Preconception care for diabetic women for improving maternal and infant health
Abstract
Background
Infants born to mothers with pre‐existing type 1 or type 2 diabetes mellitus are at greater risk of congenital anomalies, perinatal mortality and significant morbidity in the short and long term. Pregnant women with pre‐existing diabetes are at greater risk of perinatal morbidity and diabetic complications. The relationship between glycaemic control and health outcomes for both mothers and infants indicates the potential for preconception care for these women to be of benefit. This is an update of the original review, which was first published in 2010.
Objectives
To assess the effects of preconception care in women with diabetes on health outcomes for mothers and their infants.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (31 January 2017) and reference lists of retrieved articles.
Selection criteria
Randomised controlled trials (RCTs) assessing the effects of preconception care for diabetic women. Cluster‐RCTs and quasi‐RCTs were eligible for inclusion but none were identified.
Data collection and analysis
Two reviewers independently assessed study eligibility, extracted data and assessed the risk of bias of the included studies. We checked data for accuracy and assessed the quality of the evidence using the GRADE approach.
Main results
We included three trials involving 254 adolescent girls with type 1 or type 2 diabetes, with an overall unclear to high risk of bias. The three trials were conducted at diabetes clinics in the USA, and assessed the READY‐Girls (Reproductive‐health Education and Awareness of Diabetes in Youth for Girls) programme versus standard care.
Considering primary outcomes, one trial reported no pregnancies in the trial period (12 months) (very low‐quality evidence, with downgrading based on study limitations (risk of bias) and imprecision); in the other two trials, pregnancy was an exclusion criterion, or was not clearly reported on. None of the trials reported on the other primary maternal outcomes, hypertensive disorders of pregnancy and caesarean section; or primary infant outcomes, large‐for‐gestational age, perinatal mortality, death or morbidity composite, or congenital malformations. Similarly, none of the trials reported on the secondary outcomes, for which we had planned to assess the quality of the evidence using the GRADE approach (maternal: induction of labour; perineal trauma; gestational weight gain; long‐term cardiovascular health; infant: adiposity; type 1 or 2 diabetes; neurosensory disability).
The majority of secondary maternal and infant outcomes, and outcomes relating to the use and costs of health services were not reported by the three included trials. Regarding behaviour changes associated with the intervention, in one trial, participants in the preconception care group had a slightly higher score for the actual initiation of discussion regarding preconception care with healthcare providers at follow‐up (nine months), compared with those in the standard care group (mean difference 0.40, 95% confidence interval ‐0.02 to 0.82 (on a scale of 0 to 4 points); participants = 87) (a summation of four dichotomous items; possible range 0 to 4, with 0 being no discussion).
Authors' conclusions
There are insufficient RCT data available to assess the effects of preconception care for diabetic women on health outcomes for mothers and their infants.
More high‐quality evidence is needed to determine the effects of different protocols of preconception care for diabetic women. Future trials should be powered to evaluate effects on short‐ and long‐term maternal and infant outcomes, and outcomes relating to the use and costs of health services. We have identified three ongoing studies that we will consider in the next review update.
PICOs
Plain language summary
Preconception care for women with diabetes to improve maternal and infant health
What is the issue?
The aim of this Cochrane review was to find out if giving women with diabetes specialised care before they become pregnant has an impact on their health, and on the health of their future babies. We collected and analysed all relevant studies to answer this question (date of search: January 2017).
Why is this important?
If a woman has type 1 or type 2 diabetes, and she becomes pregnant, she is at a greater risk of high blood pressure, and her baby has a greater risk of being born early (preterm ‐ before 37 weeks). In addition, her pregnancy makes it more likely she will develop one or more of the known complications of diabetes, such as heart disease, problems with the nervous system and eyesight problems. Babies born to mothers with type 1 or type 2 diabetes may be larger, and they have a higher risk of death and abnormality of the spinal column or brain. They are also at risk of developing type 2 diabetes in the long term.
Effective control of blood sugar level (glycaemic control) is part of diabetes care. The relationship between glycaemic control and better health outcomes for mothers and their babies indicates that specialist care before pregnancy (preconception care) could be of benefit. This involves education and support, and help with self‐monitoring of blood sugar levels, and self‐care.
We searched for studies which looked at preconception care in diabetes clinics.
What evidence did we find?
We found three randomised controlled trials, conducted at diabetes clinics in the USA. The total number of participants in the studies was 254. The participants were all adolescent girls involved in the programme READY‐Girls (Reproductive‐health Education and Awareness of Diabetes in Youth for Girls). Their care was compared with standard care.
None of these three trials gave us the information on the health outcomes we needed. In one trial, there were no pregnancies among the participants during the period of the study, and the other two trials’ reporting of pregnancy was not sufficient. There were no data about short and long term outcomes for the mothers and their babies, or about the use of the health service and related costs.
What does this mean?
Because the information is lacking, we have no evidence from this Cochrane review to guide practice on this topic. Further large, well‐designed, randomised controlled trials are required. Three trials are ongoing and will be considered in the next update of this review.
