Antibióticos para la otitis media aguda en niños
Resumen
Antecedentes
La otitis media aguda (OMA) es una de las enfermedades más frecuentes en la infancia temprana y en la niñez. El uso de antibióticos para la OMA varía desde el 56% en los Países Bajos hasta el 95% en los EE.UU., Canadá y Australia. Esta es una actualización de una revisión Cochrane publicada por primera vez en The Cochrane Library , Número 1, 1997 y actualizada previamente en 1999, 2005, 2009 y 2013.
Objetivos
Evaluar los efectos de los antibióticos para los niños con OMA.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL (2015, Número 3), MEDLINE (1966 hasta la semana tres de abril 2015), OLDMEDLINE (1958 hasta 1965), EMBASE (enero 1990 hasta abril 2015), Current Contents (1966 hasta abril 2015), CINAHL (2008 hasta abril 2015) y LILACS (2008 hasta abril 2015).
Criterios de selección
Ensayos controlados aleatorizados (ECA) que compararan 1) fármacos antimicrobianos con placebo y 2) tratamiento inmediato con antibióticos con observación expectante (incluida la prescripción tardía de antibióticos) en niños con OMA.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente la calidad de los ensayos y extrajeron los datos.
Resultados principales
Para el examen de los antibióticos frente a placebo se seleccionaron 13 ECA (3401 niños y 3938 episodios de OMA) de países de altos ingresos, que en general presentaban un bajo riesgo de sesgo. Los resultados combinados de los ensayos mostraron que a las 24 horas del inicio del tratamiento, el 60% de los niños se habían recuperado después de recibir o no placebo o antibióticos. El dolor no se redujo con los antibióticos a las 24 horas (riesgo relativo [RR] 0,89; intervalo de confianza [IC] del 95%: 0,78 a 1,01) pero casi un tercio menos tuvo dolor residual a los dos o tres días (CR 0,70; IC del 95%: 0,57 a 0,86; número necesario a tratar para un resultado beneficioso adicional [NNTB] 20). Una cuarta parte menos tuvo dolor a los cuatro a siete días (RR 0,76; IC del 95%: 0,63 a 0,91; NNTB 16) y dos tercios menos tuvieron dolor a los diez a 12 días (RR 0,33; IC del 95%: 0,17 a 0,66; NNTB 7), en comparación con placebo. Los antibióticos redujeron el número de niños con hallazgos anormales en la timpanometría de dos a cuatro semanas (RR 0,82, IC del 95%: 0,74 a 0,90; NNTB 11), de seis a ocho semanas (RR 0,88, IC del 95%: 0,78 a 1.00; NNTB 16) y el número de niños con perforaciones en la membrana timpánica (RR 0,37; IC del 95%: 0,18 a 0,76; NNTB 33) y redujeron a la mitad los episodios de otitis contralateral (RR 0,49; IC del 95%: 0,25 a 0,95; NNTB 11) en comparación con placebo. Sin embargo, los antibióticos no redujeron el número de niños con hallazgos anormales en la timpanometría a los tres meses (RR 0,97; IC del 95%: 0,76 a 1,24) ni el número de niños con recurrencias tardías de la OMA (RR 0,93; IC del 95%: 0,78 a 1,10), en comparación con el placebo. Las complicaciones graves fueron poco frecuentes y no difirieron entre los niños tratados con antibióticos y los tratados con placebo. Los eventos adversos (como vómitos, diarrea o erupción cutánea) ocurrieron con mayor frecuencia en los niños que tomaron antibióticos (RR 1,38; IC del 95%: 1,19 a 1,59; número necesario a tratar para un resultado perjudicial adicional [NNTD] 14). Los gráficos en embudo no indican sesgo de publicación. El metanálisis de los datos de los pacientes individuales de un subconjunto de ensayos incluidos encontró que los antibióticos fueron más beneficiosos en los niños menores de dos años de edad con OMA bilateral, o con OMA y otorrea.
Para el análisis antibióticos inmediatos frente a observación expectante, cinco ensayos (1149 niños) de países de altos ingresos fueron elegibles y tuvieron un riesgo de sesgo de bajo a moderado. Cuatro ensayos (1007 niños) informaron datos de resultados que fue posible utilizar para esta revisión. A partir de estos ensayos fue posible extraer los datos de 959 niños para el metanálisis del dolor a los tres a siete días. No se detectaron diferencias en cuanto al dolor a los tres a siete días (RR 0,75; IC del 95%: 0,50 a 1,12). Un ensayo (247 niños) informó de datos sobre el dolor a los 11 a 14 días. Los antibióticos inmediatos no se asociaron con una reducción del número de niños con dolor (RR 0,91; IC del 95%: 0,75 a 1,10), en comparación con la observación expectante. Además, no se observaron diferencias entre los grupos en cuanto al número de niños con hallazgos anormales en la timpanometría a las cuatro semanas, perforaciones de la membrana timpánica y recurrencia de la AOM. No ocurrieron complicaciones graves en el grupo de antibióticos ni en el grupo de observación expectante. Los antibióticos inmediatos se asociaron con un aumento significativo del riesgo de vómitos, diarrea o erupción cutánea, en comparación con la observación expectante (RR 1,71; IC del 95%: 1,24 a 2,36; NNTD 9).
Los resultados de un metanálisis de datos de pacientes individuales que incluyó datos de seis ensayos de alta calidad (1643 niños), y que también se incluyeron como ensayos individuales en esta revisión, mostraron que los antibióticos parecen ser más beneficiosos en niños menores de dos años de edad con OMA bilateral (NNTB 4) y en niños con OMA y otorrea (NNTB 3).
Conclusiones de los autores
Esta revisión muestra que los antibióticos no tienen un efecto temprano sobre el dolor, un efecto leve sobre el dolor en los días siguientes y solo un efecto moderado sobre el número de niños con perforaciones timpánicas, episodios de otitis contralateral y hallazgos anormales en la timpanometría a las dos o cuatro semanas y a las seis u ocho semanas, en comparación con placebo, en los niños con OMA. En los países de altos ingresos, la mayoría de los casos de OMA remiten espontáneamente sin complicaciones. Los efectos beneficiosos de los antibióticos se deben sopesar contra los posibles efectos perjudiciales: por cada 14 niños tratados con antibióticos un niño experimentó un evento adverso (como vómitos, diarrea o sarpullido), que no habría ocurrido si se hubiera diferido la administración de los antibióticos. Por lo tanto, en el tratamiento se debe enfatizar el asesoramiento sobre la analgesia adecuada y la limitada función de los antibióticos. Los antibióticos parecen ser más beneficiosos en niños menores de dos años de edad con OMA bilateral y en los niños que presentan OMA y otorrea. En el caso de la mayoría de otros niños con enfermedades leves en los países de altos ingresos, parece justificado un enfoque de observación expectante.
PICOs
Resumen en términos sencillos
Antibióticos para la infección del oído medio (otitis media aguda) en niños
Preguntas de la revisión
Esta revisión comparó 1) la efectividad y seguridad clínicas de los antibióticos contra placebo en niños con una infección aguda del oído medio (otitis media aguda [OMA]) y 2) la efectividad y seguridad clínicas de los antibióticos contra la observación expectante (enfoques de observación en los que se pueden o no prescribir) en niños con OMA.
Antecedentes
La OMA es una de las infecciones más frecuentes en la primera infancia y la niñez que causa dolor y síntomas generales de enfermedad como fiebre, irritabilidad y problemas con la alimentación y el sueño. Alrededor de los tres años de edad la mayoría de los niños han tenido al menos un episodio de OMA. Aunque la OMA generalmente se resuelve sin tratamiento, con frecuencia se trata con antibióticos.
Características de los estudios
La evidencia de esta revisión están actualizada hasta el 26 de abril de 2015.
Para la revisión de los antibióticos en comparación con placebo se incluyeron 13 ensayos (3401 niños entre dos meses y 15 años de edad) de países de altos ingresos, con un riesgo de sesgo generalmente bajo. Se realizaron tres ensayos en un entorno de práctica general (PG), seis en un entorno de hospital ambulatorio y cuatro en ambos entornos.
Para el análisis de los antibióticos en relación con la observación expectante se seleccionaron cinco ensayos (1149 niños) de países de altos ingresos, con un riesgo de sesgo de bajo a moderado. Dos ensayos se realizaron en un entorno de PG y tres en un entorno de hospital ambulatorio. Cuatro ensayos (1007 niños) informaron datos de resultados que fue posible utilizar para esta revisión.
