Vacunas para la prevención de la gripe en adultos sanos
Resumen
Antecedentes
En la actualidad, se producen diferentes tipos de vacunas contra la gripe en todo el mundo. La vacunación de las mujeres embarazadas se recomienda internacionalmente, aunque en Norteamérica está indicada en los adultos sanos.
Objetivos
Identificar, recuperar y evaluar todos los estudios que evalúan los efectos (eficacia, efectividad y efectos perjudiciales) de las vacunas contra la gripe en adultos sanos, incluidas las mujeres embarazadas.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) (The Cochrane Library 2013, número 2), MEDLINE (enero 1966 hasta mayo 2013) y en EMBASE (1990 hasta mayo 2013).
Criterios de selección
Ensayos controlados aleatorios (ECA) o ensayos controlados cuasialeatorios que compararan las vacunas contra la gripe con placebo o ninguna intervención en individuos sanos de entre 16 a 65 años de edad con gripe adquirida de forma natural. También se incluyeron estudios comparativos que evaluaron daños graves y poco frecuentes.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos.
Resultados principales
Se incluyeron 90 informes que contenían 116 grupos de datos; entre éstos, 69 eran ensayos clínicos con más de 70 000 personas, 27 eran estudios comparativos de cohortes (cerca de ocho millones de personas) y 20 eran estudios de casos y controles (casi 25 000 personas). Se recuperaron 23 informes de la efectividad y la seguridad de la administración de las vacunas en las mujeres embarazadas (cerca de 1 600 000 parejas madre‐feto).
La efectividad general de la vacuna inactivada que se administra por vía parenteral contra las enfermedades tipo gripe es limitada, correspondiente a un número necesario a vacunar (NNV) de 40 (intervalo de confianza [IC] del 95%: 26 a 128). La eficacia general de las vacunas inactivadas para prevenir la gripe confirmada tiene un NNV de 71 (IC del 95%: 64 a 80). La diferencia entre estos dos valores depende de la incidencia diferente de las enfermedades tipo gripe y la gripe confirmada entre las poblaciones de estudio: el 15,6% de los participantes no vacunados versus el 9,9% de los participantes vacunados presentaron síntomas de enfermedades tipo gripe, aunque solamente el 2,4% y el 1,1%, respectivamente, desarrollaron gripe confirmada por laboratorio.
No se encontraron ECA que evaluaran la vacunación en mujeres embarazadas. Las únicas pruebas disponibles provienen de estudios observacionales con calidad metodológica modesta. Sobre esta base, la vacunación muestra efectos muy limitados: NNV 92 (IC del 95%: 63 a 201) contra las enfermedades tipo gripe en mujeres embarazadas y NNV 27 (IC del 95%: 18 a 185) contra la gripe confirmada por laboratorio en recién nacidos de pacientes vacunadas.
Las vacunas de virus vivos administradas en forma de aerosol tienen una efectividad general correspondiente a un NNV de 46 (IC del 95%: 29 a 115).
El rendimiento de las vacunas pandémicas de virus enteros de una dosis o dos dosis fue mayor y muestra un NNV de 16 (IC del 95%: 14 a 20) contra las enfermedades tipo gripe y un NNV de 35 (IC del 95%: 33 a 47) contra la gripe, aunque se observó una repercusión limitada sobre la hospitalización (NNV 94; IC del 95%: 70 a 1022).
La vacunación tuvo un efecto moderado en el tiempo de ausentismo laboral y no tuvo ningún efecto en los ingresos al hospital ni en las tasas de complicaciones. Las vacunas inactivadas ocasionaron daños locales. No se encontraron pruebas de asociación con eventos adversos graves, pero la base de pruebas sobre los efectos adversos fue limitada.
El riesgo general de sesgo en los ensayos incluidos no está claro, ya que no fue posible evaluar el impacto real del sesgo.
Conclusiones de los autores
Las vacunas contra la gripe tienen un efecto muy moderado en la reducción de los síntomas de gripe y los días laborales perdidos en la población general, incluidas las mujeres embarazadas. En los estudios comparativos considerados en la revisión, no se encontraron pruebas de asociación entre la vacunación contra la gripe y eventos adversos graves. Esta revisión incluye 90 estudios y 24 (26,7%) fueron patrocinados total o parcialmente por la industria. De los 48 ECA, 17 fueron patrocinados por la industria (35,4%).
PICOs
Resumen en términos sencillos
Vacunas para la prevención de la gripe en adultos sanos
Pregunta de la revisión
Se evaluó el efecto de la inmunización con vacunas contra la gripe sobre la prevención de las infecciones por gripe A o B (eficacia), las enfermedades tipo gripe y las consecuencias (efectividad) y se determinó si la exposición a las vacunas contra la gripe se asocia con efectos perjudiciales serios o graves. Las poblaciones destinatarias fueron adultos sanos, incluidas mujeres embarazadas y recién nacidos.
Antecedentes
Más de 200 virus causan gripe y enfermedades tipo gripe que producen los mismos síntomas (fiebre, cefalea, malestar, dolores, tos y rinorrea). Sin pruebas de laboratorio, los médicos no pueden distinguir entre ellos y ambos duran días y rara vez provocan la muerte o una enfermedad grave. En el mejor de los casos, las vacunas podrían ser efectivas sólo contra la gripe A y B, que representan cerca del 10% de todos los virus circulantes. Anualmente, la Organización Mundial de la Salud determina qué cepas virales se deben incluir en las vacunaciones de la próxima estación.
La vacuna inactivada es preparada al tratar los virus de la gripe con un agente químico específico que "mata" el virus. Las preparaciones finales pueden contener virus completos (vacuna entera) o la parte activa de ellos (vacunas fraccionadas o de subunidades). Estas clases de vacunas normalmente se administran de forma intramuscular (vía parenteral)
Las vacunas de virus vivos atenuados se preparan mediante el cultivo de los virus de la gripe a través de una serie de cultivos de células o embriones animales. Con cada proceso, los virus pierden la capacidad de reproducirse en las células humanas, pero todavía pueden estimular el sistema inmunológico. Las vacunas de virus vivos atenuados se administran en forma de aerosol en los orificios nasales (vía intranasal).
Habitualmente, las cepas de los virus contenidas en la vacuna son las que se espera que circulen en las siguientes temporadas epidémicas (dos cepas Tipo A y una B), según las recomendaciones de la Organización Mundial de la Salud (vacuna estacional).
La vacuna pandémica solamente contiene la cepa del virus que es responsable de la pandemia (es decir, el Tipo A H1N1 para la pandemia de 2009/2010).
Características de los estudios
Las pruebas están actualizadas hasta mayo de 2013. En esta actualización, 90 informes de 116 estudios compararon el efecto de la vacuna contra la gripe con placebo o ninguna intervención. Sesenta y nueve informes eran ensayos clínicos (más de 70 000 personas), 27 eran estudios comparativos de cohortes (cerca de ocho millones de personas) y 20 eran estudios de casos y controles (casi 25 000 personas). De los 116 estudios, 23 (tres estudios de casos y controles y 20 de cohortes) se realizaron durante el embarazo (cerca de 1 600 000 parejas madre‐feto).
Resultados clave
El efecto preventivo de la vacuna inactivada administrada por vía parenteral contra la gripe sobre los adultos sanos es pequeño: al menos 40 personas necesitarían vacunarse para evitar un caso de enfermedad tipo gripe (intervalo de confianza [IC] del 95%: 26 a 128) y 71 personas necesitarían vacunarse para prevenir un caso de gripe (IC del 95%: 64 a 80). La vacunación no muestra efectos considerables sobre los días laborales perdidos o la hospitalización.
La protección contra las enfermedades tipo gripe que proporciona la administración de la vacuna inactivada contra la gripe en las mujeres embarazadas es incierta o al menos muy limitada; el efecto sobre los recién nacidos no es estadísticamente significativo.
La efectividad de las vacunas de virus vivos administradas en forma de aerosol en adultos sanos es similar a la de las vacunas inactivadas: 46 personas (IC del 95%: 29 a 115) necesitarían recibir la inmunización para evitar un caso de enfermedad tipo gripe.
La administración de la vacuna inactivada estacional contra la gripe no se asocia con la aparición de esclerosis múltiple, neuritis óptica (inflamación del nervio óptico del ojo) o púrpura trombocitopénica inmune (una enfermedad que afecta las plaquetas de la sangre). La administración de la vacuna inactivada pandémica monovalente H1N1 no se asocia con el síndrome de Guillain‐Barré (una enfermedad que afecta los nervios de los miembros y el cuerpo).
Las pruebas indican que la administración de las vacunas estacional y pandémica del 2009 durante el embarazo no tiene efectos significativos sobre el aborto o la muerte neonatal.
Calidad de la evidencia
No fue posible determinar la repercusión real de los sesgos en cerca del 70% de los estudios incluidos (p.ej., detalles del informe insuficientes, puntuaciones muy diferentes entre los ítems evaluados). Cerca del 20% de los estudios incluidos (principalmente de cohortes) tenían un alto riesgo de sesgo. Menos del 10% tuvo buena calidad metodológica.
Conclusiones de los autores
Antecedentes
Descripción de la afección
Las enfermedades respiratorias virales imponen una gran carga a la sociedad. La mayor parte de las enfermedades respiratorias virales (enfermedades tipo gripe) son causadas por numerosos agentes diferentes, clínicamente indistinguibles unos de otros. Una proporción variable de enfermedades tipo gripe (7% al 15% como promedio) es causada por los virus de la gripe y se conoce como gripe (Jefferson 2009b).
La gripe es una infección respiratoria aguda causada por un virus de la familia Orthomyxoviridae. Se conocen tres serotipos (A, B y C). La gripe causa un cuadro febril agudo con mialgia, cefalea y tos. A pesar de que la duración promedio del cuadro agudo es de tres días, la tos y el malestar pueden persistir durante semanas. Las complicaciones de la gripe incluyen otitis media, neumonía, neumonía bacteriana secundaria, exacerbaciones de la enfermedad respiratoria crónica y bronquiolitis en los niños. Además, la gripe puede provocar diversas complicaciones no respiratorias, que incluyen convulsiones febriles, síndrome de Reye y miocarditis (Wiselka 1994). Los esfuerzos para prevenir o minimizar la repercusión de la gripe estacional en la segunda parte del siglo XX se han concentrado en el uso de las vacunas. Debido a los cambios anuales en la configuración antigénica viral y la falta de protección que se prolongue de año en año, es necesario organizar anualmente una nueva campaña de vacunación, con un esfuerzo científico y logístico enorme para asegurar la producción y la entrega de las vacunas.
Descripción de la intervención
En la actualidad existen tres tipos de vacunas contra la gripe:
-
vacunas inactivas de virus enteros, que consisten en virus completos a los que se ha "matado" o inactivado, de modo que no son infecciosos pero retienen sus propiedades antigénicas específicas de la cepa;
-
vacunas de subunidades de virus que se elaboran sólo con antígenos superficiales (H y N);
-
vacunas de virus fraccionados, en las que se fracciona la estructura viral mediante un agente separador.
Estas vacunas contienen tanto antígenos superficiales como internos. Además, varios fabricantes no europeos producen vacunas de virus vivos atenuados. Tradicionalmente, se cree que las vacunas de virus enteros no son tan bien toleradas debido a la presencia de un estrato lipídico en la superficie de las partículas virales (un residuo de la membrana celular huésped que recubre el virus, cuando se reproducen de la célula huésped).
Las vacunas contra la gripe se fabrican en todo el mundo. Las variaciones antigénicas menores y los cambios antigénicos periódicos plantean problemas para la producción y la adquisición de vacunas, ya que se debe producir y adquirir una vacuna nueva que sea estrechamente compatible con la configuración antigénica circulante para el comienzo de cada nueva "estación" de gripe. Para lograr estos requerimientos, la Organización Mundial de la Salud (OMS) ha establecido un sistema de vigilancia mundial que permite identificar y aislar las cepas virales que circulan en las diferentes regiones del mundo. Las prácticas centinelas recuperan las partículas virales de la nasofaringe de los pacientes con síntomas asociados a la gripe, y las muestras se envían rápidamente a los laboratorios de los centros nacionales de gripe (110 laboratorios en 79 países). Cuando se detectan cepas nuevas, se envían las muestras a uno de los cuatro centros de referencia de la OMS (Londres, Atlanta, Tokio y Melbourne) para realizarles un análisis antigénico. Posteriormente, la información sobre la cepa circulante se envía a la OMS, que en febrero de cada año recomienda, a través de un comité, las cepas que se deben incluir en la vacuna para la próxima "estación". Los gobiernos individuales pueden o no seguir las recomendaciones de la OMS. Australia, Nueva Zelanda y más recientemente Sudáfrica, siguen sus propias recomendaciones para el contenido de la vacuna. Por lo tanto, la vigilancia y la identificación temprana desempeñan un papel central en la composición de la vacuna.
De qué manera podría funcionar la intervención
Cada campaña de vacunación ha establecido objetivos con los que se deben medir los efectos de la campaña. Quizás el documento más detallado que presenta la justificación de un programa preventivo integral es el del Advisory Committee on Immunization Practices (ACIP) de los EE.UU., publicado en 2006 (ACIP 2006). El documento identifica 11 categorías de personas con alto riesgo de complicaciones de la gripe, entre las que se encuentran los adultos sanos de 50 a 65 años de edad y los trabajadores de los servicios de salud. La justificación para la selección de políticas se basa en la gran carga que la gripe impone sobre las poblaciones y los efectos beneficiosos derivados de la vacunación. La disminución de los casos y las complicaciones (como el número excesivo de hospitalizaciones, el ausentismo laboral, la mortalidad y las visitas a los servicios sanitarios) y la interrupción de la transmisión son los principales argumentos para extender la vacunación a los adultos sanos de 50 a 65 años de edad (ACIP 2006).
La actualización del documento ACIP 2010 recomienda la vacunación sistemática de todos los participantes con edades de seis meses de vida y más. Subraya la importancia de centrar los esfuerzos de vacunación, cuando los suministros para la vacunación son limitados, en los adultos sanos que tienen mayor riesgo de desarrollar complicaciones graves de la gripe, como:
-
personas de 50 años o más;
-
mujeres que están o estarán embarazadas durante la temporada de gripe;
-
personal sanitario;
-
contactos familiares y cuidadores de niños con edades inferiores a los cinco años y adultos de 50 años de edad o más, con particular énfasis en vacunar los contactos de los niños menores de seis meses; y
-
contactos familiares y cuidadores de pacientes con enfermedades que los exponen a un riesgo mayor de complicaciones graves a causa de la gripe (ACIP 2010).
Las mujeres embarazadas están incluidas entre los receptores prioritarios para la inmunización estacional contra la gripe en muchos países (AIH 2013; Green Book 2013; NACI 2012; STIKO 2010), debido al riesgo de morbilidad asociada con la gripe durante el embarazo, los posibles resultados neonatales adversos asociados con infecciones maternas de gripe, y según las pruebas de que la vacunación de las mujeres embarazadas protege a los recién nacidos de la gripe y de las hospitalizaciones relacionadas con la gripe (NACI 2012).
La vacuna inactivada contra la gripe podría ser administrada en cualquier estadio del embarazo, mientras que la vacuna de virus vivos no se autoriza para utilizarla durante el embarazo, ya que los datos disponibles acerca de la seguridad y eficacia en las madres y los recién nacidos son muy limitados (ACIP 2010; Green Book 2013).
Por qué es importante realizar esta revisión
Debido al costo muy elevado de la vacunación anual de gran parte de la población, la extrema variabilidad de la incidencia de la gripe durante cada "estación" y la heterogeneidad de las recomendaciones para la salud pública, se realizó una revisión sistemática de las pruebas. En la actualización de 2007 de la revisión, se incluyeron estudios comparativos no aleatorios que informaron pruebas de efectos perjudiciales graves o poco frecuentes (o ambos) para aumentar su importancia entre los encargados de adoptar decisiones (Jefferson 2007). En la presente actualización (2013), también se han incluido pruebas acerca de la vacunación contra la gripe en mujeres embarazadas y recién nacidos.
Objetivos
Identificar, recuperar y evaluar todos los estudios que evalúan los efectos (eficacia, efectividad y efectos perjudiciales) de las vacunas contra la gripe en adultos sanos, incluidas las mujeres embarazadas.
Los "efectos" se definieron como sigue:
-
la eficacia se definió como la capacidad de las vacunas para prevenir la gripe A o B y sus complicaciones;
-
la efectividad, como la capacidad de las vacunas para prevenir las enfermedades tipo gripe y sus consecuencias; y
-
los efectos perjudiciales como cualquier evento perjudicial potencialmente asociado con la exposición a las vacunas contra la gripe.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Cualquier estudio aleatorio (ECA) o cuasialeatorio que compare las vacunas contra la gripe en humanos versus placebo o ninguna intervención, o que comparen tipos, dosis o esquemas de administración de la vacuna contra la gripe. Solamente se consideraron los estudios que evaluaron la protección a la gripe adquirida por exposición natural.
Los estudios comparativos no aleatorios se incluyeron si informaron pruebas sobre la asociación entre las vacunas contra la gripe y efectos adversos graves, como el síndrome de Guillain‐Barré o síndromes óculo‐respiratorios, o si informaron datos de efectividad o eficacia de la administración de vacunas durante el embarazo.
Se definieron como ECA los estudios en los que pareció que los individuos (u otras unidades experimentales) incluidos en el estudio se asignaron definitiva o posiblemente de forma prospectiva a una de dos (o más) formas alternativas de atención sanitaria mediante asignación aleatoria. Un estudio se considera cuasialeatorio cuando al parecer los individuos (u otras unidades experimentales), seguidos durante el mismo, han sido definitiva o posiblemente asignados al azar de forma prospectiva a una de dos (o más) alternativas de atención sanitaria, con el uso de un método cuasialeatorio de asignación (como la alternancia, la fecha de nacimiento o el número de historia clínica).
Tipos de participantes
Individuos sanos de 16 a 65 años de edad, independientemente de su estado de inmunidad a la gripe. Se excluyeron de la revisión los estudios que incluían más del 25% de los individuos fuera de este intervalo de edad. También se incluyeron mujeres embarazadas junto con los recién nacidos.
Tipos de intervenciones
Vacunas para virus vivos, atenuados o inactivados o fracciones de los mismos administradas por cualquier vía, independientemente de la configuración antigénica.
Tipos de medida de resultado
Resultados primarios
Clínicas
-
Número y gravedad (complicaciones y días de trabajo perdidos) de los casos de gripe sintomática y de enfermedades tipo gripe que ocurrieron en los grupos de vacuna y placebo.
Efectos perjudiciales
-
Número y gravedad de los efectos adversos (sistémicos y graves). Los efectos adversos sistémicos incluyen casos de malestar, náuseas, fiebre, artralgias, erupción cutánea, cefalea y signos más generalizados y graves, como efectos neurológicos.
-
Resultados maternos y resultados relacionados con el curso del embarazo. Éstos incluyen aborto (espontáneo, interno, muerte fetal, mortinatalidad), parto de prematuros (menos de 37 semanas), muerte materna.
-
Resultados neonatales: malformaciones congénitas (leves e importantes), muerte neonatal.
Resultados secundarios
-
Los efectos secundarios locales incluyen induración, dolor y enrojecimiento en el lugar de inoculación.
Results
Description of studies
Results of the search
The first publication of this review contained 20 trials (Demicheli 1999). The second publication added five more trials (Demicheli 2004). The third publication included 48 trials in total (Jefferson 2007). The fourth published update (Jefferson 2010) included two new trials (aa Beran 2009a; aa Beran 2009b) and excluded three new trials (Belongia 2009; Chou 2007; Khazeni 2009). In this 2013 update, 41 new study reports have been included and 63 new trials have been excluded.
Some of the included studies had more than two arms, comparing different vaccines, routes of administration, schedules or dosages, or reported data from different settings and epidemic seasons. We split these studies into sub‐studies (data sets). For the remainder of this review, the term 'study report' refers to the original study report, while the word 'data set' refers to the sub‐study; these sub‐studies could refer either to different study arms, to different influenza seasons or to different study designs. Risk of bias can be independently assessed for each sub‐study (or data set) study design.
More information about the division of study reports into data sets is given in the Characteristics of included studies table. In this 2013 update, 90 studies (116 data sets) are now included in the review (Figure 1).

