护士主导滴定血管紧张素转换酶抑制剂、β‐肾上腺素能阻滞剂和血管紧张素受体阻滞剂用于射血分数降低的心衰患者
摘要
研究背景
心力衰竭与高死亡率、再入院有关。β‐肾上腺素能阻滞剂、血管紧张素转换酶抑制剂(angiotensin converting enzyme inhibitors, ACEIs)和血管紧张素受体阻滞剂(angiotensin receptor blockers, ARBs)可以提高生存率并减少再入院率,被推荐作为心力衰竭治疗的一线治疗药物。证据还表明,这些药物与患者预后存在剂量反应关系。尽管有这些证据,初级保健医生仍不愿意上调这些药物的剂量。旨在促进这种剂量上调的新策略是必要的。护士主导滴定(Nurse‐led titration, NLT)就是这样的策略之一。
研究目的
旨在评价β‐肾上腺素能阻滞剂、ACEI和ARB的NLT对射血分数降低的心力衰竭(heart failure with reduced ejection fraction, HFrEF)的安全性和对患者结局的影响。
检索策略
我们检索了Cochrane图书馆的Cochrane对照试验中心注册库(Cochrane Central Register of Controlled Trials, CENTRAL第11期,共12期,2014年12月19日)、MEDLINE OVID(1946年至2014年11月第3周)以及EMBASE Classic和EMBASE OVID(1947年至2014年第50周)。我们还检索了相关初步研究、系统综述、临床试验登记和未发表的论文资源的参考文献列表。我们未设置任何语言限制。
纳入排除标准
比较β‐肾上腺素能阻断剂、ACEI和/或ARB的NLT,比较护士对HFrEF患者的这些药物的优化与另一位卫生专业人员的优化的随机对照试验(Randomised controlled trials, RCT)。
资料收集与分析
两位综述作者(AD和JC)独立评价研究的合格性和偏倚风险。如果我们需要更多信息,我们会联系主要作者。我们使用RCT的GRADE评级工具评价了证据的质量。我们通过二分数据的风险比(risk ratio, RR)及其95%置信区间(confidence interval, CI)分析提取的资料,以衡量干预组与常规护理组相比的效果大小。meta分析使用固定效应Mantel‐Haenszel方法。我们使用Chi 2 和I 2 评价了研究之间的异质性。
主要结果
我们在本综述中纳入了七项研究(1684名受试者)。一项研究招募了来自寄宿护理机构的受试者,其他六项研究的受试者则来自初级保健和门诊诊所。四项研究(556名受试者)提供了全因入院资料。与常规护理组相比,NLT组的受试者全因入院率较低(RR=0.80, 95% CI [0.72, 0.88],高质量证据),且与心力衰竭相关的入院率较低(RR=0.51, 95% CI [0.36, 0.72],中等质量证据)。六项研究(902名受试者)调查了全因死亡率。与常规治疗相比,NLT组的全因死亡率也较低(RR=0.66, 95% CI [0.48, 0.92],中等质量证据)。接受β‐肾上腺素能阻断剂、ACEI和ARB的NLT治疗的患者中,每1000人大约可以避免27人死亡。只有三项研究(370名受试者)报告了全因和心力衰竭相关的无事件生存结局。与常规护理组的受试者相比,NLT组的受试者更有可能保持无事件(RR=0.60, 95% CI [0.46, 0.77],中等质量证据)。五项研究(966名受试者)报告了达到β‐肾上腺素能阻滞剂目标剂量的受试者人数。与常规治疗相比,NLT组的这一比例也更高(RR=1.99, 95% CI [1.61, 2.47],低质量证据)。然而,该汇总分析存在很大程度的异质性。我们将这些研究的偏倚风险评为高,主要是由于不完整结局数据、缺乏与干预相关的不良事件报告以及无法对受试者和人员进行盲法。NLT组的受试者达到β‐肾上腺素能阻断剂的最大剂量的时间为常规治疗组的一半。两项研究报告了不良事件;其中一项研究报告称没有不良事件,另一项研究发现了一项不良事件,但没有具体说明不良事件的类型或严重程度。
作者结论
NLT组的受试者因任何原因入院的次数减少,存活率增加,并且在更短的时间内达到目标剂量的受试者数量增加。然而,有关达到目标剂量的受试者比例的证据质量较低,应谨慎解释。我们发现高质量的证据支持NLT作为一种可以改善β‐肾上腺素能阻滞剂的优化,从而减少入院率的策略。尽管有证据表明β‐肾上腺素能阻滞剂、ACEIs和ARB与改善HFrEF患者的预后存在剂量反应关系,但这一证据转化为临床实践的情况很差。NLT是一种促进将这些证据付诸实践的策略。
PICOs
简语概要
护士主导的心力衰竭药物优化
研究问题
评价护士主导滴定(nurse‐led titration, NLT)β‐肾上腺素能阻滞剂、血管紧张素转换酶抑制剂(angiotensin converting enzyme inhibitors, ACEI)和血管紧张素受体阻滞剂(angiotensin receptor blockers, ARB)对心力衰竭患者的安全性和患者预后的影响。
研究背景
心力衰竭的住院率和死亡率很高。大型临床试验表明,使用β‐肾上腺素能阻滞剂、ACEI和ARB将改善这些结局。此外,这之间还存在剂量反应关系,因此这些药物的剂量越高,患者治疗效果的改善就越大。然而,初级保健医生通常不愿意增加这些药物的剂量。需要新的方法来增加这些药物的剂量。这些药物用药的优化可以由执业护士或高级执业护士在医疗监督下完成。
研究特征
我们对七项随机对照试验(1684名受试者)进行了评价,这些试验比较护士滴定β‐肾上腺素能阻断剂、ACEI和ARB与初级保健医生滴定这些药物的情况。每项研究受试者的人口统计特征相似。其中四项研究的男性和女性人数相同。受试者的平均年龄为59岁至81岁。证据检索日期截至2014年12月。
主要研究结果
本综述发现,接受护士药物滴定的受试者入院或死亡的可能性较小,与那些由初级保健医生滴定这些药物的受试者相比,更多的受试者达到了最大剂量。在医疗监督下的护士或执业护士对这些药物进行滴定时,每1000名患者中可以避免大约27例死亡。关于ACEI和ARB滴定的数据资料很少。两项研究报告了不良事件;其中一项研究报告称没有不良事件,另一项研究发现了一项不良事件,但没有具体说明不良事件的类型或严重程度。
总之,医疗监督下的护士或执业护士对这些药物的滴定可能会促进剂量上调,从而改善患者的治疗效果。
证据质量
我们将有关达到这些药物最佳剂量的受试者比例的证据质量评为低质量。这表明我们无法确定达到β‐肾上腺素能阻断剂最佳剂量的受试者数量是否因NLT或常规治疗而不同。我们发现高质量的证据表明,与常规治疗相比,NLT减少了因任何原因住院的情况。这表明我们确信全因住院率的减少是NLT造成的,进一步的研究不太可能改变这一发现。
Authors' conclusions
Summary of findings
| Nurse‐led titration versus usual care for people with heart failure with reduced ejection fraction | ||||||
| Patient or population: people with heart failure with reduced ejection fraction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nurse‐led titration versus usual care | |||||
| All‐cause hospital admissions | Study population | RR 0.80 | 560 | ⊕⊕⊕⊕ | ||
| 763 per 1000 | 610 per 1000 | |||||
| Moderate | ||||||
| 437 per 1000 | 350 per 1000 | |||||
| Heart failure‐related hospital admissions | Study population | RR 0.51 | 642 | ⊕⊕⊕⊝ | ||
| 248 per 1000 | 126 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 93 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.66 | 902 | ⊕⊕⊕⊝ | ||
| 166 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 163 per 1000 | 108 per 1000 | |||||
| All‐cause event‐free survival | Study population | RR 0.60 | 370 | ⊕⊕⊕⊝ | ||
