grimaldi‐Bensouda 2012, the case‐control study bb Garbe 2012 considered as exposed those cases that were immunised up until 28 days before immune thrombocytopenic purpura onset.
Serious and rare harms
Oculo‐respiratory syndrome
On the basis of one randomised trial in 651 healthy adults aged around 45, trivalent split inactivated vaccine caused mild oculo‐respiratory syndrome in people with no previous history of oculo‐respiratory syndrome (ab Scheifele 2003). Oculo‐respiratory syndrome was defined as bilateral conjunctivitis, facial swelling (lip, lid, or mouth), difficulty in breathing and chest discomfort (including cough, wheeze, dysphagia, or sore throat). Oculo‐respiratory syndrome (attributable risk 2.9%, 95% CI 0.6 to 5.2), hoarseness (1.3%, 95% CI 0.3 to 1.3), and coughing (1.2%, 95% CI 0.2 to 1.6) occurred within six days of vaccination. The association did not appear to be specific to any type of trivalent inactivated vaccine. One register‐based case‐control study carried out in Quebec showed an increased risk (adjusted OR 2.71, 95% CI 1.80 to 4.08) of oculo‐respiratory syndrome during the first four weeks of the 2009 pandemic vaccination campaign (monovalent, AS03‐adjuvanted pH1N1 vaccine) (bb Rouleau 2014).
Bell's palsy
One case‐control study and case series based in the German‐speaking regions of Switzerland assessed the association between an intranasal inactivated virosomal influenza vaccine and Bell's palsy (bb Mutsch 2004). Two hundred and fifty cases that could be evaluated (from an original 773 cases identified) were matched to 722 controls. All were aged around 50. The study reported a massive increase in risk (adjusted OR 84, 95% CI 20.1 to 351.9) within 1 to 91 days from vaccination. Despite the many limitations of this study (case attrition: 187 cases could not be identified; ascertainment bias: physicians picked controls for their own cases; confounding by indication: different vaccine exposure rate between controls and the reference population), it is unlikely that such a large OR could have been affected significantly by systematic error. The authors called for larger pre‐licence harms trials, given the rarity of Bell's palsy. On the basis of this study the vaccine was withdrawn from sale.
Rheumatoid arthritis
One case‐control study used the register of the Northern California Kaiser Permanente Health Plan (NCKPHP) in order to identify cases of rheumatoid arthritis diagnosed during a three‐year period (1 January 1997 to 31 December 1999) among members of NCKPHP for at least two years (i.e. since 1 January 1995) and aged between 15 and 59 (bb Ray 2011). After reviewing clinical cards, 415 cases of definite or probable rheumatoid arthritis were included with 1245 randomly selected controls matched for age within one year and for a categorical utilisation variable based on the number of clinic visits during the year prior to the rheumatoid arthritis symptom onset date (none, one to two, three to five, six to nine, or 10+ visits). The Kaiser Immunisation Tracking System and chart review were used to determine vaccination status of cases and controls. Different time intervals between immunisation and rheumatoid arthritis onset were considered for analysis: 90, 180, 365, and 730 days. No significant association between vaccination and rheumatoid arthritis could be determined for any time interval, even after adjustment for confounders (sex, race, and exact number of utilisation visits). The authors of this study performed a data analysis by using a person‐time cohort design, in which vaccinated cases contributed to the unexposed follow‐up time until they were immunised and to the exposed follow‐up time thereafter. Unlike case‐control analysis, person‐time cohort analysis was performed by excluding cases who showed symptoms in 1996. Even if a significant association for exposure to vaccine occurred within 180 and 365 days before disease onset (OR adjusted for race, sex, and number of clinic visits 1.36, 95% CI 1.03 to 1.80 and 1.34, 95% CI 1.06 to 1.69, respectively), the authors note that it is very difficult to estimate with sufficient precision the true onset date of rheumatoid arthritis, as the first symptoms could already be present for some time before people present for medical care. This is the most important limitation of this study and could have significantly affected the estimates.
Neurological and autoimmune disorders
The study of cb Bardage 2011 was a large, prospective cohort study carried out in a Stockholm population (n = 1,945,024) during the vaccination campaign with monovalent A (H1N1) pandemic vaccine Pandemrix (GlaxoSmithKline, containing adjuvants AS03 and squalene) to evaluate the presence of an association between Pandemrix and neurological and/or autoimmune diseases (Guillain‐Barré syndrome, multiple sclerosis, Bell's palsy, narcolepsy, polyneuropathy, an/hypoaesthesia, paraesthesia, rheumatological disease and inflammatory bowel disease). During the first 45 days, participants with high‐risk conditions were preferentially vaccinated; vaccination was then offered to the remainder of the population in a second phase of the campaign (see Characteristics of included studies’ table for more details).
The analysis of the HR adjusted for age, sex, socioeconomic status, and healthcare consumption (number of hospital admissions and visits to specialist care one year before the pandemic period) showed that in participants immunised during the early phase of the campaign, there was a significantly increased risk of Bell's palsy (HR 1.34, 95% CI 1.11 to 1.64), paraesthesia (HR 1.25, 95% CI 1.10 to 1.41), and inflammatory bowel disease (HR 1.25, 95% CI 1.04 to 1.50). For the participants vaccinated in the late phase of the campaign (> 45 days), HR estimates showed there was no statistically different incidence in the investigated diseases between vaccinated and unvaccinated participants.
A further stratification was performed considering the time since first vaccination (six weeks or less and more than six weeks), which showed that in participants immunised during the first phase of the campaign, an increased incidence of Bell's palsy and paraesthesia was most pronounced, as well as within six weeks of vaccination (HR 1.74, 95% CI 1.16 to 2.59 for Bell's palsy and HR 1.60, 95% CI 1.25 to 2.05 for paraesthesia) and thereafter (HR 1.26, 95% CI 1.01 to 1.57 for Bell's palsy and HR 1.17, 95% CI 1.02 to 1.34 for paraesthesia). An increased risk of inflammatory bowel disease among those vaccinated in the early phase was only observed more than six weeks after vaccination (HR 1.29, 95% CI 1.06 to 1.58). Formal tests to determine whether risks differed further between those within and more than six weeks from vaccination were only statistically significant for paraesthesia (P = 0.005). In participants immunised during the second phase of the campaign, polyneuropathy was significantly more common within six weeks of immunisation (HR 1.79, 95% CI 1.16 to 2.77).
The study by cb Persson 2014 consisted of an extension of the Bardage study to more Swedish regions, namely the healthcare regions of Skåne and Västra Götaland and the counties of Kalmar, Östergötland, Värmland, and Norrbotten. The study included over 5.8 million participants, corresponding to about 61% of the whole Swedish population in 2009. In all, 207 cases of narcolepsy were confirmed, with the exclusion of eight cases with prodromal conditions during the last five years. The overall risk of narcolepsy after immunisation with Pandemrix assessed by Cox regression after adjusting for age, gender, county, education, income, number of hospital admissions and ambulatory care visits, pregnancy status, and presence of other diagnoses was not statistically relevant in the population aged above 20 years (HR 1.35, 95% CI 0.93 to 1.95). A significant association was instead found in those aged below 20 (HR 2.92, 95% CI 1.78 to 4.79), in whom most cases of narcolepsy had occurred (n = 126).
A population‐based cohort study carried out in Ireland identified only three cases of narcolepsy in the whole Irish adult population aged above 20 years during the pandemic season 2009 to 2010; two of them received Pandremix and one did not (cb O'Flanagan 2014). The risk estimate was extremely imprecise and did not allow us to draw any conclusions (RR 20.4, 95% CI 1.8 to 225). One case‐control study (bb Dauvilliers 2013), performed across the institutions of 14 French expert orphan disease narcolepsy centres, identified 25 narcolepsy cases and 73 matched controls (age, sex, and geographical location) in the study population aged at least 18 years. An association between exposure to H1N1 vaccination and narcolepsy‐catalepsy (crude OR 4.7, 95% CI 2.1 to 13.9) was found and was also confirmed after the performance of a sensitivity analysis and adjusting for smoking habits and family history of excessive daytime sleepiness (OR 4.1, 95% CI 1.4 to 12.2).
Cutaneous melanoma
A case‐control study assessed the association between influenza vaccines and cutaneous melanoma in 99 cases and 104 controls (bb Mastrangelo 2000). The authors reported a protective effect of repeated influenza vaccination on risk of cutaneous melanoma (OR 0.43, 95% CI 0.19 to 1.00). The study was at high risk of bias due to the selective nature of cases (all patients in the authors' hospital), attrition bias (four cases and four controls eliminated due to "failure to collaborate"), recall bias (up to five years' exposure data were based on patients' recollection), and ascertainment bias (non‐blinded exposure survey).
Primary cardiac arrest
A case‐control study assessed the association between influenza vaccination the previous year and the risk of primary cardiac arrest (i.e. occurring in people with no previous history of cardiac disease) in 360 cases and 418 controls (bb Siscovick 2000). The authors concluded that vaccination is protective against primary cardiac arrest (OR 0.51, 95% CI 0.33 to 0.79). The difficulty of case ascertainment (77% of potential cases had no medical examiner report and/or autopsy) and recall bias (spouses provided exposure data for 304 cases, while 56 survivor cases provided data jointly with their spouses) make the conclusions of this study unreliable. It is impossible to judge the reliability of this study because of a lack of detail on the circulation of influenza in the study areas in the 12 months preceding cardiac arrest (the causal hypothesis is based on the effects of influenza infection on the oxygen supply to the myocardium through lung infection and inflammation).
Acute myocardial infarction
One case‐control study performed in Australia assessed whether exposure to influenza vaccine provides protection against acute myocardial infarction in an adult population aged over 40 (bb MacIntyre 2013). Cases of acute myocardial infarction admitted to the cardiology unit of a tertiary hospital in Sydney during three consecutive epidemic seasons (2008, 2009, and 2010) were compared to unmatched controls attending the orthopaedic or ophthalmic outpatient clinics during the same time period with respect to their exposure to influenza vaccine (176 cases and 72 controls aged below 64 were included). From multivariate analysis, after adjusting for several confounders, influenza vaccination did not confer significant protection against acute myocardial infarction in an adult population aged between 40 and 64 years (OR 0.55, 95% CI 0.27 to 1.15).
Pulmonary function
A double‐blind, placebo‐controlled randomised trial in 72 healthy volunteers aged around 26 assessed the effects of different types of live attenuated cold recombinant influenza vaccination on pulmonary function (data on 17 asthmatics were not extracted) (ab Atmar 1990). The authors reported several non‐significant drops in lung function up to seven days postinoculation and a higher incidence of ILI (17/46 versus 4/26) in the vaccinated arms.
Other serious adverse events
The study of cb Baxter 2012 is a large, retrospective cohort performed among members of Kaiser Permanente Health Plans of Northern California, Hawaii, and Colorado aged between 18 and 59 years, who were immunised with live attenuated, inactivated influenza vaccine or who did not receive vaccination. The study retrospectively investigated the occurrence of adverse events (see Characteristics of included studies’ table for more details) during five subsequent epidemics, but did not identify any unexpected serious risks when the live attenuated vaccine was used in approved populations.
Vaccines for the 1968 to 1969 (H3N2) influenza pandemic (Comparisons 13 to 17)
Five studies yielded 12 data sets (aa Eddy 1970; aa Mogabgab 1970a; aa Mogabgab 1970b; aa Sumarokow 1971; aa Waldman 1969a; aa Waldman 1969b; aa Waldman 1969c; aa Waldman 1969d; aa Waldman 1972a; aa Waldman 1972b; aa Waldman 1972c; aa Waldman 1972d). As one would expect, vaccine performance was poor when the content did not match the pandemic strain (Analysis 13.1; Analysis 13.2). However, one‐ or two‐dose monovalent whole‐virion (i.e. containing dead complete viruses) vaccines achieved a VE of 65% (95% CI 52% to 75%) protection against ILI (NNV 16, 95% CI 14 to 20), a VE of 93% (95% CI 69% to 98%) with NNV 35 (95% CI 33 to 47) protection against influenza, and a VE of 65% (95% CI 6% to 87%) with NNV 94 (95% CI 70 to 1022) against hospitalisation (Analysis 14.1; Analysis 14.2; Analysis 14.3).
Approximately half a working day and half a day of illness were saved (Analysis 14.5; Analysis 14.6), but no effect was observed on pneumonia (Analysis 14.4). All comparisons except for ILI were based on a single study (Analysis 14.4). The large effect on ILI is coherent with the high proportion of these illnesses caused by influenza viruses in a pandemic (i.e. the gap between the efficacy and effectiveness of the vaccines is narrow). Aerosol polyvalent or monovalent vaccines had a modest effect.
Discusión
Resumen de los resultados principales
En los adultos sanos, las vacunas parenterales con virus vivos probablemente reducen la gripe del 2,3% al 1%, sobre la base de una eficacia de la vacuna del 59% (evidencia de certeza moderada). Lo anterior corresponde a un NNV de 71. La efectividad de la vacuna parenteral con virus vivos contra la ESG fue menor (16%), con un NNV de 29 basado en un supuesto riesgo del grupo de control de 21,5%. Se encontró una mayor variación en los riesgos del grupo de control de las ESG en comparación con la gripe (Resumen de resultados, tabla 1). Los riesgos bajos y altos del grupo de control (4% y 91%) correspondían a los NNV de 167 y 7, respectivamente. La eficacia general de las vacunas inactivadas para prevenir la gripe es del 59% (IC del 95%: 51% a 66%) con un NNV de 77. Cuando el contenido de la vacuna coincide con la cepa circulante la eficacia es del 59% (IC del 95%: 53% a 64%). Sobre la base de los resultados de un único estudio (aa Bridges 2000b), las visitas al médico parecen tener una frecuencia de un 42% menos en los participantes inmunizados con vacunas preparadas con cepas que coinciden con los virus en circulación, mientras que no se encontraron diferencias significativas cuando el grado de coincidencia era desconocido o estaba ausente (RR 1,28; IC del 95%: 0,90 a 1,83). Nuevamente, el efecto general no es significativo (RR 0,87; IC del 95%: 0,40 a 1,89). No parece haber efectos sobre el momento en que se prescribe un antibiótico o un fármaco. Cuatro ensayos evaluaron el tiempo de ausentismo laboral y estimaron que la vacunación ahorra un promedio de cerca de 0,04 días laborales. Este resultado se vio afectado por altos niveles de heterogeneidad y cambios de acuerdo a si se utilizó un modelo de efectos fijos (DM ‐0,04; IC del 95%: ‐0,06 a ‐0,01) o de efectos aleatorios (DM ‐0,04; IC del 95%: ‐0,14 a 0,06).
Las vacunas con virus vivos en aerosol tienen una efectividad general contra la ESG del 10% (IC del 95%: 4% a 16%) y un NNV de 46. El contenido y la coincidencia no parecen afectar el rendimiento de manera significativa. La eficacia global contra la gripe es del 53% (IC del 95%: 38% a 65%) y el NNV es de 39. Igualmente, ni el contenido ni la compatibilidad parecen afectar su rendimiento de forma significativa. Muchos más receptores a los que se les administró la vacuna presentaron síntomas locales en comparación con los que recibieron placebo.
Un ECA evaluó la eficacia de la vacuna en aerosol con virus inactivado en la prevención de la gripe (Análisis 3.1.1) (aa Langley 2011). Los resultados no mostraron un efecto protector estadísticamente significativo (RR 0,38; IC del 95%: 0,14 a 1,02).
Un ECA investigó los efectos de la administración de la vacuna contra la gripe en embarazadas y sus recién nacidos (paa Madhi 2014). Una vacuna inactivada trivalente que contenía el pH1N1 presentó una protección débil contra la gripe confirmada en ambas madres (RR 0,50; IC del 95%: 0,29 a 0,86; EV 50%, IC del 95%: 14% a 71%; NNV 55; IC del 95%: 39 a 198; Análisis 4.1.1) y en los niños (RR 0,51, IC del 95%: 0,30 a 0,88; EV 49%, IC del 95%: 12% a 70%; NNV 56; Análisis 4.3.1). La protección contra la ESG no fue estadísticamente significativa. El resto de la evidencia sobre la vacunación durante el embarazo se basó en estudios de observación (estudios de casos y controles y estudios de cohortes); la efectividad de la vacunación con la vacuna parenteral inactivada estacional durante el embarazo para prevenir las ESG en los recién nacidos no fue estadísticamente significativa. La evidencia proviene de dos estudios de cohortes que utilizaron estimaciones ajustadas del CRI o el RR. Los datos agrupados de tres estudios de cohortes (dos de ellos en riesgo alto de sesgo) muestran un efecto moderado de la vacunación contra la ESG en las embarazadas cuando se aplica el modelo de efectos fijos al análisis (NNV 92; IC del 95%: 63 a 201). Un estudio de cohortes mostró un efecto protector moderado contra la gripe en los recién nacidos de madres vacunadas (NNV 27; IC del 95%: 18 a 185).
El análisis agrupado de tres ECA y un ECC mostró que la inmunización con al menos una dosis de las vacunas monovalentes inactivadas con virus enteros antipandémicas de 1968 a 1969 logró una EV del 65% (IC del 95%: 52% a 75%) contra la ESG (NNV 16; IC del 95%: 14 a 20). Un ECA demostró que la eficacia de las vacunas monovalentes inactivadas con virus enteros antipandémicas para la prevención de la gripe de 1968 a 1969 fue del 93% (IC del 95%: 69% a 98%; NNV 35). Otro ECA mostró una eficacia del 65% (IC del 95%: 6% a 87%); NNV 94 (IC del 95%: 70 a 1022) en la prevención de la hospitalización. Un ECC aportó evidencia del efecto de la inmunización con las vacunas monovalentes inactivadas con virus enteros antipandémicas de 1968 a 1969 en los días de ausentismo laboral y los días de enfermedad: se ahorró aproximadamente medio día laborable y medio día de enfermedad (DM ‐0,45; IC del 95%: ‐0,60 a ‐0,30). El efecto de esta vacuna en la prevención de la neumonía no fue estadísticamente significativo (RR 0,59; IC del 95%: 0,05 a 6,51).
Sobre la base de la evidencia de estudios observacionales, la administración de la vacuna inactivada estacional o de la vacuna monovalente contra la pandemia de H1N1 durante el embarazo no se asocia con un mayor riesgo de aborto, malformación congénita, prematuridad o muerte neonatal, pero los IC son amplios.
No se encontró evidencia de una asociación entre las vacunas inactivadas estacionales y el síndrome de Guillain‐Barré ni de la vacuna pandémica H1N1 y el síndrome de Guillain‐Barré.
No hubo evidencia de una asociación entre la exposición a la vacuna inactivada estacional contra la gripe y otros eventos adversos graves (esclerosis múltiple, neuritis óptica y púrpura trombocitopénica inmune).
Compleción y aplicabilidad general de las pruebas
Se deben tener en cuenta varias cuestiones al interpretar los resultados de esta revisión.
-
Los métodos de estandarización de vacunas han cambiado significativamente.
-
Las vacunas recientes presentan diferencias significativas en cuanto a su pureza en comparación con las más antiguas.
-
Se agruparon diferentes dosis y esquemas en el análisis.
Esta revisión indica que según la evidencia aleatoria, las vacunas inactivadas tienen un efecto pequeño para prevenir los síntomas de la gripe y lograr que las personas retornen al trabajo más rápidamente. Si se observan los NNV para la gripe y las ESG para la vacuna parenteral inactivada, parece que la efectividad contra las ESG es mayor que la eficacia contra la gripe confirmada con pruebas de laboratorio (NNV‐ESG 29; NNV‐gripe 71). Estos resultados paradójicos muestran una efectividad no específica aparentemente mayor y una eficacia específica menor. Este hecho representa las diferentes tasas de ESG y de gripe confirmada en las poblaciones de estudio en los respectivos resultados. El porcentaje de participantes no vacunados que presentaron síntomas de ESG fue del 21,5%, mientras que el 2,3% de los participantes en los brazos no vacunados de los ensayos presentaron gripe confirmada con pruebas de laboratorio.
Calidad de la evidencia
Se calificó la calidad de la evidencia de la enfermedad similar a la gripe y de la gripe como moderada; la hospitalización, el tiempo de ausencia del trabajo y el aumento del riesgo de náuseas o vómitos como bajos; y la fiebre como alta (Resumen de resultados, tabla 1). El impacto del sesgo varió a través de los resultados, lo que dio lugar a la disminución de la calidad de la evidencia para la hospitalización, el tiempo fuera del trabajo y las náuseas. En cuanto a otros resultados, los análisis se basaron más en los estudios en riesgo bajo de sesgo o en los que el impacto probable del sesgo en los estudios era pequeño. La variación en la definición de la ESG dio lugar a que se presentara el riesgo de forma estratificada y a disminuir la calidad debido a la inconsistencia a causa de cierta discordancia en la dirección del efecto y a la heterogeneidad estadística alta. La decisión de disminuir la calidad de la evidencia de la gripe debido a su carácter indirecto representa la falta de certeza en cuanto a los métodos para determinar el resultado en estudios más antiguos y el impacto que tiene en la aplicabilidad de la evidencia a los entornos actuales. Los datos de la hospitalización estuvieron dominados por el estudio de aa Leibovitz de 1971 en el Análisis 1.8. Aunque la dirección general del efecto indicó una pequeña reducción en términos absolutos con la vacuna, no es posible descartar que no haya ningún efecto de la intervención. El IC del efecto sobre las náuseas/vómitos fue amplio, aunque este hecho puede representar la incorporación de variación en los resultados del estudio, en lugar de un poder estadístico bajo.
Sesgos potenciales en el proceso de revisión
Las conclusiones de esta revisión son inciertas con respecto al perfil de seguridad de las vacunas inactivadas, lo cual representa el tamaño de la base de la evidencia.
Una revisión anterior de 274 estudios de vacunas contra la gripe en todos los grupos etarios (que incluyó la mayoría de los estudios de esta revisión) mostró una relación inversa entre el riesgo de sesgo y la dirección de las conclusiones de los estudios. Las conclusiones favorables al uso de vacunas contra la gripe se asociaron con un mayor riesgo de sesgo. Los autores de los estudios de esta revisión hicieron afirmaciones y establecieron conclusiones que no estaban respaldadas por los datos que presentaban. Además, los estudios financiados por la industria tienen mayores probabilidades de establecer conclusiones favorables, de ser publicados en revistas con un factor de impacto significativamente mayor y de tener tasas mayores de citas que los estudios no financiados por la industria. Esta diferencia no se explica al tener en cuenta el tamaño ni la calidad metodológica (Jefferson 2009b). Cualquier interpretación del conjunto de la evidencia de esta revisión debe hacerse teniendo en cuenta estos resultados.
Se debe tener más cuidado al interpretar los resultados de los estudios de observación en el embarazo, ya que la posible presencia y los efectos del sesgo de tiempo inmortal no se analizaron en las versiones anteriores de esta revisión. El sesgo de tiempo inmortal se produce cuando una exposición dependiente del tiempo (en este caso la vacunación) no se incluye de forma adecuada en el análisis de un resultado de supervivencia. El término «sesgo de tiempo inmortal» se utiliza debido a que en los estudios de observación los pacientes deben sobrevivir el tiempo suficiente para recibir tratamiento; por lo tanto, son inmortales por definición antes de la exposición. Este tipo de sesgo, a veces denominado sesgo dependiente del tiempo, no suele ser un problema en los estudios aleatorizados, ya que el tratamiento (incluido el placebo) se suele administrar al principio del estudio. Por el contrario, en los estudios de observación, la exposición a la vacuna con frecuencia ha tenido lugar antes de que se inicie el estudio, con la consiguiente clasificación errónea de la exposición. Un sesgo de este tipo puede afectar las conclusiones del estudio (Jones 2016).
Acuerdos y desacuerdos con otros estudios o revisiones
Revisiones sistemáticas que realizan una estimación de la eficacia de la vacunación contra la gripe
DiazGranados 2012 realizó un metanálisis que incluyó ECA sobre las vacunas inactivadas estacionales o las vacunas de virus vivos atenuados contra la gripe, y presentó la gripe (con confirmación de la infección por la reacción en cadena de la polimerasa [RCP] o serológica) como el resultado de eficacia. El metanálisis incluyó 30 estudios en niños y adultos. Los autores proporcionaron estimaciones de la eficacia (RR con IC del 95%) estratificadas por el grado de compatibilidad entre la vacuna y las cepas circulantes (adecuada, deficiente, ninguna compatibilidad, compatibilidad) y por tipo de cepa (A H1N1; A H3N2; B). DiazGranados 2012 calculó que en una población adulta, la eficacia de la vacuna inactivada contra la gripe confirmada por laboratorio es del 59% (IC del 95%: 50% a 66%). La estimación de la eficacia de la vacuna con virus vivos atenuados es del 39% (IC del 95%: 16% a 55%).
La revisión sistemática Osterholm 2012 incluye evidencia de la eficacia de las vacunas de virus vivos atenuados y las vacunas inactivadas para prevenir la infección por gripe confirmada por laboratorio, evaluada exclusivamente por la RCP o un cultivo positivo. Al considerar los estudios realizados en adultos solamente, la estimación agrupada de la eficacia de seis estudios (ocho grupos de datos) fue del 59% (IC del 95%: 51% a 67%). Aunque se incluyeron tres ECA que calcularon la eficacia de las vacunas con virus vivos atenuados, los autores no realizaron un análisis debido a que ninguna de las estimaciones únicas fue estadísticamente significativa. También se incluyeron y analizaron los estudios observacionales.
Revisiones sistemáticas que evaluaron la eficacia / efectividad o aspectos de seguridad de las vacunas contra la gripe cuando se administraron durante el embarazo
La revisión Skowronski 2009 es la primera publicación exhaustiva que ha analizado con rigor la evidencia de la efectividad y los aspectos de seguridad de la vacunación durante el embarazo. En la primera parte del artículo, los autores consideran la carga de morbilidad durante el embarazo, el riesgo de muerte y el riesgo relacionado con la gripe para el feto, y resumen cómo han cambiado las recomendaciones del Advisory Committee on Immunization Practice (ACIP) de los EE.UU. durante las últimas cuatro décadas. La evidencia disponible sobre la protección (de la madre y el recién nacido) y los aspectos de seguridad de la vacunación se ilustran de forma descriptiva, se analizan y se comparan con las declaraciones de las políticas actuales de vacunación informadas. En la opinión de los autores, la inmunización contra la gripe en cualquier estadio del embarazo puede autorizarse durante las pandemias o en las pacientes con comorbilidades. La inmunización estacional con la vacuna inactivada trivalente puede estar justificada en el embarazo, sin complicaciones potenciales durante la segunda mitad del mismo. Finalmente, la evidencia disponible no es suficiente para recomendar la vacunación sistemática estándar en las primeras etapas del embarazo.
Revisiones sistemáticas de la evidencia de efectos perjudiciales
Farez 2011 evaluó el riesgo de desarrollar esclerosis múltiple o de presentar esclerosis múltiple recurrente después de la inmunización con algunas vacunaciones, incluida la de la gripe. El metanálisis realizado mediante el agrupamiento de los resultados de cuatro estudios de casos y controles excluiría un mayor riesgo de desarrollar esclerosis múltiple tras la administración de la vacuna contra la gripe (OR 0,97, IC del 95%: 0,77 a 1,23) (bb DeStefano 2003; bb Hernan 2004; bb Payne 2006; bb Zorzon 2003).
Otros problemas
En Toback 2012, hay evidencia que apoya la introducción de una nueva vacuna cuadrivalente de virus vivos atenuados (Q‐LAIV, ya autorizada en los EE.UU. donde estará disponible para la temporada 2013 a 2014) que contiene dos cepas B diferentes de diferente linaje (B/Yamagata/16/88 y B/Victoria/2/87). Esta evidencia proviene de dos ECA que compararon la inmunogenicidad y las reacciones locales y sistémicas después de la administración de las vacunas Q‐LAIV, trivalente inactivada o trivalente de virus vivos atenuados. Un ECA se realizó en adultos, el otro en una población pediátrica. La presencia de dos cepas B no afectaría significativamente la respuesta de los anticuerpos contra cada cepa B. Los eventos adversos locales y sistémicos inducidos por la administración de Q‐LAIV no difirieron de forma significativa de los registrados después de recibir otras vacunas ya en uso.
En resumen, las conclusiones de las revisiones citadas son ampliamente comparables con las de esta revisión, pero los resultados se comunican con estimaciones basadas en los efectos relativos. Además, ninguna de las revisiones ha identificado los efectos de las vacunas en resultados importantes como las complicaciones, las hospitalizaciones y las muertes. Estos hallazgos también son similares a los de esta revisión.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
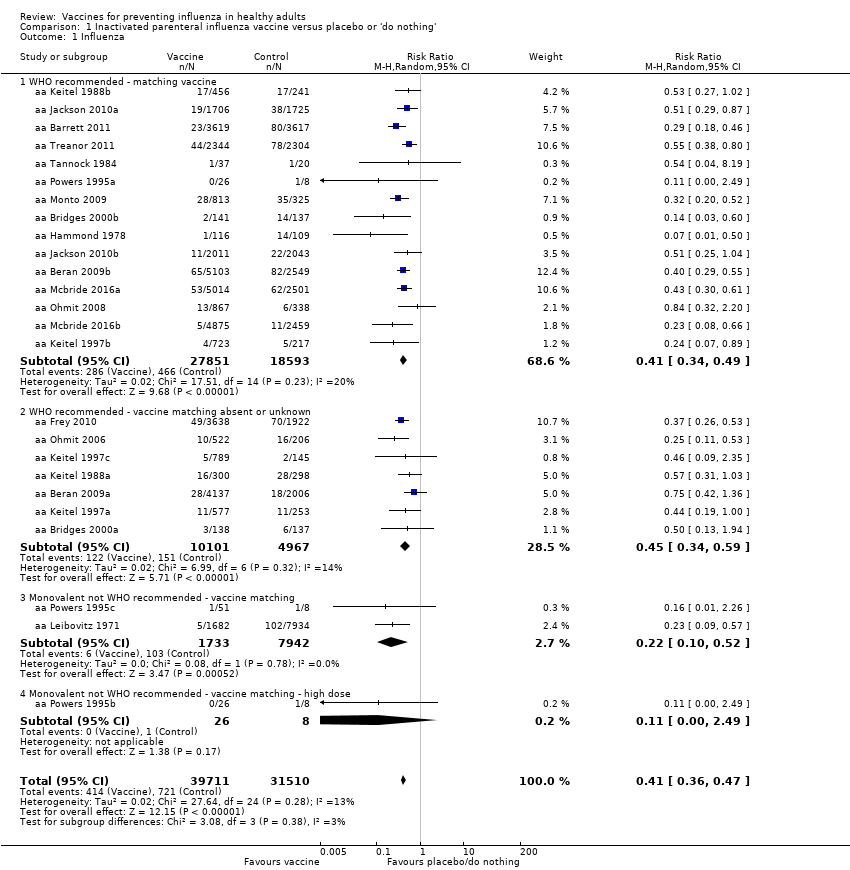
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 1 Influenza.

Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 2 Influenza‐like illness.
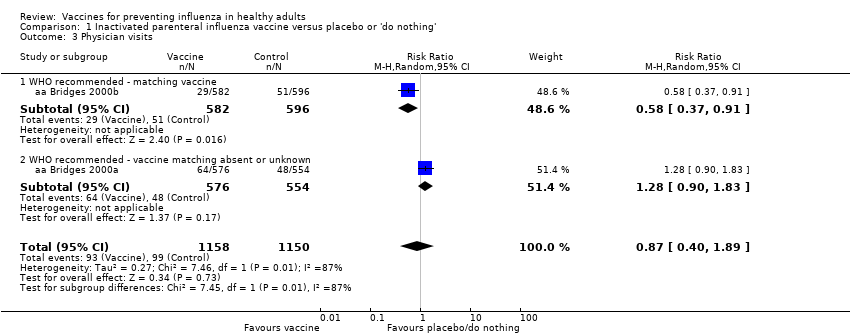
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 3 Physician visits.
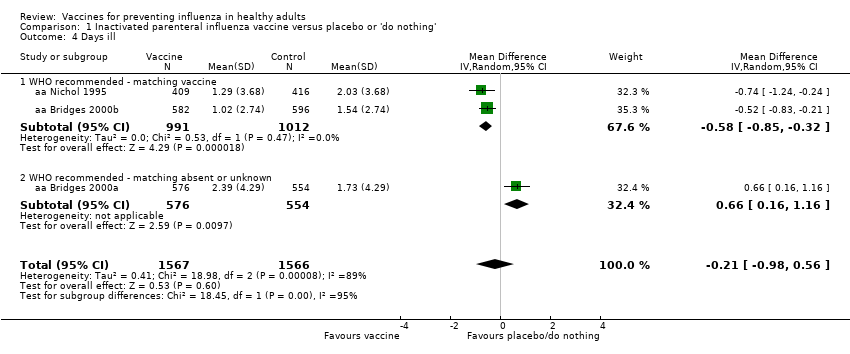
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 4 Days ill.

Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 5 Times any drugs were prescribed.
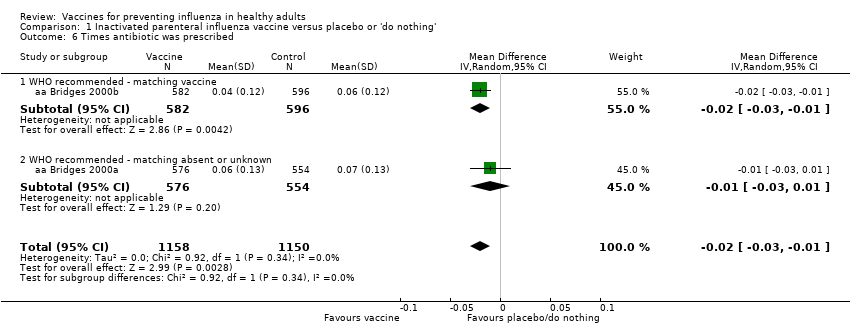
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 6 Times antibiotic was prescribed.
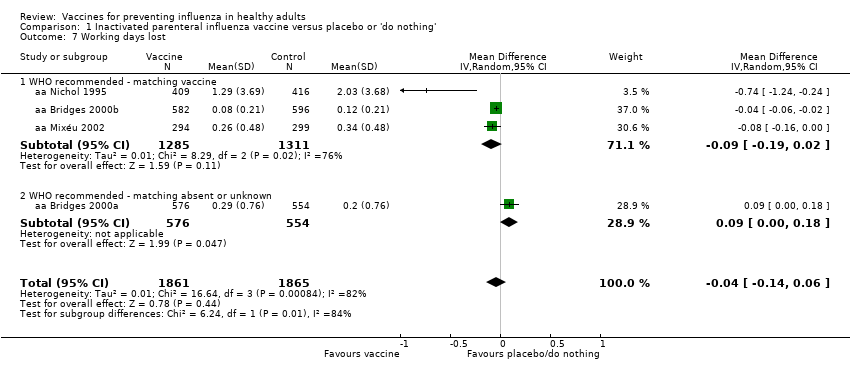
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 7 Working days lost.

Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 8 Hospitalisations.

Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 9 Clinical cases (clinically defined without clear definition).
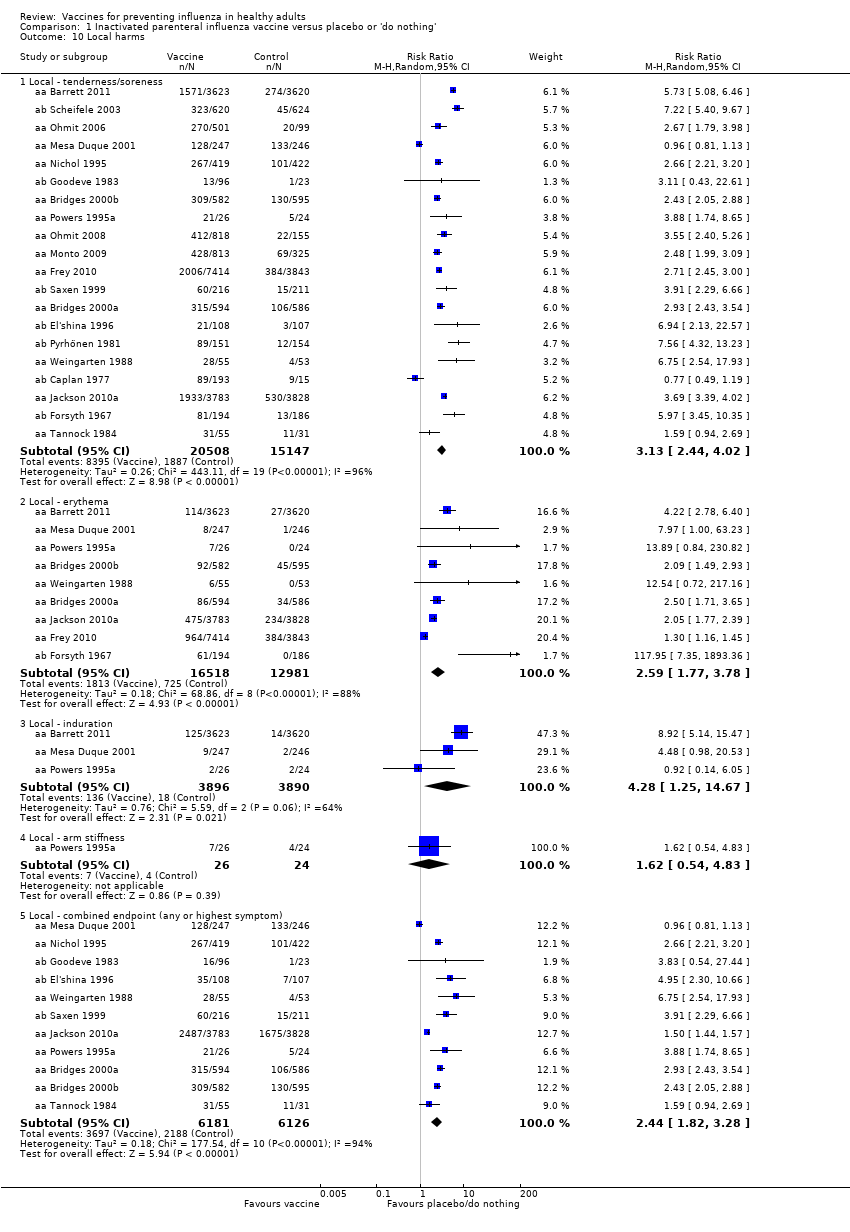
Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 10 Local harms.

Comparison 1 Inactivated parenteral influenza vaccine versus placebo or 'do nothing', Outcome 11 Systemic harms.

Comparison 2 Live aerosol influenza vaccine versus placebo or 'do nothing', Outcome 1 Influenza.
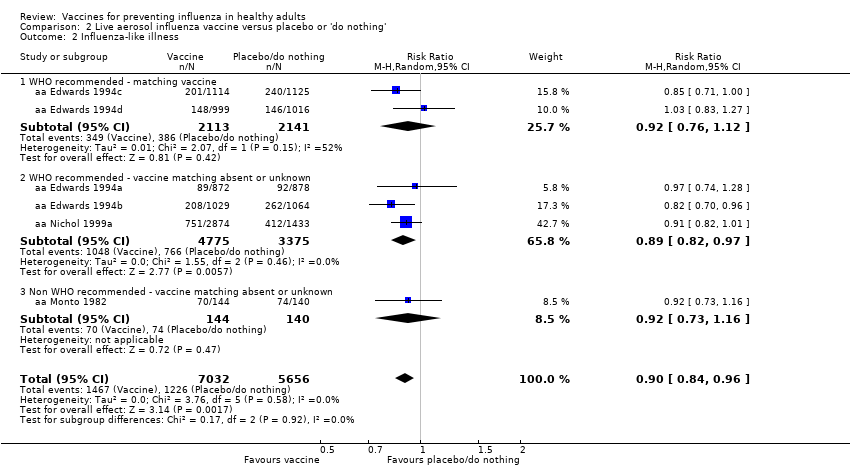
Comparison 2 Live aerosol influenza vaccine versus placebo or 'do nothing', Outcome 2 Influenza‐like illness.
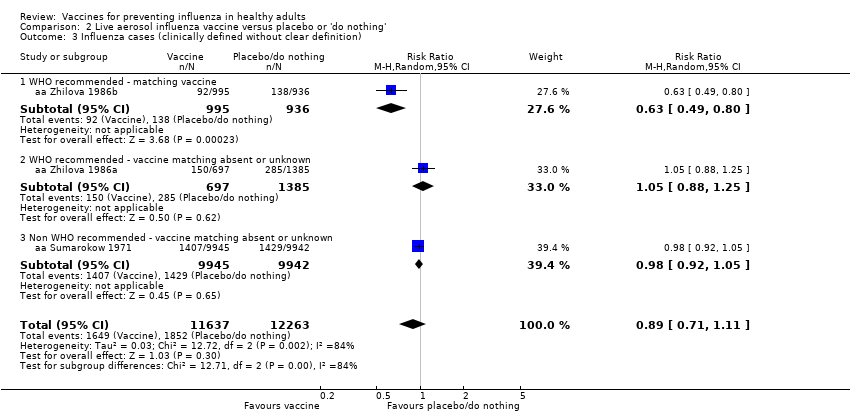
Comparison 2 Live aerosol influenza vaccine versus placebo or 'do nothing', Outcome 3 Influenza cases (clinically defined without clear definition).