Study flow diagram
Included studies
We have coded each trial on the basis of study design and the type of data contributed to the review as follows. The letter preceding the study represents the study design: (a) denotes RCTs, (b) denotes case‐control studies and (c) denotes cohort studies. The second letter indicates the contribution to the evidence in the data set: (a) efficacy/effectiveness or (b) harms. So, for example, a case‐control study contributing safety or harms data is coded as (bb) and a trial contributing efficacy/effectiveness data is coded as (aa). A (p) code has been added to refer to the studies on vaccination during pregnancy.
Seasonal vaccines: efficacy or effectiveness
-
RCTs on inactivated parenteral vaccine: (20 studies/29 data sets) (aa Barrett 2011; aa Beran 2009a; aa Beran 2009b; aa Bridges 2000a; aa Bridges 2000b; aa Eddy 1970; aa Frey 2010; aa Hammond 1978; aa Jackson 2010a; aa Jackson 2010b; aa Keitel 1988a; aa Keitel 1988b; aa Keitel 1997a; aa Keitel 1997b; aa Keitel 1997c; aa Leibovitz 1971; aa Mesa Duque 2001; aa Mixéu 2002; aa Monto 2009; aa Nichol 1995; aa Ohmit 2006; aa Ohmit 2008; aa Powers 1995a; aa Powers 1995b; aa Powers 1995c; aa Tannock 1984; aa Weingarten 1988; aa Zhilova 1986a; aa Zhilova 1986b).
-
RCTs on live aerosol vaccine: (eight studies/12 data sets) (aa Edwards 1994a; aa Edwards 1994b; aa Edwards 1994c; aa Edwards 1994d; aa Monto 1982; aa Monto 2009; aa Nichol 1999a; aa Ohmit 2006; aa Ohmit 2008; aa Rytel 1977; aa Zhilova 1986a; aa Zhilova 1986b).
-
RCTs on inactivated aerosol vaccine: (one study/one data set) (aa Langley 2011).
Seasonal vaccines: safety (local and systemic harms)
-
RCTs on inactivated parenteral vaccine: (20 studies/21 data sets) (aa Barrett 2011; aa Bridges 2000a; aa Bridges 2000b; aa Frey 2010; aa Jackson 2010a; aa Mesa Duque 2001; aa Monto 2009; aa Nichol 1995; aa Ohmit 2006; aa Ohmit 2008; aa Powers 1995a; aa Tannock 1984; aa Weingarten 1988; ab Caplan 1977; ab El'shina 1996; ab Forsyth 1967; ab Goodeve 1983; ab Pyrhönen 1981; ab Rocchi 1979a; ab Saxen 1999; ab Scheifele 2003).
-
RCTs on live aerosol vaccine: (13 studies/14 data sets) (ab Atmar 1990; ab Betts 1977a; ab Evans 1976; ab Hrabar 1977; ab Keitel 1993a; ab Keitel 1993b; ab Lauteria 1974; ab Miller 1977; aa Monto 1982; aa Nichol 1999a; aa Ohmit 2006; aa Ohmit 2008; ab Rocchi 1979b; aa Rytel 1977).
-
RCTs on inactivated aerosol vaccine: (three studies/three data sets) (ab Boyce 2000; ab Langley 2005; aa Langley 2011).
Two studies with live aerosol vaccine (ab Reeve 1982; ab Spencer 1977) (each one a data set) could not be introduced into the harms analysis (secondary effects) because the data did not allow for quantitative analysis (systemic and local harms were reported given as cumulative in ab Spencer 1977 and data were not clearly reported in ab Reeve 1982).
Administration during pregnancy ‐ efficacy/effectiveness in mothers
-
Seasonal inactivated vaccine ‐ cohort studies: (two studies/two data sets) (pca Black 2004; pca Hulka 1964).
-
2009 to 2010 pandemic: inactivated vaccines ‐ cohort studies: (one study/one data set) (pca Yamada 2012).
Administration during pregnancy ‐ efficacy/effectiveness in newborns
-
Seasonal inactivated vaccine ‐ cohort studies on effectiveness (ILI): (three studies/three data sets) (pca Black 2004; pca Eick 2011; pca France 2006).
-
Seasonal inactivated vaccine ‐ cohort studies on efficacy (laboratory‐confirmed): (one study/one data set) (pca Eick 2011).
-
Seasonal inactivated vaccine ‐ case‐control on effectiveness (ILI): (two studies/two data sets) (pba Benowitz 2010; pba Poehling 2011).
Administration during pregnancy ‐ pregnancy‐related outcomes (abortion, congenital malformation, prematurity, neonatal death)
-
Seasonal inactivated vaccine ‐ cohort studies: (four studies/four data sets) (pca Black 2004; pca Munoz 2005; pcb Omer 2011; pcb Sheffield 2012).
-
2009 to 2010 pandemic: inactivated vaccine ‐ cohort studies: (nine studies/nine data sets) (pcb Fell 2012; pcb Håberg 2013; pcb Heikkinen 2012; pcb Källén 2012; pcb Launay 2012; pcb Lin 2012; pcb Oppermann 2012; pcb Pasternak 2012; pcb Richards 2013).
-
Seasonal inactivated vaccine ‐ case‐control: (one study/one data set) (pbb Irving 2013).
One study has not been introduced in the quantitative synthesis because it is the only study about the A/NJ/8/76 vaccine (pcb Deinard 1981). The retrospective cohort of pcb Toback 2012 was also not included in the analysis because it did not contain useful outcomes.
Administration during pregnancy ‐ severe harms
One cohort study was introduced (pcb Nordin 2013), assessing the association between seasonal vaccine exposure during pregnancy and the following harms within 42 days from administration: Guillain‐Barré syndrome, demyelinating diseases and immune thrombocytopenic purpura.
Severe harms ‐ general population
Guillain‐Barré syndrome
-
2009 to 2010 pandemic ‐ case‐control: (two studies/six data sets) (bb Grimaldi Bensouda 2011; bb Dieleman 2011a; bb Dieleman 2011b; bb Dieleman 2011c; bb Dieleman 2011d; bb Dieleman 2011e).
-
Seasonal inactivated vaccine ‐ case‐control: (one study/one data set) (bb Galeotti 2013).
-
Seasonal inactivated vaccine ‐ cohort studies: (two studies/four data sets) (cb Kaplan 1982; cb Lasky 1998).
One cohort study assessing the association between the A/NJ/8/76 vaccine and Guillain‐Barré syndrome was not introduced into the analysis (cb Shonberger 1979).
Demyelinating diseases (optic neuritis or multiple sclerosis)
-
Seasonal inactivated vaccine ‐ case‐control: (four studies/four data sets) (bb DeStefano 2003; bb Hernan 2004; bb Payne 2006; bb Zorzon 2003).
-
2009 to 2010 pandemic ‐ cohort study: (one study/one data set) (cb Moro 2013).
Immune thrombocytopenic purpura
-
Seasonal inactivated vaccine ‐ case‐control: (two studies/two data sets) (bb Garbe 2012; bb Grimaldi‐Bensouda 2012).
Other serious adverse events
-
Oculo‐respiratory syndrome: RCT ‐ cross‐over (one study) (ab Scheifele 2003).
-
Respiratory function: RCT (ab Atmar 1990).
-
Cutaneous melanoma: case‐control (bb Mastrangelo 2000).
-
Bell's palsy: case‐control (bb Mutsch 2004).
-
Cardiac arrest: case‐control (bb Siscovick 2000).
-
Rheumatoid arthritis: case‐control (bb Ray 2011).
-
Neurological and autoimmune disorders: cohort study (cb Bardage 2011).
-
Other serious adverse events: cohort study (cb Baxter 2012).
Pandemic vaccine: efficacy or effectiveness
-
RCT on inactivated parenteral vaccine: (four studies/seven data sets) (aa Eddy 1970; aa Mogabgab 1970a; aa Mogabgab 1970b; aa Waldman 1969a; aa Waldman 1969b; aa Waldman 1972b; aa Waldman 1972d).
-
RCT on inactivated aerosol vaccine: (two studies/four data sets) (aa Waldman 1969c; aa Waldman 1969d; aa Waldman 1972a; aa Waldman 1972c).
-
RCT on live aerosol vaccine (one study/one data set) (aa Sumarokow 1971).
Excluded studies
We excluded 155 studies (see Characteristics of excluded studies table).
Risk of bias in included studies
Out of the 116 included studies (sub‐study or data set), we classified 9.5% (11/116) as low risk of bias (nine RCTs, two case‐control); 19.8% (23/116) as high risk of bias (six RCTs, 14 cohorts, three case‐control) and finally 70.6% (82/116) did not present either sufficient information in one or more key domains or, although presenting a low risk of bias in a specific domain, scored a high risk of bias in one or more items used in the quality evaluation. Table 1 shows the summary quality assessment of all included studies and a graphical display of the quality assessment is presented in Figure 2 and Figure 3. We have highlighted that each 'paper' could include more than one study (data set) and these different studies required separate quality assessment. On the other hand, the funding source can be referred only to a single paper.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
| Study design | High risk | Low risk | Unclear risk | Total |
| Case‐control | 3 | 2 | 15 | 20 |
| Cohort | 14 | 0 | 13 | 27 |
| RCT/CCT | 6 | 9 | 54 | 69 |
| Total | 23 | 11 | 82 | 116 |
Allocation
In the included trials allocation concealment was adequate (low risk of bias) in 18 studies (26.1%), inadequate (high risk of bias) in six (8.7%) and unclear (unclear risk of bias) in 45 (65.2%).
Blinding
We judged blinding as low risk of bias in 16 RCTs (23.2%), as high risk of bias in two studies (2.9%) and as unclear in 51 studies (73.9%).
Incomplete outcome data
The majority of the included RCTs/CCTs did not report sufficient information about loss to follow‐up (63 studies; 91.3%).
Selective reporting
The assessment of selective reporting bias presents several difficulties and would require review of the original study protocols for the included studies, which are mainly unavailable.
Other potential sources of bias
Few studies reported information on influenza circulation in the surrounding community, making interpretation of the results and assessment of their generalisability difficult.
It is now known that industry funding of influenza vaccine studies determines publication in high‐prestige journals and higher citation rates than other types of funding. In addition, industry funding is associated with optimistic conclusions, but the quality of the majority of influenza vaccine studies is low, irrespective of funding (see Table 2). A previously cited review showed a complex web of interrelationships between these variables (Jefferson 2009a), but how this impacts on policy‐making is not known.
| Study design | Government, institutional or public | Industry | Mixed | Total |
| Case‐control | 13 | 1 | 1 | 15 |
| Cohort | 22 | 3 | 2 | 27 |
| RCT/CCT | 31 | 12 | 5 | 48 |
| Total | 66 | 16 | 8 | 90 |
Case‐control studies ‐ quality assessment
-
Case selection (definition/representativeness): case identification is mainly performed by means of registers maintained at several healthcare organisations (HMO, Kaiser Permanente) or by hospital or GP (general practice) registers. A further case ascertainment is conducted by specialists in order to verify the agreement with the chosen case definition. In studies assessing vaccine efficacy, cases were identified by using a laboratory test performed on all participants having symptoms. For 19 out of 20 (95%), we classified case selection and definition as low risk of bias.
-
Control selection (definition): controls were selected from within the same registers used for case identification or from among participants living in the same catchment area of the hospitals in which the cases were identified. For 10 out of 20 studies (50%), we classified control selection and definition as low risk of bias and for 8 out of 20 (40%) we classified this as unclear risk of bias.
-
Comparability: the most frequent method used to ensure comparability between cases and controls consisted of matching for age, gender and index date (onset of symptoms for cases and GP visit for controls). Less frequently matching was also done for other possible parameters, such as the number of GP visits within a certain time interval, or by resorting to the use of a propensity score or multivariate models in order to reduce the impact of other possible confounders. Nevertheless many studies (16 out of 20 (80%)) did not provide sufficient information to be able to tell how comparable cases and controls effectively are.
-
Exposure ascertainment (same method of ascertainment for cases and controls/non‐response rate): for studies based on healthcare organisations or insurance registers assessment of vaccine exposure was certified in the same registers. In other studies vaccine exposure was ascertained with a structured interview and less frequently also with the recovering of the vaccination records. In many studies (14 out of 20 (70%)), ascertainment of the vaccine exposure was not fully reliable. For 5 out of 20 (25%), we judged exposure ascertainment as low risk of bias.
Cohort studies ‐ quality assessment
-
Selection exposed cohort (definition/representativeness): the majority of the studies are retrospective and use a data linkage method to select the exposed cohort. In 17 out of 27 studies (63%) this procedure was insufficiently described.
-
Selection non‐exposed cohort (definition/ascertainment): most of the studies are based on record linkage and the identification of the non‐exposed cohort was done by considering the absence of vaccination records. However, insufficient detail was provided and therefore we classified these kinds of studies as unclear risk of bias (17 out of 27 (63%)).
-
Comparability: in most of the included cohort studies matching procedures for the most probable confounders were applied by using a multivariate model to ensure comparability between exposed and unexposed cohorts. Sometimes a propensity score procedure was also used. Therefore in many studies only a few confounders were used to ensure comparability between exposed and non‐exposed cohorts, thus we classified no studies as low risk of bias.
-
Assessment of outcome (demonstration that outcome of interest was not present at the start of the study/whether follow‐up was long enough for outcomes to occur/adequacy of follow‐up of cohorts): outcomes of interest were generally documented in the registries used to identify the study population and consequently were almost always retrospectively assessed, thus we classified 9 out of 27 as low risk of bias.