Resultados clave
Se comprobó que los antibióticos no fueron muy útiles para la mayoría de los niños con OMA; los antibióticos no disminuyeron el número de niños con dolor a las 24 horas (cuando el 60% de los niños estaban mejor de todos modos), solo redujeron ligeramente el número de niños con dolor en los días siguientes y no redujeron el número de niños con recurrencias tardías de OMA y pérdida de audición (que puede durar varias semanas) a los tres meses, en comparación con placebo. Sin embargo, los antibióticos redujeron ligeramente el número de niños con perforaciones del tímpano y episodios de AOM en el oído inicialmente no afectado, en comparación con placebo. Los resultados de un metanálisis de datos de pacientes individuales que incluyó datos de seis ensayos de alta calidad (1643 niños), que también se incluyeron como ensayos individuales en esta revisión, mostraron que los antibióticos parecen ser más beneficiosos en los niños menores de dos años con infección en ambos oídos y en los niños con OMA y secreción auditiva.
No se encontraron diferencias entre la administración inmediata de antibióticos y los enfoques de observación expectante en cuanto al número de niños con dolor tres a siete días y 11 a 14 días después de la evaluación. Además, no se observaron diferencias entre los grupos en cuanto al número de niños con pérdida de audición a las cuatro semanas, perforaciones del tímpano y recurrencias tardías de la OMA.
No hubo información suficiente para determinar si los antibióticos redujeron complicaciones poco frecuentes como la mastoiditis (infección del hueso que rodea el oído). Todos los estudios incluidos en esta revisión se realizaron en países de altos ingresos. Existe una falta de datos sobre las poblaciones en las cuales la incidencia de OMA y el riesgo de progresión a la mastoiditis es mayor.
Los antibióticos provocaron efectos indeseables como diarrea, vómitos y erupciones cutáneas, y también puede aumentar la resistencia a los antibióticos en la comunidad. Es difícil sopesar los efectos beneficiosos pequeños con los daños menores que causan los antibióticos en la mayoría de los niños con OMA. Sin embargo, para la mayoría de los niños con enfermedades leves en los países de altos ingresos, parece justificado un enfoque de observación expectante.
Calidad de la evidencia
Se considera que la calidad de la evidencia fue alta para la mayoría de los resultados de la revisión de antibióticos contra placebo (este hecho significa que es muy poco probable que los estudios de investigación adicionales cambien nuestra confianza en la estimación del efecto).
Para al análisis antibióticos inmediatos versus observación expectante, se consideró que la evidencia fue de calidad moderada para la mayoría de los resultados (este hecho significa que es probable que los estudios de investigación adicionales tenga un impacto importante en la confianza en los resultados, y que pueden cambiar esos resultados). La calidad se vio afectada por las preocupaciones sobre el tamaño de la muestra (perforación del tímpano, complicaciones poco frecuentes) y el gran número de niños que se "perdieron durante el seguimiento" (dolor los días 11 a 14, pérdida de audición a las cuatro semanas y recurrencias tardías de la OMA).
Authors' conclusions
Summary of findings
| Antibiotics versus placebo for acute otitis media in children | ||||||
| Patient or population: children with acute otitis media | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
| Pain ‐ pain at 24 hours | Study population | RR 0.89 | 1394 | ⊕⊕⊕⊕ | ||
| 426 per 1000 | 379 per 1000 | |||||
| Pain ‐ pain at 2 to 3 days | Study population | RR 0.70 | 2320 | ⊕⊕⊕⊕ | ||
| 159 per 1000 | 111 per 1000 | |||||
| Pain ‐ pain at 4 to 7 days | Study population | RR 0.76 | 1347 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 183 per 1000 | |||||
| Pain ‐ pain at 10 to 12 days | Study population | RR 0.33 | 278 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 71 per 1000 | |||||
| Abnormal tympanometry ‐ 2 to 4 weeks | Study population | RR 0.82 | 2138 | ⊕⊕⊕⊕ | ||
| 481 per 1000 | 395 per 1000 | |||||
| Abnormal tympanometry ‐ 3 months | Study population | RR 0.97 | 809 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 234 per 1000 | |||||
| Vomiting, diarrhoea or rash | Study population | RR 1.38 | 2107 | ⊕⊕⊕⊕ | ||
| 196 per 1000 | 270 per 1000 | |||||
| *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The number of studies reported in the 'Summary of findings' table for the outcomes 'Pain at 24 hours' and 'Pain at 4 to 7 days' differ slightly from those reported in the Data Analysis Table 1 ‐ Antibiotics versus placebo (five versus six studies and seven versus eight studies, respectively). This is due to the van Buchem trial. This trial is included as one study in our review (and in the 'Summary of findings' table), but we included data from two different comparisons from this 2 x 2 factorial design trial in our analyses (van Buchem 1981a; van Buchem 1981b). 2We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis). | ||||||
Background
Description of the condition
Acute otitis media (AOM) is one of the most frequent diseases in early infancy and childhood. AOM is defined as the presence of middle‐ear effusion and a rapid onset of signs or symptoms of middle‐ear inflammation, such as ear pain, otorrhoea or fever (AAP 2013), and has a high morbidity and low mortality (Stool 1989). Approximately 10% of children have an episode of AOM by three months of age and, by three years of age, approximately 50% to 85% of all children have experienced at least one AOM episode (Teele 1989). The peak age‐specific incidence is between six and 15 months (Klein 1989).
Description of the intervention
Despite a large number of published clinical trials, there is no consensus regarding the most appropriate therapy for AOM; for example, the rates of use of antibiotics for AOM vary from 56% in the Netherlands (Akkerman 2005) to 95% in the USA and Canada (Froom 2001). One meta‐analysis emphasises that AOM resolves spontaneously in most children (Rosenfeld 1994). However, one semi‐randomised trial of 1365 participants conducted in Sweden in 1954 reported a rate of mastoiditis of 17% in the untreated group versus none in the penicillin‐treated groups (Rudberg 1954). Over recent years, prescription strategies in which antibiotic treatment for acute respiratory infections such as AOM is delayed and instituted only if symptoms persist or worsen after several days have been advocated (AAP 2013).
How the intervention might work
AOM has a multifactorial pathogenesis. Mucosal swelling of the nasopharynx and Eustachian tube due to a viral upper respiratory tract infection can lead to Eustachian tube dysfunction with impaired clearance and pressure regulation of the middle ear. Prolonged dysfunction may be followed by aspiration of potential viral and bacterial pathogens from the nasopharynx to the middle ear. These pathogens might in turn provoke a host inflammatory response, which leads to the clinical manifestations of AOM such as ear pain, otorrhoea, fever and irritability. Streptococcus pneumoniae (S. pneumoniae) has been the predominant pathogen related to AOM for many years, next to Moraxella catarrhalis (M. catarrhalis) and non‐typeable Haemophilus influenzae (H. influenzae). However, recent studies suggest that widespread implementation of pneumococcal conjugate vaccination has changed the frequency of otopathogens related to AOM with non‐typeable H. influenzae and non‐vaccine S. pneumoniae serotypes becoming more prevalent (Casey 2013; Coker 2010). Additionally, viral (co‐)infection is known to worsen the clinical and bacteriological outcome of AOM (Arola 1990; Chonmaitree 1992). As bacteria are considered to play a predominant role in the causation of AOM‐related symptoms, antibiotic treatment may accelerate clinical recovery and may reduce the number of complications related to AOM.
Why it is important to do this review
Although numerous randomised clinical trials (RCTs) on the effectiveness of antibiotic treatment in children with AOM have been performed over the decades, consensus regarding the most appropriate treatment strategy is lacking. As symptoms consistent with AOM resolve spontaneously in the majority of children, an expectant observational approach might be justified. We therefore performed a systematic review to examine the effects of both immediate antibiotic treatment and an expectant observational approach in children with AOM. This is an update of a Cochrane review first published in The Cochrane Library in Issue 1, 1997 (Glasziou 1997) and updated in 1999 (Glasziou 1999), 2005 (Glasziou 2005), 2009 (Sanders 2009), and 2013 (Venekamp 2013).
Objectives
To assess the effects of antibiotics for children with AOM.
We attempted to determine to what extent antibiotic therapy was more effective than placebo and what, if any, advantages it offered to children in terms of symptom relief (pain), avoidance of complications (such as tympanic membrane perforations and severe complications such as mastoiditis) and longer‐term hearing problems from middle‐ear effusion (as measured by tympanometry or audiometry). We also assessed the effect of immediate antibiotic versus expectant observation on AOM. Moreover, we aimed to provide information on subgroups of children with AOM that benefit more or less from antibiotics.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of antimicrobial drugs versus placebo control. We also included RCTs comparing immediate antibiotic versus expectant observation.
Types of participants
Studies including children (aged from one month to 15 years) of either gender without ventilation tubes, suffering from AOM irrespective of the setting from which they were recruited.
Types of interventions
Antimicrobial drugs versus placebo control.
Immediate antibiotic versus expectant observation (also known as 'wait and see' or 'watchful waiting' or 'observation therapy'). This includes expectant observational approaches in which prescriptions may or may not be provided.