Authors' conclusions
Summary of findings
| Preconception care versus standard care for diabetic women for improving maternal and infant health: women's outcomes | ||||||
| Patient or population: adolescent girls with type 1 or type 2 diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with preconception care | |||||
| Pregnancy | Study population | not estimable | 109 | ⊕⊝⊝⊝ | No pregnancies reported in 1 RCT In 2 additional RCTs pregnancy was an exclusion criterion or was not clearly reported | |
| 0 per 1000 | 0 per 1000 | |||||
| Hypertensive disorders of pregnancy | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Caesarean section | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Perineal trauma | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Gestational weight gain | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Cardiovascular health | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Induction of labour | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The study had design limitations (‐1). | ||||||
| Preconception care versus standard care for diabetic women for improving maternal and infant health: child outcomes | ||||||
| Patient or population: adolescent girls with type 1 or 2 diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with preconception care | |||||
| Large‐for‐gestational age | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Perinatal mortality | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Death or morbidity composite | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Congenital malformations | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adiposity | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Type 1 or 2 diabetes | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Neurosensory disability | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
The system of glucose control by insulin can be disrupted in two ways. A problem with insulin release can occur, such as in type 1 diabetes mellitus. Alternatively, insulin may not act as effectively in promoting glucose uptake. This is known as insulin resistance, and is seen in type 2 diabetes mellitus and gestational diabetes mellitus. Pre‐existing diabetes refers to pregnant women who have previously been diagnosed with type 1 or type 2 diabetes mellitus, or other rare types of diabetes mellitus. Interconception care for women who have a history of gestational diabetes mellitus in a previous pregnancy is the topic of another Cochrane Review (Tieu 2013). Routine preconception care is evaluated in the Whitworth 2009 Cochrane Review.
Infant effects
It is widely acknowledged that pre‐existing diabetes in pregnant women is associated with increased risk of stillbirth, neonatal mortality and congenital anomalies (Abell 2016; Negrato 2012; Ornoy 2015). Major congenital malformations among infants born to women with pre‐existing diabetes may be found in the following systems: cardiovascular (e.g. cardiac transportation of the great arteries; ventricular septal defects), central nervous (e.g. neural tube defects), gastrointestinal (e.g. duodenal atresia), musculoskeletal (e.g. arthrogryposis), urinary tract (e.g. hydronephrosis), as well as caudal regression syndrome, cleft lips and palate anomalies (Negrato 2012).
Fetal hyperinsulinaemia associated with pre‐existing diabetes can contribute to neonatal morbidity in a similar way to its effect in gestational diabetes. As well as an increased risk of perinatal mortality, pre‐existing diabetes has been associated with adverse outcomes including macrosomia, large‐for‐gestational age, shoulder dystocia, neonatal hypoglycaemia, preterm birth, hyperbilirubinaemia, hypocalcaemia and neonatal intensive care unit admission (Abell 2016; Abell 2017; Negrato 2012).
Infants born to women with pre‐existing diabetes are also at an increased risk of developing obesity, type 2 diabetes and cardiovascular disease in the long term (Abell 2016; Negrato 2012). This effect is attributed to the intrauterine environment and genetic predisposition (Abell 2016; Negrato 2012). Infants born to diabetic women may also have an increased risk of long term adverse neurodevelopmental outcomes, such as gross and fine motor abnormalities, attention deficit hyperactivity disorder, learning difficulties, and possibly autism spectrum disorder (Ornoy 2015).
Maternal effects
Pre‐existing diabetes has been associated with adverse outcomes for women including caesarean section, pregnancy‐induced hypertension and pre‐eclampsia (Abell 2016; Negrato 2012; Ornoy 2015). Pregnancy may also accelerate the effects of diabetes on renal function in women with moderate to severe renal insufficiency and uncontrolled hypertension (Negrato 2012; Ornoy 2015). Pregnancy is also a risk factor for progression of diabetic retinopathy (Negrato 2012). Additionally, diabetes‐induced heart disease may worsen in women with pre‐existing diabetes during pregnancy (Ornoy 2015).
Description of the intervention
Education
Clinical guidelines have placed a strong emphasis on patient education on the risks of diabetes and pregnancy (ADA 2017; ADIPS 2005; NICE 2015; Thompson 2013). They explain the risks to both mother and infant, and the potential for these risks to be reduced through adequate glycaemic control, and they stress that women should continue contraception until target glycaemic control has been reached (ADA 2017; ADIPS 2005; NICE 2015; Thompson 2013).
Management of diabetes
Because of the strong association between glycaemic control, as measured by glycated haemoglobin (HbA1c), and congenital anomalies, glycaemic targets are central to preconception care. Glucose and HbA1c levels are achieved through adequate nutritional therapy, (short‐acting) insulin therapy and self‐monitoring of blood glucose concentrations (ADA 2017; ADIPS 2005; NICE 2015). Insulin is preferred for diabetes management due to limited evidence on the safety and efficacy of oral anti‐diabetic agents (ADA 2017; ADIPS 2005; NICE 2015). The use of an oral agent (e.g. metformin) may be suggested as an alternative to insulin therapy (NICE 2015). While aiming to achieve glycaemic targets, women are also warned to avoid hypoglycaemia.
Diabetes complication assessments
An assessment of diabetes‐related complications is commonly recommended as part of determining the progression of diabetes (ADA 2017; ADIPS 2005; NICE 2015). This includes investigations and examinations for retinal, renal and vascular complications (ADA 2017; ADIPS 2005; NICE 2015; Thompson 2013). These assessments may also provide an indication of how pregnancy may affect the progression of diabetes in the mother.