Effects of interventions
Inactivated parenteral vaccines (Analysis 01)
The overall effectiveness of parenteral inactivated parenteral vaccine against influenza‐like illness (ILI) is 16% (95% confidence interval (CI) 5% to 25%), with a corresponding number needed to vaccinate (NNV) of 40 (95% CI 26 to 128). Heterogeneity amongst the studies in this comparison is relatively low (I2 statistic = 26%) and a sensitivity analysis made by comparing estimates obtained using the random‐effects model versus the fixed‐effect model does not change the conclusion. The CI of the NNV becomes narrower by applying the fixed‐effect model (NNV 38, 95% CI 29 to 49).
Inactivated parenteral vaccines are 16% effective (95% CI 9% to 23%) in preventing ILI symptoms when strains contained in the vaccine antigenically match those circulating (Analysis 1.1.1). The estimated NNV for this comparison is 17 (95% CI 12 to 29). On the other hand, inactivated vaccines are not significantly protective against ILI when the degree of matching between the vaccine and circulating influenza strains is absent or unknown (risk ratio (RR) 0.90, 95% CI 0.69 to 1.18, Analysis 1.1.2). In the subgroup Analysis 1.1.2 heterogeneity is particularly high (I2 statistic = 82%) and estimates using the fixed‐effect model show statistical significance: Vaccine Effectiveness 18% (95% CI 10% to 25%) and NNV 59 (95% CI 43 to 106).
The overall efficacy of inactivated vaccines in preventing confirmed influenza (Analysis 1.2) is 60% (95% CI 53% to 66%) with a NNV of 71 (95% CI 64 to 80). When the vaccine content matches the circulating strain, the efficacy is 62% (95% CI 52% to 69%) and the NNV is 58 (95% CI 52 to 69). The results are very similar when matching is absent or unknown (Vaccine Efficacy 55%, 95% CI 41% to 66% and NNV 60, 95% CI 50 to 80). Since heterogeneity was very low (I2 statistic = 17% for Analysis 1.2.1; I2 statistic = 14% for Analysis 1.2.2 and I2 statistic = 11% overall), there were no differences when comparing the estimates obtained by using a fixed‐effect model with those from a random‐effects model.
Looking at the NNV in Analysis 1.1 and Analysis 1.2, it seems that effectiveness against ILI is higher than efficacy against laboratory‐confirmed influenza (NNV‐ILI 40; NNV‐influenza 71). These paradoxical results, showing an apparently higher aspecific effectiveness and a lower specific efficacy, are mainly due to the fact that ILI and confirmed influenza have a very different incidence among the study population. We note that 15.6% of unvaccinated participants versus 9.9% of vaccinated participants developed ILI symptoms, whilst the corresponding figures for participants who developed laboratory‐confirmed influenza are 2.4% and 1.1% for unvaccinated and vaccinated people, respectively.
Based on the results from a single study (aa Bridges 2000b), physician visits appear 42% less frequent (95% CI 9% to 63%) in participants immunised with vaccines prepared with strains matching circulating viruses (Analysis 1.3.1), whereas there are no significant results when the degree of matching is unknown or absent (RR 1.28, 95% CI 0.90 to 1.83; Analysis 1.3.2). The overall effect is also not significant (RR 0.87, 95% CI 0.40 to 1.89) (Analysis 1.3). Even though the two data sets of aa Bridges 2000b showed very high heterogeneity (I2 statistic = 87%), no difference arose when comparing the results from the fixed‐effect with the random‐effects model analysis.
A similar conflicting result is observed when analysing the effect of inactivated vaccine administration on days of illness (Analysis 1.4), when the estimate (mean difference (MD)) obtained in good match conditions was compared with that where there was an unknown or absent degree of matching. As a consequence of the high overall heterogeneity (I2 statistic = 87%), the result obtained from the fixed‐effect model analysis (MD ‐0.31, 95% CI ‐0.54 to ‐0.07) differs substantially from that resulting from the application of a random‐effects model (MD ‐0.21, 95% CI ‐0.98 to 0.56).
There seems to be no effect on the time an antibiotic or drug was prescribed (Analysis 1.5; Analysis 1.6).
Four trials evaluated time off work, estimating that vaccination saves around 0.04 working days on average. This result is affected by high levels of heterogeneity (I2 statistic = 82%) and changes depending on whether a fixed‐effect (MD ‐0.04, 95% CI ‐0.06 to ‐0.01) or random‐effects model (MD ‐0.04, 95% CI ‐0.14 to 0.06) is used.
The effect on hospitalisation (Analysis 1.8) was evaluated in two trials (aa Bridges 2000a; aa Leibovitz 1971), but it was not statistically significant. No evidence was found for cases of pneumonia.
Harms
Local tenderness and soreness are more than three times as common among parenteral vaccine recipients than among those in the placebo group (RR 3.13, 95% CI 2.44 to 4.02) (Analysis 1.10.1). There are also increases in erythema (RR 2.59, 95% CI 1.77 to 3.78, Analysis 1.10.2) and induration (RR 4.28, 95% CI 1.25 to 14.67) but not in arm stiffness. The combined local effects endpoint was significantly higher for those receiving the vaccine (RR 2.44, 95% CI 1.82 to 3.28; Analysis 1.10.5).
Myalgia (Analysis 1.11.1) is significantly associated with vaccination (RR 1.77, 95% CI 1.40 to 2.24), as well as systemic fever (RR 1.54, 95% CI 1.22 to 1.95), headache (RR 1.17, 95% CI 1.01 to 1.36), fatigue or indisposition (RR 1.23, 95% CI 1.07 to 1.42) and malaise (RR 1.51, 95% CI 1.18 to 1.92). The combined endpoint was not increased (RR 1.16, 95% CI 0.87 to 1.53; Analysis 1.11.7).
Live aerosol vaccines (Analysis 02)
Live aerosol vaccines have an overall effectiveness of 10% (95% CI 4% to 16%; NNV 46, 95% CI 29 to 115) and content and matching appear not to affect their performance significantly (Analysis 2.1). Overall efficacy (Analysis 2.2) is 53% (95% CI 38% to 65%) and the NNV is 39 (95% CI 32 to 54). Again, neither content nor matching appear to affect their performance significantly.
No evidence is available on complications (e.g. bronchitis, otitis, pneumonia).
The effectiveness of the aerosol vaccines against ILI (with no clear definition) is significant only for vaccines with absent or unknown matching (37%, 95% CI 20% to 51%) and the NNV is 69 (95% CI 23 to 46) (Analysis 2.3).
The conclusions of this comparison were unaffected by analysis using either the fixed‐effect or random‐effects models.
Harms
Significantly more recipients experienced local symptoms after vaccine administration than after placebo administration (Analysis 2.4).
-
Upper respiratory infection (RR 1.66, 95% CI 1.22 to 2.27).
-
Cough (RR 1.51, 95% CI 1.08 to 2.10).
-
Coryza (RR 1.56, 95% CI 1.26 to 1.94).
-
Sore throat (RR 1.66, 95% CI 1.49 to 1.86).
-
Combined endpoint (any or highest symptom) (RR 1.56, 95% CI 1.31 to 1.87).
There is no significant increase in systemic harms (combined endpoint: any or highest symptom RR 1.40, 95% CI 0.82 to 2.38), although rates of myalgia (RR 2.47, 95% CI 1.26 to 4.85) and headache (RR 1.54, 95% CI 1.09 to 2.18) are higher in the vaccine than in the placebo groups (Analysis 2.5).
Inactivated aerosol vaccines (Analysis 03)
No RCTs assessing the effectiveness of inactivated aerosol vaccines in preventing ILI could be included; the only available evidence comes from studies carried out during the 1968 to 1969 pandemic (Analyses 12 to 16).
The efficacy of inactivated aerosol vaccine in preventing laboratory‐confirmed influenza (Analysis 3.1.1) is assessed in one RCT (aa Langley 2011), whose results do not show a statistically significant protective effect (RR 0.38, 95% CI 0.14 to 1.02).
Harms
None of the trials on inactivated aerosol vaccines reported significant harms.
Inactivated parenteral vaccines ‐ cohort studies (Analysis 04)
In this analysis, we have considered the effects of vaccine administration in pregnant women and their newborns.
Based on unadjusted data from a cohort study (high risk of bias), 2009/2010 H1N1 monovalent pandemic vaccines (Analysis 4.1.1) provide a significant protective effect against ILI in pregnant women (Vaccine Effectiveness 89%, 95% CI 79% to 94%; NNV 54, 95% CI 51 to 61). Seasonal inactivated vaccine is not effective against ILI (RR 0.54, 95% CI 0.22 to 1.32; Analysis 4.1.2). Sensitivity analysis performed using the fixed‐effect model showed statistical significance, even for a modest, protective effect (RR 0.76, 95% CI 0.65 to 0.89; NNV 92, 95% CI 63 to 201; VE 24%, 95% CI 11% to 35%).
The effectiveness of vaccination with seasonal inactivated vaccine during pregnancy for preventing ILI in newborns is not statistically significant, as it results from two cohort studies using either hazard ratio (HR) or RR adjusted estimates (Analysis 4.2.1 and Analysis 4.3.1, respectively). Efficacy against confirmed influenza (Analysis 4.3.2) is really modest but has statistical significance (adjusted RR 0.59, 95% CI 0.37 to 0.94; NNV 27, 95% CI 18 to 185; VE 41%, 95% CI 6% to 63%).
It seems that vaccination with the 2009/2010 H1N1 monovalent pandemic vaccine during pregnancy is not associated with a higher risk of abortion (Analysis 4.4.1 and Analysis 4.4.2), congenital malformation (Analysis 4.4.3) or neonatal death (Analysis 4.4.5).
Cases of neonatal death and abortion have been observed less frequently among women immunised with seasonal influenza vaccine (Analysis 4.5.1 and Analysis 4.5.4, both unadjusted estimates).
The results of pcb Deinard 1981 are based on the follow‐up results of 189 pregnant women immunised with monovalent pandemic A/New Jersey/8/76 (either in split or whole virus formulation) and 517 pregnant women who did not receive vaccination. The time of observation was extended up to the first eight weeks of life of the newborns. No statistically different incidence of maternal pregnancy outcomes and infant deaths was observed between vaccinated and unvaccinated groups. Statistical analysis (Chi2 test) shows no relation between immunisation history and presence of anomalities at the 8th week of life. This cohort study has not been included in the analysis as the vaccine studied is no longer in use.
Inactivated parenteral vaccines ‐ case‐control studies (Analysis 05)
This analysis only includes studies assessing the effect of vaccination against influenza during pregnancy. The incidence of ILI in pregnant women who were immunised with inactivated seasonal vaccine during pregnancy was not statistically different when compared with that observed among unvaccinated pregnant women (Analysis 5.1.1). However, in sensitivity analysis using the fixed‐effect model, the results of the analysis become statistically significant. In conclusion, the results of this comparison were affected by the model used to perform the analysis.
One further case‐control study did not find a statistically significant association between exposure to seasonal inactivated vaccine in pregnancy and abortion cases (Analysis 5.2.1).
One retrospective cohort study tried to assess the effect of live attenuated vaccine during pregnancy, based on data from a health insurance database during six subsequent influenza seasons (pcb Toback 2012). A total of 834,999 pregnant women were identified, out of whom 138 received live attenuated vaccine at any time during pregnancy. Claims for hospitalisation or visits to the emergency department within 42 days after immunisation were searched for but all observed events were considered to be related to a normal physiological pregnancy and not to immunisation. The system used (claim data) would be unable to detect birth outcomes.
Serious adverse events ‐ Guillain‐Barré syndrome ‐ cohort studies (Analysis 06)
The possible association between exposure to seasonal inactivated vaccine in healthy adults and Guillain‐Barré syndrome onset within six weeks following immunisation was investigated by two cohort studies performed during two subsequent epidemic seasons. No significant association was found Analysis 6.1.1). Administration of seasonal inactivated vaccine during pregnancy was not associated with Guillain‐Barré syndrome onset within six weeks from immunisation (Analysis 6.1.2).
The cohort of cb Shonberger 1979 was the first study that compared Guillain‐Barré syndrome cases by vaccination status and the national incidence in vaccinated and unvaccinated national cohorts after the suspension of the National Influenza Immunisation Program in the winter of 1976 to 1977. At that time the monovalent inactivated swine vaccine A/New Jersey/8/76 had been administered. The attributable risk from vaccination was just below one case of Guillain‐Barré syndrome in every 100,000 vaccinations. This cohort study has not been included in the analysis as the vaccine studied is no longer in use.
Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control studies (Analysis 07)
In an analysis performed using the mean of unadjusted data relative to six data sets, exposure to monovalent H1N1 pandemic inactivated vaccine resulted in an apparent statistically significant association with Guillain‐Barré syndrome onset when administration took place within six weeks before symptoms occurred (odds ratio (OR) 2.22, 95% CI 1.14 to 4.31, Analysis 7.1.1). Thus, it should be taken into account that only one out of the six data sets showed a statistically significant association between vaccine exposure and Guillain‐Barré syndrome onset (bb Dieleman 2011e). When we performed a sensitivity analysis excluding this data set from the pooled estimate, the result was no longer significant. When the analysis was performed considering that vaccine exposure occurred at any time before disease onset, there was no significant association (Analysis 7.1.2).