| 487 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 385 per 1000 | 231 per 1000 | |||||
| Proportion reaching target dose of medications | Study population | RR 1.99 | 966 | ⊕⊕⊝⊝ | ||
| 171 per 1000 | 340 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 362 per 1000 | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1,2 I = 68% and P = 0.03 with a high Chi2 in relation to degrees of freedom. | ||||||
Background
Heart failure with reduced ejection fraction (HFrEF) is associated with a high morbidity and mortality (Levy 2002; Najafi 2007). It is the most frequent cause of hospitalisation in people aged 65 years or older. Approximately 50% of people with severe heart failure die within five years of diagnosis (Levy 2002; Najafi 2007; Roger 2004). It has been well established that pharmacotherapy involving beta‐adrenergic blocking agents, angiotensin converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) can improve morbidity and survival (CIBIS II Investigators and Committees 2003; Cohn 2001; Dulin 2005; Freemantle 1999; Garg 1995; MERIT‐HF Study Group 1999; Packer 1996). These medications are recommended as first‐line therapy in Australian, National Heart Foundation & CSANZ 2011, and international, McMurray 2012 and Yancy 2013, guidelines for the management of patients with chronic heart failure. Studies have also found that there is a dose‐dependent relationship, with an improvement in left ventricular function as the dose increases (Bristow 1996; Simon 2003; Wikstrand 2002). Despite this evidence, many people diagnosed with heart failure receive suboptimal doses, and so are denied full benefits of therapy (Krum 2001; Phillips 2004). In light of the poor uptake of expert guidelines and the reluctance of primary care physicians to up‐titrate these medications (Phillips 2004), new strategies are required to fill this treatment gap.
Description of the condition
Heart failure is a clinical syndrome that arises due to the heart’s inability to pump an adequate volume of blood around the body to meet the metabolic demand of the tissues either at rest or during exercise (Francis 2001). The inadequate volume of blood is due to poor cardiac filling and/or impaired contractility and emptying. Compensatory mechanisms attempt to increase or maintain cardiac output through increasing blood volume (through redistribution of blood flow, heart rate, cardiac contractility, and cardiac muscle mass) (Francis 2001). After a period of time, despite the compensatory mechanisms, the heart begins to fail and the ability of the myocardium to contract and relax deteriorates, resulting in worsening of heart failure. Heart failure is a potential complication of nearly all types of heart disease (Givertz 2001). Classical symptomatology includes dyspnoea, fatigue, and exercise intolerance because of left ventricular dysfunction (Francis 2001; Laurent‐Bopp 2000).
There are two broad types of heart failure: heart with reduced ejection fraction (HFrEF), and heart failure with preserved ejection function (HFpEF). HFrEF refers to the inability of the ventricle to contract adequately to eject a volume of blood that meets the body’s metabolic demands (National Heart Foundation & CSANZ 2011). This is the most common form of heart failure. People with HFrEF have reduced ejection fraction and may be symptomatic (overt heart failure) or asymptomatic (covert heart failure) (Francis 2001). HFrEF is also often referred to as chronic heart failure, which describes the long‐term inability of the heart to meet metabolic demands. This is opposed to acute heart failure, which refers to exacerbations of chronic heart failure but also includes the initial hospitalisation for the diagnosis of heart failure (Francis 2001). Both forms of heart failure are a debilitating condition with a poor prognosis and are associated with a high hospital readmission and mortality rate.