Types of outcome measures
We focused our data extraction on patient‐relevant outcomes, that is, those symptoms or problems that are important to the patient's sense of well‐being. While other endpoints, such as microbiological cure, may enhance medical understanding of the disease process, decisions about treatment should focus on helping the patient. We analysed the outcomes listed below in this review, but these outcomes were not used as a basis for including or excluding studies.
Primary outcomes
-
Proportion of children with pain at various time points (24 hours, two to three days, four to seven days, 10 to 14 days).
-
Adverse effects likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash.
Secondary outcomes
-
Abnormal tympanometry findings at various time points (two to four weeks, six to eight weeks, and three months) as a surrogate measure for hearing problems caused by middle‐ear fluid.
-
Tympanic membrane perforation.
-
Contralateral otitis (in unilateral cases).
-
AOM recurrences.
-
Serious complications related to AOM such as mastoiditis and meningitis.
-
Long‐term effects (including the number of parent‐reported AOM‐symptom episodes, antibiotic prescriptions and health care utilisation as assessed at least one year after randomisation).
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 3) (accessed 26 April 2015), which contains the Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (October 2012 to April week 3, 2015), EMBASE (November 2012 to April 2015), Current Contents (2012 to April 2015), CINAHL (October 2012 to April 2015) and LILACS (2012 to April 2015). Our previous update using the same search strategies covered the period 2008 to November 2012. See Appendix 1 for details of earlier searches.
We used the search strategy described in Appendix 2 to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search EMBASE (Appendix 3), Current Contents (Appendix 4), CINAHL (Appendix 5) and LILACS (Appendix 6).
There were no language or publication restrictions.
Searching other resources
We checked ClinicalTrials.gov (clinicaltrials.gov/) for ongoing trials (11 May 2015). To increase the yield of relevant studies, we inspected the reference lists of all identified studies and reviews.
Data collection and analysis
Selection of studies
One review author (RPV) screened titles and abstracts obtained from the database searches. Two review authors (RPV, MMR) reviewed the full text of the potentially relevant titles and abstracts against the inclusion criteria.
Data extraction and management
Two review authors (RPV, MMR) extracted data from the included studies. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Two review authors (RPV, MMR) independently assessed the methodological quality of the included trials. We resolved any disagreements by discussion. We assessed the methodological quality of the included studies as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As a consequence, methodological quality assessment was based on random sequence generation, allocation concealment, blinding, completeness of data and outcome assessment. Results of the 'Risk of bias' assessment are presented in a 'Risk of bias' summary (Figure 1) and a 'Risk of bias' graph (Figure 2).
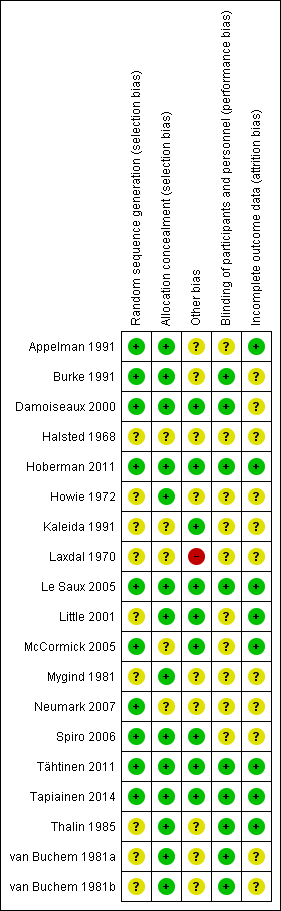
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We expressed dichotomous outcomes as risk ratio (RR) and risk difference (RD) with 95% confidence intervals (CIs). Additionally, we calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) (1/(absolute risk in exposed minus absolute risk in unexposed)).
Unit of analysis issues
We did not identify any studies with non‐standard designs, such as cross‐over trials and cluster‐randomised trials.
Dealing with missing data
We tried to contact the trial authors to provide additional information in case of missing data.
Assessment of heterogeneity
We assessed the level of clinical heterogeneity between the trials by reviewing differences across trials in study population, setting, intervention and outcome measures used. In the absence of substantial clinical heterogeneity, we performed meta‐analyses. We used the Chi2 test, the I2 statistic and visual inspection of the forest plots to assess statistical heterogeneity. When statistical heterogeneity was present (P value < 0.1), we re‐analysed the data using the random‐effects model. For the outcome of pain, we explored the magnitude of baseline risk and heterogeneity using L'Abbé plots (a graph of the proportion of participants with an outcome by the proportion of participants without an outcome).
Assessment of reporting biases
We assessed reporting bias using a funnel plot.
Data synthesis
We analysed the data according to the intention‐to‐treat (ITT) principle, whereby all participants are analysed in the groups to which they were randomly allocated. We performed meta‐analysis where we judged clinical heterogeneity to be minimal, to ensure that we would derive clinically meaningful results. We calculated treatment differences by the Mantel‐Haenszel method using a fixed‐effect or random‐effects (when statistical heterogeneity was present) model. We presented results separately for the reviews of antibiotics against placebo and immediate antibiotics versus expectant observation.
Subgroup analysis and investigation of heterogeneity
The publication of Rovers 2006 describes the results of an individual patient data (IPD) meta‐analysis that was performed on a subset of trials included in this review (six trials including 1643 children aged six months to 12 years with AOM) to identify subgroups of children with AOM who might benefit more than others from treatment with antibiotics. Extensive details on the methods and results of this IPD meta‐analysis can be found in the original article (Rovers 2006). The primary outcome was a prolonged course of AOM defined as having either residual pain or fever (> 38 ºC) at three to seven days. Potential subgroups were selected on the basis of a multivariable prediction tool. The independent baseline predictors, that is, age (< two years versus > two years), fever and bilateral AOM (yes versus no), were used to study whether those at risk of a prolonged course also benefited more from treatment with antibiotics. In addition, otorrhoea (yes versus no) at baseline was studied as this is a clinically relevant outcome that occurred too infrequently to be identified as an independent predictor. To assess whether the effect of antibiotics was modified by age, bilateral disease, otorrhoea or a combination of these, a fixed‐effect logistic regression analysis. In this model, antibiotics (yes versus no), the potential effect modifier (age, bilateral disease, otorrhoea, or a combination of these), a dummy for the particular study and an interaction term (antibiotics * potential effect modifier) were included as independent variables and a prolonged course at three to seven days was the dependent variable. If a significant interaction effect was found, stratified analyses were performed to study the rate ratios and rate differences within each stratum of the subgroups.
Sensitivity analysis
We did not perform sensitivity analysis.
GRADE and ’Summary of findings'
For each outcome, we rated the overall quality of evidence as high, moderate, low and very low using the GRADE approach. Randomised controlled trials that do not have serious limitations are rated as high quality. However, we downgraded the evidence to moderate, low or very low depending on the presence of each of the following factors:
-
study limitations (risk of bias);
-
indirectness of evidence (directness of evidence);
-
imprecision (precision of results);
-
inconsistency (consistency of results); and
-
publication bias (existence of publication bias).
We included a 'Summary of findings' table (summary of findings Table for the main comparison) for the review of antibiotics against placebo, constructed according to the descriptions as described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included our primary outcomes and important secondary outcomes in the 'Summary of findings' table:
-
pain at 24 hours;
-
pain at two to three days;
-
pain at four to seven days;
-
pain at 10 to 12 days;
-
adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash);
-
abnormal tympanometry findings at two to four weeks;
-
abnormal tympanometry findings at three months.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
This is an update of a Cochrane review first published in The Cochrane Library in Issue 1, 1997 (Glasziou 1997) and updated in 1999 (Glasziou 1999), 2005 (Glasziou 2005), 2009 (Sanders 2009), and 2013 (Venekamp 2013). In the 2013 update of our review (Venekamp 2013), we identified 12 RCTs for the review of antibiotics against placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Thalin 1985; van Buchem 1981a and van Buchem 1981b), while we judged five RCTs eligible for the review of immediate antibiotics versus expectant observation (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). We excluded a total of 11 studies for various reasons (Arguedas 2011; Casey 2012; Chaput 1982; Engelhard 1989; Liu 2011; Ostfeld 1987; Rudberg 1954; Ruohola 2003; Sarrell 2003; Tähtinen 2012; van Buchem 1985).
With the updated search (November week 2, 2012 to April week 3, 2015), we retrieved a total of 1065 records. Removing duplicates left 937. After screening titles and abstracts, we identified four potentially eligible articles. After reviewing the full text, all articles appeared to be relevant for this review. However, three articles were additional analyses of previously included trials (Damoiseaux 2000; Hoberman 2011; Little 2001), providing additional data on pain at 10 to 12 days (Hoberman 2011) and long‐term effects (Damoiseaux 2000) for the review of antibiotics against placebo and data on long‐term effects (Little 2001) for the review of immediate antibiotics versus expectant observation. We did not identify any additional trials after reviewing the reference lists of the full‐text papers and relevant systematic reviews. This left one new trial eligible for inclusion in the review of antibiotics against placebo (Tapiainen 2014). We identified one ongoing trial (ACTRN12608000424303).