The analyses performed by pooling authors' estimates adjusted for several confounders (i.e. receipt of other vaccines, family history of autoimmune diseases, physician consultation during the previous year and use of antibiotic, antiviral or antipyretic agents) do not show a statistical association for exposure within six weeks (Analysis 7.2.1) before disease onset or for exposure at any time (Analysis 7.2.2).
Data from one other case‐control study confirm that immunisation with seasonal inactivated vaccine is not significantly associated with the onset of Guillain‐Barré syndrome within six weeks after inoculation (bb Galeotti 2013) (Analysis 7.3).
Serious adverse events ‐ demyelinating diseases ‐ cohort studies (Analysis 08)
In one cohort study the authors tried to assess whether there is an association between exposure to inactivated trivalent seasonal influenza vaccine during pregnancy and several pathologies (e.g. Guillain‐Barré syndrome, demyelinating diseases, immune thrombocytopaenic purpura) within six weeks after immunisation. Unadjusted estimates were calculated for an association with demyelinating diseases by using the number of cases observed among exposed and unexposed hemi‐cohorts and indicate that there is no association (Analysis 8.1.2).
One cohort study assessed the safety of the H1N1 vaccine. No statistical association was found between vaccination with H1N1 monovalent pandemic vaccine and demyelinating diseases.
Serious adverse events ‐ demyelinating diseases ‐ case‐control studies (Analysis 09)
An association between exposure to seasonal inactivated vaccine and demyelinating diseases (including both multiple sclerosis and optic neuritis case definitions) in a healthy adult population was not statistically significant when we pooled unadjusted data from four case‐control studies (OR 0.96, 95% CI 0.79 to 1.17) (Analysis 9.1). Also, when we analysed adjusted data for each of the case definitions separately, the estimates remained non‐statistically significant for multiple sclerosis (Analysis 9.2) and for optic neuritis (Analysis 9.3).
Serious adverse events ‐ immune thrombocytopenic purpura ‐ cohort studies (Analysis 10)
One cohort study aimed to assess whether there is an association between exposure to inactivated trivalent seasonal influenza vaccine during pregnancy and several pathologies (e.g. Guillain‐Barré syndrome, demyelinating diseases, immune thrombocytopaenic purpura) within six weeks after immunisation. Neither the unadjusted (Analysis 10.2.2) nor adjusted estimates (Analysis 10.1.2) for an association with immune thrombocytopenic purpura were statistically significant.
Serious adverse events ‐ immune thrombocytopenic purpura ‐ case‐control studies (Analysis 11)
Data analysis of two case‐control studies (bb Garbe 2012; bb Grimaldi‐Bensouda 2012) did not show a statistically significant association between immune thrombocytopaenic purpura and seasonal influenza vaccine in any of the time frames considered (i.e. less than two months, six or 12 months between immunisation and disease onset), or when the data were pooled together (Analysis 11.2). The same conclusions could be drawn when analysis was performed by using estimates adjusted for confounders (Analysis 11.1) and are further confirmed by the fact that a sensitivity analysis carried out by using either a random‐effects or fixed‐effect model did not change them in any way. It should be observed that no data sets included in this comparison, with the exception of bb Garbe 2012, showed a statistical association between disease and influenza vaccination. It is possible that the ages of the participants (cases and controls) were different in these two studies and that some elderly participants could have been included. Unlike bb Grimaldi‐Bensouda 2012, the case‐control study (bb Garbe 2012) considered as exposed those cases that were immunised up until 28 days before immune thrombocytopaenic purpura onset.
Serious and rare harms
Oculo‐respiratory syndrome
On the basis of one randomised trial in 651 healthy adults aged around 45, trivalent split inactivated vaccine (TIV) caused mild oculo‐respiratory syndrome in people with no previous history of oculo‐respiratory syndrome (ab Scheifele 2003). Oculo‐respiratory syndrome was defined as bilateral conjunctivitis, facial swelling (lip, lid or mouth), difficulty in breathing and chest discomfort (including cough, wheeze, dysphagia or sore throat). Oculo‐respiratory syndrome (attributable risk 2.9%, 95% CI 0.6 to 5.2), hoarseness (1.3%, 95% CI 0.3 to 1.3) and coughing (1.2%, 95% CI 0.2 to 1.6) occurred within six days of vaccination. The association did not appear to be specific to any type of TIV.
Bell's palsy
One case‐control study and case series, based in the German‐speaking regions of Switzerland, assessed the association between an intranasal inactivated virosomal influenza vaccine and Bell's palsy (bb Mutsch 2004). Two hundred and fifty cases that could be evaluated (from an original 773 cases identified) were matched to 722 controls. All were aged around 50. The study reports a massive increase in risk (adjusted OR 84, 95% CI 20.1 to 351.9) within 1 to 91 days from vaccination. Despite its many limitations (case attrition: 187 cases could not be identified; ascertainment bias: physicians picked controls for their own cases; confounding by indication: different vaccine exposure rate between controls and the reference population), it is unlikely that such a large OR could have been affected significantly by systematic error. The authors called for larger pre‐licence harms trials, given the rarity of Bell's palsy. On the basis of this study the vaccine was withdrawn from sale.
Rheumatoid arthritis
One case‐control study used the register of the Northern California Kaiser Permanente Health Plan (NCKPHP) in order to identify cases of rheumatoid arthritis diagnosed during a three‐year period (1 January 1997 to 31 December 1999) among members of NCKPHP for at least two years (i.e. since 1 January 1995) and aged between 15 and 59 (bb Ray 2011). After reviewing clinical cards, 415 cases of definite or probable rheumatoid arthritis were included together with 1245 randomly selected controls matched for age within one year and for a categorical utilisation variable based on the number of clinic visits during the year prior to the rheumatoid arthritis symptom onset date (none, one to two, three to five, six to nine or 10+ visits). The Kaiser Immunisation Tracking System and chart review were used to determine vaccination status of cases and controls. Different time intervals between immunisation and rheumatoid arthritis onset were considered for analysis: 90, 180, 365 and 730 days. No significant association between vaccination and rheumatoid arthritis could be determined for any time interval, even after adjustment for confounders (sex, race and exact number of utilisation visits). The authors of this study performed a data analysis by using a person‐time cohort design, in which vaccinated cases contributed to the unexposed follow‐up time until they were immunised and to the exposed follow‐up time thereafter. Unlike case‐control analysis, person‐time cohort analysis was performed by excluding cases who showed symptoms in 1996. Even if a significant association for exposure to vaccine occurred within 180 and 365 days before disease onset was found (OR adjusted for race, sex and number of clinic visits 1.36, 95% CI 1.03 to 1.80 and 1.34, 95% CI 1.06 to 1.69, respectively), the authors point out that it is very difficult to estimate with sufficient precision the true onset date of rheumatoid arthritis, as the first symptoms could already be present some time before participants present for medical care. This is the most important limitation of this study and could have affected the estimates in a significant manner.
Neurological and autoimmune disorders
The study of cb Bardage 2011 is a large, prospective cohort study carried out in a Stockholm population (n = 1,945,024) during the vaccination campaign with monovalent A (H1N1) pandemic vaccine Pandemrix (GlaxoSmithKline, containing adjuvants AS03 and squalene) to evaluate the presence of an association between Pandemrix and neurological and/or autoimmune diseases (Guillain‐Barré syndrome, multiple sclerosis, Bell's palsy, narcolepsy, polyneuropathy, an/hypoaesthesia, paraesthesia, rheumatological disease and inflammatory bowel disease). During the first 45 days, participants with high‐risk conditions were preferentially vaccinated; vaccination was then offered to the remainder of the population in a second phase of the campaign (see description for more details).
The analysis of the hazard ratio (HR) adjusted for age, sex, socioeconomic status and healthcare consumption (number of hospital admissions and visits to specialist care one year before the pandemic period) showed that in participants immunised during the early phase of the campaign there was a significantly increased risk of Bell's palsy (HR 1.34, 95% CI 1.11 to 1.64), paraesthesia (HR 1.25, 95% CI 1.10 to 1.41) and inflammatory bowel disease (HR 1.25, 95% CI 1.04 to 1.50). For the participants vaccinated in the late phase of the campaign (> 45 days), HR estimates showed that the investigated diseases had been observed with no statistically different incidence between the vaccinated and unvaccinated participants.
A further stratification was performed, considering the time since first vaccination (six weeks or less and more than six weeks). This showed that in participants immunised during the first phase of the campaign, an increased incidence of Bell's palsy and paraesthesia was most pronounced, as well as within six weeks of vaccination (HR 1.74, 95% CI 1.16 to 2.59 for Bell's palsy and HR 1.60, 95% CI 1.25 to 2.05 for paraesthesia) and thereafter (HR 1.26, 95% CI 1.01 to 1.57 for Bell's palsy and 1.17, 95% CI 1.02 to 1.34 for paraesthesia). The increased risk of inflammatory bowel disease among those vaccinated in the early phase was only observed more than six weeks after vaccination (HR 1.29, 95% CI 1.06 to 1.58). Formal tests to determine whether risks further differed between those within and more than six weeks from vaccination were only statistically significant for paraesthesia (P = 0.005). In participants immunised during the second phase of the campaign, polyneuropathy was significantly more common within six weeks of immunisation (HR 1.79, 95% CI 1.16 to 2.77).
Cutaneous melanoma
The association between influenza vaccines and cutaneous melanoma was assessed by a case‐control study in 99 cases and 104 controls (bb Mastrangelo 2000). The authors reported a protective effect of repeated influenza vaccination on the risk of cutaneous melanoma (OR 0.43, 95% CI 0.19 to 1.00). The study is at high risk of bias because of the selective nature of cases (all patients in the authors' hospital), attrition bias (four cases and four controls eliminated because of "failure to collaborate"), recall bias (up to five years exposure data were based on patients' recollection) and ascertainment bias (non‐blinded exposure survey).
Primary cardiac arrest
The association between influenza vaccination the previous year and the risk of primary cardiac arrest (i.e. occurring in people with no previous history of cardiac disease) was assessed by a case‐control study in 360 cases and 418 controls (bb Siscovick 2000). The authors concluded that vaccination is protective against primary cardiac arrest (OR 0.51, 95% CI 0.33 to 0.79). The difficulty of case ascertainment (77% of potential cases had no medical examiner report and/or autopsy) and recall bias (spouses provided exposure data for 304 cases, while 56 survivor cases provided data jointly with their spouses) make the conclusions of this study unreliable. It is impossible to judge the reliability of this study because of a lack of detail on the circulation of influenza in the study areas in the 12 months preceding cardiac arrest (the causal hypothesis is based on the effects of influenza infection on the oxygen supply to the myocardium through lung infection and inflammation).
Pulmonary function
The effects of different types of live attenuated cold recombinant influenza vaccination on pulmonary function were assessed by a double‐blind, placebo‐controlled randomised trial in 72 healthy volunteers aged around 26 (ab Atmar 1990) (data on 17 asthmatics were not extracted). The authors report several non‐significant drops in lung function up to seven days post‐inoculation and a higher incidence of influenza‐like illness (17/46 versus 4/26) in the vaccinated arms.
Other serious adverse events
The study of cb Baxter 2012 is a large, retrospective cohort performed among members of Kaiser Permanente Health Plans of Northern California, Hawaii and Colorado aged between 18 and 59 years, who were immunised with live attenuated, inactivated influenza vaccine or did not receive vaccination. The study retrospectively investigated the occurrence of adverse events (see description) during five subsequent epidemics, but did not identify any unexpected serious risks when the live attenuated vaccine was used in approved populations.