Many people diagnosed with heart failure may be asymptomatic for a number of years. However, as their condition worsens, they may experience exertional dyspnoea, lethargy, dizziness, palpitations, ascities, peripheral oedema, and/or orthopnoea. Unfortunately, the lifetime risk of developing heart failure at 40 years of age is one in five for both men and women, and at 80 years remains at 20% despite the shorter life expectancy (Lloyd‐Jones 2002).
Description of the intervention
In the outpatient setting, optimisation of ACEIs, beta‐adrenergic blocking agents, and/or ARBs is usually done by cardiologists or the patient's primary care physician. However, this has resulted in prolonged and extracted time delays in patients reaching their optimal dose. This is mainly due to appointments in the cardiologist clinic being quarterly. Also, primary care physicians have been reluctant to up‐titrate these medications (Phillips 2004). Several strategies have been implemented to increase the uptake of evidence‐based pharmacotherapy for the management of HFrEF, in particular the titration of ACEIs, beta‐adrenergic blocking agents, and ARBs. However, this continues to be problematic. Several studies have investigated the utility of nurse‐led titration (NLT) of beta‐adrenergic blocking agents and ACEIs in hospital‐based clinics and reported an increase in utilisation rates of key therapeutic agents and in the number of patients receiving target doses (Gustafsson 2007; Jain 2005; Phillips 2005; Ryder 2003; Stromberg 2003).
How the intervention might work
NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs can occur in a hospital‐based outpatient clinic or in the community when the heart failure nurse visits the patient at home. In the outpatient clinic, the patient attends the clinic to visit the heart failure nurse. The nurse assesses the patient, reviews blood test results, and educates the patient and carer about heart failure. Based on the findings from the clinical assessment and blood test results, the heart failure nurse or nurse practitioner will titrate the beta‐adrenergic blocking agents or ACEI according to a predetermined protocol. Depending on hospital policy, the heart failure nurse may or may not consult with a cardiologist prior to titration of these medications. In the home visit setting, similar processes are undertaken as in the outpatient clinic but the heart failure nurse may or may not consult with a cardiologist over the telephone. Nurses that undertake optimisation of beta‐adrenergic blocking agents, ACEIs, and/or ARBs are advanced practice nurses and employed as a nurse practitioner or senior cardiac nurse. They must have institutional approval to titrate these medications. None of the studies described the training undertaken by the heart failure nurses. In clinical practice, provided the nurses are employed in an advanced practice role, no additional training is required.
Stromberg 2003 investigated the titration of medications in a nurse‐led heart failure clinic. They randomly assigned 106 participants to follow‐up in the nurse‐led heart failure clinic or to usual care, which was follow‐up in primary health care. The intervention consisted of an appointment in the nurse‐led heart failure clinic two to three weeks postdischarge. The clinic was staffed by specially educated and experienced cardiac nurses who were able to make protocol‐led changes to medications. The majority of participants had three to eight visits postdischarge. During each visit the nurse examined the participant to assess their heart failure status and treatment. The participant and their carer received education about heart failure and social support. At 12 months, the intervention group had fewer hospital admissions (33 versus 56, P = 0.047) and deaths (7 versus 20, P = 0.005) compared to the usual‐care group (Stromberg 2003). Another study by Driscoll 2011 examined the effect of NLT of beta‐adrenergic blocking agents in the community. Thirty‐three heart failure home visit nurses recruited 484 consecutive patients diagnosed with HFrEF. In this study, the heart failure nurses visited the participant in their home, assessed the participant for heart failure status and response to treatment, delivered education about heart failure, and provided social support to the participant and their carer (Driscoll 2011). In programs that enabled the heart failure nurse to titrate medications (14 programs and 229 participants), beta‐adrenergic blocking agents were adjusted according to a predetermined protocol. In the usual‐care programs (19 programs and 255 participants), all participants were visited by the heart failure nurse at home, but titration of medications was done by the primary care physician. Driscoll and colleagues (2011) found that patients participating in programs that allowed NLT were more likely to reach target dose (48% versus 36%, P = 0.05) and had lower all‐cause hospitalisations and mortality (hazard ratio 0.58, 95% confidence interval 0.42 to 0.81, P = 0.001) at six months compared to usual‐care group.
The adverse events associated with NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs are primarily due to inappropriate titration of these medications. The main symptoms are dizziness, bradycardia, deterioration in renal function, and abnormal serum electrolyte results. Two studies reported adverse events of NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs.
Why it is important to do this review
The optimisation of beta‐adrenergic blocking agents, ACEIs, and ARBs should result in a further improvement in patient outcomes. Observational studies have found an increase in utilisation rates and titration to optimal doses (Driscoll 2011; Jain 2005; Ryder 2003). However, no meta‐analysis or systematic review of randomised control trials has been done to investigate the utility of NLT to date. This review collated evidence of the benefits of NLT as an effective and safe strategy to ensure that patients receive the optimal benefits of beta‐adrenergic blocking agents, ACEIs, and ARBs through dose titration.
Objectives
We systematically reviewed the evidence from randomised controlled trials (RCTs) of NLT of ACEIs, beta‐adrenergic blocking agents, and ARBs in people with HFrEF for efficacy and risk of hospitalisations and mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that compared NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs with optimisation by another health professional in people with HFrEF. We considered parallel or cross‐over trials. We excluded uncontrolled and non‐randomised studies.
Types of participants
People aged 18 years or older diagnosed with symptomatic HFrEF and prescribed beta‐adrenergic blocking agents, ACEIs, and/or ARBs.
Types of interventions
NLT of ACEIs, beta‐adrenergic blocking agents, and/or ARBs. NLT refers to heart failure nurses or nurse practitioners, or both visiting the patient at home or in an outpatient clinic. The heart failure nurses have been delegated the responsibility for making protocol‐led changes in the dosage of beta‐adrenergic blocking agents, ACEIs, and ARBs. The nurse practitioners are able to titrate the medications as part of their scope of practice.