Comparison 2 Live aerosol influenza vaccine versus placebo or 'do nothing', Outcome 4 Local harms.
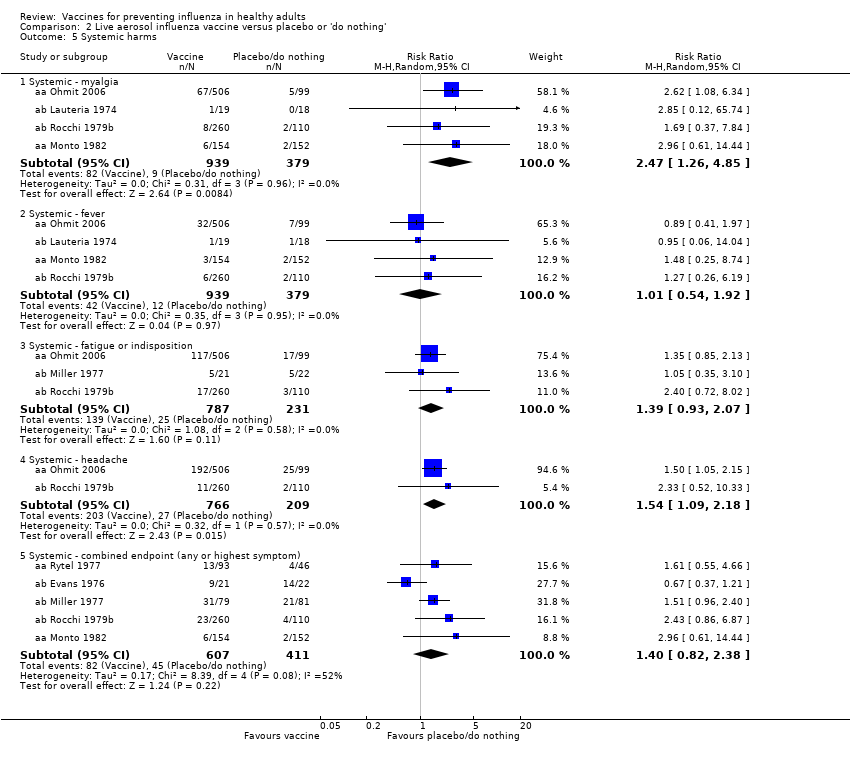
Comparison 2 Live aerosol influenza vaccine versus placebo or 'do nothing', Outcome 5 Systemic harms.

Comparison 3 Inactivated aerosol influenza vaccine versus placebo or 'do nothing', Outcome 1 Influenza.
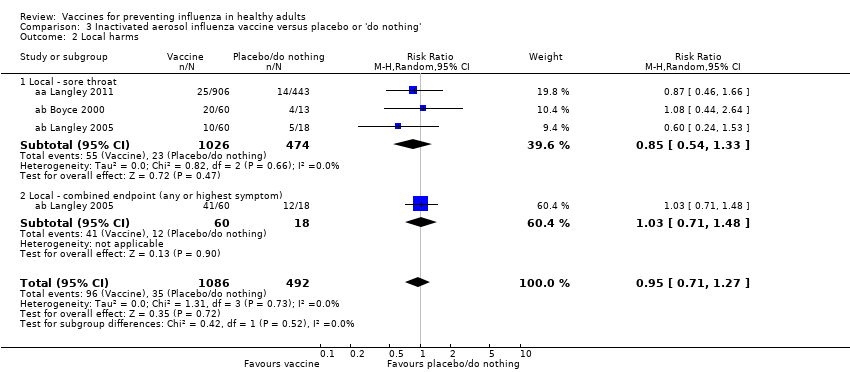
Comparison 3 Inactivated aerosol influenza vaccine versus placebo or 'do nothing', Outcome 2 Local harms.

Comparison 3 Inactivated aerosol influenza vaccine versus placebo or 'do nothing', Outcome 3 Systemic harms.
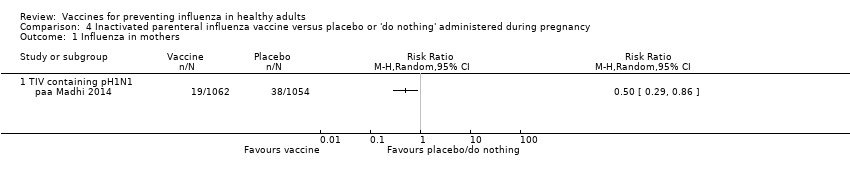
Comparison 4 Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy, Outcome 1 Influenza in mothers.

Comparison 4 Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy, Outcome 2 Influenza‐like illness in mothers.

Comparison 4 Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy, Outcome 3 Influenza in newborn.

Comparison 4 Inactivated parenteral influenza vaccine versus placebo or 'do nothing' administered during pregnancy, Outcome 4 Influenza‐like illness in newborn.

Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women.

Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.

Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.

Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.

Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.
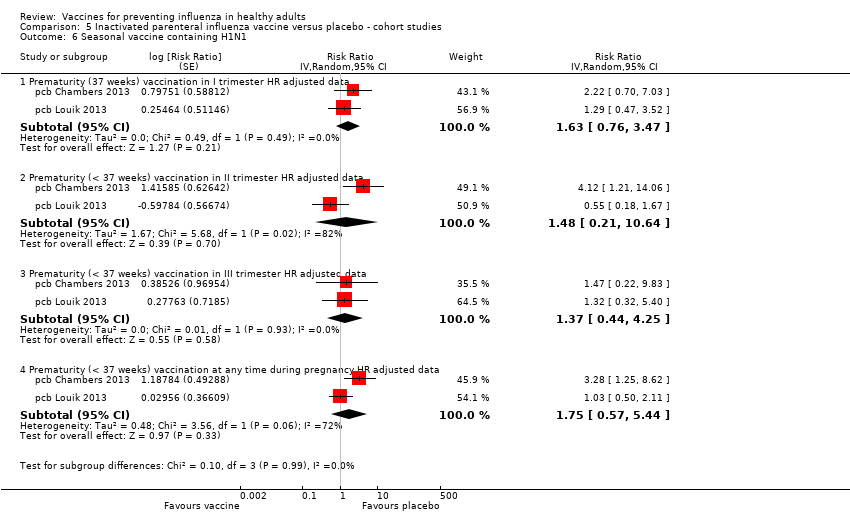
Comparison 5 Inactivated parenteral influenza vaccine versus placebo ‐ cohort studies, Outcome 6 Seasonal vaccine containing H1N1.

Comparison 6 Inactivated parenteral influenza vaccine versus placebo ‐ case‐control studies, Outcome 1 Effectiveness in newborns ‐ pregnant women (adjusted data).

Comparison 6 Inactivated parenteral influenza vaccine versus placebo ‐ case‐control studies, Outcome 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data).
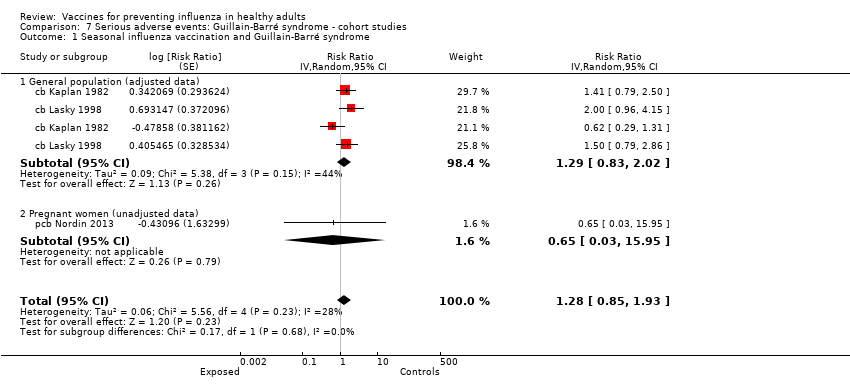
Comparison 7 Serious adverse events: Guillain‐Barré syndrome ‐ cohort studies, Outcome 1 Seasonal influenza vaccination and Guillain‐Barré syndrome.

Comparison 8 Serious adverse events: Guillain‐Barré syndrome ‐ case‐control studies, Outcome 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data).

Comparison 8 Serious adverse events: Guillain‐Barré syndrome ‐ case‐control studies, Outcome 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data).
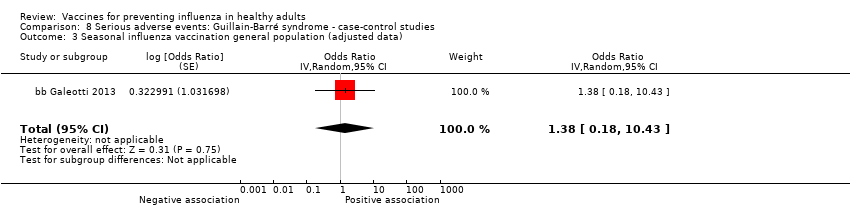
Comparison 8 Serious adverse events: Guillain‐Barré syndrome ‐ case‐control studies, Outcome 3 Seasonal influenza vaccination general population (adjusted data).
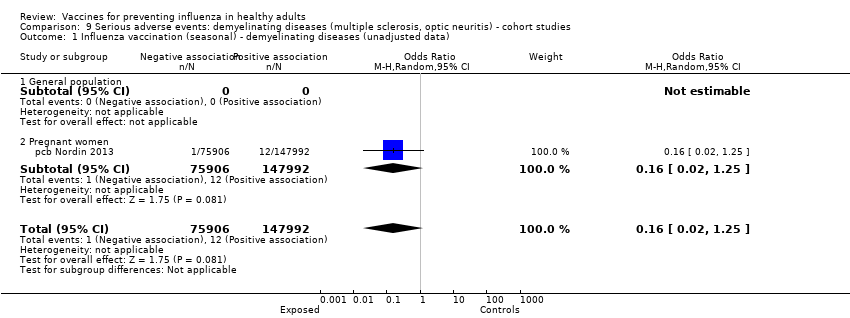
Comparison 9 Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data).