Vaccines for the 1968 to 1969 (H3N2) influenza pandemic (Analyses 12 to 16)
Five studies yielded 12 data sets (aa Eddy 1970; aa Mogabgab 1970a; aa Mogabgab 1970b; aa Sumarokow 1971; aa Waldman 1969a; aa Waldman 1969b; aa Waldman 1969c; aa Waldman 1969d; aa Waldman 1972a; aa Waldman 1972b; aa Waldman 1972c; aa Waldman 1972d). As one would expect, vaccine performance was poor when the content did not match the pandemic strain (Analysis 12.1; Analysis 12.2). However, one‐dose or two‐dose monovalent whole virion (i.e. containing dead complete viruses) vaccines achieved a VE of 65% (95% CI 52% to 75%) protection against ILI (NNV 16, 95% CI 14 to 20), a VE of 93% (95% CI 69% to 98%; NNV 35, 95% CI 33 to 47) protection against influenza and a VE of 65% (95% CI 6% to 87%) with NNV 94 (95% CI 70 to 1022) against hospitalisation (Analysis 13.1; Analysis 13.2; Analysis 13.3).
Approximately half a working day and half a day of illness (Analysis 13.5; Analysis 13.6) were saved but no effect was observed on pneumonia (Analysis 13.4). All comparisons except for ILI are based on a single study (Analysis 13.4). The large effect on ILI is coherent with the high proportion of these illnesses caused by influenza viruses in a pandemic (i.e. the gap between the efficacy and effectiveness of the vaccines is narrow). Aerosol polyvalent or monovalent vaccines had a modest effect.
Discusión
Resumen de los resultados principales
La efectividad general de la vacuna inactivada que se administra por vía parenteral contra enfermedades tipo gripe es del 16% (intervalo de confianza [IC] del 95%: 5% a 25%), con un número correspondiente necesario a vacunar (NNV) de 40 (IC del 95%: 26 a 128). La eficacia general de las vacunas inactivadas para prevenir la gripe es del 60% (IC del 95%: 53% a 66%) con un NNV de 71 (IC del 95%: 64 a 80). Cuando el contenido de la vacuna coincide con la cepa circulante, la eficacia es del 62% (IC del 95%: 52% a 69%) y el NNV es 58 (IC del 95%: 52 a 69). Según los resultados de un único estudio (aa Bridges 2000b), las visitas a los médicos parecen ser 42% menos frecuentes (IC del 95%: 9% a 63%) en los participantes inmunizados con vacunas preparadas con cepas compatibles con los virus circulantes, mientras que no se encuentran diferencias significativas cuando se desconoce el grado de compatibilidad o no existe (cociente de riesgos [CR] 1,28; IC del 95%: 0,90 a 1,83). Nuevamente, el efecto general no es significativo (CR 0,87; IC del 95%: 0,40 a 1,89). No parece haber efectos sobre el momento en que se prescribe un antibiótico o un fármaco. Cuatro ensayos evaluaron el tiempo de ausencia del trabajo y estimaron que la vacunación ahorra un promedio de alrededor de 0,04 días de trabajo. Este resultado está afectado por niveles altos de heterogeneidad y cambia en dependencia de si se utiliza un modelo de efectos fijos (diferencia de medias [DM] ‐0,04; IC del 95%: ‐0,06 a ‐0,01) o de efectos aleatorios (DM ‐0,04; IC del 95%: ‐0,14 a 0,06).
Las vacunas de virus vivos administradas en forma de aerosol tienen una efectividad general del 10% (IC del 95%: 4% a 16%) y un NNV de 46 (IC del 95%: 29 a 115) y el contenido y la compatibilidad parecen no afectar el rendimiento de forma significativa. La eficacia general es del 53% (IC del 95%: 38% a 65%) y el NNV es 39 (IC del 95%: 32 a 54). Igualmente, ni el contenido ni la compatibilidad parecen afectar su rendimiento de forma significativa. Muchos más receptores presentaron síntomas locales después de la administración de las vacunas que de la administración de placebo.
No se pudieron incluir ensayos controlados aleatorios (ECA) que evaluaran la efectividad de las vacunas inactivadas administradas en forma de aerosol para prevenir las enfermedades tipo gripe. Las únicas pruebas disponibles provienen de estudios realizados durante la pandemia de 1968 a 1969. En un ECA (aa Langley 2011), se evaluó la eficacia de las vacunas inactivadas administradas en forma de aerosol para prevenir la gripe confirmada por laboratorio (Análisis 3.1.1) y los resultados no mostraron un efecto protector estadísticamente significativo (CR 0,38; IC del 95%: 0,14 a 1,02).
Los efectos de la administración de las vacunas contra la gripe en mujeres embarazadas y recién nacidos se investigaron en un ECA (Zaman 2008) en el que al grupo control se le administró la vacuna antineumocócica 23 valente. Por este motivo, el ECA se excluyó de la revisión y las pruebas de efectividad y eficacia se basan solamente en estudios observacionales (estudios de casos y controles y estudios de cohortes).
La efectividad de la vacunación con la vacuna inactivada estacional que se administra por vía parenteral durante el embarazo para la prevención de las enfermedades tipo gripe en los recién nacidos no fue estadísticamente significativa. Las pruebas provienen de dos estudios de cohortes que utilizaron estimaciones ajustadas de CRI o CR. Sin embargo, parece que la vacunación tiene un efecto moderado contra las enfermedades tipo gripe en las mujeres embarazadas (NNV 92; IC del 95%: 63 a 201) y contra la gripe confirmada por laboratorio en los recién nacidos de las pacientes vacunadas (NNV 27; IC del 95%: 18 a 185).
No se encontraron pruebas de una asociación entre las vacunas inactivadas estacionales y el síndrome de Guillain‐Barré ni de la vacuna pandémica H1N1 y el síndrome de Guillain‐Barré.
No hubo pruebas de una asociación entre la exposición a la vacuna inactivada estacional contra la gripe y otros eventos adversos graves (esclerosis múltiple, neuritis óptica y púrpura trombocitopénica inmune).
Compleción y aplicabilidad general de las pruebas
Se deben tener en cuenta varias cuestiones al interpretar los resultados de esta revisión.
-
Los métodos de estandarización de vacunas han cambiado significativamente.
-
Las vacunas recientes presentan diferencias significativas en cuanto a su pureza en comparación con las más antiguas.
-
Se combinaron diferentes dosis y esquemas en el análisis.
Esta revisión indica que según las pruebas aleatorias, las vacunas inactivadas tienen un efecto pequeño para prevenir los síntomas de la gripe y lograr que las personas retornen al trabajo más rápidamente.
Calidad de la evidencia
Se encontraron pruebas de más de 70 000 personas en 69 estudios aleatorios. Independientemente de la calidad, ningún estudio logró informar pruebas del efecto sobre las complicaciones. La base de pruebas de seguridad de los ensayos aleatorios de vacunas inactivadas es muy pequeña, lo que probablemente indica menos preocupación por los efectos perjudiciales. Las vacunas inactivadas provocan efectos perjudiciales importantes poco frecuentes que parecen estar vinculados principalmente con productos o lotes específicos.
Sesgos potenciales en el proceso de revisión
Las conclusiones de esta revisión son inciertas con respecto al perfil de seguridad de las vacunas inactivadas, que es un reflejo del tamaño de la base de pruebas.
Una revisión anterior de 274 estudios de vacunas contra la gripe en todos los grupos etarios (que incluyó la mayoría de los estudios de esta revisión) mostró una relación inversa entre el riesgo de sesgo y la dirección de las conclusiones de los estudios. Las conclusiones favorables al uso de vacunas contra la gripe se asociaron con un mayor riesgo de sesgo. En estos estudios, los autores hicieron afirmaciones y establecieron conclusiones que no fueron apoyadas por los datos que presentaron. Además, los estudios patrocinados por la industria tienen mayores probabilidades de establecer conclusiones favorables para ser publicados en revistas con un factor impacto significativamente mayor y tener tasas mayores de citas que los estudios no patrocinados por la industria. Esta diferencia no se explica por el tamaño ni la calidad metodológica (Jefferson 2009a). Cualquier interpretación del grupo de pruebas de esta revisión debe hacerse con estos resultados en mente.
Acuerdos y desacuerdos con otros estudios o revisiones
Revisiones sistemáticas que hacen una estimación de la eficacia de la vacunación para la gripe
DiazGranados 2012 realizó un metanálisis que incluyó ECA sobre las vacunas inactivadas estacionales o las vacunas de virus vivos atenuados de la gripe, con la gripe confirmada por laboratorio (con confirmación de la infección por la reacción en cadena de la polimerasa [RCP] o serológica) como el resultado de eficacia. Se incluyeron 30 estudios en niños y adultos. Los autores proporcionaron estimaciones de la eficacia (CR con IC del 95%) estratificadas por el grado de compatibilidad entre la vacuna y las cepas circulantes (bueno, pobre, ninguna compatibilidad, compatibilidad) y por tipo de cepa (A H1N1, A H3N2, B). DiazGranados 2012 calculó que en una población adulta, la eficacia de la vacuna inactivada contra la gripe confirmada por laboratorio es del 59% (IC del 95%: 50% a 66%). La estimación de la eficacia de la vacuna de virus vivos atenuados es del 39% (IC del 95%: 16% a 55%).
La revisión sistemática Osterholm 2012 incluye pruebas de la eficacia de las vacunas de virus vivos atenuados y las vacunas inactivadas para prevenir la infección por gripe confirmada por laboratorio, evaluada exclusivamente por la RCP o un cultivo positivo. Al considerar exclusivamente los estudios realizados en adultos, la estimación agrupada de la eficacia de seis estudios (ocho grupos de datos) fue del 59% (IC del 95%: 51% a 67%). Incluso aunque se incluyeron tres ECA que calcularon la eficacia de las vacunas de virus vivos atenuados, los autores no realizaron un análisis debido a que ninguna de las estimaciones únicas fue estadísticamente significativa. También se incluyeron y analizaron los estudios observacionales.
Revisiones sistemáticas que evaluaron la eficacia / efectividad o aspectos de seguridad de las vacunas contra la gripe cuando se administró durante el embarazo
La revisión Skowronski 2009 es la primera publicación exhaustiva que ha analizado con rigor las pruebas de la efectividad y los aspectos de seguridad de la vacunación durante el embarazo. En la primera parte del artículo, los autores consideran la carga de morbilidad durante el embarazo, el riesgo de muerte y el riesgo relacionado con la gripe para el feto, y resumen como han cambiado las recomendaciones del Advisory Committee on Immunization Practice (ACIP) de los EE.UU. durante las últimas cuatro décadas. Las pruebas disponibles sobre la protección (de la madre y el recién nacido) y los aspectos de seguridad de la vacunación se ilustran de forma descriptiva, se analizan y se comparan con las declaraciones de las políticas actuales de vacunación informadas. En opinión de los autores, la inmunización contra la gripe en cualquier estadio del embarazo puede autorizarse durante las pandemias o en las pacientes con comorbilidades. La inmunización estacional con TIV se puede autorizar en el embarazo, sin complicaciones potenciales durante la segunda mitad del embarazo. Finalmente, las pruebas disponibles no son suficientes para recomendar la vacunación sistemática estándar en las primeras etapas del embarazo.
Revisiones sistemáticas de las pruebas de efectos perjudiciales graves
Farez 2011 evalúa el riesgo de desarrollar esclerosis múltiple o presentar esclerosis múltiple recurrente después de la inmunización con algunas vacunaciones, incluida la de la gripe. El metanálisis realizado mediante el agrupamiento de los resultados de cuatro estudios de casos y controles (bb DeStefano 2003; bb Hernan 2004; Ramagopalan 2009; bb Zorzon 2003)excluiría un aumento en el riesgo de desarrollar esclerosis múltiple después de la administración de vacunas contra la gripe (odds ratio [OR] 0,97; IC del 95%: 0,77 a 1,23).
Otras cuestiones
En Toback 2012, hay pruebas que apoyan la introducción de una nueva vacuna cuadrivalente de virus vivos atenuados (Q‐LAIV, ya autorizada en los EE.UU. donde estará disponible para la estación 2013 a 2014) que contiene dos cepas B diferentes de diferente linaje (B/Yamagata/16/88 y B/Victoria/2/87). Estas pruebas provienen de dos ECA que compararon la inmunogenicidad y las reacciones locales y sistémicas después de la administración de las vacunas Q‐LAIV, trivalente inactivada o trivalente de virus vivos atenuados. Uno de ellos se realizó en adultos, el otro en una población pediátrica. La presencia de dos cepas B no afectaría significativamente la respuesta de los anticuerpos contra cada cepa B. Los eventos adversos locales y sistémicos inducidos por la administración de Q‐LAIV no difirieron significativamente de los registrados después de recibir otras vacunas ya en uso.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 2 Influenza.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 3 Physician visits.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 4 Days ill.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 5 Times any drugs were prescribed.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 6 Times antibiotic was prescribed.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 7 Working days lost.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 8 Hospitalisations.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 9 Clinical cases (clinically defined without clear definition).