Included studies
Methods, participants, interventions and outcomes of the included studies are described in more detail in the table of Characteristics of included studies.
Antibiotics versus placebo
Thirteen trials including 3401 children (3938 AOM episodes) were eligible for the review of antibiotics against placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b).
Design
Twelve trials were double‐blind, placebo‐controlled, parallel‐group randomised clinical trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985), while one trial had a 2 x 2 factorial design (van Buchem 1981a and van Buchem 1981b).
Participants and settings
The sample size of the 13 individual trials ranged from 84 children (Tapiainen 2014) to 536 children (Kaleida 1991). The children were aged between two months and 15 years and 50% to 60% of included children were male. Three trials were performed in primary care (Burke 1991; Damoiseaux 2000; Tähtinen 2011), six in secondary care (Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Thalin 1985), and four in both primary and secondary care (Appelman 1991; Mygind 1981; Tapiainen 2014; van Buchem 1981a and van Buchem 1981b). AOM was diagnosed by the presence of acute symptoms and otoscopic signs in nine trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Halsted 1968; Hoberman 2011; Howie 1972; Kaleida 1991; Mygind 1981; van Buchem 1981a and van Buchem 1981b), and by the presence of middle‐ear effusion at pneumatic otoscopy and/or tympanometry in three trials (Le Saux 2005; Tähtinen 2011; Tapiainen 2014), while the criteria were not clearly described in one trial (Thalin 1985).
Interventions and comparators
Two trials compared penicillin for seven days with placebo (Mygind 1981; Thalin 1985), four trials compared amoxicillin for seven to 14 days with or without myringotomy with placebo (Burke 1991; Damoiseaux 2000; Kaleida 1991; Le Saux 2005), and four trials compared amoxicillin/clavulanate for seven to 10 days with placebo (Appelman 1991; Hoberman 2011; Tähtinen 2011; Tapiainen 2014). In one trial, ampicillin for 10 days was compared with pheneticillin and sulfisoxazole and placebo (Halsted 1968), while another trial compared erythromycin and triple sulphonamide with ampicillin, triple sulphonamide, erythromycin and placebo (Howie 1972). One trial, van Buchem 1981a and van Buchem 1981b, had a 2 x 2 factorial design resulting in four treatment groups: (1) sham myringotomy plus antibiotics; (2) sham myringotomy plus placebo; (3) myringotomy plus antibiotics; and (4) myringotomy plus placebo. We used all arms of this trial: van Buchem 1981a includes the sham myringotomy plus antibiotic and the sham myringotomy plus placebo arms, whereas van Buchem 1981b includes the myringotomy plus antibiotic and myringotomy plus placebo arms.
Outcomes
Pain
Five trials (1394 children) reported data on pain at 24 hours (Burke 1991; Le Saux 2005; Thalin 1985; Tähtinen 2011; van Buchem 1981a and van Buchem 1981b), seven (2320 children) on pain at two to three days (Appelman 1991; Halsted 1968; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Thalin 1985), seven (1347 children) on pain at four to seven days (Burke 1991; Damoiseaux 2000; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b), and one (278 children) on pain at 10 to 12 days (Hoberman 2011).
Adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash)
Eight trials (2107 children) reported data on adverse events likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash (Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985).
Abnormal tympanometry findings as a surrogate measure for hearing problems
Seven trials (2138 children) reported data on abnormal tympanometry findings at two to four weeks (Appelman 1991; Burke 1991; Kaleida 1991; Le Saux 2005; Mygind 1981; Tapiainen 2014; Thalin 1985), three (953 children) on abnormal tympanometry findings at six to eight weeks (Damoiseaux 2000; Kaleida 1991; Tapiainen 2014), and three (809 children) on abnormal tympanometry findings at three months (Burke 1991; Le Saux 2005; Mygind 1981), as a surrogate measure for hearing problems caused by middle‐ear fluid.
Tympanic membrane perforation
Five trials (1075 children) reported data on tympanic membrane perforation (Burke 1991; Hoberman 2011; Mygind 1981; Tähtinen 2011; Tapiainen 2014).
Progression of symptoms (contralateral otitis or late AOM recurrences)
Four trials (906 children) reported data on contralateral otitis (in unilateral cases) (Burke 1991; Hoberman 2011; Mygind 1981; Thalin 1985), while six trials (2200 children) reported data on late AOM recurrences (Hoberman 2011; Kaleida 1991; Le Saux 2005; Mygind 1981; Thalin 1985; van Buchem 1981a).
Serious complications
Ten trials reported on serious complications including mastoiditis or meningitis (Burke 1991; Damoiseaux 2000; Hoberman 2011; Howie 1972; Kaleida 1991; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; van Buchem 1981a and van Buchem 1981b), while information on complications was not explicitly reported in three trials (Appelman 1991; Halsted 1968; Thalin 1985).
Long‐term effects
One trial reported data on secondary care referrals at one year after randomisation as assessed by reviewing the children's notes (Burke 1991). Four children in the antibiotic group (4%) and seven in the placebo group (6%) were lost to follow‐up.
One trial reported data on the proportion of children with AOM recurrences, secondary care referrals and ENT surgery at approximately 3.5 years after randomisation (Damoiseaux 2000). These long‐term outcome data were collected by questionnaires. Questionnaires were returned in 168 of the 240 children (70%) that were originally randomised.
Immediate antibiotics versus expectant observation
Five trials including a total of 1149 children were eligible for the review of immediate antibiotics versus expectant observation (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006).
Design
All trials were open‐label, parallel‐group randomised clinical trials.
Participants and settings
The sample size of the five individual trials ranged from 142 children (Laxdal 1970) to 315 children (Little 2001). The children were aged 15 years and younger and 50% to 60% of included children were male. Two trials were performed in primary care (Little 2001; Neumark 2007), and three in secondary care (Laxdal 1970; McCormick 2005; Spiro 2006). AOM was diagnosed by the presence of acute symptoms and otoscopic signs in three trials (Laxdal 1970; Little 2001; McCormick 2005), by pneumatic otoscopy or preferably an aural microscope in one trial (Neumark 2007), while diagnostic criteria were unclear in one trial (AOM diagnosis was made at the discretion of the clinician) (Spiro 2006).
Intervention and comparators
In two of these trials provision of an immediate antibiotic script was compared with an antibiotic script with instructions not to commence antibiotic treatment unless the child was not better or was worse at 48 hours (Spiro 2006) or 72 hours (Little 2001). In these trials, 24% (36/150) and 38% (50/132) of children in the delayed arms reported using antibiotics at some stage during the illness.
The other three trials compared immediate antibiotics with a watchful waiting approach (Laxdal 1970; McCormick 2005; Neumark 2007). In the Laxdal 1970 trial, children in the control group were closely monitored, especially during the first 48 hours and particularly when severe involvement was evident. In the McCormick 2005 trial, antibiotics were administered to the watchful waiting group if a child returned to the office with a treatment failure or recurrence (four children in the expectant observation group had received antibiotics by day four). In the Neumark 2007 trial, 5% (4/87) of children randomised to the watchful waiting group received antibiotics due to treatment failure.
Outcomes
One trial did not report any data on our primary or secondary outcomes (Laxdal 1970), leaving four trials from which relevant data could be extracted (Little 2001; McCormick 2005; Neumark 2007; Spiro 2006).
Pain
Data on pain at three to seven days could be derived from four trials (959 children) (Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). The data on pain from the Little 2001 trial have been derived from data from the IPD meta‐analysis (Rovers 2006), while the data on pain from the McCormick 2005 trial have been provided by the author. One trial (247 children) reported data on pain at 11 to 14 days (Spiro 2006).
Adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash)
Two trials (550 children) reported data on adverse events likely to be related to the use of antibiotics such as vomiting, diarrhoea or rash (Little 2001; Spiro 2006).
Abnormal tympanometry findings as a surrogate measure for hearing problems
One trial (207 children) reported data on abnormal tympanometry findings at two to four weeks (McCormick 2005).
Tympanic membrane perforation
One trial (179 children) reported data on tympanic membrane perforation (Neumark 2007).
Progression of symptoms (contralateral otitis or late AOM recurrences)
None of the trials reported data on contralateral otitis (in unilateral cases), while one trial (209 children) reported data on late AOM recurrences (McCormick 2005).
Serious complications
Three trials reported on serious complications including mastoiditis or meningitis (McCormick 2005; Neumark 2007; Spiro 2006), while information on complications was not explicitly reported in one trial (Little 2001).