Multidisciplinary approach
While there is a strong focus on glycaemic control, preconception care is multidisciplinary. The healthcare team may include an obstetrician, endocrinologist or other physician with experience of diabetes in pregnancy, dietitian, diabetes educator and other health professionals as required (ADA 2017; ADIPS 2005). This approach is suggested to ensure adequate support from preconception through to the postnatal period in terms of diabetes and pregnancy complications but also to provide psychosocial support (ADA 2017).
Cost effectiveness
A recent study (estimating the preconception care‐preventable health and cost burden of adverse birth outcomes associated with diagnosed and undiagnosed diabetes preconception in the United States), suggested a substantial health and cost burden that could be prevented by universal preconception care, thus offsetting the cost of providing such care (Peterson 2015).
How the intervention might work
The exact pathogenesis of diabetes‐related pregnancy complications is believed to be multifactorial. While the risk of congenital malformations has been strongly associated with glycaemic control (with high glucose concentrations during critical periods of development believed to be the major teratogen in diabetic pregnancies), the precise mechanisms by which malformations occur have not been conclusively determined (Negrato 2012; Ornoy 2015). In addition to hyperglycaemia itself, hyperketonaemia, and disordered metabolism of arachidonic acid, myo‐inositol and prostaglandin, as well as increased oxidative stress, have all been associated with changes in embryonic development in the pregnancies of diabetic women (Negrato 2012; Ornoy 2015).
Glycaemic control in pregnancy
Glycaemic control can be measured in several ways, but is most commonly reflected by blood glucose concentrations or HbA1c levels. Blood glucose, while easy to measure, is highly variable and dependent on many factors. HbA1c, however, provides a picture of glycaemic control over a prolonged period of several weeks or months prior to the test and is, therefore, commonly considered a better indicator of overall glycaemic control. Both have been used in the preconception care of diabetic women, and a strong focus is often placed on achieving target HbA1c levels (ADA 2017; ADIPS 2005; NICE 2015; Thompson 2013).
In women with pre‐existing diabetes, pregnancy planning, and improved glycaemic control prior to and during pregnancy, have been associated with reductions in adverse outcomes, including pregnancy loss and congenital malformations (Kekäläinen 2016; Kitzmiller 2010; Pearson 2007). A systematic review and meta‐analysis of observational evidence supported an association between preconception care for diabetic women and improved glycaemic control (lower HbA1c in the first trimester of pregnancy), as well as reductions in congential malformations, preterm birth and perinatal mortality (Wahabi 2010). Thus, achieving and maintaining adequate glycaemic control is likely to be an important component of preconception care for diabetic women.
Why it is important to do this review
Type 1 and type 2 diabetes are becoming increasingly prevalent worldwide. With the potential adverse effects of diabetes on mothers and their infants, there is an urgent need for adequate management of diabetic women in the preconceptual period. This is an update of the original review, which was first published in 2010 (Tieu 2010).
Objectives
To assess the effects of preconception care in women with diabetes on health outcomes for mothers and their infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, quasi‐randomised controlled trials and cluster‐randomised trials were eligible for inclusion. Due to the nature of the topic being considered, cross‐over trials were not eligible for inclusion.
Types of participants
Women of reproductive age with diabetes mellitus (type 1 or type 2) who were not pregnant.
Types of interventions
Any protocol for preconception care of diabetic women compared with no preconception care, or one protocol of preconception care compared with another protocol of preconception care.
Types of outcome measures
For this update, we modified, as appropriate, the standard outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus (GDM) and pre‐existing diabetes.
Primary outcomes
For the woman
-
Pregnancy
-
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
-
Caesarean section
For the child
-
Large‐for‐gestational age
-
Perinatal mortality (stillbirth and neonatal mortality)
-
Death or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
-
Congenital malformations (e.g. neural tube defects including anencephaly, spina bifida)
Secondary outcomes
For the woman
All women (preconception ‐ before and after intervention commenced, antenatal, postnatal)
-
Adherence to the intervention
-
Behaviour changes associated with the intervention
-
Sense of well‐being and quality of life
-
Views of the intervention
-
Use of pharmacotherapy (insulin use; oral anti‐diabetic agent use)
-
Glycaemic control during/end of intervention (HbA1c or blood glucose)
-
Hypoglycaemia
-
Mortality
-
Diabetic complications (e.g. retinopathy, nephropathy, neuropathy, ischaemic heart disease, cerebrovascular disease, peripheral vascular disease)
-
Body mass index
If pregnant during trial period
-
Operative vaginal birth
-
Induction of labour
-
Perineal trauma
-
Placental abruption
-
Postpartum haemorrhage
-
Postpartum infection
-
Gestational weight gain
-
Breastfeeding (e.g. at discharge, six weeks postpartum)
Long‐term
-
Postnatal depression
-
Postnatal weight retention or return to pre‐pregnancy weight
-
Cardiovascular health (e.g. blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
For the child
Neonatal/infant
-
Abortion (spontaneous or therapeutic)/miscarriage
-
Stillbirth
-
Neonatal mortality
-
Postneonatal mortality
-
Gestational age at birth
-
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
-
Apgar score (less than seven at five minutes)
-
Macrosomia
-
Small‐for‐gestational age
-
Birthweight and z score
-
Length and z score
-
Head circumference and z score
-
Ponderal index
-
Adiposity
-
Shoulder dystocia
-
Bone fracture
-
Nerve palsy
-
Respiratory distress syndrome
-
Hypoglycaemia
-
Hyperbilirubinaemia
-
Hypocalcaemia
-
Polycythaemia
Later infant, childhood/adulthood
-
Weight and z scores
-
Height and z scores
-
Head circumference and z scores
-
Adiposity
-
Type 1 diabetes
-
Type 2 diabetes
-
Impaired glucose tolerance
-
Cardiovascular health (e.g. blood pressure, hypertension, cardiovascular disease, dyslipidaemia, metabolic syndrome)
-
Employment, education and social status/achievement
-
Neurosensory disability
Health service use
-
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
-
Number of antenatal visits or admissions
-
Length of antenatal stay
-
Neonatal intensive care unit admission
-
Length of postnatal stay (mother)
-
Length of postnatal stay (baby)
-
Costs to families associated with the management provided
-
Costs associated with the intervention
-
Cost of maternal care
-
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 January 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Cochrane Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
-
scoping searches of ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP).