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 10 Local harms.

Comparison 1 Inactivated parenteral vaccine versus placebo or 'do nothing', Outcome 11 Systemic harms.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza‐like illness.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 2 Influenza.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 3 Influenza cases (clinically defined without clear definition).
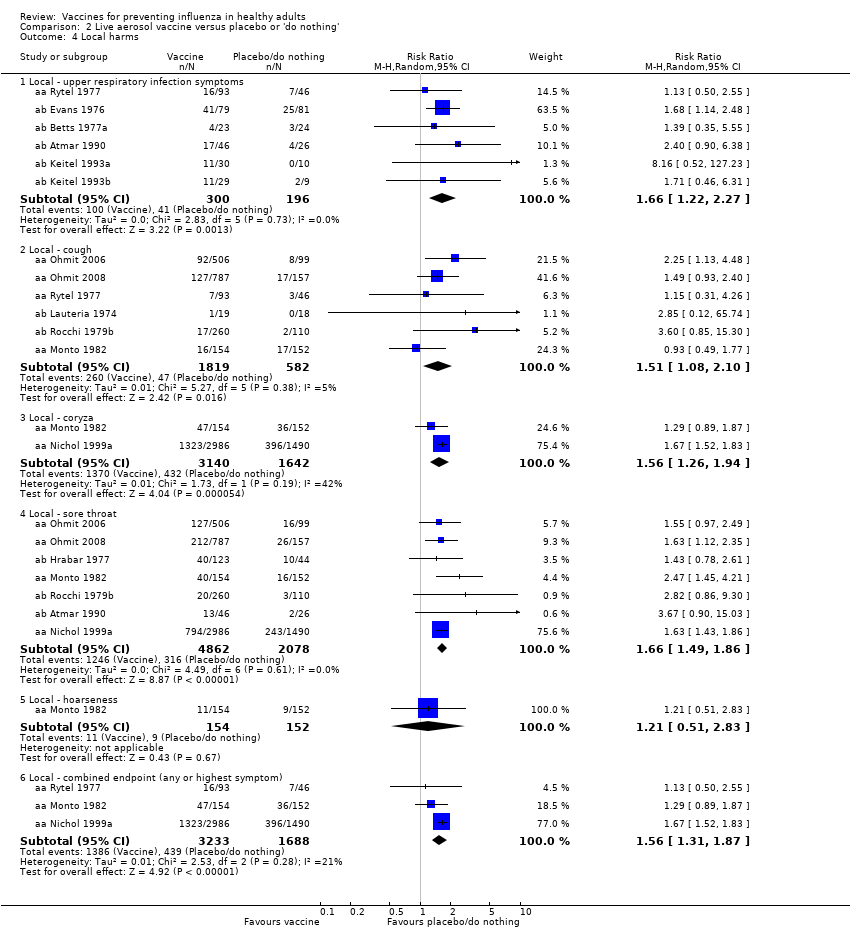
Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 4 Local harms.