Long‐term effects
One trial reported data on the further ear pain episodes at three months and one year after randomisation (Little 2001). These long‐term outcome data were collected by questionnaires. Questionnaires were returned in 219 of the 315 children (70%) that were originally randomised at one year.
Excluded studies
We excluded 11 studies after reviewing the full text. Three were non‐randomised studies (Ostfeld 1987; Rudberg 1954; van Buchem 1985), while three other studies had no comparison of antibiotic with placebo or expectant observation (Casey 2012; Engelhard 1989; Sarrell 2003). Two trials studied the effectiveness of short‐ versus long‐course antibiotics (Arguedas 2011; Chaput 1982), one trial studied a single‐dose antibiotic with slow versus immediate‐release formulations (Liu 2011), whereas another trial was conducted in children with ventilation tubes (Ruohola 2003). Moreover, we excluded one trial report as this study reported on the effectiveness of immediate versus delayed antibiotic prescription based on a secondary analysis of a placebo‐controlled trial (Tähtinen 2012).
Risk of bias in included studies
The methodological quality of the included studies was generally high. For further details on the risk of bias in included studies see the 'Risk of bias' summary (Figure 1) and 'Risk of bias' graph (Figure 2).
Allocation
Concealment of allocation was adequately described in 11 of the 13 included trials comparing antibiotics with placebo (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Howie 1972; Le Saux 2005; Mygind 1981; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b), and two out of five trials comparing immediate antibiotics with expectant observation (Little 2001; Spiro 2006). Random sequence generation was adequate in seven of the 13 trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Tähtinen 2011; Tapiainen 2014), and in three of the five included trials (McCormick 2005; Neumark 2007; Spiro 2006), respectively.
Blinding
All included trials in the review of antibiotics against placebo stated that they were double‐blinded. However, we judged blinding to be adequate in eight of the 13 included trials (Burke 1991; Damoiseaux 2000; Hoberman 2011; Le Saux 2005; Tähtinen 2011; Tapiainen 2014; Thalin 1985; van Buchem 1981a and van Buchem 1981b). All five trials comparing immediate antibiotics with expectant observation were open‐label trials (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). As a consequence, reporting of the child's symptoms by parents was not blinded in these trials. However, investigators were blinded in two of the five trials (McCormick 2005; Spiro 2006).
Incomplete outcome data
The loss to follow‐up was below 5% in eight of the 13 trials comparing antibiotics with placebo (Appelman 1991; Burke 1991; Hoberman 2011; Howie 1972; Le Saux 2005; Tähtinen 2011; Tapiainen 2014; Thalin 1985). Loss to follow‐up was high in three trials with a total loss to follow‐up of 15% (van Buchem 1981a and van Buchem 1981b), 7% (Kaleida 1991), and 12% (Damoiseaux 2000), respectively. However, one of these trials included all randomised patients in the primary analysis at day four (Damoiseaux 2000). In two of the 13 trials the total number of loss to follow‐up/exclusions are described but it was unclear from which treatment group children were excluded (Halsted 1968; Mygind 1981). For the review of immediate antibiotics against expectant observation, the loss to follow‐up was below 5% in two of the five trials (McCormick 2005; Neumark 2007). The total loss to follow‐up in the other trials was 11% (Laxdal 1970), 10% (Little 2001), and 6% (Spiro 2006), respectively.
Selective reporting
Eight of the 13 included trials comparing antibiotics with placebo used intention‐to‐treat (ITT) analyses, while in the other five this was not clear (Halsted 1968; Howie 1972; Mygind 1981; Thalin 1985; van Buchem 1981a and van Buchem 1981b). For the review of immediate antibiotics versus expectant observation, three of the five included trials used ITT analyses, while this was not clear in the other two trials (Laxdal 1970; Neumark 2007).
Other potential sources of bias
No other potential sources of bias could be detected in the included trials, except for the Laxdal 1970 trial, which we judged as having a high risk of detection bias since children in the control group were subjected to very close scrutiny, especially during the first 48 hours and particularly when severe involvement was evident. However, this trial did not report any data on our primary or secondary outcomes.
Effects of interventions
Antibiotics versus placebo
Primary outcomes
1. Proportion of children with pain at various time points
The combined results of the trials revealed that by 24 hours from the start of treatment, 60% of the children had recovered whether or not they had placebo or antibiotics. The proportion of children that recovered spontaneously at two to three days, four to seven days and 10 to 12 days was 84%, 76% and 78%, respectively. Antibiotics achieved a 30% (95% confidence interval (CI) 14% to 43%) relative reduction in the risk of pain at two to three days, 24% (95% CI 9% to 37%) relative reduction in the risk of pain at four to seven days and 67% (95% CI 34% to 83%) relative reduction in the risk of pain at 10 to 12 days (Analysis 1.1). This means 5% (95% CI 2% to 7%) fewer children had pain after two to three days (number needed to treat for an additional beneficial outcome (NNTB) 20, 95% CI 14 to 50), 6% (95% CI 2% to 9%) fewer children had pain after four to seven days (NNTB 16, 95% CI 11 to 50) and 14% (95% CI 6% to 22%) fewer children had pain after 10 to 12 days (NNTB 7, 95% CI 4 to 16), respectively. Plots of the event rate (pain) in the treatment and control groups for each study at 24 hours and two to three days are reported in Figure 3 and Figure 4. The funnel plot for pain at the various time points did not reveal asymmetry (Figure 5).

L'Abbé plot of the rates of pain at 24 hours for the placebo (control) versus antibiotic (experimental) group.

L'Abbé plot of the rates of pain at two to three days for the placebo (control) versus antibiotic (experimental) group.

Funnel plot of comparison: 1 Antibiotic versus placebo, outcome: 1.1 Pain.
Quality of the evidence
We judged the data on pain at 24 hours, two to three days and four to seven days to be of high quality, while we judged the data on pain at 10 to 12 days to be of moderate quality. We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis).
2. Adverse effects likely to be related to the use of antibiotics
Antibiotics resulted in a 38% (95% CI 15% to 73%) relative increase in the risk of adverse effects likely to be related to the use of antibiotics (defined as vomiting, diarrhoea or rash) compared with placebo; 27% (283/1044) of children treated with antibiotics versus 20% (208/1063) of children treated with placebo experienced vomiting, diarrhoea or rash (Analysis 1.2). The number needed to treat for an additional harmful outcome (NNTH) was 14 (9 to 26).
Quality of the evidence
We judged the evidence for adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash) to be of high quality.
Secondary outcomes
1. Abnormal tympanometry findings at various time points
Antibiotics achieved an 18% (95% CI 10% to 26%) relative reduction in the risk of abnormal tympanometry findings at two to four weeks, and a 12% (95% CI 0% to 22%) relative reduction in the risk of abnormal tympanometry findings at six to eight weeks (Analysis 1.3). This means 9% (95% CI 5% to 13%) fewer children had abnormal tympanometry findings at two to four weeks (NNTB 11, 95% CI 7 to 20) and 6% (95% CI 0% to 12%) fewer children had abnormal tympanometry findings at six to eight weeks (NNTB 16, 95% CI 8 to 277), respectively.
However, antibiotics were not associated with a statistically significant reduction in the risk of abnormal tympanometry findings at three months compared with placebo (Analysis 1.3). Furthermore, audiometry was done in only two studies and incompletely reported. The two studies that used audiograms were van Buchem 1981a and Kaleida 1991: (i) van Buchem 1981a reported that, "After one month, 31% of the patients showed an air/bone gap of more than 20 dB. After two months, this was still the case with 19% of the patients. Here again, there were no significant differences between the groups"; (ii) Kaleida 1991 stated that "Analysis of hearing acuity in children two years of age and older indicated that elevated hearing thresholds ... bore no apparent relationship ... to mode of treatment (amoxicillin versus placebo)".
Quality of the evidence
We judged the evidence for abnormal tympanometry findings at the various time points to be of high quality.
2. Tympanic membrane perforation
Antibiotic treatment was associated with a 63% (95% CI 24% to 82%) relative reduction in the risk of tympanic membrane perforation compared with placebo (Analysis 1.4). However, absolute benefits of antibiotics appeared to be small: 3% (95% CI 1% to 5%) fewer children had a tympanic membrane perforation. Therefore, 33 children (95% CI 20 to 100) needed to be treated to prevent one child experiencing a tympanic membrane perforation.
Quality of the evidence
We judged the evidence for tympanic membrane perforation to be of high quality.
3. Contralateral otitis
Antibiotics were associated with a 51% (5% to 75%) relative reduction in the development of contralateral otitis compared with placebo (Analysis 1.5). This means 9% (95% CI 5% to 13%) fewer children had contralateral otitis (NNTB 11, 95% CI 7 to 20).
Quality of the evidence
We judged the evidence for contralateral otitis to be of high quality.
