Resección y ablación del endometrio versus histerectomía para el sangrado menstrual abundante
Resumen
Antecedentes
El sangrado menstrual abundante (SMA), que incluye la menorragia y la metrorragia, es una causa importante de enfermedad en las pacientes. Con frecuencia, el tratamiento quirúrgico del SMA sigue al tratamiento médico que fracasa o no es efectivo. El tratamiento definitivo es la histerectomía, pero éste es un procedimiento quirúrgico mayor con complicaciones emocionales y físicas significativas, así como con costos sociales y económicos. Se han desarrollado varias técnicas quirúrgicas menos invasivas (p.ej., resección transcervical del endometrio (RTCE), procedimientos con láser) y diversos métodos de ablación del endometrio con el objetivo de mejorar los síntomas menstruales al eliminar o extirpar todo el espesor del endometrio.
Objetivos
El objetivo de esta revisión es comparar la efectividad, la aceptabilidad y la seguridad de las técnicas de destrucción del endometrio por cualquier medio versus la histerectomía por cualquier medio para el tratamiento del sangrado menstrual abundante.
Métodos de búsqueda
Las búsquedas electrónicas de ensayos controlados aleatorios relevantes (ECA) se dirigieron, pero no se limitaron a los siguientes: registro de ensayos del Grupo Cochrane de Trastornos Menstruales y Subfertilidad (Cochrane Menstrual Disorders and Subfertility Group), MEDLINE, EMBASE, PsycINFO y el registro de ensayos Cochrane CENTRAL. Se hicieron intentos por identificar ensayos mediante el examen de las listas de citas de artículos de revisión y guías, así como la realización de búsquedas manuales. Se hicieron búsquedas en 2007, 2008 y 2013.
Criterios de selección
En la revisión, se incluyó cualquier ECA que comparara técnicas de destrucción del endometrio por cualquier medio con histerectomía por cualquier medio para el tratamiento del sangrado menstrual abundante en pacientes premenopáusicas.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, buscaron los estudios, extrajeron los datos y evaluaron el riesgo de sesgo. Se calcularon los cocientes de riesgos (CR) para los resultados dicotómicos y las diferencias de medias (DM) para los resultados continuos. Los resultados analizados incluyeron mejoría en la pérdida de sangre menstrual, satisfacción, cambio en la calidad de vida, duración de la cirugía y la estancia hospitalaria, tiempo para retornar al trabajo, eventos adversos y necesidad de cirugía repetida debido al fracaso del tratamiento quirúrgico inicial.
Resultados principales
Se identificaron ocho ECA que cumplieron con los criterios de inclusión de esta revisión. De dos ensayos los revisores identificaron publicaciones múltiples que evaluaron resultados diferentes en puntos temporales postoperatorios diferentes en las mismas pacientes.
Se observaron ventajas a favor de la histerectomía en comparación con la ablación del endometrio en diversas medidas de mejoría en los síntomas de hemorragia y las tasas de satisfacción. Una proporción ligeramente inferior de pacientes a las que se les realizó la ablación del endometrio percibió mejoría en los síntomas de sangrado al año (CR 0,89; intervalo de confianza [IC] del 95%: 0,85 a 0,93; cuatro estudios, 650 pacientes, I2 = 31%), a los dos años (CR 0,92; IC del 95%: 0,86 a 0,99; dos estudios, 292 pacientes, I2 = 53%) y a los cuatro años (CR 0,93; IC del 95%: 0,88 a 0,99; dos estudios, 237 pacientes, I2 = 79%). El mismo grupo de pacientes también mostró mejorías en la puntuación del gráfico pictórico de evaluación de la pérdida de sangre (GPES) al año (DM 24,40; IC del 95%: 16,01 a 32,79; un estudio, 68 pacientes) y a los dos años (DM 44,00; IC del 95%: 36,09 a 51,91; un estudio, 68 pacientes). La necesidad de cirugía repetida debido al fracaso del tratamiento inicial fue más probable después de la ablación del endometrio que después de la histerectomía al año (CR 14,9; IC del 95%: 5,2 a 42,6; seis estudios, 887 pacientes, I2 = 0%), a los dos años (CR 23,4; IC del 95%: 8,3 a 65,8; seis estudios, 930 pacientes, I2 = 0%), a los tres años (CR 11,1; IC del 95%: 1,5 a 80,1; un estudio, 172 pacientes) y a los cuatro años (CR 36,4; IC del 95%: 5,1 a 259,2; un estudio, 197 pacientes). En su mayoría, los eventos adversos graves y leves fueron significativamente más probables después de la histerectomía durante la estancia hospitalaria. Las pacientes a las que se les realizó histerectomía tuvieron mayores probabilidades de presentar sepsis (CR 0,2; IC del 95%: 0,1 a 0,3; cuatro estudios, 621 pacientes, I2 = 62%), transfusión de sangre (CR 0,2; IC del 95%: 0,1 a 0,6; cuatro estudios, 751 pacientes, I2 = 0%), pirexia (CR 0,2; IC del 95%: 0,1 a 0,4; tres estudios, 605 pacientes, I2 = 66%), hematoma de la cúpula vaginal (CR 0,1; IC del 95%: 0,04 a 0,3; cinco estudios, 858 pacientes, I2= 0%) y hematoma de la herida (CR 0,03; IC del 95%: 0,00 a 0,5; un estudio, 202 pacientes) antes del alta hospitalaria. Después del alta hospitalaria, la única diferencia que se informó para este grupo fue una tasa mayor de infección (CR 0,2; IC del 95%: 0,1 a 0,5; un estudio, 172 pacientes).
En algunos resultados (como la percepción de la paciente del sangrado y la proporción de pacientes que necesitaron cirugía adicional por SMA), se generó una puntuación GRADE baja, lo que indica que es probable que estudios de investigación adicionales en estas áreas cambien las estimaciones.
Conclusiones de los autores
La resección y la ablación del endometrio ofrecen una alternativa a la histerectomía como tratamiento quirúrgico para el sangrado menstrual abundante. Ambos procedimientos son efectivos y las tasas de satisfacción son altas. Aunque la histerectomía se asocia con un tiempo quirúrgico más largo (en particular por vía laparoscópica), un período de recuperación más largo y tasas mayores de complicaciones postoperatorias, ofrece alivio permanente del sangrado menstrual abundante. El costo inicial de la destrucción del endometrio es significativamente inferior que el de la histerectomía, pero debido a que a menudo es necesaria la repetición del tratamiento, la diferencia de costo se reduce con el transcurso del tiempo.
PICOs
Resumen en términos sencillos
Una comparación de la efectividad y la seguridad de dos tratamientos quirúrgicos diferentes para el sangrado menstrual abundante
Pregunta de la revisión
Esta revisión Cochrane se refiere a las pacientes con sangrado menstrual abundante (SMA), que es la pérdida de sangre menstrual que la mujer siente que es excesiva y que a menudo interfiere con su calidad de vida. Los investigadores de la Colaboración Cochrane compararon la resección o ablación del endometrio versus la histerectomía para las pacientes con SMA. Los factores principales (que se consideran los de mayor importancia) fueron lo bien que cada operación pudo tratar los síntomas de SMA, cómo se sintieron las pacientes con respecto a la realización de cada operación y cuáles fueron las tasas de complicación. Los factores adicionales estudiados fueron cuánto tiempo tomó realizar cada operación, cuánto tiempo tardaron las pacientes en recuperarse de la operación y cuánto cuesta la operación al hospital y a la paciente.
Antecedentes
Los tratamientos quirúrgicos para el SMA incluyen la extracción o destrucción del recubrimiento interior (endometrio) de la matriz (resección o ablación del endometrio) y la extracción quirúrgica de la matriz completa (histerectomía). Ambos métodos son ofrecidos con frecuencia por los ginecólogos, habitualmente pero no siempre después de que un tratamiento no quirúrgico no ha logrado corregir el problema. La resección / ablación del endometrio se realiza mediante la entrada a la matriz, sin la necesidad de un corte quirúrgico. Durante la histerectomía, el útero se puede extraer mediante un corte quirúrgico al abdomen, por la vagina o por cirugía de "mínimo acceso" que incluye cortes quirúrgicos muy pequeños al abdomen (laparoscopía); este último enfoque es una forma más moderna de realizar la histerectomía. La histerectomía es efectiva para detener de forma permanente el SMA, pero elimina la fertilidad y se asocia con todos los riesgos de una cirugía mayor, incluida la infección y la pérdida de sangre. Estos riesgos son más pequeños con la resección / ablación del endometrio.
Fecha de la búsqueda
Los investigadores de la Colaboración Cochrane actualizaron recientemente, en octubre de 2013, la revisión sistemática de los estudios de investigación que comparan la resección y la ablación del endometrio versus la histerectomía para el tratamiento del sangrado menstrual abundante. Después de buscar todos los estudios pertinentes, los revisores incluyeron ocho estudios con 1260 pacientes.
Características de los estudios
En las revisiones Cochrane solamente se incluyen ensayos controlados aleatorios (ECA). Los ECA son estudios en los que los participantes se asignan al azar a uno de dos grupos y cada uno recibe una intervención diferente (en este caso, ablación / resección del endometrio o histerectomía). Luego se comparan los dos grupos. Los ECA que compararon estas dos intervenciones se incluyeron en esta revisión si estudiaban pacientes con SMA que no estaban en la etapa de menopausia y que no presentaban cáncer ni precáncer de útero.
Resultados clave y conclusiones
La revisión de los estudios mostró que la ablación / resección del endometrio es una alternativa efectiva y posiblemente más barata a la histerectomía y con una recuperación más rápida, aunque a veces se necesita la repetición del tratamiento con cirugía adicional. La histerectomía se asocia con la resolución más definitiva de los síntomas, pero con tiempos quirúrgicos más largos y mayores posibilidades de complicaciones quirúrgicas. Generalmente, en ambas operaciones las pacientes informaron que la realización del procedimiento fue aceptable y que estaban satisfechas con la experiencia.
Debido a que la histerectomía laparoscópica es cada vez más utilizada, varias de las desventajas anteriormente descritas de los tipos tradicionales de histerectomía han mejorado y algunos resultados como la duración de la estancia hospitalaria, el tiempo para retornar al trabajo y para realizar las actividades normales se han vuelto más comparables a los de la ablación del endometrio. Sin embargo, la histerectomía laparoscópica se asocia con un tiempo quirúrgico más largo que otras formas de histerectomía y requiere pericia quirúrgica y un equipamiento más complejo.
Solamente tres de los ocho ensayos incluyeron histerectomía laparoscópica en la comparación. Se necesitarán más ensayos en esta área, ya que esta forma de histerectomía está cada vez más disponible.
