Fármacos catecolaminérgicos no antipsicóticos para la discinesia tardía inducida por antipsicóticos
Resumen
Antecedentes
La discinesia tardía (DT) es un trastorno de movimiento incapacitante asociado al uso prolongado de fármacos antipsicóticos. Se han examinado varias estrategias en el tratamiento de la DT. Sin embargo, en la actualidad no hay evidencia clara de la efectividad de estos fármacos en la DT y se han asociado con muchos efectos secundarios. Una estrategia particular sería utilizar agentes farmacéuticos que se sabe que influyen en el sistema catecolaminérgico en diversas coyunturas.
Objetivos
1. Determinar los efectos de cualquiera de los siguientes fármacos para la DT inducida por antipsicóticos en pacientes con esquizofrenia u otras enfermedades mentales crónicas.
i. Fármacos que influyen en el sistema noradrenérgico.
ii. Agonistas de los receptores de dopamina.
iii. Antagonistas de los receptores de dopamina.
iv. Fármacos que provocan depleción de la dopamina.
v. Fármacos que aumentan la producción o la liberación de dopamina.
2. Examinar si aparecía alguna mejoría con períodos cortos de intervención (menos de seis semanas) y, en caso de que esto sucediera, si este efecto se mantuvo durante períodos de seguimiento más prolongados.
3. Examinar si existía un efecto diferencial entre los diversos componentes.
4. Examinar si el uso de los fármacos catecolaminérgicos no antipsicóticos es más eficaz en los pacientes que presentan DT de aparición más reciente (menos de cinco años).
Métodos de búsqueda
Se recuperaron 712 referencias a partir de las búsquedas en el Registro de ensayos del Grupo Cochrane de Esquizofrenia (Cochrane Schizophrenia Group) (julio de 2015 y abril de 2017). Además, se examinaron las referencias de todos los estudios identificados para obtener ensayos adicionales y se estableció contacto con autores de ensayos para obtener más información.
Criterios de selección
Los estudios se seleccionaron si eran ensayos controlados aleatorizados centrados en pacientes con esquizofrenia u otras enfermedades mentales crónicas y discinesia tardía inducida por antipsicóticos. Se comparó el uso de intervenciones con catecolaminérgicos versus placebo, ninguna intervención o cualquier otra intervención para el tratamiento de la discinesia tardía inducida por antipsicóticos.
Obtención y análisis de los datos
Se extrajeron de forma independiente los datos de los ensayos incluidos y se calcularon los riesgos relativos (RR) con intervalos de confianza (IC) del 95%. Se asumió que los pacientes que abandonaron los estudios de manera temprana no presentaron mejoría.
Resultados principales
Se incluyeron diez ensayos (n = 261) publicados entre 1973 y 2010; ocho son nuevos a partir de las búsquedas de actualización de 2015 y 2017. Se excluyen 48 estudios. Los participantes fueron en su mayoría pacientes de 50 años de edad hospitalizados con enfermedades mentales crónicas y los estudios fueron principalmente de corta duración (dos a seis semanas). El riesgo general de sesgo en estos estudios no estuvo claro, principalmente debido a la escasa información sobre la ocultación de la asignación y la generación de la secuencia. Los estudios tampoco fueron claramente cegados y no hay seguridad con respecto a si los datos están incompletos o se informaron de manera selectiva o si hubo otros sesgos.
Un pequeño ensayo de tres brazos encontró que la alfametildopa (n = 20; RR 0,33; IC del 95%: 0,14 a 0,80; evidencia de calidad baja) y la reserpina (n = 20; RR 0,52; IC del 95%: 0,29 a 0.96; evidencia de calidad baja) pueden dar lugar a una mejoría clínicamente importante de los síntomas de discinesia tardía en comparación con placebo después de dos semanas de tratamiento, pero no se encontró evidencia de una diferencia entre la alfametildopa y la reserpina (n = 20; RR 0,60; IC del 95%: 0,19 a 1,86; evidencia de calidad muy baja). Otro ensayo pequeño comparó la tetrabenazina y el haloperidol después de 18 semanas de tratamiento y no encontró evidencia de una diferencia en la mejoría clínicamente importante de los síntomas de discinesia tardía (n = 13; RR 0,93; IC del 95%: 0,45 a 1,95; evidencia de calidad muy baja). Ningún estudio informó sobre los efectos adversos.
Para los resultados restantes no hubo evidencia de una diferencia entre ninguna de las intervenciones: alfametildopa versus placebo para el deterioro de los síntomas de discinesia tardía (un ECA; n = 20; RR 0,33; IC del 95%: 0,02 a 7,32; evidencia de calidad muy baja), celiprolol versus placebo para el abandono temprano del estudio (un ECA; n = 35; RR 5,28; IC del 95%: 0,27 a 102.58; evidencia de calidad muy baja) y la calidad de vida (un ECA; n = 35; RR 0,87; IC del 95%: 0,68 a 1,12; evidencia de calidad muy baja), alfametildopa versus reserpina para el deterioro de los síntomas de discinesia tardía (un ECA; n = 20; no estimable, ningún evento informado; evidencia de calidad muy baja), reserpina o carbidopa/levodopa versus placebo para el deterioro de los síntomas de discinesia tardía (dos ECA; n = 37; RR 1,18; IC del 95%: 0,35 a 3,99; evidencia de calidad muy baja), oxipertina versus placebo para el deterioro del estado mental (un ECA; n = 42; RR 2,20; IC del 95%: 0,22 a 22,45; evidencia de calidad muy baja), fármacos dopaminérgicos (amantadina, bromocriptina, tiapride, oxipertina, carbidopa/levodopa) versus placebo para el abandono temprano del estudio (seis ECA; n = 163; RR 1,29; IC del 95%: 0,65 a 2,54; evidencia de calidad muy baja), y tetrabenazina versus haloperidol para el deterioro de los síntomas de discinesia tardía (un ECA; n = 13; RR 1,17; IC del 95%: 0,09 a 14,92) y el abandono temprano del estudio (un ECA; n = 13; RR 0,23; IC del 95%: 0,01 a 4,00).
Conclusiones de los autores
Aunque se ha realizado una gran cantidad de estudios de investigación en esta área, muchos estudios se excluyeron debido a problemas inherentes a la naturaleza de sus diseños cruzados. Por lo general no se informan los datos antes del cruzamiento y la naturaleza de la DT y su probable respuesta a los tratamientos hacen que sea poco prudente utilizar estos datos. La revisión proporciona poca información útil para los usuarios de los servicios o los profesionales y se indica la realización de más estudios bien diseñados y bien informados.
PICOs
Resumen en términos sencillos
Fármacos catecolaminérgicos no antipsicóticos para la discinesia tardía inducida por antipsicóticos
Pregunta de la revisión.
Determinar si los fármacos catecolaminérgicos ayudan en el tratamiento de la discinesia tardía en pacientes con esquizofrenia o problemas de salud mental similares.
Antecedentes.
Los pacientes con esquizofrenia a menudo oyen voces y ven cosas (alucinaciones) y tienen creencias extrañas (delirios). El tratamiento principal para la esquizofrenia son los fármacos antipsicóticos. Sin embargo, estos fármacos pueden tener efectos secundarios debilitantes. La discinesia tardía es un movimiento involuntario que causa que la cara, la boca, la lengua y la mandíbula presenten convulsiones, espasmos y muecas. Es causada por el uso a largo plazo o las dosis altas de los fármacos antipsicóticos, es difícil de tratar y puede ser incurable. Un tratamiento indicado es el uso de fármacos que afectan al sistema catecolaminérgico, que es un grupo de sustancias químicas del cerebro.
Características de los estudios.
La revisión incluye 10 pequeños estudios breves publicados principalmente en la década de 1980, en los que participaron un total de 261 pacientes.
Resultados clave.
Un pequeño estudio encontró que después de dos semanas de tratamiento tanto la alfametildopa como la reserpina pueden producir una mejora clínicamente importante de los síntomas de discinesia tardía en comparación con el placebo, pero la calidad de la evidencia era baja. No existe seguridad acerca del efecto de la reserpina frente a la alfametildopa; la calidad de la evidencia fue muy baja. Otro pequeño ensayo comparó la tetrabenazina y el haloperidol después de 18 semanas de tratamiento, pero una vez más, no se sabe con certeza cuál es el efecto, ya que la calidad de la evidencia es muy baja. Los estudios incluidos no informaron sobre efectos perjudiciales de los fármacos.
Calidad de la evidencia.
La evidencia es débil, limitada, a corto plazo y a pequeña escala. No es posible recomendar estos fármacos como tratamiento para la discinesia tardía en base a los hallazgos. Se necesitan estudios de investigación más amplios y rigurosos en el área.
Este resumen en términos sencillos fue adaptado por los autores de la revisión a partir de un resumen escrito originalmente por Ben Gray, Investigador Superior Externo, McPin Foundation (mcpin.org/).
Authors' conclusions
Summary of findings
| NORADRENERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 330 per 1000 | RR 0.33 | 20 | ⊕⊕⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Tardive dyskinesia: deterioration follow‐up: 2 weeks | 100 per 1000 | 33 per 1000 | RR 0.33 | 20 | ⊕⊝⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Mental state ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 13 weeks | 0 per 1000 | 0 per 1000 | RR 5.28 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| No improvement in quality of life follow‐up: 13 weeks | 944 per 1000 | 822 per 1000 | RR 0.87 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| NORADRENERGIC DRUGS compared to DOPAMINERGIC DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with DOPAMINERGIC DRUGS | Risk with NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | Study population | RR 0.60 | 20 | ⊕⊝⊝⊝ | ||
| 500 per 1,000 | 300 per 1,000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 2 weeks | Study population | not estimable | 20 | ⊕⊝⊝⊝ | Among the 20 participants no events were reported. | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| DOPAMINERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 520 per 1000 | RR 0.52 | 20 | ⊕⊕⊝⊝ | The included study evaluated reserpine. |
| Tardive dyskinesia: Deterioration follow‐up: 2‐6 weeks | 167 per 1000 | 197 per 1000 | RR 1.18 | 37 | ⊕⊝⊝⊝ | The included studies evaluated reserpine and carbidopa/levodopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| General mental state: Deterioration follow‐up: 24 weeks | 45 per 1000 | 100 per 1000 | RR 2.2 | 42 | ⊕⊝⊝⊝ | The included study evaluated oxypertine. |
| Acceptability of treatment: Leaving the study early follow‐up: 2‐24 weeks | 111 per 1000 | 143 per 1000 | RR 1.29 | 163 | ⊕⊝⊝⊝ | Only two studies (59 participants) evaluating carbidopa/levodopa and oxypertine reported any events for this outcome. 4 studies evaluating amantadine, bromocriptine, and tiapride reported no events and consequently no estimates could be made for these 3 compounds. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | This outcome was designated to be of importance, especially to patients. We found no studies rating this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| DOPAMINERGIC DRUGS compared to OTHER DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER DRUGS | Risk with DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 18 weeks | Study population | RR 0.93 | 13 | ⊕⊝⊝⊝ | ||
| 714 per 1000 | 664 per 1000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 18 weeks | Study population | RR 1.17 | 13 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 167 per 1000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 18 weeks | Study population | RR 0.23 | 13 | ⊕⊝⊝⊝ | ||
| 286 per 1000 | 66 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
Background
Description of the condition
Since the 1950s, antipsychotic (or neuroleptic) medication has been extensively used to treat people with chronic mental illnesses, such as schizophrenia. These drugs can effectively control symptoms such as abnormal perceptions (hallucinations), disordered thinking and fixed false beliefs (delusions). In addition, maintenance therapy with antipsychotics is associated with a reduced risk of relapses (Schooler 1993). Antipsychotic medication, however, has also been associated with a wide range of adverse effects, including movement disorders. The appearance of these movement disorders can contribute to poor compliance with antipsychotic treatment and hence relapse (Barnes 1993).
Tardive dyskinesia (TD) is one such movement disorder and is characterised by abnormal, repetitive and involuntary movements (APA 1992). The clinical features include tongue protrusion, side‐to‐side or rotatory movement of the jaw, lip smacking, puckering and pursing, and rapid eye blinking (Casey 1994). In some people rapid movements of the arms, legs, and trunk may also occur. TD is a chronic condition of insidious onset, the severity of which spontaneously fluctuates (APA 1992). Studies on the natural history of tardive dyskinesia have reported widely variable remission rates (1% to 62%) depending on patient age, psychiatric diagnosis, course of the psychiatric disorder, and duration of therapy (Bergen 1989; Fernandez 2001; Glazer 1990).
Although the most frequent cause of TD is the use of antipsychotic medication, it is clinically striking that dose reduction can lead to a temporary exacerbation in symptoms. Conversely, increasing the dose is often associated with a temporary remission (Cavallaro 1993; Smith 1980). The exact mechanisms of the pathophysiology of TD are unknown. Antipsychotic drugs block certain chemical receptor sites in the brain — one of these is specific for dopamine (Casey 1994). One hypothesis explaining the cause of antipsychotic‐induced TD is that chronic blockade of dopamine receptors in specific cells of the brain (neurones from the nigrostriatum) causes an overgrowth of these receptors (Casey 1994). There is also suggestion that the chronic use of antipsychotics may also cause an abnormal production of highly active atoms and chemical groups (cytotoxic free radicals), which may damage specific cells in the brain. This, in turn, could be responsible for the appearance of TD (Cadet 1989; Sachdev 2000).
TD occurs in more than 20% of those using antipsychotic medication continually for longer than three months (Glazer 2000; Kane 1982; Tarsy 2011). Every year 4% to 5% of adults and 25% to 30% of elderly persons who continually use these drugs begin to show signs of TD (APA 1992; Correll 2004). Advancing age is a risk factor for both TD's prevalence and severity, with those who are under 60 years of age being three times more likely to spontaneously remit (Jeste 2000; Smith 1980).
The prevalence of tardive dyskinesia is often thought to be decreasing based on the use of atypical antipsychotics in place of typical antipsychotics (Cloud 2014). A systematic review found that the incidence of tardive dyskinesia associated with atypical drugs (2% to 4%) was significantly lower than that for typicals (5% to 8%) (Correll 2008). Despite this, the widespread use of atypical drugs in clinical settings, increased off‐label use, and an ageing population may still result in an overall increase in the number of cases of TD (Cloud 2014; Glazer 2000; Maher 2012). The problem will be considerably greater for people in countries where use of newer drugs is less prevalent (Ballesteros 2000; Martins 2011).
Description of the intervention
Catecholamines occur naturally in the body. They are synthesised from the amino acid tyrosine, and examples include epinephrine (adrenaline), norepinephrine and dopamine. There are several pharmaceutical compounds acting as catecholamine analogues that have been tested as treatment for tardive dyskinesia, especially during the 1980s (Jeste 1988). This review will present data on pharmaceutical compounds affecting catecholamiergic pathways in different ways.
The catecholaminergic systems involve a complex cascade of steps that can be modified by pharmaceutical compounds at various junctures. Several strategies have been examined in the treatment of TD. These include: i. increasing the presynaptic release of dopamine (e.g. amantadine); ii. increasing the production of dopamine (e.g. L‐dopa); iii. dopamine receptor antagonists (e.g. alpha‐methyl‐paratyrosine (AMTP)); iv. dopamine receptor agonists (e.g. apomorphine); v. agents that deplete dopamine (e.g. tetrabenazine); vi. agents that block the beta‐adrenergic receptors (e.g. propanolol); vii. agents that act as 'false neurotransmitters' (e.g. methyldopa) (see Types of interventions for the full list of drugs).
How the intervention might work
One of the most influential theories to explain the appearance of TD suggests that long‐term use of antipsychotic medication leads to an increase in the number of dopamine and dopamine‐related receptors. This hypothesis is usually referred to as the dopamine supersensitivity theory (Browne 1986b; Casey 1994); and hence drugs that influence the catecholaminergic (noradrenergic and dopaminergic) function in the extrapyramidal system have been used as treatments for antipsychotic‐induced TD. It was thought that these compounds could reverse dopamine supersensitivity by increasing the levels of available dopamine, thus overcoming the antipsychotic‐induced dopamine blockade (Friedhoff 1977).
Currently, however, there is no clear evidence of the effectiveness of these drugs in treating TD. Nevertheless, they have been associated with many side effects, including drowsiness, confusion, postural hypotension, depression and worsening of psychosis (Turjanski 2005). In addition, an excess of dopamine has itself been associated with movement disorders (choreoathetoid).
Why it is important to do this review
Several atypical antipsychotic drugs have been produced in the last decades that claim to cause less or no TD (Lieberman 1996). These claims may or may not be true, and certainly evidence does point to the fact that thoughtful use of older‐generation drugs is not associated with any more problems of TD than are newer treatments (Chouinard 2008). However, in a global context, it is likely that the less expensive and more familiar drugs — such as chlorpromazine or haloperidol — will continue to be the mainstay of treatment of people with schizophrenia (WHO Essential List 2010). Use of drugs such as these is associated with emergence of TD and, therefore, this condition will remain a problem for years to come.
TD can result in considerable social and physical disability (Barnes 1993); and symptoms are often irreversible (Bergen 1989; Fernandez 2001; Glazer 1990). Additionally, TD is frequently associated with lower quality of life (Ascher‐Svanum 2008); and a greater mortality rate (Chong 2009). Given the high incidence and prevalence of TD among people taking antipsychotic medication, the need for prevention or treatment is clear. Unfortunately, there has been sparse evidence to guide clinicians (NICE 2014; Taylor 2009). Although many treatments have been tested, no one intervention has been shown clearly to be effective. Cessation or reduction of the dose of antipsychotic medication would be the ideal management for TD. In clinical practice this is not always possible, not least because in many individuals such a reduction would lead to relapse. This review focuses on whether the addition of different types of catecholaminergic medications to those already receiving antipsychotic medication is likely to help TD.
This review is one in a series of Cochrane Reviews evaluating treatments for antipsychotic‐induced TD (see Table 1), and is an update of a Cochrane Review first published in 2006 (El‐Sayeh 2006).
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | This review |
| Vitamin E |
Objectives
1. To determine the effects of any of the following drugs for antipsychotic‐induced TD in people with schizophrenia or other chronic mental illnesses.
i. Drugs which influence the noradrenergic system.
ii. Dopamine receptor agonists.
iii. Dopamine receptor antagonists.
iv. Dopamine‐depletor drugs.
v. Drugs that increase the production or release of dopamine.
2. To examine whether any improvement occurred with short periods of intervention (less than 6 weeks) and, if this did occur, whether this effect was maintained at longer periods of follow‐up.
3. To examine if there was a differential effect for the various compounds.
4. To examine whether the use of non‐antipsychotic catecholaminergic drugs are most effective in those with more recent onset TD (less than five years).
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. Where a trial was described as 'double‐blind' but it was implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these 'implied randomisation' studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
People with schizophrenia or any other chronic mental illness, diagnosed by any criteria, irrespective of gender, age or nationality who:
i. required the use of antipsychotics for more than three months;
ii. developed tardive dyskinesia (diagnosed by any criteria) during antipsychotic treatment; and
iii. for whom the dose of antipsychotic medication had been stable for one month or more before the trial (the same applies for those free of antipsychotics).
Types of interventions
A. Noradrenergic drugs
i. Celiprolol, clonidine, disulfiram, fusaric acid, methyldopa, pindolol, propanolol, oxprenolol or yohimbine, compared with placebo or no intervention. For the 2017 update a post hoc decision was made to also include studies evaluating the above‐mentioned noradrenergic drugs compared to any other intervention for the treatment of tardive dyskinesia.
B. Dopaminergic drugs
i. The dopamine receptor agonists (apomorphine, bromocriptine, CF25‐397, dopamine, hydergine, lisuride);
ii. the dopamine receptor antagonists (AMTP, oxiperomide, metoclopramide, papaverine, tiapride);
iii. the dopamine‐depleting drugs (oxypertine, reserpine, tetrabenazine);
iv. drugs that increase the release (amantadine, amphetamine) or production (L‐dopa) of dopamine;
all compared with placebo or no intervention. For the 2017 update a post hoc decision was made to also include studies evaluating the above mentioned dopaminergic drugs compared to any other intervention for the treatment of tardive dyskinesia.
Types of outcome measures
We have defined clinical efficacy as an improvement in the symptoms of TD of more than 50%, on any scale. We grouped outcomes into short term (less than six weeks), medium term (between six weeks and six months) and long term (more than six months).
Primary outcomes
1. Tardive dyskinesia
No clinically important improvement in the symptoms of individuals, defined as more than 50% improvement on any tardive dyskinesia scale ‒ any time period.
2. Adverse effects
No clinically significant extrapyramidal adverse effects ‒ any time period.
Secondary outcomes
1. Tardive dyskinesia (TD)
1.1 Any improvement in the symptoms of individuals on any TD scale, as opposed to no improvement.
1.2 Deterioration in the symptoms of individuals, defined as any deleterious change on any TD scale.
1.3 Average change in severity of TD during the trial period.
1.4 Average difference in severity of TD at the end of the trial.
2. General mental state changes
2.1 Deterioration in general psychiatric symptoms (such as delusions and hallucinations) defined as any deleterious change on any scale.
2.2 Average difference in severity of psychiatric symptoms at the end of the trial.
3. Acceptability of the treatment
3.1 Acceptability of the intervention to the participant group as measured by numbers of people dropping out during the trial.
4. Adverse effects
4.1 Use of any anti‐parkinsonism drugs.
4.2 Average score/change in extrapyramidal adverse effects.
4.3 Acute dystonia.
5. Other adverse effects, general and specific
6. Hospital and service utilisation outcomes
6.1 Hospital admission.
6.2 Average change in days in hospital.
6.3 Improvement in hospital status (for example: change from formal to informal admission status, use of seclusion, level of observation).
7. Economic outcomes
7.1 Average change in total cost of medical and mental health care.
7.2 Total indirect and direct costs.
8. Social confidence, social inclusion, social networks, or personalised quality of life measures
8.1. No significant change in social confidence, social inclusion, social networks, or personalised quality of life measures.
8.2 Average score/change in social confidence, social inclusion, social networks, or personalised quality of life measures.
9. Behaviour
9.1 Clinically significant agitation.
9.2 Use of adjunctive medication for sedation.
9.3 Aggression to self or others.
10. Cognitive state
10.1 No clinically important change.
10.2 No change, general and specific.
'Summary of findings' table
We used the GRADE approach to interpret findings (Schünemann 2011) and used GRADEpro to export data from this review to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effects of interventions examined and the sum of available data on all outcomes rated as important to patient care and decision making. This summary was used to guide our conclusions. We selected the following main outcomes for inclusion in the 'Summary of findings' table.
1. Tardive dyskinesia
1.1 Improved to a clinically important extent
1.2 Deteriorated
2. Mental state
2.1 Deteriorated
3. Adverse effect
3.1 Any adverse event
3.2 Adverse effects: no clinically significant extrapyramidal adverse effects
4. Acceptability of treatment
4.1 Leaving the study early
5. Social confidence, social inclusion, social networks, or personalised quality of life measures*
5.1 No significant change in social confidence, social inclusion, social networks, or personalised quality of life measures for either recipients of care or caregivers
* Outcome designated important to patients. We wished to add perspectives from people’s personal experience with TD to the research agenda. A consultation with service users was planned where the previously published version of another review in the tardive dyskinesia series and a lay overview of that review gave the foundation for the discussions (Soares‐Weiser 2011; Table 1). The session was planned to provide time to reflect on current research on TD and consider gaps in knowledge. The report is published in the Health Technology Assessment (HTA) report for the UK National Institute of Health Research (Appendix 1, Bergman 2017). We have added one figure showing a service user's expression of frustration concerning this neglected area of research (Figure 1). Informed by the results of the consultation, for this review we updated outcomes for the 'Summary of findings' table.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.
Search methods for identification of studies
Electronic searches
The 2017 review update was carried out in parallel with updating eight other TD reviews; see Table 1 for details. The search covered all nine tardive dyskinesia reviews.
1. Cochrane Schizophrenia Group’s Register
We searched Cochrane Schizophrenia Group’s Study‐Based Register of Trials on 16 July 2015 and 26 April 2017 using the following string: *Tardive Dyskinesia* in Healthcare Condition Field of Study. In a study‐based register such as this, searching the major concept retrieves all the synonym keywords and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics. The Cochrane Schizophrenia Group’s Register of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
3. Details of previous electronic searches
See Appendix 1.
Searching other resources
1. Reference searching
We inspected references of all identified studies for further relevant studies.
2. Personal contact
We contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
For the 2017 update, reviewers RA and AG (see Acknowledgements) inspected all abstracts of studies identified as above and identified potentially relevant reports. We resolved disagreement by discussion, or where there was still doubt, we acquired the full article for further inspection. We acquired the full articles of relevant reports/abstracts meeting initial criteria for reassessment and carefully inspected for a final decision on inclusion (see Criteria for considering studies for this review). RA and AG were not blinded to the names of the authors, institutions or journal of publication. Where difficulties or disputes arose, we asked author HB for help and where it was impossible to decide or if adequate information was not available to make a decision, we added these studies to those awaiting assessment and contacted the authors of the papers for clarification.
Data extraction and management
1. Extraction
For the 2017 update, reviewers RA and HB independently extracted data from all included studies. Again, we discussed any disagreement and documented decisions. With remaining problems KSW helped clarify issues and we documented these final decisions. We extracted data presented only in graphs and figures whenever possible, but included only if two reviewers independently had the same result. We attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible we extracted data relevant to each component centre separately.
2. Management
2.1 Forms
For the 2017 update we extracted data online in Covidence. Extracted data are available here with a link to the original source PDF for each item.
2.2 Scale‐derived data
We included continuous data from rating scales only if:
a) the psychometric properties of the measuring instrument have been described in a peer‐reviewed journal (Marshall 2000); and
b) the measuring instrument has not been written or modified by one of the trialists for that particular trial.
Ideally the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly; we noted in Description of studies if this was the case or not.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to primarily use endpoint data, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we preferred to use mean differences (MD) rather than standardised mean differences throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant data before inclusion (see (a), (b) and (c) below).
Please note: we entered data from studies of at least 200 participants in the analysis, because skewed data pose less of a problem in large studies. We also entered all relevant change data as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not.
For endpoint data from studies with fewer than 200 participants:
(a) when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation. If this value was lower than 1, it strongly suggests a skew and we excluded these data. If this ratio was higher than 1 but below 2, there is suggestion of skew. We entered these data and tested whether their inclusion or exclusion changed the results substantially. Finally, if the ratio was larger than 2 we included these data, because skew is less likely (Altman 1996; Higgins 2011).
(b) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) (Kay 1986), which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
2.5 Common measure
Where relevant, to facilitate comparison between trials we converted variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we converted continuous outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this can be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
Assessment of risk of bias in included studies
Reviewers RA (see Acknowledgements) and HB independently assessed risk of bias within the included studies by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess trial quality (Higgins 2011). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, we made the final rating by consensus, with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. If non‐concurrence occurred, we reported this.
We noted the level of risk of bias in the text of the review and in Figure 2, Figure 3, summary of findings Table for the main comparison and summary of findings Table 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
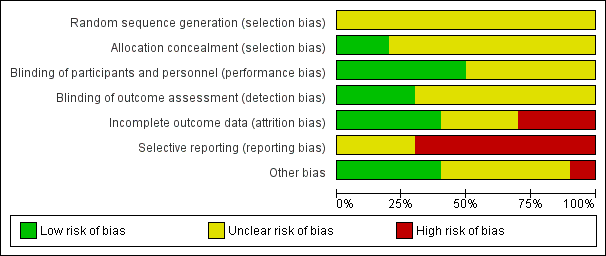
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive than odds ratios (Boissel 1999), as odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes we estimated mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference (SMD)). However, if scales of very considerable similarity were used, we presumed there is a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
If any of the included trials had randomised participants by clusters, and where clustering is not accounted for in primary studies, we would have presented such data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intra‐class correlation coefficients for their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjust for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation coefficient (ICC) (Design effect = 1 + (m − 1) * ICC] (Donner 2002)). If the ICC is not reported it will be assumed to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account intra‐class correlation coefficients and relevant data documented in the report, synthesis with other studies would be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we only used data of the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involves more than two treatment arms, if relevant we presented the additional treatment arms in comparisons. If data were binary we simply added and combined within the two‐by‐two table. If data were continuous we combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not use data where the additional treatment arms were not relevant.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss to follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we addressed this within the 'Summary of findings' table/s by down‐rating quality. We also downgraded quality within the 'Summary of findings' table/s should loss be 25% to 50% in total.
2. Binary
In the case where attrition for a binary outcome is between 0% and 50% and where these data are not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). We assumed all those leaving the study early had no improvement. We undertook a sensitivity analysis testing how prone the primary outcomes were to change by comparing data only from people who completed the study to that point to the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
We reported and used data where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported.
3.2 Standard deviations
If standard deviations were not reported, we first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error and confidence intervals available for group means, and either P value or t value available for differences in mean, we calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): when only the standard error (SE) is reported, standard deviations (SDs) are calculated by the formula SD = SE * √(n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions present detailed formulae for estimating SDs from P, t or F values, confidence intervals, ranges or other statistics (Higgins 2011). If these formulae did not apply, we calculated the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Assumptions about participants who left the trials early or were lost to follow‐up
Various methods are available to account for participants who left the trials early or were lost to follow‐up. Some trials just present the results of study completers; others use the method of last observation carried forward (LOCF); while more recently, methods such as 'multiple imputation' or 'mixed effects' models for repeated measurements (MMRM) have become more of a standard. While the last two methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences in the reasons for leaving the studies early between groups is often the core problem in randomised schizophrenia trials. We therefore did not exclude studies which used the statistical approach. However, we preferred to use the more sophisticated approaches (e.g. MMRM or 'multiple imputation') and only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item 'Incomplete outcome data' of the 'Risk of bias' tool.
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for clearly outlying people or situations which we had not predicted would arise; and discussed in the text if they arose.
2. Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods which we had not predicted would arise; and discussed in the text if they arose.
3. Statistical heterogeneity
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I² statistic
We investigated heterogeneity between studies by considering the I² method alongside the Chi² P value. The I² provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I² depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. P value from Chi² test, or a confidence interval for I²). An I² estimate greater than or equal to around 50% accompanied by a statistically significant Chi² statistic can be interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 Cochrane Handbook for Systematic Reviews of Interventions;Higgins 2011). We explored and discussed in the text potential reasons for substantial levels of heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there are 10 or fewer studies, or where all studies were of similar sizes. If funnel plots are possible in future versions of this review, we will seek statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for fixed‐effect over random‐effects models, or vice versa. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random effects model: it puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size. We chose the fixed‐effect model for all analyses.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
1.1 Type of compound
As different non‐antipsychotic catecholaminergic compounds may have differential effects on antipsychotic‐induced tardive dyskinesia, we performed a subgroup analysis to compare the effects of different non‐antipsychotic catecholaminergic drugs. We proposed to undertake comparisons only for primary outcomes to minimise the risk of multiple comparisons.
1.2 Duration of treatment
We also anticipated a sub‐group analysis to examine whether any improvement occurred with short periods of intervention (less than six weeks); and if this did occur, whether this effect was maintained at longer periods of follow‐up.
1.3 Clinical state, stage or problem: recent onset TD
We proposed to undertake this review and provide an overview of the effects of non‐antipsychotic catecholaminergic drugs for people with schizophrenia in general. In addition, however, we tried to report data on subgroups of people in the same clinical state, stage and with similar problems. We anticipated testing the hypothesis that the use of non‐antipsychotic catecholaminergic drugs is most effective for those with more recent onset TD (less than five years). We had hoped to present data for this subgroup for the primary outcomes.
2. Investigation of heterogeneity
We reported when inconsistency was high. First we investigated whether data were entered correctly. Second, if data were correct, we visually inspected the graph and successively removed studies from the rest to see if homogeneity was restored. For this review we decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present data. If not, we did not pool such data and discussed issues. We know of no supporting research for this 10% cut‐off but we are investigating use of prediction intervals as an alternative to this unsatisfactory state.
When unanticipated clinical or methodological heterogeneity were obvious, we simply discussed. We did not undertake sensitivity analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
If trials were described in some way as to imply randomisation we undertook sensitivity analyses for the primary outcomes. We included these studies in the analyses and if there was no substantive difference when the implied randomised studies were added to those with better description of randomisation, then we used relevant data from these studies.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data) we compared the findings of the primary outcomes when we used our assumption compared with completer data only. If there was a substantial difference, we reported and discussed these results but continued to employ our assumption.
Where assumptions have to be made regarding missing SDs data (see Dealing with missing data), we compared the findings on primary outcomes when we used our assumption with completer data only. We undertook a sensitivity analysis, testing how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we reported and discussed these results but continued to employ our assumption.
3. Risk of bias
We analysed the effects of excluding trials that we judged to be at high risk of bias across one or more of the domains of randomisation (implied as randomised with no further details available), allocation concealment, blinding and outcome reporting for the meta‐analysis of the primary outcome. If the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates, we included data from these trials in the analysis.
4. Imputed values
Had cluster trials been included, we would have undertaken a sensitivity analysis to assess the effects of including data from trials where we used imputed values for ICC in calculating the design effect.
If we found substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed above, we did not pool data from the excluded trials with the other trials contributing to the outcome, but presented them separately
5. Fixed and random effects
We synthesised data using a fixed‐effect model; however, we also synthesised data for the primary outcome using a random‐effects model to evaluate whether this altered the significance of the results.
Results
Description of studies
Please see Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
The 2015 and 2017 update searches were part of an update search of nine Cochrane Reviews; see Table 1. The 2015 search retrieved 704 references for 344 studies; see Figure 4 for study flow diagram. After having excluded irrelevant references at title and abstract screening, we screened full texts of 71 references (58 studies). Forty‐eight studies (57 references) were excluded, and 29 of these are new excluded studies for the 2017 update. Two studies were awaiting assessment in the previous version of the review and have since been assessed in Chinese and Portuguese, found to have met inclusion criteria, and included (Chen 1995; Karniol 1983). Another six new studies were included from the 2015 search (Huang 1981; Kazamatsuri 1973; Pappa 2010; Rust 1984; Simpson 1988; Soni 1986). Ten studies are now included in this review.

Study flow diagram for 2015 and 2017 searching
The 2017 search found eight records (five studies). The Editorial base of Cochrane Schizophrenia screened these records and no new studies were relevant to this review. They could be relevant to the other reviews in this series of TD reviews (see Table 1), and have been put into 'Studies awaiting classification' of the Soares‐Weiser 2006 miscellaneous treatments review.
Included studies
Overall the review now includes 10 studies with 261 participants published between 1973 and 2010. Eight of these studies were added at the 2017 update (Chen 1995; Huang 1981; Karniol 1983; Kazamatsuri 1973; Pappa 2010; Rust 1984; Simpson 1988; Soni 1986).
1. Methods
All studies were stated to be randomised and double blind. For further details, please see sections below on Allocation (selection bias) and Blinding (performance bias and detection bias).
2. Design
All included studies presented a parallel longitudinal design. Three of the 10 studies used a cross‐over design with two periods (Buruma 1982; Chen 1995; Pappa 2010). We had considered this possibility when embarking on the review and have used only the data from before the first cross‐over for the reasons outlined above (see Unit of analysis issues).
3. Duration
Treatment phases of five studies were of short duration (2 to 5 weeks) (Buruma 1982; Chen 1995; Huang 1981; Karniol 1983; Pappa 2010); and treatment phases of the remaining five studies were of medium duration (6 to 24 weeks) (Hebenstreit 1986; Kazamatsuri 1973; Rust 1984; Simpson 1988; Soni 1986).
4. Participants
Participants, now totalling 261 people, were mostly men in their 50s, with diagnoses of various chronic psychiatric disorders, but mainly schizophrenia. All had antipsychotic‐induced tardive dyskinesia (TD), though only four studies reported the specific diagnostic criteria used (Hebenstreit 1986; Pappa 2010; Simpson 1988; Soni 1986). The number of participants ranged from 12 to 50 (median 21).
5. Setting
One trial was conducted with outpatients in Greece (Pappa 2010); and the rest with psychiatric inpatients in the USA (Huang 1981; Kazamatsuri 1973; Simpson 1988), Austria (Hebenstreit 1986), Brazil (Karniol 1983), China (Chen 1995), France (Rust 1984), the Netherlands (Buruma 1982), and the UK (Soni 1986).
6. Interventions
6.1 Noradrenergic drugs
6.1.1 Alpha‐methyldopa
Huang 1981 used alpha‐methyldopa in a dose ranging from 750 to 1500 mg/day. Methyldopa inhibits dopamine production and is also an adrenergic receptor agonist, and is used to treat hypertension and pregnancy‐induced hypertension.
6.1.2 Celiprolol
Hebenstreit 1986 used celiprolol in a 200 mg/day dose. Celiprolol is a cardioselective beta blocker reported to possess intrinsic sympathomimetic activity and direct vasodilator activity. Celiprolol is used as the hydrochloride in the management of hypertension and angina pectoris.
6.2 Dopaminergic drugs
6.2.1 Amantadine
Pappa 2010 used amantadine in a dose of 100 mg/day. Amantadine is a glutamate receptor antagonist and anticholinergic that increases dopamine release and blocks dopamine reuptake. It can be used both as an antiviral and antiparkinsonian drug.
6.2.2 Bromocriptine
Chen 1995 used bromocriptine one capsule twice per day (exact dose unknown). Bromocriptine is a dopamine agonist used to treat various conditions including pituitary tumours, Parkinson's disease, type 2 diabetes, and cocaine withdrawal.
6.2.3 Carbidopa/levodopa (L‐dopa)
Simpson 1988 used carbidopa/levodopa in a dose of 50/350 mg/day. Karniol 1983 used levodopa in a dose ranging from 500 mg to 2000 mg. Carbidopa is used in Parkinson's disease in combination with levodopa to make levodopa more accessible. L‐dopa is the precursor to the catecholaminergic neurotransmitters dopamine, noradrenaline and adrenaline. L‐dopa can also be manufactured and is used as a drug to treat Parkinson's disease.
6.2.4 Oxypertine
Soni 1986 used oxypertine in a dose ranging from 80 mg/day to 240 mg/day. Oxypertine is a dopamine depleter drug used in the treatment of mania, disturbed behaviour, psychosis and schizophrenia.
6.2.5 Reserpine
Huang 1981 used reserpine in a dose ranging from 0.75 to 1.5 mg/day. Reserpine is a dopamine depleter drug that has been used in the past to treat psychosis and hypertension. Today it is mainly used as a horse tranquilliser.
6.2.6 Tetrabenazine
Kazamatsuri 1973 used tetrabenazine in a dose ranging from 50 mg to 200 mg/day. Tetrabenazine is a dopamine depleter drug approved to treat symptoms of Huntington's disease chorea.
6.2.7 Tiapride
Two studies used tiapride in a dose ranging from 300 mg to 600 mg/day (Buruma 1982; Rust 1984). Tiapride is a substituted benzamide with general properties similar to those of the antipsychotic sulpiride. It is usually given as the hydrochloride in the management of behavioural disorders and to treat dyskinesias. Tiapride has been tried in the treatment of Tourette's syndrome and chorea such as Huntington's chorea.
6.3 Comparison group
In most of the studies a placebo was used as a comparison group, with no further details given. In one study the comparison group was haloperidol (Kazamatsuri 1973). Another trial compared groups with different doses of L‐dopa and placebo (Karniol 1983); and Huang 1981 included three arms: celiprolol (noradrenergic), reserpine (dopamine depleter) and placebo.
Participants remained on stable schizophrenia treatment antipsychotic medication during the trials.
7. Outcomes
7.1 General
Some outcomes were presented in graphs, inexact P values of differences, or a statement of significant or non‐significant difference. This made it impossible to acquire raw data for synthesis. Some continuous outcomes could not be extracted due to missing number of participants or missing means, standard deviations, or standard errors.
7.2 Scales used to measure TD symptoms
We have shown details of the scales that provided usable data below. We have provided reasons for exclusions of data under 'Outcomes' in the Characteristics of included studies table.
7.2.1 Abnormal Involuntary Movement Scale (AIMS)
Simpson 1988 reported using AIMS to assess TD symptoms, and Hebenstreit 1986 reported using SKAUB, the German version of AIMS. The AIMS is a 12‐item scale consisting of a standardised examination followed by questions rating the orofacial, extremity and trunk movements, as well as three global measurements (Guy 1976). Each of these 10 items can be scored from 0 (none) to 4 (severe). Two additional items assess dental status. The AIMS ranges from 0 to 40, with higher scores indicating greater severity.
7.2.2 Extrapyramidal Bilan scale (EBS)
Karniol 1983 used the EBS. The EBS is a nine‐item rating scale for use by neurologists, to measure severity of symptoms such as facial mask, tremor, rigidity, akathisia, dystonia, dyskinesia and others (Tetreault 1969). Each item can be scored from 0 to 3, such that the overall score can range from 0 (no symptoms) to a possible 27 (severe symptoms of all types).
7.2.3 Clinical assessment
Two studies reported using a frequency count of mouth movements, performed by a psychiatrist, to assess oral dyskinesia (Huang 1981; Kazamatsuri 1973).
Excluded studies
There are 48 excluded studies (57 references). Thirteen studies were not randomised and we therefore excluded them (Asher 1981; Chouza 1982; Delwaide 1980; Fahn 1983; Ferrari 1972; Gerlach 1976; Kazamatsuri 1972; Konig 1996; Leblhuber 1987; Levy 1984; Ringwald 1978; Rondot 1987; Smith 1977). Seven RCTs did not meet inclusion criteria because they recruited participants without tardive dyskinesia (Adler 1990; DiMascio 1976; Fann 1976; Gutierrez 1979; NCT00310661 2006; NCT00845000 2009; O'Suilleabhain 2003). Participants in two RCTs were not on stable antipsychotic medication before and during the study and were consequently not eligible for inclusion (Jankovic 1982; Lieberman 1989). Two RCTs evaluated selegiline, an intervention that is not relevant for this review: Goff 1993 is included in the update of the 'Miscellaneous treatments for antipsychotic‐induced tardive dyskinesia' Cochrane Review (Soares‐Weiser 2003); and Stearns 1996 also reported no usable data so was excluded from the Soares‐Weiser 2003 review as well as from this review.
Twenty‐four studies had to be excluded because data were all unusable, in 18 of these as a result of failure to report outcomes from the first phase before cross‐over. We contacted authors of six of these 18 studies but received no reply (Doongaji 1982; Hemnani 1982; Jeste 1983; Lieberman 1988; Nasrallah 1986; Tamminga 1980); and since they were all published over 25 years ago and we assumed we would be very unlikely to receive a reply with data so many years later, they were excluded. We did not identify up‐to‐date contact details of authors for 12 of 18 cross‐over studies and decided to also exclude them as they were published 20 to 45 years ago and again we assumed we would be very unlikely to receive a reply with data so many years later (Angus 1997; Auberger 1985; Bateman 1979; Braun 1989; Browne 1986a; Chien 1978; Delwaide 1979; Freeman 1980; Gardos 1979; Glover 1980; Godwin Austen 1971; Viukari 1975). No usable outcome data were reported in the six remaining studies. We contacted authors of Alpert 1983 and Diehl 1999 but received no reply. We could not identify up‐to‐date contact details for authors of Greendyke 1988, Ludatscher 1989, Reker 1982 and Silver 1995. These six studies were also excluded as they were published 15 to 30 years ago and again we assumed we would be very unlikely to receive a reply with data so many years later.
See Characteristics of excluded studies for more details on each excluded study.
Studies awaiting classification
There are currently no studies awaiting classification.
Ongoing studies
As far as we are aware, there are currently no ongoing studies.
Risk of bias in included studies
Please refer to Figure 2 and Figure 3 for graphical overviews of the risk of bias in the included studies, and Characteristics of included studies for details.
Allocation
Reporting of randomisation and allocation concealment was poor overall. No study explicitly reported the method for sequence generation other than using the word "randomized" and consequently all studies were rated at unclear risk of bias for sequence generation. Only two studies were rated at low risk of bias for allocation concealment. Chen 1995 reported the allocation of participants by an external site while Karniol 1983 used sealed opaque envelopes. The remaining studies were rated at unclear risk of bias for allocation concealment.
Blinding
Although all studies were stated to be conducted on a double‐blind basis, not all explicitly described how this was undertaken and none tested the blindness of raters, clinicians and trial participants. Chen 1995, Hebenstreit 1986, Karniol 1983, Pappa 2010 and Simpson 1988 described how the participants and personnel were blinded and were rated at low risk of performance bias. Kazamatsuri 1973, Pappa 2010, and Soni 1986 described how the raters were blinded and were rated at low risk of detection bias. The remaining studies were rated at unclear risk of performance or detection bias, or both.
Incomplete outcome data
In four studies all randomised participants completed the study and were included in analyses; these were rated at low risk of attrition bias (Buruma 1982; Chen 1995; Pappa 2010; Rust 1984). Three studies did not report fully on attrition and were at unclear risk of bias (Hebenstreit 1986; Huang 1981; Karniol 1983). Three studies had 30% or greater loss to follow‐up (Soni 1986), or unbalanced loss to follow‐up between groups (Kazamatsuri 1973; Simpson 1988), and did not report outcomes for participants lost to follow‐up. These studies were rated at high risk of attrition bias. In all cases, however, we tried to ensure that every person randomised was analysed.
Selective reporting
Data in this review originates from published reports. Expected outcomes (impact on tardive dyskinesia symptoms, adverse events) were not reported sufficiently for most of the trials. In addition, we have had no opportunity to see protocols of these trials to compare the outcomes reported in the full publications with what was planned and measured during the conduct of the trial. Three studies were rated at unclear risk of reporting bias as it was unclear whether all outcomes were fully reported (Chen 1995; Kazamatsuri 1973; Rust 1984). The remaining seven studies were at high risk of reporting bias as they failed to fully report all measured outcomes.
Other potential sources of bias
All studies had small or very small sample sizes. Three of the studies used a cross‐over design (Buruma 1982; Chen 1995; Pappa 2010); four of the studies had the drugs used in the trials provided by pharmaceutical companies (Buruma 1982; Kazamatsuri 1973; Simpson 1988; Soni 1986); and in six studies no details of funding were given (Chen 1995; Hebenstreit 1986; Huang 1981; Karniol 1983; Pappa 2010; Rust 1984).
Nevertheless, we rated four studies at low risk bias as they seemed to be free from other sources of bias and baseline characteristics were balanced between groups (Chen 1995; Hebenstreit 1986; Karniol 1983; Soni 1986). Five studies were at unclear risk of other bias as insufficient information was available to make a judgement otherwise (Huang 1981; Kazamatsuri 1973; Pappa 2010; Rust 1984; Simpson 1988). Finally, Buruma 1982 was at high risk of other bias as the placebo group contained participants more severely affected by TD at baseline.
Effects of interventions
See: Summary of findings for the main comparison NORADRENERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia; Summary of findings 2 NORADRENERGIC DRUGS compared to DOPAMINERGIC DRUGS for antipsychotic‐induced tardive dyskinesia; Summary of findings 3 DOPAMINERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia; Summary of findings 4 DOPAMINERGIC DRUGS compared to OTHER DRUGS for antipsychotic‐induced tardive dyskinesia
1. Comparison 1: noradrenergic drugs versus placebo
1.1 TD symptoms
We had chosen 'any improvement in TD symptoms of more than 50% on any TD scale – any time period' as a primary outcome. Although the data we found in trials did not fit this exactly we feel that the outcome 'not improved to a clinically important extent' fits best with what we had hoped to find.
1.1.1 Not improved to a clinically important extent
The overall results for 'clinically relevant improvement' found a significant benefit of alpha‐methyldopa over placebo after 2 weeks' treatment (low‐quality evidence, 1 trial, 20 people; RR 0.33, 95% CI 0.14 to 0.80; Analysis 1.1).
1.1.2 Not any improvement
For the outcome of 'any improvement in TD symptoms' we found no significant difference between noradrenergic drugs (alpha‐methyldopa, celiprolol) and placebo after 2 to 13 weeks' treatment (2 trials, 55 people; RR 0.91, 95% CI 0.65 to 1.27; I² = 0%, Analysis 1.2).
1.1.3 Deterioration of symptoms
There was no significant difference in deterioration of symptoms between people allocated to alpha‐methyldopa or placebo after 2 weeks' treatment (very low quality evidence, 1 trial, 20 people; RR 0.33, 95% CI 0.02 to 7.32; Analysis 1.3).
1.2 Leaving the study early
Using celiprolol did not significantly increase the chances of a person leaving the study early compared with placebo after 13 weeks' treatment (very low quality evidence, 1 trial, 35 people; RR 5.28, 95% CI 0.27 to 102.58; Analysis 1.4).
1.3 Quality of life
There was no significant difference in quality of life between people allocated to celiprolol or placebo after 13 weeks' treatment (very low quality evidence, 1 trial, 35 people; RR 0.87, 95% CI 0.68 to 1.12; Analysis 1.5).
We did not identify any studies that reported on hospital and service utilisation outcomes, economic outcomes, behaviour, or cognitive state.
1.4 Subgroup analysis
1.4.1 Type of compound
There were no significant subgroup differences (I² = 0%, P = 0.52, Analysis 1.2) for alpha‐methyldopa versus placebo (RR 0.33, 95% CI 0.02 to 7.32; 20 participants, 1 study) and celiprolol versus placebo (RR 0.92, 95% CI 0.66 to 1.28; 35 participants, 1 study) on 'not any improvement in TD symptoms', the only outcome for this comparison that evaluated more than one non‐antipsychotic catecholaminergic compound.
1.4.2 Duration of follow‐up
Any effects that noradrenergic drugs may have did not clearly change in relation to duration of follow‐up compared with placebo.
1.4.3 Clinical stage: recent onset TD
It was not possible to evaluate whether those with recent onset TD responded differently to those with more established problems, since no trial reported data for groups with different durations of TD that could be extracted for separate analyses.
1.5 Heterogeneity
Data were homogeneous. We did not detect clinical, methodological or statistical heterogeneity as described in Assessment of heterogeneity.
1.6 Sensitivity analyses
1.6.1 Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. Only one study was included for the primary outcome: consequently this sensitivity analysis could not be performed.
1.6.2 Assumptions for lost binary data
The above results are based on data as presented in the original study reports, with the assumption that those who left early before the end of the trial had not improved (see Dealing with missing data). We planned to test the sensitivity of the results to this assumption, but all randomised participants were reported for the primary outcome 'no clinically important improvement in TD symptoms'. Therefore, we could not undertake this sensitivity analysis. If there had been a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
1.6.3 Risk of bias
We planned to exclude trials that we judged to be at high risk of bias across one or more of the domains, but only one study was included for the primary outcome. Consequently this sensitivity analysis could not be performed.
1.6.4 Imputed values
We would have undertaken a sensitivity analysis to assess the effects of including data from cluster randomised trials where we used imputed values for ICC in calculating the design effect. No cluster randomised trials were included.
1.6.5 Fixed and random effects
We also synthesised data using a random effects model. This did not alter the effect estimates or CIs (analysis not shown).
2. Comparison 2: noradrenergic drugs versus dopaminergic drugs
2.1 TD symptoms
2.1.1 Not improved to a clinically important extent
The overall results for 'clinically relevant improvement' found no significant benefit of alpha‐methyldopa over reserpine after 2 weeks' treatment (1 trial, 20 people; RR 0.60, 95% CI 0.19 to 1.86; Analysis 2.1).
2.1.2 Not any improvement
We could not estimate the effect of alpha‐methyldopa compared with reserpine on any improvement in TD symptoms as no events were reported (1 trial, 20 participants, Analysis 2.2).
2.1.3 Deterioration of symptoms
We could not estimate the effect of alpha‐methyldopa compared with reserpine on deterioration of TD symptoms as no events were reported (1 trial, 20 participants, Analysis 2.3).
2.2 Heterogeneity, subgroup‐ and sensitivity analyses
Only one study was included in this comparison: consequently subgroup and sensitivity analyses could not be undertaken and there was no heterogeneity.
3. Comparison 3: dopaminergic drugs versus placebo
3.1 TD symptoms
3.1.1 Not improved to a clinically important extent
The overall results for 'clinically relevant improvement' found a significant benefit of reserpine over placebo after 2 weeks' treatment (low‐quality evidence, 1 trial, 20 people; RR 0.52, 95% CI 0.29 to 0.96; Analysis 3.1).
3.1.2 Not any improvement
For the outcome of 'any improvement in TD symptoms' we found no significant difference between dopaminergic drugs (Carbidopa/levodopa, L‐dopa, reserpine) and placebo after 2 to 6 weeks' treatment (3 trials, 57 people;RR 0.60, 95% CI 0.35 to 1.03; I² = 0%, Analysis 3.2).
3.1.3 Deterioration of symptoms
There was no significant difference in deterioration of symptoms between people allocated to dopaminergic drugs (carbidopa/levodopa, reserpine) or placebo after 2 to 6 weeks' treatment (very low quality evidence, 2 trials, 37 people; RR 1.18, 95% CI 0.35 to 3.99; I² = 0%, Analysis 3.3).
3.2 Mental state
There was no significant difference between oxypertine and placebo on deterioration of mental state after 24 weeks' treatment (very low quality evidence, 1 trial, 42 people; RR 2.20, 95% CI 0.22 to 22.45; Analysis 3.4).
3.3 Leaving the study early
Using dopaminergic drugs (amantadine, bromocriptine, carbidopa/levodopa, oxypertine, tiapride) did not significantly affect the chances of a person leaving the study early compared with placebo after 2 to 24 weeks' treatment (very low quality evidence, 6 trials, 163 people; RR 1.29, 95% CI 0.65 to 2.54; I² = 58%, Analysis 3.5).
3.4 Subgroup analysis
3.4.1 Type of compound
There were no significant subgroup differences (I² = 0%, P = 0.90, Analysis 3.2) for reserpine versus placebo (RR 0.33, 95% CI 0.02 to 7.32; 20 participants, 1 study), L‐dopa versus placebo (RR 0.67, 95% CI 0.35 to 1.27; 20 participants, 1 study), and carbidopa/levodopa versus placebo (RR 0.59, 95% CI 0.26 to 1.36; 17 participants, 1 study) on 'not any improvement in TD symptoms'. For 'deterioration of TD symptoms' there were no significant subgroup differences (I² = 0%, P = 0.32, Analysis 3.3) for reserpine versus placebo (RR 0.33, 95% CI 0.02 to 7.32; 20 participants, 1 study) and carbidopa/levodopa versus placebo (RR 1.78, 95% CI 0.44 to 7.25; 17 participants, 1 study). Finally, for 'acceptability of treatment: leaving the study early', there were subgroup differences (I² = 54.5%, P = 0.14, Analysis 3.5) for the subgroups that reported events, oxypertine versus placebo (RR 1.73, 95% CI 0.83 to 3.58; 42 participants, 1 study) and carbidopa/levodopa versus placebo (RR 0.18, 95% CI 0.01 to 3.27; 17 participants, 1 study; see '3.5 Heterogeneity' below).
3.4.2 Duration of follow‐up
Any effects that dopaminergic drugs may have did not clearly change in relation to duration of follow‐up compared with placebo.
3.4.3 Clinical stage: recent onset TD
It was not possible to evaluate whether those with recent onset TD responded differently to those with more established problems, since no trial reported data for groups with different durations of TD that could be extracted for separate analyses.
3.5 Heterogeneity
Data were mostly homogeneous. We detected statistical heterogeneity (I² = 58%, P = 0.12) as described in Assessment of heterogeneity for the outcome 'acceptability of treatment: leaving the study early'. Six studies reported on this outcome, but only two reported any events. One of these two studies reported an effect estimate favouring placebo over oxypertine after 24 weeks' treatment and the other study reported an effect estimate favouring carbidopa/levodopa over placebo after 6 weeks' treatment, but none of the studies reported statistically significant differences between groups (see Analysis 3.5 and '3.4.1 Type of compound' above).
3.6 Sensitivity analysis
3.6.1 Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. Only one study was included for the primary outcome: consequently this sensitivity analysis could not be performed.
3.6.2 Assumptions for lost binary data
The above results are based on data as presented in the original study reports, with the assumption that those who left early before the end of the trial had not improved (see Dealing with missing data). We planned to test the sensitivity of the results to this assumption, but all randomised participants were reported for the primary outcome 'no clinically important improvement in TD symptoms'. Therefore we could not undertake this sensitivity analysis. If there had been a substantial difference, we would have reported results and discussed them but continued to employ our assumption.
3.6.3 Risk of bias
We planned to exclude trials that we judged to be at high risk of bias across one or more of the domains, but only one study was included for the primary outcome. Consequently this sensitivity analysis could not be performed.
3.6.4 Imputed values
We would have undertaken a sensitivity analysis to assess the effects of including data from cluster randomised trials where we used imputed values for ICC in calculating the design effect. No cluster randomised trials were included.
3.6.5 Fixed and random effects
We also synthesised data using a random‐effects model. This did not alter the effect estimate or CIs for the primary outcome (analyses not shown).
4. Comparison 4: dopaminergic drugs versus other drugs
4.1 TD symptoms
4.1.1 Not improved to a clinically important extent
We found no significant benefit of tetrabenazine over haloperidol for 'no clinically relevant improvement after 18 weeks' treatment' (1 trial, 13 people; RR 0.93, 95% CI 0.45 to 1.95; Analysis 4.1).
4.1.2 Not any improvement
For the outcome of 'any improvement in TD symptoms', we found no significant difference between tetrabenazine and haloperidol after 18 weeks' treatment (1 trial, 13 people; RR 0.39, 95% CI 0.05 to 2.83; Analysis 4.2).
4.1.3 Deterioration of symptoms
There was no significant difference in deterioration of TD symptoms between people allocated to tetrabenazine or haloperidol after 18 weeks' treatment (1 trial, 13 people; RR 1.17, 95% CI 0.09 to 14.92; Analysis 4.3).
4.2 Leaving the study early
There was no significant difference between tetrabenazine and haloperidol in the chances of a person leaving the study early after 18 weeks' treatment (1 trial, 13 people; RR 0.23, 95% CI 0.01 to 4.00; Analysis 4.4).
4.3 Heterogeneity, and subgroup and sensitivity analyses
Only one study was included in this comparison. Consequently, subgroup and sensitivity analyses could not be undertaken; and there was no heterogeneity.
Discussion
Summary of main results
1. The search
This area of research does not seem to be active. The 2017 update has identified additional data, but most trials predate the year 2000: only one was carried out after, published in 2010. This could be because of reasons such as less concern with TD, or less emergence of the problem in research‐active communities because of more thoughtful use of antipsychotic drugs or loss of faith in non‐antipsychotic catecholaminergic drugs as a potential treatment.
2. Few data
Only a little over 250 people have been included in this review. It is possible that real, and important, effects have not been highlighted because of the necessarily wide CIs of the findings. Many outcomes were not measured at all (see Overall completeness and applicability of evidence), including one of our pre‐stated outcome measures. We may have been overambitious in hoping for some of these outcomes in TD trials but simple reporting of satisfaction with care or quality of life still does not seem too demanding and does remain of interest.
3. Comparison 1: noradrenergic drugs versus placebo
3.1 TD symptoms
Results from one study show that significantly more participants on alpha‐methyldopa than on placebo improved to a clinically important level at short term; however, our confidence in the evidence is low so further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
3.2 Acceptability of treatment
It is always unclear what leaving the study early means. It could be to do with the participant not accepting treatment for a series of reasons, or of participants finding the trial intolerable. It also could be a function of a trial design in which willing participants are still asked to leave because of some degree of protocol violation. In any event, one study reported that 2/17 participants left the celiprolol group compared with a 'not significantly different' 0/18 in the placebo group.
3.3 Social confidence, social inclusion, social networks, or personalised quality of life
This group of outcomes was selected as being of importance to patients for the 2017 review update following a service user consultation. One study reported on 'no improvement in quality of life' and found no difference between celiprolol and placebo; however, we are uncertain about the results as the evidence is of very low quality.
No studies comparing noradrenergic drugs versus placebo were identified that reported on adverse events or mental state. See summary of findings Table for the main comparison for a summary of the evidence.
4. Comparison 2: noradrenergic drugs versus dopaminergic drugs
4.1 TD symptoms
Only one small, short duration trial reported on this comparison and found no difference between alpha‐methyldopa and reserpine on 'no clinically important improvement in TD'. The size and duration of the trial were so limited that only a treatment of very great potency could have really shown up as effective.
No studies were identified that reported on adverse events, mental state, acceptability of treatment or social confidence, social inclusion, social networks, or personalised quality of life.
5. Comparison 3: dopaminergic drugs versus placebo
3.1 TD symptoms
Results from one small study show that significantly more participants on reserpine than on placebo improved to a clinically important level at short term; however, our confidence in the evidence is low so further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We are uncertain about the results on 'not any improvement in TD' and 'deterioration of TD'; evidence was of very low quality.
3.2 Mental state
We are uncertain about the results on 'deterioration in mental state'; evidence was of very low quality.
3.3 Acceptability of treatment
We are uncertain about the results on 'acceptability of treatment', measured by the number of participants leaving the study early; evidence was of very low quality.
No studies were identified that reported on adverse events or social confidence, social inclusion, social networks, or personalised quality of life. See summary of findings Table 3 for a summary of the evidence.
6. Comparison 4: dopaminergic drugs versus other drugs
3.1 TD symptoms
Only one small, short‐duration trial reported on this comparison and found no difference between tetrabenazine and haloperidol on 'no clinically important improvement in TD', on 'not any improvement in TD', or on 'deterioration of TD'. The size and duration of the trial were so limited that only a treatment of very great potency could have really shown up as effective.
3.2 Acceptability of treatment
None out of six participants left the tetrabenazine group compared with a 'not significantly different' two out of seven in the haloperidol group.
No studies were identified that reported on adverse events, mental state, or social confidence, social inclusion, social networks, or personalised quality of life.
Overall completeness and applicability of evidence
1. Completeness
Although we identified a large number of studies on the initial database search, the number of relevant studies was very small in comparison. The majority of studies selected were excluded because of inherent problems in the nature of their cross‐over design. That only eight studies were included in the final review does not give full justice to the fact that there has obviously been a reasonable amount of research in this area. This may have implications for future studies as discussed below.
No outcomes in this review involve large numbers of people, and many outcomes reported no events. None of the studies reported on adverse events. Even if these small studies only set out to show efficacy, not reporting on adverse events is unexpected as non‐antipsychotic catecholaminergic compounds are associated with several side effects (Turjanski 2005). In addition, there were very few data on the patient‐designated important outcomes of social confidence, social inclusion, social networks, or personalised quality of life; and there were no data on hospital and service utilisation outcomes, economic outcomes, behaviour or cognitive response.
2. Applicability
All but one trial were hospital‐based but were nevertheless on people who would be recognisable in everyday care. Trials were set in North and South America, Asia, and Europe. Outcomes are understandable in terms of clinical practice. However, most of the interventions in question are experimental in the treatment of tardive dyskinesia. Therefore, should any of the non‐antipsychotic catecholaminergic compounds have had important effects, findings might only have been applicable for those compounds accepted as treatment of TD.
Quality of the evidence
Overall, the quality of the evidence is low to very low. This means that we have limited to very little confidence in the effect estimates, and the true effect may be, or is likely to be, substantially different from the estimate of the effect. The main reasons for our low confidence in the evidence were as follows.
-
Poor study methodology and reporting of methods resulting in downgrading evidence for risk of bias. Overall the quality of reporting of these trials was poor (see Figure 3). Allocation concealment was not described; generation of the sequence was not explicit; studies were not clearly blinded and we are unsure if data are incomplete or selectively reported or if other biases were operating.
-
Very small sample sizes resulting in downgrading evidence for imprecision. The largest trial in this review randomised only 50 people. A trial of this size is unable to detect subtle, yet important, differences due to an intervention with any confidence. In order to detect a 20% difference between groups, probably about 150 people are needed in each arm of the study (alpha 0.05, beta 0.8).
-
Wide CIs (often due to low event rates) that included appreciable benefit or harm for the intervention as well as no effect, resulting in downgrading evidence for imprecision.
The small trial sizes, along with the poor reporting of trials, is associated with an exaggeration of effect of the experimental treatment where an effect is detected (Jűni 2001). This is only evident for the outcome of ‘no clinically important improvement in TD' where there is indeed an effect favouring the noradrenergic and dopaminergic drugs compared with placebo (see summary of findings Table for the main comparison and summary of findings Table 3). This finding may be real — but could equally be a function of biases or of chance.
Potential biases in the review process
1. Missing studies
We made every effort to identify relevant trials. However, these studies are all small and it is likely that we have failed to identify other studies of limited power. It is likely that such studies would also not be in favour of the intervention group: if they had been so, it is more likely that they would have been published in accessible literature. We do not, however, think it likely that we have failed to identify large relevant studies.
2. Introducing bias
We have tried to be balanced in our appraisal of the evidence but could have inadvertently introduced bias. We welcome comments or criticisms. New methods and innovations now make it possible to report data where, in the past, we could not report data at all or had to report data in a different way. We think the 'Summary of findings' tables to be a valuable innovation — but problematic to those not ‘blind’ to the outcome data. It is possible to ‘cherry pick’ significant findings for presentation in this table. We have tried to decrease the chance of doing this by asking a new reviewer (HB) to select outcomes relevant for this table before becoming familiar with the data.
Agreements and disagreements with other studies or reviews
The only other relevant quantitative review we know of is the previous Cochrane Review (El‐Sayeh 2006). This update expands and improves this review but does not substantially change the conclusions.

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Study flow diagram for 2015 and 2017 searching
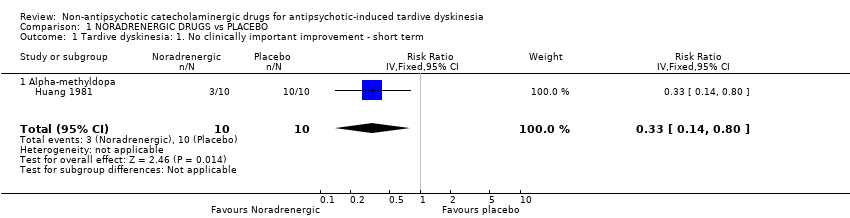
Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.

Comparison 1 NORADRENERGIC DRUGS vs PLACEBO, Outcome 5 Quality of life: No improvement ‐ medium term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ short term.

Comparison 2 NORADRENERGIC DRUGS vs DOPAMINERGIC DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ short term.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 2 Tardive dyskinesia: 2. Not any improvement.
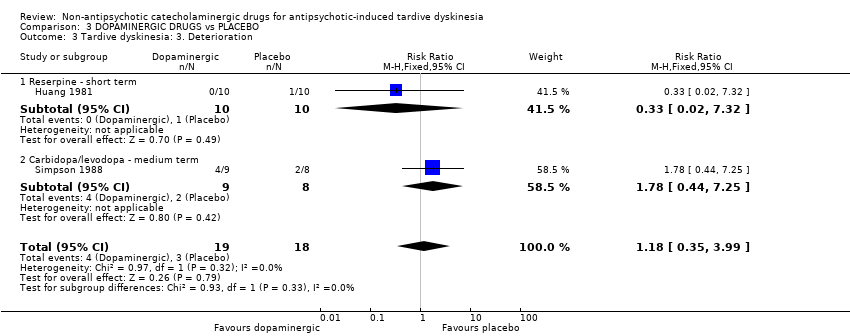
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 3 Tardive dyskinesia: 3. Deterioration.

Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 4 Mental state: Deterioration ‐ medium term.
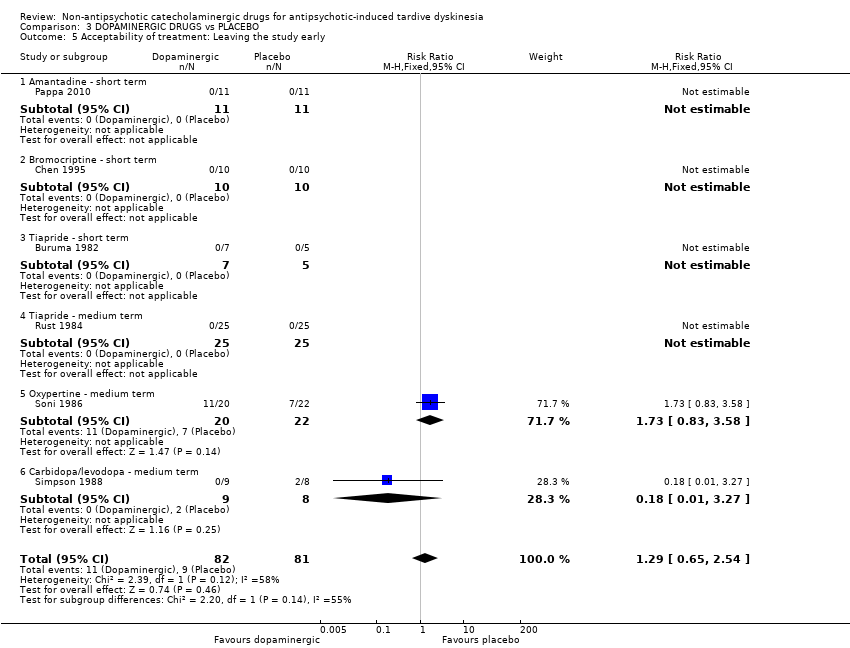
Comparison 3 DOPAMINERGIC DRUGS vs PLACEBO, Outcome 5 Acceptability of treatment: Leaving the study early.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term.

Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term.
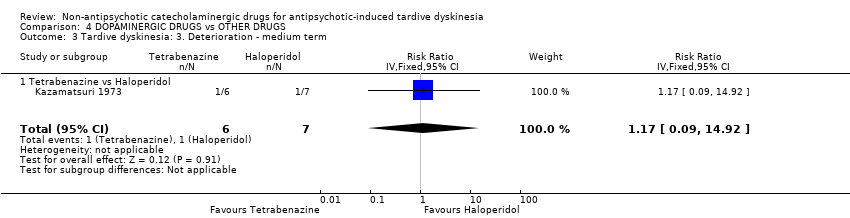
Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 3 Tardive dyskinesia: 3. Deterioration ‐ medium term.
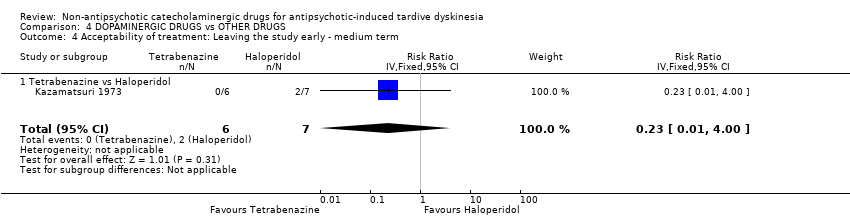
Comparison 4 DOPAMINERGIC DRUGS vs OTHER DRUGS, Outcome 4 Acceptability of treatment: Leaving the study early ‐ medium term.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Non‐antipsychotic catecholaminergic compound. N = 150. |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting all participants could be screened using operational criteria, otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| NORADRENERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 330 per 1000 | RR 0.33 | 20 | ⊕⊕⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Tardive dyskinesia: deterioration follow‐up: 2 weeks | 100 per 1000 | 33 per 1000 | RR 0.33 | 20 | ⊕⊝⊝⊝ | The included study evaluated alpha‐methyldopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Mental state ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 13 weeks | 0 per 1000 | 0 per 1000 | RR 5.28 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| No improvement in quality of life follow‐up: 13 weeks | 944 per 1000 | 822 per 1000 | RR 0.87 | 35 | ⊕⊝⊝⊝ | The included study evaluated celiprolol. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| NORADRENERGIC DRUGS compared to DOPAMINERGIC DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with DOPAMINERGIC DRUGS | Risk with NORADRENERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | Study population | RR 0.60 | 20 | ⊕⊝⊝⊝ | ||
| 500 per 1,000 | 300 per 1,000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 2 weeks | Study population | not estimable | 20 | ⊕⊝⊝⊝ | Among the 20 participants no events were reported. | |
| 0 per 1,000 | 0 per 1,000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| DOPAMINERGIC DRUGS compared to PLACEBO for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PLACEBO | DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 2 weeks | 1000 per 1000 | 520 per 1000 | RR 0.52 | 20 | ⊕⊕⊝⊝ | The included study evaluated reserpine. |
| Tardive dyskinesia: Deterioration follow‐up: 2‐6 weeks | 167 per 1000 | 197 per 1000 | RR 1.18 | 37 | ⊕⊝⊝⊝ | The included studies evaluated reserpine and carbidopa/levodopa. |
| Adverse events ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | We found no studies rating this outcome. |
| General mental state: Deterioration follow‐up: 24 weeks | 45 per 1000 | 100 per 1000 | RR 2.2 | 42 | ⊕⊝⊝⊝ | The included study evaluated oxypertine. |
| Acceptability of treatment: Leaving the study early follow‐up: 2‐24 weeks | 111 per 1000 | 143 per 1000 | RR 1.29 | 163 | ⊕⊝⊝⊝ | Only two studies (59 participants) evaluating carbidopa/levodopa and oxypertine reported any events for this outcome. 4 studies evaluating amantadine, bromocriptine, and tiapride reported no events and consequently no estimates could be made for these 3 compounds. |
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | Not estimable | 0 | See comment | This outcome was designated to be of importance, especially to patients. We found no studies rating this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately, blinding of outcome assessors was not described. | ||||||
| DOPAMINERGIC DRUGS compared to OTHER DRUGS for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with OTHER DRUGS | Risk with DOPAMINERGIC DRUGS | |||||
| Tardive dyskinesia: No clinically important improvement follow‐up: 18 weeks | Study population | RR 0.93 | 13 | ⊕⊝⊝⊝ | ||
| 714 per 1000 | 664 per 1000 | |||||
| Tardive dyskinesia: Deterioration follow‐up: 18 weeks | Study population | RR 1.17 | 13 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 167 per 1000 | |||||
| Adverse events ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Mental state ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| Acceptability of treatment: Leaving the study early follow‐up: 18 weeks | Study population | RR 0.23 | 13 | ⊕⊝⊝⊝ | ||
| 286 per 1000 | 66 per 1000 | |||||
| Social confidence, social inclusion, social networks, or personalised quality of life ‐ not reported | See comment | See comment | not estimable | 0 | See comment | We found no studies reporting on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one step for risk of bias: unclear whether randomisation procedure and allocation concealment were carried out adequately. | ||||||
| Interventions | Reference |
| Anticholinergic medication | |
| Benzodiazepines | |
| Calcium channel blockers | |
| Cholinergic medication | |
| Gamma‐aminobutyric acid agonists | |
| Miscellaneous treatments | |
| Neuroleptic reduction and/or cessation and neuroleptics | |
| Non‐neuroleptic catecholaminergic drugs | This review |
| Vitamin E |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 1.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.14, 0.80] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 2 | 55 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.65, 1.27] |
| 2.1 Alpha‐methyldopa ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 Celiprolol ‐ medium term | 1 | 35 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.1 Alpha‐methyldopa | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 4.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.27, 102.58] |
| 5 Quality of life: No improvement ‐ medium term Show forest plot | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| 5.1 Celiprolol | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.68, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 1.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.19, 1.86] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ short term Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Alpha‐methyldopa versus Reserpine | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement Show forest plot | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 1.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.52 [0.29, 0.96] |
| 2 Tardive dyskinesia: 2. Not any improvement Show forest plot | 3 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.03] |
| 2.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 2.2 L‐DOPA ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.27] |
| 2.3 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.36] |
| 3 Tardive dyskinesia: 3. Deterioration Show forest plot | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.35, 3.99] |
| 3.1 Reserpine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3.2 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.44, 7.25] |
| 4 Mental state: Deterioration ‐ medium term Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oxypertine | 1 | 42 | Risk Ratio (IV, Fixed, 95% CI) | 2.2 [0.22, 22.45] |
| 5 Acceptability of treatment: Leaving the study early Show forest plot | 6 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.65, 2.54] |
| 5.1 Amantadine ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Bromocriptine ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tiapride ‐ short term | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Tiapride ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Oxypertine ‐ medium term | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.83, 3.58] |
| 5.6 Carbidopa/levodopa ‐ medium term | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tardive dyskinesia: 1. No clinically important improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 1.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.93 [0.45, 1.95] |
| 2 Tardive dyskinesia: 2. Not any improvement ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 2.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.39 [0.05, 2.83] |
| 3 Tardive dyskinesia: 3. Deterioration ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 3.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 4 Acceptability of treatment: Leaving the study early ‐ medium term Show forest plot | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |
| 4.1 Tetrabenazine vs Haloperidol | 1 | 13 | Risk Ratio (IV, Fixed, 95% CI) | 0.23 [0.01, 4.00] |