Comparison 2 Live aerosol vaccine versus placebo or 'do nothing', Outcome 5 Systemic harms.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 1 Influenza.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 2 Local harms.

Comparison 3 Inactivated aerosol vaccine versus placebo or 'do nothing', Outcome 3 Systemic harms.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.

Comparison 4 Inactivated parenteral vaccine versus placebo ‐ cohort studies, Outcome 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women.

Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 1 Effectiveness in newborns ‐ pregnant women (adjusted data).

Comparison 5 Inactivated parenteral vaccine versus placebo ‐ case‐control, Outcome 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data).

Comparison 6 Serious adverse events ‐ Guillain‐Barré syndrome ‐ cohort studies, Outcome 1 Seasonal influenza vaccination and Guillain‐Barré syndrome.

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data).

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data).

Comparison 7 Serious adverse events ‐ Guillain‐Barré syndrome ‐ case‐control, Outcome 3 Seasonal influenza vaccination general population (adjusted data).

Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data).

Comparison 8 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ cohort studies, Outcome 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data).

Comparison 9 Serious adverse events ‐ demyelinating diseases (multiple sclerosis, optic neuritis) ‐ case‐control studies, Outcome 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data).

Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 1 Seasonal influenza vaccine ‐ HR (adjusted data).

Comparison 10 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ cohort studies, Outcome 2 Seasonal influenza vaccine (unadjusted data).

Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 1 Seasonal influenza vaccine ‐ general population (adjusted data).

Comparison 11 Serious adverse events ‐ immune thrombocytopaenic purpura ‐ case‐control studies, Outcome 2 Seasonal influenza vaccine ‐ general population (unadjusted data).

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 2 Influenza.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 12 1968 to 1969 pandemic: inactivated polyvalent parenteral vaccine versus placebo, Outcome 4 Pneumonia.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 2 Influenza.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 3 Hospitalisations.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 4 Pneumonia.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 5 Working days lost.

Comparison 13 1968 to 1969 pandemic: inactivated monovalent parenteral vaccine versus placebo, Outcome 6 Days ill.

Comparison 14 1968 to 1969 pandemic: inactivated polyvalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 15 1968 to 1969 pandemic: inactivated monovalent aerosol vaccine versus placebo, Outcome 1 Influenza‐like illness.

Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 1 Influenza cases (clinically defined without clear definition).