Identificación de los efectos perjudiciales
Generalmente, ambos tratamientos quirúrgicos se consideran seguros y se informan bajas tasas de complicación. Sin embargo, la histerectomía se asocia con mayores tasas de infección y de necesidad de transfusión de sangre.
Calidad de la evidencia
Las pruebas informadas en esta revisión fueron ocasionalmente de calidad baja, lo que indica que probablemente los estudios de investigación adicionales cambien el resultado. Éste fue el caso de resultados como la percepción de la paciente del sangrado y la proporción de pacientes que necesitan cirugía adicional por SMA.
Authors' conclusions
Summary of findings
| Endometrial resection/ablation for heavy menstrual bleeding | ||||||
| Patient or population: women with heavy menstrual bleeding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Endometrial resection/ablation | |||||
| Woman's perception (proportion with improvement in bleeding symptoms)—within one year of follow‐up | 978 per 1,000 | 890 per 1,000 | RR 0.91 | 650 | ⊕⊕⊝⊝ | |
| PBAC score (continuous data)—at one year of follow‐up | Mean PBAC score (continuous data) at one year of follow‐up in the intervention groups was | 68 | ⊕⊕⊕⊝ | |||
| Adverse events—short term (intraoperative and immediate postoperative)—sepsis | 319 per 1,000 | 61 per 1,000 | RR 0.19 | 621 | ⊕⊕⊕⊝ | |
| Proportion requiring further surgery for HMB—within one year after surgery | 2 per 1,0003 | 36 per 1,000 | RR 14.94 | 887 | ⊕⊕⊝⊝ | |
| Proportion satisfied with treatment—at one year of follow‐up | 820 per 1,000 | 771 per 1,000 | RR 0.94 | 739 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Lack of blinding (although unfeasible) is likely to bias women's responses. 3Result suggests incomplete operation—rare. | ||||||
Background
Description of the condition
Heavy menstrual bleeding (HMB) is a common presenting complaint for primary care services and a frequent reason for secondary referral, with 5% of women between 30 and 49 years of age seeking medical attention for the problem (Vessey 1992; Cooper 2011).
Unacceptably problematic bleeding is most commonly determined by the woman herself if the amount or frequency of blood loss interferes with her physical or psychosocial well‐being. This personal perception is often what determines the need for treatment and assessment of outcomes afterwards.
Many alternative definitions for HMB are used, including menorrhagia, abnormal menstrual bleeding, abnormal uterine bleeding and disordered uterine bleeding. These terms have not always been universally defined, and considerable overlap and confusion between them have been noted in the existing literature (Woolcock 2008). In response to this confusion, the International Federation of Gynaecology and Obstetrics (FIGO) formally defined HMB as ‘the woman's perspective of increased menstrual volume, regardless of regularity, frequency, or duration’ (Munro 2011).
Intervention for HMB should be aimed at correction of identified underlying causes, control of bleeding, amelioration of anaemia and improvement in quality of life measures, with the woman’s contraceptive requirements or desire for future fertility taken into account, along with individual preferences for treatment.
Description of the intervention
Many well‐established treatment options for HMB are available, including hormonal and non‐hormonal medications, but among women who do not desire future fertility or for whom medical treatment may be problematic, inconvenient or prolonged, surgical management may be preferred.
Until the mid‐1980s, hysterectomy was the only option for such women, and 60% of women presenting with HMB during this time underwent hysterectomy as a first‐line treatment (NICE 2007). Hysterectomy continues to be performed via the more traditional abdominal and vaginal routes, but minimally invasive techniques have been introduced for women with benign disease, and these techniques continue to be developed. Such approaches include the introduction of laparoscopic hysterectomy (total laparoscopic hysterectomy and laparoscopically assisted vaginal hysterectomy) in 1989 (Reich 1989) and single‐port laparoscopic hysterectomy in 2009 (Li 2012).
Considerable differences between types of approach have been noted with regard to intraoperative complications, duration of hospital stay, length of recovery and cost. However, as this review aims to compare hysterectomy with endometrial resection and ablation, the results for hysterectomy by all approaches will be pooled.
Endometrial ablation is a minimally invasive hysteroscopic surgical intervention for HMB involving removal or destruction of the endometrium. Since it was introduced in the 1990s, its popularity as an alternative to hysterectomy has grown. An examination of the UK National Health Service (NHS) statistics in 2005 revealed that endometrial destruction was being performed more frequently than hysterectomy for benign HMB in the UK (Reid 2007).
Since its invention, techniques for endometrial resection and ablation have evolved considerably as technologies have advanced (Sharp 2012). Currently, no clear evidence of differences in effectiveness or safety between ‘first’‐ and ‘second’‐generation techniques has been found (Lethaby 2005; Fernandez 2011).
‘First‐generation’ techniques included desiccation of the endometrium by way of rollerball endometrial ablation (REA) and transcervical resection of the endometrium with loop electrode. These techniques require direct hysteroscopic vision throughout the procedure, whereby other endometrial pathology such as polyps and fibroids can be diagnosed and, if necessary, removed. A potential complication of endometrial resection is systemic fluid overload due to absorption of hysteroscopic fluid; therefore careful intraoperative input/output fluid balance requires attention and documentation. The efficacy and safety of first‐generation techniques are often operator dependent.
Newer technologies were subsequently introduced, including thermal balloon ablation, microwave ablation, free‐fluid thermal ablation, radiofrequency ablation and cryotherapy. These ‘second‐generation’ techniques have allowed widespread use of endometrial ablation, as they require less advanced hysteroscopic skill and do not necessitate intraoperative fluid balance. However, these procedures depend heavily on the surgical equipment itself for efficacy and safety. In some (but not all) instances, they can be performed without the hysteroscope altogether, making these approaches simpler, quicker and safer.
How the intervention might work
Hysterectomy is regarded as ‘definitive’ treatment for HMB with guaranteed amenorrhoea and cessation of fertility and little need for future treatment. However, hysterectomy by any route is associated with all the complications of major surgery, including intraoperative bleeding, infection and venous thromboembolism. Other reported complications include urinary incontinence and dyspareunia.
Although a successful outcome after endometrial ablation is not guaranteed and further surgery is occasionally required, many women still choose a less invasive surgical option (Nagele 1998; Bourdrez 2004). Endometrial resection/ablation is often chosen because of perceived short hospitalisation, quick return to normal functioning and avoidance of major surgery (Nagele 1998). Vaginal discharge and increased period pain are common complications following endometrial resection/ablation. Uncommon complications include intraoperative uterine perforation and associated pelvic organ injury from direct perforation or electrosurgical burns.
Why it is important to do this review
HMB is a common reason for women to seek treatment, and many choose a surgical option as first‐ or second‐line management. Surgical methods for treatment of HMB show a trend in favour of endometrial resection/ablation and towards newer endometrial resection/ablation techniques. In the late 1980s and early 1990s, 60% of women with HMB who were referred to a gynaecologist in the UK were treated with hysterectomy (Coulter 1991), but an examination of NHS statistics in 2005 revealed that endometrial destruction was being performed more frequently than hysterectomy for benign HMB in the UK (Reid 2007).
Despite this trend, many women still express a preference for first‐line hysterectomy (Kennedy 2002), and rates of different methods of hysterectomy are evolving.
As surgical techniques for both hysterectomy and endometrial resection/ablation continue to evolve, corresponding changes in success rates, outcomes and complications will be noted, necessitating regular comparison and review. At the time of the 2008 review, robust evidence in the literature comparing less invasive hysterectomy techniques versus second‐generation methods of endometrial ablation was lacking.
Objectives
The objective of this review is to compare the effectiveness, acceptability and safety of techniques of endometrial destruction by any means versus hysterectomy by any means for the treatment of heavy menstrual bleeding.
Methods
Criteria for considering studies for this review
Types of studies
Published and non‐published randomised controlled trials (RCTs) were eligible for inclusion. Quasi‐randomised trials were excluded.
Types of participants
Source of recruitment
Primary care, family planning and specialist clinics.
Inclusion criterion
-
Women of reproductive years with heavy menstrual bleeding (including both heavy regular periods (menorrhagia) and heavy irregular periods (metrorrhagia)), measured objectively or subjectively.
Exclusion criteria
-
Postmenopausal bleeding (> 1 year from the last period).
-
HMB caused by uterine malignancy or endometrial hyperplasia.
-
Iatrogenic causes of HMB (e.g. intrauterine coil devices).
Types of interventions
-
Endometrial resection and ablation (including first‐generation techniques, such as transcervical resection of the endometrium with loop electrode or rollerball, and second‐generation techniques, such as endometrial ablation by thermal balloon, microwave, thermal free‐fluid, radiofrequency and cryotherapy).
-
Hysterectomy (by abdominal, vaginal and laparoscopic/laparoscopically assisted routes).
Types of outcome measures
Primary outcomes
Effectiveness (improvement in bleeding)
1.1 Woman's perception (proportion with improvement in bleeding symptoms).
1.2 PBAC (Pictorial Blood Loss Assessment Chart) score: a visual measure of amount of blood loss (clinically significant HMB correlates with a score > 100).*
1.3 Requirement for further surgery.
Acceptability
1.4 Proportion satisfied with treatment.
Safety (adverse outcomes)
1.5 Adverse events: short term (intraoperative and immediately postoperative).
1.6 Adverse events: long term (after hospital discharge).
Secondary outcomes
1.7 Quality of life scores (continuous data).
1.8 Quality of life (proportion with improvement).
1.9 Duration of surgery.
1.10 Duration of hospital stay.
1.11 Time to return to normal activity.
1.12 Time to return to work.
1.13 Total health service cost per woman.
1.14 Total individual cost per woman.
*PBAC score: A score over 100 suggests significantly heavy menstrual bleeding. A reduction from over 100 to under 100 would be clinically significant at an individual level.
Search methods for identification of studies
We searched all publications that would potentially describe RCTs comparing surgical techniques to resect or ablate the endometrium versus hysterectomy for the treatment of heavy menstrual bleeding. Search strategies for identifying potential studies for inclusion were employed in consultation with the Cochrane Menstrual Disorders and Subfertility Group Trials Search Co‐ordinator.
Electronic searches
The original search was performed in 1999. Updated searches were performed in 2008 and 2013.
1. The principal and second review authors searched the trial register of the Menstrual Disorders and Subfertility Group using the search strategy developed by the Menstrual Disorders and Subfertility Group as follows:
Keywords CONTAINS "menorrhagia" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or "dysfunctional bleeding" or Title CONTAINS "menorrhagia" or "heavy bleeding" or "heavy menstrual bleeding" or "heavy menstrual loss" or "dysfunctional uterine bleeding" or "dysfunctional bleeding".
AND
Keywords CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal" or Title CONTAINS "Hysterectomy" or "Hysterectomy, abdominal" or "hysterectomy, laparoscopically assisted vaginal" or "Hysterectomy, subtotal" or "Hysterectomy, Vaginal".