4. AOM recurrences
Antibiotics were not associated with a statistically significant reduction in the occurrence of late AOM recurrences compared with placebo (Analysis 1.6). AOM recurrences were common. Burke 1991 stated "The mean number of recorded recurrences of otitis media or acute red ear was 0.70 (range 0 to 4) in the antibiotic group and 0.63 (range 0 to 7) in the placebo group and this difference was not significant (difference 0.06, 95% CI ‐0.22 to 0.339)." Six other trials reported the proportions who relapsed; combined, these give a risk ratio (RR) of 0.93 (95% CI 0.78 to 1.10), which is consistent with Burke's findings.
Quality of the evidence
We judged the evidence for late AOM recurrences to be of high quality.
5. Serious complications related to AOM
Few serious complications occurred in either the antibiotic treatment group or the control group. In just over 3000 children studied, only one case of mastoiditis occurred in both the antibiotic group (Mygind 1981) and the placebo group (Hoberman 2011). Moreover, one child suffered from meningitis (Damoiseaux 2000), pneumococcal bacteraemia and radiologically confirmed pneumonia (Hoberman 2011) in the placebo group and one child had transient facial paralysis in the antibiotic group (Kaleida 1991). Hence, the applicability of these findings to groups of children in whom serious complications such as mastoiditis is common is uncertain. One of the excluded studies did report high rates of mastoiditis (Rudberg 1954). This was an open, semi‐randomised study conducted in Sweden in 1954. Participants were randomised by case‐sheet number but a proportion (about 30 of 220) requested, and were granted, entry to the penicillin group. The rate of mastoiditis was 17% in the untreated group versus 1.5% in the sulphonamide‐treated group and 0% in the penicillin‐treated group. The biases of this study (semi‐randomisation and unblinded outcome assessment) are unlikely to explain such a large difference.
Quality of the evidence
We judged the evidence for serious complications to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data and due to the conflicting results found in an open, semi‐randomised study that was not included in our review.
6. Long‐term effects
Based on reviewing children's notes, antibiotics were not associated with a statistically significant reduction in the number of secondary care referrals at one year after randomisation: 7/110 (6%) in the antibiotic group and 9/111 (8%) in the placebo group (RR 0.78, 95% 0.30 to 2.03).
Based on questionnaires returned by parents approximately 3.5 years after initial randomisation, antibiotics were associated with a 46% (95% CI 8% to 97%) relative increase in the risk of AOM recurrences. This means 20% (95% CI 5% to 35%) fewer children had AOM recurrences (NNTB 5, 95% CI 2 to 20). No between‐group differences were observed for secondary care referrals. Furthermore, antibiotics were not associated with a statistically significant reduction in the number of ear, nose and throat surgeries (RR 0.68, 95% CI 0.40 to 1.17).
Quality of the evidence
We judged the evidence for long‐term effects at one year to be of high quality, while we judged the 3.5 years data to be of moderate quality. We mainly downgraded the evidence from high quality because of the high proportion of children that were not included in the analysis (30%), which introduced a significant risk of (attrition) bias.
Immediate antibiotics versus expectant observation
Primary outcomes
1. Proportion of children with pain at various time points
Immediate antibiotics were not associated with a statistically significant reduction in the risk of pain at three to seven days (RR 0.75, 95% CI 0.50 to 1.12) and 11 to 14 days (RR 0.91, 95% CI 0.75 to 1.10) compared with expectant observation (observation with or without an antibiotic prescription) (Analysis 2.1).
Quality of the evidence
We judged the data on pain at three to seven days to be of high quality, while we judged the data on pain at 11 to 14 days to be of moderate quality. We downgraded the evidence for pain at days 11 to 14 from high quality because of the substantial number of children that were 'lost to follow‐up' (13%), which introduced a risk of (attrition) bias.
2. Adverse effects likely to be related to the use of antibiotics
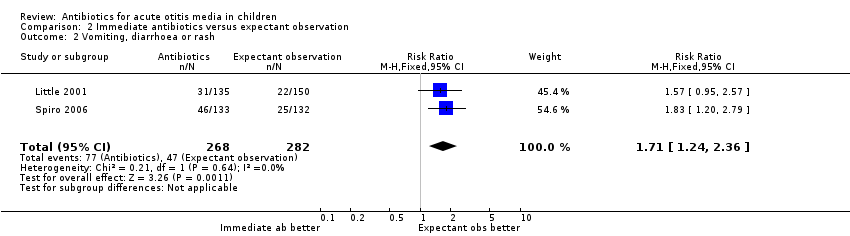
Immediate antibiotics were associated with a 71% (95% CI 24% to 136%) relative increase in the risk of adverse effects likely to be related to the use of antibiotics (defined as vomiting, diarrhoea or rash) compared with expectant observation; 29% (77/268) of children treated with immediate antibiotics versus 17% (47/282) of children treated with expectant observation experienced vomiting, diarrhoea or rash (Analysis 2.2). The NNTH was 9 (6 to 20).
Quality of the evidence
We judged the evidence for adverse effects likely to be related to the use of antibiotics (vomiting, diarrhoea or rash) to be of high quality.
Secondary outcomes
1. Abnormal tympanometry findings at various time points
In one trial (207 children), the proportion of children with abnormal tympanometry findings at four weeks did not substantially differ between those receiving immediate antibiotics and expectant observation (RR 1.03, 95% CI 0.78 to 1.35) (Analysis 2.3).
Quality of the evidence
We judged the data on abnormal tympanometry findings at four weeks to be of moderate quality. We downgraded the evidence from high quality as the number of children that were 'lost to follow‐up' in the immediate antibiotics group was substantially lower than in the expectant observation group (4% versus 11%), thereby introducing a risk of (attrition) bias.
2. Tympanic membrane perforation
No tympanic membrane perforations were observed in either group in the only trial (179 children) reporting on this outcome (Analysis 2.4).
Quality of the evidence
We judged the data on tympanic membrane perforation to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data
3. Contralateral otitis
None of the trials reported data on contralateral otitis.
4. AOM recurrences
In one trial (209 children), immediate antibiotics were associated with a non‐statistically significant 41% (95% CI ‐26% to 169%) relative increase in the risk of AOM recurrences (Analysis 2.5).
Quality of the evidence
We judged the data on late AOM recurrences to be of moderate quality. We downgraded the evidence from high quality as the number of children that were 'lost to follow‐up' in the immediate antibiotics group was substantially lower than in the expectant observation group (3% versus 10%), thereby introducing a risk of (attrition) bias.
5. Serious complications related to AOM
No serious complications occurred in either the immediate antibiotic group or the expectant observation group.
Quality of the evidence
We judged the evidence for serious complications to be of moderate quality. We downgraded the evidence from high quality as we considered the sample size to be insufficient to draw any definite conclusions based on these data.
6. Long‐term effects
No statistically significant differences were observed between the immediate antibiotics and the delayed antibiotics group in parent‐reported ear pain episodes at one year (odds ratio (OR) 1.03, 95% 0.60 to 1.78).
Quality of the evidence
We judged the evidence for long‐term effects to be of moderate quality. We mainly downgraded the evidence from high quality as this evidence was derived from a secondary analysis and because of the high proportion of children that were not included in the analysis at one year (30%), which introduced a significant risk of (attrition) bias.
Individual patient data (IPD) meta‐analysis to identify children most likely to benefit from antibiotic treatment
In 2006, an individual patient data (IPD) meta‐analysis was performed, Rovers 2006, using data from six high‐quality RCTs, including a total of 1643 children, which were also included in this review as individual trials (Appelman 1991; Burke 1991; Damoiseaux 2000; Le Saux 2005; Little 2001; McCormick 2005). The main findings of this IPD meta‐analysis were that significant effect modifications were noted for age and bilateral AOM and for otorrhoea; in children aged less than two years with bilateral AOM, 55% of the control group and 30% of the antibiotics group still had pain, fever or both at three to seven days (absolute risk reduction of 25%, 95% CI 14% to 36%; NNTB 4). In children aged two years or older with bilateral AOM the absolute risk reduction was 12% (95% CI ‐1% to 25%; P value for interaction = 0.022). Among children with otorrhoea, 60% of those in the control group had pain, fever or both at three to seven days versus 24% in the antibiotics group (risk reduction of 36%, 95% CI 19% to 53%; NNTB 3). The absolute reduction in risk among those without otorrhoea was 14% (95% CI 5% to 23%; NNTB 8; P value for interaction = 0.039). No differences were identified for age alone.
Quality of the evidence
We judged the evidence for subgroup analyses based on the IPD meta‐analysis to be of high quality.
Discussion
Summary of main results
This review reveals that antibiotics have no early effect on pain, a slight effect on pain in the days following and only a modest effect on the number of children with tympanic perforations, contralateral otitis episodes and abnormal tympanometry findings at two to four weeks and at six to eight weeks, compared with placebo in children with acute otitis media (AOM). However, in applying these results, there are a number of issues to consider, including the individual potential for serious complications and subgroups of children in whom there may be greater benefits.