Comparison 9 Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted).
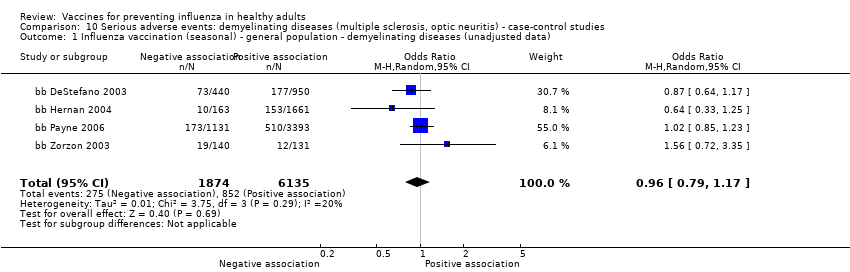
Comparison 10 Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data).

Comparison 10 Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data).
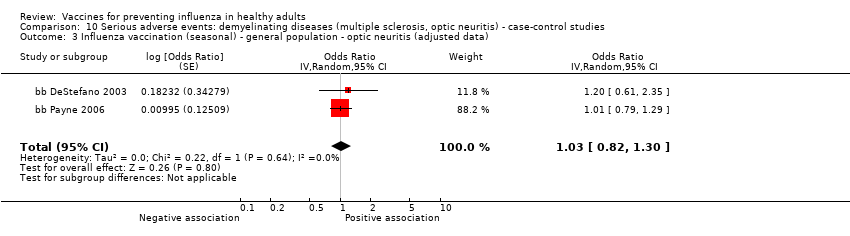
Comparison 10 Serious adverse events: demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data).

Comparison 11 Serious adverse events: immune thrombocytopenic purpura ‐ cohort studies, Outcome 1 Seasonal influenza vaccine ‐ HR (adjusted data).

Comparison 11 Serious adverse events: immune thrombocytopenic purpura ‐ cohort studies, Outcome 2 Seasonal influenza vaccine (unadjusted data).
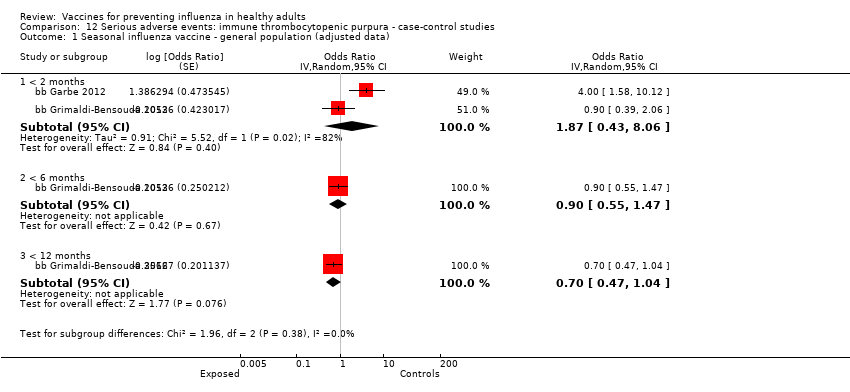
Comparison 12 Serious adverse events: immune thrombocytopenic purpura ‐ case‐control studies, Outcome 1 Seasonal influenza vaccine ‐ general population (adjusted data).
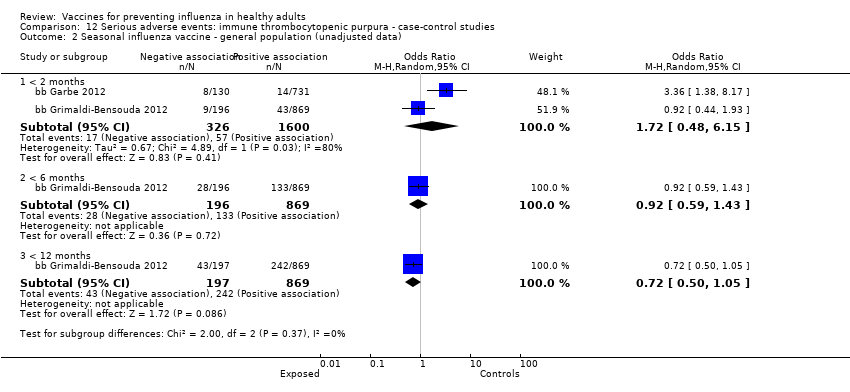
Comparison 12 Serious adverse events: immune thrombocytopenic purpura ‐ case‐control studies, Outcome 2 Seasonal influenza vaccine ‐ general population (unadjusted data).
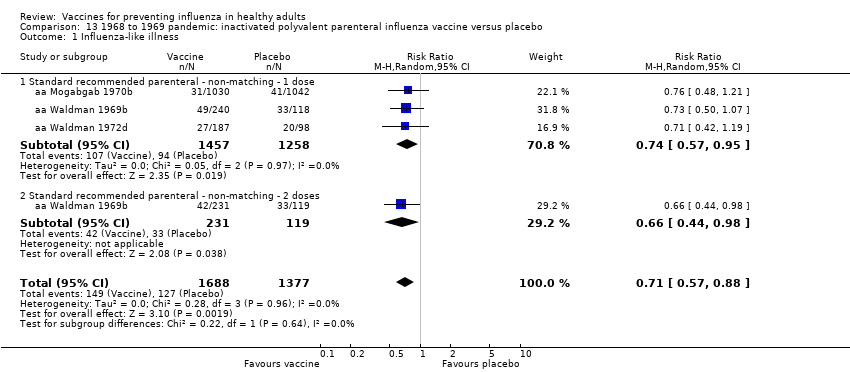
Comparison 13 1968 to 1969 pandemic: inactivated polyvalent parenteral influenza vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 13 1968 to 1969 pandemic: inactivated polyvalent parenteral influenza vaccine versus placebo, Outcome 2 Influenza.
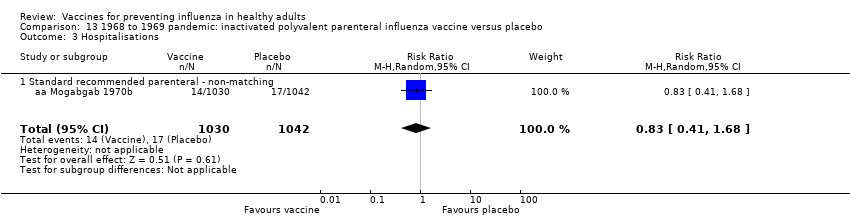
Comparison 13 1968 to 1969 pandemic: inactivated polyvalent parenteral influenza vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 13 1968 to 1969 pandemic: inactivated polyvalent parenteral influenza vaccine versus placebo, Outcome 4 Pneumonia.
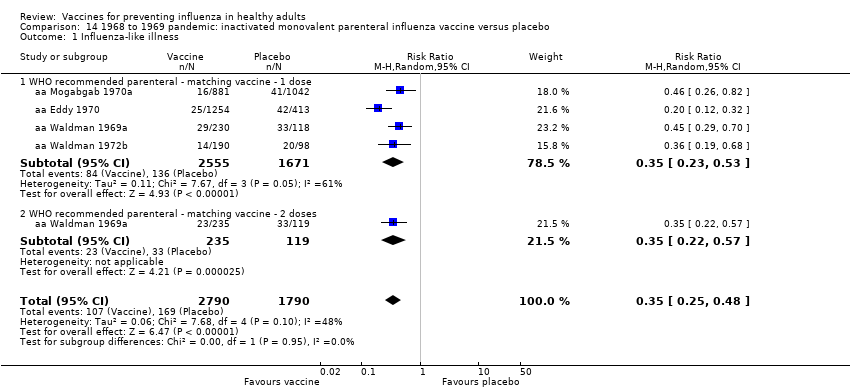
Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 1 Influenza‐like illness.
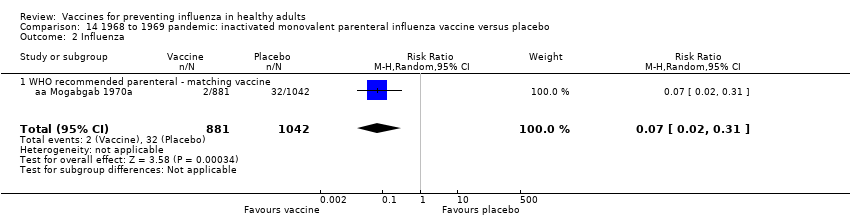
Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 2 Influenza.

Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 4 Pneumonia.

Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 5 Working days lost.
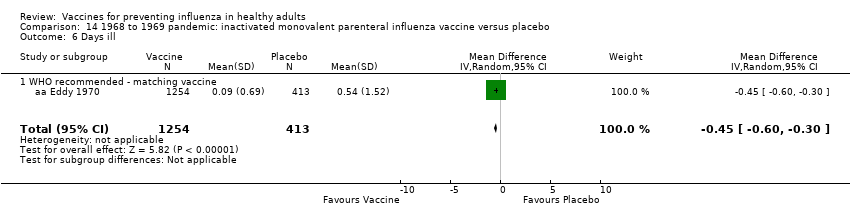
Comparison 14 1968 to 1969 pandemic: inactivated monovalent parenteral influenza vaccine versus placebo, Outcome 6 Days ill.