(See Review Group details for additional information.)
2. The two review authors then searched MEDLINE (1966 to October 2013; Appendix 1), EMBASE (1980 to October 2013; Appendix 2), PsycINFO (1972 to October 2013; Appendix 3) and the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 10, 2013; Appendix 4), using the search strategy developed by The Cochrane Collaboration.
Searching other resources
-
The principal review author searched all registers of the metaRegister of Controlled Trials in October 2013 to identify ongoing trials, trials that may have been missed by the search of electronic databases and trials that were unpublished.
-
Citation lists of all studies that appeared to meet the inclusion criteria of the review, studies found in the literature review stage of the introduction, other relevant studies and evidence‐based guidelines on the management of abnormal bleeding were also searched.
Data collection and analysis
Statistical analysis was performed in accordance with guidelines from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When possible, outcomes were pooled statistically.
Selection of studies
Trials for inclusion in the review were selected by two of the review authors (AL and IC or AL and SS in 2008, and RJF and AL in 2013) after the search strategy described previously was employed. This task was undertaken independently, and the final list of included studies reflected consensus between the two review authors. Details of the screening and selection process are shown in Figure 1.
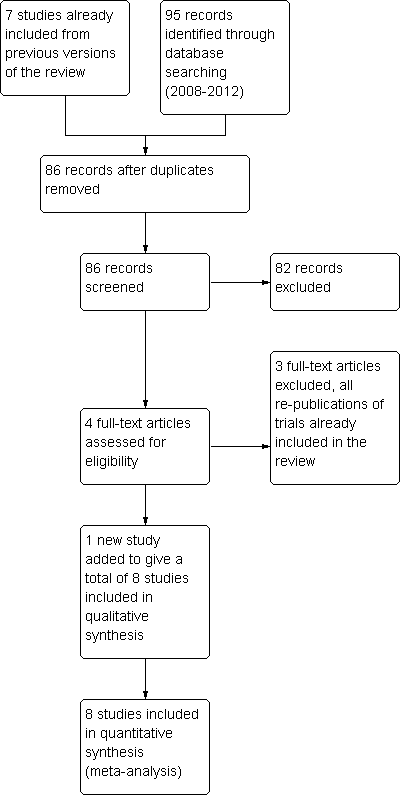
Study screening and selection process (2008 to 2013).
Data extraction and management
All assessments of characteristics of the included studies were performed independently by two review authors (AL and IC in 2008, and RJF and AL in 2013) using forms designed according to Cochrane guidelines and described in Higgins 2011
When necessary, additional information on trial methodology or original trial data were sought from the principal or corresponding author of any trials that appeared to meet the eligibility criteria (see Acknowledgements section for details of the study authors who provided clarification of data beyond that reported in the publications).
Quality criteria and methodological details were extracted from each study as follows.
Trial characteristics
-
Study design.
-
Numbers of women randomly assigned, excluded and lost to follow‐up.
-
Whether an intention‐to‐treat analysis was done.
-
Whether a power calculation was done.
-
Duration, timing and location of the study.
-
Number of centres.
-
Source of funding.
Characteristics of study participants
-
Age and any other recorded characteristics of participants in the study.
-
Other inclusion criteria.
-
Exclusion criteria.
-
Type of surgery.
-
Source of participants.
-
Proportion participating of those eligible.
Interventions used
-
Type of endometrial destruction technique used and route of hysterectomy performed.
Outcomes
-
Methods used to evaluate menstrual symptoms (e.g. PBAC score, woman‐defined 'improvement in symptoms').
-
Methods used to measure requirement for further surgery for HMB.
-
Methods used to evaluate participant satisfaction and change in quality of life postsurgery.
-
Methods used to record adverse surgical events.
-
Methods used to measure resource and individual costs.
-
Methods used to measure duration of surgery, hospital stay and recovery time.
Assessment of risk of bias in included studies
Included trials were assessed for risk of bias using the 'Risk of bias tool' developed by The Cochrane Collaboration and described in Higgins 2011.
-
Sequence generation (whether the allocation sequence was adequately generated to produce comparable groups).
-
Allocation concealment (whether the allocation was adequately concealed).
-
Blinding of participants, personnel and outcome assessors (whether knowledge of the allocated intervention was adequately controlled during the study).
-
Incomplete outcome data (whether incomplete outcome data were adequately addressed).
-
Selective outcome reporting (whether reports of the study were free of the suggestion of selective outcome reporting).
-
Other sources of bias (whether the study was apparently free of other problems that could put it at high risk of bias, e.g. baseline imbalance, bias related to study design, early termination of the study).
Details of the risk of bias for each included study are displayed in Characteristics of included studies and are summarised in Figure 2 and Figure 3.
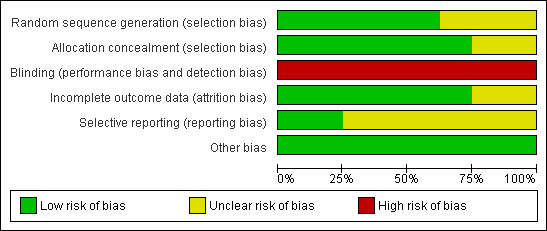
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
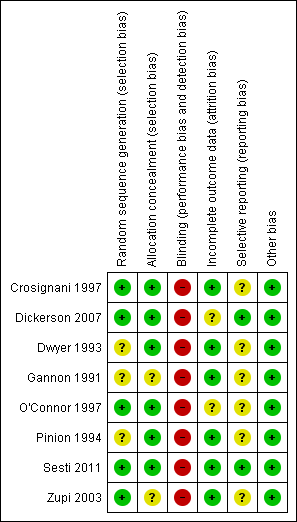
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
For dichotomous data (e.g. number of adverse outcomes), we will use the numbers of events in the control and intervention groups of each study to calculate risk ratios (RRs). For continuous data (e.g. PBAC score), if all studies report exactly the same outcomes, we will calculate mean differences (MDs) between treatment groups. If similar outcomes are reported on different scales, we will calculate the standardised mean difference (SMD). We will reverse the direction of effect of individual studies, if required, to ensure consistency across trials. We will treat ordinal data (e.g. quality of life scores) as continuous data. We will present 95% confidence intervals for all outcomes. When data needed to calculate RRs or MDs are not available, we will utilise the most detailed numerical data available that may facilitate similar analyses of included studies (e.g. test statistics, P values). We will compare the magnitude and direction of effect reported by studies versus how they are presented in the review, while taking account of legitimate differences.
For dichotomous data (e.g. proportion of participants satisfied with their treatment), results of each study were expressed as a risk ratio. For some dichotomous outcomes (e.g. proportion of participants requiring further surgery), a higher proportion represented a negative consequence of that treatment, and for other outcomes (e.g. proportion with improvement in menstrual blood loss), a higher proportion was considered a benefit of treatment. This approach to the categorising of outcomes should be noted when summary graphs for the meta‐analysis are viewed for assessment of benefits as opposed to harms of treatment. Thus, for some dichotomous outcomes, treatment benefit has been displayed as risk ratios and confidence intervals to the left of the centre line, but for others, a treatment benefit has been shown to the right of the centre line. Each outcome was labelled for clarification.
For other outcomes for which high values were considered a negative consequence of treatment, for example, duration of surgery, hospital stay and return to work, evaluation of the summary graphs reveals that means and confidence intervals to the left were considered a benefit of endometrial destruction.
With one exception, quality of life scores were entered as continuous data—mean plus standard deviation values post‐treatment. One study, however, recorded the percentage mean change of Euroqol scores from baseline to four months after treatment. Some scales measured general quality of life summary score; others reported results separately in different categories, representing general quality of life but without a summary score; still others used more specific measurements of aspects of quality of life, such as anxiety, social adjustment and depression.
For some outcomes, women were assessed at different periods of follow‐up after surgery.
-
Menstrual symptoms were recorded at one, two, three and four years after surgery, PBAC scores at one and two years after surgery, and quality of life at four months and one, two and four years post‐treatment.
-
Adverse events were recorded before and after discharge from hospital.
-
Requirement for further surgery was measured separately within the first year and at two, three and four years after treatment.
Unit of analysis issues
The unit of analysis was the woman with HMB who was randomly assigned to endometrial resection/ablation or to hysterectomy.
Dealing with missing data
Data were analysed on an intention‐to‐treat basis as far as possible; otherwise only available data were analysed. When data were found to be missing, attempts were made to obtain this information from the original trial authors.
Assessment of heterogeneity
Heterogeneity (variations) between the results of different studies was examined by visual inspection of scatter of data points on the graphs and overlap in their confidence intervals and, more formally, by examination of the results of the I2 quantity (a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error; Higgins 2008). When statistical heterogeneity was found to be very substantial (> 90%), calculation of a summary effect measure was not considered appropriate, and study data were not pooled. However, results of these studies can be viewed in forest plots that portray the range of values for comparison in each study.
Assessment of reporting biases
Avoidance of reporting bias was attempted by using a robust search strategy with no restrictions on language or publication forum. Multiple publications of the same study were identified for several of the included studies and are referenced as such. The use of funnel plots was planned to explore the possibility of small study effects if 10 or more studies had been included in the analysis.
Data synthesis
Combination of data was not always possible, as some outcomes were measured differently (e.g. some trials used PBAC score as a measure of improvement in bleeding, whilst others used proportion reporting improvement in bleeding symptoms, proportion with excessive bleeding, etc.). Such data were displayed in the forest plots; however, often only one trial contributed data to each plot.
For continuous data (e.g. PBAC score), if all studies reported exactly the same outcomes, mean differences (MDs) between treatment groups were calculated. Continuous outcomes were also displayed differently according to whether benefit or harm was measured. For most quality of life scores, a high score represented a benefit of treatment, but for the Hospital Anxiety and Depression Scale (HAD), a high score represented a greater degree of anxiety or depression. To display on the same graph quality of life scales that differed in this way, HAD scores were displayed as minus values, so that all quality of life continuous outcomes could be included in a single forest plot.
Subgroup analysis and investigation of heterogeneity
A priori, it was planned to explore the possible contribution of differences in trial design to any heterogeneity identified in the manner described above under 'Assessment of heterogeneity'. As a guide, I2 values ≤ 25%, = 50% and ≥ 75% correspond to low, moderate and high levels of heterogeneity (Higgins 2008).
Sensitivity analysis
To assess the robustness of pooled estimates, the following sensitivity analyses were conducted.
-
Estimates whereby all types of hysterectomy were combined versus estimates for which each individual surgical approach was used.
-
Estimates based on all relevant trials regardless of evidence of allocation concealment versus estimates based on trials that provided clear evidence that allocation was concealed.
The following analysis was planned but was not conducted.
-
Estimates based on all relevant trials regardless of missing data and loss to follow‐up versus estimates based on trials in which incomplete outcome assessment was not likely to cause bias.