Two people screen the search results and review the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Tieu 2010.
For this update, we used the following methods for assessing the 12 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two reviewers independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted a third reviewer.
Data extraction and management
We designed a form to extract data. For eligible studies, two reviewers extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third reviewer. We entered data into Review Manager 5 (RevMan 5) software (RevMan 2014) and checked them for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two reviewers independently assessed risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third reviewer.
(1) Random sequence generation (checking for possible selection bias)
We described for each included trial the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included trial the methods used, if any, to blind trial participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included trial the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included trial, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included trial how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the trial’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the trial’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; trial failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included trial any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update we planned to use the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison. The following outcomes are taken from the GRADE standard outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus (GDM) and pre‐existing diabetes.
For the woman
-
Pregnancy
-
Hypertensive disorders of pregnancy
-
Caesarean section
-
Perineal trauma
-
Induction of labour
-
Gestational weight gain
-
Cardiovascular health
For the child
-
Large‐for‐gestational age
-
Perinatal mortality
-
Death or morbidity composite
-
Congenital malformations
-
Adiposity
-
Type 1 or 2 diabetes
-
Neurosensory disability
We used GRADEpro Guideline Development Tool (GRADEproGDT) to import data from RevMan 5 (RevMan 2014) in order to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach, which uses five considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually randomised trials. In future updates of this review, if we include cluster‐randomised trials, we will adjust their sample sizes using the methods described in the Cochrane Handbook of Systematic Reviews of Interventions, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from another source. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the trial designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity and subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We planned to exclude any cross‐over trials due to the nature of the outcomes we are considering.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if we include more eligible studies, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to assess statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics. We planned to regard heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we had identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis (Deeks 2011).
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using RevMan 5 software (RevMan 2014). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. We planned to treat the random‐effects summary as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we planned to not combine trials. If we had used random‐effects analyses, the results would have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, we planned to use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses.
-
Intensity of intervention programme (how often, how long, type of health professional(s) ‐ trials would be characterised as reported and categorised where there was sufficient information)
-
Type of intervention programme (variations include supports ‐ individual or group intervention and partner support, mode ‐ verbal or written, content ‐ glycaemic control targets, dietary and lifestyle advice, etc)
We planned to use primary outcomes in subgroup analyses.
We planned to assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We planned to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² statistic value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
The updated searches identified 13 new trial reports. Following the application of the eligibility criteria, we included two new trials (Charron‐Prochownik 2013; Fischl 2010), excluded one new trial (Mathiesen 2012), and identified three new ongoing trials (NCT01788527; NTR2742; NCT02508779).
Previously, we had included one trial (Charron‐Prochownik 2008) and excluded two trials (DCCT 1996; Mathiesen 2007).
See: Figure 1.

Study flow diagram
Included studies
The three included studies were randomised controlled trials (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010).
Settings
All three trials were conducted at diabetes clinics in the USA, with dates of recruitment not reported (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010).
Participants
In total 254 participants were randomised in the three trials (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010). All trials recruited adolescent girls, between 13 and 19.9 years (Fischl 2010), 16 and 19.9 years (Charron‐Prochownik 2008) and 13 to less than 20 years (Charron‐Prochownik 2013), with either type 1 (Charron‐Prochownik 2008; Fischl 2010) or type 1 or 2 diabetes (Charron‐Prochownik 2013). Two trials did not report any exclusion criteria (Charron‐Prochownik 2013; Fischl 2010). Charron‐Prochownik 2008 excluded girls with pregnancies during the trial period.
Interventions
All three trials assessed the READY‐Girls (Reproductive‐health Education and Awareness of Diabetes in Youth for Girls) programme, which was modified over the course of the studies. First, Charron‐Prochownik 2008 assessed a CD‐ROM and book in two intervention groups, whereby participants received either a self‐instructional, developmentally appropriate, evidence‐based CD‐ROM or a book, as well as one comprehensive counselling session before a routine diabetes clinic visit, with three‐month follow‐up. Fischl 2010 then assessed the effects of a multiple session intervention of the READY‐Girls programme, with the intervention group viewing two CD‐ROMs (sessions one and two), reading a book version (session three), and having a brief counselling session with a research nurse during three consecutive diabetes clinic visits over a nine‐month period. In Charron‐Prochownik 2013, the programme was further modified, with the intervention group viewing two DVDs (sessions one and two), reading a book version (session three), with 12‐month follow‐up.