Comparison: Usual care, in which participants are under the management of a primary care physician who is responsible for titration of ACEIs, ARBs, and/or beta‐adrenergic blocking agents. We also included studies where the participant was under the management of a heart failure nurse who did not alter medication, but analysed these studies as a subgroup.
Types of outcome measures
Primary outcomes
-
All‐cause hospital admissions
-
Heart failure‐related hospital admissions
-
All‐cause mortality
-
All‐cause event‐free survival
Secondary outcomes
-
Time to maximum dose
-
Adverse events associated with titration of ACEIs, beta‐adrenergic blocking agents, and/or ARBs
-
Proportion reaching target dose of medications
-
Change in quality‐of‐life scores
-
Cost‐effectiveness
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for RCTs of nurse‐led titration of beta‐adrenergic blocking agents, ACEIs, and ARBs in people with heart failure:
-
The Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11 of 12, searched 19 December 2014, results: 120)
-
MEDLINE (Ovid, 1946 to November week 3 2014, searched 19 December 2014, results: 317)
-
EMBASE (Ovid, 1947 to 2014 week 50, searched 19 December 2014, results: 277)
We used the Cochrane Highly Sensitive Search Strategy for MEDLINE and an adaptation of it for EMBASE (Lefebvre 2011).
See Appendix 1 for details of the search strategies. We applied no date or language restrictions to any of the searches.
Searching other resources
We searched the following clinical trial registries: WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch) and ClinicalTrials.gov (www.clinicaltrials.gov) (searched 30 July 2015).
We searched full reference lists of all eligible papers and review articles to identify potential papers. We also searched the grey literature for unpublished theses and abstracts. We searched reference lists of heart failure guidelines (national and professional) and other relevant systematic review articles. We used Science Citation Index to forward search citations of key papers. We also contacted primary authors for additional information if required.
Data collection and analysis
Selection of studies
Using predetermined criteria outlined in this protocol, two review authors (AD & JC) independently assessed all titles and abstracts for eligibility. Both review authors are experts in NLT. If the title and abstract contained sufficient information to determine exclusion, the article was rejected.
Where an intervention or study type was not clear from the title and abstract, we obtained the full text of the paper. Two review authors (AD & JC) independently assessed the full text of all eligible papers. We reviewed any potential papers identified from reference lists of eligible papers and personal communication. We documented a list of rejected papers and reasons for rejection. Disagreements were resolved by discussion between the two review authors (AD & JC).
Data extraction and management
Two review authors (AD & JC) independently extracted data from all eligible studies using a predesigned data extraction form. The review authors (AD & JC) are experts in NLT and were not blinded to study authors or journals. Any disagreements were resolved by discussion between the two review authors (AD & JC).
We extracted information about participants (demographic data and severity of heart failure), sample size at baseline and follow‐up, medications (beta‐adrenergic blocking agents, ACEIs, and ARBs), method of titration (outpatient clinic, in‐hospital, community setting), comparison group (no intervention, usual care), length of follow‐up, descriptive statistics and inferential statistics of primary and secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (AD & JC) independently assessed the risk of bias using The Cochrane Collaboration's tool for 'Risk of bias' assessment (Higgins 2011). We assessed each study in terms of: selection bias (systematic differences between groups), performance bias (systematic differences in the care provided apart from the intervention being studied), attrition bias (systematic differences in withdrawals), and detection bias (systematic differences in outcome assessment). We judged the risk of bias in all studies (low risk of bias, high risk of bias, or unclear risk of bias). We presented this in a ‘Risk of bias' summary figure. In the NLT intervention, blinding of heart failure nurses and participants was not feasible and was not evaluated. However, blinding of the outcome assessor was evaluated.
Study quality was not a reason for exclusion from the whole review.
Measures of treatment effect
The measures of treatment effect for continuous variables were standardised mean difference, risk ratios for dichotomous variables with a 95% confidence interval, and hazard ratios for survival data. The number needed to treat (NNT) to benefit was also calculated.
Unit of analysis issues
The review included all RCTs where the participant was the unit of analysis and they were randomised to either the treatment or the control group. The number of observations matched the number of units randomised.
Dealing with missing data
Where data were missing from included studies, the review authors contacted the study authors to request the missing data. This included authors of published RCTs and/or unpublished studies with a published abstract. We contacted two authors of published abstracts (Doyon 2010; Guder 2015). One author provided us with the in‐press article, which has since been published (Guder 2015). We have included this in the review. The other author has not published her results, and we were unable to obtain a copy of her PhD thesis (Doyon 2010). We have excluded this abstract from the review due to the large amount of missing data.
Assessment of heterogeneity
We tested heterogeneity for statistical significance using the Q‐statistics with a 95% confidence interval and forest plots. We calculated the I2 statistic to determine the proportion of variability in the results due to heterogeneity. We performed Chi2 test and considered heterogeneity to be significant if P < 0.1. Where there was significant between‐study heterogeneity, we further interrogated the data for possible explanations.
Assessment of reporting biases
We included seven studies from the literature review in the analysis. Due to the limited number of included studies (less than 10), we did not use funnel plots to determine the risk of reporting bias.
Data synthesis
We pooled the outcome of homogenous studies in a meta‐analysis. We used a fixed‐effect model for the studies. We presented the overall effect sizes in a forest plot. We also performed sensitivity analyses in studies considered at risk of introducing bias; in trials where no intention‐to‐treat analysis was conducted; and in studies with a high rate of missing data and participant attrition.