Overall completeness and applicability of evidence
Does the effect vary in different clinical groups? Our number needed to treat for an additional beneficial outcome (NNTB) of 20 for pain at days two to three days, 16 for pain at four to seven days and seven for pain at 10 to 12 days is for the 'average' case and may vary in subgroups. Several studies reported higher rates of failure of placebo treatment among children less than two years of age and those with bilateral disease (Appelman 1991; Burke 1991; Damoiseaux 2000; Hoberman 2011; Tähtinen 2011), and another trial has suggested that most benefit is seen in children with high fever or vomiting (Little 2001). Moreover, some studies found that children with bilateral AOM differ with regards to clinical and microbiological (increased presence of (non‐typeable) H. influenzae) characteristics compared with children with unilateral AOM (Barkai 2009; McCormick 2007). However, the individual patient data (IPD) meta‐analysis demonstrated that the relative effects of antibiotics were not significantly modified by either age or bilateral disease alone but the absolute differences were larger in the younger patients (less than two years) with bilateral disease and in children with both AOM and otorrhoea (Rovers 2006). Further analysis of these data has shown that age younger than two years is an independent predictor of the development of asymptomatic middle‐ear effusion (Koopman 2008). This analysis also found that antibiotic therapy has a marginal effect on the development of asymptomatic middle‐ear effusion in children with AOM.
Does the impact vary by duration and dose of antibiotics? Most trials use seven days of antibiotic treatment. One recent meta‐analysis of a short (less than seven days) versus long (more than seven days) course of antibiotics reported that risk of treatment failure at one month was higher with short courses of antibiotics (odds ratio (OR) 1.34, 95% confidence interval (CI) 1.15 to 1.55) (Kozyrskyj 2010). However, the absolute difference in treatment effect was small (3%) and short courses of antibiotics were associated with a statistically significant reduction in gastrointestinal adverse events compared with longer courses. A recommendation regarding the most appropriate dose of antibiotics is not possible due to a lack of sufficient data.
What are the potential consequences of not using antibiotics? Besides the immediate pain associated with AOM, there are some more serious complications. Though only two cases of mastoiditis were reported in the included trials (one child received antibiotics and one child was assigned to placebo), a semi‐randomised trial in Sweden in 1954 reported a rate of 17% in the untreated group versus none in the penicillin‐treated groups (Rudberg 1954). In populations or sub‐populations where mastoiditis is still judged a frequent problem, such as in some low‐income countries, antibiotic treatment would be strongly advised (Berman 1995).
Of note is an article that revealed that doctors commonly over‐diagnose AOM (Rothman 2003). What effect might this have on the efficacy of antibiotics (or any treatment)? One effect will be to blunt any treatment effect by dilution (from the cases of non‐AOM). The results of two recently performed trials (Hoberman 2011; Tähtinen 2011), in which AOM has been diagnosed with the use of stringent criteria (including pneumatic otoscopic examination in one trial (Tähtinen 2011), underline this phenomenon. Nevertheless, physicians in daily practice are likely to use the same diagnostic methods (perhaps even less stringent) as used in the majority of the included trials in this review. As a consequence, the effectiveness of antibiotics reported in this review is likely to be a true reflection of the effectiveness in actual clinical practice. However, if new and more accurate diagnostic procedures are introduced in future daily practice, then the current estimate of effectiveness will have to be reconsidered.
Quality of the evidence
The methodological quality of the included studies was generally high. We judged the evidence to be of high quality for most of the outcomes in the review of antibiotics against placebo. We judged the quality of evidence to be moderate for pain at 10 to 12 days, serious complications and long‐term effects (3.5 years data). We downgraded the evidence mainly because of the risk of reporting bias (pain at 10 to 12 days), sample size considerations (serious complications) and the risk of attrition bias (long‐term effects).
For the review of immediate antibiotics versus expectant observation, we judged the evidence to be of moderate quality for most of the outcomes. We downgraded the evidence mainly because of sample size considerations (tympanic membrane perforation, serious complications) and the risk of attrition bias (pain at days 11 to 14, abnormal tympanometry findings at four weeks, late AOM recurrences, long‐term effects). We judged the evidence to be of high quality for pain at days four to seven and adverse effects likely to be related to the use of antibiotics.
Potential biases in the review process
There was some clinical heterogeneity among the included trials. For example, patients were recruited from different settings (general practice, ear, nose and throat and paediatric clinics). However, the majority of included trials did use a diagnostic method (clinical diagnosis of AOM as inclusion criteria) that resembles daily clinical practice. Besides, duration and dosage of the antibiotic treatment varied to some extent. For the review of antibiotics against placebo, the duration of antibiotic treatment varied from seven to 14 days. However, we do not consider this as a major drawback since most trials used seven days of antibiotic treatment and current evidence indicates only a small absolute treatment difference (3%) in treatment failure at one month in favour of a long (more than seven days) versus a short (less than seven days) course of antibiotics. Moreover, the primary outcome of this review (proportions of children with pain) is reported within the first seven days of antibiotic treatment. In addition, we assessed funnel plots for potential reporting biases for the primary analysis (Figure 5). No asymmetry could be detected in the included trials.
Agreements and disagreements with other studies or reviews
This review demonstrated that at 24 hours pain had recovered spontaneously in 60% of children and that the majority had recovered in the following two to 12 days regardless of whether they had received placebo or antibiotics. However, the IPD meta‐analysis, which included six of the trials included in this review, revealed a slower rate of recovery (Figure 6) with only 22% of children experiencing spontaneous recovery at 24 hours (Rovers 2006). There are a number of possible explanations for this. First, data from older trials were not included in the IPD meta‐analysis and consequently the study population may reflect a higher threshold of doctor visitation; for example, the children may be 'sicker' or presenting to the doctor later in the course of their illness. Variation in the definitions of pain/no pain cut‐offs among the trials included in the reviews may also explain some of this variation. From the IPD meta‐analysis survival curve (Figure 6) it can be seen that antibiotics had greatest effect compared with placebo at day three.

Percentage with pain based on the subset of six studies included in the IPD meta‐analysis (Rovers 2006).
A previous meta‐analysis has examined the question of whether antibiotics were indicated for AOM in children and concluded that the answer is a qualified 'yes' (Rosenfeld 1994). It estimated a NNTB of seven for "primary control" (complete clinical resolution), compared with our NNTB of 20 for symptom relief. The difference may be the consequence of our focus on patient‐oriented outcomes, such as pain, rather than clinical signs, such as eardrum appearance. The previous systematic review suggests that where mastoiditis is not a concern, primary care physicians could weigh the benefits against the risks of adverse effects from antibiotics with their patients. This statement is in agreement with the findings of our review as adverse events such as diarrhoea, vomiting or rash were more common in children receiving antibiotics. In the IPD meta‐analysis the most commonly described adverse effect of antibiotic treatment was diarrhoea, ranging from 2% to 14% in controls and from 4% to 21% in those given antibiotics (Rovers 2006). Occurrence of rash ranged from 2% to 6% in the control groups and from 1% to 8% in the antibiotic groups. A recent systematic review and meta‐analysis on common harms of amoxicillin revealed that harms were poorly reported in most placebo‐controlled trials (Gillies 2014). In this review, diarrhoea was attributed to amoxicillin only in the form of amoxicillin/clavulanate. Amoxicillin did increase the risk of candidiasis compared with placebo, but no association between amoxicillin and rash or vomiting was observed (Gillies 2014). Bacterial resistance to antibiotics is also a consideration, with an association between antibiotic use and resistant bacteria demonstrated for many important pathogens (Arnold 2005).
Several trials evaluated a management approach for AOM in which an expectant observational approach is used (Laxdal 1970; Little 2001; McCormick 2005; Neumark 2007; Spiro 2006). In one of these trials pain and malaise at day three were greater among those randomised to receive an antibiotic prescription with advice to fill it only if there was no improvement after 72 hours compared with those receiving immediate antibiotics (Little 2001). In a secondary analysis of the trial no difference was found between delayed and immediate treatment groups in ear function and ear pain at three and 12 months (Little 2006). Another study using a similar prescribing approach and examining clinical outcomes at four to six days found no difference between immediate and delayed antibiotic groups (Spiro 2006). In the third study (McCormick 2005), immediate antibiotic treatment was associated with decreased numbers of treatment failures and improved symptom control at day four and day 12 compared with those allocated to expectant observation with no prescription. Neumark 2007, in a similar comparison, found that immediate antibiotics provided some symptomatic benefit; children who received antibiotics had less pain, used fewer analgesics and consulted less during the first seven days. Meta‐analysis of data from these four trials found no difference in pain between immediate antibiotics and expectant observational approaches at three to seven days. Another review (Spurling 2013), which evaluated the effect of delayed versus immediate or no antibiotics for respiratory infections and which included two studies on AOM (Little 2001; Spiro 2006), concluded that immediate antibiotics was the strategy most likely to provide the best clinical outcomes for AOM. One randomised study found that observation therapy with or without a prescription in children with AOM was well accepted by parents (Chao 2008). Antibiotic use was less in those randomised to observation without prescription and no complications were reported.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

L'Abbé plot of the rates of pain at 24 hours for the placebo (control) versus antibiotic (experimental) group.

L'Abbé plot of the rates of pain at two to three days for the placebo (control) versus antibiotic (experimental) group.

Funnel plot of comparison: 1 Antibiotic versus placebo, outcome: 1.1 Pain.

Percentage with pain based on the subset of six studies included in the IPD meta‐analysis (Rovers 2006).
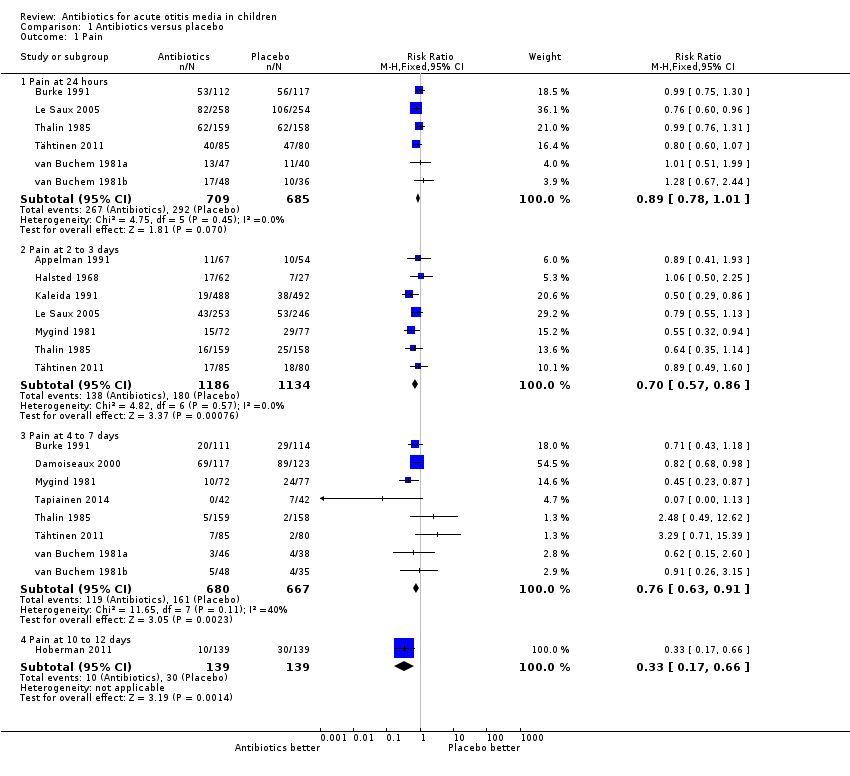
Comparison 1 Antibiotics versus placebo, Outcome 1 Pain.
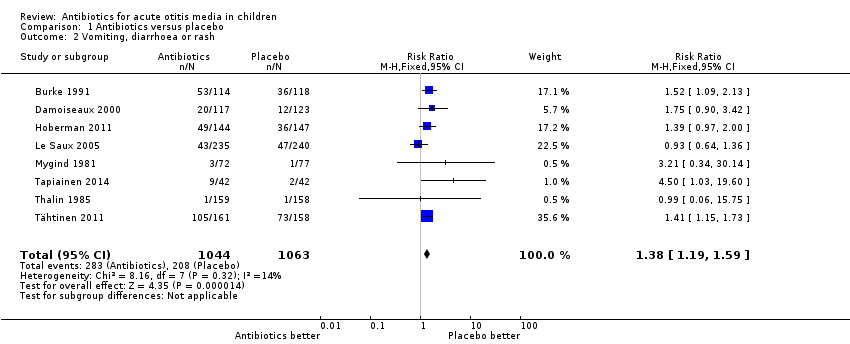
Comparison 1 Antibiotics versus placebo, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 1 Antibiotics versus placebo, Outcome 3 Abnormal tympanometry.

Comparison 1 Antibiotics versus placebo, Outcome 4 Tympanic membrane perforation.
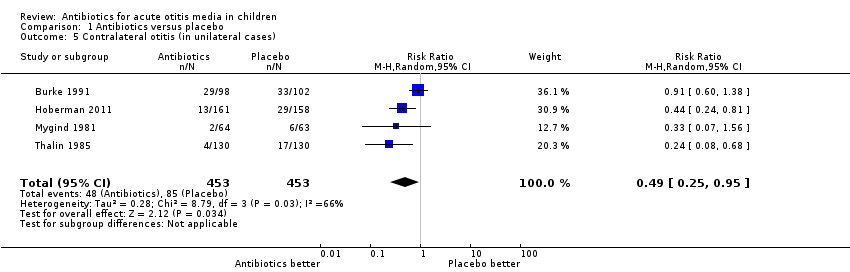
Comparison 1 Antibiotics versus placebo, Outcome 5 Contralateral otitis (in unilateral cases).

Comparison 1 Antibiotics versus placebo, Outcome 6 Late AOM recurrences.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 1 Pain.
Comparison 2 Immediate antibiotics versus expectant observation, Outcome 2 Vomiting, diarrhoea or rash.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 3 Abnormal tympanometry at 4 weeks.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 4 Tympanic membrane perforation.

Comparison 2 Immediate antibiotics versus expectant observation, Outcome 5 AOM recurrences.
| Antibiotics versus placebo for acute otitis media in children | ||||||
| Patient or population: children with acute otitis media | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics versus placebo | |||||
| Pain ‐ pain at 24 hours | Study population | RR 0.89 | 1394 | ⊕⊕⊕⊕ | ||
| 426 per 1000 | 379 per 1000 | |||||
| Pain ‐ pain at 2 to 3 days | Study population | RR 0.70 | 2320 | ⊕⊕⊕⊕ | ||
| 159 per 1000 | 111 per 1000 | |||||
| Pain ‐ pain at 4 to 7 days | Study population | RR 0.76 | 1347 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 183 per 1000 | |||||
| Pain ‐ pain at 10 to 12 days | Study population | RR 0.33 | 278 | ⊕⊕⊕⊝ | ||
| 216 per 1000 | 71 per 1000 | |||||
| Abnormal tympanometry ‐ 2 to 4 weeks | Study population | RR 0.82 | 2138 | ⊕⊕⊕⊕ | ||
| 481 per 1000 | 395 per 1000 | |||||
| Abnormal tympanometry ‐ 3 months | Study population | RR 0.97 | 809 | ⊕⊕⊕⊕ | ||
| 241 per 1000 | 234 per 1000 | |||||
| Vomiting, diarrhoea or rash | Study population | RR 1.38 | 2107 | ⊕⊕⊕⊕ | ||
| 196 per 1000 | 270 per 1000 | |||||
| *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The number of studies reported in the 'Summary of findings' table for the outcomes 'Pain at 24 hours' and 'Pain at 4 to 7 days' differ slightly from those reported in the Data Analysis Table 1 ‐ Antibiotics versus placebo (five versus six studies and seven versus eight studies, respectively). This is due to the van Buchem trial. This trial is included as one study in our review (and in the 'Summary of findings' table), but we included data from two different comparisons from this 2 x 2 factorial design trial in our analyses (van Buchem 1981a; van Buchem 1981b). 2We downgraded the evidence for pain at days 10 to 12 from high quality as this outcome was not specified a priori in this trial (secondary analysis). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain at 24 hours | 6 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 1.2 Pain at 2 to 3 days | 7 | 2320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.86] |
| 1.3 Pain at 4 to 7 days | 8 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 1.4 Pain at 10 to 12 days | 1 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.17, 0.66] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 8 | 2107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.19, 1.59] |
| 3 Abnormal tympanometry Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2 to 4 weeks | 7 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.90] |
| 3.2 6 to 8 weeks | 3 | 953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 3.3 3 months | 3 | 809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 4 Tympanic membrane perforation Show forest plot | 5 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.18, 0.76] |
| 5 Contralateral otitis (in unilateral cases) Show forest plot | 4 | 906 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.95] |
| 6 Late AOM recurrences Show forest plot | 6 | 2200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pain at 3 to 7 days | 4 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.50, 1.12] |
| 1.2 Pain at 11 to 14 days | 1 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.10] |
| 2 Vomiting, diarrhoea or rash Show forest plot | 2 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.24, 2.36] |
| 3 Abnormal tympanometry at 4 weeks Show forest plot | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.35] |
| 4 Tympanic membrane perforation Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 AOM recurrences Show forest plot | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.74, 2.69] |