Comparison 16 1968 to 1969 pandemic: live aerosol vaccine versus placebo, Outcome 2 Complications (bronchitis, otitis, pneumonia).
| Study design | High risk | Low risk | Unclear risk | Total |
| Case‐control | 3 | 2 | 15 | 20 |
| Cohort | 14 | 0 | 13 | 27 |
| RCT/CCT | 6 | 9 | 54 | 69 |
| Total | 23 | 11 | 82 | 116 |
| Study design | Government, institutional or public | Industry | Mixed | Total |
| Case‐control | 13 | 1 | 1 | 15 |
| Cohort | 22 | 3 | 2 | 27 |
| RCT/CCT | 31 | 12 | 5 | 48 |
| Total | 66 | 16 | 8 | 90 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 16 | 25795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.78, 0.87] |
| 1.1 WHO recommended ‐ matching vaccine | 7 | 4760 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.89] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 20942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.75, 0.90] |
| 1.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.70] |
| 1.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.30] |
| 2 Influenza Show forest plot | 22 | 51724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.33, 0.44] |
| 2.1 WHO recommended ‐ matching vaccine | 12 | 26947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.31, 0.45] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 7 | 15068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.35, 0.56] |
| 2.3 Monovalent not WHO recommended ‐ vaccine matching | 2 | 9675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.54] |
| 2.4 Monovalent not WHO recommended ‐ vaccine matching ‐ high dose | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.00, 2.49] |
| 3 Physician visits Show forest plot | 2 | 2308 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.40, 1.89] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.90, 1.83] |
| 4 Days ill Show forest plot | 3 | 3133 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.98, 0.56] |
| 4.1 WHO recommended ‐ matching vaccine | 2 | 2003 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.85, ‐0.32] |
| 4.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.66 [0.16, 1.16] |
| 5 Times any drugs were prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 5.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.04, ‐0.00] |
| 5.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.00, 0.00] |
| 6 Times antibiotic was prescribed Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.03, ‐0.01] |
| 6.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 7 Working days lost Show forest plot | 4 | 3726 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 7.1 WHO recommended ‐ matching vaccine | 3 | 2596 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.19, 0.02] |
| 7.2 WHO recommended ‐ matching absent or unknown | 1 | 1130 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.00, 0.18] |
| 8 Hospitalisations Show forest plot | 3 | 11924 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 8.1 WHO recommended ‐ matching vaccine | 1 | 1178 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1130 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [0.12, 70.68] |
| 8.3 Monovalent not WHO recommended ‐ vaccine matching | 1 | 9616 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 9 Clinical cases (clinically defined without clear definition) Show forest plot | 3 | 4259 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 9.1 WHO recommended ‐ matching vaccine | 2 | 2056 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.25] |
| 9.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2203 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.99] |
| 10 Local harms Show forest plot | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Local ‐ tenderness/soreness | 20 | 35655 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [2.44, 4.02] |
| 10.2 Local ‐ erythema | 9 | 29499 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.77, 3.78] |
| 10.3 Local ‐ induration | 3 | 7786 | Risk Ratio (M‐H, Random, 95% CI) | 4.28 [1.25, 14.67] |
| 10.4 Local ‐ arm stiffness | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.54, 4.83] |
| 10.5 Local ‐ combined endpoint (any or highest symptom) | 11 | 12307 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [1.82, 3.28] |
| 11 Systemic harms Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Systemic ‐ myalgia | 10 | 30360 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.40, 2.24] |
| 11.2 Systemic ‐ fever | 12 | 19202 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.22, 1.95] |
| 11.3 Systemic ‐ headache | 13 | 31351 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.36] |
| 11.4 Systemic ‐ fatigue or indisposition | 11 | 31140 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.07, 1.42] |
| 11.5 Systemic ‐ nausea/vomiting | 3 | 1667 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [0.55, 13.08] |
| 11.6 Systemic ‐ malaise | 3 | 26111 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.18, 1.92] |
| 11.7 Systemic ‐ combined endpoint (any or highest symptom) | 6 | 2128 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 6 | 12688 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.84, 0.96] |
| 1.1 WHO recommended ‐ matching vaccine | 2 | 4254 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.12] |
| 1.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 8150 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.82, 0.97] |
| 1.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 284 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 2 Influenza Show forest plot | 9 | 11579 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.35, 0.62] |
| 2.1 WHO recommended ‐ matching vaccine | 4 | 6584 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.37, 0.82] |
| 2.2 WHO recommended ‐ vaccine matching absent or unknown | 3 | 4568 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| 2.3 Non WHO recommended ‐ vaccine matching absent or unknown | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 3 Influenza cases (clinically defined without clear definition) Show forest plot | 3 | 23900 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.11] |
| 3.1 WHO recommended ‐ matching vaccine | 1 | 1931 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.49, 0.80] |
| 3.2 WHO recommended ‐ vaccine matching absent or unknown | 1 | 2082 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 3.3 Non WHO recommended ‐ vaccine matching absent or unknown | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4 Local harms Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Local ‐ upper respiratory infection symptoms | 6 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.22, 2.27] |
| 4.2 Local ‐ cough | 6 | 2401 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.08, 2.10] |
| 4.3 Local ‐ coryza | 2 | 4782 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.94] |
| 4.4 Local ‐ sore throat | 7 | 6940 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.49, 1.86] |
| 4.5 Local ‐ hoarseness | 1 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.51, 2.83] |
| 4.6 Local ‐ combined endpoint (any or highest symptom) | 3 | 4921 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.31, 1.87] |
| 5 Systemic harms Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Systemic ‐ myalgia | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [1.26, 4.85] |
| 5.2 Systemic ‐ fever | 4 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.92] |
| 5.3 Systemic ‐ fatigue or indisposition | 3 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.93, 2.07] |
| 5.4 Systemic ‐ headache | 2 | 975 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.09, 2.18] |
| 5.5 Systemic ‐ combined endpoint (any or highest symptom) | 5 | 1018 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.82, 2.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza Show forest plot | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.1 WHO recommended ‐ vaccine matching absent or unknown | 1 | 1348 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.14, 1.02] |
| 1.2 WHO recommended ‐ matching vaccine | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Local harms Show forest plot | 3 | 1578 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.27] |
| 2.1 Local ‐ sore throat | 3 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 2.2 Local ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.71, 1.48] |
| 3 Systemic harms Show forest plot | 3 | 1880 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.71, 1.62] |
| 3.1 Systemic ‐ myalgia | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.36, 2.25] |
| 3.2 Systemic ‐ fatigue or indisposition | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.52, 3.75] |
| 3.3 Systemic ‐ headache | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.85, 2.72] |
| 3.4 Systemic ‐ fever | 1 | 1349 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.03, 7.80] |
| 3.5 Systemic ‐ combined endpoint (any or highest symptom) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal inactivated vaccine effectiveness in mothers ‐ pregnant women Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 H1N1 ‐ vaccine ‐ effectiveness ILI (unadjusted data) | 1 | 7328 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.21] |
| 1.2 Seasonal ‐ vaccine ‐ effectiveness ILI ‐ (unadjusted data) | 2 | 50129 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.22, 1.32] |
| 2 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Seasonal vaccine effectiveness ILI (HR adjusted data) | 2 | Hazard Ratio (Random, 95% CI) | 0.96 [0.90, 1.03] | |
| 3 Seasonal inactivated vaccine effectiveness in newborns ‐ pregnant women Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Seasonal vaccine effectiveness ILI (RR adjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.92 [0.73, 1.16] | |
| 3.2 Seasonal vaccine efficacy influenza ‐ laboratory‐confirmed | 1 | Risk Ratio (Random, 95% CI) | 0.59 [0.37, 0.94] | |
| 4 H1N1 vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 9 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Abortion (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 0.75 [0.62, 0.90] | |
| 4.2 Abortion (HR ‐ adjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.88 [0.67, 1.16] | |
| 4.3 Congenital malformation (OR ‐ adjusted data) | 5 | Odds Ratio (Random, 95% CI) | 1.06 [0.90, 1.25] | |
| 4.4 Prematurity (< 37 weeks) (OR adjusted data) | 8 | Odds Ratio (Random, 95% CI) | 0.86 [0.76, 0.97] | |
| 4.5 Neonatal death (OR adjusted data) | 1 | Odds Ratio (Random, 95% CI) | 1.81 [0.16, 20.35] | |
| 5 Seasonal vaccine ‐ safety ‐ pregnancy‐related outcomes ‐ pregnant women Show forest plot | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Abortion (OR ‐ unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.60 [0.41, 0.86] | |
| 5.2 Congenital malformation (OR unadjusted data) | 2 | Odds Ratio (Random, 95% CI) | 0.55 [0.08, 3.73] | |
| 5.3 Prematurity (OR unadjusted data) | 4 | Odds Ratio (Random, 95% CI) | 0.96 [0.79, 1.17] | |
| 5.4 Neonatal death (OR unadjusted data) | 1 | Odds Ratio (Random, 95% CI) | 0.55 [0.35, 0.88] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Effectiveness in newborns ‐ pregnant women (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 1.1 Seasonal vaccine ‐ effectiveness ‐ ILI ‐ pregnant women | 2 | Odds Ratio (Random, 95% CI) | 0.24 [0.04, 1.40] | |
| 2 Seasonal vaccine safety ‐ pregnancy‐related outcomes (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| 2.1 Abortion | 1 | Odds Ratio (Random, 95% CI) | 0.80 [0.36, 1.78] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccination and Guillain‐Barré syndrome Show forest plot | 3 | Risk Ratio (Random, 95% CI) | 1.28 [0.85, 1.93] | |
| 1.1 General population (adjusted data) | 2 | Risk Ratio (Random, 95% CI) | 1.29 [0.83, 2.02] | |
| 1.2 Pregnant women (unadjusted data) | 1 | Risk Ratio (Random, 95% CI) | 0.65 [0.03, 15.95] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 2009 to 2010 A/H1N1 ‐ general population (unadjusted data) Show forest plot | 6 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 7 weeks | 6 | 1528 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [1.14, 4.31] |
| 1.2 At any time | 6 | 1656 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.87, 3.29] |
| 2 2009 to 2010 A/H1N1 ‐ general population (adjusted data) Show forest plot | 4 | Odds Ratio (Random, 95% CI) | 0.83 [0.39, 1.75] | |
| 2.1 < 7 weeks | 4 | Odds Ratio (Random, 95% CI) | 0.92 [0.35, 2.40] | |
| 2.2 > 6 weeks | 3 | Odds Ratio (Random, 95% CI) | 0.71 [0.22, 2.32] | |
| 3 Seasonal influenza vaccination general population (adjusted data) Show forest plot | 1 | Odds Ratio (Random, 95% CI) | 1.38 [0.18, 10.43] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ demyelinating diseases (unadjusted data) Show forest plot | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 1.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.16 [0.02, 1.25] |
| 2 Influenza vaccination (H1N1) ‐ demyelinating diseases (unadjusted) Show forest plot | 1 | 144252 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.51, 8.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza vaccination (seasonal) ‐ general population ‐ demyelinating diseases (unadjusted data) Show forest plot | 4 | 8009 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.79, 1.17] |
| 2 Influenza vaccination (seasonal) ‐ general population ‐ multiple sclerosis (adjusted data) Show forest plot | 2 | (Random, 95% CI) | 0.76 [0.54, 1.08] | |
| 3 Influenza vaccination (seasonal) ‐ general population ‐ optic neuritis (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | 1.03 [0.82, 1.30] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ HR (adjusted data) Show forest plot | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 General population | 0 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pregnant women | 1 | Hazard Ratio (Random, 95% CI) | 0.90 [0.68, 1.19] | |
| 2 Seasonal influenza vaccine (unadjusted data) Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 General population | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Pregnant women | 1 | 223898 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.70, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seasonal influenza vaccine ‐ general population (adjusted data) Show forest plot | 2 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 < 2 months | 2 | Odds Ratio (Random, 95% CI) | 1.87 [0.43, 8.06] | |
| 1.2 < 6 months | 1 | Odds Ratio (Random, 95% CI) | 0.90 [0.55, 1.47] | |
| 1.3 < 12 months | 1 | Odds Ratio (Random, 95% CI) | 0.70 [0.47, 1.04] | |
| 2 Seasonal influenza vaccine ‐ general population (unadjusted data) Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2 months | 2 | 1926 | Odds Ratio (M‐H, Random, 95% CI) | 1.72 [0.48, 6.15] |
| 2.2 < 6 months | 1 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.59, 1.43] |
| 2.3 < 12 months | 1 | 1066 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 3 | 3065 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.88] |
| 1.1 Standard recommended parenteral ‐ non‐matching ‐ 1 dose | 3 | 2715 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.57, 0.95] |
| 1.2 Standard recommended parenteral ‐ non‐matching ‐ 2 doses | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.44, 0.98] |
| 2 Influenza Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 2.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.26, 0.87] |
| 3 Hospitalisations Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 3.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.41, 1.68] |
| 4 Pneumonia Show forest plot | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| 4.1 Standard recommended parenteral ‐ non‐matching | 1 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.14, 7.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 4 | 4580 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.25, 0.48] |
| 1.1 WHO recommended parenteral ‐ matching vaccine ‐ 1 dose | 4 | 4226 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.53] |
| 1.2 WHO recommended parenteral ‐ matching vaccine ‐ 2 doses | 1 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.22, 0.57] |
| 2 Influenza Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 2.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.31] |
| 3 Hospitalisations Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 3.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.13, 0.94] |
| 4 Pneumonia Show forest plot | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 4.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.05, 6.51] |
| 5 Working days lost Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 5.1 WHO recommended parenteral ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6 Days ill Show forest plot | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| 6.1 WHO recommended ‐ matching vaccine | 1 | 1667 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.60, ‐0.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 1.1 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 1 dose | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.27] |
| 1.2 Inactivated polyvalent aerosol vaccine versus placebo ‐ non‐matching ‐ 2 doses | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza‐like illness Show forest plot | 2 | 1009 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.32, 0.91] |
| 1.1 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 1 dose | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.41] |
| 1.2 Inactivated monovalent aerosol vaccine versus placebo ‐ matching ‐ 2 doses | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Influenza cases (clinically defined without clear definition) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 1.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 2 Complications (bronchitis, otitis, pneumonia) Show forest plot | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |
| 2.1 Non‐matching | 1 | 19887 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.03, 2.24] |