In heterogeneous studies where I2 > 40%, we used a random‐effects model to determine if the conclusions were different. For time‐to‐event data, hazard ratios and 95% confidence intervals were converted to log rank observed minus expected events and variance of the log rank.
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was undertaken. There were no RCTs where the patient was managed by a heart failure nurse but the titration of medications was done by another health professional.
Sensitivity analysis
We performed sensitivity analysis to examine factors in the included studies that may lead to potential bias. We defined a good quality study as one with a low risk of bias in: adequate allocation concealment, blinding of outcome assessment, and data analysis based on intention‐to‐treat.We did not create funnel plots due to the low number of included studies.
Results
Description of studies
Results of the search
We identified a total of 1016 studies from the literature search. After reviewing the titles, we retrieved 100 abstracts for possible inclusion. Of these, we identified 18 full‐text articles for retrieval. From the full‐text articles we excluded 11 studies. We excluded one additional study late in the review as only the abstract was available, which did not include absolute numbers. We included seven articles in the final analysis.
The PRISMA flow diagram in Figure 1 summarises the selection of RCTs.

Study flow diagram.
Included studies
We included seven studies (1684 participants) comparing NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs with usual care. All of the included studies were RCTs. One study randomised health professionals into three arms comparing education about titration of beta‐blockers, NLT of beta‐blockers, and a computer‐generated alert about potential patients for titration of beta‐blockers (Ansari 2003). The other studies compared two arms of usual care and NLT of medications (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Hancock 2012; Sisk 2006; Stromberg 2003).
In one study, Doyon 2010, we retrieved information from the abstract. However, we have not included this study in the analysis as the abstract did not contain absolute values, only P values. This abstract was sourced from a conference abstract publication. We contacted the author to ascertain if the full study had been published; to date the original study has not been published, and we were unable to access the thesis.
Definition of heart failure
Inclusion criteria for all of the included studies included a definition of heart failure. However, the definition of heart failure varied between the studies. One study defined HFrEF as a left ventricular ejection fraction (LVEF) less than 45% and meeting the Framingham criteria for heart failure (Ansari 2003). Two studies defined HFrEF as a LVEF less than 40% (Driscoll 2014; Guder 2015). Three studies did not stipulate a numerical limit for LVEF (Hancock 2012; Sisk 2006; Stromberg 2003).
Study setting
The setting and type of nurse‐led medication titration service differed between the studies. Four studies were implemented in an outpatient clinic environment in a tertiary hospital (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Stromberg 2003). Ansari and colleagues (2003) implemented a NLT clinic in primary care (Ansari 2003). In one study optimisation of medications was done via telephone follow‐up according to a pre‐approved titration protocol (Sisk 2006). Hancock and authors developed a NLT service in a residential care facility (Hancock 2012).
Intervention
All of the included studies involved the optimisation of key medications for the treatment of heart failure. However, there was heterogeneity in the medication titrated. Three studies optimised beta‐adrenergic blockers (Ansari 2003; Driscoll 2014; Sisk 2006), and one of these studies also titrated diuretics and hydralazine (Sisk 2006). Two studies titrated beta‐adrenergic blockers and ACEIs (Hancock 2012; Stromberg 2003). Only two studies titrated all three medications: beta‐adrenergic blockers, ACEIs, and ARBs (Bruggink‐Andre de la Porte 2007; Guder 2015). Bruggink‐Andre De La and colleagues (2007) also titrated spironolactone.
Two studies stipulated specific beta‐adrenergic blockers, ACEIs, or ARBs to be titrated (Ansari 2003; Hancock 2012). Hancock and colleagues (2012) investigated the titration of ramipril and bisoprolol, which they stated was mainly due to cost and ease of titration. In this study a heart failure nurse specialist visited the residential care facility to optimise medications according to a pre‐approved protocol. In another study, carvedilol and metoprolol tartrate were the only medications titrated (Ansari 2003). In this study a nurse practitioner optimised the medications; no pre‐approved protocol was followed.
In two other studies (Driscoll 2014; Sisk 2006), the titration of medication was based on a key therapeutic group such as beta‐adrenergic blocking agents, ACEIs, or ARBs, rather than a specific medication. Optimisation of medications in Sisk 2006 was done by a registered nurse according to a pre‐approved protocol over the telephone. In other studies (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003), the titration of medications was done by a heart failure nurse specialist in an outpatient setting and according to a pre‐approved protocol.
Outcomes reported
Reported outcomes varied across the seven included studies. The length of follow‐up varied between studies. Two studies had a six‐month follow‐up (Driscoll 2014; Hancock 2012). Four studies had a 12‐month follow‐up (Ansari 2003; Bruggink‐Andre de la Porte 2007; Sisk 2006; Stromberg 2003), and Guder and colleagues (2015) followed up participants for 18 months (Guder 2015).
Five studies reported all‐cause hospitalisation (Ansari 2003; Driscoll 2014; Hancock 2012; Sisk 2006; Stromberg 2003), but of these, only four studies reported heart failure hospitalisation (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003).
All of the included studies reported all‐cause mortality except the study by Guder 2015. Four studies reported the outcome of heart failure‐related hospitalisation (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003). Only three studies reported all‐cause hospitalisation or mortality, or both (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003).
Regarding titration of beta‐adrenergic blocking agents, ACEIs, and ARBs, six studies reported on the percentage of participants who were prescribed these medications. Of these, three studies focused on only the titration of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Sisk 2006). Four studies reported the proportion of participants reaching target dose of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Hancock 2012; Stromberg 2003), with three studies reporting on their time to optimal dose (Ansari 2003; Driscoll 2014; Hancock 2012). One study reported on the number of participants receiving optimal dose of ACEIs (Hancock 2012), and no studies reported on optimal dose of ARBs.