Outcomes
The three trials focused on similar outcomes related to reproductive health and preconception care knowledge, attitudes and beliefs, intentions and actual behaviours regarding initiating preconception counselling discussions, and preventing unplanned pregnancies (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010), though variably reported on additional outcomes, such as metabolic control (HbA1c). An additional report (Thurheimer 2016) presented pooled data from two of the trials (Charron‐Prochownik 2008; Fischl 2010) on general risk‐taking behaviours, condom use, and sexually transmitted infections. Outcomes were assessed at three‐ (Charron‐Prochownik 2008), nine‐ (Fischl 2010) and 12‐month follow‐up (Charron‐Prochownik 2013).
Funding and declarations of interest
All three trials reported funding support including from the American Diabetes Association (Charron‐Prochownik 2008; Fischl 2010), the General Clinical Research Center of the Children's Hospital of Pittsburgh (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010), the National Institutes of Health/National Institute of Nursing Research/Center for Research in Chronic Disorders (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010), and the Pediatric Clinical and Translational Research Center (Charron‐Prochownik 2013; Fischl 2010).
Charron‐Prochownik 2008 did not report on declarations of interest, and both Fischl 2010and Charron‐Prochownik 2013 reported that the authors had no conflicts of interest to declare.
Excluded studies
We excluded three trials (DCCT 1996; Mathiesen 2007; Mathiesen 2012), published as 12 reports, from this review. We excluded the Diabetes Complications and Control Trial (DCCT 1996), since all women planning pregnancy or who became pregnant received the same preconception care. Both Mathiesen 2007 and Mathiesen 2012 evaluated different types of insulin rather than preconception care.
Risk of bias in included studies
Allocation
We judged all three trials to be at unclear risk of selection bias overall. Both Charron‐Prochownik 2008 and Fischl 2010 did not report on methods for sequence generation or allocation concealment. Charron‐Prochownik 2013 reported that they used a 'minimisation algorithm' to generate the random sequence, but provided no further detail regarding how they had concealed allocation.
Blinding
As it was not feasible to blind participants or trial personnel (due to the nature of the interventions), we judged all three trials to be at high risk of performance bias (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010). We judged the three trials to be at unclear risk of detection bias, as they did not discuss whether it was possible to blind any of the outcome assessments (and/or the impact of lack of blinding of outcome assessments) (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010).
Incomplete outcome data
We judged one trial to be at low risk of attrition bias, with only one out of 88 of the participants (from the intervention group) dropping out over the course of the trial (Fischl 2010). We judged one trial to be at high risk of attrition bias, with data not reported for one out of 17 and five out of 20 participants in the intervention and control groups respectively (Charron‐Prochownik 2008). The third trial we judged to be at unclear risk of attrition bias, as, while complete data were provided for 109 out of 113 of the participants, the number randomised and lost from each group, and reasons for incomplete data, were not reported (Charron‐Prochownik 2013).
Selective reporting
We judged all three trials to be at unclear risk of selective reporting, with none of the trials providing access to a trial registration or published trial protocol to assess reporting bias (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010). None of the included trials provided the majority of data in a way that allowed us to extract them for inclusion in meta‐analyses (that is, mean values reported in figures, with no measures of variance).
Other potential sources of bias
Two of the trials did not provide baseline characteristics by group (Charron‐Prochownik 2008; Fischl 2010), while Charron‐Prochownik 2013 did report on some limited baseline characteristics, it was difficult to judge comparability, with small numbers in each group (for example, for the characteristic '≥ 1 episode unprotected sex', this was reported for 64% of intervention group participants versus 36% of control group participants).
Overall risk of bias
In general, assessment of the included trials (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010) for methodological quality revealed an unclear to high risk of bias (Figure 2; Figure 3). Allocation concealment, blinding and selective reporting biases were unclear.
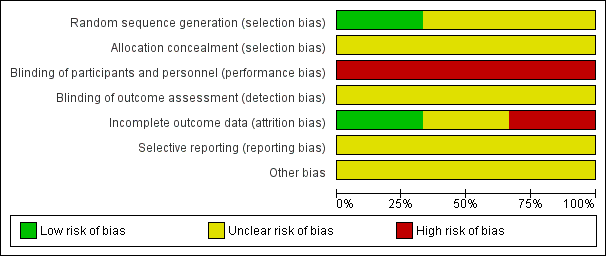
Methodological quality graph: reviewers' judgements about each methodological quality item presented as percentages across all included studies
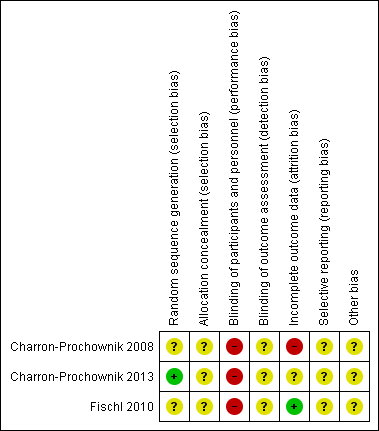
Methodological quality summary: reviewers' judgements about each methodological quality item for each included study
Effects of interventions
See: Summary of findings for the main comparison Preconception care versus standard care for diabetic women: outcomes for the woman; Summary of findings 2 Preconception care versus standard care for diabetic women: outcomes for the child
Preconception care versus standard care for diabetic women
Primary outcomes
For the woman
Pregnancy
Charron‐Prochownik 2008 excluded participants with pregnancies during the trial period. Fischl 2010 detailed that "Two participants reported a pregnancy," however it was not reported whether these were previous pregnancies, or pregnancies during the trial period, and from which trial group(s) the adolescent girls with pregnancies came. Charron‐Prochownik 2013 reported that there were no pregnancies among the adolescent girls throughout the trial period (12 months) (Analysis 1.1) (very low‐quality evidence).