Overall quality of the body of evidence: summary of findings table
summary of findings Table for the main comparison was generated using GRADEPRO software. This table evaluates the overall quality of the body of evidence for the main review outcomes, using GRADE criteria.
-
Study limitations (i.e. risk of bias).
-
Consistency of effect.
-
Imprecision.
-
Indirectness.
-
Publication bias.
Judgements about evidence quality (high, moderate, low or very low) were justified, documented and incorporated into reporting of results for each of these main outcomes.
Results
Description of studies
Eight RCTs comparing techniques of removal or ablation of endometrium versus hysterectomy by any route for the treatment of heavy menstrual bleeding were included. Two of these trials had multiple publications, each based on the same study population but providing assessment of different outcomes and different follow‐up times, as well as cost‐utility analyses of previously published data (Dwyer 1993; Pinion 1994). Trials comparing different types of endometrial destruction were not considered in this review but are assessed in Lethaby 2009. Details of included studies can be found in the Characteristics of included studies table.
Study design
All included studies used a parallel‐group design. Six of these studies were carried out at a single centre (three in Italy, three in the UK), one study was performed at nine centres throughout the UK and one study was completed at 25 centres in the USA and Canada.
Participants
Participants in all included studies were premenopausal, had symptomatic heavy menstrual bleeding (regular or irregular prolonged or excessive bleeding) and were eligible for (i.e. had shown no response to medical treatment) or were awaiting hysterectomy. Participants in five of the included studies had received a diagnosis of menorrhagia (heavy regular bleeding), and participants in two of the studies had been given a diagnosis of dysfunctional uterine bleeding (which was defined as both regular and irregular ovulatory heavy bleeding and anovulatory abnormal bleeding not due to pathology). Exclusion criteria included large fibroids, large uterine size, pelvic inflammatory disease (PID) and endometriosis and abnormal pathology. Three studies excluded participants with submucosal fibroids.
Interventions
Available data mostly compared first‐generation ablation techniques (predominantly transcervical resection of the endometrium (TCRE)) versus total hysterectomy. Two trials (Gannon 1991; Dwyer 1993) compared TCRE versus abdominal hysterectomy; the latter trial also included a preoperative procedure (medroxyprogesterone acetate injection four to six weeks before surgery) to reduce the thickness of the endometrium for participants undergoing endometrial resection. One trial compared endometrial resection versus vaginal hysterectomy (Crosignani 1997), another trial compared endometrial resection versus hysterectomy (50% abdominal, 50% vaginal) (O'Connor 1997) and another trial compared endometrial destruction after preoperative gonadotrophin‐releasing hormone (GnRH) treatment (50% endometrial resection and 50% laser ablation) versus hysterectomy (88% abdominal and 12% vaginal) (Pinion 1994). One study compared endometrial resection or thermal balloon ablation (according to surgeon choice) versus total hysterectomy (vaginal, laparoscopic or abdominal approach, according to surgeon choice) (Dickerson 2007), another study compared endometrial resection after preoperative GnRH agonist (GnRHa) treatment versus laparoscopic supracervical hysterectomy (Zupi 2003) and the final study compared thermal balloon endometrial ablation versus laparoscopic supracervical hysterectomy (Sesti 2011).
Sensitivity analysis was undertaken to compare trial results with all types of hysterectomy included in the estimates versus exclusion of the more invasive types of hysterectomy to assess effects in the trials in which solely vaginal hysterectomy surgery or laparoscopic supracervical hysterectomy was performed (Crosignani 1997; Zupi 2003; Sesti 2011).
Outcomes
Follow‐up after surgery for all included studies ranged from four months to four years. Six studies assessed change in menstrual bleeding patterns; four of these assessed whether menorrhagia‐like symptoms had resolved (amount and frequency of bleeding), one study (in which participants had a complaint of dysfunctional bleeding) measured separate components of bleeding excess (excessive amount and duration) and another study used PBAC questionnaires. Four studies assessed time to return to normal activities in days. Two studies, in which participants had a diagnosis of dysfunctional bleeding, assessed whether an improvement in overall symptoms had occurred (recorded in one trial as 'problem solved'). Three studies measured costs of treatment.
All but three studies assessed satisfaction with surgery (although this was reported at different follow‐up times) and return to work, all but two studies assessed postoperative complication rate and duration of surgery and all studies assessed hospital stay and requirement for further surgery.
Some outcomes measured time of surgery or recovery time and strictly speaking were time to event outcomes, such as duration of surgery, length of stay in hospital and time to return to normal activities and work. However, these were analysed as continuous data, as all participants had initial and end values representing the time that had elapsed. Time to event analysis is mandatory when censoring is performed and only a subset of participants have an event, but in this review, comparison of means was considered by the review authors to be an acceptable analysis.
Seven studies assessed quality of life after surgery, but several different scales were used. This review has assessed quality of life as measured by the Golombok Rust Inventory of Marital State (one year after surgery), the Short Form‐36 Scale (SF‐36), the Euroqol Visual Analogue Scale, the Hospital Anxiety and Depression Scale (HAD) and the Sabbatsberg Sexual Rating Scale (all one or two years after surgery). The SF‐36 is a generic measure of subjective health in the form of a profile with eight multi‐item dimensions (including physical and emotional role limitation, physical and social functioning, mental health, energy, pain and general health perception) developed in the USA and shown to be an acceptable tool when used by women with HMB (Ware 1993; Coulter 1994). The Euroqol health instrument is a generic single index measure of health‐related quality of life validated in several European countries, including the UK (Euroqol Group 1990; Brazier 1993). The Golombok Rust Inventory was modified by the investigators to obtain a brief measure of the overall quality of the marital relationship (Rust 1986), and the Sabbatsberg Sexual Rating Scale was designed to provide a self assessment of sexual functioning by women engaging in intercourse (Garrat 1995). The Hospital Anxiety and Depression Scale is a self‐assessment mood scale specifically designed to identify states of anxiety and depression and is regarded as a valid measure of the severity of these mood disorders (Zigmond 1983). Several other scales were used to evaluate aspects of quality of life in some of the trials, including the General Health Questionnaire, the Psychosocial Adjustment to Illness Scale, a psychiatric mood scale, a modified social adjustment scale and an unvalidated questionnaire, but these outcomes were not entered into the review because the data were not obtained in a suitable form for inclusion in a meta‐analysis (quantitative data provided in graphical form indicated significant skew; data could not be obtained from study authors).
Five publications from three trials (Cameron 1996 and Aberdeen 1999 from the Pinion 1994 study; Gannon 1991; and Sculpher 1998 and Sculpher 1996 from the Dwyer 1993 study) compared costs to the health service of the two techniques. Two of these trials had two publications reporting total health resource costs of the procedures (including the need for retreatment) at different follow‐up times (Cameron 1996 and Aberdeen 1999 (Pinion 1994); Sculpher 1993 and 1996 (Dwyer 1993)). One trial measured direct costs to the participant after one year (Cameron 1996 (Pinion 1994)).
Two trials (Sculpher 1993 and Sculpher 1996 from Dwyer 1993, and Cameron 1996 and Aberdeen 1999 from Pinion 1994) calculated cost per participant based on resource use. The third trial calculated costs by summing the average costs of variable resources and then adding a factor of 100% to allow for fixed costs (Gannon 1991).
Results of the search
The search was updated in October 2013 and included 62 additional potential references. Titles and abstracts were screened by two review authors (RJF and AL). Full‐text copies of two references that appeared to meet the inclusion criteria were obtained; one was the re‐publication of a trial already included in the 2008 review (Dickerson 2007), and the other was an additional new trial to be included (Sesti 2011), leading to a total of eight trials in the updated review.
Included studies
Eight RCTs of endometrial resection/ablation versus hysterectomy with a total of 1,260 randomly assigned participants met the criteria for inclusion in the review, although not all participants contributed to the assessment of every outcome.
Excluded studies
In the 2008 update of this review, two studies were retrieved for closer inspection and were excluded from the review (Paddison 2003; Lin 2006). One study had allocation according to date of admission and did not satisfy the criteria for true randomisation, and the other was a review of ablation versus hysterectomy.
No further studies were retrieved for closer inspection but subsequently excluded in the 2013 update of the review.
Risk of bias in included studies
All included studies were assessed separately for risk of bias (Higgins 2011). A summary of these assessments is provided in Figure 2 and Figure 3.
Allocation
Five of the eight included studies provided sufficient detail on the adequacy of the randomisation method (Crosignani 1997; O'Connor 1997; Zupi 2003; Dickerson 2007; Sesti 2011), all at low risk of bias. The other three studies did not describe how randomisation was undertaken and were therefore at unclear risk of bias in this category.
All but two studies (Gannon 1991; Zupi 2003) provided sufficient details of allocation concealment; these two studies were therefore at unclear risk of bias, and all other studies were at low risk of bias in this category.
Blinding
Three of the more recent studies used single blinding for assessment of some outcomes (Zupi 2003; Dickerson 2007; Sesti 2011). The other five studies did not appear to have any blinding of participants, investigators or assessors. All studies were therefore at high risk of bias in this category.
Incomplete outcome data
In two studies (O'Connor 1997; Dickerson 2007), withdrawal from the study and/or missing data were greater than 10%, and explanations were not provided to enable judgement of whether this could have biased the results. In the former trial, primary outcomes were analysed by an intention‐to‐treat method (satisfaction rate, quality of life and bleeding outcomes), but intraoperative and perioperative outcomes (adverse events, requirement for further surgery and hospital stay) were analysed according to surgery received. This trial also reported outcomes at three and four years' follow‐up, but as women who were assigned later during the trial had shorter follow‐up, these assessments are likely to be underpowered. The other six studies had withdrawals of less than 10% at the time of calculation of outcomes at the first time point. However, in studies with longer follow‐up, additional loss to follow‐up increased with the duration of the trial.
Selective reporting
Sufficient information was provided in only one study (Dickerson 2007) to indicate that it was free of selective outcome reporting. Before publication of the study results, an earlier publication provided details of the study protocol and changes made throughout the study (Dickerson 2007: other study cited under this ID). The other studies did not provide any evidence of measures taken to prevent selective outcome reporting.
Other potential sources of bias
In all of the studies, groups were balanced at baseline. In three studies (Gannon 1991; O'Connor 1997; Zupi 2003), the prior experience of the operating surgeon was described. However, this was not described in the remainder of the studies and could be a potential source of bias, as a less experienced surgeon for either treatment group could alter results such as duration of surgery, adverse outcomes and, in the case of endometrial ablation, effectiveness.
Effects of interventions
Endometrial resection and ablation versus hysterectomy
Primary outcomes
Effectiveness
A significant advantage favoured hysterectomy for improvement of bleeding outcomes. These were assessed in different ways in the trials, and not all outcomes could be pooled.