Comparison 15 1968 to 1969 pandemic: inactivated polyvalent aerosol influenza vaccine versus placebo, Outcome 1 Influenza‐like illness.
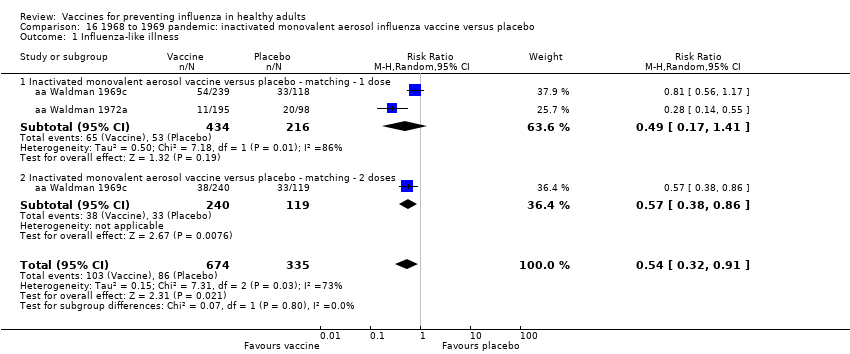
Comparison 16 1968 to 1969 pandemic: inactivated monovalent aerosol influenza vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 17 1968 to 1969 pandemic: live aerosol influenza vaccine versus placebo, Outcome 1 Influenza cases (clinically defined without clear definition).