As the three trials (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010) did not report on any pregnancies among their participants in the trial periods, there were no data available to report on the remaining review outcomes related to pregnancy, for women or their children.
Hypertensive disorders of pregnancy
None of the included trials reported on this outcome.
Caesarean section
None of the included trials reported on this outcome.
For the child
Large‐for‐gestational age
None of the included trials reported on this outcome.
Perinatal mortality
None of the included trials reported on this outcome.
Death or morbidity composite
None of the included trials reported on this outcome.
Congenital malformations
None of the included trials reported on this outcome.
Secondary outcomes
For the woman
Behaviour changes associated with the intervention
Fischl 2010 reported on the actual initiation of discussion with healthcare provider (a summation of four dichotomous items; possible range 0 to 4, with 0 being no discussion). Adolescent girls in the preconception care group had a higher score for the actual initiation of discussion at nine months, compared with girls in the standard care group (mean difference 0.40, 95% confidence interval ‐0.02 to 0.82; participants = 87; studies = 1; P = 0.06) (Analysis 1.2). In the text, it was reported that "Only the [intervention group] significantly increased over time with... actual initiation of discussion about (P 0.001) preconception counselling and reproductive health with their diabetes health care team... [intervention group] teens showed only a marginal effect in their consistency to use highly effective birth control methods over time compared with the [standard care] teens (P 0.10)".
Charron‐Prochownik 2008 detailed in text that "Actual behaviours (seeking [preconception counselling] and using effective family planning) had no significant group‐by‐time effect".
An additional report (Thurheimer 2016) detailed a secondary analysis of pooled data from Charron‐Prochownik 2008 and Fischl 2010 relating to general risk‐taking behaviours, condom use, and sexually transmitted infections (STIs) at three‐month follow‐up. While it was stated that 136 of the 141 participants from the two trials were included, it was also noted that "Attrition of 28 subjects (21%) occurred for the 3‐month follow‐up". It was not clear how many girls were in each group at follow‐up, and thus we could not include these data in meta‐analyses: "When the groups were compared over time, there were no significant interactions or main effects between them on sexual or other risk‐taking behaviors" ("ever" unprotected sex, P = 0.498; > 1 sex partner, P = 0.604; "ever" smoked cigarettes, P = 0.883; "ever" used alcohol, P = 0.572; "ever" used illicit drugs, P = 0.502); "When the groups were compared over time, there were no significant differences between them on most frequent birth control use" ("ever" used condoms, P = 0.179); "At 3 months, an additional subject in the RG‐C reported an STI... Therefore, there were no significant interactions or main effects between groups" ("ever" diagnosed with STI, P = 0.775).
Charron‐Prochownik 2013 reported on behaviour changes at 12 months, however it was not clear how many girls were in each group at follow‐up; thus we could not include these data in meta‐analyses: "[intervention group] participants, compared with [control group], showed a trend towards lower rates of overall sexual activity; less sexual debut (35 vs. 41%)… and increased abstinence (44 vs. 32%). Although not significant, these patterns were consistent. As expected over time, both groups showed an increase in becoming sexually active (X2 [3] = 18.36, P = 0.0004). There were no significant group, time, or group‐by‐time effects for abstinence. With regards to risk taking behaviors, there were no significant group‐by‐time effects of group or time differences at 12 months on the number of partners, unprotected sexual intercourse, or condom use; although fewer IG participants tended to engage in these risky behaviors at the 12‐month follow‐up visit (for example, 11% [n = 4] of CG vs. 0% of IG had multiple partners at 12 months)...none of the teens reported any actual [preconception counselling] seeking behavior to plan a pregnancy".
Views of the intervention
Charron‐Prochownik 2008 reported in text that "Both [the CD and the book] were rated (94‐100%) as having helpful, easy‐to‐understand information".
Glycaemic control during/end of intervention (HbA1c or blood glucose)
Charron‐Prochownik 2008 reported on mean percentage change in HbA1C from baseline to three months, however did not provide measures of variance, and thus we could not include these data in the review; the manuscript reports: "A1C had no significant group differences from baseline to 3‐month follow‐up (P = 0.134). However, those who received the CD had an average decrease of ‐1% compared with those who received the book (average increase 2%) and control subjects (average increase 8%)".
Outcomes not reported by included trials
None of the included trials reported on any of the other secondary outcomes for the woman pre‐specified in this review, including: adherence to the intervention; sense of well‐being and quality of life; use of pharmacotherapy; hypoglycaemia; mortality; diabetic complications; body mass index; operative vaginal birth; induction of labour; perineal trauma; placental abruption; postpartum haemorrhage; postpartum infection; weight gain during pregnancy; breastfeeding; postnatal depression; postnatal weight retention or return to pre‐pregnancy weight; cardiovascular health
For the child
Outcomes not reported by included trials
None of the included trials reported on any of the secondary outcomes for the child pre‐specified in this review, including: abortion/miscarriage; stillbirth; neonatal mortality; postneonatal mortality; gestational age at birth; preterm birth; Apgar score; macrosomia; small‐for‐gestational age; birthweight; length; head circumference; ponderal index; adiposity; shoulder dystocia; bone fracture; nerve palsy; respiratory distress syndrome; hypoglycaemia; hyperbilirubinaemia; hypocalcaemia; polycythaemia; childhood/adulthood weight; height; head circumference; adiposity; type 1 diabetes; type 2 diabetes; impaired glucose tolerance; cardiovascular health; employment, education and social status/achievement; neurosensory disability.
Health service use
Costs associated with the intervention
Fischl 2010 reported that "the READY‐Girls intervention cost [USD] $18 per participant. Thus, it would cost approximately $1,800 to deliver the program to 100 teens… one unplanned pregnancy for a teen in the U.S. would cost approximately $2,800… Thus, if pregnancy were prevented in 1 year for 100 teens, the program costs would be offset. If > 0.6 pregnancies were prevented in 1 year, the program would be cost‐saving".
Charron‐Prochownik 2013 detailed that "To determine cost‐effectiveness, we computed program delivery costs and compared the [intervention group] to [control group] on the probability of becoming pregnant. Self‐reported outcome measures included a weighted probability of becoming pregnant calculated for each subject at each time point using an algorithm on the effectiveness and frequency of their [birth control] methods used in the past 3 months. There appeared to be a trend in the direction of decreasing the probability of becoming pregnant for the [intervention group] teens and increasing for the [control group] teens (t = 1.715, P = 0.09)".
Outcomes not reported by included trials
None of the included trials reported on any of the other secondary outcomes relating to the use of health services pre‐specified in this review, including: number of hospital or health professional visits; number of antenatal visits or admissions; length of antenatal stay; neonatal intensive care unit admission; length of postnatal stay; costs to families associated with the management provided; cost of maternal care; cost of offspring care.
Discussion
Summary of main results
The effect of pre‐existing diabetes on health outcomes for mothers and their infants combined with observational evidence of the benefits of preconception care have prompted the implementation of preconceptual care for women with diabetes (ADA 2017; ADIPS 2005; NICE 2015; Thompson 2013). Various guidelines agree on the need for multidisciplinary care in the management of glycaemic control and diabetic complications. However, there is a lack of high‐quality, randomised evidence to support providing preconception care over standard care, or what type of care should be offered.
This review included three small randomised controlled trials, involving 254 adolescent girls with type 1 or type 2 diabetes.
Only one trial (Charron‐Prochownik 2013) reported on the maternal primary outcome of 'pregnancy', and reported no pregnancies in the preconception care or standard care groups across the 12‐month duration of the trial. One trial (Charron‐Prochownik 2008) excluded girls with pregnancies during the trial period, and one (Fischl 2010) did not clearly report on the outcome, pregnancy. Thus, none of the other maternal or child primary outcomes for this review were reported by the included trials. Regarding behaviour changes associated with the intervention, one trial (Fischl 2010) reported a higher score for the actual initiation of discussion at nine months for adolescent girls in the preconception care group, compared with those in the standard care group.
As it was not possible to extract outcome data in a format suitable for input into meta‐analyses, we took additional narrative results from the three included trials relating to the review outcomes, behaviour changes associated with the intervention, views of the intervention, glycaemic control, and costs associated with the intervention.
Regarding behaviour changes: Charron‐Prochownik 2008, reported no clear differences between groups for seeking preconception counselling and using effective family planning; in Fischl 2010, there was no clear difference for consistency to use highly effective birth control methods over time between groups; and Charron‐Prochownik 2013 showed no clear differences between groups for overall sexual activity, sexual debut and abstinence, nor for risk‐taking behaviours (number of partners, unprotected sexual intercourse, condom use). Considering views of the intervention, the adolescent girls receiving preconception care in Charron‐Prochownik 2008 considered both the CD and book resources to be helpful and easy to understand. In regards to glycaemic control, Charron‐Prochownik 2008 reported no clear difference between groups in HbA1c over time. Finally, considering costs associated with the intervention, Fischl 2010 reported that the intervention would be 'cost‐saving' if at least 0.6 pregnancies were prevented in one year (based on the intervention costing USD 18 per participant, and one unplanned pregnancy costing approximately USD 2800 in the USA); and in relation to cost‐effectiveness Charron‐Prochownik 2013 detailed a 'trend' in the direction of decreasing the probability of becoming pregnant for the preconception care group, and increasing the probability for the standard care group.
Overall completeness and applicability of evidence
The evidence is limited in terms of the number of trials available, and in the lack of data reported for the majority of outcomes pre‐specified for this review. The trials were all conducted at diabetes clinics in the USA with adolescent girls with type 1 or type 2 diabetes, and assessed the READY‐Girls (Reproductive‐health Education and Awareness of Diabetes in Youth for Girls) programme (Charron‐Prochownik 2008; Charron‐Prochownik 2013; Fischl 2010). Thus, the results are unlikely to be applicable to all settings or countries worldwide.
Trials involving preconception care are difficult to design and implement. For example, it is important to consider that outcomes applicable for women receiving preconception care vary significantly depending on whether or not they become pregnant and consequently outcomes for their infants. Future research should not only aim to include health outcomes for mothers and their infants during and after pregnancy, but also collect data on long‐term outcomes for both. Outcomes should also include the views of women on the preconception care and the cost‐effectiveness of the intervention. The outcomes important to measure in mothers with pre‐existing diabetes in pregnancy can have very small prevalence rates, requiring trials to have very large numbers of women. Furthermore, the intensity of care required in multidisciplinary preconception care and long‐term follow‐up present further hurdles in trial design.
Given the recommendation for preconception care from various guidelines and the existing observational evidence, it may be inappropriate for sites with a preconception care programme as standard to offer a no preconception care arm. It may still be possible, however, to evaluate the provision of preconception care compared with standard care where no preconception care programme exists. Future trials should also consider comparing different protocols of preconception care, which would be appropriate for sites where preconception care is provided as standard care.
Quality of the evidence
Overall, we judged the risk of bias of the included studies to be unclear to high due to lack of key methodological information.
We assessed the quality of the evidence for one outcome only, pregnancy, and we judged it to be very low. Downgrading was due to trial limitations (risk of bias) and imprecision (summary of findings Table for the main comparison). None of the other outcomes selected for judgement using GRADE had reported data.
Potential biases in the review process
A detailed, systematic search was conducted by Cochrane Pregnancy and Childbirth's Information Specialist, without language or publication status restrictions. It is possible that additional trials assessing preconception care for diabetic women have been published but not identified; or that further trials have been conducted but are not yet published. Should any such studies be identified, we will include them in future updates of this review.
In order to minimise bias throughout the review process, two reviewers independently assessed eligibility for inclusion, extracted data and assessed risk of bias.
Agreements and disagreements with other studies or reviews
We identified one additional systematic review of preconception care for diabetic women for improving maternal and fetal outcomes (Wahabi 2010). This systematic review included one controlled trial (DCCT 1996, which we excluded from our review), 11 prospective cohort studies, seven retrospective cohort studies, and one case control study (Wahabi 2010). Meta‐analyses of the included observational studies suggested reductions in HbA1c in the first trimester of pregnancy, congenital malformations, preterm birth and perinatal mortality with preconception care.
We also identified a review of the quality and content of clinical guidelines concerned with preconception care of women with diabetes (Mahmud 2010). This review included five guidelines (all judged to be high quality), and determined these to be consistent in their recommendations, including about the risks of congential malformation related to uncontrolled glucose preconceptually, use of contraception prior to achieving adequate glycaemic control, the use of HbA1c to monitor glycaemic control, and when to commence insulin (Mahmud 2010). Differences in recommendations related to optimal targets for metabolic control, and the use of metformin preconceptually and during pregnancy (Mahmud 2010).

Methodological quality graph: reviewers' judgements about each methodological quality item presented as percentages across all included studies

Methodological quality summary: reviewers' judgements about each methodological quality item for each included study

Comparison 1 Preconception care versus standard care for diabetic women, Outcome 1 Pregnancy.
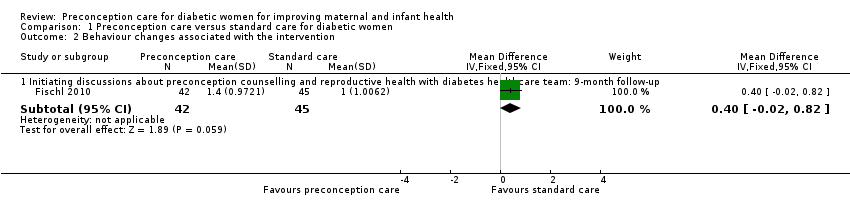
Comparison 1 Preconception care versus standard care for diabetic women, Outcome 2 Behaviour changes associated with the intervention.
| Preconception care versus standard care for diabetic women for improving maternal and infant health: women's outcomes | ||||||
| Patient or population: adolescent girls with type 1 or type 2 diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with preconception care | |||||
| Pregnancy | Study population | not estimable | 109 | ⊕⊝⊝⊝ | No pregnancies reported in 1 RCT In 2 additional RCTs pregnancy was an exclusion criterion or was not clearly reported | |
| 0 per 1000 | 0 per 1000 | |||||
| Hypertensive disorders of pregnancy | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Caesarean section | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Perineal trauma | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Gestational weight gain | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Cardiovascular health | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Induction of labour | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The study had design limitations (‐1). | ||||||
| Preconception care versus standard care for diabetic women for improving maternal and infant health: child outcomes | ||||||
| Patient or population: adolescent girls with type 1 or 2 diabetes | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with standard care | Risk with preconception care | |||||
| Large‐for‐gestational age | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Perinatal mortality | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Death or morbidity composite | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Congenital malformations | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Adiposity | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Type 1 or 2 diabetes | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Neurosensory disability | Study population | not estimable | (0 studies) | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pregnancy Show forest plot | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Behaviour changes associated with the intervention Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Initiating discussions about preconception counselling and reproductive health with diabetes healthcare team: 9‐month follow‐up | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.02, 0.82] |