Two studies reported adverse events associated with NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs (Driscoll 2014; Hancock 2012). One of these studies stated there were no adverse events (Driscoll 2014), and the other study found one adverse event but did not specify the type or severity of adverse event (Hancock 2012). Only two studies discussed the safety of NLT under the supervision of a cardiologist (Ansari 2003; Driscoll 2014). However, Ansari and colleagues did not report whether they had an adverse event related to the NLT intervention (Ansari 2003). Driscoll and colleagues reported that there were no safety issues related to the NLT intervention (Driscoll 2014).
One study reported on the maximal dose titrated of beta‐adrenergic blocking agents, ACEIs, and ARBs (Bruggink‐Andre de la Porte 2007). However, the authors did not comment on how this figure was calculated and whether it was a mean dose or median dose.
Two studies reported quality‐of‐life scores (Bruggink‐Andre de la Porte 2007; Sisk 2006), and both studies used the Minnesota Living with Heart Failure questionnaire.
No studies reported on the cost‐effectiveness of the NLT intervention.
More details of the included studies are described in Characteristics of included studies.
Excluded studies
We excluded two studies (Lowery 2012; Spaeder 2006). One study was a RCT, however both arms involved titration of beta‐adrenergic blocking agents (Spaeder 2006). The other study had a quasi‐experimental design (Lowery 2012).
Risk of bias in included studies
Figure 2 and Figure 3 display the review authors' judgements about the risk of bias across all of the included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We judged all of the studies to be at high risk of performance bias due to the inability to blind participants and personnel to group allocation.
Regarding random sequence generation, we judged six studies to be at low risk of bias as they discussed their randomisation process (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Hancock 2012; Sisk 2006; Stromberg 2003). All of the studies except Guder 2015 reported on all‐cause hospitalisations or mortality, or both, so there was a low risk of reporting bias for these outcomes. However, only two studies reported on adverse events associated with the intervention, so there was a high risk of reporting bias for this outcome.
Allocation
The risk of bias for allocation concealment was unclear in six studies as there was insufficient information to make a judgement (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Sisk 2006; Stromberg 2003).
Blinding
In all of the studies the risk of bias for blinding of participants and health professionals was high as this was not possible due to the nature of the intervention.
In three studies, the risk of bias of blinding of outcome assessment was unclear as there was insufficient information provided (Driscoll 2014; Guder 2015; Stromberg 2003). The other four studies all provided information about a researcher/cardiologist who was blinded to treatment allocation assessing outcomes (Ansari 2003; Bruggink‐Andre de la Porte 2007; Hancock 2012; Sisk 2006).
Incomplete outcome data
We assessed five studies as at unclear risk of bias in incomplete outcome reporting as no information was provided about incomplete outcome data (Ansari 2003; Driscoll 2014; Guder 2015; Hancock 2012; Stromberg 2003). We assessed two studies as at low risk as the authors discussed the management of missing data (Driscoll 2014; Sisk 2006).
Selective reporting
We assessed two studies as at low risk of bias of selective outcome reporting as all outcomes were reported.
We assessed five studies as at high risk of bias as adverse events associated with the intervention were not reported (Ansari 2003; Bruggink‐Andre de la Porte 2007; Guder 2015; Sisk 2006; Stromberg 2003).
Effects of interventions
The baseline characteristics of participants enrolled in the usual‐care and NLT groups were comparable in all of the studies (Ansari 2003; Bruggink‐Andre de la Porte 2007; Driscoll 2014; Guder 2015; Hancock 2012; Sisk 2006; Stromberg 2003). A summary of the main results of primary and secondary outcomes is described in the summary of findings Table for the main comparison.
Primary outcomes
All‐cause hospital admissions
Four studies reported on all‐cause hospitalisation (Ansari 2003; Driscoll 2014; Hancock 2012; Sisk 2006). The four studies were homogenous (I2 = 0%), with two of the studies showing wide confidence intervals due to small sample sizes (Driscoll 2014; Hancock 2012). Pooled analysis suggested that participants randomised to the NLT group had a 21% reduction in all‐cause hospitalisation (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.71 to 0.88) (Analysis 1.1). However, these results should be interpreted with caution as the pooled analysis was mainly influenced by one large study (weight 85%), Sisk 2006, due to the small sample sizes in the other studies.
Heart failure‐related hospital admissions
We included four studies in the pooled analysis (Ansari 2003; Hancock 2012; Sisk 2006; Stromberg 2003), which suggested that participants receiving NLT were 39% less likely to experience a heart failure‐related hospital admission (RR 0.51, 95% CI 0.36 to 0.72) (summary of findings Table for the main comparison). One study reported that no participants experienced a heart failure‐related hospital admission (Hancock 2012).
All‐cause mortality
Six of the seven included studies reported on all‐cause mortality. Pooled analysis suggested that participants in the NLT were 34% less likely to die (RR 0.66, 95% CI 0.48 to 0.92) (Analysis 1.3). However, one small study suggested that participants in the NLT group were more likely to die (Driscoll 2014). Driscoll and colleagues (2014) reported one death in the NLT group and none in the usual‐care group. Due to the size of this study (n = 24) and wide confidence interval (0.16 to 18.19), it should be interpreted with caution. As I2 was 15%, we did not consider the level of heterogeneity to be important; also, Chi2 and degrees of freedom were similar despite a P value of 0.31.
All‐cause event‐free survival
Three studies reported on event‐free survival (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Stromberg 2003). Participants in the NLT group were 40% more likely to remain event‐free compared to participants in usual care (RR 0.60, 95% CI 0.46 to 0.77).
Secondary outcomes
Time to maximum dose
Three studies reported on the time to maximal dose (Ansari 2003; Driscoll 2014; Hancock 2012). However, in one study (Hancock 2012), the time to maximal dose was reported for the NLT group and not for the usual‐care group. In the other two studies, the time to maximal dose was reduced in the NLT group compared to usual care. We did not undertake pooled analysis because in one study no standard deviation was reported (Ansari 2003). Driscoll 2014 was the only study to report the mean and standard deviation for both groups.
Adverse events associated with titration of ACEIs, beta‐adrenergic blocking agents, and/or ARBs
Two of the included studies reported on adverse events associated with NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs (Driscoll 2014; Hancock 2012). One study reported no adverse events (Driscoll 2014). The other study reported an adverse event in the NLT group (Hancock 2012), but they did not specify what type or severity of adverse event.
Proportion reaching target dose of medications
Five studies comparing NLT with usual care reported on the proportion of participants reaching maximal dose of beta‐adrenergic blocking agents (Ansari 2003; Driscoll 2014; Guder 2015; Hancock 2012; Stromberg 2003). Pooled analysis suggested a 99% improvement in participants reaching maximal dose (RR 1.99, 95% CI 1.61 to 2.47) (Analysis 1.5). We used a random‐effects model as there was a substantial degree of heterogeneity in the pooled analysis (I2 = 72%). Also, three of the studies had wide confidence intervals.
We planned a subgroup analysis of the proportion of participants receiving optimal dose of ACEIs and ARBs. One study reported on maximal dose of ACEIs and found that it was achieved in 100% of participants in the NLT group and 75% in the usual‐care group (Hancock 2012). Another study reported on the combined dose of ACEIs/ARBs (Guder 2015), finding the maximal dose of ACEI/ARBs in 39% of participants in the NLT group compared with 9% of participants in the usual‐care group.
Change in quality‐of‐life scores
Three studies reported on quality‐of‐life scores (Bruggink‐Andre de la Porte 2007; Driscoll 2014; Sisk 2006). All studies suggested an improvement in quality of life in participants in the NLT group.
Cost‐effectiveness
No studies reported on the cost‐effectiveness of NLT.
Discussion
Summary of main results
We included seven studies in the review. All of the included studies randomised participants diagnosed with HFrEF to NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs or to usual care, which was medication titration by their primary care physician. The meta‐analysis found evidence suggesting that NLT of beta‐adrenergic blocking agents, ACEIs, and/or ARBs may result in a significant reduction in hospital admissions, improvement in survival, increase in number of participants reaching optimal dose, and reduction in time to maximal dose (summary of findings Table for the main comparison). This will have an impact on clinical practice and NLT is one strategy that may be of benefit to patients diagnosed with heart failure.
Fewer participants in the NLT group experienced a hospital admission for any cause (RR 0.80, 95% CI 0.72 to 0.88) or heart failure‐related (RR 0.51, 95% CI 0.36 to 0.72) (summary of findings Table for the main comparison). The meta‐analysis on all‐cause hospitalisation included four studies of 560 participants, and we rated the quality of the evidence as high. There was a good overlap of confidence intervals in each of the studies, with 0% heterogeneity between studies. However, the results should be interpreted with caution, as 85% of the effect was due to a large study of 400 participants (Sisk 2006).
The pooled analysis of heart failure‐related hospitalisations should be interpreted with caution. We downgraded the quality of the evidence for heart failure‐related hospital admissions to moderate due to the overall large effect size and low event rate. We detected no heterogeneity between the studies.
Pooled analysis suggested that participants in the NLT group had a 34% reduction in mortality. If this estimate is correct, approximately 56 deaths could be avoided for every 1000 patients receiving NLT of key therapeutic agents. This was based on the assumed risk minus corresponding risk in the Summary of Findings table, in 902 participants from six studies. All of the studies were homogenous.
Participants in the NLT group experienced fewer hospital admissions and/or deaths compared to participants in the usual‐care group (RR 0.60, 95% CI 0.46 to 0.77). Despite the homogenous nature of the studies, we downgraded the quality of the evidence to moderate due to the large effect size and low event rate.
Participants in the NLT group were more likely to reach target dose of beta‐adrenergic blocking agents (RR 1.99, 95% CI 1.61 to 2.47) and in a shorter period of time compared to the usual‐care group. Pooled analysis of the number of participants reaching maximal dose of beta‐adrenergic blocking agents showed a substantial degree of heterogeneity, so we used a random‐effects model. One study was not statistically significant (Hancock 2012). This study (Hancock 2012) was conducted in a residential care facility. Prescribing guidelines recommend that caution be used when prescribing and titration of beta‐adrenergic blocking agents in the elderly. This may have impacted on the low number of participants reaching maximal dose. The age of participants in this study was higher than the ages of participants in the other three studies (Ansari 2003; Driscoll 2014; Guder 2015) (mean age 81 ± 7.1 years compared to a mean age of 70 years respectively).
We did not undertake a pooled analysis of time to maximal dose due to incomplete data.
Only two studies (Driscoll 2014; Hancock 2012) reported that there were no adverse events related to NLT. The other five studies did not report on adverse events.
Overall completeness and applicability of evidence
We included all eligible RCTs up to 19 December 2014. The demographic characteristics of participants within each study were comparable. Four of the included studies enrolled equal numbers of men and women compared to one study that enrolled 79% women. The mean age of participants ranged from 59 to 81 years, with five of the seven included studies enrolling participants with a mean age of 70 years. The majority of the participants were white. In one study (Sisk 2006), the majority of participants were Hispanic (78%).
Two of the studies had small sample sizes with wide confidence intervals so we could not be confident that there was not a type II error. A type II error occurs when one believes that no relationship exists when in fact there is a relationship. Larger sample sizes would reduce the risk of a type II error.
There was a large degree of heterogeneity in the pooled analysis of participants reaching target dose of beta‐adrenergic blocking agents. In this analysis, one study had a small sample size with a wide confidence interval (RR 1.23, 95% CI 0.29 to 5.25) (Driscoll 2014), and another small study had low event rates and was conducted in a residential care facility (Hancock 2012).
We could not conduct a pooled analysis of time to maximal dose due to incomplete data in two of the three studies. Two studies did report a significant reduction in time to maximal dose in the NLT group compared to the usual‐care group. However, this should be interpreted with caution as one study had a small sample and the other study did not report standard deviations.
Quality of the evidence
The findings of our review are limited by the quality of the included studies. Our review followed a peer‐reviewed published Cochrane protocol and the GRADE methodology to explore the quality of evidence. The initial literature search identified 714 studies, of which only seven were included in the meta‐analysis. We rated our primary outcome of all‐cause hospital admissions as a high quality of evidence. This was mainly due to the small confidence intervals, and further research is unlikely to have an effect on our confidence in the estimate of effect. There was a low quality of evidence in our third secondary outcome of the proportion of participants reaching maximal dose. This was attributable to a high risk of methodological bias, and two of these studies had a total sample size of less than 25 participants, resulting in wide confidence intervals. Further research involving large RCTs is warranted and will have an impact on our confidence in the estimate of effect and is likely to change our estimate.
Potential biases in the review process
We judged all of the included studies to be at high risk of performance bias. Unfortunately, with NLT it is not feasible to blind participants and study personnel to group allocation.
In six of the seven included studies selection bias was unclear as allocation concealment was not reported. We rated reporting bias as high as only two studies reported on adverse events associated with the intervention. We excluded one potential study as we were unable to access their thesis, so it is possible that we excluded an important study. We did not create funnel plots due to the low number of included studies (less than 10).
Agreements and disagreements with other studies or reviews
A systematic review or meta‐analysis on NLT in people diagnosed with HFrEF has not been previously undertaken.
Observational studies investigating NLT of beta‐adrenergic blocking agents, ACEIs, and ARBs have found results similar to this review. Several observational studies reporting on the feasibility of NLT clinics suggest an increase in utilisation and dosage of these key therapeutic medications (Gustafsson 2007; Jain 2005; Phillips 2005; Ryder 2003). One study investigated the feasibility of NLT in the community, such as during a home visit (Driscoll 2011). They found NLT to be a safe and cost‐effective strategy to increase utilisation and dosage in maximising the best benefit‐risk ratio for people with heart failure.
A quasi‐experimental study (Lowery 2012), excluded from our review, reported similar results. They suggested a reduction in all‐cause and heart failure‐related hospital admissions and mortality and an increase in prescribing of beta‐adrenergic blocking agents by a nurse practitioner.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Nurse‐led titration versus usual care, Outcome 1 All‐cause hospital admissions.

Comparison 1 Nurse‐led titration versus usual care, Outcome 2 Heart failure‐related hospital admissions.
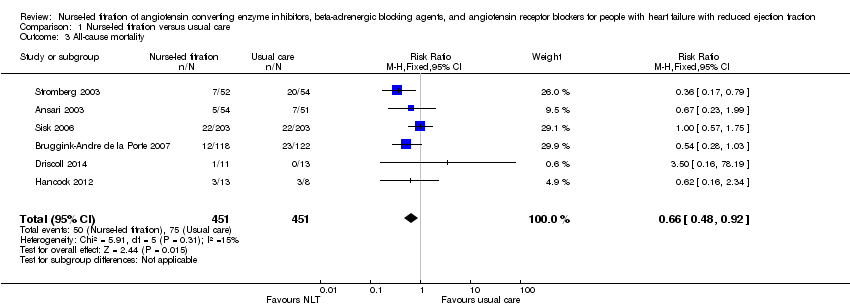
Comparison 1 Nurse‐led titration versus usual care, Outcome 3 All‐cause mortality.

Comparison 1 Nurse‐led titration versus usual care, Outcome 4 All‐cause event free survival.

Comparison 1 Nurse‐led titration versus usual care, Outcome 5 Proportion reaching target dose of medications.
| Nurse‐led titration versus usual care for people with heart failure with reduced ejection fraction | ||||||
| Patient or population: people with heart failure with reduced ejection fraction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nurse‐led titration versus usual care | |||||
| All‐cause hospital admissions | Study population | RR 0.80 | 560 | ⊕⊕⊕⊕ | ||
| 763 per 1000 | 610 per 1000 | |||||
| Moderate | ||||||
| 437 per 1000 | 350 per 1000 | |||||
| Heart failure‐related hospital admissions | Study population | RR 0.51 | 642 | ⊕⊕⊕⊝ | ||
| 248 per 1000 | 126 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 93 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.66 | 902 | ⊕⊕⊕⊝ | ||
| 166 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 163 per 1000 | 108 per 1000 | |||||
| All‐cause event‐free survival | Study population | RR 0.60 | 370 | ⊕⊕⊕⊝ | ||
| 487 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 385 per 1000 | 231 per 1000 | |||||
| Proportion reaching target dose of medications | Study population | RR 1.99 | 966 | ⊕⊕⊝⊝ | ||
| 171 per 1000 | 340 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 362 per 1000 | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1,2 I = 68% and P = 0.03 with a high Chi2 in relation to degrees of freedom. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause hospital admissions Show forest plot | 4 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.72, 0.88] |
| 2 Heart failure‐related hospital admissions Show forest plot | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| 3 All‐cause mortality Show forest plot | 6 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.92] |
| 4 All‐cause event free survival Show forest plot | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.77] |
| 5 Proportion reaching target dose of medications Show forest plot | 5 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.61, 2.47] |