Comparison 17 1968 to 1969 pandemic: live aerosol influenza vaccine versus placebo, Outcome 2 Complications (bronchitis, otitis, pneumonia).
| Inactivated parenteral influenza vaccine compared to placebo or 'do nothing' for preventing influenza in healthy adults | ||||||
| Patient or population: healthy adults | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or 'do nothing' | Risk with inactivated parenteral influenza vaccine | |||||
| Influenza Timing of assessment: most studies tested vaccines over a single influenza season | Study population1 | RR 0.41 | 71,221 | ⊕⊕⊕⊝ | ||
| 23 per 1000 | 9 per 1000 | |||||
| Influenza‐like illness Timing of assessment: most studies tested vaccines over a single influenza season | Low1 | RR 0.84 | 25,795 | ⊕⊕⊕⊝ | ||
| 40 per 1000 | 34 per 1000 | |||||
| Moderate | ||||||
| 215 per 1000 | 181 per 1000 | |||||
| High | ||||||
| 910 per 1000 | 764 per 1000 | |||||
| Hospitalisations Timing of assessment: single influenza season | Study population1 | RR 0.96 | 11,924 | ⊕⊕⊝⊝ | ||
| 147 per 1000 | 141 per 1000 | |||||
| Time off work Timing of assessment: single influenza season | Study population1 | NA | 3726 (4 RCTs) | ⊕⊕⊝⊝ | ||
| Average number of days lost per person ranged from 0.2 to 2 days over the season. | Average reduction in working days lost following vaccination was 0.04 days fewer (0.14 fewer to 0.06 days more) | |||||
| Fever assessed by subjective report Timing of assessment: single influenza season | Study population1 | RR 1.55 | 23,850 | ⊕⊕⊕⊕ | ||
| 15 per 1000 | 23 per 1000 | |||||
| Nausea or vomiting Timing of assessment: single influenza season | Study population1 | RR 1.80 | 6315 | ⊕⊕⊝⊝ | ||
| 37 per 1000 | 66 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk calculated as the sum of events over total sample size from the control groups. For the outcome of influenza‐like illness, control group risk was stratified as low, moderate (or median), and high due to variation in risk groups across the studies. For the remaining outcomes, the control group risk was taken as aggregate. | ||||||
| Review version (searches date) | Number of included trials (RCTs/CCTs) | Number of included observational studies | Estimates of effect (RCTs/CCTs only) | Conclusions (1‐2 lines from abstract) |
| Version 1 (6 July 1999) | 20 | 0 | Clinical influenza TIV = 24% (95% CI 15% to 32%) LAIV = 13% (95% CI 5% to 20%) IAV = 40% (95% CI 13% to 59%) Laboratory‐confirmed influenza TIV = 68% (95% CI 49% to 79%) LAIV = 48% (95% CI 24% to 64%) IAV = no evidence | Influenza vaccines are effective in reducing serologically confirmed cases of influenza A. However, they are not as effective in reducing cases of clinical influenza. The use of WHO recommended vaccines appears to enhance their effectiveness in practice. |
| Version 2 (24 May 2004) | 25 | 0 | Clinical influenza TIV = 25% (95% CI 13% to 35%) LAIV = 15% (95% CI 8% to 21%) IAV = 40% (95% CI 13% to 59%) Laboratory‐confirmed influenza TIV = 70% (95% CI 56% to 80%) LAIV = 48% (95% CI 24% to 64%) IAV = no evidence | Influenza vaccines are effective in reducing serologically confirmed cases of influenza. However, they are not as effective in reducing cases of clinical influenza and number of working days lost. Universal immunisation of healthy adults is not supported by the results of this review. |
| Version 3 (16 February 2007) | 38 | 10 (for harms only) | ILI TIV = 30% (95% CI 17% to 41%) LAIV = n.s. IAV = n.s. Influenza TIV = 80% (95% CI 56% to 81%) LAIV = 56% (95% CI 19% to 76%) IAV = no evidence | Influenza vaccines are effective in reducing cases of influenza, especially when the content accurately predicts circulating types and circulation is high. However, they are less effective in reducing cases of influenza‐like illness and have a modest impact on working days lost. There is insufficient evidence to assess their impact on complications. Whole‐virion monovalent vaccines may perform best in a pandemic. |
| Version 4 (15 June 2010) | 40 | 10 (for harms only) | ILI TIV = 30% (95% CI 17% to 41%) LAIV = n.s. IAV = n.s. Influenza TIV = 73% (95% CI 54% to 84%) LAIV = 56% (95% CI 19% to 76%) IAV = no evidence | Influenza vaccines have a modest effect in reducing influenza symptoms and working days lost. There is no evidence that they affect complications, such as pneumonia, or transmission. |
| Version 5 (4 March 2014) | 48 | 42 | ILI TIV = 17% (95% CI 11% to 23%) LAIV = n.s. IAV = n.s. Influenza TIV = 63% (95% CI 55% to 69%) LAIV = 45% (95% CI 18% to 63%) IAV = n.s. | Influenza vaccines have a very modest effect in reducing influenza symptoms and working days lost in the general population, including pregnant women. No evidence of association between influenza vaccination and serious adverse events was found in the comparative studies considered in the review. |
| CCT: controlled clinical trial Effect estimates are from Comparison 02 (At least one vaccine recommended for that year versus placebo or other vaccine). A clinically defined case was assumed as any case definition based on symptoms without further specification. A clinically defined case (specific definition) was defined as:
When more than one definition was given for the same trial, data related to the more specific definition were included. In Analysis 2.1 from versions 1 and 2, studies with both definitions are included. Evidence about effectiveness of aerosol inactivated vaccine comes only from studies carried out during the 1968‐69 pandemic. From version 3 onwards, specific comparisons have been added. Versions 3, 4, 5 Recommended vaccine matching circulating strains. Version 5 Out of the 42 included observational studies, 8 assessed efficacy or effectiveness of vaccine, or both, when administered during pregnancy (6 cohort and 2 case‐control studies). Version 6 (current) In two new RCTs included in this version, vaccination was performed during pregnancy. Regarding efficacy/effectiveness of TIV administered in general population, estimates assessed by applying random‐effects model were 16% (95% CI 9% to 23%) against ILI and 62% (95% CI 52% to 69%) against influenza, respectively. In a previous interim unpublished update before the decision to stabilise the review was made, a further 16 observational studies were included: 3 case‐control and 2 cohort studies assessing the safety of influenza vaccine administration in general population, 10 cohort studies assessing the safety of influenza vaccine administration during pregnancy, and one cohort study assessing efficacy/effectiveness of the vaccine administration during pregnancy. In this 2016 updated review, we included a total of 160 studies (137 data sets), while we no longer updated searches for observational comparative studies. | ||||
| Study design | High risk | Low risk | Unclear risk | Total |
| Case‐control | 3 | 2 | 18 | 23 |
| Cohort | 14 | 8 | 18 | 40 |
| RCT/CCT | 7 | 12 | 55 | 74 |
| Total | 24 | 22 | 91 | 137 |
| CCT: controlled clinical trial This table displays the overall methodological quality assessment of the included studies described in the text and represented in extended form (with all items of the tools) in Figure 1. | ||||
| Study design | Government, institutional, or public | Industry | Mixed | Total |
| Case‐control | 14 | 2 | 2 | 18 |
| Cohort | 33 | 5 | 2 | 40 |
| RCT/CCT | 32 | 15 | 5 | 52 |
| Total | 79 | 22 | 9 | 110 |
| CCT: controlled clinical trial | ||||
| Outcome (analysis) | All studies (primary analysis) | Studies at low risk of bias (sensitivity analysis) |
| Influenza (Analysis 1.1) | RR 0.41 (0.36 to 0.47) | RR 0.34 (0.25 to 0.45) |
| Influenza‐like illness (Analysis 1.2) | RR 0.84 (0.75 to 0.95) | RR 0.82 (0.69 to 0.98) |
| Hospitalisations (Analysis 1.8) | RR 0.96 (0.85 to 1.08) | RR 2.89 (0.12 to 70.68) |
| Fever (Analysis 1.11.2) | RR 1.55 (1.26 to 1.91) | RR 1.59 (1 to 2.53) |
| Nausea/vomiting (Analysis 1.11.5) | RR 1.80 (0.65 to 5.04) | RR 7.05 (1.61 to 30.87) |
| RR: risk ratio | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 25 | 71221 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.36, 0.47] |
| 1.1 WHO recommended ‐ matching vaccine | 15 | 46444 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.34, 0.49] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 15068 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.34, 0.59] |
| 1.3 Monovalent not WHO recommended ‐ vaccine matching | 2 | 9675 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.10, 0.52] |
| 1.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.00, 2.49] |
| 2 Influenza‐like illness Show forest plot | 16 | 25795 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.75, 0.95] |
| 2.1 WHO recommended ‐ matching vaccine | 7 | 4760 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.77, 0.91] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 20942 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.18] |
| 2.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.28, 3.70] |
| 2.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.09, 2.30] |
| 3 Physician visits Show forest plot | 2 | 2308 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.89] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.90, 1.83] |
| 4 Days ill Show forest plot | 3 | 3133 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.98, 0.56] |
| 4.1 WHO recommended ‐ matching vaccine | 2 | 2003 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.85, ‐0.32] |
| 4.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.16, 1.16] |
| 5 Times any drugs were prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 5.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 5.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 6 Times antibiotic was prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7 Working days lost Show forest plot | 4 | 3726 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 7.1 WHO recommended ‐ matching vaccine | 3 | 2596 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.02] |
| 7.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.18] |
| 8 Hospitalisations Show forest plot | 3 | 11924 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 8.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.68] |
| 8.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 9616 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 9 Clinical cases (clinically defined without clear definition) Show forest plot | 3 | 4259 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 9.1 WHO recommended ‐ matching vaccine | 2 | 2056 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 9.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 10 Local harms Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Local ‐ tenderness/soreness | 20 | 35655 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.44, 4.02] |
| 10.2 Local ‐ erythema | 9 | 29499 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.77, 3.78] |
| 10.3 Local ‐ induration | 3 | 7786 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.25, 14.67] |
| 10.4 Local ‐ arm stiffness | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.54, 4.83] |
| 10.5 Local ‐ combined endpoint (any or highest symptom) | 11 | 12307 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.28] |
| 11 Systemic harms Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Systemic ‐ myalgia | 11 | 35008 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.41, 2.14] |
| 11.2 Systemic ‐ fever | 13 | 23850 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.26, 1.91] |
| 11.3 Systemic ‐ headache | 14 | 35999 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.99, 1.30] |
| 11.4 Systemic ‐ fatigue or indisposition | 12 | 35788 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.05, 1.36] |
| 11.5 Systemic ‐ nausea/vomiting | 4 | 6315 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.65, 5.04] |
| 11.6 Systemic ‐ malaise | 3 | 26111 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.18, 1.92] |
| 11.7 Systemic ‐ combined endpoint (any or highest symptom) | 6 | 2128 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 9 | 11579 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.35, 0.62] |
| 1.1 WHO recommended ‐ matching vaccine | 4 | 6584 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 4568 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| 1.3 Non WHO recommended ‐ vaccine matching absent or unknown | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 2 Influenza‐like illness Show forest plot | 6 | 12688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 2.1 WHO recommended ‐ matching vaccine | 2 | 4254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.12] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 2.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 3 Influenza cases (clinically defined without clear definition) Show forest plot | 3 | 23900 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.11] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2082 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 3.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4 Local harms Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Local ‐ upper respiratory infection symptoms | 6 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.22, 2.27] |
| 4.2 Local ‐ cough | 6 | 2401 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.08, 2.10] |
| 4.3 Local ‐ coryza | 2 | 4782 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.94] |
| 4.4 Local ‐ sore throat | 7 | 6940 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.49, 1.86] |
| 4.5 Local ‐ hoarseness | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.51, 2.83] |
| 4.6 Local ‐ combined endpoint (any or highest symptom) | 3 | 4921 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.31, 1.87] |
| 5 Systemic harms Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Systemic ‐ myalgia | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.26, 4.85] |
| 5.2 Systemic ‐ fever | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.92] |
| 5.3 Systemic ‐ fatigue or indisposition | 3 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.93, 2.07] |
| 5.4 Systemic ‐ headache | 2 | 975 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.09, 2.18] |
| 5.5 Systemic ‐ combined endpoint (any or highest symptom) | 5 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.82, 2.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.1 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.2 WHO recommended ‐ matching vaccine | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Local harms Show forest plot | 3 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.27] |
| 2.1 Local ‐ sore throat | 3 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 2.2 Local ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.48] |
| 3 Systemic harms Show forest plot | 3 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.62] |
| 3.1 Systemic ‐ myalgia | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.25] |
| 3.2 Systemic ‐ fatigue or indisposition | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.52, 3.75] |
| 3.3 Systemic ‐ headache | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.85, 2.72] |
| 3.4 Systemic ‐ fever | 1 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.80] |
| 3.5 Systemic ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza in mothers Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 TIV containing pH1N1 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Influenza‐like illness in mothers Show forest plot | 2 | 2342 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.20, 1.95] |
| 2.1 TIV containing pH1N1 | 1 | 2116 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.16] |
| 2.2 Monovalent pH1N1 | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.08, 1.02] |
| 3 Influenza in newborn Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 TIV containing pH1N1 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Influenza‐like illness in newborn Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 TIV containing pH1N1 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 H1N1 ‐ vaccine ‐ effectiveness ILI (unadjusted data) | 1 | 7328 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.21] |
| 1.2 Seasonal ‐ vaccine ‐ effectiveness ILI ‐ (unadjusted data) | 3 | 50507 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.18] |
| 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Seasonal vaccine effectiveness ILI (HR adjusted data) | 2 | Hazard Ratio (Random, 95% CI) | 0.96 [0.90, 1.03] | |
| 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Seasonal vaccine effectiveness ILI (RR adjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.92 [0.73, 1.16] | |
| 3.2 Seasonal vaccine efficacy influenza ‐ laboratory‐confirmed | 1 | Risk Ratio (Random, 95% CI) | 0.59 [0.37, 0.94] | |
| 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 15 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Abortion (OR adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 0.75 [0.62, 0.90] | |
| 4.2 Abortion (HR adjusted data) | 3 | Odds Ratio (Random, 95% CI) | 0.81 [0.63, 1.04] | |
| 4.3 Congenital malformation (OR adjusted data) | 6 | Odds Ratio (Random, 95% CI) | 1.11 [0.99, 1.23] | |
| 4.4 Prematurity (< 37 weeks) (OR unadjusted data) | 11 | Odds Ratio (Random, 95% CI) | 0.76 [0.67, 0.85] | |
| 4.5 Prematurity (< 37 weeks) (OR adjusted data) | 7 | Odds Ratio (Random, 95% CI) | 0.84 [0.76, 0.93] | |
| 4.6 Prematurity (< 37 weeks) (HR adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 1.11 [0.46, 2.68] | |
| 4.7 Prematurity (< 37 weeks) vaccination in I trimester OR adjusted data | 2 | Odds Ratio (Random, 95% CI) | 1.08 [0.92, 1.28] | |
| 4.8 Prematurity (< 37 weeks) vaccination in II/III trimester OR adjusted data | 2 | Odds Ratio (Random, 95% CI) | 0.96 [0.87, 1.06] | |
| 4.9 Neonatal death (OR adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 1.09 [0.40, 2.95] | |
| 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 7 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Abortion (OR unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.60 [0.41, 0.86] | |
| 5.2 Congenital malformation (OR unadjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.55 [0.08, 3.73] | |
| 5.3 Prematurity (OR unadjusted data) | 6 | Odds Ratio (Random, 95% CI) | 0.95 [0.82, 1.10] | |
| 5.4 Prematurity (OR adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.93 [0.82, 1.06] | |
| 5.5 Neonatal death (OR unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.55 [0.35, 0.88] | |
| 6 Seasonal vaccine containing H1N1 Show forest plot | 2 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Prematurity (37 weeks) vaccination in I trimester HR adjusted data | 2 | Risk Ratio (Random, 95% CI) | 1.63 [0.76, 3.47] | |
| 6.2 Prematurity (< 37 weeks) vaccination in II trimester HR adjusted data | 2 | Risk Ratio (Random, 95% CI) | 1.48 [0.21, 10.64] | |
| 6.3 Prematurity (< 37 weeks) vaccination in III trimester HR adjusted data | 2 | Risk Ratio (Random, 95% CI) | 1.37 [0.44, 4.25] | |
| 6.4 Prematurity (< 37 weeks) vaccination at any time during pregnancy HR adjusted data | 2 | Risk Ratio (Random, 95% CI) | 1.75 [0.57, 5.44] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effectiveness in newborns ‐ pregnant women (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 1.1 Seasonal vaccine ‐ effectiveness ‐ ILI ‐ pregnant women | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| 2.1 Abortion | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccination and Guillain‐Barré syndrome Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 1.28 [0.85, 1.93] | |
| 1.1 General population (adjusted data) | 2 | Risk Ratio (Random, 95% CI) | 1.29 [0.83, 2.02] | |
| 1.2 Pregnant women (unadjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.65 [0.03, 15.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data) Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 7 weeks | 6 | 1528 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [1.14, 4.31] |
| 1.2 At any time | 6 | 1656 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.87, 3.29] |
| 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data) Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 0.83 [0.39, 1.75] | |
| 2.1 < 7 weeks | 4 | Odds Ratio (Random, 95% CI) | 0.92 [0.35, 2.40] | |
| 2.2 > 6 weeks (i.e. at any time) | 3 | Odds Ratio (Random, 95% CI) | 0.71 [0.22, 2.32] | |
| 3 Seasonal influenza vaccination general population (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.38 [0.18, 10.43] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data) Show forest plot | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 1.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted) Show forest plot | 1 | 144252 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.51, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data) Show forest plot | 4 | 8009 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.76 [0.54, 1.08] | |
| 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.03 [0.82, 1.30] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ HR (adjusted data) Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 General population | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pregnant women | 1 | Hazard Ratio (Random, 95% CI) | 0.90 [0.68, 1.19] | |
| 2 Seasonal influenza vaccine (unadjusted data) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ general population (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 < 2 months | 2 | Odds Ratio (Random, 95% CI) | 1.87 [0.43, 8.06] | |
| 1.2 < 6 months | 1 | Odds Ratio (Random, 95% CI) | 0.90 [0.55, 1.47] | |
| 1.3 < 12 months | 1 | Odds Ratio (Random, 95% CI) | 0.70 [0.47, 1.04] | |
| 2 Seasonal influenza vaccine ‐ general population (unadjusted data) Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2 months | 2 | 1926 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.48, 6.15] |
| 2.2 < 6 months | 1 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 2.3 < 12 months | 1 | 1066 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 3 | 3065 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.88] |
| 1.1 Standard recommended parenteral ‐ non‐matching ‐ 1 dose | 3 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.57, 0.95] |
| 1.2 Standard recommended parenteral ‐ non‐matching ‐ 2 doses | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.98] |
| 2 Influenza Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 2.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 3 Hospitalisations Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 3.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 4 Pneumonia Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| 4.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 4 | 4580 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| 1.1 WHO recommended parenteral ‐ matching vaccine ‐ 1 dose | 4 | 4226 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 1.2 WHO recommended parenteral ‐ matching vaccine ‐ 2 doses | 1 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.57] |
| 2 Influenza Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 2.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 3 Hospitalisations Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 3.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 4 Pneumonia Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 4.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 5 Working days lost Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 5.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6 Days ill Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 1.1 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 1 dose | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.27] |
| 1.2 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 2 doses | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| 1.1 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 1 dose | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.41] |
| 1.2 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 2 doses | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza cases (clinically defined without clear definition) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 1.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2 Complications (bronchitis, otitis, pneumonia) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| 2.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |

