Ventilación mecánica sincronizada para la asistencia respiratoria en recién nacidos
Resumen
Antecedentes
Durante la ventilación mecánica sincronizada, coinciden la presión positiva en las vías respiratorias y la inspiración espontánea. Si se provoca la ventilación sincronizada, se debe alcanzar el intercambio de gases adecuado a presiones máximas de las vías respiratorias inferiores, lo que reduce potencialmente el baro/volutrauma, la pérdida de aire y la displasia broncopulmonar. La ventilación sincronizada se puede lograr mediante la manipulación de la velocidad y del tiempo de inspiración durante la ventilación convencional y con el empleo de la ventilación activada por el paciente.
Objetivos
Comparar la eficacia de:
(i) la ventilación mecánica sincronizada, administrada como ventilación con presión positiva de alta frecuencia (VPPAF) o la ventilación activada por el paciente (ventilación asistida con control, VAC) y la ventilación intermitente sincronizada obligatoria (VISO), con ventilación convencional u oscilación de alta frecuencia [OAF]);
(ii) diferentes tipos de ventilación activada por el paciente (VAC, VISO, ventilación con presión regulada con control de volumen [VPRCV], VISO con asistencia de presión [AP] y ventilación con asistencia de presión [VAP]).
Métodos de búsqueda
Se utilizó la estrategia de búsqueda estándar del Grupo Cochrane de Neonatología (Cochrane Neonatal Review group) para buscar en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL 2016, número 5), MEDLINE vía PubMed (1966 hasta 5 junio 2016), EMBASE (1980 hasta 5 junio 2016) y en CINAHL (1982 hasta 5 junio 2016). También se buscaron ensayos controlados aleatorizados y cuasialeatorizados en las bases de datos de ensayos clínicos, las actas de congresos y las listas de referencias de los artículos recuperados.
Criterios de selección
Ensayos clínicos aleatorizados o cuasialeatorizados que compararan la ventilación sincronizada administrada como VPPAF con VMC, o VAC/VISO con VMC u OAF en recién nacidos. Los ensayos aleatorizados que comparaban diferentes modalidades de ventilación activada (VAC, VISO, VISO más AP y VAP) en recién nacidos.
Obtención y análisis de los datos
Se recopilaron datos de los resultados clínicos, incluidos la mortalidad, las pérdidas de aire (neumotórax o enfisema intersticial pulmonar [EIP]), la hemorragia intraventricular severa (grado 3 y 4), la displasia broncopulmonar (DBP) (dependencia de oxígeno más allá de los 28 días), DBP moderada/severa (dependencia de oxígeno/asistencia respiratoria más allá de las 36 semanas de edad posmenstrual [EPM]) y la duración del retiro/ventilación.
Se hicieron ocho comparaciones: i) VPPAF versus VMC; ii) VAC/VISO versus VMC; iii) VISO o VISO + AP versus OAF; iv) VAC versus VISO; v) VISO más AP versus VISO; vi) VISO versus VPRCV; vii) VISO vs VAP; viii) VAC versus VAP. Se realizó el análisis de los datos con el riesgo relativo para los resultados categóricos y la diferencia de medias para los resultados medidos en una escala continua.
Resultados principales
Se incluyen 22 estudios en esta revisión. El metanálisis demuestra que la VPPAF en comparación con la VMC se asoció con una reducción del riesgo de pérdida de aire (riesgo relativo [RR] típico de neumotórax 0,69; intervalo de confianza [IC] del 95%: 0,51 a 0,93). La VAC/VISO en comparación con la VMC se asoció con una menor duración de la ventilación (diferencia de medias [DM] ‐38,3 horas; IC del 95%: ‐53,90 a ‐22,69). La VISO o VISO + AP se asoció con un riesgo mayor de DBP moderada/grave en comparación con OAF (CR 1,33; IC del 95%: 1,07 a 1,65) y una duración más larga de la ventilación mecánica en comparación con la OAF (DM 1,89 días, IC del 95%: 1,04 a 2,74).
La VAC en comparación con la VISO se asoció con una tendencia a una menor duración del retiro (DM ‐42,38 horas; IC del 95%: ‐94,35 a 9,60). Ni la VPPAF ni la ventilación activada se asociaron con una reducción significativa de la incidencia de DBP. Hubo una tendencia no significativa hacia una tasa de mortalidad inferior al administrar VPPAF versus VMC y una tendencia no significativa hacia una tasa de mortalidad mayor al administrar ventilación activada versus VMC. No se observaron desventajas de la VPPAF ni de la ventilación activada con respecto a otros resultados.
Conclusiones de los autores
En comparación con la ventilación convencional, se demuestra un beneficio de la VPPAF y de la ventilación activada con respecto a una reducción de la pérdida de aire y una menor duración de la ventilación, respectivamente. En ningún ensayo se realizó una monitorización respiratoria compleja y, por lo tanto, no es posible concluir que el mecanismo de producción de esos beneficios es la ventilación sincronizada. La ventilación activada en forma de VISO ± AP dio lugar a un riesgo mayor de DBP y a una mayor duración de la ventilación en comparación con la OAF. Se debe optimizar el diseño del disparador y del ventilador en lo que se refiere al diagnóstico respiratorio antes de realizar ensayos adicionales. Es esencial que se prueben formas más nuevas de ventilación activada en ensayos aleatorizados con el poder estadístico suficiente para evaluar los resultados a largo plazo antes de incorporarlos a la práctica clínica habitual.
PICOs
Resumen en términos sencillos
Ventilación mecánica sincronizada para la asistencia respiratoria en recién nacidos
La mayoría de los recién nacidos que necesitan asistencia mecánica para apoyar su respiración, también, hasta cierto punto, respiraban por cuenta propia. Es posible que se necesite menos presión si los intentos del recién nacido por respirar se sincronizan con los ciclos mecánicos del respirador. Esta sincronización puede reducir la probabilidad de que se produzcan pérdidas de aire o variaciones en el flujo sanguíneo al cerebro. La revisión de los ensayos halló que la ventilación con presión positiva de alta frecuencia (VPPAF) en comparación con la ventilación mecánica convencional (VMC), redujo el riesgo de pérdidas de aire y que la ventilación activada se asoció con una menor duración de la ventilación. En comparación con la oscilación de alta frecuencia, sin embargo, ciertas modalidades de ventilación activada dieron lugar a un riesgo mayor de enfermedad pulmonar crónica moderada a grave y una duración mayor de la ventilación. Las formas más nuevas de la ventilación activada sólo se han evaluado en ensayos aleatorizados pequeños y no se han demostrado ventajas en resultados clínicos importantes.
Authors' conclusions
Background
Description of the condition
The majority of neonates breathe during mechanical ventilation. Those that actively exhale against positive pressure inflation develop pneumothoraces; whereas if positive pressure inflation and spontaneous inspiration coincide (synchrony), oxygenation and carbon dioxide elimination improve. If synchrony occurs, it should be possible to achieve adequate gas exchange at lower inflating pressures, reducing barotrauma, a known risk factor for bronchopulmonary dysplasia. Active expiration is more common if infants have non‐compliant lungs and are ventilated with long inspiratory times or relatively slow ventilator rates (30 to 40 bpm), or both. Increasing ventilator rate and reducing inspiratory time, mimicking more closely the preterm infant's respiratory pattern, has been shown in a proportion of infants to stop them actively expiring.
Description of the intervention
Synchronised ventilation can be achieved by HFPPV during which the ventilator rate more closely resembles the spontaneous respiratory rate of very prematurely born infants, and this is more likely to be associated with synchronous ventilation. It can also be achieved by PTV (ACV, SIMV, PRVCV or PSV), during which the infant's respiratory efforts exceeding a critical level trigger a positive pressure inflation. In SIMV only a preset number of the infant's respiratory efforts trigger positive pressure inflations.
How the intervention might work
During mechanical ventilation, infants actively exhale against positive pressure inflation and develop pneumothoraces. Theoretically, by increasing synchronous ventilation, either HFPPV or PTV could reduce air leaks and associated intraventricular haemorrhage and BPD. Indeed, 'triggered ventilation' compared to conventional mechanical ventilation (CMV) has been shown to improve tidal volume (Jarreau 1996) and oxygenation (Cleary 1995) and reduce blood pressure fluctuations (Hummler 1996). Synchronised mechanical ventilation might also reduce baro trauma and hence BPD.
Why it is important to do this review
The aim of this review was to determine whether HFPPV or triggered ventilation were associated with positive outcomes for prematurely born neonates. This review updates the existing review of synchronised ventilation which was published in the Cochrane Database of Systematic Reviews Issue 1, 2008 (Greenough 2008).
Objectives
To compare the efficacy of:
(i) synchronised mechanical ventilation, delivered as high‐frequency positive pressure ventilation (HFPPV) or patient‐triggered ventilation (assist control ventilation (ACV) and synchronous intermittent mandatory ventilation (SIMV)), with conventional ventilation or high‐frequency oscillation (HFO);
(ii) different types of triggered ventilation (ACV, SIMV, pressure‐regulated volume control ventilation (PRVCV), SIMV with pressure support (PS) and pressure support ventilation (PSV))
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials comparing the use of synchronised ventilation (HFPPV or patient‐triggered ventilation) to conventional ventilation or high‐frequency oscillation, and randomised trials of different modes of triggered ventilation in neonates were considered for this review.
Types of participants
Neonates (less than four weeks of age) requiring assisted ventilation.
Types of interventions
We considered two forms of ventilation likely to induce synchrony:
high‐frequency positive pressure ventilation (HFPPV, ventilator rates ≥ 60 bpm); and triggered ventilation.
Triggered ventilation was divided into:
-
assist control ventilation (ACV), otherwise known as synchronous intermittent positive pressure ventilation (SIPPV), the infant being able to trigger a positive pressure inflation with each breath;
-
synchronised intermittent mandatory ventilation (SIMV), the infant being able to trigger only a pre‐set number of positive pressure inflations;
-
pressure‐regulated volume control ventilation (PRVCV), a synchronised pressure‐limited assist control mode that sequentially varies the delivered pressure to approximate a target inspiratory tidal volume;
-
pressure support ventilation, the initiation and end of inflation triggered by the infant's respiratory effort;
-
SIMV with pressure support (PS); PS assists every additional spontaneous breath beyond the set SIMV rate.
These modes of ventilation were compared to non‐synchronised ventilation either in the form of conventional ventilation (CMV), which for the purpose of this review is defined as pressure pre‐set, time‐limited ventilation delivered at rates of fewer than 60 bpm, or high‐frequency oscillatory ventilation (HFO).
Infants were randomly allocated to receive one or other forms of ventilation (except in Heicher 1981 when alternate allocation was used):
-
HFPPV versus CMV;
-
ACV or SIMV versus CMV;
-
SIMV or SIMV + PS versus HFO.
Triggered modes of ventilation were compared:
-
ACV versus SIMV;
-
SIMV plus PS versus SIMV;
-
SIMV versus PRVCV;
-
SIMV versus PSV;
-
ACV versus PSV.
Types of outcome measures
Primary outcomes
Data regarding clinical outcomes included:
-
mortality;
-
air leaks (pneumothorax or pulmonary interstitial emphysema (PIE));
-
severe intraventricular haemorrhage (grades 3 and 4);
-
bronchopulmonary dysplasia (BPD) (oxygen dependency beyond 28 days);
-
moderate/severe BPD (oxygen dependency or respiratory support dependency (or both) at 36 weeks' postmenstrual age);
-
duration of weaning/ventilation.
Search methods for identification of studies
Electronic searches
For the 2016 update we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 5) in The Cochrane Library; MEDLINE via PubMed (1996 to June 5 2016); EMBASE (1980 to June 5 2016); and CINAHL (1982 to June 5 2016) using the following search terms: (mechanical ventilation[MeSH] OR respiration, artificial[MeSH] OR mechanical ventilation OR triggered ventilation OR SIMV), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry).
For the previous version of this review we searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2008); Oxford Database of Perinatal Trials; MEDLINE from 1966 to 2008; and EMBASE 1996 to 2008 (MeSH terms: mechanical ventilation; triggered ventilation; artificial respiration; newborn infant).
Searching other resources
We searched previous reviews, abstracts, symposia proceedings as well as conducting handsearches of journals in the English language and establishing contact with expert informants.
Data collection and analysis
Three of the review authors (AS, TR, VM) identified trials that might be included. Each trial was then assessed independently by each review author who completed data collection forms that the review authors had previously agreed upon. The results were then compared and if there was disagreement a fourth and fifth review author (AG, ADM) assessed the results independently. For each included trial, we collected information regarding the method of randomisation, blinding, stratification, number of centres participating in the study, trial inclusion and exclusion criteria and sample size. We also collected demographic data of the trial participants (e.g. gestational age, birth weight, postnatal age, primary diagnosis). We analysed information on clinical outcomes including death, air leaks, intraventricular haemorrhage, chronic lung disease, duration of ventilation or weaning, ventilation mode failure and extubation failure. The denominator for each outcome was the number randomised. In the meta‐analyses involving comparison with CMV or HFO, either HFPPV or ACV/SIMV was designated the experimental therapy. In the meta‐analyses of ACV or PRVCV versus SIMV, then ACV or PRVCV was designated the experimental therapy. In the meta‐analyses of SIMV with PS versus SIMV, SIMV with PS was designated the experimental therapy, and in the meta‐analysis of ACV versus PSV, ACV was designated the experimental therapy.
Results
Description of studies
Included studies
We identified twenty‐two studies for inclusion in this review (see Figure 1).
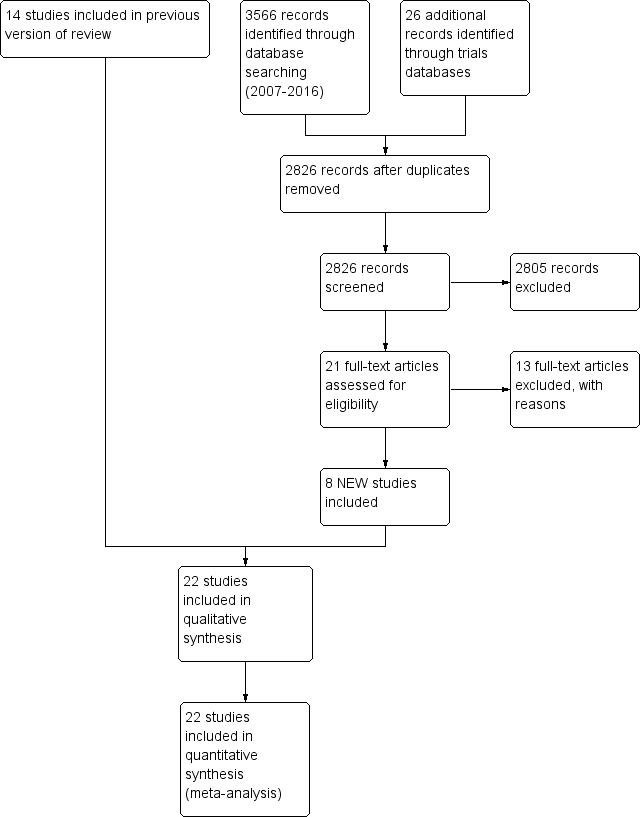
Study flow diagram: review update
1. HFPPV versus CMV (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013).
2. ACV/SIMV versus CMV: Chan 1993 (ACV versus CMV); Donn 1994 (SIMV versus CMV); Bernstein 1996 (SIMV versus CMV); Chen 1997 (SIMV versus CMV); Baumer 2000 (ACV versus CMV); Beresford 2000 (ACV versus CMV); Liu 2011 (ACV versus CMV).
3. SIMV or SIMV + PS versus HFO (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014)
4. ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b)
5. SIMV plus PS versus SIMV (Reyes 2006)
6. SIMV versus PRVCV (D'Angio 2005)
7. SIMV versus PSV (Erdemir 2014)
8. ACV versus PSV (Patel 2012)
Pohlandt 1992, Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Baumer 2000 and Beresford 2000 included only preterm infants. Donn 1994 included preterm infants with a birth weight between 1100 grams and 1500 grams. Courtney 2002a included infants with a birth weight between 601 grams and 1200 grams. Reyes 2006 included preterm infants with a birth weight of 500 grams to 1000 grams. Singh 2012 included infants with a birth weight of greater than 750 grams. D'Angio 2005 included preterm infants with a birth weight of 500 grams to 1249 grams. Erdemir 2014 and Sun 2014 included preterm infants with birth weight less than 1500 grams. Craft 2003a included preterm infants with birth weight less than 1000 grams. Bernstein 1996, Heicher 1981 and OCTAVE 1991 studied mainly premature neonates. Chen 1997 included term and preterm infants but analysed the groups separately; only the results from the 63 infants with RDS are included in the meta‐analysis. Amini 2013 does not make it clear whether term as well as preterm infants were included. Patel 2012 included infants of any gestation who were less than 14 days old.
Information regarding use of antenatal steroids was given only in Baumer 2000, Beresford 2000, Courtney 2002a, Craft 2003a, D'Angio 2005, Reyes 2006, Patel 2012, Singh 2012, Erdemir 2014 and Sun 2014. Data regarding surfactant usage were available in the trials of Donn 1994, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Bernstein 1996, Chen 1997, Baumer 2000, Beresford 2000, Courtney 2002a, Craft 2003a, D'Angio 2005, Reyes 2006, Patel 2012, Singh 2012, Amini 2013, Erdemir 2014 and Sun 2014. The age at entry varied between studies; the trials of Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Reyes 2006, Patel 2012 and Erdemir 2014 were weaning studies. Data for the duration of ventilation or weaning were obtained by personal communication with the investigators for Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Baumer 2000 and Beresford 2000.
Excluded studies
Seventy‐two additional studies were detected, but were found to be not eligible for inclusion in this review (see table Characteristics of excluded studies).
Risk of bias in included studies
All the studies but one were randomised; Heicher 1981 used alternate allocation. The method of randomisation is reported in all but one trial (Chen 1997). Certain outcomes were only available in some of the trials and therefore only presented for the subgroups in which they were reported for at least two trials, except for the trials assessing PSV, PRVCV and SIMV plus PS.
HFPPV versus CMV: In Heicher 1981 and OCTAVE 1991 analysis was by intention to treat, but in Pohlandt 1992 infants not ventilated strictly according to the technique to which they were randomised were excluded from the analysis.
SIMV/ACV versus CMV: Analysis was according to intention to treat in Chan 1993 and Baumer 2000; in Bernstein 1996, 6% of infants erroneously randomised because of problems including seizures, non‐viability and birth weight of less than 500 grams were excluded from the analysis.
SIMV or SIMV + PS versus HFO: Analysis was according to intention to treat in Courtney 2002a and Sun 2014. In Craft 2003a an ad‐hoc interim analysis was performed due to declining recruitment and the study was curtailed.
ACV versus SIMV: Dimitriou 1995 is presented as two studies (Dimitriou 1995a; Dimitriou 1995b), as two separate consecutive randomised trials were performed in which ACV was compared to two different methods of delivering SIMV. In Chan 1994, Dimitriou 1995a and Dimitriou 1995b weaning failure was defined as no reduction in ventilator settings over a 24 or 48 hour period respectively; extubation failure was defined as a requirement for re‐intubation using standardised criteria within a 48 hour period.
SIMV plus PS versus SIMV: In Reyes 2006 analysis was by intention to treat; five infants who were randomised met exclusion criteria and their results were not included in the analysis.
PRVCV versus SIMV: In D'Angio 2005 analysis was by intention to treat; one infant did not receive the allocated intervention.
SIMV vs PSV: In Erdemir 2014 weaning and extubation was performed according to standardised criteria. Analysis was not stated to be by intention to treat; however there were no failures of weaning reported in either arm.
ACV vs PSV: In Patel 2012 analysis was by intention to treat. Weaning was by standardised criteria.
Effects of interventions
HFPPV versus CMV (comparison 1)
Death (Analysis 1.1): Four trials reported this outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013). None demonstrated a significant effect. The meta‐analysis indicates a trend towards reduction in death rate using HFPPV but this does not reach statistical significance (relative risk (RR) 0.78, 95% confidence interval (CI) 0.61 to 1.00).
Air leaks (Analysis 1.2 and Analysis 1.3): Three trials reported pneumothorax as an outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992); one trial reported a significant effect, with a lower rate of pneumothorax in the HFPPV group (Heicher 1981). The meta‐analysis supports a significant reduction in the risk of pneumothorax (typical RR for pneumothorax was 0.69, 95% CI 0.51 to 0.93). Pohlandt 1992 reported a significant reduction in PIE in the HFPPV group (RR 0.68, 95% CI 0.49 to 0.94). Amini 2013 reported total air leaks as outcome variable, with a non‐significant trend favouring HFPPV (RR 0.25, 95% CI 0.06 to 1.08).
BPD (oxygen dependency at 28 days) (Analysis 1.4): Four trials reported this outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
IVH (Analysis 1.5): One trial reported this outcome, with a significantly lower rate of IVH in the HFPPV group (RR 0.13, 95% CI 0.02 to 0.94) (Amini 2013).
Other outcomes: The incidence of PDA post randomisation was given in two trials (Heicher 1981; Pohlandt 1992); in neither did it differ significantly.
ACV/SIMV versus CMV (comparison 2)
Death (Analysis 2.1): Six trials reported this outcome (Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis indicates a trend towards an increase in death rate using ACV/SIMV but this does not reach statistical significance (typical RR 1.17, 95% CI 0.94 to 1.47).
Air leaks (Analysis 2.2): Seven trials reported this outcome (Chan 1993; Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
Duration of ventilation (hours) (Analysis 2.3): Five trials reported this outcome (Donn 1994; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). Bernstein 1996 also reported the duration of ventilation, but the data are presented as the median and 95% CIs (SIMV 103 hours, 95% CI 94 to 118 versus CMV 120 hours, 95% CI 101 to 142), and the results, therefore, could not be meta‐analysed in the relevant Outcome (2.3). A significantly shorter duration of ventilation was noted in Chen's study (Chen 1997) and Donn's study (Donn 1994). The meta‐analysis of the five studies supported a significant reduction in ventilation duration (MD −38.30 hours, 95% CI −53.90 to −22.69). Chan 1993 reported the duration of weaning: ACV mean 39 hours, SD 45 versus CMV mean 65 hours, SD 75 (the results are not presented in Outcome 2.3).
Extubation failure (Analysis 2.4): Four trials reported this outcome (Chan 1993; Donn 1994; Chen 1997; Baumer 2000). One trial reported a significant effect in favour of ACV/SIMV (Chen 1997). The meta‐analysis of the results of the four trials, however, did not demonstrate a significant effect, the typical RR being 0.93 (95% CI 0.68 to 1.28).
Severe IVH (Analysis 2.5): Six trials reported this outcome (Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
BPD (oxygen dependency at 28 days) (Analysis 2.6): Four trials reported this outcome (Baumer 2000; Bernstein 1996; Chen 1997; Donn 1994). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
Moderate/severe BPD (Oxygen dependency at 36 weeks postmenstrual age) (Analysis 2.7): Two trials reported this outcome (Baumer 2000; Beresford 2000). Neither demonstrated a significant effect. The meta‐analysis found no evidence of effect.
Other outcomes: In two trials the incidence of PDA is given post randomisation (Chen 1997; Beresford 2000). In one trial only the incidence of PDA requiring indomethacin and/or ligation was higher in the conventional group for both survivors (P < 0.05) and the whole population of infants (P < 0.05) (Beresford 2000).
SIMV OR SIMV + PS versus HFO (comparison 3)
Death (Analysis 3.1): Four trials of SIMV OR SIMV + PS VS HFO reported this outcome (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014). Sun 2014 reported a significant effect in favour of HFO (RR 3.21, 95% CI 1.07 to 9.67). No other trials reported a difference in this outcome, and the meta‐analysis found no evidence of effect.
BPD: oxygen dependency at 28 days (Analysis 3.2): One trial reported this outcome with no significant effect (Singh 2012).
Moderate/Severe BPD (Analysis 3.3): Three trials reported this outcome (Courtney 2002a; Craft 2003a; Sun 2014). Sun 2014 reported a significant effect in favour of HFO (RR 2.24, 95% CI 1.20 to 4.18) and Courtney 2002a reported a trend in favour of HFO (RR 1.24, 95% CI 0.96 to 1.60). The meta‐analysis showed a significant effect in favour of HFO (RR 1.33, 95% CI 1.07 to 1.65).
Death or BPD (Analysis 3.4): Only one study reported this combined outcome, with a significant effect in favour of HFO (RR 2.38, 95% CI 1.41 to 4.03) (Sun 2014).
Duration of mechanical ventilation (Analysis 3.5): Two trials reported this outcome with both showing a significant effect in favour of HFO (Singh 2012; Sun 2014). The meta‐analysis demonstrated a significant effect in favour of HFO (Mean difference 1.89 days, 95% CI 1.04 to 2.74).
Air Leaks (Analysis 3.6): One trial reported PIE as an outcome and showed a significant decrease of this outcome in the SIMV group (RR 0.66, CI 0.44 to 0.99) (Courtney 2002a). Three trials reported pneumothorax as an outcome with no significant difference (Courtney 2002a; Craft 2003a; Sun 2014).
IVH Grade 3 or 4 (Analysis 3.7): Four trials reported this outcome (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014). No trial found a significant difference.
ACV versus SIMV (comparison 4)
Duration of weaning (hours) (Analysis 4.1): Three trials of ACV versus SIMV reported this outcome (Chan 1994; Dimitriou 1995a; Dimitriou 1995b). None demonstrated a significant effect. In all three, however, the duration of weaning tended to be shorter in infants supported by ACV rather than SIMV. The meta‐analysis supported a trend in this direction which, however, did not reach statistical significance.
Weaning failure (Analysis 4.2): Three trials of ACV versus SIMV reported this outcome (Chan 1994; Dimitriou 1995a; Dimitriou 1995b). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
Extubation failure (Analysis 4.3): Three trials of ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b) reported this outcome. None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
Air leaks (Analysis 4.4): Three trials of ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b) reported this outcome. None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
SIMV PLUS PS versus SIMV (comparison 5)
Only Reyes 2006 reports on SIMV plus PS compared to SIMV alone.
Death (Analysis 5.1): There was no significant effect with regard to death at 28 days or death prior to discharge.
Air leaks (Analysis 5.2): The occurrence of PIE and pneumothorax are reported separately; there were no significant differences in either outcome.
BPD, oxygen dependency at 28 days (Outcome 5.3.1): There was no significant effect.
Moderate/severe BPD, oxygen dependency at 36 weeks postmenstrual age (Outcome 5.3.2): There was no overall significant effect.
Severe IVH (Grade III and IV) (Analysis 5.4): There was no significant effect.
Other outcomes: There was no significant effect re PDA. Days on mechanical ventilation and supplementary oxygen did not differ by ventilation status, but in the subgroup of infants with birth weight of 700 grams to 1000 grams, the days of supplementary oxygen were lower in the SIMV plus PS group (P = 0.034).
PRVCV versus SIMV (comparison 6)
Death prior to discharge (Analysis 6.1): One trial (D'Angio 2005) reported this with no significant difference between intervention.
BPD, oxygen requirement at 36 weeks' postmenstrual age in survivors (Analysis 6.2): One trial reported this with no significant difference between intervention (D'Angio 2005).
Airleak (PIE (Outcome 6.3.1) or Pneumothorax (Outcome 6.3.2)): In the PRVCV versus SIMV trial there were no significant effects with regard to either pneumothorax or PIE (D'Angio 2005).
Severe IVH (Analysis 6.4): One trial reported this outcome with no significant difference between intervention (D'Angio 2005).
SIMV versus PSV (comparison 7)
Duration of weaning (Analysis 7.1): One trial reported this outcome (Erdemir 2014). There was no significant difference.
Extubation failure (Analysis 7.2): One trial reported this outcome (Erdemir 2014). There was no significant difference.
Air leaks (total) (Analysis 7.3): One trial reported this outcome (Erdemir 2014). There was no significant difference.
Moderate/Severe BPD; Oxygen requirement at 36 weeks postmenstrual age (Analysis 7.4): One trial reported this outcome (Erdemir 2014). There was no significant difference.
ACV versus PSV (comparison 8)
Duration of weaning (Outcome 8.1): One trial reported this outcome (Patel 2012). The data were reported as median and range. No significant difference was reported.
Discussion
Physiological studies have demonstrated in prematurely born neonates that both high‐frequency positive pressure ventilation and triggered ventilation are more likely to provoke a synchronous respiratory interaction; that is, the infant's inspiratory efforts coincide with positive pressure inflations. When compared to CMV, those ventilation modes were shown to be associated with improved ventilation. Unfortunately, in none of the subsequent randomised trials is it reported whether synchronous ventilation was achieved and few outcome measures are consistently reported in all relevant trials. Nevertheless, the meta‐analyses demonstrate a significant decrease in air leak and a shorter duration of ventilation with HFPPV and triggered ventilation respectively. However, no significant effect on the incidence of BPD or death has been shown using either HFPPV or trigger ventilation modes.
No deleterious effects of those two ventilatory modes were highlighted. Some positive effects have been demonstrated of the newer triggered modes PRVCV and SIMV plus PS, but both modes have each only been examined in one randomised trial; further trials are required which incorporate long‐term outcomes.
A meta‐analysis of the studies comparing SIMV or SIMV + PS with HFO show a significant decrease in moderate or severe BPD, and significantly shorter duration of mechanical ventilation with HFO. Whether other trigger modes such as ACV have similar results to HFO requires investigating.

Study flow diagram: review update

Comparison 1: HFPPV vs CMV, Outcome 1: Death

Comparison 1: HFPPV vs CMV, Outcome 2: Air leaks

Comparison 1: HFPPV vs CMV, Outcome 3: Total air leak

Comparison 1: HFPPV vs CMV, Outcome 4: BPD (oxygen dependency at 28 days)

Comparison 1: HFPPV vs CMV, Outcome 5: IVH

Comparison 2: ACV/SIMV vs CMV, Outcome 1: Death

Comparison 2: ACV/SIMV vs CMV, Outcome 2: Air leaks

Comparison 2: ACV/SIMV vs CMV, Outcome 3: Duration of ventilation (hours)

Comparison 2: ACV/SIMV vs CMV, Outcome 4: Extubation failure

Comparison 2: ACV/SIMV vs CMV, Outcome 5: Severe IVH

Comparison 2: ACV/SIMV vs CMV, Outcome 6: BPD (oxygen dependency at 28 days)

Comparison 2: ACV/SIMV vs CMV, Outcome 7: Moderate/Severe BPD (oxygen dependent at 36 weeks PCA)

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 1: Death

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 2: BPD: oxygen requirement at 28 days

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 3: Moderate/ Severe BPD: Oxygen requirement at 36 weeks PCA

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 4: Death or BPD

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 5: Duration of mechanical ventilation

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 6: Air leaks

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 7: IVH Grade 3/4

Comparison 4: ACV vs SIMV, Outcome 1: Duration of weaning (hours)

Comparison 4: ACV vs SIMV, Outcome 2: Weaning failure

Comparison 4: ACV vs SIMV, Outcome 3: Extubation failure

Comparison 4: ACV vs SIMV, Outcome 4: Air leaks

Comparison 5: PS + SIMV versus SIMV, Outcome 1: Death

Comparison 5: PS + SIMV versus SIMV, Outcome 2: Air leaks

Comparison 5: PS + SIMV versus SIMV, Outcome 3: BPD

Comparison 5: PS + SIMV versus SIMV, Outcome 4: Severe IVH (grade III and IV)

Comparison 6: PRVCV vs SIMV, Outcome 1: Death prior to discharge

Comparison 6: PRVCV vs SIMV, Outcome 2: BPD: oxygen requirement at 36 weeks in survivors

Comparison 6: PRVCV vs SIMV, Outcome 3: Air leak

Comparison 6: PRVCV vs SIMV, Outcome 4: Severe IVH

Comparison 7: PSV vs SIMV, Outcome 1: Duration of weaning

Comparison 7: PSV vs SIMV, Outcome 2: Extubation failure

Comparison 7: PSV vs SIMV, Outcome 3: Air leaks (total)

Comparison 7: PSV vs SIMV, Outcome 4: Moderate/Severe BPD: oxygen requirement at 36 weeks PCA
| Duration of weaning | ||||
| Study | Participants | Duration of weaning ACV (Median (range)) | Duration of weaning PSV (Median(range)) | Significance |
|---|---|---|---|---|
| Patel 2012 | 36 | 34 (7‐100) | 27 (10‐169) | p=0.88 |
Comparison 8: ACV versus PSV, Outcome 1: Duration of weaning
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Death Show forest plot | 4 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 1.2 Air leaks Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Pneumothorax | 3 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 1.2.2 Pulmonary interstitial emphysema | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 1.3 Total air leak Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.08] |
| 1.4 BPD (oxygen dependency at 28 days) Show forest plot | 4 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 1.5 IVH Show forest plot | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Death Show forest plot | 6 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.47] |
| 2.2 Air leaks Show forest plot | 7 | 1830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 2.3 Duration of ventilation (hours) Show forest plot | 5 | 1463 | Mean Difference (IV, Fixed, 95% CI) | ‐38.30 [‐53.90, ‐22.69] |
| 2.4 Extubation failure Show forest plot | 4 | 1056 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 2.5 Severe IVH Show forest plot | 6 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 2.6 BPD (oxygen dependency at 28 days) Show forest plot | 4 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.12] |
| 2.7 Moderate/Severe BPD (oxygen dependent at 36 weeks PCA) Show forest plot | 2 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Death Show forest plot | 4 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.91, 1.71] |
| 3.2 BPD: oxygen requirement at 28 days Show forest plot | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.37, 27.83] |
| 3.3 Moderate/ Severe BPD: Oxygen requirement at 36 weeks PCA Show forest plot | 3 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.07, 1.65] |
| 3.4 Death or BPD Show forest plot | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.41, 4.03] |
| 3.5 Duration of mechanical ventilation Show forest plot | 2 | 466 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [1.04, 2.74] |
| 3.6 Air leaks Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.19] |
| 3.6.1 Pulmonary interstitial emphysema | 1 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.99] |
| 3.6.2 Pneumothorax | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.82, 1.64] |
| 3.7 IVH Grade 3/4 Show forest plot | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Duration of weaning (hours) Show forest plot | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐42.38 [‐94.35, 9.60] |
| 4.2 Weaning failure Show forest plot | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.93] |
| 4.3 Extubation failure Show forest plot | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.37, 2.67] |
| 4.4 Air leaks Show forest plot | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.23, 2.83] |
| 4.4.1 Total air leaks | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.23, 2.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Death Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Death during first 28 days | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.01] |
| 5.1.2 Death prior to discharge | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.31, 2.43] |
| 5.2 Air leaks Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Pneumothorax | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2.2 Pulmonary interstitial emphysema | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.15] |
| 5.3 BPD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 BPD (oxygen dependency at 28 days) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.84, 1.23] |
| 5.3.2 Moderate/Severe BPD (oxygen dependency at 36 weeks PMA) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.18] |
| 5.4 Severe IVH (grade III and IV) Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Death prior to discharge Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.11] |
| 6.2 BPD: oxygen requirement at 36 weeks in survivors Show forest plot | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.55, 1.27] |
| 6.3 Air leak Show forest plot | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.51, 2.13] |
| 6.3.1 PIE | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.56, 4.91] |
| 6.3.2 Pneumothorax | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.26, 1.88] |
| 6.4 Severe IVH Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Duration of weaning Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐26.53, 3.93] |
| 7.2 Extubation failure Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.57, 2.07] |
| 7.3 Air leaks (total) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.00] |
| 7.4 Moderate/Severe BPD: oxygen requirement at 36 weeks PCA Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.46, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Duration of weaning Show forest plot | 1 | Other data | No numeric data | |