1.1 Woman's perception (proportion with improvement in bleeding symptoms)
Five trials assessed whether bleeding symptoms were perceived as improved; women randomly assigned to endometrial ablation were slightly less likely to show improvement in bleeding symptoms when compared with those randomly assigned to hysterectomy at one year (RR 0.89, 95% confidence interval (CI) 0.85 to 0.93, four studies, 650 women, I2 = 31%). Differences were also noted at later stages of follow‐up, but these findings were only just within the range of statistical significance: at two years (RR 0.92, 95% CI 0.86 to 0.99, two studies, 292 women, I2 = 53%) and at four years (RR 0.93, 95% CI 0.88 to 0.99, two studies, 237 women, I2 = 79%; Figure 4; Analysis 1.1).
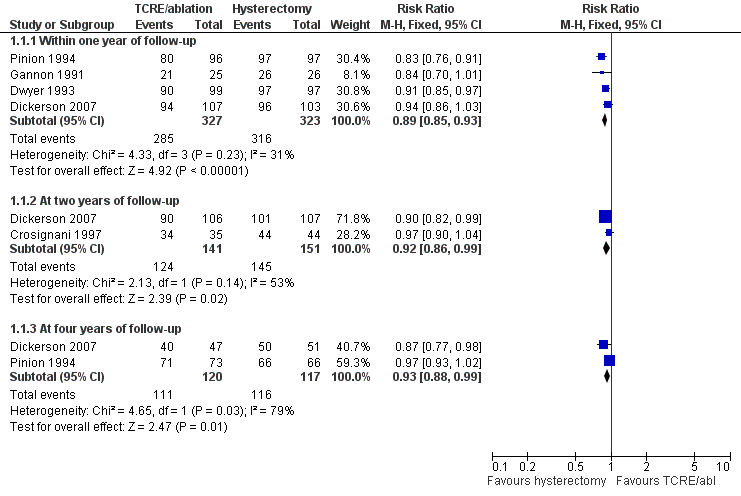
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.1 Woman's perception (proportion with improvement in bleeding symptoms).
1.2 PBAC score
One trial assessed menstrual blood loss using the PBAC (Pictorial Blood Loss Assessment Chart) score. Compared with pretreatment scores, the PBAC score was significantly reduced in both groups at one and two years postoperatively; however, this finding overall significantly favoured women randomly assigned to hysterectomy at one year (MD 24.40, 95% CI 16.01 to 32.79, one study, 68 women), and even more so at two years (MD 44.00, 95% CI 36.09 to 51.91, one study, 68 women; Analysis 1.2).
1.3 Requirement for further surgery
Risk of repeat surgery for failure of the initial surgical treatment was significantly more likely for TCRE/ablation than for hysterectomy at all follow‐up periods (all eight trials contributed data for at least one time point): within the first year (RR 14.9, 95% CI 5.2 to 42.6, six studies, 887 women, I2 = 0%), at two years (RR 23.4, 95% CI 8.3 to 65.8, six studies, 930 women, I2 = 0%), at three years (RR 11.1, 95% CI 1.5 to 80.1, one study, 172 women) and at four years (RR 36.4, 95% CI 5.1 to 259.2, one study, 197 women; Figure 5; Analysis 1.3).
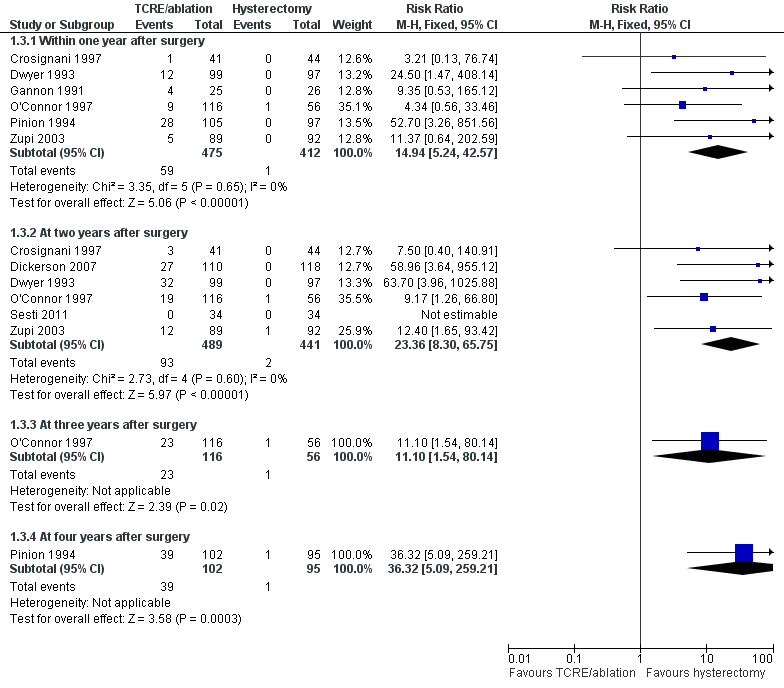
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.3 Proportion requiring further surgery for HMB.
Acceptability
1.4 Proportion satisfied with treatment
Satisfaction (very or moderately satisfied) rates were comparable (five trials) although lower amongst those who had endometrial ablation at two years after surgery (RR 0.87, 95% CI 0.80 to 0.95, four studies, 567 women, I2 = 0%).
No significant differences were noted between post‐treatment satisfaction rates in groups at other follow‐up times (one and four years), although the trend favoured hysterectomy (Figure 6; Analysis 1.4).
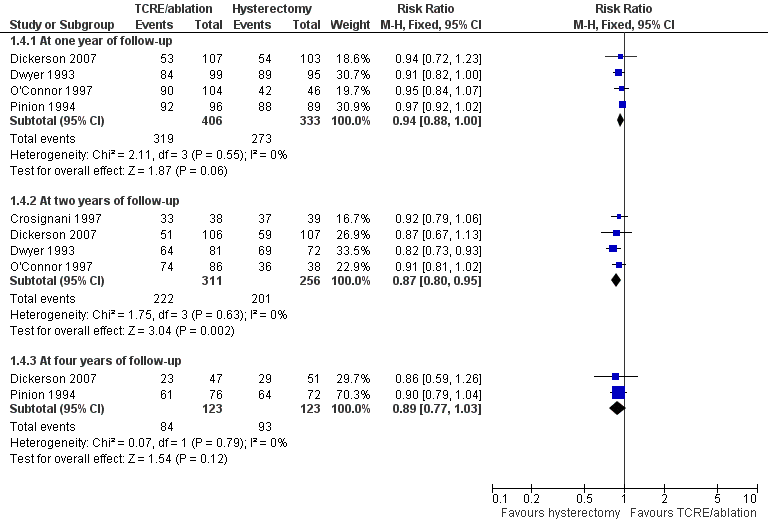
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.4 Proportion satisfied with treatment.
Safety (adverse events)
1.5 Adverse events: short term (intraoperative and immediately postoperative)
Most short‐term complications were more frequent after hysterectomy than after endometrial resection and ablation techniques. Women who had a hysterectomy were more likely to experience sepsis (RR 0.2, 95% CI 0.1 to 0.3, four studies, 621 women, I2 = 62%), blood transfusion (RR 0.2, 95% CI 0.1 to 0.6, four studies, 751 women, I2 = 0%), pyrexia (RR 0.2, 95% CI 0.1 to 0.4, three studies, 605 women, I2 = 66%), vault haematoma (RR 0.1, 95% CI 0.04 to 0.3, five studies, 858 women, I2 = 0%) and wound haematoma (RR 0.03, 95% CI 0.00 to 0.5, one study, 202 women) before their hospital discharge.
However, women who underwent TCRE/ablation were more likely to have fluid overload (RR 9.3, 95% CI 2.2 to 39.6, three studies, 611 women, I2 = 0%) when compared with those who had a hysterectomy.
No differences between groups were reported for haemorrhage, anaesthesia, perforation, gastrointestinal obstruction, laparotomy, cystotomy, cervical laceration, cardiorespiratory event, thromboembolic event or return to surgery as causes of postoperative complications, although some of these short‐term results were based on the findings of only one study (Pinion 1994 or Dickerson 2007; Figure 7; Analysis 1.5).
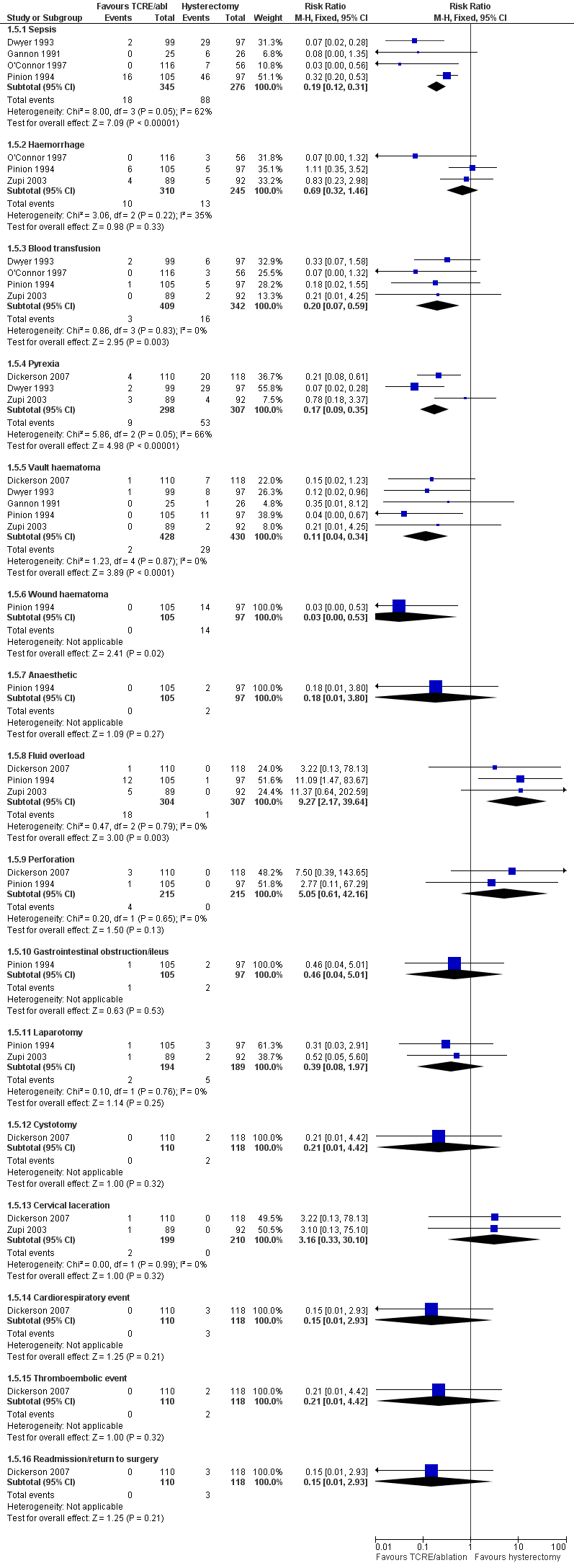
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.5 Adverse events—short term (intraoperative and immediate postoperative).
1.6 Adverse events: long term (after hospital discharge)
After hospital discharge, sepsis was more likely following hysterectomy in one study (RR 0.2, 95% CI 0.1 to 0.5, one study, 172 women), but no other significant differences were reported (Figure 8; Analysis 1.6).
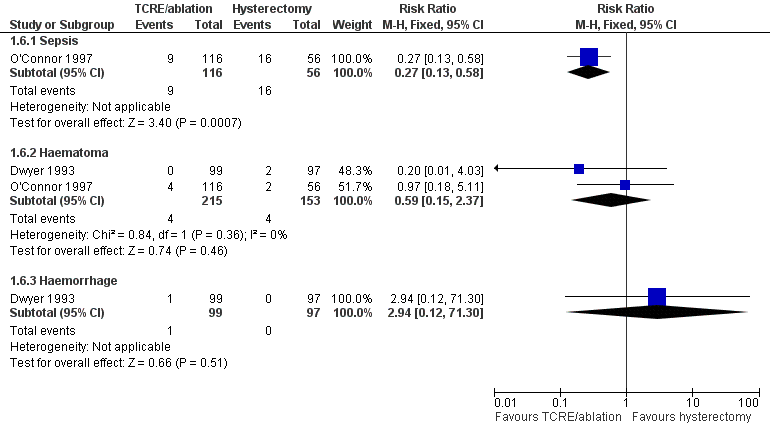
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.6 Adverse events—long term (after hospital discharge).
Secondary outcomes
Quality of life
Quality of life was measured by a number of validated scales. It was not possible to combine scales, as the domains differed, measuring different aspects of quality of life.
1.7 Quality of life scores (continuous data)
Significant differences were detected in five domains of the SF‐36 Scale measured one and two years after surgery. Women randomly assigned to hysterectomy reported significantly higher scores on the social functioning (one and two years), pain (two years), energy (one year) and general health perception (one and two years) domains (social functioning: one year: MD ‐21.2, 95% CI ‐24.7 to ‐17.7, one study, 181 women; two years: MD ‐10.1, 95% CI ‐13.55 to ‐6.58, three studies, 300 women, I2 = 25%) (pain: two years: MD ‐9.5, 95% CI ‐12.8 to ‐6.2, four studies, 513 women, I2 = 63%) (energy: one year: MD ‐11.0, 95% CI ‐14.5 to ‐7.5, two studies, 211 women, I2 = 0%) (general health perception: one year: MD ‐7.3, 95% CI ‐10.7 to ‐3.8, two studies, 385 women, I2 = 81%; two years: MD ‐7.4, 95% CI ‐12.8 to ‐6.21, four studies, 509 women, I2 = 41%).
However, women randomly assigned to TCRE/ablation reported significantly higher scores for emotional role limitation at two years (MD 10.2, 95% CI 5.5 to 15.0, three studies, 300 women, I2 = 92%).
No statistically significant differences in SF‐36 scores were noted between the two interventions in the domains of physical role limitation, mental health and physical functioning (Figure 9; Analysis 1.7).
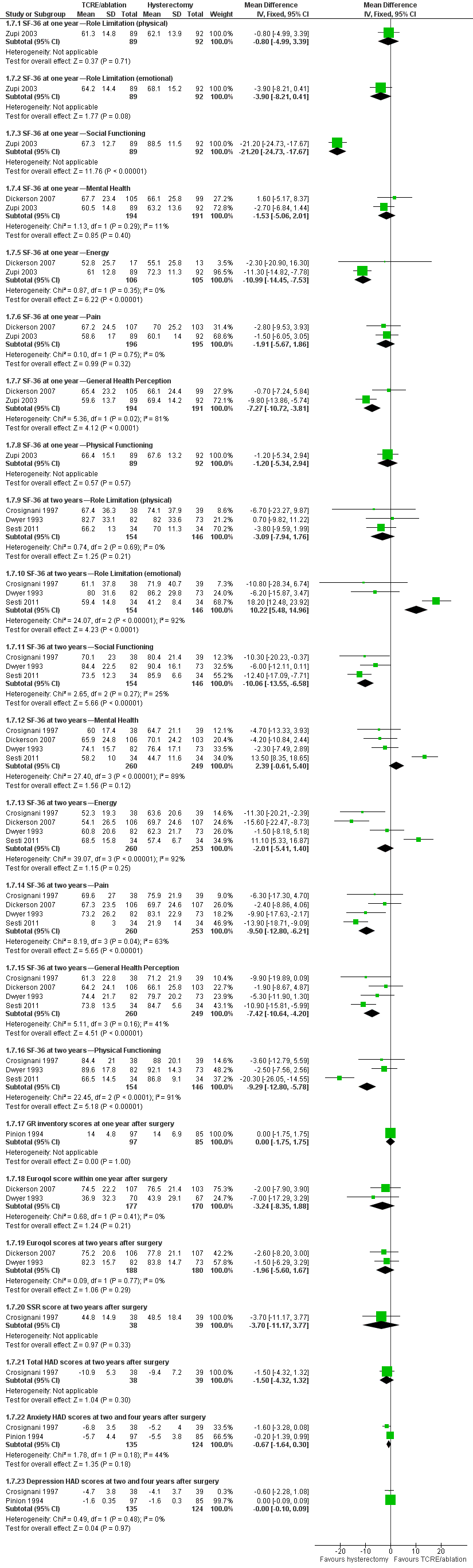
Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.7 Quality of life scores (continuous data).
1.8 Quality of life (proportion with improvement)
Differences between groups were not reported in quality of life dimensions as measured by any of the other scales: Golombok Inventory, Euroqol Scale, Sabbatsberg Scale or HAD Scale. The dichotomous outcome of proportion with improvement in pain at two years was not significantly different between surgical groups. However, a greater proportion of those who had undergone a hysterectomy reported an improvement in their general health one year after surgery when compared with those who had received TCRE/ablation (RR 4.2, 95% CI 1.5 to 11.9, one study, 185 women). At four years, this difference between groups had narrowed and was just outside the level of significance (Analysis 1.8).
1.9 Duration of surgery
The duration of surgery was significantly longer for hysterectomy in all seven trials, especially when compared with laparoscopic hysterectomy. A pooled estimate was not included in this analysis, as a high degree of heterogeneity was noted, as discussed below under 'Heterogeneity' (Analysis 1.9).
1.10. Duration of hospital stay
Seven trials evaluated this outcome, and the results were significantly longer after hysterectomy in all trials. A pooled estimate was not included in this analysis, as a high degree of heterogeneity was noted, as discussed below under 'Heterogeneity' (Analysis 1.12).
1.11. Time to return to normal activity
Time to return to normal activity was significantly longer after hysterectomy in all four trials. A pooled estimate was not included in this analysis, as a high degree of heterogeneity was noted, as discussed below under 'Heterogeneity' (Analysis 1.11).
1.12. Time to return to work
Time to return to work was significantly longer after hysterectomy in four of the five trials (the fifth showed no significant difference). A pooled estimate was not included in this analysis, as a high degree of heterogeneity was noted, as discussed below under 'Heterogeneity' (Analysis 1.10).
1.13. Total health service cost per woman
All three trials evaluating costs reported that endometrial resection cost the health service less than hysterectomy for the management of heavy menstrual bleeding. This difference continued over a prolonged follow‐up time, but the cost gap narrowed primarily because of the retreatment rate for women who underwent endometrial resection. By four years, ablation techniques cost between 5% and 11% less than a hysterectomy in one study that evaluated long‐term follow‐up (Aberdeen 1999 (Pinion 1994); Analysis 1.13).
1.14. Total individual cost per woman
One trial (Cameron 1996 (Pinion 1994)) measured costs to the women. At one year of follow‐up, total personal costs, in terms of travel, loss of pay and child care, were higher for women who had a hysterectomy than for women who underwent endometrial ablation. However, women who had a hysterectomy estimated significantly greater savings in the cost of sanitary protection when compared with those who underwent endometrial ablation (savings of UK £85.10 per year vs UK £58.30 per year; Analysis 1.14).
Funnel Plots
Insufficient studies were included in the review for funnel plots to have sufficient power to distinguish chance from true asymmetry.
Heterogeneity
For some outcomes, a high level of heterogeneity was observed.
For many outcomes, only one trial contributed data, and analysis of heterogeneity was therefore not applicable. However, for four outcomes, data were contributed from several or all trials, and for these outcomes, the forest plots were visually inspected. The estimate for proportion requiring further surgery showed a low level of heterogeneity (all confidence intervals overlapping and I2 = 0% at one and two years of follow‐up). However, a very high degree of heterogeneity was evident for outcomes such as duration of surgery (I2 = 99%), time to return to work (I2 = 100%) and time to return to normal activities (I2 = 97%).
The level of clinical heterogeneity for these outcomes may be explained in part by differences in interventions (most notably, mode of hysterectomy) throughout the trials. Two trials (Dwyer and Gannon) included abdominal hysterectomies only; one trial (Crosignani) included only vaginal hysterectomies; and two trials (Zupi and Sesti) included only laparoscopic hysterectomies. All other studies included two or all modes of hysterectomy. To investigate this further, a sensitivity analysis was conducted as below.
All trials had similar risk profiles for most measures in the risk of bias table. It is therefore assumed that no significant methodological heterogeneity was present.
Sensitivity Analysis
The first analysis was performed to determine whether mode of hysterectomy altered the estimates as compared with estimates when all types of hysterectomy were pooled. TCRE/ablation was compared individually against each mode of hysterectomy.
For many of the outcomes, the estimates were largely unchanged when this analysis was conducted. Mode of hysterectomy did not change the estimates of comparisons for bleeding outcomes, requirement for further surgery, quality of life or satisfaction rates. In some instances, this occurred because only one trial contributed data to the particular outcome for the pooled estimate.
Mode of hysterectomy appeared to influence some aspects of surgical safety and several secondary outcomes. When laparoscopic hysterectomy was compared against TCRE/ablation, the risk ratios for the following short‐term adverse outcomes were no longer statistically significant: blood transfusion (RR 0.20, 95% CI 0.07 to 0.59, to RR 0.21, 95% CI 0.01 to 4.27), pyrexia (RR 0.17, 95% CI 0.09 to 0.35, to RR 0.78, 95% CI 0.18 to 3.37) and vault haematoma (RR 0.11, 95% CI 0.04 to 0.34, to RR 0.21, 95% CI 0.01 to 4.25); however, the trend still favoured TCRE/ablation.
All modes of hysterectomy were still associated with longer operating times, but this was most marked for laparoscopic hysterectomy, followed by vaginal hysterectomy, and least so for abdominal hysterectomy (mean difference (MD) for all modes pooled 39.6, 95% CI 55.1 to 24.1; for laparoscopic route comparison MD 75.3, 95% CI 165.4 to 14.8; for vaginal route comparison MD 57.6, 95% CI 60.3 to 54.9; and for abdominal route comparison MD 15.6, 95% CI 26.13 to 4.97).
Mean differences in hospital stay remained significantly longer for both abdominal and vaginal hysterectomy when compared with TCRE/ablation (MD 4.9 days, 95% CI 6.5 to 3.2; MD 4.3 days, 95% CI 4.5 to 4.1, respectively), but the mean difference in hospital stay between TCRE/ablation and laparoscopic hysterectomy was only just statistically significant (MD 0.3 days, 95% CI 0.7 to 0.1).
Abdominal hysterectomy was associated with the greatest difference in time to return to normal activities and time to return to work when compared with TCRE/ablation (MD 21 days, 95% CI 24.8 to 17.2; MD 8.2 weeks, 95% CI 9.5 to 6.8, respectively). For the same outcomes, when TCRE/ablation was compared against vaginal hysterectomy (MD 5 days, 95% CI 7.3 to 2.7; MD 2.4 weeks, 95% CI 2.7 to 2.1, respectively), the difference was less, particularly when compared against laparoscopic hysterectomy (MD 1.5 days, 95% CI 3.1 to 0.1; MD 0.04 weeks, 95% CI 0.3 to 0.25, respectively).
A further analysis was performed to investigate the effect of clear evidence of allocation concealment on estimates. For six of the eight trials, clear evidence indicated that allocation was concealed, but for two trials (Gannon 1991 and Zupi 2003), this was not the case. When only trials with clear evidence of allocation concealment were included in the meta‐analysis, no significant change was seen in the estimates compared with when all trials were included.
The following analysis was planned but was not conducted: estimates based on all relevant trials regardless of missing data and loss to follow‐up versus estimates based on trials for which incomplete outcome assessment was not likely to cause bias. This analysis was not conducted, as no data were missing from the included trials.
Discussion
Summary of main results
This review assessed the benefits and harms of two surgical procedures for the treatment of heavy menstrual bleeding. When the primary outcomes of this review—effectiveness (woman's perception of improvement in bleeding outcomes, PBAC score, requirement for further surgery), acceptability and safety (adverse outcomes)—are considered, this review shows that both hysterectomy and TCRE/ablation are effective, acceptable and safe surgical treatments for heavy menstrual bleeding.
Whilst hysterectomy is more effective at reducing bleeding symptoms and improving quality of life and is associated with less repeated surgery than endometrial ablation, considerable short‐term benefits are associated with endometrial ablation techniques when compared with hysterectomy, mainly in the areas of recovery time and cost.
Hysterectomy was completely successful in treating bleeding problems and led to very high levels (> 95% in most trials) of participant satisfaction up to two years after surgery. Satisfaction levels were also high for women who underwent endometrial ablation; they were significantly lower for these women when compared with hysterectomy at two years of follow‐up, although the trend strongly favoured hysterectomy at earlier follow‐up. Endometrial destruction techniques were also highly successful in reducing menstrual blood loss in most participants, but a proportion of participants (ranging from 3% to 18%) showed no improvement in their bleeding complaints. At most periods of follow‐up, regardless of how bleeding symptoms were measured, women in the hysterectomy groups showed a significantly greater reduction in symptoms than those in the endometrial ablation groups. For some outcomes, it was suggested that these differences were no longer experienced at longer periods of follow‐up, possibly because of reduced numbers and/or retreatment in the TCRE/ablation group.
Most quality of life measures were not markedly different between the two types of surgery, although evidence from analysis of SF‐36 scores shows that women who had a hysterectomy perceived greater benefit for their general health, social functioning and energy levels at both one and two years and for pain levels at two years after surgery was completed compared with those having endometrial ablation. All other aspects of quality of life as measured by different instruments did not differ between groups.
Both surgical treatments were considered to be safe, with low complication rates reported. However, hysterectomy was associated with higher rates of sepsis, pyrexia, requirement for blood transfusion, vault haematoma and wound haematoma.
Duration of surgery, hospital stay, time to return to normal activities and time to return to work were shorter for those who had TCRE/ablation when compared with those who underwent hysterectomy. Although the findings from the included studies could not be pooled, almost all of the individual trials consistently reported a significant benefit for TCRE/ablation in these areas. Likely causes of heterogeneity for duration of surgery were variations in surgical route (e.g. vaginal vs abdominal vs laparoscopic hysterectomy or first‐generation vs second‐generation endometrial resection/ablation techniques), surgical technique and the expertise of surgeons carrying out the procedures.
Duration of hospital stay was likely to be affected by changes outside the control of investigators. Hospital policy for maximum stay can vary significantly between hospitals and could have accounted for the heterogeneity observed in the results for this outcome. Route of surgery (e.g. vaginal vs abdominal vs laparoscopic) partially explains the extreme heterogeneity in the time taken for recovery after discharge from hospital. Many hospitals worldwide are adopting a policy of 'enhanced recovery' following elective surgery, including shorter times to discharge for major operations such as abdominal hysterectomy. Future updates of this review may include trials that reflect this change.
Significant differences in operating time, hospital stay and time to return to normal activities and work can be seen in most of the trials, but the magnitude of these differences varies, depending on which mode of hysterectomy is being compared. Although precise estimates cannot be given, TCRE/ablation for abnormal bleeding takes less time to perform and has a shorter recovery time than hysterectomy. These differences are associated with a reduction in health service costs.
Evaluation of comparative costs between the two surgical procedures is affected by the increasing retreatment rate. Initially, treatment costs are much lower for women undergoing endometrial ablation than for those undergoing hysterectomy, but the difference in costs between the groups narrows over time because of the cost of retreatment. In one study with a minimum of four years of follow‐up, endometrial ablation was reported to be only 5% to 11% less costly than hysterectomy compared with 24% and 29% less costly at one and two years of follow‐up. Again here, 'enhanced recovery' policies may reflect a narrower difference in duration of hospital stay and therefore a corresponding narrower difference in patient costs.
As described previously, outcomes may be altered by the prior experience of the surgeon for both surgical procedures. A less experienced surgeon for either treatment group could alter results such as duration of surgery, adverse outcomes and, in the case of endometrial ablation, effectiveness.
Overall completeness and applicability of evidence
The included studies adequately addressed the review question: to assess the relative effectiveness, acceptability and safety of endometrial resection/ablation by any means and hysterectomy via any route for the treatment of heavy menstrual bleeding, as well as the secondary outcomes described above. The inclusion and exclusion criteria were largely met, and the interventions well described. Unfortunately, the outcomes (particularly measures of improvement in bleeding symptoms) were measured in several different ways, and it was not possible for all estimates to be pooled, making meta‐analysis impossible for some outcomes.
On the whole, the review findings were consistent with currently accepted clinical advice and are in agreement with current guidelines. National Institute for Health and Care Excellence (NICE) guidelines for menorrhagia (NICE 2007) suggest that hysterectomy should not be used as a first‐line treatment for benign HMB and should be considered only when other treatment options have failed, are contraindicated or are declined by the woman; when there is a wish for amenorrhoea; when the woman (who has been fully informed) requests it; and/or when the woman no longer wishes to retain her uterus and fertility. NICE 2007 guidelines are in agreement with the findings of this review with regard to potential complications associated with both modes of surgical treatment.
Quality of the evidence
In total, eight studies were included with a total of 1,260 randomly assigned participants.
All studies were at high risk of performance/detection bias, as it is not feasible to blind women or the surgeon against the type of operation that was performed. Several of the older studies were at high risk of selection bias.
As the studies used many different measurements for the outcomes (particularly for effectiveness of treatment), it was not possible to pool data for every outcome. However, the results were largely comparable; both treatments are considered to be effective and safe, hysterectomy was generally superior in improving symptoms and in leading to less requirement for future surgery and endometrial ablation was superior in terms of fewer adverse outcomes, shorter operating times and faster return to activities/work.
For many outcomes, assessment of heterogeneity was not relevant, as only one trial contributed data. For outcomes that displayed a high level of clinical heterogeneity, this was explained in part by differences in outcomes for different modes of hysterectomy (further described under 'Sensitivity analysis').
Quality of evidence was low to moderate, depending on the outcome. PBAC score, adverse events and proportion satisfied with treatment generated a moderate GRADE score, suggesting that further research may change the estimate. Outcomes that generated a low GRADE score included the woman’s perception of bleeding and the proportion of women requiring further surgery for HMB. For these two outcomes, further evidence is likely to change the estimate.
Potential biases in the review process
Electronic searches in combination with handsearches performed by the review authors identified all relevant studies known to be available currently. The inability to pool data for particular outcomes is likely to decrease the power of results; this may change if future studies are able to add data to the existing outcomes.
Agreements and disagreements with other studies or reviews
Bhattacharya 2011 performed a systematic review that compared clinical effectiveness and cost‐effectiveness analyses of hysterectomy, endometrial ablation and Mirena for heavy menstrual bleeding. These findings were in accordance with the findings of this review; satisfaction rates at 12 months were highest for hysterectomy, and rates of further surgery after endometrial resection/ablation were comparable (8.5%). The authors also noted longer hospital stay and longer time to return to normal activities for hysterectomy.
Women who have undergone hysterectomy have experienced more postoperative complications than women who have received TCRE/ablation, although for some types of complications, no differences were reported. Because some complications are rare, these rates are best compared with those of large audits, such as the Mistletoe audit in the UK (Overton 1997). The low rate of complications for TCRE/ablation in this review confirms the findings of the Mistletoe study.
A comparison of costs of endometrial resection and ablation techniques versus those of hysterectomy cannot adequately provide information on the relative value in terms of money of these two surgical procedures. The availability of TCRE/ablation as a treatment for HMB may result in an earlier recourse to surgery than a woman would have considered if the only surgery available was hysterectomy (Bridgman 1994; Coulter 1994), and this will have a significant impact on costs. Additional studies have provided assessments of cost‐effectiveness based on the Dwyer 1993 trial through a cost‐utility analysis (Sculpher 1998), preference‐based treatment allocation (woman allocated to the treatment that she prefers) (Sculpher 1998) and four other cost‐utility analyses (Garside 2004; You 2006; Clegg 2007; Roberts 2011). Garside 2004 concluded that abdominal hysterectomy is likely to be more cost‐effective than endometrial resection if healthcare purchasers are willing to pay an additional cost of at least UK £6,500 per extra quality‐adjusted life‐year (QALY) generated by hysterectomy, although an area of uncertainty is attached to this conclusion in the form of variation in the parameters used in the analysis. Clegg 2007 found that a preference‐based treatment allocation was more cost‐effective than reliance on a single intervention for all women requiring surgery for HMB. Garside 2004 reported that hysterectomy was more expensive than endometrial ablation but accrued more QALYs over 10 years. The incremental cost per QALY of hysterectomy compared with two second‐generation endometrial ablation techniques was approximately UK £2,000. You 2006 also confirmed that hysterectomy was a more expensive option than endometrial ablation (cost per woman: US $6,878 vs US $6,185 over five years) but was more effective (4.725 QALYs vs 4.624 QALYs). Roberts 2011 found that hysterectomy produced more QALYs relative to second‐generation endometrial ablation, with an incremental cost‐effectiveness ratio of £970 per additional QALY. Data from the Clegg 2007 cost‐utility analysis contradicted this finding; the authors reported that second‐generation methods accrued marginally more QALYs than hysterectomy over five years (4.13 vs 4.01 QALYs). Evaluation of the comparative cost‐effectiveness of endometrial destruction techniques and hysterectomy is complex, and the simple conclusion that endometrial destruction is cheaper than hysterectomy, with the difference narrowing over time, may not represent an adequate economic assessment.

Study screening and selection process (2008 to 2013).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.1 Woman's perception (proportion with improvement in bleeding symptoms).

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.3 Proportion requiring further surgery for HMB.

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.4 Proportion satisfied with treatment.

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.5 Adverse events—short term (intraoperative and immediate postoperative).

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.6 Adverse events—long term (after hospital discharge).

Forest plot of comparison: 1 Endometrial resection/ablation versus hysterectomy, outcome: 1.7 Quality of life scores (continuous data).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 1 Woman's perception (proportion with improvement in bleeding symptoms).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 2 PBAC score (continuous data).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 3 Proportion requiring further surgery for HMB.

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 4 Proportion satisfied with treatment.

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 5 Adverse events—short term (intraoperative and immediate postoperative).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 6 Adverse events—long term (after hospital discharge).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 7 Quality of life scores (continuous data).
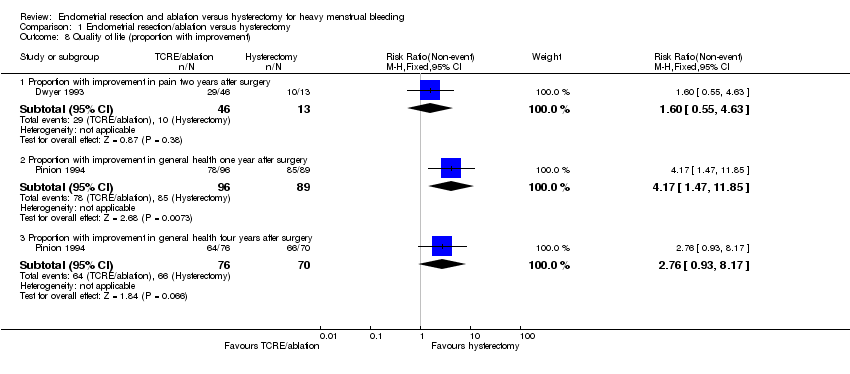
Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 8 Quality of life (proportion with improvement).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 9 Duration of surgery (minutes).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 10 Time to return to work (weeks).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 11 Time to return to normal activity (days).

Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 12 Duration of hospital stay (days).
| Study | |
| Dwyer 1993 | FOUR MONTHS FOLLOW UP 2.2 YEARS OF FOLLOW UP |
| Gannon 1991 | INITIAL COSTS (NHS) |
| Pinion 1994 | ONE YEAR OF FOLLOW UP FOUR YEARS OF FOLLOW UP |
Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 13 Total health service cost per woman.
| Study | |
| Pinion 1994 | ONE YEAR OF FOLLOW UP Mean annual savings from sanitary protection after treatment in UK pounds in 1994: |
Comparison 1 Endometrial resection/ablation versus hysterectomy, Outcome 14 Total individual cost per woman.
| Endometrial resection/ablation for heavy menstrual bleeding | ||||||
| Patient or population: women with heavy menstrual bleeding | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Endometrial resection/ablation | |||||
| Woman's perception (proportion with improvement in bleeding symptoms)—within one year of follow‐up | 978 per 1,000 | 890 per 1,000 | RR 0.91 | 650 | ⊕⊕⊝⊝ | |
| PBAC score (continuous data)—at one year of follow‐up | Mean PBAC score (continuous data) at one year of follow‐up in the intervention groups was | 68 | ⊕⊕⊕⊝ | |||
| Adverse events—short term (intraoperative and immediate postoperative)—sepsis | 319 per 1,000 | 61 per 1,000 | RR 0.19 | 621 | ⊕⊕⊕⊝ | |
| Proportion requiring further surgery for HMB—within one year after surgery | 2 per 1,0003 | 36 per 1,000 | RR 14.94 | 887 | ⊕⊕⊝⊝ | |
| Proportion satisfied with treatment—at one year of follow‐up | 820 per 1,000 | 771 per 1,000 | RR 0.94 | 739 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Lack of blinding (although unfeasible) is likely to bias women's responses. 3Result suggests incomplete operation—rare. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Woman's perception (proportion with improvement in bleeding symptoms) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within one year of follow‐up | 4 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.85, 0.93] |
| 1.2 At two years of follow‐up | 2 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.86, 0.99] |
| 1.3 At four years of follow‐up | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.99] |
| 2 PBAC score (continuous data) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At one year of follow‐up | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 24.4 [16.01, 32.79] |
| 2.2 At two years of follow‐up | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 44.0 [36.09, 51.91] |
| 3 Proportion requiring further surgery for HMB Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Within one year after surgery | 6 | 887 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.94 [5.24, 42.57] |
| 3.2 At two years after surgery | 6 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 23.36 [8.30, 65.75] |
| 3.3 At three years after surgery | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.10 [1.54, 80.14] |
| 3.4 At four years after surgery | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 36.32 [5.09, 259.21] |
| 4 Proportion satisfied with treatment Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At one year of follow‐up | 4 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.00] |
| 4.2 At two years of follow‐up | 4 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 4.3 At four years of follow‐up | 2 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 5 Adverse events—short term (intraoperative and immediate postoperative) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Sepsis | 4 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.12, 0.31] |
| 5.2 Haemorrhage | 3 | 555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.46] |
| 5.3 Blood transfusion | 4 | 751 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.59] |
| 5.4 Pyrexia | 3 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.09, 0.35] |
| 5.5 Vault haematoma | 5 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.34] |
| 5.6 Wound haematoma | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.53] |
| 5.7 Anaesthetic | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.80] |
| 5.8 Fluid overload | 3 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.27 [2.17, 39.64] |
| 5.9 Perforation | 2 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.05 [0.61, 42.16] |
| 5.10 Gastrointestinal obstruction/ileus | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.01] |
| 5.11 Laparotomy | 2 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.97] |
| 5.12 Cystotomy | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.42] |
| 5.13 Cervical laceration | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.33, 30.10] |
| 5.14 Cardiorespiratory event | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.93] |
| 5.15 Thromboembolic event | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.42] |
| 5.16 Readmission/return to surgery | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.93] |
| 6 Adverse events—long term (after hospital discharge) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Sepsis | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.13, 0.58] |
| 6.2 Haematoma | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.37] |
| 6.3 Haemorrhage | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.12, 71.30] |
| 7 Quality of life scores (continuous data) Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 SF‐36 at one year—Role Limitation (physical) | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐4.99, 3.39] |
| 7.2 SF‐36 at one year—Role Limitation (emotional) | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐8.21, 0.41] |
| 7.3 SF‐36 at one year—Social Functioning | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐21.20 [‐24.73, ‐17.67] |
| 7.4 SF‐36 at one year—Mental Health | 2 | 385 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐5.06, 2.01] |
| 7.5 SF‐36 at one year—Energy | 2 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐10.99 [‐14.45, ‐7.53] |
| 7.6 SF‐36 at one year—Pain | 2 | 391 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐5.67, 1.86] |
| 7.7 SF‐36 at one year—General Health Perception | 2 | 385 | Mean Difference (IV, Fixed, 95% CI) | ‐7.27 [‐10.72, ‐3.81] |
| 7.8 SF‐36 at one year—Physical Functioning | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐5.34, 2.94] |
| 7.9 SF‐36 at two years—Role Limitation (physical) | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐3.09 [‐7.94, 1.76] |
| 7.10 SF‐36 at two years—Role Limitation (emotional) | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | 10.22 [5.48, 14.96] |
| 7.11 SF‐36 at two years—Social Functioning | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐10.06 [‐13.55, ‐6.58] |
| 7.12 SF‐36 at two years—Mental Health | 4 | 509 | Mean Difference (IV, Fixed, 95% CI) | 2.39 [‐0.61, 5.40] |
| 7.13 SF‐36 at two years—Energy | 4 | 513 | Mean Difference (IV, Fixed, 95% CI) | ‐2.01 [‐5.41, 1.40] |
| 7.14 SF‐36 at two years—Pain | 4 | 513 | Mean Difference (IV, Fixed, 95% CI) | ‐9.50 [‐12.80, ‐6.21] |
| 7.15 SF‐36 at two years—General Health Perception | 4 | 509 | Mean Difference (IV, Fixed, 95% CI) | ‐7.42 [‐10.64, ‐4.20] |
| 7.16 SF‐36 at two years—Physical Functioning | 3 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐9.29 [‐12.80, ‐5.78] |
| 7.17 GR inventory scores at one year after surgery | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.75, 1.75] |
| 7.18 Euroqol score within one year after surgery | 2 | 347 | Mean Difference (IV, Fixed, 95% CI) | ‐3.24 [‐8.35, 1.88] |
| 7.19 Euroqol scores at two years after surgery | 2 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐1.96 [‐5.60, 1.67] |
| 7.20 SSR score at two years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐11.17, 3.77] |
| 7.21 Total HAD scores at two years after surgery | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐4.32, 1.32] |
| 7.22 Anxiety HAD scores at two and four years after surgery | 2 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.64, 0.30] |
| 7.23 Depression HAD scores at two and four years after surgery | 2 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.09] |
| 8 Quality of life (proportion with improvement) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Proportion with improvement in pain two years after surgery | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.55, 4.63] |
| 8.2 Proportion with improvement in general health one year after surgery | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.17 [1.47, 11.85] |
| 8.3 Proportion with improvement in general health four years after surgery | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.93, 8.17] |
| 9 Duration of surgery (minutes) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Time to return to work (weeks) Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Time to return to normal activity (days) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Duration of hospital stay (days) Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Total health service cost per woman Show forest plot | Other data | No numeric data | ||
| 14 Total individual cost per woman Show forest plot | Other data | No numeric data | ||

